US20040068230A1 - System for providing blood glucose measurements to an infusion device - Google Patents
System for providing blood glucose measurements to an infusion deviceDownload PDFInfo
- Publication number
- US20040068230A1 US20040068230A1US10/624,389US62438903AUS2004068230A1US 20040068230 A1US20040068230 A1US 20040068230A1US 62438903 AUS62438903 AUS 62438903AUS 2004068230 A1US2004068230 A1US 2004068230A1
- Authority
- US
- United States
- Prior art keywords
- infusion
- user
- determining device
- communication system
- infusion device
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Abandoned
Links
- 238000001802infusionMethods0.000titleclaimsabstractdescription536
- 239000008280bloodSubstances0.000titleclaimsdescription30
- 210000004369bloodAnatomy0.000titleclaimsdescription30
- WQZGKKKJIJFFOK-GASJEMHNSA-NGlucoseNatural productsOC[C@H]1OC(O)[C@H](O)[C@@H](O)[C@@H]1OWQZGKKKJIJFFOK-GASJEMHNSA-N0.000titleclaimsdescription22
- 239000008103glucoseSubstances0.000titleclaimsdescription22
- 238000005259measurementMethods0.000titledescription153
- 238000004891communicationMethods0.000claimsabstractdescription425
- 239000012491analyteSubstances0.000claimsabstractdescription152
- 239000012530fluidSubstances0.000claimsabstractdescription65
- 238000012360testing methodMethods0.000claimsabstractdescription22
- NOESYZHRGYRDHS-UHFFFAOYSA-NinsulinChemical compoundN1C(=O)C(NC(=O)C(CCC(N)=O)NC(=O)C(CCC(O)=O)NC(=O)C(C(C)C)NC(=O)C(NC(=O)CN)C(C)CC)CSSCC(C(NC(CO)C(=O)NC(CC(C)C)C(=O)NC(CC=2C=CC(O)=CC=2)C(=O)NC(CCC(N)=O)C(=O)NC(CC(C)C)C(=O)NC(CCC(O)=O)C(=O)NC(CC(N)=O)C(=O)NC(CC=2C=CC(O)=CC=2)C(=O)NC(CSSCC(NC(=O)C(C(C)C)NC(=O)C(CC(C)C)NC(=O)C(CC=2C=CC(O)=CC=2)NC(=O)C(CC(C)C)NC(=O)C(C)NC(=O)C(CCC(O)=O)NC(=O)C(C(C)C)NC(=O)C(CC(C)C)NC(=O)C(CC=2NC=NC=2)NC(=O)C(CO)NC(=O)CNC2=O)C(=O)NCC(=O)NC(CCC(O)=O)C(=O)NC(CCCNC(N)=N)C(=O)NCC(=O)NC(CC=3C=CC=CC=3)C(=O)NC(CC=3C=CC=CC=3)C(=O)NC(CC=3C=CC(O)=CC=3)C(=O)NC(C(C)O)C(=O)N3C(CCC3)C(=O)NC(CCCCN)C(=O)NC(C)C(O)=O)C(=O)NC(CC(N)=O)C(O)=O)=O)NC(=O)C(C(C)CC)NC(=O)C(CO)NC(=O)C(C(C)O)NC(=O)C1CSSCC2NC(=O)C(CC(C)C)NC(=O)C(NC(=O)C(CCC(N)=O)NC(=O)C(CC(N)=O)NC(=O)C(NC(=O)C(N)CC=1C=CC=CC=1)C(C)C)CC1=CN=CN1NOESYZHRGYRDHS-UHFFFAOYSA-N0.000claimsdescription84
- 102000004877InsulinHuman genes0.000claimsdescription42
- 108090001061InsulinProteins0.000claimsdescription42
- 229940125396insulinDrugs0.000claimsdescription42
- 238000000034methodMethods0.000claimsdescription31
- 230000001351cycling effectEffects0.000claimsdescription27
- 230000004044responseEffects0.000claimsdescription24
- 238000012545processingMethods0.000claimsdescription14
- 239000000463materialSubstances0.000claimsdescription11
- 230000008878couplingEffects0.000claimsdescription7
- 238000010168coupling processMethods0.000claimsdescription7
- 238000005859coupling reactionMethods0.000claimsdescription7
- 230000007246mechanismEffects0.000claimsdescription7
- 150000001720carbohydratesChemical class0.000claimsdescription6
- 235000014633carbohydratesNutrition0.000claimsdescription6
- 230000005540biological transmissionEffects0.000description14
- 229940079593drugDrugs0.000description7
- 239000003814drugSubstances0.000description7
- 238000010586diagramMethods0.000description6
- 230000009471actionEffects0.000description5
- 210000001124body fluidAnatomy0.000description5
- 230000003287optical effectEffects0.000description5
- 239000010839body fluidSubstances0.000description4
- HVYWMOMLDIMFJA-DPAQBDIFSA-NcholesterolChemical compoundC1C=C2C[C@@H](O)CC[C@]2(C)[C@@H]2[C@@H]1[C@@H]1CC[C@H]([C@H](C)CCCC(C)C)[C@@]1(C)CC2HVYWMOMLDIMFJA-DPAQBDIFSA-N0.000description4
- 238000012790confirmationMethods0.000description4
- 230000006870functionEffects0.000description4
- 230000002641glycemic effectEffects0.000description4
- 230000008569processEffects0.000description4
- 239000000853adhesiveSubstances0.000description3
- 230000001070adhesive effectEffects0.000description3
- 230000002255enzymatic effectEffects0.000description3
- 229940088597hormoneDrugs0.000description3
- 239000005556hormoneSubstances0.000description3
- 230000002218hypoglycaemic effectEffects0.000description3
- 238000002347injectionMethods0.000description3
- 239000007924injectionSubstances0.000description3
- 238000005070samplingMethods0.000description3
- 208000013016HypoglycemiaDiseases0.000description2
- XEEYBQQBJWHFJM-UHFFFAOYSA-NIronChemical compound[Fe]XEEYBQQBJWHFJM-UHFFFAOYSA-N0.000description2
- JVTAAEKCZFNVCJ-UHFFFAOYSA-MLactateChemical compoundCC(O)C([O-])=OJVTAAEKCZFNVCJ-UHFFFAOYSA-M0.000description2
- QVGXLLKOCUKJST-UHFFFAOYSA-Natomic oxygenChemical compound[O]QVGXLLKOCUKJST-UHFFFAOYSA-N0.000description2
- 230000008859changeEffects0.000description2
- 239000003795chemical substances by applicationSubstances0.000description2
- 235000012000cholesterolNutrition0.000description2
- 210000003722extracellular fluidAnatomy0.000description2
- 230000036541healthEffects0.000description2
- 201000001421hyperglycemiaDiseases0.000description2
- 238000012986modificationMethods0.000description2
- 230000004048modificationEffects0.000description2
- 229910052760oxygenInorganic materials0.000description2
- 239000001301oxygenSubstances0.000description2
- 230000036387respiratory rateEffects0.000description2
- 210000003296salivaAnatomy0.000description2
- 210000004243sweatAnatomy0.000description2
- 210000001138tearAnatomy0.000description2
- 238000002560therapeutic procedureMethods0.000description2
- 210000002700urineAnatomy0.000description2
- 230000003612virological effectEffects0.000description2
- 102000004190EnzymesHuman genes0.000description1
- 108090000790EnzymesProteins0.000description1
- 206010022489Insulin ResistanceDiseases0.000description1
- 241001465754MetazoaSpecies0.000description1
- 206010067584Type 1 diabetes mellitusDiseases0.000description1
- 230000003466anti-cipated effectEffects0.000description1
- 238000011394anticancer treatmentMethods0.000description1
- 239000000427antigenSubstances0.000description1
- 102000036639antigensHuman genes0.000description1
- 108091007433antigensProteins0.000description1
- 230000008901benefitEffects0.000description1
- 230000009920chelationEffects0.000description1
- 125000004122cyclic groupChemical group0.000description1
- 230000003247decreasing effectEffects0.000description1
- 230000001419dependent effectEffects0.000description1
- 238000011161developmentMethods0.000description1
- 239000000284extractSubstances0.000description1
- 230000003345hyperglycaemic effectEffects0.000description1
- 229910052742ironInorganic materials0.000description1
- 230000005055memory storageEffects0.000description1
- 229940124583pain medicationDrugs0.000description1
- 230000002093peripheral effectEffects0.000description1
- 208000002815pulmonary hypertensionDiseases0.000description1
- 238000012552reviewMethods0.000description1
- 239000000126substanceSubstances0.000description1
- 230000001360synchronised effectEffects0.000description1
- 238000013518transcriptionMethods0.000description1
- 230000035897transcriptionEffects0.000description1
- 238000012546transferMethods0.000description1
- 208000001072type 2 diabetes mellitusDiseases0.000description1
- 229940088594vitaminDrugs0.000description1
- 239000011782vitaminSubstances0.000description1
- 229930003231vitaminNatural products0.000description1
- 235000013343vitaminNutrition0.000description1
Images
Classifications
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61B—DIAGNOSIS; SURGERY; IDENTIFICATION
- A61B5/00—Measuring for diagnostic purposes; Identification of persons
- A61B5/0002—Remote monitoring of patients using telemetry, e.g. transmission of vital signals via a communication network
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M5/00—Devices for bringing media into the body in a subcutaneous, intra-vascular or intramuscular way; Accessories therefor, e.g. filling or cleaning devices, arm-rests
- A61M5/14—Infusion devices, e.g. infusing by gravity; Blood infusion; Accessories therefor
- A61M5/142—Pressure infusion, e.g. using pumps
- A61M5/14244—Pressure infusion, e.g. using pumps adapted to be carried by the patient, e.g. portable on the body
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61B—DIAGNOSIS; SURGERY; IDENTIFICATION
- A61B5/00—Measuring for diagnostic purposes; Identification of persons
- A61B5/145—Measuring characteristics of blood in vivo, e.g. gas concentration or pH-value ; Measuring characteristics of body fluids or tissues, e.g. interstitial fluid or cerebral tissue
- A61B5/14532—Measuring characteristics of blood in vivo, e.g. gas concentration or pH-value ; Measuring characteristics of body fluids or tissues, e.g. interstitial fluid or cerebral tissue for measuring glucose, e.g. by tissue impedance measurement
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M5/00—Devices for bringing media into the body in a subcutaneous, intra-vascular or intramuscular way; Accessories therefor, e.g. filling or cleaning devices, arm-rests
- A61M5/14—Infusion devices, e.g. infusing by gravity; Blood infusion; Accessories therefor
- A61M2005/1401—Functional features
- A61M2005/1405—Patient controlled analgesia [PCA]
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M2205/00—General characteristics of the apparatus
- A61M2205/35—Communication
- A61M2205/3576—Communication with non implanted data transmission devices, e.g. using external transmitter or receiver
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M2205/00—General characteristics of the apparatus
- A61M2205/35—Communication
- A61M2205/3576—Communication with non implanted data transmission devices, e.g. using external transmitter or receiver
- A61M2205/3584—Communication with non implanted data transmission devices, e.g. using external transmitter or receiver using modem, internet or bluetooth
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M2230/00—Measuring parameters of the user
- A61M2230/20—Blood composition characteristics
- A61M2230/201—Glucose concentration
Definitions
- This inventionrelates generally to infusion systems that are used for infusing a fluid into a user, and in particular, to apparatuses and methods for providing blood glucose measurements to an infusion device.
- BGblood glucose
- a patient's BG levelis too high, the patient can inject a “bolus” (dose) of insulin to lower his/her BG level from its present level to a desired target level.
- patientsmay inject a bolus of insulin in anticipation of ingesting carbohydrates, thus heading off a sharp rise in their BG level.
- Patientsemploy various calculations to determine the amount of insulin to inject.
- Bolus estimation softwareis available for calculating an insulin bolus. Patients may use these software programs on an electronic computing device, such as a computer, the Internet, a personal digital assistant (PDA), or an insulin delivery device.
- PDApersonal digital assistant
- Insulin delivery devicesinclude infusion pumps, injection pens, and IV meters.
- the best bolus estimation softwaretakes into account the patient's present BG level.
- a patientmust measure his/her blood glucose using a BG measurement device, such as a test strip meter, a continuous glucose measurement system, or a hospital hemacue.
- BG measurement devicesuse various methods to measure the BG level of a patient, such as a sample of the patient's blood, a sensor in contact with a bodily fluid, an optical sensor, an enzymatic sensor, or a fluorescent sensor.
- the BG measurement devicehas generated a BG measurement, the measurement is displayed on the BG measurement device.
- the patientmay visually read the BG measurement and physically enter the BG measurement into an electronic computing device to calculate a bolus estimate.
- the patientmust inject the insulin bolus or program an insulin delivery device to deliver the bolus into their body.
- this processis cumbersome and is subject to transcribing errors—for example, the patient may inaccurately enter the BG measurement that is displayed on the BG measurement device into the electronic computing device.
- the bolus estimateis not accurate, which may lead to the delivery of an inappropriate insulin dose.
- an infusion system for infusing a fluid into a body of a userincludes a characteristic determining device and an infusion device.
- the characteristic determining deviceincludes a housing adapted to be carried by the user, a receptacle coupled to the housing for receiving and testing an analyte from the user to determine a concentration of the analyte in the user, a processor contained in the housing and coupled to the receptacle for processing the determined concentration of the analyte from the receptacle, and a communication system contained in the housing and coupled to the processor for transmitting a communication including data indicative of the determined concentration of the analyte in the user.
- the characteristic determining devicemay also include a lancing device coupled to the receptacle for obtaining the analyte from the user.
- the infusion deviceincludes a housing adapted to be carried by the user, a drive mechanism contained in the housing and operatively coupled with a reservoir containing the fluid for infusing the fluid into the body of the user, a communication system contained in the housing for receiving the communication including the data indicative of the determined concentration of the analyte in the user from the determining device, and a processor contained in the housing and coupled to the communication system for processing the data indicative of the determined concentration of the analyte in the user and controlling the infusion device.
- the infusion devicefurther includes a bolus estimator used in conjunction with the processor for calculating an estimated amount of fluid to be infused into the body of the user based upon the received data indicative of the determined concentration of the analyte in the user and a target concentration of the analyte in the user, and an indicator to indicate when the estimated amount of fluid to be infused has been calculated.
- the infusion devicemay include a user input device for inputting an estimate of a material to be ingested by the user, and the bolus estimator may include the capability to calculate the estimated amount of fluid to be infused into the body of the user based upon the inputted estimate of the material to be ingested by the user.
- the infusion devicemay also include a memory for storing the data indicative of the determined concentration of the analyte in the user received by the infusion device communication system from the determining device communication system.
- the characteristic determining deviceautomatically transmits the communication including the data indicative of the determined concentration of the analyte in the user to the infusion device.
- the characteristic determining devicefurther includes a user input device for inputting commands, and transmits the communication including the data indicative of the determined concentration of the analyte in the user to the infusion device in response to a command from the user input device.
- the characteristic determining devicefurther includes an indicator to indicate a status of the communication including the data indicative of the determined concentration of the analyte in the user being transmitted from the determining device communication system to the infusion device communication system.
- the communication transmitted from the characteristic determining device to the infusion devicefurther includes a time at which the concentration of the analyte in the user was determined.
- the processor of the characteristic determining devicedetermines an amount of time that has elapsed since the concentration of the analyte in the user was determined, and the communication transmitted from the determining device to the infusion device further includes the elapsed amount of time. Further, the processor of the characteristic determining device may cause the communication system of the characteristic determining device not to transmit the communication including the data indicative of the determined concentration of the analyte in the user if the elapsed amount of time exceeds a predetermined amount of time.
- the infusion device processordetermines an amount of time that has elapsed since the data indicative of the determined concentration of the analyte in the user was received, and causes the bolus estimator not to calculate the estimated amount of fluid to be infused based upon the determined concentration of the analyte if the elapsed amount of time exceeds a predetermined amount of time.
- the processor of the infusion devicedetermines an amount of time that has elapsed since the concentration of the analyte in the user was determined, and causes the bolus estimator not to calculate the estimated amount of fluid to be infused based upon the determined concentration of the analyte if the elapsed amount of time exceeds a predetermined amount of time.
- the determining device communication systemis capable of being deactivated and reactivated.
- the characteristic determining deviceincludes a user input device for inputting commands, and the communication system of the characteristic determining device is capable of being deactivated in response to a first command from the user input device and being reactivated in response to a second command from the user input device.
- the communication system of the characteristic determining devicemay be automatically reactivated after a predetermined amount of time has elapsed or at a predetermined time of day.
- the characteristic determining devicemay include a memory for storing data indicative of the determined concentration of the analyte in the user that is determined when the determining device communication system is deactivated, and the determining device communication system may transmit a communication including the stored data to the infusion device communication system when the determining device communication system is reactivated.
- the processor of the characteristic determining devicehas unique identification information
- the communication transmitted from the characteristic determining device to the infusion devicefurther includes the unique identification information of the determining device processor such that the infusion device is capable of discerning whether the communication is intended for receipt by the infusion device.
- the processor of the infusion devicehas unique identification information
- the communication transmitted from the characteristic determining device to the infusion devicefurther includes the unique identification information of the infusion device processor such that the infusion device is capable of discerning whether the communication is intended for receipt by the infusion device.
- the processor of the infusion deviceuses power cycling whereby power is periodically supplied to the communication system of the infusion device until a communication is received from the characteristic determining device.
- the processor of the infusion devicediscontinues using power cycling whereby the power is continuously supplied to the infusion device communication system.
- the infusion device processormay then resume using power cycling upon completing the receipt of the communication including the data indicative of the determined concentration of the analyte in the user from the determining device communication system.
- the infusion systemfurther includes a connector for coupling the characteristic determining device to a computer and downloading data from the characteristic determining device to the computer.
- the communication system of the infusion deviceis further capable of transmitting a communication including infusion device data to be downloaded
- the communication system of the characteristic determining deviceis further capable of receiving the communication including the infusion device data to be downloaded from the infusion device.
- the received infusion device datais then downloaded from the characteristic determining device through the connector to the computer.
- the characteristic determining devicemay further include a memory for storing data, and the received infusion device data may be stored in the memory of the characteristic determining device for subsequent downloading through the connector to the computer.
- the characteristic determining devicefurther includes a user input device for inputting remote control commands for controlling the infusion device.
- the communication system of the characteristic determining devicefurther transmits a communication including the remote control commands, and the communication system of the infusion device further receives the communication including the remote control commands from the characteristic determining device.
- the processor of the infusion devicethen controls the infusion device in accordance with the received remote control commands.
- the infusion devicefurther includes a user input device for inputting remote control commands for controlling the characteristic determining device.
- the communication system of the infusion devicefurther transmits a communication including the remote control commands
- the communication system of the characteristic determining devicefurther receives the communication including the remote control commands from the infusion device.
- the processor of the characteristic determining devicethen controls the characteristic determining device in accordance with the received remote control commands.
- the characteristic determining devicefurther includes a determining device clock
- the infusion devicefurther includes an infusion device clock.
- the infusion device communication systemfurther transmits a communication including a time of the infusion device clock
- the determining device communication systemfurther receives the communication including the time of the infusion device clock from the infusion device communication system.
- the determining device clockis then set to the received time of the infusion device clock.
- the determining device communication systemfurther transmits a communication including a time of the determining device clock
- the infusion device communication systemfurther receives the communication including the time of the determining device clock from the determining device communication system.
- the infusion device clockis then set to the received time of the determining device clock.
- an infusion deviceinfuses a fluid into a body of a user and is capable of communicating with a characteristic determining device, which is adapted for determining a concentration of an analyte in the user.
- the infusion deviceincludes a housing adapted to be carried by the user, a drive mechanism contained in the housing and operatively coupled with a reservoir containing the fluid for infusing the fluid into the body of the user, a communication system contained in the housing for receiving a communication including data indicative of the determined concentration of the analyte in the user from the characteristic determining device, and a processor contained in the housing and coupled to the communication system for processing the data indicative of the determined concentration of the analyte in the user and controlling the infusion device.
- the infusion devicealso includes a bolus estimator used in conjunction with the processor for calculating an estimated amount of fluid to be infused into the body of the user based upon the received data indicative of the determined concentration of the analyte in the user and a target concentration of the analyte in the user.
- the infusion devicefurther includes an indicator to indicate when the estimated amount of fluid to be infused has been calculated.
- a characteristic determining devicedetermines a concentration of an analyte in a body of a user and is capable of communicating with an infusion device, which is adapted for infusing a fluid into the body of the user and calculating an estimated amount of the fluid to be infused into the body of the user based upon the determined concentration of the analyte in the user and a target concentration of the analyte in the user.
- the characteristic determining deviceincludes a housing adapted to be carried by the user, a receptacle coupled to the housing for receiving and testing an analyte from the user to determine the concentration of the analyte in the user, a processor contained in the housing and coupled to the receptacle for processing the determined concentration of the analyte from the receptacle, and a communication system contained in the housing and coupled to the processor for transmitting a communication including data indicative of the determined concentration of the analyte in the user to the infusion device.
- an infusion systemincludes a characteristic determining device and an infusion device, and a method for infusing a fluid into a body of a user.
- the methodincludes the steps of: receiving and testing an analyte from the user to determine a concentration of the analyte in the user, transmitting with the characteristic determining device a communication including data indicative of the determined concentration of the analyte in the user, and receiving with the infusion device the communication including the data indicative of the determined concentration of the analyte in the user.
- the data indicative of the determined concentration of the analyte in the user received by the infusion device from the characteristic determining devicemay then be stored in a memory of the infusion device.
- the methodfurther includes the steps of calculating an estimated amount of fluid to be infused into the body of the user based upon the received data indicative of the determined concentration of the analyte in the user and a target concentration of the analyte in the user, and indicating when the estimated amount of fluid to be infused has been calculated. Additionally, the method may include the step of inputting an estimate of a material to be ingested by the user, and the estimated amount of fluid to be infused into the body of the user is calculated further based upon the inputted estimate of the material to be ingested by the user.
- the communication including the data indicative of the determined concentration of the analyte in the useris automatically transmitted from the characteristic determining device to the infusion device.
- the communication including the data indicative of the determined concentration of the analyte in the useris transmitted from the characteristic determining device to the infusion device in response to an inputted command.
- the systemindicates a status of the communication including the data indicative of the determined concentration of the analyte in the user being transmitted from the characteristic determining device to the infusion device.
- the communication including the data indicative of the determined concentration of the analyte in the user transmitted from the characteristic determining device to the infusion devicefurther includes a time at which the concentration of the analyte in the user was determined.
- the systemalso determines an amount of time that has elapsed since the concentration of the analyte in the user was determined.
- the systemdetermines an amount of time that has elapsed since the communication including the data indicative of the determined concentration of the analyte in the user was received by the infusion device.
- the methodfurther includes the steps of transmitting with the infusion device a communication including a clock time of the infusion device, receiving with the characteristic determining device the communication including the clock time of the infusion device, and setting a clock time of the characteristic determining device to the received clock time of the infusion device.
- the methodmay include the steps of transmitting with the characteristic determining device a communication including a clock time of the characteristic determining device, receiving with the infusion device the communication including the clock time of the characteristic determining device, and setting a clock time of the infusion device to the received clock time of the characteristic determining device.
- an infusion system for infusing a fluid into a body of a userincludes a characteristic determining device and an infusion device.
- the characteristic determining deviceincludes a determining device housing adapted to be carried by the user, a sensor coupled to the determining device housing for determining a concentration of an analyte in the user, a determining device processor contained in the determining device housing and coupled to the sensor for processing the determined concentration of the analyte from the sensor, and a determining device communication system contained in the determining device housing and coupled to the determining device processor for transmitting a communication including data indicative of the determined concentration of the analyte in the user.
- the infusion deviceincludes an infusion device housing adapted to be carried by the user, a drive mechanism contained in the infusion device housing and operatively coupled with a reservoir containing the fluid for infusing the fluid into the body of the user, an infusion device communication system contained in the infusion device housing for receiving the communication including the data indicative of the determined concentration of the analyte in the user from the determining device communication system, and an infusion device processor contained in the infusion device housing and coupled to the infusion device communication system for processing the data indicative of the determined concentration of the analyte in the user and controlling the infusion device.
- the determining device communication systemautomatically transmits the communication including the data indicative of the determined concentration of the analyte in the user to the infusion device communication system.
- the characteristic determining devicefurther includes a user input device for inputting commands, and the determining device communication system transmits the communication including the data indicative of the determined concentration of the analyte in the user to the infusion device communication system in response to a command from the user input device.
- the characteristic determining deviceincludes an indicator to indicate a status of the communication including the data indicative of the determined concentration of the analyte in the user being transmitted from the determining device communication system to the infusion device communication system.
- the infusion devicefurther includes a bolus estimator used in conjunction with the infusion device processor for calculating an estimated amount of fluid to be infused into the body of the user based upon the received data indicative of the determined concentration of the analyte in the user and a target concentration of the analyte in the user.
- the infusion devicealso includes an infusion device indicator to indicate when the estimated amount of fluid to be infused has been calculated.
- the infusion devicefurther includes a memory for storing data, and the data indicative of the determined concentration of the analyte in the user received by the infusion device communication system from the determining device communication system is stored in the memory of the infusion device.
- the determining device processorhas unique identification information
- the communication transmitted from the determining device communication system to the infusion device communication systemfurther includes the unique identification information of the determining device processor such that the infusion device is capable of discerning whether the communication is intended for receipt by the infusion device.
- the infusion device processorhas unique identification information
- the communication transmitted from the determining device communication system to the infusion device communication systemfurther includes the unique identification information of the infusion device processor such that the infusion device is capable of discerning whether the communication is intended for receipt by the infusion device.
- the determining device communication systemis capable of being deactivated and reactivated.
- the characteristic determining devicemay also include a memory for storing data indicative of the determined concentration of the analyte in the user that is determined when the determining device communication system is deactivated.
- the determining device communication systemthen transmits a communication including the stored data to the infusion device communication system when the determining device communication system is reactivated.
- the infusion device processoruses power cycling whereby power is periodically supplied to the infusion device communication system until a communication is received from the determining device communication system.
- the infusion device processordiscontinues using power cycling whereby the power is continuously supplied to the infusion device communication system when the communication including the data indicative of the determined concentration of the analyte in the user is received from the determining device communication system.
- the infusion device processorresumes using power cycling upon completing the receipt of the communication including the data indicative of the determined concentration of the analyte in the user from the determining device communication system.
- the infusion systemfurther includes a connector for coupling the characteristic determining device to a computer and downloading data from the characteristic determining device to the computer.
- the infusion device communication systemis further capable of transmitting a communication including infusion device data to be downloaded
- the determining device communication systemis further capable of receiving the communication including the infusion device data to be downloaded from the infusion device communication system. The received infusion device data is then downloaded from the characteristic determining device through the connector to the computer.
- the characteristic determining devicefurther includes a determining device clock
- the infusion devicefurther includes an infusion device clock.
- the infusion device communication systemfurther transmits a communication including a time of the infusion device clock
- the determining device communication systemfurther receives the communication including the time of the infusion device clock from the infusion device communication system.
- the determining device clockis then set to the received time of the infusion device clock.
- the determining device communication systemfurther transmits a communication including a time of the determining device clock
- the infusion device communication systemfurther receives the communication including the time of the determining device clock from the determining device communication system.
- the infusion device clockis then set to the received time of the determining device clock.
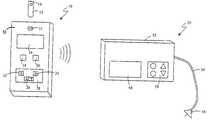
- FIG. 1is a perspective view of a blood glucose meter and an infusion pump in accordance with an embodiment of the present invention.
- FIG. 2is a simplified block diagram of an infusion pump in accordance with an embodiment of the present invention.
- FIG. 3( a )is a block diagram of an RF communication system in the infusion pump in accordance with an embodiment of the present invention.
- FIG. 3( b )is a block diagram of an RF communication system in the infusion pump in accordance with another embodiment of the present invention.
- FIG. 4( a )is a simplified block diagram of a blood glucose meter in accordance with an embodiment of the present invention.
- FIG. 4( b )is a simplified block diagram of a blood glucose meter in accordance with another embodiment of the present invention.
- FIG. 5is a simplified block diagram of a blood glucose meter in accordance with still another embodiment of the present invention.
- the inventionis embodied in a system for communicating blood glucose measurements from a blood glucose measurement device to an electronic computing device, which utilizes the blood glucose measurements to calculate a bolus estimate.
- the blood glucose (BG) measurement deviceis a blood glucose (BG) test strip meter
- the electronic computing deviceis an insulin delivery device, preferably an external insulin infusion pump.
- the BG meterutilizes a test strip with a sample of the user's blood to measure the user's BG level, and then transmits the BG measurement to the infusion pump using a communication system that includes, for example, a radio frequency (RF) transmitter or transceiver.
- RFradio frequency
- the infusion pumpreceives the BG measurement from the BG meter, and includes bolus estimation software to calculate a bolus estimate using the received BG measurement. The infusion pump may then deliver a bolus amount to the user based on the calculated bolus estimate. Transmission of the BG measurement from the BG meter to the infusion pump eliminates user transcription errors (i.e., the user may not accurately enter the BG measurement into the infusion pump) and simplifies the use of a bolus estimator.
- the BG metermay also function as a remote controller for the infusion pump, so the user can initiate a bolus delivery (without the bolus estimator) or stop a bolus delivery using buttons located on the BG meter.
- the BG metermay further function as a communications link for downloading data from the infusion pump to a computer or the like.
- the BG measurement devicemay be a continuous glucose measurement system, a hospital hemacue, an automated intermittent blood glucose measurement system, and the like, and/or the BG measurement device may use other methods for measuring the user's BG level, such as a sensor in contact with a body fluid, an optical sensor, an enzymatic sensor, a fluorescent sensor, a blood sample placed in a receptacle, or the like.
- the electronic computing devicemay be another type of insulin delivery device, such as an implantable insulin infusion pump or system that uses a combination of implantable and external components, an injection pen, an IV meter, and the like.
- the electronic computing devicemay be a computer, the Internet, a personal digital assistant (PDA), a portable telephone, a custom computing device, and the like.
- the BG measurement devicemay use samples from body fluids other than blood, such as interstitial fluid, spinal fluid, saliva, urine, tears, sweat, or the like.
- other measurement devicesmay be utilized to determine the concentrations, levels, or quantities of other characteristics, analytes, or agents in the user, such as hormones, cholesterol, oxygen, pH, lactate, heart rate, respiratory rate, medication concentrations, viral loads (e.g., HIV), or the like.
- other fluidsmay be delivered to the user, such as medication other than insulin (e.g., HIV drugs, drugs to treat pulmonary hypertension, iron chelation drugs, pain medications, and anti-cancer treatments), chemicals, enzymes, antigens, hormones, vitamins, or the like.
- medication other than insuline.g., HIV drugs, drugs to treat pulmonary hypertension, iron chelation drugs, pain medications, and anti-cancer treatments
- chemicals, enzymes, antigens, hormones, vitamins, or the likee.g., HIV drugs, drugs to treat pulmonary hypertension, iron chelation drugs, pain medications, and anti-cancer treatments
- enzymese.g., antigens, hormones, vitamins, or the like.
- the infusion devicesmay be used in animals.
- a blood glucose (BG) measurement devicemeasures a user's BG level and then communicates the BG measurement to an electronic computing device, which utilizes the BG measurement to calculate a bolus estimate.
- the BG measurement deviceis a BG test strip meter 10
- the electronic computing deviceis an insulin delivery device, preferably an external insulin infusion pump 50 .
- a housing 13 of the BG meter 10preferably includes a test strip receptacle or port 11 for receiving and analyzing a test strip 12 or the like with a sample of the user's blood 14 on the test strip 12 to obtain a BG measurement.
- the BG meter 10is adapted to be carried by the user, for example, in the hand, on the body, in a clothing pocket, attached to clothing (e.g., using a clip, strap, adhesive, or fastener), and the like.
- the usermay utilize a separate lancing device (not shown) to obtain a blood sample, and then apply the sample onto the test strip 12 .
- the BG meter 10may incorporate a lancing device (not shown) that obtains and automatically applies the blood sample onto the test strip 12 .
- the BG measurement devicemay be a continuous glucose measurement system, a hospital hemacue, an automated intermittent blood glucose measurement system, and the like, and/or the BG measurement device may use other methods for measuring the user's BG level, such as a sensor in contact with a body fluid, an optical sensor, an enzymatic sensor, a fluorescent sensor, a blood sample placed in a receptacle, or the like.
- the BG measurement devicemay generally be of the type and/or include features disclosed in U.S. patent application Ser. No. 09/377,472 filed Aug. 19, 1999 and entitled “Telemetered Characteristic Monitor System and Method of Using the Same,” Ser. No. 09/334,996 filed Jun.
- Such BG measurement devicesmay be adapted to be carried by the user, for example, in the hand, on the body, in a clothing pocket, attached to clothing (e.g., using a clip, strap, adhesive, or fastener), and the like.
- the BG measurement devicemay use samples from body fluids other than blood, such as interstitial fluid, spinal fluid, saliva, urine, tears, sweat, or the like.
- other characteristic determining or measuring devicesmay be utilized to determine or measure the concentrations, levels, or quantities of other characteristics, analytes, or agents in the user, such as hormones, cholesterol, oxygen, pH, lactate, heart rate, respiratory rate, medication concentrations, viral loads (e.g., HIV), or the like.
- the BG measurementis transmitted to the infusion pump 50 using a communication system, which includes a radio frequency (RF) transmitter 15 , as will be described below.
- RFradio frequency
- the RF transmitter 15may be replaced with an RF transceiver 19 (as shown in FIG. 4( b )) or 36 (as shown in FIG. 5), and the BG measurement may be transmitted to the infusion pump 50 using the RF transceiver 19 or 36 .
- the test strip port 11 and RF transmitter 15are coupled to a processor 17 contained in the housing 13 of the BG meter 10 .
- the processor 17runs programs and controls the BG meter 10 , and is also connected to a memory 30 for storing programs, history data, user defined information and parameters, and the like.
- the BG meter 10also preferably includes a display 16 for providing the BG measurement and/or messages, such as status or error messages, to the user.
- the display 16may include a backlight for reading the display 16 in the dark.
- the BG meter 10includes one or more buttons 18 and 20 for operation of the meter 10 , such as turning on/off the meter 10 , reviewing previous BG measurements, transmitting BG measurements to the infusion pump 50 , turning off the transmitter 15 (or transceiver 19 (shown in FIG. 4( b )) or 36 (shown in FIG. 5)) in the BG meter 10 so that it does not send a BG measurement to the infusion pump 50 , and the like.
- the BG meter 10may further include a keypad 28 with one or more buttons 22 , 24 , and 26 that are preferably dedicated to remotely controlling the infusion pump 50 , for example, via the RF transmitter 15 (or RF transceiver 19 (as shown in FIG. 4( b )) or 36 (as shown in FIG. 5)), as will be described below.
- the buttons 22 , 24 , and 26may also be used to transmit BG measurements to the infusion pump 50 .
- the buttons 22 , 24 , and 26may be labeled ‘S’ for “suspend”, ‘B’ for “bolus”, and ‘ACT’ for “activate”.
- buttons for operating the meter 10 and/or remotely controlling the infusion pump 50may be included on the meter 10 , and the buttons may be labeled other than as illustrated in FIG. 1.
- the BG meter 10may include an additional button for operating a lancing device (not shown) that is incorporated into the meter 10 .
- the buttons 22 , 24 , and 26may be omitted, and the buttons 18 and 20 may be used to remotely control the infusion pump 50 .
- the buttons 18 and 20may be omitted, and the buttons 22 , 24 , and 26 may be used to operate the BG meter 10 , or alternatively, no buttons may be needed to operate the meter 10 .
- the meter 10may include no buttons or other user interface or input device, and may be controlled using an external device, such as a remote programmer (not shown), the infusion pump 50 , a PDA, or the like.
- a remote programmernot shown
- the infusion pump 50a PDA
- one or more of the buttons 18 , 20 , 22 , 24 , and 26may be omitted, and the user may utilize other input devices to interface with the BG meter 10 , such as selecting a menu item, utilizing the display 16 as a touch screen, pressing multi-function keys, or the like.
- the BG meter 10In addition to transmitting the BG measurement to the infusion pump 50 , the BG meter 10 also preferably stores the BG measurement in the memory 30 of the BG meter 10 for subsequent analysis and review. A history of alarms or error messages generated by the BG meter 10 , as well as remote control commands sent to and/or information received from the infusion pump 50 , may also be stored in the memory 30 of the BG meter 10 . Further, the user may periodically cause the BG meter 10 to download the stored data through an interface (such as the RF transmitter 15 (or RF transceiver 19 (as shown in FIG. 4( b )) or 36 (as shown in FIG.
- an interfacesuch as the RF transmitter 15 (or RF transceiver 19 (as shown in FIG. 4( b )) or 36 (as shown in FIG.
- a connector 32may be inserted into the test strip port 11 to provide a wired connection to a USB, serial, or the like port of the computer 34 , and data may be downloaded from the BG meter 10 through the connector 32 to the computer 34 .
- the user or a caregivere.g., the user's parent, health care professional, educator
- can evaluate the user's therapyby accessing the historical BG measurements and insulin delivery information downloaded from the pump 50 , as will be described below.
- the electronic computing deviceis an insulin delivery device, preferably an external insulin infusion pump 50 .
- the infusion pump 50regulates the flow of fluid from the infusion pump 50 , through a flexible tube 54 , and into an infusion set 56 or the like that is adhered to the individual.
- Infusion sets 56that may be used as a delivery device are described in, but not limited to, U.S. Pat. Nos. 4,723,947; 4,755,173; 5,176,662; 5,584,813; and 6,056,718, which are herein incorporated by reference.
- the infusion pump 50may be of the type described in U.S. Pat. Nos.
- infusion pumps 50may be adapted to be carried by the user, for example, in the hand, on the body, in a clothing pocket, attached to clothing (e.g., using a clip, strap, adhesive, or fastener), and the like.
- infusion pumps 50may be used for delivery of fluid through an infusion set 56 into an individual's body.
- devices other than infusion pumps 50may be used for delivery of fluid into an individual's body, such as an implantable insulin infusion pump or system that uses a combination of implantable and external components, an injection pen, an IV meter, and the like.
- the electronic computing devicemay be a computer, the Internet, a personal digital assistant (PDA), a portable telephone, a custom computing device, and the like.
- PDApersonal digital assistant
- the infusion pump 50include an RF communication system 60 and a bolus estimator 62 .
- the RF communication system 60includes an RF receiver 80 , as shown in FIG. 3( a ), which allows one-way communication from the BG meter 10 (or other external devices such as a remote programmer for the infusion pump 50 ) to the infusion pump 50 .
- the RF communication system 60 ′may include an RF transceiver 81 , as shown in FIG. 3( b ), which allows two-way communication between the BG meter 10 (or other external devices such as a remote programmer for the infusion pump 50 ) and the infusion pump 50 .
- the RF communication system 60 and bolus estimator 62communicate with a processor 64 contained in a housing 52 of the infusion pump 50 .
- the processor 64is used to run programs and control the infusion pump 50 , and is connected to an internal memory device 66 that stores programs, history data, user defined information and parameters.
- the memory device 66is a ROM and DRAM; however, in alternative embodiments, the memory device 66 may include other memory storage devices, such as RAM, EPROM, dynamic storage such as flash memory, energy efficient hard-drive, or the like.
- the processor 64is also coupled to a drive mechanism 72 that is connected to a fluid reservoir 74 containing fluid, which is delivered through an outlet 76 in the reservoir 74 and housing 52 , and then into the user's body through the tubing 54 and the infusion set 56 .
- the infusion pump 50is preferably programmed through a user input device such as a keypad 58 on the housing 52 , or alternatively, by commands received from an RF programmer (not shown) through the RF communication system 60 .
- the infusion pump 50may also be programmed through the keypad 58 on the BG meter 10 , for example, through the RF communication system 60 , as will be described below.
- Feedback to the infusion pump 50 on status or programming changesare shown on a display 68 , audibly through a speaker 70 , and/or tactilely through a vibration alarm 78 .
- the infusion pump 50may also provide the user with an alarm either audibly via the speaker 70 and/or tactilely via the vibration alarm 78 , such as a warning that is indicative of a low reservoir situation or low battery. Alarms may start out at a low level and escalate until acknowledged by the user.
- the keypad 58may include more or less keys or different key arrangements than those illustrated in FIG. 1.
- the keypad 58may be omitted, and the display 68 may be used as a touch screen input device.
- the keypad 58 , display 68 , speaker 70 , and/or vibration alarm 78may be omitted, and all programming and data transfer may be handled through the RF communication system 60 .
- one-way communicationis provided from the BG meter 10 to the infusion pump 50 .
- the BG meter 10includes the RF transmitter 15 (shown in FIG. 4( a )), and the infusion pump 50 includes an RF receiver 80 (shown in FIG. 3( a )).
- two-way communicationis provided between the BG meter 10 and the infusion pump 50 .
- the RF transmitter 15 in the BG meter 10is replaced with an RF transceiver 19 (shown in FIG. 4( b )) or 36 (shown in FIG. 5), and the RF receiver 80 in the infusion pump 50 is replaced with an RF transceiver 81 (shown in FIG. 3( b )).
- the infusion pump 50may provide several programming options, including the bolus estimator 62 , as well as remote and on-device programming.
- the infusion pump 50may also be configured through an interface, such as a cable or communication station, using a computer or the like. Additionally, the infusion pump 50 may allow the user to download information in the memory 66 through the interface to a computer or the like, or alternatively, over the Internet to a remote server, for storage. Further description of a communication station of this general type may be found in U.S. Pat. No. 5,376,070, which is herein incorporated by reference.
- the user or a caregivere.g., the user's parent, health care professional, educator
- can evaluate the user's therapyby accessing the historical BG measurements downloaded from the BG meter 10 and insulin delivery information downloaded from the pump 50 .
- the RF communication system 60 ′may include the RF transceiver 81 for transmitting information to and receiving information from external devices.
- an external communication link(not shown) may be connected to a serial, USB, or the like port of a computer.
- Informationmay be transmitted from the RF transceiver 81 in the infusion pump 50 to an RF transceiver in the external communication link (not shown), which then downloads the information through a wired connection to the computer or the like.
- the communication linkmay draw power from the computer through the serial, USB, or the like port.
- the connector 32may be inserted into the test strip port of the BG meter 10 ′ to provide a wired connection to a USB, serial, or the like port of the computer 34 , as shown in FIG. 4( b ).
- Informationmay be transmitted from the RF transceiver 81 in the infusion pump 50 to the RF transceiver 19 in the BG meter 10 ′, and may then be downloaded through the connector 32 to the computer 34 .
- the BG meter 10 ′merely functions as a “pass through” connection between the infusion pump 50 and the computer 34 .
- powermay be drawn from the power supply (not shown) for the BG meter 10 ′ (e.g., battery or the like), or alternatively, from the USB, serial, or the like port of the computer 34 .
- informationmay be transmitted from the RF transceiver 81 in the infusion pump 50 to the RF transceiver 36 in the BG meter 10 ′′, as shown in FIG. 5.
- the informationmay be transmitted from the infusion pump 50 to the BG meter 10 ′′ at a rate higher than can be handled by the meter processor 17 ′′.
- the BG meter 10 ′′may include a communications microcontroller or processor 38 with a higher processing speed (e.g., 10 MHz) than the meter processor 17 ′′ with a lower processing speed (e.g., 1-4 MHz).
- the transmitted informationis first processed by the communications processor 38 , then processed by the meter processor 17 ′′, and finally downloaded through the connector 32 to the computer 34 .
- the BG meter 10 ′merely functions as a “pass through” connection between the infusion pump 50 and the computer 34 .
- informationmay be transmitted from the infusion pump 50 and stored in the memory 30 ′ or 30 ′′ of the BG meter 10 ′ or 10 ′′ for subsequent downloading from the BG meter 10 ′ or 10 ′′ to the computer 34 .
- informationmay be transmitted from the infusion pump 50 through the BG meter to the computer 34 using other modes of communication, such as infrared, optical, or the like.
- the infusion pump 50communicates with various external devices, such as the BG meter 10 , a remote programmer, and a communication station, using the RF communication system 60 , which will be described below.
- the infusion pump 50also provides a confirmation to the user upon receipt of a communication from another device (e.g., the BG meter 10 ).
- the infusion pump 50provides one or more audible signals when it has received a communication. More than one audible signal may be used, and each audible signal indicates the type of communication that was received.
- the infusion pump 50may beep 4 times when it has received a communication to deliver 0.4 units of insulin in a bolus, provide a long low tone when it has received a communication to suspend insulin delivery, and/or sound off with a two-tone “door bell” sound when a new BG measurement has been communicated.
- the infusion pump 50may provide other forms of confirmation when a communication has been received, such as one or more vibrations via the vibration alarm 78 , messages on the display 68 , lights or flashing lights, or the like.
- the BG meter 10automatically transmits the BG measurement to the infusion pump 50 .
- the BG meter 10analyzes the blood sample 14 on the test strip 12 to calculate a BG measurement and then transmits the BG measurement to the infusion pump 50 without additional effort by the user.
- the BG measurementis transmitted when the test strip 12 is removed from the BG meter 10 .
- the BG meter 10transmits the BG measurement in response to an action by the user.
- the BG meter 10may also retransmit the BG measurement to the infusion pump 50 in response to a user action, such as pressing a button, selecting a menu item, or holding down a button on the BG meter 10 , aligning the BG meter 10 and the infusion pump 50 , or the like.
- the BG meter 10is notified by the infusion pump 50 to transmit or retransmit the BG measurement.
- the infusion pump 50may provide an alarm or warning to the user if the received BG measurement is above or below glycemic limits.
- the glycemic limitsare preferably programmable, such as 120 mg/dl for hyperglycemia and 60 mg/dl for hypoglycemia.
- the user, a caregiver, a physician, a parent, a guardian, a child, or the likemay program other limits into the infusion pump 50 .
- the glycemic limitsare not programmable.
- the infusion pump 50will suspend insulin delivery if the received BG measurement is below the hypoglycemic limit.
- the infusion pump 50may also notify the user to activate a bolus delivery if the received BG measurement is above the hyperglycemic limit. In alternative embodiments, the infusion pump 50 does not compare the received BG measurement to glycemic limits, and does not suspend insulin delivery in the event of hypoglycemia or notify the user to activate bolus delivery in the event of hyperglycemia.
- the infusion pump 50also stores the received BG measurement in its memory 66 .
- the bolus estimator 62 in the infusion pump 50may utilize the received BG measurement to calculate a bolus estimate, either automatically or in response to user input, such as through the keypad 58 , a remote programmer, or the like. Once the bolus estimate is calculated and provided to the user, for example, on the display 68 , the user may then approve the recommended estimate for delivery into the body, modify the recommended estimate for delivery into the body, or reject the recommended estimate.
- the bolus estimator 62may generally be of the type and/or include features disclosed in U.S. patent application Ser. No. 09/334,858 filed Jun. 16, 1999, now issued U.S. Pat. No. 6,554,798 issued Apr. 29, 2003.
- the BG meter 10preferably informs the user of the status of the BG measurement calculation and/or transmission. If the infusion pump 50 is capable of only one-way communication, such notification is preferable because no confirmation is received from the infusion pump 50 indicating that the transmitted data has been received by the pump 50 .
- the BG meter 10may notify the user when a blood sample is being analyzed to obtain a BG measurement.
- the BG meter 10may also notify the user when the BG measurement is being transmitted to the infusion pump 50 .
- the BG meter 10may further notify the user when the transmission of the BG measurement is complete.
- the usermay access the bolus estimator 62 in the infusion pump 50 to view the BG measurement and calculate a bolus.
- the BG meter 10displays the status on the display 16 , for example, as an alphanumeric message, a graphical icon, or the like.
- the statusis communicated to the user in other ways, such as using one or more light emitting diodes, one or more audible tones, a speaker, a piezo electric device, a vibrator or other tactile device, or the like.
- the BG meter 10may not provide the status to the user. For example, if the BG measurement device provides continuous or automatic intermittent BG measurements, the user is not perpetually notified regarding the status of the calculations and/or transmissions.
- the BG meter 10keeps track of the elapsed time between when a BG measurement is collected and when it is communicated to the infusion pump 50 for calculating a bolus estimate.
- a BG measurementis preferably used to calculate a bolus estimate only if the BG measurement is recent enough.
- the bolus estimationis at least partially dependent on the difference between the user's present BG level and a desired target BG level. Since a user's BG level varies over time, using an old BG measurement to calculate a bolus estimation might result in a bolus estimation that is inappropriate for the user.
- a BG measurementis expired (and is not used for bolus estimation) when it is too old to be considered representative of the user's present BG level.
- the BG meter 10does not transmit a BG measurement to the infusion pump 50 for use in a bolus estimation calculation if the BG measurement is expired.
- the BG meter 10may also indicate to the user that a new BG measurement is required because the BG measurement is expired or unavailable.
- the BG measurementexpires at 10 minutes.
- the BG measurementmay expire in an amount of time greater or less than 10 minutes, such as 5 or 7 minutes, 15 or 30 minutes, 1 hour, or the like.
- the time required for a BG measurement to expiremay be set by the user, a caregiver, a physician, a parent, a guardian, a child, and the like.
- a child's BG levelmay change more quickly than that of a heavy adult, so the BG meter 10 may be set so that BG measurements older than 5 minutes cannot be communicated to the infusion pump 50 for use in a bolus estimation.
- an adultmight program the BG meter 10 so that BG measurements expire after 12 minutes.
- the time required for a BG measurement to expiremay be set depending on the time of the user's most recent bolus dose of medication.
- a first periodmay be set if the user has taken a bolus within a specified duration of time, and a second period may be set if the user has not taken a bolus within the specified duration of time.
- the time required for a BG measurement to expiremay be set to 5 minutes if the user has taken a bolus within the past 2 hours, and to 15 minutes if the user has not taken a bolus within the past 2 hours.
- the infusion pump 50does not use an expired BG measurement in a bolus estimation calculation.
- the infusion pump 50preferably keeps track of the time between when a new BG measurement is received from the BG meter 10 and when the new BG measurement is used in a bolus estimation calculation.
- the BG measurementis immediately transmitted to the infusion pump 50 , either automatically or in response to a user action.
- the pump 50when the infusion pump 50 receives a BG measurement, the pump 50 knows that the BG measurement was recent, and can calculate the approximate age of the BG measurement simply by determining the amount of time that has elapsed between when the BG measurement was received from the BG meter 10 and when the BG measurement is used in a bolus estimation calculation. In other particular embodiments, the infusion pump 50 is told the age of the BG measurements it receives. In other words, the elapsed time between when a BG measurement is collected and when it is communicated to the infusion pump 50 is transmitted along with each BG measurement.
- the infusion pump 50can calculate the age of the BG measurement by adding the age of BG measurement at the time it was transmitted to the time that has passed since the BG measurement was received. Since the infusion pump 50 knows the age of the BG measurement, the infusion pump 50 can eliminate BG measurements that are expired and/or prevent expired BG measurements from being used in a bolus estimation calculation. In particular embodiments, the infusion pump 50 will request a new BG measurement from the user when the user attempts to use a bolus estimator and the BG measurement is expired or unavailable.
- an estimate of the user's BG levelis used for bolus estimation.
- the user's BG levelis estimated using the last BG measurement, the age of the BG measurement, the amount of insulin that has been delivered, the insulin action time, the number of carbohydrates consumed, the carbohydrate/insulin ratio, and the like.
- the estimate of the user's BG levelwill expire if not used soon enough.
- the estimate of the user's BG levelmay only be calculated for a certain period after a BG measurement is collected.
- the length of time that a BG estimate may be calculated since a BG measurement was collectedis determined by the amount of insulin that has been delivered, the amount of carbohydrates the user has ingested, the user's insulin sensitivity, and/or by the user's insulin action time. For example, estimates of BG levels may be calculated for a longer period if the user has not eaten lately and is using only basal insulin. If the user has eaten or taken a bolus of insulin, then the period of time that an estimate of the user's BG level might be calculated is shorter.
- the BG meter 10communicates with the infusion pump 50 using RF communication. In alternative embodiments, other modes of communication may be used, such as infrared (IR), wired, ultrasonic, sonic, optical, and the like.
- the BG meter 10transmits one or more BG measurements to the infusion pump 50 .
- the BG meter 10may also communicate one or more remote control commands to the infusion pump 50 .
- the available commandspreferably include a bolus amount of insulin, a command to begin insulin delivery, and a command to suspend insulin delivery. In alternative embodiments, more or less remote control commands may be provided between the BG meter 10 and the infusion pump 50 .
- the RF transmitter 15(or RF transceiver 19 (as shown in FIG. 4( b )) or 36 (as shown in FIG. 5)) in the BG meter 10 transmits the data (e.g., BG measurements or remote control commands) to the RF communication system 60 in the infusion pump 50 .
- the infusion pump 50may communicate one or more user-defined parameters to the BG meter 10 (e.g., the time required for a BG measurement to expire).
- the RF transceiver 81 in the infusion pump 50(shown in FIG. 3( b )) transmits such parameters to the RF transceiver 19 (as shown in FIG. 4( b )) or 36 (as shown in FIG. 5) in the BG meter 10 ′ or 10 ′′.
- communication between the BG meter 10 and the infusion pump 50contains unique identifying information about the BG meter 10 and/or infusion pump 50 , such as the BG meter's 10 and/or infusion pump's 50 serial number, identification number, a password, a code, or the like.
- the unique identifying information about the BG meter 10 and/or infusion pump 50 included in the communication between the BG meter 10 and the infusion pump 50is used by the respective devices (i.e., BG meter 10 and/or infusion pump 50 ) to discern between communications that are intended for the device and those that are not.
- other codesmay be included in communications between the BG meter 10 and the infusion pump 50 that are used by the respective devices to recognize which communications are intended for the device, such as an identification code for the device, a password, a bit sequence, a special frequency, timing between communications, or the like.
- the communication system in the BG meter 10may be deactivated, preferably by the user.
- the BG meter 10will not attempt to communicate with other devices, including the infusion pump 50 .
- the BG meter 10will not communicate the BG measurement to another device, such as the infusion pump 50 .
- the BG meter 10includes an RF transmitter 15 (shown in FIG. 4( a )) (or RF transceiver 19 (shown in FIG. 4( b )) or 36 (shown in FIG. 5)) that can be deactivated and reactivated by the user.
- the BG meter 10transmits at frequencies that might disrupt an airplane during take-off.
- other devicesmay be used to deactivate and reactivate the communication system in the BG meter 10 , such as the infusion pump 50 , other insulin delivery device, a computer, PDA, portable telephone, or the like.
- the BG meter 10may be programmed to reactivate its communication system after a certain duration.
- the useris prompted to enter a duration for how long communication system is to be deactivated, and the communication system will automatically become active at the end of the duration.
- the usermay specify a time of day for the communication system to become active.
- all of the BG measurements that have been generated while the communication system was deactivatedare transmitted to the infusion pump 50 when the communication system is reactivated.
- Other datamay also be transmitted to the infusion pump 50 , such as the BG meter's clock time when the BG measurement was generated (i.e., the timestamp for the BG measurement), the age of the BG measurement, and the like.
- One-way communicationis preferably used between the BG meter 10 and the infusion pump 50 .
- the BG meter 10includes a transmitter 15
- the infusion pump 50includes a receiver 80 .
- the BG meter 10transmits data (e.g., BG measurements or remote control commands), and the infusion pump 50 receives this data.
- the benefits of one-way communicationinclude cheaper unit costs, less development time, and decreased battery power requirements.
- the drawback of one-way communicationis that there is no confirmation that the BG meter 10 has transmitted the data to the infusion pump 50 .
- two-way communicationmay be used, and the BG meter 10 may include a transceiver 19 (as shown in FIG. 4( b )) or 36 (as shown in FIG. 5), and the infusion pump 50 may include a transceiver 81 (as shown in FIG. 3( b )).
- the infusion pump 50uses power cycling to periodically supply power to its communication system.
- the infusion pump 50may not use power cycling, and instead, may continuously supply power to its communication system.
- the power cyclewhich is one period that the communication system is off plus one period that the communication system is on, is preferably 8 seconds.
- the power cyclemay be shorter or longer than 8 seconds, such as 2 or 4 seconds, 12 or 15 seconds, or the like.
- the period that the communication system is on during each power cycleis preferably 48 milliseconds (ms).
- the period that the communication system is on during each power cyclemay be greater or less than 48 ms, depending on the length of the message to be received, the communication frequency, the speed of the communication system electronics, and the like.
- the BG meter 10sends repeated signals to the infusion pump 50 for a period longer than the power cycle.
- the signal sent from the BG meter 10 to the infusion pump 50preferably includes a command that is short enough to be captured during the on-time of the infusion pump's communication system.
- the commandis short enough to be captured multiple times (i.e., two, three, or more times) during the on-time of the infusion pump's communication system.
- the time that the infusion pump's communication system must be on to capture the command from the BG meter 10is short compared to the power cycle.
- the commandis short compared to a string of information.
- the infusion pump 50stops power cycling the communication system and turns the communication system on continuously.
- the infusion pump 50may continue to use power cycling unless the command indicates that the pump 50 should prepare to receive a string of information.
- short commandsmay be used to activate the infusion pump's communication system so that one or more longer strings of information may be received by the infusion pump 50 .
- the infusion pump 50prepares to receive a string of information longer than a command.
- the string of informationpreferably includes a BG measurement.
- the string of informationmay further include an elapsed time since the BG measurement was taken.
- the string of informationmay include a clock time.
- the BG meter 10may transmit a clock time to the infusion pump 50 so that the infusion pump 50 can determine the difference between the BG meter's clock and the infusion pump's clock.
- the infusion pump 50may use the BG meter's clock time to reset the infusion pump's clock time.
- the infusion pump 50returns to power cycling the communication system after information has been received from the BG meter 10 .
- the infusion pump 50returns to power cycling after it receives a complete signal containing a BG measurement from the BG meter 10 .
- the infusion pump 50returns to power cycling at a predetermined period after a signal from the BG meter 10 has stopped.
- the infusion pump 50returns to power cycling at a predetermined period after receiving a signal from the BG meter 10 .
- the infusion pump 50preferably communicates with various external devices, such as the BG meter 10 , using the RF communication system 60 .
- the RF communication system 60includes an RF receiver 80 , an RF microcontroller 82 (RF PIC), and an application specific integrated circuit 84 (ASIC), as shown in FIG. 3( a ).
- the RF receiver 80may be replaced with an RF transceiver 81 , as shown in FIG. 3( b ).
- the RF PIC 82may hold a 7-byte word, although in alternative embodiments, the RF PIC 82 may hold other lengths of data.
- the processor 64communicates with the RF PIC 82 and the ASIC 84 using synchronous peripheral interfaces (SPI interfaces).
- SPI interfacessynchronous peripheral interfaces
- the RF receiver 80receives and demodulates RF signals, extracts a data packet from the RF signal, and passes the data packet to the RF PIC 82 .
- the RF PIC 82accepts and decodes the data packet and checks for format. If the format of the data packet is valid, the RF PIC 82 sends an interrupt signal to the ASIC 84 .
- the ASIC 84receives an interrupt signal from the RF PIC 82 , the ASIC 84 sends an interrupt to the processor 64 , triggering the processor 64 to notify the RF PIC 82 to pass the contents of its buffer to the processor 64 .
- the processor 64acquires the decoded data packet from the RF PIC 82 and evaluates the content, which may include a command or information to be stored. In response to some data packets, the processor 64 will send a command to the ASIC 84 to change the power conditions on the RF receiver 80 .
- the processor 64also processes the commands and information received from the BG meter 10 , which may result in changing the bolus delivery on the infusion pump 50 or entering a BG measurement into the bolus estimator 62 .
- One of the main tasks for the ASIC 84is to enable and disable power on the RF receiver 80 . Generally, the ASIC 84 cycles the power on the RF receiver 80 to save energy. If commanded by the processor 64 , however, the ASIC 84 will enable the RF receiver 80 to be powered continuously.
- Each RF transmission sent to the pumppreferably includes an RF signal header followed by a command packet or an information packet. Since the pump's RF receiver 80 is likely to wake up in the middle of a command packet, the RF signal header at the start of each transmission helps the pump 50 to synchronize its data sampling and identify the first byte of a new command packet or information packet.
- the RF signal headeris preferably the same for each transmission, and is transmitted at the start of each RF transmission.
- the RF signal headermay include two parts: a preamble and a start signature.
- the preambleis a series of pulses used to train the pump's digital signal sampling, and allows the pump 50 to synchronize its pulse sampling with the pulse bits in the new transmission.
- the start signaturenotifies the pump RF PIC 82 when the first byte of a new packet is starting.
- the RF signal headermay include other data.
- the RF signal headermay be omitted.
- command packetsare 7 bytes in length, and information packets are 71 bytes in length.
- the command packets and/or information packetsmay be of different lengths.
- the last byte of every command or information packetis an 8-bit cyclic redundancy check (CRC) calculated on all the preceding bytes in the packet.
- CRCcyclic redundancy check
- a command or information packetis sent by the BG meter 10 to the infusion pump 50 , it is encoded using a DC balanced encoding scheme, which translates 4 bits of data into 6 for transmission as follows: HEX DC HEX DC 0 010101 8 011010 1 110001 9 011001 2 110010 A 101010 3 100011 B 001011 4 110100 C 101100 5 100101 D 001101 6 100110 E 001110 7 010110 F 011100
- the result of the encodingis that the 7-byte command packets require transmission of 11 bytes and the 71-byte data packets require transmission of 107 bytes.
- the pump RF PIC 82 in the infusion pump 50decodes the packet into the 7-byte command packet or the 71-byte information packet.
- the processor 64checks all packets for valid identification of the infusion pump 50 (e.g., identification or serial number) and CRC. If the identification of the infusion pump 50 is not valid, the packet is ignored. If the CRC of the first command packet is not valid, the command is ignored. Otherwise, the processor 64 sends a negative acknowledge (NAK) response to any packet with an invalid CRC.
- NAKnegative acknowledge
- Information packets(71 bytes) are much larger than command packets (7 bytes), and cannot be stored in the pump RF PIC 82 , and thus, cannot be used to “wake up” the pump 50 . Instead, a command packet must be sent to the pump 50 to turn on the pump's RF receiver 80 and prepare the pump 50 to receive an information packet. While power to the infusion pump's communication system (i.e. RF receiver 80 ) is being cycled, a command packet is repeatedly transmitted from the BG meter 10 to the infusion pump 50 . If an RF signal (i.e. including the first command packet) is present when the pump's RF receiver 80 comes on, the pump 50 will attempt to store contents of the signal in the pump RF PIC 82 .
- RF signali.e. including the first command packet
- the processor 64will verify whether the content of the signal is a valid command packet. If the command packet is valid, then the pump 50 will stop power cycling and power the RF receiver 80 continuously. Only the first command packet must be transmitted repeatedly. After the RF receiver 80 is on full-time, other command packets can be sent to the pump 50 in quick succession (for example, as quickly as the user can press buttons on the BG meter 10 or other external device to send the new command packets). Additional command packets or an information packet may also be transmitted to the pump 50 .
- the pump 50preferably recognizes two categories of command packets: remote control or bolus commands and BG measurement commands.
- Remote control or bolus commandsdirectly control the pump's insulin bolus delivery.
- BG measurement commandsmay transmit a new BG measurement(s) from the BG meter 10 to the pump 50 , or alternatively, prepare the pump 50 to receive an information packet containing a new BG measurement value as well as other related data (e.g., a clock time or timestamp of the BG measurement, the age of the BG measurement, or the like) from the BG meter 10 .
- the pump 50may receive a bolus command from the BG meter 10 or a remote programmer associated with the pump 50 .
- the bolus commandpreferably includes a type code indicating the type of device transmitting the message (e.g., the BG meter 10 or the remote programmer), unique identifying information about the pump 50 (e.g., serial number, identification number, password, or the like), a key code indicating which bolus command button has been pressed (e.g., button “S” 22 , button “B” 24 , or button “ACT” 26 on the BG meter 10 ), and a counter indicating the number of times that the button has been pressed.
- a type codeindicating the type of device transmitting the message
- unique identifying information about the pump 50e.g., serial number, identification number, password, or the like
- a key code indicating which bolus command button has been pressede.g., button “S” 22 , button “B” 24 , or button “ACT”
- the bolus commandmay include other information and/or omit some of this data.
- the processor 64filters the command to discern the counter value so that the pump 50 can respond to the number of times the user has pressed the button to adjust a bolus.
- the pump 50may also receive a BG measurement command from the BG meter 10 .
- the BG measurement commandis transmitted to the pump 50 to send a new BG measurement(s) from the BG meter 10 to the pump 50 , or alternatively, to prepare the pump 50 to receive an information packet containing a new BG measurement as well as other related data (e.g., a clock time or timestamp of the BG measurement, the age of the BG measurement, or the like) from the BG meter 10 .
- the commandpreferably includes a type code indicating the type of device transmitting the message (e.g., the BG meter 10 ), the BG measurement value(s), and unique identifying information about the meter 10 and/or pump 50 (e.g., serial number, identification number, password, or the like).
- a type codeindicating the type of device transmitting the message (e.g., the BG meter 10 ), the BG measurement value(s), and unique identifying information about the meter 10 and/or pump 50 (e.g., serial number, identification number, password, or the like).
- the commandpreferably includes a type code indicating the type of device transmitting the message (e.g., the BG meter 10 ), unique identifying information about the meter 10 and/or pump 50 (e.g., serial number, identification number, password, or the like), and a key code indicating that a new BG measurement is about to be transmitted.
- the BG measurement commandmay include other information and/or omit some of this data.
- the pump 50In response to communications from the BG meter 10 , the pump 50 typically sends an acknowledge (ACK) response.
- the BG meter 10does not include an RF receiver, and the pump 50 does not include an RF transmitter, and thus, the pump 50 does not send an ACK response if the type code in the command (e.g., bolus or BG measurement command) indicates that the device transmitting the message is the BG meter 10 .
- both the BG meter 10 and the pump 50may include an RF transmitter and receiver (i.e. transceiver 19 (shown in FIG. 4( b )) or 36 (shown in FIG.
- the pump 50may send an ACK response to the BG meter 10 .
- the pump 50may send its clock time to the BG meter 10 , and the BG meter 10 may use the pump's clock time to reset the BG meter's clock time if the devices' clock times do not correspond with one another. Further, if the meter 10 does not receive an ACK response from the pump 50 , the meter 10 may attempt to retransmit the communication to the pump 50 , either immediately or at a later time.
- the processor 64will send a data packet through the ASIC 84 , commanding the RF receiver 80 to remain on full-time for a specified number of minutes, to receive other command packets or an information packet.
- the RF receiver 80may return to power cycling after the information packet has been received, a certain period of time after receiving a BG measurement command (in the event that the anticipated information packet does not arrive), a certain period of time after receiving a bolus command, or after the battery in the pump 50 has been removed and replaced.
- the pump RF PIC 82remains in receive mode unless it has received a command to send from the processor 64 , in which case it shall switch to transmit mode until the transmission is complete. Once the data has been transmitted, the pump RF PIC 82 automatically switches back to receive mode.
Landscapes
- Health & Medical Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Engineering & Computer Science (AREA)
- Public Health (AREA)
- General Health & Medical Sciences (AREA)
- Heart & Thoracic Surgery (AREA)
- Biomedical Technology (AREA)
- Veterinary Medicine (AREA)
- Animal Behavior & Ethology (AREA)
- Computer Networks & Wireless Communication (AREA)
- Vascular Medicine (AREA)
- Anesthesiology (AREA)
- Hematology (AREA)
- Physics & Mathematics (AREA)
- Biophysics (AREA)
- Pathology (AREA)
- Medical Informatics (AREA)
- Molecular Biology (AREA)
- Surgery (AREA)
- Infusion, Injection, And Reservoir Apparatuses (AREA)
- Measurement Of The Respiration, Hearing Ability, Form, And Blood Characteristics Of Living Organisms (AREA)
Abstract
Description
- This application claims priority from U.S. Provisional Applications No. 60/398,199 filed Jul. 24, 2002 and No. 60/412,998 filed Sep. 23, 2002, which are herein incorporated by reference.[0001]
- This invention relates generally to infusion systems that are used for infusing a fluid into a user, and in particular, to apparatuses and methods for providing blood glucose measurements to an infusion device.[0002]
- Patients with[0003]
Type 1 diabetes and some patients with Type 2 diabetes use insulin to control their blood glucose (BG) level. Typically, if a patient's BG level is too high, the patient can inject a “bolus” (dose) of insulin to lower his/her BG level from its present level to a desired target level. Furthermore, patients may inject a bolus of insulin in anticipation of ingesting carbohydrates, thus heading off a sharp rise in their BG level. Patients employ various calculations to determine the amount of insulin to inject. Bolus estimation software is available for calculating an insulin bolus. Patients may use these software programs on an electronic computing device, such as a computer, the Internet, a personal digital assistant (PDA), or an insulin delivery device. Insulin delivery devices include infusion pumps, injection pens, and IV meters. The best bolus estimation software takes into account the patient's present BG level. Presently, a patient must measure his/her blood glucose using a BG measurement device, such as a test strip meter, a continuous glucose measurement system, or a hospital hemacue. BG measurement devices use various methods to measure the BG level of a patient, such as a sample of the patient's blood, a sensor in contact with a bodily fluid, an optical sensor, an enzymatic sensor, or a fluorescent sensor. When the BG measurement device has generated a BG measurement, the measurement is displayed on the BG measurement device. Then the patient may visually read the BG measurement and physically enter the BG measurement into an electronic computing device to calculate a bolus estimate. Finally, once the bolus estimate is calculated, the patient must inject the insulin bolus or program an insulin delivery device to deliver the bolus into their body. Unfortunately, this process is cumbersome and is subject to transcribing errors—for example, the patient may inaccurately enter the BG measurement that is displayed on the BG measurement device into the electronic computing device. Thus, if the BG measurement is not entered correctly, the bolus estimate is not accurate, which may lead to the delivery of an inappropriate insulin dose. - In preferred embodiments of the present invention, an infusion system for infusing a fluid into a body of a user includes a characteristic determining device and an infusion device. The characteristic determining device includes a housing adapted to be carried by the user, a receptacle coupled to the housing for receiving and testing an analyte from the user to determine a concentration of the analyte in the user, a processor contained in the housing and coupled to the receptacle for processing the determined concentration of the analyte from the receptacle, and a communication system contained in the housing and coupled to the processor for transmitting a communication including data indicative of the determined concentration of the analyte in the user. In particular embodiments, the characteristic determining device may also include a lancing device coupled to the receptacle for obtaining the analyte from the user. In preferred embodiments, the infusion device includes a housing adapted to be carried by the user, a drive mechanism contained in the housing and operatively coupled with a reservoir containing the fluid for infusing the fluid into the body of the user, a communication system contained in the housing for receiving the communication including the data indicative of the determined concentration of the analyte in the user from the determining device, and a processor contained in the housing and coupled to the communication system for processing the data indicative of the determined concentration of the analyte in the user and controlling the infusion device. The infusion device further includes a bolus estimator used in conjunction with the processor for calculating an estimated amount of fluid to be infused into the body of the user based upon the received data indicative of the determined concentration of the analyte in the user and a target concentration of the analyte in the user, and an indicator to indicate when the estimated amount of fluid to be infused has been calculated. Additionally, the infusion device may include a user input device for inputting an estimate of a material to be ingested by the user, and the bolus estimator may include the capability to calculate the estimated amount of fluid to be infused into the body of the user based upon the inputted estimate of the material to be ingested by the user. The infusion device may also include a memory for storing the data indicative of the determined concentration of the analyte in the user received by the infusion device communication system from the determining device communication system.[0004]
- In particular embodiments, the characteristic determining device automatically transmits the communication including the data indicative of the determined concentration of the analyte in the user to the infusion device. In other particular embodiments, the characteristic determining device further includes a user input device for inputting commands, and transmits the communication including the data indicative of the determined concentration of the analyte in the user to the infusion device in response to a command from the user input device. In additional embodiments, the characteristic determining device further includes an indicator to indicate a status of the communication including the data indicative of the determined concentration of the analyte in the user being transmitted from the determining device communication system to the infusion device communication system.[0005]
- In some embodiments, the communication transmitted from the characteristic determining device to the infusion device further includes a time at which the concentration of the analyte in the user was determined. In additional embodiments, the processor of the characteristic determining device determines an amount of time that has elapsed since the concentration of the analyte in the user was determined, and the communication transmitted from the determining device to the infusion device further includes the elapsed amount of time. Further, the processor of the characteristic determining device may cause the communication system of the characteristic determining device not to transmit the communication including the data indicative of the determined concentration of the analyte in the user if the elapsed amount of time exceeds a predetermined amount of time. In other embodiments, the infusion device processor determines an amount of time that has elapsed since the data indicative of the determined concentration of the analyte in the user was received, and causes the bolus estimator not to calculate the estimated amount of fluid to be infused based upon the determined concentration of the analyte if the elapsed amount of time exceeds a predetermined amount of time. In still other embodiments, the processor of the infusion device determines an amount of time that has elapsed since the concentration of the analyte in the user was determined, and causes the bolus estimator not to calculate the estimated amount of fluid to be infused based upon the determined concentration of the analyte if the elapsed amount of time exceeds a predetermined amount of time.[0006]
- In further embodiments, the determining device communication system is capable of being deactivated and reactivated. The characteristic determining device includes a user input device for inputting commands, and the communication system of the characteristic determining device is capable of being deactivated in response to a first command from the user input device and being reactivated in response to a second command from the user input device. Alternatively, the communication system of the characteristic determining device may be automatically reactivated after a predetermined amount of time has elapsed or at a predetermined time of day. Additionally, the characteristic determining device may include a memory for storing data indicative of the determined concentration of the analyte in the user that is determined when the determining device communication system is deactivated, and the determining device communication system may transmit a communication including the stored data to the infusion device communication system when the determining device communication system is reactivated.[0007]
- In still other embodiments, the processor of the characteristic determining device has unique identification information, and the communication transmitted from the characteristic determining device to the infusion device further includes the unique identification information of the determining device processor such that the infusion device is capable of discerning whether the communication is intended for receipt by the infusion device. In yet other embodiments, the processor of the infusion device has unique identification information, and the communication transmitted from the characteristic determining device to the infusion device further includes the unique identification information of the infusion device processor such that the infusion device is capable of discerning whether the communication is intended for receipt by the infusion device.[0008]
- In preferred embodiments, the processor of the infusion device uses power cycling whereby power is periodically supplied to the communication system of the infusion device until a communication is received from the characteristic determining device. When a communication is received from the characteristic determining device, the processor of the infusion device discontinues using power cycling whereby the power is continuously supplied to the infusion device communication system. The infusion device processor may then resume using power cycling upon completing the receipt of the communication including the data indicative of the determined concentration of the analyte in the user from the determining device communication system.[0009]
- In particular embodiments, the infusion system further includes a connector for coupling the characteristic determining device to a computer and downloading data from the characteristic determining device to the computer. The communication system of the infusion device is further capable of transmitting a communication including infusion device data to be downloaded, and the communication system of the characteristic determining device is further capable of receiving the communication including the infusion device data to be downloaded from the infusion device. The received infusion device data is then downloaded from the characteristic determining device through the connector to the computer. Alternatively, the characteristic determining device may further include a memory for storing data, and the received infusion device data may be stored in the memory of the characteristic determining device for subsequent downloading through the connector to the computer.[0010]
- In other particular embodiments, the characteristic determining device further includes a user input device for inputting remote control commands for controlling the infusion device. The communication system of the characteristic determining device further transmits a communication including the remote control commands, and the communication system of the infusion device further receives the communication including the remote control commands from the characteristic determining device. The processor of the infusion device then controls the infusion device in accordance with the received remote control commands.[0011]
- In yet other particular embodiments, the infusion device further includes a user input device for inputting remote control commands for controlling the characteristic determining device. The communication system of the infusion device further transmits a communication including the remote control commands, and the communication system of the characteristic determining device further receives the communication including the remote control commands from the infusion device. The processor of the characteristic determining device then controls the characteristic determining device in accordance with the received remote control commands.[0012]
- In additional embodiments, the characteristic determining device further includes a determining device clock, and the infusion device further includes an infusion device clock. The infusion device communication system further transmits a communication including a time of the infusion device clock, and the determining device communication system further receives the communication including the time of the infusion device clock from the infusion device communication system. The determining device clock is then set to the received time of the infusion device clock. Alternatively, the determining device communication system further transmits a communication including a time of the determining device clock, and the infusion device communication system further receives the communication including the time of the determining device clock from the determining device communication system. The infusion device clock is then set to the received time of the determining device clock.[0013]
- In accordance with another embodiment of the present invention, an infusion device infuses a fluid into a body of a user and is capable of communicating with a characteristic determining device, which is adapted for determining a concentration of an analyte in the user. The infusion device includes a housing adapted to be carried by the user, a drive mechanism contained in the housing and operatively coupled with a reservoir containing the fluid for infusing the fluid into the body of the user, a communication system contained in the housing for receiving a communication including data indicative of the determined concentration of the analyte in the user from the characteristic determining device, and a processor contained in the housing and coupled to the communication system for processing the data indicative of the determined concentration of the analyte in the user and controlling the infusion device. The infusion device also includes a bolus estimator used in conjunction with the processor for calculating an estimated amount of fluid to be infused into the body of the user based upon the received data indicative of the determined concentration of the analyte in the user and a target concentration of the analyte in the user. The infusion device further includes an indicator to indicate when the estimated amount of fluid to be infused has been calculated.[0014]
- In accordance with still another embodiment of the present invention, a characteristic determining device determines a concentration of an analyte in a body of a user and is capable of communicating with an infusion device, which is adapted for infusing a fluid into the body of the user and calculating an estimated amount of the fluid to be infused into the body of the user based upon the determined concentration of the analyte in the user and a target concentration of the analyte in the user. The characteristic determining device includes a housing adapted to be carried by the user, a receptacle coupled to the housing for receiving and testing an analyte from the user to determine the concentration of the analyte in the user, a processor contained in the housing and coupled to the receptacle for processing the determined concentration of the analyte from the receptacle, and a communication system contained in the housing and coupled to the processor for transmitting a communication including data indicative of the determined concentration of the analyte in the user to the infusion device.[0015]
- According to yet another embodiment of the present invention, an infusion system includes a characteristic determining device and an infusion device, and a method for infusing a fluid into a body of a user is provided. The method includes the steps of: receiving and testing an analyte from the user to determine a concentration of the analyte in the user, transmitting with the characteristic determining device a communication including data indicative of the determined concentration of the analyte in the user, and receiving with the infusion device the communication including the data indicative of the determined concentration of the analyte in the user. The data indicative of the determined concentration of the analyte in the user received by the infusion device from the characteristic determining device may then be stored in a memory of the infusion device. The method further includes the steps of calculating an estimated amount of fluid to be infused into the body of the user based upon the received data indicative of the determined concentration of the analyte in the user and a target concentration of the analyte in the user, and indicating when the estimated amount of fluid to be infused has been calculated. Additionally, the method may include the step of inputting an estimate of a material to be ingested by the user, and the estimated amount of fluid to be infused into the body of the user is calculated further based upon the inputted estimate of the material to be ingested by the user.[0016]
- In some embodiments, the communication including the data indicative of the determined concentration of the analyte in the user is automatically transmitted from the characteristic determining device to the infusion device. In other embodiments, the communication including the data indicative of the determined concentration of the analyte in the user is transmitted from the characteristic determining device to the infusion device in response to an inputted command. In still other embodiments, the system indicates a status of the communication including the data indicative of the determined concentration of the analyte in the user being transmitted from the characteristic determining device to the infusion device.[0017]
- In particular embodiments, the communication including the data indicative of the determined concentration of the analyte in the user transmitted from the characteristic determining device to the infusion device further includes a time at which the concentration of the analyte in the user was determined. In other particular embodiments, the system also determines an amount of time that has elapsed since the concentration of the analyte in the user was determined. In yet other particular embodiments, the system determines an amount of time that has elapsed since the communication including the data indicative of the determined concentration of the analyte in the user was received by the infusion device.[0018]
- In additional embodiments, the method further includes the steps of transmitting with the infusion device a communication including a clock time of the infusion device, receiving with the characteristic determining device the communication including the clock time of the infusion device, and setting a clock time of the characteristic determining device to the received clock time of the infusion device. Alternatively, the method may include the steps of transmitting with the characteristic determining device a communication including a clock time of the characteristic determining device, receiving with the infusion device the communication including the clock time of the characteristic determining device, and setting a clock time of the infusion device to the received clock time of the characteristic determining device.[0019]
- In accordance with a further embodiment of the present invention, an infusion system for infusing a fluid into a body of a user includes a characteristic determining device and an infusion device. The characteristic determining device includes a determining device housing adapted to be carried by the user, a sensor coupled to the determining device housing for determining a concentration of an analyte in the user, a determining device processor contained in the determining device housing and coupled to the sensor for processing the determined concentration of the analyte from the sensor, and a determining device communication system contained in the determining device housing and coupled to the determining device processor for transmitting a communication including data indicative of the determined concentration of the analyte in the user. The infusion device includes an infusion device housing adapted to be carried by the user, a drive mechanism contained in the infusion device housing and operatively coupled with a reservoir containing the fluid for infusing the fluid into the body of the user, an infusion device communication system contained in the infusion device housing for receiving the communication including the data indicative of the determined concentration of the analyte in the user from the determining device communication system, and an infusion device processor contained in the infusion device housing and coupled to the infusion device communication system for processing the data indicative of the determined concentration of the analyte in the user and controlling the infusion device.[0020]
- In particular embodiments, the determining device communication system automatically transmits the communication including the data indicative of the determined concentration of the analyte in the user to the infusion device communication system. In other particular embodiments, the characteristic determining device further includes a user input device for inputting commands, and the determining device communication system transmits the communication including the data indicative of the determined concentration of the analyte in the user to the infusion device communication system in response to a command from the user input device. In further particular embodiments, the characteristic determining device includes an indicator to indicate a status of the communication including the data indicative of the determined concentration of the analyte in the user being transmitted from the determining device communication system to the infusion device communication system.[0021]
- In some embodiments, the infusion device further includes a bolus estimator used in conjunction with the infusion device processor for calculating an estimated amount of fluid to be infused into the body of the user based upon the received data indicative of the determined concentration of the analyte in the user and a target concentration of the analyte in the user. The infusion device also includes an infusion device indicator to indicate when the estimated amount of fluid to be infused has been calculated. In other embodiments, the infusion device further includes a memory for storing data, and the data indicative of the determined concentration of the analyte in the user received by the infusion device communication system from the determining device communication system is stored in the memory of the infusion device.[0022]
- In additional embodiments, the determining device processor has unique identification information, and the communication transmitted from the determining device communication system to the infusion device communication system further includes the unique identification information of the determining device processor such that the infusion device is capable of discerning whether the communication is intended for receipt by the infusion device. In yet additional embodiments, the infusion device processor has unique identification information, and the communication transmitted from the determining device communication system to the infusion device communication system further includes the unique identification information of the infusion device processor such that the infusion device is capable of discerning whether the communication is intended for receipt by the infusion device.[0023]
- In further embodiments, the determining device communication system is capable of being deactivated and reactivated. The characteristic determining device may also include a memory for storing data indicative of the determined concentration of the analyte in the user that is determined when the determining device communication system is deactivated. The determining device communication system then transmits a communication including the stored data to the infusion device communication system when the determining device communication system is reactivated.[0024]
- In still further embodiments, the infusion device processor uses power cycling whereby power is periodically supplied to the infusion device communication system until a communication is received from the determining device communication system. The infusion device processor discontinues using power cycling whereby the power is continuously supplied to the infusion device communication system when the communication including the data indicative of the determined concentration of the analyte in the user is received from the determining device communication system. Further, the infusion device processor resumes using power cycling upon completing the receipt of the communication including the data indicative of the determined concentration of the analyte in the user from the determining device communication system.[0025]
- In other embodiments, the infusion system further includes a connector for coupling the characteristic determining device to a computer and downloading data from the characteristic determining device to the computer. The infusion device communication system is further capable of transmitting a communication including infusion device data to be downloaded, and the determining device communication system is further capable of receiving the communication including the infusion device data to be downloaded from the infusion device communication system. The received infusion device data is then downloaded from the characteristic determining device through the connector to the computer.[0026]
- In yet other embodiments, the characteristic determining device further includes a determining device clock, and the infusion device further includes an infusion device clock. The infusion device communication system further transmits a communication including a time of the infusion device clock, and the determining device communication system further receives the communication including the time of the infusion device clock from the infusion device communication system. The determining device clock is then set to the received time of the infusion device clock. Alternatively, the determining device communication system further transmits a communication including a time of the determining device clock, and the infusion device communication system further receives the communication including the time of the determining device clock from the determining device communication system. The infusion device clock is then set to the received time of the determining device clock.[0027]
- A detailed description of embodiments of the invention will be made with reference to the accompanying drawings, wherein like numerals designate corresponding parts in the several figures.[0028]
- FIG. 1 is a perspective view of a blood glucose meter and an infusion pump in accordance with an embodiment of the present invention.[0029]
- FIG. 2 is a simplified block diagram of an infusion pump in accordance with an embodiment of the present invention.[0030]
- FIG. 3([0031]a) is a block diagram of an RF communication system in the infusion pump in accordance with an embodiment of the present invention.
- FIG. 3([0032]b) is a block diagram of an RF communication system in the infusion pump in accordance with another embodiment of the present invention.
- FIG. 4([0033]a) is a simplified block diagram of a blood glucose meter in accordance with an embodiment of the present invention.
- FIG. 4([0034]b) is a simplified block diagram of a blood glucose meter in accordance with another embodiment of the present invention.
- FIG. 5 is a simplified block diagram of a blood glucose meter in accordance with still another embodiment of the present invention.[0035]
- As shown in the drawings for purposes of illustration, the invention is embodied in a system for communicating blood glucose measurements from a blood glucose measurement device to an electronic computing device, which utilizes the blood glucose measurements to calculate a bolus estimate. In preferred embodiments, the blood glucose (BG) measurement device is a blood glucose (BG) test strip meter, and the electronic computing device is an insulin delivery device, preferably an external insulin infusion pump. The BG meter utilizes a test strip with a sample of the user's blood to measure the user's BG level, and then transmits the BG measurement to the infusion pump using a communication system that includes, for example, a radio frequency (RF) transmitter or transceiver. The infusion pump receives the BG measurement from the BG meter, and includes bolus estimation software to calculate a bolus estimate using the received BG measurement. The infusion pump may then deliver a bolus amount to the user based on the calculated bolus estimate. Transmission of the BG measurement from the BG meter to the infusion pump eliminates user transcription errors (i.e., the user may not accurately enter the BG measurement into the infusion pump) and simplifies the use of a bolus estimator. In particular embodiments, the BG meter may also function as a remote controller for the infusion pump, so the user can initiate a bolus delivery (without the bolus estimator) or stop a bolus delivery using buttons located on the BG meter. The BG meter may further function as a communications link for downloading data from the infusion pump to a computer or the like.[0036]
- However, in alternative embodiments of the present invention, the BG measurement device may be a continuous glucose measurement system, a hospital hemacue, an automated intermittent blood glucose measurement system, and the like, and/or the BG measurement device may use other methods for measuring the user's BG level, such as a sensor in contact with a body fluid, an optical sensor, an enzymatic sensor, a fluorescent sensor, a blood sample placed in a receptacle, or the like. In further alternative embodiments, the electronic computing device may be another type of insulin delivery device, such as an implantable insulin infusion pump or system that uses a combination of implantable and external components, an injection pen, an IV meter, and the like. In other alternative embodiments, the electronic computing device may be a computer, the Internet, a personal digital assistant (PDA), a portable telephone, a custom computing device, and the like. In still further alternative embodiments, the BG measurement device may use samples from body fluids other than blood, such as interstitial fluid, spinal fluid, saliva, urine, tears, sweat, or the like. In yet other alternative embodiments, other measurement devices may be utilized to determine the concentrations, levels, or quantities of other characteristics, analytes, or agents in the user, such as hormones, cholesterol, oxygen, pH, lactate, heart rate, respiratory rate, medication concentrations, viral loads (e.g., HIV), or the like. In still other alternative embodiments, other fluids may be delivered to the user, such as medication other than insulin (e.g., HIV drugs, drugs to treat pulmonary hypertension, iron chelation drugs, pain medications, and anti-cancer treatments), chemicals, enzymes, antigens, hormones, vitamins, or the like. Particular embodiments are directed towards the use in humans; however, in alternative embodiments, the infusion devices may be used in animals.[0037]
- In preferred embodiments of the present invention, a blood glucose (BG) measurement device measures a user's BG level and then communicates the BG measurement to an electronic computing device, which utilizes the BG measurement to calculate a bolus estimate. In the embodiment illustrated in FIG. 1, the BG measurement device is a BG[0038]
test strip meter 10, and the electronic computing device is an insulin delivery device, preferably an externalinsulin infusion pump 50. - Referring to FIGS. 1 and 4([0039]a), a
housing 13 of theBG meter 10 preferably includes a test strip receptacle orport 11 for receiving and analyzing atest strip 12 or the like with a sample of the user'sblood 14 on thetest strip 12 to obtain a BG measurement. TheBG meter 10 is adapted to be carried by the user, for example, in the hand, on the body, in a clothing pocket, attached to clothing (e.g., using a clip, strap, adhesive, or fastener), and the like. In particular embodiments, the user may utilize a separate lancing device (not shown) to obtain a blood sample, and then apply the sample onto thetest strip 12. In other particular embodiments, theBG meter 10 may incorporate a lancing device (not shown) that obtains and automatically applies the blood sample onto thetest strip 12. - In alternative embodiments, the BG measurement device may be a continuous glucose measurement system, a hospital hemacue, an automated intermittent blood glucose measurement system, and the like, and/or the BG measurement device may use other methods for measuring the user's BG level, such as a sensor in contact with a body fluid, an optical sensor, an enzymatic sensor, a fluorescent sensor, a blood sample placed in a receptacle, or the like. The BG measurement device may generally be of the type and/or include features disclosed in U.S. patent application Ser. No. 09/377,472 filed Aug. 19, 1999 and entitled “Telemetered Characteristic Monitor System and Method of Using the Same,” Ser. No. 09/334,996 filed Jun. 17, 1999 and entitled “Characteristic Monitor with a Characteristic Meter and Method of Using the Same,” Ser. No. 09/487,423 filed Jan. 20, 2000 and entitled “Handheld Personal Data Assistant (PDA) with a Medical Device and Method of Using the Same,” and Ser. No. 09/935,827 filed Aug. 23, 2001 and entitled “Handheld Personal Data Assistant (PDA) with a Medical Device and Method of Using the Same,” which are herein incorporated by reference. Such BG measurement devices may be adapted to be carried by the user, for example, in the hand, on the body, in a clothing pocket, attached to clothing (e.g., using a clip, strap, adhesive, or fastener), and the like. In further alternative embodiments, the BG measurement device may use samples from body fluids other than blood, such as interstitial fluid, spinal fluid, saliva, urine, tears, sweat, or the like. In yet other alternative embodiments, other characteristic determining or measuring devices may be utilized to determine or measure the concentrations, levels, or quantities of other characteristics, analytes, or agents in the user, such as hormones, cholesterol, oxygen, pH, lactate, heart rate, respiratory rate, medication concentrations, viral loads (e.g., HIV), or the like.[0040]
- In particular embodiments, once the[0041]
BG meter 10 obtains a BG measurement, the BG measurement is transmitted to theinfusion pump 50 using a communication system, which includes a radio frequency (RF)transmitter 15, as will be described below. In other particular embodiments, theRF transmitter 15 may be replaced with an RF transceiver19 (as shown in FIG. 4(b)) or36 (as shown in FIG. 5), and the BG measurement may be transmitted to theinfusion pump 50 using theRF transceiver - The[0042]
test strip port 11 andRF transmitter 15 are coupled to aprocessor 17 contained in thehousing 13 of theBG meter 10. Theprocessor 17 runs programs and controls theBG meter 10, and is also connected to amemory 30 for storing programs, history data, user defined information and parameters, and the like. TheBG meter 10 also preferably includes adisplay 16 for providing the BG measurement and/or messages, such as status or error messages, to the user. In particular embodiments, thedisplay 16 may include a backlight for reading thedisplay 16 in the dark. - In preferred embodiments, the[0043]
BG meter 10 includes one ormore buttons meter 10, such as turning on/off themeter 10, reviewing previous BG measurements, transmitting BG measurements to theinfusion pump 50, turning off the transmitter15 (or transceiver19 (shown in FIG. 4(b)) or36 (shown in FIG. 5)) in theBG meter 10 so that it does not send a BG measurement to theinfusion pump 50, and the like. TheBG meter 10 may further include akeypad 28 with one ormore buttons infusion pump 50, for example, via the RF transmitter15 (or RF transceiver19 (as shown in FIG. 4(b)) or36 (as shown in FIG. 5)), as will be described below. Thebuttons infusion pump 50. Thebuttons meter 10 and/or remotely controlling theinfusion pump 50 may be included on themeter 10, and the buttons may be labeled other than as illustrated in FIG. 1. For example, theBG meter 10 may include an additional button for operating a lancing device (not shown) that is incorporated into themeter 10. In further alternative embodiments, thebuttons buttons infusion pump 50. In other alternative embodiments, thebuttons buttons BG meter 10, or alternatively, no buttons may be needed to operate themeter 10. For example, themeter 10 may include no buttons or other user interface or input device, and may be controlled using an external device, such as a remote programmer (not shown), theinfusion pump 50, a PDA, or the like. In yet other alternative embodiments, one or more of thebuttons BG meter 10, such as selecting a menu item, utilizing thedisplay 16 as a touch screen, pressing multi-function keys, or the like. - In addition to transmitting the BG measurement to the[0044]
infusion pump 50, theBG meter 10 also preferably stores the BG measurement in thememory 30 of theBG meter 10 for subsequent analysis and review. A history of alarms or error messages generated by theBG meter 10, as well as remote control commands sent to and/or information received from theinfusion pump 50, may also be stored in thememory 30 of theBG meter 10. Further, the user may periodically cause theBG meter 10 to download the stored data through an interface (such as the RF transmitter15 (or RF transceiver19 (as shown in FIG. 4(b)) or36 (as shown in FIG. 5)), a cable, a communication station, or the like), to acomputer 34, or alternatively, over the Internet to a remote server for storage. In particular embodiments, aconnector 32 may be inserted into thetest strip port 11 to provide a wired connection to a USB, serial, or the like port of thecomputer 34, and data may be downloaded from theBG meter 10 through theconnector 32 to thecomputer 34. The user or a caregiver (e.g., the user's parent, health care professional, educator) can evaluate the user's therapy by accessing the historical BG measurements and insulin delivery information downloaded from thepump 50, as will be described below. - In the embodiment illustrated in FIGS. 1 and 2, the electronic computing device is an insulin delivery device, preferably an external[0045]
insulin infusion pump 50. Theinfusion pump 50 regulates the flow of fluid from theinfusion pump 50, through aflexible tube 54, and into an infusion set56 or the like that is adhered to the individual. Infusion sets56 that may be used as a delivery device are described in, but not limited to, U.S. Pat. Nos. 4,723,947; 4,755,173; 5,176,662; 5,584,813; and 6,056,718, which are herein incorporated by reference. Theinfusion pump 50 may be of the type described in U.S. Pat. Nos. 4,562,751; 4,685,903; 5,080,653; 5,097,122; 5,505,709; and 6,248,093; and disclosed in U.S. patent application Ser. No. 09/334,858 (attorney docket PD-0294), filed Jun. 17, 1999 and entitled “Infusion Pump With Remote Programming and Carbohydrate Calculator Capabilities,” which are herein incorporated by reference. Such infusion pumps50 may be adapted to be carried by the user, for example, in the hand, on the body, in a clothing pocket, attached to clothing (e.g., using a clip, strap, adhesive, or fastener), and the like. Alternatively, other infusion pumps50 may be used for delivery of fluid through an infusion set56 into an individual's body. In further alternative embodiments, devices other than infusion pumps50 may be used for delivery of fluid into an individual's body, such as an implantable insulin infusion pump or system that uses a combination of implantable and external components, an injection pen, an IV meter, and the like. In other alternative embodiments, the electronic computing device may be a computer, the Internet, a personal digital assistant (PDA), a portable telephone, a custom computing device, and the like. - As illustrated in FIGS. 1 and 2, preferred embodiments of the[0046]
infusion pump 50 include anRF communication system 60 and abolus estimator 62. In particular embodiments, theRF communication system 60 includes anRF receiver 80, as shown in FIG. 3(a), which allows one-way communication from the BG meter10 (or other external devices such as a remote programmer for the infusion pump50) to theinfusion pump 50. In other particular embodiments, theRF communication system 60′ may include anRF transceiver 81, as shown in FIG. 3(b), which allows two-way communication between the BG meter10 (or other external devices such as a remote programmer for the infusion pump50) and theinfusion pump 50. - The[0047]
RF communication system 60 andbolus estimator 62 communicate with aprocessor 64 contained in ahousing 52 of theinfusion pump 50. Theprocessor 64 is used to run programs and control theinfusion pump 50, and is connected to aninternal memory device 66 that stores programs, history data, user defined information and parameters. In preferred embodiments, thememory device 66 is a ROM and DRAM; however, in alternative embodiments, thememory device 66 may include other memory storage devices, such as RAM, EPROM, dynamic storage such as flash memory, energy efficient hard-drive, or the like. In the illustrated embodiment, theprocessor 64 is also coupled to adrive mechanism 72 that is connected to afluid reservoir 74 containing fluid, which is delivered through an outlet76 in thereservoir 74 andhousing 52, and then into the user's body through thetubing 54 and the infusion set56. - The[0048]
infusion pump 50 is preferably programmed through a user input device such as akeypad 58 on thehousing 52, or alternatively, by commands received from an RF programmer (not shown) through theRF communication system 60. Theinfusion pump 50 may also be programmed through thekeypad 58 on theBG meter 10, for example, through theRF communication system 60, as will be described below. Feedback to theinfusion pump 50 on status or programming changes are shown on adisplay 68, audibly through aspeaker 70, and/or tactilely through a vibration alarm78. Theinfusion pump 50 may also provide the user with an alarm either audibly via thespeaker 70 and/or tactilely via the vibration alarm78, such as a warning that is indicative of a low reservoir situation or low battery. Alarms may start out at a low level and escalate until acknowledged by the user. In alternative embodiments, thekeypad 58 may include more or less keys or different key arrangements than those illustrated in FIG. 1. In further alternative embodiments, thekeypad 58 may be omitted, and thedisplay 68 may be used as a touch screen input device. In other alternative embodiments, thekeypad 58,display 68,speaker 70, and/or vibration alarm78 may be omitted, and all programming and data transfer may be handled through theRF communication system 60. - In particular embodiments, one-way communication is provided from the[0049]
BG meter 10 to theinfusion pump 50. TheBG meter 10 includes the RF transmitter15 (shown in FIG. 4(a)), and theinfusion pump 50 includes an RF receiver80 (shown in FIG. 3(a)). In other particular embodiments, two-way communication is provided between theBG meter 10 and theinfusion pump 50. TheRF transmitter 15 in theBG meter 10 is replaced with an RF transceiver19 (shown in FIG. 4(b)) or36 (shown in FIG. 5), and theRF receiver 80 in theinfusion pump 50 is replaced with an RF transceiver81 (shown in FIG. 3(b)). - The[0050]
infusion pump 50 may provide several programming options, including thebolus estimator 62, as well as remote and on-device programming. Theinfusion pump 50 may also be configured through an interface, such as a cable or communication station, using a computer or the like. Additionally, theinfusion pump 50 may allow the user to download information in thememory 66 through the interface to a computer or the like, or alternatively, over the Internet to a remote server, for storage. Further description of a communication station of this general type may be found in U.S. Pat. No. 5,376,070, which is herein incorporated by reference. The user or a caregiver (e.g., the user's parent, health care professional, educator) can evaluate the user's therapy by accessing the historical BG measurements downloaded from theBG meter 10 and insulin delivery information downloaded from thepump 50. - Information may also be downloaded from the[0051]
infusion pump 50 through theRF communication system 60. Referring to FIG. 3(b), theRF communication system 60′ may include theRF transceiver 81 for transmitting information to and receiving information from external devices. In particular embodiments, an external communication link (not shown) may be connected to a serial, USB, or the like port of a computer. Information may be transmitted from theRF transceiver 81 in theinfusion pump 50 to an RF transceiver in the external communication link (not shown), which then downloads the information through a wired connection to the computer or the like. During the download process, the communication link may draw power from the computer through the serial, USB, or the like port. In other particular embodiments, theconnector 32 may be inserted into the test strip port of theBG meter 10′ to provide a wired connection to a USB, serial, or the like port of thecomputer 34, as shown in FIG. 4(b). Information may be transmitted from theRF transceiver 81 in theinfusion pump 50 to theRF transceiver 19 in theBG meter 10′, and may then be downloaded through theconnector 32 to thecomputer 34. TheBG meter 10′ merely functions as a “pass through” connection between theinfusion pump 50 and thecomputer 34. During the download process, power may be drawn from the power supply (not shown) for theBG meter 10′ (e.g., battery or the like), or alternatively, from the USB, serial, or the like port of thecomputer 34. In still other particular embodiments, information may be transmitted from theRF transceiver 81 in theinfusion pump 50 to theRF transceiver 36 in theBG meter 10″, as shown in FIG. 5. The information may be transmitted from theinfusion pump 50 to theBG meter 10″ at a rate higher than can be handled by themeter processor 17″. Accordingly, theBG meter 10″ may include a communications microcontroller orprocessor 38 with a higher processing speed (e.g., 10 MHz) than themeter processor 17″ with a lower processing speed (e.g., 1-4 MHz). The transmitted information is first processed by thecommunications processor 38, then processed by themeter processor 17″, and finally downloaded through theconnector 32 to thecomputer 34. Again, theBG meter 10′ merely functions as a “pass through” connection between theinfusion pump 50 and thecomputer 34. In alternative embodiments, information may be transmitted from theinfusion pump 50 and stored in thememory 30′ or30″ of theBG meter 10′ or10″ for subsequent downloading from theBG meter 10′ or10″ to thecomputer 34. In further alternative embodiments, information may be transmitted from theinfusion pump 50 through the BG meter to thecomputer 34 using other modes of communication, such as infrared, optical, or the like. - In preferred embodiments, the[0052]
infusion pump 50 communicates with various external devices, such as theBG meter 10, a remote programmer, and a communication station, using theRF communication system 60, which will be described below. Theinfusion pump 50 also provides a confirmation to the user upon receipt of a communication from another device (e.g., the BG meter10). In particular embodiments, theinfusion pump 50 provides one or more audible signals when it has received a communication. More than one audible signal may be used, and each audible signal indicates the type of communication that was received. For example, theinfusion pump 50 may beep 4 times when it has received a communication to deliver 0.4 units of insulin in a bolus, provide a long low tone when it has received a communication to suspend insulin delivery, and/or sound off with a two-tone “door bell” sound when a new BG measurement has been communicated. In alternative embodiments, theinfusion pump 50 may provide other forms of confirmation when a communication has been received, such as one or more vibrations via the vibration alarm78, messages on thedisplay 68, lights or flashing lights, or the like. - In preferred embodiments, once the[0053]
BG meter 10 obtains a BG measurement, theBG meter 10 automatically transmits the BG measurement to theinfusion pump 50. In particular embodiments, theBG meter 10 analyzes theblood sample 14 on thetest strip 12 to calculate a BG measurement and then transmits the BG measurement to theinfusion pump 50 without additional effort by the user. In alternative embodiments, the BG measurement is transmitted when thetest strip 12 is removed from theBG meter 10. In other alternative embodiments, theBG meter 10 transmits the BG measurement in response to an action by the user. TheBG meter 10 may also retransmit the BG measurement to theinfusion pump 50 in response to a user action, such as pressing a button, selecting a menu item, or holding down a button on theBG meter 10, aligning theBG meter 10 and theinfusion pump 50, or the like. In alternative embodiments, theBG meter 10 is notified by theinfusion pump 50 to transmit or retransmit the BG measurement. - Once the[0054]
infusion pump 50 receives the BG measurement, theinfusion pump 50 may provide an alarm or warning to the user if the received BG measurement is above or below glycemic limits. The glycemic limits are preferably programmable, such as 120 mg/dl for hyperglycemia and 60 mg/dl for hypoglycemia. The user, a caregiver, a physician, a parent, a guardian, a child, or the like may program other limits into theinfusion pump 50. In alternative embodiments, the glycemic limits are not programmable. In preferred embodiments, theinfusion pump 50 will suspend insulin delivery if the received BG measurement is below the hypoglycemic limit. Theinfusion pump 50 may also notify the user to activate a bolus delivery if the received BG measurement is above the hyperglycemic limit. In alternative embodiments, theinfusion pump 50 does not compare the received BG measurement to glycemic limits, and does not suspend insulin delivery in the event of hypoglycemia or notify the user to activate bolus delivery in the event of hyperglycemia. - In preferred embodiments, the[0055]
infusion pump 50 also stores the received BG measurement in itsmemory 66. Further, thebolus estimator 62 in theinfusion pump 50 may utilize the received BG measurement to calculate a bolus estimate, either automatically or in response to user input, such as through thekeypad 58, a remote programmer, or the like. Once the bolus estimate is calculated and provided to the user, for example, on thedisplay 68, the user may then approve the recommended estimate for delivery into the body, modify the recommended estimate for delivery into the body, or reject the recommended estimate. Thebolus estimator 62 may generally be of the type and/or include features disclosed in U.S. patent application Ser. No. 09/334,858 filed Jun. 16, 1999, now issued U.S. Pat. No. 6,554,798 issued Apr. 29, 2003. - The[0056]
BG meter 10 preferably informs the user of the status of the BG measurement calculation and/or transmission. If theinfusion pump 50 is capable of only one-way communication, such notification is preferable because no confirmation is received from theinfusion pump 50 indicating that the transmitted data has been received by thepump 50. TheBG meter 10 may notify the user when a blood sample is being analyzed to obtain a BG measurement. TheBG meter 10 may also notify the user when the BG measurement is being transmitted to theinfusion pump 50. TheBG meter 10 may further notify the user when the transmission of the BG measurement is complete. Once notified that the transmission of the BG measurement is complete, the user may access thebolus estimator 62 in theinfusion pump 50 to view the BG measurement and calculate a bolus. In preferred embodiments, theBG meter 10 displays the status on thedisplay 16, for example, as an alphanumeric message, a graphical icon, or the like. In alternative embodiments, the status is communicated to the user in other ways, such as using one or more light emitting diodes, one or more audible tones, a speaker, a piezo electric device, a vibrator or other tactile device, or the like. In further alternative embodiments, theBG meter 10 may not provide the status to the user. For example, if the BG measurement device provides continuous or automatic intermittent BG measurements, the user is not perpetually notified regarding the status of the calculations and/or transmissions. - In preferred embodiments, the[0057]
BG meter 10 keeps track of the elapsed time between when a BG measurement is collected and when it is communicated to theinfusion pump 50 for calculating a bolus estimate. A BG measurement is preferably used to calculate a bolus estimate only if the BG measurement is recent enough. The bolus estimation is at least partially dependent on the difference between the user's present BG level and a desired target BG level. Since a user's BG level varies over time, using an old BG measurement to calculate a bolus estimation might result in a bolus estimation that is inappropriate for the user. A BG measurement is expired (and is not used for bolus estimation) when it is too old to be considered representative of the user's present BG level. TheBG meter 10 does not transmit a BG measurement to theinfusion pump 50 for use in a bolus estimation calculation if the BG measurement is expired. TheBG meter 10 may also indicate to the user that a new BG measurement is required because the BG measurement is expired or unavailable. In preferred embodiments, the BG measurement expires at 10 minutes. In alternative embodiments, the BG measurement may expire in an amount of time greater or less than 10 minutes, such as 5 or 7 minutes, 15 or 30 minutes, 1 hour, or the like. In further alternative embodiments, the time required for a BG measurement to expire may be set by the user, a caregiver, a physician, a parent, a guardian, a child, and the like. For example, a child's BG level may change more quickly than that of a heavy adult, so theBG meter 10 may be set so that BG measurements older than 5 minutes cannot be communicated to theinfusion pump 50 for use in a bolus estimation. To continue the example, an adult might program theBG meter 10 so that BG measurements expire after 12 minutes. Furthermore, the time required for a BG measurement to expire may be set depending on the time of the user's most recent bolus dose of medication. A first period may be set if the user has taken a bolus within a specified duration of time, and a second period may be set if the user has not taken a bolus within the specified duration of time. For example, the time required for a BG measurement to expire may be set to 5 minutes if the user has taken a bolus within the past 2 hours, and to 15 minutes if the user has not taken a bolus within the past 2 hours. - In preferred embodiments, the[0058]
infusion pump 50 does not use an expired BG measurement in a bolus estimation calculation. The infusion pump50 preferably keeps track of the time between when a new BG measurement is received from theBG meter 10 and when the new BG measurement is used in a bolus estimation calculation. In particular embodiments, once theBG meter 10 obtains a BG measurement, the BG measurement is immediately transmitted to theinfusion pump 50, either automatically or in response to a user action. Thus, when theinfusion pump 50 receives a BG measurement, thepump 50 knows that the BG measurement was recent, and can calculate the approximate age of the BG measurement simply by determining the amount of time that has elapsed between when the BG measurement was received from theBG meter 10 and when the BG measurement is used in a bolus estimation calculation. In other particular embodiments, theinfusion pump 50 is told the age of the BG measurements it receives. In other words, the elapsed time between when a BG measurement is collected and when it is communicated to theinfusion pump 50 is transmitted along with each BG measurement. Then, theinfusion pump 50 can calculate the age of the BG measurement by adding the age of BG measurement at the time it was transmitted to the time that has passed since the BG measurement was received. Since theinfusion pump 50 knows the age of the BG measurement, theinfusion pump 50 can eliminate BG measurements that are expired and/or prevent expired BG measurements from being used in a bolus estimation calculation. In particular embodiments, theinfusion pump 50 will request a new BG measurement from the user when the user attempts to use a bolus estimator and the BG measurement is expired or unavailable. - In alternative embodiments, an estimate of the user's BG level is used for bolus estimation. In particular alternative embodiments, the user's BG level is estimated using the last BG measurement, the age of the BG measurement, the amount of insulin that has been delivered, the insulin action time, the number of carbohydrates consumed, the carbohydrate/insulin ratio, and the like. In further alternative embodiments, the estimate of the user's BG level will expire if not used soon enough. In still further alternative embodiments, the estimate of the user's BG level may only be calculated for a certain period after a BG measurement is collected. In other alternative embodiments, the length of time that a BG estimate may be calculated since a BG measurement was collected is determined by the amount of insulin that has been delivered, the amount of carbohydrates the user has ingested, the user's insulin sensitivity, and/or by the user's insulin action time. For example, estimates of BG levels may be calculated for a longer period if the user has not eaten lately and is using only basal insulin. If the user has eaten or taken a bolus of insulin, then the period of time that an estimate of the user's BG level might be calculated is shorter.[0059]
- In preferred embodiments, the[0060]
BG meter 10 communicates with theinfusion pump 50 using RF communication. In alternative embodiments, other modes of communication may be used, such as infrared (IR), wired, ultrasonic, sonic, optical, and the like. TheBG meter 10 transmits one or more BG measurements to theinfusion pump 50. TheBG meter 10 may also communicate one or more remote control commands to theinfusion pump 50. The available commands preferably include a bolus amount of insulin, a command to begin insulin delivery, and a command to suspend insulin delivery. In alternative embodiments, more or less remote control commands may be provided between theBG meter 10 and theinfusion pump 50. The RF transmitter15 (or RF transceiver19 (as shown in FIG. 4(b)) or36 (as shown in FIG. 5)) in theBG meter 10 transmits the data (e.g., BG measurements or remote control commands) to theRF communication system 60 in theinfusion pump 50. Additionally, theinfusion pump 50 may communicate one or more user-defined parameters to the BG meter10 (e.g., the time required for a BG measurement to expire). TheRF transceiver 81 in the infusion pump50 (shown in FIG. 3(b)) transmits such parameters to the RF transceiver19 (as shown in FIG. 4(b)) or36 (as shown in FIG. 5) in theBG meter 10′ or10″. - In preferred embodiments, communication between the[0061]
BG meter 10 and theinfusion pump 50 contains unique identifying information about theBG meter 10 and/orinfusion pump 50, such as the BG meter's10 and/or infusion pump's50 serial number, identification number, a password, a code, or the like. In particular embodiments, the unique identifying information about theBG meter 10 and/or infusion pump50 included in the communication between theBG meter 10 and theinfusion pump 50 is used by the respective devices (i.e.,BG meter 10 and/or infusion pump50) to discern between communications that are intended for the device and those that are not. In alternative embodiments, other codes may be included in communications between theBG meter 10 and theinfusion pump 50 that are used by the respective devices to recognize which communications are intended for the device, such as an identification code for the device, a password, a bit sequence, a special frequency, timing between communications, or the like. - In preferred embodiments, the communication system in the[0062]
BG meter 10 may be deactivated, preferably by the user. When the communication system is deactivated, theBG meter 10 will not attempt to communicate with other devices, including theinfusion pump 50. For example, when a new BG measurement is available, theBG meter 10 will not communicate the BG measurement to another device, such as theinfusion pump 50. In particular embodiments, theBG meter 10 includes an RF transmitter15 (shown in FIG. 4(a)) (or RF transceiver19 (shown in FIG. 4(b)) or36 (shown in FIG. 5)) that can be deactivated and reactivated by the user. This is especially useful if theBG meter 10 transmits at frequencies that might disrupt an airplane during take-off. In alternative embodiments, other devices may be used to deactivate and reactivate the communication system in theBG meter 10, such as theinfusion pump 50, other insulin delivery device, a computer, PDA, portable telephone, or the like. In preferred embodiments, theBG meter 10 may be programmed to reactivate its communication system after a certain duration. In particular embodiments, when the user deactivates the BG meter's communication system, the user is prompted to enter a duration for how long communication system is to be deactivated, and the communication system will automatically become active at the end of the duration. In alternative embodiments, the user may specify a time of day for the communication system to become active. In particular embodiments, all of the BG measurements that have been generated while the communication system was deactivated are transmitted to theinfusion pump 50 when the communication system is reactivated. Other data may also be transmitted to theinfusion pump 50, such as the BG meter's clock time when the BG measurement was generated (i.e., the timestamp for the BG measurement), the age of the BG measurement, and the like. - One-way communication is preferably used between the[0063]
BG meter 10 and theinfusion pump 50. TheBG meter 10 includes atransmitter 15, and theinfusion pump 50 includes areceiver 80. For example, theBG meter 10 transmits data (e.g., BG measurements or remote control commands), and theinfusion pump 50 receives this data. The benefits of one-way communication (compared to two-way) include cheaper unit costs, less development time, and decreased battery power requirements. However, the drawback of one-way communication is that there is no confirmation that theBG meter 10 has transmitted the data to theinfusion pump 50. Accordingly, in alternative embodiments, two-way communication may be used, and theBG meter 10 may include a transceiver19 (as shown in FIG. 4(b)) or36 (as shown in FIG. 5), and theinfusion pump 50 may include a transceiver81 (as shown in FIG. 3(b)). - In preferred embodiments, the[0064]
infusion pump 50 uses power cycling to periodically supply power to its communication system. In alternative embodiments, theinfusion pump 50 may not use power cycling, and instead, may continuously supply power to its communication system. The power cycle, which is one period that the communication system is off plus one period that the communication system is on, is preferably 8 seconds. In alternative embodiments, the power cycle may be shorter or longer than 8 seconds, such as 2 or 4 seconds, 12 or 15 seconds, or the like. Further, the period that the communication system is on during each power cycle is preferably 48 milliseconds (ms). In alternative embodiments, the period that the communication system is on during each power cycle may be greater or less than 48 ms, depending on the length of the message to be received, the communication frequency, the speed of the communication system electronics, and the like. In preferred embodiments, theBG meter 10 sends repeated signals to theinfusion pump 50 for a period longer than the power cycle. The signal sent from theBG meter 10 to theinfusion pump 50 preferably includes a command that is short enough to be captured during the on-time of the infusion pump's communication system. In particular embodiments, the command is short enough to be captured multiple times (i.e., two, three, or more times) during the on-time of the infusion pump's communication system. - In preferred embodiments, the time that the infusion pump's communication system must be on to capture the command from the[0065]
BG meter 10 is short compared to the power cycle. In further embodiments, the command is short compared to a string of information. When theinfusion pump 50 receives a command, theinfusion pump 50 stops power cycling the communication system and turns the communication system on continuously. Alternatively, when theinfusion pump 50 receives a command, theinfusion pump 50 may continue to use power cycling unless the command indicates that thepump 50 should prepare to receive a string of information. Thus, short commands may be used to activate the infusion pump's communication system so that one or more longer strings of information may be received by theinfusion pump 50. - In particular embodiments, the[0066]
infusion pump 50 prepares to receive a string of information longer than a command. The string of information preferably includes a BG measurement. The string of information may further include an elapsed time since the BG measurement was taken. In alternative embodiments, the string of information may include a clock time. In further alternative embodiments, theBG meter 10 may transmit a clock time to theinfusion pump 50 so that theinfusion pump 50 can determine the difference between the BG meter's clock and the infusion pump's clock. In other alternative embodiments, theinfusion pump 50 may use the BG meter's clock time to reset the infusion pump's clock time. - In preferred embodiments, the[0067]
infusion pump 50 returns to power cycling the communication system after information has been received from theBG meter 10. In particular embodiments, theinfusion pump 50 returns to power cycling after it receives a complete signal containing a BG measurement from theBG meter 10. In alternative embodiments, theinfusion pump 50 returns to power cycling at a predetermined period after a signal from theBG meter 10 has stopped. In other alternative embodiments, theinfusion pump 50 returns to power cycling at a predetermined period after receiving a signal from theBG meter 10. - As described above, the[0068]
infusion pump 50 preferably communicates with various external devices, such as theBG meter 10, using theRF communication system 60. In particular embodiments, theRF communication system 60 includes anRF receiver 80, an RF microcontroller82 (RF PIC), and an application specific integrated circuit84 (ASIC), as shown in FIG. 3(a). In other particular embodiments, theRF receiver 80 may be replaced with anRF transceiver 81, as shown in FIG. 3(b). TheRF PIC 82 may hold a 7-byte word, although in alternative embodiments, theRF PIC 82 may hold other lengths of data. Theprocessor 64 communicates with theRF PIC 82 and theASIC 84 using synchronous peripheral interfaces (SPI interfaces). - The[0069]
RF receiver 80 receives and demodulates RF signals, extracts a data packet from the RF signal, and passes the data packet to theRF PIC 82. TheRF PIC 82 accepts and decodes the data packet and checks for format. If the format of the data packet is valid, theRF PIC 82 sends an interrupt signal to theASIC 84. When theASIC 84 receives an interrupt signal from theRF PIC 82, theASIC 84 sends an interrupt to theprocessor 64, triggering theprocessor 64 to notify theRF PIC 82 to pass the contents of its buffer to theprocessor 64. Theprocessor 64 acquires the decoded data packet from theRF PIC 82 and evaluates the content, which may include a command or information to be stored. In response to some data packets, theprocessor 64 will send a command to theASIC 84 to change the power conditions on theRF receiver 80. Theprocessor 64 also processes the commands and information received from theBG meter 10, which may result in changing the bolus delivery on theinfusion pump 50 or entering a BG measurement into thebolus estimator 62. One of the main tasks for theASIC 84 is to enable and disable power on theRF receiver 80. Generally, theASIC 84 cycles the power on theRF receiver 80 to save energy. If commanded by theprocessor 64, however, theASIC 84 will enable theRF receiver 80 to be powered continuously. - Each RF transmission sent to the pump preferably includes an RF signal header followed by a command packet or an information packet. Since the pump's[0070]
RF receiver 80 is likely to wake up in the middle of a command packet, the RF signal header at the start of each transmission helps thepump 50 to synchronize its data sampling and identify the first byte of a new command packet or information packet. The RF signal header is preferably the same for each transmission, and is transmitted at the start of each RF transmission. The RF signal header may include two parts: a preamble and a start signature. The preamble is a series of pulses used to train the pump's digital signal sampling, and allows thepump 50 to synchronize its pulse sampling with the pulse bits in the new transmission. The start signature notifies thepump RF PIC 82 when the first byte of a new packet is starting. In alternative embodiments, the RF signal header may include other data. In further alternative embodiments, the RF signal header may be omitted. - In particular embodiments, command packets are 7 bytes in length, and information packets are 71 bytes in length. In alternative embodiments, the command packets and/or information packets may be of different lengths. The last byte of every command or information packet is an 8-bit cyclic redundancy check (CRC) calculated on all the preceding bytes in the packet. Before a command or information packet is sent by the[0071]
BG meter 10 to theinfusion pump 50, it is encoded using a DC balanced encoding scheme, which translates 4 bits of data into 6 for transmission as follows:HEX DC HEX DC 0 010101 8 011010 1 110001 9 011001 2 110010 A 101010 3 100011 B 001011 4 110100 C 101100 5 100101 D 001101 6 100110 E 001110 7 010110 F 011100 - The result of the encoding is that the 7-byte command packets require transmission of 11 bytes and the 71-byte data packets require transmission of 107 bytes. Upon receipt of the 11-byte or 107-byte packets from the[0072]
BG meter 10, thepump RF PIC 82 in theinfusion pump 50 decodes the packet into the 7-byte command packet or the 71-byte information packet. Theprocessor 64 then checks all packets for valid identification of the infusion pump50 (e.g., identification or serial number) and CRC. If the identification of theinfusion pump 50 is not valid, the packet is ignored. If the CRC of the first command packet is not valid, the command is ignored. Otherwise, theprocessor 64 sends a negative acknowledge (NAK) response to any packet with an invalid CRC. - Information packets (71 bytes) are much larger than command packets (7 bytes), and cannot be stored in the[0073]
pump RF PIC 82, and thus, cannot be used to “wake up” thepump 50. Instead, a command packet must be sent to thepump 50 to turn on the pump'sRF receiver 80 and prepare thepump 50 to receive an information packet. While power to the infusion pump's communication system (i.e. RF receiver80) is being cycled, a command packet is repeatedly transmitted from theBG meter 10 to theinfusion pump 50. If an RF signal (i.e. including the first command packet) is present when the pump'sRF receiver 80 comes on, thepump 50 will attempt to store contents of the signal in thepump RF PIC 82. Theprocessor 64 will verify whether the content of the signal is a valid command packet. If the command packet is valid, then thepump 50 will stop power cycling and power theRF receiver 80 continuously. Only the first command packet must be transmitted repeatedly. After theRF receiver 80 is on full-time, other command packets can be sent to thepump 50 in quick succession (for example, as quickly as the user can press buttons on theBG meter 10 or other external device to send the new command packets). Additional command packets or an information packet may also be transmitted to thepump 50. - The[0074]
pump 50 preferably recognizes two categories of command packets: remote control or bolus commands and BG measurement commands. Remote control or bolus commands directly control the pump's insulin bolus delivery. BG measurement commands may transmit a new BG measurement(s) from theBG meter 10 to thepump 50, or alternatively, prepare thepump 50 to receive an information packet containing a new BG measurement value as well as other related data (e.g., a clock time or timestamp of the BG measurement, the age of the BG measurement, or the like) from theBG meter 10. - The[0075]
pump 50 may receive a bolus command from theBG meter 10 or a remote programmer associated with thepump 50. The bolus command preferably includes a type code indicating the type of device transmitting the message (e.g., theBG meter 10 or the remote programmer), unique identifying information about the pump50 (e.g., serial number, identification number, password, or the like), a key code indicating which bolus command button has been pressed (e.g., button “S”22, button “B”24, or button “ACT”26 on the BG meter10), and a counter indicating the number of times that the button has been pressed. In alternative embodiments, the bolus command may include other information and/or omit some of this data. When thepump 50 receives the bolus command, theprocessor 64 filters the command to discern the counter value so that thepump 50 can respond to the number of times the user has pressed the button to adjust a bolus. - The[0076]
pump 50 may also receive a BG measurement command from theBG meter 10. The BG measurement command is transmitted to thepump 50 to send a new BG measurement(s) from theBG meter 10 to thepump 50, or alternatively, to prepare thepump 50 to receive an information packet containing a new BG measurement as well as other related data (e.g., a clock time or timestamp of the BG measurement, the age of the BG measurement, or the like) from theBG meter 10. If the BG measurement command transmits a new BG measurement(s) from theBG meter 10 to theinfusion pump 50, the command preferably includes a type code indicating the type of device transmitting the message (e.g., the BG meter10), the BG measurement value(s), and unique identifying information about themeter 10 and/or pump50 (e.g., serial number, identification number, password, or the like). If the BG measurement command is transmitted to prepare thepump 50 to receive an information packet containing a BG measurement and other related data from theBG meter 10, the command preferably includes a type code indicating the type of device transmitting the message (e.g., the BG meter10), unique identifying information about themeter 10 and/or pump50 (e.g., serial number, identification number, password, or the like), and a key code indicating that a new BG measurement is about to be transmitted. In alternative embodiments, the BG measurement command may include other information and/or omit some of this data. - In response to communications from the[0077]
BG meter 10, thepump 50 typically sends an acknowledge (ACK) response. However, in particular embodiments, theBG meter 10 does not include an RF receiver, and thepump 50 does not include an RF transmitter, and thus, thepump 50 does not send an ACK response if the type code in the command (e.g., bolus or BG measurement command) indicates that the device transmitting the message is theBG meter 10. In alternative embodiments, both theBG meter 10 and thepump 50 may include an RF transmitter and receiver (i.e. transceiver19 (shown in FIG. 4(b)) or36 (shown in FIG. 5) in theBG meter 10′ or10″, and transceiver81 (shown in FIG. 3(b)) in the infusion pump50), and thus, thepump 50 may send an ACK response to theBG meter 10. Additionally, thepump 50 may send its clock time to theBG meter 10, and theBG meter 10 may use the pump's clock time to reset the BG meter's clock time if the devices' clock times do not correspond with one another. Further, if themeter 10 does not receive an ACK response from thepump 50, themeter 10 may attempt to retransmit the communication to thepump 50, either immediately or at a later time. - When the[0078]
pump 50 receives a command packet from theBG meter 10, theprocessor 64 will send a data packet through theASIC 84, commanding theRF receiver 80 to remain on full-time for a specified number of minutes, to receive other command packets or an information packet. TheRF receiver 80 may return to power cycling after the information packet has been received, a certain period of time after receiving a BG measurement command (in the event that the anticipated information packet does not arrive), a certain period of time after receiving a bolus command, or after the battery in thepump 50 has been removed and replaced. - The[0079]
pump RF PIC 82 remains in receive mode unless it has received a command to send from theprocessor 64, in which case it shall switch to transmit mode until the transmission is complete. Once the data has been transmitted, thepump RF PIC 82 automatically switches back to receive mode. - While the description above refers to particular embodiments of the present invention, it will be understood that many modifications may be made without departing from the spirit thereof. The accompanying claims are intended to cover such modifications as would fall within the true scope and spirit of the present invention.[0080]
- The presently disclosed embodiments are therefore to be considered in all respects as illustrative and not restrictive, the scope of the invention being indicated by the appended claims, rather than the foregoing description, and all changes which come within the meaning and range of equivalency of the claims are therefore intended to be embraced therein.[0081]
Claims (94)
1. An infusion system for infusing a fluid into a body of a user, the infusion system comprising:
a characteristic determining device including:
a determining device housing adapted to be carried by the user;
a receptacle coupled to the determining device housing for receiving and testing an analyte from the user to determine a concentration of the analyte in the user;
a determining device processor contained in the determining device housing and coupled to the receptacle for processing the determined concentration of the analyte from the receptacle; and
a determining device communication system contained in the determining device housing and coupled to the determining device processor for transmitting a communication including data indicative of the determined concentration of the analyte in the user; and
an infusion device including:
an infusion device housing adapted to be carried by the user;
a drive mechanism contained in the infusion device housing and operatively coupled with a reservoir containing the fluid for infusing the fluid into the body of the user;
an infusion device communication system contained in the infusion device housing for receiving the communication including the data indicative of the determined concentration of the analyte in the user from the determining device communication system;
an infusion device processor contained in the infusion device housing and coupled to the infusion device communication system for processing the data indicative of the determined concentration of the analyte in the user and controlling the infusion device;
a bolus estimator used in conjunction with the infusion device processor for calculating an estimated amount of fluid to be infused into the body of the user based upon the received data indicative of the determined concentration of the analyte in the user-and a target concentration of the analyte in the user; and
an infusion device indicator to indicate when the estimated amount of fluid to be infused has been calculated.
2. The infusion system according toclaim 1 , wherein the determining device communication system automatically transmits the communication including the data indicative of the determined concentration of the analyte in the user to the infusion device communication system.
3. The infusion system according toclaim 1 , wherein the characteristic determining device further includes a user input device for inputting commands, and the determining device communication system transmits the communication including the data indicative of the determined concentration of the analyte in the user to the infusion device communication system in response to a command from the user input device.
4. The infusion system according toclaim 1 , wherein the characteristic determining device further includes an indicator to indicate a status of the communication including the data indicative of the determined concentration of the analyte in the user being transmitted from the determining device communication system to the infusion device communication system.
5. The infusion system according toclaim 1 , wherein the communication transmitted from the determining device communication system to the infusion device communication system further includes a time at which the concentration of the analyte in the user was determined.
6. The infusion system according toclaim 1 , wherein the determining device processor determines an amount of time that has elapsed since the concentration of the analyte in the user was determined, and wherein the communication transmitted from the determining device communication system to the infusion device communication system further includes the elapsed amount of time.
7. The infusion system according toclaim 1 , wherein the determining device processor determines an amount of time that has elapsed since the concentration of the analyte in the user was determined, and causes the determining device communication system not to transmit the communication including the data indicative of the determined concentration of the analyte in the user if the elapsed amount of time exceeds a predetermined amount of time.
8. The infusion system according toclaim 1 , wherein the infusion device processor determines an amount of time that has elapsed since the data indicative of the determined concentration of the analyte in the user was received, and causes the bolus estimator not to calculate the estimated amount of fluid to be infused based upon the determined concentration of the analyte if the elapsed amount of time exceeds a predetermined amount of time.
9. The infusion system according toclaim 1 , wherein the infusion device processor determines an amount of time that has elapsed since the concentration of the analyte in the user was determined, and causes the bolus estimator not to calculate the estimated amount of fluid to be infused based upon the determined concentration of the analyte if the elapsed amount of time exceeds a predetermined amount of time.
10. The infusion system according toclaim 1 , wherein the determining device processor has unique identification information, and further wherein the communication transmitted from the determining device communication system to the infusion device communication system further includes the unique identification information of the determining device processor such that the infusion device is capable of discerning whether the communication is intended for receipt by the infusion device.
11. The infusion system according toclaim 1 , wherein the infusion device processor has unique identification information, and further wherein the communication transmitted from the determining device communication system to the infusion device communication system further includes the unique identification information of the infusion device processor such that the infusion device is capable of discerning whether the communication is intended for receipt by the infusion device.
12. The infusion system according toclaim 1 , wherein the determining device communication system is capable of being deactivated and reactivated.
13. The infusion system according toclaim 12 , wherein the characteristic determining device further includes a user input device for inputting commands, and further wherein the determining device communication system is capable of being deactivated in response to a first command from the user input device and being reactivated in response to a second command from the user input device.
14. The infusion system according toclaim 12 , wherein the characteristic determining device further includes a user input device for inputting commands, and the determining device communication system is capable of being deactivated in response to a command from the user input device, and further wherein the determining device processor causes the determining device communication system to be automatically reactivated after a predetermined amount of time has elapsed.
15. The infusion system according toclaim 12 , wherein the characteristic determining device further includes a user input device for inputting commands, and the determining device communication system is capable of being deactivated in response to a command from the user input device, and further wherein the determining device processor causes the determining device communication system to be automatically reactivated at a predetermined time of day.
16. The infusion system according toclaim 12 , wherein the characteristic determining device further includes a memory for storing data indicative of the determined concentration of the analyte in the user that is determined when the determining device communication system is deactivated, and further wherein the determining device communication system transmits a communication including the stored data to the infusion device communication system when the determining device communication system is reactivated.
17. The infusion system according toclaim 1 , wherein the infusion device processor uses power cycling whereby power is periodically supplied to the infusion device communication system until a communication is received from the determining device communication system, and further wherein the infusion device processor discontinues using power cycling whereby the power is continuously supplied to the infusion device communication system when a communication is received from the determining device communication system.
18. The infusion system according toclaim 1 , wherein the infusion device processor uses power cycling whereby power is periodically supplied to the infusion device communication system until a communication is received from the determining device communication system, and further wherein the infusion device processor discontinues using power cycling whereby the power is continuously supplied to the infusion device communication system when the communication including the data indicative of the determined concentration of the analyte in the user is received from the determining device communication system.
19. The infusion system according toclaim 18 , wherein the infusion device processor resumes using power cycling upon completing the receipt of the communication including the data indicative of the determined concentration of the analyte in the user from the determining device communication system.
20. The infusion system according toclaim 1 , further including a connector for coupling the characteristic determining device to a computer and downloading data from the characteristic determining device to the computer, wherein the infusion device communication system is further capable of transmitting a communication including infusion device data to be downloaded, and the determining device communication system is further capable of receiving the communication including the infusion device data to be downloaded from the infusion device communication system, and further wherein the received infusion device data is downloaded from the characteristic determining device through the connector to the computer.
21. The infusion system according toclaim 1 , further including a connector for coupling the characteristic determining device to a computer and downloading data from the characteristic determining device to the computer, wherein the infusion device communication system is further capable of transmitting a communication including infusion device data to be downloaded, and the determining device communication system is further capable of receiving the communication including the infusion device data to be downloaded from the infusion device communication system, wherein the characteristic determining device further includes a memory for storing data, and further wherein the received infusion device data is stored in the memory of the characteristic determining device for subsequent downloading through the connector to the computer.
22. The infusion system according toclaim 1 , wherein the infusion device indicator further indicates when the determined concentration of the analyte in the user is above or below a predetermined level of the analyte in the user.
23. The infusion system according toclaim 1 , wherein the determining device communication system and the infusion device communication system communicate using one of radio frequencies and infrared frequencies.
24. The infusion system according toclaim 1 , wherein the concentration of the analyte in the user determined by the characteristic determining device is a blood glucose level of the user, and the fluid infused by the infusion device is insulin.
25. The infusion system according toclaim 24 , wherein the characteristic determining device is a blood glucose test strip meter, and the infusion device is an insulin infusion pump.
26. The infusion system according toclaim 1 , wherein the characteristic determining device further includes a user input device for inputting remote control commands for controlling the infusion device, and wherein the determining device communication system further transmits a communication including the remote control commands, and the infusion device communication system further receives the communication including the remote control commands from the determining device communication system, and further wherein the infusion device processor controls the infusion device in accordance with the received remote control commands.
27. The infusion system according toclaim 1 , wherein the infusion device further includes a user input device for inputting remote control commands for controlling the characteristic determining device, and wherein the infusion device communication system further transmits a communication including the remote control commands, and the determining device communication system further receives the communication including the remote control commands from the infusion device communication system, and further wherein the determining device processor controls the characteristic determining device in accordance with the received remote control commands.
28. The infusion system according toclaim 1 , wherein the determining device communication system includes one of a transmitter and a transceiver, and the infusion device communication system includes one of a receiver and a transceiver.
29. The infusion system according toclaim 1 , wherein the infusion device further includes a user input device for inputting an estimate of a material to be ingested by the user, and further wherein the bolus estimator includes the capability to calculate the estimated amount of fluid to be infused into the body of the user based upon the inputted estimate of the material to be ingested by the user.
30. The infusion system according toclaim 29 , wherein the fluid to be infused is insulin, and the material to be ingested is carbohydrates.
31. The infusion system according toclaim 1 , wherein the characteristic determining device further includes a lancing device coupled to the receptacle for obtaining the analyte from the user.
32. The infusion system according toclaim 1 , wherein the characteristic determining device further includes a determining device clock, and the infusion device further includes an infusion device clock, and wherein the infusion device communication system further transmits a communication including a time of the infusion device clock, and the determining device communication system further receives the communication including the time of the infusion device clock from the infusion device communication system, and further wherein the determining device clock is set to the received time of the infusion device clock.
33. The infusion system according toclaim 1 , wherein the characteristic determining device further includes a determining device clock, and the infusion device further includes an infusion device clock, and wherein the determining device communication system further transmits a communication including a time of the determining device clock, and the infusion device communication system further receives the communication including the time of the determining device clock from the determining device communication system, and further wherein the infusion device clock is set to the received time of the determining device clock.
34. The infusion system according toclaim 1 , wherein the infusion device further includes a memory for storing data, and further wherein the data indicative of the determined concentration of the analyte in the user received by the infusion device communication system from the determining device communication system is stored in the memory of the infusion device.
35. An infusion device for infusing a fluid into a body of a user, wherein the infusion device is capable of communicating with a characteristic determining device adapted for determining a concentration of an analyte in the user, the infusion device comprising:
a housing adapted to be carried by the user;
a drive mechanism contained in the housing and operatively coupled with a reservoir containing the fluid for infusing the fluid into the body of the user;
a communication system contained in the housing for receiving a communication from the characteristic determining device, the communication including data indicative of the determined concentration of the analyte in the user;
a processor contained in the housing and coupled to the communication system for processing the data indicative of the determined concentration of the analyte in the user and controlling the infusion device;
a bolus estimator used in conjunction with the processor for calculating an estimated amount of fluid to be infused into the body of the user based upon the received data indicative of the determined concentration of the analyte in the user and a target concentration of the analyte in the user; and
an indicator to indicate when the estimated amount of fluid to be infused has been calculated.
36. The infusion device according toclaim 35 , wherein the communication received by the communication system from the characteristic determining device further includes a time at which the concentration of the analyte in the user was determined.
37. The infusion device according toclaim 35 , wherein the processor determines an amount of time that has elapsed since the data indicative of the determined concentration of the analyte in the user was received from the characteristic determining device, and causes the bolus estimator not to calculate the estimated amount of fluid to be infused based upon the determined, concentration of the analyte if the elapsed amount of time exceeds a predetermined amount of time.
38. The infusion device according toclaim 35 , wherein the processor has unique identification information, and further wherein the communication received by the communication system from the characteristic determining device further includes the unique identification information of the infusion device such that the infusion device is capable of discerning whether the communication is intended for receipt by the infusion device.
39. The infusion device according toclaim 35 , wherein the communication received by the communication system from the characteristic determining device further includes unique identification information of the characteristic determining device such that the infusion device is capable of discerning whether the communication is intended for receipt by the infusion device.
40. The infusion device according toclaim 35 , wherein the processor uses power cycling whereby power is periodically supplied to the communication system until a communication is received from the characteristic determining device, and further wherein the processor discontinues using power cycling whereby the power is continuously supplied to the communication system when the communication including data indicative of the determined concentration of the analyte in the user is received from the characteristic determining device.
41. The infusion device according toclaim 40 , wherein the processor resumes using power cycling upon completing the receipt of the communication including the data indicative of the determined concentration of the analyte in the user from the characteristic determining device.
42. The infusion device according toclaim 35 , wherein the indicator further indicates when the determined concentration of the analyte in the user is above or below a predetermined level of the analyte in the user.
43. The infusion device according toclaim 35 , wherein the communication system communicates with the characteristic determining device using one of radio frequencies and infrared frequencies.
44. The infusion device according toclaim 35 , wherein the communication system includes one of a receiver and a transceiver.
45. The infusion device according toclaim 35 , further comprising a user input device for inputting an estimate of a material to be ingested by the user, wherein the bolus estimator includes the capability to calculate the estimated amount of fluid to be infused into the body of the user based upon the inputted estimate of the material to be ingested by the user.
46. The infusion device according toclaim 35 , further comprising a clock, wherein the communication system further transmits a communication including a clock time of the infusion device to the characteristic determining device, the clock time of the infusion device being adapted for setting a clock time of the characteristic determining device.
47. The infusion device according toclaim 35 , further comprising a clock, wherein the communication system further receives from the characteristic determining device a communication including a clock time of the characteristic determining device, the time of the characteristic determining device being adapted for setting a clock time of the infusion device.
48. The infusion device according toclaim 35 , further comprising a memory for storing data, wherein the data indicative of the determined concentration of the analyte in the user received by the communication system from the characteristic determining device is stored in the memory of the infusion device.
49. A characteristic determining device for determining a concentration of an analyte in a body of a user, wherein the characteristic determining device is capable of communicating with an infusion device adapted for infusing a fluid into the body of the user and calculating an estimated amount of the fluid to be infused into the body of the user based upon the determined concentration of the analyte in the user and a target concentration of the analyte in the user, the characteristic determining device comprising:
a housing adapted to be carried by the user;
a receptacle coupled to the housing for receiving and testing an analyte from the user to determine the concentration of the analyte in the user;
a processor contained in the housing and coupled to the receptacle for processing the determined concentration of the analyte from the receptacle; and
a communication system contained in the housing and coupled to the processor for transmitting a communication including data indicative of the determined concentration of the analyte in the user to the infusion device.
50. The characteristic determining device according toclaim 49 , wherein the communication system automatically transmits the communication including the data indicative of the determined concentration of the analyte in the user to the infusion device.
51. The characteristic determining device according toclaim 49 , further comprising a user input device for inputting commands, wherein the communication system transmits the communication including the data indicative of the determined concentration of the analyte in the user to the infusion device in response to a command from the user input device.
52. The characteristic determining device according toclaim 49 , further comprising an indicator to indicate a status of the communication including the data indicative of the determined concentration of the analyte in the user being transmitted from the communication system to the infusion device.
53. The characteristic determining device according toclaim 49 , wherein the communication transmitted by the communication system to the infusion device further includes a time at which the concentration of the analyte in the user was determined.
54. The characteristic determining device according toclaim 49 , wherein the processor has unique identification information, and the communication transmitted by the communication system to the infusion device further includes the unique identification information of the processor such that the infusion device is capable of discerning whether the communication is intended for receipt by the infusion device.
55. The characteristic determining device according toclaim 49 , wherein the communication system is capable of being deactivated and reactivated.
56. The characteristic determining device according toclaim 55 , further comprising a memory for storing the data indicative of the determined concentration of the analyte in the user that is determined when the communication system is deactivated, and wherein the stored data is transmitted by the communication system to the infusion device when the communication system is reactivated.
57. The characteristic determining device according toclaim 49 , further comprising a port adapted to receive a connector for coupling the characteristic determining device to a computer and downloading data from the characteristic determining device to the computer, wherein the communication system is further capable of receiving a communication including data to be downloaded from the infusion device, and further wherein the received data is downloaded from the characteristic determining device through the connector to the computer.
58. The characteristic determining device according toclaim 49 , further comprising:
a port adapted to receive a connector for coupling the characteristic determining device to a computer and downloading data from the characteristic determining device to the computer; and
a memory for storing data,
wherein the communication system is further capable of receiving a communication including data to be downloaded from the infusion device, and further wherein the received data is stored in the memory for subsequent downloading through the connector to the computer.
59. The characteristic determining device according toclaim 49 , wherein the communication system includes one of a transmitter and a transceiver.
60. The characteristic determining device according toclaim 49 , further comprising a lancing device coupled to the receptacle for obtaining the analyte from the user.
61. The characteristic determining device according toclaim 49 , further comprising a clock, wherein the communication system further transmits a communication including a clock time of the characteristic determining device to the infusion device, the time of the characteristic determining device being adapted for setting a clock time of the infusion device.
62. The characteristic determining device according toclaim 49 , further comprising a clock, wherein the communication system further receives from the infusion device a communication including a clock time of the infusion device, the clock time of the infusion device being adapted for setting a clock time of the characteristic determining device.
63. In an infusion system including a characteristic determining device and an infusion device, a method for infusing a fluid into a body of a user, the method comprising the steps of:
receiving and testing an analyte from the user to determine a concentration of the analyte in the user;
transmitting with the characteristic determining device a communication including data indicative of the determined concentration of the analyte in the user;
receiving with the infusion device the communication including the data indicative of the determined concentration of the analyte in the user;
calculating an estimated amount of fluid to be infused into the body of the user based upon the received data indicative of the determined concentration of the analyte in the user and a target concentration of the analyte in the user; and
indicating when the estimated amount of fluid to be infused has been calculated.
64. The method according toclaim 63 , wherein the communication including the data indicative of the determined concentration of the analyte in the user is automatically transmitted from the characteristic determining device to the infusion device.
65. The method according toclaim 63 , further comprising the step of inputting a command on the characteristic determining device, wherein the communication including the data indicative of the determined concentration of the analyte in the user is transmitted from the characteristic determining device to the infusion device in response to the inputted command.
66. The method according toclaim 63 , further comprising the step of indicating a status of the communication including the data indicative of the determined concentration of the analyte in the user being transmitted from the characteristic determining device to the infusion device.
67. The method according toclaim 63 , wherein the communication including the data indicative of the determined concentration of the analyte in the user transmitted from the characteristic determining device to the infusion device further includes a time at which the concentration of the analyte in the user was determined.
68. The method according toclaim 63 , further comprising the step of determining an amount of time that has elapsed since the concentration of the analyte in the user was determined.
69. The method according toclaim 63 , further comprising the step of determining an amount of time that has elapsed since the communication including the data indicative of the determined concentration of the analyte in the user was received by the infusion device.
70. The method according toclaim 63 , further comprising the steps of:
transmitting with the infusion device a communication including data from the infusion device to be downloaded;
receiving with the characteristic determining device the communication including data from the infusion device to be downloaded; and
downloading the received data from the characteristic determining device to a computer.
71. The method according toclaim 63 , further comprising the step of inputting an estimate of a material to be ingested by the user, wherein the estimated amount of fluid to be infused into the body of the user is calculated further based upon the inputted estimate of the material to be ingested by the user.
72. The method according toclaim 63 , further comprising the steps of:
transmitting with the infusion device a communication including a clock time of the infusion device;
receiving with the characteristic determining device the communication including the clock time of the infusion device; and
setting a clock time of the characteristic determining device to the received clock time of the infusion device.
73. The method according toclaim 63 , further comprising the steps of:
transmitting with the characteristic determining device a communication including a clock time of the characteristic determining device;
receiving with the infusion device the communication including the clock time of the characteristic determining device; and
setting a clock time of the infusion device to the received clock time of the characteristic determining device.
74. The method according toclaim 63 , further comprising the step of storing in a memory of the infusion device the data indicative of the determined concentration of the analyte in the user received by the infusion device from the characteristic determining device.
75. An infusion system for infusing a fluid into a body of a user, the infusion system comprising:
a characteristic determining device including:
a determining device housing adapted to be carried by the user;
a sensor coupled to the determining device housing for determining a concentration of an analyte in the user;
a determining device processor contained in the determining device housing and coupled to the sensor for processing the determined concentration of the analyte from the sensor; and
a determining device communication system contained in the determining device housing and coupled to the determining device processor for transmitting a communication including data indicative of the determined concentration of the analyte in the user; and
an infusion device including:
an infusion device housing adapted to be carried by the user;
a drive mechanism contained in the infusion device housing and operatively coupled with a reservoir containing the fluid for infusing the fluid into the body of the user;
an infusion device communication system contained in the infusion device housing for receiving the communication including the data indicative of the determined concentration of the analyte in the user from the determining device communication system; and
an infusion device processor contained in the infusion device housing and coupled to the infusion device communication system for processing the data indicative of the determined concentration of the analyte in the user and controlling the infusion device.
76. The infusion system according toclaim 75 , wherein the determining device communication system automatically transmits the communication including the data indicative of the determined concentration of the analyte in the user to the infusion device communication system.
77. The infusion system according toclaim 75 , wherein the characteristic determining device further includes a user input device for inputting commands, and the determining device communication system transmits the communication including the data indicative of the determined concentration of the analyte in the user to the infusion device communication system in response to a command from the user input device.
78. The infusion system according toclaim 75 , wherein the characteristic determining device further includes an indicator to indicate a status of the communication including the data indicative of the determined concentration of the analyte in the user being transmitted from the determining device communication system to the infusion device communication system.
79. The infusion system according toclaim 75 , wherein the communication transmitted from the determining device communication system to the infusion device communication system further includes a time at which the concentration of the analyte in the user was determined.
80. The infusion system according toclaim 75 , wherein the infusion device further includes:
a bolus estimator used in conjunction with the infusion device processor for calculating an estimated amount of fluid to be infused into the body of the user based upon the received data indicative of the determined concentration of the analyte in the user and a target concentration of the analyte in the user; and
an infusion device indicator to indicate when the estimated amount of fluid to be infused has been calculated.
81. The infusion system according toclaim 80 , wherein the infusion device processor determines an amount of time that has elapsed since the concentration of the analyte in the user was determined, and causes the bolus estimator not to calculate the estimated amount of fluid to be infused based upon the determined concentration of the analyte if the elapsed amount of time exceeds a predetermined amount of time.
82. The infusion system according toclaim 75 , wherein the determining device processor has unique identification information, and further wherein the communication transmitted from the determining device communication system to the infusion device communication system further includes the unique identification information of the determining device processor such that the infusion device is capable of discerning whether the communication is intended for receipt by the infusion device.
83. The infusion system according toclaim 75 , wherein the infusion device processor has unique identification information, and further wherein the communication transmitted from the determining device communication system to the infusion device communication system further includes the unique identification information of the infusion device processor such that the infusion device is capable of discerning whether the communication is intended for receipt by the infusion device.
84. The infusion system according toclaim 75 , wherein the determining device communication system is capable of being deactivated and reactivated.
85. The infusion system according toclaim 84 , wherein the characteristic determining device further includes a memory for storing data indicative of the determined concentration of the analyte in the user that is determined when the determining device communication system is deactivated, and further wherein the determining device communication system transmits a communication including the stored data to the infusion device communication system when the determining device communication system is reactivated.
86. The infusion system according toclaim 75 , wherein the infusion device processor uses power cycling whereby power is periodically supplied to the infusion device communication system until a communication is received from the determining device communication system, and further wherein the infusion device processor discontinues using power cycling whereby the power is continuously supplied to the infusion device communication system when the communication including the data indicative of the determined concentration of the analyte in the user is received from the determining device communication system.
87. The infusion system according toclaim 86 , wherein the infusion device processor resumes using power cycling upon completing the receipt of the communication including the data indicative of the determined concentration of the analyte in the user from the determining device communication system.
88. The infusion system according toclaim 75 , further including a connector for coupling the characteristic determining device to a computer and downloading data from the characteristic determining device to the computer, wherein the infusion device communication system is further capable of transmitting a communication including infusion device data to be downloaded, and the determining device communication system is further capable of receiving the communication including the infusion device data to be downloaded from the infusion device communication system, and further wherein the received infusion device data is downloaded from the characteristic determining device through the connector to the computer.
89. The infusion system according toclaim 75 , wherein the determining device communication system and the infusion device communication system communicate using one of radio frequencies and infrared frequencies.
90. The infusion system according toclaim 75 , wherein the concentration of the analyte in the user determined by the characteristic determining device is a blood glucose level of the user, and the fluid infused by the infusion device is insulin.
91. The infusion system according toclaim 75 , wherein the determining device communication system includes one of a transmitter and a transceiver, and the infusion device communication system includes one of a receiver and a transceiver.
92. The infusion system according toclaim 75 , wherein the characteristic determining device further includes a determining device clock, and the infusion device further includes an infusion device clock, and wherein the infusion device communication system further transmits a communication including a time of the infusion device clock, and the determining device communication system further receives the communication including the time of the infusion device clock from the infusion device communication system, and further wherein the determining device clock is set to the received time of the infusion device clock.
93. The infusion system according toclaim 75 , wherein the characteristic determining device further includes a determining device clock, and the infusion device further includes an infusion device clock, and wherein the determining device communication system further transmits a communication including a time of the determining device clock, and the infusion device communication system further receives the communication including the time of the determining device clock from the determining device communication system, and further wherein the infusion device clock is set to the received time of the determining device clock.
94. The infusion system according toclaim 75 , wherein the infusion device further includes a memory for storing data, and further wherein the data indicative of the determined concentration of the analyte in the user received by the infusion device communication system from the determining device communication system is stored in the memory of the infusion device.
Priority Applications (18)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US10/624,389US20040068230A1 (en) | 2002-07-24 | 2003-07-22 | System for providing blood glucose measurements to an infusion device |
| EP10176392AEP2253347A1 (en) | 2002-07-24 | 2003-07-23 | System for providing blood glucose measurements to an infusion device |
| EP10176391AEP2253346A1 (en) | 2002-07-24 | 2003-07-23 | System for providing blood glucose measurements to an infusion device |
| PCT/US2003/022862WO2004008956A2 (en) | 2002-07-24 | 2003-07-23 | System for providing blood glucose measurements to an infusion device |
| AU2003252104AAU2003252104A1 (en) | 2002-07-24 | 2003-07-23 | System for providing blood glucose measurements to an infusion device |
| DK03765896.0TDK1525014T3 (en) | 2002-07-24 | 2003-07-23 | System for providing blood glucose measurements to an infusion apparatus |
| EP03765896AEP1525014B1 (en) | 2002-07-24 | 2003-07-23 | System for providing blood glucose measurements to an infusion device |
| US10/867,529US8512276B2 (en) | 2002-07-24 | 2004-06-14 | System for providing blood glucose measurements to an infusion device |
| US12/333,639US20090099506A1 (en) | 2002-07-24 | 2008-12-12 | System for Providing Blood Glucose Measurements to an Infusion Device |
| US12/333,690US20090112076A1 (en) | 2002-07-24 | 2008-12-12 | System for Providing Blood Glucose Measurements to an Infusion Device |
| US12/333,966US20090118665A1 (en) | 2002-07-24 | 2008-12-12 | System for Providing Blood Glucose Measurements to an Infusion Device |
| US12/333,731US20090118664A1 (en) | 2002-07-24 | 2008-12-12 | System for Providing Blood Glucose Measurements to an Infusion Device |
| US12/333,655US20090143662A1 (en) | 2002-07-24 | 2008-12-12 | System for Providing Blood Glucose Measurements to an Infusion Device |
| US12/333,667US20090149803A1 (en) | 2002-07-24 | 2008-12-12 | System for Providing Blood Glucose Measurements to an Infusion Device |
| US12/334,001US20090099509A1 (en) | 2002-07-24 | 2008-12-12 | System for Providing Blood Glucose Measurements to an Infusion Device |
| US12/756,544US8192395B2 (en) | 2002-07-24 | 2010-04-08 | System for providing blood glucose measurements to an infusion device |
| US13/465,037US8568357B2 (en) | 2002-07-24 | 2012-05-07 | System for providing blood glucose measurements to an infusion device |
| US13/944,630US9636456B2 (en) | 2002-07-24 | 2013-07-17 | System for providing blood glucose measurements to an infusion device |
Applications Claiming Priority (3)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US39819902P | 2002-07-24 | 2002-07-24 | |
| US41299802P | 2002-09-23 | 2002-09-23 | |
| US10/624,389US20040068230A1 (en) | 2002-07-24 | 2003-07-22 | System for providing blood glucose measurements to an infusion device |
Related Child Applications (9)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US10/867,529Continuation-In-PartUS8512276B2 (en) | 2002-07-24 | 2004-06-14 | System for providing blood glucose measurements to an infusion device |
| US12/333,690DivisionUS20090112076A1 (en) | 2002-07-24 | 2008-12-12 | System for Providing Blood Glucose Measurements to an Infusion Device |
| US12/333,667DivisionUS20090149803A1 (en) | 2002-07-24 | 2008-12-12 | System for Providing Blood Glucose Measurements to an Infusion Device |
| US12/333,639DivisionUS20090099506A1 (en) | 2002-07-24 | 2008-12-12 | System for Providing Blood Glucose Measurements to an Infusion Device |
| US12/333,655DivisionUS20090143662A1 (en) | 2002-07-24 | 2008-12-12 | System for Providing Blood Glucose Measurements to an Infusion Device |
| US12/334,001DivisionUS20090099509A1 (en) | 2002-07-24 | 2008-12-12 | System for Providing Blood Glucose Measurements to an Infusion Device |
| US12/333,731DivisionUS20090118664A1 (en) | 2002-07-24 | 2008-12-12 | System for Providing Blood Glucose Measurements to an Infusion Device |
| US12/333,966DivisionUS20090118665A1 (en) | 2002-07-24 | 2008-12-12 | System for Providing Blood Glucose Measurements to an Infusion Device |
| US12/756,544ContinuationUS8192395B2 (en) | 2002-07-24 | 2010-04-08 | System for providing blood glucose measurements to an infusion device |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| US20040068230A1true US20040068230A1 (en) | 2004-04-08 |
Family
ID=30773527
Family Applications (10)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US10/624,389AbandonedUS20040068230A1 (en) | 2002-07-24 | 2003-07-22 | System for providing blood glucose measurements to an infusion device |
| US12/334,001AbandonedUS20090099509A1 (en) | 2002-07-24 | 2008-12-12 | System for Providing Blood Glucose Measurements to an Infusion Device |
| US12/333,690AbandonedUS20090112076A1 (en) | 2002-07-24 | 2008-12-12 | System for Providing Blood Glucose Measurements to an Infusion Device |
| US12/333,667AbandonedUS20090149803A1 (en) | 2002-07-24 | 2008-12-12 | System for Providing Blood Glucose Measurements to an Infusion Device |
| US12/333,731AbandonedUS20090118664A1 (en) | 2002-07-24 | 2008-12-12 | System for Providing Blood Glucose Measurements to an Infusion Device |
| US12/333,655AbandonedUS20090143662A1 (en) | 2002-07-24 | 2008-12-12 | System for Providing Blood Glucose Measurements to an Infusion Device |
| US12/333,639AbandonedUS20090099506A1 (en) | 2002-07-24 | 2008-12-12 | System for Providing Blood Glucose Measurements to an Infusion Device |
| US12/333,966AbandonedUS20090118665A1 (en) | 2002-07-24 | 2008-12-12 | System for Providing Blood Glucose Measurements to an Infusion Device |
| US12/756,544Expired - Fee RelatedUS8192395B2 (en) | 2002-07-24 | 2010-04-08 | System for providing blood glucose measurements to an infusion device |
| US13/465,037Expired - LifetimeUS8568357B2 (en) | 2002-07-24 | 2012-05-07 | System for providing blood glucose measurements to an infusion device |
Family Applications After (9)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US12/334,001AbandonedUS20090099509A1 (en) | 2002-07-24 | 2008-12-12 | System for Providing Blood Glucose Measurements to an Infusion Device |
| US12/333,690AbandonedUS20090112076A1 (en) | 2002-07-24 | 2008-12-12 | System for Providing Blood Glucose Measurements to an Infusion Device |
| US12/333,667AbandonedUS20090149803A1 (en) | 2002-07-24 | 2008-12-12 | System for Providing Blood Glucose Measurements to an Infusion Device |
| US12/333,731AbandonedUS20090118664A1 (en) | 2002-07-24 | 2008-12-12 | System for Providing Blood Glucose Measurements to an Infusion Device |
| US12/333,655AbandonedUS20090143662A1 (en) | 2002-07-24 | 2008-12-12 | System for Providing Blood Glucose Measurements to an Infusion Device |
| US12/333,639AbandonedUS20090099506A1 (en) | 2002-07-24 | 2008-12-12 | System for Providing Blood Glucose Measurements to an Infusion Device |
| US12/333,966AbandonedUS20090118665A1 (en) | 2002-07-24 | 2008-12-12 | System for Providing Blood Glucose Measurements to an Infusion Device |
| US12/756,544Expired - Fee RelatedUS8192395B2 (en) | 2002-07-24 | 2010-04-08 | System for providing blood glucose measurements to an infusion device |
| US13/465,037Expired - LifetimeUS8568357B2 (en) | 2002-07-24 | 2012-05-07 | System for providing blood glucose measurements to an infusion device |
Country Status (5)
| Country | Link |
|---|---|
| US (10) | US20040068230A1 (en) |
| EP (3) | EP1525014B1 (en) |
| AU (1) | AU2003252104A1 (en) |
| DK (1) | DK1525014T3 (en) |
| WO (1) | WO2004008956A2 (en) |
Cited By (192)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US20040215492A1 (en)* | 2003-01-30 | 2004-10-28 | Choi Soo Bong | Method for controlling insulin pump through internet |
| US20050027181A1 (en)* | 2003-08-01 | 2005-02-03 | Goode Paul V. | System and methods for processing analyte sensor data |
| US20050090607A1 (en)* | 2003-10-28 | 2005-04-28 | Dexcom, Inc. | Silicone composition for biocompatible membrane |
| US20050112169A1 (en)* | 2003-05-21 | 2005-05-26 | Dexcom, Inc. | Porous membranes for use with implantable devices |
| US20050137573A1 (en)* | 2003-12-19 | 2005-06-23 | Animas Corporation | System, method, and communication hub for controlling external infusion device |
| US20050154271A1 (en)* | 2003-11-19 | 2005-07-14 | Andrew Rasdal | Integrated receiver for continuous analyte sensor |
| US20050192557A1 (en)* | 2004-02-26 | 2005-09-01 | Dexcom | Integrated delivery device for continuous glucose sensor |
| US20060020189A1 (en)* | 2004-07-13 | 2006-01-26 | Dexcom, Inc. | Transcutaneous analyte sensor |
| US20060137695A1 (en)* | 2004-12-23 | 2006-06-29 | Robert Hellwig | System and method for determining insulin bolus quantities |
| WO2006066038A3 (en)* | 2004-12-17 | 2006-08-03 | Bayer Healthcare Llc | Analyte test device having a trend-indicating display |
| WO2006108809A1 (en) | 2005-04-13 | 2006-10-19 | Novo Nordisk A/S | Medical skin mountable device and system |
| US20060264835A1 (en)* | 2003-10-21 | 2006-11-23 | Novo Nordisk A/S | Medical skin mountable device |
| US20070032891A1 (en)* | 2003-05-23 | 2007-02-08 | Choi Soo B | Method for controlling insulin pump through internet |
| US20070066873A1 (en)* | 2003-08-22 | 2007-03-22 | Apurv Kamath | Systems and methods for processing analyte sensor data |
| US20070078818A1 (en)* | 2005-06-09 | 2007-04-05 | Roche Diagnostics Operations, Inc. | Device and method for insulin dosing |
| US20070112298A1 (en)* | 2005-11-17 | 2007-05-17 | Medtronic Minimed, Inc. | External infusion device with programmable capabilities to time-shift basal insulin and method of using the same |
| US20070229257A1 (en)* | 2005-03-18 | 2007-10-04 | Olle Bliding | Wake-up device and method for generating a control signal |
| US20070233051A1 (en)* | 2006-03-31 | 2007-10-04 | David Hohl | Drug delivery systems and methods |
| US7291107B2 (en) | 2004-08-26 | 2007-11-06 | Roche Diagnostics Operations, Inc. | Insulin bolus recommendation system |
| US20080020794A1 (en)* | 2004-10-22 | 2008-01-24 | Mark Garon | Mobile electronic device with fluid delivery system |
| US20080214919A1 (en)* | 2006-12-26 | 2008-09-04 | Lifescan, Inc. | System and method for implementation of glycemic control protocols |
| US20080228056A1 (en)* | 2007-03-13 | 2008-09-18 | Michael Blomquist | Basal rate testing using frequent blood glucose input |
| US20080256738A1 (en)* | 2007-04-23 | 2008-10-23 | Malone Randolph W | Frameless, Heated Wiper Assembly And System Utilizing Same |
| US20080262469A1 (en)* | 2004-02-26 | 2008-10-23 | Dexcom. Inc. | Integrated medicament delivery device for use with continuous analyte sensor |
| US20080287870A1 (en)* | 2005-10-17 | 2008-11-20 | Nov Nordisk A/S | Vented Drug Reservoir Unit |
| US20080300534A1 (en)* | 2007-05-30 | 2008-12-04 | Michael Blomquist | Insulin pump based expert system |
| US20080306443A1 (en)* | 2007-06-06 | 2008-12-11 | Mallinckrodt Inc. | Medical Fluid Injector Having Wireless Pressure Monitoring Feature |
| US20080312512A1 (en)* | 2007-06-15 | 2008-12-18 | Animas Corporation | Methods to pair a medical device and at least a remote controller for such medical device |
| US20080312585A1 (en)* | 2007-06-15 | 2008-12-18 | Animas Corporation | Method of operating a medical device and at least a remote controller for such medical device |
| US20080312584A1 (en)* | 2007-06-15 | 2008-12-18 | Animas Corporation | Systems and methods to pair a medical device and a remote controller for such medical device |
| US7471972B2 (en) | 2001-07-27 | 2008-12-30 | Dexcom, Inc. | Sensor head for use with implantable devices |
| US7494465B2 (en) | 2004-07-13 | 2009-02-24 | Dexcom, Inc. | Transcutaneous analyte sensor |
| US20090118682A1 (en)* | 2005-09-13 | 2009-05-07 | Novo Nordisk A/S | Reservoir Device With Inspection Aid For Detection Of Drug Condition |
| US20090118592A1 (en)* | 2005-12-08 | 2009-05-07 | Novo Nordisk A/S | Medical System Comprising a Sensor Device |
| US20090124877A1 (en)* | 2003-08-22 | 2009-05-14 | Dexcom, Inc. | Systems and methods for replacing signal artifacts in a glucose sensor data stream |
| US20090177154A1 (en)* | 2008-01-08 | 2009-07-09 | Michael Blomquist | Insulin pump with convenience features |
| US20090216103A1 (en)* | 2004-07-13 | 2009-08-27 | Dexcom, Inc. | Transcutaneous analyte sensor |
| US20090240385A1 (en)* | 2008-03-21 | 2009-09-24 | Denso Corporation | Electronic control apparatus for vehicle |
| US20090271021A1 (en)* | 2008-04-28 | 2009-10-29 | Popp Shane M | Execution system for the monitoring and execution of insulin manufacture |
| WO2009146379A1 (en)* | 2008-05-28 | 2009-12-03 | Pelikan Technologies, Inc. | Devices and methods for glucose measurement using rechargeable battery energy sources |
| US20090307520A1 (en)* | 2008-06-06 | 2009-12-10 | Bruno Anselmi | Apparatus and Method for Processing Wirelessly Communicated Information Within an Electronic Device |
| US20090305317A1 (en)* | 2008-06-05 | 2009-12-10 | Brauer Jacob S | User interface for testing device |
| US7632228B2 (en) | 2001-07-27 | 2009-12-15 | Dexcom, Inc. | Membrane for use with implantable devices |
| US7640048B2 (en) | 2004-07-13 | 2009-12-29 | Dexcom, Inc. | Analyte sensor |
| US20090326722A1 (en)* | 2008-06-30 | 2009-12-31 | Animas Corporation | Method and System for Using Status Indicators in Wireless Communication with Medical Devices |
| US20100030485A1 (en)* | 2003-12-09 | 2010-02-04 | Dexcom, Inc. | Signal processing for continuous analyte sensor |
| US20100100048A1 (en)* | 2003-10-27 | 2010-04-22 | Novo Nordisk A/S | Medical Skin Mountable Device |
| US7711402B2 (en) | 1997-03-04 | 2010-05-04 | Dexcom, Inc. | Device and method for determining analyte levels |
| US7774145B2 (en) | 2003-08-01 | 2010-08-10 | Dexcom, Inc. | Transcutaneous analyte sensor |
| US7783333B2 (en) | 2004-07-13 | 2010-08-24 | Dexcom, Inc. | Transcutaneous medical device with variable stiffness |
| US20100222765A1 (en)* | 2007-01-24 | 2010-09-02 | Smiths Medical Asd, Inc. | Correction factor testing using frequent blood glucose input |
| US20100274751A1 (en)* | 2007-05-24 | 2010-10-28 | Smith Medical Asd, Inc. | Expert system for insulin pump therapy |
| US7860544B2 (en) | 1998-04-30 | 2010-12-28 | Abbott Diabetes Care Inc. | Analyte monitoring device and methods of use |
| US7860545B2 (en) | 1997-03-04 | 2010-12-28 | Dexcom, Inc. | Analyte measuring device |
| US20110014954A1 (en)* | 2005-10-27 | 2011-01-20 | Dossas Vasilios D | Emergency Medical Diagnosis and Communications Device |
| US7875293B2 (en) | 2003-05-21 | 2011-01-25 | Dexcom, Inc. | Biointerface membranes incorporating bioactive agents |
| US20110033833A1 (en)* | 2008-01-07 | 2011-02-10 | Michael Blomquist | Pump with therapy coaching |
| US20110040251A1 (en)* | 2008-01-09 | 2011-02-17 | Michael Blomquist | Infusion pump with add-on modules |
| US7896809B2 (en) | 2003-07-25 | 2011-03-01 | Dexcom, Inc. | Dual electrode system for a continuous analyte sensor |
| US7914450B2 (en) | 2003-08-01 | 2011-03-29 | Dexcom, Inc. | System and methods for processing analyte sensor data |
| US7920907B2 (en) | 2006-06-07 | 2011-04-05 | Abbott Diabetes Care Inc. | Analyte monitoring system and method |
| US20110137255A1 (en)* | 2003-10-27 | 2011-06-09 | Novo Nordisk A/S | Medical Skin Mountable Device |
| US7976778B2 (en) | 2001-04-02 | 2011-07-12 | Abbott Diabetes Care Inc. | Blood glucose tracking apparatus |
| US20110201911A1 (en)* | 2010-02-12 | 2011-08-18 | Dexcom, Inc. | Receivers for analyzing and displaying sensor data |
| US20110208013A1 (en)* | 2010-02-24 | 2011-08-25 | Edwards Lifesciences Corporation | Body Parameter Sensor and Monitor Interface |
| US8016789B2 (en) | 2008-10-10 | 2011-09-13 | Deka Products Limited Partnership | Pump assembly with a removable cover assembly |
| US8034026B2 (en) | 2001-05-18 | 2011-10-11 | Deka Products Limited Partnership | Infusion pump assembly |
| US20110282173A1 (en)* | 2007-08-29 | 2011-11-17 | Brighter Ab | Portable medical apparatus |
| US8066672B2 (en) | 2008-10-10 | 2011-11-29 | Deka Products Limited Partnership | Infusion pump assembly with a backup power supply |
| US8115635B2 (en) | 2005-02-08 | 2012-02-14 | Abbott Diabetes Care Inc. | RF tag on test strips, test strip vials and boxes |
| US8113244B2 (en) | 2006-02-09 | 2012-02-14 | Deka Products Limited Partnership | Adhesive and peripheral systems and methods for medical devices |
| US8132037B2 (en) | 2008-06-06 | 2012-03-06 | Roche Diagnostics International Ag | Apparatus and method for processing wirelessly communicated data and clock information within an electronic device |
| US8133178B2 (en) | 2006-02-22 | 2012-03-13 | Dexcom, Inc. | Analyte sensor |
| US8160669B2 (en) | 2003-08-01 | 2012-04-17 | Dexcom, Inc. | Transcutaneous analyte sensor |
| US8223028B2 (en) | 2008-10-10 | 2012-07-17 | Deka Products Limited Partnership | Occlusion detection system and method |
| US8229535B2 (en) | 2008-02-21 | 2012-07-24 | Dexcom, Inc. | Systems and methods for blood glucose monitoring and alert delivery |
| US8260393B2 (en) | 2003-07-25 | 2012-09-04 | Dexcom, Inc. | Systems and methods for replacing signal data artifacts in a glucose sensor data stream |
| US8262616B2 (en) | 2008-10-10 | 2012-09-11 | Deka Products Limited Partnership | Infusion pump assembly |
| US8267892B2 (en) | 2008-10-10 | 2012-09-18 | Deka Products Limited Partnership | Multi-language / multi-processor infusion pump assembly |
| US8275437B2 (en) | 2003-08-01 | 2012-09-25 | Dexcom, Inc. | Transcutaneous analyte sensor |
| US8280475B2 (en) | 2004-07-13 | 2012-10-02 | Dexcom, Inc. | Transcutaneous analyte sensor |
| US8287454B2 (en) | 1998-04-30 | 2012-10-16 | Abbott Diabetes Care Inc. | Analyte monitoring device and methods of use |
| US8290559B2 (en) | 2007-12-17 | 2012-10-16 | Dexcom, Inc. | Systems and methods for processing sensor data |
| US8287495B2 (en) | 2009-07-30 | 2012-10-16 | Tandem Diabetes Care, Inc. | Infusion pump system with disposable cartridge having pressure venting and pressure feedback |
| US8346399B2 (en) | 2002-02-28 | 2013-01-01 | Tandem Diabetes Care, Inc. | Programmable insulin pump |
| US8346337B2 (en) | 1998-04-30 | 2013-01-01 | Abbott Diabetes Care Inc. | Analyte monitoring device and methods of use |
| US8369919B2 (en) | 2003-08-01 | 2013-02-05 | Dexcom, Inc. | Systems and methods for processing sensor data |
| US8386004B2 (en) | 2003-12-05 | 2013-02-26 | Dexcom, Inc. | Calibration techniques for a continuous analyte sensor |
| US8414563B2 (en) | 2007-12-31 | 2013-04-09 | Deka Products Limited Partnership | Pump assembly with switch |
| US8417312B2 (en) | 2007-10-25 | 2013-04-09 | Dexcom, Inc. | Systems and methods for processing sensor data |
| US8423113B2 (en) | 2003-07-25 | 2013-04-16 | Dexcom, Inc. | Systems and methods for processing sensor data |
| US8465425B2 (en) | 1998-04-30 | 2013-06-18 | Abbott Diabetes Care Inc. | Analyte monitoring device and methods of use |
| US8496646B2 (en) | 2007-02-09 | 2013-07-30 | Deka Products Limited Partnership | Infusion pump assembly |
| US8548553B2 (en) | 2003-08-01 | 2013-10-01 | Dexcom, Inc. | System and methods for processing analyte sensor data |
| US8562558B2 (en) | 2007-06-08 | 2013-10-22 | Dexcom, Inc. | Integrated medicament delivery device for use with continuous analyte sensor |
| US8612159B2 (en) | 1998-04-30 | 2013-12-17 | Abbott Diabetes Care Inc. | Analyte monitoring device and methods of use |
| US8622905B2 (en) | 2003-08-01 | 2014-01-07 | Dexcom, Inc. | System and methods for processing analyte sensor data |
| US8652043B2 (en) | 2001-01-02 | 2014-02-18 | Abbott Diabetes Care Inc. | Analyte monitoring device and methods of use |
| US8688188B2 (en) | 1998-04-30 | 2014-04-01 | Abbott Diabetes Care Inc. | Analyte monitoring device and methods of use |
| US8708376B2 (en) | 2008-10-10 | 2014-04-29 | Deka Products Limited Partnership | Medium connector |
| US8777853B2 (en) | 2003-08-22 | 2014-07-15 | Dexcom, Inc. | Systems and methods for replacing signal artifacts in a glucose sensor data stream |
| US8845536B2 (en) | 2003-08-01 | 2014-09-30 | Dexcom, Inc. | Transcutaneous analyte sensor |
| US8886273B2 (en) | 2003-08-01 | 2014-11-11 | Dexcom, Inc. | Analyte sensor |
| US8974386B2 (en) | 1998-04-30 | 2015-03-10 | Abbott Diabetes Care Inc. | Analyte monitoring device and methods of use |
| WO2015047693A1 (en) | 2013-09-30 | 2015-04-02 | Animas Corporation | Methods for secure communication and pairing of a medical infusion device and a remote controller for such medical device |
| US9066695B2 (en) | 1998-04-30 | 2015-06-30 | Abbott Diabetes Care Inc. | Analyte monitoring device and methods of use |
| US20150182695A1 (en)* | 2013-12-26 | 2015-07-02 | Tandem Diabetes Care, Inc. | Integration of infusion pump with remote electronic device |
| US9135402B2 (en) | 2007-12-17 | 2015-09-15 | Dexcom, Inc. | Systems and methods for processing sensor data |
| US9173996B2 (en) | 2001-05-18 | 2015-11-03 | Deka Products Limited Partnership | Infusion set for a fluid pump |
| US9180245B2 (en) | 2008-10-10 | 2015-11-10 | Deka Products Limited Partnership | System and method for administering an infusible fluid |
| US9247900B2 (en) | 2004-07-13 | 2016-02-02 | Dexcom, Inc. | Analyte sensor |
| US20160038675A1 (en)* | 2014-08-06 | 2016-02-11 | Bigfoot Biomedical, Inc. | Infusion pump assembly and method |
| US9282925B2 (en) | 2002-02-12 | 2016-03-15 | Dexcom, Inc. | Systems and methods for replacing signal artifacts in a glucose sensor data stream |
| US9330237B2 (en) | 2008-12-24 | 2016-05-03 | Medtronic Minimed, Inc. | Pattern recognition and filtering in a therapy management system |
| US20160184511A1 (en)* | 2007-12-12 | 2016-06-30 | Bigfoot Biomedical, Inc. | Portable Infusion Pump and Media Player |
| US9391670B2 (en) | 2007-06-15 | 2016-07-12 | Animas Corporation | Methods for secure communication and pairing of a medical infusion device and a remote controller for such medical device |
| US9451908B2 (en) | 2006-10-04 | 2016-09-27 | Dexcom, Inc. | Analyte sensor |
| US9486571B2 (en) | 2013-12-26 | 2016-11-08 | Tandem Diabetes Care, Inc. | Safety processor for wireless control of a drug delivery device |
| EP2757951B1 (en) | 2011-09-23 | 2016-11-09 | Dexcom, Inc. | Systems and methods for processing and transmitting sensor data |
| EP2644088B1 (en) | 2009-09-29 | 2017-01-18 | Lifescan Scotland Limited | Analyte testing method and device for diabetes management |
| JP2017032574A (en)* | 2012-03-12 | 2017-02-09 | パナソニックヘルスケアホールディングス株式会社 | Biological information measuring instrument |
| US9623173B2 (en) | 2012-03-05 | 2017-04-18 | Becton, Dickinson And Company | Wireless communication for on-body medical devices |
| US9669160B2 (en) | 2014-07-30 | 2017-06-06 | Tandem Diabetes Care, Inc. | Temporary suspension for closed-loop medicament therapy |
| US20170212039A1 (en)* | 2016-01-21 | 2017-07-27 | Wellysun Inc. | Extre occult blood inspection method and apparatus thereof |
| EP3263033A1 (en)* | 2005-02-02 | 2018-01-03 | F. Hoffmann-La Roche AG | Ambulatory medical device and method of communication between medical devices |
| US9901292B2 (en) | 2013-11-07 | 2018-02-27 | Dexcom, Inc. | Systems and methods for a continuous monitoring of analyte values |
| US9962486B2 (en) | 2013-03-14 | 2018-05-08 | Tandem Diabetes Care, Inc. | System and method for detecting occlusions in an infusion pump |
| US9986942B2 (en) | 2004-07-13 | 2018-06-05 | Dexcom, Inc. | Analyte sensor |
| US10016561B2 (en) | 2013-03-15 | 2018-07-10 | Tandem Diabetes Care, Inc. | Clinical variable determination |
| US10016559B2 (en) | 2009-12-04 | 2018-07-10 | Smiths Medical Asd, Inc. | Advanced step therapy delivery for an ambulatory infusion pump and system |
| US10112011B2 (en) | 2013-07-19 | 2018-10-30 | Dexcom, Inc. | Time averaged basal rate optimizer |
| US10258736B2 (en) | 2012-05-17 | 2019-04-16 | Tandem Diabetes Care, Inc. | Systems including vial adapter for fluid transfer |
| US10357606B2 (en) | 2013-03-13 | 2019-07-23 | Tandem Diabetes Care, Inc. | System and method for integration of insulin pumps and continuous glucose monitoring |
| US10463786B2 (en) | 2013-03-15 | 2019-11-05 | Tandem Diabetes Care, Inc. | Method and device utilizing insulin delivery protocols |
| US10471208B2 (en)* | 2015-08-14 | 2019-11-12 | Baxter International Inc. | Medical device data integration apparatus and methods |
| US10569016B2 (en) | 2015-12-29 | 2020-02-25 | Tandem Diabetes Care, Inc. | System and method for switching between closed loop and open loop control of an ambulatory infusion pump |
| US10610136B2 (en) | 2005-03-10 | 2020-04-07 | Dexcom, Inc. | System and methods for processing analyte sensor data for sensor calibration |
| US10653835B2 (en) | 2007-10-09 | 2020-05-19 | Dexcom, Inc. | Integrated insulin delivery system with continuous glucose sensor |
| US10980461B2 (en) | 2008-11-07 | 2021-04-20 | Dexcom, Inc. | Advanced analyte sensor calibration and error detection |
| US10987468B2 (en) | 2016-01-05 | 2021-04-27 | Bigfoot Biomedical, Inc. | Operating multi-modal medicine delivery systems |
| US11000215B1 (en) | 2003-12-05 | 2021-05-11 | Dexcom, Inc. | Analyte sensor |
| US11147914B2 (en) | 2013-07-19 | 2021-10-19 | Bigfoot Biomedical, Inc. | Infusion pump system and method |
| US20220008688A1 (en)* | 2018-09-26 | 2022-01-13 | Cary Kochman | Method and system for facilitating the transition between a conscious and unconscious state |
| US11294407B2 (en) | 2001-04-27 | 2022-04-05 | Roche Diabetes Care, Inc. | Device and method for insulin dosing |
| US11331022B2 (en) | 2017-10-24 | 2022-05-17 | Dexcom, Inc. | Pre-connected analyte sensors |
| US11350862B2 (en) | 2017-10-24 | 2022-06-07 | Dexcom, Inc. | Pre-connected analyte sensors |
| US11364335B2 (en) | 2006-02-09 | 2022-06-21 | Deka Products Limited Partnership | Apparatus, system and method for fluid delivery |
| US11382539B2 (en) | 2006-10-04 | 2022-07-12 | Dexcom, Inc. | Analyte sensor |
| US11395877B2 (en) | 2006-02-09 | 2022-07-26 | Deka Products Limited Partnership | Systems and methods for fluid delivery |
| US11404776B2 (en) | 2007-12-31 | 2022-08-02 | Deka Products Limited Partnership | Split ring resonator antenna adapted for use in wirelessly controlled medical device |
| US11399745B2 (en) | 2006-10-04 | 2022-08-02 | Dexcom, Inc. | Dual electrode system for a continuous analyte sensor |
| US11406755B1 (en) | 2021-02-19 | 2022-08-09 | Fresenius Kabi Deutschland Gmbh | Sensing fluid flow irregularities in an on-body injector |
| US11413394B1 (en)* | 2021-02-19 | 2022-08-16 | Fresenius Kabi Deutschland Gmbh | Display for wearable drug delivery device |
| US11426512B2 (en) | 2006-02-09 | 2022-08-30 | Deka Products Limited Partnership | Apparatus, systems and methods for an infusion pump assembly |
| US11464908B2 (en) | 2019-02-18 | 2022-10-11 | Tandem Diabetes Care, Inc. | Methods and apparatus for monitoring infusion sites for ambulatory infusion pumps |
| US11464899B2 (en) | 2014-08-28 | 2022-10-11 | Becton, Dickinson And Company | Wireless communication for on-body medical devices |
| US11464906B2 (en) | 2013-12-02 | 2022-10-11 | Bigfoot Biomedical, Inc. | Infusion pump system and method |
| US11471598B2 (en) | 2015-04-29 | 2022-10-18 | Bigfoot Biomedical, Inc. | Operating an infusion pump system |
| US11478623B2 (en) | 2006-02-09 | 2022-10-25 | Deka Products Limited Partnership | Infusion pump assembly |
| US11484652B2 (en) | 2017-08-02 | 2022-11-01 | Diabeloop | Closed-loop blood glucose control systems and methods |
| US11497846B2 (en) | 2006-02-09 | 2022-11-15 | Deka Products Limited Partnership | Patch-sized fluid delivery systems and methods |
| US11497847B1 (en) | 2021-02-19 | 2022-11-15 | Fresenius Kabi Deutschland Gmbh | Wearable injector with adhesive substrate |
| US11497686B2 (en) | 2007-12-31 | 2022-11-15 | Deka Products Limited Partnership | Apparatus, system and method for fluid delivery |
| US11508476B2 (en) | 2006-10-31 | 2022-11-22 | Abbott Diabetes Care, Inc. | Infusion devices and methods |
| US11523972B2 (en) | 2018-04-24 | 2022-12-13 | Deka Products Limited Partnership | Apparatus, system and method for fluid delivery |
| US11524151B2 (en) | 2012-03-07 | 2022-12-13 | Deka Products Limited Partnership | Apparatus, system and method for fluid delivery |
| US11534542B2 (en) | 2007-12-31 | 2022-12-27 | Deka Products Limited Partnership | Apparatus, system and method for fluid delivery |
| US11559260B2 (en) | 2003-08-22 | 2023-01-24 | Dexcom, Inc. | Systems and methods for processing analyte sensor data |
| US11597541B2 (en) | 2013-07-03 | 2023-03-07 | Deka Products Limited Partnership | Apparatus, system and method for fluid delivery |
| US11633133B2 (en) | 2003-12-05 | 2023-04-25 | Dexcom, Inc. | Dual electrode system for a continuous analyte sensor |
| US11642283B2 (en) | 2007-12-31 | 2023-05-09 | Deka Products Limited Partnership | Method for fluid delivery |
| US11676694B2 (en) | 2012-06-07 | 2023-06-13 | Tandem Diabetes Care, Inc. | Device and method for training users of ambulatory medical devices |
| US11678821B2 (en) | 2007-06-29 | 2023-06-20 | Abbott Diabetes Care Inc. | Analyte monitoring and management device and method to analyze the frequency of user interaction with the device |
| US11723841B2 (en) | 2007-12-31 | 2023-08-15 | Deka Products Limited Partnership | Apparatus, system and method for fluid delivery |
| US11854693B2 (en) | 2009-06-04 | 2023-12-26 | Abbott Diabetes Care Inc. | Method and system for updating a medical device |
| US11865299B2 (en) | 2008-08-20 | 2024-01-09 | Insulet Corporation | Infusion pump systems and methods |
| US11872368B2 (en) | 2018-04-10 | 2024-01-16 | Tandem Diabetes Care, Inc. | System and method for inductively charging a medical device |
| US11890448B2 (en) | 2006-02-09 | 2024-02-06 | Deka Products Limited Partnership | Method and system for shape-memory alloy wire control |
| US11964126B2 (en) | 2006-02-09 | 2024-04-23 | Deka Products Limited Partnership | Infusion pump assembly |
| US12064590B2 (en) | 2006-02-09 | 2024-08-20 | Deka Products Limited Partnership | Patch-sized fluid delivery systems and methods |
| US12070574B2 (en) | 2006-02-09 | 2024-08-27 | Deka Products Limited Partnership | Apparatus, systems and methods for an infusion pump assembly |
| US12102791B1 (en) | 2021-04-30 | 2024-10-01 | Fresenius Kabi Deutschland Gmbh | Wearable drug delivery device with pressurized fluid dispensing |
| US12106837B2 (en) | 2016-01-14 | 2024-10-01 | Insulet Corporation | Occlusion resolution in medication delivery devices, systems, and methods |
| US12151080B2 (en) | 2006-02-09 | 2024-11-26 | Deka Products Limited Partnership | Adhesive and peripheral systems and methods for medical devices |
| US12186531B2 (en) | 2008-10-10 | 2025-01-07 | Deka Products Limited Partnership | Infusion pump assembly |
| US12239825B1 (en) | 2021-02-19 | 2025-03-04 | Fresenius Kabi Deutschland Gmbh | Drug delivery assembly including an actuator |
| US12274857B2 (en) | 2006-02-09 | 2025-04-15 | Deka Products Limited Partnership | Method and system for shape-memory alloy wire control |
| US12337138B1 (en) | 2021-02-19 | 2025-06-24 | Fresenius Kabi Deutschland Gmbh | Drug delivery assembly including a pre-filled cartridge |
| US12370327B2 (en) | 2008-10-10 | 2025-07-29 | Deka Products Limited Partnership | Infusion pump methods, systems and apparatus |
| US12370305B2 (en) | 2006-02-09 | 2025-07-29 | Deka Products Limited Partnership | Patch-sized fluid delivery systems and methods |
| US12377209B1 (en) | 2021-04-30 | 2025-08-05 | Fresenius Kabi Deutschland Gmbh | Deformable drug reservoir for wearable drug delivery device |
| US12420033B1 (en) | 2021-02-19 | 2025-09-23 | Fresenius Kabi Deutschland Gmbh | Wearable injector with sterility sensors |
Families Citing this family (314)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US6577899B2 (en) | 2000-01-21 | 2003-06-10 | Medtronic Minimed, Inc. | Microprocessor controlled ambulatory medical apparatus with hand held communication device |
| US8152789B2 (en) | 2001-10-23 | 2012-04-10 | Medtronic Minimed, Inc. | System and method for providing closed loop infusion formulation delivery |
| US7399277B2 (en) | 2001-12-27 | 2008-07-15 | Medtronic Minimed, Inc. | System for monitoring physiological characteristics |
| US10080529B2 (en) | 2001-12-27 | 2018-09-25 | Medtronic Minimed, Inc. | System for monitoring physiological characteristics |
| US7022072B2 (en) | 2001-12-27 | 2006-04-04 | Medtronic Minimed, Inc. | System for monitoring physiological characteristics |
| US20050027182A1 (en) | 2001-12-27 | 2005-02-03 | Uzair Siddiqui | System for monitoring physiological characteristics |
| US20080172026A1 (en) | 2006-10-17 | 2008-07-17 | Blomquist Michael L | Insulin pump having a suspension bolus |
| US8512276B2 (en)* | 2002-07-24 | 2013-08-20 | Medtronic Minimed, Inc. | System for providing blood glucose measurements to an infusion device |
| US20040068230A1 (en)* | 2002-07-24 | 2004-04-08 | Medtronic Minimed, Inc. | System for providing blood glucose measurements to an infusion device |
| US9123077B2 (en) | 2003-10-07 | 2015-09-01 | Hospira, Inc. | Medication management system |
| US8065161B2 (en) | 2003-11-13 | 2011-11-22 | Hospira, Inc. | System for maintaining drug information and communicating with medication delivery devices |
| EP2256495B1 (en) | 2004-06-03 | 2015-07-29 | Medtronic MiniMed, Inc. | System for monitoring physiological characteristics according to the user biological state |
| WO2007056592A2 (en) | 2005-11-08 | 2007-05-18 | M2 Medical A/S | Method and system for manual and autonomous control of an infusion pump |
| ES2336360T3 (en)* | 2006-04-20 | 2010-04-12 | Lifescan Scotland Ltd | METHOD FOR TRANSMITTING DATA IN A GLUCOSE SYSTEM IN BLOOD AND GLUCOSE SYSTEM IN BLOOD CORRESPONDING. |
| US9056165B2 (en) | 2006-09-06 | 2015-06-16 | Medtronic Minimed, Inc. | Intelligent therapy recommendation algorithm and method of using the same |
| AU2007317669A1 (en) | 2006-10-16 | 2008-05-15 | Hospira, Inc. | System and method for comparing and utilizing activity information and configuration information from mulitple device management systems |
| US8231048B2 (en)* | 2006-11-20 | 2012-07-31 | Bayer Healthcare Llc | Test-sensor cartridge |
| EP2120680A2 (en) | 2007-02-06 | 2009-11-25 | Glumetrics, Inc. | Optical systems and methods for rationmetric measurement of blood glucose concentration |
| WO2008141241A1 (en) | 2007-05-10 | 2008-11-20 | Glumetrics, Inc. | Equilibrium non-consuming fluorescence sensor for real time intravascular glucose measurement |
| US9968742B2 (en) | 2007-08-29 | 2018-05-15 | Medtronic Minimed, Inc. | Combined sensor and infusion set using separated sites |
| US20120046533A1 (en) | 2007-08-29 | 2012-02-23 | Medtronic Minimed, Inc. | Combined sensor and infusion sets |
| USD612279S1 (en) | 2008-01-18 | 2010-03-23 | Lifescan Scotland Limited | User interface in an analyte meter |
| USD612275S1 (en) | 2008-03-21 | 2010-03-23 | Lifescan Scotland, Ltd. | Analyte test meter |
| USD611151S1 (en) | 2008-06-10 | 2010-03-02 | Lifescan Scotland, Ltd. | Test meter |
| US8700114B2 (en) | 2008-07-31 | 2014-04-15 | Medtronic Minmed, Inc. | Analyte sensor apparatuses comprising multiple implantable sensor elements and methods for making and using them |
| US9566383B2 (en)* | 2008-10-16 | 2017-02-14 | Roche Diabetes Care, Inc. | Method and system for adaptive communication transmission |
| EP2241344B1 (en)* | 2009-04-16 | 2013-12-11 | F. Hoffmann-La Roche AG | Ambulatory infusion device with sensor testing unit |
| US8271106B2 (en) | 2009-04-17 | 2012-09-18 | Hospira, Inc. | System and method for configuring a rule set for medical event management and responses |
| RU2012103000A (en)* | 2009-06-30 | 2013-08-10 | Лайфскен, Инк. | METHOD AND SYSTEM FOR ANALYTES TESTING |
| EP2455875A3 (en)* | 2009-06-30 | 2013-01-16 | Lifescan Scotland Limited | System and method for diabetes management |
| EP3973855A1 (en)* | 2009-06-30 | 2022-03-30 | Lifescan, Inc. | Analyte testing methods and device for calculating basal insulin therapy |
| US8545693B2 (en)* | 2009-09-29 | 2013-10-01 | Lifescan Scotland Limited | Analyte measurment method and system |
| US20110082356A1 (en) | 2009-10-01 | 2011-04-07 | Medtronic Minimed, Inc. | Analyte sensor apparatuses having interference rejection membranes and methods for making and using them |
| US20110288388A1 (en) | 2009-11-20 | 2011-11-24 | Medtronic Minimed, Inc. | Multi-conductor lead configurations useful with medical device systems and methods for making and using them |
| US8660628B2 (en) | 2009-12-21 | 2014-02-25 | Medtronic Minimed, Inc. | Analyte sensors comprising blended membrane compositions and methods for making and using them |
| US8070723B2 (en) | 2009-12-31 | 2011-12-06 | Medtronic Minimed, Inc. | Activity guard |
| US20110184653A1 (en)* | 2010-01-22 | 2011-07-28 | Lifescan, Inc. | Analyte testing method and system |
| BR112012021572A2 (en) | 2010-02-25 | 2016-10-25 | Lifescan Scotland Ltd | analyte testing method and system with notification of high and low blood glucose trends. |
| US10448872B2 (en) | 2010-03-16 | 2019-10-22 | Medtronic Minimed, Inc. | Analyte sensor apparatuses having improved electrode configurations and methods for making and using them |
| US8246573B2 (en)* | 2010-04-27 | 2012-08-21 | Medtronic, Inc. | Detecting empty medical pump reservoir |
| US9215995B2 (en) | 2010-06-23 | 2015-12-22 | Medtronic Minimed, Inc. | Sensor systems having multiple probes and electrode arrays |
| US8401194B2 (en) | 2010-10-15 | 2013-03-19 | Roche Diagnostics Operations, Inc. | Diabetes care kit that is preconfigured to establish a secure bidirectional communication link between a blood glucose meter and insulin pump |
| US8454554B2 (en)* | 2010-10-15 | 2013-06-04 | Roche Diagnostics Operations, Inc. | Use of a handheld medical device as a communications mediator between a personal computer-based configurator and another networked medical device |
| US8861731B2 (en)* | 2010-10-15 | 2014-10-14 | Roche Diagnostics Operations, Inc. | Efficient procedure for pairing medical devices for wireless communication with limited user interaction |
| US8427817B2 (en)* | 2010-10-15 | 2013-04-23 | Roche Diagnostics Operations, Inc. | Handheld diabetes manager with touch screen display |
| US9192719B2 (en) | 2010-11-01 | 2015-11-24 | Medtronic, Inc. | Implantable medical pump diagnostics |
| WO2012059209A1 (en) | 2010-11-01 | 2012-05-10 | Roche Diagnostics Gmbh | Fluid dispensing device with a flow detector |
| US8672874B2 (en) | 2010-12-22 | 2014-03-18 | Roche Diagnoistics Operations, Inc. | Communication protocol that supports pass-thru communication |
| US8628510B2 (en) | 2010-12-22 | 2014-01-14 | Medtronic Minimed, Inc. | Monitoring the operating health of a force sensor in a fluid infusion device |
| US9339639B2 (en) | 2011-02-22 | 2016-05-17 | Medtronic Minimed, Inc. | Sealing assembly for a fluid reservoir of a fluid infusion device |
| US11266823B2 (en) | 2011-02-22 | 2022-03-08 | Medtronic Minimed, Inc. | Retractable sealing assembly for a fluid reservoir of a fluid infusion device |
| US8454581B2 (en) | 2011-03-16 | 2013-06-04 | Asante Solutions, Inc. | Infusion pump systems and methods |
| US9008744B2 (en) | 2011-05-06 | 2015-04-14 | Medtronic Minimed, Inc. | Method and apparatus for continuous analyte monitoring |
| US8795231B2 (en) | 2011-05-10 | 2014-08-05 | Medtronic Minimed, Inc. | Automated reservoir fill system |
| WO2013049068A1 (en) | 2011-09-27 | 2013-04-04 | Glumetrics, Inc. | Method for functionalizing a porous membrane covering of an optical sensor to facilitate coupling of an antithrom-bogenic agent |
| CA2852271A1 (en) | 2011-10-21 | 2013-04-25 | Hospira, Inc. | Medical device update system |
| US9989522B2 (en) | 2011-11-01 | 2018-06-05 | Medtronic Minimed, Inc. | Methods and materials for modulating start-up time and air removal in dry sensors |
| US8999720B2 (en) | 2011-11-17 | 2015-04-07 | Medtronic Minimed, Inc. | Aqueous radiation protecting formulations and methods for making and using them |
| US9136939B2 (en) | 2011-12-29 | 2015-09-15 | Roche Diabetes Care, Inc. | Graphical user interface pertaining to a bolus calculator residing on a handheld diabetes management device |
| US9610401B2 (en) | 2012-01-13 | 2017-04-04 | Medtronic Minimed, Inc. | Infusion set component with modular fluid channel element |
| US9493807B2 (en) | 2012-05-25 | 2016-11-15 | Medtronic Minimed, Inc. | Foldover sensors and methods for making and using them |
| US9632060B2 (en) | 2012-06-08 | 2017-04-25 | Medtronic Minimed, Inc. | Application of electrochemical impedance spectroscopy in sensor systems, devices, and related methods |
| US9682188B2 (en) | 2012-08-21 | 2017-06-20 | Medtronic Minimed, Inc. | Reservoir fluid volume estimator and medical device incorporating same |
| US9662445B2 (en) | 2012-08-30 | 2017-05-30 | Medtronic Minimed, Inc. | Regulating entry into a closed-loop operating mode of an insulin infusion system |
| US10130767B2 (en) | 2012-08-30 | 2018-11-20 | Medtronic Minimed, Inc. | Sensor model supervisor for a closed-loop insulin infusion system |
| WO2014074621A1 (en) | 2012-11-07 | 2014-05-15 | Glumetrics, Inc. | Dry insertion and one-point in vivo calibration of an optical analyte sensor |
| US9265455B2 (en) | 2012-11-13 | 2016-02-23 | Medtronic Minimed, Inc. | Methods and systems for optimizing sensor function by the application of voltage |
| US10194840B2 (en) | 2012-12-06 | 2019-02-05 | Medtronic Minimed, Inc. | Microarray electrodes useful with analyte sensors and methods for making and using them |
| US10426383B2 (en) | 2013-01-22 | 2019-10-01 | Medtronic Minimed, Inc. | Muting glucose sensor oxygen response and reducing electrode edge growth with pulsed current plating |
| WO2014138446A1 (en) | 2013-03-06 | 2014-09-12 | Hospira,Inc. | Medical device communication method |
| US9338819B2 (en) | 2013-05-29 | 2016-05-10 | Medtronic Minimed, Inc. | Variable data usage personal medical system and method |
| US10194864B2 (en) | 2013-06-21 | 2019-02-05 | Medtronic Minimed, Inc. | Anchoring apparatus and method for attaching device on body |
| US9880528B2 (en) | 2013-08-21 | 2018-01-30 | Medtronic Minimed, Inc. | Medical devices and related updating methods and systems |
| EP3039596A4 (en) | 2013-08-30 | 2017-04-12 | Hospira, Inc. | System and method of monitoring and managing a remote infusion regimen |
| US9662436B2 (en) | 2013-09-20 | 2017-05-30 | Icu Medical, Inc. | Fail-safe drug infusion therapy system |
| US8979799B1 (en) | 2013-10-14 | 2015-03-17 | Medtronic Minimed, Inc. | Electronic injector |
| US8979808B1 (en) | 2013-10-14 | 2015-03-17 | Medtronic Minimed, Inc. | On-body injector and method of use |
| US9265881B2 (en) | 2013-10-14 | 2016-02-23 | Medtronic Minimed, Inc. | Therapeutic agent injection device |
| US9375537B2 (en) | 2013-10-14 | 2016-06-28 | Medtronic Minimed, Inc. | Therapeutic agent injection device |
| US9226709B2 (en) | 2013-11-04 | 2016-01-05 | Medtronic Minimed, Inc. | ICE message system and method |
| US10311972B2 (en) | 2013-11-11 | 2019-06-04 | Icu Medical, Inc. | Medical device system performance index |
| EP3071253B1 (en) | 2013-11-19 | 2019-05-22 | ICU Medical, Inc. | Infusion pump automation system and method |
| US9267875B2 (en) | 2013-11-21 | 2016-02-23 | Medtronic Minimed, Inc. | Accelerated life testing device and method |
| US9750877B2 (en) | 2013-12-11 | 2017-09-05 | Medtronic Minimed, Inc. | Predicted time to assess and/or control a glycemic state |
| US9849240B2 (en) | 2013-12-12 | 2017-12-26 | Medtronic Minimed, Inc. | Data modification for predictive operations and devices incorporating same |
| US10105488B2 (en) | 2013-12-12 | 2018-10-23 | Medtronic Minimed, Inc. | Predictive infusion device operations and related methods and systems |
| US9603561B2 (en) | 2013-12-16 | 2017-03-28 | Medtronic Minimed, Inc. | Methods and systems for improving the reliability of orthogonally redundant sensors |
| US10638947B2 (en) | 2013-12-16 | 2020-05-05 | Medtronic Minimed, Inc. | Use of electrochemical impedance spectroscopy (EIS) in intelligent diagnostics |
| US9779226B2 (en) | 2013-12-18 | 2017-10-03 | Medtronic Minimed, Inc. | Fingerprint enhanced authentication for medical devices in wireless networks |
| US9143941B2 (en) | 2013-12-18 | 2015-09-22 | Medtronic Minimed, Inc. | Secure communication by user selectable communication range |
| US9694132B2 (en) | 2013-12-19 | 2017-07-04 | Medtronic Minimed, Inc. | Insertion device for insertion set |
| US9861748B2 (en) | 2014-02-06 | 2018-01-09 | Medtronic Minimed, Inc. | User-configurable closed-loop notifications and infusion systems incorporating same |
| US9388805B2 (en) | 2014-03-24 | 2016-07-12 | Medtronic Minimed, Inc. | Medication pump test device and method of use |
| US9689830B2 (en) | 2014-04-03 | 2017-06-27 | Medtronic Minimed, Inc. | Sensor detection pads with integrated fuse |
| US9707336B2 (en) | 2014-04-07 | 2017-07-18 | Medtronic Minimed, Inc. | Priming detection system and method of using the same |
| US10232113B2 (en) | 2014-04-24 | 2019-03-19 | Medtronic Minimed, Inc. | Infusion devices and related methods and systems for regulating insulin on board |
| EP3138032B1 (en) | 2014-04-30 | 2024-07-24 | ICU Medical, Inc. | Patient care system with conditional alarm forwarding |
| US9681828B2 (en) | 2014-05-01 | 2017-06-20 | Medtronic Minimed, Inc. | Physiological characteristic sensors and methods for forming such sensors |
| US9901305B2 (en) | 2014-06-13 | 2018-02-27 | Medtronic Minimed, Inc. | Physiological sensor history backfill system and method |
| US9724470B2 (en) | 2014-06-16 | 2017-08-08 | Icu Medical, Inc. | System for monitoring and delivering medication to a patient and method of using the same to minimize the risks associated with automated therapy |
| US9452255B2 (en) | 2014-07-21 | 2016-09-27 | Medtronic Minimed, Inc. | Smart connection interface |
| US9717845B2 (en) | 2014-08-19 | 2017-08-01 | Medtronic Minimed, Inc. | Geofencing for medical devices |
| US20160051755A1 (en) | 2014-08-25 | 2016-02-25 | Medtronic Minimed, Inc. | Low cost fluid delivery device |
| US9539383B2 (en) | 2014-09-15 | 2017-01-10 | Hospira, Inc. | System and method that matches delayed infusion auto-programs with manually entered infusion programs and analyzes differences therein |
| US9839753B2 (en) | 2014-09-26 | 2017-12-12 | Medtronic Minimed, Inc. | Systems for managing reservoir chamber pressure |
| US10279126B2 (en) | 2014-10-07 | 2019-05-07 | Medtronic Minimed, Inc. | Fluid conduit assembly with gas trapping filter in the fluid flow path |
| US9841014B2 (en) | 2014-10-20 | 2017-12-12 | Medtronic Minimed, Inc. | Insulin pump data acquisition device and system |
| US9592335B2 (en) | 2014-10-20 | 2017-03-14 | Medtronic Minimed, Inc. | Insulin pump data acquisition device |
| US9731067B2 (en) | 2014-11-25 | 2017-08-15 | Medtronic Minimed, Inc. | Mechanical injection pump and method of use |
| US9901675B2 (en) | 2014-11-25 | 2018-02-27 | Medtronic Minimed, Inc. | Infusion set insertion device and method of use |
| US9943645B2 (en) | 2014-12-04 | 2018-04-17 | Medtronic Minimed, Inc. | Methods for operating mode transitions and related infusion devices and systems |
| US9636453B2 (en) | 2014-12-04 | 2017-05-02 | Medtronic Minimed, Inc. | Advance diagnosis of infusion device operating mode viability |
| US10307535B2 (en) | 2014-12-19 | 2019-06-04 | Medtronic Minimed, Inc. | Infusion devices and related methods and systems for preemptive alerting |
| US9717848B2 (en) | 2015-01-22 | 2017-08-01 | Medtronic Minimed, Inc. | Data derived pre-bolus delivery |
| US9872954B2 (en) | 2015-03-02 | 2018-01-23 | Medtronic Minimed, Inc. | Belt clip |
| US10307528B2 (en) | 2015-03-09 | 2019-06-04 | Medtronic Minimed, Inc. | Extensible infusion devices and related methods |
| US10449298B2 (en) | 2015-03-26 | 2019-10-22 | Medtronic Minimed, Inc. | Fluid injection devices and related methods |
| US10130757B2 (en) | 2015-05-01 | 2018-11-20 | Medtronic Minimed, Inc. | Method and system for leakage detection in portable medical devices |
| EP3304370B1 (en) | 2015-05-26 | 2020-12-30 | ICU Medical, Inc. | Infusion pump system and method with multiple drug library editor source capability |
| US9999721B2 (en) | 2015-05-26 | 2018-06-19 | Medtronic Minimed, Inc. | Error handling in infusion devices with distributed motor control and related operating methods |
| US10201657B2 (en) | 2015-08-21 | 2019-02-12 | Medtronic Minimed, Inc. | Methods for providing sensor site rotation feedback and related infusion devices and systems |
| US10463297B2 (en) | 2015-08-21 | 2019-11-05 | Medtronic Minimed, Inc. | Personalized event detection methods and related devices and systems |
| US10293108B2 (en) | 2015-08-21 | 2019-05-21 | Medtronic Minimed, Inc. | Infusion devices and related patient ratio adjustment methods |
| US10478557B2 (en) | 2015-08-21 | 2019-11-19 | Medtronic Minimed, Inc. | Personalized parameter modeling methods and related devices and systems |
| US10117992B2 (en) | 2015-09-29 | 2018-11-06 | Medtronic Minimed, Inc. | Infusion devices and related rescue detection methods |
| US9992818B2 (en) | 2015-10-06 | 2018-06-05 | Medtronic Minimed, Inc. | Protocol translation device |
| US11666702B2 (en) | 2015-10-19 | 2023-06-06 | Medtronic Minimed, Inc. | Medical devices and related event pattern treatment recommendation methods |
| US11501867B2 (en) | 2015-10-19 | 2022-11-15 | Medtronic Minimed, Inc. | Medical devices and related event pattern presentation methods |
| US9757511B2 (en) | 2015-10-19 | 2017-09-12 | Medtronic Minimed, Inc. | Personal medical device and method of use with restricted mode challenge |
| US10146911B2 (en) | 2015-10-23 | 2018-12-04 | Medtronic Minimed, Inc. | Medical devices and related methods and systems for data transfer |
| US10037722B2 (en) | 2015-11-03 | 2018-07-31 | Medtronic Minimed, Inc. | Detecting breakage in a display element |
| US10827959B2 (en) | 2015-11-11 | 2020-11-10 | Medtronic Minimed, Inc. | Sensor set |
| US10350380B2 (en) | 2015-12-17 | 2019-07-16 | Soniphi Llc | Systems and methods for distal control of health effectors |
| US9848805B2 (en) | 2015-12-18 | 2017-12-26 | Medtronic Minimed, Inc. | Biostable glucose permeable polymer |
| US10349872B2 (en) | 2015-12-28 | 2019-07-16 | Medtronic Minimed, Inc. | Methods, systems, and devices for sensor fusion |
| US20170181672A1 (en) | 2015-12-28 | 2017-06-29 | Medtronic Minimed, Inc. | Sensor systems, devices, and methods for continuous glucose monitoring |
| US10327686B2 (en) | 2015-12-28 | 2019-06-25 | Medtronic Minimed, Inc. | Sensor systems, devices, and methods for continuous glucose monitoring |
| US20170184527A1 (en) | 2015-12-28 | 2017-06-29 | Medtronic Minimed, Inc. | Sensor systems, devices, and methods for continuous glucose monitoring |
| US10327680B2 (en) | 2015-12-28 | 2019-06-25 | Medtronic Minimed, Inc. | Sensor systems, devices, and methods for continuous glucose monitoring |
| US10449294B1 (en) | 2016-01-05 | 2019-10-22 | Bigfoot Biomedical, Inc. | Operating an infusion pump system |
| US20230368046A9 (en) | 2016-01-28 | 2023-11-16 | Medtronic Minimed, Inc. | Activation of Ancillary Sensor Systems Based on Triggers from a Wearable Gesture Sensing Device |
| US10790054B1 (en) | 2016-12-07 | 2020-09-29 | Medtronic Minimed, Inc. | Method and apparatus for tracking of food intake and other behaviors and providing relevant feedback |
| EP3407780A4 (en) | 2016-01-28 | 2019-09-18 | Klue, Inc. | METHOD AND APPARATUS FOR MONITORING FOOD RECEPTACLE AND OTHER BEHAVIOR AND PROVIDING RETURN OF RELEVANT INFORMATION |
| US10631787B2 (en) | 2016-04-08 | 2020-04-28 | Medtronic Minimed, Inc. | Sensor and transmitter product |
| US12419547B2 (en) | 2016-04-08 | 2025-09-23 | Medtronic Minimed, Inc. | Sensor and transmitter product |
| US10765369B2 (en) | 2016-04-08 | 2020-09-08 | Medtronic Minimed, Inc. | Analyte sensor |
| US10765348B2 (en) | 2016-04-08 | 2020-09-08 | Medtronic Minimed, Inc. | Sensor and transmitter product |
| US10589038B2 (en) | 2016-04-27 | 2020-03-17 | Medtronic Minimed, Inc. | Set connector systems for venting a fluid reservoir |
| US10426389B2 (en) | 2016-04-28 | 2019-10-01 | Medtronic Minimed, Inc. | Methods, systems, and devices for electrode capacitance calculation and application |
| US10324058B2 (en) | 2016-04-28 | 2019-06-18 | Medtronic Minimed, Inc. | In-situ chemistry stack for continuous glucose sensors |
| US9970893B2 (en) | 2016-04-28 | 2018-05-15 | Medtronic Minimed, Inc. | Methods, systems, and devices for electrode capacitance calculation and application |
| US10086133B2 (en) | 2016-05-26 | 2018-10-02 | Medtronic Minimed, Inc. | Systems for set connector assembly with lock |
| US10086134B2 (en) | 2016-05-26 | 2018-10-02 | Medtronic Minimed, Inc. | Systems for set connector assembly with lock |
| US9968737B2 (en) | 2016-05-26 | 2018-05-15 | Medtronic Minimed, Inc. | Systems for set connector assembly with lock |
| US11179078B2 (en) | 2016-06-06 | 2021-11-23 | Medtronic Minimed, Inc. | Polycarbonate urea/urethane polymers for use with analyte sensors |
| US11134872B2 (en) | 2016-06-06 | 2021-10-05 | Medtronic Minimed, Inc. | Thermally stable glucose limiting membrane for glucose sensors |
| CA3030786A1 (en) | 2016-07-14 | 2018-01-18 | Icu Medical, Inc. | Multi-communication path selection and security system for a medical device |
| US11238133B1 (en) | 2016-07-14 | 2022-02-01 | Bigfoot Biomedical, Inc. | Systems and methods for monitoring use of and ensuring continuity of functionality of insulin infusion pumps, glucose monitors, and other diabetes treatment equipment |
| US10449291B2 (en) | 2016-09-06 | 2019-10-22 | Medtronic Minimed, Inc. | Pump clip for a fluid infusion device |
| US10709834B2 (en) | 2016-12-21 | 2020-07-14 | Medtronic Minimed, Inc. | Medication fluid infusion set component with integrated physiological analyte sensor, and corresponding fluid infusion device |
| US10861591B2 (en) | 2016-12-21 | 2020-12-08 | Medtronic Minimed, Inc. | Infusion systems and methods for pattern-based therapy adjustments |
| US10272201B2 (en) | 2016-12-22 | 2019-04-30 | Medtronic Minimed, Inc. | Insertion site monitoring methods and related infusion devices and systems |
| US11197949B2 (en) | 2017-01-19 | 2021-12-14 | Medtronic Minimed, Inc. | Medication infusion components and systems |
| US10821225B2 (en) | 2017-01-20 | 2020-11-03 | Medtronic Minimed, Inc. | Cannulas for drug delivery devices |
| US10552580B2 (en) | 2017-02-07 | 2020-02-04 | Medtronic Minimed, Inc. | Infusion system consumables and related calibration methods |
| US10646649B2 (en) | 2017-02-21 | 2020-05-12 | Medtronic Minimed, Inc. | Infusion devices and fluid identification apparatuses and methods |
| US11986288B2 (en) | 2017-03-06 | 2024-05-21 | Medtronic Minimed, Inc. | Colorometric sensor for the non-invasive screening of glucose in sweat in pre and type 2 diabetes |
| US11134868B2 (en) | 2017-03-17 | 2021-10-05 | Medtronic Minimed, Inc. | Metal pillar device structures and methods for making and using them in electrochemical and/or electrocatalytic applications |
| US11350886B2 (en) | 2017-03-24 | 2022-06-07 | Medtronic Minimed, Inc. | Context-sensitive infusion devices, systems and methods |
| USD853583S1 (en) | 2017-03-29 | 2019-07-09 | Becton, Dickinson And Company | Hand-held device housing |
| AU2018261124B2 (en) | 2017-05-05 | 2021-01-28 | Ypsomed Ag | Closed loop control of physiological glucose |
| US20180328877A1 (en) | 2017-05-11 | 2018-11-15 | Medtronic Minimed, Inc. | Analyte sensors and methods for fabricating analyte sensors |
| US10856784B2 (en) | 2017-06-30 | 2020-12-08 | Medtronic Minimed, Inc. | Sensor initialization methods for faster body sensor response |
| US11412960B2 (en) | 2017-08-28 | 2022-08-16 | Medtronic Minimed, Inc. | Pedestal for sensor assembly packaging and sensor introducer removal |
| US10596295B2 (en) | 2017-08-28 | 2020-03-24 | Medtronic Minimed, Inc. | Adhesive patch arrangement for a physiological characteristic sensor, and related sensor assembly |
| US11344235B2 (en) | 2017-09-13 | 2022-05-31 | Medtronic Minimed, Inc. | Methods, systems, and devices for calibration and optimization of glucose sensors and sensor output |
| US10874300B2 (en) | 2017-09-26 | 2020-12-29 | Medtronic Minimed, Inc. | Waferscale physiological characteristic sensor package with integrated wireless transmitter |
| US10524730B2 (en) | 2017-09-28 | 2020-01-07 | Medtronic Minimed, Inc. | Medical devices with microneedle arrays and methods for operating such medical devices |
| US10525244B2 (en) | 2017-09-28 | 2020-01-07 | Medtronic Minimed, Inc. | Microneedle arrays and methods for fabricating microneedle arrays |
| US11676734B2 (en) | 2017-11-15 | 2023-06-13 | Medtronic Minimed, Inc. | Patient therapy management system that leverages aggregated patient population data |
| US11213230B2 (en) | 2017-12-13 | 2022-01-04 | Medtronic Minimed, Inc. | Optional sensor calibration in continuous glucose monitoring |
| US11471082B2 (en) | 2017-12-13 | 2022-10-18 | Medtronic Minimed, Inc. | Complex redundancy in continuous glucose monitoring |
| US11901060B2 (en) | 2017-12-21 | 2024-02-13 | Ypsomed Ag | Closed loop control of physiological glucose |
| US11439352B2 (en) | 2018-01-17 | 2022-09-13 | Medtronic Minimed, Inc. | Medical device with adhesive patch longevity |
| US12042284B2 (en) | 2018-01-23 | 2024-07-23 | Medtronic Minimed, Inc. | Implantable polymer surfaces exhibiting reduced in vivo inflammatory responses |
| US11186859B2 (en) | 2018-02-07 | 2021-11-30 | Medtronic Minimed, Inc. | Multilayer electrochemical analyte sensors and methods for making and using them |
| US11583213B2 (en) | 2018-02-08 | 2023-02-21 | Medtronic Minimed, Inc. | Glucose sensor electrode design |
| US11220735B2 (en) | 2018-02-08 | 2022-01-11 | Medtronic Minimed, Inc. | Methods for controlling physical vapor deposition metal film adhesion to substrates and surfaces |
| US11672446B2 (en) | 2018-03-23 | 2023-06-13 | Medtronic Minimed, Inc. | Insulin delivery recommendations based on nutritional information |
| US11158413B2 (en) | 2018-04-23 | 2021-10-26 | Medtronic Minimed, Inc. | Personalized closed loop medication delivery system that utilizes a digital twin of the patient |
| US11147919B2 (en) | 2018-04-23 | 2021-10-19 | Medtronic Minimed, Inc. | Methodology to recommend and implement adjustments to a fluid infusion device of a medication delivery system |
| US11367526B2 (en) | 2018-05-07 | 2022-06-21 | Medtronic Minimed, Inc. | Proactive patient guidance using augmented reality |
| EP3794135A1 (en) | 2018-05-16 | 2021-03-24 | Medtronic MiniMed, Inc. | Thermally stable glucose limiting membrane for glucose sensors |
| CA3103479A1 (en) | 2018-06-22 | 2019-12-26 | Eli Lilly And Company | Insulin and pramlintide delivery systems, methods, and devices |
| US11483402B2 (en) | 2018-07-17 | 2022-10-25 | Icu Medical, Inc. | Maintaining clinical messaging during an internet outage |
| ES2985889T3 (en) | 2018-07-17 | 2024-11-07 | Icu Medical Inc | Updating infusion pump drug libraries and operational software in a networked environment |
| CA3106519A1 (en) | 2018-07-17 | 2020-01-23 | Icu Medical, Inc. | Systems and methods for facilitating clinical messaging in a network environment |
| US11139058B2 (en) | 2018-07-17 | 2021-10-05 | Icu Medical, Inc. | Reducing file transfer between cloud environment and infusion pumps |
| EP3827337A4 (en) | 2018-07-26 | 2022-04-13 | ICU Medical, Inc. | Drug library management system |
| US10692595B2 (en) | 2018-07-26 | 2020-06-23 | Icu Medical, Inc. | Drug library dynamic version management |
| US11761077B2 (en) | 2018-08-01 | 2023-09-19 | Medtronic Minimed, Inc. | Sputtering techniques for biosensors |
| US11122697B2 (en) | 2018-08-07 | 2021-09-14 | Medtronic Minimed, Inc. | Method of fabricating an electronic medical device, including overmolding an assembly with thermoplastic material |
| US11021731B2 (en) | 2018-08-23 | 2021-06-01 | Medtronic Minimed, Inc. | Analyte sensing layers, analyte sensors and methods for fabricating the same |
| US10828419B2 (en) | 2018-09-04 | 2020-11-10 | Medtronic Minimed, Inc. | Infusion set with pivoting metal cannula and strain relief |
| US11547799B2 (en) | 2018-09-20 | 2023-01-10 | Medtronic Minimed, Inc. | Patient day planning systems and methods |
| US11097052B2 (en) | 2018-09-28 | 2021-08-24 | Medtronic Minimed, Inc. | Insulin infusion device with configurable target blood glucose value for automatic basal insulin delivery operation |
| US11071821B2 (en) | 2018-09-28 | 2021-07-27 | Medtronic Minimed, Inc. | Insulin infusion device with efficient confirmation routine for blood glucose measurements |
| US10894126B2 (en) | 2018-09-28 | 2021-01-19 | Medtronic Minimed, Inc. | Fluid infusion system that automatically determines and delivers a correction bolus |
| US10980942B2 (en) | 2018-09-28 | 2021-04-20 | Medtronic Minimed, Inc. | Infusion devices and related meal bolus adjustment methods |
| US10946140B2 (en) | 2018-10-11 | 2021-03-16 | Medtronic Minimed, Inc. | Systems and methods for measurement of fluid delivery |
| US20200116748A1 (en) | 2018-10-11 | 2020-04-16 | Medtronic Minimed, Inc. | Systems and methods for measurement of fluid delivery |
| US11367516B2 (en) | 2018-10-31 | 2022-06-21 | Medtronic Minimed, Inc. | Automated detection of a physical behavior event and corresponding adjustment of a medication dispensing system |
| US20200289373A1 (en) | 2018-10-31 | 2020-09-17 | Medtronic Minimed, Inc. | Automated detection of a physical behavior event and corresponding adjustment of a physiological characteristic sensor device |
| US11363986B2 (en) | 2018-10-31 | 2022-06-21 | Medtronic Minimed, Inc. | Automated detection of a physical behavior event and corresponding adjustment of a medication dispensing system |
| US11367517B2 (en) | 2018-10-31 | 2022-06-21 | Medtronic Minimed, Inc. | Gesture-based detection of a physical behavior event based on gesture sensor data and supplemental information from at least one external source |
| US11382541B2 (en) | 2018-11-16 | 2022-07-12 | Medtronic Minimed, Inc. | Miniaturized analyte sensor |
| US11540750B2 (en) | 2018-12-19 | 2023-01-03 | Medtronic Minimed, Inc | Systems and methods for physiological characteristic monitoring |
| US12114972B2 (en) | 2019-02-01 | 2024-10-15 | Medtronic Minimed, Inc. | Methods, systems, and devices for continuous glucose monitoring |
| US11439752B2 (en) | 2019-02-01 | 2022-09-13 | Medtronic Minimed, Inc. | Methods and devices for occlusion detection using actuator sensors |
| US11389587B2 (en) | 2019-02-06 | 2022-07-19 | Medtronic Minimed, Inc. | Patient monitoring systems and related presentation methods |
| US12082910B2 (en) | 2019-02-12 | 2024-09-10 | Medtronic Minimed, Inc. | Miniaturized noninvasive glucose sensor and continuous glucose monitoring system |
| US11191899B2 (en) | 2019-02-12 | 2021-12-07 | Medtronic Minimed, Inc. | Infusion systems and related personalized bolusing methods |
| US11311215B2 (en) | 2019-04-04 | 2022-04-26 | Medtronic Minimed, Inc. | Measurement of device materials using non-Faradaic electrochemical impedance spectroscopy |
| US11986629B2 (en) | 2019-06-11 | 2024-05-21 | Medtronic Minimed, Inc. | Personalized closed loop optimization systems and methods |
| US11224361B2 (en) | 2019-04-23 | 2022-01-18 | Medtronic Minimed, Inc. | Flexible physiological characteristic sensor assembly |
| US11317867B2 (en) | 2019-04-23 | 2022-05-03 | Medtronic Minimed, Inc. | Flexible physiological characteristic sensor assembly |
| CA3138528A1 (en) | 2019-05-08 | 2020-11-12 | Icu Medical, Inc. | Threshold signature based medical device management |
| US10939488B2 (en) | 2019-05-20 | 2021-03-02 | Medtronic Minimed, Inc. | Method and system for controlling communication between devices of a wireless body area network for an medical device system |
| US11642454B2 (en) | 2019-06-06 | 2023-05-09 | Medtronic Minimed, Inc. | Fluid infusion systems |
| US11448611B2 (en) | 2019-07-03 | 2022-09-20 | Medtronic Minimed, Inc. | Structurally reinforced sensor and method for manufacturing the same |
| EP4000075A4 (en) | 2019-07-16 | 2023-10-04 | Beta Bionics, Inc. | BLOOD GLUCOSE CONTROL SYSTEM |
| US11617828B2 (en) | 2019-07-17 | 2023-04-04 | Medtronic Minimed, Inc. | Reservoir connection interface with detectable signature |
| US11718865B2 (en) | 2019-07-26 | 2023-08-08 | Medtronic Minimed, Inc. | Methods to improve oxygen delivery to implantable sensors |
| US11523757B2 (en) | 2019-08-01 | 2022-12-13 | Medtronic Minimed, Inc. | Micro-pillar working electrodes design to reduce backflow of hydrogen peroxide in glucose sensor |
| US11617522B2 (en) | 2019-08-06 | 2023-04-04 | Medtronic Minimed, Inc. | Sensor inserter with disposal lockout state |
| US11883208B2 (en) | 2019-08-06 | 2024-01-30 | Medtronic Minimed, Inc. | Machine learning-based system for estimating glucose values based on blood glucose measurements and contextual activity data |
| US20220039755A1 (en) | 2020-08-06 | 2022-02-10 | Medtronic Minimed, Inc. | Machine learning-based system for estimating glucose values |
| US11724045B2 (en) | 2019-08-21 | 2023-08-15 | Medtronic Minimed, Inc. | Connection of a stopper and piston in a fluid delivery device |
| US20210060244A1 (en) | 2019-08-28 | 2021-03-04 | Medtronic Minimed, Inc. | Method and system for verifying whether a non-medical client device is operating correctly with a medical device controlled by the non-medical client device and causing a notification to be generated |
| US11571515B2 (en) | 2019-08-29 | 2023-02-07 | Medtronic Minimed, Inc. | Controlling medical device operation and features based on detected patient sleeping status |
| US11654235B2 (en) | 2019-09-12 | 2023-05-23 | Medtronic Minimed, Inc. | Sensor calibration using fabrication measurements |
| US11565044B2 (en) | 2019-09-12 | 2023-01-31 | Medtronic Minimed, Inc. | Manufacturing controls for sensor calibration using fabrication measurements |
| US11213623B2 (en) | 2019-09-20 | 2022-01-04 | Medtronic Minimed, Inc. | Infusion systems and related personalized bolusing methods |
| US11241537B2 (en) | 2019-09-20 | 2022-02-08 | Medtronic Minimed, Inc. | Contextual personalized closed-loop adjustment methods and systems |
| US11511099B2 (en) | 2019-10-08 | 2022-11-29 | Medtronic Minimed, Inc. | Apparatus for detecting mating of a cap with a fluid delivery device and method |
| US12329926B2 (en) | 2019-10-10 | 2025-06-17 | Medtronic Minimed, Inc. | Adjustable insertion device |
| US11638545B2 (en) | 2019-10-16 | 2023-05-02 | Medtronic Minimed, Inc. | Reducing sensor foreign body response via high surface area metal structures |
| US12315631B2 (en) | 2019-11-07 | 2025-05-27 | Medtronic Minimed, Inc. | Medical device site monitoring methods and related devices and systems |
| US12263327B2 (en) | 2019-11-14 | 2025-04-01 | Medtronic Minimed, Inc. | Synergistic features and functions related to operation of a medication delivery system and a meal transaction application |
| US11496083B2 (en) | 2019-11-15 | 2022-11-08 | Medtronic Minimed, Inc. | Devices and methods for controlling electromechanical actuators |
| US11944784B2 (en) | 2019-11-18 | 2024-04-02 | Medtronic Minimed, Inc. | Combined analyte sensor and infusion set |
| US11559624B2 (en) | 2019-11-21 | 2023-01-24 | Medtronic Minimed, Inc. | Systems for wearable infusion port and associated pump |
| US11324881B2 (en) | 2019-11-21 | 2022-05-10 | Medtronic Minimed, Inc. | Systems for wearable infusion port and associated pump |
| US12247941B2 (en) | 2019-11-21 | 2025-03-11 | Medtronic Minimed, Inc. | Glucose biosensor encasement, glucose biosensor package, and method |
| US12119119B2 (en) | 2019-12-09 | 2024-10-15 | Medtronic Minimed, Inc. | Methods and systems for real-time sensor measurement simulation |
| US11488700B2 (en) | 2019-12-13 | 2022-11-01 | Medtronic Minimed, Inc. | Medical device configuration procedure guidance responsive to detected gestures |
| US11887712B2 (en) | 2019-12-13 | 2024-01-30 | Medtronic Minimed, Inc. | Method and system for classifying detected events as labeled event combinations for processing at a client application |
| US11938301B2 (en) | 2019-12-13 | 2024-03-26 | Medtronic Minimed, Inc. | Controlling medication delivery system operation and features based on automatically detected muscular movements |
| US20210178063A1 (en) | 2019-12-13 | 2021-06-17 | Medtronic Minimed, Inc. | Controlling medication delivery system operation and features based on automatically detected user stress level |
| US12133969B2 (en) | 2019-12-13 | 2024-11-05 | Medtronic Minimed, Inc. | Translating therapy parameters of an insulin therapy system to translated therapy parameters for use at a different insulin therapy system |
| US11786655B2 (en) | 2019-12-13 | 2023-10-17 | Medtronic Minimed, Inc. | Context-sensitive predictive operation of a medication delivery system in response to gesture-indicated activity changes |
| US11690573B2 (en) | 2019-12-18 | 2023-07-04 | Medtronic Minimed, Inc. | Systems for skin patch gravity resistance |
| US11375955B2 (en) | 2019-12-18 | 2022-07-05 | Medtronic Minimed, Inc. | Systems for skin patch gravity resistance |
| US11821022B2 (en) | 2019-12-23 | 2023-11-21 | Medtronic Minimed, Inc. | Ethylene oxide absorption layer for analyte sensing and method |
| US11529466B2 (en) | 2020-01-08 | 2022-12-20 | Beta Bionics, Inc. | Cloud-connected ambulatory pump integration |
| US11244753B2 (en) | 2020-01-30 | 2022-02-08 | Medtronic Minimed, Inc. | Activity monitoring systems and methods |
| US12186098B2 (en) | 2020-02-07 | 2025-01-07 | Medtronic Minimed, Inc. | Systems for medical device breathability |
| US11957488B2 (en) | 2020-02-07 | 2024-04-16 | Medtronic Minimed, Inc. | Systems for medical device breathability |
| CA3165932A1 (en)* | 2020-02-14 | 2021-08-19 | Dexcom, Inc. | Decision support and treatment administration systems |
| US11833327B2 (en) | 2020-03-06 | 2023-12-05 | Medtronic Minimed, Inc. | Analyte sensor configuration and calibration based on data collected from a previously used analyte sensor |
| USD1032624S1 (en) | 2020-03-10 | 2024-06-25 | Beta Bionics, Inc. | Display screen with animated graphical user interface |
| US11278661B2 (en) | 2020-03-10 | 2022-03-22 | Beta Bionics, Inc. | Infusion system and components thereof |
| USD958167S1 (en) | 2020-03-23 | 2022-07-19 | Companion Medical, Inc. | Display screen with graphical user interface |
| US12191017B2 (en) | 2020-03-23 | 2025-01-07 | Medtronic Minimed, Inc. | Systems and methods for medicine administration and tracking |
| USD958817S1 (en) | 2020-03-31 | 2022-07-26 | Medtronic Minimed, Inc. | Display screen with graphical user interface |
| US11590057B2 (en) | 2020-04-03 | 2023-02-28 | Icu Medical, Inc. | Systems, methods, and components for transferring medical fluids |
| US11596359B2 (en) | 2020-04-09 | 2023-03-07 | Medtronic Minimed, Inc. | Methods and systems for mitigating sensor error propagation |
| US11690955B2 (en) | 2020-04-23 | 2023-07-04 | Medtronic Minimed, Inc. | Continuous analyte sensor quality measures and related therapy actions for an automated therapy delivery system |
| US11583631B2 (en) | 2020-04-23 | 2023-02-21 | Medtronic Minimed, Inc. | Intuitive user interface features and related functionality for a therapy delivery system |
| US20210377726A1 (en) | 2020-05-27 | 2021-12-02 | Medtronic Minimed, Inc. | Method and system for automatically associating a non-medical device with a medical device |
| US12213782B2 (en) | 2020-06-04 | 2025-02-04 | Medtronic Minimed, Inc. | Physiological characteristic sensor system |
| US12433538B2 (en) | 2020-06-04 | 2025-10-07 | Medtronic Minimed, Inc. | Liner for adhesive skin patch |
| US11272884B2 (en) | 2020-06-04 | 2022-03-15 | Medtronic Minimed, Inc. | Liner for adhesive skin patch |
| US12064236B2 (en) | 2020-06-11 | 2024-08-20 | Medtronic Minimed, Inc. | Methods, systems, and devices for improved sensors for continuous glucose monitoring |
| US11650248B2 (en) | 2020-07-28 | 2023-05-16 | Medtronic Minimed, Inc. | Electrical current measurement system |
| US11960311B2 (en) | 2020-07-28 | 2024-04-16 | Medtronic Minimed, Inc. | Linear voltage regulator with isolated supply current |
| US12082924B2 (en) | 2020-07-31 | 2024-09-10 | Medtronic Minimed, Inc. | Sensor identification and integrity check design |
| US11445807B2 (en) | 2020-07-31 | 2022-09-20 | Medtronic Minimed, Inc. | Pump clip with tube clamp for a fluid infusion device |
| EP4208798A4 (en) | 2020-09-05 | 2024-10-09 | ICU Medical, Inc. | Identity-based secure medical device communications |
| US11839743B2 (en) | 2020-10-07 | 2023-12-12 | Medtronic Minimed, Inc. | Graphic user interface for automated infusate delivery |
| US11737783B2 (en) | 2020-10-16 | 2023-08-29 | Medtronic Minimed, Inc. | Disposable medical device introduction system |
| US11806503B2 (en) | 2020-10-29 | 2023-11-07 | Medtronic Minimed, Inc. | Removable wearable device and related attachment methods |
| US11844930B2 (en) | 2020-10-29 | 2023-12-19 | Medtronic Minimed, Inc. | User-mountable electronic device with accelerometer-based activation feature |
| US11534086B2 (en) | 2020-10-30 | 2022-12-27 | Medtronic Minimed, Inc. | Low-profile wearable medical device |
| US11951281B2 (en) | 2020-11-11 | 2024-04-09 | Medtronic Minimed, Inc. | Fluid conduit insertion devices |
| US20220188388A1 (en) | 2020-12-07 | 2022-06-16 | Beta Bionics, Inc. | Ambulatory medicament pump with safe access control |
| US20220199218A1 (en) | 2020-12-07 | 2022-06-23 | Beta Bionics, Inc. | Ambulatory medicament pump with integrated medicament ordering interface |
| US20220265143A1 (en) | 2020-12-07 | 2022-08-25 | Beta Bionics, Inc. | Ambulatory medicament pumps with selective alarm muting |
| US12138047B2 (en) | 2021-01-22 | 2024-11-12 | Medtronic Minimed, Inc. | Micro models and layered prediction models for estimating sensor glucose values and reducing sensor glucose signal blanking |
| US12423490B2 (en) | 2021-01-29 | 2025-09-23 | Medtronic Minimed, Inc. | Model mosaic framework for modeling glucose sensitivity |
| US11998330B2 (en) | 2021-01-29 | 2024-06-04 | Medtronic Minimed, Inc. | Interference rejection membranes useful with analyte sensors |
| CN116830208A (en) | 2021-02-02 | 2023-09-29 | 美敦力迷你迈德公司 | Dynamic adjustment of physiological data |
| US11904146B2 (en) | 2021-06-08 | 2024-02-20 | Medtronic Minimed, Inc. | Medicine injection devices, systems, and methods for medicine administration and tracking |
| US12214173B2 (en) | 2021-06-08 | 2025-02-04 | Medtronic Minimed, Inc. | Injection pens for medicine administration and tracking |
| US11792714B2 (en) | 2021-06-16 | 2023-10-17 | Medtronic Minimed, Inc. | Medicine administration in dynamic networks |
| US12370320B2 (en) | 2021-06-21 | 2025-07-29 | Medtronic Minimed, Inc. | Accessory devices, systems, and methods for medicine administration and tracking |
| US12433515B2 (en) | 2021-08-13 | 2025-10-07 | Medtronic Minimed, Inc. | Dry electrochemical impedance spectroscopy metrology for conductive chemical layers |
| US11817285B2 (en) | 2021-09-02 | 2023-11-14 | Medtronic Minimed, Inc. | Ingress-tolerant input devices comprising sliders |
| US11587742B1 (en) | 2021-09-02 | 2023-02-21 | Medtronic Minimed, Inc. | Ingress-tolerant input devices |
| US12201421B2 (en) | 2021-10-08 | 2025-01-21 | Medtronic Minimed, Inc. | Immunosuppressant releasing coatings |
| US11896447B2 (en) | 2022-03-14 | 2024-02-13 | Medtronic Minimed, Inc. | Safeguards against separation from portable medicine delivery devices |
| US11997806B2 (en) | 2022-04-26 | 2024-05-28 | Medtronic Minimed, Inc. | Energy management based on an open switch configuration |
| US12011293B2 (en) | 2022-04-26 | 2024-06-18 | Medtronic Minimed, Inc. | Energy management based on a closed switch configuration |
| US20240023849A1 (en) | 2022-07-20 | 2024-01-25 | Medtronic Minimed, Inc. | Acrylate hydrogel membrane for dual function of diffusion limiting membrane as well as attenuation to the foreign body response |
Citations (59)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US3631847A (en)* | 1966-03-04 | 1972-01-04 | James C Hobbs | Method and apparatus for injecting fluid into the vascular system |
| US4270532A (en)* | 1977-12-28 | 1981-06-02 | Siemens Aktiengesellschaft | Device for the pre-programmable infusion of liquids |
| US4282872A (en)* | 1977-12-28 | 1981-08-11 | Siemens Aktiengesellschaft | Device for the pre-programmable infusion of liquids |
| US4373527A (en)* | 1979-04-27 | 1983-02-15 | The Johns Hopkins University | Implantable, programmable medication infusion system |
| US4392849A (en)* | 1981-07-27 | 1983-07-12 | The Cleveland Clinic Foundation | Infusion pump controller |
| US4395259A (en)* | 1980-09-22 | 1983-07-26 | Siemens Aktiengesellschaft | Device for the infusion of fluids into the human or animal body |
| US4433072A (en)* | 1978-12-15 | 1984-02-21 | Hospal-Sodip, S.A. | Mixtures of polymers for medical use |
| US4443218A (en)* | 1982-09-09 | 1984-04-17 | Infusaid Corporation | Programmable implantable infusate pump |
| US4469481A (en)* | 1981-06-23 | 1984-09-04 | Terumo Corporation | Apparatus for infusing medication |
| US4475901A (en)* | 1980-07-23 | 1984-10-09 | The Garvan Research Development Ltd. | Apparatus for improving blood sugar control in diabetics |
| US4494950A (en)* | 1982-01-19 | 1985-01-22 | The Johns Hopkins University | Plural module medication delivery system |
| US4529401A (en)* | 1982-01-11 | 1985-07-16 | Cardiac Pacemakers, Inc. | Ambulatory infusion pump having programmable parameters |
| US4542532A (en)* | 1984-03-09 | 1985-09-17 | Medtronic, Inc. | Dual-antenna transceiver |
| US4550731A (en)* | 1984-03-07 | 1985-11-05 | Cordis Corporation | Acquisition circuit for cardiac pacer |
| US4559037A (en)* | 1977-12-28 | 1985-12-17 | Siemens Aktiengesellschaft | Device for the pre-programmable infusion of liquids |
| US4562751A (en)* | 1984-01-06 | 1986-01-07 | Nason Clyde K | Solenoid drive apparatus for an external infusion pump |
| US4671288A (en)* | 1985-06-13 | 1987-06-09 | The Regents Of The University Of California | Electrochemical cell sensor for continuous short-term use in tissues and blood |
| US4678408A (en)* | 1984-01-06 | 1987-07-07 | Pacesetter Infusion, Ltd. | Solenoid drive apparatus for an external infusion pump |
| US4685903A (en)* | 1984-01-06 | 1987-08-11 | Pacesetter Infusion, Ltd. | External infusion pump apparatus |
| US4696671A (en)* | 1984-02-08 | 1987-09-29 | Omni-Flow, Inc. | Infusion system having plural fluid input ports and at least one patient output port |
| US4703756A (en)* | 1986-05-06 | 1987-11-03 | The Regents Of The University Of California | Complete glucose monitoring system with an implantable, telemetered sensor module |
| US4731726A (en)* | 1986-05-19 | 1988-03-15 | Healthware Corporation | Patient-operated glucose monitor and diabetes management system |
| US4731051A (en)* | 1979-04-27 | 1988-03-15 | The Johns Hopkins University | Programmable control means for providing safe and controlled medication infusion |
| US4781798A (en)* | 1985-04-19 | 1988-11-01 | The Regents Of The University Of California | Transparent multi-oxygen sensor array and method of using same |
| US4803625A (en)* | 1986-06-30 | 1989-02-07 | Buddy Systems, Inc. | Personal health monitor |
| US4809697A (en)* | 1987-10-14 | 1989-03-07 | Siemens-Pacesetter, Inc. | Interactive programming and diagnostic system for use with implantable pacemaker |
| US4826810A (en)* | 1983-12-16 | 1989-05-02 | Aoki Thomas T | System and method for treating animal body tissues to improve the dietary fuel processing capabilities thereof |
| US4865584A (en)* | 1984-02-08 | 1989-09-12 | Omni-Flow, Inc. | Cassette for programable multiple input infusion system |
| US4871351A (en)* | 1984-09-28 | 1989-10-03 | Vladimir Feingold | Implantable medication infusion system |
| US4898578A (en)* | 1988-01-26 | 1990-02-06 | Baxter International Inc. | Drug infusion system with calculator |
| US4935105A (en)* | 1987-02-24 | 1990-06-19 | Imperial Chemical Industries Plc | Methods of operating enzyme electrode sensors |
| US4953552A (en)* | 1989-04-21 | 1990-09-04 | Demarzo Arthur P | Blood glucose monitoring system |
| US5019974A (en)* | 1987-05-01 | 1991-05-28 | Diva Medical Systems Bv | Diabetes management system and apparatus |
| US5034004A (en)* | 1987-06-19 | 1991-07-23 | The University Of Melbourne | Infusion pump and drive systems therefor |
| US5050612A (en)* | 1989-09-12 | 1991-09-24 | Matsumura Kenneth N | Device for computer-assisted monitoring of the body |
| US5078683A (en)* | 1990-05-04 | 1992-01-07 | Block Medical, Inc. | Programmable infusion system |
| US5080653A (en)* | 1990-04-16 | 1992-01-14 | Pacesetter Infusion, Ltd. | Infusion pump with dual position syringe locator |
| US5097122A (en)* | 1990-04-16 | 1992-03-17 | Pacesetter Infusion, Ltd. | Medication infusion system having optical motion sensor to detect drive mechanism malfunction |
| US5100380A (en)* | 1984-02-08 | 1992-03-31 | Abbott Laboratories | Remotely programmable infusion system |
| US5101814A (en)* | 1989-08-11 | 1992-04-07 | Palti Yoram Prof | System for monitoring and controlling blood glucose |
| US5108819A (en)* | 1990-02-14 | 1992-04-28 | Eli Lilly And Company | Thin film electrical component |
| US5113869A (en)* | 1990-08-21 | 1992-05-19 | Telectronics Pacing Systems, Inc. | Implantable ambulatory electrocardiogram monitor |
| US5153827A (en)* | 1989-01-30 | 1992-10-06 | Omni-Flow, Inc. | An infusion management and pumping system having an alarm handling system |
| US5165407A (en)* | 1990-04-19 | 1992-11-24 | The University Of Kansas | Implantable glucose sensor |
| US5211626A (en)* | 1987-05-01 | 1993-05-18 | Product Innovation Holdings Ltd. | Medical infusion apparatus |
| US5216597A (en)* | 1987-05-01 | 1993-06-01 | Diva Medical Systems Bv | Diabetes therapy management system, apparatus and method |
| US5262035A (en)* | 1989-08-02 | 1993-11-16 | E. Heller And Company | Enzyme electrodes |
| US5262305A (en)* | 1991-03-04 | 1993-11-16 | E. Heller & Company | Interferant eliminating biosensors |
| US5264104A (en)* | 1989-08-02 | 1993-11-23 | Gregg Brian A | Enzyme electrodes |
| US5264105A (en)* | 1989-08-02 | 1993-11-23 | Gregg Brian A | Enzyme electrodes |
| US5266013A (en)* | 1990-03-23 | 1993-11-30 | Asulab S.A. | Portable pump for the administration of a therapeutic |
| US5904708A (en)* | 1998-03-19 | 1999-05-18 | Medtronic, Inc. | System and method for deriving relative physiologic signals |
| US20020019606A1 (en)* | 2000-01-21 | 2002-02-14 | Lebel Ronald J. | Microprocessor controlled ambulatory medical apparatus with hand held communication device |
| US20030060765A1 (en)* | 2000-02-16 | 2003-03-27 | Arthur Campbell | Infusion device menu structure and method of using the same |
| US6544212B2 (en)* | 2001-07-31 | 2003-04-08 | Roche Diagnostics Corporation | Diabetes management system |
| US20030114836A1 (en)* | 2001-12-19 | 2003-06-19 | Estes Mark C. | Medication delivery system and monitor |
| US20030211617A1 (en)* | 2002-05-07 | 2003-11-13 | International Business Machines Corporation | Blood glucose meter that reminds the user to test after a hypoglycemic event |
| US7025743B2 (en)* | 1998-08-18 | 2006-04-11 | Medtronic Minimed, Inc. | External infusion device with remote programming, bolus estimator and/or vibration alarm capabilities |
| US7278983B2 (en)* | 2002-07-24 | 2007-10-09 | Medtronic Minimed, Inc. | Physiological monitoring device for controlling a medication infusion device |
Family Cites Families (196)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US562144A (en)* | 1896-06-16 | Water meter | ||
| US5935099A (en) | 1992-09-09 | 1999-08-10 | Sims Deltec, Inc. | Drug pump systems and methods |
| US5338157B1 (en)* | 1992-09-09 | 1999-11-02 | Sims Deltec Inc | Systems and methods for communicating with ambulat |
| FR2385406A1 (en)* | 1977-03-28 | 1978-10-27 | Akzo Nv | ARTIFICIAL KIDNEY |
| EP0098592A3 (en) | 1982-07-06 | 1985-08-21 | Fujisawa Pharmaceutical Co., Ltd. | Portable artificial pancreas |
| US5364346A (en) | 1985-12-20 | 1994-11-15 | Schrezenmeir Juergen | Process for the continuous and discontinuous administration of insulin to the human body |
| US5003298A (en)* | 1986-01-15 | 1991-03-26 | Karel Havel | Variable color digital display for emphasizing position of decimal point |
| US4755173A (en) | 1986-02-25 | 1988-07-05 | Pacesetter Infusion, Ltd. | Soft cannula subcutaneous injection set |
| US4723947A (en) | 1986-04-09 | 1988-02-09 | Pacesetter Infusion, Ltd. | Insulin compatible infusion set |
| US5011468A (en)* | 1987-05-29 | 1991-04-30 | Retroperfusion Systems, Inc. | Retroperfusion and retroinfusion control apparatus, system and method |
| US5041086A (en) | 1987-12-04 | 1991-08-20 | Pacesetter Infusion, Ltd. | Clinical configuration of multimode medication infusion system |
| US5025374A (en)* | 1987-12-09 | 1991-06-18 | Arch Development Corp. | Portable system for choosing pre-operative patient test |
| GB2218831A (en) | 1988-05-17 | 1989-11-22 | Mark John Newland | Personal medical apparatus |
| US5320725A (en)* | 1989-08-02 | 1994-06-14 | E. Heller & Company | Electrode and method for the detection of hydrogen peroxide |
| US5176662A (en) | 1990-08-23 | 1993-01-05 | Minimed Technologies, Ltd. | Subcutaneous injection set with improved cannula mounting arrangement |
| EP0550517B1 (en)* | 1990-08-31 | 1998-12-23 | The General Hospital Corporation | A system for managing multiple devices, for example, portable patient monitoring devices in a network |
| US5593852A (en)* | 1993-12-02 | 1997-01-14 | Heller; Adam | Subcutaneous glucose electrode |
| JPH04278450A (en) | 1991-03-04 | 1992-10-05 | Adam Heller | Biosensor and method for analyzing subject |
| US5247434A (en)* | 1991-04-19 | 1993-09-21 | Althin Medical, Inc. | Method and apparatus for kidney dialysis |
| US5322063A (en)* | 1991-10-04 | 1994-06-21 | Eli Lilly And Company | Hydrophilic polyurethane membranes for electrochemical glucose sensors |
| EP0665025B1 (en)* | 1991-10-23 | 2002-02-27 | Terumo Kabushiki Kaisha | Medical pump driving device |
| US5284140A (en)* | 1992-02-11 | 1994-02-08 | Eli Lilly And Company | Acrylic copolymer membranes for biosensors |
| JPH08275927A (en)* | 1992-02-13 | 1996-10-22 | Seta:Kk | Homestay medical care system and medical device used in this system |
| US5382232A (en)* | 1992-03-13 | 1995-01-17 | Ivac Corporation | Infusion system with air-in-line clear function |
| FR2690622B1 (en)* | 1992-04-29 | 1995-01-20 | Chronotec | Programmable ambulatory infusion pump system. |
| US5788669A (en) | 1995-11-22 | 1998-08-04 | Sims Deltec, Inc. | Pump tracking system |
| US5376070A (en) | 1992-09-29 | 1994-12-27 | Minimed Inc. | Data transfer system for an infusion pump |
| JP3660678B2 (en)* | 1992-10-15 | 2005-06-15 | ザ ゼネラル ホスピタル コーポレーション | Infusion pump with electronically loadable drug library |
| US5832448A (en)* | 1996-10-16 | 1998-11-03 | Health Hero Network | Multiple patient monitoring system for proactive health management |
| US5918603A (en) | 1994-05-23 | 1999-07-06 | Health Hero Network, Inc. | Method for treating medical conditions using a microprocessor-based video game |
| US5940801A (en) | 1994-04-26 | 1999-08-17 | Health Hero Network, Inc. | Modular microprocessor-based diagnostic measurement apparatus and method for psychological conditions |
| US6101478A (en)* | 1997-04-30 | 2000-08-08 | Health Hero Network | Multi-user remote health monitoring system |
| US5960403A (en) | 1992-11-17 | 1999-09-28 | Health Hero Network | Health management process control system |
| US5997476A (en) | 1997-03-28 | 1999-12-07 | Health Hero Network, Inc. | Networked system for interactive communication and remote monitoring of individuals |
| US5933136A (en) | 1996-12-23 | 1999-08-03 | Health Hero Network, Inc. | Network media access control system for encouraging patient compliance with a treatment plan |
| US5879163A (en)* | 1996-06-24 | 1999-03-09 | Health Hero Network, Inc. | On-line health education and feedback system using motivational driver profile coding and automated content fulfillment |
| US5913310A (en) | 1994-05-23 | 1999-06-22 | Health Hero Network, Inc. | Method for diagnosis and treatment of psychological and emotional disorders using a microprocessor-based video game |
| US5899855A (en)* | 1992-11-17 | 1999-05-04 | Health Hero Network, Inc. | Modular microprocessor-based health monitoring system |
| US5897493A (en)* | 1997-03-28 | 1999-04-27 | Health Hero Network, Inc. | Monitoring system for remotely querying individuals |
| US5307263A (en)* | 1992-11-17 | 1994-04-26 | Raya Systems, Inc. | Modular microprocessor-based health monitoring system |
| US5956501A (en) | 1997-01-10 | 1999-09-21 | Health Hero Network, Inc. | Disease simulation system and method |
| US5371687A (en) | 1992-11-20 | 1994-12-06 | Boehringer Mannheim Corporation | Glucose test data acquisition and management system |
| ZA938555B (en)* | 1992-11-23 | 1994-08-02 | Lilly Co Eli | Technique to improve the performance of electrochemical sensors |
| US5499243A (en)* | 1993-01-22 | 1996-03-12 | Hall; Dennis R. | Method and apparatus for coordinating transfer of information between a base station and a plurality of radios |
| US5299571A (en)* | 1993-01-22 | 1994-04-05 | Eli Lilly And Company | Apparatus and method for implantation of sensors |
| US5357427A (en) | 1993-03-15 | 1994-10-18 | Digital Equipment Corporation | Remote monitoring of high-risk patients using artificial intelligence |
| US5350411A (en) | 1993-06-28 | 1994-09-27 | Medtronic, Inc. | Pacemaker telemetry system |
| US5368562A (en) | 1993-07-30 | 1994-11-29 | Pharmacia Deltec, Inc. | Systems and methods for operating ambulatory medical devices such as drug delivery devices |
| DE4329229A1 (en) | 1993-08-25 | 1995-03-09 | Meditech Medizintechnik Gmbh | Adaptive controlled pump control, in particular for adaptive patient-controlled analgesia (APCA) |
| US5497772A (en)* | 1993-11-19 | 1996-03-12 | Alfred E. Mann Foundation For Scientific Research | Glucose monitoring system |
| US5791344A (en) | 1993-11-19 | 1998-08-11 | Alfred E. Mann Foundation For Scientific Research | Patient monitoring system |
| US5660176A (en)* | 1993-12-29 | 1997-08-26 | First Opinion Corporation | Computerized medical diagnostic and treatment advice system |
| US5594638A (en)* | 1993-12-29 | 1997-01-14 | First Opinion Corporation | Computerized medical diagnostic system including re-enter function and sensitivity factors |
| US5417222A (en)* | 1994-01-21 | 1995-05-23 | Hewlett-Packard Company | Patient monitoring system |
| FR2716286A1 (en) | 1994-02-16 | 1995-08-18 | Debiotech Sa | Installation of remote monitoring of controllable equipment. |
| US5543326A (en)* | 1994-03-04 | 1996-08-06 | Heller; Adam | Biosensor including chemically modified enzymes |
| US5536249A (en)* | 1994-03-09 | 1996-07-16 | Visionary Medical Products, Inc. | Pen-type injector with a microprocessor and blood characteristic monitor |
| US5630710A (en)* | 1994-03-09 | 1997-05-20 | Baxter International Inc. | Ambulatory infusion pump |
| US5482446A (en)* | 1994-03-09 | 1996-01-09 | Baxter International Inc. | Ambulatory infusion pump |
| US5478211A (en) | 1994-03-09 | 1995-12-26 | Baxter International Inc. | Ambulatory infusion pump |
| US5391250A (en)* | 1994-03-15 | 1995-02-21 | Minimed Inc. | Method of fabricating thin film sensors |
| US5390671A (en)* | 1994-03-15 | 1995-02-21 | Minimed Inc. | Transcutaneous sensor insertion set |
| EP0672427A1 (en) | 1994-03-17 | 1995-09-20 | Siemens-Elema AB | System for infusion of medicine into the body of a patient |
| US5609575A (en)* | 1994-04-11 | 1997-03-11 | Graseby Medical Limited | Infusion pump and method with dose-rate calculation |
| US5569186A (en) | 1994-04-25 | 1996-10-29 | Minimed Inc. | Closed loop infusion pump system with removable glucose sensor |
| US5370622A (en) | 1994-04-28 | 1994-12-06 | Minimed Inc. | Proctective case for a medication infusion pump |
| DE4415896A1 (en)* | 1994-05-05 | 1995-11-09 | Boehringer Mannheim Gmbh | Analysis system for monitoring the concentration of an analyte in the blood of a patient |
| US5545191A (en)* | 1994-05-06 | 1996-08-13 | Alfred E. Mann Foundation For Scientific Research | Method for optimally positioning and securing the external unit of a transcutaneous transducer of the skin of a living body |
| US5482473A (en)* | 1994-05-09 | 1996-01-09 | Minimed Inc. | Flex circuit connector |
| US5704366A (en)* | 1994-05-23 | 1998-01-06 | Enact Health Management Systems | System for monitoring and reporting medical measurements |
| US5472317A (en) | 1994-06-03 | 1995-12-05 | Minimed Inc. | Mounting clip for a medication infusion pump |
| US5582593A (en) | 1994-07-21 | 1996-12-10 | Hultman; Barry W. | Ambulatory medication delivery system |
| US5569187A (en) | 1994-08-16 | 1996-10-29 | Texas Instruments Incorporated | Method and apparatus for wireless chemical supplying |
| US5505709A (en) | 1994-09-15 | 1996-04-09 | Minimed, Inc., A Delaware Corporation | Mated infusion pump and syringe |
| US5840026A (en) | 1994-09-21 | 1998-11-24 | Medrad, Inc. | Patient specific dosing contrast delivery systems and methods |
| US6397098B1 (en)* | 1994-09-21 | 2002-05-28 | Medrad, Inc. | Data communication and control for medical imaging systems |
| US5687734A (en) | 1994-10-20 | 1997-11-18 | Hewlett-Packard Company | Flexible patient monitoring system featuring a multiport transmitter |
| US6355018B1 (en)* | 1995-11-25 | 2002-03-12 | I-Flow Corporation Inc. | Remotely programmable infusion system |
| US5573506A (en)* | 1994-11-25 | 1996-11-12 | Block Medical, Inc. | Remotely programmable infusion system |
| US5685844A (en) | 1995-01-06 | 1997-11-11 | Abbott Laboratories | Medicinal fluid pump having multiple stored protocols |
| US5584814A (en) | 1995-01-26 | 1996-12-17 | Schuster; John H. | Syringe actuation system |
| US5586553A (en) | 1995-02-16 | 1996-12-24 | Minimed Inc. | Transcutaneous sensor insertion set |
| US5814015A (en) | 1995-02-24 | 1998-09-29 | Harvard Clinical Technology, Inc. | Infusion pump for at least one syringe |
| US5647853A (en)* | 1995-03-03 | 1997-07-15 | Minimed Inc. | Rapid response occlusion detector for a medication infusion pump |
| US5620312A (en)* | 1995-03-06 | 1997-04-15 | Sabratek Corporation | Infusion pump with dual-latching mechanism |
| US5713856A (en)* | 1995-03-13 | 1998-02-03 | Alaris Medical Systems, Inc. | Modular patient care system |
| US5882494A (en) | 1995-03-27 | 1999-03-16 | Minimed, Inc. | Polyurethane/polyurea compositions containing silicone for biosensor membranes |
| US5786439A (en) | 1996-10-24 | 1998-07-28 | Minimed Inc. | Hydrophilic, swellable coatings for biosensors |
| US5609060A (en)* | 1995-04-28 | 1997-03-11 | Dentsleeve Pty Limited | Multiple channel perfused manometry apparatus and a method of operation of such a device |
| US5772635A (en) | 1995-05-15 | 1998-06-30 | Alaris Medical Systems, Inc. | Automated infusion system with dose rate calculator |
| US5665065A (en) | 1995-05-26 | 1997-09-09 | Minimed Inc. | Medication infusion device with blood glucose data input |
| US5584813A (en) | 1995-06-07 | 1996-12-17 | Minimed Inc. | Subcutaneous injection set |
| US6018289A (en) | 1995-06-15 | 2000-01-25 | Sekura; Ronald D. | Prescription compliance device and method of using device |
| US5750926A (en)* | 1995-08-16 | 1998-05-12 | Alfred E. Mann Foundation For Scientific Research | Hermetically sealed electrical feedthrough for use with implantable electronic devices |
| US5972199A (en) | 1995-10-11 | 1999-10-26 | E. Heller & Company | Electrochemical analyte sensors using thermostable peroxidase |
| US6689265B2 (en)* | 1995-10-11 | 2004-02-10 | Therasense, Inc. | Electrochemical analyte sensors using thermostable soybean peroxidase |
| US5665222A (en)* | 1995-10-11 | 1997-09-09 | E. Heller & Company | Soybean peroxidase electrochemical sensor |
| US5944659A (en) | 1995-11-13 | 1999-08-31 | Vitalcom Inc. | Architecture for TDMA medical telemetry system |
| US5748103A (en)* | 1995-11-13 | 1998-05-05 | Vitalcom, Inc. | Two-way TDMA telemetry system with power conservation features |
| AUPN707195A0 (en) | 1995-12-12 | 1996-01-11 | University Of Melbourne, The | Field programmable intravenous infusion system |
| FI960636A7 (en) | 1996-02-12 | 1997-08-13 | Nokia Corp | Method for monitoring patient health status |
| FI118509B (en) | 1996-02-12 | 2007-12-14 | Nokia Oyj | Method and apparatus for predicting glucose levels in the blood of a patient |
| US6790178B1 (en) | 1999-09-24 | 2004-09-14 | Healthetech, Inc. | Physiological monitor and associated computation, display and communication unit |
| FR2748588B1 (en) | 1996-05-07 | 1998-08-07 | Soc Et Tech Set | DEVICE COMPRISING AT LEAST ONE ARRAY OF NEURONES FOR DETERMINING THE QUANTITY OF A SUBSTANCE TO BE ADMINISTERED TO A PATIENT, IN PARTICULAR INSULIN |
| US5861018A (en)* | 1996-05-28 | 1999-01-19 | Telecom Medical Inc. | Ultrasound transdermal communication system and method |
| US5885245A (en)* | 1996-08-02 | 1999-03-23 | Sabratek Corporation | Medical apparatus with remote virtual input device |
| US6689091B2 (en)* | 1996-08-02 | 2004-02-10 | Tuan Bui | Medical apparatus with remote control |
| US5807336A (en) | 1996-08-02 | 1998-09-15 | Sabratek Corporation | Apparatus for monitoring and/or controlling a medical device |
| WO1998020439A1 (en) | 1996-11-08 | 1998-05-14 | Roman Linda L | System for providing comprehensive health care and support |
| AU5461298A (en) | 1996-12-04 | 1998-06-29 | Enact Health Management Systems | System for downloading and reporting medical information |
| US6043437A (en) | 1996-12-20 | 2000-03-28 | Alfred E. Mann Foundation | Alumina insulation for coating implantable components and other microminiature devices |
| US6032119A (en)* | 1997-01-16 | 2000-02-29 | Health Hero Network, Inc. | Personalized display of health information |
| AU6157898A (en) | 1997-02-06 | 1998-08-26 | E. Heller & Company | Small volume (in vitro) analyte sensor |
| US6009339A (en)* | 1997-02-27 | 1999-12-28 | Terumo Cardiovascular Systems Corporation | Blood parameter measurement device |
| WO1998042407A1 (en) | 1997-03-27 | 1998-10-01 | Medtronic, Inc. | Concepts to implement medconnect |
| US5960085A (en)* | 1997-04-14 | 1999-09-28 | De La Huerga; Carlos | Security badge for automated access control and secure data gathering |
| US5961451A (en) | 1997-04-07 | 1999-10-05 | Motorola, Inc. | Noninvasive apparatus having a retaining member to retain a removable biosensor |
| US5779665A (en) | 1997-05-08 | 1998-07-14 | Minimed Inc. | Transdermal introducer assembly |
| TW357517B (en) | 1997-05-29 | 1999-05-01 | Koji Akai | Monitoring system |
| US6558351B1 (en)* | 1999-06-03 | 2003-05-06 | Medtronic Minimed, Inc. | Closed loop system for controlling insulin infusion |
| US5954643A (en) | 1997-06-09 | 1999-09-21 | Minimid Inc. | Insertion set for a transcutaneous sensor |
| AU8165498A (en) | 1997-06-23 | 1999-01-04 | Enact Health Management Systems | Improved system for downloading and reporting medical information |
| ES2224420T3 (en)* | 1997-08-01 | 2005-03-01 | Alfred E. Mann Foundation For Scientific Research | IMPLANTABLE DEVICE WITH IMPROVED POWER AND BATTERY RECHARGE CONFIGURATION. |
| US6171276B1 (en)* | 1997-08-06 | 2001-01-09 | Pharmacia & Upjohn Ab | Automated delivery device and method for its operation |
| US6130620A (en) | 1997-08-11 | 2000-10-10 | Electronic Monitoring Systems, Inc. | Remote monitoring system |
| AU8826498A (en) | 1997-08-22 | 1999-03-16 | Apex Inc. | Remote computer control system |
| US6259937B1 (en)* | 1997-09-12 | 2001-07-10 | Alfred E. Mann Foundation | Implantable substrate sensor |
| US5999848A (en) | 1997-09-12 | 1999-12-07 | Alfred E. Mann Foundation | Daisy chainable sensors and stimulators for implantation in living tissue |
| US5917346A (en) | 1997-09-12 | 1999-06-29 | Alfred E. Mann Foundation | Low power current to frequency converter circuit for use in implantable sensors |
| US6071391A (en)* | 1997-09-12 | 2000-06-06 | Nok Corporation | Enzyme electrode structure |
| US5999849A (en)* | 1997-09-12 | 1999-12-07 | Alfred E. Mann Foundation | Low power rectifier circuit for implantable medical device |
| US6556963B1 (en)* | 1997-09-24 | 2003-04-29 | International Business Machines Corporation | User state sensitive system and method for nutrient analysis using natural language interface |
| US6183412B1 (en)* | 1997-10-02 | 2001-02-06 | Micromed Technology, Inc. | Implantable pump system |
| US6119028A (en) | 1997-10-20 | 2000-09-12 | Alfred E. Mann Foundation | Implantable enzyme-based monitoring systems having improved longevity due to improved exterior surfaces |
| US6088608A (en)* | 1997-10-20 | 2000-07-11 | Alfred E. Mann Foundation | Electrochemical sensor and integrity tests therefor |
| US6081736A (en)* | 1997-10-20 | 2000-06-27 | Alfred E. Mann Foundation | Implantable enzyme-based monitoring systems adapted for long term use |
| WO1999021596A1 (en)* | 1997-10-23 | 1999-05-06 | Mernoee Morten | An infusion pump system and an infusion pump unit |
| FI107080B (en) | 1997-10-27 | 2001-05-31 | Nokia Mobile Phones Ltd | measuring device |
| US6579690B1 (en)* | 1997-12-05 | 2003-06-17 | Therasense, Inc. | Blood analyte monitoring through subcutaneous measurement |
| WO2000029047A1 (en) | 1998-11-18 | 2000-05-25 | Phiscience Gmbh, Entwicklung Von Sensoren | Portable device and method for the mobile supply of medicaments with wireless transmission of data for control or programming purposes |
| US6056718A (en) | 1998-03-04 | 2000-05-02 | Minimed Inc. | Medication infusion set |
| US6103033A (en) | 1998-03-04 | 2000-08-15 | Therasense, Inc. | Process for producing an electrochemical biosensor |
| US6134461A (en) | 1998-03-04 | 2000-10-17 | E. Heller & Company | Electrochemical analyte |
| WO1999047190A1 (en)* | 1998-03-16 | 1999-09-23 | Medtronic, Inc. | Hemostatic system and components for extracorporeal circuit |
| US6175752B1 (en)* | 1998-04-30 | 2001-01-16 | Therasense, Inc. | Analyte monitoring device and methods of use |
| US6142008A (en) | 1998-06-12 | 2000-11-07 | Abbott Laboratories | Air bubble sensor |
| US6294281B1 (en)* | 1998-06-17 | 2001-09-25 | Therasense, Inc. | Biological fuel cell and method |
| US6558320B1 (en)* | 2000-01-20 | 2003-05-06 | Medtronic Minimed, Inc. | Handheld personal data assistant (PDA) with a medical device and method of using the same |
| US6248067B1 (en)* | 1999-02-05 | 2001-06-19 | Minimed Inc. | Analyte sensor and holter-type monitor system and method of using the same |
| US5951521A (en) | 1998-09-25 | 1999-09-14 | Minimed Inc. | Subcutaneous implantable sensor set having the capability to remove deliver fluids to an insertion site |
| US6254586B1 (en)* | 1998-09-25 | 2001-07-03 | Minimed Inc. | Method and kit for supplying a fluid to a subcutaneous placement site |
| US6591125B1 (en)* | 2000-06-27 | 2003-07-08 | Therasense, Inc. | Small volume in vitro analyte sensor with diffusible or non-leachable redox mediator |
| US6338790B1 (en)* | 1998-10-08 | 2002-01-15 | Therasense, Inc. | Small volume in vitro analyte sensor with diffusible or non-leachable redox mediator |
| CA2345043C (en)* | 1998-10-08 | 2009-08-11 | Minimed, Inc. | Telemetered characteristic monitor system |
| US6248093B1 (en) | 1998-10-29 | 2001-06-19 | Minimed Inc. | Compact pump drive system |
| US20040158193A1 (en) | 1999-02-10 | 2004-08-12 | Baxter International Inc. | Medical apparatus using selective graphical interface |
| WO2000047109A1 (en)* | 1999-02-12 | 2000-08-17 | Cygnus, Inc. | Devices and methods for frequent measurement of an analyte present in a biological system |
| US6560741B1 (en)* | 1999-02-24 | 2003-05-06 | Datastrip (Iom) Limited | Two-dimensional printed code for storing biometric information and integrated off-line apparatus for reading same |
| US6669663B1 (en)* | 1999-04-30 | 2003-12-30 | Medtronic, Inc. | Closed loop medicament pump |
| US6461331B1 (en) | 1999-05-21 | 2002-10-08 | Minimed Inc. | Device and method for infusion of small molecule insulin mimetic materials |
| US6270481B1 (en)* | 1999-06-16 | 2001-08-07 | Breg, Inc. | Patient-controlled medication delivery system |
| EP1191875A1 (en) | 1999-06-17 | 2002-04-03 | Medtronic MiniMed, Inc. | Characteristic monitor system for use with analyte sensor |
| EP1192269A2 (en)* | 1999-06-18 | 2002-04-03 | Therasense, Inc. | MASS TRANSPORT LIMITED i IN VIVO /i ANALYTE SENSOR |
| US7247138B2 (en) | 1999-07-01 | 2007-07-24 | Medtronic Minimed, Inc. | Reusable analyte sensor site and method of using the same |
| US6804558B2 (en)* | 1999-07-07 | 2004-10-12 | Medtronic, Inc. | System and method of communicating between an implantable medical device and a remote computer system or health care provider |
| US6250309B1 (en)* | 1999-07-21 | 2001-06-26 | Medtronic Inc | System and method for transferring information relating to an implantable medical device to a remote location |
| US6478736B1 (en) | 1999-10-08 | 2002-11-12 | Healthetech, Inc. | Integrated calorie management system |
| US6616819B1 (en) | 1999-11-04 | 2003-09-09 | Therasense, Inc. | Small volume in vitro analyte sensor and methods |
| EP1230248B1 (en)* | 1999-11-15 | 2007-06-06 | Therasense, Inc. | Transition metal complexes attached to a polymer via a flexible chain |
| JP2003534581A (en) | 1999-11-24 | 2003-11-18 | ヘルセテック インコーポレイテッド | Health management system with connection to remote computer system |
| US6513532B2 (en) | 2000-01-19 | 2003-02-04 | Healthetech, Inc. | Diet and activity-monitoring device |
| US6629934B2 (en) | 2000-02-02 | 2003-10-07 | Healthetech, Inc. | Indirect calorimeter for medical applications |
| US6895263B2 (en) | 2000-02-23 | 2005-05-17 | Medtronic Minimed, Inc. | Real time self-adjusting calibration algorithm |
| US6623501B2 (en)* | 2000-04-05 | 2003-09-23 | Therasense, Inc. | Reusable ceramic skin-piercing device |
| WO2001088524A1 (en)* | 2000-05-12 | 2001-11-22 | Therasense, Inc. | Electrodes with multilayer membranes and methods of using and making the electrodes |
| EP1332440B1 (en)* | 2000-10-04 | 2012-04-11 | Insulet Corporation | Data collection assembly for patient infusion system |
| AU2002239709B2 (en)* | 2000-12-21 | 2007-02-15 | Insulet Corporation | Medical apparatus remote control and method |
| US6560471B1 (en) | 2001-01-02 | 2003-05-06 | Therasense, Inc. | Analyte monitoring device and methods of use |
| EP1397068A2 (en) | 2001-04-02 | 2004-03-17 | Therasense, Inc. | Blood glucose tracking apparatus and methods |
| US6676816B2 (en)* | 2001-05-11 | 2004-01-13 | Therasense, Inc. | Transition metal complexes with (pyridyl)imidazole ligands and sensors using said complexes |
| US6932894B2 (en)* | 2001-05-15 | 2005-08-23 | Therasense, Inc. | Biosensor membranes composed of polymers containing heterocyclic nitrogens |
| US6671554B2 (en)* | 2001-09-07 | 2003-12-30 | Medtronic Minimed, Inc. | Electronic lead for a medical implant device, method of making same, and method and apparatus for inserting same |
| US7025760B2 (en)* | 2001-09-07 | 2006-04-11 | Medtronic Minimed, Inc. | Method and system for non-vascular sensor implantation |
| US7052591B2 (en) | 2001-09-21 | 2006-05-30 | Therasense, Inc. | Electrodeposition of redox polymers and co-electrodeposition of enzymes by coordinative crosslinking |
| US20030212379A1 (en)* | 2002-02-26 | 2003-11-13 | Bylund Adam David | Systems and methods for remotely controlling medication infusion and analyte monitoring |
| US20040068230A1 (en)* | 2002-07-24 | 2004-04-08 | Medtronic Minimed, Inc. | System for providing blood glucose measurements to an infusion device |
| US20040061232A1 (en)* | 2002-09-27 | 2004-04-01 | Medtronic Minimed, Inc. | Multilayer substrate |
| US7162289B2 (en)* | 2002-09-27 | 2007-01-09 | Medtronic Minimed, Inc. | Method and apparatus for enhancing the integrity of an implantable sensor device |
| US7736309B2 (en)* | 2002-09-27 | 2010-06-15 | Medtronic Minimed, Inc. | Implantable sensor method and system |
| US7138330B2 (en)* | 2002-09-27 | 2006-11-21 | Medtronic Minimed, Inc. | High reliability multilayer circuit substrates and methods for their formation |
| DE60336834D1 (en)* | 2002-10-09 | 2011-06-01 | Abbott Diabetes Care Inc | FUEL FEEDING DEVICE, SYSTEM AND METHOD |
| US20040074785A1 (en)* | 2002-10-18 | 2004-04-22 | Holker James D. | Analyte sensors and methods for making them |
| US6931328B2 (en)* | 2002-11-08 | 2005-08-16 | Optiscan Biomedical Corp. | Analyte detection system with software download capabilities |
| US6932584B2 (en)* | 2002-12-26 | 2005-08-23 | Medtronic Minimed, Inc. | Infusion device and driving mechanism and process for same with actuator for multiple infusion uses |
| JP4754474B2 (en)* | 2003-02-25 | 2011-08-24 | エシコン・エンド−サージェリィ・インコーポレイテッド | Biopsy device with variable speed cutter |
| US7201977B2 (en) | 2004-03-23 | 2007-04-10 | Seagate Technology Llc | Anti-ferromagnetically coupled granular-continuous magnetic recording media |
- 2003
- 2003-07-22USUS10/624,389patent/US20040068230A1/ennot_activeAbandoned
- 2003-07-23AUAU2003252104Apatent/AU2003252104A1/ennot_activeAbandoned
- 2003-07-23WOPCT/US2003/022862patent/WO2004008956A2/ennot_activeApplication Discontinuation
- 2003-07-23EPEP03765896Apatent/EP1525014B1/ennot_activeExpired - Lifetime
- 2003-07-23EPEP10176391Apatent/EP2253346A1/ennot_activeWithdrawn
- 2003-07-23DKDK03765896.0Tpatent/DK1525014T3/enactive
- 2003-07-23EPEP10176392Apatent/EP2253347A1/ennot_activeWithdrawn
- 2008
- 2008-12-12USUS12/334,001patent/US20090099509A1/ennot_activeAbandoned
- 2008-12-12USUS12/333,690patent/US20090112076A1/ennot_activeAbandoned
- 2008-12-12USUS12/333,667patent/US20090149803A1/ennot_activeAbandoned
- 2008-12-12USUS12/333,731patent/US20090118664A1/ennot_activeAbandoned
- 2008-12-12USUS12/333,655patent/US20090143662A1/ennot_activeAbandoned
- 2008-12-12USUS12/333,639patent/US20090099506A1/ennot_activeAbandoned
- 2008-12-12USUS12/333,966patent/US20090118665A1/ennot_activeAbandoned
- 2010
- 2010-04-08USUS12/756,544patent/US8192395B2/ennot_activeExpired - Fee Related
- 2012
- 2012-05-07USUS13/465,037patent/US8568357B2/ennot_activeExpired - Lifetime
Patent Citations (60)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US3631847A (en)* | 1966-03-04 | 1972-01-04 | James C Hobbs | Method and apparatus for injecting fluid into the vascular system |
| US4270532A (en)* | 1977-12-28 | 1981-06-02 | Siemens Aktiengesellschaft | Device for the pre-programmable infusion of liquids |
| US4282872A (en)* | 1977-12-28 | 1981-08-11 | Siemens Aktiengesellschaft | Device for the pre-programmable infusion of liquids |
| US4559037A (en)* | 1977-12-28 | 1985-12-17 | Siemens Aktiengesellschaft | Device for the pre-programmable infusion of liquids |
| US4433072A (en)* | 1978-12-15 | 1984-02-21 | Hospal-Sodip, S.A. | Mixtures of polymers for medical use |
| US4373527A (en)* | 1979-04-27 | 1983-02-15 | The Johns Hopkins University | Implantable, programmable medication infusion system |
| US4373527B1 (en)* | 1979-04-27 | 1995-06-27 | Univ Johns Hopkins | Implantable programmable medication infusion system |
| US4731051A (en)* | 1979-04-27 | 1988-03-15 | The Johns Hopkins University | Programmable control means for providing safe and controlled medication infusion |
| US4475901A (en)* | 1980-07-23 | 1984-10-09 | The Garvan Research Development Ltd. | Apparatus for improving blood sugar control in diabetics |
| US4395259A (en)* | 1980-09-22 | 1983-07-26 | Siemens Aktiengesellschaft | Device for the infusion of fluids into the human or animal body |
| US4469481A (en)* | 1981-06-23 | 1984-09-04 | Terumo Corporation | Apparatus for infusing medication |
| US4392849A (en)* | 1981-07-27 | 1983-07-12 | The Cleveland Clinic Foundation | Infusion pump controller |
| US4529401A (en)* | 1982-01-11 | 1985-07-16 | Cardiac Pacemakers, Inc. | Ambulatory infusion pump having programmable parameters |
| US4494950A (en)* | 1982-01-19 | 1985-01-22 | The Johns Hopkins University | Plural module medication delivery system |
| US4443218A (en)* | 1982-09-09 | 1984-04-17 | Infusaid Corporation | Programmable implantable infusate pump |
| US4826810A (en)* | 1983-12-16 | 1989-05-02 | Aoki Thomas T | System and method for treating animal body tissues to improve the dietary fuel processing capabilities thereof |
| US4562751A (en)* | 1984-01-06 | 1986-01-07 | Nason Clyde K | Solenoid drive apparatus for an external infusion pump |
| US4678408A (en)* | 1984-01-06 | 1987-07-07 | Pacesetter Infusion, Ltd. | Solenoid drive apparatus for an external infusion pump |
| US4685903A (en)* | 1984-01-06 | 1987-08-11 | Pacesetter Infusion, Ltd. | External infusion pump apparatus |
| US4865584A (en)* | 1984-02-08 | 1989-09-12 | Omni-Flow, Inc. | Cassette for programable multiple input infusion system |
| US4696671A (en)* | 1984-02-08 | 1987-09-29 | Omni-Flow, Inc. | Infusion system having plural fluid input ports and at least one patient output port |
| US5100380A (en)* | 1984-02-08 | 1992-03-31 | Abbott Laboratories | Remotely programmable infusion system |
| US4550731A (en)* | 1984-03-07 | 1985-11-05 | Cordis Corporation | Acquisition circuit for cardiac pacer |
| US4542532A (en)* | 1984-03-09 | 1985-09-17 | Medtronic, Inc. | Dual-antenna transceiver |
| US4871351A (en)* | 1984-09-28 | 1989-10-03 | Vladimir Feingold | Implantable medication infusion system |
| US4781798A (en)* | 1985-04-19 | 1988-11-01 | The Regents Of The University Of California | Transparent multi-oxygen sensor array and method of using same |
| US4671288A (en)* | 1985-06-13 | 1987-06-09 | The Regents Of The University Of California | Electrochemical cell sensor for continuous short-term use in tissues and blood |
| US4703756A (en)* | 1986-05-06 | 1987-11-03 | The Regents Of The University Of California | Complete glucose monitoring system with an implantable, telemetered sensor module |
| US4731726A (en)* | 1986-05-19 | 1988-03-15 | Healthware Corporation | Patient-operated glucose monitor and diabetes management system |
| US4803625A (en)* | 1986-06-30 | 1989-02-07 | Buddy Systems, Inc. | Personal health monitor |
| US4935105A (en)* | 1987-02-24 | 1990-06-19 | Imperial Chemical Industries Plc | Methods of operating enzyme electrode sensors |
| US5216597A (en)* | 1987-05-01 | 1993-06-01 | Diva Medical Systems Bv | Diabetes therapy management system, apparatus and method |
| US5019974A (en)* | 1987-05-01 | 1991-05-28 | Diva Medical Systems Bv | Diabetes management system and apparatus |
| US5211626A (en)* | 1987-05-01 | 1993-05-18 | Product Innovation Holdings Ltd. | Medical infusion apparatus |
| US5034004A (en)* | 1987-06-19 | 1991-07-23 | The University Of Melbourne | Infusion pump and drive systems therefor |
| US4809697A (en)* | 1987-10-14 | 1989-03-07 | Siemens-Pacesetter, Inc. | Interactive programming and diagnostic system for use with implantable pacemaker |
| US4898578A (en)* | 1988-01-26 | 1990-02-06 | Baxter International Inc. | Drug infusion system with calculator |
| US5153827A (en)* | 1989-01-30 | 1992-10-06 | Omni-Flow, Inc. | An infusion management and pumping system having an alarm handling system |
| US4953552A (en)* | 1989-04-21 | 1990-09-04 | Demarzo Arthur P | Blood glucose monitoring system |
| US5264105A (en)* | 1989-08-02 | 1993-11-23 | Gregg Brian A | Enzyme electrodes |
| US5264104A (en)* | 1989-08-02 | 1993-11-23 | Gregg Brian A | Enzyme electrodes |
| US5262035A (en)* | 1989-08-02 | 1993-11-16 | E. Heller And Company | Enzyme electrodes |
| US5101814A (en)* | 1989-08-11 | 1992-04-07 | Palti Yoram Prof | System for monitoring and controlling blood glucose |
| US5050612A (en)* | 1989-09-12 | 1991-09-24 | Matsumura Kenneth N | Device for computer-assisted monitoring of the body |
| US5108819A (en)* | 1990-02-14 | 1992-04-28 | Eli Lilly And Company | Thin film electrical component |
| US5266013A (en)* | 1990-03-23 | 1993-11-30 | Asulab S.A. | Portable pump for the administration of a therapeutic |
| US5097122A (en)* | 1990-04-16 | 1992-03-17 | Pacesetter Infusion, Ltd. | Medication infusion system having optical motion sensor to detect drive mechanism malfunction |
| US5080653A (en)* | 1990-04-16 | 1992-01-14 | Pacesetter Infusion, Ltd. | Infusion pump with dual position syringe locator |
| US5165407A (en)* | 1990-04-19 | 1992-11-24 | The University Of Kansas | Implantable glucose sensor |
| US5078683A (en)* | 1990-05-04 | 1992-01-07 | Block Medical, Inc. | Programmable infusion system |
| US5113869A (en)* | 1990-08-21 | 1992-05-19 | Telectronics Pacing Systems, Inc. | Implantable ambulatory electrocardiogram monitor |
| US5262305A (en)* | 1991-03-04 | 1993-11-16 | E. Heller & Company | Interferant eliminating biosensors |
| US5904708A (en)* | 1998-03-19 | 1999-05-18 | Medtronic, Inc. | System and method for deriving relative physiologic signals |
| US7025743B2 (en)* | 1998-08-18 | 2006-04-11 | Medtronic Minimed, Inc. | External infusion device with remote programming, bolus estimator and/or vibration alarm capabilities |
| US20020019606A1 (en)* | 2000-01-21 | 2002-02-14 | Lebel Ronald J. | Microprocessor controlled ambulatory medical apparatus with hand held communication device |
| US20030060765A1 (en)* | 2000-02-16 | 2003-03-27 | Arthur Campbell | Infusion device menu structure and method of using the same |
| US6544212B2 (en)* | 2001-07-31 | 2003-04-08 | Roche Diagnostics Corporation | Diabetes management system |
| US20030114836A1 (en)* | 2001-12-19 | 2003-06-19 | Estes Mark C. | Medication delivery system and monitor |
| US20030211617A1 (en)* | 2002-05-07 | 2003-11-13 | International Business Machines Corporation | Blood glucose meter that reminds the user to test after a hypoglycemic event |
| US7278983B2 (en)* | 2002-07-24 | 2007-10-09 | Medtronic Minimed, Inc. | Physiological monitoring device for controlling a medication infusion device |
Cited By (667)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US7711402B2 (en) | 1997-03-04 | 2010-05-04 | Dexcom, Inc. | Device and method for determining analyte levels |
| US7860545B2 (en) | 1997-03-04 | 2010-12-28 | Dexcom, Inc. | Analyte measuring device |
| US10478108B2 (en) | 1998-04-30 | 2019-11-19 | Abbott Diabetes Care Inc. | Analyte monitoring device and methods of use |
| US9326714B2 (en) | 1998-04-30 | 2016-05-03 | Abbott Diabetes Care Inc. | Analyte monitoring device and methods of use |
| US9072477B2 (en) | 1998-04-30 | 2015-07-07 | Abbott Diabetes Care Inc. | Analyte monitoring device and methods of use |
| US9066695B2 (en) | 1998-04-30 | 2015-06-30 | Abbott Diabetes Care Inc. | Analyte monitoring device and methods of use |
| US9066694B2 (en) | 1998-04-30 | 2015-06-30 | Abbott Diabetes Care Inc. | Analyte monitoring device and methods of use |
| US9066697B2 (en) | 1998-04-30 | 2015-06-30 | Abbott Diabetes Care Inc. | Analyte monitoring device and methods of use |
| US9042953B2 (en) | 1998-04-30 | 2015-05-26 | Abbott Diabetes Care Inc. | Analyte monitoring device and methods of use |
| US9014773B2 (en) | 1998-04-30 | 2015-04-21 | Abbott Diabetes Care Inc. | Analyte monitoring device and methods of use |
| US9011331B2 (en) | 1998-04-30 | 2015-04-21 | Abbott Diabetes Care Inc. | Analyte monitoring device and methods of use |
| US8974386B2 (en) | 1998-04-30 | 2015-03-10 | Abbott Diabetes Care Inc. | Analyte monitoring device and methods of use |
| US8880137B2 (en) | 1998-04-30 | 2014-11-04 | Abbott Diabetes Care Inc. | Analyte monitoring device and methods of use |
| US8840553B2 (en) | 1998-04-30 | 2014-09-23 | Abbott Diabetes Care Inc. | Analyte monitoring device and methods of use |
| US8774887B2 (en) | 1998-04-30 | 2014-07-08 | Abbott Diabetes Care Inc. | Analyte monitoring device and methods of use |
| US8744545B2 (en) | 1998-04-30 | 2014-06-03 | Abbott Diabetes Care Inc. | Analyte monitoring device and methods of use |
| US8738109B2 (en) | 1998-04-30 | 2014-05-27 | Abbott Diabetes Care Inc. | Analyte monitoring device and methods of use |
| US8734346B2 (en) | 1998-04-30 | 2014-05-27 | Abbott Diabetes Care Inc. | Analyte monitoring device and methods of use |
| US8734348B2 (en) | 1998-04-30 | 2014-05-27 | Abbott Diabetes Care Inc. | Analyte monitoring device and methods of use |
| US8688188B2 (en) | 1998-04-30 | 2014-04-01 | Abbott Diabetes Care Inc. | Analyte monitoring device and methods of use |
| US8672844B2 (en) | 1998-04-30 | 2014-03-18 | Abbott Diabetes Care Inc. | Analyte monitoring device and methods of use |
| US8670815B2 (en) | 1998-04-30 | 2014-03-11 | Abbott Diabetes Care Inc. | Analyte monitoring device and methods of use |
| US8666469B2 (en) | 1998-04-30 | 2014-03-04 | Abbott Diabetes Care Inc. | Analyte monitoring device and methods of use |
| US8660627B2 (en) | 1998-04-30 | 2014-02-25 | Abbott Diabetes Care Inc. | Analyte monitoring device and methods of use |
| US8649841B2 (en) | 1998-04-30 | 2014-02-11 | Abbott Diabetes Care Inc. | Analyte monitoring device and methods of use |
| US8641619B2 (en) | 1998-04-30 | 2014-02-04 | Abbott Diabetes Care Inc. | Analyte monitoring device and methods of use |
| US8622906B2 (en) | 1998-04-30 | 2014-01-07 | Abbott Diabetes Care Inc. | Analyte monitoring device and methods of use |
| US8617071B2 (en) | 1998-04-30 | 2013-12-31 | Abbott Diabetes Care Inc. | Analyte monitoring device and methods of use |
| US8612159B2 (en) | 1998-04-30 | 2013-12-17 | Abbott Diabetes Care Inc. | Analyte monitoring device and methods of use |
| US8597189B2 (en) | 1998-04-30 | 2013-12-03 | Abbott Diabetes Care Inc. | Analyte monitoring device and methods of use |
| US8480580B2 (en) | 1998-04-30 | 2013-07-09 | Abbott Diabetes Care Inc. | Analyte monitoring device and methods of use |
| US8473021B2 (en) | 1998-04-30 | 2013-06-25 | Abbott Diabetes Care Inc. | Analyte monitoring device and methods of use |
| US8465425B2 (en) | 1998-04-30 | 2013-06-18 | Abbott Diabetes Care Inc. | Analyte monitoring device and methods of use |
| US8409131B2 (en) | 1998-04-30 | 2013-04-02 | Abbott Diabetes Care Inc. | Analyte monitoring device and methods of use |
| US8391945B2 (en) | 1998-04-30 | 2013-03-05 | Abbott Diabetes Care Inc. | Analyte monitoring device and methods of use |
| US8380273B2 (en) | 1998-04-30 | 2013-02-19 | Abbott Diabetes Care Inc. | Analyte monitoring device and methods of use |
| US8372005B2 (en) | 1998-04-30 | 2013-02-12 | Abbott Diabetes Care Inc. | Analyte monitoring device and methods of use |
| US8366614B2 (en) | 1998-04-30 | 2013-02-05 | Abbott Diabetes Care Inc. | Analyte monitoring device and methods of use |
| US8357091B2 (en) | 1998-04-30 | 2013-01-22 | Abbott Diabetes Care Inc. | Analyte monitoring device and methods of use |
| US8353829B2 (en) | 1998-04-30 | 2013-01-15 | Abbott Diabetes Care Inc. | Analyte monitoring device and methods of use |
| US8346337B2 (en) | 1998-04-30 | 2013-01-01 | Abbott Diabetes Care Inc. | Analyte monitoring device and methods of use |
| US8346336B2 (en) | 1998-04-30 | 2013-01-01 | Abbott Diabetes Care Inc. | Analyte monitoring device and methods of use |
| US8306598B2 (en) | 1998-04-30 | 2012-11-06 | Abbott Diabetes Care Inc. | Analyte monitoring device and methods of use |
| US8287454B2 (en) | 1998-04-30 | 2012-10-16 | Abbott Diabetes Care Inc. | Analyte monitoring device and methods of use |
| US8275439B2 (en) | 1998-04-30 | 2012-09-25 | Abbott Diabetes Care Inc. | Analyte monitoring device and methods of use |
| US8273022B2 (en) | 1998-04-30 | 2012-09-25 | Abbott Diabetes Care Inc. | Analyte monitoring device and methods of use |
| US8265726B2 (en) | 1998-04-30 | 2012-09-11 | Abbott Diabetes Care Inc. | Analyte monitoring device and methods of use |
| US8260392B2 (en) | 1998-04-30 | 2012-09-04 | Abbott Diabetes Care Inc. | Analyte monitoring device and methods of use |
| US8255031B2 (en) | 1998-04-30 | 2012-08-28 | Abbott Diabetes Care Inc. | Analyte monitoring device and methods of use |
| US8235896B2 (en) | 1998-04-30 | 2012-08-07 | Abbott Diabetes Care Inc. | Analyte monitoring device and methods of use |
| US8231532B2 (en) | 1998-04-30 | 2012-07-31 | Abbott Diabetes Care Inc. | Analyte monitoring device and methods of use |
| US8226558B2 (en) | 1998-04-30 | 2012-07-24 | Abbott Diabetes Care Inc. | Analyte monitoring device and methods of use |
| US8226555B2 (en) | 1998-04-30 | 2012-07-24 | Abbott Diabetes Care Inc. | Analyte monitoring device and methods of use |
| US8226557B2 (en) | 1998-04-30 | 2012-07-24 | Abbott Diabetes Care Inc. | Analyte monitoring device and methods of use |
| US8224413B2 (en) | 1998-04-30 | 2012-07-17 | Abbott Diabetes Care Inc. | Analyte monitoring device and methods of use |
| US8177716B2 (en) | 1998-04-30 | 2012-05-15 | Abbott Diabetes Care Inc. | Analyte monitoring device and methods of use |
| US8175673B2 (en) | 1998-04-30 | 2012-05-08 | Abbott Diabetes Care Inc. | Analyte monitoring device and methods of use |
| US8162829B2 (en) | 1998-04-30 | 2012-04-24 | Abbott Diabetes Care Inc. | Analyte monitoring device and methods of use |
| US7885699B2 (en) | 1998-04-30 | 2011-02-08 | Abbott Diabetes Care Inc. | Analyte monitoring device and methods of use |
| US7869853B1 (en) | 1998-04-30 | 2011-01-11 | Abbott Diabetes Care Inc. | Analyte monitoring device and methods of use |
| US7860544B2 (en) | 1998-04-30 | 2010-12-28 | Abbott Diabetes Care Inc. | Analyte monitoring device and methods of use |
| US9011332B2 (en) | 2001-01-02 | 2015-04-21 | Abbott Diabetes Care Inc. | Analyte monitoring device and methods of use |
| US8652043B2 (en) | 2001-01-02 | 2014-02-18 | Abbott Diabetes Care Inc. | Analyte monitoring device and methods of use |
| US9498159B2 (en) | 2001-01-02 | 2016-11-22 | Abbott Diabetes Care Inc. | Analyte monitoring device and methods of use |
| US8668645B2 (en) | 2001-01-02 | 2014-03-11 | Abbott Diabetes Care Inc. | Analyte monitoring device and methods of use |
| US9610034B2 (en) | 2001-01-02 | 2017-04-04 | Abbott Diabetes Care Inc. | Analyte monitoring device and methods of use |
| US7976778B2 (en) | 2001-04-02 | 2011-07-12 | Abbott Diabetes Care Inc. | Blood glucose tracking apparatus |
| US9477811B2 (en) | 2001-04-02 | 2016-10-25 | Abbott Diabetes Care Inc. | Blood glucose tracking apparatus and methods |
| US8236242B2 (en) | 2001-04-02 | 2012-08-07 | Abbott Diabetes Care Inc. | Blood glucose tracking apparatus and methods |
| US8268243B2 (en) | 2001-04-02 | 2012-09-18 | Abbott Diabetes Care Inc. | Blood glucose tracking apparatus and methods |
| US8765059B2 (en) | 2001-04-02 | 2014-07-01 | Abbott Diabetes Care Inc. | Blood glucose tracking apparatus |
| US11294407B2 (en) | 2001-04-27 | 2022-04-05 | Roche Diabetes Care, Inc. | Device and method for insulin dosing |
| US8034026B2 (en) | 2001-05-18 | 2011-10-11 | Deka Products Limited Partnership | Infusion pump assembly |
| US9173996B2 (en) | 2001-05-18 | 2015-11-03 | Deka Products Limited Partnership | Infusion set for a fluid pump |
| US9328371B2 (en) | 2001-07-27 | 2016-05-03 | Dexcom, Inc. | Sensor head for use with implantable devices |
| US7471972B2 (en) | 2001-07-27 | 2008-12-30 | Dexcom, Inc. | Sensor head for use with implantable devices |
| US10039480B2 (en) | 2001-07-27 | 2018-08-07 | Dexcom, Inc. | Membrane for use with implantable devices |
| US9532741B2 (en) | 2001-07-27 | 2017-01-03 | Dexcom, Inc. | Membrane for use with implantable devices |
| US9804114B2 (en) | 2001-07-27 | 2017-10-31 | Dexcom, Inc. | Sensor head for use with implantable devices |
| US8840552B2 (en) | 2001-07-27 | 2014-09-23 | Dexcom, Inc. | Membrane for use with implantable devices |
| US8509871B2 (en) | 2001-07-27 | 2013-08-13 | Dexcom, Inc. | Sensor head for use with implantable devices |
| US7632228B2 (en) | 2001-07-27 | 2009-12-15 | Dexcom, Inc. | Membrane for use with implantable devices |
| US9282925B2 (en) | 2002-02-12 | 2016-03-15 | Dexcom, Inc. | Systems and methods for replacing signal artifacts in a glucose sensor data stream |
| US8346399B2 (en) | 2002-02-28 | 2013-01-01 | Tandem Diabetes Care, Inc. | Programmable insulin pump |
| US10049768B2 (en) | 2002-02-28 | 2018-08-14 | Tandem Diabetes Care, Inc. | Programmable insulin pump |
| US20040215492A1 (en)* | 2003-01-30 | 2004-10-28 | Choi Soo Bong | Method for controlling insulin pump through internet |
| US20050112169A1 (en)* | 2003-05-21 | 2005-05-26 | Dexcom, Inc. | Porous membranes for use with implantable devices |
| US7875293B2 (en) | 2003-05-21 | 2011-01-25 | Dexcom, Inc. | Biointerface membranes incorporating bioactive agents |
| US8118877B2 (en) | 2003-05-21 | 2012-02-21 | Dexcom, Inc. | Porous membranes for use with implantable devices |
| US7192450B2 (en) | 2003-05-21 | 2007-03-20 | Dexcom, Inc. | Porous membranes for use with implantable devices |
| US20070032891A1 (en)* | 2003-05-23 | 2007-02-08 | Choi Soo B | Method for controlling insulin pump through internet |
| US7231263B2 (en)* | 2003-05-23 | 2007-06-12 | Soo Bong Choi | Method for controlling insulin pump through internet |
| US8423113B2 (en) | 2003-07-25 | 2013-04-16 | Dexcom, Inc. | Systems and methods for processing sensor data |
| US7896809B2 (en) | 2003-07-25 | 2011-03-01 | Dexcom, Inc. | Dual electrode system for a continuous analyte sensor |
| US8260393B2 (en) | 2003-07-25 | 2012-09-04 | Dexcom, Inc. | Systems and methods for replacing signal data artifacts in a glucose sensor data stream |
| US7979104B2 (en) | 2003-08-01 | 2011-07-12 | Dexcom, Inc. | System and methods for processing analyte sensor data |
| US7797028B2 (en) | 2003-08-01 | 2010-09-14 | Dexcom, Inc. | System and methods for processing analyte sensor data |
| US8622905B2 (en) | 2003-08-01 | 2014-01-07 | Dexcom, Inc. | System and methods for processing analyte sensor data |
| US7933639B2 (en) | 2003-08-01 | 2011-04-26 | Dexcom, Inc. | System and methods for processing analyte sensor data |
| US8548553B2 (en) | 2003-08-01 | 2013-10-01 | Dexcom, Inc. | System and methods for processing analyte sensor data |
| US7914450B2 (en) | 2003-08-01 | 2011-03-29 | Dexcom, Inc. | System and methods for processing analyte sensor data |
| US7276029B2 (en) | 2003-08-01 | 2007-10-02 | Dexcom, Inc. | System and methods for processing analyte sensor data |
| US7955261B2 (en) | 2003-08-01 | 2011-06-07 | Dexcom, Inc. | System and methods for processing analyte sensor data |
| US8676287B2 (en) | 2003-08-01 | 2014-03-18 | Dexcom, Inc. | System and methods for processing analyte sensor data |
| US7959569B2 (en) | 2003-08-01 | 2011-06-14 | Dexcom, Inc. | System and methods for processing analyte sensor data |
| US9895089B2 (en) | 2003-08-01 | 2018-02-20 | Dexcom, Inc. | System and methods for processing analyte sensor data |
| US8700117B2 (en) | 2003-08-01 | 2014-04-15 | Dexcom, Inc. | System and methods for processing analyte sensor data |
| US8588882B2 (en) | 2003-08-01 | 2013-11-19 | Dexcom, Inc. | System and methods for processing analyte sensor data |
| US20080195967A1 (en)* | 2003-08-01 | 2008-08-14 | Dexcom, Inc. | System and methods for processing analyte sensor data |
| US20050027181A1 (en)* | 2003-08-01 | 2005-02-03 | Goode Paul V. | System and methods for processing analyte sensor data |
| US7986986B2 (en) | 2003-08-01 | 2011-07-26 | Dexcom, Inc. | System and methods for processing analyte sensor data |
| US8000901B2 (en) | 2003-08-01 | 2011-08-16 | Dexcom, Inc. | Transcutaneous analyte sensor |
| US8442610B2 (en) | 2003-08-01 | 2013-05-14 | Dexcom, Inc. | System and methods for processing analyte sensor data |
| US8428679B2 (en) | 2003-08-01 | 2013-04-23 | Dexcom, Inc. | System and methods for processing analyte sensor data |
| US8761856B2 (en) | 2003-08-01 | 2014-06-24 | Dexcom, Inc. | System and methods for processing analyte sensor data |
| US8771187B2 (en) | 2003-08-01 | 2014-07-08 | Dexcom, Inc. | System and methods for processing analyte sensor data |
| US8774888B2 (en) | 2003-08-01 | 2014-07-08 | Dexcom, Inc. | System and methods for processing analyte sensor data |
| US20080194937A1 (en)* | 2003-08-01 | 2008-08-14 | Dexcom, Inc. | System and methods for processing analyte sensor data |
| US10786185B2 (en) | 2003-08-01 | 2020-09-29 | Dexcom, Inc. | System and methods for processing analyte sensor data |
| US8052601B2 (en) | 2003-08-01 | 2011-11-08 | Dexcom, Inc. | System and methods for processing analyte sensor data |
| US8060173B2 (en) | 2003-08-01 | 2011-11-15 | Dexcom, Inc. | System and methods for processing analyte sensor data |
| US8394021B2 (en) | 2003-08-01 | 2013-03-12 | Dexcom, Inc. | System and methods for processing analyte sensor data |
| US8788007B2 (en) | 2003-08-01 | 2014-07-22 | Dexcom, Inc. | Transcutaneous analyte sensor |
| US8788008B2 (en) | 2003-08-01 | 2014-07-22 | Dexcom, Inc. | System and methods for processing analyte sensor data |
| US8788006B2 (en) | 2003-08-01 | 2014-07-22 | Dexcom, Inc. | System and methods for processing analyte sensor data |
| US8369919B2 (en) | 2003-08-01 | 2013-02-05 | Dexcom, Inc. | Systems and methods for processing sensor data |
| US8801612B2 (en) | 2003-08-01 | 2014-08-12 | Dexcom, Inc. | System and methods for processing analyte sensor data |
| US8808182B2 (en) | 2003-08-01 | 2014-08-19 | Dexcom, Inc. | System and methods for processing analyte sensor data |
| US8332008B2 (en) | 2003-08-01 | 2012-12-11 | Dexcom, Inc. | System and methods for processing analyte sensor data |
| US8321149B2 (en) | 2003-08-01 | 2012-11-27 | Dexcom, Inc. | Transcutaneous analyte sensor |
| US8311749B2 (en) | 2003-08-01 | 2012-11-13 | Dexcom, Inc. | Transcutaneous analyte sensor |
| US8290562B2 (en) | 2003-08-01 | 2012-10-16 | Dexcom, Inc. | System and methods for processing analyte sensor data |
| US7925321B2 (en) | 2003-08-01 | 2011-04-12 | Dexcom, Inc. | System and methods for processing analyte sensor data |
| US8845536B2 (en) | 2003-08-01 | 2014-09-30 | Dexcom, Inc. | Transcutaneous analyte sensor |
| US8160669B2 (en) | 2003-08-01 | 2012-04-17 | Dexcom, Inc. | Transcutaneous analyte sensor |
| US8886273B2 (en) | 2003-08-01 | 2014-11-11 | Dexcom, Inc. | Analyte sensor |
| US8915849B2 (en) | 2003-08-01 | 2014-12-23 | Dexcom, Inc. | Transcutaneous analyte sensor |
| US8285354B2 (en) | 2003-08-01 | 2012-10-09 | Dexcom, Inc. | System and methods for processing analyte sensor data |
| US7583990B2 (en) | 2003-08-01 | 2009-09-01 | Dexcom, Inc. | System and methods for processing analyte sensor data |
| US7599726B2 (en) | 2003-08-01 | 2009-10-06 | Dexcom, Inc. | System and methods for processing analyte sensor data |
| US8986209B2 (en) | 2003-08-01 | 2015-03-24 | Dexcom, Inc. | Transcutaneous analyte sensor |
| US8206297B2 (en) | 2003-08-01 | 2012-06-26 | Dexcom, Inc. | System and methods for processing analyte sensor data |
| US7774145B2 (en) | 2003-08-01 | 2010-08-10 | Dexcom, Inc. | Transcutaneous analyte sensor |
| US8275437B2 (en) | 2003-08-01 | 2012-09-25 | Dexcom, Inc. | Transcutaneous analyte sensor |
| US7778680B2 (en) | 2003-08-01 | 2010-08-17 | Dexcom, Inc. | System and methods for processing analyte sensor data |
| US9510782B2 (en) | 2003-08-22 | 2016-12-06 | Dexcom, Inc. | Systems and methods for replacing signal artifacts in a glucose sensor data stream |
| US8073519B2 (en) | 2003-08-22 | 2011-12-06 | Dexcom, Inc. | Systems and methods for replacing signal artifacts in a glucose sensor data stream |
| US7935057B2 (en) | 2003-08-22 | 2011-05-03 | Dexcom, Inc. | Systems and methods for replacing signal artifacts in a glucose sensor data stream |
| US8657747B2 (en) | 2003-08-22 | 2014-02-25 | Dexcom, Inc. | Systems and methods for processing analyte sensor data |
| US8229536B2 (en) | 2003-08-22 | 2012-07-24 | Dexcom, Inc. | Systems and methods for replacing signal artifacts in a glucose sensor data stream |
| US8491474B2 (en) | 2003-08-22 | 2013-07-23 | Dexcom, Inc. | Systems and methods for replacing signal artifacts in a glucose sensor data stream |
| US8672845B2 (en) | 2003-08-22 | 2014-03-18 | Dexcom, Inc. | Systems and methods for processing analyte sensor data |
| US8843187B2 (en) | 2003-08-22 | 2014-09-23 | Dexcom, Inc. | Systems and methods for replacing signal artifacts in a glucose sensor data stream |
| US8812073B2 (en) | 2003-08-22 | 2014-08-19 | Dexcom, Inc. | Systems and methods for replacing signal artifacts in a glucose sensor data stream |
| US9247901B2 (en) | 2003-08-22 | 2016-02-02 | Dexcom, Inc. | Systems and methods for replacing signal artifacts in a glucose sensor data stream |
| US20070066873A1 (en)* | 2003-08-22 | 2007-03-22 | Apurv Kamath | Systems and methods for processing analyte sensor data |
| US8233959B2 (en) | 2003-08-22 | 2012-07-31 | Dexcom, Inc. | Systems and methods for processing analyte sensor data |
| US20090124877A1 (en)* | 2003-08-22 | 2009-05-14 | Dexcom, Inc. | Systems and methods for replacing signal artifacts in a glucose sensor data stream |
| US11589823B2 (en) | 2003-08-22 | 2023-02-28 | Dexcom, Inc. | Systems and methods for replacing signal artifacts in a glucose sensor data stream |
| US8292810B2 (en) | 2003-08-22 | 2012-10-23 | Dexcom, Inc. | Systems and methods for replacing signal artifacts in a glucose sensor data stream |
| US9420968B2 (en) | 2003-08-22 | 2016-08-23 | Dexcom, Inc. | Systems and methods for replacing signal artifacts in a glucose sensor data stream |
| US7998071B2 (en) | 2003-08-22 | 2011-08-16 | Dexcom, Inc. | Systems and methods for replacing signal artifacts in a glucose sensor data stream |
| US9149219B2 (en) | 2003-08-22 | 2015-10-06 | Dexcom, Inc. | Systems and methods for replacing signal artifacts in a glucose sensor data stream |
| US8435179B2 (en) | 2003-08-22 | 2013-05-07 | Dexcom, Inc. | Systems and methods for replacing signal artifacts in a glucose sensor data stream |
| US9750460B2 (en) | 2003-08-22 | 2017-09-05 | Dexcom, Inc. | Systems and methods for replacing signal artifacts in a glucose sensor data stream |
| US8005525B2 (en) | 2003-08-22 | 2011-08-23 | Dexcom, Inc. | Systems and methods for replacing signal artifacts in a glucose sensor data stream |
| US8128562B2 (en) | 2003-08-22 | 2012-03-06 | Dexcom, Inc. | Systems and methods for replacing signal artifacts in a glucose sensor data stream |
| US8010174B2 (en) | 2003-08-22 | 2011-08-30 | Dexcom, Inc. | Systems and methods for replacing signal artifacts in a glucose sensor data stream |
| US9724045B1 (en) | 2003-08-22 | 2017-08-08 | Dexcom, Inc. | Systems and methods for replacing signal artifacts in a glucose sensor data stream |
| US8412301B2 (en) | 2003-08-22 | 2013-04-02 | Dexcom, Inc. | Systems and methods for replacing signal artifacts in a glucose sensor data stream |
| US8777853B2 (en) | 2003-08-22 | 2014-07-15 | Dexcom, Inc. | Systems and methods for replacing signal artifacts in a glucose sensor data stream |
| US9649069B2 (en) | 2003-08-22 | 2017-05-16 | Dexcom, Inc. | Systems and methods for replacing signal artifacts in a glucose sensor data stream |
| US9427183B2 (en) | 2003-08-22 | 2016-08-30 | Dexcom, Inc. | Systems and methods for replacing signal artifacts in a glucose sensor data stream |
| US8346338B2 (en) | 2003-08-22 | 2013-01-01 | Dexcom, Inc. | System and methods for replacing signal artifacts in a glucose sensor data stream |
| US11559260B2 (en) | 2003-08-22 | 2023-01-24 | Dexcom, Inc. | Systems and methods for processing analyte sensor data |
| US8195265B2 (en) | 2003-08-22 | 2012-06-05 | Dexcom, Inc. | Systems and methods for replacing signal artifacts in a glucose sensor data stream |
| US8821400B2 (en) | 2003-08-22 | 2014-09-02 | Dexcom, Inc. | Systems and methods for replacing signal artifacts in a glucose sensor data stream |
| US8790260B2 (en) | 2003-08-22 | 2014-07-29 | Dexcom, Inc. | Systems and methods for replacing signal artifacts in a glucose sensor data stream |
| US8073520B2 (en) | 2003-08-22 | 2011-12-06 | Dexcom, Inc. | Systems and methods for replacing signal artifacts in a glucose sensor data stream |
| US8795177B2 (en) | 2003-08-22 | 2014-08-05 | Dexcom, Inc. | Systems and methods for replacing signal artifacts in a glucose sensor data stream |
| US8150488B2 (en) | 2003-08-22 | 2012-04-03 | Dexcom, Inc. | Systems and methods for replacing signal artifacts in a glucose sensor data stream |
| US8167801B2 (en) | 2003-08-22 | 2012-05-01 | Dexcom, Inc. | Systems and methods for replacing signal artifacts in a glucose sensor data stream |
| US9585607B2 (en) | 2003-08-22 | 2017-03-07 | Dexcom, Inc. | Systems and methods for replacing signal artifacts in a glucose sensor data stream |
| US8062253B2 (en) | 2003-10-21 | 2011-11-22 | Novo Nordisk A/S | Medical skin mountable device |
| US20060264835A1 (en)* | 2003-10-21 | 2006-11-23 | Novo Nordisk A/S | Medical skin mountable device |
| US9592336B2 (en) | 2003-10-27 | 2017-03-14 | Novo Nordisk A/S | Medical skin mountable device |
| US20100100048A1 (en)* | 2003-10-27 | 2010-04-22 | Novo Nordisk A/S | Medical Skin Mountable Device |
| US20110137255A1 (en)* | 2003-10-27 | 2011-06-09 | Novo Nordisk A/S | Medical Skin Mountable Device |
| US20050090607A1 (en)* | 2003-10-28 | 2005-04-28 | Dexcom, Inc. | Silicone composition for biocompatible membrane |
| US9538946B2 (en) | 2003-11-19 | 2017-01-10 | Dexcom, Inc. | Integrated receiver for continuous analyte sensor |
| US8282550B2 (en) | 2003-11-19 | 2012-10-09 | Dexcom, Inc. | Integrated receiver for continuous analyte sensor |
| US7927274B2 (en) | 2003-11-19 | 2011-04-19 | Dexcom, Inc. | Integrated receiver for continuous analyte sensor |
| US11564602B2 (en) | 2003-11-19 | 2023-01-31 | Dexcom, Inc. | Integrated receiver for continuous analyte sensor |
| US7519408B2 (en) | 2003-11-19 | 2009-04-14 | Dexcom, Inc. | Integrated receiver for continuous analyte sensor |
| US20050154271A1 (en)* | 2003-11-19 | 2005-07-14 | Andrew Rasdal | Integrated receiver for continuous analyte sensor |
| USRE44695E1 (en) | 2003-12-05 | 2014-01-07 | Dexcom, Inc. | Dual electrode system for a continuous analyte sensor |
| US11000215B1 (en) | 2003-12-05 | 2021-05-11 | Dexcom, Inc. | Analyte sensor |
| US8483793B2 (en) | 2003-12-05 | 2013-07-09 | Dexcom, Inc. | Dual electrode system for a continuous analyte sensor |
| US11020031B1 (en) | 2003-12-05 | 2021-06-01 | Dexcom, Inc. | Analyte sensor |
| US11633133B2 (en) | 2003-12-05 | 2023-04-25 | Dexcom, Inc. | Dual electrode system for a continuous analyte sensor |
| US8386004B2 (en) | 2003-12-05 | 2013-02-26 | Dexcom, Inc. | Calibration techniques for a continuous analyte sensor |
| US8257259B2 (en) | 2003-12-09 | 2012-09-04 | Dexcom, Inc. | Signal processing for continuous analyte sensor |
| US11638541B2 (en) | 2003-12-09 | 2023-05-02 | Dexconi, Inc. | Signal processing for continuous analyte sensor |
| US8374667B2 (en) | 2003-12-09 | 2013-02-12 | Dexcom, Inc. | Signal processing for continuous analyte sensor |
| US8282549B2 (en) | 2003-12-09 | 2012-10-09 | Dexcom, Inc. | Signal processing for continuous analyte sensor |
| US9351668B2 (en) | 2003-12-09 | 2016-05-31 | Dexcom, Inc. | Signal processing for continuous analyte sensor |
| US9498155B2 (en) | 2003-12-09 | 2016-11-22 | Dexcom, Inc. | Signal processing for continuous analyte sensor |
| US10898113B2 (en) | 2003-12-09 | 2021-01-26 | Dexcom, Inc. | Signal processing for continuous analyte sensor |
| US8657745B2 (en) | 2003-12-09 | 2014-02-25 | Dexcom, Inc. | Signal processing for continuous analyte sensor |
| US9420965B2 (en) | 2003-12-09 | 2016-08-23 | Dexcom, Inc. | Signal processing for continuous analyte sensor |
| US8216139B2 (en) | 2003-12-09 | 2012-07-10 | Dexcom, Inc. | Signal processing for continuous analyte sensor |
| US8801610B2 (en) | 2003-12-09 | 2014-08-12 | Dexcom, Inc. | Signal processing for continuous analyte sensor |
| US8265725B2 (en) | 2003-12-09 | 2012-09-11 | Dexcom, Inc. | Signal processing for continuous analyte sensor |
| US9364173B2 (en) | 2003-12-09 | 2016-06-14 | Dexcom, Inc. | Signal processing for continuous analyte sensor |
| US9750441B2 (en) | 2003-12-09 | 2017-09-05 | Dexcom, Inc. | Signal processing for continuous analyte sensor |
| US8290561B2 (en) | 2003-12-09 | 2012-10-16 | Dexcom, Inc. | Signal processing for continuous analyte sensor |
| US8469886B2 (en) | 2003-12-09 | 2013-06-25 | Dexcom, Inc. | Signal processing for continuous analyte sensor |
| US20100030485A1 (en)* | 2003-12-09 | 2010-02-04 | Dexcom, Inc. | Signal processing for continuous analyte sensor |
| US8233958B2 (en) | 2003-12-09 | 2012-07-31 | Dexcom, Inc. | Signal processing for continuous analyte sensor |
| US9107623B2 (en) | 2003-12-09 | 2015-08-18 | Dexcom, Inc. | Signal processing for continuous analyte sensor |
| US8251906B2 (en) | 2003-12-09 | 2012-08-28 | Dexcom, Inc. | Signal processing for continuous analyte sensor |
| US8747315B2 (en) | 2003-12-09 | 2014-06-10 | Dexcom. Inc. | Signal processing for continuous analyte sensor |
| US9192328B2 (en) | 2003-12-09 | 2015-11-24 | Dexcom, Inc. | Signal processing for continuous analyte sensor |
| US20050137573A1 (en)* | 2003-12-19 | 2005-06-23 | Animas Corporation | System, method, and communication hub for controlling external infusion device |
| US20140288494A1 (en)* | 2004-02-26 | 2014-09-25 | Dexcom, Inc. | Integrated medicament delivery device for use with continuous analyte sensor |
| US11246990B2 (en) | 2004-02-26 | 2022-02-15 | Dexcom, Inc. | Integrated delivery device for continuous glucose sensor |
| US10278580B2 (en)* | 2004-02-26 | 2019-05-07 | Dexcom, Inc. | Integrated medicament delivery device for use with continuous analyte sensor |
| US8721585B2 (en) | 2004-02-26 | 2014-05-13 | Dex Com, Inc. | Integrated delivery device for continuous glucose sensor |
| US20210068658A1 (en)* | 2004-02-26 | 2021-03-11 | Dexcom, Inc. | Integrated medicament delivery device for use with continuous analyte sensor |
| US7976492B2 (en) | 2004-02-26 | 2011-07-12 | Dexcom, Inc. | Integrated delivery device for continuous glucose sensor |
| US20210186328A1 (en)* | 2004-02-26 | 2021-06-24 | Dexcom, Inc. | Integrated medicament delivery device for use with continuous analyte sensor |
| US7591801B2 (en) | 2004-02-26 | 2009-09-22 | Dexcom, Inc. | Integrated delivery device for continuous glucose sensor |
| US20210212563A1 (en)* | 2004-02-26 | 2021-07-15 | Dexcom, Inc. | Integrated medicament delivery device for use with continuous analyte sensor |
| US20080262469A1 (en)* | 2004-02-26 | 2008-10-23 | Dexcom. Inc. | Integrated medicament delivery device for use with continuous analyte sensor |
| US9937293B2 (en) | 2004-02-26 | 2018-04-10 | Dexcom, Inc. | Integrated delivery device for continuous glucose sensor |
| US12102410B2 (en)* | 2004-02-26 | 2024-10-01 | Dexcom, Inc | Integrated medicament delivery device for use with continuous analyte sensor |
| US8808228B2 (en) | 2004-02-26 | 2014-08-19 | Dexcom, Inc. | Integrated medicament delivery device for use with continuous analyte sensor |
| US20050192557A1 (en)* | 2004-02-26 | 2005-09-01 | Dexcom | Integrated delivery device for continuous glucose sensor |
| US12115357B2 (en) | 2004-02-26 | 2024-10-15 | Dexcom, Inc. | Integrated delivery device for continuous glucose sensor |
| US10966609B2 (en) | 2004-02-26 | 2021-04-06 | Dexcom, Inc. | Integrated medicament delivery device for use with continuous analyte sensor |
| US12226617B2 (en) | 2004-02-26 | 2025-02-18 | Dexcom, Inc. | Integrated delivery device for continuous glucose sensor |
| US10835672B2 (en) | 2004-02-26 | 2020-11-17 | Dexcom, Inc. | Integrated insulin delivery system with continuous glucose sensor |
| US8926585B2 (en) | 2004-02-26 | 2015-01-06 | Dexcom, Inc. | Integrated delivery device for continuous glucose sensor |
| US20090299276A1 (en)* | 2004-02-26 | 2009-12-03 | Dexcom, Inc. | Integrated delivery device for continuous glucose sensor |
| US11045120B2 (en) | 2004-07-13 | 2021-06-29 | Dexcom, Inc. | Analyte sensor |
| US10722152B2 (en) | 2004-07-13 | 2020-07-28 | Dexcom, Inc. | Analyte sensor |
| US8565849B2 (en) | 2004-07-13 | 2013-10-22 | Dexcom, Inc. | Transcutaneous analyte sensor |
| US11883164B2 (en) | 2004-07-13 | 2024-01-30 | Dexcom, Inc. | System and methods for processing analyte sensor data for sensor calibration |
| US8571625B2 (en) | 2004-07-13 | 2013-10-29 | Dexcom, Inc. | Transcutaneous analyte sensor |
| US7654956B2 (en) | 2004-07-13 | 2010-02-02 | Dexcom, Inc. | Transcutaneous analyte sensor |
| US7713574B2 (en) | 2004-07-13 | 2010-05-11 | Dexcom, Inc. | Transcutaneous analyte sensor |
| US8565848B2 (en) | 2004-07-13 | 2013-10-22 | Dexcom, Inc. | Transcutaneous analyte sensor |
| US7640048B2 (en) | 2004-07-13 | 2009-12-29 | Dexcom, Inc. | Analyte sensor |
| US7310544B2 (en) | 2004-07-13 | 2007-12-18 | Dexcom, Inc. | Methods and systems for inserting a transcutaneous analyte sensor |
| US8615282B2 (en) | 2004-07-13 | 2013-12-24 | Dexcom, Inc. | Analyte sensor |
| US20070265515A1 (en)* | 2004-07-13 | 2007-11-15 | Mark Brister | Transcutaneous analyte sensor |
| US8548551B2 (en) | 2004-07-13 | 2013-10-01 | Dexcom, Inc. | Transcutaneous analyte sensor |
| US7783333B2 (en) | 2004-07-13 | 2010-08-24 | Dexcom, Inc. | Transcutaneous medical device with variable stiffness |
| US11064917B2 (en) | 2004-07-13 | 2021-07-20 | Dexcom, Inc. | Analyte sensor |
| US8515519B2 (en) | 2004-07-13 | 2013-08-20 | Dexcom, Inc. | Transcutaneous analyte sensor |
| US20070232879A1 (en)* | 2004-07-13 | 2007-10-04 | Mark Brister | Transcutaneous analyte sensor |
| US8515516B2 (en) | 2004-07-13 | 2013-08-20 | Dexcom, Inc. | Transcutaneous analyte sensor |
| US11026605B1 (en) | 2004-07-13 | 2021-06-08 | Dexcom, Inc. | Analyte sensor |
| US7857760B2 (en) | 2004-07-13 | 2010-12-28 | Dexcom, Inc. | Analyte sensor |
| US8663109B2 (en) | 2004-07-13 | 2014-03-04 | Dexcom, Inc. | Transcutaneous analyte sensor |
| US10993641B2 (en) | 2004-07-13 | 2021-05-04 | Dexcom, Inc. | Analyte sensor |
| US8483791B2 (en) | 2004-07-13 | 2013-07-09 | Dexcom, Inc. | Transcutaneous analyte sensor |
| US10993642B2 (en) | 2004-07-13 | 2021-05-04 | Dexcom, Inc. | Analyte sensor |
| US8475373B2 (en) | 2004-07-13 | 2013-07-02 | Dexcom, Inc. | Transcutaneous analyte sensor |
| US9044199B2 (en) | 2004-07-13 | 2015-06-02 | Dexcom, Inc. | Transcutaneous analyte sensor |
| US10980452B2 (en) | 2004-07-13 | 2021-04-20 | Dexcom, Inc. | Analyte sensor |
| US8690775B2 (en) | 2004-07-13 | 2014-04-08 | Dexcom, Inc. | Transcutaneous analyte sensor |
| US8474397B2 (en) | 2004-07-13 | 2013-07-02 | Dexcom, Inc. | Transcutaneous analyte sensor |
| US7497827B2 (en) | 2004-07-13 | 2009-03-03 | Dexcom, Inc. | Transcutaneous analyte sensor |
| US20060020189A1 (en)* | 2004-07-13 | 2006-01-26 | Dexcom, Inc. | Transcutaneous analyte sensor |
| US8231531B2 (en) | 2004-07-13 | 2012-07-31 | Dexcom, Inc. | Analyte sensor |
| US10932700B2 (en) | 2004-07-13 | 2021-03-02 | Dexcom, Inc. | Analyte sensor |
| US20060020187A1 (en)* | 2004-07-13 | 2006-01-26 | Dexcom, Inc. | Transcutaneous analyte sensor |
| US8731630B2 (en) | 2004-07-13 | 2014-05-20 | Dexcom, Inc. | Transcutaneous analyte sensor |
| US8463350B2 (en) | 2004-07-13 | 2013-06-11 | Dexcom, Inc. | Transcutaneous analyte sensor |
| US8457708B2 (en) | 2004-07-13 | 2013-06-04 | Dexcom, Inc. | Transcutaneous analyte sensor |
| US10918314B2 (en) | 2004-07-13 | 2021-02-16 | Dexcom, Inc. | Analyte sensor |
| US10918315B2 (en) | 2004-07-13 | 2021-02-16 | Dexcom, Inc. | Analyte sensor |
| US8452368B2 (en) | 2004-07-13 | 2013-05-28 | Dexcom, Inc. | Transcutaneous analyte sensor |
| US8750955B2 (en) | 2004-07-13 | 2014-06-10 | Dexcom, Inc. | Analyte sensor |
| US10918313B2 (en) | 2004-07-13 | 2021-02-16 | Dexcom, Inc. | Analyte sensor |
| US7885697B2 (en) | 2004-07-13 | 2011-02-08 | Dexcom, Inc. | Transcutaneous analyte sensor |
| US10827956B2 (en) | 2004-07-13 | 2020-11-10 | Dexcom, Inc. | Analyte sensor |
| US10813576B2 (en) | 2004-07-13 | 2020-10-27 | Dexcom, Inc. | Analyte sensor |
| US10799158B2 (en) | 2004-07-13 | 2020-10-13 | Dexcom, Inc. | Analyte sensor |
| US10799159B2 (en) | 2004-07-13 | 2020-10-13 | Dexcom, Inc. | Analyte sensor |
| US9055901B2 (en) | 2004-07-13 | 2015-06-16 | Dexcom, Inc. | Transcutaneous analyte sensor |
| US10709362B2 (en) | 2004-07-13 | 2020-07-14 | Dexcom, Inc. | Analyte sensor |
| US10709363B2 (en) | 2004-07-13 | 2020-07-14 | Dexcom, Inc. | Analyte sensor |
| US10524703B2 (en) | 2004-07-13 | 2020-01-07 | Dexcom, Inc. | Transcutaneous analyte sensor |
| US7494465B2 (en) | 2004-07-13 | 2009-02-24 | Dexcom, Inc. | Transcutaneous analyte sensor |
| US20060036139A1 (en)* | 2004-07-13 | 2006-02-16 | Dexcom, Inc. | Transcutaneous analyte sensor |
| US10314525B2 (en) | 2004-07-13 | 2019-06-11 | Dexcom, Inc. | Analyte sensor |
| US8792953B2 (en) | 2004-07-13 | 2014-07-29 | Dexcom, Inc. | Transcutaneous analyte sensor |
| US8229534B2 (en) | 2004-07-13 | 2012-07-24 | Dexcom, Inc. | Transcutaneous analyte sensor |
| US8989833B2 (en) | 2004-07-13 | 2015-03-24 | Dexcom, Inc. | Transcutaneous analyte sensor |
| US7905833B2 (en) | 2004-07-13 | 2011-03-15 | Dexcom, Inc. | Transcutaneous analyte sensor |
| US8313434B2 (en) | 2004-07-13 | 2012-11-20 | Dexcom, Inc. | Analyte sensor inserter system |
| US10022078B2 (en) | 2004-07-13 | 2018-07-17 | Dexcom, Inc. | Analyte sensor |
| US8812072B2 (en) | 2004-07-13 | 2014-08-19 | Dexcom, Inc. | Transcutaneous medical device with variable stiffness |
| US9986942B2 (en) | 2004-07-13 | 2018-06-05 | Dexcom, Inc. | Analyte sensor |
| US7949381B2 (en) | 2004-07-13 | 2011-05-24 | Dexcom, Inc. | Transcutaneous analyte sensor |
| US7946984B2 (en) | 2004-07-13 | 2011-05-24 | Dexcom, Inc. | Transcutaneous analyte sensor |
| US9247900B2 (en) | 2004-07-13 | 2016-02-02 | Dexcom, Inc. | Analyte sensor |
| US8825127B2 (en) | 2004-07-13 | 2014-09-02 | Dexcom, Inc. | Transcutaneous analyte sensor |
| US20060036141A1 (en)* | 2004-07-13 | 2006-02-16 | Dexcom, Inc. | Transcutaneous analyte sensor |
| US9833176B2 (en) | 2004-07-13 | 2017-12-05 | Dexcom, Inc. | Transcutaneous analyte sensor |
| US9775543B2 (en) | 2004-07-13 | 2017-10-03 | Dexcom, Inc. | Transcutaneous analyte sensor |
| US9668677B2 (en) | 2004-07-13 | 2017-06-06 | Dexcom, Inc. | Analyte sensor |
| US8290560B2 (en) | 2004-07-13 | 2012-10-16 | Dexcom, Inc. | Transcutaneous analyte sensor |
| US9603557B2 (en) | 2004-07-13 | 2017-03-28 | Dexcom, Inc. | Transcutaneous analyte sensor |
| US8858434B2 (en) | 2004-07-13 | 2014-10-14 | Dexcom, Inc. | Transcutaneous analyte sensor |
| US8280475B2 (en) | 2004-07-13 | 2012-10-02 | Dexcom, Inc. | Transcutaneous analyte sensor |
| US8886272B2 (en) | 2004-07-13 | 2014-11-11 | Dexcom, Inc. | Analyte sensor |
| US9414777B2 (en) | 2004-07-13 | 2016-08-16 | Dexcom, Inc. | Transcutaneous analyte sensor |
| US8170803B2 (en) | 2004-07-13 | 2012-05-01 | Dexcom, Inc. | Transcutaneous analyte sensor |
| US20090216103A1 (en)* | 2004-07-13 | 2009-08-27 | Dexcom, Inc. | Transcutaneous analyte sensor |
| US7291107B2 (en) | 2004-08-26 | 2007-11-06 | Roche Diagnostics Operations, Inc. | Insulin bolus recommendation system |
| US7553281B2 (en) | 2004-08-26 | 2009-06-30 | Roche Diagnostices Operations, Inc. | Insulin bolus recommendation system |
| US20080058628A1 (en)* | 2004-08-26 | 2008-03-06 | Robert Hellwig | Insulin bolus recommendation system |
| US20080020794A1 (en)* | 2004-10-22 | 2008-01-24 | Mark Garon | Mobile electronic device with fluid delivery system |
| US20080133146A1 (en)* | 2004-12-17 | 2008-06-05 | Chang Kc Shu Kun | Device Having a Trend-Indicating Display |
| WO2006066038A3 (en)* | 2004-12-17 | 2006-08-03 | Bayer Healthcare Llc | Analyte test device having a trend-indicating display |
| US20060137695A1 (en)* | 2004-12-23 | 2006-06-29 | Robert Hellwig | System and method for determining insulin bolus quantities |
| EP1834266B1 (en)* | 2004-12-23 | 2019-06-05 | Roche Diabetes Care GmbH | System and method for determining insulin bolus quantities |
| US7869851B2 (en) | 2004-12-23 | 2011-01-11 | Roche Diagnostics Operations, Inc. | System and method for determining insulin bolus quantities |
| EP3263033A1 (en)* | 2005-02-02 | 2018-01-03 | F. Hoffmann-La Roche AG | Ambulatory medical device and method of communication between medical devices |
| US8358210B2 (en) | 2005-02-08 | 2013-01-22 | Abbott Diabetes Care Inc. | RF tag on test strips, test strip vials and boxes |
| US8542122B2 (en) | 2005-02-08 | 2013-09-24 | Abbott Diabetes Care Inc. | Glucose measurement device and methods using RFID |
| US8390455B2 (en) | 2005-02-08 | 2013-03-05 | Abbott Diabetes Care Inc. | RF tag on test strips, test strip vials and boxes |
| US8115635B2 (en) | 2005-02-08 | 2012-02-14 | Abbott Diabetes Care Inc. | RF tag on test strips, test strip vials and boxes |
| US8223021B2 (en) | 2005-02-08 | 2012-07-17 | Abbott Diabetes Care Inc. | RF tag on test strips, test strip vials and boxes |
| US10610137B2 (en) | 2005-03-10 | 2020-04-07 | Dexcom, Inc. | System and methods for processing analyte sensor data for sensor calibration |
| US10925524B2 (en) | 2005-03-10 | 2021-02-23 | Dexcom, Inc. | System and methods for processing analyte sensor data for sensor calibration |
| US10918318B2 (en) | 2005-03-10 | 2021-02-16 | Dexcom, Inc. | System and methods for processing analyte sensor data for sensor calibration |
| US10709364B2 (en) | 2005-03-10 | 2020-07-14 | Dexcom, Inc. | System and methods for processing analyte sensor data for sensor calibration |
| US10716498B2 (en) | 2005-03-10 | 2020-07-21 | Dexcom, Inc. | System and methods for processing analyte sensor data for sensor calibration |
| US10898114B2 (en) | 2005-03-10 | 2021-01-26 | Dexcom, Inc. | System and methods for processing analyte sensor data for sensor calibration |
| US10918317B2 (en) | 2005-03-10 | 2021-02-16 | Dexcom, Inc. | System and methods for processing analyte sensor data for sensor calibration |
| US10743801B2 (en) | 2005-03-10 | 2020-08-18 | Dexcom, Inc. | System and methods for processing analyte sensor data for sensor calibration |
| US10610135B2 (en) | 2005-03-10 | 2020-04-07 | Dexcom, Inc. | System and methods for processing analyte sensor data for sensor calibration |
| US10856787B2 (en) | 2005-03-10 | 2020-12-08 | Dexcom, Inc. | System and methods for processing analyte sensor data for sensor calibration |
| US10918316B2 (en) | 2005-03-10 | 2021-02-16 | Dexcom, Inc. | System and methods for processing analyte sensor data for sensor calibration |
| US10617336B2 (en) | 2005-03-10 | 2020-04-14 | Dexcom, Inc. | System and methods for processing analyte sensor data for sensor calibration |
| US11051726B2 (en) | 2005-03-10 | 2021-07-06 | Dexcom, Inc. | System and methods for processing analyte sensor data for sensor calibration |
| US11000213B2 (en) | 2005-03-10 | 2021-05-11 | Dexcom, Inc. | System and methods for processing analyte sensor data for sensor calibration |
| US10610136B2 (en) | 2005-03-10 | 2020-04-07 | Dexcom, Inc. | System and methods for processing analyte sensor data for sensor calibration |
| US20090184801A1 (en)* | 2005-03-18 | 2009-07-23 | Olle Bliding | Method for Unlocking a Lock by a Lock Device Enabled for Short-Range Wireless Data Communication in Compliance With a Communication Standard and Associated Device |
| US20070229257A1 (en)* | 2005-03-18 | 2007-10-04 | Olle Bliding | Wake-up device and method for generating a control signal |
| US8593249B2 (en)* | 2005-03-18 | 2013-11-26 | Phoniro Ab | Method for unlocking a lock by a lock device enabled for short-range wireless data communication in compliance with a communication standard and associated device |
| US8298172B2 (en) | 2005-04-13 | 2012-10-30 | Novo Nordisk A/S | Medical skin mountable device and system |
| WO2006108809A1 (en) | 2005-04-13 | 2006-10-19 | Novo Nordisk A/S | Medical skin mountable device and system |
| US8747363B2 (en) | 2005-04-13 | 2014-06-10 | Novo Nordisk A/S | Medical skin mountable device and system |
| US20090131860A1 (en)* | 2005-04-13 | 2009-05-21 | Novo Nordisk A/S | Medical Skin Mountable Device And System |
| US10311209B2 (en) | 2005-06-09 | 2019-06-04 | Roche Diabetes Care, Inc. | Device and method for insulin dosing |
| US20070078818A1 (en)* | 2005-06-09 | 2007-04-05 | Roche Diagnostics Operations, Inc. | Device and method for insulin dosing |
| US8251904B2 (en) | 2005-06-09 | 2012-08-28 | Roche Diagnostics Operations, Inc. | Device and method for insulin dosing |
| US10813577B2 (en) | 2005-06-21 | 2020-10-27 | Dexcom, Inc. | Analyte sensor |
| US20090118682A1 (en)* | 2005-09-13 | 2009-05-07 | Novo Nordisk A/S | Reservoir Device With Inspection Aid For Detection Of Drug Condition |
| US20110160670A1 (en)* | 2005-10-17 | 2011-06-30 | Novo Nordisk A/S | Vented drug reservoir unit |
| US8394060B2 (en) | 2005-10-17 | 2013-03-12 | Novo Nordisk A/S | Vented drug reservoir unit |
| US20080287870A1 (en)* | 2005-10-17 | 2008-11-20 | Nov Nordisk A/S | Vented Drug Reservoir Unit |
| US20110014954A1 (en)* | 2005-10-27 | 2011-01-20 | Dossas Vasilios D | Emergency Medical Diagnosis and Communications Device |
| US11272867B2 (en) | 2005-11-01 | 2022-03-15 | Abbott Diabetes Care Inc. | Analyte monitoring device and methods of use |
| US10952652B2 (en) | 2005-11-01 | 2021-03-23 | Abbott Diabetes Care Inc. | Analyte monitoring device and methods of use |
| US11911151B1 (en) | 2005-11-01 | 2024-02-27 | Abbott Diabetes Care Inc. | Analyte monitoring device and methods of use |
| US9078607B2 (en) | 2005-11-01 | 2015-07-14 | Abbott Diabetes Care Inc. | Analyte monitoring device and methods of use |
| US11103165B2 (en) | 2005-11-01 | 2021-08-31 | Abbott Diabetes Care Inc. | Analyte monitoring device and methods of use |
| US9326716B2 (en) | 2005-11-01 | 2016-05-03 | Abbott Diabetes Care Inc. | Analyte monitoring device and methods of use |
| US10231654B2 (en) | 2005-11-01 | 2019-03-19 | Abbott Diabetes Care Inc. | Analyte monitoring device and methods of use |
| US8920319B2 (en) | 2005-11-01 | 2014-12-30 | Abbott Diabetes Care Inc. | Analyte monitoring device and methods of use |
| US8915850B2 (en) | 2005-11-01 | 2014-12-23 | Abbott Diabetes Care Inc. | Analyte monitoring device and methods of use |
| US10201301B2 (en) | 2005-11-01 | 2019-02-12 | Abbott Diabetes Care Inc. | Analyte monitoring device and methods of use |
| US11363975B2 (en) | 2005-11-01 | 2022-06-21 | Abbott Diabetes Care Inc. | Analyte monitoring device and methods of use |
| US11399748B2 (en) | 2005-11-01 | 2022-08-02 | Abbott Diabetes Care Inc. | Analyte monitoring device and methods of use |
| US20070112298A1 (en)* | 2005-11-17 | 2007-05-17 | Medtronic Minimed, Inc. | External infusion device with programmable capabilities to time-shift basal insulin and method of using the same |
| US7704226B2 (en)* | 2005-11-17 | 2010-04-27 | Medtronic Minimed, Inc. | External infusion device with programmable capabilities to time-shift basal insulin and method of using the same |
| US20090118592A1 (en)* | 2005-12-08 | 2009-05-07 | Novo Nordisk A/S | Medical System Comprising a Sensor Device |
| US11406753B2 (en) | 2006-02-09 | 2022-08-09 | Deka Products Limited Partnership | Adhesive and peripheral systems and methods for medical devices |
| US11738139B2 (en) | 2006-02-09 | 2023-08-29 | Deka Products Limited Partnership | Patch-sized fluid delivery systems and methods |
| US11992650B2 (en) | 2006-02-09 | 2024-05-28 | Deka Products Limited Partnership | Adhesive and peripheral systems and methods for medical devices |
| US11559625B2 (en) | 2006-02-09 | 2023-01-24 | Deka Products Limited Partnership | Patch-sized fluid delivery systems and methods |
| US12233236B2 (en) | 2006-02-09 | 2025-02-25 | Deka Products Limited Partnership | Adhesive and peripheral systems and methods for medical devices |
| US8545445B2 (en) | 2006-02-09 | 2013-10-01 | Deka Products Limited Partnership | Patch-sized fluid delivery systems and methods |
| US11534543B2 (en) | 2006-02-09 | 2022-12-27 | Deka Products Limited Partnership | Method for making patch-sized fluid delivery systems |
| US12311143B2 (en) | 2006-02-09 | 2025-05-27 | Deka Products Limited Partnership | Adhesive and peripheral systems and methods for medical devices |
| US11690952B2 (en) | 2006-02-09 | 2023-07-04 | Deka Products Limited Partnership | Pumping fluid delivery systems and methods using force application assembly |
| US11904134B2 (en) | 2006-02-09 | 2024-02-20 | Deka Products Limited Partnership | Patch-sized fluid delivery systems and methods |
| US11497846B2 (en) | 2006-02-09 | 2022-11-15 | Deka Products Limited Partnership | Patch-sized fluid delivery systems and methods |
| US11712513B2 (en) | 2006-02-09 | 2023-08-01 | Deka Products Limited Partnership | Adhesive and peripheral systems and methods for medical devices |
| US11890448B2 (en) | 2006-02-09 | 2024-02-06 | Deka Products Limited Partnership | Method and system for shape-memory alloy wire control |
| US11339774B2 (en) | 2006-02-09 | 2022-05-24 | Deka Products Limited Partnership | Adhesive and peripheral systems and methods for medical devices |
| US11964126B2 (en) | 2006-02-09 | 2024-04-23 | Deka Products Limited Partnership | Infusion pump assembly |
| US11491273B2 (en) | 2006-02-09 | 2022-11-08 | Deka Products Limited Partnership | Adhesive and peripheral systems and methods for medical devices |
| US11844926B2 (en) | 2006-02-09 | 2023-12-19 | Deka Products Limited Partnership | Adhesive and peripheral systems and methods for medical devices |
| US12070574B2 (en) | 2006-02-09 | 2024-08-27 | Deka Products Limited Partnership | Apparatus, systems and methods for an infusion pump assembly |
| US8414522B2 (en) | 2006-02-09 | 2013-04-09 | Deka Products Limited Partnership | Fluid delivery systems and methods |
| US11478623B2 (en) | 2006-02-09 | 2022-10-25 | Deka Products Limited Partnership | Infusion pump assembly |
| US8585377B2 (en) | 2006-02-09 | 2013-11-19 | Deka Products Limited Partnership | Pumping fluid delivery systems and methods using force application assembly |
| US11364335B2 (en) | 2006-02-09 | 2022-06-21 | Deka Products Limited Partnership | Apparatus, system and method for fluid delivery |
| US12274857B2 (en) | 2006-02-09 | 2025-04-15 | Deka Products Limited Partnership | Method and system for shape-memory alloy wire control |
| US11426512B2 (en) | 2006-02-09 | 2022-08-30 | Deka Products Limited Partnership | Apparatus, systems and methods for an infusion pump assembly |
| US12370305B2 (en) | 2006-02-09 | 2025-07-29 | Deka Products Limited Partnership | Patch-sized fluid delivery systems and methods |
| US11413391B2 (en) | 2006-02-09 | 2022-08-16 | Deka Products Limited Partnership | Patch-sized fluid delivery systems and methods |
| US8113244B2 (en) | 2006-02-09 | 2012-02-14 | Deka Products Limited Partnership | Adhesive and peripheral systems and methods for medical devices |
| US12151080B2 (en) | 2006-02-09 | 2024-11-26 | Deka Products Limited Partnership | Adhesive and peripheral systems and methods for medical devices |
| US11391273B2 (en) | 2006-02-09 | 2022-07-19 | Deka Products Limited Partnership | Adhesive and peripheral systems and methods for medical devices |
| US11786651B2 (en) | 2006-02-09 | 2023-10-17 | Deka Products Limited Partnership | Patch-sized fluid delivery system |
| US11395877B2 (en) | 2006-02-09 | 2022-07-26 | Deka Products Limited Partnership | Systems and methods for fluid delivery |
| US11617826B2 (en) | 2006-02-09 | 2023-04-04 | Deka Products Limited Partnership | Patch-sized fluid delivery systems and methods |
| US11408414B2 (en) | 2006-02-09 | 2022-08-09 | Deka Products Limited Partnership | Adhesive and peripheral systems and methods for medical devices |
| US12036387B2 (en) | 2006-02-09 | 2024-07-16 | Deka Products Limited Partnership | Device to determine volume of fluid dispensed |
| US11717609B2 (en) | 2006-02-09 | 2023-08-08 | Deka Products Limited Partnership | Adhesive and peripheral systems and methods for medical devices |
| US12064590B2 (en) | 2006-02-09 | 2024-08-20 | Deka Products Limited Partnership | Patch-sized fluid delivery systems and methods |
| US8133178B2 (en) | 2006-02-22 | 2012-03-13 | Dexcom, Inc. | Analyte sensor |
| US9724028B2 (en) | 2006-02-22 | 2017-08-08 | Dexcom, Inc. | Analyte sensor |
| US20070233051A1 (en)* | 2006-03-31 | 2007-10-04 | David Hohl | Drug delivery systems and methods |
| US7920907B2 (en) | 2006-06-07 | 2011-04-05 | Abbott Diabetes Care Inc. | Analyte monitoring system and method |
| US11432772B2 (en) | 2006-08-02 | 2022-09-06 | Dexcom, Inc. | Systems and methods for replacing signal artifacts in a glucose sensor data stream |
| US11382539B2 (en) | 2006-10-04 | 2022-07-12 | Dexcom, Inc. | Analyte sensor |
| US9451908B2 (en) | 2006-10-04 | 2016-09-27 | Dexcom, Inc. | Analyte sensor |
| US11399745B2 (en) | 2006-10-04 | 2022-08-02 | Dexcom, Inc. | Dual electrode system for a continuous analyte sensor |
| US10349873B2 (en) | 2006-10-04 | 2019-07-16 | Dexcom, Inc. | Analyte sensor |
| US11508476B2 (en) | 2006-10-31 | 2022-11-22 | Abbott Diabetes Care, Inc. | Infusion devices and methods |
| US11837358B2 (en) | 2006-10-31 | 2023-12-05 | Abbott Diabetes Care Inc. | Infusion devices and methods |
| US12073941B2 (en) | 2006-10-31 | 2024-08-27 | Abbott Diabetes Care Inc. | Infusion device and methods |
| US20080214919A1 (en)* | 2006-12-26 | 2008-09-04 | Lifescan, Inc. | System and method for implementation of glycemic control protocols |
| US20100222765A1 (en)* | 2007-01-24 | 2010-09-02 | Smiths Medical Asd, Inc. | Correction factor testing using frequent blood glucose input |
| US8208984B2 (en) | 2007-01-24 | 2012-06-26 | Smiths Medical Asd, Inc. | Correction factor testing using frequent blood glucose input |
| US8496646B2 (en) | 2007-02-09 | 2013-07-30 | Deka Products Limited Partnership | Infusion pump assembly |
| US20080228056A1 (en)* | 2007-03-13 | 2008-09-18 | Michael Blomquist | Basal rate testing using frequent blood glucose input |
| US20080256738A1 (en)* | 2007-04-23 | 2008-10-23 | Malone Randolph W | Frameless, Heated Wiper Assembly And System Utilizing Same |
| US12249412B2 (en) | 2007-05-24 | 2025-03-11 | Tandem Diabetes Care, Inc. | Expert system for insulin pump therapy |
| US8219222B2 (en) | 2007-05-24 | 2012-07-10 | Smiths Medical Asd, Inc. | Expert system for pump therapy |
| US10943687B2 (en) | 2007-05-24 | 2021-03-09 | Tandem Diabetes Care, Inc. | Expert system for insulin pump therapy |
| US11257580B2 (en) | 2007-05-24 | 2022-02-22 | Tandem Diabetes Care, Inc. | Expert system for insulin pump therapy |
| US20100274751A1 (en)* | 2007-05-24 | 2010-10-28 | Smith Medical Asd, Inc. | Expert system for insulin pump therapy |
| US11848089B2 (en) | 2007-05-24 | 2023-12-19 | Tandem Diabetes Care, Inc. | Expert system for insulin pump therapy |
| US9833177B2 (en) | 2007-05-30 | 2017-12-05 | Tandem Diabetes Care, Inc. | Insulin pump based expert system |
| US20080300534A1 (en)* | 2007-05-30 | 2008-12-04 | Michael Blomquist | Insulin pump based expert system |
| US11986292B2 (en) | 2007-05-30 | 2024-05-21 | Tandem Diabetes Care, Inc. | Insulin pump based expert system |
| US11576594B2 (en) | 2007-05-30 | 2023-02-14 | Tandem Diabetes Care, Inc. | Insulin pump based expert system |
| US11298053B2 (en) | 2007-05-30 | 2022-04-12 | Tandem Diabetes Care, Inc. | Insulin pump based expert system |
| US8221345B2 (en) | 2007-05-30 | 2012-07-17 | Smiths Medical Asd, Inc. | Insulin pump based expert system |
| US20080306443A1 (en)* | 2007-06-06 | 2008-12-11 | Mallinckrodt Inc. | Medical Fluid Injector Having Wireless Pressure Monitoring Feature |
| US9741139B2 (en) | 2007-06-08 | 2017-08-22 | Dexcom, Inc. | Integrated medicament delivery device for use with continuous analyte sensor |
| US8562558B2 (en) | 2007-06-08 | 2013-10-22 | Dexcom, Inc. | Integrated medicament delivery device for use with continuous analyte sensor |
| US11373347B2 (en) | 2007-06-08 | 2022-06-28 | Dexcom, Inc. | Integrated medicament delivery device for use with continuous analyte sensor |
| US10403012B2 (en) | 2007-06-08 | 2019-09-03 | Dexcom, Inc. | Integrated medicament delivery device for use with continuous analyte sensor |
| US12394120B2 (en) | 2007-06-08 | 2025-08-19 | Dexcom, Inc. | Integrated medicament delivery device for use with continuous analyte sensor |
| US20080312512A1 (en)* | 2007-06-15 | 2008-12-18 | Animas Corporation | Methods to pair a medical device and at least a remote controller for such medical device |
| US9391670B2 (en) | 2007-06-15 | 2016-07-12 | Animas Corporation | Methods for secure communication and pairing of a medical infusion device and a remote controller for such medical device |
| US9049982B2 (en) | 2007-06-15 | 2015-06-09 | Animas Corporation | Methods to pair a medical device and at least a remote controller for such medical device |
| US8444595B2 (en)* | 2007-06-15 | 2013-05-21 | Animas Corporation | Methods to pair a medical device and at least a remote controller for such medical device |
| US20080312585A1 (en)* | 2007-06-15 | 2008-12-18 | Animas Corporation | Method of operating a medical device and at least a remote controller for such medical device |
| US8449523B2 (en) | 2007-06-15 | 2013-05-28 | Animas Corporation | Method of operating a medical device and at least a remote controller for such medical device |
| US20080312584A1 (en)* | 2007-06-15 | 2008-12-18 | Animas Corporation | Systems and methods to pair a medical device and a remote controller for such medical device |
| US20110178499A1 (en)* | 2007-06-15 | 2011-07-21 | Animas Corporation | Methods to pair a medical device and at least a remote controller for such medical device |
| US8932250B2 (en)* | 2007-06-15 | 2015-01-13 | Animas Corporation | Systems and methods to pair a medical device and a remote controller for such medical device |
| US11678821B2 (en) | 2007-06-29 | 2023-06-20 | Abbott Diabetes Care Inc. | Analyte monitoring and management device and method to analyze the frequency of user interaction with the device |
| US12426812B2 (en) | 2007-06-29 | 2025-09-30 | Abbott Diabetes Care Inc. | Analyte monitoring and management device and method to analyze the frequency of user interaction with the device |
| US20110282173A1 (en)* | 2007-08-29 | 2011-11-17 | Brighter Ab | Portable medical apparatus |
| US9737253B2 (en)* | 2007-08-29 | 2017-08-22 | Brighter Ab | Portable medical apparatus including sampling, determining and injecting components |
| US10653835B2 (en) | 2007-10-09 | 2020-05-19 | Dexcom, Inc. | Integrated insulin delivery system with continuous glucose sensor |
| US12246166B2 (en) | 2007-10-09 | 2025-03-11 | Dexcom, Inc. | Integrated insulin delivery system with continuous glucose sensor |
| US11160926B1 (en) | 2007-10-09 | 2021-11-02 | Dexcom, Inc. | Pre-connected analyte sensors |
| US11744943B2 (en) | 2007-10-09 | 2023-09-05 | Dexcom, Inc. | Integrated insulin delivery system with continuous glucose sensor |
| US12397110B2 (en) | 2007-10-09 | 2025-08-26 | Dexcom, Inc. | Integrated insulin delivery system with continuous glucose sensor |
| US12397113B2 (en) | 2007-10-09 | 2025-08-26 | Dexcom, Inc. | Integrated insulin delivery system with continuous glucose sensor |
| US9717449B2 (en) | 2007-10-25 | 2017-08-01 | Dexcom, Inc. | Systems and methods for processing sensor data |
| US10182751B2 (en) | 2007-10-25 | 2019-01-22 | Dexcom, Inc. | Systems and methods for processing sensor data |
| US8417312B2 (en) | 2007-10-25 | 2013-04-09 | Dexcom, Inc. | Systems and methods for processing sensor data |
| US11272869B2 (en) | 2007-10-25 | 2022-03-15 | Dexcom, Inc. | Systems and methods for processing sensor data |
| US10376634B2 (en)* | 2007-12-12 | 2019-08-13 | Bigfoot Biomedical, Inc. | Portable infusion pump and media player |
| US20160184511A1 (en)* | 2007-12-12 | 2016-06-30 | Bigfoot Biomedical, Inc. | Portable Infusion Pump and Media Player |
| US10827980B2 (en) | 2007-12-17 | 2020-11-10 | Dexcom, Inc. | Systems and methods for processing sensor data |
| US9149233B2 (en) | 2007-12-17 | 2015-10-06 | Dexcom, Inc. | Systems and methods for processing sensor data |
| US9149234B2 (en) | 2007-12-17 | 2015-10-06 | Dexcom, Inc. | Systems and methods for processing sensor data |
| US10506982B2 (en) | 2007-12-17 | 2019-12-17 | Dexcom, Inc. | Systems and methods for processing sensor data |
| US11342058B2 (en) | 2007-12-17 | 2022-05-24 | Dexcom, Inc. | Systems and methods for processing sensor data |
| US9135402B2 (en) | 2007-12-17 | 2015-09-15 | Dexcom, Inc. | Systems and methods for processing sensor data |
| US9339238B2 (en) | 2007-12-17 | 2016-05-17 | Dexcom, Inc. | Systems and methods for processing sensor data |
| US8290559B2 (en) | 2007-12-17 | 2012-10-16 | Dexcom, Inc. | Systems and methods for processing sensor data |
| US9839395B2 (en) | 2007-12-17 | 2017-12-12 | Dexcom, Inc. | Systems and methods for processing sensor data |
| US12165757B2 (en) | 2007-12-17 | 2024-12-10 | Dexcom, Inc. | Systems and methods for processing sensor data |
| US9901307B2 (en) | 2007-12-17 | 2018-02-27 | Dexcom, Inc. | Systems and methods for processing sensor data |
| US8414563B2 (en) | 2007-12-31 | 2013-04-09 | Deka Products Limited Partnership | Pump assembly with switch |
| US11894609B2 (en) | 2007-12-31 | 2024-02-06 | Deka Products Limited Partnership | Split ring resonator antenna adapted for use in wirelessly controlled medical device |
| US11723841B2 (en) | 2007-12-31 | 2023-08-15 | Deka Products Limited Partnership | Apparatus, system and method for fluid delivery |
| US11497686B2 (en) | 2007-12-31 | 2022-11-15 | Deka Products Limited Partnership | Apparatus, system and method for fluid delivery |
| US12415031B2 (en) | 2007-12-31 | 2025-09-16 | Deka Products Limited Partnership | Wearable pump assembly |
| US8491570B2 (en) | 2007-12-31 | 2013-07-23 | Deka Products Limited Partnership | Infusion pump assembly |
| US11701300B2 (en) | 2007-12-31 | 2023-07-18 | Deka Products Limited Partnership | Method for fluid delivery |
| US11642283B2 (en) | 2007-12-31 | 2023-05-09 | Deka Products Limited Partnership | Method for fluid delivery |
| US11404776B2 (en) | 2007-12-31 | 2022-08-02 | Deka Products Limited Partnership | Split ring resonator antenna adapted for use in wirelessly controlled medical device |
| US9526830B2 (en) | 2007-12-31 | 2016-12-27 | Deka Products Limited Partnership | Wearable pump assembly |
| US12121497B2 (en) | 2007-12-31 | 2024-10-22 | Deka Products Limited Partnership | Method for fluid delivery |
| US12128006B2 (en) | 2007-12-31 | 2024-10-29 | Deka Products Limited Partnership | Apparatus, system and method for fluid delivery |
| US11534542B2 (en) | 2007-12-31 | 2022-12-27 | Deka Products Limited Partnership | Apparatus, system and method for fluid delivery |
| US20110033833A1 (en)* | 2008-01-07 | 2011-02-10 | Michael Blomquist | Pump with therapy coaching |
| US10052049B2 (en) | 2008-01-07 | 2018-08-21 | Tandem Diabetes Care, Inc. | Infusion pump with blood glucose alert delay |
| US8801657B2 (en) | 2008-01-07 | 2014-08-12 | Tandem Diabetes Care, Inc. | Pump with therapy coaching |
| US8718949B2 (en) | 2008-01-07 | 2014-05-06 | Tandem Diabetes Care, Inc. | Insulin pump with blood glucose modules |
| US11302433B2 (en) | 2008-01-07 | 2022-04-12 | Tandem Diabetes Care, Inc. | Diabetes therapy coaching |
| US20090177154A1 (en)* | 2008-01-08 | 2009-07-09 | Michael Blomquist | Insulin pump with convenience features |
| WO2009089028A3 (en)* | 2008-01-08 | 2009-12-30 | Smiths Medical Md, Inc. | Insulin pump with convenience features |
| US8414523B2 (en) | 2008-01-09 | 2013-04-09 | Tandem Diabetes Care, Inc. | Infusion pump with add-on modules |
| US20110040251A1 (en)* | 2008-01-09 | 2011-02-17 | Michael Blomquist | Infusion pump with add-on modules |
| US9889250B2 (en) | 2008-01-09 | 2018-02-13 | Tandem Diabetes Care, Inc. | Infusion pump with temperature monitoring |
| US8840582B2 (en) | 2008-01-09 | 2014-09-23 | Tandem Diabetes Care, Inc. | Infusion pump with activity monitoring |
| US11850394B2 (en) | 2008-01-09 | 2023-12-26 | Tandem Diabetes Care, Inc. | Infusion pump with add-on modules |
| US10773015B2 (en) | 2008-01-09 | 2020-09-15 | Tandem Diabetes Care, Inc. | Infusion pump incorporating information from personal information manager devices |
| US9143569B2 (en) | 2008-02-21 | 2015-09-22 | Dexcom, Inc. | Systems and methods for processing, transmitting and displaying sensor data |
| US11102306B2 (en) | 2008-02-21 | 2021-08-24 | Dexcom, Inc. | Systems and methods for processing, transmitting and displaying sensor data |
| US9020572B2 (en) | 2008-02-21 | 2015-04-28 | Dexcom, Inc. | Systems and methods for processing, transmitting and displaying sensor data |
| US8591455B2 (en) | 2008-02-21 | 2013-11-26 | Dexcom, Inc. | Systems and methods for customizing delivery of sensor data |
| US8229535B2 (en) | 2008-02-21 | 2012-07-24 | Dexcom, Inc. | Systems and methods for blood glucose monitoring and alert delivery |
| US20090240385A1 (en)* | 2008-03-21 | 2009-09-24 | Denso Corporation | Electronic control apparatus for vehicle |
| US20090271021A1 (en)* | 2008-04-28 | 2009-10-29 | Popp Shane M | Execution system for the monitoring and execution of insulin manufacture |
| WO2009146379A1 (en)* | 2008-05-28 | 2009-12-03 | Pelikan Technologies, Inc. | Devices and methods for glucose measurement using rechargeable battery energy sources |
| US20090305317A1 (en)* | 2008-06-05 | 2009-12-10 | Brauer Jacob S | User interface for testing device |
| US8117481B2 (en) | 2008-06-06 | 2012-02-14 | Roche Diagnostics International Ag | Apparatus and method for processing wirelessly communicated information within an electronic device |
| US20090307520A1 (en)* | 2008-06-06 | 2009-12-10 | Bruno Anselmi | Apparatus and Method for Processing Wirelessly Communicated Information Within an Electronic Device |
| US8738957B2 (en) | 2008-06-06 | 2014-05-27 | Roche Diagnostics Operations, Inc. | Apparatus and method for processing a wirelessly received information encoded in form of embedded data and clock |
| US8132037B2 (en) | 2008-06-06 | 2012-03-06 | Roche Diagnostics International Ag | Apparatus and method for processing wirelessly communicated data and clock information within an electronic device |
| US20090326722A1 (en)* | 2008-06-30 | 2009-12-31 | Animas Corporation | Method and System for Using Status Indicators in Wireless Communication with Medical Devices |
| EP2502641A2 (en) | 2008-06-30 | 2012-09-26 | Animas Corporation | A method and system for using status indicators in wireless communications with medical devices |
| US8502662B2 (en) | 2008-06-30 | 2013-08-06 | Animas Corporation | Method and system for using status indicators in wireless communication with medical devices |
| EP2140893A1 (en) | 2008-06-30 | 2010-01-06 | Animas Corporation | System for using status indicators in wireless communications with medical devices |
| US12296139B2 (en) | 2008-08-20 | 2025-05-13 | Insulet Corporation | Infusion pump systems and methods |
| US11865299B2 (en) | 2008-08-20 | 2024-01-09 | Insulet Corporation | Infusion pump systems and methods |
| US8066672B2 (en) | 2008-10-10 | 2011-11-29 | Deka Products Limited Partnership | Infusion pump assembly with a backup power supply |
| US12186531B2 (en) | 2008-10-10 | 2025-01-07 | Deka Products Limited Partnership | Infusion pump assembly |
| US8223028B2 (en) | 2008-10-10 | 2012-07-17 | Deka Products Limited Partnership | Occlusion detection system and method |
| US8708376B2 (en) | 2008-10-10 | 2014-04-29 | Deka Products Limited Partnership | Medium connector |
| US8267892B2 (en) | 2008-10-10 | 2012-09-18 | Deka Products Limited Partnership | Multi-language / multi-processor infusion pump assembly |
| US9180245B2 (en) | 2008-10-10 | 2015-11-10 | Deka Products Limited Partnership | System and method for administering an infusible fluid |
| US8016789B2 (en) | 2008-10-10 | 2011-09-13 | Deka Products Limited Partnership | Pump assembly with a removable cover assembly |
| US8262616B2 (en) | 2008-10-10 | 2012-09-11 | Deka Products Limited Partnership | Infusion pump assembly |
| US12370327B2 (en) | 2008-10-10 | 2025-07-29 | Deka Products Limited Partnership | Infusion pump methods, systems and apparatus |
| US10980461B2 (en) | 2008-11-07 | 2021-04-20 | Dexcom, Inc. | Advanced analyte sensor calibration and error detection |
| US9330237B2 (en) | 2008-12-24 | 2016-05-03 | Medtronic Minimed, Inc. | Pattern recognition and filtering in a therapy management system |
| US11854693B2 (en) | 2009-06-04 | 2023-12-26 | Abbott Diabetes Care Inc. | Method and system for updating a medical device |
| US12322505B2 (en) | 2009-06-04 | 2025-06-03 | Abbott Diabetes Care Inc. | Method and system for updating a medical device |
| US10434253B2 (en) | 2009-07-30 | 2019-10-08 | Tandem Diabetes Care, Inc. | Infusion pump system with disposable cartridge having pressure venting and pressure feedback |
| US8287495B2 (en) | 2009-07-30 | 2012-10-16 | Tandem Diabetes Care, Inc. | Infusion pump system with disposable cartridge having pressure venting and pressure feedback |
| US8758323B2 (en) | 2009-07-30 | 2014-06-24 | Tandem Diabetes Care, Inc. | Infusion pump system with disposable cartridge having pressure venting and pressure feedback |
| US11135362B2 (en) | 2009-07-30 | 2021-10-05 | Tandem Diabetes Care, Inc. | Infusion pump systems and methods |
| US12144964B2 (en) | 2009-07-30 | 2024-11-19 | Tandem Diabetes Care, Inc | Infusion pump system with disposable cartridge having pressure venting and pressure feedback |
| US9211377B2 (en) | 2009-07-30 | 2015-12-15 | Tandem Diabetes Care, Inc. | Infusion pump system with disposable cartridge having pressure venting and pressure feedback |
| US12042627B2 (en) | 2009-07-30 | 2024-07-23 | Tandem Diabetes Care, Inc. | Infusion pump systems and methods |
| US8298184B2 (en) | 2009-07-30 | 2012-10-30 | Tandem Diabetes Care, Inc. | Infusion pump system with disposable cartridge having pressure venting and pressure feedback |
| US8926561B2 (en) | 2009-07-30 | 2015-01-06 | Tandem Diabetes Care, Inc. | Infusion pump system with disposable cartridge having pressure venting and pressure feedback |
| US11285263B2 (en) | 2009-07-30 | 2022-03-29 | Tandem Diabetes Care, Inc. | Infusion pump systems and methods |
| EP2644088B1 (en) | 2009-09-29 | 2017-01-18 | Lifescan Scotland Limited | Analyte testing method and device for diabetes management |
| US11090432B2 (en) | 2009-12-04 | 2021-08-17 | Smiths Medical Asd, Inc. | Advanced step therapy delivery for an ambulatory infusion pump and system |
| US12233239B2 (en) | 2009-12-04 | 2025-02-25 | Icu Medical, Inc. | Advanced step therapy delivery for an ambulatory infusion pump and system |
| US10016559B2 (en) | 2009-12-04 | 2018-07-10 | Smiths Medical Asd, Inc. | Advanced step therapy delivery for an ambulatory infusion pump and system |
| US9833199B2 (en) | 2010-02-12 | 2017-12-05 | Dexcom, Inc. | Receivers for analyzing and displaying sensor data |
| US10278650B2 (en) | 2010-02-12 | 2019-05-07 | Dexcom, Inc. | Receivers for analyzing and displaying sensor data |
| US9041730B2 (en) | 2010-02-12 | 2015-05-26 | Dexcom, Inc. | Receivers for analyzing and displaying sensor data |
| US9504430B2 (en) | 2010-02-12 | 2016-11-29 | Dexcom, Inc. | Receivers for analyzing and displaying sensor data |
| US20110201911A1 (en)* | 2010-02-12 | 2011-08-18 | Dexcom, Inc. | Receivers for analyzing and displaying sensor data |
| US9498165B2 (en) | 2010-02-12 | 2016-11-22 | Dexcom, Inc. | Receivers for analyzing and displaying sensor data |
| US9498164B2 (en) | 2010-02-12 | 2016-11-22 | Dexcom, Inc. | Receivers for analyzing and displaying sensor data |
| US12183460B2 (en) | 2010-02-12 | 2024-12-31 | Dexcom, Inc. | Receivers for analyzing and displaying sensor data |
| US11769589B2 (en) | 2010-02-12 | 2023-09-26 | Dexcom, Inc. | Receivers for analyzing and displaying sensor data |
| US10165986B2 (en) | 2010-02-12 | 2019-01-01 | Dexcom, Inc. | Receivers for analyzing and displaying sensor data |
| US10265030B2 (en) | 2010-02-12 | 2019-04-23 | Dexcom, Inc. | Receivers for analyzing and displaying sensor data |
| US20110208013A1 (en)* | 2010-02-24 | 2011-08-25 | Edwards Lifesciences Corporation | Body Parameter Sensor and Monitor Interface |
| US10111169B2 (en) | 2011-09-23 | 2018-10-23 | Dexcom, Inc. | Systems and methods for processing and transmitting sensor data |
| US12382389B2 (en) | 2011-09-23 | 2025-08-05 | Dexcom, Inc. | Systems and methods for processing and transmitting sensor data |
| US9974018B2 (en) | 2011-09-23 | 2018-05-15 | Dexcom, Inc. | Systems and methods for processing and transmitting sensor data |
| EP2757951B1 (en) | 2011-09-23 | 2016-11-09 | Dexcom, Inc. | Systems and methods for processing and transmitting sensor data |
| US9980223B2 (en) | 2011-09-23 | 2018-05-22 | Dexcom, Inc. | Systems and methods for processing and transmitting sensor data |
| US10625017B2 (en) | 2012-03-05 | 2020-04-21 | Becton, Dickinson And Company | Wireless communication for on-body medical devices |
| US9623173B2 (en) | 2012-03-05 | 2017-04-18 | Becton, Dickinson And Company | Wireless communication for on-body medical devices |
| US11524151B2 (en) | 2012-03-07 | 2022-12-13 | Deka Products Limited Partnership | Apparatus, system and method for fluid delivery |
| US9804147B2 (en) | 2012-03-12 | 2017-10-31 | Panasonic Healthcare Holdings Co., Ltd. | Charging device for biological information measurement device and biological information measurement device charged using same |
| US10209237B2 (en) | 2012-03-12 | 2019-02-19 | Phc Holdings Corporation | Charging device for biological information measurement device and biological information measurement device charged using same |
| JP2017032574A (en)* | 2012-03-12 | 2017-02-09 | パナソニックヘルスケアホールディングス株式会社 | Biological information measuring instrument |
| US10018616B2 (en) | 2012-03-12 | 2018-07-10 | Phc Holdings Corporation | Charging device for biological information measurement device and biological information measurement device charged using same |
| US10258736B2 (en) | 2012-05-17 | 2019-04-16 | Tandem Diabetes Care, Inc. | Systems including vial adapter for fluid transfer |
| US11676694B2 (en) | 2012-06-07 | 2023-06-13 | Tandem Diabetes Care, Inc. | Device and method for training users of ambulatory medical devices |
| US11607492B2 (en) | 2013-03-13 | 2023-03-21 | Tandem Diabetes Care, Inc. | System and method for integration and display of data of insulin pumps and continuous glucose monitoring |
| US10357606B2 (en) | 2013-03-13 | 2019-07-23 | Tandem Diabetes Care, Inc. | System and method for integration of insulin pumps and continuous glucose monitoring |
| US12251536B2 (en) | 2013-03-13 | 2025-03-18 | Tandem Diabetes Care, Inc. | System and method for integration and display of data of insulin pumps and continuous glucose monitoring |
| US9962486B2 (en) | 2013-03-14 | 2018-05-08 | Tandem Diabetes Care, Inc. | System and method for detecting occlusions in an infusion pump |
| US12226608B2 (en) | 2013-03-15 | 2025-02-18 | Tandem Diabetes Care, Inc. | Method and device utilizing medicament delivery protocols |
| US10016561B2 (en) | 2013-03-15 | 2018-07-10 | Tandem Diabetes Care, Inc. | Clinical variable determination |
| US10463786B2 (en) | 2013-03-15 | 2019-11-05 | Tandem Diabetes Care, Inc. | Method and device utilizing insulin delivery protocols |
| US11596731B2 (en) | 2013-03-15 | 2023-03-07 | Tandem Diabetes Care, Inc. | Method and device utilizing medicament delivery protocols |
| US11597541B2 (en) | 2013-07-03 | 2023-03-07 | Deka Products Limited Partnership | Apparatus, system and method for fluid delivery |
| US12012241B2 (en) | 2013-07-03 | 2024-06-18 | Deka Products Limited Partnership | Apparatus, system and method for fluid delivery |
| US11147914B2 (en) | 2013-07-19 | 2021-10-19 | Bigfoot Biomedical, Inc. | Infusion pump system and method |
| US11813433B2 (en) | 2013-07-19 | 2023-11-14 | Dexcom, Inc. | Time averaged basal rate optimizer |
| US11957877B2 (en) | 2013-07-19 | 2024-04-16 | Dexcom, Inc. | Time averaged basal rate optimizer |
| US10112011B2 (en) | 2013-07-19 | 2018-10-30 | Dexcom, Inc. | Time averaged basal rate optimizer |
| US10821229B2 (en) | 2013-07-19 | 2020-11-03 | Dexcom, Inc. | Time averaged basal rate optimizer |
| US12138427B2 (en) | 2013-07-19 | 2024-11-12 | Dexcom, Inc. | Time averaged basal rate optimizer |
| US12064591B2 (en) | 2013-07-19 | 2024-08-20 | Insulet Corporation | Infusion pump system and method |
| WO2015047693A1 (en) | 2013-09-30 | 2015-04-02 | Animas Corporation | Methods for secure communication and pairing of a medical infusion device and a remote controller for such medical device |
| EP3869699A1 (en) | 2013-09-30 | 2021-08-25 | Animas Corporation | Methods for secure communication and pairing of a medical infusion device and a remote controller for such medical device |
| US9999379B2 (en) | 2013-11-07 | 2018-06-19 | Dexcom, Inc. | Systems and methods for a continuous monitoring of analyte values |
| US11730402B2 (en) | 2013-11-07 | 2023-08-22 | Dexcom, Inc. | Systems and methods for a continuous monitoring of analyte values |
| US9901292B2 (en) | 2013-11-07 | 2018-02-27 | Dexcom, Inc. | Systems and methods for a continuous monitoring of analyte values |
| US10226205B2 (en) | 2013-11-07 | 2019-03-12 | Dexcom, Inc. | Systems and methods for a continuous monitoring of analyte values |
| US12070306B2 (en) | 2013-11-07 | 2024-08-27 | Dexcom, Inc. | Systems and methods for a continuous monitoring of analyte values |
| US11399742B2 (en) | 2013-11-07 | 2022-08-02 | Dexcom, Inc. | Systems and methods for a continuous monitoring of analyte values |
| US10863931B2 (en) | 2013-11-07 | 2020-12-15 | Dexcom, Inc. | Systems and methods for a continuous monitoring of analyte values |
| US9974470B2 (en) | 2013-11-07 | 2018-05-22 | Dexcom, Inc. | Systems and methods for a continuous monitoring of analyte values |
| US11464906B2 (en) | 2013-12-02 | 2022-10-11 | Bigfoot Biomedical, Inc. | Infusion pump system and method |
| US10806851B2 (en) | 2013-12-26 | 2020-10-20 | Tandem Diabetes Care, Inc. | Wireless control of a drug delivery device |
| US11911590B2 (en)* | 2013-12-26 | 2024-02-27 | Tandem Diabetes Care, Inc. | Integration of infusion pump with remote electronic device |
| US20150182695A1 (en)* | 2013-12-26 | 2015-07-02 | Tandem Diabetes Care, Inc. | Integration of infusion pump with remote electronic device |
| US10213547B2 (en) | 2013-12-26 | 2019-02-26 | Tandem Diabetes Care, Inc. | Safety processor for a drug delivery device |
| US11383027B2 (en) | 2013-12-26 | 2022-07-12 | Tandem Diabetes Care, Inc. | Integration of infusion pump with remote electronic device |
| US10478551B2 (en)* | 2013-12-26 | 2019-11-19 | Tandem Diabetes Care, Inc. | Integration of infusion pump with remote electronic device |
| US20210146041A1 (en)* | 2013-12-26 | 2021-05-20 | Tandem Diabetes Care, Inc. | Integration of infusion pump with remote electronic device |
| US9486571B2 (en) | 2013-12-26 | 2016-11-08 | Tandem Diabetes Care, Inc. | Safety processor for wireless control of a drug delivery device |
| US10918785B2 (en)* | 2013-12-26 | 2021-02-16 | Tandem Diabetes Care, Inc. | Integration of infusion pump with remote electronic device |
| US9737656B2 (en)* | 2013-12-26 | 2017-08-22 | Tandem Diabetes Care, Inc. | Integration of infusion pump with remote electronic device |
| US20170312423A1 (en)* | 2013-12-26 | 2017-11-02 | Tandem Diabetes Care, Inc. | Integration of infusion pump with remote electronic device |
| US9669160B2 (en) | 2014-07-30 | 2017-06-06 | Tandem Diabetes Care, Inc. | Temporary suspension for closed-loop medicament therapy |
| US20160038675A1 (en)* | 2014-08-06 | 2016-02-11 | Bigfoot Biomedical, Inc. | Infusion pump assembly and method |
| US10137246B2 (en)* | 2014-08-06 | 2018-11-27 | Bigfoot Biomedical, Inc. | Infusion pump assembly and method |
| US12053615B2 (en) | 2014-08-06 | 2024-08-06 | Insulet Corporation | Infusion pump assembly and method |
| US10994078B2 (en) | 2014-08-06 | 2021-05-04 | Bigfoot Biomedical, Inc. | Infusion pump assembly and method |
| US11464899B2 (en) | 2014-08-28 | 2022-10-11 | Becton, Dickinson And Company | Wireless communication for on-body medical devices |
| US11471598B2 (en) | 2015-04-29 | 2022-10-18 | Bigfoot Biomedical, Inc. | Operating an infusion pump system |
| US10471208B2 (en)* | 2015-08-14 | 2019-11-12 | Baxter International Inc. | Medical device data integration apparatus and methods |
| US12128216B2 (en) | 2015-08-14 | 2024-10-29 | Baxter International Inc. | Medical device data integration apparatus and methods |
| US10780224B2 (en) | 2015-08-14 | 2020-09-22 | Baxter International Inc. | Medical device data integration apparatus and methods |
| US11511041B2 (en) | 2015-08-14 | 2022-11-29 | Baxter International Inc. | Medical device data integration apparatus and methods |
| US11738145B2 (en) | 2015-08-14 | 2023-08-29 | Baxter International Inc. | Medical device data integration apparatus and methods |
| US10569016B2 (en) | 2015-12-29 | 2020-02-25 | Tandem Diabetes Care, Inc. | System and method for switching between closed loop and open loop control of an ambulatory infusion pump |
| US12318578B2 (en) | 2015-12-29 | 2025-06-03 | Tandem Diabetes Care, Inc. | System and method for switching between closed loop and open loop control of an ambulatory infusion pump |
| US11638781B2 (en) | 2015-12-29 | 2023-05-02 | Tandem Diabetes Care, Inc. | System and method for switching between closed loop and open loop control of an ambulatory infusion pump |
| US10987468B2 (en) | 2016-01-05 | 2021-04-27 | Bigfoot Biomedical, Inc. | Operating multi-modal medicine delivery systems |
| US12106837B2 (en) | 2016-01-14 | 2024-10-01 | Insulet Corporation | Occlusion resolution in medication delivery devices, systems, and methods |
| US20170212039A1 (en)* | 2016-01-21 | 2017-07-27 | Wellysun Inc. | Extre occult blood inspection method and apparatus thereof |
| US10145787B2 (en)* | 2016-01-21 | 2018-12-04 | Wellysun Inc. | Excreta occult blood inspection method and apparatus thereof |
| US11484652B2 (en) | 2017-08-02 | 2022-11-01 | Diabeloop | Closed-loop blood glucose control systems and methods |
| US11350862B2 (en) | 2017-10-24 | 2022-06-07 | Dexcom, Inc. | Pre-connected analyte sensors |
| US11382540B2 (en) | 2017-10-24 | 2022-07-12 | Dexcom, Inc. | Pre-connected analyte sensors |
| US12150250B2 (en) | 2017-10-24 | 2024-11-19 | Dexcom, Inc. | Pre-connected analyte sensors |
| US11706876B2 (en) | 2017-10-24 | 2023-07-18 | Dexcom, Inc. | Pre-connected analyte sensors |
| US11331022B2 (en) | 2017-10-24 | 2022-05-17 | Dexcom, Inc. | Pre-connected analyte sensors |
| US11943876B2 (en) | 2017-10-24 | 2024-03-26 | Dexcom, Inc. | Pre-connected analyte sensors |
| US11872368B2 (en) | 2018-04-10 | 2024-01-16 | Tandem Diabetes Care, Inc. | System and method for inductively charging a medical device |
| US11523972B2 (en) | 2018-04-24 | 2022-12-13 | Deka Products Limited Partnership | Apparatus, system and method for fluid delivery |
| US20220008688A1 (en)* | 2018-09-26 | 2022-01-13 | Cary Kochman | Method and system for facilitating the transition between a conscious and unconscious state |
| US11464908B2 (en) | 2019-02-18 | 2022-10-11 | Tandem Diabetes Care, Inc. | Methods and apparatus for monitoring infusion sites for ambulatory infusion pumps |
| US12337138B1 (en) | 2021-02-19 | 2025-06-24 | Fresenius Kabi Deutschland Gmbh | Drug delivery assembly including a pre-filled cartridge |
| US11497847B1 (en) | 2021-02-19 | 2022-11-15 | Fresenius Kabi Deutschland Gmbh | Wearable injector with adhesive substrate |
| US11413394B1 (en)* | 2021-02-19 | 2022-08-16 | Fresenius Kabi Deutschland Gmbh | Display for wearable drug delivery device |
| US11406755B1 (en) | 2021-02-19 | 2022-08-09 | Fresenius Kabi Deutschland Gmbh | Sensing fluid flow irregularities in an on-body injector |
| US12420033B1 (en) | 2021-02-19 | 2025-09-23 | Fresenius Kabi Deutschland Gmbh | Wearable injector with sterility sensors |
| US12239825B1 (en) | 2021-02-19 | 2025-03-04 | Fresenius Kabi Deutschland Gmbh | Drug delivery assembly including an actuator |
| US12102791B1 (en) | 2021-04-30 | 2024-10-01 | Fresenius Kabi Deutschland Gmbh | Wearable drug delivery device with pressurized fluid dispensing |
| US12377209B1 (en) | 2021-04-30 | 2025-08-05 | Fresenius Kabi Deutschland Gmbh | Deformable drug reservoir for wearable drug delivery device |
| US12357750B1 (en) | 2021-04-30 | 2025-07-15 | Fresenius Kabi Deutschland Gmbh | Wearable drug delivery device with drug delivery indication |
Also Published As
| Publication number | Publication date |
|---|---|
| AU2003252104A1 (en) | 2004-02-09 |
| EP2253346A1 (en) | 2010-11-24 |
| WO2004008956A3 (en) | 2004-04-22 |
| US20090149803A1 (en) | 2009-06-11 |
| US8568357B2 (en) | 2013-10-29 |
| US20090112076A1 (en) | 2009-04-30 |
| US20090118664A1 (en) | 2009-05-07 |
| US20090143662A1 (en) | 2009-06-04 |
| AU2003252104A8 (en) | 2004-02-09 |
| DK1525014T3 (en) | 2013-03-04 |
| EP2253347A1 (en) | 2010-11-24 |
| US20090099506A1 (en) | 2009-04-16 |
| US8192395B2 (en) | 2012-06-05 |
| EP1525014A2 (en) | 2005-04-27 |
| US20090118665A1 (en) | 2009-05-07 |
| US20120220928A1 (en) | 2012-08-30 |
| US20100198143A1 (en) | 2010-08-05 |
| EP1525014B1 (en) | 2012-11-14 |
| US20090099509A1 (en) | 2009-04-16 |
| WO2004008956A2 (en) | 2004-01-29 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| US8192395B2 (en) | System for providing blood glucose measurements to an infusion device | |
| US9636456B2 (en) | System for providing blood glucose measurements to an infusion device | |
| US7901394B2 (en) | Physiological monitoring device for controlling a medication infusion device | |
| US8622954B2 (en) | Relay device for transferring information between a sensor system and a fluid delivery system | |
| US8663201B2 (en) | Infusion device | |
| US8663103B2 (en) | Handheld medical device programmer | |
| EP1924307B1 (en) | Hand-held controller device for an infusion pump | |
| US20120227737A1 (en) | Analyte sensor and method of using the same | |
| US20070060870A1 (en) | Controller device for an infusion pump | |
| HK1116032A1 (en) | Ambulatory medical device and method of communication between medical devices | |
| HK1116032B (en) | Ambulatory medical device and method of communication between medical devices |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| AS | Assignment | Owner name:MEDTRONIC MINIMED, INC., CALIFORNIA Free format text:ASSIGNMENT OF ASSIGNORS INTEREST;ASSIGNORS:ESTES, MARK C.;TALBOT, CARY D.;TOLLE, MIKE CHARLES VALLET;AND OTHERS;REEL/FRAME:014723/0738;SIGNING DATES FROM 20031016 TO 20031029 | |
| STCB | Information on status: application discontinuation | Free format text:ABANDONED -- FAILURE TO RESPOND TO AN OFFICE ACTION |