US20040018577A1 - Multiple hybrid immunoassay - Google Patents
Multiple hybrid immunoassayDownload PDFInfo
- Publication number
- US20040018577A1 US20040018577A1US10/208,560US20856002AUS2004018577A1US 20040018577 A1US20040018577 A1US 20040018577A1US 20856002 AUS20856002 AUS 20856002AUS 2004018577 A1US2004018577 A1US 2004018577A1
- Authority
- US
- United States
- Prior art keywords
- analyte
- antibodies
- binding
- different
- immunoassay
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Abandoned
Links
- 238000003018immunoassayMethods0.000titleclaimsabstractdescription77
- 239000012491analyteSubstances0.000claimsabstractdescription193
- 238000000034methodMethods0.000claimsabstractdescription38
- 239000000203mixtureSubstances0.000claimsabstractdescription31
- 238000001514detection methodMethods0.000claimsabstractdescription15
- 239000003153chemical reaction reagentSubstances0.000claimsdescription69
- 239000011859microparticleSubstances0.000claimsdescription22
- 102000004190EnzymesHuman genes0.000claimsdescription20
- 108090000790EnzymesProteins0.000claimsdescription20
- 229940088598enzymeDrugs0.000claimsdescription20
- 102000004169proteins and genesHuman genes0.000claimsdescription19
- 108090000623proteins and genesProteins0.000claimsdescription19
- -1TSHProteins0.000claimsdescription18
- 102000002260Alkaline PhosphataseHuman genes0.000claimsdescription16
- 108020004774Alkaline PhosphataseProteins0.000claimsdescription16
- 239000004816latexSubstances0.000claimsdescription15
- 229920000126latexPolymers0.000claimsdescription15
- 208000010125myocardial infarctionDiseases0.000claimsdescription12
- 239000000126substanceSubstances0.000claimsdescription12
- 108010054477Immunoglobulin Fab FragmentsProteins0.000claimsdescription8
- 102000001706Immunoglobulin Fab FragmentsHuman genes0.000claimsdescription8
- 102000007592ApolipoproteinsHuman genes0.000claimsdescription7
- 108010071619ApolipoproteinsProteins0.000claimsdescription7
- VYPSYNLAJGMNEJ-UHFFFAOYSA-NSilicium dioxideChemical compoundO=[Si]=OVYPSYNLAJGMNEJ-UHFFFAOYSA-N0.000claimsdescription7
- 229910052751metalInorganic materials0.000claimsdescription7
- 239000002184metalSubstances0.000claimsdescription7
- 230000003287optical effectEffects0.000claimsdescription7
- 102000036675MyoglobinHuman genes0.000claimsdescription6
- 108010062374MyoglobinProteins0.000claimsdescription6
- 239000011324beadSubstances0.000claimsdescription6
- 229920003023plasticPolymers0.000claimsdescription6
- 239000004033plasticSubstances0.000claimsdescription6
- 108010010803GelatinProteins0.000claimsdescription5
- 239000004793PolystyreneSubstances0.000claimsdescription5
- 229920000159gelatinPolymers0.000claimsdescription5
- 235000019322gelatineNutrition0.000claimsdescription5
- 235000011852gelatine dessertsNutrition0.000claimsdescription5
- 229920002223polystyrenePolymers0.000claimsdescription5
- 239000004971Cross linkerSubstances0.000claimsdescription4
- 239000003431cross linking reagentSubstances0.000claimsdescription4
- 239000011521glassSubstances0.000claimsdescription4
- BASFCYQUMIYNBI-UHFFFAOYSA-NplatinumChemical compound[Pt]BASFCYQUMIYNBI-UHFFFAOYSA-N0.000claimsdescription4
- 239000004065semiconductorSubstances0.000claimsdescription4
- 108010015776Glucose oxidaseProteins0.000claimsdescription3
- 239000004366Glucose oxidaseSubstances0.000claimsdescription3
- SXRSQZLOMIGNAQ-UHFFFAOYSA-NGlutaraldehydeChemical compoundO=CCCCC=OSXRSQZLOMIGNAQ-UHFFFAOYSA-N0.000claimsdescription3
- 108010021625Immunoglobulin FragmentsProteins0.000claimsdescription3
- 102000008394Immunoglobulin FragmentsHuman genes0.000claimsdescription3
- 102000003425TyrosinaseHuman genes0.000claimsdescription3
- 108060008724TyrosinaseProteins0.000claimsdescription3
- 108010005774beta-GalactosidaseProteins0.000claimsdescription3
- 239000000835fiberSubstances0.000claimsdescription3
- 230000005669field effectEffects0.000claimsdescription3
- 239000008273gelatinSubstances0.000claimsdescription3
- 229940116332glucose oxidaseDrugs0.000claimsdescription3
- 235000019420glucose oxidaseNutrition0.000claimsdescription3
- 239000000377silicon dioxideSubstances0.000claimsdescription3
- 235000012239silicon dioxideNutrition0.000claimsdescription3
- 238000010897surface acoustic wave methodMethods0.000claimsdescription3
- RBAFCMJBDZWZIV-UHFFFAOYSA-N(2,5-dioxopyrrolidin-1-yl) 4-azido-2-hydroxybenzoateChemical compoundOC1=CC(N=[N+]=[N-])=CC=C1C(=O)ON1C(=O)CCC1=ORBAFCMJBDZWZIV-UHFFFAOYSA-N0.000claimsdescription2
- AASYSXRGODIQGY-UHFFFAOYSA-N1-[1-(2,5-dioxopyrrol-1-yl)hexyl]pyrrole-2,5-dioneChemical compoundO=C1C=CC(=O)N1C(CCCCC)N1C(=O)C=CC1=OAASYSXRGODIQGY-UHFFFAOYSA-N0.000claimsdescription2
- FPKVOQKZMBDBKP-UHFFFAOYSA-N1-[4-[(2,5-dioxopyrrol-1-yl)methyl]cyclohexanecarbonyl]oxy-2,5-dioxopyrrolidine-3-sulfonic acidChemical compoundO=C1C(S(=O)(=O)O)CC(=O)N1OC(=O)C1CCC(CN2C(C=CC2=O)=O)CC1FPKVOQKZMBDBKP-UHFFFAOYSA-N0.000claimsdescription2
- SMZOUWXMTYCWNB-UHFFFAOYSA-N2-(2-methoxy-5-methylphenyl)ethanamineChemical compoundCOC1=CC=C(C)C=C1CCNSMZOUWXMTYCWNB-UHFFFAOYSA-N0.000claimsdescription2
- NIXOWILDQLNWCW-UHFFFAOYSA-N2-Propenoic acidNatural productsOC(=O)C=CNIXOWILDQLNWCW-UHFFFAOYSA-N0.000claimsdescription2
- SHKUUQIDMUMQQK-UHFFFAOYSA-N2-[4-(oxiran-2-ylmethoxy)butoxymethyl]oxiraneChemical compoundC1OC1COCCCCOCC1CO1SHKUUQIDMUMQQK-UHFFFAOYSA-N0.000claimsdescription2
- HJBUBXIDMQBSQW-UHFFFAOYSA-N4-(4-diazoniophenyl)benzenediazoniumChemical compoundC1=CC([N+]#N)=CC=C1C1=CC=C([N+]#N)C=C1HJBUBXIDMQBSQW-UHFFFAOYSA-N0.000claimsdescription2
- BQCADISMDOOEFD-UHFFFAOYSA-NSilverChemical compound[Ag]BQCADISMDOOEFD-UHFFFAOYSA-N0.000claimsdescription2
- IBVAQQYNSHJXBV-UHFFFAOYSA-Nadipic acid dihydrazideChemical compoundNNC(=O)CCCCC(=O)NNIBVAQQYNSHJXBV-UHFFFAOYSA-N0.000claimsdescription2
- 239000000975dyeSubstances0.000claimsdescription2
- 239000007850fluorescent dyeSubstances0.000claimsdescription2
- PCHJSUWPFVWCPO-UHFFFAOYSA-NgoldChemical compound[Au]PCHJSUWPFVWCPO-UHFFFAOYSA-N0.000claimsdescription2
- 229910052737goldInorganic materials0.000claimsdescription2
- 239000010931goldSubstances0.000claimsdescription2
- 229910052741iridiumInorganic materials0.000claimsdescription2
- GKOZUEZYRPOHIO-UHFFFAOYSA-Niridium atomChemical compound[Ir]GKOZUEZYRPOHIO-UHFFFAOYSA-N0.000claimsdescription2
- 239000002923metal particleSubstances0.000claimsdescription2
- 229910052697platinumInorganic materials0.000claimsdescription2
- 229910052709silverInorganic materials0.000claimsdescription2
- 239000004332silverSubstances0.000claimsdescription2
- 101100537532Rattus norvegicus Tnni3 geneProteins0.000claims10
- 102000003992PeroxidasesHuman genes0.000claims1
- 102000005936beta-GalactosidaseHuman genes0.000claims1
- 108040007629peroxidase activity proteinsProteins0.000claims1
- 239000003550markerSubstances0.000abstractdescription17
- 208000030090Acute DiseaseDiseases0.000abstractdescription12
- 238000003745diagnosisMethods0.000abstractdescription8
- 201000010099diseaseDiseases0.000abstractdescription8
- 208000037265diseases, disorders, signs and symptomsDiseases0.000abstractdescription8
- 102100036859Troponin I, cardiac muscleHuman genes0.000description59
- 101710128251Troponin I, cardiac muscleProteins0.000description59
- 238000003556assayMethods0.000description22
- 235000018102proteinsNutrition0.000description18
- 238000002360preparation methodMethods0.000description14
- 238000006243chemical reactionMethods0.000description13
- 239000008280bloodSubstances0.000description12
- 239000000243solutionSubstances0.000description12
- 108010065729Troponin IProteins0.000description11
- 210000004369bloodAnatomy0.000description11
- 239000002245particleSubstances0.000description11
- LMDZBCPBFSXMTL-UHFFFAOYSA-N1-ethyl-3-(3-dimethylaminopropyl)carbodiimideChemical compoundCCN=C=NCCCN(C)CLMDZBCPBFSXMTL-UHFFFAOYSA-N0.000description10
- 238000002965ELISAMethods0.000description10
- 239000000047productSubstances0.000description10
- 238000001179sorption measurementMethods0.000description10
- 210000002966serumAnatomy0.000description8
- 230000001154acute effectEffects0.000description7
- 230000000747cardiac effectEffects0.000description7
- 230000021615conjugationEffects0.000description7
- 238000012360testing methodMethods0.000description7
- 239000012634fragmentSubstances0.000description6
- 239000000463materialSubstances0.000description6
- 239000004005microsphereSubstances0.000description6
- PPBRXRYQALVLMV-UHFFFAOYSA-NStyreneChemical compoundC=CC1=CC=CC=C1PPBRXRYQALVLMV-UHFFFAOYSA-N0.000description5
- 150000001413amino acidsChemical class0.000description5
- 238000010668complexation reactionMethods0.000description5
- 229920000642polymerPolymers0.000description5
- 210000002027skeletal muscleAnatomy0.000description5
- 239000000758substrateSubstances0.000description5
- IAZDPXIOMUYVGZ-UHFFFAOYSA-NDimethylsulphoxideChemical compoundCS(C)=OIAZDPXIOMUYVGZ-UHFFFAOYSA-N0.000description4
- 239000007987MES bufferSubstances0.000description4
- 108010029485Protein IsoformsProteins0.000description4
- 102000001708Protein IsoformsHuman genes0.000description4
- 238000004458analytical methodMethods0.000description4
- 238000007385chemical modificationMethods0.000description4
- 238000010168coupling processMethods0.000description4
- LOKCTEFSRHRXRJ-UHFFFAOYSA-Idipotassium trisodium dihydrogen phosphate hydrogen phosphate dichlorideChemical compoundP(=O)(O)(O)[O-].[K+].P(=O)(O)([O-])[O-].[Na+].[Na+].[Cl-].[K+].[Cl-].[Na+]LOKCTEFSRHRXRJ-UHFFFAOYSA-I0.000description4
- 230000007274generation of a signal involved in cell-cell signalingEffects0.000description4
- 230000003993interactionEffects0.000description4
- 210000004165myocardiumAnatomy0.000description4
- 239000002953phosphate buffered salineSubstances0.000description4
- 229920001184polypeptidePolymers0.000description4
- 108090000765processed proteins & peptidesProteins0.000description4
- 102000004196processed proteins & peptidesHuman genes0.000description4
- 108010074051C-Reactive ProteinProteins0.000description3
- 102100032752C-reactive proteinHuman genes0.000description3
- 238000012286ELISA AssayMethods0.000description3
- 102000057297Pepsin AHuman genes0.000description3
- 108090000284Pepsin AProteins0.000description3
- 239000004372Polyvinyl alcoholSubstances0.000description3
- 102000013394Troponin IHuman genes0.000description3
- 235000001014amino acidNutrition0.000description3
- 239000000872bufferSubstances0.000description3
- 229920001577copolymerPolymers0.000description3
- 230000008878couplingEffects0.000description3
- 238000005859coupling reactionMethods0.000description3
- 238000011534incubationMethods0.000description3
- 229940111202pepsinDrugs0.000description3
- 229920002451polyvinyl alcoholPolymers0.000description3
- 230000017854proteolysisEffects0.000description3
- 230000035945sensitivityEffects0.000description3
- 102100026189Beta-galactosidaseHuman genes0.000description2
- DHMQDGOQFOQNFH-UHFFFAOYSA-NGlycineChemical compoundNCC(O)=ODHMQDGOQFOQNFH-UHFFFAOYSA-N0.000description2
- 101000851334Homo sapiens Troponin I, cardiac muscleProteins0.000description2
- 108010001336Horseradish PeroxidaseProteins0.000description2
- 239000004698PolyethyleneSubstances0.000description2
- 239000004743PolypropyleneSubstances0.000description2
- 239000004365ProteaseSubstances0.000description2
- 239000012506Sephacryl®Substances0.000description2
- 229920005654SephadexPolymers0.000description2
- 239000012507Sephadex™Substances0.000description2
- XUIMIQQOPSSXEZ-UHFFFAOYSA-NSiliconChemical compound[Si]XUIMIQQOPSSXEZ-UHFFFAOYSA-N0.000description2
- 102000004903TroponinHuman genes0.000description2
- 108090001027TroponinProteins0.000description2
- 102100026893Troponin T, cardiac muscleHuman genes0.000description2
- 101710165323Troponin T, cardiac muscleProteins0.000description2
- 125000000539amino acid groupChemical group0.000description2
- 239000002981blocking agentSubstances0.000description2
- 125000004432carbon atomChemical groupC*0.000description2
- 230000015556catabolic processEffects0.000description2
- 229920002678cellulosePolymers0.000description2
- 239000001913celluloseSubstances0.000description2
- 238000005119centrifugationMethods0.000description2
- 239000003638chemical reducing agentSubstances0.000description2
- 238000003776cleavage reactionMethods0.000description2
- 150000001875compoundsChemical class0.000description2
- 230000001268conjugating effectEffects0.000description2
- 238000006731degradation reactionMethods0.000description2
- 238000011033desaltingMethods0.000description2
- 238000010828elutionMethods0.000description2
- 238000013467fragmentationMethods0.000description2
- 238000006062fragmentation reactionMethods0.000description2
- 229940127121immunoconjugateDrugs0.000description2
- MYWUZJCMWCOHBA-VIFPVBQESA-NmethamphetamineChemical compoundCN[C@@H](C)CC1=CC=CC=C1MYWUZJCMWCOHBA-VIFPVBQESA-N0.000description2
- 238000002156mixingMethods0.000description2
- 230000004048modificationEffects0.000description2
- 238000012986modificationMethods0.000description2
- 239000000178monomerSubstances0.000description2
- 230000003647oxidationEffects0.000description2
- 238000007254oxidation reactionMethods0.000description2
- 230000026731phosphorylationEffects0.000description2
- 238000006366phosphorylation reactionMethods0.000description2
- 229920002285poly(styrene-co-acrylonitrile)Polymers0.000description2
- 229920000573polyethylenePolymers0.000description2
- 229920001155polypropylenePolymers0.000description2
- 239000002243precursorSubstances0.000description2
- 230000006337proteolytic cleavageEffects0.000description2
- 238000006722reduction reactionMethods0.000description2
- 230000007017scissionEffects0.000description2
- 239000010703siliconSubstances0.000description2
- 229910052710siliconInorganic materials0.000description2
- 239000004094surface-active agentSubstances0.000description2
- 239000000725suspensionSubstances0.000description2
- 125000003396thiol groupChemical group[H]S*0.000description2
- 229920002554vinyl polymerPolymers0.000description2
- 235000012431wafersNutrition0.000description2
- SXGZJKUKBWWHRA-UHFFFAOYSA-N2-(N-morpholiniumyl)ethanesulfonateChemical compound[O-]S(=O)(=O)CC[NH+]1CCOCC1SXGZJKUKBWWHRA-UHFFFAOYSA-N0.000description1
- IEQAICDLOKRSRL-UHFFFAOYSA-N2-[2-[2-[2-[2-[2-[2-[2-[2-[2-[2-[2-[2-[2-[2-[2-[2-[2-[2-[2-[2-[2-(2-dodecoxyethoxy)ethoxy]ethoxy]ethoxy]ethoxy]ethoxy]ethoxy]ethoxy]ethoxy]ethoxy]ethoxy]ethoxy]ethoxy]ethoxy]ethoxy]ethoxy]ethoxy]ethoxy]ethoxy]ethoxy]ethoxy]ethoxy]ethanolChemical compoundCCCCCCCCCCCCOCCOCCOCCOCCOCCOCCOCCOCCOCCOCCOCCOCCOCCOCCOCCOCCOCCOCCOCCOCCOCCOCCOCCOIEQAICDLOKRSRL-UHFFFAOYSA-N0.000description1
- 108091006112ATPasesProteins0.000description1
- NIXOWILDQLNWCW-UHFFFAOYSA-MAcrylateChemical compound[O-]C(=O)C=CNIXOWILDQLNWCW-UHFFFAOYSA-M0.000description1
- 108010043137ActomyosinProteins0.000description1
- 108010052875Adenine deaminaseProteins0.000description1
- 102000057290Adenosine TriphosphatasesHuman genes0.000description1
- 229920000936AgarosePolymers0.000description1
- 102000007698Alcohol dehydrogenaseHuman genes0.000description1
- 108010021809Alcohol dehydrogenaseProteins0.000description1
- 102000013142AmylasesHuman genes0.000description1
- 108010065511AmylasesProteins0.000description1
- 108010032595Antibody Binding SitesProteins0.000description1
- 102000004452ArginaseHuman genes0.000description1
- 108700024123ArginasesProteins0.000description1
- 102000004625Aspartate AminotransferasesHuman genes0.000description1
- 108010003415Aspartate AminotransferasesProteins0.000description1
- 208000010392Bone FracturesDiseases0.000description1
- OYPRJOBELJOOCE-UHFFFAOYSA-NCalciumChemical compound[Ca]OYPRJOBELJOOCE-UHFFFAOYSA-N0.000description1
- 102100035882CatalaseHuman genes0.000description1
- 108010053835CatalaseProteins0.000description1
- 206010008479Chest PainDiseases0.000description1
- 102000004420Creatine KinaseHuman genes0.000description1
- 108010042126Creatine kinaseProteins0.000description1
- 229920002307DextranPolymers0.000description1
- 108090000270FicainProteins0.000description1
- 108010068561Fructose-Bisphosphate AldolaseProteins0.000description1
- 102000001390Fructose-Bisphosphate AldolaseHuman genes0.000description1
- 239000004471GlycineSubstances0.000description1
- 108090000288GlycoproteinsProteins0.000description1
- 102000003886GlycoproteinsHuman genes0.000description1
- 229930189936GlyoxalaseNatural products0.000description1
- 206010018852HaematomaDiseases0.000description1
- 102000005548HexokinaseHuman genes0.000description1
- 108700040460HexokinasesProteins0.000description1
- 108010093096Immobilized EnzymesProteins0.000description1
- 108010044467IsoenzymesProteins0.000description1
- 102000003855L-lactate dehydrogenaseHuman genes0.000description1
- 108700023483L-lactate dehydrogenasesProteins0.000description1
- 102000004882LipaseHuman genes0.000description1
- 108090001060LipaseProteins0.000description1
- 239000004367LipaseSubstances0.000description1
- 108090000362Lymphotoxin-betaProteins0.000description1
- PEEHTFAAVSWFBL-UHFFFAOYSA-NMaleimideChemical compoundO=C1NC(=O)C=C1PEEHTFAAVSWFBL-UHFFFAOYSA-N0.000description1
- CERQOIWHTDAKMF-UHFFFAOYSA-MMethacrylateChemical compoundCC(=C)C([O-])=OCERQOIWHTDAKMF-UHFFFAOYSA-M0.000description1
- 208000029549Muscle injuryDiseases0.000description1
- 239000000020NitrocelluloseSubstances0.000description1
- 239000004677NylonSubstances0.000description1
- 108090000526PapainProteins0.000description1
- 108091093037Peptide nucleic acidProteins0.000description1
- 102000045595Phosphoprotein PhosphatasesHuman genes0.000description1
- 108700019535Phosphoprotein PhosphatasesProteins0.000description1
- 102000004160Phosphoric Monoester HydrolasesHuman genes0.000description1
- 108090000608Phosphoric Monoester HydrolasesProteins0.000description1
- 102000009097PhosphorylasesHuman genes0.000description1
- 108010073135PhosphorylasesProteins0.000description1
- 239000004642PolyimideSubstances0.000description1
- 239000004721Polyphenylene oxideSubstances0.000description1
- 229920001213Polysorbate 20Polymers0.000description1
- 229920001328Polyvinylidene chloridePolymers0.000description1
- 229920000297RayonPolymers0.000description1
- 108010071390Serum AlbuminProteins0.000description1
- 102000007562Serum AlbuminHuman genes0.000description1
- 241000191940StaphylococcusSpecies0.000description1
- 102000019259Succinate DehydrogenaseHuman genes0.000description1
- 108010012901Succinate DehydrogenaseProteins0.000description1
- 229920004890Triton X-100Polymers0.000description1
- 108010030743TropomyosinProteins0.000description1
- 102000005937TropomyosinHuman genes0.000description1
- 102000013534Troponin CHuman genes0.000description1
- 102000004987Troponin THuman genes0.000description1
- 108090001108Troponin TProteins0.000description1
- 102000004142TrypsinHuman genes0.000description1
- 108090000631TrypsinProteins0.000description1
- 230000002159abnormal effectEffects0.000description1
- 150000001252acrylic acid derivativesChemical class0.000description1
- 206010000891acute myocardial infarctionDiseases0.000description1
- 239000002390adhesive tapeSubstances0.000description1
- 230000032683agingEffects0.000description1
- 125000005210alkyl ammonium groupChemical group0.000description1
- 125000002947alkylene groupChemical group0.000description1
- 229910045601alloyInorganic materials0.000description1
- 239000000956alloySubstances0.000description1
- 235000019418amylaseNutrition0.000description1
- 229940025131amylasesDrugs0.000description1
- 239000000427antigenSubstances0.000description1
- 230000000890antigenic effectEffects0.000description1
- 102000036639antigensHuman genes0.000description1
- 108091007433antigensProteins0.000description1
- 238000013459approachMethods0.000description1
- 239000008346aqueous phaseSubstances0.000description1
- 239000007864aqueous solutionSubstances0.000description1
- 239000013060biological fluidSubstances0.000description1
- 210000004556brainAnatomy0.000description1
- 229920005549butyl rubberPolymers0.000description1
- 229910052791calciumInorganic materials0.000description1
- 239000011575calciumSubstances0.000description1
- 125000003178carboxy groupChemical group[H]OC(*)=O0.000description1
- 229920002301cellulose acetatePolymers0.000description1
- 239000004568cementSubstances0.000description1
- 238000010382chemical cross-linkingMethods0.000description1
- 239000007795chemical reaction productSubstances0.000description1
- 239000003795chemical substances by applicationSubstances0.000description1
- 238000002967competitive immunoassayMethods0.000description1
- 239000012141concentrateSubstances0.000description1
- 230000002596correlated effectEffects0.000description1
- 239000007822coupling agentSubstances0.000description1
- 238000004132cross linkingMethods0.000description1
- 230000009260cross reactivityEffects0.000description1
- ATDGTVJJHBUTRL-UHFFFAOYSA-Ncyanogen bromideChemical compoundBrC#NATDGTVJJHBUTRL-UHFFFAOYSA-N0.000description1
- NZNMSOFKMUBTKW-UHFFFAOYSA-Ncyclohexanecarboxylic acidChemical compoundOC(=O)C1CCCCC1NZNMSOFKMUBTKW-UHFFFAOYSA-N0.000description1
- UFULAYFCSOUIOV-UHFFFAOYSA-NcysteamineChemical compoundNCCSUFULAYFCSOUIOV-UHFFFAOYSA-N0.000description1
- 235000018417cysteineNutrition0.000description1
- XUJNEKJLAYXESH-UHFFFAOYSA-NcysteineNatural productsSCC(N)C(O)=OXUJNEKJLAYXESH-UHFFFAOYSA-N0.000description1
- 230000007423decreaseEffects0.000description1
- 230000007812deficiencyEffects0.000description1
- 238000011161developmentMethods0.000description1
- 230000018109developmental processEffects0.000description1
- 238000002405diagnostic procedureMethods0.000description1
- 229910003460diamondInorganic materials0.000description1
- 239000010432diamondSubstances0.000description1
- 230000029087digestionEffects0.000description1
- 229960001760dimethyl sulfoxideDrugs0.000description1
- 238000004090dissolutionMethods0.000description1
- 239000002934diureticSubstances0.000description1
- 230000000694effectsEffects0.000description1
- 239000002158endotoxinSubstances0.000description1
- 238000005516engineering processMethods0.000description1
- 230000002255enzymatic effectEffects0.000description1
- 230000007717exclusionEffects0.000description1
- POTUGHMKJGOKRI-UHFFFAOYSA-NficinChemical compoundFI=CI=NPOTUGHMKJGOKRI-UHFFFAOYSA-N0.000description1
- 235000019836ficinNutrition0.000description1
- 239000000499gelSubstances0.000description1
- 102000034356gene-regulatory proteinsHuman genes0.000description1
- 108091006104gene-regulatory proteinsProteins0.000description1
- 229920000578graft copolymerPolymers0.000description1
- 210000005003heart tissueAnatomy0.000description1
- 229940088597hormoneDrugs0.000description1
- 239000005556hormoneSubstances0.000description1
- 230000002209hydrophobic effectEffects0.000description1
- 208000014674injuryDiseases0.000description1
- 229920000592inorganic polymerPolymers0.000description1
- 102000027411intracellular receptorsHuman genes0.000description1
- 108091008582intracellular receptorsProteins0.000description1
- 239000012948isocyanateSubstances0.000description1
- IQPQWNKOIGAROB-UHFFFAOYSA-Nisocyanate groupChemical group[N-]=C=OIQPQWNKOIGAROB-UHFFFAOYSA-N0.000description1
- 150000002513isocyanatesChemical class0.000description1
- 210000000265leukocyteAnatomy0.000description1
- 125000005647linker groupChemical group0.000description1
- 235000019421lipaseNutrition0.000description1
- 229920006008lipopolysaccharidePolymers0.000description1
- 239000007788liquidSubstances0.000description1
- 239000000696magnetic materialSubstances0.000description1
- 238000004519manufacturing processMethods0.000description1
- 238000013507mappingMethods0.000description1
- 229960003151mercaptamineDrugs0.000description1
- 150000002734metacrylic acid derivativesChemical class0.000description1
- 229910044991metal oxideInorganic materials0.000description1
- 150000004706metal oxidesChemical class0.000description1
- 229910052752metalloidInorganic materials0.000description1
- 150000002738metalloidsChemical class0.000description1
- 150000002739metalsChemical class0.000description1
- 238000003541multi-stage reactionMethods0.000description1
- 230000009756muscle regenerationEffects0.000description1
- 230000035772mutationEffects0.000description1
- 208000037891myocardial injuryDiseases0.000description1
- 230000001452natriuretic effectEffects0.000description1
- 229920001220nitrocellulosPolymers0.000description1
- 229920001778nylonPolymers0.000description1
- 229920000620organic polymerPolymers0.000description1
- 229940055729papainDrugs0.000description1
- 235000019834papainNutrition0.000description1
- 239000008188pelletSubstances0.000description1
- 239000012071phaseSubstances0.000description1
- 150000003904phospholipidsChemical class0.000description1
- 229920002401polyacrylamidePolymers0.000description1
- 229920001515polyalkylene glycolPolymers0.000description1
- 229920001748polybutylenePolymers0.000description1
- 229920000728polyesterPolymers0.000description1
- 229920000570polyetherPolymers0.000description1
- 229920001721polyimidePolymers0.000description1
- 102000040430polynucleotideHuman genes0.000description1
- 108091033319polynucleotideProteins0.000description1
- 239000002157polynucleotideSubstances0.000description1
- 229920000098polyolefinPolymers0.000description1
- 239000000256polyoxyethylene sorbitan monolaurateSubstances0.000description1
- 235000010486polyoxyethylene sorbitan monolaurateNutrition0.000description1
- 229920006324polyoxymethylenePolymers0.000description1
- 229920002689polyvinyl acetatePolymers0.000description1
- 239000011118polyvinyl acetateSubstances0.000description1
- 229920000915polyvinyl chloridePolymers0.000description1
- 239000004800polyvinyl chlorideSubstances0.000description1
- 229920002620polyvinyl fluoridePolymers0.000description1
- 239000005033polyvinylidene chlorideSubstances0.000description1
- 239000002244precipitateSubstances0.000description1
- 239000003755preservative agentSubstances0.000description1
- 230000002335preservative effectEffects0.000description1
- 230000005855radiationEffects0.000description1
- 239000000941radioactive substanceSubstances0.000description1
- 239000002964rayonSubstances0.000description1
- 239000000376reactantSubstances0.000description1
- 239000011535reaction bufferSubstances0.000description1
- 230000008707rearrangementEffects0.000description1
- 230000004044responseEffects0.000description1
- 230000002441reversible effectEffects0.000description1
- 238000000926separation methodMethods0.000description1
- 229920000260silasticPolymers0.000description1
- 239000000741silica gelSubstances0.000description1
- 229910002027silica gelInorganic materials0.000description1
- 150000004760silicatesChemical class0.000description1
- 229920002379silicone rubberPolymers0.000description1
- VUFNRPJNRFOTGK-UHFFFAOYSA-Msodium;1-[4-[(2,5-dioxopyrrol-1-yl)methyl]cyclohexanecarbonyl]oxy-2,5-dioxopyrrolidine-3-sulfonateChemical compound[Na+].O=C1C(S(=O)(=O)[O-])CC(=O)N1OC(=O)C1CCC(CN2C(C=CC2=O)=O)CC1VUFNRPJNRFOTGK-UHFFFAOYSA-M0.000description1
- 238000001228spectrumMethods0.000description1
- 230000006641stabilisationEffects0.000description1
- 238000011105stabilizationMethods0.000description1
- 239000012536storage bufferSubstances0.000description1
- 210000003699striated muscleAnatomy0.000description1
- 229920003051synthetic elastomerPolymers0.000description1
- 239000005061synthetic rubberSubstances0.000description1
- 230000008685targetingEffects0.000description1
- 230000026683transductionEffects0.000description1
- 238000010361transductionMethods0.000description1
- 230000001052transient effectEffects0.000description1
- 230000008736traumatic injuryEffects0.000description1
- 239000012588trypsinSubstances0.000description1
- 125000000391vinyl groupChemical group[H]C([*])=C([H])[H]0.000description1
Images
Classifications
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01L—CHEMICAL OR PHYSICAL LABORATORY APPARATUS FOR GENERAL USE
- B01L3/00—Containers or dishes for laboratory use, e.g. laboratory glassware; Droppers
- B01L3/50—Containers for the purpose of retaining a material to be analysed, e.g. test tubes
- B01L3/502—Containers for the purpose of retaining a material to be analysed, e.g. test tubes with fluid transport, e.g. in multi-compartment structures
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01L—CHEMICAL OR PHYSICAL LABORATORY APPARATUS FOR GENERAL USE
- B01L3/00—Containers or dishes for laboratory use, e.g. laboratory glassware; Droppers
- B01L3/50—Containers for the purpose of retaining a material to be analysed, e.g. test tubes
- B01L3/502—Containers for the purpose of retaining a material to be analysed, e.g. test tubes with fluid transport, e.g. in multi-compartment structures
- B01L3/5027—Containers for the purpose of retaining a material to be analysed, e.g. test tubes with fluid transport, e.g. in multi-compartment structures by integrated microfluidic structures, i.e. dimensions of channels and chambers are such that surface tension forces are important, e.g. lab-on-a-chip
- B01L3/50273—Containers for the purpose of retaining a material to be analysed, e.g. test tubes with fluid transport, e.g. in multi-compartment structures by integrated microfluidic structures, i.e. dimensions of channels and chambers are such that surface tension forces are important, e.g. lab-on-a-chip characterised by the means or forces applied to move the fluids
- G—PHYSICS
- G01—MEASURING; TESTING
- G01N—INVESTIGATING OR ANALYSING MATERIALS BY DETERMINING THEIR CHEMICAL OR PHYSICAL PROPERTIES
- G01N33/00—Investigating or analysing materials by specific methods not covered by groups G01N1/00 - G01N31/00
- G01N33/48—Biological material, e.g. blood, urine; Haemocytometers
- G01N33/50—Chemical analysis of biological material, e.g. blood, urine; Testing involving biospecific ligand binding methods; Immunological testing
- G01N33/53—Immunoassay; Biospecific binding assay; Materials therefor
- G—PHYSICS
- G01—MEASURING; TESTING
- G01N—INVESTIGATING OR ANALYSING MATERIALS BY DETERMINING THEIR CHEMICAL OR PHYSICAL PROPERTIES
- G01N33/00—Investigating or analysing materials by specific methods not covered by groups G01N1/00 - G01N31/00
- G01N33/48—Biological material, e.g. blood, urine; Haemocytometers
- G01N33/50—Chemical analysis of biological material, e.g. blood, urine; Testing involving biospecific ligand binding methods; Immunological testing
- G01N33/53—Immunoassay; Biospecific binding assay; Materials therefor
- G01N33/5302—Apparatus specially adapted for immunological test procedures
- G—PHYSICS
- G01—MEASURING; TESTING
- G01N—INVESTIGATING OR ANALYSING MATERIALS BY DETERMINING THEIR CHEMICAL OR PHYSICAL PROPERTIES
- G01N33/00—Investigating or analysing materials by specific methods not covered by groups G01N1/00 - G01N31/00
- G01N33/48—Biological material, e.g. blood, urine; Haemocytometers
- G01N33/50—Chemical analysis of biological material, e.g. blood, urine; Testing involving biospecific ligand binding methods; Immunological testing
- G01N33/53—Immunoassay; Biospecific binding assay; Materials therefor
- G01N33/543—Immunoassay; Biospecific binding assay; Materials therefor with an insoluble carrier for immobilising immunochemicals
- G—PHYSICS
- G01—MEASURING; TESTING
- G01N—INVESTIGATING OR ANALYSING MATERIALS BY DETERMINING THEIR CHEMICAL OR PHYSICAL PROPERTIES
- G01N33/00—Investigating or analysing materials by specific methods not covered by groups G01N1/00 - G01N31/00
- G01N33/48—Biological material, e.g. blood, urine; Haemocytometers
- G01N33/50—Chemical analysis of biological material, e.g. blood, urine; Testing involving biospecific ligand binding methods; Immunological testing
- G01N33/53—Immunoassay; Biospecific binding assay; Materials therefor
- G01N33/543—Immunoassay; Biospecific binding assay; Materials therefor with an insoluble carrier for immobilising immunochemicals
- G01N33/54313—Immunoassay; Biospecific binding assay; Materials therefor with an insoluble carrier for immobilising immunochemicals the carrier being characterised by its particulate form
- G—PHYSICS
- G01—MEASURING; TESTING
- G01N—INVESTIGATING OR ANALYSING MATERIALS BY DETERMINING THEIR CHEMICAL OR PHYSICAL PROPERTIES
- G01N33/00—Investigating or analysing materials by specific methods not covered by groups G01N1/00 - G01N31/00
- G01N33/48—Biological material, e.g. blood, urine; Haemocytometers
- G01N33/50—Chemical analysis of biological material, e.g. blood, urine; Testing involving biospecific ligand binding methods; Immunological testing
- G01N33/53—Immunoassay; Biospecific binding assay; Materials therefor
- G01N33/543—Immunoassay; Biospecific binding assay; Materials therefor with an insoluble carrier for immobilising immunochemicals
- G01N33/54353—Immunoassay; Biospecific binding assay; Materials therefor with an insoluble carrier for immobilising immunochemicals with ligand attached to the carrier via a chemical coupling agent
- G—PHYSICS
- G01—MEASURING; TESTING
- G01N—INVESTIGATING OR ANALYSING MATERIALS BY DETERMINING THEIR CHEMICAL OR PHYSICAL PROPERTIES
- G01N33/00—Investigating or analysing materials by specific methods not covered by groups G01N1/00 - G01N31/00
- G01N33/48—Biological material, e.g. blood, urine; Haemocytometers
- G01N33/50—Chemical analysis of biological material, e.g. blood, urine; Testing involving biospecific ligand binding methods; Immunological testing
- G01N33/53—Immunoassay; Biospecific binding assay; Materials therefor
- G01N33/543—Immunoassay; Biospecific binding assay; Materials therefor with an insoluble carrier for immobilising immunochemicals
- G01N33/54366—Apparatus specially adapted for solid-phase testing
- G01N33/54386—Analytical elements
- G—PHYSICS
- G01—MEASURING; TESTING
- G01N—INVESTIGATING OR ANALYSING MATERIALS BY DETERMINING THEIR CHEMICAL OR PHYSICAL PROPERTIES
- G01N33/00—Investigating or analysing materials by specific methods not covered by groups G01N1/00 - G01N31/00
- G01N33/48—Biological material, e.g. blood, urine; Haemocytometers
- G01N33/50—Chemical analysis of biological material, e.g. blood, urine; Testing involving biospecific ligand binding methods; Immunological testing
- G01N33/53—Immunoassay; Biospecific binding assay; Materials therefor
- G01N33/543—Immunoassay; Biospecific binding assay; Materials therefor with an insoluble carrier for immobilising immunochemicals
- G01N33/544—Immunoassay; Biospecific binding assay; Materials therefor with an insoluble carrier for immobilising immunochemicals the carrier being organic
- G01N33/545—Synthetic resin
- G—PHYSICS
- G01—MEASURING; TESTING
- G01N—INVESTIGATING OR ANALYSING MATERIALS BY DETERMINING THEIR CHEMICAL OR PHYSICAL PROPERTIES
- G01N33/00—Investigating or analysing materials by specific methods not covered by groups G01N1/00 - G01N31/00
- G01N33/48—Biological material, e.g. blood, urine; Haemocytometers
- G01N33/50—Chemical analysis of biological material, e.g. blood, urine; Testing involving biospecific ligand binding methods; Immunological testing
- G01N33/68—Chemical analysis of biological material, e.g. blood, urine; Testing involving biospecific ligand binding methods; Immunological testing involving proteins, peptides or amino acids
- G01N33/6887—Chemical analysis of biological material, e.g. blood, urine; Testing involving biospecific ligand binding methods; Immunological testing involving proteins, peptides or amino acids from muscle, cartilage or connective tissue
- G—PHYSICS
- G01—MEASURING; TESTING
- G01N—INVESTIGATING OR ANALYSING MATERIALS BY DETERMINING THEIR CHEMICAL OR PHYSICAL PROPERTIES
- G01N33/00—Investigating or analysing materials by specific methods not covered by groups G01N1/00 - G01N31/00
- G01N33/48—Biological material, e.g. blood, urine; Haemocytometers
- G01N33/50—Chemical analysis of biological material, e.g. blood, urine; Testing involving biospecific ligand binding methods; Immunological testing
- G01N33/68—Chemical analysis of biological material, e.g. blood, urine; Testing involving biospecific ligand binding methods; Immunological testing involving proteins, peptides or amino acids
- G01N33/6893—Chemical analysis of biological material, e.g. blood, urine; Testing involving biospecific ligand binding methods; Immunological testing involving proteins, peptides or amino acids related to diseases not provided for elsewhere
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01L—CHEMICAL OR PHYSICAL LABORATORY APPARATUS FOR GENERAL USE
- B01L2400/00—Moving or stopping fluids
- B01L2400/06—Valves, specific forms thereof
- B01L2400/0677—Valves, specific forms thereof phase change valves; Meltable, freezing, dissolvable plugs; Destructible barriers
- B01L2400/0683—Valves, specific forms thereof phase change valves; Meltable, freezing, dissolvable plugs; Destructible barriers mechanically breaking a wall or membrane within a channel or chamber
- G—PHYSICS
- G01—MEASURING; TESTING
- G01N—INVESTIGATING OR ANALYSING MATERIALS BY DETERMINING THEIR CHEMICAL OR PHYSICAL PROPERTIES
- G01N2333/00—Assays involving biological materials from specific organisms or of a specific nature
- G01N2333/435—Assays involving biological materials from specific organisms or of a specific nature from animals; from humans
- G01N2333/46—Assays involving biological materials from specific organisms or of a specific nature from animals; from humans from vertebrates
- G01N2333/47—Assays involving proteins of known structure or function as defined in the subgroups
- G—PHYSICS
- G01—MEASURING; TESTING
- G01N—INVESTIGATING OR ANALYSING MATERIALS BY DETERMINING THEIR CHEMICAL OR PHYSICAL PROPERTIES
- G01N2333/00—Assays involving biological materials from specific organisms or of a specific nature
- G01N2333/435—Assays involving biological materials from specific organisms or of a specific nature from animals; from humans
- G01N2333/46—Assays involving biological materials from specific organisms or of a specific nature from animals; from humans from vertebrates
- G01N2333/47—Assays involving proteins of known structure or function as defined in the subgroups
- G01N2333/4701—Details
- G01N2333/4712—Muscle proteins, e.g. myosin, actin, protein
- G—PHYSICS
- G01—MEASURING; TESTING
- G01N—INVESTIGATING OR ANALYSING MATERIALS BY DETERMINING THEIR CHEMICAL OR PHYSICAL PROPERTIES
- G01N2800/00—Detection or diagnosis of diseases
- G01N2800/32—Cardiovascular disorders
- G01N2800/324—Coronary artery diseases, e.g. angina pectoris, myocardial infarction
Definitions
- the present inventionrelates to the diagnosis of diseases and medical events, notably myocardial infarction (“MI”). More specifically, the present invention relates to the detection of a clinical marker of a disease or medical event, in particular MI, using multiple antibodies, each antibody having a different specificity for the clinical marker. The present invention also relates to reagents and apparatuses used in the diagnosis of a disease or medical event, in particular MI.
- MImyocardial infarction
- ELISAenzyme-linked immunosorbent assay
- CK-MBas a clinical marker of MI is that the level of CK-MB in the skeletal muscle varies with the degree of skeletal muscle regeneration, information which may often not be known when administering a test or analyzing a test result for MI.
- Another disadvantage of testing for CK-MBis that CK-MB levels remain elevated for only 2-3 days after the onset of chest pain. For patients admitted after that time, the CK-MB test will be of limited value. Thus, due to the lack of specificity when assaying CK-MB, and the limited time frame for its use as a diagnostic tool, CK-MB is not an ideal clinical marker for diagnosing MI.
- Cardiac troponin I(“cTnI”) is now used as an accurate cardiac-specific biological parameter detectable in serum very soon after MI and remaining present for more than 2-3 days after the onset of MI.
- Troponinis present in cardiac tissue as a complex of three subunits: Troponin T (“TnT”), the tropomyosin binding subunit, Troponin C (“TnC”), the Ca 2+ binding subunit; and Troponin I (“TnI”), the sub-unit which inhibits the actomyosin Mg 2+ ATPase.
- TnIis a thin filament regulatory protein complex, which confers calcium sensitivity to the cardiac and striated muscle.
- Cardiac TnIis uniquely located in the myocardium where it is the only isoform present.
- Cardiac TnIrapidly appears in human serum (within approximately 4 to 6 hours) following a MI. It reaches a peak level after 18-24 hours and remains at elevated levels in the blood stream for up to 6-10 days. As a result, cTnT released into the circulation from the myocardium is very specific for myocardial injury.
- Elevated cTnI levels in bloodmay be used to diagnose MI and distinguish other heart related events and diseases.
- Immunoassay systems capable of accurately detecting human cTnIwould be valuable to the medical community for diagnosing the occurrence of MI.
- Apple and WuMyocardial infarction redefined: Role of cardiac troponin testing.
- Cardiac TnIexists in multiple subforms in blood as a result of modifications such as proteolytic cleavage, phosphorylation, chemical oxidation, chemical reduction, cleavage of amino acid residues, and chemical modification of amino acid moieties.
- amino acids 1 to 25 and 150 to 209are generally not found on cTnI subforms in serum as a result of proteolysis.
- the many different subforms of cTnI circulating in the bloodstreamare predominantly found in complexes with other proteins. See Katrukha (1997).
- cTnImay be complexed with cTnT and cTnC (“cITC”).
- the present inventionis related to the detection of an analyte of interest.
- the analyte of interestmay be cTnI.
- an immunoassay composition for detecting an analyte of interestmay comprise three or more antibodies, wherein each antibody is capable of binding to a different epitope on the analyte. At least two of the three different antibodies which bind to the analyte are also capable of binding to at least two different epitopes on a subform of the analyte. At least one of the epitopes on the analyte of interest is unavailable for binding on the subform.
- an immunoassay device for detecting an analyte of interestmay comprise two or more antibodies, wherein each antibody binds to a different epitope on the analyte and wherein the two or more antibodies are bound to at least one surface. At least one of the two different antibodies which bind to the analyte is also capable of binding to at least one different epitope on a subform of the analyte. At least one of the epitopes on the analyte of interest is unavailable for binding on the subform.
- an immunoassay kit for detecting an analyte of interestmay comprise three or more antibodies, wherein each antibody is capable of binding to a different epitope on the analyte. At least two of the three different antibodies which bind to the analyte are also capable of binding to at least two different epitopes on a subform of the analyte. At least one of the epitopes on the analyte of interest is unavailable for binding on the subform.
- a sandwich immunoassay productmay comprise an analyte of interest and a subform of the analyte. At least three different epitopes on the analyte are available for binding by at least three different antibodies. At least three different antibodies are bound to different epitopes on the analyte. At least two of the three epitopes on the analyte are available for binding on the subform. At least two of the at least three antibodie are bound to different epitopes on the subform. At least one of the epitopes on the analyte of interest is unavailable for binding on the subform.
- a patientmay be diagnosed for the occurrence of an acute disease, such as myocardial infarction, by applying a sample obtained from the patient to a surface comprising at least two antibodies which bind to different epitopes on cTnI, wherein at least one of the two antibodies is capable of binding to a different epitope on a subform of cTnI, and wherein at least one epitope of the at least two antibodies is unavailable for binding on the subform.
- a third antibodyis then added which binds to yet another epitope on cTnI and the subform. The extent of binding of the third antibody to cTnI and the subform is then measured.
- FIG. 1shows the standard sandwich assay for detecting an analyte of interest, wherein a capture antibody (Ab 1 ) and signal antibody (Ab 2 ) are directed to a first and second epitope (e 1 and e 2 ), respectively, of an analyte of interest (An).
- the signal antibodyis labeled with an enzyme (Enz), which is used for signal generation by converting substrate (S) to product (P).
- FIG. 2is a representation of an analyte of interest (An) with three epitopes (e 1 , e 2 and e 3 ).
- analyte of interestAn
- three epitopese 1 , e 2 and e 3
- a subformis produced with one or more of the epitopes unavailable for binding by antibodies.
- Each epitope that is unavailable for bindingis identified by a circle, whereas each epitope that is available for binding is identified without a circle.
- Each subformmay be further modified so that additional epitopes are unavailable for binding by antibodies.
- FIG. 3shows a modified sandwich assay for detecting an analyte of interest (An) and subforms thereof using one capture antibody (Ab 1 ) and more than one signal antibody (Ab 2 and Ab 3 ).
- the assayis able to detect the presence of the analyte and all subforms thereof that have at least one epitope for the capture antibody (e 1 ) and at least one epitope for a signal antibody (e 2 or e 3 ). All epitopes for binding are shown bound by an antibody; however, the analyte or subforms thereof need to be bound by only one capture antibody and one signal antibody to be detected.
- FIG. 4shows a modified sandwich assay for detecting an analyte of interest (An) and subforms thereof using more than one capture antibody (Ab 1 and Ab 2 ) and one signal antibody (Ab 3 ).
- the assayis able to detect the presence of the analyte and all subforms thereof that have at least one epitope for the signal antibody (e 3 ) and at least one epitope for a capture antibody (e 1 or e 2 ). All epitopes for binding are shown bound by an antibody; however, the analyte or subforms thereof need to be bound by only one capture antibody and one signal antibody to be detected.
- FIG. 5shows a modified sandwich assay for detecting an analyte of interest (An) using more than one capture antibody (Ab 1 and Ab 2 ) and more than one signal antibody (Ab 3 and Ab 4 ).
- the assayis able to detect the presence of the analyte and all subforms thereof that have at least one epitope for a capture antibody (e 1 or e 2 ) and at least one epitope for a signal antibody (e 3 or e 4 ). All epitopes for binding are shown bound by an antibody; however, the analyte or subforms thereof need to be bound by only one capture antibody and one signal antibody to be detected.
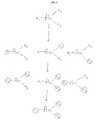
- FIG. 6shows one antibody (Ab 1 ) or more than one antibody (Ab 1 and Ab 2 ) binding to a different epitope (e 1 or e 2 ) on the analyte of interest (An) and conjugated to a common member, wherein the common member is a surface (FIG. 6A), microparticle (FIG. 6B), and a polypeptide, such as an enzyme (FIG. 6C).
- FIG. 7shows a mixture of common members individually conjugated with a different antibody (Ab 1 or Ab 2 ), each antibody binding to a different epitope (e 1 or e 2 ) on the analyte being assayed (An), wherein the common member is a microparticle (FIG. 7A) or polypeptide, such as an enzyme (FIG. 7B).
- FIG. 8shows a common member, such as a microparticle, conjugated to two or more antibodies (Ab 1 and Ab 2 ), conjugated to another common member, such as a surface, wherein two or more capture antibodies are either (i) individually conjugated to different microparticles (FIG. 8A), or (ii) conjugated together on a microparticle (FIG. 8B).
- FIG. 9depicts the primary structure of subform of cardiac troponin I (cTnI) without amino acids 1 to 25 and 150 to 209, and shows the location for epitopes of the cTnI antibodies.
- FIG. 10shows the amperometric signals from different single-use assays prepared with a first capture antibody (CB1), a second capture antibody (CB2), or a combination of first and second capture antibodies (CB12) to a whole blood sample spiked with free cTnI and two levels of cITC complex.
- CB1first capture antibody
- CB2second capture antibody
- CB12combination of first and second capture antibodies
- FIG. 11shows a plot of the average signal obtained from a whole blood sample spiked with 6.0 ng/mL cITC complex immediately after preparation and after one-day incubation at room temperature using cartridges prepared with either CB1 or CB12 coated sensors and with a signal producing enzyme conjugated to a first antibody (EC1), a second antibody (EC2) or a mixture of EC1 and EC2 (EC12).
- EC1first antibody
- EC2second antibody
- EC12a mixture of EC1 and EC2
- FIG. 12shows elution profiles for Fab-ALP conjugates with different ratios of ALP to Fab.
- Alytemeans a biological or chemical substance that is capable of being bound by at least three different antibodies, and includes subforms thereof that may be bound by at least three different antibodies.
- Antibodymeans a polypeptide or derivative thereof that specifically binds to an epitope on an analyte of interest or subform thereof.
- Capture antibodyor “capture reagent” means an antibody that specifically binds to an analyte of interest or subform thereof, wherein said antibody is immobilized on a surface either (i) before, (ii) after or (iii) during binding to said analyte or subform thereof.
- Detectwhen used to describe a signal of a signal-generating element, means a signal capable of being distinguished from background.
- Epitopemeans a binding site for an antibody or other binding member on an analyte of interest or subform thereof.
- An epitopeis “unavailable for binding” if the epitope is either not present or inaccessible for binding by an antibody.
- Immunoassayor “sandwich immunoassay” means a method of simultaneous sandwich, forward sandwich and reverse sandwich immunoassays, and includes competitive immunoassays thereof, all of which are well understood by those skilled in the art.
- Subformwhen used to describe an analyte, means the product of a chemical reaction, a member of a complex with other biological or chemical substances, alternative conformations, or combinations thereof that may be bound by at least two different antibodies.
- Sensing elementmeans any device or apparatus that is capable of detecting a signal.
- “Signal antibody” or “signal reagent”means an antibody that specifically binds to an analyte of interest or subform thereof, wherein said antibody is attached to a signal-generating element via a covalent linkage, hydrophobic interactions, hydrophilic interaction, ionic interactions, Van der Waal forces, or a combination thereof.
- “Signal-generating element”means a biological, chemical, or radioactive substance that (i) produces a detectable signal, directly or indirectly, or (ii) is itself detectable.
- “Surface”means a support that may be separated from a solution.
- the present inventionrelates to the detection of an analyte and subforms thereof.
- the analytemay be a clinical marker of a disease state from a patient believed to have suffered an acute disease event, wherein the clinical marker is also present in one or more subforms.
- the clinical markermay be a transiently elevated substance in the blood which is released in a significant quantity at, after, or before the time of the acute disease event of interest.
- the elevated concentration of the clinical marker analytedecreases as endogenous conversion factors act upon the clinical marker to produce subforms of the analyte.
- Subforms derived from the clinical markermay be transiently elevated in a serial manner, as each is first created then metabolized by endogenous conversion factors. The period of transient elevation may be for as short as a few hours to as long as several weeks.
- the analytemay be a biological substance such as a protein, glycoprotein, enzyme, etc., which is released in small quantities at the occasion of an acute disease event such as a heart attack, stroke, or at the occasion of a traumatic injury such as a broken bone or a hematoma.
- the analytemay be normally absent in such increased quantities and may be converted to a one or more subforms over time by endogenous conversion factors including, but not limited to proteolytic cleavage, phosphorylation, chemical oxidation, chemical reduction, cleavage of amino acid residues, and chemical modification of amino acid moieties.
- the analytemay be a protein including, but not limited to, TnI, TnT, CK-M, CK-B, myoglobin, hCG, TSH, FSH, pneumococcal PCA, apolipoproteins, C-reactive protein (CRP), brain natriuretic protein (BNP) and its pro-form pro-BNP, human leukocyte antigen and human apolipoproteins.
- a preferred analyteis cTnI.
- the analytehas at least three different epitopes that are available for binding by an antibody (FIG. 2).
- a subform of the analytehas at least two different epitopes that are available for binding by an antibody and the epitopes of the subform are also present on the analyte (FIG. 2).
- At least one epitope of the analyteis unavailable for binding on the subform.
- An epitope of the analyte unavailable for binding on the subformmay be as a result of complexation or alternative conformations, as well as the endogenous conversion factors described above.
- the analytemay be in a native or natural form.
- the analytemay also be a derivative of the native or natural form.
- the analytemay be full length cTnI monomer or a derivative thereof that is either free or part of cITC complex.
- the analytemay be any form of cTnI that is released into serum in connection with an MI.
- the analytemay also be any modified form of cTnI present in serum including, but not limited to, cTnI as shown in FIG. 9.
- the analytemay also be any modified form of cTnI that has one or more epitopes that are unavailable for binding on any precursor of the analyte.
- the modified or derivative form of cTnImust have at least three epitopes available for binding by three different antibodies.
- the present inventionmay also be used to detect an analyte and subforms thereof that are present at low concentration where high sensitivity is required.
- Analyteshave traditionally been detected using a system based on the sandwich assay depicted in FIG. 1 wherein a first monoclonal or polyclonal antibody for capture is attached to a surface, and a second monoclonal or polyclonal antibody is labeled with a signal-generating element (e.g., an enzyme).
- a signal-generating elemente.g., an enzyme
- the present inventionrelates to the use of a sandwich immunoassay for detecting an analyte and one or more subforms thereof (FIG. 2).
- Subforms of the analytemay be due to causes including mutations, complexation, chemical modification, proteolysis or rearrangement.
- the present inventionmay also be used to detect more than one related analyte, such as an active protein and its inactive precursor or a class of similar analytes for which a single detection assay is developed.
- the analyte of interest and one or more subforms thereofmay be detected by performing an immunoassay using antibodies specific for more than one epitope on the analyte either (i) on the capture side of the sandwich (FIG. 4), (ii) signal side of the sandwich (FIG. 3), (iii) or both (FIG. 5).
- the multiple sandwich assay of the present inventionmay detect the presence of the analyte and subforms thereof even if certain epitopes are unavailable for binding on the subform, so long as there is at least one epitope capable of binding by a capture antibody and at least one epitope available for binding by a signal antibody.
- the present inventionis therefore able to detect analytes and multiple subforms thereof that appear within a sample.
- Binding membersinclude, but are not limited to, extracellular or intracellular receptors, polynucleotides, peptide nucleic acids, and derivatives thereof.
- the use of multiple antibodies in the practice of the present inventionis contrasted with the use of polyclonal antibodies in traditional sandwich assays; in addition, the present invention is superior.
- the specific epitopes recognized and the ratio of various antibodies in the preparationis generally unknown.
- the epitopes recognized and the ratio of various antibodiesvary between polyclonal preparations.
- Another deficiency in the polyclonal approachis that the binding of individual antibodies within the polyclonal preparation will have diverse binding affinities.
- a significant portion of polyclonal preparationsare antibodies which have sub-standard analytical performances or which bind epitopes that compete with other antibodies in the preparation.
- the present inventionallows the preparation of controlled multi-epitopic reagents with more efficient assay characteristics because of better binding affinities and less reagent required for a given assay signal.
- the immunoassayis based on a first antibody attached to a surface (i.e., “capture antibody” or “capture reagent”) and at least a second and third antibody, wherein each antibody binds to a different epitope on the analyte.
- the antibodies not attached to a surfacemay be labeled with a signal-generating element (i.e., “signal antibody” or “signal reagent”). See FIG. 3.
- the immunoassayis based on at least a first and second capture antibody and a third antibody.
- the antibody not attached to a surfacemay be a signal antibody. See FIG. 4.
- the immunoassayis based on at least a first and second capture antibody and at least a third and fourth antibody.
- the antibodies not attached to a surfacemay be signal antibodies. See FIG. 5.
- the antibodies of the present inventionmay be any antibody that specifically binds to an epitope available for binding on an analyte of interest or a subform thereof.
- the antibodies of the present inventioninclude antibodies of classes IgG, IgM, IgA, IgD, and IgE, and fragments and derivatives thereof including Fab, F(ab′) 2 and single chain antibodies.
- the antibodies of the present inventioninclude monoclonal antibodies, polyclonal antibodies, affinity purified antibodies, or mixtures thereof which exhibit sufficient binding specificity to an epitope of the analyte or subform thereof. Monoclonal antibodies and fragments and derivatives thereof are preferred in the practice of the invention.
- the antibodies of the present inventionpreferably bind to epitopes of the analyte sufficiently removed from each other such that the antibodies do not mutually interfere with binding to the analyte or subform thereof.
- Appropriate antibodies for an analyte of interestmay be chosen by means of mapping combinations of available antibodies to known epitope sites on the analyte using methods known in the art.
- the antibodies of the present inventionmay be specific for human cTnI.
- the antibodiesmay specifically recognize epitopes located on the primary sequence of cTnI as indicated in FIG. 9.
- the antibodiesmay also be chosen from those antibodies indicated in FIG. 9.
- Capture antibodies of the present inventionmay be immobilized by conjugating one or more antibodies to a surface. See, e.g., FIG. 6A and FIG. 6B.
- a wide variety of compoundsmay be employed as the surface, the primary consideration being the binding of the antibody to the surface, the absence of interference with the signal generating element, and the absence of interference with the examination of the label. In particular, if a fluorescence or chromogenic spectrum is being measured, the surface should not provide interference.
- Organic and inorganic polymersmay be employed as the surface.
- suitable polymersinclude polyethylene, polypropylene, polybutylene, poly(4-methylbutylene), butyl rubber and other synthetic rubbers, silicone rubbers and silastic polymers, polyesters, polyimides, cellulose and cellulose derivatives (such as cellulose acetate, nitrocellulose and the like), acrylates, methacrylates, vinyl polymers (such as polyvinyl acetate, polyvinyl chloride, polyvinylidene chloride, polyvinyl fluoride, and the like), polystyrene and styrene graft copolymers, styrene-acrylonitrile copolymers, rayon, nylon, polyvinylbutyrate, polyformaldehyde, etc.
- silica gelsilicon wafers, glass, paper, insoluble protein, metals, metalloids, metal oxides, magnetic materials, semiconductive materials, cements or the like.
- substances that form gelsi.e., proteins such as gelatins, lipopolysaccharides, silicates, agarose, polyacrylamides or polymers which form several aqueous phases such as dextrans, polyalkylene glycols (alkylene with 2 to 3 carbon atoms) or surfactants, e.g. amphophilic compounds such as phospholipids, long chain (12-24 carbon atoms) alkyl ammonium salts and the like.
- the surfacemay comprise polystyrene, styrene copolymers including styrene(vinyl monomer) copolymers such as styrene-acrylonitrile copolymers, polyolefins such as polyethylene and polypropylene, and acrylate and methacrylate polymers and copolymers and mixtures thereof.
- styrene copolymersincluding styrene(vinyl monomer) copolymers such as styrene-acrylonitrile copolymers, polyolefins such as polyethylene and polypropylene, and acrylate and methacrylate polymers and copolymers and mixtures thereof.
- the surfacemay also comprise magnetizable materials in particulate form. Traditional interference by such magnetizable materials may be minimized by adding magnetizable particles to each of the reaction steps. Magnetic interference produced at each step may be made nearly equal, and thus is effectively cancelled. Magnetizable particles may be easily separated from the serum or other solution by application of a magnetic field to concentrate the particles.
- the capture antibodymay be bound to the surface by any method of bonding which does not significantly reduce the antibody binding sites and which binds sufficiently to permit separation of the surface from the liquids and rinse solutions without significant detachment of antibody from the surface.
- Non-covalent bondingmay be achieved by adsorption, ionic bonding, van der Waals adsorption, electrostatic bonding, and other non-covalent bonding.
- the antibodymay also be bound to the surface by covalent bonding.
- the antibodymay be coupled to a hydroxylated material by means of cyanogen bromide as described in U.S. Pat. No. 3,720,760.
- Staphylococcus Protein Amay be bound to the surface, and the F c chain of the antibody can be conjugated with the Protein A.
- Capture antibodiesmay be attached to a surface by adhesion followed by chemical crosslinking.
- Crosslinking agentswhich may be used include, but are not limited to, 1-ethyl-3-(3-dimethylaminopropyl)-carbodiimide (EDC), glutaraldehyde, adipic acid dihydrazide, bis-diazotized benzidine, 1,4-butane diglycidyl ether, bis-maleimido hexane, sulfosuccinimidyl 4-(N-maleimidomethyl)-cyclohexane-1-carboxylate, and N-hydroxysuccinimidyl 4-azidosalicylic acid.
- EDCmay be used for any surface that has free carboxyl groups. These and many other similar reagents are well known in the art.
- the same or different antibodiesmay be conjugated to a common member, using any of the conjugation methods described above.
- Common membersinclude, but are not limited to, a particle, microparticle (FIG. 6B), polypeptide (FIG. 6C), chemical linker, or a surface (FIG. 6A) as described above.
- a common membermay be conjugated with more than one antibody, each antibody binding to a different epitope on the analyte being assayed. See FIG. 6.
- the multiple antibodiesmay be conjugated to the common member at controlled molar ratios, for example using methods where the stoichiometry of the conjugation reaction is controlled.
- multiple common membersmay be individually conjugated with a different antibody, each antibody binding to a different epitope on the analyte being assayed. See FIG. 7. Molar ratios of the different antibodies may be controlled, using methods such as the mixing of multiple common members with conjugated antibodies.
- Antibodiesmay be attached to microparticles using any of the conjugation methods described above. Various factors may be considered when choosing the coupling method including, but not limited to type and size of particle, coupling reagents, concentration of reactants, single or multi-step reaction, reaction buffer and pH, storage buffer, and blocking agents.
- the coupling methodincluding, but not limited to type and size of particle, coupling reagents, concentration of reactants, single or multi-step reaction, reaction buffer and pH, storage buffer, and blocking agents.
- Signal reagentsmay be prepared by conjugating an antibody to a signal-generating element by any of a number of common methods.
- Conjugatesmay be prepared, for example, by utilizing free sulfhydryl groups, generating sulflhydryl groups from available disulfide bonds, or introducing additional sulfhydryls onto the antibody.
- Linking agentssuch as succinimydyl 4-(N-maleimidomethyl)cyclohexane-1-carboxylate (SMCC) may then used to conjugate the activated antibody to the signal-generating element.
- SMCCsuccinimydyl 4-(N-maleimidomethyl)cyclohexane-1-carboxylate
- Signal-generating elements of the present inventioninclude, but are not limited to, radiolabels, metal particles, chromogens, fluorescent dyes, labeled proteins and enzymes.
- the enzymemay catalyze a reaction wherein the depletion of substrate or the production of product can be detected.
- Exemplary enzymesinclude, but are not limited to, amylases, polynucleotidase, arginase, adenase, aminopolypeptidase, pepsin, lipases, catalase, tyrosinases, alcohol dehydrogenase, succinic dehydrogenase, diaphorase, glyoxalase, aldolase, glucose oxidase, horseradish peroxidase, beta-galactosidase, phosphatases, phosphorylases and hexokinases.
- Exemplary enzymesalso include alkaline phosphatase, glucose oxidase, horseradish peroxidase, ⁇ -galactosidase and phenol oxidase.
- the enzymatic conversion of a substrate to a productmay be measured by for example optical and electrochemical means.
- the sensitivity of a sandwich assayis limited largely by the level of non-specific adsorption of the signal reagent to the capture reagent and surfaces within the detection region of the analysis device.
- Such surfacesmay include the walls of a cuvette, a wicking element, an electrode and the like.
- the propensity of the signal reagent to non-specifically adsorb to surfaces within the analysis devicemay be a function of the inherent properties of the antibody and signal-generating element used to form the signal antibody, as well as the crosslinker.
- the materials used in the analysis devicee.g., types of plastics
- the composition of the wash and substrate-containing solutionsare generally optimized to minimize such non-specific protein adsorption.
- various surfactantse.g. Tween 20, Brij 35, Triton X100
- Non-enzymatic proteinssuch as serum albumin, denatured proteins such as gelatins, and deactivated enzymes may also be added as surface blocking agents in order to lower the level of non-specific adsorption of the signal reagent.
- the signal reagentmay comprise fragments of antibodies, such as Fab fragments, which may contribute to lowering the level of background signal. By lowering the molecular weight of the signal reagent, the time required for the binding step may be reduced due to diffusional considerations.
- the signal reagentmay also comprise more than one antibody, which may also lower background signal levels by minimizing the overall size of the signal reagent while maximizing its ability to generate a signal in response to the presence of the analyte of interest.
- the more than one antibody of the signal reagentmay bind to two or more different epitopes of the analyte, which may lead to stabilization of the signal antibody-analyte complex when more than one epitope of the analyte is bound by more than one antibody of the signal reagent.
- the signal reagentcomprises more than one antibody
- said antibodiesmay bind to one or more different epitopes.
- a signal-generating elementmay be conjugated with more than one antibody, each antibody binding to a different epitope on the analyte of interest. See FIG. 6C.
- the molar ratio of antibodies conjugated to the signal-generating elementmay be controlled by selecting the reaction conditions and stoichiometry for the conjugation reaction.
- a first antibodymay be conjugated to a first signal-generating element and a second antibody may be conjugated to a second signal-generating element, each at a ratio greater than 1:1, and then mixed, wherein the first and second antibodies each bind to a different epitope on the analyte of interest. See FIG. 7B.
- the molar ratio of the first and second antibodiesmay also be controlled.
- the signal-generating elementmay be conjugated to a single antibody, or two or more antibodies to different epitopes on the same analyte, wherein the signal antibody comprises a ratio of signal-generating element to a population of antibodies from about 1:100 to about 1:1.001.
- the signal-generating elementmay also be conjugated to a population of antibodies at a ratio from about 1:90 to about 1:1.001.
- the signal-generating elementmay also be conjugated to a population of antibodies at a ratio from about 1:80 to about 1:1.001.
- the signal-generating elementmay also be conjugated to a population of antibodies at a ratio from about 1:70 to about 1:1.001.
- the signal-generating elementmay also be conjugated to a population of antibodies at a ratio from about 1:60 to about 1:1.001.
- the signal-generating elementmay also be conjugated to a population of antibodies at a ratio from about 1:50 to about 1:1.001.
- the signal-generating elementmay also be conjugated to a population of antibodies at a ratio from about 1:40 to about 1:1.001.
- the signal-generating elementmay also be conjugated to a population of antibodies at a ratio from about 1:30 to about 1:1.001.
- the signal-generating elementmay also be conjugated to a population of antibodies at a ratio from about 1:20 to about 1:1.001.
- the signal-generating elementmay also be conjugated to a population of antibodies at a ratio from about 1:10 to about 1:1.001, about 1:20 to about 1:10, about 1:30 to about 1:20, about 1:40 to about 1:30, about 1:50 to about 1:40, about 1:60 to about 1:50, about 1:70 to about 1:60, about 1:80 to about 1:70, about 1:90 to about 1:80, and about 1:100 to about 1:90.
- the signal-generating elementmay also be conjugated to a population of antibodies at a ratio from about 1:5 to about 1:1.001, about 1:10 to about 1:5, about 1:15 to about 1:10, about 1:20 to about 1:15, about 1:25 to about 1:20, about 1:30 to about 1:25, about 1:35 to about 1:30, about 1:40 to about 1:35, about 1:45 to about 1:40, and about 1:50 to about 1:45.
- the signal-generating elementmay also be conjugated to a population of antibodies at a ratio of about 1:3.3.
- the signal-generating elementmay be conjugated to a first and second antibody, each capable of binding to different epitopes on an analyte or subforms thereof, wherein the ratio of signal-generating element to total antibody is as disclosed above, and wherein the ratio of the first antibody to the second antibody is from about 1:50 to about 50:1.
- the ration of the first antibody to the second antibodymay also be from about 1:10 to about 10:1, from about 1:5 to about 5:1, from about 1:3 to about 3:1, and from about 1:2 to about 2:1.
- a signalmay be produced by the signal-generating element, which may be measurable by a sensing element using methods which include, but are not limited to, radiation, optically, electrochemically, or by some other transduction means known in the art.
- a sensing elementmay be an electrode, biosensor, field-effect transistor, surface acoustic wave device, optical wave-guide, fiber optic, cuvette, radioactivity detector, reagents that facilitate, physical, nuclear, chemical, biochemical, electrical, or optical detection, or combinations thereof.
- the immunoassay of an analyte of interestmay be conducted in an immunoassay device comprising one or more sensing elements and a surface on which a modified sandwich immunoassay is conducted as described above.
- the device surfacemay comprise two or more antibodies that are each capable of binding to different epitopes on the analyte. At least one of the two antibodies is also capable of binding to the same epitope on a subform of the analyte. At least one of the epitopes on the analyte is unavailable for binding on the subform.
- Devices of the present inventioninclude a cartridge, columns, syringe, cuvette, or other analytical device or system known in the art.
- the cartridgemay be of a type as described in U.S. Pat. No. 5,096,669. Examples of other cartridge configurations are found in U.S. Pat. Nos. 5,416,026, 5,593,638, 5,447,440, 5,628,961, 5,514,253, 5,609,824, 5,605,664, 5,614,416, 5,789,253, 6,030,827, and 6,379,883. Still other cartridge configurations are described in PCT/US00/31158, PCT/US01/04345 and copending U.S. patent application Ser. No. 10/087,730. The disclosures of the foregoing are hereby incorporated by reference.
- the surface of the immunoassay devicemay be of any appropriate surface known to those of skill in the art including, but not limited to, glass, semiconductor, plastic, silicon dioxide, photoformable PVA, photoformable gelatin, film forming latex, and conductive metal.
- a conductive metal surfacemay be silver, iridium, gold, platinum, and alloys thereof.
- Antibodiesmay be absorbed, adsorbed or covalently attached to the surface of the immunoassay device. Antibodies may be absorbed into PVA, gelatin and latex layers or absorbed to their surfaces. Antibodies may be adsorbed to glass, metal, semiconductor, plastic, silicon dioxide, photoformable PVA, and conductive metal. The two or more antibodies may also be attached to a microparticle as discussed above, wherein the microparticle is attached to the surface as discussed above.
- the immunoassay devicemay also contain a third antibody which binds to a different epitope on the analyte.
- the sensing element of the immunoassay devicemay be of any type known in the art including, but not limited to, an electrode, biosensor, field-effect transistor, surface acoustic wave device, optical wave-guide, fiber optic, cuvette, radioactivity detector, immunochromatographic device, reagents that facilitate, physical, nuclear, chemical, biochemical, electrical, optical detection, or combinations thereof.
- An immunoassay kitmay be used to detect an analyte of interest and subforms thereof in a modified sandwich immunoassay as described above.
- the kitmay comprise three or more antibodies that are each capable of binding to different epitopes on the analyte. At least two of the three antibodies are also capable of binding to the same epitopes on a subform of the analyte. At least one of the epitopes on the analyte is unavailable for binding on the subform.
- a sandwich immunoassay productmay comprise an analyte of interest and a subform thereof. At least three epitopes on the analyte are available for binding by at least three different antibodies.
- the analytemay be bound by at least three different antibodies with each antibody binding to a different epitope on the analyte. At least two of the three epitopes on the analyte are available for binding on the subform by at least two of the at least three different antibodies.
- the subformmay be bound by at least two of the three different antibodies that bind to the analyte with each antibody binding to a different epitope on the subform. At least one epitope of the analyte is unavailable for binding on the subform. At least one of the antibodies bound to the analyte and the subform of the analyte may be a signal antibody.
- a samplemay be immunoassayed for the presence of an analyte and subforms thereof by performing a modified sandwich immunoassay as described above. At least three different antibodies are used, wherein each antibody is capable of binding to a different epitope on the analyte. At least two of the antibodies capable of binding to the analyte are capture antibodies or signal antibodies, or a combination thereof. At least one of the epitopes on the analyte is unavailable for binding on the subform.
- the sample to be immunoassayedis added to a surface comprising one or more capture antibodies.
- the one or more signal antibodiesare then added.
- one or more signal antibodiesmay be added to the sample before or during application of the sample to the surface comprising one or more capture antibodies. After removing unbound signal antibodies, the extent of binding of the one or more signal antibodies is determined as described above.
- a signalwill be produced by analyte as well as all subforms of the analyte that have at least one epitope that is present or capable of being bound by a capture antibody and at least a second epitope that is present or capable of being bound by a signal antibody.
- the analyte of interest and subforms thereofmay be immunoassayed to determine whether a patient has suffered an acute medical event.
- a samplemay be obtained from the patient and immunoassayed for the presence of the analyte of interest and subforms thereof by using the method of assaying described above.
- Signal produced by signal antibodiesmay be compared to control values.
- a signal, or a sequence of signals from a series of assays over a period of hours or days, detectable above controlmay indicate that the patient has suffered an acute medical event.
- the acute medical eventmay be any disease state that is associated with a clinical marker, wherein the clinical marker is an analyte in the modified immunoassay described herein.
- the medical eventmay be for example a myocardial infarction.
- the analyte of interestmay be for example TnI, TnT, TnC, CK-M, CK-B, CK-MB, myoglobin, TSH, FSH, CRP, BNP, pro-BNP, PSA, PCA, apolipoprotein, and combinations thereof.
- the analyte of interest and subforms thereofmay be immunoassayed to determine when a patient suffered an acute medical event.
- a sample, or set of samples obtained at different times,may be obtained from the patient and immunoassayed for the presence of the analyte of interest and subforms thereof by using the method of assaying described above.
- the amount of signal produced by signal antibodiesmay be correlated with a standard curve of amount of analyte vs. time to determine when the patient suffered the acute medical event.
- One of ordinary skillmay produce the standard curve by using the methods described herein.
- the analyte of interest and subforms thereofmay be immunoassayed to determine the severity of an acute medical event suffered by a patient.
- a sample, or set of samples obtained at different times,may be obtained from the patient and immunoassayed for the presence of the analyte of interest and subforms thereof by using the method of assaying described above.
- the amount of signal produced by signal antibodiesmay correlate to the severity of a medical event suffered by the patient.
- Bead particles conjugated with antibodies specific for cTnIwere prepared by first exchanging polystyrene/acrylic acid latex 0.2 ⁇ m diameter spheres (Seradyn) into 0.05 M 2-(N-morpholino)ethanesulfonic acid (MES, Sigma Aldrich) buffer at pH 6.2. To a 2% w/w solution of the latex spheres was added cTnI antibodies (buffer exchanged into the same 0.05 M MES buffer) at a controlled mass ratio relative to the mass of the latex spheres. The solution was stirred for 15 minutes at 4° C. and the antibody coated beads separated via controlled centrifugation.
- MES2-(N-morpholino)ethanesulfonic acid
- microparticle centrifuged pelletwas then resuspended into MES buffer at 1% w/v density and 1 to 10 mM of EDC was added to covalently crosslink the adsorbed antibody to the latex spheres.
- the suspensionwas allowed to react for 4 to 12 hours at which time the latex particles were separated by centrifugation, resuspended at 1% in the MES buffer and the EDC addition was repeated.
- microparticle capture reagentswere made comprising either CB1 (19C7, Hytest Inc.) or CB2 (34503228P, Biospacific Inc.).
- CB12 microparticlescomprising both CB1 and CB2 were prepared using the above method by adding a mixture of the two capture antibodies.
- alkaline phosphatase (ALP) labeled Fab conjugateswas used as signal reagents.
- Alkaline phosphataseBiozyme
- PBSphosphate buffered saline
- a 1 mg/ml to 15 mg/ml solution of the ALPwas reacted with 25 weight percent (relative to the ALP weight in the solution) of the heterobifunctional crosslinker succinimidyl-4-[N-maleimidomethyl]-cyclohexane-1-carboxy[6-amidocaproate]
- LCSMCCphosphate buffered saline
- Piercethe heterobifunctional crosslinker succinimidyl-4-[N-maleimidomethyl]-cyclohexane-1-carboxy[6-amidocaproate]
- the crosslinkerwas added as a solution in dimethyl sulphoxide (DMSO, Sigma). This solution was allowed to react at room temperature for 45 minutes, after which it was centrifuged to remove
- Fab fragments of the antibodies to be incorporated into the signal reagentwere formed via pepsin (Sigma) digestion of the whole antibodies and separated using a S200 sephacryl size exclusion column (Amersham Pharmacia). The resulting F(ab) 2 fragments were chemically reduced in 60 mM mercaptoethylamine (MEA, Sigma Aldrich) for 45 minutes at 3 7 ° C. to produce two Fab fragments, which were separated from excess MEA by desalting using a sephadex G50 column. The Fab fragments were then added to activated ALP and allowed to react at 4° C. for 2 to 12 hours.
- FIG. 12shows the elution profile for ALP-Fab signal reagents with ALP:Fab ratios of 1:1, 1:3, 1:6 and 1:10.
- ALP-labeled signal reagentswere made comprising either EC1 (G129C, Biospacific Inc.) or EC2 (G130C, Biospacific Inc.).
- EC12 signal reagentscomprising both EC1 and EC2 were prepared using the above method by mixing the two fab fragments at a molar ratio of 1:1 before adding the mixture to activated ALP
- an analyte detectorcomprising an immobilized layer of antibody labeled particles on an ELISA sensor.
- amperometric sensors used in the analyte detectorare fabricated using traditional semiconductor thin layer techniques on oxidized silicon wafers.
- the cTnI capture regionsare produced by microdispensing solutions of the capture antibodies described in Example 1 using methods described in U.S. Pat. No. 5,554,339. Droplets of the particle-containing suspension are allowed to dry to form cTnI capture regions on the sensor surface.
- the sensor chipsare assembled with a separated electrochemical grounding chip into a plastic base covered with a two sided adhesive tape gasket, laser cut with openings for the sensors, ground chip and fluidic channels.
- the enzyme labeled signal reagent of Example 2is printed onto the same ELISA sensor as the capture antibodies.
- the signal reagentis formulated in a 1% to 50% sugar solution containing PBS and a preservative. This composition was found to provide rapid dissolution of the signal reagent into a blood sample.
- the assembled cartridgeis pressed to yield the disposable cartridge, which may be used in a portable electrochemical analyzer.
- FIG. 10shows high signal levels for cartridges comprising the CB1 capture reagent for both free cTnI (diamond points) and the two levels of ITC complex (square and triangle points).
- cartridges comprising the CB2 capture reagentyield lower signals, especially for the ITC complex.
- Cartridges using the CB12 hybrid capture reagentshow improved signal generation for both forms of cTnI relative to either CB1 or CB2 capture reagents.
- the improved performance of the CB12 capture reagentis surprising, because the hybrid reagent comprises the CB2 antibody that by itself has markedly lower analytical performance than the CB1 antibody.
- cTnIwas assayed in a whole blood sample spiked with a 6.0 ng/mL cITC complex immediately after preparation and after a one-day incubation at room temperature using cartridges with sensors coated with latex microsphere comprising CB1 and CB12 capture reagents, as described in Example 3, and EC1, EC2 or EC12 signal reagents as described in Example 2.
- FIG. 11demonstrates that the EC1 signal reagent shows a higher signal generation than the EC2 signal reagent. After a one day incubation of the sample to simulate aging of cTnI (e.g., proteolytic degradation), the signal level for the EC1 signal reagent drops significantly. In contrast, the EC2 signal reagent show only a modest drop in the signal level.
- the signal generation of the hybrid signal reagent EC12is surprisingly superior to each of the two single antibody signal reagents, especially after incubating the sample for one day.
- Whole blood samplesare obtained from patients suspected of suffering a myocardial infarction, as well as from control individuals.
- cTnIis assayed in the whole blood samples using cartridges with sensors coated with latex microsphere comprising CB12 capture reagents, as described in Example 3, and EC12 signal reagents as described in Example 2.
- the signal generated in samples derived from patients suspected of suffering an MIis compared to the level of signal from controls. Levels of signal greater than control indicate that the patient has suffered a MI.
Landscapes
- Health & Medical Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Immunology (AREA)
- Engineering & Computer Science (AREA)
- Chemical & Material Sciences (AREA)
- Biomedical Technology (AREA)
- Hematology (AREA)
- Molecular Biology (AREA)
- Urology & Nephrology (AREA)
- General Health & Medical Sciences (AREA)
- Analytical Chemistry (AREA)
- Food Science & Technology (AREA)
- Pathology (AREA)
- Microbiology (AREA)
- Medicinal Chemistry (AREA)
- Physics & Mathematics (AREA)
- Cell Biology (AREA)
- Biochemistry (AREA)
- Biotechnology (AREA)
- General Physics & Mathematics (AREA)
- Proteomics, Peptides & Aminoacids (AREA)
- Clinical Laboratory Science (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Dispersion Chemistry (AREA)
- Peptides Or Proteins (AREA)
Abstract
Description
- The present invention relates to the diagnosis of diseases and medical events, notably myocardial infarction (“MI”). More specifically, the present invention relates to the detection of a clinical marker of a disease or medical event, in particular MI, using multiple antibodies, each antibody having a different specificity for the clinical marker. The present invention also relates to reagents and apparatuses used in the diagnosis of a disease or medical event, in particular MI.[0001]
- Diagnosis of acute disease is often based on immunoassay, e.g. enzyme-linked immunosorbent assay (“ELISA”), detection of abnormal levels of clinical markers, such as proteins, enzymes and hormones in biological fluids, particularly when the concentration changes quickly during the acute phase of disease. ELISA systems allowing for the rapid and simple diagnosis of the occurrence of an acute disease, such as the occurrence of an MI, are therefore extremely important.[0002]
- In the past, clinical markers for diagnosing the occurrence of an MI included lactate dehydrogenase (“LDH”) and glutamic oxaloacetic transaminase (“GOT”), though these were not very specific. Problems with LDH and GDH led to the use of the MB isoenzyme of creatine kinase (“CK-MB”) as a clinical marker for the diagnosis of MI. However, CK-MB can also be found in skeletal muscle and in blood after skeletal muscle injury. Thus, CK-MB is not completely specific for cardiac muscle. Another disadvantage of CK-MB as a clinical marker of MI is that the level of CK-MB in the skeletal muscle varies with the degree of skeletal muscle regeneration, information which may often not be known when administering a test or analyzing a test result for MI. Another disadvantage of testing for CK-MB is that CK-MB levels remain elevated for only 2-3 days after the onset of chest pain. For patients admitted after that time, the CK-MB test will be of limited value. Thus, due to the lack of specificity when assaying CK-MB, and the limited time frame for its use as a diagnostic tool, CK-MB is not an ideal clinical marker for diagnosing MI.[0003]
- Cardiac troponin I (“cTnI”) is now used as an accurate cardiac-specific biological parameter detectable in serum very soon after MI and remaining present for more than 2-3 days after the onset of MI. Troponin is present in cardiac tissue as a complex of three subunits: Troponin T (“TnT”), the tropomyosin binding subunit, Troponin C (“TnC”), the Ca[0004]2+ binding subunit; and Troponin I (“TnI”), the sub-unit which inhibits the actomyosin Mg2+ ATPase. TnI is a thin filament regulatory protein complex, which confers calcium sensitivity to the cardiac and striated muscle.
- Human Troponin I exists in three isoforms: two skeletal muscle isoforms (fast and slow) (MW=19.8 kDa) and a cardiac TnI isoform (“cTnI”) with an additional 31 residues on the N-terminus resulting in a molecular weight of 23 kDa (209 amino acids). Cardiac TnI is uniquely located in the myocardium where it is the only isoform present. Cardiac TnI rapidly appears in human serum (within approximately 4 to 6 hours) following a MI. It reaches a peak level after 18-24 hours and remains at elevated levels in the blood stream for up to 6-10 days. As a result, cTnT released into the circulation from the myocardium is very specific for myocardial injury.[0005]
- Elevated cTnI levels in blood may be used to diagnose MI and distinguish other heart related events and diseases. Immunoassay systems capable of accurately detecting human cTnI would be valuable to the medical community for diagnosing the occurrence of MI. For more information on the utility of cTnI testing, see Apple and Wu, Myocardial infarction redefined: Role of cardiac troponin testing. Clinical Chemistry 47, 377-9, (2001), which is hereby incorporated by reference.[0006]
- Cardiac TnI exists in multiple subforms in blood as a result of modifications such as proteolytic cleavage, phosphorylation, chemical oxidation, chemical reduction, cleavage of amino acid residues, and chemical modification of amino acid moieties. For example,[0007]
amino acids 1 to 25 and 150 to 209 are generally not found on cTnI subforms in serum as a result of proteolysis. The many different subforms of cTnI circulating in the bloodstream are predominantly found in complexes with other proteins. See Katrukha (1997). For example, cTnI may be complexed with cTnT and cTnC (“cITC”). - In many instances, the chemical modifications and complexation of cTnI in the bloodstream eliminate or block the binding sites for ELISA reagents on some subforms of cTnI, thereby making the epitopes of the ELISA reagents unavailable for binding. For more information on cTnI epitopes and cTnI instability, see Morjana et al., Degradation of Human cardiac troponin I after myocardial infarction: Biotechnol. Appl. Biochem. (1998), 28, 105-111; Gaelle Ferrieres et al. Human cardiac troponin I: precise identification of antigenic epitopes and prediction of secondary structure: Clin. Chem. (1998), 44, 487-493; Katrukha et al., Troponin I is released in bloodstream of patients with acute myocardial infarction not in free form but as complex: Clin. Chem. (1997), 43, 1379-1385; and Katrukha et al., Degradation of cardiac troponin I: implication for reliable immunodetection: Clin. Chem. (1998), 44, 2433-244, which are incorporated herein by reference.[0008]
- For most commercially important clinical marker analytes, a number of efficient monoclonal antibodies have been developed which bind a particular epitope of an analyte. The difference between antibodies are the specific location of binding to the analyte, affinity for the analyte, and cross-reactivity with other potential interferents within the sample. ELISA assays have been traditionally developed by pairwise testing of available antibodies for use either as the capture reagent or signal reagent.[0009]
- In the development of ELISA assays for cTnI, much effort has been expended in optimizing the antibody and enzyme reagents used. However, the likelihood of epitopes being unavailable for binding on the many subforms of cTnI present in serum and complexation with other proteins makes detection of cTnI significantly difficult and typically cause current ELISA reagents to underestimate actual cTnI levels in a sample. Furthermore, the many different subforms of cTnI with different epitopes available for binding cause current ELISA assays to give significantly different results from sample to sample, from reagent set to reagent set, and as a function of time.[0010]
- Thus, there exists a need to develop methods of detecting a clinical marker and subforms thereof, in particular cTnI, for the diagnosis of an acute disease event, in particular MI. The present invention satisfies this need and provides reagents, kits, and apparatuses for detecting a clinical marker and subforms thereof, in particular cTnI.[0011]
- The present invention is related to the detection of an analyte of interest. The analyte of interest may be cTnI. In an exemplary embodiment of the present invention, an immunoassay composition for detecting an analyte of interest may comprise three or more antibodies, wherein each antibody is capable of binding to a different epitope on the analyte. At least two of the three different antibodies which bind to the analyte are also capable of binding to at least two different epitopes on a subform of the analyte. At least one of the epitopes on the analyte of interest is unavailable for binding on the subform.[0012]
- In another exemplary embodiment of the present invention, an immunoassay device for detecting an analyte of interest may comprise two or more antibodies, wherein each antibody binds to a different epitope on the analyte and wherein the two or more antibodies are bound to at least one surface. At least one of the two different antibodies which bind to the analyte is also capable of binding to at least one different epitope on a subform of the analyte. At least one of the epitopes on the analyte of interest is unavailable for binding on the subform.[0013]
- In another exemplary embodiment of the present invention, an immunoassay kit for detecting an analyte of interest may comprise three or more antibodies, wherein each antibody is capable of binding to a different epitope on the analyte. At least two of the three different antibodies which bind to the analyte are also capable of binding to at least two different epitopes on a subform of the analyte. At least one of the epitopes on the analyte of interest is unavailable for binding on the subform.[0014]
- In another exemplary embodiment of the present invention, a sandwich immunoassay product may comprise an analyte of interest and a subform of the analyte. At least three different epitopes on the analyte are available for binding by at least three different antibodies. At least three different antibodies are bound to different epitopes on the analyte. At least two of the three epitopes on the analyte are available for binding on the subform. At least two of the at least three antibodie are bound to different epitopes on the subform. At least one of the epitopes on the analyte of interest is unavailable for binding on the subform.[0015]
- In another exemplary embodiment of the present invention, a patient may be diagnosed for the occurrence of an acute disease, such as myocardial infarction, by applying a sample obtained from the patient to a surface comprising at least two antibodies which bind to different epitopes on cTnI, wherein at least one of the two antibodies is capable of binding to a different epitope on a subform of cTnI, and wherein at least one epitope of the at least two antibodies is unavailable for binding on the subform. A third antibody is then added which binds to yet another epitope on cTnI and the subform. The extent of binding of the third antibody to cTnI and the subform is then measured.[0016]
- FIG. 1 shows the standard sandwich assay for detecting an analyte of interest, wherein a capture antibody (Ab[0017]1) and signal antibody (Ab2) are directed to a first and second epitope (e1and e2), respectively, of an analyte of interest (An). The signal antibody is labeled with an enzyme (Enz), which is used for signal generation by converting substrate (S) to product (P).
- FIG. 2 is a representation of an analyte of interest (An) with three epitopes (e[0018]1, e2and e3). As the analyte is modified, forms complexes or adopts alternative conformations, a subform is produced with one or more of the epitopes unavailable for binding by antibodies. Each epitope that is unavailable for binding is identified by a circle, whereas each epitope that is available for binding is identified without a circle. Each subform may be further modified so that additional epitopes are unavailable for binding by antibodies.
- FIG. 3 shows a modified sandwich assay for detecting an analyte of interest (An) and subforms thereof using one capture antibody (Ab[0019]1) and more than one signal antibody (Ab2and Ab3). The assay is able to detect the presence of the analyte and all subforms thereof that have at least one epitope for the capture antibody (e1) and at least one epitope for a signal antibody (e2or e3). All epitopes for binding are shown bound by an antibody; however, the analyte or subforms thereof need to be bound by only one capture antibody and one signal antibody to be detected.
- FIG. 4 shows a modified sandwich assay for detecting an analyte of interest (An) and subforms thereof using more than one capture antibody (Ab[0020]1and Ab2) and one signal antibody (Ab3). The assay is able to detect the presence of the analyte and all subforms thereof that have at least one epitope for the signal antibody (e3) and at least one epitope for a capture antibody (e1or e2). All epitopes for binding are shown bound by an antibody; however, the analyte or subforms thereof need to be bound by only one capture antibody and one signal antibody to be detected.
- FIG. 5 shows a modified sandwich assay for detecting an analyte of interest (An) using more than one capture antibody (Ab[0021]1and Ab2) and more than one signal antibody (Ab3and Ab4). The assay is able to detect the presence of the analyte and all subforms thereof that have at least one epitope for a capture antibody (e1or e2) and at least one epitope for a signal antibody (e3or e4). All epitopes for binding are shown bound by an antibody; however, the analyte or subforms thereof need to be bound by only one capture antibody and one signal antibody to be detected.
- FIG. 6 shows one antibody (Ab[0022]1) or more than one antibody (Ab1and Ab2) binding to a different epitope (e1or e2) on the analyte of interest (An) and conjugated to a common member, wherein the common member is a surface (FIG. 6A), microparticle (FIG. 6B), and a polypeptide, such as an enzyme (FIG. 6C).
- FIG. 7 shows a mixture of common members individually conjugated with a different antibody (Ab[0023]1or Ab2), each antibody binding to a different epitope (e1or e2) on the analyte being assayed (An), wherein the common member is a microparticle (FIG. 7A) or polypeptide, such as an enzyme (FIG. 7B).
- FIG. 8 shows a common member, such as a microparticle, conjugated to two or more antibodies (Ab[0024]1and Ab2), conjugated to another common member, such as a surface, wherein two or more capture antibodies are either (i) individually conjugated to different microparticles (FIG. 8A), or (ii) conjugated together on a microparticle (FIG. 8B). [00251 FIG. 9 depicts the primary structure of subform of cardiac troponin I (cTnI) without
amino acids 1 to 25 and 150 to 209, and shows the location for epitopes of the cTnI antibodies. - FIG. 10 shows the amperometric signals from different single-use assays prepared with a first capture antibody (CB1), a second capture antibody (CB2), or a combination of first and second capture antibodies (CB12) to a whole blood sample spiked with free cTnI and two levels of cITC complex.[0025]
- FIG. 11 shows a plot of the average signal obtained from a whole blood sample spiked with 6.0 ng/mL cITC complex immediately after preparation and after one-day incubation at room temperature using cartridges prepared with either CB1 or CB12 coated sensors and with a signal producing enzyme conjugated to a first antibody (EC1), a second antibody (EC2) or a mixture of EC1 and EC2 (EC12).[0026]
- FIG. 12 shows elution profiles for Fab-ALP conjugates with different ratios of ALP to Fab.[0027]
- 1. Definitions[0028]
- To aid in the understanding of the present invention, several terms are defined below.[0029]
- “Analyte” means a biological or chemical substance that is capable of being bound by at least three different antibodies, and includes subforms thereof that may be bound by at least three different antibodies.[0030]
- “Antibody” means a polypeptide or derivative thereof that specifically binds to an epitope on an analyte of interest or subform thereof.[0031]
- “Capture antibody” or “capture reagent” means an antibody that specifically binds to an analyte of interest or subform thereof, wherein said antibody is immobilized on a surface either (i) before, (ii) after or (iii) during binding to said analyte or subform thereof.[0032]
- “Detect”, “detected,” or “detectable,” when used to describe a signal of a signal-generating element, means a signal capable of being distinguished from background.[0033]
- “Epitope” means a binding site for an antibody or other binding member on an analyte of interest or subform thereof. An epitope is “unavailable for binding” if the epitope is either not present or inaccessible for binding by an antibody.[0034]
- “Immunoassay” or “sandwich immunoassay” means a method of simultaneous sandwich, forward sandwich and reverse sandwich immunoassays, and includes competitive immunoassays thereof, all of which are well understood by those skilled in the art.[0035]
- “Subform,” when used to describe an analyte, means the product of a chemical reaction, a member of a complex with other biological or chemical substances, alternative conformations, or combinations thereof that may be bound by at least two different antibodies.[0036]
- “Sensing element” means any device or apparatus that is capable of detecting a signal.[0037]
- “Signal antibody” or “signal reagent” means an antibody that specifically binds to an analyte of interest or subform thereof, wherein said antibody is attached to a signal-generating element via a covalent linkage, hydrophobic interactions, hydrophilic interaction, ionic interactions, Van der Waal forces, or a combination thereof.[0038]
- “Signal-generating element” means a biological, chemical, or radioactive substance that (i) produces a detectable signal, directly or indirectly, or (ii) is itself detectable.[0039]
- “Surface” means a support that may be separated from a solution.[0040]
- 2. Analyte[0041]
- The present invention relates to the detection of an analyte and subforms thereof. The analyte may be a clinical marker of a disease state from a patient believed to have suffered an acute disease event, wherein the clinical marker is also present in one or more subforms. In an acute disease state which is amenable to analysis by this invention, the clinical marker may be a transiently elevated substance in the blood which is released in a significant quantity at, after, or before the time of the acute disease event of interest. The elevated concentration of the clinical marker analyte decreases as endogenous conversion factors act upon the clinical marker to produce subforms of the analyte. Subforms derived from the clinical marker may be transiently elevated in a serial manner, as each is first created then metabolized by endogenous conversion factors. The period of transient elevation may be for as short as a few hours to as long as several weeks.[0042]
- The analyte may be a biological substance such as a protein, glycoprotein, enzyme, etc., which is released in small quantities at the occasion of an acute disease event such as a heart attack, stroke, or at the occasion of a traumatic injury such as a broken bone or a hematoma. The analyte may be normally absent in such increased quantities and may be converted to a one or more subforms over time by endogenous conversion factors including, but not limited to proteolytic cleavage, phosphorylation, chemical oxidation, chemical reduction, cleavage of amino acid residues, and chemical modification of amino acid moieties. The analyte may be a protein including, but not limited to, TnI, TnT, CK-M, CK-B, myoglobin, hCG, TSH, FSH, pneumococcal PCA, apolipoproteins, C-reactive protein (CRP), brain natriuretic protein (BNP) and its pro-form pro-BNP, human leukocyte antigen and human apolipoproteins. A preferred analyte is cTnI.[0043]
- The analyte has at least three different epitopes that are available for binding by an antibody (FIG. 2). A subform of the analyte has at least two different epitopes that are available for binding by an antibody and the epitopes of the subform are also present on the analyte (FIG. 2). At least one epitope of the analyte is unavailable for binding on the subform. An epitope of the analyte unavailable for binding on the subform may be as a result of complexation or alternative conformations, as well as the endogenous conversion factors described above.[0044]
- The analyte may be in a native or natural form. The analyte may also be a derivative of the native or natural form. For example, in the case of cTnI the analyte may be full length cTnI monomer or a derivative thereof that is either free or part of cITC complex. In addition, the analyte may be any form of cTnI that is released into serum in connection with an MI. The analyte may also be any modified form of cTnI present in serum including, but not limited to, cTnI as shown in FIG. 9. The analyte may also be any modified form of cTnI that has one or more epitopes that are unavailable for binding on any precursor of the analyte. In order to be an analyte, the only requirement is that the modified or derivative form of cTnI must have at least three epitopes available for binding by three different antibodies.[0045]
- The present invention may also be used to detect an analyte and subforms thereof that are present at low concentration where high sensitivity is required.[0046]
- 3. Immunoassay[0047]
- Analytes have traditionally been detected using a system based on the sandwich assay depicted in FIG. 1 wherein a first monoclonal or polyclonal antibody for capture is attached to a surface, and a second monoclonal or polyclonal antibody is labeled with a signal-generating element (e.g., an enzyme). For more information on commercial immunoassay products and the technology on which they are based, see Wild, (Ed), The Immunoassay Handbook, Stockton Press N.Y., 1994, which is incorporated herein by reference. The traditional sandwich assay fails, however, to adequately detect analytes that undergo complexation, alternative conformations or modification that make epitopes for the capture antibody, signal antibody, or both unavailable for binding.[0048]
- The present invention relates to the use of a sandwich immunoassay for detecting an analyte and one or more subforms thereof (FIG. 2). Subforms of the analyte may be due to causes including mutations, complexation, chemical modification, proteolysis or rearrangement. The present invention may also be used to detect more than one related analyte, such as an active protein and its inactive precursor or a class of similar analytes for which a single detection assay is developed.[0049]
- The analyte of interest and one or more subforms thereof may be detected by performing an immunoassay using antibodies specific for more than one epitope on the analyte either (i) on the capture side of the sandwich (FIG. 4), (ii) signal side of the sandwich (FIG. 3), (iii) or both (FIG. 5). By targeting at least three epitopes, in total, on the analyte of interest, the multiple sandwich assay of the present invention may detect the presence of the analyte and subforms thereof even if certain epitopes are unavailable for binding on the subform, so long as there is at least one epitope capable of binding by a capture antibody and at least one epitope available for binding by a signal antibody. The present invention is therefore able to detect analytes and multiple subforms thereof that appear within a sample.[0050]
- One of skill in the art will recognize that the invention described herein may detect an analyte of interest by using any binding member that is capable of specifically binding to an epitope on the analyte of interest. Binding members include, but are not limited to, extracellular or intracellular receptors, polynucleotides, peptide nucleic acids, and derivatives thereof.[0051]
- The use of multiple antibodies in the practice of the present invention is contrasted with the use of polyclonal antibodies in traditional sandwich assays; in addition, the present invention is superior. Within a given polyclonal preparation, the specific epitopes recognized and the ratio of various antibodies in the preparation is generally unknown. Furthermore, the epitopes recognized and the ratio of various antibodies vary between polyclonal preparations. Another deficiency in the polyclonal approach is that the binding of individual antibodies within the polyclonal preparation will have diverse binding affinities. As a result, a significant portion of polyclonal preparations are antibodies which have sub-standard analytical performances or which bind epitopes that compete with other antibodies in the preparation. By contrast, the present invention allows the preparation of controlled multi-epitopic reagents with more efficient assay characteristics because of better binding affinities and less reagent required for a given assay signal.[0052]
- In one exemplary embodiment of the invention, the immunoassay is based on a first antibody attached to a surface (i.e., “capture antibody” or “capture reagent”) and at least a second and third antibody, wherein each antibody binds to a different epitope on the analyte. The antibodies not attached to a surface may be labeled with a signal-generating element (i.e., “signal antibody” or “signal reagent”). See FIG. 3.[0053]
- In another exemplary embodiment of the invention, the immunoassay is based on at least a first and second capture antibody and a third antibody. The antibody not attached to a surface may be a signal antibody. See FIG. 4.[0054]
- In another exemplary embodiment of the invention, the immunoassay is based on at least a first and second capture antibody and at least a third and fourth antibody. The antibodies not attached to a surface may be signal antibodies. See FIG. 5.[0055]
- 4. Antibodies[0056]
- The antibodies of the present invention may be any antibody that specifically binds to an epitope available for binding on an analyte of interest or a subform thereof. The antibodies of the present invention include antibodies of classes IgG, IgM, IgA, IgD, and IgE, and fragments and derivatives thereof including Fab, F(ab′)[0057]2and single chain antibodies. The antibodies of the present invention include monoclonal antibodies, polyclonal antibodies, affinity purified antibodies, or mixtures thereof which exhibit sufficient binding specificity to an epitope of the analyte or subform thereof. Monoclonal antibodies and fragments and derivatives thereof are preferred in the practice of the invention. The antibodies of the present invention preferably bind to epitopes of the analyte sufficiently removed from each other such that the antibodies do not mutually interfere with binding to the analyte or subform thereof. Appropriate antibodies for an analyte of interest may be chosen by means of mapping combinations of available antibodies to known epitope sites on the analyte using methods known in the art.
- Numerous methods exist for preparing antibody fragments, including the use of common enzymes such as pepsin, papain, ficin, and trypsin. For more information on antibody preparation and antibody fragmentation, see Harlow and Lane, Antibodies, A Laboratory Manual, Cold Spring Harbor Laboratory, N.Y., 1988, which is hereby incorporated by reference.[0058]
- The antibodies of the present invention may be specific for human cTnI. The antibodies may specifically recognize epitopes located on the primary sequence of cTnI as indicated in FIG. 9. The antibodies may also be chosen from those antibodies indicated in FIG. 9.[0059]
- 5. Capture[0060]
- Capture antibodies of the present invention may be immobilized by conjugating one or more antibodies to a surface. See, e.g., FIG. 6A and FIG. 6B. A wide variety of compounds may be employed as the surface, the primary consideration being the binding of the antibody to the surface, the absence of interference with the signal generating element, and the absence of interference with the examination of the label. In particular, if a fluorescence or chromogenic spectrum is being measured, the surface should not provide interference.[0061]
- Organic and inorganic polymers, both natural and synthetic, may be employed as the surface. Examples of suitable polymers include polyethylene, polypropylene, polybutylene, poly(4-methylbutylene), butyl rubber and other synthetic rubbers, silicone rubbers and silastic polymers, polyesters, polyimides, cellulose and cellulose derivatives (such as cellulose acetate, nitrocellulose and the like), acrylates, methacrylates, vinyl polymers (such as polyvinyl acetate, polyvinyl chloride, polyvinylidene chloride, polyvinyl fluoride, and the like), polystyrene and styrene graft copolymers, styrene-acrylonitrile copolymers, rayon, nylon, polyvinylbutyrate, polyformaldehyde, etc. Other materials which may be employed as the surface are silica gel, silicon wafers, glass, paper, insoluble protein, metals, metalloids, metal oxides, magnetic materials, semiconductive materials, cements or the like. In addition are included substances that form gels, i.e., proteins such as gelatins, lipopolysaccharides, silicates, agarose, polyacrylamides or polymers which form several aqueous phases such as dextrans, polyalkylene glycols (alkylene with 2 to 3 carbon atoms) or surfactants, e.g. amphophilic compounds such as phospholipids, long chain (12-24 carbon atoms) alkyl ammonium salts and the like.[0062]
- The surface may comprise polystyrene, styrene copolymers including styrene(vinyl monomer) copolymers such as styrene-acrylonitrile copolymers, polyolefins such as polyethylene and polypropylene, and acrylate and methacrylate polymers and copolymers and mixtures thereof.[0063]
- The surface may also comprise magnetizable materials in particulate form. Traditional interference by such magnetizable materials may be minimized by adding magnetizable particles to each of the reaction steps. Magnetic interference produced at each step may be made nearly equal, and thus is effectively cancelled. Magnetizable particles may be easily separated from the serum or other solution by application of a magnetic field to concentrate the particles.[0064]
- The capture antibody may be bound to the surface by any method of bonding which does not significantly reduce the antibody binding sites and which binds sufficiently to permit separation of the surface from the liquids and rinse solutions without significant detachment of antibody from the surface. Non-covalent bonding may be achieved by adsorption, ionic bonding, van der Waals adsorption, electrostatic bonding, and other non-covalent bonding. The antibody may also be bound to the surface by covalent bonding.[0065]
- Procedures for covalently adhering antibodies to surfaces are described by I. Chibata in IMMOBILIZED ENZYMES, Halsted Press, New York, 1978, and by A. Cuatrecasas, J.Bio.Chem. 245:3059 (1970), the entire contents of which are hereby incorporated by reference. The surface may be coated with a protein and coupled with antibody using the procedures described in U.S. Pat. No. 4,210,418 using glutaraldehyde as a coupling agent, for example. In an alternate procedure, the surface may be coated with a layer having free isocyanate groups such as a polyether isocyanate. Application of the antibody in aqueous solution thereto effects the requisite bonding. In another procedure, the antibody may be coupled to a hydroxylated material by means of cyanogen bromide as described in U.S. Pat. No. 3,720,760. In a still further procedure, Staphylococcus Protein A may be bound to the surface, and the F[0066]cchain of the antibody can be conjugated with the Protein A.
- Capture antibodies may be attached to a surface by adhesion followed by chemical crosslinking. Crosslinking agents which may be used include, but are not limited to, 1-ethyl-3-(3-dimethylaminopropyl)-carbodiimide (EDC), glutaraldehyde, adipic acid dihydrazide, bis-diazotized benzidine, 1,4-butane diglycidyl ether, bis-maleimido hexane, sulfosuccinimidyl 4-(N-maleimidomethyl)-cyclohexane-1-carboxylate, and N-hydroxysuccinimidyl 4-azidosalicylic acid. EDC may be used for any surface that has free carboxyl groups. These and many other similar reagents are well known in the art.[0067]
- 6. Conjugation of Antibodies to a Common Member[0068]
- In another exemplary embodiment, the same or different antibodies may be conjugated to a common member, using any of the conjugation methods described above. Common members include, but are not limited to, a particle, microparticle (FIG. 6B), polypeptide (FIG. 6C), chemical linker, or a surface (FIG. 6A) as described above.[0069]
- A common member may be conjugated with more than one antibody, each antibody binding to a different epitope on the analyte being assayed. See FIG. 6. The multiple antibodies may be conjugated to the common member at controlled molar ratios, for example using methods where the stoichiometry of the conjugation reaction is controlled.[0070]
- In addition, multiple common members may be individually conjugated with a different antibody, each antibody binding to a different epitope on the analyte being assayed. See FIG. 7. Molar ratios of the different antibodies may be controlled, using methods such as the mixing of multiple common members with conjugated antibodies.[0071]
- a. Microparticle[0072]
- Antibodies may be attached to microparticles using any of the conjugation methods described above. Various factors may be considered when choosing the coupling method including, but not limited to type and size of particle, coupling reagents, concentration of reactants, single or multi-step reaction, reaction buffer and pH, storage buffer, and blocking agents. For more information on using microparticles, see Bangs TechNote #201 “Working with Microspheres”; Bangs TechNote #204 “Adsorption to Microspheres”; Bangs TechNote #205 “Covalent Coupling”; Seradyn Technical Method Bulletin “Recommended Adsorption and Covalent Coupling Procedure” (1999); Hager, H. J. “Latex Polymer Reagents for Diagnostic Tests”; U.S. Pat. No. 3,857,931; and Wong, Chemistry of Protein Conjugation and Cross-Linking, 1991, CRC Press, Boca Raton, Fla., which are incorporated herein by reference.[0073]
- 7. Signal[0074]
- Signal reagents may be prepared by conjugating an antibody to a signal-generating element by any of a number of common methods. Conjugates may be prepared, for example, by utilizing free sulfhydryl groups, generating sulflhydryl groups from available disulfide bonds, or introducing additional sulfhydryls onto the antibody. Linking agents, such as succinimydyl 4-(N-maleimidomethyl)cyclohexane-1-carboxylate (SMCC) may then used to conjugate the activated antibody to the signal-generating element. For more information on preparing antibody conjugates, see Pierce Instructions “ImmunoPure IgG1 Fab and F(ab′)2 Preparation Kit” #44880; Pierce Instructions “SMCC, Sulfo-SMCC” #22360, #22322; Pierce Instructions “EZ-Link Maleimide Activated Phosphatase Kit” # 31493; Beale, D. (1987) Molecular fragmentation: Some applications in immunology, Exp Comp Immunology 11, 287-296; Lamoyi, E. (1986) Preparation of F(ab′)2 fragments from mouse IgG of various subclasses, Meth Enz 121, 652-663; King, T P, Kochoumian, L. (1979), A comparison of different enzyme-antibody conjugates for enzyme-linked immunosorbent assay, J Immun Meth 28, 201-210; Brinklay, Mass. (1992), A survey of methods for preparing protein conjugates with dyes, haptens and crosslinking reagents,[0075]
Bioconj Chem 3, 2-13; and TechNote #204, “Adsorption to Microspheres”, Bangs Laboratories Inc Rev. #001 Aug. 4, 1999, which are incorporated herein by reference. - Signal-generating elements of the present invention include, but are not limited to, radiolabels, metal particles, chromogens, fluorescent dyes, labeled proteins and enzymes. When the signal-generating element is an enzyme, the enzyme may catalyze a reaction wherein the depletion of substrate or the production of product can be detected. Exemplary enzymes include, but are not limited to, amylases, polynucleotidase, arginase, adenase, aminopolypeptidase, pepsin, lipases, catalase, tyrosinases, alcohol dehydrogenase, succinic dehydrogenase, diaphorase, glyoxalase, aldolase, glucose oxidase, horseradish peroxidase, beta-galactosidase, phosphatases, phosphorylases and hexokinases. Exemplary enzymes also include alkaline phosphatase, glucose oxidase, horseradish peroxidase, β-galactosidase and phenol oxidase. The enzymatic conversion of a substrate to a product may be measured by for example optical and electrochemical means.[0076]
- a. Multiple Antibodies Conjugated to a Signal-Generating Element[0077]
- The sensitivity of a sandwich assay is limited largely by the level of non-specific adsorption of the signal reagent to the capture reagent and surfaces within the detection region of the analysis device. Such surfaces may include the walls of a cuvette, a wicking element, an electrode and the like. When the analyte is at low levels in a sample, a significant amount of the signal measured is background, and thus not related to the concentration of the analyte in the sample. The propensity of the signal reagent to non-specifically adsorb to surfaces within the analysis device may be a function of the inherent properties of the antibody and signal-generating element used to form the signal antibody, as well as the crosslinker. Additionally, the materials used in the analysis device (e.g., types of plastics) and the composition of the wash and substrate-containing solutions are generally optimized to minimize such non-specific protein adsorption. In particular, various surfactants,[0078]
e.g. Tween 20,Brij 35, Triton X100, are almost invariably used in sandwich assays to minimize unwanted interactions of the signal reagents with other components within the assay device. Non-enzymatic proteins such as serum albumin, denatured proteins such as gelatins, and deactivated enzymes may also be added as surface blocking agents in order to lower the level of non-specific adsorption of the signal reagent. Even when all components of the assay are optimized, there is still usually a detectable level of non-specific signal reagent adsorption. The level of this non-specific adsorption is proportional to the amount of signal reagent used in the assay. Therefore, using less signal reagent or using the same molar amount of a lower molecular weight signal reagent will usually result in a lower background signal in the assay. - The signal reagent may comprise fragments of antibodies, such as Fab fragments, which may contribute to lowering the level of background signal. By lowering the molecular weight of the signal reagent, the time required for the binding step may be reduced due to diffusional considerations. The signal reagent may also comprise more than one antibody, which may also lower background signal levels by minimizing the overall size of the signal reagent while maximizing its ability to generate a signal in response to the presence of the analyte of interest. The more than one antibody of the signal reagent may bind to two or more different epitopes of the analyte, which may lead to stabilization of the signal antibody-analyte complex when more than one epitope of the analyte is bound by more than one antibody of the signal reagent.[0079]
- When the signal reagent comprises more than one antibody, said antibodies may bind to one or more different epitopes.[0080]
- A signal-generating element may be conjugated with more than one antibody, each antibody binding to a different epitope on the analyte of interest. See FIG. 6C. The molar ratio of antibodies conjugated to the signal-generating element may be controlled by selecting the reaction conditions and stoichiometry for the conjugation reaction.[0081]
- A first antibody may be conjugated to a first signal-generating element and a second antibody may be conjugated to a second signal-generating element, each at a ratio greater than 1:1, and then mixed, wherein the first and second antibodies each bind to a different epitope on the analyte of interest. See FIG. 7B. The molar ratio of the first and second antibodies may also be controlled.[0082]
- The signal-generating element may be conjugated to a single antibody, or two or more antibodies to different epitopes on the same analyte, wherein the signal antibody comprises a ratio of signal-generating element to a population of antibodies from about 1:100 to about 1:1.001. The signal-generating element may also be conjugated to a population of antibodies at a ratio from about 1:90 to about 1:1.001. The signal-generating element may also be conjugated to a population of antibodies at a ratio from about 1:80 to about 1:1.001. The signal-generating element may also be conjugated to a population of antibodies at a ratio from about 1:70 to about 1:1.001. The signal-generating element may also be conjugated to a population of antibodies at a ratio from about 1:60 to about 1:1.001. The signal-generating element may also be conjugated to a population of antibodies at a ratio from about 1:50 to about 1:1.001. The signal-generating element may also be conjugated to a population of antibodies at a ratio from about 1:40 to about 1:1.001. The signal-generating element may also be conjugated to a population of antibodies at a ratio from about 1:30 to about 1:1.001. The signal-generating element may also be conjugated to a population of antibodies at a ratio from about 1:20 to about 1:1.001. The signal-generating element may also be conjugated to a population of antibodies at a ratio from about 1:10 to about 1:1.001, about 1:20 to about 1:10, about 1:30 to about 1:20, about 1:40 to about 1:30, about 1:50 to about 1:40, about 1:60 to about 1:50, about 1:70 to about 1:60, about 1:80 to about 1:70, about 1:90 to about 1:80, and about 1:100 to about 1:90. The signal-generating element may also be conjugated to a population of antibodies at a ratio from about 1:5 to about 1:1.001, about 1:10 to about 1:5, about 1:15 to about 1:10, about 1:20 to about 1:15, about 1:25 to about 1:20, about 1:30 to about 1:25, about 1:35 to about 1:30, about 1:40 to about 1:35, about 1:45 to about 1:40, and about 1:50 to about 1:45. The signal-generating element may also be conjugated to a population of antibodies at a ratio of about 1:3.3.[0083]
- The signal-generating element may be conjugated to a first and second antibody, each capable of binding to different epitopes on an analyte or subforms thereof, wherein the ratio of signal-generating element to total antibody is as disclosed above, and wherein the ratio of the first antibody to the second antibody is from about 1:50 to about 50:1. The ration of the first antibody to the second antibody may also be from about 1:10 to about 10:1, from about 1:5 to about 5:1, from about 1:3 to about 3:1, and from about 1:2 to about 2:1.[0084]
- 8. Signal and Detection[0085]
- A signal may be produced by the signal-generating element, which may be measurable by a sensing element using methods which include, but are not limited to, radiation, optically, electrochemically, or by some other transduction means known in the art. A sensing element may be an electrode, biosensor, field-effect transistor, surface acoustic wave device, optical wave-guide, fiber optic, cuvette, radioactivity detector, reagents that facilitate, physical, nuclear, chemical, biochemical, electrical, or optical detection, or combinations thereof.[0086]
- 9. Immunoassay Device[0087]
- The immunoassay of an analyte of interest may be conducted in an immunoassay device comprising one or more sensing elements and a surface on which a modified sandwich immunoassay is conducted as described above. The device surface may comprise two or more antibodies that are each capable of binding to different epitopes on the analyte. At least one of the two antibodies is also capable of binding to the same epitope on a subform of the analyte. At least one of the epitopes on the analyte is unavailable for binding on the subform.[0088]
- Devices of the present invention include a cartridge, columns, syringe, cuvette, or other analytical device or system known in the art. The cartridge may be of a type as described in U.S. Pat. No. 5,096,669. Examples of other cartridge configurations are found in U.S. Pat. Nos. 5,416,026, 5,593,638, 5,447,440, 5,628,961, 5,514,253, 5,609,824, 5,605,664, 5,614,416, 5,789,253, 6,030,827, and 6,379,883. Still other cartridge configurations are described in PCT/US00/31158, PCT/US01/04345 and copending U.S. patent application Ser. No. 10/087,730. The disclosures of the foregoing are hereby incorporated by reference.[0089]
- The surface of the immunoassay device may be of any appropriate surface known to those of skill in the art including, but not limited to, glass, semiconductor, plastic, silicon dioxide, photoformable PVA, photoformable gelatin, film forming latex, and conductive metal. A conductive metal surface may be silver, iridium, gold, platinum, and alloys thereof.[0090]
- Antibodies may be absorbed, adsorbed or covalently attached to the surface of the immunoassay device. Antibodies may be absorbed into PVA, gelatin and latex layers or absorbed to their surfaces. Antibodies may be adsorbed to glass, metal, semiconductor, plastic, silicon dioxide, photoformable PVA, and conductive metal. The two or more antibodies may also be attached to a microparticle as discussed above, wherein the microparticle is attached to the surface as discussed above. The immunoassay device may also contain a third antibody which binds to a different epitope on the analyte.[0091]
- The sensing element of the immunoassay device may be of any type known in the art including, but not limited to, an electrode, biosensor, field-effect transistor, surface acoustic wave device, optical wave-guide, fiber optic, cuvette, radioactivity detector, immunochromatographic device, reagents that facilitate, physical, nuclear, chemical, biochemical, electrical, optical detection, or combinations thereof.[0092]
- 10. Immunoassay Kit[0093]
- An immunoassay kit may be used to detect an analyte of interest and subforms thereof in a modified sandwich immunoassay as described above. The kit may comprise three or more antibodies that are each capable of binding to different epitopes on the analyte. At least two of the three antibodies are also capable of binding to the same epitopes on a subform of the analyte. At least one of the epitopes on the analyte is unavailable for binding on the subform.[0094]
- 11. Sandwich Immunoassay Product[0095]
- A sandwich immunoassay product may comprise an analyte of interest and a subform thereof. At least three epitopes on the analyte are available for binding by at least three different antibodies. The analyte may be bound by at least three different antibodies with each antibody binding to a different epitope on the analyte. At least two of the three epitopes on the analyte are available for binding on the subform by at least two of the at least three different antibodies. The subform may be bound by at least two of the three different antibodies that bind to the analyte with each antibody binding to a different epitope on the subform. At least one epitope of the analyte is unavailable for binding on the subform. At least one of the antibodies bound to the analyte and the subform of the analyte may be a signal antibody.[0096]
- 12. Method of Assaying for an Analyte[0097]
- A sample may be immunoassayed for the presence of an analyte and subforms thereof by performing a modified sandwich immunoassay as described above. At least three different antibodies are used, wherein each antibody is capable of binding to a different epitope on the analyte. At least two of the antibodies capable of binding to the analyte are capture antibodies or signal antibodies, or a combination thereof. At least one of the epitopes on the analyte is unavailable for binding on the subform.[0098]
- The sample to be immunoassayed is added to a surface comprising one or more capture antibodies. The one or more signal antibodies are then added. Alternatively, one or more signal antibodies may be added to the sample before or during application of the sample to the surface comprising one or more capture antibodies. After removing unbound signal antibodies, the extent of binding of the one or more signal antibodies is determined as described above. A signal will be produced by analyte as well as all subforms of the analyte that have at least one epitope that is present or capable of being bound by a capture antibody and at least a second epitope that is present or capable of being bound by a signal antibody.[0099]
- 13. Method of Diagnosing an Acute Disease[0100]
- The analyte of interest and subforms thereof may be immunoassayed to determine whether a patient has suffered an acute medical event. A sample may be obtained from the patient and immunoassayed for the presence of the analyte of interest and subforms thereof by using the method of assaying described above. Signal produced by signal antibodies may be compared to control values. A signal, or a sequence of signals from a series of assays over a period of hours or days, detectable above control may indicate that the patient has suffered an acute medical event.[0101]
- The acute medical event may be any disease state that is associated with a clinical marker, wherein the clinical marker is an analyte in the modified immunoassay described herein. The medical event may be for example a myocardial infarction. The analyte of interest may be for example TnI, TnT, TnC, CK-M, CK-B, CK-MB, myoglobin, TSH, FSH, CRP, BNP, pro-BNP, PSA, PCA, apolipoprotein, and combinations thereof.[0102]
- 14. Method of Diagnosing the Time of the Occurrence of an Acute Disease[0103]
- The analyte of interest and subforms thereof may be immunoassayed to determine when a patient suffered an acute medical event. A sample, or set of samples obtained at different times, may be obtained from the patient and immunoassayed for the presence of the analyte of interest and subforms thereof by using the method of assaying described above. The amount of signal produced by signal antibodies may be correlated with a standard curve of amount of analyte vs. time to determine when the patient suffered the acute medical event. One of ordinary skill may produce the standard curve by using the methods described herein.[0104]
- 15. Method of Diagnosing the Severity of an Acute Disease[0105]
- The analyte of interest and subforms thereof may be immunoassayed to determine the severity of an acute medical event suffered by a patient. A sample, or set of samples obtained at different times, may be obtained from the patient and immunoassayed for the presence of the analyte of interest and subforms thereof by using the method of assaying described above. The amount of signal produced by signal antibodies may correlate to the severity of a medical event suffered by the patient.[0106]
- Having now generally described the invention, the following examples are provided in order to more fully illustrate the invention. These examples are for purposes of illustration only and are not intended to limit the scope of the invention.[0107]
- Conjugation of cTnI Capture Antibodies to a Particle[0108]
- Bead particles conjugated with antibodies specific for cTnI were prepared by first exchanging polystyrene/acrylic acid latex 0.2 μm diameter spheres (Seradyn) into 0.05 M 2-(N-morpholino)ethanesulfonic acid (MES, Sigma Aldrich) buffer at pH 6.2. To a 2% w/w solution of the latex spheres was added cTnI antibodies (buffer exchanged into the same 0.05 M MES buffer) at a controlled mass ratio relative to the mass of the latex spheres. The solution was stirred for 15 minutes at 4° C. and the antibody coated beads separated via controlled centrifugation. Typically sufficient antibody was added to fully saturate the latex bead surface (8% to 15% of the mass of the latex particles). The microparticle centrifuged pellet was then resuspended into MES buffer at 1% w/v density and 1 to 10 mM of EDC was added to covalently crosslink the adsorbed antibody to the latex spheres. The suspension was allowed to react for 4 to 12 hours at which time the latex particles were separated by centrifugation, resuspended at 1% in the MES buffer and the EDC addition was repeated. Using the above method, microparticle capture reagents were made comprising either CB1 (19C7, Hytest Inc.) or CB2 (34503228P, Biospacific Inc.). CB12 microparticles comprising both CB1 and CB2 were prepared using the above method by adding a mixture of the two capture antibodies.[0109]
- Preparation of Signal Antibodies[0110]
- The following method was used to prepare alkaline phosphatase (ALP) labeled Fab conjugates to be used as signal reagents. Alkaline phosphatase (Biozyme) was buffer exchanged into phosphate buffered saline (PBS). A 1 mg/ml to 15 mg/ml solution of the ALP was reacted with 25 weight percent (relative to the ALP weight in the solution) of the heterobifunctional crosslinker succinimidyl-4-[N-maleimidomethyl]-cyclohexane-1-carboxy[6-amidocaproate] (LCSMCC, Pierce). The crosslinker was added as a solution in dimethyl sulphoxide (DMSO, Sigma). This solution was allowed to react at room temperature for 45 minutes, after which it was centrifuged to remove precipitates. The activated ALP was separated from unreacted LCSMCC via a Sephadex G50 desalting column (Sigma Aldrich).[0111]
- Fab fragments of the antibodies to be incorporated into the signal reagent were formed via pepsin (Sigma) digestion of the whole antibodies and separated using a S200 sephacryl size exclusion column (Amersham Pharmacia). The resulting F(ab)[0112]2fragments were chemically reduced in 60 mM mercaptoethylamine (MEA, Sigma Aldrich) for 45 minutes at 37° C. to produce two Fab fragments, which were separated from excess MEA by desalting using a sephadex G50 column. The Fab fragments were then added to activated ALP and allowed to react at 4° C. for 2 to 12 hours. The reaction was quenched with an equal volume of 0.1 M glycine (Sigma), 0.01 M cysteine (Pierce) in PBS pH 7.4. The reaction products were then fractionated on a Sephacryl S200 column to yield the signal reagent comprising Fab fragments. Signal reagents with specific molar ratios of Fab fragments to ALP were obtained by varying the ratio of Fab2fragments to ALP in the reaction above. FIG. 12 shows the elution profile for ALP-Fab signal reagents with ALP:Fab ratios of 1:1, 1:3, 1:6 and 1:10.
- Using the above method, ALP-labeled signal reagents were made comprising either EC1 (G129C, Biospacific Inc.) or EC2 (G130C, Biospacific Inc.). EC12 signal reagents comprising both EC1 and EC2 were prepared using the above method by mixing the two fab fragments at a molar ratio of 1:1 before adding the mixture to activated ALP[0113]
- Preparation Of An ELISA Sensor With cTnI Capture Antibodies[0114]
- The following method was used to prepare an analyte detector comprising an immobilized layer of antibody labeled particles on an ELISA sensor. Briefly, amperometric sensors used in the analyte detector are fabricated using traditional semiconductor thin layer techniques on oxidized silicon wafers. The cTnI capture regions are produced by microdispensing solutions of the capture antibodies described in Example 1 using methods described in U.S. Pat. No. 5,554,339. Droplets of the particle-containing suspension are allowed to dry to form cTnI capture regions on the sensor surface. The sensor chips are assembled with a separated electrochemical grounding chip into a plastic base covered with a two sided adhesive tape gasket, laser cut with openings for the sensors, ground chip and fluidic channels. To this base assembly a plastic cover is added to form fluidic conduits. The enzyme labeled signal reagent of Example 2 is printed onto the same ELISA sensor as the capture antibodies. The signal reagent is formulated in a 1% to 50% sugar solution containing PBS and a preservative. This composition was found to provide rapid dissolution of the signal reagent into a blood sample. The assembled cartridge is pressed to yield the disposable cartridge, which may be used in a portable electrochemical analyzer.[0115]
- Comparison Of Detection Of cTnI Using Single or Multiple Capture Antibodies[0116]
- An analytical system for measuring cTnI was used of the general type described in U.S. Pat. No. 5,096,669, which is hereby incorporated by reference. Briefly, cartridges for assaying cTnI were produced using sensors coated with latex microsphere comprising CB1, CB2 and CB12 capture reagents, as described in Example 3. cTnI was assayed in three whole-blood samples to which either free cTnI (Scripps Laboratories) or ITC complex (Hytest Ltd) was added. The signal reagent EC12, as described in Example 2, was used to produce an amperometric signal based on dephosporylation of a substrate by ALP to produce an electroactive product. The amount of electroactive product is proportional to the amount of detectable cTnI.[0117]
- FIG. 10 shows high signal levels for cartridges comprising the CB1 capture reagent for both free cTnI (diamond points) and the two levels of ITC complex (square and triangle points). By contrast, cartridges comprising the CB2 capture reagent yield lower signals, especially for the ITC complex. Cartridges using the CB12 hybrid capture reagent show improved signal generation for both forms of cTnI relative to either CB1 or CB2 capture reagents. The improved performance of the CB12 capture reagent is surprising, because the hybrid reagent comprises the CB2 antibody that by itself has markedly lower analytical performance than the CB1 antibody.[0118]
- Comparison of Detection of cTnI Using Single or Multiple Signal Antibodies[0119]
- cTnI was assayed in a whole blood sample spiked with a 6.0 ng/mL cITC complex immediately after preparation and after a one-day incubation at room temperature using cartridges with sensors coated with latex microsphere comprising CB1 and CB12 capture reagents, as described in Example 3, and EC1, EC2 or EC12 signal reagents as described in Example 2. FIG. 11 demonstrates that the EC1 signal reagent shows a higher signal generation than the EC2 signal reagent. After a one day incubation of the sample to simulate aging of cTnI (e.g., proteolytic degradation), the signal level for the EC1 signal reagent drops significantly. In contrast, the EC2 signal reagent show only a modest drop in the signal level. The signal generation of the hybrid signal reagent EC12 is surprisingly superior to each of the two single antibody signal reagents, especially after incubating the sample for one day.[0120]
- Detection of cTnI in Serum as a Diagnosis of Myocardial Infarction[0121]
- Whole blood samples are obtained from patients suspected of suffering a myocardial infarction, as well as from control individuals. cTnI is assayed in the whole blood samples using cartridges with sensors coated with latex microsphere comprising CB12 capture reagents, as described in Example 3, and EC12 signal reagents as described in Example 2. The signal generated in samples derived from patients suspected of suffering an MI is compared to the level of signal from controls. Levels of signal greater than control indicate that the patient has suffered a MI.[0122]
Claims (41)
1. An immunoassay composition for detecting an analyte comprising at least three different antibodies, the at least three different antibodies being capable of binding to at least three different epitopes on an analyte, in which at least two of the at least three different antibodies are capable of binding to at least two different epitopes on a subform of the analyte, and in which at least one of the at least three different epitopes on the analyte is unavailable for binding on the subform.
2. An immunoassay composition for detecting an analyte comprising m different antibodies,
(a) in which at least n of the m different antibodies are capable of binding to n different epitopes on an analyte, and
(b) in which no more than n−1 of the n different epitopes on the analyte are available for binding on a subform of the analyte,
provided that m and n are greater than or equal to 3, and m is greater than or equal to n.
3. The immunoassay composition ofclaim 1 in which the analyte comprises TnI, TnT, TnC, CK-M, CK-B, CK-MB, myoglobin, TSH, FSH, CRP, BNP, pro-BNP, PSA, PCA, apolipoprotein, or combinations thereof.
4. The immunoassay composition ofclaim 1 in which at least one of the at least three antibodies comprises a full length antibody, a single-chain antibody, or an antibody fragment.
5. The immunoassay composition ofclaim 4 in which the antibody fragment comprises a Fab fragment.
6. The immunoassay composition ofclaim 1 in which the analyte is cTnI.
7. The immunoassay composition ofclaim 1 which further comprises a common member conjugated to at least one of the at least three antibodies.
8. The immunoassay composition ofclaim 7 in which the at least one of the at least three antibodies is covalently attached to the common member.
9. The immunoassay composition ofclaim 8 in which the covalent attachment comprises a crosslinker, which is derived from a crosslinking agent.
10. The immunoassay composition ofclaim 9 in which the crosslinking agent comprises succinimidyl-4-[N-maleimidomethyl]-cyclohexane-1-carboxy[6-amidocaproate], glutaraldehyde, adipic acid dihydrazide, bis-diazotized benzidine, 1,4-butane diglycidyl ether, bis-maleimido hexane, sulfosuccinimidyl 4-(N-maleimidomethyl)-cyclohexane-1-carboxylate, or N-hydroxysuccinimidyl 4-azidosalicylic acid.
11. The immunoassay composition ofclaim 7 in which the common member is a microparticle.
12. The immunoassay composition ofclaim 11 in which the microparticle comprises latex beads, polystyrene beads, polystyrene/acrylic acid beads, or combinations thereof.
13. The immunoassay composition ofclaim 7 in which the common member comprises a signal-generating element.
14. The immunoassay composition ofclaim 13 in which the signal-generating element comprises a radiolabel, metal particle, fluorescent dye, chromogenic dye, labeled protein, enzyme, or combinations thereof.
15. The immunoassay composition ofclaim 14 in which the enzyme comprises peroxidase, glucose oxidase, phenol oxidase, β-galactosidase, alkaline phosphatase, or combinations thereof.
16. The immunoassay composition ofclaim 13 in which the molar ratio of the at least one of the at least three antibodies conjugated to the signal-generating element is from about 1:1 to about 10:1.
17. The immunoassay composition ofclaim 16 in which the molar ratio of the at least one of the at least three antibodies conjugated to the signal-generating element is about 3:1.
18. The immunoassay composition ofclaim 13 in which at least two of the at least three antibodies are conjugated to the signal-generating element.
19. The immunoassay composition ofclaim 18 , in which the molar ratio of the at least two of the at least three antibodies conjugated to the signal-generating element is from 1:1 to 10:1.
20. An immunoassay composition for detecting an analyte comprising four different antibodies, the four different antibodies being capable of binding to four different epitopes on an analyte, in which at least one of four different epitopes on the analyte is unavailable for binding on a subform of the analyte, in which a first and second of the four different antibodies are conjugated to a surface, in which a third and fourth of the four different antibodies are each conjugated to an enzyme, in which at least one of the first and second antibodies is capable of binding to at least one epitope on the subform of the analyte, and in which at least one of the third and fourth antibodies is capable of binding to at least one epitope on the subform.
21. An immunoassay composition for detecting an analyte comprising m different antibodies,
(a) in which n of the m different antibodies are capable of binding to n different epitopes on an analyte,
(b) in which no more than n−1 of the n different epitopes on the analyte are available for binding on a subform of the analyte,
(c) in which p of the n different antibodies are conjugated to a surface,
(d) in which p of the n different antibodies are each conjugated to an enzyme,
(e) in which at least p−1 of the p antibodies in step (c) is capable of binding to at least p−1 different epitopes on the subform of the analyte, and
(f) in which at least p−1 of the p antibodies in step (d), is capable of binding to at least p−1 different epitopes on the subform of the analyte.
provided that m and n are 4, and that p is 2.
22. An immunoassay device comprising one or more sensing elements and at least one surface on which an immunoassay can be conducted for detecting an analyte, which at least one surface comprises at least two different antibodies, the at least two different antibodies being capable of binding to at least two different epitopes on the analyte, in which at least one of the at least two different antibodies is capable of binding to at least one epitope on a subform of the analyte, in which at least one of the at least two different epitopes on the analyte is unavailable for binding on the subform, and in which the at least two different antibodies are bound to the at least one surface.
23. An immunoassay device comprising one or more sensing elements and at least one surface on which an immunoassay can be conducted for detecting an analyte,
(a) in which at least one surface comprises m different antibodies,
(b) in which at least n of the m different antibodies are capable of binding to n different epitopes on the analyte
(c) in which no more than n−1 of the n different epitopes on the analyte are available for binding on a subform of the analyte, and
(d) in which at least n of the m different antibodies are bound to the at least one surface,
provided that m and n are greater than or equal to 2, and m is greater than or equal to n.
24. The immunoassay device of claim21(a) in which the analyte comprises TnI, TnT, TnC, CK-M, CK-B, CK-MB, myoglobin, TSH, FSH, CRP, BNP, pro-BNP, PSA, PCA, apolipoprotein, or combinations thereof.
25. The immunoassay device of claim21(a) in which the at least one surface comprises glass, semiconductor, plastic, silicon dioxide, photoformable PVA, photoformable gelatin, film forming latex, conductive metal, or combinations thereof.
26. The immunoassay device ofclaim 25 in which the conductive metal comprises silver, iridium, gold, platinum, or combinations thereof.
27. The immunoassay device of claim21(a) in which at least one of the at least two antibodies is absorbed or adsorbed to the at least one surface.
28. The immunoassay device of claim21(a) in which at least one of the at least two antibodies is covalently attached to the at least one surface.
29. The immunoassay device of claim21(a) which further comprises a microparticle in which the at least two antibodies are bound to the microparticle and in which the microparticle is bound to the at least one surface.
30. The immunoassay device of claim21(a) which further comprises at least two microparticles in which only one of the at least two antibodies is bound to one of the at least two microparticles and in which the at least two microparticles are bound to the at least one surface.
31. The immunoassay device of claim21(a) which further comprises a third antibody which binds to a different epitope on the analyte and the subform of the analyte.
32. The immunoassay device of claim21(a) in which the sensing element comprises an electrode, biosensor, field-effect transistor, surface acoustic wave device, optical wave-guide, fiber optic, cuvette, radioactivity detector, immunochromatographic device, reagents that facilitate physical, nuclear, chemical, biochemical, electrical, or optical detection, or combinations thereof.
33. An immunoassay kit comprising in a suitable container, at least three different antibodies, the at least three antibodies being capable of binding to at least three different epitopes on an analyte, in which at least two of the at least three different antibodies are capable of binding to at least two different epitopes on a subform of the analyte, and in which at least one of the at least three different epitopes on the analyte is unavailable for binding on the subform.
34. An immunoassay kit comprising in a suitable container m different antibodies,
(a) in which at least n of the m different antibodies are capable of binding to n different epitopes on an analyte, and
(b) in which no more than n−1 of the n different epitopes on the analyte are available for binding on a subform of the analyte,
provided that m and n are greater than or equal to 3, and m is greater than or equal to n.
35. The immunoassay kit ofclaim 33 in which the analyte comprises TnI, TnT, TnC, CK-M, CK-B, CK-MB, myoglobin, TSH, FSH, CRP, BNP, pro-BNP, PSA, PCA, apolipoprotein, or combinations thereof.
36. A sandwich immunoassay product comprising an analyte and a subform of the analyte, in which at least three different epitopes on the analyte are available for binding by at least three different antibodies, in which at least two of the three different antibodies are bound to a different epitope on the analyte, in which two of the three different epitopes on the analyte are available for binding on the subform, in which at least two of the three different antibodies are bound to a different epitope on the subform, and in which at least one of the at least three different epitopes on the analyte is unavailable for binding on the subform.
37. A sandwich immunoassay product comprising an analyte, a subform of the analyte, and at least 2 of m different antibodies,
(a) in which the analyte includes at least n different epitopes each capable of being bound by one of the m different antibodies,
(b) in which at least 2 of the m different antibodies are each bound to a different epitope on the analyte,
(c) in which no more than n−1 of the n different epitopes on the analyte are available for binding on a subform of the analyte, and
(d) in which at least 2 of the m different antibodies are each bound to a different epitope on the subform of the analyte,
provided that m and n are greater than or equal to 3, and that m can be greater than or equal to n.
38. The sandwich immunoassay product ofclaim 36 in which the analyte comprises TnI, TnT, TnC, CK-M, CK-B, CK-MB, myoglobin, TSH, FSH, CRP, BNP, pro-BNP, PSA, PCA, apolipoprotein, or combinations thereof.
39. The sandwich immunoassay product ofclaim 36 in which at least one of the at least three antibodies and least one of the at least two antibodies comprises a signal-generating element.
40. A method of determining whether a patient has suffered a myocardial infarction comprising:
(a) applying a sample from a patient suspected of suffering a myocardial infarction to a surface to which is bound at least two antibodies, in which the at least two antibodies are capable of binding to at least two different epitopes on cTnI, in which at least one of the at least two antibodies is capable of binding to at least one different epitope on a subform of cTnI, and in which at least one epitope of cTnI is unavailable for binding on the subform;
(b) adding a reagent comprising a third antibody which binds to yet another epitope on cTnI and the subform; and
(c) determining the extent of binding of the third antibody.
41. A method of determining whether a patient has suffered a myocardial infarction comprising:
(a) applying a sample from a patient suspected of suffering a myocardial infarction to a surface to which is bound a first antibody which is capable of binding to a first epitope on cTnI and a subform of cTnI;
(b) adding separately or together at least a second and third antibody, in which the at least second and third antibodies are capable of binding to at least two different epitopes on cTnI, in which the at least second and third antibodies are not capable of binding to the first epitope, in which at least one of the at least second and third antibodies are each capable of binding to at least one different epitope on the subform of cTnI, and in which at least one epitope of cTnI is unavailable for binding on the subform; and
(c) determining the extent of binding of the at least second and third antibodies.
Priority Applications (7)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US10/208,560US20040018577A1 (en) | 2002-07-29 | 2002-07-29 | Multiple hybrid immunoassay |
| EP03771940.8AEP1525480B1 (en) | 2002-07-29 | 2003-07-29 | Multiple hybrid immunoassay |
| JP2004524914AJP4507879B2 (en) | 2002-07-29 | 2003-07-29 | Multiple hybrid immunoassay |
| PCT/US2003/023483WO2004011947A1 (en) | 2002-07-29 | 2003-07-29 | Multiple hybrid immunoassay |
| US14/081,309US9267939B2 (en) | 2002-07-29 | 2013-11-15 | Multiple hybrid immunoassay |
| US14/994,713US9995744B2 (en) | 2002-07-29 | 2016-01-13 | Multiple hybrid immunoassay |
| US15/975,145US10641767B2 (en) | 2002-07-29 | 2018-05-09 | Multiple hybrid immunoassay |
Applications Claiming Priority (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US10/208,560US20040018577A1 (en) | 2002-07-29 | 2002-07-29 | Multiple hybrid immunoassay |
Related Child Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US14/081,309ContinuationUS9267939B2 (en) | 2002-07-29 | 2013-11-15 | Multiple hybrid immunoassay |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| US20040018577A1true US20040018577A1 (en) | 2004-01-29 |
Family
ID=30770571
Family Applications (4)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US10/208,560AbandonedUS20040018577A1 (en) | 2002-07-29 | 2002-07-29 | Multiple hybrid immunoassay |
| US14/081,309Expired - Fee RelatedUS9267939B2 (en) | 2002-07-29 | 2013-11-15 | Multiple hybrid immunoassay |
| US14/994,713Expired - LifetimeUS9995744B2 (en) | 2002-07-29 | 2016-01-13 | Multiple hybrid immunoassay |
| US15/975,145Expired - Fee RelatedUS10641767B2 (en) | 2002-07-29 | 2018-05-09 | Multiple hybrid immunoassay |
Family Applications After (3)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US14/081,309Expired - Fee RelatedUS9267939B2 (en) | 2002-07-29 | 2013-11-15 | Multiple hybrid immunoassay |
| US14/994,713Expired - LifetimeUS9995744B2 (en) | 2002-07-29 | 2016-01-13 | Multiple hybrid immunoassay |
| US15/975,145Expired - Fee RelatedUS10641767B2 (en) | 2002-07-29 | 2018-05-09 | Multiple hybrid immunoassay |
Country Status (4)
| Country | Link |
|---|---|
| US (4) | US20040018577A1 (en) |
| EP (1) | EP1525480B1 (en) |
| JP (1) | JP4507879B2 (en) |
| WO (1) | WO2004011947A1 (en) |
Cited By (201)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US20040197841A1 (en)* | 2003-04-02 | 2004-10-07 | Apffel James Alexander | Methods and reagents for multiplexed analyses |
| US20040222091A1 (en)* | 2002-12-02 | 2004-11-11 | Imants Lauks | Diagnostic devices incorporating fluidics and methods of manufacture |
| US20040231984A1 (en)* | 2002-12-02 | 2004-11-25 | Imants Lauks | Heterogeneous membrane electrodes |
| US20050047972A1 (en)* | 2003-08-28 | 2005-03-03 | Imants Lauks | Lateral flow diagnostic devices with instrument controlled fluidics |
| US20050137481A1 (en)* | 2003-12-18 | 2005-06-23 | Paul Sheard | Monitoring method and apparatus |
| US20050150761A1 (en)* | 2001-06-04 | 2005-07-14 | Epocal Inc. | Electrode module |
| AT500800A1 (en)* | 2004-09-08 | 2006-03-15 | Biomedica Medizinprodukte Gmbh | PROCESS FOR DETERMINING PROBNP |
| US20060204999A1 (en)* | 2005-03-14 | 2006-09-14 | Stephen Macevicz | Detecting molecular complexes |
| US20060286680A1 (en)* | 2004-07-26 | 2006-12-21 | University Of Louisville Research Foundation | Fiber-optic biosensor and biosensing methods |
| US20070170062A1 (en)* | 2001-06-04 | 2007-07-26 | Epocal Inc. | Integrated electrokinetic devices and methods of manufacture |
| WO2008051761A2 (en) | 2006-10-26 | 2008-05-02 | Abbott Laboratories | Assay for cardiac troponin autoantibodies |
| US20080153109A1 (en)* | 2003-04-30 | 2008-06-26 | Susann Eriksson | Immunoassay |
| WO2008082974A2 (en) | 2006-12-29 | 2008-07-10 | Abbott Laboratories | Non-denaturing lysis reagent |
| WO2008082984A2 (en) | 2006-12-29 | 2008-07-10 | Abbott Laboratories | Non-denaturing lysis reagent for use with capture-in-solution immunoassay |
| WO2008137991A1 (en) | 2007-05-08 | 2008-11-13 | Abbott Laboratories | Human b-type natriuretic peptide assay having reduced cross-reactivity with other peptide forms |
| US20080305512A1 (en)* | 2006-10-26 | 2008-12-11 | Mattingly Phillip G | Assay for cardiac troponin autoantibodies |
| US20080311676A1 (en)* | 2007-05-24 | 2008-12-18 | Abbott Laboratories | Immunoassays exhibiting reduced cross-reactivity with hydrophobic drug analyte metabolites |
| US20090053736A1 (en)* | 2007-08-21 | 2009-02-26 | Mattingly Phillip G | Homogeneous Chemiluminescent Immunoassay for Analysis of Iron Metalloproteins |
| US20090054741A1 (en)* | 2005-03-29 | 2009-02-26 | Inverness Medical Switzerland Gmbh | Device and method of monitoring a patient |
| US20090053596A1 (en)* | 2007-08-22 | 2009-02-26 | Stauffer John E | Alkali Metal Battery |
| WO2009036228A2 (en) | 2007-09-13 | 2009-03-19 | Abbott Laboratories | Hepatitis b pre-s2 nucleic acid |
| US20090098531A1 (en)* | 2007-09-13 | 2009-04-16 | Abbott Laboratories | Detecting hepatitis b virus |
| US20090111123A1 (en)* | 2007-10-25 | 2009-04-30 | Birkenmeyer Larry G | Compositions for detecting antibodies to babesia microti and methods of uses thereof |
| WO2009078875A1 (en) | 2007-12-19 | 2009-06-25 | Abbott Laboratories | Immunosuppressant drug extraction reagent for immunoassays |
| US20090203037A1 (en)* | 2007-12-27 | 2009-08-13 | Abbott Laboratories | Anti-T. Cruzi Antibodies and Methods of Use |
| US20090226445A1 (en)* | 2008-03-06 | 2009-09-10 | Abbott Laboratories | Plasmodium malariae and plasmodium ovale genes and uses thereof |
| WO2009111599A2 (en) | 2008-03-06 | 2009-09-11 | Abbott Laboratories | Plasmodium malariae and plasmodium ovale genes and uses thereof |
| US20090246800A1 (en)* | 2006-10-26 | 2009-10-01 | Abbott Laboratories | Immunoassay of analytes in samples containing endogenous anti-analyte antibodies |
| US20090286256A1 (en)* | 2008-05-19 | 2009-11-19 | Abbott Laboratories | Isolated human autoantibodies to natriuretic peptides and methods and kits for detecting human autoantibodies to natriuretic peptides |
| US20090286329A1 (en)* | 2008-05-19 | 2009-11-19 | Abbott Laboratoires | Isolated human autoantibodies to natriuretic peptides and methods and kits for detecting human autoantibodies to natriuretic peptides |
| US20090297527A1 (en)* | 2005-09-30 | 2009-12-03 | Muller Bernhard K | Binding Domains of Proteins of the Repulsive Guidance Molecule (RGM) Protein Family and Functional Fragments Thereof, and Their Use |
| WO2009149185A2 (en) | 2008-06-03 | 2009-12-10 | Abbott Laboratories | Dual variable domain immunoglobulins and uses thereof |
| WO2009155324A2 (en) | 2008-06-18 | 2009-12-23 | Abbott Laboratories | Pigf-1 assay and kits and components thereof |
| US20100015655A1 (en)* | 2008-07-17 | 2010-01-21 | Abbott Laboratories | Methods and Kits for Detecting and Quantifying Damage Caused by a Parasite |
| US20100028340A1 (en)* | 2008-02-29 | 2010-02-04 | Abbott Gmbh & Co. Kg | Antibodies against the rgm a protein and uses thereof |
| WO2010028014A1 (en) | 2008-09-03 | 2010-03-11 | Abbott Laboratories | Assays and kits for determining hiv-1 tropism |
| US20100105150A1 (en)* | 2008-10-24 | 2010-04-29 | Abbott Laboratories | Isolated human autoantibodies to neutrophil gelatinase-associated lipocalin (ngal) and methods and kits for the detection of human autoantibodies to ngal |
| US20100136590A1 (en)* | 2007-03-07 | 2010-06-03 | Biomedica Medizinprodukte Gmbh & Co Kg | Method of Determining NT-proBNP |
| US20100136520A1 (en)* | 2007-09-13 | 2010-06-03 | Abbott Laboratories | Detecting hepatitis b virus |
| US20100151490A1 (en)* | 2006-09-01 | 2010-06-17 | Abbott Laboratories | Antigenic Protein Conjugates and Process for Preparing Same |
| WO2010075460A1 (en) | 2008-12-23 | 2010-07-01 | Abbott Laboratories | Alkylated interleukin-18 and related compositions, kits, and methods of making and using same |
| WO2010075475A1 (en) | 2008-12-23 | 2010-07-01 | Abbott Laboratories | SOLUBLE FMS-LIKE TYROSINE KINASE-1 (sFLT-1) ANTIBODY AND RELATED COMPOSITION, KIT, METHODS OF USING, AND MATERIALS AND METHOD FOR MAKING |
| WO2010078443A1 (en) | 2008-12-31 | 2010-07-08 | Abbott Point Of Care Inc. | Method and device for immunoassay using nucleotide conjugates |
| US20100178660A1 (en)* | 2009-01-14 | 2010-07-15 | Abbott Laboratories | Methods and kits for detecting hemoglobin in test samples |
| WO2010088470A1 (en) | 2009-01-31 | 2010-08-05 | Abbott Laboratories | Markers to predict and monitor response to aurora kinase b inhibitor therapy |
| WO2010093742A1 (en) | 2009-02-11 | 2010-08-19 | Abbott Laboratories | Methods and compositions for identifying, classifying and monitoring subject having bcl-2 family inhibitor-resistant tumors and cancers |
| US20100216720A1 (en)* | 2009-02-24 | 2010-08-26 | Abbott Laboratories | Antibodies to troponin i and methods of use thereof |
| WO2010104815A1 (en) | 2009-03-10 | 2010-09-16 | Abbott Laboratories | Antibodies binding to pivka-ii amino acids 13-27 |
| US20100248273A1 (en)* | 2009-03-25 | 2010-09-30 | Abbott Point Of Care Inc. | Amelioration of heterophile antibody immunosensor interference |
| WO2010111220A1 (en) | 2009-03-27 | 2010-09-30 | Abbott Laboratories | Nucleotide and amino acid sequences encoding an exported protein 1 derived from plasmodium vivax and uses thereof |
| US20100266531A1 (en)* | 2009-03-05 | 2010-10-21 | Abbott Laboratories | Il-17 binding proteins |
| US20100267055A1 (en)* | 2007-10-21 | 2010-10-21 | Abbott Laboratories | One-step immunoassays exhibiting increased sensitivity and specificity |
| US20100291709A1 (en)* | 2009-05-13 | 2010-11-18 | Abbott Laboratories | Human nt-pro b-type natriuretic peptide assay having reduced cross-reactivity with other peptide forms |
| US20100322948A1 (en)* | 2007-09-06 | 2010-12-23 | Abbott Gmbh & Co. Kg | Bone morphogenetic protein (BMP)-binding domains of proteins of the repulsive guidance molecule (RGM) protein family and functional fragments thereof, and use of same |
| WO2011002936A2 (en) | 2009-06-30 | 2011-01-06 | Abbott Laboratories | Markers of xmrv infection and uses thereof |
| WO2011026017A1 (en) | 2009-08-31 | 2011-03-03 | Abbott Laboratories | Biomarkers for prediction of major adverse cardiac events and uses thereof |
| US20110076697A1 (en)* | 2009-04-28 | 2011-03-31 | Innovative Laboratory Technologies, Inc. | Lateral-flow immuno-chromatographic assay devices |
| US20110117581A1 (en)* | 2009-11-17 | 2011-05-19 | Abbott Point Of Care Inc. | Reducing leukocyte interference in competitive immunoassays |
| US20110117580A1 (en)* | 2009-11-17 | 2011-05-19 | Abbott Point Of Care Inc. | Reducing leukocyte interference in non-competitive immunoassays |
| WO2011062931A1 (en) | 2009-11-21 | 2011-05-26 | Abbott Laboratories | Assays for human nt-pro b-type natriuretic peptide, human pro b-type natriuretic peptide and human b-type natriuretic peptide |
| US20110129818A1 (en)* | 2009-12-02 | 2011-06-02 | Abbott Laboratories | ASSAY FOR CARDIAC TROPONIN-T (cTnT) |
| US20110129854A1 (en)* | 2009-12-02 | 2011-06-02 | Abbott Laboratories | Assay for diagnosis of cardiac myocyte damage |
| US20110136141A1 (en)* | 2009-12-03 | 2011-06-09 | Abbott Laboratories | Peptide reagents and method for inhibiting autoantibody antigen binding |
| WO2011068863A1 (en) | 2009-12-04 | 2011-06-09 | Abbott Laboratories | Combination therapy for treating cancer and diagnostic assays for use therein |
| US20110136103A1 (en)* | 2009-12-03 | 2011-06-09 | Abbott Laboratories | Autoantibody enhanced immunoassays and kits |
| US20110135664A1 (en)* | 2009-12-08 | 2011-06-09 | Abbott Gmbh & Co. Kg | Monoclonal antibodies against the rgm a protein for use in the treatment of retinal nerve fiber layer degeneration |
| WO2011116023A1 (en) | 2010-03-18 | 2011-09-22 | Abbott Laboratories | METHODS OF ASSAYING URINARY NEUTROPHIL GELATINASE-ASSOCIATED LIPOCALIN (uNGAL) IN THE PROGNOSIS OF CADAVERIC KIDNEY TRANSPLANT FUNCTION IN A PATIENT, INCLUDING A PATIENT DIAGNOSED WITH DELAYED GRAFT FUNCTION (DGF), A METHOD OF ASSAYING uNGAL IN THE ASSESSMENT OF RISK OF DGF IN A PATIENT DIAGNOSED WITH EARLY GRAFT FUNCTION (EGF), AND RELATED KITS |
| US20110256640A1 (en)* | 2008-12-22 | 2011-10-20 | Koninklijke Philips Electronics N.V. | Assay for troponin i using magnetic labels |
| WO2011137165A1 (en) | 2010-04-30 | 2011-11-03 | Abbott Point Of Care Inc. | Reagents for reducing leukocyte interference in immunoassays |
| CN102279269A (en)* | 2011-04-12 | 2011-12-14 | 王贤俊 | Preparation method of cystatin C detection kit |
| WO2011159707A1 (en) | 2010-06-14 | 2011-12-22 | Abbott Point Of Care Inc. | Magnetic beads for reducing leukocyte interference in immunoassays |
| WO2011163558A1 (en) | 2010-06-25 | 2011-12-29 | Abbott Laboratories | Materials and methods for assay of anti-hepatitis c virus (hcv) antibodies |
| WO2012006500A2 (en) | 2010-07-08 | 2012-01-12 | Abbott Laboratories | Monoclonal antibodies against hepatitis c virus core protein |
| WO2012018476A1 (en) | 2010-07-26 | 2012-02-09 | Abbott Laboratories | Antibodies relating to pivka-ii and uses thereof |
| WO2012024513A2 (en) | 2010-08-18 | 2012-02-23 | Abbott Laboratories | Molecular detection of xmrv infection |
| WO2012024518A1 (en) | 2010-08-18 | 2012-02-23 | Abbott Laboratories | Molecular detection of xmrv infection |
| WO2012064674A1 (en) | 2010-11-09 | 2012-05-18 | Abbott Laboratories | Materials and methods for immunoassay of pterins |
| CN102539784A (en)* | 2011-12-26 | 2012-07-04 | 宁波美康生物科技股份有限公司 | Method for detecting cardiac troponin I and application thereof |
| WO2012121775A2 (en) | 2010-12-21 | 2012-09-13 | Abbott Laboratories | Dual variable domain immunoglobulins and uses thereof |
| CN102749460A (en)* | 2012-07-27 | 2012-10-24 | 北京恩济和生物科技有限公司 | Troponin detection kit and preparing method thereof |
| WO2012166198A1 (en) | 2011-05-27 | 2012-12-06 | Abbott Point Of Care Inc. | Tsh antibodies for point-of-care immunoassay formats |
| WO2012166199A1 (en) | 2011-05-27 | 2012-12-06 | Abbott Point Of Care Inc. | Tsh immunoassays and processes for performing tsh immunoassays in the presence of endogenous contaminants in restricted wash formats |
| WO2012166200A1 (en) | 2011-05-27 | 2012-12-06 | Abbott Point Of Care Inc. | Tsh immunoassays employing scavenging reagents for cross-reacting endocrine glycoprotein hormone analogues |
| WO2013063110A1 (en) | 2011-10-24 | 2013-05-02 | Abbvie Inc. | Bispecific immunobinders directed against tnf and il-17 |
| WO2013063095A1 (en) | 2011-10-24 | 2013-05-02 | Abbvie Inc. | Immunobinders directed against sclerostin |
| WO2013090633A2 (en) | 2011-12-14 | 2013-06-20 | AbbVie Deutschland GmbH & Co. KG | Composition and method for the diagnosis and treatment of iron-related disorders |
| WO2013090635A2 (en) | 2011-12-14 | 2013-06-20 | AbbVie Deutschland GmbH & Co. KG | Composition and method for the diagnosis and treatment of iron-related disorders |
| WO2013102149A1 (en) | 2011-12-31 | 2013-07-04 | Abbott Laboratories | Truncated human vitamin d binding protein and mutation and fusion thereof and related materials and methods of use |
| WO2013102042A2 (en) | 2011-12-30 | 2013-07-04 | Abbvie Inc. | Dual specific binding proteins directed against il-13 and/or il-17 |
| US8481333B2 (en) | 2011-05-31 | 2013-07-09 | Idexx Laboratories, Inc. | Detection of degradation products of feline NT-proBNP |
| CN103217535A (en)* | 2013-03-18 | 2013-07-24 | 杭州德安奇生物工程有限公司 | Immunochromatography detection card for detecting troponin I |
| US8664367B2 (en) | 2010-05-14 | 2014-03-04 | Abbvie, Inc. | IL-I binding proteins |
| US8680239B2 (en) | 2000-12-22 | 2014-03-25 | Max-Planck-Gesellschaft Zur Foerderung Der Wissenschaften E.V. | Use of RGM and its modulators |
| WO2014071074A2 (en) | 2012-11-01 | 2014-05-08 | Abbvie Inc. | Anti-vegf/dll4 dual variable domain immunoglobulins and uses thereof |
| US20140141484A1 (en)* | 2002-07-29 | 2014-05-22 | Abbott Point Of Care Inc. | Multiple Hybrid Immunoassay |
| WO2014089209A2 (en) | 2012-12-04 | 2014-06-12 | Abbvie, Inc. | Blood-brain barrier (bbb) penetrating dual specific binding proteins |
| WO2014106001A2 (en) | 2012-12-28 | 2014-07-03 | Abbvie, Inc. | Dual specific binding proteins having a receptor sequence |
| CN103913580A (en)* | 2014-04-23 | 2014-07-09 | 齐鲁工业大学 | Nanotechnology for myocardial infarction diagnosis and device adopting same |
| US8778699B2 (en) | 2010-08-04 | 2014-07-15 | Idexx Laboratories, Inc. | Detection of degradation products of canine NT-proBNP |
| CN103940986A (en)* | 2014-03-24 | 2014-07-23 | 安徽省煦棠医疗科技有限公司 | Preparation of troponin I specific locus antibody and detection kit thereof |
| WO2014116846A2 (en) | 2013-01-23 | 2014-07-31 | Abbvie, Inc. | Methods and compositions for modulating an immune response |
| US20140257833A1 (en)* | 2013-03-08 | 2014-09-11 | Adventive Ipbank | Cloud Based System For Remote Medical Checkup And Physician Managed Biometric Data |
| WO2014144355A2 (en) | 2013-03-15 | 2014-09-18 | Abbott Laboratories | Anti-gp73 monoclonal antibodies and methods of obtaining the same |
| WO2014144299A2 (en) | 2013-03-15 | 2014-09-18 | Abbvie Inc. | DUAL SPECIFIC BINDING PROTEINS DIRECTED AGAINST TNFα |
| WO2014152991A1 (en) | 2013-03-14 | 2014-09-25 | Abbott Laboratories | Methods for the early detection of lung cancer |
| US20140304796A1 (en)* | 2006-04-28 | 2014-10-09 | Microsoft Corporation | Providing guest users network access based on information read from a credit card or other object |
| WO2015031626A1 (en) | 2013-08-28 | 2015-03-05 | Abbvie Inc. | Soluble cmet assay |
| US8987418B2 (en) | 2013-03-15 | 2015-03-24 | Abbvie Inc. | Dual specific binding proteins directed against IL-1β and/or IL-17 |
| WO2015051379A2 (en) | 2013-10-06 | 2015-04-09 | Abbvie Inc. | Dual specific binding proteins directed against immune cell receptors and autoantigens |
| US9005901B2 (en) | 2013-03-15 | 2015-04-14 | Abbott Laboratories | Assay with internal calibration |
| US9035027B2 (en) | 2008-06-03 | 2015-05-19 | Abbvie Inc. | Dual variable domain immunoglobulins and uses thereof |
| US9046513B2 (en) | 2010-08-26 | 2015-06-02 | Abbvie Inc. | Dual variable domain immunoglobulins and uses thereof |
| WO2015103072A1 (en) | 2013-12-30 | 2015-07-09 | Epimab Biotherapeutics | Fabs-in-tandem immunoglobulin and uses thereof |
| EP2899209A1 (en) | 2008-04-29 | 2015-07-29 | Abbvie Inc. | Dual Variable Domain Immunoglobulins and uses thereof |
| US9102722B2 (en) | 2012-01-27 | 2015-08-11 | AbbVie Deutschland GmbH & Co. KG | Composition and method for the diagnosis and treatment of diseases associated with neurite degeneration |
| US9115195B2 (en) | 2010-03-02 | 2015-08-25 | Abbvie Inc. | Therapeutic DLL4 binding proteins |
| EP2910949A1 (en) | 2006-09-01 | 2015-08-26 | Abbott Laboratories | Combination hepatitis C virus antigen and antibody detection method |
| EP2915818A2 (en) | 2011-12-30 | 2015-09-09 | AbbVie Inc. | Dual variable domain immunoglobulins and uses thereof |
| US9132190B2 (en) | 2009-08-29 | 2015-09-15 | Abbvie Inc. | Therapeutic DLL4 binding proteins |
| EP2921177A2 (en) | 2010-07-09 | 2015-09-23 | AbbVie Inc. | Dual variable domain immunoglobulins and uses thereof |
| US9157910B2 (en) | 2013-03-15 | 2015-10-13 | Abbott Laboratories | Assay with increased dynamic range |
| US9194873B2 (en) | 2013-03-14 | 2015-11-24 | Abbott Laboratories | HCV antigen-antibody combination assay and methods and compositions for use therein |
| CN105137066A (en)* | 2015-07-31 | 2015-12-09 | 江苏巨珩新材料科技有限公司 | Polystyrene latex based cancer marker immuno-chromatography kit and application method thereof |
| WO2015191934A2 (en) | 2014-06-11 | 2015-12-17 | Abbvie Inc. | Blood-brain barrier (bbb) penetrating dual specific binding proteins for treating brain and neurological diseases |
| WO2016069762A2 (en) | 2014-10-29 | 2016-05-06 | Abbott Laboratories | Subject anti-hcv antibody detection assays employing ns3 capture peptides |
| US9371374B2 (en) | 2013-03-14 | 2016-06-21 | Abbott Laboratories | HCV core lipid binding domain monoclonal antibodies |
| US20160202250A1 (en)* | 2015-01-09 | 2016-07-14 | Council Of Scientific & Industrial Research | Chemiresistive Biosensor for the Quantitative Detection of Human Cardiac Biomarker and a Process Thereof |
| WO2016160976A2 (en) | 2015-03-30 | 2016-10-06 | Abbvie Inc. | Monovalent tnf binding proteins |
| US9488663B2 (en) | 2013-03-14 | 2016-11-08 | Abbott Point Of Care Inc. | Electrochemical methods and devices for amending urine samples for immunosensor detection |
| US9493560B2 (en) | 2010-08-03 | 2016-11-15 | Abbvie Inc. | Dual variable domain immunoglobulins and uses thereof |
| WO2016205427A2 (en) | 2015-06-15 | 2016-12-22 | Abbvie Inc. | Binding proteins against vegf, pdgf, and/or their receptors |
| US9528998B2 (en) | 2010-04-16 | 2016-12-27 | Abbott Laboratories | Methods and reagents for diagnosing rheumatoid arthrtis |
| US9651547B2 (en) | 2013-03-14 | 2017-05-16 | Abbott Point Of Care Inc. | Electrochemical methods and devices for amending urine samples for immunosensor detection |
| US9670276B2 (en) | 2012-07-12 | 2017-06-06 | Abbvie Inc. | IL-1 binding proteins |
| WO2017136820A2 (en) | 2016-02-06 | 2017-08-10 | Epimab Biotherapeutics, Inc. | Fabs-in-tandem immunoglobulin and uses thereof |
| US9777340B2 (en) | 2014-06-27 | 2017-10-03 | Abbott Laboratories | Compositions and methods for detecting human Pegivirus 2 (HPgV-2) |
| US9790478B2 (en) | 2013-03-14 | 2017-10-17 | Abbott Laboratories | HCV NS3 recombinant antigens and mutants thereof for improved antibody detection |
| WO2017218911A1 (en) | 2016-06-17 | 2017-12-21 | Abbott Laboratories | BIOMARKERS TO PREDICT NEW ONSET HEART FAILURE WITH PRESERVED EJECTION FRACTION (HFpEF) |
| US9856319B2 (en) | 2012-12-28 | 2018-01-02 | Abbvie Inc. | Monovalent binding proteins |
| EP3279667A1 (en) | 2016-08-02 | 2018-02-07 | Abbott Laboratories | Cardiac troponin i and copeptin as biomarkers for short-term mortality in acute exacerbation of chronic obstructive pulmonary disease (aecopd) |
| WO2018067474A1 (en) | 2016-10-03 | 2018-04-12 | Abbott Laboratories | Improved methods of assessing gfap status in patient samples |
| US10030268B2 (en) | 2014-11-11 | 2018-07-24 | Abbott Molecular Inc. | Hybridization probes and methods |
| CN108535491A (en)* | 2018-03-22 | 2018-09-14 | 北京九强生物技术股份有限公司 | A kind of latex enhancing immune of Troponin I is than turbid detection kit |
| WO2018175942A1 (en) | 2017-03-23 | 2018-09-27 | Abbott Laboratories | Methods for aiding in the diagnosis and determination of the extent of traumatic brain injury in a human subject using the early biomarker ubiquitin carboxy-terminal hydrolase l1 |
| US10093733B2 (en) | 2014-12-11 | 2018-10-09 | Abbvie Inc. | LRP-8 binding dual variable domain immunoglobulin proteins |
| WO2018191531A1 (en) | 2017-04-15 | 2018-10-18 | Abbott Laboratories | Methods for aiding in the hyperacute diagnosis and determination of traumatic brain injury in a human subject using early biomarkers |
| US10107826B2 (en) | 2011-05-20 | 2018-10-23 | Abbott Japan Co. Ltd. | Immunoassay methods and reagents for decreasing nonspecific binding |
| WO2018200823A1 (en) | 2017-04-28 | 2018-11-01 | Abbott Laboratories | Methods for aiding in the hyperacute diagnosis and determination of traumatic brain injury using early biomarkers on at least two samples from the same human subject |
| WO2018218169A1 (en) | 2017-05-25 | 2018-11-29 | Abbott Laboratories | Methods for aiding in the determination of whether to perform imaging on a human subject who has sustained or may have sustained an injury to the head using early biomarkers |
| CN108918883A (en)* | 2018-05-18 | 2018-11-30 | 瑞莱生物科技江苏有限公司 | A kind of immunofluorescent reagent box of rapid quantitative detection cardiac muscle troponin I, creatine kinase isozyme and myoglobins |
| WO2018222784A1 (en) | 2017-05-30 | 2018-12-06 | Abbott Laboratories | Methods for aiding in diagnosing and evaluating a mild traumatic brain injury in a human subject using cardiac troponin i |
| WO2019010131A1 (en) | 2017-07-03 | 2019-01-10 | Abbott Laboratories | Improved methods for measuring ubiquitin carboxy-terminal hydrolase l1 levels in blood |
| WO2019113525A2 (en) | 2017-12-09 | 2019-06-13 | Abbott Laboratories | Methods for aiding in the diagnosis and evaluation of a subject who has sustained an orthopedic injury and that has or may have sustained an injury to the head, such as mild traumatic brain injury (tbi), using glial fibrillary acidic protein (gfap) and/or ubiquitin carboxy-terminal hydrolase l1 (uch-l1) |
| WO2019112860A1 (en) | 2017-12-09 | 2019-06-13 | Abbott Laboratories | Methods for aiding in diagnosing and evaluating a traumatic brain injury in a human subject using a combination of gfap and uch-l1 |
| WO2019133717A1 (en) | 2017-12-29 | 2019-07-04 | Abbott Laboratories | Novel biomarkers and methods for diagnosing and evaluating traumatic brain injury |
| WO2019211665A1 (en) | 2018-04-30 | 2019-11-07 | Takeda Pharmaceutical Company Limited | Cannabinoid receptor type 1 (cb1) binding proteins and uses thereof |
| WO2019213619A1 (en) | 2018-05-04 | 2019-11-07 | Abbott Laboratories | Hbv diagnostic, prognostic, and therapeutic methods and products |
| EP3657174A1 (en) | 2015-09-28 | 2020-05-27 | Abbott Japan Co., Ltd. | Use of laminin 2 for diagnosing hepatocellular carcinoma and pancreatic cancer |
| US10670611B2 (en) | 2014-09-26 | 2020-06-02 | Somalogic, Inc. | Cardiovascular risk event prediction and uses thereof |
| CN111308085A (en)* | 2019-12-30 | 2020-06-19 | 菲鹏生物股份有限公司 | A kind of high-sensitivity cardiac troponin I detection method and kit |
| CN111308084A (en)* | 2019-12-30 | 2020-06-19 | 菲鹏生物股份有限公司 | A kind of high-sensitivity cardiac troponin I detection method and kit |
| US10720255B2 (en) | 2016-07-06 | 2020-07-21 | Clemson University | Radiation delivery devices and applications thereof |
| WO2020180695A1 (en) | 2019-03-01 | 2020-09-10 | Abbott Laboratories | Methods for predicting major adverse cardiovascular events in subjects with coronary artery disease |
| CN111781385A (en)* | 2020-08-19 | 2020-10-16 | 武汉生之源生物科技股份有限公司 | NT-proBNP detection kit and preparation method thereof |
| US10871485B2 (en) | 2018-04-13 | 2020-12-22 | Rarecyte, Inc. | Kits for labeling of biomarkers and methods of using the same |
| WO2021026403A1 (en) | 2019-08-07 | 2021-02-11 | Abbott Laboratories | Methods for detecting assay interferents and increasing dynamic range |
| WO2021061272A1 (en) | 2019-09-26 | 2021-04-01 | Pacesetter, Inc. | Method and device for managing energy usage by a medical device |
| CN112710855A (en)* | 2020-12-15 | 2021-04-27 | 深圳天辰医疗科技有限公司 | BNP detection kit and BNP detection method |
| WO2021211331A1 (en) | 2020-04-13 | 2021-10-21 | Abbott Point Of Care Inc. | METHODS, COMPLEXES AND KITS FOR DETECTING OR DETERMINING AN AMOUNT OF A ß-CORONAVIRUS ANTIBODY IN A SAMPLE |
| EP3906843A1 (en) | 2020-05-08 | 2021-11-10 | Pacesetter, Inc. | System for verifying a pathologic episode using an accelerometer |
| WO2022031804A1 (en) | 2020-08-04 | 2022-02-10 | Abbott Laboratories | Improved methods and kits for detecting sars-cov-2 protein in a sample |
| WO2022029494A1 (en) | 2020-08-04 | 2022-02-10 | Abbott Rapid Diagnostics International Unlimited Company | Assays for detecting sars-cov-2 |
| EP3991787A1 (en) | 2020-10-30 | 2022-05-04 | Pacesetter, Inc. | Implantable medical device for managing a physical layer utilized during a wireless connection |
| CN114544978A (en)* | 2022-03-04 | 2022-05-27 | 美康生物科技股份有限公司 | IGF-1 detection kit, preparation method and detection method thereof |
| WO2022119841A1 (en) | 2020-12-01 | 2022-06-09 | Abbott Laboratories | Use of one or more biomarkers to determine traumatic brain injury (tbi) in a subject having received a head computerized tomography scan that is negative for a tbi |
| US20220187330A1 (en)* | 2019-04-16 | 2022-06-16 | Aligned Genetics, Inc. | Analyte collecting device, and analyte collecting method and analyte inspection system using same |
| WO2022147147A1 (en) | 2020-12-30 | 2022-07-07 | Abbott Laboratories | Methods for determining sars-cov-2 antigen and anti-sars-cov-2 antibody in a sample |
| WO2022147178A1 (en) | 2020-12-30 | 2022-07-07 | Abbott Laboratories | Improved methods, reagents and kits for detergent-based inactivation of betacoronavirus prior to and/or while assessing a biological sample for sars-cov-2 antigen or antibody |
| US11493507B2 (en) | 2009-09-14 | 2022-11-08 | Siemens Healthineers Nederland B.V. | Highly sensitive immunoassay with large particle labels |
| WO2022245920A1 (en) | 2021-05-18 | 2022-11-24 | Abbott Laboratories | Methods of evaluating brain injury in a pediatric subject |
| WO2022266034A1 (en) | 2021-06-14 | 2022-12-22 | Abbott Laboratories | Methods of diagnosing or aiding in diagnosis of brain injury caused by acoustic energy, electromagnetic energy, an over pressurization wave, and/or blast wind |
| EP4111948A1 (en) | 2021-06-28 | 2023-01-04 | Pacesetter, Inc. | Method and system for optimizing filter settings of an implantable medical device |
| WO2023028186A1 (en) | 2021-08-27 | 2023-03-02 | Abbott Laboratories | Methods for detecting immunoglobulin g, subclass 4 (igg4) in a biological sample |
| WO2023034777A1 (en) | 2021-08-31 | 2023-03-09 | Abbott Laboratories | Methods and systems of diagnosing brain injury |
| WO2023056268A1 (en) | 2021-09-30 | 2023-04-06 | Abbott Laboratories | Methods and systems of diagnosing brain injury |
| WO2023102384A1 (en) | 2021-11-30 | 2023-06-08 | Abbott Laboratories | Use of one or more biomarkers to determine traumatic brain injury (tbi) in a subject having received a head computerized tomography scan that is negative for a tbi |
| WO2023129942A1 (en) | 2021-12-28 | 2023-07-06 | Abbott Laboratories | Use of biomarkers to determine sub-acute traumatic brain injury (tbi) in a subject having received a head computerized tomography (ct) scan that is negative for a tbi or no head ct scan |
| EP4268711A1 (en) | 2022-04-28 | 2023-11-01 | Pacesetter, Inc. | System for determining change in position of an implanted medical device within an implant pocket |
| WO2024044288A1 (en) | 2022-08-26 | 2024-02-29 | Abbott Laboratories | Use of cardiac troponin and galectin-3 to differentiate myocardial infarction type i and type ii |
| WO2024059708A1 (en) | 2022-09-15 | 2024-03-21 | Abbott Laboratories | Biomarkers and methods for differentiating between mild and supermild traumatic brain injury |
| WO2024059692A1 (en) | 2022-09-15 | 2024-03-21 | Abbott Laboratories | Hbv diagnostic, prognostic, and therapeutic methods and products |
| WO2024102383A1 (en) | 2022-11-10 | 2024-05-16 | Abbott Laboratories | Methods of identifying macrotroponin in biological samples |
| US12060425B2 (en) | 2018-05-03 | 2024-08-13 | Shanghai Epimab Biotherapeutics Co., Ltd. | High affinity antibodies to PD-1 and LAG-3 and bispecific binding proteins made therefrom |
| WO2024211475A1 (en) | 2023-04-04 | 2024-10-10 | Abbott Laboratories | Use of biomarkers to determine whether a subject has sustained, may have sustained or is suspected of sustaining a subacute acquired brain injury (abi) |
| WO2024226899A1 (en) | 2023-04-28 | 2024-10-31 | Abbott Laboratories | Diagnosis of late-stage hepatocellular carcinoma |
| WO2024226969A1 (en) | 2023-04-28 | 2024-10-31 | Abbott Point Of Care Inc. | Improved assays, cartridges, and kits for detection of biomarkers, including brain injury biomarkers |
| US12192285B2 (en) | 2020-10-30 | 2025-01-07 | Abbott Diabetes Care Inc. | Method for managing a physical layer utilized during a wireless connection with medical devices |
| WO2025085348A1 (en) | 2023-10-16 | 2025-04-24 | Abbott Laboratories | Methods and systems for diagnosing orthopoxvirus infection |
| WO2025136783A1 (en) | 2023-12-21 | 2025-06-26 | Tc1 Llc | Methods and systems for displaying healthcare metrics associated with patient populations |
| US12365729B2 (en) | 2020-05-13 | 2025-07-22 | Disc Medicine, Inc. | Anti-hemojuvelin (HJV) antibodies for treating myelofibrosis |
| WO2025193825A1 (en) | 2024-03-13 | 2025-09-18 | Abbott Laboratories | Diagnosis and monitoring of liver disease |
Families Citing this family (23)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP6080163B2 (en) | 2013-10-02 | 2017-02-15 | 古河電気工業株式会社 | Target substance detection method |
| WO2008105814A2 (en)* | 2006-08-22 | 2008-09-04 | Los Alamos National Security, Llc | Miniturized lateral flow device for rapid and sensitive detection of proteins or nucleic acids |
| WO2008140581A2 (en)* | 2006-11-22 | 2008-11-20 | 3M Innovative Properties Company | Systems and methods for preparing and analyzing samples |
| US11730407B2 (en) | 2008-03-28 | 2023-08-22 | Dexcom, Inc. | Polymer membranes for continuous analyte sensors |
| US8682408B2 (en) | 2008-03-28 | 2014-03-25 | Dexcom, Inc. | Polymer membranes for continuous analyte sensors |
| US8583204B2 (en) | 2008-03-28 | 2013-11-12 | Dexcom, Inc. | Polymer membranes for continuous analyte sensors |
| EP2279403B1 (en) | 2008-05-05 | 2016-03-16 | Los Alamos National Security, LLC | Highly simplified lateral flow-based nucleic acid sample preparation and passive fluid flow control |
| JP2010122205A (en)* | 2008-08-29 | 2010-06-03 | Sysmex Corp | Method for detecting measles virus, membrane assay test device, and membrane assay test kit |
| EP2504466B1 (en) | 2009-11-23 | 2019-11-20 | Proxim Diagnostics Corporation | Controlled electrochemical activation of carbon-based electrodes |
| CN102103143B (en)* | 2011-02-24 | 2013-09-25 | 南京基蛋生物科技有限公司 | Colloidal gold test strip for double-index detection and preparation method thereof |
| JP5731066B2 (en) | 2011-04-20 | 2015-06-10 | メサ テック インターナショナル,インク. | Cycle-variable amplification reactions for nucleic acids |
| EP2737315B1 (en) | 2011-07-25 | 2022-03-16 | Proxim Diagnostics Corporation | Cartridge for diagnostic testing |
| CN103267855A (en)* | 2013-05-06 | 2013-08-28 | 南京凯基生物科技发展有限公司 | CTnI, Myo and CK-MB three-in-one colloidal gold test strip, kit thereof, and making methods of test strip and kit |
| JP6080164B2 (en) | 2013-10-02 | 2017-02-15 | 古河電気工業株式会社 | Fluorescently labeled particles |
| EP4282532A3 (en) | 2015-04-24 | 2024-02-28 | Mesa Biotech, Inc. | Fluidic test cassette |
| CN105842440B (en)* | 2016-04-12 | 2017-08-04 | 江苏晶红生物医药科技股份有限公司 | People's C reactive protein fluorogenic quantitative detection test cards |
| CN105954523A (en)* | 2016-07-14 | 2016-09-21 | 宏葵生物(中国)有限公司 | Cardiac troponin I detection kit |
| EP3655160A4 (en) | 2017-07-19 | 2021-04-07 | Evanostics, LLC | ORAL LIQUID ANALYSIS CARTRIDGES AND METHOD OF USE |
| CA3086538A1 (en) | 2017-12-15 | 2019-06-20 | Evanostics Llc | Optical reader for analyte testing |
| JP7434144B2 (en)* | 2018-02-27 | 2024-02-20 | 株式会社島津製作所 | Antibody that specifically recognizes the N-terminus of APP669-x and immunoassay |
| WO2020132146A1 (en) | 2018-12-18 | 2020-06-25 | Evanostics Llc | Optical analyte detection |
| US11567068B2 (en)* | 2019-03-28 | 2023-01-31 | Autonomous Medical Devices Inc. | Detection of cardiac troponin or biological markers via shear horizontal surface acoustic wave biosensor using a wet-dry bioanalytical technique |
| CN118076895A (en)* | 2021-10-08 | 2024-05-24 | 万迈医疗仪器有限公司 | Method for testing solid surface repeatedly in biochemical determination |
Citations (43)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US3720960A (en)* | 1971-05-21 | 1973-03-20 | J Bond | Spring attachment clip |
| US3857931A (en)* | 1971-02-01 | 1974-12-31 | Hoffmann La Roche | Latex polymer reagents for diagnostic tests |
| US4210418A (en)* | 1976-08-30 | 1980-07-01 | Mallinckrodt, Inc. | Container for immunochemical and enzymatical determinations or procedures |
| US4353982A (en)* | 1980-04-10 | 1982-10-12 | Hoffmann-La Roche Inc. | Immunochemical assay for creatine kinase-MB isoenzyme |
| US4411868A (en)* | 1981-12-11 | 1983-10-25 | Becton, Dickinson And Company | Multiple tube rack |
| US4433059A (en)* | 1981-09-08 | 1984-02-21 | Ortho Diagnostic Systems Inc. | Double antibody conjugate |
| US4514505A (en)* | 1982-05-21 | 1985-04-30 | The Trustees Of Columbia University In The City Of New York | Monoclonal antibody mixtures and use thereof for enhanced sensitivity immunoassays |
| US4595661A (en)* | 1983-11-18 | 1986-06-17 | Beckman Instruments, Inc. | Immunoassays and kits for use therein which include low affinity antibodies for reducing the hook effect |
| US4624930A (en)* | 1982-07-05 | 1986-11-25 | Boehringer Mannheim Gmbh | Immunochemical process |
| US4657853A (en)* | 1984-09-14 | 1987-04-14 | E. I. Du Pont De Nemours And Company | Immunoassays utilizing covalent conjugates of polymerized enzyme and antibody |
| US4737456A (en)* | 1985-05-09 | 1988-04-12 | Syntex (U.S.A.) Inc. | Reducing interference in ligand-receptor binding assays |
| US4804626A (en)* | 1986-10-22 | 1989-02-14 | The General Hospital Corporation | Immunometric assay for the detection of human chorionic gonadotropin |
| US4808521A (en)* | 1985-01-23 | 1989-02-28 | Serone Diagnostics Partners | Double-labeled enzymeimmunoassay methods |
| US4880731A (en)* | 1986-03-19 | 1989-11-14 | Boehringer Mannheim Gmbh | Process and reagent for the determination of a reaction partner of an immunological reaction |
| US4885255A (en)* | 1987-04-28 | 1989-12-05 | Boehringer Mannheim Gmbh | Immunochemical process and reagent for the determination of a polyvalent antigen in a liquid sample |
| US5011771A (en)* | 1984-04-12 | 1991-04-30 | The General Hospital Corporation | Multiepitopic immunometric assay |
| US5026653A (en)* | 1985-04-02 | 1991-06-25 | Leeco Diagnostic, Inc. | Scavenger antibody mixture and its use for immunometric assay |
| US5096669A (en)* | 1988-09-15 | 1992-03-17 | I-Stat Corporation | Disposable sensing device for real time fluid analysis |
| US5108896A (en)* | 1986-03-21 | 1992-04-28 | Applied Research Systems Ars Holding N.V. | Simultaneous immunoassay of two analytes using dual enzyme labelled antibodies |
| US5108933A (en)* | 1988-09-16 | 1992-04-28 | Immunicon Corporation | Manipulation of colloids for facilitating magnetic separations |
| US5120660A (en)* | 1989-12-05 | 1992-06-09 | International Canine Genetics, Inc. | Method for canine fertility detection |
| US5187067A (en)* | 1986-12-15 | 1993-02-16 | Teijin Limited | Immunological determination of free human protein S and C4bp-protein S complex |
| US5202234A (en)* | 1987-07-21 | 1993-04-13 | Internationl Immunoassay Laboratories, Inc. | Myocardial infarction immunoassay |
| US5324650A (en)* | 1990-03-20 | 1994-06-28 | E. I. Du Pont De Nemours And Company | Situ process for production of conjugates |
| US5416026A (en)* | 1993-10-04 | 1995-05-16 | I-Stat Corporation | Method for detecting the change in an analyte due to hemolysis in a fluid sample |
| US5447440A (en)* | 1993-10-28 | 1995-09-05 | I-Stat Corporation | Apparatus for assaying viscosity changes in fluid samples and method of conducting same |
| US5468846A (en)* | 1993-06-30 | 1995-11-21 | Kirin-Amgen Inc. | Monoclonal antibodies and method for the determination of human G-CSF |
| US5514253A (en)* | 1994-07-13 | 1996-05-07 | I-Stat Corporation | Method of measuring gas concentrations and microfabricated sensing device for practicing same |
| US5554339A (en)* | 1988-11-14 | 1996-09-10 | I-Stat Corporation | Process for the manufacture of wholly microfabricated biosensors |
| US5599677A (en)* | 1993-12-29 | 1997-02-04 | Abbott Laboratories | Immunoassays for prostate specific antigen |
| US5605664A (en)* | 1994-07-13 | 1997-02-25 | I-Stat Corporation | Methods and apparatus for rapid equilibration of dissolved gas composition |
| US5661040A (en)* | 1993-07-13 | 1997-08-26 | Abbott Laboratories | Fluorescent polymer labeled conjugates and intermediates |
| US5795725A (en)* | 1995-04-18 | 1998-08-18 | Biosite Diagnostics Incorporated | Methods for the assay of troponin I and T and selection of antibodies for use in immunoassays |
| US5821061A (en)* | 1995-01-12 | 1998-10-13 | Toyo Boseki Kabushiki Kaisha | Method and reagent for detecting a ligand in a sample |
| US5834220A (en)* | 1993-05-17 | 1998-11-10 | Fortron Bioscience, Inc. | Assay for cardiac troponin I |
| US5869348A (en)* | 1996-05-15 | 1999-02-09 | Medinox, Inc. | Methods for the preparation of antibodies directed against dithiocarbamates and the use thereof for detection of nitric oxide in body fluids |
| US5871905A (en)* | 1996-09-04 | 1999-02-16 | Epitope, Inc. | Reduction of false positives in oral-fluid based immunoassays |
| US5947124A (en)* | 1997-03-11 | 1999-09-07 | Biosite Diagnostics Incorporated | Diagnostic for determining the time of a heart attack |
| US6030827A (en)* | 1998-01-23 | 2000-02-29 | I-Stat Corporation | Microfabricated aperture-based sensor |
| US6114180A (en)* | 1995-07-06 | 2000-09-05 | Bayer Aktiengesellschaft | Synthetic calibrators for use in immunoassays, comprising the analytes or partial sequences thereof which are conjugated to inert carrier molecules |
| US6140474A (en)* | 1995-05-02 | 2000-10-31 | Japan Tobacco, Inc. | Monoclonal antibody reactive with human-origin CETP and method of quantifying human-origin CETP |
| US6180340B1 (en)* | 1997-10-31 | 2001-01-30 | Gen-Probe Incorporated | Extended dynamic range assays |
| US7419821B2 (en)* | 2002-03-05 | 2008-09-02 | I-Stat Corporation | Apparatus and methods for analyte measurement and immunoassay |
Family Cites Families (12)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US687851A (en) | 1897-11-05 | 1901-12-03 | Frederick C Austin | Impact-tool. |
| US3720760A (en) | 1968-09-06 | 1973-03-13 | Pharmacia Ab | Method for determining the presence of reagin-immunoglobulins(reagin-ig)directed against certain allergens,in aqueous samples |
| DK546988A (en)* | 1987-10-02 | 1989-04-03 | Du Pont | IMMUNE DETERMINATION AND THE REAGENT SITE USED HEREIN |
| SE504798C2 (en)* | 1995-06-16 | 1997-04-28 | Ulf Landegren | Immunoassay and test kits with two reagents that can be cross-linked if adsorbed to the analyte |
| AU1274097A (en)* | 1995-11-29 | 1997-06-19 | Dade International Inc. | Human heart cnbr troponin i isoform and use of same |
| US6942771B1 (en)* | 1999-04-21 | 2005-09-13 | Clinical Micro Sensors, Inc. | Microfluidic systems in the electrochemical detection of target analytes |
| DE60034479T2 (en) | 1999-11-15 | 2007-12-27 | I-Stat Corporation | DEVICE AND METHOD FOR DETERMINING COAGULATION IN LIQUID SAMPLES |
| US6438498B1 (en) | 2000-02-10 | 2002-08-20 | I-Stat Corporation | System, method and computer implemented process for assaying coagulation in fluid samples |
| DE10064827A1 (en)* | 2000-12-22 | 2002-06-27 | Dade Behring Marburg Gmbh | Sandwich assay for detecting analyte, useful e.g. for hormones, with detection or correction of the hook effect by measuring detection signals twice |
| US20040018577A1 (en)* | 2002-07-29 | 2004-01-29 | Emerson Campbell John Lewis | Multiple hybrid immunoassay |
| FI20030652A0 (en) | 2003-04-30 | 2003-04-30 | Susann Eriksson | Improved immune determination |
| AU2009334505B2 (en)* | 2008-12-31 | 2013-05-09 | Abbott Point Of Care Inc. | Method and device for immunoassay using nucleotide conjugates |
- 2002
- 2002-07-29USUS10/208,560patent/US20040018577A1/ennot_activeAbandoned
- 2003
- 2003-07-29WOPCT/US2003/023483patent/WO2004011947A1/enactiveApplication Filing
- 2003-07-29EPEP03771940.8Apatent/EP1525480B1/ennot_activeExpired - Lifetime
- 2003-07-29JPJP2004524914Apatent/JP4507879B2/ennot_activeExpired - Fee Related
- 2013
- 2013-11-15USUS14/081,309patent/US9267939B2/ennot_activeExpired - Fee Related
- 2016
- 2016-01-13USUS14/994,713patent/US9995744B2/ennot_activeExpired - Lifetime
- 2018
- 2018-05-09USUS15/975,145patent/US10641767B2/ennot_activeExpired - Fee Related
Patent Citations (50)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US3857931A (en)* | 1971-02-01 | 1974-12-31 | Hoffmann La Roche | Latex polymer reagents for diagnostic tests |
| US3720960A (en)* | 1971-05-21 | 1973-03-20 | J Bond | Spring attachment clip |
| US4210418A (en)* | 1976-08-30 | 1980-07-01 | Mallinckrodt, Inc. | Container for immunochemical and enzymatical determinations or procedures |
| US4353982A (en)* | 1980-04-10 | 1982-10-12 | Hoffmann-La Roche Inc. | Immunochemical assay for creatine kinase-MB isoenzyme |
| US4433059A (en)* | 1981-09-08 | 1984-02-21 | Ortho Diagnostic Systems Inc. | Double antibody conjugate |
| US4411868A (en)* | 1981-12-11 | 1983-10-25 | Becton, Dickinson And Company | Multiple tube rack |
| US4514505A (en)* | 1982-05-21 | 1985-04-30 | The Trustees Of Columbia University In The City Of New York | Monoclonal antibody mixtures and use thereof for enhanced sensitivity immunoassays |
| US4624930A (en)* | 1982-07-05 | 1986-11-25 | Boehringer Mannheim Gmbh | Immunochemical process |
| US4595661A (en)* | 1983-11-18 | 1986-06-17 | Beckman Instruments, Inc. | Immunoassays and kits for use therein which include low affinity antibodies for reducing the hook effect |
| US5011771A (en)* | 1984-04-12 | 1991-04-30 | The General Hospital Corporation | Multiepitopic immunometric assay |
| US4657853A (en)* | 1984-09-14 | 1987-04-14 | E. I. Du Pont De Nemours And Company | Immunoassays utilizing covalent conjugates of polymerized enzyme and antibody |
| US4808521A (en)* | 1985-01-23 | 1989-02-28 | Serone Diagnostics Partners | Double-labeled enzymeimmunoassay methods |
| US5026653A (en)* | 1985-04-02 | 1991-06-25 | Leeco Diagnostic, Inc. | Scavenger antibody mixture and its use for immunometric assay |
| US4737456A (en)* | 1985-05-09 | 1988-04-12 | Syntex (U.S.A.) Inc. | Reducing interference in ligand-receptor binding assays |
| US4880731A (en)* | 1986-03-19 | 1989-11-14 | Boehringer Mannheim Gmbh | Process and reagent for the determination of a reaction partner of an immunological reaction |
| US5108896A (en)* | 1986-03-21 | 1992-04-28 | Applied Research Systems Ars Holding N.V. | Simultaneous immunoassay of two analytes using dual enzyme labelled antibodies |
| US4804626A (en)* | 1986-10-22 | 1989-02-14 | The General Hospital Corporation | Immunometric assay for the detection of human chorionic gonadotropin |
| US5187067A (en)* | 1986-12-15 | 1993-02-16 | Teijin Limited | Immunological determination of free human protein S and C4bp-protein S complex |
| US4885255A (en)* | 1987-04-28 | 1989-12-05 | Boehringer Mannheim Gmbh | Immunochemical process and reagent for the determination of a polyvalent antigen in a liquid sample |
| US5202234A (en)* | 1987-07-21 | 1993-04-13 | Internationl Immunoassay Laboratories, Inc. | Myocardial infarction immunoassay |
| US5096669A (en)* | 1988-09-15 | 1992-03-17 | I-Stat Corporation | Disposable sensing device for real time fluid analysis |
| US5108933A (en)* | 1988-09-16 | 1992-04-28 | Immunicon Corporation | Manipulation of colloids for facilitating magnetic separations |
| US5554339A (en)* | 1988-11-14 | 1996-09-10 | I-Stat Corporation | Process for the manufacture of wholly microfabricated biosensors |
| US5120660A (en)* | 1989-12-05 | 1992-06-09 | International Canine Genetics, Inc. | Method for canine fertility detection |
| US5324650A (en)* | 1990-03-20 | 1994-06-28 | E. I. Du Pont De Nemours And Company | Situ process for production of conjugates |
| US5834220A (en)* | 1993-05-17 | 1998-11-10 | Fortron Bioscience, Inc. | Assay for cardiac troponin I |
| US5468846A (en)* | 1993-06-30 | 1995-11-21 | Kirin-Amgen Inc. | Monoclonal antibodies and method for the determination of human G-CSF |
| US5661040A (en)* | 1993-07-13 | 1997-08-26 | Abbott Laboratories | Fluorescent polymer labeled conjugates and intermediates |
| US5416026A (en)* | 1993-10-04 | 1995-05-16 | I-Stat Corporation | Method for detecting the change in an analyte due to hemolysis in a fluid sample |
| US5593638A (en)* | 1993-10-04 | 1997-01-14 | I-Stat Corporation | Apparatus for estimating the change in an analyte from hemolysis in a fluid sample |
| US5628961A (en)* | 1993-10-28 | 1997-05-13 | I-Stat Corporation | Apparatus for assaying viscosity changes in fluid samples and method of conducting same |
| US5447440A (en)* | 1993-10-28 | 1995-09-05 | I-Stat Corporation | Apparatus for assaying viscosity changes in fluid samples and method of conducting same |
| US5599677A (en)* | 1993-12-29 | 1997-02-04 | Abbott Laboratories | Immunoassays for prostate specific antigen |
| US5605664A (en)* | 1994-07-13 | 1997-02-25 | I-Stat Corporation | Methods and apparatus for rapid equilibration of dissolved gas composition |
| US5609824A (en)* | 1994-07-13 | 1997-03-11 | I-Stat Corporation | Methods and apparatus for rapid equilibration of dissolved gas composition |
| US5614416A (en)* | 1994-07-13 | 1997-03-25 | I-Stat Corporation | Methods and apparatus for rapid equilibration of dissolved gas composition |
| US5514253A (en)* | 1994-07-13 | 1996-05-07 | I-Stat Corporation | Method of measuring gas concentrations and microfabricated sensing device for practicing same |
| US5789253A (en)* | 1994-07-13 | 1998-08-04 | I-Stat Corporation | Methods for rapid equalibration of dissolved gas composition |
| US5821095A (en)* | 1995-01-12 | 1998-10-13 | Toyo Boseki Kabushiki Kaisha | Alkaline phosphatase |
| US5821061A (en)* | 1995-01-12 | 1998-10-13 | Toyo Boseki Kabushiki Kaisha | Method and reagent for detecting a ligand in a sample |
| US5795725A (en)* | 1995-04-18 | 1998-08-18 | Biosite Diagnostics Incorporated | Methods for the assay of troponin I and T and selection of antibodies for use in immunoassays |
| US6140474A (en)* | 1995-05-02 | 2000-10-31 | Japan Tobacco, Inc. | Monoclonal antibody reactive with human-origin CETP and method of quantifying human-origin CETP |
| US6114180A (en)* | 1995-07-06 | 2000-09-05 | Bayer Aktiengesellschaft | Synthetic calibrators for use in immunoassays, comprising the analytes or partial sequences thereof which are conjugated to inert carrier molecules |
| US5869348A (en)* | 1996-05-15 | 1999-02-09 | Medinox, Inc. | Methods for the preparation of antibodies directed against dithiocarbamates and the use thereof for detection of nitric oxide in body fluids |
| US5871905A (en)* | 1996-09-04 | 1999-02-16 | Epitope, Inc. | Reduction of false positives in oral-fluid based immunoassays |
| US5947124A (en)* | 1997-03-11 | 1999-09-07 | Biosite Diagnostics Incorporated | Diagnostic for determining the time of a heart attack |
| US6180340B1 (en)* | 1997-10-31 | 2001-01-30 | Gen-Probe Incorporated | Extended dynamic range assays |
| US6030827A (en)* | 1998-01-23 | 2000-02-29 | I-Stat Corporation | Microfabricated aperture-based sensor |
| US6379883B2 (en)* | 1998-01-23 | 2002-04-30 | I-Stat Corporation | Microfabricated aperture-based sensor |
| US7419821B2 (en)* | 2002-03-05 | 2008-09-02 | I-Stat Corporation | Apparatus and methods for analyte measurement and immunoassay |
Cited By (365)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US8680239B2 (en) | 2000-12-22 | 2014-03-25 | Max-Planck-Gesellschaft Zur Foerderung Der Wissenschaften E.V. | Use of RGM and its modulators |
| US20050150761A1 (en)* | 2001-06-04 | 2005-07-14 | Epocal Inc. | Electrode module |
| US7824529B2 (en) | 2001-06-04 | 2010-11-02 | Epocal Inc. | Electrode module |
| US20070170062A1 (en)* | 2001-06-04 | 2007-07-26 | Epocal Inc. | Integrated electrokinetic devices and methods of manufacture |
| US8007648B2 (en) | 2001-06-04 | 2011-08-30 | Lauks Imants R | Integrated electrokinetic devices and methods of manufacture |
| US20140141484A1 (en)* | 2002-07-29 | 2014-05-22 | Abbott Point Of Care Inc. | Multiple Hybrid Immunoassay |
| US10641767B2 (en) | 2002-07-29 | 2020-05-05 | Abbott Point Of Care Inc. | Multiple hybrid immunoassay |
| US9995744B2 (en) | 2002-07-29 | 2018-06-12 | Abbott Point Of Care Inc. | Multiple hybrid immunoassay |
| US9267939B2 (en)* | 2002-07-29 | 2016-02-23 | Abbott Point Of Care Inc. | Multiple hybrid immunoassay |
| US7842234B2 (en) | 2002-12-02 | 2010-11-30 | Epocal Inc. | Diagnostic devices incorporating fluidics and methods of manufacture |
| US9753003B2 (en) | 2002-12-02 | 2017-09-05 | Epocal Inc. | Diagnostic devices incorporating fluidics and methods of manufacture |
| US20040222091A1 (en)* | 2002-12-02 | 2004-11-11 | Imants Lauks | Diagnostic devices incorporating fluidics and methods of manufacture |
| US10031099B2 (en) | 2002-12-02 | 2018-07-24 | Siemens Healthcare Diagnostics Inc. | Heterogeneous membrane electrodes |
| US20040231984A1 (en)* | 2002-12-02 | 2004-11-25 | Imants Lauks | Heterogeneous membrane electrodes |
| US20100321004A1 (en)* | 2002-12-02 | 2010-12-23 | Epocal Inc. | Diagnostic devices incorporating fluidics and methods of manufacture |
| US10852266B2 (en) | 2002-12-02 | 2020-12-01 | Siemens Healthcare Diagnostics Inc. | Heterogeneous membrane electrodes |
| US8506778B2 (en) | 2002-12-02 | 2013-08-13 | Epocal Inc. | Diagnostic devices incorporating fluidics and methods of manufacture |
| US10436735B2 (en) | 2002-12-02 | 2019-10-08 | Siemens Healthcare Diagnostics Inc. | Diagnostic devices incorporating fluidics and methods of manufacture |
| US7767068B2 (en) | 2002-12-02 | 2010-08-03 | Epocal Inc. | Heterogeneous membrane electrodes |
| US20040197841A1 (en)* | 2003-04-02 | 2004-10-07 | Apffel James Alexander | Methods and reagents for multiplexed analyses |
| US7638292B2 (en)* | 2003-04-30 | 2009-12-29 | University Of Turku | Immunoassay |
| US20080153109A1 (en)* | 2003-04-30 | 2008-06-26 | Susann Eriksson | Immunoassay |
| US20050047972A1 (en)* | 2003-08-28 | 2005-03-03 | Imants Lauks | Lateral flow diagnostic devices with instrument controlled fluidics |
| US20100202926A1 (en)* | 2003-08-28 | 2010-08-12 | Epocal Inc. | Lateral flow diagnostic devices with instrument controlled fluidics |
| US8124026B2 (en) | 2003-08-28 | 2012-02-28 | Epocal Inc. | Lateral flow diagnostic devices with instrument controlled fluidics |
| US7722817B2 (en) | 2003-08-28 | 2010-05-25 | Epocal Inc. | Lateral flow diagnostic devices with instrument controlled fluidics |
| US8129191B2 (en)* | 2003-12-18 | 2012-03-06 | Inverness Medical Switzerland Gmbh | Monitoring method and apparatus |
| US20050137481A1 (en)* | 2003-12-18 | 2005-06-23 | Paul Sheard | Monitoring method and apparatus |
| US8563328B2 (en)* | 2004-07-26 | 2013-10-22 | University Of Louisville Research Foundation, Inc. | Fiber-optic biosensor and biosensing methods |
| US20060286680A1 (en)* | 2004-07-26 | 2006-12-21 | University Of Louisville Research Foundation | Fiber-optic biosensor and biosensing methods |
| US20090170135A1 (en)* | 2004-09-08 | 2009-07-02 | Wolfgang Woloszczuk | DETERMINING FELINE proBNP |
| AT500800B1 (en)* | 2004-09-08 | 2008-07-15 | Biomedica Medizinprodukte Gmbh | PROCESS FOR DETERMINING PROBNP |
| AT500800A1 (en)* | 2004-09-08 | 2006-03-15 | Biomedica Medizinprodukte Gmbh | PROCESS FOR DETERMINING PROBNP |
| US9605068B2 (en) | 2004-09-08 | 2017-03-28 | The Antibody Lab Gmbh | Determining feline proBNP |
| US9005984B2 (en) | 2004-09-08 | 2015-04-14 | Biomedica Medizinprodukte Gmbh & Co Kg | Determining feline proBNP |
| US20090170136A1 (en)* | 2004-09-08 | 2009-07-02 | Wolfgang Woloszczuk | DETERMINING FELINE proBNP |
| US20090170138A1 (en)* | 2004-09-08 | 2009-07-02 | Wolfgang Woloszczuk | DETERMINING CANINE proBNP |
| US20090170137A1 (en)* | 2004-09-08 | 2009-07-02 | Wolfgang Woloszczuk | DETERMINING CANINE proBNP |
| US8628973B2 (en) | 2004-09-08 | 2014-01-14 | Biomedica Medizinprodukte Gmbh & Co Kg | Identification of canine proBNP by immunoassay |
| US20070161041A1 (en)* | 2004-09-08 | 2007-07-12 | Biomedica Medizinprodukte Gmbh & Co Kg | Identification of feline or canine probnp |
| US20060204999A1 (en)* | 2005-03-14 | 2006-09-14 | Stephen Macevicz | Detecting molecular complexes |
| US20090054741A1 (en)* | 2005-03-29 | 2009-02-26 | Inverness Medical Switzerland Gmbh | Device and method of monitoring a patient |
| US8906864B2 (en) | 2005-09-30 | 2014-12-09 | AbbVie Deutschland GmbH & Co. KG | Binding domains of proteins of the repulsive guidance molecule (RGM) protein family and functional fragments thereof, and their use |
| US20090297527A1 (en)* | 2005-09-30 | 2009-12-03 | Muller Bernhard K | Binding Domains of Proteins of the Repulsive Guidance Molecule (RGM) Protein Family and Functional Fragments Thereof, and Their Use |
| US20140304796A1 (en)* | 2006-04-28 | 2014-10-09 | Microsoft Corporation | Providing guest users network access based on information read from a credit card or other object |
| EP2910949A1 (en) | 2006-09-01 | 2015-08-26 | Abbott Laboratories | Combination hepatitis C virus antigen and antibody detection method |
| US20100151490A1 (en)* | 2006-09-01 | 2010-06-17 | Abbott Laboratories | Antigenic Protein Conjugates and Process for Preparing Same |
| US20090246800A1 (en)* | 2006-10-26 | 2009-10-01 | Abbott Laboratories | Immunoassay of analytes in samples containing endogenous anti-analyte antibodies |
| US8357495B2 (en) | 2006-10-26 | 2013-01-22 | Abbott Laboratories | Immunoassay of analytes in samples containing endogenous anti-analyte antibodies |
| US20080305512A1 (en)* | 2006-10-26 | 2008-12-11 | Mattingly Phillip G | Assay for cardiac troponin autoantibodies |
| US8173382B2 (en) | 2006-10-26 | 2012-05-08 | Abbott Laboratories | Assay for cardiac troponin autoantibodies |
| US20100311079A1 (en)* | 2006-10-26 | 2010-12-09 | Abbott Laboratories | Assay for cardiac troponin autoantibodies |
| WO2008051761A2 (en) | 2006-10-26 | 2008-05-02 | Abbott Laboratories | Assay for cardiac troponin autoantibodies |
| WO2008082984A2 (en) | 2006-12-29 | 2008-07-10 | Abbott Laboratories | Non-denaturing lysis reagent for use with capture-in-solution immunoassay |
| WO2008082974A2 (en) | 2006-12-29 | 2008-07-10 | Abbott Laboratories | Non-denaturing lysis reagent |
| US20100136590A1 (en)* | 2007-03-07 | 2010-06-03 | Biomedica Medizinprodukte Gmbh & Co Kg | Method of Determining NT-proBNP |
| US9103839B2 (en) | 2007-03-07 | 2015-08-11 | Biomedica Medizinprodukte Gmbh & Co Kg | Method of determining equine NT-proBNP |
| WO2008137991A1 (en) | 2007-05-08 | 2008-11-13 | Abbott Laboratories | Human b-type natriuretic peptide assay having reduced cross-reactivity with other peptide forms |
| US20080311676A1 (en)* | 2007-05-24 | 2008-12-18 | Abbott Laboratories | Immunoassays exhibiting reduced cross-reactivity with hydrophobic drug analyte metabolites |
| US8288111B2 (en) | 2007-05-24 | 2012-10-16 | Abbott Laboratories | Immunoassays exhibiting reduced cross-reactivity with hydrophobic drug analyte metabolites |
| US20090053736A1 (en)* | 2007-08-21 | 2009-02-26 | Mattingly Phillip G | Homogeneous Chemiluminescent Immunoassay for Analysis of Iron Metalloproteins |
| US20090053596A1 (en)* | 2007-08-22 | 2009-02-26 | Stauffer John E | Alkali Metal Battery |
| US20100322948A1 (en)* | 2007-09-06 | 2010-12-23 | Abbott Gmbh & Co. Kg | Bone morphogenetic protein (BMP)-binding domains of proteins of the repulsive guidance molecule (RGM) protein family and functional fragments thereof, and use of same |
| US20090098531A1 (en)* | 2007-09-13 | 2009-04-16 | Abbott Laboratories | Detecting hepatitis b virus |
| US8138318B2 (en) | 2007-09-13 | 2012-03-20 | Abbott Laboratories | Hepatitis B pre-S2 nucleic acid |
| US20100136520A1 (en)* | 2007-09-13 | 2010-06-03 | Abbott Laboratories | Detecting hepatitis b virus |
| WO2009036228A2 (en) | 2007-09-13 | 2009-03-19 | Abbott Laboratories | Hepatitis b pre-s2 nucleic acid |
| US20100267055A1 (en)* | 2007-10-21 | 2010-10-21 | Abbott Laboratories | One-step immunoassays exhibiting increased sensitivity and specificity |
| US20090111123A1 (en)* | 2007-10-25 | 2009-04-30 | Birkenmeyer Larry G | Compositions for detecting antibodies to babesia microti and methods of uses thereof |
| US8283124B2 (en) | 2007-10-25 | 2012-10-09 | Abbott Laboratories | Compositions for detecting antibodies to Babesia microti and methods of uses thereof |
| WO2009078875A1 (en) | 2007-12-19 | 2009-06-25 | Abbott Laboratories | Immunosuppressant drug extraction reagent for immunoassays |
| US20090203037A1 (en)* | 2007-12-27 | 2009-08-13 | Abbott Laboratories | Anti-T. Cruzi Antibodies and Methods of Use |
| US9073984B2 (en) | 2007-12-27 | 2015-07-07 | Abbott Laboratories | Anti-T. cruzi antibodies and methods of use |
| US9482667B2 (en) | 2007-12-27 | 2016-11-01 | Abbott Laboratories | Anti-T. cruzi antibodies and methods of use |
| US10215754B2 (en) | 2007-12-27 | 2019-02-26 | Abbott Laboratories | Anti-T. cruzi antibodies and methods of use |
| US8962803B2 (en) | 2008-02-29 | 2015-02-24 | AbbVie Deutschland GmbH & Co. KG | Antibodies against the RGM A protein and uses thereof |
| US9605069B2 (en) | 2008-02-29 | 2017-03-28 | AbbVie Deutschland GmbH & Co. KG | Antibodies against the RGM a protein and uses thereof |
| US20100028340A1 (en)* | 2008-02-29 | 2010-02-04 | Abbott Gmbh & Co. Kg | Antibodies against the rgm a protein and uses thereof |
| WO2009111599A2 (en) | 2008-03-06 | 2009-09-11 | Abbott Laboratories | Plasmodium malariae and plasmodium ovale genes and uses thereof |
| US20090232797A1 (en)* | 2008-03-06 | 2009-09-17 | Abbott Laboratories | Plasmodium malariae and plasmodium ovale genes and uses thereof |
| US8268981B2 (en) | 2008-03-06 | 2012-09-18 | Abbott Laboratories | Plasmodium malariae and plasmodium ovale genes and uses thereof |
| US20090226445A1 (en)* | 2008-03-06 | 2009-09-10 | Abbott Laboratories | Plasmodium malariae and plasmodium ovale genes and uses thereof |
| US8030471B2 (en) | 2008-03-06 | 2011-10-04 | Abbott Laboratories | Plasmodium malariae and Plasmodium ovale genes and uses thereof |
| EP2899209A1 (en) | 2008-04-29 | 2015-07-29 | Abbvie Inc. | Dual Variable Domain Immunoglobulins and uses thereof |
| US20090286256A1 (en)* | 2008-05-19 | 2009-11-19 | Abbott Laboratories | Isolated human autoantibodies to natriuretic peptides and methods and kits for detecting human autoantibodies to natriuretic peptides |
| US20090286329A1 (en)* | 2008-05-19 | 2009-11-19 | Abbott Laboratoires | Isolated human autoantibodies to natriuretic peptides and methods and kits for detecting human autoantibodies to natriuretic peptides |
| EP3002299A1 (en) | 2008-06-03 | 2016-04-06 | AbbVie Inc. | Dual variable domain immunoglobulins and uses thereof |
| US9035027B2 (en) | 2008-06-03 | 2015-05-19 | Abbvie Inc. | Dual variable domain immunoglobulins and uses thereof |
| WO2009149185A2 (en) | 2008-06-03 | 2009-12-10 | Abbott Laboratories | Dual variable domain immunoglobulins and uses thereof |
| WO2009155324A2 (en) | 2008-06-18 | 2009-12-23 | Abbott Laboratories | Pigf-1 assay and kits and components thereof |
| US20100015655A1 (en)* | 2008-07-17 | 2010-01-21 | Abbott Laboratories | Methods and Kits for Detecting and Quantifying Damage Caused by a Parasite |
| WO2010028014A1 (en) | 2008-09-03 | 2010-03-11 | Abbott Laboratories | Assays and kits for determining hiv-1 tropism |
| US20100240024A1 (en)* | 2008-09-03 | 2010-09-23 | Abbott Laboratories | Assays And Kits For Determining HIV-1 Tropism |
| US20100105150A1 (en)* | 2008-10-24 | 2010-04-29 | Abbott Laboratories | Isolated human autoantibodies to neutrophil gelatinase-associated lipocalin (ngal) and methods and kits for the detection of human autoantibodies to ngal |
| US20110256640A1 (en)* | 2008-12-22 | 2011-10-20 | Koninklijke Philips Electronics N.V. | Assay for troponin i using magnetic labels |
| US9720003B2 (en)* | 2008-12-22 | 2017-08-01 | Koninklijke Philips Electronics N.V. | Assay for Troponin I using magnetic labels |
| WO2010075475A1 (en) | 2008-12-23 | 2010-07-01 | Abbott Laboratories | SOLUBLE FMS-LIKE TYROSINE KINASE-1 (sFLT-1) ANTIBODY AND RELATED COMPOSITION, KIT, METHODS OF USING, AND MATERIALS AND METHOD FOR MAKING |
| WO2010075460A1 (en) | 2008-12-23 | 2010-07-01 | Abbott Laboratories | Alkylated interleukin-18 and related compositions, kits, and methods of making and using same |
| US20100166702A1 (en)* | 2008-12-23 | 2010-07-01 | Abbott Laboratories | Alkylated interleukin-18 and related compositions, kits, and methods of making and using same |
| US8168165B2 (en) | 2008-12-23 | 2012-05-01 | Abbott Laboratories | Alkylated interleukin-18 compositions |
| US8445199B2 (en) | 2008-12-31 | 2013-05-21 | Abbott Point Of Care Inc. | Method and device for immunoassay using nucleotide conjugates |
| WO2010078443A1 (en) | 2008-12-31 | 2010-07-08 | Abbott Point Of Care Inc. | Method and device for immunoassay using nucleotide conjugates |
| CN102272600A (en)* | 2008-12-31 | 2011-12-07 | 雅培医护站股份有限公司 | Methods and devices for immunoassays using nucleotide conjugates |
| US9964537B2 (en) | 2008-12-31 | 2018-05-08 | Abbott Point Of Care Inc. | Method and device for immunoassay using nucleotide conjugates |
| US9207246B2 (en) | 2008-12-31 | 2015-12-08 | Abbott Point Of Care Inc. | Method and device for immunoassay using nucleotide conjugates |
| US8241915B2 (en) | 2009-01-14 | 2012-08-14 | Abbott Laboratories | Methods and kits for detecting hemoglobin in test samples |
| US20100178660A1 (en)* | 2009-01-14 | 2010-07-15 | Abbott Laboratories | Methods and kits for detecting hemoglobin in test samples |
| US20100196907A1 (en)* | 2009-01-31 | 2010-08-05 | Abbott Laboratories | Markers to predict and monitor response to aurora kinase b inhibitor therapy |
| WO2010088470A1 (en) | 2009-01-31 | 2010-08-05 | Abbott Laboratories | Markers to predict and monitor response to aurora kinase b inhibitor therapy |
| EP2479288A1 (en) | 2009-01-31 | 2012-07-25 | Abbott Laboratories | Markers to predict and monitor response to Aurora kinase B inhibitor therapy |
| US9045800B2 (en) | 2009-02-11 | 2015-06-02 | Abbvie Inc. | Methods and compositions for identifying, classifying and monitoring subject having Bcl-2 family inhibitor-resistant tumors and cancers |
| US20100234239A1 (en)* | 2009-02-11 | 2010-09-16 | Abbott Laboratories | Methods and compositions for identifying, classifying and monitoring subject having bcl-2 family inhibitor-resistant tumors and cancers |
| WO2010093742A1 (en) | 2009-02-11 | 2010-08-19 | Abbott Laboratories | Methods and compositions for identifying, classifying and monitoring subject having bcl-2 family inhibitor-resistant tumors and cancers |
| WO2010099079A1 (en) | 2009-02-24 | 2010-09-02 | Abbott Laboratories | Antibodies to troponin i and methods of use thereof |
| EP2853540A1 (en) | 2009-02-24 | 2015-04-01 | Abbott Laboratories | Antibodies to troponin I and methods of use therof |
| US8030026B2 (en) | 2009-02-24 | 2011-10-04 | Abbott Laboratories | Antibodies to troponin I and methods of use thereof |
| US20100216720A1 (en)* | 2009-02-24 | 2010-08-26 | Abbott Laboratories | Antibodies to troponin i and methods of use thereof |
| USRE45763E1 (en) | 2009-02-24 | 2015-10-20 | Abbott Laboratories | Antibodies to troponin I and methods of use thereof |
| US20100266531A1 (en)* | 2009-03-05 | 2010-10-21 | Abbott Laboratories | Il-17 binding proteins |
| US8835610B2 (en) | 2009-03-05 | 2014-09-16 | Abbvie Inc. | IL-17 binding proteins |
| US9481735B2 (en) | 2009-03-05 | 2016-11-01 | Abbvie Inc. | IL-17 binding proteins |
| US9481736B2 (en) | 2009-03-05 | 2016-11-01 | Abbvie, Inc. | IL-17 binding proteins |
| EP2810652A2 (en) | 2009-03-05 | 2014-12-10 | AbbVie Inc. | IL-17 binding proteins |
| US9663587B2 (en) | 2009-03-05 | 2017-05-30 | Abbvie Inc. | IL-17 binding proteins |
| US8779101B2 (en) | 2009-03-05 | 2014-07-15 | Abbvie, Inc. | IL-17 binding proteins |
| EP2772269A2 (en) | 2009-03-05 | 2014-09-03 | Abbvie Inc. | IL-17 binding proteins |
| WO2010104815A1 (en) | 2009-03-10 | 2010-09-16 | Abbott Laboratories | Antibodies binding to pivka-ii amino acids 13-27 |
| US8283162B2 (en) | 2009-03-10 | 2012-10-09 | Abbott Laboratories | Antibodies relating to PIVKAII and uses thereof |
| US20100233175A1 (en)* | 2009-03-10 | 2010-09-16 | Abbott Laboratories | Antibodies relating to pivkaii and uses thereof |
| US8084272B2 (en) | 2009-03-25 | 2011-12-27 | Abbott Point Of Care Inc. | Amelioration of heterophile antibody immunosensor interference |
| US20100248273A1 (en)* | 2009-03-25 | 2010-09-30 | Abbott Point Of Care Inc. | Amelioration of heterophile antibody immunosensor interference |
| US8828738B2 (en) | 2009-03-25 | 2014-09-09 | Abbott Point Of Care Inc. | Amelioration of heterophile antibody immunosensor interference |
| WO2010111382A1 (en) | 2009-03-25 | 2010-09-30 | Abbott Point Of Care Inc. | Amelioration of heterophile antibody immunosensor interference |
| US8318897B2 (en) | 2009-03-27 | 2012-11-27 | Abbott Laboratories | Nucleotide and amino acid sequences encoding an exported protein 1 derived from Plasmodium vivax and uses thereof |
| WO2010111220A1 (en) | 2009-03-27 | 2010-09-30 | Abbott Laboratories | Nucleotide and amino acid sequences encoding an exported protein 1 derived from plasmodium vivax and uses thereof |
| US8063193B2 (en) | 2009-03-27 | 2011-11-22 | Abbott Laboratories | Nucleotide and amino acid sequences encoding an exported protein 1 derived from Plasmodium vivax and uses thereof |
| US20110076697A1 (en)* | 2009-04-28 | 2011-03-31 | Innovative Laboratory Technologies, Inc. | Lateral-flow immuno-chromatographic assay devices |
| US20100291709A1 (en)* | 2009-05-13 | 2010-11-18 | Abbott Laboratories | Human nt-pro b-type natriuretic peptide assay having reduced cross-reactivity with other peptide forms |
| WO2011002936A2 (en) | 2009-06-30 | 2011-01-06 | Abbott Laboratories | Markers of xmrv infection and uses thereof |
| US9132190B2 (en) | 2009-08-29 | 2015-09-15 | Abbvie Inc. | Therapeutic DLL4 binding proteins |
| US9469688B2 (en) | 2009-08-29 | 2016-10-18 | Abbvie Inc. | Therapeutic DLL4 binding proteins |
| WO2011026017A1 (en) | 2009-08-31 | 2011-03-03 | Abbott Laboratories | Biomarkers for prediction of major adverse cardiac events and uses thereof |
| US20110053179A1 (en)* | 2009-08-31 | 2011-03-03 | Abbott Laboratories | Biomarkers For Prediction Of Major Adverse Cardiac Events And Uses Thereof |
| US8501420B2 (en) | 2009-08-31 | 2013-08-06 | Abbott Laboratories | Biomarkers for prediction of major adverse cardiac events and uses thereof |
| US11493507B2 (en) | 2009-09-14 | 2022-11-08 | Siemens Healthineers Nederland B.V. | Highly sensitive immunoassay with large particle labels |
| US8389293B2 (en) | 2009-11-17 | 2013-03-05 | Abbott Point Of Care Inc. | Reducing leukocyte interference in competitive immunoassays |
| US20110117580A1 (en)* | 2009-11-17 | 2011-05-19 | Abbott Point Of Care Inc. | Reducing leukocyte interference in non-competitive immunoassays |
| US20110117581A1 (en)* | 2009-11-17 | 2011-05-19 | Abbott Point Of Care Inc. | Reducing leukocyte interference in competitive immunoassays |
| US8377669B2 (en) | 2009-11-17 | 2013-02-19 | Abbott Point Of Care Inc. | Reducing leukocyte interference in non-competitive immunoassays |
| WO2011063012A1 (en) | 2009-11-17 | 2011-05-26 | Abbott Point Of Care Inc. | Reducing leukocyte interference in competitive immunoassays |
| WO2011063010A1 (en) | 2009-11-17 | 2011-05-26 | Abbott Point Of Care Inc. | Reducing leukocyte interference in non-competitive immunoassays |
| US20110124012A1 (en)* | 2009-11-21 | 2011-05-26 | Abbott Laboratories | Assays for human nt-pro b-type natriuretic peptide, human pro b-type natriuretic peptide and human b-type natriuretic peptide |
| US8455212B2 (en) | 2009-11-21 | 2013-06-04 | Abbott Laboratories | Assays for human NT-pro B-type natriuretic peptide, human pro B-type natriuretic peptide and human B-type natriuretic peptide |
| US20130164767A1 (en)* | 2009-11-21 | 2013-06-27 | Abbott Laboratories | Assays for human nt-pro b-type natriuretic peptide, human pro b-type natriuretic peptide and human b-type natriuretic peptide |
| WO2011062931A1 (en) | 2009-11-21 | 2011-05-26 | Abbott Laboratories | Assays for human nt-pro b-type natriuretic peptide, human pro b-type natriuretic peptide and human b-type natriuretic peptide |
| US20110129854A1 (en)* | 2009-12-02 | 2011-06-02 | Abbott Laboratories | Assay for diagnosis of cardiac myocyte damage |
| US8835120B2 (en) | 2009-12-02 | 2014-09-16 | Abbott Laboratories | Assay for cardiac troponin-T (cTnT) |
| US8652788B2 (en) | 2009-12-02 | 2014-02-18 | Abbott Laboratories | Assay for diagnosis of cardiac myocyte damage |
| WO2011068681A1 (en) | 2009-12-02 | 2011-06-09 | Abbott Laboratories | ASSAY FOR CARDIAC TROPONIN-T (cTnT) |
| US20110129818A1 (en)* | 2009-12-02 | 2011-06-02 | Abbott Laboratories | ASSAY FOR CARDIAC TROPONIN-T (cTnT) |
| WO2011068676A1 (en) | 2009-12-03 | 2011-06-09 | Abbott Laboratories | Peptide reagents and method for inhibiting autoantibody antigen binding |
| WO2011068720A1 (en) | 2009-12-03 | 2011-06-09 | Abbott Laboratories | Detecting hepatitis b virus mutants |
| WO2011068680A1 (en) | 2009-12-03 | 2011-06-09 | Abbott Laboratories | Assay for diagnosis of cardiac myocyte damage |
| US8183002B2 (en) | 2009-12-03 | 2012-05-22 | Abbott Laboratories | Autoantibody enhanced immunoassays and kits |
| US20110136103A1 (en)* | 2009-12-03 | 2011-06-09 | Abbott Laboratories | Autoantibody enhanced immunoassays and kits |
| US8304201B2 (en) | 2009-12-03 | 2012-11-06 | Abbott Laboratories | Autoantibody enhanced immunoassays and kits |
| US20110136141A1 (en)* | 2009-12-03 | 2011-06-09 | Abbott Laboratories | Peptide reagents and method for inhibiting autoantibody antigen binding |
| US8574858B2 (en) | 2009-12-03 | 2013-11-05 | Abbott Laboratories | Autoantibody enhanced immunoassays and kits |
| WO2011068863A1 (en) | 2009-12-04 | 2011-06-09 | Abbott Laboratories | Combination therapy for treating cancer and diagnostic assays for use therein |
| US20110135664A1 (en)* | 2009-12-08 | 2011-06-09 | Abbott Gmbh & Co. Kg | Monoclonal antibodies against the rgm a protein for use in the treatment of retinal nerve fiber layer degeneration |
| US9175075B2 (en) | 2009-12-08 | 2015-11-03 | AbbVie Deutschland GmbH & Co. KG | Methods of treating retinal nerve fiber layer degeneration with monoclonal antibodies against a retinal guidance molecule (RGM) protein |
| US9115195B2 (en) | 2010-03-02 | 2015-08-25 | Abbvie Inc. | Therapeutic DLL4 binding proteins |
| US9469689B2 (en) | 2010-03-02 | 2016-10-18 | Abbvie Inc. | Therapeutic DLL4 binding proteins |
| WO2011116023A1 (en) | 2010-03-18 | 2011-09-22 | Abbott Laboratories | METHODS OF ASSAYING URINARY NEUTROPHIL GELATINASE-ASSOCIATED LIPOCALIN (uNGAL) IN THE PROGNOSIS OF CADAVERIC KIDNEY TRANSPLANT FUNCTION IN A PATIENT, INCLUDING A PATIENT DIAGNOSED WITH DELAYED GRAFT FUNCTION (DGF), A METHOD OF ASSAYING uNGAL IN THE ASSESSMENT OF RISK OF DGF IN A PATIENT DIAGNOSED WITH EARLY GRAFT FUNCTION (EGF), AND RELATED KITS |
| US20110229921A1 (en)* | 2010-03-18 | 2011-09-22 | Abbott Laboratories | METHODS OF ASSAYING URINARY NEUTROPHIL GELATINASE-ASSOCIATED LIPOCALIN (uNGAL) IN THE PROGNOSIS OF CADAVERIC KIDNEY TRANSPLANT FUNCTION IN A PATIENT, INCLUDING A PATIENT DIAGNOSED WITH DELAYED GRAFT FUNCTION (DGF), A METHOD OF ASSAYING uNGAL IN THE ASSESSMENT OF RISK OF DGF IN A PATIENT DIAGNOSED WITH EARLY GRAFT FUNCTION (EGF), AND RELATED KITS |
| US9528998B2 (en) | 2010-04-16 | 2016-12-27 | Abbott Laboratories | Methods and reagents for diagnosing rheumatoid arthrtis |
| WO2011137165A1 (en) | 2010-04-30 | 2011-11-03 | Abbott Point Of Care Inc. | Reagents for reducing leukocyte interference in immunoassays |
| US8476079B2 (en) | 2010-04-30 | 2013-07-02 | Abbott Point Of Care Inc. | Reagents for reducing leukocyte interference in immunoassays |
| US9409986B2 (en) | 2010-05-14 | 2016-08-09 | Abbvie Inc. | IL-1 binding proteins |
| US9441038B2 (en) | 2010-05-14 | 2016-09-13 | Abbvie Inc. | IL-1 binding proteins |
| US8664367B2 (en) | 2010-05-14 | 2014-03-04 | Abbvie, Inc. | IL-I binding proteins |
| US9447184B2 (en) | 2010-05-14 | 2016-09-20 | Abbvie Inc. | IL-1 binding proteins |
| US9303085B2 (en) | 2010-05-14 | 2016-04-05 | Abbvie Inc. | IL-1 binding proteins |
| US8841417B2 (en) | 2010-05-14 | 2014-09-23 | Abbvie Inc. | IL-1 binding proteins |
| US9447183B2 (en) | 2010-05-14 | 2016-09-20 | Abbvie Inc. | IL-1 binding proteins |
| US8486721B2 (en) | 2010-06-14 | 2013-07-16 | Abbott Point Of Care Inc. | Magnetic beads for reducing leukocyte interference in immunoassays |
| US8394325B2 (en) | 2010-06-14 | 2013-03-12 | Abbott Point Of Care Inc. | Magnetic beads for reducing leukocyte interference in immunoassays |
| WO2011159707A1 (en) | 2010-06-14 | 2011-12-22 | Abbott Point Of Care Inc. | Magnetic beads for reducing leukocyte interference in immunoassays |
| US9551714B2 (en) | 2010-06-25 | 2017-01-24 | Abbott Laboratories | Materials and methods for assay of anti-hepatitis C virus (HCV) antibodies |
| WO2011163558A1 (en) | 2010-06-25 | 2011-12-29 | Abbott Laboratories | Materials and methods for assay of anti-hepatitis c virus (hcv) antibodies |
| WO2012006500A2 (en) | 2010-07-08 | 2012-01-12 | Abbott Laboratories | Monoclonal antibodies against hepatitis c virus core protein |
| EP2921177A2 (en) | 2010-07-09 | 2015-09-23 | AbbVie Inc. | Dual variable domain immunoglobulins and uses thereof |
| US9120862B2 (en) | 2010-07-26 | 2015-09-01 | Abbott Laboratories | Antibodies relating to PIVKA-II and uses thereof |
| WO2012018476A1 (en) | 2010-07-26 | 2012-02-09 | Abbott Laboratories | Antibodies relating to pivka-ii and uses thereof |
| EP3252072A2 (en) | 2010-08-03 | 2017-12-06 | AbbVie Inc. | Dual variable domain immunoglobulins and uses thereof |
| US9493560B2 (en) | 2010-08-03 | 2016-11-15 | Abbvie Inc. | Dual variable domain immunoglobulins and uses thereof |
| US8778699B2 (en) | 2010-08-04 | 2014-07-15 | Idexx Laboratories, Inc. | Detection of degradation products of canine NT-proBNP |
| WO2012024513A2 (en) | 2010-08-18 | 2012-02-23 | Abbott Laboratories | Molecular detection of xmrv infection |
| WO2012024518A1 (en) | 2010-08-18 | 2012-02-23 | Abbott Laboratories | Molecular detection of xmrv infection |
| US9046513B2 (en) | 2010-08-26 | 2015-06-02 | Abbvie Inc. | Dual variable domain immunoglobulins and uses thereof |
| WO2012064674A1 (en) | 2010-11-09 | 2012-05-18 | Abbott Laboratories | Materials and methods for immunoassay of pterins |
| WO2012121775A2 (en) | 2010-12-21 | 2012-09-13 | Abbott Laboratories | Dual variable domain immunoglobulins and uses thereof |
| CN102279269A (en)* | 2011-04-12 | 2011-12-14 | 王贤俊 | Preparation method of cystatin C detection kit |
| US10107826B2 (en) | 2011-05-20 | 2018-10-23 | Abbott Japan Co. Ltd. | Immunoassay methods and reagents for decreasing nonspecific binding |
| US9201078B2 (en) | 2011-05-27 | 2015-12-01 | Abbott Point Of Care Inc. | TSH immunoassays and processes for performing TSH immunoassays in the presence of endogenous contaminants in restricted wash formats |
| WO2012166200A1 (en) | 2011-05-27 | 2012-12-06 | Abbott Point Of Care Inc. | Tsh immunoassays employing scavenging reagents for cross-reacting endocrine glycoprotein hormone analogues |
| US8617826B2 (en) | 2011-05-27 | 2013-12-31 | Abbott Point Of Care Inc. | TSH immunoassays employing scavenging reagents for cross-reacting endocrine glycoprotein hormone analogues |
| WO2012166199A1 (en) | 2011-05-27 | 2012-12-06 | Abbott Point Of Care Inc. | Tsh immunoassays and processes for performing tsh immunoassays in the presence of endogenous contaminants in restricted wash formats |
| US9575077B2 (en) | 2011-05-27 | 2017-02-21 | Abbott Point Of Care Inc. | TSH antibodies for point-of-care immunoassay formats |
| US9199234B2 (en) | 2011-05-27 | 2015-12-01 | Abbott Point Of Care Inc. | TSH antibodies for point-of-care immunoassay formats |
| US9068994B2 (en) | 2011-05-27 | 2015-06-30 | Abbott Point Of Care Inc. | TSH immunoassays employing scavenging reagents for cross-reacting endocrine glycoprotein hormone analogues |
| WO2012166198A1 (en) | 2011-05-27 | 2012-12-06 | Abbott Point Of Care Inc. | Tsh antibodies for point-of-care immunoassay formats |
| US9575076B2 (en) | 2011-05-27 | 2017-02-21 | Abbott Point Of Care Inc. | TSH immunoassays and processes for performing TSH immunoassays in the presence of endogenous contaminants in restricted wash formats |
| US8481333B2 (en) | 2011-05-31 | 2013-07-09 | Idexx Laboratories, Inc. | Detection of degradation products of feline NT-proBNP |
| WO2013063110A1 (en) | 2011-10-24 | 2013-05-02 | Abbvie Inc. | Bispecific immunobinders directed against tnf and il-17 |
| WO2013063095A1 (en) | 2011-10-24 | 2013-05-02 | Abbvie Inc. | Immunobinders directed against sclerostin |
| WO2013090635A2 (en) | 2011-12-14 | 2013-06-20 | AbbVie Deutschland GmbH & Co. KG | Composition and method for the diagnosis and treatment of iron-related disorders |
| US10118958B2 (en) | 2011-12-14 | 2018-11-06 | AbbVie Deutschland GmbH & Co. KG | Composition and method for the diagnosis and treatment of iron-related disorders |
| US9636398B2 (en) | 2011-12-14 | 2017-05-02 | AbbVie Deutschland GmbH & Co. KG | Composition and method for the diagnosis and treatment of iron-related disorders |
| US10822403B2 (en) | 2011-12-14 | 2020-11-03 | AbbVie Deutschland GmbH & Co. KG | Composition and method for the diagnosis and treatment of iron-related disorders |
| US12098189B2 (en) | 2011-12-14 | 2024-09-24 | AbbVie Deutschland GmbH & Co. KG | Composition and method for the diagnosis and treatment of iron-related disorders |
| EP3800200A1 (en) | 2011-12-14 | 2021-04-07 | AbbVie Deutschland GmbH & Co. KG | Composition and method for the diagnosis and treatment of iron-related disorders |
| WO2013090633A2 (en) | 2011-12-14 | 2013-06-20 | AbbVie Deutschland GmbH & Co. KG | Composition and method for the diagnosis and treatment of iron-related disorders |
| US12098192B2 (en) | 2011-12-14 | 2024-09-24 | AbbVie Deutschland GmbH & Co. KG | Composition and method for the diagnosis and treatment of iron-related disorders |
| CN102539784A (en)* | 2011-12-26 | 2012-07-04 | 宁波美康生物科技股份有限公司 | Method for detecting cardiac troponin I and application thereof |
| EP2915818A2 (en) | 2011-12-30 | 2015-09-09 | AbbVie Inc. | Dual variable domain immunoglobulins and uses thereof |
| WO2013102042A2 (en) | 2011-12-30 | 2013-07-04 | Abbvie Inc. | Dual specific binding proteins directed against il-13 and/or il-17 |
| US9120870B2 (en) | 2011-12-30 | 2015-09-01 | Abbvie Inc. | Dual specific binding proteins directed against IL-13 and IL-17 |
| WO2013102149A1 (en) | 2011-12-31 | 2013-07-04 | Abbott Laboratories | Truncated human vitamin d binding protein and mutation and fusion thereof and related materials and methods of use |
| US10106602B2 (en) | 2012-01-27 | 2018-10-23 | AbbVie Deutschland GmbH & Co. KG | Isolated monoclonal anti-repulsive guidance molecule A antibodies and uses thereof |
| US9365643B2 (en) | 2012-01-27 | 2016-06-14 | AbbVie Deutschland GmbH & Co. KG | Antibodies that bind to repulsive guidance molecule A (RGMA) |
| US9102722B2 (en) | 2012-01-27 | 2015-08-11 | AbbVie Deutschland GmbH & Co. KG | Composition and method for the diagnosis and treatment of diseases associated with neurite degeneration |
| US9670276B2 (en) | 2012-07-12 | 2017-06-06 | Abbvie Inc. | IL-1 binding proteins |
| CN102749460A (en)* | 2012-07-27 | 2012-10-24 | 北京恩济和生物科技有限公司 | Troponin detection kit and preparing method thereof |
| WO2014071074A2 (en) | 2012-11-01 | 2014-05-08 | Abbvie Inc. | Anti-vegf/dll4 dual variable domain immunoglobulins and uses thereof |
| US9944720B2 (en) | 2012-11-01 | 2018-04-17 | Abbvie Inc. | Anti-DLL4/VEGF dual variable domain immunoglobulin and uses thereof |
| US9045551B2 (en) | 2012-11-01 | 2015-06-02 | Abbvie Inc. | Anti-DLL4/VEGF dual variable domain immunoglobulin and uses thereof |
| US9163093B2 (en) | 2012-11-01 | 2015-10-20 | Abbvie Inc. | Anti-DLL4/VEGF dual variable domain immunoglobulin and uses thereof |
| WO2014089209A2 (en) | 2012-12-04 | 2014-06-12 | Abbvie, Inc. | Blood-brain barrier (bbb) penetrating dual specific binding proteins |
| US9856319B2 (en) | 2012-12-28 | 2018-01-02 | Abbvie Inc. | Monovalent binding proteins |
| WO2014106001A2 (en) | 2012-12-28 | 2014-07-03 | Abbvie, Inc. | Dual specific binding proteins having a receptor sequence |
| WO2014116846A2 (en) | 2013-01-23 | 2014-07-31 | Abbvie, Inc. | Methods and compositions for modulating an immune response |
| US20140257833A1 (en)* | 2013-03-08 | 2014-09-11 | Adventive Ipbank | Cloud Based System For Remote Medical Checkup And Physician Managed Biometric Data |
| US10444242B2 (en) | 2013-03-14 | 2019-10-15 | Abbott Laboratories | Detection methods employing HCV core lipid and DNA binding domain monoclonal antibodies |
| US9371374B2 (en) | 2013-03-14 | 2016-06-21 | Abbott Laboratories | HCV core lipid binding domain monoclonal antibodies |
| US9651547B2 (en) | 2013-03-14 | 2017-05-16 | Abbott Point Of Care Inc. | Electrochemical methods and devices for amending urine samples for immunosensor detection |
| US10345311B2 (en) | 2013-03-14 | 2019-07-09 | Abbott Laboratories | Detection methods employing HCV core lipid and DNA binding domain monoclonal antibodies |
| US9488663B2 (en) | 2013-03-14 | 2016-11-08 | Abbott Point Of Care Inc. | Electrochemical methods and devices for amending urine samples for immunosensor detection |
| US10234450B2 (en) | 2013-03-14 | 2019-03-19 | Abbott Point Of Care Inc. | Electrochemical methods and devices for amending urine samples for immunosensor detection |
| EP3564384A1 (en) | 2013-03-14 | 2019-11-06 | Abbott Laboratories | Hcv core lipid binding domain monoclonal antibodies |
| US9194873B2 (en) | 2013-03-14 | 2015-11-24 | Abbott Laboratories | HCV antigen-antibody combination assay and methods and compositions for use therein |
| US10209251B2 (en) | 2013-03-14 | 2019-02-19 | Abbott Point Of Care Inc. | Electrochemical methods and devices for amending urine samples for immunosensor detection |
| US9790478B2 (en) | 2013-03-14 | 2017-10-17 | Abbott Laboratories | HCV NS3 recombinant antigens and mutants thereof for improved antibody detection |
| EP3916103A1 (en) | 2013-03-14 | 2021-12-01 | Abbott Laboratories | Hcv core lipid binding domain monoclonal antibodies |
| US9841427B2 (en) | 2013-03-14 | 2017-12-12 | Abbott Laboratories | HCV antigen-antibody combination assay and methods and compositions for use therein |
| US10197573B2 (en) | 2013-03-14 | 2019-02-05 | Abbott Laboratories | HCV core lipid binding domain monoclonal antibodies |
| WO2014152991A1 (en) | 2013-03-14 | 2014-09-25 | Abbott Laboratories | Methods for the early detection of lung cancer |
| US11428694B2 (en) | 2013-03-14 | 2022-08-30 | Abbott Laboratories | Detection methods employing HCV core lipid and DNA binding domain monoclonal antibodies |
| US9005901B2 (en) | 2013-03-15 | 2015-04-14 | Abbott Laboratories | Assay with internal calibration |
| US10942179B2 (en) | 2013-03-15 | 2021-03-09 | Abbott Laboratories | Assay with increased dynamic range |
| US9062108B2 (en) | 2013-03-15 | 2015-06-23 | Abbvie Inc. | Dual specific binding proteins directed against IL-1 and/or IL-17 |
| WO2014144355A2 (en) | 2013-03-15 | 2014-09-18 | Abbott Laboratories | Anti-gp73 monoclonal antibodies and methods of obtaining the same |
| US10308709B2 (en) | 2013-03-15 | 2019-06-04 | Abbott Laboratories | Anti-GP73 monoclonal antibodies and methods of obtaining the same |
| EP3527586A1 (en) | 2013-03-15 | 2019-08-21 | Abbott Laboratories | Anti-gp73 monoclonal antibodies and methods of obtaining the same |
| US8987418B2 (en) | 2013-03-15 | 2015-03-24 | Abbvie Inc. | Dual specific binding proteins directed against IL-1β and/or IL-17 |
| WO2014144299A2 (en) | 2013-03-15 | 2014-09-18 | Abbvie Inc. | DUAL SPECIFIC BINDING PROTEINS DIRECTED AGAINST TNFα |
| US9157910B2 (en) | 2013-03-15 | 2015-10-13 | Abbott Laboratories | Assay with increased dynamic range |
| US10073090B2 (en) | 2013-03-15 | 2018-09-11 | Abbott Laboratories | Assay with increased dynamic range |
| US11421023B2 (en) | 2013-03-15 | 2022-08-23 | Abbott Laboratories | Anti-GP73 monoclonal antibodies and methods of obtaining the same |
| EP3124499A1 (en) | 2013-03-15 | 2017-02-01 | Abbott Laboratories | Anti-gp73 monoclonal antibodies and methods of obtaining the same |
| US9469686B2 (en) | 2013-03-15 | 2016-10-18 | Abbott Laboratories | Anti-GP73 monoclonal antibodies and methods of obtaining the same |
| CN103217535A (en)* | 2013-03-18 | 2013-07-24 | 杭州德安奇生物工程有限公司 | Immunochromatography detection card for detecting troponin I |
| WO2015031626A1 (en) | 2013-08-28 | 2015-03-05 | Abbvie Inc. | Soluble cmet assay |
| WO2015051379A2 (en) | 2013-10-06 | 2015-04-09 | Abbvie Inc. | Dual specific binding proteins directed against immune cell receptors and autoantigens |
| EP4071177A1 (en) | 2013-12-30 | 2022-10-12 | Epimab Biotherapeutics, Inc. | Fabs-in-tandem immunoglobulin and uses thereof |
| US10519251B2 (en) | 2013-12-30 | 2019-12-31 | Epimab Biotherapeutics, Inc. | Fabs-in-tandem immunoglobulin and uses thereof |
| US10266608B2 (en) | 2013-12-30 | 2019-04-23 | Epimab Biotherapeutics, Inc. | Fabs-in-tandem immunoglobulin and uses thereof |
| WO2015103072A1 (en) | 2013-12-30 | 2015-07-09 | Epimab Biotherapeutics | Fabs-in-tandem immunoglobulin and uses thereof |
| CN103940986A (en)* | 2014-03-24 | 2014-07-23 | 安徽省煦棠医疗科技有限公司 | Preparation of troponin I specific locus antibody and detection kit thereof |
| CN103913580A (en)* | 2014-04-23 | 2014-07-09 | 齐鲁工业大学 | Nanotechnology for myocardial infarction diagnosis and device adopting same |
| WO2015191934A2 (en) | 2014-06-11 | 2015-12-17 | Abbvie Inc. | Blood-brain barrier (bbb) penetrating dual specific binding proteins for treating brain and neurological diseases |
| US9938589B2 (en) | 2014-06-27 | 2018-04-10 | Abbott Laboratories | Compositions and methods for detecting human pegivirus 2 (HPgV-2) |
| US10501816B2 (en) | 2014-06-27 | 2019-12-10 | Abbott Laboratories | Compositions and methods for detecting human pegivirus 2 (HPgV-2) |
| US9777340B2 (en) | 2014-06-27 | 2017-10-03 | Abbott Laboratories | Compositions and methods for detecting human Pegivirus 2 (HPgV-2) |
| EP3594684A1 (en) | 2014-06-27 | 2020-01-15 | Abbott Laboratories | Compositions and methods for detecting human pegivirus 2 (hpgv-2) |
| US10670611B2 (en) | 2014-09-26 | 2020-06-02 | Somalogic, Inc. | Cardiovascular risk event prediction and uses thereof |
| US11340230B2 (en) | 2014-10-29 | 2022-05-24 | Abbott Laboratories | Subject anti-HCV antibody detection assays employing NS3 capture peptides |
| WO2016069762A2 (en) | 2014-10-29 | 2016-05-06 | Abbott Laboratories | Subject anti-hcv antibody detection assays employing ns3 capture peptides |
| US10088483B2 (en) | 2014-10-29 | 2018-10-02 | Abbott Laboratories | Subject anti-HCV antibody detection assays employing NS3 capture peptides |
| US10816551B2 (en) | 2014-10-29 | 2020-10-27 | Abbott Laboratories | Subject anti-HCV antibody detection assays employing NS3 capture peptides |
| US10030268B2 (en) | 2014-11-11 | 2018-07-24 | Abbott Molecular Inc. | Hybridization probes and methods |
| US10093733B2 (en) | 2014-12-11 | 2018-10-09 | Abbvie Inc. | LRP-8 binding dual variable domain immunoglobulin proteins |
| US20160202250A1 (en)* | 2015-01-09 | 2016-07-14 | Council Of Scientific & Industrial Research | Chemiresistive Biosensor for the Quantitative Detection of Human Cardiac Biomarker and a Process Thereof |
| WO2016160976A2 (en) | 2015-03-30 | 2016-10-06 | Abbvie Inc. | Monovalent tnf binding proteins |
| WO2016205427A2 (en) | 2015-06-15 | 2016-12-22 | Abbvie Inc. | Binding proteins against vegf, pdgf, and/or their receptors |
| US9840554B2 (en) | 2015-06-15 | 2017-12-12 | Abbvie Inc. | Antibodies against platelet-derived growth factor (PDGF) |
| CN105137066A (en)* | 2015-07-31 | 2015-12-09 | 江苏巨珩新材料科技有限公司 | Polystyrene latex based cancer marker immuno-chromatography kit and application method thereof |
| EP3657174A1 (en) | 2015-09-28 | 2020-05-27 | Abbott Japan Co., Ltd. | Use of laminin 2 for diagnosing hepatocellular carcinoma and pancreatic cancer |
| US11340229B2 (en) | 2015-09-28 | 2022-05-24 | Abbott Japan Llc | Methods of diagnosing hepatocellular carcinoma and pancreatic cancer |
| US11421028B2 (en) | 2016-02-06 | 2022-08-23 | Epimab Biotherapeutics, Inc. | Fabs-in-tandem immunoglobulin and uses thereof |
| WO2017136820A2 (en) | 2016-02-06 | 2017-08-10 | Epimab Biotherapeutics, Inc. | Fabs-in-tandem immunoglobulin and uses thereof |
| WO2017218911A1 (en) | 2016-06-17 | 2017-12-21 | Abbott Laboratories | BIOMARKERS TO PREDICT NEW ONSET HEART FAILURE WITH PRESERVED EJECTION FRACTION (HFpEF) |
| US10720255B2 (en) | 2016-07-06 | 2020-07-21 | Clemson University | Radiation delivery devices and applications thereof |
| EP3279667A1 (en) | 2016-08-02 | 2018-02-07 | Abbott Laboratories | Cardiac troponin i and copeptin as biomarkers for short-term mortality in acute exacerbation of chronic obstructive pulmonary disease (aecopd) |
| EP4521116A2 (en) | 2016-10-03 | 2025-03-12 | Abbott Laboratories | Improved methods of assessing uch-l1 status in patient samples |
| WO2018067474A1 (en) | 2016-10-03 | 2018-04-12 | Abbott Laboratories | Improved methods of assessing gfap status in patient samples |
| WO2018067468A1 (en) | 2016-10-03 | 2018-04-12 | Abbott Laboratories | Improved methods of assessing uch-l1 status in patient samples |
| WO2018175942A1 (en) | 2017-03-23 | 2018-09-27 | Abbott Laboratories | Methods for aiding in the diagnosis and determination of the extent of traumatic brain injury in a human subject using the early biomarker ubiquitin carboxy-terminal hydrolase l1 |
| WO2018191531A1 (en) | 2017-04-15 | 2018-10-18 | Abbott Laboratories | Methods for aiding in the hyperacute diagnosis and determination of traumatic brain injury in a human subject using early biomarkers |
| WO2018200823A1 (en) | 2017-04-28 | 2018-11-01 | Abbott Laboratories | Methods for aiding in the hyperacute diagnosis and determination of traumatic brain injury using early biomarkers on at least two samples from the same human subject |
| WO2018218169A1 (en) | 2017-05-25 | 2018-11-29 | Abbott Laboratories | Methods for aiding in the determination of whether to perform imaging on a human subject who has sustained or may have sustained an injury to the head using early biomarkers |
| WO2018222784A1 (en) | 2017-05-30 | 2018-12-06 | Abbott Laboratories | Methods for aiding in diagnosing and evaluating a mild traumatic brain injury in a human subject using cardiac troponin i |
| WO2018222783A1 (en) | 2017-05-30 | 2018-12-06 | Abbott Laboratories | Methods for aiding in diagnosing and evaluating a mild traumatic brain injury in a human subject using cardiac troponin i and early biomarkers |
| WO2019010131A1 (en) | 2017-07-03 | 2019-01-10 | Abbott Laboratories | Improved methods for measuring ubiquitin carboxy-terminal hydrolase l1 levels in blood |
| WO2019113525A2 (en) | 2017-12-09 | 2019-06-13 | Abbott Laboratories | Methods for aiding in the diagnosis and evaluation of a subject who has sustained an orthopedic injury and that has or may have sustained an injury to the head, such as mild traumatic brain injury (tbi), using glial fibrillary acidic protein (gfap) and/or ubiquitin carboxy-terminal hydrolase l1 (uch-l1) |
| WO2019112860A1 (en) | 2017-12-09 | 2019-06-13 | Abbott Laboratories | Methods for aiding in diagnosing and evaluating a traumatic brain injury in a human subject using a combination of gfap and uch-l1 |
| WO2019133717A1 (en) | 2017-12-29 | 2019-07-04 | Abbott Laboratories | Novel biomarkers and methods for diagnosing and evaluating traumatic brain injury |
| CN108535491A (en)* | 2018-03-22 | 2018-09-14 | 北京九强生物技术股份有限公司 | A kind of latex enhancing immune of Troponin I is than turbid detection kit |
| US10871485B2 (en) | 2018-04-13 | 2020-12-22 | Rarecyte, Inc. | Kits for labeling of biomarkers and methods of using the same |
| WO2019211665A1 (en) | 2018-04-30 | 2019-11-07 | Takeda Pharmaceutical Company Limited | Cannabinoid receptor type 1 (cb1) binding proteins and uses thereof |
| US12060425B2 (en) | 2018-05-03 | 2024-08-13 | Shanghai Epimab Biotherapeutics Co., Ltd. | High affinity antibodies to PD-1 and LAG-3 and bispecific binding proteins made therefrom |
| WO2019213619A1 (en) | 2018-05-04 | 2019-11-07 | Abbott Laboratories | Hbv diagnostic, prognostic, and therapeutic methods and products |
| CN108918883A (en)* | 2018-05-18 | 2018-11-30 | 瑞莱生物科技江苏有限公司 | A kind of immunofluorescent reagent box of rapid quantitative detection cardiac muscle troponin I, creatine kinase isozyme and myoglobins |
| US11079395B2 (en) | 2019-03-01 | 2021-08-03 | Abbott Laboratories | Methods for predicting major adverse cardiovascular events in subjects with coronary artery disease |
| WO2020180695A1 (en) | 2019-03-01 | 2020-09-10 | Abbott Laboratories | Methods for predicting major adverse cardiovascular events in subjects with coronary artery disease |
| US20220187330A1 (en)* | 2019-04-16 | 2022-06-16 | Aligned Genetics, Inc. | Analyte collecting device, and analyte collecting method and analyte inspection system using same |
| WO2021026403A1 (en) | 2019-08-07 | 2021-02-11 | Abbott Laboratories | Methods for detecting assay interferents and increasing dynamic range |
| WO2021061272A1 (en) | 2019-09-26 | 2021-04-01 | Pacesetter, Inc. | Method and device for managing energy usage by a medical device |
| CN111308084A (en)* | 2019-12-30 | 2020-06-19 | 菲鹏生物股份有限公司 | A kind of high-sensitivity cardiac troponin I detection method and kit |
| CN111308085A (en)* | 2019-12-30 | 2020-06-19 | 菲鹏生物股份有限公司 | A kind of high-sensitivity cardiac troponin I detection method and kit |
| WO2021211331A1 (en) | 2020-04-13 | 2021-10-21 | Abbott Point Of Care Inc. | METHODS, COMPLEXES AND KITS FOR DETECTING OR DETERMINING AN AMOUNT OF A ß-CORONAVIRUS ANTIBODY IN A SAMPLE |
| EP3906843A1 (en) | 2020-05-08 | 2021-11-10 | Pacesetter, Inc. | System for verifying a pathologic episode using an accelerometer |
| US12365729B2 (en) | 2020-05-13 | 2025-07-22 | Disc Medicine, Inc. | Anti-hemojuvelin (HJV) antibodies for treating myelofibrosis |
| WO2022029494A1 (en) | 2020-08-04 | 2022-02-10 | Abbott Rapid Diagnostics International Unlimited Company | Assays for detecting sars-cov-2 |
| US11988671B2 (en) | 2020-08-04 | 2024-05-21 | Abbott Rapid Diagnostics International Unlimited Company | Assays for detecting SARS-CoV-2 |
| WO2022031804A1 (en) | 2020-08-04 | 2022-02-10 | Abbott Laboratories | Improved methods and kits for detecting sars-cov-2 protein in a sample |
| CN111781385A (en)* | 2020-08-19 | 2020-10-16 | 武汉生之源生物科技股份有限公司 | NT-proBNP detection kit and preparation method thereof |
| EP3991787A1 (en) | 2020-10-30 | 2022-05-04 | Pacesetter, Inc. | Implantable medical device for managing a physical layer utilized during a wireless connection |
| US12192285B2 (en) | 2020-10-30 | 2025-01-07 | Abbott Diabetes Care Inc. | Method for managing a physical layer utilized during a wireless connection with medical devices |
| WO2022119841A1 (en) | 2020-12-01 | 2022-06-09 | Abbott Laboratories | Use of one or more biomarkers to determine traumatic brain injury (tbi) in a subject having received a head computerized tomography scan that is negative for a tbi |
| CN112710855A (en)* | 2020-12-15 | 2021-04-27 | 深圳天辰医疗科技有限公司 | BNP detection kit and BNP detection method |
| WO2022147178A1 (en) | 2020-12-30 | 2022-07-07 | Abbott Laboratories | Improved methods, reagents and kits for detergent-based inactivation of betacoronavirus prior to and/or while assessing a biological sample for sars-cov-2 antigen or antibody |
| WO2022147147A1 (en) | 2020-12-30 | 2022-07-07 | Abbott Laboratories | Methods for determining sars-cov-2 antigen and anti-sars-cov-2 antibody in a sample |
| WO2022245920A1 (en) | 2021-05-18 | 2022-11-24 | Abbott Laboratories | Methods of evaluating brain injury in a pediatric subject |
| WO2022266034A1 (en) | 2021-06-14 | 2022-12-22 | Abbott Laboratories | Methods of diagnosing or aiding in diagnosis of brain injury caused by acoustic energy, electromagnetic energy, an over pressurization wave, and/or blast wind |
| EP4528280A2 (en) | 2021-06-14 | 2025-03-26 | Abbott Laboratories | Methods of diagnosing or aiding in diagnosis of brain injury caused by acoustic energy, electromagnetic energy, an over pressurization wave, and/or blast wind |
| EP4111948A1 (en) | 2021-06-28 | 2023-01-04 | Pacesetter, Inc. | Method and system for optimizing filter settings of an implantable medical device |
| WO2023028186A1 (en) | 2021-08-27 | 2023-03-02 | Abbott Laboratories | Methods for detecting immunoglobulin g, subclass 4 (igg4) in a biological sample |
| WO2023034777A1 (en) | 2021-08-31 | 2023-03-09 | Abbott Laboratories | Methods and systems of diagnosing brain injury |
| WO2023056268A1 (en) | 2021-09-30 | 2023-04-06 | Abbott Laboratories | Methods and systems of diagnosing brain injury |
| WO2023102384A1 (en) | 2021-11-30 | 2023-06-08 | Abbott Laboratories | Use of one or more biomarkers to determine traumatic brain injury (tbi) in a subject having received a head computerized tomography scan that is negative for a tbi |
| WO2023129942A1 (en) | 2021-12-28 | 2023-07-06 | Abbott Laboratories | Use of biomarkers to determine sub-acute traumatic brain injury (tbi) in a subject having received a head computerized tomography (ct) scan that is negative for a tbi or no head ct scan |
| CN114544978A (en)* | 2022-03-04 | 2022-05-27 | 美康生物科技股份有限公司 | IGF-1 detection kit, preparation method and detection method thereof |
| EP4268711A1 (en) | 2022-04-28 | 2023-11-01 | Pacesetter, Inc. | System for determining change in position of an implanted medical device within an implant pocket |
| WO2024044288A1 (en) | 2022-08-26 | 2024-02-29 | Abbott Laboratories | Use of cardiac troponin and galectin-3 to differentiate myocardial infarction type i and type ii |
| WO2024059708A1 (en) | 2022-09-15 | 2024-03-21 | Abbott Laboratories | Biomarkers and methods for differentiating between mild and supermild traumatic brain injury |
| WO2024059692A1 (en) | 2022-09-15 | 2024-03-21 | Abbott Laboratories | Hbv diagnostic, prognostic, and therapeutic methods and products |
| WO2024102383A1 (en) | 2022-11-10 | 2024-05-16 | Abbott Laboratories | Methods of identifying macrotroponin in biological samples |
| WO2024211475A1 (en) | 2023-04-04 | 2024-10-10 | Abbott Laboratories | Use of biomarkers to determine whether a subject has sustained, may have sustained or is suspected of sustaining a subacute acquired brain injury (abi) |
| WO2024226969A1 (en) | 2023-04-28 | 2024-10-31 | Abbott Point Of Care Inc. | Improved assays, cartridges, and kits for detection of biomarkers, including brain injury biomarkers |
| WO2024226971A1 (en) | 2023-04-28 | 2024-10-31 | Abbott Point Of Care Inc. | Improved assays, cartridges, and kits for detection of biomarkers, including brain injury biomarkers |
| WO2024226899A1 (en) | 2023-04-28 | 2024-10-31 | Abbott Laboratories | Diagnosis of late-stage hepatocellular carcinoma |
| WO2025085348A1 (en) | 2023-10-16 | 2025-04-24 | Abbott Laboratories | Methods and systems for diagnosing orthopoxvirus infection |
| WO2025136783A1 (en) | 2023-12-21 | 2025-06-26 | Tc1 Llc | Methods and systems for displaying healthcare metrics associated with patient populations |
| WO2025193825A1 (en) | 2024-03-13 | 2025-09-18 | Abbott Laboratories | Diagnosis and monitoring of liver disease |
Also Published As
| Publication number | Publication date |
|---|---|
| US9995744B2 (en) | 2018-06-12 |
| WO2004011947A1 (en) | 2004-02-05 |
| US20160123976A1 (en) | 2016-05-05 |
| US9267939B2 (en) | 2016-02-23 |
| EP1525480A1 (en) | 2005-04-27 |
| US10641767B2 (en) | 2020-05-05 |
| EP1525480B1 (en) | 2017-09-13 |
| US20140141484A1 (en) | 2014-05-22 |
| US20180259511A1 (en) | 2018-09-13 |
| JP4507879B2 (en) | 2010-07-21 |
| JP2005534907A (en) | 2005-11-17 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| US10641767B2 (en) | Multiple hybrid immunoassay | |
| EP1591789B1 (en) | Method for improving the recovery of Troponin I and T | |
| KR920005963B1 (en) | Method for the determination of a specific binding substance | |
| US4894347A (en) | Erythrocyte agglutination assay | |
| US20020106708A1 (en) | Assays reagents and kits for detecting or determining the concentration of analytes | |
| US5362655A (en) | Process for the determination of a specifically bindable substance | |
| US5814461A (en) | Method for the determination of anti-TSH receptor autoantibodies | |
| JP2001505999A (en) | Receptor binding assay for detecting TSH receptor autoantibodies | |
| US5437981A (en) | Method for the immunological determination of ligands | |
| US5620861A (en) | Method and kit for pyridinium crosslink assay | |
| JPH0646893A (en) | Method and means for measuring analyte | |
| US5639627A (en) | Method for assaying specific antibody | |
| JPH02124462A (en) | Improved immunoassay | |
| JPS6223823B2 (en) | ||
| EP0125368A1 (en) | A method of immunoassay detection by reaction-rate potentiometry using fluoride ion-selective electrode | |
| EP0188608A1 (en) | Two site enzyme labeled cross reaction immunometric sandwich assay method | |
| JPH10319017A (en) | Measuring method for substance utilizing fluorescent energy transfer and reagent therefor | |
| JPS61243363A (en) | Highly sensitive assay of crp | |
| KR940008091B1 (en) | Immunological Detection of Ligands | |
| AU7721094A (en) | Immobilization of chemically cross-linked proteins on solid supports | |
| JP2006105910A (en) | Target substance measurement method and reagent | |
| JPH08114595A (en) | Specific binding assay | |
| JP2025006951A (en) | Hapten measurement method | |
| JP2000235032A (en) | Immunoassey and dry immunoassey reagent | |
| Ricchiuti | IMMUNOASSAY-BASED TECHNOL-OGIES F OR THE MEASUREMENT OF BIOLOGICAL MATERIALS USED FOR BIOMARKERS DISCOVERY AND TRANSLATIONAL RESEARCH |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| AS | Assignment | Owner name:I-STAT CORPORATION, NEW JERSEY Free format text:ASSIGNMENT OF ASSIGNORS INTEREST;ASSIGNORS:CAMPBELL, JOHN LEWIS EMERSON;FRANK, PAMELA ANNE;HAWKES, MEGHAN ELIZABETH;AND OTHERS;REEL/FRAME:014265/0566;SIGNING DATES FROM 20030623 TO 20030625 | |
| AS | Assignment | Owner name:ABBOTT POINT OF CARE INC., NEW JERSEY Free format text:CHANGE OF NAME;ASSIGNOR:I-STAT CORPORATION;REEL/FRAME:022416/0268 Effective date:20060914 Owner name:ABBOTT POINT OF CARE INC.,NEW JERSEY Free format text:CHANGE OF NAME;ASSIGNOR:I-STAT CORPORATION;REEL/FRAME:022416/0268 Effective date:20060914 | |
| STCB | Information on status: application discontinuation | Free format text:ABANDONED -- FAILURE TO RESPOND TO AN OFFICE ACTION |