US20040118704A1 - Analyte test intrument having improved versatility - Google Patents
Analyte test intrument having improved versatilityDownload PDFInfo
- Publication number
- US20040118704A1 US20040118704A1US10/326,008US32600802AUS2004118704A1US 20040118704 A1US20040118704 A1US 20040118704A1US 32600802 AUS32600802 AUS 32600802AUS 2004118704 A1US2004118704 A1US 2004118704A1
- Authority
- US
- United States
- Prior art keywords
- test
- analyte
- strip
- test strip
- instrument
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Abandoned
Links
- 238000012360testing methodMethods0.000titleclaimsabstractdescription489
- 239000012491analyteSubstances0.000titleclaimsabstractdescription203
- 238000003556assayMethods0.000claimsabstractdescription104
- 238000000034methodMethods0.000claimsdescription49
- 239000000523sampleSubstances0.000claimsdescription29
- 238000006243chemical reactionMethods0.000claimsdescription23
- WQZGKKKJIJFFOK-GASJEMHNSA-NGlucoseNatural productsOC[C@H]1OC(O)[C@H](O)[C@@H](O)[C@@H]1OWQZGKKKJIJFFOK-GASJEMHNSA-N0.000claimsdescription18
- 239000008103glucoseSubstances0.000claimsdescription18
- 230000004044responseEffects0.000claimsdescription16
- 239000012472biological sampleSubstances0.000claimsdescription14
- 238000005259measurementMethods0.000claimsdescription14
- 239000004020conductorSubstances0.000claimsdescription11
- 150000002576ketonesChemical class0.000claimsdescription11
- WQZGKKKJIJFFOK-VFUOTHLCSA-Nbeta-D-glucoseChemical compoundOC[C@H]1O[C@@H](O)[C@H](O)[C@@H](O)[C@@H]1OWQZGKKKJIJFFOK-VFUOTHLCSA-N0.000claimsdescription9
- JVTAAEKCZFNVCJ-UHFFFAOYSA-MLactateChemical compoundCC(O)C([O-])=OJVTAAEKCZFNVCJ-UHFFFAOYSA-M0.000claimsdescription5
- 230000005284excitationEffects0.000claimsdescription4
- 238000011534incubationMethods0.000claimsdescription4
- 238000003780insertionMethods0.000claimsdescription4
- 230000037431insertionEffects0.000claimsdescription4
- 230000006870functionEffects0.000description15
- 238000007812electrochemical assayMethods0.000description8
- 238000012545processingMethods0.000description7
- 230000014509gene expressionEffects0.000description6
- 239000008280bloodSubstances0.000description5
- 210000004369bloodAnatomy0.000description5
- 230000008859changeEffects0.000description5
- 239000003153chemical reaction reagentSubstances0.000description4
- 238000004458analytical methodMethods0.000description3
- 239000011248coating agentSubstances0.000description3
- 238000000576coating methodMethods0.000description3
- 238000004891communicationMethods0.000description3
- 238000010586diagramMethods0.000description3
- 238000003487electrochemical reactionMethods0.000description3
- 239000012530fluidSubstances0.000description3
- 238000001514detection methodMethods0.000description2
- 238000000840electrochemical analysisMethods0.000description2
- 238000004519manufacturing processMethods0.000description2
- 238000010998test methodMethods0.000description2
- 230000004075alterationEffects0.000description1
- 230000005540biological transmissionEffects0.000description1
- 210000001124body fluidAnatomy0.000description1
- 239000010839body fluidSubstances0.000description1
- 230000001276controlling effectEffects0.000description1
- 230000002596correlated effectEffects0.000description1
- 230000000875corresponding effectEffects0.000description1
- 230000003247decreasing effectEffects0.000description1
- 230000007812deficiencyEffects0.000description1
- 230000009977dual effectEffects0.000description1
- 238000002848electrochemical methodMethods0.000description1
- 239000007788liquidSubstances0.000description1
- 239000000203mixtureSubstances0.000description1
- 238000012986modificationMethods0.000description1
- 230000004048modificationEffects0.000description1
- 238000012544monitoring processMethods0.000description1
- 230000007935neutral effectEffects0.000description1
- 238000007254oxidation reactionMethods0.000description1
- 230000008569processEffects0.000description1
- 238000006722reduction reactionMethods0.000description1
- 238000011160researchMethods0.000description1
- 239000004065semiconductorSubstances0.000description1
- 238000007920subcutaneous administrationMethods0.000description1
- 239000000126substanceSubstances0.000description1
- 238000006276transfer reactionMethods0.000description1
- 230000000007visual effectEffects0.000description1
Images
Classifications
- G—PHYSICS
- G01—MEASURING; TESTING
- G01N—INVESTIGATING OR ANALYSING MATERIALS BY DETERMINING THEIR CHEMICAL OR PHYSICAL PROPERTIES
- G01N27/00—Investigating or analysing materials by the use of electric, electrochemical, or magnetic means
- G01N27/26—Investigating or analysing materials by the use of electric, electrochemical, or magnetic means by investigating electrochemical variables; by using electrolysis or electrophoresis
- G01N27/28—Electrolytic cell components
- G01N27/30—Electrodes, e.g. test electrodes; Half-cells
- G01N27/327—Biochemical electrodes, e.g. electrical or mechanical details for in vitro measurements
- G01N27/3271—Amperometric enzyme electrodes for analytes in body fluids, e.g. glucose in blood
- G01N27/3272—Test elements therefor, i.e. disposable laminated substrates with electrodes, reagent and channels
- G—PHYSICS
- G01—MEASURING; TESTING
- G01N—INVESTIGATING OR ANALYSING MATERIALS BY DETERMINING THEIR CHEMICAL OR PHYSICAL PROPERTIES
- G01N27/00—Investigating or analysing materials by the use of electric, electrochemical, or magnetic means
- G01N27/26—Investigating or analysing materials by the use of electric, electrochemical, or magnetic means by investigating electrochemical variables; by using electrolysis or electrophoresis
- G01N27/28—Electrolytic cell components
- G01N27/30—Electrodes, e.g. test electrodes; Half-cells
- G01N27/327—Biochemical electrodes, e.g. electrical or mechanical details for in vitro measurements
- G01N27/3271—Amperometric enzyme electrodes for analytes in body fluids, e.g. glucose in blood
- G01N27/3273—Devices therefor, e.g. test element readers, circuitry
- G—PHYSICS
- G01—MEASURING; TESTING
- G01N—INVESTIGATING OR ANALYSING MATERIALS BY DETERMINING THEIR CHEMICAL OR PHYSICAL PROPERTIES
- G01N33/00—Investigating or analysing materials by specific methods not covered by groups G01N1/00 - G01N31/00
- G01N33/48—Biological material, e.g. blood, urine; Haemocytometers
- G01N33/483—Physical analysis of biological material
- G01N33/487—Physical analysis of biological material of liquid biological material
- G01N33/48785—Electrical and electronic details of measuring devices for physical analysis of liquid biological material not specific to a particular test method, e.g. user interface or power supply
- G—PHYSICS
- G01—MEASURING; TESTING
- G01N—INVESTIGATING OR ANALYSING MATERIALS BY DETERMINING THEIR CHEMICAL OR PHYSICAL PROPERTIES
- G01N33/00—Investigating or analysing materials by specific methods not covered by groups G01N1/00 - G01N31/00
- G01N33/48—Biological material, e.g. blood, urine; Haemocytometers
- G01N33/483—Physical analysis of biological material
- G01N33/487—Physical analysis of biological material of liquid biological material
- G01N33/4875—Details of handling test elements, e.g. dispensing or storage, not specific to a particular test method
- G01N33/48771—Coding of information, e.g. calibration data, lot number
Definitions
- This inventionrelates to analyte test instruments that perform electrochemical assays on biological samples. More particularly, the invention relates to analyte test instruments that can perform electrochemical assays by using different modes of operation.
- Electrochemical assays for determining the concentrations of analytes in samples comprising complex mixtures of liquidshave been developed. Such electrochemical assays can be performed with test strips, i.e., biosensors in the form of strips. Test strips can function in an invasive manner (i.e., as probes that come into contact with a body fluid, such as whole blood or subcutaneous fluid). Test strips can function in a non-invasive manner (i.e., as strips that come into contact with blood withdrawn by a syringe or a lancing device). In particular, test strips for biomedical applications (e.g., whole blood analyses) have been developed for the determination of glucose levels in biological samples.
- test strips for biomedical applicationse.g., whole blood analyses
- An analyte test instrumentis an instrument can be used to perform electrochemical assays to determine the concentration of an analyte (e.g., glucose) in a biological sample (e.g., blood).
- an analytee.g., glucose
- a biological samplee.g., blood
- a userinserts a test strip into a test port in the instrument.
- the instrumentdisplays a “ready” indication to the user and allows sufficient time for the user to deposit a biological sample on the test strip.
- an electrochemical reactionoccurs.
- the reactionproduces an electrical response, such as a change in current.
- the electrical responseis detectable by the analyte test instrument.
- the analyte test instrumentconverts the detected signal into data that corresponds to information relating to the analyte and displays the information to the user.
- the instrumentmay be able to store a series of such measurements and provide the stored information to the user via a display or to an external processor via a data link.
- test strips having two electrodesAll commercially available electrochemical assays employing test strips for determining the concentration of glucose employ test strips having two electrodes. See, for example, WO 99/19507, incorporated herein by reference, which describes a test strip having two electrodes.
- the test striphas (1) a working electrode and (2) a dual-purpose reference/counter electrode.
- the reaction that takes place at the working electrodeis the reaction that is required to be monitored and controlled.
- the second electrodeis called a dual-purpose reference/counter electrode because this electrode acts as a reference electrode as well as a counter electrode.
- Electrochemical assays employing test strips having three electrodesemploy a test strip having (1) a working electrode, (2) a reference electrode, and (3) a counter electrode. See, for example, U.S. Ser. No. 10/062,313, filed Feb. 1, 2002, incorporated herein by reference.
- the reaction that takes place at the working electrodeis the reaction that is required to be monitored and controlled.
- the functions of the reference electrode and the counter electrodeare to ensure that the working electrode actually experiences the conditions desired, i.e. the correct potential intended to be applied.
- the function of the reference electrodeis to measure the potential at the interface of the working electrode and the sample as accurately as possible. In an ideal situation, no current passes through the reference electrode.
- the function of the counter electrodeis to ensure that the correct potential difference between the reference electrode and the working electrode is being applied.
- the potential difference between the working electrode and the reference electrodeis assumed to be the same as the desired potential at the working electrode. If the potential measured at the working electrode is not the potential desired at the working electrode, the potential that is applied between the counter electrode and the working electrode is altered accordingly, i.e., the potential is either increased or decreased.
- the reaction at the counter electrode, as measured by the currentis also equal and opposite to the charge transfer reaction, as measured by the current, occurring at the working electrode, i.e., if an oxidation reaction is occurring at the working electrode then a reduction reaction will take place at the counter electrode, thereby allowing the sample to remain electrically neutral.
- An analyte test instrument designed for test strips having two electrodescould not be used if an assay employing a test strip having three electrodes needs to be performed. The user would have to use a separate analyte test instrument. If the user wanted to perform a set of assays that required strips having two electrodes and a set of assays that required strips having three electrodes, these assays could not be performed on the same analyte test instrument.
- An analyte test instrument for electrochemical assaysoften requires the user to calibrate the instrument for each batch of test strips.
- U.S. Pat. No. 5,366,609describes a calibration technique that requires a read-only-memory (ROM) key for operation and calibration of an analyte test instrument.
- ROMread-only-memory
- a ROM keyis inserted into a port (i.e., the ROM key port) that is distinct from the port for a test strip (i.e., the test port).
- a test stripis inserted into the test port after the ROM key is inserted into the ROM key port.
- the ROM keymust remain in the ROM key port during both the calibration and the operation of the instrument.
- the ROM keycontains specific data, including algorithms, for carrying out procedures for determining the concentration of an analyte in a biological sample applied to one of a batch of test strips associated with the ROM key.
- the data stored in the ROM keyinclude measurement delay times, incubation times, the number of measurements to be taken during a measurement period, various thresholds against which voltage levels can be compared, values of excitation voltage levels applied to the strip during a test procedure, glucose value conversion factors, and a variety of failsafe test threshold values.
- the ROM keycan contain some or all of the code for the microprocessor that controls the performing of the assay.
- a microprocessor in the analyte test instrumentuses the algorithms, the conversion factors, and the code provided by the ROM key as needed.
- U.S. Pat. No. 6,377,894incorporated herein by reference, describes an instrument requiring a ROM key for operation and calibration of the instrument.
- the ROM keyis inserted into the test port of the instrument and data is downloaded from the ROM key by the instrument and stored in the memory of the instrument.
- the ROM keycontains data needed for carrying out procedures for determining the concentration of an analyte in a biological sample applied to a test strip.
- the ROM keyis removed so that test strips can be inserted into the test port to perform assays.
- Different ROM keyscan be inserted into the instrument to provide data for the testing of different analytes on the same instrument.
- the instrumentcan communicate with the ROM key to determine the analyte for which the ROM key contains information.
- Calibration informationcan be stored in different locations in the memory of the instrument for each analyte the instrument is capable of testing.
- the instrumentWhen a test strip is inserted into the test port, the instrument has the ability to recognize which analyte is being tested. The microprocessor in the instrument then recalls the instructions for carrying out procedures for determining the concentration of that analyte, and the instrument then performs the appropriate test.
- the aforementioned patentsdo not describe how the electrical circuitry of the instrument can be reconfigured so that analytical tests that require different circuit configurations can be performed on the same instrument.
- the aforementioned patentsdo not describe how stored information relating to the configuration of the electrical circuitry of the instrument can be modified when an assay for a specific analyte needs to be modified.
- the aforementioned patentsdo not describe how stored information can be used to reconfigure the electrical circuitry of the instrument while a test strip is being used. Accordingly, it would be desirable to provide an analyte test instrument that addresses the foregoing deficiencies.
- this inventionprovides an analyte test instrument that has test strip circuitry that can be placed into different configurations by means of information provided by a calibration strip to perform assays with test strips having two electrodes and test strips having three electrodes.
- this inventionprovides methods for using the analyte test instrument to perform assays with test strips having two electrodes and test strips having three electrodes.
- the analyte test instrument of this inventioncomprises:
- test strip circuitcapable of having a plurality of configurations, the configurations being set by the microprocessor, whereby an assay can be performed using a test strip that has been inserted into the test port.
- the analyte test instrumentfurther includes a memory for storing instructions and information required for the operation of the instrument.
- the memorycan be removably attached to the instrument, as described previously in U.S. Pat. No. 5,366,609.
- the inventionprovides an analyte test instrument that can perform assays on a variety of different analytes.
- a calibration stripis inserted into the test port.
- informationi.e., data or programs or both
- the analyte test instrumenthas a memory, preferably stored in the memory of the analyte test instrument.
- the informationis stored in the analyte test instrument after the calibration strip is removed. The stored information specifies whether the method(s) of the assay(s) requires a test strip having two electrodes or test strip having three electrodes.
- a test stripis inserted into the test port, and the identity of the assay is indicated, preferably by means of a pattern of conductive material applied to a major surface of the test strip, preferably the major surface that does not support the electrodes.
- the analyte test instrumentdetermines from the downloaded information whether the assay calls for a test strip having two electrodes or for a test strip having three electrodes.
- the appropriate electrical switches in the test strip circuit of the analyte test instrumentare then opened or closed to establish the configuration of the test strip circuit appropriate for the test strip utilized in the assay, that is, a test strip having two electrodes or a test strip having three electrodes.
- a sample to be analyzedis then applied to the test strip, and a reaction that generates an electrical response occurs.
- the electrical responseis detected and measured by the analyte test instrument, and the concentration of the analyte tested is determined by means of the downloaded calibration information.
- the analyte test instrumentdisplays the concentration of the analyte.
- Assays that call for a test strip having two electrodes and assays that call for a test strip having three electrodescan be performed on the same analyte test instrument.
- the analyte test instrument of this inventionfeatures the capability of changing the method for performing an assay to determine the concentration of a particular analyte.
- a new calibration stripis inserted into the test port.
- the instructions for the performing the new method of the assay for the particular analyteare then downloaded to the analyte test instrument, and, if the analyte test instrument has a memory, preferably stored in the memory of the analyte test instrument.
- the identity of the assayis determined.
- the appropriate electrical switches in the test strip circuit of the analyte test instrumentare then opened or closed to establish the appropriate circuit configuration for the test strip utilized in the assay, that is, a test strip having two electrodes or a test strip having three electrodes.
- the circuit configurationsare based on the information from the calibration strip most recently downloaded to the analyte test instrument.
- the same analyte test instrumentcan be used to perform an assay even if the test method is changed one employing a test strip having two electrodes to one employing a test strip having three electrodes, and vice versa.
- the analyte test instrumentcan employ both a two-electrode mode and a three-electrode mode during the same assay.
- the expression “two-electrode mode”refers to the test strip circuitry employed for operating an analyte test instrument with a test strip having two electrodes.
- the expression “three-electrode mode”refers to the test strip circuitry employed for operating an analyte test instrument with a test strip having three electrodes.
- the information previously downloaded from the calibration stripspecifies what portion of the assay employs a test strip circuit configuration in a two-electrode mode and what portion of the assay employs a test strip circuit configuration in a three-electrode mode.
- a test stripis inserted into the test port, and the identity of the assasy is indicated, preferably from a pattern of conductive material that has been applied to a major surface of the test strip, preferably the major surface that does not support the electrodes.
- the analyte test instrumentdetermines from the aforementioned downloaded information whether the assay requires a test strip circuit configuration in a two-electrode mode or a test strip circuit configuration in a three-electrode mode at the start of the assay.
- the appropriate electrical switches in the analyte test instrumentare then opened or closed to establish the appropriate electrode mode.
- a sample to be analyzedtypically a biological sample, is then applied to the test strip, and a reaction that generates an electrical response occurs.
- the appropriate electrical switches in the analyte test instrumentare then opened or closed to establish the test strip circuit configuration for the appropriate electrode mode, which is a different electrode mode than was used at the start of the assay.
- the electrical responseis detected and measured by the analyte test instrument, and the concentration of the analyte is determined by means of the downloaded calibration information.
- the analyte test instrumentcan then display the concentration of the analyte.
- test strip circuit configurationis switched during an assay involves an assay in which it may be preferred to use a test strip having three electrodes for the advantages provided by the use of a test strip having three electrodes, such as, for example, improved control of voltage at the working electrode.
- the test strip circuit for the two-electrode modeis preferred during this sample detection phase of the assay. Accordingly, test strip circuit configurations for both the two-electrode mode and the three-electrode mode are desired within the course of an assay. It is assumed that an assay involves operational steps beginning with the insertion of the test strip into the analyte test instrument and obtaining the result of the assay.
- the analyte test instrument of this inventionmakes it possible for the user to perform assays with test strips having two electrodes and test strips having three electrodes with the same instrument.
- the analyte test instrument of this inventionmakes it possible for an assay to be modified without having to discard the instrument.
- the analyte test instrument of this inventionmakes it possible for the mode of operation to change during the performance of an assay without intervention from the user.
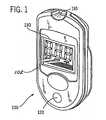
- FIG. 1is a perspective view of an embodiment of an analyte test instrument suitable for use in this invention.
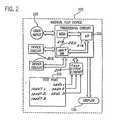
- FIG. 2is a block diagram that illustrates electronic components of an analyte test instrument suitable for use in this invention.
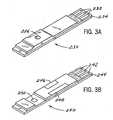
- FIG. 3Ais a perspective view of a test strip that is suitable for use with the analyte test instrument of this invention.
- FIG. 3Bis a perspective view of a calibration strip that is suitable for use with the analyte test instrument of this invention.
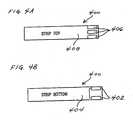
- FIG. 4Aillustrates a top plan view of test strip that is suitable for use with the analyte test instrument of this invention.
- FIG. 4Billustrates a bottom plan view of test strip that is suitable for use with the analyte test instrument of this invention.
- FIG. 5is a flow chart illustrating a method for calibrating the analyte test instrument of this invention.
- FIG. 6is a flow chart illustrating a method for calibrating the analyte test instrument of this invention.
- FIGS. 7A, 7B, and 8are schematic diagrams that illustrates a test strip circuit that can be used to perform assays with two different types of test strips.
- test strip having two electrodesand other expressions relating to tests strips having two electrodes refer to test strips that have a working electrode and a dual-purpose reference/counter electrode.
- test strip having three electrodesand other expressions relating to tests strips having three electrodes refer to test strips that have a working electrode, a counter electrode, and a reference electrode, the reference electrode being separate from the counter electrode.
- a “test strip having two electrodes”can have one or more additional electrodes, so long as the strip has a dual-purpose reference/counter electrode that performs the functions of both a reference electrode and a counter electrode.
- a test strip having two electrodescan have a trigger electrode, which is an electrode that detects when a sufficient quantity of sample has been applied to the test strip.
- a “test strip having three electrodes”can have one or more additional electrodes, so long as the test strip has one electrode for performing the function of a reference electrode and another electrode for performing the function of a counter electrode.
- a test strip having three electrodescan have a dummy electrode, which is an electrode that is similar to the working electrode, but lacks the substance that reacts with the analyte (see, for example, U.S. Pat. No. 5,628,890), or a trigger electrode dedicated to the sole function of detecting when a sufficient quantity of sample has been applied to the test strip (see, for example, U.S. Pat. No. 5,582,697).
- electrochemical test strips for determining the concentration of glucoseemploy two electrodes—(1) a working electrode and (2) a dual-purpose reference/ counter electrode.
- electrochemical systems having three electrodesemploy (1) a working electrode, (2) a reference electrode, and (3) a counter electrode.
- Electrochemical systems employing test strips having three electrodeshave the requirement that little or no current pass between the working electrode and the reference electrode. This requirement is achieved by using high impedance operational amplifiers in the electrical circuits of these systems. High impedance operational amplifiers are expensive; consequently, electrochemical systems that perform assays with test strips having three electrodes are expensive. These expensive systems are generally used only in research and are not practical from a cost standpoint for use by diabetics for glucose monitoring at home.
- a test strip having three electrodeswould be preferred in any electrochemical measurement that involves the application of an external voltage and measurement of current.
- all electrochemical test strips commercially availableuse only two electrodes. Precise control of the voltage difference between the working electrode and the reference electrode must be maintained, but such control is difficult to achieve in a test strip having two electrodes.
- the electrical components of an analyte test instrument designed for test strips employing two electrodeswould not operate with test strips employing three electrodes.
- an analyte test instrument 100comprises a housing 102 , which contains the electrical and electronic components of the analyte test instrument.
- the analyte test instrument 100comprises a test port 110 , a push button 120 , and a display 130 .
- the test port 110is a multi-purpose test port, which comprises a slot into which a user inserts test strips and calibration strips.
- the test port 110comprises a slot assembly capable of receiving a strip, such as a test strip or a calibration strip.
- the test port 110can have a plurality of electrical contacts capable of electrically engaging such a strip when the strip is inserted into the test port 110 .
- the push button 120allows the user to control the analyte test instrument 100 .
- the push button 120is used to turn the instrument on and off, to recall information stored in the instrument, to respond to messages displayed, and to set some of the configuration control parameters for the instrument.
- the push button 120can also provide access to menus generated by software contained in the analyte test instrument 100 .
- the display 130is a device that gives information in a visual form.
- the display 130is typically a screen.
- the information giventypically includes, but is not limited to, test results, messages to the user, information stored in the memory of the analyte test instrument.
- one or more replaceable batteriesinstalled via a battery compartment at the rear of the analyte test instrument 100 (not shown) provide power for the analyte test instrument 100 . It should be understood, however, that any source of power capable of providing a suitable direct (DC) voltage can provide power to the analyte test instrument 100 .
- DCdirect
- FIG. 2is a block diagram that shows the interrelationship among the electronic components of an analyte test instrument 100 .
- the analyte test instrument 100comprises a processing circuit 210 , at least one device circuit 212 , at least one test strip circuit 214 , a microprocessor 216 , and a memory 218 .
- the purpose of the processing circuit 210is to enable a strip that is engaged in the test port 110 to communicate with the microprocessor 216 and the memory 218 .
- the processing circuit 210can send signals to the test port 110 to determine the identity of the strip inserted therein, i.e., to determine whether the strip is a calibration strip or a test strip.
- the device circuit(s) 212 and the test strip circuit(s) 214can comprise analog, digital, or mixed-signal circuits, application-specific integrated circuits (ASICS), and passive and active electrical components.
- the device circuit(s) 212can perform various electrical functions required by the analyte test instrument 100 , such as driving the display function 130 and the clock functions for a microprocessor 216 .
- the device circuit(s)carries instructions from the microprocessor 216 to various functional components of the analyte test instrument 100 so that these components can perform their intended functions.
- Test strip circuit(s) 214can perform analog-to-digital (A/D) conversion of signals received at the test port 110 from a test strip and can perform digital-to-analog (D/A) conversion of signals received from the microprocessor 216 .
- the test strip circuit(s)transmits information between the microprocessor 216 and the test strip.
- the test strip circuit(s)is used to ensure that the proper voltage is being applied to the test strip and that the proper value of current generated at the test strip is being measured by the microprocessor 216 .
- the microprocessor 216is an integrated circuit that contains the entire central processing unit of a computer.
- the memory 218is a unit of a computer that preserves information for the purpose of retrieval. Such information may include, but is not limited to, measurement delay time(s), sample incubation time(s), number of measurements to be taken during an assay, threshold(s) against which voltage level(s) can be compared, value(s) of excitation voltage level(s) applied to a test strip during an assay, analyte value conversion factors, failsafe assay threshold value(s), and configurations of circuitry of the analyte test instrument.
- the memory 218comprises at least 1K of random access memory (RAM). In more preferred embodiments, the memory 218 has sufficient additional capacity to store a multiplicity of assay results.
- RAMrandom access memory
- Instrument software 220is responsive to information received at the test port 110 from a calibration strip.
- the instrument software 220uses the information received to control the operation of the analyte test instrument 100 .
- the instrument software 220also controls operations of the analyte test instrument 100 that are independent of information introduced or generated at the test port 110 .
- the instrument software 220can enable the user to recall assay results and assay information, can provide various warning, error, and prompting messages, can permit setting of date and time, can control transmission of data to external devices, can monitor power level or battery level or both, and can provide indications to the user if power drops below a specified level.
- the test port 110includes six electrical contacts, which are labeled IDENT 1 , IDENT 2 , IDENT 3 , SENS 1 , SENS 2 , and SENS 3 .
- the major surfaces of the stripengage the electrical contacts of the test port 110 , thereby enabling the analyte test instrument 110 to identify a pattern of conductive material on the top major surface of the strip, on the bottom major surface of the strip, or on both major surfaces of the strip.
- the pattern of conductive material on an inserted strip that interacts with the electrical contacts IDENT 1 , IDENT 2 , and IDENT 3indicates whether the inserted strip is a calibration strip or a test strip.
- FIGS. 4A and 4BThis embodiment is shown in FIGS. 4A and 4B, which will be described later.
- the inserted stripis a test strip
- the type of analyte to be determined by the assay to be performed with the test stripis also identified (e.g., glucose, ketone bodies, etc.).
- the engagement of the electrical contacts and the strip identification processare described in more detail in U.S. Pat. No. 6,377,894, incorporated herein by reference.
- the electrical contacts labeled SENS 1 , SENS 2 , and SENS 3relate to the electrodes that are involved in performing analytical tests.
- FIG. 3Aillustrates in more detail a test strip 230 .
- a plurality of electrical contacts 232is provided at the end 234 of the test strip 230 that is inserted into the test port 110 .
- the electrical contacts 232contact the electrical contacts SENS 1 , SENS 2 , and SENS 3 .
- a samplee.g., a drop of blood, undergoing the assay is placed for testing on the reaction area 236 of the test strip 230 .
- the reaction area 236is the area where the sample contacts the electrodes of the test strip 230 (i.e., the working electrode and the dual purpose reference/counter electrode in the strip having two electrodes and the working electrode, the reference electrode, and the counter electrode in the strip having three electrodes).
- the electrodes of the test strip 230i.e., the working electrode and the dual purpose reference/counter electrode in the strip having two electrodes and the working electrode, the reference electrode, and the counter electrode in the strip having three electrodes.
- FIG. 3Billustrates a ROM-type calibration strip 240 .
- a ROM-type calibration strip 240is associated with a package (not shown) of test strips 230 .
- a plurality of electrical contacts 242is provided at the end 244 of the calibration strip 240 that is inserted into the test port 110 .
- the calibration code 246 and manufacturing lot number 248are printed on the calibration strip 240 and are visible to the user.
- the lot numberis stored in a read-only-memory (ROM) 250 in binary coded decimal (BCD) format.
- ROMread-only-memory
- BCDbinary coded decimal
- the ROM 250which is in electrical communication with the electrical contacts 242 , encodes information relating to algorithm(s) for processing data obtained in an assay with a test strip.
- the ROM 250can also encode information relating to the calibration code 246 and manufacturing lot number 248 as well as other parameters, as described in U.S. Pat. No. 6,377,894, incorporated herein by reference.
- the assaysare not performed with the calibration strip 240 . Rather, the calibration strip 240 delivers the information, the algorithms, the parameters, and the procedures that are required to characterize an assay to the analyte test instrument 100 .
- the ROM 250is capable of storing and downloading to the analyte test instrument 100 parameters that characterize an assay as having a two-electrode format or a three-electrode format.
- a test strip 400has a pattern of conductive material 402 on the major surface 404 thereof that does not support the electrodes 406 .
- the electrodes 406are supported on the major surface 408 of the test strip 400 .
- Different patterns of conductive material 402can be used to specify different assays (e.g., glucose, ketone bodies, etc.).
- the pattern of conductive material 402is disposed in such a way that the electrical contacts IDENT 1 , IDENT 2 , and IDENT 3 of the test port 110 interact with the conductive material in the pattern to identify the type of assay that will be performed by the test strip 400 , such as, for example, glucose, ketone bodies, lactate.
- a device circuit 212such as an ASIC (see FIG. 2), identifies the type of assay that will be performed by the test strip 400 by determining the pattern of connection of the conductive material 402 on the major surface 404 of the test strip 400 .
- a stripe.g., calibration strip, glucose test strip, ketone bodies test strip, etc.
- the analyte test instrument 100detects the presence of the strip and performs a procedure to determine whether the strip is a calibration strip or a test strip for determination of the concentration of an analyte.
- the instrument software 220polls the test port 110 to identify the function of the strip that has been inserted, i.e. calibration strip, test strip for determination of the concentration of an analyte.
- the instrument software 220attempts to communicate with the inserted strip by means of a protocol capable of operating with a serial EE-squared interface, such as that defined by the Dallas ROM protocol of Dallas Semiconductor, Dallas Tex. Such an interface provides single-wire communication. If the attempt to communicate is successful, the instrument software 220 proceeds to the ROM calibration procedure. If the attempt to communicate is unsuccessful, the instrument software 220 puts the analyte test instrument 100 into a brief wait mode (a predetermined time period), e.g., three to five minutes. If the analyte test instrument 100 fails to receive a signal indicating that a sample has been received during the waiting period, the analyte test instrument 100 shuts itself off automatically.
- a protocol capable of operating with a serial EE-squared interfacesuch as that defined by the Dallas ROM protocol of Dallas Semiconductor, Dallas Tex.
- a serial EE-squared interfacesuch as that defined by the Dallas ROM protocol of Dallas Semiconductor, Dallas Tex.
- the receipt of a signal by microprocessor 216indicates that the user is performing an assay for determination of the concentration of an analyte.
- the electrical contacts 232communicate with the analyte test instrument 100 .
- a sample(not shown) is added to the reaction area 236 , the sample reacts with the reagents in the reaction area, thereby causing a flow of electrons to produce an electrical response, such as a change in current.
- the responseis detectable by the analyte test instrument 100 .
- the analyte test instrument 100converts the detected signal into data corresponding to information relating to the analyte and displays the information to the user.
- FIG. 5illustrates the ROM calibration procedure when a calibration strip is introduced into the test port 110 .
- the instrument software 220identifies the calibration strip 240 (step 710 )
- data from the ROM 250is downloaded to the analyte test instrument 100 (step 720 ).
- the display 130displays the lot number downloaded from the calibration strip 240 (step 730 ), as an indication that the calibration is complete. This data is stored in the memory 218 (step 740 ). The user can then remove the calibration strip from the test port 110 (step 750 ).
- the downloaded dataremains in the memory 218 for use by the analyte test instrument 110 until a new calibration procedure is performed (step 760 ).
- the analyte test instrument 100can store more than one set of calibration data in the memory 218 .
- an analyte test instrument 100capable of performing assays with a plurality of test strips 230 (e.g., glucose, ketone bodies), can store a set of calibration data for each type of test strip 230 .
- the downloaded and stored datacomprises parameters, algorithms, operational procedures, and the like for controlling the operation of the analyte test instrument 100 .
- the datacomprise information that instructs the analyte test instrument to perform an assay with a test strip having two electrodes or with a test strip having three electrodes.
- the datacomprise information that instructs the analyte test instrument 100 to begin an assay in a mode where the circuitry anticipates a test strip having two electrodes and then switch to a mode where the circuitry is changed to accommodate a test strip having three electrodes.
- FIG. 6depicts a flow chart of a method of performing an assay with the analyte test instrument of this invention.
- a calibration strip 240is inserted into the test port 110 and information about types of assays (e.g., glucose, ketone bodies) and the configuration of the test strip circuit 214 (i.e., two electrodes or three electrodes) are downloaded and stored in the memory 218 of the analyte test instrument 100 (step 800 ).
- the calibration strip 240is removed from the test port 110 .
- a test strip 230is inserted into the test port 110 (step 810 ).
- the microprocessor 216 of the analyte test instrument 100determines whether the strip inserted into the test port 110 is a test strip 230 or a calibration strip 240 by transmitting a digital signal along a wire to the strip. If no signal is received from the strip, the microprocessor 216 has determined that the strip is a test strip 230 . The microprocessor 216 then determines the pattern of electrical contacts on the major surface of the test strip 230 that does not support the electrodes (step 820 ). The aforementioned pattern of electrical contacts provides a signal to the microprocessor 216 indicating the assay that can be performed with the test strip 230 that has been inserted into the test port 110 , such as, for example, a glucose assay, a ketone bodies assay.
- the microprocessor 216sets the switches of the test strip circuit 214 to the mode for a test strip having two electrodes (step 830 ).
- a sampleis then introduced to the reaction area 236 of the test strip 230 .
- a voltageis applied, and after a brief period of time, a small current can be detected (step 840 ).
- the currentindicates that a sample, which covers the electrodes, has been detected (step 850 ).
- the microprocessor 216instructs a switch (not shown) in the device circuit 212 to open, thereby disconnecting the electrodes on the test strip from the test strip circuit 214 for a specified period of time (step 860 ), which period has been preset by the microprocessor 216 .
- the switch (not shown) in the device circuit 212is closed and the test strip circuit 214 remains in the two-electrode mode if the test strip is one having two electrodes, or the switches (not shown) in the test strip circuit 214 are set for a test strip having three electrodes (step 870 ) if the test strip is one having three electrodes.
- the appropriate electrode modeis determined by the microprocessor 216 .
- the appropriate level of voltageis applied, and the current resulting from the electrochemical reaction between the sample and the reagents on the test strip is measured (step 880 ).
- the microprocessor 216then converts the current measured into the appropriate value of concentration of analyte by means of parameters and algorithms that had been previously supplied by the calibration strip 240 and stored in the memory 218 .
- the microprocessor 216then instructs the display 130 to show the value of the concentration of analyte (step 890 ).
- Assays for different types of analytes and assays employing different types of test strips, i.e., test strips having two electrodes and test strips having three electrodes,can be carried out on the same analyte test instrument 100 .
- test strip for a particular assayis changed, such as, for example, a new assay for glucose is developed, the instructions for the analyte test instrument can be changed merely by using a new calibration strip; the analyte test instrument need not be discarded.
- FIG. 7A, FIG. 7B, and FIG. 8illustrate a test strip circuit 214 that can be used to perform assays with two different types of test strips—a test strip having two electrodes and a test strip having three electrodes.
- FIG. 7A and FIG. 7Bshow a top view of a test strip 900 having three electrodes, the test strip inserted in the test port 110 .
- the test strip 900is shown without its insulating coating, whereby a working electrode 902 , a counter electrode 904 , and a reference electrode 906 are visible.
- the electrical contacts 908 at the end 910 of the test strip 900are also visible.
- FIG. 8shows a top view of a test strip 900 a having two electrodes, the test strip inserted in the test port 110 .
- the test strip 900 ais shown without its insulating coating, whereby a working electrode 912 , a dual-purpose reference/counter electrode 914 , and a trigger electrode 916 are visible.
- the electrical contacts 918 at the end 920 of the test strip 900 aare also visible.
- FIG. 3Ashows a test strip 230 having an insulating coating 238 present.
- the electrical contacts 908 at the end 910 of the test strip 900are shown inserted into the test port 110 , where they make contact with electrical contacts SENS 1 , SENS 2 , and SENS 3 . These electrical contacts are depicted in FIG. 2.
- the electrical contacts SENS 1 , SENS 2 , SENS 3make electrical contact with the active electrical components of the test strip circuit 214 through wires 922 , 924 , and 926 , respectively.
- the wires 922 and 924have switches 928 and 930 , respectively, controlled by the microprocessor 216 , located between the electrical contacts (not shown) of the test port 110 and the test strip circuit 214 .
- the switches 928 and 930are used to connect or disconnect the electrical contacts SENS 1 and SENS 3 from the test strip circuit 214 .
- FIG. 7Aalso shows operational amplifiers 932 and 934 ; resistors 940 , 942 , and 944 ; microprocessor-controlled switch 946 ; two analog-to-digital (A/D) converters 950 and 952 ; and two digital-to-analog (D/A) converters 954 and 956 .
- the microprocessor 216 shown in FIG. 7Ais part of the processing circuit 210 .
- the processing circuitis shown schematically in FIG. 2.
- the test strip circuit 214is first set in the two-electrode mode by the microprocessor 216 .
- FIG. 7Ashows switch 946 set in the two-electrode mode.
- the working electrode 902is disconnected from the test strip circuit 214 by means of a microprocessor-controlled switch 928 in the wire 922 .
- the D/A converter 956receives a digital voltage instruction from the microprocessor 216 and applies an analog voltage, 400 mV, between the counter electrode 904 and the reference electrode 906 by means of the operational amplifier 932 .
- the microprocessor 216continues to interrogate the A/D converter 952 .
- a currentbegins to flow between the two electrodes.
- a thresholde.g., 0.5 microamperes
- the microprocessor 216opens the switch 930 in the wire 924 leading to the reference electrode 906 for a short period of time, e.g., from about 0 to about 10 seconds.
- the next instructions from the microprocessor 216differ, depending on whether the assay employs a test strip having two electrodes or a test strip having three electrodes.
- the switch 946is set at shown in FIG. 7B.
- the D/A converter 954receives a digital voltage instruction from the microprocessor 216 and applies an analog voltage, 200 mV, to the working electrode 902 by means of the operational amplifier 934 .
- the current originating at the working electrode 902 as a result of the reaction of the sample with the reagentis converted by the A/D converter 950 to a digital signal that is received by the microprocessor 216 .
- the microprocessor 216receives the digital signal from the A/D converter 950 at a specific time or at specific times.
- the microprocessor 216can receive data from the A/D converter 950 at more than one time window, and the data from the different time windows can be used to perform error checks on the assay. Typical time windows for the microprocessor 216 to receive data are 4 to 5 seconds and 8 to 10 seconds.
- the microprocessor 216uses the digital signal to calculate a concentration of analyte in the sample by using calibration factors supplied by a calibration strip. The concentration can then be displayed on the display 130 of the analyte test instrument 100 .
- the switch 946remains in the position shown in FIG. 8.
- the test strip circuit 214is first set in the two-electrode mode by the microprocessor 216 .
- FIG. 8shows the switch 946 set in the two-electrode mode.
- the working electrode 912is disconnected from the test strip circuit 214 by means of the microprocessor-controlled switch 928 in the wire 922 .
- the D/A converter 956receives a digital voltage instruction from the microprocessor 216 and applies an analog voltage, 400 mV, between the trigger electrode 916 and the dual-purpose reference/counter electrode 914 by means of the operational amplifier 932 .
- the microprocessor 216continually interrogates the D/A converter 952 .
- a currentbegins to flow between the two electrodes.
- a thresholde.g., 0.5 microamperes
- the microprocessor 216opens the switch 930 in the wire 924 leading to the trigger electrode 916 for a short period of time, e.g., from about 0 to about 10 seconds. Because the assay employs a test strip having two electrodes, the switch 946 remains in the position shown in FIG. 8. The microprocessor-controlled switch 928 in the wire 922 is closed.
- the D/A converter 954receives a digital voltage instruction from the microprocessor 216 and applies an analog voltage, 200 mV, to the working electrode 912 by means of the operational amplifier 934 .
- the current originating from the working electrode 912 resulting from the reaction of the sample with the reagentis converted by the A/D converter 950 into a digital signal that is received by the microprocessor 216 .
- the microprocessor 216receives the digital signal from the A/D converter 950 at a specific time or at specific times.
- the microprocessor 216can receive data from the A/D converter 950 at more than one time window, and the data from the different time windows can be used to perform error checks on the assay.
- Typical time windows for the microprocessor 216 to receive dataare 4 to 5 seconds and 8 to 10 seconds.
- the microprocessor 216uses the digital signal to calculate a concentration of analyte in the sample by using calibration factors supplied by a calibration strip. The concentration can then be displayed on the display 130 of the analyte test instrument 100 .
- FIG. 7A, FIG. 7B, and FIG. 8demonstrate that the same test strip circuit 214 can be used to analyze test strips having either two electrodes or three electrodes.
- the analyte test instrument of this inventionis therefore more versatile than analyte test instruments of the prior art.
- the analyte test instrument of this inventioncan identify the type of test strip inserted into the instrument (i.e., one having two electrodes or one having three electrodes), and, by using stored calibration information, can configure the analyte test instrument appropriately without relying on input from the user.
- the analyte test instrument of this inventionis therefore easier for the user to switch from one circuit to another than are analyte test instruments of the prior art.
- test strip circuit of the analyte test instrument of this invention and the method wherein a two-electrode mode is employed at the beginning of the assay to detect when the test strip is filledallows measurements to be made with much less expensive operational amplifiers, thereby reducing the cost of the analyte test instrument while providing performance characteristics of expensive analyte test instruments.
Landscapes
- Health & Medical Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Engineering & Computer Science (AREA)
- Chemical & Material Sciences (AREA)
- Physics & Mathematics (AREA)
- Biomedical Technology (AREA)
- Biophysics (AREA)
- Hematology (AREA)
- Molecular Biology (AREA)
- Analytical Chemistry (AREA)
- Biochemistry (AREA)
- General Health & Medical Sciences (AREA)
- General Physics & Mathematics (AREA)
- Immunology (AREA)
- Pathology (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Electrochemistry (AREA)
- Human Computer Interaction (AREA)
- Urology & Nephrology (AREA)
- Food Science & Technology (AREA)
- Medicinal Chemistry (AREA)
- Investigating Or Analysing Biological Materials (AREA)
- Measurement Of The Respiration, Hearing Ability, Form, And Blood Characteristics Of Living Organisms (AREA)
- Measuring Or Testing Involving Enzymes Or Micro-Organisms (AREA)
Abstract
Description
- 1. Field of the Invention[0001]
- This invention relates to analyte test instruments that perform electrochemical assays on biological samples. More particularly, the invention relates to analyte test instruments that can perform electrochemical assays by using different modes of operation.[0002]
- 2. Discussion of the Art[0003]
- Electrochemical assays for determining the concentrations of analytes in samples comprising complex mixtures of liquids have been developed. Such electrochemical assays can be performed with test strips, i.e., biosensors in the form of strips. Test strips can function in an invasive manner (i.e., as probes that come into contact with a body fluid, such as whole blood or subcutaneous fluid). Test strips can function in a non-invasive manner (i.e., as strips that come into contact with blood withdrawn by a syringe or a lancing device). In particular, test strips for biomedical applications (e.g., whole blood analyses) have been developed for the determination of glucose levels in biological samples.[0004]
- An analyte test instrument is an instrument can be used to perform electrochemical assays to determine the concentration of an analyte (e.g., glucose) in a biological sample (e.g., blood). To operate such an instrument, a user inserts a test strip into a test port in the instrument. The instrument displays a “ready” indication to the user and allows sufficient time for the user to deposit a biological sample on the test strip. When a sufficient quantity of the sample reaches the working electrode of the test strip, an electrochemical reaction occurs. The reaction produces an electrical response, such as a change in current. The electrical response is detectable by the analyte test instrument. The analyte test instrument converts the detected signal into data that corresponds to information relating to the analyte and displays the information to the user. The instrument may be able to store a series of such measurements and provide the stored information to the user via a display or to an external processor via a data link.[0005]
- All commercially available electrochemical assays employing test strips for determining the concentration of glucose employ test strips having two electrodes. See, for example, WO 99/19507, incorporated herein by reference, which describes a test strip having two electrodes. In a test strip having two electrodes, the test strip has (1) a working electrode and (2) a dual-purpose reference/counter electrode. The reaction that takes place at the working electrode is the reaction that is required to be monitored and controlled. The second electrode is called a dual-purpose reference/counter electrode because this electrode acts as a reference electrode as well as a counter electrode. No current passes through an ideal reference electrode, and such an electrode maintains a steady potential; current does pass through a dual-purpose reference/counter electrode, and thus, the dual-purpose reference/counter electrode does not maintain a steady potential during the measurement. At low currents and/or at short durations of time for measurement, the shift in potential is small enough such that the response at the working electrode is not significantly affected, and hence the dual-purpose reference/counter electrode is designated a dual-purpose reference/counter electrode. The dual-purpose reference/counter electrode continues to carry out the function of a counter electrode; however, in this case, the potential that is applied between the dual-purpose reference/counter electrode and the working electrode cannot be altered to compensate for changes in potential at the working electrode.[0006]
- Electrochemical assays employing test strips having three electrodes employ a test strip having (1) a working electrode, (2) a reference electrode, and (3) a counter electrode. See, for example, U.S. Ser. No. 10/062,313, filed Feb. 1, 2002, incorporated herein by reference. As in the test strip having two electrodes, the reaction that takes place at the working electrode is the reaction that is required to be monitored and controlled. The functions of the reference electrode and the counter electrode are to ensure that the working electrode actually experiences the conditions desired, i.e. the correct potential intended to be applied. The function of the reference electrode is to measure the potential at the interface of the working electrode and the sample as accurately as possible. In an ideal situation, no current passes through the reference electrode. The function of the counter electrode is to ensure that the correct potential difference between the reference electrode and the working electrode is being applied. The potential difference between the working electrode and the reference electrode is assumed to be the same as the desired potential at the working electrode. If the potential measured at the working electrode is not the potential desired at the working electrode, the potential that is applied between the counter electrode and the working electrode is altered accordingly, i.e., the potential is either increased or decreased. The reaction at the counter electrode, as measured by the current, is also equal and opposite to the charge transfer reaction, as measured by the current, occurring at the working electrode, i.e., if an oxidation reaction is occurring at the working electrode then a reduction reaction will take place at the counter electrode, thereby allowing the sample to remain electrically neutral.[0007]
- An analyte test instrument designed for test strips having two electrodes could not be used if an assay employing a test strip having three electrodes needs to be performed. The user would have to use a separate analyte test instrument. If the user wanted to perform a set of assays that required strips having two electrodes and a set of assays that required strips having three electrodes, these assays could not be performed on the same analyte test instrument.[0008]
- An analyte test instrument for electrochemical assays often requires the user to calibrate the instrument for each batch of test strips. U.S. Pat. No. 5,366,609, incorporated herein by reference, describes a calibration technique that requires a read-only-memory (ROM) key for operation and calibration of an analyte test instrument. A ROM key is inserted into a port (i.e., the ROM key port) that is distinct from the port for a test strip (i.e., the test port). A test strip is inserted into the test port after the ROM key is inserted into the ROM key port. The ROM key must remain in the ROM key port during both the calibration and the operation of the instrument. The ROM key contains specific data, including algorithms, for carrying out procedures for determining the concentration of an analyte in a biological sample applied to one of a batch of test strips associated with the ROM key. The data stored in the ROM key include measurement delay times, incubation times, the number of measurements to be taken during a measurement period, various thresholds against which voltage levels can be compared, values of excitation voltage levels applied to the strip during a test procedure, glucose value conversion factors, and a variety of failsafe test threshold values. In addition, the ROM key can contain some or all of the code for the microprocessor that controls the performing of the assay. A microprocessor in the analyte test instrument uses the algorithms, the conversion factors, and the code provided by the ROM key as needed.[0009]
- U.S. Pat. No. 6,377,894, incorporated herein by reference, describes an instrument requiring a ROM key for operation and calibration of the instrument. The ROM key is inserted into the test port of the instrument and data is downloaded from the ROM key by the instrument and stored in the memory of the instrument. The ROM key contains data needed for carrying out procedures for determining the concentration of an analyte in a biological sample applied to a test strip. The ROM key is removed so that test strips can be inserted into the test port to perform assays. Different ROM keys can be inserted into the instrument to provide data for the testing of different analytes on the same instrument. The instrument can communicate with the ROM key to determine the analyte for which the ROM key contains information. Calibration information can be stored in different locations in the memory of the instrument for each analyte the instrument is capable of testing. When a test strip is inserted into the test port, the instrument has the ability to recognize which analyte is being tested. The microprocessor in the instrument then recalls the instructions for carrying out procedures for determining the concentration of that analyte, and the instrument then performs the appropriate test.[0010]
- The aforementioned patents do not describe how the electrical circuitry of the instrument can be reconfigured so that analytical tests that require different circuit configurations can be performed on the same instrument. The aforementioned patents do not describe how stored information relating to the configuration of the electrical circuitry of the instrument can be modified when an assay for a specific analyte needs to be modified. The aforementioned patents do not describe how stored information can be used to reconfigure the electrical circuitry of the instrument while a test strip is being used. Accordingly, it would be desirable to provide an analyte test instrument that addresses the foregoing deficiencies.[0011]
- In one aspect, this invention provides an analyte test instrument that has test strip circuitry that can be placed into different configurations by means of information provided by a calibration strip to perform assays with test strips having two electrodes and test strips having three electrodes. In another aspect, this invention provides methods for using the analyte test instrument to perform assays with test strips having two electrodes and test strips having three electrodes. The analyte test instrument of this invention comprises:[0012]
- (a) a test port for receiving a test strip;[0013]
- (b) a microprocessor for executing instructions downloaded into the instrument; and[0014]
- (c) a test strip circuit capable of having a plurality of configurations, the configurations being set by the microprocessor, whereby an assay can be performed using a test strip that has been inserted into the test port.[0015]
- In preferred embodiments, the analyte test instrument further includes a memory for storing instructions and information required for the operation of the instrument. However, in other embodiments, the memory can be removably attached to the instrument, as described previously in U.S. Pat. No. 5,366,609.[0016]
- In one embodiment, the invention provides an analyte test instrument that can perform assays on a variety of different analytes. In order to perform these assays, a calibration strip is inserted into the test port. After communication is established between the calibration strip and the analyte test instrument, information (i.e., data or programs or both) involving the method(s) for performing the assay(s) are downloaded from the calibration strip, and, if the analyte test instrument has a memory, preferably stored in the memory of the analyte test instrument. In the analyte test instrument having a memory, the information is stored in the analyte test instrument after the calibration strip is removed. The stored information specifies whether the method(s) of the assay(s) requires a test strip having two electrodes or test strip having three electrodes.[0017]
- In the performance of an assay, a test strip is inserted into the test port, and the identity of the assay is indicated, preferably by means of a pattern of conductive material applied to a major surface of the test strip, preferably the major surface that does not support the electrodes. The analyte test instrument then determines from the downloaded information whether the assay calls for a test strip having two electrodes or for a test strip having three electrodes. The appropriate electrical switches in the test strip circuit of the analyte test instrument are then opened or closed to establish the configuration of the test strip circuit appropriate for the test strip utilized in the assay, that is, a test strip having two electrodes or a test strip having three electrodes. A sample to be analyzed, typically a biological sample, is then applied to the test strip, and a reaction that generates an electrical response occurs. The electrical response is detected and measured by the analyte test instrument, and the concentration of the analyte tested is determined by means of the downloaded calibration information. The analyte test instrument then displays the concentration of the analyte. Assays that call for a test strip having two electrodes and assays that call for a test strip having three electrodes can be performed on the same analyte test instrument.[0018]
- In another embodiment, the analyte test instrument of this invention features the capability of changing the method for performing an assay to determine the concentration of a particular analyte. In order to change the method for performing the assay, a new calibration strip is inserted into the test port. The instructions for the performing the new method of the assay for the particular analyte are then downloaded to the analyte test instrument, and, if the analyte test instrument has a memory, preferably stored in the memory of the analyte test instrument. When the test strip is inserted into the test port, the identity of the assay is determined. The appropriate electrical switches in the test strip circuit of the analyte test instrument are then opened or closed to establish the appropriate circuit configuration for the test strip utilized in the assay, that is, a test strip having two electrodes or a test strip having three electrodes. The circuit configurations are based on the information from the calibration strip most recently downloaded to the analyte test instrument. The same analyte test instrument can be used to perform an assay even if the test method is changed one employing a test strip having two electrodes to one employing a test strip having three electrodes, and vice versa.[0019]
- In another embodiment of this invention, the analyte test instrument can employ both a two-electrode mode and a three-electrode mode during the same assay. The expression “two-electrode mode” refers to the test strip circuitry employed for operating an analyte test instrument with a test strip having two electrodes. The expression “three-electrode mode” refers to the test strip circuitry employed for operating an analyte test instrument with a test strip having three electrodes. The information previously downloaded from the calibration strip, and, preferably, stored in the memory of the analyte test instrument, specifies what portion of the assay employs a test strip circuit configuration in a two-electrode mode and what portion of the assay employs a test strip circuit configuration in a three-electrode mode. A test strip is inserted into the test port, and the identity of the assasy is indicated, preferably from a pattern of conductive material that has been applied to a major surface of the test strip, preferably the major surface that does not support the electrodes. The analyte test instrument then determines from the aforementioned downloaded information whether the assay requires a test strip circuit configuration in a two-electrode mode or a test strip circuit configuration in a three-electrode mode at the start of the assay. The appropriate electrical switches in the analyte test instrument are then opened or closed to establish the appropriate electrode mode. A sample to be analyzed, typically a biological sample, is then applied to the test strip, and a reaction that generates an electrical response occurs. During the performance of the assay, the appropriate electrical switches in the analyte test instrument are then opened or closed to establish the test strip circuit configuration for the appropriate electrode mode, which is a different electrode mode than was used at the start of the assay. The electrical response is detected and measured by the analyte test instrument, and the concentration of the analyte is determined by means of the downloaded calibration information. The analyte test instrument can then display the concentration of the analyte.[0020]
- One example wherein the test strip circuit configuration is switched during an assay involves an assay in which it may be preferred to use a test strip having three electrodes for the advantages provided by the use of a test strip having three electrodes, such as, for example, improved control of voltage at the working electrode. However, it may be desired to exclude the working electrode of the test strip having three electrodes during the sample detection phase of the assay. In this case, the test strip circuit for the two-electrode mode is preferred during this sample detection phase of the assay. Accordingly, test strip circuit configurations for both the two-electrode mode and the three-electrode mode are desired within the course of an assay. It is assumed that an assay involves operational steps beginning with the insertion of the test strip into the analyte test instrument and obtaining the result of the assay.[0021]
- The analyte test instrument of this invention makes it possible for the user to perform assays with test strips having two electrodes and test strips having three electrodes with the same instrument. The analyte test instrument of this invention makes it possible for an assay to be modified without having to discard the instrument. The analyte test instrument of this invention makes it possible for the mode of operation to change during the performance of an assay without intervention from the user.[0022]
- FIG. 1 is a perspective view of an embodiment of an analyte test instrument suitable for use in this invention.[0023]
- FIG. 2 is a block diagram that illustrates electronic components of an analyte test instrument suitable for use in this invention.[0024]
- FIG. 3A is a perspective view of a test strip that is suitable for use with the analyte test instrument of this invention.[0025]
- FIG. 3B is a perspective view of a calibration strip that is suitable for use with the analyte test instrument of this invention.[0026]
- FIG. 4A illustrates a top plan view of test strip that is suitable for use with the analyte test instrument of this invention.[0027]
- FIG. 4B illustrates a bottom plan view of test strip that is suitable for use with the analyte test instrument of this invention.[0028]
- FIG. 5 is a flow chart illustrating a method for calibrating the analyte test instrument of this invention.[0029]
- FIG. 6 is a flow chart illustrating a method for calibrating the analyte test instrument of this invention.[0030]
- FIGS. 7A, 7B, and[0031]8 are schematic diagrams that illustrates a test strip circuit that can be used to perform assays with two different types of test strips.
- As used herein, the expression “test strip having two electrodes” and other expressions relating to tests strips having two electrodes refer to test strips that have a working electrode and a dual-purpose reference/counter electrode. The expression “test strip having three electrodes” and other expressions relating to tests strips having three electrodes refer to test strips that have a working electrode, a counter electrode, and a reference electrode, the reference electrode being separate from the counter electrode. A “test strip having two electrodes” can have one or more additional electrodes, so long as the strip has a dual-purpose reference/counter electrode that performs the functions of both a reference electrode and a counter electrode. For example, a test strip having two electrodes can have a trigger electrode, which is an electrode that detects when a sufficient quantity of sample has been applied to the test strip. A “test strip having three electrodes” can have one or more additional electrodes, so long as the test strip has one electrode for performing the function of a reference electrode and another electrode for performing the function of a counter electrode. For example, a test strip having three electrodes can have a dummy electrode, which is an electrode that is similar to the working electrode, but lacks the substance that reacts with the analyte (see, for example, U.S. Pat. No. 5,628,890), or a trigger electrode dedicated to the sole function of detecting when a sufficient quantity of sample has been applied to the test strip (see, for example, U.S. Pat. No. 5,582,697).[0032]
- As stated previously, all commercially available electrochemical test strips for determining the concentration of glucose employ two electrodes—(1) a working electrode and (2) a dual-purpose reference/ counter electrode. As stated previously, electrochemical systems having three electrodes employ (1) a working electrode, (2) a reference electrode, and (3) a counter electrode.[0033]
- Electrochemical systems employing test strips having three electrodes have the requirement that little or no current pass between the working electrode and the reference electrode. This requirement is achieved by using high impedance operational amplifiers in the electrical circuits of these systems. High impedance operational amplifiers are expensive; consequently, electrochemical systems that perform assays with test strips having three electrodes are expensive. These expensive systems are generally used only in research and are not practical from a cost standpoint for use by diabetics for glucose monitoring at home.[0034]
- A test strip having three electrodes would be preferred in any electrochemical measurement that involves the application of an external voltage and measurement of current. However, due to constraints of sample volume (lower volume requirements), all electrochemical test strips commercially available use only two electrodes. Precise control of the voltage difference between the working electrode and the reference electrode must be maintained, but such control is difficult to achieve in a test strip having two electrodes. In known analyte test instruments, the electrical components of an analyte test instrument designed for test strips employing two electrodes would not operate with test strips employing three electrodes.[0035]
- Referring now to FIG. 1, an[0036]
analyte test instrument 100 comprises ahousing 102, which contains the electrical and electronic components of the analyte test instrument. Theanalyte test instrument 100 comprises atest port 110, apush button 120, and adisplay 130. Thetest port 110 is a multi-purpose test port, which comprises a slot into which a user inserts test strips and calibration strips. Thetest port 110 comprises a slot assembly capable of receiving a strip, such as a test strip or a calibration strip. Thetest port 110 can have a plurality of electrical contacts capable of electrically engaging such a strip when the strip is inserted into thetest port 110. Thepush button 120 allows the user to control theanalyte test instrument 100. In particular, thepush button 120 is used to turn the instrument on and off, to recall information stored in the instrument, to respond to messages displayed, and to set some of the configuration control parameters for the instrument. Thepush button 120 can also provide access to menus generated by software contained in theanalyte test instrument 100. Thedisplay 130 is a device that gives information in a visual form. Thedisplay 130 is typically a screen. The information given typically includes, but is not limited to, test results, messages to the user, information stored in the memory of the analyte test instrument. - In one embodiment, one or more replaceable batteries (not shown) installed via a battery compartment at the rear of the analyte test instrument[0037]100 (not shown) provide power for the
analyte test instrument 100. It should be understood, however, that any source of power capable of providing a suitable direct (DC) voltage can provide power to theanalyte test instrument 100. - FIG. 2 is a block diagram that shows the interrelationship among the electronic components of an[0038]
analyte test instrument 100. In addition to theaforementioned test port 110,push button 120, anddisplay 130, all of which are accessible from the exterior of theanalyte test instrument 100, theanalyte test instrument 100 comprises aprocessing circuit 210, at least onedevice circuit 212, at least onetest strip circuit 214, amicroprocessor 216, and amemory 218. - The purpose of the[0039]
processing circuit 210 is to enable a strip that is engaged in thetest port 110 to communicate with themicroprocessor 216 and thememory 218. For example, theprocessing circuit 210 can send signals to thetest port 110 to determine the identity of the strip inserted therein, i.e., to determine whether the strip is a calibration strip or a test strip. - The device circuit(s)[0040]212 and the test strip circuit(s)214 can comprise analog, digital, or mixed-signal circuits, application-specific integrated circuits (ASICS), and passive and active electrical components. The device circuit(s)212 can perform various electrical functions required by the
analyte test instrument 100, such as driving thedisplay function 130 and the clock functions for amicroprocessor 216. In other words, the device circuit(s) carries instructions from themicroprocessor 216 to various functional components of theanalyte test instrument 100 so that these components can perform their intended functions. Test strip circuit(s)214 can perform analog-to-digital (A/D) conversion of signals received at thetest port 110 from a test strip and can perform digital-to-analog (D/A) conversion of signals received from themicroprocessor 216. In other words, the test strip circuit(s) transmits information between themicroprocessor 216 and the test strip. For example, the test strip circuit(s) is used to ensure that the proper voltage is being applied to the test strip and that the proper value of current generated at the test strip is being measured by themicroprocessor 216. - The[0041]
microprocessor 216 is an integrated circuit that contains the entire central processing unit of a computer. Thememory 218 is a unit of a computer that preserves information for the purpose of retrieval. Such information may include, but is not limited to, measurement delay time(s), sample incubation time(s), number of measurements to be taken during an assay, threshold(s) against which voltage level(s) can be compared, value(s) of excitation voltage level(s) applied to a test strip during an assay, analyte value conversion factors, failsafe assay threshold value(s), and configurations of circuitry of the analyte test instrument. - In a preferred embodiment, the[0042]
memory 218 comprises at least 1K of random access memory (RAM). In more preferred embodiments, thememory 218 has sufficient additional capacity to store a multiplicity of assay results. - [0043]
Instrument software 220 is responsive to information received at thetest port 110 from a calibration strip. Theinstrument software 220 uses the information received to control the operation of theanalyte test instrument 100. theinstrument software 220 also controls operations of theanalyte test instrument 100 that are independent of information introduced or generated at thetest port 110. For example, theinstrument software 220 can enable the user to recall assay results and assay information, can provide various warning, error, and prompting messages, can permit setting of date and time, can control transmission of data to external devices, can monitor power level or battery level or both, and can provide indications to the user if power drops below a specified level. - In the embodiment illustrated in FIG. 2, the[0044]
test port 110 includes six electrical contacts, which are labeled IDENT1, IDENT2, IDENT3, SENS1, SENS2, and SENS3. When a strip is inserted into thetest port 110, the major surfaces of the strip engage the electrical contacts of thetest port 110, thereby enabling theanalyte test instrument 110 to identify a pattern of conductive material on the top major surface of the strip, on the bottom major surface of the strip, or on both major surfaces of the strip. In a preferred embodiment, the pattern of conductive material on an inserted strip that interacts with the electrical contacts IDENT1, IDENT2, and IDENT3 indicates whether the inserted strip is a calibration strip or a test strip. This embodiment is shown in FIGS. 4A and 4B, which will be described later. If the inserted strip is a test strip, the type of analyte to be determined by the assay to be performed with the test strip is also identified (e.g., glucose, ketone bodies, etc.). The engagement of the electrical contacts and the strip identification process are described in more detail in U.S. Pat. No. 6,377,894, incorporated herein by reference. The electrical contacts labeled SENS1, SENS2, and SENS3 relate to the electrodes that are involved in performing analytical tests. - FIG. 3A illustrates in more detail a[0045]
test strip 230. A plurality ofelectrical contacts 232 is provided at theend 234 of thetest strip 230 that is inserted into thetest port 110. Upon insertion of thetest strip 230 into thetest port 110, theelectrical contacts 232 contact the electrical contacts SENS1, SENS2, and SENS3. Typically, a sample, e.g., a drop of blood, undergoing the assay is placed for testing on thereaction area 236 of thetest strip 230. Thereaction area 236 is the area where the sample contacts the electrodes of the test strip230 (i.e., the working electrode and the dual purpose reference/counter electrode in the strip having two electrodes and the working electrode, the reference electrode, and the counter electrode in the strip having three electrodes). When a sufficient quantity of sample is deposited on thereaction area 236, an electrochemical reaction occurs, whereby a flow of electrons produces an electrical response, such as a change in current. The response is detectable by theanalyte test instrument 100. Theanalyte test instrument 100 converts the detected response into data that is correlated with information relating to the analyte and displays the information to the user. - FIG. 3B illustrates a ROM-[0046]
type calibration strip 240. In one embodiment, a ROM-type calibration strip 240 is associated with a package (not shown) oftest strips 230. A plurality ofelectrical contacts 242 is provided at theend 244 of thecalibration strip 240 that is inserted into thetest port 110. In one embodiment, thecalibration code 246 andmanufacturing lot number 248 are printed on thecalibration strip 240 and are visible to the user. In another embodiment, the lot number is stored in a read-only-memory (ROM)250 in binary coded decimal (BCD) format. - The[0047]
ROM 250, which is in electrical communication with theelectrical contacts 242, encodes information relating to algorithm(s) for processing data obtained in an assay with a test strip. TheROM 250 can also encode information relating to thecalibration code 246 andmanufacturing lot number 248 as well as other parameters, as described in U.S. Pat. No. 6,377,894, incorporated herein by reference. The assays are not performed with thecalibration strip 240. Rather, thecalibration strip 240 delivers the information, the algorithms, the parameters, and the procedures that are required to characterize an assay to theanalyte test instrument 100. TheROM 250 is capable of storing and downloading to theanalyte test instrument 100 parameters that characterize an assay as having a two-electrode format or a three-electrode format. - Referring to FIGS. 4A and 4B, a[0048]
test strip 400 has a pattern of conductive material402 on themajor surface 404 thereof that does not support theelectrodes 406. Theelectrodes 406 are supported on themajor surface 408 of thetest strip 400. Different patterns of conductive material402 can be used to specify different assays (e.g., glucose, ketone bodies, etc.). For each different assay, the pattern of conductive material402 is disposed in such a way that the electrical contacts IDENT1, IDENT2, and IDENT3 of thetest port 110 interact with the conductive material in the pattern to identify the type of assay that will be performed by thetest strip 400, such as, for example, glucose, ketone bodies, lactate. Adevice circuit 212, such as an ASIC (see FIG. 2), identifies the type of assay that will be performed by thetest strip 400 by determining the pattern of connection of the conductive material402 on themajor surface 404 of thetest strip 400. - When a strip (e.g., calibration strip, glucose test strip, ketone bodies test strip, etc.) is inserted into[0049]
test port 110 of theanalyte test instrument 100, theanalyte test instrument 100 detects the presence of the strip and performs a procedure to determine whether the strip is a calibration strip or a test strip for determination of the concentration of an analyte. First, theinstrument software 220 polls thetest port 110 to identify the function of the strip that has been inserted, i.e. calibration strip, test strip for determination of the concentration of an analyte. In one embodiment, theinstrument software 220 attempts to communicate with the inserted strip by means of a protocol capable of operating with a serial EE-squared interface, such as that defined by the Dallas ROM protocol of Dallas Semiconductor, Dallas Tex. Such an interface provides single-wire communication. If the attempt to communicate is successful, theinstrument software 220 proceeds to the ROM calibration procedure. If the attempt to communicate is unsuccessful, theinstrument software 220 puts theanalyte test instrument 100 into a brief wait mode (a predetermined time period), e.g., three to five minutes. If theanalyte test instrument 100 fails to receive a signal indicating that a sample has been received during the waiting period, theanalyte test instrument 100 shuts itself off automatically. - The receipt of a signal by[0050]
microprocessor 216 indicates that the user is performing an assay for determination of the concentration of an analyte. Referring to FIG. 3A, when atest strip 230 is inserted into thetest port 110, theelectrical contacts 232 communicate with theanalyte test instrument 100. When a sample (not shown) is added to thereaction area 236, the sample reacts with the reagents in the reaction area, thereby causing a flow of electrons to produce an electrical response, such as a change in current. The response is detectable by theanalyte test instrument 100. Theanalyte test instrument 100 converts the detected signal into data corresponding to information relating to the analyte and displays the information to the user. - FIG. 5 illustrates the ROM calibration procedure when a calibration strip is introduced into the[0051]
test port 110. When theinstrument software 220 identifies the calibration strip240 (step710), data from theROM 250 is downloaded to the analyte test instrument100 (step720). After the data from theROM 250 has been downloaded to theanalyte test instrument 100, thedisplay 130 displays the lot number downloaded from the calibration strip240 (step730), as an indication that the calibration is complete. This data is stored in the memory218 (step740). The user can then remove the calibration strip from the test port110 (step750). The downloaded data remains in thememory 218 for use by theanalyte test instrument 110 until a new calibration procedure is performed (step760). In some embodiments, theanalyte test instrument 100 can store more than one set of calibration data in thememory 218. For example, ananalyte test instrument 100 capable of performing assays with a plurality of test strips230 (e.g., glucose, ketone bodies), can store a set of calibration data for each type oftest strip 230. - As described in U.S. Pat. No. 6,377,894, incorporated herein by reference, the downloaded and stored data comprises parameters, algorithms, operational procedures, and the like for controlling the operation of the[0052]
analyte test instrument 100. In a preferred embodiment of this invention, the data comprise information that instructs the analyte test instrument to perform an assay with a test strip having two electrodes or with a test strip having three electrodes. In another preferred embodiment, the data comprise information that instructs theanalyte test instrument 100 to begin an assay in a mode where the circuitry anticipates a test strip having two electrodes and then switch to a mode where the circuitry is changed to accommodate a test strip having three electrodes. - FIG. 6 depicts a flow chart of a method of performing an assay with the analyte test instrument of this invention. A[0053]
calibration strip 240 is inserted into thetest port 110 and information about types of assays (e.g., glucose, ketone bodies) and the configuration of the test strip circuit214 (i.e., two electrodes or three electrodes) are downloaded and stored in thememory 218 of the analyte test instrument100 (step800). Thecalibration strip 240 is removed from thetest port 110. Atest strip 230 is inserted into the test port110 (step810). Themicroprocessor 216 of theanalyte test instrument 100 determines whether the strip inserted into thetest port 110 is atest strip 230 or acalibration strip 240 by transmitting a digital signal along a wire to the strip. If no signal is received from the strip, themicroprocessor 216 has determined that the strip is atest strip 230. Themicroprocessor 216 then determines the pattern of electrical contacts on the major surface of thetest strip 230 that does not support the electrodes (step820). The aforementioned pattern of electrical contacts provides a signal to themicroprocessor 216 indicating the assay that can be performed with thetest strip 230 that has been inserted into thetest port 110, such as, for example, a glucose assay, a ketone bodies assay. Themicroprocessor 216 then sets the switches of thetest strip circuit 214 to the mode for a test strip having two electrodes (step830). A sample is then introduced to thereaction area 236 of thetest strip 230. A voltage is applied, and after a brief period of time, a small current can be detected (step840). The current indicates that a sample, which covers the electrodes, has been detected (step850). When the current is detected, themicroprocessor 216 instructs a switch (not shown) in thedevice circuit 212 to open, thereby disconnecting the electrodes on the test strip from thetest strip circuit 214 for a specified period of time (step860), which period has been preset by themicroprocessor 216. After the specified period of time, the switch (not shown) in thedevice circuit 212 is closed and thetest strip circuit 214 remains in the two-electrode mode if the test strip is one having two electrodes, or the switches (not shown) in thetest strip circuit 214 are set for a test strip having three electrodes (step870) if the test strip is one having three electrodes. The appropriate electrode mode is determined by themicroprocessor 216. The appropriate level of voltage is applied, and the current resulting from the electrochemical reaction between the sample and the reagents on the test strip is measured (step880). Themicroprocessor 216 then converts the current measured into the appropriate value of concentration of analyte by means of parameters and algorithms that had been previously supplied by thecalibration strip 240 and stored in thememory 218. Themicroprocessor 216 then instructs thedisplay 130 to show the value of the concentration of analyte (step890). Assays for different types of analytes and assays employing different types of test strips, i.e., test strips having two electrodes and test strips having three electrodes, can be carried out on the sameanalyte test instrument 100. If the characteristics of test strip for a particular assay are changed, such as, for example, a new assay for glucose is developed, the instructions for the analyte test instrument can be changed merely by using a new calibration strip; the analyte test instrument need not be discarded. - FIG. 7A, FIG. 7B, and FIG. 8 illustrate a[0054]
test strip circuit 214 that can be used to perform assays with two different types of test strips—a test strip having two electrodes and a test strip having three electrodes. FIG. 7A and FIG. 7B show a top view of atest strip 900 having three electrodes, the test strip inserted in thetest port 110. Thetest strip 900 is shown without its insulating coating, whereby a workingelectrode 902, acounter electrode 904, and areference electrode 906 are visible. Theelectrical contacts 908 at theend 910 of thetest strip 900 are also visible. FIG. 8 shows a top view of a test strip900ahaving two electrodes, the test strip inserted in thetest port 110. The test strip900ais shown without its insulating coating, whereby a workingelectrode 912, a dual-purpose reference/counter electrode 914, and atrigger electrode 916 are visible. Theelectrical contacts 918 at theend 920 of the test strip900aare also visible. FIG. 3A shows atest strip 230 having an insulating coating238 present. In FIG. 7A, theelectrical contacts 908 at theend 910 of thetest strip 900 are shown inserted into thetest port 110, where they make contact with electrical contacts SENS1, SENS2, and SENS3. These electrical contacts are depicted in FIG. 2. The electrical contacts SENS1, SENS2, SENS3 make electrical contact with the active electrical components of thetest strip circuit 214 throughwires wires switches microprocessor 216, located between the electrical contacts (not shown) of thetest port 110 and thetest strip circuit 214. Theswitches test strip circuit 214. FIG. 7A also showsoperational amplifiers resistors switch 946; two analog-to-digital (A/D)converters 950 and952; and two digital-to-analog (D/A)converters microprocessor 216 shown in FIG. 7A is part of theprocessing circuit 210. The processing circuit is shown schematically in FIG. 2. - The[0055]
test strip circuit 214 is first set in the two-electrode mode by themicroprocessor 216. FIG. 7A showsswitch 946 set in the two-electrode mode. The workingelectrode 902 is disconnected from thetest strip circuit 214 by means of a microprocessor-controlledswitch 928 in thewire 922. The D/A converter 956 receives a digital voltage instruction from themicroprocessor 216 and applies an analog voltage, 400 mV, between thecounter electrode 904 and thereference electrode 906 by means of theoperational amplifier 932. Themicroprocessor 216 continues to interrogate the A/D converter952. When a sufficient quantity of the sample is applied to thetest strip 900 to result in a fluid connection between thecounter electrode 904 and thereference electrode 906, a current begins to flow between the two electrodes. When the current reaches a threshold, e.g., 0.5 microamperes, themicroprocessor 216 opens theswitch 930 in thewire 924 leading to thereference electrode 906 for a short period of time, e.g., from about 0 to about 10 seconds. The next instructions from themicroprocessor 216 differ, depending on whether the assay employs a test strip having two electrodes or a test strip having three electrodes. - If the assay involves a test strip employing three electrodes, the[0056]
switch 946 is set at shown in FIG. 7B. The microprocessor-controlledswitches wires A converter 954 receives a digital voltage instruction from themicroprocessor 216 and applies an analog voltage, 200 mV, to the workingelectrode 902 by means of theoperational amplifier 934. The current originating at the workingelectrode 902 as a result of the reaction of the sample with the reagent is converted by the A/D converter 950 to a digital signal that is received by themicroprocessor 216. Themicroprocessor 216 receives the digital signal from the A/D converter 950 at a specific time or at specific times. Themicroprocessor 216 can receive data from the A/D converter 950 at more than one time window, and the data from the different time windows can be used to perform error checks on the assay. Typical time windows for themicroprocessor 216 to receive data are 4 to 5 seconds and 8 to 10 seconds. Themicroprocessor 216 uses the digital signal to calculate a concentration of analyte in the sample by using calibration factors supplied by a calibration strip. The concentration can then be displayed on thedisplay 130 of theanalyte test instrument 100. - If the assay employs a test strip having two electrodes, the[0057]
switch 946 remains in the position shown in FIG. 8. Thetest strip circuit 214 is first set in the two-electrode mode by themicroprocessor 216. FIG. 8 shows theswitch 946 set in the two-electrode mode. The workingelectrode 912 is disconnected from thetest strip circuit 214 by means of the microprocessor-controlledswitch 928 in thewire 922. The D/A converter 956 receives a digital voltage instruction from themicroprocessor 216 and applies an analog voltage, 400 mV, between thetrigger electrode 916 and the dual-purpose reference/counter electrode 914 by means of theoperational amplifier 932. Themicroprocessor 216 continually interrogates the D/A converter952. When a sufficient quantity of sample is applied to the test strip900ato result in a fluid connection between thefill trigger electrode 916 and the dual-purpose reference/counter electrode 914, a current begins to flow between the two electrodes. When the current reaches a threshold, e.g., 0.5 microamperes, themicroprocessor 216 opens theswitch 930 in thewire 924 leading to thetrigger electrode 916 for a short period of time, e.g., from about 0 to about 10 seconds. Because the assay employs a test strip having two electrodes, theswitch 946 remains in the position shown in FIG. 8. The microprocessor-controlledswitch 928 in thewire 922 is closed. The D/A converter 954 receives a digital voltage instruction from themicroprocessor 216 and applies an analog voltage, 200 mV, to the workingelectrode 912 by means of theoperational amplifier 934. The current originating from the workingelectrode 912 resulting from the reaction of the sample with the reagent is converted by the A/D converter 950 into a digital signal that is received by themicroprocessor 216. Themicroprocessor 216 receives the digital signal from the A/D converter 950 at a specific time or at specific times. Themicroprocessor 216 can receive data from the A/D converter 950 at more than one time window, and the data from the different time windows can be used to perform error checks on the assay. Typical time windows for themicroprocessor 216 to receive data are 4 to 5 seconds and 8 to 10 seconds. Themicroprocessor 216 uses the digital signal to calculate a concentration of analyte in the sample by using calibration factors supplied by a calibration strip. The concentration can then be displayed on thedisplay 130 of theanalyte test instrument 100. - FIG. 7A, FIG. 7B, and FIG. 8 demonstrate that the same[0058]
test strip circuit 214 can be used to analyze test strips having either two electrodes or three electrodes. The analyte test instrument of this invention is therefore more versatile than analyte test instruments of the prior art. The analyte test instrument of this invention can identify the type of test strip inserted into the instrument (i.e., one having two electrodes or one having three electrodes), and, by using stored calibration information, can configure the analyte test instrument appropriately without relying on input from the user. The analyte test instrument of this invention is therefore easier for the user to switch from one circuit to another than are analyte test instruments of the prior art. - The test strip circuit of the analyte test instrument of this invention and the method wherein a two-electrode mode is employed at the beginning of the assay to detect when the test strip is filled allows measurements to be made with much less expensive operational amplifiers, thereby reducing the cost of the analyte test instrument while providing performance characteristics of expensive analyte test instruments.[0059]
- Various modifications and alterations of this invention will become apparent to those skilled in the art without departing from the scope and spirit of this invention, and it should be understood that this invention is not to be unduly limited to the illustrative embodiments set forth herein.[0060]
Claims (46)
1. An analyte test instrument suitable for performing an assay with a test strip, said analyte test instrument comprising:
(a) a test port for receiving a test strip;
(b) a microprocessor for executing instructions downloaded into said instrument; and
(c) a test strip circuit capable of having a plurality of configurations, said configurations being set by said microprocessor, whereby an assay can be performed using said test strip.
2. The analyte test instrument ofclaim 1 , further including a memory for storing instructions and information.
3. The analyte test instrument ofclaim 2 , wherein said instructions are selected from the group consisting of measurement delay time(s), sample incubation time(s), number of measurements to be taken during an assay, threshold(s) against which voltage level(s) can be compared, value(s) of excitation voltage level(s) applied to a test strip during an assay, analyte value conversion factors, failsafe assay threshold value(s), and configurations of said test strip circuit of said analyte test instrument.
4. The analyte test instrument ofclaim 2 , wherein said test port is capable of receiving a calibration strip, said calibration strip being removable from said test port to allow a test strip to be inserted into said test port.
5. The analyte test instrument ofclaim 1 , wherein at least one of said plurality of configurations can be used to perform an assay with a test strip having two electrodes.
6. The analyte test instrument ofclaim 1 , wherein at least one of said plurality of configurations can be used to perform an assay with a test strip having three electrodes.
7. The analyte test instrument ofclaim 1 , further comprising a push button.
8. The analyte test instrument ofclaim 1 , further comprising a display.
9. The analyte test instrument ofclaim 1 , wherein said test port comprises two sets of electrical contacts, whereby a first set of electrical contacts engages a first major surface of a test strip and a second set of electrical contacts engages a second major surface of said test strip.
10. The analyte test instrument ofclaim 1 , wherein said test strip is capable of performing an assay for determining concentration of an analyte in a biological sample, said analyte selected from the group consisting of glucose, lactate, and ketone bodies.
11. A method for determining the concentration of at least one analyte in a biological sample, said method comprising the steps of:
(a) providing said analyte test instrument ofclaim 1;
(b) inserting a test strip into said test port of said analyte test instrument;
(c) applying a biological sample to said test strip;
(d) allowing said analyte test instrument to set said configuration of said test strip circuit to a mode suitable for performing said determination; and
(e) measuring an electrical response provided by said test strip by means of said test strip circuit.
12. The method ofclaim 11 , wherein said configurations include a first configuration, said first configuration for use with a test strip having two electrodes, and a second configuration, said second configuration for use with a test strip having three electrodes.
13. The method ofclaim 11 , further including the step of allowing said analyte test instrument to identify said test strip when said test strip is inserted into said test port.
14. The method ofclaim 13 , wherein said identification step is performed by having electrical contacts located in said test port interact or fail to interact with electrically conductive material applied to at least one major surface of said test strip.
15. The method ofclaim 13 , wherein said identification step indicates the analyte to be determined with said test strip.
16. The method ofclaim 11 , wherein said microprocessor converts said electrical response provided by said test strip into a value that represents the concentration of said analyte.
17. The method ofclaim 16 , further including the step of reporting said concentration of said analyte to a user of said analyte test instrument.
18. The method ofclaim 11 , further including the step of calibrating said analyte test instrument for each of a plurality of assays that can be performed with said analyte test instrument.
19. The method ofclaim 18 , wherein said calibration step is performed with a calibration strip that has been inserted into said test port.
20. The method ofclaim 19 , further including the step of providing instructions during said calibration step for effecting the switching of said configuration of said test strip circuit from a first configuration, said first configuration for use with a test strip having two electrodes, and a second configuration, said second configuration for use with a test strip having three electrodes.
21. The method ofclaim 20 , wherein said instruction providing step is performed with a calibration strip inserted into said test port.
22. The method ofclaim 11 , further including the step of calibrating said analyte test instrument for each of a plurality of assays, said calibration step involving (a) insertion of a calibration strip into a port in said analyte test instrument, said calibration strip containing instructions for switching the test strip circuit configuration from a first mode to a second mode during said determination, said instructions capable of being downloaded into said analyte test instrument, and (b) removal of said calibration strip from said test port.
23. The method ofclaim 11 , wherein said analyte is selected from the group consisting of glucose, lactate, and ketone bodies.
24. An analyte test instrument suitable for performing an assay with a test strip, said analyte test instrument comprising:
(a) a test port for receiving a test strip;
(b) a microprocessor for executing instructions downloaded into said instrument; and
(c) a test strip circuit capable of having a plurality of configurations, said configurations being set by said microprocessor, wherein at least one of said instruction enables said microprocessor to switch configuration of said test strip circuit during an assay.
25. The analyte test instrument ofclaim 24 , further including a memory for storing instructions and information.
26. The analyte test instrument ofclaim 25 , wherein said instructions are selected from the group consisting of measurement delay time(s), sample incubation time(s), number of measurements to be taken during an assay, threshold(s) against which voltage level(s) can be compared, value(s) of excitation voltage level(s) applied to a test strip during an assay, analyte value conversion factors, failsafe assay threshold value(s), and configurations of said test strip circuit of said analyte test instrument.
27. The analyte test instrument ofclaim 25 , wherein said test port is capable of receiving a calibration strip, said calibration strip being removable from said test port to allow a test strip is inserted into said test port.
28. The analyte test instrument ofclaim 24 , wherein at least one of said plurality of configurations can be used to perform an assay with a test strip having two electrodes.
29. The analyte test instrument ofclaim 24 , wherein at least one of said plurality of configurations can be used to perform an assay with a test strip having three electrodes.
30. The analyte test instrument ofclaim 24 , further comprising a push button.
31. The analyte test instrument ofclaim 24 , further comprising a display.
32. The analyte test instrument ofclaim 24 , wherein said test port comprises two sets of electrical contacts, whereby a first set of electrical contacts engages a first major surface of a test strip and a second set of electrical contacts engages a second major surface of said test strip.
33. The analyte test instrument ofclaim 24 , wherein said test strip is capable of performing an assay for determining concentration of an analyte in a biological sample, said analyte selected from the group consisting of glucose, lactate, and ketone bodies.
34. A method for determining the concentration of at least one analyte in a biological sample, said method comprising the steps of:
(a) providing the analyte test instrument ofclaim 24;
(b) inserting a test strip into said test port of said analyte test instrument;
(c) applying a biological sample to said test strip;
(d) allowing said analyte test instrument to set said configuration of said test strip circuit to a mode suitable for performing said determination; and
(e) measuring an electrical response provided by said test strip by means of said test strip circuit.
35. The method ofclaim 34 , wherein said configurations include a first configuration, said first configuration for use with a test strip having two electrodes, and a second configuration, said second configuration for use with a test strip having three electrodes.
36. The method ofclaim 34 , further including the step of allowing said analyte test instrument to identify said test strip when said test strip is inserted into said test port.
37. The method ofclaim 36 , wherein said identification step is performed by having electrical contacts located in said test port interact or fail to interact with electrically conductive material applied to at least one major surface or said test strip.
38. The method ofclaim 36 , wherein said identification step indicates the analyte to be determined with said test strip.
39. The method ofclaim 34 , wherein said microprocessor converts said electrical response provided by said test strip into a value that represents the concentration of said analyte.
40. The method ofclaim 39 , further including the step of reporting said concentration of said analyte to a user of said analyte test instrument.
41. The method ofclaim 34 , further including the step of calibrating said analyte test instrument for each of a plurality of assays that can be performed with said analyte test instrument.
42. The method ofclaim 41 , wherein said calibration step is performed with a calibration strip that has been inserted into said test port.
43. The method ofclaim 42 , further including the step of providing instructions during said calibration step for effecting the switching of said configuration of said test strip circuit from a first configuration, said first configuration for use with a test strip having two electrodes, and a second configuration, said second configuration for use with a test strip having three electrodes.
44. The method ofclaim 43 , wherein said instruction providing step is performed with a calibration strip inserted into said test port.
45. The method ofclaim 34 , further including the step of calibrating said analyte test instrument for each of a plurality of assays, said calibration step involving (a) insertion of a calibration strip into a port in said analyte test instrument, said calibration strip containing instructions for switching the test strip circuit configuration from a first mode to a second mode during said determination, said instructions capable of being downloaded into said analyte test instrument, and (b) removal of said calibration strip from said test port.
46. The method ofclaim 34 , wherein said analyte is selected from the group consisting of glucose, lactate, and ketone bodies.
Priority Applications (9)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US10/326,008US20040118704A1 (en) | 2002-12-19 | 2002-12-19 | Analyte test intrument having improved versatility |
| CA2444094ACA2444094C (en) | 2002-12-19 | 2003-10-01 | Analyte test instrument having improved versatility |
| AU2003252777AAU2003252777B2 (en) | 2002-12-19 | 2003-10-02 | Analyte Test Instrument Having Improved Versatility |
| EP03790402AEP1576367B1 (en) | 2002-12-19 | 2003-12-08 | Analyte test instrument having improved versatility |
| JP2004568861AJP4836457B2 (en) | 2002-12-19 | 2003-12-08 | Sample testing equipment with improved versatility |
| PCT/US2003/038897WO2004077052A1 (en) | 2002-12-19 | 2003-12-08 | Analyte test instrument having improved versatility |
| AT03790402TATE550649T1 (en) | 2002-12-19 | 2003-12-08 | TESTING DEVICE FOR ANALYTES WITH VERSATILE USES |
| US12/623,894US8916036B2 (en) | 2002-12-19 | 2009-11-23 | Analyte test instrument having improved versatility |
| US14/575,753US9234865B2 (en) | 2002-12-19 | 2014-12-18 | Analyte test instrument having improved versatility |
Applications Claiming Priority (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US10/326,008US20040118704A1 (en) | 2002-12-19 | 2002-12-19 | Analyte test intrument having improved versatility |
Related Child Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US12/623,894ContinuationUS8916036B2 (en) | 2002-12-19 | 2009-11-23 | Analyte test instrument having improved versatility |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| US20040118704A1true US20040118704A1 (en) | 2004-06-24 |
Family
ID=32593915
Family Applications (3)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US10/326,008AbandonedUS20040118704A1 (en) | 2002-12-19 | 2002-12-19 | Analyte test intrument having improved versatility |
| US12/623,894Expired - Fee RelatedUS8916036B2 (en) | 2002-12-19 | 2009-11-23 | Analyte test instrument having improved versatility |
| US14/575,753Expired - Fee RelatedUS9234865B2 (en) | 2002-12-19 | 2014-12-18 | Analyte test instrument having improved versatility |
Family Applications After (2)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US12/623,894Expired - Fee RelatedUS8916036B2 (en) | 2002-12-19 | 2009-11-23 | Analyte test instrument having improved versatility |
| US14/575,753Expired - Fee RelatedUS9234865B2 (en) | 2002-12-19 | 2014-12-18 | Analyte test instrument having improved versatility |
Country Status (7)
| Country | Link |
|---|---|
| US (3) | US20040118704A1 (en) |
| EP (1) | EP1576367B1 (en) |
| JP (1) | JP4836457B2 (en) |
| AT (1) | ATE550649T1 (en) |
| AU (1) | AU2003252777B2 (en) |
| CA (1) | CA2444094C (en) |
| WO (1) | WO2004077052A1 (en) |
Cited By (49)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| USD534444S1 (en)* | 2002-02-15 | 2007-01-02 | Polymer Technology Systems, Inc. | Hand-held diagnostic instrument |
| US20070065342A1 (en)* | 2001-08-13 | 2007-03-22 | Brown Michael K | Mechanical mechanism for a sensor-dispensing instrument |
| WO2006130577A3 (en)* | 2005-06-01 | 2007-07-05 | Bayer Healthcare Llc | Multi-contact sensor connector with release mechanism |
| US20070163894A1 (en)* | 2005-12-30 | 2007-07-19 | Medtronic Minimed, Inc. | Real-time self-calibrating sensor system and method |
| US20080083773A1 (en)* | 2004-08-20 | 2008-04-10 | Micinski Russell J | Contact Connector Assembly For A Sensor-Dispensing Instrument |
| US20080101983A1 (en)* | 2006-10-24 | 2008-05-01 | Abbott Diabetes Care, Inc. | Embossed cell analyte sensor and methods of manufacture |
| US20080118401A1 (en)* | 2001-08-13 | 2008-05-22 | Kirchhevel G L | Button layout for a testing instrument |
| USD586678S1 (en) | 2007-10-12 | 2009-02-17 | Lifescan, Inc. | Analyte test meter |
| US20090149717A1 (en)* | 2007-12-10 | 2009-06-11 | Jacob Brauer | Interface for a health measurement and monitoring system |
| WO2009097466A1 (en)* | 2008-01-31 | 2009-08-06 | Abbott Diabetes Care Inc. | Method for automatically and rapidly distinguishing between control and sample solutions in a biosensor strip |
| US20090302873A1 (en)* | 2007-09-05 | 2009-12-10 | Lifescan Scotland Limited | System for electrochemically measuring an analyte in a sample material |
| US20100032319A1 (en)* | 2008-08-06 | 2010-02-11 | Jun Okada | Diagnostic cassette for electrochemical measuring apparatus and method of diagnosing electrochemical measuring apparatus |
| USD611151S1 (en) | 2008-06-10 | 2010-03-02 | Lifescan Scotland, Ltd. | Test meter |
| USD611372S1 (en) | 2008-09-19 | 2010-03-09 | Lifescan Scotland Limited | Analyte test meter |
| USD611489S1 (en) | 2008-07-25 | 2010-03-09 | Lifescan, Inc. | User interface display for a glucose meter |
| USD611853S1 (en) | 2008-03-21 | 2010-03-16 | Lifescan Scotland Limited | Analyte test meter |
| USD612274S1 (en) | 2008-01-18 | 2010-03-23 | Lifescan Scotland, Ltd. | User interface in an analyte meter |
| USD612275S1 (en) | 2008-03-21 | 2010-03-23 | Lifescan Scotland, Ltd. | Analyte test meter |
| US20100094110A1 (en)* | 1998-04-30 | 2010-04-15 | Abbott Diabetes Care Inc. | Analyte Monitoring Device and Methods of Use |
| USD615431S1 (en) | 2008-03-21 | 2010-05-11 | Lifescan Scotland Limited | Analyte test meter |
| USD626651S1 (en) | 2008-05-29 | 2010-11-02 | Bayer Healthcare, Llc | Analyte-determining meter |
| USD627068S1 (en) | 2009-09-22 | 2010-11-09 | Bayer Healthcare, Llc | Analyte-determining meter |
| WO2011002694A1 (en) | 2009-06-30 | 2011-01-06 | Abbott Diabetes Care Inc. | Analyte monitoring device and methods of use |
| WO2011002693A1 (en) | 2009-06-30 | 2011-01-06 | Abbott Diabetes Care Inc. | Analyte monitoring device and methods of use |
| US20110174618A1 (en)* | 2008-09-30 | 2011-07-21 | Menai Medical Technologies Limited | Sample measurement system |
| US8115635B2 (en) | 2005-02-08 | 2012-02-14 | Abbott Diabetes Care Inc. | RF tag on test strips, test strip vials and boxes |
| US20130192987A1 (en)* | 2010-09-13 | 2013-08-01 | Dai Nippon Printing Co., Ltd. | Biosensor and method for producing the same |
| US20140180050A1 (en)* | 2009-02-18 | 2014-06-26 | Panasonic Corporation | Measuring device |
| US8917184B2 (en) | 2008-03-21 | 2014-12-23 | Lifescan Scotland Limited | Analyte testing method and system |
| US8921061B2 (en) | 2012-11-02 | 2014-12-30 | Roche Diagnostics Operations, Inc. | Reagent materials and associated test elements |
| US8920628B2 (en) | 2012-11-02 | 2014-12-30 | Roche Diagnostics Operations, Inc. | Systems and methods for multiple analyte analysis |
| US8974386B2 (en) | 1998-04-30 | 2015-03-10 | Abbott Diabetes Care Inc. | Analyte monitoring device and methods of use |
| US9011332B2 (en) | 2001-01-02 | 2015-04-21 | Abbott Diabetes Care Inc. | Analyte monitoring device and methods of use |
| WO2015069563A1 (en) | 2013-11-05 | 2015-05-14 | Abbott Diabetes Care Inc. | Systems, devices, and methods for control of a power supply connection |
| US20150177176A1 (en)* | 2013-12-23 | 2015-06-25 | Lifescan Scotland Limited | Analyte meter test strip detection |
| WO2015100109A1 (en) | 2013-12-27 | 2015-07-02 | Abbott Diabetes Care Inc. | Systems, devices, and methods for authentication in an analyte monitoring environment |
| USD742524S1 (en) | 2014-11-17 | 2015-11-03 | Bayer Healthcare Llc | Analyte meter |
| DE202014010579U1 (en) | 2013-12-27 | 2016-01-05 | Abbott Diabetes Care Inc. | Application interface and display control in an analyte monitoring environment |
| EP3001194A1 (en) | 2009-08-31 | 2016-03-30 | Abbott Diabetes Care, Inc. | Medical devices and methods |
| EP2912438A4 (en)* | 2012-10-26 | 2016-11-02 | Pixie Scient Inc | HEALTH DIAGNOSTIC SYSTEMS AND METHODS |
| US9501272B2 (en) | 2010-05-24 | 2016-11-22 | Abbott Diabetes Care Inc. | Systems and methods for updating a medical device |
| US20170241996A1 (en)* | 2014-09-08 | 2017-08-24 | Indian Institute Of Science | Electrochemical biosensor and a method of sensing albumin and its complexes |
| WO2018118822A1 (en) | 2016-12-20 | 2018-06-28 | Abbott Diabetes Care Inc. | Systems, devices and methods for wireless communications in analyte monitoring devices |
| WO2019035073A2 (en) | 2017-08-18 | 2019-02-21 | Abbott Diabetes Care Inc. | Systems, devices, and methods related to the individualized calibration and/or manufacturing of medical devices |
| US10213141B2 (en) | 2013-04-30 | 2019-02-26 | Abbott Diabetes Care Inc. | Systems, devices, and methods for energy efficient electrical device activation |
| WO2019152966A1 (en) | 2018-02-05 | 2019-08-08 | Abbott Diabetes Care Inc. | Notes and event log information associated with analyte sensors |
| US10383564B2 (en) | 2015-05-22 | 2019-08-20 | Pixie Scientific, Llc | Indicator panels for incontinence products |
| WO2022164940A1 (en) | 2021-01-26 | 2022-08-04 | Abbott Diabetes Care Inc. | Systems, devices, and methods related to ketone sensors |
| US11719687B2 (en)* | 2013-03-15 | 2023-08-08 | Agamatrix, Inc. | Analyte detection meter and associated method of use |
Families Citing this family (22)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US20040118704A1 (en)* | 2002-12-19 | 2004-06-24 | Yi Wang | Analyte test intrument having improved versatility |
| ES2709991T3 (en) | 2003-08-21 | 2019-04-22 | Agamatrix Inc | Method and apparatus for the analysis of electrochemical properties |
| DE602005023433D1 (en)* | 2005-07-07 | 2010-10-21 | Asulab Sa | System for the differential determination of the amount of a proteolytic enzyme in a body fluid |
| CN101626720A (en)* | 2007-01-23 | 2010-01-13 | 拜尔保健有限公司 | Analyte-testing device |
| EP2015067A1 (en) | 2007-06-15 | 2009-01-14 | Roche Diagnostics GmbH | System for measuring the analyte concentration in a body fluid sample |
| JP5534163B2 (en)* | 2009-12-09 | 2014-06-25 | 日本電気株式会社 | Action determination device, action determination system, action determination method, and program |
| TWI456895B (en)* | 2011-10-26 | 2014-10-11 | Htc Corp | Electronic device and method for driving an internal function block of a processor of the electronic device to operate in a linear region |
| US8466817B2 (en)* | 2011-10-26 | 2013-06-18 | Htc Corporation | Electronic device and method for driving an internal function block of a processor of the electronic device to operate in a linear region |
| US20150355129A1 (en)* | 2014-06-05 | 2015-12-10 | Avails Medical, Inc. | Systems and methods for detecting substances in bodily fluids |
| WO2017035393A1 (en)* | 2015-08-25 | 2017-03-02 | eSense, LLC | Devices, systems and methods for detecting viable microorganisms in a fluid sample |
| JP6691780B2 (en)* | 2016-01-14 | 2020-05-13 | テルモ株式会社 | Component measuring device, measuring mode setting method and program for this device |
| WO2017209839A1 (en) | 2016-05-31 | 2017-12-07 | Avails Medical, Inc. | Detecting viable infectious agents in a fluid sample and susceptibility of infectious agents to anti-infectives |
| US12188893B2 (en) | 2016-08-30 | 2025-01-07 | Analog Devices International Unlimited Company | Electrochemical sensor, and a method of forming an electrochemical sensor |
| JP6834317B2 (en)* | 2016-10-03 | 2021-02-24 | 大日本印刷株式会社 | Microbial contaminant detection device and microbial contaminant detection method |
| WO2018111234A1 (en) | 2016-12-13 | 2018-06-21 | Avails Medical, Inc. | DEVICES, SYSTEMS AND METHODS TO DETECT THE PRESENCE OF ß-LACTAM ANTIBIOTIC HYDROLYZING BACTERIA IN A SAMPLE |
| WO2019005296A1 (en) | 2017-06-27 | 2019-01-03 | Avails Medical, Inc. | Apparatus, systems, and methods for determining susceptibility of microorganisms to anti-infectives |
| WO2019070739A1 (en) | 2017-10-03 | 2019-04-11 | Avails Medical, Inc. | Apparatus, systems, and methods for determining the concentration of microorganisms and the susceptibility of microorganisms to anti-infectives based on redox reactions |
| JP2019074442A (en)* | 2017-10-18 | 2019-05-16 | ルネサスエレクトロニクス株式会社 | Semiconductor circuit for impedance measurement and blood glucose meter |
| CN111712709B (en) | 2017-12-05 | 2023-11-10 | 阿威尔斯医疗公司 | Preparation of output samples containing defined concentrations of infectious agents for downstream testing |
| US11022579B2 (en) | 2018-02-05 | 2021-06-01 | Analog Devices International Unlimited Company | Retaining cap |
| WO2020117650A1 (en) | 2018-12-03 | 2020-06-11 | Avails Medical, Inc. | Apparatus, systems, and methods for quantifying infectious agents |
| CO2021005504A1 (en)* | 2021-04-27 | 2022-10-31 | Pontificia Univ Javeriana | Device for electronic and electrochemical measurement of analyte concentrations in biological samples |
Citations (9)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US5366609A (en)* | 1993-06-08 | 1994-11-22 | Boehringer Mannheim Corporation | Biosensing meter with pluggable memory key |
| US5582697A (en)* | 1995-03-17 | 1996-12-10 | Matsushita Electric Industrial Co., Ltd. | Biosensor, and a method and a device for quantifying a substrate in a sample liquid using the same |
| US5628890A (en)* | 1995-09-27 | 1997-05-13 | Medisense, Inc. | Electrochemical sensor |
| US5873990A (en)* | 1995-08-22 | 1999-02-23 | Andcare, Inc. | Handheld electromonitor device |
| US6117289A (en)* | 1996-12-20 | 2000-09-12 | Matsushita Electric Industrial Co., Ltd. | Cholesterol sensor and method for producing the same |
| US6129823A (en)* | 1997-09-05 | 2000-10-10 | Abbott Laboratories | Low volume electrochemical sensor |
| US6377894B1 (en)* | 1998-11-30 | 2002-04-23 | Abbott Laboratories | Analyte test instrument having improved calibration and communication processes |
| US6713308B1 (en)* | 1999-05-05 | 2004-03-30 | Fang Lu | System for electrochemical quantitative analysis of analytes within a solid phase |
| US6773564B1 (en)* | 1998-09-29 | 2004-08-10 | Matsushita Electric Industrial Co., Ltd. | Glucose sensor |
Family Cites Families (15)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US5509410A (en)* | 1983-06-06 | 1996-04-23 | Medisense, Inc. | Strip electrode including screen printing of a single layer |
| DE4123348A1 (en)* | 1991-07-15 | 1993-01-21 | Boehringer Mannheim Gmbh | ELECTROCHEMICAL ANALYSIS SYSTEM |
| JPH07128338A (en)* | 1993-11-02 | 1995-05-19 | Kyoto Daiichi Kagaku:Kk | Convenient blood sugar meter and data managing method therefor |
| ES2186003T3 (en)* | 1996-10-30 | 2003-05-01 | Amira Medical | SYNCHRONIZED ANALYZE ANALYSIS SYSTEM. |
| JP3375040B2 (en)* | 1997-07-29 | 2003-02-10 | 松下電器産業株式会社 | Substrate quantification method |
| CO5040209A1 (en) | 1997-10-16 | 2001-05-29 | Abbott Lab | BIOSENSOR ELECTRODES MEDIATORS OF COFACTOR REGENERATION |
| NZ524206A (en)* | 1997-12-04 | 2004-05-28 | Roche Diagnostics Corp | Instrument for determining the concentration of a medically significant component of a sample |
| US7077328B2 (en)* | 1998-07-31 | 2006-07-18 | Abbott Laboratories | Analyte test instrument system including data management system |
| US6616819B1 (en)* | 1999-11-04 | 2003-09-09 | Therasense, Inc. | Small volume in vitro analyte sensor and methods |
| JP4184573B2 (en)* | 2000-04-28 | 2008-11-19 | 松下電器産業株式会社 | Biosensor |
| JP2001281197A (en)* | 2000-03-30 | 2001-10-10 | Matsushita Electric Ind Co Ltd | Biosensor measuring device |
| JP2002156358A (en)* | 2000-11-20 | 2002-05-31 | Matsushita Electric Ind Co Ltd | Biosensor, notification device, and measurement device |
| AU2002347057A1 (en)* | 2001-10-12 | 2003-04-28 | Kurt Koch | Rescue and survival case |
| US6863800B2 (en)* | 2002-02-01 | 2005-03-08 | Abbott Laboratories | Electrochemical biosensor strip for analysis of liquid samples |
| US20040118704A1 (en)* | 2002-12-19 | 2004-06-24 | Yi Wang | Analyte test intrument having improved versatility |
- 2002
- 2002-12-19USUS10/326,008patent/US20040118704A1/ennot_activeAbandoned
- 2003
- 2003-10-01CACA2444094Apatent/CA2444094C/ennot_activeExpired - Fee Related
- 2003-10-02AUAU2003252777Apatent/AU2003252777B2/ennot_activeCeased
- 2003-12-08ATAT03790402Tpatent/ATE550649T1/enactive
- 2003-12-08JPJP2004568861Apatent/JP4836457B2/ennot_activeExpired - Fee Related
- 2003-12-08WOPCT/US2003/038897patent/WO2004077052A1/enactiveApplication Filing
- 2003-12-08EPEP03790402Apatent/EP1576367B1/ennot_activeExpired - Lifetime
- 2009
- 2009-11-23USUS12/623,894patent/US8916036B2/ennot_activeExpired - Fee Related
- 2014
- 2014-12-18USUS14/575,753patent/US9234865B2/ennot_activeExpired - Fee Related
Patent Citations (9)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US5366609A (en)* | 1993-06-08 | 1994-11-22 | Boehringer Mannheim Corporation | Biosensing meter with pluggable memory key |
| US5582697A (en)* | 1995-03-17 | 1996-12-10 | Matsushita Electric Industrial Co., Ltd. | Biosensor, and a method and a device for quantifying a substrate in a sample liquid using the same |
| US5873990A (en)* | 1995-08-22 | 1999-02-23 | Andcare, Inc. | Handheld electromonitor device |
| US5628890A (en)* | 1995-09-27 | 1997-05-13 | Medisense, Inc. | Electrochemical sensor |
| US6117289A (en)* | 1996-12-20 | 2000-09-12 | Matsushita Electric Industrial Co., Ltd. | Cholesterol sensor and method for producing the same |
| US6129823A (en)* | 1997-09-05 | 2000-10-10 | Abbott Laboratories | Low volume electrochemical sensor |
| US6773564B1 (en)* | 1998-09-29 | 2004-08-10 | Matsushita Electric Industrial Co., Ltd. | Glucose sensor |
| US6377894B1 (en)* | 1998-11-30 | 2002-04-23 | Abbott Laboratories | Analyte test instrument having improved calibration and communication processes |
| US6713308B1 (en)* | 1999-05-05 | 2004-03-30 | Fang Lu | System for electrochemical quantitative analysis of analytes within a solid phase |
Cited By (127)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US20100094110A1 (en)* | 1998-04-30 | 2010-04-15 | Abbott Diabetes Care Inc. | Analyte Monitoring Device and Methods of Use |
| US9066697B2 (en) | 1998-04-30 | 2015-06-30 | Abbott Diabetes Care Inc. | Analyte monitoring device and methods of use |
| US8974386B2 (en) | 1998-04-30 | 2015-03-10 | Abbott Diabetes Care Inc. | Analyte monitoring device and methods of use |
| US8688188B2 (en) | 1998-04-30 | 2014-04-01 | Abbott Diabetes Care Inc. | Analyte monitoring device and methods of use |
| US9610034B2 (en) | 2001-01-02 | 2017-04-04 | Abbott Diabetes Care Inc. | Analyte monitoring device and methods of use |
| US9011332B2 (en) | 2001-01-02 | 2015-04-21 | Abbott Diabetes Care Inc. | Analyte monitoring device and methods of use |
| US9498159B2 (en) | 2001-01-02 | 2016-11-22 | Abbott Diabetes Care Inc. | Analyte monitoring device and methods of use |
| US20080118401A1 (en)* | 2001-08-13 | 2008-05-22 | Kirchhevel G L | Button layout for a testing instrument |
| US20070065342A1 (en)* | 2001-08-13 | 2007-03-22 | Brown Michael K | Mechanical mechanism for a sensor-dispensing instrument |
| USD534444S1 (en)* | 2002-02-15 | 2007-01-02 | Polymer Technology Systems, Inc. | Hand-held diagnostic instrument |
| US20080083773A1 (en)* | 2004-08-20 | 2008-04-10 | Micinski Russell J | Contact Connector Assembly For A Sensor-Dispensing Instrument |
| US7416430B2 (en) | 2004-08-20 | 2008-08-26 | Bayer Healthcare Llc | Contact connector assembly for a sensor-dispensing instrument |
| US20080293278A1 (en)* | 2004-08-20 | 2008-11-27 | Bayer Healthcare Llc | Contact connector assembly for a sensor-dispensing instrument |
| US7575457B2 (en) | 2004-08-20 | 2009-08-18 | Bayer Healthcare Llc | Contact connector assembly for a sensor-dispensing instrument |
| US8358210B2 (en) | 2005-02-08 | 2013-01-22 | Abbott Diabetes Care Inc. | RF tag on test strips, test strip vials and boxes |
| US8390455B2 (en) | 2005-02-08 | 2013-03-05 | Abbott Diabetes Care Inc. | RF tag on test strips, test strip vials and boxes |
| US8542122B2 (en) | 2005-02-08 | 2013-09-24 | Abbott Diabetes Care Inc. | Glucose measurement device and methods using RFID |
| US8115635B2 (en) | 2005-02-08 | 2012-02-14 | Abbott Diabetes Care Inc. | RF tag on test strips, test strip vials and boxes |
| US8223021B2 (en) | 2005-02-08 | 2012-07-17 | Abbott Diabetes Care Inc. | RF tag on test strips, test strip vials and boxes |
| US20090100927A1 (en)* | 2005-06-01 | 2009-04-23 | D Glenn Purcell | Multi-contact sensor connector with release mechanism |
| WO2006130577A3 (en)* | 2005-06-01 | 2007-07-05 | Bayer Healthcare Llc | Multi-contact sensor connector with release mechanism |
| US10952652B2 (en) | 2005-11-01 | 2021-03-23 | Abbott Diabetes Care Inc. | Analyte monitoring device and methods of use |
| US10201301B2 (en) | 2005-11-01 | 2019-02-12 | Abbott Diabetes Care Inc. | Analyte monitoring device and methods of use |
| US11272867B2 (en) | 2005-11-01 | 2022-03-15 | Abbott Diabetes Care Inc. | Analyte monitoring device and methods of use |
| US8920319B2 (en) | 2005-11-01 | 2014-12-30 | Abbott Diabetes Care Inc. | Analyte monitoring device and methods of use |
| US8915850B2 (en) | 2005-11-01 | 2014-12-23 | Abbott Diabetes Care Inc. | Analyte monitoring device and methods of use |
| US9326716B2 (en) | 2005-11-01 | 2016-05-03 | Abbott Diabetes Care Inc. | Analyte monitoring device and methods of use |
| US11399748B2 (en) | 2005-11-01 | 2022-08-02 | Abbott Diabetes Care Inc. | Analyte monitoring device and methods of use |
| US10231654B2 (en) | 2005-11-01 | 2019-03-19 | Abbott Diabetes Care Inc. | Analyte monitoring device and methods of use |
| US11911151B1 (en) | 2005-11-01 | 2024-02-27 | Abbott Diabetes Care Inc. | Analyte monitoring device and methods of use |
| US11103165B2 (en) | 2005-11-01 | 2021-08-31 | Abbott Diabetes Care Inc. | Analyte monitoring device and methods of use |
| US9078607B2 (en) | 2005-11-01 | 2015-07-14 | Abbott Diabetes Care Inc. | Analyte monitoring device and methods of use |
| US11363975B2 (en) | 2005-11-01 | 2022-06-21 | Abbott Diabetes Care Inc. | Analyte monitoring device and methods of use |
| US20100324853A1 (en)* | 2005-12-30 | 2010-12-23 | Medtronic Minimed, Inc. | Real-time self-calibrating sensor system and method |
| US9125607B2 (en) | 2005-12-30 | 2015-09-08 | Medtronic Minimed, Inc. | Real-time self-calibrating sensor system and method |
| US9125608B2 (en) | 2005-12-30 | 2015-09-08 | Medtronic Minimed, Inc. | Real-time self-calibrating sensor system and method |
| US7774038B2 (en)* | 2005-12-30 | 2010-08-10 | Medtronic Minimed, Inc. | Real-time self-calibrating sensor system and method |
| US8249683B2 (en) | 2005-12-30 | 2012-08-21 | Medtronic Minimed, Inc. | Real-time self-calibrating sensor system and method |
| US20070163894A1 (en)* | 2005-12-30 | 2007-07-19 | Medtronic Minimed, Inc. | Real-time self-calibrating sensor system and method |
| US20110099786A1 (en)* | 2006-10-24 | 2011-05-05 | Abbott Diabetes Care Inc. | Embossed Cell Analyte Sensor and Methods of Manufacture |
| US20080101983A1 (en)* | 2006-10-24 | 2008-05-01 | Abbott Diabetes Care, Inc. | Embossed cell analyte sensor and methods of manufacture |
| WO2008051407A3 (en)* | 2006-10-24 | 2008-12-31 | Abbott Diabetes Care Inc | Embossed cell analyte sensor and methods of manufacture |
| US9638698B2 (en) | 2006-10-24 | 2017-05-02 | Abbott Diabetes Care Inc. | Embossed cell analyte sensor and methods of manufacture |
| US8632965B2 (en) | 2006-10-24 | 2014-01-21 | Abbott Diabetes Care Inc. | Embossed cell analyte sensor and methods of manufacture |
| US7771926B2 (en) | 2006-10-24 | 2010-08-10 | Abbott Diabetes Care Inc. | Embossed cell analyte sensor and methods of manufacture |
| US8211632B2 (en) | 2006-10-24 | 2012-07-03 | Abbott Diabetes Care Inc. | Embossed cell analyte sensor and methods of manufacture |
| US20090302873A1 (en)* | 2007-09-05 | 2009-12-10 | Lifescan Scotland Limited | System for electrochemically measuring an analyte in a sample material |
| USD586678S1 (en) | 2007-10-12 | 2009-02-17 | Lifescan, Inc. | Analyte test meter |
| US20090149717A1 (en)* | 2007-12-10 | 2009-06-11 | Jacob Brauer | Interface for a health measurement and monitoring system |
| US11450411B2 (en) | 2007-12-10 | 2022-09-20 | Ascensia Diabetes Care Holdings Ag | Interface for a health measurement and monitoring system |
| US9022931B2 (en) | 2007-12-10 | 2015-05-05 | Bayer Healthcare Llc | Interface for a health measurement and monitoring system |
| US10548537B2 (en) | 2007-12-10 | 2020-02-04 | Ascensia Diabetes Care Holdings Ag | Interface for a health measurement and monitoring system |
| USD612279S1 (en) | 2008-01-18 | 2010-03-23 | Lifescan Scotland Limited | User interface in an analyte meter |
| USD612274S1 (en) | 2008-01-18 | 2010-03-23 | Lifescan Scotland, Ltd. | User interface in an analyte meter |
| WO2009097466A1 (en)* | 2008-01-31 | 2009-08-06 | Abbott Diabetes Care Inc. | Method for automatically and rapidly distinguishing between control and sample solutions in a biosensor strip |
| USD615431S1 (en) | 2008-03-21 | 2010-05-11 | Lifescan Scotland Limited | Analyte test meter |
| USD611853S1 (en) | 2008-03-21 | 2010-03-16 | Lifescan Scotland Limited | Analyte test meter |
| US9626480B2 (en) | 2008-03-21 | 2017-04-18 | Lifescan Scotland Limited | Analyte testing method and system |
| USD612275S1 (en) | 2008-03-21 | 2010-03-23 | Lifescan Scotland, Ltd. | Analyte test meter |
| US8917184B2 (en) | 2008-03-21 | 2014-12-23 | Lifescan Scotland Limited | Analyte testing method and system |
| USD626651S1 (en) | 2008-05-29 | 2010-11-02 | Bayer Healthcare, Llc | Analyte-determining meter |
| USD611151S1 (en) | 2008-06-10 | 2010-03-02 | Lifescan Scotland, Ltd. | Test meter |
| USD611489S1 (en) | 2008-07-25 | 2010-03-09 | Lifescan, Inc. | User interface display for a glucose meter |
| US20100032319A1 (en)* | 2008-08-06 | 2010-02-11 | Jun Okada | Diagnostic cassette for electrochemical measuring apparatus and method of diagnosing electrochemical measuring apparatus |
| USD611372S1 (en) | 2008-09-19 | 2010-03-09 | Lifescan Scotland Limited | Analyte test meter |
| US20110174618A1 (en)* | 2008-09-30 | 2011-07-21 | Menai Medical Technologies Limited | Sample measurement system |
| US9173601B2 (en)* | 2009-02-18 | 2015-11-03 | Panasonic Healthcare Holdings Co., Ltd. | Measuring device |
| US20140180050A1 (en)* | 2009-02-18 | 2014-06-26 | Panasonic Corporation | Measuring device |
| WO2011002694A1 (en) | 2009-06-30 | 2011-01-06 | Abbott Diabetes Care Inc. | Analyte monitoring device and methods of use |
| WO2011002693A1 (en) | 2009-06-30 | 2011-01-06 | Abbott Diabetes Care Inc. | Analyte monitoring device and methods of use |
| CN102469965A (en)* | 2009-06-30 | 2012-05-23 | 雅培糖尿病护理公司 | Analyte monitoring device and methods of use |
| EP3001194A1 (en) | 2009-08-31 | 2016-03-30 | Abbott Diabetes Care, Inc. | Medical devices and methods |
| EP3923295A1 (en) | 2009-08-31 | 2021-12-15 | Abbott Diabetes Care, Inc. | Medical devices and methods |
| USD627068S1 (en) | 2009-09-22 | 2010-11-09 | Bayer Healthcare, Llc | Analyte-determining meter |
| US9501272B2 (en) | 2010-05-24 | 2016-11-22 | Abbott Diabetes Care Inc. | Systems and methods for updating a medical device |
| US12373189B2 (en) | 2010-05-24 | 2025-07-29 | Abbott Diabetes Care Inc. | Systems and methods for updating a medical device |
| US11748088B2 (en) | 2010-05-24 | 2023-09-05 | Abbott Diabetes Care Inc. | Systems and methods for updating a medical device |
| US11169794B2 (en) | 2010-05-24 | 2021-11-09 | Abbott Diabetes Care Inc. | Systems and methods for updating a medical device |
| US10255055B2 (en) | 2010-05-24 | 2019-04-09 | Abbott Diabetes Care Inc. | Systems and methods for updating a medical device |
| US20130192987A1 (en)* | 2010-09-13 | 2013-08-01 | Dai Nippon Printing Co., Ltd. | Biosensor and method for producing the same |
| US9222909B2 (en)* | 2010-09-13 | 2015-12-29 | Dai Nippon Printing Co., Ltd. | Biosensor and method for producing the same |
| EP2912438A4 (en)* | 2012-10-26 | 2016-11-02 | Pixie Scient Inc | HEALTH DIAGNOSTIC SYSTEMS AND METHODS |
| US10251602B2 (en) | 2012-10-26 | 2019-04-09 | Pixie Scientific, Llc | Health diagnostic systems and methods |
| US8920628B2 (en) | 2012-11-02 | 2014-12-30 | Roche Diagnostics Operations, Inc. | Systems and methods for multiple analyte analysis |
| US10466196B2 (en) | 2012-11-02 | 2019-11-05 | Roche Diabetes Care, Inc. | Systems and methods for multiple analyte analysis |
| US9416397B2 (en) | 2012-11-02 | 2016-08-16 | Roche Diabetes Care, Inc. | Methods of determining glucose and ketone values in a sample |
| US8921061B2 (en) | 2012-11-02 | 2014-12-30 | Roche Diagnostics Operations, Inc. | Reagent materials and associated test elements |
| US9921176B2 (en) | 2012-11-02 | 2018-03-20 | Roche Diabetes Care, Inc. | Systems and methods for multiple analyte analysis |
| US9958409B2 (en) | 2012-11-02 | 2018-05-01 | Roche Diabetes Care, Inc. | Systems and methods for multiple analyte analysis |
| US11719687B2 (en)* | 2013-03-15 | 2023-08-08 | Agamatrix, Inc. | Analyte detection meter and associated method of use |
| US12350041B2 (en) | 2013-04-30 | 2025-07-08 | Abbott Diabetes Care Inc. | Systems, devices, and methods for energy efficient electrical device activation |
| US11571149B1 (en) | 2013-04-30 | 2023-02-07 | Abbott Diabetes Care Inc. | Systems, devices, and methods for energy efficient electrical device activation |
| US10213141B2 (en) | 2013-04-30 | 2019-02-26 | Abbott Diabetes Care Inc. | Systems, devices, and methods for energy efficient electrical device activation |
| US11207006B2 (en) | 2013-04-30 | 2021-12-28 | Abbott Diabetes Care Inc. | Systems, devices, and methods for energy efficient electrical device activation |
| US10361574B2 (en) | 2013-11-05 | 2019-07-23 | Abbott Diabetes Care Inc. | Systems, devices, and methods for control of a power supply connection |
| US9590438B2 (en) | 2013-11-05 | 2017-03-07 | Abbott Diabetes Care Inc. | Systems, devices, and methods for control of a power supply connection |
| WO2015069563A1 (en) | 2013-11-05 | 2015-05-14 | Abbott Diabetes Care Inc. | Systems, devices, and methods for control of a power supply connection |
| US20150177176A1 (en)* | 2013-12-23 | 2015-06-25 | Lifescan Scotland Limited | Analyte meter test strip detection |
| US9442089B2 (en)* | 2013-12-23 | 2016-09-13 | Lifescan Scotland Limited | Analyte meter test strip detection |
| US12411930B2 (en) | 2013-12-27 | 2025-09-09 | Abbott Diabetes Care Inc. | Application interface and display control in an analyte monitoring environment |
| US11122043B2 (en) | 2013-12-27 | 2021-09-14 | Abbott Diabetes Care Inc. | Systems, devices, and methods for authentication in an analyte monitoring environment |
| US10360368B2 (en) | 2013-12-27 | 2019-07-23 | Abbott Diabetes Care Inc. | Application interface and display control in an analyte monitoring environment |
| DE202014010579U1 (en) | 2013-12-27 | 2016-01-05 | Abbott Diabetes Care Inc. | Application interface and display control in an analyte monitoring environment |
| DE202014011533U1 (en) | 2013-12-27 | 2021-12-16 | Abbott Diabetes Care, Inc. | Systems and devices for authentication in an analyte monitoring environment |
| EP4343784A2 (en) | 2013-12-27 | 2024-03-27 | Abbott Diabetes Care Inc. | Application interface and display control in an analyte monitoring environment |
| US10110603B2 (en) | 2013-12-27 | 2018-10-23 | Abbott Diabetes Care Inc. | Systems, devices, and methods for authentication in an analyte monitoring environment |
| EP3780689A1 (en) | 2013-12-27 | 2021-02-17 | Abbott Diabetes Care, Inc. | Systems, devices, and methods for authentication in an analyte monitoring environment |
| US9544313B2 (en) | 2013-12-27 | 2017-01-10 | Abbott Diabetes Care Inc. | Systems, devices, and methods for authentication in an analyte monitoring environment |
| WO2015100109A1 (en) | 2013-12-27 | 2015-07-02 | Abbott Diabetes Care Inc. | Systems, devices, and methods for authentication in an analyte monitoring environment |
| EP4404210A2 (en) | 2013-12-27 | 2024-07-24 | Abbott Diabetes Care Inc. | Application interface and display control in an analyte monitoring environment |
| US20170241996A1 (en)* | 2014-09-08 | 2017-08-24 | Indian Institute Of Science | Electrochemical biosensor and a method of sensing albumin and its complexes |
| US11435344B2 (en)* | 2014-09-08 | 2022-09-06 | Indian Institute Of Science | Electrochemical biosensor and a method of sensing albumin and its complexes |
| USD742524S1 (en) | 2014-11-17 | 2015-11-03 | Bayer Healthcare Llc | Analyte meter |
| US10383564B2 (en) | 2015-05-22 | 2019-08-20 | Pixie Scientific, Llc | Indicator panels for incontinence products |
| US11789008B2 (en) | 2016-12-20 | 2023-10-17 | Abbott Diabetes Care Inc. | Systems, devices, and methods for wireless communications in analyte monitoring systems |
| WO2018118822A1 (en) | 2016-12-20 | 2018-06-28 | Abbott Diabetes Care Inc. | Systems, devices and methods for wireless communications in analyte monitoring devices |
| US11191463B2 (en) | 2017-08-18 | 2021-12-07 | Abbott Diabetes Care Inc. | Systems, devices, and methods related to the individualized calibration and/or manufacturing of medical devices |
| EP4218568A1 (en) | 2017-08-18 | 2023-08-02 | Abbott Diabetes Care Inc. | Analyte monitoring system storing a measured electrical characteristic of the in vivo analyte sensor of the system as individualized calibration information |
| DE202018006591U1 (en) | 2017-08-18 | 2021-07-21 | Abbott Diabetes Care, Inc. | Systems and devices relating to the individualized calibration and / or manufacture of medical devices |
| US10993646B2 (en) | 2017-08-18 | 2021-05-04 | Abbott Diabetes Care Inc. | Systems, devices, and methods related to the individualized calibration and/or manufacturing of medical devices |
| US12279867B2 (en) | 2017-08-18 | 2025-04-22 | Abbott Diabetes Care Inc. | Systems, devices, and methods related to the individualized calibration and/or manufacturing of medical devices |
| US12285251B2 (en) | 2017-08-18 | 2025-04-29 | Abbott Diabetes Care Inc. | Systems, devices, and methods related to the individualized calibration and/or manufacturing of medical devices |
| US12285252B2 (en) | 2017-08-18 | 2025-04-29 | Abbott Diabetes Care Inc. | Systems, devices, and methods related to the individualized calibration and/or manufacturing of medical devices |
| WO2019035073A2 (en) | 2017-08-18 | 2019-02-21 | Abbott Diabetes Care Inc. | Systems, devices, and methods related to the individualized calibration and/or manufacturing of medical devices |
| WO2019152966A1 (en) | 2018-02-05 | 2019-08-08 | Abbott Diabetes Care Inc. | Notes and event log information associated with analyte sensors |
| EP4628014A2 (en) | 2018-02-05 | 2025-10-08 | Abbott Diabetes Care, Inc. | Notes and event log information associated with analyte sensors |
| WO2022164940A1 (en) | 2021-01-26 | 2022-08-04 | Abbott Diabetes Care Inc. | Systems, devices, and methods related to ketone sensors |
Also Published As
| Publication number | Publication date |
|---|---|
| JP4836457B2 (en) | 2011-12-14 |
| WO2004077052A1 (en) | 2004-09-10 |
| EP1576367B1 (en) | 2012-03-21 |
| ATE550649T1 (en) | 2012-04-15 |
| CA2444094C (en) | 2012-09-25 |
| US8916036B2 (en) | 2014-12-23 |
| EP1576367A1 (en) | 2005-09-21 |
| CA2444094A1 (en) | 2004-06-19 |
| JP2006511818A (en) | 2006-04-06 |
| US20150168338A1 (en) | 2015-06-18 |
| US20100126856A1 (en) | 2010-05-27 |
| AU2003252777B2 (en) | 2009-09-24 |
| AU2003252777A1 (en) | 2004-07-08 |
| US9234865B2 (en) | 2016-01-12 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| US9234865B2 (en) | Analyte test instrument having improved versatility | |
| EP0746762B2 (en) | Biosensing meter with pluggable memory key | |
| US9846140B2 (en) | Method for determination of analyte concentrations and related apparatus | |
| EP1443322B1 (en) | Concentration measuring method and concentration measuring device | |
| EP1279033B1 (en) | Electrochemical biosensor test strip with recognition electrode and readout meter using this test strip | |
| EP2149792B1 (en) | Sample analyzing method | |
| US20080093228A1 (en) | Method and apparatus for providing a stable voltage to an analytical system | |
| WO2008049074A2 (en) | Error detection in analyte measurements based on measurement of system resistance | |
| AU2006233767A1 (en) | Error detection in analyte measurements based on measurement of system resistance | |
| US20080169799A1 (en) | Method for biosensor analysis |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| AS | Assignment | Owner name:ABBOTT LABORATORIES, ILLINOIS Free format text:ASSIGNMENT OF ASSIGNORS INTEREST;ASSIGNORS:WANG, YI;KARINKA, SHRIDHARA ALVA;SANGHERA, GURDIAL;REEL/FRAME:013742/0116 Effective date:20030321 | |
| STCB | Information on status: application discontinuation | Free format text:ABANDONED -- AFTER EXAMINER'S ANSWER OR BOARD OF APPEALS DECISION |