US20040037887A1 - Bulking agent - Google Patents
Bulking agentDownload PDFInfo
- Publication number
- US20040037887A1 US20040037887A1US10/459,895US45989503AUS2004037887A1US 20040037887 A1US20040037887 A1US 20040037887A1US 45989503 AUS45989503 AUS 45989503AUS 2004037887 A1US2004037887 A1US 2004037887A1
- Authority
- US
- United States
- Prior art keywords
- bulking agent
- polyvinyl alcohol
- substantially spherical
- carrier
- bulking
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Abandoned
Links
Images
Classifications
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/74—Synthetic polymeric materials
- A61K31/765—Polymers containing oxygen
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/0012—Galenical forms characterised by the site of application
- A61K9/0019—Injectable compositions; Intramuscular, intravenous, arterial, subcutaneous administration; Compositions to be administered through the skin in an invasive manner
- A61K9/0024—Solid, semi-solid or solidifying implants, which are implanted or injected in body tissue
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61L—METHODS OR APPARATUS FOR STERILISING MATERIALS OR OBJECTS IN GENERAL; DISINFECTION, STERILISATION OR DEODORISATION OF AIR; CHEMICAL ASPECTS OF BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES; MATERIALS FOR BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES
- A61L27/00—Materials for grafts or prostheses or for coating grafts or prostheses
- A61L27/14—Macromolecular materials
- A61L27/16—Macromolecular materials obtained by reactions only involving carbon-to-carbon unsaturated bonds
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61L—METHODS OR APPARATUS FOR STERILISING MATERIALS OR OBJECTS IN GENERAL; DISINFECTION, STERILISATION OR DEODORISATION OF AIR; CHEMICAL ASPECTS OF BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES; MATERIALS FOR BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES
- A61L27/00—Materials for grafts or prostheses or for coating grafts or prostheses
- A61L27/50—Materials characterised by their function or physical properties, e.g. injectable or lubricating compositions, shape-memory materials, surface modified materials
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M25/00—Catheters; Hollow probes
- A61M25/10—Balloon catheters
- A61M25/1025—Connections between catheter tubes and inflation tubes
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M5/00—Devices for bringing media into the body in a subcutaneous, intra-vascular or intramuscular way; Accessories therefor, e.g. filling or cleaning devices, arm-rests
- A61M5/178—Syringes
- A61M5/31—Details
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P13/00—Drugs for disorders of the urinary system
- A61P13/02—Drugs for disorders of the urinary system of urine or of the urinary tract, e.g. urine acidifiers
Definitions
- the inventionrelates generally to the treatment of mammalian tissue through the process of bulking, and more specifically to the injection of bulking particles into a treatment region of a mammal.
- Surgical implantation of artificial sphinctershas often been employed to treat patients suffering from urinary incontinence.
- the surgical implantation of the artificial sphinctercommonly requires hospitalization, is relatively complex and expensive, and will usually require six to eight weeks of recovery time.
- the proceduremay be unsuccessful if the artificial sphincter malfunctions. As a result, additional surgery is required to adjust, repair, or replace the implant.
- Urinary incontinencecan also be treated using nonsurgical means.
- a common method to treat patients with urinary incontinenceis periurethral injection of a bulking material.
- a bulking compositionis a Teflon® paste known commercially as “Polytef” or “Urethrin.” This paste is comprised of a fifty-fifty (50-50) by weight mixture of a glycerin liquid with Teflon® (polytetrafluoroethylene (PTFE)) brand particles sold by DuPont.
- PTFEpolytetrafluoroethylene
- the glycerinis biodegradable, however, and over a period of time the glycerin dissipates into the body and is then metabolized or eliminated leaving only about fifty percent (50%) of the injected mixture (i.e., the Teflon® particles) at the injection site. Consequently, to achieve the desired result, the surgeon typically overcompensate for the anticipated loss of bulking material by injecting a significantly larger amount of material than initially required. At the extreme, this overcompensation can lead to complete closure of the urethra, which could put the patient into temporary urinary retention. Additionally, the eventual dissipation of the glycerin complicates the surgeon's ability to visually gauge the appropriate amount of bulking material to inject. To avoid these over-bulking side effects, the surgeon may ultimately not inject enough bulking mixture, leading to the likelihood of a second or even a third procedure to inject additional material.
- Teflon® paste bulking materialif sufficiently small may allow, the particles to migrate to other locations of the body, such as the lungs, brain, etc.
- Teflon® particleshave been known to induce undesirable tissue reaction and form Teflon® induced granulomas in certain individuals.
- the Teflon® pasteis typically highly viscous and can only be injected using a hypodermic needle held by an injection assist device.
- Use of an injection assist devicemay be required, because a surgeon would likely not have sufficient strength to force the highly viscous Teflon® paste through a needle of any acceptable size.
- Teflon® pasteTwo alternatives to the Teflon® paste are a collagen gel and carbon coated zirconium beads.
- One such commercially available productincludes Contigen®, available from C R Bard.
- the collagen gelis injected in the same manner as the Teflon® paste and forms a fibrous mass of tissue around the augmentation site. This fibrous mass created by the collagen injection, however, also dissipates over time and is eventually eliminated by the patient's body. As a result, additional injections are periodically required.
- Yet another bulking procedureincludes the injection of swollen hydrogel particles.
- the swollen hydrogel particlesexhibit relatively low injection forces by incorporating low molecular weight water-soluble organic compounds, along with water, in the particles. See, for example, U.S. Pat. Nos. 5,813,411 and 5,902,832 to Van Bladel et al., and U.S. Pat. No. 5,855,615 to Bley et al., the disclosures of which are hereby incorporated herein by reference in their entireties.
- Teflon pasteAnother alternative to the Teflon paste is a hard particle suspension.
- Durasphere®available from Carbon Medical Technologies. These hard particles, for example carbon coated zirconium beads, are injected in a beta-glucan carrier. The beta-glucan is eliminated by the patient's body over time. As a result, additional injections may be required.
- hard particle suspensionsdepending on the size of the particle, may tend not to be easily dispensed without clogging smaller gauge injection needles.
- the bulking agentis injected into a plurality of locations, causing the urethral lining to coapt.
- the newly added bulking agentmay need to be injected at a higher pressure than the pressure at which the initial bulking agent was injected.
- the higher pressure requirements for subsequent injectionsmay result from the effect of closing off the treatment region by the initial bulking agent, thereby creating backpressure when attempting to insert additional bulking agent(s).
- the bulking agentis injected at multiple locations to cause the uretheral lining to coapt with a higher opening pressure than the patient had prior to injection of the bulking agent.
- UroviveTMutilizes a plurality of silicone balloons that are inserted into the treatment region, specifically, the periphery of the sphincter. The balloons are then filled with a hydrogel to effect tissue coaptation.
- the inventiongenerally relates to an injectable bulking composition that does not degrade or dissipate in the body, has sufficiently low viscosity such that it is easily administered via injection, and will not migrate from the site of injection, thereby enabling the affected tissue to maintain the desired constriction without causing undesirable side effects.
- the inventiongenerally relates to an injection method that reduces the injection pressure required to place the bulking agents.
- the inventionrelates to the use of polymeric particles to facilitate bulking in a treatment region of a mammal's body through injection of the particles into the treatment region.
- the particlesare compliant enough to be delivered through a relatively small gauge injection device.
- the inventionis employed in the treatment of diseases requiring sphincter bulking, e.g., for treating urinary or fecal incontinence; however, the bulking method described herein can also be used for soft tissue bulking for use during, for example, plastic surgery.
- the inventionin another aspect relates to a bulking agent for medical applications.
- the bulking agentincludes a carrier and a plurality of substantially spherical polyvinyl alcohol particles dispersed within the carrier.
- the carrieraids the delivery of the substantially spherical polyvinyl alcohol particles to a site to be bulked.
- the inventionin yet another aspect, relates to a method for bulking mammalian tissue.
- the methodincludes the steps of introducing a bulking agent to the mammalian tissue to coapt the mammalian tissue with the bulking agent.
- the bulking agentincludes a carrier and a plurality of substantially spherical polyvinyl alcohol particles dispersed within the carrier.
- the carrieraids the delivery of the substantially spherical polyvinyl alcohol particles to a site to be bulked.
- the bulking agentcomprises a volume.
- the volumecould be, for example, from about 1 ml to about 30 ml, from about 20 ml to about 30 ml, or from about 2 ml to about 16 ml.
- the substantially spherical polyvinyl alcohol particlesare sized from about 40 micron to about 1500 microns in diameter, preferably from about 150 micron to about 1100 microns in diameter, and more preferably from about 500 micron to about 900 microns in diameter.
- the substantially spherical polyvinyl alcohol particlescan comprise pores and/or bioreactive agents, such as drugs, proteins, genes, chemo-therapeutic agents, and growth factors.
- the substantially spherical polyvinyl alcohol particlescan be compressible and/or substantially dimensionally stable.
- the carriercan be a water-based solution, such as saline solution.
- the carriercan include at least one of a lubricant, a biocompatible thickening agent, or a color.
- the bulking agentcan be delivered through a needle and/or a catheter. In one embodiment, the bulking agent is delivered transuretherally. In addition, the bulking agent can be delivered while viewing the tissue to be bulked with a cytoscope.
- the inventionin still another aspect, relates to an apparatus for bulking mammalian tissue.
- the apparatusincludes a needle defining a lumen, an inflation device adapted to advance through the lumen of the needle, and a bulking agent insertable via the lumen of the needle.
- the needleis adapted to penetrate the mammalian tissue.
- the inflation deviceis disposed adjacent to the mammalian tissue after being advanced through the needle.
- the inflation deviceis inflatable and subsequently deflatable to create a void in the mammalian tissue.
- the bulking agentis inserted to fill at least partially the void in the tissue, the bulking agent coapting the mammalian tissue.
- the inflation devicecan include a biocompatible balloon, and/or a color coating for visualization made from at least one of a silicone, an ethylene vinyl alcohol, a polypropylene, a latex rubber, a polyurethane, a polyester, a nylon, or a thermoplastic rubber.
- the inflation devicecan have a shape selected from the group consisting of substantially round, oval, hemi spherical, spherical, or oblong.
- the needleis sized from 16 gauge to 24 gauge, preferably from 18 gauge to 22 gauge.
- the bulking agentcomprises a plurality of polymeric particles and can be injected into the void by a syringe.
- the bulking agentincludes a carrier and a plurality of substantially spherical polyvinyl alcohol particles dispersed within the carrier.
- the carrieraids the delivery of the substantially spherical polyvinyl alcohol particles to a site to be bulked.
- the bulking agentcan further include a color.
- the inventionin yet another aspect, relates to a method for bulking mammalian tissue.
- the methodincludes the steps of inserting an inflation device within a portion of a mammal, inflating the inflation device to compress the mammalian tissue surrounding the inflated inflation device, thereby creating a void in the tissue, deflating the inflation device, removing the inflation device from the mammal, and providing a bulking agent to at least partially fill the void, the bulking agent coapting the mammalian tissue.
- the methodincludes the steps of inserting a needle with a penetration device into the mammalian tissue, removing the penetration device while retaining the inserted needle, and advancing the inflation device through the needle.
- the needlecan be sized from 16 gauge to 24 gauge, preferably 18 gauge to 22 gauge.
- the methodcan also include the step of viewing the tissue to be bulked with a cytoscope.
- the inflation devicecan include a biocompatible balloon, and/or a color coating for visualization made from at least one of a silicone, an ethylene vinyl alcohol, a polypropylene, a latex rubber, a polyurethane, a polyester, a nylon and a thermoplastic rubber. Additionally, the inflation device can have a shape selected from the group consisting of substantially round, oval, hemi spherical, spherical, or oblong.
- the bulking agentcomprises a plurality of polymeric particles and can be injected into the void by a syringe.
- the substantially spherical polyvinyl alcohol particlesare coated, embedded, or filled with a material that will aid the delivery of the particles to a site to be bulked.
- the bulking agentincludes a carrier and a plurality of substantially spherical polyvinyl alcohol particles dispersed within the carrier. The carrier aids the delivery of the substantially spherical polyvinyl alcohol particles to a site to be bulked.
- the bulking agentcan further include a color.
- FIG. 1depicts a side view of a tissue structure with an enlarged lumen surrounded by muscle tissue
- FIG. 2depicts the tissue structure of FIG. 1 immediately after a bulking agent in accordance with the invention has been injected around the enlarged lumen of the tissue;
- FIG. 3depicts the tissue structure of FIG. 1 immediately after a bulking agent in accordance with the invention has been injected around the enlarged lumen of the tissue utilizing a cystoscope-aided injection method;
- FIG. 4is a schematic plan view of a needle assembly in accordance with the invention.
- FIG. 5is a schematic plan view of the needle assembly of FIG. 4 with the trocar/obtuator assembly being removed;
- FIG. 6is a schematic plan view of the needle assembly of FIG. 4 with a balloon assembly being inserted into the needle assembly;
- FIG. 7is a schematic plan view of the needle assembly of FIG. 4 with a syringe attached to the needle assembly for inflating the balloon;
- FIG. 8is a schematic plan view of the assembly of FIG. 7 with the syringe and balloon assembly being removed;
- FIG. 9is a schematic plan view of the assembly of FIG. 4 with another syringe attached to the needle assembly for injecting a bulking agent into tissue;
- FIG. 10is a pictorial representation of a method of creating a void within a patient's tissue by inserting and inflating a balloon;
- FIG. 11is a pictorial representation of a method of filling the void within the patient's tissue with a bulking agent.
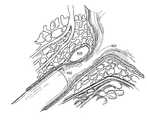
- a tissue structuremore specifically a urethra/ureter 10 , having a wall 20 and an enlarged lumen 30 surrounded by muscle tissue 40 is shown in side view.
- a cystoscope 50comprising a fiberoptic light transmitting element 60 , a working channel 70 and a viewing element 80 encased in a sheath 90 may be inserted in the urethra/ureter 10 to a distance close to the enlarged lumen 30 . The close distance is selected to allow a clear view of the enlarged lumen 30 .
- the urethra/ureter 10is shown immediately after a bulking agent in accordance with the invention has been injected around the enlarged lumen 30 of the tissue.
- a hypodermic needle 100is inserted through the tissue 40 , preferably over the enlarged lumen 30 , stopping near the wall 20 of the enlarged lumen 30 .
- a bulking agent 110 including polymeric particles 120is injected via the hypodermic needle 100 into the tissue 40 adjacent the wall 20 .
- the resultis a constricted region 130 located in the vicinity of the accumulation of the bulking agent 110 .
- the urethra/ureter 10is shown immediately after the bulking agent 110 of the present invention has been injected around the enlarged lumen 30 of the tissue 40 utilizing a cystoscope 50 aided injection method in accordance with another embodiment of the invention.
- An elongate needle 140may be inserted through the working channel 70 into the urethra/ureter 10 and the surrounding tissue 40 and the injection can be completed operating solely through the cystoscope 50 .
- Thisis generally the preferred method of operation on male patients for the area surrounding the urethra/ureter and is the preferred method for female patients for the area surrounding the ureter.
- the present inventionrelates to a bulking agent including substantially spherical polyvinyl alcohol particles used to facilitate bulking in a region of the human body through injection of the particles into the treatment region.
- the particlesare compliant enough to be delivered through a substantially small-gauge injection device.
- the particlesare 50% compressible. This is accomplished through the use of particles that are adapted to compress as they pass through the small gauge injection device.
- a 16 to 24 gauge needleis used to dispense the bulking composition without clogging. In other applications, other size needles may be preferred, for example 18-22 gauge.
- the present inventionis employed in the treatment of diseases requiring bulking, e.g., urinary or fecal incontinence.
- diseases requiring bulkinge.g., urinary or fecal incontinence.
- Some examples of conditions that can be treated by way of the present inventioninclude urinary incontinence, vesicourethral reflux, fecal incontinence and intrinsic sphincter deficiency or ISD.
- the bulking method described hereincan also be used for soft tissue bulking for use during, for example, plastic surgery.
- the method of providing a bulking agent to the human bodyincludes using polymeric particles, such as polyvinyl alcohol, as a bulking agent and injecting the particles into the treatment region of the human body.
- polymeric particlessuch as polyvinyl alcohol
- An advantage of the present inventionis that the particles are substantially non-biodegradable, thereby virtually eliminating the need for replenishing the particles to maintain efficacy.
- a further advantage of the present inventionis that the substantially spherical size and shape of the particles allows for close packing of the particles in the treatment space.
- the particlesare made of a water and polyvinyl alcohol mixture.
- a description of particles contemplated for use with the present inventionsee U.S. patent application Ser. Nos. 10/232,265, 10/215,594, 10/116,330, 10/109,966, 10/231,664, the disclosures of which are hereby incorporated by reference herein in their entirety.
- water, polyvinyl alcohol, and alginateare combined and pumped through a nozzle under pressure, generating substantially spherically-shaped droplets.
- the substantially spherically-shaped dropletsencounter a solution that promotes cross-linking of the polyvinyl alcohol.
- the alginateis removed from the outer surface. The result is a substantially spherically-shaped particle that is substantially all polyvinyl alcohol.
- bio-active agentscan be added to the particles.
- substancessuch as drugs, growth factors, proteins, genes, and chemo-therapeutic agents can be added to the particles to enhance localized treatments while still providing significant bulking benefits.
- the particlesthemselves are substantially inert in that they do not tend to react with body fluids and/or tissue. For example, many other types of bulking particles swell in use.
- the substantially spherical polyvinyl alcohol particlesare substantially dimensionally stable. Some tissue growth on, near, or around the particle surface may occur, but no biological interaction between the tissue and the particles is expected.
- the particlesare substantially solid.
- the particlesare substantially spherically-shaped and are sized in a range of about 40 microns to about 1500 microns in diameter, preferably about 150 microns to about 1100 microns in diameter, and more preferably about 500 microns to about 900 microns in diameter.
- the size of the particles chosen for a particular applicationwill be determined by a number of factors. Smaller particles are easier to inject with a smaller gauge size needle; however, embolization due to migration of the particles is a concern with the smaller particle sizes.
- the size of the particles used in a particular procedurewill include consideration of the procedure employed, disease progression, the degree of degradation of the affected region, patient size, the disposition of the patient, and the preferences and techniques of the doctor performing the procedure. Similarly, such factors must be considered when determining the proper volume of bulking agent to inject into a patient.
- the volume of bulking compositionis about 1 ml to about 30 ml, and preferably about 20 ml to about 30 ml.
- the volume of bulking composition injected into a patientis about 2 ml to about 16 ml.
- these amountscan vary significantly based on the doctor's determination as to when the target region is sufficiently bulked up.
- the porosity of the particlesmay be modified. These effects, if desired, can be enhanced by increasing pore size. For example, tissue in-growth can be encouraged by increasing pore size.
- pore sizesare within a range of about 4 microns to about 5 microns up to about 30 microns to about 50 microns. In one embodiment, the pores cover up to 80% of the surface area of the particle.
- the bulking particlesare injected through a needle.
- a cystoscopeis used to allow for viewing the injection area.
- the bulking particlescan be supplemented with a contrast agent to enhance their appearance as an aid to the doctor performing the procedure. Other methods of visual enhancement to assist in viewing of the bulking agent can also be employed. Injection of the particles can also be accomplished transuretherally by, for example, using a catheter.
- the method of providing the bulking agent to the human bodyfurther includes mixing the bulking particles with a carrier such that the particles are suspended in the carrier, and then injecting the particles-carrier mix into the treatment portion of the human body.
- the carrierserves as a lubricant for the particles thereby increasing the ease with which the particles move into the body.
- the carrieris a saline solution.
- bio-compatible thickening agentssuch as alginate, beta-glucan, glycerin, cellulose, or collagen are added to the carrier or serve as the carrier themselves to modify the viscosity of the carrier.
- the carriermay be bio-active, that is the carrier includes an anti-microbial agent, or the like.
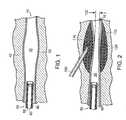
- the present inventionalso relates to a method used to dilate tissue within a treatment tissue region to facilitate injection of the bulking agent.
- the methodincludes: inserting a needle with a penetration device (e.g., a taper point obtuator or trocar) into the treatment region (e.g., the sphincter region) (FIG. 4); removing the penetration device while retaining the inserted needle (FIG. 5); advancing a balloon through the needle (FIG. 6); inflating the balloon, thereby creating a void in the treatment region (FIG. 7); deflating and removing the balloon from the treatment region (FIG.
- a penetration devicee.g., a taper point obtuator or trocar
- a needle 400such as a blunt-end hypotube or hypodermic needle having a first end and a second end, is adapted to accept a penetration device 404 , such as a taper point obtuator or a trocar, at the first end of the needle 400 (FIG. 4).
- the needle 400may range in size from about 18 gauge to about 22 gauge, and preferably about 20 gauge to about 22 gauge.
- the penetration device 404is attached to the needle 400 to enable penetration of the needle 400 into the tissue.
- the penetration device 404may be adapted to the needle 400 by way of a luer hub or fitting, and in one embodiment, a male luer hub is used.
- the needle 400is inserted with the penetration device 404 into the treatment region 420 (e.g., the sphincter region)(FIG. 10) to the desired depth.
- desired penetration depthcan be determined by striping 406 located on the penetration device 404 .
- the amount of penetration of the penetration device 404ranges from about 2 cm to about 2.5 cm (FIG. 4).
- the amount of tissue penetration of the needle 400ranges from about 0.5 cm to about 1 cm beyond the tissue line 407 (FIG. 5).
- the penetration device 404is removed while retaining the inserted needle 400 (FIG. 6).
- a luer hub 402 or fitting, or in one embodiment a female luer hub,may be adapted to the second end of the needle 400 , to which a syringe 412 , 418 (FIGS. 7 - 9 ) is adapted.
- the luer hub 402is depicted in its locked position, and in FIG. 5 the luer hub 402 is depicted in its unlocked position.
- the locked positionthe luer hub 402 can be positioned for inflating the balloon 408 or injecting a bulking agent 416 .
- the unlocked positionthe luer hub 402 can be positioned for accepting the balloon 408 for insertion or for removal of the balloon 408 after dilation.
- the balloon 408is adapted to advance through a lumen of the needle 400 , and an adapter on the balloon 408 provides a means to lock the balloon 408 to the luer hub 402 , which in turn adapts to the syringe 412 (FIG. 6).
- the balloon 408may have no tip or, alternatively, the balloon 408 may have a small stump appendage, which may remain from processing of the balloon.
- the balloon 408is affixed to an end of a plastic tube 410 (FIG. 6).
- the tip for the balloon 408is integral with a shaft.
- balloon 408includes at least one fill and/or evacuation port.
- the balloonis a colored balloon (e.g., blue) to facilitate remote visualization of the procedure and proper placement of the balloon.
- the ballooncould be clear to transparent and the inflation media could be colored, for example, a colored saline solution.
- the balloonmay be semi-compliant or non-compliant.
- the balloonmay be manufactured from any suitable material, for example, a polymer. Some examples of suitable balloon materials include: silicone, ethylene vinyl acetate (EVA), polypropylene, latex rubber, polyurethane, polyester, nylon and thermoplastic rubber.
- EVAethylene vinyl acetate
- the balloonis inflated to, for example, about 3 cm to about 5 cm in diameter.
- the balloonmay assume a variety of shapes.
- the length of the balloonmay vary depending upon the procedure.
- the inflated balloonmay have a length in the range of, for example, about 3 cm to about 10 cm.
- Other balloon configurationsmay be employed, and the types and methods used to employ the most suitable balloon configurations for a particular application of this invention will be obvious to those skilled in the art.
- the balloon 408is then inflated using an inflation device, such as the syringe 412 , creating a void in the treatment region (FIGS. 7 and 8).
- the balloonmay be colored (i.e. blue) to aid in visibility through the tissue.
- the balloon 408becomes visible to aid in proper balloon placement.
- the expanding balloon 408may become visible under the urethra as it thins.
- the balloon 408inflates to a volume of about 1 cc to about 1.5 cc, although such volumes may vary depending upon many factors inherent in the characteristics of the particular application, some of which were discussed previously.
- salineis used to inflate the balloon 408 .
- about 3 cc of salineis placed in the syringe 412 and injected into the balloon 408 for inflation.
- the balloon 408is then deflated and removed from the treatment region, resulting in a tissue void 414 where the inflated balloon 408 previously resided (FIGS. 8 and 10).
- the balloon 408is removable through the lumen of the needle 400 .
- a plastic tube or other tip 410is used to aid in removal of the balloon 408 .
- a syringe or other injection device 418 containing the bulking agent 416is then affixed to the needle 400 by way of the luer hub 402 .
- the plunger of the syringe 418is then depressed, thereby injecting the bulking agent 416 into the tissue void 414 (FIGS. 9 and 11).
Landscapes
- Health & Medical Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Veterinary Medicine (AREA)
- Public Health (AREA)
- General Health & Medical Sciences (AREA)
- Animal Behavior & Ethology (AREA)
- Chemical & Material Sciences (AREA)
- Medicinal Chemistry (AREA)
- Epidemiology (AREA)
- Dermatology (AREA)
- Engineering & Computer Science (AREA)
- Oral & Maxillofacial Surgery (AREA)
- Transplantation (AREA)
- Heart & Thoracic Surgery (AREA)
- Biomedical Technology (AREA)
- Pharmacology & Pharmacy (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Hematology (AREA)
- Anesthesiology (AREA)
- Child & Adolescent Psychology (AREA)
- Pulmonology (AREA)
- Biophysics (AREA)
- Vascular Medicine (AREA)
- Neurosurgery (AREA)
- Organic Chemistry (AREA)
- Urology & Nephrology (AREA)
- General Chemical & Material Sciences (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Materials For Medical Uses (AREA)
- Prostheses (AREA)
- Polymers & Plastics (AREA)
- Organic Low-Molecular-Weight Compounds And Preparation Thereof (AREA)
Abstract
Description
- This application incorporates by reference, and claims priority to and the benefit of, provisional U.S. patent application serial No. 60/388,446, which was filed on Jun. 12, 2002.[0001]
- The invention relates generally to the treatment of mammalian tissue through the process of bulking, and more specifically to the injection of bulking particles into a treatment region of a mammal.[0002]
- Urinary incontinence, vesicourethral reflux, fecal incontinence, and intrinsic sphincter deficiency (ISD), for example, are disorders that have responded to treatments with augmentative materials. Such disorders occur when the resistance to flow of bodily discharges decreases to the point where the resistance can no longer overcome the intra-abdominal pressure. Nearly all procedures developed to restore continence are based on restoring the lost resistance.[0003]
- Surgical implantation of artificial sphincters has often been employed to treat patients suffering from urinary incontinence. The surgical implantation of the artificial sphincter commonly requires hospitalization, is relatively complex and expensive, and will usually require six to eight weeks of recovery time. Moreover, the procedure may be unsuccessful if the artificial sphincter malfunctions. As a result, additional surgery is required to adjust, repair, or replace the implant.[0004]
- Urinary incontinence can also be treated using nonsurgical means. A common method to treat patients with urinary incontinence is periurethral injection of a bulking material. One such bulking composition is a Teflon® paste known commercially as “Polytef” or “Urethrin.” This paste is comprised of a fifty-fifty (50-50) by weight mixture of a glycerin liquid with Teflon® (polytetrafluoroethylene (PTFE)) brand particles sold by DuPont. The glycerin is biodegradable, however, and over a period of time the glycerin dissipates into the body and is then metabolized or eliminated leaving only about fifty percent (50%) of the injected mixture (i.e., the Teflon® particles) at the injection site. Consequently, to achieve the desired result, the surgeon typically overcompensate for the anticipated loss of bulking material by injecting a significantly larger amount of material than initially required. At the extreme, this overcompensation can lead to complete closure of the urethra, which could put the patient into temporary urinary retention. Additionally, the eventual dissipation of the glycerin complicates the surgeon's ability to visually gauge the appropriate amount of bulking material to inject. To avoid these over-bulking side effects, the surgeon may ultimately not inject enough bulking mixture, leading to the likelihood of a second or even a third procedure to inject additional material.[0005]
- Further, the particle size in the Teflon® paste bulking material if sufficiently small may allow, the particles to migrate to other locations of the body, such as the lungs, brain, etc. Teflon® particles have been known to induce undesirable tissue reaction and form Teflon® induced granulomas in certain individuals.[0006]
- In addition, the Teflon® paste is typically highly viscous and can only be injected using a hypodermic needle held by an injection assist device. Use of an injection assist device may be required, because a surgeon would likely not have sufficient strength to force the highly viscous Teflon® paste through a needle of any acceptable size.[0007]
- Two alternatives to the Teflon® paste are a collagen gel and carbon coated zirconium beads. One such commercially available product includes Contigen®, available from C R Bard. The collagen gel is injected in the same manner as the Teflon® paste and forms a fibrous mass of tissue around the augmentation site. This fibrous mass created by the collagen injection, however, also dissipates over time and is eventually eliminated by the patient's body. As a result, additional injections are periodically required.[0008]
- Yet another bulking procedure includes the injection of swollen hydrogel particles. The swollen hydrogel particles exhibit relatively low injection forces by incorporating low molecular weight water-soluble organic compounds, along with water, in the particles. See, for example, U.S. Pat. Nos. 5,813,411 and 5,902,832 to Van Bladel et al., and U.S. Pat. No. 5,855,615 to Bley et al., the disclosures of which are hereby incorporated herein by reference in their entireties.[0009]
- Another alternative to the Teflon paste is a hard particle suspension. One such commercially available product is Durasphere® available from Carbon Medical Technologies. These hard particles, for example carbon coated zirconium beads, are injected in a beta-glucan carrier. The beta-glucan is eliminated by the patient's body over time. As a result, additional injections may be required. Furthermore, hard particle suspensions, depending on the size of the particle, may tend not to be easily dispensed without clogging smaller gauge injection needles.[0010]
- Furthermore, available methods of injecting bulking agents require the placement of a needle at a treatment region, for example, peri-urethrally or transperenially. Assisted by visual aids, the bulking agent is injected into a plurality of locations, causing the urethral lining to coapt. In cases where additional applications of bulking agent are required (e.g., when bulking agents are dissipated within the body), the newly added bulking agent may need to be injected at a higher pressure than the pressure at which the initial bulking agent was injected. The higher pressure requirements for subsequent injections may result from the effect of closing off the treatment region by the initial bulking agent, thereby creating backpressure when attempting to insert additional bulking agent(s). Typically, the bulking agent is injected at multiple locations to cause the uretheral lining to coapt with a higher opening pressure than the patient had prior to injection of the bulking agent.[0011]
- Bulking agent delivery methods have attempted to address the issue of subsequent injection requirements. One method that has been employed is hydrodissection of tissue in the vicinity of the treatment region, thereby creating tissue voids designed to decrease the injection pressure required when adding additional bulking agent to the voids. Another method used to reduce injection pressures is the Urovive™ device available from American Medical Systems. Urovive™ utilizes a plurality of silicone balloons that are inserted into the treatment region, specifically, the periphery of the sphincter. The balloons are then filled with a hydrogel to effect tissue coaptation.[0012]
- The invention generally relates to an injectable bulking composition that does not degrade or dissipate in the body, has sufficiently low viscosity such that it is easily administered via injection, and will not migrate from the site of injection, thereby enabling the affected tissue to maintain the desired constriction without causing undesirable side effects. In addition, the invention generally relates to an injection method that reduces the injection pressure required to place the bulking agents.[0013]
- In one aspect the invention relates to the use of polymeric particles to facilitate bulking in a treatment region of a mammal's body through injection of the particles into the treatment region. The particles are compliant enough to be delivered through a relatively small gauge injection device. Generally, the invention is employed in the treatment of diseases requiring sphincter bulking, e.g., for treating urinary or fecal incontinence; however, the bulking method described herein can also be used for soft tissue bulking for use during, for example, plastic surgery.[0014]
- In another aspect the invention relates to a bulking agent for medical applications. The bulking agent includes a carrier and a plurality of substantially spherical polyvinyl alcohol particles dispersed within the carrier. The carrier aids the delivery of the substantially spherical polyvinyl alcohol particles to a site to be bulked.[0015]
- In yet another aspect, the invention relates to a method for bulking mammalian tissue. The method includes the steps of introducing a bulking agent to the mammalian tissue to coapt the mammalian tissue with the bulking agent. The bulking agent includes a carrier and a plurality of substantially spherical polyvinyl alcohol particles dispersed within the carrier. The carrier aids the delivery of the substantially spherical polyvinyl alcohol particles to a site to be bulked.[0016]
- In various embodiments of the foregoing aspects, the bulking agent comprises a volume. The volume could be, for example, from about 1 ml to about 30 ml, from about 20 ml to about 30 ml, or from about 2 ml to about 16 ml. In additional embodiments, the substantially spherical polyvinyl alcohol particles are sized from about 40 micron to about 1500 microns in diameter, preferably from about 150 micron to about 1100 microns in diameter, and more preferably from about 500 micron to about 900 microns in diameter. Further, the substantially spherical polyvinyl alcohol particles can comprise pores and/or bioreactive agents, such as drugs, proteins, genes, chemo-therapeutic agents, and growth factors. In other embodiments, the substantially spherical polyvinyl alcohol particles can be compressible and/or substantially dimensionally stable.[0017]
- In additional embodiments, the carrier can be a water-based solution, such as saline solution. In addition, the carrier can include at least one of a lubricant, a biocompatible thickening agent, or a color. Furthermore, the bulking agent can be delivered through a needle and/or a catheter. In one embodiment, the bulking agent is delivered transuretherally. In addition, the bulking agent can be delivered while viewing the tissue to be bulked with a cytoscope.[0018]
- In still another aspect, the invention relates to an apparatus for bulking mammalian tissue. The apparatus includes a needle defining a lumen, an inflation device adapted to advance through the lumen of the needle, and a bulking agent insertable via the lumen of the needle. The needle is adapted to penetrate the mammalian tissue. The inflation device is disposed adjacent to the mammalian tissue after being advanced through the needle. The inflation device is inflatable and subsequently deflatable to create a void in the mammalian tissue. The bulking agent is inserted to fill at least partially the void in the tissue, the bulking agent coapting the mammalian tissue.[0019]
- In various embodiments of the foregoing aspect of the invention, the inflation device can include a biocompatible balloon, and/or a color coating for visualization made from at least one of a silicone, an ethylene vinyl alcohol, a polypropylene, a latex rubber, a polyurethane, a polyester, a nylon, or a thermoplastic rubber. Additionally, the inflation device can have a shape selected from the group consisting of substantially round, oval, hemi spherical, spherical, or oblong. In one embodiment, the needle is sized from 16 gauge to 24 gauge, preferably from 18 gauge to 22 gauge.[0020]
- In additional embodiments, the bulking agent comprises a plurality of polymeric particles and can be injected into the void by a syringe. In one embodiment, the bulking agent includes a carrier and a plurality of substantially spherical polyvinyl alcohol particles dispersed within the carrier. The carrier aids the delivery of the substantially spherical polyvinyl alcohol particles to a site to be bulked. The bulking agent can further include a color.[0021]
- In yet another aspect, the invention relates to a method for bulking mammalian tissue. The method includes the steps of inserting an inflation device within a portion of a mammal, inflating the inflation device to compress the mammalian tissue surrounding the inflated inflation device, thereby creating a void in the tissue, deflating the inflation device, removing the inflation device from the mammal, and providing a bulking agent to at least partially fill the void, the bulking agent coapting the mammalian tissue.[0022]
- In various embodiments of this aspect of the invention, the method includes the steps of inserting a needle with a penetration device into the mammalian tissue, removing the penetration device while retaining the inserted needle, and advancing the inflation device through the needle. The needle can be sized from 16 gauge to 24 gauge, preferably 18 gauge to 22 gauge. The method can also include the step of viewing the tissue to be bulked with a cytoscope. In one embodiment, the inflation device can include a biocompatible balloon, and/or a color coating for visualization made from at least one of a silicone, an ethylene vinyl alcohol, a polypropylene, a latex rubber, a polyurethane, a polyester, a nylon and a thermoplastic rubber. Additionally, the inflation device can have a shape selected from the group consisting of substantially round, oval, hemi spherical, spherical, or oblong.[0023]
- In additional embodiments, the bulking agent comprises a plurality of polymeric particles and can be injected into the void by a syringe. In another embodiment, the substantially spherical polyvinyl alcohol particles are coated, embedded, or filled with a material that will aid the delivery of the particles to a site to be bulked. In one embodiment, the bulking agent includes a carrier and a plurality of substantially spherical polyvinyl alcohol particles dispersed within the carrier. The carrier aids the delivery of the substantially spherical polyvinyl alcohol particles to a site to be bulked. The bulking agent can further include a color.[0024]
- These and other objects, along with advantages and features of the present invention, will become apparent through reference to the following description, the accompanying drawings, and the claims. Furthermore, it is to be understood that the features of the various embodiments described herein are not mutually exclusive and can exist in various combinations and permutations.[0025]
- In the drawings, like reference characters generally refer to the same parts throughout the different views. Also, the drawings are not necessarily to scale, emphasis generally being placed upon illustrating the principles of the invention. In the following description, various embodiments of the present invention are described with reference to the following drawings, in which:[0026]
- FIG. 1 depicts a side view of a tissue structure with an enlarged lumen surrounded by muscle tissue;[0027]
- FIG. 2 depicts the tissue structure of FIG. 1 immediately after a bulking agent in accordance with the invention has been injected around the enlarged lumen of the tissue;[0028]
- FIG. 3 depicts the tissue structure of FIG. 1 immediately after a bulking agent in accordance with the invention has been injected around the enlarged lumen of the tissue utilizing a cystoscope-aided injection method;[0029]
- FIG. 4 is a schematic plan view of a needle assembly in accordance with the invention;[0030]
- FIG. 5 is a schematic plan view of the needle assembly of FIG. 4 with the trocar/obtuator assembly being removed;[0031]
- FIG. 6 is a schematic plan view of the needle assembly of FIG. 4 with a balloon assembly being inserted into the needle assembly;[0032]
- FIG. 7 is a schematic plan view of the needle assembly of FIG. 4 with a syringe attached to the needle assembly for inflating the balloon;[0033]
- FIG. 8 is a schematic plan view of the assembly of FIG. 7 with the syringe and balloon assembly being removed;[0034]
- FIG. 9 is a schematic plan view of the assembly of FIG. 4 with another syringe attached to the needle assembly for injecting a bulking agent into tissue;[0035]
- FIG. 10 is a pictorial representation of a method of creating a void within a patient's tissue by inserting and inflating a balloon; and[0036]
- FIG. 11 is a pictorial representation of a method of filling the void within the patient's tissue with a bulking agent.[0037]
- Embodiments of the present invention are described below. The invention is not limited, however, to these embodiments. For example, various embodiments of the invention are described in terms of treating incontinence; however, embodiments of the invention may be used in other applications, such as cosmetic reconstruction.[0038]
- Referring to FIG. 1, a tissue structure, more specifically a urethra/[0039]
ureter 10, having awall 20 and anenlarged lumen 30 surrounded bymuscle tissue 40 is shown in side view. Before theenlarged lumen 30 is constricted with the bulking composition, acystoscope 50 comprising a fiberopticlight transmitting element 60, a workingchannel 70 and aviewing element 80 encased in asheath 90 may be inserted in the urethra/ureter 10 to a distance close to theenlarged lumen 30. The close distance is selected to allow a clear view of theenlarged lumen 30. - Referring to FIG. 2, the urethra/[0040]
ureter 10 is shown immediately after a bulking agent in accordance with the invention has been injected around theenlarged lumen 30 of the tissue. Once theenlarged lumen 30 is readily in view, ahypodermic needle 100 is inserted through thetissue 40, preferably over theenlarged lumen 30, stopping near thewall 20 of theenlarged lumen 30. Thereafter, a bulkingagent 110 includingpolymeric particles 120 is injected via thehypodermic needle 100 into thetissue 40 adjacent thewall 20. The result is aconstricted region 130 located in the vicinity of the accumulation of the bulkingagent 110. - Alternatively, referring to FIG. 3, the urethra/[0041]
ureter 10 is shown immediately after thebulking agent 110 of the present invention has been injected around theenlarged lumen 30 of thetissue 40 utilizing acystoscope 50 aided injection method in accordance with another embodiment of the invention. Anelongate needle 140 may be inserted through the workingchannel 70 into the urethra/ureter 10 and the surroundingtissue 40 and the injection can be completed operating solely through thecystoscope 50. This is generally the preferred method of operation on male patients for the area surrounding the urethra/ureter and is the preferred method for female patients for the area surrounding the ureter. - Furthermore, the present invention relates to a bulking agent including substantially spherical polyvinyl alcohol particles used to facilitate bulking in a region of the human body through injection of the particles into the treatment region. The particles are compliant enough to be delivered through a substantially small-gauge injection device. In one embodiment, the particles are 50% compressible. This is accomplished through the use of particles that are adapted to compress as they pass through the small gauge injection device. In one embodiment, a 16 to 24 gauge needle is used to dispense the bulking composition without clogging. In other applications, other size needles may be preferred, for example 18-22 gauge.[0042]
- Filling the space surrounding the urethra/ureter allows the sphincter to be more readily coapted by the patient to maintain continence. Generally, the present invention is employed in the treatment of diseases requiring bulking, e.g., urinary or fecal incontinence. Some examples of conditions that can be treated by way of the present invention include urinary incontinence, vesicourethral reflux, fecal incontinence and intrinsic sphincter deficiency or ISD. However, the bulking method described herein can also be used for soft tissue bulking for use during, for example, plastic surgery.[0043]
- In greater detail, the method of providing a bulking agent to the human body includes using polymeric particles, such as polyvinyl alcohol, as a bulking agent and injecting the particles into the treatment region of the human body. An advantage of the present invention is that the particles are substantially non-biodegradable, thereby virtually eliminating the need for replenishing the particles to maintain efficacy. A further advantage of the present invention is that the substantially spherical size and shape of the particles allows for close packing of the particles in the treatment space.[0044]
- In one embodiment, the particles are made of a water and polyvinyl alcohol mixture. For a description of particles contemplated for use with the present invention, see U.S. patent application Ser. Nos. 10/232,265, 10/215,594, 10/116,330, 10/109,966, 10/231,664, the disclosures of which are hereby incorporated by reference herein in their entirety. Generally, water, polyvinyl alcohol, and alginate are combined and pumped through a nozzle under pressure, generating substantially spherically-shaped droplets. The substantially spherically-shaped droplets encounter a solution that promotes cross-linking of the polyvinyl alcohol. Subsequently, the alginate is removed from the outer surface. The result is a substantially spherically-shaped particle that is substantially all polyvinyl alcohol.[0045]
- To facilitate other treatments, dosages of bio-active agents can be added to the particles. For example, substances, such as drugs, growth factors, proteins, genes, and chemo-therapeutic agents can be added to the particles to enhance localized treatments while still providing significant bulking benefits. The particles themselves are substantially inert in that they do not tend to react with body fluids and/or tissue. For example, many other types of bulking particles swell in use. In contrast thereto, the substantially spherical polyvinyl alcohol particles are substantially dimensionally stable. Some tissue growth on, near, or around the particle surface may occur, but no biological interaction between the tissue and the particles is expected.[0046]
- In one embodiment, the particles are substantially solid. In a particular embodiment, the particles are substantially spherically-shaped and are sized in a range of about 40 microns to about 1500 microns in diameter, preferably about 150 microns to about 1100 microns in diameter, and more preferably about 500 microns to about 900 microns in diameter. The size of the particles chosen for a particular application will be determined by a number of factors. Smaller particles are easier to inject with a smaller gauge size needle; however, embolization due to migration of the particles is a concern with the smaller particle sizes. The size of the particles used in a particular procedure will include consideration of the procedure employed, disease progression, the degree of degradation of the affected region, patient size, the disposition of the patient, and the preferences and techniques of the doctor performing the procedure. Similarly, such factors must be considered when determining the proper volume of bulking agent to inject into a patient. In one embodiment of the invention, the volume of bulking composition is about 1 ml to about 30 ml, and preferably about 20 ml to about 30 ml. In another embodiment, the volume of bulking composition injected into a patient is about 2 ml to about 16 ml. However, these amounts can vary significantly based on the doctor's determination as to when the target region is sufficiently bulked up.[0047]
- To vary compressibility, provide for absorption of medications, or for the purpose of incorporating the particles into the surrounding tissue, the porosity of the particles may be modified. These effects, if desired, can be enhanced by increasing pore size. For example, tissue in-growth can be encouraged by increasing pore size. Preferably, pore sizes are within a range of about 4 microns to about 5 microns up to about 30 microns to about 50 microns. In one embodiment, the pores cover up to 80% of the surface area of the particle.[0048]
- In one embodiment, the bulking particles are injected through a needle. In other embodiments, a cystoscope is used to allow for viewing the injection area. The bulking particles can be supplemented with a contrast agent to enhance their appearance as an aid to the doctor performing the procedure. Other methods of visual enhancement to assist in viewing of the bulking agent can also be employed. Injection of the particles can also be accomplished transuretherally by, for example, using a catheter.[0049]
- In another embodiment, the method of providing the bulking agent to the human body further includes mixing the bulking particles with a carrier such that the particles are suspended in the carrier, and then injecting the particles-carrier mix into the treatment portion of the human body. The carrier serves as a lubricant for the particles thereby increasing the ease with which the particles move into the body. In another embodiment, the carrier is a saline solution. In other embodiments bio-compatible thickening agents such as alginate, beta-glucan, glycerin, cellulose, or collagen are added to the carrier or serve as the carrier themselves to modify the viscosity of the carrier. By varying the carrier viscosity, proper disbursement of the bulking particles can be accomplished; however, carriers must not be so viscous that their passage through an injection device is inhibited. In yet another embodiment, the carrier may be bio-active, that is the carrier includes an anti-microbial agent, or the like.[0050]
- The present invention also relates to a method used to dilate tissue within a treatment tissue region to facilitate injection of the bulking agent. The method includes: inserting a needle with a penetration device (e.g., a taper point obtuator or trocar) into the treatment region (e.g., the sphincter region) (FIG. 4); removing the penetration device while retaining the inserted needle (FIG. 5); advancing a balloon through the needle (FIG. 6); inflating the balloon, thereby creating a void in the treatment region (FIG. 7); deflating and removing the balloon from the treatment region (FIG. 8); affixing a syringe with a bulking agent to the needle and injecting the bulking agent into the tissue void (FIG. 9). This procedure can be repeated as necessary in order to maximize the effectiveness of the bulking agent and to achieve the desired results.[0051]
- The method and apparatus for carrying out the method in a method to treat urinary incontinence by bulking the urethral tissue is described generally with reference to FIGS.[0052]4-11. A
needle 400, such as a blunt-end hypotube or hypodermic needle having a first end and a second end, is adapted to accept apenetration device 404, such as a taper point obtuator or a trocar, at the first end of the needle400 (FIG. 4). Theneedle 400 may range in size from about 18 gauge to about 22 gauge, and preferably about 20 gauge to about 22 gauge. Thepenetration device 404 is attached to theneedle 400 to enable penetration of theneedle 400 into the tissue. Thepenetration device 404 may be adapted to theneedle 400 by way of a luer hub or fitting, and in one embodiment, a male luer hub is used. Theneedle 400 is inserted with thepenetration device 404 into the treatment region420 (e.g., the sphincter region)(FIG. 10) to the desired depth. In one embodiment, desired penetration depth can be determined by striping406 located on thepenetration device 404. In one embodiment, the amount of penetration of thepenetration device 404 ranges from about 2 cm to about 2.5 cm (FIG. 4). In one embodiment, the amount of tissue penetration of theneedle 400 ranges from about 0.5 cm to about 1 cm beyond the tissue line407 (FIG. 5). Thepenetration device 404 is removed while retaining the inserted needle400 (FIG. 6). - A[0053]
luer hub 402 or fitting, or in one embodiment a female luer hub, may be adapted to the second end of theneedle 400, to which asyringe 412,418 (FIGS.7-9) is adapted. Referring to FIG. 4, theluer hub 402 is depicted in its locked position, and in FIG. 5 theluer hub 402 is depicted in its unlocked position. In the locked position, theluer hub 402 can be positioned for inflating theballoon 408 or injecting abulking agent 416. In the unlocked position, theluer hub 402 can be positioned for accepting theballoon 408 for insertion or for removal of theballoon 408 after dilation. - The[0054]
balloon 408 is adapted to advance through a lumen of theneedle 400, and an adapter on theballoon 408 provides a means to lock theballoon 408 to theluer hub 402, which in turn adapts to the syringe412 (FIG. 6). Theballoon 408 may have no tip or, alternatively, theballoon 408 may have a small stump appendage, which may remain from processing of the balloon. In one embodiment, theballoon 408 is affixed to an end of a plastic tube410 (FIG. 6). In another embodiment, the tip for theballoon 408 is integral with a shaft. In yet another embodiment,balloon 408 includes at least one fill and/or evacuation port. - In one embodiment, the balloon is a colored balloon (e.g., blue) to facilitate remote visualization of the procedure and proper placement of the balloon. Alternatively, the balloon could be clear to transparent and the inflation media could be colored, for example, a colored saline solution. The balloon may be semi-compliant or non-compliant. The balloon may be manufactured from any suitable material, for example, a polymer. Some examples of suitable balloon materials include: silicone, ethylene vinyl acetate (EVA), polypropylene, latex rubber, polyurethane, polyester, nylon and thermoplastic rubber. In one embodiment, the balloon is inflated to, for example, about 3 cm to about 5 cm in diameter. The balloon may assume a variety of shapes. Some shapes that may be considered, depending upon the attendant requirements of the procedure, include substantially round, oval, hemi spherical, and oblong. The length of the balloon may vary depending upon the procedure. In one embodiment, the inflated balloon may have a length in the range of, for example, about 3 cm to about 10 cm. Other balloon configurations may be employed, and the types and methods used to employ the most suitable balloon configurations for a particular application of this invention will be obvious to those skilled in the art.[0055]
- The[0056]
balloon 408 is then inflated using an inflation device, such as thesyringe 412, creating a void in the treatment region (FIGS. 7 and 8). The balloon may be colored (i.e. blue) to aid in visibility through the tissue. As theballoon 408 expands, theballoon 408 becomes visible to aid in proper balloon placement. For example, the expandingballoon 408 may become visible under the urethra as it thins. In one embodiment, theballoon 408 inflates to a volume of about 1 cc to about 1.5 cc, although such volumes may vary depending upon many factors inherent in the characteristics of the particular application, some of which were discussed previously. In another embodiment, saline is used to inflate theballoon 408. In yet another embodiment, about 3 cc of saline is placed in thesyringe 412 and injected into theballoon 408 for inflation. - The[0057]
balloon 408 is then deflated and removed from the treatment region, resulting in atissue void 414 where theinflated balloon 408 previously resided (FIGS. 8 and 10). Theballoon 408 is removable through the lumen of theneedle 400. In one embodiment, a plastic tube orother tip 410 is used to aid in removal of theballoon 408. - A syringe or[0058]
other injection device 418 containing the bulkingagent 416 is then affixed to theneedle 400 by way of theluer hub 402. The plunger of thesyringe 418 is then depressed, thereby injecting the bulkingagent 416 into the tissue void414 (FIGS. 9 and 11). - While the invention has been shown and described with reference to specific embodiments, it should be understood by those skilled in the art that various changes in form and detail may be made therein without departing from the spirit and scope of the invention.[0059]
- Having thus described certain embodiments of the present invention, various alterations, modifications, and improvements will be apparent to those of ordinary skill. Such alterations, modifications, and improvements are within the spirit and scope of the invention, and the foregoing description of certain embodiments is not exhaustive or limiting.[0060]
Claims (33)
Priority Applications (5)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US10/459,895US20040037887A1 (en) | 2002-06-12 | 2003-06-12 | Bulking agent |
| US12/946,990US8394400B2 (en) | 2002-06-12 | 2010-11-16 | Bulking agent |
| US13/792,346US8586071B2 (en) | 2002-06-12 | 2013-03-11 | Bulking agents |
| US14/082,274US10398724B2 (en) | 2002-06-12 | 2013-11-18 | Bulking agents |
| US16/519,450US10813945B2 (en) | 2002-06-12 | 2019-07-23 | Bulking agents |
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US38844602P | 2002-06-12 | 2002-06-12 | |
| US10/459,895US20040037887A1 (en) | 2002-06-12 | 2003-06-12 | Bulking agent |
Related Child Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US12/946,990DivisionUS8394400B2 (en) | 2002-06-12 | 2010-11-16 | Bulking agent |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| US20040037887A1true US20040037887A1 (en) | 2004-02-26 |
Family
ID=29736473
Family Applications (5)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US10/459,895AbandonedUS20040037887A1 (en) | 2002-06-12 | 2003-06-12 | Bulking agent |
| US12/946,990Expired - Fee RelatedUS8394400B2 (en) | 2002-06-12 | 2010-11-16 | Bulking agent |
| US13/792,346Expired - Fee RelatedUS8586071B2 (en) | 2002-06-12 | 2013-03-11 | Bulking agents |
| US14/082,274Expired - Fee RelatedUS10398724B2 (en) | 2002-06-12 | 2013-11-18 | Bulking agents |
| US16/519,450Expired - Fee RelatedUS10813945B2 (en) | 2002-06-12 | 2019-07-23 | Bulking agents |
Family Applications After (4)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US12/946,990Expired - Fee RelatedUS8394400B2 (en) | 2002-06-12 | 2010-11-16 | Bulking agent |
| US13/792,346Expired - Fee RelatedUS8586071B2 (en) | 2002-06-12 | 2013-03-11 | Bulking agents |
| US14/082,274Expired - Fee RelatedUS10398724B2 (en) | 2002-06-12 | 2013-11-18 | Bulking agents |
| US16/519,450Expired - Fee RelatedUS10813945B2 (en) | 2002-06-12 | 2019-07-23 | Bulking agents |
Country Status (5)
| Country | Link |
|---|---|
| US (5) | US20040037887A1 (en) |
| EP (1) | EP1511522B1 (en) |
| AU (1) | AU2003240000A1 (en) |
| CA (1) | CA2492339A1 (en) |
| WO (1) | WO2003105917A2 (en) |
Cited By (25)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US20030161887A1 (en)* | 2002-02-27 | 2003-08-28 | Klein Dean A. | Lower esophagus tissue modifier |
| US20040010245A1 (en)* | 1999-06-22 | 2004-01-15 | Cerier Jeffrey C. | Method and devices for tissue reconfiguration |
| US20040193193A1 (en)* | 1999-06-22 | 2004-09-30 | Ndo Surgical, Inc., A Massachusetts Corporation | Tissue reconfiguration |
| US20040267357A1 (en)* | 2003-04-30 | 2004-12-30 | Allen Jeffrey W. | Cardiac valve modification method and device |
| US20050033328A1 (en)* | 1999-06-22 | 2005-02-10 | Ndo Surgical, Inc., A Massachusetts Corporation | Methods and devices for tissue reconfiguration |
| US20050288639A1 (en)* | 2004-06-25 | 2005-12-29 | Hibner Michael C | Instrument used in treatment of the urinary incontinence in women |
| US20060025789A1 (en)* | 1999-06-22 | 2006-02-02 | Ndo Surgical, Inc., A Massachusetts Corporation | Methods and devices for tissue reconfiguration |
| US20060058892A1 (en)* | 2004-09-16 | 2006-03-16 | Lesh Michael D | Valved tissue augmentation implant |
| US20060058735A1 (en)* | 2004-09-16 | 2006-03-16 | Lesh Michael D | Systems and devices for soft tissue augmentation |
| US20060058891A1 (en)* | 2004-09-16 | 2006-03-16 | Lesh Michael D | Transformable tissue bulking device |
| US20060088568A1 (en)* | 2004-10-26 | 2006-04-27 | Tropsha Yelena G | Implantation of tissue bulking devices |
| US20060161253A1 (en)* | 2004-09-16 | 2006-07-20 | Michael Lesh | Tissue augmentation device |
| US20060246033A1 (en)* | 2005-03-02 | 2006-11-02 | Cook Biotech Incorporated | Injectable bulking agent compositions |
| US20060251697A1 (en)* | 2005-05-09 | 2006-11-09 | Jamie Li | Injectable bulking compositions |
| US20060264912A1 (en)* | 2005-05-09 | 2006-11-23 | Mcintyre Jon T | Medical devices for treating urological and uterine conditions |
| US20080234703A1 (en)* | 2007-03-23 | 2008-09-25 | Ethicon Endo-Surgery, Inc. | Tissue approximation system |
| US20080275569A1 (en)* | 2004-09-16 | 2008-11-06 | Evera Medical, Inc | Tissue Augmentation Device |
| US7553273B2 (en) | 2006-05-01 | 2009-06-30 | Duodyn Technology, Llc | Apparatus and method for managing incontinence |
| US20090198331A1 (en)* | 2008-02-01 | 2009-08-06 | Kesten Randy J | Implantable prosthesis with open cell flow regulation |
| US7846180B2 (en) | 1999-06-22 | 2010-12-07 | Ethicon Endo-Surgery, Inc. | Tissue fixation devices and methods of fixing tissue |
| US20130096552A1 (en)* | 2011-10-14 | 2013-04-18 | Christopher L. Brace | Hydrodissection Material with Reduced Migration |
| US8852216B2 (en) | 2007-03-23 | 2014-10-07 | Ethicon Endo-Surgery, Inc. | Tissue approximation methods |
| US9333070B2 (en) | 2008-02-01 | 2016-05-10 | Evera Medical, Inc. | Breast implant with internal flow dampening |
| CN110201419A (en)* | 2019-05-09 | 2019-09-06 | 西安交通大学 | It is a kind of using polyvinyl alcohol microparticles as membrane flexibility column of carrier and preparation method thereof |
| US10448955B2 (en)* | 2006-01-30 | 2019-10-22 | Biosphere Medical, Inc. | Compressible intravascular embolization particles and related methods and delivery systems |
Families Citing this family (9)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| AU2003240000A1 (en)* | 2002-06-12 | 2003-12-31 | Boston Scientific Limited | Bulking agents |
| US7632291B2 (en) | 2003-06-13 | 2009-12-15 | Trivascular2, Inc. | Inflatable implant |
| PL2124831T3 (en) | 2007-03-15 | 2017-03-31 | Ortho-Space Ltd. | Prosthetic devices |
| US9289307B2 (en) | 2011-10-18 | 2016-03-22 | Ortho-Space Ltd. | Prosthetic devices and methods for using same |
| US10322269B1 (en) | 2015-01-19 | 2019-06-18 | Dalent, LLC | Dilator device |
| WO2017046647A1 (en) | 2015-09-18 | 2017-03-23 | Ortho-Space Ltd. | Intramedullary fixated subacromial spacers |
| CN105852918A (en)* | 2016-04-21 | 2016-08-17 | 中国人民解放军南京军区南京总医院 | Injection and expansion integrated device |
| EP3573806A4 (en) | 2017-01-30 | 2019-12-11 | Ortho-Space Ltd. | Processing machine and methods for processing dip-molded articles |
| USD877325S1 (en) | 2019-06-06 | 2020-03-03 | Dalent, LLC | Inflatable therapeutic treatment balloon device |
Citations (95)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US3663470A (en)* | 1969-06-05 | 1972-05-16 | Kanegafuchi Spinning Co Ltd | Method of producing polyvinyl acetal porous articles and the shaped porous articles made therefrom |
| US3737398A (en)* | 1969-11-13 | 1973-06-05 | D Yamaguchi | Method of making a polyvinyl acetal sponge buff |
| US3957933A (en)* | 1975-03-05 | 1976-05-18 | General Atomic Company | Apparatus for producing microspherical particles and method for operating such apparatus |
| US4025686A (en)* | 1975-06-26 | 1977-05-24 | Owens-Corning Fiberglas Corporation | Molded composite article and method for making the article |
| US4076640A (en)* | 1975-02-24 | 1978-02-28 | Xerox Corporation | Preparation of spheroidized particles |
| US4191672A (en)* | 1976-10-25 | 1980-03-04 | Berger Jenson & Nicholson Ltd. | Polymer aggregates |
| US4198318A (en)* | 1978-11-24 | 1980-04-15 | Conoco, Inc. | Production of high strength alumina spheres by hydrogelling corresponding slurries |
| US4243794A (en)* | 1978-10-10 | 1981-01-06 | Minnesota Mining And Manufacturing Company | Mixture of rough and spheroidized resin particles |
| US4246208A (en)* | 1979-03-22 | 1981-01-20 | Xerox Corporation | Dust-free plasma spheroidization |
| US4266030A (en)* | 1978-08-07 | 1981-05-05 | Basf Aktiengesellschaft | Macroporous polymeric carrier for covalently binding proteins, its preparation and its use for fixing active proteins |
| US4268495A (en)* | 1979-01-08 | 1981-05-19 | Ethicon, Inc. | Injectable embolization and occlusion solution |
| US4271281A (en)* | 1980-05-29 | 1981-06-02 | American Hoechst Corporation | Process for preparing styrenic polymer particles |
| US4427794A (en)* | 1980-08-22 | 1984-01-24 | Bayer Aktiengesellschaft | Process for the preparation of bead polymers of uniform particle size by polymerization of microencapsulated monomer |
| US4429062A (en)* | 1980-02-18 | 1984-01-31 | Emil Pasztor | Pharmaceutically acceptable silicon rubber and therapeutical set and the use thereof for surgical embolization |
| US4428869A (en)* | 1981-08-20 | 1984-01-31 | International Flavors & Fragrances Inc. | Cologne consisting of microcapsule suspension |
| US4444961A (en)* | 1980-10-30 | 1984-04-24 | The Dow Chemical Company | Process and apparatus for preparing uniform size polymer beads |
| US4492720A (en)* | 1983-11-15 | 1985-01-08 | Benjamin Mosier | Method of preparing microspheres for intravascular delivery |
| US4573967A (en)* | 1983-12-06 | 1986-03-04 | Eli Lilly And Company | Vacuum vial infusion system |
| US4657756A (en)* | 1980-11-17 | 1987-04-14 | Schering Aktiengesellschaft | Microbubble precursors and apparatus for their production and use |
| US4661137A (en)* | 1984-06-21 | 1987-04-28 | Saint Gobain Vitrage | Process for producing glass microspheres |
| US4663358A (en)* | 1985-05-01 | 1987-05-05 | Biomaterials Universe, Inc. | Porous and transparent poly(vinyl alcohol) gel and method of manufacturing the same |
| US4742086A (en)* | 1985-11-02 | 1988-05-03 | Lion Corporation | Process for manufacturing porous polymer |
| US4743507A (en)* | 1986-09-12 | 1988-05-10 | Franses Elias I | Nonspherical microparticles and method therefor |
| US4795741A (en)* | 1987-05-06 | 1989-01-03 | Biomatrix, Inc. | Compositions for therapeutic percutaneous embolization and the use thereof |
| US4801458A (en)* | 1985-06-24 | 1989-01-31 | Teijin Limited | Sustained release pharmaceutical plaster |
| US4804366A (en)* | 1987-10-29 | 1989-02-14 | Baxter International Inc. | Cartridge and adapter for introducing a beneficial agent into an intravenous delivery system |
| US4819637A (en)* | 1987-09-01 | 1989-04-11 | Interventional Therapeutics Corporation | System for artificial vessel embolization and devices for use therewith |
| US4822535A (en)* | 1985-07-12 | 1989-04-18 | Norsk Hydro A.S. | Method for producing small, spherical polymer particles |
| US4833237A (en)* | 1984-07-31 | 1989-05-23 | Fuji Spinning Co., Ltd. | Process for producing granular porous chitosan |
| US4929400A (en)* | 1986-04-28 | 1990-05-29 | California Institute Of Technology | Production of monodisperse, polymeric microspheres |
| US4981625A (en)* | 1988-03-14 | 1991-01-01 | California Institute Of Technology | Monodisperse, polymeric microspheres produced by irradiation of slowly thawing frozen drops |
| US4990340A (en)* | 1986-01-22 | 1991-02-05 | Teijin Limited | Sustained release pharmaceutical preparation |
| US4999188A (en)* | 1983-06-30 | 1991-03-12 | Solodovnik Valentin D | Methods for embolization of blood vessels |
| US5007940A (en)* | 1989-06-09 | 1991-04-16 | American Medical Systems, Inc. | Injectable polymeric bodies |
| US5011677A (en)* | 1984-11-19 | 1991-04-30 | The Curators Of The University Of Missouri | Radioactive glass microspheres |
| USH915H (en)* | 1985-07-22 | 1991-05-07 | Gibbs Marylu B | Controlled macroporous copolymer properties by removal of impurities in the diluent |
| US5079274A (en)* | 1989-03-15 | 1992-01-07 | The Dow Chemical Company | Process for preparing absorptive porous resin beads |
| US5106903A (en)* | 1984-12-17 | 1992-04-21 | Lehigh University | Preparation of large particle size monodisperse latexes |
| US5114421A (en)* | 1986-09-22 | 1992-05-19 | Polak Robert B | Medicament container/dispenser assembly |
| US5116387A (en)* | 1989-06-09 | 1992-05-26 | American Medical Systems, Inc. | Preparation of injectable polymeric bodies |
| US5190766A (en)* | 1990-04-16 | 1993-03-02 | Ken Ishihara | Method of controlling drug release by resonant sound wave |
| US5202352A (en)* | 1990-08-08 | 1993-04-13 | Takeda Chemical Industries, Ltd. | Intravascular embolizing agent containing angiogenesis-inhibiting substance |
| US5288763A (en)* | 1992-12-23 | 1994-02-22 | The Johns Hopkins University School Of Medicine | Porous, polymer beads and process of their preparation |
| US5292814A (en)* | 1987-04-29 | 1994-03-08 | Ernst Bayer | Process for the preparation of monodispersed polymer beads |
| US5314974A (en)* | 1992-04-10 | 1994-05-24 | Mitsubishi Kasei Corporation | Method for producing a spherical acrylonitrile crosslinked copolymer |
| US5316774A (en)* | 1990-06-20 | 1994-05-31 | Advanced Polymer Systems, Inc. | Blocked polymeric particles having internal pore networks for delivering active substances to selected environments |
| US5382260A (en)* | 1992-10-30 | 1995-01-17 | Interventional Therapeutics Corp. | Embolization device and apparatus including an introducer cartridge and method for delivering the same |
| US5384124A (en)* | 1988-07-21 | 1995-01-24 | Farmalyoc | Solid porous unitary form comprising micro-particles and/or nano-particles, and its preparation |
| US5385561A (en)* | 1994-01-18 | 1995-01-31 | Bard International, Inc. | Apparatus and method for injecting a viscous material into the tissue of a patient |
| US5397303A (en)* | 1993-08-06 | 1995-03-14 | River Medical, Inc. | Liquid delivery device having a vial attachment or adapter incorporated therein |
| US5398851A (en)* | 1993-08-06 | 1995-03-21 | River Medical, Inc. | Liquid delivery device |
| US5403870A (en)* | 1989-05-31 | 1995-04-04 | Kimberly-Clark Corporation | Process for forming a porous particle of an absorbent polymer |
| US5417982A (en)* | 1994-02-17 | 1995-05-23 | Modi; Pankaj | Controlled release of drugs or hormones in biodegradable polymer microspheres |
| US5484584A (en)* | 1990-10-02 | 1996-01-16 | Board Of Regents, The University Of Texas System | Therapeutic and diagnostic use of modified polymeric microcapsules |
| US5490984A (en)* | 1992-02-28 | 1996-02-13 | Jsf Consulants Ltd. | Use of injectable biomaterials for the repair and augmentation of the anal sphincters |
| US5494940A (en)* | 1991-12-20 | 1996-02-27 | Alliedsignal Inc. | Low density materials having high surface areas and articles formed therefrom |
| US5494682A (en)* | 1990-10-05 | 1996-02-27 | Massachusetts Institute Of Technology | Ionically cross-linked polymeric microcapsules |
| US5512604A (en)* | 1992-08-28 | 1996-04-30 | The Dow Chemical Company | Porous copolymers having a cellular polymeric structure suitable for preparing ion-exchange resins and adsorbents |
| US5514090A (en)* | 1990-04-24 | 1996-05-07 | Science Incorporated | Closed drug delivery system |
| US5595821A (en)* | 1994-05-04 | 1997-01-21 | Minnesota Mining And Manufacturing Company | Repulpable plastic films |
| US5622657A (en)* | 1991-10-01 | 1997-04-22 | Takeda Chemical Industries, Ltd. | Prolonged release microparticle preparation and production of the same |
| US5624685A (en)* | 1991-10-16 | 1997-04-29 | Terumo Kabushiki Kaisha | High polymer gel and vascular lesion embolizing material comprising the same |
| US5716981A (en)* | 1993-07-19 | 1998-02-10 | Angiogenesis Technologies, Inc. | Anti-angiogenic compositions and methods of use |
| US5718884A (en)* | 1992-09-16 | 1998-02-17 | Nycomed Imaging As | Microbubble-based contrast agents with crosslinked and reduced proteinaceous shells |
| US5723269A (en)* | 1992-07-24 | 1998-03-03 | Takeda Chemical Industries, Ltd. | Microparticle preparation and production thereof |
| US5725534A (en)* | 1995-01-03 | 1998-03-10 | William Cook Europe A/S | Method of manufacturing an assembly for positioning an embolization coil in the vascular system, and such an assembly |
| US5752974A (en)* | 1995-12-18 | 1998-05-19 | Collagen Corporation | Injectable or implantable biomaterials for filling or blocking lumens and voids of the body |
| US5855615A (en)* | 1996-06-07 | 1999-01-05 | Menlo Care, Inc. | Controller expansion sphincter augmentation media |
| US5863957A (en)* | 1994-06-06 | 1999-01-26 | Biopore Corporation | Polymeric microbeads |
| US5876372A (en)* | 1995-03-22 | 1999-03-02 | Abbott Laboratories | Syringe system accomodating seperate prefilled barrels for two constituents |
| US5877224A (en)* | 1995-07-28 | 1999-03-02 | Rutgers, The State University Of New Jersey | Polymeric drug formulations |
| US5885216A (en)* | 1993-10-28 | 1999-03-23 | Medrad, Inc. | Total system for contrast delivery |
| US5885547A (en)* | 1994-01-21 | 1999-03-23 | Paragon Medical Ltd. | Particulate material |
| US5891155A (en)* | 1995-01-27 | 1999-04-06 | Scimed Life Systems, Inc. | Embolizing system |
| US5899877A (en)* | 1994-04-28 | 1999-05-04 | Primed Halberstadt Medizintechnik Gmbh | One-piece dispensing device for the contamination-free administration of medicaments (cytostatica) |
| US5902832A (en)* | 1996-08-20 | 1999-05-11 | Menlo Care, Inc. | Method of synthesizing swollen hydrogel for sphincter augmentation |
| US6015546A (en)* | 1992-10-10 | 2000-01-18 | Quadrant Healthcare (Uk) Limited | Preparation of further diagnostic agents |
| US6028066A (en)* | 1997-05-06 | 2000-02-22 | Imarx Pharmaceutical Corp. | Prodrugs comprising fluorinated amphiphiles |
| US6027472A (en)* | 1992-08-13 | 2000-02-22 | Science Incorporated | Mixing and delivery syringe assembly |
| US6047861A (en)* | 1998-04-15 | 2000-04-11 | Vir Engineering, Inc. | Two component fluid dispenser |
| US6051247A (en)* | 1996-05-30 | 2000-04-18 | University Of Florida Research Foundation, Inc. | Moldable bioactive compositions |
| US6060053A (en)* | 1993-04-30 | 2000-05-09 | Children's Medical Center Corp. | Injectable chondrocyte-carrier suspension for treatment of vesicoureteral reflux and incontinence |
| US6059766A (en)* | 1998-02-27 | 2000-05-09 | Micro Therapeutics, Inc. | Gynecologic embolotherapy methods |
| US6063068A (en)* | 1997-12-04 | 2000-05-16 | Baxter International Inc. | Vial connecting device for a sliding reconstitution device with seal |
| US6191193B1 (en)* | 2000-01-06 | 2001-02-20 | Korea Institute Of Science & Technology | Microspheric embolic materials having a dual structure of poly(vinyl acetate) core and poly(vinyl alcohol) shell and method for preparing the same |
| US6214331B1 (en)* | 1995-06-06 | 2001-04-10 | C. R. Bard, Inc. | Process for the preparation of aqueous dispersions of particles of water-soluble polymers and the particles obtained |
| US6224630B1 (en)* | 1998-05-29 | 2001-05-01 | Advanced Bio Surfaces, Inc. | Implantable tissue repair device |
| US6224794B1 (en)* | 1998-05-06 | 2001-05-01 | Angiotech Pharmaceuticals, Inc. | Methods for microsphere production |
| US6235224B1 (en)* | 1995-07-21 | 2001-05-22 | Brown University Research Foundation | Process for preparing microparticles through phase inversion phenomena |
| US6238403B1 (en)* | 1999-10-04 | 2001-05-29 | Microvention, Inc. | Filamentous embolic device with expansible elements |
| US6335384B1 (en)* | 1996-01-31 | 2002-01-01 | Micro Therapeutics, Inc. | Methods for embolizing blood vessels |
| US6355275B1 (en)* | 2000-06-23 | 2002-03-12 | Carbon Medical Technologies, Inc. | Embolization using carbon coated microparticles |
| US6394965B1 (en)* | 2000-08-15 | 2002-05-28 | Carbon Medical Technologies, Inc. | Tissue marking using biocompatible microparticles |
| US6537574B1 (en)* | 1992-02-11 | 2003-03-25 | Bioform, Inc. | Soft tissue augmentation material |
| US6548592B1 (en)* | 2000-05-04 | 2003-04-15 | Kimberly-Clark Worldwide, Inc. | Ion-sensitive, water-dispersible polymers, a method of making same and items using same |
Family Cites Families (156)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US2609347A (en) | 1948-05-27 | 1952-09-02 | Wilson Christopher Lumley | Method of making expanded polyvinyl alcohol-formaldehyde reaction product and product resulting therefrom |
| CS179075B1 (en) | 1974-11-26 | 1977-10-31 | Stoy Vladimir | Mode of manufacture of spherical particles from polymer |
| US4098728A (en) | 1976-01-02 | 1978-07-04 | Solomon Rosenblatt | Medical surgical sponge and method of making same |
| US4055377A (en) | 1976-08-03 | 1977-10-25 | Minnesota Mining And Manufacturing Company | Magnetically orientable retroreflectorization particles |
| ES478736A1 (en) | 1978-03-23 | 1979-06-01 | Hoechst Ag | Polyvinyl alcohol pellets containing a plasticizer, and method for their preparation. |
| US4442843A (en)* | 1980-11-17 | 1984-04-17 | Schering, Ag | Microbubble precursors and methods for their production and use |
| US4681119A (en) | 1980-11-17 | 1987-07-21 | Schering Aktiengesellschaft | Method of production and use of microbubble precursors |
| NZ199916A (en) | 1981-03-11 | 1985-07-12 | Unilever Plc | Low density polymeric block material for use as carrier for included liquids |
| US4622362A (en) | 1981-03-30 | 1986-11-11 | California Institute Of Technology | Polyacrolein microspheres |
| US4678814A (en) | 1981-03-30 | 1987-07-07 | California Institute Of Technology | Polyacrolein microspheres |
| US4413070A (en) | 1981-03-30 | 1983-11-01 | California Institute Of Technology | Polyacrolein microspheres |
| US4456693A (en) | 1982-03-08 | 1984-06-26 | W. R. Grace & Co. | Hydrocracking catalyst |
| US4452773A (en) | 1982-04-05 | 1984-06-05 | Canadian Patents And Development Limited | Magnetic iron-dextran microspheres |
| US4472552A (en) | 1982-09-27 | 1984-09-18 | W. R. Grace & Co. | Continuous process for making solid, free-flowing water dispersible PVA-aldehyde reaction product |
| US4459145A (en) | 1982-09-30 | 1984-07-10 | The United States Of America As Represented By The United States Department Of Energy | Fabrication of glass microspheres with conducting surfaces |
| JPS59196738A (en) | 1983-04-21 | 1984-11-08 | Kanegafuchi Chem Ind Co Ltd | Adsorbent and preparation thereof |
| US4671954A (en) | 1983-12-13 | 1987-06-09 | University Of Florida | Microspheres for incorporation of therapeutic substances and methods of preparation thereof |
| US4568559A (en)* | 1984-02-06 | 1986-02-04 | Biotek, Inc. | Composite core coated microparticles and process of preparing same |
| US4551436A (en) | 1984-04-11 | 1985-11-05 | General Electric Company | Fabrication of small dense silicon carbide spheres |
| US4623706A (en) | 1984-08-23 | 1986-11-18 | The Dow Chemical Company | Process for preparing uniformly sized polymer particles by suspension polymerization of vibratorily excited monomers in a gaseous or liquid stream |
| US4675113A (en) | 1984-11-28 | 1987-06-23 | University Patents, Inc. | Affinity chromatography using dried calcium alginate-magnetite separation media in a magnetically stabilized fluidized bed |
| DE3568442D1 (en) | 1984-12-06 | 1989-04-06 | Kanegafuchi Chemical Ind | A method of preparation of droplets |
| JPS6245637A (en) | 1985-08-24 | 1987-02-27 | Bio Material Yunibaasu:Kk | Porous polyvinyl alcohol hydrogel microsphere |
| DE3543348A1 (en) | 1985-12-07 | 1987-06-11 | Bayer Ag | PEARL-SHAPED CROSS-NETWORKED MIXED POLYMERS WITH EPOXY AND BASIC AMINO GROUPS, METHOD FOR THE PRODUCTION THEREOF AND THEIR USE |
| US4803075A (en)* | 1986-06-25 | 1989-02-07 | Collagen Corporation | Injectable implant composition having improved intrudability |
| US4773393A (en)* | 1986-07-03 | 1988-09-27 | C. R. Bard, Inc. | Hypodermically implantable genitourinary prosthesis |
| JPS6317904A (en) | 1986-07-09 | 1988-01-25 | Mitsubishi Chem Ind Ltd | Production of crosslinked porous polyvinyl alcohol particle |
| CA1287459C (en) | 1986-10-01 | 1991-08-13 | Mukesh Jain | Process for the preparation of hollow microspheres |
| US4859711A (en) | 1986-10-01 | 1989-08-22 | Alcan International Limited | Hollow microspheres |
| JPH0612993B2 (en) | 1987-08-10 | 1994-02-23 | 株式会社クラレ | Method for producing spherical microbe-immobilized moldings |
| JPH0762054B2 (en) | 1987-10-13 | 1995-07-05 | 倉敷紡績株式会社 | Crosslinked polymer particles |
| US4850978A (en) | 1987-10-29 | 1989-07-25 | Baxter International Inc. | Drug delivery cartridge with protective cover |
| US5047438A (en) | 1988-09-26 | 1991-09-10 | Supelco, Inc. | Porous rigid resins and process of preparation |
| US4933372A (en) | 1988-09-26 | 1990-06-12 | Supelco, Inc. | Porous rigid resins and process of preparation |
| US5681576A (en) | 1988-11-16 | 1997-10-28 | Mdv Technologies, Inc. | Method and composition for post surgical adhesion reduction |
| DE3841401A1 (en) | 1988-12-08 | 1990-06-13 | Martin Lemperle | ALLOPLASTIC IMPLANT |
| US5258028A (en) | 1988-12-12 | 1993-11-02 | Ersek Robert A | Textured micro implants |
| GB8900376D0 (en) | 1989-01-09 | 1989-03-08 | Nycomed As | Iodinated esters |
| US5032117A (en) | 1989-01-30 | 1991-07-16 | Motta Louis J | Tandem syringe |
| US5354290A (en) | 1989-05-31 | 1994-10-11 | Kimberly-Clark Corporation | Porous structure of an absorbent polymer |
| US5158573A (en) | 1989-06-09 | 1992-10-27 | American Medical Systems, Inc. | Injectable polymeric bodies |
| US5253991A (en) | 1989-11-20 | 1993-10-19 | Sumitomo Cement Co., Ltd. | Apparatus for producing spheroidal inorganic particulate material |
| US5580575A (en) | 1989-12-22 | 1996-12-03 | Imarx Pharmaceutical Corp. | Therapeutic drug delivery systems |
| US5585112A (en) | 1989-12-22 | 1996-12-17 | Imarx Pharmaceutical Corp. | Method of preparing gas and gaseous precursor-filled microspheres |
| US5542935A (en) | 1989-12-22 | 1996-08-06 | Imarx Pharmaceutical Corp. | Therapeutic delivery systems related applications |
| US5435645A (en) | 1989-12-29 | 1995-07-25 | Tecres Spa | Process and apparatus for the mixing and direct emplacement of a two-component bone cement |
| US5147937A (en) | 1990-03-22 | 1992-09-15 | Rohm And Haas Company | Process for making controlled, uniform-sized particles in the 1 to 50 micrometer range |
| US5556610A (en) | 1992-01-24 | 1996-09-17 | Bracco Research S.A. | Gas mixtures useful as ultrasound contrast media, contrast agents containing the media and method |
| CA2016870C (en) | 1990-05-15 | 1994-03-29 | Arnie Drudik | Dispenser for storing and mixing several components |
| US6291605B1 (en) | 1990-06-06 | 2001-09-18 | Clarence S. Freeman | Polymerization process with spraying step |
| JPH0474117A (en) | 1990-07-16 | 1992-03-09 | Kokuritsu Eisei Shikenjo | New microsphere |
| US5120349A (en) | 1990-12-07 | 1992-06-09 | Landec Labs, Inc. | Microcapsule having temperature-dependent permeability profile |
| US5171214A (en) | 1990-12-26 | 1992-12-15 | Abbott Laboratories | Drug storage and delivery system |
| US5147631A (en) | 1991-04-30 | 1992-09-15 | Du Pont Merck Pharmaceutical Company | Porous inorganic ultrasound contrast agents |
| FR2676927B1 (en) | 1991-05-29 | 1995-06-23 | Ibf | MICROSPHERES FOR USE IN THERAPEUTIC VASCULAR OCCLUSIONS AND INJECTABLE SOLUTIONS CONTAINING THEM. |
| ATE151992T1 (en) | 1991-06-03 | 1997-05-15 | Nycomed Imaging As | IMPROVEMENTS REGARDING CONTRAST AGENTS |
| GB9116610D0 (en) | 1991-08-01 | 1991-09-18 | Danbiosyst Uk | Preparation of microparticles |
| US5216096A (en) | 1991-09-24 | 1993-06-01 | Japan Synthetic Rubber Co., Ltd. | Process for the preparation of cross-linked polymer particles |
| US5258042A (en) | 1991-12-16 | 1993-11-02 | Henry Ford Health System | Intravascular hydrogel implant |
| US5260002A (en) | 1991-12-23 | 1993-11-09 | Vanderbilt University | Method and apparatus for producing uniform polymeric spheres |
| DE69233638T2 (en) | 1991-12-24 | 2007-07-12 | E.I. Du Pont De Nemours And Co., Wilmington | TWO STABILIZED MICROPARTICLES |
| GB9200391D0 (en) | 1992-01-09 | 1992-02-26 | Nycomed As | Improvements in or relating to contrast agents |
| GB9200388D0 (en) | 1992-01-09 | 1992-02-26 | Nycomed As | Improvements in or relating to contrast agents |
| HUT68612A (en) | 1992-03-06 | 1995-07-28 | Nycomed Imaging As | New contrast agents |
| JPH05265477A (en) | 1992-03-23 | 1993-10-15 | Pioneer Electron Corp | Sound field correcting device |
| ES2118953T3 (en) | 1992-04-06 | 1998-10-01 | Uroplasty Inc | TREATMENT OF DYSFUNCTIONS THROUGH INJECTION OF MICROPARTICLES. |
| US6235313B1 (en) | 1992-04-24 | 2001-05-22 | Brown University Research Foundation | Bioadhesive microspheres and their use as drug delivery and imaging systems |
| JPH0657012A (en) | 1992-08-11 | 1994-03-01 | Kuraray Co Ltd | Method for producing polyvinyl alcohol gel molding |
| US6592859B1 (en) | 1992-08-20 | 2003-07-15 | Ethicon, Inc. | Controlled expansion sphincter augmentation media |
| US5282814A (en)* | 1992-09-08 | 1994-02-01 | Rajesh Srivastava | Instrument for cleaning the top of the human tongue with antiseptic strip |
| AU4926193A (en) | 1992-09-21 | 1994-04-12 | Vitaphore Corporation | Embolization plugs for blood vessels |
| KR960001417B1 (en) | 1992-09-26 | 1996-01-27 | 한국과학기술원 | Synthesis of Improved Porous Polymer Carrier |
| US5369163A (en) | 1992-11-13 | 1994-11-29 | Rohm And Haas Company | Process for preparing large dimension emulsion polymer particles, polymer product and uses thereof |
| US5690666A (en) | 1992-11-18 | 1997-11-25 | Target Therapeutics, Inc. | Ultrasoft embolism coils and process for using them |
| JP3256583B2 (en) | 1992-12-10 | 2002-02-12 | 株式会社リコー | Electrophotographic toner and method for producing the same |
| US6090925A (en) | 1993-03-09 | 2000-07-18 | Epic Therapeutics, Inc. | Macromolecular microparticles and methods of production and use |
| US5701899A (en) | 1993-05-12 | 1997-12-30 | The Board Of Regents Of The University Of Nebraska | Perfluorobutane ultrasound contrast agent and methods for its manufacture and use |
| US5695740A (en) | 1993-05-12 | 1997-12-09 | The Board Of Regents Of The University Of Nebraska | Perfluorocarbon ultrasound contrast agent comprising microbubbles containing a filmogenic protein and a saccharide |
| US5567415A (en) | 1993-05-12 | 1996-10-22 | The Board Of Regents Of The University Of Nebraska | Ultrasound contrast agents and methods for their manufacture and use |
| US5344867A (en) | 1993-06-14 | 1994-09-06 | The Bfgoodrich Company | Vinylidene chloride emulsion interpolymer composition |
| ATE420628T1 (en) | 1993-07-19 | 2009-01-15 | Angiotech Pharm Inc | ANTI-ANGIogenic AGENTS AND METHODS OF USE THEREOF |
| US5531716A (en) | 1993-09-29 | 1996-07-02 | Hercules Incorporated | Medical devices subject to triggered disintegration |
| PL182223B1 (en) | 1993-12-15 | 2001-11-30 | Bracco Research Sa | Biocompatible dispersion phase for ultrasound contrast agent preparation, ultrasound contrast agent, dry contrast agent preparation and two-component ultrasound contrast agent kit PL EN EN |
| US5569468A (en) | 1994-02-17 | 1996-10-29 | Modi; Pankaj | Vaccine delivery system for immunization, using biodegradable polymer microspheres |
| AU1702395A (en) | 1994-02-17 | 1995-09-04 | Pankaj Modi | Drugs, vaccines and hormones in polylactide coated microspheres |
| WO1995025480A1 (en) | 1994-03-18 | 1995-09-28 | Cook Incorporated | Helical embolization coil |
| US5431174A (en) | 1994-04-04 | 1995-07-11 | Via Medical Corporation | Method of fluid delivery and collection |
| JP2535785B2 (en) | 1994-06-03 | 1996-09-18 | 工業技術院長 | Vascular embolic agent |
| US5639710A (en) | 1994-07-06 | 1997-06-17 | Zeneca Limited | Solid microspheres for agriculturally active compounds and process for their production |
| JP3085570B2 (en) | 1994-07-08 | 2000-09-11 | 株式会社トクヤマ | Polymerizable composition |
| US6099864A (en) | 1994-12-02 | 2000-08-08 | The United States Of America As Represented By The Administrator Of The National Aeronautics And Space Administration | In situ activation of microcapsules |
| US5827531A (en) | 1994-12-02 | 1998-10-27 | The United States Of America As Represented By The Administrator Of The National Aeronautics And Space Administration | Microcapsules and methods for making |
| EP0730847B1 (en)* | 1995-03-07 | 2001-05-23 | Menlo Care Inc. | Controlled expansion sphincter augmentation media |
| US5785682A (en) | 1995-03-22 | 1998-07-28 | Abbott Laboratories | Pre-filled syringe drug delivery system |
| US5637087A (en) | 1995-03-22 | 1997-06-10 | Abbott Laboratories | Prefilled, two-constituent syringe |
| US5569193A (en) | 1995-03-22 | 1996-10-29 | Abbott Laboratories | Syringe system accommodating separately storable prefilled containers for two constituents |
| US5779668A (en) | 1995-03-29 | 1998-07-14 | Abbott Laboratories | Syringe barrel for lyophilization, reconstitution and administration |
| US6428771B1 (en) | 1995-05-15 | 2002-08-06 | Pharmaceutical Discovery Corporation | Method for drug delivery to the pulmonary system |
| DE69634013T2 (en) | 1995-05-26 | 2005-12-15 | SurModics, Inc., Eden Prairie | PROCESS AND IMPLANTABLE OBJECT FOR PROMOTING ENDOTHELIALIZATION |
| JPH11507679A (en) | 1995-06-06 | 1999-07-06 | シー.アール.バード,インコーポレイティド | Method for producing aqueous dispersion of water-soluble polymer particles and resulting particles |
| US5657756A (en) | 1995-06-07 | 1997-08-19 | Ctf Systems Inc. | Method and systems for obtaining higher order gradiometer measurements with lower order gradiometers |
| US5766147A (en) | 1995-06-07 | 1998-06-16 | Winfield Medical | Vial adaptor for a liquid delivery device |
| US6096344A (en) | 1995-07-28 | 2000-08-01 | Advanced Polymer Systems, Inc. | Bioerodible porous compositions |
| US5558822A (en) | 1995-08-16 | 1996-09-24 | Gas Research Institute | Method for production of spheroidized particles |
| US5833361A (en) | 1995-09-07 | 1998-11-10 | Funk; James E. | Apparatus for the production of small spherical granules |
| US5637215A (en) | 1995-10-18 | 1997-06-10 | Purolator Products Company | Fuel filter having improved communication with a contaminant container |
| JPH09110678A (en) | 1995-10-19 | 1997-04-28 | Tanabe Seiyaku Co Ltd | Coated microsphere preparation and method for producing the same |
| CA2161863A1 (en) | 1995-10-31 | 1997-05-01 | Michael Vivian Sefton | Angiogenic material and uses thereof |
| DK0928182T3 (en) | 1996-01-11 | 2002-08-26 | Duoject Inc | Delivery system for medicines packed in pharmaceutical bottles |
| JPH09316271A (en) | 1996-05-31 | 1997-12-09 | Kuraray Co Ltd | Spherical hydrogel |
| US5792478A (en) | 1996-07-08 | 1998-08-11 | Advanced Uro Science | Tissue injectable composition and method of use |
| US5695480A (en) | 1996-07-29 | 1997-12-09 | Micro Therapeutics, Inc. | Embolizing compositions |
| US5830178A (en) | 1996-10-11 | 1998-11-03 | Micro Therapeutics, Inc. | Methods for embolizing vascular sites with an emboilizing composition comprising dimethylsulfoxide |
| WO1998004616A1 (en) | 1996-07-31 | 1998-02-05 | Kanebo Limited | Porous spherical polyvinyl acetal particles, process for producing the same, and microbial carriers |
| US5823198A (en) | 1996-07-31 | 1998-10-20 | Micro Therapeutics, Inc. | Method and apparatus for intravasculer embolization |
| EP0826381A3 (en)* | 1996-08-20 | 2000-01-19 | Menlo Care Inc. | Swollen hydrogel for sphincter augmentation or for deforming tissue and method for its synthesis |
| US5813411A (en) | 1996-08-20 | 1998-09-29 | Menlo Care, Inc. | Method of deforming tissue with a swollen hydrogel |
| JP4139440B2 (en) | 1996-09-11 | 2008-08-27 | イマアーレクス・フアーマシユーチカル・コーポレーシヨン | Improved method for diagnostic imaging using contrast agents and vasodilators |
| US5785642A (en) | 1996-10-18 | 1998-07-28 | Micro Therapeutics, Inc. | Methods for treating urinary incontinence in mammals |
| JP3763904B2 (en) | 1996-10-31 | 2006-04-05 | 株式会社クラレ | Method for producing spherical hydrous gel |
| US6139963A (en) | 1996-11-28 | 2000-10-31 | Kuraray Co., Ltd. | Polyvinyl alcohol hydrogel and process for producing the same |
| WO1998045191A1 (en) | 1997-04-10 | 1998-10-15 | Johns Hopkins University | Gaz syringe and package therefor |
| US6419624B1 (en) | 1999-10-11 | 2002-07-16 | Uromedica, Inc. | Apparatus and method for inserting an adjustable implantable genitourinary device |
| US5959073A (en) | 1997-07-07 | 1999-09-28 | Southwest Research Institute | Method for preparing polymeric beads |
| WO1999012577A1 (en) | 1997-09-05 | 1999-03-18 | Nycomed Imaging As | Polymer particles made of polyvinyl alcohol and comprising a contrast agent for chemoembolization |
| US6538026B1 (en)* | 1997-09-11 | 2003-03-25 | Provasis Therapeutics, Inc. | Compositions useful for remodeling body spaces |
| US5951160A (en) | 1997-11-20 | 1999-09-14 | Biomet, Inc. | Method and apparatus for packaging, mixing and delivering bone cement |
| US6003566A (en) | 1998-02-26 | 1999-12-21 | Becton Dickinson And Company | Vial transferset and method |
| US6660301B1 (en) | 1998-03-06 | 2003-12-09 | Biosphere Medical, Inc. | Injectable microspheres for dermal augmentation and tissue bulking |
| US6267154B1 (en) | 1998-06-05 | 2001-07-31 | Abbott Laboratories | System for storing mixing and administering a drug |
| US6165193A (en) | 1998-07-06 | 2000-12-26 | Microvention, Inc. | Vascular embolization with an expansible implant |
| US6264861B1 (en) | 1998-08-05 | 2001-07-24 | Xeikon Nv | Method for producing rounded polymeric particles |
| US6315709B1 (en) | 1998-08-07 | 2001-11-13 | Stereotaxis, Inc. | Magnetic vascular defect treatment system |
| FR2784580B1 (en)* | 1998-10-16 | 2004-06-25 | Biosepra Inc | POLYVINYL-ALCOHOL MICROSPHERES AND METHODS OF MAKING THE SAME |
| KR100321854B1 (en) | 1998-12-30 | 2002-08-28 | 동국제약 주식회사 | Long-term sustained-release microspheres containing luteinizing hormone releasing hormone homologues and a method of producing the same |
| US6162377A (en) | 1999-02-23 | 2000-12-19 | Alberta Research Council Inc. | Apparatus and method for the formation of uniform spherical particles |
| US7185657B1 (en)* | 1999-04-07 | 2007-03-06 | Johnson George M | Method and device for treating gastroesophageal reflux disease |
| US6268405B1 (en)* | 1999-05-04 | 2001-07-31 | Porex Surgical, Inc. | Hydrogels and methods of making and using same |
| US6511472B1 (en) | 1999-05-21 | 2003-01-28 | Microtherapeutics, Inc. | Interface needle and method for creating a blunt interface between delivered liquids |
| JP4290363B2 (en) | 1999-06-09 | 2009-07-01 | エシコン・インコーポレイテッド | Method and apparatus for conditioning flexible medical polymer implants |
| US6277392B1 (en) | 1999-09-16 | 2001-08-21 | Carbon Medical Technologies, Inc. | Tissue injectable composition |
| US6652883B2 (en) | 2000-03-13 | 2003-11-25 | Biocure, Inc. | Tissue bulking and coating compositions |
| JP4871476B2 (en) | 2000-03-13 | 2012-02-08 | バイオコンパティブルズ ユーケー リミテッド | Embolization composition |
| US6423332B1 (en) | 2000-05-26 | 2002-07-23 | Ethicon, Inc. | Method and composition for deforming soft tissues |
| DE10026620A1 (en) | 2000-05-29 | 2002-03-07 | Gerhard Quelle | Biocompatible material for cell and tissue implantation, useful e.g. for drug release or cosmetic tissue augmentation, consisting of spherical particles having (semi-)permeable or porous outer shell and internal cavity |
| WO2002011696A2 (en) | 2000-08-08 | 2002-02-14 | Ev & M | Active tissue augmentation materials and method |
| US6478775B1 (en) | 2000-10-02 | 2002-11-12 | Genyx Medical Inc. | Device for delivering non-biodegradable bulking composition to a urological site |
| US6425854B1 (en) | 2000-10-02 | 2002-07-30 | Genyx Medical, Inc. | Method for delivering non-biodegradable bulking composition to a urological site |
| AUPR098300A0 (en) | 2000-10-25 | 2000-11-16 | Sirtex Medical Limited | Polymer based radionuclide containing microspheres |
| AUPR098400A0 (en) | 2000-10-25 | 2000-11-16 | Sirtex Medical Limited | Production of radionuclide coated microspheres and seeds |
| AUPR098200A0 (en) | 2000-10-25 | 2000-11-16 | Sirtex Medical Limited | Production of low density radionuclide containing microspheres |
| EP1335661B1 (en) | 2000-10-27 | 2009-05-06 | Baxter Healthcare S.A. | Production of microspheres |
| US7131997B2 (en)* | 2002-03-29 | 2006-11-07 | Scimed Life Systems, Inc. | Tissue treatment |
| WO2003082359A1 (en)* | 2002-03-29 | 2003-10-09 | Boston Scientific Limited | Tissue treatment |
| US7094369B2 (en) | 2002-03-29 | 2006-08-22 | Scimed Life Systems, Inc. | Processes for manufacturing polymeric microspheres |
| AU2003240000A1 (en)* | 2002-06-12 | 2003-12-31 | Boston Scientific Limited | Bulking agents |
- 2003
- 2003-06-12AUAU2003240000Apatent/AU2003240000A1/ennot_activeAbandoned
- 2003-06-12EPEP03734578Apatent/EP1511522B1/ennot_activeExpired - Lifetime
- 2003-06-12WOPCT/US2003/018625patent/WO2003105917A2/ennot_activeApplication Discontinuation
- 2003-06-12CACA002492339Apatent/CA2492339A1/ennot_activeAbandoned
- 2003-06-12USUS10/459,895patent/US20040037887A1/ennot_activeAbandoned
- 2010
- 2010-11-16USUS12/946,990patent/US8394400B2/ennot_activeExpired - Fee Related
- 2013
- 2013-03-11USUS13/792,346patent/US8586071B2/ennot_activeExpired - Fee Related
- 2013-11-18USUS14/082,274patent/US10398724B2/ennot_activeExpired - Fee Related
- 2019
- 2019-07-23USUS16/519,450patent/US10813945B2/ennot_activeExpired - Fee Related
Patent Citations (99)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US3663470A (en)* | 1969-06-05 | 1972-05-16 | Kanegafuchi Spinning Co Ltd | Method of producing polyvinyl acetal porous articles and the shaped porous articles made therefrom |
| US3737398A (en)* | 1969-11-13 | 1973-06-05 | D Yamaguchi | Method of making a polyvinyl acetal sponge buff |
| US4076640A (en)* | 1975-02-24 | 1978-02-28 | Xerox Corporation | Preparation of spheroidized particles |
| US3957933A (en)* | 1975-03-05 | 1976-05-18 | General Atomic Company | Apparatus for producing microspherical particles and method for operating such apparatus |
| US4025686A (en)* | 1975-06-26 | 1977-05-24 | Owens-Corning Fiberglas Corporation | Molded composite article and method for making the article |
| US4191672A (en)* | 1976-10-25 | 1980-03-04 | Berger Jenson & Nicholson Ltd. | Polymer aggregates |
| US4266030A (en)* | 1978-08-07 | 1981-05-05 | Basf Aktiengesellschaft | Macroporous polymeric carrier for covalently binding proteins, its preparation and its use for fixing active proteins |
| US4243794A (en)* | 1978-10-10 | 1981-01-06 | Minnesota Mining And Manufacturing Company | Mixture of rough and spheroidized resin particles |
| US4198318A (en)* | 1978-11-24 | 1980-04-15 | Conoco, Inc. | Production of high strength alumina spheres by hydrogelling corresponding slurries |
| US4268495A (en)* | 1979-01-08 | 1981-05-19 | Ethicon, Inc. | Injectable embolization and occlusion solution |
| US4246208A (en)* | 1979-03-22 | 1981-01-20 | Xerox Corporation | Dust-free plasma spheroidization |
| US4429062A (en)* | 1980-02-18 | 1984-01-31 | Emil Pasztor | Pharmaceutically acceptable silicon rubber and therapeutical set and the use thereof for surgical embolization |
| US4271281A (en)* | 1980-05-29 | 1981-06-02 | American Hoechst Corporation | Process for preparing styrenic polymer particles |
| US4427794A (en)* | 1980-08-22 | 1984-01-24 | Bayer Aktiengesellschaft | Process for the preparation of bead polymers of uniform particle size by polymerization of microencapsulated monomer |
| US4444961A (en)* | 1980-10-30 | 1984-04-24 | The Dow Chemical Company | Process and apparatus for preparing uniform size polymer beads |
| US4657756A (en)* | 1980-11-17 | 1987-04-14 | Schering Aktiengesellschaft | Microbubble precursors and apparatus for their production and use |
| US4428869A (en)* | 1981-08-20 | 1984-01-31 | International Flavors & Fragrances Inc. | Cologne consisting of microcapsule suspension |
| US4999188A (en)* | 1983-06-30 | 1991-03-12 | Solodovnik Valentin D | Methods for embolization of blood vessels |
| US4492720A (en)* | 1983-11-15 | 1985-01-08 | Benjamin Mosier | Method of preparing microspheres for intravascular delivery |
| US4573967A (en)* | 1983-12-06 | 1986-03-04 | Eli Lilly And Company | Vacuum vial infusion system |
| US4661137A (en)* | 1984-06-21 | 1987-04-28 | Saint Gobain Vitrage | Process for producing glass microspheres |
| US4833237A (en)* | 1984-07-31 | 1989-05-23 | Fuji Spinning Co., Ltd. | Process for producing granular porous chitosan |
| US5011677A (en)* | 1984-11-19 | 1991-04-30 | The Curators Of The University Of Missouri | Radioactive glass microspheres |
| US5302369A (en)* | 1984-11-19 | 1994-04-12 | The Curators Of The University Of Missouri | Microspheres for radiation therapy |
| US5106903A (en)* | 1984-12-17 | 1992-04-21 | Lehigh University | Preparation of large particle size monodisperse latexes |
| US4663358A (en)* | 1985-05-01 | 1987-05-05 | Biomaterials Universe, Inc. | Porous and transparent poly(vinyl alcohol) gel and method of manufacturing the same |
| US4801458A (en)* | 1985-06-24 | 1989-01-31 | Teijin Limited | Sustained release pharmaceutical plaster |
| US4822535A (en)* | 1985-07-12 | 1989-04-18 | Norsk Hydro A.S. | Method for producing small, spherical polymer particles |
| USH915H (en)* | 1985-07-22 | 1991-05-07 | Gibbs Marylu B | Controlled macroporous copolymer properties by removal of impurities in the diluent |
| US4742086A (en)* | 1985-11-02 | 1988-05-03 | Lion Corporation | Process for manufacturing porous polymer |
| US4990340A (en)* | 1986-01-22 | 1991-02-05 | Teijin Limited | Sustained release pharmaceutical preparation |
| US4929400A (en)* | 1986-04-28 | 1990-05-29 | California Institute Of Technology | Production of monodisperse, polymeric microspheres |
| US4743507A (en)* | 1986-09-12 | 1988-05-10 | Franses Elias I | Nonspherical microparticles and method therefor |
| US5114421A (en)* | 1986-09-22 | 1992-05-19 | Polak Robert B | Medicament container/dispenser assembly |
| US5292814A (en)* | 1987-04-29 | 1994-03-08 | Ernst Bayer | Process for the preparation of monodispersed polymer beads |
| US4795741A (en)* | 1987-05-06 | 1989-01-03 | Biomatrix, Inc. | Compositions for therapeutic percutaneous embolization and the use thereof |
| US4819637A (en)* | 1987-09-01 | 1989-04-11 | Interventional Therapeutics Corporation | System for artificial vessel embolization and devices for use therewith |
| US4804366A (en)* | 1987-10-29 | 1989-02-14 | Baxter International Inc. | Cartridge and adapter for introducing a beneficial agent into an intravenous delivery system |
| US4981625A (en)* | 1988-03-14 | 1991-01-01 | California Institute Of Technology | Monodisperse, polymeric microspheres produced by irradiation of slowly thawing frozen drops |
| US5384124A (en)* | 1988-07-21 | 1995-01-24 | Farmalyoc | Solid porous unitary form comprising micro-particles and/or nano-particles, and its preparation |
| US5079274A (en)* | 1989-03-15 | 1992-01-07 | The Dow Chemical Company | Process for preparing absorptive porous resin beads |
| US5403870A (en)* | 1989-05-31 | 1995-04-04 | Kimberly-Clark Corporation | Process for forming a porous particle of an absorbent polymer |
| US5116387A (en)* | 1989-06-09 | 1992-05-26 | American Medical Systems, Inc. | Preparation of injectable polymeric bodies |
| US5007940A (en)* | 1989-06-09 | 1991-04-16 | American Medical Systems, Inc. | Injectable polymeric bodies |
| US5190766A (en)* | 1990-04-16 | 1993-03-02 | Ken Ishihara | Method of controlling drug release by resonant sound wave |
| US5514090A (en)* | 1990-04-24 | 1996-05-07 | Science Incorporated | Closed drug delivery system |
| US5316774A (en)* | 1990-06-20 | 1994-05-31 | Advanced Polymer Systems, Inc. | Blocked polymeric particles having internal pore networks for delivering active substances to selected environments |
| US5202352A (en)* | 1990-08-08 | 1993-04-13 | Takeda Chemical Industries, Ltd. | Intravascular embolizing agent containing angiogenesis-inhibiting substance |
| US5484584A (en)* | 1990-10-02 | 1996-01-16 | Board Of Regents, The University Of Texas System | Therapeutic and diagnostic use of modified polymeric microcapsules |
| US5494682A (en)* | 1990-10-05 | 1996-02-27 | Massachusetts Institute Of Technology | Ionically cross-linked polymeric microcapsules |
| US5622657A (en)* | 1991-10-01 | 1997-04-22 | Takeda Chemical Industries, Ltd. | Prolonged release microparticle preparation and production of the same |
| US5624685A (en)* | 1991-10-16 | 1997-04-29 | Terumo Kabushiki Kaisha | High polymer gel and vascular lesion embolizing material comprising the same |
| US5494940A (en)* | 1991-12-20 | 1996-02-27 | Alliedsignal Inc. | Low density materials having high surface areas and articles formed therefrom |
| US6537574B1 (en)* | 1992-02-11 | 2003-03-25 | Bioform, Inc. | Soft tissue augmentation material |
| US5490984A (en)* | 1992-02-28 | 1996-02-13 | Jsf Consulants Ltd. | Use of injectable biomaterials for the repair and augmentation of the anal sphincters |
| US5314974A (en)* | 1992-04-10 | 1994-05-24 | Mitsubishi Kasei Corporation | Method for producing a spherical acrylonitrile crosslinked copolymer |
| US5723269A (en)* | 1992-07-24 | 1998-03-03 | Takeda Chemical Industries, Ltd. | Microparticle preparation and production thereof |
| US6027472A (en)* | 1992-08-13 | 2000-02-22 | Science Incorporated | Mixing and delivery syringe assembly |
| US5512604A (en)* | 1992-08-28 | 1996-04-30 | The Dow Chemical Company | Porous copolymers having a cellular polymeric structure suitable for preparing ion-exchange resins and adsorbents |
| US5718884A (en)* | 1992-09-16 | 1998-02-17 | Nycomed Imaging As | Microbubble-based contrast agents with crosslinked and reduced proteinaceous shells |
| US6015546A (en)* | 1992-10-10 | 2000-01-18 | Quadrant Healthcare (Uk) Limited | Preparation of further diagnostic agents |
| US6344182B1 (en)* | 1992-10-10 | 2002-02-05 | Quadrant Healthcare (Uk) Limited | Preparation of diagnostic agents by spray drying |
| US5382260A (en)* | 1992-10-30 | 1995-01-17 | Interventional Therapeutics Corp. | Embolization device and apparatus including an introducer cartridge and method for delivering the same |
| US5746734A (en)* | 1992-10-30 | 1998-05-05 | International Therapeutics Corporation | Introducer cartridge for delivering an embolization device |
| US5288763A (en)* | 1992-12-23 | 1994-02-22 | The Johns Hopkins University School Of Medicine | Porous, polymer beads and process of their preparation |
| US6060053A (en)* | 1993-04-30 | 2000-05-09 | Children's Medical Center Corp. | Injectable chondrocyte-carrier suspension for treatment of vesicoureteral reflux and incontinence |
| US5716981A (en)* | 1993-07-19 | 1998-02-10 | Angiogenesis Technologies, Inc. | Anti-angiogenic compositions and methods of use |
| US5397303A (en)* | 1993-08-06 | 1995-03-14 | River Medical, Inc. | Liquid delivery device having a vial attachment or adapter incorporated therein |
| US5398851A (en)* | 1993-08-06 | 1995-03-21 | River Medical, Inc. | Liquid delivery device |
| US5885216A (en)* | 1993-10-28 | 1999-03-23 | Medrad, Inc. | Total system for contrast delivery |
| US5385561A (en)* | 1994-01-18 | 1995-01-31 | Bard International, Inc. | Apparatus and method for injecting a viscous material into the tissue of a patient |
| US5885547A (en)* | 1994-01-21 | 1999-03-23 | Paragon Medical Ltd. | Particulate material |
| US5417982A (en)* | 1994-02-17 | 1995-05-23 | Modi; Pankaj | Controlled release of drugs or hormones in biodegradable polymer microspheres |
| US5899877A (en)* | 1994-04-28 | 1999-05-04 | Primed Halberstadt Medizintechnik Gmbh | One-piece dispensing device for the contamination-free administration of medicaments (cytostatica) |
| US5595821A (en)* | 1994-05-04 | 1997-01-21 | Minnesota Mining And Manufacturing Company | Repulpable plastic films |
| US5863957A (en)* | 1994-06-06 | 1999-01-26 | Biopore Corporation | Polymeric microbeads |
| US5725534A (en)* | 1995-01-03 | 1998-03-10 | William Cook Europe A/S | Method of manufacturing an assembly for positioning an embolization coil in the vascular system, and such an assembly |
| US5891155A (en)* | 1995-01-27 | 1999-04-06 | Scimed Life Systems, Inc. | Embolizing system |
| US5895411A (en)* | 1995-01-27 | 1999-04-20 | Scimed Life Systems Inc. | Embolizing system |
| US5876372A (en)* | 1995-03-22 | 1999-03-02 | Abbott Laboratories | Syringe system accomodating seperate prefilled barrels for two constituents |
| US6214331B1 (en)* | 1995-06-06 | 2001-04-10 | C. R. Bard, Inc. | Process for the preparation of aqueous dispersions of particles of water-soluble polymers and the particles obtained |
| US6235224B1 (en)* | 1995-07-21 | 2001-05-22 | Brown University Research Foundation | Process for preparing microparticles through phase inversion phenomena |
| US5877224A (en)* | 1995-07-28 | 1999-03-02 | Rutgers, The State University Of New Jersey | Polymeric drug formulations |
| US5752974A (en)* | 1995-12-18 | 1998-05-19 | Collagen Corporation | Injectable or implantable biomaterials for filling or blocking lumens and voids of the body |
| US6335384B1 (en)* | 1996-01-31 | 2002-01-01 | Micro Therapeutics, Inc. | Methods for embolizing blood vessels |
| US6051247A (en)* | 1996-05-30 | 2000-04-18 | University Of Florida Research Foundation, Inc. | Moldable bioactive compositions |
| US5855615A (en)* | 1996-06-07 | 1999-01-05 | Menlo Care, Inc. | Controller expansion sphincter augmentation media |
| US5902832A (en)* | 1996-08-20 | 1999-05-11 | Menlo Care, Inc. | Method of synthesizing swollen hydrogel for sphincter augmentation |
| US6028066A (en)* | 1997-05-06 | 2000-02-22 | Imarx Pharmaceutical Corp. | Prodrugs comprising fluorinated amphiphiles |
| US6063068A (en)* | 1997-12-04 | 2000-05-16 | Baxter International Inc. | Vial connecting device for a sliding reconstitution device with seal |
| US6059766A (en)* | 1998-02-27 | 2000-05-09 | Micro Therapeutics, Inc. | Gynecologic embolotherapy methods |
| US6047861A (en)* | 1998-04-15 | 2000-04-11 | Vir Engineering, Inc. | Two component fluid dispenser |
| US6224794B1 (en)* | 1998-05-06 | 2001-05-01 | Angiotech Pharmaceuticals, Inc. | Methods for microsphere production |
| US6224630B1 (en)* | 1998-05-29 | 2001-05-01 | Advanced Bio Surfaces, Inc. | Implantable tissue repair device |
| US6238403B1 (en)* | 1999-10-04 | 2001-05-29 | Microvention, Inc. | Filamentous embolic device with expansible elements |
| US6191193B1 (en)* | 2000-01-06 | 2001-02-20 | Korea Institute Of Science & Technology | Microspheric embolic materials having a dual structure of poly(vinyl acetate) core and poly(vinyl alcohol) shell and method for preparing the same |
| US6548592B1 (en)* | 2000-05-04 | 2003-04-15 | Kimberly-Clark Worldwide, Inc. | Ion-sensitive, water-dispersible polymers, a method of making same and items using same |
| US6355275B1 (en)* | 2000-06-23 | 2002-03-12 | Carbon Medical Technologies, Inc. | Embolization using carbon coated microparticles |
| US6394965B1 (en)* | 2000-08-15 | 2002-05-28 | Carbon Medical Technologies, Inc. | Tissue marking using biocompatible microparticles |
Cited By (47)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US8277468B2 (en) | 1999-06-22 | 2012-10-02 | Ethicon Endo-Surgery, Inc. | Tissue reconfiguration |
| US7736373B2 (en) | 1999-06-22 | 2010-06-15 | Ndo Surical, Inc. | Methods and devices for tissue reconfiguration |
| US20040193193A1 (en)* | 1999-06-22 | 2004-09-30 | Ndo Surgical, Inc., A Massachusetts Corporation | Tissue reconfiguration |
| US20040194790A1 (en)* | 1999-06-22 | 2004-10-07 | Ndo Surgical, Inc. | Tissue reconfiguration |
| US20090198254A1 (en)* | 1999-06-22 | 2009-08-06 | Ethicon Endo-Surgery, Inc. | Methods and Devices for Tissue Reconfiguration |
| US20050033328A1 (en)* | 1999-06-22 | 2005-02-10 | Ndo Surgical, Inc., A Massachusetts Corporation | Methods and devices for tissue reconfiguration |
| US7857823B2 (en) | 1999-06-22 | 2010-12-28 | Ethicon Endo-Surgery, Inc. | Tissue reconfiguration |
| US20060025789A1 (en)* | 1999-06-22 | 2006-02-02 | Ndo Surgical, Inc., A Massachusetts Corporation | Methods and devices for tissue reconfiguration |
| US7776057B2 (en) | 1999-06-22 | 2010-08-17 | Ethicon Endo-Surgery, Inc. | Methods and devices for tissue reconfiguration |
| US8057494B2 (en) | 1999-06-22 | 2011-11-15 | Ethicon Endo-Surgery, Inc. | Methods and devices for tissue reconfiguration |
| US7722633B2 (en) | 1999-06-22 | 2010-05-25 | Ethicon Endo-Surgery, Inc. | Tissue reconfiguration |
| US7896893B2 (en) | 1999-06-22 | 2011-03-01 | Ethicon Endo-Surgery, Inc. | Methods and devices for tissue reconfiguration |
| US7713277B2 (en) | 1999-06-22 | 2010-05-11 | Ethicon Endo-Surgery, Inc. | Tissue reconfiguration |
| US8287554B2 (en) | 1999-06-22 | 2012-10-16 | Ethicon Endo-Surgery, Inc. | Method and devices for tissue reconfiguration |
| US20040010245A1 (en)* | 1999-06-22 | 2004-01-15 | Cerier Jeffrey C. | Method and devices for tissue reconfiguration |
| US7846180B2 (en) | 1999-06-22 | 2010-12-07 | Ethicon Endo-Surgery, Inc. | Tissue fixation devices and methods of fixing tissue |
| US20030161887A1 (en)* | 2002-02-27 | 2003-08-28 | Klein Dean A. | Lower esophagus tissue modifier |
| US7491243B2 (en)* | 2002-02-27 | 2009-02-17 | Carbon Medical Technologies | Method of modifying a lower esophagus |
| US20040267357A1 (en)* | 2003-04-30 | 2004-12-30 | Allen Jeffrey W. | Cardiac valve modification method and device |
| US20050288639A1 (en)* | 2004-06-25 | 2005-12-29 | Hibner Michael C | Instrument used in treatment of the urinary incontinence in women |
| US20090024215A1 (en)* | 2004-09-16 | 2009-01-22 | Evera Medical, Inc. | Multilayer tissue implant having a compliance that simulates tissue |
| US20080275569A1 (en)* | 2004-09-16 | 2008-11-06 | Evera Medical, Inc | Tissue Augmentation Device |
| US7244270B2 (en) | 2004-09-16 | 2007-07-17 | Evera Medical | Systems and devices for soft tissue augmentation |
| US7998202B2 (en) | 2004-09-16 | 2011-08-16 | Evera Medical, Inc. | Tissue implant having a biased layer and compliance that simulates tissue |
| US7998201B2 (en) | 2004-09-16 | 2011-08-16 | Evera Medical, Inc. | Methods of forming a tissue implant having a tissue contacting layer held under compression |
| US7641688B2 (en) | 2004-09-16 | 2010-01-05 | Evera Medical, Inc. | Tissue augmentation device |
| US20060161253A1 (en)* | 2004-09-16 | 2006-07-20 | Michael Lesh | Tissue augmentation device |
| US20060058891A1 (en)* | 2004-09-16 | 2006-03-16 | Lesh Michael D | Transformable tissue bulking device |
| US20060058735A1 (en)* | 2004-09-16 | 2006-03-16 | Lesh Michael D | Systems and devices for soft tissue augmentation |
| US20060058892A1 (en)* | 2004-09-16 | 2006-03-16 | Lesh Michael D | Valved tissue augmentation implant |
| US20060088568A1 (en)* | 2004-10-26 | 2006-04-27 | Tropsha Yelena G | Implantation of tissue bulking devices |
| US7305993B2 (en) | 2004-10-26 | 2007-12-11 | Medtronic, Inc. | Implantation of tissue bulking devices |
| US20060246033A1 (en)* | 2005-03-02 | 2006-11-02 | Cook Biotech Incorporated | Injectable bulking agent compositions |
| US8753620B2 (en) | 2005-05-09 | 2014-06-17 | Boston Scientific Scimed, Inc. | Injectable bulking compositions |
| US7862552B2 (en) | 2005-05-09 | 2011-01-04 | Boston Scientific Scimed, Inc. | Medical devices for treating urological and uterine conditions |
| US20110098631A1 (en)* | 2005-05-09 | 2011-04-28 | Boston Scientific Scimed, Inc. | Medical devices for treating urological and uterine conditions |
| US20060264912A1 (en)* | 2005-05-09 | 2006-11-23 | Mcintyre Jon T | Medical devices for treating urological and uterine conditions |
| US8263109B2 (en) | 2005-05-09 | 2012-09-11 | Boston Scientific Scimed, Inc. | Injectable bulking compositions |
| US20060251697A1 (en)* | 2005-05-09 | 2006-11-09 | Jamie Li | Injectable bulking compositions |
| US10448955B2 (en)* | 2006-01-30 | 2019-10-22 | Biosphere Medical, Inc. | Compressible intravascular embolization particles and related methods and delivery systems |
| US7553273B2 (en) | 2006-05-01 | 2009-06-30 | Duodyn Technology, Llc | Apparatus and method for managing incontinence |
| US20080234703A1 (en)* | 2007-03-23 | 2008-09-25 | Ethicon Endo-Surgery, Inc. | Tissue approximation system |
| US8852216B2 (en) | 2007-03-23 | 2014-10-07 | Ethicon Endo-Surgery, Inc. | Tissue approximation methods |
| US9333070B2 (en) | 2008-02-01 | 2016-05-10 | Evera Medical, Inc. | Breast implant with internal flow dampening |
| US20090198331A1 (en)* | 2008-02-01 | 2009-08-06 | Kesten Randy J | Implantable prosthesis with open cell flow regulation |
| US20130096552A1 (en)* | 2011-10-14 | 2013-04-18 | Christopher L. Brace | Hydrodissection Material with Reduced Migration |
| CN110201419A (en)* | 2019-05-09 | 2019-09-06 | 西安交通大学 | It is a kind of using polyvinyl alcohol microparticles as membrane flexibility column of carrier and preparation method thereof |
Also Published As
| Publication number | Publication date |
|---|---|
| US20190343872A1 (en) | 2019-11-14 |
| CA2492339A1 (en) | 2003-12-24 |
| US20140072636A1 (en) | 2014-03-13 |
| EP1511522A2 (en) | 2005-03-09 |
| US20110064774A1 (en) | 2011-03-17 |
| EP1511522B1 (en) | 2011-08-10 |
| US10398724B2 (en) | 2019-09-03 |
| WO2003105917A3 (en) | 2004-02-19 |
| US8394400B2 (en) | 2013-03-12 |
| US8586071B2 (en) | 2013-11-19 |
| US20130190720A1 (en) | 2013-07-25 |
| AU2003240000A1 (en) | 2003-12-31 |
| WO2003105917A2 (en) | 2003-12-24 |
| US10813945B2 (en) | 2020-10-27 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| US10813945B2 (en) | Bulking agents | |
| US6419701B1 (en) | Adjustable implantable genitourinary device | |
| US8882654B2 (en) | Method for treating fecal incontinence | |
| US20060189940A1 (en) | Implant positioning system and method | |
| US20080275569A1 (en) | Tissue Augmentation Device | |
| US20080015498A1 (en) | Systems and devices for soft tissue augmentation | |
| US6645138B2 (en) | Adjustable implantable genitourinary device | |
| US6572532B1 (en) | Implant positioning system and method | |
| US20060058890A1 (en) | Methods for soft tissue augmentation | |
| US20030171646A1 (en) | Implant positioning system and method | |
| CN101072545A (en) | Valved tissue augmentation implant | |
| Yoo et al. | Detachable self-sealing membrane system for the endoscopic treatment of incontinence | |
| AU2004201529A1 (en) | Method for treating gastrointestinal tract | |
| US9320588B2 (en) | Method for treating fecal incontinence |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| AS | Assignment | Owner name:SCIMED LIFE SYSTEMS, INC., MINNESOTA Free format text:ASSIGNMENT OF ASSIGNORS INTEREST;ASSIGNORS:BOURNE, GEORGE;MADDEN, MIKE;MADENFIAN, ART;AND OTHERS;REEL/FRAME:014513/0570;SIGNING DATES FROM 20030612 TO 20040127 Owner name:SCIMED LIFE SYSTEMS, INC., MINNESOTA Free format text:ASSIGNMENT OF ASSIGNORS INTEREST;ASSIGNORS:BOURNE, GEORGE;MADDEN, MIKE;MADENJIAN, ART;AND OTHERS;REEL/FRAME:014513/0644;SIGNING DATES FROM 20030612 TO 20040127 | |
| AS | Assignment | Owner name:BOSTON SCIENTIFIC SCIMED, INC., MINNESOTA Free format text:CHANGE OF NAME;ASSIGNOR:SCIMED LIFE SYSTEMS, INC.;REEL/FRAME:018505/0868 Effective date:20050101 Owner name:BOSTON SCIENTIFIC SCIMED, INC.,MINNESOTA Free format text:CHANGE OF NAME;ASSIGNOR:SCIMED LIFE SYSTEMS, INC.;REEL/FRAME:018505/0868 Effective date:20050101 | |
| STCB | Information on status: application discontinuation | Free format text:ABANDONED -- FAILURE TO RESPOND TO AN OFFICE ACTION |