US20230393088A1 - System and method for optimizing insulin dosages for diabetic subjects - Google Patents
System and method for optimizing insulin dosages for diabetic subjectsDownload PDFInfo
- Publication number
- US20230393088A1 US20230393088A1US18/329,451US202318329451AUS2023393088A1US 20230393088 A1US20230393088 A1US 20230393088A1US 202318329451 AUS202318329451 AUS 202318329451AUS 2023393088 A1US2023393088 A1US 2023393088A1
- Authority
- US
- United States
- Prior art keywords
- patient
- meal
- insulin
- current
- display
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Pending
Links
Images
Classifications
- G—PHYSICS
- G01—MEASURING; TESTING
- G01N—INVESTIGATING OR ANALYSING MATERIALS BY DETERMINING THEIR CHEMICAL OR PHYSICAL PROPERTIES
- G01N27/00—Investigating or analysing materials by the use of electric, electrochemical, or magnetic means
- G01N27/26—Investigating or analysing materials by the use of electric, electrochemical, or magnetic means by investigating electrochemical variables; by using electrolysis or electrophoresis
- G01N27/28—Electrolytic cell components
- G01N27/30—Electrodes, e.g. test electrodes; Half-cells
- G01N27/327—Biochemical electrodes, e.g. electrical or mechanical details for in vitro measurements
- G01N27/3271—Amperometric enzyme electrodes for analytes in body fluids, e.g. glucose in blood
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61B—DIAGNOSIS; SURGERY; IDENTIFICATION
- A61B5/00—Measuring for diagnostic purposes; Identification of persons
- A61B5/145—Measuring characteristics of blood in vivo, e.g. gas concentration or pH-value ; Measuring characteristics of body fluids or tissues, e.g. interstitial fluid or cerebral tissue
- A61B5/14532—Measuring characteristics of blood in vivo, e.g. gas concentration or pH-value ; Measuring characteristics of body fluids or tissues, e.g. interstitial fluid or cerebral tissue for measuring glucose, e.g. by tissue impedance measurement
- G—PHYSICS
- G16—INFORMATION AND COMMUNICATION TECHNOLOGY [ICT] SPECIALLY ADAPTED FOR SPECIFIC APPLICATION FIELDS
- G16H—HEALTHCARE INFORMATICS, i.e. INFORMATION AND COMMUNICATION TECHNOLOGY [ICT] SPECIALLY ADAPTED FOR THE HANDLING OR PROCESSING OF MEDICAL OR HEALTHCARE DATA
- G16H10/00—ICT specially adapted for the handling or processing of patient-related medical or healthcare data
- G16H10/40—ICT specially adapted for the handling or processing of patient-related medical or healthcare data for data related to laboratory analysis, e.g. patient specimen analysis
- G—PHYSICS
- G16—INFORMATION AND COMMUNICATION TECHNOLOGY [ICT] SPECIALLY ADAPTED FOR SPECIFIC APPLICATION FIELDS
- G16H—HEALTHCARE INFORMATICS, i.e. INFORMATION AND COMMUNICATION TECHNOLOGY [ICT] SPECIALLY ADAPTED FOR THE HANDLING OR PROCESSING OF MEDICAL OR HEALTHCARE DATA
- G16H10/00—ICT specially adapted for the handling or processing of patient-related medical or healthcare data
- G16H10/60—ICT specially adapted for the handling or processing of patient-related medical or healthcare data for patient-specific data, e.g. for electronic patient records
- G—PHYSICS
- G16—INFORMATION AND COMMUNICATION TECHNOLOGY [ICT] SPECIALLY ADAPTED FOR SPECIFIC APPLICATION FIELDS
- G16H—HEALTHCARE INFORMATICS, i.e. INFORMATION AND COMMUNICATION TECHNOLOGY [ICT] SPECIALLY ADAPTED FOR THE HANDLING OR PROCESSING OF MEDICAL OR HEALTHCARE DATA
- G16H20/00—ICT specially adapted for therapies or health-improving plans, e.g. for handling prescriptions, for steering therapy or for monitoring patient compliance
- G16H20/10—ICT specially adapted for therapies or health-improving plans, e.g. for handling prescriptions, for steering therapy or for monitoring patient compliance relating to drugs or medications, e.g. for ensuring correct administration to patients
- G16H20/17—ICT specially adapted for therapies or health-improving plans, e.g. for handling prescriptions, for steering therapy or for monitoring patient compliance relating to drugs or medications, e.g. for ensuring correct administration to patients delivered via infusion or injection
- G—PHYSICS
- G16—INFORMATION AND COMMUNICATION TECHNOLOGY [ICT] SPECIALLY ADAPTED FOR SPECIFIC APPLICATION FIELDS
- G16H—HEALTHCARE INFORMATICS, i.e. INFORMATION AND COMMUNICATION TECHNOLOGY [ICT] SPECIALLY ADAPTED FOR THE HANDLING OR PROCESSING OF MEDICAL OR HEALTHCARE DATA
- G16H40/00—ICT specially adapted for the management or administration of healthcare resources or facilities; ICT specially adapted for the management or operation of medical equipment or devices
- G16H40/60—ICT specially adapted for the management or administration of healthcare resources or facilities; ICT specially adapted for the management or operation of medical equipment or devices for the operation of medical equipment or devices
- G16H40/63—ICT specially adapted for the management or administration of healthcare resources or facilities; ICT specially adapted for the management or operation of medical equipment or devices for the operation of medical equipment or devices for local operation
- G—PHYSICS
- G16—INFORMATION AND COMMUNICATION TECHNOLOGY [ICT] SPECIALLY ADAPTED FOR SPECIFIC APPLICATION FIELDS
- G16H—HEALTHCARE INFORMATICS, i.e. INFORMATION AND COMMUNICATION TECHNOLOGY [ICT] SPECIALLY ADAPTED FOR THE HANDLING OR PROCESSING OF MEDICAL OR HEALTHCARE DATA
- G16H40/00—ICT specially adapted for the management or administration of healthcare resources or facilities; ICT specially adapted for the management or operation of medical equipment or devices
- G16H40/60—ICT specially adapted for the management or administration of healthcare resources or facilities; ICT specially adapted for the management or operation of medical equipment or devices for the operation of medical equipment or devices
- G16H40/67—ICT specially adapted for the management or administration of healthcare resources or facilities; ICT specially adapted for the management or operation of medical equipment or devices for the operation of medical equipment or devices for remote operation
Definitions
- This inventionis directed to a system and method for optimizing the control of blood glucose for diabetic subjects.
- this inventionis directed to a system incorporating a processor whose logic optimizes the control of the blood glucose of the diabetic subject based upon a subject blood glucose level reading at a particular time period and a meal type having been ingested or to be ingested.
- this inventionpertains to an optimized control of the blood glucose level for developing a recommended insulin dosage dependent upon a time period defined as a pre-meal, post-meal, bedtime, mid-sleep, or miscellaneous blood glucose reading taken with a standard glucometer.
- this inventionpertains to a system which takes into account both the meal type such as breakfast, lunch, dinner, or snack.
- this inventionis directed to a system which utilizes the past history of the subject for a particular meal type and time period to optimize the blood glucose level for the diabetic subject. Still further, this invention pertains to optimization of blood glucose levels based upon estimated carbohydrates to be ingested by the subject at a particular meal type in association with whether or not the subject is on a meal plan.
- Diabetesis a growing problem in the world.
- Conventional treatment for diabetesrequires that the patient measures his/her blood glucose several times a day with a glucometer. The patient then estimates the number of units of insulin that should be injected to prevent either hypoglycemia (too low blood glucose) or hyperglycemia (too high blood glucose) based upon the blood glucose reading and the type of food that the patient has ingested or expects to ingest. This generally is a trial and error solution which may have deleterious effects.
- Diabetes or diabetes mellitusis a generally chronic disorder of glucose or sugar metabolism. This is generally caused by the inadequate production of insulin or inadequate use of the insulin generated. Insulin is a hormone produced in specialized cells in the pancreas which permits the body to use and store glucose. Diabetes is a leading cause of death in the World.
- diabetesSymptoms of diabetes or diabetes mellitus results in elevated sugar levels in the urine and blood, as well as increased urination, thirst, hunger, weakness, and weight loss. Prolonged excess blood glucose levels (hypoglycemia) leads to increased protein and fat catabolism which may cause premature vascular degeneration and atherosclerosis. Where diabetes is not controlled, such leads to diabetic acidosis where ketones are built up in the blood.
- Diabetesaffects the body handling of fats which may lead to fat accumulation in the arteries and potential damage to the kidneys, eyes, heart, and brain.
- fatswhich may lead to fat accumulation in the arteries and potential damage to the kidneys, eyes, heart, and brain.
- the optimization of blood glucose levels for a diabetic subjectis a function of numerous interdependent parameters associated with time periods, meal types, ingested food products, prior history of the diabetic subject at respective time periods and meal types being ingested or to be ingested, as well as the physical condition of the subject.
- the interdependent parameterseach having an effect on the other, have to be taken into account to produce a recommended insulin dosage level.
- the subject systemhas been developed in order to optimize the insulin dosage recommended for a subject at a particular time period and further associated with a meal type based upon the above-referenced interrelated parameters.
- Prior systems for recommending insulin dosagesuffer from the fact that the previous history of the subject are not generally taken into account with respect to a particular time period and meal type either ingested or to be ingested
- Conventional treatments for diabetesrequire that the patient measure his/her blood glucose a predetermined number of times during a day with a standard glucometer. The patient may then estimate the number of units of insulin that he/she should inject for prevention of either hypoglycemia or hyperglycemia.
- One aspect of the disclosureprovides [an independent claim].
- the subject systemprovides a system and method for optimizing the glycemic control of a diabetic subject based upon his/her past glycemic history and provides an optimized guidance as to the number of units of insulin or insulin dosage to be injected at any time depending on an extended set of factors. Each of these factors can affect the patient's need for insulin.
- the diabetic patient or caregiverinserts base data into a computer system for estimating the number of grams of carbohydrates that the patient is about to ingest or has ingested at a particular meal type.
- the meal type and time periodis further input as manual data inserted into the computer system.
- the subject systemBased upon the input data as to the current blood glucose reading and the aforementioned input data, the subject system calculates an insulin dosage which is optimized for the patient's particular condition at the current time.
- One of the features of the subject systemis that such provides for the patient the optimum number of units of insulin to be injected dependent on the subject's past history of blood glucose results under similar circumstances.
- a blood glucose rating of 100 mg/dlsuch is input into the system and indicates to the system that there is an estimated ingestion of 30 grams of carbohydrates to be ingested or has previously been ingested, and the system states that 15 units of insulin should be injected, and then the next reading of blood glucose is 70 mg/dl, such would result in an undesirably low reading.
- the systemknowing that 15 units of insulin is too high a dose, the system may suggest 13 units of insulin to prevent a low reading of blood glucose.
- an important feature of the subject systemis that the patient's prior glycemic history is stored by the system and subsequently used in calculations for recommending insulin to be injected dependent upon prior results experienced by the specific subject.
- FIG. 1is a broad flow block diagram of the computer system for processing and calculating insulin dosages to be administered to a subject responsive to a particular meal type and a predetermined time period;
- FIG. 2is a logic block diagram providing a broad logic flow of the logic associated with the computer system and modules dependent upon whether the meal type is pre-meal, post-meal, bedtime, mid-sleep, or miscellaneous;
- FIG. 3is an information flow block diagram associated with processing a physical condition of the subject
- FIG. 4is a flow block diagram associated with determining a correction dosage to be administered to the subject dependent upon input data provided by the subject associated with the meal type and the time period;
- FIG. 5is a logic flow diagram for calculation of the current bolus associated with an adjustment factor
- FIG. 6is a flow block diagram showing the processing for calculating updated basal dosages.
- FIG. 7is a flow block diagram showing the adjustment factor calculations based upon current blood glucose levels.
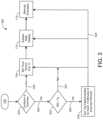
- FIG. 1there is shown blood glucose in insulin dosage administering system 100 for determining an insulin dosage value to be administered to a subject.
- system 100is directed to calculating, processing and recommending blood glucose levels for diabetic subjects at specific times prior to or subsequent to ingestion of food or other nutrients and calculating a recommended insulin dose to be administered.
- System 100is designed to provide the subject with calculated insulin dosage instructions based upon nutritional and physical information in combination with the personal history of previous insulin administration and resulting blood glucose levels.
- Mid-point target blood glucose rangeshall refer to the mid-point of a target blood glucose range (or other blood glucose value within the range) inserted into remote processor 114 by a physician or caregiver for a subject. Although referring to “mid-point” of the blood glucose range, the mid-point target data may be inserted as a function of the mid-point of the mid-point target blood glucose range or some other input deemed appropriate by the subject's physician or caregiver.
- Time periodsshall refer to the time that a subject is taking a blood glucose reading with a standard glucometer and further refers to a pre-meal time period, a post-meal time period, a bedtime period, a mid-sleep time period, or some miscellaneous time period when the subject is taking the blood glucose reading.
- Meal typeshall refer to either breakfast, lunch, dinner, snack, or miscellaneous associated with when the subject is taking the subject's blood glucose reading.
- Blood glucose readingshall be the blood glucose reading taken at a predetermined time period and associated with a meal type.
- Bolusshall refer to recommended insulin dose administered for a meal type and a time period.
- Basal Doseshall refer to a total basal dosage of insulin to be taken for one day.
- Hypoglycemia thresholdshall refer to a lower blood glucose value for a particular subject provided by a physician or other caregiver.
- Prior blood glucose doses and/or levelsshall refer to previous blood glucose doses and/or levels taken or calculated at previous time periods associated with a respective meal type.
- Basal insulin typeshall refer to the type or brand of long acting insulin used with basal dose calculations.
- Bolus insulin typeshall refer to the type or brand of short acting insulin used with meal bolus and correction doses of insulin.
- Basal dose distributionshall refer to the frequency and distribution of basal doses for a particular day such as (1) once a day (SID); (2) twice a day (BID); or, (3) three times a day (TID).
- SIDonce a day
- BIDtwice a day
- TIDthree times a day
- Physical condition parametershall refer to a physical condition of the subject at the time that the blood glucose reading is being taken such as whether or not the subject is exercising or plans to exercise.
- Intermediate blood glucose correction dosageshall refer to a first calculation by processor 116 shown in FIG. 1 .
- Carbohydrate to insulin ratiois a subject specific factor based upon a function of the total daily dose of insulin based upon the subject's weight at the time of initialization of the system 100 processes.
- Meal planshall refer to whether or not the subject is limited to ingesting a known number of carbohydrates for each meal type. When a subject is “on” a meal plan, the subject is generally prescribed a predetermined number of carbohydrates to be ingested at a selected meal type.
- Miscellaneous time periodshall refer to blood glucose calculations at a time period which is not associated with the time periods of breakfast, lunch, dinner, or snack. Such a miscellaneous time period may be associated with a subject fasting period when blood glucose calculations are being processed.
- Mid-sleep time periodshall refer to blood glucose readings taken at a time during a time period when the subject is normally asleep, generally at some point during a sleeping cycle of the subject.
- Insulin sensitivity factorshall refer to a subject specific sensitivity to insulin, generally determined by a physician or care giver and inserted as a portion of the data stored in the remote processor.
- System processorshall refer to an on-site processor which calculates a user's recommended insulin dosage value to be taken at a selected time period and a selected meal type.
- Remote processorshall refer to a processor which is coupled to the system processor and stores a first set of a subject's blood glucose parameters and includes but is not limited to prior basal and bolus dosages, prior or previous blood glucose readings for selected meal types and time periods, subject specific hypoglycemia thresholds, prescribed mid-point of a subject's target range, a subject specific insulin sensitivity factor, basal insulin type, bolus insulin type, basal dose distributions, and the number of carbohydrates a subject is recommended to ingest for a selected meal type.
- the remote processoris generally locationally removed (but in communication) with the system processor, however in some cases the remote processor may be incorporated with the system processor.
- FIG. 1there is shown blood glucose system 100 for calculating, processing, determining, and displaying a recommended insulin dosage value (bolus) to be administered to a subject.
- the broad block diagram shown in FIG. 1includes a glucometer reading (BG) which is inserted by the subject in block 102 .
- the subjecttakes his/her blood glucose value with a standard glucometer well-known in the art and commercially available.
- the glucometergenerally provides the subject's current blood glucose reading in mg/dl.
- datais inserted by the subject in block 101 as to the physical condition of the subject at the time of the taking of the blood glucose value.
- the data inserted in block 101will further be described throughout the flow process and in particular with regard to FIG. 3 .
- data inserted into block 101includes whether the subject is currently exercising or plans to exercise.
- datais stored in remote processor 114 associated with prior basal dosages, prior blood glucose doses administered for particular meal types and time periods (bolus), a subject specific hypoglycemia threshold determined by the physician.
- Data to be included in block 105is the estimated number of carbohydrates the subject will be ingesting at a particular meal type if the subject is not on a meal plan, as well as the number of carbohydrates recommended to be ingested for a particular meal type if the subject is on a prescribed meal plan. Further included in the data stored in remote processor 114 is the mid-point target blood glucose range and the mid-point (T m ) inserted by a physician or other caregiver for a particular subject.
- Block 103directs processor 116 to decision block 302 in FIG. 3 where the subject indicates whether his condition is exercise. If the condition in decision block 302 is that the subject is not exercising and does not plan to exercise, the information flows on information line 320 back to block 104 in FIG. 1 for further calculations to be further described in following paragraphs. From block 104 , the information then flows to dosing adjustment 108 detailed in FIG. 4 and then to subject display 110 and to remote processor 114 for storing the data.
- the logicmoves on line 326 to decision block 320 where it is determined whether the blood glucose level read in block 102 from the glucometer reading is less than or equal to the mid-point target blood glucose range stored in remote processor 114 . If the blood glucose level is equal to or greater than the mid-point target blood glucose range, information is directed on line 322 to block 104 in FIG. 1 for further calculations and passes subsequently to display 110 and remote processor 114 .
- the blood glucose level value in decision block 320is found to be less than the mid-point target blood glucose range, information is directed on line 326 to block 318 where the subject is instructed to eat a predetermined amount of carbohydrates for each predetermined minutes of exercise being planned or having been accomplished. This instruction is then provided to the patient on subject display 110 on line 324 and the information is additionally sent to remote processor 114 for storage of the instructions.
- System processor 116 and subject data display 110may be incorporated within a standard Personal Computer System which has a standard monitor screen for permitting the subject to visually obtain the recommended insulin dosage value being calculated within the system processor 116 and/or the remote processor 114 .
- the subject display monitor 110generally provides visual data to the user, however, as is known, audio information may also be transmitted to the subject.
- the logic initiallyis directed to FIG. 2 where a decision is made as to whether the time period at which the blood glucose level has been taken is determined to be pre-meal, post-meal, bedtime, mid-sleep, or miscellaneous.
- Information flow from within block 104 of FIG. 1is inserted on line 260 to decision block 202 for determining whether the blood glucose reading taken is pre-meal. If the blood glucose reading is taken prior to breakfast, lunch, dinner, or snack, then information flows on line 262 to decision block 204 to determine whether the meal type of the pre-meal time period is breakfast.
- Block 212includes the processing of the logic blocks in FIG. 4 .
- the information in block 212is inserted into decision block 402 on line 424 for determination of whether insulin has been administered within a predetermined time period which is generally 2.0 hours, however, this is adjustable by a physician for a specific subject. If insulin has been administered within a predetermined time period, the logic then moves on line 426 to block 408 where “no correction dose” is recommended and the information returns to FIG. 2 for further processing in block 220 .
- decision block 412information is directed to decision block 412 on line 430 for determination of whether the instant or current blood glucose level reading from the glucometer in block 102 is less than the hypoglycemia threshold value stored in block 114 . If the blood glucose reading is equal to or greater than the hypoglycemia threshold value, information is transported on line 432 to decision block 404 where a determination is made whether the blood glucose reading is greater than the mid-point of the target blood glucose range (T M ).
- the logicpasses on line 436 to calculation block 410 where the intermediate correction or correction insulin dosage is calculated.
- the intermediate blood glucose correction dosage calculated in block 410is a function of the blood glucose reading, the mid-point of the blood glucose target range, and the subject sensitivity factor in accordance with the formula:
- CD( BG - T m ) ( 1 ⁇ 7 ⁇ 0 ⁇ 0 ⁇ ( ( T m - 60 ) ⁇ S 1 ⁇ 24 ) ) ( 1 )
- CDcorrection ⁇ dose ⁇ calculated ⁇ ( units ⁇ of ⁇ insulin )
- BGblood ⁇ glucose ⁇ reading ⁇ ( mg / dl )
- T mmid ⁇ ⁇ ⁇ point ⁇ of ⁇ blood ⁇ glucose ⁇ target ⁇ range ⁇ ( mg / dl )
- S 1patient ⁇ insulin ⁇ sensitivity ⁇ factor ⁇ ( units / mg / dl )
- the blood glucose correction dosageis determined in calculation block 410 , information is directed to decision block 480 on line 438 . Since the correction dosage and associated logic of FIG. 4 is used in conjunction with all time periods where the blood glucose value is taken including pre-meal, post-meal, bedtime, mid-sleep, and miscellaneous, as well as meal types, breakfast, lunch, dinner, snack, bedtime and mid-sleep, the information on line 438 is inserted into the decision block 480 where it is once again determined whether the meal type and the time period is breakfast and pre-meal.
- information from transitional block 422passes on line 424 into decision block 426 to determine whether a previous mid-sleep blood glucose level has been determined and stored in either system processor 116 and/or remote processor 114 . If there is no previous mid-sleep blood glucose level available or the subject does not take mid-sleep blood glucose readings, information passes on line 428 to transfer block 642 for further processing in FIG. 5 .
- Block 604 calculations decision blocksare made in 702 , 706 , 710 , and 714 , as well as calculation block 718 which provides for a particular adjustment factor associated with the blood glucose reading.
- the informationis then passed to block 608 in FIG. 6 for a Basal dose to be calculated based upon the adjustment factor.
- processing block 606If the previous mid-sleep blood glucose level is greater than the previous breakfast blood glucose level in decision block 602 , information is transported on line 630 to processing block 606 where the adjustment factor is calculated using the previous breakfast blood glucose level in accordance with the adjustment factor found in FIG. 7 . Thus, in both processing block 604 and 606 , an adjustment factor is calculated in the logic flow associated with FIG. 7 .
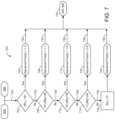
- Calculation blocks 604 and 606are calculated in FIG. 7 where the information flows on line 722 to initial decision block 702 to determine whether the blood glucose level is greater than or equal to 181 mg/dl. If the blood glucose level is greater than 181 mg/dl, then an adjustment factor is set in block 704 as being 1.2. If the blood glucose level is not greater than or equal to 181 mg/dl, then information flows on line 724 to decision block 706 where it is determined whether the blood glucose level is within the range of 141 mg/dl to 180 mg/dl. If the blood glucose level is within the range defined in decision block 706 , the adjustment factor is set to be 1.1 in block 708 .
- the adjustment factoris set in block 712 as 1.0. If the blood glucose level does not fall within the range associated with decision block 710 , information is directed on line 728 to decision block 714 where it is determined whether the blood glucose level is within the range of 71 mg/dl to 100 mg/dl. If the blood glucose level is within the range defined in block 714 , the adjustment factor is set in block 716 to be 0.8.
- the adjustment factoris set in block 720 as 0.8.
- the adjustment factors set in blocks 704 , 708 , 712 , 716 , and 720are dimensionless.
- the adjustment factorafter being calculated in FIG. 7 , the information returns to FIG. 6 and in particular to blocks 604 and 606 .
- the information in block 604 and 606respectively pass on lines 632 or 634 to calculation block 608 where the new basal dose is calculated.
- the new basal dose calculated in block 608is the previous basal dose multiplied by the adjustment factor and this value is inserted into block 610 to recommend the basal dose at the configured time interval.
- Informationthen flows on line 636 to block 638 to insert the recommended basal dose to the subject data display 110 and storage in the system processor 116 and/or remote processor 114 , as well as being returned on line 640 for further calculations of either the breakfast, lunch, dinner, or snack bolus associated with the logic flow in FIG. 5 .
- decision block 490determines whether the time period is pre-meal. If the time period is pre-meal the logic moves on line 496 to transfer block 488 for processing in FIG. 5 . If the time period is not pre-meal then the logic flow is directed to block 110 in FIG. 1 and the correction dose is inserted in accordance with the calculations made in calculation block 410 .
- decision block 414it is determined whether the subject has impaired consciousness, and if the subject does not have impaired consciousness information flows on line 452 to block 420 where the subject is instructed to be given a predetermined dosage of oral glucose and data is then sent directly to data display block 110 . If the subject has impaired consciousness found in decision block 414 , information flows on line 454 to decision block 416 where it is determined whether there is IV access.
- Information from FIG. 2 processing blocks 228 , 222 , 224 , and 226are calculated in accordance with the logic flow in FIG. 5 .
- Calculation of the breakfast, lunch, dinner, or snack bolusis shown in FIG. 5 with information passing from blocks 228 , 222 , 224 , and 226 on line 530 to decision block 502 where it is once again determined whether the pre-meal time period is breakfast. If the pre-meal time period is breakfast, information passes to calculation block 510 on line 532 for calculation of the adjustment factor as previously detailed in the logic flow provided for FIG. 7 .
- calculation of the adjustment factoris made in block 510 in accordance with FIG. 7 as previously discussed.
- Informationthen passes to decision block 562 where there is a determination of whether the subject is on a fixed meal plan. If it is determined that the subject is on a fixed meal plan, such as substantially the same number of carbohydrates to be ingested at each time period and meal type, information then passes on line 564 to calculation block 518 which calculates the current bolus in accordance with the equation:
- the current bolusis then passed on line 554 to subject data display 110 and eventually to remote processor 114 as provided in FIG. 1 . If it is determined that the subject is not on a fixed meal plan in decision block 562 , information is directed through line 566 to calculation block 586 where a number of calculations are performed. Initially, the total prescribed daily basal dose of insulin in units of insulin per day is calculated (TDD) in accordance with the formula:
- TDDTDD M ⁇ W S (3)
- the meal of bolusis calculated by first calculating the carbohydrate to insulin ratio (dimensionless) in accordance with the formula:
- decision block 502If it is determined in decision block 502 that the meal is not breakfast, information is directed on line 536 to decision block 504 where a decision is made as to whether the meal is lunch. If the pre-meal is lunch, then information is passed on line 538 to calculation block 512 for calculation of the adjustment factor in FIG. 7 as previously discussed. Once the adjustment factor has been determined from the logic flow in FIG. 7 , information then is transported on line 540 to fixed meal plan decision block 568 . Decision block 568 , similar to decision block 562 , determines whether the subject is on a fixed meal plan and if the subject is on a fixed meal plan, information passes on line 570 to calculation block 520 where the current bolus is calculated in accordance with Equation 2.
- decision block 504information is transported on line 542 to decision block 506 where it is determined whether the meal type is dinner. If the meal type is dinner, information is inserted to calculation block 514 on line 544 for calculation of the adjustment factor provided by the logic in FIG. 7 . Once the adjustment factor in FIG. 7 has been calculated, information passes on line 546 to decision block 574 determining whether the subject is on a fixed meal plan. The decision block 574 is similar to decision blocks 562 and 568 . If it is determined that the subject is on a fixed meal plan, information is then sent to calculation block 522 on line 576 for calculation of the current bolus (CB) in accordance with Equation 2.
- CBcurrent bolus
- calculation block 590for calculation of the dinner meal bolus in accordance with Equations 3, 4, 5 and 6.
- Informationis then sent from either calculation block 522 or block 590 on respective lines 558 and 559 to subject data display 110 and then to remote processor 114 .
- decision block 506If it is determined in decision block 506 that the meal is not dinner, information then flows on line 548 to decision block 508 where it is determined whether the meal type is a snack. If it determined in decision block 508 that the meal is a snack, information passes on line 550 to calculation block 516 where the adjustment factor is calculated in accordance with FIG. 7 . Information then passes on line 552 to decision block 580 which determines whether the subject is on a fixed meal plan. If the subject is on a fixed meal plan as determined in decision block 580 , information passes on line 582 to calculation block 524 where the current bolus is calculated based upon equation 2.
- the logicflows through line 584 to calculation block 592 where the current meal bolus is calculated in accordance with Equations 3, 4, and 6.
- Information from block 524 or block 592is then transported on either Line 560 or 562 to subject data display system 110 and then to remote processor 114 .
- the time periodis miscellaneous and passes on line 598 to transfer block 599 where logic is transferred to line 288 in FIG. 2 . Processing is then provided in calculation block 258 in accordance with the logic flow in FIG. 4 .
- the informationpasses on line 280 to decision block 230 where decision block 230 determines whether the time period is a post-meal time. If the time period is determined to be post-meal, information is transported on line 282 to decision block 232 where a decision is determined whether this is a breakfast post-meal glucometer reading. If the inputs provided by the subject is to a time period which is post-meal and the meal type is breakfast, information is then transmitted to calculation block 240 in FIG. 2 . In this instance, there is no adjustment of the basal dose as was the case when the time period was pre-meal (previously described) and the meal type was breakfast.
- Calculation block 240directs the information to FIG. 4 where a correction dose is calculated in calculation block 410 .
- All logic blockshave been previously detailed, however, in overview, if insulin has not been given within a predetermined period of time, for example two hours as indicated in decision block 402 , and the blood glucose reading is equal to or greater than the hypoglycemia threshold value (Hi) as determined in decision block 412 , the information is directed to decision block 404 and if the blood glucose reading is determined to be greater than the mid-point target blood glucose range reading, the correction dose is calculated in calculation block 410 . Responsively, subsequent to the calculations provided in calculation block 240 , the results and calculation of the post-meal breakfast correction is transmitted on line 284 to subject display 110 and remote processor 114 for storage of the data calculated.
- Hihypoglycemia threshold value
- a decisionis made as to the fact whether the post-meal blood glucose reading is taken subsequent to lunch in decision block 234 , dinner in decision block 236 , or a snack in decision block 238 . If it is determined that the post-meal blood glucose reading is subsequent to lunch in decision block 234 , the information then is inserted into calculation block 242 for calculation of the post-meal lunch correction as associated with the logic flow previously described for FIG. 4 .
- decision block 234If the decision in decision block 234 is that the post-meal was not lunch, the information then is directed to decision block 236 for determination of whether the post-meal blood glucose reading was dinner and if it is dinner, the logic flows to block 244 and correction dosage as well as the subject meal bolus is made in association with FIG. 4 .
- the informationis directed to calculation block 246 for calculation in the same manner as previously described for the post-meal breakfast, lunch and dinner decisions.
- Information from blocks 240 , 242 , 244 , and 246are then provided on line 284 to both subject display 110 and remote processor 114 for storage of the data and display of the recommended correction reading.
- decision block 230If it is determined in decision block 230 that the blood glucose time period is neither a pre-meal nor a post-meal, the information is directed on line 290 to decision block 248 where it is determined whether the blood glucose taken is at the time period of bedtime (prior to sleep).
- the informationis directed to calculation block 254 for insert into the logic flow of FIG. 4 .
- the logic in FIG. 4in overall view, passes into correction dose calculation block 410 .
- the bolus for bedtimeis then provided on line 286 ( FIG. 2 ) to both subject display system 110 and remote processor 114 as shown in FIG. 1 .
- the informationpasses on line 294 to calculation block 252 where the meal type is defined as miscellaneous since it is neither for a breakfast, lunch, dinner, or snack reading.
- the information in 252is then directed to calculation block 258 where the bolus is calculated in accordance with FIGS. 4 , 5 , and 7 .
- the meal typemust be “miscellaneous” and the information passes on line 288 into block 252 and 258 for calculation of the correction dosage.
- the informationis directed on line 288 to 252 since the reading must be a “miscellaneous” reading.
- information calculatedis then inserted for display in system display 110 and stored in remote processor 114 for further use.
- FIGS. 1 - 7a system for determining the insulin dosage value to be administered to a subject dependent on many interrelated parameters.
- Input to system 100includes a glucometer reading taken by the subject at a time period defined by whether the blood glucose reading is taken pre-meal, post-meal, bedtime, or at some miscellaneous time.
- Remote processor 114maintains in storage, prior basal dosages, hypoglycemia thresholds, target ranges and mid-points of target ranges, and subject insulin sensitivity factor.
- the subjectprovides a manual input on line 118 as represented by block 105 as to the particular time period, whether such is pre-meal, post-meal, bedtime, or at some miscellaneous time.
- the meal typesuch as breakfast, lunch, dinner, or snack is inserted as represented by block 105 for insert into processor 116 for determination of the appropriate correction factors and bolus to be calculated.
- System 100provides the patient with calculated insulin dosage instructions based on nutritional and physical information, as well as personal history of insulin administration and resulting blood glucose levels as previously described.
- the calculated insulin dosage instructionsare output to the subject on subject data display 110 which can be the monitor of a PC or through some other type of audio or sensory indication to the subject.
- subject data display 110can be the monitor of a PC or through some other type of audio or sensory indication to the subject.
- the resulting datais then inserted into remote processor 114 for storage of the data where prior basal dosages, prior blood glucose doses, hypoglycemia thresholds, subject insulin sensitivity factor, whether a meal plan is in effect, and mid-point of target ranges are maintained in storage.
- the subjectfurther includes input as to a physical condition from block 101 . All of this data is then inserted into processor 116 where the physical condition is initially calculated independent of the further processing to be accomplished by processor 116 .
- the physical conditionmay require administration of a predetermined amount of carbohydrates as calculated in FIG. 3 for each time period of exercise which has been accomplished or is being planned and such is inserted into subject data display 110 .
- Prior basal dosages and prior BG doses of the subject for previous time periods of pre-meal, post-meal, bedtime, or miscellaneous as well as prior BG doses associated with specific time periods and meal typesis stored in remote processor 114 along with the hypoglycemia threshold and the mid-point of the target range (T m ). All of this is inserted into processor 116 on line 124 for calculations in blocks 104 and 108 .
- System 100then processes all data drawing on the preset conditions and subject history for determining optimum dosage levels of the subject's current condition where all calculated data is then displayed as represented by block 110 and the calculated data is then stored in remote processor 114 .
- FIG. 2is representative of the calculation blocks 104 and 108 in a further breakdown of the processor calculation procedures.
- the system 100processes patient input of dietary events in FIG. 2 where initially the subject indicates whether the current blood glucose level read from glucometer reading 102 is a time period of a pre-meal (decision block 202 ), post-meal (decision block 230 ), prior to bedtime (decision block 248 ), or mid-sleep cycle (decision block 250 ). If the time period is neither pre-meal, post-meal, bedtime, or during the mid-sleep cycle, then the time period is miscellaneous as represented by input block 252 . Thus, all time periods are then represented and appropriate calculations can be processed.
- Each of the decision blocks 202 , 204 , 206 , 208 , 210 or 203 , 232 , 234 , 236 , 238 , or 248 and 250define individual series of decision blocks.
- a positive indication for one decision blockimplies a negative indication for other decision blocks in each series. This type of event oriented organization permits the subject to expeditiously enter important information.
- the patientelects or indicates whether the pre-meal reading is breakfast as shown in decision block 204 . As previously described, if the pre-meal is not breakfast, the election is made for lunch in decision block 206 , dinner in block 208 , or a snack in decision block 210 .
- An algorithm within processor 116calculates the dosage correction for the planned meal using the calculation algorithm as previously described in FIG. 4 in association with sub-algorithms provided in FIGS. 5 - 7 and in overall block diagram shown in blocks 212 , 214 , 216 , and 218 of FIG. 2 .
- the basal doseis adjusted as indicated in block 220 in association with the logic flow shown in FIG. 6 .
- the pre-meal bolus or recommended insulin dosageis calculated in associated blocks 228 , 222 , 224 , and 226 . If the meal type is neither breakfast, lunch, dinner, or a snack, then it is defined as a miscellaneous time period and the calculations for the bolus are input into block 252 and the calculated correction is made in block 258 as previously detailed. All recommended optimum doses to be taken in any of the time periods is then displayed to the subject on display 110 and the data inserted into remote processor 114 for further use for subsequent blood glucose readings at specific meal types and time periods.
- Mealtime nutritional informationmay be input by the subject and a post-meal bolus correction is calculated for correcting unacceptable blood glucose levels within the logic of processor 116 as indicated by block 108 in FIG. 1 in association with FIG. 5 . logic.
- the meal typeis determined from the decision blocks 232 , 234 , 236 , or 238 for respective calculation of the post-meal type correction in respective blocks 240 , 242 , 244 , and 246 .
- Each of the decision blocks 230 , 232 , 234 , 236 , and 238determine a series of decision blocks where a positive indication for one decision block defines a negative indication for other decision blocks in this series.
- a pre-sleep blood glucose correction doseis calculated in calculation block 254 associated with calculations performed in the logic steps as provided in FIG. 4 .
- the logicblows into decision block 250 where it is determined whether the time period is mid-sleep and if the time period is mid-sleep, calculations are made in block 256 in accordance with the logic flow in FIG. 4 . All information is then inserted on line 286 for insert into subject display 110 and remote processor 114 .
- the meal typeis defaulted to input block 252 where it is determined that the meal type is miscellaneous and then passes to calculation block 258 for calculation in accordance with the calculations processed in FIG. 4 .
- the information from block 258is inserted onto line 286 for display and storage of the data in respective blocks 110 and 114 .
- the information exiting decision blocks 210 and 238indicate that the meal type was neither breakfast, lunch, dinner, or a snack, the information is directed to input block 252 and then inserted into block 258 for calculation in accordance with the logic associated with FIG. 4 .
- FIG. 4is a sub-system which takes information from FIG. 2 and is associated with the calculation blocks 212 , 214 , 216 , and 218 for the pre-meal blood glucose reading time period, as well as logic blocks 240 , 242 , 244 , and 246 for the post-meal time period and blocks 254 , 256 , and 258 for the time periods of bedtime, mid-sleep or miscellaneous.
- the calculation blocks of FIG. 2are read into decision block 402 for determination of whether insulin has been administered within a predetermined time interval of the taking of the blood glucose reading and if insulin has been given within this predetermined time, there is no correction dosage recommended by system 100 and the information is returned to FIG. 2 for further processing.
- decision block 412determines whether the subject's blood glucose level is below a pre-set hypoglycemia risk level (Hi) (hypoglycemia threshold). If it is not below the Hi, information then is directed to decision block 404 where it is determined whether the blood glucose reading is greater than the mid-point of the target range and if it is not, information is then sent back to block 408 where no correction dose is recommended and the system returns to FIG. 2 .
- Hihypoglycemia risk level
- the informationthen is directed to block 410 where a correction dosage is calculated as previously discussed in relation to the correction dosage equation.
- the correction dosageis then inserted into decision block 480 where it is determined whether the time period is pre-meal and whether the meal type is breakfast. If the data corresponds to both of these two criteria, the information is then inserted into FIG. 6 for calculation of the recommended basal dose based upon previous mid-sleep blood glucose levels and adjustment factors in FIG. 7 .
- the logicthen flows on line 486 to FIG. 5 as shown by transfer block 488 . If the information does not correspond to both a breakfast and pre-meal time period in decision block 420 , the information then goes directly to FIG. 5 for further calculations as previously discussed.
- the systemrequests input in decision block 414 regarding the consciousness of the subject. If consciousness is not impaired, the data then flows to block 420 for administration of a predetermined amount of oral glucose (generally 15 grams). If the subject does have impaired consciousness, the physician or caregiver is then instructed to either administer glucogen in block 420 or if there is IV access, for intravenous insertion of an insulin based upon a 50% saline solution and insulin in accordance with the previously defined equations.
- Sub-system 500 shown in FIG. 5illustrates the logic flow within processor 116 associated with adjustment factors calculated in sub-system 700 shown in FIG. 7 which are incorporated into the meal time bolus calculations in the respective calculation blocks 228 , 222 , 224 , and 226 of FIG. 2 .
- calculation adjustment factorsare calculated in the logic flow of FIG. 7 and then the current bolus is calculated as a function of the previous meal type bolus times the adjustment factor for each of the meal types in respective blocks 518 , 520 , 522 , and 524 as well as a determination of whether the subject is on a meal plan.
- Informationis then sent to subject display 110 and remote processor 114 subsequent to the calculations made.
- Sub-system 600 shown in FIG. 6describes the system 100 processing for incorporating the patient's personal fasting glucose levels into the adjustment factor ( FIG. 7 ) for an increased defective recommended basal dose.
- a determinationis made if it is determined that this is a breakfast and pre-meal meal type and time period in FIG. 4 , the information is sent to block 422 where it then is transmitted on line 424 to the decision block 426 to determine whether a mid-sleep glucose level has been taken and in decision block 426 and if it has not, such returns to FIG. 2 for calculation of the breakfast bolus in calculation block 228 .
- the adjustment factorit is determined whether the previous mid-sleep blood glucose level is less than the previous breakfast blood glucose level in decision block 602 and if it is then the adjustment factor is calculated in block 604 from the adjustment factors in FIG. 7 . If the previous mid-sleep blood glucose level is equal to or greater than the previous breakfast blood glucose level, then the adjustment factor is calculated from FIG. 7 in block 606 and in this case, the adjustment factor is calculated using the previous breakfast blood glucose level. In either cases, information flows from either block 604 or 606 into block 608 on respective lines 632 and 634 for calculation of the new basal dosage being the previous basal dose multiplied by the adjustment factor. Once again, the recommended basal dose at a particular time period is then provided in data block 610 which is then again sent to the subject display 110 and remote processor 114 as well as back to insertion into the system in FIG. 5 .
Landscapes
- Health & Medical Sciences (AREA)
- Engineering & Computer Science (AREA)
- General Health & Medical Sciences (AREA)
- Medical Informatics (AREA)
- Public Health (AREA)
- Biomedical Technology (AREA)
- Primary Health Care (AREA)
- Epidemiology (AREA)
- Life Sciences & Earth Sciences (AREA)
- Physics & Mathematics (AREA)
- Chemical & Material Sciences (AREA)
- Business, Economics & Management (AREA)
- General Business, Economics & Management (AREA)
- Molecular Biology (AREA)
- Pathology (AREA)
- Veterinary Medicine (AREA)
- Animal Behavior & Ethology (AREA)
- Surgery (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Medicinal Chemistry (AREA)
- Heart & Thoracic Surgery (AREA)
- Biophysics (AREA)
- Optics & Photonics (AREA)
- Emergency Medicine (AREA)
- Hematology (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Electrochemistry (AREA)
- Analytical Chemistry (AREA)
- Biochemistry (AREA)
- General Physics & Mathematics (AREA)
- Immunology (AREA)
- Measurement Of The Respiration, Hearing Ability, Form, And Blood Characteristics Of Living Organisms (AREA)
- Infusion, Injection, And Reservoir Apparatuses (AREA)
Abstract
Description
- This U.S. patent application is a continuation of, and claims priority under 35 U.S.C. § 120 from, U.S. patent application Ser. No. 17/474,001, filed on Sep. 13, 2021, which is a continuation of U.S. patent application Ser. No. 15/862,819, filed on Jan. 5, 2018, which is a continuation of U.S. patent application Ser. No. 13/610,287, filed on Sep. 11, 2012. The disclosures of these prior applications are considered part of the disclosure of this application and are hereby incorporated by reference in their entireties.
- This invention is directed to a system and method for optimizing the control of blood glucose for diabetic subjects. In particular, this invention is directed to a system incorporating a processor whose logic optimizes the control of the blood glucose of the diabetic subject based upon a subject blood glucose level reading at a particular time period and a meal type having been ingested or to be ingested. Still further, this invention pertains to an optimized control of the blood glucose level for developing a recommended insulin dosage dependent upon a time period defined as a pre-meal, post-meal, bedtime, mid-sleep, or miscellaneous blood glucose reading taken with a standard glucometer. Additionally, this invention pertains to a system which takes into account both the meal type such as breakfast, lunch, dinner, or snack. Further, this invention is directed to a system which utilizes the past history of the subject for a particular meal type and time period to optimize the blood glucose level for the diabetic subject. Still further, this invention pertains to optimization of blood glucose levels based upon estimated carbohydrates to be ingested by the subject at a particular meal type in association with whether or not the subject is on a meal plan.
- Diabetes is a growing problem in the world. Conventional treatment for diabetes requires that the patient measures his/her blood glucose several times a day with a glucometer. The patient then estimates the number of units of insulin that should be injected to prevent either hypoglycemia (too low blood glucose) or hyperglycemia (too high blood glucose) based upon the blood glucose reading and the type of food that the patient has ingested or expects to ingest. This generally is a trial and error solution which may have deleterious effects.
- Diabetes or diabetes mellitus is a generally chronic disorder of glucose or sugar metabolism. This is generally caused by the inadequate production of insulin or inadequate use of the insulin generated. Insulin is a hormone produced in specialized cells in the pancreas which permits the body to use and store glucose. Diabetes is a leading cause of death in the World.
- Lack of insulin for a subject results in the inability to metabolize glucose and the capacity to store glycogen in the liver and active transport of glucose across cell membranes are impaired.
- Symptoms of diabetes or diabetes mellitus results in elevated sugar levels in the urine and blood, as well as increased urination, thirst, hunger, weakness, and weight loss. Prolonged excess blood glucose levels (hypoglycemia) leads to increased protein and fat catabolism which may cause premature vascular degeneration and atherosclerosis. Where diabetes is not controlled, such leads to diabetic acidosis where ketones are built up in the blood.
- Diabetes affects the body handling of fats which may lead to fat accumulation in the arteries and potential damage to the kidneys, eyes, heart, and brain. Thus, there is a great need for accuracy in controlling the blood glucose level of a diabetic subject to optimize the blood glucose level and have the subject maintain a stable blood glucose level within safe limits.
- The optimization of blood glucose levels for a diabetic subject is a function of numerous interdependent parameters associated with time periods, meal types, ingested food products, prior history of the diabetic subject at respective time periods and meal types being ingested or to be ingested, as well as the physical condition of the subject. In order to optimize the insulin dosage to be administered to a subject, the interdependent parameters each having an effect on the other, have to be taken into account to produce a recommended insulin dosage level.
- Thus, the subject system has been developed in order to optimize the insulin dosage recommended for a subject at a particular time period and further associated with a meal type based upon the above-referenced interrelated parameters.
- Prior systems for recommending insulin dosage suffer from the fact that the previous history of the subject are not generally taken into account with respect to a particular time period and meal type either ingested or to be ingested
- Conventional treatments for diabetes require that the patient measure his/her blood glucose a predetermined number of times during a day with a standard glucometer. The patient may then estimate the number of units of insulin that he/she should inject for prevention of either hypoglycemia or hyperglycemia.
- It is difficult for the diabetic subject to accurately estimate the number of grams of carbohydrates that he/she will ingest and in general the interrelated aforementioned parameters are not taken into account for optimizing glycemic control.
- Thus, there is a great need for a system which calculates an optimized blood glucose level and insulin dosage to be recommended to the subject based upon a current blood glucose reading in association with a meal type, time period, physical condition, and previous history of blood glucose levels.
- One aspect of the disclosure provides [an independent claim].
- The subject system provides a system and method for optimizing the glycemic control of a diabetic subject based upon his/her past glycemic history and provides an optimized guidance as to the number of units of insulin or insulin dosage to be injected at any time depending on an extended set of factors. Each of these factors can affect the patient's need for insulin.
- The diabetic patient or caregiver inserts base data into a computer system for estimating the number of grams of carbohydrates that the patient is about to ingest or has ingested at a particular meal type. The meal type and time period is further input as manual data inserted into the computer system.
- Based upon the input data as to the current blood glucose reading and the aforementioned input data, the subject system calculates an insulin dosage which is optimized for the patient's particular condition at the current time.
- One of the features of the subject system is that such provides for the patient the optimum number of units of insulin to be injected dependent on the subject's past history of blood glucose results under similar circumstances. As an example, if the subject measures a blood glucose rating of 100 mg/dl, such is input into the system and indicates to the system that there is an estimated ingestion of 30 grams of carbohydrates to be ingested or has previously been ingested, and the system states that 15 units of insulin should be injected, and then the next reading of blood glucose is 70 mg/dl, such would result in an undesirably low reading. At a subsequent time that the subject takes the same or similar blood glucose reading and the number of grams of carbohydrates to be eaten is inserted, then the system knowing that 15 units of insulin is too high a dose, the system may suggest 13 units of insulin to prevent a low reading of blood glucose. Thus, an important feature of the subject system is that the patient's prior glycemic history is stored by the system and subsequently used in calculations for recommending insulin to be injected dependent upon prior results experienced by the specific subject.
FIG.1 is a broad flow block diagram of the computer system for processing and calculating insulin dosages to be administered to a subject responsive to a particular meal type and a predetermined time period;FIG.2 is a logic block diagram providing a broad logic flow of the logic associated with the computer system and modules dependent upon whether the meal type is pre-meal, post-meal, bedtime, mid-sleep, or miscellaneous;FIG.3 is an information flow block diagram associated with processing a physical condition of the subject;FIG.4 is a flow block diagram associated with determining a correction dosage to be administered to the subject dependent upon input data provided by the subject associated with the meal type and the time period;FIG.5 is a logic flow diagram for calculation of the current bolus associated with an adjustment factor;FIG.6 is a flow block diagram showing the processing for calculating updated basal dosages; and,FIG.7 is a flow block diagram showing the adjustment factor calculations based upon current blood glucose levels.- Referring now to
FIG.1 , there is shown blood glucose in insulindosage administering system 100 for determining an insulin dosage value to be administered to a subject. In particular,system 100 is directed to calculating, processing and recommending blood glucose levels for diabetic subjects at specific times prior to or subsequent to ingestion of food or other nutrients and calculating a recommended insulin dose to be administered.System 100 is designed to provide the subject with calculated insulin dosage instructions based upon nutritional and physical information in combination with the personal history of previous insulin administration and resulting blood glucose levels. - The following definitions of the terminology used in the following paragraphs are as follows:
- Mid-point target blood glucose range (Tm) shall refer to the mid-point of a target blood glucose range (or other blood glucose value within the range) inserted into
remote processor 114 by a physician or caregiver for a subject. Although referring to “mid-point” of the blood glucose range, the mid-point target data may be inserted as a function of the mid-point of the mid-point target blood glucose range or some other input deemed appropriate by the subject's physician or caregiver. - Time periods shall refer to the time that a subject is taking a blood glucose reading with a standard glucometer and further refers to a pre-meal time period, a post-meal time period, a bedtime period, a mid-sleep time period, or some miscellaneous time period when the subject is taking the blood glucose reading.
- Meal type shall refer to either breakfast, lunch, dinner, snack, or miscellaneous associated with when the subject is taking the subject's blood glucose reading.
- Blood glucose reading shall be the blood glucose reading taken at a predetermined time period and associated with a meal type.
- Bolus shall refer to recommended insulin dose administered for a meal type and a time period.
- Basal Dose shall refer to a total basal dosage of insulin to be taken for one day.
- Hypoglycemia threshold shall refer to a lower blood glucose value for a particular subject provided by a physician or other caregiver.
- Prior blood glucose doses and/or levels shall refer to previous blood glucose doses and/or levels taken or calculated at previous time periods associated with a respective meal type.
- Basal insulin type shall refer to the type or brand of long acting insulin used with basal dose calculations.
- Bolus insulin type shall refer to the type or brand of short acting insulin used with meal bolus and correction doses of insulin.
- Basal dose distribution shall refer to the frequency and distribution of basal doses for a particular day such as (1) once a day (SID); (2) twice a day (BID); or, (3) three times a day (TID).
- Physical condition parameter shall refer to a physical condition of the subject at the time that the blood glucose reading is being taken such as whether or not the subject is exercising or plans to exercise.
- Intermediate blood glucose correction dosage shall refer to a first calculation by
processor 116 shown inFIG.1 . - Carbohydrate to insulin ratio is a subject specific factor based upon a function of the total daily dose of insulin based upon the subject's weight at the time of initialization of the
system 100 processes. - Meal plan shall refer to whether or not the subject is limited to ingesting a known number of carbohydrates for each meal type. When a subject is “on” a meal plan, the subject is generally prescribed a predetermined number of carbohydrates to be ingested at a selected meal type.
- Miscellaneous time period shall refer to blood glucose calculations at a time period which is not associated with the time periods of breakfast, lunch, dinner, or snack. Such a miscellaneous time period may be associated with a subject fasting period when blood glucose calculations are being processed.
- Mid-sleep time period shall refer to blood glucose readings taken at a time during a time period when the subject is normally asleep, generally at some point during a sleeping cycle of the subject.
- Insulin sensitivity factor shall refer to a subject specific sensitivity to insulin, generally determined by a physician or care giver and inserted as a portion of the data stored in the remote processor.
- System processor shall refer to an on-site processor which calculates a user's recommended insulin dosage value to be taken at a selected time period and a selected meal type.
- Remote processor shall refer to a processor which is coupled to the system processor and stores a first set of a subject's blood glucose parameters and includes but is not limited to prior basal and bolus dosages, prior or previous blood glucose readings for selected meal types and time periods, subject specific hypoglycemia thresholds, prescribed mid-point of a subject's target range, a subject specific insulin sensitivity factor, basal insulin type, bolus insulin type, basal dose distributions, and the number of carbohydrates a subject is recommended to ingest for a selected meal type. The remote processor is generally locationally removed (but in communication) with the system processor, however in some cases the remote processor may be incorporated with the system processor.
- Referring now to
FIG.1 , there is shownblood glucose system 100 for calculating, processing, determining, and displaying a recommended insulin dosage value (bolus) to be administered to a subject. The broad block diagram shown inFIG.1 includes a glucometer reading (BG) which is inserted by the subject inblock 102. The subject takes his/her blood glucose value with a standard glucometer well-known in the art and commercially available. The glucometer generally provides the subject's current blood glucose reading in mg/dl. - Further, data is inserted by the subject in
block 101 as to the physical condition of the subject at the time of the taking of the blood glucose value. The data inserted inblock 101 will further be described throughout the flow process and in particular with regard toFIG.3 . In general, data inserted intoblock 101 includes whether the subject is currently exercising or plans to exercise. Further, data is stored inremote processor 114 associated with prior basal dosages, prior blood glucose doses administered for particular meal types and time periods (bolus), a subject specific hypoglycemia threshold determined by the physician. Data to be included inblock 105 is the estimated number of carbohydrates the subject will be ingesting at a particular meal type if the subject is not on a meal plan, as well as the number of carbohydrates recommended to be ingested for a particular meal type if the subject is on a prescribed meal plan. Further included in the data stored inremote processor 114 is the mid-point target blood glucose range and the mid-point (Tm) inserted by a physician or other caregiver for a particular subject. - The blood glucose reading taken in
block 102 and the subject physical condition inblock 101 is inserted intoprocessor 116 online 118. Withinblock 103, a determination of the physical condition of the subject is made independent of further calculations withinprocessor 116 to be further detailed in relation toFIG.3 .Block 103 directsprocessor 116 to decision block302 inFIG.3 where the subject indicates whether his condition is exercise. If the condition indecision block 302 is that the subject is not exercising and does not plan to exercise, the information flows oninformation line 320 back to block104 inFIG.1 for further calculations to be further described in following paragraphs. Fromblock 104, the information then flows todosing adjustment 108 detailed inFIG.4 and then tosubject display 110 and toremote processor 114 for storing the data. - If the condition is an exercise condition, found in
decision block 302 ofFIG.3 , the logic moves online 326 to decision block320 where it is determined whether the blood glucose level read inblock 102 from the glucometer reading is less than or equal to the mid-point target blood glucose range stored inremote processor 114. If the blood glucose level is equal to or greater than the mid-point target blood glucose range, information is directed online 322 to block104 inFIG.1 for further calculations and passes subsequently to display110 andremote processor 114. - If the blood glucose level value in
decision block 320 is found to be less than the mid-point target blood glucose range, information is directed online 326 to block318 where the subject is instructed to eat a predetermined amount of carbohydrates for each predetermined minutes of exercise being planned or having been accomplished. This instruction is then provided to the patient onsubject display 110 online 324 and the information is additionally sent toremote processor 114 for storage of the instructions. - Thus, whether the condition is exercise determined in
decision block 302, or whether or not the blood glucose level is less than the mid-point of the target blood glucose range determined indecision block 320, all logic then passes to blood glucosetime period block 104 shown inFIG.1 where the processing ofblock 104 is initiated inFIG.2 . - Once an intermediate processing or correction dosage calculation is completed in
FIG.2 for a particular meal type and time period, the logic flows on line120 (FIG.1 ) todosing adjustment block 108 which is calculated inFIG.4 to be further detailed and described. Once the dosing adjustment inblock 108 has been made byprocessor 116, information flows online 122 tosubject data display 110 for providing a visual, audio or other type of sensory indication to the subject as to the recommended insulin dosage to be administered. In overall concept, the information provided online 122 todata display 110 is then transported toremote processor 114 online 124 for storage of all data calculated.Remote processor 114 stores prior basal dosages, prior administered blood glucose doses (bolus), hypoglycemia threshold, and mid-point target blood glucose range (TM) which are transmitted toprocessor 116 online 130 for processing. - Returning back to block103, which has been detailed in the description of
FIG.3 , all information with regard to the physical condition of the subject is additionally transported online 126 to subject data display110 simultaneous with the information flowing online 128 intoblock 104 for determination of the blood glucose time period. System processor 116 andsubject data display 110 may be incorporated within a standard Personal Computer System which has a standard monitor screen for permitting the subject to visually obtain the recommended insulin dosage value being calculated within thesystem processor 116 and/or theremote processor 114. Thesubject display monitor 110 generally provides visual data to the user, however, as is known, audio information may also be transmitted to the subject.- Referring now to
FIGS.2 and4-7 , when the information flows intoblock 104, the logic initially is directed toFIG.2 where a decision is made as to whether the time period at which the blood glucose level has been taken is determined to be pre-meal, post-meal, bedtime, mid-sleep, or miscellaneous. - Information flow from within
block 104 ofFIG.1 is inserted on line260 to decision block202 for determining whether the blood glucose reading taken is pre-meal. If the blood glucose reading is taken prior to breakfast, lunch, dinner, or snack, then information flows online 262 to decision block204 to determine whether the meal type of the pre-meal time period is breakfast. - If it is determined in
decision block 204 that the pre-meal type is breakfast, then the logic is transported online 264 to block212 for calculation of a blood glucose correction dosage or intermediate blood glucose correction dosage.Block 212 includes the processing of the logic blocks inFIG.4 . The information inblock 212 is inserted intodecision block 402 online 424 for determination of whether insulin has been administered within a predetermined time period which is generally 2.0 hours, however, this is adjustable by a physician for a specific subject. If insulin has been administered within a predetermined time period, the logic then moves online 426 to block408 where “no correction dose” is recommended and the information returns toFIG.2 for further processing inblock 220. - Where insulin has not been administered within a predetermined time period found in
decision block 402, information is directed to decision block412 online 430 for determination of whether the instant or current blood glucose level reading from the glucometer inblock 102 is less than the hypoglycemia threshold value stored inblock 114. If the blood glucose reading is equal to or greater than the hypoglycemia threshold value, information is transported online 432 to decision block404 where a determination is made whether the blood glucose reading is greater than the mid-point of the target blood glucose range (TM). - If it is determined that the blood glucose reading is less than the mid-point of the target blood glucose range, information is directed on
line 434 back to block408 where there is “no correction dose recommended” and the information flows back toFIG.2 for further processing online 428 inblock 220. - Where it is determined that the blood glucose reading is greater than the mid-point of the target blood glucose range in
block 404, the logic then passes online 436 to calculation block410 where the intermediate correction or correction insulin dosage is calculated. The intermediate blood glucose correction dosage calculated inblock 410 is a function of the blood glucose reading, the mid-point of the blood glucose target range, and the subject sensitivity factor in accordance with the formula: - Once the blood glucose correction dosage is determined in
calculation block 410, information is directed to decision block480 online 438. Since the correction dosage and associated logic ofFIG.4 is used in conjunction with all time periods where the blood glucose value is taken including pre-meal, post-meal, bedtime, mid-sleep, and miscellaneous, as well as meal types, breakfast, lunch, dinner, snack, bedtime and mid-sleep, the information online 438 is inserted into thedecision block 480 where it is once again determined whether the meal type and the time period is breakfast and pre-meal. - If both of the conditions are met (e.g., meal type is pre-meal and time period is breakfast), information then is directed on
line 440 to transfer block422 which is representative ofFIG.6 . Referring now toFIG.6 , information fromtransitional block 422 passes online 424 intodecision block 426 to determine whether a previous mid-sleep blood glucose level has been determined and stored in eithersystem processor 116 and/orremote processor 114. If there is no previous mid-sleep blood glucose level available or the subject does not take mid-sleep blood glucose readings, information passes online 428 to transferblock 642 for further processing inFIG.5 . - If there is a previous mid-sleep blood glucose level availability, information is directed on
line 430 to decision block602 to determine whether the previous mid-sleep blood glucose level was less than the previous breakfast blood glucose level reading stored inremote processor 114. If the previous mid-sleep blood glucose level is less than or equal to the previous breakfast blood glucose level, the logic passes online 614 to calculation block604 for calculating an adjustment factor using the previous mid-sleep blood glucose level. - Calculation of the adjustment factor using the previous mid-sleep blood glucose level is shown in
FIG.7 to be further detailed.Block 604 calculations decision blocks are made in702,706,710, and714, as well ascalculation block 718 which provides for a particular adjustment factor associated with the blood glucose reading. The information is then passed to block608 inFIG.6 for a Basal dose to be calculated based upon the adjustment factor. - If the previous mid-sleep blood glucose level is greater than the previous breakfast blood glucose level in
decision block 602, information is transported online 630 to processing block606 where the adjustment factor is calculated using the previous breakfast blood glucose level in accordance with the adjustment factor found inFIG.7 . Thus, in bothprocessing block FIG.7 . - Calculation blocks604 and606 are calculated in
FIG.7 where the information flows online 722 toinitial decision block 702 to determine whether the blood glucose level is greater than or equal to 181 mg/dl. If the blood glucose level is greater than 181 mg/dl, then an adjustment factor is set inblock 704 as being 1.2. If the blood glucose level is not greater than or equal to 181 mg/dl, then information flows online 724 to decision block706 where it is determined whether the blood glucose level is within the range of 141 mg/dl to 180 mg/dl. If the blood glucose level is within the range defined indecision block 706, the adjustment factor is set to be 1.1 inblock 708. If the blood glucose level is not within the range determined indecision block 706, information is transported online 726 to decision block710 where it is determined whether the blood glucose level is greater than or equal to 101 mg/dl and less than or equal to 140 mg/dl. If the blood glucose level is within the range defined inblock 710, the adjustment factor is set inblock 712 as 1.0. If the blood glucose level does not fall within the range associated withdecision block 710, information is directed online 728 to decision block714 where it is determined whether the blood glucose level is within the range of 71 mg/dl to 100 mg/dl. If the blood glucose level is within the range defined inblock 714, the adjustment factor is set inblock 716 to be 0.8. If the blood glucose level is not within the range associated with the decision made indecision block 714, the blood glucose level must be less than or equal to 70 mg/dl as shown inblock 718 and in this case, the adjustment factor is set inblock 720 as 0.8. The adjustment factors set inblocks - Once the proper adjustment factor is defined in
blocks respective lines blocks FIG.6 . - As stated, the adjustment factor after being calculated in
FIG.7 , the information returns toFIG.6 and in particular toblocks block lines block 608 is the previous basal dose multiplied by the adjustment factor and this value is inserted intoblock 610 to recommend the basal dose at the configured time interval. Information then flows online 636 to block638 to insert the recommended basal dose to thesubject data display 110 and storage in thesystem processor 116 and/orremote processor 114, as well as being returned online 640 for further calculations of either the breakfast, lunch, dinner, or snack bolus associated with the logic flow inFIG.5 . - Thus, as shown in
FIG.4 , if it is determined that both conditions of the time period being pre-meal and the meal type is breakfast, information is passed online 440 to transferblock 422 for calculations inFIG.6 and then the information is inserted intotransfer block 488 for processing in accordance with the logic described inFIG.5 . - Returning now to
FIG.4 , where once the correction dosage has been calculated inblock 410, and the information passed to decision block480, if it is determined inblock 480 that both conditions of the time period being pre-meal and the meal type being breakfast are not met, logic flows online 492 todecision block 490.Decision block 490 determines whether the time period is pre-meal. If the time period is pre-meal the logic moves online 496 to transferblock 488 for processing inFIG.5 . If the time period is not pre-meal then the logic flow is directed to block110 inFIG.1 and the correction dose is inserted in accordance with the calculations made incalculation block 410. - Returning back to
FIG.4 anddecision block 412, if it is determined indecision block 412 that the blood glucose level is less than the hypoglycemia threshold level, information flows online 450 to decision block414. In decision block414, it is determined whether the subject has impaired consciousness, and if the subject does not have impaired consciousness information flows online 452 to block420 where the subject is instructed to be given a predetermined dosage of oral glucose and data is then sent directly todata display block 110. If the subject has impaired consciousness found in decision block414, information flows online 454 to decision block416 where it is determined whether there is IV access. If there is IV access, information online 456 is inserted intoblock 418 where instructions are provided to give a D50IV=(100−BG)×0.04 amount to the subject. If there is no IV access, glucogen is then recommended to be administered inblock 422. Information fromblocks lines data display 110 and subsequently inserted intoremote processor 114 ofFIG.1 . - Returning now to
FIG.2 , once the basal dose has been adjusted inblock 220 as associated with the processing inFIGS.6 and7 , for a time period which is pre-meal and a meal type which is breakfast, information is directed to block228 for calculation of the recommended insulin dosage at breakfast or breakfast bolus. - Similarly, if the time period is pre-meal and meal type is lunch, calculations of the intermediate blood glucose correction dosage for lunch is calculated in
FIG.4 . If the time period is pre-meal and the meal type is dinner, calculation of the intermediate blood glucose correction dosage is made inblock 216. Similarly, if it is determined that the time period is pre-meal and that the meal type is a snack indecision block 210, a calculation of the blood glucose correction dosage for the snack is calculated inblock 218. - In all processing and calculation blocks212,214,216, and218, the calculations are provided in association with the previous logic flow description given for the logic blocks in
FIG.4 . - Information from
FIG.2 processing blocks228,222,224, and226 are calculated in accordance with the logic flow inFIG.5 . Calculation of the breakfast, lunch, dinner, or snack bolus is shown inFIG.5 with information passing fromblocks line 530 to decision block502 where it is once again determined whether the pre-meal time period is breakfast. If the pre-meal time period is breakfast, information passes to calculation block510 online 532 for calculation of the adjustment factor as previously detailed in the logic flow provided forFIG.7 . - If the time period is pre-meal and the meal type is breakfast, calculation of the adjustment factor is made in
block 510 in accordance withFIG.7 as previously discussed. Information then passes to decision block562 where there is a determination of whether the subject is on a fixed meal plan. If it is determined that the subject is on a fixed meal plan, such as substantially the same number of carbohydrates to be ingested at each time period and meal type, information then passes online 564 to calculation block518 which calculates the current bolus in accordance with the equation:
CB=CBi×AF (2)- Where:
- CB=current bolus (units of insulin)
- CBi=previous bolus administered at the previous Meal type and time period (units of insulin)
- AF=adjustment factor (dimensionless)
- The current bolus is then passed on
line 554 tosubject data display 110 and eventually toremote processor 114 as provided inFIG.1 . If it is determined that the subject is not on a fixed meal plan indecision block 562, information is directed throughline 566 to calculation block586 where a number of calculations are performed. Initially, the total prescribed daily basal dose of insulin in units of insulin per day is calculated (TDD) in accordance with the formula:
TDD=TDDM×WS (3)- Where:
- TDD=total prescribed daily basal dose of insulin (units of insulin)
- WS=weight of subject (Kg.)
- TDDM=subject's Total Daily Dose Multiplier (a weighting factor having dimensions of (units per Kg/day). Typically 0.25 for pediatric subjects, 0.3 for subjects with renal insufficiency, 0.5 for adult subjects, or another subject specific number)
- Once the total prescribed daily basal dose is calculated in equation (3), within
block 586, the meal of bolus (CB) is calculated by first calculating the carbohydrate to insulin ratio (dimensionless) in accordance with the formula:
CIR=450×TDD (4)- Where:
- CIR=current carbohydrate to insulin ratio (dimensionless)
- TDD=total prescribed basal dose of insulin (units of insulin)
- Using the previous selected pre-meal CIR to calculate the instant CIR for a particular meal type is made in accordance with the formula:
- Where: CIRB,L,D,S=instant carbohydrate to insulin ratio for a selected meal type of breakfast, lunch, dinner, or snack
- CIRB,L,D,S=previous carbohydrate to insulin ratio for previous selected meal type of breakfast, lunch, dinner or snack
- AF=adjustment factor Finally, the current bolus to be recommended is derived from the Equation:
- Where: CEST=estimated number of carbohydrates to be ingested at the pre-meal time period for the current meal type (mg.)
- CIRB,L,D,S=calculated carbohydrate to insulin ratio calculated in Equation 5
- Subsequent to the calculation of the current bolus in
block 518 or block586, information passes onrespective lines subject data display 110 and then toremote processor 114. - If it is determined in
decision block 502 that the meal is not breakfast, information is directed online 536 to decision block504 where a decision is made as to whether the meal is lunch. If the pre-meal is lunch, then information is passed online 538 to calculation block512 for calculation of the adjustment factor inFIG.7 as previously discussed. Once the adjustment factor has been determined from the logic flow inFIG.7 , information then is transported online 540 to fixed mealplan decision block 568.Decision block 568, similar to decision block562, determines whether the subject is on a fixed meal plan and if the subject is on a fixed meal plan, information passes online 570 to calculation block520 where the current bolus is calculated in accordance withEquation 2. Where the subject is not on a fixed meal plan as determined indecision block 568, information passes online 572 to calculation block588 which calculates the lunch bolus in accordance with Equations 3, 4, 5 and 6 as previously discussed. Subsequently, information passes either online subject display data 110 andremote processor 114. - If it is determined that the meal type is not lunch in
decision block 504, information is transported online 542 to decision block506 where it is determined whether the meal type is dinner. If the meal type is dinner, information is inserted to calculation block514 online 544 for calculation of the adjustment factor provided by the logic inFIG.7 . Once the adjustment factor inFIG.7 has been calculated, information passes online 546 to decision block574 determining whether the subject is on a fixed meal plan. Thedecision block 574 is similar to decision blocks562 and568. If it is determined that the subject is on a fixed meal plan, information is then sent to calculation block522 online 576 for calculation of the current bolus (CB) in accordance withEquation 2. If the subject is not on a fixed meal plan as determined indecision block 574, the information enterscalculation block 590 for calculation of the dinner meal bolus in accordance with Equations 3, 4, 5 and 6. Information is then sent from either calculation block522 or block590 onrespective lines subject data display 110 and then toremote processor 114. - If it is determined in
decision block 506 that the meal is not dinner, information then flows online 548 to decision block508 where it is determined whether the meal type is a snack. If it determined indecision block 508 that the meal is a snack, information passes online 550 to calculation block516 where the adjustment factor is calculated in accordance withFIG.7 . Information then passes online 552 to decision block580 which determines whether the subject is on a fixed meal plan. If the subject is on a fixed meal plan as determined indecision block 580, information passes online 582 to calculation block524 where the current bolus is calculated based uponequation 2. If the subject is not on a fixed meal plan, the logic flows throughline 584 to calculation block592 where the current meal bolus is calculated in accordance with Equations 3, 4, and 6. Information fromblock 524 or block592 is then transported on eitherLine data display system 110 and then toremote processor 114. - In this manner, when the blood glucometer reading is taken as represented by
block 102, and the physical condition is input by the subject as represented byblock 101, when the time period of the blood glucose reading is taken is pre-meal as is determined indecision block 202, a breakfast, lunch, dinner, and snack bolus is calculated bysystem 100. - If the meal type is not a snack, then the time period is miscellaneous and passes on
line 598 to transfer block599 where logic is transferred toline 288 inFIG.2 . Processing is then provided incalculation block 258 in accordance with the logic flow inFIG.4 . - Returning to
FIG.2 , assuming that the blood glucose time period has been determined not to be a pre-meal time period indecision block 202, the information passes online 280 to decision block230 wheredecision block 230 determines whether the time period is a post-meal time. If the time period is determined to be post-meal, information is transported online 282 to decision block232 where a decision is determined whether this is a breakfast post-meal glucometer reading. If the inputs provided by the subject is to a time period which is post-meal and the meal type is breakfast, information is then transmitted to calculation block240 inFIG.2 . In this instance, there is no adjustment of the basal dose as was the case when the time period was pre-meal (previously described) and the meal type was breakfast. Calculation block 240 directs the information toFIG.4 where a correction dose is calculated incalculation block 410. All logic blocks have been previously detailed, however, in overview, if insulin has not been given within a predetermined period of time, for example two hours as indicated indecision block 402, and the blood glucose reading is equal to or greater than the hypoglycemia threshold value (Hi) as determined indecision block 412, the information is directed to decision block404 and if the blood glucose reading is determined to be greater than the mid-point target blood glucose range reading, the correction dose is calculated incalculation block 410. Responsively, subsequent to the calculations provided incalculation block 240, the results and calculation of the post-meal breakfast correction is transmitted online 284 tosubject display 110 andremote processor 114 for storage of the data calculated.- Similarly, as has previously been described for the pre-meal type calculations in decision blocks206,208, and210, a decision is made as to the fact whether the post-meal blood glucose reading is taken subsequent to lunch in
decision block 234, dinner indecision block 236, or a snack indecision block 238. If it is determined that the post-meal blood glucose reading is subsequent to lunch indecision block 234, the information then is inserted intocalculation block 242 for calculation of the post-meal lunch correction as associated with the logic flow previously described forFIG.4 . - If the decision in
decision block 234 is that the post-meal was not lunch, the information then is directed to decision block236 for determination of whether the post-meal blood glucose reading was dinner and if it is dinner, the logic flows to block244 and correction dosage as well as the subject meal bolus is made in association withFIG.4 . - If the blood glucose post-meal reading is a snack determined in
decision block 238, similarly as previously described, the information is directed to calculation block246 for calculation in the same manner as previously described for the post-meal breakfast, lunch and dinner decisions. Information fromblocks line 284 to bothsubject display 110 andremote processor 114 for storage of the data and display of the recommended correction reading. - If it is determined in
decision block 230 that the blood glucose time period is neither a pre-meal nor a post-meal, the information is directed online 290 to decision block248 where it is determined whether the blood glucose taken is at the time period of bedtime (prior to sleep). - With the blood glucose reading provided in
block 101, the information is directed to calculation block254 for insert into the logic flow ofFIG.4 . The logic inFIG.4 in overall view, passes into correctiondose calculation block 410. The bolus for bedtime is then provided on line286 (FIG.2 ) to bothsubject display system 110 andremote processor 114 as shown inFIG.1 . - Assuming that the blood glucose type is not found to be bedtime in
decision block 248, information is then inserted online 292 to decision block250 where the blood glucose reading time period is taken as “mid-sleep”. If the blood glucose reading is taken as a mid-sleep type reading, information then is inserted intocalculation block 256 where the calculation correction is transmitted to the logic previously detailed forFIG.4 and then inserted online 286 tosubject display system 110 andremote processor 114 as shown inFIG.2 . - In the event that the blood glucose reading provided in
block 101 is not a mid-sleep reading as determined indecision block 250, the information then passes online 294 to calculation block252 where the meal type is defined as miscellaneous since it is neither for a breakfast, lunch, dinner, or snack reading. The information in252 is then directed to calculation block258 where the bolus is calculated in accordance withFIGS.4,5, and7 . - In the event that the blood glucose reading meets the time criteria period of a pre-meal, but is not at breakfast, lunch, dinner, or snack as determined in decision blocks204,206,208, and210, then the meal type must be “miscellaneous” and the information passes on
line 288 intoblock FIG.2 , if the blood glucose reading is post-meal, but is not for breakfast as determined indecision block 232, lunch as determined indecision block 234, dinner as determined indecision block 236, or the snack as determined indecision block 238, again, the information is directed online 288 to252 since the reading must be a “miscellaneous” reading. In all cases subsequent to the bolus being determined in254,256, and258, information calculated is then inserted for display insystem display 110 and stored inremote processor 114 for further use. - In overall concept, there is provided in
FIGS.1-7 a system for determining the insulin dosage value to be administered to a subject dependent on many interrelated parameters. Input tosystem 100 includes a glucometer reading taken by the subject at a time period defined by whether the blood glucose reading is taken pre-meal, post-meal, bedtime, or at some miscellaneous time.Remote processor 114 maintains in storage, prior basal dosages, hypoglycemia thresholds, target ranges and mid-points of target ranges, and subject insulin sensitivity factor. The subject provides a manual input online 118 as represented byblock 105 as to the particular time period, whether such is pre-meal, post-meal, bedtime, or at some miscellaneous time. Additionally, the meal type such as breakfast, lunch, dinner, or snack is inserted as represented byblock 105 for insert intoprocessor 116 for determination of the appropriate correction factors and bolus to be calculated. System 100 provides the patient with calculated insulin dosage instructions based on nutritional and physical information, as well as personal history of insulin administration and resulting blood glucose levels as previously described. The calculated insulin dosage instructions are output to the subject onsubject data display 110 which can be the monitor of a PC or through some other type of audio or sensory indication to the subject. The resulting data is then inserted intoremote processor 114 for storage of the data where prior basal dosages, prior blood glucose doses, hypoglycemia thresholds, subject insulin sensitivity factor, whether a meal plan is in effect, and mid-point of target ranges are maintained in storage.- Once the user has manually input the current glucometer reading of his/her blood glucose level from
block 102 along with the time period and meal type as represented inblock 105, the subject further includes input as to a physical condition fromblock 101. All of this data is then inserted intoprocessor 116 where the physical condition is initially calculated independent of the further processing to be accomplished byprocessor 116. The physical condition may require administration of a predetermined amount of carbohydrates as calculated inFIG.3 for each time period of exercise which has been accomplished or is being planned and such is inserted intosubject data display 110. Prior basal dosages and prior BG doses of the subject for previous time periods of pre-meal, post-meal, bedtime, or miscellaneous as well as prior BG doses associated with specific time periods and meal types is stored inremote processor 114 along with the hypoglycemia threshold and the mid-point of the target range (Tm). All of this is inserted intoprocessor 116 online 124 for calculations inblocks System 100 then processes all data drawing on the preset conditions and subject history for determining optimum dosage levels of the subject's current condition where all calculated data is then displayed as represented byblock 110 and the calculated data is then stored inremote processor 114.FIG.2 is representative of the calculation blocks104 and108 in a further breakdown of the processor calculation procedures. Thesystem 100 processes patient input of dietary events inFIG.2 where initially the subject indicates whether the current blood glucose level read from glucometer reading102 is a time period of a pre-meal (decision block202), post-meal (decision block230), prior to bedtime (decision block248), or mid-sleep cycle (decision block250). If the time period is neither pre-meal, post-meal, bedtime, or during the mid-sleep cycle, then the time period is miscellaneous as represented byinput block 252. Thus, all time periods are then represented and appropriate calculations can be processed. Each of the decision blocks202,204,206,208,210 or203,232,234,236,238, or248 and250 define individual series of decision blocks. A positive indication for one decision block implies a negative indication for other decision blocks in each series. This type of event oriented organization permits the subject to expeditiously enter important information.- If the time period is pre-meal as determined in
decision block 202, the patient elects or indicates whether the pre-meal reading is breakfast as shown indecision block 204. As previously described, if the pre-meal is not breakfast, the election is made for lunch indecision block 206, dinner inblock 208, or a snack indecision block 210. An algorithm withinprocessor 116 calculates the dosage correction for the planned meal using the calculation algorithm as previously described inFIG.4 in association with sub-algorithms provided inFIGS.5-7 and in overall block diagram shown inblocks FIG.2 . - In the time period of pre-meal and breakfast, the basal dose is adjusted as indicated in
block 220 in association with the logic flow shown inFIG.6 . - For all pre-meals such as breakfast, lunch, dinner, snack, or miscellaneous, the pre-meal bolus or recommended insulin dosage is calculated in associated
blocks block 252 and the calculated correction is made inblock 258 as previously detailed. All recommended optimum doses to be taken in any of the time periods is then displayed to the subject ondisplay 110 and the data inserted intoremote processor 114 for further use for subsequent blood glucose readings at specific meal types and time periods. - Mealtime nutritional information may be input by the subject and a post-meal bolus correction is calculated for correcting unacceptable blood glucose levels within the logic of
processor 116 as indicated byblock 108 inFIG.1 in association withFIG.5 . logic. - In the event that the time period of the blood glucose reading is post-meal and determined in
decision block 230, once again the meal type is determined from the decision blocks232,234,236, or238 for respective calculation of the post-meal type correction inrespective blocks - As shown in
FIG.2 , if the time period is bedtime as determined indecision block 248, a pre-sleep blood glucose correction dose is calculated incalculation block 254 associated with calculations performed in the logic steps as provided inFIG.4 . In the event that the blood glucose reading is mid-sleep as determined indecision block 250, where it has been determined indecision block 248 that the time period is not bedtime, the logic blows intodecision block 250 where it is determined whether the time period is mid-sleep and if the time period is mid-sleep, calculations are made inblock 256 in accordance with the logic flow inFIG.4 . All information is then inserted online 286 for insert intosubject display 110 andremote processor 114. - In the event that one of the meal types previously discussed are found for either the pre-meal, post-meal, mid-sleep or bedtime calculations, the meal type is defaulted to input block252 where it is determined that the meal type is miscellaneous and then passes to calculation block258 for calculation in accordance with the calculations processed in
FIG.4 . Once again, the information fromblock 258 is inserted ontoline 286 for display and storage of the data inrespective blocks block 258 for calculation in accordance with the logic associated withFIG.4 . FIG.4 is a sub-system which takes information fromFIG.2 and is associated with the calculation blocks212,214,216, and218 for the pre-meal blood glucose reading time period, as well as logic blocks240,242,244, and246 for the post-meal time period and blocks254,256, and258 for the time periods of bedtime, mid-sleep or miscellaneous. The calculation blocks ofFIG.2 are read intodecision block 402 for determination of whether insulin has been administered within a predetermined time interval of the taking of the blood glucose reading and if insulin has been given within this predetermined time, there is no correction dosage recommended bysystem 100 and the information is returned toFIG.2 for further processing.- If the insulin has not been given within the predetermined period of time (which is generally two hours), it is determined in
decision block 412 whether the subject's blood glucose level is below a pre-set hypoglycemia risk level (Hi) (hypoglycemia threshold). If it is not below the Hi, information then is directed to decision block404 where it is determined whether the blood glucose reading is greater than the mid-point of the target range and if it is not, information is then sent back to block408 where no correction dose is recommended and the system returns toFIG.2 . - If the blood glucose reading is greater than the mid-point of the target range as determined in
decision block 404, the information then is directed to block410 where a correction dosage is calculated as previously discussed in relation to the correction dosage equation. The correction dosage is then inserted intodecision block 480 where it is determined whether the time period is pre-meal and whether the meal type is breakfast. If the data corresponds to both of these two criteria, the information is then inserted intoFIG.6 for calculation of the recommended basal dose based upon previous mid-sleep blood glucose levels and adjustment factors inFIG.7 . The logic then flows online 486 toFIG.5 as shown bytransfer block 488. If the information does not correspond to both a breakfast and pre-meal time period indecision block 420, the information then goes directly toFIG.5 for further calculations as previously discussed. - In overall concept, if the decision in
decision block 412 determines that the blood glucose level is below Hi, the system requests input in decision block414 regarding the consciousness of the subject. If consciousness is not impaired, the data then flows to block420 for administration of a predetermined amount of oral glucose (generally 15 grams). If the subject does have impaired consciousness, the physician or caregiver is then instructed to either administer glucogen inblock 420 or if there is IV access, for intravenous insertion of an insulin based upon a 50% saline solution and insulin in accordance with the previously defined equations. Sub-system 500 shown inFIG.5 illustrates the logic flow withinprocessor 116 associated with adjustment factors calculated insub-system 700 shown inFIG.7 which are incorporated into the meal time bolus calculations in the respective calculation blocks228,222,224, and226 ofFIG.2 . For respective meal types, calculation adjustment factors are calculated in the logic flow ofFIG.7 and then the current bolus is calculated as a function of the previous meal type bolus times the adjustment factor for each of the meal types inrespective blocks subject display 110 andremote processor 114 subsequent to the calculations made.Sub-system 600 shown inFIG.6 describes thesystem 100 processing for incorporating the patient's personal fasting glucose levels into the adjustment factor (FIG.7 ) for an increased defective recommended basal dose. A determination is made if it is determined that this is a breakfast and pre-meal meal type and time period inFIG.4 , the information is sent to block422 where it then is transmitted online 424 to thedecision block 426 to determine whether a mid-sleep glucose level has been taken and indecision block 426 and if it has not, such returns toFIG.2 for calculation of the breakfast bolus incalculation block 228. If the mid-sleep glucose level has been taken, the adjustment factor it is determined whether the previous mid-sleep blood glucose level is less than the previous breakfast blood glucose level indecision block 602 and if it is then the adjustment factor is calculated inblock 604 from the adjustment factors inFIG.7 . If the previous mid-sleep blood glucose level is equal to or greater than the previous breakfast blood glucose level, then the adjustment factor is calculated fromFIG.7 inblock 606 and in this case, the adjustment factor is calculated using the previous breakfast blood glucose level. In either cases, information flows from either block604 or606 intoblock 608 onrespective lines subject display 110 andremote processor 114 as well as back to insertion into the system inFIG.5 .- Although this invention has been described in connection with specific forms and embodiments thereof, it will be appreciated that various modifications other than those discussed above may be resorted to without departing from the spirit or scope of the invention as defined in the appended claims. For example, functionally equivalent elements may be substituted for those specifically shown and described, certain features may be used independently of other features, and in certain cases, particular locations of elements, steps, or processes may be reversed or interposed, all without departing from the spirit or scope of the invention as defined in the appended claims.
Claims (20)
Priority Applications (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US18/329,451US20230393088A1 (en) | 2012-09-11 | 2023-06-05 | System and method for optimizing insulin dosages for diabetic subjects |
Applications Claiming Priority (4)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US13/610,287US9897565B1 (en) | 2012-09-11 | 2012-09-11 | System and method for optimizing insulin dosages for diabetic subjects |
| US15/862,819US11131643B2 (en) | 2012-09-11 | 2018-01-05 | Method and system for optimizing insulin dosages for diabetic subjects |
| US17/474,001US11733196B2 (en) | 2012-09-11 | 2021-09-13 | System and method for optimizing insulin dosages for diabetic subjects |
| US18/329,451US20230393088A1 (en) | 2012-09-11 | 2023-06-05 | System and method for optimizing insulin dosages for diabetic subjects |
Related Parent Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US17/474,001ContinuationUS11733196B2 (en) | 2012-09-11 | 2021-09-13 | System and method for optimizing insulin dosages for diabetic subjects |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| US20230393088A1true US20230393088A1 (en) | 2023-12-07 |
Family
ID=61188107
Family Applications (4)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US13/610,287Active2035-03-14US9897565B1 (en) | 2012-09-11 | 2012-09-11 | System and method for optimizing insulin dosages for diabetic subjects |
| US15/862,819ActiveUS11131643B2 (en) | 2012-09-11 | 2018-01-05 | Method and system for optimizing insulin dosages for diabetic subjects |
| US17/474,001ActiveUS11733196B2 (en) | 2012-09-11 | 2021-09-13 | System and method for optimizing insulin dosages for diabetic subjects |
| US18/329,451PendingUS20230393088A1 (en) | 2012-09-11 | 2023-06-05 | System and method for optimizing insulin dosages for diabetic subjects |
Family Applications Before (3)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US13/610,287Active2035-03-14US9897565B1 (en) | 2012-09-11 | 2012-09-11 | System and method for optimizing insulin dosages for diabetic subjects |
| US15/862,819ActiveUS11131643B2 (en) | 2012-09-11 | 2018-01-05 | Method and system for optimizing insulin dosages for diabetic subjects |
| US17/474,001ActiveUS11733196B2 (en) | 2012-09-11 | 2021-09-13 | System and method for optimizing insulin dosages for diabetic subjects |
Country Status (1)
| Country | Link |
|---|---|
| US (4) | US9897565B1 (en) |
Families Citing this family (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| TW201333870A (en) | 2011-12-21 | 2013-08-16 | 艾登工具股份有限公司 | Systems and methods for determining insulin therapy for a patient |
| US10387623B2 (en)* | 2014-01-28 | 2019-08-20 | Debiotech S.A. | Control device with recommendations |
| EP3500960A1 (en)* | 2016-08-17 | 2019-06-26 | Novo Nordisk A/S | Systems and methods for optimization of bolus timing relative to meal events |
| EP3928323A1 (en)* | 2019-02-21 | 2021-12-29 | Companion Medical, Inc. | Methods, systems and devices for a medicament dose calculator |
Citations (6)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US20030028089A1 (en)* | 2001-07-31 | 2003-02-06 | Galley Paul J. | Diabetes management system |
| US20030208113A1 (en)* | 2001-07-18 | 2003-11-06 | Mault James R | Closed loop glycemic index system |
| US20060253296A1 (en)* | 2003-10-29 | 2006-11-09 | Novo Nordisk A/S | Medical advisory system |
| US20070078314A1 (en)* | 2005-09-30 | 2007-04-05 | Grounsell Richard L | System and method for measuring and predicting insulin dosing rates |
| US20070179434A1 (en)* | 2005-12-08 | 2007-08-02 | Stefan Weinert | System and method for determining drug administration information |
| US20090006061A1 (en)* | 2007-06-27 | 2009-01-01 | Roche Diagnostics Operations, Inc. | System for developing patient specific therapies based on dynamic modeling of patient physiology and method thereof |
Family Cites Families (264)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US4055175A (en) | 1976-05-07 | 1977-10-25 | Miles Laboratories, Inc. | Blood glucose control apparatus |
| FR2387659A1 (en) | 1977-04-21 | 1978-11-17 | Armines | GLYCEMIA CONTROL AND REGULATION DEVICE |
| US4151845A (en) | 1977-11-25 | 1979-05-01 | Miles Laboratories, Inc. | Blood glucose control apparatus |
| US4464170A (en) | 1982-09-29 | 1984-08-07 | Miles Laboratories, Inc. | Blood glucose control apparatus and method |
| US4850959A (en) | 1988-08-02 | 1989-07-25 | Bioresearch, Inc. | Bioelectrochemical modulation of biological functions using resonant/non-resonant fields synergistically |
| US5101814A (en) | 1989-08-11 | 1992-04-07 | Palti Yoram Prof | System for monitoring and controlling blood glucose |
| US5091190A (en) | 1989-09-05 | 1992-02-25 | Alza Corporation | Delivery system for administration blood-glucose lowering drug |
| US5251126A (en) | 1990-10-29 | 1993-10-05 | Miles Inc. | Diabetes data analysis and interpretation method |
| GB9024771D0 (en) | 1990-11-14 | 1991-01-02 | Axis Research | Assay |
| GB9104037D0 (en) | 1991-02-26 | 1991-04-10 | Hospital For Sick The Children | Process for the phosphorylation of insulin |
| SE9101381D0 (en) | 1991-05-07 | 1991-05-07 | Tomas Moks | PEPTIDE HORMONE SOLUTION |
| US5956501A (en) | 1997-01-10 | 1999-09-21 | Health Hero Network, Inc. | Disease simulation system and method |
| US5917052A (en) | 1994-09-28 | 1999-06-29 | Shaman Pharmaceuticals, Inc. | Hypoglycemic agent from cryptolepis |
| US5614224A (en) | 1995-04-20 | 1997-03-25 | Womack; Rick W. | Nutritional supplement for diabetics |
| MY115050A (en) | 1995-10-16 | 2003-03-31 | Mead Johnson Nutrition Co | Diabetic nutritional product having controlled absorption of carbohydrate |
| US5821217A (en) | 1995-10-27 | 1998-10-13 | Beth Israel Deaconess Medical Center, Inc. | Enteral formulation: low in fat and containing protein hydrolysates |
| EP0862648B1 (en) | 1995-11-22 | 2004-10-06 | Medtronic MiniMed, Inc. | Detection of biological molecules using chemical amplification and optical sensors |
| CA2246431A1 (en) | 1996-01-19 | 1997-07-24 | Board Of Regents, The University Of Texas System | Recombinant expression of proteins from secretory cell lines |
| AU718500B2 (en) | 1997-01-23 | 2000-04-13 | Sumitomo Pharmaceuticals Company, Limited | Remedies for diabetes |
| US20010002269A1 (en) | 1997-05-06 | 2001-05-31 | Zhao Iris Ginron | Multi-phase food & beverage |
| US6558351B1 (en) | 1999-06-03 | 2003-05-06 | Medtronic Minimed, Inc. | Closed loop system for controlling insulin infusion |
| US5929055A (en) | 1997-06-23 | 1999-07-27 | The Research Foundation Of State University Of New York | Therapeutic method for management of diabetes mellitus |
| JP2001518316A (en) | 1997-10-02 | 2001-10-16 | ザ・レジェンツ・オブ・ザ・ユニバーシティー・オブ・カリフォルニア | Protein delivery by secretory gland expression |
| CO4970787A1 (en) | 1997-12-23 | 2000-11-07 | Lilly Co Eli | INSOLUBLE COMPOSITIONS OF INSULIN AND INSULIN DERIVATIVES THAT CONTROL BLOOD GLUCOSE |
| AU5916298A (en) | 1998-01-09 | 1999-07-26 | Amylin Pharmaceuticals, Inc. | Amylin agonist pharmaceutical compositions containing insulin |
| AU2989699A (en) | 1998-03-05 | 1999-09-20 | Universal Health-Watch, Inc. | Apparatus to non-invasively measure glucose or other constituents in aqueous humor using infra-red spectroscopy |
| AU748432B2 (en) | 1998-04-02 | 2002-06-06 | Merck & Co., Inc. | Antidiabetic agents |
| EP1087753A4 (en) | 1998-05-19 | 2004-06-30 | Sdg Inc | Targeted liposomal drug delivery system |
| CN1324234A (en) | 1998-09-25 | 2001-11-28 | 格利科克斯有限公司 | Fructosamine oxidase: antagonists and inhibitors |
| US6615081B1 (en) | 1998-10-26 | 2003-09-02 | Birinder R. Boveja | Apparatus and method for adjunct (add-on) treatment of diabetes by neuromodulation with an external stimulator |
| CA2352295C (en) | 1998-11-30 | 2008-07-15 | Novo Nordisk A/S | A method and a system for assisting a user in a medical self treatment, said self treatment comprising a plurality of actions |
| US8700161B2 (en) | 1999-03-05 | 2014-04-15 | Metacure Limited | Blood glucose level control |
| AU4450700A (en) | 1999-04-27 | 2000-11-10 | Eli Lilly And Company | Insulin crystals for pulmonary administration |
| US6890568B2 (en) | 1999-05-24 | 2005-05-10 | Grant Pierce | Method for management of blood glucose levels |
| US7806886B2 (en) | 1999-06-03 | 2010-10-05 | Medtronic Minimed, Inc. | Apparatus and method for controlling insulin infusion with state variable feedback |
| AU5844300A (en) | 1999-06-04 | 2000-12-28 | Indian Council Of Medical Research | Eugenia jambolina fruit extracts for treating diabetes |
| AU5453300A (en) | 1999-06-29 | 2001-01-31 | Eli Lilly And Company | Insulin crystals for pulmonary administration |
| CA2381539A1 (en) | 1999-09-15 | 2001-03-22 | Medtronic Minimed, Inc. | Glucose sensing molecules having selected fluorescent properties |
| DE19954233A1 (en) | 1999-11-11 | 2001-05-31 | Nutricia Nv | Diabetic food |
| US6572542B1 (en) | 2000-03-03 | 2003-06-03 | Medtronic, Inc. | System and method for monitoring and controlling the glycemic state of a patient |
| JP4580542B2 (en) | 2000-05-17 | 2010-11-17 | 株式會社バイオニア | Microorganism for treating obesity or diabetes and pharmaceutical composition containing the microorganism |
| WO2001089368A2 (en) | 2000-05-25 | 2001-11-29 | Healthetech, Inc. | Physiological monitoring using wrist-mounted device |
| US6605039B2 (en) | 2000-07-24 | 2003-08-12 | Medtronic, Inc. | Cell-based biosensors suitable for implantable medical device applications |
| JP2004505042A (en) | 2000-08-02 | 2004-02-19 | ファーマニュートリエンツ | Methods and compositions for prevention and / or treatment of diabetes and glucose degeneration |
| US6365176B1 (en) | 2000-08-08 | 2002-04-02 | Functional Foods, Inc. | Nutritional supplement for patients with type 2 diabetes mellitus for lipodystrophy |
| WO2002036139A1 (en) | 2000-11-01 | 2002-05-10 | Biovan, Inc. | Method for management of blood glucose levels |
| AU2002251944A1 (en) | 2001-02-15 | 2002-09-04 | Medtronic Minimed, Inc. | Polymers functionalized with fluorescent boronate motifs |
| ATE419858T1 (en) | 2001-06-18 | 2009-01-15 | Neptune Technologies & Bioress | KRILL EXTRACTS FOR THE PREVENTION AND/OR TREATMENT OF CARDIOVASCULAR DISEASES |
| JP3775263B2 (en) | 2001-08-10 | 2006-05-17 | ニプロ株式会社 | Recording medium and blood glucose measurement system using the recording medium |
| MY135783A (en) | 2001-09-07 | 2008-06-30 | Meiji Dairies Corp | Nutritional composition for controlling blood sugar level |
| WO2003024468A1 (en) | 2001-09-19 | 2003-03-27 | Bioneer Corporation | Substances extracted from corn which can inhibit the activities of amylase, pharmaceutical compositions and food additives containing the same extracts for treatment or prevention of obesity and diabetes mellitus, and processes for their preparations |
| WO2003053460A1 (en) | 2001-12-19 | 2003-07-03 | Eli Lilly And Company | Crystalline compositions for controlling blood glucose |
| DK1482919T3 (en) | 2002-01-25 | 2007-07-30 | Silanes Sa De Cv Lab | Pharmaceutical composition for blood sugar control in patients with type 2 diabetes |
| US20030199445A1 (en) | 2002-02-07 | 2003-10-23 | Knudsen Lotte Bjerre | Use of GLP-1 compound for treatment of critically ill patients |
| TW200304372A (en) | 2002-03-20 | 2003-10-01 | Kanegafuchi Chemical Ind | Compositions for diabetes |
| BRPI0309689B1 (en) | 2002-04-30 | 2021-06-29 | Unigen, Inc | COMPOSITIONS COMPRISING A MIXTURE OF B-RING-FREE FLAVONOIDS AND FLAVANES AND USES THEREOF |
| JP2003319758A (en) | 2002-05-07 | 2003-11-11 | Unitika Ltd | Method and inhibitor for inhibiting d-glucose absorption |
| US20040018533A1 (en) | 2002-06-04 | 2004-01-29 | Adam Gail Isabel Reid | Diagnosing predisposition to fat deposition and therapeutic methods for reducing fat deposition and treatment of associated conditions |
| US20050014158A1 (en) | 2002-06-27 | 2005-01-20 | Adam Gail Isabel Reid | Therapeutic methods for reducing fat deposition and treating associated conditions |
| KR100709528B1 (en) | 2002-06-28 | 2007-04-20 | 깃세이 야쿠힌 고교 가부시키가이샤 | Pharmaceutical composition for blood sugar control |
| EP1382363A1 (en) | 2002-07-15 | 2004-01-21 | Novo Nordisk A/S | Closed loop system for controlling blood glucose levels |
| FR2842735B1 (en) | 2002-07-26 | 2006-01-06 | Flamel Tech Sa | MODIFIED RELEASE MICROCAPSULES OF LOW SOLUBLE ACTIVE PRINCIPLES FOR PER OS ADMINISTRATION |
| US8571801B2 (en) | 2002-08-28 | 2013-10-29 | Atkins Nutritionals, Inc. | Methods and systems for determining and controlling glycemic responses |
| US20040092590A1 (en) | 2002-09-27 | 2004-05-13 | Linda Arterburn | Glycemic control for prediabetes and/or diabetes Type II using docosahexaenoic acid |
| US7504118B2 (en) | 2003-04-11 | 2009-03-17 | Fhg Corporation | Dietary supplements containing extracts of cinnamon and methods of using same to enhance creatine transport |
| JP2005029570A (en) | 2003-06-16 | 2005-02-03 | Tokiwa Shokubutsu Kagaku Kenkyusho:Kk | Method for production of corosolic acid |
| US20050054818A1 (en) | 2003-07-02 | 2005-03-10 | Brader Mark Laurence | Crystalline compositions for controlling blood glucose |
| US20080119703A1 (en) | 2006-10-04 | 2008-05-22 | Mark Brister | Analyte sensor |
| KR100567837B1 (en) | 2003-10-24 | 2006-04-05 | 케이제이헬스케어 주식회사 | Insulin pump combined with mobile which detects a blood glucose, network system for transmitting control imformation of the insulin pump |
| US20050096637A1 (en) | 2003-10-31 | 2005-05-05 | Medtronic, Inc. | Sensing food intake |
| US20080119421A1 (en) | 2003-10-31 | 2008-05-22 | Jack Tuszynski | Process for treating a biological organism |
| AU2004285368A1 (en) | 2003-11-04 | 2005-05-12 | Meiji Dairies Corporation | Alpha-glucosidase activity inhibitor |
| SG179411A1 (en) | 2003-11-06 | 2012-04-27 | Lifescan Inc | Drug delivery pen with event notification means |
| EP1711101A1 (en) | 2004-01-15 | 2006-10-18 | Glucon Inc. | Wearable glucometer |
| WO2005081171A2 (en) | 2004-02-19 | 2005-09-01 | Edward Henry Mathews | Insulin bolus calculator for mobile communication device |
| CA2547934C (en) | 2004-02-19 | 2013-05-21 | Abbott Laboratories | Methods of using gamma cyclodextrin to control blood glucose and insulin secretion |
| WO2005081173A1 (en) | 2004-02-19 | 2005-09-01 | Edward Henry Mathews | Diabetes slide ruler for blood glucose control |
| WO2005081119A2 (en) | 2004-02-19 | 2005-09-01 | Edward Henry Mathews | Interactive diabetes education system |
| WO2005081170A2 (en) | 2004-02-19 | 2005-09-01 | Edward Henry Mathews | Diabetes management and patient database system via mobile communication device |
| WO2006006286A1 (en) | 2004-07-09 | 2006-01-19 | Meiji Dairies Corporation | Composition for enhancing the production of ppar and/or ppar-associated factor |
| WO2006022638A1 (en) | 2004-07-22 | 2006-03-02 | Sequenom, Inc. | Methods for identifying risk of type ii diabetes and treatments thereof |
| WO2006022619A2 (en) | 2004-07-22 | 2006-03-02 | Sequenom, Inc. | Methods for identifying risk of type ii diabetes and treatments thereof |
| WO2006022629A1 (en) | 2004-07-22 | 2006-03-02 | Sequenom, Inc. | Methods of identifying risk of type ii diabetes and treatments thereof |
| WO2006022633A1 (en) | 2004-07-22 | 2006-03-02 | Sequenom, Inc. | Methods for identifying a risk of type ii diabetes and treatments thereof |
| WO2006022636A1 (en) | 2004-07-22 | 2006-03-02 | Sequenom, Inc. | Methods for identifying risk of type ii diabetes and treatments thereof |
| US20080199480A1 (en) | 2004-07-22 | 2008-08-21 | Sequenom, Inc. | Methods for Identifying Risk of Type II Diabetes and Treatments Thereof |
| WO2006022634A1 (en) | 2004-07-22 | 2006-03-02 | Sequenom, Inc. | Methods for identifying risk of type ii diabetes and treatments thereof |
| US20070060549A1 (en) | 2004-08-10 | 2007-03-15 | Friesen Albert D | Combination therapies employing ace inhibitors and uses thereof for the treatment of diabetic disorders |
| US20060040003A1 (en) | 2004-08-10 | 2006-02-23 | Alvin Needleman | Dietary supplement for supressing appetite, enhancing and extending satiety, improving glycemic control, and stimulant free |
| US20060046962A1 (en) | 2004-08-25 | 2006-03-02 | Aegis Therapeutics Llc | Absorption enhancers for drug administration |
| US20120213886A1 (en) | 2004-08-25 | 2012-08-23 | U.S. Dept. Of Veterans Affairs | Compositions and Methods to Lower Glycohemoglobin Levels |
| EP1698898A3 (en) | 2004-09-21 | 2006-10-11 | Foundation of Biomedical Research of the Academy of Athens | Prognostic, diagnostic and therapeutic significance in detecting and using TIN-antigen (Tubulo-Interstitial nephritis antigen) and its segments |
| US20060078593A1 (en) | 2004-09-27 | 2006-04-13 | Strozier Deborah C | Nutritional compostions comprising a soluble viscous fiber in a solid crisp matrix |
| WO2006044556A2 (en) | 2004-10-14 | 2006-04-27 | Galileo Pharmaceuticals, Inc. | Dual inhibitors of lipoxygenase for treating diabetes |
| US8117020B2 (en) | 2004-12-03 | 2012-02-14 | Roche Diagnostics Operations, Inc. | Method to determine the degree and stability of blood glucose control in patients with diabetes mellitus via creation and continuous updating of new statistical indicators |
| ITMI20050010A1 (en) | 2005-01-05 | 2006-07-06 | Aboca S P A | NUTRACEUTICAL PHARMACEUTICAL COMPOSITIONS NUTRITIONAL DIETS BASED ON VEGETABLE FIBERS |
| CA2491763A1 (en) | 2005-01-06 | 2006-07-06 | Tassos P. Anastassiades | Blood glucose control with n-acylated glucosamines |
| WO2006079124A2 (en) | 2005-01-24 | 2006-07-27 | Edward Henry Mathews | Apparatus and method for predicting the effect of ingested foodstuff or exercise on blood sugar level of patient and suggesting a corrective action |
| WO2006091918A2 (en) | 2005-02-23 | 2006-08-31 | Streck, Inc. | Process, composition and kit for providing a stable whole blood calibrator/control |
| US20060188995A1 (en) | 2005-02-24 | 2006-08-24 | Ryan Wayne L | Process, composition and kit for providing a stable whole blood calibrator/control |
| KR100750988B1 (en) | 2005-03-18 | 2007-08-22 | 주식회사 유니젠 | Pharmaceutical composition for the prevention or treatment of diabetes or glycemic control abnormalities, including ginsenoside derived from natural products |
| BRPI0500959A (en) | 2005-03-23 | 2006-11-21 | Unicamp | pharmacological use of peroxisome proliferator-activated receptor (pgc-1 (alpha)) coactivating protein 1 alpha expression inhibitor for the treatment of diabetes mellitus, insulin resistance and metabolic syndrome, its compound and its composition |
| RU2007143510A (en) | 2005-04-27 | 2009-06-10 | Яллал МЕССАДЕК (BE) | INSULIN COMPOSITIONS |
| US20060263450A1 (en) | 2005-05-17 | 2006-11-23 | Julius Oben | Method and composition for reducing body weight and improving control of body lipids |
| US7509156B2 (en) | 2005-05-18 | 2009-03-24 | Clarian Health Partners, Inc. | System for managing glucose levels in patients with diabetes or hyperglycemia |
| WO2006130901A1 (en) | 2005-06-07 | 2006-12-14 | Bayer Healthcare Ag | Control of metabolic abnormalities |
| US20090239944A1 (en) | 2005-06-24 | 2009-09-24 | D Orazio Daniel | Novel Use of Organic Compounds |
| BRPI0614489A2 (en) | 2005-08-01 | 2011-03-29 | Mannkind Corp | method for preserving the function of insulin producing cells |
| US7704226B2 (en) | 2005-11-17 | 2010-04-27 | Medtronic Minimed, Inc. | External infusion device with programmable capabilities to time-shift basal insulin and method of using the same |
| US8557274B2 (en) | 2005-12-06 | 2013-10-15 | Purdue Research Foundation | Slowly digesting starch and fermentable fiber |
| US8057840B2 (en) | 2006-01-25 | 2011-11-15 | Tate & Lyle Ingredients Americas Llc | Food products comprising a slowly digestible or digestion resistant carbohydrate composition |
| US7824333B2 (en) | 2006-03-31 | 2010-11-02 | Lifescan, Inc. | Diabetes management methods and systems |
| US20070282186A1 (en) | 2006-05-02 | 2007-12-06 | Adrian Gilmore | Blood glucose monitor with an integrated data management system |
| US8548544B2 (en) | 2006-06-19 | 2013-10-01 | Dose Safety | System, method and article for controlling the dispensing of insulin |
| CA2655566A1 (en) | 2006-06-30 | 2008-01-10 | Healthy Interactions, Inc. | System, method, and device for providing health information |
| KR101080955B1 (en) | 2006-07-14 | 2011-11-08 | 포항공과대학교 산학협력단 | Epidermal growth factor increases insulin secretion and lowers blood glucose |
| WO2008013324A1 (en) | 2006-07-28 | 2008-01-31 | National University Corporation Kanazawa University | Use of diabetes-related, liver-derived secreted protein in diagnosis or treatment of type-2 diabetes or vascular disorder |
| WO2008073609A2 (en) | 2006-11-01 | 2008-06-19 | Philip Michael Sher | Device for predicting and managing blood glucose concentration by analyzing the effect of, and controlling, pharmacodynamic insulin unit equivalents |
| US20080139910A1 (en) | 2006-12-06 | 2008-06-12 | Metronic Minimed, Inc. | Analyte sensor and method of using the same |
| US7946985B2 (en) | 2006-12-29 | 2011-05-24 | Medtronic Minimed, Inc. | Method and system for providing sensor redundancy |
| WO2008124478A1 (en) | 2007-04-04 | 2008-10-16 | Pronia Medical Systems, Llc | Systems, methods, and computer program product for improved management of medical procedures for patients on medical protocols |
| CA2684457A1 (en) | 2007-04-19 | 2008-10-30 | C.G.M.3 Ltd | Device system and method for monitoring and controlling blood analyte levels |
| US8417311B2 (en) | 2008-09-12 | 2013-04-09 | Optiscan Biomedical Corporation | Fluid component analysis system and method for glucose monitoring and control |
| EP2178546A1 (en) | 2007-08-21 | 2010-04-28 | ATP Marketing & Promotion AG | Herbal formulations for controlling blood glucose levels in patients with diabetes |
| US7943163B2 (en) | 2007-08-22 | 2011-05-17 | Response Scientific, Inc. | Medical food or nutritional supplement, method of manufacturing same, and method of managing diabetes |
| TWI348910B (en) | 2007-10-29 | 2011-09-21 | Univ Taipei Medical | Composition for controlling blood glucose and method thereof |
| WO2009081403A2 (en) | 2007-12-26 | 2009-07-02 | Medingo Ltd. | Maintaining glycemic control during exercise |
| US20090177147A1 (en) | 2008-01-07 | 2009-07-09 | Michael Blomquist | Insulin pump with insulin therapy coaching |
| US20090238879A1 (en) | 2008-01-24 | 2009-09-24 | Northwestern University | Delivery scaffolds and related methods of use |
| US20090214511A1 (en) | 2008-02-21 | 2009-08-27 | Trung Hong Tran | Digestible compositions of inulin for managing blood glucose levels |
| US8317699B2 (en) | 2008-02-29 | 2012-11-27 | Roche Diagnostics Operations, Inc. | Device and method for assessing blood glucose control |
| US8396528B2 (en) | 2008-03-25 | 2013-03-12 | Dexcom, Inc. | Analyte sensor |
| US20090242399A1 (en) | 2008-03-25 | 2009-10-01 | Dexcom, Inc. | Analyte sensor |
| US8185412B1 (en) | 2008-03-28 | 2012-05-22 | Mahesh Harpale | Method and apparatus for chronic care treatment control with custom named-type factors and user estimation error correction |
| WO2009124111A2 (en) | 2008-04-01 | 2009-10-08 | Trustees Of Boston University | Glucose sensor employing semiconductor nanoelectronic device |
| ES2670793T3 (en) | 2008-04-04 | 2018-06-01 | Hygieia, Inc. | Apparatus for optimizing an insulin dosage regimen for a patient |
| TWI394580B (en) | 2008-04-28 | 2013-05-01 | Halozyme Inc | Super fast-acting insulin compositions |
| BRPI0822909B8 (en) | 2008-06-26 | 2021-05-25 | Laboratorios Silanes S A De C V | metformin glycinate salt for blood glucose control |
| IT1396465B1 (en) | 2008-07-10 | 2012-12-14 | B G Informatica S R L | METHOD FOR THE DEFINITION AND INTERACTIVE MANAGEMENT OF A TREATMENT FOR THE CONTROL OF GLYCEMIC RATE IN THE DIABETIC PATIENT AND INTEGRATED DEVICE THAT IMPLEMENTS THIS METHOD |
| KR100918776B1 (en) | 2009-04-20 | 2009-09-24 | 계명대학교 산학협력단 | Blood Glucose Inhibition Composition Using Polyethylene Glycol and Gallate Catechin |
| EP2352456A1 (en) | 2008-08-20 | 2011-08-10 | Bayer HealthCare LLC | Health management supply organizing system and methods |
| US20110177175A1 (en) | 2008-09-23 | 2011-07-21 | Rubicon Research Private Limited | Dietary fiber compositions |
| EP2179661A1 (en) | 2008-10-23 | 2010-04-28 | Generale Biscuit | Biscuit comprising guar gum in a rod-like form |
| WO2010056718A2 (en) | 2008-11-11 | 2010-05-20 | Hygieia, Inc. | Apparatus and system for diabetes management |
| US8527208B2 (en) | 2008-11-17 | 2013-09-03 | Roche Diagnostics International Ag | Prandial blood glucose excursion optimization method via computation of time-varying optimal insulin profiles and system thereof |
| US20120011125A1 (en) | 2008-12-23 | 2012-01-12 | Roche Diagnostics Operations, Inc. | Management method and system for implementation, execution, data collection, and data analysis of a structured collection procedure which runs on a collection device |
| US8198320B2 (en) | 2008-12-31 | 2012-06-12 | Taipei Medical University | Hypoglycemic activity of osthole |
| WO2010078842A1 (en) | 2009-01-07 | 2010-07-15 | Huang Haidong | Gene encoding human glucokinase mutant, enzyme encoded by the same, recombinant vectors and hosts, pharmaceutical compositions and uses thereof, methods for treating and preventing diseases |
| US20120053222A1 (en) | 2009-01-23 | 2012-03-01 | Mark Gorrell | Novel Metabolic Disease Therapy |
| AU2010236680A1 (en) | 2009-04-14 | 2011-11-10 | Abbott Laboratories | High fiber nutritional emulsions for blood glucose control |
| WO2010129375A1 (en) | 2009-04-28 | 2010-11-11 | Abbott Diabetes Care Inc. | Closed loop blood glucose control algorithm analysis |
| GB2471066A (en) | 2009-06-10 | 2010-12-22 | Dna Electronics Ltd | A glucagon pump controller |
| US9218453B2 (en) | 2009-06-29 | 2015-12-22 | Roche Diabetes Care, Inc. | Blood glucose management and interface systems and methods |
| CN102018766B (en) | 2009-09-16 | 2013-03-06 | 博仲盛景医药技术(北京)有限公司 | Plant extractive, extraction method and application thereof as well as composite comprising same |
| KR101632308B1 (en) | 2009-09-23 | 2016-06-21 | 삼성전자주식회사 | Method and apparatus for providing blood glucose management information |
| EP2644088B1 (en) | 2009-09-29 | 2017-01-18 | Lifescan Scotland Limited | Analyte testing method and device for diabetes management |
| KR20120102048A (en)* | 2009-09-30 | 2012-09-17 | 모르 리서치 애플리케이션즈 리미티드 | Monitoring device for management of insulin delivery |
| US20110098548A1 (en) | 2009-10-22 | 2011-04-28 | Abbott Diabetes Care Inc. | Methods for modeling insulin therapy requirements |
| US20110115894A1 (en) | 2009-11-19 | 2011-05-19 | Daniel Rogers Burnett | Device and method for diagnosis and monitoring of neural, opthalmologic and retinal disorders |
| WO2011073788A2 (en) | 2009-12-18 | 2011-06-23 | Dr Reddy's Laboratories, Limited | Balaglitazone compositions and methods |
| US8759284B2 (en) | 2009-12-24 | 2014-06-24 | Rani Therapeutics, Llc | Therapeutic agent preparations for delivery into a lumen of the intestinal tract using a swallowable drug delivery device |
| US20110173308A1 (en) | 2010-01-14 | 2011-07-14 | Brent Gutekunst | System and method for medical surveillance through personal communication device |
| AR080029A1 (en) | 2010-01-27 | 2012-03-07 | Baylor Res Inst | COMPOSITION OF MICROBUBBLES THAT INCLUDES A PLASMIDIC DNA CODIFYING A GROWTH FACTOR OF THE HUMAN VASCULAR ENDOTHELY |
| US8579879B2 (en) | 2010-02-19 | 2013-11-12 | Medtronic Minimed, Inc. | Closed-loop glucose control startup |
| US9089292B2 (en) | 2010-03-26 | 2015-07-28 | Medtronic Minimed, Inc. | Calibration of glucose monitoring sensor and/or insulin delivery system |
| US9144586B2 (en) | 2010-04-07 | 2015-09-29 | Incube Labs, Llc | Method for treating glucose related disorders using stem cell-derived gastro-intestinal cells |
| US20120197358A1 (en) | 2010-04-09 | 2012-08-02 | Prescott Marvin A | Light Therapy for Treating or Managing Diabetes and Metabolic Syndrome |
| JP2011237408A (en) | 2010-04-14 | 2011-11-24 | Arkray Inc | Biological information measuring device, lighting method in the same and biological information measuring method |
| US9387279B2 (en) | 2010-05-05 | 2016-07-12 | The Governing Council Of The University Of Toronto | Implantable-glucose responsive insulin delivery device |
| US10561785B2 (en) | 2010-06-22 | 2020-02-18 | Medtronic Minimed, Inc. | Method and/or system for closed-loop control of glucose to a treatment range |
| US8543354B2 (en) | 2010-06-23 | 2013-09-24 | Medtronic Minimed, Inc. | Glucose sensor signal stability analysis |
| US20120003339A1 (en) | 2010-07-02 | 2012-01-05 | Minacapelli Pompeo | Compositions and methods for controlling blood glucose levels |
| US20120006100A1 (en) | 2010-07-06 | 2012-01-12 | Medtronic Minimed, Inc. | Method and/or system for determining blood glucose reference sample times |
| US8828390B2 (en) | 2010-07-09 | 2014-09-09 | Universitat Zurich | Uses of NOGO-A inhibitors and related methods |
| US8870807B2 (en) | 2010-07-19 | 2014-10-28 | Surag Mantri | Gastro-intestinal compression device |
| WO2012010298A1 (en) | 2010-07-23 | 2012-01-26 | Roche Diagnostics Gmbh | System and method considering the effect of physical activity on the glucoregulatory system |
| AU2011280986B2 (en) | 2010-07-23 | 2015-11-12 | Avacen, Inc. | Methods and apparatus for therapeutic application of thermal energy |
| EP2603133A1 (en) | 2010-08-10 | 2013-06-19 | Roche Diagnostics GmbH | Method and system for improving glycemic control |
| US20120100248A1 (en) | 2010-08-17 | 2012-04-26 | Abbott Laboratories | Nutritional composition comprising cereal beta-glucan and salacia extract |
| US20120058942A1 (en) | 2010-09-03 | 2012-03-08 | John Dupre | Methods for controlling blood-glucose levels in insulin-requiring subjects |
| WO2012047800A1 (en) | 2010-10-06 | 2012-04-12 | Abbott Laboratories | Methods for increasing muscle glucose uptake and decreasing muscle recovery time using amino acid blend |
| US20130225683A1 (en) | 2010-10-27 | 2013-08-29 | Prometic Biosciences Inc. | Compounds and Pharmaceutical Compositions for Uses in Diabetes |
| US8919180B2 (en) | 2010-10-28 | 2014-12-30 | Medtronic Minimed, Inc. | Determination and application of glucose sensor reliability indicator and/or metric |
| US8657746B2 (en) | 2010-10-28 | 2014-02-25 | Medtronic Minimed, Inc. | Glucose sensor signal purity analysis |
| WO2012058694A2 (en) | 2010-10-31 | 2012-05-03 | Trustees Of Boston University | Blood glucose control system |
| US8349612B2 (en) | 2010-11-15 | 2013-01-08 | Roche Diagnostics Operations, Inc. | Guided structured testing kit |
| EP2640373A4 (en) | 2010-11-16 | 2014-04-23 | Univ New Jersey Med | TREATMENT OF TYPE II DIABETES AND DISEASES ASSOCIATED WITH DIABETES THROUGH SAFE CHEMICAL MITOCHONDRIAL DECOUPLERS |
| US20120295985A1 (en) | 2010-11-19 | 2012-11-22 | Miller Guy M | Methods for improving blood glucose control |
| CN102462736B (en) | 2010-11-19 | 2015-07-15 | 北京唐安营养保健品有限公司 | Preparation method of cinnamon water extract |
| US8329232B2 (en) | 2010-11-23 | 2012-12-11 | Beijing Tang-An Nutrition & Healthcare Products Co. Ltd. | Process for preparing water extract of cinnamon |
| ES2396844B1 (en) | 2010-12-01 | 2014-01-27 | Universitat Politècnica De Catalunya | System and method for simultaneous and non-invasive estimation of blood glucose, glucocorticoid level and blood pressure |
| US9238802B2 (en) | 2010-12-01 | 2016-01-19 | Kikkoman Corporation | E. coli transformant, method for producing flavin-bound glucose dehydrogenase using the same, and mutant flavin-bound glucose dehydrogenases |
| US9469844B2 (en) | 2010-12-02 | 2016-10-18 | Kikkoman Corporation | Flavin-bound glucose dehydrogenases, a method for producing a flavin-bound glucose dehydrogenase, and yeast transformant used for the same |
| EP2654777A4 (en) | 2010-12-21 | 2014-07-09 | Nestec Sa | METHODS AND COMPOSITIONS SUITABLE FOR MANAGING GLYCEMIA IN ANIMALS |
| US20120173151A1 (en) | 2010-12-29 | 2012-07-05 | Roche Diagnostics Operations, Inc. | Methods of assessing diabetes treatment protocols based on protocol complexity levels and patient proficiency levels |
| WO2012097064A1 (en) | 2011-01-13 | 2012-07-19 | Abbott Laboratories | Nutritional compositions and methods for controlling blood glucose |
| MX350611B (en) | 2011-01-31 | 2017-09-11 | Cadila Healthcare Ltd | Treatment for lipodystrophy. |
| CN107019515B (en)* | 2011-02-28 | 2021-02-26 | 雅培糖尿病护理公司 | Method of displaying sensor readings and analyte monitoring device and method of operating the same |
| EP2685895B1 (en) | 2011-03-17 | 2018-10-10 | University of Newcastle Upon Tyne | System for the self-monitoring and regulation of blood glucose |
| MX339374B (en) | 2011-04-29 | 2016-05-13 | Inst De Investigación En Química Aplic S A De C V | Metformin-based ionic co-crystals. |
| US8690934B2 (en) | 2011-05-09 | 2014-04-08 | The Invention Science Fund I, Llc | Method, device and system for modulating an activity of brown adipose tissue in a vertebrate subject |
| US8755938B2 (en) | 2011-05-13 | 2014-06-17 | Roche Diagnostics Operations, Inc. | Systems and methods for handling unacceptable values in structured collection protocols |
| US8766803B2 (en) | 2011-05-13 | 2014-07-01 | Roche Diagnostics Operations, Inc. | Dynamic data collection |
| EP3293197B1 (en) | 2011-06-07 | 2020-01-08 | Wisconsin Alumini Research Foundation | Hepatocyte based insulin gene therapy for diabetes |
| JP5890516B2 (en) | 2011-06-17 | 2016-03-22 | ハロザイム インコーポレイテッド | Continuous subcutaneous insulin infusion method using hyaluronan degrading enzyme |
| WO2012178134A2 (en) | 2011-06-23 | 2012-12-27 | University Of Virginia Patent Foundation | Unified platform for monitoring and control of blood glucose levels in diabetic patients |
| KR20140082642A (en) | 2011-07-26 | 2014-07-02 | 글리젠스 인코포레이티드 | Tissue implantable sensor with hermetically sealed housing |
| US8667293B2 (en) | 2011-08-11 | 2014-03-04 | Roche Diagnostics Operations, Inc. | Cryptographic data distribution and revocation for handheld medical devices |
| GB2493712B (en) | 2011-08-12 | 2014-07-02 | Gene Onyx Ltd | Insulin pump |
| JP2014524297A (en) | 2011-08-18 | 2014-09-22 | ノボ・ノルデイスク・エー/エス | Drug delivery device having means for handling data |
| WO2013040712A1 (en) | 2011-09-20 | 2013-03-28 | Afexa Life Sciences Inc. | Composition comprising ku ding cha and ginseng for managing blood glucose levels |
| WO2013049381A1 (en) | 2011-09-28 | 2013-04-04 | Abbott Diabetes Care Inc. | Methods for analyte monitoring management and analyte measurement data management, and articles of manufacture related thereto |
| US20130085682A1 (en) | 2011-10-04 | 2013-04-04 | Roche Diagnostics Operations, Inc. | Memory card usage with blood glucose devices |
| EP2763722B1 (en) | 2011-10-07 | 2016-08-10 | Novo Nordisk A/S | System for determining position of element based on three-axial magnetic sensors |
| US20130144283A1 (en) | 2011-12-02 | 2013-06-06 | Medtronic Ardian Luxembourg S.A.R.L. | Treatment of polycystic ovary syndrome using renal neuromodulation and associated systems and methods |
| US20140315162A1 (en) | 2011-12-09 | 2014-10-23 | Joel Ehrenkranz | System and methods for monitoring food consumption |
| JP2015503565A (en) | 2011-12-29 | 2015-02-02 | ラティチュード ファーマシューティカルズ インコーポレイテッドLatitude Pharmaceuticals, Inc. | Stabilized glucagon nanoemulsion |
| US20130172688A1 (en) | 2011-12-29 | 2013-07-04 | Roche Diagnostics Operations, Inc. | Diabetes management application for mobile phone |
| WO2013108262A1 (en) | 2012-01-18 | 2013-07-25 | Zota Health Care Ltd | Synergistic combination for the treatment of diabetic neuropathy |
| US10694983B2 (en) | 2012-01-19 | 2020-06-30 | Medtronic Minimed, Inc. | Method and/or system for assessing a patient's glycemic response |
| WO2013108122A1 (en) | 2012-01-20 | 2013-07-25 | Mueller-Wolf Martin | "indima apparatus" system, method and computer program product for individualized and collaborative health care |
| KR101166907B1 (en) | 2012-02-08 | 2012-07-19 | 최병철 | The extract of rhynchosia volubilis and its manufacturing method bearing the character of bioactivity such as blood sugar |
| EP2812706A1 (en) | 2012-02-10 | 2014-12-17 | Alere Switzerland GmbH | Assay and method for determining insulin-resistance |
| US9010477B2 (en) | 2012-02-16 | 2015-04-21 | Aonec, Llc | In vehicle glucose apparatus and vehicular operation inhibitor |
| ES2421956B1 (en) | 2012-03-02 | 2014-09-29 | Moehs Ibérica S.L. | NEW CRYSTAL FORM OF SITAGLIPTINA SULFATE |
| US9080939B2 (en) | 2012-03-06 | 2015-07-14 | National Central University | Method of measuring glycosylated protein proportion by AC impedance method |
| WO2013134469A1 (en) | 2012-03-07 | 2013-09-12 | Medtronic Ardian Luxembourg Sarl | Selective modulation of renal nerves |
| AU2013230781B2 (en) | 2012-03-08 | 2015-12-03 | Medtronic Af Luxembourg S.A.R.L. | Ovarian neuromodulation and associated systems and methods |
| US20140337041A1 (en) | 2012-03-30 | 2014-11-13 | Joseph Madden | Mobile Application for Defining, Sharing and Rewarding Compliance with a Blood Glucose Level Monitoring Regimen |
| US20130281796A1 (en) | 2012-04-20 | 2013-10-24 | Broadmaster Biotech Corp. | Biosensor with exercise amount measuring function and remote medical system thereof |
| PH12013500989B1 (en) | 2012-05-16 | 2019-10-16 | Melaleuca Inc | Dietary supplement compositions |
| US20150174209A1 (en) | 2012-05-25 | 2015-06-25 | Amylin Pharmaceuticals. Llc | Insulin-pramlintide compositions and methods for making and using them |
| US20130317316A1 (en) | 2012-05-25 | 2013-11-28 | City Of Hope | Carbohydrate modeling methods, systems, and devices |
| US9150482B2 (en) | 2012-06-06 | 2015-10-06 | Development Center For Biotechnology | GLP-1 potentiators from hedychium coronarium and their applications |
| US9536449B2 (en) | 2013-05-23 | 2017-01-03 | Medibotics Llc | Smart watch and food utensil for monitoring food consumption |
| US9254099B2 (en) | 2013-05-23 | 2016-02-09 | Medibotics Llc | Smart watch and food-imaging member for monitoring food consumption |
| US9861744B2 (en) | 2012-06-25 | 2018-01-09 | International Business Machines Corporation | Managing blood glucose levels |
| US20140012118A1 (en) | 2012-07-09 | 2014-01-09 | Dexcom, Inc. | Systems and methods for leveraging smartphone features in continuous glucose monitoring |
| US8492426B1 (en) | 2012-07-12 | 2013-07-23 | Anis Ahmad | Use of carvedilol for treatment of diabetes mellitus |
| WO2014024201A1 (en) | 2012-08-06 | 2014-02-13 | Ramchandran T S | Regulate and manage blood glucose levels of a subject by promoting cell metabolism with the application of low frequency (20 khz to 70 khz ) airborne ultrasound waves and a machine to produce the airborne ultrasound waves therefor |
| AR092077A1 (en) | 2012-08-10 | 2015-03-18 | Sanofi Aventis Deutschland | MEDICAL SYSTEM |
| WO2014028607A1 (en) | 2012-08-14 | 2014-02-20 | Abbott Laboratories | Low glycemic index nutritional compositions for preserving muscle mass and improving body composition in diabetics |
| US9504411B2 (en) | 2012-08-28 | 2016-11-29 | Roche Diabetes Care, Inc. | Diabetes manager for glucose testing and continuous glucose monitoring |
| US9662445B2 (en) | 2012-08-30 | 2017-05-30 | Medtronic Minimed, Inc. | Regulating entry into a closed-loop operating mode of an insulin infusion system |
| US9171343B1 (en)* | 2012-09-11 | 2015-10-27 | Aseko, Inc. | Means and method for improved glycemic control for diabetic patients |
| US9278200B2 (en) | 2012-09-19 | 2016-03-08 | Mackay Memorial Hospital | Flow control device, system and method for controlling the flow of body fluid |
| EP2727587A1 (en) | 2012-10-30 | 2014-05-07 | Pharnext | Compositions, methods and uses for the treatment of diabetes and related conditions by controlling blood glucose level |
| PE20151518A1 (en) | 2012-11-13 | 2015-11-25 | Gordagen Pharmaceuticals Pty Ltd | TRANSMUCOSE ADMINISTRATION OF TOCOTRIENOL |
| US9744211B2 (en) | 2012-12-17 | 2017-08-29 | MYGALAXY Limited Company | Uses of BT lipopeptides as therapeutics for obesity and related diseases |
| US9351670B2 (en) | 2012-12-31 | 2016-05-31 | Abbott Diabetes Care Inc. | Glycemic risk determination based on variability of glucose levels |
| US9192509B2 (en) | 2013-03-11 | 2015-11-24 | Avacen, Inc. | Methods and apparatus for therapeutic application of thermal energy including blood viscosity adjustment |
| WO2014149781A1 (en) | 2013-03-15 | 2014-09-25 | Cercacor Laboratories, Inc. | Cloud-based physiological monitoring system |
| US9795737B2 (en) | 2013-03-15 | 2017-10-24 | Animas Corporation | Method and system for closed-loop control of an artificial pancreas |
| CN105377118B (en) | 2013-03-15 | 2019-09-17 | 雅培糖尿病护理公司 | Devices, systems and methods associated with analyte monitoring devices and devices incorporating the same |
| EP2967506B1 (en) | 2013-03-15 | 2020-04-29 | Becton, Dickinson and Company | Smart adapter for infusion devices |
| WO2014162549A1 (en) | 2013-04-04 | 2014-10-09 | テルモ株式会社 | Blood glucose management system |
| US9237866B2 (en) | 2013-04-29 | 2016-01-19 | Birch Narrows Development, LLC | Blood glucose management |
| US20160089500A1 (en) | 2013-05-21 | 2016-03-31 | Novo Nordisk A/S | Drug Delivery Device with Means for Disabling Activation of Expelling Mechanism |
| CA2913334A1 (en) | 2013-05-23 | 2014-11-27 | Iphenotype Llc | Method and system for maintaining or improving wellness |
| US9529385B2 (en) | 2013-05-23 | 2016-12-27 | Medibotics Llc | Smart watch and human-to-computer interface for monitoring food consumption |
| US9056049B2 (en) | 2013-05-30 | 2015-06-16 | Chin Yuan Huang | Micro-particle comprising a protein extract from sweet potato for extending satiety and controlling blood glucose and lipid levels |
| CN103432572B (en) | 2013-06-21 | 2018-09-18 | 四川大学华西医院 | Preparation method of rhesus monkey diabetic nephropathy model |
| US9482635B2 (en) | 2013-06-25 | 2016-11-01 | Animas Corporation | Glucose-measurement systems and methods presenting icons |
| WO2014209630A2 (en) | 2013-06-27 | 2014-12-31 | Inspark Technologies, Inc. | Systems, devices, and/or methods for identifying time periods of insufficient blood glucose testing |
- 2012
- 2012-09-11USUS13/610,287patent/US9897565B1/enactiveActive
- 2018
- 2018-01-05USUS15/862,819patent/US11131643B2/enactiveActive
- 2021
- 2021-09-13USUS17/474,001patent/US11733196B2/enactiveActive
- 2023
- 2023-06-05USUS18/329,451patent/US20230393088A1/enactivePending
Patent Citations (6)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US20030208113A1 (en)* | 2001-07-18 | 2003-11-06 | Mault James R | Closed loop glycemic index system |
| US20030028089A1 (en)* | 2001-07-31 | 2003-02-06 | Galley Paul J. | Diabetes management system |
| US20060253296A1 (en)* | 2003-10-29 | 2006-11-09 | Novo Nordisk A/S | Medical advisory system |
| US20070078314A1 (en)* | 2005-09-30 | 2007-04-05 | Grounsell Richard L | System and method for measuring and predicting insulin dosing rates |
| US20070179434A1 (en)* | 2005-12-08 | 2007-08-02 | Stefan Weinert | System and method for determining drug administration information |
| US20090006061A1 (en)* | 2007-06-27 | 2009-01-01 | Roche Diagnostics Operations, Inc. | System for developing patient specific therapies based on dynamic modeling of patient physiology and method thereof |
Non-Patent Citations (3)
| Title |
|---|
| Davidson et al. (Diabetes Care, 2005, Vol. 28, No. 10, pp.2418-23) (Year: 2005)* |
| Kaufman et al. (Diabetes Metab. Res. Rev., 1999, Vol. 15, p.338-352). (Year: 1999)* |
| Steil et al. (Diabetes, 2006, Vol. 55, pp.3344-3350) (Year: 2006)* |
Also Published As
| Publication number | Publication date |
|---|---|
| US20220074883A1 (en) | 2022-03-10 |
| US11131643B2 (en) | 2021-09-28 |
| US20180128765A1 (en) | 2018-05-10 |
| US11733196B2 (en) | 2023-08-22 |
| US9897565B1 (en) | 2018-02-20 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| US11733196B2 (en) | System and method for optimizing insulin dosages for diabetic subjects | |
| US10629294B2 (en) | Means and method for improved glycemic control for diabetic patients | |
| CN110869075B (en) | Calculation of residual insulin Activity content in Artificial islet systems | |
| JP3594897B2 (en) | Extrapolation system for glucose concentration | |
| US6582366B1 (en) | Medical devices for contemporaneous decision support in metabolic control | |
| US9486172B2 (en) | Estimation of insulin sensitivity from CGM and subcutaneous insulin delivery in type 1 diabetes | |
| US8454510B2 (en) | Method and device for assessing carbohydrate-to-insulin ratio | |
| Magdelaine et al. | A long-term model of the glucose–insulin dynamics of type 1 diabetes | |
| US6544212B2 (en) | Diabetes management system | |
| CN102596307B (en) | Utilize nominal open loop characteristic in diabetes, carry out system, the method and computer program product of insulin feed adjustment (AID) | |
| US20200015760A1 (en) | Method to determine individualized insulin sensitivity and optimal insulin dose by linear regression, and related systems | |
| WO2016061308A1 (en) | Human metabolic condition management | |
| Hussain et al. | Insulin Pumps and Continuous Glucose Monitoring Made Easy E-Book: Insulin Pumps and Continuous Glucose Monitoring Made Easy E-Book | |
| US9652595B1 (en) | Kit that improves impaired hepatic glucose processing in subjects | |
| US10533990B2 (en) | Physiologic insulin-sensitivity improvement | |
| Bhat et al. | Automated glucose control: A review | |
| Reiterer et al. | Identification of mixed-meal effects on insulin needs and glycemic control | |
| EP4120281A1 (en) | Method for modification of insulin delivery during pregnancy in automatic insulin delivery systems | |
| Hu et al. | Type 1 Diabetes in Children and Adolescents | |
| Kleck | The Efficacy of a Bi-hormonal Closed-loop System at Preventing Hypoglycemia during and after Exercise | |
| Ronca | Estimation of insulin sensitivity from continuous glucose monitoring and insulin pump data in type 1 diabetes |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| AS | Assignment | Owner name:HAYFIN SERVICES LLP, AS THE AGENT, UNITED KINGDOM Free format text:SECURITY INTEREST;ASSIGNOR:ASEKO, INC.;REEL/FRAME:064517/0872 Effective date:20230804 | |
| AS | Assignment | Owner name:BOOTH, ROBERT C, MASSACHUSETTS Free format text:ASSIGNMENT OF ASSIGNORS INTEREST;ASSIGNOR:BOOTH, ROBERT C;REEL/FRAME:065520/0324 Effective date:20120911 | |
| STPP | Information on status: patent application and granting procedure in general | Free format text:NON FINAL ACTION MAILED | |
| STPP | Information on status: patent application and granting procedure in general | Free format text:RESPONSE TO NON-FINAL OFFICE ACTION ENTERED AND FORWARDED TO EXAMINER | |
| AS | Assignment | Owner name:GLYTEC, LLC, MASSACHUSETTS Free format text:ASSIGNMENT OF ASSIGNORS INTEREST;ASSIGNOR:ASEKO, INC.;REEL/FRAME:067320/0764 Effective date:20240228 Owner name:ASEKO, INC., SOUTH CAROLINA Free format text:CORRECTIVE ASSIGNMENT TO CORRECT THE ASSIGNEE FROM BOOTH, ROBERT C TO ASEKO, INC. PREVIOUSLY RECORDED ON REEL 65520 FRAME 324. ASSIGNOR(S) HEREBY CONFIRMS THE ASSIGNMENT;ASSIGNOR:BOOTH, ROBERT C.;REEL/FRAME:066975/0110 Effective date:20120911 | |
| STPP | Information on status: patent application and granting procedure in general | Free format text:FINAL REJECTION MAILED | |
| STPP | Information on status: patent application and granting procedure in general | Free format text:RESPONSE AFTER FINAL ACTION FORWARDED TO EXAMINER | |
| STPP | Information on status: patent application and granting procedure in general | Free format text:ADVISORY ACTION MAILED | |
| STPP | Information on status: patent application and granting procedure in general | Free format text:DOCKETED NEW CASE - READY FOR EXAMINATION | |
| STPP | Information on status: patent application and granting procedure in general | Free format text:NON FINAL ACTION MAILED | |
| STPP | Information on status: patent application and granting procedure in general | Free format text:RESPONSE TO NON-FINAL OFFICE ACTION ENTERED AND FORWARDED TO EXAMINER | |
| STPP | Information on status: patent application and granting procedure in general | Free format text:NON FINAL ACTION MAILED | |
| STPP | Information on status: patent application and granting procedure in general | Free format text:RESPONSE TO NON-FINAL OFFICE ACTION ENTERED AND FORWARDED TO EXAMINER |