US20050281860A1 - Surgically implanted devices having reduced scar tissue formation - Google Patents
Surgically implanted devices having reduced scar tissue formationDownload PDFInfo
- Publication number
- US20050281860A1 US20050281860A1US11/084,948US8494805AUS2005281860A1US 20050281860 A1US20050281860 A1US 20050281860A1US 8494805 AUS8494805 AUS 8494805AUS 2005281860 A1US2005281860 A1US 2005281860A1
- Authority
- US
- United States
- Prior art keywords
- rapamycin
- vascular structure
- sleeve
- drug
- proliferative
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Abandoned
Links
- 231100000241scarToxicity0.000titledescription23
- 230000009772tissue formationEffects0.000titledescription14
- 230000001028anti-proliverative effectEffects0.000claimsabstractdescription73
- 229960002930sirolimusDrugs0.000claimsabstractdescription31
- QFJCIRLUMZQUOT-HPLJOQBZSA-NsirolimusChemical compoundC1C[C@@H](O)[C@H](OC)C[C@@H]1C[C@@H](C)[C@H]1OC(=O)[C@@H]2CCCCN2C(=O)C(=O)[C@](O)(O2)[C@H](C)CC[C@H]2C[C@H](OC)/C(C)=C/C=C/C=C/[C@@H](C)C[C@@H](C)C(=O)[C@H](OC)[C@H](O)/C(C)=C/[C@@H](C)C(=O)C1QFJCIRLUMZQUOT-HPLJOQBZSA-N0.000claimsabstractdescription20
- ZAHRKKWIAAJSAO-UHFFFAOYSA-NrapamycinNatural productsCOCC(O)C(=C/C(C)C(=O)CC(OC(=O)C1CCCCN1C(=O)C(=O)C2(O)OC(CC(OC)C(=CC=CC=CC(C)CC(C)C(=O)C)C)CCC2C)C(C)CC3CCC(O)C(C3)OC)CZAHRKKWIAAJSAO-UHFFFAOYSA-N0.000claimsabstractdescription19
- 238000000034methodMethods0.000claimsdescription20
- 230000002792vascularEffects0.000claimsdescription14
- 201000010099diseaseDiseases0.000claims6
- 208000037265diseases, disorders, signs and symptomsDiseases0.000claims6
- HTTJABKRGRZYRN-UHFFFAOYSA-NHeparinChemical compoundOC1C(NC(=O)C)C(O)OC(COS(O)(=O)=O)C1OC1C(OS(O)(=O)=O)C(O)C(OC2C(C(OS(O)(=O)=O)C(OC3C(C(O)C(O)C(O3)C(O)=O)OS(O)(=O)=O)C(CO)O2)NS(O)(=O)=O)C(C(O)=O)O1HTTJABKRGRZYRN-UHFFFAOYSA-N0.000claims5
- 229960002897heparinDrugs0.000claims5
- 229920000669heparinPolymers0.000claims5
- 239000011159matrix materialSubstances0.000claims4
- 238000010276constructionMethods0.000claims3
- 230000001225therapeutic effectEffects0.000claims3
- 206010003226Arteriovenous fistulaDiseases0.000claims2
- 230000002195synergetic effectEffects0.000claims2
- 239000003814drugSubstances0.000abstractdescription79
- 229940079593drugDrugs0.000abstractdescription78
- 239000000463materialSubstances0.000abstractdescription15
- 239000007943implantSubstances0.000abstractdescription12
- 229930012538PaclitaxelNatural products0.000abstractdescription6
- 229960001592paclitaxelDrugs0.000abstractdescription6
- RCINICONZNJXQF-MZXODVADSA-NtaxolChemical compoundO([C@@H]1[C@@]2(C[C@@H](C(C)=C(C2(C)C)[C@H](C([C@]2(C)[C@@H](O)C[C@H]3OC[C@]3([C@H]21)OC(C)=O)=O)OC(=O)C)OC(=O)[C@H](O)[C@@H](NC(=O)C=1C=CC=CC=1)C=1C=CC=CC=1)O)C(=O)C1=CC=CC=C1RCINICONZNJXQF-MZXODVADSA-N0.000abstractdescription6
- 230000003872anastomosisEffects0.000abstractdescription2
- 210000001519tissueAnatomy0.000description25
- 239000003795chemical substances by applicationSubstances0.000description16
- 238000001356surgical procedureMethods0.000description15
- AOJJSUZBOXZQNB-TZSSRYMLSA-NDoxorubicinChemical compoundO([C@H]1C[C@@](O)(CC=2C(O)=C3C(=O)C=4C=CC=C(C=4C(=O)C3=C(O)C=21)OC)C(=O)CO)[C@H]1C[C@H](N)[C@H](O)[C@H](C)O1AOJJSUZBOXZQNB-TZSSRYMLSA-N0.000description12
- 210000000481breastAnatomy0.000description10
- 230000004663cell proliferationEffects0.000description8
- NWIBSHFKIJFRCO-WUDYKRTCSA-NMytomycinChemical compoundC1N2C(C(C(C)=C(N)C3=O)=O)=C3[C@@H](COC(N)=O)[C@@]2(OC)[C@@H]2[C@H]1N2NWIBSHFKIJFRCO-WUDYKRTCSA-N0.000description6
- OGWKCGZFUXNPDA-XQKSVPLYSA-NvincristineChemical compoundC([N@]1C[C@@H](C[C@]2(C(=O)OC)C=3C(=CC4=C([C@]56[C@H]([C@@]([C@H](OC(C)=O)[C@]7(CC)C=CCN([C@H]67)CC5)(O)C(=O)OC)N4C=O)C=3)OC)C[C@@](C1)(O)CC)CC1=C2NC2=CC=CC=C12OGWKCGZFUXNPDA-XQKSVPLYSA-N0.000description6
- 229960004528vincristineDrugs0.000description6
- OGWKCGZFUXNPDA-UHFFFAOYSA-NvincristineNatural productsC1C(CC)(O)CC(CC2(C(=O)OC)C=3C(=CC4=C(C56C(C(C(OC(C)=O)C7(CC)C=CCN(C67)CC5)(O)C(=O)OC)N4C=O)C=3)OC)CN1CCC1=C2NC2=CC=CC=C12OGWKCGZFUXNPDA-UHFFFAOYSA-N0.000description6
- 230000015572biosynthetic processEffects0.000description5
- 239000003356suture materialSubstances0.000description5
- 230000009471actionEffects0.000description4
- 210000003462veinAnatomy0.000description4
- OUNADCPYEMRCEK-AHTHDSRYSA-N(1R,9S,12S,15R,16E,18R,19R,21R,23S,24Z,26E,28E,32S,35R)-1,18-dihydroxy-12-[(2R)-1-[(1S,3R,4R)-4-hydroxy-3-methoxycyclohexyl]propan-2-yl]-19-methoxy-15,17,21,23,29,35-hexamethyl-11,36-dioxa-4-azatricyclo[30.3.1.04,9]hexatriaconta-16,24,26,28-tetraene-2,3,10,14,20-pentoneChemical compoundC1C[C@@H](O)[C@H](OC)C[C@@H]1C[C@@H](C)[C@H]1OC(=O)[C@@H]2CCCCN2C(=O)C(=O)[C@](O)(O2)[C@H](C)CC[C@H]2CC\C(C)=C\C=C\C=C/[C@@H](C)C[C@@H](C)C(=O)[C@H](OC)[C@H](O)/C(C)=C/[C@@H](C)C(=O)C1OUNADCPYEMRCEK-AHTHDSRYSA-N0.000description3
- VSNHCAURESNICA-NJFSPNSNSA-N1-oxidanylureaChemical compoundN[14C](=O)NOVSNHCAURESNICA-NJFSPNSNSA-N0.000description3
- DLGOEMSEDOSKAD-UHFFFAOYSA-NCarmustineChemical compoundClCCNC(=O)N(N=O)CCClDLGOEMSEDOSKAD-UHFFFAOYSA-N0.000description3
- CMSMOCZEIVJLDB-UHFFFAOYSA-NCyclophosphamideChemical compoundClCCN(CCCl)P1(=O)NCCCO1CMSMOCZEIVJLDB-UHFFFAOYSA-N0.000description3
- HKVAMNSJSFKALM-GKUWKFKPSA-NEverolimusChemical compoundC1C[C@@H](OCCO)[C@H](OC)C[C@@H]1C[C@@H](C)[C@H]1OC(=O)[C@@H]2CCCCN2C(=O)C(=O)[C@](O)(O2)[C@H](C)CC[C@H]2C[C@H](OC)/C(C)=C/C=C/C=C/[C@@H](C)C[C@@H](C)C(=O)[C@H](OC)[C@H](O)/C(C)=C/[C@@H](C)C(=O)C1HKVAMNSJSFKALM-GKUWKFKPSA-N0.000description3
- GHASVSINZRGABV-UHFFFAOYSA-NFluorouracilChemical compoundFC1=CNC(=O)NC1=OGHASVSINZRGABV-UHFFFAOYSA-N0.000description3
- XDXDZDZNSLXDNA-TZNDIEGXSA-NIdarubicinChemical compoundC1[C@H](N)[C@H](O)[C@H](C)O[C@H]1O[C@@H]1C2=C(O)C(C(=O)C3=CC=CC=C3C3=O)=C3C(O)=C2C[C@@](O)(C(C)=O)C1XDXDZDZNSLXDNA-TZNDIEGXSA-N0.000description3
- FBOZXECLQNJBKD-ZDUSSCGKSA-NL-methotrexateChemical compoundC=1N=C2N=C(N)N=C(N)C2=NC=1CN(C)C1=CC=C(C(=O)N[C@@H](CCC(O)=O)C(O)=O)C=C1FBOZXECLQNJBKD-ZDUSSCGKSA-N0.000description3
- ONIBWKKTOPOVIA-UHFFFAOYSA-NProlineNatural productsOC(=O)C1CCCN1ONIBWKKTOPOVIA-UHFFFAOYSA-N0.000description3
- CBPNZQVSJQDFBE-FUXHJELOSA-NTemsirolimusChemical compoundC1C[C@@H](OC(=O)C(C)(CO)CO)[C@H](OC)C[C@@H]1C[C@@H](C)[C@H]1OC(=O)[C@@H]2CCCCN2C(=O)C(=O)[C@](O)(O2)[C@H](C)CC[C@H]2C[C@H](OC)/C(C)=C/C=C/C=C/[C@@H](C)C[C@@H](C)C(=O)[C@H](OC)[C@H](O)/C(C)=C/[C@@H](C)C(=O)C1CBPNZQVSJQDFBE-FUXHJELOSA-N0.000description3
- 229940009456adriamycinDrugs0.000description3
- 229940098174alkeranDrugs0.000description3
- 230000008901benefitEffects0.000description3
- 229940108502bicnuDrugs0.000description3
- JCKYGMPEJWAADB-UHFFFAOYSA-NchlorambucilChemical compoundOC(=O)CCCC1=CC=C(N(CCCl)CCCl)C=C1JCKYGMPEJWAADB-UHFFFAOYSA-N0.000description3
- 239000011248coating agentSubstances0.000description3
- 238000000576coating methodMethods0.000description3
- HPXRVTGHNJAIIH-UHFFFAOYSA-NcyclohexanolChemical compoundOC1CCCCC1HPXRVTGHNJAIIH-UHFFFAOYSA-N0.000description3
- STQGQHZAVUOBTE-VGBVRHCVSA-NdaunorubicinChemical compoundO([C@H]1C[C@@](O)(CC=2C(O)=C3C(=O)C=4C=CC=C(C=4C(=O)C3=C(O)C=21)OC)C(C)=O)[C@H]1C[C@H](N)[C@H](O)[C@H](C)O1STQGQHZAVUOBTE-VGBVRHCVSA-N0.000description3
- 229960004679doxorubicinDrugs0.000description3
- VJJPUSNTGOMMGY-MRVIYFEKSA-NetoposideChemical compoundCOC1=C(O)C(OC)=CC([C@@H]2C3=CC=4OCOC=4C=C3[C@@H](O[C@H]3[C@@H]([C@@H](O)[C@@H]4O[C@H](C)OC[C@H]4O3)O)[C@@H]3[C@@H]2C(OC3)=O)=C1VJJPUSNTGOMMGY-MRVIYFEKSA-N0.000description3
- 229960005420etoposideDrugs0.000description3
- 229960002949fluorouracilDrugs0.000description3
- SDUQYLNIPVEERB-QPPQHZFASA-NgemcitabineChemical compoundO=C1N=C(N)C=CN1[C@H]1C(F)(F)[C@H](O)[C@@H](CO)O1SDUQYLNIPVEERB-QPPQHZFASA-N0.000description3
- 229940020967gemzarDrugs0.000description3
- 229940099279idamycinDrugs0.000description3
- UWKQSNNFCGGAFS-XIFFEERXSA-NirinotecanChemical compoundC1=C2C(CC)=C3CN(C(C4=C([C@@](C(=O)OC4)(O)CC)C=4)=O)C=4C3=NC2=CC=C1OC(=O)N(CC1)CCC1N1CCCCC1UWKQSNNFCGGAFS-XIFFEERXSA-N0.000description3
- 229960004768irinotecanDrugs0.000description3
- 229940063725leukeranDrugs0.000description3
- 229940087732matulaneDrugs0.000description3
- SGDBTWWWUNNDEQ-LBPRGKRZSA-NmelphalanChemical compoundOC(=O)[C@@H](N)CC1=CC=C(N(CCCl)CCCl)C=C1SGDBTWWWUNNDEQ-LBPRGKRZSA-N0.000description3
- 229960000485methotrexateDrugs0.000description3
- CFCUWKMKBJTWLW-BKHRDMLASA-NmithramycinChemical compoundO([C@@H]1C[C@@H](O[C@H](C)[C@H]1O)OC=1C=C2C=C3C[C@H]([C@@H](C(=O)C3=C(O)C2=C(O)C=1C)O[C@@H]1O[C@H](C)[C@@H](O)[C@H](O[C@@H]2O[C@H](C)[C@H](O)[C@H](O[C@@H]3O[C@H](C)[C@@H](O)[C@@](C)(O)C3)C2)C1)[C@H](OC)C(=O)[C@@H](O)[C@@H](C)O)[C@H]1C[C@@H](O)[C@H](O)[C@@H](C)O1CFCUWKMKBJTWLW-BKHRDMLASA-N0.000description3
- 229960004857mitomycinDrugs0.000description3
- 239000002674ointmentSubstances0.000description3
- 229910052697platinumInorganic materials0.000description3
- BASFCYQUMIYNBI-UHFFFAOYSA-NplatinumSubstances[Pt]BASFCYQUMIYNBI-UHFFFAOYSA-N0.000description3
- 229960003171plicamycinDrugs0.000description3
- CPTBDICYNRMXFX-UHFFFAOYSA-NprocarbazineChemical compoundCNNCC1=CC=C(C(=O)NC(C)C)C=C1CPTBDICYNRMXFX-UHFFFAOYSA-N0.000description3
- 229960000235temsirolimusDrugs0.000description3
- NRUKOCRGYNPUPR-QBPJDGROSA-NteniposideChemical compoundCOC1=C(O)C(OC)=CC([C@@H]2C3=CC=4OCOC=4C=C3[C@@H](O[C@H]3[C@@H]([C@@H](O)[C@@H]4O[C@@H](OC[C@H]4O3)C=3SC=CC=3)O)[C@@H]3[C@@H]2C(OC3)=O)=C1NRUKOCRGYNPUPR-QBPJDGROSA-N0.000description3
- UCFGDBYHRUNTLO-QHCPKHFHSA-NtopotecanChemical compoundC1=C(O)C(CN(C)C)=C2C=C(CN3C4=CC5=C(C3=O)COC(=O)[C@]5(O)CC)C4=NC2=C1UCFGDBYHRUNTLO-QHCPKHFHSA-N0.000description3
- 206010016717FistulaDiseases0.000description2
- 206010060932Postoperative adhesionDiseases0.000description2
- 206010038848Retinal detachmentDiseases0.000description2
- 208000002847Surgical WoundDiseases0.000description2
- 210000001367arteryAnatomy0.000description2
- 239000008280bloodSubstances0.000description2
- 210000004369bloodAnatomy0.000description2
- 208000002925dental cariesDiseases0.000description2
- 238000000502dialysisMethods0.000description2
- 230000000694effectsEffects0.000description2
- 230000003890fistulaEffects0.000description2
- 239000002874hemostatic agentSubstances0.000description2
- 238000002513implantationMethods0.000description2
- 208000015181infectious diseaseDiseases0.000description2
- 230000002062proliferating effectEffects0.000description2
- 230000035755proliferationEffects0.000description2
- 230000009467reductionEffects0.000description2
- 238000007910systemic administrationMethods0.000description2
- 230000009885systemic effectEffects0.000description2
- 208000031737Tissue AdhesionsDiseases0.000description1
- 230000003187abdominal effectEffects0.000description1
- 230000006978adaptationEffects0.000description1
- 239000002390adhesive tapeSubstances0.000description1
- 238000002399angioplastyMethods0.000description1
- 239000000730antalgic agentSubstances0.000description1
- 239000003242anti bacterial agentSubstances0.000description1
- 230000002421anti-septic effectEffects0.000description1
- 229940088710antibiotic agentDrugs0.000description1
- 239000004599antimicrobialSubstances0.000description1
- 230000003190augmentative effectEffects0.000description1
- 230000003115biocidal effectEffects0.000description1
- 239000000560biocompatible materialSubstances0.000description1
- 230000017531blood circulationEffects0.000description1
- 239000003560cancer drugSubstances0.000description1
- 230000010261cell growthEffects0.000description1
- 239000006071creamSubstances0.000description1
- 230000003247decreasing effectEffects0.000description1
- 230000002526effect on cardiovascular systemEffects0.000description1
- 239000000806elastomerSubstances0.000description1
- 229920001971elastomerPolymers0.000description1
- 230000037406food intakeEffects0.000description1
- 210000003734kidneyAnatomy0.000description1
- 238000002483medicationMethods0.000description1
- 238000012986modificationMethods0.000description1
- 230000004048modificationEffects0.000description1
- 210000003101oviductAnatomy0.000description1
- 206010033675panniculitisDiseases0.000description1
- 239000004033plasticSubstances0.000description1
- 230000002265preventionEffects0.000description1
- 210000002307prostateAnatomy0.000description1
- 239000004627regenerated celluloseSubstances0.000description1
- 210000001525retinaAnatomy0.000description1
- 230000002207retinal effectEffects0.000description1
- 210000004304subcutaneous tissueAnatomy0.000description1
- 239000000829suppositorySubstances0.000description1
- 230000008467tissue growthEffects0.000description1
- 210000000626ureterAnatomy0.000description1
- 210000003708urethraAnatomy0.000description1
Images
Classifications
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61L—METHODS OR APPARATUS FOR STERILISING MATERIALS OR OBJECTS IN GENERAL; DISINFECTION, STERILISATION OR DEODORISATION OF AIR; CHEMICAL ASPECTS OF BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES; MATERIALS FOR BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES
- A61L27/00—Materials for grafts or prostheses or for coating grafts or prostheses
- A61L27/50—Materials characterised by their function or physical properties, e.g. injectable or lubricating compositions, shape-memory materials, surface modified materials
- A61L27/54—Biologically active materials, e.g. therapeutic substances
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61B—DIAGNOSIS; SURGERY; IDENTIFICATION
- A61B17/00—Surgical instruments, devices or methods
- A61B17/04—Surgical instruments, devices or methods for suturing wounds; Holders or packages for needles or suture materials
- A61B17/06—Needles ; Sutures; Needle-suture combinations; Holders or packages for needles or suture materials
- A61B17/06166—Sutures
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61L—METHODS OR APPARATUS FOR STERILISING MATERIALS OR OBJECTS IN GENERAL; DISINFECTION, STERILISATION OR DEODORISATION OF AIR; CHEMICAL ASPECTS OF BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES; MATERIALS FOR BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES
- A61L15/00—Chemical aspects of, or use of materials for, bandages, dressings or absorbent pads
- A61L15/16—Bandages, dressings or absorbent pads for physiological fluids such as urine or blood, e.g. sanitary towels, tampons
- A61L15/42—Use of materials characterised by their function or physical properties
- A61L15/44—Medicaments
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61L—METHODS OR APPARATUS FOR STERILISING MATERIALS OR OBJECTS IN GENERAL; DISINFECTION, STERILISATION OR DEODORISATION OF AIR; CHEMICAL ASPECTS OF BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES; MATERIALS FOR BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES
- A61L31/00—Materials for other surgical articles, e.g. stents, stent-grafts, shunts, surgical drapes, guide wires, materials for adhesion prevention, occluding devices, surgical gloves, tissue fixation devices
- A61L31/14—Materials characterised by their function or physical properties, e.g. injectable or lubricating compositions, shape-memory materials, surface modified materials
- A61L31/16—Biologically active materials, e.g. therapeutic substances
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61B—DIAGNOSIS; SURGERY; IDENTIFICATION
- A61B17/00—Surgical instruments, devices or methods
- A61B17/064—Surgical staples, i.e. penetrating the tissue
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61B—DIAGNOSIS; SURGERY; IDENTIFICATION
- A61B17/00—Surgical instruments, devices or methods
- A61B2017/00831—Material properties
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F2/00—Filters implantable into blood vessels; Prostheses, i.e. artificial substitutes or replacements for parts of the body; Appliances for connecting them with the body; Devices providing patency to, or preventing collapsing of, tubular structures of the body, e.g. stents
- A61F2/0063—Implantable repair or support meshes, e.g. hernia meshes
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F13/00—Bandages or dressings; Absorbent pads
- A61F2013/00361—Plasters
- A61F2013/00365—Plasters use
- A61F2013/00451—Plasters use for surgical sutures, e.g. butterfly type
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61L—METHODS OR APPARATUS FOR STERILISING MATERIALS OR OBJECTS IN GENERAL; DISINFECTION, STERILISATION OR DEODORISATION OF AIR; CHEMICAL ASPECTS OF BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES; MATERIALS FOR BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES
- A61L2300/00—Biologically active materials used in bandages, wound dressings, absorbent pads or medical devices
- A61L2300/40—Biologically active materials used in bandages, wound dressings, absorbent pads or medical devices characterised by a specific therapeutic activity or mode of action
- A61L2300/416—Anti-neoplastic or anti-proliferative or anti-restenosis or anti-angiogenic agents, e.g. paclitaxel, sirolimus
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61L—METHODS OR APPARATUS FOR STERILISING MATERIALS OR OBJECTS IN GENERAL; DISINFECTION, STERILISATION OR DEODORISATION OF AIR; CHEMICAL ASPECTS OF BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES; MATERIALS FOR BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES
- A61L2300/00—Biologically active materials used in bandages, wound dressings, absorbent pads or medical devices
- A61L2300/40—Biologically active materials used in bandages, wound dressings, absorbent pads or medical devices characterised by a specific therapeutic activity or mode of action
- A61L2300/424—Anti-adhesion agents
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61L—METHODS OR APPARATUS FOR STERILISING MATERIALS OR OBJECTS IN GENERAL; DISINFECTION, STERILISATION OR DEODORISATION OF AIR; CHEMICAL ASPECTS OF BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES; MATERIALS FOR BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES
- A61L2300/00—Biologically active materials used in bandages, wound dressings, absorbent pads or medical devices
- A61L2300/60—Biologically active materials used in bandages, wound dressings, absorbent pads or medical devices characterised by a special physical form
- A61L2300/602—Type of release, e.g. controlled, sustained, slow
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61L—METHODS OR APPARATUS FOR STERILISING MATERIALS OR OBJECTS IN GENERAL; DISINFECTION, STERILISATION OR DEODORISATION OF AIR; CHEMICAL ASPECTS OF BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES; MATERIALS FOR BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES
- A61L2300/00—Biologically active materials used in bandages, wound dressings, absorbent pads or medical devices
- A61L2300/60—Biologically active materials used in bandages, wound dressings, absorbent pads or medical devices characterised by a special physical form
- A61L2300/602—Type of release, e.g. controlled, sustained, slow
- A61L2300/604—Biodegradation
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61L—METHODS OR APPARATUS FOR STERILISING MATERIALS OR OBJECTS IN GENERAL; DISINFECTION, STERILISATION OR DEODORISATION OF AIR; CHEMICAL ASPECTS OF BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES; MATERIALS FOR BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES
- A61L2300/00—Biologically active materials used in bandages, wound dressings, absorbent pads or medical devices
- A61L2300/60—Biologically active materials used in bandages, wound dressings, absorbent pads or medical devices characterised by a special physical form
- A61L2300/62—Encapsulated active agents, e.g. emulsified droplets
- A61L2300/622—Microcapsules
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61L—METHODS OR APPARATUS FOR STERILISING MATERIALS OR OBJECTS IN GENERAL; DISINFECTION, STERILISATION OR DEODORISATION OF AIR; CHEMICAL ASPECTS OF BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES; MATERIALS FOR BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES
- A61L2300/00—Biologically active materials used in bandages, wound dressings, absorbent pads or medical devices
- A61L2300/60—Biologically active materials used in bandages, wound dressings, absorbent pads or medical devices characterised by a special physical form
- A61L2300/62—Encapsulated active agents, e.g. emulsified droplets
- A61L2300/626—Liposomes, micelles, vesicles
Definitions
- This inventionis in the field of materials used to prevent the formation of scar tissue subsequent to a surgical procedure or accidental skin cut of a human subject.
- a first embodiment of this inventionis a device consisting of a drug impregnated into, coated onto or placed onto a material sheet or mesh designed to be placed between internal body tissues that have been surgically separated to prevent the formation of post-operative adhesions, which adhesions are really scar tissue formation.
- a drug that is impregnated into a gauze-like material or coated onto the material or joined to the material by adhesion and/or capillary actionis defined herein as a drug “attached” to a mesh.
- This mesh or gauze onto which the drug is attachedmay be either a permanent implant or it may be biodegradable.
- the drugcan be attached to an existing product such as the Johnson & Johnson SURGICELTM absorbable hemostat gauze-like sheet.
- an anti-proliferative drugsuch as Rapamycin or Taxol which have a known effect on proliferating cells
- the biodegradable meshwould decrease cellular proliferation and hence be a deterrent to the formation of adhesions.
- an anti-proliferative drug attached to a bandagecould be placed onto a cut in the skin for reducing scar tissue formation. This cut could be accidental or a result of a surgical incision.
- an anti-proliferative drugcould be attached to surgical suture material that is used (for example) to join together two blood generally cylindrical cavitys, i.e., an anastomosis, with the attached drug causing a reduction in cellular proliferation in the vicinity where the sutures penetrate through the human tissue.
- suture materialcould be either soluble or insoluble and could be used for any application for which sutures are used.
- Still another embodiment of the present inventionis an anti-proliferative drug coated onto a surgical staple thus reducing scar tissue around that staple.
- Still another embodiment of this inventionis to attach an anti-proliferative drug to a device such as a buckle that is used for the treatment of a detached retina. Since scar tissue formation is one of the main complications of a retinal attachment procedure, by attaching an anti-proliferative drug to the buckle that is placed around the eye, there can be some reduction in scar tissue formation.
- a generally cylindrical cavitymight be a nostril after an operation for a deviated septum, a fallopian tube, a billiary duct, a urethra, (for example after prostate surgery) a ureter, a bronchial tube, etc.
- the tube with the attached anti-proliferative drugcould be biodegradable, remain implanted or it could be removed after a few days or weeks.
- Another device that would benefit from a coating of an anti-proliferative agent such as Rapamycinis a prosthetic implant that is placed into a woman's breast after reconstructive or augmentative surgery.
- Breast implantstypically form significant scar tissue around their surface after implantation. Coating the surface of the breast implant with a slowly releasing anti-proliferative agent can significantly reduce this scar tissue formation.
- Still another application of these conceptsis for aterio-venous fistulas that are used for kidney dialysis patients.
- These devices(which are also called a-v shunts) are used to connect an artery in an arm to a large vein in the same arm.
- the plastic a-v shuntis then penetrated by comparatively large needles through which the patient's blood is cleansed typically every other day.
- a frequent cause of failure for these shuntsis caused by proliferative cell growth at the anastamosis where the shunt is joined to a vein.
- an anti-proliferative drugcould be applied by oral ingestion, by a transdermal patch, by a cream or ointment applied to the skin, by inhalation or by a suppository. Any of these methods being a systemic application of an anti-proliferative drug. It should be understood that such a drug should be applied systemically starting at least one day prior to a surgical procedure but could be started as long as 5 days prior to a surgical procedure.
- the drugshould be applied for a period of at least one day after the procedure and for some cases as long as 60 days. It should be understood that an anti-proliferative drug could be given systemically without using any of the devices described herein. Preferably, the anti-proliferative drug would be given systemically in addition to the application of an anti-proliferative drug attached to any one or more of the devices described herein. It should also be understood that an optimum result might be obtained with using one anti-proliferative drug attached to a device with a second and/or third drug being used for systemic administration. A typical dose for a patient, for example with Rapomycin, would be 1.5 mg/kg per day. The dose would of course depend on the anti-proliferative drug that was used.
- Another object of this inventionis to have a biodegradable sheet of material or mesh suitable for placement between body tissues including an attached drug that prevents the cellular proliferation associated with post-surgical adhesions.
- Still another object of the inventionis to have a bandage to which an anti-proliferative drug is attached that is placed onto a cut in the skin to reduce scar tissue formation.
- Still another object of the inventionis to have a suture material or surgical staple to which an anti-proliferative drug is attached.
- Still another object of the inventionis to have an anti-proliferative drug attached to the exterior of a cylindrical tube that is placed into a generally cylindrical cavity of the human body after a surgical procedure on that generally cylindrical cavity.
- Still another object of the inventionis to have a device implanted in a human subject, the device having an anti-proliferative agent attached; the device being a breast implant, an a-v shunt or an equivalent device for implantation into the human subject.
- Still another object of this inventionis to have the anti-proliferative drug be Rapamycin or an equivalent drug.
- Still another object of this inventionis to have the anti-proliferative drug be Taxol or an equivalent drug.
- Still another object of the inventionis to employ a device placed into or onto the body of a human subject, which device has an attached anti-proliferative drug, plus using the same or a different anti-proliferative drug as a medication to be applied systemically to the human subject from some time prior to a surgical procedure to some time after that procedure.
- FIG. 1is a plan view of a sheet or mesh onto which an anti-proliferative drug has been attached.
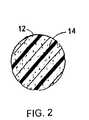
- FIG. 2is an enlargement of the cross section of a single strand of the mesh where the drug is embedded within the strand.
- FIG. 3is an enlargement of the cross section of a single strand of the mesh where the drug is coated onto the strand.
- FIG. 4is an enlargement of two strands of the mesh that have been dipped into a solution of an anti-proliferative drug thereby attaching the drug to the strands by adhesion and capillary action.
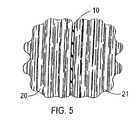
- FIG. 5shows a cross section of the mesh to which an anti-proliferative drug has been attached, the mesh being placed between two layers of tissue of the human body.
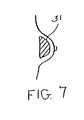
- FIG. 7is a cross section of a human breast into which a breast implant has been placed.
- FIG. 1shows an absorbable hemostat mesh sheet 10 with mesh strands 12 and open spaces 11 .
- the sheet 10is designed to be placed post-operatively between internal body tissues that have been separated by a surgical procedure.
- the mesh strands 12can be made from oxidized regenerated cellulose or other biodegradable materials with the anti-proliferative drug either embedded within the strands, coated onto the outer surfaces of the strands or held onto the strands by adhesion or capillary action. Any of these possibilities will be described herein as the drug being attached to the mesh or attached to the strand of the mesh.
- FIG. 2is an enlargement of a cross section of a single strand 12 of the mesh 10 in which the anti-proliferative drug 14 is embedded within the strand 12 .
- FIG. 3is an enlargement of the cross section of a single strand 12 of the mesh where the anti-proliferative drug 17 is coated onto the exterior surface of the strand.
- FIG. 4is an enlargement of two adjacent strands 12 of the mesh 10 onto which an anti-proliferative drug 18 is attached by means of adhesion and capillary action.
- FIG. 5shows the anti-proliferative drug attached to the mesh 10 placed between two adjacent tissues 20 and 21 of a human body.
- the mesh 10would be inserted during a surgical procedure typically just before closing of the surgical incision.
- the biodegradable mesh 10dissolves or is absorbed into the tissues 20 and 21 , the anti-proliferative drug attached to the mesh 10 will become dispersed into the tissues 20 and 21 .
- the biocompatible sheet of materialis not biodegradable, the anti-proliferative drug will remain at the site where it is placed for a longer period of time than if the material sheet is biodegradable.
- the drug itselfmay be produced in a soluble or insoluble form. An insoluble form would remain at the treatment site longer than a soluble form.
- the anti-proliferative drugs that may be usedinclude cancer drugs such as Taxol and other known anti-proliferative drugs such as Rapamycin.
- Other drugs that could be usedare Alkeran, Cytoxan, Leukeran, Cis-platinum, BiCNU, Adriamycin, Doxorubicin, Cerubidine, Idamycin, Mithracin, Mutamycin, Fluorouracil, Methotrexate, Thoguanine, Toxotere, Etoposide, Vincristine, Irinotecan, Hycamptin, Matulane, Vumon, Hexalin, Hydroxyurea, Gemzar, Oncovin and Etophophos, taclolimus (FK506), and the following analogs of sirolimus: SDZ-RAD, CCI-779, 7-epi-rapamycin, 7-thiomethyl-rapamycin, 7-epi-trimethoxyphenyl-rapamycin, 7-epi-thiomethyl-rapa
- an anti-proliferative drugcan be made to be part of any sheet of material that is or is not biodegradable, as long as the sheet of material is biocompatible. In any case the effect of the anti-proliferative drug that is attached to at least part of the sheet of material will decrease cellular proliferation and therefore decrease the formation of scar tissue and adhesions.
- the mesh 10could be rolled into a cylinder and placed into a generally cylindrical cavity of the human body that has undergone a surgical procedure.
- the mesh 10in a cylindrical form, could also be placed around an elastomer tube prior to placement in the human generally cylindrical cavity.
- FIG. 6is a cross section of a cut 23 in the skin 22 that is situated above the subcutaneous tissue 24 .
- a bandage 25 to which an anti-proliferative drug has been attachedis shown attached to the skin 22 by means of an adhesive tape 26 .
- the purpose of the anti-proliferative drugis to reduce scar tissue formation in order to have an improved appearance of the skin.
- the bandagemay also include an antiseptic agent to decrease the possibility of infection.
- an ointment that includes an anti-proliferative agentcould be used separately from the bandage 25 of FIG. 6 .
- the anti-proliferative agentwould be selected from the group that includes Alkeran, Cytoxan, Leukeran, Cis-platinum, BiCNU, Adriamycin, Doxorubicin, Cerubidine, Idamycin, Mithracin, Mutamycin, Fluorouracil, Methotrexate, Thoguanine, Toxotere, Etoposide, Vincristine, Irinotecan, Hycamptin, Matulane, Vumon, Hexalin, Hydroxyurea, Gemzar, Oncovin and Etophophos, taclolimus (FK506), and the following analogs of sirolimus: SDZ-RAD, CCI-779, 7-epi-rapamycin, 7-thiomethyl-rapamycin, 7-epi-trimethoxyphenyl-rapamycin, 7-epi
- FIG. 2Another alternative embodiment of the invention is a suture material to which an anti-proliferative drug is attached.
- a drawing of a highly enlarged cross section of such a suturewould be shown by FIG. 2 or 3 . That is, FIG. 2 could be considered to be a cross section of a suture 12 into which is embedded an anti-proliferative drug 14 .
- FIG. 3could be considered a highly enlarged cross section of a suture 12 that is coated with an anti-proliferative drug 17 .
- the object of attaching an anti-proliferative drug to a suturewould be to reduce scar tissue formation where the suture penetrates through human tissue. This would be particularly true for the use a suture to join together two generally cylindrical cavitys, i.e., an anastamosis.
- an anti-proliferative drugcould be attached to any surgical staple that is used to join together human tissue after a surgical procedure. It should be understood that sutures or staples with an anti-proliferative agent attached could be used for joining any tissue of a human subject where it is desired to reduce cellular proliferation, i.e., the formation of adhesions or scar tissue.
- FIG. 7illustrates the implant into the breast of a human subject of a breast implant 31 .
- Attached to the breast implant 31would be an anti-proliferative agent selected from the group that includes Rapamycin, Taxol, Alkeran, Cytoxan, Leukeran, Cis-platinum, BiCNU, Adriamycin, Doxorubicin, Cerubidine, Idamycin, Mithracin, Mutamycin, Fluorouracil, Methotrexate, Thoguanine, Toxotere, Etoposide, Vincristine, Irinotecan, Hycamptin, Matulane, Vumon, Hexalin, Hydroxyurea, Gemzar, Oncovin and Etophophos, taclolimus (FK506), and the following analogs of sirolimus: SDZ-RAD, CCI-779, 7-epi-rapamycin, 7-thiomethyl-rapamycin, 7-epi-trimethoxyphenyl-rapamycin,
- an arterio-venus fistula shuntis placed into the arm of a dialysis patient, then the same type of anti-proliferative agent(s) as described above could be attached to that implanted device to increase the time during which the associated vein in the arm would remain patent.
- Another application of the present inventionis for prevention of scar tissue formation subsequent to a procedure for attaching a detached retina.
- This procedureuses what is called a “buckle” placed around the eye to cause re-attachment of the retina.
- the extent of scar tissue formation after this procedure is performedcan be decreased by attaching an anti-proliferative drug to the buckle. The anti-proliferative drug would then find its way into the eye to decrease scar tissue formation.
- the systemic application of one or more of the anti-proliferative agents that have been describedcould be used conjunctively to further minimize the creation of scar tissue.
- an antiseptic, and/or anti-biotic, and/or analgesic agentcould be added to an anti-proliferative ointment to prevent infection and/or to decrease pain.
- an antiseptic, and/or anti-biotic, and/or analgesic agentcould be added to an anti-proliferative ointment to prevent infection and/or to decrease pain.
- analgesic agentcould be added to an anti-proliferative ointment to prevent infection and/or to decrease pain.
- these other agentscould also be applied for any other use of the anti-proliferative drugs that are described herein.
- any human subject in whom an anti-proliferative agent is used plus at least one of the other drugs listed abovecould also benefit from the systemic administration of one or more anti-proliferative agent that has been listed herein.
Landscapes
- Health & Medical Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Animal Behavior & Ethology (AREA)
- Chemical & Material Sciences (AREA)
- Engineering & Computer Science (AREA)
- Veterinary Medicine (AREA)
- Public Health (AREA)
- General Health & Medical Sciences (AREA)
- Epidemiology (AREA)
- Medicinal Chemistry (AREA)
- Molecular Biology (AREA)
- Biomedical Technology (AREA)
- Surgery (AREA)
- Heart & Thoracic Surgery (AREA)
- Transplantation (AREA)
- Dermatology (AREA)
- Oral & Maxillofacial Surgery (AREA)
- Medical Informatics (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Vascular Medicine (AREA)
- Hematology (AREA)
- Materials Engineering (AREA)
- Materials For Medical Uses (AREA)
- Pharmaceuticals Containing Other Organic And Inorganic Compounds (AREA)
- Prostheses (AREA)
- Medicinal Preparation (AREA)
Abstract
Description
- This is a continuation-in-part application of the patent application Ser. No. 09/705,999 filed on Nov. 6, 2000.
- This invention is in the field of materials used to prevent the formation of scar tissue subsequent to a surgical procedure or accidental skin cut of a human subject.
- Post-operative adhesions are a major problem following abdominal and other surgical procedures. These adhesions are caused by the unwanted proliferation of scar tissue between internal tissues and structures of the human body generally after surgery. Several companies have developed sheets of biodegradable mesh that can be placed between these structures to reduce the tissue growth. None are entirely effective as some scar tissue typically grows through the mesh. U.S. Pat. No. 5,795,286 describes the use of a beta emitting radioisotope to reduce the proliferation of tissue through a biocompatible material placed into the human body. Although radioisotopes may be effective at preventing the cell proliferation associated with adhesions, the limited shelf life and safety issues associated with radioisotopes makes them less than ideal for this purpose.
- Recent publications (Transcutaneous Cardiovascular Therapeutics 2000 Abstracts) report a greatly reduced cell proliferation within angioplasty injured arteries when vascular stents used for recannalization are coated with an anti-proliferative drug such as Rapamycin (Sirolmus) or Taxol. However, these drugs have never been used for reducing cellular proliferation of tissues separated by a surgical procedure.
- A first embodiment of this invention is a device consisting of a drug impregnated into, coated onto or placed onto a material sheet or mesh designed to be placed between internal body tissues that have been surgically separated to prevent the formation of post-operative adhesions, which adhesions are really scar tissue formation. A drug that is impregnated into a gauze-like material or coated onto the material or joined to the material by adhesion and/or capillary action is defined herein as a drug “attached” to a mesh. This mesh or gauze onto which the drug is attached may be either a permanent implant or it may be biodegradable. The drug can be attached to an existing product such as the Johnson & Johnson SURGICEL™ absorbable hemostat gauze-like sheet. With an anti-proliferative drug such as Rapamycin or Taxol which have a known effect on proliferating cells, the biodegradable mesh would decrease cellular proliferation and hence be a deterrent to the formation of adhesions. It is also envisioned that an anti-proliferative drug attached to a bandage could be placed onto a cut in the skin for reducing scar tissue formation. This cut could be accidental or a result of a surgical incision. It is also envisioned that an anti-proliferative drug could be attached to surgical suture material that is used (for example) to join together two blood generally cylindrical cavitys, i.e., an anastomosis, with the attached drug causing a reduction in cellular proliferation in the vicinity where the sutures penetrate through the human tissue. It should be understood that the suture material could be either soluble or insoluble and could be used for any application for which sutures are used. Still another embodiment of the present invention is an anti-proliferative drug coated onto a surgical staple thus reducing scar tissue around that staple. Still another embodiment of this invention is to attach an anti-proliferative drug to a device such as a buckle that is used for the treatment of a detached retina. Since scar tissue formation is one of the main complications of a retinal attachment procedure, by attaching an anti-proliferative drug to the buckle that is placed around the eye, there can be some reduction in scar tissue formation. It is also envisioned to attach an anti-proliferative drug attached to the outside of a cylindrical tube that is placed within a generally cylindrical cavity of the human body to decrease scar tissue formation after a surgical procedure on that generally cylindrical cavity. Such a generally cylindrical cavity might be a nostril after an operation for a deviated septum, a fallopian tube, a billiary duct, a urethra, (for example after prostate surgery) a ureter, a bronchial tube, etc. For such an application, the tube with the attached anti-proliferative drug could be biodegradable, remain implanted or it could be removed after a few days or weeks.
- Another device that would benefit from a coating of an anti-proliferative agent such as Rapamycin is a prosthetic implant that is placed into a woman's breast after reconstructive or augmentative surgery. Breast implants typically form significant scar tissue around their surface after implantation. Coating the surface of the breast implant with a slowly releasing anti-proliferative agent can significantly reduce this scar tissue formation.
- Still another application of these concepts is for aterio-venous fistulas that are used for kidney dialysis patients. These devices (which are also called a-v shunts) are used to connect an artery in an arm to a large vein in the same arm. The plastic a-v shunt is then penetrated by comparatively large needles through which the patient's blood is cleansed typically every other day. A frequent cause of failure for these shunts is caused by proliferative cell growth at the anastamosis where the shunt is joined to a vein. By having sutures coated with an anti-proliferative agent and by coating the interior and/or exterior of the a-v shunt with an anti-proliferative agent it is expected that the time for maintaining adequate blood flow through the vein will be extended.
- In addition to applying the anti-proliferative drug by means of a device to which the anti-proliferative drug is attached, it is also envisioned to apply the anti-proliferative drug systemically by any one or more of the well known means for introducing a drug into a human subject. For example, an anti-proliferative drug could be applied by oral ingestion, by a transdermal patch, by a cream or ointment applied to the skin, by inhalation or by a suppository. Any of these methods being a systemic application of an anti-proliferative drug. It should be understood that such a drug should be applied systemically starting at least one day prior to a surgical procedure but could be started as long as 5 days prior to a surgical procedure. Furthermore, the drug should be applied for a period of at least one day after the procedure and for some cases as long as 60 days. It should be understood that an anti-proliferative drug could be given systemically without using any of the devices described herein. Preferably, the anti-proliferative drug would be given systemically in addition to the application of an anti-proliferative drug attached to any one or more of the devices described herein. It should also be understood that an optimum result might be obtained with using one anti-proliferative drug attached to a device with a second and/or third drug being used for systemic administration. A typical dose for a patient, for example with Rapomycin, would be 1.5 mg/kg per day. The dose would of course depend on the anti-proliferative drug that was used.
- Thus it is an object of this invention to have a sheet of material that can be placed between internal body tissues, the material having an anti-proliferative drug attached to reduce scar tissue formation between adjacent layers of the human tissue.
- Another object of this invention is to have a biodegradable sheet of material or mesh suitable for placement between body tissues including an attached drug that prevents the cellular proliferation associated with post-surgical adhesions.
- Still another object of the invention is to have a bandage to which an anti-proliferative drug is attached that is placed onto a cut in the skin to reduce scar tissue formation.
- Still another object of the invention is to have a suture material or surgical staple to which an anti-proliferative drug is attached.
- Still another object of the invention is to have an anti-proliferative drug attached to the exterior of a cylindrical tube that is placed into a generally cylindrical cavity of the human body after a surgical procedure on that generally cylindrical cavity.
- Still another object of the invention is to have a device implanted in a human subject, the device having an anti-proliferative agent attached; the device being a breast implant, an a-v shunt or an equivalent device for implantation into the human subject.
- Still another object of this invention is to have the anti-proliferative drug be Rapamycin or an equivalent drug.
- Still another object of this invention is to have the anti-proliferative drug be Taxol or an equivalent drug.
- Still another object of the invention is to employ a device placed into or onto the body of a human subject, which device has an attached anti-proliferative drug, plus using the same or a different anti-proliferative drug as a medication to be applied systemically to the human subject from some time prior to a surgical procedure to some time after that procedure.
- These and other objects and advantages of this invention will become obvious to a person of ordinary skill in this art upon reading of the detailed description of this invention including the associated drawings.
FIG. 1 is a plan view of a sheet or mesh onto which an anti-proliferative drug has been attached.FIG. 2 is an enlargement of the cross section of a single strand of the mesh where the drug is embedded within the strand.FIG. 3 is an enlargement of the cross section of a single strand of the mesh where the drug is coated onto the strand.FIG. 4 is an enlargement of two strands of the mesh that have been dipped into a solution of an anti-proliferative drug thereby attaching the drug to the strands by adhesion and capillary action.FIG. 5 shows a cross section of the mesh to which an anti-proliferative drug has been attached, the mesh being placed between two layers of tissue of the human body.FIG. 6 is a cross section of the skin onto which is taped a bandage to which an anti-proliferative drug has been attached.FIG. 7 is a cross section of a human breast into which a breast implant has been placed.FIG. 1 shows an absorbablehemostat mesh sheet 10 withmesh strands 12 and open spaces11. Thesheet 10 is designed to be placed post-operatively between internal body tissues that have been separated by a surgical procedure. Themesh strands 12 can be made from oxidized regenerated cellulose or other biodegradable materials with the anti-proliferative drug either embedded within the strands, coated onto the outer surfaces of the strands or held onto the strands by adhesion or capillary action. Any of these possibilities will be described herein as the drug being attached to the mesh or attached to the strand of the mesh.FIG. 2 is an enlargement of a cross section of asingle strand 12 of themesh 10 in which theanti-proliferative drug 14 is embedded within thestrand 12.FIG. 3 is an enlargement of the cross section of asingle strand 12 of the mesh where theanti-proliferative drug 17 is coated onto the exterior surface of the strand.FIG. 4 is an enlargement of twoadjacent strands 12 of themesh 10 onto which an anti-proliferative drug18 is attached by means of adhesion and capillary action.FIG. 5 shows the anti-proliferative drug attached to themesh 10 placed between twoadjacent tissues mesh 10 would be inserted during a surgical procedure typically just before closing of the surgical incision. When thebiodegradable mesh 10 dissolves or is absorbed into thetissues mesh 10 will become dispersed into thetissues - The anti-proliferative drugs that may be used include cancer drugs such as Taxol and other known anti-proliferative drugs such as Rapamycin. Other drugs that could be used are Alkeran, Cytoxan, Leukeran, Cis-platinum, BiCNU, Adriamycin, Doxorubicin, Cerubidine, Idamycin, Mithracin, Mutamycin, Fluorouracil, Methotrexate, Thoguanine, Toxotere, Etoposide, Vincristine, Irinotecan, Hycamptin, Matulane, Vumon, Hexalin, Hydroxyurea, Gemzar, Oncovin and Etophophos, taclolimus (FK506), and the following analogs of sirolimus: SDZ-RAD, CCI-779, 7-epi-rapamycin, 7-thiomethyl-rapamycin, 7-epi-trimethoxyphenyl-rapamycin, 7-epi-thiomethyl-rapamycin, 7-demethoxy-rapamycin, 32-demethoxy, 2-desmethyl and proline.
- Although a mesh has been discussed herein, more generally, an anti-proliferative drug can be made to be part of any sheet of material that is or is not biodegradable, as long as the sheet of material is biocompatible. In any case the effect of the anti-proliferative drug that is attached to at least part of the sheet of material will decrease cellular proliferation and therefore decrease the formation of scar tissue and adhesions.
- It should also be understood that the
mesh 10 could be rolled into a cylinder and placed into a generally cylindrical cavity of the human body that has undergone a surgical procedure. Themesh 10, in a cylindrical form, could also be placed around an elastomer tube prior to placement in the human generally cylindrical cavity. FIG. 6 is a cross section of acut 23 in theskin 22 that is situated above thesubcutaneous tissue 24. Abandage 25 to which an anti-proliferative drug has been attached is shown attached to theskin 22 by means of anadhesive tape 26. The purpose of the anti-proliferative drug is to reduce scar tissue formation in order to have an improved appearance of the skin. The bandage may also include an antiseptic agent to decrease the possibility of infection.- It should also be understood that an ointment that includes an anti-proliferative agent could be used separately from the
bandage 25 ofFIG. 6 . The anti-proliferative agent would be selected from the group that includes Alkeran, Cytoxan, Leukeran, Cis-platinum, BiCNU, Adriamycin, Doxorubicin, Cerubidine, Idamycin, Mithracin, Mutamycin, Fluorouracil, Methotrexate, Thoguanine, Toxotere, Etoposide, Vincristine, Irinotecan, Hycamptin, Matulane, Vumon, Hexalin, Hydroxyurea, Gemzar, Oncovin and Etophophos, taclolimus (FK506), and the following analogs of sirolimus: SDZ-RAD, CCI-779, 7-epi-rapamycin, 7-thiomethyl-rapamycin, 7-epi-trimethoxyphenyl-rapamycin, 7-epi-thiomethyl-rapamycin, 7-demethoxy-rapamycin, 32-demethoxy, 2-desmethyl and proline. - Another alternative embodiment of the invention is a suture material to which an anti-proliferative drug is attached. A drawing of a highly enlarged cross section of such a suture would be shown by
FIG. 2 or3. That is,FIG. 2 could be considered to be a cross section of asuture 12 into which is embedded ananti-proliferative drug 14.FIG. 3 could be considered a highly enlarged cross section of asuture 12 that is coated with ananti-proliferative drug 17. The object of attaching an anti-proliferative drug to a suture would be to reduce scar tissue formation where the suture penetrates through human tissue. This would be particularly true for the use a suture to join together two generally cylindrical cavitys, i.e., an anastamosis. This could be used for both soluble and insoluble suture materials. Furthermore, an anti-proliferative drug could be attached to any surgical staple that is used to join together human tissue after a surgical procedure. It should be understood that sutures or staples with an anti-proliferative agent attached could be used for joining any tissue of a human subject where it is desired to reduce cellular proliferation, i.e., the formation of adhesions or scar tissue. FIG. 7 illustrates the implant into the breast of a human subject of abreast implant 31. Attached to thebreast implant 31 would be an anti-proliferative agent selected from the group that includes Rapamycin, Taxol, Alkeran, Cytoxan, Leukeran, Cis-platinum, BiCNU, Adriamycin, Doxorubicin, Cerubidine, Idamycin, Mithracin, Mutamycin, Fluorouracil, Methotrexate, Thoguanine, Toxotere, Etoposide, Vincristine, Irinotecan, Hycamptin, Matulane, Vumon, Hexalin, Hydroxyurea, Gemzar, Oncovin and Etophophos, taclolimus (FK506), and the following analogs of sirolimus: SDZ-RAD, CCI-779, 7-epi-rapamycin, 7-thiomethyl-rapamycin, 7-epi-trimethoxyphenyl-rapamycin, 7-epi-thiomethyl-rapamycin, 7-demethoxy-rapamycin, 32-demethoxy, 2-desmethyl and proline. When a breast implant has an attached anti-proliferative agent, the scar tissue that typically forms around such an implant will be significantly reduced.- If an arterio-venus fistula shunt is placed into the arm of a dialysis patient, then the same type of anti-proliferative agent(s) as described above could be attached to that implanted device to increase the time during which the associated vein in the arm would remain patent.
- Another application of the present invention is for prevention of scar tissue formation subsequent to a procedure for attaching a detached retina. This procedure uses what is called a “buckle” placed around the eye to cause re-attachment of the retina. The extent of scar tissue formation after this procedure is performed can be decreased by attaching an anti-proliferative drug to the buckle. The anti-proliferative drug would then find its way into the eye to decrease scar tissue formation.
- For any of the applications described herein, the systemic application of one or more of the anti-proliferative agents that have been described could be used conjunctively to further minimize the creation of scar tissue.
- Although only the use of certain anti-proliferative agents has been discussed herein, it should be understood that other medications could be added to the anti-proliferative drugs to provide an improved outcome for the patients. Specifically, for applications on the skin, an antiseptic, and/or anti-biotic, and/or analgesic agent could be added to an anti-proliferative ointment to prevent infection and/or to decrease pain. These other agents could also be applied for any other use of the anti-proliferative drugs that are described herein. It is further understood that any human subject in whom an anti-proliferative agent is used plus at least one of the other drugs listed above could also benefit from the systemic administration of one or more anti-proliferative agent that has been listed herein.
- Various other modifications, adaptations, and alternative designs are of course possible in light of the above teachings. Therefore, it should be understood at this time that within the scope of the appended claims, the invention can be practiced otherwise than as specifically described herein.
Claims (10)
Priority Applications (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US11/084,948US20050281860A1 (en) | 2000-11-06 | 2005-03-21 | Surgically implanted devices having reduced scar tissue formation |
Applications Claiming Priority (4)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US70599900A | 2000-11-06 | 2000-11-06 | |
| US09/772,693US6534693B2 (en) | 2000-11-06 | 2001-01-31 | Surgically implanted devices having reduced scar tissue formation |
| US10/351,207US20040006296A1 (en) | 2000-11-06 | 2003-01-24 | Surgically implanted devices having reduced scar tissue formation |
| US11/084,948US20050281860A1 (en) | 2000-11-06 | 2005-03-21 | Surgically implanted devices having reduced scar tissue formation |
Related Parent Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US10/351,207ContinuationUS20040006296A1 (en) | 2000-11-06 | 2003-01-24 | Surgically implanted devices having reduced scar tissue formation |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| US20050281860A1true US20050281860A1 (en) | 2005-12-22 |
Family
ID=24835796
Family Applications (3)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US09/772,693Expired - LifetimeUS6534693B2 (en) | 2000-11-06 | 2001-01-31 | Surgically implanted devices having reduced scar tissue formation |
| US10/351,207AbandonedUS20040006296A1 (en) | 2000-11-06 | 2003-01-24 | Surgically implanted devices having reduced scar tissue formation |
| US11/084,948AbandonedUS20050281860A1 (en) | 2000-11-06 | 2005-03-21 | Surgically implanted devices having reduced scar tissue formation |
Family Applications Before (2)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US09/772,693Expired - LifetimeUS6534693B2 (en) | 2000-11-06 | 2001-01-31 | Surgically implanted devices having reduced scar tissue formation |
| US10/351,207AbandonedUS20040006296A1 (en) | 2000-11-06 | 2003-01-24 | Surgically implanted devices having reduced scar tissue formation |
Country Status (8)
| Country | Link |
|---|---|
| US (3) | US6534693B2 (en) |
| EP (1) | EP1385458A4 (en) |
| JP (1) | JP2004529667A (en) |
| AU (2) | AU2002224318B2 (en) |
| BR (1) | BR0115457A (en) |
| CA (1) | CA2428082A1 (en) |
| MX (1) | MXPA03003906A (en) |
| WO (1) | WO2002036054A1 (en) |
Cited By (11)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US20040146546A1 (en)* | 2002-09-26 | 2004-07-29 | Angiotech Pharmaceuticals, Inc. | Perivascular wraps |
| US20050149158A1 (en)* | 2003-11-10 | 2005-07-07 | Angiotech International Ag | Medical implants and anti-scarring agents |
| US7798385B2 (en) | 2007-05-16 | 2010-09-21 | The Invention Science Fund I, Llc | Surgical stapling instrument with chemical sealant |
| US7810691B2 (en) | 2007-05-16 | 2010-10-12 | The Invention Science Fund I, Llc | Gentle touch surgical stapler |
| US7823761B2 (en) | 2007-05-16 | 2010-11-02 | The Invention Science Fund I, Llc | Maneuverable surgical stapler |
| US7832611B2 (en) | 2007-05-16 | 2010-11-16 | The Invention Science Fund I, Llc | Steerable surgical stapler |
| US7922064B2 (en) | 2007-05-16 | 2011-04-12 | The Invention Science Fund, I, LLC | Surgical fastening device with cutter |
| US20110237542A1 (en)* | 2008-12-01 | 2011-09-29 | Shin Poong Pharmaceutical Co., Ltd. | Composition for preventing adhesion |
| US8485411B2 (en) | 2007-05-16 | 2013-07-16 | The Invention Science Fund I, Llc | Gentle touch surgical stapler |
| EP3597206A1 (en) | 2011-06-21 | 2020-01-22 | BVW Holding AG | Medical device comprising boswellic acid |
| US11717593B2 (en) | 2013-03-13 | 2023-08-08 | Avery Dennison Corporation | Improving adhesive properties |
Families Citing this family (138)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US7963997B2 (en)* | 2002-07-19 | 2011-06-21 | Kensey Nash Corporation | Device for regeneration of articular cartilage and other tissue |
| US8795242B2 (en)* | 1994-05-13 | 2014-08-05 | Kensey Nash Corporation | Resorbable polymeric device for localized drug delivery |
| CN1219872A (en)* | 1996-05-24 | 1999-06-16 | 血管技术药物公司 | Compositions and methods for treating or preventing diseases of the body passage |
| US7747325B2 (en)* | 1998-08-05 | 2010-06-29 | Neurovista Corporation | Systems and methods for monitoring a patient's neurological disease state |
| US7324851B1 (en) | 1998-08-05 | 2008-01-29 | Neurovista Corporation | Closed-loop feedback-driven neuromodulation |
| US7209787B2 (en)* | 1998-08-05 | 2007-04-24 | Bioneuronics Corporation | Apparatus and method for closed-loop intracranial stimulation for optimal control of neurological disease |
| US9415222B2 (en) | 1998-08-05 | 2016-08-16 | Cyberonics, Inc. | Monitoring an epilepsy disease state with a supervisory module |
| US9320900B2 (en) | 1998-08-05 | 2016-04-26 | Cyberonics, Inc. | Methods and systems for determining subject-specific parameters for a neuromodulation therapy |
| US9375573B2 (en) | 1998-08-05 | 2016-06-28 | Cyberonics, Inc. | Systems and methods for monitoring a patient's neurological disease state |
| US7403820B2 (en)* | 1998-08-05 | 2008-07-22 | Neurovista Corporation | Closed-loop feedback-driven neuromodulation |
| US8762065B2 (en)* | 1998-08-05 | 2014-06-24 | Cyberonics, Inc. | Closed-loop feedback-driven neuromodulation |
| US7974696B1 (en) | 1998-08-05 | 2011-07-05 | Dilorenzo Biomedical, Llc | Closed-loop autonomic neuromodulation for optimal control of neurological and metabolic disease |
| US7277758B2 (en)* | 1998-08-05 | 2007-10-02 | Neurovista Corporation | Methods and systems for predicting future symptomatology in a patient suffering from a neurological or psychiatric disorder |
| US7242984B2 (en) | 1998-08-05 | 2007-07-10 | Neurovista Corporation | Apparatus and method for closed-loop intracranial stimulation for optimal control of neurological disease |
| US9042988B2 (en) | 1998-08-05 | 2015-05-26 | Cyberonics, Inc. | Closed-loop vagus nerve stimulation |
| US7231254B2 (en)* | 1998-08-05 | 2007-06-12 | Bioneuronics Corporation | Closed-loop feedback-driven neuromodulation |
| US6955661B1 (en)* | 1999-01-25 | 2005-10-18 | Atrium Medical Corporation | Expandable fluoropolymer device for delivery of therapeutic agents and method of making |
| US6616876B1 (en) | 2000-10-03 | 2003-09-09 | Atrium Medical Corporation | Method for treating expandable polymer materials |
| US6923927B2 (en) | 2000-10-03 | 2005-08-02 | Atrium Medical Corporation | Method for forming expandable polymers having drugs or agents included therewith |
| US20040241211A9 (en)* | 2000-11-06 | 2004-12-02 | Fischell Robert E. | Devices and methods for reducing scar tissue formation |
| US20040018228A1 (en)* | 2000-11-06 | 2004-01-29 | Afmedica, Inc. | Compositions and methods for reducing scar tissue formation |
| AU2002249958B2 (en)* | 2001-01-16 | 2007-11-08 | Vascular Therapies, Inc. | Implantable device containing resorbable matrix material and anti-proliferative drugs for preventing or treating failure of hemodialysis vascular access and other vascular grafts |
| US6629988B2 (en)* | 2001-08-28 | 2003-10-07 | Ethicon, Inc. | Composite staple for completing an anastomosis |
| AU2003209297A1 (en)* | 2002-01-18 | 2003-09-02 | Massachusetts Eye And Ear Infirmary | Methods and compositions for preserving the viability of photoreceptor cells |
| DE60310383T2 (en)* | 2002-01-29 | 2007-09-20 | Vlaams Interuniversitair Instituut Voor Biotechnologie Vzw. | PREVENTION OF TISSUE ADHESION |
| US20040087613A1 (en)* | 2002-01-29 | 2004-05-06 | Molmenti Ernesto P. | Prevention of adhesions with rapamycin |
| AU2003280437A1 (en)* | 2002-06-27 | 2004-01-19 | Microport Medical (Shanghai) Co., Ltd. | Drug eluting stent |
| US7300942B2 (en)* | 2002-07-16 | 2007-11-27 | Biotica Technology Limited | Production of polyketides and other natural products |
| US7101381B2 (en)* | 2002-08-02 | 2006-09-05 | C.R. Bard, Inc. | Implantable prosthesis |
| US8163726B2 (en)* | 2002-09-18 | 2012-04-24 | University Of Pennsylvania | Method of inhibiting choroidal neovascularization |
| CA2500067A1 (en)* | 2002-09-26 | 2004-04-08 | Endovascular Devices, Inc. | Apparatus and method for delivery of mitomycin through an eluting biocompatible implantable medical device |
| US8455458B2 (en) | 2002-10-16 | 2013-06-04 | Arthrodynamic Technologies, Animal Health Division, Inc. | Composition and method for treating connective tissue damage |
| US7354574B2 (en)* | 2002-11-07 | 2008-04-08 | Advanced Ocular Systems Limited | Treatment of ocular disease |
| WO2004060283A2 (en) | 2002-12-16 | 2004-07-22 | Nitromed, Inc. | Nitrosated and nitrosylated rapamycin compounds, compositions and methods of use |
| WO2004060405A2 (en)* | 2002-12-30 | 2004-07-22 | Angiotech International Ag | Tissue reactive compounds and compositions and uses thereof |
| DE10305811A1 (en) | 2003-02-12 | 2004-08-26 | Ethicon Gmbh | Surgical implant, preferably in net form, comprises basic structure containing anabolic steroid and corticosteroid to accelerate healing and reduce scar contraction |
| US20040167572A1 (en)* | 2003-02-20 | 2004-08-26 | Roth Noah M. | Coated medical devices |
| US7083802B2 (en)* | 2003-07-31 | 2006-08-01 | Advanced Ocular Systems Limited | Treatment of ocular disease |
| US8021331B2 (en)* | 2003-09-15 | 2011-09-20 | Atrium Medical Corporation | Method of coating a folded medical device |
| AU2004274026A1 (en)* | 2003-09-18 | 2005-03-31 | Macusight, Inc. | Transscleral delivery |
| US7087237B2 (en)* | 2003-09-19 | 2006-08-08 | Advanced Ocular Systems Limited | Ocular solutions |
| US20050181018A1 (en)* | 2003-09-19 | 2005-08-18 | Peyman Gholam A. | Ocular drug delivery |
| US7083803B2 (en)* | 2003-09-19 | 2006-08-01 | Advanced Ocular Systems Limited | Ocular solutions |
| US9066912B2 (en)* | 2003-11-17 | 2015-06-30 | Ethicon, Inc. | Drug-enhanced adhesion prevention |
| AU2004293075A1 (en)* | 2003-11-20 | 2005-06-09 | Angiotech International Ag | Soft tissue implants and anti-scarring agents |
| US20050208095A1 (en)* | 2003-11-20 | 2005-09-22 | Angiotech International Ag | Polymer compositions and methods for their use |
| US8277831B2 (en) | 2004-02-17 | 2012-10-02 | Advanced Technologies And Regenerative Medicine, Llc. | Drug-enhanced adhesion prevention |
| US20050232972A1 (en) | 2004-04-15 | 2005-10-20 | Steven Odrich | Drug delivery via punctal plug |
| US7758654B2 (en) | 2004-05-20 | 2010-07-20 | Kensey Nash Corporation | Anti-adhesion device |
| KR100596218B1 (en)* | 2004-06-10 | 2006-07-03 | (주)액세스 플러스 | Tube for arteriovenous connection of drug-treated hemodialysis patients |
| EP2377569A1 (en) | 2004-07-02 | 2011-10-19 | QLT Plug Delivery, Inc. | Treatment medium delivery device and methods for delivery of such treatment mediums to the eye using such a delivery device |
| US8367099B2 (en)* | 2004-09-28 | 2013-02-05 | Atrium Medical Corporation | Perforated fatty acid films |
| WO2006036970A2 (en)* | 2004-09-28 | 2006-04-06 | Atrium Medical Corporation | Method of thickening a coating using a drug |
| US9801982B2 (en) | 2004-09-28 | 2017-10-31 | Atrium Medical Corporation | Implantable barrier device |
| US9012506B2 (en) | 2004-09-28 | 2015-04-21 | Atrium Medical Corporation | Cross-linked fatty acid-based biomaterials |
| US9801913B2 (en) | 2004-09-28 | 2017-10-31 | Atrium Medical Corporation | Barrier layer |
| US8124127B2 (en) | 2005-10-15 | 2012-02-28 | Atrium Medical Corporation | Hydrophobic cross-linked gels for bioabsorbable drug carrier coatings |
| US9000040B2 (en) | 2004-09-28 | 2015-04-07 | Atrium Medical Corporation | Cross-linked fatty acid-based biomaterials |
| US8312836B2 (en)* | 2004-09-28 | 2012-11-20 | Atrium Medical Corporation | Method and apparatus for application of a fresh coating on a medical device |
| US20090011116A1 (en)* | 2004-09-28 | 2009-01-08 | Atrium Medical Corporation | Reducing template with coating receptacle containing a medical device to be coated |
| WO2006036982A2 (en) | 2004-09-28 | 2006-04-06 | Atrium Medical Corporation | Drug delivery coating for use with a stent |
| US8637070B2 (en)* | 2005-02-09 | 2014-01-28 | Santen Pharmaceutical Co., Ltd. | Rapamycin formulations and methods of their use |
| US8663639B2 (en)* | 2005-02-09 | 2014-03-04 | Santen Pharmaceutical Co., Ltd. | Formulations for treating ocular diseases and conditions |
| ATE489052T1 (en)* | 2005-05-18 | 2010-12-15 | Gt Urological Llc | ELUTION OF ACTIVE SUBSTANCES FOR IMPLANTABLE INCONTINENCE DEVICES |
| US20070014760A1 (en)* | 2005-07-18 | 2007-01-18 | Peyman Gholam A | Enhanced recovery following ocular surgery |
| AU2006270041B2 (en) | 2005-07-18 | 2011-08-18 | Minu, Llc | Enhanced ocular neuroprotection/neurostimulation |
| US7592330B2 (en)* | 2005-08-08 | 2009-09-22 | Massachusetts Eye And Ear Infirmary | Methods and compositions for preserving the viability of photoreceptor cells |
| JP4994379B2 (en)* | 2005-09-01 | 2012-08-08 | ブリストル−マイヤーズ スクイブ カンパニー | Biomarkers and methods for determining sensitivity to vascular endothelial growth factor receptor-2 modulators |
| US9278161B2 (en)* | 2005-09-28 | 2016-03-08 | Atrium Medical Corporation | Tissue-separating fatty acid adhesion barrier |
| US9427423B2 (en) | 2009-03-10 | 2016-08-30 | Atrium Medical Corporation | Fatty-acid based particles |
| US8725243B2 (en) | 2005-12-28 | 2014-05-13 | Cyberonics, Inc. | Methods and systems for recommending an appropriate pharmacological treatment to a patient for managing epilepsy and other neurological disorders |
| US20070149952A1 (en)* | 2005-12-28 | 2007-06-28 | Mike Bland | Systems and methods for characterizing a patient's propensity for a neurological event and for communicating with a pharmacological agent dispenser |
| US8868172B2 (en)* | 2005-12-28 | 2014-10-21 | Cyberonics, Inc. | Methods and systems for recommending an appropriate action to a patient for managing epilepsy and other neurological disorders |
| WO2007089878A2 (en)* | 2006-01-31 | 2007-08-09 | Angiotech Pharmaceuticals, Inc. | Sutures and anti-scarring agents |
| EP2001438A2 (en) | 2006-02-09 | 2008-12-17 | Macusight, Inc. | Stable formulations, and methods of their preparation and use |
| US20070287931A1 (en)* | 2006-02-14 | 2007-12-13 | Dilorenzo Daniel J | Methods and systems for administering an appropriate pharmacological treatment to a patient for managing epilepsy and other neurological disorders |
| BRPI0709016A2 (en) | 2006-03-23 | 2011-06-21 | Macusight Inc | formulations and methods for diseases or conditions related to vascular permeability |
| NZ572193A (en) | 2006-03-31 | 2011-10-28 | Quadra Logic Tech Inc | Nasolacrimal drainage system implants for drug therapy with non-fluid swellable retention structure around drug core |
| US20080021341A1 (en)* | 2006-06-23 | 2008-01-24 | Neurovista Corporation A Delware Corporation | Methods and Systems for Facilitating Clinical Trials |
| US9492596B2 (en) | 2006-11-06 | 2016-11-15 | Atrium Medical Corporation | Barrier layer with underlying medical device and one or more reinforcing support structures |
| EP2083875B1 (en) | 2006-11-06 | 2013-03-27 | Atrium Medical Corporation | Coated surgical mesh |
| US8295934B2 (en) | 2006-11-14 | 2012-10-23 | Neurovista Corporation | Systems and methods of reducing artifact in neurological stimulation systems |
| US8668703B2 (en) | 2006-12-01 | 2014-03-11 | Wake Forest University Health Sciences | Medical devices incorporating collagen inhibitors |
| EP2126785A2 (en) | 2007-01-25 | 2009-12-02 | NeuroVista Corporation | Systems and methods for identifying a contra-ictal condition in a subject |
| WO2008092133A2 (en) | 2007-01-25 | 2008-07-31 | Neurovista Corporation | Methods and systems for measuring a subject's susceptibility to a seizure |
| US7938286B2 (en)* | 2007-02-13 | 2011-05-10 | Gateway Plastics, Inc. | Container system |
| US8036736B2 (en) | 2007-03-21 | 2011-10-11 | Neuro Vista Corporation | Implantable systems and methods for identifying a contra-ictal condition in a subject |
| US20080265343A1 (en)* | 2007-04-26 | 2008-10-30 | International Business Machines Corporation | Field effect transistor with inverted t shaped gate electrode and methods for fabrication thereof |
| US20080287987A1 (en)* | 2007-05-16 | 2008-11-20 | Searete Llc, A Limited Liability Corporation Of The State Of Delaware | Dispensing system for tissue sealants |
| US8721711B2 (en)* | 2007-06-20 | 2014-05-13 | Oregon Health & Science University | Graft having microporous membrane for uniform fluid infusion |
| US9788744B2 (en) | 2007-07-27 | 2017-10-17 | Cyberonics, Inc. | Systems for monitoring brain activity and patient advisory device |
| WO2009035567A2 (en)* | 2007-09-07 | 2009-03-19 | Qlt Plug Delivery, Inc | Insertion and extraction tools for lacrimal implants |
| CN101984745B (en) | 2007-09-07 | 2013-08-14 | Qlt股份有限公司 | Drug Cores for Sustained Release of Therapeutics |
| CN101861135A (en) | 2007-09-07 | 2010-10-13 | Qlt栓塞输送公司 | Lacrimal implant testing |
| US20090112243A1 (en)* | 2007-10-25 | 2009-04-30 | Searete Llc, A Limited Liability Corporation Of The State Of Delaware | Surgical cutter with dispensing system for tissue sealants |
| US20090112256A1 (en)* | 2007-10-30 | 2009-04-30 | Searete Llc, A Limited Liability Corporation Of The State Of Delaware | Suturing device with tissue sealant dispenser |
| US20090143816A1 (en)* | 2007-11-30 | 2009-06-04 | Searete Llc, A Limited Liability Corporation Of The State Of Delaware | Grasper with surgical sealant dispenser |
| US8808255B2 (en)* | 2007-12-14 | 2014-08-19 | Oregon Health & Science University | Drug delivery cuff |
| US9259591B2 (en) | 2007-12-28 | 2016-02-16 | Cyberonics, Inc. | Housing for an implantable medical device |
| US20090171168A1 (en) | 2007-12-28 | 2009-07-02 | Leyde Kent W | Systems and Method for Recording Clinical Manifestations of a Seizure |
| US10376261B2 (en)* | 2008-04-01 | 2019-08-13 | Covidien Lp | Anchoring suture |
| CN104623741A (en) | 2008-04-30 | 2015-05-20 | 马缇医疗股份有限公司 | Composite lacrimal insert and related methods |
| WO2009137085A2 (en)* | 2008-05-09 | 2009-11-12 | Qlt Plug Delivery, Inc. | Sustained release delivery of active agents to treat glaucoma and ocular hypertension |
| CA2728623A1 (en)* | 2008-06-24 | 2010-01-21 | Qlt Plug Delivery, Inc. | Combination treatment of glaucoma |
| US8849390B2 (en) | 2008-12-29 | 2014-09-30 | Cyberonics, Inc. | Processing for multi-channel signals |
| US8588933B2 (en) | 2009-01-09 | 2013-11-19 | Cyberonics, Inc. | Medical lead termination sleeve for implantable medical devices |
| RU2011134043A (en) | 2009-01-23 | 2013-02-20 | Клт Инк. | DELIVERY OF ONE OR MORE AGENT WITH LONG-TERM RELEASE |
| US8786624B2 (en) | 2009-06-02 | 2014-07-22 | Cyberonics, Inc. | Processing for multi-channel signals |
| DE102009030931A1 (en) | 2009-06-24 | 2010-12-30 | Eberhard-Karls-Universität Tübingen Universitätsklinikum | Surgical wound closure element |
| US20110038910A1 (en) | 2009-08-11 | 2011-02-17 | Atrium Medical Corporation | Anti-infective antimicrobial-containing biomaterials |
| US9643019B2 (en) | 2010-02-12 | 2017-05-09 | Cyberonics, Inc. | Neurological monitoring and alerts |
| EP2538876A4 (en)* | 2010-02-22 | 2014-09-03 | Rudy A Mazzocchi | MINIMALLY INVASIVE METHOD AND APPARATUS FOR RESTRUCTURING THE RETINA |
| US20110219325A1 (en)* | 2010-03-02 | 2011-09-08 | Himes David M | Displaying and Manipulating Brain Function Data Including Enhanced Data Scrolling Functionality |
| WO2012009707A2 (en) | 2010-07-16 | 2012-01-19 | Atrium Medical Corporation | Composition and methods for altering the rate of hydrolysis of cured oil-based materials |
| TW201311303A (en)* | 2011-06-21 | 2013-03-16 | Bvw Holding Ag | Implant localization device |
| US8776716B2 (en) | 2011-08-09 | 2014-07-15 | Biomet Biologics, Llc | Surgical mesh spray and delivery system |
| CN107669399B (en) | 2011-08-29 | 2019-11-19 | 麦提疗法有限公司 | Sustained release delivery activating agent is to treat glaucoma and intraocular hypertension |
| US9974685B2 (en) | 2011-08-29 | 2018-05-22 | Mati Therapeutics | Drug delivery system and methods of treating open angle glaucoma and ocular hypertension |
| US9474685B2 (en) | 2011-09-28 | 2016-10-25 | Sure-Shot Medical Device Inc. | Apparatus for localized dermatological treatment |
| US20130202656A1 (en) | 2012-02-03 | 2013-08-08 | Dynasil Biomedical Corporation | Systems and kits for the fabrication of tissue patches |
| KR102119375B1 (en) | 2012-02-16 | 2020-06-09 | 신세스 게엠바하 | Drug eluting insert for implantable body |
| CN104349726B (en)* | 2012-03-28 | 2017-11-24 | 伊西康内外科公司 | Tissue thickness compensator and manufacturing method thereof |
| EP2844224B1 (en) | 2012-05-03 | 2018-04-11 | Mati Therapeutics Inc. | Drug delivery system for treating open angle glaucoma and ocular hypertension |
| US9867880B2 (en) | 2012-06-13 | 2018-01-16 | Atrium Medical Corporation | Cured oil-hydrogel biomaterial compositions for controlled drug delivery |
| WO2014169301A1 (en)* | 2013-04-12 | 2014-10-16 | Bui The Duy | Systems and methods for delivering cross-linked halyuronic acid into a patient |
| US11432990B2 (en) | 2013-08-30 | 2022-09-06 | ISOS Solutions, LLC | Textured apparatus with therapeutic material incorporated therein and methods of manufacturing same |
| US10022475B2 (en)* | 2014-05-01 | 2018-07-17 | Bao Tran | Body augmentation device |
| US9833538B2 (en) | 2015-08-07 | 2017-12-05 | Xcede Technologies, Inc. | Adhesive compositions and related methods |
| CA2994959A1 (en) | 2015-08-07 | 2017-02-16 | Xcede Technologies, Inc. | Adhesive compositions and related methods |
| US9540548B1 (en) | 2015-08-07 | 2017-01-10 | Xcede Technologies, Inc. | Adhesive compositions and related methods |
| FR3041236B1 (en)* | 2015-09-21 | 2017-10-20 | Vygon | SYSTEM FOR TREATING EPISTAXIS |
| US10932523B2 (en) | 2015-11-30 | 2021-03-02 | Nike, Inc. | Electrorheological fluid structure with attached conductor and method of fabrication |
| CZ307155B6 (en)* | 2016-12-16 | 2018-02-07 | Synthesia, A. S. | A supramolecular complex of an oxycellulose matrix with an anthracycline cytostatic with sequential release of an anthracycline cytostatic and its use |
| WO2019044765A1 (en)* | 2017-08-28 | 2019-03-07 | 川澄化学工業株式会社 | Adhesion prevention material |
| CN111263597B (en) | 2017-08-31 | 2022-04-01 | 耐克创新有限合伙公司 | Recliner with multiple discrete chambers |
| JP6965443B2 (en) | 2017-10-13 | 2021-11-10 | ナイキ イノベイト シーブイ | Footwear midsole with electrorheological fluid housing |
| WO2019157048A1 (en)* | 2018-02-06 | 2019-08-15 | The Trustees Of The University Of Pennsylvania | The kirigami modification of biomedical tissue reinforcing meshes and matrices for expansile two-to-three dimensional conversion |
| CN117815229A (en)* | 2023-12-20 | 2024-04-05 | 中国医学科学院整形外科医院 | Use of rapamycin in products of envelope proliferation |
Citations (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US6726923B2 (en)* | 2001-01-16 | 2004-04-27 | Vascular Therapies, Llc | Apparatus and methods for preventing or treating failure of hemodialysis vascular access and other vascular grafts |
Family Cites Families (55)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US4176664A (en)* | 1978-03-13 | 1979-12-04 | Stanley Kalish | Impregnated bandage |
| US4747845A (en)* | 1983-10-17 | 1988-05-31 | Enquay Pharmaceutical Associates | Synthetic resin matrix system for the extended delivery of drugs |
| US4619913A (en) | 1984-05-29 | 1986-10-28 | Matrix Pharmaceuticals, Inc. | Treatments employing drug-containing matrices for introduction into cellular lesion areas |
| US4835061A (en)* | 1984-11-09 | 1989-05-30 | Konishiroku Photo Industry Co., Ltd. | Conductive laminate |
| US4865031A (en)* | 1985-07-12 | 1989-09-12 | Keeffe Paul J O | Fabric and method of use for treatment of scars |
| US5250516A (en)* | 1986-04-17 | 1993-10-05 | Uab Research Foundation | Bioelastomeric materials suitable for the protection of burn areas or the protection of wound repair sites from the occurrence of adhesions |
| US4895566A (en) | 1986-07-25 | 1990-01-23 | C. R. Bard, Inc. | Coating medical devices with cationic antibiotics |
| US4863457A (en)* | 1986-11-24 | 1989-09-05 | Lee David A | Drug delivery device |
| US4952403A (en)* | 1987-06-19 | 1990-08-28 | President And Fellows Of Harvard College | Implants for the promotion of healing of meniscal tissue |
| US5843156A (en) | 1988-08-24 | 1998-12-01 | Endoluminal Therapeutics, Inc. | Local polymeric gel cellular therapy |
| US5540931A (en)* | 1989-03-03 | 1996-07-30 | Charles W. Hewitt | Methods for inducing site-specific immunosuppression and compositions of site specific immunosuppressants |
| US5134229A (en)* | 1990-01-12 | 1992-07-28 | Johnson & Johnson Medical, Inc. | Process for preparing a neutralized oxidized cellulose product and its method of use |
| US6117425A (en) | 1990-11-27 | 2000-09-12 | The American National Red Cross | Supplemented and unsupplemented tissue sealants, method of their production and use |
| IL102414A (en) | 1991-07-25 | 1996-08-04 | Univ Louisville Res Found | Pharmaceutical compositions for treating ocular inflammation comprising rapamycin |
| US5708002A (en) | 1991-09-05 | 1998-01-13 | Abbott Laboratories | Macrocyclic immunomodulators |
| US5366504A (en)* | 1992-05-20 | 1994-11-22 | Boston Scientific Corporation | Tubular medical prosthesis |
| US5151413A (en) | 1991-11-06 | 1992-09-29 | American Home Products Corporation | Rapamycin acetals as immunosuppressant and antifungal agents |
| US5516781A (en)* | 1992-01-09 | 1996-05-14 | American Home Products Corporation | Method of treating restenosis with rapamycin |
| CA2146973C (en)* | 1992-10-29 | 2008-09-02 | Patricia R. Segarini | Uses of tgf-.beta. receptor fragment as a therapeutic agent |
| US5981568A (en) | 1993-01-28 | 1999-11-09 | Neorx Corporation | Therapeutic inhibitor of vascular smooth muscle cells |
| US5552162A (en)* | 1993-02-09 | 1996-09-03 | Arch Development Corporation | Method for improvement of scar size and appearance |
| FR2707860B1 (en)* | 1993-07-23 | 1995-09-08 | Bour Jean | Apparatus for measuring and processing physiological signals and automatic method implemented by said apparatus. |
| US5624893A (en) | 1993-10-14 | 1997-04-29 | Alcon Laboratories, Inc. | Pharmaceutical compositions and methods of treatment of the cornea following laser irradiation |
| US5443505A (en)* | 1993-11-15 | 1995-08-22 | Oculex Pharmaceuticals, Inc. | Biocompatible ocular implants |
| JP3745772B2 (en) | 1993-12-17 | 2006-02-15 | ノバルティス アクチエンゲゼルシャフト | Rapamycin derivatives useful as immunosuppressants |
| US6063396A (en) | 1994-10-26 | 2000-05-16 | Houston Biotechnology Incorporated | Methods and compositions for the modulation of cell proliferation and wound healing |
| US6063116A (en)* | 1994-10-26 | 2000-05-16 | Medarex, Inc. | Modulation of cell proliferation and wound healing |
| US5665591A (en) | 1994-12-06 | 1997-09-09 | Trustees Of Boston University | Regulation of smooth muscle cell proliferation |
| US5563145A (en)* | 1994-12-07 | 1996-10-08 | American Home Products Corporation | Rapamycin 42-oximes and hydroxylamines |
| US5496832A (en) | 1995-03-09 | 1996-03-05 | American Home Products Corporation | Method of treating cardiac inflammatory disease |
| US5609629A (en)* | 1995-06-07 | 1997-03-11 | Med Institute, Inc. | Coated implantable medical device |
| SK284529B6 (en) | 1995-06-09 | 2005-05-05 | Novartis Ag | Rapamycin derivatives, process of their preparation, pharmaceutical composition containing thereof and kit or package for use in immunosuppression, inflammation or infections |
| WO1996041818A1 (en) | 1995-06-09 | 1996-12-27 | Drohan William N | Chitin hydrogels, methods of their production and use |
| US5798334A (en)* | 1995-09-28 | 1998-08-25 | Colla-Gene, Inc. | Pharmaceutical compositions for scarless tissue repair and regeneration and methods related thereto |
| US5767135A (en)* | 1995-12-29 | 1998-06-16 | Fernandez-Pol; Jose Alberto | Antiviral agent |
| US5912224A (en) | 1996-02-22 | 1999-06-15 | The General Hospital Corporation | Methods and compositions for enhancing cellular response to TGF-β ligands |
| US5916585A (en)* | 1996-06-03 | 1999-06-29 | Gore Enterprise Holdings, Inc. | Materials and method for the immobilization of bioactive species onto biodegradable polymers |
| US6143037A (en)* | 1996-06-12 | 2000-11-07 | The Regents Of The University Of Michigan | Compositions and methods for coating medical devices |
| US5795286A (en)* | 1996-08-15 | 1998-08-18 | Cathco, Inc. | Radioisotope impregnated sheet of biocompatible material for preventing scar tissue formation |
| EP0975340B2 (en)* | 1997-03-31 | 2009-10-28 | Boston Scientific Limited | Therapeutic inhibitor of vascular smooth muscle cells |
| DK1319651T3 (en)* | 1997-04-04 | 2005-08-01 | Mitsubishi Pharma Corp | 2-aminopropane-1,3-diol compounds, their medical use and intermediates for their synthesis |
| US6273913B1 (en)* | 1997-04-18 | 2001-08-14 | Cordis Corporation | Modified stent useful for delivery of drugs along stent strut |
| US6015815A (en) | 1997-09-26 | 2000-01-18 | Abbott Laboratories | Tetrazole-containing rapamycin analogs with shortened half-lives |
| US6273908B1 (en)* | 1997-10-24 | 2001-08-14 | Robert Ndondo-Lay | Stents |
| US6015432A (en)* | 1998-02-25 | 2000-01-18 | Cordis Corporation | Wire reinforced vascular prosthesis |
| US6063369A (en)* | 1998-03-16 | 2000-05-16 | Alterna, Inc. | Quaternized hemp seed oil |
| AU761058B2 (en)* | 1998-04-07 | 2003-05-29 | Macropore, Inc. | Membrane with tissue-guiding surface corrugations |
| US8029561B1 (en) | 2000-05-12 | 2011-10-04 | Cordis Corporation | Drug combination useful for prevention of restenosis |
| US6312713B1 (en)* | 1998-06-12 | 2001-11-06 | Bernard Korol | Polymer matrices for storage and sustained release of drugs and chemicals |
| US6060474A (en)* | 1998-11-05 | 2000-05-09 | New York Society For The Relief Of The Ruptured And Crippled Maintaining The Hospital For Special Surgery | Method for preventing scar tissue formation |
| US6187024B1 (en)* | 1998-11-10 | 2001-02-13 | Target Therapeutics, Inc. | Bioactive coating for vaso-occlusive devices |
| US6702828B2 (en)* | 1999-09-01 | 2004-03-09 | Converge Medical, Inc. | Anastomosis system |
| JP2003520830A (en)* | 2000-01-25 | 2003-07-08 | エドワーズ ライフサイエンシーズ コーポレイション | Delivery system for treatment of restenosis and anastomotic intimal hyperplasia |
| US6776796B2 (en)* | 2000-05-12 | 2004-08-17 | Cordis Corportation | Antiinflammatory drug and delivery device |
| US7419678B2 (en)* | 2000-05-12 | 2008-09-02 | Cordis Corporation | Coated medical devices for the prevention and treatment of vascular disease |
- 2001
- 2001-01-31USUS09/772,693patent/US6534693B2/ennot_activeExpired - Lifetime
- 2001-10-16WOPCT/US2001/027771patent/WO2002036054A1/enactiveIP Right Grant
- 2001-10-16AUAU2002224318Apatent/AU2002224318B2/ennot_activeCeased
- 2001-10-16EPEP01992543Apatent/EP1385458A4/ennot_activeCeased
- 2001-10-16AUAU2431802Apatent/AU2431802A/enactivePending
- 2001-10-16BRBR0115457-5Apatent/BR0115457A/ennot_activeApplication Discontinuation
- 2001-10-16JPJP2002538866Apatent/JP2004529667A/enactivePending
- 2001-10-16MXMXPA03003906Apatent/MXPA03003906A/ennot_activeApplication Discontinuation
- 2001-10-16CACA002428082Apatent/CA2428082A1/ennot_activeAbandoned
- 2003
- 2003-01-24USUS10/351,207patent/US20040006296A1/ennot_activeAbandoned
- 2005
- 2005-03-21USUS11/084,948patent/US20050281860A1/ennot_activeAbandoned
Patent Citations (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US6726923B2 (en)* | 2001-01-16 | 2004-04-27 | Vascular Therapies, Llc | Apparatus and methods for preventing or treating failure of hemodialysis vascular access and other vascular grafts |
Cited By (17)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US20040146546A1 (en)* | 2002-09-26 | 2004-07-29 | Angiotech Pharmaceuticals, Inc. | Perivascular wraps |
| US20080085855A1 (en)* | 2002-09-26 | 2008-04-10 | Angiotech International Ag | Perivascular wraps |
| US20050149158A1 (en)* | 2003-11-10 | 2005-07-07 | Angiotech International Ag | Medical implants and anti-scarring agents |
| US20050149080A1 (en)* | 2003-11-10 | 2005-07-07 | Angiotech International Ag | Medical implants and anti-scarring agents |
| US7922064B2 (en) | 2007-05-16 | 2011-04-12 | The Invention Science Fund, I, LLC | Surgical fastening device with cutter |
| US7810691B2 (en) | 2007-05-16 | 2010-10-12 | The Invention Science Fund I, Llc | Gentle touch surgical stapler |
| US7823761B2 (en) | 2007-05-16 | 2010-11-02 | The Invention Science Fund I, Llc | Maneuverable surgical stapler |
| US7832611B2 (en) | 2007-05-16 | 2010-11-16 | The Invention Science Fund I, Llc | Steerable surgical stapler |
| US7798385B2 (en) | 2007-05-16 | 2010-09-21 | The Invention Science Fund I, Llc | Surgical stapling instrument with chemical sealant |
| US7931182B2 (en) | 2007-05-16 | 2011-04-26 | The Invention Science Fund I, Llc | Steerable surgical stapler |
| US7975894B2 (en) | 2007-05-16 | 2011-07-12 | The Invention Science Fund I, Llc | Sensing surgical fastener |
| US8485411B2 (en) | 2007-05-16 | 2013-07-16 | The Invention Science Fund I, Llc | Gentle touch surgical stapler |
| US9445809B2 (en) | 2007-05-16 | 2016-09-20 | Deep Science, Llc | Gentle touch surgical stapler |
| US20110237542A1 (en)* | 2008-12-01 | 2011-09-29 | Shin Poong Pharmaceutical Co., Ltd. | Composition for preventing adhesion |
| US8703740B2 (en) | 2008-12-01 | 2014-04-22 | Shin Poong Pharmaceutical Co., Ltd. | Composition for preventing adhesion |
| EP3597206A1 (en) | 2011-06-21 | 2020-01-22 | BVW Holding AG | Medical device comprising boswellic acid |
| US11717593B2 (en) | 2013-03-13 | 2023-08-08 | Avery Dennison Corporation | Improving adhesive properties |
Also Published As
| Publication number | Publication date |
|---|---|
| US6534693B2 (en) | 2003-03-18 |
| MXPA03003906A (en) | 2004-09-10 |
| EP1385458A4 (en) | 2008-04-09 |
| CA2428082A1 (en) | 2002-05-10 |
| JP2004529667A (en) | 2004-09-30 |
| AU2431802A (en) | 2002-05-15 |
| BR0115457A (en) | 2004-06-15 |
| AU2002224318B2 (en) | 2005-10-06 |
| US20040006296A1 (en) | 2004-01-08 |
| WO2002036054A1 (en) | 2002-05-10 |
| EP1385458A1 (en) | 2004-02-04 |
| US20020055701A1 (en) | 2002-05-09 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| US6534693B2 (en) | Surgically implanted devices having reduced scar tissue formation | |
| AU2002224318A1 (en) | Surgically implanted devices having reduced scar tissue | |
| US20040071756A1 (en) | Devices and methods for reducing scar tissue formation | |
| US20050084514A1 (en) | Combination drug therapy for reducing scar tissue formation | |
| CA2529754C (en) | Medical devices and methods for regulating the tissue response to vascular closure devices | |
| CA2467797A1 (en) | Increased biocompatibility of implantable medical devices | |
| US20080039362A1 (en) | Combination drug therapy for reducing scar tissue formation | |
| US20060286063A1 (en) | Combination drug therapy for reducing scar tissue formation | |
| WO2014087325A1 (en) | Smart Coating for Implantable Devices | |
| US20140249618A1 (en) | Site specific drug delivery wraps, systems and methods of use thereof | |
| AU2008201020A1 (en) | Combination drug therapy for reducing scar tissue formation |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| AS | Assignment | Owner name:AFMEDICA, INC., MICHIGAN Free format text:ASSIGNMENT OF ASSIGNORS INTEREST;ASSIGNORS:FISCHELL, ROBERT E.;FISCHELL, DAVID R.;FISCHELL, TIM A;AND OTHERS;REEL/FRAME:016713/0246 Effective date:20030511 | |
| AS | Assignment | Owner name:CREDIT SUISSE, AS COLLATERAL AGENT, NEW YORK Free format text:SECURITY AGREEMENT;ASSIGNOR:AFMEDICA, INC.;REEL/FRAME:017448/0676 Effective date:20060323 | |
| AS | Assignment | Owner name:AFMEDICA, INC., WASHINGTON Free format text:RELEASE BY SECURED PARTY;ASSIGNOR:CREDIT SUISSE;REEL/FRAME:018606/0626 Effective date:20061211 | |
| AS | Assignment | Owner name:WELLS FARGO FOOTHILL, LLC AS AGENT, GEORGIA Free format text:SECURITY AGREEMENT;ASSIGNORS:ANGIOTECH PHARMACEUTICALS, INC.;AFMEDICA, INC.;AMERICAN MEDICAL INSTRUMENTS HOLDINGS, INC.;AND OTHERS;REEL/FRAME:022329/0310 Effective date:20090227 Owner name:WELLS FARGO FOOTHILL, LLC AS AGENT,GEORGIA Free format text:SECURITY AGREEMENT;ASSIGNORS:ANGIOTECH PHARMACEUTICALS, INC.;AFMEDICA, INC.;AMERICAN MEDICAL INSTRUMENTS HOLDINGS, INC.;AND OTHERS;REEL/FRAME:022329/0310 Effective date:20090227 | |
| STCB | Information on status: application discontinuation | Free format text:ABANDONED -- FAILURE TO RESPOND TO AN OFFICE ACTION |