US20130197607A1 - Dual patient controllers - Google Patents
Dual patient controllersDownload PDFInfo
- Publication number
- US20130197607A1 US20130197607A1US13/800,729US201313800729AUS2013197607A1US 20130197607 A1US20130197607 A1US 20130197607A1US 201313800729 AUS201313800729 AUS 201313800729AUS 2013197607 A1US2013197607 A1US 2013197607A1
- Authority
- US
- United States
- Prior art keywords
- medical device
- controller
- charger
- controller charger
- control
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Abandoned
Links
- 230000009977dual effectEffects0.000titledescription6
- 230000006870functionEffects0.000claimsabstractdescription52
- 230000000638stimulationEffects0.000claimsdescription141
- 238000004891communicationMethods0.000claimsdescription57
- 230000005540biological transmissionEffects0.000claimsdescription14
- 230000001939inductive effectEffects0.000claimsdescription12
- 239000007943implantSubstances0.000claimsdescription4
- 238000012544monitoring processMethods0.000claimsdescription4
- 238000000034methodMethods0.000abstractdescription18
- 238000011282treatmentMethods0.000description14
- 230000002354daily effectEffects0.000description8
- 238000002560therapeutic procedureMethods0.000description8
- 230000004044responseEffects0.000description7
- 230000001537neural effectEffects0.000description6
- 238000010586diagramMethods0.000description5
- 210000001519tissueAnatomy0.000description4
- 238000012546transferMethods0.000description4
- 208000002193PainDiseases0.000description3
- 230000008859changeEffects0.000description3
- 238000012423maintenanceMethods0.000description3
- 238000007726management methodMethods0.000description3
- 230000004048modificationEffects0.000description3
- 238000012986modificationMethods0.000description3
- 210000000115thoracic cavityAnatomy0.000description3
- 208000000094Chronic PainDiseases0.000description2
- 230000009286beneficial effectEffects0.000description2
- 230000005684electric fieldEffects0.000description2
- 230000036541healthEffects0.000description2
- 210000004705lumbosacral regionAnatomy0.000description2
- 230000007257malfunctionEffects0.000description2
- 238000004519manufacturing processMethods0.000description2
- 229920001690polydopaminePolymers0.000description2
- 210000000954sacrococcygeal regionAnatomy0.000description2
- 210000000278spinal cordAnatomy0.000description2
- 208000024891symptomDiseases0.000description2
- 206010010904ConvulsionDiseases0.000description1
- 208000018737Parkinson diseaseDiseases0.000description1
- 230000008901benefitEffects0.000description1
- 230000033228biological regulationEffects0.000description1
- 210000000746body regionAnatomy0.000description1
- 230000036760body temperatureEffects0.000description1
- 239000003086colorantSubstances0.000description1
- 230000003750conditioning effectEffects0.000description1
- 230000008878couplingEffects0.000description1
- 238000010168coupling processMethods0.000description1
- 238000005859coupling reactionMethods0.000description1
- 230000007812deficiencyEffects0.000description1
- 238000001514detection methodMethods0.000description1
- 210000001951dura materAnatomy0.000description1
- 206010015037epilepsyDiseases0.000description1
- 230000003203everyday effectEffects0.000description1
- 239000003292glueSubstances0.000description1
- 230000003993interactionEffects0.000description1
- 229910001416lithium ionInorganic materials0.000description1
- 210000003205muscleAnatomy0.000description1
- 210000005036nerveAnatomy0.000description1
- 210000000944nerve tissueAnatomy0.000description1
- 238000013021overheatingMethods0.000description1
- 230000000737periodic effectEffects0.000description1
- 230000002265preventionEffects0.000description1
- 230000009467reductionEffects0.000description1
- 238000009877renderingMethods0.000description1
- 230000001225therapeutic effectEffects0.000description1
- 230000002618waking effectEffects0.000description1
- 230000003442weekly effectEffects0.000description1
- 238000004260weight controlMethods0.000description1
Images
Classifications
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61N—ELECTROTHERAPY; MAGNETOTHERAPY; RADIATION THERAPY; ULTRASOUND THERAPY
- A61N1/00—Electrotherapy; Circuits therefor
- A61N1/18—Applying electric currents by contact electrodes
- A61N1/32—Applying electric currents by contact electrodes alternating or intermittent currents
- A61N1/36—Applying electric currents by contact electrodes alternating or intermittent currents for stimulation
- A61N1/3605—Implantable neurostimulators for stimulating central or peripheral nerve system
- A61N1/36128—Control systems
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61N—ELECTROTHERAPY; MAGNETOTHERAPY; RADIATION THERAPY; ULTRASOUND THERAPY
- A61N1/00—Electrotherapy; Circuits therefor
- A61N1/18—Applying electric currents by contact electrodes
- A61N1/32—Applying electric currents by contact electrodes alternating or intermittent currents
- A61N1/36—Applying electric currents by contact electrodes alternating or intermittent currents for stimulation
- A61N1/372—Arrangements in connection with the implantation of stimulators
- A61N1/378—Electrical supply
- A61N1/3787—Electrical supply from an external energy source
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61N—ELECTROTHERAPY; MAGNETOTHERAPY; RADIATION THERAPY; ULTRASOUND THERAPY
- A61N1/00—Electrotherapy; Circuits therefor
- A61N1/18—Applying electric currents by contact electrodes
- A61N1/32—Applying electric currents by contact electrodes alternating or intermittent currents
- A61N1/36—Applying electric currents by contact electrodes alternating or intermittent currents for stimulation
- A61N1/372—Arrangements in connection with the implantation of stimulators
- A61N1/37211—Means for communicating with stimulators
- A61N1/37235—Aspects of the external programmer
- A61N1/37247—User interfaces, e.g. input or presentation means
Definitions
- This disclosureis directed to dual patient controllers for controlling an electrical stimulation implantable device.
- Neurostimulation devicesdeliver therapy in the form of electrical stimulation pulses to treat symptoms and conditions, such as chronic pain, Parkinson's disease, or epilepsy, for example.
- Implantable neurostimulation devicesfor example, deliver neurostimulation therapy via leads that include electrodes located proximate to the muscles and nerves of a patient. Treatments frequently require two external devices: a neurostimulator controller and a neurostimulation device charger. Neurostimulator controllers are frequently used to adjust treatment parameters, select programs, and even to program treatment platforms into the implantable device. External neurostimulator device chargers are used to recharge batteries on the implanted device.
- Conventional neurostimulator controllersare approximately the size of hand-held gaming system controllers, smartphones, or PDAs. While small, they are too large to comfortably be carried around in one's pocket and must be carried in a belt pouch or purse. Because of the size of these devices, they are not easily concealed and frequently result in unwanted attention.
- Known controllersprovide so many features or functions that they sometimes overwhelm the patient to the point where the trial fails.
- a typical patientwill only use a very small subset of the available features on the controllers. Most patients only use their controller to turn the neurostimulator on or off, select which neurostimulation program to run, and adjust their stimulation amplitude, while a very small percentage of patients utilize the advance controls to adjust program frequency and individual pulse/area stimulation parameters such as pulse width.
- Existing chargersare typically about the same size as the neurostimulator controllers and are used for the sole purpose of recharging the implantable device's battery. These chargers are usually kept in the patient's house and used once a week or so depending upon stimulation parameters.
- the present disclosureis directed to devices, systems, and methods that address one or more deficiencies in the prior art.
- This disclosureis directed to a system with dual patient controllers that control an implantable medical device. It includes a pocket controller and a separate integrated controller charger.
- the present disclosureis directed to an integrated controller charger operable to both charge a rechargeable power source of an implantable medical device and control the implantable medical device.
- the controller chargerincludes a communication module configured to transmit information from the controller charger to the implantable medical device and configured to receive information from the implanted medical device. It also includes a power charging module configured to transmit energy storable at the rechargeable power source on the implantable medical device.
- a control moduleis configured to control both the communication module and the power charging module.
- the control modulemay include a processor and a memory and is operable to store the information received from the implanted medical device and generate signals to activate the stimulation programs on the implanted medical device.
- the present disclosureis directed to an assembly for controlling an implantable medical device configured to transmit and receive information.
- the implantable medical deviceincludes a rechargeable power source.
- the assemblyincludes a controller charger operable to both charge the implantable medical device and control the implantable medical device and includes a limited purpose controller sized smaller than the controller charger.
- the limited purpose controllerincludes a communication module configured to transmit information from the limited purpose controller to the implantable medical device and configured to receive information from the implanted medical device. It also includes a control module configured to transmit to and receive information from the communication module in communication with the implantable medical device.
- the controllermay be configured to permit a user to control only a subset of the features controllable with the controller charger, including, for example, 1 ) electrical stimulation on/off selection, 2 ) stimulation program amplitude adjustment, and 3 ) electrical stimulation program selection.
- the assemblyincludes a controller charger operable to both charge the implantable medical device and control the implantable medical device.
- the controller chargerincludes a communication module configured to transmit information from the controller charger to the implantable medical device and configured to receive information from the implanted medical device. It also includes a power charging module configured to emit energy storable at the rechargeable power source on the implantable medical device.
- a control moduleinterfaces with both the communication module and the power charging module. The control module is configured to permit a user to control features on the implantable medical device including a) electrical stimulation on/off selection, b) stimulation program amplitude adjustment, c) electrical stimulation program selection, and d) adjusting stimulation program frequency, as well as providing additional status information relating to the implanted medical device.
- the assemblyalso includes a limited purpose controller sized smaller than the controller charger.
- the limited purpose controllerincludes a communication module configured to transmit information from the controller to the implantable medical device and configured to receive information from the implanted medical device. It also includes a control module configured to transmit to and receive information from the implantable medical device.
- the limited purpose controlleris configured to permit a user to control only a subset of the features controllable with the controller charger. The subset of the features includes 1) electrical stimulation on/off selection, 2) stimulation program amplitude adjustment, and 3) electrical stimulation program selection.
- the present disclosureis directed to a method performed by a limited purpose controller in communication with an implanted implantable pulse generator.
- the methodincludes steps of receiving from the implantable pulse generator data representing enabled stimulation programs; storing the data representing the enabled stimulation programs on the limited purpose controller; receiving an input from a user to activate stimulation from the implantable pulse generator according to one of the enabled stimulation programs, the limited purpose controller being configured in a manner not permitting the user to modify the enabled stimulation program other than to adjust a global amplitude of pulses emitted by the implantable pulse generator according to an enabled stimulation program; and transmitting data representing the selected stimulation program from the limited purpose controller to the implantable pulse generator.
- the present disclosureis directed to a method performed by a patient controller charger in communication with an implanted implantable pulse generator.
- the methodincludes steps of receiving from the implantable pulse generator data representing enabled stimulation programs; storing the data representing the enabled stimulation programs on the limited purpose controller; receiving an input from a user to adjust a frequency and pulse width of an electrical stimulation treatment; transmitting data representing the adjusted frequency and pulse from the patient controller charger to the implantable pulse generator; receiving an input from a user to charge a battery on an implanted implantable pulse generator with the patient controller charger; and emitting energy from the patient controller charger configured to charge the implantable pulse generator.
- the present disclosureis directed to an integrated controller charger operable to both charge a rechargeable power source of an implantable medical device and control the implantable medical device.
- the controller chargerincludes a controller charger portion configured to wirelessly communicate with the implantable medical device, configured to receive inputs from a user, configured to send control signals indicative of the received inputs to the implantable medical device, and configured to receive information from the implantable medical device and display the information to a user.
- the controller chargeralso includes a coil portion configured to generate an inductive field to wirelessly charge a battery in the implantable medical device, and includes a flexible cable extending between and electrically connecting the controller charger portion and the coil portion, the flexible cable electrically connecting the controller charger and the coil portion.
- the present disclosureis directed toward a pocket controller that is operable by a patient to adjust a plurality of operational parameters defining a stimulation program to be performed by an implantable medical device within the patient.
- the pocket controllerincludes a housing comprising a smaller form factor than a housing of a patient controller that offers a greater number of control options than the pocket controller.
- the pocket controlleralso communicates with the implantable medical device to adjust a plurality of the operational parameters of the implantable medical device.
- a number of the operational parameters that are adjustable with the pocket controlleris less than a number of the operational parameters that are adjustable with the patient controller.
- a user interfaceis also provided to the pocket controller to receive input from the patient, allowing the patient to select, from the plurality of operational parameters adjustable with the pocket controller, a selected operational parameter that is to be adjusted by the patient using the pocket controller.
- the pocket controlleralso receives input from the patient indicating an adjustment to the selected operational parameter.
- the pocket controlleralso includes a communication module that is compatible with a receiver provided to the implantable medical device to transmit a signal indicative of the adjustment from the pocket controller to the implantable medical device and receive information from the implantable medical device.
- a control module including a processoris configured to convey the signal indicative of the adjustment entered via the user interface to the communication module to be transmitted to the implantable medical device.
- the present disclosureis directed toward a system for treating a patient.
- the systemincludes an implantable medical device that is to be implanted in the patient to execute an internal treatment routine on the patient, the internal treatment routine being governed by a plurality of operational parameters.
- a patient controlleris provided to communicate with the implantable medical device and is to be placed in possession of the patient to allow the patient to adjust a plurality of the operational parameters while the implantable medical device is implanted in the patient.
- a pocket controllerthat communicates with the implantable medical device and is also to be placed in possession of the patient.
- the pocket controllerincludes a smaller form factor than a form factor of the patient controller and is usable by the patient to adjust a plurality of, but less than all of the operational parameters that are adjustable with the patient controller.
- the pocket controllerincludes a user interface that is usable by the patient to select, from the plurality of operational parameters, a selected operational parameter that is to be adjusted by the patient using the pocket controller, and to input or select an adjustment to the selected operational parameter.
- the present disclosureis directed toward a system for treating a patient.
- the systemincludes an implantable electrical stimulation device that is to be implanted in the patient that executes an electrical stimulation program to be performed on the patient.
- the electrical stimulation programis governed by a plurality of operational parameters.
- a patient controlleris provided to communicate with the implantable electrical stimulation device and is to be placed in possession of the patient to allow the patient to adjust the plurality of operational parameters while the implantable electrical stimulation device is implanted in the patient.
- the plurality of operational parameters adjustable via the patient controllerinclude an amplitude of an individual electrical pulse emitted as part of a stimulation program performed by the implantable electrical stimulation device relative to an amplitude of another electrical pulse to be emitted as part of the same stimulation program performed by the implantable electrical stimulation device.
- a pocket controlleris also provided to communicate with the implantable electrical stimulation device and is to be placed in possession of the patient.
- the pocket controllerhas a smaller form factor than a form factor of the patient controller.
- the pocket controlleris usable by the patient to adjust a plurality of, but less than all of the operational parameters that are adjustable with the patient controller.
- One of the operational parameters adjustable via the pocket controllerincludes a global amplitude of electrical pulses in a series of electrical pulses to be emitted by the implantable electrical stimulation device as part of a stimulation program.
- the pocket controllerfurther includes a user interface that is usable by the patient to input an adjustment of each of the plurality of operational parameters, and a display device.
- the display devicedisplays at least one of a status of a battery provided to power the implantable electrical stimulation device, a status of a battery provided to power the pocket controller, an operational status of the implantable electrical stimulation device, and information concerning one or more of the operational parameters.
- the present disclosureis directed toward a method of familiarizing a patient with a programmer for controlling execution of a treatment routine to be performed on the patient, where the treatment routine is governed by a plurality of operational parameters.
- the methodincludes providing the patient with a first pocket controller that communicates with a temporary, trial electrical stimulation device supported externally of the patient during a trial period to perform the treatment routine.
- the trial periodis to determine whether the treatment routine performed by the external electrical stimulation device is beneficial to the patient.
- the methodincludes establishing communication between the first pocket controller and an implanted electrical stimulation device or establishing communication between a second pocket controller, which is substantially-similar to the first pocket controller, and the electrical stimulation device to allow the first or second pocket controllers to change a plurality of the operational parameters. Communications are also established between a patient controller to be provided to the patient and the internal electrical stimulation device to change a greater number of the operational parameters than a number of the operational parameters that are adjustable using the first or second pocket controllers.
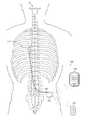
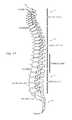
- FIGS. 1A and 1Bare illustrations of a patient's spine with an exemplary electrical stimulator treatment system disposed to treat a particular region of the spine in accordance with one aspect of the present disclosure.
- FIG. 2is an illustration of an exemplary pocket controller in accordance with one aspect of the present disclosure.
- FIG. 3is an illustration of a side of the exemplary pocket controller of FIG. 2 in accordance with one aspect of the present disclosure.
- FIG. 4is block diagram of components of the exemplary pocket controller of FIG. 2 in accordance with one aspect of the present disclosure.
- FIG. 5is an illustration of an exemplary patient controller charger in accordance with one aspect of the present disclosure.
- FIG. 6is an illustration of a top view of the exemplary patient controller charger of FIG. 5 in accordance with one aspect of the present disclosure.
- FIG. 7is an illustration of an exemplary alternatively programmed patient controller charger in accordance with one aspect of the present disclosure.
- FIG. 8is block diagram of components of the exemplary patient controller charger of FIGS. 5-7 in accordance with one aspect of the present disclosure.
- FIG. 9is block diagram of alternative components of the exemplary patient controller charger of FIGS. 5-7 in accordance with one aspect of the present disclosure.
- FIG. 10is a flow chart showing method steps performed by the pocket controller according to one exemplary aspect of the present disclosure.
- FIG. 11is a flow chart showing method steps performed by the patient controller charger according to one exemplary aspect of the present disclosure.
- the devices, systems, and methods described hereinintroduce an improved way for controlling and charging an implanted medical device. They use a dual controller system that includes a pocket controller and a separate controller charger.
- the most-used functions of an electrical stimulator's controllerare incorporated into a small, thin pocket controller that is not only comfortable to carry in a pocket, but can also be attached to a key ring, lanyard, or other such carrying device for ease of daily use.
- These most-used functionsare a subset of features commonly found on an electrical stimulator's controller, and include features such as turning the electrical stimulation on and off, selecting which electrical stimulation program to run, and adjusting the stimulation amplitude.
- this deviceBy limiting the functions of this device to those most commonly used on a daily basis, the device becomes much less intimidating to the patient thereby increasing patient compliance, and the size of the device is substantially reduced, rendering it less obtrusive and therefore making the patient more comfortable with having and using an implanted electrical stimulator.
- the devices, systems, and methods described hereinintroduce a separate patient controller charger for the implanted medical device that provides advanced control features and includes an integrated charger for the implantable power source. Since the advanced features are necessary only for performing periodic (non-daily) maintenance or stimulation adjustment, and performing other less frequently required functions, these advanced features, including the subset features for redundancy, are maintained on a device separate and apart from the pocket controller. These features are integrated with the also-infrequently used charger for the implantable power source. Therefore, where a conventional charger performs only a function of charging the implant's battery and may include powering stimulation on and off, the system disclosed herein includes a multi-function, full-featured advance patient controller charger combination.
- patient controller chargerincorporates a full color graphical user interface with touch screen that presents a simplistic, but rich feature set for the more advanced, while maintaining the charging functions. Since the larger-sized patient controller charger can be left at home, its larger size (approximate size of a PDA) will not raise undesired interest from others. Users have the smaller unobtrusive pocket controller to take with them when they are on the go, and have comfort of the advanced features available for use when in the privacy of their home.
- the dual patient controllers disclosed hereinprovide a level of redundancy and risk management not achieved with a single controller. For example, if one controller malfunctions or is misplaced, a patient can still use the second controller for important control functions until the malfunctioning or misplaced controller is replaced. Accordingly, the patient can continue with his or her scheduled therapy, select another program as desired for effectiveness, and control the stimulation amplitude in the event that it becomes painful or undesired.
- stimulation programrefers to a series of one or more stimulation pulses having a defined relationship used to treat a specific therapy or condition.
- a modification to the stimulation programinvolves adding or removing a pulse from the program, or changing the defined relationship of one pulse relative to the next pulse, but does not include adjusting the pulse amplitude or the pulse width of individual pulses or the series of pulses.
- the pulse amplitude and/or the pulse width of individual pulses or the series of pulsescan optionally be adjusted using a full-feature controller such as the patient controller, but can not be adjusted using the pocket controller.
- FIG. 1Ais a side view of a spine 10
- FIG. 1Bis a posterior view of the spine 10
- FIG. 1Bshows an exemplary electrical stimulator treatment system 100 disposed to treat a spinal region for treating a symptom, such as chronic pain.
- the systemincludes an implantable pulse generator (IPG) 102 that delivers electrical stimulation therapy to the patient, and dual patient controllers shown and described as a pocket controller 104 and a patient controller charger (PPC) 106 .
- IPGimplantable pulse generator
- PPCpatient controller charger
- the spine 10includes a cervical region 11 , a thoracic region 12 , a lumbar region 14 , and a sacrococcygeal region 16 .
- the cervical region 11includes the top seven vertebrae, which may be designated with C 1 -C 7 .
- the thoracic region 12includes the next twelve vertebrae below the cervical region 11 , which may be designated with T 1 -T 12 .
- the lumbar region 14includes the final five “true” vertebrae, which may be designated with L 1 -L 5 .
- the sacrococcygeal region 16includes nine fused vertebrae that make up the sacrum and the coccyx. The fused vertebrae of the sacrum may be designated with S 1 -S 5 .
- Neural tissuebranches off from the spinal cord through spaces between the vertebrae.
- the neural tissue, along with the cord itself,can be individually and selectively stimulated in accordance with various aspects of the present disclosure.
- the IPG 102is implanted inside the body.
- a conductive lead 108is electrically coupled to the circuitry inside the IPG 102 .
- the conductive lead 108may be removably coupled to the IPG 102 through a connector, for example.
- a distal end of the conductive lead 108is attached to one or more electrodes 110 .
- the electrodes 110are implanted adjacent to a desired nerve tissue in the thoracic region 12 .
- the distal end of the lead 108 with its accompanying electrodesmay be positioned beneath the dura mater using well-established and known techniques in the art.
- the electrodes 110deliver current drawn from the IPG 102 , thereby generating an electric field near the neural tissue.
- the electric fieldstimulates the neural tissue to accomplish its intended functions.
- the neural stimulationmay alleviate pain in an embodiment.
- a stimulator as described abovemay be placed in different locations throughout the body and may be programmed to address a variety of problems, including for example but without limitation; prevention or reduction of epileptic seizures, bladder control, weight control or regulation of heart beats.
- the IPG 102 , the lead 108 , and the electrodes 110may be implanted completely inside the body, may be positioned completely outside the body or may have only one or more components implanted within the body while other components remain outside the body. When they are implanted inside the body, the implant location may be adjusted (e.g., anywhere along the spine 10 ) to deliver the intended therapeutic effects of spinal cord electrical stimulation in a desired region of the spine.
- the IPG 102 in this systemis a fully implantable, battery-powered neurostimulation device for providing electrical stimulation to a body region of a patient. In the example shown in FIG. 1B , the IPG 102 is configured to provide neural stimulation to the spine.
- IPG 102may be a different type of pulse generator, including, for example, a pacemaker, a defibrillator, a trial stimulator or any other type of medical device.
- the IPG 102is structurally configured and arranged for wireless programming and control through the skin of the patient. Accordingly, it includes a transmitter and receiver capable of communicating with external programming and control devices, such as the pocket controller 104 , the PPC 106 , and a separate clinician programmer (not shown). It also includes a rechargeable power source, such as a battery configured to be wirelessly recharged through the patient's skin when the PPC 106 is externally placed in the proximity of the IPG 102 .
- a rechargeable power sourcesuch as a battery configured to be wirelessly recharged through the patient's skin when the PPC 106 is externally placed in the proximity of the IPG 102 .
- the pocket controller 104provides only limited functionality for controlling the IPG 102 , and allows a user to control the most-used, such as daily-used, functions of the IPG 102 . It is sized and configured for simple convenience and discreetness.
- the PPC 106performs all the functions of the pocket controller 104 , but also includes more advanced features and functionality for controlling the IPG 102 that are used less frequently than a daily basis, such as, for example, perhaps weekly. In addition, it is an integrated charger for recharging the power source in the IPG 102 .
- the PPC 106can be left at home, as its functions are typically not required for daily use.
- a separate clinician programmer(not shown) is a device typically maintained in a health care provider's possession and can be used to program the IPG 102 during office visits.
- the clinician controllercan define the available stimulation programs for the device by enabling and disabling particular stimulation programs, can define the actual stimulation programs by creating defined relationships between pulses, and perform other functions.
- FIGS. 2-4show the pocket controller 104 in greater detail and FIGS. 5-9 show the PPC 106 in greater detail.
- the pocket controller 104comprises an outer housing 120 having an on-off switch 122 , a plurality of control buttons 124 , and a display 126 .
- the housing 120is sized for discreetness and may be sized to fit easily in a pocket and may be about the same size as a key fob.
- the housing 120 forming the pocket controller 104has a thickness of less than about 1.5 inch, a width of less than about 1.5 inch, and a height of less than about 3 inches.
- the housing 120 forming the pocket controller 104has a thickness of about 0.8 inch, a width of about 1.4 inch, and a height of about 2.56 inch. However, both larger and smaller sizes are contemplated.
- the control buttons 124include two adjustment buttons 128 a, 128 b, a select button 130 , and an emergency off button (not shown, but disposed on a side of the housing 120 opposing the on-off switch 122 ).
- the two adjustment buttons 128 a, 128 ballow a user to scroll or highlight available options and increase or decrease values shown on the display 126 .
- the select button 130allows a user to enter the value or select the highlighted options.
- the buttons 128 a, 128 bare used to navigate to one of the three available functions: 1) electrical stimulation on/off, 2) control stimulation amplitude adjustment, and 3) electrical stimulation program selection. Once the desired function is highlighted, the select button is pushed to allow changes (i.e.
- the IPG control functions of the pocket controller 104consist of these functions.
- the emergency off buttonis disposed for easy access for a patient to turn off stimulation from the IPG 102 if the IPG provides too much stimulation or stimulation becomes uncomfortable for the patient.
- the display 126is an LCD display arranged to convey information to the user regarding selectable options, present settings, operating parameters and other information about the IPG 102 or the pocket controller 104 .
- the display 126shows the pocket controller's battery status at 132 , the IPG's battery status at 134 , the IPG's on or off status at 136 , the currently selected electrical stimulation program at 138 , and the amplitude setting of the running electrical stimulation program at 140 .
- Other types of displaysare also contemplated.
- FIG. 4shows a block diagram of components making up the pocket controller 104 . It includes a user interface 150 , a control module 152 , a communication module 154 , and a power storing controller 156 .
- the user interface 150is comprised of the buttons 128 a, 128 b, 130 and the display 126 described above with reference to FIG. 2 .
- the user interface 150is in communication with the control module 152 .
- the control module 152comprises a processor 158 , memory, an analog-digital converter 162 , and a watch dog circuit 164 .
- the processor 158may include a microprocessor, a controller, a digital signal processor (DSP), an application specific integrated circuit (ASIC), a field programmable gate array (FPGA), discrete logic circuitry, or the like.
- the processor 158is configured to execute code or instructions provided in the memory.
- the memoryis comprised of flash memory 166 and RAM memory 168 .
- the memorymay include any volatile or non-volatile media, such as a random access memory (RAM), read only memory (ROM), non-volatile RAM (NVRAM), electrically erasable programmable ROM (EEPROM), flash memory, and the like.
- the memorystores sets of stimulation control parameters that are available to be selected for delivery through the communication module 154 to the IPG 102 for electrical stimulation therapy.
- the AD converter 162performs known functions of converting signals and the WD 164 is arranged to time out when necessary, such as in an event where the software becomes stuck in a loop.
- the control module 152comprises integrated circuits disposed on a PC board.
- the communication module 154comprises a medical implant communication service (MICS) RF transceiver 172 used to communicate with the IPG 102 to communicate desired changes and to receive status updates from and relating to the IPG 102 , such as battery status and any error information.
- the MICS RF transceiver 172utilizes a loop antenna 179 a for the communications with the IPG 102 .
- Other antennassuch as, for example, dipole, chip antennas, or other known in the art also may be used.
- the communication module 154also includes a wake up transmitter 174 , an amplifier 176 , and matching networks 178 .
- the wake up transmitter 174operates on a high frequency and is configured to send a short signal burst to wake up the IPG 102 when it is in a power-saving mode.
- a communications linkcan be established between the IPG 102 and pocket controller 104 , and communications can then occur over the MICS transceiver 172 using a standard frequency for a medical device transmission.
- the matching networks 178tunes the antenna for optimum transmission power for the frequency selected.
- the pocket controller 104also includes a programming interface 182 . This may be used during manufacturing to load an operating system and program the pocket controller 104 .
- the wake up transmitter 174may utilize the same antenna as the MICS Transceiver, or it may have its own separate antenna 179 b, which may be preferable because the wake up transmitter 174 operates at a substantially higher frequency range (in the GHz range) than the MICS Transceiver which operates in a different frequency range (which typically operates in the hundreds of MHz range). Hence, by using separate antennas for each transmitter, the antennas can be optimized for their respective transmission frequencies.
- the higher frequency capability of the wake-up transmitter 174is more efficient at waking up the IPG due to its use of the higher frequency range, which can be monitored by the IPG using a lower power level than the alternative of monitoring for MICS transceiver transmission at the lower frequency range.
- the wake-up transmitter 174 , the transceiver 172 , and the power charging module 256are individually (independently) controlled by the control module 152 such that the control module controls the transmission of the wake-up signal, the information, and the charging energy, and are all connected to the control module 152 for such control.
- the power storing controller 156is configured to convert power to recharge one or more rechargeable batteries 180 .
- the batteries 180provide power to operate the pocket controller 104 allowing it to receive user inputs and transmit control signals to the IPG 102 .
- Some embodimentsuse primary cell batteries instead of rechargeable batteries.
- this pocket controller 104is part of a larger system that contains the PPC 106 with a rich feature set for controlling the IPG 102 and includes an integrated battery charger used to charge the IPG's battery. By providing both the pocket controller 104 and the PPC 106 , the patient can have a small unobtrusive device to carry around as they go about their daily business and a larger more full featured device which they can use in the comfort and privacy of their homes.
- the pocket controller 104is not only comfortable to carry in a pocket, but can also be attached to a key ring, lanyard, or other such carrying device for ease of daily use. Its functions are a subset of functions found on the PPC 106 , and permit a user to power the IPG on and off (i.e., the IPG 102 remains on, but stimulation is toggled between the on state when the IPG 102 is emitting electrical pulses and the off state when the IPG 102 is not emitting electrical pulses but remains in the standby mode for additional communications from the pocket controller 104 , the PPC 106 , or both), select which electrical stimulation program to run, and adjust their stimulation amplitude.
- the deviceBy limiting the functions of the pocket controller to those most commonly used on a daily basis, the device becomes much less intimidating to the patient, and allows it to be kept very small. By keeping the device small, such as about key fob size, it becomes unobtrusive and the patient is more comfortable with having and using an implanted device.
- FIGS. 5-7show the PPC 106 in greater detail.
- FIG. 5is a front view of the PPC and
- FIG. 6is a top view of FIG. 5 .
- the PPC 106performs all the same operating functions as the pocket controller 104 , but includes additional operating functions making it a multi-function full-featured, advanced patient controller charger.
- the PPC 106provides a simple but rich feature set to the more advanced user, along with the charging functions.
- the PPC 106includes a controller-charger portion 200 and a coil portion 202 connected by a flexible cable 204 and sharing components as described below.
- the controller-charger portion 200comprises an outer housing 206 having an on-off switch 208 on its side, a plurality of control buttons 210 , and a display 212 , and an emergency off button (not shown, but disposed on a side of the housing 206 opposing the on-off switch 208 ).
- the control buttons 210are icons on the display 212
- the displayis a full color, touch screen, graphical user interface.
- the controller-charger portion 200includes a home button 214 configured to return the displayed images to a home screen.
- the controller-charger portion 200is larger than the pocket controller 104 and in one embodiment is sized with a height greater than about 3 inches, a width greater than about 2.5 inches, and a thickness greater than about 0.8 inch. In another embodiment, the controller-charger portion is sized with a width of about 3.1 inches, a height of about 4.5 inches, and thickness of about 0.96 inches, although both larger and smaller sizes are contemplated.
- the example coil portion 202is of a rounded rectangular shape, and has a pair of loop portions 205 , with each loop portion forming an oblong hole 206 that can be used to receive a strap, such as a belt, in order to mount the coil portion to the body of the patient when charging the IPG.
- the coil portioncould be mounted to the patient using one or more suction cups, hook and loop fasteners, a non-permanent glue, etc.
- control buttons 210allow a user to select a desired feature for control or further display.
- the control buttons 210enable functions of the PPC 106 that are the same as those of the pocket controller 104 (stimulation on/off, program stimulation amplitude adjustment, and stimulation program selection) along with additional features including: charging IPG battery, individual pulse stimulation amplitude adjustment, stimulation program frequency adjustment, individual pulse width adjustment, detailed IPG status, detailed PPC status, PPC setup/configuration, a PPC battery status indicator, PPC to IPG communication status indicator, and other items and functions.
- the detailed IPG statusmay include, for example, IPG serial number and IPG software revision level.
- Detailed PPC statusmay include, for example, date and time setting, brightness control, audio volume and mute control, and PPC serial number and software revision level.
- a usercan quickly and easily identify and select the features that are most commonly used.
- Features that are used less frequently, such as IPG recharge,are only included on the full-featured PPC.
- Features that are seldom accessed, or not accessed at all by some users, including individual pulse amplitude adjust, pulse width adjust, stimulation program frequency adjust, or serial number and software revision information,are also not included on the limited-feature pocket controller, but are included on the PPC. This allows the pocket controller to be significantly smaller, with a very simple and easy to user interface, as compared to systems that need to support all of these features.
- the touch screen display 212is arranged to convey information to the user regarding selectable options, current settings, operating parameters and other information about the IPG 102 or the PPC 106 .
- the display 212shows a MICS communication indicator 220 , the PPC's battery status at 222 , the IPG's battery status at 224 , the IPG's on or off status at 226 , the currently selected electrical stimulation program at 228 , and the amplitude setting of the active electrical stimulation program at 230 .
- the display 212shows the frequency 232 , the pulse width setting 234 , a selectable status icon for accessing detailed PPC information 236 , a selectable status icon for accessing detailed IPG information 238 , and a selectable icon for enabling IPG charging 240 . Selecting any single icon may activate another menu within that selected subject area.
- the controller-charger portion 200may include a rechargeable battery whose charge status is shown by the PPC's battery status at 222 .
- the coil portion 202is configured to wirelessly charge the batteries in the IPG 102 .
- the coil portion 202is applied against the patient's skin externally so that energy can be inductively transmitted and stored in the IPG battery.
- the coil portion 202is connected with the integrated controller-charger portion 200 .
- the controller-charger portion 200can simultaneously display the current status of the coil portion 204 , the battery power level of the IPG 102 , as well as the battery power level of the PPC. Accordingly, controlling and charging can occur in a more simplistic, time-effective manner, where the patient can perform all IPG maintenance in a single sitting.
- the PPC 106since the most commonly used features of the PPC 106 are already functional on the pocket controller, the PPC 106 may be left at home when the user does not desire to carry the larger, more bulky PPC.
- FIG. 7shows an alternative display configuration of the PPC 106 , including fewer available functions for user control.
- the userhas been denied access to the frequency and pulse width buttons shown in FIG. 5 .
- the health care providermay program the IPG 102 with the clinician controller (not shown) so that the IPG 102 allows the PPC 106 to control certain parameters as in the device in FIG. 5 or may program the IPG 102 to deny control of certain parameters from the PPC 106 as in the device in FIG. 7 .
- the absence of the buttonsindicates that those options are not available.
- FIG. 8shows a block diagram of the components making up the PPC 106 . It includes a user interface 250 , a control module 252 , a communication module 254 , an IPG power charging module 256 , and a power storing module 258 .
- the user interface 250is comprised of the buttons 210 and the display 212 described above.
- the user interface 250also includes one or more LEDs 266 signifying whether the PPC 106 is charging or powered on and a backlight 268 that illuminates the color display. In some embodiments, these LEDs may have colors symbolizing the occurring function.
- An LED driver 270 and a speaker or amplifier 272also form a part of the user interface 250 .
- the user interface 250is in communication with the control module 252 .
- the control module 252comprises a processor 276 , memory 278 , and a power management integrated circuit (PMIC)/real time clock (RTC) 280 .
- the control module 252also includes a Wi-Fi RF transceiver 282 that allows the PPC 106 to connect to a wireless network for data transfer. For example, it may permit doctor-patient interaction via the internet, remote access to PPC log files, remote diagnostics, and other information transfer functions.
- the PMIC 280is configured to control the charging aspects of the PPC 106 .
- the Wi-FI transceiver 282enables Wi-Fi data transfer for programming the PPC 106 , and may permit wireless access to stored data and operating parameters. Some embodiments also include a Bluetooth RF transceiver for communication with, for example, a Bluetooth enabled printer, a keyboard, etc.
- control module 252also includes an AD converter and a watch dog circuit as described above with reference to the control module 252 .
- the memory 278is comprised of flash memory and RAM memory, but may be other memory as described above.
- the processor 276is an embedded processor running a WinCE operating system (or any real time OS) with the graphics interface 250 , and the memory 278 stores sets of stimulation control parameters that are available to be selected for delivery through the communication module 254 to the IPG 102 for electrical stimulation therapy.
- the control module 252comprises integrated circuits disposed on a PC board.
- the communication module 254comprises a MICS RF transceiver 290 , a wake up transmitter 292 , an amplifier 294 , and matching networks 296 .
- the communication module 254may be similar to the communication module 154 discussed above, and will not be further described here in any detail, except to note that for this embodiment, the transceiver 290 is shown sharing an antenna with the wake-up transmitter 292 , although separate antennas as discussed for the 154 embodiment shown in FIG. 4 could be utilized for this embodiment as well.
- the PPC 206also includes a programming interface 298 that may be used during manufacturing to load an operating system and program the PPC 206 .
- the power storing module 258is configured to convert power to recharge one or more rechargeable batteries 302 .
- the batteries 302are lithium-ion cells that provide power to operate the PPC 106 allowing it to receive user inputs, transmit control signals to, and charge the IPG 102 .
- the power storing module 258includes a connector 304 for connecting to a power source, a power protection detection circuit 306 for protecting the PPC from power surges, and linear power supplies 308 for assisting with the electric transfer to charge the batteries 302 .
- the control module 252aids with the charging and is configured to monitor and send the battery charge level to the user interface 250 for display.
- the connector 304connects the PPC, directly or indirectly, to a power source (not shown) such as a conventional wall outlet for receiving electrical current.
- the connector 304comprises a cradle.
- the power charging module 256communicates with the control module 252 and is arranged to magnetically or inductively charge the IPG 102 . In the embodiments shown, it is magnetically or inductively coupled to the IPG 102 to charge rechargeable batteries on the IPG 102 .
- the charging module 256includes components in both the controller-charger portion 200 and the coil portion 202 ( FIG. 5 ). It includes switch boost circuitry 316 , a load power monitor 318 , an LSK demodulator 320 , a ASK modulator 322 , a current mode transmitter 324 , an ADC 326 , and coils 328 .
- the control module 252aids with the charging and is configured to monitor and send the IPG battery charge level to the user interface 250 for display.
- the coils 328are disposed in the coil portion 202 and are configured to create magnetic or inductive coupling with components in the IPG 102 . Since the coil portion 202 is integrated with the controller-charger portion 200 , both operate from a single battery 302 . Accordingly, as can be seen by the circuitry, the battery 302 powers the control module 252 and all its associated components. In addition, the battery 302 powers the power charging module 256 for recharging the IPG 102 .
- the coils 328are provided with a temperature sensor for monitoring the temperature of the coil portion 202 , in which the coils 328 are installed. This can be important as the coils 328 may generate substantial heat when charging the IPG battery, and hence the temperature should be monitored in order to ensure that the coil portion 202 does not overheat and potentially injure the patient.
- the temperature sensorcan be configured to send data back to the control module 252 via the ADC 326 ,
- the control modulecan then monitor the temperature of the coil portion 202 , and adjust the charging rate based on the measured temperature, such as by reducing the energy transmitted by the coils 328 , for example, when the temperature rises above a threshold value, such as a value that may be considered uncomfortable or harmful to the patient wearing the charger (e.g, a value more than a few degrees above normal body temperatures).
- a threshold valuesuch as a value that may be considered uncomfortable or harmful to the patient wearing the charger (e.g, a value more than a few degrees above normal body temperatures).
- the chargingcan be stopped to prevent overheating, and then resumed when the coil portion sufficiently cools down.
- the control module 252provides a single control interface and a single user interface for performing both functions of controlling the IPG 102 and of charging the IPG 102 .
- the controller-charger portion 200 and the coil portion 202are integrated, the controller-charger portion 200 simultaneously controls both the current status of the charger, the battery power level of the IPG 102 , as well as the battery power level of the PPC. Accordingly, controlling and charging can occur in a more simplistic, time-effective manner, where the patient can perform all IPG maintenance in a single sitting.
- the PPC 106since the most commonly used features of the PPC 106 are already functional on the pocket controller, the PPC 106 may be left at home when the user does not desire to carry the larger, more bulky PPC.
- FIG. 9shows an alternative embodiment of a PPC, referenced herein by the numeral 106 a.
- the functions of the control moduleare divided into processors splitting the functions required by the single processor embodiment in FIG. 8 .
- similar itemsmaintain the same reference numeral.
- the PPC 106 aincludes the user interface 250 , the communication module 254 , and the power storing module 258 . It also includes a power charging module 256 a and a control module that in this embodiment includes both a controller 340 and a controller 342 .
- the controller 340is similar in some ways to the control module 152 in FIG. 4
- the controller 342is similar in some ways to the control module 252 described in FIG. 8 .
- the controllers 340 , 342have divided the functionality of the PPC into separate portions however.
- the controller 340is arranged, and contains instructions for controlling the power charging module 256 a and MICS communications module 254
- the controller 342is arranged and contains instructions for controlling the power storing module 258 and the user interface 250 .
- the PPCcontains all the features available in the pocket controller 104 (stimulation on/off, stimulation program amplitude adjustment, and stimulation program selection). It also includes additional features including, for example, charge IPG battery, individual pulse/area stimulation amplitude adjustment, stimulation program frequency adjustment, individual pulse/area pulse width adjustment, detailed IPG status, detailed PPC status, PPC setup/configuration, PPC battery status indicator, PPC to IPG communication status indicator, and other items.
- FIG. 10is a flow chart showing a method of operation taken by the pocket controller 104 according to one aspect of the present disclosure.
- the processor 158 and memory 160contain all the necessary software and programming in order to communicate with the IPG 102 and to receive user inputs performing the available functions of: 1) electrical stimulation on/off, 2) program stimulation amplitude adjustment, and 3) electrical stimulation program selection.
- the control functions of the pocket controllerconsist only of these functions, while in other embodiments, the control functions contain more or fewer functions, but in all cases is a limited function device having fewer control functional capabilities than the PPC.
- the pocket controller 104When the pocket controller 104 is powered on using the on-off switch 122 and within communication range of the IPG 102 , the pocket controller 104 interrogates the IPG 102 , as indicated at step 402 in FIG. 10 . In response, the IPG 102 transmits to the pocket controller information regarding its current settings including which stimulation programs are available for implementation. As indicated above, some embodiments of the pocket controller communicate only with the IPG 102 , and not with the PPC 106 . In these embodiments, the pocket controller 104 must receive its data from the IPG. In one example, the information received from the IPG includes the current nuerostimulation on-off state, the amplitude setting or level, and the stimulation programs available for implementation.
- the pocket controller 104may receive data representing a stimulation program identifier uniquely representing each stimulation program that the IPG is capable of carrying out.
- the stimulation program identifieris a numerical number with a “p” as shown at 138 .
- a clinicianhowever, using a clinician programmer or other device, may have only enabled certain stimulation programs on the IPG 102 , but not all of the available stimulation programs. In these cases, in response to the interrogation, the pocket controller receives data indicating which stimulation programs are enabled on the IPG 102 .
- the pocket controller 104stores the received data at step 406 .
- the pocket controller 104receives data indicative of the power charge level of the IPG 102 , and at step 410 , the pocket controller 104 displays the current settings and data indicative of the power charge level. An example of this is shown in FIG. 2 , where the pocket controller 104 displays the current on-off state of the electrical stimulation, the amplitude setting, the currently set stimulation program, and the power level of both the IPG 102 and the pocket controller 104 .
- the pocket controller 104receives an input from a user to modify the current settings. To do this, the pocket controller 104 receives inputs via the adjustment buttons 128 a, 128 b. In response to the adjustment buttons, the pocket controller scrolls through the available function options and highlights each in turn.
- the select button 130allows a user to select the desired highlighted function. Accordingly, when the pocket controller 104 receives a select input via the select button 130 , the pocket controller permits modification of the selected function setting using the adjustment buttons 128 a, 128 b and the select button 130 .
- the pocket controllerenters an adjustment mode allowing the user to increase or decrease the amplitude level (shown as a numerical value in FIG. 2 ) using the adjustment buttons 128 a, 128 b.
- the usermay select it using the select button 130 , or alternatively, the controller may automatically enter the displayed value.
- the pocket controller 104may be programmed to display for selection only stimulation programs 1 , 5 , and 10 , and not display the remaining, non-enabled stimulation programs. Accordingly, the pocket controller may be enabled to only display options available to the user.
- the pocket controller 104transmits data representing the selected adjustment to the IPG 102 at step 414 , and the IPG 102 responds by modifying its settings to carry out the command of the pocket controller 104 .
- FIG. 11is a flow chart showing a method of operation taken by the PPC 106 according to one aspect of the present disclosure. Although described with reference to PPC 106 in FIG. 8 , it should be understood that the method may be performed by any PPC within this scope of this disclosure.
- the processor 256 and memory 278contain all the necessary software and programming in order to communicate with the IPG 102 , to charge the IPG, and to receive user inputs performing the same control functions as the pocket controller 104 , plus additional control functions.
- these additional control functions performed by the PPC 106may include charging the IPG battery, adjusting individual pulse/area stimulation amplitude, adjusting stimulation program frequency, adjusting individual pulse/area pulse width, providing detailed IPG status, providing detailed PPC status, enabling PPC setup/configuration, indicating PPC and IPG battery status, displaying PPC to IPG communication status indicator, and other items.
- the PPC 106when the PPC 106 is powered on using the on-off switch 208 and within communication range of the IPG 102 , the PPC 106 interrogates the IPG 102 , as indicated at step 454 . In response, the IPG 102 transmits to the PPC information regarding its current settings including stimulation programs available for implementation, at step 456 .
- the PPC 106includes a single user interface for performing both functions of controlling the IPG 102 and of charging the IPG 102 .
- the controller-charger portion 200 and the coil portion 202are integrated, the controller-charger portion 200 simultaneously controls both the current status of the charger, the battery power level of the IPG 102 , as well as the battery power level of the PPC.
- the PPC 106may receive data representing a stimulation program identifier uniquely representing each stimulation program that the IPG is capable of carrying out or programmed to carry out, similar to the operation of the pocket controller 104 above.
- the PPC 106stores the received data at step 458 .
- the PPC 106receives data indicative of the power charge level of the IPG 102 , and at step 462 , the PPC 106 displays the current settings and data indicative of the power charge level.
- FIG. 5An example of this is shown in FIG. 5 , where the PPC displays the current on-off state of the electrical stimulation, the amplitude setting, the currently set stimulation program, and the power level of both the IPG 102 and the PPC 104 , the communication status of the PPC to the IPG.
- the PPC 106displays the current stimulation program frequency, the individual pulse/area pulse width, and other information.
- the PPC 106receives an input from a user to modify the current settings.
- the PPC 106includes a touch screen display, and the inputs are received via a user touching an icon displayed on the touch screen display. Once a particular function is selected, the PPC permits modification of the selected function setting using the touch screen.
- the PPCtransmits signals to the IPG to modify the settings and control.
- the PPC 106receives instructions from a user to charge the IPG 102 .
- the PPCresponds by directing current through the coil 328 for creating an inductive or a magnetic field. Since the coil portion 202 is integrated with the controller-charger portion 200 , in the examples shown, both operate from a single battery 302 . Accordingly, as can be seen by the circuitry, the battery 302 powers the control module 252 and all its associated components. In addition, the battery 302 powers the power charging module 256 for recharging the IPG 102 .
- the pocket controllerIn addition to providing the advantage of a discrete, smaller, functionally limited device, the pocket controller also provides controller redundancy to the patient. Accordingly, the patient is more likely to carry the pocket controller for use, but also serves as a backup to turn off treatments if they become uncomfortable.
- the devices, systems, and methods described hereinintroduce an improved way for controlling and charging an implanted medical device by using a full-featured PPC and a smaller, less intimidating, limited feature, device for everyday use.
- the system disclosed hereinprovides a level of risk management not achieved with a single controller. For example, if one controller malfunctions or is misplaced, the patient still will have the second controller to manage at least the more important and immediate functions of the IPG until the malfunctioning or misplaced controller is replaced. Therefore, the patient can continue with his or her scheduled therapy, modify the stimulation treatment as desired for effectiveness, and control the stimulation in the event that it becomes painful or undesired.
Landscapes
- Health & Medical Sciences (AREA)
- Engineering & Computer Science (AREA)
- Animal Behavior & Ethology (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Radiology & Medical Imaging (AREA)
- Life Sciences & Earth Sciences (AREA)
- Biomedical Technology (AREA)
- General Health & Medical Sciences (AREA)
- Public Health (AREA)
- Veterinary Medicine (AREA)
- Human Computer Interaction (AREA)
- Neurology (AREA)
- Neurosurgery (AREA)
- Electrotherapy Devices (AREA)
Abstract
Description
- This application is a continuation-in-part of application Ser. No. 13/170,775, filed on Jun. 28, 2011, and incorporated herein by reference.
- This disclosure is directed to dual patient controllers for controlling an electrical stimulation implantable device.
- Neurostimulation devices deliver therapy in the form of electrical stimulation pulses to treat symptoms and conditions, such as chronic pain, Parkinson's disease, or epilepsy, for example. Implantable neurostimulation devices, for example, deliver neurostimulation therapy via leads that include electrodes located proximate to the muscles and nerves of a patient. Treatments frequently require two external devices: a neurostimulator controller and a neurostimulation device charger. Neurostimulator controllers are frequently used to adjust treatment parameters, select programs, and even to program treatment platforms into the implantable device. External neurostimulator device chargers are used to recharge batteries on the implanted device.
- Conventional neurostimulator controllers are approximately the size of hand-held gaming system controllers, smartphones, or PDAs. While small, they are too large to comfortably be carried around in one's pocket and must be carried in a belt pouch or purse. Because of the size of these devices, they are not easily concealed and frequently result in unwanted attention. Known controllers provide so many features or functions that they sometimes overwhelm the patient to the point where the trial fails. A typical patient will only use a very small subset of the available features on the controllers. Most patients only use their controller to turn the neurostimulator on or off, select which neurostimulation program to run, and adjust their stimulation amplitude, while a very small percentage of patients utilize the advance controls to adjust program frequency and individual pulse/area stimulation parameters such as pulse width.
- Existing chargers are typically about the same size as the neurostimulator controllers and are used for the sole purpose of recharging the implantable device's battery. These chargers are usually kept in the patient's house and used once a week or so depending upon stimulation parameters.
- The present disclosure is directed to devices, systems, and methods that address one or more deficiencies in the prior art.
- This disclosure is directed to a system with dual patient controllers that control an implantable medical device. It includes a pocket controller and a separate integrated controller charger.
- In one exemplary aspect, the present disclosure is directed to an integrated controller charger operable to both charge a rechargeable power source of an implantable medical device and control the implantable medical device. The controller charger includes a communication module configured to transmit information from the controller charger to the implantable medical device and configured to receive information from the implanted medical device. It also includes a power charging module configured to transmit energy storable at the rechargeable power source on the implantable medical device. A control module is configured to control both the communication module and the power charging module. The control module may include a processor and a memory and is operable to store the information received from the implanted medical device and generate signals to activate the stimulation programs on the implanted medical device.
- In another exemplary aspect, the present disclosure is directed to an assembly for controlling an implantable medical device configured to transmit and receive information. The implantable medical device includes a rechargeable power source. The assembly includes a controller charger operable to both charge the implantable medical device and control the implantable medical device and includes a limited purpose controller sized smaller than the controller charger. The limited purpose controller includes a communication module configured to transmit information from the limited purpose controller to the implantable medical device and configured to receive information from the implanted medical device. It also includes a control module configured to transmit to and receive information from the communication module in communication with the implantable medical device. The controller may be configured to permit a user to control only a subset of the features controllable with the controller charger, including, for example,1) electrical stimulation on/off selection,2) stimulation program amplitude adjustment, and3) electrical stimulation program selection.
- In some exemplary aspects the assembly includes a controller charger operable to both charge the implantable medical device and control the implantable medical device. The controller charger includes a communication module configured to transmit information from the controller charger to the implantable medical device and configured to receive information from the implanted medical device. It also includes a power charging module configured to emit energy storable at the rechargeable power source on the implantable medical device. A control module interfaces with both the communication module and the power charging module. The control module is configured to permit a user to control features on the implantable medical device including a) electrical stimulation on/off selection, b) stimulation program amplitude adjustment, c) electrical stimulation program selection, and d) adjusting stimulation program frequency, as well as providing additional status information relating to the implanted medical device. The assembly also includes a limited purpose controller sized smaller than the controller charger. The limited purpose controller includes a communication module configured to transmit information from the controller to the implantable medical device and configured to receive information from the implanted medical device. It also includes a control module configured to transmit to and receive information from the implantable medical device. The limited purpose controller is configured to permit a user to control only a subset of the features controllable with the controller charger. The subset of the features includes 1) electrical stimulation on/off selection, 2) stimulation program amplitude adjustment, and 3) electrical stimulation program selection.
- In another exemplary aspect, the present disclosure is directed to a method performed by a limited purpose controller in communication with an implanted implantable pulse generator. The method includes steps of receiving from the implantable pulse generator data representing enabled stimulation programs; storing the data representing the enabled stimulation programs on the limited purpose controller; receiving an input from a user to activate stimulation from the implantable pulse generator according to one of the enabled stimulation programs, the limited purpose controller being configured in a manner not permitting the user to modify the enabled stimulation program other than to adjust a global amplitude of pulses emitted by the implantable pulse generator according to an enabled stimulation program; and transmitting data representing the selected stimulation program from the limited purpose controller to the implantable pulse generator.
- In another exemplary aspect, the present disclosure is directed to a method performed by a patient controller charger in communication with an implanted implantable pulse generator. The method includes steps of receiving from the implantable pulse generator data representing enabled stimulation programs; storing the data representing the enabled stimulation programs on the limited purpose controller; receiving an input from a user to adjust a frequency and pulse width of an electrical stimulation treatment; transmitting data representing the adjusted frequency and pulse from the patient controller charger to the implantable pulse generator; receiving an input from a user to charge a battery on an implanted implantable pulse generator with the patient controller charger; and emitting energy from the patient controller charger configured to charge the implantable pulse generator.
- In another exemplary aspect, the present disclosure is directed to an integrated controller charger operable to both charge a rechargeable power source of an implantable medical device and control the implantable medical device. The controller charger includes a controller charger portion configured to wirelessly communicate with the implantable medical device, configured to receive inputs from a user, configured to send control signals indicative of the received inputs to the implantable medical device, and configured to receive information from the implantable medical device and display the information to a user. The controller charger also includes a coil portion configured to generate an inductive field to wirelessly charge a battery in the implantable medical device, and includes a flexible cable extending between and electrically connecting the controller charger portion and the coil portion, the flexible cable electrically connecting the controller charger and the coil portion.
- In another exemplary aspect, the present disclosure is directed toward a pocket controller that is operable by a patient to adjust a plurality of operational parameters defining a stimulation program to be performed by an implantable medical device within the patient. The pocket controller includes a housing comprising a smaller form factor than a housing of a patient controller that offers a greater number of control options than the pocket controller. The pocket controller also communicates with the implantable medical device to adjust a plurality of the operational parameters of the implantable medical device. A number of the operational parameters that are adjustable with the pocket controller is less than a number of the operational parameters that are adjustable with the patient controller. A user interface is also provided to the pocket controller to receive input from the patient, allowing the patient to select, from the plurality of operational parameters adjustable with the pocket controller, a selected operational parameter that is to be adjusted by the patient using the pocket controller. The pocket controller also receives input from the patient indicating an adjustment to the selected operational parameter. The pocket controller also includes a communication module that is compatible with a receiver provided to the implantable medical device to transmit a signal indicative of the adjustment from the pocket controller to the implantable medical device and receive information from the implantable medical device. A control module including a processor is configured to convey the signal indicative of the adjustment entered via the user interface to the communication module to be transmitted to the implantable medical device.
- In another exemplary aspect, the present disclosure is directed toward a system for treating a patient. The system includes an implantable medical device that is to be implanted in the patient to execute an internal treatment routine on the patient, the internal treatment routine being governed by a plurality of operational parameters. A patient controller is provided to communicate with the implantable medical device and is to be placed in possession of the patient to allow the patient to adjust a plurality of the operational parameters while the implantable medical device is implanted in the patient. A pocket controller that communicates with the implantable medical device and is also to be placed in possession of the patient. The pocket controller includes a smaller form factor than a form factor of the patient controller and is usable by the patient to adjust a plurality of, but less than all of the operational parameters that are adjustable with the patient controller. The pocket controller includes a user interface that is usable by the patient to select, from the plurality of operational parameters, a selected operational parameter that is to be adjusted by the patient using the pocket controller, and to input or select an adjustment to the selected operational parameter.
- In another exemplary aspect, the present disclosure is directed toward a system for treating a patient. The system includes an implantable electrical stimulation device that is to be implanted in the patient that executes an electrical stimulation program to be performed on the patient. The electrical stimulation program is governed by a plurality of operational parameters. A patient controller is provided to communicate with the implantable electrical stimulation device and is to be placed in possession of the patient to allow the patient to adjust the plurality of operational parameters while the implantable electrical stimulation device is implanted in the patient. The plurality of operational parameters adjustable via the patient controller include an amplitude of an individual electrical pulse emitted as part of a stimulation program performed by the implantable electrical stimulation device relative to an amplitude of another electrical pulse to be emitted as part of the same stimulation program performed by the implantable electrical stimulation device. A pocket controller is also provided to communicate with the implantable electrical stimulation device and is to be placed in possession of the patient. The pocket controller has a smaller form factor than a form factor of the patient controller. The pocket controller is usable by the patient to adjust a plurality of, but less than all of the operational parameters that are adjustable with the patient controller. One of the operational parameters adjustable via the pocket controller includes a global amplitude of electrical pulses in a series of electrical pulses to be emitted by the implantable electrical stimulation device as part of a stimulation program. The pocket controller further includes a user interface that is usable by the patient to input an adjustment of each of the plurality of operational parameters, and a display device. The display device displays at least one of a status of a battery provided to power the implantable electrical stimulation device, a status of a battery provided to power the pocket controller, an operational status of the implantable electrical stimulation device, and information concerning one or more of the operational parameters.
- In another exemplary aspect, the present disclosure is directed toward a method of familiarizing a patient with a programmer for controlling execution of a treatment routine to be performed on the patient, where the treatment routine is governed by a plurality of operational parameters. The method includes providing the patient with a first pocket controller that communicates with a temporary, trial electrical stimulation device supported externally of the patient during a trial period to perform the treatment routine. The trial period is to determine whether the treatment routine performed by the external electrical stimulation device is beneficial to the patient. In response to a determination that the treatment routine is beneficial to the patient, the method includes establishing communication between the first pocket controller and an implanted electrical stimulation device or establishing communication between a second pocket controller, which is substantially-similar to the first pocket controller, and the electrical stimulation device to allow the first or second pocket controllers to change a plurality of the operational parameters. Communications are also established between a patient controller to be provided to the patient and the internal electrical stimulation device to change a greater number of the operational parameters than a number of the operational parameters that are adjustable using the first or second pocket controllers.
- Note that U.S. patent application Ser. No. 13/170,558, titled “KEY FOB CONTROLLER FOR AN IMPLANTABLE NEUROSTIMULATOR” and filed on the same day as the present application and having attorney docket number QIG-47477, is incorporated herein in its entirety by reference.
- Aspects of the present disclosure are best understood from the following detailed description when read with the accompanying figures.
FIGS. 1A and 1B are illustrations of a patient's spine with an exemplary electrical stimulator treatment system disposed to treat a particular region of the spine in accordance with one aspect of the present disclosure.FIG. 2 is an illustration of an exemplary pocket controller in accordance with one aspect of the present disclosure.FIG. 3 is an illustration of a side of the exemplary pocket controller ofFIG. 2 in accordance with one aspect of the present disclosure.FIG. 4 is block diagram of components of the exemplary pocket controller ofFIG. 2 in accordance with one aspect of the present disclosure.FIG. 5 is an illustration of an exemplary patient controller charger in accordance with one aspect of the present disclosure.FIG. 6 is an illustration of a top view of the exemplary patient controller charger ofFIG. 5 in accordance with one aspect of the present disclosure.FIG. 7 is an illustration of an exemplary alternatively programmed patient controller charger in accordance with one aspect of the present disclosure.FIG. 8 is block diagram of components of the exemplary patient controller charger ofFIGS. 5-7 in accordance with one aspect of the present disclosure.FIG. 9 is block diagram of alternative components of the exemplary patient controller charger ofFIGS. 5-7 in accordance with one aspect of the present disclosure.FIG. 10 is a flow chart showing method steps performed by the pocket controller according to one exemplary aspect of the present disclosure.FIG. 11 is a flow chart showing method steps performed by the patient controller charger according to one exemplary aspect of the present disclosure.- The following disclosure provides many different embodiments, or examples, for implementing different features of various embodiments. Specific examples of components and arrangements are described below to simplify the present disclosure. These are, of course, merely examples and are not intended to be limiting. In addition, the present disclosure may repeat reference numerals and/or letters in the various examples. This repetition is for the purpose of simplicity and clarity and does not in itself dictate a relationship between the various embodiments and/or configurations discussed.
- The devices, systems, and methods described herein introduce an improved way for controlling and charging an implanted medical device. They use a dual controller system that includes a pocket controller and a separate controller charger. The most-used functions of an electrical stimulator's controller are incorporated into a small, thin pocket controller that is not only comfortable to carry in a pocket, but can also be attached to a key ring, lanyard, or other such carrying device for ease of daily use. These most-used functions are a subset of features commonly found on an electrical stimulator's controller, and include features such as turning the electrical stimulation on and off, selecting which electrical stimulation program to run, and adjusting the stimulation amplitude. By limiting the functions of this device to those most commonly used on a daily basis, the device becomes much less intimidating to the patient thereby increasing patient compliance, and the size of the device is substantially reduced, rendering it less obtrusive and therefore making the patient more comfortable with having and using an implanted electrical stimulator.
- In addition, the devices, systems, and methods described herein introduce a separate patient controller charger for the implanted medical device that provides advanced control features and includes an integrated charger for the implantable power source. Since the advanced features are necessary only for performing periodic (non-daily) maintenance or stimulation adjustment, and performing other less frequently required functions, these advanced features, including the subset features for redundancy, are maintained on a device separate and apart from the pocket controller. These features are integrated with the also-infrequently used charger for the implantable power source. Therefore, where a conventional charger performs only a function of charging the implant's battery and may include powering stimulation on and off, the system disclosed herein includes a multi-function, full-featured advance patient controller charger combination. In the embodiment shown, patient controller charger incorporates a full color graphical user interface with touch screen that presents a simplistic, but rich feature set for the more advanced, while maintaining the charging functions. Since the larger-sized patient controller charger can be left at home, its larger size (approximate size of a PDA) will not raise undesired interest from others. Users have the smaller unobtrusive pocket controller to take with them when they are on the go, and have comfort of the advanced features available for use when in the privacy of their home.
- Further, the dual patient controllers disclosed herein provide a level of redundancy and risk management not achieved with a single controller. For example, if one controller malfunctions or is misplaced, a patient can still use the second controller for important control functions until the malfunctioning or misplaced controller is replaced. Accordingly, the patient can continue with his or her scheduled therapy, select another program as desired for effectiveness, and control the stimulation amplitude in the event that it becomes painful or undesired.
- As used herein, the term “stimulation program” refers to a series of one or more stimulation pulses having a defined relationship used to treat a specific therapy or condition. A modification to the stimulation program involves adding or removing a pulse from the program, or changing the defined relationship of one pulse relative to the next pulse, but does not include adjusting the pulse amplitude or the pulse width of individual pulses or the series of pulses. According to alternate embodiments, the pulse amplitude and/or the pulse width of individual pulses or the series of pulses can optionally be adjusted using a full-feature controller such as the patient controller, but can not be adjusted using the pocket controller.
FIG. 1A is a side view of aspine 10, andFIG. 1B is a posterior view of thespine 10.FIG. 1B shows an exemplary electricalstimulator treatment system 100 disposed to treat a spinal region for treating a symptom, such as chronic pain. The system includes an implantable pulse generator (IPG)102 that delivers electrical stimulation therapy to the patient, and dual patient controllers shown and described as apocket controller 104 and a patient controller charger (PPC)106.- Referring now to
FIGS. 1A and 1B , thespine 10 includes acervical region 11, athoracic region 12, alumbar region 14, and asacrococcygeal region 16. Thecervical region 11 includes the top seven vertebrae, which may be designated with C1-C7. Thethoracic region 12 includes the next twelve vertebrae below thecervical region 11, which may be designated with T1-T12. Thelumbar region 14 includes the final five “true” vertebrae, which may be designated with L1-L5. Thesacrococcygeal region 16 includes nine fused vertebrae that make up the sacrum and the coccyx. The fused vertebrae of the sacrum may be designated with S1-S5. - Neural tissue (not illustrated for the sake of simplicity) branches off from the spinal cord through spaces between the vertebrae. The neural tissue, along with the cord itself, can be individually and selectively stimulated in accordance with various aspects of the present disclosure. For example, referring to
FIG. 1B , theIPG 102 is implanted inside the body. Aconductive lead 108 is electrically coupled to the circuitry inside theIPG 102. Theconductive lead 108 may be removably coupled to theIPG 102 through a connector, for example. A distal end of theconductive lead 108 is attached to one ormore electrodes 110. In the example shown, theelectrodes 110 are implanted adjacent to a desired nerve tissue in thethoracic region 12. The distal end of thelead 108 with its accompanying electrodes may be positioned beneath the dura mater using well-established and known techniques in the art. - The
electrodes 110 deliver current drawn from theIPG 102, thereby generating an electric field near the neural tissue. The electric field stimulates the neural tissue to accomplish its intended functions. For example, the neural stimulation may alleviate pain in an embodiment. In other embodiments, a stimulator as described above may be placed in different locations throughout the body and may be programmed to address a variety of problems, including for example but without limitation; prevention or reduction of epileptic seizures, bladder control, weight control or regulation of heart beats. - It is understood that the
IPG 102, thelead 108, and theelectrodes 110 may be implanted completely inside the body, may be positioned completely outside the body or may have only one or more components implanted within the body while other components remain outside the body. When they are implanted inside the body, the implant location may be adjusted (e.g., anywhere along the spine10) to deliver the intended therapeutic effects of spinal cord electrical stimulation in a desired region of the spine. TheIPG 102 in this system is a fully implantable, battery-powered neurostimulation device for providing electrical stimulation to a body region of a patient. In the example shown inFIG. 1B , theIPG 102 is configured to provide neural stimulation to the spine. However, in other embodiments,IPG 102 may be a different type of pulse generator, including, for example, a pacemaker, a defibrillator, a trial stimulator or any other type of medical device. Here, theIPG 102 is structurally configured and arranged for wireless programming and control through the skin of the patient. Accordingly, it includes a transmitter and receiver capable of communicating with external programming and control devices, such as thepocket controller 104, thePPC 106, and a separate clinician programmer (not shown). It also includes a rechargeable power source, such as a battery configured to be wirelessly recharged through the patient's skin when thePPC 106 is externally placed in the proximity of theIPG 102. - The
pocket controller 104 provides only limited functionality for controlling theIPG 102, and allows a user to control the most-used, such as daily-used, functions of theIPG 102. It is sized and configured for simple convenience and discreetness. ThePPC 106 performs all the functions of thepocket controller 104, but also includes more advanced features and functionality for controlling theIPG 102 that are used less frequently than a daily basis, such as, for example, perhaps weekly. In addition, it is an integrated charger for recharging the power source in theIPG 102. ThePPC 106 can be left at home, as its functions are typically not required for daily use. A separate clinician programmer (not shown) is a device typically maintained in a health care provider's possession and can be used to program theIPG 102 during office visits. For example only, the clinician controller can define the available stimulation programs for the device by enabling and disabling particular stimulation programs, can define the actual stimulation programs by creating defined relationships between pulses, and perform other functions.FIGS. 2-4 show thepocket controller 104 in greater detail andFIGS. 5-9 show thePPC 106 in greater detail. - Turning first to
FIGS. 2 and 3 , thepocket controller 104 comprises anouter housing 120 having an on-off switch 122, a plurality ofcontrol buttons 124, and adisplay 126. In this embodiment, thehousing 120 is sized for discreetness and may be sized to fit easily in a pocket and may be about the same size as a key fob. In one example, thehousing 120 forming thepocket controller 104 has a thickness of less than about 1.5 inch, a width of less than about 1.5 inch, and a height of less than about 3 inches. In another example, thehousing 120 forming thepocket controller 104 has a thickness of about 0.8 inch, a width of about 1.4 inch, and a height of about 2.56 inch. However, both larger and smaller sizes are contemplated. - In this example, the
control buttons 124 include twoadjustment buttons select button 130, and an emergency off button (not shown, but disposed on a side of thehousing 120 opposing the on-off switch122). The twoadjustment buttons display 126. Theselect button 130 allows a user to enter the value or select the highlighted options. In this example, thebuttons pocket controller 104 consist of these functions. The emergency off button is disposed for easy access for a patient to turn off stimulation from theIPG 102 if the IPG provides too much stimulation or stimulation becomes uncomfortable for the patient. - In the embodiment shown, the
display 126 is an LCD display arranged to convey information to the user regarding selectable options, present settings, operating parameters and other information about theIPG 102 or thepocket controller 104. In this example, thedisplay 126 shows the pocket controller's battery status at132, the IPG's battery status at134, the IPG's on or off status at136, the currently selected electrical stimulation program at138, and the amplitude setting of the running electrical stimulation program at140. Other types of displays are also contemplated. FIG. 4 shows a block diagram of components making up thepocket controller 104. It includes auser interface 150, acontrol module 152, acommunication module 154, and apower storing controller 156. Theuser interface 150 is comprised of thebuttons display 126 described above with reference toFIG. 2 .- As can be seen, the
user interface 150 is in communication with thecontrol module 152. Thecontrol module 152 comprises aprocessor 158, memory, an analog-digital converter 162, and awatch dog circuit 164. Theprocessor 158 may include a microprocessor, a controller, a digital signal processor (DSP), an application specific integrated circuit (ASIC), a field programmable gate array (FPGA), discrete logic circuitry, or the like. Theprocessor 158 is configured to execute code or instructions provided in the memory. Here, the memory is comprised offlash memory 166 andRAM memory 168. However, the memory may include any volatile or non-volatile media, such as a random access memory (RAM), read only memory (ROM), non-volatile RAM (NVRAM), electrically erasable programmable ROM (EEPROM), flash memory, and the like. In some embodiments, the memory stores sets of stimulation control parameters that are available to be selected for delivery through thecommunication module 154 to theIPG 102 for electrical stimulation therapy. TheAD converter 162 performs known functions of converting signals and theWD 164 is arranged to time out when necessary, such as in an event where the software becomes stuck in a loop. In one embodiment, thecontrol module 152 comprises integrated circuits disposed on a PC board. - The
communication module 154 comprises a medical implant communication service (MICS)RF transceiver 172 used to communicate with theIPG 102 to communicate desired changes and to receive status updates from and relating to theIPG 102, such as battery status and any error information. In this example, theMICS RF transceiver 172 utilizes aloop antenna 179afor the communications with theIPG 102. Other antennas, such as, for example, dipole, chip antennas, or other known in the art also may be used. Thecommunication module 154 also includes a wake uptransmitter 174, anamplifier 176, and matchingnetworks 178. The wake uptransmitter 174 operates on a high frequency and is configured to send a short signal burst to wake up theIPG 102 when it is in a power-saving mode. Once theIPG 102 is ready, a communications link can be established between theIPG 102 andpocket controller 104, and communications can then occur over theMICS transceiver 172 using a standard frequency for a medical device transmission. The matchingnetworks 178 tunes the antenna for optimum transmission power for the frequency selected. Thepocket controller 104 also includes aprogramming interface 182. This may be used during manufacturing to load an operating system and program thepocket controller 104. - The wake up
transmitter 174 may utilize the same antenna as the MICS Transceiver, or it may have its ownseparate antenna 179b,which may be preferable because the wake uptransmitter 174 operates at a substantially higher frequency range (in the GHz range) than the MICS Transceiver which operates in a different frequency range (which typically operates in the hundreds of MHz range). Hence, by using separate antennas for each transmitter, the antennas can be optimized for their respective transmission frequencies. The higher frequency capability of the wake-uptransmitter 174 is more efficient at waking up the IPG due to its use of the higher frequency range, which can be monitored by the IPG using a lower power level than the alternative of monitoring for MICS transceiver transmission at the lower frequency range. Hence, by adding the wake-uptransmitter 174 and its accompanyingamplifier 176 andantenna 179b(and any other conditioning circuits that may be desired), IPG power drain on itsinternal battery 302 can be reduced. The wake-uptransmitter 174, thetransceiver 172, and thepower charging module 256 are individually (independently) controlled by thecontrol module 152 such that the control module controls the transmission of the wake-up signal, the information, and the charging energy, and are all connected to thecontrol module 152 for such control. - The
power storing controller 156 is configured to convert power to recharge one or morerechargeable batteries 180. Thebatteries 180 provide power to operate thepocket controller 104 allowing it to receive user inputs and transmit control signals to theIPG 102. Some embodiments use primary cell batteries instead of rechargeable batteries. As indicated above, thispocket controller 104 is part of a larger system that contains thePPC 106 with a rich feature set for controlling theIPG 102 and includes an integrated battery charger used to charge the IPG's battery. By providing both thepocket controller 104 and thePPC 106, the patient can have a small unobtrusive device to carry around as they go about their daily business and a larger more full featured device which they can use in the comfort and privacy of their homes. - The
pocket controller 104 is not only comfortable to carry in a pocket, but can also be attached to a key ring, lanyard, or other such carrying device for ease of daily use. Its functions are a subset of functions found on thePPC 106, and permit a user to power the IPG on and off (i.e., theIPG 102 remains on, but stimulation is toggled between the on state when theIPG 102 is emitting electrical pulses and the off state when theIPG 102 is not emitting electrical pulses but remains in the standby mode for additional communications from thepocket controller 104, thePPC 106, or both), select which electrical stimulation program to run, and adjust their stimulation amplitude. By limiting the functions of the pocket controller to those most commonly used on a daily basis, the device becomes much less intimidating to the patient, and allows it to be kept very small. By keeping the device small, such as about key fob size, it becomes unobtrusive and the patient is more comfortable with having and using an implanted device. FIGS. 5-7 show thePPC 106 in greater detail.FIG. 5 is a front view of the PPC andFIG. 6 is a top view ofFIG. 5 . Referring toFIGS. 5-7 , thePPC 106 performs all the same operating functions as thepocket controller 104, but includes additional operating functions making it a multi-function full-featured, advanced patient controller charger. In the embodiment shown, thePPC 106 provides a simple but rich feature set to the more advanced user, along with the charging functions.- The
PPC 106 includes a controller-charger portion 200 and acoil portion 202 connected by aflexible cable 204 and sharing components as described below. The controller-charger portion 200 comprises anouter housing 206 having an on-off switch 208 on its side, a plurality ofcontrol buttons 210, and adisplay 212, and an emergency off button (not shown, but disposed on a side of thehousing 206 opposing the on-off switch208). In this embodiment, thecontrol buttons 210 are icons on thedisplay 212, and the display is a full color, touch screen, graphical user interface. In addition, the controller-charger portion 200 includes ahome button 214 configured to return the displayed images to a home screen. The controller-charger portion 200 is larger than thepocket controller 104 and in one embodiment is sized with a height greater than about 3 inches, a width greater than about 2.5 inches, and a thickness greater than about 0.8 inch. In another embodiment, the controller-charger portion is sized with a width of about 3.1 inches, a height of about 4.5 inches, and thickness of about 0.96 inches, although both larger and smaller sizes are contemplated. - The
example coil portion 202 is of a rounded rectangular shape, and has a pair ofloop portions 205, with each loop portion forming anoblong hole 206 that can be used to receive a strap, such as a belt, in order to mount the coil portion to the body of the patient when charging the IPG. Alternatively, the coil portion could be mounted to the patient using one or more suction cups, hook and loop fasteners, a non-permanent glue, etc. - In this example, the
control buttons 210 allow a user to select a desired feature for control or further display. Particularly, thecontrol buttons 210 enable functions of thePPC 106 that are the same as those of the pocket controller104 (stimulation on/off, program stimulation amplitude adjustment, and stimulation program selection) along with additional features including: charging IPG battery, individual pulse stimulation amplitude adjustment, stimulation program frequency adjustment, individual pulse width adjustment, detailed IPG status, detailed PPC status, PPC setup/configuration, a PPC battery status indicator, PPC to IPG communication status indicator, and other items and functions. The detailed IPG status may include, for example, IPG serial number and IPG software revision level. Detailed PPC status may include, for example, date and time setting, brightness control, audio volume and mute control, and PPC serial number and software revision level. - By having a pocket controller that includes only three controls (stimulation on/off, program amplitude adjust, and stimulation program selection), a user can quickly and easily identify and select the features that are most commonly used. Features that are used less frequently, such as IPG recharge, are only included on the full-featured PPC. Features that are seldom accessed, or not accessed at all by some users, including individual pulse amplitude adjust, pulse width adjust, stimulation program frequency adjust, or serial number and software revision information, are also not included on the limited-feature pocket controller, but are included on the PPC. This allows the pocket controller to be significantly smaller, with a very simple and easy to user interface, as compared to systems that need to support all of these features.
- Referring to the example shown in
FIG. 5 , thetouch screen display 212 is arranged to convey information to the user regarding selectable options, current settings, operating parameters and other information about theIPG 102 or thePPC 106. In this example, thedisplay 212 shows aMICS communication indicator 220, the PPC's battery status at222, the IPG's battery status at224, the IPG's on or off status at226, the currently selected electrical stimulation program at228, and the amplitude setting of the active electrical stimulation program at230. In addition, thedisplay 212 shows thefrequency 232, the pulse width setting234, a selectable status icon for accessingdetailed PPC information 236, a selectable status icon for accessingdetailed IPG information 238, and a selectable icon for enabling IPG charging240. Selecting any single icon may activate another menu within that selected subject area. The controller-charger portion 200 may include a rechargeable battery whose charge status is shown by the PPC's battery status at222. - The
coil portion 202 is configured to wirelessly charge the batteries in theIPG 102. In use, thecoil portion 202 is applied against the patient's skin externally so that energy can be inductively transmitted and stored in the IPG battery. As noted above, thecoil portion 202 is connected with the integrated controller-charger portion 200. Accordingly, the controller-charger portion 200 can simultaneously display the current status of thecoil portion 204, the battery power level of theIPG 102, as well as the battery power level of the PPC. Accordingly, controlling and charging can occur in a more simplistic, time-effective manner, where the patient can perform all IPG maintenance in a single sitting. In addition, since the most commonly used features of thePPC 106 are already functional on the pocket controller, thePPC 106 may be left at home when the user does not desire to carry the larger, more bulky PPC. FIG. 7 shows an alternative display configuration of thePPC 106, including fewer available functions for user control. In this embodiment, the user has been denied access to the frequency and pulse width buttons shown inFIG. 5 . Accordingly, in some examples, the health care provider may program theIPG 102 with the clinician controller (not shown) so that theIPG 102 allows thePPC 106 to control certain parameters as in the device inFIG. 5 or may program theIPG 102 to deny control of certain parameters from thePPC 106 as in the device inFIG. 7 . InFIG. 7 , the absence of the buttons indicates that those options are not available.FIG. 8 shows a block diagram of the components making up thePPC 106. It includes auser interface 250, acontrol module 252, acommunication module 254, an IPGpower charging module 256, and apower storing module 258. Theuser interface 250 is comprised of thebuttons 210 and thedisplay 212 described above. In this embodiment however, theuser interface 250 also includes one ormore LEDs 266 signifying whether thePPC 106 is charging or powered on and abacklight 268 that illuminates the color display. In some embodiments, these LEDs may have colors symbolizing the occurring function. AnLED driver 270 and a speaker oramplifier 272 also form a part of theuser interface 250.- As can be seen, the
user interface 250 is in communication with thecontrol module 252. Thecontrol module 252 comprises aprocessor 276,memory 278, and a power management integrated circuit (PMIC)/real time clock (RTC)280. In the example shown, thecontrol module 252 also includes a Wi-Fi RF transceiver 282 that allows thePPC 106 to connect to a wireless network for data transfer. For example, it may permit doctor-patient interaction via the internet, remote access to PPC log files, remote diagnostics, and other information transfer functions. ThePMIC 280 is configured to control the charging aspects of thePPC 106. The Wi-FI transceiver 282 enables Wi-Fi data transfer for programming thePPC 106, and may permit wireless access to stored data and operating parameters. Some embodiments also include a Bluetooth RF transceiver for communication with, for example, a Bluetooth enabled printer, a keyboard, etc. - In one embodiment, the
control module 252 also includes an AD converter and a watch dog circuit as described above with reference to thecontrol module 252. Here, thememory 278 is comprised of flash memory and RAM memory, but may be other memory as described above. In some embodiments, theprocessor 276 is an embedded processor running a WinCE operating system (or any real time OS) with thegraphics interface 250, and thememory 278 stores sets of stimulation control parameters that are available to be selected for delivery through thecommunication module 254 to theIPG 102 for electrical stimulation therapy. In one embodiment, thecontrol module 252 comprises integrated circuits disposed on a PC board. - The
communication module 254 comprises aMICS RF transceiver 290, a wake uptransmitter 292, anamplifier 294, and matchingnetworks 296. Thecommunication module 254 may be similar to thecommunication module 154 discussed above, and will not be further described here in any detail, except to note that for this embodiment, thetransceiver 290 is shown sharing an antenna with the wake-uptransmitter 292, although separate antennas as discussed for the154 embodiment shown inFIG. 4 could be utilized for this embodiment as well. ThePPC 206 also includes aprogramming interface 298 that may be used during manufacturing to load an operating system and program thePPC 206. - The
power storing module 258 is configured to convert power to recharge one or morerechargeable batteries 302. In this embodiment, thebatteries 302 are lithium-ion cells that provide power to operate thePPC 106 allowing it to receive user inputs, transmit control signals to, and charge theIPG 102. Thepower storing module 258 includes aconnector 304 for connecting to a power source, a powerprotection detection circuit 306 for protecting the PPC from power surges, andlinear power supplies 308 for assisting with the electric transfer to charge thebatteries 302. As can be seen, thecontrol module 252 aids with the charging and is configured to monitor and send the battery charge level to theuser interface 250 for display. Theconnector 304 connects the PPC, directly or indirectly, to a power source (not shown) such as a conventional wall outlet for receiving electrical current. In some embodiments, theconnector 304 comprises a cradle. - The
power charging module 256 communicates with thecontrol module 252 and is arranged to magnetically or inductively charge theIPG 102. In the embodiments shown, it is magnetically or inductively coupled to theIPG 102 to charge rechargeable batteries on theIPG 102. Thecharging module 256 includes components in both the controller-charger portion 200 and the coil portion202 (FIG. 5 ). It includesswitch boost circuitry 316, a load power monitor318, anLSK demodulator 320, aASK modulator 322, acurrent mode transmitter 324, anADC 326, and coils328. As can be seen, thecontrol module 252 aids with the charging and is configured to monitor and send the IPG battery charge level to theuser interface 250 for display. - In this embodiment, the
coils 328 are disposed in thecoil portion 202 and are configured to create magnetic or inductive coupling with components in theIPG 102. Since thecoil portion 202 is integrated with the controller-charger portion 200, both operate from asingle battery 302. Accordingly, as can be seen by the circuitry, thebattery 302 powers thecontrol module 252 and all its associated components. In addition, thebattery 302 powers thepower charging module 256 for recharging theIPG 102. - Furthermore, the
coils 328 are provided with a temperature sensor for monitoring the temperature of thecoil portion 202, in which thecoils 328 are installed. This can be important as thecoils 328 may generate substantial heat when charging the IPG battery, and hence the temperature should be monitored in order to ensure that thecoil portion 202 does not overheat and potentially injure the patient. The temperature sensor can be configured to send data back to thecontrol module 252 via theADC 326, The control module can then monitor the temperature of thecoil portion 202, and adjust the charging rate based on the measured temperature, such as by reducing the energy transmitted by thecoils 328, for example, when the temperature rises above a threshold value, such as a value that may be considered uncomfortable or harmful to the patient wearing the charger (e.g, a value more than a few degrees above normal body temperatures). In a worst-case scenario, the charging can be stopped to prevent overheating, and then resumed when the coil portion sufficiently cools down. - Because the
coil portion 202 is integrated with the controller-charger portion 200, thecontrol module 252 provides a single control interface and a single user interface for performing both functions of controlling theIPG 102 and of charging theIPG 102. In addition, because the controller-charger portion 200 and thecoil portion 202 are integrated, the controller-charger portion 200 simultaneously controls both the current status of the charger, the battery power level of theIPG 102, as well as the battery power level of the PPC. Accordingly, controlling and charging can occur in a more simplistic, time-effective manner, where the patient can perform all IPG maintenance in a single sitting. In addition, since the most commonly used features of thePPC 106 are already functional on the pocket controller, thePPC 106 may be left at home when the user does not desire to carry the larger, more bulky PPC. FIG. 9 shows an alternative embodiment of a PPC, referenced herein by the numeral106a.In this embodiment, instead of having a single processor in the PPC, the functions of the control module are divided into processors splitting the functions required by the single processor embodiment inFIG. 8 . For reference, similar items maintain the same reference numeral.- As can be seen, the
PPC 106aincludes theuser interface 250, thecommunication module 254, and thepower storing module 258. It also includes apower charging module 256aand a control module that in this embodiment includes both acontroller 340 and acontroller 342. Thecontroller 340 is similar in some ways to thecontrol module 152 inFIG. 4 , and thecontroller 342 is similar in some ways to thecontrol module 252 described inFIG. 8 . Thecontrollers controller 340 is arranged, and contains instructions for controlling thepower charging module 256aandMICS communications module 254, while thecontroller 342 is arranged and contains instructions for controlling thepower storing module 258 and theuser interface 250. - As discussed above, the PPC contains all the features available in the pocket controller104 (stimulation on/off, stimulation program amplitude adjustment, and stimulation program selection). It also includes additional features including, for example, charge IPG battery, individual pulse/area stimulation amplitude adjustment, stimulation program frequency adjustment, individual pulse/area pulse width adjustment, detailed IPG status, detailed PPC status, PPC setup/configuration, PPC battery status indicator, PPC to IPG communication status indicator, and other items.
FIG. 10 is a flow chart showing a method of operation taken by thepocket controller 104 according to one aspect of the present disclosure. Theprocessor 158 and memory160 contain all the necessary software and programming in order to communicate with theIPG 102 and to receive user inputs performing the available functions of: 1) electrical stimulation on/off, 2) program stimulation amplitude adjustment, and 3) electrical stimulation program selection. In some examples, the control functions of the pocket controller consist only of these functions, while in other embodiments, the control functions contain more or fewer functions, but in all cases is a limited function device having fewer control functional capabilities than the PPC.- When the
pocket controller 104 is powered on using the on-off switch 122 and within communication range of theIPG 102, thepocket controller 104 interrogates theIPG 102, as indicated atstep 402 inFIG. 10 . In response, theIPG 102 transmits to the pocket controller information regarding its current settings including which stimulation programs are available for implementation. As indicated above, some embodiments of the pocket controller communicate only with theIPG 102, and not with thePPC 106. In these embodiments, thepocket controller 104 must receive its data from the IPG. In one example, the information received from the IPG includes the current nuerostimulation on-off state, the amplitude setting or level, and the stimulation programs available for implementation. - In this embodiment, the
pocket controller 104 may receive data representing a stimulation program identifier uniquely representing each stimulation program that the IPG is capable of carrying out. InFIG. 2 , the stimulation program identifier is a numerical number with a “p” as shown at138. A clinician however, using a clinician programmer or other device, may have only enabled certain stimulation programs on theIPG 102, but not all of the available stimulation programs. In these cases, in response to the interrogation, the pocket controller receives data indicating which stimulation programs are enabled on theIPG 102. - Once received, the
pocket controller 104 stores the received data atstep 406. Atstep 408, thepocket controller 104 receives data indicative of the power charge level of theIPG 102, and atstep 410, thepocket controller 104 displays the current settings and data indicative of the power charge level. An example of this is shown inFIG. 2 , where thepocket controller 104 displays the current on-off state of the electrical stimulation, the amplitude setting, the currently set stimulation program, and the power level of both theIPG 102 and thepocket controller 104. - At
step 412, thepocket controller 104 receives an input from a user to modify the current settings. To do this, thepocket controller 104 receives inputs via theadjustment buttons select button 130 allows a user to select the desired highlighted function. Accordingly, when thepocket controller 104 receives a select input via theselect button 130, the pocket controller permits modification of the selected function setting using theadjustment buttons select button 130. For example, if the user highlights and selects the stimulation amplitude adjustment function, the pocket controller enters an adjustment mode allowing the user to increase or decrease the amplitude level (shown as a numerical value inFIG. 2 ) using theadjustment buttons select button 130, or alternatively, the controller may automatically enter the displayed value. - In some examples, when the user scrolls to and selects the program function, further inputs by the
adjustment buttons only stimulation programs pocket controller 104 may be programmed to display for selection onlystimulation programs - In response to receiving an input at the
select button 130, thepocket controller 104 transmits data representing the selected adjustment to theIPG 102 atstep 414, and theIPG 102 responds by modifying its settings to carry out the command of thepocket controller 104. FIG. 11 is a flow chart showing a method of operation taken by thePPC 106 according to one aspect of the present disclosure. Although described with reference toPPC 106 inFIG. 8 , it should be understood that the method may be performed by any PPC within this scope of this disclosure. Theprocessor 256 andmemory 278 contain all the necessary software and programming in order to communicate with theIPG 102, to charge the IPG, and to receive user inputs performing the same control functions as thepocket controller 104, plus additional control functions. For example, these additional control functions performed by thePPC 106 may include charging the IPG battery, adjusting individual pulse/area stimulation amplitude, adjusting stimulation program frequency, adjusting individual pulse/area pulse width, providing detailed IPG status, providing detailed PPC status, enabling PPC setup/configuration, indicating PPC and IPG battery status, displaying PPC to IPG communication status indicator, and other items.- Turning to
FIG. 11 , when thePPC 106 is powered on using the on-off switch 208 and within communication range of theIPG 102, thePPC 106 interrogates theIPG 102, as indicated atstep 454. In response, theIPG 102 transmits to the PPC information regarding its current settings including stimulation programs available for implementation, atstep 456. In some embodiments, as described above, thePPC 106 includes a single user interface for performing both functions of controlling theIPG 102 and of charging theIPG 102. In addition, because the controller-charger portion 200 and thecoil portion 202 are integrated, the controller-charger portion 200 simultaneously controls both the current status of the charger, the battery power level of theIPG 102, as well as the battery power level of the PPC. - In some embodiments, the
PPC 106 may receive data representing a stimulation program identifier uniquely representing each stimulation program that the IPG is capable of carrying out or programmed to carry out, similar to the operation of thepocket controller 104 above. - The
PPC 106 stores the received data atstep 458. Atstep 460, thePPC 106 receives data indicative of the power charge level of theIPG 102, and atstep 462, thePPC 106 displays the current settings and data indicative of the power charge level. An example of this is shown inFIG. 5 , where the PPC displays the current on-off state of the electrical stimulation, the amplitude setting, the currently set stimulation program, and the power level of both theIPG 102 and thePPC 104, the communication status of the PPC to the IPG. In some embodiments, thePPC 106 displays the current stimulation program frequency, the individual pulse/area pulse width, and other information. - At
step 464, thePPC 106 receives an input from a user to modify the current settings. In the exemplary embodiment shown, thePPC 106 includes a touch screen display, and the inputs are received via a user touching an icon displayed on the touch screen display. Once a particular function is selected, the PPC permits modification of the selected function setting using the touch screen. At astep 466, the PPC transmits signals to the IPG to modify the settings and control. - At a
step 468, thePPC 106 receives instructions from a user to charge theIPG 102. In response, atstep 470, the PPC responds by directing current through thecoil 328 for creating an inductive or a magnetic field. Since thecoil portion 202 is integrated with the controller-charger portion 200, in the examples shown, both operate from asingle battery 302. Accordingly, as can be seen by the circuitry, thebattery 302 powers thecontrol module 252 and all its associated components. In addition, thebattery 302 powers thepower charging module 256 for recharging theIPG 102. - In addition to providing the advantage of a discrete, smaller, functionally limited device, the pocket controller also provides controller redundancy to the patient. Accordingly, the patient is more likely to carry the pocket controller for use, but also serves as a backup to turn off treatments if they become uncomfortable.
- The devices, systems, and methods described herein introduce an improved way for controlling and charging an implanted medical device by using a full-featured PPC and a smaller, less intimidating, limited feature, device for everyday use. In addition, because of the redundant nature of the two controllers, the system disclosed herein provides a level of risk management not achieved with a single controller. For example, if one controller malfunctions or is misplaced, the patient still will have the second controller to manage at least the more important and immediate functions of the IPG until the malfunctioning or misplaced controller is replaced. Therefore, the patient can continue with his or her scheduled therapy, modify the stimulation treatment as desired for effectiveness, and control the stimulation in the event that it becomes painful or undesired.
- Other embodiments of the invention will be apparent to those skilled in the art from consideration of the specification and practice of the invention disclosed herein. It is intended that the specification and examples be considered as exemplary only, with a true scope and spirit of the invention being indicated by the following claims.
Claims (35)
Priority Applications (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US13/800,729US20130197607A1 (en) | 2011-06-28 | 2013-03-13 | Dual patient controllers |
| US15/962,556US11191964B2 (en) | 2011-06-28 | 2018-04-25 | Dual patient controllers |
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US13/170,775US20130006330A1 (en) | 2011-06-28 | 2011-06-28 | Dual patient controllers |
| US13/800,729US20130197607A1 (en) | 2011-06-28 | 2013-03-13 | Dual patient controllers |
Related Parent Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US13/170,775Continuation-In-PartUS20130006330A1 (en) | 2011-06-28 | 2011-06-28 | Dual patient controllers |
Related Child Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US15/962,556DivisionUS11191964B2 (en) | 2011-06-28 | 2018-04-25 | Dual patient controllers |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| US20130197607A1true US20130197607A1 (en) | 2013-08-01 |
Family
ID=48870913
Family Applications (2)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US13/800,729AbandonedUS20130197607A1 (en) | 2011-06-28 | 2013-03-13 | Dual patient controllers |
| US15/962,556Active2032-06-30US11191964B2 (en) | 2011-06-28 | 2018-04-25 | Dual patient controllers |
Family Applications After (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US15/962,556Active2032-06-30US11191964B2 (en) | 2011-06-28 | 2018-04-25 | Dual patient controllers |
Country Status (1)
| Country | Link |
|---|---|
| US (2) | US20130197607A1 (en) |
Cited By (54)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US20140067015A1 (en)* | 2012-08-29 | 2014-03-06 | Boston Scientific Neuromodulation Corporation | System and method for identifying availability of clinician defined programming settings for a patient |
| EP2865412A1 (en)* | 2013-10-23 | 2015-04-29 | Mainstay Medical Limited | System for restoring muscle function to the lumbar spine |
| WO2015070200A1 (en) | 2013-11-11 | 2015-05-14 | Thoratec Corporation | Resonant power transfer systems with communications |
| US9072897B2 (en) | 2007-03-09 | 2015-07-07 | Mainstay Medical Limited | Systems and methods for restoring muscle function to the lumbar spine |
| US20160033308A1 (en)* | 2014-08-04 | 2016-02-04 | Infineon Technologies Ag | Intelligent gauge devices and related systems and methods |
| USD752763S1 (en)* | 2014-08-08 | 2016-03-29 | Inspire Medical Systems, Inc. | Patient control |
| US20160211064A1 (en)* | 2015-01-19 | 2016-07-21 | Industry-Academic Cooperation Foundation Chosun University | Wireless power charging apparatus using superconducting coil |
| US20170033567A1 (en)* | 2015-07-27 | 2017-02-02 | Apple Inc. | Charging apparatus for wearable electronic device |
| US9780596B2 (en) | 2013-07-29 | 2017-10-03 | Alfred E. Mann Foundation For Scientific Research | Microprocessor controlled class E driver |
| USD801538S1 (en) | 2015-12-16 | 2017-10-31 | Inspire Medical Systems, Inc. | Patient control |
| US9805863B2 (en) | 2012-07-27 | 2017-10-31 | Thoratec Corporation | Magnetic power transmission utilizing phased transmitter coil arrays and phased receiver coil arrays |
| US9825471B2 (en) | 2012-07-27 | 2017-11-21 | Thoratec Corporation | Resonant power transfer systems with protective algorithm |
| CN107427675A (en)* | 2015-01-09 | 2017-12-01 | 艾克索尼克斯调制技术股份有限公司 | Patient remote control and associated method for use with a neurostimulation system |
| US9855436B2 (en) | 2013-07-29 | 2018-01-02 | Alfred E. Mann Foundation For Scientific Research | High efficiency magnetic link for implantable devices |
| US9855437B2 (en) | 2013-11-11 | 2018-01-02 | Tc1 Llc | Hinged resonant power transfer coil |
| US9861811B2 (en) | 2010-03-11 | 2018-01-09 | Mainstay Medical Limited | Electrical stimulator for treatment of back pain and methods of use |
| USD810306S1 (en) | 2016-03-24 | 2018-02-13 | Inspire Medical Systems, Inc. | Patient control |
| US9950159B2 (en) | 2013-10-23 | 2018-04-24 | Mainstay Medical Limited | Systems and methods for restoring muscle function to the lumbar spine and kits for implanting the same |
| US9981122B2 (en) | 2012-06-13 | 2018-05-29 | Mainstay Medical Limited | Systems and methods for implanting electrode leads for use with implantable neuromuscular electrical stimulator |
| US9997928B2 (en) | 2012-07-27 | 2018-06-12 | Tc1 Llc | Self-tuning resonant power transfer systems |
| US9999763B2 (en) | 2012-06-13 | 2018-06-19 | Mainstay Medical Limited | Apparatus and methods for anchoring electrode leads adjacent to nervous tissue |
| US10148126B2 (en) | 2015-08-31 | 2018-12-04 | Tc1 Llc | Wireless energy transfer system and wearables |
| US10177604B2 (en) | 2015-10-07 | 2019-01-08 | Tc1 Llc | Resonant power transfer systems having efficiency optimization based on receiver impedance |
| US10186760B2 (en) | 2014-09-22 | 2019-01-22 | Tc1 Llc | Antenna designs for communication between a wirelessly powered implant to an external device outside the body |
| US10195419B2 (en) | 2012-06-13 | 2019-02-05 | Mainstay Medical Limited | Electrode leads for use with implantable neuromuscular electrical stimulator |
| USD840358S1 (en)* | 2016-11-10 | 2019-02-12 | Qingdao Bright Medical Manufacturing Co., Ltd. | Control device |
| USD840357S1 (en)* | 2016-08-30 | 2019-02-12 | Qingdao Bright Medical Manufacturing Co., Ltd. | Control device |
| US10251987B2 (en) | 2012-07-27 | 2019-04-09 | Tc1 Llc | Resonant power transmission coils and systems |
| US10265450B2 (en) | 2014-10-06 | 2019-04-23 | Tc1 Llc | Multiaxial connector for implantable devices |
| US10291067B2 (en) | 2012-07-27 | 2019-05-14 | Tc1 Llc | Computer modeling for resonant power transfer systems |
| US10327810B2 (en) | 2016-07-05 | 2019-06-25 | Mainstay Medical Limited | Systems and methods for enhanced implantation of electrode leads between tissue layers |
| US10373756B2 (en) | 2013-03-15 | 2019-08-06 | Tc1 Llc | Malleable TETs coil with improved anatomical fit |
| US10383990B2 (en) | 2012-07-27 | 2019-08-20 | Tc1 Llc | Variable capacitor for resonant power transfer systems |
| US10434235B2 (en) | 2012-07-27 | 2019-10-08 | Tci Llc | Thermal management for implantable wireless power transfer systems |
| US10476317B2 (en) | 2013-03-15 | 2019-11-12 | Tci Llc | Integrated implantable TETs housing including fins and coil loops |
| US10471268B2 (en) | 2014-10-16 | 2019-11-12 | Mainstay Medical Limited | Systems and methods for monitoring muscle rehabilitation |
| US10525181B2 (en) | 2012-07-27 | 2020-01-07 | Tc1 Llc | Resonant power transfer system and method of estimating system state |
| US10615642B2 (en) | 2013-11-11 | 2020-04-07 | Tc1 Llc | Resonant power transfer systems with communications |
| US10610692B2 (en) | 2014-03-06 | 2020-04-07 | Tc1 Llc | Electrical connectors for implantable devices |
| US10770923B2 (en) | 2018-01-04 | 2020-09-08 | Tc1 Llc | Systems and methods for elastic wireless power transmission devices |
| US10898292B2 (en) | 2016-09-21 | 2021-01-26 | Tc1 Llc | Systems and methods for locating implanted wireless power transmission devices |
| US11103706B2 (en) | 2007-03-09 | 2021-08-31 | Mainstay Medical Limited | Systems and methods for enhancing function of spine stabilization muscles associated with a spine surgery intervention |
| US11197990B2 (en) | 2017-01-18 | 2021-12-14 | Tc1 Llc | Systems and methods for transcutaneous power transfer using microneedles |
| US11324953B2 (en) | 2013-08-09 | 2022-05-10 | Inspire Medical Systems, Inc. | Patient control for implantable medical device |
| US11331488B2 (en) | 2007-03-09 | 2022-05-17 | Mainstay Medical Limited | Systems and methods for enhancing function of spine stabilization muscles associated with a spine surgery intervention |
| WO2022162582A1 (en)* | 2021-01-27 | 2022-08-04 | Cochlear Limited | Heat reduction associated with prostheses |
| US11642537B2 (en) | 2019-03-11 | 2023-05-09 | Axonics, Inc. | Charging device with off-center coil |
| US11679262B2 (en) | 2007-03-09 | 2023-06-20 | Mainstay Medical Limited | Systems and methods for restoring muscle function to the lumbar spine |
| US11679261B2 (en) | 2007-03-09 | 2023-06-20 | Mainstay Medical Limited | Systems and methods for enhancing function of spine stabilization muscles associated with a spine surgery intervention |
| US11684774B2 (en) | 2010-03-11 | 2023-06-27 | Mainstay Medical Limited | Electrical stimulator for treatment of back pain and methods of use |
| CN116764094A (en)* | 2023-07-11 | 2023-09-19 | 四川君健万峰医疗器械有限责任公司 | Safety control method for electric shock dual-redundancy host |
| US11786725B2 (en) | 2012-06-13 | 2023-10-17 | Mainstay Medical Limited | Systems and methods for restoring muscle function to the lumbar spine and kits for implanting the same |
| USD1010831S1 (en) | 2021-02-23 | 2024-01-09 | Inspire Medical Systems, Inc. | Patient control |
| US12097365B2 (en) | 2010-03-11 | 2024-09-24 | Mainstay Medical Limited | Electrical stimulator for the treatment of back pain and methods of use |
Families Citing this family (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP7191873B2 (en)* | 2020-01-17 | 2022-12-19 | 株式会社東芝 | Charge/discharge control device, charge/discharge system, charge/discharge control method, and charge/discharge control program |
Citations (12)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US3727616A (en)* | 1971-06-15 | 1973-04-17 | Gen Dynamics Corp | Electronic system for the stimulation of biological systems |
| US5342408A (en)* | 1993-01-07 | 1994-08-30 | Incontrol, Inc. | Telemetry system for an implantable cardiac device |
| US20050075693A1 (en)* | 2003-10-02 | 2005-04-07 | Medtronic, Inc. | Driver circuitry switchable between energy transfer and telemetry for an implantable medical device |
| US7272445B2 (en)* | 2003-10-02 | 2007-09-18 | Medtronic, Inc. | Medical device programmer with faceplate |
| US7330762B2 (en)* | 2004-06-07 | 2008-02-12 | Neuro And Cardiac Technologies, Llc | Method and system for providing pulsed electrical stimulation to provide therapy for erectile/sexual dysfunction, prostatitis, prostatitis pain, and chronic pelvic pain |
| US20090118796A1 (en)* | 2007-11-05 | 2009-05-07 | Advanced Bionics Corporation | External controller for an implantable medical device system with coupleable external charging coil assembly |
| US20100204756A1 (en)* | 2009-02-10 | 2010-08-12 | Boston Scientific Neuromodulation Corporation | External Device for Communicating with an Implantable Medical Device Having Data Telemetry and Charging Integrated in a Single Housing |
| US20110046698A1 (en)* | 2009-08-24 | 2011-02-24 | Medtronic, Inc. | Recovery of a wireless communication session with an implantable medical device |
| US20110184491A1 (en)* | 2010-01-28 | 2011-07-28 | Medtronic, Inc. | Wireless communication with an implantable medical device |
| US20110202103A1 (en)* | 2010-02-18 | 2011-08-18 | Birgitte Wikman | Wakeup of implantable communication circuitry |
| US8346361B2 (en)* | 2003-10-02 | 2013-01-01 | Medtronic, Inc. | User interface for external charger for implantable medical device |
| US8447411B2 (en)* | 2008-07-11 | 2013-05-21 | Medtronic, Inc. | Patient interaction with posture-responsive therapy |
Family Cites Families (85)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US5383915A (en) | 1991-04-10 | 1995-01-24 | Angeion Corporation | Wireless programmer/repeater system for an implanted medical device |
| CA2157600A1 (en) | 1993-03-05 | 1994-09-15 | Roy J. Mankovitz | Apparatus and method using compressed codes for television program record scheduling |
| US6249703B1 (en) | 1994-07-08 | 2001-06-19 | Medtronic, Inc. | Handheld patient programmer for implantable human tissue stimulator |
| US5626630A (en) | 1994-10-13 | 1997-05-06 | Ael Industries, Inc. | Medical telemetry system using an implanted passive transponder |
| US5973592A (en) | 1996-01-04 | 1999-10-26 | Flick; Kenneth E. | Vehicle security system including a remote unit that emulates security system condition local indications and related method |
| US6393325B1 (en) | 1999-01-07 | 2002-05-21 | Advanced Bionics Corporation | Directional programming for implantable electrode arrays |
| US6312378B1 (en) | 1999-06-03 | 2001-11-06 | Cardiac Intelligence Corporation | System and method for automated collection and analysis of patient information retrieved from an implantable medical device for remote patient care |
| US6516227B1 (en) | 1999-07-27 | 2003-02-04 | Advanced Bionics Corporation | Rechargeable spinal cord stimulator system |
| US7177690B2 (en) | 1999-07-27 | 2007-02-13 | Advanced Bionics Corporation | Implantable system having rechargeable battery indicator |
| US6553263B1 (en) | 1999-07-30 | 2003-04-22 | Advanced Bionics Corporation | Implantable pulse generators using rechargeable zero-volt technology lithium-ion batteries |
| US6820019B1 (en) | 1999-07-31 | 2004-11-16 | Medtronic, Inc. | Device and method for determining and communicating the remaining life of a battery in an implantable neurological tissue stimulating device |
| US6850803B1 (en) | 2000-06-16 | 2005-02-01 | Medtronic, Inc. | Implantable medical device with a recharging coil magnetic shield |
| EP1166820B1 (en) | 2000-06-19 | 2009-09-30 | Medtronic, Inc. | Implantable medical device with external recharging coil |
| US6842647B1 (en) | 2000-10-20 | 2005-01-11 | Advanced Bionics Corporation | Implantable neural stimulator system including remote control unit for use therewith |
| WO2002034331A2 (en) | 2000-10-26 | 2002-05-02 | Medtronic, Inc. | Externally worn transceiver for use with an implantable medical device |
| US7045628B2 (en) | 2000-11-28 | 2006-05-16 | Eli Lilly And Company | Synthesis of 2-aryl-1-naphthol derivatives via a tandem palladium catalyzed arylation and dehydrogenation |
| US7369897B2 (en) | 2001-04-19 | 2008-05-06 | Neuro And Cardiac Technologies, Llc | Method and system of remotely controlling electrical pulses provided to nerve tissue(s) by an implanted stimulator system for neuromodulation therapies |
| US6662052B1 (en) | 2001-04-19 | 2003-12-09 | Nac Technologies Inc. | Method and system for neuromodulation therapy using external stimulator with wireless communication capabilites |
| JP4176011B2 (en) | 2001-07-10 | 2008-11-05 | シャープ株式会社 | AV data transmitter, AV data receiver, AV data display / playback device |
| US20050137480A1 (en) | 2001-10-01 | 2005-06-23 | Eckhard Alt | Remote control of implantable device through medical implant communication service band |
| EP1481561A1 (en) | 2002-03-04 | 2004-12-01 | Nokia Corporation | Method for intermediate unlocking of a keypad on a mobile electronic device |
| US7209790B2 (en) | 2002-09-30 | 2007-04-24 | Medtronic, Inc. | Multi-mode programmer for medical device communication |
| US20040064166A1 (en) | 2002-09-30 | 2004-04-01 | Thompson David L. | Multi-mode programmer for medical device communication |
| US7181286B2 (en) | 2002-10-31 | 2007-02-20 | Medtronic, Inc. | Distributed system for neurostimulation therapy programming |
| US20040133079A1 (en) | 2003-01-02 | 2004-07-08 | Mazar Scott Thomas | System and method for predicting patient health within a patient management system |
| US7647116B2 (en) | 2003-03-13 | 2010-01-12 | Medtronic, Inc. | Context-sensitive collection of neurostimulation therapy data |
| AU2004224345B2 (en) | 2003-03-21 | 2010-02-18 | Welch Allyn, Inc. | Personal status physiologic monitor system and architecture and related monitoring methods |
| US7617002B2 (en) | 2003-09-15 | 2009-11-10 | Medtronic, Inc. | Selection of neurostimulator parameter configurations using decision trees |
| US7203549B2 (en) | 2003-10-02 | 2007-04-10 | Medtronic, Inc. | Medical device programmer with internal antenna and display |
| US9259584B2 (en)* | 2003-10-02 | 2016-02-16 | Medtronic, Inc. | External unit for implantable medical device coupled by cord |
| US8086318B2 (en) | 2004-02-12 | 2011-12-27 | Ndi Medical, Llc | Portable assemblies, systems, and methods for providing functional or therapeutic neurostimulation |
| US20070016090A1 (en) | 2004-04-28 | 2007-01-18 | Transoma Medical, Inc. | Implantable medical devices and related methods |
| GB0409769D0 (en) | 2004-04-30 | 2004-06-09 | Algotec Ltd | Electrical nerve stimulation device |
| US7359751B1 (en) | 2004-05-05 | 2008-04-15 | Advanced Neuromodulation Systems, Inc. | Clinician programmer for use with trial stimulator |
| US7761167B2 (en) | 2004-06-10 | 2010-07-20 | Medtronic Urinary Solutions, Inc. | Systems and methods for clinician control of stimulation systems |
| US20070060979A1 (en) | 2004-06-10 | 2007-03-15 | Ndi Medical, Llc | Implantable pulse generator systems and methods for providing functional and / or therapeutic stimulation of muscles and / or nerves and / or central nervous system tissue |
| US8428712B2 (en) | 2004-07-20 | 2013-04-23 | Medtronic, Inc. | Concurrent delivery of treatment therapy with telemetry in an implantable medical device |
| US7565200B2 (en) | 2004-11-12 | 2009-07-21 | Advanced Neuromodulation Systems, Inc. | Systems and methods for selecting stimulation sites and applying treatment, including treatment of symptoms of Parkinson's disease, other movement disorders, and/or drug side effects |
| US7460912B2 (en) | 2004-11-19 | 2008-12-02 | Cardiac Pacemakers, Inc. | System and method for temporary programming for implanted medical devices |
| US8001975B2 (en) | 2004-12-29 | 2011-08-23 | Depuy Products, Inc. | Medical device communications network |
| US20060206024A1 (en) | 2005-03-09 | 2006-09-14 | Invivo Corporation | Wireless in-bore patient monitor for MRI |
| US7991467B2 (en) | 2005-04-26 | 2011-08-02 | Medtronic, Inc. | Remotely enabled pacemaker and implantable subcutaneous cardioverter/defibrillator system |
| US7389147B2 (en) | 2005-04-29 | 2008-06-17 | Medtronic, Inc. | Therapy delivery mode selection |
| US7505816B2 (en) | 2005-04-29 | 2009-03-17 | Medtronic, Inc. | Actively cooled external energy source, external charger, system of transcutaneous energy transfer, system of transcutaneous charging and method therefore |
| US7711419B2 (en) | 2005-07-13 | 2010-05-04 | Cyberonics, Inc. | Neurostimulator with reduced size |
| US8027727B2 (en) | 2005-08-29 | 2011-09-27 | Cardiac Pacemakers, Inc. | Pacemaker RF telemetry repeater and method |
| US20070156207A1 (en) | 2006-01-04 | 2007-07-05 | Sridhar Kothandaraman | Expanding single channel stimulator capability on multi-area stimulation programs |
| US8612024B2 (en) | 2006-02-24 | 2013-12-17 | Medtronic, Inc. | User interface with 3D environment for configuring stimulation therapy |
| CN101401314B (en) | 2006-03-13 | 2013-04-24 | 诺沃-诺迪斯克有限公司 | Medical system with dual purpose communication device comprising first and second units |
| US9480846B2 (en) | 2006-05-17 | 2016-11-01 | Medtronic Urinary Solutions, Inc. | Systems and methods for patient control of stimulation systems |
| US20100165993A1 (en) | 2006-06-09 | 2010-07-01 | Henrik Basilier | Operator Managed Virtual Home Network |
| US7623922B2 (en) | 2006-07-12 | 2009-11-24 | Cardiac Pacemakers, Inc. | Implantable medical device telemetry with periodic frequency hopping |
| US20080140163A1 (en) | 2006-12-06 | 2008-06-12 | Keacher Jeffrey T | Telemetry device for a medical device programmer |
| US9289149B2 (en) | 2007-05-10 | 2016-03-22 | General Electric Company | Apparatus and method for wirelessly transferring and storing medical data |
| GB0709834D0 (en) | 2007-05-22 | 2007-07-04 | Gillbe Ivor S | Array stimulator |
| US8805508B2 (en) | 2007-05-30 | 2014-08-12 | Medtronic, Inc. | Collecting activity data for evaluation of patient incontinence |
| DE102007033993A1 (en) | 2007-07-19 | 2009-01-22 | Biotronik Crm Patent Ag | Arrangement and method for the remote programming of a programmable personal device |
| US9452288B2 (en) | 2007-12-06 | 2016-09-27 | Boston Scientific Neuromodulation Corporation | Multimodal neurostimulation systems and methods |
| US8332040B1 (en) | 2008-03-10 | 2012-12-11 | Advanced Neuromodulation Systems, Inc. | External charging device for charging an implantable medical device and methods of regulating duty of cycle of an external charging device |
| US8193766B2 (en) | 2008-04-30 | 2012-06-05 | Medtronic, Inc. | Time remaining to charge an implantable medical device, charger indicator, system and method therefore |
| WO2010014259A1 (en) | 2008-08-01 | 2010-02-04 | Ndi Medical, Llc | Portable assemblies, systems, and methods for providing functional or therapeutic neurostimulation |
| US8401660B2 (en) | 2008-08-06 | 2013-03-19 | Texas Instruments Incorporated | Polling mechanism in a medical implant based system |
| US8249701B2 (en) | 2008-10-15 | 2012-08-21 | Spinal Modulation, Inc. | Methods, devices and systems for programming neurostimulation |
| WO2010111324A1 (en) | 2009-03-27 | 2010-09-30 | Medtronic, Inc. | Conditional electrical stimulation in response to user input for pelvic health |
| EP3228350B1 (en) | 2009-04-22 | 2025-09-24 | Nevro Corporation | Device for selective high frequency spinal cord modulation for inhibiting pain with reduced side effects, and associated systems and methods |
| US8214042B2 (en) | 2009-05-26 | 2012-07-03 | Boston Scientific Neuromodulation Corporation | Techniques for controlling charging of batteries in an external charger and an implantable medical device |
| US20100305663A1 (en) | 2009-06-02 | 2010-12-02 | Boston Scientific Neuromodulation Corporation | Implantable medical device system having short range communication link between an external controller and an external charger |
| US8260432B2 (en) | 2009-06-30 | 2012-09-04 | Boston Scientific Neuromodulation Corporation | Moldable charger with shape-sensing means for an implantable pulse generator |
| US8473066B2 (en) | 2009-07-06 | 2013-06-25 | Boston Scientific Neuromodulation Company | External charger for a medical implantable device using field sensing coils to improve coupling |
| EP2456515A4 (en) | 2009-07-20 | 2013-01-23 | Nat Ict Australia Ltd | NEURO-STIMULATION |
| US8463392B2 (en) | 2009-11-11 | 2013-06-11 | Boston Scientific Neuromodulation Corporation | External controller/charger system for an implantable medical device capable of automatically providing data telemetry through a charging coil during a charging session |
| US9956418B2 (en) | 2010-01-08 | 2018-05-01 | Medtronic, Inc. | Graphical manipulation of posture zones for posture-responsive therapy |
| US8401663B2 (en) | 2010-01-19 | 2013-03-19 | Boston Scientific Neuromodulation Corporation | Pressure-sensitive external charger for an implantable medical device |
| US8994325B2 (en) | 2010-11-17 | 2015-03-31 | Boston Scientific Neuromodulation Corporation | External charger for an implantable medical device having at least one moveable charging coil |
| US10201702B2 (en) | 2010-11-30 | 2019-02-12 | Medtronic, Inc. | Pelvic floor muscle training |
| EP2658606B1 (en) | 2010-12-27 | 2016-07-27 | Boston Scientific Neuromodulation Corporation | Neurostimulation system for selectively estimating volume of activation and providing therapy |
| US9113473B2 (en) | 2011-04-06 | 2015-08-18 | Spinal Modulation, Inc. | Power efficient wireless RF communication between a base station and a medical device |
| US8594804B2 (en) | 2011-04-28 | 2013-11-26 | Cyberonics, Inc. | Implantable medical device charging |
| US8401664B2 (en) | 2011-04-29 | 2013-03-19 | Cyberonics, Inc. | System and method for charging a power cell in an implantable medical device |
| EP2707089A2 (en) | 2011-05-13 | 2014-03-19 | Boston Scientific Neuromodulation Corporation | Neurostimulation system with on-effector programmer control |
| US8954148B2 (en) | 2011-06-28 | 2015-02-10 | Greatbatch, Ltd. | Key fob controller for an implantable neurostimulator |
| WO2013095799A1 (en) | 2011-12-21 | 2013-06-27 | Boston Scientific Neuromodulation Corporation | A system for an implantable medical device having an external charger coupleable to accessory charging coils |
| US9362774B2 (en) | 2012-01-27 | 2016-06-07 | Medtronic, Inc. | Battery charging top-off |
| US9653935B2 (en)* | 2012-04-20 | 2017-05-16 | Medtronic, Inc. | Sensing temperature within medical devices |
| US8818523B2 (en)* | 2012-04-25 | 2014-08-26 | Medtronic, Inc. | Recharge of an implantable device in the presence of other conductive objects |
- 2013
- 2013-03-13USUS13/800,729patent/US20130197607A1/ennot_activeAbandoned
- 2018
- 2018-04-25USUS15/962,556patent/US11191964B2/enactiveActive
Patent Citations (12)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US3727616A (en)* | 1971-06-15 | 1973-04-17 | Gen Dynamics Corp | Electronic system for the stimulation of biological systems |
| US5342408A (en)* | 1993-01-07 | 1994-08-30 | Incontrol, Inc. | Telemetry system for an implantable cardiac device |
| US20050075693A1 (en)* | 2003-10-02 | 2005-04-07 | Medtronic, Inc. | Driver circuitry switchable between energy transfer and telemetry for an implantable medical device |
| US7272445B2 (en)* | 2003-10-02 | 2007-09-18 | Medtronic, Inc. | Medical device programmer with faceplate |
| US8346361B2 (en)* | 2003-10-02 | 2013-01-01 | Medtronic, Inc. | User interface for external charger for implantable medical device |
| US7330762B2 (en)* | 2004-06-07 | 2008-02-12 | Neuro And Cardiac Technologies, Llc | Method and system for providing pulsed electrical stimulation to provide therapy for erectile/sexual dysfunction, prostatitis, prostatitis pain, and chronic pelvic pain |
| US20090118796A1 (en)* | 2007-11-05 | 2009-05-07 | Advanced Bionics Corporation | External controller for an implantable medical device system with coupleable external charging coil assembly |
| US8447411B2 (en)* | 2008-07-11 | 2013-05-21 | Medtronic, Inc. | Patient interaction with posture-responsive therapy |
| US20100204756A1 (en)* | 2009-02-10 | 2010-08-12 | Boston Scientific Neuromodulation Corporation | External Device for Communicating with an Implantable Medical Device Having Data Telemetry and Charging Integrated in a Single Housing |
| US20110046698A1 (en)* | 2009-08-24 | 2011-02-24 | Medtronic, Inc. | Recovery of a wireless communication session with an implantable medical device |
| US20110184491A1 (en)* | 2010-01-28 | 2011-07-28 | Medtronic, Inc. | Wireless communication with an implantable medical device |
| US20110202103A1 (en)* | 2010-02-18 | 2011-08-18 | Birgitte Wikman | Wakeup of implantable communication circuitry |
Cited By (96)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US9474906B2 (en) | 2007-03-09 | 2016-10-25 | Mainstay Medical Limited | Systems and methods for restoring muscle function to the lumbar spine |
| US11679262B2 (en) | 2007-03-09 | 2023-06-20 | Mainstay Medical Limited | Systems and methods for restoring muscle function to the lumbar spine |
| US12168130B2 (en) | 2007-03-09 | 2024-12-17 | Mainstay Medical Limited | Systems and methods for restoring muscle function to the lumbar spine |
| US11951310B2 (en) | 2007-03-09 | 2024-04-09 | Mainstay Medical Limited | Systems and methods for restoring muscle function to the lumbar spine |
| US9072897B2 (en) | 2007-03-09 | 2015-07-07 | Mainstay Medical Limited | Systems and methods for restoring muscle function to the lumbar spine |
| US11331488B2 (en) | 2007-03-09 | 2022-05-17 | Mainstay Medical Limited | Systems and methods for enhancing function of spine stabilization muscles associated with a spine surgery intervention |
| US11103706B2 (en) | 2007-03-09 | 2021-08-31 | Mainstay Medical Limited | Systems and methods for enhancing function of spine stabilization muscles associated with a spine surgery intervention |
| US10016603B2 (en) | 2007-03-09 | 2018-07-10 | Mainstay Medical Limited | Systems and methods for restoring muscle function to the lumbar spine |
| US12285612B1 (en) | 2007-03-09 | 2025-04-29 | Mainstay Medical Limited | Systems and methods for restoring muscle function to the lumbar spine |
| US10828490B2 (en) | 2007-03-09 | 2020-11-10 | Mainstay Medical Limited | Systems and methods for restoring muscle function to the lumbar spine |
| US12121728B2 (en) | 2007-03-09 | 2024-10-22 | Mainstay Medical Limited | Systems and methods for enhancing function of spine stabilization muscles associated with a spine surgery intervention |
| US11679261B2 (en) | 2007-03-09 | 2023-06-20 | Mainstay Medical Limited | Systems and methods for enhancing function of spine stabilization muscles associated with a spine surgery intervention |
| US11684774B2 (en) | 2010-03-11 | 2023-06-27 | Mainstay Medical Limited | Electrical stimulator for treatment of back pain and methods of use |
| US9861811B2 (en) | 2010-03-11 | 2018-01-09 | Mainstay Medical Limited | Electrical stimulator for treatment of back pain and methods of use |
| US10661078B2 (en) | 2010-03-11 | 2020-05-26 | Mainstay Medical Limited | Modular stimulator for treatment of back pain, implantable RF ablation system and methods of use |
| US10926083B2 (en) | 2010-03-11 | 2021-02-23 | Mainstay Medical Limited | Stimulator for treatment of back pain utilizing feedback |
| US11471670B2 (en) | 2010-03-11 | 2022-10-18 | Mainstay Medical Limited | Electrical stimulator for treatment of back pain and methods of use |
| US12048844B2 (en) | 2010-03-11 | 2024-07-30 | Mainstay Medical Limited | Modular stimulator for treatment of back pain, implantable RF ablation system and methods of use |
| US12097365B2 (en) | 2010-03-11 | 2024-09-24 | Mainstay Medical Limited | Electrical stimulator for the treatment of back pain and methods of use |
| US11786725B2 (en) | 2012-06-13 | 2023-10-17 | Mainstay Medical Limited | Systems and methods for restoring muscle function to the lumbar spine and kits for implanting the same |
| US11376427B2 (en) | 2012-06-13 | 2022-07-05 | Mainstay Medical Limited | Systems and methods for restoring muscle function to the lumbar spine and kits for implanting the same |
| US10449355B2 (en) | 2012-06-13 | 2019-10-22 | Mainstay Medical Limited | Systems and methods for restoring muscle function to the lumbar spine and kits for implanting the same |
| US9981122B2 (en) | 2012-06-13 | 2018-05-29 | Mainstay Medical Limited | Systems and methods for implanting electrode leads for use with implantable neuromuscular electrical stimulator |
| US10195419B2 (en) | 2012-06-13 | 2019-02-05 | Mainstay Medical Limited | Electrode leads for use with implantable neuromuscular electrical stimulator |
| US9999763B2 (en) | 2012-06-13 | 2018-06-19 | Mainstay Medical Limited | Apparatus and methods for anchoring electrode leads adjacent to nervous tissue |
| US10644514B2 (en) | 2012-07-27 | 2020-05-05 | Tc1 Llc | Resonant power transfer systems with protective algorithm |
| US10291067B2 (en) | 2012-07-27 | 2019-05-14 | Tc1 Llc | Computer modeling for resonant power transfer systems |
| US10383990B2 (en) | 2012-07-27 | 2019-08-20 | Tc1 Llc | Variable capacitor for resonant power transfer systems |
| US10668197B2 (en) | 2012-07-27 | 2020-06-02 | Tc1 Llc | Resonant power transmission coils and systems |
| US10434235B2 (en) | 2012-07-27 | 2019-10-08 | Tci Llc | Thermal management for implantable wireless power transfer systems |
| US9805863B2 (en) | 2012-07-27 | 2017-10-31 | Thoratec Corporation | Magnetic power transmission utilizing phased transmitter coil arrays and phased receiver coil arrays |
| US9997928B2 (en) | 2012-07-27 | 2018-06-12 | Tc1 Llc | Self-tuning resonant power transfer systems |
| US10637303B2 (en) | 2012-07-27 | 2020-04-28 | Tc1 Llc | Magnetic power transmission utilizing phased transmitter coil arrays and phased receiver coil arrays |
| US10525181B2 (en) | 2012-07-27 | 2020-01-07 | Tc1 Llc | Resonant power transfer system and method of estimating system state |
| US10251987B2 (en) | 2012-07-27 | 2019-04-09 | Tc1 Llc | Resonant power transmission coils and systems |
| US9825471B2 (en) | 2012-07-27 | 2017-11-21 | Thoratec Corporation | Resonant power transfer systems with protective algorithm |
| US10277039B2 (en) | 2012-07-27 | 2019-04-30 | Tc1 Llc | Resonant power transfer systems with protective algorithm |
| US12017077B2 (en) | 2012-08-29 | 2024-06-25 | Boston Scientific Neuromodulation Corporation | System and method for identifying availability of clinician defined programming settings for a patient |
| US20140067015A1 (en)* | 2012-08-29 | 2014-03-06 | Boston Scientific Neuromodulation Corporation | System and method for identifying availability of clinician defined programming settings for a patient |
| US10828495B2 (en)* | 2012-08-29 | 2020-11-10 | Boston Scientific Neuromodulation Corporation | System and method for identifying availability of clinician defined programming settings for a patient |
| US10476317B2 (en) | 2013-03-15 | 2019-11-12 | Tci Llc | Integrated implantable TETs housing including fins and coil loops |
| US10373756B2 (en) | 2013-03-15 | 2019-08-06 | Tc1 Llc | Malleable TETs coil with improved anatomical fit |
| US10636566B2 (en) | 2013-03-15 | 2020-04-28 | Tc1 Llc | Malleable TETS coil with improved anatomical fit |
| US11722007B2 (en) | 2013-07-29 | 2023-08-08 | The Alfred E. Mann Foundation For Scientific Rsrch | Microprocessor controlled class E driver |
| US10971950B2 (en) | 2013-07-29 | 2021-04-06 | The Alfred E. Mann Foundation For Scientific Research | Microprocessor controlled class E driver |
| US10449377B2 (en) | 2013-07-29 | 2019-10-22 | The Alfred E. Mann Foundation For Scientific Research | High efficiency magnetic link for implantable devices |
| US9855436B2 (en) | 2013-07-29 | 2018-01-02 | Alfred E. Mann Foundation For Scientific Research | High efficiency magnetic link for implantable devices |
| US9780596B2 (en) | 2013-07-29 | 2017-10-03 | Alfred E. Mann Foundation For Scientific Research | Microprocessor controlled class E driver |
| US10447083B2 (en) | 2013-07-29 | 2019-10-15 | The Alfred E. Mann Foundation For Scientific Research | Microprocessor controlled class E driver |
| US11324953B2 (en) | 2013-08-09 | 2022-05-10 | Inspire Medical Systems, Inc. | Patient control for implantable medical device |
| US9950159B2 (en) | 2013-10-23 | 2018-04-24 | Mainstay Medical Limited | Systems and methods for restoring muscle function to the lumbar spine and kits for implanting the same |
| EP2865412A1 (en)* | 2013-10-23 | 2015-04-29 | Mainstay Medical Limited | System for restoring muscle function to the lumbar spine |
| EP3560552A1 (en)* | 2013-10-23 | 2019-10-30 | Mainstay Medical Limited | System for restoring muscle function to the lumbar spine |
| WO2015059570A1 (en)* | 2013-10-23 | 2015-04-30 | Mainstay Medical Limited | Systems and methods for restoring muscle function to the lumbar spine |
| US10695476B2 (en) | 2013-11-11 | 2020-06-30 | Tc1 Llc | Resonant power transfer systems with communications |
| JP2018201332A (en)* | 2013-11-11 | 2018-12-20 | ソーラテック エルエルシー | Resonance power transmission system with communication |
| JP2016535974A (en)* | 2013-11-11 | 2016-11-17 | ソーラテック コーポレイション | Resonant power transmission system with communication |
| WO2015070200A1 (en) | 2013-11-11 | 2015-05-14 | Thoratec Corporation | Resonant power transfer systems with communications |
| EP3072211A4 (en)* | 2013-11-11 | 2017-07-05 | Thoratec Corporation | Resonant power transfer systems with communications |
| US11179559B2 (en) | 2013-11-11 | 2021-11-23 | Tc1 Llc | Resonant power transfer systems with communications |
| US10615642B2 (en) | 2013-11-11 | 2020-04-07 | Tc1 Llc | Resonant power transfer systems with communications |
| US10873220B2 (en) | 2013-11-11 | 2020-12-22 | Tc1 Llc | Resonant power transfer systems with communications |
| US9855437B2 (en) | 2013-11-11 | 2018-01-02 | Tc1 Llc | Hinged resonant power transfer coil |
| US10610692B2 (en) | 2014-03-06 | 2020-04-07 | Tc1 Llc | Electrical connectors for implantable devices |
| US20160033308A1 (en)* | 2014-08-04 | 2016-02-04 | Infineon Technologies Ag | Intelligent gauge devices and related systems and methods |
| USD752763S1 (en)* | 2014-08-08 | 2016-03-29 | Inspire Medical Systems, Inc. | Patient control |
| US11245181B2 (en) | 2014-09-22 | 2022-02-08 | Tc1 Llc | Antenna designs for communication between a wirelessly powered implant to an external device outside the body |
| US10186760B2 (en) | 2014-09-22 | 2019-01-22 | Tc1 Llc | Antenna designs for communication between a wirelessly powered implant to an external device outside the body |
| US10265450B2 (en) | 2014-10-06 | 2019-04-23 | Tc1 Llc | Multiaxial connector for implantable devices |
| US10471268B2 (en) | 2014-10-16 | 2019-11-12 | Mainstay Medical Limited | Systems and methods for monitoring muscle rehabilitation |
| US11123569B2 (en) | 2015-01-09 | 2021-09-21 | Axonics, Inc. | Patient remote and associated methods of use with a nerve stimulation system |
| CN107427675A (en)* | 2015-01-09 | 2017-12-01 | 艾克索尼克斯调制技术股份有限公司 | Patient remote control and associated method for use with a neurostimulation system |
| US20160211064A1 (en)* | 2015-01-19 | 2016-07-21 | Industry-Academic Cooperation Foundation Chosun University | Wireless power charging apparatus using superconducting coil |
| US20170033567A1 (en)* | 2015-07-27 | 2017-02-02 | Apple Inc. | Charging apparatus for wearable electronic device |
| US10020668B2 (en)* | 2015-07-27 | 2018-07-10 | Apple Inc. | Charging apparatus for wearable electronic device |
| US10148126B2 (en) | 2015-08-31 | 2018-12-04 | Tc1 Llc | Wireless energy transfer system and wearables |
| US10770919B2 (en) | 2015-08-31 | 2020-09-08 | Tc1 Llc | Wireless energy transfer system and wearables |
| US10177604B2 (en) | 2015-10-07 | 2019-01-08 | Tc1 Llc | Resonant power transfer systems having efficiency optimization based on receiver impedance |
| US10804744B2 (en) | 2015-10-07 | 2020-10-13 | Tc1 Llc | Resonant power transfer systems having efficiency optimization based on receiver impedance |
| USD940880S1 (en) | 2015-12-16 | 2022-01-11 | Inspire Medical Systems, Inc. | Patient control |
| USD849252S1 (en) | 2015-12-16 | 2019-05-21 | Inspire Medical Systems, Inc. | Patient control |
| USD801538S1 (en) | 2015-12-16 | 2017-10-31 | Inspire Medical Systems, Inc. | Patient control |
| USD810306S1 (en) | 2016-03-24 | 2018-02-13 | Inspire Medical Systems, Inc. | Patient control |
| US11937847B2 (en) | 2016-07-05 | 2024-03-26 | Mainstay Medical Limited | Systems and methods for enhanced implantation of electrode leads between tissue layers |
| US10327810B2 (en) | 2016-07-05 | 2019-06-25 | Mainstay Medical Limited | Systems and methods for enhanced implantation of electrode leads between tissue layers |
| US11406421B2 (en) | 2016-07-05 | 2022-08-09 | Mainstay Medical Limited | Systems and methods for enhanced implantation of electrode leads between tissue layers |
| USD840357S1 (en)* | 2016-08-30 | 2019-02-12 | Qingdao Bright Medical Manufacturing Co., Ltd. | Control device |
| US10898292B2 (en) | 2016-09-21 | 2021-01-26 | Tc1 Llc | Systems and methods for locating implanted wireless power transmission devices |
| US11317988B2 (en) | 2016-09-21 | 2022-05-03 | Tc1 Llc | Systems and methods for locating implanted wireless power transmission devices |
| USD840358S1 (en)* | 2016-11-10 | 2019-02-12 | Qingdao Bright Medical Manufacturing Co., Ltd. | Control device |
| US11197990B2 (en) | 2017-01-18 | 2021-12-14 | Tc1 Llc | Systems and methods for transcutaneous power transfer using microneedles |
| US10770923B2 (en) | 2018-01-04 | 2020-09-08 | Tc1 Llc | Systems and methods for elastic wireless power transmission devices |
| US11642537B2 (en) | 2019-03-11 | 2023-05-09 | Axonics, Inc. | Charging device with off-center coil |
| WO2022162582A1 (en)* | 2021-01-27 | 2022-08-04 | Cochlear Limited | Heat reduction associated with prostheses |
| USD1010831S1 (en) | 2021-02-23 | 2024-01-09 | Inspire Medical Systems, Inc. | Patient control |
| CN116764094A (en)* | 2023-07-11 | 2023-09-19 | 四川君健万峰医疗器械有限责任公司 | Safety control method for electric shock dual-redundancy host |
Also Published As
| Publication number | Publication date |
|---|---|
| US11191964B2 (en) | 2021-12-07 |
| US20180236236A1 (en) | 2018-08-23 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| US11191964B2 (en) | Dual patient controllers | |
| US9878165B2 (en) | Patient programmer having a key-fob-sized form factor | |
| US8954148B2 (en) | Key fob controller for an implantable neurostimulator | |
| US10080897B2 (en) | IPG configured to deliver different pulse regimes to different leads for pudendal nerve stimulation | |
| US10245434B2 (en) | Systems, methods, and devices for evaluating lead placement based on patient physiological responses | |
| EP2762198B1 (en) | Techniques for controlling charging of batteries in an external charger and an implantable medical device | |
| US20100010582A1 (en) | Medical system and method for setting programmable heat limits | |
| US9345892B2 (en) | Transceiver duty cycled operational mode | |
| US9968795B2 (en) | Implantable neurostimulator devices including both non-rechargeable and rechargeable batteries and methods of use therewith | |
| WO2010087868A1 (en) | Transceiver duty cycled operational mode | |
| US20190105494A1 (en) | Portable electronic device with tens function | |
| WO2024259194A1 (en) | Wireless communication with medical devices |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| AS | Assignment | Owner name:GREATBATCH LTD., NEW YORK Free format text:ASSIGNMENT OF ASSIGNORS INTEREST;ASSIGNORS:WILDER, STEVEN;LABBE, MICHAEL;GAGNON, JEFF;AND OTHERS;SIGNING DATES FROM 20130314 TO 20130402;REEL/FRAME:030143/0527 | |
| AS | Assignment | Owner name:MANUFACTURERS AND TRADERS TRUST COMPANY, NEW YORK Free format text:SECURITY INTEREST;ASSIGNORS:GREATBATCH, INC.;GREATBATCH LTD.;ELECTROCHEM SOLUTIONS, INC.;AND OTHERS;REEL/FRAME:036980/0482 Effective date:20151027 | |
| AS | Assignment | Owner name:QIG GROUP, LLC, NEW YORK Free format text:ASSIGNMENT OF ASSIGNORS INTEREST;ASSIGNOR:GREATBATCH LTD.;REEL/FRAME:037810/0051 Effective date:20160212 | |
| AS | Assignment | Owner name:NUVECTRA CORPORATION, TEXAS Free format text:CHANGE OF NAME;ASSIGNOR:QIG GROUP, LLC;REEL/FRAME:038722/0050 Effective date:20160314 | |
| AS | Assignment | Owner name:QIG GROUP LLC, TEXAS Free format text:RELEASE BY SECURED PARTY;ASSIGNOR:MANUFACTURERS AND TRADERS TRUST COMPANY;REEL/FRAME:039132/0773 Effective date:20160418 Owner name:MICRO POWER ELECTRONICS, INC., OREGON Free format text:RELEASE BY SECURED PARTY;ASSIGNOR:MANUFACTURERS AND TRADERS TRUST COMPANY;REEL/FRAME:039132/0773 Effective date:20160418 Owner name:NEURONEXUS TECHNOLOGIES, INC., MICHIGAN Free format text:RELEASE BY SECURED PARTY;ASSIGNOR:MANUFACTURERS AND TRADERS TRUST COMPANY;REEL/FRAME:039132/0773 Effective date:20160418 Owner name:GREATBATCH INC., NEW YORK Free format text:RELEASE BY SECURED PARTY;ASSIGNOR:MANUFACTURERS AND TRADERS TRUST COMPANY;REEL/FRAME:039132/0773 Effective date:20160418 Owner name:GREATBATCH LTD., NEW YORK Free format text:RELEASE BY SECURED PARTY;ASSIGNOR:MANUFACTURERS AND TRADERS TRUST COMPANY;REEL/FRAME:039132/0773 Effective date:20160418 Owner name:ELECTROCHEM SOLUTIONS, INC., NEW YORK Free format text:RELEASE BY SECURED PARTY;ASSIGNOR:MANUFACTURERS AND TRADERS TRUST COMPANY;REEL/FRAME:039132/0773 Effective date:20160418 | |
| STCB | Information on status: application discontinuation | Free format text:ABANDONED -- AFTER EXAMINER'S ANSWER OR BOARD OF APPEALS DECISION | |
| AS | Assignment | Owner name:MICRO POWER ELECTRONICS, INC., NEW YORK Free format text:RELEASE BY SECURED PARTY;ASSIGNOR:MANUFACTURERS AND TRADERS TRUST COMPANY (AS ADMINISTRATIVE AGENT);REEL/FRAME:060938/0069 Effective date:20210903 Owner name:PRECIMED INC., NEW YORK Free format text:RELEASE BY SECURED PARTY;ASSIGNOR:MANUFACTURERS AND TRADERS TRUST COMPANY (AS ADMINISTRATIVE AGENT);REEL/FRAME:060938/0069 Effective date:20210903 Owner name:GREATBATCH-GLOBE TOOL, INC., NEW YORK Free format text:RELEASE BY SECURED PARTY;ASSIGNOR:MANUFACTURERS AND TRADERS TRUST COMPANY (AS ADMINISTRATIVE AGENT);REEL/FRAME:060938/0069 Effective date:20210903 Owner name:NEURONEXUS TECHNOLOGIES, INC., NEW YORK Free format text:RELEASE BY SECURED PARTY;ASSIGNOR:MANUFACTURERS AND TRADERS TRUST COMPANY (AS ADMINISTRATIVE AGENT);REEL/FRAME:060938/0069 Effective date:20210903 Owner name:ELECTROCHEM SOLUTIONS, INC., NEW YORK Free format text:RELEASE BY SECURED PARTY;ASSIGNOR:MANUFACTURERS AND TRADERS TRUST COMPANY (AS ADMINISTRATIVE AGENT);REEL/FRAME:060938/0069 Effective date:20210903 Owner name:GREATBATCH LTD., NEW YORK Free format text:RELEASE BY SECURED PARTY;ASSIGNOR:MANUFACTURERS AND TRADERS TRUST COMPANY (AS ADMINISTRATIVE AGENT);REEL/FRAME:060938/0069 Effective date:20210903 Owner name:GREATBATCH, INC., NEW YORK Free format text:RELEASE BY SECURED PARTY;ASSIGNOR:MANUFACTURERS AND TRADERS TRUST COMPANY (AS ADMINISTRATIVE AGENT);REEL/FRAME:060938/0069 Effective date:20210903 | |
| AS | Assignment | Owner name:MICRO POWER ELECTRONICS, INC., NEW YORK Free format text:RELEASE BY SECURED PARTY;ASSIGNOR:MANUFACTURERS AND TRADERS TRUST COMPANY (AS ADMINISTRATIVE AGENT);REEL/FRAME:061659/0858 Effective date:20210903 Owner name:PRECIMED INC., NEW YORK Free format text:RELEASE BY SECURED PARTY;ASSIGNOR:MANUFACTURERS AND TRADERS TRUST COMPANY (AS ADMINISTRATIVE AGENT);REEL/FRAME:061659/0858 Effective date:20210903 Owner name:GREATBATCH-GLOBE TOOL, INC., NEW YORK Free format text:RELEASE BY SECURED PARTY;ASSIGNOR:MANUFACTURERS AND TRADERS TRUST COMPANY (AS ADMINISTRATIVE AGENT);REEL/FRAME:061659/0858 Effective date:20210903 Owner name:NEURONEXUS TECHNOLOGIES, INC., NEW YORK Free format text:RELEASE BY SECURED PARTY;ASSIGNOR:MANUFACTURERS AND TRADERS TRUST COMPANY (AS ADMINISTRATIVE AGENT);REEL/FRAME:061659/0858 Effective date:20210903 Owner name:ELECTROCHEM SOLUTIONS, INC., NEW YORK Free format text:RELEASE BY SECURED PARTY;ASSIGNOR:MANUFACTURERS AND TRADERS TRUST COMPANY (AS ADMINISTRATIVE AGENT);REEL/FRAME:061659/0858 Effective date:20210903 Owner name:GREATBATCH LTD., NEW YORK Free format text:RELEASE BY SECURED PARTY;ASSIGNOR:MANUFACTURERS AND TRADERS TRUST COMPANY (AS ADMINISTRATIVE AGENT);REEL/FRAME:061659/0858 Effective date:20210903 Owner name:GREATBATCH, INC., NEW YORK Free format text:RELEASE BY SECURED PARTY;ASSIGNOR:MANUFACTURERS AND TRADERS TRUST COMPANY (AS ADMINISTRATIVE AGENT);REEL/FRAME:061659/0858 Effective date:20210903 |