US20200093617A1 - Stent with shaped wires - Google Patents
Stent with shaped wiresDownload PDFInfo
- Publication number
- US20200093617A1 US20200093617A1US16/577,567US201916577567AUS2020093617A1US 20200093617 A1US20200093617 A1US 20200093617A1US 201916577567 AUS201916577567 AUS 201916577567AUS 2020093617 A1US2020093617 A1US 2020093617A1
- Authority
- US
- United States
- Prior art keywords
- stent
- wire
- wires
- length
- adjacent
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Granted
Links
- 238000000034methodMethods0.000claimsdescription10
- 238000005520cutting processMethods0.000claimsdescription9
- 238000004519manufacturing processMethods0.000claimsdescription6
- 238000005304joiningMethods0.000claimsdescription5
- 230000010355oscillationEffects0.000claimsdescription5
- 239000002184metalSubstances0.000claimsdescription4
- 238000011282treatmentMethods0.000description17
- 239000000463materialSubstances0.000description6
- 206010002329AneurysmDiseases0.000description3
- 230000037237body shapeEffects0.000description3
- 230000008901benefitEffects0.000description2
- 230000003073embolic effectEffects0.000description2
- 238000012986modificationMethods0.000description2
- 230000004048modificationEffects0.000description2
- HLXZNVUGXRDIFK-UHFFFAOYSA-Nnickel titaniumChemical compound[Ti].[Ti].[Ti].[Ti].[Ti].[Ti].[Ti].[Ti].[Ti].[Ti].[Ti].[Ni].[Ni].[Ni].[Ni].[Ni].[Ni].[Ni].[Ni].[Ni].[Ni].[Ni].[Ni].[Ni].[Ni]HLXZNVUGXRDIFK-UHFFFAOYSA-N0.000description2
- 229910001000nickel titaniumInorganic materials0.000description2
- 239000011148porous materialSubstances0.000description2
- 238000012800visualizationMethods0.000description2
- 238000004804windingMethods0.000description2
- 238000004026adhesive bondingMethods0.000description1
- 229910045601alloyInorganic materials0.000description1
- 239000000956alloySubstances0.000description1
- 210000003484anatomyAnatomy0.000description1
- 238000004873anchoringMethods0.000description1
- 238000005219brazingMethods0.000description1
- 230000006870functionEffects0.000description1
- 239000007943implantSubstances0.000description1
- 238000003698laser cuttingMethods0.000description1
- 238000005476solderingMethods0.000description1
- 210000005166vasculatureAnatomy0.000description1
- 238000009941weavingMethods0.000description1
- 238000003466weldingMethods0.000description1
Images
Classifications
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F2/00—Filters implantable into blood vessels; Prostheses, i.e. artificial substitutes or replacements for parts of the body; Appliances for connecting them with the body; Devices providing patency to, or preventing collapsing of, tubular structures of the body, e.g. stents
- A61F2/82—Devices providing patency to, or preventing collapsing of, tubular structures of the body, e.g. stents
- A61F2/86—Stents in a form characterised by the wire-like elements; Stents in the form characterised by a net-like or mesh-like structure
- A61F2/88—Stents in a form characterised by the wire-like elements; Stents in the form characterised by a net-like or mesh-like structure the wire-like elements formed as helical or spiral coils
- A61F2/885—Stents in a form characterised by the wire-like elements; Stents in the form characterised by a net-like or mesh-like structure the wire-like elements formed as helical or spiral coils comprising a coil including a plurality of spiral or helical sections with alternate directions around a central axis
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F2/00—Filters implantable into blood vessels; Prostheses, i.e. artificial substitutes or replacements for parts of the body; Appliances for connecting them with the body; Devices providing patency to, or preventing collapsing of, tubular structures of the body, e.g. stents
- A61F2/82—Devices providing patency to, or preventing collapsing of, tubular structures of the body, e.g. stents
- A61F2/86—Stents in a form characterised by the wire-like elements; Stents in the form characterised by a net-like or mesh-like structure
- A61F2/90—Stents in a form characterised by the wire-like elements; Stents in the form characterised by a net-like or mesh-like structure characterised by a net-like or mesh-like structure
- A61F2/91—Stents in a form characterised by the wire-like elements; Stents in the form characterised by a net-like or mesh-like structure characterised by a net-like or mesh-like structure made from perforated sheets or tubes, e.g. perforated by laser cuts or etched holes
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F2/00—Filters implantable into blood vessels; Prostheses, i.e. artificial substitutes or replacements for parts of the body; Appliances for connecting them with the body; Devices providing patency to, or preventing collapsing of, tubular structures of the body, e.g. stents
- A61F2/82—Devices providing patency to, or preventing collapsing of, tubular structures of the body, e.g. stents
- A61F2/86—Stents in a form characterised by the wire-like elements; Stents in the form characterised by a net-like or mesh-like structure
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F2/00—Filters implantable into blood vessels; Prostheses, i.e. artificial substitutes or replacements for parts of the body; Appliances for connecting them with the body; Devices providing patency to, or preventing collapsing of, tubular structures of the body, e.g. stents
- A61F2/82—Devices providing patency to, or preventing collapsing of, tubular structures of the body, e.g. stents
- A61F2/86—Stents in a form characterised by the wire-like elements; Stents in the form characterised by a net-like or mesh-like structure
- A61F2/90—Stents in a form characterised by the wire-like elements; Stents in the form characterised by a net-like or mesh-like structure characterised by a net-like or mesh-like structure
- A61F2/91—Stents in a form characterised by the wire-like elements; Stents in the form characterised by a net-like or mesh-like structure characterised by a net-like or mesh-like structure made from perforated sheets or tubes, e.g. perforated by laser cuts or etched holes
- A61F2/915—Stents in a form characterised by the wire-like elements; Stents in the form characterised by a net-like or mesh-like structure characterised by a net-like or mesh-like structure made from perforated sheets or tubes, e.g. perforated by laser cuts or etched holes with bands having a meander structure, adjacent bands being connected to each other
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F2/00—Filters implantable into blood vessels; Prostheses, i.e. artificial substitutes or replacements for parts of the body; Appliances for connecting them with the body; Devices providing patency to, or preventing collapsing of, tubular structures of the body, e.g. stents
- A61F2/82—Devices providing patency to, or preventing collapsing of, tubular structures of the body, e.g. stents
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F2/00—Filters implantable into blood vessels; Prostheses, i.e. artificial substitutes or replacements for parts of the body; Appliances for connecting them with the body; Devices providing patency to, or preventing collapsing of, tubular structures of the body, e.g. stents
- A61F2/82—Devices providing patency to, or preventing collapsing of, tubular structures of the body, e.g. stents
- A61F2/844—Devices providing patency to, or preventing collapsing of, tubular structures of the body, e.g. stents folded prior to deployment
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F2/00—Filters implantable into blood vessels; Prostheses, i.e. artificial substitutes or replacements for parts of the body; Appliances for connecting them with the body; Devices providing patency to, or preventing collapsing of, tubular structures of the body, e.g. stents
- A61F2/82—Devices providing patency to, or preventing collapsing of, tubular structures of the body, e.g. stents
- A61F2/86—Stents in a form characterised by the wire-like elements; Stents in the form characterised by a net-like or mesh-like structure
- A61F2/90—Stents in a form characterised by the wire-like elements; Stents in the form characterised by a net-like or mesh-like structure characterised by a net-like or mesh-like structure
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61L—METHODS OR APPARATUS FOR STERILISING MATERIALS OR OBJECTS IN GENERAL; DISINFECTION, STERILISATION OR DEODORISATION OF AIR; CHEMICAL ASPECTS OF BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES; MATERIALS FOR BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES
- A61L31/00—Materials for other surgical articles, e.g. stents, stent-grafts, shunts, surgical drapes, guide wires, materials for adhesion prevention, occluding devices, surgical gloves, tissue fixation devices
- A61L31/02—Inorganic materials
- A61L31/022—Metals or alloys
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61L—METHODS OR APPARATUS FOR STERILISING MATERIALS OR OBJECTS IN GENERAL; DISINFECTION, STERILISATION OR DEODORISATION OF AIR; CHEMICAL ASPECTS OF BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES; MATERIALS FOR BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES
- A61L31/00—Materials for other surgical articles, e.g. stents, stent-grafts, shunts, surgical drapes, guide wires, materials for adhesion prevention, occluding devices, surgical gloves, tissue fixation devices
- A61L31/14—Materials characterised by their function or physical properties, e.g. injectable or lubricating compositions, shape-memory materials, surface modified materials
- D—TEXTILES; PAPER
- D03—WEAVING
- D03D—WOVEN FABRICS; METHODS OF WEAVING; LOOMS
- D03D1/00—Woven fabrics designed to make specified articles
- D—TEXTILES; PAPER
- D03—WEAVING
- D03D—WOVEN FABRICS; METHODS OF WEAVING; LOOMS
- D03D3/00—Woven fabrics characterised by their shape
- D03D3/02—Tubular fabrics
- D—TEXTILES; PAPER
- D03—WEAVING
- D03D—WOVEN FABRICS; METHODS OF WEAVING; LOOMS
- D03D9/00—Open-work fabrics
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F2/00—Filters implantable into blood vessels; Prostheses, i.e. artificial substitutes or replacements for parts of the body; Appliances for connecting them with the body; Devices providing patency to, or preventing collapsing of, tubular structures of the body, e.g. stents
- A61F2/82—Devices providing patency to, or preventing collapsing of, tubular structures of the body, e.g. stents
- A61F2/86—Stents in a form characterised by the wire-like elements; Stents in the form characterised by a net-like or mesh-like structure
- A61F2/90—Stents in a form characterised by the wire-like elements; Stents in the form characterised by a net-like or mesh-like structure characterised by a net-like or mesh-like structure
- A61F2/91—Stents in a form characterised by the wire-like elements; Stents in the form characterised by a net-like or mesh-like structure characterised by a net-like or mesh-like structure made from perforated sheets or tubes, e.g. perforated by laser cuts or etched holes
- A61F2/915—Stents in a form characterised by the wire-like elements; Stents in the form characterised by a net-like or mesh-like structure characterised by a net-like or mesh-like structure made from perforated sheets or tubes, e.g. perforated by laser cuts or etched holes with bands having a meander structure, adjacent bands being connected to each other
- A61F2002/91508—Stents in a form characterised by the wire-like elements; Stents in the form characterised by a net-like or mesh-like structure characterised by a net-like or mesh-like structure made from perforated sheets or tubes, e.g. perforated by laser cuts or etched holes with bands having a meander structure, adjacent bands being connected to each other the meander having a difference in amplitude along the band
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F2/00—Filters implantable into blood vessels; Prostheses, i.e. artificial substitutes or replacements for parts of the body; Appliances for connecting them with the body; Devices providing patency to, or preventing collapsing of, tubular structures of the body, e.g. stents
- A61F2/82—Devices providing patency to, or preventing collapsing of, tubular structures of the body, e.g. stents
- A61F2/86—Stents in a form characterised by the wire-like elements; Stents in the form characterised by a net-like or mesh-like structure
- A61F2/90—Stents in a form characterised by the wire-like elements; Stents in the form characterised by a net-like or mesh-like structure characterised by a net-like or mesh-like structure
- A61F2/91—Stents in a form characterised by the wire-like elements; Stents in the form characterised by a net-like or mesh-like structure characterised by a net-like or mesh-like structure made from perforated sheets or tubes, e.g. perforated by laser cuts or etched holes
- A61F2/915—Stents in a form characterised by the wire-like elements; Stents in the form characterised by a net-like or mesh-like structure characterised by a net-like or mesh-like structure made from perforated sheets or tubes, e.g. perforated by laser cuts or etched holes with bands having a meander structure, adjacent bands being connected to each other
- A61F2002/91525—Stents in a form characterised by the wire-like elements; Stents in the form characterised by a net-like or mesh-like structure characterised by a net-like or mesh-like structure made from perforated sheets or tubes, e.g. perforated by laser cuts or etched holes with bands having a meander structure, adjacent bands being connected to each other within the whole structure different bands showing different meander characteristics, e.g. frequency or amplitude
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F2/00—Filters implantable into blood vessels; Prostheses, i.e. artificial substitutes or replacements for parts of the body; Appliances for connecting them with the body; Devices providing patency to, or preventing collapsing of, tubular structures of the body, e.g. stents
- A61F2/82—Devices providing patency to, or preventing collapsing of, tubular structures of the body, e.g. stents
- A61F2/86—Stents in a form characterised by the wire-like elements; Stents in the form characterised by a net-like or mesh-like structure
- A61F2/90—Stents in a form characterised by the wire-like elements; Stents in the form characterised by a net-like or mesh-like structure characterised by a net-like or mesh-like structure
- A61F2/91—Stents in a form characterised by the wire-like elements; Stents in the form characterised by a net-like or mesh-like structure characterised by a net-like or mesh-like structure made from perforated sheets or tubes, e.g. perforated by laser cuts or etched holes
- A61F2/915—Stents in a form characterised by the wire-like elements; Stents in the form characterised by a net-like or mesh-like structure characterised by a net-like or mesh-like structure made from perforated sheets or tubes, e.g. perforated by laser cuts or etched holes with bands having a meander structure, adjacent bands being connected to each other
- A61F2002/9155—Adjacent bands being connected to each other
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F2210/00—Particular material properties of prostheses classified in groups A61F2/00 - A61F2/26 or A61F2/82 or A61F9/00 or A61F11/00 or subgroups thereof
- A61F2210/0014—Particular material properties of prostheses classified in groups A61F2/00 - A61F2/26 or A61F2/82 or A61F9/00 or A61F11/00 or subgroups thereof using shape memory or superelastic materials, e.g. nitinol
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F2230/00—Geometry of prostheses classified in groups A61F2/00 - A61F2/26 or A61F2/82 or A61F9/00 or A61F11/00 or subgroups thereof
- A61F2230/0002—Two-dimensional shapes, e.g. cross-sections
- A61F2230/0004—Rounded shapes, e.g. with rounded corners
- A61F2230/0013—Horseshoe-shaped, e.g. crescent-shaped, C-shaped, U-shaped
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F2240/00—Manufacturing or designing of prostheses classified in groups A61F2/00 - A61F2/26 or A61F2/82 or A61F9/00 or A61F11/00 or subgroups thereof
- A61F2240/001—Designing or manufacturing processes
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F2250/00—Special features of prostheses classified in groups A61F2/00 - A61F2/26 or A61F2/82 or A61F9/00 or A61F11/00 or subgroups thereof
- A61F2250/0014—Special features of prostheses classified in groups A61F2/00 - A61F2/26 or A61F2/82 or A61F9/00 or A61F11/00 or subgroups thereof having different values of a given property or geometrical feature, e.g. mechanical property or material property, at different locations within the same prosthesis
- A61F2250/0036—Special features of prostheses classified in groups A61F2/00 - A61F2/26 or A61F2/82 or A61F9/00 or A61F11/00 or subgroups thereof having different values of a given property or geometrical feature, e.g. mechanical property or material property, at different locations within the same prosthesis differing in thickness
- D—TEXTILES; PAPER
- D10—INDEXING SCHEME ASSOCIATED WITH SUBLASSES OF SECTION D, RELATING TO TEXTILES
- D10B—INDEXING SCHEME ASSOCIATED WITH SUBLASSES OF SECTION D, RELATING TO TEXTILES
- D10B2509/00—Medical; Hygiene
- D10B2509/06—Vascular grafts; stents
Definitions
- the present inventiongenerally relates to implantable stent medical devices, methods for manufacturing the same, and more particularly, to novel stent structures.
- Medical stentsare used for supporting, maintaining, or repairing a lumen, passageway or opening in a living body.
- Stent designis unique to location and objective of the treatment as the stent must be flexible enough in a collapsed state to navigate body lumen to arrive at a treatment site, structurally robust enough in an implanted state to provide the required structural support to repair the treatment site, and flexibly expandable to have high conformity in treatment sites having tapered, bent, or other non-linear or non-tubular shapes. Flexibility, structural integrity, and conformability are often competing design goals that will vary depending on the location of the treatment site, goal of the treatment, and geometry of the treatment site.
- Wire, or braided stentsare typically braided from flexible wires to form a tube of wires that wrap helically around a center axis of the stent, with roughly half of the wires wrapping clockwise, and the other half wrapping counterclockwise such that wires extending in opposite direction wrap over and under each other diagonally in an alternating fashion.
- Wire stentscan be very flexible, can achieve high conformity within a body lumen containing a bend, and can resist kinking; however, the wire stents typically lack structural integrity to apply outward force radially against a lumen wall that is required in some treatments.
- a general strategy for stent design when structural integrity is desiredinvolves laser cutting patterns from a length of elastic tubing, typically made of a memory shape metal such as Nitinol or a Nitinol alloy.
- materialis removed from the tubing to form a cell pattern.
- enough materialmust remain so that the overall structural integrity of the laser cut stent is sufficient to apply the required outward force against a lumen wall once implanted.
- Materialis strategically removed to increase the flexibility of the stent for delivery to a treatment site and conformity to lumen walls in treatment sites including a bend or other non-uniform wall structure.
- patternsare cut to form rings that are substantially circumferential connected longitudinally by bridge struts. In such designs, bridge struts are added to achieve greater structural integrity and taken away to achieve greater flexibility.
- stentshaving greater flexibility and kink resistance compared to stents cut from elastic tubing and greater structural integrity compared to wire stents.
- One such strategyinvolves cutting elastic tubing to for a single helical structure that wraps circumferentially around the body of the stent such that adjacent windings of the helix are longitudinally interconnected with bridge struts (e.g. U.S. Pat. No. 5,925,061).
- Another strategyinvolves cutting a sheet of material to form a lattice strand that can be wrapped as a single helix about a mandrel and adjacent windings are subsequently interconnected (e.g. U.S. Pat. No. 5,370,683).
- the stentsgenerally can include a tubular structure having circumferentially positioned undulating wires that extend over a majority of a length of the stent such that the undulations oscillate circumferentially, and the undulations of the wires collectively define a circumference of the stent.
- the undulationscan wrap over and under adjacent undulations to form an interwoven structure. Additionally, or alternatively, adjacent wires can be joined.
- An example stentcan include a stent length measured from a first open end to a second open end, two or more wires each having a three-dimensional oscillating portion that extends over most of the stent length and is movable independent of the oscillating portion of every other of the one or more wires.
- the oscillating portion for each wirecan have an oscillating portion length measured parallel to a z-axis, a curvature extending circumferentially through an arc of less than 360° about the z-axis at a constant radius from the z-axis, and a waveform that oscillates over the length of the oscillating portion through the arc.
- the oscillating portion of each of the one or more wirescan be movable independent of every other of the one or more wires.
- Another example stent having a circumference and a lengthcan include two or more wires, each wire having a three-dimensional waveform that oscillates circumferentially within an arc about a z-axis parallel to the stent length, such that each waveform extends parallel to the z-axis through a majority of the stent length, maintaining a substantially constant radial distance from the z-axis.
- the wires of the stentcan be positioned circumferentially adjacent each other about the circumference of the stent to define the circumference of the stent.
- Each wirecan be movable along a majority of the length of the stent independently of every other wire in the stent.
- Another example stent having a tubular structure with a circumference and a lengthcan include a plurality of wires positioned around the circumference of the stent.
- Each wirecan be independently formed, can undulate circumferentially to form a wave pattern that extends over a majority of the length of the stent, can pass under an over an adjacent wire in a repeated fashion while maintaining an adjacent position to the adjacent wire over the majority of the length of the stent, and can intertwine circumferentially with other wires to form the tubular stent.
- Another example stent having tubular structurecan include circumferentially positioned undulating wires that extend over a majority of a length of the stent.
- the wirescan each have undulations that oscillate circumferentially.
- the undulations of each wirecan recess circumferentially within undulations of an adjacent wire such that the circumferential positioning of the undulating wires can define a circumference of the stent, and the circumferential positioning of the undulating wires can solely define the circumference of the stent absent any additional structures to define the circumference of the stent.
- each wire of the stentcan be independently formed from every other wire of the stent.
- Each wire of the stentcan be joined at one or more locations to a circumferentially adjacent wire.
- the stentcan include a first joint affixing a first wire to a second wire near the first open end.
- the first jointcan be the only affixed joint between the first wire and the second wire.
- the first wirecan cross under and cross over the second wire within one period of oscillation of the waveform of the first wire.
- the stentcan include a first end structure positioned adjacent the first open end, extending between the first open end and the oscillating portion of the stent, and the stent can include a second end structure positioned adjacent the second open end, extending between the second open end and the oscillating portion.
- One or both of the first and second end structurescan have an atraumatic shape.
- a wire of the stentcan have a width that varies along the length of the stent.
- one or more of the wirescan include a memory shape material. At least one wire can have a pre-determined three-dimensional shape curved along an arc, the three-dimensional shape having a wave pattern that undulates within the arc.
- An example method for manufacturing a stent including any of the example stents described hereincan include the steps of providing an elastic tubing, cutting the tubing to form a plurality of substantially similar wave patterns, separating each of the plurality of wave patterns from the tubing, positioning each wave pattern to extend across a majority of the length of the stent, and forming a majority of a tubular stent body from the plurality of wave patterns.
- Each wave patterncan have an amplitude extending circumferentially through an arc within a circumference of the tubing from which it is cut, an axis extending along at least a portion of a length of the tubing over which the wave pattern repeats, and a curved inner surface being the cut portion of the luminal surface of the tubing.
- Another example method for manufacturing a stent including any of the example stents described hereincan include the steps of providing an elastic tubing having a circumference and a length, cutting individual wires from the tubing such that each wire has a wave pattern oscillating peak-to-peak across a portion of the circumference, and weaving the wires to form a tube shape.
- Any of the example methodscan include any combination of the steps of cutting individual wires from the provided tubing such that each wire has a wave pattern that oscillates peak-to-peak across a portion of the circumference, separating the cut wires from the tubing such that the wires are disconnected from each other, joining a first wire to a second wire at one or more locations, joining a first wire to a second wire near an end of the stent body, and/or intertwining each wire with a clockwise adjacent wire and a counterclockwise adjacent wire.
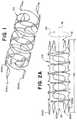
- FIG. 1is a three-dimensional perspective view of a stent according to the present invention
- FIG. 2Ais a side view of a stent in an expanded state according to the present invention.
- FIG. 2Bis a side view of the stent of FIG. 2A in a collapsed state according to the present invention
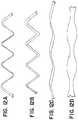
- FIG. 3Ais a two-dimensional depiction of a wire of a stent according to the present invention.
- FIG. 3Bis a three-dimensional depiction of the wire of FIG. 3A according to the present invention.
- FIG. 3Cis a schematic of a coordinate system for describing the three-dimension shape of a wire of a stent such as the wire depicted in FIG. 3B ;
- FIGS. 4 to 9are two-dimensional depictions of wires of a stent illustrating example placements of wires around a circumference of the stent according to the present invention
- FIGS. 10A to 10Eillustrate example waveform patterns or undulating patterns for a wire of a stent according to the present invention
- FIG. 11illustrates an example wire weave pattern of a stent according to the present invention.
- FIGS. 12A to 12Dillustrate example wire segments having a variable width according to the present invention.
- a stentcan have a generally tubular structure with circumferentially positioned undulating wires extending over a majority of a length of the stent.
- Undulations of each wirecan oscillate circumferentially, and the undulations of a wire can recess within undulations of adjacent wires, such that the recession of each wire into each other defines a circumference of the stent.
- Adjacent wirescan be joined at few locations, such as at the ends, or not at all, such that individual wires are movable independent from every other wire. Additionally, or alternatively, the undulations can wrap over and under adjacent undulations to form an interwoven structure.
- example stents described hereincan be cut from a metal tube and can be prepared by cutting the tube into separate wires that retain a helical curvature from the tube wall from which they are cut.
- Wirescan be cut from the tube to be substantially independent from one another, and these wires can be braided, woven, or otherwise intertwined to form a tubular shape. In some applications it may be desirable to utilize between eight and sixteen laser cut wires to form a stent with desired flexibility, structural integrity, and conformability.
- Wires in some example stentscan move independently of each other to some extent; for example, the wires of an example stent can be movable like how wires in known braided or woven structures are generally movable independent of each other.
- wires in some example stentscan be welded or otherwise joined to each other at one or more locations along a length of each of the wires. Joined wires can form an interlocking structure and can increase structural integrity of the stent.
- some example stentscan be made substantially movable to each other, some example stents can have improved flexibility and kink resistance compared to known laser cut tube designs and can achieve flexibility and kink resistance like known wire braid stent designs. Because the wires of some example stents can be cut from a metal tube, the wires of an example stent can provide greater radial force compared to wires of known wire braid stent designs, the wires of the stent can be designed to have an atraumatic end structure (which is typically not achievable by using cut wires in known wire braid stent designs), and the stent can have wires that vary in thickness and shape along the length of the stent (which is generally not possible in known wire braid stent designs that utilize constant diameter wires).
- a potential application of some example stentscan be supporting embolic coils within an aneurysm at a treatment site that requires navigation of torturous anatomy to reach.
- FIG. 1is a three-dimensional perspective view of a stent 100 having four wires 200 a , 200 b , 200 c , 200 d .
- a stent 100can include as few or as many wires as required to achieve a desired flexibility, structural integrity, and conformability.
- a stent having a greater number of wiresmay be required, for example, when treating a body lumen having a larger diameter.
- a stentcan also be designed to have a space between wires through which a microcatheter for delivering an embolic implant can passthrough; in which case, it may be desirable to create pores between wires that are small enough to provide enough structural support but large enough so a coiling microcatheter can fit through a pore. In some applications it may be desirable to utilize between eight and sixteen laser cut wires.
- FIG. 2Ais a side view of a stent 100 in an expanded state like that of FIG. 1 having four wires 200 a , 200 b , 200 c , 200 d .
- the stent 100can have a substantially tubular body shape with a first open end 112 , a second open end 114 , and a length 110 extending from the first open end 112 to the second open end 114 in a longitudinal direction 10 .
- the stent 100can be designed to have a length to meet the needs of the treatment, for example, the length 110 can be sized larger than a neck of an aneurysm such that aneurysms having a larger neck opening could be treated with a longer stent.
- the tubular body shape of the stent 100can have a substantially uniform circumference 20 along its length 110 in an expanded state.
- a stentcan have a tubular body shape having a large circumference at a first open end that tapers to a smaller circumference at a second open end.
- Such a tapered designcan be advantageous for treating tapered body lumens, for example.
- FIG. 2Bis a side view of the stent of FIG. 2A in a collapsed state.
- the stent 100can be sized to be delivered through a microcatheter to a treatment site within a vasculature.
- the stent 100can be made of a small number of wires, and each wire 200 a , 200 b , 200 c , 200 d can be stretched in the longitudinal direction 10 to occupy a small cross-sectional profile, an advantage of the stent 100 is that it can be collapsed to a thinner dimension than a laser cut tubular type stent. Laser cut tubular type stents typically have little ability to stretch lengthwise. The thinner collapsed dimension, in some applications, can allow the stent 100 to be delivered to a treatment site through a smaller catheter, therefore reaching treatment sites that may be challenging or impossible to reach with other stent designs.
- a wire 200is illustrated in FIG. 3A in two dimensions and in FIG. 3B in three dimensions.
- the wire 200has a three-dimensional shape as illustrated in FIG. 3B ; the two-dimensional illustration of FIG. 3A is provided to aid visualization and discussion of the wire 200 .
- a wire 200can have an oscillating portion 210 having an oscillating portion length 211 measured parallel to a z-axis 12 .
- the oscillating portion 210can have a waveform that repeats over the length 211 of the oscillating portion 210 , repeating with a period of oscillation 212 .
- the waveformcan be sinusoidal having a series of peaks 202 , troughs 204 , and intermediate segments 206 extending between the peaks 202 and troughs 204 .
- the oscillating portion 210can extend over a majority of a length of the stent, and the wire 200 can also have end structures 220 at the ends of each wire 200 .
- Each end structure 220can have an atraumatic shape.
- FIG. 3Cis a schematic of a cylindrical coordinate system for describing the three-dimension shape of a wire 200 of a stent 100 such as the wire depicted in FIG. 3B .
- the z-axis 12is understood to be perpendicular to the page, positioned at the center of the circle illustrated in FIG. 3C .
- a position of a point in the cylindrical coordinate systemcan be defined by the coordinates r, ⁇ , and z, where the r coordinate defines a distance from the z-axis, the ⁇ coordinate defines an angle from the r-axis, and the z coordinate defines a linear position along the z-axis.
- a tubewould therefore include points where r is equal to a constant, R over given length in the z-axis, and an arc in said tube would be confined to an angle, or range of values for ⁇ , the angle being less than 360°.
- the example wire 200 illustrated in FIG. 3Bcan therefore be described as having a curvature extending circumferentially through an arc 216 of approximately 180° that maintains a substantially constant radius 214 from the z-axis.
- the waveformcan be described as oscillating circumferentially, confined within the arc of the curvature, extending in the z-direction over the length 211 of the oscillating portion 210 .
- an amplitude of the waveformcan be described as the distance between the peaks 202 and the troughs 204 .
- the amplitude of the waveformcan therefore be expressed as a function of the arc of the curvature 216 and the radius 214 .
- the curvaturecould be wider or narrower to achieve desired properties of the stent. For example, a waveform having a larger amplitude can result in an overall stent design having greater flexibility while a waveform having a smaller amplitude can result in an overall stent design that is easier to deliver through a microcatheter.
- the wirecan be formed by cutting the wire from a portion of elastic tubing.
- the tubingcan have a radius 214 , and the tubing can be cut so that the resulting wire 200 has an oscillating portion that maintains the radius 214 of the tubing.
- the wire 200can be cut from an arc defined by at least a portion of the circumference of the tubing so that the resulting wire 200 oscillates within the arc 216 , and the resulting wire can have a wave pattern that oscillates peak-to-peak across the cut portion of the circumference of the tube.
- the tubingcan have a lumen with an inner luminal surface.
- the wire 200can be cut from the tubing to have an inner surface that is cut from the luminal surface of the tubing.
- the wire 200can be cut from the tubing such that a majority of the oscillating portion 210 is movable independent of oscillating portions 210 of other wires.
- the wire 200once cut, can be separated from the tubing to form an independently formed wire 200 .
- FIGS. 4 to 9are two-dimensional depictions of wires of a stent illustrating example placements of wires around a circumference of the stent. The illustrations are provided in two dimensions for discussion and visualization. Referring collectively to FIGS. 4 to 8 , the illustrations depict the placement of four wires 200 a , 200 b , 200 c , 200 d circumferentially to form a stent.
- the bottom wire 200 ais redrawn as a dashed wire 200 a ′ above the top wire 200 d
- the top wire 200 dis redrawn as a dashed wire 200 d ′ below the bottom wire 200 a to illustrate the placement and connection of the wires shown as the top and bottom wires 200 d , 200 a in a three-dimensional stent.
- the wires 200 a , 200 b , 200 c , 200 dcan be cut from tubing, and each wire can be positioned to define a circumference of a stent. Wires can be positioned such that each oscillating portion 210 extends across a length of the stent. Inner curved surface of each oscillating portion 210 of each wire 200 a , 200 b , 200 c , 200 d can be aligned to collectively form the circumference of the stent. Wires 200 a , 200 b , 200 c , 200 d can be joined and/or woven to form a tube shape.
- FIG. 4is a two-dimensional illustration of four wires placed to define a circumference of a stent like the stents depicted in FIGS. 1 and 2A .
- Each wirecan be recessed circumferentially within undulations of circumferentially adjacent wires such that the circumferential positioning of the undulating wires defines a circumference of the stent.
- Each wirecan be independently formed and at least to some extent, movable compared to every other wire.
- FIG. 5is a two-dimensional illustration of four wires placed to define a circumference of a stent like the pattern shown in FIG. 4 .
- Each wirecan be connected to each adjacent wire by a single joint 230 a , 230 b , 230 c , 230 d , 230 d ′.
- a first joint 230 ccan join a first wire 200 c to a second wire 200 d adjacent to the first wire 200 c
- the first joint 230 ccan be the only affixed joint between the first wire 200 c and the second wire 200 d .
- the first wire 200 ccan also be joined to a third wire 200 b with a second joint 230 b .
- each wire 200 a , 200 b , 200 c , 200 dcan be joined to its two adjacent neighbors by a single joint for each neighbor.
- Minimal connections 230 a , 230 b , 230 c , 230 dcan allow the wires to move independently of each other over a majority of the length of the stent.
- each jointcan be formed by any conventional means such as welding, brazing, soldering, gluing, tying, etc.
- a stentcan be cut from a single piece of tubing such that the joints 230 a , 230 b , 230 c , 230 d are uncut portions of the tubing.
- a wire 200 a , 200 b , 200 c , 200 d joined to a neighboring wire by an uncut tubing portionwould not be completely separated from the neighbor during manufacturing.
- the uncut portioncan be placed at a joint location 230 a , 230 b , 230 c , 230 d like those shown in FIGS. 5 and 6 and described herein to allow the wire 200 a , 200 b , 200 c , 200 d to be movable independently of the neighbor wire and other wires over a majority of the length of the stent.
- FIG. 6is a two-dimensional illustration of four wires placed to define a circumference of a stent having one joint 230 a , 230 b , 230 c , 230 d , 230 d ′ connecting each pair of adjacent wires.
- the joints 230 a , 230 b , 230 c , 230 d , 230 d ′can be positioned at any number of locations.
- FIG. 6illustrates an alternative configuration of the four wires 200 a , 200 b , 200 c , 200 d , illustrated in FIG. 5 and the four joints 230 a , 230 b , 230 c , 230 d .
- Minimal connections 230 a , 230 b , 230 c , 230 dcan allow the wires 200 a , 200 b , 200 c , 200 d to move independently of each other over a majority of the length of the stent.
- FIG. 7is a two-dimensional illustration of four wires placed to define a circumference of a stent such that the wires are interwoven to define a circumference of the stent.
- Each wirecan cross over and under each neighboring wire.
- a first wire 200 acan cross under 244 a first neighboring wire 200 d ′ and over 246 the same wire 200 d ′ within one period of oscillation of the first wire 200 a .
- the first wire 200 acan cross under 242 a second neighboring wire 200 b and over 248 the second neighboring wire 200 b within the same period of oscillation of the first wire 200 a .
- the interwoven structure of the wires 200 a , 200 b , 200 c , 200 dcan be sufficient to maintain the structural integrity of the stent absent any joints to affix wires.
- the wirescan therefore be independently formed and independently movable.
- wires 200 a , 200 b , 200 c , 200 dcan be intertwined with other wave patterns in any number of patterns.
- FIG. 8is a two-dimensional illustration of four wires placed to define a circumference of a stent such that the wires are interwoven to define a circumference of the stent like the one as illustrated in FIG. 7 .
- a stentcan have both interwoven wires 200 a , 200 b , 200 c , 200 d , and joints 230 a , 230 b , 230 c , 230 d to join neighboring wires.
- a joint 230 bcan be placed to connect a first wire 200 c and a second wire 200 b at a cross-over point.
- the joints 230 a , 230 b , 230 c , 230 dcan be placed near an end of the wire, and only one joint can be used to connect each wire to each adjacent wire. As will be appreciated and understood, any number of joints can be used to connect wires and various locations along the length of the stent. Minimal connections 230 a , 230 b , 230 c , 230 d can allow the wires 200 a , 200 b , 200 c , 200 d to move independently of each other over a majority of the length of the stent.
- FIG. 9is a two-dimensional illustration of four wire segments 200 a , 200 b , 200 c , 200 d joined by three bends 230 , wherein the wire segments are placed to define a circumference of a stent and each wire segment 200 a , 200 b , 200 c , 200 d is joined to a neighboring segment by a bend 230 .
- each bend 230can be positioned at either a first or second open end of the stent to connect a first wire 200 a and a second wire 200 b .
- the wire segments 200 a , 200 b , 200 c , 200 dcan be joined to form a contiguous wire characterized by longitudinal undulating segments 200 a , 200 b , 200 c , 200 d that are joined alternatively at bends 230 positioned at each end of the stent.
- the contiguous wirecan include atraumatic ends 220 positioned at an end of a first wire segment 200 a and an end of a last wire segment 200 d in the chain of segments.
- the stentcan be constructed with multiple independent wires consisting of wire segments joined at bends. Wires and wire segments can be otherwise joined or interwoven as described in other examples presented herein or as known in the art.
- FIGS. 10A to 10Eillustrate example waveform patterns or undulating patterns for a wire of a stent that can be used in addition to or in place of other waveforms depicted and described herein.
- waveform patternscan be utilized to achieve a desired flexibility, structural integrity, and conformability for the stent, including those not shown, and as known in the art.
- FIG. 11illustrates an example wire weave pattern that could be utilized to form a stent in addition to or in place of other weave patterns depicted and described herein.
- FIGS. 12A to 12Dillustrate example wire segments having a variable width that could be utilized to form a stent in addition to or in place of other wire features depicted and described herein.
- a wirecan have an undulating pattern having thicker segments near each end of the wire and thinner segments positioned in the middle of the wire. Thinner central segments can allow the stent to pass more easily through a microcatheter while thicker end segments can improve structural integrity at the stent ends for anchoring within a body lumen.
- a wirecan have thin segments near one end and thicker segments at the other end. As illustrated, the segments can become increasingly thicker or thinner from one end of the wire to the other. Stents formed from wire segments with progressively changing segment thickness can be formed to have a tapered structure that can be advantageous for achieving conformity to the walls of a body lumen when implanted in a body lumen that is tapered.
- the inventioncontemplates many variations and modifications of the stent, including alternative shapes for oscillating portions of wires, alternate shapes for atraumatic end segments of wires, alternative means of joining or connecting wires, alternative patterns for interlacing wires to form the stent, forming stents with any number of wires, or utilizing any of numerous materials or manufacturing means for the stent, for example. These modifications would be apparent to those having ordinary skill in the art to which this invention relates and are intended to be within the scope of the claims which follow.
Landscapes
- Health & Medical Sciences (AREA)
- Engineering & Computer Science (AREA)
- Biomedical Technology (AREA)
- Heart & Thoracic Surgery (AREA)
- Vascular Medicine (AREA)
- Life Sciences & Earth Sciences (AREA)
- Animal Behavior & Ethology (AREA)
- General Health & Medical Sciences (AREA)
- Public Health (AREA)
- Veterinary Medicine (AREA)
- Oral & Maxillofacial Surgery (AREA)
- Cardiology (AREA)
- Transplantation (AREA)
- Optics & Photonics (AREA)
- Physics & Mathematics (AREA)
- Epidemiology (AREA)
- Surgery (AREA)
- Chemical & Material Sciences (AREA)
- Inorganic Chemistry (AREA)
- Media Introduction/Drainage Providing Device (AREA)
- Prostheses (AREA)
- Textile Engineering (AREA)
Abstract
Description
- This application claims the benefit of U.S. Provisional Application No. 62/734,128 titled “STENT WITH SHAPED WIRES” filed on Sep. 20, 2018 which prior application is hereby incorporated by reference in its entirety herein into this application as if set forth in full.
- The present invention generally relates to implantable stent medical devices, methods for manufacturing the same, and more particularly, to novel stent structures.
- Medical stents are used for supporting, maintaining, or repairing a lumen, passageway or opening in a living body. Stent design is unique to location and objective of the treatment as the stent must be flexible enough in a collapsed state to navigate body lumen to arrive at a treatment site, structurally robust enough in an implanted state to provide the required structural support to repair the treatment site, and flexibly expandable to have high conformity in treatment sites having tapered, bent, or other non-linear or non-tubular shapes. Flexibility, structural integrity, and conformability are often competing design goals that will vary depending on the location of the treatment site, goal of the treatment, and geometry of the treatment site.
- Wire, or braided stents, are typically braided from flexible wires to form a tube of wires that wrap helically around a center axis of the stent, with roughly half of the wires wrapping clockwise, and the other half wrapping counterclockwise such that wires extending in opposite direction wrap over and under each other diagonally in an alternating fashion. Wire stents can be very flexible, can achieve high conformity within a body lumen containing a bend, and can resist kinking; however, the wire stents typically lack structural integrity to apply outward force radially against a lumen wall that is required in some treatments.
- A general strategy for stent design when structural integrity is desired involves laser cutting patterns from a length of elastic tubing, typically made of a memory shape metal such as Nitinol or a Nitinol alloy. In general, material is removed from the tubing to form a cell pattern. Generally speaking, enough material must remain so that the overall structural integrity of the laser cut stent is sufficient to apply the required outward force against a lumen wall once implanted. Material is strategically removed to increase the flexibility of the stent for delivery to a treatment site and conformity to lumen walls in treatment sites including a bend or other non-uniform wall structure. In many stent designs, patterns are cut to form rings that are substantially circumferential connected longitudinally by bridge struts. In such designs, bridge struts are added to achieve greater structural integrity and taken away to achieve greater flexibility.
- Attempts have been made to design a stent having greater flexibility and kink resistance compared to stents cut from elastic tubing and greater structural integrity compared to wire stents. One such strategy involves cutting elastic tubing to for a single helical structure that wraps circumferentially around the body of the stent such that adjacent windings of the helix are longitudinally interconnected with bridge struts (e.g. U.S. Pat. No. 5,925,061). Another strategy involves cutting a sheet of material to form a lattice strand that can be wrapped as a single helix about a mandrel and adjacent windings are subsequently interconnected (e.g. U.S. Pat. No. 5,370,683). Although such designs typically can achieve greater flexibility compared to a laser cut tubular stent utilizing circumferential rings, it is at the cost of structural integrity; and although such designs can achieve greater structural integrity compared to wire stents, they cannot achieve the conformability and kink resistance of most wire stents.
- There therefore exists a need for alternative stent designs for achieving flexibility, structural integrity, and conformability to meet the needs of a variety of treatment goals at a variety of treatment sites having a variety of anatomical geometries.
- Disclosed herein are various exemplary stents of the present invention that can address the above needs. The stents generally can include a tubular structure having circumferentially positioned undulating wires that extend over a majority of a length of the stent such that the undulations oscillate circumferentially, and the undulations of the wires collectively define a circumference of the stent. The undulations can wrap over and under adjacent undulations to form an interwoven structure. Additionally, or alternatively, adjacent wires can be joined.
- An example stent can include a stent length measured from a first open end to a second open end, two or more wires each having a three-dimensional oscillating portion that extends over most of the stent length and is movable independent of the oscillating portion of every other of the one or more wires. The oscillating portion for each wire can have an oscillating portion length measured parallel to a z-axis, a curvature extending circumferentially through an arc of less than 360° about the z-axis at a constant radius from the z-axis, and a waveform that oscillates over the length of the oscillating portion through the arc. The oscillating portion of each of the one or more wires can be movable independent of every other of the one or more wires.
- Another example stent having a circumference and a length can include two or more wires, each wire having a three-dimensional waveform that oscillates circumferentially within an arc about a z-axis parallel to the stent length, such that each waveform extends parallel to the z-axis through a majority of the stent length, maintaining a substantially constant radial distance from the z-axis. The wires of the stent can be positioned circumferentially adjacent each other about the circumference of the stent to define the circumference of the stent. Each wire can be movable along a majority of the length of the stent independently of every other wire in the stent.
- Another example stent having a tubular structure with a circumference and a length can include a plurality of wires positioned around the circumference of the stent. Each wire can be independently formed, can undulate circumferentially to form a wave pattern that extends over a majority of the length of the stent, can pass under an over an adjacent wire in a repeated fashion while maintaining an adjacent position to the adjacent wire over the majority of the length of the stent, and can intertwine circumferentially with other wires to form the tubular stent.
- Another example stent having tubular structure can include circumferentially positioned undulating wires that extend over a majority of a length of the stent. The wires can each have undulations that oscillate circumferentially. The undulations of each wire can recess circumferentially within undulations of an adjacent wire such that the circumferential positioning of the undulating wires can define a circumference of the stent, and the circumferential positioning of the undulating wires can solely define the circumference of the stent absent any additional structures to define the circumference of the stent.
- In any of the example stents, each wire of the stent can be independently formed from every other wire of the stent. Each wire of the stent can be joined at one or more locations to a circumferentially adjacent wire. The stent can include a first joint affixing a first wire to a second wire near the first open end. The first joint can be the only affixed joint between the first wire and the second wire. Additionally, or alternatively, to affixing adjacent wires, the first wire can cross under and cross over the second wire within one period of oscillation of the waveform of the first wire.
- In any of the example stents, the stent can include a first end structure positioned adjacent the first open end, extending between the first open end and the oscillating portion of the stent, and the stent can include a second end structure positioned adjacent the second open end, extending between the second open end and the oscillating portion. One or both of the first and second end structures can have an atraumatic shape.
- In any of the example stents, a wire of the stent can have a width that varies along the length of the stent.
- In any of the example stents, one or more of the wires can include a memory shape material. At least one wire can have a pre-determined three-dimensional shape curved along an arc, the three-dimensional shape having a wave pattern that undulates within the arc.
- An example method for manufacturing a stent including any of the example stents described herein can include the steps of providing an elastic tubing, cutting the tubing to form a plurality of substantially similar wave patterns, separating each of the plurality of wave patterns from the tubing, positioning each wave pattern to extend across a majority of the length of the stent, and forming a majority of a tubular stent body from the plurality of wave patterns. Each wave pattern can have an amplitude extending circumferentially through an arc within a circumference of the tubing from which it is cut, an axis extending along at least a portion of a length of the tubing over which the wave pattern repeats, and a curved inner surface being the cut portion of the luminal surface of the tubing.
- Another example method for manufacturing a stent including any of the example stents described herein can include the steps of providing an elastic tubing having a circumference and a length, cutting individual wires from the tubing such that each wire has a wave pattern oscillating peak-to-peak across a portion of the circumference, and weaving the wires to form a tube shape.
- Any of the example methods can include any combination of the steps of cutting individual wires from the provided tubing such that each wire has a wave pattern that oscillates peak-to-peak across a portion of the circumference, separating the cut wires from the tubing such that the wires are disconnected from each other, joining a first wire to a second wire at one or more locations, joining a first wire to a second wire near an end of the stent body, and/or intertwining each wire with a clockwise adjacent wire and a counterclockwise adjacent wire.
- The above and further aspects of this invention are further discussed with reference to the following description in conjunction with the accompanying drawings, in which like numerals indicate like structural elements and features in various figures. The drawings are not necessarily to scale, emphasis instead being placed upon illustrating principles of the invention. The figures depict one or more implementations of the inventive devices, by way of example only, not by way of limitation.
FIG. 1 is a three-dimensional perspective view of a stent according to the present invention;FIG. 2A is a side view of a stent in an expanded state according to the present invention;FIG. 2B is a side view of the stent ofFIG. 2A in a collapsed state according to the present invention;FIG. 3A is a two-dimensional depiction of a wire of a stent according to the present invention;FIG. 3B is a three-dimensional depiction of the wire ofFIG. 3A according to the present invention;FIG. 3C is a schematic of a coordinate system for describing the three-dimension shape of a wire of a stent such as the wire depicted inFIG. 3B ;FIGS. 4 to 9 are two-dimensional depictions of wires of a stent illustrating example placements of wires around a circumference of the stent according to the present invention;FIGS. 10A to 10E illustrate example waveform patterns or undulating patterns for a wire of a stent according to the present invention;FIG. 11 illustrates an example wire weave pattern of a stent according to the present invention; andFIGS. 12A to 12D illustrate example wire segments having a variable width according to the present invention.- Various exemplary stents are described herein that can address the above needs. In general, a stent can have a generally tubular structure with circumferentially positioned undulating wires extending over a majority of a length of the stent. Undulations of each wire can oscillate circumferentially, and the undulations of a wire can recess within undulations of adjacent wires, such that the recession of each wire into each other defines a circumference of the stent. Adjacent wires can be joined at few locations, such as at the ends, or not at all, such that individual wires are movable independent from every other wire. Additionally, or alternatively, the undulations can wrap over and under adjacent undulations to form an interwoven structure.
- Generally, example stents described herein can be cut from a metal tube and can be prepared by cutting the tube into separate wires that retain a helical curvature from the tube wall from which they are cut. Wires can be cut from the tube to be substantially independent from one another, and these wires can be braided, woven, or otherwise intertwined to form a tubular shape. In some applications it may be desirable to utilize between eight and sixteen laser cut wires to form a stent with desired flexibility, structural integrity, and conformability. Wires in some example stents can move independently of each other to some extent; for example, the wires of an example stent can be movable like how wires in known braided or woven structures are generally movable independent of each other. Additionally, or alternatively, wires in some example stents can be welded or otherwise joined to each other at one or more locations along a length of each of the wires. Joined wires can form an interlocking structure and can increase structural integrity of the stent.
- Because wires of some example stents can be made substantially movable to each other, some example stents can have improved flexibility and kink resistance compared to known laser cut tube designs and can achieve flexibility and kink resistance like known wire braid stent designs. Because the wires of some example stents can be cut from a metal tube, the wires of an example stent can provide greater radial force compared to wires of known wire braid stent designs, the wires of the stent can be designed to have an atraumatic end structure (which is typically not achievable by using cut wires in known wire braid stent designs), and the stent can have wires that vary in thickness and shape along the length of the stent (which is generally not possible in known wire braid stent designs that utilize constant diameter wires). A potential application of some example stents can be supporting embolic coils within an aneurysm at a treatment site that requires navigation of torturous anatomy to reach.
FIG. 1 is a three-dimensional perspective view of astent 100 having fourwires stent 100 can include as few or as many wires as required to achieve a desired flexibility, structural integrity, and conformability. A stent having a greater number of wires may be required, for example, when treating a body lumen having a larger diameter. A stent can also be designed to have a space between wires through which a microcatheter for delivering an embolic implant can passthrough; in which case, it may be desirable to create pores between wires that are small enough to provide enough structural support but large enough so a coiling microcatheter can fit through a pore. In some applications it may be desirable to utilize between eight and sixteen laser cut wires.FIG. 2A is a side view of astent 100 in an expanded state like that ofFIG. 1 having fourwires stent 100 can have a substantially tubular body shape with a firstopen end 112, a secondopen end 114, and alength 110 extending from the firstopen end 112 to the secondopen end 114 in alongitudinal direction 10. Thestent 100 can be designed to have a length to meet the needs of the treatment, for example, thelength 110 can be sized larger than a neck of an aneurysm such that aneurysms having a larger neck opening could be treated with a longer stent. The tubular body shape of thestent 100 can have a substantiallyuniform circumference 20 along itslength 110 in an expanded state. Alternatively, (not shown) a stent can have a tubular body shape having a large circumference at a first open end that tapers to a smaller circumference at a second open end. Such a tapered design can be advantageous for treating tapered body lumens, for example.FIG. 2B is a side view of the stent ofFIG. 2A in a collapsed state. In the collapsed state, thestent 100 can be sized to be delivered through a microcatheter to a treatment site within a vasculature. Because thestent 100 can be made of a small number of wires, and eachwire longitudinal direction 10 to occupy a small cross-sectional profile, an advantage of thestent 100 is that it can be collapsed to a thinner dimension than a laser cut tubular type stent. Laser cut tubular type stents typically have little ability to stretch lengthwise. The thinner collapsed dimension, in some applications, can allow thestent 100 to be delivered to a treatment site through a smaller catheter, therefore reaching treatment sites that may be challenging or impossible to reach with other stent designs.- A
wire 200 is illustrated inFIG. 3A in two dimensions and inFIG. 3B in three dimensions. Thewire 200 has a three-dimensional shape as illustrated inFIG. 3B ; the two-dimensional illustration ofFIG. 3A is provided to aid visualization and discussion of thewire 200. Referring collectively toFIGS. 3A and 3B , awire 200 can have anoscillating portion 210 having anoscillating portion length 211 measured parallel to a z-axis 12. Theoscillating portion 210 can have a waveform that repeats over thelength 211 of theoscillating portion 210, repeating with a period ofoscillation 212. The waveform can be sinusoidal having a series ofpeaks 202,troughs 204, andintermediate segments 206 extending between thepeaks 202 andtroughs 204. Theoscillating portion 210 can extend over a majority of a length of the stent, and thewire 200 can also haveend structures 220 at the ends of eachwire 200. Eachend structure 220 can have an atraumatic shape. FIG. 3C is a schematic of a cylindrical coordinate system for describing the three-dimension shape of awire 200 of astent 100 such as the wire depicted inFIG. 3B . In the cylindrical coordinate system, the z-axis 12 is understood to be perpendicular to the page, positioned at the center of the circle illustrated inFIG. 3C . In general, a position of a point in the cylindrical coordinate system can be defined by the coordinates r, θ, and z, where the r coordinate defines a distance from the z-axis, the θ coordinate defines an angle from the r-axis, and the z coordinate defines a linear position along the z-axis. A tube would therefore include points where r is equal to a constant, R over given length in the z-axis, and an arc in said tube would be confined to an angle, or range of values for θ, the angle being less than 360°. Theexample wire 200 illustrated inFIG. 3B can therefore be described as having a curvature extending circumferentially through anarc 216 of approximately 180° that maintains a substantiallyconstant radius 214 from the z-axis. The waveform can be described as oscillating circumferentially, confined within the arc of the curvature, extending in the z-direction over thelength 211 of theoscillating portion 210.- Referring to
FIG. 3A , an amplitude of the waveform can be described as the distance between thepeaks 202 and thetroughs 204. Referring toFIGS. 3B and 3C , the amplitude of the waveform can therefore be expressed as a function of the arc of thecurvature 216 and theradius 214. As will be appreciated and understood, the curvature could be wider or narrower to achieve desired properties of the stent. For example, a waveform having a larger amplitude can result in an overall stent design having greater flexibility while a waveform having a smaller amplitude can result in an overall stent design that is easier to deliver through a microcatheter. - Referring to
FIGS. 3B and 3C , the wire can be formed by cutting the wire from a portion of elastic tubing. The tubing can have aradius 214, and the tubing can be cut so that the resultingwire 200 has an oscillating portion that maintains theradius 214 of the tubing. Thewire 200 can be cut from an arc defined by at least a portion of the circumference of the tubing so that the resultingwire 200 oscillates within thearc 216, and the resulting wire can have a wave pattern that oscillates peak-to-peak across the cut portion of the circumference of the tube. - The tubing can have a lumen with an inner luminal surface. The
wire 200 can be cut from the tubing to have an inner surface that is cut from the luminal surface of the tubing. Thewire 200 can be cut from the tubing such that a majority of theoscillating portion 210 is movable independent of oscillatingportions 210 of other wires. Thewire 200, once cut, can be separated from the tubing to form an independently formedwire 200. FIGS. 4 to 9 are two-dimensional depictions of wires of a stent illustrating example placements of wires around a circumference of the stent. The illustrations are provided in two dimensions for discussion and visualization. Referring collectively toFIGS. 4 to 8 , the illustrations depict the placement of fourwires bottom wire 200ais redrawn as a dashedwire 200a′ above thetop wire 200d, and thetop wire 200dis redrawn as a dashedwire 200d′ below thebottom wire 200ato illustrate the placement and connection of the wires shown as the top andbottom wires - The
wires oscillating portion 210 extends across a length of the stent. Inner curved surface of eachoscillating portion 210 of eachwire Wires FIG. 4 is a two-dimensional illustration of four wires placed to define a circumference of a stent like the stents depicted inFIGS. 1 and 2A . Each wire can be recessed circumferentially within undulations of circumferentially adjacent wires such that the circumferential positioning of the undulating wires defines a circumference of the stent. Each wire can be independently formed and at least to some extent, movable compared to every other wire.FIG. 5 is a two-dimensional illustration of four wires placed to define a circumference of a stent like the pattern shown inFIG. 4 . Each wire can be connected to each adjacent wire by a single joint230a,230b,230c,230d,230d′. As shown, a first joint230ccan join afirst wire 200cto asecond wire 200dadjacent to thefirst wire 200c, and the first joint230ccan be the only affixed joint between thefirst wire 200cand thesecond wire 200d. Thefirst wire 200ccan also be joined to athird wire 200bwith a second joint230b. As shown, eachwire Minimal connections - As will be appreciated and understood, each joint can be formed by any conventional means such as welding, brazing, soldering, gluing, tying, etc. Alternatively, or additionally, a stent can be cut from a single piece of tubing such that the
joints wire joint location FIGS. 5 and 6 and described herein to allow thewire FIG. 6 is a two-dimensional illustration of four wires placed to define a circumference of a stent having one joint230a,230b,230c,230d,230d′ connecting each pair of adjacent wires. As will be appreciated and understood, thejoints FIG. 6 illustrates an alternative configuration of the fourwires FIG. 5 and the fourjoints Minimal connections wires FIG. 7 is a two-dimensional illustration of four wires placed to define a circumference of a stent such that the wires are interwoven to define a circumference of the stent. Each wire can cross over and under each neighboring wire. As illustrated, afirst wire 200acan cross under244 a firstneighboring wire 200d′ and over246 thesame wire 200d′ within one period of oscillation of thefirst wire 200a. Thefirst wire 200acan cross under242 a secondneighboring wire 200band over248 the secondneighboring wire 200bwithin the same period of oscillation of thefirst wire 200a. The interwoven structure of thewires wires FIG. 8 is a two-dimensional illustration of four wires placed to define a circumference of a stent such that the wires are interwoven to define a circumference of the stent like the one as illustrated inFIG. 7 . A stent can have both interwovenwires first wire 200cand asecond wire 200bat a cross-over point. Thejoints Minimal connections wires FIG. 9 is a two-dimensional illustration of fourwire segments bends 230, wherein the wire segments are placed to define a circumference of a stent and eachwire segment bend 230. As illustrated, eachbend 230 can be positioned at either a first or second open end of the stent to connect afirst wire 200aand asecond wire 200b. Connected thusly, thewire segments segments bends 230 positioned at each end of the stent. The contiguous wire can includeatraumatic ends 220 positioned at an end of afirst wire segment 200aand an end of alast wire segment 200din the chain of segments. Alternatively (not shown), the stent can be constructed with multiple independent wires consisting of wire segments joined at bends. Wires and wire segments can be otherwise joined or interwoven as described in other examples presented herein or as known in the art.FIGS. 10A to 10E illustrate example waveform patterns or undulating patterns for a wire of a stent that can be used in addition to or in place of other waveforms depicted and described herein. As will be appreciated and understood, and number of waveform patterns can be utilized to achieve a desired flexibility, structural integrity, and conformability for the stent, including those not shown, and as known in the art.FIG. 11 illustrates an example wire weave pattern that could be utilized to form a stent in addition to or in place of other weave patterns depicted and described herein.FIGS. 12A to 12D illustrate example wire segments having a variable width that could be utilized to form a stent in addition to or in place of other wire features depicted and described herein. As illustrated inFIG. 12A , a wire can have an undulating pattern having thicker segments near each end of the wire and thinner segments positioned in the middle of the wire. Thinner central segments can allow the stent to pass more easily through a microcatheter while thicker end segments can improve structural integrity at the stent ends for anchoring within a body lumen. As illustrated inFIG. 12B , a wire can have thin segments near one end and thicker segments at the other end. As illustrated, the segments can become increasingly thicker or thinner from one end of the wire to the other. Stents formed from wire segments with progressively changing segment thickness can be formed to have a tapered structure that can be advantageous for achieving conformity to the walls of a body lumen when implanted in a body lumen that is tapered.- The descriptions contained herein are examples of embodiments of the invention and are not intended in any way to limit the scope of the invention. As described herein, the invention contemplates many variations and modifications of the stent, including alternative shapes for oscillating portions of wires, alternate shapes for atraumatic end segments of wires, alternative means of joining or connecting wires, alternative patterns for interlacing wires to form the stent, forming stents with any number of wires, or utilizing any of numerous materials or manufacturing means for the stent, for example. These modifications would be apparent to those having ordinary skill in the art to which this invention relates and are intended to be within the scope of the claims which follow.
Claims (20)
Priority Applications (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US16/577,567US11590007B2 (en) | 2018-09-20 | 2019-09-20 | Stent with shaped wires |
| US18/111,164US12396870B2 (en) | 2018-09-20 | 2023-02-17 | Stent with shaped wires |
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US201862734128P | 2018-09-20 | 2018-09-20 | |
| US16/577,567US11590007B2 (en) | 2018-09-20 | 2019-09-20 | Stent with shaped wires |
Related Child Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US18/111,164DivisionUS12396870B2 (en) | 2018-09-20 | 2023-02-17 | Stent with shaped wires |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| US20200093617A1true US20200093617A1 (en) | 2020-03-26 |
| US11590007B2 US11590007B2 (en) | 2023-02-28 |
Family
ID=67998194
Family Applications (2)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US16/577,567Active2040-11-26US11590007B2 (en) | 2018-09-20 | 2019-09-20 | Stent with shaped wires |
| US18/111,164ActiveUS12396870B2 (en) | 2018-09-20 | 2023-02-17 | Stent with shaped wires |
Family Applications After (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US18/111,164ActiveUS12396870B2 (en) | 2018-09-20 | 2023-02-17 | Stent with shaped wires |
Country Status (6)
| Country | Link |
|---|---|
| US (2) | US11590007B2 (en) |
| EP (1) | EP3626212A3 (en) |
| JP (2) | JP2020044335A (en) |
| KR (1) | KR20200033757A (en) |
| CN (1) | CN110916861B (en) |
| BR (1) | BR102019019522A2 (en) |
Families Citing this family (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| EP3626212A3 (en)* | 2018-09-20 | 2020-07-22 | DePuy Synthes Products, Inc. | Stent with shaped wires |
Citations (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US4800882A (en)* | 1987-03-13 | 1989-01-31 | Cook Incorporated | Endovascular stent and delivery system |
| US6027526A (en)* | 1996-04-10 | 2000-02-22 | Advanced Cardiovascular Systems, Inc. | Stent having varied amounts of structural strength along its length |
Family Cites Families (151)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US4969458A (en) | 1987-07-06 | 1990-11-13 | Medtronic, Inc. | Intracoronary stent and method of simultaneous angioplasty and stent implant |
| US5370683A (en) | 1992-03-25 | 1994-12-06 | Cook Incorporated | Vascular stent |
| DE4424242A1 (en)* | 1994-07-09 | 1996-01-11 | Ernst Peter Prof Dr M Strecker | Endoprosthesis implantable percutaneously in a patient's body |
| US6331188B1 (en) | 1994-08-31 | 2001-12-18 | Gore Enterprise Holdings, Inc. | Exterior supported self-expanding stent-graft |
| US5707387A (en) | 1996-03-25 | 1998-01-13 | Wijay; Bandula | Flexible stent |
| EP0808614B1 (en)* | 1996-05-23 | 2003-02-26 | Samsung Electronics Co., Ltd. | Flexible self-expandable stent and method for making the same |
| JPH09313617A (en) | 1996-05-30 | 1997-12-09 | Piolax Inc | Stent |
| US5925061A (en) | 1997-01-13 | 1999-07-20 | Gore Enterprise Holdings, Inc. | Low profile vascular stent |
| DE19703482A1 (en) | 1997-01-31 | 1998-08-06 | Ernst Peter Prof Dr M Strecker | Stent |
| US6656218B1 (en)* | 1998-07-24 | 2003-12-02 | Micrus Corporation | Intravascular flow modifier and reinforcement device |
| US20020173839A1 (en)* | 1998-07-24 | 2002-11-21 | Leopold Eric W. | Intravascular flow modifier and reinforcement device with connected segments |
| US6551351B2 (en) | 1999-07-02 | 2003-04-22 | Scimed Life Systems | Spiral wound stent |
| EP1242004B1 (en) | 1999-12-21 | 2004-12-08 | Imperial College of Science, Technology and Medicine | Vascular stents |
| US6391037B1 (en) | 2000-03-02 | 2002-05-21 | Prodesco, Inc. | Bag for use in the intravascular treatment of saccular aneurysms |
| US6632241B1 (en)* | 2000-03-22 | 2003-10-14 | Endovascular Technologies, Inc. | Self-expanding, pseudo-braided intravascular device |
| US8715312B2 (en) | 2001-07-20 | 2014-05-06 | Microvention, Inc. | Aneurysm treatment device and method of use |
| US8252040B2 (en) | 2001-07-20 | 2012-08-28 | Microvention, Inc. | Aneurysm treatment device and method of use |
| US20030171801A1 (en)* | 2002-03-06 | 2003-09-11 | Brian Bates | Partially covered intraluminal support device |
| US9039755B2 (en) | 2003-06-27 | 2015-05-26 | Medinol Ltd. | Helical hybrid stent |
| US7371228B2 (en) | 2003-09-19 | 2008-05-13 | Medtronic Vascular, Inc. | Delivery of therapeutics to treat aneurysms |
| US9308382B2 (en) | 2004-06-10 | 2016-04-12 | Medtronic Urinary Solutions, Inc. | Implantable pulse generator systems and methods for providing functional and/or therapeutic stimulation of muscles and/or nerves and/or central nervous system tissue |
| US20060074480A1 (en) | 2004-09-01 | 2006-04-06 | Pst, Llc | Stent and method for manufacturing the stent |
| US9655633B2 (en) | 2004-09-10 | 2017-05-23 | Penumbra, Inc. | System and method for treating ischemic stroke |
| WO2006052322A2 (en) | 2004-09-22 | 2006-05-18 | Guterman Lee R | Cranial aneurysm treatment arrangement |
| US20060089637A1 (en) | 2004-10-14 | 2006-04-27 | Werneth Randell L | Ablation catheter |
| US8562672B2 (en) | 2004-11-19 | 2013-10-22 | Medtronic, Inc. | Apparatus for treatment of cardiac valves and method of its manufacture |
| US8292944B2 (en)* | 2004-12-17 | 2012-10-23 | Reva Medical, Inc. | Slide-and-lock stent |
| US9636115B2 (en) | 2005-06-14 | 2017-05-02 | Stryker Corporation | Vaso-occlusive delivery device with kink resistant, flexible distal end |
| EP2759276A1 (en) | 2005-06-20 | 2014-07-30 | Medtronic Ablation Frontiers LLC | Ablation catheter |
| US8066036B2 (en) | 2005-11-17 | 2011-11-29 | Microvention, Inc. | Three-dimensional complex coil |
| US20070208409A1 (en) | 2006-03-01 | 2007-09-06 | Boston Scientific Scimed, Inc. | Flexible stent-graft devices and methods of producing the same |
| US9757260B2 (en) | 2006-03-30 | 2017-09-12 | Medtronic Vascular, Inc. | Prosthesis with guide lumen |
| US9615832B2 (en) | 2006-04-07 | 2017-04-11 | Penumbra, Inc. | Aneurysm occlusion system and method |
| CA2655026C (en) | 2006-06-15 | 2016-08-02 | Microvention, Inc. | Embolization device constructed from expansible polymer |
| US9622888B2 (en) | 2006-11-16 | 2017-04-18 | W. L. Gore & Associates, Inc. | Stent having flexibly connected adjacent stent elements |
| US20080281350A1 (en) | 2006-12-13 | 2008-11-13 | Biomerix Corporation | Aneurysm Occlusion Devices |
| US8623070B2 (en) | 2007-03-08 | 2014-01-07 | Thomas O. Bales | Tapered helical stent and method for manufacturing the stent |
| EP2211773A4 (en) | 2007-11-30 | 2015-07-29 | Reva Medical Inc | Axially-radially nested expandable device |
| WO2009077845A2 (en)* | 2007-12-14 | 2009-06-25 | Welldone Weartec N.V. | Support implant, in particular a stent, and implantation catheter for the support implant |
| WO2009086208A2 (en) | 2007-12-21 | 2009-07-09 | Microvention, Inc. | Hydrogel filaments for biomedical uses |
| US8974518B2 (en) | 2008-03-25 | 2015-03-10 | Medtronic Vascular, Inc. | Eversible branch stent-graft and deployment method |
| EP2192947A1 (en) | 2008-04-30 | 2010-06-09 | Medtronic, Inc. | Techniques for placing medical leads for electrical stimulation of nerve tissue |
| US8070694B2 (en) | 2008-07-14 | 2011-12-06 | Medtronic Vascular, Inc. | Fiber based medical devices and aspiration catheters |
| US8333796B2 (en) | 2008-07-15 | 2012-12-18 | Penumbra, Inc. | Embolic coil implant system and implantation method |
| US9232992B2 (en) | 2008-07-24 | 2016-01-12 | Aga Medical Corporation | Multi-layered medical device for treating a target site and associated method |
| US8721714B2 (en) | 2008-09-17 | 2014-05-13 | Medtronic Corevalve Llc | Delivery system for deployment of medical devices |
| EP2331014B1 (en) | 2008-10-10 | 2017-08-09 | Reva Medical, Inc. | Expandable slide and lock stent |
| US9717500B2 (en) | 2009-04-15 | 2017-08-01 | Microvention, Inc. | Implant delivery system |
| US8758423B2 (en) | 2009-06-18 | 2014-06-24 | Graftcraft I Goteborg Ab | Device and method for treating ruptured aneurysms |
| US8425586B2 (en)* | 2009-09-02 | 2013-04-23 | Novostent Corporation | Vascular prosthesis with stress relief slots |
| US8911487B2 (en) | 2009-09-22 | 2014-12-16 | Penumbra, Inc. | Manual actuation system for deployment of implant |
| US8454535B2 (en) | 2010-01-29 | 2013-06-04 | Cordis Corporation | Highly flexible tubular device for medical use |
| JP5809237B2 (en) | 2010-04-10 | 2015-11-10 | レヴァ メディカル、 インコーポレイテッドReva Medical, Inc. | Expandable slide lock stent |
| AU2011240927B2 (en) | 2010-04-14 | 2015-07-16 | Microvention, Inc. | Implant delivery device |
| US8764811B2 (en) | 2010-04-20 | 2014-07-01 | Medtronic Vascular, Inc. | Controlled tip release stent graft delivery system and method |
| US8876878B2 (en) | 2010-07-23 | 2014-11-04 | Medtronic, Inc. | Attachment mechanism for stent release |
| US8616040B2 (en) | 2010-09-17 | 2013-12-31 | Medtronic Vascular, Inc. | Method of forming a drug-eluting medical device |
| US20130226282A1 (en)* | 2010-10-29 | 2013-08-29 | Medisourceplus Co., Ltd. | Stent wires, and method for manufacturing such stent wires and stents |
| WO2012088162A1 (en) | 2010-12-20 | 2012-06-28 | Microvention, Inc. | Polymer stents and methods of manufacture |
| US9839540B2 (en) | 2011-01-14 | 2017-12-12 | W. L. Gore & Associates, Inc. | Stent |
| ES2807348T3 (en) | 2011-03-07 | 2021-02-22 | Stryker Corp | Balloon catheter and support shaft for it |
| US20120283768A1 (en) | 2011-05-05 | 2012-11-08 | Sequent Medical Inc. | Method and apparatus for the treatment of large and giant vascular defects |
| US9486604B2 (en) | 2011-05-12 | 2016-11-08 | Medtronic, Inc. | Packaging and preparation tray for a delivery system |
| WO2012158668A1 (en) | 2011-05-17 | 2012-11-22 | Stryker Corporation | Method of fabricating an implantable medical device that includes one or more thin film polymer support layers |
| WO2012166467A1 (en) | 2011-05-27 | 2012-12-06 | Stryker Corporation | Assembly for percutaneously inserting an implantable medical device, steering the device to a target location and deploying the device |
| US9750565B2 (en) | 2011-09-30 | 2017-09-05 | Medtronic Advanced Energy Llc | Electrosurgical balloons |
| CA2867181C (en) | 2012-03-16 | 2020-08-11 | Microvention, Inc. | Stent and stent delivery device |
| US9717421B2 (en) | 2012-03-26 | 2017-08-01 | Medtronic, Inc. | Implantable medical device delivery catheter with tether |
| US9833625B2 (en) | 2012-03-26 | 2017-12-05 | Medtronic, Inc. | Implantable medical device delivery with inner and outer sheaths |
| US9242290B2 (en) | 2012-04-03 | 2016-01-26 | Medtronic Vascular, Inc. | Method and apparatus for creating formed elements used to make wound stents |
| US9549832B2 (en) | 2012-04-26 | 2017-01-24 | Medtronic Vascular, Inc. | Apparatus and methods for filling a drug eluting medical device via capillary action |
| US9700399B2 (en) | 2012-04-26 | 2017-07-11 | Medtronic Vascular, Inc. | Stopper to prevent graft material slippage in a closed web stent-graft |
| US9149190B2 (en) | 2012-07-17 | 2015-10-06 | Stryker Corporation | Notification system of deviation from predefined conditions |
| EP2882350B1 (en) | 2012-08-13 | 2019-09-25 | MicroVention, Inc. | Shaped removal device |
| US9504476B2 (en) | 2012-10-01 | 2016-11-29 | Microvention, Inc. | Catheter markers |
| EP2906254B1 (en) | 2012-10-15 | 2020-01-08 | Microvention, Inc. | Polymeric treatment compositions |
| US9717592B2 (en)* | 2012-10-29 | 2017-08-01 | Aneumed, Inc. | Personalized aortic valve prosthesis |
| US10327781B2 (en) | 2012-11-13 | 2019-06-25 | Covidien Lp | Occlusive devices |
| US9539022B2 (en) | 2012-11-28 | 2017-01-10 | Microvention, Inc. | Matter conveyance system |
| WO2014089392A1 (en) | 2012-12-07 | 2014-06-12 | Medtronic, Inc. | Minimally invasive implantable neurostimulation system |
| US10342546B2 (en) | 2013-01-14 | 2019-07-09 | Microvention, Inc. | Occlusive device |
| US9539382B2 (en) | 2013-03-12 | 2017-01-10 | Medtronic, Inc. | Stepped catheters with flow restrictors and infusion systems using the same |
| WO2014150824A1 (en) | 2013-03-14 | 2014-09-25 | Stryker Corporation | Vaso-occlusive device delivery system |
| US9408732B2 (en) | 2013-03-14 | 2016-08-09 | Reva Medical, Inc. | Reduced-profile slide and lock stent |
| EP2967573B1 (en) | 2013-03-14 | 2021-04-21 | Stryker Corporation | Vaso-occlusive device delivery system |
| CN105228534B (en) | 2013-03-14 | 2018-03-30 | 斯瑞克公司 | Vaso-Occlusive Device Delivery System |
| US8690907B1 (en) | 2013-03-15 | 2014-04-08 | Insera Therapeutics, Inc. | Vascular treatment methods |
| WO2014151123A1 (en) | 2013-03-15 | 2014-09-25 | Microvention, Inc. | Multi-component obstruction removal system and method |
| US9398966B2 (en) | 2013-03-15 | 2016-07-26 | Medtronic Vascular, Inc. | Welded stent and stent delivery system |
| BR112015023602A2 (en) | 2013-03-15 | 2017-07-18 | Microvention Inc | embolic protection device |
| US9662425B2 (en) | 2013-04-22 | 2017-05-30 | Stryker European Holdings I, Llc | Method for drug loading hydroxyapatite coated implant surfaces |
| US9375311B2 (en) | 2013-05-03 | 2016-06-28 | Medtronic, Inc. | Prosthetic valves and associated appartuses, systems and methods |
| US9445928B2 (en) | 2013-05-30 | 2016-09-20 | Medtronic Vascular, Inc. | Delivery system having a single handed deployment handle for a retractable outer sheath |
| US9675782B2 (en) | 2013-10-10 | 2017-06-13 | Medtronic Vascular, Inc. | Catheter pull wire actuation mechanism |
| US9955978B2 (en) | 2013-10-25 | 2018-05-01 | Medtronic Vascular, Inc. | Tissue compression device with multi-chamber bladder |
| EP3082939B1 (en) | 2013-12-20 | 2020-12-02 | MicroVention, Inc. | Delivery adapter |
| KR102211335B1 (en) | 2013-12-20 | 2021-02-03 | 마이크로벤션, 인코포레이티드 | Device delivery system |
| WO2015157181A1 (en) | 2014-04-08 | 2015-10-15 | Stryker Corporation | Implant delivery system |
| WO2015167997A1 (en) | 2014-04-30 | 2015-11-05 | Stryker Corporation | Implant delivery system and method of use |
| US9060777B1 (en) | 2014-05-28 | 2015-06-23 | Tw Medical Technologies, Llc | Vaso-occlusive devices and methods of use |
| US9668898B2 (en) | 2014-07-24 | 2017-06-06 | Medtronic Vascular, Inc. | Stent delivery system having dynamic deployment and methods of manufacturing same |
| US9770577B2 (en) | 2014-09-15 | 2017-09-26 | Medtronic Xomed, Inc. | Pressure relief for a catheter balloon device |
| US9579484B2 (en) | 2014-09-19 | 2017-02-28 | Medtronic Vascular, Inc. | Sterile molded dispenser |
| JP6177259B2 (en)* | 2015-01-13 | 2017-08-09 | 日本ライフライン株式会社 | Stents and stent grafts |
| US9692557B2 (en) | 2015-02-04 | 2017-06-27 | Stryker European Holdings I, Llc | Apparatus and methods for administering treatment within a bodily duct of a patient |
| US10307168B2 (en) | 2015-08-07 | 2019-06-04 | Terumo Corporation | Complex coil and manufacturing techniques |
| US10154905B2 (en) | 2015-08-07 | 2018-12-18 | Medtronic Vascular, Inc. | System and method for deflecting a delivery catheter |
| US10492938B2 (en) | 2015-08-11 | 2019-12-03 | Terumo Corporation | System and method for implant delivery |
| JP6854282B2 (en) | 2015-09-18 | 2021-04-07 | テルモ株式会社 | Pressable implant delivery system |
| JP6816126B2 (en) | 2015-09-18 | 2021-01-20 | マイクロベンション インコーポレイテッドMicrovention, Inc. | Releasable delivery system |
| EP3349669B1 (en) | 2015-09-18 | 2020-10-21 | Terumo Corporation | Vessel prosthesis |
| CN113952095B (en) | 2015-09-18 | 2025-06-06 | 微仙美国有限公司 | Implant retention, detachment and delivery systems |
| CN108024821B (en) | 2015-09-21 | 2020-10-30 | 斯瑞克公司 | Embolectomy device |
| JP6591664B2 (en) | 2015-09-21 | 2019-10-16 | ストライカー コーポレイションStryker Corporation | Embolization removal device |
| US10172632B2 (en) | 2015-09-22 | 2019-01-08 | Medtronic Vascular, Inc. | Occlusion bypassing apparatus with a re-entry needle and a stabilization tube |
| US10327791B2 (en) | 2015-10-07 | 2019-06-25 | Medtronic Vascular, Inc. | Occlusion bypassing apparatus with a re-entry needle and a distal stabilization balloon |
| WO2017062383A1 (en) | 2015-10-07 | 2017-04-13 | Stryker Corporation | Multiple barrel clot removal devices |
| US10786302B2 (en) | 2015-10-09 | 2020-09-29 | Medtronic, Inc. | Method for closure and ablation of atrial appendage |
| US10271873B2 (en) | 2015-10-26 | 2019-04-30 | Medtronic Vascular, Inc. | Sheathless guide catheter assembly |
| WO2017087816A1 (en) | 2015-11-19 | 2017-05-26 | Penumbra, Inc. | Systems and methods for treatment of stroke |
| US10631946B2 (en) | 2015-11-30 | 2020-04-28 | Penumbra, Inc. | System for endoscopic intracranial procedures |
| EP3386580B1 (en) | 2015-12-09 | 2023-11-01 | Medtronic Vascular Inc. | Catheter with a lumen shaped as an identification symbol |
| US10500046B2 (en) | 2015-12-14 | 2019-12-10 | Medtronic, Inc. | Delivery system having retractable wires as a coupling mechanism and a deployment mechanism for a self-expanding prosthesis |
| US10159568B2 (en) | 2015-12-14 | 2018-12-25 | Medtronic, Inc. | Delivery system having retractable wires as a coupling mechanism and a deployment mechanism for a self-expanding prosthesis |
| WO2017117284A1 (en) | 2015-12-30 | 2017-07-06 | Stryker Corporation | Embolic devices and methods of manufacturing same |
| US20170189033A1 (en) | 2016-01-06 | 2017-07-06 | Microvention, Inc. | Occlusive Embolic Coil |
| US10070950B2 (en) | 2016-02-09 | 2018-09-11 | Medtronic Vascular, Inc. | Endoluminal prosthetic assemblies, and associated systems and methods for percutaneous repair of a vascular tissue defect |
| KR102837372B1 (en) | 2016-02-10 | 2025-07-24 | 마이크로벤션, 인코포레이티드 | Intravascular treatment site access |
| AU2017218115B2 (en) | 2016-02-10 | 2020-03-05 | Microvention, Inc. | Devices for vascular occlusion |
| US10188500B2 (en) | 2016-02-12 | 2019-01-29 | Medtronic Vascular, Inc. | Stent graft with external scaffolding and method |
| CN114432008A (en) | 2016-03-31 | 2022-05-06 | 美敦力瓦斯科尔勒公司 | Expandable introducer sheath with steering mechanism |
| US20170281331A1 (en) | 2016-03-31 | 2017-10-05 | Medtronic Vascular, Inc. | Endoluminal prosthetic devices having fluid-absorbable compositions for repair of a vascular tissue defect |
| US10695542B2 (en) | 2016-04-04 | 2020-06-30 | Medtronic Vascular, Inc. | Drug coated balloon |
| US10252024B2 (en) | 2016-04-05 | 2019-04-09 | Stryker Corporation | Medical devices and methods of manufacturing same |
| US10441407B2 (en) | 2016-04-12 | 2019-10-15 | Medtronic Vascular, Inc. | Gutter filling stent-graft and method |
| US9987122B2 (en) | 2016-04-13 | 2018-06-05 | Medtronic Vascular, Inc. | Iliac branch device and method |
| US10010403B2 (en) | 2016-04-18 | 2018-07-03 | Medtronic Vascular, Inc. | Stent-graft prosthesis and method of manufacture |
| US20170304097A1 (en) | 2016-04-21 | 2017-10-26 | Medtronic Vascular, Inc. | Stent-graft delivery system having an inner shaft component with a loading pad or covering on a distal segment thereof for stent retention |
| US10940294B2 (en) | 2016-04-25 | 2021-03-09 | Medtronic Vascular, Inc. | Balloon catheter including a drug delivery sheath |
| US10517711B2 (en) | 2016-04-25 | 2019-12-31 | Medtronic Vascular, Inc. | Dissection prosthesis system and method |
| EP3590446B1 (en) | 2016-04-25 | 2021-01-06 | Stryker Corporation | Anti-jamming and macerating thrombectomy apparatuses |
| EP3448276B1 (en) | 2016-04-25 | 2020-03-04 | Stryker Corporation | Clot-engulfing mechanical thrombectomy apparatuses |
| WO2017189591A1 (en) | 2016-04-25 | 2017-11-02 | Stryker Corporation | Inverting mechanical thrombectomy apparatuses and methods of use in the vasculature |
| JP7222881B2 (en)* | 2016-04-25 | 2023-02-15 | ソリナス メディカル インコーポレイテッド | Self-sealing tubular grafts, patches, methods of making and using same |
| US10191615B2 (en) | 2016-04-28 | 2019-01-29 | Medtronic Navigation, Inc. | Method and apparatus for image-based navigation |
| US11147952B2 (en) | 2016-04-28 | 2021-10-19 | Medtronic Vascular, Inc. | Drug coated inflatable balloon having a thermal dependent release layer |
| US10406011B2 (en) | 2016-04-28 | 2019-09-10 | Medtronic Vascular, Inc. | Implantable medical device delivery system |
| US10292844B2 (en) | 2016-05-17 | 2019-05-21 | Medtronic Vascular, Inc. | Method for compressing a stented prosthesis |
| US10786659B2 (en) | 2016-06-01 | 2020-09-29 | Microvention, Inc. | Reinforced balloon catheter |
| CN109561903B (en) | 2016-06-03 | 2021-07-27 | 斯瑞克公司 | Flip thrombectomy device |
| EP3626212A3 (en)* | 2018-09-20 | 2020-07-22 | DePuy Synthes Products, Inc. | Stent with shaped wires |
- 2019
- 2019-09-19EPEP19198448.3Apatent/EP3626212A3/enactivePending
- 2019-09-19KRKR1020190115567Apatent/KR20200033757A/ennot_activeCeased
- 2019-09-19JPJP2019170161Apatent/JP2020044335A/enactivePending
- 2019-09-19BRBR102019019522Apatent/BR102019019522A2/ennot_activeIP Right Cessation
- 2019-09-20CNCN201910891829.8Apatent/CN110916861B/enactiveActive
- 2019-09-20USUS16/577,567patent/US11590007B2/enactiveActive
- 2023
- 2023-02-17USUS18/111,164patent/US12396870B2/enactiveActive
- 2023-11-13JPJP2023193007Apatent/JP7640056B2/enactiveActive
Patent Citations (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US4800882A (en)* | 1987-03-13 | 1989-01-31 | Cook Incorporated | Endovascular stent and delivery system |
| US6027526A (en)* | 1996-04-10 | 2000-02-22 | Advanced Cardiovascular Systems, Inc. | Stent having varied amounts of structural strength along its length |
Also Published As
| Publication number | Publication date |
|---|---|
| JP2024009069A (en) | 2024-01-19 |
| KR20200033757A (en) | 2020-03-30 |
| EP3626212A3 (en) | 2020-07-22 |
| US12396870B2 (en) | 2025-08-26 |
| JP2020044335A (en) | 2020-03-26 |
| EP3626212A2 (en) | 2020-03-25 |
| CN110916861A (en) | 2020-03-27 |
| CN110916861B (en) | 2024-03-22 |
| US11590007B2 (en) | 2023-02-28 |
| US20230190498A1 (en) | 2023-06-22 |
| BR102019019522A2 (en) | 2020-04-07 |
| JP7640056B2 (en) | 2025-03-05 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| AU776110B2 (en) | Multi-section filamentary endoluminal stent | |
| JP4801838B2 (en) | Intraluminal stent | |
| US8038707B2 (en) | Helical stent having improved flexibility and expandability | |
| EP1871292B1 (en) | Flexible stent | |
| CN103784222B (en) | A kind of self-expandable stent | |
| JP2022180588A (en) | Stent device having reduced foreshortening and recoil and method of making the same | |
| US12109133B2 (en) | Weaving method for nasal sinus stent and stent obtained thereof | |
| KR20120018772A (en) | Flexible devices | |
| US12310867B2 (en) | Stent with longitudinal variable width struts | |
| US12396870B2 (en) | Stent with shaped wires | |
| JP2021522020A (en) | Expansion members and related systems and methods for implantable devices | |
| KR101602400B1 (en) | Method for manufacturing stent | |
| EP3313329B1 (en) | Endoluminal prosthesis systems | |
| JP4159962B2 (en) | Stent and stent graft | |
| JP2003334255A (en) | Stents and stent grafts |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| AS | Assignment | Owner name:DEPUY SYNTHES PRODUCTS, INC., MASSACHUSETTS Free format text:ASSIGNMENT OF ASSIGNORS INTEREST;ASSIGNOR:GOROCHOW, LACEY;REEL/FRAME:050446/0162 Effective date:20180920 | |
| FEPP | Fee payment procedure | Free format text:ENTITY STATUS SET TO UNDISCOUNTED (ORIGINAL EVENT CODE: BIG.); ENTITY STATUS OF PATENT OWNER: LARGE ENTITY | |
| STPP | Information on status: patent application and granting procedure in general | Free format text:DOCKETED NEW CASE - READY FOR EXAMINATION | |
| STPP | Information on status: patent application and granting procedure in general | Free format text:NON FINAL ACTION MAILED | |
| STPP | Information on status: patent application and granting procedure in general | Free format text:RESPONSE TO NON-FINAL OFFICE ACTION ENTERED AND FORWARDED TO EXAMINER | |
| STPP | Information on status: patent application and granting procedure in general | Free format text:NON FINAL ACTION MAILED | |
| STPP | Information on status: patent application and granting procedure in general | Free format text:RESPONSE TO NON-FINAL OFFICE ACTION ENTERED AND FORWARDED TO EXAMINER | |
| STPP | Information on status: patent application and granting procedure in general | Free format text:FINAL REJECTION MAILED | |
| STPP | Information on status: patent application and granting procedure in general | Free format text:RESPONSE AFTER FINAL ACTION FORWARDED TO EXAMINER | |
| STPP | Information on status: patent application and granting procedure in general | Free format text:ADVISORY ACTION MAILED | |
| STPP | Information on status: patent application and granting procedure in general | Free format text:DOCKETED NEW CASE - READY FOR EXAMINATION | |
| STPP | Information on status: patent application and granting procedure in general | Free format text:AWAITING TC RESP., ISSUE FEE NOT PAID | |
| STPP | Information on status: patent application and granting procedure in general | Free format text:NOTICE OF ALLOWANCE MAILED -- APPLICATION RECEIVED IN OFFICE OF PUBLICATIONS | |
| STCF | Information on status: patent grant | Free format text:PATENTED CASE |