US5246454A - Encapsulated implant - Google Patents
Encapsulated implantDownload PDFInfo
- Publication number
- US5246454A US5246454AUS07/733,813US73381391AUS5246454AUS 5246454 AUS5246454 AUS 5246454AUS 73381391 AUS73381391 AUS 73381391AUS 5246454 AUS5246454 AUS 5246454A
- Authority
- US
- United States
- Prior art keywords
- pouches
- bags
- saline
- breast implant
- implant
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Expired - Fee Related
Links
- 239000007943implantSubstances0.000titleclaimsabstractdescription59
- FAPWRFPIFSIZLT-UHFFFAOYSA-MSodium chlorideChemical compound[Na+].[Cl-]FAPWRFPIFSIZLT-UHFFFAOYSA-M0.000claimsabstractdescription51
- 239000011780sodium chlorideSubstances0.000claimsabstractdescription35
- 239000012528membraneSubstances0.000claimsabstractdescription30
- 210000000481breastAnatomy0.000claimsabstractdescription21
- 238000005538encapsulationMethods0.000claimsabstractdescription8
- 239000000463materialSubstances0.000claimsabstractdescription8
- 239000012530fluidSubstances0.000claimsdescription14
- 230000003190augmentative effectEffects0.000claims1
- 230000002708enhancing effectEffects0.000claims1
- 239000000499gelSubstances0.000description12
- 210000001519tissueAnatomy0.000description10
- 230000008901benefitEffects0.000description6
- 229920001296polysiloxanePolymers0.000description6
- 239000000243solutionSubstances0.000description6
- 239000011324beadSubstances0.000description4
- 210000002976pectoralis muscleAnatomy0.000description3
- 231100000241scarToxicity0.000description3
- 229920002379silicone rubberPolymers0.000description3
- 239000004945silicone rubberSubstances0.000description3
- 206010062575Muscle contractureDiseases0.000description2
- 208000006111contractureDiseases0.000description2
- 238000003780insertionMethods0.000description2
- 230000037431insertionEffects0.000description2
- 239000000203mixtureSubstances0.000description2
- 230000004048modificationEffects0.000description2
- 238000012986modificationMethods0.000description2
- 206010016275FearDiseases0.000description1
- 206010028980NeoplasmDiseases0.000description1
- 239000008156Ringer's lactate solutionSubstances0.000description1
- 239000004809TeflonSubstances0.000description1
- 229920006362Teflon®Polymers0.000description1
- 230000004888barrier functionEffects0.000description1
- 239000013060biological fluidSubstances0.000description1
- 230000015572biosynthetic processEffects0.000description1
- ZEWYCNBZMPELPF-UHFFFAOYSA-Jcalcium;potassium;sodium;2-hydroxypropanoic acid;sodium;tetrachlorideChemical group[Na].[Na+].[Cl-].[Cl-].[Cl-].[Cl-].[K+].[Ca+2].CC(O)C(O)=OZEWYCNBZMPELPF-UHFFFAOYSA-J0.000description1
- 201000011510cancerDiseases0.000description1
- 210000000038chestAnatomy0.000description1
- 238000001514detection methodMethods0.000description1
- 230000000694effectsEffects0.000description1
- 238000005516engineering processMethods0.000description1
- 208000014674injuryDiseases0.000description1
- 230000036210malignancyEffects0.000description1
- 238000004519manufacturing processMethods0.000description1
- 230000002265preventionEffects0.000description1
- 238000007788rougheningMethods0.000description1
- 238000007789sealingMethods0.000description1
- 238000004513sizingMethods0.000description1
- PUZPDOWCWNUUKD-UHFFFAOYSA-Msodium fluorideChemical compound[F-].[Na+]PUZPDOWCWNUUKD-UHFFFAOYSA-M0.000description1
- 239000000126substanceSubstances0.000description1
- 238000001356surgical procedureMethods0.000description1
- 230000008467tissue growthEffects0.000description1
- 230000008733traumaEffects0.000description1
Images
Classifications
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F2/00—Filters implantable into blood vessels; Prostheses, i.e. artificial substitutes or replacements for parts of the body; Appliances for connecting them with the body; Devices providing patency to, or preventing collapsing of, tubular structures of the body, e.g. stents
- A61F2/02—Prostheses implantable into the body
- A61F2/12—Mammary prostheses
Definitions
- This inventionconcerns implants for human bodies, and particularly breast implants.
- Implantsare used to augment or re-shape body portions, or to replace or restore diseased or injured body parts or parts removed by surgery or trauma.
- breast prosthesis or implantswhich are used to augment, re-shape or replace human breast tissue.
- breast implantsare used for placement between pectoral muscles and breast tissue.
- the implantscan also be placed under pectoral muscles between pectoral muscles and the rib cage.
- implantsare made of soft fluid impermeable rupture-preventing material such as silicone rubber. Layers of material may be used.
- the bagsmay be filled or inflated with fluid materials, usually a gel and more recently a or saline solution.
- a gelhas been preferred because of its ability to match the weight and feel of tissue which the implants are designed to augment, supplant or replace, and because saline implants deflate rapidly if a leak develops.
- the present inventionis directed toward a solution of those problems.
- Prior art implant devicesmay experience contracture, causing unwanted firmness of the implant which is intended to be soft and flexible. That is because scar tissue may tend to surround and may tend to compress the implant.
- Prior art implant deviceshave approached the problem by constructing outer surfaces of Teflon and roughening the outer surfaces to redirect scar tissue.
- Prior art saline-filled implantsare prone to rapid deflation.
- the present inventionsolves that problem by encapsulation of the saline.
- Prior art saline-filled implantshad an unnatural feel.
- the present design implantby encapsulating the saline, gives a more viscous feel to the saline, matching the feel of normal breast tissue.
- the present inventionuses the non-smooth outer surfaces made of the prior art structure and composition to reduce the problem, and also uniquely tends to solve the problem by virtue of a changed inner structure.
- the present inventionprovides surgical implants for human breasts, which have small, 5-10 cc pouches containing saline material without voids or gas.
- the small pouchesare held within an outer membrane. About 20-50 pouches fill each outer membrane.
- the encapsulation of the saline solution within the small pouchesprovides an apparent increase of viscosity of saline.
- the small pouchesPreferably have flat disc shapes, which provide protection against rupture.
- a preferred human breast implantcomprises a flexible outer membrane containing small individual pouches within the membrane and fluid material sealed within the pouches.
- the fluid with the breast implant pouchesis saline solution.
- the preferred poucheshave a generally flat form and round peripheries.
- the pouchesare loosely positioned within the outer membrane and the pouches fill the membrane.
- the encapsulation of the saline in the small poucheseffectively increases apparent viscosity of the saline.
- the preferred pouchesare small bags, about 2 to 20 cubic centimeters in volume and preferably about 5 to 10 cubic centimeters in volume.
- the pouchesare formed as a skin around the encapsulated fluid. Encapsulating about 5 to 10 cubic centimeters of saline solution in each pouch increases apparent viscosity of saline, enhances the stability of the implants and provides a more natural feel.
- the bagsare disc-shaped for increasing surface area to volume ratio of the bags and making the bags relatively rupture-proof. From about 40 to 48 disc-shaped bags are held within the outer membrane in a preferred implant.
- Encapsulationhas the additional benefit of increasing the apparent viscosity of the saline. That enhances the stability of the implant, giving it a more natural feel.
- Salineis an excellent solution for filing the pouches, but other options may be slightly better.
- One exampleis Ringer's Lactate.
- salineis slightly hypertonic, the volume involved is small. Saline has the advantage of being extremely stable, non-reactive and inexpensive.
- the small beadsare not spherical. Spherical beads might be more easily ruptured. To diminish the problem of rupture, the beads are disc-shaped. That increases their surface area-to-volume ratio and makes them relatively rupture-proof.
- a preferred form of the inventionuses 5 cc disc/bags, which simplifies sizing of the implants.
- a 200 cc implantcontains 40 discs, a 240 cc implant contains 48 discs, etc.
- the advantages of the encapsulated saline implantare many.
- the implantoffers significant advantages in safety and in perceived safety. Leakage of saline within the implant and deflation of individual pouches would be highly unlikely, and perfectly safe. Leaking of saline from a ruptured outer membrane and a few or more than a few pouches would be safe.
- the implantis radiographically isodense with saline. That should make mammographic detection of malignancy easier in patients with this implant than in those with silicone implants.
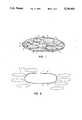
- FIG. 1is a cross-section of one embodiment of an implant outer membrane filled with disc-shaped saline-filled pouches.
- FIG. 2shows an implant shell and uniform saline packets before assembly.
- FIG. 3shows inserting the packets into an open shell before closing.
- FIG. 4shows the nested packets with spaces still existing within the shell.
- FIG. 5shows a preferred embodiment of the sealed shell, wherein spaces within have been evacuated.
- a preferred breast implant 1is made with a relatively thick outer membrane 3 which is made of any suitable material, for example silicone rubber.
- An outer surface 5may be coated and roughened to redirect scar tissue growth.
- Within the relatively thick outer membraneare a plurality of small saline-filled pouches 7.
- Each pouchis made of a relatively thin disc-shaped outer layer 9 and is filled with saline solution 11 or other biologically compatible solution or gel.
- all discsare of a similar size, holding about 5 cc of solution.
- all of the small bagsare of an equal size and hold about 10 cc of solution.
- the individual bags 7may be made of varied size such as small bags 15, intermediate bags 19, and larger bags 21.
- there may be larger bags 23which may have an elliptical or elongated shape. It is preferable for the bags to hold between 2 cc and 20 cc of saline solution.
- all of the small bagsare generally flat so the bags may readily deform upon localized contact or pressure without increasing internal pressure, which might otherwise influence the bags to rupture.
- the bags 7 in a preferred arrangementare loosely arranged within the thick outer membrane 3.
- the bagsmay be fused, bonded or welded to surfaces of adjacent bags.
- all of the bagsare formed with a similar saline solution.
- the bagsmay be filled with gels or with differing solutions, or with gels of different compositions or physical qualities.
- the bagsmay be part-filled with gel and part-filled with fluids which do not dissolve the gel.
- the individual bags 7are free to move within the outer membrane 3.
- the bagsmay be shaped or positioned in such a way as to restrict or prevent their movement within the outer membrane 3.
- Outer surfaces of the bagsmay be bonded or welded to the inner surface of the membrane, when freedom of movement of the bags is not desired.
- the bagsmay be interconnected with orifices which permit flow between adjacent bags in groups of two or more.
- surfaces of the bags and an inner surface of the outer membraneare smooth.
- surfaces of the bagsmay be textured, roughened or formed in a non-slip manner to discourage or retard relative movement of the bags.
- An inner surface of the thick outer membrane 3may be similarly formed.
- spaces 25 among the bags and between the bags and the thick outer membrane 3may be evacuated and refilled with inert biocompatible gas at or below atmospheric pressure or slightly above atmospheric pressure.
- the space 25may be filled with saline solution or with a biocompatible gel.
- the individual bagsare made with soft, flexible substances such as silicone rubber with fluid impermeable skins 9.
- the disc-shaped bags 7may be inserted into a preformed outer membrane 3, such as through a slit which is resealed, or the thick outer membrane may be formed after the bags are arranged in a desired number and relationship.
- FIGS. 2-5A preferred embodiment of the invention is assembled as shown in FIGS. 2-5.
- FIG. 2shows the formed saline packets 7 and shell 3 having opening 4 for insertion of the packets.
- FIG. 3shows insertion of the packets 7 and subsequent nesting.
- the packetsare uniformly constructed 5 cc saline bags which nest within the open shell, FIG. 4, leaving spaces prior to sealing of the shell.
- FIG. 5shows the sealed shell wherein air spaces have been evacuated, causing the saline packets to be tightly packed within the shell.
- the shape of the shelltherefore, depends upon forces applied to the implant with less lateral movement allowed because of evacuation of spaces 25 shown in FIG. 1.
- the outer containermay be net-like or porous so that the small pouches are held in place without a fluid or tissue barrier between the pouches and the surrounding fluid and tissue.
Landscapes
- Health & Medical Sciences (AREA)
- Cardiology (AREA)
- Oral & Maxillofacial Surgery (AREA)
- Transplantation (AREA)
- Engineering & Computer Science (AREA)
- Biomedical Technology (AREA)
- Heart & Thoracic Surgery (AREA)
- Vascular Medicine (AREA)
- Life Sciences & Earth Sciences (AREA)
- Animal Behavior & Ethology (AREA)
- General Health & Medical Sciences (AREA)
- Public Health (AREA)
- Veterinary Medicine (AREA)
- Prostheses (AREA)
Abstract
Description
This invention concerns implants for human bodies, and particularly breast implants.
Implants are used to augment or re-shape body portions, or to replace or restore diseased or injured body parts or parts removed by surgery or trauma.
Of particular interest are breast prosthesis or implants which are used to augment, re-shape or replace human breast tissue.
Particularly, breast implants are used for placement between pectoral muscles and breast tissue. The implants can also be placed under pectoral muscles between pectoral muscles and the rib cage. Typically, implants are made of soft fluid impermeable rupture-preventing material such as silicone rubber. Layers of material may be used. The bags may be filled or inflated with fluid materials, usually a gel and more recently a or saline solution. A gel has been preferred because of its ability to match the weight and feel of tissue which the implants are designed to augment, supplant or replace, and because saline implants deflate rapidly if a leak develops.
At the time of this invention, concern with rupturing of the bag or leakage of the gel and its possible effect on long-range health has been expressed. It is believed that the concerns may be unfounded, since the preferred gel is stable and non-reactive with human biological fluids or tissue.
Simple replacement of the gel by saline solution is not satisfactory because saline does not have the weight and feel of the gel and saline solution flows more freely in the implant than the tissue which it replaces. In addition, even a minute crack or hole in the shell leads to rapid deflation of the implant, exposing the patient to the risks and expense of reparation.
Some of the biggest problems with the current saline implants are threefold: They deflate rapidly when the membrane is ruptured. They have a less natural feel than silicone implants. They are prone to compressive capsular formation.
The present invention is directed toward a solution of those problems.
Prior art implant devices may experience contracture, causing unwanted firmness of the implant which is intended to be soft and flexible. That is because scar tissue may tend to surround and may tend to compress the implant. Prior art implant devices have approached the problem by constructing outer surfaces of Teflon and roughening the outer surfaces to redirect scar tissue.
Prior art saline-filled implants are prone to rapid deflation. The present invention solves that problem by encapsulation of the saline.
Prior art saline-filled implants had an unnatural feel. The present design implant, by encapsulating the saline, gives a more viscous feel to the saline, matching the feel of normal breast tissue.
The present invention uses the non-smooth outer surfaces made of the prior art structure and composition to reduce the problem, and also uniquely tends to solve the problem by virtue of a changed inner structure.
The present invention provides surgical implants for human breasts, which have small, 5-10 cc pouches containing saline material without voids or gas. The small pouches are held within an outer membrane. About 20-50 pouches fill each outer membrane. The encapsulation of the saline solution within the small pouches provides an apparent increase of viscosity of saline. Preferably the small pouches have flat disc shapes, which provide protection against rupture.
A preferred human breast implant comprises a flexible outer membrane containing small individual pouches within the membrane and fluid material sealed within the pouches.
Preferably the fluid with the breast implant pouches is saline solution. The preferred pouches have a generally flat form and round peripheries. Preferably the pouches are loosely positioned within the outer membrane and the pouches fill the membrane.
The encapsulation of the saline in the small pouches effectively increases apparent viscosity of the saline. The preferred pouches are small bags, about 2 to 20 cubic centimeters in volume and preferably about 5 to 10 cubic centimeters in volume. The pouches are formed as a skin around the encapsulated fluid. Encapsulating about 5 to 10 cubic centimeters of saline solution in each pouch increases apparent viscosity of saline, enhances the stability of the implants and provides a more natural feel. The bags are disc-shaped for increasing surface area to volume ratio of the bags and making the bags relatively rupture-proof. From about 40 to 48 disc-shaped bags are held within the outer membrane in a preferred implant.
Problems of the prior art are avoided by modification of the implant to encapsulate the saline according to the present invention. The manufacture of saline implants using encapsulation of saline is one of the objects of the invention.
By encapsulation of the saline into small bags similar to bath oil beads within the implant membrane, rapid deflation is impossible, even with disruption of the outer membrane. Small bags of about 2-20 cc and preferably 5-10 cc are packaged into an outer, larger membrane.
Encapsulation has the additional benefit of increasing the apparent viscosity of the saline. That enhances the stability of the implant, giving it a more natural feel.
Prevention of capsular contracture relies on the same technologies that are currently employed for the silicone prostheses; texturing of the surface.
Saline is an excellent solution for filing the pouches, but other options may be slightly better. One example is Ringer's Lactate. Although saline is slightly hypertonic, the volume involved is small. Saline has the advantage of being extremely stable, non-reactive and inexpensive.
Preferably the small beads are not spherical. Spherical beads might be more easily ruptured. To diminish the problem of rupture, the beads are disc-shaped. That increases their surface area-to-volume ratio and makes them relatively rupture-proof.
A preferred form of the invention uses 5 cc disc/bags, which simplifies sizing of the implants. A 200 cc implant contains 40 discs, a 240 cc implant contains 48 discs, etc.
The advantages of the encapsulated saline implant are many. The implant offers significant advantages in safety and in perceived safety. Leakage of saline within the implant and deflation of individual pouches would be highly unlikely, and perfectly safe. Leaking of saline from a ruptured outer membrane and a few or more than a few pouches would be safe.
The implant is radiographically isodense with saline. That should make mammographic detection of malignancy easier in patients with this implant than in those with silicone implants.
Concerns regarding silicone implants may prove in future years to be unfounded. At the present, however, there is insufficient data to allay the public's fears. Since there is no advantage of silicone implants over encapsulated saline ones, there is no reason to use silicone implants. Conversely, there are significant advantages of the present implants over all current prostheses, and there are compelling safety reasons for preferring them.
These and further and other objects and features of the invention are apparent in the disclosure, which includes the above and ongoing written specification, with the claims and the drawings.
FIG. 1 is a cross-section of one embodiment of an implant outer membrane filled with disc-shaped saline-filled pouches.
FIG. 2 shows an implant shell and uniform saline packets before assembly.
FIG. 3 shows inserting the packets into an open shell before closing.
FIG. 4 shows the nested packets with spaces still existing within the shell.
FIG. 5 shows a preferred embodiment of the sealed shell, wherein spaces within have been evacuated.
Referring to FIG. 1, a preferred breast implant 1 is made with a relatively thickouter membrane 3 which is made of any suitable material, for example silicone rubber. Anouter surface 5 may be coated and roughened to redirect scar tissue growth. Within the relatively thick outer membrane are a plurality of small saline-filledpouches 7. Each pouch is made of a relatively thin disc-shapedouter layer 9 and is filled with saline solution 11 or other biologically compatible solution or gel. In one preferred embodiment all discs are of a similar size, holding about 5 cc of solution. In another embodiment, all of the small bags are of an equal size and hold about 10 cc of solution. In another embodiment, as shown in the drawings, theindividual bags 7 may be made of varied size such assmall bags 15,intermediate bags 19, andlarger bags 21. In addition, there may belarger bags 23 which may have an elliptical or elongated shape. It is preferable for the bags to hold between 2 cc and 20 cc of saline solution.
In preferred embodiments, all of the small bags are generally flat so the bags may readily deform upon localized contact or pressure without increasing internal pressure, which might otherwise influence the bags to rupture.
Thebags 7 in a preferred arrangement are loosely arranged within the thickouter membrane 3. Alternatively, the bags may be fused, bonded or welded to surfaces of adjacent bags.
In one embodiment, all of the bags are formed with a similar saline solution. In other embodiments of the invention, the bags may be filled with gels or with differing solutions, or with gels of different compositions or physical qualities. The bags may be part-filled with gel and part-filled with fluids which do not dissolve the gel.
In a preferred embodiment, theindividual bags 7 are free to move within theouter membrane 3. Alternatively, the bags may be shaped or positioned in such a way as to restrict or prevent their movement within theouter membrane 3. Outer surfaces of the bags may be bonded or welded to the inner surface of the membrane, when freedom of movement of the bags is not desired.
The bags may be interconnected with orifices which permit flow between adjacent bags in groups of two or more.
In a preferred embodiment, surfaces of the bags and an inner surface of the outer membrane are smooth. In an alternate embodiment, surfaces of the bags may be textured, roughened or formed in a non-slip manner to discourage or retard relative movement of the bags. An inner surface of the thickouter membrane 3 may be similarly formed.
In one embodiment of the invention,spaces 25 among the bags and between the bags and the thickouter membrane 3 may be evacuated and refilled with inert biocompatible gas at or below atmospheric pressure or slightly above atmospheric pressure. Thespace 25 may be filled with saline solution or with a biocompatible gel.
In one form of the invention, the individual bags are made with soft, flexible substances such as silicone rubber with fluidimpermeable skins 9.
The disc-shapedbags 7 may be inserted into a preformedouter membrane 3, such as through a slit which is resealed, or the thick outer membrane may be formed after the bags are arranged in a desired number and relationship.
A preferred embodiment of the invention is assembled as shown in FIGS. 2-5. FIG. 2 shows the formedsaline packets 7 andshell 3 having opening 4 for insertion of the packets. FIG. 3 shows insertion of thepackets 7 and subsequent nesting. In one embodiment, the packets are uniformly constructed 5 cc saline bags which nest within the open shell, FIG. 4, leaving spaces prior to sealing of the shell.
FIG. 5 shows the sealed shell wherein air spaces have been evacuated, causing the saline packets to be tightly packed within the shell. The shape of the shell, therefore, depends upon forces applied to the implant with less lateral movement allowed because of evacuation ofspaces 25 shown in FIG. 1.
The outer container may be net-like or porous so that the small pouches are held in place without a fluid or tissue barrier between the pouches and the surrounding fluid and tissue.
While the invention has been described with reference to specific embodiments, modifications and variations of the invention may be constructed without departing from the scope of the invention, which is defined in the following claims.
Claims (9)
1. A bio-compatible human breast implant for augmenting, reshaping or replacing human breast tissue, comprising a flexible outer membrane containing a plurality of small individual pouches which are stackably layered within the membrane and fluid material sealed within the pouches, wherein each of the pouches is disc-shaped and has a generally flat form with a height less than a width.
2. The breast implant of claim 1, wherein the fluid in the pouches is saline solution.
3. The breast implant of claim 1 wherein the pouches have round peripheries.
4. The breast implant of claim 1, wherein the pouches are loosely positioned within the outer membrane.
5. The breast implant of claim 4, wherein the pouches fill the membrane.
6. The breast implant of claim 5, wherein the fluid is saline solution and encapsulation of the saline solution in the small pouches effectively increases apparent viscosity of the saline solution in the entire implant.
7. The breast implant of claim 1, wherein the pouches form a skin around the encapsulated fluid.
8. The breast implant of claim 1, wherein each of the pouches is a small bag of about 2 to 20 cubic centimeters in volume, and wherein about 2 to 20 cubic centimeters of saline solution is encapsulated in each pouch for increasing apparent viscosity of saline, and enhancing the feel of the implants and providing a more natural feel, wherein each of the bags is disc-shaped for increasing surface area to volume ratio of the bags and making the bags relatively rupture-proof, and wherein from about 10 to 80 disc-shaped bags are held within the outer membrane.
9. The breast implant of claim 8, wherein about 40 to 48 bags each contain about 5 to 10 cubic centimeters of fluid.
Priority Applications (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US07/733,813US5246454A (en) | 1991-07-22 | 1991-07-22 | Encapsulated implant |
Applications Claiming Priority (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US07/733,813US5246454A (en) | 1991-07-22 | 1991-07-22 | Encapsulated implant |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| US5246454Atrue US5246454A (en) | 1993-09-21 |
Family
ID=24949208
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US07/733,813Expired - Fee RelatedUS5246454A (en) | 1991-07-22 | 1991-07-22 | Encapsulated implant |
Country Status (1)
| Country | Link |
|---|---|
| US (1) | US5246454A (en) |
Cited By (88)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US5496370A (en)* | 1992-03-13 | 1996-03-05 | Robert S. Hamas | Gel-like prosthetic device |
| US5496367A (en)* | 1993-01-13 | 1996-03-05 | Fisher; Jack | Breast implant with baffles |
| US5496368A (en)* | 1992-06-12 | 1996-03-05 | Wiese; K. Guenter | Tissue expander inflating due to osmotic driving forces of a shaped body of hydrogel and an aqueous solution |
| US5549671A (en)* | 1994-12-28 | 1996-08-27 | Mcghan Medical Corporation | Adjunctive filler material for fluid-filled prosthesis |
| US5824081A (en)* | 1996-09-13 | 1998-10-20 | Lipomatrix Incorporated | Hydraulic foam tissue implant |
| US5843189A (en)* | 1995-06-13 | 1998-12-01 | Laboratoire Perouse Implant | Breast prosthesis |
| US6083262A (en)* | 1994-06-16 | 2000-07-04 | Caravel; Jean-Baudoin | Supple implantable prosthesis used in surgery for increasing the volume of or reconstructing soft tissue, notably a breast prosthesis, and its method of manufacture |
| US6099565A (en)* | 1995-06-07 | 2000-08-08 | Sakura, Jr.; Chester Y. | Prosthetic tissue implant and filler therefor |
| US6146418A (en)* | 1997-02-28 | 2000-11-14 | Berman; Mark | Body implant and method of implanting |
| US6183514B1 (en)* | 1999-08-16 | 2001-02-06 | Hilton Becker | Self positioning breast prosthesis |
| US6203570B1 (en) | 1999-11-24 | 2001-03-20 | John L. Baeke | Breast implant with position lock |
| US6312466B1 (en)* | 1995-05-22 | 2001-11-06 | Board Of Regents, University Of Texas System | Prosthesis containing a solution of polyethylene glycol |
| US6464726B1 (en)* | 2000-07-13 | 2002-10-15 | Jenna Heljenek | Breast implant system and method of augmentation |
| US20040249457A1 (en)* | 2003-06-09 | 2004-12-09 | Smith Lane Fielding | Mastopexy stabilization apparatus and method |
| US20050049701A1 (en)* | 2003-09-03 | 2005-03-03 | Brennan William A. | System and method for breast augmentation |
| US20060282164A1 (en)* | 2005-06-08 | 2006-12-14 | Joann Seastrom | Implant shell and filler apparatus |
| US20070123983A1 (en)* | 2003-09-03 | 2007-05-31 | Brennan William A | System and method for breast augmentation |
| US20080306590A1 (en)* | 2004-01-29 | 2008-12-11 | Smart Implant Plc | Prosthesis and Method of Manufacturing a Prosthesis |
| FR2921250A1 (en)* | 2007-09-20 | 2009-03-27 | Philippe Bellity | PROTHETIC UNIT, METHOD OF MANUFACTURE AND USE |
| US20090299473A1 (en)* | 2005-04-25 | 2009-12-03 | Jacky Govrin-Yehudian | Lightweight implantable prosthetic device |
| US7879091B1 (en)* | 2007-08-06 | 2011-02-01 | Martin Inell O | Inflatable prosthetic breast assembly and associated method |
| US20120116509A1 (en)* | 2009-07-17 | 2012-05-10 | Milux Holding Sa | Breast implant system |
| US20130150962A1 (en)* | 2011-11-04 | 2013-06-13 | Freddy Sanabria Scharf | Mammary prosthesis filled with expanded polymer microspheres |
| US8487012B2 (en) | 2010-01-28 | 2013-07-16 | Allergan, Inc. | Open celled foams, implants including them and processes for making same |
| US8506627B2 (en) | 2008-08-13 | 2013-08-13 | Allergan, Inc. | Soft filled prosthesis shell with discrete fixation surfaces |
| US20130231743A1 (en)* | 2011-10-19 | 2013-09-05 | Hilton Becker | Hybrid breast implant |
| US8546458B2 (en) | 2010-12-07 | 2013-10-01 | Allergan, Inc. | Process for texturing materials |
| US8679279B2 (en) | 2010-11-16 | 2014-03-25 | Allergan, Inc. | Methods for creating foam-like texture |
| US8679570B2 (en) | 2010-04-27 | 2014-03-25 | Allergan, Inc. | Foam-like materials and methods for producing same |
| US8685296B2 (en) | 2010-05-11 | 2014-04-01 | Allergan, Inc. | Porogen compositions, method of making and uses |
| US8801782B2 (en) | 2011-12-15 | 2014-08-12 | Allergan, Inc. | Surgical methods for breast reconstruction or augmentation |
| US8877822B2 (en) | 2010-09-28 | 2014-11-04 | Allergan, Inc. | Porogen compositions, methods of making and uses |
| US8889751B2 (en) | 2010-09-28 | 2014-11-18 | Allergan, Inc. | Porous materials, methods of making and uses |
| US8951596B2 (en) | 2009-10-16 | 2015-02-10 | Allergan, Inc. | Implants and methods for manufacturing same |
| US9044897B2 (en) | 2010-09-28 | 2015-06-02 | Allergan, Inc. | Porous materials, methods of making and uses |
| EP2240116B1 (en) | 2008-01-28 | 2015-07-01 | Biomet 3I, LLC | Implant surface with increased hydrophilicity |
| US9072821B2 (en) | 2010-02-05 | 2015-07-07 | Allergan, Inc. | Biocompatible structures and compositions |
| US9138310B2 (en) | 2007-11-05 | 2015-09-22 | Allergan, Inc. | Soft prosthesis shell texturing method |
| US9138308B2 (en) | 2010-02-03 | 2015-09-22 | Apollo Endosurgery, Inc. | Mucosal tissue adhesion via textured surface |
| US9138309B2 (en) | 2010-02-05 | 2015-09-22 | Allergan, Inc. | Porous materials, methods of making and uses |
| US9205577B2 (en) | 2010-02-05 | 2015-12-08 | Allergan, Inc. | Porogen compositions, methods of making and uses |
| US9370414B2 (en) | 2008-10-28 | 2016-06-21 | Implite Ltd. | Reconstructive breast prostheses |
| US9539086B2 (en) | 2014-05-16 | 2017-01-10 | Allergan, Inc. | Soft filled prosthesis shell with variable texture |
| US9688006B2 (en) | 2012-12-13 | 2017-06-27 | Allergan, Inc. | Device and method for making a variable surface breast implant |
| US9713524B2 (en) | 2013-01-30 | 2017-07-25 | Implite Ltd. | Human implantable tissue expanders |
| US9848972B2 (en) | 2008-08-13 | 2017-12-26 | Allergan, Inc. | Dual plane breast implant |
| US20170367850A1 (en)* | 2016-06-23 | 2017-12-28 | American Breast Care, Lp | Breast Prostheses with Phase Change Material |
| US10092392B2 (en) | 2014-05-16 | 2018-10-09 | Allergan, Inc. | Textured breast implant and methods of making same |
| US10213293B2 (en) | 2010-01-18 | 2019-02-26 | G & G Biotechnology Ltd | Lightweight breast implant material |
| US20190307598A1 (en)* | 2016-08-16 | 2019-10-10 | Purewick Corporation | Using wicking material to collect urine from a male for transport |
| US10933165B2 (en) | 2015-03-12 | 2021-03-02 | G & G Biotechnology Ltd | Composite implant material |
| US20210093469A1 (en)* | 2016-06-23 | 2021-04-01 | American Breast Care, Lp | Cooling Cushion for Breast Form |
| US11202853B2 (en) | 2010-05-11 | 2021-12-21 | Allergan, Inc. | Porogen compositions, methods of making and uses |
| US11801186B2 (en) | 2020-09-10 | 2023-10-31 | Purewick Corporation | Urine storage container handle and lid accessories |
| US11865030B2 (en) | 2021-01-19 | 2024-01-09 | Purewick Corporation | Variable fit fluid collection devices, systems, and methods |
| US11925575B2 (en) | 2021-02-26 | 2024-03-12 | Purewick Corporation | Fluid collection devices having a sump between a tube opening and a barrier, and related systems and methods |
| US11938053B2 (en) | 2018-05-01 | 2024-03-26 | Purewick Corporation | Fluid collection devices, systems, and methods |
| US11944740B2 (en) | 2018-05-01 | 2024-04-02 | Purewick Corporation | Fluid collection devices, related systems, and related methods |
| US12029678B2 (en) | 2016-07-27 | 2024-07-09 | Purewick Corporation | Male urine collection device using wicking material |
| US12029677B2 (en) | 2021-04-06 | 2024-07-09 | Purewick Corporation | Fluid collection devices having a collection bag, and related systems and methods |
| US12042423B2 (en) | 2020-10-07 | 2024-07-23 | Purewick Corporation | Fluid collection systems including at least one tensioning element |
| US12048644B2 (en) | 2020-11-03 | 2024-07-30 | Purewick Corporation | Apparatus for receiving discharged urine |
| US12048643B2 (en) | 2020-05-27 | 2024-07-30 | Purewick Corporation | Fluid collection assemblies including at least one inflation device and methods and systems of using the same |
| US12053364B2 (en) | 2018-02-18 | 2024-08-06 | G & G Biotechnology Ltd | Implants with enhanced shell adhesion |
| US12070432B2 (en) | 2020-11-11 | 2024-08-27 | Purewick Corporation | Urine collection system including a flow meter and related methods |
| US12121468B2 (en) | 2014-03-19 | 2024-10-22 | Purewick Corporation | Apparatus and methods for receiving discharged urine |
| US12138195B2 (en) | 2020-04-10 | 2024-11-12 | Purewick Corporation | Fluid collection assemblies including one or more leak prevention features |
| US12138196B2 (en) | 2014-03-19 | 2024-11-12 | Purewick Corporation | Apparatus and methods for receiving discharged urine |
| US12150885B2 (en) | 2021-05-26 | 2024-11-26 | Purewick Corporation | Fluid collection system including a cleaning system and methods |
| US12156792B2 (en) | 2020-09-10 | 2024-12-03 | Purewick Corporation | Fluid collection assemblies including at least one inflation device |
| US12161579B2 (en) | 2014-03-19 | 2024-12-10 | Purewick Corporation | Apparatus and methods for receiving discharged urine |
| US12178735B2 (en) | 2021-02-09 | 2024-12-31 | Purewick Corporation | Noise reduction for a urine suction system |
| US12193962B2 (en) | 2016-06-02 | 2025-01-14 | Purewick Corporation | Using wicking material to collect liquid for transport |
| US12208031B2 (en) | 2020-10-21 | 2025-01-28 | Purewick Corporation | Adapters for fluid collection devices |
| US12233003B2 (en) | 2021-04-29 | 2025-02-25 | Purewick Corporation | Fluid collection assemblies including at least one length adjusting feature |
| US12245967B2 (en) | 2020-11-18 | 2025-03-11 | Purewick Corporation | Fluid collection assemblies including an adjustable spine |
| US12251333B2 (en) | 2021-05-21 | 2025-03-18 | Purewick Corporation | Fluid collection assemblies including at least one inflation device and methods and systems of using the same |
| US12257173B2 (en) | 2017-01-31 | 2025-03-25 | Purewick Corporation | Apparatus and methods for receiving discharged urine |
| US12257174B2 (en) | 2020-10-21 | 2025-03-25 | Purewick Corporation | Fluid collection assemblies including at least one of a protrusion or at least one expandable material |
| US12268627B2 (en) | 2021-01-06 | 2025-04-08 | Purewick Corporation | Fluid collection assemblies including at least one securement body |
| US12274638B2 (en) | 2018-05-01 | 2025-04-15 | Purewick Corporation | Fluid collection devices, related systems, and related methods |
| US12295876B2 (en) | 2018-05-01 | 2025-05-13 | Purewick Corporation | Fluid collection devices and methods of using the same |
| US12324767B2 (en) | 2021-05-24 | 2025-06-10 | Purewick Corporation | Fluid collection assembly including a customizable external support and related methods |
| US12329364B2 (en) | 2019-07-19 | 2025-06-17 | Purewick Corporation | Fluid collection devices including at least one shape memory material |
| US12350190B2 (en) | 2020-01-03 | 2025-07-08 | Purewick Corporation | Urine collection devices having a relatively wide portion and an elongated portion and related methods |
| US12350187B2 (en) | 2020-08-11 | 2025-07-08 | Purewick Corporation | Fluid collection assemblies defining waist and leg openings |
| US12419778B2 (en) | 2019-06-21 | 2025-09-23 | Purewick Corporation | Fluid collection devices including a base securement area, and related systems and methods |
| US12440370B2 (en) | 2021-10-19 | 2025-10-14 | Purewick Corporation | Apparatus with compressible casing for receiving discharged urine |
Citations (9)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US2542619A (en)* | 1948-05-22 | 1951-02-20 | Ella H Bernhardt | Breast form |
| US2636182A (en)* | 1951-08-23 | 1953-04-28 | Freedman Ruth | Artificial body bulge |
| US4507810A (en)* | 1979-02-19 | 1985-04-02 | Polar-Plastik Hb | Implantable breast prostheses |
| US4650487A (en)* | 1980-10-27 | 1987-03-17 | Memorial Hospital For Cancer And Allied Diseases | Multi-lumen high profile mammary implant |
| US4790848A (en)* | 1987-11-27 | 1988-12-13 | Dow Corning Wright | Breast prosthesis with multiple lumens |
| US4955909A (en)* | 1989-01-31 | 1990-09-11 | Bioplasty, Inc. | Textured silicone implant prosthesis |
| US4960425A (en)* | 1987-05-27 | 1990-10-02 | Mentor Corporation | Textured surface frosthesis implants |
| US4963150A (en)* | 1984-08-30 | 1990-10-16 | Daniel Brauman | Implantable prosthetic devices |
| US4984585A (en)* | 1983-02-17 | 1991-01-15 | Austad Eric D | Tissue expander |
- 1991
- 1991-07-22USUS07/733,813patent/US5246454A/ennot_activeExpired - Fee Related
Patent Citations (10)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US2542619A (en)* | 1948-05-22 | 1951-02-20 | Ella H Bernhardt | Breast form |
| US2636182A (en)* | 1951-08-23 | 1953-04-28 | Freedman Ruth | Artificial body bulge |
| US4507810A (en)* | 1979-02-19 | 1985-04-02 | Polar-Plastik Hb | Implantable breast prostheses |
| US4650487A (en)* | 1980-10-27 | 1987-03-17 | Memorial Hospital For Cancer And Allied Diseases | Multi-lumen high profile mammary implant |
| US4984585A (en)* | 1983-02-17 | 1991-01-15 | Austad Eric D | Tissue expander |
| US4963150A (en)* | 1984-08-30 | 1990-10-16 | Daniel Brauman | Implantable prosthetic devices |
| US4963150B1 (en)* | 1984-08-30 | 1994-10-04 | Daniel Brauman | Implantable prosthetic device |
| US4960425A (en)* | 1987-05-27 | 1990-10-02 | Mentor Corporation | Textured surface frosthesis implants |
| US4790848A (en)* | 1987-11-27 | 1988-12-13 | Dow Corning Wright | Breast prosthesis with multiple lumens |
| US4955909A (en)* | 1989-01-31 | 1990-09-11 | Bioplasty, Inc. | Textured silicone implant prosthesis |
Cited By (125)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US5496370A (en)* | 1992-03-13 | 1996-03-05 | Robert S. Hamas | Gel-like prosthetic device |
| US5496368A (en)* | 1992-06-12 | 1996-03-05 | Wiese; K. Guenter | Tissue expander inflating due to osmotic driving forces of a shaped body of hydrogel and an aqueous solution |
| US5496367A (en)* | 1993-01-13 | 1996-03-05 | Fisher; Jack | Breast implant with baffles |
| US6083262A (en)* | 1994-06-16 | 2000-07-04 | Caravel; Jean-Baudoin | Supple implantable prosthesis used in surgery for increasing the volume of or reconstructing soft tissue, notably a breast prosthesis, and its method of manufacture |
| US5549671A (en)* | 1994-12-28 | 1996-08-27 | Mcghan Medical Corporation | Adjunctive filler material for fluid-filled prosthesis |
| US6312466B1 (en)* | 1995-05-22 | 2001-11-06 | Board Of Regents, University Of Texas System | Prosthesis containing a solution of polyethylene glycol |
| US6099565A (en)* | 1995-06-07 | 2000-08-08 | Sakura, Jr.; Chester Y. | Prosthetic tissue implant and filler therefor |
| US5843189A (en)* | 1995-06-13 | 1998-12-01 | Laboratoire Perouse Implant | Breast prosthesis |
| US5824081A (en)* | 1996-09-13 | 1998-10-20 | Lipomatrix Incorporated | Hydraulic foam tissue implant |
| US6146418A (en)* | 1997-02-28 | 2000-11-14 | Berman; Mark | Body implant and method of implanting |
| US6183514B1 (en)* | 1999-08-16 | 2001-02-06 | Hilton Becker | Self positioning breast prosthesis |
| US6203570B1 (en) | 1999-11-24 | 2001-03-20 | John L. Baeke | Breast implant with position lock |
| US6464726B1 (en)* | 2000-07-13 | 2002-10-15 | Jenna Heljenek | Breast implant system and method of augmentation |
| US20040249457A1 (en)* | 2003-06-09 | 2004-12-09 | Smith Lane Fielding | Mastopexy stabilization apparatus and method |
| US20060036333A1 (en)* | 2003-06-09 | 2006-02-16 | Smith Lane F | Mastopexy stabilization apparatus and method |
| US7081135B2 (en) | 2003-06-09 | 2006-07-25 | Lane Fielding Smith | Mastopexy stabilization apparatus and method |
| US20050049701A1 (en)* | 2003-09-03 | 2005-03-03 | Brennan William A. | System and method for breast augmentation |
| US20050055093A1 (en)* | 2003-09-03 | 2005-03-10 | Brennan William A. | System and method for breast augmentation |
| US7846205B2 (en)* | 2003-09-03 | 2010-12-07 | Brennan William A | System and method for breast augmentation |
| US7169180B2 (en) | 2003-09-03 | 2007-01-30 | Brennan William A | System and method for breast augmentation |
| US20070123983A1 (en)* | 2003-09-03 | 2007-05-31 | Brennan William A | System and method for breast augmentation |
| US8092527B2 (en) | 2003-09-03 | 2012-01-10 | Brennan William A | System and method for breast augmentation |
| US20080306590A1 (en)* | 2004-01-29 | 2008-12-11 | Smart Implant Plc | Prosthesis and Method of Manufacturing a Prosthesis |
| US20090299473A1 (en)* | 2005-04-25 | 2009-12-03 | Jacky Govrin-Yehudian | Lightweight implantable prosthetic device |
| US10052191B2 (en) | 2005-04-25 | 2018-08-21 | G & G Biotechnology Ltd | Lightweight implantable prosthetic device |
| US20110060411A1 (en)* | 2005-04-25 | 2011-03-10 | Jacky Govrin-Yehudian | Lightweight implantable prosthetic device |
| US7988731B2 (en) | 2005-04-25 | 2011-08-02 | G & G Biotechnology Ltd | Lightweight implantable prosthetic device |
| US9452043B2 (en) | 2005-04-25 | 2016-09-27 | G & Biotechnology Ltd | Lightweight implantable prosthetic device |
| US20060282164A1 (en)* | 2005-06-08 | 2006-12-14 | Joann Seastrom | Implant shell and filler apparatus |
| US7879091B1 (en)* | 2007-08-06 | 2011-02-01 | Martin Inell O | Inflatable prosthetic breast assembly and associated method |
| WO2009071770A3 (en)* | 2007-09-20 | 2009-11-05 | Philippe Bellity | Prosthetic unit, method for its production, and its use |
| FR2921250A1 (en)* | 2007-09-20 | 2009-03-27 | Philippe Bellity | PROTHETIC UNIT, METHOD OF MANUFACTURE AND USE |
| US9138310B2 (en) | 2007-11-05 | 2015-09-22 | Allergan, Inc. | Soft prosthesis shell texturing method |
| EP2240116B1 (en) | 2008-01-28 | 2015-07-01 | Biomet 3I, LLC | Implant surface with increased hydrophilicity |
| US9393106B2 (en) | 2008-08-13 | 2016-07-19 | Allergan, Inc. | Soft filled prosthesis shell with discrete fixation surfaces |
| US10675144B2 (en) | 2008-08-13 | 2020-06-09 | Allergan, Inc. | Soft filled prosthesis shell with discrete fixation surfaces |
| US9918829B2 (en) | 2008-08-13 | 2018-03-20 | Allergan, Inc. | Soft filled prosthesis shell with discrete fixation surfaces |
| US10765501B2 (en) | 2008-08-13 | 2020-09-08 | Allergan, Inc. | Dual plane breast implant |
| US9848972B2 (en) | 2008-08-13 | 2017-12-26 | Allergan, Inc. | Dual plane breast implant |
| US9138311B2 (en) | 2008-08-13 | 2015-09-22 | Allergan, Inc. | Soft filled prosthesis shell with discrete fixation surfaces |
| US8506627B2 (en) | 2008-08-13 | 2013-08-13 | Allergan, Inc. | Soft filled prosthesis shell with discrete fixation surfaces |
| US9370414B2 (en) | 2008-10-28 | 2016-06-21 | Implite Ltd. | Reconstructive breast prostheses |
| US9724189B2 (en)* | 2009-07-17 | 2017-08-08 | Peter Forsell | Breast implant system |
| US20120116509A1 (en)* | 2009-07-17 | 2012-05-10 | Milux Holding Sa | Breast implant system |
| US8951596B2 (en) | 2009-10-16 | 2015-02-10 | Allergan, Inc. | Implants and methods for manufacturing same |
| US10213293B2 (en) | 2010-01-18 | 2019-02-26 | G & G Biotechnology Ltd | Lightweight breast implant material |
| US8487012B2 (en) | 2010-01-28 | 2013-07-16 | Allergan, Inc. | Open celled foams, implants including them and processes for making same |
| US9138308B2 (en) | 2010-02-03 | 2015-09-22 | Apollo Endosurgery, Inc. | Mucosal tissue adhesion via textured surface |
| US9205577B2 (en) | 2010-02-05 | 2015-12-08 | Allergan, Inc. | Porogen compositions, methods of making and uses |
| US9138309B2 (en) | 2010-02-05 | 2015-09-22 | Allergan, Inc. | Porous materials, methods of making and uses |
| US10624997B2 (en) | 2010-02-05 | 2020-04-21 | Allergan, Inc. | Porogen compositions, methods of making and uses |
| US10391199B2 (en) | 2010-02-05 | 2019-08-27 | Allergan, Inc. | Porous materials, methods of making and uses |
| US9072821B2 (en) | 2010-02-05 | 2015-07-07 | Allergan, Inc. | Biocompatible structures and compositions |
| US8679570B2 (en) | 2010-04-27 | 2014-03-25 | Allergan, Inc. | Foam-like materials and methods for producing same |
| US11202853B2 (en) | 2010-05-11 | 2021-12-21 | Allergan, Inc. | Porogen compositions, methods of making and uses |
| US8685296B2 (en) | 2010-05-11 | 2014-04-01 | Allergan, Inc. | Porogen compositions, method of making and uses |
| US9593224B2 (en) | 2010-09-28 | 2017-03-14 | Allergan, Inc. | Porogen compositions, methods of making and uses |
| US8889751B2 (en) | 2010-09-28 | 2014-11-18 | Allergan, Inc. | Porous materials, methods of making and uses |
| US9522502B2 (en) | 2010-09-28 | 2016-12-20 | Allergan, Inc. | Porous materials, methods of making and uses |
| US8877822B2 (en) | 2010-09-28 | 2014-11-04 | Allergan, Inc. | Porogen compositions, methods of making and uses |
| US9044897B2 (en) | 2010-09-28 | 2015-06-02 | Allergan, Inc. | Porous materials, methods of making and uses |
| US9155613B2 (en) | 2010-11-16 | 2015-10-13 | Allergan, Inc. | Methods for creating foam-like texture |
| US8679279B2 (en) | 2010-11-16 | 2014-03-25 | Allergan, Inc. | Methods for creating foam-like texture |
| US8546458B2 (en) | 2010-12-07 | 2013-10-01 | Allergan, Inc. | Process for texturing materials |
| US20130231743A1 (en)* | 2011-10-19 | 2013-09-05 | Hilton Becker | Hybrid breast implant |
| US20130150962A1 (en)* | 2011-11-04 | 2013-06-13 | Freddy Sanabria Scharf | Mammary prosthesis filled with expanded polymer microspheres |
| US8932353B2 (en)* | 2011-11-04 | 2015-01-13 | Freddy Sanabria Scharf | Mammary prosthesis filled with expanded polymer microspheres |
| US9393107B2 (en) | 2011-11-04 | 2016-07-19 | Freddy Sanabria Scharf | Implantable prosthesis filled with expanded polymer microspheres |
| US8801782B2 (en) | 2011-12-15 | 2014-08-12 | Allergan, Inc. | Surgical methods for breast reconstruction or augmentation |
| US9688006B2 (en) | 2012-12-13 | 2017-06-27 | Allergan, Inc. | Device and method for making a variable surface breast implant |
| US10864661B2 (en) | 2012-12-13 | 2020-12-15 | Allergan, Inc. | Device and method for making a variable surface breast implant |
| US9713524B2 (en) | 2013-01-30 | 2017-07-25 | Implite Ltd. | Human implantable tissue expanders |
| US12239567B2 (en) | 2014-03-19 | 2025-03-04 | Purewick Corporation | Apparatus and methods for receiving discharged urine |
| US12121468B2 (en) | 2014-03-19 | 2024-10-22 | Purewick Corporation | Apparatus and methods for receiving discharged urine |
| US12138196B2 (en) | 2014-03-19 | 2024-11-12 | Purewick Corporation | Apparatus and methods for receiving discharged urine |
| US12324765B2 (en) | 2014-03-19 | 2025-06-10 | Purewick Corporation | Apparatus and methods for receiving discharged urine |
| US12161579B2 (en) | 2014-03-19 | 2024-12-10 | Purewick Corporation | Apparatus and methods for receiving discharged urine |
| US12171685B2 (en) | 2014-03-19 | 2024-12-24 | Purewick Corporation | Apparatus and methods for receiving discharged urine |
| US9808338B2 (en) | 2014-05-16 | 2017-11-07 | Allergan, Inc. | Soft filled prosthesis shell with variable texture |
| US10092392B2 (en) | 2014-05-16 | 2018-10-09 | Allergan, Inc. | Textured breast implant and methods of making same |
| US9539086B2 (en) | 2014-05-16 | 2017-01-10 | Allergan, Inc. | Soft filled prosthesis shell with variable texture |
| US10350055B2 (en) | 2014-05-16 | 2019-07-16 | Allergan, Inc. | Textured breast implant and methods of making same |
| US10933165B2 (en) | 2015-03-12 | 2021-03-02 | G & G Biotechnology Ltd | Composite implant material |
| US12193962B2 (en) | 2016-06-02 | 2025-01-14 | Purewick Corporation | Using wicking material to collect liquid for transport |
| US10912660B2 (en)* | 2016-06-23 | 2021-02-09 | American Breast Care, Lp | Breast prostheses with phase change material |
| US20170367850A1 (en)* | 2016-06-23 | 2017-12-28 | American Breast Care, Lp | Breast Prostheses with Phase Change Material |
| US20210093469A1 (en)* | 2016-06-23 | 2021-04-01 | American Breast Care, Lp | Cooling Cushion for Breast Form |
| US10307270B2 (en)* | 2016-06-23 | 2019-06-04 | American Breast Care, Lp | Breast prostheses with phase change material |
| US12029678B2 (en) | 2016-07-27 | 2024-07-09 | Purewick Corporation | Male urine collection device using wicking material |
| US20190307598A1 (en)* | 2016-08-16 | 2019-10-10 | Purewick Corporation | Using wicking material to collect urine from a male for transport |
| US12257173B2 (en) | 2017-01-31 | 2025-03-25 | Purewick Corporation | Apparatus and methods for receiving discharged urine |
| US12053364B2 (en) | 2018-02-18 | 2024-08-06 | G & G Biotechnology Ltd | Implants with enhanced shell adhesion |
| US11944740B2 (en) | 2018-05-01 | 2024-04-02 | Purewick Corporation | Fluid collection devices, related systems, and related methods |
| US12285352B2 (en) | 2018-05-01 | 2025-04-29 | Purewick Corporation | Fluid collection devices, systems, and methods |
| US12295876B2 (en) | 2018-05-01 | 2025-05-13 | Purewick Corporation | Fluid collection devices and methods of using the same |
| US12274638B2 (en) | 2018-05-01 | 2025-04-15 | Purewick Corporation | Fluid collection devices, related systems, and related methods |
| US11938053B2 (en) | 2018-05-01 | 2024-03-26 | Purewick Corporation | Fluid collection devices, systems, and methods |
| US12419778B2 (en) | 2019-06-21 | 2025-09-23 | Purewick Corporation | Fluid collection devices including a base securement area, and related systems and methods |
| US12329364B2 (en) | 2019-07-19 | 2025-06-17 | Purewick Corporation | Fluid collection devices including at least one shape memory material |
| US12350190B2 (en) | 2020-01-03 | 2025-07-08 | Purewick Corporation | Urine collection devices having a relatively wide portion and an elongated portion and related methods |
| US12138195B2 (en) | 2020-04-10 | 2024-11-12 | Purewick Corporation | Fluid collection assemblies including one or more leak prevention features |
| US12048643B2 (en) | 2020-05-27 | 2024-07-30 | Purewick Corporation | Fluid collection assemblies including at least one inflation device and methods and systems of using the same |
| US12350187B2 (en) | 2020-08-11 | 2025-07-08 | Purewick Corporation | Fluid collection assemblies defining waist and leg openings |
| US12156792B2 (en) | 2020-09-10 | 2024-12-03 | Purewick Corporation | Fluid collection assemblies including at least one inflation device |
| US11801186B2 (en) | 2020-09-10 | 2023-10-31 | Purewick Corporation | Urine storage container handle and lid accessories |
| US12042423B2 (en) | 2020-10-07 | 2024-07-23 | Purewick Corporation | Fluid collection systems including at least one tensioning element |
| US12257174B2 (en) | 2020-10-21 | 2025-03-25 | Purewick Corporation | Fluid collection assemblies including at least one of a protrusion or at least one expandable material |
| US12208031B2 (en) | 2020-10-21 | 2025-01-28 | Purewick Corporation | Adapters for fluid collection devices |
| US12048644B2 (en) | 2020-11-03 | 2024-07-30 | Purewick Corporation | Apparatus for receiving discharged urine |
| US12070432B2 (en) | 2020-11-11 | 2024-08-27 | Purewick Corporation | Urine collection system including a flow meter and related methods |
| US12290485B2 (en) | 2020-11-11 | 2025-05-06 | Purewick Corporation | Urine collection system including a flow meter and related methods |
| US12245967B2 (en) | 2020-11-18 | 2025-03-11 | Purewick Corporation | Fluid collection assemblies including an adjustable spine |
| US12268627B2 (en) | 2021-01-06 | 2025-04-08 | Purewick Corporation | Fluid collection assemblies including at least one securement body |
| US12186229B2 (en) | 2021-01-19 | 2025-01-07 | Purewick Corporation | Variable fit fluid collection devices, systems, and methods |
| US11865030B2 (en) | 2021-01-19 | 2024-01-09 | Purewick Corporation | Variable fit fluid collection devices, systems, and methods |
| US12178735B2 (en) | 2021-02-09 | 2024-12-31 | Purewick Corporation | Noise reduction for a urine suction system |
| US12245966B2 (en) | 2021-02-26 | 2025-03-11 | Purewick Corporation | Fluid collection devices having a sump between a tube opening and a barrier, and related systems and methods |
| US11925575B2 (en) | 2021-02-26 | 2024-03-12 | Purewick Corporation | Fluid collection devices having a sump between a tube opening and a barrier, and related systems and methods |
| US12029677B2 (en) | 2021-04-06 | 2024-07-09 | Purewick Corporation | Fluid collection devices having a collection bag, and related systems and methods |
| US12233003B2 (en) | 2021-04-29 | 2025-02-25 | Purewick Corporation | Fluid collection assemblies including at least one length adjusting feature |
| US12251333B2 (en) | 2021-05-21 | 2025-03-18 | Purewick Corporation | Fluid collection assemblies including at least one inflation device and methods and systems of using the same |
| US12324767B2 (en) | 2021-05-24 | 2025-06-10 | Purewick Corporation | Fluid collection assembly including a customizable external support and related methods |
| US12150885B2 (en) | 2021-05-26 | 2024-11-26 | Purewick Corporation | Fluid collection system including a cleaning system and methods |
| US12440371B2 (en) | 2021-08-05 | 2025-10-14 | Purewick Corporation | Fluid collection system including a garment and a fluid collection device |
| US12440370B2 (en) | 2021-10-19 | 2025-10-14 | Purewick Corporation | Apparatus with compressible casing for receiving discharged urine |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| US5246454A (en) | Encapsulated implant | |
| EP0320133B1 (en) | Breast prosthesis with multiple lumens | |
| US5496370A (en) | Gel-like prosthetic device | |
| US10052191B2 (en) | Lightweight implantable prosthetic device | |
| EP0322194B1 (en) | Implantable prosthetic device | |
| US5171269A (en) | Mammary prosthesis | |
| US4955907A (en) | Implantable prosthetic device | |
| US7625405B2 (en) | Breast implant and method of manufacture | |
| US5358521A (en) | Multiple-layer prosthesis implant with tissue tactility | |
| US5425762A (en) | Prosthetic implants and process for obtaining the same | |
| US5447535A (en) | Mammary prosthesis | |
| JP2004519312A (en) | Breast prosthesis | |
| US5092882A (en) | Multiple compartment breast prosthesis | |
| US5534023A (en) | Fluid filled prosthesis excluding gas-filled beads | |
| US6802861B1 (en) | Structured breast implant | |
| AU2006306193B2 (en) | Variable cohesive gel form-stable breast implant | |
| US8236054B2 (en) | Breast implants and methods of manufacture | |
| US20030093151A1 (en) | Implantable mammary prosthesis with flexible sheet | |
| WO2006028864A1 (en) | Implant device | |
| US9393107B2 (en) | Implantable prosthesis filled with expanded polymer microspheres | |
| US20170367809A1 (en) | Human implantable tissue expanders | |
| WO2007084285A2 (en) | Implantable nipple prosthetic device | |
| US20130116786A1 (en) | Breast prosthesis filled with microspheres of thermoexpanded polymer | |
| JPH03140155A (en) | Complementarily forming material | |
| CA1094252A (en) | Deflatable tissue augmentation prosthesis |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| FPAY | Fee payment | Year of fee payment:4 | |
| REMI | Maintenance fee reminder mailed | ||
| LAPS | Lapse for failure to pay maintenance fees | ||
| FP | Lapsed due to failure to pay maintenance fee | Effective date:20010921 | |
| STCH | Information on status: patent discontinuation | Free format text:PATENT EXPIRED DUE TO NONPAYMENT OF MAINTENANCE FEES UNDER 37 CFR 1.362 |