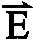US20050161331A1 - Fractionation of macro-molecules using asymmetric pulsed field electrophoresis - Google Patents
Fractionation of macro-molecules using asymmetric pulsed field electrophoresisDownload PDFInfo
- Publication number
- US20050161331A1 US20050161331A1US11/075,682US7568205AUS2005161331A1US 20050161331 A1US20050161331 A1US 20050161331A1US 7568205 AUS7568205 AUS 7568205AUS 2005161331 A1US2005161331 A1US 2005161331A1
- Authority
- US
- United States
- Prior art keywords
- molecules
- electric
- pulses
- time
- matrix
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Granted
Links
- 229920002521macromoleculePolymers0.000titleclaimsabstractdescription15
- 238000005194fractionationMethods0.000titleclaimsabstractdescription14
- 238000001853pulsed-field electrophoresisMethods0.000titledescription3
- 230000005684electric fieldEffects0.000claimsabstractdescription62
- 238000000034methodMethods0.000claimsabstractdescription38
- 239000011159matrix materialSubstances0.000claimsabstractdescription29
- 108020004414DNAProteins0.000claimsdescription64
- 239000013598vectorSubstances0.000claimsdescription13
- 102000053602DNAHuman genes0.000claimsdescription7
- 230000036962time dependentEffects0.000claims2
- 238000000605extractionMethods0.000claims1
- 239000012634fragmentSubstances0.000description6
- 239000000463materialSubstances0.000description5
- 239000000523sampleSubstances0.000description4
- 239000000758substrateSubstances0.000description4
- 239000000203mixtureSubstances0.000description3
- 239000010453quartzSubstances0.000description3
- VYPSYNLAJGMNEJ-UHFFFAOYSA-Nsilicon dioxideInorganic materialsO=[Si]=OVYPSYNLAJGMNEJ-UHFFFAOYSA-N0.000description3
- 238000010586diagramMethods0.000description2
- 238000006073displacement reactionMethods0.000description2
- 238000001962electrophoresisMethods0.000description2
- 238000001502gel electrophoresisMethods0.000description2
- 238000001208nuclear magnetic resonance pulse sequenceMethods0.000description2
- 238000010561standard procedureMethods0.000description2
- OSBLTNPMIGYQGY-UHFFFAOYSA-N2-amino-2-(hydroxymethyl)propane-1,3-diol;2-[2-[bis(carboxymethyl)amino]ethyl-(carboxymethyl)amino]acetic acid;boric acidChemical compoundOB(O)O.OCC(N)(CO)CO.OC(=O)CN(CC(O)=O)CCN(CC(O)=O)CC(O)=OOSBLTNPMIGYQGY-UHFFFAOYSA-N0.000description1
- 239000008051TBE bufferSubstances0.000description1
- 238000004458analytical methodMethods0.000description1
- 239000011521glassSubstances0.000description1
- 230000003993interactionEffects0.000description1
- 238000002032lab-on-a-chipMethods0.000description1
- 238000012177large-scale sequencingMethods0.000description1
- 230000002093peripheral effectEffects0.000description1
- 238000003906pulsed field gel electrophoresisMethods0.000description1
- 239000012488sample solutionSubstances0.000description1
- 238000010408sweepingMethods0.000description1
Images
Classifications
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N15/00—Mutation or genetic engineering; DNA or RNA concerning genetic engineering, vectors, e.g. plasmids, or their isolation, preparation or purification; Use of hosts therefor
- C12N15/09—Recombinant DNA-technology
- C12N15/10—Processes for the isolation, preparation or purification of DNA or RNA
- C12N15/1003—Extracting or separating nucleic acids from biological samples, e.g. pure separation or isolation methods; Conditions, buffers or apparatuses therefor
- C12N15/1006—Extracting or separating nucleic acids from biological samples, e.g. pure separation or isolation methods; Conditions, buffers or apparatuses therefor by means of a solid support carrier, e.g. particles, polymers
- C12N15/101—Extracting or separating nucleic acids from biological samples, e.g. pure separation or isolation methods; Conditions, buffers or apparatuses therefor by means of a solid support carrier, e.g. particles, polymers by chromatography, e.g. electrophoresis, ion-exchange, reverse phase
- Y—GENERAL TAGGING OF NEW TECHNOLOGICAL DEVELOPMENTS; GENERAL TAGGING OF CROSS-SECTIONAL TECHNOLOGIES SPANNING OVER SEVERAL SECTIONS OF THE IPC; TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y10—TECHNICAL SUBJECTS COVERED BY FORMER USPC
- Y10T—TECHNICAL SUBJECTS COVERED BY FORMER US CLASSIFICATION
- Y10T436/00—Chemistry: analytical and immunological testing
- Y10T436/10—Composition for standardization, calibration, simulation, stabilization, preparation or preservation; processes of use in preparation for chemical testing
- Y—GENERAL TAGGING OF NEW TECHNOLOGICAL DEVELOPMENTS; GENERAL TAGGING OF CROSS-SECTIONAL TECHNOLOGIES SPANNING OVER SEVERAL SECTIONS OF THE IPC; TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y10—TECHNICAL SUBJECTS COVERED BY FORMER USPC
- Y10T—TECHNICAL SUBJECTS COVERED BY FORMER US CLASSIFICATION
- Y10T436/00—Chemistry: analytical and immunological testing
- Y10T436/11—Automated chemical analysis
- Y—GENERAL TAGGING OF NEW TECHNOLOGICAL DEVELOPMENTS; GENERAL TAGGING OF CROSS-SECTIONAL TECHNOLOGIES SPANNING OVER SEVERAL SECTIONS OF THE IPC; TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y10—TECHNICAL SUBJECTS COVERED BY FORMER USPC
- Y10T—TECHNICAL SUBJECTS COVERED BY FORMER US CLASSIFICATION
- Y10T436/00—Chemistry: analytical and immunological testing
- Y10T436/25—Chemistry: analytical and immunological testing including sample preparation
Definitions
- the present inventionrelates to a method and apparatus for fractionating charged macro-molecules such as DNA using asymmetric pulsed field electrophoresis.
- sample loading and fractionationare used to fractionate DNA molecules according to their sizes.
- This methodincludes two steps: sample loading and fractionation. First, sample solution containing DNA is loaded into loading wells in the gel slab before the electric field is turned on. Then, an electric field is applied. The DNA molecules move in the opposite direction of the electric field because they are negatively charged. As the electric field is applied, DNA molecules travel at different speeds according to their sizes, but the directions in which they migrate are always the same. Eventually, sample DNA molecules are separated into different bands, each of which contains DNA molecules of the same size, as shown in FIG. 1 . Shorter DNA fragments move faster than longer ones. Therefore, they are separated according to their sizes.
- the present inventionrelates to a method and apparatus for fractionation of charged macro-molecules such as DNA.
- DNA solutionis loaded into a matrix including an array of obstacles.
- An alternating electric field having two different fields at different orientationsis applied.
- the alternating electric fieldis asymmetric in that one field is stronger in duration or intensity than the other field, or is otherwise asymmetric.
- the DNA moleculesare thereby fractionated according to size and are driven to a far side of the matrix where the fractionated DNA is recovered.
- the fractionating electric fieldcan be used to load and recover the DNA to operate the process continuously.
- FIG. 1shows conventional gel electrophoresis.
- FIG. 2is a diagram showing asymmetric pulsed-field electrophoresis in micro/nano-fabricated matrices according to the present invention.
- FIG. 3is a diagram showing the basic principle of asymmetrical pulsed electrophoresis of the present invention.
- FIG. 4shows the way stretched DNA molecules move under asymmetrical pulsed electric field.
- FIG. 5shows a support material (matrix) for use in fractionation of DNA according to the present invention.
- FIG. 6Ais a top view and FIG. 6B is a side view of the microfabricated support material shown in FIG. 5 .
- FIG. 7shows fractionation of T4 and T7 DNA.
- the present inventionrelates to a method and apparatus for fractionation of charged macro-molecules such as DNA.
- DNA solutionis loaded into a matrix including an array of obstacles.
- An alternating electric field having two different fields at different orientationsis applied.
- the alternating electric fieldis asymmetric in that one field is stronger in duration or intensity than the other field, or is otherwise asymmetric.
- the DNA moleculesare thereby fractionated according to size and are driven to a far side of the matrix where the fractionated DNA is recovered.
- the fractionating electric fieldcan be used to load and recover the DNA to operate the process continuously.
- the present inventionprovides a method and apparatus for the fractionation of macro-molecules on micro/nano-fabricated support materials (a.k.a. matrices). Because the motion of DNA molecules can be accurately controlled in micro/nano-fabricated environments, the fractionation of DNA can be achieved with very high resolution in a short time (i.e. seconds), even for DNA molecules larger than 100 kbp. In addition, the process can be operated continuously, i.e., DNA is loaded, fractionated, and recovered at the same time. Moreover, because this method exploits micro/nano-fabricated structure, it can be readily integrated into lab-on-a-chip devices as a component.
- DNA moleculesenter from one point or loading channel 14 on the boundary 12 of the matrix 10 as shown in FIG. 2 .
- the moleculesare subsequently fractionated into different bands at different orientations, according to their sizes, as they are driven towards the other side 13 of the matrix 10 , where the purified DNA molecules 30 are finally recovered.
- the DNA moleculesare fractionated into short fragments 32 at one end, long fragments 36 at the other end, and medium fragments 34 therebetween.
- the electric field (E 1 and E 2 ) that fractionates the DNA samplecan also be used to load and recover the sample, enabling the process to be operated continuously.
- the support materialcomprises a micro/nano-fabricated porous structure, in which DNA molecules can move.
- An alternating electric field, shown in E 1 and E 2is applied across the whole matrix.
- E 1 and E 2are at an angle with respect to each other, preferably an obtuse angle, and have different intensities and/or durations. Because DNA molecules are stretched and moving in a zigzag way under the alternating field, shorter fragments move at an angle to longer fragments.
- DNA moleculesWhen DNA molecules are subject to an alternating electric field between two orientations at an angle such as an obtuse angle, they are stretched to different lengths according to their molecular weight. Referring to FIG. 3 , let the end-to-end length of a stretched DNA molecule be x. Assume that electric field E 1 displaces every DNA molecules by approximately the same displacement ⁇ e 1 , whereas E 2 displaces every DNA molecules by approximately ⁇ e 2 (e 1 and e 2 are unit vectors, and both ⁇ and ⁇ are positive numbers, since DNA molecules are negatively charged and move opposite to an applied electric field).
- an alternating electric fieldnot only stretches DNA molecules to a linear conformation, but also makes them to move in a zigzag way.
- the initial position of a DNA moleculeis labeled as 0 .
- the big dot on one end of the DNArepresents the “head” 42 of the molecule.
- the other end of the moleculeis referred to as the “tail” 40 .
- E 1When E 1 is applied, the DNA molecule moves to position 1 .
- the tail 40leads the motion as the electric field is switched to E 2 .
- the end of one cyclethe molecule moves to position 2 , and the net displacement in one cycle ( ⁇ x)e 1 +( ⁇ x)e 2 .
- electric fieldwhat is meant is the spatial average of the field around a location over a length scale of several obstacles, not the microscopic field distribution around a single obstacle.
- Any electric field at a given locationwhose direction varys with time, can be resolved uniquely into two sequences of electric pulses according to the instantaneous direction of the field.
- the first sequence of electric pulsescomprises the electric field pointing to one side of the average field vector over the whole period of time when the field is applied to fractionate the molecules.
- the second sequence of electric pulsescomprises the electric field pointing to the other side of the average field vector. If the field vector at a moment is at the same direction or at the opposite direction of the average field vector, it is excluded in either of the pulse sequence.
- asymmetrical electric fieldwhat is meant is that the two sequences of electric pulses, resolved from a given electric field, as a function of time, have vector integrals over time that is not symmetric about the time-averaged field direction. Said another way, the electric fields, fields (t) whose odd-order integrals over time, ⁇
- Asymmetric fieldscan also be generated by sweeping signals in terms of orientation, duration and intensity. In the past, the field has first and second pulse sequences whose vector integrals over time are symmetrical about the average field.
- the matrix 10uses a microfabricated matrix 10 .
- the matrix 10consists of two parts: a microfabricated array of obstacles 20 in quartz, and a cap layer 18 that is hermetically bonded to the microfabricated side of the quartz substrate 16 .
- the quartz substrate 16is surface-micromachined using standard microfabrication techniques.
- the substrateis subsequently bonded to a glass cap layer 18 hermetically.
- the cavities between the substrate and the cap layerbecome microfluidic channels in which DNA molecules are fractionated.
- FIGS. 6 a and 6 bThe dimensions of this microfabricated device are depicted in FIGS. 6 a and 6 b .
- FIG. 6 ais the top view of the matrix 10
- FIG. 6 bis a side view of the matrix 10 .
- the matrix 10 in this caseis a hexagonal array of obstacles 20 .
- Each obstacle 20comprises a cylindrical post 2 ⁇ m in diameter.
- the center-to-center distance between neighboring obstaclesis 4 ⁇ m.
- the uniformity of the electric field across the whole matrixis controlled accurately by the peripheral structures surrounding the matrix.
- FIG. 7shows the fractionation of T4 (169 kbp) and T7 (40 kbp) DNA molecules.
- the DNA injected into the matrixis 10 ⁇ g/ml of T4 DNA and 10 ⁇ g/ml of T7 DNA in 1 ⁇ 2 TBE buffer.
- the duration of E 1is identical to that of E 2 , which is 166 msec.
- the frequency at which the electric field alternatesis 3 Hz.
- the DNA mixtureseparates into two bands.
Landscapes
- Chemical & Material Sciences (AREA)
- Health & Medical Sciences (AREA)
- Engineering & Computer Science (AREA)
- Life Sciences & Earth Sciences (AREA)
- Biomedical Technology (AREA)
- Genetics & Genomics (AREA)
- Zoology (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Wood Science & Technology (AREA)
- Biotechnology (AREA)
- General Engineering & Computer Science (AREA)
- Organic Chemistry (AREA)
- Analytical Chemistry (AREA)
- Molecular Biology (AREA)
- Plant Pathology (AREA)
- Microbiology (AREA)
- Biophysics (AREA)
- Physics & Mathematics (AREA)
- Biochemistry (AREA)
- General Health & Medical Sciences (AREA)
- Crystallography & Structural Chemistry (AREA)
- Apparatus Associated With Microorganisms And Enzymes (AREA)
- Measuring Or Testing Involving Enzymes Or Micro-Organisms (AREA)
- Saccharide Compounds (AREA)
Abstract
Description
- This application claims the priority of Provisional Application Ser. No. 60/256,298, filed Dec. 18, 2000, the entire disclosure of which is expressly incorporated herein by reference.
- The present invention has been made under Federal Contract Grant No. MDA 972-00-1-0031 and the government may have certain rights to the subject invention.
- 1. Field of the Invention
- The present invention relates to a method and apparatus for fractionating charged macro-molecules such as DNA using asymmetric pulsed field electrophoresis.
- 2. Related Art
- The analysis and fractionation of large DNA molecules is a central step in large scale sequencing projects. Conventionally, gel electrophoresis is used to fractionate DNA molecules according to their sizes. This method includes two steps: sample loading and fractionation. First, sample solution containing DNA is loaded into loading wells in the gel slab before the electric field is turned on. Then, an electric field is applied. The DNA molecules move in the opposite direction of the electric field because they are negatively charged. As the electric field is applied, DNA molecules travel at different speeds according to their sizes, but the directions in which they migrate are always the same. Eventually, sample DNA molecules are separated into different bands, each of which contains DNA molecules of the same size, as shown in
FIG. 1 . Shorter DNA fragments move faster than longer ones. Therefore, they are separated according to their sizes. However, this standard method only works effectively for DNA molecules smaller than 40 kbp. Above this range, the standard method has to be modified. In particular, the applied electric field can no longer be DC, but is made to alternate between two different orientations. This modified scheme (pulsed-field gel electrophoresis) is routinely used in modern molecular biology laboratories, but it typically takes a few days to fractionate one set of DNA samples. - What is needed, and has not heretofore been provided, is a method and apparatus for quickly, or even continuously, fractionating charged macro-molecules.
- It is an object of the present invention to provide a method and apparatus for quickly fractionating charged macro-molecules.
- It is an additional object of the present invention to provide a method and apparatus for continuously fractionating charged macro-molecules.
- It is a further object of the present invention to provide a method and apparatus for fractionating macro-molecules using asymmetric pulsed electrophoresis wherein an alternating electric field having two different orientations is applied, and one of the fields is stronger than the other in terms of duration or intensity, or the field is otherwise asymmetric.
- The present invention relates to a method and apparatus for fractionation of charged macro-molecules such as DNA. DNA solution is loaded into a matrix including an array of obstacles. An alternating electric field having two different fields at different orientations is applied. The alternating electric field is asymmetric in that one field is stronger in duration or intensity than the other field, or is otherwise asymmetric. The DNA molecules are thereby fractionated according to size and are driven to a far side of the matrix where the fractionated DNA is recovered. The fractionating electric field can be used to load and recover the DNA to operate the process continuously.
- Other important objects and features of the invention will be apparent from the following Detailed Description of the Invention taken in connection with the accompanying drawings in which:
FIG. 1 shows conventional gel electrophoresis.FIG. 2 is a diagram showing asymmetric pulsed-field electrophoresis in micro/nano-fabricated matrices according to the present invention.FIG. 3 is a diagram showing the basic principle of asymmetrical pulsed electrophoresis of the present invention.FIG. 4 shows the way stretched DNA molecules move under asymmetrical pulsed electric field.FIG. 5 shows a support material (matrix) for use in fractionation of DNA according to the present invention.FIG. 6A is a top view andFIG. 6B is a side view of the microfabricated support material shown inFIG. 5 .FIG. 7 shows fractionation of T4 and T7 DNA.- The present invention relates to a method and apparatus for fractionation of charged macro-molecules such as DNA. DNA solution is loaded into a matrix including an array of obstacles. An alternating electric field having two different fields at different orientations is applied. The alternating electric field is asymmetric in that one field is stronger in duration or intensity than the other field, or is otherwise asymmetric. The DNA molecules are thereby fractionated according to size and are driven to a far side of the matrix where the fractionated DNA is recovered. The fractionating electric field can be used to load and recover the DNA to operate the process continuously.
- The present invention provides a method and apparatus for the fractionation of macro-molecules on micro/nano-fabricated support materials (a.k.a. matrices). Because the motion of DNA molecules can be accurately controlled in micro/nano-fabricated environments, the fractionation of DNA can be achieved with very high resolution in a short time (i.e. seconds), even for DNA molecules larger than 100 kbp. In addition, the process can be operated continuously, i.e., DNA is loaded, fractionated, and recovered at the same time. Moreover, because this method exploits micro/nano-fabricated structure, it can be readily integrated into lab-on-a-chip devices as a component.
- According to the present invention, DNA molecules enter from one point or loading
channel 14 on theboundary 12 of thematrix 10 as shown inFIG. 2 . The molecules are subsequently fractionated into different bands at different orientations, according to their sizes, as they are driven towards theother side 13 of thematrix 10, where the purifiedDNA molecules 30 are finally recovered. The DNA molecules are fractionated intoshort fragments 32 at one end,long fragments 36 at the other end, andmedium fragments 34 therebetween. The electric field (E1and E2) that fractionates the DNA sample can also be used to load and recover the sample, enabling the process to be operated continuously. - A mixture of DNA molecules emerges continuously from the loading channel. The support material comprises a micro/nano-fabricated porous structure, in which DNA molecules can move. An alternating electric field, shown in E1and E2, is applied across the whole matrix. E1and E2are at an angle with respect to each other, preferably an obtuse angle, and have different intensities and/or durations. Because DNA molecules are stretched and moving in a zigzag way under the alternating field, shorter fragments move at an angle to longer fragments.
- When DNA molecules are subject to an alternating electric field between two orientations at an angle such as an obtuse angle, they are stretched to different lengths according to their molecular weight. Referring to
FIG. 3 , let the end-to-end length of a stretched DNA molecule be x. Assume that electric field E1displaces every DNA molecules by approximately the same displacement α e1, whereas E2displaces every DNA molecules by approximately β e2(e1and e2are unit vectors, and both α and β are positive numbers, since DNA molecules are negatively charged and move opposite to an applied electric field). This is a valid assumption because it is known that all DNA molecules have virtually the same mobility due to the fact that the long range hydrodynamic interaction is shielded by the counter ion layers. For the simplicity, let α be larger than β. This can be achieved by pulsing along −e1longer than along −e2, and/or by making the electric field stronger along −e1than along −e2. Because the electric field is alternating between two different directions, the DNA molecules will move in a zigzag way. Ideally, the electric field is chosen so that x<β<α. The net motion of very short DNA molecules (x<<β) in one pulsing cycle (a cycle refers to applying E1, then E2) is simply α e1+β e2. On the contrary, very long molecules (x>β) travel (α−β)e1in a cycle. Even though this could be rather surprising at first glance, it is not hard to understand if it is realized that when the field is switched from one to the other, thetails 40 of DNA strands become the ends that lead the motion and theheads 42 follow, as shown inFIG. 4 . In principle, we can predict the angles of the bands into which DNA mixtures are fractionated by this technique, if the stretched lengths of DNA molecules are smaller than or equal to β. Within this range (x<β or x=β), the net motion of DNA molecules in one cycle is (α−x)e1+(β−x)e2. Purified DNA molecules can be recovered at the bottom of the support material, after many cycles. In one cycle, a DNA molecule stretched to length x will travel (α−x)e1+(β−x)e2. - As shown in
FIG. 4 , an alternating electric field not only stretches DNA molecules to a linear conformation, but also makes them to move in a zigzag way. The initial position of a DNA molecule is labeled as0. The big dot on one end of the DNA represents the “head”42 of the molecule. The other end of the molecule is referred to as the “tail”40. When E1is applied, the DNA molecule moves to position1. Thetail 40 leads the motion as the electric field is switched to E2. By the end of one cycle, the molecule moves toposition 2, and the net displacement in one cycle (α−x)e1+(β−x)e2. - By electric field, what is meant is the spatial average of the field around a location over a length scale of several obstacles, not the microscopic field distribution around a single obstacle. Any electric field at a given location, whose direction varys with time, can be resolved uniquely into two sequences of electric pulses according to the instantaneous direction of the field. The first sequence of electric pulses comprises the electric field pointing to one side of the average field vector over the whole period of time when the field is applied to fractionate the molecules. The second sequence of electric pulses comprises the electric field pointing to the other side of the average field vector. If the field vector at a moment is at the same direction or at the opposite direction of the average field vector, it is excluded in either of the pulse sequence. By asymmetrical electric field, what is meant is that the two sequences of electric pulses, resolved from a given electric field, as a function of time, have vector integrals over time that is not symmetric about the time-averaged field direction. Said another way, the electric fields, fields(t) whose odd-order integrals over time, ∫|(t)|n(t)dt, are not at the time-average field orientation for every n, where n is any positive even integer. As such, by applying electric fields with different orientations and different strengths, i.e. different durations or different intensities or both, one applies an asymmetric field. Asymmetric fields can also be generated by sweeping signals in terms of orientation, duration and intensity. In the past, the field has first and second pulse sequences whose vector integrals over time are symmetrical about the average field.
- The following example uses a
microfabricated matrix 10. As shown inFIG. 5 , thematrix 10 consists of two parts: a microfabricated array ofobstacles 20 in quartz, and acap layer 18 that is hermetically bonded to the microfabricated side of thequartz substrate 16. Thequartz substrate 16 is surface-micromachined using standard microfabrication techniques. The substrate is subsequently bonded to aglass cap layer 18 hermetically. The cavities between the substrate and the cap layer become microfluidic channels in which DNA molecules are fractionated. The dimensions of this microfabricated device are depicted inFIGS. 6 aand6b.FIG. 6 ais the top view of thematrix 10, andFIG. 6 bis a side view of thematrix 10. Thematrix 10 in this case is a hexagonal array ofobstacles 20. Eachobstacle 20 comprises acylindrical post 2 μm in diameter. The center-to-center distance between neighboring obstacles is 4 μm. The uniformity of the electric field across the whole matrix is controlled accurately by the peripheral structures surrounding the matrix.FIG. 7 shows the fractionation of T4 (169 kbp) and T7 (40 kbp) DNA molecules. The pulse condition is E1=120 V/cm at 60° with respect to the horizontal boundary, and E2=60 V/cm at −60°. The DNA injected into the matrix is 10 μg/ml of T4 DNA and 10 μg/ml of T7 DNA in ½ TBE buffer. The duration of E1is identical to that of E2, which is 166 msec. The frequency at which the electric field alternates is 3 Hz. Clearly, the DNA mixture separates into two bands. - Having thus described the invention in detail, it is to be understood that the foregoing description is not intended to limit the spirit and scope thereof.
Claims (46)
1. A method of continuously fractionating charged macro-molecules comprising:
loading molecules into a matrix of obstacles;
applying an assymetric electric field to the matrix to separate the molecules according to size along a horizontal direction of the matrix; and
collecting separated molecules at a plurality of locations along a bottom edge of the matrix.
2. (canceled)
3. The method ofclaim 1 wherein the step of applying an asymmetric electric field to the matrix comprises applying to the matrix time-dependent electric fields(t) whose odd-order integrals over time, ∫|(t)|n(t)dt, are not at the time-average field orientation for every n, where n is any positive even integer.
4. The method ofclaim 1 wherein the step of applying an asymmetric electric field comprises:
alternating first and second electric pulses of first and second waveforms;
maintaining the integral of one of the first or second pulses' amplitude over time larger than that of the other pulse;
varying the orientation of the first electric pulse within first and second orientations, and the orientation of the second electric pulse within third and forth orientations.
5. The method ofclaim 4 wherein the first and second waveforms are square pulses.
6. The method ofclaim 5 wherein one of the square pulses is of higher amplitude than the other.
7. The method ofclaim 5 wherein one of the square pulses is of longer duration than the other.
8. The method ofclaim 1 wherein the step of applying an asymmetric electric field comprises:
alternating first and second electric pulses of first and second waveforms;
maintaining the integral over time of one of the first or second pulses' amplitudes larger thank that of the other pulse; and
applying the first and second electric pulses at first and second fixed orientations.
9. The method ofclaim 8 wherein the first and second waveforms are square pulses.
10. The method ofclaim 9 wherein one of the square pulses is of higher amplitude than the other.
11. The method ofclaim 9 wherein one of the square pulses is of longer duration than the other.
12. The method ofclaim 1 wherein the charged macro-molecules are deoxyribonucleic acid (a.k.a. DNA).
13. (canceled)
14. (canceled)
15. The method ofclaim 1 wherein the molecules are loaded using electric fields.
16. The method ofclaim 1 wherein the molecules are extracted from the array of obstacles using electric fields.
17. The method ofclaim 1 wherein the molecules are routed to the next processing step after fractionation.
18. A method of continuously fractionating charged macro-molecules comprising:
loading molecules into a matrix with an array of obstacles;
applying to the matrix electric fields whose amplitudes are constant in time;
varying field orientations of the electric fields with time to create an asymmetrical electric field to separate the molecules according to size along a horizontal direction of the matrix; and
collecting separated molecules at a plurality of locations along a bottom edge of the matrix.
19. (canceled)
20. The method ofclaim 18 wherein the fields alternate between two fixed orientations.
21. The method ofclaim 18 wherein the charged macro-molecules are deoxyribonucleic acid (a.k.a. DNA).
22. (canceled)
23. (canceled)
24. The method ofclaim 18 wherein the molecules are loaded using electric fields.
25. The method ofclaim 18 wherein the molecules are extracted from the array of obstacles using electric fields.
26. The method ofclaim 18 wherein the molecules are routed to the next processing step after fractionation.
27. An apparatus for continuously fractionating charged macro-molecules comprising:
an array of obstacles;
asymmetrically alternating electric fields applied to the array of obstacles to separate molecules according to size along a horizontal direction of the array; and
a plurality of locations along a bottom edge of the array for collecting separated molecules.
28. The apparatus ofclaim 27 wherein the asymmetrically alternating electric fields comprise:
an electric field which is alternating in direction as a function of time at a location in the matrix, and which has a time average of an electric field vector over many cycles, whereby the time integral of the vector at the same location over a part of the cycles when the electric field is instantaneously pointing to one side of the vector is not spatially symmetric about the vector with the time integral of the vector over another part of the cycles at the same location when the electric field is instantaneously pointing to another side of the vector.
30. The apparatus ofclaim 27 wherein the asymmetrically alternating electric fields comprise:
first and second electric pulses of first and second waveforms;
the integral over time of one of the first or second pulses' amplitude larger than that of the other pulse;
the orientation of the first electric pulse varying between a first orientation and second orientation, and the orientation of the second electric pulse varying between a third orientation and forth orientation.
31. The apparatus ofclaim 30 wherein the first and second waveforms are square pulses.
32. The apparatus ofclaim 31 wherein one of the square pulses is of higher amplitude than the other.
33. The apparatus ofclaim 31 wherein one of the square pulses is of longer duration than the other.
34. The apparatus ofclaim 27 wherein the asymmetrically alternating electric fields comprise:
first and second alternating electric pulses of first and second waveforms;
the integral over time of one of the first or second pulses' amplitudes larger than that of the other pulse;
the first and second electric pulses applied at first and second fixed orientations.
35. The apparatus ofclaim 34 wherein the first and second waveforms are square pulses.
36. The apparatus ofclaim 35 wherein one of the square pulses is of higher amplitude than the other.
37. The apparatus ofclaim 35 wherein one of the square pulses is of longer duration than the other.
38. The apparatus ofclaim 27 wherein the asymmetrically alternating electric fields comprise:
electric fields whose amplitudes are constant in time;
the field orientation varying with time in such a manner that ∫[θ(t)]n+1dt are not zero for every n, where θ(t) is field orientation with respect to the time-average field orientation, and n is any even integer larger than zero.
39. The apparatus ofclaim 38 wherein the fields alternate between two fixed orientations.
40. The apparatus ofclaim 27 wherein the charged molecules are deoxyribonucleic acid (a.k.a. DNA).
41. (canceled)
42. The apparatus ofclaim 27 further comprising extraction structures for extracting fractionated molecules from the array of obstacles.
43. The apparatus ofclaim 27 further comprising one or more loading channels for loading molecules.
44. The apparatus ofclaim 27 wherein the molecules are extracted from the array of obstacles using electric fields.
45. The apparatus ofclaim 27 wherein the molecules are loaded into the array of obstacles using electric fields.
46. The apparatus ofclaim 27 wherein the molecules are routed to the next processing step after fractionation.
Priority Applications (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US11/075,682US20050161331A1 (en) | 2000-12-18 | 2005-03-09 | Fractionation of macro-molecules using asymmetric pulsed field electrophoresis |
| US12/181,827US20090014332A1 (en) | 2000-12-18 | 2008-07-29 | Fractionation of Macro-Molecules Using Asymmetric Pulsed Field Electrophoresis |
Applications Claiming Priority (3)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US25629800P | 2000-12-18 | 2000-12-18 | |
| US10/022,189US6881317B2 (en) | 2000-12-18 | 2001-12-18 | Fractionation of macro-molecules using asymmetric pulsed field electrophoresis |
| US11/075,682US20050161331A1 (en) | 2000-12-18 | 2005-03-09 | Fractionation of macro-molecules using asymmetric pulsed field electrophoresis |
Related Parent Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US10/022,189ContinuationUS6881317B2 (en) | 2000-12-18 | 2001-12-18 | Fractionation of macro-molecules using asymmetric pulsed field electrophoresis |
Related Child Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US12/181,827DivisionUS20090014332A1 (en) | 2000-12-18 | 2008-07-29 | Fractionation of Macro-Molecules Using Asymmetric Pulsed Field Electrophoresis |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| US20050161331A1true US20050161331A1 (en) | 2005-07-28 |
Family
ID=22971713
Family Applications (3)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US10/022,189Expired - Fee RelatedUS6881317B2 (en) | 2000-12-18 | 2001-12-18 | Fractionation of macro-molecules using asymmetric pulsed field electrophoresis |
| US11/075,682GrantedUS20050161331A1 (en) | 2000-12-18 | 2005-03-09 | Fractionation of macro-molecules using asymmetric pulsed field electrophoresis |
| US12/181,827AbandonedUS20090014332A1 (en) | 2000-12-18 | 2008-07-29 | Fractionation of Macro-Molecules Using Asymmetric Pulsed Field Electrophoresis |
Family Applications Before (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US10/022,189Expired - Fee RelatedUS6881317B2 (en) | 2000-12-18 | 2001-12-18 | Fractionation of macro-molecules using asymmetric pulsed field electrophoresis |
Family Applications After (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US12/181,827AbandonedUS20090014332A1 (en) | 2000-12-18 | 2008-07-29 | Fractionation of Macro-Molecules Using Asymmetric Pulsed Field Electrophoresis |
Country Status (5)
| Country | Link |
|---|---|
| US (3) | US6881317B2 (en) |
| EP (1) | EP1343800A4 (en) |
| JP (1) | JP2004518663A (en) |
| AU (1) | AU2002230979A1 (en) |
| WO (1) | WO2002050095A1 (en) |
Cited By (9)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US20030075444A1 (en)* | 2001-10-19 | 2003-04-24 | Huang Lotien Richard | Method and apparatus for generating electric fields and flow distributions for rapidly separating molecules |
| US20070156129A1 (en)* | 2006-01-03 | 2007-07-05 | Alcon, Inc. | System For Dissociation and Removal of Proteinaceous Tissue |
| US20110118734A1 (en)* | 2009-11-16 | 2011-05-19 | Alcon Research, Ltd. | Capsularhexis device using pulsed electric fields |
| US20110118729A1 (en)* | 2009-11-13 | 2011-05-19 | Alcon Research, Ltd | High-intensity pulsed electric field vitrectomy apparatus with load detection |
| US20110135626A1 (en)* | 2009-12-08 | 2011-06-09 | Alcon Research, Ltd. | Localized Chemical Lysis of Ocular Tissue |
| US20110144562A1 (en)* | 2009-12-14 | 2011-06-16 | Alcon Research, Ltd. | Localized Pharmacological Treatment of Ocular Tissue Using High-Intensity Pulsed Electrical Fields |
| US20110144641A1 (en)* | 2009-12-15 | 2011-06-16 | Alcon Research, Ltd. | High-Intensity Pulsed Electric Field Vitrectomy Apparatus |
| US8546979B2 (en) | 2010-08-11 | 2013-10-01 | Alcon Research, Ltd. | Self-matching pulse generator with adjustable pulse width and pulse frequency |
| US8679751B2 (en) | 2009-12-23 | 2014-03-25 | Cytovera Inc. | System and method for particle filtration |
Families Citing this family (17)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US7198701B2 (en) | 2000-11-29 | 2007-04-03 | Japan Science And Technology Corporation | Nucleic acid analyzing method |
| EP1343800A4 (en)* | 2000-12-18 | 2005-06-29 | Univ Princeton | FRACTIONATION OF MACROMOLECULES USING ASYMMETRIC CHANGE ELECTROPHORESIS |
| US10337054B2 (en) | 2004-02-02 | 2019-07-02 | Quantum-Si Incorporated | Enrichment of nucleic acid targets |
| US8518228B2 (en) | 2011-05-20 | 2013-08-27 | The University Of British Columbia | Systems and methods for enhanced SCODA |
| WO2007149111A2 (en)* | 2005-10-06 | 2007-12-27 | Massachusetts Institute Of Technology | Continuous biomolecule separation in a nanofilter |
| EP2304414B1 (en)* | 2008-07-24 | 2017-03-15 | The Trustees of Princeton University | Bump array device having asymmetric gaps for segregation of particles |
| US20110144638A1 (en)* | 2009-12-14 | 2011-06-16 | Alcon Research, Ltd. | Localized Shockwave-Induced Tissue Disruption |
| CN102140462B (en)* | 2010-04-29 | 2013-06-12 | 苏州吉玛基因股份有限公司 | Human miR-1260 antisense nucleic acid and application thereof |
| CN105247042B (en) | 2013-03-15 | 2021-06-11 | 普林斯顿大学理事会 | Method and apparatus for high throughput purification |
| CN110186835B (en) | 2013-03-15 | 2022-05-31 | Gpb科学有限公司 | On-chip microfluidic processing of particles |
| US20150064153A1 (en) | 2013-03-15 | 2015-03-05 | The Trustees Of Princeton University | High efficiency microfluidic purification of stem cells to improve transplants |
| WO2016019393A1 (en) | 2014-08-01 | 2016-02-04 | Gpb Scientific, Llc | Methods and systems for processing particles |
| US11130986B2 (en) | 2015-05-20 | 2021-09-28 | Quantum-Si Incorporated | Method for isolating target nucleic acid using heteroduplex binding proteins |
| US10976232B2 (en) | 2015-08-24 | 2021-04-13 | Gpb Scientific, Inc. | Methods and devices for multi-step cell purification and concentration |
| US10850276B2 (en) | 2017-02-24 | 2020-12-01 | VisuGen Global LLC | Systems and methods for capture and detection of low copy targets from large sample volumes |
| US10844353B2 (en) | 2017-09-01 | 2020-11-24 | Gpb Scientific, Inc. | Methods for preparing therapeutically active cells using microfluidics |
| CA3159566A1 (en) | 2019-10-29 | 2021-05-06 | Quantum-Si Incorporated | Peristaltic pumping of fluids and associated methods, systems, and devices |
Citations (35)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US2555487A (en)* | 1948-02-27 | 1951-06-05 | United Shoe Machinery Corp | Chromatographic process and apparatus |
| US3450624A (en)* | 1966-07-20 | 1969-06-17 | Fisher Scientific Co | Apparatus for the separation of chemical components by the combination of electrophoresis and gel filtration |
| US3458427A (en)* | 1965-12-08 | 1969-07-29 | Beckman Instruments Inc | Continuous flow electrophoresis apparatus |
| US3498905A (en)* | 1965-06-18 | 1970-03-03 | Beckman Instruments Inc | Continuous flow electrophoresis apparatus |
| US3519549A (en)* | 1963-11-18 | 1970-07-07 | Wolfgang Grassmann | Apparatus for performance of carrier-free,continuous electrophoresis in vertical cells |
| US3563872A (en)* | 1968-08-28 | 1971-02-16 | Beckman Instruments Inc | Voltage gradient control system for electrophoresis apparatus |
| US3847773A (en)* | 1973-06-11 | 1974-11-12 | Technicon Instr | Method and apparatus for curtain electrophoresis |
| US4061560A (en)* | 1975-02-28 | 1977-12-06 | Max-Planck-Gesellschaft Zur Forderung Der Wissenschaften E.V. | Apparatus for deflection electrophoresis |
| US4148703A (en)* | 1976-02-11 | 1979-04-10 | Morton Weintraub | Method of electrophoretic purification of enzymes and peptides by means of an adjustable, specialized, geometrically located electrode system |
| US4315812A (en)* | 1980-05-28 | 1982-02-16 | Karlson Eskil L | Apparatus for continuous electrochromatographic separation |
| US4473452A (en)* | 1982-11-18 | 1984-09-25 | The Trustees Of Columbia University In The City Of New York | Electrophoresis using alternating transverse electric fields |
| US4523320A (en)* | 1983-01-14 | 1985-06-11 | Northrop Corporation | Uniform-field electrode |
| US4693804A (en)* | 1984-12-19 | 1987-09-15 | Board Of Regents, The University Of Texas System | Apparatus for bidimensional electrophoretic separations |
| US4732656A (en)* | 1985-10-25 | 1988-03-22 | Bios Corporation | Apparatus and process for resolving sample species |
| US4737251A (en)* | 1985-09-27 | 1988-04-12 | Washington University | Field-inversion gel electrophoresis |
| US4740283A (en)* | 1986-02-27 | 1988-04-26 | University Patents, Inc. | Pulsed-field gradient gel electrophoretic apparatus |
| US4830726A (en)* | 1988-02-03 | 1989-05-16 | The Wistar Institute | Separation of large DNA molecules in alternating asymmetric electric fields |
| US5011586A (en)* | 1988-08-12 | 1991-04-30 | Mj Research, Inc. | Constrained uniform field gel electrophoresis |
| US5084157A (en)* | 1988-03-21 | 1992-01-28 | California Institute Of Technology | Gel electrophoresis using time dependent contour controlled electric fields |
| US5165898A (en)* | 1986-08-13 | 1992-11-24 | The Board Of Trustees Of The Leland Stanford Junior University | Electrophoresis using contour-clamped electric fields |
| US5180480A (en)* | 1991-01-28 | 1993-01-19 | Ciba-Geigy Corporation | Apparatus for the preparation of samples, especially for analytical purposes |
| US5427663A (en)* | 1993-06-08 | 1995-06-27 | British Technology Group Usa Inc. | Microlithographic array for macromolecule and cell fractionation |
| US5671086A (en)* | 1995-04-18 | 1997-09-23 | The Regents, University Of California | Method and apparatus for accurately manipulating an object during microelectrophoresis |
| US5972190A (en)* | 1996-11-19 | 1999-10-26 | Mcdonnell Douglas Corporation | Continuous flow electrophoresis apparatus |
| US6110339A (en)* | 1995-06-08 | 2000-08-29 | Visible Genetics Inc. | Nanofabricated separation matrix for analysis of biopolymers and methods of making and using same |
| US6127623A (en)* | 1998-07-03 | 2000-10-03 | Sharp Kabushiki Kaisha | Solar cell and production process therefor |
| US6156273A (en)* | 1997-05-27 | 2000-12-05 | Purdue Research Corporation | Separation columns and methods for manufacturing the improved separation columns |
| US6176990B1 (en)* | 1995-06-08 | 2001-01-23 | Visible Genetics Inc. | Micro-electrophoresis chip for moving and separating nucleic acids and other charged molecules |
| US6254754B1 (en)* | 1998-07-29 | 2001-07-03 | Agilent Technologies, Inc. | Chip for performing an electrophoretic separation of molecules and method using same |
| US6280590B1 (en)* | 1996-09-06 | 2001-08-28 | Nanogen, Inc. | Channel-less separation of bioparticles on a bioelectronic chip by dielectrophoresis |
| US6328868B1 (en)* | 1997-03-21 | 2001-12-11 | Gerhard Weber | Method for carrier-free deflection electrophoresis |
| US6540896B1 (en)* | 1998-08-05 | 2003-04-01 | Caliper Technologies Corp. | Open-Field serial to parallel converter |
| US20030075444A1 (en)* | 2001-10-19 | 2003-04-24 | Huang Lotien Richard | Method and apparatus for generating electric fields and flow distributions for rapidly separating molecules |
| US6685810B2 (en)* | 2000-02-22 | 2004-02-03 | California Institute Of Technology | Development of a gel-free molecular sieve based on self-assembled nano-arrays |
| US6881317B2 (en)* | 2000-12-18 | 2005-04-19 | The Trustees Of Princeton University | Fractionation of macro-molecules using asymmetric pulsed field electrophoresis |
Family Cites Families (6)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US5106468A (en) | 1985-12-30 | 1992-04-21 | Exxon Research And Engineering Company | Electrophoretic separation |
| JPH02176457A (en) | 1988-09-15 | 1990-07-09 | Carnegie Inst Of Washington | Pulse oriented electrophoresis |
| US5122248A (en)* | 1990-05-18 | 1992-06-16 | Northeastern University | Pulsed field capillary electrophoresis |
| US5116471A (en) | 1991-10-04 | 1992-05-26 | Varian Associates, Inc. | System and method for improving sample concentration in capillary electrophoresis |
| US5178737A (en) | 1991-12-04 | 1993-01-12 | The University Of North Carolina At Chapel Hill | Electrophoretic resolution of single strand DNA by asymmetric field inversion |
| US6027623A (en)* | 1998-04-22 | 2000-02-22 | Toyo Technologies, Inc. | Device and method for electrophoretic fraction |
- 2001
- 2001-12-18EPEP01991239Apatent/EP1343800A4/ennot_activeWithdrawn
- 2001-12-18WOPCT/US2001/048881patent/WO2002050095A1/enactiveApplication Filing
- 2001-12-18USUS10/022,189patent/US6881317B2/ennot_activeExpired - Fee Related
- 2001-12-18JPJP2002551988Apatent/JP2004518663A/enactivePending
- 2001-12-18AUAU2002230979Apatent/AU2002230979A1/ennot_activeAbandoned
- 2005
- 2005-03-09USUS11/075,682patent/US20050161331A1/enactiveGranted
- 2008
- 2008-07-29USUS12/181,827patent/US20090014332A1/ennot_activeAbandoned
Patent Citations (37)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US2555487A (en)* | 1948-02-27 | 1951-06-05 | United Shoe Machinery Corp | Chromatographic process and apparatus |
| US3519549A (en)* | 1963-11-18 | 1970-07-07 | Wolfgang Grassmann | Apparatus for performance of carrier-free,continuous electrophoresis in vertical cells |
| US3498905A (en)* | 1965-06-18 | 1970-03-03 | Beckman Instruments Inc | Continuous flow electrophoresis apparatus |
| US3458427A (en)* | 1965-12-08 | 1969-07-29 | Beckman Instruments Inc | Continuous flow electrophoresis apparatus |
| US3450624A (en)* | 1966-07-20 | 1969-06-17 | Fisher Scientific Co | Apparatus for the separation of chemical components by the combination of electrophoresis and gel filtration |
| US3563872A (en)* | 1968-08-28 | 1971-02-16 | Beckman Instruments Inc | Voltage gradient control system for electrophoresis apparatus |
| US3847773A (en)* | 1973-06-11 | 1974-11-12 | Technicon Instr | Method and apparatus for curtain electrophoresis |
| US4061560A (en)* | 1975-02-28 | 1977-12-06 | Max-Planck-Gesellschaft Zur Forderung Der Wissenschaften E.V. | Apparatus for deflection electrophoresis |
| US4148703A (en)* | 1976-02-11 | 1979-04-10 | Morton Weintraub | Method of electrophoretic purification of enzymes and peptides by means of an adjustable, specialized, geometrically located electrode system |
| US4315812A (en)* | 1980-05-28 | 1982-02-16 | Karlson Eskil L | Apparatus for continuous electrochromatographic separation |
| US4473452A (en)* | 1982-11-18 | 1984-09-25 | The Trustees Of Columbia University In The City Of New York | Electrophoresis using alternating transverse electric fields |
| US4523320A (en)* | 1983-01-14 | 1985-06-11 | Northrop Corporation | Uniform-field electrode |
| US4693804A (en)* | 1984-12-19 | 1987-09-15 | Board Of Regents, The University Of Texas System | Apparatus for bidimensional electrophoretic separations |
| US4737251A (en)* | 1985-09-27 | 1988-04-12 | Washington University | Field-inversion gel electrophoresis |
| US4732656A (en)* | 1985-10-25 | 1988-03-22 | Bios Corporation | Apparatus and process for resolving sample species |
| US4740283A (en)* | 1986-02-27 | 1988-04-26 | University Patents, Inc. | Pulsed-field gradient gel electrophoretic apparatus |
| US5165898A (en)* | 1986-08-13 | 1992-11-24 | The Board Of Trustees Of The Leland Stanford Junior University | Electrophoresis using contour-clamped electric fields |
| US4830726A (en)* | 1988-02-03 | 1989-05-16 | The Wistar Institute | Separation of large DNA molecules in alternating asymmetric electric fields |
| US5084157A (en)* | 1988-03-21 | 1992-01-28 | California Institute Of Technology | Gel electrophoresis using time dependent contour controlled electric fields |
| US5011586A (en)* | 1988-08-12 | 1991-04-30 | Mj Research, Inc. | Constrained uniform field gel electrophoresis |
| US5180480A (en)* | 1991-01-28 | 1993-01-19 | Ciba-Geigy Corporation | Apparatus for the preparation of samples, especially for analytical purposes |
| US5427663A (en)* | 1993-06-08 | 1995-06-27 | British Technology Group Usa Inc. | Microlithographic array for macromolecule and cell fractionation |
| US5837115A (en)* | 1993-06-08 | 1998-11-17 | British Technology Group Usa Inc. | Microlithographic array for macromolecule and cell fractionation |
| US5671086A (en)* | 1995-04-18 | 1997-09-23 | The Regents, University Of California | Method and apparatus for accurately manipulating an object during microelectrophoresis |
| US6176990B1 (en)* | 1995-06-08 | 2001-01-23 | Visible Genetics Inc. | Micro-electrophoresis chip for moving and separating nucleic acids and other charged molecules |
| US6110339A (en)* | 1995-06-08 | 2000-08-29 | Visible Genetics Inc. | Nanofabricated separation matrix for analysis of biopolymers and methods of making and using same |
| US6280590B1 (en)* | 1996-09-06 | 2001-08-28 | Nanogen, Inc. | Channel-less separation of bioparticles on a bioelectronic chip by dielectrophoresis |
| US5972190A (en)* | 1996-11-19 | 1999-10-26 | Mcdonnell Douglas Corporation | Continuous flow electrophoresis apparatus |
| US6328868B1 (en)* | 1997-03-21 | 2001-12-11 | Gerhard Weber | Method for carrier-free deflection electrophoresis |
| US6156273A (en)* | 1997-05-27 | 2000-12-05 | Purdue Research Corporation | Separation columns and methods for manufacturing the improved separation columns |
| US6596144B1 (en)* | 1997-05-27 | 2003-07-22 | Purdue Research Foundation | Separation columns and methods for manufacturing the improved separation columns |
| US6127623A (en)* | 1998-07-03 | 2000-10-03 | Sharp Kabushiki Kaisha | Solar cell and production process therefor |
| US6254754B1 (en)* | 1998-07-29 | 2001-07-03 | Agilent Technologies, Inc. | Chip for performing an electrophoretic separation of molecules and method using same |
| US6540896B1 (en)* | 1998-08-05 | 2003-04-01 | Caliper Technologies Corp. | Open-Field serial to parallel converter |
| US6685810B2 (en)* | 2000-02-22 | 2004-02-03 | California Institute Of Technology | Development of a gel-free molecular sieve based on self-assembled nano-arrays |
| US6881317B2 (en)* | 2000-12-18 | 2005-04-19 | The Trustees Of Princeton University | Fractionation of macro-molecules using asymmetric pulsed field electrophoresis |
| US20030075444A1 (en)* | 2001-10-19 | 2003-04-24 | Huang Lotien Richard | Method and apparatus for generating electric fields and flow distributions for rapidly separating molecules |
Cited By (13)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US20030075444A1 (en)* | 2001-10-19 | 2003-04-24 | Huang Lotien Richard | Method and apparatus for generating electric fields and flow distributions for rapidly separating molecules |
| US7597791B2 (en) | 2001-10-19 | 2009-10-06 | The Trustees Of Princeton University | Method and apparatus for generating electric fields and flow distributions for rapidly separating molecules |
| US20070156129A1 (en)* | 2006-01-03 | 2007-07-05 | Alcon, Inc. | System For Dissociation and Removal of Proteinaceous Tissue |
| US7824870B2 (en) | 2006-01-03 | 2010-11-02 | Alcon, Inc. | System for dissociation and removal of proteinaceous tissue |
| US20100331911A1 (en)* | 2006-01-03 | 2010-12-30 | Kovalcheck Steven W | System for Dissociation and Removal of Proteinaceous Tissue |
| US20110118729A1 (en)* | 2009-11-13 | 2011-05-19 | Alcon Research, Ltd | High-intensity pulsed electric field vitrectomy apparatus with load detection |
| US20110118734A1 (en)* | 2009-11-16 | 2011-05-19 | Alcon Research, Ltd. | Capsularhexis device using pulsed electric fields |
| US20110135626A1 (en)* | 2009-12-08 | 2011-06-09 | Alcon Research, Ltd. | Localized Chemical Lysis of Ocular Tissue |
| US20110144562A1 (en)* | 2009-12-14 | 2011-06-16 | Alcon Research, Ltd. | Localized Pharmacological Treatment of Ocular Tissue Using High-Intensity Pulsed Electrical Fields |
| US20110144641A1 (en)* | 2009-12-15 | 2011-06-16 | Alcon Research, Ltd. | High-Intensity Pulsed Electric Field Vitrectomy Apparatus |
| US8679751B2 (en) | 2009-12-23 | 2014-03-25 | Cytovera Inc. | System and method for particle filtration |
| US9174212B2 (en) | 2009-12-23 | 2015-11-03 | Cytovera Inc. | System and method for particle filtration |
| US8546979B2 (en) | 2010-08-11 | 2013-10-01 | Alcon Research, Ltd. | Self-matching pulse generator with adjustable pulse width and pulse frequency |
Also Published As
| Publication number | Publication date |
|---|---|
| WO2002050095A1 (en) | 2002-06-27 |
| US20090014332A1 (en) | 2009-01-15 |
| EP1343800A1 (en) | 2003-09-17 |
| EP1343800A4 (en) | 2005-06-29 |
| US6881317B2 (en) | 2005-04-19 |
| AU2002230979A1 (en) | 2002-07-01 |
| JP2004518663A (en) | 2004-06-24 |
| US20020098504A1 (en) | 2002-07-25 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| US20090014332A1 (en) | Fractionation of Macro-Molecules Using Asymmetric Pulsed Field Electrophoresis | |
| Cantor et al. | Pulsed-field gel electrophoresis of very large DNA molecules | |
| US9534304B2 (en) | Scodaphoresis and methods and apparatus for moving and concentrating particles | |
| JP3103031B2 (en) | Method and apparatus for moving molecules by applying an electric field | |
| US20090047681A1 (en) | Entropic trapping and sieving of molecules | |
| Slater et al. | Recent developments in DNA electrophoretic separations | |
| US8778157B2 (en) | Detecting analytes | |
| Bromberg et al. | Multichannel homogeneous immunoassay for detection of 2, 4, 6‐trinitrotoluene (TNT) using a microfabricated capillary array electrophoresis chip | |
| WO2000073780A1 (en) | Methods and apparatus for nonlinear mobility electrophoresis separation | |
| JP2007526823A5 (en) | ||
| KR102796115B1 (en) | Droplet interface within an electrowetting device | |
| Inatomi et al. | Electrophoresis of DNA in micro-pillars fabricated in polydimethylsiloxane | |
| US20100203540A1 (en) | Device for separating and/or analyzing several molecular targets dissolved in a complex mixture | |
| US7198701B2 (en) | Nucleic acid analyzing method | |
| EP0327363A2 (en) | Separation of large DNA molecules in alternating asymmetric electric fields | |
| EP0644420A2 (en) | Method and apparatus for gel electrophoresis | |
| CA2082906C (en) | Electrophoretic resolution of single strand dna by asymmetric field inversion | |
| US20040175299A1 (en) | Microscale affinity purification system | |
| Han et al. | From microfluidics to nanofluidics: DNA separation using nanofluidic entropic trap array device | |
| JP3420311B2 (en) | Electrophoresis device | |
| Craighead et al. | Separation of DNA in Microfluidic System | |
| Sudor et al. | Pulsed-field capillary electrophoresis of large DNA | |
| Kaji et al. | Fast separation of large DNA by nanopillar chip | |
| Schulze et al. | Detecting analytes | |
| JPH10206381A (en) | Sample introduction device to capillary |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| AS | Assignment | Owner name:TRUSTEES OF PRINCETON UNIVERSITY, THE, NEW JERSEY Free format text:ASSIGNMENT OF ASSIGNORS INTEREST;ASSIGNORS:HUANG, LOTIEN RICHARD;STURM, JAMES CHRISTOPHER;AUSTIN, ROBERT HAMILTON;REEL/FRAME:016368/0496;SIGNING DATES FROM 20020110 TO 20020125 | |
| STCB | Information on status: application discontinuation | Free format text:ABANDONED -- FAILURE TO RESPOND TO AN OFFICE ACTION |