US20040015224A1 - Endoluminal expansion system - Google Patents
Endoluminal expansion systemDownload PDFInfo
- Publication number
- US20040015224A1 US20040015224A1US10/201,172US20117202AUS2004015224A1US 20040015224 A1US20040015224 A1US 20040015224A1US 20117202 AUS20117202 AUS 20117202AUS 2004015224 A1US2004015224 A1US 2004015224A1
- Authority
- US
- United States
- Prior art keywords
- sheath
- endoprosthesis
- expansion system
- substantially tubular
- protrusion
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Abandoned
Links
- 238000003780insertionMethods0.000claimsabstractdescription10
- 230000037431insertionEffects0.000claimsabstractdescription10
- 239000000463materialSubstances0.000claimsdescription16
- 230000006835compressionEffects0.000claimsdescription14
- 238000007906compressionMethods0.000claimsdescription14
- 238000000034methodMethods0.000claimsdescription13
- 229920000295expanded polytetrafluoroethylenePolymers0.000claimsdescription12
- 238000004806packaging method and processMethods0.000claimsdescription7
- 230000000977initiatory effectEffects0.000claimsdescription5
- 210000004204blood vesselAnatomy0.000abstractdescription3
- 239000000835fiberSubstances0.000description10
- 230000007246mechanismEffects0.000description8
- 229920000139polyethylene terephthalatePolymers0.000description7
- 239000005020polyethylene terephthalateSubstances0.000description7
- 230000008439repair processEffects0.000description6
- 239000004952PolyamideSubstances0.000description5
- 229920002647polyamidePolymers0.000description5
- 229920001343polytetrafluoroethylenePolymers0.000description4
- 239000004810polytetrafluoroethyleneSubstances0.000description4
- 238000002513implantationMethods0.000description3
- 229910001220stainless steelInorganic materials0.000description3
- 238000002560therapeutic procedureMethods0.000description3
- 230000002792vascularEffects0.000description3
- 239000004812Fluorinated ethylene propyleneSubstances0.000description2
- 239000000853adhesiveSubstances0.000description2
- 230000001070adhesive effectEffects0.000description2
- 230000002411adverseEffects0.000description2
- 238000013459approachMethods0.000description2
- 230000008901benefitEffects0.000description2
- 239000002131composite materialSubstances0.000description2
- 238000010276constructionMethods0.000description2
- 239000003814drugSubstances0.000description2
- HQQADJVZYDDRJT-UHFFFAOYSA-Nethene;prop-1-eneChemical groupC=C.CC=CHQQADJVZYDDRJT-UHFFFAOYSA-N0.000description2
- 238000010438heat treatmentMethods0.000description2
- 230000023597hemostasisEffects0.000description2
- 238000012986modificationMethods0.000description2
- 230000004048modificationEffects0.000description2
- 229920009441perflouroethylene propylenePolymers0.000description2
- 229920000642polymerPolymers0.000description2
- 229940124597therapeutic agentDrugs0.000description2
- 230000001225therapeutic effectEffects0.000description2
- 230000032258transportEffects0.000description2
- 210000005166vasculatureAnatomy0.000description2
- 208000031481Pathologic ConstrictionDiseases0.000description1
- 239000004830Super GlueSubstances0.000description1
- 230000004913activationEffects0.000description1
- 210000000013bile ductAnatomy0.000description1
- 210000001124body fluidAnatomy0.000description1
- 239000010839body fluidSubstances0.000description1
- 239000011248coating agentSubstances0.000description1
- 238000000576coating methodMethods0.000description1
- 238000005520cutting processMethods0.000description1
- 238000013461designMethods0.000description1
- 238000006073displacement reactionMethods0.000description1
- FGBJXOREULPLGL-UHFFFAOYSA-Nethyl cyanoacrylateChemical compoundCCOC(=O)C(=C)C#NFGBJXOREULPLGL-UHFFFAOYSA-N0.000description1
- 230000002068genetic effectEffects0.000description1
- 238000004519manufacturing processMethods0.000description1
- 239000000155meltSubstances0.000description1
- 239000002184metalSubstances0.000description1
- RVTZCBVAJQQJTK-UHFFFAOYSA-Noxygen(2-);zirconium(4+)Chemical compound[O-2].[O-2].[Zr+4]RVTZCBVAJQQJTK-UHFFFAOYSA-N0.000description1
- 239000004033plasticSubstances0.000description1
- 229920003023plasticPolymers0.000description1
- -1polyethylene terephthalatePolymers0.000description1
- 230000002028prematureEffects0.000description1
- 230000002787reinforcementEffects0.000description1
- 238000000926separation methodMethods0.000description1
- 230000002269spontaneous effectEffects0.000description1
- 239000010935stainless steelSubstances0.000description1
- 239000000126substanceSubstances0.000description1
- 238000011282treatmentMethods0.000description1
- 238000012800visualizationMethods0.000description1
- 238000003466weldingMethods0.000description1
Images
Classifications
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F2/00—Filters implantable into blood vessels; Prostheses, i.e. artificial substitutes or replacements for parts of the body; Appliances for connecting them with the body; Devices providing patency to, or preventing collapsing of, tubular structures of the body, e.g. stents
- A61F2/95—Instruments specially adapted for placement or removal of stents or stent-grafts
- A61F2/958—Inflatable balloons for placing stents or stent-grafts
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F2/00—Filters implantable into blood vessels; Prostheses, i.e. artificial substitutes or replacements for parts of the body; Appliances for connecting them with the body; Devices providing patency to, or preventing collapsing of, tubular structures of the body, e.g. stents
- A61F2/95—Instruments specially adapted for placement or removal of stents or stent-grafts
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F2/00—Filters implantable into blood vessels; Prostheses, i.e. artificial substitutes or replacements for parts of the body; Appliances for connecting them with the body; Devices providing patency to, or preventing collapsing of, tubular structures of the body, e.g. stents
- A61F2/95—Instruments specially adapted for placement or removal of stents or stent-grafts
- A61F2/962—Instruments specially adapted for placement or removal of stents or stent-grafts having an outer sleeve
- A61F2/97—Instruments specially adapted for placement or removal of stents or stent-grafts having an outer sleeve the outer sleeve being splittable
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F2/00—Filters implantable into blood vessels; Prostheses, i.e. artificial substitutes or replacements for parts of the body; Appliances for connecting them with the body; Devices providing patency to, or preventing collapsing of, tubular structures of the body, e.g. stents
- A61F2/95—Instruments specially adapted for placement or removal of stents or stent-grafts
- A61F2002/9505—Instruments specially adapted for placement or removal of stents or stent-grafts having retaining means other than an outer sleeve, e.g. male-female connector between stent and instrument
- A61F2002/9511—Instruments specially adapted for placement or removal of stents or stent-grafts having retaining means other than an outer sleeve, e.g. male-female connector between stent and instrument the retaining means being filaments or wires
Definitions
- the present inventionrelates to the transcatheter delivery and remote deployment of implantable medical devices and more particularly to a system for the expansion and deployment of endoprostheses.
- Endoluminal therapiestypically involve the insertion of a delivery catheter that transports an implantable prosthetic device into a body conduit through a small, often percutaneous, remote access site. Once access to the body conduit is achieved, the delivery catheter is used to mediate intraluminal delivery and subsequent deployment of the prosthesis via one of several techniques. In this fashion, the prosthesis can be remotely implanted to achieve a therapeutic outcome.
- endoluminal treatmentsare distinguished by their “minimally invasive” nature.
- Self-expanding endoprosthesesare generally comprised of a stent component with or without a graft covering over the stent interstices. They are designed to spontaneous dilate (i.e., elastically recover) from their delivery diameter, through a range of intermediary diameters, up to a maximal, pre-determined functional diameter.
- the endoluminal delivery and deployment of self-expanding endoprosthesespose several unique problems. First, the endoprosthesis itself must be radially compacted to a suitable introductory size (or delivery diameter) to allow insertion into the vasculature, then it must be constrained in that compacted state and mounted onto a delivery device such as a catheter shaft.
- the constraintmust be removed in order to allow the endoprosthesis to expand to its functional diameter and achieve the desired therapeutic outcome.
- the means of constraintwill not adversely affect the delivery catheter performance (e.g., detracting from the flexibility of the delivery system) or add significantly to introductory profile.
- the constraintmust also incorporate some type of release mechanism or scheme that can be remotely actuated by the implanting clinician. Consequently, deployment methodologies that are consistent with conventional interventional practices are preferred.
- Delivery mechanisms for self-expanding endoprostheses of the prior artmay be generally classified into one of two general categories, either coaxial sheaths or fiber-based constraints. Delivery systems also exist that use both of these types of mechanisms in combination.
- tubular coaxial sheathsare one approach used to constrain the compacted self-expanding endoprosthesis. Normally, these coaxial sheaths extend over the entire length of an inner delivery catheter onto which the endoprosthesis is mounted near the catheter tip (i.e., leading end). Deployment is typically initiated by pulling on a handle or knob located near the hub (i.e., trailing end) of the catheter, which retracts the constraining sheath and allows the device to expand. During this procedure, the clinician maintains the position of the device by holding the inner (delivery) catheter in a stationary position.
- tubular coaxial sheath type of delivery systemExisting problems and/or complications with the tubular coaxial sheath type of delivery system include friction between compacted device and constraining sheath, friction between the constraining sheath and delivery catheter, and friction between the delivery catheter and constraining sheath hemostasis valve, all of which can hinder deployment accuracy, speed and control. Additionally, a tubular coaxial constraining sheath can also reduce flexibility and add introductory profile due to the thickness of the constraining sheath.
- the self-expanding endoprosthesisis constrained in the delivery profile by one or more removable fibrous strands, with or without an additional implantable constraint element.
- the endoprosthesisis released from its compacted-state through tension applied to a deployment “cord” that normally runs through an additional lumen within the delivery catheter.
- tension applied to the deployment cordinitiates the release of the fiber constraint by, for example, unlacing linear slip knots (see Lau, et al., U.S. Pat. No. 5,919,225), removing circumferential croquet knots (e.g., Strecker, U.S. Pat. No.
- Another variant of the fiber-based delivery systemsis the mechanism employed in the EXCLUDER® endoprosthesis marketed by W. L. Gore and Associates, Inc (Flagstaff, Ariz.).
- This mechanismentails a “chain-stitch” sewn into the seam of a biocompatible constraining tube that contains the compacted endoprosthesis. Applying tension to the fibrous constraint in this mechanism allows the seam in the biocompatible constraining tube to be open, and the self-expanding endoprosthesis to deploy.
- the biocompatible constraining tubeis implanted along with the endoprosthesis, trapped between the abluminal surface of the device and the wall of the host vessel. See WO98/27894.
- U.S. Pat. Nos. 5,755,769 and 6,019,787 to Richard et al.teach another constraining sheath around a self-expanding stent.
- the sheathis cut longitudinally into several segments by cutting wires or fibers actuated by pulling a handle at the opposite end of the delivery system.
- the sheathis attached to or integral to the delivery catheter with the result that the segments are removed with the catheter following stent deployment.
- No catheter balloon or other means for exerting a circumferential disrupting force to the sheathis suggested, nor are materials appropriate for the sheath suggested. This design requires lines to run over the length of the catheter.
- U.S. Pat. No. 6,086,610 to Duerig et al.teaches a self-expanding stent provided with a tubular constraining sheath that is plastically deformable by a circumferential distending force such as a catheter balloon.
- This sheathremains implanted with the stent following deployment and fully covers the entire circumference of the stent in the fashion of a conventional stent covering, i.e., the tubular sheath is not disrupted.
- the Duerig et al. deviceis delivered from a conventional balloon catheter, but thought to have limitations, including radial recoil of the sheath after the balloon is pressurized and deflated, which can compromise luminal gain. Further, the presence of the cover may adversely affect the ability of the stent to fully deploy, and the balloon length must be equal to or longer than the stent, and this long balloon can potentially damage the vessel.
- the present inventionrelates to an endoprosthesis expansion system comprising, in combination, a delivery component such as a length of catheter tubing having at its distal end an intermediate sheath component, and an inner elongate actuation member that is preferably an inner tube located within the full length of the delivery catheter and intermediate sheath component.
- the inner elongate actuation membere.g., inner tube
- the inner elongate actuation memberhas a protrusion affixed to its distal end, and an expandable endoprosthesis is fitted in a compacted state about the intermediate sheath, proximal to the protrusion.
- the endoprosthesisis a self-expanding endoprosthesis (as is preferred)
- an exterior constraining sheathis required around the outer surface of the endoprosthesis to contain the endoprosthesis in a compacted configuration.
- the endoprosthesisis deployed by axially moving the protrusion through the system, thereby applying a radially directed outward force and causing simultaneous dilatation of the intermediate sheath and disruption of the exterior constraining sheath.
- axial movement of the elongate actuation member against the end of the intermediate sheath, applying axial compression to the intermediate sheathmay cause the intermediate sheath to shorten and simultaneously increase in diameter, thereby initiating expansion and deployment of the endoprosthesis.
- Disruption of the exterior constraining sheathin the case of a self-expanding prosthesis, releases the stored energy in the formerly constrained prosthesis, allowing it to spontaneously expand and accomplish full deployment against the luminal surface of the body conduit at the desired site.
- the exterior constraining sheathis preferably made of an implantable material and may be left captured between the endoprosthesis and the luminal surface of the body conduit. Alternatively, the exterior constraining sheath may be secured to the adjacent delivery catheter and withdrawn from between the endoprosthesis and the wall of the body conduit when the delivery catheter is withdrawn.
- a non-self-expanding endoprosthesise.g., a balloon-expandable stent
- diametrical expansionmay be accomplished by moving the protrusion axially through the stent, thereby enlarging the diameter by plastically deforming the stent.
- the application of axial compression against one end of the intermediate sheath by the protrusioncan cause an increase in the diameter of the intermediate sheath, forcing a corresponding diametrical increase in the balloon expandable stent.
- the endoprostheses utilized with the present inventionmay also be stent-grafts.
- stent-graftis used herein to describe a stent provided with a covering, typically of a vascular graft material such as porous expanded polytetrafluoroethylene (ePTFE) or polyethylene terephthalate (PET).
- ePTFEporous expanded polytetrafluoroethylene
- PETpolyethylene terephthalate
- the coveringmay be provided over either or both of the inner and outer surfaces of the stent.
- the coveringmay cover a portion of the otherwise open stent interstices or it may cover all of the stent interstices.
- system of the present inventionis intended primarily for stents and stent-grafts for use in vascular repairs, it is also useful for expandable devices for other applications in other body conduits, e.g., esophageal or biliary duct repairs.
- a protrusioncan be used to initiate deployment without an intermediate sheath inside the endoprosthesis
- the use of the intermediate sheathmade from a thin, strong and lubricious material, prevents the protrusion from damaging the endoprosthesis (particularly if the endoprosthesis is a stent-graft with a covering on the luminal surface). It also reduces the likelihood of “bunching” of the endoprosthesis due to the application of an axial force.
- Both the exterior constraining sheath and the intermediate sheathmay be made to be dilatable or disruptable by various and similar means.
- the exterior constraining sheathit is preferred to provide a line of perforations partially or entirely through the wall of the tubular constraining sheath, parallel to the longitudinal axis of the tubular constraining sheath.
- the constraining sheathmay be caused to disrupt by splitting along this line of perforations, upon the application of an outwardly directed radial force from within the sheath (and within the contained endoprosthesis).
- the intermediate sheath located within the endoprosthesisit is preferred that it is of a substantially tubular form and is dilatable via one or more, equally radially-spaced apart splits are used along the length of that sheath.
- the intermediate sheathmay be elastically or plastically deformable by the protrusion.
- the intermediate sheathmay be caused to be split, ripped, torn or otherwise changed in proportion by the movement of the protrusion against and/or through the intermediate sheath. Any of these mechanisms are considered to constitute dilatation of the intermediate sheath.
- the tubular form of the intermediate sheathincludes various embodiments and as such is considered to be a substantially tubular sheath.
- the present inventionalso provides a means of controlling the radial dynamics of device deployment.
- the present inventioncan be configured to ‘pop’ open to allow rapid device deployment, or alternatively to undergo more gradual, controlled, stepwise release during device deployment, or a combination of both.
- the constraining sheathmay be imbibed with various pharmaceutical, biological, or genetic therapies for targeted luminal delivery of these substances. Following deployment of the endoprosthesis, these therapeutic agents can be released over time.
- An advantage of this approachis that the loading of the sheath with any of these therapeutic agents can be performed independent of the endoprosthesis manufacture.
- radiopaque elementsmay be incorporated into the constraining sheath (or other system components, notably the catheter tubes) to facilitate fluoroscopic visualization.
- the present inventionmay also be used to deliver and deploy multiple devices positioned in sequential order on the delivery catheter.
- the constraining sheathcan be made to be extremely thin, or “delicate,” for minimal implantation profile.
- a delicate constraining sheathis not adequate, without further exterior support, to constrain the endoprosthesis assembly (particularly when the assembly includes a self-expanding endoprosthesis) for very long periods of time or for shorter periods when exposed to elevated temperatures.
- the use of such a delicate constraining sheathis made practically possible when the assembly is provided with an additional tubular packaging sheath that prevents inadvertent disruption of the constraining sheath or undesirable increase in diameter of the assembly (e.g., in an amount of 0.15 mm or more).
- the tubular packaging sheathfitted coaxially about the exterior of the “delicate” constraining sheath, is removed prior to implantation and accordingly is not required to be made of an implantable material or a material with a thin wall.
- the endoprosthesis assemblymay incorporate such a delicate constraining sheath without the use of a packaging sheath if it is stored at reduced temperatures, such as 5° C. or less, prior to implantation.
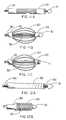
- FIG. 1is a longitudinal cross section of a compacted and constrained endoprosthesis mounted on an intermediate sheath, which is mounted upon an inner tube incorporating a protrusion at the distal end that, when moved axially through or against the intermediate sheath and endoprosthesis, applies a force directed radially outward to initiate deployment of the endoprosthesis.
- FIG. 2Ais a transverse cross sectional view taken at section A-A of FIG. 1.
- FIG. 2Bis a transverse cross sectional view taken at section A-A of FIG. 1 wherein, in an alternative embodiment, the intermediate sheath is split in one place, allowing it to enlarge diametrically when the protrusion of FIG. 1 is passed through it or applies compression against the end of the intermediate sheath.
- FIG. 2Cis a transverse cross sectional view taken at section A-A of FIG. 1 wherein, in another alternative, the intermediate sheath is split and coiled upon itself (in jelly-roll fashion).
- FIG. 2Dis a transverse cross sectional view taken at section A-A of FIG. 1 wherein, in another alternative, the intermediate sheath is split in several places.
- FIG. 2Eis a transverse cross section of a preferred constraining sheath construction.
- FIG. 3is a longitudinal cross section of a compacted and constrained endoprosthesis describing a preferred embodiment of the system of the present invention.
- FIG. 4Ais a longitudinal cross section of the endoprosthesis being deployed within a body conduit.
- FIG. 4Bis an end view of endoprosthesis deployment depicted in FIG. 4A.
- FIGS. 5 A and 5 C- 5 Hare longitudinal cross-sections of the inner tube and protrusion, describing various embodiments of the protrusion.
- FIG. 5Bis a transverse cross-sectional view taken at section B-B of FIG. 5A of the protrusion of FIG. 1, describing an embodiment wherein the transverse cross-section of the protrusion is not round.
- FIG. 6Ais a longitudinal cross-section of the protrusion of FIG. 1, wherein the protrusion is attached to a guide wire.
- FIG. 6Bis a longitudinal cross-section of the protrusion of FIG. 1, wherein the protrusion is integral to a guide wire.
- FIG. 6Cis a longitudinal cross-section of the protrusion of FIG. 1, wherein the protrusion is attached to an elongate tensile member, such as a fiber, strand or wire.
- an elongate tensile membersuch as a fiber, strand or wire.
- FIG. 7Ais a longitudinal cross section of a compacted and constrained endoprosthesis incorporating a tether element with an enlargement at the distal end of the tether element, intended to prevent unintentional axial movement of the endoprosthesis.
- FIG. 7Bis a transverse cross-section taken at section C-C of FIG. 7 describing the tether element.
- FIGS. 8 A- 8 Care longitudinal cross sections describing alternative embodiments of the tether element.
- FIG. 9is a longitudinal cross section of a compacted and constrained endoprosthesis incorporating a distensible collar positioned at the end of the endoprosthesis and intended to prevent unintentional axial movement of the endoprosthesis
- FIG. 10is a transverse cross sectional view taken at section D-D of FIG. 9, describing the distensible collar element.
- FIGS. 11 A- 11 C, 12 A and 12 Bare perspective views describing alternative embodiments of the present invention, primarily with regard to variations of the substantially tubular, intermediate sheath.
- FIG. 1is a longitudinal cross section of the endoprosthesis expansion system 10 of the present invention.
- the system 10has a proximal end 10 a and a distal end 10 b , wherein proximal end 10 a is considered to be the end from which a delivery catheter extends to a site at which the system was originally inserted into a body conduit.
- Endoprosthesis 11is indicative of any type of endoluminal medical device which might be usefully contained at a smaller diameter for insertion into a body conduit and subsequently deployed to a larger diameter at a desired location within the body conduit.
- the endoprosthesisis preferably a self-expanding device, and is most preferably a stent of the self-expanding type.
- stentsWhile these stents are most commonly used for repair of the vasculature (e.g., repair of stenoses or aneurysmal repair), they are also used for other applications in other body conduits (e.g., esophageal or bile duct repairs).
- These self-expanding stentsare typically of nitinol wire although other materials may be used, e.g., stainless steels or polymeric materials including resorbable polymers.
- balloon expandable devicesmay be expanded with the present inventive system, without requiring an exterior constraining sheath as do self-expanding devices.
- a self expanding device in use as a part of the present endoprosthesis expansion systemis provided with an exterior constraining sheath to retain the self-expanding endoprosthesis at its small, compacted diameter at which it is intended to be inserted into a body conduit for subsequent expansion and deployment.
- the constraining sheathis disrupted by activation of the expansion and deployment mechanism of the present invention.
- the constraining sheathis preferably made from an implantable material, most preferably a tube of porous expanded PTFE (hereinafter ePTFE), made generally as taught by U.S. Pat. Nos. 3,953,566 and 4,187,390 to Gore.
- the tubeis most preferably provided with a line of perforations through the wall, parallel to the length of the tube.
- the line of perforationsprovides a yield point along which the constraining sheath will disrupt by splitting. Forcibly expanding the endoprosthesis in a radially outward direction, using force applied from within the lumen of the endoprosthesis, causes the perforation line to disrupt, initiating expansion and deployment of the self-expanding endoprosthesis.
- the disrupted, now-split constraining sheathbeing of an implantable material such as ePTFE, preferably remains implanted with the deployed endoprosthesis, held captive between the endoprosthesis and the wall of the body conduit at the site of deployment.
- the implantable constraining sheathmay optionally be attached to the exterior surface of the self-expanding stent, preferably along an axially-oriented line that is 180° opposite the line of perforations.
- the constraining sheathcan be configured to be removable following deployment of the endoprosthesis, by having previously secured it to a component of the delivery system such as a catheter shaft and withdrawing it from between the endoprosthesis and the wall of the body conduit when the catheter is withdrawn.
- the endoprosthesismay be a stent-graft having a stent component and a covering over some or all of the open interstices of the stent.
- the coveringmay be provided over either or both of the inner and outer surfaces of the stent. It is preferably ePTFE, and can be attached to the stent by any of various means known in the art. Any such stent covering is in addition to and preferably separate from the constraining sheath used with a self-expanding endoprosthesis.
- FIG. 1shows a compacted and constrained endoprosthesis 11 mounted on an intermediate sheath 20 , which is in turn mounted upon inner tube 30 .
- a constraining sheath 13As a self-expanding endoprosthesis is described by the figures, it is shown with a constraining sheath 13 about its exterior surface. Constraining sheath 13 , as will be described, is disruptable to allow for expansion and deployment of endoprosthesis 11 at a desired site within a body conduit.
- Inner tube 30possesses at its distal end a protrusion 31 having a maximum diameter 32 (taken perpendicular to the longitudinal axis 12 of the system 10 ) that is larger than the inner diameter 22 of intermediate sheath 20 (also taken perpendicular to longitudinal axis 12 ).
- Intermediate sheath 20is preferably an extension of a delivery catheter extending beyond the insertion site at which endoprosthesis and associated delivery system entered the body conduit.
- Intermediate sheath 20is preferably made from a thin, strong and lubricious polymeric material such as PET.
- Intermediate sheath 20is preferably as thin or thinner than about 0.12 mm. In one alternative, the intermediate sheath may be an integral part of the delivery catheter tube.
- inner tube 30comprises a composite tube having an inner PTFE lining 24 of about 0.03 mm thickness, and an outer jacket 25 of polyamide, about 0.18 mm thick, having a braided stainless steel wire reinforcement (24 picks/cm, rectangular cross section wire 0.01 ⁇ 0.07 mm, Fluorotek, Easton Pa.) embedded in the wall of the jacket 25 .
- Protrusion 31is preferably a separate component, also of polyamide, that is melt-bonded to the exterior surface of the inner tube at a desired location at one end of a length of the inner tube.
- Melt bondingis accomplished by placing a mandrel inside inner tube 30 , fitting the protrusion 31 over the inner tube 30 , fitting a short length of fluorinated ethylene propylene shrink tubing over the protrusion 31 , and heating the assembly above the melt temperature of polyamide thereby causing simultaneous shrinking of the shrink-tubing. After heating, the shrink tubing is carefully removed with the aid of a scalpel blade, taking care not to damage the exterior surface of the protrusion 31 or the polyamide tubing 30 . Finally, the mandrel is removed from within the tube. Multiple melt steps may be required to adequately increase the protrusion diameter to the extent desired.
- FIG. 2Ais a transverse cross sectional view taken at section A-A of FIG. 1 wherein the intermediate sheath 20 is diametrically distensible when compressed axially and distended diametrically when protrusion 31 is moved axially against one end of the intermediate sheath or through its lumen.
- Endoprosthesis 11in this instance a self-expanding endoprosthesis, is shown enclosed by constraining sheath 13 .
- Constraining sheath 13is provided with a line of perforations 14 that extends for the length of the constraining sheath 13 , enabling it to subsequently disrupt along this split line 14 when the endoprosthesis is deployed.
- the direct contact of the protrusion 31 against the inner surface of the endoprosthesis 11may result in bunching up of the prosthesis axially and possible damage to the endoprosthesis, particularly if it is a stent-graft with a covering on the luminal surface of the stent that is vulnerable to damage from the protrusion 31 .
- the use of intermediate sheath 20provides for more uniform axial compression resistance against the force exerted by the protrusion 31 , thereby improving uniformity of the expansion and deployment of the endoprosthesis.
- the use of a lubricious material such as PET for the intermediate sheath 20aids in reducing and improving the uniformity of the axial effort that must be applied via a guidewire and/or catheter shaft to cause expansion and deployment of endoprosthesis 11 .
- FIG. 2Bis a transverse cross sectional view taken at section A-A of FIG. 1 showing an alternative embodiment of intermediate sheath 20 wherein the intermediate sheath 20 is provided with a longitudinally-oriented split 21 in one place, in a direction parallel to longitudinal axis 12 , allowing it to enlarge when the protrusion 31 applies axial compression against one end or is passed through it. While the intermediate sheath 20 is most preferably split entirely through its thickness, it may also be split through only a portion of the thickness if the remaining unsplit thickness will yield reliably when the protrusion 31 is forcibly pulled through the center of the intermediate sheath 20 .
- FIG. 2Cis a transverse cross sectional view taken at the same location with respect to FIG. 1, showing another alternative embodiment of sheath 20 wherein sheath 20 is coiled upon itself (in jelly-roll fashion), thereby allowing it to enlarge when the protrusion 31 is passed through it or applies axial compression against one end.
- FIG. 2Dis a transverse cross sectional view taken at section A-A of FIG. 1 wherein the intermediate sheath 20 is provided with multiple splits 21 in several places, thereby enabling it to expand when the protrusion of FIG. 1 is passed through it or applies axial compression against one end.
- FIG. 2Eis a transverse cross section describing a preferred construction of a constraining sheath 13 for use around the exterior surfaces of a self-expanding endoprosthesis 11 .
- Constraining sheath 13comprises a wrap of a thin ePTFE film 26 , made as taught by U.S. Pat. No. 5,814,405 to Branca et al.
- the particular ePTFE film usedhas a bulk density of about 0.25 g/cc and is provided with a discontinuous, porous coating of fluorinated ethylene propylene. It has a thickness of about 0.02 mm and a width that is greater than the length of the endoprosthesis 11 intended to be constrained.
- this film 26Four layers of this film 26 are sequentially wrapped about the surface of a stainless steel mandrel having a diameter equal to the outside diameter of the compacted endoprosthesis 11 intended to be constrained, with the circumferential wrapping of the mandrel accomplished in the machine direction of the film.
- a line of perforations 14are provided for the full length of the tube through the thickness of this film 26 using a laser, after which the helical wrapping is completed with a fifth layer of the film.
- the resulting five layer tube, still on the mandrel,is restrained against the surface of the mandrel at the tube ends and placed into an oven set at a temperature of about 320° C. for about five minutes, after which it is removed from the oven and allowed to cool.
- the end restraintsare removed and the five layer tube is then removed from the mandrel and trimmed to the desired length equivalent to the length of the endoprosthesis 11 .
- the resulting constraining sheath 13is ready to be fitted about the exterior surface of compacted endoprosthesis 11 .
- the endoprosthesismay be compacted by drawing it through a funnel with the aid of a fiber temporarily attached to the endoprosthesis.
- the endprosthesisis drawn through the funnel into a length of metal or plastic tubing of constant diameter and finally into the constraining sheath.
- FIG. 3is a longitudinal cross section of a preferred embodiment wherein a separate intermediate sheath 20 is affixed to the distal end of a delivery catheter tube 23 .
- the delivery catheter tube 23preferably is a length of the composite tubing described above having the PTFE lining within a wire-reinforced polyamide jacket. The inside diameter of this tubing is such that it provides a slight clearance for the outside diameter of the inner tube 30 .
- the PTFE liner of both tubesallows for smooth axial operation of other components (tubes or wires) within the lumen of either tube.
- Intermediate sheath 20comprises a length of heat shrinkable PET tubing of about 0.03 mm thickness (Advanced Polymers Inc., Salem N.H.), fitted over the distal end of the delivery catheter tube 23 , and joined to that tube with cyanoacrylate adhesive. After the adhesive has set, tension and heat are applied to the length of thin PET tubing to cause it to shrink in diameter in an amount to allow it to fit snugly over the outer surface of the inner tube 30 . An approximately 5 mm long length of the PET tubing is left unshrunk to accommodate at least a portion of the protrusion 31 when it is subsequently drawn through the endoprosthesis 11 .
- the length of the intermediate sheath 20is cut off transversely to a length that allows it to extend beyond the distal end of endoprosthesis 11 .
- the full length of inner sheath 20 extending beyond the end delivery catheter tube 23is slit in a direction parallel to longitudinal axis 12 , forming intermediate sheath split 21 (FIG. 2B).
- Compacted endoprosthesis 11is fitted around the slit intermediate sheath 20 , and protrusion 31 is then fitted about the distal end of inner tube 30 as previously described.
- FIG. 4Ais a longitudinal cross section of the endoprosthesis 11 being deployed within a body conduit 50 ;
- FIG. 4Bis an end view looking in a proximal direction of this deployment.
- Protrusion 31is moved axially through intermediate sheath 20 and prosthesis 11 via tension applied to intermediate sheath 20 .
- Intermediate sheath 20is preferably the distal end of a tubular catheter shaft which supplies resistance compressively to the inner tube 30 and protrusion 31 (affixed to the inner tube 30 ) when those components are pulled axially through the endoprosthesis in a proximal direction.
- the mechanical advantage offered by inclined plane 33 on the leading edge or proximal side of protrusion 31is utilized to apply a radial force to intermediate sheath 20 , thereby causing disruption of the intermediate sheath 20 .
- This radial forceis at first resisted compressively by intermediate sheath 20 , and quickly results in disruption of intermediate sheath 20 , thereby initiating deployment of the self-expanding endoprosthesis 11 .
- the expansion of endoprosthesis 11progresses axially toward the proximal end 10 a of the system 10 .
- FIGS. 4A and 4BThe embodiment described in FIGS. 4A and 4B is that of FIG. 2D, wherein intermediate sheath is provided with multiple splits 21 along its length, in this instance 4 parallel and equally spaced splits.
- the end view of FIG. 4Bshows the separation of these splits as the deployment progresses toward the proximal end of the system.
- FIGS. 5 A and 5 C- 5 Hare longitudinal cross sectional views of the inner tube 30 showing various embodiments of protrusion 31 . These show that protrusion 31 may take any of various forms and are intended as exemplary and are not therefore limiting. The fundamental requirement is that the maximum diameter 32 of the protrusion 31 (taken perpendicular to the longitudinal axis 12 ) is larger than the inside diameter 22 of intermediate sheath 20 , necessary to enable the protrusion 31 to disrupt the intermediate sheath 20 .
- FIG. 5Adescribes an embodiment wherein the protrusion 31 is fundamentally spherical in shape. However, as shown by the embodiment described in the end view of FIG. 5B, it is apparent that the protrusion is not required to be symmetrical in transverse cross section. As shown in FIG. 5B, the protrusion 31 may optionally be eccentric, having a maximum diameter 32 that is larger than a diameter taken through the longitudinal axis normal to the maximum diameter.
- the protrusion 31must have a maximum diameter 32 that is larger than the inside diameter 22 of intermediate sheath 20 , and is thereby capable of disrupting intermediate sheath 20 when an axial force is applied to the protrusion 31 to cause it to move axially against and/or through intermediate sheath 20 and endoprosthesis 11 .
- FIG. 5Cis a longitudinal cross-section of the protrusion of FIG. 1, wherein the protrusion is similar to the round shape of FIG. 5A, but is provided with a more pronounced inclined plane 33 , wherein the protrusion 31 merges with the inner tube in a less perpendicular fashion in order to reduce the axial force required to initiate disruption of the intermediate sheath 20 and cause expansion of the endoprosthesis 11 .
- FIGS. 5D and 5Eare longitudinal cross-sections of alternative embodiments of protrusion 31 .
- FIG. 5Fis a longitudinal cross-section of an alternative embodiment of protrusion 31 and inner tube 30 , wherein the protrusion is enlargable such as in axial compression of the braided tubular form shown.
- Application of tension to a guidewire used within the lumen of the tubular braidcan be utilized to create the protrusion 31 .
- the location of the protrusion (along the length of the braided wire) and its maximum diametercan be pre-determined.
- the braided tubular formcan be of length about equal to or slightly greater than the length of the constrained endoprosthesis.
- This braided tubular formi.e., the entire length of the intermediate sheath
- an elongate actuation membere.g., inner tube 30
- FIG. 5Gis a longitudinal cross-section of the protrusion 31 , wherein the protrusion is an enlargeable, inflatable member.
- the protrusion of FIG. 1the protrusion is made up of a material dissolvable in body fluids.
- FIGS. 6A and 6Bare longitudinal cross-sections that show, respectively, that protrusion 31 may be attached to, or made integral with a guide wire 40 , or other forms of elongate tensile members such as wires of other types, cables, strands, etc.
- FIG. 6Cis a longitudinal cross-section indicating that protrusion 31 may be attached to the very end of an elongate tensile member if appropriate for particular applications.
- FIG. 7Ais a longitudinal cross section of a compacted and constrained endoprosthesis incorporating a tether element 60 having enlargement 70 at the distal end of the tether element 60 , intended to prevent unintentional axial movement of the endoprosthesis.
- Enlargement 70aids in holding the various components in the desired axial relationship until it is desired to actually expand and deploy endoprosthesis 11 by the use of relative tension applied to the inner tube 30 and protrusion 31 .
- FIG. 7Bis a transverse cross-section taken at section C-C of FIG. 7A describing the tether element.
- FIGS. 8A and 8Bare longitudinal cross sections describing alternative tethers 60 wherein the tether 60 is used to secure an endoprosthesis 11 to another device such as a delivery catheter 25 .
- One or both ends of tether 60are secured to delivery catheter 25 .
- One or more enlargements 70are provided as restraints resisting any inadvertent displacement between the catheter 25 and the endoprosthesis 11 .
- Tether 60is held captive between endoprosthesis 11 and balloon 81 .
- balloon 81When balloon 81 is inflated to expand and deploy endoprosthesis 11 , the tether 60 remains captive.
- tether 61is freed from endoprosthesis 11 and may be withdrawn from the body conduit into which the endoprosthesis 11 has been inserted along with catheter 25 and attached balloon 81 .
- FIG. 8Cis a longitudinal cross section of a tether 60 passed through an endoprosthesis 11 that is compacted onto a catheter balloon 81 .
- FIG. 8Cincludes a side view of a hub 82 at the proximal end of the system, showing the tether 60 having both ends extending out of hub 82 wherein following inflation of balloon 81 and deployment of endoprosthesis 11 , tether 60 may be withdrawn by pulling on one end.
- FIG. 9is a longitudinal cross section of a compacted and constrained endoprosthesis 11 incorporating a distensible collar 80 preferably positioned at the distal end of the endoprosthesis 11 , intended to prevent unintentional axial movement of endoprosthesis 11 .
- Collar 80may be a separate component affixed to the exterior of intermediate sheath 20 at the distal end thereof, immediately proximal to protrusion 31 , or may be made to be integral to the intermediate sheath 20 .
- the collar 80may simply be of the form of any sort of enlargement in the diameter of the distal end of the intermediate sheath 20 that interferes with axial movement of the endoprosthesis 11 and thus prevents an unintentional movement of the endoprosthesis.
- the enlargementis not required to extend entirely around the circumference of the distal end of intermediate sheath 20 in the fashion of collar 80 .
- Such an enlargementmay be integral with the distal end of intermediate sheath 20 or separately affixed.
- either the collar or another form of enlargementmay be positioned beneath the endoprosthesis anywhere within the length of the endoprosthesis, thereby making difficult any unintended movement of the endoprosthesis with respect to the elongate tensile member 30 .
- FIG. 10is a transverse cross section taken at section D-D of FIG. 9, describing the distensible collar 80 .
- FIGS. 11A and 11Bare perspective views of an alternative embodiment of the present invention wherein the substantially tubular sheath 20 contains multiple parallel splits adjacent to its distal end that do not extend entirely to the end. As shown by FIG. 11B, this form of the intermediate sheath 20 would increase in diameter when the protrusion 31 is pulled against its distal end by elongate actuation member 30 , thereby exerting a radially outward directed force against endoprosthesis 11 (not shown) and initiating expansion and deployment of the endoprosthesis.
- FIG. 11Bthis form of the intermediate sheath 20 would increase in diameter when the protrusion 31 is pulled against its distal end by elongate actuation member 30 , thereby exerting a radially outward directed force against endoprosthesis 11 (not shown) and initiating expansion and deployment of the endoprosthesis.
- protrusion 31is no longer required and the distal end of the substantially tubular sheath is secured to the distal end of the elongate actuation member 30 , whereby axial movement of the elongate tensile member 30 with respect to the substantially tubular sheath 20 results in a compressive force applied to the substantially tubular sheath 20 , causing it to deform outwardly and exert a radially outward force against the endoprosthesis 11 (not shown) to initiate expansion and deployment of the endoprosthesis.
- the distal end of elongate actuation member 30may be secured to the distal end of the substantially tubular sheath 20 by any of various means including compression ring 111 , or by the use of an adhesive, welding, etc.
- FIGS. 12A and 12Bare alternative embodiments to those of FIGS. 11A and 11B wherein the substantially tubular sheath 20 is made to increase in diameter in a corrugated or accordion-fashion when protrusion 31 is moved axially against the substantially tubular sheath 20 by the application of tension to the elongate actuation member 30 . As with the embodiment of FIG. 11C, this can also be accomplished without requiring a protrusion 31 by securing the distal end of the substantially tubular sheath 20 to the distal end of the elongate actuation member 30 .
- the protrusionmay be fitted at the proximal end of the system and moved axially in a distal direction to initiate endoprosthesis expansion in a proximal-to-distal direction.
- the protrusionmay be fitted at the proximal end of the system and moved axially in a distal direction to initiate endoprosthesis expansion in a proximal-to-distal direction.
Landscapes
- Health & Medical Sciences (AREA)
- Engineering & Computer Science (AREA)
- Biomedical Technology (AREA)
- Cardiology (AREA)
- Oral & Maxillofacial Surgery (AREA)
- Transplantation (AREA)
- Heart & Thoracic Surgery (AREA)
- Vascular Medicine (AREA)
- Life Sciences & Earth Sciences (AREA)
- Animal Behavior & Ethology (AREA)
- General Health & Medical Sciences (AREA)
- Public Health (AREA)
- Veterinary Medicine (AREA)
- Prostheses (AREA)
- Media Introduction/Drainage Providing Device (AREA)
Abstract
Description
- The present invention relates to the transcatheter delivery and remote deployment of implantable medical devices and more particularly to a system for the expansion and deployment of endoprostheses.[0001]
- Endoluminal therapies typically involve the insertion of a delivery catheter that transports an implantable prosthetic device into a body conduit through a small, often percutaneous, remote access site. Once access to the body conduit is achieved, the delivery catheter is used to mediate intraluminal delivery and subsequent deployment of the prosthesis via one of several techniques. In this fashion, the prosthesis can be remotely implanted to achieve a therapeutic outcome. In contrast to conventional surgical therapies, endoluminal treatments are distinguished by their “minimally invasive” nature.[0002]
- Self-expanding endoprostheses are generally comprised of a stent component with or without a graft covering over the stent interstices. They are designed to spontaneous dilate (i.e., elastically recover) from their delivery diameter, through a range of intermediary diameters, up to a maximal, pre-determined functional diameter. The endoluminal delivery and deployment of self-expanding endoprostheses pose several unique problems. First, the endoprosthesis itself must be radially compacted to a suitable introductory size (or delivery diameter) to allow insertion into the vasculature, then it must be constrained in that compacted state and mounted onto a delivery device such as a catheter shaft. Subsequently, the constraint must be removed in order to allow the endoprosthesis to expand to its functional diameter and achieve the desired therapeutic outcome. Preferably, the means of constraint will not adversely affect the delivery catheter performance (e.g., detracting from the flexibility of the delivery system) or add significantly to introductory profile. The constraint must also incorporate some type of release mechanism or scheme that can be remotely actuated by the implanting clinician. Consequently, deployment methodologies that are consistent with conventional interventional practices are preferred.[0003]
- Delivery mechanisms for self-expanding endoprostheses of the prior art may be generally classified into one of two general categories, either coaxial sheaths or fiber-based constraints. Delivery systems also exist that use both of these types of mechanisms in combination.[0004]
- Tubular coaxial sheaths are one approach used to constrain the compacted self-expanding endoprosthesis. Normally, these coaxial sheaths extend over the entire length of an inner delivery catheter onto which the endoprosthesis is mounted near the catheter tip (i.e., leading end). Deployment is typically initiated by pulling on a handle or knob located near the hub (i.e., trailing end) of the catheter, which retracts the constraining sheath and allows the device to expand. During this procedure, the clinician maintains the position of the device by holding the inner (delivery) catheter in a stationary position. Existing problems and/or complications with the tubular coaxial sheath type of delivery system include friction between compacted device and constraining sheath, friction between the constraining sheath and delivery catheter, and friction between the delivery catheter and constraining sheath hemostasis valve, all of which can hinder deployment accuracy, speed and control. Additionally, a tubular coaxial constraining sheath can also reduce flexibility and add introductory profile due to the thickness of the constraining sheath.[0005]
- In the fiber-based delivery systems, the self-expanding endoprosthesis is constrained in the delivery profile by one or more removable fibrous strands, with or without an additional implantable constraint element. The endoprosthesis is released from its compacted-state through tension applied to a deployment “cord” that normally runs through an additional lumen within the delivery catheter. Typically, applying tension to the deployment cord initiates the release of the fiber constraint by, for example, unlacing linear slip knots (see Lau, et al., U.S. Pat. No. 5,919,225), removing circumferential croquet knots (e.g., Strecker, U.S. Pat. No. 5,405,378), or detaching the interlocking loops of a warp-knitted constraint (e.g., Armstrong et al., U.S. Pat. No. 6,224,627). Other fiber-based delivery systems are described by Lindemann, U.S. Pat. No. 4,878,906, and Hillstead, U.S. Pat. No. 5,019,085.[0006]
- Another variant of the fiber-based delivery systems is the mechanism employed in the EXCLUDER® endoprosthesis marketed by W. L. Gore and Associates, Inc (Flagstaff, Ariz.). This mechanism entails a “chain-stitch” sewn into the seam of a biocompatible constraining tube that contains the compacted endoprosthesis. Applying tension to the fibrous constraint in this mechanism allows the seam in the biocompatible constraining tube to be open, and the self-expanding endoprosthesis to deploy. The biocompatible constraining tube is implanted along with the endoprosthesis, trapped between the abluminal surface of the device and the wall of the host vessel. See WO98/27894.[0007]
- Problems with fiber-based type of delivery systems include possible premature deployment during introduction to the vascular system through hemostasis valves, extra lumens required on the delivery catheter which can increase profile, possible snagging of fiber(s) on the compacted implantable device, the possibility of emboli resulting from moving lines between the catheter and the blood vessel, and possible breakage of the deployment cord itself.[0008]
- U.S. Pat. Nos. 5,755,769 and 6,019,787 to Richard et al. teach another constraining sheath around a self-expanding stent. The sheath is cut longitudinally into several segments by cutting wires or fibers actuated by pulling a handle at the opposite end of the delivery system. The sheath is attached to or integral to the delivery catheter with the result that the segments are removed with the catheter following stent deployment. No catheter balloon or other means for exerting a circumferential disrupting force to the sheath is suggested, nor are materials appropriate for the sheath suggested. This design requires lines to run over the length of the catheter.[0009]
- U.S. Pat. No. 6,086,610 to Duerig et al. teaches a self-expanding stent provided with a tubular constraining sheath that is plastically deformable by a circumferential distending force such as a catheter balloon. This sheath remains implanted with the stent following deployment and fully covers the entire circumference of the stent in the fashion of a conventional stent covering, i.e., the tubular sheath is not disrupted. The Duerig et al. device is delivered from a conventional balloon catheter, but thought to have limitations, including radial recoil of the sheath after the balloon is pressurized and deflated, which can compromise luminal gain. Further, the presence of the cover may adversely affect the ability of the stent to fully deploy, and the balloon length must be equal to or longer than the stent, and this long balloon can potentially damage the vessel.[0010]
- The present invention relates to an endoprosthesis expansion system comprising, in combination, a delivery component such as a length of catheter tubing having at its distal end an intermediate sheath component, and an inner elongate actuation member that is preferably an inner tube located within the full length of the delivery catheter and intermediate sheath component. The inner elongate actuation member (e.g., inner tube) has a protrusion affixed to its distal end, and an expandable endoprosthesis is fitted in a compacted state about the intermediate sheath, proximal to the protrusion. If the endoprosthesis is a self-expanding endoprosthesis (as is preferred), an exterior constraining sheath is required around the outer surface of the endoprosthesis to contain the endoprosthesis in a compacted configuration. Following insertion of the endoprosthesis and delivery system into a body conduit (such as a blood vessel) and transport of the endoprosthesis to the desired site within the body conduit, the endoprosthesis is deployed by axially moving the protrusion through the system, thereby applying a radially directed outward force and causing simultaneous dilatation of the intermediate sheath and disruption of the exterior constraining sheath. Alternatively, axial movement of the elongate actuation member against the end of the intermediate sheath, applying axial compression to the intermediate sheath, may cause the intermediate sheath to shorten and simultaneously increase in diameter, thereby initiating expansion and deployment of the endoprosthesis. Disruption of the exterior constraining sheath, in the case of a self-expanding prosthesis, releases the stored energy in the formerly constrained prosthesis, allowing it to spontaneously expand and accomplish full deployment against the luminal surface of the body conduit at the desired site.[0011]
- The exterior constraining sheath is preferably made of an implantable material and may be left captured between the endoprosthesis and the luminal surface of the body conduit. Alternatively, the exterior constraining sheath may be secured to the adjacent delivery catheter and withdrawn from between the endoprosthesis and the wall of the body conduit when the delivery catheter is withdrawn.[0012]
- If a non-self-expanding endoprosthesis is used (e.g., a balloon-expandable stent), diametrical expansion may be accomplished by moving the protrusion axially through the stent, thereby enlarging the diameter by plastically deforming the stent. Likewise, as described with the self-expanding stent embodiment, the application of axial compression against one end of the intermediate sheath by the protrusion can cause an increase in the diameter of the intermediate sheath, forcing a corresponding diametrical increase in the balloon expandable stent.[0013]
- In addition to stent devices, the endoprostheses utilized with the present invention may also be stent-grafts. The phrase “stent-graft” is used herein to describe a stent provided with a covering, typically of a vascular graft material such as porous expanded polytetrafluoroethylene (ePTFE) or polyethylene terephthalate (PET). The covering may be provided over either or both of the inner and outer surfaces of the stent. The covering may cover a portion of the otherwise open stent interstices or it may cover all of the stent interstices.[0014]
- While the system of the present invention is intended primarily for stents and stent-grafts for use in vascular repairs, it is also useful for expandable devices for other applications in other body conduits, e.g., esophageal or biliary duct repairs.[0015]
- While a protrusion can be used to initiate deployment without an intermediate sheath inside the endoprosthesis, the use of the intermediate sheath, made from a thin, strong and lubricious material, prevents the protrusion from damaging the endoprosthesis (particularly if the endoprosthesis is a stent-graft with a covering on the luminal surface). It also reduces the likelihood of “bunching” of the endoprosthesis due to the application of an axial force. It can likewise reduce the amount of axial force required as well as reducing the variability of the axial force (as the protrusion moves along the internal length of the endoprosthesis), by providing a uniform compression resistance against the protrusion as opposed to the variable resistance provided by the wire surface of the interior of an endoprosthesis.[0016]
- Both the exterior constraining sheath and the intermediate sheath may be made to be dilatable or disruptable by various and similar means. For the exterior constraining sheath, it is preferred to provide a line of perforations partially or entirely through the wall of the tubular constraining sheath, parallel to the longitudinal axis of the tubular constraining sheath. The constraining sheath may be caused to disrupt by splitting along this line of perforations, upon the application of an outwardly directed radial force from within the sheath (and within the contained endoprosthesis).[0017]
- For the intermediate sheath located within the endoprosthesis, it is preferred that it is of a substantially tubular form and is dilatable via one or more, equally radially-spaced apart splits are used along the length of that sheath. Alternatively, the intermediate sheath may be elastically or plastically deformable by the protrusion. In other alternatives, the intermediate sheath may be caused to be split, ripped, torn or otherwise changed in proportion by the movement of the protrusion against and/or through the intermediate sheath. Any of these mechanisms are considered to constitute dilatation of the intermediate sheath. It is apparent that the tubular form of the intermediate sheath includes various embodiments and as such is considered to be a substantially tubular sheath.[0018]
- The present invention also provides a means of controlling the radial dynamics of device deployment. For example, the present invention can be configured to ‘pop’ open to allow rapid device deployment, or alternatively to undergo more gradual, controlled, stepwise release during device deployment, or a combination of both.[0019]
- The constraining sheath may be imbibed with various pharmaceutical, biological, or genetic therapies for targeted luminal delivery of these substances. Following deployment of the endoprosthesis, these therapeutic agents can be released over time. An advantage of this approach is that the loading of the sheath with any of these therapeutic agents can be performed independent of the endoprosthesis manufacture. Further, radiopaque elements may be incorporated into the constraining sheath (or other system components, notably the catheter tubes) to facilitate fluoroscopic visualization.[0020]
- The present invention may also be used to deliver and deploy multiple devices positioned in sequential order on the delivery catheter.[0021]
- In a preferred embodiment, the constraining sheath can be made to be extremely thin, or “delicate,” for minimal implantation profile. Such a delicate constraining sheath is not adequate, without further exterior support, to constrain the endoprosthesis assembly (particularly when the assembly includes a self-expanding endoprosthesis) for very long periods of time or for shorter periods when exposed to elevated temperatures. The use of such a delicate constraining sheath is made practically possible when the assembly is provided with an additional tubular packaging sheath that prevents inadvertent disruption of the constraining sheath or undesirable increase in diameter of the assembly (e.g., in an amount of 0.15 mm or more). The tubular packaging sheath, fitted coaxially about the exterior of the “delicate” constraining sheath, is removed prior to implantation and accordingly is not required to be made of an implantable material or a material with a thin wall. Alternatively, the endoprosthesis assembly may incorporate such a delicate constraining sheath without the use of a packaging sheath if it is stored at reduced temperatures, such as 5° C. or less, prior to implantation.[0022]
- FIG. 1 is a longitudinal cross section of a compacted and constrained endoprosthesis mounted on an intermediate sheath, which is mounted upon an inner tube incorporating a protrusion at the distal end that, when moved axially through or against the intermediate sheath and endoprosthesis, applies a force directed radially outward to initiate deployment of the endoprosthesis.[0023]
- FIG. 2A is a transverse cross sectional view taken at section A-A of FIG. 1.[0024]
- FIG. 2B is a transverse cross sectional view taken at section A-A of FIG. 1 wherein, in an alternative embodiment, the intermediate sheath is split in one place, allowing it to enlarge diametrically when the protrusion of FIG. 1 is passed through it or applies compression against the end of the intermediate sheath.[0025]
- FIG. 2C is a transverse cross sectional view taken at section A-A of FIG. 1 wherein, in another alternative, the intermediate sheath is split and coiled upon itself (in jelly-roll fashion).[0026]
- FIG. 2D is a transverse cross sectional view taken at section A-A of FIG. 1 wherein, in another alternative, the intermediate sheath is split in several places.[0027]
- FIG. 2E is a transverse cross section of a preferred constraining sheath construction.[0028]
- FIG. 3 is a longitudinal cross section of a compacted and constrained endoprosthesis describing a preferred embodiment of the system of the present invention.[0029]
- FIG. 4A is a longitudinal cross section of the endoprosthesis being deployed within a body conduit.[0030]
- FIG. 4B is an end view of endoprosthesis deployment depicted in FIG. 4A.[0031]
- FIGS.[0032]5A and5C-5H are longitudinal cross-sections of the inner tube and protrusion, describing various embodiments of the protrusion.
- FIG. 5B is a transverse cross-sectional view taken at section B-B of FIG. 5A of the protrusion of FIG. 1, describing an embodiment wherein the transverse cross-section of the protrusion is not round.[0033]
- FIG. 6A is a longitudinal cross-section of the protrusion of FIG. 1, wherein the protrusion is attached to a guide wire.[0034]
- FIG. 6B is a longitudinal cross-section of the protrusion of FIG. 1, wherein the protrusion is integral to a guide wire.[0035]
- FIG. 6C is a longitudinal cross-section of the protrusion of FIG. 1, wherein the protrusion is attached to an elongate tensile member, such as a fiber, strand or wire.[0036]
- FIG. 7A is a longitudinal cross section of a compacted and constrained endoprosthesis incorporating a tether element with an enlargement at the distal end of the tether element, intended to prevent unintentional axial movement of the endoprosthesis.[0037]
- FIG. 7B is a transverse cross-section taken at section C-C of FIG. 7 describing the tether element.[0038]
- FIGS.[0039]8A-8C are longitudinal cross sections describing alternative embodiments of the tether element.
- FIG. 9 is a longitudinal cross section of a compacted and constrained endoprosthesis incorporating a distensible collar positioned at the end of the endoprosthesis and intended to prevent unintentional axial movement of the endoprosthesis[0040]
- FIG. 10 is a transverse cross sectional view taken at section D-D of FIG. 9, describing the distensible collar element.[0041]
- FIGS.[0042]11A-11C,12A and12B are perspective views describing alternative embodiments of the present invention, primarily with regard to variations of the substantially tubular, intermediate sheath.
- FIG. 1 is a longitudinal cross section of the[0043]
endoprosthesis expansion system 10 of the present invention. Thesystem 10 has aproximal end 10aand adistal end 10b, whereinproximal end 10ais considered to be the end from which a delivery catheter extends to a site at which the system was originally inserted into a body conduit.Endoprosthesis 11 is indicative of any type of endoluminal medical device which might be usefully contained at a smaller diameter for insertion into a body conduit and subsequently deployed to a larger diameter at a desired location within the body conduit. The endoprosthesis is preferably a self-expanding device, and is most preferably a stent of the self-expanding type. While these stents are most commonly used for repair of the vasculature (e.g., repair of stenoses or aneurysmal repair), they are also used for other applications in other body conduits (e.g., esophageal or bile duct repairs). These self-expanding stents are typically of nitinol wire although other materials may be used, e.g., stainless steels or polymeric materials including resorbable polymers. - Alternatively, some balloon expandable devices may be expanded with the present inventive system, without requiring an exterior constraining sheath as do self-expanding devices.[0044]
- According to the present invention, a self expanding device in use as a part of the present endoprosthesis expansion system is provided with an exterior constraining sheath to retain the self-expanding endoprosthesis at its small, compacted diameter at which it is intended to be inserted into a body conduit for subsequent expansion and deployment. The constraining sheath is disrupted by activation of the expansion and deployment mechanism of the present invention. The constraining sheath is preferably made from an implantable material, most preferably a tube of porous expanded PTFE (hereinafter ePTFE), made generally as taught by U.S. Pat. Nos. 3,953,566 and 4,187,390 to Gore. The tube is most preferably provided with a line of perforations through the wall, parallel to the length of the tube. The line of perforations provides a yield point along which the constraining sheath will disrupt by splitting. Forcibly expanding the endoprosthesis in a radially outward direction, using force applied from within the lumen of the endoprosthesis, causes the perforation line to disrupt, initiating expansion and deployment of the self-expanding endoprosthesis. The disrupted, now-split constraining sheath, being of an implantable material such as ePTFE, preferably remains implanted with the deployed endoprosthesis, held captive between the endoprosthesis and the wall of the body conduit at the site of deployment. As such, the implantable constraining sheath may optionally be attached to the exterior surface of the self-expanding stent, preferably along an axially-oriented line that is 180° opposite the line of perforations. Alternatively, the constraining sheath can be configured to be removable following deployment of the endoprosthesis, by having previously secured it to a component of the delivery system such as a catheter shaft and withdrawing it from between the endoprosthesis and the wall of the body conduit when the catheter is withdrawn.[0045]
- The endoprosthesis may be a stent-graft having a stent component and a covering over some or all of the open interstices of the stent. The covering may be provided over either or both of the inner and outer surfaces of the stent. It is preferably ePTFE, and can be attached to the stent by any of various means known in the art. Any such stent covering is in addition to and preferably separate from the constraining sheath used with a self-expanding endoprosthesis.[0046]
- FIG. 1 shows a compacted and[0047]
constrained endoprosthesis 11 mounted on anintermediate sheath 20, which is in turn mounted uponinner tube 30. As a self-expanding endoprosthesis is described by the figures, it is shown with a constrainingsheath 13 about its exterior surface. Constrainingsheath 13, as will be described, is disruptable to allow for expansion and deployment ofendoprosthesis 11 at a desired site within a body conduit. - [0048]
Inner tube 30 possesses at its distal end aprotrusion 31 having a maximum diameter32 (taken perpendicular to thelongitudinal axis 12 of the system10) that is larger than theinner diameter 22 of intermediate sheath20 (also taken perpendicular to longitudinal axis12).Intermediate sheath 20 is preferably an extension of a delivery catheter extending beyond the insertion site at which endoprosthesis and associated delivery system entered the body conduit.Intermediate sheath 20 is preferably made from a thin, strong and lubricious polymeric material such as PET.Intermediate sheath 20 is preferably as thin or thinner than about 0.12 mm. In one alternative, the intermediate sheath may be an integral part of the delivery catheter tube. - Most preferably,[0049]
inner tube 30 comprises a composite tube having an inner PTFE lining24 of about 0.03 mm thickness, and anouter jacket 25 of polyamide, about 0.18 mm thick, having a braided stainless steel wire reinforcement (24 picks/cm, rectangular cross section wire 0.01×0.07 mm, Fluorotek, Easton Pa.) embedded in the wall of thejacket 25. - [0050]
Protrusion 31 is preferably a separate component, also of polyamide, that is melt-bonded to the exterior surface of the inner tube at a desired location at one end of a length of the inner tube. Melt bonding is accomplished by placing a mandrel insideinner tube 30, fitting theprotrusion 31 over theinner tube 30, fitting a short length of fluorinated ethylene propylene shrink tubing over theprotrusion 31, and heating the assembly above the melt temperature of polyamide thereby causing simultaneous shrinking of the shrink-tubing. After heating, the shrink tubing is carefully removed with the aid of a scalpel blade, taking care not to damage the exterior surface of theprotrusion 31 or thepolyamide tubing 30. Finally, the mandrel is removed from within the tube. Multiple melt steps may be required to adequately increase the protrusion diameter to the extent desired. - FIG. 2A is a transverse cross sectional view taken at section A-A of FIG. 1 wherein the[0051]
intermediate sheath 20 is diametrically distensible when compressed axially and distended diametrically whenprotrusion 31 is moved axially against one end of the intermediate sheath or through its lumen.Endoprosthesis 11, in this instance a self-expanding endoprosthesis, is shown enclosed by constrainingsheath 13. Constrainingsheath 13 is provided with a line ofperforations 14 that extends for the length of the constrainingsheath 13, enabling it to subsequently disrupt along thissplit line 14 when the endoprosthesis is deployed. - While it is possible to initiate expansion and deployment of a constrained, self-expanding endoprosthesis without the use of[0052]
intermediate sheath 20, the use of this additional component has been found to aid in the practical expansion of an endoprosthesis via axial movement of aprotrusion 31 against the end of theintermediate sheath 20 or through the center of theintermediate sheath 20 andendoprosthesis 11. Without theintermediate sheath 20, the direct contact of theprotrusion 31 against the inner surface of theendoprosthesis 11 may result in bunching up of the prosthesis axially and possible damage to the endoprosthesis, particularly if it is a stent-graft with a covering on the luminal surface of the stent that is vulnerable to damage from theprotrusion 31. The use ofintermediate sheath 20 provides for more uniform axial compression resistance against the force exerted by theprotrusion 31, thereby improving uniformity of the expansion and deployment of the endoprosthesis. Likewise, the use of a lubricious material such as PET for theintermediate sheath 20 aids in reducing and improving the uniformity of the axial effort that must be applied via a guidewire and/or catheter shaft to cause expansion and deployment ofendoprosthesis 11. - FIG. 2B is a transverse cross sectional view taken at section A-A of FIG. 1 showing an alternative embodiment of[0053]
intermediate sheath 20 wherein theintermediate sheath 20 is provided with a longitudinally-oriented split21 in one place, in a direction parallel tolongitudinal axis 12, allowing it to enlarge when theprotrusion 31 applies axial compression against one end or is passed through it. While theintermediate sheath 20 is most preferably split entirely through its thickness, it may also be split through only a portion of the thickness if the remaining unsplit thickness will yield reliably when theprotrusion 31 is forcibly pulled through the center of theintermediate sheath 20. - FIG. 2C is a transverse cross sectional view taken at the same location with respect to FIG. 1, showing another alternative embodiment of[0054]
sheath 20 whereinsheath 20 is coiled upon itself (in jelly-roll fashion), thereby allowing it to enlarge when theprotrusion 31 is passed through it or applies axial compression against one end. - FIG. 2D is a transverse cross sectional view taken at section A-A of FIG. 1 wherein the[0055]
intermediate sheath 20 is provided withmultiple splits 21 in several places, thereby enabling it to expand when the protrusion of FIG. 1 is passed through it or applies axial compression against one end. - FIG. 2E is a transverse cross section describing a preferred construction of a constraining[0056]
sheath 13 for use around the exterior surfaces of a self-expandingendoprosthesis 11. Constrainingsheath 13 comprises a wrap of athin ePTFE film 26, made as taught by U.S. Pat. No. 5,814,405 to Branca et al. The particular ePTFE film used has a bulk density of about 0.25 g/cc and is provided with a discontinuous, porous coating of fluorinated ethylene propylene. It has a thickness of about 0.02 mm and a width that is greater than the length of theendoprosthesis 11 intended to be constrained. Four layers of thisfilm 26 are sequentially wrapped about the surface of a stainless steel mandrel having a diameter equal to the outside diameter of the compactedendoprosthesis 11 intended to be constrained, with the circumferential wrapping of the mandrel accomplished in the machine direction of the film. A line ofperforations 14 are provided for the full length of the tube through the thickness of thisfilm 26 using a laser, after which the helical wrapping is completed with a fifth layer of the film. The resulting five layer tube, still on the mandrel, is restrained against the surface of the mandrel at the tube ends and placed into an oven set at a temperature of about 320° C. for about five minutes, after which it is removed from the oven and allowed to cool. The end restraints are removed and the five layer tube is then removed from the mandrel and trimmed to the desired length equivalent to the length of theendoprosthesis 11. The resulting constrainingsheath 13 is ready to be fitted about the exterior surface of compactedendoprosthesis 11. The endoprosthesis may be compacted by drawing it through a funnel with the aid of a fiber temporarily attached to the endoprosthesis. The endprosthesis is drawn through the funnel into a length of metal or plastic tubing of constant diameter and finally into the constraining sheath. - FIG. 3 is a longitudinal cross section of a preferred embodiment wherein a separate[0057]
intermediate sheath 20 is affixed to the distal end of adelivery catheter tube 23. Thedelivery catheter tube 23 preferably is a length of the composite tubing described above having the PTFE lining within a wire-reinforced polyamide jacket. The inside diameter of this tubing is such that it provides a slight clearance for the outside diameter of theinner tube 30. The PTFE liner of both tubes allows for smooth axial operation of other components (tubes or wires) within the lumen of either tube. - [0058]
Intermediate sheath 20 comprises a length of heat shrinkable PET tubing of about 0.03 mm thickness (Advanced Polymers Inc., Salem N.H.), fitted over the distal end of thedelivery catheter tube 23, and joined to that tube with cyanoacrylate adhesive. After the adhesive has set, tension and heat are applied to the length of thin PET tubing to cause it to shrink in diameter in an amount to allow it to fit snugly over the outer surface of theinner tube 30. An approximately 5 mm long length of the PET tubing is left unshrunk to accommodate at least a portion of theprotrusion 31 when it is subsequently drawn through theendoprosthesis 11. The length of theintermediate sheath 20 is cut off transversely to a length that allows it to extend beyond the distal end ofendoprosthesis 11. Preferably, the full length ofinner sheath 20 extending beyond the enddelivery catheter tube 23 is slit in a direction parallel tolongitudinal axis 12, forming intermediate sheath split21 (FIG. 2B).Compacted endoprosthesis 11 is fitted around the slitintermediate sheath 20, andprotrusion 31 is then fitted about the distal end ofinner tube 30 as previously described. - FIG. 4A is a longitudinal cross section of the[0059]
endoprosthesis 11 being deployed within abody conduit 50; FIG. 4B is an end view looking in a proximal direction of this deployment.Protrusion 31 is moved axially throughintermediate sheath 20 andprosthesis 11 via tension applied tointermediate sheath 20.Intermediate sheath 20 is preferably the distal end of a tubular catheter shaft which supplies resistance compressively to theinner tube 30 and protrusion31 (affixed to the inner tube30) when those components are pulled axially through the endoprosthesis in a proximal direction. The mechanical advantage offered byinclined plane 33 on the leading edge or proximal side ofprotrusion 31, is utilized to apply a radial force tointermediate sheath 20, thereby causing disruption of theintermediate sheath 20. This radial force is at first resisted compressively byintermediate sheath 20, and quickly results in disruption ofintermediate sheath 20, thereby initiating deployment of the self-expandingendoprosthesis 11. The expansion ofendoprosthesis 11 progresses axially toward theproximal end 10aof thesystem 10. - The embodiment described in FIGS. 4A and 4B is that of FIG. 2D, wherein intermediate sheath is provided with[0060]
multiple splits 21 along its length, in this instance4 parallel and equally spaced splits. The end view of FIG. 4B shows the separation of these splits as the deployment progresses toward the proximal end of the system. - FIGS.[0061]5A and5C-5H are longitudinal cross sectional views of the
inner tube 30 showing various embodiments ofprotrusion 31. These show that protrusion31 may take any of various forms and are intended as exemplary and are not therefore limiting. The fundamental requirement is that themaximum diameter 32 of the protrusion31 (taken perpendicular to the longitudinal axis12) is larger than theinside diameter 22 ofintermediate sheath 20, necessary to enable theprotrusion 31 to disrupt theintermediate sheath 20. - FIG. 5A describes an embodiment wherein the[0062]
protrusion 31 is fundamentally spherical in shape. However, as shown by the embodiment described in the end view of FIG. 5B, it is apparent that the protrusion is not required to be symmetrical in transverse cross section. As shown in FIG. 5B, theprotrusion 31 may optionally be eccentric, having amaximum diameter 32 that is larger than a diameter taken through the longitudinal axis normal to the maximum diameter. Theprotrusion 31 must have amaximum diameter 32 that is larger than theinside diameter 22 ofintermediate sheath 20, and is thereby capable of disruptingintermediate sheath 20 when an axial force is applied to theprotrusion 31 to cause it to move axially against and/or throughintermediate sheath 20 andendoprosthesis 11. - FIG. 5C is a longitudinal cross-section of the protrusion of FIG. 1, wherein the protrusion is similar to the round shape of FIG. 5A, but is provided with a more pronounced[0063]
inclined plane 33, wherein theprotrusion 31 merges with the inner tube in a less perpendicular fashion in order to reduce the axial force required to initiate disruption of theintermediate sheath 20 and cause expansion of theendoprosthesis 11. - FIGS. 5D and 5E are longitudinal cross-sections of alternative embodiments of[0064]
protrusion 31. - FIG. 5F is a longitudinal cross-section of an alternative embodiment of[0065]
protrusion 31 andinner tube 30, wherein the protrusion is enlargable such as in axial compression of the braided tubular form shown. Application of tension to a guidewire used within the lumen of the tubular braid (guidewire omitted from FIG. 5F for clarity) can be utilized to create theprotrusion 31. By pre-forming the protrusion at the desired site in the length of braided wire, the location of the protrusion (along the length of the braided wire) and its maximum diameter can be pre-determined. In an alternative of this embodiment, the braided tubular form can be of length about equal to or slightly greater than the length of the constrained endoprosthesis. The entire length of this braided tubular form (i.e., the entire length of the intermediate sheath) can be caused to increase in diameter in a relatively uniform fashion when axial compression is applied to the braided tubular form by an elongate actuation member (e.g., inner tube30) within the tubular braided wire. - FIG. 5G is a longitudinal cross-section of the[0066]
protrusion 31, wherein the protrusion is an enlargeable, inflatable member. In another embodiment described by the longitudinal cross-section of FIG. 5H, the protrusion of FIG. 1, the protrusion is made up of a material dissolvable in body fluids. - FIGS. 6A and 6B are longitudinal cross-sections that show, respectively, that[0067]
protrusion 31 may be attached to, or made integral with aguide wire 40, or other forms of elongate tensile members such as wires of other types, cables, strands, etc. FIG. 6C is a longitudinal cross-section indicating thatprotrusion 31 may be attached to the very end of an elongate tensile member if appropriate for particular applications. - FIG. 7A is a longitudinal cross section of a compacted and constrained endoprosthesis incorporating a[0068]
tether element 60 havingenlargement 70 at the distal end of thetether element 60, intended to prevent unintentional axial movement of the endoprosthesis.Enlargement 70 aids in holding the various components in the desired axial relationship until it is desired to actually expand and deployendoprosthesis 11 by the use of relative tension applied to theinner tube 30 andprotrusion 31. - FIG. 7B is a transverse cross-section taken at section C-C of FIG. 7A describing the tether element.[0069]
- FIGS. 8A and 8B are longitudinal cross sections describing[0070]
alternative tethers 60 wherein thetether 60 is used to secure anendoprosthesis 11 to another device such as adelivery catheter 25. One or both ends oftether 60 are secured todelivery catheter 25. One ormore enlargements 70 are provided as restraints resisting any inadvertent displacement between thecatheter 25 and theendoprosthesis 11.Tether 60 is held captive betweenendoprosthesis 11 andballoon 81. Whenballoon 81 is inflated to expand and deployendoprosthesis 11, thetether 60 remains captive. Whenballoon 81 is subsequently deflated, tether61 is freed fromendoprosthesis 11 and may be withdrawn from the body conduit into which theendoprosthesis 11 has been inserted along withcatheter 25 and attachedballoon 81. - FIG. 8C is a longitudinal cross section of a[0071]
tether 60 passed through anendoprosthesis 11 that is compacted onto acatheter balloon 81. FIG. 8C includes a side view of ahub 82 at the proximal end of the system, showing thetether 60 having both ends extending out ofhub 82 wherein following inflation ofballoon 81 and deployment ofendoprosthesis 11,tether 60 may be withdrawn by pulling on one end. - FIG. 9 is a longitudinal cross section of a compacted and[0072]
constrained endoprosthesis 11 incorporating adistensible collar 80 preferably positioned at the distal end of theendoprosthesis 11, intended to prevent unintentional axial movement ofendoprosthesis 11.Collar 80 may be a separate component affixed to the exterior ofintermediate sheath 20 at the distal end thereof, immediately proximal toprotrusion 31, or may be made to be integral to theintermediate sheath 20. It is also apparent that thecollar 80 may simply be of the form of any sort of enlargement in the diameter of the distal end of theintermediate sheath 20 that interferes with axial movement of theendoprosthesis 11 and thus prevents an unintentional movement of the endoprosthesis. As such, the enlargement is not required to extend entirely around the circumference of the distal end ofintermediate sheath 20 in the fashion ofcollar 80. Such an enlargement may be integral with the distal end ofintermediate sheath 20 or separately affixed. In an alternative, either the collar or another form of enlargement may be positioned beneath the endoprosthesis anywhere within the length of the endoprosthesis, thereby making difficult any unintended movement of the endoprosthesis with respect to the elongatetensile member 30. - FIG. 10 is a transverse cross section taken at section D-D of FIG. 9, describing the[0073]
distensible collar 80. - FIGS. 11A and 11B are perspective views of an alternative embodiment of the present invention wherein the substantially[0074]
tubular sheath 20 contains multiple parallel splits adjacent to its distal end that do not extend entirely to the end. As shown by FIG. 11B, this form of theintermediate sheath 20 would increase in diameter when theprotrusion 31 is pulled against its distal end byelongate actuation member 30, thereby exerting a radially outward directed force against endoprosthesis11 (not shown) and initiating expansion and deployment of the endoprosthesis. In an alternative embodiment shown by FIG. 11C,protrusion 31 is no longer required and the distal end of the substantially tubular sheath is secured to the distal end of theelongate actuation member 30, whereby axial movement of the elongatetensile member 30 with respect to the substantiallytubular sheath 20 results in a compressive force applied to the substantiallytubular sheath 20, causing it to deform outwardly and exert a radially outward force against the endoprosthesis11 (not shown) to initiate expansion and deployment of the endoprosthesis. The distal end ofelongate actuation member 30 may be secured to the distal end of the substantiallytubular sheath 20 by any of various means including compression ring111, or by the use of an adhesive, welding, etc. - FIGS. 12A and 12B are alternative embodiments to those of FIGS. 11A and 11B wherein the substantially[0075]
tubular sheath 20 is made to increase in diameter in a corrugated or accordion-fashion whenprotrusion 31 is moved axially against the substantiallytubular sheath 20 by the application of tension to theelongate actuation member 30. As with the embodiment of FIG. 11C, this can also be accomplished without requiring aprotrusion 31 by securing the distal end of the substantiallytubular sheath 20 to the distal end of theelongate actuation member 30. - While the principles of the invention have been made clear in the illustrative embodiments set forth herein, it will be obvious to those skilled in the art to make various modifications to the structure, arrangement, proportion, elements, materials and components used in the practice of the invention. For example, the protrusion may be fitted at the proximal end of the system and moved axially in a distal direction to initiate endoprosthesis expansion in a proximal-to-distal direction. To the extent that these various modifications do not depart from the spirit and scope of the appended claims, they are intended to be encompassed therein.[0076]
Claims (50)
1. An endoluminal expansion system comprising:
a) a protrusion affixed to an elongate actuation member, said protrusion having a maximum diameter of D2;
b) a substantially tubular sheath mounted coaxially about the elongate actuation member, said elongate actuation member being axially movable with respect to the substantially tubular sheath, said substantially tubular sheath having an inside diameter of D1, wherein D2is greater than D1; and
c) an expandable endoprosthesis affixed coaxially about the substantially tubular sheath wherein one end of said expandable endoprosthesis is adjacent to said protrusion.
2. An endoluminal expansion system according toclaim 1 wherein said endoprosthesis is a self-expanding endoprosthesis and is contained within a disruptable constraining sheath.
3. An endoluminal expansion system according toclaim 2 wherein said constraining sheath is configured to be an implantable constraining sheath.
4. An endoluminal expansion system according toclaim 2 wherein said constraining sheath is configured to be a removable constraining sheath.
5. An endoluminal expansion system according toclaim 2 wherein said constraining sheath is disruptable via a row of perforations.
6. An endoluminal expansion system according toclaim 2 wherein said constraining sheath comprises porous expanded polytetrafluoroethylene.
7. An endoluminal expansion system according toclaim 6 wherein said constraining sheath comprises a delicate constraining sheath contained within a packaging sheath that is removable prior to insertion of the endoluminal expansion system into a body conduit.
8. An endoluminal expansion system according toclaim 1 wherein said substantially tubular sheath includes at least one split along its length.
9. An endoluminal expansion system according toclaim 1 wherein said substantially tubular sheath comprises a polymeric material less than about 0.12 mm thick.
10. An endoluminal expansion system according toclaim 1 wherein said protrusion comprises a length of tubularly braided wire.
11. An endoluminal expansion system according toclaim 1 wherein said protrusion comprises an inflatable member.
12. An endoluminal expansion system according toclaim 1 wherein said elongate actuation member comprises a tube.
13. An endoluminal expansion system according toclaim 1 wherein said elongate actuation member comprises an elongate tensile member
14. An endoluminal expansion system according toclaim 13 wherein said elongate tensile member comprises a guidewire.
15. An endoluminal expansion system according toclaim 1 wherein said system incorporates a tether element.
16. An endoluminal expansion system according toclaim 1 wherein said substantially tubular sheath includes an enlargement at its distal end.
17. An endoluminal expansion system according toclaim 16 wherein said enlargement is a collar.
18. An endoluminal expansion system according toclaim 1 wherein, following the application of axial compression against an end of the substantially tubular sheath by the protrusion, the substantially tubular sheath assumes a corrugated shape.
19. An endoluminal expansion device comprising:
a protrusion and an associated actuation member, the protrusion having a maximum diameter of D1;
a substantially tubular sheath through which the actuation member moves, the substantially tubular sheath having a maximum diameter of D2, D2being less than D1,
wherein when the protrusion is moved against the substantially tubular sheath, the substantially tubular sheath is dilated to cause an expansion force.
20. An endoluminal expansion device according toclaim 19 wherein the substantially tubular sheath includes at least one split along at least a portion of its length.
21. An expandable endoprosthesis and delivery device comprising the endoprosthesis mounted on a substantially tubular sheath; wherein the delivery device comprises a protrusion and an associated actuation member, the protrusion being proportioned to enlarge the endoprosthesis when the protrusion is actuated against the substantially tubular sheath and endoprosthesis.
22. An endoluminal expansion system according toclaim 21 wherein said endoprosthesis is a self-expanding endoprosthesis and is contained within a disruptable constraining sheath.
23. An endoluminal expansion system according toclaim 22 wherein said constraining sheath is disruptable via a row of perforations.
24. An endoluminal expansion system according toclaim 23 wherein said constraining sheath comprises porous expanded polytetrafluoroethylene.
25. An endoluminal expansion system according toclaim 24 wherein said constraining sheath comprises a delicate constraining sheath contained within a packaging sheath that is removable prior to insertion of the endoluminal expansion system into a body conduit.
26. An endoluminal expansion system according toclaim 21 wherein said substantially tubular sheath includes at least one split along its length.
27. An endoluminal expansion system according toclaim 21 wherein said substantially tubular sheath comprises a polymeric material less than about 0.12 mm thick.
28. A method of expanding an expandable endoluminal prosthesis wherein said endoprosthesis is part of a delivery system comprising:
a) a protrusion affixed to an elongate actuation member, said protrusion having a maximum diameter of D2;
b) a substantially tubular sheath mounted coaxially about the elongate actuation member, said elongate actuation member being axially movable with respect to the substantially tubular sheath, said substantially tubular sheath having an inside diameter of D1, wherein D2is greater than D1; and
c) an expandable endoprosthesis affixed coaxially about the substantially tubular sheath wherein one end of said expandable endoprosthesis is adjacent said protrusion;
said method comprising moving said elongate actuation member and protrusion against said substantially tubular sheath and expandable endoprosthesis, thereby applying a radially outwardly directed force to the endoprosthesis and thereby initiating expansion of the endoprosthesis.
29. A method according toclaim 28 wherein said substantially tubular sheath includes at least one split along its length.
30. A method according toclaim 28 wherein said expandable endoprosthesis is a self-expanding endoprosthesis contained within a disruptable constraining sheath.
31. A method according toclaim 30 wherein said constraining sheath is configured as an implantable constraining sheath.
32. A method according toclaim 30 wherein said constraining sheath is configured as a removable constraining sheath.
33. A method according toclaim 30 wherein said constraining sheath comprises porous expanded polytetrafluoroethylene.
34. A method according toclaim 30 wherein said constraining sheath is disruptable via a row of perforations extending along a length of the constraining sheath.
35. A method according toclaim 34 wherein said constraining sheath comprises porous expanded polytetrafluoroethylene.
36. A method according toclaim 30 wherein said constraining sheath comprises a delicate constraining sheath contained within a packaging sheath that is removable prior to insertion of said endoprosthesis into a body conduit.
37. An endoluminal expansion system comprising:
a) an elongate actuation member having a distal end;
b) a substantially tubular sheath mounted coaxially about the elongate actuation member, said elongate actuation member being axially movable with respect to the substantially tubular sheath, wherein the substantially tubular sheath has a distal end that is secured to the distal end of the elongate actuation member; and
c) an expandable endoprosthesis affixed coaxially about the substantially tubular sheath;
wherein the application of tension to the elongate actuation member applies compression to the substantially tubular sheath whereby a radially outward force is applied to the expandable endoprosthesis by the substantially tubular sheath.
38. An endoluminal expansion system according toclaim 37 wherein said endoprosthesis is a self-expanding endoprosthesis and is contained within a disruptable constraining sheath.
39. An endoluminal expansion system according toclaim 38 wherein said constraining sheath is disruptable via a row of perforations.
40. An endoluminal expansion system according toclaim 38 wherein said constraining sheath comprises porous expanded polytetrafluoroethylene.
41. An endoluminal expansion system according toclaim 38 wherein said constraining sheath comprises a delicate constraining sheath contained within a packaging sheath that is removable prior to insertion of the endoluminal expansion system into a body conduit.
42. An endoluminal expansion system according toclaim 37 wherein said elongate actuation member comprises a tube.
43. An endoluminal expansion system according toclaim 37 wherein said elongate actuation member comprises an elongate tensile member
44. An endoluminal expansion system according toclaim 43 wherein said elongate tensile member comprises a guidewire.
45. An endoluminal expansion system according toclaim 37 wherein said system incorporates a tether element.
46. An endoluminal expansion system according toclaim 37 wherein said substantially tubular sheath includes an enlargement at its distal end.
47. An endoluminal expansion system according toclaim 46 wherein said enlargement is a collar.
48. An endoluminal expansion system according toclaim 37 wherein the application of compression to the substantially tubular sheath causes the substantially tubular sheath to assume a corrugated shape.
49. An endoluminal expansion system comprising an endoprosthesis and a delivery catheter having a balloon affixed at a distal end thereof, said system including a tether secured to the delivery catheter, wherein said tether includes a length that is captive between the balloon and the endoprosthesis.
50. An endoluminal expansion system according toclaim 49 wherein inflation of the balloon results in expansion and deployment of the endoprosthesis and wherein said length of tether is not captive following inflation and subsequent deflation of the balloon.
Priority Applications (8)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US10/201,172US20040015224A1 (en) | 2002-07-22 | 2002-07-22 | Endoluminal expansion system |
| PCT/US2003/021850WO2004008993A1 (en) | 2002-07-22 | 2003-07-11 | Endoluminal expansion system |
| CA002492984ACA2492984C (en) | 2002-07-22 | 2003-07-11 | Endoluminal expansion system |
| JP2004523112AJP2005533557A (en) | 2002-07-22 | 2003-07-11 | Intraluminal dilation system |
| EP03765548AEP1542618A1 (en) | 2002-07-22 | 2003-07-11 | Endoluminal expansion system |
| AU2003247865AAU2003247865A1 (en) | 2002-07-22 | 2003-07-11 | Endoluminal expansion system |
| US11/407,426US20060190071A1 (en) | 2002-07-22 | 2006-04-19 | Endoluminal expansion system |
| US12/876,779US8177832B2 (en) | 2002-07-22 | 2010-09-07 | Endoluminal expansion system |
Applications Claiming Priority (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US10/201,172US20040015224A1 (en) | 2002-07-22 | 2002-07-22 | Endoluminal expansion system |
Related Child Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US11/407,426DivisionUS20060190071A1 (en) | 2002-07-22 | 2006-04-19 | Endoluminal expansion system |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| US20040015224A1true US20040015224A1 (en) | 2004-01-22 |
Family
ID=30443596
Family Applications (3)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US10/201,172AbandonedUS20040015224A1 (en) | 2002-07-22 | 2002-07-22 | Endoluminal expansion system |
| US11/407,426AbandonedUS20060190071A1 (en) | 2002-07-22 | 2006-04-19 | Endoluminal expansion system |
| US12/876,779Expired - Fee RelatedUS8177832B2 (en) | 2002-07-22 | 2010-09-07 | Endoluminal expansion system |
Family Applications After (2)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US11/407,426AbandonedUS20060190071A1 (en) | 2002-07-22 | 2006-04-19 | Endoluminal expansion system |
| US12/876,779Expired - Fee RelatedUS8177832B2 (en) | 2002-07-22 | 2010-09-07 | Endoluminal expansion system |
Country Status (6)
| Country | Link |
|---|---|
| US (3) | US20040015224A1 (en) |
| EP (1) | EP1542618A1 (en) |
| JP (1) | JP2005533557A (en) |
| AU (1) | AU2003247865A1 (en) |
| CA (1) | CA2492984C (en) |
| WO (1) | WO2004008993A1 (en) |
Cited By (64)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2005074837A1 (en)* | 2004-01-30 | 2005-08-18 | Boston Scientific Scimed, Inc. | Clamping fixture for coating stents, system using the fixture, and method of using the fixture |
| US20050203614A1 (en)* | 2004-02-27 | 2005-09-15 | Cardiacmd, Inc. | Prosthetic heart valves, scaffolding structures, and systems and methods for implantation of same |
| US20060206187A1 (en)* | 2005-03-09 | 2006-09-14 | Cook Incorporated | Stent delivery system |
| US20070016243A1 (en)* | 2005-06-28 | 2007-01-18 | Venkatesh Ramaiah | Non-occlusive, retrievable dilation system |
| US20070073387A1 (en)* | 2004-02-27 | 2007-03-29 | Forster David C | Prosthetic Heart Valves, Support Structures And Systems And Methods For Implanting The Same |
| US20070156225A1 (en)* | 2003-12-23 | 2007-07-05 | Xtent, Inc. | Automated control mechanisms and methods for custom length stent apparatus |
| US20070167974A1 (en)* | 2006-01-13 | 2007-07-19 | Cully Edward H | Removable blood conduit filter |
| US20070203561A1 (en)* | 2006-02-27 | 2007-08-30 | Cardiacmd, Inc. A California Corporation | Methods and devices for delivery of prosthetic heart valves and other prosthetics |
| US20070203575A1 (en)* | 2006-02-27 | 2007-08-30 | Cardiacmd, Inc., A California Corporation | Methods and devices for delivery of prosthetic heart valves and other prosthetics |
| US20070219612A1 (en)* | 2006-03-20 | 2007-09-20 | Xtent, Inc. | Apparatus and methods for deployment of linked prosthetic segments |
| US20080091257A1 (en)* | 2003-12-23 | 2008-04-17 | Xtent, Inc. | Devices and methods for controlling and indicating the length of an interventional element |
| US20080097299A1 (en)* | 2004-03-30 | 2008-04-24 | Xtent, Inc. | Rapid exchange interventional devices and methods |
| US20080114439A1 (en)* | 2005-06-28 | 2008-05-15 | Venkatesh Ramaiah | Non-occluding dilation device |
| US20080125850A1 (en)* | 2001-12-03 | 2008-05-29 | Xtent, Inc. | Stent delivery apparatus and method |
| US20080132989A1 (en)* | 2004-06-28 | 2008-06-05 | Xtent, Inc. | Devices and methods for controlling expandable prostheses during deployment |
| US20080140175A1 (en)* | 2006-12-07 | 2008-06-12 | Boucher Donald D | Spring stop for stent delivery system and delivery system provided with same |
| US20090099554A1 (en)* | 2006-06-20 | 2009-04-16 | Forster David C | Elongate Flexible Torque Instruments And Methods Of Use |
| US20090132035A1 (en)* | 2004-02-27 | 2009-05-21 | Roth Alex T | Prosthetic Heart Valves, Support Structures and Systems and Methods for Implanting the Same |
| US20090149863A1 (en)* | 2003-10-14 | 2009-06-11 | Xtent, Inc. | Fixed stent delivery devices and methods |
| WO2009073894A1 (en)* | 2007-12-07 | 2009-06-11 | Lander, Harry | Variable-diameter biopsy punch |
| US20090210052A1 (en)* | 2006-06-20 | 2009-08-20 | Forster David C | Prosthetic heart valves, support structures and systems and methods for implanting same |
| US20090228098A1 (en)* | 2006-06-21 | 2009-09-10 | Forster David C | Prosthetic valve implantation systems |
| US20090234428A1 (en)* | 2004-06-28 | 2009-09-17 | Xtent, Inc. | Devices and methods for controlling expandable prostheses during deployment |
| US20090264979A1 (en)* | 2003-01-17 | 2009-10-22 | Xtent, Inc. | Multiple independent nested stent structures and methods for their preparation and deployment |
| US20090281611A1 (en)* | 2004-03-02 | 2009-11-12 | Cardiomind, Inc. | Sliding restraint stent delivery systems |
| WO2009140437A1 (en)* | 2008-05-13 | 2009-11-19 | Nfocus Neuromedical, Inc. | Braid implant delivery systems |
| US20100023105A1 (en)* | 2008-07-22 | 2010-01-28 | Micro Therapeutics, Inc. | Vascular remodeling device |
| US20100023113A1 (en)* | 2006-07-24 | 2010-01-28 | Cardiatis S.A. | Reversible applicator for an intraluminal endoprosthesis |
| US20100057185A1 (en)* | 2008-09-04 | 2010-03-04 | Cook Incorporated | Sliding Split-Sleeve Implant Compressor |
| US20100256752A1 (en)* | 2006-09-06 | 2010-10-07 | Forster David C | Prosthetic heart valves, support structures and systems and methods for implanting the same, |
| US20110022148A1 (en)* | 2007-02-20 | 2011-01-27 | Xtent, Inc. | Thermo-mechanically controlled implants and methods of use |
| US20110046663A1 (en)* | 2009-08-24 | 2011-02-24 | St. Jude Medical Puerto Rico Llc | Polymer membrane locator with built-in stress relief structure |
| US20110082490A1 (en)* | 2009-05-15 | 2011-04-07 | Lemaitre Vascular, Inc. | Non-Occlusive Dilation Devices |
| US20110093056A1 (en)* | 2006-06-02 | 2011-04-21 | Xtent, Inc. | Use of Plasma in Formation of Biodegradable Stent Coating |
| US20110125248A1 (en)* | 2001-12-03 | 2011-05-26 | Xtent, Inc. | Custom length stent apparatus |
| US20110152996A1 (en)* | 2001-12-03 | 2011-06-23 | Xtent, Inc. | Apparatus and methods for delivery of multiple distributed stents |
| US20110178589A1 (en)* | 2001-12-03 | 2011-07-21 | Xtent, Inc. | Apparatus and methods for delivery of braided prostheses |
| US20110184453A1 (en)* | 2010-01-28 | 2011-07-28 | Micro Therapeutics, Inc. | Vascular remodeling device |
| US20110184452A1 (en)* | 2010-01-28 | 2011-07-28 | Micro Therapeutics, Inc. | Vascular remodeling device |
| EP2497447A1 (en)* | 2011-03-08 | 2012-09-12 | Cook Medical Technologies LLC | Introducer assembly and carrier element for a medical device |
| US20130150945A1 (en)* | 2011-12-08 | 2013-06-13 | W. L. Gore & Associates, Inc. | Systems and methods for delivery of a medical device |
| WO2013096545A1 (en)* | 2011-12-20 | 2013-06-27 | Boston Scientific Scimed, Inc. | Medical device delivery systems |
| US8486132B2 (en) | 2007-03-22 | 2013-07-16 | J.W. Medical Systems Ltd. | Devices and methods for controlling expandable prostheses during deployment |
| US20130245752A1 (en)* | 2010-09-14 | 2013-09-19 | Transcatheter Technologies Gmbh | Device intended to be attached to or interconnected with a catheter, catheter and method |
| US8696701B2 (en) | 2008-04-21 | 2014-04-15 | Covidien Lp | Braid-ball embolic devices |
| US20140107758A1 (en)* | 2012-10-12 | 2014-04-17 | St. Jude Medical, Cardiology Division, Inc. | Retaining cage to permit resheathing of a tavi aortic-first transapical system |
| US20140257246A1 (en)* | 2010-11-16 | 2014-09-11 | W. L. Gore & Associated, Inc. | Medical apparatus and method of making the same |
| US20140343684A1 (en)* | 2010-06-26 | 2014-11-20 | Scott M. Epstein | Catheter or stent delivery system |
| US9060886B2 (en) | 2011-09-29 | 2015-06-23 | Covidien Lp | Vascular remodeling device |
| US9089332B2 (en) | 2011-03-25 | 2015-07-28 | Covidien Lp | Vascular remodeling device |
| US9095342B2 (en) | 2009-11-09 | 2015-08-04 | Covidien Lp | Braid ball embolic device features |
| US9101503B2 (en) | 2008-03-06 | 2015-08-11 | J.W. Medical Systems Ltd. | Apparatus having variable strut length and methods of use |
| US20150246210A1 (en)* | 2005-07-14 | 2015-09-03 | Cappella, Inc. | Delivery system and method of use for deployment of self-expandable vascular device |
| US9295571B2 (en) | 2013-01-17 | 2016-03-29 | Covidien Lp | Methods and apparatus for luminal stenting |
| US9314248B2 (en) | 2012-11-06 | 2016-04-19 | Covidien Lp | Multi-pivot thrombectomy device |
| US9393022B2 (en) | 2011-02-11 | 2016-07-19 | Covidien Lp | Two-stage deployment aneurysm embolization devices |
| US9463105B2 (en) | 2013-03-14 | 2016-10-11 | Covidien Lp | Methods and apparatus for luminal stenting |
| WO2017011358A1 (en)* | 2015-07-10 | 2017-01-19 | Boston Scientific Scimed, Inc. | Detachable implantable devices |
| US20170042669A1 (en)* | 2015-08-12 | 2017-02-16 | Boston Scientific Scimed, Inc. | Pinless release mechanism |
| US10478194B2 (en) | 2015-09-23 | 2019-11-19 | Covidien Lp | Occlusive devices |
| US10736758B2 (en) | 2013-03-15 | 2020-08-11 | Covidien | Occlusive device |
| US10751206B2 (en)* | 2010-06-26 | 2020-08-25 | Scott M. Epstein | Catheter or stent delivery system |
| WO2022060710A1 (en)* | 2020-09-21 | 2022-03-24 | Boston Scientific Scimed, Inc. | Stents with liner facilitating stent removal |
| CN116849750A (en)* | 2023-09-02 | 2023-10-10 | 杭州亿科医疗科技有限公司 | Aneurysm turbulent flow device capable of being mechanically released |
Families Citing this family (10)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| TR200605770A2 (en)* | 2006-10-16 | 2007-10-22 | Ykk Saglik Hizmetleri Anonim Sirketi | Flexible and rigid catheter resector balloon |
| JP5604110B2 (en)* | 2007-02-05 | 2014-10-08 | ボストン サイエンティフィック リミテッド | System for delivering a valve |
| JP2010183957A (en)* | 2009-02-10 | 2010-08-26 | Olympus Corp | Stent expansion device |
| DE102009055969A1 (en)* | 2009-11-27 | 2011-06-01 | Transcatheter Technologies Gmbh | Device and set for folding or unfolding a medical implant and method |
| EP2702964B1 (en)* | 2011-04-27 | 2017-07-19 | Piolax Medical Devices, Inc. | Stent |
| US10357388B2 (en)* | 2011-06-03 | 2019-07-23 | Cook Medical Technologies Llc | Prosthesis delivery system |
| KR101419019B1 (en)* | 2012-09-28 | 2014-07-11 | 신경민 | Plastic stent insertion device Plastic stent insertion device |
| US10258492B2 (en) | 2017-03-03 | 2019-04-16 | Cook Medical Technologies Llc | Prosthesis delivery system with axially collapsible sheath |
| US10905577B2 (en) | 2017-04-28 | 2021-02-02 | Covidien Lp | Stent delivery system |
| US11045339B2 (en) | 2018-12-20 | 2021-06-29 | lnnAVasc Medical, Inc. | Apparatus and method for implanting an arteriovenous graft |
Citations (5)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US5026377A (en)* | 1989-07-13 | 1991-06-25 | American Medical Systems, Inc. | Stent placement instrument and method |
| US6174327B1 (en)* | 1998-02-27 | 2001-01-16 | Scimed Life Systems, Inc. | Stent deployment apparatus and method |
| US6355060B1 (en)* | 1994-06-08 | 2002-03-12 | Medtronic Ave, Inc. | Apparatus and method for deployment release of intraluminal prostheses |
| US6447540B1 (en)* | 1996-11-15 | 2002-09-10 | Cook Incorporated | Stent deployment device including splittable sleeve containing the stent |
| US20020188341A1 (en)* | 2001-05-10 | 2002-12-12 | Elliott Christopher J. | Stent with detachable tethers and method of using same |
Family Cites Families (36)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US3585707A (en)* | 1966-04-13 | 1971-06-22 | Cordis Corp | Method of making tubular products |
| CA962021A (en) | 1970-05-21 | 1975-02-04 | Robert W. Gore | Porous products and process therefor |
| US4878906A (en) | 1986-03-25 | 1989-11-07 | Servetus Partnership | Endoprosthesis for repairing a damaged vessel |
| US5019085A (en) | 1988-10-25 | 1991-05-28 | Cordis Corporation | Apparatus and method for placement of a stent within a subject vessel |
| US5571169A (en)* | 1993-06-07 | 1996-11-05 | Endovascular Instruments, Inc. | Anti-stenotic method and product for occluded and partially occluded arteries |
| FR2688688A1 (en)* | 1992-03-12 | 1993-09-24 | Richard Thierry | Tool for fitting an autoexpansible endoprosthesis for human or animal tubular organ |
| FR2688401B1 (en) | 1992-03-12 | 1998-02-27 | Thierry Richard | EXPANDABLE STENT FOR HUMAN OR ANIMAL TUBULAR MEMBER, AND IMPLEMENTATION TOOL. |
| US5405378A (en) | 1992-05-20 | 1995-04-11 | Strecker; Ernst P. | Device with a prosthesis implantable in the body of a patient |
| EP0746375B1 (en) | 1993-10-22 | 2005-02-02 | SciMed Life Systems, Inc. | Improved stent delivery apparatus and method |
| AU1333895A (en)* | 1993-11-30 | 1995-06-19 | Raymond R. Burke | Computer system for allowing a consumer to purchase packaged goods at home |
| US5403341A (en)* | 1994-01-24 | 1995-04-04 | Solar; Ronald J. | Parallel flow endovascular stent and deployment apparatus therefore |
| US6015429A (en) | 1994-09-08 | 2000-01-18 | Gore Enterprise Holdings, Inc. | Procedures for introducing stents and stent-grafts |
| NL9401633A (en)* | 1994-10-04 | 1996-05-01 | Surgical Innovations Vof | Assembly for the treatment of blood vessels and a method thereof. |
| US5647857A (en)* | 1995-03-16 | 1997-07-15 | Endotex Interventional Systems, Inc. | Protective intraluminal sheath |
| US5814405A (en) | 1995-08-04 | 1998-09-29 | W. L. Gore & Associates, Inc. | Strong, air permeable membranes of polytetrafluoroethylene |
| JPH11511352A (en) | 1995-08-24 | 1999-10-05 | インプラ・インコーポレーテッド | Covered intraluminal stent and method of assembling the same |
| US5906606A (en) | 1995-12-04 | 1999-05-25 | Target Therapuetics, Inc. | Braided body balloon catheter |
| US5690643A (en)* | 1996-02-20 | 1997-11-25 | Leocor, Incorporated | Stent delivery system |
| US6217585B1 (en)* | 1996-08-16 | 2001-04-17 | Converge Medical, Inc. | Mechanical stent and graft delivery system |
| US6123712A (en) | 1996-08-23 | 2000-09-26 | Scimed Life Systems, Inc. | Balloon catheter with stent securement means |
| US5797879A (en)* | 1996-08-26 | 1998-08-25 | Decampli; William M. | Apparatus and methods for providing selectively adjustable blood flow through a vascular graft |
| US6086610A (en) | 1996-10-22 | 2000-07-11 | Nitinol Devices & Components | Composite self expanding stent device having a restraining element |
| US6352561B1 (en) | 1996-12-23 | 2002-03-05 | W. L. Gore & Associates | Implant deployment apparatus |
| IT1294549B1 (en)* | 1997-09-05 | 1999-04-12 | Invatec Srl | INTRODUCTION SYSTEM AND POSITIONING OF EXPANDABLE STENTS |
| US6027519A (en)* | 1997-12-15 | 2000-02-22 | Stanford; Ulf Harry | Catheter with expandable multiband segment |
| US5971990A (en)* | 1998-04-20 | 1999-10-26 | Invatec S.R.L. | System for introducing and positioning expandable stents |
| US6450989B2 (en)* | 1998-04-27 | 2002-09-17 | Artemis Medical, Inc. | Dilating and support apparatus with disease inhibitors and methods for use |
| US6224627B1 (en) | 1998-06-15 | 2001-05-01 | Gore Enterprise Holdings, Inc. | Remotely removable covering and support |
| US6673102B1 (en)* | 1999-01-22 | 2004-01-06 | Gore Enterprises Holdings, Inc. | Covered endoprosthesis and delivery system |
| US6200325B1 (en) | 1999-03-31 | 2001-03-13 | Advanced Cardiovascular Systems, Inc. | Balloon catheter and stent deploying catheter system |
| US6398802B1 (en)* | 1999-06-21 | 2002-06-04 | Scimed Life Systems, Inc. | Low profile delivery system for stent and graft deployment |
| US6432129B2 (en) | 2000-02-22 | 2002-08-13 | Scimed Life Systems, Inc. | Stent delivery system |
| US6702843B1 (en)* | 2000-04-12 | 2004-03-09 | Scimed Life Systems, Inc. | Stent delivery means with balloon retraction means |
| US6432130B1 (en) | 2000-04-20 | 2002-08-13 | Scimed Life Systems, Inc. | Fully sheathed balloon expandable stent delivery system |
| US6657289B1 (en)* | 2001-07-13 | 2003-12-02 | Alien Technology Corporation | Apparatus relating to block configurations and fluidic self-assembly processes |
| WO2007053823A2 (en)* | 2005-10-31 | 2007-05-10 | Biolucent, Inc. | Brachytherapy apparatus and methods of using same |
- 2002
- 2002-07-22USUS10/201,172patent/US20040015224A1/ennot_activeAbandoned
- 2003
- 2003-07-11WOPCT/US2003/021850patent/WO2004008993A1/enactiveApplication Filing
- 2003-07-11JPJP2004523112Apatent/JP2005533557A/enactivePending
- 2003-07-11CACA002492984Apatent/CA2492984C/ennot_activeExpired - Fee Related
- 2003-07-11EPEP03765548Apatent/EP1542618A1/ennot_activeWithdrawn
- 2003-07-11AUAU2003247865Apatent/AU2003247865A1/ennot_activeAbandoned
- 2006
- 2006-04-19USUS11/407,426patent/US20060190071A1/ennot_activeAbandoned
- 2010
- 2010-09-07USUS12/876,779patent/US8177832B2/ennot_activeExpired - Fee Related
Patent Citations (5)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US5026377A (en)* | 1989-07-13 | 1991-06-25 | American Medical Systems, Inc. | Stent placement instrument and method |
| US6355060B1 (en)* | 1994-06-08 | 2002-03-12 | Medtronic Ave, Inc. | Apparatus and method for deployment release of intraluminal prostheses |
| US6447540B1 (en)* | 1996-11-15 | 2002-09-10 | Cook Incorporated | Stent deployment device including splittable sleeve containing the stent |
| US6174327B1 (en)* | 1998-02-27 | 2001-01-16 | Scimed Life Systems, Inc. | Stent deployment apparatus and method |
| US20020188341A1 (en)* | 2001-05-10 | 2002-12-12 | Elliott Christopher J. | Stent with detachable tethers and method of using same |
Cited By (148)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US8702781B2 (en) | 2001-12-03 | 2014-04-22 | J.W. Medical Systems Ltd. | Apparatus and methods for delivery of multiple distributed stents |
| US20110152996A1 (en)* | 2001-12-03 | 2011-06-23 | Xtent, Inc. | Apparatus and methods for delivery of multiple distributed stents |
| US20110125248A1 (en)* | 2001-12-03 | 2011-05-26 | Xtent, Inc. | Custom length stent apparatus |
| US8177831B2 (en) | 2001-12-03 | 2012-05-15 | Xtent, Inc. | Stent delivery apparatus and method |
| US8574282B2 (en) | 2001-12-03 | 2013-11-05 | J.W. Medical Systems Ltd. | Apparatus and methods for delivery of braided prostheses |
| US9326876B2 (en) | 2001-12-03 | 2016-05-03 | J.W. Medical Systems Ltd. | Apparatus and methods for delivery of multiple distributed stents |
| US8956398B2 (en) | 2001-12-03 | 2015-02-17 | J.W. Medical Systems Ltd. | Custom length stent apparatus |
| US20080125850A1 (en)* | 2001-12-03 | 2008-05-29 | Xtent, Inc. | Stent delivery apparatus and method |
| US20110178589A1 (en)* | 2001-12-03 | 2011-07-21 | Xtent, Inc. | Apparatus and methods for delivery of braided prostheses |
| US8282680B2 (en) | 2003-01-17 | 2012-10-09 | J. W. Medical Systems Ltd. | Multiple independent nested stent structures and methods for their preparation and deployment |
| US20090264979A1 (en)* | 2003-01-17 | 2009-10-22 | Xtent, Inc. | Multiple independent nested stent structures and methods for their preparation and deployment |
| US8740968B2 (en) | 2003-01-17 | 2014-06-03 | J.W. Medical Systems Ltd. | Multiple independent nested stent structures and methods for their preparation and deployment |
| US20090149863A1 (en)* | 2003-10-14 | 2009-06-11 | Xtent, Inc. | Fixed stent delivery devices and methods |
| US9566179B2 (en) | 2003-12-23 | 2017-02-14 | J.W. Medical Systems Ltd. | Devices and methods for controlling and indicating the length of an interventional element |
| US20070156225A1 (en)* | 2003-12-23 | 2007-07-05 | Xtent, Inc. | Automated control mechanisms and methods for custom length stent apparatus |
| US20080091257A1 (en)* | 2003-12-23 | 2008-04-17 | Xtent, Inc. | Devices and methods for controlling and indicating the length of an interventional element |
| US8585747B2 (en) | 2003-12-23 | 2013-11-19 | J.W. Medical Systems Ltd. | Devices and methods for controlling and indicating the length of an interventional element |
| US7306677B2 (en) | 2004-01-30 | 2007-12-11 | Boston Scientific Corporation | Clamping fixture for coating stents, system using the fixture, and method of using the fixture |
| WO2005074837A1 (en)* | 2004-01-30 | 2005-08-18 | Boston Scientific Scimed, Inc. | Clamping fixture for coating stents, system using the fixture, and method of using the fixture |
| US20090132035A1 (en)* | 2004-02-27 | 2009-05-21 | Roth Alex T | Prosthetic Heart Valves, Support Structures and Systems and Methods for Implanting the Same |
| US20110082540A1 (en)* | 2004-02-27 | 2011-04-07 | Forster David C | Prosthetic Heart Valves, Scaffolding Structures, and Systems and Methods for Implantation of Same |
| US20050203615A1 (en)* | 2004-02-27 | 2005-09-15 | Cardiacmd, Inc. | Prosthetic heart valves, scaffolding structures, and systems and methods for implantation of same |
| US8608770B2 (en) | 2004-02-27 | 2013-12-17 | Cardiacmd, Inc. | Prosthetic heart valves, scaffolding structures, and systems and methods for implantation of same |
| US8128692B2 (en) | 2004-02-27 | 2012-03-06 | Aortx, Inc. | Prosthetic heart valves, scaffolding structures, and systems and methods for implantation of same |
| US20050203617A1 (en)* | 2004-02-27 | 2005-09-15 | Cardiacmd, Inc. | Prosthetic heart valves, scaffolding structures, and systems and methods for implantation of same |
| US7785341B2 (en) | 2004-02-27 | 2010-08-31 | Aortx, Inc. | Prosthetic heart valves, scaffolding structures, and systems and methods for implantation of same |
| US9168134B2 (en) | 2004-02-27 | 2015-10-27 | Cardiacmd, Inc. | Method for delivering a prosthetic heart valve with an expansion member |
| US20100256724A1 (en)* | 2004-02-27 | 2010-10-07 | Forster David C | Prosthetic Heart Valves, Scaffolding Structures, and Systems and Methods for Implantation of Same |
| US8430925B2 (en) | 2004-02-27 | 2013-04-30 | Cardiacmd, Inc. | Prosthetic heart valves, scaffolding structures, and systems and methods for implantation of same |
| US20070073387A1 (en)* | 2004-02-27 | 2007-03-29 | Forster David C | Prosthetic Heart Valves, Support Structures And Systems And Methods For Implanting The Same |
| US20050203614A1 (en)* | 2004-02-27 | 2005-09-15 | Cardiacmd, Inc. | Prosthetic heart valves, scaffolding structures, and systems and methods for implantation of same |
| US8728156B2 (en) | 2004-02-27 | 2014-05-20 | Cardiac MD, Inc. | Prosthetic heart valves, scaffolding structures, and systems and methods for implantation of same |
| US20100305691A1 (en)* | 2004-02-27 | 2010-12-02 | Forster David C | Prosthetic Heart Valves, Scaffolding Structures, and Systems and Methods for Implantation of Same |
| US20090281611A1 (en)* | 2004-03-02 | 2009-11-12 | Cardiomind, Inc. | Sliding restraint stent delivery systems |
| US8460358B2 (en) | 2004-03-30 | 2013-06-11 | J.W. Medical Systems, Ltd. | Rapid exchange interventional devices and methods |
| US20080097299A1 (en)* | 2004-03-30 | 2008-04-24 | Xtent, Inc. | Rapid exchange interventional devices and methods |
| US20090234428A1 (en)* | 2004-06-28 | 2009-09-17 | Xtent, Inc. | Devices and methods for controlling expandable prostheses during deployment |
| US8986362B2 (en) | 2004-06-28 | 2015-03-24 | J.W. Medical Systems Ltd. | Devices and methods for controlling expandable prostheses during deployment |
| US20080132989A1 (en)* | 2004-06-28 | 2008-06-05 | Xtent, Inc. | Devices and methods for controlling expandable prostheses during deployment |
| US9700448B2 (en) | 2004-06-28 | 2017-07-11 | J.W. Medical Systems Ltd. | Devices and methods for controlling expandable prostheses during deployment |
| US8317859B2 (en)* | 2004-06-28 | 2012-11-27 | J.W. Medical Systems Ltd. | Devices and methods for controlling expandable prostheses during deployment |
| US20060206187A1 (en)* | 2005-03-09 | 2006-09-14 | Cook Incorporated | Stent delivery system |
| WO2006099049A3 (en)* | 2005-03-09 | 2006-11-23 | Cook Inc | Stent delivery system |
| US20100148409A1 (en)* | 2005-03-09 | 2010-06-17 | Cook Incorporated | Method for manufacturing a stent delivery system |
| US7799266B2 (en) | 2005-03-09 | 2010-09-21 | Cook Incorporated | Method for manufacturing a stent delivery system |
| US20110009945A1 (en)* | 2005-03-09 | 2011-01-13 | Cook Incorporated | Method for deploying a stent delivery system having a blowmolded holder |
| US20070016243A1 (en)* | 2005-06-28 | 2007-01-18 | Venkatesh Ramaiah | Non-occlusive, retrievable dilation system |
| US20080114439A1 (en)* | 2005-06-28 | 2008-05-15 | Venkatesh Ramaiah | Non-occluding dilation device |
| US10369033B2 (en) | 2005-07-14 | 2019-08-06 | Merit Medical Ireland Limited | Delivery system and method of use for deployment of self-expandable vascular device |
| US20150246210A1 (en)* | 2005-07-14 | 2015-09-03 | Cappella, Inc. | Delivery system and method of use for deployment of self-expandable vascular device |
| US20070167974A1 (en)* | 2006-01-13 | 2007-07-19 | Cully Edward H | Removable blood conduit filter |
| US9107733B2 (en)* | 2006-01-13 | 2015-08-18 | W. L. Gore & Associates, Inc. | Removable blood conduit filter |
| EP1988852A4 (en)* | 2006-02-27 | 2010-04-14 | Aortx Inc | Methods and devices for delivery of prosthetic heart valves and other prosthetics |
| US20070203561A1 (en)* | 2006-02-27 | 2007-08-30 | Cardiacmd, Inc. A California Corporation | Methods and devices for delivery of prosthetic heart valves and other prosthetics |
| US20070203575A1 (en)* | 2006-02-27 | 2007-08-30 | Cardiacmd, Inc., A California Corporation | Methods and devices for delivery of prosthetic heart valves and other prosthetics |
| US20100179634A1 (en)* | 2006-02-27 | 2010-07-15 | Forster David C | Methods and Devices for Delivery of Prosthetic Heart Valves and Other Prosthetics |
| US8403981B2 (en) | 2006-02-27 | 2013-03-26 | CardiacMC, Inc. | Methods and devices for delivery of prosthetic heart valves and other prosthetics |
| WO2007101159A2 (en) | 2006-02-27 | 2007-09-07 | Aortx, Inc. | Methods and devices for delivery of prosthetic heart valves and other prosthetics |
| US8147541B2 (en) | 2006-02-27 | 2012-04-03 | Aortx, Inc. | Methods and devices for delivery of prosthetic heart valves and other prosthetics |
| US20070219612A1 (en)* | 2006-03-20 | 2007-09-20 | Xtent, Inc. | Apparatus and methods for deployment of linked prosthetic segments |
| US9883957B2 (en) | 2006-03-20 | 2018-02-06 | J.W. Medical Systems Ltd. | Apparatus and methods for deployment of linked prosthetic segments |
| US8652198B2 (en) | 2006-03-20 | 2014-02-18 | J.W. Medical Systems Ltd. | Apparatus and methods for deployment of linked prosthetic segments |
| US20110093056A1 (en)* | 2006-06-02 | 2011-04-21 | Xtent, Inc. | Use of Plasma in Formation of Biodegradable Stent Coating |
| US20090210052A1 (en)* | 2006-06-20 | 2009-08-20 | Forster David C | Prosthetic heart valves, support structures and systems and methods for implanting same |
| US8376865B2 (en) | 2006-06-20 | 2013-02-19 | Cardiacmd, Inc. | Torque shaft and torque shaft drive |
| US20090099554A1 (en)* | 2006-06-20 | 2009-04-16 | Forster David C | Elongate Flexible Torque Instruments And Methods Of Use |
| US8500799B2 (en) | 2006-06-20 | 2013-08-06 | Cardiacmd, Inc. | Prosthetic heart valves, support structures and systems and methods for implanting same |
| US20090228098A1 (en)* | 2006-06-21 | 2009-09-10 | Forster David C | Prosthetic valve implantation systems |
| US8142492B2 (en) | 2006-06-21 | 2012-03-27 | Aortx, Inc. | Prosthetic valve implantation systems |
| US8048139B2 (en)* | 2006-07-24 | 2011-11-01 | Cardiatis, S.A. | Reversible applicator for an intraluminal endoprosthesis |
| US20100023113A1 (en)* | 2006-07-24 | 2010-01-28 | Cardiatis S.A. | Reversible applicator for an intraluminal endoprosthesis |
| US20100256752A1 (en)* | 2006-09-06 | 2010-10-07 | Forster David C | Prosthetic heart valves, support structures and systems and methods for implanting the same, |
| US20080140175A1 (en)* | 2006-12-07 | 2008-06-12 | Boucher Donald D | Spring stop for stent delivery system and delivery system provided with same |
| US8980297B2 (en) | 2007-02-20 | 2015-03-17 | J.W. Medical Systems Ltd. | Thermo-mechanically controlled implants and methods of use |
| US9457133B2 (en) | 2007-02-20 | 2016-10-04 | J.W. Medical Systems Ltd. | Thermo-mechanically controlled implants and methods of use |
| US20110022148A1 (en)* | 2007-02-20 | 2011-01-27 | Xtent, Inc. | Thermo-mechanically controlled implants and methods of use |
| US9339404B2 (en) | 2007-03-22 | 2016-05-17 | J.W. Medical Systems Ltd. | Devices and methods for controlling expandable prostheses during deployment |
| US8486132B2 (en) | 2007-03-22 | 2013-07-16 | J.W. Medical Systems Ltd. | Devices and methods for controlling expandable prostheses during deployment |
| WO2009073894A1 (en)* | 2007-12-07 | 2009-06-11 | Lander, Harry | Variable-diameter biopsy punch |
| US20090149775A1 (en)* | 2007-12-07 | 2009-06-11 | Harry Lander | Variable-Diameter Punch Biopsy |
| US9101503B2 (en) | 2008-03-06 | 2015-08-11 | J.W. Medical Systems Ltd. | Apparatus having variable strut length and methods of use |
| US9585669B2 (en) | 2008-04-21 | 2017-03-07 | Covidien Lp | Multiple layer filamentary devices for treatment of vascular defects |
| US8696701B2 (en) | 2008-04-21 | 2014-04-15 | Covidien Lp | Braid-ball embolic devices |
| US8747597B2 (en) | 2008-04-21 | 2014-06-10 | Covidien Lp | Methods for making braid-ball occlusion devices |
| US11844528B2 (en) | 2008-04-21 | 2023-12-19 | Covidien Lp | Multiple layer filamentary devices for treatment of vascular defects |
| WO2009140437A1 (en)* | 2008-05-13 | 2009-11-19 | Nfocus Neuromedical, Inc. | Braid implant delivery systems |
| US10610389B2 (en)* | 2008-05-13 | 2020-04-07 | Covidien Lp | Braid implant delivery systems |
| US9675482B2 (en) | 2008-05-13 | 2017-06-13 | Covidien Lp | Braid implant delivery systems |
| US20200197203A1 (en)* | 2008-05-13 | 2020-06-25 | Covidien Lp | Braid implant delivery systems |
| US20170252190A1 (en)* | 2008-05-13 | 2017-09-07 | Covidien Lp | Braid Implant Delivery Systems |
| US20090287292A1 (en)* | 2008-05-13 | 2009-11-19 | Becking Frank P | Braid Implant Delivery Systems |
| US11707371B2 (en)* | 2008-05-13 | 2023-07-25 | Covidien Lp | Braid implant delivery systems |
| US20100023105A1 (en)* | 2008-07-22 | 2010-01-28 | Micro Therapeutics, Inc. | Vascular remodeling device |
| US9179918B2 (en) | 2008-07-22 | 2015-11-10 | Covidien Lp | Vascular remodeling device |
| US8359721B2 (en)* | 2008-09-04 | 2013-01-29 | Cook Medical Technologies Llc | Sliding split-sleeve implant compressor |
| US20100057185A1 (en)* | 2008-09-04 | 2010-03-04 | Cook Incorporated | Sliding Split-Sleeve Implant Compressor |
| US8784467B2 (en) | 2009-05-15 | 2014-07-22 | Lemaitre Vascular, Inc. | Non-occlusive dilation devices |
| US20110082490A1 (en)* | 2009-05-15 | 2011-04-07 | Lemaitre Vascular, Inc. | Non-Occlusive Dilation Devices |
| US20110046663A1 (en)* | 2009-08-24 | 2011-02-24 | St. Jude Medical Puerto Rico Llc | Polymer membrane locator with built-in stress relief structure |
| US9820726B2 (en)* | 2009-08-24 | 2017-11-21 | St. Jude Medical Puerto Rico Llc | Polymer membrane locator with built-in stress relief structure |
| US9095342B2 (en) | 2009-11-09 | 2015-08-04 | Covidien Lp | Braid ball embolic device features |
| US8926681B2 (en) | 2010-01-28 | 2015-01-06 | Covidien Lp | Vascular remodeling device |
| US20110184453A1 (en)* | 2010-01-28 | 2011-07-28 | Micro Therapeutics, Inc. | Vascular remodeling device |
| US9468442B2 (en) | 2010-01-28 | 2016-10-18 | Covidien Lp | Vascular remodeling device |
| US20110184452A1 (en)* | 2010-01-28 | 2011-07-28 | Micro Therapeutics, Inc. | Vascular remodeling device |
| US10568753B2 (en)* | 2010-06-26 | 2020-02-25 | Scott M. Epstein | Catheter or stent delivery system |
| US10751206B2 (en)* | 2010-06-26 | 2020-08-25 | Scott M. Epstein | Catheter or stent delivery system |
| US20140343684A1 (en)* | 2010-06-26 | 2014-11-20 | Scott M. Epstein | Catheter or stent delivery system |
| US9918839B2 (en)* | 2010-09-14 | 2018-03-20 | Venus Medtech (Hangzhou), Inc. | Device intended to be attached to or interconnected with a catheter, catheter and method |
| US20130245752A1 (en)* | 2010-09-14 | 2013-09-19 | Transcatheter Technologies Gmbh | Device intended to be attached to or interconnected with a catheter, catheter and method |
| US20140257246A1 (en)* | 2010-11-16 | 2014-09-11 | W. L. Gore & Associated, Inc. | Medical apparatus and method of making the same |
| US11534576B2 (en) | 2010-11-16 | 2022-12-27 | W. L. Gore & Associates, Inc. | Medical apparatus and method of making the same |
| US10426922B2 (en)* | 2010-11-16 | 2019-10-01 | W. L. Gore & Associates, Inc. | Medical apparatus and method of making the same |
| US9393022B2 (en) | 2011-02-11 | 2016-07-19 | Covidien Lp | Two-stage deployment aneurysm embolization devices |
| EP2497447A1 (en)* | 2011-03-08 | 2012-09-12 | Cook Medical Technologies LLC | Introducer assembly and carrier element for a medical device |
| US11147563B2 (en) | 2011-03-25 | 2021-10-19 | Covidien Lp | Vascular remodeling device |
| US9089332B2 (en) | 2011-03-25 | 2015-07-28 | Covidien Lp | Vascular remodeling device |
| US10004511B2 (en) | 2011-03-25 | 2018-06-26 | Covidien Lp | Vascular remodeling device |
| US11654037B2 (en) | 2011-09-29 | 2023-05-23 | Covidien Lp | Vascular remodeling device |
| US9060886B2 (en) | 2011-09-29 | 2015-06-23 | Covidien Lp | Vascular remodeling device |
| US10828182B2 (en) | 2011-09-29 | 2020-11-10 | Covidien Lp | Vascular remodeling device |
| US20130150945A1 (en)* | 2011-12-08 | 2013-06-13 | W. L. Gore & Associates, Inc. | Systems and methods for delivery of a medical device |
| US9364359B2 (en)* | 2011-12-08 | 2016-06-14 | W. L. Gore & Associates, Inc. | Systems and methods for delivery of a medical device |
| US9277993B2 (en) | 2011-12-20 | 2016-03-08 | Boston Scientific Scimed, Inc. | Medical device delivery systems |
| CN104125816A (en)* | 2011-12-20 | 2014-10-29 | 波士顿科学国际有限公司 | Medical Device Delivery Systems |
| WO2013096545A1 (en)* | 2011-12-20 | 2013-06-27 | Boston Scientific Scimed, Inc. | Medical device delivery systems |
| US20140107758A1 (en)* | 2012-10-12 | 2014-04-17 | St. Jude Medical, Cardiology Division, Inc. | Retaining cage to permit resheathing of a tavi aortic-first transapical system |
| US10524909B2 (en)* | 2012-10-12 | 2020-01-07 | St. Jude Medical, Cardiology Division, Inc. | Retaining cage to permit resheathing of a tavi aortic-first transapical system |
| US9924959B2 (en) | 2012-11-06 | 2018-03-27 | Covidien Lp | Multi-pivot thrombectomy device |
| US12089863B2 (en) | 2012-11-06 | 2024-09-17 | Covidien Lp | Multi-pivot thrombectomy device |
| US9314248B2 (en) | 2012-11-06 | 2016-04-19 | Covidien Lp | Multi-pivot thrombectomy device |
| US11406405B2 (en) | 2012-11-06 | 2022-08-09 | Covidien Lp | Multi-pivot thrombectomy device |
| US9901472B2 (en) | 2013-01-17 | 2018-02-27 | Covidien Lp | Methods and apparatus for luminal stenting |
| US9295571B2 (en) | 2013-01-17 | 2016-03-29 | Covidien Lp | Methods and apparatus for luminal stenting |
| US9463105B2 (en) | 2013-03-14 | 2016-10-11 | Covidien Lp | Methods and apparatus for luminal stenting |
| US10736758B2 (en) | 2013-03-15 | 2020-08-11 | Covidien | Occlusive device |
| US12150871B2 (en) | 2013-03-15 | 2024-11-26 | Covidien Lp | Occlusive device |
| US11389309B2 (en) | 2013-03-15 | 2022-07-19 | Covidien Lp | Occlusive device |
| WO2017011358A1 (en)* | 2015-07-10 | 2017-01-19 | Boston Scientific Scimed, Inc. | Detachable implantable devices |
| US10856877B2 (en) | 2015-07-10 | 2020-12-08 | Boston Scientific Scimed, Inc. | Detachable implantable devices |
| CN107920823A (en)* | 2015-07-10 | 2018-04-17 | 波士顿科学国际有限公司 | Dismountable implantable device |
| US20170042669A1 (en)* | 2015-08-12 | 2017-02-16 | Boston Scientific Scimed, Inc. | Pinless release mechanism |
| US10179041B2 (en)* | 2015-08-12 | 2019-01-15 | Boston Scientific Scimed Icn. | Pinless release mechanism |
| US11357510B2 (en) | 2015-09-23 | 2022-06-14 | Covidien Lp | Occlusive devices |
| US10478194B2 (en) | 2015-09-23 | 2019-11-19 | Covidien Lp | Occlusive devices |
| WO2022060710A1 (en)* | 2020-09-21 | 2022-03-24 | Boston Scientific Scimed, Inc. | Stents with liner facilitating stent removal |
| US12150875B2 (en) | 2020-09-21 | 2024-11-26 | Boston Scientific Scimed, Inc. | Stents with liner facilitating stent removal and methods of use |
| CN116849750A (en)* | 2023-09-02 | 2023-10-10 | 杭州亿科医疗科技有限公司 | Aneurysm turbulent flow device capable of being mechanically released |
Also Published As
| Publication number | Publication date |
|---|---|
| CA2492984A1 (en) | 2004-01-29 |
| EP1542618A1 (en) | 2005-06-22 |
| JP2005533557A (en) | 2005-11-10 |
| US20060190071A1 (en) | 2006-08-24 |
| US8177832B2 (en) | 2012-05-15 |
| CA2492984C (en) | 2008-09-23 |
| US20100331956A1 (en) | 2010-12-30 |
| AU2003247865A1 (en) | 2004-02-09 |
| WO2004008993A1 (en) | 2004-01-29 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| US8177832B2 (en) | Endoluminal expansion system | |
| US6827731B2 (en) | Deployment system for intraluminal devices | |
| AU2005275353B2 (en) | Deployment system for intraluminal devices | |
| AU2008244607B2 (en) | Side branched endoluminal prostheses and methods of delivery thereof | |
| EP2323595B1 (en) | Controlled deployable medical device | |
| CA2683011C (en) | Catheter having guidewire channel | |
| EP0858299B1 (en) | Braided stent | |
| AU2002248370A1 (en) | Deployment system for intraluminal devices | |
| US20060142838A1 (en) | Medical devices including metallic films and methods for loading and deploying same | |
| US20070016280A1 (en) | Delivery System And Method Of Use For Deployment Of Self-Expandable Vascular Device | |
| CA2701576A1 (en) | Delivery system for a self-expanding device for placement in a bodily lumen | |
| HK1153114A (en) | Controlled deployable medical device | |
| HK1153114B (en) | Controlled deployable medical device |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| AS | Assignment | Owner name:GORE ENTERPRISE HOLDINGS, INC., DELAWARE Free format text:ASSIGNMENT OF ASSIGNORS INTEREST;ASSIGNORS:ARMSTRONG, JOSEPH R.;CULLY, EDWARD H.;NORDHAUSEN, CRAIG T.;AND OTHERS;REEL/FRAME:013766/0388;SIGNING DATES FROM 20020722 TO 20020808 | |
| STCB | Information on status: application discontinuation | Free format text:ABANDONED -- FAILURE TO RESPOND TO AN OFFICE ACTION | |
| AS | Assignment | Owner name:W. L. GORE & ASSOCIATES, INC., DELAWARE Free format text:ASSIGNMENT OF ASSIGNORS INTEREST;ASSIGNOR:GORE ENTERPRISE HOLDINGS, INC.;REEL/FRAME:027906/0508 Effective date:20120130 |