US4840615A - Self-sealing injection reservoir - Google Patents
Self-sealing injection reservoirDownload PDFInfo
- Publication number
- US4840615A US4840615AUS07/167,409US16740988AUS4840615AUS 4840615 AUS4840615 AUS 4840615AUS 16740988 AUS16740988 AUS 16740988AUS 4840615 AUS4840615 AUS 4840615A
- Authority
- US
- United States
- Prior art keywords
- reservoir
- chamber
- dome
- self
- base
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Expired - Lifetime
Links
Images
Classifications
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M39/00—Tubes, tube connectors, tube couplings, valves, access sites or the like, specially adapted for medical use
- A61M39/02—Access sites
- A61M39/0208—Subcutaneous access sites for injecting or removing fluids
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M39/00—Tubes, tube connectors, tube couplings, valves, access sites or the like, specially adapted for medical use
- A61M2039/0036—Tubes, tube connectors, tube couplings, valves, access sites or the like, specially adapted for medical use characterised by a septum having particular features, e.g. having venting channels or being made from antimicrobial or self-lubricating elastomer
- A61M2039/0054—Multiple layers
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M39/00—Tubes, tube connectors, tube couplings, valves, access sites or the like, specially adapted for medical use
- A61M2039/0036—Tubes, tube connectors, tube couplings, valves, access sites or the like, specially adapted for medical use characterised by a septum having particular features, e.g. having venting channels or being made from antimicrobial or self-lubricating elastomer
- A61M2039/0072—Means for increasing tightness of the septum, e.g. compression rings, special materials, special constructions
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M39/00—Tubes, tube connectors, tube couplings, valves, access sites or the like, specially adapted for medical use
- A61M39/02—Access sites
- A61M39/0208—Subcutaneous access sites for injecting or removing fluids
- A61M2039/0226—Subcutaneous access sites for injecting or removing fluids having means for protecting the interior of the access site from damage due to the insertion of a needle
Definitions
- the present inventionis directed to an injection reservoir which is self-sealing to punctures by a hypodermic needle. More particularly, the present invention is directed to a self-sealing injection reservoir of the type which is implanted under the skin of humans and domestic animals in connection with tissue expanders and various drug delivery systems.
- tissue expanding deviceswhich are temporarily implanted under the skin of humans and domestic animals, and which are gradually inflated by injection of saline or similar liquids for the purpose of causing the growth of a skin flap or enlargement.
- tissue expanderscomprise a surgically implantable bag into which the liquid is introduced to gradually enlarge the bag and thereby to cause the desired skin enlargement or flap formation. After the skin flap has formed, the bag is surgically removed.
- the skin flapis either used, after severance, for purposes of plastic surgery in other parts of the body, or accommodates a permanent implantation directly beneath the flap. Neither the manner of using the skin flap, nor the permanent implants, form part of the present invention.
- the temporarily implantable tissue expander bagmust not leak, and must not be punctured to form a leak, when, from time to time, additional liquid is introduced into the bag. In some situations, liquid must even be withdrawn from the bag without causing a leak.
- Such addition or withdrawal of liquidis accomplished, in accordance with standard practice in the art, through a substantially non-expandable bioimplantable injection button or reservoir, which is self-sealing to punctures by a hypodermic needle.
- the self-sealing, non-expandable injection button or reservoiris usually connected to the bag, through a suitable bioimplantable duct or narrow tubing.
- the injection reservoiris incorporated in the wall of the bag. In other words, to gradually inflate the tissue expander bag liquid is introduced into the bag through a distinct, non-expandable but self-sealing injection reservoir, which is temporarily implanted beneath the skin together with the tissue expander bag.
- the injection reservoir of U.S. Pat. No. 4,190,040includes a substantially flat base and a generally dome-shaped, double-walled upper portion, which, together with the base, forms an enclosure or reservoir chamber for the injected liquid.
- the enclosureis connected to the tissue expander bag with a suitable tubing or duct.
- the self-sealing capability of the double-walled upper portionis attained by a silicone gel sealant layer which is contained between the two walls.
- a non-puncturable plate memberis contained within the enclosure to cover the base.
- the injection reservoir of U.S. Pat. No. 4,428,364is contained within the tissue expander bag itself.
- a generally dome-shaped wall of the reservoirhas multiple layers of fabric reinforced silicone rubber sheets, with the weave of the fabric in the several layers being oriented in multiple directions.
- a swelling agentis present in the dome-shaped wall. The forces created by the swelling of the silicone rubber sheets and the restraining force of the fabric cause a compressive stress in the wall, which, in turn, renders the wall self-sealing to punctures by a hypodermic needle.
- an injection button or reservoirhaving a body, including a base, which defines a chamber for holding liquid, and wherein a wall of the chamber is a sheet of resilient elastic rubber disposed in a curvilinear bent configuration in the location where a hypodermic needle is used to puncture the reservoir for the purpose of injecting or withdrawing liquid therefrom.
- the sheet of resilient elastic rubberis bent into its curvilinear configuration from a substantially flat sheet, so that the interior of the bent sheet is in compression and thereby provides self-sealing capability.
- the bent sheetcomprises only an inner wall of the injection reservoir, the outer wall being a single molded rubber piece which is affixed to a substantially flat base.
- a metal guard plateis disposed within the interior of the chamber to guard the base against puncture by an accidentally overextended hypodermic needle.
- a wall of the chamber of the injection reservoiris self-sealing because it is in compression, comprising a substantially dome-shaped member which has been inverted after molding.
- the wall of the chamber of the injection reservoiris self-sealing because it is derived from a wall of a cylindrical tubing which has been cut into a substantially flat disc, and inverted so that its interior is in compression.
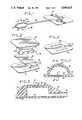
- FIG. 1is a perspective view of a first preferred embodiment of the injection reservoir of the present invention, the figure also showing a tissue expander bag to which the injection reservoir is fluidly connected;
- FIG. 2is an exploded perspective view of the first preferred embodiment of the injection reservoir of the present invention
- FIG. 3is a perspective view of a top part of the first preferred embodiment of the injection reservoir of the present invention.
- FIG. 4is a cross-sectional view taken on lines 4,4 of FIG. 1;
- FIG. 5is a cross-sectional view taken on lines 5,5 of FIG. 4;
- FIG. 6is a perspective view of a second preferred embodiment of the injection reservoir of the present invention, the figure also showing a tissue expander bag to which the injection reservoir is fluidly connected;
- FIG. 7is an exploded perspective view of the second preferred embodiment of the injection reservoir of the present invention.
- FIG. 8includes a series of schematic cross-sectional views showing a molded, substantially dome-shaped member being inverted for the purpose a self-sealing wall of the second preferred embodiment of the injection reservoir of the present invention
- FIG. 9is a cross-sectional view of the second preferred embodiment of the injection reservoir of the present invention, the cross-section being taken on lines 9,9 of FIG. 6;
- FIG. 10is a perspective view of a tubing comprising resilient rubber material from which a sealing plate or sheet of a third preferred embodiment of the injection reservoir of the present invention is fabricated;
- FIG. 11is a perspective view of an intermediate in the process of fabricating the sealing plate of the third preferred embodiment.
- FIG. 12is a perspective view of intermediate in the process of fabricating the sealing plate of the third preferred embodiment.
- FIG. 13is a perspective view of still another intermediate in the process of fabricating the sealing plate of the third preferred embodiment
- FIG. 14is a perspective view of yet another intermediate in the process of fabricating the sealing plate of the third preferred embodiment
- FIG. 15is a perspective view of a further intermediate in the process of fabricating the sealing plate of the third preferred embodiment
- FIG. 16is a cross-sectional view of a still further intermediate in the process of fabricating the sealing plate of the third preferred embodiment
- FIG. 17is a cross-sectional view of the sealing plate of the third preferred embodiment, FIGS. 16 and 17 showing the inversion of the sealing plate from the configuration obtained as the sealing plate is cut from the tubing shown on FIG. 10;
- FIG. 18is an exploded perspective view of the third preferred embodiment.
- FIG. 19is a cross-sectional view of the third preferred embodiment.
- FIGS. 1 through 5 of the appended drawingsa first preferred embodiment 20 of the injection button or reservoir of the present invention is disclosed.
- the injection reservoir or button 20 of the present inventionis used primarily in connection with a surgically implantable tissue expander, such as the tissue expander 22 shown on FIG. 1.
- a surgically implantable tissue expandersuch as the tissue expander 22 shown on FIG. 1.
- a detailed description of a tissue expander and of its role in generating skin flaps (not shown) or enlargements (not shown), primarily for the purposes of plastic or reconstructive surgery,is found in U.S. Pat. No. 4,217,889, the specification of which is expressly incorporated herein by reference.
- the injection reservoir or button 20must be biocompatible for temporary implantation under the skin, and must be self-sealing to repeated punctures by a hypodermic needle and also capable of withstanding the hydrostatic pressure of the fluid within the expansion bag, whereby liquid can be either introduced or withdrawn from the tissue expander 22 through the injection reservoir 20.
- the injection reservoir 20 of the present invention(as some other prior art injection reservoirs as well) can also be used in conjunction with certain drug delivery devices (not shown).
- a base 24, an upper or top portion 26, and a bent sealing plate 28are shown as important components of the first preferred embodiment 20 of the injection reservoir of the present invention.
- the base 24is substantially flat, and preferably comprises fabric reinforced bioimplantable silicone rubber. DACRON material is highly suitable to serve as the reinforcing fabric (not shown) of the base 24.
- the upper or top portion 28 of the injection button 20preferably has the configuration shown on FIGS. 1-5, i.e., it is preferably elongated, having a middle section 30 of substantially cylindrical curvature and two curvilinear end sections 32 and 34, which are surrounded by a substantially flat flange 36.
- the entire top portion 26preferably comprises a single, unitary piece of biocompatible silicone rubber, which can be manufactured as such, for example, by molding or casting, with molding being preferred.
- the top portion 26is attached to the base 24 through an intermediate sheet (not shown) of thin silicone rubber, which in the herein-described preferred embodiment is only approximately 0.010" (0.25 mm) thick.
- the thin sheet(not shown) is applied as raw silicone rubber, and is thereafter vulcanized to affix the top portion 26 to the base 24.
- the top portion 26may be glued to the base 24 with a suitable glue (not shown) capable of bonding silicone rubber.
- the base 24 and the top portion 26 of the injection reservoir 20jointly define a chamber 38 into which liquid, such as saline solution (not shown) can be injected, and retained.
- liquidsuch as saline solution (not shown)
- other biocompatible materialssuch as certain latex, polycarbonate and polyurethanes are also suitable.
- the sealing plate or sheet 28which comprises a principal novel feature of the present invention, is shown.
- the sealing plate or sheet 28is an elastic rubber sheet, which is originally flat, as is shown on FIG. 2, and is bent into a curvilinear configuration to become at least an inner wall of the chamber 38.
- the sealing plate 28must comprise elastic material, and although preferably it is made of biocompatible silicone rubber, it does not need to be biocompatible unless it is exposed for contact with living tissue (not shown).
- the sheet 28may be approximately 0.005 to 0.5 inch thick, although in the herein-described specific preferred embodiment, the sheet 28 is approximately 0.075 inch thick.
- FIGS. 2, 3 and 4indicate that before the top portion 26 is affixed to the base 24, the sealing sheet 28 is inserted into the top portion 26 of the injection reservoir 20 to be affixed to the middle section 30 thereof.
- the bent sealing plate or sheet 28is affixed to the interior wall of the middle section 30 through a thin silicone rubber sheet (not shown), which is applied as raw rubber and thereafter vulcanized to bond the sealing plate 28 and the middle section 30 together.
- a thin silicone rubber sheet(not shown) which is utilized to bond the top portion 26 to the base 24
- the thin sheet of rubber (not shown) used to bond the sealing plate 28 to the middle section 30is also approximately 0.010" (0.25 mm) thick.
- the sealing plate 28may also be glued to the middle section 30 with a suitable glue (not shown).
- the sealing plate 28is preferably manufactured by molding, although it can also be made by suitable casting techniques. As it should be already apparent from the foregoing description, the important feature of the sealing plate 28 is that it is made as a flat sheet (as shown on FIG. 2), and is thereafter incorporated in the injection button 20 in a bent configuration. Consequently, the inner portion of the sheet 28 is under compression, and renders the sheet 28 self-sealing to small punctures of the type which are caused by insertion of a hypodermic needle (not shown).
- a metal guard plate 40is shown incorporated in the injection button 20.
- the guard plate 40is configured to protect the base 24 and the two end portions 32 of the injection reservoir 20 from being punctured by an accidentally overextended hypodermic needle (not shown).
- the guard plate 40essentially conforms to the shape of the injection button 20, and includes a flat elongated base plate 42 and two side plates 44.
- the side plates 44are optional, in that the entire guard plate 40 may also be flat so as to have the configuration of the flat base 42.
- the herein-described first specific embodiment 20 of the injection button or reservoir of the present inventionis assembled by placed the guard plate 40 within the interior and by applying the thin raw silicone rubber sheets (not shown) between the interior of the middle section 30 and the bent sealing plate 28, and between the top portion 26 and the base 24, respectively. Thereafter, the assembly is subjected to vulcanizing conditions (approximately 300 Fahrenheit and a pressure of approximately 10 to 20 PSI) to vulcanize the thin raw silicone rubber sheets (not shown) and bond the assembly together.
- a tube or conduit 46 integrally molded with the top portion 26connects the injection reservoir 20 with the inflatable bag 22 of the tissue expander.
- a second preferred embodiment 48 of the injection button or reservoir of the present inventionis disclosed.
- Important components of the second preferred embodimentare a base 50, a guard plate 52, and a substantially dome-shaped member 54 which acts as a sealing plate.
- the dome-shaped member 54comprises elastic rubber material and is self-sealing to punctures by hypodermic needles (not shown) by virtue of having been inverted from the original configuration in which the dome-shaped member 54 was made.
- the dome-shaped sealing plate 54 of the second embodimentis also preferably manufactured by molding.
- FIG. 8indicates schematically the inversion of the originally manufactured, preferably molded, dome-shaped member to provide the sealing plate 54 which has its interior (concave) side in compression so that it is self-sealing to small punctures.
- the range of the wall thickness of the dome-shaped sealing plate 54is approximately 0.005 to 0.5 inch; in the herein-described second preferred embodiment, the dome-shaped sealing plate 54 is approximately 0.075 inch thick.
- the dome-shaped sealing plate 54comprises an interior wall of the injection reservoir 48, and is affixed to an exterior, dome-shaped wall 56 with a suitable adhesive. All components of the second preferred embodiment 48 of the injection reservoir which come into contact with living tissue (not shown) must be made of biocompatible material to permit implantation. As it is discussed above in connection with the first preferred embodiment 20, medical grade silicone rubber is highly suitable for this purpose, although other materials are also suitable.
- the dome-shaped sealing platemust be made of an elastic rubber, although if it is contained entirely within the interior of the injection reservoir 54, it need not be made of a biocompatible material. Nevertheless, medical grade silicone rubber is highly suitable, and is the preferred material for the dome-shaped sealing plate 54 as well.
- the guard plate 52which is contained in the interior of the injection reservoir 48, protects the base 50 from accidental puncture by a hypodermic needle (not shown).
- a tube 58which may be integrally molded with the external wall 56, or may be affixed thereto by gluing, connects the injection button 48 with the inflatable bag 22 of the tissue expander.
- the injection reservoirmay also be mounted directly to the inflatable bag with openings being placed in the guard plate 52 and in the base 50 for fluid passage.
- the second preferred embodiment 48 of the injection button or reservoir of the present inventionjust like the first preferred embodiment 20, can be used not only in conjunction with tissue expanders, but also in conjunction with bioimplantable drug delivery and like devices.
- a third preferred embodiment 60 of the injection reservoir of the present inventionis disclosed.
- a resilient sealing member 62is kept in a configuration where its side abutting the interior of the injection reservoir is in structural compression, whereby the sealing member 62 is self-sealing to punctures by a hypodermic needle (not shown).
- the sealing member 62 of the third preferred embodimentis fabricated from a hollow cylinder or tube 64, shown on FIG. 10, which is made of a resilient elastic material, preferably silicone rubber.
- the tube 64has a diameter of approximately 0.2 to 1.00", and a wall thickness of approximately 0.010 to 0.5".
- the tube 64has a 0.375" diameter and a wall thickness of 0.025".
- a slot or cut 66is placed into the tube 64 substantially parallel with its longitudinal axis, as is shown on FIG. 11. Thereafter, the slotted tube 68 is unfolded to provide a rectangular piece 70, and the sealing member 62 is die-cut from the rectangular piece 70.
- FIGS. 12 and 13show the natural tendency of the rectangular piece 70 to curl into its original tubular configuration.
- the injection reservoir 60includes a biocompatible silicone rubber base 74, a substantially flat metal guard plate 76, a metal guard cylinder 78, the inverted sealing member or disc 62 and a biocompatible silicone rubber outer housing 80.
- the guard cylinder 78includes an aperture 82 which permits fluid communication from the interior of the injection reservoir 60 to an expansion bag 22 through a tubing 46.
- the inverted sealing disc 62is placed on top of the metal guard cylinder 78, as is best shown in the cross-sectional view of FIG. 19.
- the outer housing 80has a flange 84 which is affixed to the base 74 either through a thin sheet of silicone rubber (not shown) in a vulcanization step, or by gluing.
- the herein-described third specific embodiment 60 of the injection reservoiris approximately 0.600" in diameter, and approximately 0.25" high.
Landscapes
- Health & Medical Sciences (AREA)
- Heart & Thoracic Surgery (AREA)
- Pulmonology (AREA)
- Engineering & Computer Science (AREA)
- Anesthesiology (AREA)
- Biomedical Technology (AREA)
- Hematology (AREA)
- Life Sciences & Earth Sciences (AREA)
- Animal Behavior & Ethology (AREA)
- General Health & Medical Sciences (AREA)
- Public Health (AREA)
- Veterinary Medicine (AREA)
- Media Introduction/Drainage Providing Device (AREA)
- Infusion, Injection, And Reservoir Apparatuses (AREA)
Abstract
Description
Claims (9)
Priority Applications (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US07/167,409US4840615A (en) | 1985-09-30 | 1988-03-14 | Self-sealing injection reservoir |
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US06/781,965US4738657A (en) | 1985-09-30 | 1985-09-30 | Self-sealing injection reservoir |
| US07/167,409US4840615A (en) | 1985-09-30 | 1988-03-14 | Self-sealing injection reservoir |
Related Parent Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US06/781,965DivisionUS4738657A (en) | 1985-09-30 | 1985-09-30 | Self-sealing injection reservoir |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| US4840615Atrue US4840615A (en) | 1989-06-20 |
Family
ID=26863144
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US07/167,409Expired - LifetimeUS4840615A (en) | 1985-09-30 | 1988-03-14 | Self-sealing injection reservoir |
Country Status (1)
| Country | Link |
|---|---|
| US (1) | US4840615A (en) |
Cited By (107)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US4908029A (en)* | 1989-04-25 | 1990-03-13 | Medical Engineering Corporation | Flexible needle stop |
| US5102389A (en)* | 1989-03-28 | 1992-04-07 | Cordis Corporation | Membrane composite |
| US5112303A (en)* | 1991-05-02 | 1992-05-12 | Pudenz-Schulte Medical Research Corporation | Tumor access device and method for delivering medication into a body cavity |
| US5213574A (en)* | 1991-09-06 | 1993-05-25 | Device Labs, Inc. | Composite implantable biocompatible vascular access port device |
| US5387192A (en)* | 1994-01-24 | 1995-02-07 | Sims Deltec, Inc. | Hybrid portal and method |
| US5425760A (en)* | 1992-05-04 | 1995-06-20 | Rosenberg; Paul H. | Tissue expander apparatus, and methods of constructing and utilizing same |
| US5480430A (en)* | 1993-06-04 | 1996-01-02 | Mcghan Medical Corporation | Shape-retaining shell for a fluid filled prosthesis |
| US5630843A (en)* | 1994-06-30 | 1997-05-20 | Rosenberg; Paul H. | Double chamber tissue expander |
| US5725507A (en)* | 1994-03-04 | 1998-03-10 | Mentor Corporation | Self-sealing injection sites and plugs |
| US5935164A (en)* | 1997-02-25 | 1999-08-10 | Pmt Corporaton | Laminated prosthesis and method of manufacture |
| USD413672S (en) | 1997-03-27 | 1999-09-07 | Hudson Design Group | Implantable injection port |
| US5964803A (en)* | 1993-07-27 | 1999-10-12 | Pmt Corporation | Enhanced surface implant and method of manufacture |
| US5989216A (en)* | 1995-06-29 | 1999-11-23 | Sims Deltec, Inc. | Access portal and method |
| US6039712A (en)* | 1997-11-04 | 2000-03-21 | Terence M. Fogarty | Implantable injection port |
| US6060639A (en)* | 1994-03-04 | 2000-05-09 | Mentor Corporation | Testicular prosthesis and method of manufacturing and filling |
| EP1082946A1 (en) | 1999-09-09 | 2001-03-14 | Grampp, Stephan, Dr. med. | Intraluminal graft with variable internal diameter |
| WO2001060444A1 (en)* | 2000-02-18 | 2001-08-23 | Compagnie Europeenne D'etude Et De Recherche De Dispositifs Pour L'implantation Par Laparoscopie | Implantable device for injecting medical substances |
| FR2805167A1 (en)* | 2000-02-18 | 2001-08-24 | Cie Euro Etude Rech Paroscopie | Subcutaneous implant for injecting medical substances has outer casing of elastomer material with integral membrane for piercing with needle |
| US6588432B1 (en)* | 2001-02-15 | 2003-07-08 | Pmt Corporation | Tissue expander magnetic injection port |
| US20030181878A1 (en)* | 1998-12-07 | 2003-09-25 | Tallarida Steven J. | Implantable vascular access device |
| US20030229337A1 (en)* | 2002-04-08 | 2003-12-11 | Broden David A. | Implantable pressure-activated micro-valve |
| US20040185421A1 (en)* | 2003-03-18 | 2004-09-23 | Cagenix, Inc. | Method of using a tissue contourer |
| US20040254537A1 (en)* | 2003-06-16 | 2004-12-16 | Conlon Sean P. | Subcutaneous self attaching injection port with integral moveable retention members |
| US20050131388A1 (en)* | 2003-12-16 | 2005-06-16 | Henrich Cheng | Embedded spinal injector |
| US20050283119A1 (en)* | 2003-06-16 | 2005-12-22 | Joshua Uth | Implantable medical device with reversible attachment mechanism and method |
| US20060058744A1 (en)* | 2000-04-26 | 2006-03-16 | Tallarida Steven J | Implantable hemodialysis access device |
| US20060058892A1 (en)* | 2004-09-16 | 2006-03-16 | Lesh Michael D | Valved tissue augmentation implant |
| US20060058891A1 (en)* | 2004-09-16 | 2006-03-16 | Lesh Michael D | Transformable tissue bulking device |
| US20060058890A1 (en)* | 2004-09-16 | 2006-03-16 | Lesh Michael D | Methods for soft tissue augmentation |
| US20060058735A1 (en)* | 2004-09-16 | 2006-03-16 | Lesh Michael D | Systems and devices for soft tissue augmentation |
| US20060100592A1 (en)* | 2004-07-13 | 2006-05-11 | Kenneth Eliasen | Infusion port |
| FR2877582A1 (en)* | 2004-11-05 | 2006-05-12 | Cie Euro Etude Rech Paroscopie | IMPLANTABLE MEDICAL SITE WITH MULTI-LAYER PUNCTURE AREA |
| US20060161253A1 (en)* | 2004-09-16 | 2006-07-20 | Michael Lesh | Tissue augmentation device |
| US20060293626A1 (en)* | 2005-06-24 | 2006-12-28 | Byrum Randal T | Applier for implantable medical device |
| US20060293628A1 (en)* | 2005-06-24 | 2006-12-28 | Hunt John V | Implantable medical device with indicator |
| JP2007000643A (en)* | 2005-06-24 | 2007-01-11 | Ethicon Endo Surgery Inc | Injection port |
| US20070067041A1 (en)* | 2005-09-16 | 2007-03-22 | Kotoske Thomas G | Inflatable facial implant and associated method |
| US20070078416A1 (en)* | 2005-10-04 | 2007-04-05 | Kenneth Eliasen | Two-piece inline vascular access portal |
| US20070149947A1 (en)* | 2003-12-19 | 2007-06-28 | Byrum Randal T | Audible and tactile feedback |
| US20080140025A1 (en)* | 2005-03-04 | 2008-06-12 | C. R. Bard, Inc. | Access port identification systems and methods |
| US20080275569A1 (en)* | 2004-09-16 | 2008-11-06 | Evera Medical, Inc | Tissue Augmentation Device |
| US20090005703A1 (en)* | 2007-06-27 | 2009-01-01 | Codman & Shurtleff, Inc. | Medical Monitor User Interface |
| US7513892B1 (en)* | 1997-10-01 | 2009-04-07 | Navilyst Medical, Inc. | Guidewire compatible port and method for inserting same |
| US20090156928A1 (en)* | 2007-11-07 | 2009-06-18 | C. R. Bard, Inc. | Radiopaque and septum-based indicators for a multi-lumen implantable port |
| US20090198331A1 (en)* | 2008-02-01 | 2009-08-06 | Kesten Randy J | Implantable prosthesis with open cell flow regulation |
| US20090264901A1 (en)* | 2008-04-17 | 2009-10-22 | Ethan Franklin | Implantable access port device and attachment system |
| US20100049316A1 (en)* | 2008-08-20 | 2010-02-25 | Allergan, Inc. | Self-sealing shell for inflatable prostheses |
| US7731700B1 (en) | 2007-06-29 | 2010-06-08 | Walter Samuel Schytte | Subdermal injection port |
| US20100234808A1 (en)* | 2003-06-16 | 2010-09-16 | Uth Joshua R | Injection Port Applier with Downward Force Actuation |
| US20100268165A1 (en)* | 2005-03-04 | 2010-10-21 | C. R. Bard, Inc. | Systems and methods for radiographically identifying an access port |
| US7947022B2 (en) | 2005-03-04 | 2011-05-24 | C. R. Bard, Inc. | Access port identification systems and methods |
| US8021324B2 (en) | 2007-07-19 | 2011-09-20 | Medical Components, Inc. | Venous access port assembly with X-ray discernable indicia |
| US8025639B2 (en) | 2005-04-27 | 2011-09-27 | C. R. Bard, Inc. | Methods of power injecting a fluid through an access port |
| US20110251453A1 (en)* | 2009-08-26 | 2011-10-13 | Allergan, Inc. | Implantable coupling device |
| US20120041341A1 (en)* | 2001-06-08 | 2012-02-16 | Juergen Rasch-Menges | Control solution packets and methods for calibrating bodily fluid sampling devices |
| US20120083751A1 (en)* | 2005-10-17 | 2012-04-05 | Dalton Michael J | Implantable drug delivery depot for subcutaneous delivery of fluids |
| US8177762B2 (en) | 1998-12-07 | 2012-05-15 | C. R. Bard, Inc. | Septum including at least one identifiable feature, access ports including same, and related methods |
| US20120123537A1 (en)* | 2010-11-08 | 2012-05-17 | Allergan, Inc. | Tissue expander with self-healing anterior side |
| US8202259B2 (en) | 2005-03-04 | 2012-06-19 | C. R. Bard, Inc. | Systems and methods for identifying an access port |
| US8257325B2 (en) | 2007-06-20 | 2012-09-04 | Medical Components, Inc. | Venous access port with molded and/or radiopaque indicia |
| USD676955S1 (en) | 2010-12-30 | 2013-02-26 | C. R. Bard, Inc. | Implantable access port |
| US8409221B2 (en) | 2008-04-17 | 2013-04-02 | Allergan, Inc. | Implantable access port device having a safety cap |
| USD682416S1 (en) | 2010-12-30 | 2013-05-14 | C. R. Bard, Inc. | Implantable access port |
| US8506532B2 (en) | 2009-08-26 | 2013-08-13 | Allergan, Inc. | System including access port and applicator tool |
| US8636797B2 (en) | 2010-02-05 | 2014-01-28 | Allergan, Inc. | Inflatable prostheses and methods of making same |
| US8641676B2 (en) | 2005-04-27 | 2014-02-04 | C. R. Bard, Inc. | Infusion apparatuses and methods of use |
| US8715244B2 (en) | 2009-07-07 | 2014-05-06 | C. R. Bard, Inc. | Extensible internal bolster for a medical device |
| US8715158B2 (en) | 2009-08-26 | 2014-05-06 | Apollo Endosurgery, Inc. | Implantable bottom exit port |
| US8801597B2 (en) | 2011-08-25 | 2014-08-12 | Apollo Endosurgery, Inc. | Implantable access port with mesh attachment rivets |
| US8821373B2 (en) | 2011-05-10 | 2014-09-02 | Apollo Endosurgery, Inc. | Directionless (orientation independent) needle injection port |
| US8858421B2 (en) | 2011-11-15 | 2014-10-14 | Apollo Endosurgery, Inc. | Interior needle stick guard stems for tubes |
| US8882655B2 (en) | 2010-09-14 | 2014-11-11 | Apollo Endosurgery, Inc. | Implantable access port system |
| US8882728B2 (en) | 2010-02-10 | 2014-11-11 | Apollo Endosurgery, Inc. | Implantable injection port |
| US8905916B2 (en) | 2010-08-16 | 2014-12-09 | Apollo Endosurgery, Inc. | Implantable access port system |
| US8932271B2 (en) | 2008-11-13 | 2015-01-13 | C. R. Bard, Inc. | Implantable medical devices including septum-based indicators |
| US8992415B2 (en) | 2010-04-30 | 2015-03-31 | Apollo Endosurgery, Inc. | Implantable device to protect tubing from puncture |
| US9079004B2 (en) | 2009-11-17 | 2015-07-14 | C. R. Bard, Inc. | Overmolded access port including anchoring and identification features |
| US9089395B2 (en) | 2011-11-16 | 2015-07-28 | Appolo Endosurgery, Inc. | Pre-loaded septum for use with an access port |
| US9125718B2 (en) | 2010-04-30 | 2015-09-08 | Apollo Endosurgery, Inc. | Electronically enhanced access port for a fluid filled implant |
| US9192501B2 (en) | 2010-04-30 | 2015-11-24 | Apollo Endosurgery, Inc. | Remotely powered remotely adjustable gastric band system |
| US9199069B2 (en) | 2011-10-20 | 2015-12-01 | Apollo Endosurgery, Inc. | Implantable injection port |
| US9265912B2 (en) | 2006-11-08 | 2016-02-23 | C. R. Bard, Inc. | Indicia informative of characteristics of insertable medical devices |
| US9333070B2 (en) | 2008-02-01 | 2016-05-10 | Evera Medical, Inc. | Breast implant with internal flow dampening |
| US9474888B2 (en) | 2005-03-04 | 2016-10-25 | C. R. Bard, Inc. | Implantable access port including a sandwiched radiopaque insert |
| US9480831B2 (en) | 2009-12-04 | 2016-11-01 | Versago Vascular Access, Inc. | Vascular access port |
| US9492315B2 (en) | 2010-08-05 | 2016-11-15 | Forsight Vision4, Inc. | Implantable therapeutic device |
| US9522082B2 (en) | 2001-06-12 | 2016-12-20 | The Johns Hopkins University | Reservoir device for intraocular drug delivery |
| US9526654B2 (en)* | 2013-03-28 | 2016-12-27 | Forsight Vision4, Inc. | Ophthalmic implant for delivering therapeutic substances |
| US9610432B2 (en) | 2007-07-19 | 2017-04-04 | Innovative Medical Devices, Llc | Venous access port assembly with X-ray discernable indicia |
| US9636070B2 (en) | 2013-03-14 | 2017-05-02 | DePuy Synthes Products, Inc. | Methods, systems, and devices for monitoring and displaying medical parameters for a patient |
| US9642986B2 (en) | 2006-11-08 | 2017-05-09 | C. R. Bard, Inc. | Resource information key for an insertable medical device |
| US9764124B2 (en) | 2014-03-31 | 2017-09-19 | Versago Vascular Access, Inc. | Vascular access port |
| US9851351B2 (en) | 2009-01-29 | 2017-12-26 | Forsight Vision4, Inc. | Posterior segment drug delivery |
| US10052190B2 (en) | 2010-02-05 | 2018-08-21 | Allergan, Inc. | Inflatable prostheses and methods of making same |
| US10238851B2 (en) | 2015-07-14 | 2019-03-26 | Versago Vascular Access, Inc. | Medical access ports, transfer devices and methods of use thereof |
| US10307581B2 (en) | 2005-04-27 | 2019-06-04 | C. R. Bard, Inc. | Reinforced septum for an implantable medical device |
| US10369345B2 (en) | 2014-03-31 | 2019-08-06 | Versago Vascular Access, Inc. | Medical access port, systems and methods of use thereof |
| US10512734B2 (en) | 2014-04-03 | 2019-12-24 | Versago Vascular Access, Inc. | Devices and methods for installation and removal of a needle tip of a needle |
| USD896383S1 (en) | 2018-09-13 | 2020-09-15 | Allergan, Inc. | Tissue expansion device |
| US10813788B2 (en) | 2009-01-29 | 2020-10-27 | Forsight Vision4, Inc. | Implantable therapeutic device |
| US10905866B2 (en) | 2014-12-18 | 2021-02-02 | Versago Vascular Access, Inc. | Devices, systems and methods for removal and replacement of a catheter for an implanted access port |
| US11058815B2 (en) | 2017-12-21 | 2021-07-13 | Versago Vascular Access, Inc. | Medical access ports, transfer devices and methods of use thereof |
| US11154687B2 (en) | 2014-12-18 | 2021-10-26 | Versago Vascular Access, Inc. | Catheter patency systems and methods |
| US11160630B2 (en) | 2018-09-13 | 2021-11-02 | Allergan, Inc. | Tissue expansion device |
| US11432959B2 (en) | 2015-11-20 | 2022-09-06 | Forsight Vision4, Inc. | Porous structures for extended release drug delivery devices |
| US11617680B2 (en) | 2016-04-05 | 2023-04-04 | Forsight Vision4, Inc. | Implantable ocular drug delivery devices |
| US11890443B2 (en) | 2008-11-13 | 2024-02-06 | C. R. Bard, Inc. | Implantable medical devices including septum-based indicators |
Citations (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US4190040A (en)* | 1978-07-03 | 1980-02-26 | American Hospital Supply Corporation | Resealable puncture housing for surgical implantation |
| US4543088A (en)* | 1983-11-07 | 1985-09-24 | American Hospital Supply Corporation | Self-sealing subcutaneous injection site |
| US4557724A (en)* | 1981-02-17 | 1985-12-10 | University Of Utah Research Foundation | Apparatus and methods for minimizing cellular adhesion on peritoneal injection catheters |
| US4685447A (en)* | 1985-03-25 | 1987-08-11 | Pmt Corporation | Tissue expander system |
- 1988
- 1988-03-14USUS07/167,409patent/US4840615A/ennot_activeExpired - Lifetime
Patent Citations (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US4190040A (en)* | 1978-07-03 | 1980-02-26 | American Hospital Supply Corporation | Resealable puncture housing for surgical implantation |
| US4557724A (en)* | 1981-02-17 | 1985-12-10 | University Of Utah Research Foundation | Apparatus and methods for minimizing cellular adhesion on peritoneal injection catheters |
| US4543088A (en)* | 1983-11-07 | 1985-09-24 | American Hospital Supply Corporation | Self-sealing subcutaneous injection site |
| US4685447A (en)* | 1985-03-25 | 1987-08-11 | Pmt Corporation | Tissue expander system |
Cited By (238)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US5102389A (en)* | 1989-03-28 | 1992-04-07 | Cordis Corporation | Membrane composite |
| US4908029A (en)* | 1989-04-25 | 1990-03-13 | Medical Engineering Corporation | Flexible needle stop |
| US5112303A (en)* | 1991-05-02 | 1992-05-12 | Pudenz-Schulte Medical Research Corporation | Tumor access device and method for delivering medication into a body cavity |
| US5213574A (en)* | 1991-09-06 | 1993-05-25 | Device Labs, Inc. | Composite implantable biocompatible vascular access port device |
| US5425760A (en)* | 1992-05-04 | 1995-06-20 | Rosenberg; Paul H. | Tissue expander apparatus, and methods of constructing and utilizing same |
| US5480430A (en)* | 1993-06-04 | 1996-01-02 | Mcghan Medical Corporation | Shape-retaining shell for a fluid filled prosthesis |
| US5964803A (en)* | 1993-07-27 | 1999-10-12 | Pmt Corporation | Enhanced surface implant and method of manufacture |
| US5558641A (en)* | 1994-01-24 | 1996-09-24 | Sims Deltec, Inc. | Hybrid portal and method |
| US5387192A (en)* | 1994-01-24 | 1995-02-07 | Sims Deltec, Inc. | Hybrid portal and method |
| US6060639A (en)* | 1994-03-04 | 2000-05-09 | Mentor Corporation | Testicular prosthesis and method of manufacturing and filling |
| US5725507A (en)* | 1994-03-04 | 1998-03-10 | Mentor Corporation | Self-sealing injection sites and plugs |
| US5935362A (en)* | 1994-03-04 | 1999-08-10 | Mentor Corporation | Method for manufacturing improved self-sealing injection sites and plugs |
| US5630843A (en)* | 1994-06-30 | 1997-05-20 | Rosenberg; Paul H. | Double chamber tissue expander |
| US5989216A (en)* | 1995-06-29 | 1999-11-23 | Sims Deltec, Inc. | Access portal and method |
| US5935164A (en)* | 1997-02-25 | 1999-08-10 | Pmt Corporaton | Laminated prosthesis and method of manufacture |
| USD413672S (en) | 1997-03-27 | 1999-09-07 | Hudson Design Group | Implantable injection port |
| US7513892B1 (en)* | 1997-10-01 | 2009-04-07 | Navilyst Medical, Inc. | Guidewire compatible port and method for inserting same |
| US6039712A (en)* | 1997-11-04 | 2000-03-21 | Terence M. Fogarty | Implantable injection port |
| US20100280465A1 (en)* | 1998-12-07 | 2010-11-04 | Std Med, Inc | Implantable Vascular Access Device |
| US8608713B2 (en) | 1998-12-07 | 2013-12-17 | C. R. Bard, Inc. | Septum feature for identification of an access port |
| US7713251B2 (en)* | 1998-12-07 | 2010-05-11 | Std Med, Inc. | Implantable vascular access device with ceramic needle guard insert |
| US8409153B2 (en) | 1998-12-07 | 2013-04-02 | Std Med, Inc. | Implantable vascular access device |
| US20030181878A1 (en)* | 1998-12-07 | 2003-09-25 | Tallarida Steven J. | Implantable vascular access device |
| US8177762B2 (en) | 1998-12-07 | 2012-05-15 | C. R. Bard, Inc. | Septum including at least one identifiable feature, access ports including same, and related methods |
| EP1082946A1 (en) | 1999-09-09 | 2001-03-14 | Grampp, Stephan, Dr. med. | Intraluminal graft with variable internal diameter |
| WO2001060444A1 (en)* | 2000-02-18 | 2001-08-23 | Compagnie Europeenne D'etude Et De Recherche De Dispositifs Pour L'implantation Par Laparoscopie | Implantable device for injecting medical substances |
| US6878137B2 (en) | 2000-02-18 | 2005-04-12 | Compagnie Europeenne D'etude Et De Recherche De Dispositifs Pour L'implantation Par Laparoscopie | Implantable device for injecting medical substances |
| US20030135168A1 (en)* | 2000-02-18 | 2003-07-17 | Salomon Benchetrit | Implantable device for injecting medical substances |
| FR2805168A1 (en)* | 2000-02-18 | 2001-08-24 | Cie Euro Etude Rech Paroscopie | IMPLANTABLE DEVICE FOR INJECTING MEDICAL SUBSTANCES |
| FR2805167A1 (en)* | 2000-02-18 | 2001-08-24 | Cie Euro Etude Rech Paroscopie | Subcutaneous implant for injecting medical substances has outer casing of elastomer material with integral membrane for piercing with needle |
| US20060058744A1 (en)* | 2000-04-26 | 2006-03-16 | Tallarida Steven J | Implantable hemodialysis access device |
| US7803143B2 (en) | 2000-04-26 | 2010-09-28 | Std Med, Inc. | Implantable hemodialysis access device |
| US6588432B1 (en)* | 2001-02-15 | 2003-07-08 | Pmt Corporation | Tissue expander magnetic injection port |
| US8772034B2 (en)* | 2001-06-08 | 2014-07-08 | Roche Diagnostics Operations, Inc. | Control solution packets and methods for calibrating bodily fluid sampling devices |
| US20120041341A1 (en)* | 2001-06-08 | 2012-02-16 | Juergen Rasch-Menges | Control solution packets and methods for calibrating bodily fluid sampling devices |
| US10470924B2 (en) | 2001-06-12 | 2019-11-12 | The Johns Hopkins University | Reservoir device for intraocular drug delivery |
| US9522082B2 (en) | 2001-06-12 | 2016-12-20 | The Johns Hopkins University | Reservoir device for intraocular drug delivery |
| US7115118B2 (en)* | 2002-04-08 | 2006-10-03 | Rosemount Inc. | Implantable pressure-activated micro-valve |
| US20030229337A1 (en)* | 2002-04-08 | 2003-12-11 | Broden David A. | Implantable pressure-activated micro-valve |
| US20040185421A1 (en)* | 2003-03-18 | 2004-09-23 | Cagenix, Inc. | Method of using a tissue contourer |
| US20110082426A1 (en)* | 2003-06-16 | 2011-04-07 | Ethicon Endo-Surgery, Inc. | Subcutaneous self attaching injection port with integral moveable retention members |
| US8715243B2 (en) | 2003-06-16 | 2014-05-06 | Ethicon Endo-Surgery, Inc. | Injection port applier with downward force actuation |
| US20100234808A1 (en)* | 2003-06-16 | 2010-09-16 | Uth Joshua R | Injection Port Applier with Downward Force Actuation |
| US20100130941A1 (en)* | 2003-06-16 | 2010-05-27 | Conlon Sean P | Audible And Tactile Feedback |
| US8007474B2 (en) | 2003-06-16 | 2011-08-30 | Ethicon Endo-Surgery, Inc. | Implantable medical device with reversible attachment mechanism and method |
| US20050283119A1 (en)* | 2003-06-16 | 2005-12-22 | Joshua Uth | Implantable medical device with reversible attachment mechanism and method |
| US8211127B2 (en) | 2003-06-16 | 2012-07-03 | Ethicon Endo-Surgery, Inc. | Injection port with extendable and retractable fasteners |
| US7862546B2 (en) | 2003-06-16 | 2011-01-04 | Ethicon Endo-Surgery, Inc. | Subcutaneous self attaching injection port with integral moveable retention members |
| US20040254537A1 (en)* | 2003-06-16 | 2004-12-16 | Conlon Sean P. | Subcutaneous self attaching injection port with integral moveable retention members |
| US8758303B2 (en) | 2003-06-16 | 2014-06-24 | Ethicon Endo-Surgery, Inc. | Injection port with applier |
| US8864717B2 (en) | 2003-06-16 | 2014-10-21 | Ethicon Endo-Surgery, Inc. | Subcutaneous self attaching injection port with integral moveable retention members |
| US20100217199A1 (en)* | 2003-06-16 | 2010-08-26 | Ethicon Endo-Surgery, Inc. | Method of Repositioning an Injection Port |
| US8764713B2 (en) | 2003-06-16 | 2014-07-01 | Ethicon Endo-Surgery, Inc. | Method of repositioning an injection port |
| US20050131388A1 (en)* | 2003-12-16 | 2005-06-16 | Henrich Cheng | Embedded spinal injector |
| US20060293627A1 (en)* | 2003-12-19 | 2006-12-28 | Byrum Randal T | Applier with safety for implantable medical device |
| US8162897B2 (en) | 2003-12-19 | 2012-04-24 | Ethicon Endo-Surgery, Inc. | Audible and tactile feedback |
| US20050283118A1 (en)* | 2003-12-19 | 2005-12-22 | Joshua Uth | Implantable medical device with simulataneous attachment mechanism and method |
| US8029477B2 (en) | 2003-12-19 | 2011-10-04 | Ethicon Endo-Surgery, Inc. | Applier with safety for implantable medical device |
| US7850660B2 (en) | 2003-12-19 | 2010-12-14 | Ethicon Endo-Surgery, Inc. | Implantable medical device with simultaneous attachment mechanism and method |
| US20060293625A1 (en)* | 2003-12-19 | 2006-12-28 | Hunt John V | Implantable medical device with cover and method |
| US7553298B2 (en) | 2003-12-19 | 2009-06-30 | Ethicon Endo-Surgery, Inc. | Implantable medical device with cover and method |
| US20070149947A1 (en)* | 2003-12-19 | 2007-06-28 | Byrum Randal T | Audible and tactile feedback |
| US11224729B2 (en) | 2004-07-13 | 2022-01-18 | Primo Medical Group, Inc. | Volume reducing reservoir insert for an infusion port |
| US8182453B2 (en) | 2004-07-13 | 2012-05-22 | Std Med, Inc. | Volume reducing reservoir insert for an infusion port |
| US20110054419A1 (en)* | 2004-07-13 | 2011-03-03 | Std Med, Inc. | Volume Reducing Reservoir Insert for an Infusion Port |
| US9744343B2 (en) | 2004-07-13 | 2017-08-29 | Primo Medical Group, Inc. | Volume reducing reservoir insert for an infusion port |
| US20180126140A1 (en)* | 2004-07-13 | 2018-05-10 | Primo Medical Group, Inc. | Volume Reducing Reservoir Insert for an Infusion Port |
| US7811266B2 (en) | 2004-07-13 | 2010-10-12 | Std Med, Inc. | Volume reducing reservoir insert for an infusion port |
| US20060100592A1 (en)* | 2004-07-13 | 2006-05-11 | Kenneth Eliasen | Infusion port |
| US7998201B2 (en) | 2004-09-16 | 2011-08-16 | Evera Medical, Inc. | Methods of forming a tissue implant having a tissue contacting layer held under compression |
| US20060058890A1 (en)* | 2004-09-16 | 2006-03-16 | Lesh Michael D | Methods for soft tissue augmentation |
| US7998202B2 (en) | 2004-09-16 | 2011-08-16 | Evera Medical, Inc. | Tissue implant having a biased layer and compliance that simulates tissue |
| US20090024215A1 (en)* | 2004-09-16 | 2009-01-22 | Evera Medical, Inc. | Multilayer tissue implant having a compliance that simulates tissue |
| US7244270B2 (en) | 2004-09-16 | 2007-07-17 | Evera Medical | Systems and devices for soft tissue augmentation |
| US20080015498A1 (en)* | 2004-09-16 | 2008-01-17 | Evera Medical, Inc. | Systems and devices for soft tissue augmentation |
| US20060058735A1 (en)* | 2004-09-16 | 2006-03-16 | Lesh Michael D | Systems and devices for soft tissue augmentation |
| US20060161253A1 (en)* | 2004-09-16 | 2006-07-20 | Michael Lesh | Tissue augmentation device |
| US20060058892A1 (en)* | 2004-09-16 | 2006-03-16 | Lesh Michael D | Valved tissue augmentation implant |
| US20060058891A1 (en)* | 2004-09-16 | 2006-03-16 | Lesh Michael D | Transformable tissue bulking device |
| US7641688B2 (en) | 2004-09-16 | 2010-01-05 | Evera Medical, Inc. | Tissue augmentation device |
| US20080275569A1 (en)* | 2004-09-16 | 2008-11-06 | Evera Medical, Inc | Tissue Augmentation Device |
| US20090099538A1 (en)* | 2004-11-05 | 2009-04-16 | Compagnie Europeenne D'etudeet De Recherche De Dispositifs Pour L' Implantation Parlaparoscopie | Medical implantable site having a multi-layer puncture zone |
| FR2877582A1 (en)* | 2004-11-05 | 2006-05-12 | Cie Euro Etude Rech Paroscopie | IMPLANTABLE MEDICAL SITE WITH MULTI-LAYER PUNCTURE AREA |
| WO2006051192A1 (en) | 2004-11-05 | 2006-05-18 | Compagnie Europeenne D'etude Et De Recherche De Dispositifs Pour L'implantation Par Laparoscopie | Medical site which can be implanted in a multilayered puncture area |
| US7985207B2 (en) | 2004-11-05 | 2011-07-26 | Compagnie Europeenne D'etude Et De Recherche De Dispositifs Pour L'implantation Par Laparoscopie | Medical implantable site having a multi-layer puncture zone |
| US20080140025A1 (en)* | 2005-03-04 | 2008-06-12 | C. R. Bard, Inc. | Access port identification systems and methods |
| US7959615B2 (en) | 2005-03-04 | 2011-06-14 | C. R. Bard, Inc. | Access port identification systems and methods |
| US7947022B2 (en) | 2005-03-04 | 2011-05-24 | C. R. Bard, Inc. | Access port identification systems and methods |
| US8998860B2 (en) | 2005-03-04 | 2015-04-07 | C. R. Bard, Inc. | Systems and methods for identifying an access port |
| US8939947B2 (en) | 2005-03-04 | 2015-01-27 | C. R. Bard, Inc. | Systems and methods for radiographically identifying an access port |
| US9474888B2 (en) | 2005-03-04 | 2016-10-25 | C. R. Bard, Inc. | Implantable access port including a sandwiched radiopaque insert |
| US8029482B2 (en) | 2005-03-04 | 2011-10-04 | C. R. Bard, Inc. | Systems and methods for radiographically identifying an access port |
| US20100268165A1 (en)* | 2005-03-04 | 2010-10-21 | C. R. Bard, Inc. | Systems and methods for radiographically identifying an access port |
| US20110311337A1 (en)* | 2005-03-04 | 2011-12-22 | C.R. Bard, Inc. | Access port identification systems and methods |
| US7785302B2 (en) | 2005-03-04 | 2010-08-31 | C. R. Bard, Inc. | Access port identification systems and methods |
| US9603992B2 (en) | 2005-03-04 | 2017-03-28 | C. R. Bard, Inc. | Access port identification systems and methods |
| US9603993B2 (en) | 2005-03-04 | 2017-03-28 | C. R. Bard, Inc. | Access port identification systems and methods |
| US9682186B2 (en) | 2005-03-04 | 2017-06-20 | C. R. Bard, Inc. | Access port identification systems and methods |
| US10179230B2 (en) | 2005-03-04 | 2019-01-15 | Bard Peripheral Vascular, Inc. | Systems and methods for radiographically identifying an access port |
| US10238850B2 (en) | 2005-03-04 | 2019-03-26 | Bard Peripheral Vascular, Inc. | Systems and methods for radiographically identifying an access port |
| US8202259B2 (en) | 2005-03-04 | 2012-06-19 | C. R. Bard, Inc. | Systems and methods for identifying an access port |
| US8585663B2 (en) | 2005-03-04 | 2013-11-19 | C. R. Bard, Inc. | Access port identification systems and methods |
| US10265512B2 (en) | 2005-03-04 | 2019-04-23 | Bard Peripheral Vascular, Inc. | Implantable access port including a sandwiched radiopaque insert |
| US8382724B2 (en) | 2005-03-04 | 2013-02-26 | C. R. Bard, Inc. | Systems and methods for radiographically identifying an access port |
| US10675401B2 (en) | 2005-03-04 | 2020-06-09 | Bard Peripheral Vascular, Inc. | Access port identification systems and methods |
| US8382723B2 (en) | 2005-03-04 | 2013-02-26 | C. R. Bard, Inc. | Access port identification systems and methods |
| US10857340B2 (en) | 2005-03-04 | 2020-12-08 | Bard Peripheral Vascular, Inc. | Systems and methods for radiographically identifying an access port |
| US10905868B2 (en) | 2005-03-04 | 2021-02-02 | Bard Peripheral Vascular, Inc. | Systems and methods for radiographically identifying an access port |
| US11077291B2 (en) | 2005-03-04 | 2021-08-03 | Bard Peripheral Vascular, Inc. | Implantable access port including a sandwiched radiopaque insert |
| US8603052B2 (en) | 2005-03-04 | 2013-12-10 | C. R. Bard, Inc. | Access port identification systems and methods |
| US8641676B2 (en) | 2005-04-27 | 2014-02-04 | C. R. Bard, Inc. | Infusion apparatuses and methods of use |
| US8805478B2 (en) | 2005-04-27 | 2014-08-12 | C. R. Bard, Inc. | Methods of performing a power injection procedure including identifying features of a subcutaneously implanted access port for delivery of contrast media |
| US8545460B2 (en) | 2005-04-27 | 2013-10-01 | C. R. Bard, Inc. | Infusion apparatuses and related methods |
| US8475417B2 (en) | 2005-04-27 | 2013-07-02 | C. R. Bard, Inc. | Assemblies for identifying a power injectable access port |
| US9937337B2 (en) | 2005-04-27 | 2018-04-10 | C. R. Bard, Inc. | Assemblies for identifying a power injectable access port |
| US9421352B2 (en) | 2005-04-27 | 2016-08-23 | C. R. Bard, Inc. | Infusion apparatuses and methods of use |
| US10016585B2 (en) | 2005-04-27 | 2018-07-10 | Bard Peripheral Vascular, Inc. | Assemblies for identifying a power injectable access port |
| US8641688B2 (en) | 2005-04-27 | 2014-02-04 | C. R. Bard, Inc. | Assemblies for identifying a power injectable access port |
| US10052470B2 (en) | 2005-04-27 | 2018-08-21 | Bard Peripheral Vascular, Inc. | Assemblies for identifying a power injectable access port |
| US10183157B2 (en) | 2005-04-27 | 2019-01-22 | Bard Peripheral Vascular, Inc. | Assemblies for identifying a power injectable access port |
| US10307581B2 (en) | 2005-04-27 | 2019-06-04 | C. R. Bard, Inc. | Reinforced septum for an implantable medical device |
| US10625065B2 (en) | 2005-04-27 | 2020-04-21 | Bard Peripheral Vascular, Inc. | Assemblies for identifying a power injectable access port |
| US8025639B2 (en) | 2005-04-27 | 2011-09-27 | C. R. Bard, Inc. | Methods of power injecting a fluid through an access port |
| US10661068B2 (en) | 2005-04-27 | 2020-05-26 | Bard Peripheral Vascular, Inc. | Assemblies for identifying a power injectable access port |
| US10780257B2 (en) | 2005-04-27 | 2020-09-22 | Bard Peripheral Vascular, Inc. | Assemblies for identifying a power injectable access port |
| JP2007000643A (en)* | 2005-06-24 | 2007-01-11 | Ethicon Endo Surgery Inc | Injection port |
| US20060293628A1 (en)* | 2005-06-24 | 2006-12-28 | Hunt John V | Implantable medical device with indicator |
| US7651483B2 (en) | 2005-06-24 | 2010-01-26 | Ethicon Endo-Surgery, Inc. | Injection port |
| US20060293626A1 (en)* | 2005-06-24 | 2006-12-28 | Byrum Randal T | Applier for implantable medical device |
| US20070010790A1 (en)* | 2005-06-24 | 2007-01-11 | Byrum Randal T | Injection port |
| US7561916B2 (en) | 2005-06-24 | 2009-07-14 | Ethicon Endo-Surgery, Inc. | Implantable medical device with indicator |
| US7918844B2 (en) | 2005-06-24 | 2011-04-05 | Ethicon Endo-Surgery, Inc. | Applier for implantable medical device |
| EP1736196A3 (en)* | 2005-06-24 | 2007-02-14 | Ethicon Endo-Surgery, Inc. | Injection port |
| US20070067041A1 (en)* | 2005-09-16 | 2007-03-22 | Kotoske Thomas G | Inflatable facial implant and associated method |
| US20070078416A1 (en)* | 2005-10-04 | 2007-04-05 | Kenneth Eliasen | Two-piece inline vascular access portal |
| US20120083751A1 (en)* | 2005-10-17 | 2012-04-05 | Dalton Michael J | Implantable drug delivery depot for subcutaneous delivery of fluids |
| US11878137B2 (en) | 2006-10-18 | 2024-01-23 | Medical Components, Inc. | Venous access port assembly with X-ray discernable indicia |
| US9265912B2 (en) | 2006-11-08 | 2016-02-23 | C. R. Bard, Inc. | Indicia informative of characteristics of insertable medical devices |
| US9642986B2 (en) | 2006-11-08 | 2017-05-09 | C. R. Bard, Inc. | Resource information key for an insertable medical device |
| US10092725B2 (en) | 2006-11-08 | 2018-10-09 | C. R. Bard, Inc. | Resource information key for an insertable medical device |
| US10556090B2 (en) | 2006-11-08 | 2020-02-11 | C. R. Bard, Inc. | Resource information key for an insertable medical device |
| US11938296B2 (en) | 2007-06-20 | 2024-03-26 | Medical Components, Inc. | Venous access port with molded and/or radiopaque indicia |
| US11406808B2 (en) | 2007-06-20 | 2022-08-09 | Medical Components, Inc. | Venous access port with molded and/or radiopaque indicia |
| US9533133B2 (en) | 2007-06-20 | 2017-01-03 | Medical Components, Inc. | Venous access port with molded and/or radiopaque indicia |
| US8852160B2 (en) | 2007-06-20 | 2014-10-07 | Medical Components, Inc. | Venous access port with molded and/or radiopaque indicia |
| US8257325B2 (en) | 2007-06-20 | 2012-09-04 | Medical Components, Inc. | Venous access port with molded and/or radiopaque indicia |
| US11478622B2 (en) | 2007-06-20 | 2022-10-25 | Medical Components, Inc. | Venous access port with molded and/or radiopaque indicia |
| US10702174B2 (en) | 2007-06-27 | 2020-07-07 | Integra Lifesciences Corporation | Medical monitor user interface |
| US20090005703A1 (en)* | 2007-06-27 | 2009-01-01 | Codman & Shurtleff, Inc. | Medical Monitor User Interface |
| US7731700B1 (en) | 2007-06-29 | 2010-06-08 | Walter Samuel Schytte | Subdermal injection port |
| US8021324B2 (en) | 2007-07-19 | 2011-09-20 | Medical Components, Inc. | Venous access port assembly with X-ray discernable indicia |
| US9517329B2 (en) | 2007-07-19 | 2016-12-13 | Medical Components, Inc. | Venous access port assembly with X-ray discernable indicia |
| US10874842B2 (en) | 2007-07-19 | 2020-12-29 | Medical Components, Inc. | Venous access port assembly with X-ray discernable indicia |
| US11547843B2 (en) | 2007-07-19 | 2023-01-10 | Innovative Medical Devices, Llc | Venous access port assembly with x-ray discernable indicia |
| US9610432B2 (en) | 2007-07-19 | 2017-04-04 | Innovative Medical Devices, Llc | Venous access port assembly with X-ray discernable indicia |
| US10639465B2 (en) | 2007-07-19 | 2020-05-05 | Innovative Medical Devices, Llc | Venous access port assembly with X-ray discernable indicia |
| US12274850B2 (en) | 2007-07-19 | 2025-04-15 | Medical Components, Inc. | Venous access port assembly with X-ray discernable indicia |
| US10792485B2 (en) | 2007-11-07 | 2020-10-06 | C. R. Bard, Inc. | Radiopaque and septum-based indicators for a multi-lumen implantable port |
| US11638810B2 (en) | 2007-11-07 | 2023-05-02 | C. R. Bard, Inc. | Radiopaque and septum-based indicators for a multi-lumen implantable port |
| US10086186B2 (en) | 2007-11-07 | 2018-10-02 | C. R. Bard, Inc. | Radiopaque and septum-based indicators for a multi-lumen implantable port |
| US9579496B2 (en) | 2007-11-07 | 2017-02-28 | C. R. Bard, Inc. | Radiopaque and septum-based indicators for a multi-lumen implantable port |
| US20090156928A1 (en)* | 2007-11-07 | 2009-06-18 | C. R. Bard, Inc. | Radiopaque and septum-based indicators for a multi-lumen implantable port |
| US20090198331A1 (en)* | 2008-02-01 | 2009-08-06 | Kesten Randy J | Implantable prosthesis with open cell flow regulation |
| US9333070B2 (en) | 2008-02-01 | 2016-05-10 | Evera Medical, Inc. | Breast implant with internal flow dampening |
| US9023062B2 (en) | 2008-04-17 | 2015-05-05 | Apollo Endosurgery, Inc. | Implantable access port device and attachment system |
| US20090264901A1 (en)* | 2008-04-17 | 2009-10-22 | Ethan Franklin | Implantable access port device and attachment system |
| US8409221B2 (en) | 2008-04-17 | 2013-04-02 | Allergan, Inc. | Implantable access port device having a safety cap |
| US9023063B2 (en) | 2008-04-17 | 2015-05-05 | Apollo Endosurgery, Inc. | Implantable access port device having a safety cap |
| US8398654B2 (en) | 2008-04-17 | 2013-03-19 | Allergan, Inc. | Implantable access port device and attachment system |
| US8968400B2 (en) | 2008-08-20 | 2015-03-03 | Allergan, Inc. | Self-sealing shell for inflatable prostheses |
| US9630366B2 (en) | 2008-08-20 | 2017-04-25 | Allergan, Inc. | Self-sealing shell for inflatable prostheses |
| US20100049316A1 (en)* | 2008-08-20 | 2010-02-25 | Allergan, Inc. | Self-sealing shell for inflatable prostheses |
| US8690943B2 (en) | 2008-08-20 | 2014-04-08 | Allergan, Inc. | Self-sealing shell for inflatable prostheses |
| US9387068B2 (en) | 2008-08-20 | 2016-07-12 | Allergan, Inc. | Self-sealing shell for inflatable prostheses |
| US10052471B2 (en) | 2008-11-13 | 2018-08-21 | C. R. Bard, Inc. | Implantable medical devices including septum-based indicators |
| US10773066B2 (en) | 2008-11-13 | 2020-09-15 | C. R. Bard, Inc. | Implantable medical devices including septum-based indicators |
| US8932271B2 (en) | 2008-11-13 | 2015-01-13 | C. R. Bard, Inc. | Implantable medical devices including septum-based indicators |
| US11890443B2 (en) | 2008-11-13 | 2024-02-06 | C. R. Bard, Inc. | Implantable medical devices including septum-based indicators |
| US10656152B2 (en) | 2009-01-29 | 2020-05-19 | Forsight Vision4, Inc. | Posterior segment drug delivery |
| US11642310B2 (en) | 2009-01-29 | 2023-05-09 | Forsight Vision4, Inc. | Posterior segment drug delivery |
| US9851351B2 (en) | 2009-01-29 | 2017-12-26 | Forsight Vision4, Inc. | Posterior segment drug delivery |
| US10813788B2 (en) | 2009-01-29 | 2020-10-27 | Forsight Vision4, Inc. | Implantable therapeutic device |
| US8715244B2 (en) | 2009-07-07 | 2014-05-06 | C. R. Bard, Inc. | Extensible internal bolster for a medical device |
| US8708979B2 (en)* | 2009-08-26 | 2014-04-29 | Apollo Endosurgery, Inc. | Implantable coupling device |
| US20110251453A1 (en)* | 2009-08-26 | 2011-10-13 | Allergan, Inc. | Implantable coupling device |
| US8715158B2 (en) | 2009-08-26 | 2014-05-06 | Apollo Endosurgery, Inc. | Implantable bottom exit port |
| US8506532B2 (en) | 2009-08-26 | 2013-08-13 | Allergan, Inc. | System including access port and applicator tool |
| US9079004B2 (en) | 2009-11-17 | 2015-07-14 | C. R. Bard, Inc. | Overmolded access port including anchoring and identification features |
| US11759615B2 (en) | 2009-11-17 | 2023-09-19 | Bard Peripheral Vascular, Inc. | Overmolded access port including anchoring and identification features |
| US10912935B2 (en) | 2009-11-17 | 2021-02-09 | Bard Peripheral Vascular, Inc. | Method for manufacturing a power-injectable access port |
| US9717895B2 (en) | 2009-11-17 | 2017-08-01 | C. R. Bard, Inc. | Overmolded access port including anchoring and identification features |
| US9248268B2 (en) | 2009-11-17 | 2016-02-02 | C. R. Bard, Inc. | Overmolded access port including anchoring and identification features |
| US10155101B2 (en) | 2009-11-17 | 2018-12-18 | Bard Peripheral Vascular, Inc. | Overmolded access port including anchoring and identification features |
| US10300262B2 (en) | 2009-12-04 | 2019-05-28 | Versago Vascular Access, Inc. | Vascular access port |
| US10835728B2 (en) | 2009-12-04 | 2020-11-17 | Versago Vascular Access, Inc. | Vascular access port |
| US9480831B2 (en) | 2009-12-04 | 2016-11-01 | Versago Vascular Access, Inc. | Vascular access port |
| US8636797B2 (en) | 2010-02-05 | 2014-01-28 | Allergan, Inc. | Inflatable prostheses and methods of making same |
| US10052190B2 (en) | 2010-02-05 | 2018-08-21 | Allergan, Inc. | Inflatable prostheses and methods of making same |
| US10765506B2 (en) | 2010-02-05 | 2020-09-08 | Allergan, Inc. | Inflatable prostheses and methods of making same |
| US8882728B2 (en) | 2010-02-10 | 2014-11-11 | Apollo Endosurgery, Inc. | Implantable injection port |
| US9125718B2 (en) | 2010-04-30 | 2015-09-08 | Apollo Endosurgery, Inc. | Electronically enhanced access port for a fluid filled implant |
| US9192501B2 (en) | 2010-04-30 | 2015-11-24 | Apollo Endosurgery, Inc. | Remotely powered remotely adjustable gastric band system |
| US9241819B2 (en) | 2010-04-30 | 2016-01-26 | Apollo Endosurgery, Inc. | Implantable device to protect tubing from puncture |
| US8992415B2 (en) | 2010-04-30 | 2015-03-31 | Apollo Endosurgery, Inc. | Implantable device to protect tubing from puncture |
| US9492315B2 (en) | 2010-08-05 | 2016-11-15 | Forsight Vision4, Inc. | Implantable therapeutic device |
| US8905916B2 (en) | 2010-08-16 | 2014-12-09 | Apollo Endosurgery, Inc. | Implantable access port system |
| US8882655B2 (en) | 2010-09-14 | 2014-11-11 | Apollo Endosurgery, Inc. | Implantable access port system |
| US20120123537A1 (en)* | 2010-11-08 | 2012-05-17 | Allergan, Inc. | Tissue expander with self-healing anterior side |
| USD676955S1 (en) | 2010-12-30 | 2013-02-26 | C. R. Bard, Inc. | Implantable access port |
| USD682416S1 (en) | 2010-12-30 | 2013-05-14 | C. R. Bard, Inc. | Implantable access port |
| US8821373B2 (en) | 2011-05-10 | 2014-09-02 | Apollo Endosurgery, Inc. | Directionless (orientation independent) needle injection port |
| US8801597B2 (en) | 2011-08-25 | 2014-08-12 | Apollo Endosurgery, Inc. | Implantable access port with mesh attachment rivets |
| US9199069B2 (en) | 2011-10-20 | 2015-12-01 | Apollo Endosurgery, Inc. | Implantable injection port |
| US8858421B2 (en) | 2011-11-15 | 2014-10-14 | Apollo Endosurgery, Inc. | Interior needle stick guard stems for tubes |
| US9089395B2 (en) | 2011-11-16 | 2015-07-28 | Appolo Endosurgery, Inc. | Pre-loaded septum for use with an access port |
| US9636070B2 (en) | 2013-03-14 | 2017-05-02 | DePuy Synthes Products, Inc. | Methods, systems, and devices for monitoring and displaying medical parameters for a patient |
| US20170165110A1 (en)* | 2013-03-28 | 2017-06-15 | Forsight Vision4, Inc. | Ophthalmic implant for delivering therapeutic substances |
| US10398593B2 (en)* | 2013-03-28 | 2019-09-03 | Forsight Vision4, Inc. | Ophthalmic implant for delivering therapeutic substances |
| US9526654B2 (en)* | 2013-03-28 | 2016-12-27 | Forsight Vision4, Inc. | Ophthalmic implant for delivering therapeutic substances |
| US11510810B2 (en) | 2013-03-28 | 2022-11-29 | Forsight Vision4, Inc. | Ophthalmic implant for delivering therapeutic substances |
| US12115102B2 (en) | 2013-03-28 | 2024-10-15 | Forsight Vision4, Inc. | Ophthalmic implant for delivering therapeutic substances |
| US9764124B2 (en) | 2014-03-31 | 2017-09-19 | Versago Vascular Access, Inc. | Vascular access port |
| US10369345B2 (en) | 2014-03-31 | 2019-08-06 | Versago Vascular Access, Inc. | Medical access port, systems and methods of use thereof |
| US11628261B2 (en) | 2014-04-03 | 2023-04-18 | Primo Medical Group, Inc. | Devices and methods for installation and removal of a needle tip of a needle |
| US10512734B2 (en) | 2014-04-03 | 2019-12-24 | Versago Vascular Access, Inc. | Devices and methods for installation and removal of a needle tip of a needle |
| US11154687B2 (en) | 2014-12-18 | 2021-10-26 | Versago Vascular Access, Inc. | Catheter patency systems and methods |
| US10905866B2 (en) | 2014-12-18 | 2021-02-02 | Versago Vascular Access, Inc. | Devices, systems and methods for removal and replacement of a catheter for an implanted access port |
| US10238851B2 (en) | 2015-07-14 | 2019-03-26 | Versago Vascular Access, Inc. | Medical access ports, transfer devices and methods of use thereof |
| US11229781B2 (en) | 2015-07-14 | 2022-01-25 | Versago Vascular Access, Inc. | Medical access ports, transfer devices and methods of use thereof |
| US11432959B2 (en) | 2015-11-20 | 2022-09-06 | Forsight Vision4, Inc. | Porous structures for extended release drug delivery devices |
| US12201556B2 (en) | 2015-11-20 | 2025-01-21 | Forsight Vision4, Inc. | Porous structures for extended release drug delivery devices |
| US11617680B2 (en) | 2016-04-05 | 2023-04-04 | Forsight Vision4, Inc. | Implantable ocular drug delivery devices |
| US12102560B2 (en) | 2016-04-05 | 2024-10-01 | Forsight Vision4, Inc. | Implantable ocular drug delivery devices |
| US11058815B2 (en) | 2017-12-21 | 2021-07-13 | Versago Vascular Access, Inc. | Medical access ports, transfer devices and methods of use thereof |
| USD977647S1 (en) | 2018-09-13 | 2023-02-07 | Allergan, Inc. | Tissue expansion device |
| US11160630B2 (en) | 2018-09-13 | 2021-11-02 | Allergan, Inc. | Tissue expansion device |
| USD926984S1 (en) | 2018-09-13 | 2021-08-03 | Allergan, Inc. | Tissue expansion device |
| USD896383S1 (en) | 2018-09-13 | 2020-09-15 | Allergan, Inc. | Tissue expansion device |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| US4840615A (en) | Self-sealing injection reservoir | |
| US4738657A (en) | Self-sealing injection reservoir | |
| US4798584A (en) | Self-sealing injection reservoir | |
| US4671255A (en) | Tissue expander with self-contained injection reservoir and reinforcing insert | |
| US4841992A (en) | Tissue expander and method of making and using | |
| US4205401A (en) | Mammary prosthesis which resists capsular contracture | |
| US4899764A (en) | Tissue expander and method of making and using | |
| US5035249A (en) | Method of making envelope for tissue expander | |
| CA1249103A (en) | Multiple envelope tissue expander device | |
| US4428364A (en) | Self-sealing injection button and method of making same | |
| US4800901A (en) | Balloon-type Tissue expansion device | |
| US4019499A (en) | Compression implant for urinary incontinence | |
| EP0196821A2 (en) | Tissue expander system | |
| AU701672B2 (en) | Testicular prosthesis and method of manufacturing and filling | |
| US6315796B1 (en) | Flexible seamless memory tissue expanding implant | |
| US4944750A (en) | Composite shell material for prosthesis | |
| US4190040A (en) | Resealable puncture housing for surgical implantation | |
| US5066303A (en) | Self-sealing tissue expander and method | |
| US4950292A (en) | Tissue expanders | |
| US4823815A (en) | Tissue expanding device and method of making same | |
| US3730186A (en) | Adjustable implantable artery-constricting device | |
| US3831583A (en) | Implantable bulb for inflation of surgical implements | |
| US4662357A (en) | Inflatable surgical implant with variable inflation position | |
| US10779840B2 (en) | Hemostatic device | |
| US4662883A (en) | Self-sealing valve for fluid fillable device |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| STCF | Information on status: patent grant | Free format text:PATENTED CASE | |
| FPAY | Fee payment | Year of fee payment:4 | |
| REMI | Maintenance fee reminder mailed | ||
| FPAY | Fee payment | Year of fee payment:8 | |
| SULP | Surcharge for late payment | ||
| AS | Assignment | Owner name:SANTA BARBARA BANK & TRUST, CALIFORNIA Free format text:SECURITY INTEREST;ASSIGNORS:INAMED CORPORATION;INAMED DEVELOPMENT CORPORATION;MCGHAN MEDICAL CORPORATION;AND OTHERS;REEL/FRAME:008660/0777 Effective date:19970731 | |
| AS | Assignment | Owner name:APPALOOSA MANAGEMENT L.P., NEW JERSEY Free format text:ASSIGNMENT OF ASSIGNORS INTEREST;ASSIGNOR:SANTA BARBARA BANK & TRUST;REEL/FRAME:009580/0595 Effective date:19980930 | |
| AS | Assignment | Owner name:ABLECO FINANCE LLC, AS AGENT, NEW YORK Free format text:SECURITY AGREEMENT;ASSIGNOR:MCGHAN MEDICAL CORPORATION;REEL/FRAME:010321/0967 Effective date:19990901 | |
| AS | Assignment | Owner name:MCGHAN MEDICAL CORPORATION, CALIFORNIA Free format text:RELEASE OF SECURITY INTEREST;ASSIGNOR:SANTA BARBARA BANK & TRUST, AS TRUSTEE;REEL/FRAME:010470/0258 Effective date:19990903 Owner name:BIOENTERICS CORPORATION, CALIFORNIA Free format text:RELEASE OF SECURITY INTEREST;ASSIGNOR:SANTA BARBARA BANK & TRUST, AS TRUSTEE;REEL/FRAME:010470/0258 Effective date:19990903 Owner name:INAMED DEVELOPMENT COMPANY, CALIFORNIA Free format text:RELEASE OF SECURITY INTEREST;ASSIGNOR:SANTA BARBARA BANK & TRUST, AS TRUSTEE;REEL/FRAME:010470/0258 Effective date:19990903 Owner name:CUI CORPORATION, CALIFORNIA Free format text:RELEASE OF SECURITY INTEREST;ASSIGNOR:SANTA BARBARA BANK & TRUST, AS TRUSTEE;REEL/FRAME:010470/0258 Effective date:19990903 Owner name:BIOPLEXUS CORPORATION, CALIFORNIA Free format text:RELEASE OF SECURITY INTEREST;ASSIGNOR:SANTA BARBARA BANK & TRUST, AS TRUSTEE;REEL/FRAME:010470/0258 Effective date:19990903 | |
| AS | Assignment | Owner name:MCGHAN MEDICAL CORPORATION, CALIFORNIA Free format text:RELEASE BY SECURED PARTY;ASSIGNOR:APPALOOSA MANAGEMENT L.P.;REEL/FRAME:010628/0941 Effective date:20000128 Owner name:BIOENTERICS CORPORATION, CALIFORNIA Free format text:RELEASE BY SECURED PARTY;ASSIGNOR:APPALOOSA MANAGEMENT L.P.;REEL/FRAME:010628/0941 Effective date:20000128 Owner name:INAMED DEVELOPMENT CO., CALIFORNIA Free format text:RELEASE BY SECURED PARTY;ASSIGNOR:APPALOOSA MANAGEMENT L.P.;REEL/FRAME:010628/0941 Effective date:20000128 Owner name:CUI CORPORATION, CALIFORNIA Free format text:RELEASE BY SECURED PARTY;ASSIGNOR:APPALOOSA MANAGEMENT L.P.;REEL/FRAME:010628/0941 Effective date:20000128 Owner name:BIOPLEXUS CORPORATION, CALIFORNIA Free format text:RELEASE BY SECURED PARTY;ASSIGNOR:APPALOOSA MANAGEMENT L.P.;REEL/FRAME:010628/0941 Effective date:20000128 | |
| FEPP | Fee payment procedure | Free format text:PAT HLDR NO LONGER CLAIMS SMALL ENT STAT AS INDIV INVENTOR (ORIGINAL EVENT CODE: LSM1); ENTITY STATUS OF PATENT OWNER: LARGE ENTITY | |
| FPAY | Fee payment | Year of fee payment:12 | |
| AS | Assignment | Owner name:FIRST UNION NATIONAL BANK, AS ADMINISTRATIVE AGENT Free format text:SECURITY AGREEMENT;ASSIGNORS:INAMED CORPORATION;BIODERMIS CORPORATION;BIOENTERICS CORPORATION;AND OTHERS;REEL/FRAME:013718/0599 Effective date:20000201 | |
| AS | Assignment | Owner name:INAMED MEDICAL PRODUCTS CORPORATION, CALIFORNIA Free format text:RELEASE OF SECURITY INTEREST;ASSIGNOR:ABLECO FINANCE LLC, AS AGENT;REEL/FRAME:014007/0459 Effective date:20030724 Owner name:UNION BANK OF CALIFORNIA, N.A., AS ADMINISTRATIVE Free format text:NOTICE OF GRANT OF SECURITY INTEREST;ASSIGNOR:INAMED MEDICAL PRODUCTS CORPORATION;REEL/FRAME:014007/0442 Effective date:20030725 | |
| AS | Assignment | Owner name:INAMED MEDICAL PRODUCTS CORPORATION, CALIFORNIA Free format text:CHANGE OF NAME;ASSIGNOR:MCGHAN MEDICAL CORPORATION;REEL/FRAME:014022/0567 Effective date:20011129 | |
| AS | Assignment | Owner name:INAMED MEDICAL PRODUCTS CORPORATION (F/K/A MCGHAN Free format text:TERMINATION OF SECURITY INTEREST;ASSIGNOR:UNION BANK OF CALIFORNIA, N.A., AS ADMINISTRATIVE AGENT;REEL/FRAME:017527/0436 Effective date:20060310 |