US5843090A - Stent delivery device - Google Patents
Stent delivery deviceDownload PDFInfo
- Publication number
- US5843090A US5843090AUS08/743,204US74320496AUS5843090AUS 5843090 AUS5843090 AUS 5843090AUS 74320496 AUS74320496 AUS 74320496AUS 5843090 AUS5843090 AUS 5843090A
- Authority
- US
- United States
- Prior art keywords
- catheter
- stent
- distal portion
- lumen
- annular space
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Expired - Lifetime
Links
Images
Classifications
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F2/00—Filters implantable into blood vessels; Prostheses, i.e. artificial substitutes or replacements for parts of the body; Appliances for connecting them with the body; Devices providing patency to, or preventing collapsing of, tubular structures of the body, e.g. stents
- A61F2/95—Instruments specially adapted for placement or removal of stents or stent-grafts
- A61F2/958—Inflatable balloons for placing stents or stent-grafts
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F2/00—Filters implantable into blood vessels; Prostheses, i.e. artificial substitutes or replacements for parts of the body; Appliances for connecting them with the body; Devices providing patency to, or preventing collapsing of, tubular structures of the body, e.g. stents
- A61F2/95—Instruments specially adapted for placement or removal of stents or stent-grafts
Definitions
- the present inventionrelates to a delivery system for delivering and deploying a stent to a treatment site within a vessel of the body of a living animal or living human.
- the delivery system of this inventionincludes a balloon catheter for dilating the vessel before deploying the stent and also after deploying the stent, if desired, without complete removal and insertion of separate catheters as was typically required in the prior art.
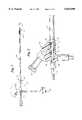
- FIG. 1is an overall view of a stent delivery apparatus useful in the practice of the present invention.
- FIG. 2is an enlarged view partly in section of detail 2 of FIG. 1.
- FIG. 3is a section view of a distal end of the apparatus of FIG. 1 showing inner and outer catheters, a stent in a radially inwardly collapsed condition and a balloon carried on the apparatus and in a deflated condition.
- FIG. 4is a section view taken along line 4--4 of FIG. 3.
- FIG. 5is a perspective view of a portion of the apparatus of FIG. 3, but with the balloon inflated.
- FIG. 6is a view similar to FIG. 3, but with the outer catheter partially retracted and the stent partially deployed.
- FIG. 7is a view similar to that of FIG. 3, but with the stent fully deployed and with the outer catheter returned to the position shown in FIG. 3 and with the balloon inflated in post-deployment dilation.
- System or device 10includes an inner catheter 12 having a proximal portion 14 and a distal portion 16.
- System 10further has an outer catheter 20 having a proximal portion 22 and a distal portion 24.
- the outer catheter 20is disposed about the inner catheter 12 and forms an annular space 18 between at least the distal portions 16 and 24 of the inner and outer catheters 12, 20.
- Device 10also has a dilation balloon 26 disposed about and secured by conventional means to the distal portion 24 of the outer catheter, and device 10 further has a stent 30 disposed in the annular space 18 between the distal portion 24 of the outer catheter 20 and the distal portion 16 of the inner catheter 12.
- Device 10also has a valve body or manifold 32 secured (by conventional means) to the outer catheter 20.
- Manifold 32has a through lumen 34 aligned with the through lumen 36 of outer catheter 20.
- Inner catheter 12is received in lumen 36.
- a flushing port 38is formed as part of manifold 32 and is in fluid communication with lumen 34, and annular space 18 when inner catheter is received in device 10.
- the flushing port 38may be used to introduce a flushing or hydrophilic-coating-activating liquid (such as a saline solution), or to introduce a conventional lubricating liquid into the annular space 18 if desired.
- the flushing fluidis useful to eliminate air in the annular space 18, reducing the chance of air emboli therein.
- Valve body 32also preferably has an inflation port 40 in fluid communication with a auxiliary or second lumen 42 of outer catheter 20.
- auxiliary or second lumen 42is fluidly coupled to an inflation plenum 44 between balloon 26 and outer catheter 20 via a skive 46.
- a guide wire(not shown) may be inserted in lumen 34 of manifold 32 to extend into lumen 28 of inner catheter 12, and through a tip 50, as desired.
- Tip 50 of system 10is preferably made of a soft elastomeric material to provide a relatively streamlined leading surface for the stent delivery system to ease insertion into the vessel.
- a pair of radiopaque bands 52, 54are preferably located at proximal and distal ends, respectively, of the stent as may be seen most clearly in FIG. 3. Bands 52 and 54 provide pronounced demarcation of the ends of stent 30 to aid in the radiographically assisted or directed placement of the stent 30 in a vessel 60 as, for example, shown in phantom in FIGS. 6 and 7.
- the balloonmay be axially configured within bands 52 and 54 so that the bands provide an indication of the balloon location for the purpose of positioning the balloon at a desired dilatation site.
- a radiopaque bandwill define the proximal and/or distal end of the balloon.
- the stent 30 of the delivery system 10is preferably a self-expanding type carried in a collapsed condition between the inner catheter 12 and the outer catheter 20.
- U.S. Pat. No. 4,655,771 B1as re-examined, for a PROSTHESIS COMPRISING AN EXPANSIBLE OR CONTRACTILE TUBULAR BODY, naming Hans I. Wallsten as inventor, is hereby expressly incorporated by reference as an example of such a self-expanding prosthesis or stent.
- Balloon 26is preferably located directly radially outwardly of stent 30 exteriorly of outer catheter 20.
- the device 10is preferably maneuvered to position the distal portion 16 carrying stent 30 at a treatment site (typically using a radiographic techniques, either with or without bands 52 and 54) followed by inflating the balloon 26 to dilate the vessel 60 and thereafter deflating the balloon 26.
- the balloon 26is inflated or pressurized (typically by injecting saline solution via inflation port 40) using the separate second lumen 42 in the outer catheter 20.
- the outer catheter 20is retracted (after the balloon 26 is deflated by extracting the saline solution via port 40) while the position of the inner catheter 12 is maintained such that the stent 30 is deployed at the treatment site.
- the distal portion 16may be positioned slightly distal of the treatment site to allow for slight proximal migration of the stent 30 during deployment.
- the inner catheter 12preferably has a radially outwardly directed stop means or element (which in the embodiment shown is combined in function with radiopaque band 52) located adjacent a proximal end of the stent 30 to restrain axial movement of the stent 30 as the outer catheter 20 is retracted in deploying the stent 30.
- the device 10is withdrawn from the vessel or canal 60 either with or without returning the outer catheter 20 distally towards tip 50. It is to be understood that pre-deployment inflation of balloon 26 may be omitted, if desired.
- outer catheter 20may be moved to position balloon 26 radially interiorly of stent 30 and inflated after deployment of stent 30 as shown in FIG. 7, if desired. Balloon 26 is thereafter deflated and device 10 withdrawn from vessel 60.
- the present inventionincludes a method of using the combined stent and delivery device to both dilate a partially occluded portion of a body canal and deploy a stent therein without the necessity of removing the device between those two operations where the operations include dilating a partial occluded portion of a body canal using a balloon carried on a distal portion of the device and the further operation of retracting an outer sleeve or catheter overlying a self-expanding stent such that the stent is released in the dilated portion of the body canal.
- the distal region of the delivery devicemay be preferably located slightly distal of the treatment site prior to stent deployment so that the stent will be deployed at the desired location with respect to the treatment site. This will allow for the known propensity of certain self-expanding stents to migrate slightly proximally during deployment in certain situations.
- the methodincludes inserting the stent delivery device 10 into the vessel 60 wherein the device has a self-expanding stent 30 carried in a collapsed condition between the inner and outer catheters 12, 20 and restrained against proximal axial movement by a step or element 52 and the device 10 further has a dilation balloon 26 carried thereon, all at a distal region of the device 10.
- the methodalso includes manoeuvering the device 10 to position the stent 30 at a treatment site, typically a partially occluded portion of the vessel 60, whereupon the outer catheter 20 is retracted such that the stent 30 is deployed at the treatment site, and the device 10 is thereafter withdrawn from the vessel 60.
- aspects of the method of the present inventioninclude the additional steps of positioning the balloon 26 at the treatment site, inflating the balloon 26 to dilate the vessel 60, and deflating the balloon 26, all after manoeuvering the device 10 to position the stent 30 at the treatment site and before deploying the stent 30. Still further (optional) aspects of the method of the present invention include the additional steps of advancing the outer catheter 20 distally to position the balloon 26 interiorly (or radially inwardly) of the stent 30 at the treatment site, inflating the balloon 26 within the stent 30 (to further expand or "set” the stent 30) and thereafter deflating the balloon 26, in this case all after deploying the stent 30 and before withdrawing the device 10 from the vessel 60.
- both pre- and post-deployment balloon inflation or vessel dilationmay be utilized in the practice of the present invention.
Landscapes
- Health & Medical Sciences (AREA)
- Engineering & Computer Science (AREA)
- Biomedical Technology (AREA)
- Cardiology (AREA)
- Oral & Maxillofacial Surgery (AREA)
- Transplantation (AREA)
- Heart & Thoracic Surgery (AREA)
- Vascular Medicine (AREA)
- Life Sciences & Earth Sciences (AREA)
- Animal Behavior & Ethology (AREA)
- General Health & Medical Sciences (AREA)
- Public Health (AREA)
- Veterinary Medicine (AREA)
- Media Introduction/Drainage Providing Device (AREA)
Abstract
Description
The present invention relates to a delivery system for delivering and deploying a stent to a treatment site within a vessel of the body of a living animal or living human. The delivery system of this invention includes a balloon catheter for dilating the vessel before deploying the stent and also after deploying the stent, if desired, without complete removal and insertion of separate catheters as was typically required in the prior art.
FIG. 1 is an overall view of a stent delivery apparatus useful in the practice of the present invention.
FIG. 2 is an enlarged view partly in section of detail 2 of FIG. 1.
FIG. 3 is a section view of a distal end of the apparatus of FIG. 1 showing inner and outer catheters, a stent in a radially inwardly collapsed condition and a balloon carried on the apparatus and in a deflated condition.
FIG. 4 is a section view taken alongline 4--4 of FIG. 3.
FIG. 5 is a perspective view of a portion of the apparatus of FIG. 3, but with the balloon inflated.
FIG. 6 is a view similar to FIG. 3, but with the outer catheter partially retracted and the stent partially deployed.
FIG. 7 is a view similar to that of FIG. 3, but with the stent fully deployed and with the outer catheter returned to the position shown in FIG. 3 and with the balloon inflated in post-deployment dilation.
Referring to the Figures and most particularly to FIGS. 1, 2, and 3, a stent delivery system or medical device 10 may be seen. System or device 10 includes aninner catheter 12 having aproximal portion 14 and adistal portion 16. System 10 further has anouter catheter 20 having aproximal portion 22 and adistal portion 24. Theouter catheter 20 is disposed about theinner catheter 12 and forms anannular space 18 between at least thedistal portions outer catheters dilation balloon 26 disposed about and secured by conventional means to thedistal portion 24 of the outer catheter, and device 10 further has astent 30 disposed in theannular space 18 between thedistal portion 24 of theouter catheter 20 and thedistal portion 16 of theinner catheter 12.
Device 10 also has a valve body ormanifold 32 secured (by conventional means) to theouter catheter 20. Manifold 32 has a throughlumen 34 aligned with the throughlumen 36 ofouter catheter 20.Inner catheter 12 is received inlumen 36. Aflushing port 38 is formed as part ofmanifold 32 and is in fluid communication withlumen 34, andannular space 18 when inner catheter is received in device 10. The flushingport 38 may be used to introduce a flushing or hydrophilic-coating-activating liquid (such as a saline solution), or to introduce a conventional lubricating liquid into theannular space 18 if desired. The flushing fluid is useful to eliminate air in theannular space 18, reducing the chance of air emboli therein. Valvebody 32 also preferably has aninflation port 40 in fluid communication with a auxiliary orsecond lumen 42 ofouter catheter 20. Referring now also to FIGS. 4 and 5, auxiliary orsecond lumen 42 is fluidly coupled to aninflation plenum 44 betweenballoon 26 andouter catheter 20 via askive 46. A guide wire (not shown) may be inserted inlumen 34 ofmanifold 32 to extend intolumen 28 ofinner catheter 12, and through atip 50, as desired.
Thestent 30 of the delivery system 10 is preferably a self-expanding type carried in a collapsed condition between theinner catheter 12 and theouter catheter 20. U.S. Pat. No. 4,655,771 B1, as re-examined, for a PROSTHESIS COMPRISING AN EXPANSIBLE OR CONTRACTILE TUBULAR BODY, naming Hans I. Wallsten as inventor, is hereby expressly incorporated by reference as an example of such a self-expanding prosthesis or stent.Balloon 26 is preferably located directly radially outwardly ofstent 30 exteriorly ofouter catheter 20. In use, the device 10 is preferably maneuvered to position thedistal portion 16 carryingstent 30 at a treatment site (typically using a radiographic techniques, either with or withoutbands 52 and 54) followed by inflating theballoon 26 to dilate the vessel 60 and thereafter deflating theballoon 26. Theballoon 26 is inflated or pressurized (typically by injecting saline solution via inflation port 40) using the separatesecond lumen 42 in theouter catheter 20. Next theouter catheter 20 is retracted (after theballoon 26 is deflated by extracting the saline solution via port 40) while the position of theinner catheter 12 is maintained such that thestent 30 is deployed at the treatment site. Alternatively, thedistal portion 16 may be positioned slightly distal of the treatment site to allow for slight proximal migration of thestent 30 during deployment. Theinner catheter 12 preferably has a radially outwardly directed stop means or element (which in the embodiment shown is combined in function with radiopaque band 52) located adjacent a proximal end of thestent 30 to restrain axial movement of thestent 30 as theouter catheter 20 is retracted in deploying thestent 30. Finally, the device 10 is withdrawn from the vessel or canal 60 either with or without returning theouter catheter 20 distally towardstip 50. It is to be understood that pre-deployment inflation ofballoon 26 may be omitted, if desired. Furthermore,outer catheter 20 may be moved to positionballoon 26 radially interiorly ofstent 30 and inflated after deployment ofstent 30 as shown in FIG. 7, if desired.Balloon 26 is thereafter deflated and device 10 withdrawn from vessel 60.
It may thus be seen that the present invention includes a method of using the combined stent and delivery device to both dilate a partially occluded portion of a body canal and deploy a stent therein without the necessity of removing the device between those two operations where the operations include dilating a partial occluded portion of a body canal using a balloon carried on a distal portion of the device and the further operation of retracting an outer sleeve or catheter overlying a self-expanding stent such that the stent is released in the dilated portion of the body canal. According to one aspect of the method of the present invention, in certain procedures the distal region of the delivery device may be preferably located slightly distal of the treatment site prior to stent deployment so that the stent will be deployed at the desired location with respect to the treatment site. This will allow for the known propensity of certain self-expanding stents to migrate slightly proximally during deployment in certain situations.
More particularly, the method includes inserting the stent delivery device 10 into the vessel 60 wherein the device has a self-expandingstent 30 carried in a collapsed condition between the inner andouter catheters element 52 and the device 10 further has adilation balloon 26 carried thereon, all at a distal region of the device 10. The method also includes manoeuvering the device 10 to position thestent 30 at a treatment site, typically a partially occluded portion of the vessel 60, whereupon theouter catheter 20 is retracted such that thestent 30 is deployed at the treatment site, and the device 10 is thereafter withdrawn from the vessel 60. Further aspects of the method of the present invention include the additional steps of positioning theballoon 26 at the treatment site, inflating theballoon 26 to dilate the vessel 60, and deflating theballoon 26, all after manoeuvering the device 10 to position thestent 30 at the treatment site and before deploying thestent 30. Still further (optional) aspects of the method of the present invention include the additional steps of advancing theouter catheter 20 distally to position theballoon 26 interiorly (or radially inwardly) of thestent 30 at the treatment site, inflating theballoon 26 within the stent 30 (to further expand or "set" the stent 30) and thereafter deflating theballoon 26, in this case all after deploying thestent 30 and before withdrawing the device 10 from the vessel 60. Of course, it is to be understood that both pre- and post-deployment balloon inflation or vessel dilation may be utilized in the practice of the present invention.
The invention is not to be taken as limited to all of the details thereof as modifications and variations thereof may be made without departing from the spirit or scope of the invention.
Claims (15)
1. A medical device comprising:
a) an inner catheter having a proximal portion and a distal portion;
b) an outer catheter having a first lumen therethrough and a proximal portion and a distal portion, the outer catheter disposed around the inner catheter and forming an annular space between the distal portion of the outer catheter and the distal portion of the inner catheter;
c) a dilation balloon disposed around the distal portion of the outer catheter; and
d) a stent disposed in the annular space between the distal portion of the outer catheter and the distal portion of the inner catheter; and
e) a manifold having
i) a flushing port fluidly coupled to the annular space between the inner catheter and first lumen of the outer catheter, and
ii) an inflation port fluidly coupled to a second lumen in the outer catheter to permit inflation of the balloon.
2. The device of claim 1 wherein the inner catheter further comprises a radially outwardly directed element adjacent a proximal end of the stent to restrain axial movement of the stent as the outer catheter is retracted.
3. The device of claim 2 wherein the element comprises a first radiopaque band.
4. The device of claim 3 further comprising a second radiopaque band adjacent a distal end of the stent.
5. The device of claim 1 wherein the stent is a self-expanding stent.
6. The device of claim 5 wherein the annular space between the inner and outer catheters is sized to retain the stent in a radially collapsed condition.
7. The device of claim 1 wherein the manifold further comprises:
iii) a through lumen for permitting passage of a guide wire therethrough.
8. A stent delivery system comprising:
a) an inner catheter having a proximal portion and a distal portion, the inner catheter forming a lumen at least in its distal portion;
b) an outer catheter disposed around the inner catheter and forming an annular space between the inner and outer catheters, the outer catheter having a dilation balloon disposed around a distal region thereof, and a lumen fluidly coupled to the interior of the dilation balloon; and
c) a stent located at a distal region of the annular space between the inner and outer catheters,
wherein the balloon is positioned radially outwardly of the stent and further wherein the balloon is inflatable for pre-stent-deployment dilation of a vessel containing the device.
9. The stent delivery system of claim 8 wherein the inner catheter further comprises a tapered tip at an end of the distal portion thereof.
10. The stent delivery system of claim 8 wherein the stent is releasable from the annular space between the inner and outer catheters by retraction of the outer catheter away from the distal end of the inner catheter.
11. A medical device comprising:
a) an inner catheter having a proximal portion and a distal portion;
b) an outer catheter having a first lumen therethrough and a proximal portion and a distal portion, the outer catheter disposed around the inner catheter and forming an annular space between the distal portion of the outer catheter and the distal portion of the inner catheter;
c) a dilation balloon disposed around the distal portion of the outer catheter; and
d) a stent disposed in the annular space between the distal portion of the outer catheter and the distal portion of the inner catheter
wherein the dilation balloon is positioned radially outwardly of the stent and further wherein the balloon is inflatable for pre-stent-deployment dilation of a vessel containing the device.
12. A stent delivery system comprising:
a) an inner catheter having a proximal portion and a distal portion, the inner catheter forming a lumen at least in its distal portion;
b) an outer catheter disposed around the inner catheter and forming an annular space between the inner and outer catheters, the outer catheter having a dilation balloon disposed around a distal region thereof, and a lumen fluidly coupled to the interior of the dilation balloon; and
c) a stent located at a distal region of the annular space between the inner and outer catheters
d) a manifold having
i) a flushing port fluidly coupled to the annular space between the inner catheter and first lumen of the outer catheter, and
ii) an inflation port fluidly coupled to a second lumen in the outer catheter to permit inflation of the balloon.
13. The stent delivery system of claim 12 wherein the inner catheter further comprises a tapered tip at an end of the distal portion thereof.
14. The stent delivery system of claim 12 wherein the stent is releasable from the annular space between the inner and outer catheters by retraction of the outer catheter away from the distal end of the inner catheter.
15. The device of claim 12 wherein the manifold further comprises:
iii) a through lumen for permitting passage of a guide wire therethrough.
Priority Applications (5)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US08/743,204US5843090A (en) | 1996-11-05 | 1996-11-05 | Stent delivery device |
| DE69730666TDE69730666T2 (en) | 1996-11-05 | 1997-10-02 | The stent delivery system |
| EP97307803AEP0839504B1 (en) | 1996-11-05 | 1997-10-02 | Stent delivery device |
| CA002218972ACA2218972C (en) | 1996-11-05 | 1997-10-22 | Stent delivery device |
| JP30318297AJP2999165B2 (en) | 1996-11-05 | 1997-11-05 | Stent supply device |
Applications Claiming Priority (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US08/743,204US5843090A (en) | 1996-11-05 | 1996-11-05 | Stent delivery device |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| US5843090Atrue US5843090A (en) | 1998-12-01 |
Family
ID=24987905
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US08/743,204Expired - LifetimeUS5843090A (en) | 1996-11-05 | 1996-11-05 | Stent delivery device |
Country Status (5)
| Country | Link |
|---|---|
| US (1) | US5843090A (en) |
| EP (1) | EP0839504B1 (en) |
| JP (1) | JP2999165B2 (en) |
| CA (1) | CA2218972C (en) |
| DE (1) | DE69730666T2 (en) |
Cited By (91)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US5910145A (en)* | 1997-03-31 | 1999-06-08 | Fischell; Robert E. | Stent delivery catheter system |
| US6015420A (en)* | 1997-03-06 | 2000-01-18 | Scimed Life Systems, Inc. | Atherectomy device for reducing damage to vessels and/or in-vivo stents |
| US6042589A (en)* | 1998-03-17 | 2000-03-28 | Medicorp, S.A. | Reversible-action endoprosthesis delivery device |
| DE20009204U1 (en) | 2000-05-22 | 2000-08-17 | Jomed GmbH, 72414 Rangendingen | Stent implantation catheter assembly |
| US6168616B1 (en) | 1997-06-02 | 2001-01-02 | Global Vascular Concepts | Manually expandable stent |
| US6168617B1 (en)* | 1999-06-14 | 2001-01-02 | Scimed Life Systems, Inc. | Stent delivery system |
| US6221097B1 (en)* | 1999-03-22 | 2001-04-24 | Scimed Life System, Inc. | Lubricated sleeve material for stent delivery |
| DE19954330A1 (en)* | 1999-11-11 | 2001-07-26 | Angiomed Ag | Percutaneous transluminal stent delivery system, has distal tip zone which is contiguous with distal end of tube, in which tip zone both luminal surface and abluminal surface taper radially inwardly nearer to distal end of tube |
| US6315790B1 (en) | 1999-06-07 | 2001-11-13 | Scimed Life Systems, Inc. | Radiopaque marker bands |
| US6368301B1 (en) | 1999-12-21 | 2002-04-09 | Advanced Cardiovascular Systems, Inc. | Catheter having a soft distal tip |
| US6379365B1 (en)* | 1999-03-29 | 2002-04-30 | Alexis Diaz | Stent delivery catheter system having grooved shaft |
| US6425898B1 (en)* | 1998-03-13 | 2002-07-30 | Cordis Corporation | Delivery apparatus for a self-expanding stent |
| US6443980B1 (en) | 1999-03-22 | 2002-09-03 | Scimed Life Systems, Inc. | End sleeve coating for stent delivery |
| US20020183826A1 (en)* | 2000-11-13 | 2002-12-05 | Angiomed Gmbh & Co. | Implant delivery device |
| US20030060872A1 (en)* | 2001-09-26 | 2003-03-27 | Gary Gomringer | Stent with radiopaque characteristics |
| US20030144670A1 (en)* | 2001-11-29 | 2003-07-31 | Cook Incorporated | Medical device delivery system |
| US6652578B2 (en)* | 1999-12-31 | 2003-11-25 | Abps Venture One, Ltd. | Endoluminal cardiac and venous valve prostheses and methods of manufacture and delivery thereof |
| US6692461B2 (en) | 2001-08-07 | 2004-02-17 | Advanced Cardiovascular Systems, Inc. | Catheter tip |
| US6702802B1 (en) | 1999-11-10 | 2004-03-09 | Endovascular Technologies, Inc. | Catheters with improved transition |
| US6702843B1 (en)* | 2000-04-12 | 2004-03-09 | Scimed Life Systems, Inc. | Stent delivery means with balloon retraction means |
| US6743219B1 (en) | 2000-08-02 | 2004-06-01 | Cordis Corporation | Delivery apparatus for a self-expanding stent |
| US6786918B1 (en)* | 2000-10-17 | 2004-09-07 | Medtronic Vascular, Inc. | Stent delivery system |
| US20040188304A1 (en)* | 2002-12-31 | 2004-09-30 | Bonnette Michael J. | Packaging system with oxygen sensor |
| US20050049668A1 (en)* | 2003-08-29 | 2005-03-03 | Jones Donald K. | Self-expanding stent and stent delivery system for treatment of vascular stenosis |
| US20050049669A1 (en)* | 2003-08-29 | 2005-03-03 | Jones Donald K. | Self-expanding stent and stent delivery system with distal protection |
| US20050049670A1 (en)* | 2003-08-29 | 2005-03-03 | Jones Donald K. | Self-expanding stent and stent delivery system for treatment of vascular disease |
| US20050154439A1 (en)* | 2004-01-08 | 2005-07-14 | Gunderson Richard C. | Medical device delivery systems |
| US20050278011A1 (en)* | 2004-06-10 | 2005-12-15 | Peckham John E | Stent delivery system |
| US20060025844A1 (en)* | 2004-07-28 | 2006-02-02 | Majercak David C | Reduced deployment force delivery device |
| US20060036309A1 (en)* | 2002-02-28 | 2006-02-16 | Counter Clockwise, Inc. | Guidewire loaded stent for delivery through a catheter |
| US20060253184A1 (en)* | 2005-05-04 | 2006-11-09 | Kurt Amplatz | System for the controlled delivery of stents and grafts |
| US7169170B2 (en) | 2002-02-22 | 2007-01-30 | Cordis Corporation | Self-expanding stent delivery system |
| US20070100420A1 (en)* | 2005-11-02 | 2007-05-03 | Kavanagh Joseph T | Guided stent delivery systems of minimal diameter |
| US20070118207A1 (en)* | 2005-05-04 | 2007-05-24 | Aga Medical Corporation | System for controlled delivery of stents and grafts |
| US20070198076A1 (en)* | 2006-02-13 | 2007-08-23 | Stephen Hebert | System for delivering a stent |
| US20070233232A1 (en)* | 2006-03-31 | 2007-10-04 | St Germain Jon | Stent and system and method for deploying a stent |
| US20080119879A1 (en)* | 2005-06-30 | 2008-05-22 | Rox Medical, Inc. | Devices, systems and methods for creation of a peripherally located fistula |
| US20080234796A1 (en)* | 2005-05-09 | 2008-09-25 | Angiomed Gmbh & Co. Medizintechnik Kg | Implant Delivery Device |
| US20080255654A1 (en)* | 2007-03-22 | 2008-10-16 | Bay Street Medical | System for delivering a stent |
| US20090024205A1 (en)* | 2007-07-19 | 2009-01-22 | Bay Street Medical | Radially Expandable Stent |
| US20090062835A1 (en)* | 2001-11-01 | 2009-03-05 | Advanced Cardovascular Systems, Inc. | Catheter having an improved distal tip |
| US7651521B2 (en) | 2004-03-02 | 2010-01-26 | Cardiomind, Inc. | Corewire actuated delivery system with fixed distal stent-carrying extension |
| US7699884B2 (en) | 2006-03-22 | 2010-04-20 | Cardiomind, Inc. | Method of stenting with minimal diameter guided delivery systems |
| US7771463B2 (en) | 2003-03-26 | 2010-08-10 | Ton Dai T | Twist-down implant delivery technologies |
| US7785361B2 (en)* | 2003-03-26 | 2010-08-31 | Julian Nikolchev | Implant delivery technologies |
| US20100256600A1 (en)* | 2009-04-04 | 2010-10-07 | Ferrera David A | Neurovascular otw pta balloon catheter and delivery system |
| US7862602B2 (en) | 2005-11-02 | 2011-01-04 | Biosensors International Group, Ltd | Indirect-release electrolytic implant delivery systems |
| US20110022148A1 (en)* | 2007-02-20 | 2011-01-27 | Xtent, Inc. | Thermo-mechanically controlled implants and methods of use |
| US20110125248A1 (en)* | 2001-12-03 | 2011-05-26 | Xtent, Inc. | Custom length stent apparatus |
| US8016869B2 (en)* | 2003-03-26 | 2011-09-13 | Biosensors International Group, Ltd. | Guidewire-less stent delivery methods |
| US8029555B2 (en) | 2004-03-31 | 2011-10-04 | Cook Medical Technologies Llc | Stent introducer system |
| US8657870B2 (en) | 2009-06-26 | 2014-02-25 | Biosensors International Group, Ltd. | Implant delivery apparatus and methods with electrolytic release |
| US8784468B2 (en) | 2010-11-17 | 2014-07-22 | Boston Scientific Scimed, Inc. | Stent delivery systems and locking members for use with stent delivery systems |
| US8986362B2 (en) | 2004-06-28 | 2015-03-24 | J.W. Medical Systems Ltd. | Devices and methods for controlling expandable prostheses during deployment |
| US9084692B2 (en) | 2010-11-17 | 2015-07-21 | Boston Scientific Scimed, Inc. | Stent delivery system |
| US9101503B2 (en) | 2008-03-06 | 2015-08-11 | J.W. Medical Systems Ltd. | Apparatus having variable strut length and methods of use |
| US9220619B2 (en) | 2010-11-17 | 2015-12-29 | Boston Scientific Scimed, Inc. | Stent delivery system |
| US9233015B2 (en) | 2012-06-15 | 2016-01-12 | Trivascular, Inc. | Endovascular delivery system with an improved radiopaque marker scheme |
| US9326876B2 (en) | 2001-12-03 | 2016-05-03 | J.W. Medical Systems Ltd. | Apparatus and methods for delivery of multiple distributed stents |
| US9339404B2 (en) | 2007-03-22 | 2016-05-17 | J.W. Medical Systems Ltd. | Devices and methods for controlling expandable prostheses during deployment |
| US9566179B2 (en) | 2003-12-23 | 2017-02-14 | J.W. Medical Systems Ltd. | Devices and methods for controlling and indicating the length of an interventional element |
| US20170128207A1 (en)* | 2008-03-18 | 2017-05-11 | Medtronic Ventor Technologies, Ltd. | Valve suturing and implantation procedures |
| US9700448B2 (en) | 2004-06-28 | 2017-07-11 | J.W. Medical Systems Ltd. | Devices and methods for controlling expandable prostheses during deployment |
| US20180000619A1 (en)* | 2015-01-29 | 2018-01-04 | Intact Vascular, Inc. | Delivery device and method of delivery |
| US9883957B2 (en) | 2006-03-20 | 2018-02-06 | J.W. Medical Systems Ltd. | Apparatus and methods for deployment of linked prosthetic segments |
| US10058443B2 (en) | 2011-11-02 | 2018-08-28 | Boston Scientific Scimed, Inc. | Stent delivery systems and methods for use |
| US10159587B2 (en) | 2015-01-16 | 2018-12-25 | Boston Scientific Scimed, Inc. | Medical device delivery system with force reduction member |
| WO2019023258A1 (en)* | 2017-07-26 | 2019-01-31 | Intact Vascular, Inc. | Delivery device and method of delivery |
| US10245167B2 (en) | 2015-01-29 | 2019-04-02 | Intact Vascular, Inc. | Delivery device and method of delivery |
| AU2016380347B2 (en)* | 2016-01-01 | 2019-11-21 | Intact Vascular, Inc. | Delivery device and method of delivery |
| WO2020056234A1 (en)* | 2018-09-14 | 2020-03-19 | 4C Medical Technologies, Inc. | Repositioning wires and methods for repositioning prosthetic heart valve devices within a heart chamber and related systems, devices and methods |
| US10940167B2 (en) | 2012-02-10 | 2021-03-09 | Cvdevices, Llc | Methods and uses of biological tissues for various stent and other medical applications |
| US10993824B2 (en)* | 2016-01-01 | 2021-05-04 | Intact Vascular, Inc. | Delivery device and method of delivery |
| US11013627B2 (en) | 2018-01-10 | 2021-05-25 | Boston Scientific Scimed, Inc. | Stent delivery system with displaceable deployment mechanism |
| US11065137B2 (en) | 2016-02-26 | 2021-07-20 | Boston Scientific Scimed, Inc. | Stent delivery systems with a reduced profile |
| US11351048B2 (en) | 2015-11-16 | 2022-06-07 | Boston Scientific Scimed, Inc. | Stent delivery systems with a reinforced deployment sheath |
| US11406495B2 (en) | 2013-02-11 | 2022-08-09 | Cook Medical Technologies Llc | Expandable support frame and medical device |
| US11602447B2 (en) | 2019-02-13 | 2023-03-14 | Boston Scientific Scimed Inc. | Stent delivery systems |
| US11660218B2 (en) | 2017-07-26 | 2023-05-30 | Intact Vascular, Inc. | Delivery device and method of delivery |
| US11857441B2 (en) | 2018-09-04 | 2024-01-02 | 4C Medical Technologies, Inc. | Stent loading device |
| US11931253B2 (en) | 2020-01-31 | 2024-03-19 | 4C Medical Technologies, Inc. | Prosthetic heart valve delivery system: ball-slide attachment |
| US11944537B2 (en) | 2017-01-24 | 2024-04-02 | 4C Medical Technologies, Inc. | Systems, methods and devices for two-step delivery and implantation of prosthetic heart valve |
| US11957577B2 (en) | 2017-01-19 | 2024-04-16 | 4C Medical Technologies, Inc. | Systems, methods and devices for delivery systems, methods and devices for implanting prosthetic heart valves |
| US11992403B2 (en) | 2020-03-06 | 2024-05-28 | 4C Medical Technologies, Inc. | Devices, systems and methods for improving recapture of prosthetic heart valve device with stent frame having valve support with inwardly stent cells |
| US12029647B2 (en) | 2017-03-07 | 2024-07-09 | 4C Medical Technologies, Inc. | Systems, methods and devices for prosthetic heart valve with single valve leaflet |
| US12036113B2 (en) | 2017-06-14 | 2024-07-16 | 4C Medical Technologies, Inc. | Delivery of heart chamber prosthetic valve implant |
| US12053375B2 (en) | 2020-03-05 | 2024-08-06 | 4C Medical Technologies, Inc. | Prosthetic mitral valve with improved atrial and/or annular apposition and paravalvular leakage mitigation |
| US12133797B2 (en) | 2020-01-31 | 2024-11-05 | 4C Medical Technologies, Inc. | Prosthetic heart valve delivery system: paddle attachment feature |
| US12232991B2 (en) | 2019-04-15 | 2025-02-25 | 4C Medical Technologies, Inc. | Loading systems for collapsible prosthetic heart valve devices and methods thereof |
| US12408907B1 (en) | 2019-11-14 | 2025-09-09 | Edwards Lifesciences Corporation | Method of reducing left atrial pressure |
| US12414797B2 (en) | 2019-08-22 | 2025-09-16 | Edwards Lifesciences Corporation | Puncture needles |
Families Citing this family (7)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US7208002B2 (en)* | 2001-01-04 | 2007-04-24 | Boston Scientific Scimed, Inc. | Expansion-assisting delivery system for self-expanding stent |
| US6623491B2 (en)* | 2001-01-18 | 2003-09-23 | Ev3 Peripheral, Inc. | Stent delivery system with spacer member |
| US20020095203A1 (en)* | 2001-01-18 | 2002-07-18 | Intra Therapeutics, Inc. | Catheter system with spacer member |
| DE60201905T2 (en) | 2002-09-09 | 2005-11-10 | Abbott Laboratories Vascular Enterprises Ltd. | System for introducing a self-expanding stent |
| US7871396B2 (en) | 2006-10-30 | 2011-01-18 | Boston Scientific Scimed, Inc. | Bifurcation catheter assembly and method |
| DE102010006187B4 (en)* | 2010-01-29 | 2017-11-16 | Acandis Gmbh & Co. Kg | Medical catheter for delivering a self-expanding non-preloaded stent |
| WO2012088089A1 (en) | 2010-12-22 | 2012-06-28 | Boston Scientific Scimed, Inc. | Stent deployment system with integrated digital camera and dilation balloon |
Citations (30)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US4655771A (en)* | 1982-04-30 | 1987-04-07 | Shepherd Patents S.A. | Prosthesis comprising an expansible or contractile tubular body |
| US4848343A (en)* | 1986-10-31 | 1989-07-18 | Medinvent S.A. | Device for transluminal implantation |
| US4886062A (en)* | 1987-10-19 | 1989-12-12 | Medtronic, Inc. | Intravascular radially expandable stent and method of implant |
| US4950227A (en)* | 1988-11-07 | 1990-08-21 | Boston Scientific Corporation | Stent delivery system |
| US4990151A (en)* | 1988-09-28 | 1991-02-05 | Medinvent S.A. | Device for transluminal implantation or extraction |
| US5026377A (en)* | 1989-07-13 | 1991-06-25 | American Medical Systems, Inc. | Stent placement instrument and method |
| US5061275A (en)* | 1986-04-21 | 1991-10-29 | Medinvent S.A. | Self-expanding prosthesis |
| US5071407A (en)* | 1990-04-12 | 1991-12-10 | Schneider (U.S.A.) Inc. | Radially expandable fixation member |
| US5201757A (en)* | 1992-04-03 | 1993-04-13 | Schneider (Usa) Inc. | Medial region deployment of radially self-expanding stents |
| US5266073A (en)* | 1987-12-08 | 1993-11-30 | Wall W Henry | Angioplasty stent |
| WO1994024961A1 (en)* | 1993-04-23 | 1994-11-10 | Schneider (Usa) Inc. | Covered stent and stent delivery device |
| WO1995026777A1 (en)* | 1994-04-01 | 1995-10-12 | Localmed, Inc. | Method and apparatus for performing multiple procedures |
| US5464408A (en)* | 1991-06-14 | 1995-11-07 | Duc; Jerome | Transluminal implantation or extraction device |
| US5476476A (en)* | 1989-06-06 | 1995-12-19 | Cordis Corporation | Dilatation balloon assembly |
| US5484444A (en)* | 1992-10-31 | 1996-01-16 | Schneider (Europe) A.G. | Device for the implantation of self-expanding endoprostheses |
| EP0699451A2 (en)* | 1994-08-29 | 1996-03-06 | Robert E. Fischell | Catheter |
| US5534007A (en)* | 1995-05-18 | 1996-07-09 | Scimed Life Systems, Inc. | Stent deployment catheter with collapsible sheath |
| EP0720837A1 (en)* | 1994-12-07 | 1996-07-10 | Robert E. Fischell | Integrated dual-function catheter system for balloon angioplasty and stent delivery |
| US5571135A (en)* | 1993-10-22 | 1996-11-05 | Scimed Life Systems Inc. | Stent delivery apparatus and method |
| US5591172A (en)* | 1991-06-14 | 1997-01-07 | Ams Medinvent S.A. | Transluminal implantation device |
| US5603698A (en)* | 1993-04-13 | 1997-02-18 | Boston Scientific Corporation | Prosthesis delivery system |
| US5628754A (en)* | 1995-08-01 | 1997-05-13 | Medtronic, Inc. | Stent delivery guide catheter |
| US5628755A (en)* | 1995-02-20 | 1997-05-13 | Schneider (Europe) A.G. | Balloon catheter and stent delivery system |
| US5645559A (en)* | 1992-05-08 | 1997-07-08 | Schneider (Usa) Inc | Multiple layer stent |
| US5653689A (en)* | 1995-09-30 | 1997-08-05 | Abacus Design & Development, Inc. | Infusion catheter |
| US5658311A (en)* | 1996-07-05 | 1997-08-19 | Schneider (Usa) Inc. | High pressure expander bundle for large diameter stent deployment |
| US5662703A (en)* | 1995-04-14 | 1997-09-02 | Schneider (Usa) Inc. | Rolling membrane stent delivery device |
| US5690644A (en)* | 1992-12-30 | 1997-11-25 | Schneider (Usa) Inc. | Apparatus for deploying body implantable stent |
| US5695499A (en)* | 1994-10-27 | 1997-12-09 | Schneider (Usa) Inc. | Medical device supported by spirally wound wire |
| US5709703A (en)* | 1995-11-14 | 1998-01-20 | Schneider (Europe) A.G. | Stent delivery device and method for manufacturing same |
Family Cites Families (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2514527Y2 (en) | 1990-08-27 | 1996-10-23 | 株式会社パイオラックス | Tubular organ dilator |
| WO1994023669A1 (en) | 1993-04-13 | 1994-10-27 | Boston Scientific Corporation | Prosthesis delivery system with dilating tip |
- 1996
- 1996-11-05USUS08/743,204patent/US5843090A/ennot_activeExpired - Lifetime
- 1997
- 1997-10-02EPEP97307803Apatent/EP0839504B1/ennot_activeExpired - Lifetime
- 1997-10-02DEDE69730666Tpatent/DE69730666T2/ennot_activeExpired - Lifetime
- 1997-10-22CACA002218972Apatent/CA2218972C/ennot_activeExpired - Fee Related
- 1997-11-05JPJP30318297Apatent/JP2999165B2/ennot_activeExpired - Fee Related
Patent Citations (35)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US4655771B1 (en)* | 1982-04-30 | 1996-09-10 | Medinvent Ams Sa | Prosthesis comprising an expansible or contractile tubular body |
| US4954126A (en)* | 1982-04-30 | 1990-09-04 | Shepherd Patents S.A. | Prosthesis comprising an expansible or contractile tubular body |
| US4954126B1 (en)* | 1982-04-30 | 1996-05-28 | Ams Med Invent S A | Prosthesis comprising an expansible or contractile tubular body |
| US4655771A (en)* | 1982-04-30 | 1987-04-07 | Shepherd Patents S.A. | Prosthesis comprising an expansible or contractile tubular body |
| US5061275A (en)* | 1986-04-21 | 1991-10-29 | Medinvent S.A. | Self-expanding prosthesis |
| US4848343A (en)* | 1986-10-31 | 1989-07-18 | Medinvent S.A. | Device for transluminal implantation |
| US4886062A (en)* | 1987-10-19 | 1989-12-12 | Medtronic, Inc. | Intravascular radially expandable stent and method of implant |
| US5266073A (en)* | 1987-12-08 | 1993-11-30 | Wall W Henry | Angioplasty stent |
| US4990151A (en)* | 1988-09-28 | 1991-02-05 | Medinvent S.A. | Device for transluminal implantation or extraction |
| US4950227A (en)* | 1988-11-07 | 1990-08-21 | Boston Scientific Corporation | Stent delivery system |
| US5476476A (en)* | 1989-06-06 | 1995-12-19 | Cordis Corporation | Dilatation balloon assembly |
| US5026377A (en)* | 1989-07-13 | 1991-06-25 | American Medical Systems, Inc. | Stent placement instrument and method |
| US5071407A (en)* | 1990-04-12 | 1991-12-10 | Schneider (U.S.A.) Inc. | Radially expandable fixation member |
| US5591172A (en)* | 1991-06-14 | 1997-01-07 | Ams Medinvent S.A. | Transluminal implantation device |
| US5464408A (en)* | 1991-06-14 | 1995-11-07 | Duc; Jerome | Transluminal implantation or extraction device |
| US5201757A (en)* | 1992-04-03 | 1993-04-13 | Schneider (Usa) Inc. | Medial region deployment of radially self-expanding stents |
| US5645559A (en)* | 1992-05-08 | 1997-07-08 | Schneider (Usa) Inc | Multiple layer stent |
| US5484444A (en)* | 1992-10-31 | 1996-01-16 | Schneider (Europe) A.G. | Device for the implantation of self-expanding endoprostheses |
| US5690644A (en)* | 1992-12-30 | 1997-11-25 | Schneider (Usa) Inc. | Apparatus for deploying body implantable stent |
| US5603698A (en)* | 1993-04-13 | 1997-02-18 | Boston Scientific Corporation | Prosthesis delivery system |
| WO1994024961A1 (en)* | 1993-04-23 | 1994-11-10 | Schneider (Usa) Inc. | Covered stent and stent delivery device |
| US5571135A (en)* | 1993-10-22 | 1996-11-05 | Scimed Life Systems Inc. | Stent delivery apparatus and method |
| WO1995026777A1 (en)* | 1994-04-01 | 1995-10-12 | Localmed, Inc. | Method and apparatus for performing multiple procedures |
| EP0699451A2 (en)* | 1994-08-29 | 1996-03-06 | Robert E. Fischell | Catheter |
| US5743874A (en)* | 1994-08-29 | 1998-04-28 | Fischell; Robert E. | Integrated catheter for balloon angioplasty and stent delivery |
| US5695499A (en)* | 1994-10-27 | 1997-12-09 | Schneider (Usa) Inc. | Medical device supported by spirally wound wire |
| US5634928A (en)* | 1994-12-07 | 1997-06-03 | Fischell Robert | Integrated dual-function catheter system and method for balloon angioplasty and stent delivery |
| EP0720837A1 (en)* | 1994-12-07 | 1996-07-10 | Robert E. Fischell | Integrated dual-function catheter system for balloon angioplasty and stent delivery |
| US5628755A (en)* | 1995-02-20 | 1997-05-13 | Schneider (Europe) A.G. | Balloon catheter and stent delivery system |
| US5662703A (en)* | 1995-04-14 | 1997-09-02 | Schneider (Usa) Inc. | Rolling membrane stent delivery device |
| US5534007A (en)* | 1995-05-18 | 1996-07-09 | Scimed Life Systems, Inc. | Stent deployment catheter with collapsible sheath |
| US5628754A (en)* | 1995-08-01 | 1997-05-13 | Medtronic, Inc. | Stent delivery guide catheter |
| US5653689A (en)* | 1995-09-30 | 1997-08-05 | Abacus Design & Development, Inc. | Infusion catheter |
| US5709703A (en)* | 1995-11-14 | 1998-01-20 | Schneider (Europe) A.G. | Stent delivery device and method for manufacturing same |
| US5658311A (en)* | 1996-07-05 | 1997-08-19 | Schneider (Usa) Inc. | High pressure expander bundle for large diameter stent deployment |
Non-Patent Citations (2)
| Title |
|---|
| European Search Report, 16 Feb. 1998, for appl. No. 973078033 2305.* |
| European Search Report, 16 Feb. 1998, for appl. No. 973078033-2305. |
Cited By (142)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US6156048A (en)* | 1997-03-06 | 2000-12-05 | Scimed Life Systems, Inc. | Atherectomy device for reducing damage to vessels and/or in-vivo stents |
| US6015420A (en)* | 1997-03-06 | 2000-01-18 | Scimed Life Systems, Inc. | Atherectomy device for reducing damage to vessels and/or in-vivo stents |
| US5910145A (en)* | 1997-03-31 | 1999-06-08 | Fischell; Robert E. | Stent delivery catheter system |
| US6168616B1 (en) | 1997-06-02 | 2001-01-02 | Global Vascular Concepts | Manually expandable stent |
| US6425898B1 (en)* | 1998-03-13 | 2002-07-30 | Cordis Corporation | Delivery apparatus for a self-expanding stent |
| US6042589A (en)* | 1998-03-17 | 2000-03-28 | Medicorp, S.A. | Reversible-action endoprosthesis delivery device |
| AU758842B2 (en)* | 1999-02-03 | 2003-04-03 | Cordis Corporation | A delivery apparatus for a self-expanding stent |
| EP1025813B2 (en)† | 1999-02-03 | 2015-07-08 | Cordis Corporation | A delivery apparatus for a self-expanding stent |
| US6221097B1 (en)* | 1999-03-22 | 2001-04-24 | Scimed Life System, Inc. | Lubricated sleeve material for stent delivery |
| US6443980B1 (en) | 1999-03-22 | 2002-09-03 | Scimed Life Systems, Inc. | End sleeve coating for stent delivery |
| US6379365B1 (en)* | 1999-03-29 | 2002-04-30 | Alexis Diaz | Stent delivery catheter system having grooved shaft |
| US6315790B1 (en) | 1999-06-07 | 2001-11-13 | Scimed Life Systems, Inc. | Radiopaque marker bands |
| US6168617B1 (en)* | 1999-06-14 | 2001-01-02 | Scimed Life Systems, Inc. | Stent delivery system |
| US6626934B2 (en)* | 1999-06-14 | 2003-09-30 | Scimed Life Systems, Inc. | Stent delivery system |
| US6702802B1 (en) | 1999-11-10 | 2004-03-09 | Endovascular Technologies, Inc. | Catheters with improved transition |
| DE19954330C2 (en)* | 1999-11-11 | 2002-12-05 | Angiomed Ag | Stent delivery system for delivery of flexible stents |
| US8092509B2 (en) | 1999-11-11 | 2012-01-10 | Angiomed Gmbh & Co. Medizintechnik Kg | Implant delivery device |
| US20100280596A1 (en)* | 1999-11-11 | 2010-11-04 | C. R. Bard, Inc. | Implant delivery device |
| DE19954330A1 (en)* | 1999-11-11 | 2001-07-26 | Angiomed Ag | Percutaneous transluminal stent delivery system, has distal tip zone which is contiguous with distal end of tube, in which tip zone both luminal surface and abluminal surface taper radially inwardly nearer to distal end of tube |
| US6368301B1 (en) | 1999-12-21 | 2002-04-09 | Advanced Cardiovascular Systems, Inc. | Catheter having a soft distal tip |
| US6837869B2 (en) | 1999-12-21 | 2005-01-04 | Advanced Cardiovascular Systems, Inc. | Catheter having a soft distal tip |
| US20020082550A1 (en)* | 1999-12-21 | 2002-06-27 | Advanced Cardiovascular Systems, Inc. | Catheter having a soft distal tip |
| US8992597B2 (en) | 1999-12-31 | 2015-03-31 | Abps Venture One, Ltd. | Endoluminal cardiac and venous valve prostheses and methods of manufacture and delivery thereof |
| US7799069B2 (en) | 1999-12-31 | 2010-09-21 | Abps Venture One, Ltd. | Endoluminal cardiac and venous valve prostheses and methods of manufacture and delivery thereof |
| US6652578B2 (en)* | 1999-12-31 | 2003-11-25 | Abps Venture One, Ltd. | Endoluminal cardiac and venous valve prostheses and methods of manufacture and delivery thereof |
| US9421100B2 (en) | 1999-12-31 | 2016-08-23 | ABPS Venture One, Ltd., a wholly owned subsidiary of Palmaz Scientific, Inc. | Endoluminal cardiac and venous valve prostheses and methods of manufacture and delivery thereof |
| US8221493B2 (en) | 1999-12-31 | 2012-07-17 | Abps Venture One, Ltd. | Endoluminal cardiac and venous valve prostheses and methods of manufacture and delivery thereof |
| US6702843B1 (en)* | 2000-04-12 | 2004-03-09 | Scimed Life Systems, Inc. | Stent delivery means with balloon retraction means |
| DE20009204U1 (en) | 2000-05-22 | 2000-08-17 | Jomed GmbH, 72414 Rangendingen | Stent implantation catheter assembly |
| US6743219B1 (en) | 2000-08-02 | 2004-06-01 | Cordis Corporation | Delivery apparatus for a self-expanding stent |
| US6773446B1 (en) | 2000-08-02 | 2004-08-10 | Cordis Corporation | Delivery apparatus for a self-expanding stent |
| US20050027306A1 (en)* | 2000-10-17 | 2005-02-03 | Medtronic Ave, Inc. | Stent delivery system |
| US7356903B2 (en) | 2000-10-17 | 2008-04-15 | Medtronic Vascular, Inc. | Stent delivery system |
| US6786918B1 (en)* | 2000-10-17 | 2004-09-07 | Medtronic Vascular, Inc. | Stent delivery system |
| US20020183826A1 (en)* | 2000-11-13 | 2002-12-05 | Angiomed Gmbh & Co. | Implant delivery device |
| US7758624B2 (en) | 2000-11-13 | 2010-07-20 | C. R. Bard, Inc. | Implant delivery device |
| US6692461B2 (en) | 2001-08-07 | 2004-02-17 | Advanced Cardiovascular Systems, Inc. | Catheter tip |
| US20030060872A1 (en)* | 2001-09-26 | 2003-03-27 | Gary Gomringer | Stent with radiopaque characteristics |
| US20090062835A1 (en)* | 2001-11-01 | 2009-03-05 | Advanced Cardovascular Systems, Inc. | Catheter having an improved distal tip |
| US8221444B2 (en) | 2001-11-01 | 2012-07-17 | Abbott Cardiovascular Systems Inc. | Catheter having an improved distal tip |
| US20030144670A1 (en)* | 2001-11-29 | 2003-07-31 | Cook Incorporated | Medical device delivery system |
| US7871430B2 (en)* | 2001-11-29 | 2011-01-18 | Cook Incorporated | Medical device delivery system |
| US20110125248A1 (en)* | 2001-12-03 | 2011-05-26 | Xtent, Inc. | Custom length stent apparatus |
| US9326876B2 (en) | 2001-12-03 | 2016-05-03 | J.W. Medical Systems Ltd. | Apparatus and methods for delivery of multiple distributed stents |
| US8956398B2 (en)* | 2001-12-03 | 2015-02-17 | J.W. Medical Systems Ltd. | Custom length stent apparatus |
| US7169170B2 (en) | 2002-02-22 | 2007-01-30 | Cordis Corporation | Self-expanding stent delivery system |
| US20060116750A1 (en)* | 2002-02-28 | 2006-06-01 | Counter Clockwise, Inc. | Guidewire loaded stent for delivery through a catheter |
| US20060036309A1 (en)* | 2002-02-28 | 2006-02-16 | Counter Clockwise, Inc. | Guidewire loaded stent for delivery through a catheter |
| US8696728B2 (en) | 2002-02-28 | 2014-04-15 | Bay Street Medical, Inc. | Guidewire loaded stent for delivery through a catheter |
| US8641748B2 (en) | 2002-02-28 | 2014-02-04 | Bay Street Medical, Inc. | Guidewire loaded stent for delivery through a catheter |
| US9114038B2 (en) | 2002-02-28 | 2015-08-25 | Back Bay Medical Inc. | Method of delivering a stent |
| US20070299501A1 (en)* | 2002-02-28 | 2007-12-27 | Counter Clockwise, Inc. | Guidewire loaded stent for delivery through a catheter |
| US20040188304A1 (en)* | 2002-12-31 | 2004-09-30 | Bonnette Michael J. | Packaging system with oxygen sensor |
| US8016869B2 (en)* | 2003-03-26 | 2011-09-13 | Biosensors International Group, Ltd. | Guidewire-less stent delivery methods |
| US7771463B2 (en) | 2003-03-26 | 2010-08-10 | Ton Dai T | Twist-down implant delivery technologies |
| US7785361B2 (en)* | 2003-03-26 | 2010-08-31 | Julian Nikolchev | Implant delivery technologies |
| US20050049669A1 (en)* | 2003-08-29 | 2005-03-03 | Jones Donald K. | Self-expanding stent and stent delivery system with distal protection |
| US20050049668A1 (en)* | 2003-08-29 | 2005-03-03 | Jones Donald K. | Self-expanding stent and stent delivery system for treatment of vascular stenosis |
| EP1516601A2 (en) | 2003-08-29 | 2005-03-23 | Cordis Neurovascular, Inc. | Self-expanding stent and stent and delivery system with distal protection |
| US20050049670A1 (en)* | 2003-08-29 | 2005-03-03 | Jones Donald K. | Self-expanding stent and stent delivery system for treatment of vascular disease |
| US9566179B2 (en) | 2003-12-23 | 2017-02-14 | J.W. Medical Systems Ltd. | Devices and methods for controlling and indicating the length of an interventional element |
| US20050154439A1 (en)* | 2004-01-08 | 2005-07-14 | Gunderson Richard C. | Medical device delivery systems |
| US8152818B2 (en) | 2004-01-08 | 2012-04-10 | Boston Scientific Scimed, Inc. | Medical device delivery systems |
| US7651521B2 (en) | 2004-03-02 | 2010-01-26 | Cardiomind, Inc. | Corewire actuated delivery system with fixed distal stent-carrying extension |
| US8029555B2 (en) | 2004-03-31 | 2011-10-04 | Cook Medical Technologies Llc | Stent introducer system |
| US20050278011A1 (en)* | 2004-06-10 | 2005-12-15 | Peckham John E | Stent delivery system |
| US8986362B2 (en) | 2004-06-28 | 2015-03-24 | J.W. Medical Systems Ltd. | Devices and methods for controlling expandable prostheses during deployment |
| US9700448B2 (en) | 2004-06-28 | 2017-07-11 | J.W. Medical Systems Ltd. | Devices and methods for controlling expandable prostheses during deployment |
| US7794487B2 (en)* | 2004-07-28 | 2010-09-14 | Cordis Corporation | Reduced deployment force delivery device |
| US20060025844A1 (en)* | 2004-07-28 | 2006-02-02 | Majercak David C | Reduced deployment force delivery device |
| US20060253184A1 (en)* | 2005-05-04 | 2006-11-09 | Kurt Amplatz | System for the controlled delivery of stents and grafts |
| US20070118207A1 (en)* | 2005-05-04 | 2007-05-24 | Aga Medical Corporation | System for controlled delivery of stents and grafts |
| US8652193B2 (en) | 2005-05-09 | 2014-02-18 | Angiomed Gmbh & Co. Medizintechnik Kg | Implant delivery device |
| US20080234796A1 (en)* | 2005-05-09 | 2008-09-25 | Angiomed Gmbh & Co. Medizintechnik Kg | Implant Delivery Device |
| US8641724B2 (en)* | 2005-06-30 | 2014-02-04 | Rox Medical, Inc. | Devices, systems and methods for creation of a peripherally located fistula |
| US9179916B2 (en) | 2005-06-30 | 2015-11-10 | Rox Medical Inc. | Devices, systems and methods for creation of a peripherally located fistula |
| US20080119879A1 (en)* | 2005-06-30 | 2008-05-22 | Rox Medical, Inc. | Devices, systems and methods for creation of a peripherally located fistula |
| US9955970B2 (en) | 2005-06-30 | 2018-05-01 | Rox Medical, Inc. | Devices, systems and methods for creation of peripherally located fistula |
| US8579954B2 (en) | 2005-11-02 | 2013-11-12 | Biosensors International Group, Ltd. | Untwisting restraint implant delivery system |
| US8273116B2 (en) | 2005-11-02 | 2012-09-25 | Biosensors International Group, Ltd. | Indirect-release electrolytic implant delivery systems |
| US7862602B2 (en) | 2005-11-02 | 2011-01-04 | Biosensors International Group, Ltd | Indirect-release electrolytic implant delivery systems |
| US20070100420A1 (en)* | 2005-11-02 | 2007-05-03 | Kavanagh Joseph T | Guided stent delivery systems of minimal diameter |
| US8900285B2 (en) | 2005-11-02 | 2014-12-02 | Biosensors International Group, Ltd. | Covering electrolytic restraint implant delivery systems |
| US8974509B2 (en) | 2005-11-02 | 2015-03-10 | Biosensors International Group, Ltd. | Pass-through restraint electrolytic implant delivery systems |
| US20070198076A1 (en)* | 2006-02-13 | 2007-08-23 | Stephen Hebert | System for delivering a stent |
| US9883957B2 (en) | 2006-03-20 | 2018-02-06 | J.W. Medical Systems Ltd. | Apparatus and methods for deployment of linked prosthetic segments |
| US7699884B2 (en) | 2006-03-22 | 2010-04-20 | Cardiomind, Inc. | Method of stenting with minimal diameter guided delivery systems |
| US8652192B2 (en) | 2006-03-31 | 2014-02-18 | St. Jude Medical, Cardiology Division, Inc. | Stent and system and method for deploying a stent |
| US20070233232A1 (en)* | 2006-03-31 | 2007-10-04 | St Germain Jon | Stent and system and method for deploying a stent |
| US8980297B2 (en) | 2007-02-20 | 2015-03-17 | J.W. Medical Systems Ltd. | Thermo-mechanically controlled implants and methods of use |
| US20110022148A1 (en)* | 2007-02-20 | 2011-01-27 | Xtent, Inc. | Thermo-mechanically controlled implants and methods of use |
| US9457133B2 (en) | 2007-02-20 | 2016-10-04 | J.W. Medical Systems Ltd. | Thermo-mechanically controlled implants and methods of use |
| US9339404B2 (en) | 2007-03-22 | 2016-05-17 | J.W. Medical Systems Ltd. | Devices and methods for controlling expandable prostheses during deployment |
| US20080255654A1 (en)* | 2007-03-22 | 2008-10-16 | Bay Street Medical | System for delivering a stent |
| US9144508B2 (en) | 2007-07-19 | 2015-09-29 | Back Bay Medical Inc. | Radially expandable stent |
| US20090024205A1 (en)* | 2007-07-19 | 2009-01-22 | Bay Street Medical | Radially Expandable Stent |
| US9101503B2 (en) | 2008-03-06 | 2015-08-11 | J.W. Medical Systems Ltd. | Apparatus having variable strut length and methods of use |
| US11602430B2 (en) | 2008-03-18 | 2023-03-14 | Medtronic Ventor Technologies Ltd. | Valve suturing and implantation procedures |
| US10856979B2 (en)* | 2008-03-18 | 2020-12-08 | Medtronic Ventor Technologies Ltd. | Valve suturing and implantation procedures |
| US20170128207A1 (en)* | 2008-03-18 | 2017-05-11 | Medtronic Ventor Technologies, Ltd. | Valve suturing and implantation procedures |
| US11278408B2 (en) | 2008-03-18 | 2022-03-22 | Medtronic Venter Technologies, Ltd. | Valve suturing and implantation procedures |
| US11458016B2 (en)* | 2008-03-18 | 2022-10-04 | Medtronic Ventor Technologies, Ltd. | Valve suturing and implantation procedures |
| US20100256600A1 (en)* | 2009-04-04 | 2010-10-07 | Ferrera David A | Neurovascular otw pta balloon catheter and delivery system |
| US8657870B2 (en) | 2009-06-26 | 2014-02-25 | Biosensors International Group, Ltd. | Implant delivery apparatus and methods with electrolytic release |
| US8784468B2 (en) | 2010-11-17 | 2014-07-22 | Boston Scientific Scimed, Inc. | Stent delivery systems and locking members for use with stent delivery systems |
| US9974679B2 (en) | 2010-11-17 | 2018-05-22 | Boston Scientific Scimed, Inc. | Stent delivery system |
| US9220619B2 (en) | 2010-11-17 | 2015-12-29 | Boston Scientific Scimed, Inc. | Stent delivery system |
| US9084692B2 (en) | 2010-11-17 | 2015-07-21 | Boston Scientific Scimed, Inc. | Stent delivery system |
| US10058443B2 (en) | 2011-11-02 | 2018-08-28 | Boston Scientific Scimed, Inc. | Stent delivery systems and methods for use |
| US10940167B2 (en) | 2012-02-10 | 2021-03-09 | Cvdevices, Llc | Methods and uses of biological tissues for various stent and other medical applications |
| US11013626B2 (en) | 2012-06-15 | 2021-05-25 | Trivascular, Inc. | Endovascular delivery system with an improved radiopaque marker scheme |
| US10034787B2 (en) | 2012-06-15 | 2018-07-31 | Trivascular, Inc. | Endovascular delivery system with an improved radiopaque marker scheme |
| US9233015B2 (en) | 2012-06-15 | 2016-01-12 | Trivascular, Inc. | Endovascular delivery system with an improved radiopaque marker scheme |
| US11406495B2 (en) | 2013-02-11 | 2022-08-09 | Cook Medical Technologies Llc | Expandable support frame and medical device |
| US10159587B2 (en) | 2015-01-16 | 2018-12-25 | Boston Scientific Scimed, Inc. | Medical device delivery system with force reduction member |
| US10245167B2 (en) | 2015-01-29 | 2019-04-02 | Intact Vascular, Inc. | Delivery device and method of delivery |
| US11304836B2 (en) | 2015-01-29 | 2022-04-19 | Intact Vascular, Inc. | Delivery device and method of delivery |
| US20180000619A1 (en)* | 2015-01-29 | 2018-01-04 | Intact Vascular, Inc. | Delivery device and method of delivery |
| US11351048B2 (en) | 2015-11-16 | 2022-06-07 | Boston Scientific Scimed, Inc. | Stent delivery systems with a reinforced deployment sheath |
| US10993824B2 (en)* | 2016-01-01 | 2021-05-04 | Intact Vascular, Inc. | Delivery device and method of delivery |
| AU2016380347B2 (en)* | 2016-01-01 | 2019-11-21 | Intact Vascular, Inc. | Delivery device and method of delivery |
| US11065137B2 (en) | 2016-02-26 | 2021-07-20 | Boston Scientific Scimed, Inc. | Stent delivery systems with a reduced profile |
| US11957577B2 (en) | 2017-01-19 | 2024-04-16 | 4C Medical Technologies, Inc. | Systems, methods and devices for delivery systems, methods and devices for implanting prosthetic heart valves |
| US11944537B2 (en) | 2017-01-24 | 2024-04-02 | 4C Medical Technologies, Inc. | Systems, methods and devices for two-step delivery and implantation of prosthetic heart valve |
| US12029647B2 (en) | 2017-03-07 | 2024-07-09 | 4C Medical Technologies, Inc. | Systems, methods and devices for prosthetic heart valve with single valve leaflet |
| US12036113B2 (en) | 2017-06-14 | 2024-07-16 | 4C Medical Technologies, Inc. | Delivery of heart chamber prosthetic valve implant |
| WO2019023258A1 (en)* | 2017-07-26 | 2019-01-31 | Intact Vascular, Inc. | Delivery device and method of delivery |
| US11660218B2 (en) | 2017-07-26 | 2023-05-30 | Intact Vascular, Inc. | Delivery device and method of delivery |
| US11013627B2 (en) | 2018-01-10 | 2021-05-25 | Boston Scientific Scimed, Inc. | Stent delivery system with displaceable deployment mechanism |
| US11857441B2 (en) | 2018-09-04 | 2024-01-02 | 4C Medical Technologies, Inc. | Stent loading device |
| CN112702979A (en)* | 2018-09-14 | 2021-04-23 | 4C医学技术有限公司 | Repositioning wire and method for repositioning a prosthetic heart valve device in a heart chamber and related systems, devices, and methods |
| WO2020056234A1 (en)* | 2018-09-14 | 2020-03-19 | 4C Medical Technologies, Inc. | Repositioning wires and methods for repositioning prosthetic heart valve devices within a heart chamber and related systems, devices and methods |
| US11000000B2 (en) | 2018-09-14 | 2021-05-11 | 4C Medical Technologies, Inc. | Repositioning wires and methods for repositioning prosthetic heart valve devices within a heart chamber and related systems, devices and methods |
| US12144752B2 (en) | 2019-02-13 | 2024-11-19 | Boston Scientific Scimed, Inc. | Stent delivery systems |
| US11602447B2 (en) | 2019-02-13 | 2023-03-14 | Boston Scientific Scimed Inc. | Stent delivery systems |
| US12232991B2 (en) | 2019-04-15 | 2025-02-25 | 4C Medical Technologies, Inc. | Loading systems for collapsible prosthetic heart valve devices and methods thereof |
| US12414797B2 (en) | 2019-08-22 | 2025-09-16 | Edwards Lifesciences Corporation | Puncture needles |
| US12408907B1 (en) | 2019-11-14 | 2025-09-09 | Edwards Lifesciences Corporation | Method of reducing left atrial pressure |
| US12133797B2 (en) | 2020-01-31 | 2024-11-05 | 4C Medical Technologies, Inc. | Prosthetic heart valve delivery system: paddle attachment feature |
| US11931253B2 (en) | 2020-01-31 | 2024-03-19 | 4C Medical Technologies, Inc. | Prosthetic heart valve delivery system: ball-slide attachment |
| US12053375B2 (en) | 2020-03-05 | 2024-08-06 | 4C Medical Technologies, Inc. | Prosthetic mitral valve with improved atrial and/or annular apposition and paravalvular leakage mitigation |
| US11992403B2 (en) | 2020-03-06 | 2024-05-28 | 4C Medical Technologies, Inc. | Devices, systems and methods for improving recapture of prosthetic heart valve device with stent frame having valve support with inwardly stent cells |
Also Published As
| Publication number | Publication date |
|---|---|
| CA2218972C (en) | 2002-12-24 |
| DE69730666T2 (en) | 2005-09-22 |
| JPH10137344A (en) | 1998-05-26 |
| EP0839504A1 (en) | 1998-05-06 |
| JP2999165B2 (en) | 2000-01-17 |
| CA2218972A1 (en) | 1998-05-05 |
| EP0839504B1 (en) | 2004-09-15 |
| DE69730666D1 (en) | 2004-10-21 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| US5843090A (en) | Stent delivery device | |
| US10912665B2 (en) | Balloon catheter for multiple adjustable stent deployment | |
| US6280412B1 (en) | Stent securement by balloon modification | |
| US6602226B1 (en) | Low-profile stent delivery system and apparatus | |
| US5078720A (en) | Stent placement instrument and method | |
| US7563270B2 (en) | Rotating stent delivery system for side branch access and protection and method of using same | |
| US5980530A (en) | Stent delivery system | |
| US6056759A (en) | Fluid actuated stent delivery system | |
| US6391032B2 (en) | Stent delivery system having stent securement means | |
| JPH10277159A (en) | Stent supply catheter system | |
| JP2004516886A (en) | Expandable assisted delivery system for self-expanding stent | |
| US6117104A (en) | Stent deployment system and method of use | |
| JP7361684B2 (en) | Delivery balloon with retractable retention cuff | |
| US6645174B1 (en) | Stent delivery system | |
| US20240139007A1 (en) | Integrated deployment system (delivery mechanism) for intracranial atherosclerosis stent | |
| AU2756502A (en) | Fluid actuated stent delivery system |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| AS | Assignment | Owner name:SCHNEIDER (USA) INC., MINNESOTA Free format text:ASSIGNMENT OF ASSIGNORS INTEREST;ASSIGNOR:SCHUETZ, WALLACE J.;REEL/FRAME:008299/0441 Effective date:19961105 | |
| STCF | Information on status: patent grant | Free format text:PATENTED CASE | |
| FPAY | Fee payment | Year of fee payment:4 | |
| REMI | Maintenance fee reminder mailed | ||
| FPAY | Fee payment | Year of fee payment:8 | |
| AS | Assignment | Owner name:BOSTON SCIENTIFIC SCIMED, INC., MINNESOTA Free format text:CHANGE OF NAME;ASSIGNOR:SCIMED LIFE SYSTEMS, INC.;REEL/FRAME:018454/0866 Effective date:20050101 Owner name:BOSTON SCIENTIFIC SCIMED, INC., MINNESOTA Free format text:CHANGE OF NAME;ASSIGNOR:SCHNEIDER (USA) INC.;REEL/FRAME:018454/0858 Effective date:19990427 Owner name:SCIMED LIFE SYSTEMS, INC., MINNESOTA Free format text:MERGER;ASSIGNOR:BOSTON SCIENTIFIC SCIMED, INC.;REEL/FRAME:018454/0834 Effective date:20050101 | |
| FPAY | Fee payment | Year of fee payment:12 |