US20160317711A1 - Reinforced biologic material - Google Patents
Reinforced biologic materialDownload PDFInfo
- Publication number
- US20160317711A1 US20160317711A1US15/210,309US201615210309AUS2016317711A1US 20160317711 A1US20160317711 A1US 20160317711A1US 201615210309 AUS201615210309 AUS 201615210309AUS 2016317711 A1US2016317711 A1US 2016317711A1
- Authority
- US
- United States
- Prior art keywords
- biologic
- elements
- elongate
- elongate non
- component
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Abandoned
Links
Images
Classifications
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61L—METHODS OR APPARATUS FOR STERILISING MATERIALS OR OBJECTS IN GENERAL; DISINFECTION, STERILISATION OR DEODORISATION OF AIR; CHEMICAL ASPECTS OF BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES; MATERIALS FOR BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES
- A61L27/00—Materials for grafts or prostheses or for coating grafts or prostheses
- A61L27/40—Composite materials, i.e. containing one material dispersed in a matrix of the same or different material
- A61L27/44—Composite materials, i.e. containing one material dispersed in a matrix of the same or different material having a macromolecular matrix
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61L—METHODS OR APPARATUS FOR STERILISING MATERIALS OR OBJECTS IN GENERAL; DISINFECTION, STERILISATION OR DEODORISATION OF AIR; CHEMICAL ASPECTS OF BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES; MATERIALS FOR BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES
- A61L27/00—Materials for grafts or prostheses or for coating grafts or prostheses
- A61L27/36—Materials for grafts or prostheses or for coating grafts or prostheses containing ingredients of undetermined constitution or reaction products thereof, e.g. transplant tissue, natural bone, extracellular matrix
- A61L27/3604—Materials for grafts or prostheses or for coating grafts or prostheses containing ingredients of undetermined constitution or reaction products thereof, e.g. transplant tissue, natural bone, extracellular matrix characterised by the human or animal origin of the biological material, e.g. hair, fascia, fish scales, silk, shellac, pericardium, pleura, renal tissue, amniotic membrane, parenchymal tissue, fetal tissue, muscle tissue, fat tissue, enamel
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61L—METHODS OR APPARATUS FOR STERILISING MATERIALS OR OBJECTS IN GENERAL; DISINFECTION, STERILISATION OR DEODORISATION OF AIR; CHEMICAL ASPECTS OF BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES; MATERIALS FOR BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES
- A61L27/00—Materials for grafts or prostheses or for coating grafts or prostheses
- A61L27/36—Materials for grafts or prostheses or for coating grafts or prostheses containing ingredients of undetermined constitution or reaction products thereof, e.g. transplant tissue, natural bone, extracellular matrix
- A61L27/3683—Materials for grafts or prostheses or for coating grafts or prostheses containing ingredients of undetermined constitution or reaction products thereof, e.g. transplant tissue, natural bone, extracellular matrix subjected to a specific treatment prior to implantation, e.g. decellularising, demineralising, grinding, cellular disruption/non-collagenous protein removal, anti-calcification, crosslinking, supercritical fluid extraction, enzyme treatment
- A61L27/3691—Materials for grafts or prostheses or for coating grafts or prostheses containing ingredients of undetermined constitution or reaction products thereof, e.g. transplant tissue, natural bone, extracellular matrix subjected to a specific treatment prior to implantation, e.g. decellularising, demineralising, grinding, cellular disruption/non-collagenous protein removal, anti-calcification, crosslinking, supercritical fluid extraction, enzyme treatment characterised by physical conditions of the treatment, e.g. applying a compressive force to the composition, pressure cycles, ultrasonic/sonication or microwave treatment, lyophilisation
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61L—METHODS OR APPARATUS FOR STERILISING MATERIALS OR OBJECTS IN GENERAL; DISINFECTION, STERILISATION OR DEODORISATION OF AIR; CHEMICAL ASPECTS OF BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES; MATERIALS FOR BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES
- A61L27/00—Materials for grafts or prostheses or for coating grafts or prostheses
- A61L27/40—Composite materials, i.e. containing one material dispersed in a matrix of the same or different material
- A61L27/42—Composite materials, i.e. containing one material dispersed in a matrix of the same or different material having an inorganic matrix
- A61L27/427—Composite materials, i.e. containing one material dispersed in a matrix of the same or different material having an inorganic matrix of other specific inorganic materials not covered by A61L27/422 or A61L27/425
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61L—METHODS OR APPARATUS FOR STERILISING MATERIALS OR OBJECTS IN GENERAL; DISINFECTION, STERILISATION OR DEODORISATION OF AIR; CHEMICAL ASPECTS OF BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES; MATERIALS FOR BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES
- A61L27/00—Materials for grafts or prostheses or for coating grafts or prostheses
- A61L27/40—Composite materials, i.e. containing one material dispersed in a matrix of the same or different material
- A61L27/44—Composite materials, i.e. containing one material dispersed in a matrix of the same or different material having a macromolecular matrix
- A61L27/446—Composite materials, i.e. containing one material dispersed in a matrix of the same or different material having a macromolecular matrix with other specific inorganic fillers other than those covered by A61L27/443 or A61L27/46
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61L—METHODS OR APPARATUS FOR STERILISING MATERIALS OR OBJECTS IN GENERAL; DISINFECTION, STERILISATION OR DEODORISATION OF AIR; CHEMICAL ASPECTS OF BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES; MATERIALS FOR BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES
- A61L27/00—Materials for grafts or prostheses or for coating grafts or prostheses
- A61L27/40—Composite materials, i.e. containing one material dispersed in a matrix of the same or different material
- A61L27/44—Composite materials, i.e. containing one material dispersed in a matrix of the same or different material having a macromolecular matrix
- A61L27/48—Composite materials, i.e. containing one material dispersed in a matrix of the same or different material having a macromolecular matrix with macromolecular fillers
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61L—METHODS OR APPARATUS FOR STERILISING MATERIALS OR OBJECTS IN GENERAL; DISINFECTION, STERILISATION OR DEODORISATION OF AIR; CHEMICAL ASPECTS OF BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES; MATERIALS FOR BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES
- A61L27/00—Materials for grafts or prostheses or for coating grafts or prostheses
- A61L27/50—Materials characterised by their function or physical properties, e.g. injectable or lubricating compositions, shape-memory materials, surface modified materials
- B—PERFORMING OPERATIONS; TRANSPORTING
- B29—WORKING OF PLASTICS; WORKING OF SUBSTANCES IN A PLASTIC STATE IN GENERAL
- B29C—SHAPING OR JOINING OF PLASTICS; SHAPING OF MATERIAL IN A PLASTIC STATE, NOT OTHERWISE PROVIDED FOR; AFTER-TREATMENT OF THE SHAPED PRODUCTS, e.g. REPAIRING
- B29C63/00—Lining or sheathing, i.e. applying preformed layers or sheathings of plastics; Apparatus therefor
- B29C63/02—Lining or sheathing, i.e. applying preformed layers or sheathings of plastics; Apparatus therefor using sheet or web-like material
- B29C63/04—Lining or sheathing, i.e. applying preformed layers or sheathings of plastics; Apparatus therefor using sheet or web-like material by folding, winding, bending or the like
- B29C63/06—Lining or sheathing, i.e. applying preformed layers or sheathings of plastics; Apparatus therefor using sheet or web-like material by folding, winding, bending or the like around tubular articles
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F2/00—Filters implantable into blood vessels; Prostheses, i.e. artificial substitutes or replacements for parts of the body; Appliances for connecting them with the body; Devices providing patency to, or preventing collapsing of, tubular structures of the body, e.g. stents
- A61F2/02—Prostheses implantable into the body
- A61F2/08—Muscles; Tendons; Ligaments
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61L—METHODS OR APPARATUS FOR STERILISING MATERIALS OR OBJECTS IN GENERAL; DISINFECTION, STERILISATION OR DEODORISATION OF AIR; CHEMICAL ASPECTS OF BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES; MATERIALS FOR BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES
- A61L2430/00—Materials or treatment for tissue regeneration
- A61L2430/10—Materials or treatment for tissue regeneration for reconstruction of tendons or ligaments
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61L—METHODS OR APPARATUS FOR STERILISING MATERIALS OR OBJECTS IN GENERAL; DISINFECTION, STERILISATION OR DEODORISATION OF AIR; CHEMICAL ASPECTS OF BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES; MATERIALS FOR BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES
- A61L2430/00—Materials or treatment for tissue regeneration
- A61L2430/34—Materials or treatment for tissue regeneration for soft tissue reconstruction
- B—PERFORMING OPERATIONS; TRANSPORTING
- B29—WORKING OF PLASTICS; WORKING OF SUBSTANCES IN A PLASTIC STATE IN GENERAL
- B29C—SHAPING OR JOINING OF PLASTICS; SHAPING OF MATERIAL IN A PLASTIC STATE, NOT OTHERWISE PROVIDED FOR; AFTER-TREATMENT OF THE SHAPED PRODUCTS, e.g. REPAIRING
- B29C53/00—Shaping by bending, folding, twisting, straightening or flattening; Apparatus therefor
- B29C53/80—Component parts, details or accessories; Auxiliary operations
- B29C53/8008—Component parts, details or accessories; Auxiliary operations specially adapted for winding and joining
- B29C53/8016—Storing, feeding or applying winding materials, e.g. reels, thread guides, tensioners
- B29C2053/8025—Storing, feeding or applying winding materials, e.g. reels, thread guides, tensioners tensioning
- B—PERFORMING OPERATIONS; TRANSPORTING
- B29—WORKING OF PLASTICS; WORKING OF SUBSTANCES IN A PLASTIC STATE IN GENERAL
- B29C—SHAPING OR JOINING OF PLASTICS; SHAPING OF MATERIAL IN A PLASTIC STATE, NOT OTHERWISE PROVIDED FOR; AFTER-TREATMENT OF THE SHAPED PRODUCTS, e.g. REPAIRING
- B29C63/00—Lining or sheathing, i.e. applying preformed layers or sheathings of plastics; Apparatus therefor
- B29C63/26—Lining or sheathing of internal surfaces
- B29C63/34—Lining or sheathing of internal surfaces using tubular layers or sheathings
- B29C63/341—Lining or sheathing of internal surfaces using tubular layers or sheathings pressed against the wall by mechanical means
- B—PERFORMING OPERATIONS; TRANSPORTING
- B29—WORKING OF PLASTICS; WORKING OF SUBSTANCES IN A PLASTIC STATE IN GENERAL
- B29K—INDEXING SCHEME ASSOCIATED WITH SUBCLASSES B29B, B29C OR B29D, RELATING TO MOULDING MATERIALS OR TO MATERIALS FOR MOULDS, REINFORCEMENTS, FILLERS OR PREFORMED PARTS, e.g. INSERTS
- B29K2305/00—Use of metals, their alloys or their compounds, as reinforcement
- B—PERFORMING OPERATIONS; TRANSPORTING
- B29—WORKING OF PLASTICS; WORKING OF SUBSTANCES IN A PLASTIC STATE IN GENERAL
- B29K—INDEXING SCHEME ASSOCIATED WITH SUBCLASSES B29B, B29C OR B29D, RELATING TO MOULDING MATERIALS OR TO MATERIALS FOR MOULDS, REINFORCEMENTS, FILLERS OR PREFORMED PARTS, e.g. INSERTS
- B29K2905/00—Use of metals, their alloys or their compounds, as mould material
- B29K2905/08—Transition metals
- B29K2905/14—Noble metals, e.g. silver, gold or platinum
- B—PERFORMING OPERATIONS; TRANSPORTING
- B29—WORKING OF PLASTICS; WORKING OF SUBSTANCES IN A PLASTIC STATE IN GENERAL
- B29K—INDEXING SCHEME ASSOCIATED WITH SUBCLASSES B29B, B29C OR B29D, RELATING TO MOULDING MATERIALS OR TO MATERIALS FOR MOULDS, REINFORCEMENTS, FILLERS OR PREFORMED PARTS, e.g. INSERTS
- B29K2995/00—Properties of moulding materials, reinforcements, fillers, preformed parts or moulds
- B29K2995/0037—Other properties
- B29K2995/0059—Degradable
- B29K2995/006—Bio-degradable, e.g. bioabsorbable, bioresorbable or bioerodible
- B—PERFORMING OPERATIONS; TRANSPORTING
- B29—WORKING OF PLASTICS; WORKING OF SUBSTANCES IN A PLASTIC STATE IN GENERAL
- B29L—INDEXING SCHEME ASSOCIATED WITH SUBCLASS B29C, RELATING TO PARTICULAR ARTICLES
- B29L2031/00—Other particular articles
- B29L2031/753—Medical equipment; Accessories therefor
- B—PERFORMING OPERATIONS; TRANSPORTING
- B29—WORKING OF PLASTICS; WORKING OF SUBSTANCES IN A PLASTIC STATE IN GENERAL
- B29L—INDEXING SCHEME ASSOCIATED WITH SUBCLASS B29C, RELATING TO PARTICULAR ARTICLES
- B29L2031/00—Other particular articles
- B29L2031/753—Medical equipment; Accessories therefor
- B29L2031/7532—Artificial members, protheses
- Y—GENERAL TAGGING OF NEW TECHNOLOGICAL DEVELOPMENTS; GENERAL TAGGING OF CROSS-SECTIONAL TECHNOLOGIES SPANNING OVER SEVERAL SECTIONS OF THE IPC; TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y10—TECHNICAL SUBJECTS COVERED BY FORMER USPC
- Y10T—TECHNICAL SUBJECTS COVERED BY FORMER US CLASSIFICATION
- Y10T29/00—Metal working
- Y10T29/49—Method of mechanical manufacture
- Y10T29/49826—Assembling or joining
Definitions
- the present disclosuregenerally relates to implantable medical devices and methods for making the same.
- Synthetic ligaments and tendonshave been made from steel, polyester, polyurethane, polyethylene, Nylons, polytetrafluoroethylene, carbon fiber and other man-made materials. Combinations of anyone or more of the aforementioned materials have also been used to manufacture synthetic ligaments.
- syntheticstypically experience decreasing functional capability over time and can wear out, fray, and/or particulate in relatively short time periods after implantation.
- natural ligament or tendon tissue harvested from autografts and/or allograft sourcesmay also be used in ligament or tendon replacement procedures.
- functional parameterse.g., failure load, linear and tangential stiffness, failure stress, and strain at failure
- an implantable medical devicecomprising a plurality of first elongate non-biologic elements, at least a portion of which are under a tensile or compressive stress prior to implantation; at least one biologic component surrounding at least a portion of the plurality of first elongate elements; and at least one second elongate non-biologic element, wherein the at least one second element secures at least one end portion of the plurality of first elongate non-biologic elements.
- a method of making a composite prosthesiscomprises providing a plurality of first elongate non-biologic elements; applying a load to the plurality of first elongate non-biologic elements; covering at least a portion of the plurality of first elongate non-biologic elements with at least one biologic component; and securing the plurality of first elongate non-biologic elements with at least one second elongate non-biologic element.
- an implantable medical devicecomprising at least one non-biologic core material under tensile stress; and at least one biologic element disposed about the at least one non-biologic core, the at least one biologic element comprising a biomatrix; wherein the at least one non-biologic core bears a greater tensile load at a time of implantation than the at least one biologic element, while transmitting stress to the at least one biologic element; and after implantation, the at least one non-biologic core gradually weakens, thereby dynamically transferring additional tensile load to the at least one biologic element.
- a method of making a composite prosthesiscomprises providing at least one non-biologic core; applying a tensile load to the non-biologic core; and disposing at least one biologic element about the at least one non-biologic core, the at least one biologic element comprising a biomatrix; wherein the at least one non-biologic core bears a greater tensile load at a time of implantation than the at least one biologic element, while transmitting stress to the at least one biologic element; and after implantation, the at least one non-biologic core gradually weakens, thereby dynamically transferring additional tensile load to the at least one biologic element.
- FIG. 1is an exemplary stress-strain curve for various graft materials.
- FIG. 2is a graph illustrating the concept of functional target loading of a composite graft material.
- FIGS. 3 and 3Ashow an exemplary embodiment of a composite graft material.
- FIG. 4Ashows an exemplary embodiment of a composite graft material employing a non-biologic component in the form of a flat sheet.
- FIG. 48shows an exemplary embodiment of a rolled composite graft material.
- FIG. 5shows an exemplary embodiment of an interference screw for anchoring a composite graft material.
- FIG. 6is a graph depicting the load capacity over time of certain components of a composite graft material, according to certain embodiments.
- FIG. 7is a graph illustrating the effect of pre-tensioning on the stress-strain curve of various graft materials, according to certain embodiments.
- FIG. 8Aillustrates a plurality of elongate non-biologic components, according to certain embodiments.
- FIG. 8Billustrates a sheath of bioabsorbable material, according to certain embodiments.
- FIG. 9illustrates a plurality of elongate non-biologic components, according to certain embodiments.
- FIGS. 10A-10Gillustrate exemplary embodiments of composite grafts, according to certain embodiments.
- the medical devicesinclude composite materials/tissues.
- the composite materialscomprise both biologic and non-biologic components suitable for use as a tissue implant or replacement for a ligament, tendon, or soft tissue structures.
- the compositeis constructed with at least two materials, for example, a non-biologic component (e.g., a synthetic polymer) and a biologic component (e.g., a biomatrix).
- the non-biologic componentis combined with the biologic component to create a composite tissue that utilizes certain properties of the constituent materials.
- the non-biologic componentsuch as a synthetic polymer, is designed to provide appropriate mechanical properties to the composite structure immediately after implantation and to transmit higher loads over traditional biologic implants when the material is implanted.
- the non-biologic componenttransmits some load and motion to the biologic component.
- the biologic componentis designed to assist in longer-term healing.
- the motion/load distribution between the non-biologic component and the biologic component of the composite materialcontributes to an environment suited for tissue healing.
- the biologic componentvia a biomatrix, facilitates a process of becoming or transforming from a basic biologic tissue scaffold to a tissue similar to the native tissue being replaced (e.g., a ligament-like tissue) by encouraging or allowing the in-growth of native cells within the matrix structure of the biologic component.
- a tissue similar to the native tissue being replacede.g., a ligament-like tissue
- Tissue graftsgenerally experience some change or deterioration in mechanical characteristics within the first month after implantation.
- Such mechanical performance characteristicsmay include, for example, load performance, elasticity and stiffness. Recovery of some or all of the mechanical performance characteristics typically progresses over one to two years after implantation.
- synthetic materials used in tissue replacementimpart an initial load capacity at the time of implantation that can be equal to or higher than natural tissue implants.
- synthetic tissue implantstypically experience a continual, and at times, significant loss in load capacity over the first two years after implantation.
- Natural fiber tissue implantssuch as autografts and allografts, experience a significant drop in load capacity soon after implantation, with an ultimate recovery of load capacity and other mechanical performance characteristics of between 50-60% of the starting capacity of the natural graft tissue.
- the composite graft material of the present disclosurecombines the benefits of typical synthetic polymer tissue grafts (i.e., relatively high initial mechanical performance characteristics) with the prohealing and better long-term mechanical characteristics of natural tissue grafts.
- the composite tissuemay, for example, perform as a summation of its individual components, or better than a summation of the individual components.
- a typical synthetic implantexperiences degradation with decreasing physical performance over time.
- the composite tissue of the present disclosureprovides a layer of biomatrix around or over the synthetic component, which can result in slower degradation than would be exhibited by a typical, uncoated synthetic implant.
- the first biologic component and the second non-biologic component of the composite graft materialare constructed to produce mechanical performance parameters desired for the specific tissue being replaced. For example, in constructing a composite material for ligament replacement, it may be desirable to have an ultimate load failure of approximately 1800 N. If the constructed first component biomatrix material provides an ultimate failure load of only 400 N, then the synthetic second component can be configured to provide the remaining 1400 N to produce a composite graft material having the desired performance characteristics. Similarly, if the desired stiffness for the ACL replacement graft is 200 N/mm and the biomatrix of the first component provides only 50 N/mm, then the polymeric material of the second component can be configured to provide the remaining stiffness of 150 N/mm.
- FIG. 1illustrates an exemplary stress-strain curve of various materials.
- Line 2illustrates a desired curve for an ideal graft.
- Line 4illustrates an exemplary actual stress-strain curve for either a synthetic or natural tissue graft. As can be seen, the actual grafts are not capable of reaching the desired stress levels for the same amount of strain sought in a desired graft.
- the composite graft material described hereinincludes both a biologic and a non-biologic material (e.g., preparation of a weave, or of a braid or of a crimp, or of some other configuration such as a layered or rolled configuration), which raises the stress-strain curve for the composite graft material along the y axis. Consequently, line 6 illustrates a modified, composite graft materials having improved performance compared to synthetic or biologic grafts.
- a biologic and a non-biologic materiale.g., preparation of a weave, or of a braid or of a crimp, or of some other configuration such as a layered or rolled configuration
- FIG. 2illustrates the load capacity versus time since implantation of the composite graft material to demonstrate the concept of functional target loading of the composite graft material.
- the biologic materialalone has a relatively low load capacity at the time of implantation, but as the body subsequently heals, the load capacity of the biologic component increases over time.
- a polymer graft's load-bearing capacityis relatively high at implantation, but decreases subsequently.
- Hybrida composite graft material consistent with the present disclosure
- a biologic component having a biomatrix and a non-biologic componentsuch as a polymer
- the non-biologic componentcan be coupled with the biologic component in a variety of ways.
- the biologic componentmay be disposed around the non-biologic component (as exemplified in FIG. 3 ), or vice versa.
- the biologic componentmay be embedded within a coating, a knit, weave, braid or other structure of the non-biologic component.
- the two componentsmay, for example, be comingled or layered and rolled tightly around one another. In some embodiments, such structures create friction between the components.
- compressive forceis added to the layered construct, e.g., by including securing straps similar to a belt and hoop design.
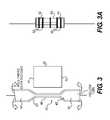
- FIG. 3depicts an exemplary embodiment of the composite graft material of the present disclosure.
- tubular non-biologic component 40is provided having upper portion 42 , upper taper region 43 , lower portion 44 , lower taper region 45 , and neck portion 46 .
- Biologic component 50is also provided and is illustrated as a sheet with an upper edge 51 and a lower edge 52 .
- biologic component 50is wrapped around neck portion 46 of non-biologic component 40 so that upper edge 51 is in contact proximal to or with upper taper region 43 and lower edge 52 is in contact proximal to or with lower taper region 45 .
- Biologic component 50can wrap or encircle neck portion 46 one or more times to form a multi-layer wrap.
- biologic component 50can wrap around neck portion 46 of non-biologic component 40 one time, two times, three times, four times, five times, or more.
- Biologic component 50can similarly wrap around upper portion 42 and lower portion 43 of non-biologic component 40 one or more times.
- upper portion 42 of non-biologic component 40may be rolled or folded over wrapped biologic component 50 and toward lower portion 44 such that at least some of upper portion 42 extends below the upper taper region 43 and overlaps neck portion 46 .
- lower portion 44is rolled or folded over biologic component 50 and toward upper portion 42 such that at least some of lower portion 44 extends above lower taper region 45 and overlaps neck portion 46 , as depicted in FIG. 3A .
- securing straps and/or tethers 60are provided to apply compressive force to the rolled composite graft material and provide frictional contact between the biologic component and the non-biologic component.
- the securing strapsmay, for example, be constructed of the same material as non-biologic component 40 .
- Tethers 60may also be made from the same material as the non-biologic component of the composite material or from other materials such as stainless steel or non-bioabsorbable polymers.
- Tethers 60may be useful during implantation or construction of the composite graft material.
- tethers 60may be used to pull the composite graft material into a bone tunnel.
- Tethers 60may also be used, for example, to anchor non-biologic component 40 while biologic component 50 is wrapped around non-biologic component 40 .
- Tethers 60can be attached to non-biologic component 40 in various ways.
- tethers 60may be woven, knitted, or braided into non-biologic component 40 .
- Tethers 60may also be integrated into the non-biologic component 40 , and may be configured to detach from non-biologic component 40 .
- tethers 60are used as a radiopaque marker.
- a composite graft material in accordance with the present disclosureis constructed with a non-biologic component in the form of a flat sheet, as illustrated in FIG. 4A .
- Non-biologic component 80includes upper portion 82 , lower portion 84 , neck portion 85 , and neck lateral edge 86 .
- Upper portion 82 and lower portion 84may be made of same or different non-biologic material as neck portion 85 .
- neck portion 85may comprise a high tensile strength textile of a bioabsorbable polymer
- upper portion 82 and lower portion 84comprise a relatively low tensile strength textile of a bioabsorbable polymer.
- Non-biologic component 80may also, for example, comprise non-textile polymers as previously described.
- biologic component 50may, for example, be provided as a flat sheet of biologic material comprising an acellular tissue matrix having an upper edge 51 , a lower edge 52 , and a lateral edge 53 .
- non-biologic component 80is placed on top of biologic component 50 such that neck lateral edge 86 of non-biologic component 80 aligns with lateral edge 53 of biologic component 50 , and the two layered components are then rolled such that neck lateral edge 86 and lateral edge 53 remain aligned and form the innermost portion of the roll.
- FIG. 4Bshows a non-limiting embodiment of a rolled composite graft material in accordance with the present disclosure.
- This rolled composite graft materialis constructed as described above, with the biologic component 50 forming the outside surface of the rolled structure and neck lateral edge 86 forming the inner most portion of the rolled structure.
- Upper portion 82 and lower portion 84form a multi-layered non-biologic material within the rolled structure and can provide added strength and material to secure the composite graft material to surrounding native tissue, for example, by interference screws.
- the composite graft materialis designed to match the size (length, width, thickness) of the natural structure (i.e., ligament or tendon) it will replace.
- the composite graft materialmay be designed to be about 6 to 12 mm in diameter for a unibody device or 3 to 6 mm in diameter if separated into two bundles. It is known that within the body, specific ligament sizes slide and fit between bony structures. A ligament that is too small may not distribute stress evenly, and a ligament that is too large may interfere with or rub against one or both bony structures. Thus, matching the size of the implant material with the native tissue to be removed can reduce complications.
- the size of the composite graft materialis customized to the patient and the tissue being replaced.
- one or more rolls of biologic and non-biologic componentscan be added or removed by varying the size or length of the individual components. The longer the pre-rolled composite construct, the more rolls are possible, thereby producing a finished composite graft material having a greater diameter.
- the number of layers of individual componentscan be altered to adjust the final size of the graft material. For example, a composite construct comprising a tissue layer, a polymer layer, and another tissue layer would provide a larger diameter graft than a composite construct of only two layers.
- interference screwsare used for anchoring cadaver or autograft materials.
- an interference screwis provided that anchors the composite graft material. This is shown in FIG. 5 , wherein interference screw 90 is inserted into the core of graft 94 such that as interference screw 90 advances, screw 90 expands the diameter of graft 94 to exert outwardly radial pressure against the surround bone tunnel.
- Other common fixation devicesmay be used, including cross-pins, endobuttons, sutures, or staples.
- screws or other anchoring devicescan be made of titanium, stainless steel, biodegradable metals, biodegradable or bioabsorbable polymers such as polylactic acid (PIA), polyglycolic acid (PGA), polylactideglycolide acid (PLGA), polydioxanone (PDO), or polycaprolactone (PCL).
- PIApolylactic acid
- PGApolyglycolic acid
- PLGApolylactideglycolide acid
- PDOpolydioxanone
- PCLpolycaprolactone
- the biologic component comprising the biomatrix material of the disclosed composite graft materialmay benefit from the stress of ordinary movement following implantation.
- the stresses and strains caused by ordinary activitymay cause the biologic tissue to be stronger and recover to a greater ultimate strength due to normal remodeling facilitated by mechanical forces.
- the non-biologic component of the composite tissue graft material described hereinis preloaded with a tensile load ranging from greater than 0 N to about 1800 N (e.g., from about 0.1 to about 1700 N; about 10 to about 1600 N, about 100 to about 1500 N, about 150 to 1400 N, about 200 to 1300 N, etc.).
- the initial stress on strain on the non-biologic materialis partially transferred to the biologic component, with the non-biologic component assuming more of the applied stress after reaching a preloaded limit.
- FIG. 1illustrates an exemplary stress-strain curve of various tissue grafts.
- Line 2illustrates a desired curve for an ideal graft.
- Line 4illustrates an exemplary actual stress-strain curve for either a synthetic or natural tissue graft.
- natural graftsare not capable of reaching the desired stress levels for the same amount of strain sought in a desired graft.
- some embodiments of the composite graft materials described hereinmay exhibit a stress-strain curve that is shifted along the y axis. Consequently, line 6 of FIG. 1 illustrates a composite graft material having improved load capacity performance over actual synthetic or biologic grafts.
- the non-biologic component, the biologic component, or both the non-biologic and biologic componentscan be placed under tensile stress prior to implantation. This has the effect of moving the stress-strain curve of the material along the x-axis as depicted in FIG. 7 .
- Line 2illustrates a desired curve for an ideal graft material.
- Line 5illustrates an actual curve for synthetic or natural tissue graft, and
- line 7illustrates a curve of a composite graft material that has been subjected to preloading with a tensile stress.
- a portion of the tensile load applied to the non-biologic componentis transferred to the biologic component.
- the initial strain appliedis selected to be high enough to prevent or retard resorption of the biologic component upon implantation, but low enough to avoid physically damaging the biologic tissue.
- the strain applied to the biologic componentmay range from less than or equal to 40%, less than or equal to 35%, less than or equal to 30%, less than or equal to 25%, less than or equal to 20%, less than or equal to 15%, less than or equal to 10%, less than or equal to 5%, and less than or equal to 1% of the initial strain applied to the non-biologic material by an initial tensile load.
- the initial strain applied to the biologic componentmay also fall within any range specified by a combination of the above recited endpoints, e.g., from greater than or equal to 1% to less than or equal to 40%, from greater than or equal to 5% to less than or equal to 35%, from greater than or equal to 10% to less than or equal to 30%, and from greater than or equal to 15% to less than or equal to 25% of the initial strain imparted to the non-biologic component by an initial tensile load.
- a composite graft materialcan be made using a plurality of elongate non-biologic components wrapped with at least one layer of a biologic component.
- the elongate non-biologic componentscan be placed under a tensile stress ranging from greater than 0 N to 1800 N (e.g., from about 100 to about 1700 N; about 200 to about 1600 N, about 300 to about 1500 N, etc.), prior to wrapping with the biologic component.
- the plurality of elongate non-biologic componentsmay also be pre-wrapped in a sheath comprising non-biologic components.
- At least one end of the non-biologic componentscan be secured (e.g., by whipping, wrapping, and/or winding) with an additional elongate component to form multiple layers with a raised surface at the end.
- the biologic componentmay, for example, be wrapped around the secured plurality of elongate non-biologic components and secured with a smaller fastening or whipping adjacent to the raised surface formed by the secured biologic component.
- all of the plurality of elongate non-biologic componentscan be placed under a tensile stress prior to forming the composite graft material. In other embodiments, a percentage of the elongate non-biologic components can be placed under tensile stress prior to forming the composite graft material, while a percentage of the elongate biologic components can be free of tensile stress prior to forming the composite graft material. Further, in some embodiments, the plurality of elongate non-biologic components and the biologic component can be placed under a tensile stress prior to final assembly of the graft.
- FIG. 8Aillustrates a plurality of elongate non-biologic components 810 .
- the plurality of elongate componentscan be bundled as a group having between 200 and 1200 individual elongate components, depending on the size and mechanical requirements of the composite graft material. In some embodiments, the number of individual components can be between 400 and 1000, 600 and 800, or approximately 700 individual components.
- the elongate non-biologic componentsmay also be bioabsorbable.
- the plurality of elongate components 810can have a proximal end 812 and a distal end 814 .
- Securing elements 816e.g., whipping elements
- the plurality of elongate components 810can be placed under a tensile load prior to securing.
- Securing elements 816are attached to the plurality of components 810 in such a manner as to retain the preloaded tensile stress in the plurality of elongate components.
- FIG. 8Billustrates a non-limiting embodiment of the present disclosure, wherein sheath 815 of bioabsorbable non-biologic material is wrapped around the plurality of elongate components 810 .
- the plurality of elongate componentsmay be loaded with a tensile stress independently of the sheath, or the sheath and plurality of elongate components can be loaded together.
- Securing elements 816can be wrapped around and over the sheath 815 of non-biologic material.
- a biologic component containing a biomatrixis used to cover or coat at least a portion of the plurality of elongate non-biologic components.
- the biologic componentmay be in the form of a sheet wrapped around a secured plurality of elongate non-biologic components.
- FIG. 9illustrates a plurality of elongate non-biologic components 910 having a proximal end 912 and distal end 914 .
- Securing elements 916are wrapped around the plurality of elongate components to secure the elongate components as a bundle 918 and maintain a preloaded tensile stress in the elongate bundle 918 .
- Securing elements 916can be wrapped in multiple layers to form a raised surface 917 .
- biologic component 920may be wrapped in one or more layers around the elongate bundle 918 and secured by second securing elements 922 proximate the raised surface 917 formed by securing elements 916 .
- Second securing elements 922can be secured about the biologic component 920 and the elongate bundle 918 such that the biologic component is free from tensile stress.
- the biologic component 920can be placed under tensile stress and secured by second securing elements 922 to maintain the tensile load in the biologic component 920 .
- the tensile load in the biologic componentcan range from greater than 0 N to about 1800 N, from greater than 0 to about 600 N, from about 50 to about 300 N, or from about 100 to about 200 N.
- the biologic component 920may, be pre-loaded to the same tensile stress as the plurality of elongate components 910 .
- the biologic component 920may also be pre-loaded to tensile stress less than that of the plurality of elongate components 910 .
- the biologic component 920can be pre-loaded to a tensile stress more than that of the plurality of elongate components 910 .
- the biologic componentcan be paired with the plurality of elongate elements in a variety of ways, including: as a single layer sheet wrapped about the elongate non-biologic components; in a jellyroll manner wherein the biologic component is wrapped in multiple layers; as a non-uniform sheet such that multiple layers of biologic component are not concentric about the plurality of elongate elements; as a top sheath wrapped or coated over an inner sheath of non-biologic material; as a coating about the outer surface of the bundle of elongate non-biologic elements; and/or as a coating interspersed throughout the bundle of elongate non-biologic components.
- FIG. 10Ashows composite graft material 1000 comprising a plurality of elongate non-biologic elements 1010 and biologic component 1020 , wherein biologic component 1020 is wrapped as a sheet in a single layer over the plurality of elongate elements 1010 .
- FIG. 10Bshows a composite graft material 1001 comprising a plurality of elongate non-biologic elements 1011 and biologic component 1021 , wherein biologic component 1021 is wrapped as a sheet in a jellyroll fashion with multiple layers over the plurality of elongate elements 1011 .
- FIG. 10Ashows composite graft material 1000 comprising a plurality of elongate non-biologic elements 1010 and biologic component 1020 , wherein biologic component 1020 is wrapped as a sheet in a single layer over the plurality of elongate elements 1010 .
- FIG. 10Bshows a composite graft material 1001 comprising a plurality of elongate non-biologic elements 1011 and biologic component 1021 , where
- FIG. 10Cshows a composite graft material 1002 comprising a plurality of elongate non-biologic elements 1012 and biologic component 1022 , wherein biologic component 1022 is wrapped as a non-uniform sheet in a jellyroll fashion with multiple non-concentric layers over the plurality of elongate elements 1012 .
- FIG. 10Dshows a composite graft material 1003 comprising a plurality of elongate non-biologic elements 1013 and biologic component 1023 , wherein biologic component 1023 is wrapped as a single layer sheet forming an outer sheath over inner sheath 1035 of non-biologic material. Inner sheath 1035 is wrapped about the plurality of elongate non-biologic components.
- Biologic component 1020may also be applied as a coating.
- the coatingcan, for example, be in the form of a liquid, a powder, or a spray, and may be applied using any suitable technique.
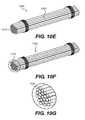
- FIG. 10Eshows a composite graft material 1004 comprising a plurality of elongate non-biologic elements 1014 and biologic component 1024 , wherein biologic component 1024 coats the outer surface of the plurality of elongate elements 1015 .
- FIG. 10Fshows a composite graft material 1005 comprising a plurality of elongate non-biologic elements and biologic component 1025 , wherein biologic component 1025 is interspersed between and coats the outer surfaces of the individual elongate elements in the plurality of elongate elements 1015 , as shown in FIG. 10G .
- the plurality of elongate non-biologic elementsare secured by a securing material (e.g., a whipping material) such that the securing material is wound about the plurality of elongate non-biologic elements in a manner that is engageable with an anchor screw.
- a securing materiale.g., a whipping material
- the securing materialmay, for example, be wound to create a screw-thread pattern having a pitch compatible with an anchoring screw to better engage and lock the composite graft material to the anchoring screw upon implantation in the patient.
- the material used to secure the tissue or biologic component to the plurality of elongate non-biologic elementscan also be wound so as to have a screw-screw thread pattern engageable with an anchoring screw.
- the securing materialcan form the male or female thread to the threads of the anchoring screw.
- the composite graft materialmay be used for ACL replacement.
- the composite graft materialis designed to have the properties of a typical ACL, e.g., failure load (1200-2400 N); stiffness (150-300 N/mm); failure stress (18-28 MPa); strain at failure (20-35%); and modulus of elasticity (75-180 MPa).
- the natural mechanical and biologic properties of native ACL tissuemay be matched, for example, by selecting and constructing each component of a device to meet specific design requirements.
- a device meeting the general characteristics of an ACLmay be made with a non-biologic component having a modulus of elasticity of about 140 MPa, a maximum rupture load or ultimate load failure of about 1200 N, and degradation resistance through 9 to 16 months before construction of the composite graft material.
- the biologic componentexhibits a modulus of elasticity of about 55 MPa at the time of implantation and a maximum load at rupture of approximately 600 N before construction of the composite graft material.
- a composite graft material suitable for ACL repairmay, for example, exhibit a maximum rupture load of approximately 1400 N before implantation.
- the ultimate failure load of the non-biologic component of such an implantmay decrease after implantation, while the failure load of the biologic component will increase over time as native cells proliferate through the biomatrix.
- the implantmay have an ultimate failure load of approximately 600 N within four months of implantation, approximately 400 N within eight months of implantation, and approximately 1000 N within 12 months of implantation.
- the composite graft material for ACL replacementmay have a stiffness of approximately 85 N/mm before implantation, approximately 106 N/mm within four months of implantation, approximately 78 N/mm within eight months of fixation, and approximately 176 N/mm within twelve months of fixation.
- FIG. 6depicts the load capacity over time of the first component, the second component and the composite graft material of a non-limiting example of a composite ACL graft according to various embodiments.
- the first component, line 8exhibited a drop in load capacity immediately following implantation with an increase in load capacity thereafter as native cells proliferated throughout the biomatrix.
- Line 10illustrates the initially high load capacity of the second component after implantation with a steady decrease in load capacity as the second component degraded over time.
- Line 12shows the load capacity of the composite graft containing both the first component and the second component.
- Biologic components that may be suitably used to produce composite graft materialscan include any biologic material (e.g., whole tissue or tissue-derived material) with the properties described herein.
- biologic materialsinclude biomatrices, such as acellular tissue matrices.
- ATMacellular tissue matrix
- tissue-derived biomatrix structurethat is made from any of a wide range of collagen-containing tissues by removing all or substantially all viable cells and all detectable subcellular components and/or debris generated by killing cells.
- an ATM lacking “substantially all viable cells”is an ATM in which the concentration of viable cells is less than 1% of that in the tissue or organ from which the ATM was made.
- the ATMs of the present disclosurecontain epithelial basement membrane.
- the composite grafts disclosed hereinlack or substantially lack epithelial basement membrane.
- the ATMsinclude a vascular basement membrane that may facilitate ingrowth of vascular endothelial cells.
- ATM's suitable for use in the present disclosuremay, for example, retain certain biologic functions, such as cell recognition, cell binding, the ability to support cell spreading, cell proliferation, cellular in-growth and cell differentiation. Such functions may be provided, for example, by undenatured collagenous proteins (e.g., type I collagen) and a variety of non-collagenous molecules (e.g., proteins that serve as ligands for either molecules such as integrin receptors, molecules with high charge density such as glycosaminoglycans (e.g., hyaluronan) or proteoglycans, or other adhesins).
- the ATM'smay retain certain structural functions, including maintenance of histological architecture and maintenance of the three-dimensional array of the tissue's components.
- the ATM's described hereinmay also, for example, exhibit desirable physical characteristics such as strength, elasticity, and durability, defined porosity, and retention of macromolecules.
- ATMs suitable for use in the present disclosuremay be crosslinked or uncrosslinked.
- the composite graftincludes an uncrosslinked ATM.
- the efficiency of the biologic functions of an ATMcan be measured, for example, by the ability of the ATM to support cell proliferation.
- the ATMexhibits at least 50% (e.g., at least: 50%; 60%; 70%; 80%; 90%; 95%; 98%; 99%; 99.5%; 100%; or more than 100%, or any ranges between 50%-100%) of that of the native tissue or organ from which the ATM is made.
- the biologic componentwhen implanted, is amenable to being remodeled by infiltrating cells such as differentiated cells of the relevant host tissue, stem cells such as mesenchymal stem cells, or progenitor cells. Remodeling may be directed by the above-described ATM components and signals from the surrounding host tissue (such as cytokines, extracellular matrix components, biomechanical stimuli, and bioelectrical stimuli).
- cytokinessuch as cytokines, extracellular matrix components, biomechanical stimuli, and bioelectrical stimuli.
- the graftwill provide some degree (greater than threshold) of tensile and biomechanical strength during the remodeling process.
- an ATM in accordance with the present disclosuremay be manufactured from a variety of source tissues.
- the ATMmay be produced from any collagen-containing soft tissue and musculo-skeletonal tissue (e.g., dermis, fascia, pericardium, dura, umbilical cords, placentae, cardiac valves, ligaments, tendons, vascular tissue (arteries and veins such as saphenous veins), neural connective tissue, urinary bladder tissue, ureter tissue, or intestinal tissue), as long as the above-described properties are retained by the matrix.
- the tissues in which the matrices containing the ATM are placedmay include any tissue that can be remodeled by invading or infiltrating cells.
- Non-limiting examples of such tissuesinclude skeletal tissues such as bone, cartilage, ligaments, fascia, and tendon.
- Other tissues in which any of the above grafts can be placedinclude, for example, skin, gingiva, dura, myocardium, vascular tissue, neural tissue, striated muscle, smooth muscle, bladder wall, ureter tissue, intestine, and urethra tissue.
- an acellular tissue matrixmay be made from one or more individuals of the same species as the recipient of the acellular tissue matrix graft, this is not necessarily the case.
- an acellular tissue matrixmay be made from porcine tissue and implanted in a human patient.
- Species that can serve as recipients of acellular tissue matrix and donors of tissues or organs for the production of the acellular tissue matrixinclude, without limitation, mammals, such as humans, nonhuman primates (e.g., monkeys, baboons, or chimpanzees), pigs, cows, horses, goats, sheep, dogs, cats, rabbits, guinea pigs, gerbils, hamsters, rats, or mice.
- porcine-derived tissuenon-limiting mention is StratticeTM, which is a porcine dermal tissue produced by Lifecell Corp, Branchburg, N.J.
- the tissue matrixmay be derived from porcine skin by removing the epidermis while leaving the dermal matrix substantially intact.
- the porcine-derived tissue matrixmay facilitate tissue ingrowth and remodeling with the patient's own cells.
- the materialcan include a collagenous matrix derived from human cadaver skin (e.g. AlloDermTM, Lifecell Corp, Branchburg, N.J.) that has been processed to remove both the epidermis and cells.
- the steps involved in the production of an acellular tissue matrixinclude harvesting the tissue from a donor (e.g., a human cadaver or animal source) and cell removal under conditions that preserve biologic and structural function.
- the processincludes chemical treatment to stabilize the tissue and avoid biochemical and structural degradation together with or before cell removal.
- the stabilizing solutionarrests and prevents osmotic, hypoxic, autolytic, and proteolytic degradation, protects against microbial contamination, and reduces mechanical damage that can occur with tissues that contain, for example, smooth muscle components (e.g., blood vessels).
- the stabilizing solutionmay contain an appropriate buffer, one or more antioxidants, one or more oncotic agents, one or more antibiotics, one or more protease inhibitors, and/or one or more a smooth muscle relaxant.
- the tissueis then placed in a decellularization solution to remove viable cells (e.g., epithelial cells, endothelial cells, smooth muscle cells, and fibroblasts) from the structural matrix without damaging the biologic and structural integrity of the collagen matrix.
- the decellularization solutionmay contain an appropriate buffer, salt, an antibiotic, one or more detergents (e.g., TRITON X-100TM, sodium deoxycholate, polyoxyethylene (20) sorbitan mono-oleate), one or more agents to prevent cross-linking, one or more protease inhibitors, and/or one or more enzymes.
- the decellularization solutioncomprises 1% TRITON X-100TM in RPMI media with Gentamicin and 25 mM EDTA (ethylenediaminetetraacetic acid).
- the tissueis incubated in the decellularization solution overnight at 37 DC with gentle shaking at 90 rpm.
- additional detergentsmay be used to remove fat from the tissue sample. For example, in some embodiments, 2% sodium deoxycholate is added to the decellularization solution.
- the tissue sampleis washed thoroughly with saline.
- the decellularized tissueis then treated overnight at room temperature with a deoxyribonuclease (DNase) solution.
- DNasedeoxyribonuclease
- the tissue sampleis treated with a DNase solution prepared in DNase buffer (20 mM HEPES (4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid), 20 mM CaCl2 and 20 mM MgCl2).
- an antibiotic solutione.g., Gentamicin
- Any suitable buffercan be used as long as the buffer provides suitable DNase activity.
- Elimination of the ⁇ -gal epitopes from the collagen-containing materialmay diminish the immune response against the collagen-containing material.
- the ⁇ -gal epitopeis expressed in non-primate mammals and in New World monkeys (monkeys of South America) as well as on macromolecules such as proteoglycans of the extracellular components.
- Anti-gal antibodiesare produced in humans and primates as a result of an immune response to ⁇ -gal epitope carbohydrate structures on gastrointestinal bacteria.
- non-primate mammalse.g., pigs
- xenotransplantation of collagen-containing material from these mammals into primatesoften results in rejection because of primate anti-Gal binding to these epitopes on the collagen-containing material.
- the bindingresults in the destruction of the collagen-containing material by complement fixation and by antibody dependent cell cytotoxicity.
- U. Galili et al.Immunology Today 14:480 (1993); M. Sandrin et al., Proc. Natl. Acad. Sci. USA 90: 11391 (1993); H. Good et al., Transplant. Proc. 24: 559 (1992); B. H. Collins et al., J. Immunol.
- xenotransplantationresults in major activation of the immune system to produce increased amounts of high affinity anti-gal antibodies.
- tissue sourcewhen animals that produce ⁇ -gal epitopes are used as the tissue source, the substantial elimination of ⁇ -gal epitopes from cells and from extracellular components of the collagen-containing material, and the prevention of re-expression of cellular ⁇ -gal epitopes can diminish the immune response against the collagen-containing material associated with anti-gal antibody binding to ⁇ -gal epitopes.
- the tissue samplemay be subjected to one or more enzymatic treatments to remove certain immunogenic antigens, if present in the sample.
- the tissue samplemay be treated with an ⁇ -galactosidase enzyme to eliminate ⁇ -gal epitopes if present in the tissue.
- the tissue sampleis treated with ⁇ -galactosidase at a concentration of 300 U/L prepared in 100 mM phosphate buffer at pH 6.0
- the concentration of ⁇ -galactosidaseis increased to 400 u/L for adequate removal of the ⁇ -gal epitopes from the harvested tissue. Any suitable enzyme concentration and buffer can be used as long as sufficient removal of antigens is achieved.
- animals that have been genetically modified to lack one or more antigenic epitopesmay be selected as the tissue source.
- animalse.g., pigs
- animalsthat have been genetically engineered to lack the terminal ⁇ -galactose moiety can be selected as the tissue source.
- appropriate animalssee co-pending U.S. application Ser. No. 10/896,594 and U.S. Pat. No. 6,166,288, the disclosures of which are incorporated herein by reference in their entirety.
- histocompatible, viable cellsmay optionally be seeded in the acellular tissue matrix to produce a graft that may be further remodeled by the host.
- histocompatible viable cellsmay be added to the matrices by standard in vitro cell co-culturing techniques prior to transplantation, or by in vivo repopulation following transplantation. In vivo repopulation can be by the recipient's own cells migrating into the acellular tissue matrix or by infusing or injecting cells obtained from the recipient or histocompatible cells from another donor into the acellular tissue matrix in situ.
- Various cell typescan be used, including embryonic stem cells, adult stem cells (e.g.
- the cellscan be directly applied to the inner portion of the acellular tissue matrix just before or after implantation. In certain embodiments, the cells can be placed within the acellular tissue matrix to be implanted, and cultured prior to implantation.
- Particulate ATMcan be made from any of the above described non-particulate ATMs by any process that results in the preservation of the biologic and structural functions described above.
- particulate ATMsare those particulate or pulverized (powdered) matrices having a longest dimension of 1.0 mm or less.
- particulate ATM used in the present disclosureis manufactured so as to minimize damage to collagen fibers, including sheared fiber ends.
- a suitable method for making particulate ATMis described in U.S. Pat. No. 6,933,326.
- the particle size for Cymetrais in the range of about 60 microns to about 150 microns as determined by mass spectrophotometry.
- Non-Biologic Component
- the at least one non-biologic component of the present disclosuremay, for example, comprise biocompatible natural and/or synthetic materials.
- Biocompatible natural materialsmay include, for example, collagen, fibrin, and silk.
- Biocompatible synthetic materialsmay include, for example, bioabsorbable polymers, non-bioabsorbable polymers, and metallic alloys or compositions.
- a non-biologic component that is biocompatible and bioabsorbableis used. Utilizing bioabsorbable polymers may allow for a transfer of loads from the non-biologic component (e.g., the polymer) to the biologic component as native tissue regenerates throughout the matrix structure of the biologic component.
- a “biocompatible” compositionis one that has the ability to support cellular activity necessary for complete or partial tissue regeneration, but does not stimulate an unacceptable inflammatory or immunological response in the host.
- unacceptable local inflammatory or immunological response in the hostmeans a local or systemic inflammatory or immunologic response that prevents tissue regeneration.
- bioabsorbablemeans that a material can be absorbed by a mammalian body via biologically mediated degradation processes, such as enzymatic and cellular processes and/or chemically mediated degradation processes. Such processes include, for example, degradation processes wherein the degradation products are excreted through one of the body's organ systems or in which the degradation products are incorporated into normal metabolic pathways.
- a suitable bioabsorbable material for use in the present disclosureis made of a poly-hydroxybutyrate (a polyhydroxyalkanoate), such as the TephaFlex polymer produced by Tepha, Inc. of Cambridge, Mass.
- useful bioabsorbable materialsinclude, for example, polylactic acid (PLA), polyglycolic acid (PGA), polylactideglycolide acid (PLGA), polydioxanone (PDO), or polycaprolactone (PCL).
- bioabsorbable materials suitable for use in the present disclosureinclude polyanhydrides, polyorthoesters, poly(amino acids), polypeptides, polydepsipeptides, nylon-2/nylon-6coplyamides, poly(alkylene succinates), poly(hydroxyl butyrate) (PHB), poly (butylene diglocolate), poly ( ⁇ -caprolactone), polydihydropyrans, polyphosphazenes, poly (ortho ester), poly (cyano acrylates), modified polysaccharides, cellulose, starch, chitin, modified proteins, collagen, fibrin, and combinations and copolymers thereof.
- Non-limiting examples of non-bioabsorbable materialsinclude noble metals such as gold, as well as the near noble metals.
- the non-biologic components used hereinmay be provided in any form.
- the non-biologic componentsare in the form of a molded shape (e.g., as a single contiguous polymeric piece).
- the non-biologic componentsare in the form of a textile comprised of multiple yarns, the yarn being either a monofilament or a multifilament structure (such as a braid). Textile manufacturing methods can then make final structures that are knitted, woven, braided, nonwoven, or combinations thereof.
- the composite materialmay be used for many applications where soft tissues need to be replaced and yet provide specific load-carrying or biomechanical characteristics, including ligament, tendon or soft tissue replacement about the knees, ankles, shoulders, neck, and spine.
- Other hybrid systemscan be developed utilizing the same basic ideas as described above.
- Examplesinclude: artificial meniscus replacement or repair; abdominal wall (e.g., hernia) repair; cartilage repair in, for example, knees, shoulders and hips; joint resurfacing (instead of removing joints, the joint articulating surface can simply be covered with a composite material reinforced with fabric using appropriately designed anchors or sutures); and pace maker pouches (a simple bag/pouch used to contain pacers or pain manager systems would make periodic replacement much simpler and would create a more stable anchor pacer implant).
- abdominal walle.g., hernia
- cartilage repairin, for example, knees, shoulders and hips
- joint resurfacinginstead of removing joints, the joint articulating surface can simply be covered with a composite material reinforced with fabric using appropriately designed anchors or sutures
- pace maker pouchesa simple bag/pouch used to contain pacers or pain manager systems would make periodic replacement much simpler and would create a more stable anchor pacer implant).
Landscapes
- Health & Medical Sciences (AREA)
- Chemical & Material Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Engineering & Computer Science (AREA)
- Public Health (AREA)
- Veterinary Medicine (AREA)
- Animal Behavior & Ethology (AREA)
- General Health & Medical Sciences (AREA)
- Dermatology (AREA)
- Medicinal Chemistry (AREA)
- Oral & Maxillofacial Surgery (AREA)
- Transplantation (AREA)
- Epidemiology (AREA)
- Materials Engineering (AREA)
- Composite Materials (AREA)
- Biomedical Technology (AREA)
- Inorganic Chemistry (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Botany (AREA)
- Molecular Biology (AREA)
- Urology & Nephrology (AREA)
- Zoology (AREA)
- Manufacturing & Machinery (AREA)
- Prostheses (AREA)
- Materials For Medical Uses (AREA)
- Laminated Bodies (AREA)
Abstract
Description
- This application is a continuation of application Ser. No. 13/616,556, filed Sep. 14, 2012 (allowed), which is a continuation of application Ser. No. 12/621,890, filed Nov. 19, 2009 and issued as U.S. Pat. No. 8,333,803, and claims priority under 35 U.S.C. §119 to U.S. Provisional Patent Application No. 61/117,068, which was filed on Nov. 21, 2008, each of which are hereby incorporated by reference.
- This invention was made with government support under U.S. Army Contract Number W81XWH-06-1-0136. Accordingly, the government has certain rights in the invention.
- The present disclosure generally relates to implantable medical devices and methods for making the same.
- Surgeons performing ligament and tendon replacement in mammals have long sought a material that approximates the load transmission and performance of the native ligament and tendon structures. Synthetic ligaments and tendons have been made from steel, polyester, polyurethane, polyethylene, Nylons, polytetrafluoroethylene, carbon fiber and other man-made materials. Combinations of anyone or more of the aforementioned materials have also been used to manufacture synthetic ligaments. However, synthetics typically experience decreasing functional capability over time and can wear out, fray, and/or particulate in relatively short time periods after implantation.
- As an alternative to synthetic materials, natural ligament or tendon tissue harvested from autografts and/or allograft sources may also be used in ligament or tendon replacement procedures. As with synthetic materials, for both autografts and allografts, long-term recovery of functional parameters (e.g., failure load, linear and tangential stiffness, failure stress, and strain at failure) remains significantly reduced compared to native ligament, tendon or other soft tissue structures.
- There is a need for a material for ligament, tendon, and other soft tissue repair and replacement that is free of donor site morbidity associated with autografts, has improved failure rates over traditional allografts and synthetic tissues, and better approximates native tissue biomechanical performance.
- This discussion of the background disclosure is included to place the present disclosure in context. It is not an admission that any of the background material previously described was published, known, or part of the common general knowledge as at the priority date of the present disclosure and claims.
- As used herein, the term, “comprise” and variations thereof, such as “comprising” and “comprises,” is not intended to exclude other additives, components, integers or steps.
- In some embodiments, an implantable medical device is provided. The device comprises a plurality of first elongate non-biologic elements, at least a portion of which are under a tensile or compressive stress prior to implantation; at least one biologic component surrounding at least a portion of the plurality of first elongate elements; and at least one second elongate non-biologic element, wherein the at least one second element secures at least one end portion of the plurality of first elongate non-biologic elements.
- In some embodiments, a method of making a composite prosthesis is provided. The method comprises providing a plurality of first elongate non-biologic elements; applying a load to the plurality of first elongate non-biologic elements; covering at least a portion of the plurality of first elongate non-biologic elements with at least one biologic component; and securing the plurality of first elongate non-biologic elements with at least one second elongate non-biologic element.
- In some embodiments, an implantable medical device is provided. The device comprises at least one non-biologic core material under tensile stress; and at least one biologic element disposed about the at least one non-biologic core, the at least one biologic element comprising a biomatrix; wherein the at least one non-biologic core bears a greater tensile load at a time of implantation than the at least one biologic element, while transmitting stress to the at least one biologic element; and after implantation, the at least one non-biologic core gradually weakens, thereby dynamically transferring additional tensile load to the at least one biologic element.
- In some embodiments, a method of making a composite prosthesis is provided. The method comprises providing at least one non-biologic core; applying a tensile load to the non-biologic core; and disposing at least one biologic element about the at least one non-biologic core, the at least one biologic element comprising a biomatrix; wherein the at least one non-biologic core bears a greater tensile load at a time of implantation than the at least one biologic element, while transmitting stress to the at least one biologic element; and after implantation, the at least one non-biologic core gradually weakens, thereby dynamically transferring additional tensile load to the at least one biologic element.
FIG. 1 is an exemplary stress-strain curve for various graft materials.FIG. 2 is a graph illustrating the concept of functional target loading of a composite graft material.FIGS. 3 and 3A show an exemplary embodiment of a composite graft material.FIG. 4A shows an exemplary embodiment of a composite graft material employing a non-biologic component in the form of a flat sheet.FIG. 48 shows an exemplary embodiment of a rolled composite graft material.FIG. 5 shows an exemplary embodiment of an interference screw for anchoring a composite graft material.FIG. 6 is a graph depicting the load capacity over time of certain components of a composite graft material, according to certain embodiments.FIG. 7 is a graph illustrating the effect of pre-tensioning on the stress-strain curve of various graft materials, according to certain embodiments.FIG. 8A illustrates a plurality of elongate non-biologic components, according to certain embodiments.FIG. 8B illustrates a sheath of bioabsorbable material, according to certain embodiments.FIG. 9 illustrates a plurality of elongate non-biologic components, according to certain embodiments.FIGS. 10A-10G illustrate exemplary embodiments of composite grafts, according to certain embodiments.- The present disclosure relates to implantable medical devices and methods of producing and using such devices. In certain embodiments, the medical devices include composite materials/tissues. In some embodiments, the composite materials comprise both biologic and non-biologic components suitable for use as a tissue implant or replacement for a ligament, tendon, or soft tissue structures. In some embodiments, the composite is constructed with at least two materials, for example, a non-biologic component (e.g., a synthetic polymer) and a biologic component (e.g., a biomatrix). In some embodiments, the non-biologic component is combined with the biologic component to create a composite tissue that utilizes certain properties of the constituent materials.
- The non-biologic component, such as a synthetic polymer, is designed to provide appropriate mechanical properties to the composite structure immediately after implantation and to transmit higher loads over traditional biologic implants when the material is implanted. In some embodiments, the non-biologic component transmits some load and motion to the biologic component. In some embodiments, the biologic component is designed to assist in longer-term healing. In some embodiments, the motion/load distribution between the non-biologic component and the biologic component of the composite material contributes to an environment suited for tissue healing. In some embodiments, the biologic component, via a biomatrix, facilitates a process of becoming or transforming from a basic biologic tissue scaffold to a tissue similar to the native tissue being replaced (e.g., a ligament-like tissue) by encouraging or allowing the in-growth of native cells within the matrix structure of the biologic component.
- Tissue grafts generally experience some change or deterioration in mechanical characteristics within the first month after implantation. Such mechanical performance characteristics may include, for example, load performance, elasticity and stiffness. Recovery of some or all of the mechanical performance characteristics typically progresses over one to two years after implantation.
- Generally, synthetic materials used in tissue replacement impart an initial load capacity at the time of implantation that can be equal to or higher than natural tissue implants. But, synthetic tissue implants typically experience a continual, and at times, significant loss in load capacity over the first two years after implantation.
- Natural fiber tissue implants, such as autografts and allografts, experience a significant drop in load capacity soon after implantation, with an ultimate recovery of load capacity and other mechanical performance characteristics of between 50-60% of the starting capacity of the natural graft tissue.
- In some embodiments, the composite graft material of the present disclosure combines the benefits of typical synthetic polymer tissue grafts (i.e., relatively high initial mechanical performance characteristics) with the prohealing and better long-term mechanical characteristics of natural tissue grafts. The composite tissue may, for example, perform as a summation of its individual components, or better than a summation of the individual components. For example, a typical synthetic implant experiences degradation with decreasing physical performance over time. The composite tissue of the present disclosure provides a layer of biomatrix around or over the synthetic component, which can result in slower degradation than would be exhibited by a typical, uncoated synthetic implant.
- In some embodiments, the first biologic component and the second non-biologic component of the composite graft material are constructed to produce mechanical performance parameters desired for the specific tissue being replaced. For example, in constructing a composite material for ligament replacement, it may be desirable to have an ultimate load failure of approximately 1800 N. If the constructed first component biomatrix material provides an ultimate failure load of only 400 N, then the synthetic second component can be configured to provide the remaining 1400 N to produce a composite graft material having the desired performance characteristics. Similarly, if the desired stiffness for the ACL replacement graft is 200 N/mm and the biomatrix of the first component provides only 50 N/mm, then the polymeric material of the second component can be configured to provide the remaining stiffness of 150 N/mm.
FIG. 1 illustrates an exemplary stress-strain curve of various materials.Line 2 illustrates a desired curve for an ideal graft.Line 4 illustrates an exemplary actual stress-strain curve for either a synthetic or natural tissue graft. As can be seen, the actual grafts are not capable of reaching the desired stress levels for the same amount of strain sought in a desired graft.- In some embodiments, the composite graft material described herein includes both a biologic and a non-biologic material (e.g., preparation of a weave, or of a braid or of a crimp, or of some other configuration such as a layered or rolled configuration), which raises the stress-strain curve for the composite graft material along the y axis. Consequently,
line 6 illustrates a modified, composite graft materials having improved performance compared to synthetic or biologic grafts. FIG. 2 illustrates the load capacity versus time since implantation of the composite graft material to demonstrate the concept of functional target loading of the composite graft material. The biologic material alone has a relatively low load capacity at the time of implantation, but as the body subsequently heals, the load capacity of the biologic component increases over time. Conversely, a polymer graft's load-bearing capacity is relatively high at implantation, but decreases subsequently. It has been found that a composite graft material consistent with the present disclosure (labeled “Hybrid”), which comprises both a biologic component having a biomatrix and a non-biologic component (such as a polymer), has a relatively stable load-bearing capacity over time (that is, the load bearing capacity starts out, and remains, in the “Target Range”).- The non-biologic component can be coupled with the biologic component in a variety of ways. For example, the biologic component may be disposed around the non-biologic component (as exemplified in
FIG. 3 ), or vice versa. Alternatively, the biologic component may be embedded within a coating, a knit, weave, braid or other structure of the non-biologic component. To provide load sharing between the non-biologic component and the biologic component, the two components may, for example, be comingled or layered and rolled tightly around one another. In some embodiments, such structures create friction between the components. In some embodiments, compressive force is added to the layered construct, e.g., by including securing straps similar to a belt and hoop design. FIG. 3 depicts an exemplary embodiment of the composite graft material of the present disclosure. As shown, tubularnon-biologic component 40 is provided havingupper portion 42,upper taper region 43,lower portion 44,lower taper region 45, andneck portion 46.Biologic component 50 is also provided and is illustrated as a sheet with anupper edge 51 and alower edge 52. In some embodiments,biologic component 50 is wrapped aroundneck portion 46 ofnon-biologic component 40 so thatupper edge 51 is in contact proximal to or withupper taper region 43 andlower edge 52 is in contact proximal to or withlower taper region 45.Biologic component 50 can wrap or encircleneck portion 46 one or more times to form a multi-layer wrap. For example,biologic component 50 can wrap aroundneck portion 46 ofnon-biologic component 40 one time, two times, three times, four times, five times, or more.Biologic component 50 can similarly wrap aroundupper portion 42 andlower portion 43 ofnon-biologic component 40 one or more times.- In some embodiments, after wrapping
biologic component 50 aroundnon-biologic component 40,upper portion 42 ofnon-biologic component 40 may be rolled or folded over wrappedbiologic component 50 and towardlower portion 44 such that at least some ofupper portion 42 extends below theupper taper region 43 and overlapsneck portion 46. In some embodiments,lower portion 44 is rolled or folded overbiologic component 50 and towardupper portion 42 such that at least some oflower portion 44 extends abovelower taper region 45 and overlapsneck portion 46, as depicted inFIG. 3A . - In some embodiments, securing straps and/or
tethers 60 are provided to apply compressive force to the rolled composite graft material and provide frictional contact between the biologic component and the non-biologic component. The securing straps may, for example, be constructed of the same material asnon-biologic component 40.Tethers 60 may also be made from the same material as the non-biologic component of the composite material or from other materials such as stainless steel or non-bioabsorbable polymers. Tethers 60 may be useful during implantation or construction of the composite graft material. For example, tethers60 may be used to pull the composite graft material into a bone tunnel.Tethers 60 may also be used, for example, to anchornon-biologic component 40 whilebiologic component 50 is wrapped aroundnon-biologic component 40.Tethers 60 can be attached tonon-biologic component 40 in various ways. For example, tethers60 may be woven, knitted, or braided intonon-biologic component 40.Tethers 60 may also be integrated into thenon-biologic component 40, and may be configured to detach fromnon-biologic component 40. In some embodiments, tethers60 are used as a radiopaque marker.- In some embodiments, a composite graft material in accordance with the present disclosure is constructed with a non-biologic component in the form of a flat sheet, as illustrated in
FIG. 4A .Non-biologic component 80 includesupper portion 82,lower portion 84,neck portion 85, and necklateral edge 86.Upper portion 82 andlower portion 84 may be made of same or different non-biologic material asneck portion 85. For example,neck portion 85 may comprise a high tensile strength textile of a bioabsorbable polymer, whileupper portion 82 andlower portion 84 comprise a relatively low tensile strength textile of a bioabsorbable polymer.Non-biologic component 80 may also, for example, comprise non-textile polymers as previously described. - Referring still to
FIG. 4A ,biologic component 50 may, for example, be provided as a flat sheet of biologic material comprising an acellular tissue matrix having anupper edge 51, alower edge 52, and alateral edge 53. In some embodiments,non-biologic component 80 is placed on top ofbiologic component 50 such that necklateral edge 86 ofnon-biologic component 80 aligns withlateral edge 53 ofbiologic component 50, and the two layered components are then rolled such that necklateral edge 86 andlateral edge 53 remain aligned and form the innermost portion of the roll. FIG. 4B shows a non-limiting embodiment of a rolled composite graft material in accordance with the present disclosure. This rolled composite graft material is constructed as described above, with thebiologic component 50 forming the outside surface of the rolled structure and necklateral edge 86 forming the inner most portion of the rolled structure.Upper portion 82 andlower portion 84 form a multi-layered non-biologic material within the rolled structure and can provide added strength and material to secure the composite graft material to surrounding native tissue, for example, by interference screws.- In some embodiments, the composite graft material is designed to match the size (length, width, thickness) of the natural structure (i.e., ligament or tendon) it will replace. For example, for an ACL, the composite graft material may be designed to be about 6 to 12 mm in diameter for a unibody device or 3 to 6 mm in diameter if separated into two bundles. It is known that within the body, specific ligament sizes slide and fit between bony structures. A ligament that is too small may not distribute stress evenly, and a ligament that is too large may interfere with or rub against one or both bony structures. Thus, matching the size of the implant material with the native tissue to be removed can reduce complications.
- In some embodiments, the size of the composite graft material is customized to the patient and the tissue being replaced. For example, one or more rolls of biologic and non-biologic components can be added or removed by varying the size or length of the individual components. The longer the pre-rolled composite construct, the more rolls are possible, thereby producing a finished composite graft material having a greater diameter. Alternatively the number of layers of individual components can be altered to adjust the final size of the graft material. For example, a composite construct comprising a tissue layer, a polymer layer, and another tissue layer would provide a larger diameter graft than a composite construct of only two layers.
- Various techniques may be employed to anchor or fix the composite graft material to an implantation site. For example, in an ACL replacement, interference screws are used for anchoring cadaver or autograft materials. In some embodiments, an interference screw is provided that anchors the composite graft material. This is shown in
FIG. 5 , whereininterference screw 90 is inserted into the core ofgraft 94 such that as interference screw90 advances, screw90 expands the diameter ofgraft 94 to exert outwardly radial pressure against the surround bone tunnel. Other common fixation devices may be used, including cross-pins, endobuttons, sutures, or staples. - In some embodiments, screws or other anchoring devices can be made of titanium, stainless steel, biodegradable metals, biodegradable or bioabsorbable polymers such as polylactic acid (PIA), polyglycolic acid (PGA), polylactideglycolide acid (PLGA), polydioxanone (PDO), or polycaprolactone (PCL). As a non-limiting example of a typical interference screw, non-limiting mention is made of the RCI screw manufactured by Smith and Nephew, Andover Mass., 01810.
- The biologic component comprising the biomatrix material of the disclosed composite graft material may benefit from the stress of ordinary movement following implantation. For example, the stresses and strains caused by ordinary activity may cause the biologic tissue to be stronger and recover to a greater ultimate strength due to normal remodeling facilitated by mechanical forces.
- In some embodiments, the non-biologic component of the composite tissue graft material described herein is preloaded with a tensile load ranging from greater than 0 N to about 1800 N (e.g., from about 0.1 to about 1700 N; about 10 to about 1600 N, about 100 to about 1500 N, about 150 to 1400 N, about 200 to 1300 N, etc.). The initial stress on strain on the non-biologic material is partially transferred to the biologic component, with the non-biologic component assuming more of the applied stress after reaching a preloaded limit.
- As previously discussed,
FIG. 1 illustrates an exemplary stress-strain curve of various tissue grafts.Line 2 illustrates a desired curve for an ideal graft.Line 4 illustrates an exemplary actual stress-strain curve for either a synthetic or natural tissue graft. As can be seen, natural grafts are not capable of reaching the desired stress levels for the same amount of strain sought in a desired graft. In contrast, because it incorporates both biologic and non-biologic materials, some embodiments of the composite graft materials described herein may exhibit a stress-strain curve that is shifted along the y axis. Consequently,line 6 ofFIG. 1 illustrates a composite graft material having improved load capacity performance over actual synthetic or biologic grafts. - In some embodiments, the non-biologic component, the biologic component, or both the non-biologic and biologic components can be placed under tensile stress prior to implantation. This has the effect of moving the stress-strain curve of the material along the x-axis as depicted in
FIG. 7 .Line 2 illustrates a desired curve for an ideal graft material.Line 5 illustrates an actual curve for synthetic or natural tissue graft, and line7 illustrates a curve of a composite graft material that has been subjected to preloading with a tensile stress. - In some embodiments, a portion of the tensile load applied to the non-biologic component is transferred to the biologic component. The initial strain applied is selected to be high enough to prevent or retard resorption of the biologic component upon implantation, but low enough to avoid physically damaging the biologic tissue. For example, the strain applied to the biologic component may range from less than or equal to 40%, less than or equal to 35%, less than or equal to 30%, less than or equal to 25%, less than or equal to 20%, less than or equal to 15%, less than or equal to 10%, less than or equal to 5%, and less than or equal to 1% of the initial strain applied to the non-biologic material by an initial tensile load. The initial strain applied to the biologic component may also fall within any range specified by a combination of the above recited endpoints, e.g., from greater than or equal to 1% to less than or equal to 40%, from greater than or equal to 5% to less than or equal to 35%, from greater than or equal to 10% to less than or equal to 30%, and from greater than or equal to 15% to less than or equal to 25% of the initial strain imparted to the non-biologic component by an initial tensile load.
- In some embodiments, a composite graft material can be made using a plurality of elongate non-biologic components wrapped with at least one layer of a biologic component. The elongate non-biologic components can be placed under a tensile stress ranging from greater than 0 N to 1800 N (e.g., from about 100 to about 1700 N; about 200 to about 1600 N, about 300 to about 1500 N, etc.), prior to wrapping with the biologic component. The plurality of elongate non-biologic components may also be pre-wrapped in a sheath comprising non-biologic components. At least one end of the non-biologic components can be secured (e.g., by whipping, wrapping, and/or winding) with an additional elongate component to form multiple layers with a raised surface at the end. The biologic component may, for example, be wrapped around the secured plurality of elongate non-biologic components and secured with a smaller fastening or whipping adjacent to the raised surface formed by the secured biologic component.
- In some embodiments, all of the plurality of elongate non-biologic components can be placed under a tensile stress prior to forming the composite graft material. In other embodiments, a percentage of the elongate non-biologic components can be placed under tensile stress prior to forming the composite graft material, while a percentage of the elongate biologic components can be free of tensile stress prior to forming the composite graft material. Further, in some embodiments, the plurality of elongate non-biologic components and the biologic component can be placed under a tensile stress prior to final assembly of the graft.
FIG. 8A illustrates a plurality of elongatenon-biologic components 810. The plurality of elongate components can be bundled as a group having between 200 and 1200 individual elongate components, depending on the size and mechanical requirements of the composite graft material. In some embodiments, the number of individual components can be between 400 and 1000, 600 and 800, or approximately 700 individual components. The elongate non-biologic components may also be bioabsorbable. The plurality ofelongate components 810 can have aproximal end 812 and adistal end 814. Securing elements816 (e.g., whipping elements) can be wrapped around the plurality of components to secure thecomponents 810 as abundle 818. The plurality ofelongate components 810 can be placed under a tensile load prior to securing. Securingelements 816 are attached to the plurality ofcomponents 810 in such a manner as to retain the preloaded tensile stress in the plurality of elongate components.FIG. 8B illustrates a non-limiting embodiment of the present disclosure, whereinsheath 815 of bioabsorbable non-biologic material is wrapped around the plurality ofelongate components 810. The plurality of elongate components may be loaded with a tensile stress independently of the sheath, or the sheath and plurality of elongate components can be loaded together. Securingelements 816 can be wrapped around and over thesheath 815 of non-biologic material.- In some embodiments, a biologic component containing a biomatrix is used to cover or coat at least a portion of the plurality of elongate non-biologic components. In some embodiments, the biologic component may be in the form of a sheet wrapped around a secured plurality of elongate non-biologic components. As a non-limiting example,
FIG. 9 illustrates a plurality of elongatenon-biologic components 910 having aproximal end 912 anddistal end 914. Securingelements 916 are wrapped around the plurality of elongate components to secure the elongate components as abundle 918 and maintain a preloaded tensile stress in theelongate bundle 918. Securingelements 916 can be wrapped in multiple layers to form a raisedsurface 917. - In some embodiments,
biologic component 920 may be wrapped in one or more layers around theelongate bundle 918 and secured by second securingelements 922 proximate the raisedsurface 917 formed by securingelements 916. Second securingelements 922 can be secured about thebiologic component 920 and theelongate bundle 918 such that the biologic component is free from tensile stress. In some embodiments, thebiologic component 920 can be placed under tensile stress and secured by second securingelements 922 to maintain the tensile load in thebiologic component 920. In some embodiments, the tensile load in the biologic component can range from greater than 0 N to about 1800 N, from greater than 0 to about 600 N, from about 50 to about 300 N, or from about 100 to about 200 N. In some embodiments, thebiologic component 920 may, be pre-loaded to the same tensile stress as the plurality ofelongate components 910. In some embodiments, thebiologic component 920 may also be pre-loaded to tensile stress less than that of the plurality ofelongate components 910. In some embodiments, thebiologic component 920 can be pre-loaded to a tensile stress more than that of the plurality ofelongate components 910. - In some embodiments, the biologic component can be paired with the plurality of elongate elements in a variety of ways, including: as a single layer sheet wrapped about the elongate non-biologic components; in a jellyroll manner wherein the biologic component is wrapped in multiple layers; as a non-uniform sheet such that multiple layers of biologic component are not concentric about the plurality of elongate elements; as a top sheath wrapped or coated over an inner sheath of non-biologic material; as a coating about the outer surface of the bundle of elongate non-biologic elements; and/or as a coating interspersed throughout the bundle of elongate non-biologic components.
FIG. 10A showscomposite graft material 1000 comprising a plurality of elongatenon-biologic elements 1010 andbiologic component 1020, whereinbiologic component 1020 is wrapped as a sheet in a single layer over the plurality ofelongate elements 1010.FIG. 10B shows acomposite graft material 1001 comprising a plurality of elongatenon-biologic elements 1011 and biologic component1021, wherein biologic component1021 is wrapped as a sheet in a jellyroll fashion with multiple layers over the plurality ofelongate elements 1011.FIG. 10C shows a composite graft material1002 comprising a plurality of elongatenon-biologic elements 1012 andbiologic component 1022, whereinbiologic component 1022 is wrapped as a non-uniform sheet in a jellyroll fashion with multiple non-concentric layers over the plurality ofelongate elements 1012.FIG. 10D shows acomposite graft material 1003 comprising a plurality of elongatenon-biologic elements 1013 andbiologic component 1023, whereinbiologic component 1023 is wrapped as a single layer sheet forming an outer sheath overinner sheath 1035 of non-biologic material.Inner sheath 1035 is wrapped about the plurality of elongate non-biologic components.Biologic component 1020 may also be applied as a coating. The coating can, for example, be in the form of a liquid, a powder, or a spray, and may be applied using any suitable technique.FIG. 10E shows acomposite graft material 1004 comprising a plurality of elongatenon-biologic elements 1014 andbiologic component 1024, whereinbiologic component 1024 coats the outer surface of the plurality ofelongate elements 1015.FIG. 10F shows acomposite graft material 1005 comprising a plurality of elongate non-biologic elements andbiologic component 1025, whereinbiologic component 1025 is interspersed between and coats the outer surfaces of the individual elongate elements in the plurality ofelongate elements 1015, as shown inFIG. 10G .- In some embodiments, the plurality of elongate non-biologic elements are secured by a securing material (e.g., a whipping material) such that the securing material is wound about the plurality of elongate non-biologic elements in a manner that is engageable with an anchor screw. The securing material may, for example, be wound to create a screw-thread pattern having a pitch compatible with an anchoring screw to better engage and lock the composite graft material to the anchoring screw upon implantation in the patient. The material used to secure the tissue or biologic component to the plurality of elongate non-biologic elements can also be wound so as to have a screw-screw thread pattern engageable with an anchoring screw. The securing material can form the male or female thread to the threads of the anchoring screw.
- In some embodiments, the composite graft material may be used for ACL replacement. In some embodiments, the composite graft material is designed to have the properties of a typical ACL, e.g., failure load (1200-2400 N); stiffness (150-300 N/mm); failure stress (18-28 MPa); strain at failure (20-35%); and modulus of elasticity (75-180 MPa).
- In some embodiments, the natural mechanical and biologic properties of native ACL tissue may be matched, for example, by selecting and constructing each component of a device to meet specific design requirements. For example, a device meeting the general characteristics of an ACL may be made with a non-biologic component having a modulus of elasticity of about 140 MPa, a maximum rupture load or ultimate load failure of about 1200 N, and degradation resistance through 9 to 16 months before construction of the composite graft material. In some embodiments, the biologic component exhibits a modulus of elasticity of about 55 MPa at the time of implantation and a maximum load at rupture of approximately 600 N before construction of the composite graft material.
- In some embodiments, a composite graft material suitable for ACL repair may, for example, exhibit a maximum rupture load of approximately 1400 N before implantation. In some embodiments, the ultimate failure load of the non-biologic component of such an implant may decrease after implantation, while the failure load of the biologic component will increase over time as native cells proliferate through the biomatrix. In some embodiments, the implant may have an ultimate failure load of approximately 600 N within four months of implantation, approximately 400 N within eight months of implantation, and approximately 1000 N within 12 months of implantation. In some embodiments, the composite graft material for ACL replacement may have a stiffness of approximately 85 N/mm before implantation, approximately 106 N/mm within four months of implantation, approximately 78 N/mm within eight months of fixation, and approximately 176 N/mm within twelve months of fixation.
FIG. 6 depicts the load capacity over time of the first component, the second component and the composite graft material of a non-limiting example of a composite ACL graft according to various embodiments. As shown, the first component,line 8, exhibited a drop in load capacity immediately following implantation with an increase in load capacity thereafter as native cells proliferated throughout the biomatrix.Line 10 illustrates the initially high load capacity of the second component after implantation with a steady decrease in load capacity as the second component degraded over time.Line 12 shows the load capacity of the composite graft containing both the first component and the second component.- Biologic components that may be suitably used to produce composite graft materials can include any biologic material (e.g., whole tissue or tissue-derived material) with the properties described herein. Non-limiting examples of such biologic materials include biomatrices, such as acellular tissue matrices.
- As used herein, the term “acellular tissue matrix” (“ATM”) refers to a tissue-derived biomatrix structure that is made from any of a wide range of collagen-containing tissues by removing all or substantially all viable cells and all detectable subcellular components and/or debris generated by killing cells. As used herein, an ATM lacking “substantially all viable cells” is an ATM in which the concentration of viable cells is less than 1% of that in the tissue or organ from which the ATM was made.
- Accordingly, in some non-limiting embodiments, the ATMs of the present disclosure contain epithelial basement membrane. In other non-limiting embodiments, the composite grafts disclosed herein lack or substantially lack epithelial basement membrane. In some embodiments, the ATMs include a vascular basement membrane that may facilitate ingrowth of vascular endothelial cells.
- ATM's suitable for use in the present disclosure may, for example, retain certain biologic functions, such as cell recognition, cell binding, the ability to support cell spreading, cell proliferation, cellular in-growth and cell differentiation. Such functions may be provided, for example, by undenatured collagenous proteins (e.g., type I collagen) and a variety of non-collagenous molecules (e.g., proteins that serve as ligands for either molecules such as integrin receptors, molecules with high charge density such as glycosaminoglycans (e.g., hyaluronan) or proteoglycans, or other adhesins). In some embodiments, the ATM's may retain certain structural functions, including maintenance of histological architecture and maintenance of the three-dimensional array of the tissue's components. The ATM's described herein may also, for example, exhibit desirable physical characteristics such as strength, elasticity, and durability, defined porosity, and retention of macromolecules.
- ATMs suitable for use in the present disclosure may be crosslinked or uncrosslinked. In some non-limiting embodiments, the composite graft includes an uncrosslinked ATM. The efficiency of the biologic functions of an ATM can be measured, for example, by the ability of the ATM to support cell proliferation. In some embodiments of the present disclosure, the ATM exhibits at least 50% (e.g., at least: 50%; 60%; 70%; 80%; 90%; 95%; 98%; 99%; 99.5%; 100%; or more than 100%, or any ranges between 50%-100%) of that of the native tissue or organ from which the ATM is made.
- In some embodiments, the biologic component, when implanted, is amenable to being remodeled by infiltrating cells such as differentiated cells of the relevant host tissue, stem cells such as mesenchymal stem cells, or progenitor cells. Remodeling may be directed by the above-described ATM components and signals from the surrounding host tissue (such as cytokines, extracellular matrix components, biomechanical stimuli, and bioelectrical stimuli). For example, the presence of mesenchymal stem cells in the bone marrow and the peripheral circulation has been documented in the literature and shown to regenerate a variety of musculoskeletal tissues [Caplan (1991) J. Orthop. Res. 9:641-650; Caplan (1994) Clin. Plast. Surg. 21:429-435; and Caplan et al. (1997) Clin. Orthop. 342:254-269]. Additionally, in some embodiments, the graft will provide some degree (greater than threshold) of tensile and biomechanical strength during the remodeling process.
- An ATM in accordance with the present disclosure may be manufactured from a variety of source tissues. For example, the ATM may be produced from any collagen-containing soft tissue and musculo-skeletonal tissue (e.g., dermis, fascia, pericardium, dura, umbilical cords, placentae, cardiac valves, ligaments, tendons, vascular tissue (arteries and veins such as saphenous veins), neural connective tissue, urinary bladder tissue, ureter tissue, or intestinal tissue), as long as the above-described properties are retained by the matrix. Moreover, the tissues in which the matrices containing the ATM are placed may include any tissue that can be remodeled by invading or infiltrating cells. Non-limiting examples of such tissues include skeletal tissues such as bone, cartilage, ligaments, fascia, and tendon. Other tissues in which any of the above grafts can be placed include, for example, skin, gingiva, dura, myocardium, vascular tissue, neural tissue, striated muscle, smooth muscle, bladder wall, ureter tissue, intestine, and urethra tissue.
- While an acellular tissue matrix may be made from one or more individuals of the same species as the recipient of the acellular tissue matrix graft, this is not necessarily the case. Thus, for example, an acellular tissue matrix may be made from porcine tissue and implanted in a human patient. Species that can serve as recipients of acellular tissue matrix and donors of tissues or organs for the production of the acellular tissue matrix include, without limitation, mammals, such as humans, nonhuman primates (e.g., monkeys, baboons, or chimpanzees), pigs, cows, horses, goats, sheep, dogs, cats, rabbits, guinea pigs, gerbils, hamsters, rats, or mice.
- As an example of suitable porcine-derived tissue, non-limiting mention is Strattice™, which is a porcine dermal tissue produced by Lifecell Corp, Branchburg, N.J. The tissue matrix may be derived from porcine skin by removing the epidermis while leaving the dermal matrix substantially intact. In some embodiments, the porcine-derived tissue matrix may facilitate tissue ingrowth and remodeling with the patient's own cells. In other embodiments, the material can include a collagenous matrix derived from human cadaver skin (e.g. AlloDerm™, Lifecell Corp, Branchburg, N.J.) that has been processed to remove both the epidermis and cells.
- In general, the steps involved in the production of an acellular tissue matrix include harvesting the tissue from a donor (e.g., a human cadaver or animal source) and cell removal under conditions that preserve biologic and structural function. In certain embodiments, the process includes chemical treatment to stabilize the tissue and avoid biochemical and structural degradation together with or before cell removal. In various embodiments, the stabilizing solution arrests and prevents osmotic, hypoxic, autolytic, and proteolytic degradation, protects against microbial contamination, and reduces mechanical damage that can occur with tissues that contain, for example, smooth muscle components (e.g., blood vessels). The stabilizing solution may contain an appropriate buffer, one or more antioxidants, one or more oncotic agents, one or more antibiotics, one or more protease inhibitors, and/or one or more a smooth muscle relaxant.
- The tissue is then placed in a decellularization solution to remove viable cells (e.g., epithelial cells, endothelial cells, smooth muscle cells, and fibroblasts) from the structural matrix without damaging the biologic and structural integrity of the collagen matrix. The decellularization solution may contain an appropriate buffer, salt, an antibiotic, one or more detergents (e.g., TRITON X-100™, sodium deoxycholate, polyoxyethylene (20) sorbitan mono-oleate), one or more agents to prevent cross-linking, one or more protease inhibitors, and/or one or more enzymes. In some embodiments, the decellularization solution comprises 1% TRITON X-100™ in RPMI media with Gentamicin and 25 mM EDTA (ethylenediaminetetraacetic acid). In some embodiments, the tissue is incubated in the decellularization solution overnight at 37 DC with gentle shaking at 90 rpm. In certain embodiments, additional detergents may be used to remove fat from the tissue sample. For example, in some embodiments, 2% sodium deoxycholate is added to the decellularization solution.
- After the decellularization process, the tissue sample is washed thoroughly with saline. In some exemplary embodiments, e.g., when xenogenic material is used, the decellularized tissue is then treated overnight at room temperature with a deoxyribonuclease (DNase) solution. In some embodiments, the tissue sample is treated with a DNase solution prepared in DNase buffer (20 mM HEPES (4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid), 20 mM CaCl2 and 20 mM MgCl2). Optionally, an antibiotic solution (e.g., Gentamicin) may be added to the DNase solution. Any suitable buffer can be used as long as the buffer provides suitable DNase activity.
- Elimination of the α-gal epitopes from the collagen-containing material may diminish the immune response against the collagen-containing material. The α-gal epitope is expressed in non-primate mammals and in New World monkeys (monkeys of South America) as well as on macromolecules such as proteoglycans of the extracellular components. U. Galili et al., J. Biol. Chem. 263: 17755 (1988). This epitope is absent in Old World primates (monkeys of Asia and Africa and apes) and humans, however. Id. Anti-gal antibodies are produced in humans and primates as a result of an immune response to α-gal epitope carbohydrate structures on gastrointestinal bacteria. U. Galili et al., Infect. Immun. 56: 1730 (1988); R. M. Hamadeh et al., J. Clin. Invest. 89: 1223 (1992).
- Since non-primate mammals (e.g., pigs) produce α-gal epitopes, xenotransplantation of collagen-containing material from these mammals into primates often results in rejection because of primate anti-Gal binding to these epitopes on the collagen-containing material. The binding results in the destruction of the collagen-containing material by complement fixation and by antibody dependent cell cytotoxicity. U. Galili et al., Immunology Today 14:480 (1993); M. Sandrin et al., Proc. Natl. Acad. Sci. USA 90: 11391 (1993); H. Good et al., Transplant. Proc. 24: 559 (1992); B. H. Collins et al., J. Immunol. 154: 5500 (1995). Furthermore, xenotransplantation results in major activation of the immune system to produce increased amounts of high affinity anti-gal antibodies. Accordingly, in some embodiments, when animals that produce α-gal epitopes are used as the tissue source, the substantial elimination of α-gal epitopes from cells and from extracellular components of the collagen-containing material, and the prevention of re-expression of cellular α-gal epitopes can diminish the immune response against the collagen-containing material associated with anti-gal antibody binding to α-gal epitopes.
- To remove α-gal epitopes, after washing the tissue thoroughly with saline to remove the DNase solution, the tissue sample may be subjected to one or more enzymatic treatments to remove certain immunogenic antigens, if present in the sample. In some embodiments, the tissue sample may be treated with an α-galactosidase enzyme to eliminate α-gal epitopes if present in the tissue. In some embodiments, the tissue sample is treated with α-galactosidase at a concentration of 300 U/L prepared in 100 mM phosphate buffer at pH 6.0 In other embodiments, the concentration of α-galactosidase is increased to 400 u/L for adequate removal of the α-gal epitopes from the harvested tissue. Any suitable enzyme concentration and buffer can be used as long as sufficient removal of antigens is achieved.
- Alternatively, rather than treating the tissue with enzymes, animals that have been genetically modified to lack one or more antigenic epitopes may be selected as the tissue source. For example, animals (e.g., pigs) that have been genetically engineered to lack the terminal α-galactose moiety can be selected as the tissue source. For descriptions of appropriate animals see co-pending U.S. application Ser. No. 10/896,594 and U.S. Pat. No. 6,166,288, the disclosures of which are incorporated herein by reference in their entirety.
- After the acellular tissue matrix is formed, histocompatible, viable cells may optionally be seeded in the acellular tissue matrix to produce a graft that may be further remodeled by the host. In some embodiments, histocompatible viable cells may be added to the matrices by standard in vitro cell co-culturing techniques prior to transplantation, or by in vivo repopulation following transplantation. In vivo repopulation can be by the recipient's own cells migrating into the acellular tissue matrix or by infusing or injecting cells obtained from the recipient or histocompatible cells from another donor into the acellular tissue matrix in situ. Various cell types can be used, including embryonic stem cells, adult stem cells (e.g. mesenchymal stem cells), and/or neuronal cells. In various embodiments, the cells can be directly applied to the inner portion of the acellular tissue matrix just before or after implantation. In certain embodiments, the cells can be placed within the acellular tissue matrix to be implanted, and cultured prior to implantation.
- Particulate ATM can be made from any of the above described non-particulate ATMs by any process that results in the preservation of the biologic and structural functions described above. As used herein, particulate ATMs are those particulate or pulverized (powdered) matrices having a longest dimension of 1.0 mm or less.
- In some embodiments, particulate ATM used in the present disclosure is manufactured so as to minimize damage to collagen fibers, including sheared fiber ends. As a non-limiting example of a suitable method for making particulate ATM is described in U.S. Pat. No. 6,933,326. The particle size for Cymetra is in the range of about 60 microns to about 150 microns as determined by mass spectrophotometry.
- The at least one non-biologic component of the present disclosure may, for example, comprise biocompatible natural and/or synthetic materials. Biocompatible natural materials may include, for example, collagen, fibrin, and silk. Biocompatible synthetic materials may include, for example, bioabsorbable polymers, non-bioabsorbable polymers, and metallic alloys or compositions. In some embodiments of the present disclosure, a non-biologic component that is biocompatible and bioabsorbable is used. Utilizing bioabsorbable polymers may allow for a transfer of loads from the non-biologic component (e.g., the polymer) to the biologic component as native tissue regenerates throughout the matrix structure of the biologic component.
- As used herein, a “biocompatible” composition is one that has the ability to support cellular activity necessary for complete or partial tissue regeneration, but does not stimulate an unacceptable inflammatory or immunological response in the host. The term, “unacceptable local inflammatory or immunological response in the host” means a local or systemic inflammatory or immunologic response that prevents tissue regeneration.
- As used herein, the term “bioabsorbable” means that a material can be absorbed by a mammalian body via biologically mediated degradation processes, such as enzymatic and cellular processes and/or chemically mediated degradation processes. Such processes include, for example, degradation processes wherein the degradation products are excreted through one of the body's organ systems or in which the degradation products are incorporated into normal metabolic pathways.
- In some embodiments, a suitable bioabsorbable material for use in the present disclosure, is made of a poly-hydroxybutyrate (a polyhydroxyalkanoate), such as the TephaFlex polymer produced by Tepha, Inc. of Cambridge, Mass. In some embodiments, useful bioabsorbable materials include, for example, polylactic acid (PLA), polyglycolic acid (PGA), polylactideglycolide acid (PLGA), polydioxanone (PDO), or polycaprolactone (PCL). In some embodiments, bioabsorbable materials suitable for use in the present disclosure include polyanhydrides, polyorthoesters, poly(amino acids), polypeptides, polydepsipeptides, nylon-2/nylon-6coplyamides, poly(alkylene succinates), poly(hydroxyl butyrate) (PHB), poly (butylene diglocolate), poly (ε-caprolactone), polydihydropyrans, polyphosphazenes, poly (ortho ester), poly (cyano acrylates), modified polysaccharides, cellulose, starch, chitin, modified proteins, collagen, fibrin, and combinations and copolymers thereof. Non-limiting examples of non-bioabsorbable materials include noble metals such as gold, as well as the near noble metals.
- In some embodiments, synthetic polymers that may be used in accordance with the present disclosure include those listed in U.S. Pat. No. 5,885,829, the disclosure of which is incorporated herein by reference in its entirety.
- The non-biologic components used herein may be provided in any form. In some embodiments, the non-biologic components are in the form of a molded shape (e.g., as a single contiguous polymeric piece). Further, in some embodiments, the non-biologic components are in the form of a textile comprised of multiple yarns, the yarn being either a monofilament or a multifilament structure (such as a braid). Textile manufacturing methods can then make final structures that are knitted, woven, braided, nonwoven, or combinations thereof.
- Although the present disclosure has been described with reference to certain non-limiting embodiments, other implementations are possible. For example, the composite material may be used for many applications where soft tissues need to be replaced and yet provide specific load-carrying or biomechanical characteristics, including ligament, tendon or soft tissue replacement about the knees, ankles, shoulders, neck, and spine. Other hybrid systems can be developed utilizing the same basic ideas as described above. Examples include: artificial meniscus replacement or repair; abdominal wall (e.g., hernia) repair; cartilage repair in, for example, knees, shoulders and hips; joint resurfacing (instead of removing joints, the joint articulating surface can simply be covered with a composite material reinforced with fabric using appropriately designed anchors or sutures); and pace maker pouches (a simple bag/pouch used to contain pacers or pain manager systems would make periodic replacement much simpler and would create a more stable anchor pacer implant).
- Accordingly, other embodiments will be apparent to those skilled in the art from consideration of the specification disclosed herein. It is intended that the specification and embodiments disclosed herein be considered as exemplary only, with a true scope being indicated by the following claims.
Claims (27)
Priority Applications (3)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US15/210,309US20160317711A1 (en) | 2008-11-21 | 2016-07-14 | Reinforced biologic material |
| US16/227,814US20190184062A1 (en) | 2008-11-21 | 2018-12-20 | Reinforced biologic material |
| US16/418,401US20190275207A1 (en) | 2008-11-21 | 2019-05-21 | Reinforced biologic material |
Applications Claiming Priority (4)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US11706808P | 2008-11-21 | 2008-11-21 | |
| US12/621,890US8333803B2 (en) | 2008-11-21 | 2009-11-19 | Reinforced biologic material |
| US13/616,556US9421306B2 (en) | 2008-11-21 | 2012-09-14 | Reinforced biologic material |
| US15/210,309US20160317711A1 (en) | 2008-11-21 | 2016-07-14 | Reinforced biologic material |
Related Parent Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US13/616,556ContinuationUS9421306B2 (en) | 2008-11-21 | 2012-09-14 | Reinforced biologic material |
Related Child Applications (2)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US16/227,814ContinuationUS20190184062A1 (en) | 2008-11-21 | 2018-12-20 | Reinforced biologic material |
| US16/418,401ContinuationUS20190275207A1 (en) | 2008-11-21 | 2019-05-21 | Reinforced biologic material |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| US20160317711A1true US20160317711A1 (en) | 2016-11-03 |
Family
ID=42198794
Family Applications (5)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US12/621,890Expired - Fee RelatedUS8333803B2 (en) | 2008-11-21 | 2009-11-19 | Reinforced biologic material |
| US13/616,556Expired - Fee RelatedUS9421306B2 (en) | 2008-11-21 | 2012-09-14 | Reinforced biologic material |
| US15/210,309AbandonedUS20160317711A1 (en) | 2008-11-21 | 2016-07-14 | Reinforced biologic material |
| US16/227,814AbandonedUS20190184062A1 (en) | 2008-11-21 | 2018-12-20 | Reinforced biologic material |
| US16/418,401AbandonedUS20190275207A1 (en) | 2008-11-21 | 2019-05-21 | Reinforced biologic material |
Family Applications Before (2)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US12/621,890Expired - Fee RelatedUS8333803B2 (en) | 2008-11-21 | 2009-11-19 | Reinforced biologic material |
| US13/616,556Expired - Fee RelatedUS9421306B2 (en) | 2008-11-21 | 2012-09-14 | Reinforced biologic material |
Family Applications After (2)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US16/227,814AbandonedUS20190184062A1 (en) | 2008-11-21 | 2018-12-20 | Reinforced biologic material |
| US16/418,401AbandonedUS20190275207A1 (en) | 2008-11-21 | 2019-05-21 | Reinforced biologic material |
Country Status (6)
| Country | Link |
|---|---|
| US (5) | US8333803B2 (en) |
| EP (1) | EP2349089A4 (en) |
| JP (1) | JP5784500B2 (en) |
| AU (1) | AU2009316588B2 (en) |
| CA (1) | CA2744232C (en) |
| WO (1) | WO2010059783A2 (en) |
Cited By (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US10639398B2 (en) | 2016-07-05 | 2020-05-05 | Lifecell Corporation | Tissue matrices incorporating multiple tissue types |
Families Citing this family (61)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US6497726B1 (en) | 2000-01-11 | 2002-12-24 | Regeneration Technologies, Inc. | Materials and methods for improved bone tendon bone transplantation |
| US20030023304A1 (en)* | 2000-01-11 | 2003-01-30 | Carter Kevin C. | Materials and methods for improved bone tendon bone transplantation |
| US7195642B2 (en)* | 2001-03-13 | 2007-03-27 | Mckernan Daniel J | Method and apparatus for fixing a graft in a bone tunnel |
| US20090062909A1 (en) | 2005-07-15 | 2009-03-05 | Micell Technologies, Inc. | Stent with polymer coating containing amorphous rapamycin |
| WO2007011707A2 (en) | 2005-07-15 | 2007-01-25 | Micell Technologies, Inc. | Polymer coatings containing drug powder of controlled morphology |
| EP2944382A1 (en) | 2006-04-26 | 2015-11-18 | Micell Technologies, Inc. | Coatings containing multiple drugs |
| EP2111184B1 (en) | 2007-01-08 | 2018-07-25 | Micell Technologies, Inc. | Stents having biodegradable layers |
| US11426494B2 (en) | 2007-01-08 | 2022-08-30 | MT Acquisition Holdings LLC | Stents having biodegradable layers |
| US8753391B2 (en)* | 2007-02-12 | 2014-06-17 | The Trustees Of Columbia University In The City Of New York | Fully synthetic implantable multi-phased scaffold |
| US20130116799A1 (en) | 2008-03-27 | 2013-05-09 | The Cleveland Clinic Foundation | Reinforced tissue graft |
| US20110014153A1 (en) | 2008-03-27 | 2011-01-20 | Kathleen Derwin | Reinforced tissue graft |
| MX350637B (en) | 2008-04-17 | 2017-09-11 | Micell Technologies Inc | Stents having bioabsorbable layers. |
| EP2349089A4 (en) | 2008-11-21 | 2014-01-15 | Lifecell Corp | Reinforced biologic material |
| US8469779B1 (en) | 2009-01-02 | 2013-06-25 | Lifecell Corporation | Method for debristling animal skin |
| US9981072B2 (en) | 2009-04-01 | 2018-05-29 | Micell Technologies, Inc. | Coated stents |
| EP2453834A4 (en) | 2009-07-16 | 2014-04-16 | Micell Technologies Inc | Drug delivery medical device |
| US9649408B1 (en) | 2009-11-05 | 2017-05-16 | Lifecell Corporation | Systems and methods for sterilization of bone or bone components |
| US8449612B2 (en) | 2009-11-16 | 2013-05-28 | Arthrocare Corporation | Graft pulley and methods of use |
| US20110190886A1 (en)* | 2010-01-29 | 2011-08-04 | Wisconsin Alumni Research Foundation | Braided tertiary nanofibrous structure for ligament, tendon, and muscle tissue implant |
| EP2531140B1 (en) | 2010-02-02 | 2017-11-01 | Micell Technologies, Inc. | Stent and stent delivery system with improved deliverability |
| WO2011115229A1 (en)* | 2010-03-17 | 2011-09-22 | 株式会社ビー・アイ・テック | Stem structure for composite prosthetic hip and method for manufacturing the same |
| US9757132B2 (en)* | 2010-03-24 | 2017-09-12 | Biorez, Inc. | Mechanically competent scaffold for rotator cuff and tendon augmentation |
| AU2011232374B2 (en)* | 2010-03-25 | 2015-06-25 | Lifecell Corporation | Preparation of regenerative tissue scaffolds |
| WO2012006390A1 (en) | 2010-07-08 | 2012-01-12 | Lifecell Corporation | Method for shaping tissue matrices |
| EP2593039B1 (en) | 2010-07-16 | 2022-11-30 | Micell Technologies, Inc. | Drug delivery medical device |
| WO2012142419A1 (en) | 2011-04-14 | 2012-10-18 | Lifecell Corporation | Regenerative materials |
| US10117972B2 (en) | 2011-07-15 | 2018-11-06 | Micell Technologies, Inc. | Drug delivery medical device |
| US9089523B2 (en) | 2011-07-28 | 2015-07-28 | Lifecell Corporation | Natural tissue scaffolds as tissue fillers |
| CA2854885C (en) | 2011-11-10 | 2020-08-25 | Lifecell Corporation | Device for tendon and ligament treatment |
| AU2012355463C1 (en) | 2011-12-20 | 2016-09-22 | Lifecell Corporation | Sheet tissue products |
| ES2726105T3 (en) | 2011-12-20 | 2019-10-01 | Lifecell Corp | Fluidizable fabric products |
| AU2013212592B2 (en) | 2012-01-24 | 2016-06-30 | Lifecell Corporation | Elongated tissue matrices |
| BR112014026088B1 (en) | 2012-04-24 | 2019-11-05 | Lifecell Corp | tissue treatment product |
| EP2841116A1 (en) | 2012-04-24 | 2015-03-04 | Lifecell Corporation | Flowable tissue matrices |
| US10363343B2 (en) | 2012-07-05 | 2019-07-30 | Lifecell Corporation | Tissue-based drain manifolds |
| AU2013289045B2 (en) | 2012-07-13 | 2017-02-16 | Lifecell Corporation | Methods for improved treatment of adipose tissue |
| US10246763B2 (en) | 2012-08-24 | 2019-04-02 | The Regents Of The University Of California | Magnesium-zinc-strontium alloys for medical implants and devices |
| US9370536B2 (en) | 2012-09-26 | 2016-06-21 | Lifecell Corporation | Processed adipose tissue |
| EP2953658B1 (en) | 2013-02-06 | 2020-01-01 | LifeCell Corporation | Methods for localized modification of tissue products |
| JP6330024B2 (en) | 2013-03-12 | 2018-05-23 | マイセル・テクノロジーズ,インコーポレイテッド | Bioabsorbable biomedical implant |
| WO2014152252A1 (en) | 2013-03-14 | 2014-09-25 | Active Implants Corporation | Meniscus prosthetic devices with anti-migration or radiopaque features |
| KR102312720B1 (en) | 2013-03-15 | 2021-10-13 | 알로소스 | Cell repopulated collagen matrix for soft tissue repair and regeneration |
| KR20180059584A (en)* | 2013-05-15 | 2018-06-04 | 미셀 테크놀로지즈, 인코포레이티드 | Bioabsorbable biomedical implants |
| CN104208750B (en)* | 2013-12-02 | 2016-08-17 | 四川大学华西医院 | Preparation method and application of acellular tendon material |
| EP3104809B1 (en)* | 2014-02-13 | 2017-12-27 | Antonio Sambusseti | Non-absorbable tissue reconstruction device, in particular for tissues such as ligaments |
| US9801910B2 (en) | 2014-03-17 | 2017-10-31 | Ethicon, Inc. | Decellularized pleural matrix |
| CA2974120A1 (en)* | 2015-01-21 | 2016-07-28 | Lifecell Corporation | Tissue matrices with controlled porosity or mechanical properties |
| US9931196B2 (en)* | 2015-08-26 | 2018-04-03 | Albert Einstein Healthcare Network | Connector for attaching tissue to bone |
| US20170273680A1 (en) | 2016-02-01 | 2017-09-28 | DePuy Synthes Products, Inc. | Tissue augmentation tacks for use with soft tissue fixation repair systems and methods |
| US11484401B2 (en) | 2016-02-01 | 2022-11-01 | Medos International Sarl | Tissue augmentation scaffolds for use in soft tissue fixation repair |
| US11523812B2 (en) | 2016-02-01 | 2022-12-13 | Medos International Sarl | Soft tissue fixation repair methods using tissue augmentation constructs |
| WO2017139102A1 (en) | 2016-02-11 | 2017-08-17 | Lifecell Corporation | Methods for stabilizing collagen-containing tissue products against enzymatic degradation |
| US11160906B2 (en) | 2016-02-18 | 2021-11-02 | The Curators Of The University Of Missouri | Injectable nanomaterial-extracellular matrix constructs |
| WO2017210109A1 (en) | 2016-06-03 | 2017-12-07 | Lifecell Corporation | Methods for localized modification of tissue products |
| US11045583B2 (en) | 2016-12-22 | 2021-06-29 | Lifecell Corporation | Devices and methods for tissue cryomilling |
| JP7297739B2 (en) | 2017-10-18 | 2023-06-26 | ライフセル コーポレーション | Adipose tissue products and manufacturing methods |
| US11123375B2 (en) | 2017-10-18 | 2021-09-21 | Lifecell Corporation | Methods of treating tissue voids following removal of implantable infusion ports using adipose tissue products |
| US11246994B2 (en) | 2017-10-19 | 2022-02-15 | Lifecell Corporation | Methods for introduction of flowable acellular tissue matrix products into a hand |
| AU2018351314A1 (en) | 2017-10-19 | 2020-03-19 | Lifecell Corporation | Flowable acellular tissue matrix products and methods of production |
| US11998654B2 (en) | 2018-07-12 | 2024-06-04 | Bard Shannon Limited | Securing implants and medical devices |
| EP3976127B1 (en) | 2019-05-30 | 2025-09-24 | LifeCell Corporation | Biologic breast implant |
Citations (41)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US3805667A (en)* | 1970-08-21 | 1974-04-23 | Columbian Rope Co | Braided rope |
| US4149277A (en)* | 1977-06-22 | 1979-04-17 | General Atomic Company | Artificial tendon prostheses |
| US4187558A (en)* | 1977-10-25 | 1980-02-12 | Cutter Laboratories, Inc. | Prosthetic ligament |
| US4662886A (en)* | 1984-06-04 | 1987-05-05 | A. W. Showell (Surgicraft) Limited | Surgical element |
| US4713075A (en)* | 1981-06-10 | 1987-12-15 | Kurland Kenneth Z | Method for the repair of connective tissue |
| US4792336A (en)* | 1986-03-03 | 1988-12-20 | American Cyanamid Company | Flat braided ligament or tendon implant device having texturized yarns |
| US4917699A (en)* | 1988-05-16 | 1990-04-17 | Zimmer, Inc. | Prosthetic ligament |
| US4917700A (en)* | 1988-08-01 | 1990-04-17 | Zimmer, Inc. | Prosthetic ligament |
| US5176708A (en)* | 1990-03-12 | 1993-01-05 | Sulzer Brothers Limited | Prosthetic implant |
| US5197983A (en)* | 1988-04-19 | 1993-03-30 | W. L. Gore & Associates, Inc. | Ligament and tendon prosthesis |
| US5258040A (en)* | 1982-09-10 | 1993-11-02 | W. L. Gore & Associates | Prosthesis for tensile load-carrying tissue and method of manufacture |
| US5458636A (en)* | 1994-07-20 | 1995-10-17 | U.S. Biomaterials Corporation | Prosthetic device for repair and replacement of fibrous connective tissue |
| US5514181A (en)* | 1993-09-29 | 1996-05-07 | Johnson & Johnson Medical, Inc. | Absorbable structures for ligament and tendon repair |
| US5800543A (en)* | 1993-03-31 | 1998-09-01 | Surgicraft Limited | Artificial ligament |
| US6143029A (en)* | 1996-03-11 | 2000-11-07 | Rippstein; Pascal Francois | Tendon guide device |
| US6206931B1 (en)* | 1996-08-23 | 2001-03-27 | Cook Incorporated | Graft prosthesis materials |
| US6214047B1 (en)* | 1998-03-10 | 2001-04-10 | University Of Cincinnati | Article and method for coupling muscle to a prosthetic device |
| US20010044659A1 (en)* | 1996-11-20 | 2001-11-22 | Jacques-Phillippe Laboureau | Pre-adjusted prosthetic ligament and method of manufacture |
| US20020038151A1 (en)* | 2000-08-04 | 2002-03-28 | Plouhar Pamela L. | Reinforced small intestinal submucosa (SIS) |
| US20030023316A1 (en)* | 2000-08-04 | 2003-01-30 | Brown Laura Jean | Hybrid biologic-synthetic bioabsorable scaffolds |
| US6592623B1 (en)* | 1999-08-31 | 2003-07-15 | Virginia Commonwealth University Intellectual Property Foundation | Engineered muscle |
| US6599319B2 (en)* | 2001-12-14 | 2003-07-29 | Celanese Advanced Materials, Inc. | Prosthetic ligament |
| US6733510B1 (en)* | 1999-01-12 | 2004-05-11 | University Of Cincinnati | Article and method for coupling muscle to a prosthetic device |
| US20040267362A1 (en)* | 2003-06-30 | 2004-12-30 | Julia Hwang | Scaffold for connective tissue repair |
| US7070558B2 (en)* | 2001-07-27 | 2006-07-04 | Boston Scientific Scimed, Inc. | Medical slings |
| US20070118217A1 (en)* | 2004-01-23 | 2007-05-24 | Lars-Laboratoire D' Application Et De Recherche Scientifique | Method for fixing traction threads to ends of a prosthetic ligament |
| US20070162121A1 (en)* | 2006-01-12 | 2007-07-12 | Tarrant Laurence J | Method for repair and reconstruction of ruptured ligaments or tendons and for treatment of ligament and tendon injuries |
| US20080300683A1 (en)* | 2007-03-20 | 2008-12-04 | Altman Gregory H | Prosthetic device and method of manufacturing the same |
| US20100298937A1 (en)* | 2009-05-22 | 2010-11-25 | Soft Tissue Regeneration, Inc. | Mechanically competent scaffold for ligament and tendon regeneration |
| US20110257744A1 (en)* | 2010-04-14 | 2011-10-20 | The University Of Tokyo | Method for Producing Artificial Bone and Artificial Bone Produced by the Method |
| US8052753B2 (en)* | 2005-01-07 | 2011-11-08 | University Of Cincinnati | Prosthetic anchor and method of making same |
| US20110288565A1 (en)* | 2010-05-19 | 2011-11-24 | University Of Utah Research Foundation | Tissue fixation |
| US20110307059A1 (en)* | 2010-06-11 | 2011-12-15 | AFcell Medical | Method of tendon repair with amnion and chorion constructs |
| US20120071975A1 (en)* | 2010-09-10 | 2012-03-22 | Eduardo Gonzalez-Hernandez | Device and method for assisting in flexor tendon repair and rehabilitation |
| US8147546B2 (en)* | 2007-03-13 | 2012-04-03 | Biomet Sports Medicine, Llc | Method and apparatus for graft fixation |
| US8333803B2 (en)* | 2008-11-21 | 2012-12-18 | Lifecell Corporation | Reinforced biologic material |
| US20130345810A1 (en)* | 2012-06-22 | 2013-12-26 | Feg Textiltechnik Forschungs-Und Entwicklungsgesellschaft Mbh | Band-like structure for the augmentation of a ligament |
| US20140039620A1 (en)* | 2011-01-20 | 2014-02-06 | Georgia Tech Research Corporation -Georgia Instittute Of Technology | Device for tissue repair |
| US20140067063A1 (en)* | 2004-10-26 | 2014-03-06 | P Tech, Llc | Devices and methods for stabilizing tissue and implants |
| US8945218B2 (en)* | 2000-03-24 | 2015-02-03 | Drexel University | Ligament and tendon replacement constructs and methods for production and use thereof |
| US20160157992A1 (en)* | 2013-07-19 | 2016-06-09 | National University Of Singapore | Tissue interface augmentation device for ligament/tendon reconstruction |
Family Cites Families (80)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US3176316A (en)* | 1963-01-07 | 1965-04-06 | Bruce R Bodell | Plastic prosthetic tendon |
| US3463158A (en) | 1963-10-31 | 1969-08-26 | American Cyanamid Co | Polyglycolic acid prosthetic devices |
| US3545008A (en)* | 1968-05-27 | 1970-12-08 | Karl F Bader Jr | Tendon prosthesis |
| US3962153A (en)* | 1970-05-21 | 1976-06-08 | W. L. Gore & Associates, Inc. | Very highly stretched polytetrafluoroethylene and process therefor |
| US4255820A (en)* | 1979-07-24 | 1981-03-17 | Rothermel Joel E | Artificial ligaments |
| CH644748A5 (en)* | 1980-06-03 | 1984-08-31 | Sulzer Ag | STRING AND / OR TAPE REPLACEMENT MATERIAL. |
| US4455690A (en)* | 1980-11-06 | 1984-06-26 | Homsy Charles A | Structure for in vivo implanation |
| US4400833A (en)* | 1981-06-10 | 1983-08-30 | Kurland Kenneth Z | Means and method of implanting bioprosthetics |
| IL65855A (en)* | 1982-05-24 | 1986-09-30 | Yeda Res & Dev | Prosthetic tendon |
| US5049155A (en)* | 1982-09-10 | 1991-09-17 | W. L. Gore & Associates, Inc. | Prosthesis for tensile-load-carrying tissue and method of manufacture |
| GB8304264D0 (en)* | 1983-02-16 | 1983-03-23 | Seedhom B B | Prosthetic ligament |
| US4599084A (en) | 1983-05-24 | 1986-07-08 | American Hospital Supply Corp. | Method of using biological tissue to promote even bone growth |
| US4633873A (en) | 1984-04-26 | 1987-01-06 | American Cyanamid Company | Surgical repair mesh |
| GB8418018D0 (en)* | 1984-07-16 | 1984-08-22 | Johnson & Johnson | Connective tissue prosthesis |
| IL76079A (en) | 1985-08-13 | 1991-03-10 | Univ Ramot | Collagen implants |
| US4731084A (en)* | 1986-03-14 | 1988-03-15 | Richards Medical Company | Prosthetic ligament |
| FR2598311B1 (en) | 1986-05-07 | 1988-09-09 | Laboureau Jacques | SURGICAL INSTRUMENT FOR FOCUSING AND PLACING THE PLASTY (OR PROSTHETIC REPLACEMENT) OF THE LIGAMENT CROSS POSTERIOR KNEE |
| US5425766A (en) | 1987-03-09 | 1995-06-20 | Astra Tech Aktiebolag | Resorbable prosthesis |
| US5263984A (en)* | 1987-07-20 | 1993-11-23 | Regen Biologics, Inc. | Prosthetic ligaments |
| JPH066115B2 (en)* | 1987-08-27 | 1994-01-26 | 新技術事業団 | Electrodes for implantation in the body |
| US5078744A (en) | 1987-09-04 | 1992-01-07 | Bio-Products, Inc. | Method of using tendon/ligament substitutes composed of long, parallel, non-antigenic tendon/ligament fibers |
| US5024669A (en)* | 1988-09-09 | 1991-06-18 | Baxter International Inc. | Artificial ligament of differential weave structure |
| US5376118A (en)* | 1989-05-10 | 1994-12-27 | United States Surgical Corporation | Support material for cell impregnation |
| US5217495A (en)* | 1989-05-10 | 1993-06-08 | United States Surgical Corporation | Synthetic semiabsorbable composite yarn |
| US4946377A (en)* | 1989-11-06 | 1990-08-07 | W. L. Gore & Associates, Inc. | Tissue repair device |
| AU650045B2 (en) | 1990-09-12 | 1994-06-09 | Lifecell Corporation | Method and apparatus for cryopreparation dry stabilization and rehydration of biological suspensions |
| US5336616A (en) | 1990-09-12 | 1994-08-09 | Lifecell Corporation | Method for processing and preserving collagen-based tissues for transplantation |
| US8067149B2 (en) | 1990-09-12 | 2011-11-29 | Lifecell Corporation | Acellular dermal matrix and method of use thereof for grafting |
| CA2060635A1 (en) | 1991-02-12 | 1992-08-13 | Keith D'alessio | Bioabsorbable medical implants |
| US5374539A (en)* | 1991-06-17 | 1994-12-20 | Nimni; Marcel E. | Process for purifying collagen and generating bioprosthesis |
| US6503277B2 (en)* | 1991-08-12 | 2003-01-07 | Peter M. Bonutti | Method of transplanting human body tissue |
| WO1993017635A1 (en)* | 1992-03-04 | 1993-09-16 | C.R. Bard, Inc. | Composite prosthesis and method for limiting the incidence of postoperative adhesions |
| EP0642773B1 (en)* | 1993-09-14 | 2003-05-28 | Lanny L. Johnson | Biological replacement ligament |
| US5595571A (en) | 1994-04-18 | 1997-01-21 | Hancock Jaffe Laboratories | Biological material pre-fixation treatment |
| ATE310839T1 (en)* | 1994-04-29 | 2005-12-15 | Scimed Life Systems Inc | STENT WITH COLLAGEN |
| CA2192103C (en) | 1994-06-06 | 2002-02-05 | Arnold I. Caplan | Biomatrix for tissue regeneration |
| US6802862B1 (en)* | 1995-01-24 | 2004-10-12 | Smith & Nephew, Inc. | Method for soft tissue reconstruction |
| US6166288A (en) | 1995-09-27 | 2000-12-26 | Nextran Inc. | Method of producing transgenic animals for xenotransplantation expressing both an enzyme masking or reducing the level of the gal epitope and a complement inhibitor |
| EP0915967A1 (en) | 1996-05-28 | 1999-05-19 | The Board Of Regents Of The University Of Michigan | Engineering oral tissues |
| US5981827A (en)* | 1996-11-12 | 1999-11-09 | Regents Of The University Of California | Carbon based prosthetic devices |
| US6468300B1 (en)* | 1997-09-23 | 2002-10-22 | Diseno Y Desarrollo Medico, S.A. De C.V. | Stent covered heterologous tissue |
| US6254627B1 (en)* | 1997-09-23 | 2001-07-03 | Diseno Y Desarrollo Medico S.A. De C.V. | Non-thrombogenic stent jacket |
| EP1082006B1 (en) | 1998-05-26 | 2006-02-01 | Lifecell Corporation | Cryopreservation of human red blood cells |
| US6933326B1 (en) | 1998-06-19 | 2005-08-23 | Lifecell Coporation | Particulate acellular tissue matrix |
| WO2000016822A1 (en) | 1998-09-21 | 2000-03-30 | The Brigham And Women's Hospital, Inc. | Compositions and methods for tissue repair |
| US20030225355A1 (en)* | 1998-10-01 | 2003-12-04 | Butler Charles E. | Composite material for wound repair |
| US6497726B1 (en) | 2000-01-11 | 2002-12-24 | Regeneration Technologies, Inc. | Materials and methods for improved bone tendon bone transplantation |
| US6203572B1 (en)* | 1999-02-09 | 2001-03-20 | Linvatec Corporation | Device and method for ligament reconstruction |
| GB9912240D0 (en)* | 1999-05-27 | 1999-07-28 | Smith & Nephew | Implantable medical devices |
| US6893462B2 (en)* | 2000-01-11 | 2005-05-17 | Regeneration Technologies, Inc. | Soft and calcified tissue implants |
| US6746483B1 (en)* | 2000-03-16 | 2004-06-08 | Smith & Nephew, Inc. | Sheaths for implantable fixation devices |
| WO2001089422A1 (en)* | 2000-05-24 | 2001-11-29 | Sklar Joseph H | Method and apparatus for making a ligament repair using compressed tendons |
| US6592622B1 (en) | 2000-10-24 | 2003-07-15 | Depuy Orthopaedics, Inc. | Apparatus and method for securing soft tissue to an artificial prosthesis |
| US6752831B2 (en)* | 2000-12-08 | 2004-06-22 | Osteotech, Inc. | Biocompatible osteogenic band for repair of spinal disorders |
| CA2365376C (en) | 2000-12-21 | 2006-03-28 | Ethicon, Inc. | Use of reinforced foam implants with enhanced integrity for soft tissue repair and regeneration |
| US6827743B2 (en)* | 2001-02-28 | 2004-12-07 | Sdgi Holdings, Inc. | Woven orthopedic implants |
| US8025896B2 (en)* | 2001-07-16 | 2011-09-27 | Depuy Products, Inc. | Porous extracellular matrix scaffold and method |
| US20050027307A1 (en)* | 2001-07-16 | 2005-02-03 | Schwartz Herbert Eugene | Unitary surgical device and method |
| EP1446015B1 (en) | 2001-10-18 | 2018-03-14 | Lifecell Corporation | Remodeling of tissues and organs |
| WO2003101312A1 (en) | 2002-06-03 | 2003-12-11 | Nmt Medical, Inc. | Device with biological tissue scaffold for intracardiac defect closure |
| US20040034418A1 (en) | 2002-07-23 | 2004-02-19 | Shu-Tung Li | Membrane-reinforced implants |
| US20040078090A1 (en)* | 2002-10-18 | 2004-04-22 | Francois Binette | Biocompatible scaffolds with tissue fragments |
| US6974862B2 (en)* | 2003-06-20 | 2005-12-13 | Kensey Nash Corporation | High density fibrous polymers suitable for implant |
| US20050028228A1 (en) | 2003-07-21 | 2005-02-03 | Lifecell Corporation | Acellular tissue matrices made from alpa-1,3-galactose-deficient tissue |
| US20060073592A1 (en) | 2004-10-06 | 2006-04-06 | Wendell Sun | Methods of storing tissue matrices |
| US7905903B2 (en)* | 2006-02-03 | 2011-03-15 | Biomet Sports Medicine, Llc | Method for tissue fixation |
| US7749250B2 (en) | 2006-02-03 | 2010-07-06 | Biomet Sports Medicine, Llc | Soft tissue repair assembly and associated method |
| US7252832B1 (en) | 2004-12-13 | 2007-08-07 | Biomet Sports Medicine, Inc. | Composite collagen material and method of forming same |
| US7727278B2 (en)* | 2005-03-04 | 2010-06-01 | Rti Biologics, Inc. | Self fixing assembled bone-tendon-bone graft |
| US7981023B2 (en)* | 2005-07-25 | 2011-07-19 | Boston Scientific Scimed, Inc. | Elastic sling system and related methods |
| CN1903144A (en)* | 2005-07-29 | 2007-01-31 | 广东冠昊生物科技有限公司 | Biological artificial ligamentum and method for preparing same |
| AU2006294654B2 (en) | 2005-09-26 | 2012-05-24 | Lifecell Corporation | Dry platelet composition |
| US20070162124A1 (en)* | 2005-12-19 | 2007-07-12 | Whittaker Gregory R | Arthroscopic implants with integral fixation devices and method for use |
| US7959650B2 (en)* | 2006-09-29 | 2011-06-14 | Biomet Sports Medicine, Llc | Adjustable knotless loops |
| US20070248575A1 (en) | 2006-04-19 | 2007-10-25 | Jerome Connor | Bone graft composition |
| WO2007134134A2 (en)* | 2006-05-09 | 2007-11-22 | Lifecell Corporation | Reinforced biological tissue |
| AU2007339257B2 (en)* | 2006-12-27 | 2013-01-10 | Shriners Hospitals For Children | Woven and/or braided fiber implants and methods of making same |
| US8007533B2 (en)* | 2007-02-12 | 2011-08-30 | Rti Biologics, Inc. | Progressive grip assembled bone-tendon-bone grafts, methods of making, and methods of use |
| US7905918B2 (en)* | 2007-08-23 | 2011-03-15 | William Wayne Cimino | Elastic metallic replacement ligament |
| AU2009256193B2 (en) | 2008-06-06 | 2015-04-23 | Lifecell Corporation | Elastase treatment of tissue matrices |
- 2009
- 2009-11-19EPEP09828197.5Apatent/EP2349089A4/ennot_activeWithdrawn
- 2009-11-19WOPCT/US2009/065080patent/WO2010059783A2/enactiveApplication Filing
- 2009-11-19AUAU2009316588Apatent/AU2009316588B2/ennot_activeCeased
- 2009-11-19CACA2744232Apatent/CA2744232C/ennot_activeExpired - Fee Related
- 2009-11-19JPJP2011537607Apatent/JP5784500B2/ennot_activeExpired - Fee Related
- 2009-11-19USUS12/621,890patent/US8333803B2/ennot_activeExpired - Fee Related
- 2012
- 2012-09-14USUS13/616,556patent/US9421306B2/ennot_activeExpired - Fee Related
- 2016
- 2016-07-14USUS15/210,309patent/US20160317711A1/ennot_activeAbandoned
- 2018
- 2018-12-20USUS16/227,814patent/US20190184062A1/ennot_activeAbandoned
- 2019
- 2019-05-21USUS16/418,401patent/US20190275207A1/ennot_activeAbandoned
Patent Citations (47)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US3805667A (en)* | 1970-08-21 | 1974-04-23 | Columbian Rope Co | Braided rope |
| US4149277A (en)* | 1977-06-22 | 1979-04-17 | General Atomic Company | Artificial tendon prostheses |
| US4187558A (en)* | 1977-10-25 | 1980-02-12 | Cutter Laboratories, Inc. | Prosthetic ligament |
| US4713075A (en)* | 1981-06-10 | 1987-12-15 | Kurland Kenneth Z | Method for the repair of connective tissue |
| US5258040A (en)* | 1982-09-10 | 1993-11-02 | W. L. Gore & Associates | Prosthesis for tensile load-carrying tissue and method of manufacture |
| US4662886A (en)* | 1984-06-04 | 1987-05-05 | A. W. Showell (Surgicraft) Limited | Surgical element |
| US4792336A (en)* | 1986-03-03 | 1988-12-20 | American Cyanamid Company | Flat braided ligament or tendon implant device having texturized yarns |
| US5197983A (en)* | 1988-04-19 | 1993-03-30 | W. L. Gore & Associates, Inc. | Ligament and tendon prosthesis |
| US4917699A (en)* | 1988-05-16 | 1990-04-17 | Zimmer, Inc. | Prosthetic ligament |
| US4917700A (en)* | 1988-08-01 | 1990-04-17 | Zimmer, Inc. | Prosthetic ligament |
| US5176708A (en)* | 1990-03-12 | 1993-01-05 | Sulzer Brothers Limited | Prosthetic implant |
| US5800543A (en)* | 1993-03-31 | 1998-09-01 | Surgicraft Limited | Artificial ligament |
| US5514181A (en)* | 1993-09-29 | 1996-05-07 | Johnson & Johnson Medical, Inc. | Absorbable structures for ligament and tendon repair |
| US5595621A (en)* | 1993-09-29 | 1997-01-21 | Johnson & Johnson Medical, Inc. | Method of making absorbable structures for ligament and tendon repair |
| US5458636A (en)* | 1994-07-20 | 1995-10-17 | U.S. Biomaterials Corporation | Prosthetic device for repair and replacement of fibrous connective tissue |
| US6143029A (en)* | 1996-03-11 | 2000-11-07 | Rippstein; Pascal Francois | Tendon guide device |
| US6206931B1 (en)* | 1996-08-23 | 2001-03-27 | Cook Incorporated | Graft prosthesis materials |
| US20010044659A1 (en)* | 1996-11-20 | 2001-11-22 | Jacques-Phillippe Laboureau | Pre-adjusted prosthetic ligament and method of manufacture |
| US6214047B1 (en)* | 1998-03-10 | 2001-04-10 | University Of Cincinnati | Article and method for coupling muscle to a prosthetic device |
| US6733510B1 (en)* | 1999-01-12 | 2004-05-11 | University Of Cincinnati | Article and method for coupling muscle to a prosthetic device |
| US6592623B1 (en)* | 1999-08-31 | 2003-07-15 | Virginia Commonwealth University Intellectual Property Foundation | Engineered muscle |
| US8945218B2 (en)* | 2000-03-24 | 2015-02-03 | Drexel University | Ligament and tendon replacement constructs and methods for production and use thereof |
| US20020038151A1 (en)* | 2000-08-04 | 2002-03-28 | Plouhar Pamela L. | Reinforced small intestinal submucosa (SIS) |
| US20030023316A1 (en)* | 2000-08-04 | 2003-01-30 | Brown Laura Jean | Hybrid biologic-synthetic bioabsorable scaffolds |
| US6638312B2 (en)* | 2000-08-04 | 2003-10-28 | Depuy Orthopaedics, Inc. | Reinforced small intestinal submucosa (SIS) |
| US7070558B2 (en)* | 2001-07-27 | 2006-07-04 | Boston Scientific Scimed, Inc. | Medical slings |
| US6599319B2 (en)* | 2001-12-14 | 2003-07-29 | Celanese Advanced Materials, Inc. | Prosthetic ligament |
| US20040267362A1 (en)* | 2003-06-30 | 2004-12-30 | Julia Hwang | Scaffold for connective tissue repair |
| US20070118217A1 (en)* | 2004-01-23 | 2007-05-24 | Lars-Laboratoire D' Application Et De Recherche Scientifique | Method for fixing traction threads to ends of a prosthetic ligament |
| US20140067063A1 (en)* | 2004-10-26 | 2014-03-06 | P Tech, Llc | Devices and methods for stabilizing tissue and implants |
| US8052753B2 (en)* | 2005-01-07 | 2011-11-08 | University Of Cincinnati | Prosthetic anchor and method of making same |
| US20070162121A1 (en)* | 2006-01-12 | 2007-07-12 | Tarrant Laurence J | Method for repair and reconstruction of ruptured ligaments or tendons and for treatment of ligament and tendon injuries |
| US8147546B2 (en)* | 2007-03-13 | 2012-04-03 | Biomet Sports Medicine, Llc | Method and apparatus for graft fixation |
| US8172901B2 (en)* | 2007-03-20 | 2012-05-08 | Allergan, Inc. | Prosthetic device and method of manufacturing the same |
| US20080300683A1 (en)* | 2007-03-20 | 2008-12-04 | Altman Gregory H | Prosthetic device and method of manufacturing the same |
| US9421306B2 (en)* | 2008-11-21 | 2016-08-23 | Lifecell Corporation | Reinforced biologic material |
| US8333803B2 (en)* | 2008-11-21 | 2012-12-18 | Lifecell Corporation | Reinforced biologic material |
| US8758437B2 (en)* | 2009-05-22 | 2014-06-24 | Soft Tissue Regeneration, Inc. | Mechanically competent scaffold for ligament and tendon regeneration |
| US8486143B2 (en)* | 2009-05-22 | 2013-07-16 | Soft Tissue Regeneration, Inc. | Mechanically competent scaffold for ligament and tendon regeneration |
| US20100298937A1 (en)* | 2009-05-22 | 2010-11-25 | Soft Tissue Regeneration, Inc. | Mechanically competent scaffold for ligament and tendon regeneration |
| US20110257744A1 (en)* | 2010-04-14 | 2011-10-20 | The University Of Tokyo | Method for Producing Artificial Bone and Artificial Bone Produced by the Method |
| US20110288565A1 (en)* | 2010-05-19 | 2011-11-24 | University Of Utah Research Foundation | Tissue fixation |
| US20110307059A1 (en)* | 2010-06-11 | 2011-12-15 | AFcell Medical | Method of tendon repair with amnion and chorion constructs |
| US20120071975A1 (en)* | 2010-09-10 | 2012-03-22 | Eduardo Gonzalez-Hernandez | Device and method for assisting in flexor tendon repair and rehabilitation |
| US20140039620A1 (en)* | 2011-01-20 | 2014-02-06 | Georgia Tech Research Corporation -Georgia Instittute Of Technology | Device for tissue repair |
| US20130345810A1 (en)* | 2012-06-22 | 2013-12-26 | Feg Textiltechnik Forschungs-Und Entwicklungsgesellschaft Mbh | Band-like structure for the augmentation of a ligament |
| US20160157992A1 (en)* | 2013-07-19 | 2016-06-09 | National University Of Singapore | Tissue interface augmentation device for ligament/tendon reconstruction |
Cited By (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US10639398B2 (en) | 2016-07-05 | 2020-05-05 | Lifecell Corporation | Tissue matrices incorporating multiple tissue types |
Also Published As
| Publication number | Publication date |
|---|---|
| CA2744232C (en) | 2017-05-02 |
| US20130053960A1 (en) | 2013-02-28 |
| US9421306B2 (en) | 2016-08-23 |
| CA2744232A1 (en) | 2010-05-27 |
| AU2009316588A1 (en) | 2010-05-27 |
| US20190275207A1 (en) | 2019-09-12 |
| WO2010059783A2 (en) | 2010-05-27 |
| JP5784500B2 (en) | 2015-09-24 |
| WO2010059783A3 (en) | 2010-09-23 |
| AU2009316588B2 (en) | 2014-09-11 |
| US8333803B2 (en) | 2012-12-18 |
| US20190184062A1 (en) | 2019-06-20 |
| US20100161054A1 (en) | 2010-06-24 |
| JP2012509710A (en) | 2012-04-26 |
| EP2349089A4 (en) | 2014-01-15 |
| EP2349089A2 (en) | 2011-08-03 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| US20190275207A1 (en) | Reinforced biologic material | |
| US20210161646A1 (en) | Reinforced biological tissue | |
| CA2674456C (en) | Tendon or ligament bioprostheses and methods of making same | |
| JP4815554B2 (en) | Medical device based on immune neutral silk fiber | |
| TW298565B (en) | ||
| US4772288A (en) | Method for producing implantable ligament and tendon prostheses and prostheses produced thereby | |
| US20220305173A1 (en) | Mesh Compositions and Methods of Production | |
| WO2018119493A1 (en) | A synthetic implantable scaffold | |
| Melvin et al. | Extended healing validation of an artificial tendon to connect the quadriceps muscle to the Tibia: 180‐day study | |
| NZ755737A (en) | A synthetic implantable scaffold |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| AS | Assignment | Owner name:WILMINGTON TRUST, NATIONAL ASSOCIATION, AS COLLATERAL AGENT, MINNESOTA Free format text:SECOND LIEN SECURITY AGREEMENT;ASSIGNORS:KCI USA, INC.;LIFECELL CORPORATION;KCI LICENSING, INC.;REEL/FRAME:040098/0268 Effective date:20160920 Owner name:WILMINGTON TRUST, NATIONAL ASSOCIATION, AS COLLATE Free format text:SECOND LIEN SECURITY AGREEMENT;ASSIGNORS:KCI USA, INC.;LIFECELL CORPORATION;KCI LICENSING, INC.;REEL/FRAME:040098/0268 Effective date:20160920 | |
| AS | Assignment | Owner name:WILMINGTON TRUST, NATIONAL ASSOCIATION, AS COLLATERAL AGENT, MINNESOTA Free format text:LIMITED THIRD LIEN INTELLECTUAL PROPERTY SECURITY AGREEMENT;ASSIGNORS:KCI USA, INC.;LIFECELL CORPORATION;KCI LICENSING, INC.;REEL/FRAME:040291/0237 Effective date:20161006 Owner name:WILMINGTON TRUST, NATIONAL ASSOCIATION, AS COLLATE Free format text:LIMITED THIRD LIEN INTELLECTUAL PROPERTY SECURITY AGREEMENT;ASSIGNORS:KCI USA, INC.;LIFECELL CORPORATION;KCI LICENSING, INC.;REEL/FRAME:040291/0237 Effective date:20161006 | |
| AS | Assignment | Owner name:LIFECELL CORPORATION, NEW JERSEY Free format text:RELEASE OF SECURITY INTEREST 037845/0497;ASSIGNOR:WILMINGTON TRUST;REEL/FRAME:041608/0603 Effective date:20170131 Owner name:LIFECELL CORPORATION, NEW JERSEY Free format text:RELEASE OF SECURITY INTEREST 040098/0268;ASSIGNOR:WILMINGTON TRUST;REEL/FRAME:041608/0554 Effective date:20170131 Owner name:LIFECELL CORPORATION, NEW JERSEY Free format text:RELEASE OF SECURITY INTEREST 040291/0237;ASSIGNOR:WILMINGTON TRUST;REEL/FRAME:041608/0702 Effective date:20170131 | |
| AS | Assignment | Owner name:LIFECELL CORPORATION, NEW JERSEY Free format text:ASSIGNMENT OF ASSIGNORS INTEREST;ASSIGNORS:PARK, SANGWOOK;BARERE, AARON;WAGNER, CHRISTOPHER T.;REEL/FRAME:047800/0084 Effective date:20100107 | |
| STPP | Information on status: patent application and granting procedure in general | Free format text:NOTICE OF ALLOWANCE MAILED -- APPLICATION RECEIVED IN OFFICE OF PUBLICATIONS | |
| STCB | Information on status: application discontinuation | Free format text:ABANDONED -- FAILURE TO PAY ISSUE FEE | |
| AS | Assignment | Owner name:STOUT MEDICAL GROUP, L.P., PENNSYLVANIA Free format text:ASSIGNMENT OF ASSIGNORS INTEREST;ASSIGNOR:LIFECELL CORPORATION;REEL/FRAME:055359/0582 Effective date:20210219 | |
| AS | Assignment | Owner name:STOUT MEDICAL GROUP, L.P., PENNSYLVANIA Free format text:ASSIGNMENT OF ASSIGNORS INTEREST;ASSIGNORS:KIEFER, ROBERT A.;GREENHALGH, E. SKOTT;SIGNING DATES FROM 20210216 TO 20210225;REEL/FRAME:055427/0943 |