US4381776A - Anticoagulant dispensing apparatus and method of use - Google Patents
Anticoagulant dispensing apparatus and method of useDownload PDFInfo
- Publication number
- US4381776A US4381776AUS06/254,078US25407881AUS4381776AUS 4381776 AUS4381776 AUS 4381776AUS 25407881 AUS25407881 AUS 25407881AUS 4381776 AUS4381776 AUS 4381776A
- Authority
- US
- United States
- Prior art keywords
- container
- anticoagulant
- spike
- rigid
- blood
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Expired - Fee Related
Links
- 239000003146anticoagulant agentSubstances0.000titleclaimsabstractdescription73
- 229940127219anticoagulant drugDrugs0.000titleclaimsabstractdescription73
- 238000000034methodMethods0.000titleclaimsdescription10
- 210000004369bloodAnatomy0.000claimsabstractdescription36
- 239000008280bloodSubstances0.000claimsabstractdescription36
- XLYOFNOQVPJJNP-UHFFFAOYSA-NwaterChemical compoundOXLYOFNOQVPJJNP-UHFFFAOYSA-N0.000claimsabstractdescription7
- 239000004033plasticSubstances0.000claimsdescription20
- 229920003023plasticPolymers0.000claimsdescription20
- 230000001954sterilising effectEffects0.000claimsdescription11
- 239000000463materialSubstances0.000claimsdescription7
- 230000012953feeding on blood of other organismEffects0.000claimsdescription6
- 239000011888foilSubstances0.000claimsdescription5
- 239000012530fluidSubstances0.000claimsdescription4
- 230000008569processEffects0.000claimsdescription3
- IAYPIBMASNFSPL-UHFFFAOYSA-NEthylene oxideChemical compoundC1CO1IAYPIBMASNFSPL-UHFFFAOYSA-N0.000abstractdescription17
- 230000004888barrier functionEffects0.000abstractdescription6
- 239000007788liquidSubstances0.000abstractdescription5
- 238000009792diffusion processMethods0.000abstractdescription3
- 230000002939deleterious effectEffects0.000abstractdescription2
- 239000000243solutionSubstances0.000description35
- XAGFODPZIPBFFR-UHFFFAOYSA-NaluminiumChemical compound[Al]XAGFODPZIPBFFR-UHFFFAOYSA-N0.000description5
- 229910052782aluminiumInorganic materials0.000description5
- 238000003780insertionMethods0.000description4
- 230000037431insertionEffects0.000description4
- 230000037361pathwayEffects0.000description4
- 238000004659sterilization and disinfectionMethods0.000description4
- KRKNYBCHXYNGOX-UHFFFAOYSA-Ncitric acidChemical compoundOC(=O)CC(O)(C(O)=O)CC(O)=OKRKNYBCHXYNGOX-UHFFFAOYSA-N0.000description3
- 238000003860storageMethods0.000description3
- 229920001875EbonitePolymers0.000description2
- 230000009471actionEffects0.000description2
- 229920001971elastomerPolymers0.000description2
- 238000005538encapsulationMethods0.000description2
- 230000036512infertilityEffects0.000description2
- 230000000977initiatory effectEffects0.000description2
- 238000004519manufacturing processMethods0.000description2
- 238000005086pumpingMethods0.000description2
- 206010053567CoagulopathiesDiseases0.000description1
- WQZGKKKJIJFFOK-GASJEMHNSA-NGlucoseNatural productsOC[C@H]1OC(O)[C@H](O)[C@@H](O)[C@@H]1OWQZGKKKJIJFFOK-GASJEMHNSA-N0.000description1
- 239000007864aqueous solutionSubstances0.000description1
- 230000008901benefitEffects0.000description1
- WQZGKKKJIJFFOK-VFUOTHLCSA-Nbeta-D-glucoseChemical compoundOC[C@H]1O[C@@H](O)[C@H](O)[C@@H](O)[C@@H]1OWQZGKKKJIJFFOK-VFUOTHLCSA-N0.000description1
- 210000000601blood cellAnatomy0.000description1
- 230000017531blood circulationEffects0.000description1
- 230000035602clottingEffects0.000description1
- 238000004891communicationMethods0.000description1
- 238000011109contaminationMethods0.000description1
- 230000008878couplingEffects0.000description1
- 238000010168coupling processMethods0.000description1
- 238000005859coupling reactionMethods0.000description1
- 238000002788crimpingMethods0.000description1
- 239000008121dextroseSubstances0.000description1
- 238000006073displacement reactionMethods0.000description1
- 230000006872improvementEffects0.000description1
- 230000003993interactionEffects0.000description1
- 230000002452interceptive effectEffects0.000description1
- 230000009972noncorrosive effectEffects0.000description1
- 238000004806packaging method and processMethods0.000description1
- 230000035515penetrationEffects0.000description1
- 229920002635polyurethanePolymers0.000description1
- 239000004814polyurethaneSubstances0.000description1
- 230000035939shockEffects0.000description1
- 229920002379silicone rubberPolymers0.000description1
- 239000001509sodium citrateSubstances0.000description1
- NLJMYIDDQXHKNR-UHFFFAOYSA-Ksodium citrateChemical compoundO.O.[Na+].[Na+].[Na+].[O-]C(=O)CC(O)(CC([O-])=O)C([O-])=ONLJMYIDDQXHKNR-UHFFFAOYSA-K0.000description1
Images
Classifications
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61J—CONTAINERS SPECIALLY ADAPTED FOR MEDICAL OR PHARMACEUTICAL PURPOSES; DEVICES OR METHODS SPECIALLY ADAPTED FOR BRINGING PHARMACEUTICAL PRODUCTS INTO PARTICULAR PHYSICAL OR ADMINISTERING FORMS; DEVICES FOR ADMINISTERING FOOD OR MEDICINES ORALLY; BABY COMFORTERS; DEVICES FOR RECEIVING SPITTLE
- A61J1/00—Containers specially adapted for medical or pharmaceutical purposes
- A61J1/05—Containers specially adapted for medical or pharmaceutical purposes for collecting, storing or administering blood, plasma or medical fluids ; Infusion or perfusion containers
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M1/00—Suction or pumping devices for medical purposes; Devices for carrying-off, for treatment of, or for carrying-over, body-liquids; Drainage systems
- A61M1/36—Other treatment of blood in a by-pass of the natural circulatory system, e.g. temperature adaptation, irradiation ; Extra-corporeal blood circuits
- A61M1/3672—Means preventing coagulation
- Y—GENERAL TAGGING OF NEW TECHNOLOGICAL DEVELOPMENTS; GENERAL TAGGING OF CROSS-SECTIONAL TECHNOLOGIES SPANNING OVER SEVERAL SECTIONS OF THE IPC; TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y10—TECHNICAL SUBJECTS COVERED BY FORMER USPC
- Y10S—TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y10S128/00—Surgery
- Y10S128/24—Medical-surgical bags
Definitions
- the anti-coagulantmay be mixed in one of several ways. For example, as shown in U.S. Pat. No. 4,086,924, it may be mixed at the distal end of the phlebotomy needle, or as shown in U.S. Pat. No. 3,221,741, it may be simply collected in a sterile container, containing a predetermined amount of anticoagulant, such as an aqueous solution of citric acid, sodium citrate, and dextrose, commonly known as an A.C.D. solution.
- a predetermined amount of anticoagulantsuch as an aqueous solution of citric acid, sodium citrate, and dextrose, commonly known as an A.C.D. solution.
- the anticoagulantis ratioed into the whole blood as it is drawn, and thus the anticoagulated whole blood which is withdrawn into the whole blood receiving bag always contains an acceptable concentration of anticoagulant.
- the ratio of anticoagulant to bloodis initially very high, thus in some cases, causing damage called "anticoagulant shock" to the blood cells.
- the present inventionis principally concerned with a blood donation system in which the anticoagulant is mixed with the donated whole blood in a predetermined ratio.
- a customary method of sterilizing the anticoagulant containers and the whole blood containers and related tubingutilizes either pressure steam autoclaving or treatment with a high diffusivity sterilant such as, ethylene oxide, or a combination of both.
- a high diffusivity sterilantsuch as, ethylene oxide, or a combination of both.
- pressure steam autoclavingis suitable for sterilizing the anticoagulant containers and tubing, it is not desirable for use with empty whole blood flexible containers, since it can cause the containers to collapse and stick together and it leads to the problem of riding the container of condensed steam following sterilization.
- ethylene oxideis suitable for the whole blood containers, it is not acceptable for sterilizing collapsible pouches with anticoagulant solution, inasmuch as the ethylene oxide reacts with the anticoagulant.
- an aseptically pure dispensing anticoagulant devicewhich is coupled to the blood collecting unit and wherein the entire unit may be aseptically treated by ethylene oxide.
- This inventiontherefore, relates to a new and unique device and method for aseptically dispensing anticoagulant.
- the essential feature of the inventionis to encapsulate the anticoagulant solution in a vapor-tight container which will be contained inside the plastic walls of the blood collection set. This will assure that the plastic walls of the blood collection set are not exposed to water vapor from the anticoagulant at any time during storage, and therefore, will retain the clarity of the plastic originally used in their manufacture.
- the blood collection setmay be sterilized using ethylene oxide and because of the vaportight barrier around the anticoagulant, the ethylene oxide will not react with the anticoagulant.
- Two distinct different containersare shown as preferred embodiments of the encapsulation method for the anticoagulant.
- Oneconsists of a collapsible pouch made from materials which provide both a barrier against diffusion of water vapor and a barrier against diffusion of ethylene oxide.
- the other embodimentcomprises a rigid container made of a material which serves as a barrier to both water vapor and ethylene oxide.
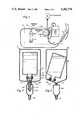
- FIG. 1is a diagramatic illustration of a blood donor system showing the location of the anticoagulant with respect to the collection bag.
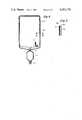
- FIG. 2is a cross-sectional view showing the details of an encapsulated rigid anticoagulant container of the invention.
- FIG. 3is a cross-sectional view of a further embodiment of the invention showing an encapsulated collapsible pouch within which is disposed a rigid anticoagulant container having a tear tab opening.
- FIG. 4is a cross-sectional view of a further embodiment of the invention in which the exterior encapsulating bag and the interior anticoagulant container are both made of collapsible plastic.
- FIG. 5is a view taken along lines 5--5 in FIG. 4.
- FIG. 6is a cross-sectional view of a further embodiment of the invention.
- FIG. 1illustrates a typical blood donor system in which the anticoagulant dispenser of this invention may be utilized.
- FIG. 1corresponds to FIG. 1 of copending U.S. Pat. application Ser. No. 182,510 filed on Aug. 29, 1980.
- the apparatus as shown in FIG. 1, which is of relevance to this invention,may be briefly described as follows.
- Anticoagulant from container 22is supplied to a Y connector 15 on a phlebotomy needle 14 via tubing or conduit 16.
- the Y connector 15is coupled on one side to the distal end of the phlebotomy needle 14.
- the other side of Y connector 15is coupled to tubing 42 which passes through a sensing device 28 to a roller pump 24.
- conduit 42After passing through the roller pump, conduit 42 is coupled to whole blood collection bag 38.
- Tubing or conduit 16 from the anticoagulant pouchis likewise passed through the rollers of pump 24.
- Pump 24is a roller-type pump as described in U.S. Pat. No. 2,565,286, having a movable platen 26 which clamps tubing 18 and tubing 42 against the rollers of the pump when the platen is in its closed position.
- phlebotomy needle 14Prior to making a venipuncture, phlebotomy needle 14 is primed with anticoagulant by opening platen 26 so that the rollers of the anticoagulant pump 24 do not apply their pumping pulses to tubing 16. With platen 26 in the open position, the outlet of anticoagulant pouch 22 is opened without interfering with the sterility of the system.
- tubing 16After venipuncture is made, the pump platen 26 is closed, thereby clamping tube 16 onto the rollers in pump 24 and initiating pumping action. Freshly withdrawn blood flows from the donor through tubing 42 into pressure bag 28 and thence to pump 24. Tubing 42 after it passes through pump 24 is connected to collector bag 38.
- the inner dimensions of tubing 16is made smaller in proportion to the ratio desired of anticoagulant to whole blood. Thus, if the ratio desired is 8 to 1, the inner cross sectional area of the tubing 16 is made eight times smaller than the inner cross sectional area of tubing 42, and since both pass through the same rollers in pump 24, a uniform metering of anticoagulant to whole blood is achieved at the Y junction 15.
- an anticoagulant dispenser 22as used in the invention.
- an anticoagulant solutionis contained in a rigid solution bottle 227, which has been previously sterilized using a steam pressure autoclave.
- the mouth of the containeris closed by a puncturable closure 226 which may comprise aluminum foil or other readily puncturable closure means which is impermeable to moisture or air and, in particularly, to ethylene oxide.
- a rigid guide sleeve 224is disposed coaxially about the outer periphery of the mouth of the solution bottle 227 in airtight relationship.
- a vented spike 223is slidably disposed within guide sleeve 224 and adjacent the puncturable closure 226.
- the vented spike 223may comprise a rigid plastic or hard rubber spike having a pointed end opposite the closure 226.
- a lengthwise slot or holeis provided in vented spike 223 at the location designated 250. This hole provides a passageway through spike 223 for flow of anticoagulant from the solution bottle 227 when the spike has pierced the closure 226.
- a shorter L-shaped fluid passageway 252is provided on vented spike 223 having an opening into chamber 255 at one end and at the other end connecting to air passage 225 in guide sleeve 224.
- a technicianmanipulates vented spike 223 so as to penetrate the puncturable closure 226 of the bottle immediately before starting to use the solution.
- the vented spikehas an air pathway 252 with a side opening to allow passage of air from the air volume, between the outside of the bottle and the inside of the collapsible pouch 228, through a small aperture 225 in an enlarged section 255 near the top of the guide sleeve 252, to the side opening of the air pathway in the spike. This then allows air to pass from the main air volume space 229 into the bottle 227 as solution is passing down the full length pathway 250 into the flexible drip chamber 222.
- FIG. 2An important feature of the embodiment of FIG. 2 is the rigid guide sleeve 224 which is securely attached to the neck of the bottle by crimping the tip of the large end over the retainer 226 of the bottle closure. In this manner, the vented spike is kept well oriented so that the risk of accidental penetration of the wall of the collapsible pouch is eliminated.
- FIG. 3shows a further embodiment of the invention in which the solution bottle 227 of the previous embodiment is replaced by solution bottle 337 having a retainer 341 which may be made of non-corrosive metallic or plastic material which secures a rubber closure 340 over the mouth of the solution bottle 337.
- Tear tab 339is provided on the retainer 341 which is sufficiently large to be grasped by a technician through the wall of the collapsible pouch 338 when it is the proper time to open the bottle.
- the solution bottlehas been previously sterilized in a steam autoclave before insertion in the collapsible pouch 338 and the pouch and bottle are then sterilized in an ethylene oxide solution as a complete entity.
- the technicianremoves the retainer by stripping the tear tab 339 there is no possibility of contamination occurring in this process.
- the pump platen 26must be opened by the technician to permit delivery of the anticoagulant to the delivery tube 316.
- FIG. 4shows a further embodiment of the invention wherein the rigid inner container for the anticoagulant is replaced by a collapsible plastic-surface aluminum foil solution pouch 439.
- Both of the collapsible pouches 438 and 439are made of suitable haemo compatible plastic materials such as polyurethane.
- the inner pouch 439is made of a sandwich of materials which is shown more clearly in FIG. 5, to consist of an outer plastic layer 439 and an inner plastic layer 441 and sandwiched therebetween is a liner of aluminum foil 440.
- the aluminumacts as a vapor barrier to prevent interaction of the anticoagulant and the ethylene oxide during sterilization.
- the lower end of the collapsible plastic-surface aluminum foil solution pouch 439is provided with a burstable seal so that initiation of use of the solution is accomplished simply by squeezing the exterior of outer pouch 438 sufficiently hard to cause the seal to rupture. In the alternative, a simple squeezing tool might be provided for this purpose.
- the collapsible inner solution pouch 439may be sterilized by a steam autoclave after the container is filled with anticoagulant and prior to encapsulation within the plastic pouch 438.
- ethylene oxide sterilizationis used in a conventional way to assure sterility throughout the interior of the set except for the anticoagulant itself which has already been sterilized by steam autoclaving while inside collapsible pouch 438.
- the apparatus shown in FIG. 6is an improved embodiment of the invention shown in FIG. 2.
- the air in the space 229 between the anticoagulant solution bottle and collapsible pouch 228should be of sufficient volume to displace the liquid in the solution bottle as the solution passes into the set through tube 216 after vented spike 223 is inserted through closure 226.
- air in space 229may escape into the set through aperture 225, pathway 250 and tube 216 unless means are provided to block this passageway.
- valve meanswould be provided in tube 216 which would normally be closed to prevent air from escaping into the set and which would be manipulated open by the technician just prior to insertion of spike 223.
- a sterile anticoagulant dispenser 622consisting of an anticoagulant bottle 627 containing anticoagulant solution and a puncturable closure 626.
- the dispenser 622also includes a spike 623 mounted adjacent the closure 626 and an outlet tube 616 coupling the spike in fluid communication with an external software set, not shown.
- the external software setmay consist of a standard plastic disposable blood collection bag (not shown).
- a plastic collapsible pouch 628totally encapsulating the spike 623 and bottle 627, completes the essential elements of the dispenser 622.
- the bottle 627 with anticoagulant contained thereinis sealed by puncturable closure 626 and sterilized using a steam pressure autoclave.
- guide 624 with spike 623 and tubing 616is coaxially mounted about the outer periphery of the mouth of the bottle and attached thereto.
- the pouch 628is then placed over the entire assembly and heat sealed around the periphery in a conventional manner.
- the dispenser 622 with a conventional blood collection software set attached theretomay then be packaged and the interior of the set sterilized by ethylene oxide in a conventional way; it being understood that the ethylene oxide will not have a deleterious effect on the anticoagulant within the bottle.
- spike 623An important element in the embodiment of FIG. 6, in contrast to the embodiment of FIG. 2 is spike 623.
- Spike 623is slidably disposed within guide 624 and adjacent the puncturable closure 626.
- the spike 623may comprise a rigid plastic or hard rubber spike having a pointed end opposite the closure 626.
- the spikeis slightly tapered from the front end (spike end) to the back end, with the smaller outer diameter being closer to the spike end.
- a side-vented slot or hole 650is provided in spike 623. This hole provides a passageway through spike 623 for flow of anticoagulant from the solution bottle 627 when the spike has pierced the closure 626.
- a second fluid passageway 652is provided lengthwise along spike 623.
- Passageway 652opens into air space 629 at the lower end of spike 623.
- an air passage between the air volume inside space 629 (between the collapsible pouch 628 and bottle 627)is provided through passageway 652 into the interior of the solution bottle.
- the air volume in space 629is made sufficiently large so as to permit the air in this volume to displace the liquid in the solution bottle as it passes into the set through passageway 650 and tube 616.
- Sleeve 700in the form of a section of silicon rubber tubing is pressed onto the outside of spike 623 between guide 624 and the exterior tubular surface of spike 623.
- Sleeve 700abuts closure 626 and may preferably be made integral with the closure to guarantee an airtight seal at this juncture.
- the sleeve 700Prior to insertion of spike 623 into closure 626, the sleeve 700 is disposed as shown in FIG. 6 with resepct to spike 623 and thus maintains an airtight seal over the side port of passageway 650.
- sleeve 700is expanded (because of the slight taper of the external diameter of spike 623) thereby providing a gripping action within guide 624 helping to immobilize the spike 623 after the closure 626 is punctured and the spike 623 is in its operational position. Note that until shortly after the closure 626 is actually punctured, the side port entrance to passageway 650 is sealed by sleeve 700 and that prior to puncture of closure 626, air in space 629 cannot exit tube 616 since the side port of passageway 650 is sealed by sleeve 700.
- the spike 623may be moved so as to puncture the closure 626 by manually compressing the pouch 628 and pushing the spike or by pushing the bottle 627 down on the spike.
- the embodiment of FIG. 6, as thus described,has the advantage that loss of air by way of the anticoagulant passageway 650 is prevented during storage and handling, and the anticoagulant passageway 650 is opened automatically during the process of pushing the spike 623 through the puncturable closure 626.
- the pouch 628 in the region of the spike 623it will be readily possible to provide for positive pressure within the pouch 628 at the time that the spike 623 is being pushed through the puncturable closure 628.
Landscapes
- Health & Medical Sciences (AREA)
- Public Health (AREA)
- Vascular Medicine (AREA)
- Life Sciences & Earth Sciences (AREA)
- Animal Behavior & Ethology (AREA)
- General Health & Medical Sciences (AREA)
- Hematology (AREA)
- Veterinary Medicine (AREA)
- Heart & Thoracic Surgery (AREA)
- Cardiology (AREA)
- Pharmacology & Pharmacy (AREA)
- Engineering & Computer Science (AREA)
- Anesthesiology (AREA)
- Biomedical Technology (AREA)
- Medical Preparation Storing Or Oral Administration Devices (AREA)
Abstract
Description
Claims (11)
Priority Applications (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US06/254,078US4381776A (en) | 1980-06-20 | 1981-04-14 | Anticoagulant dispensing apparatus and method of use |
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US16128580A | 1980-06-20 | 1980-06-20 | |
| US06/254,078US4381776A (en) | 1980-06-20 | 1981-04-14 | Anticoagulant dispensing apparatus and method of use |
Related Parent Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US16128580AContinuation-In-Part | 1980-06-20 | 1980-06-20 |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| US4381776Atrue US4381776A (en) | 1983-05-03 |
Family
ID=26857691
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US06/254,078Expired - Fee RelatedUS4381776A (en) | 1980-06-20 | 1981-04-14 | Anticoagulant dispensing apparatus and method of use |
Country Status (1)
| Country | Link |
|---|---|
| US (1) | US4381776A (en) |
Cited By (67)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US4432755A (en)* | 1982-04-06 | 1984-02-21 | Baxter Travenol Laboratories, Inc. | Sterile coupling |
| EP0145825A1 (en)* | 1983-09-08 | 1985-06-26 | Fiab System Ab | Arrangement for infusion bags |
| US4532936A (en)* | 1981-08-21 | 1985-08-06 | Leveen Eric G | Automatic urine flow meter |
| US4567999A (en)* | 1981-05-07 | 1986-02-04 | International Nutritional Research Institute Ab | Self-adhesive connecting device |
| US4583971A (en)* | 1984-02-10 | 1986-04-22 | Travenol European Research And Development Centre (Teradec) | Closed drug delivery system |
| US4600040A (en)* | 1983-03-21 | 1986-07-15 | Naeslund Jan Ingemar | Arrangement in apparatus for preparing solutions from harmful substances |
| US4645073A (en)* | 1985-04-02 | 1987-02-24 | Survival Technology, Inc. | Anti-contamination hazardous material package |
| WO1989004639A1 (en)* | 1987-11-16 | 1989-06-01 | Baxter International Inc. | Multiple bag system |
| WO1989004688A1 (en)* | 1987-11-16 | 1989-06-01 | Baxter International Inc. | Ampule for controlled administration of beneficial agent |
| WO1989004689A1 (en)* | 1987-11-16 | 1989-06-01 | Baxter International Inc. | Glassy matrix for administration of beneficial agent |
| US4910147A (en)* | 1988-09-21 | 1990-03-20 | Baxter International Inc. | Cell culture media flexible container |
| US4968624A (en)* | 1989-04-25 | 1990-11-06 | Baxter International Inc. | Large volume flexible containers |
| US5152281A (en)* | 1990-07-11 | 1992-10-06 | Walter Koll | Massaging device |
| WO1993020772A1 (en)* | 1992-04-22 | 1993-10-28 | Sherwood Medical Company | Fluid container and connection component |
| US5304130A (en)* | 1992-02-26 | 1994-04-19 | Baxter International Inc. | Container for the controlled administration of a beneficial agent |
| US5334180A (en)* | 1993-04-01 | 1994-08-02 | Abbott Laboratories | Sterile formed, filled and sealed flexible container |
| US5358501A (en)* | 1989-11-13 | 1994-10-25 | Becton Dickinson France S.A. | Storage bottle containing a constituent of a medicinal solution |
| US5391163A (en)* | 1992-01-31 | 1995-02-21 | Inpaco Corporation | Pouch for administering medical fluids |
| US5458593A (en)* | 1993-11-24 | 1995-10-17 | Bayer Corporation | Dockable bag system and method |
| US5510115A (en)* | 1987-11-16 | 1996-04-23 | Baxter Travenol Laboratories, Inc. | Method and composition for administration of beneficial agent by controlled dissolution |
| US5683658A (en)* | 1995-07-14 | 1997-11-04 | Avl Medical Instruments Ag | Reagent bottle |
| EP0919216A1 (en)* | 1997-11-20 | 1999-06-02 | Nutrichem Diät + Pharma GmbH | Double pouch for delivering a fluid substance |
| US20030093116A1 (en)* | 1997-12-16 | 2003-05-15 | Closys Corporation | Clotting cascade initiating apparatus and methods of use and methods of closing wounds |
| US20030140928A1 (en)* | 2002-01-29 | 2003-07-31 | Tuan Bui | Medical treatment verification system and method |
| US20030146170A1 (en)* | 2002-02-01 | 2003-08-07 | Frank Corbin | Whole blood collection and processing method |
| US20040123563A1 (en)* | 1997-10-27 | 2004-07-01 | Otsuka Pharmaceutical Factory, Inc. | Packaged ocular irrigating solution bad |
| US20050268416A1 (en)* | 2004-06-03 | 2005-12-08 | Sommers J E | Foldable lotion applicator |
| US20050281132A1 (en)* | 2004-05-20 | 2005-12-22 | Armstrong William D | Mixing system |
| US20070244456A1 (en)* | 2006-04-12 | 2007-10-18 | Fangrow Thomas F | Vial adaptor for regulating pressure |
| US20080249498A1 (en)* | 2007-03-09 | 2008-10-09 | Icu Medical, Inc. | Vial adaptors and vials for regulating pressure |
| US20100049157A1 (en)* | 2008-08-20 | 2010-02-25 | Fangrow Thomas F | Anti-reflux vial adaptors |
| US8234128B2 (en) | 2002-04-30 | 2012-07-31 | Baxter International, Inc. | System and method for verifying medical device operational parameters |
| US20120226236A1 (en)* | 2009-11-04 | 2012-09-06 | Massimo Fini | Medicament application adapter comprising a gas blocking element for a hemodialysis tube set |
| US20130060226A1 (en)* | 2010-05-14 | 2013-03-07 | Massimo Fini | Tubing set having a gate for the connection of vials |
| US20130112575A1 (en)* | 2011-11-07 | 2013-05-09 | General Electric Company | Portable microbubble and drug mixing device |
| US8562582B2 (en) | 2006-05-25 | 2013-10-22 | Bayer Healthcare Llc | Reconstitution device |
| US8775196B2 (en) | 2002-01-29 | 2014-07-08 | Baxter International Inc. | System and method for notification and escalation of medical data |
| US8986238B2 (en) | 2012-08-15 | 2015-03-24 | Cyclone Medtech, Inc. | Systems and methods for salvaging red blood cells for autotransfusion |
| US9089475B2 (en) | 2013-01-23 | 2015-07-28 | Icu Medical, Inc. | Pressure-regulating vial adaptors |
| US9132062B2 (en) | 2011-08-18 | 2015-09-15 | Icu Medical, Inc. | Pressure-regulating vial adaptors |
| US20160331893A1 (en)* | 2015-05-14 | 2016-11-17 | Carefusion 303, Inc. | Priming apparatus and method |
| WO2017019139A1 (en)* | 2015-07-30 | 2017-02-02 | Intravena, Llc | Convenience kits for transporting and accessing medical vials |
| US9610217B2 (en) | 2012-03-22 | 2017-04-04 | Icu Medical, Inc. | Pressure-regulating vial adaptors |
| US9615997B2 (en) | 2013-01-23 | 2017-04-11 | Icu Medical, Inc. | Pressure-regulating vial adaptors |
| US9808552B1 (en) | 1997-12-16 | 2017-11-07 | St. Croix Surgical Systems, Llc | Apparatus for closing wounds |
| US9987195B2 (en) | 2012-01-13 | 2018-06-05 | Icu Medical, Inc. | Pressure-regulating vial adaptors and methods |
| US10016554B2 (en) | 2008-07-09 | 2018-07-10 | Baxter International Inc. | Dialysis system including wireless patient data |
| US10061899B2 (en) | 2008-07-09 | 2018-08-28 | Baxter International Inc. | Home therapy machine |
| US10173008B2 (en) | 2002-01-29 | 2019-01-08 | Baxter International Inc. | System and method for communicating with a dialysis machine through a network |
| US10201476B2 (en) | 2014-06-20 | 2019-02-12 | Icu Medical, Inc. | Pressure-regulating vial adaptors |
| US10231721B2 (en) | 2010-06-24 | 2019-03-19 | St. Croix Surgical Systems, Llc | Failsafe percutaneous wound barrier |
| US10292904B2 (en) | 2016-01-29 | 2019-05-21 | Icu Medical, Inc. | Pressure-regulating vial adaptors |
| US10347374B2 (en) | 2008-10-13 | 2019-07-09 | Baxter Corporation Englewood | Medication preparation system |
| WO2019150355A1 (en)* | 2018-01-30 | 2019-08-08 | Reddress Ltd. | Blood applicator for tissue treatment |
| US10406072B2 (en) | 2013-07-19 | 2019-09-10 | Icu Medical, Inc. | Pressure-regulating fluid transfer systems and methods |
| US10552577B2 (en) | 2012-08-31 | 2020-02-04 | Baxter Corporation Englewood | Medication requisition fulfillment system and method |
| US10646405B2 (en) | 2012-10-26 | 2020-05-12 | Baxter Corporation Englewood | Work station for medical dose preparation system |
| US10818387B2 (en) | 2014-12-05 | 2020-10-27 | Baxter Corporation Englewood | Dose preparation data analytics |
| US10971257B2 (en) | 2012-10-26 | 2021-04-06 | Baxter Corporation Englewood | Image acquisition for medical dose preparation system |
| US11107574B2 (en) | 2014-09-30 | 2021-08-31 | Baxter Corporation Englewood | Management of medication preparation with formulary management |
| US11367533B2 (en) | 2014-06-30 | 2022-06-21 | Baxter Corporation Englewood | Managed medical information exchange |
| US11495334B2 (en) | 2015-06-25 | 2022-11-08 | Gambro Lundia Ab | Medical device system and method having a distributed database |
| US11516183B2 (en) | 2016-12-21 | 2022-11-29 | Gambro Lundia Ab | Medical device system including information technology infrastructure having secure cluster domain supporting external domain |
| US11575673B2 (en) | 2014-09-30 | 2023-02-07 | Baxter Corporation Englewood | Central user management in a distributed healthcare information management system |
| US11744775B2 (en) | 2016-09-30 | 2023-09-05 | Icu Medical, Inc. | Pressure-regulating vial access devices and methods |
| US11948112B2 (en) | 2015-03-03 | 2024-04-02 | Baxter Corporation Engelwood | Pharmacy workflow management with integrated alerts |
| US12412644B2 (en) | 2014-10-24 | 2025-09-09 | Baxter Corporation Englewood | Automated exchange of healthcare information for fulfillment of medication doses |
Citations (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US2690179A (en)* | 1950-01-20 | 1954-09-28 | Fox Dorothy Brown | Syringe |
| US3696919A (en)* | 1970-10-08 | 1972-10-10 | Colgate Palmolive Co | Double container with mixing means |
| US3856138A (en)* | 1973-05-31 | 1974-12-24 | Shionogi & Co | Compartmentalized container |
| US3977555A (en)* | 1974-05-07 | 1976-08-31 | Pharmaco, Inc. | Protective safety cap for medicament vial |
- 1981
- 1981-04-14USUS06/254,078patent/US4381776A/ennot_activeExpired - Fee Related
Patent Citations (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US2690179A (en)* | 1950-01-20 | 1954-09-28 | Fox Dorothy Brown | Syringe |
| US3696919A (en)* | 1970-10-08 | 1972-10-10 | Colgate Palmolive Co | Double container with mixing means |
| US3856138A (en)* | 1973-05-31 | 1974-12-24 | Shionogi & Co | Compartmentalized container |
| US3977555A (en)* | 1974-05-07 | 1976-08-31 | Pharmaco, Inc. | Protective safety cap for medicament vial |
Cited By (173)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US4567999A (en)* | 1981-05-07 | 1986-02-04 | International Nutritional Research Institute Ab | Self-adhesive connecting device |
| US4532936A (en)* | 1981-08-21 | 1985-08-06 | Leveen Eric G | Automatic urine flow meter |
| US4432755A (en)* | 1982-04-06 | 1984-02-21 | Baxter Travenol Laboratories, Inc. | Sterile coupling |
| US4600040A (en)* | 1983-03-21 | 1986-07-15 | Naeslund Jan Ingemar | Arrangement in apparatus for preparing solutions from harmful substances |
| EP0145825A1 (en)* | 1983-09-08 | 1985-06-26 | Fiab System Ab | Arrangement for infusion bags |
| US4583971A (en)* | 1984-02-10 | 1986-04-22 | Travenol European Research And Development Centre (Teradec) | Closed drug delivery system |
| US4645073A (en)* | 1985-04-02 | 1987-02-24 | Survival Technology, Inc. | Anti-contamination hazardous material package |
| AU609557B2 (en)* | 1987-11-16 | 1991-05-02 | Baxter International Inc. | Ampule for controlled administration of beneficial agent |
| WO1989004689A1 (en)* | 1987-11-16 | 1989-06-01 | Baxter International Inc. | Glassy matrix for administration of beneficial agent |
| WO1989004639A1 (en)* | 1987-11-16 | 1989-06-01 | Baxter International Inc. | Multiple bag system |
| US5030203A (en)* | 1987-11-16 | 1991-07-09 | Baxter International Inc. | Ampule for controlled administration of beneficial agent |
| US5510115A (en)* | 1987-11-16 | 1996-04-23 | Baxter Travenol Laboratories, Inc. | Method and composition for administration of beneficial agent by controlled dissolution |
| WO1989004688A1 (en)* | 1987-11-16 | 1989-06-01 | Baxter International Inc. | Ampule for controlled administration of beneficial agent |
| US4910147A (en)* | 1988-09-21 | 1990-03-20 | Baxter International Inc. | Cell culture media flexible container |
| US4968624A (en)* | 1989-04-25 | 1990-11-06 | Baxter International Inc. | Large volume flexible containers |
| US5358501A (en)* | 1989-11-13 | 1994-10-25 | Becton Dickinson France S.A. | Storage bottle containing a constituent of a medicinal solution |
| US5152281A (en)* | 1990-07-11 | 1992-10-06 | Walter Koll | Massaging device |
| US5391163A (en)* | 1992-01-31 | 1995-02-21 | Inpaco Corporation | Pouch for administering medical fluids |
| US5304130A (en)* | 1992-02-26 | 1994-04-19 | Baxter International Inc. | Container for the controlled administration of a beneficial agent |
| WO1993020772A1 (en)* | 1992-04-22 | 1993-10-28 | Sherwood Medical Company | Fluid container and connection component |
| US5334180A (en)* | 1993-04-01 | 1994-08-02 | Abbott Laboratories | Sterile formed, filled and sealed flexible container |
| US5458593A (en)* | 1993-11-24 | 1995-10-17 | Bayer Corporation | Dockable bag system and method |
| US5573527A (en)* | 1993-11-24 | 1996-11-12 | Pall Corporation | Dockable bag system and method |
| US5683658A (en)* | 1995-07-14 | 1997-11-04 | Avl Medical Instruments Ag | Reagent bottle |
| US20040123563A1 (en)* | 1997-10-27 | 2004-07-01 | Otsuka Pharmaceutical Factory, Inc. | Packaged ocular irrigating solution bad |
| US7047708B2 (en) | 1997-10-27 | 2006-05-23 | Otsuka Pharmaceutical Factory, Inc. | Method of packaging ocular irrigating solution |
| US6764481B1 (en)* | 1997-10-27 | 2004-07-20 | Otsuka Pharmaceutical Factory, Inc. | Packaged ophthalmic perfusate and cleaning fluid bag |
| EP0919216A1 (en)* | 1997-11-20 | 1999-06-02 | Nutrichem Diät + Pharma GmbH | Double pouch for delivering a fluid substance |
| US9669131B2 (en) | 1997-12-16 | 2017-06-06 | St. Croix Surgical Systems, Llc | Methods of closing wounds |
| US20060178610A1 (en)* | 1997-12-16 | 2006-08-10 | Nowakowski Karol L | Clotting cascade initiating apparatus and methods of use and methods of closing wounds |
| US8568448B2 (en) | 1997-12-16 | 2013-10-29 | Closys Corporation | Clotting cascade initiating apparatus and methods of use and methods of closing wounds |
| US8652168B2 (en) | 1997-12-16 | 2014-02-18 | Closys Corporation | Clotting cascade initiating apparatus and methods of use and methods of closing wounds |
| US8652169B2 (en) | 1997-12-16 | 2014-02-18 | Closys Corporation | Clotting cascade initiating apparatus and methods of use and methods of closing wounds |
| US6989022B2 (en)* | 1997-12-16 | 2006-01-24 | Closys Corporation | Clotting cascade initiating apparatus and methods of use and methods of closing wounds |
| US9808552B1 (en) | 1997-12-16 | 2017-11-07 | St. Croix Surgical Systems, Llc | Apparatus for closing wounds |
| US20030093116A1 (en)* | 1997-12-16 | 2003-05-15 | Closys Corporation | Clotting cascade initiating apparatus and methods of use and methods of closing wounds |
| US9839716B1 (en) | 1997-12-16 | 2017-12-12 | St. Croix Surgical Systems, Llc | Methods of closing wounds |
| US20030140928A1 (en)* | 2002-01-29 | 2003-07-31 | Tuan Bui | Medical treatment verification system and method |
| US10556062B2 (en) | 2002-01-29 | 2020-02-11 | Baxter International Inc. | Electronic medication order transfer and processing methods and apparatus |
| US10173008B2 (en) | 2002-01-29 | 2019-01-08 | Baxter International Inc. | System and method for communicating with a dialysis machine through a network |
| US8775196B2 (en) | 2002-01-29 | 2014-07-08 | Baxter International Inc. | System and method for notification and escalation of medical data |
| US6994790B2 (en)* | 2002-02-01 | 2006-02-07 | Gambro, Inc. | Whole blood collection and processing method |
| US20030146170A1 (en)* | 2002-02-01 | 2003-08-07 | Frank Corbin | Whole blood collection and processing method |
| US20050274673A1 (en)* | 2002-02-01 | 2005-12-15 | Gambro, Inc | Whole blood collection and processing method |
| US8234128B2 (en) | 2002-04-30 | 2012-07-31 | Baxter International, Inc. | System and method for verifying medical device operational parameters |
| US7441652B2 (en)* | 2004-05-20 | 2008-10-28 | Med Institute, Inc. | Mixing system |
| US20050281132A1 (en)* | 2004-05-20 | 2005-12-22 | Armstrong William D | Mixing system |
| US20050268416A1 (en)* | 2004-06-03 | 2005-12-08 | Sommers J E | Foldable lotion applicator |
| US10492993B2 (en) | 2006-04-12 | 2019-12-03 | Icu Medical, Inc. | Vial access devices and methods |
| US7658733B2 (en) | 2006-04-12 | 2010-02-09 | Icu Medical, Inc. | Vial for regulating pressure |
| US20070244456A1 (en)* | 2006-04-12 | 2007-10-18 | Fangrow Thomas F | Vial adaptor for regulating pressure |
| US20100137827A1 (en)* | 2006-04-12 | 2010-06-03 | Warren Dee E | Vial adaptors and methods for withdrawing fluid from a vial |
| US20070244464A1 (en)* | 2006-04-12 | 2007-10-18 | Fangrow Thomas F | Vial adaptor for regulating pressure |
| US11013664B2 (en) | 2006-04-12 | 2021-05-25 | Icu Medical, Inc. | Devices for transferring fluid to or from a vial |
| US7972321B2 (en) | 2006-04-12 | 2011-07-05 | Icu Medical, Inc | Vial adaptor for regulating pressure |
| US20110190723A1 (en)* | 2006-04-12 | 2011-08-04 | Fangrow Thomas F | Pressure-regulating vials and containers |
| US8206367B2 (en) | 2006-04-12 | 2012-06-26 | Icu Medical, Inc. | Medical fluid transfer devices and methods with enclosures of sterilized gas |
| US7654995B2 (en) | 2006-04-12 | 2010-02-02 | Icu Medical, Inc. | Vial adaptor for regulating pressure |
| US10327989B2 (en) | 2006-04-12 | 2019-06-25 | Icu Medical, Inc. | Devices and methods for transferring fluid to or from a vial |
| US8267913B2 (en) | 2006-04-12 | 2012-09-18 | Icu Medical, Inc. | Vial adaptors and methods for regulating pressure |
| US10327993B2 (en) | 2006-04-12 | 2019-06-25 | Icu Medical, Inc. | Vial access devices |
| US10327991B2 (en) | 2006-04-12 | 2019-06-25 | Icu Medical, Inc. | Fluid transfer apparatus with filtered air input |
| US10327992B2 (en) | 2006-04-12 | 2019-06-25 | Icu Medical, Inc. | Fluid transfer apparatus with pressure regulation |
| US20080161770A1 (en)* | 2006-04-12 | 2008-07-03 | Fangrow Thomas F | Vial adaptor for regulating pressure |
| US10071020B2 (en) | 2006-04-12 | 2018-09-11 | Icu Medical, Inc. | Devices for transferring fluid to or from a vial |
| US10022302B2 (en) | 2006-04-12 | 2018-07-17 | Icu Medical, Inc. | Devices for transferring medicinal fluids to or from a container |
| US7645271B2 (en) | 2006-04-12 | 2010-01-12 | Icu Medical, Inc. | Vial adaptor for regulating pressure |
| US7569043B2 (en) | 2006-04-12 | 2009-08-04 | Icu Medical, Inc. | Vial adaptor for regulating pressure |
| US7547300B2 (en)* | 2006-04-12 | 2009-06-16 | Icu Medical, Inc. | Vial adaptor for regulating pressure |
| US7534238B2 (en) | 2006-04-12 | 2009-05-19 | Icu Medical, Inc. | Vial adaptor for regulating pressure |
| US8827977B2 (en) | 2006-04-12 | 2014-09-09 | Icu Medical, Inc. | Vial adaptors and methods for regulating pressure |
| US8882738B2 (en) | 2006-04-12 | 2014-11-11 | Icu Medical, Inc. | Locking vial adaptors and methods |
| US9993390B2 (en) | 2006-04-12 | 2018-06-12 | Icu Medical, Inc. | Pressure-regulating vial adaptors and methods |
| US8945084B2 (en) | 2006-04-12 | 2015-02-03 | Icu Medical, Inc. | Pressure-regulating vial adaptors and methods |
| US8974433B2 (en) | 2006-04-12 | 2015-03-10 | Icu Medical, Inc. | Pressure-regulating vials and containers |
| US9993391B2 (en) | 2006-04-12 | 2018-06-12 | Icu Medical, Inc. | Devices and methods for transferring medicinal fluid to or from a container |
| US8992501B2 (en) | 2006-04-12 | 2015-03-31 | Icu Medical, Inc. | Pressure-regulating vial adaptors and methods |
| US9005179B2 (en) | 2006-04-12 | 2015-04-14 | Icu Medical, Inc. | Pressure-regulating apparatus for withdrawing medicinal fluid from a vial |
| US9005180B2 (en) | 2006-04-12 | 2015-04-14 | Icu Medical, Inc. | Vial adaptors and methods for regulating pressure |
| US9060921B2 (en) | 2006-04-12 | 2015-06-23 | Icu Medical, Inc. | Air-filtering vial adaptors and methods |
| US9072657B2 (en) | 2006-04-12 | 2015-07-07 | Icu Medical, Inc. | Pressure-regulating vial adaptors and methods |
| US12350234B2 (en) | 2006-04-12 | 2025-07-08 | Icu Medical, Inc. | Devices for accessing medicinal fluid from a container |
| US7510547B2 (en) | 2006-04-12 | 2009-03-31 | Icu Medical, Inc. | Vial adaptor for regulating pressure |
| US11696871B2 (en) | 2006-04-12 | 2023-07-11 | Icu Medical, Inc. | Devices for accessing medicinal fluid from a container |
| US7513895B2 (en) | 2006-04-12 | 2009-04-07 | Icu Medical, Inc. | Vial adaptor for regulating pressure |
| US9662272B2 (en) | 2006-04-12 | 2017-05-30 | Icu Medical, Inc. | Devices and methods for transferring fluid to or from a vial |
| US11963932B2 (en) | 2006-04-12 | 2024-04-23 | Icu Medical, Inc. | Pressure-regulating vial access devices |
| US8562582B2 (en) | 2006-05-25 | 2013-10-22 | Bayer Healthcare Llc | Reconstitution device |
| US9522098B2 (en) | 2006-05-25 | 2016-12-20 | Bayer Healthcare, Llc | Reconstitution device |
| US20080249498A1 (en)* | 2007-03-09 | 2008-10-09 | Icu Medical, Inc. | Vial adaptors and vials for regulating pressure |
| US8512307B2 (en) | 2007-03-09 | 2013-08-20 | Icu Medical, Inc. | Vial adaptors and vials for regulating pressure |
| US8540692B2 (en) | 2007-03-09 | 2013-09-24 | Icu Medical, Inc. | Adaptors for removing medicinal fluids from vials |
| US7883499B2 (en) | 2007-03-09 | 2011-02-08 | Icu Medical, Inc. | Vial adaptors and vials for regulating pressure |
| US20110073249A1 (en)* | 2007-03-09 | 2011-03-31 | Fangrow Thomas F | Vial adaptors and vials for regulating pressure |
| US9107808B2 (en) | 2007-03-09 | 2015-08-18 | Icu Medical, Inc. | Adaptors for removing medicinal fluids from a container |
| US11311658B2 (en) | 2008-07-09 | 2022-04-26 | Baxter International Inc. | Dialysis system having adaptive prescription generation |
| US10646634B2 (en) | 2008-07-09 | 2020-05-12 | Baxter International Inc. | Dialysis system and disposable set |
| US10224117B2 (en) | 2008-07-09 | 2019-03-05 | Baxter International Inc. | Home therapy machine allowing patient device program selection |
| US10068061B2 (en) | 2008-07-09 | 2018-09-04 | Baxter International Inc. | Home therapy entry, modification, and reporting system |
| US11918721B2 (en) | 2008-07-09 | 2024-03-05 | Baxter International Inc. | Dialysis system having adaptive prescription management |
| US10061899B2 (en) | 2008-07-09 | 2018-08-28 | Baxter International Inc. | Home therapy machine |
| US10272190B2 (en) | 2008-07-09 | 2019-04-30 | Baxter International Inc. | Renal therapy system including a blood pressure monitor |
| US10095840B2 (en) | 2008-07-09 | 2018-10-09 | Baxter International Inc. | System and method for performing renal therapy at a home or dwelling of a patient |
| US10016554B2 (en) | 2008-07-09 | 2018-07-10 | Baxter International Inc. | Dialysis system including wireless patient data |
| US9931275B2 (en) | 2008-08-20 | 2018-04-03 | Icu Medical, Inc. | Anti-reflux vial adaptors |
| US20100049157A1 (en)* | 2008-08-20 | 2010-02-25 | Fangrow Thomas F | Anti-reflux vial adaptors |
| US9351905B2 (en) | 2008-08-20 | 2016-05-31 | Icu Medical, Inc. | Anti-reflux vial adaptors |
| US8409164B2 (en) | 2008-08-20 | 2013-04-02 | Icu Medical, Inc. | Anti-reflux vial adaptors |
| US10347374B2 (en) | 2008-10-13 | 2019-07-09 | Baxter Corporation Englewood | Medication preparation system |
| US9744296B2 (en)* | 2009-11-04 | 2017-08-29 | Fresenius Medical Care Deutschland Gmbh | Medicament application adapter comprising a gas blocking element for a hemodialysis tube set |
| US20120226236A1 (en)* | 2009-11-04 | 2012-09-06 | Massimo Fini | Medicament application adapter comprising a gas blocking element for a hemodialysis tube set |
| EP2569032B1 (en)* | 2010-05-14 | 2018-07-11 | Fresenius Medical Care Deutschland GmbH | Tubing set having an improved gate for the connection of vials |
| US20130060226A1 (en)* | 2010-05-14 | 2013-03-07 | Massimo Fini | Tubing set having a gate for the connection of vials |
| US9623175B2 (en)* | 2010-05-14 | 2017-04-18 | Presenius Medical Care Deutschland Gmbh | Tubing set having a gate for the connection of vials |
| US10973503B2 (en) | 2010-06-24 | 2021-04-13 | St. Croix Surgical Systems, Llc | Failsafe percutaneous wound barrier |
| US10231721B2 (en) | 2010-06-24 | 2019-03-19 | St. Croix Surgical Systems, Llc | Failsafe percutaneous wound barrier |
| US11672734B2 (en) | 2011-08-18 | 2023-06-13 | Icu Medical, Inc. | Pressure-regulating vial adaptors |
| US9895291B2 (en) | 2011-08-18 | 2018-02-20 | Icu Medical, Inc. | Pressure-regulating vial adaptors |
| US10688022B2 (en) | 2011-08-18 | 2020-06-23 | Icu Medical, Inc. | Pressure-regulating vial adaptors |
| US12350237B2 (en) | 2011-08-18 | 2025-07-08 | Icu Medical, Inc. | Pressure-regulating vial adaptors |
| US9132062B2 (en) | 2011-08-18 | 2015-09-15 | Icu Medical, Inc. | Pressure-regulating vial adaptors |
| US11129773B2 (en) | 2011-08-18 | 2021-09-28 | Icu Medical, Inc. | Pressure-regulating vial adaptors |
| US12383469B2 (en) | 2011-08-18 | 2025-08-12 | Icu Medical, Inc. | Pressure-regulating vial adaptors |
| US8905992B2 (en)* | 2011-11-07 | 2014-12-09 | General Electric Company | Portable microbubble and drug mixing device |
| US20130112575A1 (en)* | 2011-11-07 | 2013-05-09 | General Electric Company | Portable microbubble and drug mixing device |
| US9987195B2 (en) | 2012-01-13 | 2018-06-05 | Icu Medical, Inc. | Pressure-regulating vial adaptors and methods |
| US12280013B2 (en) | 2012-03-22 | 2025-04-22 | Icu Medical, Inc. | Pressure-regulating vial adaptors |
| US11654086B2 (en) | 2012-03-22 | 2023-05-23 | Icu Medical, Inc. | Pressure-regulating vial adaptors |
| US10299989B2 (en) | 2012-03-22 | 2019-05-28 | Icu Medical, Inc. | Pressure-regulating vial adaptors |
| US10918573B2 (en) | 2012-03-22 | 2021-02-16 | Icu Medical, Inc. | Pressure-regulating vial adaptors |
| US9610217B2 (en) | 2012-03-22 | 2017-04-04 | Icu Medical, Inc. | Pressure-regulating vial adaptors |
| US11185471B2 (en) | 2012-03-22 | 2021-11-30 | Icu Medical, Inc. | Pressure-regulating vial adaptors |
| US10089443B2 (en) | 2012-05-15 | 2018-10-02 | Baxter International Inc. | Home medical device systems and methods for therapy prescription and tracking, servicing and inventory |
| US8986238B2 (en) | 2012-08-15 | 2015-03-24 | Cyclone Medtech, Inc. | Systems and methods for salvaging red blood cells for autotransfusion |
| US10076595B2 (en) | 2012-08-15 | 2018-09-18 | Cyclone Medtech, Inc. | Systems and methods for blood recovery from absorbent surgical materials |
| US10552577B2 (en) | 2012-08-31 | 2020-02-04 | Baxter Corporation Englewood | Medication requisition fulfillment system and method |
| US10646405B2 (en) | 2012-10-26 | 2020-05-12 | Baxter Corporation Englewood | Work station for medical dose preparation system |
| US10971257B2 (en) | 2012-10-26 | 2021-04-06 | Baxter Corporation Englewood | Image acquisition for medical dose preparation system |
| US10806672B2 (en) | 2013-01-23 | 2020-10-20 | Icu Medical, Inc. | Pressure-regulating vial adaptors |
| US9089475B2 (en) | 2013-01-23 | 2015-07-28 | Icu Medical, Inc. | Pressure-regulating vial adaptors |
| US9615997B2 (en) | 2013-01-23 | 2017-04-11 | Icu Medical, Inc. | Pressure-regulating vial adaptors |
| US11857499B2 (en) | 2013-01-23 | 2024-01-02 | Icu Medical, Inc. | Pressure-regulating vial adaptors |
| US9763855B2 (en) | 2013-01-23 | 2017-09-19 | Icu Medical, Inc. | Pressure-regulating vial adaptors |
| US10117807B2 (en) | 2013-01-23 | 2018-11-06 | Icu Medical, Inc. | Pressure-regulating devices for transferring medicinal fluid |
| US11504302B2 (en) | 2013-07-19 | 2022-11-22 | Icu Medical, Inc. | Pressure-regulating fluid transfer systems and methods |
| US10406072B2 (en) | 2013-07-19 | 2019-09-10 | Icu Medical, Inc. | Pressure-regulating fluid transfer systems and methods |
| US11648181B2 (en) | 2013-07-19 | 2023-05-16 | Icu Medical, Inc. | Pressure-regulating fluid transfer systems and methods |
| US10201476B2 (en) | 2014-06-20 | 2019-02-12 | Icu Medical, Inc. | Pressure-regulating vial adaptors |
| US12377022B2 (en) | 2014-06-20 | 2025-08-05 | Icu Medical, Inc. | Pressure-regulating vial adaptors |
| US10987277B2 (en) | 2014-06-20 | 2021-04-27 | Icu Medical, Inc. | Pressure-regulating vial adaptors |
| US11367533B2 (en) | 2014-06-30 | 2022-06-21 | Baxter Corporation Englewood | Managed medical information exchange |
| US11575673B2 (en) | 2014-09-30 | 2023-02-07 | Baxter Corporation Englewood | Central user management in a distributed healthcare information management system |
| US11107574B2 (en) | 2014-09-30 | 2021-08-31 | Baxter Corporation Englewood | Management of medication preparation with formulary management |
| US12412644B2 (en) | 2014-10-24 | 2025-09-09 | Baxter Corporation Englewood | Automated exchange of healthcare information for fulfillment of medication doses |
| US10818387B2 (en) | 2014-12-05 | 2020-10-27 | Baxter Corporation Englewood | Dose preparation data analytics |
| US11948112B2 (en) | 2015-03-03 | 2024-04-02 | Baxter Corporation Engelwood | Pharmacy workflow management with integrated alerts |
| AU2021277726B2 (en)* | 2015-05-14 | 2022-11-17 | Carefusion 303, Inc. | Priming apparatus and method |
| US11944782B2 (en) | 2015-05-14 | 2024-04-02 | Carefusion 303, Inc. | Priming apparatus and method |
| US11419981B2 (en)* | 2015-05-14 | 2022-08-23 | Carefusion 303, Inc. | Priming apparatus and method |
| AU2016261082B2 (en)* | 2015-05-14 | 2020-12-17 | Carefusion 303, Inc. | Priming apparatus and method |
| AU2021200965B2 (en)* | 2015-05-14 | 2021-10-28 | Carefusion 303, Inc. | Priming apparatus and method |
| US20160331893A1 (en)* | 2015-05-14 | 2016-11-17 | Carefusion 303, Inc. | Priming apparatus and method |
| US10413662B2 (en)* | 2015-05-14 | 2019-09-17 | Carefusion 303, Inc. | Priming apparatus and method |
| CN112190793A (en)* | 2015-05-14 | 2021-01-08 | 康尔福盛303公司 | Pre-fill apparatus and method |
| US11495334B2 (en) | 2015-06-25 | 2022-11-08 | Gambro Lundia Ab | Medical device system and method having a distributed database |
| US20170027817A1 (en)* | 2015-07-30 | 2017-02-02 | Intravena, Llc | Convenience kits for transporting and accessing medical vials |
| WO2017019139A1 (en)* | 2015-07-30 | 2017-02-02 | Intravena, Llc | Convenience kits for transporting and accessing medical vials |
| US11529289B2 (en) | 2016-01-29 | 2022-12-20 | Icu Medical, Inc. | Pressure-regulating vial adaptors |
| US10292904B2 (en) | 2016-01-29 | 2019-05-21 | Icu Medical, Inc. | Pressure-regulating vial adaptors |
| US11744775B2 (en) | 2016-09-30 | 2023-09-05 | Icu Medical, Inc. | Pressure-regulating vial access devices and methods |
| US11516183B2 (en) | 2016-12-21 | 2022-11-29 | Gambro Lundia Ab | Medical device system including information technology infrastructure having secure cluster domain supporting external domain |
| WO2019150355A1 (en)* | 2018-01-30 | 2019-08-08 | Reddress Ltd. | Blood applicator for tissue treatment |
| IL257229B (en)* | 2018-01-30 | 2022-07-01 | Reddress Ltd | A blood-conducting device for tissue treatment |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| US4381776A (en) | Anticoagulant dispensing apparatus and method of use | |
| US5769825A (en) | Self-contained syringe and pharmaceutical packaging system for enclosed mixing of pharmaceutical and diluent | |
| US3945380A (en) | Plasmapheresis assembly | |
| US2950716A (en) | Fluid handling method and apparatus | |
| US3509879A (en) | Parenteral liquid container having frangible part structure | |
| US3276447A (en) | Flexible tubing clamp | |
| US3640267A (en) | Clinical sample container | |
| US5006118A (en) | Liquid transfer assemblies | |
| US3648704A (en) | Disposable catheter apparatus | |
| US5067950A (en) | Wound drainage tube/reservoir connector | |
| US2847007A (en) | Fluid handling unit and apparatus | |
| CA2582301C (en) | Method and apparatus for blood sampling | |
| US3753432A (en) | Hypodermic syringe for blood tests | |
| US5167656A (en) | Blood container having lay-flat sample reservoir | |
| US2676591A (en) | Hypodermic unit | |
| JPS59500602A (en) | mixing device | |
| US3006341A (en) | Medical fluids handling and administering apparatus | |
| AU8932101A (en) | Sampling tube holder for blood sampling system | |
| MXPA01013377A (en) | Blood processing set including an integrated blood sampling system. | |
| JP2002539891A (en) | Containers for packing medical liquids | |
| AU2019202030B2 (en) | Sterile flexible package with pressure compensator for the dosed reconstitution of fluid medicinal or nutritional substances to be administered to patients by infusion or injection | |
| EP0078295A1 (en) | Self-adhesive connecting device. | |
| US11564389B2 (en) | Straw for cryogenic preservation of a dose of liquid-based substance, assembly comprising same, and method for empty this straw | |
| CN212490764U (en) | Safe type configurator convenient to medical personnel dispose and mix liquid medicine usefulness | |
| RU185610U1 (en) | BLOOD CONTAINER AND ITS COMPONENTS |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| AS | Assignment | Owner name:HAEMONETICS CORPORATION, 400 WOOD ROAD, BRAINTREE, Free format text:ASSIGNMENT OF ASSIGNORS INTEREST.;ASSIGNOR:LATHAM, ALLEN JR;REEL/FRAME:003931/0315 Effective date:19811125 | |
| AS | Assignment | Owner name:AMERICAN HOSPITAL SPPLY CORPORATION, ONE AMERICAN Free format text:ASSIGNMENT OF ASSIGNORS INTEREST.;ASSIGNOR:HAEMONETICS CORPORATION, A CORP. OF DE.;REEL/FRAME:004483/0821 Effective date:19850514 | |
| AS | Assignment | Owner name:FLEET CREDIT CORPORATION Free format text:SECURITY INTEREST;ASSIGNOR:LATHAM LABS, INC., A CORP. OF MA.;REEL/FRAME:004520/0794 Owner name:FLEET NATIONAL BANK, A NATIONAL BANKING ASSOCIATIO Free format text:SECURITY INTEREST;ASSIGNOR:LATHAM LABS, INC., A CORP. OF MA.;REEL/FRAME:004520/0794 | |
| AS | Assignment | Owner name:HAEMONETICS CORPORATION Free format text:CHANGE OF NAME;ASSIGNOR:LATHAM LABS, INC. (CHANGED TO);REEL/FRAME:004550/0115 Effective date:19860423 Owner name:LATHAM LABS, INC., 400 WOOD ROAD, BRAINTREE, MASSA Free format text:ASSIGNMENT OF ASSIGNORS INTEREST.;ASSIGNOR:AMERICAN HOSPITAL SUPPLY CORPORATION, A CORP OF IL.;REEL/FRAME:004550/0850 Effective date:19851120 Owner name:HAEMONETICS CORPORATION, MASSACHUSETTS Free format text:CHANGE OF NAME;ASSIGNOR:LATHAM LABS, INC. (CHANGED TO);REEL/FRAME:004550/0115 Effective date:19860423 Owner name:LATHAM LABS, INC., MASSACHUSETTS Free format text:ASSIGNMENT OF ASSIGNORS INTEREST;ASSIGNOR:AMERICAN HOSPITAL SUPPLY CORPORATION, A CORP OF IL.;REEL/FRAME:004550/0850 Effective date:19851120 | |
| AS | Assignment | Owner name:HAEMONETICS CORPORATION, A MASSACHUSETTS CORP. Free format text:ASSIGNMENT OF ASSIGNORS INTEREST.;ASSIGNOR:FLEET NATIONAL BANK;REEL/FRAME:004598/0821 Effective date:19860601 Owner name:HAEMONETICS CORPORATION, A MASSACHUSETTS CORP.,STA Free format text:ASSIGNMENT OF ASSIGNORS INTEREST;ASSIGNOR:FLEET NATIONAL BANK;REEL/FRAME:004598/0821 Effective date:19860601 | |
| FEPP | Fee payment procedure | Free format text:MAINTENANCE FEE REMINDER MAILED (ORIGINAL EVENT CODE: REM.); ENTITY STATUS OF PATENT OWNER: LARGE ENTITY | |
| LAPS | Lapse for failure to pay maintenance fees | ||
| STCH | Information on status: patent discontinuation | Free format text:PATENT EXPIRED DUE TO NONPAYMENT OF MAINTENANCE FEES UNDER 37 CFR 1.362 | |
| FP | Lapsed due to failure to pay maintenance fee | Effective date:19870503 |