US20060271169A1 - Stent with nanoporous surface - Google Patents
Stent with nanoporous surfaceDownload PDFInfo
- Publication number
- US20060271169A1 US20060271169A1US11/432,281US43228106AUS2006271169A1US 20060271169 A1US20060271169 A1US 20060271169A1US 43228106 AUS43228106 AUS 43228106AUS 2006271169 A1US2006271169 A1US 2006271169A1
- Authority
- US
- United States
- Prior art keywords
- alloy
- medical device
- porous layer
- porous
- stent
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Abandoned
Links
- 229910045601alloyInorganic materials0.000claimsabstractdescription67
- 239000000956alloySubstances0.000claimsabstractdescription67
- 238000000034methodMethods0.000claimsabstractdescription54
- 239000003814drugSubstances0.000claimsabstractdescription33
- 229940124597therapeutic agentDrugs0.000claimsabstractdescription28
- 229910052751metalInorganic materials0.000claimsabstractdescription12
- 239000002184metalSubstances0.000claimsabstractdescription12
- 239000003795chemical substances by applicationSubstances0.000claimsdescription20
- BASFCYQUMIYNBI-UHFFFAOYSA-NplatinumChemical compound[Pt]BASFCYQUMIYNBI-UHFFFAOYSA-N0.000claimsdescription20
- XEEYBQQBJWHFJM-UHFFFAOYSA-NIronChemical compound[Fe]XEEYBQQBJWHFJM-UHFFFAOYSA-N0.000claimsdescription18
- PXHVJJICTQNCMI-UHFFFAOYSA-NNickelChemical compound[Ni]PXHVJJICTQNCMI-UHFFFAOYSA-N0.000claimsdescription18
- PCHJSUWPFVWCPO-UHFFFAOYSA-NgoldChemical compound[Au]PCHJSUWPFVWCPO-UHFFFAOYSA-N0.000claimsdescription12
- 229910052737goldInorganic materials0.000claimsdescription12
- 239000010931goldSubstances0.000claimsdescription12
- KDLHZDBZIXYQEI-UHFFFAOYSA-NPalladiumChemical compound[Pd]KDLHZDBZIXYQEI-UHFFFAOYSA-N0.000claimsdescription10
- 229910052697platinumInorganic materials0.000claimsdescription10
- RTAQQCXQSZGOHL-UHFFFAOYSA-NTitaniumChemical compound[Ti]RTAQQCXQSZGOHL-UHFFFAOYSA-N0.000claimsdescription9
- 210000004204blood vesselAnatomy0.000claimsdescription9
- 229910052742ironInorganic materials0.000claimsdescription9
- 229910052759nickelInorganic materials0.000claimsdescription9
- 229910052709silverInorganic materials0.000claimsdescription9
- 239000004332silverSubstances0.000claimsdescription9
- 239000010936titaniumSubstances0.000claimsdescription9
- 229910052719titaniumInorganic materials0.000claimsdescription9
- 238000004519manufacturing processMethods0.000claimsdescription8
- VYZAMTAEIAYCRO-UHFFFAOYSA-NChromiumChemical compound[Cr]VYZAMTAEIAYCRO-UHFFFAOYSA-N0.000claimsdescription7
- 229910000831SteelInorganic materials0.000claimsdescription7
- 239000010959steelSubstances0.000claimsdescription7
- RYGMFSIKBFXOCR-UHFFFAOYSA-NCopperChemical compound[Cu]RYGMFSIKBFXOCR-UHFFFAOYSA-N0.000claimsdescription6
- BQCADISMDOOEFD-UHFFFAOYSA-NSilverChemical compound[Ag]BQCADISMDOOEFD-UHFFFAOYSA-N0.000claimsdescription6
- 229910052782aluminiumInorganic materials0.000claimsdescription6
- XAGFODPZIPBFFR-UHFFFAOYSA-NaluminiumChemical compound[Al]XAGFODPZIPBFFR-UHFFFAOYSA-N0.000claimsdescription6
- 229910052804chromiumInorganic materials0.000claimsdescription6
- 239000011651chromiumSubstances0.000claimsdescription6
- 229910052802copperInorganic materials0.000claimsdescription6
- 239000010949copperSubstances0.000claimsdescription6
- 229910001000nickel titaniumInorganic materials0.000claimsdescription6
- HLXZNVUGXRDIFK-UHFFFAOYSA-Nnickel titaniumChemical compound[Ti].[Ti].[Ti].[Ti].[Ti].[Ti].[Ti].[Ti].[Ti].[Ti].[Ti].[Ni].[Ni].[Ni].[Ni].[Ni].[Ni].[Ni].[Ni].[Ni].[Ni].[Ni].[Ni].[Ni].[Ni]HLXZNVUGXRDIFK-UHFFFAOYSA-N0.000claimsdescription6
- WAIPAZQMEIHHTJ-UHFFFAOYSA-N[Cr].[Co]Chemical group[Cr].[Co]WAIPAZQMEIHHTJ-UHFFFAOYSA-N0.000claimsdescription5
- 229910017052cobaltInorganic materials0.000claimsdescription5
- 239000010941cobaltSubstances0.000claimsdescription5
- GUTLYIVDDKVIGB-UHFFFAOYSA-Ncobalt atomChemical compound[Co]GUTLYIVDDKVIGB-UHFFFAOYSA-N0.000claimsdescription5
- 210000004351coronary vesselAnatomy0.000claimsdescription5
- 229910052758niobiumInorganic materials0.000claimsdescription5
- 239000010955niobiumSubstances0.000claimsdescription5
- GUCVJGMIXFAOAE-UHFFFAOYSA-Nniobium atomChemical compound[Nb]GUCVJGMIXFAOAE-UHFFFAOYSA-N0.000claimsdescription5
- 229910052763palladiumInorganic materials0.000claimsdescription5
- 229910052715tantalumInorganic materials0.000claimsdescription5
- GUVRBAGPIYLISA-UHFFFAOYSA-Ntantalum atomChemical compound[Ta]GUVRBAGPIYLISA-UHFFFAOYSA-N0.000claimsdescription5
- WFKWXMTUELFFGS-UHFFFAOYSA-NtungstenChemical compound[W]WFKWXMTUELFFGS-UHFFFAOYSA-N0.000claimsdescription5
- 229910052721tungstenInorganic materials0.000claimsdescription5
- 239000010937tungstenSubstances0.000claimsdescription5
- 229910052720vanadiumInorganic materials0.000claimsdescription5
- LEONUFNNVUYDNQ-UHFFFAOYSA-Nvanadium atomChemical compound[V]LEONUFNNVUYDNQ-UHFFFAOYSA-N0.000claimsdescription5
- 239000002260anti-inflammatory agentSubstances0.000claimsdescription4
- 229940121363anti-inflammatory agentDrugs0.000claimsdescription4
- 229940079593drugDrugs0.000claimsdescription4
- 208000037803restenosisDiseases0.000claimsdescription4
- 238000000151depositionMethods0.000claimsdescription3
- 229910000684Cobalt-chromeInorganic materials0.000claims2
- 239000010952cobalt-chromeSubstances0.000claims2
- 238000000137annealingMethods0.000claims1
- 239000011230binding agentSubstances0.000claims1
- 230000002401inhibitory effectEffects0.000claims1
- 239000011159matrix materialSubstances0.000abstractdescription6
- 150000002739metalsChemical class0.000abstractdescription3
- 229910052755nonmetalInorganic materials0.000abstractdescription3
- 230000002708enhancing effectEffects0.000abstract1
- 239000010410layerSubstances0.000description103
- 239000002243precursorSubstances0.000description14
- 239000011148porous materialSubstances0.000description12
- 239000000126substanceSubstances0.000description12
- 239000000463materialSubstances0.000description11
- 238000000576coating methodMethods0.000description10
- 239000011248coating agentSubstances0.000description7
- HEMHJVSKTPXQMS-UHFFFAOYSA-MSodium hydroxideChemical compound[OH-].[Na+]HEMHJVSKTPXQMS-UHFFFAOYSA-M0.000description6
- -1poly(ethylene glycol)Polymers0.000description5
- NOESYZHRGYRDHS-UHFFFAOYSA-NinsulinChemical compoundN1C(=O)C(NC(=O)C(CCC(N)=O)NC(=O)C(CCC(O)=O)NC(=O)C(C(C)C)NC(=O)C(NC(=O)CN)C(C)CC)CSSCC(C(NC(CO)C(=O)NC(CC(C)C)C(=O)NC(CC=2C=CC(O)=CC=2)C(=O)NC(CCC(N)=O)C(=O)NC(CC(C)C)C(=O)NC(CCC(O)=O)C(=O)NC(CC(N)=O)C(=O)NC(CC=2C=CC(O)=CC=2)C(=O)NC(CSSCC(NC(=O)C(C(C)C)NC(=O)C(CC(C)C)NC(=O)C(CC=2C=CC(O)=CC=2)NC(=O)C(CC(C)C)NC(=O)C(C)NC(=O)C(CCC(O)=O)NC(=O)C(C(C)C)NC(=O)C(CC(C)C)NC(=O)C(CC=2NC=NC=2)NC(=O)C(CO)NC(=O)CNC2=O)C(=O)NCC(=O)NC(CCC(O)=O)C(=O)NC(CCCNC(N)=N)C(=O)NCC(=O)NC(CC=3C=CC=CC=3)C(=O)NC(CC=3C=CC=CC=3)C(=O)NC(CC=3C=CC(O)=CC=3)C(=O)NC(C(C)O)C(=O)N3C(CCC3)C(=O)NC(CCCCN)C(=O)NC(C)C(O)=O)C(=O)NC(CC(N)=O)C(O)=O)=O)NC(=O)C(C(C)CC)NC(=O)C(CO)NC(=O)C(C(C)O)NC(=O)C1CSSCC2NC(=O)C(CC(C)C)NC(=O)C(NC(=O)C(CCC(N)=O)NC(=O)C(CC(N)=O)NC(=O)C(NC(=O)C(N)CC=1C=CC=CC=1)C(C)C)CC1=CN=CN1NOESYZHRGYRDHS-UHFFFAOYSA-N0.000description4
- 239000000203mixtureSubstances0.000description4
- 229910001220stainless steelInorganic materials0.000description4
- 239000010935stainless steelSubstances0.000description4
- 239000000758substrateSubstances0.000description4
- 239000000560biocompatible materialSubstances0.000description3
- 230000015572biosynthetic processEffects0.000description3
- 238000007887coronary angioplastyMethods0.000description3
- PQTCMBYFWMFIGM-UHFFFAOYSA-Ngold silverChemical compound[Ag].[Au]PQTCMBYFWMFIGM-UHFFFAOYSA-N0.000description3
- 239000007943implantSubstances0.000description3
- 238000001802infusionMethods0.000description3
- 229910001316Ag alloyInorganic materials0.000description2
- WQZGKKKJIJFFOK-GASJEMHNSA-NGlucoseNatural productsOC[C@H]1OC(O)[C@H](O)[C@@H](O)[C@@H]1OWQZGKKKJIJFFOK-GASJEMHNSA-N0.000description2
- 102000004877InsulinHuman genes0.000description2
- 108090001061InsulinProteins0.000description2
- GRYLNZFGIOXLOG-UHFFFAOYSA-NNitric acidChemical compoundO[N+]([O-])=OGRYLNZFGIOXLOG-UHFFFAOYSA-N0.000description2
- 208000031481Pathologic ConstrictionDiseases0.000description2
- NBIIXXVUZAFLBC-UHFFFAOYSA-NPhosphoric acidChemical compoundOP(O)(O)=ONBIIXXVUZAFLBC-UHFFFAOYSA-N0.000description2
- QAOWNCQODCNURD-UHFFFAOYSA-NSulfuric acidChemical compoundOS(O)(=O)=OQAOWNCQODCNURD-UHFFFAOYSA-N0.000description2
- 230000001464adherent effectEffects0.000description2
- QVGXLLKOCUKJST-UHFFFAOYSA-Natomic oxygenChemical compound[O]QVGXLLKOCUKJST-UHFFFAOYSA-N0.000description2
- WQZGKKKJIJFFOK-VFUOTHLCSA-Nbeta-D-glucoseChemical compoundOC[C@H]1O[C@@H](O)[C@H](O)[C@@H](O)[C@@H]1OWQZGKKKJIJFFOK-VFUOTHLCSA-N0.000description2
- 230000000694effectsEffects0.000description2
- 238000010828elutionMethods0.000description2
- 239000008103glucoseSubstances0.000description2
- 238000007654immersionMethods0.000description2
- 210000000987immune systemAnatomy0.000description2
- 229940125396insulinDrugs0.000description2
- 239000007788liquidSubstances0.000description2
- 229910017604nitric acidInorganic materials0.000description2
- 229910052760oxygenInorganic materials0.000description2
- 239000001301oxygenSubstances0.000description2
- 239000002245particleSubstances0.000description2
- ZAHRKKWIAAJSAO-UHFFFAOYSA-NrapamycinNatural productsCOCC(O)C(=C/C(C)C(=O)CC(OC(=O)C1CCCCN1C(=O)C(=O)C2(O)OC(CC(OC)C(=CC=CC=CC(C)CC(C)C(=O)C)C)CCC2C)C(C)CC3CCC(O)C(C3)OC)CZAHRKKWIAAJSAO-UHFFFAOYSA-N0.000description2
- 150000003839saltsChemical class0.000description2
- QFJCIRLUMZQUOT-HPLJOQBZSA-NsirolimusChemical compoundC1C[C@@H](O)[C@H](OC)C[C@@H]1C[C@@H](C)[C@H]1OC(=O)[C@@H]2CCCCN2C(=O)C(=O)[C@](O)(O2)[C@H](C)CC[C@H]2C[C@H](OC)/C(C)=C/C=C/C=C/[C@@H](C)C[C@@H](C)C(=O)[C@H](OC)[C@H](O)/C(C)=C/[C@@H](C)C(=O)C1QFJCIRLUMZQUOT-HPLJOQBZSA-N0.000description2
- 229960002930sirolimusDrugs0.000description2
- 208000037804stenosisDiseases0.000description2
- 239000001117sulphuric acidSubstances0.000description2
- 235000011149sulphuric acidNutrition0.000description2
- 238000002560therapeutic procedureMethods0.000description2
- 229910000851Alloy steelInorganic materials0.000description1
- 229910001020Au alloyInorganic materials0.000description1
- 229910001369BrassInorganic materials0.000description1
- 229910000881Cu alloyInorganic materials0.000description1
- 102000014150InterferonsHuman genes0.000description1
- 108010050904InterferonsProteins0.000description1
- 229930012538PaclitaxelNatural products0.000description1
- 230000004075alterationEffects0.000description1
- PNEYBMLMFCGWSK-UHFFFAOYSA-Naluminium oxideInorganic materials[O-2].[O-2].[O-2].[Al+3].[Al+3]PNEYBMLMFCGWSK-UHFFFAOYSA-N0.000description1
- 229910000147aluminium phosphateInorganic materials0.000description1
- 230000002769anti-restenotic effectEffects0.000description1
- 239000003146anticoagulant agentSubstances0.000description1
- 229940127090anticoagulant agentDrugs0.000description1
- 230000003115biocidal effectEffects0.000description1
- 239000010951brassSubstances0.000description1
- 230000001413cellular effectEffects0.000description1
- 239000000919ceramicSubstances0.000description1
- 238000005229chemical vapour depositionMethods0.000description1
- 238000013270controlled releaseMethods0.000description1
- WBLJAACUUGHPMU-UHFFFAOYSA-Ncopper platinumChemical compound[Cu].[Pt]WBLJAACUUGHPMU-UHFFFAOYSA-N0.000description1
- 230000007797corrosionEffects0.000description1
- 238000005260corrosionMethods0.000description1
- 230000008021depositionEffects0.000description1
- 238000004090dissolutionMethods0.000description1
- 238000005566electron beam evaporationMethods0.000description1
- 238000000635electron micrographMethods0.000description1
- 239000003527fibrinolytic agentSubstances0.000description1
- 239000003353gold alloySubstances0.000description1
- 238000002513implantationMethods0.000description1
- 238000011065in-situ storageMethods0.000description1
- 229940079322interferonDrugs0.000description1
- 238000005468ion implantationMethods0.000description1
- 150000002500ionsChemical class0.000description1
- 210000004153islets of langerhanAnatomy0.000description1
- 230000014759maintenance of locationEffects0.000description1
- 229910001092metal group alloyInorganic materials0.000description1
- 238000011275oncology therapyMethods0.000description1
- 230000000399orthopedic effectEffects0.000description1
- 229960001592paclitaxelDrugs0.000description1
- 238000002161passivationMethods0.000description1
- 238000005240physical vapour depositionMethods0.000description1
- 229920001223polyethylene glycolPolymers0.000description1
- 239000000843powderSubstances0.000description1
- XOFYZVNMUHMLCC-ZPOLXVRWSA-NprednisoneChemical compoundO=C1C=C[C@]2(C)[C@H]3C(=O)C[C@](C)([C@@](CC4)(O)C(=O)CO)[C@@H]4[C@@H]3CCC2=C1XOFYZVNMUHMLCC-ZPOLXVRWSA-N0.000description1
- 229960004618prednisoneDrugs0.000description1
- 239000011253protective coatingSubstances0.000description1
- 239000011241protective layerSubstances0.000description1
- 108090000623proteins and genesProteins0.000description1
- 238000004549pulsed laser depositionMethods0.000description1
- 238000001878scanning electron micrographMethods0.000description1
- 239000002904solventSubstances0.000description1
- 238000004544sputter depositionMethods0.000description1
- 229910001256stainless steel alloyInorganic materials0.000description1
- 230000036262stenosisEffects0.000description1
- 230000002966stenotic effectEffects0.000description1
- RCINICONZNJXQF-MZXODVADSA-NtaxolChemical compoundO([C@@H]1[C@@]2(C[C@@H](C(C)=C(C2(C)C)[C@H](C([C@]2(C)[C@@H](O)C[C@H]3OC[C@]3([C@H]21)OC(C)=O)=O)OC(=O)C)OC(=O)[C@H](O)[C@@H](NC(=O)C=1C=CC=CC=1)C=1C=CC=CC=1)O)C(=O)C1=CC=CC=C1RCINICONZNJXQF-MZXODVADSA-N0.000description1
- 230000001225therapeutic effectEffects0.000description1
- 238000002207thermal evaporationMethods0.000description1
- 230000002792vascularEffects0.000description1
Images
Classifications
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61L—METHODS OR APPARATUS FOR STERILISING MATERIALS OR OBJECTS IN GENERAL; DISINFECTION, STERILISATION OR DEODORISATION OF AIR; CHEMICAL ASPECTS OF BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES; MATERIALS FOR BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES
- A61L31/00—Materials for other surgical articles, e.g. stents, stent-grafts, shunts, surgical drapes, guide wires, materials for adhesion prevention, occluding devices, surgical gloves, tissue fixation devices
- A61L31/14—Materials characterised by their function or physical properties, e.g. injectable or lubricating compositions, shape-memory materials, surface modified materials
- A61L31/146—Porous materials, e.g. foams or sponges
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61L—METHODS OR APPARATUS FOR STERILISING MATERIALS OR OBJECTS IN GENERAL; DISINFECTION, STERILISATION OR DEODORISATION OF AIR; CHEMICAL ASPECTS OF BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES; MATERIALS FOR BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES
- A61L27/00—Materials for grafts or prostheses or for coating grafts or prostheses
- A61L27/02—Inorganic materials
- A61L27/04—Metals or alloys
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F2/00—Filters implantable into blood vessels; Prostheses, i.e. artificial substitutes or replacements for parts of the body; Appliances for connecting them with the body; Devices providing patency to, or preventing collapsing of, tubular structures of the body, e.g. stents
- A61F2/02—Prostheses implantable into the body
- A61F2/04—Hollow or tubular parts of organs, e.g. bladders, tracheae, bronchi or bile ducts
- A61F2/06—Blood vessels
- A61F2/07—Stent-grafts
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F2/00—Filters implantable into blood vessels; Prostheses, i.e. artificial substitutes or replacements for parts of the body; Appliances for connecting them with the body; Devices providing patency to, or preventing collapsing of, tubular structures of the body, e.g. stents
- A61F2/82—Devices providing patency to, or preventing collapsing of, tubular structures of the body, e.g. stents
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61L—METHODS OR APPARATUS FOR STERILISING MATERIALS OR OBJECTS IN GENERAL; DISINFECTION, STERILISATION OR DEODORISATION OF AIR; CHEMICAL ASPECTS OF BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES; MATERIALS FOR BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES
- A61L27/00—Materials for grafts or prostheses or for coating grafts or prostheses
- A61L27/02—Inorganic materials
- A61L27/04—Metals or alloys
- A61L27/06—Titanium or titanium alloys
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61L—METHODS OR APPARATUS FOR STERILISING MATERIALS OR OBJECTS IN GENERAL; DISINFECTION, STERILISATION OR DEODORISATION OF AIR; CHEMICAL ASPECTS OF BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES; MATERIALS FOR BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES
- A61L31/00—Materials for other surgical articles, e.g. stents, stent-grafts, shunts, surgical drapes, guide wires, materials for adhesion prevention, occluding devices, surgical gloves, tissue fixation devices
- A61L31/08—Materials for coatings
- A61L31/082—Inorganic materials
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61L—METHODS OR APPARATUS FOR STERILISING MATERIALS OR OBJECTS IN GENERAL; DISINFECTION, STERILISATION OR DEODORISATION OF AIR; CHEMICAL ASPECTS OF BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES; MATERIALS FOR BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES
- A61L31/00—Materials for other surgical articles, e.g. stents, stent-grafts, shunts, surgical drapes, guide wires, materials for adhesion prevention, occluding devices, surgical gloves, tissue fixation devices
- A61L31/14—Materials characterised by their function or physical properties, e.g. injectable or lubricating compositions, shape-memory materials, surface modified materials
- A61L31/16—Biologically active materials, e.g. therapeutic substances
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F2/00—Filters implantable into blood vessels; Prostheses, i.e. artificial substitutes or replacements for parts of the body; Appliances for connecting them with the body; Devices providing patency to, or preventing collapsing of, tubular structures of the body, e.g. stents
- A61F2/82—Devices providing patency to, or preventing collapsing of, tubular structures of the body, e.g. stents
- A61F2/86—Stents in a form characterised by the wire-like elements; Stents in the form characterised by a net-like or mesh-like structure
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61L—METHODS OR APPARATUS FOR STERILISING MATERIALS OR OBJECTS IN GENERAL; DISINFECTION, STERILISATION OR DEODORISATION OF AIR; CHEMICAL ASPECTS OF BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES; MATERIALS FOR BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES
- A61L2300/00—Biologically active materials used in bandages, wound dressings, absorbent pads or medical devices
- A61L2300/40—Biologically active materials used in bandages, wound dressings, absorbent pads or medical devices characterised by a specific therapeutic activity or mode of action
- A61L2300/41—Anti-inflammatory agents, e.g. NSAIDs
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61L—METHODS OR APPARATUS FOR STERILISING MATERIALS OR OBJECTS IN GENERAL; DISINFECTION, STERILISATION OR DEODORISATION OF AIR; CHEMICAL ASPECTS OF BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES; MATERIALS FOR BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES
- A61L2300/00—Biologically active materials used in bandages, wound dressings, absorbent pads or medical devices
- A61L2300/40—Biologically active materials used in bandages, wound dressings, absorbent pads or medical devices characterised by a specific therapeutic activity or mode of action
- A61L2300/416—Anti-neoplastic or anti-proliferative or anti-restenosis or anti-angiogenic agents, e.g. paclitaxel, sirolimus
- Y—GENERAL TAGGING OF NEW TECHNOLOGICAL DEVELOPMENTS; GENERAL TAGGING OF CROSS-SECTIONAL TECHNOLOGIES SPANNING OVER SEVERAL SECTIONS OF THE IPC; TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y10—TECHNICAL SUBJECTS COVERED BY FORMER USPC
- Y10S—TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y10S623/00—Prosthesis, i.e. artificial body members, parts thereof, or aids and accessories therefor
- Y10S623/901—Method of manufacturing prosthetic device
- Y—GENERAL TAGGING OF NEW TECHNOLOGICAL DEVELOPMENTS; GENERAL TAGGING OF CROSS-SECTIONAL TECHNOLOGIES SPANNING OVER SEVERAL SECTIONS OF THE IPC; TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y10—TECHNICAL SUBJECTS COVERED BY FORMER USPC
- Y10T—TECHNICAL SUBJECTS COVERED BY FORMER US CLASSIFICATION
- Y10T428/00—Stock material or miscellaneous articles
- Y10T428/12—All metal or with adjacent metals
- Y10T428/12458—All metal or with adjacent metals having composition, density, or hardness gradient
Definitions
- the present inventionrelates generally to medical devices and methods for making same. More specifically, the invention relates to implantable medical devices having at least one porous layer, and methods for making such devices.
- Implantable medical devicesare increasingly being used to deliver one or more therapeutic agents to a site within a body. Such agents may provide their own benefits to treatment and/or may enhance the efficacy of the implantable device. For example, much research has been conducted into the use of drug eluting stents for use in percutaneous transluminal coronary angioplasty (PTCA) procedures. Although some implantable devices are simply coated with one or more therapeutic agents, other devices include means for containing, attaching or otherwise holding therapeutic agents to provide the agents at a treatment location over a longer duration, in a controlled-release manner, or the like.
- PTCApercutaneous transluminal coronary angioplasty
- Porous materialsare commonly used in medical implants as matrices for the retention of therapeutic agents.
- Materials that have been used for this purposeinclude ceramics such as hydroxyapatites and porous alumina, as well as sintered metal powders.
- Polymeric materialssuch as poly(ethylene glycol)/poly(L-lactic acid) (PLGA) have also been used for this purpose. These materials are typically applied as coatings to the medical implant, raising issues regarding coating adhesion, mechanical properties, and material biocompatibility. Further, application of these coatings introduces additional complexity to the fabrication process, increasing overall production costs.
- a method of fabricating an implantable medical device having a porous layer for releasably containing at least one therapeutic agentincludes providing an implantable medical device comprising at least one alloy and removing at least one component of the alloy to form the porous layer.
- the componentis removed to form the porous layer as a biocompatible material, such as gold.
- the medical devicecomprises a tubular stent device having an outer surface and an inner surface.
- the stent devicemay comprise a coronary artery stent for use in a percutaneous transluminal coronary angioplasty (PTCA) procedure.
- PTCApercutaneous transluminal coronary angioplasty
- the alloyis disposed along the outer surface of the stent device.
- providing the implantable medical devicemay also include depositing the alloy on at least one surface of the medical device.
- the alloymay be disposed along an outer surface of the implantable medical device, such that the dissolving step forms the porous layer on the outer surface of the device.
- the alloyincludes one or more metals, such as but not limited to gold, silver, nitinol, steel, chromium, iron, nickel, copper, aluminum, titanium, tantalum, cobalt, tungsten, palladium, vanadium, platinum and/or niobium.
- the alloycomprises at least one metal and at least one non-metal.
- at least one substancemay be embedded within the alloy. For example, a salt or an oxide particle may be embedded in the alloy to enhance pore formation upon dissolution.
- Dissolving one or more components of the alloymay involve exposing the alloy to a dissolving substance.
- a dissolving substanceFor example, a stainless steel alloy may be exposed to sodium hydroxide in one embodiment.
- one or more of the most electrochemically active components of the alloyare dissolved.
- additional processingmay be performed.
- the devicemay be coated after the dissolving step with titanium, gold and/or platinum.
- Some embodimentsfurther include introducing at least one therapeutic agent into the porous layer.
- the therapeutic agentmay be introduced by liquid immersion, vacuum dessication, high pressure infusion or vapor loading in various embodiments.
- the therapeutic agentmay be any suitable agent or combination of agents, such as but not limited to anti-restenotic agent(s) or anti-inflammatory agent(s), such as Rapamycin, Sirolimus, Taxol, Prednisone, and/or the like.
- live cellsmay be encapsulated by the porous layer, thereby allowing transport of selected molecules, such as oxygen, glucose, or insulin, to and from the cells, while shielding the cells from the immune system of the patient.
- Some embodimentsmay optionally include multiple porous layers having various porosities and atomic compositions.
- a method for treating a blood vessel using an implantable medical device having a porous layer for releasably containing at least one therapeutic agentincludes: providing at least one implantable stent having a porous layer for releasably containing at least one therapeutic agent; and placing the stent within the blood vessel at a desired location, wherein the stent releases the at least one therapeutic agent from the porous layer after placement.
- the desired locationmay comprise an area of stenosis in the blood vessel, and the at least one therapeutic agent may inhibit re-stenosis of the blood vessel.
- the therapeutic agentin some embodiments may be one or more anti-restenosis agents, anti-inflammatory agents, or a combination of both.
- the blood vesselmay be a coronary artery.
- the placing stepmay involve placing the stent so as to contact the porous layer with at least one of a stenotic plaque in the blood vessel and an inner wall of the blood vessel.
- an implantable medical devicehas at least one porous layer comprising at least one remaining alloy component and interstitial spaces, wherein the interstitial spaces comprise at least one removed alloy component space of an alloy, the alloy comprising the at least one remaining alloy component and the at least one removed alloy component.
- the porous layercomprises a matrix.
- the implantable medical devicecomprises an implantable stent device having an outer surface and an inner surface, and the porous layer is disposed along the outer surface.
- the stent devicemay comprise a coronary artery stent for use in a percutaneous transluminal coronary angioplasty procedure.
- the alloymay comprise one or more metals selected from the group consisting of gold, silver, nitinol, steel, chromium, iron, nickel, copper, aluminum, titanium, tantalum, cobalt, tungsten, palladium, vanadium, platinum and/or niobium.
- the alloymay comprise stainless steel and the porous layer may comprise iron and nickel.
- the component (or components) that is dissolvedcomprises a most electrochemically active component of the alloy.
- the devicefurther includes at least one therapeutic agent disposed within the at least one porous layer. Any such agent or combination of agents is contemplated.
- the devicemay include a titanium or platinum coating over an outer surface of the device.
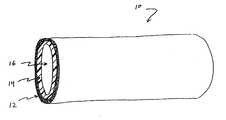
- FIG. 1is a perspective view of an implantable stent device having a porous layer according to one embodiment of the present invention.
- FIGS. 2A-2Bare electron micrographs of a porous layer formed by dissolving silver from a gold-silver alloy, according to one embodiment of the present invention.
- FIGS. 3A-3Care cross-sectional side views showing a method of making an implantable stent device having a porous layer, according to one embodiment of the present invention.
- Methods of the present inventionprovide means for fabricating an implantable medical device having at least one porous layer.
- the methodsinvolve providing an implantable medical device containing an alloy and removing at least one component of the alloy to form the porous layer.
- an alloymay first be deposited on an implantable device and one or more components of the alloy may then be removed to form the porous layer.
- dealloyingSuch methods are often referred to as “dealloying.”
- dealloying methodsFor a general description of dealloying methods, reference may be made to “Evolution of nanoporosity in dealloying,” Jonah Erlebacher et al., Nature 410, pp. 450-453, March 2001, the entire contents of which are hereby incorporated by reference.
- Dealloying a layer of an implantable deviceprovides a porous layer, which may then be infused with one or more therapeutic agents for providing delivery of an agent into a patient via the device.
- Use of dealloying methodswill typically provide more adherent and mechanically robust porous layers on medical implantables than are currently available, while also simplifying device manufacture.
- any suitable implantable medical devicemay be fabricated with methods of the invention.
- Other devicesmay include, but are not limited to, other stents, stent-grafts, implantable leads, infusion pumps, vascular access devices such as implantable ports, orthopedic implants, implantable electrodes, and the like.
- devices fabricated via methods of the present inventionmay be used to deliver any suitable therapy or combination of therapies in a patient care context, veterinary context, research setting or the like.
- Therapeutic agentsmay include, for example, drugs, genes, anti-restenosis agents, anti-thrombogenic agents, antibiotic agents, anti-clotting agents, anti-inflammatory agents, cancer therapy agents and/or the like.
- an implantable medical device fabricated by methods of the present inventionmay include an elongate stent device 10 , having two or more layers 12 , 14 and a lumen 16 .
- stent device 10includes an outer porous layer 12 and an inner non-porous layer 14 .
- Other embodimentsmay suitably include an inner porous layer 12 and an outer non-porous layer 14 , multiple porous layers 12 , multiple non-porous layers 14 , a porous coating over an entire surface of a medical device, or any combination of porous and non-porous surfaces, layers, areas or the like to provide a desired effect.
- porous layer 12 and non-porous layer 14may have any suitable thicknesses in various embodiments.
- a very thin porous layer 12may be desired, such as for delivery of a comparatively small amount of therapeutic agent.
- a thicker porous layer 12may be used for delivery of a larger quantity of therapeutic agent and/or for a longer duration of agent delivery.
- Any suitable combination and configuration of porous layer 12 and non-porous layer 14is contemplated.
- porous layer 12may comprise the entire thickness of stent device 10 , so that the device is completely porous.
- stent device 10is only one example of a device with which porous layers may be used. Other devices may not have a lumen, for example, but may still be suitable for use in the present invention.
- any medical devicemay be fabricated with one or more porous layers 12 according to embodiments of the present invention.
- the deviceis an implantable stent device 10
- any suitable type, size and configuration of stent devicemay be fabricated with one or more porous layers 12 .
- stent device 10comprises an expandable stent for implantation in a coronary artery during a PTCA procedure.
- Such a stent device 10may be fabricated from any suitable material or combination of materials.
- stent device 10comprises a stainless steel non-porous layer 14 arid an iron and nickel porous layer 12 .
- porous layer 12may be formed of a biocompatible material, such as gold.
- porous layer 12may be formed from a cobalt-chromium alloy such as L605. Any other suitable material or combination of materials is contemplated.
- stent device 10may include a layer or coating comprising a biocompatible material such as titanium, gold or platinum, which may provide biocompatibility, corrosion resistance or both.
- porous layer 12is shown in greater detail. Porous layer 12 in these figures was made by selectively dissolving silver from a gold-silver alloy. As can be seen from the scanning electron micrographs, porous layer 12 comprises a matrix of pores and structural elements. In any given embodiment, the size and density of such pores may be varied by varying one or more elements of a method for making the device and forming porous layer 12 . For example, one or more components of an alloy, a substance used to selectively dissolve the alloy, duration time of exposing the alloy to the dissolving substance, or the like may be chosen to give porous layer 12 certain desired characteristics. Thermal anneals prior or subsequent to the dealloying process may also be performed to vary pore size and density. Any suitable combination of porous layer thickness, pore size, pore density and the like is contemplated within the scope of the present invention.
- any implantable medical device of the present inventionmay include one or more therapeutic agents disposed within one or more porous layers 12 .
- any agent or combination of agentsmay be included.
- any suitable method for introducing an agent into a porous layermay be used.
- a method for fabricating an implantable medical device 20 having a porous layersuitably includes providing an implantable device comprising at least one alloy and removing at least one component of the alloy to form the porous layer.
- a medical device 20such as a stent may include a precursor alloy layer 22 , a substrate layer 24 and a lumen.
- Precursor alloy layer 22can be deposited onto substrate layer 24 by various processes, including but not limited to physical vapor deposition, ion implantation, sputter deposition, thermal or electron beam evaporation, chemical vapor deposition, pulsed laser deposition, or the like.
- precursor alloy layer 22may be synthesized in situ from various materials, as described previously, such that exposure to the dealloying process will remove the sacrificial component of precursor alloy layer 22 , leaving behind a porous matrix.
- precursor alloy layer 22 and substrate layer 24may be made from the same material.
- medical device 20may comprise any suitable stent or other device and precursor alloy layer 22 , substrate layer 24 and/or other layers may be given any suitable configurations, thicknesses and the like.
- precursor alloy layer 22is disposed along an outer surface of device 20 , while in others precursor alloy layer 22 may be disposed along an inner surface, both inner and outer surfaces, or the like.
- the alloy used to form precursor alloy layer 22may comprise any suitable alloy and may be a metal-metal alloy or a metal-non-metal alloy.
- components of precursor alloy layer 22may include steel, nitinol, chromium, brass, copper, iron, nickel, aluminum, titanium, gold, silver, tantalum, cobalt, tungsten, palladium, vanadium, platinum and/or niobium.
- one or more additional substancesmay be embedded within precursor alloy layer 22 to cause or enhance pore formation during the fabrication process. For example, a salt, an oxide particle or the like may be added to precursor alloy layer 22 to enhance pore formation.
- implantable medical device 20is typically exposed to a substance (arrows) to dissolve or otherwise remove at least one component of the alloy to form the porous layer from precursor alloy layer 22 .
- a substancearrows
- any suitable substancemay be used for removing at least one component of the alloy.
- the alloycomprises stainless steel, such as 316L stainless steel, and dissolving at least one component of the steel comprises exposing the steel to hot sodium hydroxide to dissolve chromium and leave iron and nickel as the porous layer.
- a silver-gold alloymay be exposed to nitric acid to dissolve the silver and leave the gold as the porous layer (as shown in FIGS. 2A and 2B ).
- a cobalt-chromium alloysuch as L605
- a sacrificial materialsuch as silver, copper or aluminum
- an appropriate solventsuch as nitric acid, sulphuric acid or phosphoric acid
- a platinum-copper alloyis dealloyed in the presence of sulphuric acid to produce porous platinum.
- nitinolmay be dissolved by a suitable dissolving substance to leave a porous layer. The dissolving process may include the use of electrochemical cells to bias device 20 in solution so as to facilitate the dealloying process.
- any other suitable combination of alloy and dissolving or component-removing substanceis contemplated.
- any means for exposing medical device 20 to a dissolving substanceis contemplated.
- medical device 20may be immersed in, sprayed with, coated with, etc. any suitable substance or combination of substances.
- one or more components of precursor alloy layer 22are selectively removed to form a porous layer 23 .
- removing at least one component of the alloycomprises dissolving one or more of the most electrochemically active components of the alloy.
- the chromium componentmay be dissolved, leaving the iron and nickel components.
- Additional processing of medical device 20may include introduction of one or more therapeutic agents into porous layer 23 .
- Any suitable agent(s)may be introduced and they may be introduced by any desired method.
- methods for introducing therapeutic agentsinclude, but are not limited to, liquid immersion, vacuum dessication, high pressure infusion, vapor loading, and the like.
- multiple therapeutic agentsmay be introduced into a porous matrix composed of a plurality of porous layer 23 .
- the plurality of porous layersmay vary in atomic composition, as well as in pore size and density. Compositional variations may allow for preferential binding to occur between the therapeutic agent and the coating, changing the elution kinetics of the agent. Pore size and density will also affect the transport kinetics of therapeutics from and across each layer. The use of a plurality of porous layers may thus allow for controlling elution kinetics of multiple therapeutic agents.
- live cellsmay be encapsulated within lumen 26 of device 20 .
- the entire devicemay be made porous (such that the internal lumen and the exterior of the device are separated by a porous layer).
- Live cellssuch as pancreatic islet cells
- the porosity of the layeradjusted to allow transport of selected molecules (such as oxygen, glucose; as well as therapeutic cellular products, such as insulin, interferon), while preventing access of antibodies and other immune system agents that may otherwise attack or compromise the encapsulated cells.
- a protective layer or coatingmay be formed or added to medical device 20 , such as a titanium, gold or platinum layer or coating.
- porous layer 23may not be biocompatible
- a passivation layermay be deposited into porous layer 23 to enhance biocompatibility.
- a very thin layer of goldmay be electroplated into the dealloyed porous layer 23 .
- Electroless depositionmay also be used to achieve the same effect.
- the porous coatingmay also be passivated chemically or in a reactive ion plasma.
Landscapes
- Health & Medical Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Chemical & Material Sciences (AREA)
- Veterinary Medicine (AREA)
- Public Health (AREA)
- General Health & Medical Sciences (AREA)
- Animal Behavior & Ethology (AREA)
- Heart & Thoracic Surgery (AREA)
- Epidemiology (AREA)
- Vascular Medicine (AREA)
- Surgery (AREA)
- Engineering & Computer Science (AREA)
- Biomedical Technology (AREA)
- Medicinal Chemistry (AREA)
- Inorganic Chemistry (AREA)
- Oral & Maxillofacial Surgery (AREA)
- Transplantation (AREA)
- Dermatology (AREA)
- Cardiology (AREA)
- Molecular Biology (AREA)
- Dispersion Chemistry (AREA)
- Gastroenterology & Hepatology (AREA)
- Pulmonology (AREA)
- Materials For Medical Uses (AREA)
- Prostheses (AREA)
- Laminated Bodies (AREA)
Abstract
Description
- The present application is a continuation of U.S. application Ser. No. 10/713,244 filed on Nov. 13, 2003, which claims priority under 35 U.S.C. §119(e) to U.S. Provisional Application No. 60/426,106 filed on Nov. 13, 2002, the disclosures of which are incorporated by reference herein in their entirety.
- The present invention relates generally to medical devices and methods for making same. More specifically, the invention relates to implantable medical devices having at least one porous layer, and methods for making such devices.
- Implantable medical devices are increasingly being used to deliver one or more therapeutic agents to a site within a body. Such agents may provide their own benefits to treatment and/or may enhance the efficacy of the implantable device. For example, much research has been conducted into the use of drug eluting stents for use in percutaneous transluminal coronary angioplasty (PTCA) procedures. Although some implantable devices are simply coated with one or more therapeutic agents, other devices include means for containing, attaching or otherwise holding therapeutic agents to provide the agents at a treatment location over a longer duration, in a controlled-release manner, or the like.
- Porous materials, for example, are commonly used in medical implants as matrices for the retention of therapeutic agents. Materials that have been used for this purpose include ceramics such as hydroxyapatites and porous alumina, as well as sintered metal powders. Polymeric materials such as poly(ethylene glycol)/poly(L-lactic acid) (PLGA) have also been used for this purpose. These materials are typically applied as coatings to the medical implant, raising issues regarding coating adhesion, mechanical properties, and material biocompatibility. Further, application of these coatings introduces additional complexity to the fabrication process, increasing overall production costs.
- Therefore, it would be advantageous to have improved implantable medical devices with porous layers and methods for fabricating those devices. Such methods would ideally produce a more adherent and mechanically robust porous layer while simplifying device manufacture. Methods would also ideally provide porous layers having desired pore sizes and densities. At least some of these objectives will be met by the present invention.
- Methods of the present invention provide means for fabricating an implantable medical device having at least one porous layer. In one aspect, a method of fabricating an implantable medical device having a porous layer for releasably containing at least one therapeutic agent includes providing an implantable medical device comprising at least one alloy and removing at least one component of the alloy to form the porous layer. In some embodiments, the component is removed to form the porous layer as a biocompatible material, such as gold. In some embodiments, the medical device comprises a tubular stent device having an outer surface and an inner surface. For example, the stent device may comprise a coronary artery stent for use in a percutaneous transluminal coronary angioplasty (PTCA) procedure. In some of these embodiments, the alloy is disposed along the outer surface of the stent device.
- Optionally, providing the implantable medical device may also include depositing the alloy on at least one surface of the medical device. In various embodiments, the alloy may be disposed along an outer surface of the implantable medical device, such that the dissolving step forms the porous layer on the outer surface of the device. In some embodiments, the alloy includes one or more metals, such as but not limited to gold, silver, nitinol, steel, chromium, iron, nickel, copper, aluminum, titanium, tantalum, cobalt, tungsten, palladium, vanadium, platinum and/or niobium. In other embodiments, the alloy comprises at least one metal and at least one non-metal. Optionally, before the dissolving step at least one substance may be embedded within the alloy. For example, a salt or an oxide particle may be embedded in the alloy to enhance pore formation upon dissolution.
- Dissolving one or more components of the alloy may involve exposing the alloy to a dissolving substance. For example, a stainless steel alloy may be exposed to sodium hydroxide in one embodiment. Typically, one or more of the most electrochemically active components of the alloy are dissolved. After the dissolving step, additional processing may be performed. For example, the device may be coated after the dissolving step with titanium, gold and/or platinum. Some embodiments further include introducing at least one therapeutic agent into the porous layer. For example, the therapeutic agent may be introduced by liquid immersion, vacuum dessication, high pressure infusion or vapor loading in various embodiments. The therapeutic agent may be any suitable agent or combination of agents, such as but not limited to anti-restenotic agent(s) or anti-inflammatory agent(s), such as Rapamycin, Sirolimus, Taxol, Prednisone, and/or the like. In other embodiments, live cells may be encapsulated by the porous layer, thereby allowing transport of selected molecules, such as oxygen, glucose, or insulin, to and from the cells, while shielding the cells from the immune system of the patient. Some embodiments may optionally include multiple porous layers having various porosities and atomic compositions.
- In another aspect, a method for treating a blood vessel using an implantable medical device having a porous layer for releasably containing at least one therapeutic agent includes: providing at least one implantable stent having a porous layer for releasably containing at least one therapeutic agent; and placing the stent within the blood vessel at a desired location, wherein the stent releases the at least one therapeutic agent from the porous layer after placement. For example, in one embodiment the desired location may comprise an area of stenosis in the blood vessel, and the at least one therapeutic agent may inhibit re-stenosis of the blood vessel. Again, the therapeutic agent in some embodiments may be one or more anti-restenosis agents, anti-inflammatory agents, or a combination of both. In one embodiment, the blood vessel may be a coronary artery. In such embodiments, the placing step may involve placing the stent so as to contact the porous layer with at least one of a stenotic plaque in the blood vessel and an inner wall of the blood vessel.
- In still another aspect, an implantable medical device has at least one porous layer comprising at least one remaining alloy component and interstitial spaces, wherein the interstitial spaces comprise at least one removed alloy component space of an alloy, the alloy comprising the at least one remaining alloy component and the at least one removed alloy component. In some embodiments, the porous layer comprises a matrix. Also in some embodiments, the implantable medical device comprises an implantable stent device having an outer surface and an inner surface, and the porous layer is disposed along the outer surface. For example, the stent device may comprise a coronary artery stent for use in a percutaneous transluminal coronary angioplasty procedure. As described above, the alloy may comprise one or more metals selected from the group consisting of gold, silver, nitinol, steel, chromium, iron, nickel, copper, aluminum, titanium, tantalum, cobalt, tungsten, palladium, vanadium, platinum and/or niobium. For example, the alloy may comprise stainless steel and the porous layer may comprise iron and nickel.
- In some embodiments, the component (or components) that is dissolved comprises a most electrochemically active component of the alloy. Generally, the device further includes at least one therapeutic agent disposed within the at least one porous layer. Any such agent or combination of agents is contemplated. Finally, the device may include a titanium or platinum coating over an outer surface of the device.
FIG. 1 is a perspective view of an implantable stent device having a porous layer according to one embodiment of the present invention.FIGS. 2A-2B are electron micrographs of a porous layer formed by dissolving silver from a gold-silver alloy, according to one embodiment of the present invention.FIGS. 3A-3C are cross-sectional side views showing a method of making an implantable stent device having a porous layer, according to one embodiment of the present invention.- Methods of the present invention provide means for fabricating an implantable medical device having at least one porous layer. Generally, the methods involve providing an implantable medical device containing an alloy and removing at least one component of the alloy to form the porous layer. In some embodiments, an alloy may first be deposited on an implantable device and one or more components of the alloy may then be removed to form the porous layer. Such methods are often referred to as “dealloying.” For a general description of dealloying methods, reference may be made to “Evolution of nanoporosity in dealloying,” Jonah Erlebacher et al., Nature 410, pp. 450-453, March 2001, the entire contents of which are hereby incorporated by reference. Dealloying a layer of an implantable device provides a porous layer, which may then be infused with one or more therapeutic agents for providing delivery of an agent into a patient via the device. Use of dealloying methods will typically provide more adherent and mechanically robust porous layers on medical implantables than are currently available, while also simplifying device manufacture.
- Although the following description often focuses on the example of implantable stent devices for use in PTCA procedures, any suitable implantable medical device may be fabricated with methods of the invention. Other devices may include, but are not limited to, other stents, stent-grafts, implantable leads, infusion pumps, vascular access devices such as implantable ports, orthopedic implants, implantable electrodes, and the like. Similarly, devices fabricated via methods of the present invention may be used to deliver any suitable therapy or combination of therapies in a patient care context, veterinary context, research setting or the like. Therapeutic agents may include, for example, drugs, genes, anti-restenosis agents, anti-thrombogenic agents, antibiotic agents, anti-clotting agents, anti-inflammatory agents, cancer therapy agents and/or the like. Thus, the following description of specific embodiments is provided for exemplary purposes only and should not be interpreted to limit the scope of the invention as set forth in the appended claims.
- Referring now to
FIG. 1 , an implantable medical device fabricated by methods of the present invention may include anelongate stent device 10, having two ormore layers stent device 10 includes an outerporous layer 12 and an innernon-porous layer 14. Other embodiments may suitably include an innerporous layer 12 and an outernon-porous layer 14, multipleporous layers 12, multiplenon-porous layers 14, a porous coating over an entire surface of a medical device, or any combination of porous and non-porous surfaces, layers, areas or the like to provide a desired effect. In one embodiment, for example, multiple porous layers may be layered over one another, with each layer having a different porosity and atomic composition.Porous layer 12 andnon-porous layer 14 may have any suitable thicknesses in various embodiments. In some embodiments, for example, a very thinporous layer 12 may be desired, such as for delivery of a comparatively small amount of therapeutic agent. In another embodiment, a thickerporous layer 12 may be used for delivery of a larger quantity of therapeutic agent and/or for a longer duration of agent delivery. Any suitable combination and configuration ofporous layer 12 andnon-porous layer 14 is contemplated. In one embodiment,porous layer 12 may comprise the entire thickness ofstent device 10, so that the device is completely porous. Again,stent device 10 is only one example of a device with which porous layers may be used. Other devices may not have a lumen, for example, but may still be suitable for use in the present invention. - As mentioned above, any medical device may be fabricated with one or more
porous layers 12 according to embodiments of the present invention. Where the device is animplantable stent device 10, any suitable type, size and configuration of stent device may be fabricated with one or moreporous layers 12. In one embodiment,stent device 10 comprises an expandable stent for implantation in a coronary artery during a PTCA procedure. Such astent device 10 may be fabricated from any suitable material or combination of materials. In one embodiment,stent device 10 comprises a stainless steelnon-porous layer 14 arid an iron and nickelporous layer 12. In some embodiments,porous layer 12 may be formed of a biocompatible material, such as gold. In other embodiments,porous layer 12 may be formed from a cobalt-chromium alloy such as L605. Any other suitable material or combination of materials is contemplated. Furthermore,stent device 10 may include a layer or coating comprising a biocompatible material such as titanium, gold or platinum, which may provide biocompatibility, corrosion resistance or both. - With reference now to
FIGS. 2A and 2B , aporous layer 12 is shown in greater detail.Porous layer 12 in these figures was made by selectively dissolving silver from a gold-silver alloy. As can be seen from the scanning electron micrographs,porous layer 12 comprises a matrix of pores and structural elements. In any given embodiment, the size and density of such pores may be varied by varying one or more elements of a method for making the device and formingporous layer 12. For example, one or more components of an alloy, a substance used to selectively dissolve the alloy, duration time of exposing the alloy to the dissolving substance, or the like may be chosen to giveporous layer 12 certain desired characteristics. Thermal anneals prior or subsequent to the dealloying process may also be performed to vary pore size and density. Any suitable combination of porous layer thickness, pore size, pore density and the like is contemplated within the scope of the present invention. - Although not shown in
FIGS. 1 and 2 , any implantable medical device of the present invention may include one or more therapeutic agents disposed within one or moreporous layers 12. As discussed above, any agent or combination of agents may be included. Additionally, as described further below, any suitable method for introducing an agent into a porous layer may be used. - Referring now to
FIGS. 3A through 3C , a method for fabricating an implantablemedical device 20 having a porous layer suitably includes providing an implantable device comprising at least one alloy and removing at least one component of the alloy to form the porous layer. As shown in the cross-sectionalFIG. 3A , amedical device 20 such as a stent may include aprecursor alloy layer 22, asubstrate layer 24 and a lumen.Precursor alloy layer 22 can be deposited ontosubstrate layer 24 by various processes, including but not limited to physical vapor deposition, ion implantation, sputter deposition, thermal or electron beam evaporation, chemical vapor deposition, pulsed laser deposition, or the like. Using such techniques,precursor alloy layer 22 may be synthesized in situ from various materials, as described previously, such that exposure to the dealloying process will remove the sacrificial component ofprecursor alloy layer 22, leaving behind a porous matrix. In another embodiment,precursor alloy layer 22 andsubstrate layer 24 may be made from the same material. As previously described,medical device 20 may comprise any suitable stent or other device andprecursor alloy layer 22,substrate layer 24 and/or other layers may be given any suitable configurations, thicknesses and the like. In some embodiments,precursor alloy layer 22 is disposed along an outer surface ofdevice 20, while in othersprecursor alloy layer 22 may be disposed along an inner surface, both inner and outer surfaces, or the like. The alloy used to formprecursor alloy layer 22 may comprise any suitable alloy and may be a metal-metal alloy or a metal-non-metal alloy. In various embodiments, for example, components ofprecursor alloy layer 22 may include steel, nitinol, chromium, brass, copper, iron, nickel, aluminum, titanium, gold, silver, tantalum, cobalt, tungsten, palladium, vanadium, platinum and/or niobium. In some embodiments, one or more additional substances may be embedded withinprecursor alloy layer 22 to cause or enhance pore formation during the fabrication process. For example, a salt, an oxide particle or the like may be added toprecursor alloy layer 22 to enhance pore formation. - As shown in
FIG. 3B , implantablemedical device 20 is typically exposed to a substance (arrows) to dissolve or otherwise remove at least one component of the alloy to form the porous layer fromprecursor alloy layer 22. In various embodiments, any suitable substance may be used for removing at least one component of the alloy. In one embodiment, for example, the alloy comprises stainless steel, such as 316L stainless steel, and dissolving at least one component of the steel comprises exposing the steel to hot sodium hydroxide to dissolve chromium and leave iron and nickel as the porous layer. In another embodiment, a silver-gold alloy may be exposed to nitric acid to dissolve the silver and leave the gold as the porous layer (as shown inFIGS. 2A and 2B ). In another embodiment, a cobalt-chromium alloy, such as L605, is modified by the addition of a sacrificial material such as silver, copper or aluminum, which is subsequently removed by processing in an appropriate solvent, such as nitric acid, sulphuric acid or phosphoric acid, to leave a porous film of the original cobalt-chromium alloy. In another embodiment, a platinum-copper alloy is dealloyed in the presence of sulphuric acid to produce porous platinum. In some embodiments, nitinol may be dissolved by a suitable dissolving substance to leave a porous layer. The dissolving process may include the use of electrochemical cells to biasdevice 20 in solution so as to facilitate the dealloying process. Any other suitable combination of alloy and dissolving or component-removing substance is contemplated. Furthermore, any means for exposingmedical device 20 to a dissolving substance is contemplated. For example,medical device 20 may be immersed in, sprayed with, coated with, etc. any suitable substance or combination of substances. - As shown in
FIG. 3C , one or more components ofprecursor alloy layer 22 are selectively removed to form aporous layer 23. In some embodiments, removing at least one component of the alloy comprises dissolving one or more of the most electrochemically active components of the alloy. For example, in a steel alloy the chromium component may be dissolved, leaving the iron and nickel components. Additional processing ofmedical device 20 may include introduction of one or more therapeutic agents intoporous layer 23. Any suitable agent(s) may be introduced and they may be introduced by any desired method. For example, methods for introducing therapeutic agents include, but are not limited to, liquid immersion, vacuum dessication, high pressure infusion, vapor loading, and the like. - In another embodiment, multiple therapeutic agents may be introduced into a porous matrix composed of a plurality of
porous layer 23. As described previously, the plurality of porous layers may vary in atomic composition, as well as in pore size and density. Compositional variations may allow for preferential binding to occur between the therapeutic agent and the coating, changing the elution kinetics of the agent. Pore size and density will also affect the transport kinetics of therapeutics from and across each layer. The use of a plurality of porous layers may thus allow for controlling elution kinetics of multiple therapeutic agents. In a further embodiment, live cells may be encapsulated withinlumen 26 ofdevice 20. In one such embodiment, the entire device may be made porous (such that the internal lumen and the exterior of the device are separated by a porous layer). Live cells (such a pancreatic islet cells) can be encapsulated within the internal lumen, and the porosity of the layer adjusted to allow transport of selected molecules (such as oxygen, glucose; as well as therapeutic cellular products, such as insulin, interferon), while preventing access of antibodies and other immune system agents that may otherwise attack or compromise the encapsulated cells. In some embodiments, a protective layer or coating may be formed or added tomedical device 20, such as a titanium, gold or platinum layer or coating. If there is a concern thatporous layer 23 may not be biocompatible, a passivation layer may be deposited intoporous layer 23 to enhance biocompatibility. For instance, a very thin layer of gold may be electroplated into the dealloyedporous layer 23. Electroless deposition may also be used to achieve the same effect. Depending on the composition ofporous layer 23, the porous coating may also be passivated chemically or in a reactive ion plasma. - Although the present invention has been described in full, in relation to various exemplary embodiments, various additional embodiments and alterations to the described embodiments are contemplated within the scope of the invention. Thus, no part of the foregoing description should be interpreted to limit the scope of the invention as set forth in the following claims.
Claims (20)
1. A method of fabricating an implantable medical device having at least one porous layer for releasably containing at least one therapeutic agent, the method comprising: providing an implantable medical device comprising at least one alloy; and dealloying at least a portion of the implantable medical device to form at least one porous layer.
2. A method as inclaim 1 , wherein the at least one alloy is cobalt-chromium.
3. A method as inclaim 1 , wherein providing the implantable medical device comprises providing a stent having an outer surface and an inner surface.
4. A method as inclaim 1 , wherein the stent is integrally formed from the at least one alloy.
5. A method as inclaim 4 , wherein the stent comprises cobalt-chromium.
6. A method as inclaim 1 , wherein providing the implantable medical device includes depositing the at least one alloy on at least one surface of the medical device.
7. A method as inclaim 1 , wherein the alloy is disposed along an outer surface of the implantable medical device, such that the removing step forms the porous layer on the outer surface of the device.
8. A method as inclaim 1 , wherein the alloy comprises at least one metal selected from the group consisting of gold, silver, nitinol, steel, chromium, iron, nickel, copper, aluminum, titanium, tantalum, cobalt, tungsten, palladium, vanadium, platinum and niobium.
9. A method as inclaim 1 , wherein dealloying comprises dissolving a most electrochemically active component of the alloy.
10. A method as inclaim 1 , further comprising introducing the at least one therapeutic agent into the porous layer.
11. A method as inclaim 10 , wherein the at least one therapeutic agent comprises at least one anti-restenosis agent or anti-inflammatory agent for inhibiting restenosis of a coronary artery.
12. A method as inclaim 3 , further comprising forming a porous layer on the inner surface of the stent.
13. A method for treating a blood vessel using a stent, the method comprising: providing a dealloyed stent having at least one porous layer for releasably containing at least one drug; and placing the stent within the blood vessel at a desired location, wherein the stent releases the at least one drug from the at least one porous layer after placement.
14. An implantable medical device having at least one porous layer, at least one the at least one porous layers comprising: at least one remaining metal component of an alloy comprising at least two metal components; and interstitial spaces, wherein the interstitial spaces comprise at least one removed metal component space of the alloy.
15. An implantable medical device as inclaim 14 , wherein at least one of the at least two metal components is selected from the group consisting of gold, silver, nitinol, steel, chrome, iron, nickel, copper, aluminum, titanium, tantalum, cobalt, tungsten, palladium, vanadium, platinum and niobium.
16. An implantable medical device as inclaim 14 , wherein the at least one removed metal component comprises a most electrochemically active component of the alloy.
17. A method for forming a porous medical component, comprising:
providing a medical device component comprising a metallic surface;
chemically dealloying the metallic surface; and
thermally annealing the metallic surface.
18. The method for forming a porous medical component inclaim 17 , further comprising altering the surface charge of the metallic surface.
19. The method for forming a porous medical component inclaim 17 , further comprising altering the hydrophobicity of the metallic surface.
20. The method for forming a porous medical component inclaim 17 , further comprising applying a binder to the metallic surface.
Priority Applications (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US11/432,281US20060271169A1 (en) | 2002-11-13 | 2006-05-11 | Stent with nanoporous surface |
Applications Claiming Priority (3)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US42610602P | 2002-11-13 | 2002-11-13 | |
| US10/713,244US7294409B2 (en) | 2002-11-13 | 2003-11-13 | Medical devices having porous layers and methods for making same |
| US11/432,281US20060271169A1 (en) | 2002-11-13 | 2006-05-11 | Stent with nanoporous surface |
Related Parent Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US10/713,244ContinuationUS7294409B2 (en) | 2002-11-13 | 2003-11-13 | Medical devices having porous layers and methods for making same |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| US20060271169A1true US20060271169A1 (en) | 2006-11-30 |
Family
ID=32313109
Family Applications (2)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US10/713,244Expired - LifetimeUS7294409B2 (en) | 2002-11-13 | 2003-11-13 | Medical devices having porous layers and methods for making same |
| US11/432,281AbandonedUS20060271169A1 (en) | 2002-11-13 | 2006-05-11 | Stent with nanoporous surface |
Family Applications Before (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US10/713,244Expired - LifetimeUS7294409B2 (en) | 2002-11-13 | 2003-11-13 | Medical devices having porous layers and methods for making same |
Country Status (10)
| Country | Link |
|---|---|
| US (2) | US7294409B2 (en) |
| EP (1) | EP1572032B1 (en) |
| JP (1) | JP2006514848A (en) |
| KR (1) | KR100826574B1 (en) |
| CN (1) | CN1725988A (en) |
| AT (1) | ATE402675T1 (en) |
| AU (1) | AU2003295535B2 (en) |
| CA (1) | CA2503625A1 (en) |
| DE (1) | DE60322581D1 (en) |
| WO (1) | WO2004043292A2 (en) |
Cited By (64)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US20060127442A1 (en)* | 2004-12-09 | 2006-06-15 | Helmus Michael N | Use of supercritical fluids to incorporate biologically active agents into nanoporous medical articles |
| US20070173925A1 (en)* | 2006-01-25 | 2007-07-26 | Cornova, Inc. | Flexible expandable stent |
| US20070185564A1 (en)* | 2000-03-24 | 2007-08-09 | Advanced Cardiovascular Systems, Inc. | Radiopaque intraluminal stent |
| US20070208412A1 (en)* | 2006-03-06 | 2007-09-06 | David Elmaleh | Intravascular device with netting system |
| US20080177371A1 (en)* | 2006-08-28 | 2008-07-24 | Cornova, Inc. | Implantable devices and methods of forming the same |
| US20080262598A1 (en)* | 2007-04-18 | 2008-10-23 | David Elmaleh | Intravascular device with netting system |
| US20090112315A1 (en)* | 2007-10-29 | 2009-04-30 | Zimmer, Inc. | Medical implants and methods for delivering biologically active agents |
| US7931683B2 (en) | 2007-07-27 | 2011-04-26 | Boston Scientific Scimed, Inc. | Articles having ceramic coated surfaces |
| US7938855B2 (en) | 2007-11-02 | 2011-05-10 | Boston Scientific Scimed, Inc. | Deformable underlayer for stent |
| US7942926B2 (en) | 2007-07-11 | 2011-05-17 | Boston Scientific Scimed, Inc. | Endoprosthesis coating |
| US7976915B2 (en) | 2007-05-23 | 2011-07-12 | Boston Scientific Scimed, Inc. | Endoprosthesis with select ceramic morphology |
| US7981150B2 (en) | 2006-11-09 | 2011-07-19 | Boston Scientific Scimed, Inc. | Endoprosthesis with coatings |
| US7985252B2 (en) | 2008-07-30 | 2011-07-26 | Boston Scientific Scimed, Inc. | Bioerodible endoprosthesis |
| US7998192B2 (en) | 2008-05-09 | 2011-08-16 | Boston Scientific Scimed, Inc. | Endoprostheses |
| US8002821B2 (en) | 2006-09-18 | 2011-08-23 | Boston Scientific Scimed, Inc. | Bioerodible metallic ENDOPROSTHESES |
| US8002823B2 (en) | 2007-07-11 | 2011-08-23 | Boston Scientific Scimed, Inc. | Endoprosthesis coating |
| US8029554B2 (en) | 2007-11-02 | 2011-10-04 | Boston Scientific Scimed, Inc. | Stent with embedded material |
| US8048150B2 (en) | 2006-04-12 | 2011-11-01 | Boston Scientific Scimed, Inc. | Endoprosthesis having a fiber meshwork disposed thereon |
| US8052743B2 (en) | 2006-08-02 | 2011-11-08 | Boston Scientific Scimed, Inc. | Endoprosthesis with three-dimensional disintegration control |
| US8052744B2 (en) | 2006-09-15 | 2011-11-08 | Boston Scientific Scimed, Inc. | Medical devices and methods of making the same |
| US8052745B2 (en) | 2007-09-13 | 2011-11-08 | Boston Scientific Scimed, Inc. | Endoprosthesis |
| US8057534B2 (en) | 2006-09-15 | 2011-11-15 | Boston Scientific Scimed, Inc. | Bioerodible endoprostheses and methods of making the same |
| US8067054B2 (en) | 2007-04-05 | 2011-11-29 | Boston Scientific Scimed, Inc. | Stents with ceramic drug reservoir layer and methods of making and using the same |
| US8066763B2 (en) | 1998-04-11 | 2011-11-29 | Boston Scientific Scimed, Inc. | Drug-releasing stent with ceramic-containing layer |
| US8070797B2 (en) | 2007-03-01 | 2011-12-06 | Boston Scientific Scimed, Inc. | Medical device with a porous surface for delivery of a therapeutic agent |
| US8071156B2 (en) | 2009-03-04 | 2011-12-06 | Boston Scientific Scimed, Inc. | Endoprostheses |
| US8080055B2 (en) | 2006-12-28 | 2011-12-20 | Boston Scientific Scimed, Inc. | Bioerodible endoprostheses and methods of making the same |
| US8089029B2 (en) | 2006-02-01 | 2012-01-03 | Boston Scientific Scimed, Inc. | Bioabsorbable metal medical device and method of manufacture |
| US8128689B2 (en) | 2006-09-15 | 2012-03-06 | Boston Scientific Scimed, Inc. | Bioerodible endoprosthesis with biostable inorganic layers |
| US8187620B2 (en) | 2006-03-27 | 2012-05-29 | Boston Scientific Scimed, Inc. | Medical devices comprising a porous metal oxide or metal material and a polymer coating for delivering therapeutic agents |
| US20120141820A1 (en)* | 2010-12-01 | 2012-06-07 | Hon Hai Precision Industry Co., Ltd. | Porous metal article and about method for manufacturing same |
| US8216632B2 (en) | 2007-11-02 | 2012-07-10 | Boston Scientific Scimed, Inc. | Endoprosthesis coating |
| US8221822B2 (en) | 2007-07-31 | 2012-07-17 | Boston Scientific Scimed, Inc. | Medical device coating by laser cladding |
| US8231980B2 (en) | 2008-12-03 | 2012-07-31 | Boston Scientific Scimed, Inc. | Medical implants including iridium oxide |
| US8236046B2 (en) | 2008-06-10 | 2012-08-07 | Boston Scientific Scimed, Inc. | Bioerodible endoprosthesis |
| US8267992B2 (en) | 2009-03-02 | 2012-09-18 | Boston Scientific Scimed, Inc. | Self-buffering medical implants |
| US20120244377A1 (en)* | 2011-03-23 | 2012-09-27 | Hon Hai Precision Industry Co., Ltd. | Porous metal article and about method for manufacturing same |
| US8287937B2 (en) | 2009-04-24 | 2012-10-16 | Boston Scientific Scimed, Inc. | Endoprosthese |
| US8303643B2 (en) | 2001-06-27 | 2012-11-06 | Remon Medical Technologies Ltd. | Method and device for electrochemical formation of therapeutic species in vivo |
| US20120283828A1 (en)* | 2009-11-05 | 2012-11-08 | Nonwotecc Medical Gmbh | Non-woven fabric for medical use and process for the preparation thereof |
| US8353949B2 (en) | 2006-09-14 | 2013-01-15 | Boston Scientific Scimed, Inc. | Medical devices with drug-eluting coating |
| KR101223977B1 (en) | 2010-05-26 | 2013-01-18 | 경상대학교산학협력단 | Method of manufacturing nano-structured stent inhibiting toxicity |
| US8382824B2 (en) | 2008-10-03 | 2013-02-26 | Boston Scientific Scimed, Inc. | Medical implant having NANO-crystal grains with barrier layers of metal nitrides or fluorides |
| US8431149B2 (en) | 2007-03-01 | 2013-04-30 | Boston Scientific Scimed, Inc. | Coated medical devices for abluminal drug delivery |
| US8449603B2 (en) | 2008-06-18 | 2013-05-28 | Boston Scientific Scimed, Inc. | Endoprosthesis coating |
| US8574615B2 (en) | 2006-03-24 | 2013-11-05 | Boston Scientific Scimed, Inc. | Medical devices having nanoporous coatings for controlled therapeutic agent delivery |
| US8668732B2 (en) | 2010-03-23 | 2014-03-11 | Boston Scientific Scimed, Inc. | Surface treated bioerodible metal endoprostheses |
| US8771343B2 (en) | 2006-06-29 | 2014-07-08 | Boston Scientific Scimed, Inc. | Medical devices with selective titanium oxide coatings |
| US8808726B2 (en) | 2006-09-15 | 2014-08-19 | Boston Scientific Scimed. Inc. | Bioerodible endoprostheses and methods of making the same |
| US8815275B2 (en) | 2006-06-28 | 2014-08-26 | Boston Scientific Scimed, Inc. | Coatings for medical devices comprising a therapeutic agent and a metallic material |
| US8815273B2 (en) | 2007-07-27 | 2014-08-26 | Boston Scientific Scimed, Inc. | Drug eluting medical devices having porous layers |
| US8840660B2 (en) | 2006-01-05 | 2014-09-23 | Boston Scientific Scimed, Inc. | Bioerodible endoprostheses and methods of making the same |
| US8900292B2 (en) | 2007-08-03 | 2014-12-02 | Boston Scientific Scimed, Inc. | Coating for medical device having increased surface area |
| US8920491B2 (en) | 2008-04-22 | 2014-12-30 | Boston Scientific Scimed, Inc. | Medical devices having a coating of inorganic material |
| US8932346B2 (en) | 2008-04-24 | 2015-01-13 | Boston Scientific Scimed, Inc. | Medical devices having inorganic particle layers |
| US20150133990A1 (en)* | 2013-11-13 | 2015-05-14 | Covidien Lp | Galvanically assisted attachment of medical devices to thrombus |
| US9284409B2 (en) | 2007-07-19 | 2016-03-15 | Boston Scientific Scimed, Inc. | Endoprosthesis having a non-fouling surface |
| US9566147B2 (en) | 2010-11-17 | 2017-02-14 | Abbott Cardiovascular Systems, Inc. | Radiopaque intraluminal stents comprising cobalt-based alloys containing one or more platinum group metals, refractory metals, or combinations thereof |
| US9974672B2 (en) | 2012-10-15 | 2018-05-22 | David R Elmaleh | Material structures for intravascular device |
| US10265515B2 (en) | 2015-03-27 | 2019-04-23 | Covidien Lp | Galvanically assisted aneurysm treatment |
| US11298251B2 (en) | 2010-11-17 | 2022-04-12 | Abbott Cardiovascular Systems, Inc. | Radiopaque intraluminal stents comprising cobalt-based alloys with primarily single-phase supersaturated tungsten content |
| US11806488B2 (en) | 2011-06-29 | 2023-11-07 | Abbott Cardiovascular Systems, Inc. | Medical device including a solderable linear elastic nickel-titanium distal end section and methods of preparation therefor |
| US11964337B2 (en)* | 2022-04-14 | 2024-04-23 | Shandong University | NiTi alloy surface cutting process and roughness adjustment method |
| US12151049B2 (en) | 2019-10-14 | 2024-11-26 | Abbott Cardiovascular Systems, Inc. | Methods for manufacturing radiopaque intraluminal stents comprising cobalt-based alloys with supersaturated tungsten content |
Families Citing this family (66)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US6569194B1 (en) | 2000-12-28 | 2003-05-27 | Advanced Cardiovascular Systems, Inc. | Thermoelastic and superelastic Ni-Ti-W alloy |
| US7776379B2 (en) | 2001-09-19 | 2010-08-17 | Medlogics Device Corporation | Metallic structures incorporating bioactive materials and methods for creating the same |
| US20050070989A1 (en)* | 2002-11-13 | 2005-03-31 | Whye-Kei Lye | Medical devices having porous layers and methods for making the same |
| US20060121080A1 (en)* | 2002-11-13 | 2006-06-08 | Lye Whye K | Medical devices having nanoporous layers and methods for making the same |
| US9770349B2 (en)* | 2002-11-13 | 2017-09-26 | University Of Virginia Patent Foundation | Nanoporous stents with enhanced cellular adhesion and reduced neointimal formation |
| ATE402675T1 (en) | 2002-11-13 | 2008-08-15 | Setagon Inc | MEDICAL DEVICES WITH POROUS LAYERS AND PRODUCTION PROCESSES THEREOF |
| JP4824312B2 (en)* | 2002-11-26 | 2011-11-30 | ユニバーシティ オブ ユタ リサーチ ファンデーション | Microporous material, analyte localization and quantification method, and analyte localization and quantification article |
| US7597936B2 (en)* | 2002-11-26 | 2009-10-06 | University Of Utah Research Foundation | Method of producing a pigmented composite microporous material |
| US20050096729A1 (en)* | 2003-10-31 | 2005-05-05 | Donadio James V.Iii | Methods and apparatus for intraluminal device |
| US7208172B2 (en) | 2003-11-03 | 2007-04-24 | Medlogics Device Corporation | Metallic composite coating for delivery of therapeutic agents from the surface of implantable devices |
| US20050119723A1 (en)* | 2003-11-28 | 2005-06-02 | Medlogics Device Corporation | Medical device with porous surface containing bioerodable bioactive composites and related methods |
| US20050251245A1 (en)* | 2004-05-05 | 2005-11-10 | Karl Sieradzki | Methods and apparatus with porous materials |
| US20050266040A1 (en)* | 2004-05-28 | 2005-12-01 | Brent Gerberding | Medical devices composed of porous metallic materials for delivering biologically active materials |
| ATE445424T1 (en)* | 2004-07-05 | 2009-10-15 | Ziscoat N V | BIOCOMPATIBLE COATING OF MEDICAL DEVICES USING MOLECULAR SIEVES |
| JP2008509742A (en)* | 2004-08-13 | 2008-04-03 | セタゴン インコーポレーティッド | Medical device comprising a nanoporous layer and method for making the same |
| US7901451B2 (en) | 2004-09-24 | 2011-03-08 | Biosensors International Group, Ltd. | Drug-delivery endovascular stent and method for treating restenosis |
| US20060129215A1 (en)* | 2004-12-09 | 2006-06-15 | Helmus Michael N | Medical devices having nanostructured regions for controlled tissue biocompatibility and drug delivery |
| US20060127443A1 (en)* | 2004-12-09 | 2006-06-15 | Helmus Michael N | Medical devices having vapor deposited nanoporous coatings for controlled therapeutic agent delivery |
| US9788978B2 (en)* | 2004-12-20 | 2017-10-17 | Nicholas A. Rojo | Implantable systems and stents containing cells for therapeutic uses |
| EA011594B1 (en)* | 2004-12-30 | 2009-04-28 | Синвеншен Аг | Combination comprising an agent providing a signal, an implant material and a drug |
| US8057543B2 (en)* | 2005-01-28 | 2011-11-15 | Greatbatch Ltd. | Stent coating for eluting medication |
| EP1764116A1 (en) | 2005-09-16 | 2007-03-21 | Debiotech S.A. | Porous coating process using colloidal particles |
| US20070173787A1 (en) | 2005-11-01 | 2007-07-26 | Huang Mark C T | Thin-film nitinol based drug eluting stent |
| EP1984035A2 (en) | 2006-02-13 | 2008-10-29 | Medtronic, Inc. | Medical devices having textured surfaces |
| DE102006007231B4 (en)* | 2006-02-15 | 2009-04-09 | Acandis Gmbh & Co. Kg | Method of wrapping a stent |
| US7691431B2 (en)* | 2006-03-07 | 2010-04-06 | Boston Scientific Scimed, Inc. | System and method for spray coating multiple medical devices using a rotary atomizer |
| EP1891988A1 (en)* | 2006-08-07 | 2008-02-27 | Debiotech S.A. | Anisotropic nanoporous coatings for medical implants |
| JP5290962B2 (en)* | 2006-05-17 | 2013-09-18 | デビオテック ソシエテ アノニム | Anisotropic nanoporous coating |
| EP1891995A1 (en)* | 2006-08-08 | 2008-02-27 | Debiotech S.A. | Drug loading of porous coating |
| CN100400113C (en)* | 2006-08-14 | 2008-07-09 | 董何彦 | Preparation method of drug-loaded layer in micro-blind holes on metal stent surface |
| WO2008027871A2 (en)* | 2006-08-28 | 2008-03-06 | Cornova, Inc. | Implantable devices and methods of forming the same |
| WO2008034007A2 (en)* | 2006-09-15 | 2008-03-20 | Boston Scientific Limited | Medical devices |
| US10188534B2 (en)* | 2006-11-17 | 2019-01-29 | Covidien Lp | Stent having reduced passage of emboli and stent delivery system |
| US20080138289A1 (en)* | 2006-12-08 | 2008-06-12 | Evident Technologies, Inc. | Systems and methods for detecting infrared emitting composites and medical applications therefor |
| US20080166526A1 (en)* | 2007-01-08 | 2008-07-10 | Monk Russell A | Formed panel structure |
| US8187255B2 (en)* | 2007-02-02 | 2012-05-29 | Boston Scientific Scimed, Inc. | Medical devices having nanoporous coatings for controlled therapeutic agent delivery |
| WO2008112190A1 (en)* | 2007-03-09 | 2008-09-18 | The University Of Akron | Bio-artificial pancreas and a procedure for preparation of same |
| EP2006420A1 (en)* | 2007-06-22 | 2008-12-24 | Danmarks Tekniske Universitet - DTU | A microporous layer for lowering friction in metal forming processes |
| US8205317B2 (en)* | 2007-07-16 | 2012-06-26 | Medtronic Vascular, Inc. | Method of manufacturing a controlled porosity stent |
| US20090030500A1 (en)* | 2007-07-27 | 2009-01-29 | Jan Weber | Iron Ion Releasing Endoprostheses |
| US7883736B2 (en)* | 2007-09-06 | 2011-02-08 | Boston Scientific Scimed, Inc. | Endoprostheses having porous claddings prepared using metal hydrides |
| US8105967B1 (en) | 2007-10-05 | 2012-01-31 | The United States Of America As Represented By The Secretary Of The Navy | Lightweight ballistic armor including non-ceramic-infiltrated reaction-bonded-ceramic composite material |
| US20090118821A1 (en)* | 2007-11-02 | 2009-05-07 | Boston Scientific Scimed, Inc. | Endoprosthesis with porous reservoir and non-polymer diffusion layer |
| US20090118812A1 (en)* | 2007-11-02 | 2009-05-07 | Boston Scientific Scimed, Inc. | Endoprosthesis coating |
| DE102007055018B4 (en)* | 2007-11-14 | 2021-05-12 | Fraunhofer-Gesellschaft zur Förderung der angewandten Forschung e.V. | Method for joining a noble metal surface with a polymer |
| DE102007055019B4 (en)* | 2007-11-14 | 2019-04-04 | Fraunhofer-Gesellschaft zur Förderung der angewandten Forschung e.V. | Method for producing a nanoporous layer |
| US7833266B2 (en) | 2007-11-28 | 2010-11-16 | Boston Scientific Scimed, Inc. | Bifurcated stent with drug wells for specific ostial, carina, and side branch treatment |
| US8388678B2 (en)* | 2007-12-12 | 2013-03-05 | Boston Scientific Scimed, Inc. | Medical devices having porous component for controlled diffusion |
| US7951193B2 (en) | 2008-07-23 | 2011-05-31 | Boston Scientific Scimed, Inc. | Drug-eluting stent |
| WO2010027679A2 (en)* | 2008-08-27 | 2010-03-11 | Boston Scientific Scimed, Inc. | Medical devices having coatings for therapeutic agent delivery |
| US9011706B2 (en)* | 2008-12-16 | 2015-04-21 | City University Of Hong Kong | Method of making foraminous microstructures |
| US20100183501A1 (en)* | 2009-01-16 | 2010-07-22 | Medtronic Vascular, Inc. | Medical Devices With Nanotextured Titanium Coating |
| US8734829B2 (en)* | 2009-02-13 | 2014-05-27 | Boston Scientific Scimed, Inc. | Medical devices having polymeric nanoporous coatings for controlled therapeutic agent delivery and a nonpolymeric macroporous protective layer |
| EP2512586B1 (en) | 2009-12-18 | 2017-04-19 | Advanced Bionics AG | Cochlear electrode array |
| CN102921038B (en)* | 2012-08-06 | 2014-07-09 | 西南交通大学 | Method for preparing porous scaffold with shape memory function |
| US10959715B2 (en) | 2012-10-31 | 2021-03-30 | W. L. Gore & Associates, Inc. | Devices and methods related to deposited support structures |
| US9700441B2 (en) | 2012-10-31 | 2017-07-11 | W. L. Gore & Associates, Inc. | Devices and methods related to deposited support structures |
| US11744594B2 (en) | 2012-11-16 | 2023-09-05 | W.L. Gore & Associates, Inc. | Space filling devices |
| CN107405429B (en) | 2015-04-16 | 2021-02-12 | 美国医疗设备有限公司 | Fluoropolymer coatings and related methods |
| US10709726B2 (en) | 2015-08-14 | 2020-07-14 | The University Of Sydney | Connexin 45 inhibition for therapy |
| US10385437B2 (en)* | 2016-01-13 | 2019-08-20 | Wisconsin Alumni Research Foundation | Synthesis of metal-oxygen based materials with controlled porosity by oxidative dealloying |
| CN106282962A (en)* | 2016-09-30 | 2017-01-04 | 昆山美淼环保科技有限公司 | A kind of CVD prepares the preprocess method of large area BDD electrode |
| US10481111B2 (en)* | 2016-10-21 | 2019-11-19 | Kla-Tencor Corporation | Calibration of a small angle X-ray scatterometry based metrology system |
| CN110396659B (en)* | 2019-08-30 | 2020-10-27 | 西安交通大学 | Porous material and coating preparation method |
| CN114728083B (en) | 2019-09-27 | 2024-05-14 | Isla科技公司 | Bioartificial pancreas |
| KR102290367B1 (en)* | 2020-08-28 | 2021-08-19 | (주)솔시온바이오메디칼 | Multilayer porous tubular structure with lumen containing differential quantity of bioactive material within each porous layer |
Citations (53)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US2021520A (en)* | 1932-07-15 | 1935-11-19 | Siemens Ag | Method of making bodies consisting of metallic oxides |
| US3190749A (en)* | 1963-07-23 | 1965-06-22 | Du Pont | Alloy article having a porous outer surface and process of making same |
| US3338805A (en)* | 1964-07-28 | 1967-08-29 | Theodore M Pochily | Process for anodizing titanium surfaces |
| US3923969A (en)* | 1973-06-12 | 1975-12-02 | Battelle Institut E V | Carrier system for a drug with sustained release |
| US3948254A (en)* | 1971-11-08 | 1976-04-06 | Alza Corporation | Novel drug delivery device |
| US3993072A (en)* | 1974-08-28 | 1976-11-23 | Alza Corporation | Microporous drug delivery device |
| US4218255A (en)* | 1976-08-30 | 1980-08-19 | University Of Dayton | Porous ceramic carriers for controlled release of proteins, polypeptide hormones, and other substances within human and/or other mamillian species and method |
| US4459252A (en)* | 1975-05-09 | 1984-07-10 | Macgregor David C | Method of forming a small bore flexible vascular graft involving eluting solvent-elutable particles from a polymeric tubular article |
| US4977038A (en)* | 1989-04-14 | 1990-12-11 | Karl Sieradzki | Micro- and nano-porous metallic structures |
| US5246689A (en)* | 1990-01-25 | 1993-09-21 | Mobil Oil Corporation | Synthetic porous crystalline material its synthesis and use |
| US5340614A (en)* | 1993-02-11 | 1994-08-23 | Minnesota Mining And Manufacturing Company | Methods of polymer impregnation |
| US5569198A (en)* | 1995-01-23 | 1996-10-29 | Cortrak Medical Inc. | Microporous catheter |
| US5769884A (en)* | 1996-06-27 | 1998-06-23 | Cordis Corporation | Controlled porosity endovascular implant |
| US5843289A (en)* | 1996-01-22 | 1998-12-01 | Etex Corporation | Surface modification of medical implants |
| US5843172A (en)* | 1997-04-15 | 1998-12-01 | Advanced Cardiovascular Systems, Inc. | Porous medicated stent |
| US5947893A (en)* | 1994-04-27 | 1999-09-07 | Board Of Regents, The University Of Texas System | Method of making a porous prothesis with biodegradable coatings |
| US5972027A (en)* | 1997-09-30 | 1999-10-26 | Scimed Life Systems, Inc | Porous stent drug delivery system |
| US5980551A (en)* | 1997-02-07 | 1999-11-09 | Endovasc Ltd., Inc. | Composition and method for making a biodegradable drug delivery stent |
| US5985307A (en)* | 1993-04-14 | 1999-11-16 | Emory University | Device and method for non-occlusive localized drug delivery |
| US6019784A (en)* | 1996-04-04 | 2000-02-01 | Electroformed Stents, Inc. | Process for making electroformed stents |
| US6027863A (en)* | 1991-09-05 | 2000-02-22 | Intratherapeutics, Inc. | Method for manufacturing a tubular medical device |
| US6093498A (en)* | 1997-05-22 | 2000-07-25 | Alloy Surfaces Co., Inc. | Activated metal and a method for producing the same |
| US6107004A (en)* | 1991-09-05 | 2000-08-22 | Intra Therapeutics, Inc. | Method for making a tubular stent for use in medical applications |
| US6183255B1 (en)* | 2000-03-27 | 2001-02-06 | Yoshiki Oshida | Titanium material implants |
| US6203732B1 (en)* | 1998-07-02 | 2001-03-20 | Intra Therapeutics, Inc. | Method for manufacturing intraluminal device |
| US6240616B1 (en)* | 1997-04-15 | 2001-06-05 | Advanced Cardiovascular Systems, Inc. | Method of manufacturing a medicated porous metal prosthesis |
| US6273913B1 (en)* | 1997-04-18 | 2001-08-14 | Cordis Corporation | Modified stent useful for delivery of drugs along stent strut |
| US20020032414A1 (en)* | 1998-08-20 | 2002-03-14 | Ragheb Anthony O. | Coated implantable medical device |
| US6379381B1 (en)* | 1999-09-03 | 2002-04-30 | Advanced Cardiovascular Systems, Inc. | Porous prosthesis and a method of depositing substances into the pores |
| US20020052650A1 (en)* | 2000-02-01 | 2002-05-02 | Endotex Interventional Systems, Inc. | Micro-porous mesh stent with hybrid structure |
| US20020133224A1 (en)* | 2001-03-13 | 2002-09-19 | Clara Bajgar | Drug eluting encapsulated stent |
| US20020198601A1 (en)* | 2001-06-21 | 2002-12-26 | Syntheon, Llc | Method for microporous surface modification of implantable metallic medical articles and implantable metallic medical articles having such modified surface |
| US6506437B1 (en)* | 2000-10-17 | 2003-01-14 | Advanced Cardiovascular Systems, Inc. | Methods of coating an implantable device having depots formed in a surface thereof |
| US20030060873A1 (en)* | 2001-09-19 | 2003-03-27 | Nanomedical Technologies, Inc. | Metallic structures incorporating bioactive materials and methods for creating the same |
| US20030186522A1 (en)* | 2002-04-02 | 2003-10-02 | Nanosys, Inc. | Methods of positioning and/or orienting nanostructures |
| US20040000046A1 (en)* | 2002-06-27 | 2004-01-01 | Stinson Jonathan S. | Methods of making medical devices |
| US20040026684A1 (en)* | 2002-04-02 | 2004-02-12 | Nanosys, Inc. | Nanowire heterostructures for encoding information |
| US20040039438A1 (en)* | 1998-04-11 | 2004-02-26 | Inflow Dynamics, Inc., A Delaware Corporation | Vascular and endoluminal stents with multi-layer coating including porous radiopaque layer |
| US6709379B1 (en)* | 1998-11-02 | 2004-03-23 | Alcove Surfaces Gmbh | Implant with cavities containing therapeutic agents |
| US6712845B2 (en)* | 2001-04-24 | 2004-03-30 | Advanced Cardiovascular Systems, Inc. | Coating for a stent and a method of forming the same |
| US20040095658A1 (en)* | 2002-09-05 | 2004-05-20 | Nanosys, Inc. | Nanocomposites |
| US20040118448A1 (en)* | 2002-09-05 | 2004-06-24 | Nanosys, Inc. | Nanostructure and nanocomposite based compositions and photovoltaic devices |
| US6758859B1 (en)* | 2000-10-30 | 2004-07-06 | Kenny L. Dang | Increased drug-loading and reduced stress drug delivery device |
| US20040136866A1 (en)* | 2002-06-27 | 2004-07-15 | Nanosys, Inc. | Planar nanowire based sensor elements, devices, systems and methods for using and making same |
| US20040148015A1 (en)* | 2002-11-13 | 2004-07-29 | Setagon, Inc. | Medical devices having porous layers and methods for making same |
| US20040146560A1 (en)* | 2002-09-05 | 2004-07-29 | Nanosys, Inc. | Oriented nanostructures and methods of preparing |
| US6770086B1 (en)* | 2000-11-02 | 2004-08-03 | Scimed Life Systems, Inc. | Stent covering formed of porous polytetraflouroethylene |
| US6797311B2 (en)* | 1999-11-03 | 2004-09-28 | Scimed Life Systems, Inc. | Process for impregnating a porous material with a cross-linkable composition |
| US6805898B1 (en)* | 2000-09-28 | 2004-10-19 | Advanced Cardiovascular Systems, Inc. | Surface features of an implantable medical device |
| US20050070989A1 (en)* | 2002-11-13 | 2005-03-31 | Whye-Kei Lye | Medical devices having porous layers and methods for making the same |
| US20050079200A1 (en)* | 2003-05-16 | 2005-04-14 | Jorg Rathenow | Biocompatibly coated medical implants |
| US6939376B2 (en)* | 2001-11-05 | 2005-09-06 | Sun Biomedical, Ltd. | Drug-delivery endovascular stent and method for treating restenosis |
| US20050209688A1 (en)* | 2004-03-22 | 2005-09-22 | Robert Falotico | Local vascular delivery of Panzem in combination with rapamycin to prevent restenosis following vascular injury |
Family Cites Families (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| ATE420628T1 (en) | 1993-07-19 | 2009-01-15 | Angiotech Pharm Inc | ANTI-ANGIogenic AGENTS AND METHODS OF USE THEREOF |
| US6652581B1 (en) | 1998-07-07 | 2003-11-25 | Boston Scientific Scimed, Inc. | Medical device with porous surface for controlled drug release and method of making the same |
| DE19948783C2 (en) | 1999-02-18 | 2001-06-13 | Alcove Surfaces Gmbh | Implant |
| EP1319416B1 (en)* | 2001-12-12 | 2004-11-03 | Hehrlein, Christoph, Dr. | Porous metallic stent with a ceramic coating |
- 2003
- 2003-11-12ATAT03786730Tpatent/ATE402675T1/ennot_activeIP Right Cessation
- 2003-11-12CNCNA2003801030682Apatent/CN1725988A/enactivePending
- 2003-11-12JPJP2004552227Apatent/JP2006514848A/enactivePending
- 2003-11-12AUAU2003295535Apatent/AU2003295535B2/ennot_activeCeased
- 2003-11-12DEDE60322581Tpatent/DE60322581D1/ennot_activeExpired - Lifetime
- 2003-11-12WOPCT/US2003/036451patent/WO2004043292A2/enactiveApplication Filing
- 2003-11-12EPEP03786730Apatent/EP1572032B1/ennot_activeExpired - Lifetime
- 2003-11-12CACA002503625Apatent/CA2503625A1/ennot_activeAbandoned
- 2003-11-12KRKR1020057007517Apatent/KR100826574B1/ennot_activeExpired - Lifetime
- 2003-11-13USUS10/713,244patent/US7294409B2/ennot_activeExpired - Lifetime
- 2006
- 2006-05-11USUS11/432,281patent/US20060271169A1/ennot_activeAbandoned
Patent Citations (58)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US2021520A (en)* | 1932-07-15 | 1935-11-19 | Siemens Ag | Method of making bodies consisting of metallic oxides |
| US3190749A (en)* | 1963-07-23 | 1965-06-22 | Du Pont | Alloy article having a porous outer surface and process of making same |
| US3338805A (en)* | 1964-07-28 | 1967-08-29 | Theodore M Pochily | Process for anodizing titanium surfaces |
| US3948254A (en)* | 1971-11-08 | 1976-04-06 | Alza Corporation | Novel drug delivery device |
| US3923969A (en)* | 1973-06-12 | 1975-12-02 | Battelle Institut E V | Carrier system for a drug with sustained release |
| US3993072A (en)* | 1974-08-28 | 1976-11-23 | Alza Corporation | Microporous drug delivery device |
| US4459252A (en)* | 1975-05-09 | 1984-07-10 | Macgregor David C | Method of forming a small bore flexible vascular graft involving eluting solvent-elutable particles from a polymeric tubular article |
| US4218255A (en)* | 1976-08-30 | 1980-08-19 | University Of Dayton | Porous ceramic carriers for controlled release of proteins, polypeptide hormones, and other substances within human and/or other mamillian species and method |
| US4977038A (en)* | 1989-04-14 | 1990-12-11 | Karl Sieradzki | Micro- and nano-porous metallic structures |
| US5246689A (en)* | 1990-01-25 | 1993-09-21 | Mobil Oil Corporation | Synthetic porous crystalline material its synthesis and use |
| US6027863A (en)* | 1991-09-05 | 2000-02-22 | Intratherapeutics, Inc. | Method for manufacturing a tubular medical device |
| US6107004A (en)* | 1991-09-05 | 2000-08-22 | Intra Therapeutics, Inc. | Method for making a tubular stent for use in medical applications |
| US5508060A (en)* | 1993-02-11 | 1996-04-16 | Minnesota Mining And Manufacturing Company | Method of polymer impregnation |
| US5340614A (en)* | 1993-02-11 | 1994-08-23 | Minnesota Mining And Manufacturing Company | Methods of polymer impregnation |
| US5985307A (en)* | 1993-04-14 | 1999-11-16 | Emory University | Device and method for non-occlusive localized drug delivery |
| US5947893A (en)* | 1994-04-27 | 1999-09-07 | Board Of Regents, The University Of Texas System | Method of making a porous prothesis with biodegradable coatings |
| US5569198A (en)* | 1995-01-23 | 1996-10-29 | Cortrak Medical Inc. | Microporous catheter |
| US5843289A (en)* | 1996-01-22 | 1998-12-01 | Etex Corporation | Surface modification of medical implants |
| US6019784A (en)* | 1996-04-04 | 2000-02-01 | Electroformed Stents, Inc. | Process for making electroformed stents |
| US5769884A (en)* | 1996-06-27 | 1998-06-23 | Cordis Corporation | Controlled porosity endovascular implant |
| US5980551A (en)* | 1997-02-07 | 1999-11-09 | Endovasc Ltd., Inc. | Composition and method for making a biodegradable drug delivery stent |
| US5843172A (en)* | 1997-04-15 | 1998-12-01 | Advanced Cardiovascular Systems, Inc. | Porous medicated stent |
| US6240616B1 (en)* | 1997-04-15 | 2001-06-05 | Advanced Cardiovascular Systems, Inc. | Method of manufacturing a medicated porous metal prosthesis |
| US6273913B1 (en)* | 1997-04-18 | 2001-08-14 | Cordis Corporation | Modified stent useful for delivery of drugs along stent strut |
| US6093498A (en)* | 1997-05-22 | 2000-07-25 | Alloy Surfaces Co., Inc. | Activated metal and a method for producing the same |
| US5972027A (en)* | 1997-09-30 | 1999-10-26 | Scimed Life Systems, Inc | Porous stent drug delivery system |
| US20040039438A1 (en)* | 1998-04-11 | 2004-02-26 | Inflow Dynamics, Inc., A Delaware Corporation | Vascular and endoluminal stents with multi-layer coating including porous radiopaque layer |
| US6203732B1 (en)* | 1998-07-02 | 2001-03-20 | Intra Therapeutics, Inc. | Method for manufacturing intraluminal device |
| US20020032414A1 (en)* | 1998-08-20 | 2002-03-14 | Ragheb Anthony O. | Coated implantable medical device |
| US6709379B1 (en)* | 1998-11-02 | 2004-03-23 | Alcove Surfaces Gmbh | Implant with cavities containing therapeutic agents |
| US6379381B1 (en)* | 1999-09-03 | 2002-04-30 | Advanced Cardiovascular Systems, Inc. | Porous prosthesis and a method of depositing substances into the pores |
| US6797311B2 (en)* | 1999-11-03 | 2004-09-28 | Scimed Life Systems, Inc. | Process for impregnating a porous material with a cross-linkable composition |
| US20020052650A1 (en)* | 2000-02-01 | 2002-05-02 | Endotex Interventional Systems, Inc. | Micro-porous mesh stent with hybrid structure |
| US6183255B1 (en)* | 2000-03-27 | 2001-02-06 | Yoshiki Oshida | Titanium material implants |
| US6805898B1 (en)* | 2000-09-28 | 2004-10-19 | Advanced Cardiovascular Systems, Inc. | Surface features of an implantable medical device |
| US6506437B1 (en)* | 2000-10-17 | 2003-01-14 | Advanced Cardiovascular Systems, Inc. | Methods of coating an implantable device having depots formed in a surface thereof |
| US6758859B1 (en)* | 2000-10-30 | 2004-07-06 | Kenny L. Dang | Increased drug-loading and reduced stress drug delivery device |
| US6770086B1 (en)* | 2000-11-02 | 2004-08-03 | Scimed Life Systems, Inc. | Stent covering formed of porous polytetraflouroethylene |
| US20020133224A1 (en)* | 2001-03-13 | 2002-09-19 | Clara Bajgar | Drug eluting encapsulated stent |
| US20040073298A1 (en)* | 2001-04-24 | 2004-04-15 | Hossainy Syed Faiyaz Ahmed | Coating for a stent and a method of forming the same |
| US6712845B2 (en)* | 2001-04-24 | 2004-03-30 | Advanced Cardiovascular Systems, Inc. | Coating for a stent and a method of forming the same |
| US6527938B2 (en)* | 2001-06-21 | 2003-03-04 | Syntheon, Llc | Method for microporous surface modification of implantable metallic medical articles |
| US20020198601A1 (en)* | 2001-06-21 | 2002-12-26 | Syntheon, Llc | Method for microporous surface modification of implantable metallic medical articles and implantable metallic medical articles having such modified surface |
| US20050106212A1 (en)* | 2001-09-19 | 2005-05-19 | Gertner Michael E. | Metallic structures incorporating bioactive materials and methods for creating the same |
| US20030060873A1 (en)* | 2001-09-19 | 2003-03-27 | Nanomedical Technologies, Inc. | Metallic structures incorporating bioactive materials and methods for creating the same |
| US6939376B2 (en)* | 2001-11-05 | 2005-09-06 | Sun Biomedical, Ltd. | Drug-delivery endovascular stent and method for treating restenosis |
| US20040005723A1 (en)* | 2002-04-02 | 2004-01-08 | Nanosys, Inc. | Methods of making, positioning and orienting nanostructures, nanostructure arrays and nanostructure devices |
| US20040026684A1 (en)* | 2002-04-02 | 2004-02-12 | Nanosys, Inc. | Nanowire heterostructures for encoding information |
| US20030186522A1 (en)* | 2002-04-02 | 2003-10-02 | Nanosys, Inc. | Methods of positioning and/or orienting nanostructures |
| US20040000046A1 (en)* | 2002-06-27 | 2004-01-01 | Stinson Jonathan S. | Methods of making medical devices |
| US20040136866A1 (en)* | 2002-06-27 | 2004-07-15 | Nanosys, Inc. | Planar nanowire based sensor elements, devices, systems and methods for using and making same |
| US20040095658A1 (en)* | 2002-09-05 | 2004-05-20 | Nanosys, Inc. | Nanocomposites |
| US20040146560A1 (en)* | 2002-09-05 | 2004-07-29 | Nanosys, Inc. | Oriented nanostructures and methods of preparing |
| US20040118448A1 (en)* | 2002-09-05 | 2004-06-24 | Nanosys, Inc. | Nanostructure and nanocomposite based compositions and photovoltaic devices |
| US20050070989A1 (en)* | 2002-11-13 | 2005-03-31 | Whye-Kei Lye | Medical devices having porous layers and methods for making the same |
| US20040148015A1 (en)* | 2002-11-13 | 2004-07-29 | Setagon, Inc. | Medical devices having porous layers and methods for making same |
| US20050079200A1 (en)* | 2003-05-16 | 2005-04-14 | Jorg Rathenow | Biocompatibly coated medical implants |
| US20050209688A1 (en)* | 2004-03-22 | 2005-09-22 | Robert Falotico | Local vascular delivery of Panzem in combination with rapamycin to prevent restenosis following vascular injury |
Cited By (80)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US8066763B2 (en) | 1998-04-11 | 2011-11-29 | Boston Scientific Scimed, Inc. | Drug-releasing stent with ceramic-containing layer |
| US8430923B2 (en) | 2000-03-24 | 2013-04-30 | Abbott Cardiovascular Systems, Inc. | Radiopaque intraluminal stent |
| US20070185564A1 (en)* | 2000-03-24 | 2007-08-09 | Advanced Cardiovascular Systems, Inc. | Radiopaque intraluminal stent |
| US8852264B2 (en) | 2000-03-24 | 2014-10-07 | Abbott Cardiovascular Systems, Inc. | Radiopaque intraluminal stent |
| US8303643B2 (en) | 2001-06-27 | 2012-11-06 | Remon Medical Technologies Ltd. | Method and device for electrochemical formation of therapeutic species in vivo |
| US20060127442A1 (en)* | 2004-12-09 | 2006-06-15 | Helmus Michael N | Use of supercritical fluids to incorporate biologically active agents into nanoporous medical articles |
| US8840660B2 (en) | 2006-01-05 | 2014-09-23 | Boston Scientific Scimed, Inc. | Bioerodible endoprostheses and methods of making the same |
| US20070173925A1 (en)* | 2006-01-25 | 2007-07-26 | Cornova, Inc. | Flexible expandable stent |
| US8089029B2 (en) | 2006-02-01 | 2012-01-03 | Boston Scientific Scimed, Inc. | Bioabsorbable metal medical device and method of manufacture |
| US8597341B2 (en) | 2006-03-06 | 2013-12-03 | David Elmaleh | Intravascular device with netting system |
| US20070208412A1 (en)* | 2006-03-06 | 2007-09-06 | David Elmaleh | Intravascular device with netting system |
| US8574615B2 (en) | 2006-03-24 | 2013-11-05 | Boston Scientific Scimed, Inc. | Medical devices having nanoporous coatings for controlled therapeutic agent delivery |
| US8187620B2 (en) | 2006-03-27 | 2012-05-29 | Boston Scientific Scimed, Inc. | Medical devices comprising a porous metal oxide or metal material and a polymer coating for delivering therapeutic agents |
| US8048150B2 (en) | 2006-04-12 | 2011-11-01 | Boston Scientific Scimed, Inc. | Endoprosthesis having a fiber meshwork disposed thereon |
| US8815275B2 (en) | 2006-06-28 | 2014-08-26 | Boston Scientific Scimed, Inc. | Coatings for medical devices comprising a therapeutic agent and a metallic material |
| US8771343B2 (en) | 2006-06-29 | 2014-07-08 | Boston Scientific Scimed, Inc. | Medical devices with selective titanium oxide coatings |
| US8052743B2 (en) | 2006-08-02 | 2011-11-08 | Boston Scientific Scimed, Inc. | Endoprosthesis with three-dimensional disintegration control |
| US20080177371A1 (en)* | 2006-08-28 | 2008-07-24 | Cornova, Inc. | Implantable devices and methods of forming the same |
| US20080215132A1 (en)* | 2006-08-28 | 2008-09-04 | Cornova, Inc. | Implantable devices having textured surfaces and methods of forming the same |
| US8353949B2 (en) | 2006-09-14 | 2013-01-15 | Boston Scientific Scimed, Inc. | Medical devices with drug-eluting coating |
| US8128689B2 (en) | 2006-09-15 | 2012-03-06 | Boston Scientific Scimed, Inc. | Bioerodible endoprosthesis with biostable inorganic layers |
| US8052744B2 (en) | 2006-09-15 | 2011-11-08 | Boston Scientific Scimed, Inc. | Medical devices and methods of making the same |
| US8057534B2 (en) | 2006-09-15 | 2011-11-15 | Boston Scientific Scimed, Inc. | Bioerodible endoprostheses and methods of making the same |
| US8808726B2 (en) | 2006-09-15 | 2014-08-19 | Boston Scientific Scimed. Inc. | Bioerodible endoprostheses and methods of making the same |
| US8002821B2 (en) | 2006-09-18 | 2011-08-23 | Boston Scientific Scimed, Inc. | Bioerodible metallic ENDOPROSTHESES |
| US7981150B2 (en) | 2006-11-09 | 2011-07-19 | Boston Scientific Scimed, Inc. | Endoprosthesis with coatings |
| US8715339B2 (en) | 2006-12-28 | 2014-05-06 | Boston Scientific Scimed, Inc. | Bioerodible endoprostheses and methods of making the same |
| US8080055B2 (en) | 2006-12-28 | 2011-12-20 | Boston Scientific Scimed, Inc. | Bioerodible endoprostheses and methods of making the same |
| US8070797B2 (en) | 2007-03-01 | 2011-12-06 | Boston Scientific Scimed, Inc. | Medical device with a porous surface for delivery of a therapeutic agent |
| US8431149B2 (en) | 2007-03-01 | 2013-04-30 | Boston Scientific Scimed, Inc. | Coated medical devices for abluminal drug delivery |
| US8067054B2 (en) | 2007-04-05 | 2011-11-29 | Boston Scientific Scimed, Inc. | Stents with ceramic drug reservoir layer and methods of making and using the same |
| WO2008130617A3 (en)* | 2007-04-18 | 2008-12-18 | David Elmaleh | Intravascular device with netting system |
| US8801777B2 (en) | 2007-04-18 | 2014-08-12 | David Elmaleh | Intravascular device with netting system |
| US20080262598A1 (en)* | 2007-04-18 | 2008-10-23 | David Elmaleh | Intravascular device with netting system |
| US7976915B2 (en) | 2007-05-23 | 2011-07-12 | Boston Scientific Scimed, Inc. | Endoprosthesis with select ceramic morphology |
| US8002823B2 (en) | 2007-07-11 | 2011-08-23 | Boston Scientific Scimed, Inc. | Endoprosthesis coating |
| US7942926B2 (en) | 2007-07-11 | 2011-05-17 | Boston Scientific Scimed, Inc. | Endoprosthesis coating |
| US9284409B2 (en) | 2007-07-19 | 2016-03-15 | Boston Scientific Scimed, Inc. | Endoprosthesis having a non-fouling surface |
| US8815273B2 (en) | 2007-07-27 | 2014-08-26 | Boston Scientific Scimed, Inc. | Drug eluting medical devices having porous layers |
| US7931683B2 (en) | 2007-07-27 | 2011-04-26 | Boston Scientific Scimed, Inc. | Articles having ceramic coated surfaces |
| US8221822B2 (en) | 2007-07-31 | 2012-07-17 | Boston Scientific Scimed, Inc. | Medical device coating by laser cladding |
| US8900292B2 (en) | 2007-08-03 | 2014-12-02 | Boston Scientific Scimed, Inc. | Coating for medical device having increased surface area |
| US8052745B2 (en) | 2007-09-13 | 2011-11-08 | Boston Scientific Scimed, Inc. | Endoprosthesis |
| US20090112315A1 (en)* | 2007-10-29 | 2009-04-30 | Zimmer, Inc. | Medical implants and methods for delivering biologically active agents |
| US8216632B2 (en) | 2007-11-02 | 2012-07-10 | Boston Scientific Scimed, Inc. | Endoprosthesis coating |
| US8029554B2 (en) | 2007-11-02 | 2011-10-04 | Boston Scientific Scimed, Inc. | Stent with embedded material |
| US7938855B2 (en) | 2007-11-02 | 2011-05-10 | Boston Scientific Scimed, Inc. | Deformable underlayer for stent |
| US8920491B2 (en) | 2008-04-22 | 2014-12-30 | Boston Scientific Scimed, Inc. | Medical devices having a coating of inorganic material |
| US8932346B2 (en) | 2008-04-24 | 2015-01-13 | Boston Scientific Scimed, Inc. | Medical devices having inorganic particle layers |
| US7998192B2 (en) | 2008-05-09 | 2011-08-16 | Boston Scientific Scimed, Inc. | Endoprostheses |
| US8236046B2 (en) | 2008-06-10 | 2012-08-07 | Boston Scientific Scimed, Inc. | Bioerodible endoprosthesis |
| US8449603B2 (en) | 2008-06-18 | 2013-05-28 | Boston Scientific Scimed, Inc. | Endoprosthesis coating |
| US7985252B2 (en) | 2008-07-30 | 2011-07-26 | Boston Scientific Scimed, Inc. | Bioerodible endoprosthesis |
| US8382824B2 (en) | 2008-10-03 | 2013-02-26 | Boston Scientific Scimed, Inc. | Medical implant having NANO-crystal grains with barrier layers of metal nitrides or fluorides |
| US8231980B2 (en) | 2008-12-03 | 2012-07-31 | Boston Scientific Scimed, Inc. | Medical implants including iridium oxide |
| US8267992B2 (en) | 2009-03-02 | 2012-09-18 | Boston Scientific Scimed, Inc. | Self-buffering medical implants |
| US8071156B2 (en) | 2009-03-04 | 2011-12-06 | Boston Scientific Scimed, Inc. | Endoprostheses |
| US8287937B2 (en) | 2009-04-24 | 2012-10-16 | Boston Scientific Scimed, Inc. | Endoprosthese |
| US20120283828A1 (en)* | 2009-11-05 | 2012-11-08 | Nonwotecc Medical Gmbh | Non-woven fabric for medical use and process for the preparation thereof |
| US8668732B2 (en) | 2010-03-23 | 2014-03-11 | Boston Scientific Scimed, Inc. | Surface treated bioerodible metal endoprostheses |
| KR101223977B1 (en) | 2010-05-26 | 2013-01-18 | 경상대학교산학협력단 | Method of manufacturing nano-structured stent inhibiting toxicity |
| US10441445B2 (en) | 2010-11-17 | 2019-10-15 | Abbott Cardiovascular Systems, Inc. | Radiopaque intraluminal stents comprising cobalt-based alloys containing one or more platinum group metals, refractory metals, or combinations thereof |
| US11779477B2 (en) | 2010-11-17 | 2023-10-10 | Abbott Cardiovascular Systems, Inc. | Radiopaque intraluminal stents |
| US12150872B2 (en) | 2010-11-17 | 2024-11-26 | Abbott Cardiovascular Systems, Inc. | Radiopaque intraluminal stents |
| US9566147B2 (en) | 2010-11-17 | 2017-02-14 | Abbott Cardiovascular Systems, Inc. | Radiopaque intraluminal stents comprising cobalt-based alloys containing one or more platinum group metals, refractory metals, or combinations thereof |
| US11298251B2 (en) | 2010-11-17 | 2022-04-12 | Abbott Cardiovascular Systems, Inc. | Radiopaque intraluminal stents comprising cobalt-based alloys with primarily single-phase supersaturated tungsten content |
| US20120141820A1 (en)* | 2010-12-01 | 2012-06-07 | Hon Hai Precision Industry Co., Ltd. | Porous metal article and about method for manufacturing same |
| US8470158B2 (en)* | 2010-12-01 | 2013-06-25 | Hong Fu Jin Precision Industry (Shenzhen) Co., Ltd. | Porous metal article and about method for manufacturing same |
| US20120244377A1 (en)* | 2011-03-23 | 2012-09-27 | Hon Hai Precision Industry Co., Ltd. | Porous metal article and about method for manufacturing same |
| US8512545B2 (en)* | 2011-03-23 | 2013-08-20 | Hong Fu Jin Precision Industry (Shenzhen) Co., Ltd. | Porous metal article and about method for manufacturing same |
| US11806488B2 (en) | 2011-06-29 | 2023-11-07 | Abbott Cardiovascular Systems, Inc. | Medical device including a solderable linear elastic nickel-titanium distal end section and methods of preparation therefor |
| US9974672B2 (en) | 2012-10-15 | 2018-05-22 | David R Elmaleh | Material structures for intravascular device |
| US10499939B2 (en) | 2013-11-13 | 2019-12-10 | Covidien Lp | Galvanically assisted attachment of medical devices to thrombus |
| US9795400B2 (en)* | 2013-11-13 | 2017-10-24 | Covidien Lp | Galvanically assisted attachment of medical devices to thrombus |
| US11317931B2 (en) | 2013-11-13 | 2022-05-03 | Covidien Lp | Electrically assisted attachment of medical devices to thrombus |
| US20150133990A1 (en)* | 2013-11-13 | 2015-05-14 | Covidien Lp | Galvanically assisted attachment of medical devices to thrombus |
| US12383289B2 (en) | 2013-11-13 | 2025-08-12 | Covidien Lp | Electrically assisted attachment of medical devices to thrombus |
| US10265515B2 (en) | 2015-03-27 | 2019-04-23 | Covidien Lp | Galvanically assisted aneurysm treatment |
| US12151049B2 (en) | 2019-10-14 | 2024-11-26 | Abbott Cardiovascular Systems, Inc. | Methods for manufacturing radiopaque intraluminal stents comprising cobalt-based alloys with supersaturated tungsten content |
| US11964337B2 (en)* | 2022-04-14 | 2024-04-23 | Shandong University | NiTi alloy surface cutting process and roughness adjustment method |
Also Published As
| Publication number | Publication date |
|---|---|
| WO2004043292A2 (en) | 2004-05-27 |
| EP1572032B1 (en) | 2008-07-30 |
| US20040148015A1 (en) | 2004-07-29 |
| KR20050065643A (en) | 2005-06-29 |
| AU2003295535B2 (en) | 2007-12-20 |
| US7294409B2 (en) | 2007-11-13 |
| CN1725988A (en) | 2006-01-25 |
| KR100826574B1 (en) | 2008-04-30 |
| DE60322581D1 (en) | 2008-09-11 |
| JP2006514848A (en) | 2006-05-18 |
| EP1572032A2 (en) | 2005-09-14 |
| EP1572032A4 (en) | 2006-01-04 |
| ATE402675T1 (en) | 2008-08-15 |
| AU2003295535A1 (en) | 2004-06-03 |
| CA2503625A1 (en) | 2004-05-27 |
| WO2004043292A3 (en) | 2005-02-10 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| US7294409B2 (en) | Medical devices having porous layers and methods for making same | |
| US20060276879A1 (en) | Medical devices having porous layers and methods for making the same | |
| US7208172B2 (en) | Metallic composite coating for delivery of therapeutic agents from the surface of implantable devices | |
| EP2214739B1 (en) | Endoprosthesis with porous reservoir | |
| US20050119723A1 (en) | Medical device with porous surface containing bioerodable bioactive composites and related methods | |
| US20050106212A1 (en) | Metallic structures incorporating bioactive materials and methods for creating the same | |
| JP5410440B2 (en) | Endoprosthesis with porous reservoir and non-polymeric diffusion layer | |
| US20100057188A1 (en) | Endoprostheses with porous regions and non-polymeric coating | |
| US20090118812A1 (en) | Endoprosthesis coating | |
| US20050251245A1 (en) | Methods and apparatus with porous materials | |
| KR20070063511A (en) | Medical device having a nanoporous layer and a method of manufacturing the same | |
| JP2006526426A (en) | Method for forming a porous drug delivery layer | |
| CA2574972C (en) | Metallic drug-releasing medical devices and method of making same | |
| CN101296715A (en) | A method for production of a coated endovascular device | |
| EP1839626A1 (en) | Medical devices having porous layers and methods for making same | |
| US20100274352A1 (en) | Endoprosthesis with Selective Drug Coatings |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| AS | Assignment | Owner name:UNIVERSITY OF VIRGINIA PATENT FOUNDATION, VIRGINIA Free format text:ASSIGNMENT OF ASSIGNORS INTEREST;ASSIGNOR:UNIVERSITY OF VIRGINIA;REEL/FRAME:018506/0268 Effective date:20061109 Owner name:UNIVERSITY OF VIRGINIA, VIRGINIA Free format text:ASSIGNMENT OF ASSIGNORS INTEREST;ASSIGNORS:LYE, WHYE-KEI;REED, MICHAEL L.;REEL/FRAME:018506/0216;SIGNING DATES FROM 20060824 TO 20060927 | |
| AS | Assignment | Owner name:MEDTRONIC VASCULAR, INC., CALIFORNIA Free format text:MERGER;ASSIGNOR:SETAGON, INC.;REEL/FRAME:021852/0703 Effective date:20071002 Owner name:MEDTRONIC VASCULAR, INC.,CALIFORNIA Free format text:MERGER;ASSIGNOR:SETAGON, INC.;REEL/FRAME:021852/0703 Effective date:20071002 | |
| STCB | Information on status: application discontinuation | Free format text:ABANDONED -- FAILURE TO RESPOND TO AN OFFICE ACTION |