US20040228766A1 - Point of care diagnostic platform - Google Patents
Point of care diagnostic platformDownload PDFInfo
- Publication number
- US20040228766A1 US20040228766A1US10/746,127US74612703AUS2004228766A1US 20040228766 A1US20040228766 A1US 20040228766A1US 74612703 AUS74612703 AUS 74612703AUS 2004228766 A1US2004228766 A1US 2004228766A1
- Authority
- US
- United States
- Prior art keywords
- cartridge
- modules
- cartridges
- module
- sample
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Abandoned
Links
- 210000004369bloodAnatomy0.000claimsabstractdescription31
- 239000008280bloodSubstances0.000claimsabstractdescription31
- 239000000523sampleSubstances0.000claimsdescription61
- 238000012360testing methodMethods0.000claimsdescription49
- 238000004891communicationMethods0.000claimsdescription26
- 239000012530fluidSubstances0.000claimsdescription26
- 238000012544monitoring processMethods0.000claimsdescription16
- 238000001514detection methodMethods0.000claimsdescription8
- 239000013610patient sampleSubstances0.000claimsdescription8
- 238000005345coagulationMethods0.000claimsdescription7
- 230000015271coagulationEffects0.000claimsdescription7
- 239000003792electrolyteSubstances0.000claimsdescription7
- 239000002699waste materialSubstances0.000claimsdescription6
- 238000003018immunoassayMethods0.000claimsdescription5
- 239000000969carrierSubstances0.000claimsdescription4
- 239000000383hazardous chemicalSubstances0.000claimsdescription4
- 239000000758substrateSubstances0.000claimsdescription4
- 238000004458analytical methodMethods0.000claimsdescription3
- 230000000747cardiac effectEffects0.000claimsdescription3
- 230000035558fertilityEffects0.000claimsdescription3
- 210000003734kidneyAnatomy0.000claimsdescription3
- 239000007788liquidSubstances0.000claimsdescription3
- 230000007246mechanismEffects0.000claimsdescription3
- 238000005070samplingMethods0.000claimsdescription3
- 238000005516engineering processMethods0.000claimsdescription2
- 230000000977initiatory effectEffects0.000claimsdescription2
- 238000012546transferMethods0.000claimsdescription2
- 239000000463materialSubstances0.000claims1
- 230000000717retained effectEffects0.000claims1
- 238000000034methodMethods0.000description25
- 239000003153chemical reaction reagentSubstances0.000description16
- 230000008569processEffects0.000description16
- 238000010586diagramMethods0.000description8
- 238000012545processingMethods0.000description7
- 210000004027cellAnatomy0.000description6
- 238000013022ventingMethods0.000description6
- 238000011017operating methodMethods0.000description5
- 230000003287optical effectEffects0.000description4
- 238000013459approachMethods0.000description3
- 239000000203mixtureSubstances0.000description3
- 238000003032molecular dockingMethods0.000description3
- 238000003908quality control methodMethods0.000description3
- 230000009471actionEffects0.000description2
- 239000003570airSubstances0.000description2
- 230000004888barrier functionEffects0.000description2
- 210000001124body fluidAnatomy0.000description2
- 239000010839body fluidSubstances0.000description2
- 239000003085diluting agentSubstances0.000description2
- 150000007523nucleic acidsChemical class0.000description2
- 102000039446nucleic acidsHuman genes0.000description2
- 108020004707nucleic acidsProteins0.000description2
- 238000000926separation methodMethods0.000description2
- 238000012549trainingMethods0.000description2
- 102000001554HemoglobinsHuman genes0.000description1
- 108010054147HemoglobinsProteins0.000description1
- 239000012190activatorSubstances0.000description1
- 230000003321amplificationEffects0.000description1
- 239000012491analyteSubstances0.000description1
- 238000003556assayMethods0.000description1
- 230000009286beneficial effectEffects0.000description1
- 230000008901benefitEffects0.000description1
- 230000001413cellular effectEffects0.000description1
- 230000008859changeEffects0.000description1
- 230000002950deficientEffects0.000description1
- 238000002405diagnostic procedureMethods0.000description1
- 229940079593drugDrugs0.000description1
- 239000003814drugSubstances0.000description1
- 238000005286illuminationMethods0.000description1
- 229940127121immunoconjugateDrugs0.000description1
- 239000003999initiatorSubstances0.000description1
- 210000000265leukocyteAnatomy0.000description1
- 230000002934lysing effectEffects0.000description1
- 238000005259measurementMethods0.000description1
- 239000012528membraneSubstances0.000description1
- 238000003199nucleic acid amplification methodMethods0.000description1
- 230000000149penetrating effectEffects0.000description1
- 230000000737periodic effectEffects0.000description1
- 238000012123point-of-care testingMethods0.000description1
- 239000012898sample dilutionSubstances0.000description1
- 230000011218segmentationEffects0.000description1
- 239000002910solid wasteSubstances0.000description1
- 239000000243solutionSubstances0.000description1
- 230000007704transitionEffects0.000description1
- 238000012795verificationMethods0.000description1
Images
Classifications
- G—PHYSICS
- G01—MEASURING; TESTING
- G01N—INVESTIGATING OR ANALYSING MATERIALS BY DETERMINING THEIR CHEMICAL OR PHYSICAL PROPERTIES
- G01N33/00—Investigating or analysing materials by specific methods not covered by groups G01N1/00 - G01N31/00
- G01N33/48—Biological material, e.g. blood, urine; Haemocytometers
- G01N33/50—Chemical analysis of biological material, e.g. blood, urine; Testing involving biospecific ligand binding methods; Immunological testing
- G01N33/53—Immunoassay; Biospecific binding assay; Materials therefor
- G01N33/5302—Apparatus specially adapted for immunological test procedures
- G—PHYSICS
- G01—MEASURING; TESTING
- G01N—INVESTIGATING OR ANALYSING MATERIALS BY DETERMINING THEIR CHEMICAL OR PHYSICAL PROPERTIES
- G01N21/00—Investigating or analysing materials by the use of optical means, i.e. using sub-millimetre waves, infrared, visible or ultraviolet light
- G01N21/01—Arrangements or apparatus for facilitating the optical investigation
- G01N21/03—Cuvette constructions
- G01N21/05—Flow-through cuvettes
- G—PHYSICS
- G16—INFORMATION AND COMMUNICATION TECHNOLOGY [ICT] SPECIALLY ADAPTED FOR SPECIFIC APPLICATION FIELDS
- G16H—HEALTHCARE INFORMATICS, i.e. INFORMATION AND COMMUNICATION TECHNOLOGY [ICT] SPECIALLY ADAPTED FOR THE HANDLING OR PROCESSING OF MEDICAL OR HEALTHCARE DATA
- G16H10/00—ICT specially adapted for the handling or processing of patient-related medical or healthcare data
- G16H10/40—ICT specially adapted for the handling or processing of patient-related medical or healthcare data for data related to laboratory analysis, e.g. patient specimen analysis
- Y—GENERAL TAGGING OF NEW TECHNOLOGICAL DEVELOPMENTS; GENERAL TAGGING OF CROSS-SECTIONAL TECHNOLOGIES SPANNING OVER SEVERAL SECTIONS OF THE IPC; TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y10—TECHNICAL SUBJECTS COVERED BY FORMER USPC
- Y10T—TECHNICAL SUBJECTS COVERED BY FORMER US CLASSIFICATION
- Y10T436/00—Chemistry: analytical and immunological testing
- Y10T436/11—Automated chemical analysis
- Y—GENERAL TAGGING OF NEW TECHNOLOGICAL DEVELOPMENTS; GENERAL TAGGING OF CROSS-SECTIONAL TECHNOLOGIES SPANNING OVER SEVERAL SECTIONS OF THE IPC; TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y10—TECHNICAL SUBJECTS COVERED BY FORMER USPC
- Y10T—TECHNICAL SUBJECTS COVERED BY FORMER US CLASSIFICATION
- Y10T436/00—Chemistry: analytical and immunological testing
- Y10T436/11—Automated chemical analysis
- Y10T436/115831—Condition or time responsive
- Y—GENERAL TAGGING OF NEW TECHNOLOGICAL DEVELOPMENTS; GENERAL TAGGING OF CROSS-SECTIONAL TECHNOLOGIES SPANNING OVER SEVERAL SECTIONS OF THE IPC; TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y10—TECHNICAL SUBJECTS COVERED BY FORMER USPC
- Y10T—TECHNICAL SUBJECTS COVERED BY FORMER US CLASSIFICATION
- Y10T436/00—Chemistry: analytical and immunological testing
- Y10T436/11—Automated chemical analysis
- Y10T436/117497—Automated chemical analysis with a continuously flowing sample or carrier stream
- Y—GENERAL TAGGING OF NEW TECHNOLOGICAL DEVELOPMENTS; GENERAL TAGGING OF CROSS-SECTIONAL TECHNOLOGIES SPANNING OVER SEVERAL SECTIONS OF THE IPC; TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y10—TECHNICAL SUBJECTS COVERED BY FORMER USPC
- Y10T—TECHNICAL SUBJECTS COVERED BY FORMER US CLASSIFICATION
- Y10T436/00—Chemistry: analytical and immunological testing
- Y10T436/11—Automated chemical analysis
- Y10T436/117497—Automated chemical analysis with a continuously flowing sample or carrier stream
- Y10T436/118339—Automated chemical analysis with a continuously flowing sample or carrier stream with formation of a segmented stream
Definitions
- the present inventionrelates generally to a point of care diagnostic system that has a plurality of modules and associated cartridges, and more particularly, to a point of care diagnostic system that includes a plurality of modules that share common QC protocols.
- Blood and other body fluid testsare important diagnostic methods in patient care and treatment. The reliability and the accuracy of the tests are critical in correctly diagnosing the patient and administrating proper treatment.
- the Food and Drug Administration (FDA)has established numerous quality standards for the various blood or body fluid tests. Monitoring the test process is beneficial in producing reliable and accurate test results.
- One way of monitoring the test processis periodically performing the monitoring test on standard test samples.
- the monitoring test resultsare compared with expected results to verify the accuracy of the test processes or correct the test instrument or process when appropriate.
- the test processesare assumed to generate consistent result between the monitoring tests.
- test samplesare included in the test process. This approach is suitable for a test process that performs tests on multiple samples. The test results on the standard test samples are compared with expected results to verify the accuracy of the test processes. In this approach, the test processes on real samples are assumed to generate result consistent with those on standard test samples.
- a point of care diagnostic platformthat has a plurality of modules coupled to common host computer.
- a point of care diagnostic platform with a plurality of modulesthat share common QC protocols.
- a point of care diagnostic platform with a plurality of modulescoupled to a host computer and an external communication system.
- a point of care diagnostic platform with a plurality of modules, and a plurality of analytic cartridgeswhere each cartridge is associated with a module and is configured to directly accept a blood sample from a standard blood draw tube.
- a point of care diagnostic platformthat has a plurality of modules, a host computer coupled to the modules, a common external communication interface, with each module sharing the common external communication interface.
- an object of the present inventionis to provide a point of care diagnostic platform that includes a plurality of modules that share common QC protocols. (is the common operator interface another patent?)
- Another object of the present inventionis to provide a point of care diagnostic platform with a plurality of module coupled to a common host computer.
- Yet another object of the present inventionis to provide a point of care diagnostic platform with a plurality of modules, a host computer coupled to the plurality of modules and an external communication system.
- Still another object of the present inventionis to provide a point of care diagnostic platform with a plurality of modules, and a plurality of analytic cartridges, where each cartridge is associated with a module of the plurality of modules and is configured to directly accept a blood sample from a standard blood draw tube.
- Another object of the present inventionis to provide a point of care diagnostic platform with a plurality of modules; a host computer coupled to the plurality of modules and a common external communication interface, with each module sharing the common external communication interface.
- a further object of the present inventionis to provide a point of care diagnostic platform with a plurality of modules coupled to a common external communication interface such as a least one of WAN or a LAN.
- Another object of the present inventionis to provide a point of care diagnostic platform with a plurality of modules coupled to a common external communication interface that is coupled to a wireless network.
- a further object of the present inventionis to provide a point of care diagnostic platform with a plurality of modules coupled to a hospital information network or a laboratory information network.
- Yet another object of the present inventionis to provide a point of care diagnostic platform with a plurality of modules and a plurality of analytic cartridges that are each bar-coded with information for test protocols, and lot expiration dates.
- Still a further object of the present inventionis to provide a point of care diagnostic platform with a plurality of modules and a plurality of analytic cartridges that retain and seal fluids.
- Yet another object of the present inventionis to provide a point of care diagnostic platform that has a plurality of modules and a plurality of analytic cartridges, where all fluids in a cartridge, including a patient sample, remain within the cartridge.
- a point of care diagnostic platformincludes a plurality of modules.
- a plurality of analytic cartridgesare provided. Each cartridge is associated with a module and is configured to directly accept a blood sample from a standard blood draw tube.
- a point of care diagnostic platformincludes a plurality of modules.
- a host computeris coupled to the plurality of modules and a common external communication interface. Each module shares the common external communication interface.
- a point of care diagnostic platformincludes a plurality of modules each sharing the same QC protocols.
- a plurality of analytic cartridgesare included.
- a host computeris coupled to the plurality of modules.
- the host computeris coupled to an interface.
- Each modulehas a corresponding interface component.
- a point of care diagnostic platformincludes a plurality of modules.
- a plurality of analytic cartridgesare provided that each are bar-coded with information for test protocols, and lot expiration dates.
- a point of care diagnostic platformincludes a plurality of modules.
- a plurality of analytic cartridgesare provided that retain and seal fluids.
- a point of care diagnostic platformincludes a plurality of modules.
- a plurality of analytic cartridgesare provided. All fluids in the cartridges, including patient samples, remain within the cartridges.
- a point of care diagnostic platformincludes a plurality of modules.
- a plurality of analytic cartridgesare provided. Each cartridge has wet and dry chemistries and at least one substrate that carriers a chemistry.
- FIG. 1( a )is a block diagram illustrating one embodiment of a point of care diagnostic platform of the present invention, with a user interface, host computer, multiple single-cartridge test processing modules and an external communication system.
- FIG. 1( b )is a block diagram illustrating another embodiment of a point of care diagnostic platform of the present invention, with multiple multi-cartridge test processing modules.
- FIG. 1( c )is a block diagram illustrating another embodiment of a point of care diagnostic platform of the present invention, with the host computer being integrated with multiple, multi-cartridge modules.
- FIG. 1( d )is a block diagram illustrating another embodiment of a point of care diagnostic platform of the present invention, with the host computer and user interface both integrated with multiple, multi-cartridge modules.
- FIG. 1( e )is a block diagram illustrating another embodiment of a point of care diagnostic platform of the present invention, with the host computer and user interface integrated with multiple, single-cartridge modules.
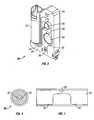
- FIG. 2is a cross-sectional view of one embodiment of a cartridge that can be utilized with the point of care diagnostic platform of the present invention.
- FIG. 3is a cross-sectional view of a sample tube that can be utilized with cartridges of the present invention.
- FIG. 4is a schematic diagram illustrating one embodiment of the docking, and the relationship between a cartridge and a module of the present invention.
- FIG. 5is a schematic diagram illustrating another embodiment of the docking, and the relationship between a cartridge and a module of the present invention.
- FIG. 6is a schematic diagram illustrating another embodiment of the docking, and the relationship between a cartridge and a module of the present invention.
- FIG. 7is a cross-sectional view of one embodiment of a cartridge utilized with the present invention, illustrating air, sample and reagent flow channels.
- FIG. 8is a flow chart illustrating an overall methodology of the point of care diagnostic platform of the present invention.
- FIG. 9is a flow chart illustrating one embodiment of a cartridge processing procedure implemented with the point of care diagnostic platform of the present invention.
- FIG. 10is a flow chart illustrating one embodiment of an immunoassay operating procedure implemented with the point of care diagnostic platform of the present invention.
- FIG. 11is a flow chart illustrating one embodiment of a hematology operating procedure implemented with the point of care diagnostic platform of the present invention.
- Point of care diagnostic platform 10includes a plurality of modules 12 .
- modules 12can be included but not limited to, immunoassay, hematology, electrolyte, molecular diagnostic, coagulation, blood gas, chemistry and the like.
- the modules 12can share at least a portion of a common functionality of operation such as fluid movement, sample introduction, and the like.
- each module 12contains common functionalities, and unique technologies that correspond to one or more selected chemistries.
- modules 12are multiple single-cartridge test processing modules.
- Platform 10can deliver a multitude of discreet testing capabilities in a standardized manner.
- Modules 12can have common operation platforms. Examples of common operation systems are user interface, quality control, calibration, training, connection to various laboratory information systems, hospital information systems, emergency room information systems, wireless communication and the like.
- a host computer 14is coupled to the plurality of modules 12 and also to a user interface 16 .
- Each module 12is coupled to the user interface 16 .
- Host computer 14has a variety of different capabilities, including but not limited to user interface, quality control, calibration, training, connection to various laboratory information systems, hospital information systems, emergency room information systems, wireless communication and the like.
- User interface 16is coupled to each module 12
- User interface 16provides uniform (automated and standardized) connectivity to the plurality of modules 12 as well as communication to other hospital and laboratory information systems. It will be appreciated that standardized includes industry standards as documented by the Connectivity Industry Consortium. User interface 16 establishes a database of analyzed samples and provides the operator with quality control options for the plurality of modules 12 .
- user interface 16includes capability for at least one of a cardiac, fertility, kidney, coagulation, electrolyte and hematology panel, molecular diagnostics and chemistry panels, and the like.
- Each module 12has a corresponding interface component for module control and sample results acquisition.
- host computer 14is also coupled to an external communication system 18 .
- external communication systemsare suitable including but not limited to a, WAN, LAN, wireless network, hospital information network, laboratory information network, and the like.
- Platform 10can be connected directly or in-directly to a emergency room/department patient management network
- each module 12shares common QC protocols.
- the QC protocolsinclude but are not limited to the following, module electronic verification, real-time process monitoring, patient record-keeping, periodic liquid control results monitoring, and the like.
- the QC protocolsare initiated in the same manner regardless of the module 12 that is tested.
- Electronic monitoring of the process at each module 12is continuous and transparent to the operator and do not require operator attention.
- Resultsare stored in module specific databases.
- Each modulecan utilize specific electronic and/or optical parameter monitoring. Changes in the electronic and optical parameters are tracked during the operation of the module 12 involved, and the outputs compared to expected thresholds/changes. These changes are indicative of correct internal operation during sample processing.
- FIG. 1( b )In another embodiment, illustrated in FIG. 1( b ), multiple, multi-modules are provided, where a module 12 can be utilized with more than one cartridge.
- host computer 14In FIG. 1( c ) host computer 14 is integrated with multiple, multi-cartridge modules 12 .
- host computer 14 and user interface 16are both integrated with multiple, multi-cartridge modules 12 .
- host computer and user interface 16are integrated with multiple, single-cartridge test processing modules 12 .
- Point of care diagnostic platform 10includes a plurality of cartridges 20 , illustrated in FIG. 2.
- Cartridges 20include but are not limited to cardiac, fertility, kidney, coagulation, electrolyte and hematology panel, molecular diagnostics and chemistry panels, and the like.
- Each cartridge 20can include a dock 22 for receiving a sample tube, an air dock 24 that can be engaged by a module 12 , a rotary valve 26 , which can also be engaged by a module 12 , a calibration chamber 28 , waste chamber 30 , sample/calibration flow path 32 which is coupled to a detector, sample out flow 34 , sample pressure channel 36 and a flow cell 38 which is a detection chamber.
- Cartridges 20can have wet and dry chemistries and at least one substrate that carriers a chemistry. Examples of various wet and dry chemistries are listed in table 1. TABLE 1 cartridge Wet Reagents Dry Reagents electrolytes calibration fluid ion specific electrode immunology — Capture antibody Conjugate antibody hemolotogy Lysing solution/white blood cell — nuclear label Hemoglobin dye Chemistry Various Various Coagulation — Initiator Blood gas — Electrode Molecular Nucleic acid label Nucleic acid capture Amplification reagents
- Cartridges 20are associated with a corresponding module 12 .
- cartridges 20can directly accept a blood sample from a standard blood draw, sample tube 40 which can include a pressure needle 42 and a sampling needle 44 , as shown in FIG. 3. This can be achieved by, (i) piercing the cap of the standard blood draw tube 40 needles 42 and 44 , which deliver low pressure air to force the sample through the other needle into the cartridge 20 , penetrating the cap with a single needle and withdrawing fluid directly using a vacuum, and the like.
- Cartridges 20can be configured to retain and seal fluids.
- modules 12can be configured to be engaged with the cartridges 20 to produce pneumatic movement of fluids in the cartridges 20 .
- the pneumatic pressureis applied by an external pump 46 through the dock 22 on cartridge 20 , FIG. 2, which is engaged by module 12 .
- Module 12can include a valve, 48 , a vent 50 to atmosphere and a channel 52 that is coupled to cartridge 20 .
- the pneumatic pressureis directed to specific reservoirs and samples in cartridge 20 using valve 48 mechanism to cause selective reagent flow.
- Cartridge 20includes a sample application area 54 .
- Optics 56are included in module 12 and an optical window 57 is included in cartridge 20 .
- excess pressureis vented through vent 50 to atmosphere to stop the flow.
- Platform 10can provide self-testing of modules 12 , to provide for monitoring and detection of fluid flow.
- Various electrical and optical properties of the samples and reagentsallow continuous monitoring of flow cell contents and are compared to expected transition values, as illustrated in FIG. 5.
- FIG. 6illustrates a cross-sectional view of one embodiment of a cartridge 20 .
- Cartridge 20can have a number of different flow channels, including but not limited to air, sample and reagent flow channels 58 , 60 and 62 .
- Flow channels 58 - 62can be created by depressions in both the top and bottom surfaces of the cartridge 20 .
- Flow paths 58 - 62can then be sealed with a vapor barrier 64 .
- pressurization of specific sample or reagent containers provided by pump 46are selectively directed to sample and reagents containers in sequence, providing an outflow directed by a valve to detection chamber 38 or other location, as needed, in sequence and with precise timing.
- the sample and reagentscan flow through an area of controlled temperature to prepare them for precise analysis prior to or during introduction to detection chamber 38 . After analysis the reagents and sample remain in the cartridge 20 in waste region 30 , although the sample tube 40 can be removed by the operator for subsequent use if desired.
- Each module 12can include a processor 56 (FIG. 1( b ).
- Host computer 16in combination with a processor 56 , determines a test protocol for a cartridge 20 .
- a fluid control mechanism in the cartridge 20is then actuated that permits a flow of a patient sample with liquid chemistries and waste materials. This can occur without exposing an operator of platform 10 and the patient, to a transfer of a patient sample into the cartridge 20 without exposure to the chemistries.
- Cartridges 20are designed to isolate biohazards in a cartridge 20 from an operator of the cartridge 20 or the patient. Blood samples from patients are introduced to the cartridges 20 while isolating biohazards in the cartridge from an operator.
- cartridges 20are designed to work with whole blood. This eliminates the requirement of a secondary process to remove the cellular components which may interfere with the testing. This additional separation is both time consuming and error prone.
- the separation of cellsis done automatically by providing a barrier which is penetrated by the analyte to be measured by excludes the cells from analytical contact, except in the case of hemotalogy, where the cells themselves are the subject of measurement.
- Cartridges 20can include electronic identifiers, including but not limited to bar-coded identifiers, with information for test protocols, and lot expiration dates. Cartridges 20 can also include serialized identification.
- placement of a cartridge 20 in a module 12begins an initiation of the module 12 .
- a cartridge 20When a cartridge 20 is inserted into a module 12 it can be sensed automatically.
- the bar code of cartridge 20with its unique sample, are read. This initiates the sequential operation of the fluid movement and detection.
- platform 10includes a plurality of modules 12 each sharing common QC protocols.
- a list of possible QC protocolsis found in table 2.
- TABLE 2 Responsibility Comments Model POCT Platform Operating Procedure Action(per cartridge) 1.
- Draw minimum of 1.5 ml whole blood OperatorExact volume sample in appropriate 5 ml vacutainer-type above minimum not draw tube, using standard draw procedure critical 2.
- Push sample tube into cartridge tube dock Operator and fully seat over needles3.
- Place patient ID bar code label in OperatorIf ED bar code designated target area on tube dock system used 4.
- LED (blue) above portflashes to indicate Platform No LED, push cartridge fully seated in port and cartridge cartridge further read in process into port 6.
- FIG. 8 through 11are flow charts illustrating point of care diagnostic platform 10 of the present invention.
- FIG. 8is a flow chart illustrating an overall methodology of the point of care diagnostic platform of the present invention.
- FIG. 9is a flow chart illustrating one embodiment of a cartridge processing procedure implemented with the point of care diagnostic platform of the present invention.
- FIG. 10is a flow chart illustrating one embodiment of an immunoassay operating procedure implemented with the point of care diagnostic platform of the present invention.
- FIG. 11is a flow chart illustrating one embodiment of a hematology operating procedure implemented with the point of care diagnostic platform of the present invention.
- cartridge 20contains the fluid flow, fluid distribution fluid segmentation and sample dilution.
- a module 12controls the fluid flow via a low pressure air connection and the fluid selection via one or more valve connections.
- platform 10provides real time QC monitoring, and real time test result threshold detection, as disclosed in U.S. Provisional No. 60/470,725, incorporated herein by reference.
Landscapes
- Health & Medical Sciences (AREA)
- Immunology (AREA)
- Life Sciences & Earth Sciences (AREA)
- Engineering & Computer Science (AREA)
- Chemical & Material Sciences (AREA)
- Biomedical Technology (AREA)
- Pathology (AREA)
- Analytical Chemistry (AREA)
- Hematology (AREA)
- General Physics & Mathematics (AREA)
- Molecular Biology (AREA)
- Urology & Nephrology (AREA)
- General Health & Medical Sciences (AREA)
- Biochemistry (AREA)
- Physics & Mathematics (AREA)
- Cell Biology (AREA)
- Medicinal Chemistry (AREA)
- Food Science & Technology (AREA)
- Microbiology (AREA)
- Biotechnology (AREA)
- Optical Measuring Cells (AREA)
- Investigating, Analyzing Materials By Fluorescence Or Luminescence (AREA)
- Investigating Or Analysing Materials By Optical Means (AREA)
- Investigating Or Analysing Biological Materials (AREA)
- Investigating Or Analysing Materials By The Use Of Chemical Reactions (AREA)
Abstract
Description
- This application claims the benefit of U.S. Ser. No. 60/470,725, and is related to co-pending application U.S. Ser. No. ______, (Attorney Docket No.: 40422-0004) filed the same day as the present application, all of which applications are fully incorporated herewith.[0001]
- 1. Field of the Invention[0002]
- The present invention relates generally to a point of care diagnostic system that has a plurality of modules and associated cartridges, and more particularly, to a point of care diagnostic system that includes a plurality of modules that share common QC protocols.[0003]
- 2. Description of the Related Art[0004]
- Blood and other body fluid tests are important diagnostic methods in patient care and treatment. The reliability and the accuracy of the tests are critical in correctly diagnosing the patient and administrating proper treatment. The Food and Drug Administration (FDA) has established numerous quality standards for the various blood or body fluid tests. Monitoring the test process is beneficial in producing reliable and accurate test results.[0005]
- One way of monitoring the test process is periodically performing the monitoring test on standard test samples. The monitoring test results are compared with expected results to verify the accuracy of the test processes or correct the test instrument or process when appropriate. In this approach, the test processes are assumed to generate consistent result between the monitoring tests.[0006]
- Another way of monitoring the test process is including standard test samples in the test process. This approach is suitable for a test process that performs tests on multiple samples. The test results on the standard test samples are compared with expected results to verify the accuracy of the test processes. In this approach, the test processes on real samples are assumed to generate result consistent with those on standard test samples.[0007]
- These monitoring processes are time and cost inefficient. They are deficient in meeting the needs of point of care, e.g., hospital emergency room/department, test processes. In addition to being reliable and accurate, an emergency room test process should be simple to operate and generate diversity of analytical results fast.[0008]
- Accordingly, there is a need for a point of care diagnostic platform that has a plurality of modules coupled to common host computer. There is another need for a point of care diagnostic platform with a plurality of modules that share common QC protocols. Yet there is another need for a point of care diagnostic platform with a plurality of modules coupled to a host computer and an external communication system. There is still another need for a point of care diagnostic platform with a plurality of modules, and a plurality of analytic cartridges, where each cartridge is associated with a module and is configured to directly accept a blood sample from a standard blood draw tube. Yet there is a further need for a point of care diagnostic platform that has a plurality of modules, a host computer coupled to the modules, a common external communication interface, with each module sharing the common external communication interface.[0009]
- Accordingly, an object of the present invention is to provide a point of care diagnostic platform that includes a plurality of modules that share common QC protocols. (is the common operator interface another patent?)[0010]
- Another object of the present invention is to provide a point of care diagnostic platform with a plurality of module coupled to a common host computer.[0011]
- Yet another object of the present invention is to provide a point of care diagnostic platform with a plurality of modules, a host computer coupled to the plurality of modules and an external communication system.[0012]
- Still another object of the present invention is to provide a point of care diagnostic platform with a plurality of modules, and a plurality of analytic cartridges, where each cartridge is associated with a module of the plurality of modules and is configured to directly accept a blood sample from a standard blood draw tube.[0013]
- Another object of the present invention is to provide a point of care diagnostic platform with a plurality of modules; a host computer coupled to the plurality of modules and a common external communication interface, with each module sharing the common external communication interface.[0014]
- A further object of the present invention is to provide a point of care diagnostic platform with a plurality of modules coupled to a common external communication interface such as a least one of WAN or a LAN.[0015]
- Another object of the present invention is to provide a point of care diagnostic platform with a plurality of modules coupled to a common external communication interface that is coupled to a wireless network.[0016]
- A further object of the present invention is to provide a point of care diagnostic platform with a plurality of modules coupled to a hospital information network or a laboratory information network.[0017]
- Yet another object of the present invention is to provide a point of care diagnostic platform with a plurality of modules and a plurality of analytic cartridges that are each bar-coded with information for test protocols, and lot expiration dates.[0018]
- Still a further object of the present invention is to provide a point of care diagnostic platform with a plurality of modules and a plurality of analytic cartridges that retain and seal fluids.[0019]
- Yet another object of the present invention is to provide a point of care diagnostic platform that has a plurality of modules and a plurality of analytic cartridges, where all fluids in a cartridge, including a patient sample, remain within the cartridge.[0020]
- These and other objects of the present invention are achieved in a point of care diagnostic platform includes a plurality of modules. A plurality of analytic cartridges are provided. Each cartridge is associated with a module and is configured to directly accept a blood sample from a standard blood draw tube.[0021]
- In another embodiment of the present invention, a point of care diagnostic platform includes a plurality of modules. A host computer is coupled to the plurality of modules and a common external communication interface. Each module shares the common external communication interface.[0022]
- In another embodiment of the present invention, a point of care diagnostic platform includes a plurality of modules each sharing the same QC protocols. A plurality of analytic cartridges are included. A host computer is coupled to the plurality of modules. The host computer is coupled to an interface. Each module has a corresponding interface component.[0023]
- In another embodiment of the present invention, a point of care diagnostic platform includes a plurality of modules. A plurality of analytic cartridges are provided that each are bar-coded with information for test protocols, and lot expiration dates.[0024]
- In another embodiment of the present invention, a point of care diagnostic platform includes a plurality of modules. A plurality of analytic cartridges are provided that retain and seal fluids.[0025]
- In another embodiment of the present invention, a point of care diagnostic platform includes a plurality of modules. A plurality of analytic cartridges are provided. All fluids in the cartridges, including patient samples, remain within the cartridges.[0026]
- In another embodiment of the present invention, a point of care diagnostic platform is provided that includes a plurality of modules. A plurality of analytic cartridges are provided. Each cartridge has wet and dry chemistries and at least one substrate that carriers a chemistry.[0027]
- FIG. 1([0028]a) is a block diagram illustrating one embodiment of a point of care diagnostic platform of the present invention, with a user interface, host computer, multiple single-cartridge test processing modules and an external communication system.
- FIG. 1([0029]b) is a block diagram illustrating another embodiment of a point of care diagnostic platform of the present invention, with multiple multi-cartridge test processing modules.
- FIG. 1([0030]c) is a block diagram illustrating another embodiment of a point of care diagnostic platform of the present invention, with the host computer being integrated with multiple, multi-cartridge modules.
- FIG. 1([0031]d) is a block diagram illustrating another embodiment of a point of care diagnostic platform of the present invention, with the host computer and user interface both integrated with multiple, multi-cartridge modules.
- FIG. 1([0032]e) is a block diagram illustrating another embodiment of a point of care diagnostic platform of the present invention, with the host computer and user interface integrated with multiple, single-cartridge modules.
- FIG. 2 is a cross-sectional view of one embodiment of a cartridge that can be utilized with the point of care diagnostic platform of the present invention.[0033]
- FIG. 3 is a cross-sectional view of a sample tube that can be utilized with cartridges of the present invention.[0034]
- FIG. 4 is a schematic diagram illustrating one embodiment of the docking, and the relationship between a cartridge and a module of the present invention.[0035]
- FIG. 5 is a schematic diagram illustrating another embodiment of the docking, and the relationship between a cartridge and a module of the present invention.[0036]
- FIG. 6 is a schematic diagram illustrating another embodiment of the docking, and the relationship between a cartridge and a module of the present invention.[0037]
- FIG. 7 is a cross-sectional view of one embodiment of a cartridge utilized with the present invention, illustrating air, sample and reagent flow channels.[0038]
- FIG. 8 is a flow chart illustrating an overall methodology of the point of care diagnostic platform of the present invention.[0039]
- FIG. 9 is a flow chart illustrating one embodiment of a cartridge processing procedure implemented with the point of care diagnostic platform of the present invention.[0040]
- FIG. 10 is a flow chart illustrating one embodiment of an immunoassay operating procedure implemented with the point of care diagnostic platform of the present invention.[0041]
- FIG. 11 is a flow chart illustrating one embodiment of a hematology operating procedure implemented with the point of care diagnostic platform of the present invention.[0042]
- As illustrated in FIG. 1([0043]a), one embodiment of the present invention is a point of care diagnostic platform, denoted generally as10, and its method of use. Point of care
diagnostic platform 10 includes a plurality ofmodules 12. A variety of different modules can be included but not limited to, immunoassay, hematology, electrolyte, molecular diagnostic, coagulation, blood gas, chemistry and the like. Themodules 12 can share at least a portion of a common functionality of operation such as fluid movement, sample introduction, and the like. In one embodiment, eachmodule 12 contains common functionalities, and unique technologies that correspond to one or more selected chemistries. In the FIG. 1(a) embodiment,modules 12 are multiple single-cartridge test processing modules. - [0044]
Platform 10 can deliver a multitude of discreet testing capabilities in a standardized manner.Modules 12 can have common operation platforms. Examples of common operation systems are user interface, quality control, calibration, training, connection to various laboratory information systems, hospital information systems, emergency room information systems, wireless communication and the like. - A[0045]
host computer 14 is coupled to the plurality ofmodules 12 and also to auser interface 16. Eachmodule 12 is coupled to theuser interface 16.Host computer 14 has a variety of different capabilities, including but not limited to user interface, quality control, calibration, training, connection to various laboratory information systems, hospital information systems, emergency room information systems, wireless communication and the like.User interface 16 is coupled to eachmodule 12User interface 16 provides uniform (automated and standardized) connectivity to the plurality ofmodules 12 as well as communication to other hospital and laboratory information systems. It will be appreciated that standardized includes industry standards as documented by the Connectivity Industry Consortium.User interface 16 establishes a database of analyzed samples and provides the operator with quality control options for the plurality ofmodules 12. This is achieved by centralizing and tracking the collective output of the plurality ofmodules 12. In one embodiment,user interface 16 includes capability for at least one of a cardiac, fertility, kidney, coagulation, electrolyte and hematology panel, molecular diagnostics and chemistry panels, and the like. - Each[0046]
module 12 has a corresponding interface component for module control and sample results acquisition. In one embodiment,host computer 14 is also coupled to anexternal communication system 18. A variety of different external communication systems are suitable including but not limited to a, WAN, LAN, wireless network, hospital information network, laboratory information network, and the like.Platform 10 can be connected directly or in-directly to a emergency room/department patient management network - In one embodiment, each[0047]
module 12 shares common QC protocols. The QC protocols include but are not limited to the following, module electronic verification, real-time process monitoring, patient record-keeping, periodic liquid control results monitoring, and the like. The QC protocols are initiated in the same manner regardless of themodule 12 that is tested. Electronic monitoring of the process at eachmodule 12 is continuous and transparent to the operator and do not require operator attention. Results are stored in module specific databases. Each module can utilize specific electronic and/or optical parameter monitoring. Changes in the electronic and optical parameters are tracked during the operation of themodule 12 involved, and the outputs compared to expected thresholds/changes. These changes are indicative of correct internal operation during sample processing. - In another embodiment, illustrated in FIG. 1([0048]b), multiple, multi-modules are provided, where a
module 12 can be utilized with more than one cartridge. In FIG. 1(c)host computer 14 is integrated with multiple,multi-cartridge modules 12. In the FIG. 1(d) embodiment,host computer 14 anduser interface 16 are both integrated with multiple,multi-cartridge modules 12. In the FIG. 1(e) embodiment, host computer anduser interface 16 are integrated with multiple, single-cartridgetest processing modules 12. - Point of care[0049]
diagnostic platform 10 includes a plurality ofcartridges 20, illustrated in FIG. 2.Cartridges 20 include but are not limited to cardiac, fertility, kidney, coagulation, electrolyte and hematology panel, molecular diagnostics and chemistry panels, and the like. - Each[0050]
cartridge 20 can include adock 22 for receiving a sample tube, anair dock 24 that can be engaged by amodule 12, arotary valve 26, which can also be engaged by amodule 12, acalibration chamber 28,waste chamber 30, sample/calibration flow path 32 which is coupled to a detector, sample outflow 34,sample pressure channel 36 and aflow cell 38 which is a detection chamber. - [0051]
Cartridges 20 can have wet and dry chemistries and at least one substrate that carriers a chemistry. Examples of various wet and dry chemistries are listed in table 1.TABLE 1 cartridge Wet Reagents Dry Reagents electrolytes calibration fluid ion specific electrode immunology — Capture antibody Conjugate antibody hemolotogy Lysing solution/white blood cell — nuclear label Hemoglobin dye Chemistry Various Various Coagulation — Initiator Blood gas — Electrode Molecular Nucleic acid label Nucleic acid capture Amplification reagents - [0052]
Cartridges 20 are associated with a correspondingmodule 12. In one embodiment,cartridges 20 can directly accept a blood sample from a standard blood draw,sample tube 40 which can include apressure needle 42 and asampling needle 44, as shown in FIG. 3. This can be achieved by, (i) piercing the cap of the standardblood draw tube 40needles cartridge 20, penetrating the cap with a single needle and withdrawing fluid directly using a vacuum, and the like.Cartridges 20 can be configured to retain and seal fluids. This can be achieved by using selective pressurization of reagent and sample reservoirs, which forces the fluids intocartridges 20 and throughflow cell 38 intowaste chamber 30, that can be an integral part ofcartridges 20. All fluids incartridges 20, including patient samples, can remain within thecartridge 20. - As illustrated in FIG. 4,[0053]
modules 12 can be configured to be engaged with thecartridges 20 to produce pneumatic movement of fluids in thecartridges 20. The pneumatic pressure is applied by anexternal pump 46 through thedock 22 oncartridge 20, FIG. 2, which is engaged bymodule 12.Module 12 can include a valve,48, avent 50 to atmosphere and achannel 52 that is coupled tocartridge 20. The pneumatic pressure is directed to specific reservoirs and samples incartridge 20 usingvalve 48 mechanism to cause selective reagent flow.Cartridge 20 includes asample application area 54.Optics 56 are included inmodule 12 and anoptical window 57 is included incartridge 20. At the cessation of reagent flow, excess pressure is vented throughvent 50 to atmosphere to stop the flow.Platform 10 can provide self-testing ofmodules 12, to provide for monitoring and detection of fluid flow. Various electrical and optical properties of the samples and reagents allow continuous monitoring of flow cell contents and are compared to expected transition values, as illustrated in FIG. 5. - FIG. 6 illustrates a cross-sectional view of one embodiment of a[0054]
cartridge 20.Cartridge 20 can have a number of different flow channels, including but not limited to air, sample andreagent flow channels cartridge 20. Flow paths58-62 can then be sealed with avapor barrier 64. - Referring again to FIG. 4, pressurization of specific sample or reagent containers provided by[0055]
pump 46 are selectively directed to sample and reagents containers in sequence, providing an outflow directed by a valve todetection chamber 38 or other location, as needed, in sequence and with precise timing. The sample and reagents can flow through an area of controlled temperature to prepare them for precise analysis prior to or during introduction todetection chamber 38. After analysis the reagents and sample remain in thecartridge 20 inwaste region 30, although thesample tube 40 can be removed by the operator for subsequent use if desired. - Each[0056]
module 12 can include a processor56 (FIG. 1(b).Host computer 16, in combination with aprocessor 56, determines a test protocol for acartridge 20. A fluid control mechanism in thecartridge 20 is then actuated that permits a flow of a patient sample with liquid chemistries and waste materials. This can occur without exposing an operator ofplatform 10 and the patient, to a transfer of a patient sample into thecartridge 20 without exposure to the chemistries.Cartridges 20 are designed to isolate biohazards in acartridge 20 from an operator of thecartridge 20 or the patient. Blood samples from patients are introduced to thecartridges 20 while isolating biohazards in the cartridge from an operator. - In one embodiment,[0057]
cartridges 20 are designed to work with whole blood. This eliminates the requirement of a secondary process to remove the cellular components which may interfere with the testing. This additional separation is both time consuming and error prone. In the cartridge, the separation of cells is done automatically by providing a barrier which is penetrated by the analyte to be measured by excludes the cells from analytical contact, except in the case of hemotalogy, where the cells themselves are the subject of measurement. - [0058]
Cartridges 20 can include electronic identifiers, including but not limited to bar-coded identifiers, with information for test protocols, and lot expiration dates.Cartridges 20 can also include serialized identification. - In one embodiment, placement of a[0059]
cartridge 20 in amodule 12 begins an initiation of themodule 12. When acartridge 20 is inserted into amodule 12 it can be sensed automatically. The bar code ofcartridge 20, with its unique sample, are read. This initiates the sequential operation of the fluid movement and detection. - In another embodiment of the present invention,[0060]
platform 10 includes a plurality ofmodules 12 each sharing common QC protocols. A list of possible QC protocols is found in table 2.TABLE 2 Responsibility Comments Model POCT Platform Operating Procedure Action (per cartridge) 1. Draw minimum of 1.5 ml whole blood Operator Exact volume sample in appropriate 5 ml vacutainer-type above minimum not draw tube, using standard draw procedure critical 2. Push sample tube into cartridge tube dock Operator and fully seat over needles 3. Place patient ID bar code label in Operator If ED bar code designated target area on tube dock system used 4. Push cartridge into module port until fully Operator* Platform in testing seated over snap-type detents mode 5. LED (blue) above port flashes to indicate Platform No LED, push cartridge fully seated in port and cartridge cartridge further read in process into port 6. LED steady illumination after 2 seconds if Platform No steady LED, cartridge read OK (lot#, exp. Date, test replace cartridge type, patient ID) OPERATOR WALK AWAY and reuse sample 7. Perform designated assay protocol Platform 10-15 minutes 8. LED extinguishes, patient, test results, Platform Downloaded to LIS reference range and QC data stored in when connected memory, displayed on screen and printed on attached printer 9. Remove cartridge and discard in Operator* biohazardous solid waste (remove and sale sample tube if required) Immunoassay 1. Action Draw minimum of 1.5 ml whole blood Operator Exact volume sample in appropriate 5 ml vacutainer-type above minimum not draw tube, using standard draw procedure critical 2. Push sample tube into cartridge tube dock Operator and fully seat over needles 3. Place patient ID bar code label in Operator If ED bar code designated target area on tube dock system used 4. Push cartridge into module port until fully Operator seated over detents 5. Pressurize sample tube and flow sample: Plafform 3X volumes for (3) 200 ul/test trip at 500 u./min. strip cartridge 6. Stop flow by: Platform Test strip manifold a. venting pressure to test strip manifold, or is a porous b. flow channel manifold if even distribution membrane 7. Read reflectance change on strip reaction Platform areas at designated intervals Hemotology 1. Draw minimum of 1.5 ml whole blood Operator Exact volume sample in appropriate 5 ml vacutainer- above minimum not type draw tube, using standard draw critical procedure 2. Push sample tube into cartridge tube Operator dock and fully seat over needles 3. Place patient ID bar code label in Operator If ED bar code designated target area on tube dock system used 4. Push cartridge into module port until fully Operator LED illuminates seated over detents above port 5. Pressurize sample tube and flow sample Platform to segment at 200 ul sample 6. Stop flow by venting pressure to sample Platform tube 7. Pressurize diluent and flow to wash Platform sample segment into mixing chamber 8. Stop flow by venting pressure Platform 9. Mix sample and diluent Platform How mix? 10. Pressurize mixed sample and flow to Platform flowcell. 11. Stop flow by venting pressure Platform 12. Repeat steps 10 and 11 (4) times Platform 13. Segment 50 ul ofsample Platform 14. Mix with 500 ul of Hb reagent Platform 15. Flow mixed sample into flowcell: 100 ul at Platform 1 ml/ min Electrolytes 1. Draw minimum of 1.5 ml whole blood Operator Exact volume sample in appropriate 5 ml vacutainer- above minimum not type draw tube, using standard draw critical procedure 2. Push sample tube into cartridge tube dock Operator and fully seat over needles 3. Place patient ID bar code label in Operator If ED bar code designated target area on tube dock system used 4. Push cartridge into module port until fully Operator seated over detents 5. Pressurize sample tube and flow sample Platform to segment: 300 ul at 2 ml/min. 6. Stop flow by venting pressure Platform 7. Pressurize sample tube and flow sample Platform through cartridge: 400 ul at 3 ml/min. 8. Stop flow by venting pressure Platform - FIG. 8 through[0061]11 are flow charts illustrating point of care
diagnostic platform 10 of the present invention. FIG. 8 is a flow chart illustrating an overall methodology of the point of care diagnostic platform of the present invention. FIG. 9 is a flow chart illustrating one embodiment of a cartridge processing procedure implemented with the point of care diagnostic platform of the present invention. FIG. 10 is a flow chart illustrating one embodiment of an immunoassay operating procedure implemented with the point of care diagnostic platform of the present invention. FIG. 11 is a flow chart illustrating one embodiment of a hematology operating procedure implemented with the point of care diagnostic platform of the present invention. - In the preceding example, all reagents and waste are contained in[0062]
cartridge 20. Fluids are moved incartridge 20 via an external pump (in the module) coupled tocartridge 20 via an air dock. Likewise the reagents and sample are directed sequentially by valve(s) with-in the cartridge but activated through physical engagement to an external activator in the module.Cartridge 20 contains the fluid flow, fluid distribution fluid segmentation and sample dilution. Amodule 12 controls the fluid flow via a low pressure air connection and the fluid selection via one or more valve connections. - In another embodiment,[0063]
platform 10 provides real time QC monitoring, and real time test result threshold detection, as disclosed in U.S. Provisional No. 60/470,725, incorporated herein by reference. - While embodiments of the invention have been illustrated and described, it is not intended that these embodiments illustrate and describe all possible forms of the invention. Rather, the words used in the specification are words of description rather than limitation, and it is understood that various changes may be made without departing from the spirit and scope of the invention.[0064]
Claims (52)
Priority Applications (7)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US10/746,127US20040228766A1 (en) | 2003-05-14 | 2003-12-23 | Point of care diagnostic platform |
| JP2006547098AJP2007516446A (en) | 2003-12-23 | 2004-12-13 | Point-of-care diagnostic platform |
| PCT/US2004/041651WO2005065157A2 (en) | 2003-12-23 | 2004-12-13 | Point of care diagnostic platform |
| CN 200480038879CN1898560A (en) | 2003-12-23 | 2004-12-13 | Point of care diagnostic platform |
| EP04813903AEP1702211A2 (en) | 2003-12-23 | 2004-12-13 | Point of care diagnostic platform |
| CA002549367ACA2549367A1 (en) | 2003-12-23 | 2004-12-13 | Point of care diagnostic platform |
| US11/517,007US20070059204A1 (en) | 2003-05-14 | 2006-09-06 | Point of care diagnostic platform |
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US47072503P | 2003-05-14 | 2003-05-14 | |
| US10/746,127US20040228766A1 (en) | 2003-05-14 | 2003-12-23 | Point of care diagnostic platform |
Related Child Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US11/517,007ContinuationUS20070059204A1 (en) | 2003-05-14 | 2006-09-06 | Point of care diagnostic platform |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| US20040228766A1true US20040228766A1 (en) | 2004-11-18 |
Family
ID=33476741
Family Applications (5)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US10/746,127AbandonedUS20040228766A1 (en) | 2003-05-14 | 2003-12-23 | Point of care diagnostic platform |
| US10/845,767Expired - Fee RelatedUS7217393B2 (en) | 2003-05-14 | 2004-05-14 | Apparatus and method for process monitoring |
| US11/064,882Expired - Fee RelatedUS7189573B2 (en) | 2003-05-14 | 2005-02-23 | Apparatus and method for process monitoring |
| US11/099,707Expired - Fee RelatedUS7192777B2 (en) | 2003-05-14 | 2005-04-05 | Apparatus and method for process monitoring |
| US11/517,007AbandonedUS20070059204A1 (en) | 2003-05-14 | 2006-09-06 | Point of care diagnostic platform |
Family Applications After (4)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US10/845,767Expired - Fee RelatedUS7217393B2 (en) | 2003-05-14 | 2004-05-14 | Apparatus and method for process monitoring |
| US11/064,882Expired - Fee RelatedUS7189573B2 (en) | 2003-05-14 | 2005-02-23 | Apparatus and method for process monitoring |
| US11/099,707Expired - Fee RelatedUS7192777B2 (en) | 2003-05-14 | 2005-04-05 | Apparatus and method for process monitoring |
| US11/517,007AbandonedUS20070059204A1 (en) | 2003-05-14 | 2006-09-06 | Point of care diagnostic platform |
Country Status (4)
| Country | Link |
|---|---|
| US (5) | US20040228766A1 (en) |
| EP (1) | EP1623226A4 (en) |
| JP (1) | JP2007501415A (en) |
| WO (1) | WO2004104552A2 (en) |
Cited By (33)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US20080009766A1 (en)* | 2005-05-09 | 2008-01-10 | Holmes Elizabeth A | Systems and methods for improving medical treatments |
| US20100100333A1 (en)* | 2008-10-15 | 2010-04-22 | Ridge Diagnostics, Inc. | Human biomarker hypermapping for depressive disorders |
| US20100136700A1 (en)* | 2008-11-18 | 2010-06-03 | John Bilello | Metabolic syndrome and hpa axis biomarkers for major depressive disorder |
| US20100280562A1 (en)* | 2009-04-06 | 2010-11-04 | Ridge Diagnostics, Inc. | Biomarkers for monitoring treatment of neuropsychiatric diseases |
| US7860727B2 (en) | 2003-07-17 | 2010-12-28 | Ventana Medical Systems, Inc. | Laboratory instrumentation information management and control network |
| US20110213219A1 (en)* | 2010-01-26 | 2011-09-01 | Ridge Diagnostics, Inc. | Multiple Biomarker Panels to Stratify Disease Severity and Monitor Treatment of Depression |
| US8158374B1 (en) | 2006-09-05 | 2012-04-17 | Ridge Diagnostics, Inc. | Quantitative diagnostic methods using multiple parameters |
| US8669047B2 (en) | 2006-05-10 | 2014-03-11 | Theranos, Inc. | Real-time detection of influenza virus |
| US8697377B2 (en) | 2007-10-02 | 2014-04-15 | Theranos, Inc. | Modular point-of-care devices, systems, and uses thereof |
| US8719053B2 (en) | 2003-07-17 | 2014-05-06 | Ventana Medical Systems, Inc. | Laboratory instrumentation information management and control network |
| US8741230B2 (en) | 2006-03-24 | 2014-06-03 | Theranos, Inc. | Systems and methods of sample processing and fluid control in a fluidic system |
| US8778665B2 (en) | 2006-11-14 | 2014-07-15 | Theranos, Inc. | Detection and quantification of analytes in bodily fluids |
| US8840838B2 (en) | 2011-09-25 | 2014-09-23 | Theranos, Inc. | Centrifuge configurations |
| US8862448B2 (en) | 2009-10-19 | 2014-10-14 | Theranos, Inc. | Integrated health data capture and analysis system |
| US8883518B2 (en) | 2007-08-06 | 2014-11-11 | Theranos, Inc. | Systems and methods of fluidic sample processing |
| US9075042B2 (en) | 2012-05-15 | 2015-07-07 | Wellstat Diagnostics, Llc | Diagnostic systems and cartridges |
| US9213043B2 (en) | 2012-05-15 | 2015-12-15 | Wellstat Diagnostics, Llc | Clinical diagnostic system including instrument and cartridge |
| US9250229B2 (en) | 2011-09-25 | 2016-02-02 | Theranos, Inc. | Systems and methods for multi-analysis |
| US9268915B2 (en) | 2011-09-25 | 2016-02-23 | Theranos, Inc. | Systems and methods for diagnosis or treatment |
| US9464981B2 (en) | 2011-01-21 | 2016-10-11 | Theranos, Inc. | Systems and methods for sample use maximization |
| US9500663B2 (en) | 2014-11-11 | 2016-11-22 | Genmark Diagnostics, Inc. | Redundant identification for sample tracking on a diagnostic device |
| CN106483125A (en)* | 2015-08-26 | 2017-03-08 | 上海依达医疗器械有限公司 | A kind of intelligent blood stasis diagnostic apparatuses and its diagnostic method |
| US9592508B2 (en) | 2011-09-25 | 2017-03-14 | Theranos, Inc. | Systems and methods for fluid handling |
| US9619627B2 (en) | 2011-09-25 | 2017-04-11 | Theranos, Inc. | Systems and methods for collecting and transmitting assay results |
| US9625465B2 (en) | 2012-05-15 | 2017-04-18 | Defined Diagnostics, Llc | Clinical diagnostic systems |
| US9632102B2 (en) | 2011-09-25 | 2017-04-25 | Theranos, Inc. | Systems and methods for multi-purpose analysis |
| US9645143B2 (en) | 2011-09-25 | 2017-05-09 | Theranos, Inc. | Systems and methods for multi-analysis |
| US9664702B2 (en) | 2011-09-25 | 2017-05-30 | Theranos, Inc. | Fluid handling apparatus and configurations |
| US10012664B2 (en) | 2011-09-25 | 2018-07-03 | Theranos Ip Company, Llc | Systems and methods for fluid and component handling |
| US11162936B2 (en) | 2011-09-13 | 2021-11-02 | Labrador Diagnostics Llc | Systems and methods for multi-analysis |
| US11215610B2 (en) | 2006-10-13 | 2022-01-04 | Labrador Diagnostics Llc | Reducing optical interference in a fluidic device |
| US11287421B2 (en) | 2006-03-24 | 2022-03-29 | Labrador Diagnostics Llc | Systems and methods of sample processing and fluid control in a fluidic system |
| US11331662B2 (en) | 2017-09-29 | 2022-05-17 | Miraplex Diagnostics Inc. | Assay preparation device |
Families Citing this family (28)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US20060233666A1 (en)* | 2005-04-15 | 2006-10-19 | Agamatrix, Inc. | Visual display for meter testing bodily fluids |
| US7991242B2 (en) | 2005-05-11 | 2011-08-02 | Optosecurity Inc. | Apparatus, method and system for screening receptacles and persons, having image distortion correction functionality |
| EP1886257A1 (en) | 2005-05-11 | 2008-02-13 | Optosecurity Inc. | Method and system for screening luggage items, cargo containers or persons |
| US20070081920A1 (en)* | 2005-10-12 | 2007-04-12 | Murphy R S | Semi-disposable optoelectronic rapid diagnostic test system |
| US7899232B2 (en) | 2006-05-11 | 2011-03-01 | Optosecurity Inc. | Method and apparatus for providing threat image projection (TIP) in a luggage screening system, and luggage screening system implementing same |
| US8494210B2 (en) | 2007-03-30 | 2013-07-23 | Optosecurity Inc. | User interface for use in security screening providing image enhancement capabilities and apparatus for implementing same |
| US20090060783A1 (en)* | 2007-09-04 | 2009-03-05 | Kenneth Charles Barrett | Polymer concentration monitoring system and use thereof |
| WO2009052501A2 (en)* | 2007-10-19 | 2009-04-23 | Midwest Medical Technologies Of America, Llc | Method and apparatus for identifying and tracking biological fluid |
| WO2009097364A1 (en)* | 2008-01-29 | 2009-08-06 | Pergenix Llc | Method and system for delivering clinical lab quality and professional interpretation to home and clinic testing |
| GB0804764D0 (en)* | 2008-03-14 | 2008-04-16 | Cheyney Design & Dev Ltd | Test apparatus |
| US20090285721A1 (en)* | 2008-05-15 | 2009-11-19 | Pason Systems Corp. | Apparatus for chemical analysis of a sample |
| WO2009152094A2 (en)* | 2008-06-09 | 2009-12-17 | Accumetrics, Inc. | Hubbed dual cannula device for closed container sampling systems |
| CN102687009B (en)* | 2009-12-18 | 2014-07-16 | 恩特格利昂公司 | Portable coagulation monitoring device and method of assessing coagulation response |
| US9074977B2 (en) | 2010-05-07 | 2015-07-07 | Stc.Unm | Multinode acoustic focusing for parallel flow cytometry analysis applications |
| US9274042B2 (en) | 2010-05-07 | 2016-03-01 | Stc.Unm | Spatially correlated light collection from multiple sample streams excited with a line focused light source |
| US8830451B1 (en) | 2010-05-07 | 2014-09-09 | Stc.Unm | Multinode acoustic focusing for parallel flow cytometry analysis applications |
| KR101973221B1 (en) | 2011-09-07 | 2019-04-26 | 라피스캔 시스템스, 인코포레이티드 | X-ray inspection system that integrates manifest data with imaging/detection processing |
| US8986527B2 (en)* | 2011-12-06 | 2015-03-24 | Edan Diagnostics | Testing cartridge for an in vitro medical diagnostic device |
| EP2864762A4 (en)* | 2012-06-21 | 2016-01-20 | Stc Unm | SPATIALLY CORRELATED LIGHT COLLECTION FROM EXCITED MULTIPLE SAMPLING STREAMS USING A LIGHT SOURCE CONCENTRATED ON A LINE |
| USD717459S1 (en) | 2012-11-12 | 2014-11-11 | Edan Diagnostics | Diagnostic device |
| USD717438S1 (en) | 2012-11-12 | 2014-11-11 | Edan Diagnostics | Fluid cartridge |
| USD706930S1 (en) | 2012-11-12 | 2014-06-10 | Edan Diagnostics | Fluid cartridge |
| CN103543185B (en)* | 2012-12-06 | 2015-06-24 | 理邦(美国)诊断有限公司 | Testing cartridge for an in vitro medical diagnostic device |
| CN116309260A (en) | 2016-02-22 | 2023-06-23 | 拉皮斯坎系统股份有限公司 | Method for evaluating average pallet size and density of goods |
| AU2017324053B2 (en) | 2016-09-08 | 2020-08-06 | Hemex Health, Inc. | Diagnostics systems and methods |
| US10349589B2 (en) | 2016-09-08 | 2019-07-16 | Hemex Health, Inc. | Diagnostics systems and methods |
| US10648909B2 (en) | 2017-05-25 | 2020-05-12 | Abbott Laboratories | Methods and systems for assessing flow cell cleanliness |
| WO2020264182A1 (en) | 2019-06-25 | 2020-12-30 | Hemex Health, Inc. | Diagnostics systems and methods |
Citations (13)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US4222744A (en)* | 1978-09-27 | 1980-09-16 | Becton Dickinson & Company | Assay for ligands |
| US5096669A (en)* | 1988-09-15 | 1992-03-17 | I-Stat Corporation | Disposable sensing device for real time fluid analysis |
| US5965456A (en)* | 1992-06-11 | 1999-10-12 | Biacore Ab | Analyte detection |
| US6218719B1 (en)* | 1998-09-18 | 2001-04-17 | Capella Microsystems, Inc. | Photodetector and device employing the photodetector for converting an optical signal into an electrical signal |
| US6222619B1 (en)* | 1997-09-18 | 2001-04-24 | University Of Utah Research Foundation | Diagnostic device and method |
| US6228652B1 (en)* | 1999-02-16 | 2001-05-08 | Coulter International Corp. | Method and apparatus for analyzing cells in a whole blood sample |
| US6326612B1 (en)* | 1998-10-13 | 2001-12-04 | Texas Instruments Incorporated | System and method for optical sensing utilizing a portable, detachable sensor cartridge |
| US6391541B1 (en)* | 1999-05-28 | 2002-05-21 | Kurt E. Petersen | Apparatus for analyzing a fluid sample |
| US6524858B1 (en)* | 1999-03-31 | 2003-02-25 | Bayer Corporation | Single channel, single dilution detection method for the identification and quantification of blood cells and platelets in a whole blood sample using an automated hematology analyzer |
| US6592822B1 (en)* | 1998-05-14 | 2003-07-15 | Luminex Corporation | Multi-analyte diagnostic system and computer implemented process for same |
| US6602469B1 (en)* | 1998-11-09 | 2003-08-05 | Lifestream Technologies, Inc. | Health monitoring and diagnostic device and network-based health assessment and medical records maintenance system |
| US6611634B2 (en)* | 1996-03-19 | 2003-08-26 | University Of Utah Research Foundation | Lens and associatable flow cell |
| US6692696B1 (en)* | 1998-06-18 | 2004-02-17 | ARETé ASSOCIATES | Biosensor |
Family Cites Families (13)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JPH0690211B2 (en)* | 1984-09-21 | 1994-11-14 | オリンパス光学工業株式会社 | Immunological analyzer and method thereof |
| JPH01500220A (en)* | 1986-07-01 | 1989-01-26 | バイオテク・インストゥルメンツ・リミテッド | automatic chemical analyzer |
| EP0504772A3 (en)* | 1991-03-18 | 1993-01-27 | Paradigm Biotechnologies Partnership | Analytical apparatus |
| WO1995011621A1 (en)* | 1993-10-28 | 1995-05-04 | I-Stat Corporation | Fluid sample collection and introduction device |
| JP2973887B2 (en)* | 1995-08-31 | 1999-11-08 | 株式会社島津製作所 | Method and apparatus for analyzing nucleic acid molecules |
| US5902982A (en)* | 1997-04-04 | 1999-05-11 | National Medical Review Office Inc. | Changeable machine readable assaying indicia |
| US6002475A (en)* | 1998-01-28 | 1999-12-14 | Careside, Inc. | Spectrophotometric analytical cartridge |
| JP3304878B2 (en)* | 1998-04-30 | 2002-07-22 | 三菱マテリアル株式会社 | Method and apparatus for measuring halogen concentration by flow analysis |
| EP1047929B1 (en)* | 1998-11-13 | 2007-07-04 | Reichert, Inc. | Method for qualitative and quantitative measurements |
| JP4647792B2 (en) | 1999-04-28 | 2011-03-09 | イジュノシッヒ テクニッヒ ホッフシューラ チューリッヒ | Polyionic coatings in analytical and sensing devices |
| CA2314398A1 (en)* | 2000-08-10 | 2002-02-10 | Edward Shipwash | Microarrays and microsystems for amino acid analysis and protein sequencing |
| JP2002148181A (en)* | 2000-11-07 | 2002-05-22 | Hioki Ee Corp | Flow injection analyzer |
| KR100426210B1 (en)* | 2000-11-11 | 2004-04-03 | 피티플러스(주) | Method for crystallizing silicone layer |
- 2003
- 2003-12-23USUS10/746,127patent/US20040228766A1/ennot_activeAbandoned
- 2004
- 2004-05-14EPEP04752415Apatent/EP1623226A4/ennot_activeWithdrawn
- 2004-05-14WOPCT/US2004/015398patent/WO2004104552A2/enactiveApplication Filing
- 2004-05-14JPJP2006533139Apatent/JP2007501415A/enactivePending
- 2004-05-14USUS10/845,767patent/US7217393B2/ennot_activeExpired - Fee Related
- 2005
- 2005-02-23USUS11/064,882patent/US7189573B2/ennot_activeExpired - Fee Related
- 2005-04-05USUS11/099,707patent/US7192777B2/ennot_activeExpired - Fee Related
- 2006
- 2006-09-06USUS11/517,007patent/US20070059204A1/ennot_activeAbandoned
Patent Citations (13)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US4222744A (en)* | 1978-09-27 | 1980-09-16 | Becton Dickinson & Company | Assay for ligands |
| US5096669A (en)* | 1988-09-15 | 1992-03-17 | I-Stat Corporation | Disposable sensing device for real time fluid analysis |
| US5965456A (en)* | 1992-06-11 | 1999-10-12 | Biacore Ab | Analyte detection |
| US6611634B2 (en)* | 1996-03-19 | 2003-08-26 | University Of Utah Research Foundation | Lens and associatable flow cell |
| US6222619B1 (en)* | 1997-09-18 | 2001-04-24 | University Of Utah Research Foundation | Diagnostic device and method |
| US6592822B1 (en)* | 1998-05-14 | 2003-07-15 | Luminex Corporation | Multi-analyte diagnostic system and computer implemented process for same |
| US6692696B1 (en)* | 1998-06-18 | 2004-02-17 | ARETé ASSOCIATES | Biosensor |
| US6218719B1 (en)* | 1998-09-18 | 2001-04-17 | Capella Microsystems, Inc. | Photodetector and device employing the photodetector for converting an optical signal into an electrical signal |
| US6326612B1 (en)* | 1998-10-13 | 2001-12-04 | Texas Instruments Incorporated | System and method for optical sensing utilizing a portable, detachable sensor cartridge |
| US6602469B1 (en)* | 1998-11-09 | 2003-08-05 | Lifestream Technologies, Inc. | Health monitoring and diagnostic device and network-based health assessment and medical records maintenance system |
| US6228652B1 (en)* | 1999-02-16 | 2001-05-08 | Coulter International Corp. | Method and apparatus for analyzing cells in a whole blood sample |
| US6524858B1 (en)* | 1999-03-31 | 2003-02-25 | Bayer Corporation | Single channel, single dilution detection method for the identification and quantification of blood cells and platelets in a whole blood sample using an automated hematology analyzer |
| US6391541B1 (en)* | 1999-05-28 | 2002-05-21 | Kurt E. Petersen | Apparatus for analyzing a fluid sample |
Cited By (105)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US7860727B2 (en) | 2003-07-17 | 2010-12-28 | Ventana Medical Systems, Inc. | Laboratory instrumentation information management and control network |
| US8812329B2 (en) | 2003-07-17 | 2014-08-19 | Ventana Medical Systems, Inc. | Laboratory instrumentation information management and control network |
| US8719053B2 (en) | 2003-07-17 | 2014-05-06 | Ventana Medical Systems, Inc. | Laboratory instrumentation information management and control network |
| US9772291B2 (en) | 2005-05-09 | 2017-09-26 | Theranos, Inc. | Fluidic medical devices and uses thereof |
| US10761030B2 (en) | 2005-05-09 | 2020-09-01 | Labrador Diagnostics Llc | System and methods for analyte detection |
| EP4483804A3 (en)* | 2005-05-09 | 2025-03-26 | Labrador Diagnostics LLC | Point-of-care fluidic systems and uses thereof |
| US20080009766A1 (en)* | 2005-05-09 | 2008-01-10 | Holmes Elizabeth A | Systems and methods for improving medical treatments |
| JP2017142265A (en)* | 2005-05-09 | 2017-08-17 | セラノス, インコーポレイテッド | Point-of-care fluid system and application of the same |
| JP2015158506A (en)* | 2005-05-09 | 2015-09-03 | セラノス, インコーポレイテッド | Point-of-care fluid system and application of the same |
| JP2019219416A (en)* | 2005-05-09 | 2019-12-26 | セラノス アイピー カンパニー エルエルシー | Point-of-care fluidic system and uses thereof |
| US8679407B2 (en)* | 2005-05-09 | 2014-03-25 | Theranos, Inc. | Systems and methods for improving medical treatments |
| EP3763824A1 (en)* | 2005-05-09 | 2021-01-13 | Labrador Diagnostics LLC | Point-of-care fluidic systems and uses thereof |
| US9182388B2 (en) | 2005-05-09 | 2015-11-10 | Theranos, Inc. | Calibration of fluidic devices |
| US9075046B2 (en) | 2005-05-09 | 2015-07-07 | Theranos, Inc. | Fluidic medical devices and uses thereof |
| JP2021193390A (en)* | 2005-05-09 | 2021-12-23 | ラブラドール ダイアグノスティクス エルエルシー | Point of Care Fluid System and Its Use |
| US11630069B2 (en) | 2005-05-09 | 2023-04-18 | Labrador Diagnostics Llc | Fluidic medical devices and uses thereof |
| EP1883341B1 (en) | 2005-05-09 | 2020-08-12 | Labrador Diagnostics LLC | Point-of-care fluidic systems and uses thereof |
| JP2015158507A (en)* | 2005-05-09 | 2015-09-03 | セラノス, インコーポレイテッド | Point-of-care fluid system and application of the same |
| US8841076B2 (en) | 2005-05-09 | 2014-09-23 | Theranos, Inc. | Systems and methods for conducting animal studies |
| US10908093B2 (en) | 2005-05-09 | 2021-02-02 | Labrador Diagnostics, LLC | Calibration of fluidic devices |
| US11287421B2 (en) | 2006-03-24 | 2022-03-29 | Labrador Diagnostics Llc | Systems and methods of sample processing and fluid control in a fluidic system |
| US9176126B2 (en) | 2006-03-24 | 2015-11-03 | Theranos, Inc. | Systems and methods of sample processing and fluid control in a fluidic system |
| US8741230B2 (en) | 2006-03-24 | 2014-06-03 | Theranos, Inc. | Systems and methods of sample processing and fluid control in a fluidic system |
| US10533994B2 (en) | 2006-03-24 | 2020-01-14 | Theranos Ip Company, Llc | Systems and methods of sample processing and fluid control in a fluidic system |
| US9885715B2 (en) | 2006-05-10 | 2018-02-06 | Theranos IP Comany, LLC | Real-time detection of influenza virus |
| US8669047B2 (en) | 2006-05-10 | 2014-03-11 | Theranos, Inc. | Real-time detection of influenza virus |
| US11162947B2 (en) | 2006-05-10 | 2021-11-02 | Labrador Diagnostics Llc | Real-time detection of influenza virus |
| US8158374B1 (en) | 2006-09-05 | 2012-04-17 | Ridge Diagnostics, Inc. | Quantitative diagnostic methods using multiple parameters |
| US8450077B2 (en) | 2006-09-05 | 2013-05-28 | Ridge Diagnostics, Inc. | Quantitative diagnostic methods using multiple parameters |
| US11442061B2 (en) | 2006-10-13 | 2022-09-13 | Labrador Diagnostics Llc | Reducing optical interference in a fluidic device |
| US11215610B2 (en) | 2006-10-13 | 2022-01-04 | Labrador Diagnostics Llc | Reducing optical interference in a fluidic device |
| US8778665B2 (en) | 2006-11-14 | 2014-07-15 | Theranos, Inc. | Detection and quantification of analytes in bodily fluids |
| US10156579B2 (en) | 2006-11-14 | 2018-12-18 | Theranos Ip Company, Llc | Methods for the detection of analytes in small-volume blood samples |
| US9303286B2 (en)* | 2006-11-14 | 2016-04-05 | Theranos, Inc. | Detection and quantification of analytes in bodily fluids |
| US11802882B2 (en) | 2006-11-14 | 2023-10-31 | Labrador Diagnostics Llc | Methods for the detection of analytes in small-volume blood samples |
| US9575058B2 (en) | 2007-08-06 | 2017-02-21 | Theranos, Inc. | Systems and methods of fluidic sample processing |
| US11754554B2 (en) | 2007-08-06 | 2023-09-12 | Labrador Diagnostics Llc | Systems and methods of fluidic sample processing |
| US8883518B2 (en) | 2007-08-06 | 2014-11-11 | Theranos, Inc. | Systems and methods of fluidic sample processing |
| US9581588B2 (en) | 2007-10-02 | 2017-02-28 | Theranos, Inc. | Modular point-of-care devices, systems, and uses thereof |
| US9285366B2 (en) | 2007-10-02 | 2016-03-15 | Theranos, Inc. | Modular point-of-care devices, systems, and uses thereof |
| US11899010B2 (en) | 2007-10-02 | 2024-02-13 | Labrador Diagnostics Llc | Modular point-of-care devices, systems, and uses thereof |
| US10670588B2 (en) | 2007-10-02 | 2020-06-02 | Theranos Ip Company, Llc | Modular point-of-care devices, systems, and uses thereof |
| US9588109B2 (en) | 2007-10-02 | 2017-03-07 | Theranos, Inc. | Modular point-of-care devices, systems, and uses thereof |
| US8697377B2 (en) | 2007-10-02 | 2014-04-15 | Theranos, Inc. | Modular point-of-care devices, systems, and uses thereof |
| US9121851B2 (en) | 2007-10-02 | 2015-09-01 | Theranos, Inc. | Modular point-of-care devices, systems, and uses thereof |
| US8822167B2 (en) | 2007-10-02 | 2014-09-02 | Theranos, Inc. | Modular point-of-care devices, systems, and uses thereof |
| US11366106B2 (en) | 2007-10-02 | 2022-06-21 | Labrador Diagnostics Llc | Modular point-of-care devices, systems, and uses thereof |
| US9012163B2 (en) | 2007-10-02 | 2015-04-21 | Theranos, Inc. | Modular point-of-care devices, systems, and uses thereof |
| US11199538B2 (en) | 2007-10-02 | 2021-12-14 | Labrador Diagnostics Llc | Modular point-of-care devices, systems, and uses thereof |
| US11143647B2 (en) | 2007-10-02 | 2021-10-12 | Labrador Diagnostics, LLC | Modular point-of-care devices, systems, and uses thereof |
| US11137391B2 (en) | 2007-10-02 | 2021-10-05 | Labrador Diagnostics Llc | Modular point-of-care devices, systems, and uses thereof |
| US11092593B2 (en) | 2007-10-02 | 2021-08-17 | Labrador Diagnostics Llc | Modular point-of-care devices, systems, and uses thereof |
| US9435793B2 (en) | 2007-10-02 | 2016-09-06 | Theranos, Inc. | Modular point-of-care devices, systems, and uses thereof |
| US10900958B2 (en) | 2007-10-02 | 2021-01-26 | Labrador Diagnostics Llc | Modular point-of-care devices, systems, and uses thereof |
| US10634667B2 (en) | 2007-10-02 | 2020-04-28 | Theranos Ip Company, Llc | Modular point-of-care devices, systems, and uses thereof |
| US11061022B2 (en) | 2007-10-02 | 2021-07-13 | Labrador Diagnostics Llc | Modular point-of-care devices, systems, and uses thereof |
| US20100100333A1 (en)* | 2008-10-15 | 2010-04-22 | Ridge Diagnostics, Inc. | Human biomarker hypermapping for depressive disorders |
| US20100136700A1 (en)* | 2008-11-18 | 2010-06-03 | John Bilello | Metabolic syndrome and hpa axis biomarkers for major depressive disorder |
| US8440418B2 (en) | 2008-11-18 | 2013-05-14 | Ridge Diagnostics, Inc. | Metabolic syndrome and HPA axis biomarkers for major depressive disorder |
| US20100280562A1 (en)* | 2009-04-06 | 2010-11-04 | Ridge Diagnostics, Inc. | Biomarkers for monitoring treatment of neuropsychiatric diseases |
| US11195624B2 (en) | 2009-10-19 | 2021-12-07 | Labrador Diagnostics Llc | Integrated health data capture and analysis system |
| US11139084B2 (en) | 2009-10-19 | 2021-10-05 | Labrador Diagnostics Llc | Integrated health data capture and analysis system |
| US9460263B2 (en) | 2009-10-19 | 2016-10-04 | Theranos, Inc. | Integrated health data capture and analysis system |
| US8862448B2 (en) | 2009-10-19 | 2014-10-14 | Theranos, Inc. | Integrated health data capture and analysis system |
| US11158429B2 (en) | 2009-10-19 | 2021-10-26 | Labrador Diagnostics Llc | Integrated health data capture and analysis system |
| US20110213219A1 (en)* | 2010-01-26 | 2011-09-01 | Ridge Diagnostics, Inc. | Multiple Biomarker Panels to Stratify Disease Severity and Monitor Treatment of Depression |
| US11199489B2 (en) | 2011-01-20 | 2021-12-14 | Labrador Diagnostics Llc | Systems and methods for sample use maximization |
| US9464981B2 (en) | 2011-01-21 | 2016-10-11 | Theranos, Inc. | Systems and methods for sample use maximization |
| US10557786B2 (en) | 2011-01-21 | 2020-02-11 | Theranos Ip Company, Llc | Systems and methods for sample use maximization |
| US11644410B2 (en) | 2011-01-21 | 2023-05-09 | Labrador Diagnostics Llc | Systems and methods for sample use maximization |
| US9677993B2 (en) | 2011-01-21 | 2017-06-13 | Theranos, Inc. | Systems and methods for sample use maximization |
| US10876956B2 (en) | 2011-01-21 | 2020-12-29 | Labrador Diagnostics Llc | Systems and methods for sample use maximization |
| US11162936B2 (en) | 2011-09-13 | 2021-11-02 | Labrador Diagnostics Llc | Systems and methods for multi-analysis |
| US9664702B2 (en) | 2011-09-25 | 2017-05-30 | Theranos, Inc. | Fluid handling apparatus and configurations |
| US9645143B2 (en) | 2011-09-25 | 2017-05-09 | Theranos, Inc. | Systems and methods for multi-analysis |
| US10518265B2 (en) | 2011-09-25 | 2019-12-31 | Theranos Ip Company, Llc | Systems and methods for fluid handling |
| US10976330B2 (en) | 2011-09-25 | 2021-04-13 | Labrador Diagnostics Llc | Fluid handling apparatus and configurations |
| US11009516B2 (en) | 2011-09-25 | 2021-05-18 | Labrador Diagnostics Llc | Systems and methods for multi-analysis |
| US11054432B2 (en) | 2011-09-25 | 2021-07-06 | Labrador Diagnostics Llc | Systems and methods for multi-purpose analysis |
| US9952240B2 (en) | 2011-09-25 | 2018-04-24 | Theranos Ip Company, Llc | Systems and methods for multi-analysis |
| US9719990B2 (en) | 2011-09-25 | 2017-08-01 | Theranos, Inc. | Systems and methods for multi-analysis |
| US10018643B2 (en) | 2011-09-25 | 2018-07-10 | Theranos Ip Company, Llc | Systems and methods for multi-analysis |
| US12146891B2 (en) | 2011-09-25 | 2024-11-19 | Labrador Diagnostics Llc | United states systems and methods for fluid and component handling |
| US9250229B2 (en) | 2011-09-25 | 2016-02-02 | Theranos, Inc. | Systems and methods for multi-analysis |
| US9128015B2 (en) | 2011-09-25 | 2015-09-08 | Theranos, Inc. | Centrifuge configurations |
| US9268915B2 (en) | 2011-09-25 | 2016-02-23 | Theranos, Inc. | Systems and methods for diagnosis or treatment |
| US12085583B2 (en) | 2011-09-25 | 2024-09-10 | Labrador Diagnostics Llc | Systems and methods for multi-analysis |
| US10534009B2 (en) | 2011-09-25 | 2020-01-14 | Theranos Ip Company, Llc | Systems and methods for multi-analysis |
| US10012664B2 (en) | 2011-09-25 | 2018-07-03 | Theranos Ip Company, Llc | Systems and methods for fluid and component handling |
| US10371710B2 (en) | 2011-09-25 | 2019-08-06 | Theranos Ip Company, Llc | Systems and methods for fluid and component handling |
| US9632102B2 (en) | 2011-09-25 | 2017-04-25 | Theranos, Inc. | Systems and methods for multi-purpose analysis |
| US10627418B2 (en) | 2011-09-25 | 2020-04-21 | Theranos Ip Company, Llc | Systems and methods for multi-analysis |
| US8840838B2 (en) | 2011-09-25 | 2014-09-23 | Theranos, Inc. | Centrifuge configurations |
| US10557863B2 (en) | 2011-09-25 | 2020-02-11 | Theranos Ip Company, Llc | Systems and methods for multi-analysis |
| US9592508B2 (en) | 2011-09-25 | 2017-03-14 | Theranos, Inc. | Systems and methods for fluid handling |
| US9619627B2 (en) | 2011-09-25 | 2017-04-11 | Theranos, Inc. | Systems and methods for collecting and transmitting assay results |
| US11524299B2 (en) | 2011-09-25 | 2022-12-13 | Labrador Diagnostics Llc | Systems and methods for fluid handling |
| US9625465B2 (en) | 2012-05-15 | 2017-04-18 | Defined Diagnostics, Llc | Clinical diagnostic systems |
| US9213043B2 (en) | 2012-05-15 | 2015-12-15 | Wellstat Diagnostics, Llc | Clinical diagnostic system including instrument and cartridge |
| US9075042B2 (en) | 2012-05-15 | 2015-07-07 | Wellstat Diagnostics, Llc | Diagnostic systems and cartridges |
| US9081001B2 (en) | 2012-05-15 | 2015-07-14 | Wellstat Diagnostics, Llc | Diagnostic systems and instruments |
| US9810704B2 (en) | 2013-02-18 | 2017-11-07 | Theranos, Inc. | Systems and methods for multi-analysis |
| US9500663B2 (en) | 2014-11-11 | 2016-11-22 | Genmark Diagnostics, Inc. | Redundant identification for sample tracking on a diagnostic device |
| CN106483125A (en)* | 2015-08-26 | 2017-03-08 | 上海依达医疗器械有限公司 | A kind of intelligent blood stasis diagnostic apparatuses and its diagnostic method |
| US11331662B2 (en) | 2017-09-29 | 2022-05-17 | Miraplex Diagnostics Inc. | Assay preparation device |
Also Published As
| Publication number | Publication date |
|---|---|
| US7189573B2 (en) | 2007-03-13 |
| US7192777B2 (en) | 2007-03-20 |
| WO2004104552A2 (en) | 2004-12-02 |
| EP1623226A2 (en) | 2006-02-08 |
| EP1623226A4 (en) | 2007-08-08 |
| US20050186682A1 (en) | 2005-08-25 |
| JP2007501415A (en) | 2007-01-25 |
| US20050186681A1 (en) | 2005-08-25 |
| WO2004104552A3 (en) | 2005-04-28 |
| US7217393B2 (en) | 2007-05-15 |
| US20040265175A1 (en) | 2004-12-30 |
| US20070059204A1 (en) | 2007-03-15 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| US20040228766A1 (en) | Point of care diagnostic platform | |
| CA2549367A1 (en) | Point of care diagnostic platform | |
| US20040228765A1 (en) | Point of care diagnostic platform | |
| US6740240B2 (en) | Method and apparatus for directly sampling a fluid for microfiltration | |
| US10261041B2 (en) | Integrated disposable chip cartridge system for mobile multiparameter analyses of chemical and/or biological substances | |
| US5919711A (en) | Analytical cartridge | |
| US9678079B2 (en) | Microfluidic LAL-reactive substances testing method and apparatus | |
| RU2579971C2 (en) | Diagnostic instrument and method for sample preparation and analysis | |
| CN101821624B (en) | Optical measurement device | |
| US10877053B2 (en) | Method of determining an analyte concentration | |
| JP2004325462A (en) | Chemical analyzer and chemical analysis system | |
| CA2710473C (en) | Automated method and apparatus for detecting erroneous sample collection in clinical assays | |
| US7288195B2 (en) | Method and apparatus for directly sampling a fluid for microfiltration | |
| CN112924706B (en) | Pipetting unit and pipetting method for closing a liquid container | |
| EP4225501A1 (en) | System for determining a biological condition and cartridge therefor | |
| US20100161267A1 (en) | Device and Method for Automatic Calibration Verification of an Analyzer | |
| US11061045B2 (en) | Sample analysis system and method | |
| HK1098822A (en) | Point of care diagnostic platform | |
| US20220390478A1 (en) | Methods and apparatus providing calibration of foreground illumination for sample container characterization | |
| CN116148484A (en) | Test tube detection assembly, sample analyzer and sample analysis system | |
| CN1898560A (en) | Point of care diagnostic platform | |
| BUN | In vitro blood gas analyzers |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| AS | Assignment | Owner name:HARTRAQ, INC., NEVADA Free format text:ASSIGNMENT OF ASSIGNORS INTEREST;ASSIGNORS:WITTY, THOMAS R.;CASE, ROBERT;REEL/FRAME:015336/0512;SIGNING DATES FROM 20040127 TO 20040128 | |
| AS | Assignment | Owner name:FASTRAQ, INC., NEVADA Free format text:CHANGE OF NAME;ASSIGNOR:HARTRAQ, INC.;REEL/FRAME:015842/0453 Effective date:20041007 | |
| AS | Assignment | Owner name:BRADDOCK FINANCIAL PARTNERS, COLORADO Free format text:SECURITY AGREEMENT;ASSIGNOR:FASTRAQ, INC.;REEL/FRAME:017829/0766 Effective date:20060602 Owner name:THE HOWARD C. BIRNDORF LIVING TRUST, CALIFORNIA Free format text:SECURITY AGREEMENT;ASSIGNOR:FASTRAQ, INC.;REEL/FRAME:017829/0766 Effective date:20060602 Owner name:FISHER SCIENTIFIC INTERNATIONAL, NEW HAMPSHIRE Free format text:SECURITY AGREEMENT;ASSIGNOR:FASTRAQ, INC.;REEL/FRAME:017829/0766 Effective date:20060602 | |
| AS | Assignment | Owner name:IN-Q-TEL EMPLOYEE FUND, LLC, VIRGINIA Free format text:SECURITY AGREEMENT;ASSIGNOR:FASTRAQ, INC.;REEL/FRAME:018621/0183 Effective date:20061129 Owner name:IN-Q-TEL, INC., VIRGINIA Free format text:SECURITY AGREEMENT;ASSIGNOR:FASTRAQ, INC.;REEL/FRAME:018621/0183 Effective date:20061129 | |
| AS | Assignment | Owner name:BRADDOCK FINANCIAL PARTNERS, LLC, COLORADO Free format text:SECURITY AGREEMENT;ASSIGNOR:FASTRAQ, INC.;REEL/FRAME:019252/0374 Effective date:20070430 Owner name:THE HOWARD C. BIRNDORF LIVING TRUST DATED SEPTEMBE Free format text:SECURITY AGREEMENT;ASSIGNOR:FASTRAQ, INC.;REEL/FRAME:019252/0374 Effective date:20070430 | |
| STCB | Information on status: application discontinuation | Free format text:ABANDONED -- FAILURE TO RESPOND TO AN OFFICE ACTION |