US6362591B1 - Method and apparatus for detection of occlusions - Google Patents
Method and apparatus for detection of occlusionsDownload PDFInfo
- Publication number
- US6362591B1 US6362591B1US09/428,411US42841199AUS6362591B1US 6362591 B1US6362591 B1US 6362591B1US 42841199 AUS42841199 AUS 42841199AUS 6362591 B1US6362591 B1US 6362591B1
- Authority
- US
- United States
- Prior art keywords
- value
- encoder count
- determining
- fluid pump
- less
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Expired - Lifetime
Links
Images
Classifications
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M5/00—Devices for bringing media into the body in a subcutaneous, intra-vascular or intramuscular way; Accessories therefor, e.g. filling or cleaning devices, arm-rests
- A61M5/14—Infusion devices, e.g. infusing by gravity; Blood infusion; Accessories therefor
- A61M5/162—Needle sets, i.e. connections by puncture between reservoir and tube ; Connections between reservoir and tube
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M39/00—Tubes, tube connectors, tube couplings, valves, access sites or the like, specially adapted for medical use
- A61M39/10—Tube connectors; Tube couplings
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M39/00—Tubes, tube connectors, tube couplings, valves, access sites or the like, specially adapted for medical use
- A61M39/10—Tube connectors; Tube couplings
- A61M39/12—Tube connectors; Tube couplings for joining a flexible tube to a rigid attachment
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M5/00—Devices for bringing media into the body in a subcutaneous, intra-vascular or intramuscular way; Accessories therefor, e.g. filling or cleaning devices, arm-rests
- A61M5/14—Infusion devices, e.g. infusing by gravity; Blood infusion; Accessories therefor
- A61M5/142—Pressure infusion, e.g. using pumps
- A61M5/14244—Pressure infusion, e.g. using pumps adapted to be carried by the patient, e.g. portable on the body
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M5/00—Devices for bringing media into the body in a subcutaneous, intra-vascular or intramuscular way; Accessories therefor, e.g. filling or cleaning devices, arm-rests
- A61M5/14—Infusion devices, e.g. infusing by gravity; Blood infusion; Accessories therefor
- A61M5/168—Means for controlling media flow to the body or for metering media to the body, e.g. drip meters, counters ; Monitoring media flow to the body
- A61M5/16831—Monitoring, detecting, signalling or eliminating infusion flow anomalies
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M5/00—Devices for bringing media into the body in a subcutaneous, intra-vascular or intramuscular way; Accessories therefor, e.g. filling or cleaning devices, arm-rests
- A61M5/14—Infusion devices, e.g. infusing by gravity; Blood infusion; Accessories therefor
- A61M5/162—Needle sets, i.e. connections by puncture between reservoir and tube ; Connections between reservoir and tube
- A61M2005/1623—Details of air intake
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M39/00—Tubes, tube connectors, tube couplings, valves, access sites or the like, specially adapted for medical use
- A61M39/10—Tube connectors; Tube couplings
- A61M2039/1033—Swivel nut connectors, e.g. threaded connectors, bayonet-connectors
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M39/00—Tubes, tube connectors, tube couplings, valves, access sites or the like, specially adapted for medical use
- A61M39/10—Tube connectors; Tube couplings
- A61M2039/1072—Tube connectors; Tube couplings with a septum present in the connector
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M39/00—Tubes, tube connectors, tube couplings, valves, access sites or the like, specially adapted for medical use
- A61M39/20—Closure caps or plugs for connectors or open ends of tubes
- A61M2039/205—Closure caps or plugs for connectors or open ends of tubes comprising air venting means
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M2205/00—General characteristics of the apparatus
- A61M2205/58—Means for facilitating use, e.g. by people with impaired vision
- A61M2205/581—Means for facilitating use, e.g. by people with impaired vision by audible feedback
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M2205/00—General characteristics of the apparatus
- A61M2205/58—Means for facilitating use, e.g. by people with impaired vision
- A61M2205/582—Means for facilitating use, e.g. by people with impaired vision by tactile feedback
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M5/00—Devices for bringing media into the body in a subcutaneous, intra-vascular or intramuscular way; Accessories therefor, e.g. filling or cleaning devices, arm-rests
- A61M5/14—Infusion devices, e.g. infusing by gravity; Blood infusion; Accessories therefor
- A61M5/142—Pressure infusion, e.g. using pumps
- A61M5/145—Pressure infusion, e.g. using pumps using pressurised reservoirs, e.g. pressurised by means of pistons
- A61M5/1452—Pressure infusion, e.g. using pumps using pressurised reservoirs, e.g. pressurised by means of pistons pressurised by means of pistons
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M5/00—Devices for bringing media into the body in a subcutaneous, intra-vascular or intramuscular way; Accessories therefor, e.g. filling or cleaning devices, arm-rests
- A61M5/14—Infusion devices, e.g. infusing by gravity; Blood infusion; Accessories therefor
- A61M5/142—Pressure infusion, e.g. using pumps
- A61M5/145—Pressure infusion, e.g. using pumps using pressurised reservoirs, e.g. pressurised by means of pistons
- A61M5/1452—Pressure infusion, e.g. using pumps using pressurised reservoirs, e.g. pressurised by means of pistons pressurised by means of pistons
- A61M5/14566—Pressure infusion, e.g. using pumps using pressurised reservoirs, e.g. pressurised by means of pistons pressurised by means of pistons with a replaceable reservoir for receiving a piston rod of the pump
- Y—GENERAL TAGGING OF NEW TECHNOLOGICAL DEVELOPMENTS; GENERAL TAGGING OF CROSS-SECTIONAL TECHNOLOGIES SPANNING OVER SEVERAL SECTIONS OF THE IPC; TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y10—TECHNICAL SUBJECTS COVERED BY FORMER USPC
- Y10T—TECHNICAL SUBJECTS COVERED BY FORMER US CLASSIFICATION
- Y10T29/00—Metal working
- Y10T29/49—Method of mechanical manufacture
- Y10T29/49764—Method of mechanical manufacture with testing or indicating
- Y—GENERAL TAGGING OF NEW TECHNOLOGICAL DEVELOPMENTS; GENERAL TAGGING OF CROSS-SECTIONAL TECHNOLOGIES SPANNING OVER SEVERAL SECTIONS OF THE IPC; TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y10—TECHNICAL SUBJECTS COVERED BY FORMER USPC
- Y10T—TECHNICAL SUBJECTS COVERED BY FORMER US CLASSIFICATION
- Y10T29/00—Metal working
- Y10T29/49—Method of mechanical manufacture
- Y10T29/49826—Assembling or joining
- Y—GENERAL TAGGING OF NEW TECHNOLOGICAL DEVELOPMENTS; GENERAL TAGGING OF CROSS-SECTIONAL TECHNOLOGIES SPANNING OVER SEVERAL SECTIONS OF THE IPC; TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y10—TECHNICAL SUBJECTS COVERED BY FORMER USPC
- Y10T—TECHNICAL SUBJECTS COVERED BY FORMER US CLASSIFICATION
- Y10T29/00—Metal working
- Y10T29/53—Means to assemble or disassemble
Definitions
- This inventionrelates generally to improvements in infusion pumps such as those used for controlled delivery of medication to a patient. More specifically, this invention relates to improved methods and apparatuses for detecting occlusions in infusion pump systems.
- Infusion pump devices and systemsare relatively well-known in the medical arts, for use in delivering or dispensing a prescribed medication such as insulin to a patient.
- such devicescomprise a relatively compact pump housing adapted to receive a syringe or reservoir carrying a prescribed medication for administration to the patient through infusion tubing and an associated catheter or infusion set.
- the infusion pumpincludes a small drive motor connected via a suitable transmission assembly for motor-driven advancement of a reservoir piston to administer the medication to the user.
- Programmable controlscan operate the drive motor continuously or at periodic intervals to obtain a closely controlled and accurate delivery of the medication over an extended period of time.
- Such infusion pumpsare utilized to administer insulin and other medications, with exemplary pump constructions being shown and described in U.S. Pat. Nos. 4,562,751; 4,678,408; 4,685,903; 5,080,653 and 5,097,122, which are incorporated by reference herein.
- Infusion pumps of the general type described abovehave provided significant advantages and benefits with respect to accurate delivery of medication or other fluids over an extended period of time.
- the infusion pumpcan be designed to be extremely compact as well as water resistant, and may thus be adapted to be carried by the user, for example, by means of a belt clip.
- important medicationcan be delivered to the user with precision and in an automated manner, without significant restriction on the user's mobility or life-style, including in some cases the ability to participate in water sports.
- These pumpsoften incorporate drive systems which use lead screws or other transmission components coupled to motors.
- the motorscan be of the DC, stepper, solenoid or other varieties.
- These drive systemstypically provide an axial displacement of the syringe or reservoir piston thereby dispensing the medication to the user.
- Powered drive systemsare advantageous since they can be electronically controlled to deliver a predetermined amount of medication by means well known in the art.
- medication infusion pumpshave included alarm systems designed to detect and indicate pump malfunction and/or nondelivery of the medication to the patient as a result of an occluded delivery line.
- alarm systemshave typically used a high pressure limit switch for activating an alarm when the force applied to the reservoir piston plunger reaches a predetermined upper limit indicative of an occluded medication delivery line.
- the high pressure switchis positioned at one end of a rotatable lead screw, wherein the mechanical reaction force or backlash between the reservoir plunger and the pressure switch is proportional to the pressure applied to the medication as a result of attempted advancement of the reservoir plunger.
- the lead screwmust move axially some distance to actuate the switch. If the concentration of the medication requires very small (0.5 microliter) dosage increments, then the axial displacement of the reservoir plunger is designed to be very small per increment. Thus, several increments may be required to initiate an alarm when there is an occlusion. This elapse of several increments before an alarm is initiated may represent less medication delivered to the user than is desired.
- the lead screw mechanism of this pump designincludes seals, a drive nut, a lead screw/motor coupling, and a bearing. All of these components have frictional properties. In systems where the high pressure switch is placed at the opposite end of the drive train from the reservoir plunger, the friction associated with these components are additive. These properties are known to change over time thereby making a fixed-setting, high pressure switch less effective.
- preferred embodimentsdisclose a method and system for detecting occlusions or other pump system failures.
- Poweris applied to a pump motor.
- a first pump motor current valueis measured and a determination made whether the first pump motor current value exceeds a second value. If it does exceed the second value, the pump system is rewound at least to the point of reducing the pressure in the system and an alarm indication is given.
- a calculationis made of a total number of alarm indications, and a determination is made whether the total number of alarm indications exceeds a third value. If it does, a system error message is given.
- input power parametersare applied to a pump motor to provide a first pump cycle.
- a first encoder countis measured during the first pump cycle and a determination is made whether the first encoder count is less than a first value. If it is less than the first value, the fluid pump system is rewound at least to the point of reducing pressure in the system, and an alarm indication is provided. A calculation is made of a total number of alarm indications which is used to determine whether this exceeds a second value. If so, a system error message is provided. To provide system feedback, a determination is made whether the first encoder count is equal to a third value.
- the input power parametersare adjusted to provide a second pump cycle which, in turn, causes a second encoder count of a different value than the first encoder count.
- the second encoder countwill be greater than the first encoder count if the first encoder count was less than the third value.
- the second encoder countwill be smaller than the first encoder count if the first encoder count was greater than the third value.
- an input power parameteris provided to a pump motor to provide a pump cycle.
- An encoder countis measured during the pump cycle.
- a torque value corresponding to the encoder count and to the input power parameteris determined.
- Another determinationis made whether the torque value is greater than a first value. If so, the fluid pump system is rewound at least to the point of reducing pressure in the fluid pump system.
- An alarm indicationis also provided.
- a calculationis made of a total number of alarm indications which is used to determine whether this total exceeds a second value. If so, a system error message is provided.
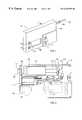
- FIG. 1is a front, perspective view of a medication infusion pump, adapted to include the occlusion detectors claimed herein.
- FIG. 2is an enlarged, side plan, partial cut-away view of the infusion pump of FIG. 1 with portions broken away to illustrate certain pump components.
- FIG. 3is a side plan, cut-away view of another infusion pump design, adapted to include the occlusion detectors claimed herein.
- FIG. 4is a block diagram illustrating a software and hardware environment in which some embodiments of the present invention may be implemented.
- FIGS. 5-7illustrate different embodiments of logic for detecting occlusions in accordance with certain embodiments of the present invention.
- a medication infusion pump referred to generally in FIG. 1 by the reference numeral 101is provided for controlled administration of medication to a patient.
- the infusion pump 101includes an improved occlusion detector for monitoring and verifying proper delivery of the medication to the patient.
- the infusion pump 101has an overall mechanical construction and mechanical operation which is generally known in the art. More specifically, with respect to FIGS. 1 and 2, the infusion pump 101 comprises a relatively compact pump housing 102 defining an elongated chamber 103 (FIG. 2) for receiving and supporting a syringe 104 charged with a selected medication, such as insulin, to be administered to a patient.
- the medication-containing syringe 104includes a syringe barrel 105 joined at the front to a luer neck 106 of reduced diametric size to seat snugly within an outlet port 107 formed in the pump housing 102 .
- a luer fitting 108is carried on the neck 106 and cooperates with the barrel 105 to fix the syringe in a seated position within the syringe chamber 103 .
- a syringe piston or plunger 109extends from the aft end of the barrel 105 and may be advanced into the barrel to deliver the medication therefrom.
- the medicationis normally dispensed to the patient through a catheter tube 110 adapted for appropriate connection to the luer fitting 108 .
- the infusion pump 101includes a compact drive motor 111 (FIG. 2) which is mechanically connected to the syringe plunger 109 for purposes of advancing the plunger in a precision controlled manner to dispense the medication.
- the drive motor 111is normally powered by a battery power supply 112 , in response to operation of a programmable controller 113 .
- the programmable controller 113can be set by the attending physician, appropriate medical personnel, or the user by an array of buttons 114 on the face or front of the pump housing 102 , with a corresponding display panel 115 providing appropriate information regarding set status and/or pump operation.
- the controller 113operates the drive motor 111 in a stepwise manner, typically on an intermittent basis, to administer discrete precision doses of the medication to the patient at programmed times.
- the mechanical connection between the drive motor 111 and the syringe plunger 109includes a lead screw assembly 116 . More specifically, the drive motor 111 has a rotary output for rotatably driving an elongated lead screw 117 mounted within the pump housing 102 . A lead screw nut 118 is carried on the lead screw 117 and includes an appropriate latch arm 119 for engaging a drive flange 120 on the end of the piston plunger 109 .
- FIG. 3shows another infusion pump design for which an improved occlusion detector may be used.
- the infusion pump 301comprises a motor 302 , drive screw 303 , and plunger slide 304 which are arranged in-line with a reservoir 305 .
- the motor 302rotates the drive screw 303 via a gear box 306 .
- the drive screw 303has external threads which engage the internal threads of the plunger slide 304 .
- the plunger slide 304is in contact with a reservoir plunger 307 , and advances it thereby forcing the medication out of a luer fitting 308 and into infusion set tubing 309 .
- an encoder 310Attached to the rear of the motor 302 is an encoder 310 , a device known in the art which is capable of counting motor rotations or fractional rotations.
- the encoderhas signal and power supply cables 311 which extend into the battery/electronics compartment 312 of the pump housing.
- the pump drive mechanism design of FIG. 3is described in greater detail in copending application Ser. No. 09/429,352, filed contemporaneously which application is incorporated by reference in its entirety.
- FIG. 4illustrates one software and hardware environment in which certain embodiments of the present invention may be implemented.
- a syringe or reservoir 401 with a luer fitting 402 and infusion set tubing 403is connected to a motor drive train.
- the drive traincomprises a motor 404 and a transmission 405 coupling the motor 404 to the reservoir 401 .
- Other reservoir and drive train systemsmay be used, however, as described herein or as known in the art or as may be developed in the future.
- At one end of the motor 404is an encoder system comprised of an encoder wheel 412 and an encoder sensor 406 .
- the encodercould be magnetic, optical, or other designs known in the art.
- the encoderWhen the encoder is attached to a motor, it provides a measurement of the rotational displacement of the motor. The accuracy of this measurement is a function of the count resolution of the encoder. If for example, the encoder had a resolution of 360 counts per motor revolution, then with each motor revolution, the sensor will provide 360 encoder signal pulses. If a pump system was designed to require one complete motor revolution to deliver the desired increment of medication, then the motor can be controlled to stop when 360 encoder counts are received.
- the encoder sensor 406transmits the encoder pulses to an encoder logic circuit 407 which in turn communicates the counts to a processing unit, such as microcontroller 408 .
- a motor drive circuit 409receives commands from the microcontroller 408 and when so instructed, causes electrical power to be transmitted to the motor 404 .
- a motor drive sensor circuit 410measures the motor operating parameters such as motor voltage, motor current, and motor operating time, and transmits these parameters to the microcontroller 408 .
- a battery 411provides power for all of these components.
- the microcontroller 408receives its operating instructions from software stored in random access memory (RAM), read only memory (ROM), or other devices known in the art for storing operating instructions for use by the microcontroller.
- the foregoing computing environmentis illustrative only. Any alternative computing environment involving a computer, a microcontroller or a microprocessor along with computer instruction code and circuitry is acceptable.
- the microporcessor controlmay include RAM or ROM programs.
- thiscan further be accomplished with a hardwired or programmable logic array driven state machine.
- a dedicated logic or a programmable logic arraycan be used to perform the operations necessary to test for an occlusion.
- the improved occlusion detector described hereincan be applied to the pump drive designs of FIGS. 1-3, or any other design incorporating a motor.
- the pumping mechanismcan use DC motors, AC motors, stepper motors, solenoid motors, lead screws, cams, ratchets, jacks, piezoelectric caterpillar drives, Nitinol effectors, electrochemical gas cells or thermally driven gas or bimetallic actuators.
- the occlusion detectormeasures increased reservoir pressure indirectly by monitoring one or more motor parameters, such as voltage, current, running time, or rotational or linear displacement.
- the pressure inside the reservoirwill exceed a predetermined threshold.
- the current necessary to drive that loadwill exceed a predetermined current threshold and the electronics will be flagged to cease further delivering.
- an audible, tactile and/or display alarmtypically is triggered.
- the improved occlusion detection system disclosed hereinprotects against this by causing the pump to rewind by some predetermined amount following each occlusion alarm. By rewinding the pump by, say, one delivery pulse, the occlusion alarm will trigger if the occlusion still exists. However it will do so at the same maximum pressure as programmed and not at above this value.
- the current measurementcan also be used as an indicator of system wear. Over the life of the product, it is expected that the torque required to drive the system will change over time due to wear of the dynamic components and their interfaces. Since the torque required to rewind a bidirectional system is due to the drive system's frictional factors, the current to rewind can be recorded and is proportional to this torque.
- the torque and therefore the current to rewindwill change.
- thiscan be used to calibrate the system.
- An averaged baseline rewind currentcan be determined and used to adjust the driving force baseline which is the torque (or current) required to advance the drive system when no other external forces, such as a syringe with fluid, are present.
- An alternative methodwould be to rewind the system, and then immediately thereafter, obtain the forward or driving baseline current by driving the system forward for some distance and recording it, after which, the system is rewound again. The advantage of using either method is that the calibration can be automatic and transparent to the user.
- FIG. 5illustrates the logic in one embodiment of the detector wherein motor current is measured for detecting a system occlusion.
- Controlbegins at block 501 where the system determines whether it is necessary to fully rewind the pump drive system. Conditions requiring such a rewind of the drive system will be discussed below. If the system is not to be rewound, then a determination is made whether it is time for an increment of medication is to be delivered (block 502 ). This determination is a function of the programming which is unique to the medical condition of each user, the type of medication being provided, or the like. If it is not time to deliver medication, then the program loops to the start for additional time to elapse or for the receipt of other control commands.
- the amount of medication delivered from the reservoiris measured (block 504 ). This can be accomplished directly or indirectly in several ways, including measuring (1) encoder counts, (2) pump operation time, (3) reservoir plunger position location, velocity or acceleration, (4) the location of any moveable component on the pump drive train, or (5) the mass or volumetric flow of the liquid.
- this motor currentcan provide feedback as to drive system characteristics, performance, and functionality, especially with the addition of an encoder. If for example, there was a failure of the gearbox causing the motor to be unable to rotate, the measured current would be high (above predetermined threshold settings) and the encoder would not increment. This would be an indication of a drive system fault. For the inline drive system shown in FIG. 3, a failure of the gearbox, screw, or slide interface would be indicated by this condition.
- the value of the average baseline currentis retrieved from a storage location in memory represented by block 520 . This value is compared with the current measured at the present time and a determination is made whether the present current exceeds the average baseline by a certain amount. If it does not, then the pump continues to run and control loops to block 504 where the amount of medication delivery is again measured. On the other hand, if the present current exceeds the average baseline by a selected amount, then the pump motor is stopped and an alarm indication, audible, tactile and/or visible, is given (blocks 509 and 510 ).
- Controlthen transfers to block 513 where an alarm flag is stored. A determination is made whether there have been an excessive number of recent alarms (block 514 ). If there have not, then control loops to the beginning (block 501 ) where the above described process is repeated. On the other hand, if there have been an excessive number of recent alarms, control transfers to block 515 where an error or reset message is displayed to the user. This message typically would be used to advise the user to contact the manufacturer or some authorized repair facility to determine the cause of the excessive number of alarms. This error message will continue to be displayed until the error is cleared (block 516 ) at which point control loops to the beginning (block 501 ) where the process is repeated.
- the pump motor currentis measured (block 518 ).
- An alternative methodwould be to obtain the forward or driving baseline current by driving the system forward (possibly immediately following rewind) for some distance and recording it, after which the system may need to be rewound again. Because the motor is running in the opposite direction (or forward following rewind), typically there is little or no fluid pressure against which the pump motor is driving. Thus the current measured during this phase can be used as a baseline for comparison in detecting occlusions.
- the value of repeatedly updating the average baseline currentis to provide a calibration against changing drive train friction forces.
- the lead screw mechanism of many pump designsincludes seals, a drive nut, a lead screw/motor coupling, and a bearing. All of these components have frictional properties. These properties are known to change over time and thus the motor torque and current required to advance a reservoir plunger are likely to change. This therefore provides a more accurate baseline against which current can be measured for the detection of an occlusion.
- motor currentother motor parameters which vary with differing fluid back pressures can be measured with like effect.
- Such parametersmay include motor voltage, linear displacement, rotary displacement, torque, rotor speed, and the like.
- one alternative embodiment of the occlusion detectorinvolves the use of a motor position encoder which can detect the motor's linear or rotational displacement. If for example, the encoder has a resolution of 360 counts per motor revolution of a rotary motor, then with each motor revolution, the sensor will provide 360 encoder signal pulses. If the pump system were designed to require one complete motor revolution to deliver the desired increment of medication, then the motor can be controlled to stop when 360 encoder counts are received. Linear displacements of linear motors may be similarly detected by suitable linear encoders or sensors.
- the slowing or decelerationcan be accomplished in several ways including: (1) coasting which simply lets the applied and frictional torque slow the motor; or (2) dynamic braking which can be accomplished for example by shorting the motor leads or applying a potential in the opposite direction.
- the applied torqueaffects the total rotational count. Thus as the applied torque varies, so will the error from the desired 360 counts.
- a feedback loopis provided whereby the input power parameters to the motor, such as motor voltage or current or the time during which power is applied to the motor, may be adjusted.
- the motoris controlled based on the results of the previous encoder count for each cycle. Thus, for example, if 360 encoder counts were desired, but only 350 were measured, then subsequent input motor parameters can be adjusted such that the running encoder average is maintained at 360 counts. If a motor system was used with a DC motor driven with a constant current source or fixed source voltage, then the motor input parameter to be adjusted for maintaining the desired encoder count for the next pump cycle would be power on time.
- a motormay be driven such that half of the rotational displacement (or 180 out of 360 counts) is due to power on time and the other half is due to the coasting down of the motor under a specified nominal load (torque). Should the load increase, then the coasting would decrease thereby reducing the total encoder count measured for a constant power input.
- the systemmay measure 350 counts rather than the target value of 360 counts. To maintain medication delivery accuracy therefore, the subsequent motor increment during the next pump cycle may be increased above the 180 encoder count for the power on time so that the running average is maintained at 360 for the entire pump cycle.
- FIG. 6illustrates an example of the logic of an embodiment of the present invention using an encoder.
- Controlbegins at block 601 , where a determination is made whether it is time for an increment of medication is to be delivered. The same considerations apply here as was described for block 502 of FIG. 5 . If it is not time to deliver medication, then the program loops to the start in order for additional time to elapse or for the receipt of other control commands.
- power parameterswould include pump power on time, operating voltage and operating current. These parameters are applied to the pump to drive it for one pump cycle. This causes an increment of medication to be delivered as the pump runs for a discrete period of time (blocks 604 & 605 ).
- the pump encoder countsare measured during this motor drive increment (block 606 ). Counts are measured both during the time that power is applied to the pump motor as well as the time during which the pump coasts down.
- the alarm process represented by blocks 612 through 618(including the incremental drive system rewind) is the same as previously described for blocks 510 through 516 of FIG. 5 .
- the alarm count table(block 608 ) can be calibrated to adjust for changes in system friction properties over time in a manner similar to that which was described in FIG. 5, blocks 517 - 520 .
- the pump drive systemperiodically could be rewound thereby removing any system fluid pressure forces from the pump drive system. Then input power parameters could be applied to the pump in either a forward or reverse direction to generate one pump cycle. The encoder count could be measured during this pump cycle and used to calculate a baseline average encoder alarm count for storage in the alarm count table (block 608 ).
- controlis transferred to block 611 where the adjustment amount is calculated and stored in the memory location represented by block 603 .
- Controlthen loops to block 601 where the above described process is repeated.
- the increment countis such that no adjustment is needed, then control loops directly to block 601 .
- the process represented by blocks 610 , 611 and 603constitutes a feedback loop for maintaining the average encoder count at the same level.
- torqueis a function of encoder count and one or more motor input power parameters.
- Motor load torquecan be determined by evaluating the stored encoder count for a known delivered amount of energy.
- the detector systemprovides a known amount of energy (i.e., power times motor on-time), and records the motor displacement via the number of encoder counts obtained. Using a look-up table or calculated value, the system determines a corresponding torque that would result from the recorded number of encoder pulses for the amount of energy supplied.
- FIG. 7illustrates an example of the logic of an embodiment of the present invention which uses an encoder to measure torque.
- Controlbegins at block 701 where a determination is made whether it is time to deliver a medication dosage increment. If it is time to deliver a dose, then control transfers to block 702 where the pump input power parameters which will be used on the upcoming cycle are stored in a storage location of an input power look up table represented by block 703 . Next, these same input power parameters are applied to the pump motor which operates for the selected time interval and delivers the dosage increment (blocks 704 & 705 ). The encoder count is measured for this cycle, including counts measured both during the pump power on time as well as the coast down of the pump motor (block 706 ).
- Controlis transferred to block 707 where the encoder count is applied to the input power parameter look up table of block 703 , and the torque corresponding to the measured encoder count is determined by retrieving the torque value from the table. Alternatively, the torque can be determined by calculating it as a function of encoder count and input power parameters. Next, a determination is made whether the torque just measured exceeds the amount of torque which would correspond to an occlusion or other drive system problem (block 708 ). If it does not, then control loops to block 701 where the above described process is repeated.
- the alarm process represented by blocks 709 through 715(including incremental drive system rewind) is the same as previously described for blocks 510 through 516 of FIG. 5 .
- the maximum allowable torque value used in block 708can be calibrated to adjust for changes in system friction properties over time in a manner similar to that which was described in FIG. 5, blocks 517 - 520 .
- the pump drive systemperiodically could be rewound thereby removing any system fluid pressure forces from the pump drive system. Then input power parameters could be applied to the pump in either a forward or reverse direction to generate one pump cycle. The encoder count could be measured during this pump cycle and used to calculate a baseline average torque value which in turn could be used to calculate the maximum allowable torque for use in block 708 to determine whether there is an occlusion.
- FIGS. 5 through 7were discussed separately, it may be advantageous to use more than one of these embodiments in one pump system. Doing so may provide enhanced system redundancy and safety.
- preferred embodimentsdisclose a method and apparatus for automatically detecting an occlusion or drive system failure in a medication infusion pump system.
- the electrical current to an infusion pumpis measured and compared against a baseline average current. If the current exceeds a threshold amount, an alarm is triggered.
- pump motor encoder pulsesare measured during a pump cycle. If the number of pulses do not correspond to a normal range, an alarm is triggered.
- a system torque valueis determined from the measurement of pump motor encoder pulses during a pump cycle. If the system torque value exceeds a maximum threshold value, an alarm is triggered. After any alarm is triggered, the pump motor is driven in reverse for an incremental distance in order to relieve the fluid pressure in the system.
Landscapes
- Health & Medical Sciences (AREA)
- Heart & Thoracic Surgery (AREA)
- Veterinary Medicine (AREA)
- Engineering & Computer Science (AREA)
- Anesthesiology (AREA)
- Biomedical Technology (AREA)
- Hematology (AREA)
- Life Sciences & Earth Sciences (AREA)
- Animal Behavior & Ethology (AREA)
- General Health & Medical Sciences (AREA)
- Public Health (AREA)
- Vascular Medicine (AREA)
- Pulmonology (AREA)
- Infusion, Injection, And Reservoir Apparatuses (AREA)
- Supply Devices, Intensifiers, Converters, And Telemotors (AREA)
- Reciprocating Pumps (AREA)
- Medical Preparation Storing Or Oral Administration Devices (AREA)
- Details Of Reciprocating Pumps (AREA)
- Details Of Connecting Devices For Male And Female Coupling (AREA)
- Cable Accessories (AREA)
- Fluid-Pressure Circuits (AREA)
- Optical Couplings Of Light Guides (AREA)
- Mechanical Coupling Of Light Guides (AREA)
- Pens And Brushes (AREA)
- Transmission Of Braking Force In Braking Systems (AREA)
Abstract
Description
This application claims priority from provisional patent application No. 60/106,237, filed Oct. 29, 1998 and which is incorporated herein by reference.
1. Field of the Invention
This invention relates generally to improvements in infusion pumps such as those used for controlled delivery of medication to a patient. More specifically, this invention relates to improved methods and apparatuses for detecting occlusions in infusion pump systems.
2. Description of the Related Art
Infusion pump devices and systems are relatively well-known in the medical arts, for use in delivering or dispensing a prescribed medication such as insulin to a patient. In one form, such devices comprise a relatively compact pump housing adapted to receive a syringe or reservoir carrying a prescribed medication for administration to the patient through infusion tubing and an associated catheter or infusion set.
The infusion pump includes a small drive motor connected via a suitable transmission assembly for motor-driven advancement of a reservoir piston to administer the medication to the user. Programmable controls can operate the drive motor continuously or at periodic intervals to obtain a closely controlled and accurate delivery of the medication over an extended period of time. Such infusion pumps are utilized to administer insulin and other medications, with exemplary pump constructions being shown and described in U.S. Pat. Nos. 4,562,751; 4,678,408; 4,685,903; 5,080,653 and 5,097,122, which are incorporated by reference herein.
Infusion pumps of the general type described above have provided significant advantages and benefits with respect to accurate delivery of medication or other fluids over an extended period of time. The infusion pump can be designed to be extremely compact as well as water resistant, and may thus be adapted to be carried by the user, for example, by means of a belt clip. As a result, important medication can be delivered to the user with precision and in an automated manner, without significant restriction on the user's mobility or life-style, including in some cases the ability to participate in water sports.
These pumps often incorporate drive systems which use lead screws or other transmission components coupled to motors. The motors can be of the DC, stepper, solenoid or other varieties. These drive systems typically provide an axial displacement of the syringe or reservoir piston thereby dispensing the medication to the user. Powered drive systems are advantageous since they can be electronically controlled to deliver a predetermined amount of medication by means well known in the art.
In the past, medication infusion pumps have included alarm systems designed to detect and indicate pump malfunction and/or nondelivery of the medication to the patient as a result of an occluded delivery line. Such alarm systems have typically used a high pressure limit switch for activating an alarm when the force applied to the reservoir piston plunger reaches a predetermined upper limit indicative of an occluded medication delivery line. In U.S. Pat. No. 4,562,751, the high pressure switch is positioned at one end of a rotatable lead screw, wherein the mechanical reaction force or backlash between the reservoir plunger and the pressure switch is proportional to the pressure applied to the medication as a result of attempted advancement of the reservoir plunger.
In actual practice, however, such high pressure limit switch systems have several disadvantages. For example, the lead screw must move axially some distance to actuate the switch. If the concentration of the medication requires very small (0.5 microliter) dosage increments, then the axial displacement of the reservoir plunger is designed to be very small per increment. Thus, several increments may be required to initiate an alarm when there is an occlusion. This elapse of several increments before an alarm is initiated may represent less medication delivered to the user than is desired. Moreover, there are frictional considerations. The lead screw mechanism of this pump design includes seals, a drive nut, a lead screw/motor coupling, and a bearing. All of these components have frictional properties. In systems where the high pressure switch is placed at the opposite end of the drive train from the reservoir plunger, the friction associated with these components are additive. These properties are known to change over time thereby making a fixed-setting, high pressure switch less effective.
There exists, therefore, a significant need for further improvements in medication infusion pumps, particularly with respect to providing an early warning of an occlusion or other pump drive system failures. The present invention fulfills this need and provides further related advantages.
To overcome the limitations in the prior art described above, preferred embodiments disclose a method and system for detecting occlusions or other pump system failures. Power is applied to a pump motor. A first pump motor current value is measured and a determination made whether the first pump motor current value exceeds a second value. If it does exceed the second value, the pump system is rewound at least to the point of reducing the pressure in the system and an alarm indication is given. A calculation is made of a total number of alarm indications, and a determination is made whether the total number of alarm indications exceeds a third value. If it does, a system error message is given.
In an alternative embodiment of the present invention, input power parameters are applied to a pump motor to provide a first pump cycle. A first encoder count is measured during the first pump cycle and a determination is made whether the first encoder count is less than a first value. If it is less than the first value, the fluid pump system is rewound at least to the point of reducing pressure in the system, and an alarm indication is provided. A calculation is made of a total number of alarm indications which is used to determine whether this exceeds a second value. If so, a system error message is provided. To provide system feedback, a determination is made whether the first encoder count is equal to a third value. If it is not equal to the third value, the input power parameters are adjusted to provide a second pump cycle which, in turn, causes a second encoder count of a different value than the first encoder count. The second encoder count will be greater than the first encoder count if the first encoder count was less than the third value. Alternatively, the second encoder count will be smaller than the first encoder count if the first encoder count was greater than the third value.
In still a further embodiment, an input power parameter is provided to a pump motor to provide a pump cycle. An encoder count is measured during the pump cycle. A torque value corresponding to the encoder count and to the input power parameter is determined. Another determination is made whether the torque value is greater than a first value. If so, the fluid pump system is rewound at least to the point of reducing pressure in the fluid pump system. An alarm indication is also provided. A calculation is made of a total number of alarm indications which is used to determine whether this total exceeds a second value. If so, a system error message is provided.
FIG. 1 is a front, perspective view of a medication infusion pump, adapted to include the occlusion detectors claimed herein.
FIG. 2 is an enlarged, side plan, partial cut-away view of the infusion pump of FIG. 1 with portions broken away to illustrate certain pump components.
FIG. 3 is a side plan, cut-away view of another infusion pump design, adapted to include the occlusion detectors claimed herein.
FIG. 4 is a block diagram illustrating a software and hardware environment in which some embodiments of the present invention may be implemented.
FIGS. 5-7 illustrate different embodiments of logic for detecting occlusions in accordance with certain embodiments of the present invention.
In the following description, reference is made to the accompanying drawings which form a part hereof and which illustrate several embodiments of the present inventions. It is understood that other embodiments may be utilized and structural and operational changes may be made without departing from the scope of the present inventions.
As shown in the exemplary drawings, a medication infusion pump referred to generally in FIG. 1 by thereference numeral 101 is provided for controlled administration of medication to a patient. In accordance with the invention, theinfusion pump 101 includes an improved occlusion detector for monitoring and verifying proper delivery of the medication to the patient.
Theinfusion pump 101 has an overall mechanical construction and mechanical operation which is generally known in the art. More specifically, with respect to FIGS. 1 and 2, theinfusion pump 101 comprises a relativelycompact pump housing 102 defining an elongated chamber103 (FIG. 2) for receiving and supporting asyringe 104 charged with a selected medication, such as insulin, to be administered to a patient. The medication-containingsyringe 104 includes asyringe barrel 105 joined at the front to aluer neck 106 of reduced diametric size to seat snugly within anoutlet port 107 formed in thepump housing 102. A luer fitting108 is carried on theneck 106 and cooperates with thebarrel 105 to fix the syringe in a seated position within thesyringe chamber 103. A syringe piston orplunger 109 extends from the aft end of thebarrel 105 and may be advanced into the barrel to deliver the medication therefrom. In this regard, the medication is normally dispensed to the patient through acatheter tube 110 adapted for appropriate connection to theluer fitting 108.
Theinfusion pump 101 includes a compact drive motor111 (FIG. 2) which is mechanically connected to thesyringe plunger 109 for purposes of advancing the plunger in a precision controlled manner to dispense the medication. In this regard, thedrive motor 111 is normally powered by abattery power supply 112, in response to operation of a programmable controller113. As known in the art, the programmable controller113 can be set by the attending physician, appropriate medical personnel, or the user by an array ofbuttons 114 on the face or front of thepump housing 102, with acorresponding display panel 115 providing appropriate information regarding set status and/or pump operation. The controller113 operates thedrive motor 111 in a stepwise manner, typically on an intermittent basis, to administer discrete precision doses of the medication to the patient at programmed times.
The mechanical connection between thedrive motor 111 and thesyringe plunger 109 includes alead screw assembly 116. More specifically, thedrive motor 111 has a rotary output for rotatably driving anelongated lead screw 117 mounted within thepump housing 102. Alead screw nut 118 is carried on thelead screw 117 and includes anappropriate latch arm 119 for engaging adrive flange 120 on the end of thepiston plunger 109. Appropriate rotation of thelead screw 117 in response to operation of themotor 111, causes thelead screw nut 118 and associatedlatch arm 119 to be translated in a precision manner to correspondingly advance thepiston plunger 109 and thereby deliver the medication from thesyringe barrel 105 and through thecatheter tube 110 to the user. Further details regarding the construction and operation of a medication infusion pump of this general type can be found in U.S. Pat. Nos. 4,562,571; 4,678,408; and 4,685,903, which are incorporated by reference herein.
FIG. 3 shows another infusion pump design for which an improved occlusion detector may be used. Theinfusion pump 301 comprises amotor 302,drive screw 303, andplunger slide 304 which are arranged in-line with areservoir 305. Themotor 302 rotates thedrive screw 303 via agear box 306. Thedrive screw 303 has external threads which engage the internal threads of theplunger slide 304. Thus the rotational torque of thedrive screw 303 is translated into axial force of theplunger slide 304. Theplunger slide 304 is in contact with areservoir plunger 307, and advances it thereby forcing the medication out of a luer fitting308 and into infusion settubing 309.
Attached to the rear of themotor 302 is anencoder 310, a device known in the art which is capable of counting motor rotations or fractional rotations. The encoder has signal andpower supply cables 311 which extend into the battery/electronics compartment312 of the pump housing. The pump drive mechanism design of FIG. 3 is described in greater detail in copending application Ser. No. 09/429,352, filed contemporaneously which application is incorporated by reference in its entirety.
FIG. 4 illustrates one software and hardware environment in which certain embodiments of the present invention may be implemented. A syringe orreservoir 401 with a luer fitting402 and infusion settubing 403 is connected to a motor drive train. The drive train comprises amotor 404 and atransmission 405 coupling themotor 404 to thereservoir 401. Other reservoir and drive train systems may be used, however, as described herein or as known in the art or as may be developed in the future. At one end of themotor 404 is an encoder system comprised of anencoder wheel 412 and anencoder sensor 406.
The encoder could be magnetic, optical, or other designs known in the art. When the encoder is attached to a motor, it provides a measurement of the rotational displacement of the motor. The accuracy of this measurement is a function of the count resolution of the encoder. If for example, the encoder had a resolution of 360 counts per motor revolution, then with each motor revolution, the sensor will provide 360 encoder signal pulses. If a pump system was designed to require one complete motor revolution to deliver the desired increment of medication, then the motor can be controlled to stop when 360 encoder counts are received.
Theencoder sensor 406 transmits the encoder pulses to anencoder logic circuit 407 which in turn communicates the counts to a processing unit, such asmicrocontroller 408. Amotor drive circuit 409 receives commands from themicrocontroller 408 and when so instructed, causes electrical power to be transmitted to themotor 404. A motordrive sensor circuit 410 measures the motor operating parameters such as motor voltage, motor current, and motor operating time, and transmits these parameters to themicrocontroller 408. Abattery 411 provides power for all of these components. Themicrocontroller 408 receives its operating instructions from software stored in random access memory (RAM), read only memory (ROM), or other devices known in the art for storing operating instructions for use by the microcontroller.
It will be appreciated that the foregoing computing environment is illustrative only. Any alternative computing environment involving a computer, a microcontroller or a microprocessor along with computer instruction code and circuitry is acceptable. For example, the microporcessor control may include RAM or ROM programs. Moreover, this can further be accomplished with a hardwired or programmable logic array driven state machine. Alternatively, a dedicated logic or a programmable logic array can be used to perform the operations necessary to test for an occlusion.
The improved occlusion detector described herein can be applied to the pump drive designs of FIGS. 1-3, or any other design incorporating a motor. The pumping mechanism can use DC motors, AC motors, stepper motors, solenoid motors, lead screws, cams, ratchets, jacks, piezoelectric caterpillar drives, Nitinol effectors, electrochemical gas cells or thermally driven gas or bimetallic actuators. The occlusion detector measures increased reservoir pressure indirectly by monitoring one or more motor parameters, such as voltage, current, running time, or rotational or linear displacement.
It is known in the art that torque developed by a brushed DC motor is directly proportional to the current supplied to it at steady state. Therefore, in a screw type drive system, as the axial load increases due to increased fluid pressure within the reservoir, more motor torque is required to drive the system.
Should there be an occlusion, the pressure inside the reservoir will exceed a predetermined threshold. Thus the current necessary to drive that load will exceed a predetermined current threshold and the electronics will be flagged to cease further delivering. In addition, an audible, tactile and/or display alarm typically is triggered.
However, care must be employed when clearing this alarm if the occlusion still exists and there is still a high pressure state in the reservoir. Since the motor must operate to obtain an indication of pressure within the reservoir, more and more pressure can potentially be developed within the system. If the motor is not in operation, there is no current flowing and negligible torque on the motor body. Therefore, when an occlusion exits distal from the reservoir due to pinched tubing for example, then the measured property will indicate this only during each motor delivery increment.
If the user clears the alarm and attempts to deliver medication again when the occlusion in fact was not removed, additional pressure will be generated within the fluid system. Assuming that the system is programmed to continue to alarm when the pressure (or motor current) is above the set point, then continued alarming will occur. Thus the user may on several occasions attempt to clear the alarm before locating and correcting the source of the occlusion.
When the occlusion is finally cleared, there could be excess pressure developed in the system which could result in the delivery of a bolus of medication larger than that which should be delivered. The improved occlusion detection system disclosed herein protects against this by causing the pump to rewind by some predetermined amount following each occlusion alarm. By rewinding the pump by, say, one delivery pulse, the occlusion alarm will trigger if the occlusion still exists. However it will do so at the same maximum pressure as programmed and not at above this value.
On a drive system that is bidirectional, such as that depicted in FIG. 3, the current measurement can also be used as an indicator of system wear. Over the life of the product, it is expected that the torque required to drive the system will change over time due to wear of the dynamic components and their interfaces. Since the torque required to rewind a bidirectional system is due to the drive system's frictional factors, the current to rewind can be recorded and is proportional to this torque.
As the system wears, the torque and therefore the current to rewind will change. By storing the rewind current, this can be used to calibrate the system. An averaged baseline rewind current can be determined and used to adjust the driving force baseline which is the torque (or current) required to advance the drive system when no other external forces, such as a syringe with fluid, are present. An alternative method would be to rewind the system, and then immediately thereafter, obtain the forward or driving baseline current by driving the system forward for some distance and recording it, after which, the system is rewound again. The advantage of using either method is that the calibration can be automatic and transparent to the user.
FIG. 5 illustrates the logic in one embodiment of the detector wherein motor current is measured for detecting a system occlusion. Control begins atblock 501 where the system determines whether it is necessary to fully rewind the pump drive system. Conditions requiring such a rewind of the drive system will be discussed below. If the system is not to be rewound, then a determination is made whether it is time for an increment of medication is to be delivered (block502). This determination is a function of the programming which is unique to the medical condition of each user, the type of medication being provided, or the like. If it is not time to deliver medication, then the program loops to the start for additional time to elapse or for the receipt of other control commands.
However, if it is time for delivery of an increment of medication, control transfers to block503 where power is applied to the pump motor thus causing medication to be dispensed from the reservoir. Next, the amount of medication delivered from the reservoir is measured (block504). This can be accomplished directly or indirectly in several ways, including measuring (1) encoder counts, (2) pump operation time, (3) reservoir plunger position location, velocity or acceleration, (4) the location of any moveable component on the pump drive train, or (5) the mass or volumetric flow of the liquid.
A determination is then made as to whether the amount of medication delivered is sufficient (block505). If it is sufficient, control is transferred to block506 where the pump is stopped and the program loops to the beginning. If on the other hand, the pump is continuing to run, but the programmed dosage has not yet been delivered, then the pump motor current is measured (block507). If there is an occlusion in the system, an increase in reservoir fluid pressure will likely result. This, in turn, can cause greater motor torque and current as the motor attempts to advance the reservoir plunger against this fluid pressure. Thus, if the measured motor current is some amount greater than a known, average baseline motor current, which may be established when there was no occlusion condition, then it is determined that an occlusion condition has likely occurred.
Not only can this current measurement indicate an occlusion condition, this motor current can provide feedback as to drive system characteristics, performance, and functionality, especially with the addition of an encoder. If for example, there was a failure of the gearbox causing the motor to be unable to rotate, the measured current would be high (above predetermined threshold settings) and the encoder would not increment. This would be an indication of a drive system fault. For the inline drive system shown in FIG. 3, a failure of the gearbox, screw, or slide interface would be indicated by this condition.
Referring to FIG. 5, atblock 508 the value of the average baseline current is retrieved from a storage location in memory represented byblock 520. This value is compared with the current measured at the present time and a determination is made whether the present current exceeds the average baseline by a certain amount. If it does not, then the pump continues to run and control loops to block504 where the amount of medication delivery is again measured. On the other hand, if the present current exceeds the average baseline by a selected amount, then the pump motor is stopped and an alarm indication, audible, tactile and/or visible, is given (blocks 509 and510).
Control transfers to block511 where the system is monitored for clearing of the alarm. If the alarm has not been cleared, then control loops to block510 where the alarm will continue to display. If the alarm has been cleared by the user, then control transfers to block512 where the drive system is rewound by an incremental amount. This rewinding serves to decrease the reservoir fluid back pressure which in turn inhibits or prevents the delivery of an excessive bolus of medication should the user experience a series of occlusion alarms before successfully clearing the occlusion.
Control then transfers to block513 where an alarm flag is stored. A determination is made whether there have been an excessive number of recent alarms (block514). If there have not, then control loops to the beginning (block501) where the above described process is repeated. On the other hand, if there have been an excessive number of recent alarms, control transfers to block515 where an error or reset message is displayed to the user. This message typically would be used to advise the user to contact the manufacturer or some authorized repair facility to determine the cause of the excessive number of alarms. This error message will continue to be displayed until the error is cleared (block516) at which point control loops to the beginning (block501) where the process is repeated.
Returning to block501, there are times when a full rewind of the drive system may be required. One instance would be when the medication reservoir in the pump housing is empty and a new reservoir must be inserted. Thus, when it has been determined that rewinding of the drive system is desired (either by user command or otherwise), control transfers to block517 where power is applied to the pump motor. As the motor is running in a rewind direction, the pump motor current is measured (block518). An alternative method would be to obtain the forward or driving baseline current by driving the system forward (possibly immediately following rewind) for some distance and recording it, after which the system may need to be rewound again. Because the motor is running in the opposite direction (or forward following rewind), typically there is little or no fluid pressure against which the pump motor is driving. Thus the current measured during this phase can be used as a baseline for comparison in detecting occlusions.
Control transfers to block519 where the previous average baseline current value is retrieved from a storage location in memory (block520) and an updated average baseline current is calculated. This updated value is then placed in the storage location, (block520), where it will be available for the next current measurement and comparison atblock 508.
The value of repeatedly updating the average baseline current is to provide a calibration against changing drive train friction forces. The lead screw mechanism of many pump designs includes seals, a drive nut, a lead screw/motor coupling, and a bearing. All of these components have frictional properties. These properties are known to change over time and thus the motor torque and current required to advance a reservoir plunger are likely to change. This therefore provides a more accurate baseline against which current can be measured for the detection of an occlusion.
Although the foregoing description involved the measurement of motor current, other motor parameters which vary with differing fluid back pressures can be measured with like effect. Such parameters may include motor voltage, linear displacement, rotary displacement, torque, rotor speed, and the like.
For example, one alternative embodiment of the occlusion detector involves the use of a motor position encoder which can detect the motor's linear or rotational displacement. If for example, the encoder has a resolution of 360 counts per motor revolution of a rotary motor, then with each motor revolution, the sensor will provide 360 encoder signal pulses. If the pump system were designed to require one complete motor revolution to deliver the desired increment of medication, then the motor can be controlled to stop when 360 encoder counts are received. Linear displacements of linear motors may be similarly detected by suitable linear encoders or sensors.
Because motors have inertia, the power supplied to them must be removed prior to the actual stopping position in order for the motor to slow and stop. The slowing or deceleration can be accomplished in several ways including: (1) coasting which simply lets the applied and frictional torque slow the motor; or (2) dynamic braking which can be accomplished for example by shorting the motor leads or applying a potential in the opposite direction.
The applied torque affects the total rotational count. Thus as the applied torque varies, so will the error from the desired 360 counts. To account for a deviation from the target encoder count, a feedback loop is provided whereby the input power parameters to the motor, such as motor voltage or current or the time during which power is applied to the motor, may be adjusted.
In one embodiment, the motor is controlled based on the results of the previous encoder count for each cycle. Thus, for example, if 360 encoder counts were desired, but only 350 were measured, then subsequent input motor parameters can be adjusted such that the running encoder average is maintained at 360 counts. If a motor system was used with a DC motor driven with a constant current source or fixed source voltage, then the motor input parameter to be adjusted for maintaining the desired encoder count for the next pump cycle would be power on time.
For example, a motor may be driven such that half of the rotational displacement (or 180 out of 360 counts) is due to power on time and the other half is due to the coasting down of the motor under a specified nominal load (torque). Should the load increase, then the coasting would decrease thereby reducing the total encoder count measured for a constant power input. For example, the system may measure 350 counts rather than the target value of 360 counts. To maintain medication delivery accuracy therefore, the subsequent motor increment during the next pump cycle may be increased above the 180 encoder count for the power on time so that the running average is maintained at 360 for the entire pump cycle.
FIG. 6 illustrates an example of the logic of an embodiment of the present invention using an encoder. Control begins atblock 601, where a determination is made whether it is time for an increment of medication is to be delivered. The same considerations apply here as was described forblock 502 of FIG.5. If it is not time to deliver medication, then the program loops to the start in order for additional time to elapse or for the receipt of other control commands.
However, if it is time for delivery of an increment of medication, control transfers to block602 where the applicable input power parameters are read from a memory storage location represented byblock 603. Such power parameters would include pump power on time, operating voltage and operating current. These parameters are applied to the pump to drive it for one pump cycle. This causes an increment of medication to be delivered as the pump runs for a discrete period of time (blocks 604 &605). The pump encoder counts are measured during this motor drive increment (block606). Counts are measured both during the time that power is applied to the pump motor as well as the time during which the pump coasts down.
Control transfers to block607 where the encoder count occlusion alarm limit number is read from the storage location represented byblock 608. This alarm limit number is compared with the encoder count just measured (block609). If the encoder count is smaller than the alarm limit amount, then it is determined that the pump motor may have experienced excessive torque which can be associated with an occlusion or a drive system problem. If this occurs, then control transfers to block612 where an alarm (audible, tactile and/or visual) is rendered. The alarm process represented byblocks 612 through618 (including the incremental drive system rewind) is the same as previously described forblocks 510 through516 of FIG.5.
Although not shown in FIG. 6, it can be appreciated that the alarm count table (block608) can be calibrated to adjust for changes in system friction properties over time in a manner similar to that which was described in FIG. 5, blocks517-520. In the embodiment of FIG. 6, the pump drive system periodically could be rewound thereby removing any system fluid pressure forces from the pump drive system. Then input power parameters could be applied to the pump in either a forward or reverse direction to generate one pump cycle. The encoder count could be measured during this pump cycle and used to calculate a baseline average encoder alarm count for storage in the alarm count table (block608).
Returning to block609 of FIG. 6, if the encoder count is not less than the alarm limit, then control transfers to block610 where a determination is made whether the encoder count is outside of an operating parameter range for purposes of adjusting the input power parameters. For example, if the encoder count should be 360 counts for the proper medication delivery increment, but during the current cycle, only 350 counts were measured, then this might not be low enough to indicate an occlusion condition. However a 350 count might indicate a need for an input power adjustment for the next increment delivery cycle. One possible adjustment might comprise an increase in the pump power on time.
If it is determined that an input power parameter adjustment is needed, control is transferred to block611 where the adjustment amount is calculated and stored in the memory location represented byblock 603. Control then loops to block601 where the above described process is repeated. On the other hand if the increment count is such that no adjustment is needed, then control loops directly to block601. Thus, the process represented byblocks
Yet another embodiment of the occlusion detector uses an encoder count to determine torque. In this embodiment, torque is a function of encoder count and one or more motor input power parameters. Motor load torque can be determined by evaluating the stored encoder count for a known delivered amount of energy. The detector system provides a known amount of energy (i.e., power times motor on-time), and records the motor displacement via the number of encoder counts obtained. Using a look-up table or calculated value, the system determines a corresponding torque that would result from the recorded number of encoder pulses for the amount of energy supplied.
For example, if the motor were running for a certain amount of time, this might result in an encoder count of 360. Later,the motor might run for the same amount of time under the same voltage and current conditions, but an encoder count of 350 may result. Thus the system would have encountered increased torque as reflected by the reduced encoder count. A lookup table or calculated value of torque vs encoder count and input power parameters can thereby be developed and used to measure motor torque.
FIG. 7 illustrates an example of the logic of an embodiment of the present invention which uses an encoder to measure torque. Control begins atblock 701 where a determination is made whether it is time to deliver a medication dosage increment. If it is time to deliver a dose, then control transfers to block702 where the pump input power parameters which will be used on the upcoming cycle are stored in a storage location of an input power look up table represented byblock 703. Next, these same input power parameters are applied to the pump motor which operates for the selected time interval and delivers the dosage increment (blocks 704 &705). The encoder count is measured for this cycle, including counts measured both during the pump power on time as well as the coast down of the pump motor (block706).
Control is transferred to block707 where the encoder count is applied to the input power parameter look up table ofblock 703, and the torque corresponding to the measured encoder count is determined by retrieving the torque value from the table. Alternatively, the torque can be determined by calculating it as a function of encoder count and input power parameters. Next, a determination is made whether the torque just measured exceeds the amount of torque which would correspond to an occlusion or other drive system problem (block708). If it does not, then control loops to block701 where the above described process is repeated.
On the other hand, if the calculated torque does exceed the amount of torque associated with an occlusion or drive system problem, control transfers to block709 where an audible, tactile and/or visual alarm is given. The alarm process represented by blocks709 through715 (including incremental drive system rewind) is the same as previously described forblocks 510 through516 of FIG.5.
Although not shown in FIG. 7, it can be appreciated that the maximum allowable torque value used inblock 708 can be calibrated to adjust for changes in system friction properties over time in a manner similar to that which was described in FIG. 5, blocks517-520. In the embodiment of FIG. 7, the pump drive system periodically could be rewound thereby removing any system fluid pressure forces from the pump drive system. Then input power parameters could be applied to the pump in either a forward or reverse direction to generate one pump cycle. The encoder count could be measured during this pump cycle and used to calculate a baseline average torque value which in turn could be used to calculate the maximum allowable torque for use inblock 708 to determine whether there is an occlusion.
In further can be appreciated that although the embodiments of FIGS. 5 through 7 were discussed separately, it may be advantageous to use more than one of these embodiments in one pump system. Doing so may provide enhanced system redundancy and safety.
In summary, preferred embodiments disclose a method and apparatus for automatically detecting an occlusion or drive system failure in a medication infusion pump system. The electrical current to an infusion pump is measured and compared against a baseline average current. If the current exceeds a threshold amount, an alarm is triggered. Alternatively, pump motor encoder pulses are measured during a pump cycle. If the number of pulses do not correspond to a normal range, an alarm is triggered. Alternatively, a system torque value is determined from the measurement of pump motor encoder pulses during a pump cycle. If the system torque value exceeds a maximum threshold value, an alarm is triggered. After any alarm is triggered, the pump motor is driven in reverse for an incremental distance in order to relieve the fluid pressure in the system.
While the description above refers to particular embodiments of the present inventions, it will be understood that many modifications may be made without departing from the spirit thereof. The accompanying claims are intended to cover such modifications as would fall within the true scope and spirit of the present inventions. The presently disclosed embodiments are therefore to be considered in all respects as illustrative and not restrictive, the scope of the inventions being indicated by the appended claims rather than the foregoing description, and all changes which come within the meaning and range of equivalency of the claims are therefore intended to be embraced therein.
Claims (17)
1. A method of detecting an occlusion in a fluid pump system, comprising:
applying input power parameters to a pump motor to provide a first pump cycle;
measuring a first encoder count during the first pump cycle;
determining whether the first encoder count is less than a first value; and
rewinding the fluid pump system at least to the point of reducing pressure in the fluid pump system after determining that the first encoder count is less than the first value.
2. The method ofclaim 1 further comprising providing an alarm indication after determining that the first encoder count is less than the first value.
3. The method ofclaim 1 further comprising:
determining whether the first encoder count is equal to a third value; and
adjusting the input power parameters to provide a second pump cycle after determining that the first encoder count does not equal the third value, said second pump cycle resulting in a second encoder count, said second encoder count being one of a value greater than the first encoder count if the first encoder count is less than the third value and a value smaller than the first encoder count if the first encoder count is greater than the third value.
4. A method of detecting an occlusion in a fluid pump system, comprising:
applying input power parameters to a pump motor to provide a first pump cycle;
measuring a first encoder count during the first pump cycle;
determining whether the first encoder count is less than a first value;
providing an alarm indication after determining that the first encoder count is less than the first value;
calculating a total number of alarm indications after determining that the first encoder count is less than the first value;
determining whether the total number of alarm indications exceeds a second value; and
providing a system error message after determining that the total number of alarm indications exceeds the second value.
5. A method of detecting an occlusion in a fluid pump system, comprising:
applying input power parameters to a pump motor to provide a first pump cycle;
measuring a first encoder count during the first pump cycle;
determining whether the first encoder count is less than a first value;
rewinding the fluid pump system at least to the point of reducing pressure in the fluid pump system after determining that the first encoder count is less than the first value;
providing an alarm indication after determining that the first encoder count is less than the first value;
calculating a total number of alarm indications after determining that the first encoder count is less than the first value;
determining whether the total number of alarm indications exceeds a second value;
providing a system error message after determining that the total number of alarm indications exceeds the second value;
determining whether the first encoder count is equal to a third value; and
adjusting the input power parameters to provide a second pump cycle after determining that the first encoder count does not equal the third value, said second pump cycle resulting in a second encoder count, said second encoder count being one of a value greater than the first encoder count if the first encoder count is less than the third value and a value smaller than the first encoder count if the first encoder count is greater than the third value.
6. A system for detecting an occlusion in a fluid pump system, comprising:
a processing unit capable of communicating with the fluid pump system; and
program logic executed by the processing unit, comprising:
means for applying input power parameters to a pump motor to provide a first pump cycle;
means for measuring a first encoder count during the first pump cycle;
means for determining whether the first encoder count is less than a first value; and
means for rewinding the fluid pump system at least to the point of reducing pressure in the fluid pump system after determining that the first encoder count is less than the first value.
7. The system ofclaim 6 wherein the program logic further comprises means for providing an alarm indication after determining that the first encoder count is less than the first value.
8. The system ofclaim 6 wherein the program logic further comprises:
means for determining whether the first encoder count is equal to a third value; and
means for adjusting the input power parameters to provide a second pump cycle after determining that the first encoder count does not equal the third value, said second pump cycle resulting in a second encoder count, said second encoder count being one of a value greater than the first encoder count if the first encoder count is less than the third value and a value smaller than the first encoder count if the first encoder count is greater than the third value.
9. A system for detecting an occlusion in a fluid pump system, comprising:
a processing unit capable of communicating with the fluid pump system; and
program logic executed by the processing unit, comprising:
means for applying input power parameters to a pump motor to provide a first pump cycle;
means for measuring a first encoder count during the first pump cycle;
means for determining whether the first encoder count is less than a first value; and
means for providing an alarm indication after determining that the first encoder count is less than the first value;
means for calculating a total number of alarm indications after determining that the first encoder count is less than the first value;
means for determining whether the total number of alarm indications exceeds a second value; and
means for providing a system error message after determining that the total number of alarm indications exceeds the second value.
10. A system for detecting an occlusion in a fluid pump system, comprising:
a processing unit capable of communicating with the fluid pump system; and
program logic executed by the processing unit, comprising:
means for applying input power parameters to a pump motor to provide a first pump cycle;
means for measuring a first encoder count during the first pump cycle;
means for determining whether the first encoder count is less than a first value;
means for rewinding the fluid pump system at least to the point of reducing pressure in the fluid pump system after determining that the first encoder count is less than the first value;
means for providing an alarm indication after determining that the first encoder count is less than the first value;
means for calculating a total number of alarm indications after determining that the first encoder count is less than the first value;
means for determining whether the total number of alarm indications exceeds a second value;
means for providing a system error message after determining that the total number of alarm indications exceeds the second value;
means for determining whether the first encoder count is equal to a third value; and
means for adjusting the input power parameters to provide a second pump cycle after determining that the first encoder count does not equal the third value, said second pump cycle resulting in a second encoder count, said second encoder count being one of a value greater than the first encoder count if the first encoder count is less than the third value and a value smaller than the first encoder count if the first encoder count is greater than the third value.
11. A method of operating a fluid pump system, comprising:
applying a power parameter to deliver a motor drive increment to the fluid pump system;
measuring a response of the fluid pump system to the power parameter so applied;
determining if the power parameter and the response of the fluid pump system are within a predetermined acceptable limit; and
rewinding the fluid pump system at least to the point of reducing pressure in the fluid pump system if the power parameter and the response of the fluid pump system are not within the predetermined acceptable limit.
12. The method ofclaim 11 , wherein the step of measuring a response of the fluid pump system to the power parameter includes reading encoder counts.
13. The method ofclaim 11 , wherein the power parameter is an amount of power and the step of determining comprises determining if the amount of power used to deliver a motor drive increment to the fluid pump system is about equal to an expected amount of power to be used to deliver a motor drive increment to the fluid pump system.
14. The method ofclaim 11 , further including adjusting the power parameter to deliver a motor drive increment to the fluid pump system if the power parameter and the response of the fluid pump system are not within the predetermined acceptable limit.
15. The method ofclaim 11 , further including providing an alarm if the power parameter and the response of the fluid pump system are not within the predetermined acceptable limit.
16. The method ofclaim 11 , wherein the step of applying the power parameter to drive the motor includes reading one or more input power parameters from a memory.
17. The method ofclaim 16 , further including modifying the one or more input power parameters in the memory if the power parameter and the response of the fluid pump system are not within the predetermined acceptable limit.
Priority Applications (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US09/428,411US6362591B1 (en) | 1998-10-29 | 1999-10-28 | Method and apparatus for detection of occlusions |
| US10/017,882US6555986B2 (en) | 1998-10-29 | 2001-12-13 | Method and apparatus for detection of occlusions |
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US10623798P | 1998-10-29 | 1998-10-29 | |
| US09/428,411US6362591B1 (en) | 1998-10-29 | 1999-10-28 | Method and apparatus for detection of occlusions |
Related Child Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US10/017,882DivisionUS6555986B2 (en) | 1998-10-29 | 2001-12-13 | Method and apparatus for detection of occlusions |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| US6362591B1true US6362591B1 (en) | 2002-03-26 |
Family
ID=22310289
Family Applications (14)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US09/428,411Expired - LifetimeUS6362591B1 (en) | 1998-10-29 | 1999-10-28 | Method and apparatus for detection of occlusions |
| US09/428,818Expired - LifetimeUS6585695B1 (en) | 1998-10-29 | 1999-10-28 | Reservoir connector |
| US10/017,882Expired - LifetimeUS6555986B2 (en) | 1998-10-29 | 2001-12-13 | Method and apparatus for detection of occlusions |
| US10/328,393Expired - LifetimeUS7658734B2 (en) | 1998-10-29 | 2002-12-23 | Reservoir connector |
| US10/607,340AbandonedUS20040003493A1 (en) | 1998-10-29 | 2003-06-26 | Reservoir connector |
| US10/923,133Expired - Fee RelatedUS7628782B2 (en) | 1998-10-29 | 2004-08-20 | Reservoir connector |
| US12/361,203Expired - Fee RelatedUS7981105B2 (en) | 1998-10-29 | 2009-01-28 | Medication reservoir |
| US12/614,229Expired - Fee RelatedUS7998131B2 (en) | 1998-10-29 | 2009-11-06 | Reservoir connector |
| US12/631,377Expired - Fee RelatedUS8257345B2 (en) | 1998-10-29 | 2009-12-04 | Reservoir connector |
| US12/693,232Expired - Fee RelatedUS7988683B2 (en) | 1998-10-29 | 2010-01-25 | Reservoir connector |
| US13/155,641Expired - Fee RelatedUS8303572B2 (en) | 1998-10-29 | 2011-06-08 | Medication reservoir |
| US13/622,183Expired - Fee RelatedUS8500716B2 (en) | 1998-10-29 | 2012-09-18 | Medication reservoir |
| US13/950,832AbandonedUS20130310807A1 (en) | 1998-10-29 | 2013-07-25 | Medication reservoir |
| US14/489,163Expired - Fee RelatedUS9579452B2 (en) | 1998-10-29 | 2014-09-17 | Medication reservoir |
Family Applications After (13)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US09/428,818Expired - LifetimeUS6585695B1 (en) | 1998-10-29 | 1999-10-28 | Reservoir connector |
| US10/017,882Expired - LifetimeUS6555986B2 (en) | 1998-10-29 | 2001-12-13 | Method and apparatus for detection of occlusions |
| US10/328,393Expired - LifetimeUS7658734B2 (en) | 1998-10-29 | 2002-12-23 | Reservoir connector |
| US10/607,340AbandonedUS20040003493A1 (en) | 1998-10-29 | 2003-06-26 | Reservoir connector |
| US10/923,133Expired - Fee RelatedUS7628782B2 (en) | 1998-10-29 | 2004-08-20 | Reservoir connector |
| US12/361,203Expired - Fee RelatedUS7981105B2 (en) | 1998-10-29 | 2009-01-28 | Medication reservoir |
| US12/614,229Expired - Fee RelatedUS7998131B2 (en) | 1998-10-29 | 2009-11-06 | Reservoir connector |
| US12/631,377Expired - Fee RelatedUS8257345B2 (en) | 1998-10-29 | 2009-12-04 | Reservoir connector |
| US12/693,232Expired - Fee RelatedUS7988683B2 (en) | 1998-10-29 | 2010-01-25 | Reservoir connector |
| US13/155,641Expired - Fee RelatedUS8303572B2 (en) | 1998-10-29 | 2011-06-08 | Medication reservoir |
| US13/622,183Expired - Fee RelatedUS8500716B2 (en) | 1998-10-29 | 2012-09-18 | Medication reservoir |
| US13/950,832AbandonedUS20130310807A1 (en) | 1998-10-29 | 2013-07-25 | Medication reservoir |
| US14/489,163Expired - Fee RelatedUS9579452B2 (en) | 1998-10-29 | 2014-09-17 | Medication reservoir |
Country Status (9)
| Country | Link |
|---|---|
| US (14) | US6362591B1 (en) |
| EP (6) | EP2204203A3 (en) |
| JP (4) | JP4267206B2 (en) |
| AT (3) | ATE498422T1 (en) |
| AU (2) | AU1330500A (en) |
| CA (5) | CA2345439C (en) |
| DE (3) | DE69923858T2 (en) |
| DK (3) | DK1716884T3 (en) |
| WO (2) | WO2000025844A1 (en) |
Cited By (216)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US20030167039A1 (en)* | 1999-10-28 | 2003-09-04 | Medtronic Minimed, Inc. | Drive system seal |
| US20030205587A1 (en)* | 2000-08-16 | 2003-11-06 | Tribe Robert James | Syringe pumps |
| US6656148B2 (en)* | 1999-06-18 | 2003-12-02 | Animas Corporation | Infusion pump with a sealed drive mechanism and improved method of occlusion detection |
| US20050123408A1 (en)* | 2003-12-08 | 2005-06-09 | Koehl Robert M. | Pump control system and method |
| US20070062250A1 (en)* | 2005-09-19 | 2007-03-22 | Lifescan, Inc. | Malfunction Detection With Derivative Calculation |
| US20070066940A1 (en)* | 2005-09-19 | 2007-03-22 | Lifescan, Inc. | Systems and Methods for Detecting a Partition Position in an Infusion Pump |
| US20070062251A1 (en)* | 2005-09-19 | 2007-03-22 | Lifescan, Inc. | Infusion Pump With Closed Loop Control and Algorithm |
| US20070149926A1 (en)* | 1998-10-29 | 2007-06-28 | Medtronic Minimed, Inc. | Method and Apparatus for Detecting Errors, Fluid Pressure, and Occlusions in an Ambulatory Infusion Pump |
| US20070163929A1 (en)* | 2004-08-26 | 2007-07-19 | Pentair Water Pool And Spa, Inc. | Filter loading |
| US20070183902A1 (en)* | 2004-08-26 | 2007-08-09 | Pentair Water Pool And Spa, Inc. | Anti-entrapment and anti-dead head function |
| US20070219597A1 (en)* | 2006-02-09 | 2007-09-20 | Dean Kamen | Adhesive and peripheral systems and methods for medical devices |
| WO2008063429A2 (en) | 2006-11-20 | 2008-05-29 | Medtronic Minimed, Inc. | Method and apparatus for detecting occlusions in an ambulatory infusion pump |
| US20080154187A1 (en)* | 2006-12-21 | 2008-06-26 | Lifescan, Inc. | Malfunction detection in infusion pumps |
| US20080281278A1 (en)* | 2007-05-09 | 2008-11-13 | E-Z-Em, Inc. | Injector device, method, and computer program product for detecting a vacuum within a syringe |
| US20090087325A1 (en)* | 2007-09-27 | 2009-04-02 | Voltenburg Jr Robert R | Peristaltic pump assembly and regulator therefor |
| US20090087327A1 (en)* | 2007-09-27 | 2009-04-02 | Voltenburg Jr Robert R | Peristaltic pump and removable cassette therefor |
| US20090087326A1 (en)* | 2007-09-27 | 2009-04-02 | Voltenburg Jr Robert R | Peristaltic pump assembly |
| US7530968B2 (en) | 2003-04-23 | 2009-05-12 | Valeritas, Inc. | Hydraulically actuated pump for long duration medicament administration |
| US20090157003A1 (en)* | 2007-12-14 | 2009-06-18 | Jones Daniel W | Method And Apparatus For Occlusion Prevention And Remediation |
| WO2010076275A1 (en)* | 2008-12-29 | 2010-07-08 | Sanofi-Aventis Deutschland Gmbh | Medical injection device with electric motor drive control |
| WO2010078073A1 (en) | 2008-12-30 | 2010-07-08 | Medtronic Minimed, Inc. | Reservoir compartment adapter for infusion device |
| WO2010096602A1 (en)* | 2009-02-20 | 2010-08-26 | Hospira, Inc. | Occlusion detection system |
| EP2229967A1 (en) | 2009-03-17 | 2010-09-22 | F. Hoffmann-La Roche AG | Cannula assembly and ambulatory infusion system with a pressure sensor made of stackes coplanar layers |
| US20100254825A1 (en)* | 2004-08-26 | 2010-10-07 | Stiles Jr Robert W | Pumping System with Power Optimization |
| US20100310382A1 (en)* | 2009-06-09 | 2010-12-09 | Melissa Drechsel Kidd | Method of Controlling a Pump and Motor |
| US20110052416A1 (en)* | 2004-08-26 | 2011-03-03 | Robert Stiles | Variable Speed Pumping System and Method |
| US7914499B2 (en) | 2006-03-30 | 2011-03-29 | Valeritas, Inc. | Multi-cartridge fluid delivery device |
| US20110076156A1 (en)* | 2004-08-26 | 2011-03-31 | Stiles Jr Robert W | Flow Control |
| US20110091329A1 (en)* | 2004-08-26 | 2011-04-21 | Stiles Jr Robert W | Pumping System with Two Way Communication |
| WO2011044706A1 (en)* | 2009-10-16 | 2011-04-21 | Tecpharma Licensing Ag | Occlusion recognition in an administering apparatus |
| US8016789B2 (en) | 2008-10-10 | 2011-09-13 | Deka Products Limited Partnership | Pump assembly with a removable cover assembly |
| US8034026B2 (en) | 2001-05-18 | 2011-10-11 | Deka Products Limited Partnership | Infusion pump assembly |
| US8066672B2 (en) | 2008-10-10 | 2011-11-29 | Deka Products Limited Partnership | Infusion pump assembly with a backup power supply |
| EP2397170A2 (en) | 2008-12-30 | 2011-12-21 | Medtronic MiniMed, Inc. | Medical fluid administration device and color coding and detection system for glucose sensor set |
| US20120078185A1 (en)* | 2010-09-24 | 2012-03-29 | Smith Roger E | Infusion pumps |
| US8223028B2 (en) | 2008-10-10 | 2012-07-17 | Deka Products Limited Partnership | Occlusion detection system and method |
| US8234128B2 (en) | 2002-04-30 | 2012-07-31 | Baxter International, Inc. | System and method for verifying medical device operational parameters |
| US8262616B2 (en) | 2008-10-10 | 2012-09-11 | Deka Products Limited Partnership | Infusion pump assembly |
| US8267892B2 (en) | 2008-10-10 | 2012-09-18 | Deka Products Limited Partnership | Multi-language / multi-processor infusion pump assembly |
| US8287495B2 (en) | 2009-07-30 | 2012-10-16 | Tandem Diabetes Care, Inc. | Infusion pump system with disposable cartridge having pressure venting and pressure feedback |
| US8414563B2 (en) | 2007-12-31 | 2013-04-09 | Deka Products Limited Partnership | Pump assembly with switch |
| US8496646B2 (en) | 2007-02-09 | 2013-07-30 | Deka Products Limited Partnership | Infusion pump assembly |
| US8564233B2 (en) | 2009-06-09 | 2013-10-22 | Sta-Rite Industries, Llc | Safety system and method for pump and motor |
| US8573952B2 (en) | 2004-08-26 | 2013-11-05 | Pentair Water Pool And Spa, Inc. | Priming protection |
| US8602743B2 (en) | 2008-10-06 | 2013-12-10 | Pentair Water Pool And Spa, Inc. | Method of operating a safety vacuum release system |
| US8628510B2 (en) | 2010-12-22 | 2014-01-14 | Medtronic Minimed, Inc. | Monitoring the operating health of a force sensor in a fluid infusion device |
| US8690855B2 (en)* | 2010-12-22 | 2014-04-08 | Medtronic Minimed, Inc. | Fluid reservoir seating procedure for a fluid infusion device |
| US8708376B2 (en) | 2008-10-10 | 2014-04-29 | Deka Products Limited Partnership | Medium connector |
| US8775196B2 (en) | 2002-01-29 | 2014-07-08 | Baxter International Inc. | System and method for notification and escalation of medical data |
| US8926542B2 (en) | 2011-04-29 | 2015-01-06 | Medtronic, Inc. | Monitoring fluid volume for patients with renal disease |
| US8961461B2 (en) | 2004-05-27 | 2015-02-24 | Baxter International Inc. | Multi-state alarm system for a medical pump |
| US8970384B2 (en) | 2009-06-14 | 2015-03-03 | Roche Diagnostics Operations, Inc. | Devices and methods for malfunctions recognition in a therapeutic dispensing device |
| US9017307B2 (en) | 2010-12-22 | 2015-04-28 | Medtronic Minimed, Inc. | Occlusion detection for a fluid infusion device |
| US9089636B2 (en) | 2004-07-02 | 2015-07-28 | Valeritas, Inc. | Methods and devices for delivering GLP-1 and uses thereof |
| US9173996B2 (en) | 2001-05-18 | 2015-11-03 | Deka Products Limited Partnership | Infusion set for a fluid pump |
| US9180245B2 (en) | 2008-10-10 | 2015-11-10 | Deka Products Limited Partnership | System and method for administering an infusible fluid |
| US9289165B2 (en) | 2005-02-07 | 2016-03-22 | Medtronic, Inc. | Ion imbalance detector |
| EP2902051A4 (en)* | 2012-09-26 | 2016-05-25 | Terumo Corp | Syringe pump |
| US9404500B2 (en) | 2004-08-26 | 2016-08-02 | Pentair Water Pool And Spa, Inc. | Control algorithm of variable speed pumping system |
| US9456755B2 (en) | 2011-04-29 | 2016-10-04 | Medtronic, Inc. | Method and device to monitor patients with kidney disease |
| US9463280B2 (en) | 2012-03-26 | 2016-10-11 | Medimop Medical Projects Ltd. | Motion activated septum puncturing drug delivery device |
| US9498573B2 (en) | 2010-09-24 | 2016-11-22 | Perqflo, Llc | Infusion pumps |
| US9526822B2 (en) | 2013-02-01 | 2016-12-27 | Medtronic, Inc. | Sodium and buffer source cartridges for use in a modular controlled compliant flow path |
| US9568005B2 (en) | 2010-12-08 | 2017-02-14 | Pentair Water Pool And Spa, Inc. | Discharge vacuum relief valve for safety vacuum release system |
| US9677555B2 (en) | 2011-12-21 | 2017-06-13 | Deka Products Limited Partnership | System, method, and apparatus for infusing fluid |
| US9675756B2 (en) | 2011-12-21 | 2017-06-13 | Deka Products Limited Partnership | Apparatus for infusing fluid |
| US9707328B2 (en) | 2013-01-09 | 2017-07-18 | Medtronic, Inc. | Sorbent cartridge to measure solute concentrations |
| US9713668B2 (en) | 2012-01-04 | 2017-07-25 | Medtronic, Inc. | Multi-staged filtration system for blood fluid removal |
| US9713665B2 (en) | 2014-12-10 | 2017-07-25 | Medtronic, Inc. | Degassing system for dialysis |
| US9744297B2 (en) | 2015-04-10 | 2017-08-29 | Medimop Medical Projects Ltd. | Needle cannula position as an input to operational control of an injection device |
| US9795534B2 (en) | 2015-03-04 | 2017-10-24 | Medimop Medical Projects Ltd. | Compliant coupling assembly for cartridge coupling of a drug delivery device |
| US9827361B2 (en) | 2013-02-02 | 2017-11-28 | Medtronic, Inc. | pH buffer measurement system for hemodialysis systems |
| US9848778B2 (en) | 2011-04-29 | 2017-12-26 | Medtronic, Inc. | Method and device to monitor patients with kidney disease |
| US9855379B2 (en) | 2013-02-02 | 2018-01-02 | Medtronic, Inc. | Sorbent cartridge configurations for improved dialysate regeneration |
| US9872949B2 (en) | 2013-02-01 | 2018-01-23 | Medtronic, Inc. | Systems and methods for multifunctional volumetric fluid control |
| US9885360B2 (en) | 2012-10-25 | 2018-02-06 | Pentair Flow Technologies, Llc | Battery backup sump pump systems and methods |
| US9889256B2 (en) | 2013-05-03 | 2018-02-13 | Medimop Medical Projects Ltd. | Sensing a status of an infuser based on sensing motor control and power input |
| US9895479B2 (en) | 2014-12-10 | 2018-02-20 | Medtronic, Inc. | Water management system for use in dialysis |
| WO2018060821A1 (en)* | 2016-09-29 | 2018-04-05 | Koninklijke Philips N.V. | System and method for predicting motor wear out in infusion systems |
| US9943633B2 (en) | 2009-09-30 | 2018-04-17 | Medtronic Inc. | System and method to regulate ultrafiltration |
| US9962486B2 (en) | 2013-03-14 | 2018-05-08 | Tandem Diabetes Care, Inc. | System and method for detecting occlusions in an infusion pump |
| US10010663B2 (en) | 2013-02-01 | 2018-07-03 | Medtronic, Inc. | Fluid circuit for delivery of renal replacement therapies |
| US10016554B2 (en) | 2008-07-09 | 2018-07-10 | Baxter International Inc. | Dialysis system including wireless patient data |
| US10022498B2 (en) | 2011-12-16 | 2018-07-17 | Icu Medical, Inc. | System for monitoring and delivering medication to a patient and method of using the same to minimize the risks associated with automated therapy |
| US10029045B2 (en) | 2010-11-20 | 2018-07-24 | Perqflo, Llc | Infusion pumps |
| US20180214634A1 (en)* | 2014-08-26 | 2018-08-02 | Debiotech S.A. | Detection of an Infusion Anomaly |
| US10061899B2 (en) | 2008-07-09 | 2018-08-28 | Baxter International Inc. | Home therapy machine |
| US10076283B2 (en) | 2013-11-04 | 2018-09-18 | Medtronic, Inc. | Method and device to manage fluid volumes in the body |
| US10098993B2 (en) | 2014-12-10 | 2018-10-16 | Medtronic, Inc. | Sensing and storage system for fluid balance |
| US10159786B2 (en) | 2014-09-30 | 2018-12-25 | Perqflo, Llc | Hybrid ambulatory infusion pumps |
| US10166328B2 (en) | 2013-05-29 | 2019-01-01 | Icu Medical, Inc. | Infusion system which utilizes one or more sensors and additional information to make an air determination regarding the infusion system |
| US10173008B2 (en) | 2002-01-29 | 2019-01-08 | Baxter International Inc. | System and method for communicating with a dialysis machine through a network |
| US10251813B2 (en) | 2015-03-04 | 2019-04-09 | West Pharma. Services IL, Ltd. | Flexibly mounted cartridge alignment collar for drug delivery device |
| US10258736B2 (en) | 2012-05-17 | 2019-04-16 | Tandem Diabetes Care, Inc. | Systems including vial adapter for fluid transfer |
| US10265463B2 (en) | 2014-09-18 | 2019-04-23 | Deka Products Limited Partnership | Apparatus and method for infusing fluid through a tube by appropriately heating the tube |
| US10272196B2 (en) | 2010-09-24 | 2019-04-30 | Perqflo, Llc | Infusion pumps |
| US10279107B2 (en) | 2015-08-20 | 2019-05-07 | Tandem Diabetes Care, Inc. | Drive mechanism for infusion pump |
| US10335545B2 (en) | 2012-01-31 | 2019-07-02 | West Pharma. Services IL, Ltd. | Time dependent drug delivery apparatus |
| US10342917B2 (en) | 2014-02-28 | 2019-07-09 | Icu Medical, Inc. | Infusion system and method which utilizes dual wavelength optical air-in-line detection |
| US10347374B2 (en) | 2008-10-13 | 2019-07-09 | Baxter Corporation Englewood | Medication preparation system |
| US10430761B2 (en) | 2011-08-19 | 2019-10-01 | Icu Medical, Inc. | Systems and methods for a graphical interface including a graphical representation of medical data |
| US10465676B2 (en) | 2011-11-01 | 2019-11-05 | Pentair Water Pool And Spa, Inc. | Flow locking system and method |
| US10463788B2 (en) | 2012-07-31 | 2019-11-05 | Icu Medical, Inc. | Patient care system for critical medications |
| US10478545B2 (en) | 2013-11-26 | 2019-11-19 | Medtronic, Inc. | Parallel modules for in-line recharging of sorbents using alternate duty cycles |
| US10543052B2 (en) | 2013-02-01 | 2020-01-28 | Medtronic, Inc. | Portable dialysis cabinet |
| US10578474B2 (en) | 2012-03-30 | 2020-03-03 | Icu Medical, Inc. | Air detection system and method for detecting air in a pump of an infusion system |
| US10583236B2 (en) | 2013-01-09 | 2020-03-10 | Medtronic, Inc. | Recirculating dialysate fluid circuit for blood measurement |
| US10596316B2 (en) | 2013-05-29 | 2020-03-24 | Icu Medical, Inc. | Infusion system and method of use which prevents over-saturation of an analog-to-digital converter |
| US10595775B2 (en) | 2013-11-27 | 2020-03-24 | Medtronic, Inc. | Precision dialysis monitoring and synchronization system |
| US10635784B2 (en) | 2007-12-18 | 2020-04-28 | Icu Medical, Inc. | User interface improvements for medical devices |
| US10646405B2 (en) | 2012-10-26 | 2020-05-12 | Baxter Corporation Englewood | Work station for medical dose preparation system |
| US10656894B2 (en) | 2017-12-27 | 2020-05-19 | Icu Medical, Inc. | Synchronized display of screen content on networked devices |
| US10668213B2 (en) | 2012-03-26 | 2020-06-02 | West Pharma. Services IL, Ltd. | Motion activated mechanisms for a drug delivery device |
| US10695481B2 (en) | 2011-08-02 | 2020-06-30 | Medtronic, Inc. | Hemodialysis system having a flow path with a controlled compliant volume |
| US10818387B2 (en) | 2014-12-05 | 2020-10-27 | Baxter Corporation Englewood | Dose preparation data analytics |
| US10850024B2 (en) | 2015-03-02 | 2020-12-01 | Icu Medical, Inc. | Infusion system, device, and method having advanced infusion features |
| US10850016B2 (en) | 2013-02-01 | 2020-12-01 | Medtronic, Inc. | Modular fluid therapy system having jumpered flow paths and systems and methods for cleaning and disinfection |
| US10857287B2 (en) | 2017-01-06 | 2020-12-08 | Trustees Of Boston University | Infusion system and components thereof |
| US10857277B2 (en) | 2011-08-16 | 2020-12-08 | Medtronic, Inc. | Modular hemodialysis system |
| US10874793B2 (en) | 2013-05-24 | 2020-12-29 | Icu Medical, Inc. | Multi-sensor infusion system for detecting air or an occlusion in the infusion system |
| US10874787B2 (en) | 2014-12-10 | 2020-12-29 | Medtronic, Inc. | Degassing system for dialysis |
| US10881789B2 (en) | 2013-10-24 | 2021-01-05 | Trustees Of Boston University | Infusion system for preventing mischanneling of multiple medicaments |
| US10905816B2 (en) | 2012-12-10 | 2021-02-02 | Medtronic, Inc. | Sodium management system for hemodialysis |
| US10926017B2 (en) | 2014-06-24 | 2021-02-23 | Medtronic, Inc. | Modular dialysate regeneration assembly |
| US10971257B2 (en) | 2012-10-26 | 2021-04-06 | Baxter Corporation Englewood | Image acquisition for medical dose preparation system |
| US10981148B2 (en) | 2016-11-29 | 2021-04-20 | Medtronic, Inc. | Zirconium oxide module conditioning |
| US10994064B2 (en) | 2016-08-10 | 2021-05-04 | Medtronic, Inc. | Peritoneal dialysate flow path sensing |
| US11013843B2 (en) | 2016-09-09 | 2021-05-25 | Medtronic, Inc. | Peritoneal dialysis fluid testing system |
| US11033667B2 (en) | 2018-02-02 | 2021-06-15 | Medtronic, Inc. | Sorbent manifold for a dialysis system |
| US11045790B2 (en) | 2014-06-24 | 2021-06-29 | Medtronic, Inc. | Stacked sorbent assembly |
| US11065381B2 (en) | 2015-10-05 | 2021-07-20 | E3D A.C.A.L. | Infusion pump device and method for use thereof |
| US11107574B2 (en) | 2014-09-30 | 2021-08-31 | Baxter Corporation Englewood | Management of medication preparation with formulary management |
| US11110215B2 (en) | 2018-02-23 | 2021-09-07 | Medtronic, Inc. | Degasser and vent manifolds for dialysis |
| US11135360B1 (en) | 2020-12-07 | 2021-10-05 | Icu Medical, Inc. | Concurrent infusion with common line auto flush |
| US11154648B2 (en) | 2013-01-09 | 2021-10-26 | Medtronic, Inc. | Fluid circuits for sorbent cartridge with sensors |
| US11167086B2 (en) | 2008-09-15 | 2021-11-09 | West Pharma. Services IL, Ltd. | Stabilized pen injector |
| US11213616B2 (en) | 2018-08-24 | 2022-01-04 | Medtronic, Inc. | Recharge solution for zirconium phosphate |
| US11219880B2 (en) | 2013-11-26 | 2022-01-11 | Medtronic, Inc | System for precision recharging of sorbent materials using patient and session data |
| US11246985B2 (en) | 2016-05-13 | 2022-02-15 | Icu Medical, Inc. | Infusion pump system and method with common line auto flush |
| US11278654B2 (en) | 2017-12-07 | 2022-03-22 | Medtronic, Inc. | Pneumatic manifold for a dialysis system |
| US11278671B2 (en) | 2019-12-04 | 2022-03-22 | Icu Medical, Inc. | Infusion pump with safety sequence keypad |
| US11278665B2 (en) | 2016-11-22 | 2022-03-22 | Eitan Medical Ltd. | Method for delivering a therapeutic substance |
| US11278661B2 (en) | 2020-03-10 | 2022-03-22 | Beta Bionics, Inc. | Infusion system and components thereof |
| US11295846B2 (en) | 2011-12-21 | 2022-04-05 | Deka Products Limited Partnership | System, method, and apparatus for infusing fluid |
| US11324888B2 (en) | 2016-06-10 | 2022-05-10 | Icu Medical, Inc. | Acoustic flow sensor for continuous medication flow measurements and feedback control of infusion |
| US11331463B2 (en) | 2015-07-08 | 2022-05-17 | Trustees Of Boston University | Infusion system and components thereof |
| US11344673B2 (en) | 2014-05-29 | 2022-05-31 | Icu Medical, Inc. | Infusion system and pump with configurable closed loop delivery rate catch-up |
| US11344668B2 (en) | 2014-12-19 | 2022-05-31 | Icu Medical, Inc. | Infusion system with concurrent TPN/insulin infusion |
| US11357909B2 (en) | 2018-10-05 | 2022-06-14 | Eitan Medical Ltd. | Triggering sequence |
| US11364335B2 (en) | 2006-02-09 | 2022-06-21 | Deka Products Limited Partnership | Apparatus, system and method for fluid delivery |
| US11367533B2 (en) | 2014-06-30 | 2022-06-21 | Baxter Corporation Englewood | Managed medical information exchange |
| US11395877B2 (en) | 2006-02-09 | 2022-07-26 | Deka Products Limited Partnership | Systems and methods for fluid delivery |
| US11395868B2 (en) | 2015-11-06 | 2022-07-26 | Medtronic, Inc. | Dialysis prescription optimization for decreased arrhythmias |
| US11404776B2 (en) | 2007-12-31 | 2022-08-02 | Deka Products Limited Partnership | Split ring resonator antenna adapted for use in wirelessly controlled medical device |
| US20220257864A1 (en)* | 2017-11-16 | 2022-08-18 | Amgen Inc. | Autoinjector with stall and end point detection |
| US11426512B2 (en) | 2006-02-09 | 2022-08-30 | Deka Products Limited Partnership | Apparatus, systems and methods for an infusion pump assembly |
| US11458246B2 (en) | 2018-02-05 | 2022-10-04 | Tandem Diabetes Care, Inc. | Methods and systems for detecting infusion pump conditions |
| US11478623B2 (en) | 2006-02-09 | 2022-10-25 | Deka Products Limited Partnership | Infusion pump assembly |
| US11495334B2 (en) | 2015-06-25 | 2022-11-08 | Gambro Lundia Ab | Medical device system and method having a distributed database |
| US11497846B2 (en) | 2006-02-09 | 2022-11-15 | Deka Products Limited Partnership | Patch-sized fluid delivery systems and methods |
| US11497686B2 (en) | 2007-12-31 | 2022-11-15 | Deka Products Limited Partnership | Apparatus, system and method for fluid delivery |
| US11504471B2 (en) | 2018-04-12 | 2022-11-22 | Diatech Diabetes, Inc. | Systems and methods for detecting disruptions in fluid delivery devices |
| US11504481B2 (en) | 2007-10-02 | 2022-11-22 | West Pharma. Services IL, Ltd. | Anti-rotation feature for infusion pump cartridge |
| US11516183B2 (en) | 2016-12-21 | 2022-11-29 | Gambro Lundia Ab | Medical device system including information technology infrastructure having secure cluster domain supporting external domain |
| US11524151B2 (en) | 2012-03-07 | 2022-12-13 | Deka Products Limited Partnership | Apparatus, system and method for fluid delivery |
| US11523972B2 (en) | 2018-04-24 | 2022-12-13 | Deka Products Limited Partnership | Apparatus, system and method for fluid delivery |
| US11534542B2 (en) | 2007-12-31 | 2022-12-27 | Deka Products Limited Partnership | Apparatus, system and method for fluid delivery |
| US11565029B2 (en) | 2013-01-09 | 2023-01-31 | Medtronic, Inc. | Sorbent cartridge with electrodes |
| US11575673B2 (en) | 2014-09-30 | 2023-02-07 | Baxter Corporation Englewood | Central user management in a distributed healthcare information management system |
| US11571507B2 (en) | 2019-07-16 | 2023-02-07 | Beta Bionics, Inc. | Ambulatory device and components thereof |
| US11597541B2 (en) | 2013-07-03 | 2023-03-07 | Deka Products Limited Partnership | Apparatus, system and method for fluid delivery |
| US11642283B2 (en) | 2007-12-31 | 2023-05-09 | Deka Products Limited Partnership | Method for fluid delivery |
| US11672909B2 (en) | 2016-02-12 | 2023-06-13 | Medtronic Minimed, Inc. | Ambulatory infusion pumps and assemblies for use with same |
| US11672904B2 (en) | 2016-01-21 | 2023-06-13 | West Pharma. Services IL, Ltd. | Needle insertion and retraction mechanism |
| US11684712B2 (en) | 2015-02-18 | 2023-06-27 | Medtronic Minimed, Inc. | Ambulatory infusion pumps and reservoir assemblies for use with same |
| US11707615B2 (en) | 2018-08-16 | 2023-07-25 | Deka Products Limited Partnership | Medical pump |
| US11723841B2 (en) | 2007-12-31 | 2023-08-15 | Deka Products Limited Partnership | Apparatus, system and method for fluid delivery |
| US11724034B2 (en) | 2015-10-09 | 2023-08-15 | West Pharma. Services, IL, Ltd. | Injector system |
| US11730892B2 (en) | 2016-08-01 | 2023-08-22 | West Pharma. Services IL, Ltd. | Partial door closure prevention spring |
| US11806456B2 (en) | 2018-12-10 | 2023-11-07 | Mozarc Medical Us Llc | Precision peritoneal dialysis therapy based on dialysis adequacy measurements |
| US11806457B2 (en) | 2018-11-16 | 2023-11-07 | Mozarc Medical Us Llc | Peritoneal dialysis adequacy meaurements |
| US11819673B2 (en) | 2016-06-02 | 2023-11-21 | West Pharma. Services, IL, Ltd. | Three position needle retraction |
| US11819666B2 (en) | 2017-05-30 | 2023-11-21 | West Pharma. Services IL, Ltd. | Modular drive train for wearable injector |
| US11850344B2 (en) | 2021-08-11 | 2023-12-26 | Mozarc Medical Us Llc | Gas bubble sensor |
| US11857767B2 (en) | 2017-12-22 | 2024-01-02 | West Pharma. Services IL, Ltd. | Injector usable with different dimension cartridges |
| US11872375B2 (en) | 2012-09-05 | 2024-01-16 | E3D Agricultural Cooperative Association Ltd. | Electronic auto-injection device |
| US11883361B2 (en) | 2020-07-21 | 2024-01-30 | Icu Medical, Inc. | Fluid transfer devices and methods of use |
| US11883794B2 (en) | 2017-06-15 | 2024-01-30 | Mozarc Medical Us Llc | Zirconium phosphate disinfection recharging and conditioning |
| US11883576B2 (en) | 2016-08-10 | 2024-01-30 | Mozarc Medical Us Llc | Peritoneal dialysis intracycle osmotic agent adjustment |
| US11890448B2 (en) | 2006-02-09 | 2024-02-06 | Deka Products Limited Partnership | Method and system for shape-memory alloy wire control |
| US11931552B2 (en) | 2015-06-04 | 2024-03-19 | West Pharma Services Il, Ltd. | Cartridge insertion for drug delivery device |
| US11948112B2 (en) | 2015-03-03 | 2024-04-02 | Baxter Corporation Engelwood | Pharmacy workflow management with integrated alerts |
| US11944733B2 (en) | 2021-11-18 | 2024-04-02 | Mozarc Medical Us Llc | Sodium and bicarbonate control |
| US11964126B2 (en) | 2006-02-09 | 2024-04-23 | Deka Products Limited Partnership | Infusion pump assembly |
| US11965763B2 (en) | 2021-11-12 | 2024-04-23 | Mozarc Medical Us Llc | Determining fluid flow across rotary pump |
| US12005237B2 (en) | 2016-01-21 | 2024-06-11 | West Pharma. Services IL, Ltd. | Medicament delivery device comprising a visual indicator |
| USD1031975S1 (en) | 2020-03-10 | 2024-06-18 | Beta Bionics, Inc. | Medicament infusion pump device |
| US12036394B2 (en) | 2015-10-09 | 2024-07-16 | West Pharma. Services IL, Ltd. | Injector needle cap and/or liner remover |
| US12064590B2 (en) | 2006-02-09 | 2024-08-20 | Deka Products Limited Partnership | Patch-sized fluid delivery systems and methods |
| US12070574B2 (en) | 2006-02-09 | 2024-08-27 | Deka Products Limited Partnership | Apparatus, systems and methods for an infusion pump assembly |
| US12097357B2 (en) | 2008-09-15 | 2024-09-24 | West Pharma. Services IL, Ltd. | Stabilized pen injector |
| US12098738B2 (en) | 2011-12-21 | 2024-09-24 | Deka Products Limited Partnership | System, method, and apparatus for clamping |
| US12128165B2 (en) | 2020-04-27 | 2024-10-29 | Mozarc Medical Us Llc | Dual stage degasser |
| US12154673B2 (en) | 2021-08-02 | 2024-11-26 | Mozarc Medical Us Llc | Artificial intelligence assisted home therapy settings for dialysis |
| US12151080B2 (en) | 2006-02-09 | 2024-11-26 | Deka Products Limited Partnership | Adhesive and peripheral systems and methods for medical devices |
| US12178992B2 (en) | 2014-09-30 | 2024-12-31 | Medtronic Minimed, Inc. | Different disposable assemblies for the same reusable assembly |
| US12186531B2 (en) | 2008-10-10 | 2025-01-07 | Deka Products Limited Partnership | Infusion pump assembly |
| US12274857B2 (en) | 2006-02-09 | 2025-04-15 | Deka Products Limited Partnership | Method and system for shape-memory alloy wire control |
| US12285552B2 (en) | 2018-08-14 | 2025-04-29 | Mozarc Medical Us Llc | Precision dialysis therapy based on sorbent effluent analysis |
| US12329892B2 (en) | 2016-04-04 | 2025-06-17 | Mozarc Medical Us Llc | Dextrose concentration sensor for a peritoneal dialysis system |
| US12350233B2 (en) | 2021-12-10 | 2025-07-08 | Icu Medical, Inc. | Medical fluid compounding systems with coordinated flow control |
| US12370327B2 (en) | 2008-10-10 | 2025-07-29 | Deka Products Limited Partnership | Infusion pump methods, systems and apparatus |
| US12397093B2 (en) | 2021-05-18 | 2025-08-26 | Mozarc Medical Us Llc | Sorbent cartridge designs |
| USD1091564S1 (en) | 2021-10-13 | 2025-09-02 | Icu Medical, Inc. | Display screen or portion thereof with graphical user interface for a medical device |
| US12412644B2 (en) | 2014-10-24 | 2025-09-09 | Baxter Corporation Englewood | Automated exchange of healthcare information for fulfillment of medication doses |
| US12433976B2 (en) | 2019-01-08 | 2025-10-07 | Mozarc Medical Us Llc | Bicarbonate sensor for dialysis |
Families Citing this family (505)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US20020173748A1 (en) | 1998-10-29 | 2002-11-21 | Mcconnell Susan | Reservoir connector |
| CA2345439C (en)* | 1998-10-29 | 2005-08-09 | Minimed, Inc. | Compact pump drive system |
| US7018401B1 (en) | 1999-02-01 | 2006-03-28 | Board Of Regents, The University Of Texas System | Woven intravascular devices and methods for making the same and apparatus for delivery of the same |
| US6577899B2 (en) | 2000-01-21 | 2003-06-10 | Medtronic Minimed, Inc. | Microprocessor controlled ambulatory medical apparatus with hand held communication device |
| US7077836B2 (en)* | 2000-07-21 | 2006-07-18 | Vein Rx, Inc. | Methods and apparatus for sclerosing the wall of a varicose vein |
| IL156245A0 (en)* | 2000-12-22 | 2004-01-04 | Dca Design Int Ltd | Drive mechanism for an injection device |
| IT1320868B1 (en)* | 2000-12-29 | 2003-12-10 | Cane Srl | PORTABLE DRUG INFUSION DEVICE. |
| US8343115B2 (en)* | 2001-01-05 | 2013-01-01 | Applied Diabetes Research, Inc. | Low profile pivoting joint infusion assembly |
| US7083597B2 (en) | 2001-01-05 | 2006-08-01 | Applied Diabetes Research, Inc. | Pivoting joint infusion system with seal |
| IL157561A0 (en) | 2001-03-04 | 2004-03-28 | Sterling Medivations Inc | Infusion hub assembly and fluid line disconnect system |
| US8152789B2 (en) | 2001-10-23 | 2012-04-10 | Medtronic Minimed, Inc. | System and method for providing closed loop infusion formulation delivery |
| US10080529B2 (en) | 2001-12-27 | 2018-09-25 | Medtronic Minimed, Inc. | System for monitoring physiological characteristics |
| US7232419B2 (en)* | 2002-02-11 | 2007-06-19 | Baxter International Inc. | Enclosure with cam action snap release |
| US7041082B2 (en)* | 2002-02-28 | 2006-05-09 | Smiths Medical Md, Inc. | Syringe pump control systems and methods |
| US7033338B2 (en)* | 2002-02-28 | 2006-04-25 | Smiths Medical Md, Inc. | Cartridge and rod for axially loading medication pump |
| US20030163089A1 (en)* | 2002-02-28 | 2003-08-28 | Bynum Gail Beth | Child safety cap for syringe pump |
| AU2003213816B2 (en) | 2002-03-08 | 2008-06-05 | Vicentra B.V. | Low profile, pivotal connection infusion assembly |
| US20030195609A1 (en)* | 2002-04-10 | 2003-10-16 | Scimed Life Systems, Inc. | Hybrid stent |
| US7175606B2 (en)* | 2002-05-24 | 2007-02-13 | Baxter International Inc. | Disposable medical fluid unit having rigid frame |
| KR100463636B1 (en)* | 2002-08-09 | 2004-12-30 | 김용년 | Cap of tube for supplying liquid |
| EP1475113A1 (en)* | 2003-05-08 | 2004-11-10 | Novo Nordisk A/S | External needle inserter |
| ATE392223T1 (en)* | 2003-05-08 | 2008-05-15 | Novo Nordisk As | INTERNAL NEEDLE INTRODUCER |
| JP4509100B2 (en)* | 2003-05-08 | 2010-07-21 | ノボ・ノルデイスク・エー/エス | Infusion device attachable to skin with removable needle insertion actuation |
| MXPA05013437A (en)* | 2003-06-09 | 2007-08-24 | Nipro Diabetes Systems Inc | Coupling system for an infusion pump. |
| ITRE20030013U1 (en)* | 2003-07-03 | 2005-01-04 | Olivetti S N C Di Olivetti E Figli Ola | SYRINGE FOR FLUID INJECTION |
| EP1502613A1 (en)* | 2003-08-01 | 2005-02-02 | Novo Nordisk A/S | Needle device with retraction means |
| KR20060099520A (en)* | 2003-10-21 | 2006-09-19 | 노보 노르디스크 에이/에스 | Medical Skin Mounting Device |
| US7309326B2 (en) | 2003-11-18 | 2007-12-18 | Icu Medical, Inc. | Infusion set |
| JP2007530860A (en)* | 2004-03-30 | 2007-11-01 | ノボ・ノルデイスク・エー/エス | Actuator system having detection means |
| CN100586495C (en)* | 2004-03-30 | 2010-02-03 | 诺和诺德公司 | Actuator system comprising lever mechanism |
| JP5100378B2 (en)* | 2004-07-07 | 2012-12-19 | ロナルド アラン グリーンベルグ | Hand tool assembly for skin polishing. |
| DE102004036106A1 (en) | 2004-07-24 | 2006-03-16 | Ina-Schaeffler Kg | Periodically actuated plunger for a valve or pump drive |
| EP1804859A1 (en)* | 2004-09-22 | 2007-07-11 | Novo Nordisk A/S | Medical device with cannula inserter |
| WO2006032689A1 (en)* | 2004-09-22 | 2006-03-30 | Novo Nordisk A/S | Medical device with transcutaneous cannula device |
| EP1824536B1 (en)* | 2004-12-06 | 2009-08-26 | Novo Nordisk A/S | Ventilated skin mountable device |
| DE102004059126B4 (en)* | 2004-12-08 | 2014-01-16 | Roche Diagnostics Gmbh | Adapter for injection device |
| US8167841B2 (en) | 2005-01-24 | 2012-05-01 | Novo Nordisk A/S | Transcutaneous device assembly |
| JP2008531093A (en)* | 2005-02-23 | 2008-08-14 | ノボ・ノルデイスク・エー/エス | Method and apparatus for reversing a piston rod in an injection device |
| US8277415B2 (en)* | 2006-08-23 | 2012-10-02 | Medtronic Minimed, Inc. | Infusion medium delivery device and method with drive device for driving plunger in reservoir |
| US7905868B2 (en) | 2006-08-23 | 2011-03-15 | Medtronic Minimed, Inc. | Infusion medium delivery device and method with drive device for driving plunger in reservoir |
| US8512288B2 (en)* | 2006-08-23 | 2013-08-20 | Medtronic Minimed, Inc. | Infusion medium delivery device and method with drive device for driving plunger in reservoir |
| CN101175516B (en)* | 2005-05-13 | 2011-02-16 | 诺和诺德公司 | Medical device suitable for detecting detachment of a percutaneous device |
| US8603034B2 (en)* | 2005-07-12 | 2013-12-10 | Applied Diabetes Research, Inc. | One piece sealing reservoir for an insulin infusion pump |
| EP1940491B1 (en)* | 2005-08-22 | 2021-10-27 | Medtronic Minimed, Inc. | Fluid delivery device |
| JP4983180B2 (en)* | 2005-09-20 | 2012-07-25 | パナソニック株式会社 | Injection device with puncture function, control method for injection device with puncture function, medicinal solution administration device, and control method for medicinal solution administration device |
| US9144645B2 (en)* | 2005-09-21 | 2015-09-29 | Applied Diabetes Research, Inc. | One piece sealing reservoir for an insulin infusion pump |
| US7534226B2 (en)* | 2005-09-26 | 2009-05-19 | M2 Group Holdings, Inc. | Dispensing fluid from an infusion pump system |
| CN101389366B (en) | 2005-11-03 | 2012-12-26 | 巴顿医疗设备有限公司 | Fluid delivery devices and system |
| KR100629994B1 (en)* | 2005-12-30 | 2006-10-02 | 한국뉴매틱(주) | Vacuum ejector pump |
| US7892216B2 (en) | 2006-02-07 | 2011-02-22 | Icu Medical, Inc. | Infusion set |
| US12370305B2 (en) | 2006-02-09 | 2025-07-29 | Deka Products Limited Partnership | Patch-sized fluid delivery systems and methods |
| EP1997233B1 (en)* | 2006-03-13 | 2014-03-05 | Novo Nordisk A/S | Secure pairing of electronic devices using dual means of communication |
| CN101401314B (en)* | 2006-03-13 | 2013-04-24 | 诺沃-诺迪斯克有限公司 | Medical system with dual purpose communication device comprising first and second units |
| JP5297372B2 (en)* | 2006-04-19 | 2013-09-25 | ノボ・ノルデイスク・エー/エス | Fluid injection system, method for assembling the system, and drug container used in the system |
| US8551045B2 (en) | 2006-04-19 | 2013-10-08 | Novo Nordisk A/S | Fluid infusion system, a method of assembling such system and drug reservoir for use in the system |
| CN101426542A (en)* | 2006-04-26 | 2009-05-06 | 诺沃-诺迪斯克有限公司 | Skin-mountable device in packaging comprising coated seal member |
| US20070255126A1 (en) | 2006-04-28 | 2007-11-01 | Moberg Sheldon B | Data communication in networked fluid infusion systems |
| CA2652206C (en) | 2006-05-25 | 2014-02-11 | Bayer Healthcare Llc | Reconstitution device |
| EP2032188A1 (en)* | 2006-06-06 | 2009-03-11 | Novo Nordisk A/S | Assembly comprising skin-mountable device and packaging therefore |
| US8636041B2 (en) | 2006-08-18 | 2014-01-28 | Roche Diagnostics Operations Inc. | Methods and devices for delivering fluid to a reservoir of a fluid delivery device |
| ATE529147T1 (en)* | 2006-08-23 | 2011-11-15 | Medtronic Minimed Inc | SYSTEMS AND METHODS FOR FILLING CONTAINERS AND DISPENSING AN INFUSION MEDIA |
| US9056165B2 (en) | 2006-09-06 | 2015-06-16 | Medtronic Minimed, Inc. | Intelligent therapy recommendation algorithm and method of using the same |
| US8202267B2 (en)* | 2006-10-10 | 2012-06-19 | Medsolve Technologies, Inc. | Method and apparatus for infusing liquid to a body |
| US7780201B2 (en)* | 2006-10-13 | 2010-08-24 | Medela Holding Ag | Tube connector with three part construction and latching component |
| US7981090B2 (en)* | 2006-10-18 | 2011-07-19 | Baxter International Inc. | Luer activated device |
| US8221363B2 (en)* | 2006-10-18 | 2012-07-17 | Baxter Healthcare S.A. | Luer activated device with valve element under tension |
| US20080097407A1 (en)* | 2006-10-18 | 2008-04-24 | Michael Plishka | Luer activated device with compressible valve element |
| MX2009004291A (en) | 2006-10-22 | 2009-09-07 | Idev Technologies Inc | Methods for securing strand ends and the resulting devices. |
| KR101659197B1 (en) | 2006-10-22 | 2016-09-22 | 이데브 테크놀로지스, 아이엔씨. | Devices and methods for stent advancement |
| US7753338B2 (en) | 2006-10-23 | 2010-07-13 | Baxter International Inc. | Luer activated device with minimal fluid displacement |
| JP5697871B2 (en)* | 2006-11-21 | 2015-04-08 | メディメトリクス ペルソナリズド ドルグ デリヴェリー ベー ヴェ | Drug delivery capsule and in vivo drug delivery or diagnostic system |
| WO2008079997A2 (en)* | 2006-12-22 | 2008-07-03 | Medtronic, Inc. | Implantable device, angiogenesis mechanism and methods |
| US20080161754A1 (en)* | 2006-12-29 | 2008-07-03 | Medsolve Technologies, Inc. | Method and apparatus for infusing liquid to a body |
| US8172799B2 (en)* | 2007-01-10 | 2012-05-08 | Acist Medical Systems, Inc. | Volumetric pump |
| EP2120680A2 (en) | 2007-02-06 | 2009-11-25 | Glumetrics, Inc. | Optical systems and methods for rationmetric measurement of blood glucose concentration |
| DK1958660T3 (en)* | 2007-02-15 | 2009-12-21 | Hoffmann La Roche | Bayonet-Luer lock connection to an insulin pump |
| WO2008106806A1 (en)* | 2007-03-02 | 2008-09-12 | Tecpharma Licensing Ag | Modular administration system |
| JP2010520409A (en)* | 2007-03-06 | 2010-06-10 | ノボ・ノルデイスク・エー/エス | Pump assembly with actuator system |
| DE102007018696A1 (en)* | 2007-04-18 | 2008-10-23 | Sanofi-Aventis Deutschland Gmbh | Injection device for dispensing a medicament |
| US8597243B2 (en)* | 2007-04-30 | 2013-12-03 | Medtronic Minimed, Inc. | Systems and methods allowing for reservoir air bubble management |
| US8613725B2 (en) | 2007-04-30 | 2013-12-24 | Medtronic Minimed, Inc. | Reservoir systems and methods |
| US7963954B2 (en) | 2007-04-30 | 2011-06-21 | Medtronic Minimed, Inc. | Automated filling systems and methods |
| JP5102350B2 (en) | 2007-04-30 | 2012-12-19 | メドトロニック ミニメド インコーポレイテッド | Reservoir filling / bubble management / infusion medium delivery system and method using the system |
| US8434528B2 (en) | 2007-04-30 | 2013-05-07 | Medtronic Minimed, Inc. | Systems and methods for reservoir filling |
| US8323250B2 (en) | 2007-04-30 | 2012-12-04 | Medtronic Minimed, Inc. | Adhesive patch systems and methods |
| US7959715B2 (en) | 2007-04-30 | 2011-06-14 | Medtronic Minimed, Inc. | Systems and methods allowing for reservoir air bubble management |
| WO2008141241A1 (en) | 2007-05-10 | 2008-11-20 | Glumetrics, Inc. | Equilibrium non-consuming fluorescence sensor for real time intravascular glucose measurement |
| CA123145S (en) | 2007-05-31 | 2008-06-26 | Danfoss Bionics As | Insulin pump |
| US9656019B2 (en) | 2007-10-02 | 2017-05-23 | Medimop Medical Projects Ltd. | Apparatuses for securing components of a drug delivery system during transport and methods of using same |
| US9345836B2 (en) | 2007-10-02 | 2016-05-24 | Medimop Medical Projects Ltd. | Disengagement resistant telescoping assembly and unidirectional method of assembly for such |
| US10420880B2 (en) | 2007-10-02 | 2019-09-24 | West Pharma. Services IL, Ltd. | Key for securing components of a drug delivery system during assembly and/or transport and methods of using same |
| US7967795B1 (en) | 2010-01-19 | 2011-06-28 | Lamodel Ltd. | Cartridge interface assembly with driving plunger |
| DE102007049446A1 (en) | 2007-10-16 | 2009-04-23 | Cequr Aps | Catheter introducer |
| CN101888859B (en)* | 2007-10-31 | 2014-09-17 | 诺沃-诺迪斯克有限公司 | Non-porous material as sterilization barrier |
| JP5252890B2 (en) | 2007-11-16 | 2013-07-31 | キヤノン株式会社 | Drug delivery device |
| US8313467B2 (en) | 2007-12-27 | 2012-11-20 | Medtronic Minimed, Inc. | Reservoir pressure equalization systems and methods |
| CN101960200A (en)* | 2008-01-28 | 2011-01-26 | 考尔得产品公司 | Quick connect/disconnect coupling assemblies |
| US8708961B2 (en)* | 2008-01-28 | 2014-04-29 | Medsolve Technologies, Inc. | Apparatus for infusing liquid to a body |
| US8366697B2 (en)* | 2008-02-08 | 2013-02-05 | Codan Us Corporation | Enteral feeding safety reservoir and system |
| US8864736B2 (en)* | 2008-02-08 | 2014-10-21 | Codan Us Corporation | Enteral feeding safety reservoir and system |
| EP2093685A1 (en) | 2008-02-21 | 2009-08-26 | F.Hoffmann-La Roche Ag | Drug administration device having a bolus administration profile controller |
| US9295776B2 (en)* | 2008-04-11 | 2016-03-29 | Medtronic Minimed, Inc. | Reservoir plunger head systems and methods |
| US8206353B2 (en)* | 2008-04-11 | 2012-06-26 | Medtronic Minimed, Inc. | Reservoir barrier layer systems and methods |
| DK2108393T3 (en) | 2008-04-11 | 2018-03-05 | Hoffmann La Roche | Administration device with patient condition monitor |
| US8858501B2 (en)* | 2008-04-11 | 2014-10-14 | Medtronic Minimed, Inc. | Reservoir barrier layer systems and methods |
| US8882700B2 (en) | 2008-05-02 | 2014-11-11 | Baxter International Inc. | Smart patient transfer set for peritoneal dialysis |
| EP3372272B1 (en)* | 2008-05-14 | 2024-11-20 | Becton, Dickinson and Company | Separatable infusion set with cleanable interface and straight line attachment |
| US8700114B2 (en) | 2008-07-31 | 2014-04-15 | Medtronic Minmed, Inc. | Analyte sensor apparatuses comprising multiple implantable sensor elements and methods for making and using them |
| US7954515B2 (en)* | 2008-08-15 | 2011-06-07 | Colder Products Company | Combination cap and plug assembly |
| US7959598B2 (en) | 2008-08-20 | 2011-06-14 | Asante Solutions, Inc. | Infusion pump systems and methods |
| USD612019S1 (en)* | 2009-01-28 | 2010-03-16 | Colder Products Company | Coupling body |
| EP2391404B1 (en) | 2009-01-30 | 2013-01-16 | Baxter International Inc. | Transfer sets for therapy optimization |
| US10441710B2 (en)* | 2009-02-09 | 2019-10-15 | Baxter International Inc. | Infusion pump and method to prevent titration errors in infusion therapies |
| US20100217233A1 (en)* | 2009-02-20 | 2010-08-26 | Ranft Elizabeth A | Method and device to anesthetize an area |
| DE102009012449A1 (en)* | 2009-03-12 | 2010-09-16 | Imp Pape Gmbh & Co. Kg | Method and device for continuously driving a syringe plunger |
| WO2010107695A1 (en) | 2009-03-16 | 2010-09-23 | Colder Products Company | Aseptic coupling devices |
| US20110023281A1 (en)* | 2009-04-30 | 2011-02-03 | Stat Medical Devices, Inc. | Pen injection device cap with integral pen needle quick release and/or removal system |
| US8672873B2 (en) | 2009-08-18 | 2014-03-18 | Cequr Sa | Medicine delivery device having detachable pressure sensing unit |
| US8547239B2 (en) | 2009-08-18 | 2013-10-01 | Cequr Sa | Methods for detecting failure states in a medicine delivery device |
| US8241018B2 (en) | 2009-09-10 | 2012-08-14 | Tyco Healthcare Group Lp | Compact peristaltic medical pump |
| US10071198B2 (en) | 2012-11-02 | 2018-09-11 | West Pharma. Servicees IL, Ltd. | Adhesive structure for medical device |
| US8157769B2 (en) | 2009-09-15 | 2012-04-17 | Medimop Medical Projects Ltd. | Cartridge insertion assembly for drug delivery system |
| US10071196B2 (en) | 2012-05-15 | 2018-09-11 | West Pharma. Services IL, Ltd. | Method for selectively powering a battery-operated drug-delivery device and device therefor |
| JP5812344B2 (en)* | 2009-10-26 | 2015-11-11 | 株式会社ジェイ・エム・エス | Medical container connector set |
| US8070723B2 (en)* | 2009-12-31 | 2011-12-06 | Medtronic Minimed, Inc. | Activity guard |
| US8348898B2 (en) | 2010-01-19 | 2013-01-08 | Medimop Medical Projects Ltd. | Automatic needle for drug pump |
| DK2371408T3 (en) | 2010-03-31 | 2013-11-04 | Hoffmann La Roche | Liquid drug degassing device and outpatient infusion system containing a degassing device |
| EP2372590A1 (en) | 2010-03-31 | 2011-10-05 | F. Hoffmann-La Roche AG | Assembly station with component backtracking features |
| EP2378119A1 (en)* | 2010-04-15 | 2011-10-19 | Mmi Ag | Plunger pump with manual insertion possibility for volumes under a microlitre |
| CN102958550B (en)* | 2010-04-23 | 2016-05-18 | 赛诺菲-安万特德国有限公司 | Cartridge case retainer and alignment interface |
| US8246573B2 (en) | 2010-04-27 | 2012-08-21 | Medtronic, Inc. | Detecting empty medical pump reservoir |
| EP2569031B1 (en) | 2010-05-10 | 2017-10-11 | Medimop Medical Projects Ltd. | Low volume accurate injector |
| US9023095B2 (en) | 2010-05-27 | 2015-05-05 | Idev Technologies, Inc. | Stent delivery system with pusher assembly |
| US20120123338A1 (en) | 2010-06-16 | 2012-05-17 | Medtronic, Inc. | Damping systems for stabilizing medications in drug delivery devices |
| US9215995B2 (en) | 2010-06-23 | 2015-12-22 | Medtronic Minimed, Inc. | Sensor systems having multiple probes and electrode arrays |
| US9675751B2 (en)* | 2010-07-31 | 2017-06-13 | Becton, Dickinson And Company | Infusion reservoir with push-on connector features and/or attachments therefor |
| WO2012019726A1 (en) | 2010-08-07 | 2012-02-16 | Roche Diagnostics Gmbh | Valve for an ambulatory infusion system and ambulatory infusion system including a valve |
| US9216249B2 (en) | 2010-09-24 | 2015-12-22 | Perqflo, Llc | Infusion pumps |
| US9211378B2 (en) | 2010-10-22 | 2015-12-15 | Cequr Sa | Methods and systems for dosing a medicament |
| US9192719B2 (en)* | 2010-11-01 | 2015-11-24 | Medtronic, Inc. | Implantable medical pump diagnostics |
| JP5990535B2 (en)* | 2010-12-22 | 2016-09-14 | サノフィ−アベンティス・ドイチュラント・ゲゼルシャフト・ミット・ベシュレンクテル・ハフツング | Dedicated cartridge |
| EP2469432A1 (en) | 2010-12-24 | 2012-06-27 | F. Hoffmann-La Roche AG | Ambulatory infusion system with alert prioritizing features |
| US9339639B2 (en) | 2011-02-22 | 2016-05-17 | Medtronic Minimed, Inc. | Sealing assembly for a fluid reservoir of a fluid infusion device |
| US11266823B2 (en) | 2011-02-22 | 2022-03-08 | Medtronic Minimed, Inc. | Retractable sealing assembly for a fluid reservoir of a fluid infusion device |
| JP2014511249A (en)* | 2011-03-04 | 2014-05-15 | デュオジェクト・メディカル・システムズ・インコーポレイテッド | Easy transfer system |
| JP5779915B2 (en)* | 2011-03-08 | 2015-09-16 | 株式会社リコー | Image forming apparatus and powder container |
| USD702834S1 (en) | 2011-03-22 | 2014-04-15 | Medimop Medical Projects Ltd. | Cartridge for use in injection device |
| US9008744B2 (en) | 2011-05-06 | 2015-04-14 | Medtronic Minimed, Inc. | Method and apparatus for continuous analyte monitoring |
| US8795231B2 (en) | 2011-05-10 | 2014-08-05 | Medtronic Minimed, Inc. | Automated reservoir fill system |
| WO2013010561A1 (en) | 2011-07-20 | 2013-01-24 | Roche Diagnostics Gmbh | Drive control for an ambulatory infusion device |
| WO2013049068A1 (en) | 2011-09-27 | 2013-04-04 | Glumetrics, Inc. | Method for functionalizing a porous membrane covering of an optical sensor to facilitate coupling of an antithrom-bogenic agent |
| DK3045187T3 (en) | 2011-10-14 | 2019-06-11 | Amgen Inc | INJECTOR AND COLLECTION PROCEDURE |
| US9989522B2 (en) | 2011-11-01 | 2018-06-05 | Medtronic Minimed, Inc. | Methods and materials for modulating start-up time and air removal in dry sensors |
| US8662540B2 (en) | 2011-11-02 | 2014-03-04 | Philip C. Whitener | Quick tube connector |
| US8999720B2 (en) | 2011-11-17 | 2015-04-07 | Medtronic Minimed, Inc. | Aqueous radiation protecting formulations and methods for making and using them |
| CN103127579B (en)* | 2011-11-21 | 2017-06-16 | 上海泽生科技开发股份有限公司 | The drive system of portable injection pump |
| US9610401B2 (en) | 2012-01-13 | 2017-04-04 | Medtronic Minimed, Inc. | Infusion set component with modular fluid channel element |
| SG11201404436XA (en) | 2012-02-02 | 2014-08-28 | Becton Dickinson Holdings Pte Ltd | Adaptor for coupling with a medical container |
| SG192310A1 (en) | 2012-02-02 | 2013-08-30 | Becton Dickinson Holdings Pte Ltd | Adaptor for coupling to a medical container |
| SG192312A1 (en) | 2012-02-02 | 2013-08-30 | Becton Dickinson Holdings Pte Ltd | Adaptor for coupling to a medical container |
| US9072827B2 (en) | 2012-03-26 | 2015-07-07 | Medimop Medical Projects Ltd. | Fail safe point protector for needle safety flap |
| JP6094829B2 (en)* | 2012-04-13 | 2017-03-15 | 株式会社ジェイ・エム・エス | Male connector with locking mechanism |
| JP6094830B2 (en)* | 2012-04-13 | 2017-03-15 | 株式会社ジェイ・エム・エス | Male connector with locking mechanism |
| US9493807B2 (en) | 2012-05-25 | 2016-11-15 | Medtronic Minimed, Inc. | Foldover sensors and methods for making and using them |
| US9555186B2 (en) | 2012-06-05 | 2017-01-31 | Tandem Diabetes Care, Inc. | Infusion pump system with disposable cartridge having pressure venting and pressure feedback |
| US9632060B2 (en) | 2012-06-08 | 2017-04-25 | Medtronic Minimed, Inc. | Application of electrochemical impedance spectroscopy in sensor systems, devices, and related methods |
| US20130338576A1 (en)* | 2012-06-15 | 2013-12-19 | Wayne C. Jaeschke, Jr. | Portable infusion pump with pressure and temperature compensation |
| US8454562B1 (en) | 2012-07-20 | 2013-06-04 | Asante Solutions, Inc. | Infusion pump system and method |
| EP2692328A1 (en) | 2012-08-03 | 2014-02-05 | Becton Dickinson France | Dose counting device for coupling with a medical container |
| US9682188B2 (en) | 2012-08-21 | 2017-06-20 | Medtronic Minimed, Inc. | Reservoir fluid volume estimator and medical device incorporating same |
| US9662445B2 (en) | 2012-08-30 | 2017-05-30 | Medtronic Minimed, Inc. | Regulating entry into a closed-loop operating mode of an insulin infusion system |
| US10130767B2 (en) | 2012-08-30 | 2018-11-20 | Medtronic Minimed, Inc. | Sensor model supervisor for a closed-loop insulin infusion system |
| EP2712651A1 (en)* | 2012-09-27 | 2014-04-02 | F. Hoffmann-La Roche AG | Venting device for use in ambulatory infusion system |
| WO2014049797A1 (en)* | 2012-09-27 | 2014-04-03 | テルモ株式会社 | Barrel for pre-filled syringe, puncture device for pre-filled syringe, pre-filled syringe and barrel packaging body for pre-filled syringe |
| JP6031113B2 (en)* | 2012-09-27 | 2016-11-24 | テルモ株式会社 | Prefilled syringe outer cylinder, puncture tool for prefilled syringe, prefilled syringe, and prefilled syringe administration preparation method |
| EP2712650A1 (en)* | 2012-09-27 | 2014-04-02 | F. Hoffmann-La Roche AG | Adapter and drug cartridge alignment device |
| US9511186B1 (en) | 2012-10-23 | 2016-12-06 | Acist Medical Systems, Inc. | Medical injection systems and pumps |
| WO2014074621A1 (en) | 2012-11-07 | 2014-05-15 | Glumetrics, Inc. | Dry insertion and one-point in vivo calibration of an optical analyte sensor |
| US9265455B2 (en) | 2012-11-13 | 2016-02-23 | Medtronic Minimed, Inc. | Methods and systems for optimizing sensor function by the application of voltage |
| EP2735300A1 (en) | 2012-11-26 | 2014-05-28 | Becton Dickinson France | Adaptor for multidose medical container |
| US10194840B2 (en) | 2012-12-06 | 2019-02-05 | Medtronic Minimed, Inc. | Microarray electrodes useful with analyte sensors and methods for making and using them |
| WO2014106008A1 (en) | 2012-12-28 | 2014-07-03 | Gambro Renal Products, Inc. | Syringe pump engagement detection apparatus and methods |
| US9421323B2 (en) | 2013-01-03 | 2016-08-23 | Medimop Medical Projects Ltd. | Door and doorstop for portable one use drug delivery apparatus |
| US10426383B2 (en) | 2013-01-22 | 2019-10-01 | Medtronic Minimed, Inc. | Muting glucose sensor oxygen response and reducing electrode edge growth with pulsed current plating |
| JP6285369B2 (en)* | 2013-02-06 | 2018-02-28 | テルモ株式会社 | Infusion pump |
| CN105008227B (en)* | 2013-02-07 | 2017-03-08 | 伊奎希尔德医疗有限公司 | Improvements to closed drug delivery systems |
| US9597260B2 (en) | 2013-03-15 | 2017-03-21 | Becton Dickinson and Company Ltd. | System for closed transfer of fluids |
| US9414990B2 (en) | 2013-03-15 | 2016-08-16 | Becton Dickinson and Company Ltd. | Seal system for cannula |
| SG11201507878SA (en) | 2013-03-22 | 2015-10-29 | Amgen Inc | Injector and method of assembly |
| US9011164B2 (en) | 2013-04-30 | 2015-04-21 | Medimop Medical Projects Ltd. | Clip contact for easy installation of printed circuit board PCB |
| US9338819B2 (en) | 2013-05-29 | 2016-05-10 | Medtronic Minimed, Inc. | Variable data usage personal medical system and method |
| US9457141B2 (en) | 2013-06-03 | 2016-10-04 | Bigfoot Biomedical, Inc. | Infusion pump system and method |
| US10194864B2 (en) | 2013-06-21 | 2019-02-05 | Medtronic Minimed, Inc. | Anchoring apparatus and method for attaching device on body |
| US9561324B2 (en) | 2013-07-19 | 2017-02-07 | Bigfoot Biomedical, Inc. | Infusion pump system and method |
| EP3024532B1 (en) | 2013-07-23 | 2019-01-02 | Colder Products Company | Aseptic coupling devices |
| ITTO20130661A1 (en) | 2013-08-02 | 2015-02-03 | Cane Spa | PORTABLE PUMP FOR THE INFUSION OF LIQUID SUBSTANCES IN THE BODY OF A LIVING AND TANK FOR USE IN THIS PUMP |
| JP1526207S (en) | 2013-08-05 | 2015-06-15 | ||
| US9880528B2 (en) | 2013-08-21 | 2018-01-30 | Medtronic Minimed, Inc. | Medical devices and related updating methods and systems |
| US8979808B1 (en) | 2013-10-14 | 2015-03-17 | Medtronic Minimed, Inc. | On-body injector and method of use |
| US8979799B1 (en) | 2013-10-14 | 2015-03-17 | Medtronic Minimed, Inc. | Electronic injector |
| US9375537B2 (en) | 2013-10-14 | 2016-06-28 | Medtronic Minimed, Inc. | Therapeutic agent injection device |
| US9265881B2 (en) | 2013-10-14 | 2016-02-23 | Medtronic Minimed, Inc. | Therapeutic agent injection device |
| EP2862586B1 (en) | 2013-10-21 | 2021-09-01 | F. Hoffmann-La Roche AG | Control unit for infusion pump units, including a controlled intervention unit |
| DK2865325T3 (en) | 2013-10-23 | 2018-02-19 | Hoffmann La Roche | STANDING ON THE SKIN OF INFUSION PUMP OR CONTINUOUS BLOOD SUGAR MONITOR WITH OPTICAL INDICATION ORGANIZATION |
| BR112016008946B1 (en) | 2013-10-24 | 2022-12-27 | Amgen Inc | INJECTORS AND METHOD FOR ASSEMBLING THE INJECTORS |
| US9226709B2 (en) | 2013-11-04 | 2016-01-05 | Medtronic Minimed, Inc. | ICE message system and method |
| ES2780857T3 (en) | 2013-11-06 | 2020-08-27 | Becton Dickinson & Co Ltd | Connection device for a medical device |
| EP3065694B1 (en) | 2013-11-06 | 2020-04-29 | Becton Dickinson and Company Limited | System for closed transfer of fluids having connector |
| ES2780856T3 (en) | 2013-11-06 | 2020-08-27 | Becton Dickinson & Co Ltd | Medical connector having locking coupling |
| CA2929476C (en) | 2013-11-06 | 2019-01-22 | Becton Dickinson and Company Limited | System for closed transfer of fluids with a locking member |
| US9267875B2 (en) | 2013-11-21 | 2016-02-23 | Medtronic Minimed, Inc. | Accelerated life testing device and method |
| US10569015B2 (en) | 2013-12-02 | 2020-02-25 | Bigfoot Biomedical, Inc. | Infusion pump system and method |
| US9750877B2 (en) | 2013-12-11 | 2017-09-05 | Medtronic Minimed, Inc. | Predicted time to assess and/or control a glycemic state |
| US9849240B2 (en) | 2013-12-12 | 2017-12-26 | Medtronic Minimed, Inc. | Data modification for predictive operations and devices incorporating same |
| US10105488B2 (en) | 2013-12-12 | 2018-10-23 | Medtronic Minimed, Inc. | Predictive infusion device operations and related methods and systems |
| US10638947B2 (en) | 2013-12-16 | 2020-05-05 | Medtronic Minimed, Inc. | Use of electrochemical impedance spectroscopy (EIS) in intelligent diagnostics |
| US9603561B2 (en) | 2013-12-16 | 2017-03-28 | Medtronic Minimed, Inc. | Methods and systems for improving the reliability of orthogonally redundant sensors |
| US9143941B2 (en) | 2013-12-18 | 2015-09-22 | Medtronic Minimed, Inc. | Secure communication by user selectable communication range |
| US9779226B2 (en) | 2013-12-18 | 2017-10-03 | Medtronic Minimed, Inc. | Fingerprint enhanced authentication for medical devices in wireless networks |
| US9694132B2 (en) | 2013-12-19 | 2017-07-04 | Medtronic Minimed, Inc. | Insertion device for insertion set |
| US10279105B2 (en) | 2013-12-26 | 2019-05-07 | Tandem Diabetes Care, Inc. | System and method for modifying medicament delivery parameters after a site change |
| CN105899246A (en)* | 2014-01-13 | 2016-08-24 | 诺和诺德股份有限公司 | Transmission arrangement for motorized drug delivery devic |
| US9861748B2 (en) | 2014-02-06 | 2018-01-09 | Medtronic Minimed, Inc. | User-configurable closed-loop notifications and infusion systems incorporating same |
| CN103768679B (en) | 2014-02-20 | 2016-08-24 | 江苏多维科技有限公司 | Precision syringe pump and manufacture method thereof |
| US9388805B2 (en) | 2014-03-24 | 2016-07-12 | Medtronic Minimed, Inc. | Medication pump test device and method of use |
| US9689830B2 (en) | 2014-04-03 | 2017-06-27 | Medtronic Minimed, Inc. | Sensor detection pads with integrated fuse |
| US9707336B2 (en) | 2014-04-07 | 2017-07-18 | Medtronic Minimed, Inc. | Priming detection system and method of using the same |
| KR101424959B1 (en) | 2014-04-08 | 2014-08-01 | 한국뉴매틱(주) | Vacuum pump |
| WO2015158230A1 (en) | 2014-04-14 | 2015-10-22 | 江苏多维科技有限公司 | Micro guiding screw pump using magnetic resistance sensor and manufacturing method therefor |
| CN106232082B (en) | 2014-04-16 | 2019-03-12 | 贝克顿迪金森有限公司 | Fluid delivery device with axially and rotatably movable parts |
| EP4091597A1 (en) | 2014-04-21 | 2022-11-23 | Becton Dickinson and Company Limited | Syringe adapter with disconnection feedback mechanism |
| BR112016024680B8 (en)* | 2014-04-21 | 2021-11-09 | Becton Dickinson & Co Ltd | Syringe adapter |
| CN110353993B (en) | 2014-04-21 | 2022-04-12 | 贝克顿迪金森有限公司 | Bottle stabilizer base with attachable bottle adapter |
| EP3134057B1 (en) | 2014-04-21 | 2018-06-27 | Becton Dickinson and Company Limited | Syringe adapter with compound motion disengagement |
| ES2792512T3 (en) | 2014-04-21 | 2020-11-11 | Becton Dickinson & Co Ltd | Fluid transfer device and packaging for it |
| EP3398583A1 (en) | 2014-04-21 | 2018-11-07 | Becton Dickinson and Company Limited | System with adapter for closed transfer of fluids |
| EP3134058B1 (en) | 2014-04-21 | 2020-03-18 | Becton Dickinson and Company Limited | Fluid transfer device and packaging therefor |
| AU2015249915B2 (en) | 2014-04-21 | 2017-11-30 | Becton Dickinson and Company Limited | System for closed transfer of fluids |
| US10232113B2 (en) | 2014-04-24 | 2019-03-19 | Medtronic Minimed, Inc. | Infusion devices and related methods and systems for regulating insulin on board |
| US9681828B2 (en) | 2014-05-01 | 2017-06-20 | Medtronic Minimed, Inc. | Physiological characteristic sensors and methods for forming such sensors |
| EP3142731A4 (en)* | 2014-05-10 | 2018-01-17 | Dr. Py Institute, LLC | Self closing and opening filling needle, needle holder, filler and method |
| EP3145561A1 (en) | 2014-05-20 | 2017-03-29 | Cequr SA | Medicine delivery device with restricted access filling port |
| US9901305B2 (en) | 2014-06-13 | 2018-02-27 | Medtronic Minimed, Inc. | Physiological sensor history backfill system and method |
| US9452255B2 (en) | 2014-07-21 | 2016-09-27 | Medtronic Minimed, Inc. | Smart connection interface |
| AU2015296088B2 (en) | 2014-07-31 | 2019-11-14 | Hospira, Inc. | Injection system |
| US10137246B2 (en) | 2014-08-06 | 2018-11-27 | Bigfoot Biomedical, Inc. | Infusion pump assembly and method |
| US9717845B2 (en) | 2014-08-19 | 2017-08-01 | Medtronic Minimed, Inc. | Geofencing for medical devices |
| US20160051755A1 (en) | 2014-08-25 | 2016-02-25 | Medtronic Minimed, Inc. | Low cost fluid delivery device |
| US9919096B2 (en) | 2014-08-26 | 2018-03-20 | Bigfoot Biomedical, Inc. | Infusion pump system and method |
| USD792584S1 (en) | 2014-09-08 | 2017-07-18 | Neomed, Inc. | Male enteral coupling |
| US11357964B2 (en) | 2014-09-08 | 2022-06-14 | Avent, Inc. | Vented connector for medical fluid vessels and tapered plug |
| USD781417S1 (en) | 2014-09-08 | 2017-03-14 | Neomed, Inc. | Male enteral coupling |
| EP3191166B1 (en) | 2014-09-08 | 2019-11-20 | Neomed, Inc. | Vented connector for medical fluid vessels |
| US11376409B2 (en) | 2014-09-08 | 2022-07-05 | Avent, Inc. | Hub component for vented connector |
| US9839753B2 (en) | 2014-09-26 | 2017-12-12 | Medtronic Minimed, Inc. | Systems for managing reservoir chamber pressure |
| US10279126B2 (en) | 2014-10-07 | 2019-05-07 | Medtronic Minimed, Inc. | Fluid conduit assembly with gas trapping filter in the fluid flow path |
| WO2016074002A2 (en)* | 2014-10-13 | 2016-05-12 | Ramey Kirk D | Connector for medication delivery system |
| US9592335B2 (en) | 2014-10-20 | 2017-03-14 | Medtronic Minimed, Inc. | Insulin pump data acquisition device |
| US9841014B2 (en) | 2014-10-20 | 2017-12-12 | Medtronic Minimed, Inc. | Insulin pump data acquisition device and system |
| NO2689315T3 (en) | 2014-10-28 | 2018-04-14 | ||
| US10792418B2 (en) | 2014-10-28 | 2020-10-06 | Bayer Healthcare Llc | Self-orienting pressure jacket and pressure jacket-to-injector interface |
| US9199033B1 (en) | 2014-10-28 | 2015-12-01 | Bayer Healthcare Llc | Self-orienting syringe and syringe interface |
| WO2016069714A1 (en) | 2014-10-28 | 2016-05-06 | Bayer Healthcare Llc | Self-orienting pressure jacket and pressure jacket-to-injector interface |
| CA2891612C (en)* | 2014-11-13 | 2021-06-08 | Cornerstone Cm, Inc. | Evacuated bottle system |
| US9731067B2 (en) | 2014-11-25 | 2017-08-15 | Medtronic Minimed, Inc. | Mechanical injection pump and method of use |
| US9901675B2 (en) | 2014-11-25 | 2018-02-27 | Medtronic Minimed, Inc. | Infusion set insertion device and method of use |
| US9833564B2 (en)* | 2014-11-25 | 2017-12-05 | Medtronic Minimed, Inc. | Fluid conduit assembly with air venting features |
| US9987420B2 (en)* | 2014-11-26 | 2018-06-05 | Medtronic Minimed, Inc. | Systems and methods for fluid infusion device with automatic reservoir fill |
| US10195341B2 (en)* | 2014-11-26 | 2019-02-05 | Medtronic Minimed, Inc. | Systems and methods for fluid infusion device with automatic reservoir fill |
| US9943645B2 (en) | 2014-12-04 | 2018-04-17 | Medtronic Minimed, Inc. | Methods for operating mode transitions and related infusion devices and systems |
| US9636453B2 (en) | 2014-12-04 | 2017-05-02 | Medtronic Minimed, Inc. | Advance diagnosis of infusion device operating mode viability |
| US10307535B2 (en) | 2014-12-19 | 2019-06-04 | Medtronic Minimed, Inc. | Infusion devices and related methods and systems for preemptive alerting |
| US9987416B2 (en)* | 2015-01-09 | 2018-06-05 | BioQuiddity Inc. | Sterile assembled liquid medicament dosage control and delivery device |
| US9717848B2 (en) | 2015-01-22 | 2017-08-01 | Medtronic Minimed, Inc. | Data derived pre-bolus delivery |
| US9872954B2 (en) | 2015-03-02 | 2018-01-23 | Medtronic Minimed, Inc. | Belt clip |
| US10307528B2 (en) | 2015-03-09 | 2019-06-04 | Medtronic Minimed, Inc. | Extensible infusion devices and related methods |
| US10624817B2 (en) | 2015-03-24 | 2020-04-21 | Neomed, Inc. | Oral administration coupler for back-of-mouth delivery |
| US10449298B2 (en) | 2015-03-26 | 2019-10-22 | Medtronic Minimed, Inc. | Fluid injection devices and related methods |
| US10293120B2 (en) | 2015-04-10 | 2019-05-21 | West Pharma. Services IL, Ltd. | Redundant injection device status indication |
| BR112017020735A2 (en) | 2015-04-15 | 2018-07-17 | Gambro Lundia Ab | infusion set pressure booster treatment system |
| US9878097B2 (en) | 2015-04-29 | 2018-01-30 | Bigfoot Biomedical, Inc. | Operating an infusion pump system |
| US10130757B2 (en) | 2015-05-01 | 2018-11-20 | Medtronic Minimed, Inc. | Method and system for leakage detection in portable medical devices |
| US9999721B2 (en) | 2015-05-26 | 2018-06-19 | Medtronic Minimed, Inc. | Error handling in infusion devices with distributed motor control and related operating methods |
| US10149943B2 (en) | 2015-05-29 | 2018-12-11 | West Pharma. Services IL, Ltd. | Linear rotation stabilizer for a telescoping syringe stopper driverdriving assembly |
| US10478557B2 (en) | 2015-08-21 | 2019-11-19 | Medtronic Minimed, Inc. | Personalized parameter modeling methods and related devices and systems |
| US10201657B2 (en) | 2015-08-21 | 2019-02-12 | Medtronic Minimed, Inc. | Methods for providing sensor site rotation feedback and related infusion devices and systems |
| US10463297B2 (en) | 2015-08-21 | 2019-11-05 | Medtronic Minimed, Inc. | Personalized event detection methods and related devices and systems |
| US10293108B2 (en) | 2015-08-21 | 2019-05-21 | Medtronic Minimed, Inc. | Infusion devices and related patient ratio adjustment methods |
| ES2955351T3 (en)* | 2015-09-03 | 2023-11-30 | Hoffmann La Roche | Dosing unit with low radial sealing forces during storage |
| US9987432B2 (en) | 2015-09-22 | 2018-06-05 | West Pharma. Services IL, Ltd. | Rotation resistant friction adapter for plunger driver of drug delivery device |
| US10086145B2 (en) | 2015-09-22 | 2018-10-02 | West Pharma Services Il, Ltd. | Rotation resistant friction adapter for plunger driver of drug delivery device |
| US10117992B2 (en) | 2015-09-29 | 2018-11-06 | Medtronic Minimed, Inc. | Infusion devices and related rescue detection methods |
| US9992818B2 (en) | 2015-10-06 | 2018-06-05 | Medtronic Minimed, Inc. | Protocol translation device |
| US9757511B2 (en) | 2015-10-19 | 2017-09-12 | Medtronic Minimed, Inc. | Personal medical device and method of use with restricted mode challenge |
| US11666702B2 (en) | 2015-10-19 | 2023-06-06 | Medtronic Minimed, Inc. | Medical devices and related event pattern treatment recommendation methods |
| US11501867B2 (en) | 2015-10-19 | 2022-11-15 | Medtronic Minimed, Inc. | Medical devices and related event pattern presentation methods |
| US10146911B2 (en) | 2015-10-23 | 2018-12-04 | Medtronic Minimed, Inc. | Medical devices and related methods and systems for data transfer |
| CA3003321C (en) | 2015-10-28 | 2023-09-26 | Bayer Healthcare Llc | System and method for fluid injector engagement with a pressure jacket and syringe cap |
| US10037722B2 (en) | 2015-11-03 | 2018-07-31 | Medtronic Minimed, Inc. | Detecting breakage in a display element |
| US10827959B2 (en) | 2015-11-11 | 2020-11-10 | Medtronic Minimed, Inc. | Sensor set |
| US11234899B2 (en) | 2017-05-11 | 2022-02-01 | Scalpal Llc | Grasping facilitators and uses thereof and kits involving the same |
| EP3167923A1 (en)* | 2015-11-13 | 2017-05-17 | Fresenius Vial SAS | Method for detecting an occlusion in an infusion line |
| WO2017083622A1 (en) | 2015-11-13 | 2017-05-18 | Bayer Healthcare Llc | Nested syringe assembly |
| US9848805B2 (en) | 2015-12-18 | 2017-12-26 | Medtronic Minimed, Inc. | Biostable glucose permeable polymer |
| US10327680B2 (en) | 2015-12-28 | 2019-06-25 | Medtronic Minimed, Inc. | Sensor systems, devices, and methods for continuous glucose monitoring |
| US20170184527A1 (en) | 2015-12-28 | 2017-06-29 | Medtronic Minimed, Inc. | Sensor systems, devices, and methods for continuous glucose monitoring |
| US10327686B2 (en) | 2015-12-28 | 2019-06-25 | Medtronic Minimed, Inc. | Sensor systems, devices, and methods for continuous glucose monitoring |
| US10349872B2 (en) | 2015-12-28 | 2019-07-16 | Medtronic Minimed, Inc. | Methods, systems, and devices for sensor fusion |
| US20170181672A1 (en) | 2015-12-28 | 2017-06-29 | Medtronic Minimed, Inc. | Sensor systems, devices, and methods for continuous glucose monitoring |
| AU2016385454B2 (en) | 2016-01-05 | 2021-12-16 | Bigfoot Biomedical, Inc. | Operating multi-modal medicine delivery systems |
| HK1256995A1 (en) | 2016-01-14 | 2019-10-11 | Bigfoot Biomedical, Inc. | Occlusion resolution in medication delivery devices, systems, and methods |
| EP3711793B1 (en) | 2016-01-21 | 2021-12-01 | West Pharma Services IL, Ltd. | A method of connecting a cartridge to an automatic injector |
| US10790054B1 (en) | 2016-12-07 | 2020-09-29 | Medtronic Minimed, Inc. | Method and apparatus for tracking of food intake and other behaviors and providing relevant feedback |
| US20230368046A9 (en) | 2016-01-28 | 2023-11-16 | Medtronic Minimed, Inc. | Activation of Ancillary Sensor Systems Based on Triggers from a Wearable Gesture Sensing Device |
| EP3407780A4 (en) | 2016-01-28 | 2019-09-18 | Klue, Inc. | METHOD AND APPARATUS FOR MONITORING FOOD RECEPTACLE AND OTHER BEHAVIOR AND PROVIDING RETURN OF RELEVANT INFORMATION |
| JP6240239B2 (en)* | 2016-03-03 | 2017-11-29 | テルモ株式会社 | Portable infusion pump |
| US20170273623A1 (en)* | 2016-03-13 | 2017-09-28 | Keepspring Medical Inc | Adjustable and Detachable Cast with Pressure Sensor |
| US11389597B2 (en) | 2016-03-16 | 2022-07-19 | West Pharma. Services IL, Ltd. | Staged telescopic screw assembly having different visual indicators |
| US10376647B2 (en) | 2016-03-18 | 2019-08-13 | West Pharma. Services IL, Ltd. | Anti-rotation mechanism for telescopic screw assembly |
| US10765369B2 (en) | 2016-04-08 | 2020-09-08 | Medtronic Minimed, Inc. | Analyte sensor |
| US10631787B2 (en) | 2016-04-08 | 2020-04-28 | Medtronic Minimed, Inc. | Sensor and transmitter product |
| US10765348B2 (en) | 2016-04-08 | 2020-09-08 | Medtronic Minimed, Inc. | Sensor and transmitter product |
| US12419547B2 (en) | 2016-04-08 | 2025-09-23 | Medtronic Minimed, Inc. | Sensor and transmitter product |
| US10589038B2 (en) | 2016-04-27 | 2020-03-17 | Medtronic Minimed, Inc. | Set connector systems for venting a fluid reservoir |
| US10426389B2 (en) | 2016-04-28 | 2019-10-01 | Medtronic Minimed, Inc. | Methods, systems, and devices for electrode capacitance calculation and application |
| US10324058B2 (en) | 2016-04-28 | 2019-06-18 | Medtronic Minimed, Inc. | In-situ chemistry stack for continuous glucose sensors |
| US9970893B2 (en) | 2016-04-28 | 2018-05-15 | Medtronic Minimed, Inc. | Methods, systems, and devices for electrode capacitance calculation and application |
| US10086133B2 (en) | 2016-05-26 | 2018-10-02 | Medtronic Minimed, Inc. | Systems for set connector assembly with lock |
| US10086134B2 (en) | 2016-05-26 | 2018-10-02 | Medtronic Minimed, Inc. | Systems for set connector assembly with lock |
| US9968737B2 (en) | 2016-05-26 | 2018-05-15 | Medtronic Minimed, Inc. | Systems for set connector assembly with lock |
| US11134872B2 (en) | 2016-06-06 | 2021-10-05 | Medtronic Minimed, Inc. | Thermally stable glucose limiting membrane for glucose sensors |
| US11179078B2 (en) | 2016-06-06 | 2021-11-23 | Medtronic Minimed, Inc. | Polycarbonate urea/urethane polymers for use with analyte sensors |
| US10583255B2 (en)* | 2016-07-27 | 2020-03-10 | Shl Medical Ag | Medicament container assembly for a medicament delivery device |
| US11338090B2 (en) | 2016-08-01 | 2022-05-24 | West Pharma. Services IL, Ltd. | Anti-rotation cartridge pin |
| US20210138461A1 (en)* | 2016-08-02 | 2021-05-13 | Qorvo Us, Inc. | Biosensor cartridge with sample acquisition |
| USD831200S1 (en)* | 2016-08-08 | 2018-10-16 | Ultradent Products, Inc. | Delivery tip |
| US10449291B2 (en) | 2016-09-06 | 2019-10-22 | Medtronic Minimed, Inc. | Pump clip for a fluid infusion device |
| JP7188877B2 (en)* | 2016-09-19 | 2022-12-13 | ウエスト・ファーマ.サービシーズ・イスラエル,リミテッド | Rotational Resistance Friction Adapter for Plunger Drivers of Drug Delivery Devices |
| US11517669B2 (en) | 2016-09-29 | 2022-12-06 | Koninklijke Philips N.V. | Piezoelectric membrane pump for the infusion of liquids |
| USD836774S1 (en)* | 2016-12-16 | 2018-12-25 | Sorrento Therapeutics, Inc. | Cartridge for a fluid delivery apparatus |
| US10861591B2 (en) | 2016-12-21 | 2020-12-08 | Medtronic Minimed, Inc. | Infusion systems and methods for pattern-based therapy adjustments |
| US10709834B2 (en) | 2016-12-21 | 2020-07-14 | Medtronic Minimed, Inc. | Medication fluid infusion set component with integrated physiological analyte sensor, and corresponding fluid infusion device |
| US10272201B2 (en) | 2016-12-22 | 2019-04-30 | Medtronic Minimed, Inc. | Insertion site monitoring methods and related infusion devices and systems |
| US11197949B2 (en) | 2017-01-19 | 2021-12-14 | Medtronic Minimed, Inc. | Medication infusion components and systems |
| US10821225B2 (en) | 2017-01-20 | 2020-11-03 | Medtronic Minimed, Inc. | Cannulas for drug delivery devices |
| GB2559553B (en)* | 2017-02-06 | 2021-10-13 | Consort Medical Plc | A medicament delivery device |
| US10552580B2 (en) | 2017-02-07 | 2020-02-04 | Medtronic Minimed, Inc. | Infusion system consumables and related calibration methods |
| US10646649B2 (en) | 2017-02-21 | 2020-05-12 | Medtronic Minimed, Inc. | Infusion devices and fluid identification apparatuses and methods |
| US11986288B2 (en) | 2017-03-06 | 2024-05-21 | Medtronic Minimed, Inc. | Colorometric sensor for the non-invasive screening of glucose in sweat in pre and type 2 diabetes |
| US11134868B2 (en) | 2017-03-17 | 2021-10-05 | Medtronic Minimed, Inc. | Metal pillar device structures and methods for making and using them in electrochemical and/or electrocatalytic applications |
| US11350886B2 (en) | 2017-03-24 | 2022-06-07 | Medtronic Minimed, Inc. | Context-sensitive infusion devices, systems and methods |
| US10413658B2 (en) | 2017-03-31 | 2019-09-17 | Capillary Biomedical, Inc. | Helical insertion infusion device |
| CN119950880A (en) | 2017-05-05 | 2025-05-09 | 里珍纳龙药品有限公司 | Auto-injectors and related methods of use |
| US11969864B2 (en) | 2017-05-11 | 2024-04-30 | Scalpal Llc | Multi-tier torque enhancer driver and/or receiver and method of using same |
| US20180328877A1 (en) | 2017-05-11 | 2018-11-15 | Medtronic Minimed, Inc. | Analyte sensors and methods for fabricating analyte sensors |
| US10856784B2 (en) | 2017-06-30 | 2020-12-08 | Medtronic Minimed, Inc. | Sensor initialization methods for faster body sensor response |
| US11504472B2 (en) | 2017-07-06 | 2022-11-22 | Quasuras, Inc. | Medical pump with flow control |
| US11412960B2 (en) | 2017-08-28 | 2022-08-16 | Medtronic Minimed, Inc. | Pedestal for sensor assembly packaging and sensor introducer removal |
| US10596295B2 (en) | 2017-08-28 | 2020-03-24 | Medtronic Minimed, Inc. | Adhesive patch arrangement for a physiological characteristic sensor, and related sensor assembly |
| US11344235B2 (en) | 2017-09-13 | 2022-05-31 | Medtronic Minimed, Inc. | Methods, systems, and devices for calibration and optimization of glucose sensors and sensor output |
| US10874300B2 (en) | 2017-09-26 | 2020-12-29 | Medtronic Minimed, Inc. | Waferscale physiological characteristic sensor package with integrated wireless transmitter |
| US10525244B2 (en) | 2017-09-28 | 2020-01-07 | Medtronic Minimed, Inc. | Microneedle arrays and methods for fabricating microneedle arrays |
| US10524730B2 (en) | 2017-09-28 | 2020-01-07 | Medtronic Minimed, Inc. | Medical devices with microneedle arrays and methods for operating such medical devices |
| US11676734B2 (en) | 2017-11-15 | 2023-06-13 | Medtronic Minimed, Inc. | Patient therapy management system that leverages aggregated patient population data |
| US11471082B2 (en) | 2017-12-13 | 2022-10-18 | Medtronic Minimed, Inc. | Complex redundancy in continuous glucose monitoring |
| US11213230B2 (en) | 2017-12-13 | 2022-01-04 | Medtronic Minimed, Inc. | Optional sensor calibration in continuous glucose monitoring |
| US11439352B2 (en) | 2018-01-17 | 2022-09-13 | Medtronic Minimed, Inc. | Medical device with adhesive patch longevity |
| US12042284B2 (en) | 2018-01-23 | 2024-07-23 | Medtronic Minimed, Inc. | Implantable polymer surfaces exhibiting reduced in vivo inflammatory responses |
| US11191893B2 (en) | 2018-01-31 | 2021-12-07 | Bayer Healthcare Llc | System and method for syringe engagement with injector |
| CA3089837A1 (en) | 2018-02-06 | 2019-08-15 | Becton, Dickinson And Company | Systems, apparatuses and methods for occlusion detection using pump operation measurement |
| US11186859B2 (en) | 2018-02-07 | 2021-11-30 | Medtronic Minimed, Inc. | Multilayer electrochemical analyte sensors and methods for making and using them |
| US11220735B2 (en) | 2018-02-08 | 2022-01-11 | Medtronic Minimed, Inc. | Methods for controlling physical vapor deposition metal film adhesion to substrates and surfaces |
| US11583213B2 (en) | 2018-02-08 | 2023-02-21 | Medtronic Minimed, Inc. | Glucose sensor electrode design |
| JP7201663B2 (en) | 2018-03-20 | 2023-01-10 | テルモ株式会社 | Cap, syringe assembly and manufacturing method thereof |
| US11672446B2 (en) | 2018-03-23 | 2023-06-13 | Medtronic Minimed, Inc. | Insulin delivery recommendations based on nutritional information |
| EP3760251A1 (en) | 2018-03-30 | 2021-01-06 | TERUMO Kabushiki Kaisha | Medicating appliance and liquid medicine administering system |
| WO2019189432A1 (en) | 2018-03-30 | 2019-10-03 | テルモ株式会社 | Administering instrument and drug solution administering system |
| US11158413B2 (en) | 2018-04-23 | 2021-10-26 | Medtronic Minimed, Inc. | Personalized closed loop medication delivery system that utilizes a digital twin of the patient |
| US11147919B2 (en) | 2018-04-23 | 2021-10-19 | Medtronic Minimed, Inc. | Methodology to recommend and implement adjustments to a fluid infusion device of a medication delivery system |
| US11367526B2 (en) | 2018-05-07 | 2022-06-21 | Medtronic Minimed, Inc. | Proactive patient guidance using augmented reality |
| US10715752B2 (en) | 2018-06-06 | 2020-07-14 | Cnh Industrial Canada, Ltd. | System and method for monitoring sensor performance on an agricultural machine |
| US11761077B2 (en) | 2018-08-01 | 2023-09-19 | Medtronic Minimed, Inc. | Sputtering techniques for biosensors |
| US11122697B2 (en) | 2018-08-07 | 2021-09-14 | Medtronic Minimed, Inc. | Method of fabricating an electronic medical device, including overmolding an assembly with thermoplastic material |
| US11021731B2 (en) | 2018-08-23 | 2021-06-01 | Medtronic Minimed, Inc. | Analyte sensing layers, analyte sensors and methods for fabricating the same |
| US10828419B2 (en) | 2018-09-04 | 2020-11-10 | Medtronic Minimed, Inc. | Infusion set with pivoting metal cannula and strain relief |
| US11547799B2 (en) | 2018-09-20 | 2023-01-10 | Medtronic Minimed, Inc. | Patient day planning systems and methods |
| US10980942B2 (en) | 2018-09-28 | 2021-04-20 | Medtronic Minimed, Inc. | Infusion devices and related meal bolus adjustment methods |
| US11071821B2 (en) | 2018-09-28 | 2021-07-27 | Medtronic Minimed, Inc. | Insulin infusion device with efficient confirmation routine for blood glucose measurements |
| US11097052B2 (en) | 2018-09-28 | 2021-08-24 | Medtronic Minimed, Inc. | Insulin infusion device with configurable target blood glucose value for automatic basal insulin delivery operation |
| US10894126B2 (en) | 2018-09-28 | 2021-01-19 | Medtronic Minimed, Inc. | Fluid infusion system that automatically determines and delivers a correction bolus |
| US20200116748A1 (en) | 2018-10-11 | 2020-04-16 | Medtronic Minimed, Inc. | Systems and methods for measurement of fluid delivery |
| US10946140B2 (en) | 2018-10-11 | 2021-03-16 | Medtronic Minimed, Inc. | Systems and methods for measurement of fluid delivery |
| US11363986B2 (en) | 2018-10-31 | 2022-06-21 | Medtronic Minimed, Inc. | Automated detection of a physical behavior event and corresponding adjustment of a medication dispensing system |
| US20200289373A1 (en) | 2018-10-31 | 2020-09-17 | Medtronic Minimed, Inc. | Automated detection of a physical behavior event and corresponding adjustment of a physiological characteristic sensor device |
| US11367517B2 (en) | 2018-10-31 | 2022-06-21 | Medtronic Minimed, Inc. | Gesture-based detection of a physical behavior event based on gesture sensor data and supplemental information from at least one external source |
| US11367516B2 (en) | 2018-10-31 | 2022-06-21 | Medtronic Minimed, Inc. | Automated detection of a physical behavior event and corresponding adjustment of a medication dispensing system |
| EP3877018A4 (en) | 2018-11-08 | 2022-08-10 | Capillary Biomedical, Inc. | LINEAR INTRODUCER WITH ROTARY DRIVE |
| US11382541B2 (en) | 2018-11-16 | 2022-07-12 | Medtronic Minimed, Inc. | Miniaturized analyte sensor |
| US11540750B2 (en) | 2018-12-19 | 2023-01-03 | Medtronic Minimed, Inc | Systems and methods for physiological characteristic monitoring |
| CH715757A2 (en) | 2019-01-17 | 2020-07-31 | Tecpharma Licensing Ag | Modular delivery device for fluid drug formulation. |
| US12114972B2 (en) | 2019-02-01 | 2024-10-15 | Medtronic Minimed, Inc. | Methods, systems, and devices for continuous glucose monitoring |
| US11439752B2 (en) | 2019-02-01 | 2022-09-13 | Medtronic Minimed, Inc. | Methods and devices for occlusion detection using actuator sensors |
| US11389587B2 (en) | 2019-02-06 | 2022-07-19 | Medtronic Minimed, Inc. | Patient monitoring systems and related presentation methods |
| US11191899B2 (en) | 2019-02-12 | 2021-12-07 | Medtronic Minimed, Inc. | Infusion systems and related personalized bolusing methods |
| US12082910B2 (en) | 2019-02-12 | 2024-09-10 | Medtronic Minimed, Inc. | Miniaturized noninvasive glucose sensor and continuous glucose monitoring system |
| US11311215B2 (en) | 2019-04-04 | 2022-04-26 | Medtronic Minimed, Inc. | Measurement of device materials using non-Faradaic electrochemical impedance spectroscopy |
| US11986629B2 (en) | 2019-06-11 | 2024-05-21 | Medtronic Minimed, Inc. | Personalized closed loop optimization systems and methods |
| US11224361B2 (en) | 2019-04-23 | 2022-01-18 | Medtronic Minimed, Inc. | Flexible physiological characteristic sensor assembly |
| US11317867B2 (en) | 2019-04-23 | 2022-05-03 | Medtronic Minimed, Inc. | Flexible physiological characteristic sensor assembly |
| FR3096040B1 (en)* | 2019-05-17 | 2021-09-24 | Aptar France Sas | Fluid dispenser device |
| US10939488B2 (en) | 2019-05-20 | 2021-03-02 | Medtronic Minimed, Inc. | Method and system for controlling communication between devices of a wireless body area network for an medical device system |
| US11642454B2 (en) | 2019-06-06 | 2023-05-09 | Medtronic Minimed, Inc. | Fluid infusion systems |
| US11448611B2 (en) | 2019-07-03 | 2022-09-20 | Medtronic Minimed, Inc. | Structurally reinforced sensor and method for manufacturing the same |
| US11617828B2 (en) | 2019-07-17 | 2023-04-04 | Medtronic Minimed, Inc. | Reservoir connection interface with detectable signature |
| US11718865B2 (en) | 2019-07-26 | 2023-08-08 | Medtronic Minimed, Inc. | Methods to improve oxygen delivery to implantable sensors |
| US11523757B2 (en) | 2019-08-01 | 2022-12-13 | Medtronic Minimed, Inc. | Micro-pillar working electrodes design to reduce backflow of hydrogen peroxide in glucose sensor |
| US11617522B2 (en) | 2019-08-06 | 2023-04-04 | Medtronic Minimed, Inc. | Sensor inserter with disposal lockout state |
| US11883208B2 (en) | 2019-08-06 | 2024-01-30 | Medtronic Minimed, Inc. | Machine learning-based system for estimating glucose values based on blood glucose measurements and contextual activity data |
| US20220039755A1 (en) | 2020-08-06 | 2022-02-10 | Medtronic Minimed, Inc. | Machine learning-based system for estimating glucose values |
| US11724045B2 (en) | 2019-08-21 | 2023-08-15 | Medtronic Minimed, Inc. | Connection of a stopper and piston in a fluid delivery device |
| EP4021557A4 (en) | 2019-08-27 | 2022-10-12 | Colder Products Company | Single-use genderless aseptic fluid couplings |
| US20210060244A1 (en) | 2019-08-28 | 2021-03-04 | Medtronic Minimed, Inc. | Method and system for verifying whether a non-medical client device is operating correctly with a medical device controlled by the non-medical client device and causing a notification to be generated |
| US11571515B2 (en) | 2019-08-29 | 2023-02-07 | Medtronic Minimed, Inc. | Controlling medical device operation and features based on detected patient sleeping status |
| US11338082B2 (en) | 2019-09-04 | 2022-05-24 | BloQ Pharma, Inc. | Variable rate dispenser with aseptic spike connector assembly |
| US11565044B2 (en) | 2019-09-12 | 2023-01-31 | Medtronic Minimed, Inc. | Manufacturing controls for sensor calibration using fabrication measurements |
| US11654235B2 (en) | 2019-09-12 | 2023-05-23 | Medtronic Minimed, Inc. | Sensor calibration using fabrication measurements |
| US11213623B2 (en) | 2019-09-20 | 2022-01-04 | Medtronic Minimed, Inc. | Infusion systems and related personalized bolusing methods |
| US11241537B2 (en) | 2019-09-20 | 2022-02-08 | Medtronic Minimed, Inc. | Contextual personalized closed-loop adjustment methods and systems |
| US11511099B2 (en)* | 2019-10-08 | 2022-11-29 | Medtronic Minimed, Inc. | Apparatus for detecting mating of a cap with a fluid delivery device and method |
| US12329926B2 (en) | 2019-10-10 | 2025-06-17 | Medtronic Minimed, Inc. | Adjustable insertion device |
| US11638545B2 (en) | 2019-10-16 | 2023-05-02 | Medtronic Minimed, Inc. | Reducing sensor foreign body response via high surface area metal structures |
| US12315631B2 (en) | 2019-11-07 | 2025-05-27 | Medtronic Minimed, Inc. | Medical device site monitoring methods and related devices and systems |
| US12263327B2 (en) | 2019-11-14 | 2025-04-01 | Medtronic Minimed, Inc. | Synergistic features and functions related to operation of a medication delivery system and a meal transaction application |
| US11496083B2 (en) | 2019-11-15 | 2022-11-08 | Medtronic Minimed, Inc. | Devices and methods for controlling electromechanical actuators |
| US11944784B2 (en) | 2019-11-18 | 2024-04-02 | Medtronic Minimed, Inc. | Combined analyte sensor and infusion set |
| US11324881B2 (en) | 2019-11-21 | 2022-05-10 | Medtronic Minimed, Inc. | Systems for wearable infusion port and associated pump |
| US11559624B2 (en) | 2019-11-21 | 2023-01-24 | Medtronic Minimed, Inc. | Systems for wearable infusion port and associated pump |
| US12247941B2 (en) | 2019-11-21 | 2025-03-11 | Medtronic Minimed, Inc. | Glucose biosensor encasement, glucose biosensor package, and method |
| WO2021113538A1 (en) | 2019-12-06 | 2021-06-10 | Quasuras, Inc. | Training cartridge for medical pump systems |
| US12119119B2 (en) | 2019-12-09 | 2024-10-15 | Medtronic Minimed, Inc. | Methods and systems for real-time sensor measurement simulation |
| US11786655B2 (en) | 2019-12-13 | 2023-10-17 | Medtronic Minimed, Inc. | Context-sensitive predictive operation of a medication delivery system in response to gesture-indicated activity changes |
| US20210178063A1 (en) | 2019-12-13 | 2021-06-17 | Medtronic Minimed, Inc. | Controlling medication delivery system operation and features based on automatically detected user stress level |
| US11938301B2 (en) | 2019-12-13 | 2024-03-26 | Medtronic Minimed, Inc. | Controlling medication delivery system operation and features based on automatically detected muscular movements |
| US11887712B2 (en) | 2019-12-13 | 2024-01-30 | Medtronic Minimed, Inc. | Method and system for classifying detected events as labeled event combinations for processing at a client application |
| US12133969B2 (en) | 2019-12-13 | 2024-11-05 | Medtronic Minimed, Inc. | Translating therapy parameters of an insulin therapy system to translated therapy parameters for use at a different insulin therapy system |
| US11488700B2 (en) | 2019-12-13 | 2022-11-01 | Medtronic Minimed, Inc. | Medical device configuration procedure guidance responsive to detected gestures |
| US11690573B2 (en) | 2019-12-18 | 2023-07-04 | Medtronic Minimed, Inc. | Systems for skin patch gravity resistance |
| US11375955B2 (en) | 2019-12-18 | 2022-07-05 | Medtronic Minimed, Inc. | Systems for skin patch gravity resistance |
| US11821022B2 (en) | 2019-12-23 | 2023-11-21 | Medtronic Minimed, Inc. | Ethylene oxide absorption layer for analyte sensing and method |
| US11244753B2 (en) | 2020-01-30 | 2022-02-08 | Medtronic Minimed, Inc. | Activity monitoring systems and methods |
| US11957488B2 (en) | 2020-02-07 | 2024-04-16 | Medtronic Minimed, Inc. | Systems for medical device breathability |
| US12186098B2 (en) | 2020-02-07 | 2025-01-07 | Medtronic Minimed, Inc. | Systems for medical device breathability |
| US11817197B2 (en) | 2020-02-07 | 2023-11-14 | Quasuras, Inc. | Medical pump electronic pairing with device |
| US11833327B2 (en) | 2020-03-06 | 2023-12-05 | Medtronic Minimed, Inc. | Analyte sensor configuration and calibration based on data collected from a previously used analyte sensor |
| US12191017B2 (en) | 2020-03-23 | 2025-01-07 | Medtronic Minimed, Inc. | Systems and methods for medicine administration and tracking |
| USD958167S1 (en) | 2020-03-23 | 2022-07-19 | Companion Medical, Inc. | Display screen with graphical user interface |
| USD958817S1 (en) | 2020-03-31 | 2022-07-26 | Medtronic Minimed, Inc. | Display screen with graphical user interface |
| US11596359B2 (en) | 2020-04-09 | 2023-03-07 | Medtronic Minimed, Inc. | Methods and systems for mitigating sensor error propagation |
| US11583631B2 (en) | 2020-04-23 | 2023-02-21 | Medtronic Minimed, Inc. | Intuitive user interface features and related functionality for a therapy delivery system |
| US11690955B2 (en) | 2020-04-23 | 2023-07-04 | Medtronic Minimed, Inc. | Continuous analyte sensor quality measures and related therapy actions for an automated therapy delivery system |
| US20210377726A1 (en) | 2020-05-27 | 2021-12-02 | Medtronic Minimed, Inc. | Method and system for automatically associating a non-medical device with a medical device |
| US12213782B2 (en) | 2020-06-04 | 2025-02-04 | Medtronic Minimed, Inc. | Physiological characteristic sensor system |
| US11272884B2 (en) | 2020-06-04 | 2022-03-15 | Medtronic Minimed, Inc. | Liner for adhesive skin patch |
| US12433538B2 (en) | 2020-06-04 | 2025-10-07 | Medtronic Minimed, Inc. | Liner for adhesive skin patch |
| US12064236B2 (en) | 2020-06-11 | 2024-08-20 | Medtronic Minimed, Inc. | Methods, systems, and devices for improved sensors for continuous glucose monitoring |
| US11650248B2 (en) | 2020-07-28 | 2023-05-16 | Medtronic Minimed, Inc. | Electrical current measurement system |
| US11960311B2 (en) | 2020-07-28 | 2024-04-16 | Medtronic Minimed, Inc. | Linear voltage regulator with isolated supply current |
| US12082924B2 (en) | 2020-07-31 | 2024-09-10 | Medtronic Minimed, Inc. | Sensor identification and integrity check design |
| US11445807B2 (en) | 2020-07-31 | 2022-09-20 | Medtronic Minimed, Inc. | Pump clip with tube clamp for a fluid infusion device |
| JP2023537497A (en)* | 2020-08-06 | 2023-09-01 | ベクトン・ディキンソン・アンド・カンパニー | Displacement pump mechanism with flexible reservoir, drug delivery system, patch pump and drug delivery device |
| EP4204066A4 (en) | 2020-08-28 | 2024-11-13 | Tandem Diabetes Care, Inc. | INSULIN INFUSION SET |
| US11839743B2 (en) | 2020-10-07 | 2023-12-12 | Medtronic Minimed, Inc. | Graphic user interface for automated infusate delivery |
| US11737783B2 (en) | 2020-10-16 | 2023-08-29 | Medtronic Minimed, Inc. | Disposable medical device introduction system |
| USD978352S1 (en) | 2020-10-28 | 2023-02-14 | Smiths Medical Asd, Inc. | Cartridge cap |
| US11844930B2 (en) | 2020-10-29 | 2023-12-19 | Medtronic Minimed, Inc. | User-mountable electronic device with accelerometer-based activation feature |
| US11806503B2 (en) | 2020-10-29 | 2023-11-07 | Medtronic Minimed, Inc. | Removable wearable device and related attachment methods |
| US11534086B2 (en) | 2020-10-30 | 2022-12-27 | Medtronic Minimed, Inc. | Low-profile wearable medical device |
| US11951281B2 (en) | 2020-11-11 | 2024-04-09 | Medtronic Minimed, Inc. | Fluid conduit insertion devices |
| AU2022206656A1 (en)* | 2021-01-05 | 2023-07-20 | Becton, Dickinson And Company | Needle hub for drug delivery device |
| US12138047B2 (en) | 2021-01-22 | 2024-11-12 | Medtronic Minimed, Inc. | Micro models and layered prediction models for estimating sensor glucose values and reducing sensor glucose signal blanking |
| US12423490B2 (en) | 2021-01-29 | 2025-09-23 | Medtronic Minimed, Inc. | Model mosaic framework for modeling glucose sensitivity |
| US11998330B2 (en) | 2021-01-29 | 2024-06-04 | Medtronic Minimed, Inc. | Interference rejection membranes useful with analyte sensors |
| CN116830208A (en) | 2021-02-02 | 2023-09-29 | 美敦力迷你迈德公司 | Dynamic adjustment of physiological data |
| KR102344214B1 (en) | 2021-05-18 | 2021-12-28 | (주)브이텍 | Vacuum ejector pump |
| US11904146B2 (en) | 2021-06-08 | 2024-02-20 | Medtronic Minimed, Inc. | Medicine injection devices, systems, and methods for medicine administration and tracking |
| US12214173B2 (en) | 2021-06-08 | 2025-02-04 | Medtronic Minimed, Inc. | Injection pens for medicine administration and tracking |
| US11792714B2 (en) | 2021-06-16 | 2023-10-17 | Medtronic Minimed, Inc. | Medicine administration in dynamic networks |
| US12370320B2 (en) | 2021-06-21 | 2025-07-29 | Medtronic Minimed, Inc. | Accessory devices, systems, and methods for medicine administration and tracking |
| EP4122511A1 (en)* | 2021-07-21 | 2023-01-25 | Ypsomed AG | Monitoring a dispensing process with a drug delivery device |
| US12433515B2 (en) | 2021-08-13 | 2025-10-07 | Medtronic Minimed, Inc. | Dry electrochemical impedance spectroscopy metrology for conductive chemical layers |
| US11587742B1 (en) | 2021-09-02 | 2023-02-21 | Medtronic Minimed, Inc. | Ingress-tolerant input devices |
| US11817285B2 (en) | 2021-09-02 | 2023-11-14 | Medtronic Minimed, Inc. | Ingress-tolerant input devices comprising sliders |
| US12201421B2 (en) | 2021-10-08 | 2025-01-21 | Medtronic Minimed, Inc. | Immunosuppressant releasing coatings |
| USD1007676S1 (en) | 2021-11-16 | 2023-12-12 | Regeneron Pharmaceuticals, Inc. | Wearable autoinjector |
| IL312892A (en)* | 2021-11-16 | 2024-07-01 | Regeneron Pharma | Patch-pump |
| US11896447B2 (en) | 2022-03-14 | 2024-02-13 | Medtronic Minimed, Inc. | Safeguards against separation from portable medicine delivery devices |
| US11997806B2 (en) | 2022-04-26 | 2024-05-28 | Medtronic Minimed, Inc. | Energy management based on an open switch configuration |
| US12011293B2 (en) | 2022-04-26 | 2024-06-18 | Medtronic Minimed, Inc. | Energy management based on a closed switch configuration |
| KR20230160013A (en) | 2022-05-16 | 2023-11-23 | 삼성전자주식회사 | Apparatus for supplying liquid and system for supplying liquid comprising the same |
| DE102022117611A1 (en)* | 2022-07-14 | 2024-01-25 | B. Braun Melsungen Aktiengesellschaft | Syringe pump with piston brake |
| JPWO2024053149A1 (en)* | 2022-09-07 | 2024-03-14 | ||
| LU103026B1 (en) | 2022-10-05 | 2024-04-05 | Stratec Se | Hose coupling and method for use in analytical systems |
| US12109387B2 (en)* | 2022-11-11 | 2024-10-08 | Carefusion 303, Inc. | Connector coupling assembly |
| WO2024184214A1 (en)* | 2023-03-03 | 2024-09-12 | Cryowrite Ag | Syringe pump |
| CN116733726A (en)* | 2023-06-19 | 2023-09-12 | 甘肃智鹏科技有限公司 | An interlocking device to avoid overpressure at the outlet of a hydraulic diaphragm metering pump |
Citations (32)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| DE2240694A1 (en) | 1971-08-20 | 1973-03-01 | Rene Derouineau | SYRINGE FOR INTRAVENOESIS OR INTRAMUSCULAR INJECTION |
| US4444546A (en) | 1980-09-19 | 1984-04-24 | Oximetrix, Inc. | Occlusion detection apparatus and method |
| US4468221A (en) | 1981-04-10 | 1984-08-28 | Parker-Hannifin Corporation | Medication infusion pump |
| US4562751A (en) | 1984-01-06 | 1986-01-07 | Nason Clyde K | Solenoid drive apparatus for an external infusion pump |
| US4601491A (en) | 1983-10-19 | 1986-07-22 | Vetco Offshore, Inc. | Pipe connector |
| US4619646A (en) | 1984-01-25 | 1986-10-28 | Fernandez Tresguerres Hernande | Device for the delivery-dosing of injectable products |
| US4678408A (en) | 1984-01-06 | 1987-07-07 | Pacesetter Infusion, Ltd. | Solenoid drive apparatus for an external infusion pump |
| US4685903A (en) | 1984-01-06 | 1987-08-11 | Pacesetter Infusion, Ltd. | External infusion pump apparatus |
| US4744790A (en) | 1986-08-19 | 1988-05-17 | The West Company | Fast action cartridge syringe holder |
| US4749109A (en) | 1983-11-15 | 1988-06-07 | Kamen Dean L | Volumetric pump with replaceable reservoir assembly |
| US4952205A (en) | 1987-04-04 | 1990-08-28 | B. Braun Melsungen Ag | Pressure infusion device |
| US5080653A (en) | 1990-04-16 | 1992-01-14 | Pacesetter Infusion, Ltd. | Infusion pump with dual position syringe locator |
| US5097122A (en) | 1990-04-16 | 1992-03-17 | Pacesetter Infusion, Ltd. | Medication infusion system having optical motion sensor to detect drive mechanism malfunction |
| US5121019A (en) | 1986-08-26 | 1992-06-09 | Josef Pradler | Rotary to linear drive unit |
| US5254096A (en) | 1992-09-23 | 1993-10-19 | Becton, Dickinson And Company | Syringe pump with graphical display or error conditions |
| US5279556A (en)* | 1989-04-28 | 1994-01-18 | Sharp Kabushiki Kaisha | Peristaltic pump with rotary encoder |
| US5292306A (en) | 1993-01-29 | 1994-03-08 | Abbott Laboratories | Method of detecting occlusions in a solution pumping system |
| USD347894S (en) | 1990-09-21 | 1994-06-14 | Novo Nordisk A/S | Adaptor top for a pen syringe ampoule |
| US5389078A (en) | 1993-10-06 | 1995-02-14 | Sims Deltec, Inc. | Programmable infusion pump for administering medication to patients |
| US5505709A (en) | 1994-09-15 | 1996-04-09 | Minimed, Inc., A Delaware Corporation | Mated infusion pump and syringe |
| US5522799A (en)* | 1993-12-17 | 1996-06-04 | Sharp Kabushiki Kaisha | Fluid infusion pump capable of detecting erroneous tube displacement |
| US5545152A (en) | 1994-10-28 | 1996-08-13 | Minimed Inc. | Quick-connect coupling for a medication infusion system |
| US5549574A (en) | 1994-08-30 | 1996-08-27 | Eli Lilly And Company | Cartridge assembly for use in a pen-type medicament injector |
| US5554134A (en) | 1990-09-21 | 1996-09-10 | Novo Nordisk A/S | Cap for an ampoule of an injection unit |
| US5584667A (en)* | 1988-05-17 | 1996-12-17 | Davis; David L. | Method of providing uniform flow from an infusion device |
| US5599323A (en) | 1991-07-12 | 1997-02-04 | Novo Nordisk A/S | Syringe system |
| US5637095A (en) | 1995-01-13 | 1997-06-10 | Minimed Inc. | Medication infusion pump with flexible drive plunger |
| USD380262S (en) | 1995-06-26 | 1997-06-24 | Minimed Inc. | Quick disconnect coupling |
| US5647853A (en) | 1995-03-03 | 1997-07-15 | Minimed Inc. | Rapid response occlusion detector for a medication infusion pump |
| WO1998000157A1 (en) | 1996-07-03 | 1998-01-08 | Alza Corporation | Aqueous formulations of peptides |
| US5722545A (en) | 1993-12-10 | 1998-03-03 | Dental-Kosmetik Gmbh | Container with twist-on-off closure cap |
| US5947935A (en) | 1996-11-12 | 1999-09-07 | Medrad, Inc. | Syringes, syringe plungers and injector systems |
Family Cites Families (72)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US462751A (en) | 1891-11-10 | Electric-railway motor | ||
| US3623474A (en)* | 1966-07-25 | 1971-11-30 | Medrad Inc | Angiographic injection equipment |
| US3701345A (en)* | 1970-09-29 | 1972-10-31 | Medrad Inc | Angiographic injector equipment |
| US4076285A (en) | 1975-08-01 | 1978-02-28 | Erika, Inc. | Laminar flow connector for conduits |
| US4084588A (en) | 1976-03-19 | 1978-04-18 | Sherwood Medical Industries Inc. | Parenteral drug storage device with closure piercing coupling member |
| US4267836A (en) | 1976-11-12 | 1981-05-19 | Whitney Douglass G | Injection device and method |
| US4351335A (en)* | 1979-04-16 | 1982-09-28 | Whitney Douglass G | Injection device and method |
| US4278188A (en)* | 1979-10-01 | 1981-07-14 | George M. Stephenson | Remote delivery nozzle assembly for pressurized container |
| US4274407A (en) | 1979-11-13 | 1981-06-23 | Med Pump, Inc. | Fluid injection system |
| US5330450A (en)* | 1983-01-24 | 1994-07-19 | Icu Medical, Inc. | Medical connector |
| US4752292A (en) | 1983-01-24 | 1988-06-21 | Icu Medical, Inc. | Medical connector |
| NL8300386A (en) | 1983-02-02 | 1984-09-03 | Steritech Bv | STERILE DEVICE CONNECTING TWO ROOMS. |
| WO1984004673A1 (en) | 1983-05-20 | 1984-12-06 | Bengt Gustavsson | A device for transferring a substance |
| DE3468173D1 (en)† | 1983-09-07 | 1988-02-04 | Disetronic Ag | Portable infusion apparatus |
| IT1173370B (en)* | 1984-02-24 | 1987-06-24 | Erba Farmitalia | SAFETY DEVICE TO CONNECT A SYRINGE TO THE MOUTH OF A BOTTLE CONTAINING A DRUG OR A TUBE FOR DISPENSING THE SYRINGE DRUG |
| US4588403A (en)† | 1984-06-01 | 1986-05-13 | American Hospital Supply Corporation | Vented syringe adapter assembly |
| US4747824A (en) | 1986-05-30 | 1988-05-31 | Spinello Ronald P | Hypodermic anesthetic injection method |
| US5251873B1 (en) | 1992-06-04 | 1995-05-02 | Vernay Laboratories | Medical coupling site. |
| GB8713810D0 (en)* | 1987-06-12 | 1987-07-15 | Hypoguard Uk Ltd | Measured dose dispensing device |
| US4834744A (en)* | 1987-11-04 | 1989-05-30 | Critikon, Inc. | Spike for parenteral solution container |
| US4919596A (en)* | 1987-12-04 | 1990-04-24 | Pacesetter Infusion, Ltd. | Fluid delivery control and monitoring apparatus for a medication infusion system |
| EP0544653B1 (en)* | 1988-01-25 | 1996-06-05 | Baxter International Inc. | Injection site |
| US5100394A (en)* | 1988-01-25 | 1992-03-31 | Baxter International Inc. | Pre-slit injection site |
| FR2628639B1 (en)* | 1988-03-21 | 1994-03-04 | Microtechnic | IMPLANTABLE DEVICE FOR PERFUSION OF MEDICAL LIQUIDS |
| IT1217595B (en)* | 1988-05-13 | 1990-03-30 | Molteni & C | ANTI-CONTACT DEVICE FOR INJECTION OF DENTAL ANESTHETIC SOLUTIONS CONTAINED IN CARTRIDGE |
| JP2909906B2 (en)† | 1988-05-30 | 1999-06-23 | ミノルタ株式会社 | Internal pressure adjustment mechanism for underwater and waterproof products |
| US5087250A (en)* | 1988-07-26 | 1992-02-11 | Gish Biomedical, Inc. | Autotransfusion unit with vacuum regulation and cardiotomy reservoir |
| DE3833821A1 (en)* | 1988-10-05 | 1990-04-12 | Braun Melsungen Ag | INJECTION DEVICE |
| CA2001732A1 (en) | 1988-10-31 | 1990-04-30 | Lawrence A. Lynn | Intravenous line coupling device |
| US4962533A (en)* | 1989-02-17 | 1990-10-09 | Texas Instrument Incorporated | Data protection for computer systems |
| US5374256A (en) | 1989-06-16 | 1994-12-20 | Science Incorporated | Fluid container for use with a fluid delivery apparatus |
| DE9013145U1 (en)* | 1989-09-22 | 1990-11-22 | B. Braun Melsungen Ag, 3508 Melsungen | Medical hose connection device |
| US5514090A (en) | 1990-04-24 | 1996-05-07 | Science Incorporated | Closed drug delivery system |
| AU645096B2 (en)* | 1990-05-07 | 1994-01-06 | Winfield Medical | Medical intravenous administration line connector |
| US5254306A (en)* | 1990-10-29 | 1993-10-19 | Toyoda Gosei Co., Ltd. | Method for making a double layer molded product using a dam in the mold cavity |
| US5201717A (en)* | 1990-12-05 | 1993-04-13 | Philip Wyatt | Safety enclosure |
| JPH0613744Y2 (en) | 1991-01-18 | 1994-04-13 | テルモ株式会社 | Puncture needle |
| US5300031A (en)* | 1991-06-07 | 1994-04-05 | Liebel-Flarsheim Company | Apparatus for injecting fluid into animals and disposable front loadable syringe therefor |
| US5219099A (en)* | 1991-09-06 | 1993-06-15 | California Institute Of Technology | Coaxial lead screw drive syringe pump |
| JP2870257B2 (en) | 1991-09-12 | 1999-03-17 | 日立電線株式会社 | Manufacturing method of electrical contact |
| US5188610A (en)* | 1991-10-18 | 1993-02-23 | Vetrisystems, Inc. | Fluid dispensing apparatus |
| US5700244A (en)* | 1992-04-17 | 1997-12-23 | Science Incorporated | Fluid dispenser with fill adapter |
| US5261572A (en)* | 1992-06-09 | 1993-11-16 | Plato Products, Inc. | Dropper bottle |
| US5334179A (en) | 1992-10-16 | 1994-08-02 | Abbott Laboratories | Latching piercing pin for use with fluid vials of varying sizes |
| WO1995001133A1 (en)* | 1993-06-30 | 1995-01-12 | Baxter International Inc. | Vial adapter |
| JP3199524B2 (en) | 1993-08-06 | 2001-08-20 | 日本メジフィジックス株式会社 | Luer needle unit and syringe |
| US5527307A (en)* | 1994-04-01 | 1996-06-18 | Minimed Inc. | Implantable medication infusion pump with discharge side port |
| FR2718645B1 (en)* | 1994-04-15 | 1996-07-12 | Nycomed Lab Sa | Rapid exchange dilation catheter. |
| ATE278218T1 (en)* | 1994-05-26 | 2004-10-15 | Commw Of Australia | SECURE COMPUTER ARCHITECTURE |
| US5881287A (en)* | 1994-08-12 | 1999-03-09 | Mast; Michael B. | Method and apparatus for copy protection of images in a computer system |
| AU697045B2 (en) | 1995-01-16 | 1998-09-24 | Baxter International Inc. | Self-supporting sheet-like material of cross-linked fibrin for preventing post- operative adhesions |
| US5647845A (en)* | 1995-02-01 | 1997-07-15 | Habley Medical Technology Corporation | Generic intravenous infusion system |
| NZ304285A (en)* | 1995-03-14 | 1998-12-23 | Siemens Ag | Ultrasonic atomizer device with a removable precision dosing unit |
| US5779675A (en)* | 1995-08-25 | 1998-07-14 | Medrad, Inc. | Front load pressure jacket system with syringe holder |
| US5810792A (en)* | 1996-04-03 | 1998-09-22 | Icu Medical, Inc. | Locking blunt cannula |
| US5897526A (en) | 1996-06-26 | 1999-04-27 | Vaillancourt; Vincent L. | Closed system medication administering system |
| US5825879A (en)* | 1996-09-30 | 1998-10-20 | Intel Corporation | System and method for copy-protecting distributed video content |
| US5817082A (en)* | 1996-11-08 | 1998-10-06 | Bracco Diagnostics Inc. | Medicament container closure with integral spike access means |
| US5895383A (en)* | 1996-11-08 | 1999-04-20 | Bracco Diagnostics Inc. | Medicament container closure with recessed integral spike access means |
| DE19717107B4 (en)† | 1997-04-23 | 2005-06-23 | Disetronic Licensing Ag | System of container and drive device for a piston, which is held in the container containing a drug fluid |
| JP2001513406A (en)† | 1997-08-22 | 2001-09-04 | コエール ラボラトリーズ,インコーポレイテッド | Angiographic injection fluid and preload injection adapter |
| DE19746595C2 (en)* | 1997-10-22 | 2001-07-12 | Bosch Gmbh Robert | Drive unit with an electric motor |
| US6090082A (en)* | 1998-02-23 | 2000-07-18 | Becton, Dickinson And Company | Vial retainer interface to a medication delivery pen |
| US5993425A (en) | 1998-04-15 | 1999-11-30 | Science Incorporated | Fluid dispenser with reservoir fill assembly |
| US6475183B1 (en) | 1998-06-03 | 2002-11-05 | Baxter International Inc. | Direct dual filling device for sealing agents |
| US5993423A (en)* | 1998-08-18 | 1999-11-30 | Choi; Soo Bong | Portable automatic syringe device and injection needle unit thereof |
| EP0980687B1 (en)† | 1998-08-20 | 2004-03-03 | Sooil Development Co., Ltd. | Portable automatic syringe device |
| US20020173748A1 (en)* | 1998-10-29 | 2002-11-21 | Mcconnell Susan | Reservoir connector |
| US6817990B2 (en)* | 1998-10-29 | 2004-11-16 | Medtronic Minimed, Inc. | Fluid reservoir piston |
| CA2345439C (en)* | 1998-10-29 | 2005-08-09 | Minimed, Inc. | Compact pump drive system |
| US6260890B1 (en)* | 1999-08-12 | 2001-07-17 | Breg, Inc. | Tubing connector |
| AU2002306833A1 (en) | 2001-03-23 | 2002-10-08 | Sterling Medivations, Inc. | Adapter for medication cartridges |
- 1999
- 1999-10-28CACA002345439Apatent/CA2345439C/ennot_activeExpired - Fee Related
- 1999-10-28DKDK05019057.8Tpatent/DK1716884T3/enactive
- 1999-10-28WOPCT/US1999/025414patent/WO2000025844A1/enactiveIP Right Grant
- 1999-10-28ATAT05026578Tpatent/ATE498422T1/ennot_activeIP Right Cessation
- 1999-10-28DKDK05026578.4Tpatent/DK1642615T3/enactive
- 1999-10-28ATAT99971335Tpatent/ATE289523T1/ennot_activeIP Right Cessation
- 1999-10-28DEDE69923858Tpatent/DE69923858T2/ennot_activeExpired - Lifetime
- 1999-10-28DEDE69928827Tpatent/DE69928827T2/ennot_activeExpired - Lifetime
- 1999-10-28CACA2669175Apatent/CA2669175C/ennot_activeExpired - Fee Related
- 1999-10-28AUAU13305/00Apatent/AU1330500A/ennot_activeAbandoned
- 1999-10-28JPJP2000579288Apatent/JP4267206B2/ennot_activeExpired - Lifetime
- 1999-10-28AUAU14567/00Apatent/AU1456700A/ennot_activeAbandoned
- 1999-10-28JPJP2000579280Apatent/JP3546015B2/ennot_activeExpired - Fee Related
- 1999-10-28USUS09/428,411patent/US6362591B1/ennot_activeExpired - Lifetime
- 1999-10-28USUS09/428,818patent/US6585695B1/ennot_activeExpired - Lifetime
- 1999-10-28EPEP10157146.1Apatent/EP2204203A3/ennot_activeWithdrawn
- 1999-10-28CACA2832936Apatent/CA2832936C/ennot_activeExpired - Fee Related
- 1999-10-28EPEP05019057.8Apatent/EP1716884B1/ennot_activeExpired - Lifetime
- 1999-10-28ATAT99956770Tpatent/ATE311925T1/ennot_activeIP Right Cessation
- 1999-10-28WOPCT/US1999/025413patent/WO2000025852A1/enactiveIP Right Grant
- 1999-10-28CACA002533850Apatent/CA2533850C/ennot_activeExpired - Fee Related
- 1999-10-28EPEP99971335Apatent/EP1124600B1/ennot_activeExpired - Lifetime
- 1999-10-28DEDE69943207Tpatent/DE69943207D1/ennot_activeExpired - Lifetime
- 1999-10-28EPEP10157144.6Apatent/EP2223713A3/ennot_activeWithdrawn
- 1999-10-28CACA002346525Apatent/CA2346525C/ennot_activeExpired - Lifetime
- 1999-10-28EPEP99956770Apatent/EP1124608B1/ennot_activeExpired - Lifetime
- 1999-10-28EPEP05026578.4Apatent/EP1642615B2/ennot_activeExpired - Lifetime
- 1999-10-28DKDK99956770Tpatent/DK1124608T3/enactive
- 2001
- 2001-12-13USUS10/017,882patent/US6555986B2/ennot_activeExpired - Lifetime
- 2002
- 2002-12-23USUS10/328,393patent/US7658734B2/ennot_activeExpired - Lifetime
- 2003
- 2003-06-26USUS10/607,340patent/US20040003493A1/ennot_activeAbandoned
- 2004
- 2004-08-20USUS10/923,133patent/US7628782B2/ennot_activeExpired - Fee Related
- 2006
- 2006-09-05JPJP2006240860Apatent/JP4489063B2/ennot_activeExpired - Lifetime
- 2009
- 2009-01-28USUS12/361,203patent/US7981105B2/ennot_activeExpired - Fee Related
- 2009-07-17JPJP2009168463Apatent/JP4759630B2/ennot_activeExpired - Lifetime
- 2009-11-06USUS12/614,229patent/US7998131B2/ennot_activeExpired - Fee Related
- 2009-12-04USUS12/631,377patent/US8257345B2/ennot_activeExpired - Fee Related
- 2010
- 2010-01-25USUS12/693,232patent/US7988683B2/ennot_activeExpired - Fee Related
- 2011
- 2011-06-08USUS13/155,641patent/US8303572B2/ennot_activeExpired - Fee Related
- 2012
- 2012-09-18USUS13/622,183patent/US8500716B2/ennot_activeExpired - Fee Related
- 2013
- 2013-07-25USUS13/950,832patent/US20130310807A1/ennot_activeAbandoned
- 2014
- 2014-09-17USUS14/489,163patent/US9579452B2/ennot_activeExpired - Fee Related
Patent Citations (32)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| DE2240694A1 (en) | 1971-08-20 | 1973-03-01 | Rene Derouineau | SYRINGE FOR INTRAVENOESIS OR INTRAMUSCULAR INJECTION |
| US4444546A (en) | 1980-09-19 | 1984-04-24 | Oximetrix, Inc. | Occlusion detection apparatus and method |
| US4468221A (en) | 1981-04-10 | 1984-08-28 | Parker-Hannifin Corporation | Medication infusion pump |
| US4601491A (en) | 1983-10-19 | 1986-07-22 | Vetco Offshore, Inc. | Pipe connector |
| US4749109A (en) | 1983-11-15 | 1988-06-07 | Kamen Dean L | Volumetric pump with replaceable reservoir assembly |
| US4562751A (en) | 1984-01-06 | 1986-01-07 | Nason Clyde K | Solenoid drive apparatus for an external infusion pump |
| US4678408A (en) | 1984-01-06 | 1987-07-07 | Pacesetter Infusion, Ltd. | Solenoid drive apparatus for an external infusion pump |
| US4685903A (en) | 1984-01-06 | 1987-08-11 | Pacesetter Infusion, Ltd. | External infusion pump apparatus |
| US4619646A (en) | 1984-01-25 | 1986-10-28 | Fernandez Tresguerres Hernande | Device for the delivery-dosing of injectable products |
| US4744790A (en) | 1986-08-19 | 1988-05-17 | The West Company | Fast action cartridge syringe holder |
| US5121019A (en) | 1986-08-26 | 1992-06-09 | Josef Pradler | Rotary to linear drive unit |
| US4952205A (en) | 1987-04-04 | 1990-08-28 | B. Braun Melsungen Ag | Pressure infusion device |
| US5584667A (en)* | 1988-05-17 | 1996-12-17 | Davis; David L. | Method of providing uniform flow from an infusion device |
| US5279556A (en)* | 1989-04-28 | 1994-01-18 | Sharp Kabushiki Kaisha | Peristaltic pump with rotary encoder |
| US5097122A (en) | 1990-04-16 | 1992-03-17 | Pacesetter Infusion, Ltd. | Medication infusion system having optical motion sensor to detect drive mechanism malfunction |
| US5080653A (en) | 1990-04-16 | 1992-01-14 | Pacesetter Infusion, Ltd. | Infusion pump with dual position syringe locator |
| US5554134A (en) | 1990-09-21 | 1996-09-10 | Novo Nordisk A/S | Cap for an ampoule of an injection unit |
| USD347894S (en) | 1990-09-21 | 1994-06-14 | Novo Nordisk A/S | Adaptor top for a pen syringe ampoule |
| US5599323A (en) | 1991-07-12 | 1997-02-04 | Novo Nordisk A/S | Syringe system |
| US5254096A (en) | 1992-09-23 | 1993-10-19 | Becton, Dickinson And Company | Syringe pump with graphical display or error conditions |
| US5292306A (en) | 1993-01-29 | 1994-03-08 | Abbott Laboratories | Method of detecting occlusions in a solution pumping system |
| US5389078A (en) | 1993-10-06 | 1995-02-14 | Sims Deltec, Inc. | Programmable infusion pump for administering medication to patients |
| US5722545A (en) | 1993-12-10 | 1998-03-03 | Dental-Kosmetik Gmbh | Container with twist-on-off closure cap |
| US5522799A (en)* | 1993-12-17 | 1996-06-04 | Sharp Kabushiki Kaisha | Fluid infusion pump capable of detecting erroneous tube displacement |
| US5549574A (en) | 1994-08-30 | 1996-08-27 | Eli Lilly And Company | Cartridge assembly for use in a pen-type medicament injector |
| US5505709A (en) | 1994-09-15 | 1996-04-09 | Minimed, Inc., A Delaware Corporation | Mated infusion pump and syringe |
| US5545152A (en) | 1994-10-28 | 1996-08-13 | Minimed Inc. | Quick-connect coupling for a medication infusion system |
| US5637095A (en) | 1995-01-13 | 1997-06-10 | Minimed Inc. | Medication infusion pump with flexible drive plunger |
| US5647853A (en) | 1995-03-03 | 1997-07-15 | Minimed Inc. | Rapid response occlusion detector for a medication infusion pump |
| USD380262S (en) | 1995-06-26 | 1997-06-24 | Minimed Inc. | Quick disconnect coupling |
| WO1998000157A1 (en) | 1996-07-03 | 1998-01-08 | Alza Corporation | Aqueous formulations of peptides |
| US5947935A (en) | 1996-11-12 | 1999-09-07 | Medrad, Inc. | Syringes, syringe plungers and injector systems |
Non-Patent Citations (1)
| Title |
|---|
| PCT Application PCT/US99/25414, Search Report mailed Feb. 2, 2000. |
Cited By (470)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US7892206B2 (en)* | 1998-10-29 | 2011-02-22 | Medtronic Minimed, Inc. | Method and apparatus for detecting errors, fluid pressure, and occlusions in an ambulatory infusion pump |
| US20110119033A1 (en)* | 1998-10-29 | 2011-05-19 | Medtronic Minimed, Inc. | Method and Apparatus for Detecting Errors, Fluid Pressure, and Occlusions in an Ambulatory Infusion Pump |
| US8715237B2 (en)* | 1998-10-29 | 2014-05-06 | Medtronic Minimed, Inc. | Method and apparatus for detecting errors, fluid pressure, and occlusions in an ambulatory infusion pump |
| US20070149926A1 (en)* | 1998-10-29 | 2007-06-28 | Medtronic Minimed, Inc. | Method and Apparatus for Detecting Errors, Fluid Pressure, and Occlusions in an Ambulatory Infusion Pump |
| US6656148B2 (en)* | 1999-06-18 | 2003-12-02 | Animas Corporation | Infusion pump with a sealed drive mechanism and improved method of occlusion detection |
| US7063684B2 (en)* | 1999-10-28 | 2006-06-20 | Medtronic Minimed, Inc. | Drive system seal |
| US20030167039A1 (en)* | 1999-10-28 | 2003-09-04 | Medtronic Minimed, Inc. | Drive system seal |
| US20030205587A1 (en)* | 2000-08-16 | 2003-11-06 | Tribe Robert James | Syringe pumps |
| US7635349B2 (en)* | 2000-08-16 | 2009-12-22 | Smiths Group Plc | Syringe pumps |
| US8034026B2 (en) | 2001-05-18 | 2011-10-11 | Deka Products Limited Partnership | Infusion pump assembly |
| US9173996B2 (en) | 2001-05-18 | 2015-11-03 | Deka Products Limited Partnership | Infusion set for a fluid pump |
| US9446188B2 (en)* | 2001-05-18 | 2016-09-20 | Deka Products Limited Partnership | Infusion pump assembly |
| US20140135695A1 (en)* | 2001-05-18 | 2014-05-15 | Deka Products Limited Partnership | Infusion pump assembly |
| US8775196B2 (en) | 2002-01-29 | 2014-07-08 | Baxter International Inc. | System and method for notification and escalation of medical data |
| US10556062B2 (en) | 2002-01-29 | 2020-02-11 | Baxter International Inc. | Electronic medication order transfer and processing methods and apparatus |
| US10173008B2 (en) | 2002-01-29 | 2019-01-08 | Baxter International Inc. | System and method for communicating with a dialysis machine through a network |
| US8234128B2 (en) | 2002-04-30 | 2012-07-31 | Baxter International, Inc. | System and method for verifying medical device operational parameters |
| US7530968B2 (en) | 2003-04-23 | 2009-05-12 | Valeritas, Inc. | Hydraulically actuated pump for long duration medicament administration |
| US8070726B2 (en) | 2003-04-23 | 2011-12-06 | Valeritas, Inc. | Hydraulically actuated pump for long duration medicament administration |
| US9125983B2 (en) | 2003-04-23 | 2015-09-08 | Valeritas, Inc. | Hydraulically actuated pump for fluid administration |
| US9072828B2 (en) | 2003-04-23 | 2015-07-07 | Valeritas, Inc. | Hydraulically actuated pump for long duration medicament administration |
| US9511187B2 (en) | 2003-04-23 | 2016-12-06 | Valeritas, Inc. | Hydraulically actuated pump for fluid administration |
| US11642456B2 (en) | 2003-04-23 | 2023-05-09 | Mannkind Corporation | Hydraulically actuated pump for fluid administration |
| US10525194B2 (en) | 2003-04-23 | 2020-01-07 | Valeritas, Inc. | Hydraulically actuated pump for fluid administration |
| US20080260540A1 (en)* | 2003-12-08 | 2008-10-23 | Koehl Robert M | Pump controller system and method |
| US10289129B2 (en) | 2003-12-08 | 2019-05-14 | Pentair Water Pool And Spa, Inc. | Pump controller system and method |
| US9371829B2 (en) | 2003-12-08 | 2016-06-21 | Pentair Water Pool And Spa, Inc. | Pump controller system and method |
| US9399992B2 (en) | 2003-12-08 | 2016-07-26 | Pentair Water Pool And Spa, Inc. | Pump controller system and method |
| US10409299B2 (en) | 2003-12-08 | 2019-09-10 | Pentair Water Pool And Spa, Inc. | Pump controller system and method |
| US20050123408A1 (en)* | 2003-12-08 | 2005-06-09 | Koehl Robert M. | Pump control system and method |
| US20100141194A1 (en)* | 2003-12-08 | 2010-06-10 | Koehl Robert M | Pump Controller System and Method |
| US8540493B2 (en) | 2003-12-08 | 2013-09-24 | Sta-Rite Industries, Llc | Pump control system and method |
| US8444394B2 (en) | 2003-12-08 | 2013-05-21 | Sta-Rite Industries, Llc | Pump controller system and method |
| US10416690B2 (en) | 2003-12-08 | 2019-09-17 | Pentair Water Pool And Spa, Inc. | Pump controller system and method |
| US10241524B2 (en) | 2003-12-08 | 2019-03-26 | Pentair Water Pool And Spa, Inc. | Pump controller system and method |
| US20110181431A1 (en)* | 2003-12-08 | 2011-07-28 | Koehl Robert M | Pump Controller System and Method |
| US7821215B2 (en)* | 2003-12-08 | 2010-10-26 | Sta-Rite Industries, Llc | Pump controller system and method |
| US10642287B2 (en) | 2003-12-08 | 2020-05-05 | Pentair Water Pool And Spa, Inc. | Pump controller system and method |
| US20080131286A1 (en)* | 2003-12-08 | 2008-06-05 | Koehl Robert M | Pump controller system and method |
| US9328727B2 (en) | 2003-12-08 | 2016-05-03 | Pentair Water Pool And Spa, Inc. | Pump controller system and method |
| US11583628B2 (en) | 2004-05-27 | 2023-02-21 | Baxter International Inc. | Medical fluid therapy system having multi-state alarm feature |
| US10518030B2 (en) | 2004-05-27 | 2019-12-31 | Baxter International Inc. | Medical fluid therapy system having multi-state alarm feature |
| US8961461B2 (en) | 2004-05-27 | 2015-02-24 | Baxter International Inc. | Multi-state alarm system for a medical pump |
| US9925334B2 (en) | 2004-05-27 | 2018-03-27 | Baxter International Inc. | Multi-state alarm system for a medical pump |
| US9089636B2 (en) | 2004-07-02 | 2015-07-28 | Valeritas, Inc. | Methods and devices for delivering GLP-1 and uses thereof |
| US10947981B2 (en) | 2004-08-26 | 2021-03-16 | Pentair Water Pool And Spa, Inc. | Variable speed pumping system and method |
| US10871163B2 (en) | 2004-08-26 | 2020-12-22 | Pentair Water Pool And Spa, Inc. | Pumping system and method having an independent controller |
| US10415569B2 (en) | 2004-08-26 | 2019-09-17 | Pentair Water Pool And Spa, Inc. | Flow control |
| US20110091329A1 (en)* | 2004-08-26 | 2011-04-21 | Stiles Jr Robert W | Pumping System with Two Way Communication |
| US9605680B2 (en) | 2004-08-26 | 2017-03-28 | Pentair Water Pool And Spa, Inc. | Control algorithm of variable speed pumping system |
| US20110076156A1 (en)* | 2004-08-26 | 2011-03-31 | Stiles Jr Robert W | Flow Control |
| US10731655B2 (en) | 2004-08-26 | 2020-08-04 | Pentair Water Pool And Spa, Inc. | Priming protection |
| US10527042B2 (en) | 2004-08-26 | 2020-01-07 | Pentair Water Pool And Spa, Inc. | Speed control |
| US9551344B2 (en) | 2004-08-26 | 2017-01-24 | Pentair Water Pool And Spa, Inc. | Anti-entrapment and anti-dead head function |
| US9777733B2 (en) | 2004-08-26 | 2017-10-03 | Pentair Water Pool And Spa, Inc. | Flow control |
| US20110052416A1 (en)* | 2004-08-26 | 2011-03-03 | Robert Stiles | Variable Speed Pumping System and Method |
| US11391281B2 (en) | 2004-08-26 | 2022-07-19 | Pentair Water Pool And Spa, Inc. | Priming protection |
| US20100254825A1 (en)* | 2004-08-26 | 2010-10-07 | Stiles Jr Robert W | Pumping System with Power Optimization |
| US10871001B2 (en) | 2004-08-26 | 2020-12-22 | Pentair Water Pool And Spa, Inc. | Filter loading |
| US8801389B2 (en) | 2004-08-26 | 2014-08-12 | Pentair Water Pool And Spa, Inc. | Flow control |
| US8840376B2 (en) | 2004-08-26 | 2014-09-23 | Pentair Water Pool And Spa, Inc. | Pumping system with power optimization |
| US10480516B2 (en) | 2004-08-26 | 2019-11-19 | Pentair Water Pool And Spa, Inc. | Anti-entrapment and anti-deadhead function |
| US10240606B2 (en) | 2004-08-26 | 2019-03-26 | Pentair Water Pool And Spa, Inc. | Pumping system with two way communication |
| US10240604B2 (en) | 2004-08-26 | 2019-03-26 | Pentair Water Pool And Spa, Inc. | Pumping system with housing and user interface |
| US10502203B2 (en) | 2004-08-26 | 2019-12-10 | Pentair Water Pool And Spa, Inc. | Speed control |
| US8602745B2 (en)* | 2004-08-26 | 2013-12-10 | Pentair Water Pool And Spa, Inc. | Anti-entrapment and anti-dead head function |
| US11073155B2 (en) | 2004-08-26 | 2021-07-27 | Pentair Water Pool And Spa, Inc. | Pumping system with power optimization |
| US9404500B2 (en) | 2004-08-26 | 2016-08-02 | Pentair Water Pool And Spa, Inc. | Control algorithm of variable speed pumping system |
| US8573952B2 (en) | 2004-08-26 | 2013-11-05 | Pentair Water Pool And Spa, Inc. | Priming protection |
| US9051930B2 (en) | 2004-08-26 | 2015-06-09 | Pentair Water Pool And Spa, Inc. | Speed control |
| US8465262B2 (en) | 2004-08-26 | 2013-06-18 | Pentair Water Pool And Spa, Inc. | Speed control |
| US8480373B2 (en) | 2004-08-26 | 2013-07-09 | Pentair Water Pool And Spa, Inc. | Filter loading |
| US20070163929A1 (en)* | 2004-08-26 | 2007-07-19 | Pentair Water Pool And Spa, Inc. | Filter loading |
| US20070183902A1 (en)* | 2004-08-26 | 2007-08-09 | Pentair Water Pool And Spa, Inc. | Anti-entrapment and anti-dead head function |
| US8500413B2 (en) | 2004-08-26 | 2013-08-06 | Pentair Water Pool And Spa, Inc. | Pumping system with power optimization |
| US9932984B2 (en) | 2004-08-26 | 2018-04-03 | Pentair Water Pool And Spa, Inc. | Pumping system with power optimization |
| US9289165B2 (en) | 2005-02-07 | 2016-03-22 | Medtronic, Inc. | Ion imbalance detector |
| US20070093752A1 (en)* | 2005-09-19 | 2007-04-26 | Lifescan, Inc. | Infusion Pumps With A Position Detector |
| US7944366B2 (en) | 2005-09-19 | 2011-05-17 | Lifescan, Inc. | Malfunction detection with derivative calculation |
| US20070093753A1 (en)* | 2005-09-19 | 2007-04-26 | Lifescan, Inc. | Malfunction Detection Via Pressure Pulsation |
| US20070062251A1 (en)* | 2005-09-19 | 2007-03-22 | Lifescan, Inc. | Infusion Pump With Closed Loop Control and Algorithm |
| US20070066939A1 (en)* | 2005-09-19 | 2007-03-22 | Lifescan, Inc. | Electrokinetic Infusion Pump System |
| US20070066940A1 (en)* | 2005-09-19 | 2007-03-22 | Lifescan, Inc. | Systems and Methods for Detecting a Partition Position in an Infusion Pump |
| US20070062250A1 (en)* | 2005-09-19 | 2007-03-22 | Lifescan, Inc. | Malfunction Detection With Derivative Calculation |
| US8414522B2 (en) | 2006-02-09 | 2013-04-09 | Deka Products Limited Partnership | Fluid delivery systems and methods |
| US11406753B2 (en) | 2006-02-09 | 2022-08-09 | Deka Products Limited Partnership | Adhesive and peripheral systems and methods for medical devices |
| US11478623B2 (en) | 2006-02-09 | 2022-10-25 | Deka Products Limited Partnership | Infusion pump assembly |
| US12274857B2 (en) | 2006-02-09 | 2025-04-15 | Deka Products Limited Partnership | Method and system for shape-memory alloy wire control |
| US11339774B2 (en) | 2006-02-09 | 2022-05-24 | Deka Products Limited Partnership | Adhesive and peripheral systems and methods for medical devices |
| US11364335B2 (en) | 2006-02-09 | 2022-06-21 | Deka Products Limited Partnership | Apparatus, system and method for fluid delivery |
| US11534543B2 (en) | 2006-02-09 | 2022-12-27 | Deka Products Limited Partnership | Method for making patch-sized fluid delivery systems |
| US12233236B2 (en) | 2006-02-09 | 2025-02-25 | Deka Products Limited Partnership | Adhesive and peripheral systems and methods for medical devices |
| US11844926B2 (en) | 2006-02-09 | 2023-12-19 | Deka Products Limited Partnership | Adhesive and peripheral systems and methods for medical devices |
| US11786651B2 (en) | 2006-02-09 | 2023-10-17 | Deka Products Limited Partnership | Patch-sized fluid delivery system |
| US11491273B2 (en) | 2006-02-09 | 2022-11-08 | Deka Products Limited Partnership | Adhesive and peripheral systems and methods for medical devices |
| US11497846B2 (en) | 2006-02-09 | 2022-11-15 | Deka Products Limited Partnership | Patch-sized fluid delivery systems and methods |
| US12036387B2 (en) | 2006-02-09 | 2024-07-16 | Deka Products Limited Partnership | Device to determine volume of fluid dispensed |
| US8585377B2 (en) | 2006-02-09 | 2013-11-19 | Deka Products Limited Partnership | Pumping fluid delivery systems and methods using force application assembly |
| US11738139B2 (en) | 2006-02-09 | 2023-08-29 | Deka Products Limited Partnership | Patch-sized fluid delivery systems and methods |
| US11717609B2 (en) | 2006-02-09 | 2023-08-08 | Deka Products Limited Partnership | Adhesive and peripheral systems and methods for medical devices |
| US11391273B2 (en) | 2006-02-09 | 2022-07-19 | Deka Products Limited Partnership | Adhesive and peripheral systems and methods for medical devices |
| US11426512B2 (en) | 2006-02-09 | 2022-08-30 | Deka Products Limited Partnership | Apparatus, systems and methods for an infusion pump assembly |
| US11413391B2 (en) | 2006-02-09 | 2022-08-16 | Deka Products Limited Partnership | Patch-sized fluid delivery systems and methods |
| US11992650B2 (en) | 2006-02-09 | 2024-05-28 | Deka Products Limited Partnership | Adhesive and peripheral systems and methods for medical devices |
| US20070228071A1 (en)* | 2006-02-09 | 2007-10-04 | Dean Kamen | Fluid delivery systems and methods |
| US20070219496A1 (en)* | 2006-02-09 | 2007-09-20 | Dean Kamen | Pumping fluid delivery systems and methods using force application assembly |
| US20070219480A1 (en)* | 2006-02-09 | 2007-09-20 | Dean Kamen | Patch-sized fluid delivery systems and methods |
| US8545445B2 (en) | 2006-02-09 | 2013-10-01 | Deka Products Limited Partnership | Patch-sized fluid delivery systems and methods |
| US20070219597A1 (en)* | 2006-02-09 | 2007-09-20 | Dean Kamen | Adhesive and peripheral systems and methods for medical devices |
| US8113244B2 (en) | 2006-02-09 | 2012-02-14 | Deka Products Limited Partnership | Adhesive and peripheral systems and methods for medical devices |
| US11712513B2 (en) | 2006-02-09 | 2023-08-01 | Deka Products Limited Partnership | Adhesive and peripheral systems and methods for medical devices |
| US11890448B2 (en) | 2006-02-09 | 2024-02-06 | Deka Products Limited Partnership | Method and system for shape-memory alloy wire control |
| US12311143B2 (en) | 2006-02-09 | 2025-05-27 | Deka Products Limited Partnership | Adhesive and peripheral systems and methods for medical devices |
| US12151080B2 (en) | 2006-02-09 | 2024-11-26 | Deka Products Limited Partnership | Adhesive and peripheral systems and methods for medical devices |
| US12064590B2 (en) | 2006-02-09 | 2024-08-20 | Deka Products Limited Partnership | Patch-sized fluid delivery systems and methods |
| US11408414B2 (en) | 2006-02-09 | 2022-08-09 | Deka Products Limited Partnership | Adhesive and peripheral systems and methods for medical devices |
| US11904134B2 (en) | 2006-02-09 | 2024-02-20 | Deka Products Limited Partnership | Patch-sized fluid delivery systems and methods |
| US11690952B2 (en) | 2006-02-09 | 2023-07-04 | Deka Products Limited Partnership | Pumping fluid delivery systems and methods using force application assembly |
| US12070574B2 (en) | 2006-02-09 | 2024-08-27 | Deka Products Limited Partnership | Apparatus, systems and methods for an infusion pump assembly |
| US11559625B2 (en) | 2006-02-09 | 2023-01-24 | Deka Products Limited Partnership | Patch-sized fluid delivery systems and methods |
| US11395877B2 (en) | 2006-02-09 | 2022-07-26 | Deka Products Limited Partnership | Systems and methods for fluid delivery |
| US11964126B2 (en) | 2006-02-09 | 2024-04-23 | Deka Products Limited Partnership | Infusion pump assembly |
| US11617826B2 (en) | 2006-02-09 | 2023-04-04 | Deka Products Limited Partnership | Patch-sized fluid delivery systems and methods |
| US8361053B2 (en) | 2006-03-30 | 2013-01-29 | Valeritas, Inc. | Multi-cartridge fluid delivery device |
| US9687599B2 (en) | 2006-03-30 | 2017-06-27 | Valeritas, Inc. | Multi-cartridge fluid delivery device |
| US12246159B2 (en) | 2006-03-30 | 2025-03-11 | Mannkind Corporation | Multi-cartridge fluid delivery device |
| US10493199B2 (en) | 2006-03-30 | 2019-12-03 | Valeritas, Inc. | Multi-cartridge fluid delivery device |
| US8821443B2 (en) | 2006-03-30 | 2014-09-02 | Valeritas, Inc. | Multi-cartridge fluid delivery device |
| US7914499B2 (en) | 2006-03-30 | 2011-03-29 | Valeritas, Inc. | Multi-cartridge fluid delivery device |
| EP3213785A1 (en) | 2006-11-20 | 2017-09-06 | Medtronic MiniMed, Inc. | Method and apparatus for detecting occlusions in an ambulatory infusion pump |
| EP3705149A1 (en) | 2006-11-20 | 2020-09-09 | Medtronic MiniMed, Inc. | Apparatus for detecting occlusions in an ambulatory infusion pump |
| WO2008063429A2 (en) | 2006-11-20 | 2008-05-29 | Medtronic Minimed, Inc. | Method and apparatus for detecting occlusions in an ambulatory infusion pump |
| US7654127B2 (en) | 2006-12-21 | 2010-02-02 | Lifescan, Inc. | Malfunction detection in infusion pumps |
| US20080154187A1 (en)* | 2006-12-21 | 2008-06-26 | Lifescan, Inc. | Malfunction detection in infusion pumps |
| US8496646B2 (en) | 2007-02-09 | 2013-07-30 | Deka Products Limited Partnership | Infusion pump assembly |
| US20080281278A1 (en)* | 2007-05-09 | 2008-11-13 | E-Z-Em, Inc. | Injector device, method, and computer program product for detecting a vacuum within a syringe |
| US9333293B2 (en)* | 2007-05-09 | 2016-05-10 | Acist Medical Systems, Inc. | Injector device, method, and computer program product for detecting a vacuum within a syringe |
| US8083503B2 (en) | 2007-09-27 | 2011-12-27 | Curlin Medical Inc. | Peristaltic pump assembly and regulator therefor |
| US20090087325A1 (en)* | 2007-09-27 | 2009-04-02 | Voltenburg Jr Robert R | Peristaltic pump assembly and regulator therefor |
| US8062008B2 (en) | 2007-09-27 | 2011-11-22 | Curlin Medical Inc. | Peristaltic pump and removable cassette therefor |
| US7934912B2 (en) | 2007-09-27 | 2011-05-03 | Curlin Medical Inc | Peristaltic pump assembly with cassette and mounting pin arrangement |
| US20090087327A1 (en)* | 2007-09-27 | 2009-04-02 | Voltenburg Jr Robert R | Peristaltic pump and removable cassette therefor |
| US20090087326A1 (en)* | 2007-09-27 | 2009-04-02 | Voltenburg Jr Robert R | Peristaltic pump assembly |
| US11504481B2 (en) | 2007-10-02 | 2022-11-22 | West Pharma. Services IL, Ltd. | Anti-rotation feature for infusion pump cartridge |
| US11590291B2 (en) | 2007-10-02 | 2023-02-28 | West Pharma. Services IL, Ltd. | External drug pump |
| US20090157003A1 (en)* | 2007-12-14 | 2009-06-18 | Jones Daniel W | Method And Apparatus For Occlusion Prevention And Remediation |
| US10635784B2 (en) | 2007-12-18 | 2020-04-28 | Icu Medical, Inc. | User interface improvements for medical devices |
| US12415031B2 (en) | 2007-12-31 | 2025-09-16 | Deka Products Limited Partnership | Wearable pump assembly |
| US8414563B2 (en) | 2007-12-31 | 2013-04-09 | Deka Products Limited Partnership | Pump assembly with switch |
| US9526830B2 (en) | 2007-12-31 | 2016-12-27 | Deka Products Limited Partnership | Wearable pump assembly |
| US8491570B2 (en) | 2007-12-31 | 2013-07-23 | Deka Products Limited Partnership | Infusion pump assembly |
| US11894609B2 (en) | 2007-12-31 | 2024-02-06 | Deka Products Limited Partnership | Split ring resonator antenna adapted for use in wirelessly controlled medical device |
| US12128006B2 (en) | 2007-12-31 | 2024-10-29 | Deka Products Limited Partnership | Apparatus, system and method for fluid delivery |
| US11404776B2 (en) | 2007-12-31 | 2022-08-02 | Deka Products Limited Partnership | Split ring resonator antenna adapted for use in wirelessly controlled medical device |
| US12121497B2 (en) | 2007-12-31 | 2024-10-22 | Deka Products Limited Partnership | Method for fluid delivery |
| US11642283B2 (en) | 2007-12-31 | 2023-05-09 | Deka Products Limited Partnership | Method for fluid delivery |
| US11497686B2 (en) | 2007-12-31 | 2022-11-15 | Deka Products Limited Partnership | Apparatus, system and method for fluid delivery |
| US11701300B2 (en) | 2007-12-31 | 2023-07-18 | Deka Products Limited Partnership | Method for fluid delivery |
| US11723841B2 (en) | 2007-12-31 | 2023-08-15 | Deka Products Limited Partnership | Apparatus, system and method for fluid delivery |
| US11534542B2 (en) | 2007-12-31 | 2022-12-27 | Deka Products Limited Partnership | Apparatus, system and method for fluid delivery |
| US10646634B2 (en) | 2008-07-09 | 2020-05-12 | Baxter International Inc. | Dialysis system and disposable set |
| US10061899B2 (en) | 2008-07-09 | 2018-08-28 | Baxter International Inc. | Home therapy machine |
| US11918721B2 (en) | 2008-07-09 | 2024-03-05 | Baxter International Inc. | Dialysis system having adaptive prescription management |
| US10224117B2 (en) | 2008-07-09 | 2019-03-05 | Baxter International Inc. | Home therapy machine allowing patient device program selection |
| US11311658B2 (en) | 2008-07-09 | 2022-04-26 | Baxter International Inc. | Dialysis system having adaptive prescription generation |
| US10016554B2 (en) | 2008-07-09 | 2018-07-10 | Baxter International Inc. | Dialysis system including wireless patient data |
| US10095840B2 (en) | 2008-07-09 | 2018-10-09 | Baxter International Inc. | System and method for performing renal therapy at a home or dwelling of a patient |
| US10272190B2 (en) | 2008-07-09 | 2019-04-30 | Baxter International Inc. | Renal therapy system including a blood pressure monitor |
| US10068061B2 (en) | 2008-07-09 | 2018-09-04 | Baxter International Inc. | Home therapy entry, modification, and reporting system |
| US11167086B2 (en) | 2008-09-15 | 2021-11-09 | West Pharma. Services IL, Ltd. | Stabilized pen injector |
| US12097357B2 (en) | 2008-09-15 | 2024-09-24 | West Pharma. Services IL, Ltd. | Stabilized pen injector |
| US9726184B2 (en) | 2008-10-06 | 2017-08-08 | Pentair Water Pool And Spa, Inc. | Safety vacuum release system |
| US8602743B2 (en) | 2008-10-06 | 2013-12-10 | Pentair Water Pool And Spa, Inc. | Method of operating a safety vacuum release system |
| US10724263B2 (en) | 2008-10-06 | 2020-07-28 | Pentair Water Pool And Spa, Inc. | Safety vacuum release system |
| US8262616B2 (en) | 2008-10-10 | 2012-09-11 | Deka Products Limited Partnership | Infusion pump assembly |
| US8223028B2 (en) | 2008-10-10 | 2012-07-17 | Deka Products Limited Partnership | Occlusion detection system and method |
| US8708376B2 (en) | 2008-10-10 | 2014-04-29 | Deka Products Limited Partnership | Medium connector |
| US8066672B2 (en) | 2008-10-10 | 2011-11-29 | Deka Products Limited Partnership | Infusion pump assembly with a backup power supply |
| US8016789B2 (en) | 2008-10-10 | 2011-09-13 | Deka Products Limited Partnership | Pump assembly with a removable cover assembly |
| US12186531B2 (en) | 2008-10-10 | 2025-01-07 | Deka Products Limited Partnership | Infusion pump assembly |
| US8267892B2 (en) | 2008-10-10 | 2012-09-18 | Deka Products Limited Partnership | Multi-language / multi-processor infusion pump assembly |
| US9180245B2 (en) | 2008-10-10 | 2015-11-10 | Deka Products Limited Partnership | System and method for administering an infusible fluid |
| US12370327B2 (en) | 2008-10-10 | 2025-07-29 | Deka Products Limited Partnership | Infusion pump methods, systems and apparatus |
| US10347374B2 (en) | 2008-10-13 | 2019-07-09 | Baxter Corporation Englewood | Medication preparation system |
| US10314968B2 (en)* | 2008-12-27 | 2019-06-11 | Sanofi-Aventis Deutschland Gmbh | Medical injection device with electric motor drive control |
| US20160271323A1 (en)* | 2008-12-27 | 2016-09-22 | Sanofi-Aventis Deutschland Gmbh | Medical Injection Device with Electric Motor Drive Control |
| US9297370B2 (en) | 2008-12-29 | 2016-03-29 | Sanofi-Aventis Deutschland Gmbh | Medical injection device with electric motor drive control |
| WO2010076275A1 (en)* | 2008-12-29 | 2010-07-08 | Sanofi-Aventis Deutschland Gmbh | Medical injection device with electric motor drive control |
| EP3216471A1 (en)* | 2008-12-29 | 2017-09-13 | Sanofi-Aventis Deutschland GmbH | Medical injection device with electric motor drive control |
| WO2010078073A1 (en) | 2008-12-30 | 2010-07-08 | Medtronic Minimed, Inc. | Reservoir compartment adapter for infusion device |
| EP2397170A2 (en) | 2008-12-30 | 2011-12-21 | Medtronic MiniMed, Inc. | Medical fluid administration device and color coding and detection system for glucose sensor set |
| WO2010096602A1 (en)* | 2009-02-20 | 2010-08-26 | Hospira, Inc. | Occlusion detection system |
| EP2229967A1 (en) | 2009-03-17 | 2010-09-22 | F. Hoffmann-La Roche AG | Cannula assembly and ambulatory infusion system with a pressure sensor made of stackes coplanar layers |
| US11493034B2 (en) | 2009-06-09 | 2022-11-08 | Pentair Flow Technologies, Llc | Method of controlling a pump and motor |
| US20100310382A1 (en)* | 2009-06-09 | 2010-12-09 | Melissa Drechsel Kidd | Method of Controlling a Pump and Motor |
| US9556874B2 (en) | 2009-06-09 | 2017-01-31 | Pentair Flow Technologies, Llc | Method of controlling a pump and motor |
| US10590926B2 (en) | 2009-06-09 | 2020-03-17 | Pentair Flow Technologies, Llc | Method of controlling a pump and motor |
| US8564233B2 (en) | 2009-06-09 | 2013-10-22 | Sta-Rite Industries, Llc | Safety system and method for pump and motor |
| US9712098B2 (en) | 2009-06-09 | 2017-07-18 | Pentair Flow Technologies, Llc | Safety system and method for pump and motor |
| US8970384B2 (en) | 2009-06-14 | 2015-03-03 | Roche Diagnostics Operations, Inc. | Devices and methods for malfunctions recognition in a therapeutic dispensing device |
| US12144964B2 (en) | 2009-07-30 | 2024-11-19 | Tandem Diabetes Care, Inc | Infusion pump system with disposable cartridge having pressure venting and pressure feedback |
| US8926561B2 (en) | 2009-07-30 | 2015-01-06 | Tandem Diabetes Care, Inc. | Infusion pump system with disposable cartridge having pressure venting and pressure feedback |
| US8287495B2 (en) | 2009-07-30 | 2012-10-16 | Tandem Diabetes Care, Inc. | Infusion pump system with disposable cartridge having pressure venting and pressure feedback |
| US11135362B2 (en) | 2009-07-30 | 2021-10-05 | Tandem Diabetes Care, Inc. | Infusion pump systems and methods |
| US9211377B2 (en) | 2009-07-30 | 2015-12-15 | Tandem Diabetes Care, Inc. | Infusion pump system with disposable cartridge having pressure venting and pressure feedback |
| US11285263B2 (en) | 2009-07-30 | 2022-03-29 | Tandem Diabetes Care, Inc. | Infusion pump systems and methods |
| US8298184B2 (en) | 2009-07-30 | 2012-10-30 | Tandem Diabetes Care, Inc. | Infusion pump system with disposable cartridge having pressure venting and pressure feedback |
| US12042627B2 (en) | 2009-07-30 | 2024-07-23 | Tandem Diabetes Care, Inc. | Infusion pump systems and methods |
| US8758323B2 (en) | 2009-07-30 | 2014-06-24 | Tandem Diabetes Care, Inc. | Infusion pump system with disposable cartridge having pressure venting and pressure feedback |
| US9943633B2 (en) | 2009-09-30 | 2018-04-17 | Medtronic Inc. | System and method to regulate ultrafiltration |
| CN102573952B (en)* | 2009-10-16 | 2014-11-19 | 特克法马许可公司 | Occlusion recognition in an administering apparatus |
| CH702075A1 (en)* | 2009-10-16 | 2011-04-29 | Tecpharma Licensing Ag | Occlusion in an administration unit. |
| WO2011044706A1 (en)* | 2009-10-16 | 2011-04-21 | Tecpharma Licensing Ag | Occlusion recognition in an administering apparatus |
| CN102573952A (en)* | 2009-10-16 | 2012-07-11 | 特克法马许可公司 | Occlusion recognition in an administering apparatus |
| US20120078184A1 (en)* | 2010-09-24 | 2012-03-29 | Smith Roger E | Infusion pumps |
| US9320849B2 (en) | 2010-09-24 | 2016-04-26 | Perqflo, Llc | Infusion pumps |
| US20120078185A1 (en)* | 2010-09-24 | 2012-03-29 | Smith Roger E | Infusion pumps |
| US10272196B2 (en) | 2010-09-24 | 2019-04-30 | Perqflo, Llc | Infusion pumps |
| US9498573B2 (en) | 2010-09-24 | 2016-11-22 | Perqflo, Llc | Infusion pumps |
| US9381300B2 (en)* | 2010-09-24 | 2016-07-05 | Perqflo, Llc | Infusion pumps |
| US9750875B2 (en)* | 2010-09-24 | 2017-09-05 | Perqflo, Llc | Infusion pumps |
| US11547792B2 (en) | 2010-09-24 | 2023-01-10 | Medtronic Minimed, Inc. | Infusion pumps |
| US12097353B2 (en) | 2010-11-20 | 2024-09-24 | Medtronic Minimed, Inc. | Infusion pumps |
| US10029045B2 (en) | 2010-11-20 | 2018-07-24 | Perqflo, Llc | Infusion pumps |
| US10967124B2 (en) | 2010-11-20 | 2021-04-06 | Medtronic Minimed, Inc. | Infusion pumps |
| US9568005B2 (en) | 2010-12-08 | 2017-02-14 | Pentair Water Pool And Spa, Inc. | Discharge vacuum relief valve for safety vacuum release system |
| US8628510B2 (en) | 2010-12-22 | 2014-01-14 | Medtronic Minimed, Inc. | Monitoring the operating health of a force sensor in a fluid infusion device |
| US9555190B2 (en) | 2010-12-22 | 2017-01-31 | Medtronic Minimed, Inc. | Fluid reservoir seating procedure for a fluid infusion device |
| US8690855B2 (en)* | 2010-12-22 | 2014-04-08 | Medtronic Minimed, Inc. | Fluid reservoir seating procedure for a fluid infusion device |
| US10071200B2 (en) | 2010-12-22 | 2018-09-11 | Medtronic Minimed, Inc. | Fluid reservoir seating procedure for a fluid infusion device |
| US9895490B2 (en) | 2010-12-22 | 2018-02-20 | Medtronic Minimed, Inc. | Occlusion detection for a fluid infusion device |
| US9017307B2 (en) | 2010-12-22 | 2015-04-28 | Medtronic Minimed, Inc. | Occlusion detection for a fluid infusion device |
| US9770553B2 (en) | 2010-12-22 | 2017-09-26 | Medtronic Minimed, Inc. | Monitoring the operating health of a force sensor in a fluid infusion device |
| US10478554B2 (en) | 2010-12-22 | 2019-11-19 | Medtronic Minimed, Inc. | Monitoring the operating health of a force sensor in a fluid infusion device |
| US9033951B2 (en) | 2010-12-22 | 2015-05-19 | Medtronic Minimed, Inc. | Occlusion detection for a fluid infusion device |
| US9050406B2 (en) | 2010-12-22 | 2015-06-09 | Medtronic Minimed, Inc. | Occlusion detection for a fluid infusion device |
| US9700661B2 (en) | 2011-04-29 | 2017-07-11 | Medtronic, Inc. | Chronic pH or electrolyte monitoring |
| US10207041B2 (en) | 2011-04-29 | 2019-02-19 | Medtronic, Inc. | Method and device to monitor patients with kidney disease |
| US9061099B2 (en) | 2011-04-29 | 2015-06-23 | Medtronic, Inc. | Cardiovascular monitoring for fluid removal processes |
| US10406268B2 (en) | 2011-04-29 | 2019-09-10 | Medtronic, Inc. | Blood fluid removal system performance monitoring |
| US9302036B2 (en) | 2011-04-29 | 2016-04-05 | Medtronic, Inc. | Blood fluid removal system performance monitoring |
| US9456755B2 (en) | 2011-04-29 | 2016-10-04 | Medtronic, Inc. | Method and device to monitor patients with kidney disease |
| US10967112B2 (en) | 2011-04-29 | 2021-04-06 | Medtronic, Inc. | Adaptive system for blood fluid removal |
| US8926542B2 (en) | 2011-04-29 | 2015-01-06 | Medtronic, Inc. | Monitoring fluid volume for patients with renal disease |
| US10064985B2 (en) | 2011-04-29 | 2018-09-04 | Medtronic, Inc. | Precision blood fluid removal therapy based on patient monitoring |
| US9642960B2 (en) | 2011-04-29 | 2017-05-09 | Medtronic, Inc. | Monitoring fluid volume for patients with renal disease |
| US11759557B2 (en) | 2011-04-29 | 2023-09-19 | Mozarc Medical Us Llc | Adaptive system for blood fluid removal |
| US10179198B2 (en) | 2011-04-29 | 2019-01-15 | Medtronic, Inc. | Electrolyte and pH monitoring for fluid removal processes |
| US9750862B2 (en) | 2011-04-29 | 2017-09-05 | Medtronic, Inc. | Adaptive system for blood fluid removal |
| US10293092B2 (en) | 2011-04-29 | 2019-05-21 | Medtronic, Inc. | Electrolyte and pH monitoring for fluid removal processes |
| US10506933B2 (en) | 2011-04-29 | 2019-12-17 | Medtronic, Inc. | Method and device to monitor patients with kidney disease |
| US10835656B2 (en) | 2011-04-29 | 2020-11-17 | Medtronic, Inc. | Method and device to monitor patients with kidney disease |
| US9968721B2 (en) | 2011-04-29 | 2018-05-15 | Medtronic, Inc. | Monitoring fluid volume for patients with renal disease |
| US9848778B2 (en) | 2011-04-29 | 2017-12-26 | Medtronic, Inc. | Method and device to monitor patients with kidney disease |
| US8951219B2 (en) | 2011-04-29 | 2015-02-10 | Medtronic, Inc. | Fluid volume monitoring for patients with renal disease |
| US10722636B2 (en) | 2011-08-02 | 2020-07-28 | Medtronic, Inc. | Hemodialysis system having a flow path with a controlled compliant volume |
| US10695481B2 (en) | 2011-08-02 | 2020-06-30 | Medtronic, Inc. | Hemodialysis system having a flow path with a controlled compliant volume |
| US10857277B2 (en) | 2011-08-16 | 2020-12-08 | Medtronic, Inc. | Modular hemodialysis system |
| US11599854B2 (en) | 2011-08-19 | 2023-03-07 | Icu Medical, Inc. | Systems and methods for a graphical interface including a graphical representation of medical data |
| US11972395B2 (en) | 2011-08-19 | 2024-04-30 | Icu Medical, Inc. | Systems and methods for a graphical interface including a graphical representation of medical data |
| US10430761B2 (en) | 2011-08-19 | 2019-10-01 | Icu Medical, Inc. | Systems and methods for a graphical interface including a graphical representation of medical data |
| US12346879B2 (en) | 2011-08-19 | 2025-07-01 | Icu Medical, Inc. | Systems and methods for a graphical interface including a graphical representation of medical data |
| US11004035B2 (en) | 2011-08-19 | 2021-05-11 | Icu Medical, Inc. | Systems and methods for a graphical interface including a graphical representation of medical data |
| US10883489B2 (en) | 2011-11-01 | 2021-01-05 | Pentair Water Pool And Spa, Inc. | Flow locking system and method |
| US10465676B2 (en) | 2011-11-01 | 2019-11-05 | Pentair Water Pool And Spa, Inc. | Flow locking system and method |
| US10022498B2 (en) | 2011-12-16 | 2018-07-17 | Icu Medical, Inc. | System for monitoring and delivering medication to a patient and method of using the same to minimize the risks associated with automated therapy |
| US11376361B2 (en) | 2011-12-16 | 2022-07-05 | Icu Medical, Inc. | System for monitoring and delivering medication to a patient and method of using the same to minimize the risks associated with automated therapy |
| US10288057B2 (en) | 2011-12-21 | 2019-05-14 | Deka Products Limited Partnership | Peristaltic pump |
| US12020798B2 (en) | 2011-12-21 | 2024-06-25 | Deka Products Limited Partnership | Peristaltic pump and related method |
| US11511038B2 (en) | 2011-12-21 | 2022-11-29 | Deka Products Limited Partnership | Apparatus for infusing fluid |
| US12098738B2 (en) | 2011-12-21 | 2024-09-24 | Deka Products Limited Partnership | System, method, and apparatus for clamping |
| US11373747B2 (en) | 2011-12-21 | 2022-06-28 | Deka Products Limited Partnership | Peristaltic pump |
| US9677555B2 (en) | 2011-12-21 | 2017-06-13 | Deka Products Limited Partnership | System, method, and apparatus for infusing fluid |
| US10202970B2 (en) | 2011-12-21 | 2019-02-12 | Deka Products Limited Partnership | System, method, and apparatus for infusing fluid |
| US9675756B2 (en) | 2011-12-21 | 2017-06-13 | Deka Products Limited Partnership | Apparatus for infusing fluid |
| US11024409B2 (en) | 2011-12-21 | 2021-06-01 | Deka Products Limited Partnership | Peristaltic pump |
| US10316834B2 (en) | 2011-12-21 | 2019-06-11 | Deka Products Limited Partnership | Peristaltic pump |
| US11348674B2 (en) | 2011-12-21 | 2022-05-31 | Deka Products Limited Partnership | Peristaltic pump |
| US10753353B2 (en) | 2011-12-21 | 2020-08-25 | Deka Products Limited Partnership | Peristaltic pump |
| US12002561B2 (en) | 2011-12-21 | 2024-06-04 | DEKA Research & Development Corp | System, method, and apparatus for infusing fluid |
| US11779703B2 (en) | 2011-12-21 | 2023-10-10 | Deka Products Limited Partnership | Apparatus for infusing fluid |
| US11295846B2 (en) | 2011-12-21 | 2022-04-05 | Deka Products Limited Partnership | System, method, and apparatus for infusing fluid |
| US10857293B2 (en) | 2011-12-21 | 2020-12-08 | Deka Products Limited Partnership | Apparatus for infusing fluid |
| US11756662B2 (en) | 2011-12-21 | 2023-09-12 | Deka Products Limited Partnership | Peristaltic pump |
| US10202971B2 (en) | 2011-12-21 | 2019-02-12 | Deka Products Limited Partnership | Peristaltic pump |
| US11705233B2 (en) | 2011-12-21 | 2023-07-18 | Deka Products Limited Partnership | Peristaltic pump |
| US12288604B2 (en) | 2011-12-21 | 2025-04-29 | Deka Products Limited Partnership | Peristaltic pump |
| US9713668B2 (en) | 2012-01-04 | 2017-07-25 | Medtronic, Inc. | Multi-staged filtration system for blood fluid removal |
| US10335545B2 (en) | 2012-01-31 | 2019-07-02 | West Pharma. Services IL, Ltd. | Time dependent drug delivery apparatus |
| US11524151B2 (en) | 2012-03-07 | 2022-12-13 | Deka Products Limited Partnership | Apparatus, system and method for fluid delivery |
| US10179204B2 (en) | 2012-03-26 | 2019-01-15 | West Pharma. Services IL, Ltd. | Motion-activated septum puncturing drug delivery device |
| US10159785B2 (en) | 2012-03-26 | 2018-12-25 | West Pharma. Services IL, Ltd. | Motion activated septum puncturing drug delivery device |
| US10668213B2 (en) | 2012-03-26 | 2020-06-02 | West Pharma. Services IL, Ltd. | Motion activated mechanisms for a drug delivery device |
| US9463280B2 (en) | 2012-03-26 | 2016-10-11 | Medimop Medical Projects Ltd. | Motion activated septum puncturing drug delivery device |
| US9878091B2 (en) | 2012-03-26 | 2018-01-30 | Medimop Medical Projects Ltd. | Motion activated septum puncturing drug delivery device |
| US11933650B2 (en) | 2012-03-30 | 2024-03-19 | Icu Medical, Inc. | Air detection system and method for detecting air in a pump of an infusion system |
| US10578474B2 (en) | 2012-03-30 | 2020-03-03 | Icu Medical, Inc. | Air detection system and method for detecting air in a pump of an infusion system |
| US10089443B2 (en) | 2012-05-15 | 2018-10-02 | Baxter International Inc. | Home medical device systems and methods for therapy prescription and tracking, servicing and inventory |
| US10258736B2 (en) | 2012-05-17 | 2019-04-16 | Tandem Diabetes Care, Inc. | Systems including vial adapter for fluid transfer |
| US11623042B2 (en) | 2012-07-31 | 2023-04-11 | Icu Medical, Inc. | Patient care system for critical medications |
| US12280239B2 (en) | 2012-07-31 | 2025-04-22 | Icu Medical, Inc. | Patient care system for critical medications |
| US10463788B2 (en) | 2012-07-31 | 2019-11-05 | Icu Medical, Inc. | Patient care system for critical medications |
| US11872375B2 (en) | 2012-09-05 | 2024-01-16 | E3D Agricultural Cooperative Association Ltd. | Electronic auto-injection device |
| EP2902051A4 (en)* | 2012-09-26 | 2016-05-25 | Terumo Corp | Syringe pump |
| US9885360B2 (en) | 2012-10-25 | 2018-02-06 | Pentair Flow Technologies, Llc | Battery backup sump pump systems and methods |
| US10646405B2 (en) | 2012-10-26 | 2020-05-12 | Baxter Corporation Englewood | Work station for medical dose preparation system |
| US10971257B2 (en) | 2012-10-26 | 2021-04-06 | Baxter Corporation Englewood | Image acquisition for medical dose preparation system |
| US10905816B2 (en) | 2012-12-10 | 2021-02-02 | Medtronic, Inc. | Sodium management system for hemodialysis |
| US12161788B2 (en) | 2013-01-09 | 2024-12-10 | Mozarc Medical Us Llc | Fluid circuits for sorbent cartridges with sensors |
| US10583236B2 (en) | 2013-01-09 | 2020-03-10 | Medtronic, Inc. | Recirculating dialysate fluid circuit for blood measurement |
| US11154648B2 (en) | 2013-01-09 | 2021-10-26 | Medtronic, Inc. | Fluid circuits for sorbent cartridge with sensors |
| US11565029B2 (en) | 2013-01-09 | 2023-01-31 | Medtronic, Inc. | Sorbent cartridge with electrodes |
| US9707328B2 (en) | 2013-01-09 | 2017-07-18 | Medtronic, Inc. | Sorbent cartridge to measure solute concentrations |
| US10881777B2 (en) | 2013-01-09 | 2021-01-05 | Medtronic, Inc. | Recirculating dialysate fluid circuit for blood measurement |
| US11857712B2 (en) | 2013-01-09 | 2024-01-02 | Mozarc Medical Us Llc | Recirculating dialysate fluid circuit for measurement of blood solute species |
| US9872949B2 (en) | 2013-02-01 | 2018-01-23 | Medtronic, Inc. | Systems and methods for multifunctional volumetric fluid control |
| US9526822B2 (en) | 2013-02-01 | 2016-12-27 | Medtronic, Inc. | Sodium and buffer source cartridges for use in a modular controlled compliant flow path |
| US10543052B2 (en) | 2013-02-01 | 2020-01-28 | Medtronic, Inc. | Portable dialysis cabinet |
| US10850016B2 (en) | 2013-02-01 | 2020-12-01 | Medtronic, Inc. | Modular fluid therapy system having jumpered flow paths and systems and methods for cleaning and disinfection |
| US10010663B2 (en) | 2013-02-01 | 2018-07-03 | Medtronic, Inc. | Fluid circuit for delivery of renal replacement therapies |
| US10532141B2 (en) | 2013-02-01 | 2020-01-14 | Medtronic, Inc. | Systems and methods for multifunctional volumetric fluid control |
| US11786645B2 (en) | 2013-02-01 | 2023-10-17 | Mozarc Medical Us Llc | Fluid circuit for delivery of renal replacement therapies |
| US10561776B2 (en) | 2013-02-01 | 2020-02-18 | Medtronic, Inc. | Fluid circuit for delivery of renal replacement therapies |
| US9827361B2 (en) | 2013-02-02 | 2017-11-28 | Medtronic, Inc. | pH buffer measurement system for hemodialysis systems |
| US9855379B2 (en) | 2013-02-02 | 2018-01-02 | Medtronic, Inc. | Sorbent cartridge configurations for improved dialysate regeneration |
| US9962486B2 (en) | 2013-03-14 | 2018-05-08 | Tandem Diabetes Care, Inc. | System and method for detecting occlusions in an infusion pump |
| US10398837B2 (en) | 2013-05-03 | 2019-09-03 | West Pharma. Services IL, Ltd. | Sensing a status of an infuser based on sensing motor control and power input |
| US9889256B2 (en) | 2013-05-03 | 2018-02-13 | Medimop Medical Projects Ltd. | Sensing a status of an infuser based on sensing motor control and power input |
| US10874793B2 (en) | 2013-05-24 | 2020-12-29 | Icu Medical, Inc. | Multi-sensor infusion system for detecting air or an occlusion in the infusion system |
| US12048831B2 (en) | 2013-05-24 | 2024-07-30 | Icu Medical, Inc. | Multi-sensor infusion system for detecting air or an occlusion in the infusion system |
| US11596737B2 (en) | 2013-05-29 | 2023-03-07 | Icu Medical, Inc. | Infusion system and method of use which prevents over-saturation of an analog-to-digital converter |
| US11433177B2 (en) | 2013-05-29 | 2022-09-06 | Icu Medical, Inc. | Infusion system which utilizes one or more sensors and additional information to make an air determination regarding the infusion system |
| US10166328B2 (en) | 2013-05-29 | 2019-01-01 | Icu Medical, Inc. | Infusion system which utilizes one or more sensors and additional information to make an air determination regarding the infusion system |
| US10596316B2 (en) | 2013-05-29 | 2020-03-24 | Icu Medical, Inc. | Infusion system and method of use which prevents over-saturation of an analog-to-digital converter |
| US12059551B2 (en) | 2013-05-29 | 2024-08-13 | Icu Medical, Inc. | Infusion system and method of use which prevents over-saturation of an analog-to-digital converter |
| US12012241B2 (en) | 2013-07-03 | 2024-06-18 | Deka Products Limited Partnership | Apparatus, system and method for fluid delivery |
| US11597541B2 (en) | 2013-07-03 | 2023-03-07 | Deka Products Limited Partnership | Apparatus, system and method for fluid delivery |
| US10881789B2 (en) | 2013-10-24 | 2021-01-05 | Trustees Of Boston University | Infusion system for preventing mischanneling of multiple medicaments |
| US11357911B2 (en) | 2013-10-24 | 2022-06-14 | Trustees Of Boston University | Infusion pump and system for preventing mischanneling of multiple medicaments |
| US11064894B2 (en) | 2013-11-04 | 2021-07-20 | Medtronic, Inc. | Method and device to manage fluid volumes in the body |
| US10076283B2 (en) | 2013-11-04 | 2018-09-18 | Medtronic, Inc. | Method and device to manage fluid volumes in the body |
| US11219880B2 (en) | 2013-11-26 | 2022-01-11 | Medtronic, Inc | System for precision recharging of sorbent materials using patient and session data |
| US10478545B2 (en) | 2013-11-26 | 2019-11-19 | Medtronic, Inc. | Parallel modules for in-line recharging of sorbents using alternate duty cycles |
| US10595775B2 (en) | 2013-11-27 | 2020-03-24 | Medtronic, Inc. | Precision dialysis monitoring and synchronization system |
| US11471099B2 (en) | 2013-11-27 | 2022-10-18 | Medtronic, Inc. | Precision dialysis monitoring and synchronization system |
| US11471100B2 (en) | 2013-11-27 | 2022-10-18 | Medtronic, Inc. | Precision dialysis monitoring and synchonization system |
| US10617349B2 (en) | 2013-11-27 | 2020-04-14 | Medtronic, Inc. | Precision dialysis monitoring and synchronization system |
| US12083310B2 (en) | 2014-02-28 | 2024-09-10 | Icu Medical, Inc. | Infusion system and method which utilizes dual wavelength optical air-in-line detection |
| US10342917B2 (en) | 2014-02-28 | 2019-07-09 | Icu Medical, Inc. | Infusion system and method which utilizes dual wavelength optical air-in-line detection |
| US11344673B2 (en) | 2014-05-29 | 2022-05-31 | Icu Medical, Inc. | Infusion system and pump with configurable closed loop delivery rate catch-up |
| US10926017B2 (en) | 2014-06-24 | 2021-02-23 | Medtronic, Inc. | Modular dialysate regeneration assembly |
| US11045790B2 (en) | 2014-06-24 | 2021-06-29 | Medtronic, Inc. | Stacked sorbent assembly |
| US11673118B2 (en) | 2014-06-24 | 2023-06-13 | Mozarc Medical Us Llc | Stacked sorbent assembly |
| US11367533B2 (en) | 2014-06-30 | 2022-06-21 | Baxter Corporation Englewood | Managed medical information exchange |
| US10668212B2 (en)* | 2014-08-26 | 2020-06-02 | Debiotech S.A. | Detection of an infusion anomaly |
| US20180214634A1 (en)* | 2014-08-26 | 2018-08-02 | Debiotech S.A. | Detection of an Infusion Anomaly |
| US11672903B2 (en) | 2014-09-18 | 2023-06-13 | Deka Products Limited Partnership | Apparatus and method for infusing fluid through a tube by appropriately heating the tube |
| US10265463B2 (en) | 2014-09-18 | 2019-04-23 | Deka Products Limited Partnership | Apparatus and method for infusing fluid through a tube by appropriately heating the tube |
| US12178992B2 (en) | 2014-09-30 | 2024-12-31 | Medtronic Minimed, Inc. | Different disposable assemblies for the same reusable assembly |
| US11107574B2 (en) | 2014-09-30 | 2021-08-31 | Baxter Corporation Englewood | Management of medication preparation with formulary management |
| US11575673B2 (en) | 2014-09-30 | 2023-02-07 | Baxter Corporation Englewood | Central user management in a distributed healthcare information management system |
| US10946137B2 (en) | 2014-09-30 | 2021-03-16 | Medtronic Minimed, Inc. | Hybrid ambulatory infusion pumps |
| US12070576B2 (en) | 2014-09-30 | 2024-08-27 | Medtronic Minimed, Inc. | Hybrid ambulatory infusion pumps |
| US10159786B2 (en) | 2014-09-30 | 2018-12-25 | Perqflo, Llc | Hybrid ambulatory infusion pumps |
| US12412644B2 (en) | 2014-10-24 | 2025-09-09 | Baxter Corporation Englewood | Automated exchange of healthcare information for fulfillment of medication doses |
| US10818387B2 (en) | 2014-12-05 | 2020-10-27 | Baxter Corporation Englewood | Dose preparation data analytics |
| US10098993B2 (en) | 2014-12-10 | 2018-10-16 | Medtronic, Inc. | Sensing and storage system for fluid balance |
| US9713665B2 (en) | 2014-12-10 | 2017-07-25 | Medtronic, Inc. | Degassing system for dialysis |
| US10195327B2 (en) | 2014-12-10 | 2019-02-05 | Medtronic, Inc. | Sensing and storage system for fluid balance |
| US10874787B2 (en) | 2014-12-10 | 2020-12-29 | Medtronic, Inc. | Degassing system for dialysis |
| US9895479B2 (en) | 2014-12-10 | 2018-02-20 | Medtronic, Inc. | Water management system for use in dialysis |
| US10420872B2 (en) | 2014-12-10 | 2019-09-24 | Medtronic, Inc. | Degassing system for dialysis |
| US11344668B2 (en) | 2014-12-19 | 2022-05-31 | Icu Medical, Inc. | Infusion system with concurrent TPN/insulin infusion |
| US11684712B2 (en) | 2015-02-18 | 2023-06-27 | Medtronic Minimed, Inc. | Ambulatory infusion pumps and reservoir assemblies for use with same |
| US12115337B2 (en) | 2015-03-02 | 2024-10-15 | Icu Medical, Inc. | Infusion system, device, and method having advanced infusion features |
| US10850024B2 (en) | 2015-03-02 | 2020-12-01 | Icu Medical, Inc. | Infusion system, device, and method having advanced infusion features |
| US11948112B2 (en) | 2015-03-03 | 2024-04-02 | Baxter Corporation Engelwood | Pharmacy workflow management with integrated alerts |
| US9795534B2 (en) | 2015-03-04 | 2017-10-24 | Medimop Medical Projects Ltd. | Compliant coupling assembly for cartridge coupling of a drug delivery device |
| US11253429B2 (en) | 2015-03-04 | 2022-02-22 | West Pharma. Services IL, Ltd. | Flexibly mounted cartridge alignment collar for drug delivery device |
| US10251813B2 (en) | 2015-03-04 | 2019-04-09 | West Pharma. Services IL, Ltd. | Flexibly mounted cartridge alignment collar for drug delivery device |
| US9744297B2 (en) | 2015-04-10 | 2017-08-29 | Medimop Medical Projects Ltd. | Needle cannula position as an input to operational control of an injection device |
| US10617819B2 (en) | 2015-04-10 | 2020-04-14 | West Pharma. Services IL, Ltd. | Needle cannula position as an input to operational control of an injection device |
| US11931552B2 (en) | 2015-06-04 | 2024-03-19 | West Pharma Services Il, Ltd. | Cartridge insertion for drug delivery device |
| US11495334B2 (en) | 2015-06-25 | 2022-11-08 | Gambro Lundia Ab | Medical device system and method having a distributed database |
| US11331463B2 (en) | 2015-07-08 | 2022-05-17 | Trustees Of Boston University | Infusion system and components thereof |
| US12201803B2 (en) | 2015-07-08 | 2025-01-21 | Trustees Of Boston University | Infusion system and components thereof |
| US11147916B2 (en) | 2015-08-20 | 2021-10-19 | Tandem Diabetes Care, Inc. | Drive mechanism for infusion pump |
| US12268839B2 (en)* | 2015-08-20 | 2025-04-08 | Tandem Diabetes Care, Inc | Drive mechanism for infusion pump |
| US20220001104A1 (en)* | 2015-08-20 | 2022-01-06 | Tandem Diabetes Care, Inc. | Drive mechanism for infusion pump |
| US10279107B2 (en) | 2015-08-20 | 2019-05-07 | Tandem Diabetes Care, Inc. | Drive mechanism for infusion pump |
| US11065381B2 (en) | 2015-10-05 | 2021-07-20 | E3D A.C.A.L. | Infusion pump device and method for use thereof |
| US12138429B2 (en) | 2015-10-09 | 2024-11-12 | West Pharma. Services IL, Ltd. | Angled syringe patch injector |
| US12208246B2 (en) | 2015-10-09 | 2025-01-28 | West Pharma. Services IL, Ltd. | Bent fluid path add on to a prefilled fluid reservoir |
| US11724034B2 (en) | 2015-10-09 | 2023-08-15 | West Pharma. Services, IL, Ltd. | Injector system |
| US11759573B2 (en) | 2015-10-09 | 2023-09-19 | West Pharma. Services, IL, Ltd. | Bent fluid path add on to a prefilled reservoir |
| US12036394B2 (en) | 2015-10-09 | 2024-07-16 | West Pharma. Services IL, Ltd. | Injector needle cap and/or liner remover |
| US11395868B2 (en) | 2015-11-06 | 2022-07-26 | Medtronic, Inc. | Dialysis prescription optimization for decreased arrhythmias |
| US12005237B2 (en) | 2016-01-21 | 2024-06-11 | West Pharma. Services IL, Ltd. | Medicament delivery device comprising a visual indicator |
| US12427261B2 (en) | 2016-01-21 | 2025-09-30 | West Pharma. Services IL, Ltd. | Medicament delivery device comprising a visual indicator |
| US11672904B2 (en) | 2016-01-21 | 2023-06-13 | West Pharma. Services IL, Ltd. | Needle insertion and retraction mechanism |
| US12397109B2 (en) | 2016-02-12 | 2025-08-26 | Medtronic Minimed, Inc. | Ambulatory infusion pumps and assemblies for use with same |
| US11672909B2 (en) | 2016-02-12 | 2023-06-13 | Medtronic Minimed, Inc. | Ambulatory infusion pumps and assemblies for use with same |
| US12329892B2 (en) | 2016-04-04 | 2025-06-17 | Mozarc Medical Us Llc | Dextrose concentration sensor for a peritoneal dialysis system |
| US12201811B2 (en) | 2016-05-13 | 2025-01-21 | Icu Medical, Inc. | Infusion pump system and method with common line auto flush |
| US11246985B2 (en) | 2016-05-13 | 2022-02-15 | Icu Medical, Inc. | Infusion pump system and method with common line auto flush |
| US11819673B2 (en) | 2016-06-02 | 2023-11-21 | West Pharma. Services, IL, Ltd. | Three position needle retraction |
| US11324888B2 (en) | 2016-06-10 | 2022-05-10 | Icu Medical, Inc. | Acoustic flow sensor for continuous medication flow measurements and feedback control of infusion |
| US12076531B2 (en) | 2016-06-10 | 2024-09-03 | Icu Medical, Inc. | Acoustic flow sensor for continuous medication flow measurements and feedback control of infusion |
| US11730892B2 (en) | 2016-08-01 | 2023-08-22 | West Pharma. Services IL, Ltd. | Partial door closure prevention spring |
| US12357767B2 (en) | 2016-08-01 | 2025-07-15 | West Pharma. Services IL, Ltd. | Partial door closure prevention spring |
| US12194214B2 (en) | 2016-08-10 | 2025-01-14 | Mozarc Medical Us Llc | Peritoneal dialysate flow path sensing |
| US11883576B2 (en) | 2016-08-10 | 2024-01-30 | Mozarc Medical Us Llc | Peritoneal dialysis intracycle osmotic agent adjustment |
| US10994064B2 (en) | 2016-08-10 | 2021-05-04 | Medtronic, Inc. | Peritoneal dialysate flow path sensing |
| US11013843B2 (en) | 2016-09-09 | 2021-05-25 | Medtronic, Inc. | Peritoneal dialysis fluid testing system |
| US11679186B2 (en) | 2016-09-09 | 2023-06-20 | Mozarc Medical Us Llc | Peritoneal dialysis fluid testing system |
| WO2018060821A1 (en)* | 2016-09-29 | 2018-04-05 | Koninklijke Philips N.V. | System and method for predicting motor wear out in infusion systems |
| CN109789266B (en)* | 2016-09-29 | 2022-05-03 | 皇家飞利浦有限公司 | System and method for predicting motor wear in an infusion system |
| CN109789266A (en)* | 2016-09-29 | 2019-05-21 | 皇家飞利浦有限公司 | System and method for predicting motor wear in an infusion system |
| US11224691B2 (en) | 2016-09-29 | 2022-01-18 | Koninklijke Philips N.V. | System and method for predicting motor wear out in infusion systems |
| US11278665B2 (en) | 2016-11-22 | 2022-03-22 | Eitan Medical Ltd. | Method for delivering a therapeutic substance |
| US11642654B2 (en) | 2016-11-29 | 2023-05-09 | Medtronic, Inc | Zirconium oxide module conditioning |
| US10981148B2 (en) | 2016-11-29 | 2021-04-20 | Medtronic, Inc. | Zirconium oxide module conditioning |
| US11516183B2 (en) | 2016-12-21 | 2022-11-29 | Gambro Lundia Ab | Medical device system including information technology infrastructure having secure cluster domain supporting external domain |
| US10857287B2 (en) | 2017-01-06 | 2020-12-08 | Trustees Of Boston University | Infusion system and components thereof |
| US11771821B2 (en) | 2017-01-06 | 2023-10-03 | Trustees Of Boston University | Infusion system and components thereof |
| US11819666B2 (en) | 2017-05-30 | 2023-11-21 | West Pharma. Services IL, Ltd. | Modular drive train for wearable injector |
| US11883794B2 (en) | 2017-06-15 | 2024-01-30 | Mozarc Medical Us Llc | Zirconium phosphate disinfection recharging and conditioning |
| US20220257864A1 (en)* | 2017-11-16 | 2022-08-18 | Amgen Inc. | Autoinjector with stall and end point detection |
| US11278654B2 (en) | 2017-12-07 | 2022-03-22 | Medtronic, Inc. | Pneumatic manifold for a dialysis system |
| US11857767B2 (en) | 2017-12-22 | 2024-01-02 | West Pharma. Services IL, Ltd. | Injector usable with different dimension cartridges |
| US11868161B2 (en) | 2017-12-27 | 2024-01-09 | Icu Medical, Inc. | Synchronized display of screen content on networked devices |
| US12333201B2 (en) | 2017-12-27 | 2025-06-17 | Icu Medical, Inc. | Synchronized display of screen content on networked devices |
| US11029911B2 (en) | 2017-12-27 | 2021-06-08 | Icu Medical, Inc. | Synchronized display of screen content on networked devices |
| US10656894B2 (en) | 2017-12-27 | 2020-05-19 | Icu Medical, Inc. | Synchronized display of screen content on networked devices |
| US11033667B2 (en) | 2018-02-02 | 2021-06-15 | Medtronic, Inc. | Sorbent manifold for a dialysis system |
| US11458246B2 (en) | 2018-02-05 | 2022-10-04 | Tandem Diabetes Care, Inc. | Methods and systems for detecting infusion pump conditions |
| US12296141B2 (en) | 2018-02-05 | 2025-05-13 | Tandem Diabetes Care, Inc | Methods and systems for detecting infusion pump conditions |
| US11110215B2 (en) | 2018-02-23 | 2021-09-07 | Medtronic, Inc. | Degasser and vent manifolds for dialysis |
| US11504471B2 (en) | 2018-04-12 | 2022-11-22 | Diatech Diabetes, Inc. | Systems and methods for detecting disruptions in fluid delivery devices |
| US11523972B2 (en) | 2018-04-24 | 2022-12-13 | Deka Products Limited Partnership | Apparatus, system and method for fluid delivery |
| US12285552B2 (en) | 2018-08-14 | 2025-04-29 | Mozarc Medical Us Llc | Precision dialysis therapy based on sorbent effluent analysis |
| US11707615B2 (en) | 2018-08-16 | 2023-07-25 | Deka Products Limited Partnership | Medical pump |
| US12251532B2 (en) | 2018-08-16 | 2025-03-18 | Deka Products Limited Partnership | Medical pump |
| US11213616B2 (en) | 2018-08-24 | 2022-01-04 | Medtronic, Inc. | Recharge solution for zirconium phosphate |
| US11357909B2 (en) | 2018-10-05 | 2022-06-14 | Eitan Medical Ltd. | Triggering sequence |
| US11701464B2 (en) | 2018-10-05 | 2023-07-18 | Eitan Medical Ltd. | Drawing drug from a vial |
| US11806457B2 (en) | 2018-11-16 | 2023-11-07 | Mozarc Medical Us Llc | Peritoneal dialysis adequacy meaurements |
| US11806456B2 (en) | 2018-12-10 | 2023-11-07 | Mozarc Medical Us Llc | Precision peritoneal dialysis therapy based on dialysis adequacy measurements |
| US12433976B2 (en) | 2019-01-08 | 2025-10-07 | Mozarc Medical Us Llc | Bicarbonate sensor for dialysis |
| US11633535B2 (en) | 2019-07-16 | 2023-04-25 | Beta Bionics, Inc. | Ambulatory device and components thereof |
| US11571507B2 (en) | 2019-07-16 | 2023-02-07 | Beta Bionics, Inc. | Ambulatory device and components thereof |
| US12268843B2 (en) | 2019-12-04 | 2025-04-08 | Icu Medical, Inc. | Infusion pump with safety sequence keypad |
| US11278671B2 (en) | 2019-12-04 | 2022-03-22 | Icu Medical, Inc. | Infusion pump with safety sequence keypad |
| US12115338B2 (en) | 2020-03-10 | 2024-10-15 | Beta Bionics, Inc. | Infusion system and components thereof |
| US11278661B2 (en) | 2020-03-10 | 2022-03-22 | Beta Bionics, Inc. | Infusion system and components thereof |
| USD1022185S1 (en) | 2020-03-10 | 2024-04-09 | Beta Bionics, Inc. | Medicament infusion pump device |
| USD1031975S1 (en) | 2020-03-10 | 2024-06-18 | Beta Bionics, Inc. | Medicament infusion pump device |
| USD1086029S1 (en) | 2020-03-10 | 2025-07-29 | Beta Bionics, Inc. | Charging device for medicament infusion pump device |
| US12128165B2 (en) | 2020-04-27 | 2024-10-29 | Mozarc Medical Us Llc | Dual stage degasser |
| US12310921B2 (en) | 2020-07-21 | 2025-05-27 | Icu Medical, Inc. | Fluid transfer devices and methods of use |
| US11883361B2 (en) | 2020-07-21 | 2024-01-30 | Icu Medical, Inc. | Fluid transfer devices and methods of use |
| US11135360B1 (en) | 2020-12-07 | 2021-10-05 | Icu Medical, Inc. | Concurrent infusion with common line auto flush |
| US12390586B2 (en) | 2020-12-07 | 2025-08-19 | Icu Medical, Inc. | Concurrent infusion with common line auto flush |
| US12397093B2 (en) | 2021-05-18 | 2025-08-26 | Mozarc Medical Us Llc | Sorbent cartridge designs |
| US12154673B2 (en) | 2021-08-02 | 2024-11-26 | Mozarc Medical Us Llc | Artificial intelligence assisted home therapy settings for dialysis |
| US11850344B2 (en) | 2021-08-11 | 2023-12-26 | Mozarc Medical Us Llc | Gas bubble sensor |
| USD1091564S1 (en) | 2021-10-13 | 2025-09-02 | Icu Medical, Inc. | Display screen or portion thereof with graphical user interface for a medical device |
| US11965763B2 (en) | 2021-11-12 | 2024-04-23 | Mozarc Medical Us Llc | Determining fluid flow across rotary pump |
| US11944733B2 (en) | 2021-11-18 | 2024-04-02 | Mozarc Medical Us Llc | Sodium and bicarbonate control |
| US12350233B2 (en) | 2021-12-10 | 2025-07-08 | Icu Medical, Inc. | Medical fluid compounding systems with coordinated flow control |
Also Published As
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| US6362591B1 (en) | Method and apparatus for detection of occlusions | |
| US9364608B2 (en) | Method and apparatus for detecting occlusions in an ambulatory infusion pump | |
| US9433732B2 (en) | Methods and apparatuses for detecting occlusions in an ambulatory infusion pump | |
| JP2801617B2 (en) | Fluid supply control / monitoring device for drug injection system | |
| US12296141B2 (en) | Methods and systems for detecting infusion pump conditions |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| AS | Assignment | Owner name:MINIMED INC., CALIFORNIA Free format text:ASSIGNMENT OF ASSIGNORS INTEREST;ASSIGNOR:MOBERG, SHELDON;REEL/FRAME:010357/0384 Effective date:19991028 | |
| AS | Assignment | Owner name:MEDTRONIC MINIMED, INC., CALIFORNIA Free format text:CHANGE OF NAME;ASSIGNOR:MINIMED INC.;REEL/FRAME:012463/0589 Effective date:20010828 | |
| STCF | Information on status: patent grant | Free format text:PATENTED CASE | |
| FPAY | Fee payment | Year of fee payment:4 | |
| FPAY | Fee payment | Year of fee payment:8 | |
| FPAY | Fee payment | Year of fee payment:12 |