US20140142968A1 - Online, interactive evaluation of research performance - Google Patents
Online, interactive evaluation of research performanceDownload PDFInfo
- Publication number
- US20140142968A1 US20140142968A1US14/075,549US201314075549AUS2014142968A1US 20140142968 A1US20140142968 A1US 20140142968A1US 201314075549 AUS201314075549 AUS 201314075549AUS 2014142968 A1US2014142968 A1US 2014142968A1
- Authority
- US
- United States
- Prior art keywords
- clinical research
- trial
- research
- investigator
- site
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Abandoned
Links
Images
Classifications
- G—PHYSICS
- G06—COMPUTING OR CALCULATING; COUNTING
- G06Q—INFORMATION AND COMMUNICATION TECHNOLOGY [ICT] SPECIALLY ADAPTED FOR ADMINISTRATIVE, COMMERCIAL, FINANCIAL, MANAGERIAL OR SUPERVISORY PURPOSES; SYSTEMS OR METHODS SPECIALLY ADAPTED FOR ADMINISTRATIVE, COMMERCIAL, FINANCIAL, MANAGERIAL OR SUPERVISORY PURPOSES, NOT OTHERWISE PROVIDED FOR
- G06Q50/00—Information and communication technology [ICT] specially adapted for implementation of business processes of specific business sectors, e.g. utilities or tourism
- G06Q50/10—Services
- G06Q50/22—Social work or social welfare, e.g. community support activities or counselling services
- G—PHYSICS
- G06—COMPUTING OR CALCULATING; COUNTING
- G06Q—INFORMATION AND COMMUNICATION TECHNOLOGY [ICT] SPECIALLY ADAPTED FOR ADMINISTRATIVE, COMMERCIAL, FINANCIAL, MANAGERIAL OR SUPERVISORY PURPOSES; SYSTEMS OR METHODS SPECIALLY ADAPTED FOR ADMINISTRATIVE, COMMERCIAL, FINANCIAL, MANAGERIAL OR SUPERVISORY PURPOSES, NOT OTHERWISE PROVIDED FOR
- G06Q10/00—Administration; Management
- G06Q10/06—Resources, workflows, human or project management; Enterprise or organisation planning; Enterprise or organisation modelling
- G06Q10/063—Operations research, analysis or management
- G06Q10/0639—Performance analysis of employees; Performance analysis of enterprise or organisation operations
- G06Q10/06393—Score-carding, benchmarking or key performance indicator [KPI] analysis
- G—PHYSICS
- G06—COMPUTING OR CALCULATING; COUNTING
- G06Q—INFORMATION AND COMMUNICATION TECHNOLOGY [ICT] SPECIALLY ADAPTED FOR ADMINISTRATIVE, COMMERCIAL, FINANCIAL, MANAGERIAL OR SUPERVISORY PURPOSES; SYSTEMS OR METHODS SPECIALLY ADAPTED FOR ADMINISTRATIVE, COMMERCIAL, FINANCIAL, MANAGERIAL OR SUPERVISORY PURPOSES, NOT OTHERWISE PROVIDED FOR
- G06Q10/00—Administration; Management
- G06Q10/10—Office automation; Time management
- G—PHYSICS
- G16—INFORMATION AND COMMUNICATION TECHNOLOGY [ICT] SPECIALLY ADAPTED FOR SPECIFIC APPLICATION FIELDS
- G16H—HEALTHCARE INFORMATICS, i.e. INFORMATION AND COMMUNICATION TECHNOLOGY [ICT] SPECIALLY ADAPTED FOR THE HANDLING OR PROCESSING OF MEDICAL OR HEALTHCARE DATA
- G16H10/00—ICT specially adapted for the handling or processing of patient-related medical or healthcare data
- G16H10/20—ICT specially adapted for the handling or processing of patient-related medical or healthcare data for electronic clinical trials or questionnaires
- G—PHYSICS
- G16—INFORMATION AND COMMUNICATION TECHNOLOGY [ICT] SPECIALLY ADAPTED FOR SPECIFIC APPLICATION FIELDS
- G16H—HEALTHCARE INFORMATICS, i.e. INFORMATION AND COMMUNICATION TECHNOLOGY [ICT] SPECIALLY ADAPTED FOR THE HANDLING OR PROCESSING OF MEDICAL OR HEALTHCARE DATA
- G16H50/00—ICT specially adapted for medical diagnosis, medical simulation or medical data mining; ICT specially adapted for detecting, monitoring or modelling epidemics or pandemics
- G16H50/70—ICT specially adapted for medical diagnosis, medical simulation or medical data mining; ICT specially adapted for detecting, monitoring or modelling epidemics or pandemics for mining of medical data, e.g. analysing previous cases of other patients
- G—PHYSICS
- G16—INFORMATION AND COMMUNICATION TECHNOLOGY [ICT] SPECIALLY ADAPTED FOR SPECIFIC APPLICATION FIELDS
- G16H—HEALTHCARE INFORMATICS, i.e. INFORMATION AND COMMUNICATION TECHNOLOGY [ICT] SPECIALLY ADAPTED FOR THE HANDLING OR PROCESSING OF MEDICAL OR HEALTHCARE DATA
- G16H70/00—ICT specially adapted for the handling or processing of medical references
Definitions
- the present inventionis directed to a system and method for evaluation of the performance and progress of research trials and more specifically to such a system and method, which provide benchmark metrics in real time.
- dataIn clinical trials for pharmaceuticals and the like, data must be taken over an extended period. For example, at intervals of time, typically 30 days, data must be taken on the number of patients who are participating in the trial, the number who have dropped out, the queries per patient, and the like.
- the present inventionis directed to an online, automated technique for collecting data not only for clinical trials but for other types of medical research including: marketing research, outcomes studies, disease management, etc.
- This type of medical research informationwould be information used to support the post approval product launches, marketing, product positioning, business development opportunities including licensing from clinical trials at intervals of e.g., 30 days and for performing automated, online evaluations based on the data.
- the evaluationscan be presented in textual or graphical formats.
- a common servercan be used to collect data from multiple trials, with the appropriate protection of the data from each trial for privacy purposes.
- the comparisons, e.g., for cost per patientcan be presented anonymously; for example, the figures for the highest and lowest costs per patient can be given without identifying the sources for those figures.
- each userprovides information to be entered into a database.
- the informationconcerns the physician's own practice and the site, if any, at which the physician is an administrator.
- the informationconcerns such things as the facilities at the site and the physicians practicing at the site.
- the informationconcerns such things as the type of organization and the sites and physicians within the organization.
- a listing of a clinical trial opportunity (blinded synopsis) or other research opportunity (blinded synopsis) with an accompanying protocol synopsis specific questionnaire (SQQ)is posted on the website.
- the listingis categorized to allow browsing by prospective researchers who are members.
- the servicecan search the database to find suitable physicians, sites or organizations who preliminarily match the criteria the sponsor has provided to the service and then select which ones are to be contacted about the clinical trial opportunity. That occurs by cross-referencing project requirements with general information about the sites, physicians and organizations. Contacting members about opportunities occurs via e-mail or facsimile to invite them to consider the opportunity.
- the researchelectronically submits the completed SQQ via email to the service.
- the servicesummarizes all the information across all completed SQQ's for the specific research opportunity and forwards qualified and matched researchers to the sponsor. The sponsor then selects which research provided by the service they will select for the clinical trial.
- information about the studyis entered into the database, e.g., at the beginning and end of the study and at 30-day intervals or less therebetween.
- the informationconcerns such things as the number of patients who have signed up and the number who have dropped out.
- benchmarkscan be computed over time.
- Study (clinical trial/protocol) specific benchmarks from one researcher, site and or organizationcan be anonymously compared with those from other researchers, sites and/or organizations.
- four values of a benchmarkare given: the highest and lowest values from the researcher, site and organization participating in the study any study, the median from all researchers, sites and/or organizations, and the actual value from the researcher, site and/or organization with which the person viewing the results is linked in which the person viewing the results is participating.
- the identities of the other researchers, sites and/or organizations from which the information is providedare not given, thus preserving confidentiality.
- the usercan thus see how their researcher, site and or organization performance results for the study compares with other researcher, site and/or organization performance results for the study and can use that information, e.g., to see where efficiency can be improved, budgets should be reallocated, patients that qualify for the study can be found, training is required or to make the case for an increase in the research budget.
- FIG. 1shows a hardware architecture on which the preferred embodiment can be implemented
- FIGS. 2 , 2 A- 2 I, 3 , 3 A- 3 D, 4 and 4 A- 4 Cshow flow charts and screen shots of the registration and data inputting process for new users;
- FIGS. 5A and 5Bshow a physician registration page
- FIG. 6shows a search page
- FIG. 7shows a search result page
- FIG. 8shows a flow chart of the processes involved in posting a CTO or ORO description
- FIGS. 8A-8Eshow screen shots of the processes of FIG. 8 ;
- FIG. 9shows a merge page
- FIGS. 10 and 11show pages used in collecting progress data
- FIGS. 12 and 13show pages used in displaying benchmark data
- FIG. 14shows a flow chart of operation of an enhancement to the service which allows physicians to obtain information on fees.
- FIG. 1shows an example of a hardware architecture on which the preferred embodiment can be implemented.
- the design of the hardware architecturehas been selected to follow the industry-standard N-tier architecture, separating site navigation and design from stored procedures and data. In that way, one part of the site can be redesigned without breaking another part of the site. For instance, the HTML which provides the user interface can be changed without affecting the stored procedures and tables of the database.
- FIG. 1shows such a hardware architecture 100 .
- Client devices 102which can be microcomputers or any other devices capable of communicating over the Internet, connect via any suitable connections 104 , such as dial-up or cable modem connections, to the Internet 106 , and thence via a full-time Internet connection 108 to a Web server 110 .
- the client devicesare used by physicians, research sponsors, research sites such as hospitals, and others participating in the operations of the preferred embodiment.
- the Web server 110provides business services, such as site navigation and design, and is connected via a full-time dedicated connection 112 to a database server 114 storing the stored procedures and database tables.
- the client devices 102access the stored procedures and database tables in the database server 114 only through the Web server 110 .
- the Web server 110implements interactive HTML pages through known technologies, such as CGI or ASP.
- the database stored in the database server 114is populated when physicians and organizations register and provide the information required for registration.
- the Web server 110provides persons accessing it from client devices 102 with several options, including physician registration and organization registration.
- a userregisters as an organization; physician registration and site registration are part of organizational registration. As noted above, a solo practitioner still registers as an organization.
- the organizational registrationhas two parts, the first part resulting in temporary status and the second part resulting in active status.
- FIG. 2shows an overview of organization registration.
- the useris prompted for the name and address of the organization and for the type of organization (see the Web page of FIG. 2A ). The latter is preferably selected through check boxes, so that multiple choices may be selected, although it may be selected through a drop-down menu or radio buttons if only one choice is allowed.
- a temporary user identification and passwordare generated and provided to the user (or the user can select them; see FIG. 2B ). The user is then allowed to view more detailed descriptions of the research opportunities; however, if any particular opportunity is selected, the user must proceed to the second part of the registration.
- That second partbegins at step 206 , in which the user is prompted to complete an organizational profile (see FIG. 2C ).
- the first page of the organization profileasks “how many site locations” at step 208 , and this automatically generates, at step 210 , a table (see FIG. 2D ) on page two of the organization profile with the necessary number of rows for all site locations. The User must complete this table in order to advance to page three of the organization profile; that will generate the correct number of site profiles.
- the usercompletes a physician profile (see FIG. 2E ) for each physician within the organization, until it is determined at step 214 (see FIG.
- step 214can be performed like step 210 .
- Identifications and passwordsare generated for the organization as a whole, for each site, and for each physician at steps 216 , 218 and 220 , respectively (see FIG. 2G ).
- the identifications and passwordsare sent to the organization.
- a responsible person at the organizationhas the option to change the identification and password for the organization (see FIG. 2I ).
- the organization representativehas the ability to change any data about the organization, sites or physicians and has complete access to all three profiles. The representative cannot add further sites or physicians or edit the name of a physician.
- FIG. 3shows an overview of the physician registration process of step 212 .
- the Web serverasks whether the physician is an administrator at the site at which the physician works. If it is determined at step 304 that the physician has answered in the affirmative, the physician is prompted at step 306 (see FIG. 3B ) for information about the site, in a manner which will be described in detail below. If not, the physician is prompted at step 308 for the name of an administrator at that site. Either way, the physician's information is taken in a physician information form at step 310 (see FIG. 3C ). A user identification (ID) and password (PW) are generated for the physician at step 312 (see FIG. 3D ).
- IDuser identification
- PWpassword
- FIG. 4shows an overview of the site registration of step 210 .
- the number of sites input by the useris received at step 402 (see FIG. 4A ).
- a table of site locations, having a sufficient number of rows,is generated at step 404 (see FIG. 4B ).
- the usercompletes the table, including information for each site, at step 406 (see FIG. 4C ).
- a data entry formcan be implemented in HTML as one page or several; if it is implemented in several pages, each page ends with “Next Page” (or if the last page of the form “Submit”), “Discard Changes and Exit” or “Save Changes and Exit” options. Also, the same forms can be used to add information about new users and to update information for existing users.
- FIG. 5Ashows the first page of the physician's form.
- the first page 502includes an area 504 for contact information, an area 506 for medical licensing and DEA information, and an area 508 in which the physician can identify specialties through drop-down menus. Other information, such as professional affiliations with one or more medical schools and hospitals, can be solicited here.
- FIG. 5Bshows a subsequent page 514 of the physician's form.
- the physicianis asked about experience as a principal investigator in a clinical trial. Detailed experience on each clinical trial can be solicited in further pages, as well as information on any FDA or sponsor audit that the physician or the site has undergone, any administrative roles that the physician plays at the site, any articles or presentations in which the physician is named as an author, and any organizations and associations in which the physician is actively involved.
- the pages requesting organization and site informationare similar.
- the organization pagesrequest the type of organization, while the site pages request what facilities the site has, among other things. Pages can also be provided to transfer a physician from one site or organization to another or to transfer a site from one organization to another. This function is accessible solely to employees of the service, not registered users.
- the administrator of the database server 114can accept the information as given or can take various steps to verify it. For example, the administrator can verify that a given facsimile number or e-mail address words, check a given medical license number, or interview either the person submitting the information or those persons named as references.
- FIG. 6shows a search page for searching for physicians.
- the search page 600uses text boxes and drop-down menus, although other suitable interface elements can be used as needed. In any text box, wildcard searching can be allowed.
- Physicianscan be searched by name, site, organization, or any combination of the three by use of the text boxes 602 , 604 , 606 .
- the drop-down menu 608allows the search to be conducted for physicians who are members, non-members, or either.
- the person conducting the searchcan select a state through a drop-down menu 610 or a region through a drop-down menu 612 .
- a specialtycan be selected through a drop-down menu 614 .
- Key wordscan be entered in a text box 616 .
- the page 600can be designed to include any other search criteria, such as those relating to clinical trial experience. Once all of the search criteria are entered, the user clicks on the “Find Physicians” button 618 .
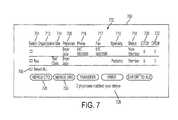
- the database server 114passes the search results to the Web server 110 which formats them as shown in FIG. 7 .
- the search results page 700shows the search results in the form of a table 702 .
- a select column 704which allows the user to select individual physicians for further consideration or to select all physicians through a “Select all” check box 706 .
- the name column 708lists the physicians by name, with links to their profiles in the database.
- the specialty column 710gives each physician's specialty.
- the organization column 712 and the site column 714identify the organization and site, respectively, with which each physician is associated and include links to those organizations' and sites' profiles.
- a telephone number column 716 and a facsimile number column 717give that information for each physician.
- the membership status column 718identifies each physician as a member or non-member.
- the CTO column 720 and the ORO column 722list CTO's and ORO's, respectively, with which the physician is or has been involved. Columns can be sorted by clicking on the name of the column.
- An indicator 726identifies the number of physicians who have matched the query.
- the merge buttons 728 and 730allow the user to merge the records for the selected physicians with a CTO or ORO in a manner to be explained below.
- An employee of the servicecan enter a CTO or ORO as shown in FIG. 8 .
- the followingare selected through drop-down menus or other suitable interface elements (see FIG. 8A ): the specialty concerned (e.g., urology), the estimated number of patients, and the duration.
- the followingare input as free-form text (see FIG. 8B ): a descriptive title of the study, one or more inclusion criteria for patients (e.g., male patient aged 40-85 years), one or more exclusion criteria for patients (e.g., no previous prostatic surgery within two weeks of base line), and a per-patient budget (this can be indicated as “to be negotiated”).
- the criteriacan also be selected through drop-down menus, in which case there will be an option to add criteria not appearing in the menus.
- the criteriacan also be assigned a sorting order.
- the relevant SQQ or PQQis designed and posted on the web at step 806 (see FIG. 8C ).
- a numberis manually assigned at step 808 (see FIG. 8D ).
- the opportunitycan be made viewable to network members only by an employee of the service at step 810 (see FIG. 8E ).
- the CTO or OROcan be made viewable to persons viewing the organization's or site's profile or in a list sorted by specialty; preferably, both are done.
- the search results from FIG. 7can be merged into the CTO or ORO.
- the button 728 or 730is clicked, the merge page 902 or 904 in the merge area 900 of FIG. 9 appears.
- the usercan select the Merge CTO or Merge ORO 902 or 904 and click the “Merge” button 906 or may cancel by closing the window. If the “Merge” button 906 is clicked, all of the selected search results from FIG. 7 are linked to the description of the CTO or ORO in the database. Checking for duplicate records is done automatically.
- the physicians, organizations, or sites merged into the listingcan be invited to consider the CTO or ORO. Since anyone found in the search results will have provided some contact information, the invitation can be made by e-mail, facsimile, or any other suitable channel.
- the service(or, in an alternative embodiment, a sponsor of a CTO or ORO) can initiate contact with a physician, organization or site which may be suited to conduct that CTO or ORO, as described above with reference to FIG. 9 .
- the questionnaireis completed and returned to the sponsor.
- the sponsorconsiders the questionnaire and can send a person to interview the physician or otherwise visit the organization or site at which the trial or other research would take place.
- a Web pagecan be provided to indicate the reasons why any particular physician, organization or site was accepted or declined. That Web page is accessible only by employees of the service or network members who have registered to use the system.
- Standard (industry) benchmarksinclude the following: revenue per primary investigator per year, patients enrolled per number of coordinator FTE's, average revenue per patient, distribution of revenue by type, average enrollment as a percentage of goal, split of patient recruitment between in-house and advertised, average number of protocols per year, average number of years in clinical research, percentage of studies through third party, percentage of studies directly from CRO or sponsor, number of sponsors with which a particular physician, site or organization has worked, and number of CRO's with which a particular physician, site or organization has worked.
- Benchmarks specific to a particular studyinclude the following: time to first patient enrolled, total patients enrolled after the first thirty days, actual enrollment as a percentage of the enrollment goal, per-patient budget, number of queries per patient, enrollment as a percentage of total patients screened, and elapsed time from notification of award of study to initiation visit.
- Data entered into the Web Form(Site Status Summary, or SSS) are used to compute the benchmarks specific to a particular study. Those data are collected on a per-protocol basis, are specific to that protocol and are not stored for future protocols. On the other hand, information collected in the profiles can be used to update the SQQ/PQQ.
- Examples of Web pages for taking data associated with a particular studyare shown in FIGS. 10 and 11 as 1000 and 1100 , respectively.
- the page 1100is only partially shown, since the data are taken not simply at the end of 30 days, but rather at the end of every 30-day interval until the end of the study; for instance, the page 1100 may ask for the number of patients screened after 720 days, etc.
- datamay be collected, such as the date of the study close-out visit and the number of queries generated.
- the benchmarksare calculated automatically by the system. The more data inputted, the more an organization can access. They do not have to wait until the study is over until they can view benchmarks, but as soon as the relevant data are input the benchmark is viewable. Once all of the data are input and the study is concluded, or once the data currently available are input if benchmarking during the study is required, benchmarking can be performed using straightforward calculations on the data. Two examples of data outputs are shown; those skilled in the art will recognize that many other types of data outputs can be provided as needed.
- FIG. 12shows a bar chart 1200 in which the total budget per patient is broken down into overhead, procedure and lab fees, the study coordinator, and the investigator fees.
- FIG. 13shows a bar chart 1300 showing a particular benchmark by its value in the user's study compared to its high, low, and average values from among all organizations participating in the same opportunity monitored by the database server 114 . The identities of the other organizations are not identified.
- Each of the bars of the bar chart 1300can be broken up like the bar in the bar chart 1200 .
- the benchmarkscan be displayed to the sponsor of the study, the physician, site or organization conducting the study, or both.
- Non-numerical informationsuch as the identities of the physicians involved in the study and information about them, can also be reported.
- An enhancement to the serviceprovides a customizable search page for the “study budget project” where physicians can search for a price range for their service.
- the searchis intuitive enough to allow physicians to select predetermined criteria to focus their search. Physicians will also be able to download their study budget information in a standard template usable in a spreadsheet program (e.g., Microsoft Excel).
- a study budget systemis provided, which is a separate module from the service previously described.
- the userlogs in. Members will pay a yearly license fee, billed monthly. Existing users will be able to retrieve their user name and password information by entering their e-mail address.
- the systemdetermines at step 1406 that the user is a new user, or is an existing member using the system for the first time, the user will be required to complete an activation form at step 1408 . On confirmation of the user's credit card, the user will receive a welcome message and information about their subscription via e-mail.
- the userwill choose from standard study questions about that user's procedure type, which sets the parameters for the query.
- the userreceives high, average, and low fees for the selected query.
- a study budget templateis provided in order to be populated with the user's information.
- the study budget templateprovides automatic calculations and a printable template.
- the informationis updated to the database.
- the site recruitergoes to the first page of the organization profile, by going to www.rapidtrials.com/orge.asp, members admin, selecting the org name from the drop down and hitting EDIT Members admin page drop down list of organizations If the organization is in black it is an active member of the network, if the org is in blue or any other color you can check the status by going to Edit and looking at the status drop down halfway down page one of the organization profile.
- Halfway down the first pageis a status drop down. The status is: Active Terminated Contact Hold Pending Inactive
- the organization statusis changed to Active. This automatically creates a second browser window with the User ID and Passwords for that organization.
- Rapidtrials.comRapid Budget Separate Web Module accessible from the current website
- Rapidtrials.comMembers pay a yearly fee, billed monthly. They can apply for membership via the website (rapidtrials.com), with payment accepted via credit card.
- a new userwill be required to complete an activation form; this will collect basic data on the physician and his organization.
- the modulewill ask them questions about the type of study they wish to budget for and which procedures are involved. Users receive a high, average, low, and your site (which allows them to view what they charged for the procedure the last time they used the system). The system then asks them to choose a price for each procedure, this feeds into a basic per patient clinical trial specific study budget template which when they have completed choosing their ranges they can print the complete budget worksheet from the website. This information then updates the underlying sequel table, so it will be available to be used in the calculation for the next user. RapidTraining Current Training Program owned by the service is for Novice Sites and or as a refresher course for experienced sites.
- Rapidtrials web modulewill suggest appropriate tactics to support the objectives. This module will also include IRB/FDA regulations governing patient recruitment advertising and recruitment guidelines. This module of the Rapidtrials website will be a portal for ordering mugs, t-shirts, ads, media buying, plaques and other promotional items through the service to enhance patient recruitment and retention in clinical trials.
- Rapid AssessmentA Rapidtrials web module that uses a psycho-behavioral assessment (similar to Myers Briggs) that has been validated by the service using historical performance and behavioral assessment response correlation's to identify sites with a high probability of succeeding in clinical trials. Investigators and coordinators will complete this behavioral assessment on- line as a predictive tool for the practice of future success in clinical trials. Gaps in attributes will be identified for Sites as a means of guiding them in clinical research program recruitment. For sponsors involved in site selection, or preparing to invest in site development it will give them another performance indicator prior to selecting sites to participate in a clinical trial.
- Type of OrganizationI.e. solo practice, SMO etc: Total number of protocols per year, Total grant value per protocol, total number of patients enrolled in a clinical trial/year, total number of Study coordinators, total number of sites doing research, Total number of Patients per study coordinator FTE's, Average number of Patients per year, Number of Physicians, Number of PI's, Overhead as a percentage of total per patient budget, Study Coordinator costs as a percentage of Total per patient budget, Investigator Fees as a percentage of total per patient budget, Procedure, lab fees as a percentage of total per patient budget, total number of sponsors worked for/year, Total number of CROs/year, Actual Enrollment/Enrollment Goal.
- any benchmarkscan be calculated, in which case the forms for collecting the data are modified accordingly. Further, the benchmarks can be displayed as text, as any suitable chart, or as a combination of the two.
- either the Web server or the database servercan incorporate the ability to handle payments from one participant to another, as well as from participants to the company operating the servers.
- any mention of a specific software packagee.g., Microsoft Excel
Landscapes
- Business, Economics & Management (AREA)
- Engineering & Computer Science (AREA)
- Human Resources & Organizations (AREA)
- Strategic Management (AREA)
- Entrepreneurship & Innovation (AREA)
- Economics (AREA)
- Health & Medical Sciences (AREA)
- Medical Informatics (AREA)
- Public Health (AREA)
- Primary Health Care (AREA)
- General Health & Medical Sciences (AREA)
- Epidemiology (AREA)
- Tourism & Hospitality (AREA)
- Educational Administration (AREA)
- Theoretical Computer Science (AREA)
- Marketing (AREA)
- Development Economics (AREA)
- Physics & Mathematics (AREA)
- General Business, Economics & Management (AREA)
- General Physics & Mathematics (AREA)
- Operations Research (AREA)
- Quality & Reliability (AREA)
- Data Mining & Analysis (AREA)
- Game Theory and Decision Science (AREA)
- Biomedical Technology (AREA)
- Databases & Information Systems (AREA)
- Pathology (AREA)
- Management, Administration, Business Operations System, And Electronic Commerce (AREA)
- Child & Adolescent Psychology (AREA)
Abstract
Description
- The present application is a continuation of U.S. patent application Ser. No. 10/118,369, filed Apr. 9, 2002, whose disclosure is hereby incorporated by reference in its entirety into the present disclosure.
- The present invention is directed to a system and method for evaluation of the performance and progress of research trials and more specifically to such a system and method, which provide benchmark metrics in real time.
- In clinical trials for pharmaceuticals and the like, data must be taken over an extended period. For example, at intervals of time, typically 30 days, data must be taken on the number of patients who are participating in the trial, the number who have dropped out, the queries per patient, and the like.
- However, the data are typically collected and tabulated by pharmaceutical companies to manage their project timelines. Such information is rarely shared with the physicians and health-care professionals conducting the research studies for purposes of understanding how they are performing, how other research sites are doing and/or learning from other clinicians participating in the study about how to improve their study management and performance. Currently no electronic mechanism exists that systematically, and automatically provides clinicians the ability to interact with the physicians and health-care professionals conducting the research studies for purposes of understanding how they are performing, how other research sites are doing and or learning from other clinicians participating in the study about how to improve their study management and performance. Currently no electronic mechanism exists that systematically and automatically provides clinicians and/or pharmaceutical sponsors with the ability to obtain interactive, real-time benchmarks and with those benchmarks better assess physician capabilities for matching physicians and research sites and with those data better assess physician capabilities for matching physicians and research sites for future clinical trials.
- One Internet-based technique for matching experts in the biological sciences with those needing their services is disclosed in WO 01/29708, published Apr. 26, 2001. That technique provides for matching based on qualifications and times of availability as well as payment. However, once the experts and their customers are matched, there is no evaluation of the work, which the former do for the latter.
- It will be readily apparent from the above that a need exists in the art for an online, interactive evaluation of research performance. It is therefore a primary object of the invention to collect data concerning the progress of research and to provide an evaluation based on the collected data.
- It is another object of the invention to provide an interactive, automated, online technique for doing so.
- It is a further object of the invention to provide such an evaluation based on data collected at various time intervals.
- It is a still further object of the invention to allow comparative evaluations among various trials.
- It is a still further object of the invention to allow comparative evaluations without divulging the identity of one trial to researchers participating in another.
- To achieve the above and other objects, the present invention is directed to an online, automated technique for collecting data not only for clinical trials but for other types of medical research including: marketing research, outcomes studies, disease management, etc. This type of medical research information would be information used to support the post approval product launches, marketing, product positioning, business development opportunities including licensing from clinical trials at intervals of e.g., 30 days and for performing automated, online evaluations based on the data. The evaluations can be presented in textual or graphical formats. A common server can be used to collect data from multiple trials, with the appropriate protection of the data from each trial for privacy purposes. The comparisons, e.g., for cost per patient, can be presented anonymously; for example, the figures for the highest and lowest costs per patient can be given without identifying the sources for those figures.
- Users register for the service as organizations. The system will then ask the user to register all sites and physicians that fall under the umbrella of the organization. If a physician is a solo practitioner, the physician still must complete all three profiles, although some information may be duplicated, since each profile focuses on collecting differing information. During the registration process, each user provides information to be entered into a database. For a physician, the information concerns the physician's own practice and the site, if any, at which the physician is an administrator. For a site, the information concerns such things as the facilities at the site and the physicians practicing at the site. For an organization, the information concerns such things as the type of organization and the sites and physicians within the organization.
- A listing of a clinical trial opportunity (blinded synopsis) or other research opportunity (blinded synopsis) with an accompanying protocol synopsis specific questionnaire (SQQ) is posted on the website. The listing is categorized to allow browsing by prospective researchers who are members. In addition, the service can search the database to find suitable physicians, sites or organizations who preliminarily match the criteria the sponsor has provided to the service and then select which ones are to be contacted about the clinical trial opportunity. That occurs by cross-referencing project requirements with general information about the sites, physicians and organizations. Contacting members about opportunities occurs via e-mail or facsimile to invite them to consider the opportunity. Following the researchers' completion of any new data not currently in the database and contained in the SQQ, the research electronically submits the completed SQQ via email to the service. The service summarizes all the information across all completed SQQ's for the specific research opportunity and forwards qualified and matched researchers to the sponsor. The sponsor then selects which research provided by the service they will select for the clinical trial.
- Once a researcher is selected for the study, information about the study is entered into the database, e.g., at the beginning and end of the study and at 30-day intervals or less therebetween. The information concerns such things as the number of patients who have signed up and the number who have dropped out. Thus, benchmarks can be computed over time.
- Study (clinical trial/protocol) specific benchmarks from one researcher, site and or organization can be anonymously compared with those from other researchers, sites and/or organizations. In one example of a comparison, four values of a benchmark are given: the highest and lowest values from the researcher, site and organization participating in the study any study, the median from all researchers, sites and/or organizations, and the actual value from the researcher, site and/or organization with which the person viewing the results is linked in which the person viewing the results is participating. The identities of the other researchers, sites and/or organizations from which the information is provided are not given, thus preserving confidentiality. The user can thus see how their researcher, site and or organization performance results for the study compares with other researcher, site and/or organization performance results for the study and can use that information, e.g., to see where efficiency can be improved, budgets should be reallocated, patients that qualify for the study can be found, training is required or to make the case for an increase in the research budget.
- A preferred embodiment of the present invention will be set forth in detail with reference to the drawings, in which:
FIG. 1 shows a hardware architecture on which the preferred embodiment can be implemented;FIGS. 2 ,2A-2I,3,3A-3D,4 and4A-4C show flow charts and screen shots of the registration and data inputting process for new users;FIGS. 5A and 5B show a physician registration page;FIG. 6 shows a search page;FIG. 7 shows a search result page;FIG. 8 shows a flow chart of the processes involved in posting a CTO or ORO description;FIGS. 8A-8E show screen shots of the processes ofFIG. 8 ;FIG. 9 shows a merge page;FIGS. 10 and 11 show pages used in collecting progress data;FIGS. 12 and 13 show pages used in displaying benchmark data; andFIG. 14 shows a flow chart of operation of an enhancement to the service which allows physicians to obtain information on fees.- A preferred embodiment of the present invention will now be set forth in detail with reference to the drawings, in which like reference numerals refer to like elements or process steps throughout.
FIG. 1 shows an example of a hardware architecture on which the preferred embodiment can be implemented. The design of the hardware architecture has been selected to follow the industry-standard N-tier architecture, separating site navigation and design from stored procedures and data. In that way, one part of the site can be redesigned without breaking another part of the site. For instance, the HTML which provides the user interface can be changed without affecting the stored procedures and tables of the database.FIG. 1 shows such ahardware architecture 100.Client devices 102, which can be microcomputers or any other devices capable of communicating over the Internet, connect via anysuitable connections 104, such as dial-up or cable modem connections, to theInternet 106, and thence via a full-time Internet connection 108 to aWeb server 110. The client devices are used by physicians, research sponsors, research sites such as hospitals, and others participating in the operations of the preferred embodiment. TheWeb server 110 provides business services, such as site navigation and design, and is connected via a full-timededicated connection 112 to a database server114 storing the stored procedures and database tables. Thus, theclient devices 102 access the stored procedures and database tables in the database server114 only through theWeb server 110. It is preferable to implement theWeb server 110 and the database server114 on separate, dedicated machines to facilitate backups and restarts with a minimum of disruption. TheWeb server 110 implements interactive HTML pages through known technologies, such as CGI or ASP.- The database stored in the database server114 is populated when physicians and organizations register and provide the information required for registration. As is known in the art, the
Web server 110 provides persons accessing it fromclient devices 102 with several options, including physician registration and organization registration. - A user registers as an organization; physician registration and site registration are part of organizational registration. As noted above, a solo practitioner still registers as an organization. The organizational registration has two parts, the first part resulting in temporary status and the second part resulting in active status.
FIG. 2 shows an overview of organization registration. Atstep 202, the user is prompted for the name and address of the organization and for the type of organization (see the Web page ofFIG. 2A ). The latter is preferably selected through check boxes, so that multiple choices may be selected, although it may be selected through a drop-down menu or radio buttons if only one choice is allowed. Atstep 204, once that information is received, a temporary user identification and password are generated and provided to the user (or the user can select them; seeFIG. 2B ). The user is then allowed to view more detailed descriptions of the research opportunities; however, if any particular opportunity is selected, the user must proceed to the second part of the registration.- That second part begins at
step 206, in which the user is prompted to complete an organizational profile (seeFIG. 2C ). When the User is completing the site location section of the organization profile, the first page of the organization profile asks “how many site locations” atstep 208, and this automatically generates, atstep 210, a table (seeFIG. 2D ) on page two of the organization profile with the necessary number of rows for all site locations. The User must complete this table in order to advance to page three of the organization profile; that will generate the correct number of site profiles. Atstep 212, the user completes a physician profile (seeFIG. 2E ) for each physician within the organization, until it is determined at step214 (seeFIG. 2F ) that profiles have been completed for all physicians within the organization; step214 can be performed likestep 210. Identifications and passwords are generated for the organization as a whole, for each site, and for each physician atsteps FIG. 2G ). Atstep 222, it is indicated in the database that the organization's status is that of an active member (seeFIG. 2H ). Atstep 224, the identifications and passwords are sent to the organization. Atstep 226, a responsible person at the organization has the option to change the identification and password for the organization (seeFIG. 2I ). The organization representative has the ability to change any data about the organization, sites or physicians and has complete access to all three profiles. The representative cannot add further sites or physicians or edit the name of a physician. FIG. 3 shows an overview of the physician registration process ofstep 212. At step302 (seeFIG. 3A ), the Web server asks whether the physician is an administrator at the site at which the physician works. If it is determined atstep 304 that the physician has answered in the affirmative, the physician is prompted at step306 (seeFIG. 3B ) for information about the site, in a manner which will be described in detail below. If not, the physician is prompted atstep 308 for the name of an administrator at that site. Either way, the physician's information is taken in a physician information form at step310 (seeFIG. 3C ). A user identification (ID) and password (PW) are generated for the physician at step312 (seeFIG. 3D ).FIG. 4 shows an overview of the site registration ofstep 210. The number of sites input by the user is received at step402 (seeFIG. 4A ). As noted above, a table of site locations, having a sufficient number of rows, is generated at step404 (seeFIG. 4B ). The user completes the table, including information for each site, at step406 (seeFIG. 4C ).- The data entry forms will now be described. In general, a data entry form can be implemented in HTML as one page or several; if it is implemented in several pages, each page ends with “Next Page” (or if the last page of the form “Submit”), “Discard Changes and Exit” or “Save Changes and Exit” options. Also, the same forms can be used to add information about new users and to update information for existing users.
FIG. 5A shows the first page of the physician's form. Thefirst page 502 includes anarea 504 for contact information, an area506 for medical licensing and DEA information, and an area508 in which the physician can identify specialties through drop-down menus. Other information, such as professional affiliations with one or more medical schools and hospitals, can be solicited here. There are also “Next Page” (or if the last page of the form “Submit”), “Main Page” and “Save Changes and Exit”buttons FIG. 5B shows a subsequent page514 of the physician's form. As shown inFIG. 5B , the physician is asked about experience as a principal investigator in a clinical trial. Detailed experience on each clinical trial can be solicited in further pages, as well as information on any FDA or sponsor audit that the physician or the site has undergone, any administrative roles that the physician plays at the site, any articles or presentations in which the physician is named as an author, and any organizations and associations in which the physician is actively involved.- The pages requesting organization and site information are similar. In addition, the organization pages request the type of organization, while the site pages request what facilities the site has, among other things. Pages can also be provided to transfer a physician from one site or organization to another or to transfer a site from one organization to another. This function is accessible solely to employees of the service, not registered users.
- The administrator of the database server114 can accept the information as given or can take various steps to verify it. For example, the administrator can verify that a given facsimile number or e-mail address words, check a given medical license number, or interview either the person submitting the information or those persons named as references.
- Once all of the data concerning participating physicians, organizations, and sites have been entered into the database, the database can be searched.
FIG. 6 shows a search page for searching for physicians. Thesearch page 600 uses text boxes and drop-down menus, although other suitable interface elements can be used as needed. In any text box, wildcard searching can be allowed. - Physicians can be searched by name, site, organization, or any combination of the three by use of the text boxes602,604,606. The drop-down menu608 allows the search to be conducted for physicians who are members, non-members, or either. The person conducting the search can select a state through a drop-
down menu 610 or a region through a drop-down menu 612. A specialty can be selected through a drop-down menu 614. Key words can be entered in atext box 616. Thepage 600 can be designed to include any other search criteria, such as those relating to clinical trial experience. Once all of the search criteria are entered, the user clicks on the “Find Physicians” button618. - Clicking on the “Find Physicians” button618 causes the database server114 to search for physician records matching the search criteria. The database server114 passes the search results to the
Web server 110 which formats them as shown inFIG. 7 . The search resultspage 700 shows the search results in the form of a table702. At the far left is aselect column 704, which allows the user to select individual physicians for further consideration or to select all physicians through a “Select all”check box 706. Thename column 708 lists the physicians by name, with links to their profiles in the database. Thespecialty column 710 gives each physician's specialty. Theorganization column 712 and thesite column 714 identify the organization and site, respectively, with which each physician is associated and include links to those organizations' and sites' profiles. Atelephone number column 716 and afacsimile number column 717 give that information for each physician. Themembership status column 718 identifies each physician as a member or non-member. TheCTO column 720 and theORO column 722 list CTO's and ORO's, respectively, with which the physician is or has been involved. Columns can be sorted by clicking on the name of the column. Anindicator 726 identifies the number of physicians who have matched the query. Themerge buttons - An employee of the service can enter a CTO or ORO as shown in
FIG. 8 . At step802, the following are selected through drop-down menus or other suitable interface elements (seeFIG. 8A ): the specialty concerned (e.g., urology), the estimated number of patients, and the duration. Atstep 804, the following are input as free-form text (seeFIG. 8B ): a descriptive title of the study, one or more inclusion criteria for patients (e.g., male patient aged 40-85 years), one or more exclusion criteria for patients (e.g., no previous prostatic surgery within two weeks of base line), and a per-patient budget (this can be indicated as “to be negotiated”). The criteria can also be selected through drop-down menus, in which case there will be an option to add criteria not appearing in the menus. The criteria can also be assigned a sorting order. Through a Design SQQ/PQQ Interface, the relevant SQQ or PQQ is designed and posted on the web at step806 (seeFIG. 8C ). A number is manually assigned at step808 (seeFIG. 8D ). The opportunity can be made viewable to network members only by an employee of the service at step810 (seeFIG. 8E ). The CTO or ORO can be made viewable to persons viewing the organization's or site's profile or in a list sorted by specialty; preferably, both are done. - Once the CTO or ORO is entered, the search results from
FIG. 7 can be merged into the CTO or ORO. Once thebutton merge page 902 or904 in the merge area900 ofFIG. 9 appears. The user can select the Merge CTO orMerge ORO 902 or904 and click the “Merge” button906 or may cancel by closing the window. If the “Merge” button906 is clicked, all of the selected search results fromFIG. 7 are linked to the description of the CTO or ORO in the database. Checking for duplicate records is done automatically. - The physicians, organizations, or sites merged into the listing can be invited to consider the CTO or ORO. Since anyone found in the search results will have provided some contact information, the invitation can be made by e-mail, facsimile, or any other suitable channel.
- Thus, the service (or, in an alternative embodiment, a sponsor of a CTO or ORO) can initiate contact with a physician, organization or site which may be suited to conduct that CTO or ORO, as described above with reference to
FIG. 9 . Either way, the questionnaire is completed and returned to the sponsor. The sponsor considers the questionnaire and can send a person to interview the physician or otherwise visit the organization or site at which the trial or other research would take place. A Web page can be provided to indicate the reasons why any particular physician, organization or site was accepted or declined. That Web page is accessible only by employees of the service or network members who have registered to use the system. - Once a physician, organization or site is selected to conduct the trial, data are collected for benchmarking. General (industry) benchmarks include the following: revenue per primary investigator per year, patients enrolled per number of coordinator FTE's, average revenue per patient, distribution of revenue by type, average enrollment as a percentage of goal, split of patient recruitment between in-house and advertised, average number of protocols per year, average number of years in clinical research, percentage of studies through third party, percentage of studies directly from CRO or sponsor, number of sponsors with which a particular physician, site or organization has worked, and number of CRO's with which a particular physician, site or organization has worked. Benchmarks specific to a particular study include the following: time to first patient enrolled, total patients enrolled after the first thirty days, actual enrollment as a percentage of the enrollment goal, per-patient budget, number of queries per patient, enrollment as a percentage of total patients screened, and elapsed time from notification of award of study to initiation visit. Data entered into the Web Form (Site Status Summary, or SSS) are used to compute the benchmarks specific to a particular study. Those data are collected on a per-protocol basis, are specific to that protocol and are not stored for future protocols. On the other hand, information collected in the profiles can be used to update the SQQ/PQQ.
- Examples of Web pages for taking data associated with a particular study are shown in
FIGS. 10 and 11 as1000 and1100, respectively. Thepage 1100 is only partially shown, since the data are taken not simply at the end of 30 days, but rather at the end of every 30-day interval until the end of the study; for instance, thepage 1100 may ask for the number of patients screened after 720 days, etc. At the end of the study, data may be collected, such as the date of the study close-out visit and the number of queries generated. - The benchmarks are calculated automatically by the system. The more data inputted, the more an organization can access. They do not have to wait until the study is over until they can view benchmarks, but as soon as the relevant data are input the benchmark is viewable. Once all of the data are input and the study is concluded, or once the data currently available are input if benchmarking during the study is required, benchmarking can be performed using straightforward calculations on the data. Two examples of data outputs are shown; those skilled in the art will recognize that many other types of data outputs can be provided as needed.
FIG. 12 shows abar chart 1200 in which the total budget per patient is broken down into overhead, procedure and lab fees, the study coordinator, and the investigator fees.FIG. 13 shows abar chart 1300 showing a particular benchmark by its value in the user's study compared to its high, low, and average values from among all organizations participating in the same opportunity monitored by the database server114. The identities of the other organizations are not identified. Each of the bars of thebar chart 1300 can be broken up like the bar in thebar chart 1200.- The benchmarks can be displayed to the sponsor of the study, the physician, site or organization conducting the study, or both. Non-numerical information, such as the identities of the physicians involved in the study and information about them, can also be reported.
- An enhancement to the service provides a customizable search page for the “study budget project” where physicians can search for a price range for their service. The search is intuitive enough to allow physicians to select predetermined criteria to focus their search. Physicians will also be able to download their study budget information in a standard template usable in a spreadsheet program (e.g., Microsoft Excel).
- At step1402, a study budget system is provided, which is a separate module from the service previously described. At
step 1404, the user logs in. Members will pay a yearly license fee, billed monthly. Existing users will be able to retrieve their user name and password information by entering their e-mail address. - If the system determines at
step 1406 that the user is a new user, or is an existing member using the system for the first time, the user will be required to complete an activation form atstep 1408. On confirmation of the user's credit card, the user will receive a welcome message and information about their subscription via e-mail. - At
step 1410, the user will choose from standard study questions about that user's procedure type, which sets the parameters for the query. Atstep 1412, the user receives high, average, and low fees for the selected query. - At
step 1414, a study budget template is provided in order to be populated with the user's information. The study budget template provides automatic calculations and a printable template. At step1416, on completion of the template, the information is updated to the database. - While the disclosure set forth above is believed to provide an enabling disclosure of the present invention and a best mode for carrying out the present invention, an administrative-level user's guide will now be set forth for the sake of completeness. For ease of understanding, the user's guide is organized in tabular form.
Question Topic Response What happens New Site If it is completed on-line, when a site Applications E-mail Copy automatically goes to the service. completes their Completing The Site Recruiter then logs onto application on- Reference Check www.rapidtrials.com/orge.asp line or hard Information on They select the organization name from the drop copy? Rapidtrials down list and hit EDIT Activating a New They move using ALT + N or Next Page to the last Site page of the profile The last page lists the references They also list whether the references were: “outstanding” “Average” “Unfavorable” Once the reference checks are done, these are changed as appropriate The date the contract is signed is inputted into the first page, as well as some other contract information. Once the site is ready to be a member of the network the site recruiter goes to the first page of the organization profile, by going to www.rapidtrials.com/orge.asp, members admin, selecting the org name from the drop down and hitting EDIT Members admin page drop down list of organizations If the organization is in black it is an active member of the network, if the org is in blue or any other color you can check the status by going to Edit and looking at the status drop down halfway down page one of the organization profile. Halfway down the first page is a status drop down. The status is: Active Terminated Contact Hold Pending Inactive The organization status is changed to Active. This automatically creates a second browser window with the User ID and Passwords for that organization. You then select Export to XLS, and these can be printed out from XLS and faxed to the organization. You then close the second browser window and select ‘Save changes and Exit’ This organization is now an Active member of the network and has full access to the member's section. How do I put a Posting a New The Project manager identifies a new CTO new clinical Clinical Trial Log on to Rapidtrials.com trial Opportunity on This will take you to CTO Admin or opportunity on Rapidtrials www.rapidtrials.com/cto/asp Rapidtrials Creating a PCRS Click New Tracking Number Enter the CTO name Enter the Tracking Number Enter if the CTO is recruiting Yes/No (When a CTO is no longer recruiting this must be changed to No) Enter if the CTO is to be shown on the web Yes/No (When the CTO is no longer to be viewed on the web must be changed to NO) Enter Project Manager Name Enter which of the following are applicable (to be update, as information becomes available over time) Sponsor Sponsor contact Name Sponsor Contract Name CRO CRO contact name CRO contract name Then select save How do I create Creating a Synopsis Click CTO description a CTO Online for a Clinical Trial This is where you create the synopsis for the (CTO) clinical trial For those choices that appear in blue, click on the blue link, type in the text that is to appear under that link, click save and click return Select Therapeutic Area and indication from the dropdown Date CTO sent to sites CTO Phase Study design Status-Proposed or awarded Study Objective Investigator specialty, up to three from dropdown Clinical Experience, do they have to have experience or can they be trial naive Is a certain number of past clinical trials necessary, indicate this here IRB Utilization-Central or Local Type of site, check all that apply Is it Inpatient/Outpatient? Key Inclusion Criteria Key exclusion Criteria Removal of patients from therapy Length in enrollment in months Note: This generates the correct amount of 30 day increments for the benchmarking Expected enrollment Duration of therapy Number of patient visits Investigator Meeting Anticipated Start Date Special request/equipment Budget There are then 5 extra criteria, which you can label and then enter text for Click Save How do I create Creating an SQQ Click SQQ design a Site Using the same If there is another SQQ already created that you Qualification template as a know is identical to the one you wish to create, or Questionnaire previously very similar so that you would change just one or (SQQ) Online developed SQQ two things- Creating Custom Click Load SQQ pattern from another CTO Questions Select CTO Name and click OK Say OK This will create the same SQQ for your clinical trial OR To create a brand new SQQ Click SQQ design There will be a list of standard questions, these are questions that if they are completed on the profiles will pre fill for the Investigators Select those you wish to delete Note: The one that is blank is actually Board Certification If you wish to create specialized questions, in addition to the standard questions, click Further Criteria Click Simple Questions A Question Interface appears Let's use some examples: How far away is the nearest airport? ___in miles Which of the following apply to your site? Small, Medium or Large Check all that your site has ECG, Sleep Lab, Study Coord What date is your next IRB meeting? Using the Interface Label- 1. Question One Label = How far away is your site After Label = miles Question Type = Text box Question Data Type = Text Multiple Choices = 2. Question Two Label = Which of the following apply to your site? After Label = Question Type = Combo Box (only one answer allowed) Question Data Type = Numeric Multiple Choices = Small, Medium, Large 3. Question Three Label = Check all that your site has After Label = Question Type = Multiple Choices (allows for more than one answer) Question Data Type = Numeric Multiple Choices = ECG, Sleep Lab, Study Coord 4. Question Four Label = What date is your next IRB meeting After Label = Question Type = Text box Question Data Type = Date Multiple Choices = Then Click Save This saves your question and it shows up under the Criteria Label Continue clicking Simple Questions and then creating questions until you are done To change any question you have already created, click the blue link and make your changes and click save When you are done Click SQQ combination, this merges the standard questions and the special questions Click re number, and then number the questions so that they appear in the correct order. To delete a question click the delete box then save To make further changes to the special questions click Edit further criteria and click on the blue links and make the change, click save and then SQQ combination When you are satisfied with the order click Save Then click Save Changes and Exit TO send The project manager will provide with the new automatic CTO a list of specialties who need to be informed emails to the about this trial orgs to inform From CTO admin, click search, this appears above them of a new the matched site window CTO This will take you to the search page Select enable-Membership Status The choices are member, non-member, or all Select member Under Investigator specialty, click enable and choose the specialty from the drop down box Note; The search by specialty is an AND search, not an OR search. So choose only one specialty at a time. Click Find Physicians At the bottom of the search results page, click select all Review the list and those that you do not wish to inform of the clinical trial unselect them by clicking on the checkmark on the left-hand side so that the box is empty. Then click merge CTO Select the CTO name Click Merge This will dump the sites you selected into the matched window on the CTO admin page Repeat with each specialty until completed From CTO admin Click Select All next to matched window Click Send SQQ and description This will create an email and fax list of all the matched organizations To send the email click send mail all One email is sent to each organization contact person that you identified, the email lists all the suitable investigators at that site. TO add a Go to CTO admin doctor that has Under CTO name drop down list, select the name submitted for a and click Edit clinical trial OR Enter tracking number on the top bar and click EDIT IF Doctor appears in matched window Doctor does not appear in matched window 1. Select his name by clicking on it Click SSS Then click EDIT under Update Compete the first page: Date of submission deadline Date of submission Date notified of submission Note: This last field sends an automatic email to the site when it is completed. See attached email Click Save changes and Exit His status will now be Submitted pending selection 2. From CTO admin halfway down Select Org Name Select Site Select Physician Click ADD He will go in to the top of the matched window Follow above steps To get a list of Go to responded window doctors Click on first name in the box submitted for a Click Shift study (not Click on last name in the box including those Click Show Info whose status is Print report not submitted) To remove a Click on his name doctor from a Click remove trial To view info for a doctor Click his name Click view info Info is shown as: Name, Site, Org, Specialty, Tel #, Fax #, Status, Last Change To update SSS Click his name Click SSS Click Edit under Update To view SQQ Click his name Click SQQ To add a new Click New next to Sponsor Sponsor Enter Info Click Save Click Return Same for Sponsor Contact, Sponsor Contract, CRO, CRO Contact, and CRO Contract Search Page Every criteria you enable limits your search It is a wild card to search: To search for Bill Jones, enable physician name and enter Bill Jones Bi Ll Jo Nes Etc Click find physicians to get results Results are shown as Org, Site, Physician, Phone, Fax, Specialty, Status (member/non-member), CTO, ORO The results can be sorted by any of the column headings, simply click on them Clicking on the physician's name takes you to his profile Clicking on the site name takes you to the site profile Clicking on the org name takes you to the organization profile To transfer a doctor If a doctor moves from one organization, or more likely someone entered him in the wrong organization profile Search for the physicians From the results page select doctor to be transferred Member Admin Page Organization Level To create a new org, click new To edit an existing org, select name from drop down list and click edit Click Transfer Select Org and Site he is to be transferred to Click Transfer To view (in read only mode) an existing org, select name from drop down list and click show To view the sites for that org, select site name from drop down and click site Site Level To create a new site, click new To edit an existing site, select name from drop down list and click edit To view (in read only mode) an existing site, select name from dropdown list and click show To view the members for that site, select site name from drop down and click members To view the SSS for a site (all the trials that they are linked with) click SSS To delete a site, select the site name from the drop down and click delete Members Level To create a new member, click new To edit an existing member, select name from drop down lists and click edit To view (in read only mode) an existing member, select name from dropdown list and click show To view the SSS for member (all the trials that they are linked with) click SSS To delete a member, select the member name from the drop down and click delete Please Note If you change the organization name at the organization level, the site level and the member level DOES NOT AUTOMATICALLY CHANGE. You must click Site and Member again to ensure the information you are looking at is relevant to the site that you are viewing To view the SSS for and org (all the trials that they are linked with) click SSS To delete an org, select the org name from the drop down and click delete SSS Search Mini search for CTO Issues Criteria Results are always shown as CTO Number, Org Name, Site Name, Physician, Specialty, Phone, Fax, Sponsor/CRO, Status, Last Change, Access to SSS You can search by Tracking Number Organization Name Site Name Physician Name The more information you enter the more limited your search After you enter the search criteria, click find To get to CTO admin, click CTO To reset search criteria, click reset Passwords Click Passwords Link Select the organization that you want to get passwords for Click Update Make changes and to save click update To print, click print To export to XLS, click export to XLS Tracking of Web These are tracked by Site Changes SSS changes SQQ changes Profile changes It tracks-Tracking number, Physician, Site, Org, Date and Time of change, Location of change (form name and page) Physician History View which CTO have been sent to a physician and whether they responded (yes or no) Search by name, site, organization or institution (for ORO doctors) Limit as much as you want, more info, more limited search, another wild card search Hit Find Then select which physician and click View History History shown as CTOs offered responded Yes/No OROs offered responded Yes/No Sponsor Requests Results of tell us about your needs Marketer Requests Results of See what we can do for you - Various other enhancements can be made to the present invention. They will now be set forth.
Functionality Description Best Practices Extension of the benchmarking capability, this would be accessible to Across All Sites members. Based on benchmarking metrics, those sites with the best Benchmarked practices will be identified by the service, contacted and interviewed on how they obtained the results in this trial. This information will be shared confidentially with other sites in the same protocol. This will promote information exchange amongst sites, help research naive sites, or poorer performing sites to achieve better results on studies, and identify problems before a study closeout situation occurs Secure Bulletin Each clinical trial listed on Rapidtrials and whose status is on-going will Board have a one (1) way (from the service outboard) Bulletin Board where sponsors can post information about a study including Q&A type information In addition, Sponsor project managers can use the bulletin Board to share information about the study on a on-going basis Rapid Budget Separate Web Module accessible from the current website (rapidtrials.com). Members pay a yearly fee, billed monthly. They can apply for membership via the website (rapidtrials.com), with payment accepted via credit card. A new user will be required to complete an activation form; this will collect basic data on the physician and his organization. The module will ask them questions about the type of study they wish to budget for and which procedures are involved. Users receive a high, average, low, and your site (which allows them to view what they charged for the procedure the last time they used the system). The system then asks them to choose a price for each procedure, this feeds into a basic per patient clinical trial specific study budget template which when they have completed choosing their ranges they can print the complete budget worksheet from the website. This information then updates the underlying sequel table, so it will be available to be used in the calculation for the next user. RapidTraining Current Training Program owned by the service is for Novice Sites and or as a refresher course for experienced sites. Currently we have the program on CD and will Migrate the CD based training program to the Web as a standalone serviced Rapid Meeting Investigator CD platform currently exists. Will be migrated to the Web. Rapidpatient This will be a guide Clinical Research sites in selecting strategies and Recruitment tactics to aid in the clinical trial patient recruitment and retention activities. Based upon a users description of the patient recruitment and retention objectives they are attempting to support, the Rapidtrials web module will suggest appropriate tactics to support the objectives. This module will also include IRB/FDA regulations governing patient recruitment advertising and recruitment guidelines. This module of the Rapidtrials website will be a portal for ordering mugs, t-shirts, ads, media buying, plaques and other promotional items through the service to enhance patient recruitment and retention in clinical trials. A catalog of items, which are IRB/FDA compliant that they can order through us. Rapid Assessment A Rapidtrials web module that uses a psycho-behavioral assessment (similar to Myers Briggs) that has been validated by the service using historical performance and behavioral assessment response correlation's to identify sites with a high probability of succeeding in clinical trials. Investigators and coordinators will complete this behavioral assessment on- line as a predictive tool for the practice of future success in clinical trials. Gaps in attributes will be identified for Sites as a means of guiding them in clinical research program recruitment. For sponsors involved in site selection, or preparing to invest in site development it will give them another performance indicator prior to selecting sites to participate in a clinical trial. Industry Generated by data entered into members organization, site and Investigator Benchmarking profiles, these benchmarks will be displayed by Type of Organization (I.e. solo practice, SMO etc): Total number of protocols per year, Total grant value per protocol, total number of patients enrolled in a clinical trial/year, total number of Study coordinators, total number of sites doing research, Total number of Patients per study coordinator FTE's, Average number of Patients per year, Number of Physicians, Number of PI's, Overhead as a percentage of total per patient budget, Study Coordinator costs as a percentage of Total per patient budget, Investigator Fees as a percentage of total per patient budget, Procedure, lab fees as a percentage of total per patient budget, total number of sponsors worked for/year, Total number of CROs/year, Actual Enrollment/Enrollment Goal. Site Capabilities Data capture approved by field based consultants to gather information on Assessment Data sites developing research programs or preparing to participate in clinical Capture and trial. Migration of proprietary data capture tools to the web. Reporting Site Operations Data capture pages used by field based consultants to gather information on Specialists Site sites developing research programs or preparing to participate in clinical Development trial. Migration of proprietary data capture tools to the web. This is the eWorkbook same as the above. Site Operations Data Capture Pages, tools and presentations to use in preparing for and Specialists Site monitoring site performance on a clinical trial. Migration of Proprietary Management Data Capture tools to the web eWorkbook - While a preferred embodiment of the present invention has been set forth in detail, those skilled in the art who have reviewed the present disclosure will readily appreciate that other embodiments can be realized within the scope of the present invention. For example, the utility of the invention is not limited to the medical field. Also, any benchmarks can be calculated, in which case the forms for collecting the data are modified accordingly. Further, the benchmarks can be displayed as text, as any suitable chart, or as a combination of the two. In addition, either the Web server or the database server can incorporate the ability to handle payments from one participant to another, as well as from participants to the company operating the servers. Moreover, any mention of a specific software package (e.g., Microsoft Excel) should be construed as illustrative rather than limiting. Therefore, the present invention should be construed as limited only by the appended claims.
Claims (19)
1. A system, comprising:
a database server for (i) providing a user interface for capturing investigator and research site metrics for a plurality of research sites and a plurality of investigators who are participating in a clinical research trial, such metrics including at least one of time to first patient enrolled, total patients enrolled after the first 30 days, actual enrollment as a percentage of the enrollment goal, enrollment rate, screen failure rate, completion rate, per-patient budget, number of queries per patient, enrollment as a percentage of patients screened, and elapsed time from irb submission to irb approval, (ii) storing said collected investigator and research site metrics useful at least for calculating benchmarking information regarding the conduct of said clinical research trial; and (iii) calculating said benchmarking information for each of said plurality of investigators and each of said plurality of clinical research sites participating in said clinical research trial using said collected investigator and research site metrics for said clinical research trial, said benchmarking information comprising information comparing each of said plurality of clinical research sites and each of said plurality of investigators to all clinical research sites and all investigators participating in said clinical research trial; and
a communication server, in communication with said database server, for providing a user interface for communicating some or all of said calculated benchmarking information to one or more of said plurality of investigators and one or more of said plurality of clinical research sites, said calculated benchmarking information being available after said investigator and research site metrics are received by said database server.
2. The system ofclaim 1 , wherein the benchmarking information comprises investigator and research metrics continually received by said database server during the clinical research trial.
3. The system ofclaim 2 , wherein the benchmarking information further comprises investigator and research site metrics received by said database server at a beginning of the clinical research trial, throughout the clinical trial, and at an end of the clinical research trial.
4. The system ofclaim 2 , wherein the benchmarking information comprises a comparison between the benchmarks of a variety of investigator and research site metrics for the clinical research trial and the benchmarks for other clinical research sites involved in the same clinical research trial.
5. The system ofclaim 2 , wherein the benchmarking information further comprises investigator and research site metrics received by said database server at a beginning of the clinical research trial, throughout the clinical trial, and at an end of the clinical research trial, aggregated for viewing by a sponsor or representatives of a sponsor.
6. An article of manufacture comprising:
a non-transitory computer-readable storage medium; and
instructions stored on the non-transitory computer-readable storage medium, the instructions comprising:
(a) providing a user interface for collecting investigator and research site metrics on a database server for a plurality of research sites and a plurality of investigators participating in a clinical research trial, such metrics including at least one of time to first patient enrolled, total patients enrolled after the first 30 days, actual enrollment as a percentage of the enrollment goal, enrollment rate, screen failure rate, completion rate, per-patient budget, number of queries per patient, enrollment as a percentage of patients screened, and elapsed time from irb submission to irb approval;
(b) storing on said database server said collected investigator and research site metrics useful at least for calculating benchmarking information regarding conduct of said clinical research trial; and
(c) automatically calculating on said database server said benchmarking information for each of said plurality of investigators and each of said plurality of clinical research sites participating in said clinical research trial using said collected investigator and research site metrics for said clinical research trial, said benchmarking information comprising information comparing each of said plurality of clinical research sites and each of said plurality of investigators to clinical research sites and investigators participating in said clinical research trial.
7. The article of manufacture ofclaim 6 , wherein said instructions further comprise:
(d) providing a user interface for communicating some or all of said calculated benchmarking information to one or more of said plurality of investigators and one or more of said plurality of clinical research sites, said calculated benchmarking information being viewable after said collected investigator and research site metrics are received by said database server.
8. The article of manufacture ofclaim 7 , wherein said collected investigator and research site metrics stored on said database server comprise metrics received by said database server at multiple times during the clinical research trial in order to update the calculated benchmarking information.
9. The article of manufacture ofclaim 8 , wherein said collected investigator and research site metrics stored on said database server comprise metrics received at regular time intervals during the clinical research trial updated at any time once the clinical research trial has begun, and wherein updated benchmarking information is viewable automatically as the benchmarking information is updated throughout the duration of the clinical research trial.
10. The article of manufacture ofclaim 9 , wherein said collected investigator and research site metrics stored on said database server comprise metrics received at a beginning of the clinical research trial, intervals throughout the clinical research trial, and at an end of the clinical research trial, aggregated for viewing by a sponsor or representatives of a sponsor.
11. The article of manufacture ofclaim 9 , wherein said collected investigator and research site metrics stored on said database server further comprise metrics received at a beginning of the clinical research trial, intervals throughout the clinical research trial, and at an end of the clinical research trial.
12. The article of manufacture ofclaim 6 , wherein the benchmarking information comprises a comparison between the benchmarks of a variety of collected investigator and research site metrics for the clinical research trial of one clinical research site to other clinical research sites involved in the same clinical research trial.
13. A method, comprising the steps of:
(a) providing a user interface for collecting investigator and research site metrics on a database server for a plurality of research sites and a plurality of investigators participating in a clinical research trial, such metrics including at least one of time to first patient enrolled, total patients enrolled after the first 30 days, actual enrollment as a percentage of the enrollment goal, enrollment rate, screen failure rate, completion rate, per-patient budget, number of queries per patient, enrollment as a percentage of patients screened, and elapsed time from irb submission to irb approval;
(b) storing on said database server said collected investigator and research site metrics useful at least for calculating benchmarking information regarding conduct of said clinical research trial; and
(c) automatically calculating on said database server said benchmarking information for each of said plurality of investigators and each of said plurality of clinical research sites participating in said clinical research trial using said collected investigator and research site metrics for said clinical research trial, said benchmarking information comprising information comparing each of said plurality of clinical research sites and each of said plurality of investigators to clinical research sites and investigators participating in said clinical research trial.
14. The method ofclaim 13 , further comprising:
(d) providing a user interface for communicating some or all of said calculated benchmarking information in real time to one or more of said plurality of investigators and one or more of said plurality of clinical research sites, said calculated benchmarking information being viewable immediately after said collected investigator and research site metrics are received by said database server.
15. The method ofclaim 14 , wherein said collected investigator and research site metrics stored on said database server comprise metrics received by said database server at multiple times during the clinical research trial in order to update the calculated benchmarking information.
16. The method ofclaim 15 , wherein said collected investigator and research site metrics stored on said database server comprise metrics received at regular time intervals during the clinical research trial updated at any time once the clinical research trial has begun, and wherein updated benchmarking information is viewable automatically as the benchmarking information is updated throughout the duration of the clinical research trial.
17. The method ofclaim 16 , wherein said collected investigator and research site metrics stored on said database server comprise metrics received at a beginning of the clinical research trial, intervals throughout the clinical research trial, and at an end of the clinical research trial, aggregated for viewing by a sponsor or representatives of a sponsor.
18. The method ofclaim 16 , wherein said collected investigator and research site metrics stored on said database server further comprise metrics received at a beginning of the clinical research trial, intervals throughout the clinical research trial, and at an end of the clinical research trial.
19. The method ofclaim 13 , wherein the benchmarking information comprises a comparison between the benchmarks of a variety of collected investigator and research site metrics for the clinical research trial of one clinical research site to other clinical research sites involved in the same clinical research trial.
Priority Applications (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US14/075,549US20140142968A1 (en) | 2002-04-09 | 2013-11-08 | Online, interactive evaluation of research performance |
| US14/621,100US20160379302A9 (en) | 2002-04-09 | 2015-02-12 | System and method for preparation of clinical trial budgets |
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US10/118,369US8620677B2 (en) | 2002-04-09 | 2002-04-09 | Online, interactive evaluation of research performance |
| US14/075,549US20140142968A1 (en) | 2002-04-09 | 2013-11-08 | Online, interactive evaluation of research performance |
Related Parent Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US10/118,369ContinuationUS8620677B2 (en) | 2002-04-09 | 2002-04-09 | Online, interactive evaluation of research performance |
Related Child Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US14/621,100ContinuationUS20160379302A9 (en) | 2002-04-09 | 2015-02-12 | System and method for preparation of clinical trial budgets |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| US20140142968A1true US20140142968A1 (en) | 2014-05-22 |
Family
ID=28674410
Family Applications (3)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US10/118,369Active2026-01-02US8620677B2 (en) | 2002-04-09 | 2002-04-09 | Online, interactive evaluation of research performance |
| US10/956,131AbandonedUS20050102162A1 (en) | 2002-04-09 | 2004-10-04 | Online, interactive evaluation of research performance |
| US14/075,549AbandonedUS20140142968A1 (en) | 2002-04-09 | 2013-11-08 | Online, interactive evaluation of research performance |
Family Applications Before (2)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US10/118,369Active2026-01-02US8620677B2 (en) | 2002-04-09 | 2002-04-09 | Online, interactive evaluation of research performance |
| US10/956,131AbandonedUS20050102162A1 (en) | 2002-04-09 | 2004-10-04 | Online, interactive evaluation of research performance |
Country Status (1)
| Country | Link |
|---|---|
| US (3) | US8620677B2 (en) |
Cited By (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2016093872A1 (en)* | 2014-12-10 | 2016-06-16 | Medidata Solutions, Inc. | System and method for assessing subject event rate reporting |
| US11308436B2 (en)* | 2020-03-17 | 2022-04-19 | King Fahd University Of Petroleum And Minerals | Web-integrated institutional research analytics platform |
Families Citing this family (47)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US20050014120A1 (en)* | 2003-06-25 | 2005-01-20 | Tim Hatton | A Method to Measure the Learning Retention of Students Engaged in a Course of Self-Study |
| US9123077B2 (en) | 2003-10-07 | 2015-09-01 | Hospira, Inc. | Medication management system |
| US8065161B2 (en) | 2003-11-13 | 2011-11-22 | Hospira, Inc. | System for maintaining drug information and communicating with medication delivery devices |
| US20050182658A1 (en)* | 2004-02-18 | 2005-08-18 | Klaus Abraham-Fuchs | Method of improving a clinical study |
| US20050228693A1 (en)* | 2004-04-09 | 2005-10-13 | Webb James D | Data exchange web services for medical device systems |
| US7548917B2 (en)* | 2005-05-06 | 2009-06-16 | Nelson Information Systems, Inc. | Database and index organization for enhanced document retrieval |
| US20070067189A1 (en)* | 2005-09-16 | 2007-03-22 | Numoda Corporation | Method and apparatus for screening, enrollment and management of patients in clinical trials |
| JP2007122295A (en)* | 2005-10-27 | 2007-05-17 | Hitachi Ltd | Clinical trial information processing server and clinical trial information processing method |
| US20070244721A1 (en)* | 2005-10-31 | 2007-10-18 | Sackner-Bernstein Jonathan D | Systems and methods for improved assessment and reporting of the efficacy and safety of drug, biologic, botanical, vitamin, medical food and medical device treatments |
| US20070100663A1 (en)* | 2005-10-31 | 2007-05-03 | Gary Zammit | Methods and systems for web based centralized patient assessment |
| WO2007067926A2 (en)* | 2005-12-06 | 2007-06-14 | Ingenix, Inc. | Analyzing administrative healthcare claims data and other data sources |
| AU2007317669A1 (en) | 2006-10-16 | 2008-05-15 | Hospira, Inc. | System and method for comparing and utilizing activity information and configuration information from mulitple device management systems |
| US20080319793A1 (en)* | 2007-06-18 | 2008-12-25 | Helmut Myritz | Method and commuication system for supporting the adherence to the study protocol of a clinical study |
| US20100023870A1 (en)* | 2008-04-28 | 2010-01-28 | Baker Matthew R | Apparatus and method for integrated management of clinical trials and studies at an irb |
| US20120130746A1 (en)* | 2008-04-28 | 2012-05-24 | Baker Matthew R | Apparatus and method for gaining approval to conduct clinical trials and studies |
| US8271106B2 (en) | 2009-04-17 | 2012-09-18 | Hospira, Inc. | System and method for configuring a rule set for medical event management and responses |
| AU2010242036A1 (en)* | 2009-04-30 | 2011-11-03 | Patientslikeme, Inc. | Systems and methods for encouragement of data submission in online communities |
| US20110238438A1 (en)* | 2010-03-25 | 2011-09-29 | Numoda Technologies, Inc. | Automated method of graphically displaying predicted patient enrollment in a clinical trial study |
| CA2852271A1 (en) | 2011-10-21 | 2013-04-25 | Hospira, Inc. | Medical device update system |
| US11392670B1 (en)* | 2011-12-09 | 2022-07-19 | Iqvia Inc. | Systems and methods for streaming normalized clinical trial capacity information |
| US20130226674A1 (en)* | 2012-02-28 | 2013-08-29 | Cognita Systems Incorporated | Integrated Educational Stakeholder Evaluation and Educational Research System |
| WO2014138446A1 (en) | 2013-03-06 | 2014-09-12 | Hospira,Inc. | Medical device communication method |
| EP3039596A4 (en) | 2013-08-30 | 2017-04-12 | Hospira, Inc. | System and method of monitoring and managing a remote infusion regimen |
| US9662436B2 (en) | 2013-09-20 | 2017-05-30 | Icu Medical, Inc. | Fail-safe drug infusion therapy system |
| US10311972B2 (en) | 2013-11-11 | 2019-06-04 | Icu Medical, Inc. | Medical device system performance index |
| EP3071253B1 (en) | 2013-11-19 | 2019-05-22 | ICU Medical, Inc. | Infusion pump automation system and method |
| EP3138032B1 (en) | 2014-04-30 | 2024-07-24 | ICU Medical, Inc. | Patient care system with conditional alarm forwarding |
| US9724470B2 (en) | 2014-06-16 | 2017-08-08 | Icu Medical, Inc. | System for monitoring and delivering medication to a patient and method of using the same to minimize the risks associated with automated therapy |
| US9539383B2 (en) | 2014-09-15 | 2017-01-10 | Hospira, Inc. | System and method that matches delayed infusion auto-programs with manually entered infusion programs and analyzes differences therein |
| US20160180275A1 (en)* | 2014-12-18 | 2016-06-23 | Medidata Solutions, Inc. | Method and system for determining a site performance index |
| EP3304370B1 (en) | 2015-05-26 | 2020-12-30 | ICU Medical, Inc. | Infusion pump system and method with multiple drug library editor source capability |
| CA3030786A1 (en) | 2016-07-14 | 2018-01-18 | Icu Medical, Inc. | Multi-communication path selection and security system for a medical device |
| WO2019055879A2 (en) | 2017-09-15 | 2019-03-21 | PatientsLikeMe Inc. | Systems and methods for collecting and analyzing comprehensive medical information |
| CA3106519A1 (en) | 2018-07-17 | 2020-01-23 | Icu Medical, Inc. | Systems and methods for facilitating clinical messaging in a network environment |
| US11483402B2 (en) | 2018-07-17 | 2022-10-25 | Icu Medical, Inc. | Maintaining clinical messaging during an internet outage |
| US11139058B2 (en) | 2018-07-17 | 2021-10-05 | Icu Medical, Inc. | Reducing file transfer between cloud environment and infusion pumps |
| ES2985889T3 (en) | 2018-07-17 | 2024-11-07 | Icu Medical Inc | Updating infusion pump drug libraries and operational software in a networked environment |
| US10692595B2 (en) | 2018-07-26 | 2020-06-23 | Icu Medical, Inc. | Drug library dynamic version management |
| EP3827337A4 (en) | 2018-07-26 | 2022-04-13 | ICU Medical, Inc. | Drug library management system |
| US11894139B1 (en) | 2018-12-03 | 2024-02-06 | Patientslikeme Llc | Disease spectrum classification |
| CA3138528A1 (en) | 2019-05-08 | 2020-11-12 | Icu Medical, Inc. | Threshold signature based medical device management |
| CN110413926B (en)* | 2019-07-24 | 2022-08-09 | 秒针信息技术有限公司 | Questionnaire survey method and device |
| CN111090735B (en)* | 2019-12-25 | 2023-03-10 | 成都航天科工大数据研究院有限公司 | Performance evaluation method of intelligent question-answering method based on knowledge graph |
| US11590057B2 (en) | 2020-04-03 | 2023-02-28 | Icu Medical, Inc. | Systems, methods, and components for transferring medical fluids |
| EP4208798A4 (en) | 2020-09-05 | 2024-10-09 | ICU Medical, Inc. | Identity-based secure medical device communications |
| US20220114518A1 (en)* | 2020-10-12 | 2022-04-14 | Dbafc, L.L.C. | Computer system and computer implemented method |
| USD1083992S1 (en)* | 2023-11-22 | 2025-07-15 | Nasdaq Technology Ab | Display screen or portion thereof with animated graphical user interface |
Citations (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US20020002474A1 (en)* | 2000-01-28 | 2002-01-03 | Michelson Leslie Dennis | Systems and methods for selecting and recruiting investigators and subjects for clinical studies |
| US20020143563A1 (en)* | 2001-04-02 | 2002-10-03 | Hufford Michael R. | System for clinical trial subject compliance |
Family Cites Families (30)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US169727A (en)* | 1875-11-09 | Improvement in reagents for testing the strength of vinegar | ||
| US130871A (en)* | 1872-08-27 | Improvement in well-tubes | ||
| US5920871A (en) | 1989-06-02 | 1999-07-06 | Macri; Vincent J. | Method of operating a general purpose digital computer for use in controlling the procedures and managing the data and information used in the operation of clinical (medical) testing and screening laboratories |
| US6196970B1 (en) | 1999-03-22 | 2001-03-06 | Stephen J. Brown | Research data collection and analysis |
| US5546564A (en) | 1993-02-09 | 1996-08-13 | Horie; Kazuhiko | Cost estimating system |
| US7133846B1 (en)* | 1995-02-13 | 2006-11-07 | Intertrust Technologies Corp. | Digital certificate support system, methods and techniques for secure electronic commerce transaction and rights management |
| US5918208A (en)* | 1995-04-13 | 1999-06-29 | Ingenix, Inc. | System for providing medical information |
| US6037940A (en) | 1995-10-20 | 2000-03-14 | Araxsys, Inc. | Graphical user interface in a medical protocol system having time delay rules and a publisher's view |
| US5826237A (en) | 1995-10-20 | 1998-10-20 | Araxsys, Inc. | Apparatus and method for merging medical protocols |
| US5832449A (en) | 1995-11-13 | 1998-11-03 | Cunningham; David W. | Method and system for dispensing, tracking and managing pharmaceutical trial products |
| US5732401A (en) | 1996-03-29 | 1998-03-24 | Intellitecs International Ltd. | Activity based cost tracking systems |
| US5862223A (en) | 1996-07-24 | 1999-01-19 | Walker Asset Management Limited Partnership | Method and apparatus for a cryptographically-assisted commercial network system designed to facilitate and support expert-based commerce |
| US6189029B1 (en) | 1996-09-20 | 2001-02-13 | Silicon Graphics, Inc. | Web survey tool builder and result compiler |
| US5893081A (en) | 1996-11-25 | 1999-04-06 | Etak, Inc. | Using multiple levels of costs for a pathfinding computation |
| US5930775A (en)* | 1997-01-14 | 1999-07-27 | Freddie Mac | Method and apparatus for determining an optimal investment plan for distressed residential real estate loans |
| US6230142B1 (en) | 1997-12-24 | 2001-05-08 | Homeopt, Llc | Health care data manipulation and analysis system |
| US6509912B1 (en)* | 1998-01-12 | 2003-01-21 | Xerox Corporation | Domain objects for use in a freeform graphics system |
| US6820235B1 (en) | 1998-06-05 | 2004-11-16 | Phase Forward Inc. | Clinical trial data management system and method |
| US7054823B1 (en) | 1999-09-10 | 2006-05-30 | Schering Corporation | Clinical trial management system |
| AU8018600A (en) | 1999-10-15 | 2001-04-30 | Biosciences Corporation | Internet-based matching service for expert consultants and customers with matching of qualifications and times of availability |
| JP2004509383A (en) | 2000-05-31 | 2004-03-25 | ファーストトラック システムズ インコーポレイテッド | Clinical trial management system and method |
| US20020038310A1 (en) | 2000-07-17 | 2002-03-28 | Reitberg Donald P. | Single-patient drug trials used with accumulated database: genomic markers |
| WO2002015085A1 (en) | 2000-08-11 | 2002-02-21 | Biosciences Corporation | A system for matching customers with consultants |
| EP1314127A2 (en)* | 2000-08-24 | 2003-05-28 | Veritas Medicine, Inc. | Recruiting a patient into a clinical trial |
| US7711580B1 (en)* | 2000-10-31 | 2010-05-04 | Emergingmed.Com | System and method for matching patients with clinical trials |
| US7076439B1 (en)* | 2001-01-10 | 2006-07-11 | Lsi Logic Corporation | Method and apparatus for managing multiple projects |
| US20020169727A1 (en) | 2001-05-11 | 2002-11-14 | Express Scripts, Inc | System and method for benefit cost plan estimation |
| US20030061096A1 (en) | 2001-09-05 | 2003-03-27 | Gallivan Gerald J. | System and method for use for linking primary market research data with secondary research data |
| US20030065669A1 (en)* | 2001-10-03 | 2003-04-03 | Fasttrack Systems, Inc. | Timeline forecasting for clinical trials |
| CA2465760A1 (en) | 2001-11-02 | 2003-05-15 | Siemens Medical Solutions Usa, Inc. | Patient data mining for quality adherence |
- 2002
- 2002-04-09USUS10/118,369patent/US8620677B2/enactiveActive
- 2004
- 2004-10-04USUS10/956,131patent/US20050102162A1/ennot_activeAbandoned
- 2013
- 2013-11-08USUS14/075,549patent/US20140142968A1/ennot_activeAbandoned
Patent Citations (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US20020002474A1 (en)* | 2000-01-28 | 2002-01-03 | Michelson Leslie Dennis | Systems and methods for selecting and recruiting investigators and subjects for clinical studies |
| US20020143563A1 (en)* | 2001-04-02 | 2002-10-03 | Hufford Michael R. | System for clinical trial subject compliance |
Cited By (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2016093872A1 (en)* | 2014-12-10 | 2016-06-16 | Medidata Solutions, Inc. | System and method for assessing subject event rate reporting |
| US11308436B2 (en)* | 2020-03-17 | 2022-04-19 | King Fahd University Of Petroleum And Minerals | Web-integrated institutional research analytics platform |
Also Published As
| Publication number | Publication date |
|---|---|
| US20050102162A1 (en) | 2005-05-12 |
| US20030191664A1 (en) | 2003-10-09 |
| US8620677B2 (en) | 2013-12-31 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| US8620677B2 (en) | Online, interactive evaluation of research performance | |
| US7844502B2 (en) | Automated shopping system and method for managing a process for selection of human entities | |
| US7487104B2 (en) | Automated system and method for managing a process for the shopping and selection of human entities | |
| US7167855B1 (en) | Internet-based matching service for expert consultants and customers with matching of qualifications and times of availability | |
| US20090222284A1 (en) | Physician office viewpoint survey system and method | |
| US20020059132A1 (en) | Online bidding for a contract to provide a good or service | |
| US20020128879A1 (en) | System and method for providing online management of medical savings accounts and benefits selection | |
| US20020055870A1 (en) | System for human capital management | |
| US20020147625A1 (en) | Method and system for managing business referrals | |
| US20050033633A1 (en) | System and method for evaluating job candidates | |
| US20040068432A1 (en) | Work force management application | |
| US20020059587A1 (en) | Method and apparatus for providing personalized services | |
| US20020173990A1 (en) | System and method for managing interactions between healthcare providers and pharma companies | |
| US20020077998A1 (en) | Web based system and method for managing sales deals | |
| US20030208388A1 (en) | Collaborative bench mark based determination of best practices | |
| US20040039601A1 (en) | Virtual file cabinet including health information method and apparatus | |
| US20060293928A1 (en) | Method and system to recommend insurance plans | |
| AU2398200A (en) | Process for consumer-directed prescription influence and health care professional information | |
| US20080091511A1 (en) | Method and system for registering, credentialing, rating, and/or cataloging businesses, organizations, and individuals on a communications network | |
| US20020013716A1 (en) | Network based integrated system of care | |
| US20140188904A1 (en) | Automated system and method for managing a process for the shopping and selection of human entities | |
| US20160379302A9 (en) | System and method for preparation of clinical trial budgets | |
| Wang et al. | The impact of internet on service quality in the banking sector | |
| US20070083415A1 (en) | Computerized internetwork system for automated training, management, and accounting of distributors | |
| US20070198572A1 (en) | Automated system and method for managing a process for the shopping and selection of human entities |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| AS | Assignment | Owner name:PCRS, INC., PENNSYLVANIA Free format text:ASSIGNMENT OF ASSIGNORS INTEREST;ASSIGNOR:BLUMENFELD, TRACY HARMON;REEL/FRAME:032392/0654 Effective date:20020408 | |
| AS | Assignment | Owner name:MEDIDATA SOLUTIONS, INC., NEW YORK Free format text:ASSIGNMENT OF ASSIGNORS INTEREST;ASSIGNOR:PCRS, INC. D/B/A RAPIDTRIALS, INC.;REEL/FRAME:035170/0626 Effective date:20150313 | |
| STCB | Information on status: application discontinuation | Free format text:ABANDONED -- FAILURE TO RESPOND TO AN OFFICE ACTION |