US20050209587A1 - Apparatus and method for cryogenic inhibition of hyperplasia - Google Patents
Apparatus and method for cryogenic inhibition of hyperplasiaDownload PDFInfo
- Publication number
- US20050209587A1 US20050209587A1US11/122,165US12216505AUS2005209587A1US 20050209587 A1US20050209587 A1US 20050209587A1US 12216505 AUS12216505 AUS 12216505AUS 2005209587 A1US2005209587 A1US 2005209587A1
- Authority
- US
- United States
- Prior art keywords
- balloon
- cooling
- cooling fluid
- lumen
- catheter
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Abandoned
Links
- 206010020718hyperplasiaDiseases0.000titleclaimsabstractdescription20
- 238000000034methodMethods0.000titledescription31
- 230000005764inhibitory processEffects0.000titledescription5
- 238000001816coolingMethods0.000claimsabstractdescription61
- 239000012530fluidSubstances0.000claimsabstractdescription48
- 239000007788liquidSubstances0.000claimsabstractdescription33
- 210000004204blood vesselAnatomy0.000claimsabstractdescription20
- 239000012809cooling fluidSubstances0.000claimsdescription42
- 238000004891communicationMethods0.000claimsdescription11
- 230000008016vaporizationEffects0.000claimsdescription9
- 210000001367arteryAnatomy0.000claimsdescription8
- 230000010261cell growthEffects0.000claimsdescription7
- 230000000694effectsEffects0.000claimsdescription6
- 206010028980NeoplasmDiseases0.000claimsdescription5
- 238000009834vaporizationMethods0.000claimsdescription5
- 230000009826neoplastic cell growthEffects0.000claimsdescription4
- 239000000110cooling liquidSubstances0.000claims2
- 239000011248coating agentSubstances0.000claims1
- 238000000576coating methodMethods0.000claims1
- 230000000916dilatatory effectEffects0.000claims1
- IJGRMHOSHXDMSA-UHFFFAOYSA-NAtomic nitrogenChemical compoundN#NIJGRMHOSHXDMSA-UHFFFAOYSA-N0.000abstractdescription39
- 229910052757nitrogenInorganic materials0.000abstractdescription19
- 238000002399angioplastyMethods0.000abstractdescription13
- 238000011282treatmentMethods0.000description30
- 239000007789gasSubstances0.000description29
- 210000001519tissueAnatomy0.000description22
- 238000011221initial treatmentMethods0.000description10
- 230000004663cell proliferationEffects0.000description9
- 239000000463materialSubstances0.000description9
- 208000037803restenosisDiseases0.000description8
- 238000002560therapeutic procedureMethods0.000description8
- 210000005166vasculatureAnatomy0.000description8
- 239000004642PolyimideSubstances0.000description7
- 229920001721polyimidePolymers0.000description7
- 230000009286beneficial effectEffects0.000description6
- 238000009826distributionMethods0.000description6
- 238000012544monitoring processMethods0.000description6
- 238000012546transferMethods0.000description6
- 230000008901benefitEffects0.000description5
- 210000004027cellAnatomy0.000description5
- 230000002401inhibitory effectEffects0.000description5
- -1polyethylenesPolymers0.000description5
- CURLTUGMZLYLDI-UHFFFAOYSA-NCarbon dioxideChemical compoundO=C=OCURLTUGMZLYLDI-UHFFFAOYSA-N0.000description4
- GQPLMRYTRLFLPF-UHFFFAOYSA-NNitrous OxideChemical compound[O-][N+]#NGQPLMRYTRLFLPF-UHFFFAOYSA-N0.000description4
- 230000003143atherosclerotic effectEffects0.000description4
- 201000010099diseaseDiseases0.000description4
- 208000037265diseases, disorders, signs and symptomsDiseases0.000description4
- 230000000670limiting effectEffects0.000description4
- 230000007246mechanismEffects0.000description4
- 230000008569processEffects0.000description4
- 239000004698PolyethyleneSubstances0.000description3
- 208000027418Wounds and injuryDiseases0.000description3
- 239000000853adhesiveSubstances0.000description3
- 230000001070adhesive effectEffects0.000description3
- 239000008280bloodSubstances0.000description3
- 210000004369bloodAnatomy0.000description3
- 238000009835boilingMethods0.000description3
- 230000006378damageEffects0.000description3
- PCHJSUWPFVWCPO-UHFFFAOYSA-NgoldChemical compound[Au]PCHJSUWPFVWCPO-UHFFFAOYSA-N0.000description3
- 208000014674injuryDiseases0.000description3
- 239000003550markerSubstances0.000description3
- 230000035407negative regulation of cell proliferationEffects0.000description3
- 229920000573polyethylenePolymers0.000description3
- 229920000642polymerPolymers0.000description3
- 230000004044responseEffects0.000description3
- 230000007704transitionEffects0.000description3
- 230000004323axial lengthEffects0.000description2
- 229910002092carbon dioxideInorganic materials0.000description2
- 239000001569carbon dioxideSubstances0.000description2
- 230000008878couplingEffects0.000description2
- 238000010168coupling processMethods0.000description2
- 238000005859coupling reactionMethods0.000description2
- 230000003511endothelial effectEffects0.000description2
- 210000001105femoral arteryAnatomy0.000description2
- 229910052737goldInorganic materials0.000description2
- 239000010931goldSubstances0.000description2
- 230000002390hyperplastic effectEffects0.000description2
- 238000013147laser angioplastyMethods0.000description2
- 229910052751metalInorganic materials0.000description2
- 239000002184metalSubstances0.000description2
- 230000017074necrotic cell deathEffects0.000description2
- 239000001272nitrous oxideSubstances0.000description2
- 230000036961partial effectEffects0.000description2
- BASFCYQUMIYNBI-UHFFFAOYSA-NplatinumChemical compound[Pt]BASFCYQUMIYNBI-UHFFFAOYSA-N0.000description2
- 229920000139polyethylene terephthalatePolymers0.000description2
- 239000005020polyethylene terephthalateSubstances0.000description2
- 229920001343polytetrafluoroethylenePolymers0.000description2
- 239000004810polytetrafluoroethyleneSubstances0.000description2
- 239000000523sampleSubstances0.000description2
- 230000002966stenotic effectEffects0.000description2
- 238000003466weldingMethods0.000description2
- 238000004804windingMethods0.000description2
- 229910052582BNInorganic materials0.000description1
- PZNSFCLAULLKQX-UHFFFAOYSA-NBoron nitrideChemical compoundN#BPZNSFCLAULLKQX-UHFFFAOYSA-N0.000description1
- 208000031481Pathologic ConstrictionDiseases0.000description1
- FAPWRFPIFSIZLT-UHFFFAOYSA-MSodium chlorideChemical compound[Na+].[Cl-]FAPWRFPIFSIZLT-UHFFFAOYSA-M0.000description1
- 208000007536ThrombosisDiseases0.000description1
- 238000002679ablationMethods0.000description1
- 230000001028anti-proliverative effectEffects0.000description1
- 229940127217antithrombotic drugDrugs0.000description1
- 230000006907apoptotic processEffects0.000description1
- 238000005452bendingMethods0.000description1
- 230000017531blood circulationEffects0.000description1
- 238000004590computer programMethods0.000description1
- 239000002826coolantSubstances0.000description1
- 239000000112cooling gasSubstances0.000description1
- 229920001577copolymerPolymers0.000description1
- 230000003247decreasing effectEffects0.000description1
- 238000011161developmentMethods0.000description1
- 230000018109developmental processEffects0.000description1
- 238000010586diagramMethods0.000description1
- 229910001873dinitrogenInorganic materials0.000description1
- 230000002708enhancing effectEffects0.000description1
- 238000000605extractionMethods0.000description1
- 238000007710freezingMethods0.000description1
- 230000008014freezingEffects0.000description1
- 238000010438heat treatmentMethods0.000description1
- 230000001939inductive effectEffects0.000description1
- 230000000977initiatory effectEffects0.000description1
- 238000009413insulationMethods0.000description1
- 230000001926lymphatic effectEffects0.000description1
- 150000002739metalsChemical class0.000description1
- 239000000203mixtureSubstances0.000description1
- 238000012986modificationMethods0.000description1
- 230000004048modificationEffects0.000description1
- 230000001613neoplastic effectEffects0.000description1
- 231100000252nontoxicToxicity0.000description1
- 230000003000nontoxic effectEffects0.000description1
- 238000004806packaging method and processMethods0.000description1
- 210000000277pancreatic ductAnatomy0.000description1
- 238000004382pottingMethods0.000description1
- 238000007639printingMethods0.000description1
- 230000002035prolonged effectEffects0.000description1
- 230000005855radiationEffects0.000description1
- 230000003134recirculating effectEffects0.000description1
- 238000007634remodelingMethods0.000description1
- 238000011160researchMethods0.000description1
- 230000000241respiratory effectEffects0.000description1
- 238000000926separation methodMethods0.000description1
- 229920002545silicone oilPolymers0.000description1
- 238000010583slow coolingMethods0.000description1
- 210000000329smooth muscle myocyteAnatomy0.000description1
- 239000011780sodium chlorideSubstances0.000description1
- 239000007787solidSubstances0.000description1
- 239000007921spraySubstances0.000description1
- 229910001220stainless steelInorganic materials0.000description1
- 208000037804stenosisDiseases0.000description1
- 230000036262stenosisEffects0.000description1
- 238000011269treatment regimenMethods0.000description1
- 230000004614tumor growthEffects0.000description1
- 210000000626ureterAnatomy0.000description1
- 230000002792vascularEffects0.000description1
Images
Classifications
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61B—DIAGNOSIS; SURGERY; IDENTIFICATION
- A61B18/00—Surgical instruments, devices or methods for transferring non-mechanical forms of energy to or from the body
- A61B18/02—Surgical instruments, devices or methods for transferring non-mechanical forms of energy to or from the body by cooling, e.g. cryogenic techniques
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61B—DIAGNOSIS; SURGERY; IDENTIFICATION
- A61B17/00—Surgical instruments, devices or methods
- A61B17/00234—Surgical instruments, devices or methods for minimally invasive surgery
- A61B2017/00292—Surgical instruments, devices or methods for minimally invasive surgery mounted on or guided by flexible, e.g. catheter-like, means
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61B—DIAGNOSIS; SURGERY; IDENTIFICATION
- A61B17/00—Surgical instruments, devices or methods
- A61B17/22—Implements for squeezing-off ulcers or the like on inner organs of the body; Implements for scraping-out cavities of body organs, e.g. bones; for invasive removal or destruction of calculus using mechanical vibrations; for removing obstructions in blood vessels, not otherwise provided for
- A61B2017/22001—Angioplasty, e.g. PCTA
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61B—DIAGNOSIS; SURGERY; IDENTIFICATION
- A61B17/00—Surgical instruments, devices or methods
- A61B17/22—Implements for squeezing-off ulcers or the like on inner organs of the body; Implements for scraping-out cavities of body organs, e.g. bones; for invasive removal or destruction of calculus using mechanical vibrations; for removing obstructions in blood vessels, not otherwise provided for
- A61B2017/22001—Angioplasty, e.g. PCTA
- A61B2017/22002—Angioplasty, e.g. PCTA preventing restenosis
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61B—DIAGNOSIS; SURGERY; IDENTIFICATION
- A61B17/00—Surgical instruments, devices or methods
- A61B17/22—Implements for squeezing-off ulcers or the like on inner organs of the body; Implements for scraping-out cavities of body organs, e.g. bones; for invasive removal or destruction of calculus using mechanical vibrations; for removing obstructions in blood vessels, not otherwise provided for
- A61B2017/22051—Implements for squeezing-off ulcers or the like on inner organs of the body; Implements for scraping-out cavities of body organs, e.g. bones; for invasive removal or destruction of calculus using mechanical vibrations; for removing obstructions in blood vessels, not otherwise provided for with an inflatable part, e.g. balloon, for positioning, blocking, or immobilisation
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61B—DIAGNOSIS; SURGERY; IDENTIFICATION
- A61B18/00—Surgical instruments, devices or methods for transferring non-mechanical forms of energy to or from the body
- A61B2018/00053—Mechanical features of the instrument of device
- A61B2018/00184—Moving parts
- A61B2018/00196—Moving parts reciprocating lengthwise
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61B—DIAGNOSIS; SURGERY; IDENTIFICATION
- A61B18/00—Surgical instruments, devices or methods for transferring non-mechanical forms of energy to or from the body
- A61B2018/00053—Mechanical features of the instrument of device
- A61B2018/00214—Expandable means emitting energy, e.g. by elements carried thereon
- A61B2018/0022—Balloons
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61B—DIAGNOSIS; SURGERY; IDENTIFICATION
- A61B18/00—Surgical instruments, devices or methods for transferring non-mechanical forms of energy to or from the body
- A61B18/02—Surgical instruments, devices or methods for transferring non-mechanical forms of energy to or from the body by cooling, e.g. cryogenic techniques
- A61B2018/0212—Surgical instruments, devices or methods for transferring non-mechanical forms of energy to or from the body by cooling, e.g. cryogenic techniques using an instrument inserted into a body lumen, e.g. catheter
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61B—DIAGNOSIS; SURGERY; IDENTIFICATION
- A61B18/00—Surgical instruments, devices or methods for transferring non-mechanical forms of energy to or from the body
- A61B18/02—Surgical instruments, devices or methods for transferring non-mechanical forms of energy to or from the body by cooling, e.g. cryogenic techniques
- A61B2018/0231—Characteristics of handpieces or probes
- A61B2018/0262—Characteristics of handpieces or probes using a circulating cryogenic fluid
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61B—DIAGNOSIS; SURGERY; IDENTIFICATION
- A61B18/00—Surgical instruments, devices or methods for transferring non-mechanical forms of energy to or from the body
- A61B18/02—Surgical instruments, devices or methods for transferring non-mechanical forms of energy to or from the body by cooling, e.g. cryogenic techniques
- A61B2018/0231—Characteristics of handpieces or probes
- A61B2018/0262—Characteristics of handpieces or probes using a circulating cryogenic fluid
- A61B2018/0268—Characteristics of handpieces or probes using a circulating cryogenic fluid with restriction of flow
- A61B2018/0275—Characteristics of handpieces or probes using a circulating cryogenic fluid with restriction of flow using porous elements
Definitions
- the present inventionrelates generally to apparatus and methods for inhibiting restenosis in arteries following angioplasty or other intravascular procedures for treating atherosclerotic disease. More particularly, the present invention relates to apparatus and methods for cryogenically treating the target site within a patient's vasculature to inhibit hyperplasia which can occur after such intravascular procedures.
- a number of percutaneous intravascular procedureshave been developed for treating atherosclerotic disease in a patient's vasculature.
- the most successful of these treatmentsis percutaneous transluminal angioplasty (PTA) which employs a catheter having an expansible distal end, usually in the form of an inflatable balloon, to dilate a stenotic region in the vasculature to restore adequate blood flow beyond the stenosis.
- PTApercutaneous transluminal angioplasty
- Other procedures for opening stenotic regionsinclude directional arthrectomy, rotational arthrectomy, laser angioplasty, stents and the like. While these procedures, particularly PTA, have gained wide acceptance, they continue to suffer from the subsequent occurrence of restenosis.
- Restenosisrefers to the re-narrowing of an artery within weeks or months following an initially successful angioplasty or other primary treatment. Restenosis afflicts up to 50% of all angioplasty patients and results at least in part from smooth muscle cell proliferation in response to the injury caused by the primary treatment, generally referred to as “hyperplasia.” Blood vessels in which significant restenosis occur will require further treatment.
- the apparatus and methodsshould be suitable for intravascular and intraluminal introduction, preferably via percutaneous access. It would be particularly desirable if the methods and apparatus were able to deliver the treatment in a very focused and specific manner with minimal effect on adjacent tissues. Such apparatus and methods should further be effective in inhibiting hyperplasia and/or neoplasia in the target tissue with minimum side affects. At least some of these objectives will be met by the invention described hereinafter.
- Balloon catheters for intravascularly cooling or heating a patientare described in U.S. Pat. No. 5,486,208 and WO 91/05528.
- a cryosurgical probe with an inflatable bladder for performing intrauterine ablationis described in U.S. Pat. No. 5,501,681.
- Cryosurgical probes relying on Joule-Thomson coolingare described in U.S. Pat. Nos. 5,275,595; 5,190,539; 5,147,355; 5,078,713; and 3,901,241.
- Catheters with heated balloons for post-angioplasty and other treatmentsare described in U.S. Pat. Nos.
- the present inventioncomprises the cryosurgical treatment of a target site within the body lumen of a patient, usually in an artery which has been previously treated for atherosclerotic disease by balloon angioplasty or any of the other primary treatment modalities described above.
- the present inventionis further suitable for treating other hyperplastic and neoplastic conditions in other body lumens, such as the ureter, the biliary duct, respiratory passages, the pancreatic duct, the lymphatic duct, and the like.
- Neoplastic cell growthwill often occur as a result of a tumor surrounding and intruding into a body lumen. Inhibition of such excessive cell growth is necessary to maintain patency of the lumen.
- Treatment according to the present inventionis effected by cooling target tissue to a temperature which is sufficiently low for a time which is sufficiently long to inhibit excessive cell proliferation.
- the cooling treatmentwill be directed against all or a portion of a circumferential surface of the body lumen, and will preferably result in cell growth inhibition, but not necessarily in significant cell necrosis. Particularly in the treatment of arteries following balloon angioplasty, cell necrosis may be undesirable if it increases the hyperplastic response.
- the present inventionwill slow or stop cell proliferation but may leave the cells which line the body lumen viable, thus lessening hyperplasia.
- Methods according to the present inventioncomprise cooling an inner surface of the body lumen to a temperature and for a time sufficient to inhibit subsequent cell growth.
- the temperature at the tissue surfacewill be in a range from about 0° C. to about ⁇ 80° C., the tissue surface temperature preferably being in a range from about ⁇ 10° C. to about ⁇ 40° C.
- the temperature at the cell surfacecan be in the range from ⁇ 20° C. to ⁇ 80° C., optionally being from ⁇ 30° C. to ⁇ 50° C.
- the tissueis typically maintained at the described temperature for a time period in the range from about 1 to about 60 seconds, often being from 1 second to 10 seconds, preferably from 2 seconds to 5 seconds.
- Hyperplasia inhibiting efficacymay be enhanced by repeating cooling in cycles, typically with from about 1 to 5 cycles, with the cycles being repeated at a rate of about one cycle every 60 seconds.
- the cooling treatmentwill usually be effected very shortly after angioplasty, arthrectomy, rotational arthrectomy, laser angioplasty, stenting, or another primary treatment procedure, preferably within one hour of the primary treatment, more preferably within thirty minutes within the primary treatment, and most preferably immediately following the primary treatment.
- cryosurgical catheterscomprising a catheter body having a proximal end, a distal end, and a primary lumen therethrough.
- the primary lumenterminates in a Joule-Thomson orifice at or near its distal end, and a balloon is disposed over the orifice on the catheter body to contain a cryogenic fluid delivered through the primary lumen.
- Suitable cryogenic fluidswill be non-toxic and include liquid nitrogen, liquid nitrous oxide, liquid carbon dioxide, and the like.
- the Joule-Thomson orificewill be spaced inwardly from each end of the balloon and the balloon will be sufficiently long so that the cooling of the balloon occurs primarily in the middle.
- the temperature of the proximal and distal ends of the balloonwill thus be much less than that of the middle, and the ends will thus act as “insulating” regions which protect luminal surfaces and other body structures from unintended cooling.
- the balloonhas a length of at least 1 cm, more preferably at least 2 cm, and typically in the range from 3 cm to 10 cm.
- the orificeis usually positioned at least 0.5 cm from each end, preferably being at least 1 cm from each end in balloons which are 2 cm or longer.
- the containment bladderwill further act to limit cooling to the central region of the balloon.
- the portions of the balloon proximal and distal to the containment bladdermay optionally be inflated with an insulating medium, such as a gas, silicone oil, saline, or the like.
- the containment bladdermay have a vent or be partially porous so that the cryogenic fluid (which is present as a gas within the containment bladder) flows at a controlled rate into the overlying balloon. By limiting the flow rate, the temperature of the cryogenic fluid will be significantly higher in the regions outside of the containment bladder but still within the balloon.
- the present inventionprovides a cryosurgical system comprising a flexible catheter body having a proximal end, a distal end, and a gas exhaust lumen defining an axis therebetween.
- An intravascular balloonis disposed near the distal end of the catheter body in fluid communication with the exhaust lumen.
- the balloonis expandable to radially engage a surrounding vessel wall.
- a cryogenic cooling fluid supplyis in fluid communication with at least one port disposed within the balloon.
- the at least one portmay optionally comprise a Joule Thompson orifice.
- the at least one portmay pass some or all of the cryogenic cooling fluid as a liquid.
- a plurality of portsmay spray the fluid radially, the liquid in some cases distributed substantially uniformly over an inner surface of the balloon wall so that enthalpy of vaporization of the liquid cools a region of the balloon wall. The vaporization of the liquid will help to inflate the balloon, while the exhaust lumen limits pressure within the balloon to safe levels.
- the inventionprovides a cryosurgical catheter for use in a blood vessel having a vessel wall.
- the cryosurgical cathetercomprises a flexible catheter body having a proximal end, a distal end, and a gas exhaust lumen defining an axis therebetween.
- a balloonis disposed at the distal end of the catheter body in fluid communication with the exhaust lumen.
- the balloonhas a balloon wall with proximal and distal ends and a radially oriented region extending therebetween.
- the wallis radially expandable to engage the surrounding vessel wall.
- At least one cooling fluid distribution portis in communication with a cryogenic cooling fluid supply. The at least one port is disposed within the balloon to cool the region of the expanded balloon wall.
- cryosurgical methods and catheters of the present inventionwill often be tailored to provide even cooling along at least a portion of a vascular wall engaged by the cooled balloon.
- the efficacy of cryogenic cell growth inhibitionmay be enhanced significantly by distributing cooling within the balloon using a plurality of cryogenic fluid ports distributed circumferentially and/or axially within the balloon so that a significant portion of the vessel wall engaging the balloon surface is cooled to the target temperature range for a time in the desired treatment period range.
- the present inventionprovides a cryosurgical catheter for use in a blood vessel having a vessel wall.
- the cryosurgical cathetercomprises a flexible catheter body having a proximal end, a distal end, and a lumen defining an axis therebetween.
- a balloonis disposed at the distal end of the catheter body. The balloon is in fluid communication with the lumen, and has a balloon wall that expands radially to engage the surrounding vessel wall.
- a plurality of cooling fluid distribution portsare in communication with a cooling fluid supply. These ports are distributed within the balloon so as to evenly cool a portion of the vessel wall.
- the diffuser headmay be moved axially within the inflated balloon by sliding a cooling fluid supply tube axially within the catheter body.
- Such a structuremay provide a variety of controllable sequential cryogenic treatment regimens, for example, multiple temperature feedback controlled cryogenic treatment cycles for inhibiting cell proliferation, or for a variety of alternative endoluminal cryogenic therapies.
- a fixed diffuser head defining an axially and circumferential distributed array of portsmay provide simultaneous even cooling throughout a significant region of the target site.
- the inventionprovides a therapy for treatment of a blood vessel having a vessel wall.
- the methodcomprises introducing a catheter into the blood vessel, and expanding a balloon of the catheter near a target site to engage the vessel wall. Fluid is expanded at a first location within the balloon. Fluid is also expanded at a second location within the balloon to cryogenically cool a portion of the engaged vessel wall, the second location being separated from the first location.
- Gas expansionmay effect cryogenic cooling via Joule-Thompson expansion as the cryogenic fluid enters the balloon and/or via the enthalpy of vaporization of a cryogenic fluid within the balloon.
- gas expansionmay be initiated while a moveable orifice head is disposed within a housing or shield at one end of the balloon.
- This housingmay conveniently be formed by extending a tubular structure distally from the catheter body into the interior of the balloon. Such a housing structure may also be used to help direct exhaust gases proximally out of the balloon without causing excessive cooling at the proximal end of the balloon, which exhaust gases might otherwise freeze blood within the vessel.
- the inventionalso provides a kit for treating hyperplasia or neoplasia in a body lumen.
- the kitcomprises a catheter having a proximal end, a distal end, and balloon near its distal end. Instructions are included in the kit for use of the catheter. These instructions comprise the step of cooling an inner surface of the body lumen with the balloon to a temperature and for a time sufficient to inhibit subsequent cell growth.
- Such a kitmay include instructions for any of the methods described herein.
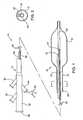
- FIG. 1illustrates a cryosurgical catheter constructed in accordance with the principles of the present invention, with a distal end shown in cross-section.
- FIG. 2is a cross-sectional view of the catheter taken along line 2 - 2 in FIG. 1 .
- FIG. 3illustrates the expansion of a cryogenic fluid within the balloon of the cryosurgical catheter of FIG. 1 .
- FIG. 4is a graph illustrating the temperature profile of the balloon of FIGS. 1 and 3 while liquid nitrogen is being expanded therein and the balloon is present in a body lumen.
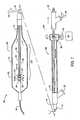
- FIG. 5illustrates the distal end of a cryosurgical catheter constructed in accordance with the principles of the present invention and having a nested containment bladder within a balloon structure.
- FIG. 6A-6Cillustrate use of the catheter of FIG. 1 in treating a target site within a patient's vasculature.
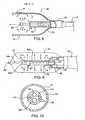
- FIG. 7is a partial cross-section of a cryosurgical catheter having a diffuser head with a plurality of radially oriented cryogenic fluid ports, in which the diffuser head can slide axially within the balloon to provide even cooling of elongate treatment sites.
- FIG. 8is a partial cross-sectional view of a cryosurgical catheter having a moveable diffuser head which can be drawn proximally into a housing within the balloon so as to avoid transients upon initiation of the cooling fluid flow.
- FIG. 9schematically illustrates an alternative fixed porous diffuser defining an axial and circumferential array of orifices.
- FIG. 10illustrates a cross-section of the catheter of FIG. 9 .
- FIG. 11illustrates a proximal end of the catheter of FIG. 9 .
- FIG. 12illustrates an alternative fixed diffuser structure having an array of axially separated cryogenic fluid ports.
- FIG. 13is a functional block diagram illustrating the operation of the catheter of FIG. 7 , including an optional feedback control loop.
- FIG. 14schematically illustrates a kit including a balloon catheter and instructions for its use according to the methods described herein.
- FIGS. 1 and 2An exemplary cryosurgical catheter 10 constructed in accordance with the principles of the present invention is illustrated in FIGS. 1 and 2 .
- the catheter 10comprises a catheter body 12 having a proximal end 14 , a distal end 16 , and an inflatable balloon 18 disposed at the distal end.
- the balloon 18is shown to be an integral extension of the catheter body 12 , but such a structure is not required by the present invention.
- the ballooncould be formed from the same or a different material and, in the latter case, attached to the distal end of the catheter body 12 by suitable adhesives, heat welding, or the like.
- the catheter bodymay be formed from conventional materials, such as polyethylenes, polyimides, and copolymers and derivatives thereof.
- the balloonmay also be formed from conventional materials used for angioplasty balloons, typically being non-distensible, such as polyethylene terephthalate (PET).
- PETpolyethylene terephthalate
- Catheter 10comprises a central shaft 20 which may be formed from polymeric material, such as polyethylene, polytetrafluoroethylene, polyimide, or from a metal, such as from hypotube.
- the coaxial shaft 20is tubular and provides a guidewire lumen for positioning of the catheter over a guidewire in a conventional manner.
- the shaft 20could have a variety of other configurations and purposes.
- the shaftcould be a solid wire or core and further optionally provide a guidewire tip at its distal end.
- the shaftcould also provide a lumen for delivering cryogenic fluid to the balloon 18 .
- the cryogenic fluidis provided by a separate cryogenic fluid delivery tube 22 which is disposed in parallel to the coaxial shaft 20 .
- the catheter 10will usually further comprise a thermocouple 24 which is optimally located near the center of balloon 18 . At this location, it can measure the temperature of the cryogenic fluid after expansion from the proximal end of the cryogenic delivery tube 22 .
- the cryogenic delivery tube 22will define an expansion orifice at its distal end 23 .
- the cryogenic fluidwill flow through the tube 22 as a liquid at an elevated pressure and (thus inhibiting flow restrictive film boiling) will expand across the orifice 23 to a gaseous state at a lower pressure within the balloon.
- the pressure within the tube 22will typically be in the range from 50 psi to 500 psi at a temperature below the associated boiling point.
- the nitrogen gas within the balloon near its centerthe location of thermocouple 24
- the pressurewill typically be in the range from 30 psi to 100 psi and the temperature in the range from ⁇ 40° C. to ⁇ 100° C.
- the temperaturemay decrease in both the radially outward direction and in both axial directions from the center of the balloon. This feature of the present invention is better described in connection with FIGS. 3 and 4 below.
- a hub 28is secured to the proximal end 14 of the catheter body 12 .
- the hubprovides for a port 30 for connecting a cryogenic fluid source to the cryogenic delivery tube 22 .
- the hubfurther provides a port 32 for exhausting the gaseous cryogenic fluid which travels from balloon 18 in a proximal direction through annular lumen 25 .
- a third port 34is provided for thermocouple wires 26 .
- a fourth port 36 at the proximal end of the hubis provided for a guidewire.
- liquid nitrogenis delivered to balloon 18 through the cryogenic delivery tube 22 .
- the liquid nitrogenis delivered at a temperature and pressure within in the ranges set forth above and expands to gaseous nitrogen (GN2) across the expansion orifice into the interior of balloon 18 .
- the gaseous nitrogenwill serve both to inflate the balloon 18 and to cool the exterior surface of the balloon in a desired temperature profile.
- the balloon dimensions and operating conditionswill be selected to provide a particular balloon temperature profile, an example of which is set forth in FIG. 4 .
- the balloon temperatureBy expanding the liquid nitrogen to its gaseous state near the center of the balloon, the balloon temperature will be lowest near the center and will decrease in both axial directions away from the center, as shown in the temperature profile of FIG. 4 .
- a balloon temperature in the range from ⁇ 20° C. to ⁇ 80° C., e.g., at about ⁇ 50° C., for a time period in the range from 1 second to 10 secondsmay be effective.
- a pressure in the range from 50 psi to 500 psi and at a temperature below the boiling pointand expanding the liquid nitrogen to a gas at a pressure in the range from 30 psi to 100 psi, a temperature in the above range at the middle of the balloon will be achieved.
- the temperatures at the ends of the balloonswill generally no lower than 0° C.
- a desired low temperaturecan be maintained at the outer surface of the balloon in a treatment region near the center of the balloon, while the distal and proximal ends of the balloon act to insulate the colder portions from non-target regions within the artery or other body lumen.
- the axial length of the treatment region of the ballooncan be varied considerably by varying the length of the balloon and controlling the volume of liquid nitrogen delivered to the balloon.
- Exemplary balloonswill have a length in the range from 3 cm to 5 cm, a diameter in the range from 1.5 mm to 4 mm, and will typically receive from 0.08 ml/sec to 1.5 ml/sec of liquid nitrogen in the temperature and pressure ranges set forth above.
- balloon assembly 50is disposed at the distal end of a catheter body 52 comprising a shaft 54 and a cryogenic fluid delivery tube 56 .
- a balloon 58is secured to the distal end of the catheter body 52 , generally as described above with respect to catheter 10 .
- balloon assembly 50comprises a containment bladder 60 nested within the balloon 58 .
- the containment bladder 50may be a second balloon formed in a manner similar to balloon 58 , except that it will be shorter and will have proximal and distal ends spaced axially inwardly from the proximal and distal ends of balloon 58 .
- the bladder 60may be disposed of different materials and have different properties.
- the containment bladderis intended to receive and contain the gaseous nitrogen after it is expanded across expansion orifice 62 into the interior thereof.
- a more distinct temperature transitionmay be effected between the cold middle region of balloon 58 and the less cold distal and proximal regions thereof.
- the balloon 58may be separately expanded with an insulating fluid to further sharpen the temperature transition between the containment bladder 60 and the remainder of balloon 58 .
- the containment bladder 60may include ports or porous regions which permit the gaseous nitrogen to pass from the interior of the bladder 60 into the interior of balloon 58 in a controlled manner to maintain the desired temperature transition.
- catheter 10for treating a target region TR within a blood vessel BV will be described.
- the target regionwill usually have been previously treated by balloon angioplasty or other primary conventional protocol for treating atherosclerotic disease.
- Such primary treatmentwill typically utilize an intravascular catheter, which catheter will have been removed leaving a guidewire GW in place, as illustrated in FIG. 6A .
- a catheter 10is then introduced over the guidewire, as illustrated in FIG. 6B .
- Liquid nitrogenis introduced to the catheter 10 from a suitable source 70 .
- the sourcemay be a Dewar flask or other conventional source.
- the liquid nitrogen (LN2)is delivered to the catheter 10 and inflates balloon 18 , as illustrated in FIG. 6C . Because of the temperature profile of the balloon, cooling of the inner wall of the blood vessel BV will be maximized over a central region CR and diminish in the proximal and distal directions from the central region, as illustrated qualitatively by the array of arrows in FIG. 6C .
- the treatmentwill be performed at the temperatures and for the times described thereabove in order to inhibit subsequent hyperplasia of the cells of the lining of the blood vessel.
- the cryogenic methods of the present inventionwill inhibit subsequent cell proliferation without inducing injury and thrombosis which can occur as a result of such injury.
- FIG. 7A catheter 80 having a moveable port head or diffuser 82 is illustrated in FIG. 7 .
- cryogenic fluid ports 23are separated circumferentially about diffuser 82 , and are oriented radially so as to enhance the heat transfer between the expanding gas and the wall of balloon 18 .
- four ports 83are provided, and are separated circumferentially from each other by about 90°.
- diffuser head 82is slidably supported on a shaft or rail 84 .
- Feed tube 22is affixed to diffuser head 82 and is slidably disposed within catheter body 12 .
- a proximal housing 86 at proximal end 14 of catheter 80contains a rack and pinion mechanism 88 which controllably moves feed tube 22 .
- feed tube 22 and diffuser 82can move from a first position 95 to a second position 96 without moving or deflating balloon 18 . This allows a relatively small fluid flow to cool an elongate central region CR.
- Inhibition of cell proliferation along elongate segments of vasculature, such as in the iliac or superior femoral arteriesshould benefit from cryosurgical treatment at repeatable temperatures and for repeatable times.
- cryosurgical treatmentat repeatable temperatures and for repeatable times.
- Safety of endoluminal cryosurgical techniquesis generally enhanced by minimizing the flow of the cooling fluid.
- Low flow rateswill generally reduce the release of gas into the body lumen in the unlikely event of a balloon rupture.
- Known balloon structurescan withstand pressures of up to about 100 psi or more. Nonetheless, safety can be enhanced by limiting maximum balloon pressures to 100 psi or less, and preferably to less than 100.
- Lower balloon pressuresnot only reduce the amount of gas released in the event of a rupture, they also help decrease the possibility of such a balloon rupture occurring. Given a constant cooling fluid pressure at port 83 , lower pressures within balloon 18 will also produce a lower balloon wall temperature. In other words, cryogenic cooling is generally enhanced by minimizing the pressure within the balloon.
- moveable diffuser head 82also allows the surgeon to selectively treat tissues in a highly controlled manner. For example, when balloon 18 extends across a branch artery, the surgeon has the option of treating the vessel proximally and distally of the branch and shutting off the gas flow when the diffuser is aligned with the branch so as to avoid freezing blood within the branch opening. Additionally, by coupling automated drive system 92 to the actuation mechanism, a wide variety of treatment cycles and times may be controllably and repeatably effected.
- Balloon 18 of moveable diffuser catheter 80may be quite elongate, the balloon typically having a length in a range from about 1 to about 10 cm.
- the balloonhas a length of about 10 cm so that proximal housing 86 and/or actuation mechanism 88 has a stroke length of about 8 cm.
- a heat transfer enhancing materialmay be included in the polymer of the balloon wall.
- the addition of between about 1 and 10% boron nitride in a polyethylene or other balloon polymercan significantly improve heat transfer of the entire system.
- a significant temperature differentialmay be found between an inner and outer surface of the balloon during cooling. Hence, improving the thermal conductivity of the balloon material may provide significant benefits.
- a fixed guidewire 94extends distally from the balloon to help when advancing catheter 80 within the vasculature.
- Fixed guidewire 94 and the distal end of balloon 18are affixed to an axial support or rail 84 which structurally supports the distal end of the catheter when the balloon is not inflated.
- Rail 84here comprises a stainless steel wire with a diameter of 0.008 inches, but may alternatively comprise a wide variety of shaft structures, optionally including one or more lumens for a moveable guidewire or the like.
- Diffuser 82includes four radially oriented ports, each having a diameter of about 0.0025 inches. These openings are in fluid communication with a central passage, which in turn is supplied by feed tube 22 .
- Diffuser head 82may have an outer diameter of about 0.032 inches, and may comprise any of a variety of alternative polymers or metals.
- Diffuser 82is affixed to feed tube 22 by adhesion bonding, heat welding, fasteners, or the like.
- diffuser 82comprises polyimide.
- Feed tube 22may also be formed from a polyimide tube, and will preferably be coated with a PTFE such as TeflonTM to avoid friction when the feed tube reciprocates within the catheter body.
- Diffuser head 82is shown affixed to rail 84 using bands which encircle the diffuser and define a channel through which rail 84 passes.
- bandswhich encircle the diffuser and define a channel through which rail 84 passes.
- alternative support arrangementsincluding a concentric support shaft or tube, a cantilevered feed tube, or the like.
- thermocouple 24 or some alternative temperature sensorsends a signal proximally via wire 26 to indicate the temperature within the balloon.
- moveable diffuser balloon catheter 80will be introduced into a blood vessel while balloon 18 is in an uninflated, small profile configuration. Balloon 18 will be maneuvered to the treatment site using fixed guidewire 94 .
- Feed tube 22will be positioned so that diffuser 82 is located at first position 95 , and cryogenic fluid will be advanced through feed tube 22 to the diffuser. This gas will inflate balloon 18 , and will also cool the interior surface of the balloon and blood vessel as described above.
- Control knob 90will be rotated so that diffuser 82 moves axially toward position 96 . As the cooling fluid exits the diffuser, the endothelial tissue engaging central region CR is cryogenically cooled.
- Cryogenic cooling fluidmay optionally pass through a Joule-Thompson orifice adjacent port 83 to effect cooling.
- at least a portion of the cryogenic cooling fluidmay exit port 83 into the balloon as a liquid.
- the liquidwill vaporize within the balloon, and the enthalpy of vaporization can help cool the surrounding vessel wall.
- the liquidmay coat at least a portion of the balloon wall so as to enhance even cooling over at least a portion of the vessel wall.
- ports 83may have a total cross section which is smaller than a cross section of the fluid supply lumen, or which is at least as large as the cross section of the fluid supply lumen.
- the surgeoncan control the cooling rate of the tissue, the temperature of the tissue, and optionally, the number of cooling cycles the tissue is subjected to while the catheter is in a single location.
- the ends of the diffuser stroke first and second positions 95 , 96may be separated from the axial ends of balloon 18 so as to limit any cooling of fluids within the vessel.
- diffuser 82may initially be parked within a housing 98 inside balloon 18 .
- Housing 98may be formed by extending a tube from catheter body 12 into balloon 18 , the housing optionally comprising an extension of the catheter body material.
- housing 98may be understood with reference to FIGS. 7, 8 , and 4 .
- cooling gasflows from diffuser 82 into balloon 18 , the expelled gases are exhausted proximally from the balloon into catheter body 12 .
- the gaseswill warm as they travel proximally, the gas flow will be accelerating from their relatively large cross-sectional diameter of the balloon into the catheter body. This may actually enhance cooling adjacent the proximal end of the balloon, and could freeze blood proximally of the balloon.
- housing 98admits gases from a central location along central region CR.
- the gases surrounding housing 98 within balloon 18are allowed to stagnate near the proximal end of the balloon, thereby limiting axial cooling at that location.
- a temperature sensor 24is mounted on the outer surface of the balloon to measure the temperature of the tissue at the target site, the tissue balloon interface, and/or the balloon outer surface temperature.
- a fixed diffuser 100includes an array of ports 83 which are distributed both axially and circumferentially around the diffuser. As ports 83 are radially oriented, diffuser 100 will achieve the desired cooling of the surrounding tissue with relatively low balloon pressures and low cooling fluid flow rates. As the cryogenic liquid or gas-liquid combination is directed perpendicularly against the wall of balloon 18 , the heat transfer coefficient between the gas and the balloon wall is quite high. This helps to reduce temperature of the balloon and provides greater heat extraction for a given flow rate of coolant into the balloon. Additionally, as ports 83 are distributed both circumferentially and axially along the balloon, diffuser 100 will distribute the cooling more uniformly over the surface of the balloon so as to produce a uniform antiproliferative response.
- Diffuser 100will generally comprise a tubular structure with radially oriented openings.
- An exemplary tubular structuremay comprise a polyimide tube having an inner diameter of about 0.032 inches and a wall thickness of 0.001 inch. Each port will again define a diameter of about 0.0025 inches.
- four axial rows of orificesare separated by about 90° from each other. The rows are axially staggered so that the orifices in a single row have centerline separations of about 4 mm, while the orifices of adjacent rows are separated by about 2 mm.
- the overall length of the porous diffuser tubeis about 2 cm.
- a central shaft 104 having a guidewire lumen 106is bonded concentrically to diffuser 100 using adhesive or the like at the distal end of the diffuser, and optionally also at the proximal end of the diffuser.
- High contrast markers 102may be provided to enhance an image of the catheter so as to facilitate positioning of balloon 18 fluroscopically, sonographically, or under any other alternative image modality (with appropriate contrast structures).
- the distal markermay optionally be formed by winding a gold or platinum wire around the central shaft and bonding the gold wire to the distal end of the diffuser tube.
- the proximal markermay similarly be formed by winding and bonding a gold or platinum wire, the proximal marker optionally being disposed over the diffuser tube so that the cryogenic cooling fluid may be introduced through the annular space between the diffuser tube and the central shaft proximally of the balloon.
- Central shaft 104will typically comprise a polyimide tube, but may alternatively comprise any of a wide variety of materials.
- the coaxial arrangement between diffuser 100 and central shaft 104(with an annular cooling fluid flow path between the tube of the diffuser and the central shaft) promotes circumferentially symmetric distribution of the cryogenic cooling fluid against the balloon wall, which in turn provides a more circumferentially even temperature distribution.
- uniform temperature distributions, both axially and circumferentially, within central region CRhelp ensure that the beneficial inhibition of cell proliferation is provided throughout a significant portion of the tissue engaged by balloon 18 .
- distal and proximal stagnant regions within the balloon flow profileare created by the shape and configuration of diffuser 100 , balloon 18 , and by the presence of housing 98 within the proximal end of the balloon, as described above. Even though no moveable diffuser will be drawn into housing 98 , this structure still helps to avoid the accelerating flow of gases along the proximally tapering balloon wall.
- a balloon pressure port 108transmits pressure proximally via a pressure monitoring lumen 110 , as can be understood with reference to FIGS. 9 and 10 . Accuracy of such pressure monitoring can be enhanced by minimizing the flow of fluid proximally within the pressure monitoring lumen.
- a pressure transducermay be mounted within the balloon with wires sending a pressure signal proximally.
- lumens for the cryogenic feed tube 22 , pressure monitoring port 108 , guidewire and the likemay be contained within an insulated jacket 112 .
- a cryogenic feed tubemay simply extend distally into an annular space between a central shaft and a jacket formed as a continuous proximal extension of the diffuser tube, with any proximal leakage of the cooling fluid within the jacket optionally being exhausted into the catheter body and removed via the exhaust lumen.
- a proximal end of the fixed diffuser catheter illustrated in FIGS. 9 and 10include many of the coupling structures described above regarding FIGS. 1 and 7 .
- Guidewire port 114provides proximal access to guidewire lumen 106
- a pressure monitoring connector 116is in fluid communication with the interior of balloon 18 via monitoring lumen 110 .
- cryogenic coolingmay optionally be controlled using an orifice disposed at exhaust port 32 .
- This proximal structurecan be assembled from commercial available components using potting adhesive 118 in a generally conventional manner.
- a series of ports 83are distributed axially so as to distributed the cooling axially within an elongate target region, as generally described above.
- a series of individual gas feed tubes 120supply the cryogenic cooling fluid to the ports, with each port optionally having an opening which has the same area as the lumen of the associated gas feed tube.
- Such individual feed tubesmay comprise polyimide tubes having an inner diameter of about 0.005 inches.
- axial distribution of coolingmay be controlled by varying the amount of fluid expelled from each port, by varying the interorifice spacing (axially and/or circumferentially), by locally varying the heat transfer coefficient or cooling fluid pattern, or the like.
- catheter 80As the actual tissue cooling may vary with pressures within the balloon, cooling fluid flow rates, and the like, and as these parameters may vary when catheter body 12 is bent in following the vasculature system, the efficacy of the cryosurgical therapy may be enhanced by adjusting the treatment based on measured characteristics of the cooling process, for example, based on temperatures measured by one or more temperature sensors 24 .
- electrical temperature signals 132 from temperature sensors 24may be directed to a controller 134 for use in a feedback control system.
- controller 134processes the temperature signals to generate cooling fluid feed signals 136 indicating the pressure or volume of cryogenic fluid to be injected into the catheter.
- Controller 134will preferably also provide electrical signals which direct diffuser drive 92 to mechanically reposition diffuser 82 , and will often provide signals varying the pressure (or vacuum) at exhaust port 32 . These signals may be used not only to vary the cooling cycle, but can also be used to control the inflation and/or deflation of the balloon, preferably based at least in part on a pressure monitored from within the balloon.
- controller 134will generally initiate, monitor, and control cooling of the tissue.
- Cryogenic system 130will often be used to effect a cooling rate of the tissue in a range from about 2 to about 30° C. per second.
- the systemwill maintain the tissue at a temperature in a range from about 0 to about ⁇ 80° C., preferably at a temperature in a range from about ⁇ 10 to about ⁇ 40° C., for a time between about 1 and about 60 seconds.
- the efficacy of the therapymay be enhanced by repeatedly cooling the tissue to these temperatures for between 1 and 5 cooling cycles, typically repeating the cooling cycles at the rate of 1 every 60 seconds.
- cryogenic liquids or liquid/gas mixturescomprising carbon dioxide, nitrous oxide, or the like may flow through the balloon at a rate in a range from about 100 to about 800 mg/sec.
- Such coolingmay inhibit cell proliferation via processes which are sometimes referred to as apoptosis and/or programmed cell growth.
- FIG. 14A kit 140 including balloon catheter 10 and instructions for its use 142 is illustrated in FIG. 14 .
- Catheter 10may be replaced by any of the balloon catheter structures described above, while instructions for use 142 may describe any of the associated method steps set forth above for inhibition of cell proliferation.
- Instructions for use 142will often be printed, optionally appearing at least in part on a sterile package 144 for balloon catheter 10 .
- instructions for use 142may comprise a machine readable code, digital or analog data graphically illustrating or demonstrating the use of balloon catheter 10 to inhibit hyperplasia, or the like. Still further alternatives are possible, including printing of the instructions for use on packaging 146 of kit 140 , and the like.
Landscapes
- Health & Medical Sciences (AREA)
- Surgery (AREA)
- Life Sciences & Earth Sciences (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Medical Informatics (AREA)
- Engineering & Computer Science (AREA)
- Biomedical Technology (AREA)
- Heart & Thoracic Surgery (AREA)
- Otolaryngology (AREA)
- Molecular Biology (AREA)
- Animal Behavior & Ethology (AREA)
- General Health & Medical Sciences (AREA)
- Public Health (AREA)
- Veterinary Medicine (AREA)
- Thermotherapy And Cooling Therapy Devices (AREA)
- Media Introduction/Drainage Providing Device (AREA)
Abstract
Description
- This application is a continuation of and claims priority from Ser. No. 09/978,253 filed on Oct. 15, 2001, which is a divisional of U.S. patent application Ser. No. 09/203,011 filed Dec. 1, 1998, which is a continuation-in-part of, and claims the benefit of priority from, co-pending U.S. patent application Ser. No. 08/982,824, filed Dec. 2, 1997, the full disclosures of which are incorporated herein by reference.
- NOT APPLICABLE
- NOT APPLICABLE
- 1. Field of the Invention
- The present invention relates generally to apparatus and methods for inhibiting restenosis in arteries following angioplasty or other intravascular procedures for treating atherosclerotic disease. More particularly, the present invention relates to apparatus and methods for cryogenically treating the target site within a patient's vasculature to inhibit hyperplasia which can occur after such intravascular procedures.
- A number of percutaneous intravascular procedures have been developed for treating atherosclerotic disease in a patient's vasculature. The most successful of these treatments is percutaneous transluminal angioplasty (PTA) which employs a catheter having an expansible distal end, usually in the form of an inflatable balloon, to dilate a stenotic region in the vasculature to restore adequate blood flow beyond the stenosis. Other procedures for opening stenotic regions include directional arthrectomy, rotational arthrectomy, laser angioplasty, stents and the like. While these procedures, particularly PTA, have gained wide acceptance, they continue to suffer from the subsequent occurrence of restenosis.
- Restenosis refers to the re-narrowing of an artery within weeks or months following an initially successful angioplasty or other primary treatment. Restenosis afflicts up to 50% of all angioplasty patients and results at least in part from smooth muscle cell proliferation in response to the injury caused by the primary treatment, generally referred to as “hyperplasia.” Blood vessels in which significant restenosis occur will require further treatment.
- A number of strategies have been proposed to treat hyperplasia and reduce restenosis. Such strategies include prolonged balloon inflation, treatment of the blood vessel with a heated balloon, treatment of the blood vessel with radiation, the administration of anti-thrombotic drugs following the primary treatment, stenting of the region following the primary treatment, and the like. While enjoying different levels of success, no one of these procedures has proven to be entirely successful in treating all occurrences of restenosis and hyperplasia.
- For these reasons, it would be desirable to provide additional apparatus and methods suitable for the treatment of restenosis and hyperplasia in blood vessels. It would be further desirable if the apparatus and methods were suitable for treatment of other conditions related to excessive cell proliferation, including neoplasms resulting from tumor growth, hyperplasia in other body lumens, and the like. The apparatus and method should be suitable for intravascular and intraluminal introduction, preferably via percutaneous access. It would be particularly desirable if the methods and apparatus were able to deliver the treatment in a very focused and specific manner with minimal effect on adjacent tissues. Such apparatus and methods should further be effective in inhibiting hyperplasia and/or neoplasia in the target tissue with minimum side affects. At least some of these objectives will be met by the invention described hereinafter.
- 2. Description of the Background Art
- Balloon catheters for intravascularly cooling or heating a patient are described in U.S. Pat. No. 5,486,208 and WO 91/05528. A cryosurgical probe with an inflatable bladder for performing intrauterine ablation is described in U.S. Pat. No. 5,501,681. Cryosurgical probes relying on Joule-Thomson cooling are described in U.S. Pat. Nos. 5,275,595; 5,190,539; 5,147,355; 5,078,713; and 3,901,241. Catheters with heated balloons for post-angioplasty and other treatments are described in U.S. Pat. Nos. 5,196,024; 5,191,883; 5,151,100; 5,106,360; 5,092,841; 5,041,089; 5,019,075; and 4,754,752. Cryogenic fluid sources are described in U.S. Pat. Nos. 5,644,502; 5,617,739; and 4,336,691.
- The full disclosures of each of the above U.S. Patents are incorporated herein by reference.
- The present invention comprises the cryosurgical treatment of a target site within the body lumen of a patient, usually in an artery which has been previously treated for atherosclerotic disease by balloon angioplasty or any of the other primary treatment modalities described above. The present invention, however, is further suitable for treating other hyperplastic and neoplastic conditions in other body lumens, such as the ureter, the biliary duct, respiratory passages, the pancreatic duct, the lymphatic duct, and the like. Neoplastic cell growth will often occur as a result of a tumor surrounding and intruding into a body lumen. Inhibition of such excessive cell growth is necessary to maintain patency of the lumen.
- Treatment according to the present invention is effected by cooling target tissue to a temperature which is sufficiently low for a time which is sufficiently long to inhibit excessive cell proliferation. The cooling treatment will be directed against all or a portion of a circumferential surface of the body lumen, and will preferably result in cell growth inhibition, but not necessarily in significant cell necrosis. Particularly in the treatment of arteries following balloon angioplasty, cell necrosis may be undesirable if it increases the hyperplastic response. Thus, the present invention will slow or stop cell proliferation but may leave the cells which line the body lumen viable, thus lessening hyperplasia.
- Methods according to the present invention comprise cooling an inner surface of the body lumen to a temperature and for a time sufficient to inhibit subsequent cell growth. Generally, the temperature at the tissue surface will be in a range from about 0° C. to about −80° C., the tissue surface temperature preferably being in a range from about −10° C. to about −40° C. In other embodiments, the temperature at the cell surface can be in the range from −20° C. to −80° C., optionally being from −30° C. to −50° C. The tissue is typically maintained at the described temperature for a time period in the range from about 1 to about 60 seconds, often being from 1 second to 10 seconds, preferably from 2 seconds to 5 seconds. Hyperplasia inhibiting efficacy may be enhanced by repeating cooling in cycles, typically with from about 1 to 5 cycles, with the cycles being repeated at a rate of about one cycle every 60 seconds. In the case of arteries, the cooling treatment will usually be effected very shortly after angioplasty, arthrectomy, rotational arthrectomy, laser angioplasty, stenting, or another primary treatment procedure, preferably within one hour of the primary treatment, more preferably within thirty minutes within the primary treatment, and most preferably immediately following the primary treatment.
- The methods of the present invention may be performed with cryosurgical catheters comprising a catheter body having a proximal end, a distal end, and a primary lumen therethrough. The primary lumen terminates in a Joule-Thomson orifice at or near its distal end, and a balloon is disposed over the orifice on the catheter body to contain a cryogenic fluid delivered through the primary lumen. Suitable cryogenic fluids will be non-toxic and include liquid nitrogen, liquid nitrous oxide, liquid carbon dioxide, and the like. By delivering the cryogenic fluid through the catheter body, the balloon can be expanded and cooled in order to effect treatments according to the present invention.
- Preferably, the Joule-Thomson orifice will be spaced inwardly from each end of the balloon and the balloon will be sufficiently long so that the cooling of the balloon occurs primarily in the middle. The temperature of the proximal and distal ends of the balloon will thus be much less than that of the middle, and the ends will thus act as “insulating” regions which protect luminal surfaces and other body structures from unintended cooling. Preferably, the balloon has a length of at least 1 cm, more preferably at least 2 cm, and typically in the range from 3 cm to 10 cm. The orifice is usually positioned at least 0.5 cm from each end, preferably being at least 1 cm from each end in balloons which are 2 cm or longer.
- While it has been found that positioning of the Joule-Thomson valve in the central region of a balloon will usually provide sufficient insulation of each end resulting from the inherent heat transfer characteristics, in some instances it will be desirable to provide a separate containment bladder nested inside the balloon to receive the cryogenic fluid. The containment bladder will further act to limit cooling to the central region of the balloon. The portions of the balloon proximal and distal to the containment bladder may optionally be inflated with an insulating medium, such as a gas, silicone oil, saline, or the like. Alternatively, the containment bladder may have a vent or be partially porous so that the cryogenic fluid (which is present as a gas within the containment bladder) flows at a controlled rate into the overlying balloon. By limiting the flow rate, the temperature of the cryogenic fluid will be significantly higher in the regions outside of the containment bladder but still within the balloon.
- In another aspect, the present invention provides a cryosurgical system comprising a flexible catheter body having a proximal end, a distal end, and a gas exhaust lumen defining an axis therebetween. An intravascular balloon is disposed near the distal end of the catheter body in fluid communication with the exhaust lumen. The balloon is expandable to radially engage a surrounding vessel wall. A cryogenic cooling fluid supply is in fluid communication with at least one port disposed within the balloon.
- As described above, the at least one port may optionally comprise a Joule Thompson orifice. Alternatively, the at least one port may pass some or all of the cryogenic cooling fluid as a liquid. In fact, a plurality of ports may spray the fluid radially, the liquid in some cases distributed substantially uniformly over an inner surface of the balloon wall so that enthalpy of vaporization of the liquid cools a region of the balloon wall. The vaporization of the liquid will help to inflate the balloon, while the exhaust lumen limits pressure within the balloon to safe levels.
- In another aspect, the invention provides a cryosurgical catheter for use in a blood vessel having a vessel wall. The cryosurgical catheter comprises a flexible catheter body having a proximal end, a distal end, and a gas exhaust lumen defining an axis therebetween. A balloon is disposed at the distal end of the catheter body in fluid communication with the exhaust lumen. The balloon has a balloon wall with proximal and distal ends and a radially oriented region extending therebetween. The wall is radially expandable to engage the surrounding vessel wall. At least one cooling fluid distribution port is in communication with a cryogenic cooling fluid supply. The at least one port is disposed within the balloon to cool the region of the expanded balloon wall.
- The cryosurgical methods and catheters of the present invention will often be tailored to provide even cooling along at least a portion of a vascular wall engaged by the cooled balloon. For example, the efficacy of cryogenic cell growth inhibition may be enhanced significantly by distributing cooling within the balloon using a plurality of cryogenic fluid ports distributed circumferentially and/or axially within the balloon so that a significant portion of the vessel wall engaging the balloon surface is cooled to the target temperature range for a time in the desired treatment period range.
- In this aspect, the present invention provides a cryosurgical catheter for use in a blood vessel having a vessel wall. The cryosurgical catheter comprises a flexible catheter body having a proximal end, a distal end, and a lumen defining an axis therebetween. A balloon is disposed at the distal end of the catheter body. The balloon is in fluid communication with the lumen, and has a balloon wall that expands radially to engage the surrounding vessel wall. A plurality of cooling fluid distribution ports are in communication with a cooling fluid supply. These ports are distributed within the balloon so as to evenly cool a portion of the vessel wall.
- To maximize cooling efficiency and minimize gas pressure within the balloon, it is generally preferable to minimize the total cooling fluid flow out of the exhaust lumen from the balloon. Efficiency can also be enhanced by directing the cooling fluid radially against the balloon wall, ideally using a plurality of ports that are separated circumferentially about a diffuser head. When treating long diseased segments of the vasculature, for example, when treating hyperplasia of the iliac or superior femoral arteries, it would be beneficial to treat the entire segment without moving or repositioning the balloon. To provide even treatment within such an elongated diseased vessel, the diffuser head may be moved axially within the inflated balloon by sliding a cooling fluid supply tube axially within the catheter body. Such a structure may provide a variety of controllable sequential cryogenic treatment regimens, for example, multiple temperature feedback controlled cryogenic treatment cycles for inhibiting cell proliferation, or for a variety of alternative endoluminal cryogenic therapies. Alternatively, a fixed diffuser head defining an axially and circumferential distributed array of ports may provide simultaneous even cooling throughout a significant region of the target site.
- In a related method aspect, the invention provides a therapy for treatment of a blood vessel having a vessel wall. The method comprises introducing a catheter into the blood vessel, and expanding a balloon of the catheter near a target site to engage the vessel wall. Fluid is expanded at a first location within the balloon. Fluid is also expanded at a second location within the balloon to cryogenically cool a portion of the engaged vessel wall, the second location being separated from the first location.
- Gas expansion may effect cryogenic cooling via Joule-Thompson expansion as the cryogenic fluid enters the balloon and/or via the enthalpy of vaporization of a cryogenic fluid within the balloon. There may be significant temperature transients when cryogenic cooling is first initiated from within the balloon catheter. To enhance the surgeon's control over the cooling rate and treatment time of these cryogenic therapies, gas expansion may be initiated while a moveable orifice head is disposed within a housing or shield at one end of the balloon. This housing may conveniently be formed by extending a tubular structure distally from the catheter body into the interior of the balloon. Such a housing structure may also be used to help direct exhaust gases proximally out of the balloon without causing excessive cooling at the proximal end of the balloon, which exhaust gases might otherwise freeze blood within the vessel.
- In yet another aspect, the invention also provides a kit for treating hyperplasia or neoplasia in a body lumen. The kit comprises a catheter having a proximal end, a distal end, and balloon near its distal end. Instructions are included in the kit for use of the catheter. These instructions comprise the step of cooling an inner surface of the body lumen with the balloon to a temperature and for a time sufficient to inhibit subsequent cell growth. Such a kit may include instructions for any of the methods described herein.
FIG. 1 illustrates a cryosurgical catheter constructed in accordance with the principles of the present invention, with a distal end shown in cross-section.FIG. 2 is a cross-sectional view of the catheter taken along line2-2 inFIG. 1 .FIG. 3 illustrates the expansion of a cryogenic fluid within the balloon of the cryosurgical catheter ofFIG. 1 .FIG. 4 is a graph illustrating the temperature profile of the balloon ofFIGS. 1 and 3 while liquid nitrogen is being expanded therein and the balloon is present in a body lumen.FIG. 5 illustrates the distal end of a cryosurgical catheter constructed in accordance with the principles of the present invention and having a nested containment bladder within a balloon structure.FIG. 6A-6C illustrate use of the catheter ofFIG. 1 in treating a target site within a patient's vasculature.FIG. 7 is a partial cross-section of a cryosurgical catheter having a diffuser head with a plurality of radially oriented cryogenic fluid ports, in which the diffuser head can slide axially within the balloon to provide even cooling of elongate treatment sites.FIG. 8 is a partial cross-sectional view of a cryosurgical catheter having a moveable diffuser head which can be drawn proximally into a housing within the balloon so as to avoid transients upon initiation of the cooling fluid flow.FIG. 9 schematically illustrates an alternative fixed porous diffuser defining an axial and circumferential array of orifices.FIG. 10 illustrates a cross-section of the catheter ofFIG. 9 .FIG. 11 illustrates a proximal end of the catheter ofFIG. 9 .FIG. 12 illustrates an alternative fixed diffuser structure having an array of axially separated cryogenic fluid ports.FIG. 13 is a functional block diagram illustrating the operation of the catheter ofFIG. 7 , including an optional feedback control loop.FIG. 14 schematically illustrates a kit including a balloon catheter and instructions for its use according to the methods described herein.- An
exemplary cryosurgical catheter 10 constructed in accordance with the principles of the present invention is illustrated inFIGS. 1 and 2 . Thecatheter 10 comprises acatheter body 12 having aproximal end 14, adistal end 16, and aninflatable balloon 18 disposed at the distal end. Theballoon 18 is shown to be an integral extension of thecatheter body 12, but such a structure is not required by the present invention. The balloon could be formed from the same or a different material and, in the latter case, attached to the distal end of thecatheter body 12 by suitable adhesives, heat welding, or the like. The catheter body may be formed from conventional materials, such as polyethylenes, polyimides, and copolymers and derivatives thereof. The balloon may also be formed from conventional materials used for angioplasty balloons, typically being non-distensible, such as polyethylene terephthalate (PET). Catheter 10 comprises acentral shaft 20 which may be formed from polymeric material, such as polyethylene, polytetrafluoroethylene, polyimide, or from a metal, such as from hypotube. In the embodiment ofcatheter 10, thecoaxial shaft 20 is tubular and provides a guidewire lumen for positioning of the catheter over a guidewire in a conventional manner. Theshaft 20, however, could have a variety of other configurations and purposes. For example, the shaft could be a solid wire or core and further optionally provide a guidewire tip at its distal end. The shaft could also provide a lumen for delivering cryogenic fluid to theballoon 18. In the illustrated embodiment ofFIG. 10 , however, the cryogenic fluid is provided by a separate cryogenicfluid delivery tube 22 which is disposed in parallel to thecoaxial shaft 20.- The
catheter 10 will usually further comprise athermocouple 24 which is optimally located near the center ofballoon 18. At this location, it can measure the temperature of the cryogenic fluid after expansion from the proximal end of thecryogenic delivery tube 22. Thecryogenic delivery tube 22 will define an expansion orifice at itsdistal end 23. Thus, the cryogenic fluid will flow through thetube 22 as a liquid at an elevated pressure and (thus inhibiting flow restrictive film boiling) will expand across theorifice 23 to a gaseous state at a lower pressure within the balloon. For liquid nitrogen, the pressure within thetube 22 will typically be in the range from 50 psi to 500 psi at a temperature below the associated boiling point. After expansion, the nitrogen gas within the balloon near its center (the location of thermocouple24) the pressure will typically be in the range from 30 psi to 100 psi and the temperature in the range from −40° C. to −100° C. The temperature may decrease in both the radially outward direction and in both axial directions from the center of the balloon. This feature of the present invention is better described in connection withFIGS. 3 and 4 below. - A
hub 28 is secured to theproximal end 14 of thecatheter body 12. The hub provides for aport 30 for connecting a cryogenic fluid source to thecryogenic delivery tube 22. The hub further provides aport 32 for exhausting the gaseous cryogenic fluid which travels fromballoon 18 in a proximal direction throughannular lumen 25. Athird port 34 is provided forthermocouple wires 26. Afourth port 36 at the proximal end of the hub is provided for a guidewire. - Referring now to
FIGS. 3 and 4 , liquid nitrogen (LN2) is delivered to balloon18 through thecryogenic delivery tube 22. The liquid nitrogen is delivered at a temperature and pressure within in the ranges set forth above and expands to gaseous nitrogen (GN2) across the expansion orifice into the interior ofballoon 18. In the single-balloon embodiment catheter 10, the gaseous nitrogen will serve both to inflate theballoon 18 and to cool the exterior surface of the balloon in a desired temperature profile. In particular, the balloon dimensions and operating conditions will be selected to provide a particular balloon temperature profile, an example of which is set forth inFIG. 4 . By expanding the liquid nitrogen to its gaseous state near the center of the balloon, the balloon temperature will be lowest near the center and will decrease in both axial directions away from the center, as shown in the temperature profile ofFIG. 4 . - For treating arterial hyperplasia, a balloon temperature in the range from −20° C. to −80° C., e.g., at about −50° C., for a time period in the range from 1 second to 10 seconds, may be effective. By delivering the liquid nitrogen at a pressure in the range from 50 psi to 500 psi and at a temperature below the boiling point, and expanding the liquid nitrogen to a gas at a pressure in the range from 30 psi to 100 psi, a temperature in the above range at the middle of the balloon will be achieved. Moreover, by extending the balloon by distances of at least 0.5 cm, preferably of at least 1 cm, in each direction from the center of the balloon, the temperatures at the ends of the balloons will generally no lower than 0° C. In this way, a desired low temperature can be maintained at the outer surface of the balloon in a treatment region near the center of the balloon, while the distal and proximal ends of the balloon act to insulate the colder portions from non-target regions within the artery or other body lumen. It will be appreciated that the axial length of the treatment region of the balloon can be varied considerably by varying the length of the balloon and controlling the volume of liquid nitrogen delivered to the balloon. Exemplary balloons will have a length in the range from 3 cm to 5 cm, a diameter in the range from 1.5 mm to 4 mm, and will typically receive from 0.08 ml/sec to 1.5 ml/sec of liquid nitrogen in the temperature and pressure ranges set forth above.
- Referring now to
FIG. 5 , analternative balloon assembly 50 will be described. Theballoon assembly 50 is disposed at the distal end of acatheter body 52 comprising ashaft 54 and a cryogenicfluid delivery tube 56. Aballoon 58 is secured to the distal end of thecatheter body 52, generally as described above with respect tocatheter 10. In contrast tocatheter 10, however,balloon assembly 50 comprises acontainment bladder 60 nested within theballoon 58. Thecontainment bladder 50 may be a second balloon formed in a manner similar toballoon 58, except that it will be shorter and will have proximal and distal ends spaced axially inwardly from the proximal and distal ends ofballoon 58. Thebladder 60, however, may be disposed of different materials and have different properties. Generally, the containment bladder is intended to receive and contain the gaseous nitrogen after it is expanded acrossexpansion orifice 62 into the interior thereof. By containing the expanded (cold) gaseous nitrogen withinbladder 60, a more distinct temperature transition may be effected between the cold middle region ofballoon 58 and the less cold distal and proximal regions thereof. - Optionally, the
balloon 58 may be separately expanded with an insulating fluid to further sharpen the temperature transition between thecontainment bladder 60 and the remainder ofballoon 58. Alternatively, thecontainment bladder 60 may include ports or porous regions which permit the gaseous nitrogen to pass from the interior of thebladder 60 into the interior ofballoon 58 in a controlled manner to maintain the desired temperature transition. - Referring now to
FIGS. 6A-6C , use ofcatheter 10 for treating a target region TR within a blood vessel BV will be described. The target region will usually have been previously treated by balloon angioplasty or other primary conventional protocol for treating atherosclerotic disease. Such primary treatment will typically utilize an intravascular catheter, which catheter will have been removed leaving a guidewire GW in place, as illustrated inFIG. 6A . Acatheter 10 is then introduced over the guidewire, as illustrated inFIG. 6B . Liquid nitrogen is introduced to thecatheter 10 from asuitable source 70. The source may be a Dewar flask or other conventional source. In some instances, it will be possible to utilize recirculating refrigerated liquid nitrogen sources, such as those described in U.S. Pat. Nos. 5,644,502 and 5,617,739, the full disclosures of which have been previously incorporated herein by reference. The liquid nitrogen (LN2) is delivered to thecatheter 10 and inflatesballoon 18, as illustrated inFIG. 6C . Because of the temperature profile of the balloon, cooling of the inner wall of the blood vessel BV will be maximized over a central region CR and diminish in the proximal and distal directions from the central region, as illustrated qualitatively by the array of arrows inFIG. 6C . The treatment will be performed at the temperatures and for the times described thereabove in order to inhibit subsequent hyperplasia of the cells of the lining of the blood vessel. Advantageously, the cryogenic methods of the present invention will inhibit subsequent cell proliferation without inducing injury and thrombosis which can occur as a result of such injury. - A
catheter 80 having a moveable port head ordiffuser 82 is illustrated inFIG. 7 . In this embodiment,cryogenic fluid ports 23 are separated circumferentially aboutdiffuser 82, and are oriented radially so as to enhance the heat transfer between the expanding gas and the wall ofballoon 18. In the embodiment illustrated here, fourports 83 are provided, and are separated circumferentially from each other by about 90°. - To enhance an axial length of a substantially evenly cooled central region CR,
diffuser head 82 is slidably supported on a shaft orrail 84.Feed tube 22 is affixed todiffuser head 82 and is slidably disposed withincatheter body 12. Aproximal housing 86 atproximal end 14 ofcatheter 80 contains a rack andpinion mechanism 88 which controllably movesfeed tube 22. By rotating control knob90 (or automatically driving rack andpinion mechanism 88 with drive system92), feedtube 22 anddiffuser 82 can move from afirst position 95 to asecond position 96 without moving or deflatingballoon 18. This allows a relatively small fluid flow to cool an elongate central region CR. - Inhibition of cell proliferation along elongate segments of vasculature, such as in the iliac or superior femoral arteries should benefit from cryosurgical treatment at repeatable temperatures and for repeatable times. To help prevent gaps between treatment regions and/or repeating treatments unintentionally, it would be advantageous to allow treatments of these elongate lumenal walls without moving or repositioning of the cryosurgical catheter. Hence, it is generally desirable to provide structures and methods which can uniformly apply radial cooling along these elongate endothelial surfaces.
- Safety of endoluminal cryosurgical techniques is generally enhanced by minimizing the flow of the cooling fluid. Low flow rates will generally reduce the release of gas into the body lumen in the unlikely event of a balloon rupture. Known balloon structures can withstand pressures of up to about 100 psi or more. Nonetheless, safety can be enhanced by limiting maximum balloon pressures to 100 psi or less, and preferably to less than 100. Lower balloon pressures not only reduce the amount of gas released in the event of a rupture, they also help decrease the possibility of such a balloon rupture occurring. Given a constant cooling fluid pressure at
port 83, lower pressures withinballoon 18 will also produce a lower balloon wall temperature. In other words, cryogenic cooling is generally enhanced by minimizing the pressure within the balloon. - As there is a limited cross-sectional area available for exhausting the expelled gases, pressure within the balloon is most easily minimized by decreasing the speed (and pressure head loss) of exhaust gases flowing proximally through
catheter body 12 to exhaustport 32. Drawing a vacuum atexhaust port 32 can encourage the flow of gases proximally and reduce balloon pressure to some extent, but this will provide limited benefits when gas velocity and pressure drops are high within the catheter body. Hence, it is beneficial to make efficient use of a relatively small cryogenic fluid flow. By movingdiffuser 82 axially withinballoon 18, an elongate region of the vessel wall can be treated sequentially with a modest cryogenic fluid supply and a low balloon pressure. As the amount of time the tissues are to be cooled is quite short, the total procedure time remains very reasonable. - The use of
moveable diffuser head 82 also allows the surgeon to selectively treat tissues in a highly controlled manner. For example, whenballoon 18 extends across a branch artery, the surgeon has the option of treating the vessel proximally and distally of the branch and shutting off the gas flow when the diffuser is aligned with the branch so as to avoid freezing blood within the branch opening. Additionally, by couplingautomated drive system 92 to the actuation mechanism, a wide variety of treatment cycles and times may be controllably and repeatably effected. Balloon 18 ofmoveable diffuser catheter 80 may be quite elongate, the balloon typically having a length in a range from about 1 to about 10 cm. In the exemplary embodiment, the balloon has a length of about 10 cm so thatproximal housing 86 and/oractuation mechanism 88 has a stroke length of about 8 cm. To enhance heat flow throughballoon 18, a heat transfer enhancing material may be included in the polymer of the balloon wall. For example, the addition of between about 1 and 10% boron nitride in a polyethylene or other balloon polymer can significantly improve heat transfer of the entire system. Surprisingly, a significant temperature differential may be found between an inner and outer surface of the balloon during cooling. Hence, improving the thermal conductivity of the balloon material may provide significant benefits.- In the embodiment of
FIG. 7 , a fixedguidewire 94 extends distally from the balloon to help when advancingcatheter 80 within the vasculature.Fixed guidewire 94 and the distal end ofballoon 18 are affixed to an axial support orrail 84 which structurally supports the distal end of the catheter when the balloon is not inflated.Rail 84 here comprises a stainless steel wire with a diameter of 0.008 inches, but may alternatively comprise a wide variety of shaft structures, optionally including one or more lumens for a moveable guidewire or the like. Diffuser 82 includes four radially oriented ports, each having a diameter of about 0.0025 inches. These openings are in fluid communication with a central passage, which in turn is supplied byfeed tube 22.Diffuser head 82 may have an outer diameter of about 0.032 inches, and may comprise any of a variety of alternative polymers or metals.Diffuser 82 is affixed to feedtube 22 by adhesion bonding, heat welding, fasteners, or the like. In the exemplary embodiment,diffuser 82 comprises polyimide.Feed tube 22 may also be formed from a polyimide tube, and will preferably be coated with a PTFE such as Teflon™ to avoid friction when the feed tube reciprocates within the catheter body.Diffuser head 82 is shown affixed to rail84 using bands which encircle the diffuser and define a channel through which rail84 passes. Clearly, a wide variety of alternative support arrangements are possible, including a concentric support shaft or tube, a cantilevered feed tube, or the like. As described above,thermocouple 24 or some alternative temperature sensor sends a signal proximally viawire 26 to indicate the temperature within the balloon.- In use, moveable
diffuser balloon catheter 80 will be introduced into a blood vessel whileballoon 18 is in an uninflated, small profile configuration.Balloon 18 will be maneuvered to the treatment site using fixedguidewire 94.Feed tube 22 will be positioned so thatdiffuser 82 is located atfirst position 95, and cryogenic fluid will be advanced throughfeed tube 22 to the diffuser. This gas will inflateballoon 18, and will also cool the interior surface of the balloon and blood vessel as described above.Control knob 90 will be rotated so thatdiffuser 82 moves axially towardposition 96. As the cooling fluid exits the diffuser, the endothelial tissue engaging central region CR is cryogenically cooled. - Cryogenic cooling fluid may optionally pass through a Joule-Thompson orifice
adjacent port 83 to effect cooling. In other embodiments, at least a portion of the cryogenic cooling fluid may exitport 83 into the balloon as a liquid. The liquid will vaporize within the balloon, and the enthalpy of vaporization can help cool the surrounding vessel wall. The liquid may coat at least a portion of the balloon wall so as to enhance even cooling over at least a portion of the vessel wall. Hence,ports 83 may have a total cross section which is smaller than a cross section of the fluid supply lumen, or which is at least as large as the cross section of the fluid supply lumen. - By controlling the rate of movement of
diffuser 82 viacontrol knob 90, the amount of cooling fluid injected viafeed tube 22, and the pressure atexhaust port 32, the surgeon can control the cooling rate of the tissue, the temperature of the tissue, and optionally, the number of cooling cycles the tissue is subjected to while the catheter is in a single location. As described above, the ends of the diffuser stroke first andsecond positions balloon 18 so as to limit any cooling of fluids within the vessel. - As can be understood with reference to
FIG. 8 , it will be desirable to control the initial rate of cooling when cryogenic fluid first starts to exitdiffuser 82. As the balloon inflates and the diffuser structure cools, significant thermal transients will occur before the desired steady state cryogenic cooling begins. To avoid unpredictable or excessively slow cooling rates,diffuser 82 may initially be parked within ahousing 98 insideballoon 18.Housing 98 may be formed by extending a tube fromcatheter body 12 intoballoon 18, the housing optionally comprising an extension of the catheter body material. - An additional benefit of
housing 98 may be understood with reference toFIGS. 7, 8 , and4. As cooling gas flows fromdiffuser 82 intoballoon 18, the expelled gases are exhausted proximally from the balloon intocatheter body 12. Although the gases will warm as they travel proximally, the gas flow will be accelerating from their relatively large cross-sectional diameter of the balloon into the catheter body. This may actually enhance cooling adjacent the proximal end of the balloon, and could freeze blood proximally of the balloon. - To avoid this enhanced proximal cooling,
housing 98 admits gases from a central location along central region CR. Thegases surrounding housing 98 withinballoon 18 are allowed to stagnate near the proximal end of the balloon, thereby limiting axial cooling at that location. - As described above, there may be a significant temperature differential between the inner surface and the outer surface of the balloon wall. To more accurately and repeatably monitor cryosurgical therapy, a
temperature sensor 24 is mounted on the outer surface of the balloon to measure the temperature of the tissue at the target site, the tissue balloon interface, and/or the balloon outer surface temperature. - Referring now to
FIG. 9 , a fixeddiffuser 100 includes an array ofports 83 which are distributed both axially and circumferentially around the diffuser. Asports 83 are radially oriented,diffuser 100 will achieve the desired cooling of the surrounding tissue with relatively low balloon pressures and low cooling fluid flow rates. As the cryogenic liquid or gas-liquid combination is directed perpendicularly against the wall ofballoon 18, the heat transfer coefficient between the gas and the balloon wall is quite high. This helps to reduce temperature of the balloon and provides greater heat extraction for a given flow rate of coolant into the balloon. Additionally, asports 83 are distributed both circumferentially and axially along the balloon,diffuser 100 will distribute the cooling more uniformly over the surface of the balloon so as to produce a uniform antiproliferative response. Diffuser 100 will generally comprise a tubular structure with radially oriented openings. An exemplary tubular structure may comprise a polyimide tube having an inner diameter of about 0.032 inches and a wall thickness of 0.001 inch. Each port will again define a diameter of about 0.0025 inches. There will typically be between about 6 and 600 orifices indiffuser 100. In the exemplary embodiment, four axial rows of orifices are separated by about 90° from each other. The rows are axially staggered so that the orifices in a single row have centerline separations of about 4 mm, while the orifices of adjacent rows are separated by about 2 mm. The overall length of the porous diffuser tube is about 2 cm.- A
central shaft 104 having aguidewire lumen 106 is bonded concentrically to diffuser100 using adhesive or the like at the distal end of the diffuser, and optionally also at the proximal end of the diffuser.High contrast markers 102 may be provided to enhance an image of the catheter so as to facilitate positioning ofballoon 18 fluroscopically, sonographically, or under any other alternative image modality (with appropriate contrast structures). The distal marker may optionally be formed by winding a gold or platinum wire around the central shaft and bonding the gold wire to the distal end of the diffuser tube. The proximal marker may similarly be formed by winding and bonding a gold or platinum wire, the proximal marker optionally being disposed over the diffuser tube so that the cryogenic cooling fluid may be introduced through the annular space between the diffuser tube and the central shaft proximally of the balloon.Central shaft 104 will typically comprise a polyimide tube, but may alternatively comprise any of a wide variety of materials. - The coaxial arrangement between
diffuser 100 and central shaft104 (with an annular cooling fluid flow path between the tube of the diffuser and the central shaft) promotes circumferentially symmetric distribution of the cryogenic cooling fluid against the balloon wall, which in turn provides a more circumferentially even temperature distribution. As generally described above, uniform temperature distributions, both axially and circumferentially, within central region CR (seeFIG. 4 ) help ensure that the beneficial inhibition of cell proliferation is provided throughout a significant portion of the tissue engaged byballoon 18. To limit cooling of tissues or fluids disposed axially of the balloon, distal and proximal stagnant regions within the balloon flow profile are created by the shape and configuration ofdiffuser 100,balloon 18, and by the presence ofhousing 98 within the proximal end of the balloon, as described above. Even though no moveable diffuser will be drawn intohousing 98, this structure still helps to avoid the accelerating flow of gases along the proximally tapering balloon wall. - To accurately control the cooling process, it is beneficial to monitor pressure within the balloon. Toward that end, a
balloon pressure port 108 transmits pressure proximally via apressure monitoring lumen 110, as can be understood with reference toFIGS. 9 and 10 . Accuracy of such pressure monitoring can be enhanced by minimizing the flow of fluid proximally within the pressure monitoring lumen. Alternatively, a pressure transducer may be mounted within the balloon with wires sending a pressure signal proximally. Within theelongate catheter body 12, lumens for thecryogenic feed tube 22,pressure monitoring port 108, guidewire and the like may be contained within aninsulated jacket 112. Asballoon 18 may elongate when inflated, and as the distal end of diffuser is affixed to the distal end of the balloon bycore shaft 104, it may be beneficial to allowjacket 112 to slide axially withincatheter body 12 to avoid axial bending of the balloon and the resulting radially uneven cooling. In alternative embodiments, a cryogenic feed tube may simply extend distally into an annular space between a central shaft and a jacket formed as a continuous proximal extension of the diffuser tube, with any proximal leakage of the cooling fluid within the jacket optionally being exhausted into the catheter body and removed via the exhaust lumen. - Referring now to
FIG. 11 , a proximal end of the fixed diffuser catheter illustrated inFIGS. 9 and 10 include many of the coupling structures described above regardingFIGS. 1 and 7 .Guidewire port 114 provides proximal access toguidewire lumen 106, while apressure monitoring connector 116 is in fluid communication with the interior ofballoon 18 viamonitoring lumen 110. Where balloon pressures are acceptable, cryogenic cooling may optionally be controlled using an orifice disposed atexhaust port 32. This proximal structure can be assembled from commercial available components usingpotting adhesive 118 in a generally conventional manner. - Referring now to
FIG. 12 , still further alternative multiple orifice diffuser structures are possible. In this embodiment (illustrated here without balloon18) a series ofports 83 are distributed axially so as to distributed the cooling axially within an elongate target region, as generally described above. In this embodiment, a series of individualgas feed tubes 120 supply the cryogenic cooling fluid to the ports, with each port optionally having an opening which has the same area as the lumen of the associated gas feed tube. Such individual feed tubes may comprise polyimide tubes having an inner diameter of about 0.005 inches. In some embodiments, axial distribution of cooling may be controlled by varying the amount of fluid expelled from each port, by varying the interorifice spacing (axially and/or circumferentially), by locally varying the heat transfer coefficient or cooling fluid pattern, or the like. - It will generally be beneficial to make use of
catheter 80 as one component of an integrated cryosurgicalendoluminal therapy system 130. As the actual tissue cooling may vary with pressures within the balloon, cooling fluid flow rates, and the like, and as these parameters may vary whencatheter body 12 is bent in following the vasculature system, the efficacy of the cryosurgical therapy may be enhanced by adjusting the treatment based on measured characteristics of the cooling process, for example, based on temperatures measured by one ormore temperature sensors 24. Hence, electrical temperature signals132 fromtemperature sensors 24 may be directed to acontroller 134 for use in a feedback control system. Preferably,controller 134 processes the temperature signals to generate cooling fluid feed signals136 indicating the pressure or volume of cryogenic fluid to be injected into the catheter.Controller 134 will preferably also provide electrical signals whichdirect diffuser drive 92 to mechanically repositiondiffuser 82, and will often provide signals varying the pressure (or vacuum) atexhaust port 32. These signals may be used not only to vary the cooling cycle, but can also be used to control the inflation and/or deflation of the balloon, preferably based at least in part on a pressure monitored from within the balloon. - To inhibit cell proliferation and/or remodeling,
controller 134 will generally initiate, monitor, and control cooling of the tissue.Cryogenic system 130 will often be used to effect a cooling rate of the tissue in a range from about 2 to about 30° C. per second. In an exemplary cell proliferation inhibition therapy, the system will maintain the tissue at a temperature in a range from about 0 to about −80° C., preferably at a temperature in a range from about −10 to about −40° C., for a time between about 1 and about 60 seconds. The efficacy of the therapy may be enhanced by repeatedly cooling the tissue to these temperatures for between 1 and 5 cooling cycles, typically repeating the cooling cycles at the rate of 1 every 60 seconds. To provide this cooling, cryogenic liquids or liquid/gas mixtures comprising carbon dioxide, nitrous oxide, or the like may flow through the balloon at a rate in a range from about 100 to about 800 mg/sec. Such cooling may inhibit cell proliferation via processes which are sometimes referred to as apoptosis and/or programmed cell growth. - A
kit 140 includingballoon catheter 10 and instructions for itsuse 142 is illustrated inFIG. 14 .Catheter 10 may be replaced by any of the balloon catheter structures described above, while instructions foruse 142 may describe any of the associated method steps set forth above for inhibition of cell proliferation. Instructions foruse 142 will often be printed, optionally appearing at least in part on asterile package 144 forballoon catheter 10. In alternative embodiments, instructions foruse 142 may comprise a machine readable code, digital or analog data graphically illustrating or demonstrating the use ofballoon catheter 10 to inhibit hyperplasia, or the like. Still further alternatives are possible, including printing of the instructions for use onpackaging 146 ofkit 140, and the like. - While the above is a complete description of the preferred embodiments of the invention, various alternatives, modifications, and equivalents may be used. For example, one or more radial orifices might move both circumferentially and axially within the balloon, optionally along a helical path, to provide cylindrically even cooling. Therefore, the above description should not be taken as limiting the scope of the invention which is defined by the appended claims.
Claims (14)
1. A system for treating a blood vessel having a vessel wall, the system comprising:
a flexible catheter body having a proximal end and a distal end with a cooling fluid lumen and an exhaust lumen therebetween;
an intravascular balloon in fluid communication with the cooling fluid lumen and the exhaust lumen near the distal end of the catheter body, the balloon expandable to radially engage a surrounding vessel wall and having an inner surface; and
a cooling fluid source coupleable to the cooling fluid lumen adjacent the proximal end of the catheter body, the source configured so that liquid cooling fluid flows from the source through the cooling fluid lumen into the balloon so as to engage at least a portion of the inner surface of the balloon when the balloon is disposed within the blood vessel, the liquid engaging the inner surface of the balloon vaporizing within the balloon to cool the vessel wall.
2. The system ofclaim 1 , wherein the vaporizing cooling fluid expands from the liquid cooling fluid to a gas cooling fluid within the balloon, and wherein the expansion of the vaporizing cooling fluid effects inflation of the balloon.
3. The system ofclaim 1 , wherein the at least a portion of the inner surface of the balloon wall is coated by the liquid cooling fluid when a cooling fluid path through the cooling fluid lumen and the exhaust lumen is open.
4. The system ofclaim 3 , wherein the liquid coats the at least a portion of the inner surface of the balloon wall so as to enhance even cooling over at least a portion of the vessel wall engaged by the balloon.
5. The system ofclaim 1 , wherein the cooling fluid supply and balloon are configured for treating hyperplasia or neoplasia in a body lumen by cooling an inner surface of the body lumen to a temperature and for a time sufficient to inhibit subsequent cell growth.
6. The system ofclaim 5 , wherein the catheter body and balloon comprise a balloon catheter configured for dilating a stenosed artery subject to hyperplasia.
7. The system ofclaim 5 , wherein the latent heat of vaporization of the cooling fluid is sufficient to cool the vessel wall to from 0° C. to −80° C., and wherein a quantity of the cooling fluid is sufficient for cooling in a range from 1 second to 10 seconds.
8. The system ofclaim 1 , wherein a pressure within the balloon is maintained at from 30 psi to 100 psi during cooling.
9. The system ofclaim 1 , wherein the temperature at each end of the balloon is above 0° C. during cooling.
10. The system ofclaim 9 , wherein a temperature profile over a length of the balloon has a temperature between the ends below 0° C. and temperatures at each end above 0° C. during cooling.
11. The system ofclaim 10 , wherein the balloon has a length of at least 1 cm.
12. The system ofclaim 1 , further comprising a thermocouple mounted outside the balloon for measuring a temperature of the vessel wall.
13. A system for treating a blood vessel having a vessel wall, the system comprising:
a flexible catheter body having a proximal end and a distal end;
an intravascular balloon disposed near the distal end of the catheter body, the balloon expandable to radially engage a surrounding vessel wall, the balloon having an inner surface; and
a cooling liquid coating at least a portion of the inner surface of the balloon, the cooling liquid vaporizing within the balloon to cool the vessel wall.
14. A cryosurgical catheter for use in a blood vessel having a vessel wall, the cryosurgical catheter comprising:
a flexible catheter body having a proximal end, a distal end, and a lumen defining an axis therebetween;
an axially elongate balloon disposed at the distal end of the catheter body in fluid communication with the lumen, the balloon having a balloon wall that can expand radially to engage the surrounding vessel wall;
a diffuser head having at least one port in fluid communication with a cooling fluid supply, the diffuser head movable axially within the balloon between a first position and a second position.
Priority Applications (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US11/122,165US20050209587A1 (en) | 1997-12-02 | 2005-05-04 | Apparatus and method for cryogenic inhibition of hyperplasia |
Applications Claiming Priority (4)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US08/982,824US5971979A (en) | 1997-12-02 | 1997-12-02 | Method for cryogenic inhibition of hyperplasia |
| US09/203,011US6355029B1 (en) | 1997-12-02 | 1998-12-01 | Apparatus and method for cryogenic inhibition of hyperplasia |
| US09/978,253US6908462B2 (en) | 1997-12-02 | 2001-10-15 | Apparatus and method for cryogenic inhibition of hyperplasia |
| US11/122,165US20050209587A1 (en) | 1997-12-02 | 2005-05-04 | Apparatus and method for cryogenic inhibition of hyperplasia |
Related Parent Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US09/978,253DivisionUS6908462B2 (en) | 1997-12-02 | 2001-10-15 | Apparatus and method for cryogenic inhibition of hyperplasia |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| US20050209587A1true US20050209587A1 (en) | 2005-09-22 |
Family
ID=25529540
Family Applications (4)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US08/982,824Expired - LifetimeUS5971979A (en) | 1997-12-02 | 1997-12-02 | Method for cryogenic inhibition of hyperplasia |
| US09/203,011Expired - LifetimeUS6355029B1 (en) | 1997-12-02 | 1998-12-01 | Apparatus and method for cryogenic inhibition of hyperplasia |
| US09/978,253Expired - LifetimeUS6908462B2 (en) | 1997-12-02 | 2001-10-15 | Apparatus and method for cryogenic inhibition of hyperplasia |
| US11/122,165AbandonedUS20050209587A1 (en) | 1997-12-02 | 2005-05-04 | Apparatus and method for cryogenic inhibition of hyperplasia |
Family Applications Before (3)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US08/982,824Expired - LifetimeUS5971979A (en) | 1997-12-02 | 1997-12-02 | Method for cryogenic inhibition of hyperplasia |
| US09/203,011Expired - LifetimeUS6355029B1 (en) | 1997-12-02 | 1998-12-01 | Apparatus and method for cryogenic inhibition of hyperplasia |
| US09/978,253Expired - LifetimeUS6908462B2 (en) | 1997-12-02 | 2001-10-15 | Apparatus and method for cryogenic inhibition of hyperplasia |
Country Status (7)
| Country | Link |
|---|---|
| US (4) | US5971979A (en) |
| EP (1) | EP1039838B1 (en) |
| JP (1) | JP2001524345A (en) |
| AU (1) | AU744106B2 (en) |
| CA (1) | CA2314352C (en) |
| DE (1) | DE69831172T2 (en) |
| WO (1) | WO1999027862A1 (en) |
Cited By (131)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US20070129714A1 (en)* | 2005-05-20 | 2007-06-07 | Echo Healthcare Llc | Subdermal cryogenic remodeling of muscles, nerves, connective tissue, and/or adipose tissue (FAT) |
| US20080154254A1 (en)* | 2006-12-21 | 2008-06-26 | Myoscience, Inc. | Dermal and Transdermal Cryogenic Microprobe Systems and Methods |
| US20080183164A1 (en)* | 2005-05-20 | 2008-07-31 | Myoscience, Inc. | Subdermal cryogenic remodeling of muscles, nerves, connective tissue, and/or adipose tissue (fat) |
| US20080200910A1 (en)* | 2007-02-16 | 2008-08-21 | Myoscience, Inc. | Replaceable and/or Easily Removable Needle Systems for Dermal and Transdermal Cryogenic Remodeling |
| US20080234809A1 (en)* | 2007-03-23 | 2008-09-25 | Medtronic Vascular, Inc. | Stent Graft System With Injection Tube |
| US20090088735A1 (en)* | 2004-03-23 | 2009-04-02 | Cryocath Technologies Inc. | Method and apparatus for inflating and deflating balloon catheters |
| WO2009067497A1 (en)* | 2007-11-21 | 2009-05-28 | Endocare, Inc. | Flexible multi-tubular cryoprobe |
| US20090287202A1 (en)* | 2008-05-15 | 2009-11-19 | Boston Scientific Scimed, Inc. | Apparatus and methods for cryogenically ablating tissue and adjusting cryogenic ablation regions |
| US20090322323A1 (en)* | 2005-05-11 | 2009-12-31 | Audrius Brazdeikis | Intraluminal Magneto Sensor System and Method of Use |
| US20110009854A1 (en)* | 2007-11-21 | 2011-01-13 | Endocare, Inc. | Expandable multi-tubular cryoprobe |
| US20110184398A1 (en)* | 2010-01-27 | 2011-07-28 | Medtronic Cryocath Lp | Cryoballoon refrigerant dispersion control |
| US20120136343A1 (en)* | 2009-06-01 | 2012-05-31 | Channel Medsystems, Inc. | Methods and apparatus for treatment of a body cavity or lumen |
| US20120245574A1 (en)* | 2011-03-25 | 2012-09-27 | Medtronic Cryocath Lp | Spray nozzle design for a catheter |
| US8298216B2 (en) | 2007-11-14 | 2012-10-30 | Myoscience, Inc. | Pain management using cryogenic remodeling |
| US8382747B2 (en) | 2004-03-23 | 2013-02-26 | Medtronic Cryocath Lp | Method and apparatus for inflating and deflating balloon catheters |
| US8473067B2 (en) | 2010-06-11 | 2013-06-25 | Boston Scientific Scimed, Inc. | Renal denervation and stimulation employing wireless vascular energy transfer arrangement |
| US8491636B2 (en) | 2004-03-23 | 2013-07-23 | Medtronic Cryopath LP | Method and apparatus for inflating and deflating balloon catheters |
| US8939970B2 (en) | 2004-09-10 | 2015-01-27 | Vessix Vascular, Inc. | Tuned RF energy and electrical tissue characterization for selective treatment of target tissues |
| US8951251B2 (en) | 2011-11-08 | 2015-02-10 | Boston Scientific Scimed, Inc. | Ostial renal nerve ablation |
| US8974451B2 (en) | 2010-10-25 | 2015-03-10 | Boston Scientific Scimed, Inc. | Renal nerve ablation using conductive fluid jet and RF energy |
| US9017318B2 (en) | 2012-01-20 | 2015-04-28 | Myoscience, Inc. | Cryogenic probe system and method |
| US9023034B2 (en) | 2010-11-22 | 2015-05-05 | Boston Scientific Scimed, Inc. | Renal ablation electrode with force-activatable conduction apparatus |
| US9028472B2 (en) | 2011-12-23 | 2015-05-12 | Vessix Vascular, Inc. | Methods and apparatuses for remodeling tissue of or adjacent to a body passage |
| US9028485B2 (en) | 2010-11-15 | 2015-05-12 | Boston Scientific Scimed, Inc. | Self-expanding cooling electrode for renal nerve ablation |
| US9050106B2 (en) | 2011-12-29 | 2015-06-09 | Boston Scientific Scimed, Inc. | Off-wall electrode device and methods for nerve modulation |
| US9060761B2 (en) | 2010-11-18 | 2015-06-23 | Boston Scientific Scime, Inc. | Catheter-focused magnetic field induced renal nerve ablation |
| US9066712B2 (en) | 2008-12-22 | 2015-06-30 | Myoscience, Inc. | Integrated cryosurgical system with refrigerant and electrical power source |
| US9079000B2 (en) | 2011-10-18 | 2015-07-14 | Boston Scientific Scimed, Inc. | Integrated crossing balloon catheter |
| US9084609B2 (en) | 2010-07-30 | 2015-07-21 | Boston Scientific Scime, Inc. | Spiral balloon catheter for renal nerve ablation |
| US9089350B2 (en) | 2010-11-16 | 2015-07-28 | Boston Scientific Scimed, Inc. | Renal denervation catheter with RF electrode and integral contrast dye injection arrangement |
| US9119632B2 (en) | 2011-11-21 | 2015-09-01 | Boston Scientific Scimed, Inc. | Deflectable renal nerve ablation catheter |
| US9119600B2 (en) | 2011-11-15 | 2015-09-01 | Boston Scientific Scimed, Inc. | Device and methods for renal nerve modulation monitoring |
| US9125666B2 (en) | 2003-09-12 | 2015-09-08 | Vessix Vascular, Inc. | Selectable eccentric remodeling and/or ablation of atherosclerotic material |
| US9125667B2 (en) | 2004-09-10 | 2015-09-08 | Vessix Vascular, Inc. | System for inducing desirable temperature effects on body tissue |
| US9155589B2 (en) | 2010-07-30 | 2015-10-13 | Boston Scientific Scimed, Inc. | Sequential activation RF electrode set for renal nerve ablation |
| US9155584B2 (en) | 2012-01-13 | 2015-10-13 | Myoscience, Inc. | Cryogenic probe filtration system |
| US9162046B2 (en) | 2011-10-18 | 2015-10-20 | Boston Scientific Scimed, Inc. | Deflectable medical devices |
| US9173696B2 (en) | 2012-09-17 | 2015-11-03 | Boston Scientific Scimed, Inc. | Self-positioning electrode system and method for renal nerve modulation |
| US9186209B2 (en) | 2011-07-22 | 2015-11-17 | Boston Scientific Scimed, Inc. | Nerve modulation system having helical guide |
| US9186210B2 (en) | 2011-10-10 | 2015-11-17 | Boston Scientific Scimed, Inc. | Medical devices including ablation electrodes |
| US9192790B2 (en) | 2010-04-14 | 2015-11-24 | Boston Scientific Scimed, Inc. | Focused ultrasonic renal denervation |
| US9192435B2 (en) | 2010-11-22 | 2015-11-24 | Boston Scientific Scimed, Inc. | Renal denervation catheter with cooled RF electrode |
| US9220561B2 (en) | 2011-01-19 | 2015-12-29 | Boston Scientific Scimed, Inc. | Guide-compatible large-electrode catheter for renal nerve ablation with reduced arterial injury |
| US9220558B2 (en) | 2010-10-27 | 2015-12-29 | Boston Scientific Scimed, Inc. | RF renal denervation catheter with multiple independent electrodes |
| US9241753B2 (en) | 2012-01-13 | 2016-01-26 | Myoscience, Inc. | Skin protection for subdermal cryogenic remodeling for cosmetic and other treatments |
| US9265969B2 (en) | 2011-12-21 | 2016-02-23 | Cardiac Pacemakers, Inc. | Methods for modulating cell function |
| US9277955B2 (en) | 2010-04-09 | 2016-03-08 | Vessix Vascular, Inc. | Power generating and control apparatus for the treatment of tissue |
| US9297845B2 (en) | 2013-03-15 | 2016-03-29 | Boston Scientific Scimed, Inc. | Medical devices and methods for treatment of hypertension that utilize impedance compensation |
| US9295512B2 (en) | 2013-03-15 | 2016-03-29 | Myoscience, Inc. | Methods and devices for pain management |
| US9314290B2 (en) | 2012-01-13 | 2016-04-19 | Myoscience, Inc. | Cryogenic needle with freeze zone regulation |
| US9326751B2 (en) | 2010-11-17 | 2016-05-03 | Boston Scientific Scimed, Inc. | Catheter guidance of external energy for renal denervation |
| US9327100B2 (en) | 2008-11-14 | 2016-05-03 | Vessix Vascular, Inc. | Selective drug delivery in a lumen |
| US9358365B2 (en) | 2010-07-30 | 2016-06-07 | Boston Scientific Scimed, Inc. | Precision electrode movement control for renal nerve ablation |
| US9364284B2 (en) | 2011-10-12 | 2016-06-14 | Boston Scientific Scimed, Inc. | Method of making an off-wall spacer cage |
| US9408661B2 (en) | 2010-07-30 | 2016-08-09 | Patrick A. Haverkost | RF electrodes on multiple flexible wires for renal nerve ablation |
| US9420955B2 (en) | 2011-10-11 | 2016-08-23 | Boston Scientific Scimed, Inc. | Intravascular temperature monitoring system and method |
| US9433760B2 (en) | 2011-12-28 | 2016-09-06 | Boston Scientific Scimed, Inc. | Device and methods for nerve modulation using a novel ablation catheter with polymeric ablative elements |
| US9439708B2 (en) | 2010-10-26 | 2016-09-13 | Medtronic Ardian Luxembourg S.A.R.L. | Neuromodulation cryotherapeutic devices and associated systems and methods |
| US9463062B2 (en) | 2010-07-30 | 2016-10-11 | Boston Scientific Scimed, Inc. | Cooled conductive balloon RF catheter for renal nerve ablation |
| US9486355B2 (en) | 2005-05-03 | 2016-11-08 | Vessix Vascular, Inc. | Selective accumulation of energy with or without knowledge of tissue topography |
| US9579030B2 (en) | 2011-07-20 | 2017-02-28 | Boston Scientific Scimed, Inc. | Percutaneous devices and methods to visualize, target and ablate nerves |
| US9610112B2 (en) | 2013-03-15 | 2017-04-04 | Myoscience, Inc. | Cryogenic enhancement of joint function, alleviation of joint stiffness and/or alleviation of pain associated with osteoarthritis |
| US9649156B2 (en) | 2010-12-15 | 2017-05-16 | Boston Scientific Scimed, Inc. | Bipolar off-wall electrode device for renal nerve ablation |
| US9668811B2 (en) | 2010-11-16 | 2017-06-06 | Boston Scientific Scimed, Inc. | Minimally invasive access for renal nerve ablation |
| US9668800B2 (en) | 2013-03-15 | 2017-06-06 | Myoscience, Inc. | Methods and systems for treatment of spasticity |
| CN106880400A (en)* | 2015-12-16 | 2017-06-23 | 上海微创电生理医疗科技有限公司 | Electrophysiologicalcatheter catheter and radio frequency ablation system |
| US9687166B2 (en) | 2013-10-14 | 2017-06-27 | Boston Scientific Scimed, Inc. | High resolution cardiac mapping electrode array catheter |
| US9693821B2 (en) | 2013-03-11 | 2017-07-04 | Boston Scientific Scimed, Inc. | Medical devices for modulating nerves |
| US9707036B2 (en) | 2013-06-25 | 2017-07-18 | Boston Scientific Scimed, Inc. | Devices and methods for nerve modulation using localized indifferent electrodes |
| US9713730B2 (en) | 2004-09-10 | 2017-07-25 | Boston Scientific Scimed, Inc. | Apparatus and method for treatment of in-stent restenosis |
| US9757193B2 (en) | 2002-04-08 | 2017-09-12 | Medtronic Ardian Luxembourg S.A.R.L. | Balloon catheter apparatus for renal neuromodulation |
| US9770606B2 (en) | 2013-10-15 | 2017-09-26 | Boston Scientific Scimed, Inc. | Ultrasound ablation catheter with cooling infusion and centering basket |
| US9808300B2 (en) | 2006-05-02 | 2017-11-07 | Boston Scientific Scimed, Inc. | Control of arterial smooth muscle tone |
| US9808311B2 (en) | 2013-03-13 | 2017-11-07 | Boston Scientific Scimed, Inc. | Deflectable medical devices |
| US20170333105A1 (en)* | 2016-05-20 | 2017-11-23 | C2 Therapeutics, Inc. | Cryogenic ablation system with rotatable and translatable catheter |
| US9827040B2 (en) | 2002-04-08 | 2017-11-28 | Medtronic Adrian Luxembourg S.a.r.l. | Methods and apparatus for intravascularly-induced neuromodulation |
| US9827039B2 (en) | 2013-03-15 | 2017-11-28 | Boston Scientific Scimed, Inc. | Methods and apparatuses for remodeling tissue of or adjacent to a body passage |
| US9833283B2 (en) | 2013-07-01 | 2017-12-05 | Boston Scientific Scimed, Inc. | Medical devices for renal nerve ablation |
| US9872718B2 (en) | 2012-04-27 | 2018-01-23 | Medtronic Adrian Luxembourg S.a.r.l. | Shafts with pressure relief in cryotherapeutic catheters and associated devices, systems, and methods |
| CN107669332A (en)* | 2013-11-01 | 2018-02-09 | C2治疗公司 | Cryogenic ablation conduit, Handleset and low temperature sacculus ablation system |
| US9895194B2 (en) | 2013-09-04 | 2018-02-20 | Boston Scientific Scimed, Inc. | Radio frequency (RF) balloon catheter having flushing and cooling capability |
| US9907609B2 (en) | 2014-02-04 | 2018-03-06 | Boston Scientific Scimed, Inc. | Alternative placement of thermal sensors on bipolar electrode |
| US9919144B2 (en) | 2011-04-08 | 2018-03-20 | Medtronic Adrian Luxembourg S.a.r.l. | Iontophoresis drug delivery system and method for denervation of the renal sympathetic nerve and iontophoretic drug delivery |
| US9925001B2 (en) | 2013-07-19 | 2018-03-27 | Boston Scientific Scimed, Inc. | Spiral bipolar electrode renal denervation balloon |
| US9943365B2 (en) | 2013-06-21 | 2018-04-17 | Boston Scientific Scimed, Inc. | Renal denervation balloon catheter with ride along electrode support |
| US9956033B2 (en) | 2013-03-11 | 2018-05-01 | Boston Scientific Scimed, Inc. | Medical devices for modulating nerves |
| US9962223B2 (en) | 2013-10-15 | 2018-05-08 | Boston Scientific Scimed, Inc. | Medical device balloon |
| US9974607B2 (en) | 2006-10-18 | 2018-05-22 | Vessix Vascular, Inc. | Inducing desirable temperature effects on body tissue |
| US10004550B2 (en) | 2010-08-05 | 2018-06-26 | Medtronic Ardian Luxembourg S.A.R.L. | Cryoablation apparatuses, systems, and methods for renal neuromodulation |
| US10022182B2 (en) | 2013-06-21 | 2018-07-17 | Boston Scientific Scimed, Inc. | Medical devices for renal nerve ablation having rotatable shafts |
| US10085799B2 (en) | 2011-10-11 | 2018-10-02 | Boston Scientific Scimed, Inc. | Off-wall electrode device and methods for nerve modulation |
| US10098686B2 (en) | 2015-05-15 | 2018-10-16 | Pentax Of America, Inc. | Cryogenic balloon ablation system |
| US10130409B2 (en) | 2013-11-05 | 2018-11-20 | Myoscience, Inc. | Secure cryosurgical treatment system |
| US10265122B2 (en) | 2013-03-15 | 2019-04-23 | Boston Scientific Scimed, Inc. | Nerve ablation devices and related methods of use |
| US10271898B2 (en) | 2013-10-25 | 2019-04-30 | Boston Scientific Scimed, Inc. | Embedded thermocouple in denervation flex circuit |
| US10321946B2 (en) | 2012-08-24 | 2019-06-18 | Boston Scientific Scimed, Inc. | Renal nerve modulation devices with weeping RF ablation balloons |
| US10342609B2 (en) | 2013-07-22 | 2019-07-09 | Boston Scientific Scimed, Inc. | Medical devices for renal nerve ablation |
| US10383676B2 (en) | 2008-11-21 | 2019-08-20 | Pentax Of America, Inc. | Cryogenic ablation system and method |
| US10398464B2 (en) | 2012-09-21 | 2019-09-03 | Boston Scientific Scimed, Inc. | System for nerve modulation and innocuous thermal gradient nerve block |
| US10413357B2 (en) | 2013-07-11 | 2019-09-17 | Boston Scientific Scimed, Inc. | Medical device with stretchable electrode assemblies |
| US10492842B2 (en) | 2014-03-07 | 2019-12-03 | Medtronic Ardian Luxembourg S.A.R.L. | Monitoring and controlling internally administered cryotherapy |
| US10543032B2 (en) | 2014-11-13 | 2020-01-28 | Adagio Medical, Inc. | Pressure modulated cryoablation system and related methods |
| US10549127B2 (en) | 2012-09-21 | 2020-02-04 | Boston Scientific Scimed, Inc. | Self-cooling ultrasound ablation catheter |
| US10588682B2 (en) | 2011-04-25 | 2020-03-17 | Medtronic Ardian Luxembourg S.A.R.L. | Apparatus and methods related to constrained deployment of cryogenic balloons for limited cryogenic ablation of vessel walls |
| US10617459B2 (en) | 2014-04-17 | 2020-04-14 | Adagio Medical, Inc. | Endovascular near critical fluid based cryoablation catheter having plurality of preformed treatment shapes |
| US10660703B2 (en) | 2012-05-08 | 2020-05-26 | Boston Scientific Scimed, Inc. | Renal nerve modulation devices |
| US10660698B2 (en) | 2013-07-11 | 2020-05-26 | Boston Scientific Scimed, Inc. | Devices and methods for nerve modulation |
| US10667854B2 (en) | 2013-09-24 | 2020-06-02 | Adagio Medical, Inc. | Endovascular near critical fluid based cryoablation catheter and related methods |
| US10695124B2 (en) | 2013-07-22 | 2020-06-30 | Boston Scientific Scimed, Inc. | Renal nerve ablation catheter having twist balloon |
| US10709490B2 (en) | 2014-05-07 | 2020-07-14 | Medtronic Ardian Luxembourg S.A.R.L. | Catheter assemblies comprising a direct heating element for renal neuromodulation and associated systems and methods |
| US10722300B2 (en) | 2013-08-22 | 2020-07-28 | Boston Scientific Scimed, Inc. | Flexible circuit having improved adhesion to a renal nerve modulation balloon |
| US10835305B2 (en) | 2012-10-10 | 2020-11-17 | Boston Scientific Scimed, Inc. | Renal nerve modulation devices and methods |
| US10864031B2 (en) | 2015-11-30 | 2020-12-15 | Adagio Medical, Inc. | Ablation method for creating elongate continuous lesions enclosing multiple vessel entries |
| US10888366B2 (en) | 2013-03-15 | 2021-01-12 | Pacira Cryotech, Inc. | Cryogenic blunt dissection methods and devices |
| US10905490B2 (en) | 2012-04-27 | 2021-02-02 | Medtronic Ardian Luxembourg S.A.R.L. | Cryotherapeutic devices for renal neuromodulation and associated systems and methods |
| US10945786B2 (en) | 2013-10-18 | 2021-03-16 | Boston Scientific Scimed, Inc. | Balloon catheters with flexible conducting wires and related methods of use and manufacture |
| US10952790B2 (en) | 2013-09-13 | 2021-03-23 | Boston Scientific Scimed, Inc. | Ablation balloon with vapor deposited cover layer |
| US10959879B2 (en) | 2011-02-01 | 2021-03-30 | Channel Medsystems, Inc. | Methods and apparatus for cryogenic treatment of a body cavity or lumen |
| US11000679B2 (en) | 2014-02-04 | 2021-05-11 | Boston Scientific Scimed, Inc. | Balloon protection and rewrapping devices and related methods of use |
| US11051867B2 (en) | 2015-09-18 | 2021-07-06 | Adagio Medical, Inc. | Tissue contact verification system |
| US11134998B2 (en) | 2017-11-15 | 2021-10-05 | Pacira Cryotech, Inc. | Integrated cold therapy and electrical stimulation systems for locating and treating nerves and associated methods |
| US11202671B2 (en) | 2014-01-06 | 2021-12-21 | Boston Scientific Scimed, Inc. | Tear resistant flex circuit assembly |
| US11246654B2 (en) | 2013-10-14 | 2022-02-15 | Boston Scientific Scimed, Inc. | Flexible renal nerve ablation devices and related methods of use and manufacture |
| US11311327B2 (en) | 2016-05-13 | 2022-04-26 | Pacira Cryotech, Inc. | Methods and systems for locating and treating nerves with cold therapy |
| WO2022261146A1 (en)* | 2021-06-07 | 2022-12-15 | Agil Therapeutics, Inc. | Cryogenic catheter probe, system, and method for selective ablation of mucosa and submucosa of the gastrointestinal tract |
| US11564725B2 (en) | 2017-09-05 | 2023-01-31 | Adagio Medical, Inc. | Ablation catheter having a shape memory stylet |
| WO2023006511A1 (en)* | 2021-07-29 | 2023-02-02 | Medtronic Ireland Manufacturing Unlimited Company | Isolated pressure monitoring for cryogenic balloon catheter |
| US11751930B2 (en) | 2018-01-10 | 2023-09-12 | Adagio Medical, Inc. | Cryoablation element with conductive liner |
| WO2024115107A1 (en)* | 2022-11-30 | 2024-06-06 | Medtronic Ireland Manufacturing Unlimited Company | Temperature-based pressure measurement in cryoballoon ablation catheters |
| WO2024123945A3 (en)* | 2022-12-06 | 2024-07-18 | Agil Therapeutics, Inc. | Cryogenic catheter, system, and method for selective ablation of mucosa and submucosa of the gastrointestinal tract |
| US12201340B2 (en) | 2011-03-25 | 2025-01-21 | Medtronic Cryocath Lp | Spray nozzle design for a catheter |
Families Citing this family (257)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US6602247B2 (en)* | 1997-02-27 | 2003-08-05 | Cryocath Technologies Inc. | Apparatus and method for performing a treatment on a selected tissue region |
| US7591814B2 (en)* | 1997-02-27 | 2009-09-22 | Cryocath Technologies Inc. | Extended treatment zone catheter |
| US7220257B1 (en) | 2000-07-25 | 2007-05-22 | Scimed Life Systems, Inc. | Cryotreatment device and method |
| US5868735A (en)* | 1997-03-06 | 1999-02-09 | Scimed Life Systems, Inc. | Cryoplasty device and method |
| US5902299A (en) | 1997-07-29 | 1999-05-11 | Jayaraman; Swaminathan | Cryotherapy method for reducing tissue injury after balloon angioplasty or stent implantation |
| US6306166B1 (en)* | 1997-08-13 | 2001-10-23 | Scimed Life Systems, Inc. | Loading and release of water-insoluble drugs |
| US6843800B1 (en) | 1998-01-23 | 2005-01-18 | Innercool Therapies, Inc. | Patient temperature regulation method and apparatus |
| US6471717B1 (en) | 1998-03-24 | 2002-10-29 | Innercool Therapies, Inc. | Selective organ cooling apparatus and method |
| US6261312B1 (en) | 1998-06-23 | 2001-07-17 | Innercool Therapies, Inc. | Inflatable catheter for selective organ heating and cooling and method of using the same |
| US6491039B1 (en) | 1998-01-23 | 2002-12-10 | Innercool Therapies, Inc. | Medical procedure |
| US6096068A (en) | 1998-01-23 | 2000-08-01 | Innercool Therapies, Inc. | Selective organ cooling catheter and method of using the same |
| US6585752B2 (en) | 1998-06-23 | 2003-07-01 | Innercool Therapies, Inc. | Fever regulation method and apparatus |
| US6991645B2 (en) | 1998-01-23 | 2006-01-31 | Innercool Therapies, Inc. | Patient temperature regulation method and apparatus |
| US6719779B2 (en) | 2000-11-07 | 2004-04-13 | Innercool Therapies, Inc. | Circulation set for temperature-controlled catheter and method of using the same |
| US6231595B1 (en) | 1998-03-31 | 2001-05-15 | Innercool Therapies, Inc. | Circulating fluid hypothermia method and apparatus |
| US6051019A (en) | 1998-01-23 | 2000-04-18 | Del Mar Medical Technologies, Inc. | Selective organ hypothermia method and apparatus |
| US6464716B1 (en) | 1998-01-23 | 2002-10-15 | Innercool Therapies, Inc. | Selective organ cooling apparatus and method |
| US6251130B1 (en) | 1998-03-24 | 2001-06-26 | Innercool Therapies, Inc. | Device for applications of selective organ cooling |
| US6325818B1 (en) | 1999-10-07 | 2001-12-04 | Innercool Therapies, Inc. | Inflatable cooling apparatus for selective organ hypothermia |
| US6558412B2 (en) | 1998-01-23 | 2003-05-06 | Innercool Therapies, Inc. | Selective organ hypothermia method and apparatus |
| US6312452B1 (en) | 1998-01-23 | 2001-11-06 | Innercool Therapies, Inc. | Selective organ cooling catheter with guidewire apparatus and temperature-monitoring device |
| US6383210B1 (en) | 2000-06-02 | 2002-05-07 | Innercool Therapies, Inc. | Method for determining the effective thermal mass of a body or organ using cooling catheter |
| US6379378B1 (en) | 2000-03-03 | 2002-04-30 | Innercool Therapies, Inc. | Lumen design for catheter |
| US7371254B2 (en) | 1998-01-23 | 2008-05-13 | Innercool Therapies, Inc. | Medical procedure |
| US6576002B2 (en) | 1998-03-24 | 2003-06-10 | Innercool Therapies, Inc. | Isolated selective organ cooling method and apparatus |
| US6599312B2 (en) | 1998-03-24 | 2003-07-29 | Innercool Therapies, Inc. | Isolated selective organ cooling apparatus |
| US6551349B2 (en) | 1998-03-24 | 2003-04-22 | Innercool Therapies, Inc. | Selective organ cooling apparatus |
| US7001378B2 (en) | 1998-03-31 | 2006-02-21 | Innercool Therapies, Inc. | Method and device for performing cooling or cryo-therapies, for, e.g., angioplasty with reduced restenosis or pulmonary vein cell necrosis to inhibit atrial fibrillation employing tissue protection |
| US6602276B2 (en) | 1998-03-31 | 2003-08-05 | Innercool Therapies, Inc. | Method and device for performing cooling- or cryo-therapies for, e.g., angioplasty with reduced restenosis or pulmonary vein cell necrosis to inhibit atrial fibrillation |
| US6905494B2 (en) | 1998-03-31 | 2005-06-14 | Innercool Therapies, Inc. | Method and device for performing cooling- or cryo-therapies for, e.g., angioplasty with reduced restenosis or pulmonary vein cell necrosis to inhibit atrial fibrillation employing tissue protection |
| US7291144B2 (en) | 1998-03-31 | 2007-11-06 | Innercool Therapies, Inc. | Method and device for performing cooling- or cryo-therapies for, e.g., angioplasty with reduced restenosis or pulmonary vein cell necrosis to inhibit atrial fibrillation |
| US6685732B2 (en) | 1998-03-31 | 2004-02-03 | Innercool Therapies, Inc. | Method and device for performing cooling- or cryo-therapies for, e.g., angioplasty with reduced restenosis or pulmonary vein cell necrosis to inhibit atrial fibrillation employing microporous balloon |
| US6338727B1 (en) | 1998-08-13 | 2002-01-15 | Alsius Corporation | Indwelling heat exchange catheter and method of using same |
| US6419643B1 (en)* | 1998-04-21 | 2002-07-16 | Alsius Corporation | Central venous catheter with heat exchange properties |
| US6575933B1 (en)* | 1998-11-30 | 2003-06-10 | Cryocath Technologies Inc. | Mechanical support for an expandable membrane |
| US6241718B1 (en)* | 1998-11-30 | 2001-06-05 | Cryocath Technologies, Inc. | Method for inhibiting restenosis |
| US20050228367A1 (en)* | 1999-01-25 | 2005-10-13 | Marwan Abboud | Leak detection system for catheter based medical device |
| US6468268B1 (en)* | 1999-01-25 | 2002-10-22 | Cryocath Technologies Inc. | Cryogenic catheter system |
| US6869440B2 (en) | 1999-02-09 | 2005-03-22 | Innercool Therapies, Inc. | Method and apparatus for patient temperature control employing administration of anti-shivering agents |
| US6830581B2 (en) | 1999-02-09 | 2004-12-14 | Innercool Therspies, Inc. | Method and device for patient temperature control employing optimized rewarming |
| US6432102B2 (en) | 1999-03-15 | 2002-08-13 | Cryovascular Systems, Inc. | Cryosurgical fluid supply |
| US6468297B1 (en)* | 1999-02-24 | 2002-10-22 | Cryovascular Systems, Inc. | Cryogenically enhanced intravascular interventions |
| US6428534B1 (en) | 1999-02-24 | 2002-08-06 | Cryovascular Systems, Inc. | Cryogenic angioplasty catheter |
| US6648879B2 (en) | 1999-02-24 | 2003-11-18 | Cryovascular Systems, Inc. | Safety cryotherapy catheter |
| US6514245B1 (en) | 1999-03-15 | 2003-02-04 | Cryovascular Systems, Inc. | Safety cryotherapy catheter |
| US7004936B2 (en)* | 2000-08-09 | 2006-02-28 | Cryocor, Inc. | Refrigeration source for a cryoablation catheter |
| US6471694B1 (en) | 2000-08-09 | 2002-10-29 | Cryogen, Inc. | Control system for cryosurgery |
| US6270493B1 (en)* | 1999-07-19 | 2001-08-07 | Cryocath Technologies, Inc. | Cryoablation structure |
| US6575966B2 (en)* | 1999-08-23 | 2003-06-10 | Cryocath Technologies Inc. | Endovascular cryotreatment catheter |
| US7527622B2 (en)* | 1999-08-23 | 2009-05-05 | Cryocath Technologies Inc. | Endovascular cryotreatment catheter |
| US6283959B1 (en)* | 1999-08-23 | 2001-09-04 | Cyrocath Technologies, Inc. | Endovascular cryotreatment catheter |
| US6551274B2 (en)* | 2000-02-29 | 2003-04-22 | Biosense Webster, Inc. | Cryoablation catheter with an expandable cooling chamber |
| US20040220559A1 (en)* | 2000-03-01 | 2004-11-04 | Kramer Hans W. | Preparation of working fluid for use in cryotherapies |
| JP2003524506A (en)* | 2000-03-01 | 2003-08-19 | イナークール セラピーズ インコーポレイテッド | Cryotherapy and device for angioplasty with restenosis |
| US6497703B1 (en) | 2000-03-02 | 2002-12-24 | Biosense Webster | Cryoablation catheter for long lesion ablations |
| US6648906B2 (en) | 2000-04-06 | 2003-11-18 | Innercool Therapies, Inc. | Method and apparatus for regulating patient temperature by irrigating the bladder with a fluid |
| US6592612B1 (en) | 2000-05-04 | 2003-07-15 | Cardeon Corporation | Method and apparatus for providing heat exchange within a catheter body |
| ATE308933T1 (en)* | 2000-06-23 | 2005-11-15 | Cryocath Technologies Inc | DEVICE FOR CRYOTREATMENT |
| US6537271B1 (en)* | 2000-07-06 | 2003-03-25 | Cryogen, Inc. | Balloon cryogenic catheter |
| US6955174B2 (en)* | 2000-08-18 | 2005-10-18 | Uryovascular Systems, Inc. | Cryotherapy method for detecting and treating vulnerable plaque |
| US6602246B1 (en) | 2000-08-18 | 2003-08-05 | Cryovascular Systems, Inc. | Cryotherapy method for detecting and treating vulnerable plaque |
| US20030149368A1 (en)* | 2000-10-24 | 2003-08-07 | Hennemann Willard W. | Method and apparatus for locating and detecting vascular plaque via impedence and conductivity measurements, and for cryogenically passivating vascular plaque and inhibiting vascular plaque progression and rupture |
| US6673066B2 (en)* | 2000-11-10 | 2004-01-06 | Cardiostream, Inc. | Apparatus and method to diagnose and treat vulnerable plaque |
| US6719723B2 (en) | 2000-12-06 | 2004-04-13 | Innercool Therapies, Inc. | Multipurpose catheter assembly |
| US6450987B1 (en) | 2001-02-01 | 2002-09-17 | Innercool Therapies, Inc. | Collapsible guidewire lumen |
| US6666858B2 (en)* | 2001-04-12 | 2003-12-23 | Scimed Life Systems, Inc. | Cryo balloon for atrial ablation |
| US6786900B2 (en)* | 2001-08-13 | 2004-09-07 | Cryovascular Systems, Inc. | Cryotherapy methods for treating vessel dissections and side branch occlusion |
| WO2003015672A1 (en) | 2001-08-15 | 2003-02-27 | Innercool Therapies, Inc. | Method and apparatus for patient temperature control employing administration of anti-shivering |
| SE524399C2 (en) | 2001-09-07 | 2004-08-03 | Synergio Ag | A system for delivering a self-expanding medical device into a body vessel |
| US6929656B1 (en)* | 2001-09-14 | 2005-08-16 | Medcool, Inc. | Method and device for reducing secondary brain injury |
| US6767346B2 (en)* | 2001-09-20 | 2004-07-27 | Endocare, Inc. | Cryosurgical probe with bellows shaft |
| AU2002336764B2 (en)* | 2001-09-24 | 2007-03-29 | Boston Scientific Limited | Optimized dosing for drug coated stents |
| US7912554B2 (en)* | 2001-09-26 | 2011-03-22 | Medtronic Cryocath Lp | Method for treatment of aneurysms |
| US20040249372A1 (en)* | 2001-09-26 | 2004-12-09 | Leonilda Capuano | Method for treatment of aneurysms |
| US6736809B2 (en) | 2001-09-26 | 2004-05-18 | Cryocath Technologies Inc. | Method and device for treatment of aneurysms |
| WO2003026719A2 (en)* | 2001-09-27 | 2003-04-03 | Galil Medical Ltd. | Cryoplasty apparatus and method |
| CA2461627A1 (en)* | 2001-09-27 | 2003-04-03 | Roni Zvuloni | Apparatus and method for cryosurgical treatment of tumors of the breast |
| US6579287B2 (en)* | 2001-10-09 | 2003-06-17 | Cryocath Technologies Inc. | Cryosurgical ablation device having sequential injection and method therefor |
| US6800068B1 (en) | 2001-10-26 | 2004-10-05 | Radiant Medical, Inc. | Intra-aortic balloon counterpulsation with concurrent hypothermia |
| US7144418B1 (en)* | 2001-11-02 | 2006-12-05 | Medcool, Inc. | Method, and system for selective cerebral hypothermia |
| US7018346B2 (en) | 2001-12-18 | 2006-03-28 | Scimed Life Systems, Inc. | Guide wire with adjustable flexibility |
| US6709431B2 (en) | 2001-12-18 | 2004-03-23 | Scimed Life Systems, Inc. | Cryo-temperature monitoring |
| US7156867B2 (en) | 2001-12-31 | 2007-01-02 | Medcool, Inc. | Uniform selective cerebral hypothermia |
| US7241307B2 (en)* | 2001-12-31 | 2007-07-10 | Medcool, Inc. | Method and apparatus for managing temperature in a patient |
| US6692487B2 (en)* | 2002-01-23 | 2004-02-17 | Endocare, Inc. | Cryosurgical monitoring system |
| US6884342B2 (en)* | 2002-02-28 | 2005-04-26 | Bioprocess Technologies Ltd. | Wastewater trickle tower biomedia strands arrangement |
| US6989009B2 (en)* | 2002-04-19 | 2006-01-24 | Scimed Life Systems, Inc. | Cryo balloon |
| US20040024392A1 (en)* | 2002-08-05 | 2004-02-05 | Lewis James D. | Apparatus and method for cryosurgery |
| US6955673B2 (en)* | 2002-08-16 | 2005-10-18 | Cryocor, Inc. | Heat transfer segment for a cryoablation catheter |
| US6929639B2 (en)* | 2002-08-30 | 2005-08-16 | Scimed Life Systems, Inc. | Cryo ablation coil |
| US6918869B2 (en) | 2002-12-02 | 2005-07-19 | Scimed Life Systems | System for administering a combination of therapies to a body lumen |
| US7378048B2 (en)* | 2002-12-03 | 2008-05-27 | Boston Scientific Scimed, Inc. | Method for forming catheter curves |
| US6893433B2 (en)* | 2002-12-11 | 2005-05-17 | Cryocor, Inc. | System and method for performing a single step cryoablation |
| US7195625B2 (en)* | 2002-12-11 | 2007-03-27 | Cryocor, Inc. | Catheter system for performing a single step cryoablation |
| US6796979B2 (en)* | 2002-12-11 | 2004-09-28 | Cryocor, Inc. | Coaxial catheter system for performing a single step cryoablation |
| US20040167466A1 (en)* | 2003-02-21 | 2004-08-26 | Drasler William J. | Delivering cooled fluid to sites inside the body |
| US20040167467A1 (en)* | 2003-02-21 | 2004-08-26 | Kent Harrison | Delivering cooled fluid to sites inside the body |
| US7758623B2 (en)* | 2003-03-17 | 2010-07-20 | The Board Of Trustees Of The Leland Stanford Junior University | Transesophageal heat exchange catheter for cooling of the heart |
| US20040204705A1 (en) | 2003-04-10 | 2004-10-14 | Scimed Life Systems, Inc. | Cryotreatment devices and methods of forming conduction blocks |
| US20040215177A1 (en)* | 2003-04-24 | 2004-10-28 | Scimed Life Systems, Inc. | Therapeutic apparatus having insulated region at the insertion area |
| US20040226556A1 (en) | 2003-05-13 | 2004-11-18 | Deem Mark E. | Apparatus for treating asthma using neurotoxin |
| US7072460B2 (en)* | 2003-05-27 | 2006-07-04 | Vtech Telecommunications Limited | System and method for retrieving telephone numbers |
| US7060062B2 (en) | 2003-06-04 | 2006-06-13 | Cryo Vascular Systems, Inc. | Controllable pressure cryogenic balloon treatment system and method |
| US11877781B2 (en)* | 2003-06-25 | 2024-01-23 | Varian Medical Systems, Inc. | Cryosurgical probe with adjustable sliding apparatus |
| US20040267338A1 (en)* | 2003-06-25 | 2004-12-30 | Kent Harrison | Targeted tissue cooling within a body |
| US7794454B2 (en)* | 2003-07-11 | 2010-09-14 | Medtronic Cryocath Lp | Method and device for epicardial ablation |
| US7326195B2 (en)* | 2003-11-18 | 2008-02-05 | Boston Scientific Scimed, Inc. | Targeted cooling of tissue within a body |
| US7157213B2 (en)* | 2004-03-01 | 2007-01-02 | Think Laboratory Co., Ltd. | Developer agent for positive type photosensitive compound |
| US7582083B2 (en) | 2004-05-10 | 2009-09-01 | Boston Scientific Scimed, Inc. | Probe based low temperature lesion formation apparatus, systems and methods |
| US7291142B2 (en)* | 2004-05-10 | 2007-11-06 | Boston Scientific Scimed, Inc. | Low temperature lesion formation apparatus, systems and methods |
| US7288088B2 (en) | 2004-05-10 | 2007-10-30 | Boston Scientific Scimed, Inc. | Clamp based low temperature lesion formation apparatus, systems and methods |
| US8177779B2 (en)* | 2004-06-02 | 2012-05-15 | Boston Scientific Scimed, Inc. | Controllable pressure cryogenic balloon treatment system and method |
| US7537580B2 (en)* | 2004-06-23 | 2009-05-26 | Boston Scientific Scimed, Inc. | Intravascular dilatation infusion catheter |
| US7156840B2 (en)* | 2004-06-29 | 2007-01-02 | Cryocor, Inc. | Pressure monitor for cryoablation catheter |
| US7163535B2 (en)* | 2004-06-30 | 2007-01-16 | Cryocor, Inc. | System for detecting leaks and occlusions in a cryoablation catheter |
| FR2873324B1 (en)* | 2004-07-22 | 2006-10-27 | Bic Sa Soc | LIQUID SUPPLY UNIT FOR A LIQUID PROJECTION INSTRUMENT AND A LIQUID PROJECTION INSTRUMENT COMPRISING SUCH A LIQUID SUPPLY |
| US20060025840A1 (en)* | 2004-08-02 | 2006-02-02 | Martin Willard | Cooling tissue inside the body |
| US8672988B2 (en)* | 2004-10-22 | 2014-03-18 | Medtronic Cryocath Lp | Method and device for local cooling within an organ using an intravascular device |
| US7828790B2 (en) | 2004-12-03 | 2010-11-09 | Boston Scientific Scimed, Inc. | Selectively flexible catheter and method of use |
| US7604631B2 (en)* | 2004-12-15 | 2009-10-20 | Boston Scientific Scimed, Inc. | Efficient controlled cryogenic fluid delivery into a balloon catheter and other treatment devices |
| US8206345B2 (en) | 2005-03-07 | 2012-06-26 | Medtronic Cryocath Lp | Fluid control system for a medical device |
| US7674256B2 (en)* | 2005-03-17 | 2010-03-09 | Boston Scientific Scimed, Inc. | Treating internal body tissue |
| US20060258981A1 (en)* | 2005-04-27 | 2006-11-16 | Tracee Eidenschink | Balloon catheter with perfusion lumen |
| US7740627B2 (en)* | 2005-04-29 | 2010-06-22 | Medtronic Cryocath Lp | Surgical method and apparatus for treating atrial fibrillation |
| US7794455B2 (en)* | 2005-04-29 | 2010-09-14 | Medtronic Cryocath Lp | Wide area ablation of myocardial tissue |
| US7442190B2 (en) | 2005-05-13 | 2008-10-28 | Cryocath Technologies Inc. | Contact assessment of balloon catheters |
| US7727191B2 (en)* | 2005-05-13 | 2010-06-01 | Medtronic Cryocath Lp | Compliant balloon catheter |
| US20060270981A1 (en)* | 2005-05-13 | 2006-11-30 | Leonilda Capuano | Coiled injection tube |
| US8992515B2 (en)* | 2005-05-13 | 2015-03-31 | Medtronic Cryocath Lp | Coolant injection tube |
| US7963940B2 (en)* | 2005-08-22 | 2011-06-21 | Boston Scientific Scimed, Inc. | Local perfusion device |
| US7625368B2 (en)* | 2005-10-17 | 2009-12-01 | Galil Medical Ltd. | Endometrial ablation device and method |
| DE102005050344A1 (en) | 2005-10-20 | 2007-05-03 | Siemens Ag | Cryocatheter for medical investigation and treatment equipment for e.g. diagnosis and treatment of heart infarcts, has image capture device that maps region of vessel around balloon arranged near catheter tip |
| US20080051775A1 (en)* | 2005-11-23 | 2008-02-28 | Evans David K | Multi-modal analgesia delivery system ("MADS") |
| US20070129682A1 (en)* | 2005-12-02 | 2007-06-07 | Tracee Eidenschink | Guidewire with perfusion capability |
| JP2007167127A (en)* | 2005-12-19 | 2007-07-05 | Atsuo Mori | Catheter for topical cooling |
| JP5199118B2 (en) | 2005-12-22 | 2013-05-15 | ザ・トラスティーズ・オブ・コロンビア・ユニバーシティ・イン・ザ・シティ・オブ・ニューヨーク | Intravascular cooling system and method |
| US8147453B2 (en) | 2006-03-13 | 2012-04-03 | Applied Medical Resources Corporation | Balloon trocar |
| US8287503B2 (en)* | 2006-03-13 | 2012-10-16 | Applied Medical Resources Corporation | Balloon trocar |
| US20070219585A1 (en)* | 2006-03-14 | 2007-09-20 | Cornet Douglas A | System for administering reduced pressure treatment having a manifold with a primary flow passage and a blockage prevention member |
| US20080097251A1 (en)* | 2006-06-15 | 2008-04-24 | Eilaz Babaev | Method and apparatus for treating vascular obstructions |
| US20070299433A1 (en)* | 2006-06-27 | 2007-12-27 | C2 Therapeutics | Barrett's Esophagus Cryogenic Ablation System |
| CA2775940A1 (en)* | 2006-07-03 | 2008-01-10 | Hemoteq Ag | Catheter balloon with polymeric coating |
| KR101198980B1 (en)* | 2007-01-21 | 2012-11-07 | 헤모텍 아게 | Medical product for treating stenosis of body passages and for preventing threatening restenosis |
| US20080312644A1 (en)* | 2007-06-14 | 2008-12-18 | Boston Scientific Scimed, Inc. | Cryogenic balloon ablation instruments and systems |
| US9192697B2 (en) | 2007-07-03 | 2015-11-24 | Hemoteq Ag | Balloon catheter for treating stenosis of body passages and for preventing threatening restenosis |
| US9717501B2 (en)* | 2007-11-21 | 2017-08-01 | St. Jude Medical, Atrial Fibrillation Division, Inc. | Methods and systems for occluding vessels during cardiac ablation including optional electroanatomical guidance |
| US8579889B2 (en) | 2008-01-11 | 2013-11-12 | Boston Scientific Scimed Inc. | Linear ablation devices and methods of use |
| US8235977B2 (en) | 2008-01-11 | 2012-08-07 | Boston Scientific Scimed, Inc. | Ablation devices and methods of use |
| US8483831B1 (en) | 2008-02-15 | 2013-07-09 | Holaira, Inc. | System and method for bronchial dilation |
| US8469919B2 (en)* | 2008-02-19 | 2013-06-25 | Boston Scientific Scimed, Inc. | Apparatus and methods for uniformly distributing coolant within a cryo-ablation device |
| WO2009114701A1 (en)* | 2008-03-13 | 2009-09-17 | Boston Scientific Scimed, Inc. | Cryo-ablation refrigerant distribution catheter |
| JP2011519699A (en) | 2008-05-09 | 2011-07-14 | インノブアトイブエ プルモナルイ ソルウトイオンス,インコーポレイティッド | Systems, assemblies and methods for treatment of bronchial trees |
| US8187261B2 (en) | 2008-05-29 | 2012-05-29 | Boston Scientific Scimed, Inc. | Regulating internal pressure of a cryotherapy balloon catheter |
| EP2147662A1 (en)* | 2008-07-23 | 2010-01-27 | Abbott Laboratories Vascular Enterprises Limited | Stent delivery system |
| WO2010019481A1 (en) | 2008-08-11 | 2010-02-18 | Conceptx Medical, Inc. | Systems and methods for treating dyspnea, including via electrical afferent signal blocking |
| US8845627B2 (en)* | 2008-08-22 | 2014-09-30 | Boston Scientific Scimed, Inc. | Regulating pressure to lower temperature in a cryotherapy balloon catheter |
| US8465481B2 (en)* | 2008-10-20 | 2013-06-18 | Boston Scientific Scimed, Inc. | Providing cryotherapy with a balloon catheter having a non-uniform thermal profile |
| CA2746114C (en) | 2008-12-23 | 2016-03-22 | Cryomedix Llc | Isotherm-based tissue ablation control system and method |
| JP5245842B2 (en)* | 2009-01-09 | 2013-07-24 | 株式会社カネカ | Balloon catheter having a detachable balloon inflation liquid injection tube |
| WO2010093603A1 (en) | 2009-02-11 | 2010-08-19 | Boston Scientific Scimed, Inc. | Insulated ablation catheter devices and methods of use |
| US9301871B2 (en) | 2009-02-26 | 2016-04-05 | Advanced Cooling Therapy, Inc. | Devices and methods for controlling patient temperature |
| US9326890B2 (en) | 2009-02-26 | 2016-05-03 | Advanced Cooling Therapy, Inc. | Devices and methods for controlling patient temperature |
| US9622909B2 (en) | 2009-02-26 | 2017-04-18 | Advanced Cooling Therapy, Inc. | Devices and methods for controlling patient temperature |
| WO2010111122A1 (en)* | 2009-03-23 | 2010-09-30 | Boston Scientific Scimed, Inc. | Systems apparatus for distributing coolant within a cryo-ablation device |
| CN102387755A (en)* | 2009-04-06 | 2012-03-21 | 克莱米迪克斯有限责任公司 | Single phase liquid refrigerant cryoablation system with multitubular distal section and related method |
| US8888768B2 (en)* | 2009-04-30 | 2014-11-18 | Cryomedix, Llc | Cryoablation system having docking station for charging cryogen containers and related method |
| EP3106116B1 (en) | 2009-06-30 | 2018-08-01 | Boston Scientific Scimed, Inc. | Map and ablate open irrigated hybrid catheter |
| US10369256B2 (en) | 2009-07-10 | 2019-08-06 | Boston Scientific Scimed, Inc. | Use of nanocrystals for drug delivery from a balloon |
| EP2453938B1 (en)* | 2009-07-17 | 2015-08-19 | Boston Scientific Scimed, Inc. | Nucleation of drug delivery balloons to provide improved crystal size and density |
| US20110092967A1 (en)* | 2009-10-21 | 2011-04-21 | Medtronic Cryocath Lp | Deflation mechanism for a medical device |
| WO2011056684A2 (en) | 2009-10-27 | 2011-05-12 | Innovative Pulmonary Solutions, Inc. | Delivery devices with coolable energy emitting assemblies |
| US8911439B2 (en) | 2009-11-11 | 2014-12-16 | Holaira, Inc. | Non-invasive and minimally invasive denervation methods and systems for performing the same |
| WO2011060200A1 (en) | 2009-11-11 | 2011-05-19 | Innovative Pulmonary Solutions, Inc. | Systems, apparatuses, and methods for treating tissue and controlling stenosis |
| US20110263921A1 (en) | 2009-12-31 | 2011-10-27 | Anthony Vrba | Patterned Denervation Therapy for Innervated Renal Vasculature |
| US20110270238A1 (en) | 2009-12-31 | 2011-11-03 | Raed Rizq | Compliant Cryoballoon Apparatus for Denervating Ostia of the Renal Arteries |
| US9089314B2 (en)* | 2010-01-27 | 2015-07-28 | Medtronic Cryocath Lp | Partially compliant balloon device |
| US8926602B2 (en)* | 2010-01-28 | 2015-01-06 | Medtronic Cryocath Lp | Triple balloon catheter |
| US9445859B2 (en) | 2010-01-29 | 2016-09-20 | Medtronic Cryocath Lp | Multifunctional ablation device |
| US9931152B2 (en)* | 2010-07-27 | 2018-04-03 | Medtronic Cryocath Lp | Dual injection tube cryocatheter and method for using same |
| US20120029512A1 (en) | 2010-07-30 | 2012-02-02 | Willard Martin R | Balloon with surface electrodes and integral cooling for renal nerve ablation |
| CN103118613A (en) | 2010-08-26 | 2013-05-22 | 克莱米迪克斯有限责任公司 | Cryoablation balloon catheter and related method |
| US8889211B2 (en) | 2010-09-02 | 2014-11-18 | Boston Scientific Scimed, Inc. | Coating process for drug delivery balloons using heat-induced rewrap memory |
| US9060754B2 (en) | 2010-10-26 | 2015-06-23 | Medtronic Ardian Luxembourg S.A.R.L. | Neuromodulation cryotherapeutic devices and associated systems and methods |
| EP2632372A4 (en) | 2010-10-27 | 2015-04-01 | Cryomedix Llc | Cryoablation apparatus with enhanced heat exchange area and related method |
| EP4032486A1 (en) | 2010-11-16 | 2022-07-27 | TVA Medical, Inc. | Devices for forming a fistula |
| US11246653B2 (en)* | 2010-12-07 | 2022-02-15 | Boaz Avitall | Catheter systems for cardiac arrhythmia ablation |
| US9089340B2 (en) | 2010-12-30 | 2015-07-28 | Boston Scientific Scimed, Inc. | Ultrasound guided tissue ablation |
| CN103781443B (en) | 2011-04-13 | 2016-07-06 | 低温疗法有限公司 | Plaque stabilization using cryogenic energy |
| WO2012166239A1 (en) | 2011-06-01 | 2012-12-06 | Boston Scientific Scimed, Inc. | Ablation probe with ultrasonic imaging capabilities |
| US9084592B2 (en) | 2011-07-11 | 2015-07-21 | C2 Therapeutics, Inc. | Focal ablation assembly |
| EP2731529B1 (en)* | 2011-07-11 | 2018-09-05 | Pentax of America, Inc. | Focal ablation assembly |
| US20130018369A1 (en)* | 2011-07-13 | 2013-01-17 | Medtronic Cryocath Lp | Systems and methods for treatment in proximity to sensitive tissue structures |
| US9387031B2 (en) | 2011-07-29 | 2016-07-12 | Medtronic Ablation Frontiers Llc | Mesh-overlayed ablation and mapping device |
| US8669360B2 (en) | 2011-08-05 | 2014-03-11 | Boston Scientific Scimed, Inc. | Methods of converting amorphous drug substance into crystalline form |
| US9056152B2 (en) | 2011-08-25 | 2015-06-16 | Boston Scientific Scimed, Inc. | Medical device with crystalline drug coating |
| US8888692B1 (en) | 2011-08-26 | 2014-11-18 | Applied Medical Resources Corporation | Trocar cannula assembly and method of manufacture |
| WO2013040201A2 (en) | 2011-09-14 | 2013-03-21 | Boston Scientific Scimed, Inc. | Ablation device with multiple ablation modes |
| US9603659B2 (en) | 2011-09-14 | 2017-03-28 | Boston Scientific Scimed Inc. | Ablation device with ionically conductive balloon |
| JP2015506209A (en) | 2011-12-28 | 2015-03-02 | ボストン サイエンティフィック サイムド,インコーポレイテッドBoston Scientific Scimed,Inc. | Ablation probe and ablation and ultrasound imaging system |
| JP2015506234A (en) | 2012-01-10 | 2015-03-02 | ボストン サイエンティフィック サイムド,インコーポレイテッドBoston Scientific Scimed,Inc. | Electrophysiology system |
| US9220556B2 (en)* | 2012-01-27 | 2015-12-29 | Medtronic Cryocath Lp | Balloon design to enhance cooling uniformity |
| EP2809253B8 (en) | 2012-01-31 | 2016-09-21 | Boston Scientific Scimed, Inc. | Ablation probe with fluid-based acoustic coupling for ultrasonic tissue imaging |
| JP2015097548A (en)* | 2012-03-09 | 2015-05-28 | テルモ株式会社 | Balloon catheter |
| WO2013163322A1 (en) | 2012-04-24 | 2013-10-31 | Cibiem, Inc. | Endovascular catheters and methods for carotid body ablation |
| US20130304180A1 (en)* | 2012-05-09 | 2013-11-14 | Michael L. Green | Catheter having dual balloon hydraulic actuator |
| US9011513B2 (en) | 2012-05-09 | 2015-04-21 | Abbott Cardiovascular Systems Inc. | Catheter having hydraulic actuator |
| WO2013181660A1 (en)* | 2012-06-01 | 2013-12-05 | Cibiem, Inc. | Methods and devices for cryogenic carotid body ablation |
| US9113911B2 (en) | 2012-09-06 | 2015-08-25 | Medtronic Ablation Frontiers Llc | Ablation device and method for electroporating tissue cells |
| US9211156B2 (en) | 2012-09-18 | 2015-12-15 | Boston Scientific Scimed, Inc. | Map and ablate closed-loop cooled ablation catheter with flat tip |
| US9370329B2 (en) | 2012-09-18 | 2016-06-21 | Boston Scientific Scimed, Inc. | Map and ablate closed-loop cooled ablation catheter |
| US20140088584A1 (en)* | 2012-09-26 | 2014-03-27 | Boston Scientific Scimed, Inc. | Medical device balloon catheter |
| WO2014055514A2 (en) | 2012-10-01 | 2014-04-10 | C.R. Bard, Inc. | Balloon catheter having multiple inflation lumens and related methods |
| US9486276B2 (en) | 2012-10-11 | 2016-11-08 | Tva Medical, Inc. | Devices and methods for fistula formation |
| ES2717013T3 (en)* | 2012-10-30 | 2019-06-18 | Nitro Medical Ltd | Apparatus and probe for a cryogenic system |
| US9095321B2 (en) | 2012-11-21 | 2015-08-04 | Medtronic Ardian Luxembourg S.A.R.L. | Cryotherapeutic devices having integral multi-helical balloons and methods of making the same |
| US9017317B2 (en) | 2012-12-06 | 2015-04-28 | Medtronic Ardian Luxembourg S.A.R.L. | Refrigerant supply system for cryotherapy including refrigerant recompression and associated devices, systems, and methods |
| US9398933B2 (en) | 2012-12-27 | 2016-07-26 | Holaira, Inc. | Methods for improving drug efficacy including a combination of drug administration and nerve modulation |
| US9522030B2 (en)* | 2013-01-23 | 2016-12-20 | Medtronic Cryocath Lp | Purge phase for cryoablation systems |
| US10039586B2 (en) | 2013-02-26 | 2018-08-07 | Cpsi Holdings Llc | Ablation probe with deployable sensors |
| US10420662B2 (en) | 2013-03-12 | 2019-09-24 | Abbott Cardiovascular Systems Inc. | Catheter having movable tubular structure and proximal stopper |
| US10531971B2 (en) | 2013-03-12 | 2020-01-14 | Abbott Cardiovascular System Inc. | Balloon catheter having hydraulic actuator |
| US9283101B2 (en) | 2013-03-12 | 2016-03-15 | Abbott Cardiovascular Systems Inc. | Catheter having hydraulic actuator and locking system |
| CN105228683B (en) | 2013-03-14 | 2022-06-10 | Tva医疗公司 | Fistula-forming device and method for forming fistula |
| KR102301914B1 (en) | 2013-03-15 | 2021-09-15 | 어플라이드 메디컬 리소시스 코포레이션 | Trocar cannula assembly with low profile insertion configuration and method of manufacture |
| US9895183B2 (en) | 2013-09-17 | 2018-02-20 | Channel Medsystems, Inc. | Liner for cryogenic treatment systems |
| WO2015048806A2 (en) | 2013-09-30 | 2015-04-02 | Nidus Medical, Llc | Apparatus and methods for treating rhinitis |
| CA2925946A1 (en) | 2013-11-06 | 2015-05-14 | Cryotherapeutics Gmbh | Catheter for plaque stablisation |
| US12114916B2 (en)* | 2013-12-12 | 2024-10-15 | Nuvaira, Inc. | Catheter and handle assembly, systems, and methods |
| EP3116408B1 (en) | 2014-03-12 | 2018-12-19 | Cibiem, Inc. | Ultrasound ablation catheter |
| US10695534B2 (en) | 2014-03-14 | 2020-06-30 | Tva Medical, Inc. | Fistula formation devices and methods therefor |
| US10610279B2 (en) | 2014-04-10 | 2020-04-07 | Channel Medsystems, Inc. | Apparatus and methods for regulating cryogenic treatment |
| US9763743B2 (en) | 2014-07-25 | 2017-09-19 | Arrinex, Inc. | Apparatus and method for treating rhinitis |
| WO2016033374A1 (en) | 2014-08-27 | 2016-03-03 | Tva Medical, Inc. | Cryolipopysis devices and methods therefor |
| JP2017529169A (en) | 2014-10-13 | 2017-10-05 | ボストン サイエンティフィック サイムド,インコーポレイテッドBoston Scientific Scimed,Inc. | Tissue diagnosis and treatment using mini-electrodes |
| WO2016065337A1 (en) | 2014-10-24 | 2016-04-28 | Boston Scientific Scimed Inc. | Medical devices with a flexible electrode assembly coupled to an ablation tip |
| US9743854B2 (en) | 2014-12-18 | 2017-08-29 | Boston Scientific Scimed, Inc. | Real-time morphology analysis for lesion assessment |
| US10603040B1 (en) | 2015-02-09 | 2020-03-31 | Tva Medical, Inc. | Methods for treating hypertension and reducing blood pressure with formation of fistula |
| JP7219090B2 (en) | 2016-01-15 | 2023-02-07 | ティーブイエー メディカル, インコーポレイテッド | Systems and methods for gluing vessels |
| CN114042224B (en) | 2016-01-15 | 2024-09-17 | Tva医疗公司 | Device and method for advancing a wire |
| US10874422B2 (en) | 2016-01-15 | 2020-12-29 | Tva Medical, Inc. | Systems and methods for increasing blood flow |
| WO2017124062A1 (en) | 2016-01-15 | 2017-07-20 | Tva Medical, Inc. | Devices and methods for forming a fistula |
| EP3413822B1 (en) | 2016-02-11 | 2023-08-30 | Arrinex, Inc. | Device for image guided post-nasal nerve ablation |
| CN109600988B (en) | 2016-06-15 | 2021-08-31 | 阿里内克斯股份有限公司 | Devices and methods for treating the lateral surface of the nasal cavity |
| US10939965B1 (en) | 2016-07-20 | 2021-03-09 | Arrinex, Inc. | Devices and methods for treating a nerve of the nasal cavity using image guidance |
| CN109982652B (en) | 2016-09-25 | 2022-08-05 | Tva医疗公司 | Vascular stent device and method |
| US11253312B2 (en) | 2016-10-17 | 2022-02-22 | Arrinex, Inc. | Integrated nasal nerve detector ablation-apparatus, nasal nerve locator, and methods of use |
| EP3614940B1 (en) | 2017-04-28 | 2024-11-20 | Arrinex, Inc. | Systems for locating blood vessels in the treatment of rhinitis |
| US11684403B2 (en)* | 2018-08-21 | 2023-06-27 | Boston Scientific Scimed, Inc. | System and method for inflating a cryoablation balloon catheter |
| WO2020092593A1 (en)* | 2018-11-01 | 2020-05-07 | Theranova, Llc | Devices and methods for treating the prostate |
| CN110464444B (en)* | 2019-08-14 | 2023-03-31 | 心诺普医疗技术(北京)有限公司 | Temperature-controllable cryoablation system |
| US12233219B2 (en)* | 2019-11-08 | 2025-02-25 | Hollister Incorporated | Methods of making sleeved and packaged hydrophilic catheter assemblies |
| CN111281527A (en)* | 2020-01-13 | 2020-06-16 | 珠海大横琴科技发展有限公司 | cryoablation catheter |
| US20220192738A1 (en)* | 2020-12-22 | 2022-06-23 | Biosense Webster (Israel) Ltd. | Balloon Catheter with Irrigated Tip Electrode |
| EP4268744A1 (en)* | 2022-04-27 | 2023-11-01 | Cryovasc GmbH | Medical cryotherapy device with heat transfer body |
| US20240225712A9 (en)* | 2022-10-20 | 2024-07-11 | Ryme Medical, Inc. | Cryotherapeutic systems and methods for targeted lung neuromodulation therapies |
| WO2024260683A1 (en)* | 2023-06-21 | 2024-12-26 | Medtronic Ireland Manufacturing Unlimted Company | Fluid flow distribution in occlusive denervation catheter balloons |
| CN116687495B (en)* | 2023-07-24 | 2023-11-03 | 上海宏普医疗器械有限公司 | Implanted saccule |
Citations (34)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US3901241A (en)* | 1973-05-31 | 1975-08-26 | Al Corp Du | Disposable cryosurgical instrument |
| US4336691A (en)* | 1979-12-13 | 1982-06-29 | The Board Of Trustees Of The Leland Stanford Junior University | Cryojet rapid freezing apparatus |
| US4754752A (en)* | 1986-07-28 | 1988-07-05 | Robert Ginsburg | Vascular catheter |
| US5019075A (en)* | 1984-10-24 | 1991-05-28 | The Beth Israel Hospital | Method and apparatus for angioplasty |
| US5041089A (en)* | 1987-12-11 | 1991-08-20 | Devices For Vascular Intervention, Inc. | Vascular dilation catheter construction |
| US5076713A (en)* | 1990-01-17 | 1991-12-31 | Nippon Thompson Co., Ltd. | Linear motion rolling guide unit made of resin |
| US5092641A (en)* | 1991-05-09 | 1992-03-03 | Brammal, Inc. | Cable locking and sealing device |
| US5106360A (en)* | 1987-09-17 | 1992-04-21 | Olympus Optical Co., Ltd. | Thermotherapeutic apparatus |
| US5147355A (en)* | 1988-09-23 | 1992-09-15 | Brigham And Womens Hospital | Cryoablation catheter and method of performing cryoablation |
| US5151100A (en)* | 1988-10-28 | 1992-09-29 | Boston Scientific Corporation | Heating catheters |
| US5190589A (en)* | 1988-04-18 | 1993-03-02 | Cedona Pharmaceuticals B.V. | Compounds and process preparing a substituted or an unsubstituted 4(5)-(ω-aminoalkyl)imidazole |
| US5191683A (en)* | 1990-12-12 | 1993-03-09 | Nhk Spring Co., Ltd. | Hose band |
| US5196024A (en)* | 1990-07-03 | 1993-03-23 | Cedars-Sinai Medical Center | Balloon catheter with cutting edge |
| US5275595A (en)* | 1992-07-06 | 1994-01-04 | Dobak Iii John D | Cryosurgical instrument |
| US5458612A (en)* | 1994-01-06 | 1995-10-17 | Origin Medsystems, Inc. | Prostatic ablation method and apparatus for perineal approach |
| US5486208A (en)* | 1993-02-10 | 1996-01-23 | Ginsburg; Robert | Method and apparatus for controlling a patient's body temperature by in situ blood temperature modification |
| US5501681A (en)* | 1993-11-12 | 1996-03-26 | Neuwirth; Robert S. | Intrauterine cryoablation cauterizing apparatus and method |
| US5520682A (en)* | 1991-09-06 | 1996-05-28 | Cryomedical Sciences, Inc. | Cryosurgical instrument with vent means and method using same |
| US5545195A (en)* | 1994-08-01 | 1996-08-13 | Boston Scientific Corporation | Interstitial heating of tissue |
| US5617739A (en)* | 1995-03-29 | 1997-04-08 | Mmr Technologies, Inc. | Self-cleaning low-temperature refrigeration system |
| US5624392A (en)* | 1990-05-11 | 1997-04-29 | Saab; Mark A. | Heat transfer catheters and methods of making and using same |
| US5644502A (en)* | 1995-05-04 | 1997-07-01 | Mmr Technologies, Inc. | Method for efficient counter-current heat exchange using optimized mixtures |
| US5733280A (en)* | 1995-11-15 | 1998-03-31 | Avitall; Boaz | Cryogenic epicardial mapping and ablation |
| US5868735A (en)* | 1997-03-06 | 1999-02-09 | Scimed Life Systems, Inc. | Cryoplasty device and method |
| US5902299A (en)* | 1997-07-29 | 1999-05-11 | Jayaraman; Swaminathan | Cryotherapy method for reducing tissue injury after balloon angioplasty or stent implantation |
| US6125096A (en)* | 1998-04-08 | 2000-09-26 | Stomage Technology Comporation | Dynamic/stationary tape guide |
| US6235019B1 (en)* | 1997-02-27 | 2001-05-22 | Cryocath Technologies, Inc. | Cryosurgical catheter |
| US6241716B1 (en)* | 1996-03-13 | 2001-06-05 | Sca Hygiene Products Aktiebolag | Waist belt for absorbent articles |
| US6283959B1 (en)* | 1999-08-23 | 2001-09-04 | Cyrocath Technologies, Inc. | Endovascular cryotreatment catheter |
| US6575966B2 (en)* | 1999-08-23 | 2003-06-10 | Cryocath Technologies Inc. | Endovascular cryotreatment catheter |
| US6624392B2 (en)* | 2001-07-11 | 2003-09-23 | Acerne Enterprises, Llc | Multifunctional cooking system |
| US6685732B2 (en)* | 1998-03-31 | 2004-02-03 | Innercool Therapies, Inc. | Method and device for performing cooling- or cryo-therapies for, e.g., angioplasty with reduced restenosis or pulmonary vein cell necrosis to inhibit atrial fibrillation employing microporous balloon |
| US6786901B2 (en)* | 1999-03-15 | 2004-09-07 | Cryovascular Systems, Inc. | Cryosurgical fluid supply |
| US20050245943A1 (en)* | 2001-09-27 | 2005-11-03 | Galil Medical Ltd. | Method of controlling the temperature of gasses passing through a Joule-Thomson orifice |
Family Cites Families (13)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US3125096A (en)* | 1964-03-17 | Compressor | ||
| US3859986A (en)* | 1973-06-20 | 1975-01-14 | Jiro Okada | Surgical device |
| US5191883A (en)* | 1988-10-28 | 1993-03-09 | Prutech Research And Development Partnership Ii | Device for heating tissue in a patient's body |
| GB2226497B (en)* | 1988-12-01 | 1992-07-01 | Spembly Medical Ltd | Cryosurgical probe |
| WO1991005528A1 (en)* | 1989-10-19 | 1991-05-02 | Granulab B.V. | Device for cooling or heating a person |
| US5092841A (en)* | 1990-05-17 | 1992-03-03 | Wayne State University | Method for treating an arterial wall injured during angioplasty |
| US5190539A (en)* | 1990-07-10 | 1993-03-02 | Texas A & M University System | Micro-heat-pipe catheter |
| US5536252A (en)* | 1994-10-28 | 1996-07-16 | Intelliwire, Inc. | Angioplasty catheter with multiple coaxial balloons |
| US6624382B2 (en)* | 1997-01-30 | 2003-09-23 | Anvik Corporation | Configured-hole high-speed drilling system for micro-via pattern formation, and resulting structure |
| US5971983A (en)* | 1997-05-09 | 1999-10-26 | The Regents Of The University Of California | Tissue ablation device and method of use |
| US6241718B1 (en) | 1998-11-30 | 2001-06-05 | Cryocath Technologies, Inc. | Method for inhibiting restenosis |
| JP2003524506A (en) | 2000-03-01 | 2003-08-19 | イナークール セラピーズ インコーポレイテッド | Cryotherapy and device for angioplasty with restenosis |
| US6620882B1 (en)* | 2000-05-02 | 2003-09-16 | Advanced Syntech, Llc | Solid support template for preparation of highly functionalized heterocycle compounds |
- 1997
- 1997-12-02USUS08/982,824patent/US5971979A/ennot_activeExpired - Lifetime
- 1998
- 1998-12-01WOPCT/US1998/025448patent/WO1999027862A1/enactiveIP Right Grant
- 1998-12-01EPEP98959646Apatent/EP1039838B1/ennot_activeExpired - Lifetime
- 1998-12-01DEDE69831172Tpatent/DE69831172T2/ennot_activeExpired - Lifetime
- 1998-12-01USUS09/203,011patent/US6355029B1/ennot_activeExpired - Lifetime
- 1998-12-01JPJP2000522851Apatent/JP2001524345A/enactivePending
- 1998-12-01AUAU15404/99Apatent/AU744106B2/ennot_activeCeased
- 1998-12-01CACA2314352Apatent/CA2314352C/ennot_activeExpired - Fee Related
- 2001
- 2001-10-15USUS09/978,253patent/US6908462B2/ennot_activeExpired - Lifetime
- 2005
- 2005-05-04USUS11/122,165patent/US20050209587A1/ennot_activeAbandoned
Patent Citations (35)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US3901241A (en)* | 1973-05-31 | 1975-08-26 | Al Corp Du | Disposable cryosurgical instrument |
| US4336691A (en)* | 1979-12-13 | 1982-06-29 | The Board Of Trustees Of The Leland Stanford Junior University | Cryojet rapid freezing apparatus |
| US5019075A (en)* | 1984-10-24 | 1991-05-28 | The Beth Israel Hospital | Method and apparatus for angioplasty |
| US4754752A (en)* | 1986-07-28 | 1988-07-05 | Robert Ginsburg | Vascular catheter |
| US5106360A (en)* | 1987-09-17 | 1992-04-21 | Olympus Optical Co., Ltd. | Thermotherapeutic apparatus |
| US5041089A (en)* | 1987-12-11 | 1991-08-20 | Devices For Vascular Intervention, Inc. | Vascular dilation catheter construction |
| US5190589A (en)* | 1988-04-18 | 1993-03-02 | Cedona Pharmaceuticals B.V. | Compounds and process preparing a substituted or an unsubstituted 4(5)-(ω-aminoalkyl)imidazole |
| US5147355A (en)* | 1988-09-23 | 1992-09-15 | Brigham And Womens Hospital | Cryoablation catheter and method of performing cryoablation |
| US5151100A (en)* | 1988-10-28 | 1992-09-29 | Boston Scientific Corporation | Heating catheters |
| US5076713A (en)* | 1990-01-17 | 1991-12-31 | Nippon Thompson Co., Ltd. | Linear motion rolling guide unit made of resin |
| US5624392A (en)* | 1990-05-11 | 1997-04-29 | Saab; Mark A. | Heat transfer catheters and methods of making and using same |
| US5196024A (en)* | 1990-07-03 | 1993-03-23 | Cedars-Sinai Medical Center | Balloon catheter with cutting edge |
| US5191683A (en)* | 1990-12-12 | 1993-03-09 | Nhk Spring Co., Ltd. | Hose band |
| US5092641A (en)* | 1991-05-09 | 1992-03-03 | Brammal, Inc. | Cable locking and sealing device |
| US5520682A (en)* | 1991-09-06 | 1996-05-28 | Cryomedical Sciences, Inc. | Cryosurgical instrument with vent means and method using same |
| US5275595A (en)* | 1992-07-06 | 1994-01-04 | Dobak Iii John D | Cryosurgical instrument |
| US5486208A (en)* | 1993-02-10 | 1996-01-23 | Ginsburg; Robert | Method and apparatus for controlling a patient's body temperature by in situ blood temperature modification |
| US5501681A (en)* | 1993-11-12 | 1996-03-26 | Neuwirth; Robert S. | Intrauterine cryoablation cauterizing apparatus and method |
| US5458612A (en)* | 1994-01-06 | 1995-10-17 | Origin Medsystems, Inc. | Prostatic ablation method and apparatus for perineal approach |
| US5545195A (en)* | 1994-08-01 | 1996-08-13 | Boston Scientific Corporation | Interstitial heating of tissue |
| US5617739A (en)* | 1995-03-29 | 1997-04-08 | Mmr Technologies, Inc. | Self-cleaning low-temperature refrigeration system |
| US5644502A (en)* | 1995-05-04 | 1997-07-01 | Mmr Technologies, Inc. | Method for efficient counter-current heat exchange using optimized mixtures |
| US5733280A (en)* | 1995-11-15 | 1998-03-31 | Avitall; Boaz | Cryogenic epicardial mapping and ablation |
| US6241716B1 (en)* | 1996-03-13 | 2001-06-05 | Sca Hygiene Products Aktiebolag | Waist belt for absorbent articles |
| US6235019B1 (en)* | 1997-02-27 | 2001-05-22 | Cryocath Technologies, Inc. | Cryosurgical catheter |
| US5868735A (en)* | 1997-03-06 | 1999-02-09 | Scimed Life Systems, Inc. | Cryoplasty device and method |
| US6290696B1 (en)* | 1997-03-06 | 2001-09-18 | Scimed Life Systems, Inc. | Cryoplasty device and method |
| US5902299A (en)* | 1997-07-29 | 1999-05-11 | Jayaraman; Swaminathan | Cryotherapy method for reducing tissue injury after balloon angioplasty or stent implantation |
| US6685732B2 (en)* | 1998-03-31 | 2004-02-03 | Innercool Therapies, Inc. | Method and device for performing cooling- or cryo-therapies for, e.g., angioplasty with reduced restenosis or pulmonary vein cell necrosis to inhibit atrial fibrillation employing microporous balloon |
| US6125096A (en)* | 1998-04-08 | 2000-09-26 | Stomage Technology Comporation | Dynamic/stationary tape guide |
| US6786901B2 (en)* | 1999-03-15 | 2004-09-07 | Cryovascular Systems, Inc. | Cryosurgical fluid supply |
| US6283959B1 (en)* | 1999-08-23 | 2001-09-04 | Cyrocath Technologies, Inc. | Endovascular cryotreatment catheter |
| US6575966B2 (en)* | 1999-08-23 | 2003-06-10 | Cryocath Technologies Inc. | Endovascular cryotreatment catheter |
| US6624392B2 (en)* | 2001-07-11 | 2003-09-23 | Acerne Enterprises, Llc | Multifunctional cooking system |
| US20050245943A1 (en)* | 2001-09-27 | 2005-11-03 | Galil Medical Ltd. | Method of controlling the temperature of gasses passing through a Joule-Thomson orifice |
Cited By (230)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US9827041B2 (en) | 2002-04-08 | 2017-11-28 | Medtronic Ardian Luxembourg S.A.R.L. | Balloon catheter apparatuses for renal denervation |
| US10105180B2 (en) | 2002-04-08 | 2018-10-23 | Medtronic Ardian Luxembourg S.A.R.L. | Methods and apparatus for intravascularly-induced neuromodulation |
| US9757193B2 (en) | 2002-04-08 | 2017-09-12 | Medtronic Ardian Luxembourg S.A.R.L. | Balloon catheter apparatus for renal neuromodulation |
| US10376311B2 (en) | 2002-04-08 | 2019-08-13 | Medtronic Ardian Luxembourg S.A.R.L. | Methods and apparatus for intravascularly-induced neuromodulation |
| US9827040B2 (en) | 2002-04-08 | 2017-11-28 | Medtronic Adrian Luxembourg S.a.r.l. | Methods and apparatus for intravascularly-induced neuromodulation |
| US10420606B2 (en) | 2002-04-08 | 2019-09-24 | Medtronic Ardian Luxembourg S.A.R.L. | Methods and apparatus for performing a non-continuous circumferential treatment of a body lumen |
| US10188457B2 (en) | 2003-09-12 | 2019-01-29 | Vessix Vascular, Inc. | Selectable eccentric remodeling and/or ablation |
| US9125666B2 (en) | 2003-09-12 | 2015-09-08 | Vessix Vascular, Inc. | Selectable eccentric remodeling and/or ablation of atherosclerotic material |
| US9510901B2 (en) | 2003-09-12 | 2016-12-06 | Vessix Vascular, Inc. | Selectable eccentric remodeling and/or ablation |
| US11357563B2 (en) | 2004-03-23 | 2022-06-14 | Medtronic Cryocath Lp | Method and apparatus for inflating and deflating balloon catheters |
| US8491636B2 (en) | 2004-03-23 | 2013-07-23 | Medtronic Cryopath LP | Method and apparatus for inflating and deflating balloon catheters |
| US8900222B2 (en) | 2004-03-23 | 2014-12-02 | Medtronic Cryocath Lp | Method and apparatus for inflating and deflating balloon catheters |
| US8545491B2 (en) | 2004-03-23 | 2013-10-01 | Medtronic Cryocath Lp | Method and apparatus for inflating and deflating balloon catheters |
| US9555223B2 (en) | 2004-03-23 | 2017-01-31 | Medtronic Cryocath Lp | Method and apparatus for inflating and deflating balloon catheters |
| US8382747B2 (en) | 2004-03-23 | 2013-02-26 | Medtronic Cryocath Lp | Method and apparatus for inflating and deflating balloon catheters |
| US20090088735A1 (en)* | 2004-03-23 | 2009-04-02 | Cryocath Technologies Inc. | Method and apparatus for inflating and deflating balloon catheters |
| US9808301B2 (en) | 2004-03-23 | 2017-11-07 | Medtronic Cryocath Lp | Method and apparatus for inflating and deflating balloon catheters |
| US9713730B2 (en) | 2004-09-10 | 2017-07-25 | Boston Scientific Scimed, Inc. | Apparatus and method for treatment of in-stent restenosis |
| US8939970B2 (en) | 2004-09-10 | 2015-01-27 | Vessix Vascular, Inc. | Tuned RF energy and electrical tissue characterization for selective treatment of target tissues |
| US9125667B2 (en) | 2004-09-10 | 2015-09-08 | Vessix Vascular, Inc. | System for inducing desirable temperature effects on body tissue |
| US9486355B2 (en) | 2005-05-03 | 2016-11-08 | Vessix Vascular, Inc. | Selective accumulation of energy with or without knowledge of tissue topography |
| US8212554B2 (en)* | 2005-05-11 | 2012-07-03 | The University Of Houston System | Intraluminal magneto sensor system and method of use |
| US20090322323A1 (en)* | 2005-05-11 | 2009-12-31 | Audrius Brazdeikis | Intraluminal Magneto Sensor System and Method of Use |
| US11963706B2 (en) | 2005-05-20 | 2024-04-23 | Pacira Cryotech, Inc. | Subdermal cryogenic remodeling of muscles, nerves, connective tissue, and/or adipose tissue (fat) |
| US7862558B2 (en) | 2005-05-20 | 2011-01-04 | Myoscience, Inc. | Subdermal cryogenic remodeling of muscles, nerves, connective tissue, and/or adipose tissue (fat) |
| US11350979B2 (en) | 2005-05-20 | 2022-06-07 | Pacira Cryotech, Inc. | Subdermal cryogenic remodeling of muscles, nerves, connective tissue, and/or adipose tissue (fat) |
| US7850683B2 (en) | 2005-05-20 | 2010-12-14 | Myoscience, Inc. | Subdermal cryogenic remodeling of muscles, nerves, connective tissue, and/or adipose tissue (fat) |
| US10363080B2 (en) | 2005-05-20 | 2019-07-30 | Pacira Cryotech, Inc. | Subdermal cryogenic remodeling of muscles, nerves, connective tissue, and/or adipose tissue (fat) |
| US20070129714A1 (en)* | 2005-05-20 | 2007-06-07 | Echo Healthcare Llc | Subdermal cryogenic remodeling of muscles, nerves, connective tissue, and/or adipose tissue (FAT) |
| US20100198207A1 (en)* | 2005-05-20 | 2010-08-05 | Myoscience, Inc. | Subdermal cryogenic remodeling of muscles, nerves, connective tissue, and/or adipose tissue (fat) |
| US20090171334A1 (en)* | 2005-05-20 | 2009-07-02 | Myoscience, Inc. | Subdermal cryogenic remodeling of muscles, nerves, connective tissue, and/or adipose tissue (fat) |
| US9345526B2 (en) | 2005-05-20 | 2016-05-24 | Myoscience, Inc. | Subdermal cryogenic remodeling of muscles, nerves, connective tissue, and/or adipose tissue (fat) |
| US7998137B2 (en) | 2005-05-20 | 2011-08-16 | Myoscience, Inc. | Subdermal cryogenic remodeling of muscles, nerves, connective tissue, and/or adipose tissue (fat) |
| US20080183164A1 (en)* | 2005-05-20 | 2008-07-31 | Myoscience, Inc. | Subdermal cryogenic remodeling of muscles, nerves, connective tissue, and/or adipose tissue (fat) |
| US7713266B2 (en) | 2005-05-20 | 2010-05-11 | Myoscience, Inc. | Subdermal cryogenic remodeling of muscles, nerves, connective tissue, and/or adipose tissue (fat) |
| US9072498B2 (en) | 2005-05-20 | 2015-07-07 | Myoscience, Inc. | Subdermal cryogenic remodeling of muscles, nerves, connective tissue, and/or adipose tissue (fat) |
| US9808300B2 (en) | 2006-05-02 | 2017-11-07 | Boston Scientific Scimed, Inc. | Control of arterial smooth muscle tone |
| US10413356B2 (en) | 2006-10-18 | 2019-09-17 | Boston Scientific Scimed, Inc. | System for inducing desirable temperature effects on body tissue |
| US10213252B2 (en) | 2006-10-18 | 2019-02-26 | Vessix, Inc. | Inducing desirable temperature effects on body tissue |
| US12161392B2 (en) | 2006-10-18 | 2024-12-10 | Boston Scientific Scimed, Inc. | System for inducing desirable temperature effects on body tissue |
| US9974607B2 (en) | 2006-10-18 | 2018-05-22 | Vessix Vascular, Inc. | Inducing desirable temperature effects on body tissue |
| US9254162B2 (en) | 2006-12-21 | 2016-02-09 | Myoscience, Inc. | Dermal and transdermal cryogenic microprobe systems |
| US20080154254A1 (en)* | 2006-12-21 | 2008-06-26 | Myoscience, Inc. | Dermal and Transdermal Cryogenic Microprobe Systems and Methods |
| US10939947B2 (en) | 2006-12-21 | 2021-03-09 | Pacira Cryotech, Inc. | Dermal and transdermal cryogenic microprobe systems |
| US8409185B2 (en) | 2007-02-16 | 2013-04-02 | Myoscience, Inc. | Replaceable and/or easily removable needle systems for dermal and transdermal cryogenic remodeling |
| US20080200910A1 (en)* | 2007-02-16 | 2008-08-21 | Myoscience, Inc. | Replaceable and/or Easily Removable Needle Systems for Dermal and Transdermal Cryogenic Remodeling |
| US9113855B2 (en) | 2007-02-16 | 2015-08-25 | Myoscience, Inc. | Replaceable and/or easily removable needle systems for dermal and transdermal cryogenic remodeling |
| US20080234809A1 (en)* | 2007-03-23 | 2008-09-25 | Medtronic Vascular, Inc. | Stent Graft System With Injection Tube |
| US11672694B2 (en) | 2007-11-14 | 2023-06-13 | Pacira Cryotech, Inc. | Pain management using cryogenic remodeling |
| US8715275B2 (en) | 2007-11-14 | 2014-05-06 | Myoscience, Inc. | Pain management using cryogenic remodeling |
| US9907693B2 (en) | 2007-11-14 | 2018-03-06 | Myoscience, Inc. | Pain management using cryogenic remodeling |
| US10869779B2 (en) | 2007-11-14 | 2020-12-22 | Pacira Cryotech, Inc. | Pain management using cryogenic remodeling |
| US8298216B2 (en) | 2007-11-14 | 2012-10-30 | Myoscience, Inc. | Pain management using cryogenic remodeling |
| US10864112B2 (en) | 2007-11-14 | 2020-12-15 | Pacira Cryotech, Inc. | Pain management using cryogenic remodeling |
| US9101346B2 (en) | 2007-11-14 | 2015-08-11 | Myoscience, Inc. | Pain management using cryogenic remodeling |
| US12178746B2 (en) | 2007-11-14 | 2024-12-31 | Pacira Cryotech, Inc. | Pain management using cryogenic remodeling |
| US8740891B2 (en) | 2007-11-21 | 2014-06-03 | Endocare, Inc. | Flexible multi-tubular cryoprobe |
| US8740892B2 (en) | 2007-11-21 | 2014-06-03 | Endocare, Inc. | Expandable multi-tubular cryoprobe |
| US20110009854A1 (en)* | 2007-11-21 | 2011-01-13 | Endocare, Inc. | Expandable multi-tubular cryoprobe |
| US20110040297A1 (en)* | 2007-11-21 | 2011-02-17 | Endocare, Inc. | Flexible multi-tubular cryoprobe |
| EP2211743A4 (en)* | 2007-11-21 | 2011-01-05 | Endocare Inc | FLEXIBLE MULTITUBULAR CRYOSONDE |
| EP3289992A1 (en)* | 2007-11-21 | 2018-03-07 | Adagio Medical, Inc. | Flexible multi-tubular cryoprobe |
| WO2009067497A1 (en)* | 2007-11-21 | 2009-05-28 | Endocare, Inc. | Flexible multi-tubular cryoprobe |
| WO2009140067A1 (en)* | 2008-05-15 | 2009-11-19 | Boston Scientific Scimed, Inc. | Apparatus for cryogenically ablating tissue and adjusting cryogenic ablation regions |
| US20090287202A1 (en)* | 2008-05-15 | 2009-11-19 | Boston Scientific Scimed, Inc. | Apparatus and methods for cryogenically ablating tissue and adjusting cryogenic ablation regions |
| US10070910B2 (en) | 2008-05-15 | 2018-09-11 | Boston Scientific Scimed, Inc. | Apparatus and methods for cryogenically ablating tissue and adjusting cryogenic ablation regions |
| US8480663B2 (en) | 2008-05-15 | 2013-07-09 | Boston Scientific Scimed, Inc. | Apparatus and methods for cryogenically ablating tissue and adjusting cryogenic ablation regions |
| US9327100B2 (en) | 2008-11-14 | 2016-05-03 | Vessix Vascular, Inc. | Selective drug delivery in a lumen |
| US10383676B2 (en) | 2008-11-21 | 2019-08-20 | Pentax Of America, Inc. | Cryogenic ablation system and method |
| US11298174B2 (en) | 2008-11-21 | 2022-04-12 | Pentax Of America, Inc. | Cryogenic ablation system and method |
| US9066712B2 (en) | 2008-12-22 | 2015-06-30 | Myoscience, Inc. | Integrated cryosurgical system with refrigerant and electrical power source |
| US9427556B2 (en)* | 2009-06-01 | 2016-08-30 | Channel Medsystems, Inc. | Methods for treatment of a body cavity or lumen |
| US10849673B2 (en) | 2009-06-01 | 2020-12-01 | Channel Medsystems, Inc. | Methods and apparatus for treatment of a body cavity or lumen |
| US10004551B2 (en) | 2009-06-01 | 2018-06-26 | Channel Medsystems, Inc. | Methods and apparatus for treatment of a body cavity or lumen |
| US20120136343A1 (en)* | 2009-06-01 | 2012-05-31 | Channel Medsystems, Inc. | Methods and apparatus for treatment of a body cavity or lumen |
| US11957398B2 (en) | 2009-06-01 | 2024-04-16 | Channel Medsystems, Inc. | Methods and apparatus for treatment of a body cavity or lumen |
| US8986293B2 (en)* | 2010-01-27 | 2015-03-24 | Medtronic Cryocath Lp | Cryoballoon refrigerant dispersion control |
| US20110184398A1 (en)* | 2010-01-27 | 2011-07-28 | Medtronic Cryocath Lp | Cryoballoon refrigerant dispersion control |
| US9277955B2 (en) | 2010-04-09 | 2016-03-08 | Vessix Vascular, Inc. | Power generating and control apparatus for the treatment of tissue |
| US9192790B2 (en) | 2010-04-14 | 2015-11-24 | Boston Scientific Scimed, Inc. | Focused ultrasonic renal denervation |
| US8880185B2 (en) | 2010-06-11 | 2014-11-04 | Boston Scientific Scimed, Inc. | Renal denervation and stimulation employing wireless vascular energy transfer arrangement |
| US8473067B2 (en) | 2010-06-11 | 2013-06-25 | Boston Scientific Scimed, Inc. | Renal denervation and stimulation employing wireless vascular energy transfer arrangement |
| US9358365B2 (en) | 2010-07-30 | 2016-06-07 | Boston Scientific Scimed, Inc. | Precision electrode movement control for renal nerve ablation |
| US9408661B2 (en) | 2010-07-30 | 2016-08-09 | Patrick A. Haverkost | RF electrodes on multiple flexible wires for renal nerve ablation |
| US9084609B2 (en) | 2010-07-30 | 2015-07-21 | Boston Scientific Scime, Inc. | Spiral balloon catheter for renal nerve ablation |
| US9155589B2 (en) | 2010-07-30 | 2015-10-13 | Boston Scientific Scimed, Inc. | Sequential activation RF electrode set for renal nerve ablation |
| US9463062B2 (en) | 2010-07-30 | 2016-10-11 | Boston Scientific Scimed, Inc. | Cooled conductive balloon RF catheter for renal nerve ablation |
| US10004550B2 (en) | 2010-08-05 | 2018-06-26 | Medtronic Ardian Luxembourg S.A.R.L. | Cryoablation apparatuses, systems, and methods for renal neuromodulation |
| US8974451B2 (en) | 2010-10-25 | 2015-03-10 | Boston Scientific Scimed, Inc. | Renal nerve ablation using conductive fluid jet and RF energy |
| US9439708B2 (en) | 2010-10-26 | 2016-09-13 | Medtronic Ardian Luxembourg S.A.R.L. | Neuromodulation cryotherapeutic devices and associated systems and methods |
| US10188445B2 (en) | 2010-10-26 | 2019-01-29 | Medtronic Ardian Luxembourg S.A.R.L. | Neuromodulation cryotherapeutic devices and associated systems and methods |
| US10842547B2 (en) | 2010-10-26 | 2020-11-24 | Medtronic Ardian Luxembourg S.A.R.L. | Neuromodulation cryotherapeutic devices and associated systems and methods |
| US9220558B2 (en) | 2010-10-27 | 2015-12-29 | Boston Scientific Scimed, Inc. | RF renal denervation catheter with multiple independent electrodes |
| US9848946B2 (en) | 2010-11-15 | 2017-12-26 | Boston Scientific Scimed, Inc. | Self-expanding cooling electrode for renal nerve ablation |
| US9028485B2 (en) | 2010-11-15 | 2015-05-12 | Boston Scientific Scimed, Inc. | Self-expanding cooling electrode for renal nerve ablation |
| US9089350B2 (en) | 2010-11-16 | 2015-07-28 | Boston Scientific Scimed, Inc. | Renal denervation catheter with RF electrode and integral contrast dye injection arrangement |
| US9668811B2 (en) | 2010-11-16 | 2017-06-06 | Boston Scientific Scimed, Inc. | Minimally invasive access for renal nerve ablation |
| US9326751B2 (en) | 2010-11-17 | 2016-05-03 | Boston Scientific Scimed, Inc. | Catheter guidance of external energy for renal denervation |
| US9060761B2 (en) | 2010-11-18 | 2015-06-23 | Boston Scientific Scime, Inc. | Catheter-focused magnetic field induced renal nerve ablation |
| US9023034B2 (en) | 2010-11-22 | 2015-05-05 | Boston Scientific Scimed, Inc. | Renal ablation electrode with force-activatable conduction apparatus |
| US9192435B2 (en) | 2010-11-22 | 2015-11-24 | Boston Scientific Scimed, Inc. | Renal denervation catheter with cooled RF electrode |
| US9649156B2 (en) | 2010-12-15 | 2017-05-16 | Boston Scientific Scimed, Inc. | Bipolar off-wall electrode device for renal nerve ablation |
| US9220561B2 (en) | 2011-01-19 | 2015-12-29 | Boston Scientific Scimed, Inc. | Guide-compatible large-electrode catheter for renal nerve ablation with reduced arterial injury |
| US11883324B2 (en) | 2011-02-01 | 2024-01-30 | Channel Medsystems, Inc. | Cryogenic treatment systems |
| US10959879B2 (en) | 2011-02-01 | 2021-03-30 | Channel Medsystems, Inc. | Methods and apparatus for cryogenic treatment of a body cavity or lumen |
| US11833076B2 (en) | 2011-02-01 | 2023-12-05 | Channel Medsystems, Inc. | Methods and apparatus for cryogenic treatment of a body cavity or lumen |
| US20120245574A1 (en)* | 2011-03-25 | 2012-09-27 | Medtronic Cryocath Lp | Spray nozzle design for a catheter |
| US12201340B2 (en) | 2011-03-25 | 2025-01-21 | Medtronic Cryocath Lp | Spray nozzle design for a catheter |
| US9439707B2 (en)* | 2011-03-25 | 2016-09-13 | Medtronic Cryocath Lp | Spray nozzle design for a catheter |
| US11806065B2 (en) | 2011-03-25 | 2023-11-07 | Medtronic Cryocath Lp | Spray nozzle design for a catheter |
| US9936999B2 (en) | 2011-03-25 | 2018-04-10 | Medtronic Cryocath Lp | Spray nozzle design for a catheter |
| US11259857B2 (en) | 2011-03-25 | 2022-03-01 | Medtronic Cryocath Lp | Spray nozzle design for a catheter |
| US9919144B2 (en) | 2011-04-08 | 2018-03-20 | Medtronic Adrian Luxembourg S.a.r.l. | Iontophoresis drug delivery system and method for denervation of the renal sympathetic nerve and iontophoretic drug delivery |
| US10588682B2 (en) | 2011-04-25 | 2020-03-17 | Medtronic Ardian Luxembourg S.A.R.L. | Apparatus and methods related to constrained deployment of cryogenic balloons for limited cryogenic ablation of vessel walls |
| US9579030B2 (en) | 2011-07-20 | 2017-02-28 | Boston Scientific Scimed, Inc. | Percutaneous devices and methods to visualize, target and ablate nerves |
| US9186209B2 (en) | 2011-07-22 | 2015-11-17 | Boston Scientific Scimed, Inc. | Nerve modulation system having helical guide |
| US9186210B2 (en) | 2011-10-10 | 2015-11-17 | Boston Scientific Scimed, Inc. | Medical devices including ablation electrodes |
| US10085799B2 (en) | 2011-10-11 | 2018-10-02 | Boston Scientific Scimed, Inc. | Off-wall electrode device and methods for nerve modulation |
| US9420955B2 (en) | 2011-10-11 | 2016-08-23 | Boston Scientific Scimed, Inc. | Intravascular temperature monitoring system and method |
| US9364284B2 (en) | 2011-10-12 | 2016-06-14 | Boston Scientific Scimed, Inc. | Method of making an off-wall spacer cage |
| US9079000B2 (en) | 2011-10-18 | 2015-07-14 | Boston Scientific Scimed, Inc. | Integrated crossing balloon catheter |
| US9162046B2 (en) | 2011-10-18 | 2015-10-20 | Boston Scientific Scimed, Inc. | Deflectable medical devices |
| US8951251B2 (en) | 2011-11-08 | 2015-02-10 | Boston Scientific Scimed, Inc. | Ostial renal nerve ablation |
| US9119600B2 (en) | 2011-11-15 | 2015-09-01 | Boston Scientific Scimed, Inc. | Device and methods for renal nerve modulation monitoring |
| US9119632B2 (en) | 2011-11-21 | 2015-09-01 | Boston Scientific Scimed, Inc. | Deflectable renal nerve ablation catheter |
| US9265969B2 (en) | 2011-12-21 | 2016-02-23 | Cardiac Pacemakers, Inc. | Methods for modulating cell function |
| US9072902B2 (en) | 2011-12-23 | 2015-07-07 | Vessix Vascular, Inc. | Methods and apparatuses for remodeling tissue of or adjacent to a body passage |
| US9402684B2 (en) | 2011-12-23 | 2016-08-02 | Boston Scientific Scimed, Inc. | Methods and apparatuses for remodeling tissue of or adjacent to a body passage |
| US9028472B2 (en) | 2011-12-23 | 2015-05-12 | Vessix Vascular, Inc. | Methods and apparatuses for remodeling tissue of or adjacent to a body passage |
| US9037259B2 (en) | 2011-12-23 | 2015-05-19 | Vessix Vascular, Inc. | Methods and apparatuses for remodeling tissue of or adjacent to a body passage |
| US9592386B2 (en) | 2011-12-23 | 2017-03-14 | Vessix Vascular, Inc. | Methods and apparatuses for remodeling tissue of or adjacent to a body passage |
| US9174050B2 (en) | 2011-12-23 | 2015-11-03 | Vessix Vascular, Inc. | Methods and apparatuses for remodeling tissue of or adjacent to a body passage |
| US9186211B2 (en) | 2011-12-23 | 2015-11-17 | Boston Scientific Scimed, Inc. | Methods and apparatuses for remodeling tissue of or adjacent to a body passage |
| US9433760B2 (en) | 2011-12-28 | 2016-09-06 | Boston Scientific Scimed, Inc. | Device and methods for nerve modulation using a novel ablation catheter with polymeric ablative elements |
| US9050106B2 (en) | 2011-12-29 | 2015-06-09 | Boston Scientific Scimed, Inc. | Off-wall electrode device and methods for nerve modulation |
| US9155584B2 (en) | 2012-01-13 | 2015-10-13 | Myoscience, Inc. | Cryogenic probe filtration system |
| US10213244B2 (en) | 2012-01-13 | 2019-02-26 | Myoscience, Inc. | Cryogenic needle with freeze zone regulation |
| US9314290B2 (en) | 2012-01-13 | 2016-04-19 | Myoscience, Inc. | Cryogenic needle with freeze zone regulation |
| US9241753B2 (en) | 2012-01-13 | 2016-01-26 | Myoscience, Inc. | Skin protection for subdermal cryogenic remodeling for cosmetic and other treatments |
| US10188444B2 (en) | 2012-01-13 | 2019-01-29 | Myoscience, Inc. | Skin protection for subdermal cryogenic remodeling for cosmetic and other treatments |
| US11857239B2 (en) | 2012-01-13 | 2024-01-02 | Pacira Cryotech, Inc. | Cryogenic needle with freeze zone regulation |
| US9017318B2 (en) | 2012-01-20 | 2015-04-28 | Myoscience, Inc. | Cryogenic probe system and method |
| US9872718B2 (en) | 2012-04-27 | 2018-01-23 | Medtronic Adrian Luxembourg S.a.r.l. | Shafts with pressure relief in cryotherapeutic catheters and associated devices, systems, and methods |
| US10905490B2 (en) | 2012-04-27 | 2021-02-02 | Medtronic Ardian Luxembourg S.A.R.L. | Cryotherapeutic devices for renal neuromodulation and associated systems and methods |
| US11751931B2 (en) | 2012-04-27 | 2023-09-12 | Medtronic Ardian Luxembourg S.A.R.L. | Cryotherapeutic devices for renal neuromodulation and associated systems and methods |
| US10660703B2 (en) | 2012-05-08 | 2020-05-26 | Boston Scientific Scimed, Inc. | Renal nerve modulation devices |
| US10321946B2 (en) | 2012-08-24 | 2019-06-18 | Boston Scientific Scimed, Inc. | Renal nerve modulation devices with weeping RF ablation balloons |
| US9173696B2 (en) | 2012-09-17 | 2015-11-03 | Boston Scientific Scimed, Inc. | Self-positioning electrode system and method for renal nerve modulation |
| US10398464B2 (en) | 2012-09-21 | 2019-09-03 | Boston Scientific Scimed, Inc. | System for nerve modulation and innocuous thermal gradient nerve block |
| US10549127B2 (en) | 2012-09-21 | 2020-02-04 | Boston Scientific Scimed, Inc. | Self-cooling ultrasound ablation catheter |
| US10835305B2 (en) | 2012-10-10 | 2020-11-17 | Boston Scientific Scimed, Inc. | Renal nerve modulation devices and methods |
| US9693821B2 (en) | 2013-03-11 | 2017-07-04 | Boston Scientific Scimed, Inc. | Medical devices for modulating nerves |
| US9956033B2 (en) | 2013-03-11 | 2018-05-01 | Boston Scientific Scimed, Inc. | Medical devices for modulating nerves |
| US9808311B2 (en) | 2013-03-13 | 2017-11-07 | Boston Scientific Scimed, Inc. | Deflectable medical devices |
| US10888366B2 (en) | 2013-03-15 | 2021-01-12 | Pacira Cryotech, Inc. | Cryogenic blunt dissection methods and devices |
| US10314739B2 (en) | 2013-03-15 | 2019-06-11 | Myoscience, Inc. | Methods and devices for pain management |
| US10085881B2 (en) | 2013-03-15 | 2018-10-02 | Myoscience, Inc. | Methods, systems, and devices for treating neuromas, fibromas, nerve entrapment, and/or pain associated therewith |
| US11253393B2 (en) | 2013-03-15 | 2022-02-22 | Pacira Cryotech, Inc. | Methods, systems, and devices for treating neuromas, fibromas, nerve entrapment, and/or pain associated therewith |
| US11134999B2 (en) | 2013-03-15 | 2021-10-05 | Pacira Cryotech, Inc. | Methods and systems for treatment of occipital neuralgia |
| US10016229B2 (en) | 2013-03-15 | 2018-07-10 | Myoscience, Inc. | Methods and systems for treatment of occipital neuralgia |
| US9668800B2 (en) | 2013-03-15 | 2017-06-06 | Myoscience, Inc. | Methods and systems for treatment of spasticity |
| US9827039B2 (en) | 2013-03-15 | 2017-11-28 | Boston Scientific Scimed, Inc. | Methods and apparatuses for remodeling tissue of or adjacent to a body passage |
| US9610112B2 (en) | 2013-03-15 | 2017-04-04 | Myoscience, Inc. | Cryogenic enhancement of joint function, alleviation of joint stiffness and/or alleviation of pain associated with osteoarthritis |
| US9297845B2 (en) | 2013-03-15 | 2016-03-29 | Boston Scientific Scimed, Inc. | Medical devices and methods for treatment of hypertension that utilize impedance compensation |
| US10596030B2 (en) | 2013-03-15 | 2020-03-24 | Pacira Cryotech, Inc. | Cryogenic enhancement of joint function, alleviation of joint stiffness and/or alleviation of pain associated with osteoarthritis |
| US11865038B2 (en) | 2013-03-15 | 2024-01-09 | Pacira Cryotech, Inc. | Methods, systems, and devices for treating nerve spasticity |
| US9295512B2 (en) | 2013-03-15 | 2016-03-29 | Myoscience, Inc. | Methods and devices for pain management |
| US11642241B2 (en) | 2013-03-15 | 2023-05-09 | Pacira Cryotech, Inc. | Cryogenic enhancement of joint function, alleviation of joint stiffness and/or alleviation of pain associated with osteoarthritis |
| US10265122B2 (en) | 2013-03-15 | 2019-04-23 | Boston Scientific Scimed, Inc. | Nerve ablation devices and related methods of use |
| US10085789B2 (en) | 2013-03-15 | 2018-10-02 | Myoscience, Inc. | Methods and systems for treatment of occipital neuralgia |
| US10022182B2 (en) | 2013-06-21 | 2018-07-17 | Boston Scientific Scimed, Inc. | Medical devices for renal nerve ablation having rotatable shafts |
| US9943365B2 (en) | 2013-06-21 | 2018-04-17 | Boston Scientific Scimed, Inc. | Renal denervation balloon catheter with ride along electrode support |
| US9707036B2 (en) | 2013-06-25 | 2017-07-18 | Boston Scientific Scimed, Inc. | Devices and methods for nerve modulation using localized indifferent electrodes |
| US9833283B2 (en) | 2013-07-01 | 2017-12-05 | Boston Scientific Scimed, Inc. | Medical devices for renal nerve ablation |
| US10413357B2 (en) | 2013-07-11 | 2019-09-17 | Boston Scientific Scimed, Inc. | Medical device with stretchable electrode assemblies |
| US10660698B2 (en) | 2013-07-11 | 2020-05-26 | Boston Scientific Scimed, Inc. | Devices and methods for nerve modulation |
| US9925001B2 (en) | 2013-07-19 | 2018-03-27 | Boston Scientific Scimed, Inc. | Spiral bipolar electrode renal denervation balloon |
| US10695124B2 (en) | 2013-07-22 | 2020-06-30 | Boston Scientific Scimed, Inc. | Renal nerve ablation catheter having twist balloon |
| US10342609B2 (en) | 2013-07-22 | 2019-07-09 | Boston Scientific Scimed, Inc. | Medical devices for renal nerve ablation |
| US10722300B2 (en) | 2013-08-22 | 2020-07-28 | Boston Scientific Scimed, Inc. | Flexible circuit having improved adhesion to a renal nerve modulation balloon |
| US12167889B2 (en) | 2013-08-22 | 2024-12-17 | Boston Scientific Scimed, Inc. | Flexible circuit having improved adhesion to a renal nerve modulation balloon |
| US9895194B2 (en) | 2013-09-04 | 2018-02-20 | Boston Scientific Scimed, Inc. | Radio frequency (RF) balloon catheter having flushing and cooling capability |
| US10952790B2 (en) | 2013-09-13 | 2021-03-23 | Boston Scientific Scimed, Inc. | Ablation balloon with vapor deposited cover layer |
| US11883085B2 (en) | 2013-09-24 | 2024-01-30 | Adagio Medical, Inc. | Endovascular near critical fluid based cryoablation catheter and related methods |
| US10667854B2 (en) | 2013-09-24 | 2020-06-02 | Adagio Medical, Inc. | Endovascular near critical fluid based cryoablation catheter and related methods |
| US11179186B2 (en) | 2013-09-24 | 2021-11-23 | Adagio Medical, Inc. | Endovascular near critical fluid based cryoablation catheter and related methods |
| US9687166B2 (en) | 2013-10-14 | 2017-06-27 | Boston Scientific Scimed, Inc. | High resolution cardiac mapping electrode array catheter |
| US11246654B2 (en) | 2013-10-14 | 2022-02-15 | Boston Scientific Scimed, Inc. | Flexible renal nerve ablation devices and related methods of use and manufacture |
| US9770606B2 (en) | 2013-10-15 | 2017-09-26 | Boston Scientific Scimed, Inc. | Ultrasound ablation catheter with cooling infusion and centering basket |
| US9962223B2 (en) | 2013-10-15 | 2018-05-08 | Boston Scientific Scimed, Inc. | Medical device balloon |
| US10945786B2 (en) | 2013-10-18 | 2021-03-16 | Boston Scientific Scimed, Inc. | Balloon catheters with flexible conducting wires and related methods of use and manufacture |
| US10271898B2 (en) | 2013-10-25 | 2019-04-30 | Boston Scientific Scimed, Inc. | Embedded thermocouple in denervation flex circuit |
| EP3062720B1 (en)* | 2013-11-01 | 2021-04-07 | Pentax of America, Inc. | Cryogenic balloon ablation system |
| CN107669332A (en)* | 2013-11-01 | 2018-02-09 | C2治疗公司 | Cryogenic ablation conduit, Handleset and low temperature sacculus ablation system |
| US10130409B2 (en) | 2013-11-05 | 2018-11-20 | Myoscience, Inc. | Secure cryosurgical treatment system |
| US10864033B2 (en) | 2013-11-05 | 2020-12-15 | Pacira Cryotech, Inc. | Secure cryosurgical treatment system |
| US11690661B2 (en) | 2013-11-05 | 2023-07-04 | Pacira Cryotech, Inc. | Secure cryosurgical treatment system |
| US11202671B2 (en) | 2014-01-06 | 2021-12-21 | Boston Scientific Scimed, Inc. | Tear resistant flex circuit assembly |
| US9907609B2 (en) | 2014-02-04 | 2018-03-06 | Boston Scientific Scimed, Inc. | Alternative placement of thermal sensors on bipolar electrode |
| US11000679B2 (en) | 2014-02-04 | 2021-05-11 | Boston Scientific Scimed, Inc. | Balloon protection and rewrapping devices and related methods of use |
| US12274483B2 (en) | 2014-03-07 | 2025-04-15 | Medtronic Ardian Luxembourg S.A.R.L. | Monitoring and controlling internally administered cryotherapy |
| US10492842B2 (en) | 2014-03-07 | 2019-12-03 | Medtronic Ardian Luxembourg S.A.R.L. | Monitoring and controlling internally administered cryotherapy |
| US11406437B2 (en) | 2014-03-07 | 2022-08-09 | Medtronic Ardian Luxembourg S.A.R.L. | Monitoring and controlling internally administered cryotherapy |
| US10617459B2 (en) | 2014-04-17 | 2020-04-14 | Adagio Medical, Inc. | Endovascular near critical fluid based cryoablation catheter having plurality of preformed treatment shapes |
| US10709490B2 (en) | 2014-05-07 | 2020-07-14 | Medtronic Ardian Luxembourg S.A.R.L. | Catheter assemblies comprising a direct heating element for renal neuromodulation and associated systems and methods |
| US10543032B2 (en) | 2014-11-13 | 2020-01-28 | Adagio Medical, Inc. | Pressure modulated cryoablation system and related methods |
| CN110051420A (en)* | 2015-05-15 | 2019-07-26 | 美国宾得公司 | Low temperature sacculus ablation system |
| EP3294170A4 (en)* | 2015-05-15 | 2019-01-30 | Pentax of America, Inc. | CRYOGENIC BALLOON ABLATION SYSTEM |
| US10098686B2 (en) | 2015-05-15 | 2018-10-16 | Pentax Of America, Inc. | Cryogenic balloon ablation system |
| US11051867B2 (en) | 2015-09-18 | 2021-07-06 | Adagio Medical, Inc. | Tissue contact verification system |
| US10864031B2 (en) | 2015-11-30 | 2020-12-15 | Adagio Medical, Inc. | Ablation method for creating elongate continuous lesions enclosing multiple vessel entries |
| CN106880400A (en)* | 2015-12-16 | 2017-06-23 | 上海微创电生理医疗科技有限公司 | Electrophysiologicalcatheter catheter and radio frequency ablation system |
| US12076069B2 (en) | 2016-05-13 | 2024-09-03 | Pacira Cryotech, Inc. | Methods and systems for locating and treating nerves with cold therapy |
| US11311327B2 (en) | 2016-05-13 | 2022-04-26 | Pacira Cryotech, Inc. | Methods and systems for locating and treating nerves with cold therapy |
| US11266456B2 (en)* | 2016-05-20 | 2022-03-08 | Pentax Of America, Inc. | Cryogenic ablation system with rotatable and translatable catheter |
| US10251693B2 (en)* | 2016-05-20 | 2019-04-09 | Pentax Of America, Inc. | Cryogenic ablation system with rotatable and translatable catheter |
| KR102366631B1 (en)* | 2016-05-20 | 2022-02-22 | 펜탁스 오브 아메리카 인코포레이티드 | Cryogenic ablation system with rotatable and translatable catheter |
| CN113893022A (en)* | 2016-05-20 | 2022-01-07 | 美国宾得公司 | Cryogenic ablation system with rotatable and translatable catheter |
| US20170333105A1 (en)* | 2016-05-20 | 2017-11-23 | C2 Therapeutics, Inc. | Cryogenic ablation system with rotatable and translatable catheter |
| AU2017267476B2 (en)* | 2016-05-20 | 2021-10-14 | Pentax Of America, Inc. | Cryogenic ablation system with rotatable and translatable catheter |
| KR20190010873A (en)* | 2016-05-20 | 2019-01-31 | 펜탁스 오브 아메리카 인코포레이티드 | Cryogenic wear system with rotatable and translatable catheters |
| US11564725B2 (en) | 2017-09-05 | 2023-01-31 | Adagio Medical, Inc. | Ablation catheter having a shape memory stylet |
| US12364530B2 (en) | 2017-09-05 | 2025-07-22 | Adagio Medical, Inc. | Ablation catheter having a shape memory stylet |
| US11134998B2 (en) | 2017-11-15 | 2021-10-05 | Pacira Cryotech, Inc. | Integrated cold therapy and electrical stimulation systems for locating and treating nerves and associated methods |
| US12167881B2 (en) | 2017-11-15 | 2024-12-17 | Pacira Cryotech, Inc. | Integrated cold therapy and electrical stimulation systems for locating and treating nerves and associated methods |
| US11751930B2 (en) | 2018-01-10 | 2023-09-12 | Adagio Medical, Inc. | Cryoablation element with conductive liner |
| WO2022261146A1 (en)* | 2021-06-07 | 2022-12-15 | Agil Therapeutics, Inc. | Cryogenic catheter probe, system, and method for selective ablation of mucosa and submucosa of the gastrointestinal tract |
| WO2023006511A1 (en)* | 2021-07-29 | 2023-02-02 | Medtronic Ireland Manufacturing Unlimited Company | Isolated pressure monitoring for cryogenic balloon catheter |
| WO2024115107A1 (en)* | 2022-11-30 | 2024-06-06 | Medtronic Ireland Manufacturing Unlimited Company | Temperature-based pressure measurement in cryoballoon ablation catheters |
| WO2024123945A3 (en)* | 2022-12-06 | 2024-07-18 | Agil Therapeutics, Inc. | Cryogenic catheter, system, and method for selective ablation of mucosa and submucosa of the gastrointestinal tract |
Also Published As
| Publication number | Publication date |
|---|---|
| US6908462B2 (en) | 2005-06-21 |
| AU744106B2 (en) | 2002-02-14 |
| WO1999027862A1 (en) | 1999-06-10 |
| EP1039838B1 (en) | 2005-08-10 |
| EP1039838A1 (en) | 2000-10-04 |
| CA2314352A1 (en) | 1999-06-10 |
| JP2001524345A (en) | 2001-12-04 |
| US5971979A (en) | 1999-10-26 |
| EP1039838A4 (en) | 2001-10-10 |
| DE69831172T2 (en) | 2006-03-30 |
| US20020026182A1 (en) | 2002-02-28 |
| DE69831172D1 (en) | 2005-09-15 |
| CA2314352C (en) | 2010-05-04 |
| AU1540499A (en) | 1999-06-16 |
| US6355029B1 (en) | 2002-03-12 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| US6908462B2 (en) | Apparatus and method for cryogenic inhibition of hyperplasia | |
| JP4833494B2 (en) | Cryotherapy apparatus and method | |
| CA2666334C (en) | Endovascular cryotreatment catheter | |
| US6283959B1 (en) | Endovascular cryotreatment catheter | |
| EP1773224B1 (en) | Endovascular cryotreatment catheter | |
| JP2002538882A (en) | Cryosurgical fluid supply |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| STCB | Information on status: application discontinuation | Free format text:ABANDONED -- FAILURE TO RESPOND TO AN OFFICE ACTION |