US7353823B2 - Powder chemical feeding device for nasal cavity - Google Patents
Powder chemical feeding device for nasal cavityDownload PDFInfo
- Publication number
- US7353823B2 US7353823B2US10/512,857US51285705AUS7353823B2US 7353823 B2US7353823 B2US 7353823B2US 51285705 AUS51285705 AUS 51285705AUS 7353823 B2US7353823 B2US 7353823B2
- Authority
- US
- United States
- Prior art keywords
- capsule
- medicine
- pump
- air
- housing
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Expired - Fee Related, expires
Links
Images
Classifications
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M15/00—Inhalators
- A61M15/0028—Inhalators using prepacked dosages, one for each application, e.g. capsules to be perforated or broken-up
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M15/00—Inhalators
- A61M15/08—Inhaling devices inserted into the nose
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M13/00—Insufflators for therapeutic or disinfectant purposes, i.e. devices for blowing a gas, powder or vapour into the body
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M15/00—Inhalators
- A61M15/0028—Inhalators using prepacked dosages, one for each application, e.g. capsules to be perforated or broken-up
- A61M15/003—Inhalators using prepacked dosages, one for each application, e.g. capsules to be perforated or broken-up using capsules, e.g. to be perforated or broken-up
- A61M15/0033—Details of the piercing or cutting means
- A61M15/0038—Cutting means
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M15/00—Inhalators
- A61M15/0028—Inhalators using prepacked dosages, one for each application, e.g. capsules to be perforated or broken-up
- A61M15/003—Inhalators using prepacked dosages, one for each application, e.g. capsules to be perforated or broken-up using capsules, e.g. to be perforated or broken-up
- A61M15/0033—Details of the piercing or cutting means
- A61M15/004—Details of the piercing or cutting means with fixed piercing or cutting means
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M2202/00—Special media to be introduced, removed or treated
- A61M2202/06—Solids
- A61M2202/064—Powder
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M2205/00—General characteristics of the apparatus
- A61M2205/07—General characteristics of the apparatus having air pumping means
- A61M2205/071—General characteristics of the apparatus having air pumping means hand operated
- A61M2205/075—Bulb type
Definitions
- the present inventionconcerns an optimal device to deliver a powdery medicine into the nasal cavity.
- a treatment method to deliver a powdery medicine into the nasal cavity of a patient suffering from asthma or nasal allergyhas been known generally.
- a powdery medicine filled in a capsuleis administered into the nasal cavity using a special delivery device.
- JP-A No. 59-34267(hereinafter referred to as prior art) has proposed a delivery device used for the treatment.
- the device of the prior artcomprises a cylindrical member having a pump on the air inlet and a concave part in which a capsule is inserted on the air exit of the cylindrical member.
- a top end partis fitted into the concave part to form a capsule housing part, and an air guide passage having a valve mechanism is formed from the capsule housing part to the pump.
- Another valve mechanismis provided to the other side of the pump, and air is supplied to the capsule housing part through the air guide passage having a valve mechanism upon pressing of the pump, and external air is sucked into the pump through the another valve upon removal of the pump pressure.
- the cylindrical memberhas, at its top end, a cap fitted to the top end and a needle is extended axially in the cap so as to perforate both axial ends of the capsule by engaging the cap in a state of fitting the concave part of the cylindrical member and the top end having an opening.
- the capsuleafter inserting the capsule filled with a powdery medicine into the concave part of the cylindrical member, the capsule is inserted and fixed in the capsule housing portion by fitting the top end. Then a cap is fitted to the top end made of a hard resin to perforate both axial top ends of the capsule by a needle built inside the cap and guided to the top end.
- a userdetaches the cap from the cylindrical member and inserts the top end into one of the nasal nostril and presses the pump. Then, when the pump is pressed, air from the pump flows through the air guide passage into the capsule to deliver the medicine in the capsule to the nasal cavity of the user. Further, by repeating the operations for both nasal cavities, the medicine is dosed to both of the nasal cavities.
- the capsuleis perforated by a needle built in the detachable cap and then the pump is pressed to deliver and dose the medicine in the capsule to the user's nasal cavity.
- the medicinewould remain in the capsule even after the frequent pump actuation, to result in a problem failing to deliver at an adequate dose for a user.
- the subject of the present inventionis to provide a device to deliver a powdery medicine into the nasal cavity capable of overcoming such a problem.
- a device to deliver a powdery medicine into the nasal cavitycomprises a capsule housing/holding part for housing and holding a capsule filled with a powdery medicine, a pump installed in the capsule housing/holding part for supplying dosing air to the capsule housing/holding part, a medicine delivery part installed in the capsule housing/holding part for delivering and dosing the medicine in the capsule from the pump to the nasal cavity of a user, and a one-way valve built in the air flow passage an opening pressure of which is controlled by a spring for separating and dispersing the medicine in the capsule by the airflow rectification in the capsule resulting from the entrance of one end thereof on the capsule side into the capsule upon the pump actuation.
- the device to deliver powdery medicine into nasal cavitycomprises a one-way valve as a movable member for separating and dispersing a medicine in a capsule which is actuated upon medicine spraying to the capsule housing/holding part and such a one-way valve can completely prevent falling and backward flowing of the medicine after perforation from falling and backwardly flowing into the pump and can deliver and dose a prescribed amount of the medicine filled in the capsule reliably to the nasal cavity of the user.
- FIG. 1is a side elevational view illustrating an embodiment of a powdery medicine delivery device according to the present invention.
- FIG. 2is a cross sectional view illustrating an embodiment of a powdery medicine delivery device according to the present invention.
- FIG. 3is a detailed view for a portion of FIG. 2 .
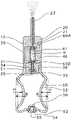
- FIG. 4is a view showing a state of drawing out a capsule setting/detaching part and placing a capsule therein in the embodiment described above.
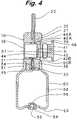
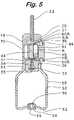
- FIG. 5is a cross sectional view showing a state in which cutting blades are cutting capsule ends in the course where the capsule setting/detaching part with the capsule placed therein is inserted into the capsule housing/holding part.
- FIG. 6is a cross sectional view showing a state in which the capsule ends are cut off by cutting blades, and the medicine in the capsule upon completion of perforation is falling and flowing backwardly to the pump in the embodiment described above.
- FIG. 7is a cross sectional view in a state where the medicine in the capsule is under delivery and dosing by pressing the pump in the embodiment described above.
- FIG. 8is a detailed for a portion of FIG. 7 .
- FIG. 1 to FIG. 8An embodiment of the present invention is shown in FIG. 1 to FIG. 8 .
- FIG. 1is a side elevational view of an embodiment of a device to deliver a powdery medicine into the nasal cavity according to the present invention
- FIG. 2is a cross sectional side elevational view of a device to deliver a powdery medicine into the nasal cavity shown in the embodiment
- FIG. 3is a detailed view for FIG. 2 .
- a device 10 to deliver a powdery medicine into the nasal cavitycomprises, generally, a capsule housing/holding part 30 for housing and holding a capsule K together with a medicine delivery part 20 , a capsule setting/detaching part 40 built drawably in the capsule housing/holding part 40 and a pump 50 installed in the air inlet side of the capsule housing/holding part 30 for supplying air to the capsule, and cutting blades 60 a , 60 b situated at both axial ends of the capsule K of the capsule setting/detaching part 40 of the capsule housing/holding part 33 for perforating both axial ends of the capsule K by setting/detaching operation of the capsule setting/detaching part 40 .
- a medicine passage 21is built in an upper part (air exit side) with respect to the axial direction of the capsule K of the capsule housing/holding part 30 , and a nozzle 22 made of a flexible tube is formed to the top end of the medicine passage 21 .
- a one-way valve 30is built to prevent falling and backward flowing of the powdery medicine from the capsule K to the pump 50 .
- the one-way valve 33is adapted to prevent back flow of air such that it opens when the pressure of air from the pump 50 reaches at or higher than a prescribed pressure and closes the air flow inlet 35 when the pressure of air from the pump 50 is lower than the prescribed pressure.
- the one-way valve end 71 as the end of the one-way valve 33 on the side of the capsule Kis below the surface where the cutting blade 60 B is in contact with the capsule K (on the side of the pump 50 ) when the one-way valve 33 is opened and does not interfere the setting/detaching operation of the capsule setting/detaching part 40 and the capsule K, whereas it intrudes as far as the inside of the capsule K when the pump 50 is pressed and the one-way valve 33 is opened upon dosage to be described later.
- the capsule setting/detaching part 40has a capsule attaching/detaching recess 41 at a position of attaching or detaching the capsule K, such that it can be set and detached drawably in the lateral direction with respect to the axial direction of the capsule K to the capsule housing/holding part 30 and a drawing end 44 of the capsule setting/detaching part 40 is regulated by the abutment of the detaching end 44 to the protrusion 37 built in the capsule housing/holding part 30 .
- the inlet endis regulated by the abutment of a setting/detaching protrusion 45 of the capsule setting/detaching part 40 against the abutment face 36 of the capsule housing/holding part 30 .
- the pump 50is formed from a resilient rubber material into a bottomed cylindrical shape having an attaching part 51 , a bottom 52 and a pressing part 53 at the circumferential surface.
- the attaching part 51is mounted sealingly to the cylindrical outer circumferential surface of the capsule housing/holding lower part 38 of the capsule housing/holding part 30 , and an air inlet valve 54 is attached to a central portion of the bottom 52 .
- the air intake valve 54produced using a resilient rubber material and comprises an air inlet form 55 and an inlet valve body 56 .
- the valveis closed when the pump 50 is pressed, while the valve is opened upon restoration of the pump 50 after pressing to supply external air to the pump 50 .
- the device to deliver a powdery medicine into the nasal cavity according to this embodimenthas been constituted as described above. Then, description is to be made to an operation upon perforation of the capsule with reference to FIG. 4 to FIG. 6 .
- the capsule Kis placed in the capsule attaching/detaching concave 41 of the capsule setting/detaching part 40 and the capsule setting/attaching end face 46 of the capsule setting/detaching part 40 is pressed so as to intrude the capsule setting/detaching part 40 into the capsule housing/holding part 30 .
- the capsule setting/detaching end face 46 of the capsule setting/detaching part 40is pushed to abut the setting/detaching protrusion 45 against the abutment surface 36 of the capsule housing/holding part 30 , the capsule K that was already perforated both axial ends pass completely through to the medicine passage 21 of the medicine delivery part 20 and the air flow passage 31 of the capsule housing/holding part 30 to be in a state ready for dosing the medicine.
- the medicine in the capsule K perforated both axial ends thereoffalls toward to the airflow passage 31 . Since the one-way valve 33 is closed by the function of the spring 34 , the medicine is prevented from falling and flowing backwardly to the pump 50 .
- holesare easily perforated at both axial ends of the capsule K only by the operation of housing the capsule K in the device 10 to deliver a powdery medicine into the nasal cavity and falling and backward flowing of the medicine to the pump after perforation is completely prevented by the one-way valve 33 .
- the pressure of air loaded on the one-way valve 33increases and, when it reaches to a predetermined pressure, the one-way valve 33 is opened in which air is supplied from the pump 50 through the one-way valve 33 and the air flow passage 31 to the capsule K.
- air from the pump 50flows from the inside of the capsule K by way of the medicine passage 21 and the nozzle 22 to the nasal cavity of the user.
- the one-way valve end 71is at a position detaching from the air flow passage 31 and entering in the capsule K and, since the valve reduced diameter part 72 is within the air flow passage 31 , it does not hinder the air flow.
- a portion of air passing the air flow passage 31hits on the flow control surface 72 succeeding to the valve reduced diameter part 73 to generate an air flow along the inner surface of the capsule K and an air flow agitating the inside of the capsule K thereby separating and dispersing the medicine coagulated in the capsule K or deposited to the inner surface of the capsule K, which is delivered and dosed to the user's nasal cavity together with other air and medicine.
- the medicine that dropped and flowed backwardly upon perforation as far as the one-way valve 33is sent by air from the pump 50 , and delivered and dosed together with the medicine in the capsule K to the nasal cavity of the user.
- a prescribed amount of the medicine filled in the capsule Kcan be reliably delivered and dosed to the user's nasal cavity.
- pressure of air loaded on the one-way valve 33is weakened and when it becomes lower than a predetermined pressure to open the one-way valve 33 , the one-way valve is closed.
- airstill flows from the pump 50 to the capsule K. Accordingly, the medicine in the capsule K and the air flow passage 31 does not fall and flow backwardly to the pump 50 and falling and backward flow of the medicine to the pump 50 can be prevented reliably.
- the pressing part 53 of the pump 50 having the rubber resiliencyrestores in the direction shown by an arrow R to cause a negative pressure in the pump 50 , so that the intake valve body 56 of the air intake valve 54 is opened by the pressure of the external air and air flows into the pump 50 from the outside by way of the air intake hole 55 to restore the pressing portion of the pump 50 to an original state as shown by dotted chains.
- the capsule housing/holding part 30comprises the one-way valve 33 for preventing falling and backward flowing of the powdery medicine falling and flowing backwardly from the capsule K to the pump 50 .
- the one-way valve 33is adapted such that it opens when the pressure of air from the pump 50 reaches a predetermined pressure, where the one-way valve end 71 detaches from the air flow channel 31 and enters in the capsule, and the valve reduced diameter part 73 is within the air flow passage 31 , so that it does not hinder the air flow.
- the one-way valve end partis equipped with the end of the one-way valve on the side of the capsule the opening pressure of which is controlled by the spring between the capsule housing/holding part and the pump for preventing falling and backward flow of the medicine falling and flowing backwardly from the capsule after perforation from falling and flowing backwardly to the pump and dosing such medicine together with the medicine in the capsule by the pump actuation
- air passing through the air flow passageabuts against the flow control surface of the one-way valve succeeding to the valve reduced diameter part to generate an air flow along the inner surface of the capsule and an air agitating the inside of the capsule to separate and disperse the medicine deposited to the inner surface of the capsule or the medicine coagulated in the capsule and they can be delivered and dosed to the nasal cavity of the user including the thus separated and dispersed medicine falling and flowing backwardly as far as the one-way valve together with air.
- the present inventioncan provide advantageous effects capable of resolving the problem for the occurrence of residues of medicine in the capsule in a case of using a medicine with poor flowability or separability or a medicine tending to deposit to the inner surface of the capsule due to static charges generated in the capsule, which was difficult to be solved in the prior art, and capable of reliably delivering and dosing a prescribed amount of the medicine filled in the capsule to the nasal cavity of the user.
Landscapes
- Health & Medical Sciences (AREA)
- Engineering & Computer Science (AREA)
- General Health & Medical Sciences (AREA)
- Public Health (AREA)
- Heart & Thoracic Surgery (AREA)
- Hematology (AREA)
- Life Sciences & Earth Sciences (AREA)
- Animal Behavior & Ethology (AREA)
- Anesthesiology (AREA)
- Biomedical Technology (AREA)
- Veterinary Medicine (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Pulmonology (AREA)
- Otolaryngology (AREA)
- Infusion, Injection, And Reservoir Apparatuses (AREA)
- Medicinal Preparation (AREA)
Abstract
Description
- 10 device to deliver powdery medicine into nasal cavity
- 20 medicine delivery part
- 21 medicine passage
- 22 nozzle
- 30 capsule housing/holding part
- 31 air flow passage
- 33 one-way valve
- 34 spring
- 35 air flow inlet
- 36 abutment surface
- 37 protrusion
- 38 lower portion of the capsule housing/holding part
- 40 capsule setting/detaching part
- 41 capsule attaching/detaching concave part
- 42A,42B capsule cut end discharge part
- 44 drawing end
- 45 protrusion for setting/detaching part
- 46 end face for capsule setting/detaching part
- 50 pump
- 51 attaching part
- 52 bottom
- 53 pressing part
- 54 air intake valve
- 55 air intake valve
- 56 intake valve body
- 60A,60B cutting blade
- 71 one-way valve end
- 72 flow control surface
- 73 reduced diameter valve portion
- K capsule
- KA, KB capsule end
Claims (8)
Applications Claiming Priority (3)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| JP2002-138058 | 2002-05-14 | ||
| JP2002138058AJP3950738B2 (en) | 2002-05-14 | 2002-05-14 | Nasal powder medicine administration device |
| PCT/JP2003/005924WO2003095008A1 (en) | 2002-05-14 | 2003-05-13 | Powder chemical feeding device for nasal cavity |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| US20050177095A1 US20050177095A1 (en) | 2005-08-11 |
| US7353823B2true US7353823B2 (en) | 2008-04-08 |
Family
ID=29416838
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US10/512,857Expired - Fee RelatedUS7353823B2 (en) | 2002-05-14 | 2003-05-13 | Powder chemical feeding device for nasal cavity |
Country Status (11)
| Country | Link |
|---|---|
| US (1) | US7353823B2 (en) |
| EP (1) | EP1504780B1 (en) |
| JP (2) | JP3950738B2 (en) |
| KR (1) | KR20050007536A (en) |
| CN (1) | CN1652836A (en) |
| AU (1) | AU2003235232A1 (en) |
| CA (1) | CA2485713A1 (en) |
| MX (1) | MXPA04011281A (en) |
| RU (1) | RU2004133346A (en) |
| TW (1) | TW200306872A (en) |
| WO (1) | WO2003095008A1 (en) |
Cited By (17)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US20070125371A1 (en)* | 2003-08-28 | 2007-06-07 | Optinose As | Delivery devices |
| US20070283955A1 (en)* | 2006-06-07 | 2007-12-13 | Bioactis Limites | Peroral powder delivery device |
| US20080124373A1 (en)* | 2006-08-02 | 2008-05-29 | Inframat Corporation | Lumen - supporting devices and methods of making and using |
| US20080260848A1 (en)* | 2004-08-10 | 2008-10-23 | Translational Research, Ltd., | Compositions that Enable Rapid-Acting and Highly Absorptive Intranasal Administration |
| US20090169640A1 (en)* | 2003-02-21 | 2009-07-02 | Translational Research, Ltd. | Compositons for nasal administration of pharmaceuticals |
| US20090178676A1 (en)* | 2006-05-16 | 2009-07-16 | Hovione Inter Ag | Simple inhaler |
| US20100178331A1 (en)* | 2006-12-26 | 2010-07-15 | Ryoichi Nagata | Preparation for transnasal application |
| US20100263666A1 (en)* | 2007-05-23 | 2010-10-21 | Ryoichi Nagata | Test substance administration system for animal experiment |
| US20110033544A1 (en)* | 2009-05-15 | 2011-02-10 | Shin Nippon Biomedical Laboratories, Ltd. | Intranasal pharmaceutical compositions with improved pharmacokinetcs |
| US20110045088A1 (en)* | 2009-07-31 | 2011-02-24 | Shin Nippon Biomedical Laboratories, Ltd. | Intranasal granisetron and nasal applicator |
| US20140060535A1 (en)* | 2011-01-31 | 2014-03-06 | Shin Nippon Biomedical Laboratories, Ltd. | Intranasal delivery devices |
| USRE45404E1 (en) | 2003-03-27 | 2015-03-03 | Shin Nippon Biomedical Laboratories, Ltd. | Powder medicine applicator for nasal cavity |
| US10828432B1 (en)* | 2019-06-24 | 2020-11-10 | De Motu Cordis Pty Ltd | Respiratory delivery device and method |
| US20220379052A1 (en) | 2019-06-24 | 2022-12-01 | De Motu Cordis Pty Ltd | Automatic dispenser for respiratory delivery device |
| US20230090739A1 (en)* | 2020-01-27 | 2023-03-23 | Aptar France Sas | Device for dispensing a pulverulent product |
| US11744967B2 (en) | 2017-09-26 | 2023-09-05 | Shin Nippon Biomedical Laboratories, Ltd. | Intranasal delivery devices |
| US11793951B2 (en) | 2019-06-24 | 2023-10-24 | De Motu Cordis Pty Ltd | Automatic dispenser for respiratory delivery device and method |
Families Citing this family (13)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| EP1671667A4 (en) | 2003-10-09 | 2013-05-01 | Shin Nippon Biomedical Lab Ltd | Powdery medicine dispensing device for nasal cavity |
| JP4508689B2 (en)* | 2004-03-19 | 2010-07-21 | 株式会社新日本科学 | Oral powder administration device |
| DE102006006647B3 (en)* | 2006-02-14 | 2007-01-18 | Braunform Gmbh | Inhaler device for inhalation of powder in container has capsule holding powder, cutting appliance with two blades to cut ends of capsule which is received by movable slide or drum |
| CN104159633B (en)* | 2011-12-16 | 2018-10-12 | 因多西斯有限公司 | unit dose kit and conveying device |
| CN104258490B (en)* | 2014-10-10 | 2016-08-03 | 唐少臣 | A kind of quantitative sprayer of multi-faceted medicine powder |
| JP5843272B1 (en)* | 2015-03-24 | 2016-01-13 | 株式会社カタリメディック | Pump type filling device |
| US11559640B2 (en) | 2017-09-15 | 2023-01-24 | Shin Nippon Biomedical Laboratories, Ltd. | Medicine storage cartridge with nozzle, sprayer therefor, and powdered medicine dispensing device for nasal cavity |
| CN109011055A (en)* | 2018-09-04 | 2018-12-18 | 佛山市禅城区热拉空间生物科技有限公司 | A kind of capsule drug delivery device |
| JP1706573S (en)* | 2021-02-15 | 2022-02-01 | Nasal sprayer | |
| CN113730747A (en)* | 2021-10-18 | 2021-12-03 | 深圳市新鸿镁医疗器械有限公司 | Dosing device and nebulizer system |
| CN113893418B (en)* | 2021-12-02 | 2022-07-12 | 时新(上海)产品设计有限公司 | Spray device |
| CN115105694A (en)* | 2022-07-25 | 2022-09-27 | 苏州新劢德医疗器械科技有限公司 | A dual powered nasal spray delivery device |
| CN115518246B (en)* | 2022-09-26 | 2025-08-19 | 万通(苏州)定量阀系统有限公司 | Dry powder quantitative drug feeder |
Citations (14)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US3888252A (en)* | 1974-01-23 | 1975-06-10 | Anthony J Side | Powder inhaler |
| US3906950A (en)* | 1973-04-04 | 1975-09-23 | Isf Spa | Inhaling device for powdered medicaments |
| US3949751A (en)* | 1970-03-03 | 1976-04-13 | Fisons Limited | Method and device for dispensing medicament to the body |
| JPS5934267A (en) | 1982-08-23 | 1984-02-24 | 帝人株式会社 | Powdery drug applying apparatus |
| US5250287A (en)* | 1991-06-14 | 1993-10-05 | Miat S.P.A. | Multi-dose insufflator for medicaments in powder form |
| US5647349A (en)* | 1995-06-01 | 1997-07-15 | Unisia Jecs Corporation | Medicine administering inhaling device |
| JPH09253208A (en) | 1996-03-21 | 1997-09-30 | Unisia Jecs Corp | Nasal administration device |
| US5810004A (en)* | 1995-10-09 | 1998-09-22 | Unisia Jecs Corporation | Medicator for a capsule filled with a powdered drug |
| US5899202A (en)* | 1995-06-30 | 1999-05-04 | Unisia Jecs Corporation | Medicine administering device for nasal cavities and method of using same |
| US5921236A (en)* | 1995-03-10 | 1999-07-13 | Unisia Jecs Corporation | Medicine administering device for nasal cavities |
| JP2001095918A (en) | 1999-09-28 | 2001-04-10 | Unisia Jecs Corp | Nasal administration device |
| US6298846B1 (en)* | 1997-01-30 | 2001-10-09 | Unisia Jecs Corporation | Suction type medicator |
| WO2004087243A1 (en) | 2003-03-27 | 2004-10-14 | Bioactis Limited | Powder medicine applicator for nasal cavity |
| US6824080B2 (en)* | 2000-06-12 | 2004-11-30 | Teijin Limited | Powdered medicine multi-dose administering device |
Family Cites Families (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| GB1392945A (en)* | 1972-08-23 | 1975-05-07 | Fisons Ltd | Inhalation device |
- 2002
- 2002-05-14JPJP2002138058Apatent/JP3950738B2/ennot_activeExpired - Fee Related
- 2003
- 2003-05-12TWTW092112816Apatent/TW200306872A/enunknown
- 2003-05-13CACA002485713Apatent/CA2485713A1/ennot_activeAbandoned
- 2003-05-13EPEP03721098Apatent/EP1504780B1/ennot_activeExpired - Lifetime
- 2003-05-13USUS10/512,857patent/US7353823B2/ennot_activeExpired - Fee Related
- 2003-05-13KRKR10-2004-7018224Apatent/KR20050007536A/ennot_activeWithdrawn
- 2003-05-13CNCNA038107465Apatent/CN1652836A/enactivePending
- 2003-05-13JPJP2004503087Apatent/JPWO2003095008A1/enactivePending
- 2003-05-13MXMXPA04011281Apatent/MXPA04011281A/ennot_activeApplication Discontinuation
- 2003-05-13RURU2004133346/14Apatent/RU2004133346A/ennot_activeApplication Discontinuation
- 2003-05-13WOPCT/JP2003/005924patent/WO2003095008A1/enactiveApplication Filing
- 2003-05-13AUAU2003235232Apatent/AU2003235232A1/ennot_activeAbandoned
Patent Citations (15)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US3949751A (en)* | 1970-03-03 | 1976-04-13 | Fisons Limited | Method and device for dispensing medicament to the body |
| US3906950A (en)* | 1973-04-04 | 1975-09-23 | Isf Spa | Inhaling device for powdered medicaments |
| US3888252A (en)* | 1974-01-23 | 1975-06-10 | Anthony J Side | Powder inhaler |
| JPS5934267A (en) | 1982-08-23 | 1984-02-24 | 帝人株式会社 | Powdery drug applying apparatus |
| US5250287A (en)* | 1991-06-14 | 1993-10-05 | Miat S.P.A. | Multi-dose insufflator for medicaments in powder form |
| US5921236A (en)* | 1995-03-10 | 1999-07-13 | Unisia Jecs Corporation | Medicine administering device for nasal cavities |
| US5647349A (en)* | 1995-06-01 | 1997-07-15 | Unisia Jecs Corporation | Medicine administering inhaling device |
| US5899202A (en)* | 1995-06-30 | 1999-05-04 | Unisia Jecs Corporation | Medicine administering device for nasal cavities and method of using same |
| US5810004A (en)* | 1995-10-09 | 1998-09-22 | Unisia Jecs Corporation | Medicator for a capsule filled with a powdered drug |
| JPH09253208A (en) | 1996-03-21 | 1997-09-30 | Unisia Jecs Corp | Nasal administration device |
| US5989217A (en)* | 1996-03-21 | 1999-11-23 | Unisia Jecs Corporation | Medicine administering device for nasal cavities |
| US6298846B1 (en)* | 1997-01-30 | 2001-10-09 | Unisia Jecs Corporation | Suction type medicator |
| JP2001095918A (en) | 1999-09-28 | 2001-04-10 | Unisia Jecs Corp | Nasal administration device |
| US6824080B2 (en)* | 2000-06-12 | 2004-11-30 | Teijin Limited | Powdered medicine multi-dose administering device |
| WO2004087243A1 (en) | 2003-03-27 | 2004-10-14 | Bioactis Limited | Powder medicine applicator for nasal cavity |
Non-Patent Citations (4)
| Title |
|---|
| English Language Abstract of JP 2001-95918. |
| English Language Abstract of JP 5934267. |
| English Language Abstract of JP 9-253208. |
| U.S. Appl. No. 10/550,490, in the name of Tatsuo Tsutsui, filed Mar. 27, 2003. |
Cited By (34)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US20090169640A1 (en)* | 2003-02-21 | 2009-07-02 | Translational Research, Ltd. | Compositons for nasal administration of pharmaceuticals |
| US9138410B2 (en) | 2003-02-21 | 2015-09-22 | Shin Nippon Biomedical Laboratories, Ltd. | Compositions for nasal administration of pharmaceuticals |
| US8435554B2 (en) | 2003-02-21 | 2013-05-07 | Shin Nippon Biomedical Laboratories, Ltd. | Compositons for nasal administration of pharmaceuticals |
| USRE45404E1 (en) | 2003-03-27 | 2015-03-03 | Shin Nippon Biomedical Laboratories, Ltd. | Powder medicine applicator for nasal cavity |
| US10737045B2 (en) | 2003-08-28 | 2020-08-11 | Optinose As | Delivery devices |
| US20070125371A1 (en)* | 2003-08-28 | 2007-06-07 | Optinose As | Delivery devices |
| US8800555B2 (en)* | 2003-08-28 | 2014-08-12 | Optinose As | Delivery devices |
| US8673360B2 (en) | 2004-08-10 | 2014-03-18 | Shin Nippon Biomedical Laboratories, Ltd. | Compositions that enable rapid-acting and highly absorptive intranasal administration |
| US20080260848A1 (en)* | 2004-08-10 | 2008-10-23 | Translational Research, Ltd., | Compositions that Enable Rapid-Acting and Highly Absorptive Intranasal Administration |
| US20090178676A1 (en)* | 2006-05-16 | 2009-07-16 | Hovione Inter Ag | Simple inhaler |
| US8109267B2 (en)* | 2006-05-16 | 2012-02-07 | Hovione International Ltd. | Simple inhaler |
| US7806117B2 (en)* | 2006-06-07 | 2010-10-05 | Shin Nippon Biomedical Laboratories, Ltd. | Peroral powder delivery device |
| US20070283955A1 (en)* | 2006-06-07 | 2007-12-13 | Bioactis Limites | Peroral powder delivery device |
| US20080124373A1 (en)* | 2006-08-02 | 2008-05-29 | Inframat Corporation | Lumen - supporting devices and methods of making and using |
| US10195139B2 (en) | 2006-12-26 | 2019-02-05 | Shin Nippon Biomedical Laboratories, Ltd. | Preparation for transnasal application |
| US8337817B2 (en) | 2006-12-26 | 2012-12-25 | Shin Nippon Biomedical Laboratories, Ltd. | Preparation for transnasal application |
| US20100178331A1 (en)* | 2006-12-26 | 2010-07-15 | Ryoichi Nagata | Preparation for transnasal application |
| US10293123B2 (en) | 2007-05-23 | 2019-05-21 | Shin Nippon Biomedical Laboratories, Ltd. | Test substance administration system for animal experiment |
| US20100263666A1 (en)* | 2007-05-23 | 2010-10-21 | Ryoichi Nagata | Test substance administration system for animal experiment |
| US9101539B2 (en) | 2009-05-15 | 2015-08-11 | Shin Nippon Biomedical Laboratories, Ltd. | Intranasal pharmaceutical compositions with improved pharmacokinetics |
| US20110033544A1 (en)* | 2009-05-15 | 2011-02-10 | Shin Nippon Biomedical Laboratories, Ltd. | Intranasal pharmaceutical compositions with improved pharmacokinetcs |
| US8827946B2 (en) | 2009-07-31 | 2014-09-09 | Shin Nippon Biomedical Laboratories, Ltd. | Intranasal granisetron and nasal applicator |
| US20110045088A1 (en)* | 2009-07-31 | 2011-02-24 | Shin Nippon Biomedical Laboratories, Ltd. | Intranasal granisetron and nasal applicator |
| US10071211B2 (en)* | 2011-01-31 | 2018-09-11 | Shin Nippon Biomedical Laboratories, Ltd. | Intranasal delivery devices |
| US20140060535A1 (en)* | 2011-01-31 | 2014-03-06 | Shin Nippon Biomedical Laboratories, Ltd. | Intranasal delivery devices |
| US11744967B2 (en) | 2017-09-26 | 2023-09-05 | Shin Nippon Biomedical Laboratories, Ltd. | Intranasal delivery devices |
| US12102754B2 (en) | 2017-09-26 | 2024-10-01 | Shin Nippon Biomedical Laboratories, Ltd. | Intranasal delivery devices |
| US20220379052A1 (en) | 2019-06-24 | 2022-12-01 | De Motu Cordis Pty Ltd | Automatic dispenser for respiratory delivery device |
| US11717621B2 (en) | 2019-06-24 | 2023-08-08 | De Motu Cordis Pty Ltd | Automatic dispenser for respiratory delivery device |
| US10828432B1 (en)* | 2019-06-24 | 2020-11-10 | De Motu Cordis Pty Ltd | Respiratory delivery device and method |
| US11793951B2 (en) | 2019-06-24 | 2023-10-24 | De Motu Cordis Pty Ltd | Automatic dispenser for respiratory delivery device and method |
| US12109356B2 (en) | 2019-06-24 | 2024-10-08 | De Motu Cordis Pty Ltd | Automatic dispenser for respiratory delivery device and method |
| US20230090739A1 (en)* | 2020-01-27 | 2023-03-23 | Aptar France Sas | Device for dispensing a pulverulent product |
| US12233243B2 (en)* | 2020-01-27 | 2025-02-25 | Aptar France Sas | Device for dispensing a pulverulent product |
Also Published As
| Publication number | Publication date |
|---|---|
| EP1504780B1 (en) | 2012-07-11 |
| MXPA04011281A (en) | 2005-07-01 |
| RU2004133346A (en) | 2005-09-10 |
| US20050177095A1 (en) | 2005-08-11 |
| AU2003235232A1 (en) | 2003-11-11 |
| JP2005168513A (en) | 2005-06-30 |
| JPWO2003095008A1 (en) | 2005-09-08 |
| EP1504780A4 (en) | 2006-06-21 |
| EP1504780A1 (en) | 2005-02-09 |
| JP3950738B2 (en) | 2007-08-01 |
| KR20050007536A (en) | 2005-01-19 |
| WO2003095008A1 (en) | 2003-11-20 |
| TW200306872A (en) | 2003-12-01 |
| CA2485713A1 (en) | 2003-11-20 |
| CN1652836A (en) | 2005-08-10 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| US7353823B2 (en) | Powder chemical feeding device for nasal cavity | |
| US7278982B2 (en) | Powder medicine applicator for nasal cavity | |
| EP0768094B1 (en) | Powder inhalation medication | |
| EP0303844B1 (en) | Insufflator for the administration of drugs in the form of a powder pre-dosed into opercula | |
| KR100187737B1 (en) | Nasal Dosing Machine | |
| US7677467B2 (en) | Methods and devices for aerosolizing medicament | |
| BG65349B1 (en) | Powdered drug aerozolizing device and method for using it | |
| JP3975327B2 (en) | Nasal powder medicine administration device | |
| US20050205089A1 (en) | Methods and devices for aerosolizing medicament | |
| US20120193377A1 (en) | Nasal spray pump | |
| KR20190123104A (en) | Upper airway spraying device | |
| RU2324502C2 (en) | Medical instrument for administration of powder therapeutical agent into nasal cavity | |
| JP2004129823A (en) | Powdery medicine dispensing device for nasal cavity | |
| HK1081472A (en) | Powder chemical feeding device for nasal cavity | |
| TWI286944B (en) | A device for applying medicine powder to nose | |
| JPH08238318A (en) | Nasal drug dispenser |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| AS | Assignment | Owner name:TATSUO TSUTSUI, JAPAN Free format text:ASSIGNMENT OF ASSIGNORS INTEREST;ASSIGNOR:TSUTSUI, TATSUO;REEL/FRAME:016152/0631 Effective date:20041110 Owner name:BIOACTIS LIMITED, JAPAN Free format text:ASSIGNMENT OF ASSIGNORS INTEREST;ASSIGNOR:TSUTSUI, TATSUO;REEL/FRAME:016152/0631 Effective date:20041110 | |
| AS | Assignment | Owner name:BIOACTIS LIMITED, JAPAN Free format text:CORRECTIV;ASSIGNOR:TSUTSUI, TATSUO;REEL/FRAME:017204/0640 Effective date:20041110 Owner name:TSUTSUI, TATSUO, JAPAN Free format text:CORRECTIV;ASSIGNOR:TSUTSUI, TATSUO;REEL/FRAME:017204/0640 Effective date:20041110 Owner name:BIOACTIS LIMITED, JAPAN Free format text:CORRECTED RECORDATION AT REEL 016152 AND FRAME 0631;ASSIGNOR:TSUTSUI, TATSUO;REEL/FRAME:017204/0249 Effective date:20041110 Owner name:TSUTSUI, TATSUO, JAPAN Free format text:CORRECTED RECORDATION AT REEL 016152 AND FRAME 0631;ASSIGNOR:TSUTSUI, TATSUO;REEL/FRAME:017204/0249 Effective date:20041110 | |
| STCF | Information on status: patent grant | Free format text:PATENTED CASE | |
| AS | Assignment | Owner name:BIOACTIS LTD., JAPAN Free format text:ASSIGNMENT OF ASSIGNORS INTEREST;ASSIGNOR:TSUTSUI, TATSUO;REEL/FRAME:022473/0694 Effective date:20081210 | |
| AS | Assignment | Owner name:SHIN NIPPON BIOMEDICAL LABORATORIES, LTD.,JAPAN Free format text:MERGER;ASSIGNOR:BIOACTIS LIMITED;REEL/FRAME:024599/0898 Effective date:20090812 Owner name:SHIN NIPPON BIOMEDICAL LABORATORIES, LTD., JAPAN Free format text:MERGER;ASSIGNOR:BIOACTIS LIMITED;REEL/FRAME:024599/0898 Effective date:20090812 | |
| FEPP | Fee payment procedure | Free format text:PAYOR NUMBER ASSIGNED (ORIGINAL EVENT CODE: ASPN); ENTITY STATUS OF PATENT OWNER: LARGE ENTITY | |
| FPAY | Fee payment | Year of fee payment:4 | |
| FPAY | Fee payment | Year of fee payment:8 | |
| FEPP | Fee payment procedure | Free format text:MAINTENANCE FEE REMINDER MAILED (ORIGINAL EVENT CODE: REM.); ENTITY STATUS OF PATENT OWNER: LARGE ENTITY | |
| LAPS | Lapse for failure to pay maintenance fees | Free format text:PATENT EXPIRED FOR FAILURE TO PAY MAINTENANCE FEES (ORIGINAL EVENT CODE: EXP.); ENTITY STATUS OF PATENT OWNER: LARGE ENTITY | |
| STCH | Information on status: patent discontinuation | Free format text:PATENT EXPIRED DUE TO NONPAYMENT OF MAINTENANCE FEES UNDER 37 CFR 1.362 | |
| FP | Lapsed due to failure to pay maintenance fee | Effective date:20200408 |