US6183497B1 - Absorbable sponge with contrasting agent - Google Patents
Absorbable sponge with contrasting agentDownload PDFInfo
- Publication number
- US6183497B1 US6183497B1US09/335,452US33545299AUS6183497B1US 6183497 B1US6183497 B1US 6183497B1US 33545299 AUS33545299 AUS 33545299AUS 6183497 B1US6183497 B1US 6183497B1
- Authority
- US
- United States
- Prior art keywords
- contrasting agent
- pledget
- sponge
- absorbable
- absorbable sponge
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Expired - Lifetime
Links
Images
Classifications
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M31/00—Devices for introducing or retaining media, e.g. remedies, in cavities of the body
- A61M31/005—Devices for introducing or retaining media, e.g. remedies, in cavities of the body for contrast media
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61B—DIAGNOSIS; SURGERY; IDENTIFICATION
- A61B10/00—Instruments for taking body samples for diagnostic purposes; Other methods or instruments for diagnosis, e.g. for vaccination diagnosis, sex determination or ovulation-period determination; Throat striking implements
- A61B10/02—Instruments for taking cell samples or for biopsy
- A61B10/0233—Pointed or sharp biopsy instruments
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61B—DIAGNOSIS; SURGERY; IDENTIFICATION
- A61B17/00—Surgical instruments, devices or methods
- A61B17/0057—Implements for plugging an opening in the wall of a hollow or tubular organ, e.g. for sealing a vessel puncture or closing a cardiac septal defect
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61B—DIAGNOSIS; SURGERY; IDENTIFICATION
- A61B6/00—Apparatus or devices for radiation diagnosis; Apparatus or devices for radiation diagnosis combined with radiation therapy equipment
- A61B6/02—Arrangements for diagnosis sequentially in different planes; Stereoscopic radiation diagnosis
- A61B6/03—Computed tomography [CT]
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61B—DIAGNOSIS; SURGERY; IDENTIFICATION
- A61B6/00—Apparatus or devices for radiation diagnosis; Apparatus or devices for radiation diagnosis combined with radiation therapy equipment
- A61B6/48—Diagnostic techniques
- A61B6/481—Diagnostic techniques involving the use of contrast agents
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61B—DIAGNOSIS; SURGERY; IDENTIFICATION
- A61B6/00—Apparatus or devices for radiation diagnosis; Apparatus or devices for radiation diagnosis combined with radiation therapy equipment
- A61B6/48—Diagnostic techniques
- A61B6/485—Diagnostic techniques involving fluorescence X-ray imaging
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61B—DIAGNOSIS; SURGERY; IDENTIFICATION
- A61B8/00—Diagnosis using ultrasonic, sonic or infrasonic waves
- A61B8/48—Diagnostic techniques
- A61B8/481—Diagnostic techniques involving the use of contrast agents, e.g. microbubbles introduced into the bloodstream
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61B—DIAGNOSIS; SURGERY; IDENTIFICATION
- A61B90/00—Instruments, implements or accessories specially adapted for surgery or diagnosis and not covered by any of the groups A61B1/00 - A61B50/00, e.g. for luxation treatment or for protecting wound edges
- A61B90/39—Markers, e.g. radio-opaque or breast lesions markers
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61B—DIAGNOSIS; SURGERY; IDENTIFICATION
- A61B17/00—Surgical instruments, devices or methods
- A61B2017/00004—(bio)absorbable, (bio)resorbable or resorptive
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61B—DIAGNOSIS; SURGERY; IDENTIFICATION
- A61B17/00—Surgical instruments, devices or methods
- A61B17/0057—Implements for plugging an opening in the wall of a hollow or tubular organ, e.g. for sealing a vessel puncture or closing a cardiac septal defect
- A61B2017/00637—Implements for plugging an opening in the wall of a hollow or tubular organ, e.g. for sealing a vessel puncture or closing a cardiac septal defect for sealing trocar wounds through abdominal wall
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61B—DIAGNOSIS; SURGERY; IDENTIFICATION
- A61B17/00—Surgical instruments, devices or methods
- A61B17/0057—Implements for plugging an opening in the wall of a hollow or tubular organ, e.g. for sealing a vessel puncture or closing a cardiac septal defect
- A61B2017/00646—Type of implements
- A61B2017/00654—Type of implements entirely comprised between the two sides of the opening
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61B—DIAGNOSIS; SURGERY; IDENTIFICATION
- A61B90/00—Instruments, implements or accessories specially adapted for surgery or diagnosis and not covered by any of the groups A61B1/00 - A61B50/00, e.g. for luxation treatment or for protecting wound edges
- A61B90/39—Markers, e.g. radio-opaque or breast lesions markers
- A61B2090/3933—Liquid markers
Definitions
- the inventionrelates to an absorbable sponge, and more particularly, the invention relates to the delivery of a contrasting agent to a specific area or site in a mammal after a surgical or interventional procedure.
- the contrasting agentfacilitates the location of the area or site even weeks or months after the initial procedure.
- Percutaneous needle biopsy of solid organsis one of the most common interventional medical procedures. Millions of percutaneous needle biopsies are performed annually in the United States and throughout the world. Percutaneous biopsy is a safe procedure which has supplanted surgical biopsy for many indications, such as skin biopsy and liver biopsy.
- Possible complications of needle biopsyinclude bleeding at the biopsy site.
- the amount of bleedingis related to a number of factors including needle size, tissue sample size, patient's coagulation status, and the location of the biopsy site.
- vascular organssuch as the liver, a common biopsy target, may bleed significantly after needle biopsy.
- Sterile spongessuch as GELFOAM
- GELFOAMSterile sponges
- the sponge sheetsare left in the surgical site after surgery to stop bleeding and are absorbed by the body.
- a number of techniqueshave used these absorbable sterile sponge materials to plug a biopsy tract to minimize or prevent bleeding.
- the absorbable spongeprovides a mechanical blockage of the tract, encourages clotting, and minimizes bleeding though the biopsy tract.
- a mechanic clip deviceis often attached to the site where tissue is removed, so that if further treatment is later required the location of the site can be identified.
- the time period between the biopsy and treatmentmay be weeks during which time the clip may become dislodged thereby making it difficult to relocate the site.
- the present inventionis based in part on the discovery that adding a contrasting agent (e.g, radiopaque agent) to an absorbable sponge provides for a material that not only facilitates hemostasis of a biopsy tract or other puncture wound but also permits precise identification of the site's location.
- a contrasting agente.g, radiopaque agent
- the inventionis directed to a method for marking a bodily site in a patient that includes the steps of:
- the exact location of the bodily sitecan be located many weeks or longer following positioning of the absorbable sponge material.
- the inventionis directed to a method for performing a biopsy that included the steps of:
- the inventionis directed to a liquid permeable, absorbable, gelatin sponge that is prepared by a process that includes the steps of:
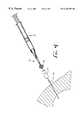
- FIG. 1is a side cross sectional view of an adaptor for delivery of a pledget to a needle
- FIG. 2is a side cross sectional view of a syringe for connection to the adaptor
- FIG. 3is a side cross sectional view of an adaptor and syringe combination with a pledget positioned within the adaptor;
- FIG. 4is a side cross sectional view of the loaded adaptor and syringe combination in preparation for connection to a biopsy needle;
- FIG. 5is a side cross sectional view of an alternative embodiment of an adaptor for delivery of a pledget including a template attached to the adaptor;
- FIG. 6is a top view of the template as it is used for cutting a pledget from an absorbable sponge sheet
- FIG. 7is a side cross sectional view of a portion of an organ and a system for delivering a pledget into a biopsy tract in the organ.
- the present inventionis directed to an absorbable sponge material containing a contrasting agent.
- the absorbable sponge materialis delivered to a specific area or site in a patient (i.e., mammal) after a surgical or interventional procedure.
- the sponge materialcan be placed in the biopsy tract or other puncture wound and the contrasting agent enables marking or identification of the area or site.
- the absorbable spongewill be described in connection with treatment of a biopsy tract after a percutaneous needle biopsy.
- the absorbable sponge materialcan also exhibit secondary benefits of, for example, facilitating hemostasis and delivering therapeutic agents.
- “Pledget”means a piece of absorbable sponge containing a contrasting agent preferably of a generally elongated shape having a size which allows injection in a hydrated state through a biopsy needle or other cannula.
- “Sponge”means a biocompatible material which is capable of being hydrated and is resiliently compressible in a hydrated state.
- the spongeis non-inmmunogenic and is absorbable.
- “Absorbable sponge”means a sponge which when implanted within a patient (i.e., human or other mammalian body) is absorbed by the body.
- the absorbable spongecontains a contrasting agent which may or may not be absorbable. Besides the contrasting agent, the sponge can also be used to deliver a beneficial agent such as thrombin, radiation treatment or the like.
- “Bodily site”means any tissue in a mammal where the absorbable sponge containing the contrasting agent can be introduced.
- the inventionis particularly suited for introducing the absorbable sponge into tissue sites where further treatment may be required, for example, as in the case following biopsy.
- “Hydrate”means to partially or fully saturate with a fluid, such as, saline, water, or the like.
- “Kneading” of the absorbable sponge materialmeans both dry and wet manipulation of the sponge material which compresses, enlarges, or changes the shape of the sponge material causing the sponge material to have improved expansion response.
- “Contrasting agent”means a biocompatible material that is capable of being detected or monitored by fluoroscopy, X-ray photography, CAT scan, ultrasound, or similar imaging techniques following placement into a mammalian subject.
- Preferred contrasting agentsare radiopaque materials.
- the contrast agentcan be either water soluble or water insoluble. Examples of water soluble contrast agents include metrizamide, iopamidol, iothalamate sodium, iodomide sodium, and meglumine. Examples of water insoluble contrast agents include tantalum, tantalum oxide, and barium sulfate, each of which is commercially available. Other water insoluble contrast agents include gold, tungsten, and platinum powders. Some radiopaque contrasting agents are available in liquid form. These include, for example, OMNIPAQUE from Nycomed, Inc., Princeton, NJ. Preferably, the contrast agent is water insoluble (i.e., has a water solubility of less than 0.01 mg/ml at 20° C.).
- the absorbable sponge material of the present inventionis preferably a liquid permeable, water insoluble gelatin based sponge that has contrasting agent incorporated in the matrix of the sponge.
- incorporatedis meant that the contrasting agent is substantially dispersed throughout the sponge so that the contrasting material is not simply found on the periphery of the sponge.
- the spongeis made by mixing a suitable organic solvent (e.g., formaldehyde) with an aqueous solution of gelatin.
- the organic solventfacilitates the cross linkage of gelatin polymers. It is expected that glutaraldehyde may also be suitable.
- the resulting solutionis then incubated typically at slightly above room temperature (30°-40° C.). Subsequently, a contrasting agent is added and the resulting mixture beaten to produce a firm foam. Thereafter, the foam is dried to produce the absorbable sponge material.
- the aqueous gelatin solution containing 3-10% gelatin by weightis prepared as a warm solution (e.g., 80° C.) to help dissolve the gelatin.
- the solutionis then allowed to cool (e.g, 35°-40° C.) before the organic solvent is added.
- a preferred organic solventis formalin (an aqueous solution of formaldehyde) .
- formalinan aqueous solution of formaldehyde
- the amount of formalin usedwill control the hardness of the sponge and its rate of absorption into the body. The more formalin used, the harder the sponge and the lower the absorption rate. Typically, the amount used is between 0.01 to 10% based on the gelatin solution.
- the beating or whipping processtakes about 5-15 or more minutes to produce a firm foam of about 4 to 8 times the volume of the original solution.
- the drying processinitially begins with oven drying in the presence of circulating air at about 30° to 33°C. or higher and 10% humidity. After the foam is thoroughly dried, the foam can be heated to an elevated temperature of about 140° C. for a sufficient length of time (e.g., 3 hrs) until the sponge is firm. Suitable absorbable sponge materials are further described in U.S. Pat. No. 2,465,357 which is incorporated herein by reference.
- the contrasting agentmust be added prior to beating the gelatin/formalin solution. The reason is that once the foam material is produced, the contrasting agent cannot be incorporated into the matrix of the sponge.
- contrasting agentsthat are liquids it is preferred that they be added to the gelatin/formalin solution prior to being beaten to form the foam product. This will insure that the contrasting agent is dispersed throughout the sponge.
- absorbable sponge materials containing different amounts of contrasting agentwere prepared and tested. Specifically, 5 grams of pork gelatin (Bloom value 275) were mixed in 100 grams of water at 80° C. and the solution was allowed to cool to 35° C. before 0.03 cc of 40% formalin was added. The resulting solution was incubated at 35° C. for 2 hours before tantalum powder (50 to 150 grams) was added. The liquid was then vigorously mixed in a malt mixer to produce a foam. The foam was then oven dried at 35° C. for 12 hours.
- the absorbable sponge materialwas examined with a fluoroscope and found to be extremely visible. Moreover, placement of the sponge material with contrasting agent in puncture sites of a swine model demonstrated that the absorbable sponge exhibited good hemostatic properties as well.
- the sponge material with contrasting agent of the present inventionis particularly suited for biopsies and other percutaneous procedures where knowledge of the site of initial treatment, e.g., tissue removal, is important.
- the absorbable sponge materialcan be employed with any suitable medical instrument, a preferred device and method for facilitating hemostasis of a biopsy tract is described herein to illustrate use of the absorbable sponge material. The technique is further described in U.S. patent application Ser. No. 09/247,880 entiled “Device and Method for Facilitating Hemostatis of a Biopsy Track” which application is incorprated herein by reference.
- FIG. 1shows the adaptor 12 in which the pledget 18 is placed for hydration and for delivery through the biopsy needle 16 .
- the adaptor 12allows pieces of absorbable sponge material with relatively large cross sections to be easily delivered through a biopsy needle 16 with a much smaller cross section.
- the adaptor 12also functions to remove air from the pledget 18 .
- the adaptor 12 which delivers the hydrated pledget 18 to the needle 16includes a first end 30 having an annular lip 32 or female luer fitting for connection to the syringe 14 .
- a second end 34 of the adaptor 12has a male luer fitting 36 for connection to a biopsy needle 16 or other cannula.
- the luer fitting 36includes a tapered external surface 38 and a retaining ring 40 with internal threads for receiving an annular lip of the biopsy needle.
- the adaptor 12has an internal lumen with a first diameter D 1 at the first end 30 and a second diameter D 2 at the second end 34 . Between the first and second ends of the adaptor 12 a tapered section 42 of the adaptor provides a funnel for compressing the hydrated pledget 18 prior to injection through the biopsy needle 16 and needle hub 28 .
- the adaptor 12may be formed in any known manner such as by molding from a plastic material.
- the adaptor 12is transparent so that the pledget 18 can be viewed through the adaptor and the user can visually monitor when the pledget is loaded within the adaptor and when the pledget has been delivered into the needle.
- the adaptor lumenmay be provided with a friction reducing coating for improved delivery. The delivery fluid also reduces friction for improved delivery by wetting the exterior surface of the pledget 18 .
- the syringe 14includes a male luer fitting 46 , a fluid chamber 48 , and a plunger 50 .
- the first end 30 of the adaptor 12is connectable to the luer fitting 46 of the conventional syringe 14 .
- the syringe 14may be provided with a spring 52 for automatic filling of the syringe 14 with a predetermined volume of fluid.
- the biopsy needle 16 used with the present inventionis preferably a co-axial biopsy needle, such as a bi-axial or a tri-axial biopsy needle.
- a co-axial biopsy needleincludes an outer needle or cannula through which a tissue sample is removed with a tissue scoop or other biopsy instrument. Once the tissue sample has been removed, the outer cannula remains in the patient as illustrated in FIG. 4 .
- the cannula for delivery of the sponge pledgethas been described as a biopsy needle, the cannula may be a catheter, sheath, or any other type of cannula.
- FIG. 3shows the loading and hydration of the pledget 18 within the adaptor 12 .
- a pledget 18is cut and placed within the adaptor 12 from the first end 30 of the adaptor.
- the syringe 14is filled with a predetermined amount of fluid, such as saline, and is connected to the first end 30 of the adaptor 12 by the luer fitting 46 .
- the plunger 50 of the syringe 14is then depressed slowly causing fluid to pass into the adaptor 12 , hydrating the pledget 18 , and filling the adaptor with a column of fluid.
- the userwaits a few seconds once the fluid is injected into the adaptor 12 until the pledget 18 is adequately hydrated creating a lubricous surface on the pledget.
- the pledget 18may expand within the adaptor to fill or nearly fill the lumen of the adaptor.
- the adaptor 12 with the pledget 18 hydrated within the proximal endis ready to inject the pledget into a biopsy tract to facilitate hemostasis within the biopsy tract.
- the adaptor 12may be loaded prior to beginning the biopsy procedure.
- the outer sheath of the biopsy needle 16 through which the biopsy has been takenis maintained in place within the biopsy tract, as shown in FIG. 4 .
- the biopsy needle 16provides preestablished targeting of the delivery site for delivery of the absorbable sponge pledget 18 and eliminates the uncertainty of re-access.
- the luer fitting 36 of the adaptor 12is connected to the biopsy needle hub 28 , as illustrated in FIG. 4 .
- the biopsy needle 16is withdrawn a short distance, such as about 1 to 20 mm, along the biopsy tract to provide space for the pledget 18 to be received in the biopsy tract. Additional fluid is then rapidly injected by the syringe to move the pledget 18 into the biopsy needle 16 .
- the adaptor lumenWhen the adaptor lumen has been blocked by the hydrated pledget 18 which has swelled within the adaptor, injection of additional fluid will push the pledget through the tapered section 42 of the adaptor. If the adaptor lumen has not been entirely blocked by the pledget 18 , the venturi effect will help draw the pledget through the tapered section 42 of the adaptor.
- the pledget 18After the pledget 18 is moved to the biopsy needle 16 , the pledget 18 is then delivered from the needle 16 to the biopsy tract by rapid injection of additional fluid by the syringe 14 .
- the hydrated pledget 18quickly expands upon delivery to fill the available space in the biopsy tract to facilitate hemostasis and provide localized compression.
- the absorbable sponge material of the present inventioncan be shaped into the required size by conventional means. Pledgets may be cut with a punch or a stencil or template and knife. Once hydrated, the pledget 18 can be easily compressed to fit into a lumen having a smaller cross sectional area than the original cross sectional area of the pledget. Additionally, the kneading of the hydrated pledget 18 during delivery encourages air trapped within the absorbable sponge to be expelled and replaced with fluid, allowing rapid expansion upon delivery.
- Pledgets 118 with increased cross sectional area proximal endsmay be prepared in a variety of manners.
- the increased proximal masscan be achieved by cutting the pledget with an enlarged proximal end.
- the pledget 118may be formed by folding, rolling, compressing, or otherwise manipulating the sponge material to the desired shape.
- the proximal pledget massmay also be increased by adding separate pieces of material to the proximal end of the pledget. This additional material may be layered, wrapped, coiled or attached to the pledget in any other manner.
- the pledgetsmay also be formed by molding, bump extruding, dipping, or the like.
- the larger cross sectional area proximal endis generally about 1.2 to 4 times the cross sectional area of the distal end.
- the proximal end with the larger cross section areapreferably extends along about 1 ⁇ 8 to 3 ⁇ 4 of the total pledget length.
- the pledget 118 illustrated in FIG. 5has been formed by cutting a strip of material from an absorbable sponge sheet 20 with the aid of the template 122 as illustrated in FIG. 6 . After the strip is cut, the proximal end of the strip is then folded back onto itself to form a pledget 118 with an increased cross sectional area and material mass at a proximal end.
- a preferred embodiment of a pledget for delivery down a 20 gauge biopsy needle or cannulahas a size of approximately 0.1 ⁇ 1.5 ⁇ 0.06 inches and is folded as illustrated in FIG. 5 to an overall length of about 0.9 inches. Placing this pledget 118 in an adaptor 112 having a largest internal diameter of 0.125 inches allows the pledget to be delivered to a 20 gauge or larger biopsy needle.
- a pledget 118maybe delivered through the cannula to the biopsy site.
- the pledget 118 for use in the system employing an 18 gauge or larger biopsy needlemay be formed from a strip which is approximately 0.11-0.12 inches wide by about 3.125 inches long with a thickness of about 0.06 inches and folded to an overall length of about 2.2 inches. This pledget having a 28 which is attached to the distal end of the adaptor.
- the pledgetmay be delivered to the biopsy tract by holding the biopsy needle or cannula 16 stationary and injecting the pledget through the biopsy needle. If additional pledgets are to be delivered, the biopsy needle 16 is withdrawn a distance sufficient to accommodate an additional pledget and the additional pledget is then injected.
- An alternative method of delivering the pledget into the biopsy tractincludes withdrawing the biopsy needle or cannula 16 during delivery of the pledget 18 to deliver the pledget in an elongated trail which follows the biopsy tract. Placing the absorbable sponge material in a trail which fills the entire biopsy tract provides the added benefit of providing hemostasis along the entire biopsy tract. This is particularly helpful for stopping the bleeding of biopsy tracts in organs which tend to have excessive bleeding such as the liver, kidney, spleen, and other vascular organs.
- one method of the present inventioninvolves the delivery of the pledget into the biopsy needle by a predetermined amount of fluid.
- the biopsy needleis then withdrawn at a velocity V while the pledget material is ejected from the biopsy needle at a velocity E with respect to the biopsy needle.
- the velocity V at which the biopsy needle is withdrawnis equal to or less than the velocity E at which the absorbable sponge material is delivered.
- the control of injection of fluid and withdrawal of the needle to achieve the desired trail of absorbable sponge material in the biopsy tractmaybe controlled with an injection controlling device.
- the adaptormay be used to deliver the pledget into the biopsy needle 16 and then the adaptor is removed from the biopsy needle.
- a plunger or stylet 80which is generally provided with the biopsy needle 16 for inserting the biopsy needle is then used to deliver the pledget from the biopsy needle.
- the biopsy needleextends through the tissue 84 and into the organ 86 for removal of a core of tissue.
- the plunger 80is placed within the biopsy needle so that a distal end of the plunger abuts the proximal end of the pledget 118 .
- the plunger 80is then held stationary while the biopsy needle 16 is withdrawn from the biopsy site.
- the plunger 80causes the pledget 118 to be delivered in a trail 88 which fills the biopsy tract.
- the trail 88preferably extends along the entire biopsy tract to or past a surface of the organ 86 .
- the delivery of the trail 88 of absorbable sponge materialprovides an advantage over the delivery of discrete blobs of material because the trail is able to provide hemostasis along the entire tract.
- a blob of absorbable sponge materialis delivered within the tract at a depth of 1-2 cm from the surface of the organs, this 1-2 cm of biopsy tract may continue to bleed significantly.
- the pledgetmay be delivered as a plug.
- the plunger 80is advanced into the needle 16 pushing the pledget out of the distal end of the needle while the needle is held stationary.
- a combination of delivery of plugs and trailsmay also be used.
- the pledget materialmay be delivered entirely within a single anatomical structure or may cross two or more anatomical structures such as an organ, surrounding tissue and facial layer.
- the biopsy needle 16is retracted a distance sufficient to provide a space to accommodate an additional pledget 18 and the injection procedure described above is repeated for the additional pledget(s).
- additional pledgets 18may be injected beside an initially injected pledget until the cavity is filled.
- biopsyis most commonly performed by biopsy needle, biopsy may also be performed through other cannulas, such as catheters, long needles, endoscopes, or the like.
- the treatment procedure according to the present inventioncan be used for facilitating hemostasis of puncture wounds through different types of cannulas including needles, catheters, endoscopes, and the like.
- the treatment procedure and systems according to the present inventionmay be used to deliver absorbable or non-absorbable sponge for other therapys.
- spongemay be delivered for cosmetic or reconstructive bulking or for temporary or permanent intravascular embolization.
- the absorbable sponge pledget 18may be used to deliver a beneficial agent, such as, thrombin, radiation treatment, or the like.
- the pledgetcan also be used to deliver therapeutic agents, such as radioactive isotopes for localized treatment of tumors, anti-cancer agents, antimetastatic agents, and the like.
- therapeutic agentssuch as radioactive isotopes for localized treatment of tumors, anti-cancer agents, antimetastatic agents, and the like.
- anti-cancer agentsinclude 5-fluorouracil, cisplatin, prednisone, and others described in U.S. Pat. No. 4,619,913 which is incorporated herein by reference.
- the absorbable sponge pledget 18may be presoaked with the beneficial agent for delivery to the biopsy tract.
- the pledget 18may be hydrated with the beneficial liquid agent or the agent may be delivered to the pledget after the pledget is placed within the biopsy tract.
- a pledget formed of inventive absorbable sponge materialpreferably will be absorbed by the body within 1 to 6 weeks. However, the pledget material may be designed to provide different rates of absorption. If the contrasting agent employed is also absorbable, the contrasting agent should be absorbed at approximately the same rate as the sponge material. Where the contrasting agent is non-absorbable, it will remain at the site.
Landscapes
- Health & Medical Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Engineering & Computer Science (AREA)
- Surgery (AREA)
- Medical Informatics (AREA)
- Biomedical Technology (AREA)
- Veterinary Medicine (AREA)
- Heart & Thoracic Surgery (AREA)
- Public Health (AREA)
- Animal Behavior & Ethology (AREA)
- General Health & Medical Sciences (AREA)
- Molecular Biology (AREA)
- Pathology (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Radiology & Medical Imaging (AREA)
- Physics & Mathematics (AREA)
- Biophysics (AREA)
- Optics & Photonics (AREA)
- High Energy & Nuclear Physics (AREA)
- Hematology (AREA)
- Cardiology (AREA)
- Oral & Maxillofacial Surgery (AREA)
- Anesthesiology (AREA)
- Surgical Instruments (AREA)
- Materials For Medical Uses (AREA)
- Medicines Containing Antibodies Or Antigens For Use As Internal Diagnostic Agents (AREA)
- Apparatus For Radiation Diagnosis (AREA)
- Ultra Sonic Daignosis Equipment (AREA)
Abstract
Description
Claims (30)
Priority Applications (11)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US09/335,452US6183497B1 (en) | 1998-05-01 | 1999-06-17 | Absorbable sponge with contrasting agent |
| EP00944716AEP1191884A1 (en) | 1999-06-17 | 2000-06-16 | Absorbable sponge with contrasting agent |
| PCT/US2000/016775WO2000078228A1 (en) | 1999-06-17 | 2000-06-16 | Absorbable sponge with contrasting agent |
| AU58772/00AAU5877200A (en) | 1999-06-17 | 2000-06-16 | Absorbable sponge with contrasting agent |
| JP2001504297AJP4460203B2 (en) | 1999-06-17 | 2000-06-16 | Method for producing absorbent sponge with contrast agent |
| CA002371841ACA2371841A1 (en) | 1999-06-17 | 2000-06-16 | Absorbable sponge with contrasting agent |
| US09/966,611US20020038133A1 (en) | 1998-05-01 | 2001-09-27 | Absorbable sponge with contrasting agent |
| US10/978,321US7618567B2 (en) | 1998-05-01 | 2004-10-29 | Absorbable sponge with contrasting agent |
| US12/578,088US20100029908A1 (en) | 1998-05-01 | 2009-10-13 | Absorbable sponge with contrasting agent |
| US12/887,945US20110014290A1 (en) | 1995-09-15 | 2010-09-22 | System and method for facilitating hemostasis with an absorbable sponge |
| US13/776,952US20130172737A1 (en) | 1998-05-01 | 2013-02-26 | Absorbable sponge with contrasting agent |
Applications Claiming Priority (3)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US09/071,284US6162192A (en) | 1998-05-01 | 1998-05-01 | System and method for facilitating hemostasis of blood vessel punctures with absorbable sponge |
| US09/071,670US6071301A (en) | 1998-05-01 | 1998-05-01 | Device and method for facilitating hemostasis of a biopsy tract |
| US09/335,452US6183497B1 (en) | 1998-05-01 | 1999-06-17 | Absorbable sponge with contrasting agent |
Related Parent Applications (2)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US09/071,670Continuation-In-PartUS6071301A (en) | 1995-09-15 | 1998-05-01 | Device and method for facilitating hemostasis of a biopsy tract |
| US09/071,284Continuation-In-PartUS6162192A (en) | 1995-09-15 | 1998-05-01 | System and method for facilitating hemostasis of blood vessel punctures with absorbable sponge |
Related Child Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US63081400ADivision | 1995-09-15 | 2000-08-02 |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| US6183497B1true US6183497B1 (en) | 2001-02-06 |
Family
ID=23311845
Family Applications (5)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US09/335,452Expired - LifetimeUS6183497B1 (en) | 1995-09-15 | 1999-06-17 | Absorbable sponge with contrasting agent |
| US09/966,611AbandonedUS20020038133A1 (en) | 1995-09-15 | 2001-09-27 | Absorbable sponge with contrasting agent |
| US10/978,321Expired - Fee RelatedUS7618567B2 (en) | 1995-09-15 | 2004-10-29 | Absorbable sponge with contrasting agent |
| US12/578,088AbandonedUS20100029908A1 (en) | 1995-09-15 | 2009-10-13 | Absorbable sponge with contrasting agent |
| US13/776,952AbandonedUS20130172737A1 (en) | 1998-05-01 | 2013-02-26 | Absorbable sponge with contrasting agent |
Family Applications After (4)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US09/966,611AbandonedUS20020038133A1 (en) | 1995-09-15 | 2001-09-27 | Absorbable sponge with contrasting agent |
| US10/978,321Expired - Fee RelatedUS7618567B2 (en) | 1995-09-15 | 2004-10-29 | Absorbable sponge with contrasting agent |
| US12/578,088AbandonedUS20100029908A1 (en) | 1995-09-15 | 2009-10-13 | Absorbable sponge with contrasting agent |
| US13/776,952AbandonedUS20130172737A1 (en) | 1998-05-01 | 2013-02-26 | Absorbable sponge with contrasting agent |
Country Status (6)
| Country | Link |
|---|---|
| US (5) | US6183497B1 (en) |
| EP (1) | EP1191884A1 (en) |
| JP (1) | JP4460203B2 (en) |
| AU (1) | AU5877200A (en) |
| CA (1) | CA2371841A1 (en) |
| WO (1) | WO2000078228A1 (en) |
Cited By (85)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US6270464B1 (en) | 1998-06-22 | 2001-08-07 | Artemis Medical, Inc. | Biopsy localization method and device |
| US20010034528A1 (en)* | 1994-09-16 | 2001-10-25 | Foerster Seth A. | Methods and devices for defining and marking tissue |
| US6356782B1 (en) | 1998-12-24 | 2002-03-12 | Vivant Medical, Inc. | Subcutaneous cavity marking device and method |
| US6427081B1 (en)* | 1999-02-02 | 2002-07-30 | Senorx, Inc. | Methods and chemical preparations for time-limited marking of biopsy sites |
| US6440153B2 (en) | 1998-05-01 | 2002-08-27 | Sub-Q, Inc. | Device and method for facilitating hemostasis of a biopsy tract |
| US6447534B2 (en) | 1998-05-01 | 2002-09-10 | Sub-Q, Inc. | Device and method for facilitating hemostasis of a biopsy tract |
| US20020156495A1 (en)* | 1995-09-15 | 2002-10-24 | Rodney Brenneman | Apparatus and method for percutaneous sealing of blood vessel punctures |
| US20020190226A1 (en)* | 2001-03-12 | 2002-12-19 | Mark Ashby | Methods for sterilizing cross-linked gelatin compositions |
| US20030013989A1 (en)* | 2001-06-29 | 2003-01-16 | Joseph Obermiller | Porous sponge matrix medical devices and methods |
| US6511457B2 (en)* | 2001-05-04 | 2003-01-28 | Garey Thompson | Airless syringe |
| US20030028140A1 (en)* | 2001-03-12 | 2003-02-06 | Greff Richard J. | Cross-linked gelatin composition comprising a wetting agent |
| US6527734B2 (en) | 1998-05-01 | 2003-03-04 | Sub-Q, Inc. | System and method for facilitating hemostasis of blood vessel punctures with absorbable sponge |
| US20030135237A1 (en)* | 1998-05-01 | 2003-07-17 | Cragg Andrew H. | Device, system and method for improving delivery of hemostatic material |
| US6610026B2 (en) | 1998-05-01 | 2003-08-26 | Sub-Q, Inc. | Method of hydrating a sponge material for delivery to a body |
| US6662041B2 (en) | 1999-02-02 | 2003-12-09 | Senorx, Inc. | Imageable biopsy site marker |
| US20030233101A1 (en)* | 2002-06-17 | 2003-12-18 | Senorx, Inc. | Plugged tip delivery tube for marker placement |
| US20040019330A1 (en)* | 2001-11-08 | 2004-01-29 | Sub-Q, Inc., A California Corporation | Sheath based blood vessel puncture locator and depth indicator |
| US6725083B1 (en)* | 1999-02-02 | 2004-04-20 | Senorx, Inc. | Tissue site markers for in VIVO imaging |
| US20040102730A1 (en)* | 2002-10-22 | 2004-05-27 | Davis Thomas P. | System and method for facilitating hemostasis of blood vessel punctures with absorbable sponge |
| US20040122350A1 (en)* | 2002-12-20 | 2004-06-24 | Sheng-Ping Zhong | Puncture hole sealing device |
| US20040122349A1 (en)* | 2002-12-20 | 2004-06-24 | Lafontaine Daniel M. | Closure device with textured surface |
| US20040176723A1 (en)* | 2001-11-08 | 2004-09-09 | Sing Eduardo Chi | Pledget-handling system and method for delivering hemostasis promoting material to a blood vessel puncture site by fluid pressure |
| US20040236211A1 (en)* | 2003-05-23 | 2004-11-25 | Senorx, Inc. | Marker or filler forming fluid |
| US20040236213A1 (en)* | 2003-05-23 | 2004-11-25 | Senorx, Inc. | Marker delivery device with releasable plug |
| US20040267155A1 (en)* | 1998-06-22 | 2004-12-30 | Fulton Richard Eustis | Biopsy localization method and device |
| US20050010248A1 (en)* | 2003-07-10 | 2005-01-13 | Scimed Life Systems, Inc. | System for closing an opening in a body cavity |
| US6846320B2 (en) | 1998-05-01 | 2005-01-25 | Sub-Q, Inc. | Device and method for facilitating hemostasis of a biopsy tract |
| US20050019262A1 (en)* | 2003-07-25 | 2005-01-27 | Rubicor Medical, Inc. | Post-biopsy cavity treatment implants and methods |
| US20050020899A1 (en)* | 2003-07-25 | 2005-01-27 | Rubicor Medical, Inc. | Post-biopsy cavity treatmetn implants and methods |
| US20050033360A1 (en)* | 2001-11-08 | 2005-02-10 | Sing Eduardo Chi | Pledget-handling system and method for delivering hemostasis promoting material to a blood vessel puncture site by fluid pressure |
| US6862470B2 (en) | 1999-02-02 | 2005-03-01 | Senorx, Inc. | Cavity-filling biopsy site markers |
| US20050059080A1 (en)* | 1998-05-01 | 2005-03-17 | Sing Eduardo Chi | Absorbable sponge with contrasting agent |
| US20050119562A1 (en)* | 2003-05-23 | 2005-06-02 | Senorx, Inc. | Fibrous marker formed of synthetic polymer strands |
| US20050234336A1 (en)* | 2004-03-26 | 2005-10-20 | Beckman Andrew T | Apparatus and method for marking tissue |
| US6964658B2 (en) | 2000-05-12 | 2005-11-15 | Sub-Q, Inc. | System and method for facilitating hemostasis of blood vessel punctures with absorbable sponge |
| US6984219B2 (en) | 1999-09-23 | 2006-01-10 | Mark Ashby | Depth and puncture control for blood vessel hemostasis system |
| US7008440B2 (en) | 2001-11-08 | 2006-03-07 | Sub-Q, Inc. | System and method for delivering hemostasis promoting material to a blood vessel puncture site by fluid pressure |
| US7029489B1 (en) | 2001-05-18 | 2006-04-18 | Sub-Q, Inc. | System and method for delivering hemostasis promoting material to a blood vessel puncture site |
| US7037322B1 (en) | 2001-11-08 | 2006-05-02 | Sub-Q, Inc. | System and method for delivering hemostasis promoting material to a blood vessel puncture with a staging tube |
| US7048710B1 (en) | 1998-05-01 | 2006-05-23 | Sub-Q, Inc. | System and method for facilitating hemostasis of blood vessel punctures with absorbable sponge |
| US7201725B1 (en) | 2000-09-25 | 2007-04-10 | Sub-Q, Inc. | Device and method for determining a depth of an incision |
| US20070129630A1 (en)* | 2005-12-07 | 2007-06-07 | Shimko Daniel A | Imaging method, device and system |
| US20070135706A1 (en)* | 2005-12-13 | 2007-06-14 | Shimko Daniel A | Debridement method, device and kit |
| WO2007045913A3 (en)* | 2005-10-22 | 2007-09-27 | Invibio Ltd | Fiducial marker |
| US20080039819A1 (en)* | 2006-08-04 | 2008-02-14 | Senorx, Inc. | Marker formed of starch or other suitable polysaccharide |
| US7335219B1 (en) | 2002-11-04 | 2008-02-26 | Sub-Q, Inc. | Hemostatic device including a capsule |
| US20080234532A1 (en)* | 2005-10-22 | 2008-09-25 | Invibio Limited | Fiducial marker |
| US20080254298A1 (en)* | 2006-02-23 | 2008-10-16 | Meadwestvaco Corporation | Method for treating a substrate |
| US20090030309A1 (en)* | 2007-07-26 | 2009-01-29 | Senorx, Inc. | Deployment of polysaccharide markers |
| US7625352B1 (en) | 1998-05-01 | 2009-12-01 | Sub-Q, Inc. | Depth and puncture control for system for hemostasis of blood vessel |
| US7695492B1 (en) | 1999-09-23 | 2010-04-13 | Boston Scientific Scimed, Inc. | Enhanced bleed back system |
| US7744852B2 (en) | 2003-07-25 | 2010-06-29 | Rubicor Medical, Llc | Methods and systems for marking post biopsy cavity sites |
| US20100234726A1 (en)* | 1998-12-24 | 2010-09-16 | Sirimanne D Laksen | Device and method for safe location and marking of a biopsy cavity |
| US7875043B1 (en) | 2003-12-09 | 2011-01-25 | Sub-Q, Inc. | Cinching loop |
| US7955353B1 (en) | 2002-11-04 | 2011-06-07 | Sub-Q, Inc. | Dissolvable closure device |
| US20110166550A1 (en)* | 2008-04-25 | 2011-07-07 | Zymogenetics, Inc. | Medical devices for delivering fluids during surgery and methods for their use |
| WO2011163591A1 (en)* | 2010-06-25 | 2011-12-29 | Kci Licensing, Inc. | Systems and methods for imaging sinuses |
| US8157862B2 (en) | 1997-10-10 | 2012-04-17 | Senorx, Inc. | Tissue marking implant |
| US8311610B2 (en) | 2008-01-31 | 2012-11-13 | C. R. Bard, Inc. | Biopsy tissue marker |
| US8317821B1 (en) | 2002-11-04 | 2012-11-27 | Boston Scientific Scimed, Inc. | Release mechanism |
| US8361082B2 (en) | 1999-02-02 | 2013-01-29 | Senorx, Inc. | Marker delivery device with releasable plug |
| US8401622B2 (en) | 2006-12-18 | 2013-03-19 | C. R. Bard, Inc. | Biopsy marker with in situ-generated imaging properties |
| US8437834B2 (en) | 2006-10-23 | 2013-05-07 | C. R. Bard, Inc. | Breast marker |
| US8486028B2 (en) | 2005-10-07 | 2013-07-16 | Bard Peripheral Vascular, Inc. | Tissue marking apparatus having drug-eluting tissue marker |
| US8498693B2 (en) | 1999-02-02 | 2013-07-30 | Senorx, Inc. | Intracorporeal marker and marker delivery device |
| US8579931B2 (en) | 1999-06-17 | 2013-11-12 | Bard Peripheral Vascular, Inc. | Apparatus for the percutaneous marking of a lesion |
| US8634899B2 (en) | 2003-11-17 | 2014-01-21 | Bard Peripheral Vascular, Inc. | Multi mode imaging marker |
| US8670818B2 (en) | 2008-12-30 | 2014-03-11 | C. R. Bard, Inc. | Marker delivery device for tissue marker placement |
| US8668737B2 (en) | 1997-10-10 | 2014-03-11 | Senorx, Inc. | Tissue marking implant |
| US8718745B2 (en) | 2000-11-20 | 2014-05-06 | Senorx, Inc. | Tissue site markers for in vivo imaging |
| USD715442S1 (en) | 2013-09-24 | 2014-10-14 | C. R. Bard, Inc. | Tissue marker for intracorporeal site identification |
| USD715942S1 (en) | 2013-09-24 | 2014-10-21 | C. R. Bard, Inc. | Tissue marker for intracorporeal site identification |
| USD716451S1 (en) | 2013-09-24 | 2014-10-28 | C. R. Bard, Inc. | Tissue marker for intracorporeal site identification |
| USD716450S1 (en) | 2013-09-24 | 2014-10-28 | C. R. Bard, Inc. | Tissue marker for intracorporeal site identification |
| US9327061B2 (en) | 2008-09-23 | 2016-05-03 | Senorx, Inc. | Porous bioabsorbable implant |
| EP3073252A1 (en)* | 2015-03-27 | 2016-09-28 | Delavan, Inc. | Systems and methods for radiographic inspection |
| US9579077B2 (en) | 2006-12-12 | 2017-02-28 | C.R. Bard, Inc. | Multiple imaging mode tissue marker |
| US9669113B1 (en) | 1998-12-24 | 2017-06-06 | Devicor Medical Products, Inc. | Device and method for safe location and marking of a biopsy cavity |
| US9820824B2 (en) | 1999-02-02 | 2017-11-21 | Senorx, Inc. | Deployment of polysaccharide markers for treating a site within a patent |
| US9848956B2 (en) | 2002-11-18 | 2017-12-26 | Bard Peripheral Vascular, Inc. | Self-contained, self-piercing, side-expelling marking apparatus |
| US20180008872A1 (en)* | 2014-02-20 | 2018-01-11 | Parsons Xtreme Golf, LLC | Golf club heads and methods to manufacture golf club heads |
| US10071181B1 (en) | 2015-04-17 | 2018-09-11 | Teleflex Innovations S.À.R.L. | Resorbable embolization spheres |
| US10342635B2 (en) | 2005-04-20 | 2019-07-09 | Bard Peripheral Vascular, Inc. | Marking device with retractable cannula |
| US10643371B2 (en) | 2014-08-11 | 2020-05-05 | Covidien Lp | Treatment procedure planning system and method |
| US11883246B2 (en)* | 2012-11-21 | 2024-01-30 | Trustees Of Boston University | Tissue markers and uses thereof |
Families Citing this family (7)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US6863680B2 (en)* | 2001-11-08 | 2005-03-08 | Sub-Q, Inc. | System and method for delivering hemostasis promoting material to a blood vessel puncture site by fluid pressure |
| EP1553877A4 (en)* | 2002-05-06 | 2007-04-04 | Univ Drexel | TISSUE BONDING DEVICES CAPABLE OF DELIVERING BIOACTIVE AGENTS AND METHODS OF USE THEREOF |
| AU2003275607A1 (en)* | 2002-10-24 | 2004-05-13 | Terumo Kabushiki Kaisha | Method of producing syringe, cap, and prefilled syringe |
| US9642658B2 (en) | 2008-10-15 | 2017-05-09 | Orthoclip Llc | Device and method for delivery of therapeutic agents via internal implants |
| JP2014503811A (en) | 2010-12-08 | 2014-02-13 | エクスプレッション、パソロジー、インコーポレイテッド | Cleaved Her2SRM / MRM assay |
| US20190247638A1 (en)* | 2018-02-13 | 2019-08-15 | Kieran P. Murphy | Delivery system for delivering a drug depot to a target site under image guidance and methods and uses of same |
| US12251491B2 (en) | 2019-03-20 | 2025-03-18 | Astellas Pharma Inc. | Thrombin-carrying hemostatic sheet |
Citations (76)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US581235A (en) | 1897-04-20 | Island | ||
| US1578517A (en) | 1924-12-23 | 1926-03-30 | George N Hein | Valve piston and barrel construction for hypodermic syringes |
| US2086580A (en) | 1935-06-24 | 1937-07-13 | Myron C Shirley | Applicator |
| US2465357A (en) | 1944-08-14 | 1949-03-29 | Upjohn Co | Therapeutic sponge and method of making |
| US2492458A (en) | 1944-12-08 | 1949-12-27 | Jr Edgar A Bering | Fibrin foam |
| US2507244A (en) | 1947-04-14 | 1950-05-09 | Upjohn Co | Surgical gelatin dusting powder and process for preparing same |
| US2558395A (en) | 1947-06-03 | 1951-06-26 | Hoffmann La Roche | Undenatured gelatin hemostatic sponge containing thrombin |
| US2597011A (en) | 1950-07-28 | 1952-05-20 | Us Agriculture | Preparation of starch sponge |
| US2680442A (en) | 1952-04-04 | 1954-06-08 | Frank L Linzmayer | Disposable suppository casing |
| US2761446A (en) | 1955-03-30 | 1956-09-04 | Chemical Specialties Co Inc | Implanter and cartridge |
| US2814294A (en) | 1953-04-17 | 1957-11-26 | Becton Dickinson Co | Unit for and method of inhibiting and controlling bleeding tendencies |
| US2824092A (en) | 1955-01-04 | 1958-02-18 | Robert E Thompson | Process of preparation of a gelatincarboxymethyl cellulose complex |
| US2899362A (en) | 1959-08-11 | Hemostatic sponges and method of | ||
| US3157524A (en) | 1960-10-25 | 1964-11-17 | Ethicon Inc | Preparation of collagen sponge |
| US4000741A (en) | 1975-11-03 | 1977-01-04 | The Kendall Company | Syringe assembly |
| GB1509023A (en) | 1973-02-12 | 1978-04-26 | Ochsner Med Found Alton | Septal defect closure apparatus |
| GB1569660A (en) | 1976-07-30 | 1980-06-18 | Medline Ab | Occlusion of body channels |
| SU782814A1 (en) | 1977-01-18 | 1980-11-30 | За витель | Prosthesis for closing defect in heart tissues |
| EP0032826A2 (en) | 1980-01-18 | 1981-07-29 | Shiley Incorporated | Vein distention apparatus |
| US4340066A (en) | 1980-02-01 | 1982-07-20 | Sherwood Medical Industries Inc. | Medical device for collecting a body sample |
| US4390018A (en) | 1980-09-15 | 1983-06-28 | Zukowski Henry J | Method for preventing loss of spinal fluid after spinal tap |
| SU1088709A1 (en) | 1981-02-10 | 1984-04-30 | Институт Клинической И Экспериментальной Хирургии | Method of treatment of stomach fistula |
| US4515637A (en) | 1983-11-16 | 1985-05-07 | Seton Company | Collagen-thrombin compositions |
| US4588395A (en) | 1978-03-10 | 1986-05-13 | Lemelson Jerome H | Catheter and method |
| US4587969A (en) | 1985-01-28 | 1986-05-13 | Rolando Gillis | Support assembly for a blood vessel or like organ |
| US4619261A (en) | 1984-08-09 | 1986-10-28 | Frederico Guerriero | Hydrostatic pressure device for bleeding control through an inflatable, stitchable and retrievable balloon-net system |
| US4619913A (en) | 1984-05-29 | 1986-10-28 | Matrix Pharmaceuticals, Inc. | Treatments employing drug-containing matrices for introduction into cellular lesion areas |
| US4645488A (en) | 1982-08-12 | 1987-02-24 | Board Of Trustees Of The University Of Alabama | Syringe for extrusion of wetted, particulate material |
| US4744364A (en) | 1987-02-17 | 1988-05-17 | Intravascular Surgical Instruments, Inc. | Device for sealing percutaneous puncture in a vessel |
| US4790819A (en) | 1987-08-24 | 1988-12-13 | American Cyanamid Company | Fibrin clot delivery device and method |
| US4829994A (en) | 1987-05-27 | 1989-05-16 | Kurth Paul A | Femoral compression device for post-catheterization hemostasis |
| US4850960A (en) | 1987-07-08 | 1989-07-25 | Joseph Grayzel | Diagonally tapered, bevelled tip introducing catheter and sheath and method for insertion |
| US4852568A (en) | 1987-02-17 | 1989-08-01 | Kensey Nash Corporation | Method and apparatus for sealing an opening in tissue of a living being |
| US4890612A (en) | 1987-02-17 | 1990-01-02 | Kensey Nash Corporation | Device for sealing percutaneous puncture in a vessel |
| US4900303A (en) | 1978-03-10 | 1990-02-13 | Lemelson Jerome H | Dispensing catheter and method |
| US4929246A (en) | 1988-10-27 | 1990-05-29 | C. R. Bard, Inc. | Method for closing and sealing an artery after removing a catheter |
| US4936835A (en) | 1988-05-26 | 1990-06-26 | Haaga John R | Medical needle with bioabsorbable tip |
| FR2641692A1 (en) | 1989-01-17 | 1990-07-20 | Nippon Zeon Co | Plug for closing an opening for a medical application, and device for the closure plug making use thereof |
| US4950234A (en) | 1987-05-26 | 1990-08-21 | Sumitomo Pharmaceuticals Company, Limited | Device for administering solid preparations |
| US5007895A (en) | 1989-04-05 | 1991-04-16 | Burnett George S | Wound packing instrument |
| US5021059A (en) | 1990-05-07 | 1991-06-04 | Kensey Nash Corporation | Plug device with pulley for sealing punctures in tissue and methods of use |
| US5053046A (en) | 1988-08-22 | 1991-10-01 | Woodrow W. Janese | Dural sealing needle and method of use |
| US5061274A (en) | 1989-12-04 | 1991-10-29 | Kensey Nash Corporation | Plug device for sealing openings and method of use |
| US5080655A (en) | 1988-05-26 | 1992-01-14 | Haaga John R | Medical biopsy needle |
| EP0476178A1 (en) | 1990-09-21 | 1992-03-25 | Bioplex Medical B.V. | Device for placing styptic material on perforated blood vessels |
| US5108421A (en) | 1990-10-01 | 1992-04-28 | Quinton Instrument Company | Insertion assembly and method of inserting a vessel plug into the body of a patient |
| EP0482350A2 (en) | 1990-09-21 | 1992-04-29 | Datascope Investment Corp. | Device for sealing puncture wounds |
| US5163904A (en) | 1991-11-12 | 1992-11-17 | Merit Medical Systems, Inc. | Syringe apparatus with attached pressure gauge |
| US5167624A (en) | 1990-11-09 | 1992-12-01 | Catheter Research, Inc. | Embolus delivery system and method |
| US5192300A (en) | 1990-10-01 | 1993-03-09 | Quinton Instrument Company | Insertion assembly and method of inserting a vessel plug into the body of a patient |
| US5195988A (en) | 1988-05-26 | 1993-03-23 | Haaga John R | Medical needle with removable sheath |
| US5221259A (en) | 1990-12-27 | 1993-06-22 | Novoste Corporation | Wound treating device and method of using same |
| US5220926A (en) | 1992-07-13 | 1993-06-22 | Jones George T | Finger mounted core biopsy guide |
| US5310407A (en) | 1991-06-17 | 1994-05-10 | Datascope Investment Corp. | Laparoscopic hemostat delivery system and method for using said system |
| US5325857A (en) | 1993-07-09 | 1994-07-05 | Hossein Nabai | Skin biopsy device and method |
| US5334216A (en) | 1992-12-10 | 1994-08-02 | Howmedica Inc. | Hemostatic plug |
| US5366480A (en) | 1990-12-24 | 1994-11-22 | American Cyanamid Company | Synthetic elastomeric buttressing pledget |
| US5383896A (en) | 1993-05-25 | 1995-01-24 | Gershony; Gary | Vascular sealing device |
| US5383899A (en) | 1993-09-28 | 1995-01-24 | Hammerslag; Julius G. | Method of using a surface opening adhesive sealer |
| US5388588A (en) | 1993-05-04 | 1995-02-14 | Nabai; Hossein | Biopsy wound closure device and method |
| US5419765A (en) | 1990-12-27 | 1995-05-30 | Novoste Corporation | Wound treating device and method for treating wounds |
| US5431639A (en) | 1993-08-12 | 1995-07-11 | Boston Scientific Corporation | Treating wounds caused by medical procedures |
| US5486195A (en) | 1993-07-26 | 1996-01-23 | Myers; Gene | Method and apparatus for arteriotomy closure |
| WO1996008208A1 (en) | 1994-09-16 | 1996-03-21 | Biopsys Medical, Inc. | Methods and devices for defining and marking tissue |
| US5522850A (en) | 1994-06-23 | 1996-06-04 | Incontrol, Inc. | Defibrillation and method for cardioverting a heart and storing related activity data |
| US5526822A (en) | 1994-03-24 | 1996-06-18 | Biopsys Medical, Inc. | Method and apparatus for automated biopsy and collection of soft tissue |
| US5540715A (en) | 1992-07-16 | 1996-07-30 | Sherwood Medical Company | Device for sealing hemostatic incisions |
| US5545178A (en) | 1994-04-29 | 1996-08-13 | Kensey Nash Corporation | System for closing a percutaneous puncture formed by a trocar to prevent tissue at the puncture from herniating |
| US5558853A (en) | 1993-01-25 | 1996-09-24 | Sonus Pharmaceuticals | Phase shift colloids as ultrasound contrast agents |
| US5645566A (en) | 1995-09-15 | 1997-07-08 | Sub Q Inc. | Apparatus and method for percutaneous sealing of blood vessel punctures |
| US5649547A (en) | 1994-03-24 | 1997-07-22 | Biopsys Medical, Inc. | Methods and devices for automated biopsy and collection of soft tissue |
| US5653730A (en) | 1993-09-28 | 1997-08-05 | Hemodynamics, Inc. | Surface opening adhesive sealer |
| US5681279A (en) | 1996-11-04 | 1997-10-28 | Roper; David H. | Pill dispensing syringe |
| WO1998006346A1 (en) | 1996-08-12 | 1998-02-19 | Biopsys Medical, Inc. | Apparatus and method for marking tissue |
| US5769086A (en) | 1995-12-06 | 1998-06-23 | Biopsys Medical, Inc. | Control system and method for automated biopsy device |
| US5810806A (en) | 1996-08-29 | 1998-09-22 | Ethicon Endo-Surgery | Methods and devices for collection of soft tissue |
Family Cites Families (119)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US451094A (en)* | 1891-04-28 | Sectional steam-generator | ||
| US2370319A (en)* | 1944-11-07 | 1945-02-27 | Dohner & Lippincott | Paper perforator |
| US2874776A (en)* | 1954-06-07 | 1959-02-24 | Royal Mcbee Corp | Punch and die mechanism |
| GB858477A (en) | 1959-07-16 | 1961-01-11 | Yat Chuen Yuen | Improvements in or relating to drinking straws |
| US3358689A (en) | 1964-06-09 | 1967-12-19 | Roehr Products Company Inc | Integral lancet and package |
| US3411505A (en) | 1965-12-15 | 1968-11-19 | Paul D. Nobis | Device for interrupting arterial flow |
| US3703174A (en) | 1970-07-14 | 1972-11-21 | Medidyne Corp | Method and apparatus for catheter injection |
| US3724465A (en)* | 1971-07-22 | 1973-04-03 | Kimberly Clark Co | Tampon coated with insertion aid and method for coating |
| US3736939A (en)* | 1972-01-07 | 1973-06-05 | Kendall & Co | Balloon catheter with soluble tip |
| US4098728A (en) | 1976-01-02 | 1978-07-04 | Solomon Rosenblatt | Medical surgical sponge and method of making same |
| US4218155A (en) | 1978-02-10 | 1980-08-19 | Etablissements Armor, S.A. | Stick for applying a liquid |
| US4238480A (en) | 1978-05-19 | 1980-12-09 | Sawyer Philip Nicholas | Method for preparing an improved hemostatic agent and method of employing the same |
| US4404970A (en) | 1978-05-19 | 1983-09-20 | Sawyer Philip Nicholas | Hemostatic article and methods for preparing and employing the same |
| US4224945A (en) | 1978-08-30 | 1980-09-30 | Jonathan Cohen | Inflatable expansible surgical pressure dressing |
| US4219026A (en) | 1978-09-15 | 1980-08-26 | The Kendall Company | Bladder hemostatic catheter |
| US4211323A (en) | 1978-12-01 | 1980-07-08 | California Medical Developments, Inc. | Disposable diagnostic swab having a stored culture medium |
| US4292972A (en) | 1980-07-09 | 1981-10-06 | E. R. Squibb & Sons, Inc. | Lyophilized hydrocolloio foam |
| US4405314A (en) | 1982-04-19 | 1983-09-20 | Cook Incorporated | Apparatus and method for catheterization permitting use of a smaller gage needle |
| EP0135334A1 (en) | 1983-08-13 | 1985-03-27 | Arthur Morris | Fountain |
| US4573576A (en)* | 1983-10-27 | 1986-03-04 | Krol Thomas C | Percutaneous gastrostomy kit |
| JPS6144825A (en)* | 1984-08-09 | 1986-03-04 | Unitika Ltd | Hemostatic agent |
| US4573573A (en)* | 1985-01-02 | 1986-03-04 | Lori Favaro | Protective covering for portable audio devices |
| US4869143A (en) | 1985-06-11 | 1989-09-26 | Merrick Industries, Inc. | Card file punch |
| US4708718A (en) | 1985-07-02 | 1987-11-24 | Target Therapeutics | Hyperthermic treatment of tumors |
| US4644649A (en)* | 1985-09-26 | 1987-02-24 | Seaman Roy C | Apparatus for trimming reeds of musical instruments |
| JPS62236560A (en)* | 1986-04-09 | 1987-10-16 | テルモ株式会社 | Catheter for repairing blood vessel |
| US4699616A (en) | 1986-06-13 | 1987-10-13 | Hollister Incorporated | Catheter retention device and method |
| US4839204A (en)* | 1987-05-19 | 1989-06-13 | Yazaki Kakoh Co., Ltd. | Resin coated metal pipe having a plane surface for a lightweight structure |
| US4836204A (en)* | 1987-07-06 | 1989-06-06 | Landymore Roderick W | Method for effecting closure of a perforation in the septum of the heart |
| US5129889A (en) | 1987-11-03 | 1992-07-14 | Hahn John L | Synthetic absorbable epidural catheter |
| US5330445A (en) | 1988-05-26 | 1994-07-19 | Haaga John R | Sheath for wound closure caused by a medical tubular device |
| US5254105A (en) | 1988-05-26 | 1993-10-19 | Haaga John R | Sheath for wound closure caused by a medical tubular device |
| US5219899A (en)* | 1989-04-22 | 1993-06-15 | Degussa Aktiengesellschaft | Pasty dental material which is an organopolysilane filler combined with a polymerizable bonding agent |
| US4973312A (en) | 1989-05-26 | 1990-11-27 | Andrew Daniel E | Method and system for inserting spinal catheters |
| US5620461A (en)* | 1989-05-29 | 1997-04-15 | Muijs Van De Moer; Wouter M. | Sealing device |
| DE3922406C1 (en)* | 1989-07-07 | 1990-10-11 | B. Braun Melsungen Ag, 3508 Melsungen, De | |
| US5232453A (en) | 1989-07-14 | 1993-08-03 | E. R. Squibb & Sons, Inc. | Catheter holder |
| GB8916781D0 (en) | 1989-07-21 | 1989-09-06 | Nycomed As | Compositions |
| US5049138A (en) | 1989-11-13 | 1991-09-17 | Boston Scientific Corporation | Catheter with dissolvable tip |
| US5092873A (en) | 1990-02-28 | 1992-03-03 | Devices For Vascular Intervention, Inc. | Balloon configuration for atherectomy catheter |
| US5299581A (en)* | 1990-07-05 | 1994-04-05 | Donnell John T | Intravaginal device |
| US5192290A (en)* | 1990-08-29 | 1993-03-09 | Applied Medical Resources, Inc. | Embolectomy catheter |
| US5458570A (en) | 1991-01-22 | 1995-10-17 | May, Jr.; James W. | Absorbable catheter and method of using the same |
| CA2078530A1 (en) | 1991-09-23 | 1993-03-24 | Jay Erlebacher | Percutaneous arterial puncture seal device and insertion tool therefore |
| JP3356447B2 (en)* | 1991-10-16 | 2002-12-16 | テルモ株式会社 | Vascular lesion embolic material composed of dried polymer gel |
| GB9123524D0 (en) | 1991-11-06 | 1992-01-02 | Tambrands Ltd | Sanitary tampon |
| US5282827A (en) | 1991-11-08 | 1994-02-01 | Kensey Nash Corporation | Hemostatic puncture closure system and method of use |
| US5676689A (en) | 1991-11-08 | 1997-10-14 | Kensey Nash Corporation | Hemostatic puncture closure system including vessel location device and method of use |
| US5433725A (en)* | 1991-12-13 | 1995-07-18 | Unisurge, Inc. | Hand-held surgical device and tools for use therewith, assembly and method |
| US6056768A (en)* | 1992-01-07 | 2000-05-02 | Cates; Christopher U. | Blood vessel sealing system |
| CA2089999A1 (en) | 1992-02-24 | 1993-08-25 | H. Jonathan Tovey | Resilient arm mesh deployer |
| IL105529A0 (en)* | 1992-05-01 | 1993-08-18 | Amgen Inc | Collagen-containing sponges as drug delivery for proteins |
| US5326350A (en)* | 1992-05-11 | 1994-07-05 | Li Shu Tung | Soft tissue closure systems |
| US5443481A (en) | 1992-07-27 | 1995-08-22 | Lee; Benjamin I. | Methods and device for percutaneous sealing of arterial puncture sites |
| US5514379A (en)* | 1992-08-07 | 1996-05-07 | The General Hospital Corporation | Hydrogel compositions and methods of use |
| CZ281454B6 (en) | 1992-11-23 | 1996-10-16 | Milan Mudr. Csc. Krajíček | Aid for non-surgical closing of a hole in a vessel wall |
| US5417699A (en)* | 1992-12-10 | 1995-05-23 | Perclose Incorporated | Device and method for the percutaneous suturing of a vascular puncture site |
| DK0604103T3 (en) | 1992-12-15 | 1999-09-27 | Johnson & Johnson Consumer | Hydrogel laminate, compounds and materials, and processes for their preparation |
| CA2095553A1 (en) | 1993-02-12 | 1994-08-13 | Kimberly-Clark Worldwide, Inc. | Encapsulated catamenial tampon with and without an applicator |
| US5370656A (en) | 1993-02-26 | 1994-12-06 | Merocel Corporation | Throat pack |
| US5320639A (en) | 1993-03-12 | 1994-06-14 | Meadox Medicals, Inc. | Vascular plug delivery system |
| US5322515A (en) | 1993-03-15 | 1994-06-21 | Abbott Laboratories | Luer adapter assembly for emergency syringe |
| US5342388A (en) | 1993-03-25 | 1994-08-30 | Sonia Toller | Method and apparatus for sealing luminal tissue |
| WO1994028800A1 (en) | 1993-06-04 | 1994-12-22 | Kensey Nash Corporation | Hemostatic vessel puncture closure with filament lock |
| ATE141481T1 (en)* | 1993-06-16 | 1996-09-15 | White Spot Ag | DEVICE FOR INTRODUCING FIBRIN GLUE INTO A STITCH CHANNEL |
| US5545175A (en) | 1993-06-18 | 1996-08-13 | Leonard Bloom | Disposable quarded finger scalpel for inserting a line in a patent and lock off therefor |
| US5352211A (en) | 1993-07-11 | 1994-10-04 | Louisville Laboratories | External stability device |
| DE4325969C1 (en) | 1993-08-03 | 1994-09-08 | Aesculap Ag | By-pass instrument |
| US5462561A (en) | 1993-08-05 | 1995-10-31 | Voda; Jan K. | Suture device |
| JPH09504719A (en) | 1993-11-03 | 1997-05-13 | クラリオン、ファーマシューティカルズ、インコーポレイテッド | Hemostatic patch |
| US5437292A (en) | 1993-11-19 | 1995-08-01 | Bioseal, Llc | Method for sealing blood vessel puncture sites |
| US5507279A (en)* | 1993-11-30 | 1996-04-16 | Fortune; John B. | Retrograde endotracheal intubation kit |
| US5385550A (en)* | 1994-03-29 | 1995-01-31 | Su; Chan-Ho | Needle protective means for prevention against stab and virus infection |
| AU1999995A (en) | 1994-04-08 | 1995-11-10 | Atrix Laboratories, Inc. | An adjunctive polymer system for use with medical device |
| US5554029A (en) | 1994-05-31 | 1996-09-10 | Medical Laser Technology, Inc. | Dental laser apparatus and method for ablating non-metallic dental material from a tooth |
| WO1995032671A1 (en) | 1994-06-01 | 1995-12-07 | Perclose, Inc. | Method and device for providing vascular hemostasis |
| WO1995032669A1 (en) | 1994-06-01 | 1995-12-07 | Perclose, Inc. | Apparatus and method for advancing surgical knots |
| WO1996004954A1 (en)* | 1994-08-17 | 1996-02-22 | Boston Scientific Corporation | Implant, and method and device for inserting the implant |
| US5931165A (en) | 1994-09-06 | 1999-08-03 | Fusion Medical Technologies, Inc. | Films having improved characteristics and methods for their preparation and use |
| US5490736A (en)* | 1994-09-08 | 1996-02-13 | Habley Medical Technology Corporation | Stylus applicator for a rehydrated multi-constituent medication |
| US5527332A (en) | 1994-11-02 | 1996-06-18 | Mectra Labs, Inc. | Tissue cutter for surgery |
| US5624402A (en)* | 1994-12-12 | 1997-04-29 | Becton, Dickinson And Company | Syringe tip cap |
| US5462194A (en) | 1995-01-11 | 1995-10-31 | Candea Inc. | Self-venting straw tip |
| WO1996022123A1 (en)* | 1995-01-18 | 1996-07-25 | Medchem Products, Inc. | Apparatus and method for applying a hemostatic agent onto a tissue |
| US5571168A (en) | 1995-04-05 | 1996-11-05 | Scimed Lifesystems Inc | Pull back stent delivery system |
| US5578097A (en) | 1995-08-28 | 1996-11-26 | Norton Company | Washable coated abrasives |
| US6183497B1 (en)* | 1998-05-01 | 2001-02-06 | Sub-Q, Inc. | Absorbable sponge with contrasting agent |
| US6071300A (en)* | 1995-09-15 | 2000-06-06 | Sub-Q Inc. | Apparatus and method for percutaneous sealing of blood vessel punctures |
| US6162192A (en) | 1998-05-01 | 2000-12-19 | Sub Q, Inc. | System and method for facilitating hemostasis of blood vessel punctures with absorbable sponge |
| US6071301A (en) | 1998-05-01 | 2000-06-06 | Sub Q., Inc. | Device and method for facilitating hemostasis of a biopsy tract |
| US5800389A (en) | 1996-02-09 | 1998-09-01 | Emx, Inc. | Biopsy device |
| US5601207A (en)* | 1996-03-13 | 1997-02-11 | Paczonay; Joseph R. | Bite valve having a plurality of slits |
| US5827218A (en) | 1996-04-18 | 1998-10-27 | Stryker Corporation | Surgical suction pool tip |
| US5858008A (en)* | 1997-04-22 | 1999-01-12 | Becton, Dickinson And Company | Cannula sealing shield assembly |
| US6066325A (en)* | 1996-08-27 | 2000-05-23 | Fusion Medical Technologies, Inc. | Fragmented polymeric compositions and methods for their use |
| US6063061A (en)* | 1996-08-27 | 2000-05-16 | Fusion Medical Technologies, Inc. | Fragmented polymeric compositions and methods for their use |
| US6706690B2 (en)* | 1999-06-10 | 2004-03-16 | Baxter Healthcare Corporation | Hemoactive compositions and methods for their manufacture and use |
| US5782861A (en) | 1996-12-23 | 1998-07-21 | Sub Q Inc. | Percutaneous hemostasis device |
| US6197327B1 (en)* | 1997-06-11 | 2001-03-06 | Umd, Inc. | Device and method for treatment of dysmenorrhea |
| US5868762A (en)* | 1997-09-25 | 1999-02-09 | Sub-Q, Inc. | Percutaneous hemostatic suturing device and method |
| US6270464B1 (en) | 1998-06-22 | 2001-08-07 | Artemis Medical, Inc. | Biopsy localization method and device |
| US6033427A (en)* | 1998-01-07 | 2000-03-07 | Lee; Benjamin I. | Method and device for percutaneous sealing of internal puncture sites |
| US6161034A (en) | 1999-02-02 | 2000-12-12 | Senorx, Inc. | Methods and chemical preparations for time-limited marking of biopsy sites |
| US6610026B2 (en) | 1998-05-01 | 2003-08-26 | Sub-Q, Inc. | Method of hydrating a sponge material for delivery to a body |
| US6315753B1 (en) | 1998-05-01 | 2001-11-13 | Sub-Q, Inc. | System and method for facilitating hemostasis of blood vessel punctures with absorbable sponge |
| US6200328B1 (en)* | 1998-05-01 | 2001-03-13 | Sub Q, Incorporated | Device and method for facilitating hemostasis of a biopsy tract |
| US20010045575A1 (en)* | 1998-05-01 | 2001-11-29 | Mark Ashby | Device and method for facilitating hemostasis of a biopsy tract |
| US6126675A (en) | 1999-01-11 | 2000-10-03 | Ethicon, Inc. | Bioabsorbable device and method for sealing vascular punctures |
| JP4271375B2 (en)* | 1999-02-10 | 2009-06-03 | サブ−キュー・インコーポレーテッド | Device and method for facilitating hemostasis in a biopsy duct |
| US6984219B2 (en)* | 1999-09-23 | 2006-01-10 | Mark Ashby | Depth and puncture control for blood vessel hemostasis system |
| DE29920949U1 (en) | 1999-11-29 | 2000-04-27 | Bugge, Mogens, Göteborg | Suction tube for surgical purposes |
| US6547806B1 (en)* | 2000-02-04 | 2003-04-15 | Ni Ding | Vascular sealing device and method of use |
| AU2001255725A1 (en)* | 2000-04-28 | 2001-11-12 | Sub-Q Inc. | Easy cutter |
| US6540735B1 (en)* | 2000-05-12 | 2003-04-01 | Sub-Q, Inc. | System and method for facilitating hemostasis of blood vessel punctures with absorbable sponge |
| US6503222B2 (en)* | 2000-09-28 | 2003-01-07 | Pfizer Inc | Oral dosage dispenser |
| US8187625B2 (en)* | 2001-03-12 | 2012-05-29 | Boston Scientific Scimed, Inc. | Cross-linked gelatin composition comprising a wetting agent |
| US6863680B2 (en)* | 2001-11-08 | 2005-03-08 | Sub-Q, Inc. | System and method for delivering hemostasis promoting material to a blood vessel puncture site by fluid pressure |
| US7008440B2 (en)* | 2001-11-08 | 2006-03-07 | Sub-Q, Inc. | System and method for delivering hemostasis promoting material to a blood vessel puncture site by fluid pressure |
| US7025748B2 (en)* | 2001-11-08 | 2006-04-11 | Boston Scientific Scimed, Inc. | Sheath based blood vessel puncture locator and depth indicator |
- 1999
- 1999-06-17USUS09/335,452patent/US6183497B1/ennot_activeExpired - Lifetime
- 2000
- 2000-06-16CACA002371841Apatent/CA2371841A1/ennot_activeAbandoned
- 2000-06-16EPEP00944716Apatent/EP1191884A1/ennot_activeWithdrawn
- 2000-06-16WOPCT/US2000/016775patent/WO2000078228A1/ennot_activeApplication Discontinuation
- 2000-06-16JPJP2001504297Apatent/JP4460203B2/ennot_activeExpired - Fee Related
- 2000-06-16AUAU58772/00Apatent/AU5877200A/ennot_activeAbandoned
- 2001
- 2001-09-27USUS09/966,611patent/US20020038133A1/ennot_activeAbandoned
- 2004
- 2004-10-29USUS10/978,321patent/US7618567B2/ennot_activeExpired - Fee Related
- 2009
- 2009-10-13USUS12/578,088patent/US20100029908A1/ennot_activeAbandoned
- 2013
- 2013-02-26USUS13/776,952patent/US20130172737A1/ennot_activeAbandoned
Patent Citations (96)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US2899362A (en) | 1959-08-11 | Hemostatic sponges and method of | ||
| US581235A (en) | 1897-04-20 | Island | ||
| US1578517A (en) | 1924-12-23 | 1926-03-30 | George N Hein | Valve piston and barrel construction for hypodermic syringes |
| US2086580A (en) | 1935-06-24 | 1937-07-13 | Myron C Shirley | Applicator |
| US2465357A (en) | 1944-08-14 | 1949-03-29 | Upjohn Co | Therapeutic sponge and method of making |
| US2492458A (en) | 1944-12-08 | 1949-12-27 | Jr Edgar A Bering | Fibrin foam |
| US2507244A (en) | 1947-04-14 | 1950-05-09 | Upjohn Co | Surgical gelatin dusting powder and process for preparing same |
| US2558395A (en) | 1947-06-03 | 1951-06-26 | Hoffmann La Roche | Undenatured gelatin hemostatic sponge containing thrombin |
| US2597011A (en) | 1950-07-28 | 1952-05-20 | Us Agriculture | Preparation of starch sponge |
| US2680442A (en) | 1952-04-04 | 1954-06-08 | Frank L Linzmayer | Disposable suppository casing |
| US2814294A (en) | 1953-04-17 | 1957-11-26 | Becton Dickinson Co | Unit for and method of inhibiting and controlling bleeding tendencies |
| US2824092A (en) | 1955-01-04 | 1958-02-18 | Robert E Thompson | Process of preparation of a gelatincarboxymethyl cellulose complex |
| US2761446A (en) | 1955-03-30 | 1956-09-04 | Chemical Specialties Co Inc | Implanter and cartridge |
| US3157524A (en) | 1960-10-25 | 1964-11-17 | Ethicon Inc | Preparation of collagen sponge |
| GB1509023A (en) | 1973-02-12 | 1978-04-26 | Ochsner Med Found Alton | Septal defect closure apparatus |
| US4000741A (en) | 1975-11-03 | 1977-01-04 | The Kendall Company | Syringe assembly |
| GB1569660A (en) | 1976-07-30 | 1980-06-18 | Medline Ab | Occlusion of body channels |
| SU782814A1 (en) | 1977-01-18 | 1980-11-30 | За витель | Prosthesis for closing defect in heart tissues |
| US4900303A (en) | 1978-03-10 | 1990-02-13 | Lemelson Jerome H | Dispensing catheter and method |
| US4588395A (en) | 1978-03-10 | 1986-05-13 | Lemelson Jerome H | Catheter and method |
| EP0032826A2 (en) | 1980-01-18 | 1981-07-29 | Shiley Incorporated | Vein distention apparatus |
| US4323072A (en) | 1980-01-18 | 1982-04-06 | Shiley, Incorporated | Cannula for a vein distention system |
| US4340066A (en) | 1980-02-01 | 1982-07-20 | Sherwood Medical Industries Inc. | Medical device for collecting a body sample |
| US4390018A (en) | 1980-09-15 | 1983-06-28 | Zukowski Henry J | Method for preventing loss of spinal fluid after spinal tap |
| SU1088709A1 (en) | 1981-02-10 | 1984-04-30 | Институт Клинической И Экспериментальной Хирургии | Method of treatment of stomach fistula |
| US4645488A (en) | 1982-08-12 | 1987-02-24 | Board Of Trustees Of The University Of Alabama | Syringe for extrusion of wetted, particulate material |
| US4515637A (en) | 1983-11-16 | 1985-05-07 | Seton Company | Collagen-thrombin compositions |
| US4619913A (en) | 1984-05-29 | 1986-10-28 | Matrix Pharmaceuticals, Inc. | Treatments employing drug-containing matrices for introduction into cellular lesion areas |
| US4619261A (en) | 1984-08-09 | 1986-10-28 | Frederico Guerriero | Hydrostatic pressure device for bleeding control through an inflatable, stitchable and retrievable balloon-net system |
| US4587969A (en) | 1985-01-28 | 1986-05-13 | Rolando Gillis | Support assembly for a blood vessel or like organ |
| US4890612A (en) | 1987-02-17 | 1990-01-02 | Kensey Nash Corporation | Device for sealing percutaneous puncture in a vessel |
| US4744364A (en) | 1987-02-17 | 1988-05-17 | Intravascular Surgical Instruments, Inc. | Device for sealing percutaneous puncture in a vessel |
| US4852568A (en) | 1987-02-17 | 1989-08-01 | Kensey Nash Corporation | Method and apparatus for sealing an opening in tissue of a living being |
| US4950234A (en) | 1987-05-26 | 1990-08-21 | Sumitomo Pharmaceuticals Company, Limited | Device for administering solid preparations |
| US4829994A (en) | 1987-05-27 | 1989-05-16 | Kurth Paul A | Femoral compression device for post-catheterization hemostasis |
| US4850960A (en) | 1987-07-08 | 1989-07-25 | Joseph Grayzel | Diagonally tapered, bevelled tip introducing catheter and sheath and method for insertion |
| US4790819A (en) | 1987-08-24 | 1988-12-13 | American Cyanamid Company | Fibrin clot delivery device and method |
| US5195988A (en) | 1988-05-26 | 1993-03-23 | Haaga John R | Medical needle with removable sheath |
| US4936835A (en) | 1988-05-26 | 1990-06-26 | Haaga John R | Medical needle with bioabsorbable tip |
| US5080655A (en) | 1988-05-26 | 1992-01-14 | Haaga John R | Medical biopsy needle |
| US5053046A (en) | 1988-08-22 | 1991-10-01 | Woodrow W. Janese | Dural sealing needle and method of use |
| US4929246A (en) | 1988-10-27 | 1990-05-29 | C. R. Bard, Inc. | Method for closing and sealing an artery after removing a catheter |
| FR2641692A1 (en) | 1989-01-17 | 1990-07-20 | Nippon Zeon Co | Plug for closing an opening for a medical application, and device for the closure plug making use thereof |
| US5192301A (en) | 1989-01-17 | 1993-03-09 | Nippon Zeon Co., Ltd. | Closing plug of a defect for medical use and a closing plug device utilizing it |
| US5007895A (en) | 1989-04-05 | 1991-04-16 | Burnett George S | Wound packing instrument |
| US5061274A (en) | 1989-12-04 | 1991-10-29 | Kensey Nash Corporation | Plug device for sealing openings and method of use |
| US5021059A (en) | 1990-05-07 | 1991-06-04 | Kensey Nash Corporation | Plug device with pulley for sealing punctures in tissue and methods of use |
| US5391183A (en) | 1990-09-21 | 1995-02-21 | Datascope Investment Corp | Device and method sealing puncture wounds |
| US5830130A (en) | 1990-09-21 | 1998-11-03 | Datascope Investment Corp. | Device and method for sealing puncture wounds |
| EP0482350A2 (en) | 1990-09-21 | 1992-04-29 | Datascope Investment Corp. | Device for sealing puncture wounds |
| US5437631A (en) | 1990-09-21 | 1995-08-01 | Datascope Investment Corp. | Percutaneous introducer set and method for sealing puncture wounds |
| EP0476178A1 (en) | 1990-09-21 | 1992-03-25 | Bioplex Medical B.V. | Device for placing styptic material on perforated blood vessels |
| US5591204A (en) | 1990-09-21 | 1997-01-07 | Datascope Investment Corp. | Device and method for sealing puncture wounds |
| US5741223A (en) | 1990-09-21 | 1998-04-21 | Datascope Investment Corp. | Device and method for sealing puncture wounds |
| US5725498A (en) | 1990-09-21 | 1998-03-10 | Datascope Investment Corp. | Device and method for sealing puncture wounds |
| US5192300A (en) | 1990-10-01 | 1993-03-09 | Quinton Instrument Company | Insertion assembly and method of inserting a vessel plug into the body of a patient |
| US5716375A (en) | 1990-10-01 | 1998-02-10 | Quinton Instrument Company | Insertion assembly and method of inserting a vessel plug into the body of a patient |
| US5601602A (en) | 1990-10-01 | 1997-02-11 | Quinton Instrument Company | Insertion assembly and method of inserting a vessel plug into the body of a patient |
| US5591205A (en) | 1990-10-01 | 1997-01-07 | Quinton Instrument Company | Insertion assembly and method of inserting a vessel plug into the body of a patient |
| US5275616A (en) | 1990-10-01 | 1994-01-04 | Quinton Instrument Company | Insertion assembly and method of inserting a vessel plug into the body of a patient |
| US5275616B1 (en) | 1990-10-01 | 1996-01-23 | Quinton Instr | Insertion assembly and method of inserting a vessel plug into the body of a patient |
| US5478352A (en) | 1990-10-01 | 1995-12-26 | Quinton Instrument Company | Insertion assembly and method of inserting a vessel plug into the body of a patient |
| US5108421A (en) | 1990-10-01 | 1992-04-28 | Quinton Instrument Company | Insertion assembly and method of inserting a vessel plug into the body of a patient |
| US5167624A (en) | 1990-11-09 | 1992-12-01 | Catheter Research, Inc. | Embolus delivery system and method |
| US5366480A (en) | 1990-12-24 | 1994-11-22 | American Cyanamid Company | Synthetic elastomeric buttressing pledget |
| US5419765A (en) | 1990-12-27 | 1995-05-30 | Novoste Corporation | Wound treating device and method for treating wounds |
| US5221259A (en) | 1990-12-27 | 1993-06-22 | Novoste Corporation | Wound treating device and method of using same |
| US5310407A (en) | 1991-06-17 | 1994-05-10 | Datascope Investment Corp. | Laparoscopic hemostat delivery system and method for using said system |
| US5163904A (en) | 1991-11-12 | 1992-11-17 | Merit Medical Systems, Inc. | Syringe apparatus with attached pressure gauge |
| US5220926A (en) | 1992-07-13 | 1993-06-22 | Jones George T | Finger mounted core biopsy guide |
| US5540715A (en) | 1992-07-16 | 1996-07-30 | Sherwood Medical Company | Device for sealing hemostatic incisions |
| US5334216A (en) | 1992-12-10 | 1994-08-02 | Howmedica Inc. | Hemostatic plug |
| US5558853A (en) | 1993-01-25 | 1996-09-24 | Sonus Pharmaceuticals | Phase shift colloids as ultrasound contrast agents |
| US5388588A (en) | 1993-05-04 | 1995-02-14 | Nabai; Hossein | Biopsy wound closure device and method |
| US5467780A (en) | 1993-05-04 | 1995-11-21 | Nabai; Hossein | Biopsy wound closure device and method |
| US5479936A (en) | 1993-05-04 | 1996-01-02 | Nabai; Hossein | Biopsy wound closure device and method |
| US5383896A (en) | 1993-05-25 | 1995-01-24 | Gershony; Gary | Vascular sealing device |
| US5325857A (en) | 1993-07-09 | 1994-07-05 | Hossein Nabai | Skin biopsy device and method |
| US5486195A (en) | 1993-07-26 | 1996-01-23 | Myers; Gene | Method and apparatus for arteriotomy closure |
| US5431639A (en) | 1993-08-12 | 1995-07-11 | Boston Scientific Corporation | Treating wounds caused by medical procedures |
| US5383899A (en) | 1993-09-28 | 1995-01-24 | Hammerslag; Julius G. | Method of using a surface opening adhesive sealer |
| US5529577A (en) | 1993-09-28 | 1996-06-25 | Hemodynamics, Inc. | Surface opening adhesive sealer |
| US5653730A (en) | 1993-09-28 | 1997-08-05 | Hemodynamics, Inc. | Surface opening adhesive sealer |
| US5665107A (en) | 1993-09-28 | 1997-09-09 | Hemodynamics, Inc. | Surface opening adhesive sealer |
| US5649547A (en) | 1994-03-24 | 1997-07-22 | Biopsys Medical, Inc. | Methods and devices for automated biopsy and collection of soft tissue |
| US5775333A (en) | 1994-03-24 | 1998-07-07 | Ethicon Endo-Surgery, Inc. | Apparatus for automated biopsy and collection of soft tissue |
| US5526822A (en) | 1994-03-24 | 1996-06-18 | Biopsys Medical, Inc. | Method and apparatus for automated biopsy and collection of soft tissue |
| US5545178A (en) | 1994-04-29 | 1996-08-13 | Kensey Nash Corporation | System for closing a percutaneous puncture formed by a trocar to prevent tissue at the puncture from herniating |
| US5522850A (en) | 1994-06-23 | 1996-06-04 | Incontrol, Inc. | Defibrillation and method for cardioverting a heart and storing related activity data |
| WO1996008208A1 (en) | 1994-09-16 | 1996-03-21 | Biopsys Medical, Inc. | Methods and devices for defining and marking tissue |
| US5645566A (en) | 1995-09-15 | 1997-07-08 | Sub Q Inc. | Apparatus and method for percutaneous sealing of blood vessel punctures |
| US5769086A (en) | 1995-12-06 | 1998-06-23 | Biopsys Medical, Inc. | Control system and method for automated biopsy device |
| WO1998006346A1 (en) | 1996-08-12 | 1998-02-19 | Biopsys Medical, Inc. | Apparatus and method for marking tissue |
| US5902310A (en) | 1996-08-12 | 1999-05-11 | Ethicon Endo-Surgery, Inc. | Apparatus and method for marking tissue |
| US5810806A (en) | 1996-08-29 | 1998-09-22 | Ethicon Endo-Surgery | Methods and devices for collection of soft tissue |
| US5681279A (en) | 1996-11-04 | 1997-10-28 | Roper; David H. | Pill dispensing syringe |
Non-Patent Citations (17)
Cited By (223)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US8277391B2 (en) | 1994-09-16 | 2012-10-02 | Devicor Medical Products, Inc. | Methods and devices for defining and marking tissue |
| US20010034528A1 (en)* | 1994-09-16 | 2001-10-25 | Foerster Seth A. | Methods and devices for defining and marking tissue |
| US20050049489A1 (en)* | 1994-09-16 | 2005-03-03 | Foerster Seth A. | Methods for marking a biopsy site |
| US7625397B2 (en) | 1994-09-16 | 2009-12-01 | Ethicon Endo-Surgery, Inc. | Methods for defining and marking tissue |
| US20050165305A1 (en)* | 1994-09-16 | 2005-07-28 | Foerster Seth A. | Methods and devices for defining and marking tissue |
| US20060074443A1 (en)* | 1994-09-16 | 2006-04-06 | Foerster Seth A | Devices and methods for marking a biopsy site |
| US7044957B2 (en) | 1994-09-16 | 2006-05-16 | Ethicon Endo-Surgery, Inc. | Devices for defining and marking tissue |
| US7229417B2 (en) | 1994-09-16 | 2007-06-12 | Ethicon Endo-Surgery, Inc. | Methods for marking a biopsy site |
| US20020193815A1 (en)* | 1994-09-16 | 2002-12-19 | Foerster Seth A. | Methods and devices for defining and marking tissue |
| US20040024304A1 (en)* | 1994-09-16 | 2004-02-05 | Foerster Seth A. | Methods and devices for defining and marking tissue |
| US7175646B2 (en) | 1995-09-15 | 2007-02-13 | Boston Scientific Scimed, Inc. | Apparatus and method for percutaneous sealing of blood vessel punctures |
| US20020156495A1 (en)* | 1995-09-15 | 2002-10-24 | Rodney Brenneman | Apparatus and method for percutaneous sealing of blood vessel punctures |
| US8668737B2 (en) | 1997-10-10 | 2014-03-11 | Senorx, Inc. | Tissue marking implant |
| US8157862B2 (en) | 1997-10-10 | 2012-04-17 | Senorx, Inc. | Tissue marking implant |
| US9039763B2 (en) | 1997-10-10 | 2015-05-26 | Senorx, Inc. | Tissue marking implant |
| US9480554B2 (en) | 1997-10-10 | 2016-11-01 | Senorx, Inc. | Tissue marking implant |
| US10058416B2 (en) | 1997-10-10 | 2018-08-28 | Senorx, Inc. | Tissue marking implant |
| US20050113737A1 (en)* | 1998-05-01 | 2005-05-26 | Mark Ashby | Device and method for facilitating hemostasis of a biopsy tract |
| US6447534B2 (en) | 1998-05-01 | 2002-09-10 | Sub-Q, Inc. | Device and method for facilitating hemostasis of a biopsy tract |
| US8050741B2 (en) | 1998-05-01 | 2011-11-01 | Boston Scientific Scimed, Inc. | Device and method for facilitating hemostasis of a biopsy tract |
| US20030135237A1 (en)* | 1998-05-01 | 2003-07-17 | Cragg Andrew H. | Device, system and method for improving delivery of hemostatic material |
| US7753872B2 (en) | 1998-05-01 | 2010-07-13 | Boston Scientific Scimed, Inc. | Device, system and method for improving delivery of hemostatic material |
| US20100036414A1 (en)* | 1998-05-01 | 2010-02-11 | Sub-Q, Inc. | System and method for facilitating hemostasis of blood vessel punctures with absorbable sponge |
| US7618567B2 (en) | 1998-05-01 | 2009-11-17 | Boston Scientific Scimed, Inc. | Absorbable sponge with contrasting agent |
| US6610026B2 (en) | 1998-05-01 | 2003-08-26 | Sub-Q, Inc. | Method of hydrating a sponge material for delivery to a body |
| US20100029908A1 (en)* | 1998-05-01 | 2010-02-04 | Boston Scientific Scimed, Inc. | Absorbable sponge with contrasting agent |
| US7625352B1 (en) | 1998-05-01 | 2009-12-01 | Sub-Q, Inc. | Depth and puncture control for system for hemostasis of blood vessel |
| US7048710B1 (en) | 1998-05-01 | 2006-05-23 | Sub-Q, Inc. | System and method for facilitating hemostasis of blood vessel punctures with absorbable sponge |
| US6440151B1 (en) | 1998-05-01 | 2002-08-27 | Sub-Q, Inc. | Device and method for facilitating hemostasis of a biopsy tract |
| US20050059080A1 (en)* | 1998-05-01 | 2005-03-17 | Sing Eduardo Chi | Absorbable sponge with contrasting agent |
| US6440153B2 (en) | 1998-05-01 | 2002-08-27 | Sub-Q, Inc. | Device and method for facilitating hemostasis of a biopsy tract |
| US6527734B2 (en) | 1998-05-01 | 2003-03-04 | Sub-Q, Inc. | System and method for facilitating hemostasis of blood vessel punctures with absorbable sponge |
| US7611479B2 (en) | 1998-05-01 | 2009-11-03 | Sub-Q, Inc. | System and method for facilitating hemostasis of blood vessel punctures with absorbable sponge |
| US6846320B2 (en) | 1998-05-01 | 2005-01-25 | Sub-Q, Inc. | Device and method for facilitating hemostasis of a biopsy tract |
| US20030088271A1 (en)* | 1998-05-01 | 2003-05-08 | Cragg Andrew M. | System and method for facilitating hemostasis of blood vessel punctures with absorbable sponge |
| US8292822B2 (en) | 1998-06-22 | 2012-10-23 | Devicor Medical Products, Inc. | Biopsy localization method and device |
| US20040267155A1 (en)* | 1998-06-22 | 2004-12-30 | Fulton Richard Eustis | Biopsy localization method and device |
| US10010380B2 (en) | 1998-06-22 | 2018-07-03 | Devicor Medical Products, Inc. | Biopsy localization method and device |
| US20050045192A1 (en)* | 1998-06-22 | 2005-03-03 | Artemis Medical, Inc. | Biopsy localization method and device |
| US6270464B1 (en) | 1998-06-22 | 2001-08-07 | Artemis Medical, Inc. | Biopsy localization method and device |
| US20040210160A1 (en)* | 1998-06-22 | 2004-10-21 | Fulton Richard E. | Biopsy localization method and device |
| US20040204660A1 (en)* | 1998-06-22 | 2004-10-14 | Artemis Medical, Inc. | Biopsy localization method and device |
| US20050033195A1 (en)* | 1998-06-22 | 2005-02-10 | Fulton Richard E. | Biopsy localization method and device |
| US20060079829A1 (en)* | 1998-06-22 | 2006-04-13 | Fulton Richard E | Biopsy localization method and device |
| US6699205B2 (en) | 1998-06-22 | 2004-03-02 | Artemis Medical, Inc. | Biopsy localization method and device |
| US20050080337A1 (en)* | 1998-12-24 | 2005-04-14 | Vivant Medical, Inc. | Biopsy site marker |
| US20060036159A1 (en)* | 1998-12-24 | 2006-02-16 | Sirimanne D L | Biopsy cavity marking device |
| US20050059888A1 (en)* | 1998-12-24 | 2005-03-17 | Sirimanne D. Laksen | Biopsy cavity marking device and method |
| US6356782B1 (en) | 1998-12-24 | 2002-03-12 | Vivant Medical, Inc. | Subcutaneous cavity marking device and method |
| US20020107437A1 (en)* | 1998-12-24 | 2002-08-08 | Sirimanne D. Laksen | Subcutaneous cavity marking device and method |
| US20050080339A1 (en)* | 1998-12-24 | 2005-04-14 | Vivant Medical, Inc. | Biopsy cavity marking device |
| US7668582B2 (en) | 1998-12-24 | 2010-02-23 | Ethicon Endo-Surgery, Inc. | Biopsy site marker |
| US20050080338A1 (en)* | 1998-12-24 | 2005-04-14 | Sirimanne D. Laksen | Biopsy cavity marking device and method |
| US20050085724A1 (en)* | 1998-12-24 | 2005-04-21 | Vivant Medical, Inc. | Biopsy cavity marking device and method |
| US20100234726A1 (en)* | 1998-12-24 | 2010-09-16 | Sirimanne D Laksen | Device and method for safe location and marking of a biopsy cavity |
| US9986974B2 (en) | 1998-12-24 | 2018-06-05 | Devicor Medical Products, Inc. | Biopsy cavity marking device |
| US8306602B2 (en) | 1998-12-24 | 2012-11-06 | Devicor Medical Products, Inc. | Biopsy cavity marking device |
| US8320993B2 (en)* | 1998-12-24 | 2012-11-27 | Devicor Medical Products, Inc. | Subcutaneous cavity marking device |
| US9380998B2 (en) | 1998-12-24 | 2016-07-05 | Devicor Medical Products, Inc. | Subcutaneous cavity marking device and method |
| US9492570B2 (en) | 1998-12-24 | 2016-11-15 | Devicor Medical Products, Inc. | Device and method for safe location and marking of a biopsy cavity |
| US9669113B1 (en) | 1998-12-24 | 2017-06-06 | Devicor Medical Products, Inc. | Device and method for safe location and marking of a biopsy cavity |
| US8320994B2 (en) | 1998-12-24 | 2012-11-27 | Devicor Medical Products, Inc. | Biopsy cavity marking device and method |
| US20060079770A1 (en)* | 1998-12-24 | 2006-04-13 | Sirimanne D L | Biopsy site marker |
| US8600481B2 (en) | 1998-12-24 | 2013-12-03 | Devicor Medical Products, Inc. | Subcutaneous cavity marking device |
| US8626270B2 (en) | 1999-02-02 | 2014-01-07 | Senorx, Inc. | Cavity-filling biopsy site markers |
| US20040101479A1 (en)* | 1999-02-02 | 2004-05-27 | Senorx, Inc. | Biopsy site marker and process and apparatus for applying it |
| US8965486B2 (en) | 1999-02-02 | 2015-02-24 | Senorx, Inc. | Cavity filling biopsy site markers |
| US20090131825A1 (en)* | 1999-02-02 | 2009-05-21 | Senorx, Inc. | Imageable biopsy site marker |
| US6427081B1 (en)* | 1999-02-02 | 2002-07-30 | Senorx, Inc. | Methods and chemical preparations for time-limited marking of biopsy sites |
| US9649093B2 (en) | 1999-02-02 | 2017-05-16 | Senorx, Inc. | Cavity-filling biopsy site markers |
| US8498693B2 (en) | 1999-02-02 | 2013-07-30 | Senorx, Inc. | Intracorporeal marker and marker delivery device |
| US20060084865A1 (en)* | 1999-02-02 | 2006-04-20 | Burbank Fred H | Imageable biopsy site marker |
| US8361082B2 (en) | 1999-02-02 | 2013-01-29 | Senorx, Inc. | Marker delivery device with releasable plug |
| US20050063908A1 (en)* | 1999-02-02 | 2005-03-24 | Senorx, Inc. | Tissue site markers for in vivo imaging |
| US7047063B2 (en) | 1999-02-02 | 2006-05-16 | Senorx, Inc. | Tissue site markers for in vivo imaging |
| US6862470B2 (en) | 1999-02-02 | 2005-03-01 | Senorx, Inc. | Cavity-filling biopsy site markers |
| US20040116806A1 (en)* | 1999-02-02 | 2004-06-17 | Senorx, Inc. | Biopsy site marker and process and apparatus for applying it |
| US20060122503A1 (en)* | 1999-02-02 | 2006-06-08 | Senorx, Inc. | Imageable biopsy site marker |
| US20060155190A1 (en)* | 1999-02-02 | 2006-07-13 | Senorx, Inc. | Imageable biopsy site marker |
| US9820824B2 (en) | 1999-02-02 | 2017-11-21 | Senorx, Inc. | Deployment of polysaccharide markers for treating a site within a patent |
| US20050143656A1 (en)* | 1999-02-02 | 2005-06-30 | Senorx, Inc. | Cavity-filling biopsy site markers |
| US20100010342A1 (en)* | 1999-02-02 | 2010-01-14 | Senorx, Inc. | Tissue site markers for in vivo imaging |
| US10172674B2 (en) | 1999-02-02 | 2019-01-08 | Senorx, Inc. | Intracorporeal marker and marker delivery device |
| US6996433B2 (en) | 1999-02-02 | 2006-02-07 | Senorx, Inc. | Imageable biopsy site marker |
| US6725083B1 (en)* | 1999-02-02 | 2004-04-20 | Senorx, Inc. | Tissue site markers for in VIVO imaging |
| US9861294B2 (en) | 1999-02-02 | 2018-01-09 | Senorx, Inc. | Marker delivery device with releasable plug |
| US8224424B2 (en) | 1999-02-02 | 2012-07-17 | Senorx, Inc. | Tissue site markers for in vivo imaging |
| US8219182B2 (en) | 1999-02-02 | 2012-07-10 | Senorx, Inc. | Cavity-filling biopsy site markers |
| US9237937B2 (en) | 1999-02-02 | 2016-01-19 | Senorx, Inc. | Cavity-filling biopsy site markers |
| US6662041B2 (en) | 1999-02-02 | 2003-12-09 | Senorx, Inc. | Imageable biopsy site marker |
| US9149341B2 (en) | 1999-02-02 | 2015-10-06 | Senorx, Inc | Deployment of polysaccharide markers for treating a site within a patient |
| US7792569B2 (en) | 1999-02-02 | 2010-09-07 | Senorx, Inc. | Cavity-filling biopsy site markers |
| US6567689B2 (en)* | 1999-02-02 | 2003-05-20 | Senorx, Inc. | Methods and chemical preparations for time-limited marking of biopsy sites |
| US9044162B2 (en) | 1999-02-02 | 2015-06-02 | Senorx, Inc. | Marker delivery device with releasable plug |
| US10463446B2 (en) | 1999-06-17 | 2019-11-05 | Bard Peripheral Vascular, Inc. | Apparatus for the percutaneous marking of a lesion |
| US8579931B2 (en) | 1999-06-17 | 2013-11-12 | Bard Peripheral Vascular, Inc. | Apparatus for the percutaneous marking of a lesion |
| US9579159B2 (en) | 1999-06-17 | 2017-02-28 | Bard Peripheral Vascular, Inc. | Apparatus for the percutaneous marking of a lesion |
| US7695492B1 (en) | 1999-09-23 | 2010-04-13 | Boston Scientific Scimed, Inc. | Enhanced bleed back system |
| US6984219B2 (en) | 1999-09-23 | 2006-01-10 | Mark Ashby | Depth and puncture control for blood vessel hemostasis system |
| US6964658B2 (en) | 2000-05-12 | 2005-11-15 | Sub-Q, Inc. | System and method for facilitating hemostasis of blood vessel punctures with absorbable sponge |
| US7201725B1 (en) | 2000-09-25 | 2007-04-10 | Sub-Q, Inc. | Device and method for determining a depth of an incision |
| US8718745B2 (en) | 2000-11-20 | 2014-05-06 | Senorx, Inc. | Tissue site markers for in vivo imaging |
| US20050106063A1 (en)* | 2001-03-12 | 2005-05-19 | Mark Ashby | Methods for sterilizing cross-linked gelatin compositions |
| US8524270B2 (en) | 2001-03-12 | 2013-09-03 | Boston Scientific Scimed, Inc. | Cross-linked gelatin composition coated with a wetting agent |
| US20020190226A1 (en)* | 2001-03-12 | 2002-12-19 | Mark Ashby | Methods for sterilizing cross-linked gelatin compositions |
| US20030028140A1 (en)* | 2001-03-12 | 2003-02-06 | Greff Richard J. | Cross-linked gelatin composition comprising a wetting agent |
| US7264772B2 (en) | 2001-03-12 | 2007-09-04 | Boston Scientific Scimed, Inc. | Methods for sterilizing cross-linked gelatin compositions |
| US6849232B2 (en) | 2001-03-12 | 2005-02-01 | Sub-Q, Inc. | Methods for sterilizing cross-linked gelatin compositions |
| US8187625B2 (en) | 2001-03-12 | 2012-05-29 | Boston Scientific Scimed, Inc. | Cross-linked gelatin composition comprising a wetting agent |
| US8821918B2 (en) | 2001-03-12 | 2014-09-02 | Boston Scientific Scimed Inc. | Cross-linked gelatin composition comprising a wetting agent |
| US6511457B2 (en)* | 2001-05-04 | 2003-01-28 | Garey Thompson | Airless syringe |
| US7029489B1 (en) | 2001-05-18 | 2006-04-18 | Sub-Q, Inc. | System and method for delivering hemostasis promoting material to a blood vessel puncture site |
| US20030013989A1 (en)* | 2001-06-29 | 2003-01-16 | Joseph Obermiller | Porous sponge matrix medical devices and methods |
| US8877233B2 (en) | 2001-06-29 | 2014-11-04 | Cook Biotech Incorporated | Porous sponge matrix medical devices and methods |
| US20050033360A1 (en)* | 2001-11-08 | 2005-02-10 | Sing Eduardo Chi | Pledget-handling system and method for delivering hemostasis promoting material to a blood vessel puncture site by fluid pressure |
| US7025748B2 (en) | 2001-11-08 | 2006-04-11 | Boston Scientific Scimed, Inc. | Sheath based blood vessel puncture locator and depth indicator |
| US7008440B2 (en) | 2001-11-08 | 2006-03-07 | Sub-Q, Inc. | System and method for delivering hemostasis promoting material to a blood vessel puncture site by fluid pressure |
| US20040176723A1 (en)* | 2001-11-08 | 2004-09-09 | Sing Eduardo Chi | Pledget-handling system and method for delivering hemostasis promoting material to a blood vessel puncture site by fluid pressure |
| US7037322B1 (en) | 2001-11-08 | 2006-05-02 | Sub-Q, Inc. | System and method for delivering hemostasis promoting material to a blood vessel puncture with a staging tube |
| US7037323B2 (en) | 2001-11-08 | 2006-05-02 | Sub-Q, Inc. | Pledget-handling system and method for delivering hemostasis promoting material to a blood vessel puncture site by fluid pressure |
| US7192436B2 (en) | 2001-11-08 | 2007-03-20 | Sub-Q, Inc. | Pledget-handling system and method for delivering hemostasis promoting material to a blood vessel puncture site by fluid pressure |
| US20040019330A1 (en)* | 2001-11-08 | 2004-01-29 | Sub-Q, Inc., A California Corporation | Sheath based blood vessel puncture locator and depth indicator |
| US8784433B2 (en) | 2002-06-17 | 2014-07-22 | Senorx, Inc. | Plugged tip delivery tube for marker placement |
| US7651505B2 (en) | 2002-06-17 | 2010-01-26 | Senorx, Inc. | Plugged tip delivery for marker placement |
| US20030233101A1 (en)* | 2002-06-17 | 2003-12-18 | Senorx, Inc. | Plugged tip delivery tube for marker placement |
| US8177792B2 (en) | 2002-06-17 | 2012-05-15 | Senorx, Inc. | Plugged tip delivery tube for marker placement |
| US20040102730A1 (en)* | 2002-10-22 | 2004-05-27 | Davis Thomas P. | System and method for facilitating hemostasis of blood vessel punctures with absorbable sponge |
| US7955353B1 (en) | 2002-11-04 | 2011-06-07 | Sub-Q, Inc. | Dissolvable closure device |
| US7335219B1 (en) | 2002-11-04 | 2008-02-26 | Sub-Q, Inc. | Hemostatic device including a capsule |
| US8317821B1 (en) | 2002-11-04 | 2012-11-27 | Boston Scientific Scimed, Inc. | Release mechanism |
| US7455680B1 (en) | 2002-11-04 | 2008-11-25 | Boston Scientific Scimed, Inc. | Apparatus and method for inhibiting blood loss |
| US10813716B2 (en) | 2002-11-18 | 2020-10-27 | Bard Peripheral Vascular, Inc. | Self-contained, self-piercing, side-expelling marking apparatus |
| US9848956B2 (en) | 2002-11-18 | 2017-12-26 | Bard Peripheral Vascular, Inc. | Self-contained, self-piercing, side-expelling marking apparatus |
| US8709038B2 (en) | 2002-12-20 | 2014-04-29 | Boston Scientific Scimed, Inc. | Puncture hole sealing device |
| US20040122350A1 (en)* | 2002-12-20 | 2004-06-24 | Sheng-Ping Zhong | Puncture hole sealing device |
| US20040122349A1 (en)* | 2002-12-20 | 2004-06-24 | Lafontaine Daniel M. | Closure device with textured surface |
| US8398677B2 (en) | 2002-12-20 | 2013-03-19 | Boston Scientific Scimed, Inc. | Closure device with textured surface |
| US20100234884A1 (en)* | 2002-12-20 | 2010-09-16 | Boston Scientific Scimed, Inc. | Closure device with textured surface |
| US20050119562A1 (en)* | 2003-05-23 | 2005-06-02 | Senorx, Inc. | Fibrous marker formed of synthetic polymer strands |
| US9801688B2 (en) | 2003-05-23 | 2017-10-31 | Senorx, Inc. | Fibrous marker and intracorporeal delivery thereof |
| US20040236211A1 (en)* | 2003-05-23 | 2004-11-25 | Senorx, Inc. | Marker or filler forming fluid |
| US7877133B2 (en) | 2003-05-23 | 2011-01-25 | Senorx, Inc. | Marker or filler forming fluid |
| US10045832B2 (en) | 2003-05-23 | 2018-08-14 | Senorx, Inc. | Marker or filler forming fluid |
| US10299881B2 (en) | 2003-05-23 | 2019-05-28 | Senorx, Inc. | Marker or filler forming fluid |
| US7970454B2 (en) | 2003-05-23 | 2011-06-28 | Senorx, Inc. | Marker delivery device with releasable plug |
| US20040236213A1 (en)* | 2003-05-23 | 2004-11-25 | Senorx, Inc. | Marker delivery device with releasable plug |
| US20040236212A1 (en)* | 2003-05-23 | 2004-11-25 | Senorx, Inc. | Fibrous marker and intracorporeal delivery thereof |
| US20090287078A1 (en)* | 2003-05-23 | 2009-11-19 | Senorx, Inc. | Marker or filler forming fluid |
| US8447386B2 (en) | 2003-05-23 | 2013-05-21 | Senorx, Inc. | Marker or filler forming fluid |
| US8880154B2 (en) | 2003-05-23 | 2014-11-04 | Senorx, Inc. | Fibrous marker and intracorporeal delivery thereof |
| US7983734B2 (en) | 2003-05-23 | 2011-07-19 | Senorx, Inc. | Fibrous marker and intracorporeal delivery thereof |
| US8626269B2 (en) | 2003-05-23 | 2014-01-07 | Senorx, Inc. | Fibrous marker and intracorporeal delivery thereof |
| US20110237943A1 (en)* | 2003-05-23 | 2011-09-29 | Senorx, Inc. | Fibrous marker and intracorporeal delivery thereof |
| US8639315B2 (en) | 2003-05-23 | 2014-01-28 | Senorx, Inc. | Marker or filler forming fluid |
| US7942897B2 (en) | 2003-07-10 | 2011-05-17 | Boston Scientific Scimed, Inc. | System for closing an opening in a body cavity |
| US20050010248A1 (en)* | 2003-07-10 | 2005-01-13 | Scimed Life Systems, Inc. | System for closing an opening in a body cavity |
| US7537788B2 (en) | 2003-07-25 | 2009-05-26 | Rubicor Medical, Inc. | Post-biopsy cavity treatment implants and methods |
| US7744852B2 (en) | 2003-07-25 | 2010-06-29 | Rubicor Medical, Llc | Methods and systems for marking post biopsy cavity sites |
| US20070299541A1 (en)* | 2003-07-25 | 2007-12-27 | Rubicor Medical, Inc. | Post-biopsy cavity treatment implants and methods |
| WO2005016112A3 (en)* | 2003-07-25 | 2005-09-01 | Rubicor Medical Inc | Post-biopsy cavity treatment implants and methods |
| US7780948B2 (en) | 2003-07-25 | 2010-08-24 | Rubicor Medical, Llc | Post biopsy cavity treatment implants and methods |
| US20050019262A1 (en)* | 2003-07-25 | 2005-01-27 | Rubicor Medical, Inc. | Post-biopsy cavity treatment implants and methods |
| US20090263442A1 (en)* | 2003-07-25 | 2009-10-22 | Rubicor Medical, Inc. | Post Biopsy Cavity Treatment Implants and Methods |
| US8092779B2 (en) | 2003-07-25 | 2012-01-10 | Rubicor Medical, Llc | Post-biopsy cavity treatment implants and methods |
| US8491630B2 (en) | 2003-07-25 | 2013-07-23 | Encapsule Medical, LLC. | Post-biopsy cavity treatment implants and methods |
| US20070299339A1 (en)* | 2003-07-25 | 2007-12-27 | Rubicor Medical, Inc. | Post-biopsy cavity treatment implants and methods |
| US20050020899A1 (en)* | 2003-07-25 | 2005-01-27 | Rubicor Medical, Inc. | Post-biopsy cavity treatmetn implants and methods |
| US20080114329A1 (en)* | 2003-10-16 | 2008-05-15 | Rubicor Medical, Inc. | Post-biopsy cavity treatment implants and methods |
| US7534452B2 (en) | 2003-10-16 | 2009-05-19 | Rubicor Medical, Inc. | Post-biopsy cavity treatment implants and methods |
| US8634899B2 (en) | 2003-11-17 | 2014-01-21 | Bard Peripheral Vascular, Inc. | Multi mode imaging marker |
| US7875043B1 (en) | 2003-12-09 | 2011-01-25 | Sub-Q, Inc. | Cinching loop |
| US20050234336A1 (en)* | 2004-03-26 | 2005-10-20 | Beckman Andrew T | Apparatus and method for marking tissue |
| US11278370B2 (en) | 2005-04-20 | 2022-03-22 | Bard Peripheral Vascular, Inc. | Marking device with retractable cannula |
| US10357328B2 (en) | 2005-04-20 | 2019-07-23 | Bard Peripheral Vascular, Inc. and Bard Shannon Limited | Marking device with retractable cannula |
| US10342635B2 (en) | 2005-04-20 | 2019-07-09 | Bard Peripheral Vascular, Inc. | Marking device with retractable cannula |
| US8486028B2 (en) | 2005-10-07 | 2013-07-16 | Bard Peripheral Vascular, Inc. | Tissue marking apparatus having drug-eluting tissue marker |
| WO2007045913A3 (en)* | 2005-10-22 | 2007-09-27 | Invibio Ltd | Fiducial marker |
| GB2438282B (en)* | 2005-10-22 | 2011-07-20 | Invibio Ltd | Fiducial marker |
| US20080234532A1 (en)* | 2005-10-22 | 2008-09-25 | Invibio Limited | Fiducial marker |
| GB2438282A (en)* | 2005-10-22 | 2007-11-21 | Invibio Ltd | Fiducial marker |
| US20080058641A1 (en)* | 2005-12-07 | 2008-03-06 | Shimko Daniel A | Imaging method, device and system |
| US20070129630A1 (en)* | 2005-12-07 | 2007-06-07 | Shimko Daniel A | Imaging method, device and system |
| US20070135706A1 (en)* | 2005-12-13 | 2007-06-14 | Shimko Daniel A | Debridement method, device and kit |
| US20080254298A1 (en)* | 2006-02-23 | 2008-10-16 | Meadwestvaco Corporation | Method for treating a substrate |
| US20080058640A1 (en)* | 2006-08-04 | 2008-03-06 | Senoxrx, Inc. | Marker formed of starch or other suitable polysaccharide |
| US20080039819A1 (en)* | 2006-08-04 | 2008-02-14 | Senorx, Inc. | Marker formed of starch or other suitable polysaccharide |
| US8437834B2 (en) | 2006-10-23 | 2013-05-07 | C. R. Bard, Inc. | Breast marker |
| US9579077B2 (en) | 2006-12-12 | 2017-02-28 | C.R. Bard, Inc. | Multiple imaging mode tissue marker |
| US10682200B2 (en) | 2006-12-12 | 2020-06-16 | C. R. Bard, Inc. | Multiple imaging mode tissue marker |
| US11471244B2 (en) | 2006-12-12 | 2022-10-18 | C.R. Bard, Inc. | Multiple imaging mode tissue marker |
| US9901415B2 (en) | 2006-12-12 | 2018-02-27 | C. R. Bard, Inc. | Multiple imaging mode tissue marker |
| US9042965B2 (en) | 2006-12-18 | 2015-05-26 | C. R. Bard, Inc. | Biopsy marker with in situ-generated imaging properties |
| US8401622B2 (en) | 2006-12-18 | 2013-03-19 | C. R. Bard, Inc. | Biopsy marker with in situ-generated imaging properties |
| US20090030309A1 (en)* | 2007-07-26 | 2009-01-29 | Senorx, Inc. | Deployment of polysaccharide markers |
| US8311610B2 (en) | 2008-01-31 | 2012-11-13 | C. R. Bard, Inc. | Biopsy tissue marker |
| US9597062B2 (en)* | 2008-04-25 | 2017-03-21 | Mallinckrodt Pharma Ip Trading D.A.C. | Medical devices for delivering fluids during surgery and methods for their use |
| US20110166550A1 (en)* | 2008-04-25 | 2011-07-07 | Zymogenetics, Inc. | Medical devices for delivering fluids during surgery and methods for their use |
| US11833275B2 (en) | 2008-09-23 | 2023-12-05 | Senorx, Inc. | Porous bioabsorbable implant |
| US9327061B2 (en) | 2008-09-23 | 2016-05-03 | Senorx, Inc. | Porous bioabsorbable implant |
| US10786604B2 (en) | 2008-09-23 | 2020-09-29 | Senorx, Inc. | Porous bioabsorbable implant |
| US10258428B2 (en) | 2008-12-30 | 2019-04-16 | C. R. Bard, Inc. | Marker delivery device for tissue marker placement |
| US8670818B2 (en) | 2008-12-30 | 2014-03-11 | C. R. Bard, Inc. | Marker delivery device for tissue marker placement |
| US11779431B2 (en) | 2008-12-30 | 2023-10-10 | C. R. Bard, Inc. | Marker delivery device for tissue marker placement |
| US9198987B2 (en) | 2010-06-25 | 2015-12-01 | Kci Licensing, Inc. | Systems and methods for imaging sinuses |
| WO2011163591A1 (en)* | 2010-06-25 | 2011-12-29 | Kci Licensing, Inc. | Systems and methods for imaging sinuses |
| US12310800B2 (en) | 2012-11-21 | 2025-05-27 | Trustees Of Boston University | Injection applicator for tissue markers |
| US11883246B2 (en)* | 2012-11-21 | 2024-01-30 | Trustees Of Boston University | Tissue markers and uses thereof |
| USD715942S1 (en) | 2013-09-24 | 2014-10-21 | C. R. Bard, Inc. | Tissue marker for intracorporeal site identification |
| USD716451S1 (en) | 2013-09-24 | 2014-10-28 | C. R. Bard, Inc. | Tissue marker for intracorporeal site identification |
| USD715442S1 (en) | 2013-09-24 | 2014-10-14 | C. R. Bard, Inc. | Tissue marker for intracorporeal site identification |
| USD716450S1 (en) | 2013-09-24 | 2014-10-28 | C. R. Bard, Inc. | Tissue marker for intracorporeal site identification |
| US20180008872A1 (en)* | 2014-02-20 | 2018-01-11 | Parsons Xtreme Golf, LLC | Golf club heads and methods to manufacture golf club heads |
| US10874921B2 (en)* | 2014-02-20 | 2020-12-29 | Parsons Xtreme Golf, LLC | Golf club heads and methods to manufacture golf club heads |
| US10643371B2 (en) | 2014-08-11 | 2020-05-05 | Covidien Lp | Treatment procedure planning system and method |
| US11227427B2 (en) | 2014-08-11 | 2022-01-18 | Covidien Lp | Treatment procedure planning system and method |
| US11238642B2 (en) | 2014-08-11 | 2022-02-01 | Covidien Lp | Treatment procedure planning system and method |
| US11769292B2 (en) | 2014-08-11 | 2023-09-26 | Covidien Lp | Treatment procedure planning system and method |
| US9909999B2 (en) | 2015-03-27 | 2018-03-06 | Delavan Inc. | Systems and methods for radiographic inspection |
| EP3073252A1 (en)* | 2015-03-27 | 2016-09-28 | Delavan, Inc. | Systems and methods for radiographic inspection |
| US10620139B2 (en) | 2015-03-27 | 2020-04-14 | Delavan Inc. | Systems and methods for radiographic inspection |
| US10179188B1 (en) | 2015-04-17 | 2019-01-15 | Teleflex Innovations S.À.R.L. | Resorbable embolization spheres |
| US11116867B1 (en) | 2015-04-17 | 2021-09-14 | Teleflex Life Sciences Limited | Resorbable embolization spheres |
| US10071181B1 (en) | 2015-04-17 | 2018-09-11 | Teleflex Innovations S.À.R.L. | Resorbable embolization spheres |
Also Published As
| Publication number | Publication date |
|---|---|
| WO2000078228A1 (en) | 2000-12-28 |
| JP4460203B2 (en) | 2010-05-12 |
| US20130172737A1 (en) | 2013-07-04 |
| WO2000078228A9 (en) | 2002-09-26 |
| EP1191884A1 (en) | 2002-04-03 |
| US7618567B2 (en) | 2009-11-17 |
| US20050059080A1 (en) | 2005-03-17 |
| US20100029908A1 (en) | 2010-02-04 |
| AU5877200A (en) | 2001-01-09 |
| CA2371841A1 (en) | 2000-12-28 |
| US20020038133A1 (en) | 2002-03-28 |
| JP2003502099A (en) | 2003-01-21 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| US6183497B1 (en) | Absorbable sponge with contrasting agent | |
| US8050741B2 (en) | Device and method for facilitating hemostasis of a biopsy tract | |
| US6440151B1 (en) | Device and method for facilitating hemostasis of a biopsy tract | |
| US6447534B2 (en) | Device and method for facilitating hemostasis of a biopsy tract | |
| US6540735B1 (en) | System and method for facilitating hemostasis of blood vessel punctures with absorbable sponge | |
| CA2356890C (en) | Device and method for safe location and marking of a cavity and sentinel lymph nodes | |
| US20130066195A1 (en) | Device and method for safe location and marking of a cavity and sentinel lymph nodes | |
| JP2002513639A (en) | System and method for facilitating hemostasis of vascular puncture with absorbable sponge | |
| EP1383452A1 (en) | Method of hydrating a sponge material for delivery to a body | |
| US9669113B1 (en) | Device and method for safe location and marking of a biopsy cavity | |
| AU2005202005A1 (en) | Absorbable sponge with contrasting agent |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| AS | Assignment | Owner name:SUB-Q, CALIFORNIA Free format text:ASSIGNMENT OF ASSIGNORS INTEREST;ASSIGNORS:SING, EDUARDO CHI;ASHBY, MARK;REEL/FRAME:010047/0025 Effective date:19990617 | |
| FEPP | Fee payment procedure | Free format text:PAYOR NUMBER ASSIGNED (ORIGINAL EVENT CODE: ASPN); ENTITY STATUS OF PATENT OWNER: LARGE ENTITY | |
| STCF | Information on status: patent grant | Free format text:PATENTED CASE | |
| AS | Assignment | Owner name:SUB-O, INC., CALIFORNIA Free format text:CORRECTIVE ASSIGNMENT TO CORRECT THE NAME OF THE ASSIGNEE, FILED ON 6/17/99, RECORDED ON REEL 010047 FRAME 0025;ASSIGNORS:SING, EDUARDO CHI;ASHBY, MARK;REEL/FRAME:012327/0733 Effective date:19990617 | |
| REMI | Maintenance fee reminder mailed | ||
| AS | Assignment | Owner name:BOSTON SCIENTIFIC CORPORATION, MASSACHUSETTS Free format text:NOTE AND WARRANT PURCHASE AGREEMENT;ASSIGNOR:SUB-Q, INC.;REEL/FRAME:015156/0484 Effective date:20040831 Owner name:PACIFIC VENTURE GROUP II, L.P., CALIFORNIA Free format text:NOTE AND WARRANT PURCHASE AGREEMENT;ASSIGNOR:SUB-Q, INC.;REEL/FRAME:015156/0484 Effective date:20040831 Owner name:PVG ASSOCIATES II, L.P., CALIFORNIA Free format text:NOTE AND WARRANT PURCHASE AGREEMENT;ASSIGNOR:SUB-Q, INC.;REEL/FRAME:015156/0484 Effective date:20040831 Owner name:PEQUOT PRIVATE EQUITY FUND II, L.P., CONNECTICUT Free format text:NOTE AND WARRANT PURCHASE AGREEMENT;ASSIGNOR:SUB-Q, INC.;REEL/FRAME:015156/0484 Effective date:20040831 Owner name:KINGSBURY CAPITAL PARTNERS, L.P. III, CALIFORNIA Free format text:NOTE AND WARRANT PURCHASE AGREEMENT;ASSIGNOR:SUB-Q, INC.;REEL/FRAME:015156/0484 Effective date:20040831 Owner name:KINGSBURY CAPITAL PARTNERS, L.P. IV, CALIFORNIA Free format text:NOTE AND WARRANT PURCHASE AGREEMENT;ASSIGNOR:SUB-Q, INC.;REEL/FRAME:015156/0484 Effective date:20040831 Owner name:HARTZLER, GEOFFREY O., KANSAS Free format text:NOTE AND WARRANT PURCHASE AGREEMENT;ASSIGNOR:SUB-Q, INC.;REEL/FRAME:015156/0484 Effective date:20040831 Owner name:WALLACE, GEORGE, CALIFORNIA Free format text:NOTE AND WARRANT PURCHASE AGREEMENT;ASSIGNOR:SUB-Q, INC.;REEL/FRAME:015156/0484 Effective date:20040831 Owner name:CRAGG, ANDREW H., MINNESOTA Free format text:NOTE AND WARRANT PURCHASE AGREEMENT;ASSIGNOR:SUB-Q, INC.;REEL/FRAME:015156/0484 Effective date:20040831 Owner name:ALLEN FAMILY TRUST DATED 10/12/81, CALIFORNIA Free format text:NOTE AND WARRANT PURCHASE AGREEMENT;ASSIGNOR:SUB-Q, INC.;REEL/FRAME:015156/0484 Effective date:20040831 Owner name:FEUCHTER, BRUCE, CALIFORNIA Free format text:NOTE AND WARRANT PURCHASE AGREEMENT;ASSIGNOR:SUB-Q, INC.;REEL/FRAME:015156/0484 Effective date:20040831 Owner name:BOSTON SCIENTIFIC CORPORATION,MASSACHUSETTS Free format text:NOTE AND WARRANT PURCHASE AGREEMENT;ASSIGNOR:SUB-Q, INC.;REEL/FRAME:015156/0484 Effective date:20040831 Owner name:PACIFIC VENTURE GROUP II, L.P.,CALIFORNIA Free format text:NOTE AND WARRANT PURCHASE AGREEMENT;ASSIGNOR:SUB-Q, INC.;REEL/FRAME:015156/0484 Effective date:20040831 Owner name:PVG ASSOCIATES II, L.P.,CALIFORNIA Free format text:NOTE AND WARRANT PURCHASE AGREEMENT;ASSIGNOR:SUB-Q, INC.;REEL/FRAME:015156/0484 Effective date:20040831 Owner name:PEQUOT PRIVATE EQUITY FUND II, L.P.,CONNECTICUT Free format text:NOTE AND WARRANT PURCHASE AGREEMENT;ASSIGNOR:SUB-Q, INC.;REEL/FRAME:015156/0484 Effective date:20040831 Owner name:KINGSBURY CAPITAL PARTNERS, L.P. III,CALIFORNIA Free format text:NOTE AND WARRANT PURCHASE AGREEMENT;ASSIGNOR:SUB-Q, INC.;REEL/FRAME:015156/0484 Effective date:20040831 Owner name:KINGSBURY CAPITAL PARTNERS, L.P. IV,CALIFORNIA Free format text:NOTE AND WARRANT PURCHASE AGREEMENT;ASSIGNOR:SUB-Q, INC.;REEL/FRAME:015156/0484 Effective date:20040831 Owner name:HARTZLER, GEOFFREY O.,KANSAS Free format text:NOTE AND WARRANT PURCHASE AGREEMENT;ASSIGNOR:SUB-Q, INC.;REEL/FRAME:015156/0484 Effective date:20040831 Owner name:WALLACE, GEORGE,CALIFORNIA Free format text:NOTE AND WARRANT PURCHASE AGREEMENT;ASSIGNOR:SUB-Q, INC.;REEL/FRAME:015156/0484 Effective date:20040831 Owner name:CRAGG, ANDREW H.,MINNESOTA Free format text:NOTE AND WARRANT PURCHASE AGREEMENT;ASSIGNOR:SUB-Q, INC.;REEL/FRAME:015156/0484 Effective date:20040831 Owner name:ALLEN FAMILY TRUST DATED 10/12/81,CALIFORNIA Free format text:NOTE AND WARRANT PURCHASE AGREEMENT;ASSIGNOR:SUB-Q, INC.;REEL/FRAME:015156/0484 Effective date:20040831 Owner name:FEUCHTER, BRUCE,CALIFORNIA Free format text:NOTE AND WARRANT PURCHASE AGREEMENT;ASSIGNOR:SUB-Q, INC.;REEL/FRAME:015156/0484 Effective date:20040831 | |
| FPAY | Fee payment | Year of fee payment:4 | |
| SULP | Surcharge for late payment | ||
| AS | Assignment | Owner name:BOSTON SCIENTIFIC SCIMED, INC.,MINNESOTA Free format text:ASSIGNMENT OF ASSIGNORS INTEREST;ASSIGNOR:SUB-Q, INC.;REEL/FRAME:018420/0029 Effective date:20050504 Owner name:BOSTON SCIENTIFIC SCIMED, INC., MINNESOTA Free format text:ASSIGNMENT OF ASSIGNORS INTEREST;ASSIGNOR:SUB-Q, INC.;REEL/FRAME:018420/0029 Effective date:20050504 | |
| FEPP | Fee payment procedure | Free format text:PAYOR NUMBER ASSIGNED (ORIGINAL EVENT CODE: ASPN); ENTITY STATUS OF PATENT OWNER: LARGE ENTITY Free format text:PAYER NUMBER DE-ASSIGNED (ORIGINAL EVENT CODE: RMPN); ENTITY STATUS OF PATENT OWNER: LARGE ENTITY | |
| FEPP | Fee payment procedure | Free format text:PAT HOLDER NO LONGER CLAIMS SMALL ENTITY STATUS, ENTITY STATUS SET TO UNDISCOUNTED (ORIGINAL EVENT CODE: STOL); ENTITY STATUS OF PATENT OWNER: LARGE ENTITY | |
| REFU | Refund | Free format text:REFUND - PAYMENT OF MAINTENANCE FEE, 8TH YR, SMALL ENTITY (ORIGINAL EVENT CODE: R2552); ENTITY STATUS OF PATENT OWNER: LARGE ENTITY | |
| FPAY | Fee payment | Year of fee payment:8 | |
| SULP | Surcharge for late payment | Year of fee payment:7 | |
| FPAY | Fee payment | Year of fee payment:12 |