US20030195553A1 - System and method for retaining vaso-occlusive devices within an aneurysm - Google Patents
System and method for retaining vaso-occlusive devices within an aneurysmDownload PDFInfo
- Publication number
- US20030195553A1 US20030195553A1US10/121,980US12198002AUS2003195553A1US 20030195553 A1US20030195553 A1US 20030195553A1US 12198002 AUS12198002 AUS 12198002AUS 2003195553 A1US2003195553 A1US 2003195553A1
- Authority
- US
- United States
- Prior art keywords
- mesh
- vaso
- aneurysmal
- aneurysm
- occlusive device
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Abandoned
Links
- 206010002329AneurysmDiseases0.000titleclaimsabstractdescription125
- 238000000034methodMethods0.000titleclaimsabstractdescription53
- 229910001000nickel titaniumInorganic materials0.000claimsabstractdescription10
- 230000003073embolic effectEffects0.000claimsabstractdescription8
- 230000004913activationEffects0.000claimsdescription14
- 229910045601alloyInorganic materials0.000claimsdescription7
- 239000000956alloySubstances0.000claimsdescription7
- 239000000463materialSubstances0.000claimsdescription7
- 230000000717retained effectEffects0.000claimsdescription3
- 229910001285shape-memory alloyInorganic materials0.000abstractdescription16
- 239000007788liquidSubstances0.000abstractdescription6
- 230000007704transitionEffects0.000abstractdescription2
- 229910000734martensiteInorganic materials0.000description26
- 229910001566austeniteInorganic materials0.000description13
- 238000012549trainingMethods0.000description11
- 230000006870functionEffects0.000description10
- 238000004519manufacturing processMethods0.000description9
- 210000004204blood vesselAnatomy0.000description7
- HZEWFHLRYVTOIW-UHFFFAOYSA-N[Ti].[Ni]Chemical compound[Ti].[Ni]HZEWFHLRYVTOIW-UHFFFAOYSA-N0.000description6
- BASFCYQUMIYNBI-UHFFFAOYSA-NplatinumChemical compound[Pt]BASFCYQUMIYNBI-UHFFFAOYSA-N0.000description5
- 230000007246mechanismEffects0.000description3
- 239000002184metalSubstances0.000description3
- 229910052751metalInorganic materials0.000description3
- 238000002360preparation methodMethods0.000description3
- PXHVJJICTQNCMI-UHFFFAOYSA-NNickelChemical compound[Ni]PXHVJJICTQNCMI-UHFFFAOYSA-N0.000description2
- 210000001367arteryAnatomy0.000description2
- 230000008901benefitEffects0.000description2
- 238000009954braidingMethods0.000description2
- 239000010931goldSubstances0.000description2
- 229910001092metal group alloyInorganic materials0.000description2
- 239000000203mixtureSubstances0.000description2
- 238000012986modificationMethods0.000description2
- 230000004048modificationEffects0.000description2
- 229910052697platinumInorganic materials0.000description2
- 238000011282treatmentMethods0.000description2
- 241000218202CoptisSpecies0.000description1
- 235000002991Coptis groenlandicaNutrition0.000description1
- 208000005189EmbolismDiseases0.000description1
- 230000001154acute effectEffects0.000description1
- 238000013459approachMethods0.000description1
- 230000000740bleeding effectEffects0.000description1
- 230000017531blood circulationEffects0.000description1
- 230000036760body temperatureEffects0.000description1
- 230000008859changeEffects0.000description1
- 239000003795chemical substances by applicationSubstances0.000description1
- 239000011248coating agentSubstances0.000description1
- 238000000576coating methodMethods0.000description1
- 238000010276constructionMethods0.000description1
- 239000000835fiberSubstances0.000description1
- PCHJSUWPFVWCPO-UHFFFAOYSA-NgoldChemical compound[Au]PCHJSUWPFVWCPO-UHFFFAOYSA-N0.000description1
- 229910052737goldInorganic materials0.000description1
- 238000002513implantationMethods0.000description1
- 238000003780insertionMethods0.000description1
- 230000037431insertionEffects0.000description1
- 230000001788irregularEffects0.000description1
- 230000014759maintenance of locationEffects0.000description1
- 229910052759nickelInorganic materials0.000description1
- 238000007747platingMethods0.000description1
- WFKWXMTUELFFGS-UHFFFAOYSA-NtungstenChemical compound[W]WFKWXMTUELFFGS-UHFFFAOYSA-N0.000description1
- 229910052721tungstenInorganic materials0.000description1
- 239000010937tungstenSubstances0.000description1
- 230000002792vascularEffects0.000description1
- 210000005166vasculatureAnatomy0.000description1
- 230000003313weakening effectEffects0.000description1
Images
Classifications
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F2/00—Filters implantable into blood vessels; Prostheses, i.e. artificial substitutes or replacements for parts of the body; Appliances for connecting them with the body; Devices providing patency to, or preventing collapsing of, tubular structures of the body, e.g. stents
- A61F2/02—Prostheses implantable into the body
- A61F2/04—Hollow or tubular parts of organs, e.g. bladders, tracheae, bronchi or bile ducts
- A61F2/06—Blood vessels
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61B—DIAGNOSIS; SURGERY; IDENTIFICATION
- A61B17/00—Surgical instruments, devices or methods
- A61B17/12—Surgical instruments, devices or methods for ligaturing or otherwise compressing tubular parts of the body, e.g. blood vessels or umbilical cord
- A61B17/12022—Occluding by internal devices, e.g. balloons or releasable wires
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61B—DIAGNOSIS; SURGERY; IDENTIFICATION
- A61B17/00—Surgical instruments, devices or methods
- A61B17/12—Surgical instruments, devices or methods for ligaturing or otherwise compressing tubular parts of the body, e.g. blood vessels or umbilical cord
- A61B17/12022—Occluding by internal devices, e.g. balloons or releasable wires
- A61B17/12099—Occluding by internal devices, e.g. balloons or releasable wires characterised by the location of the occluder
- A61B17/12109—Occluding by internal devices, e.g. balloons or releasable wires characterised by the location of the occluder in a blood vessel
- A61B17/12113—Occluding by internal devices, e.g. balloons or releasable wires characterised by the location of the occluder in a blood vessel within an aneurysm
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61B—DIAGNOSIS; SURGERY; IDENTIFICATION
- A61B17/00—Surgical instruments, devices or methods
- A61B17/12—Surgical instruments, devices or methods for ligaturing or otherwise compressing tubular parts of the body, e.g. blood vessels or umbilical cord
- A61B17/12022—Occluding by internal devices, e.g. balloons or releasable wires
- A61B17/12131—Occluding by internal devices, e.g. balloons or releasable wires characterised by the type of occluding device
- A61B17/12168—Occluding by internal devices, e.g. balloons or releasable wires characterised by the type of occluding device having a mesh structure
- A61B17/12172—Occluding by internal devices, e.g. balloons or releasable wires characterised by the type of occluding device having a mesh structure having a pre-set deployed three-dimensional shape
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61B—DIAGNOSIS; SURGERY; IDENTIFICATION
- A61B17/00—Surgical instruments, devices or methods
- A61B17/12—Surgical instruments, devices or methods for ligaturing or otherwise compressing tubular parts of the body, e.g. blood vessels or umbilical cord
- A61B17/12022—Occluding by internal devices, e.g. balloons or releasable wires
- A61B2017/1205—Introduction devices
- A61B2017/12054—Details concerning the detachment of the occluding device from the introduction device
- A61B2017/12063—Details concerning the detachment of the occluding device from the introduction device electrolytically detachable
Definitions
- the present inventionrelates generally to vaso-occlusion, and more particularly to systems and methods for retaining vaso-occlusive devices within an aneurysm.
- occlusive devicesare useful in filling vascular or other body spaces.
- Some body spaces, such as vascular aneurysms,are formed due to a weakening in the wall of an artery. Often these aneurysms are the site of internal bleeding and stroke.
- embolic agentsare known to be, at least ideally, suitable for treatment of these anomalies. These treatments are commonly known as “artificial vasoocclusion.”
- vaso-occlusive coilsImplantable metal coils that are useful as artificial occlusion devices in vasculature lumens or aneurysms are herein referred to as “vaso-occlusive coils.”
- Vaso-occlusive coilsare generally constructed of a wire, usually made of a metal or metal alloy that is wound to a helix. The vaso-occlusive coil assumes an irregular shape upon discharge of the device from the distal end of the catheter.
- a variety of vaso-occlusive coilsare known. For instance, U.S. Pat. No.
- the coilsmay be used for small vessel sites, e.g., 0.5-6 mm in diameter.
- the coilsthemselves are described as being between 0.010 and 0.030 inches in diameter.
- the length of the coil wireis typically 15 to 20 times the diameter of the vessel to be occluded.
- the wire used to make up the coilsmay be, for instance, 0.002 to 0.006 inches in diameter.
- Tungsten, platinum, and gold threads or wiresare said to be preferred.
- vaso-occlusive coilsIn addition to the various types of space filling mechanisms and geometries of vaso-occlusive coils, other particularized features of coil designs, such as mechanisms for delivering vaso-occlusive coils through delivery catheters and implanting them in a desired occlusion site, have also been described.
- the examples of categories of vaso-occlusive coils based upon their delivery mechanismsinclude pushable coils, mechanically detachable coils, and electrolytically detachable coils.
- Pushable coilsare commonly provided in a cartridge and are pushed or “plunged” from the cartridge into a delivery catheter lumen. A pusher advances the pushable coil through and out of the delivery catheter lumen and into the site for occlusion.
- Mechanically detachable vaso-occlusive coilsare typically integrated with a pusher rod and are mechanically detached from the distal end of that pusher after exiting a delivery catheter. Examples of such mechanically detachable vaso-occlusive coils are found in U.S. Pat. No. 5,261,916 to Engelson or U.S. Pat. No. 5,250,071 to Palermo.
- aneurysmspresent a particularly acute medical risk due to the dangers of potential rupture of the thin wall inherent in such aneurysms. Occlusion of aneurysms by use of vaso-occlusive coils without occluding the adjacent artery is a special challenge and is a desirable method of reducing such risk of rupture.
- These vaso-occlusive devicesare typically placed in an aneurysm in a manner described in U.S. Pat. No. 4,739,768 issued to Engelson.
- a microcatheteris initially steered into or adjacent to the entrance of an aneurysm, typically aided by the use of a steerable guidewire. The wire is then withdrawn from the microcatheter lumen and replaced by the vaso-occlusive coil. The vaso-occlusive coil is advanced through and out of the microcatheter, desirably being completely delivered into the aneurysm.
- Aneurysmcommonly known as a “wide neck aneurysm,” is known to present particular difficulty in the placement and retention of vaso-occlusive coils, because vaso-occlusive coils lacking substantial secondary shape strength may be difficult to maintain in position within an aneurysm no matter how skillfully they are placed.
- Wide neck aneurysmsare herein referred to as aneurysms of vessel walls having a neck or “entrance zone” from the adjacent vessel, wherein the entrance zone has a diameter that either: (1) is at least 80% of the largest diameter of the aneurysm; or (2) is clinically observed to be too wide effectively to retain vaso-occlusive coils that are deployed using the techniques discussed above.
- U.S. Pat. No. 6,168,622which describes a vaso-occlusive device with a secondary shape having a bulbous body portion and an anchor.
- the bulbous body portionis deployed within the aneurysm while the anchor is set just outside of the aneurysm, covering the aneurysm's neck or entrance zone.
- the deviceis integrally formed from a tube—clamped at both ends—of braided Nickel-Titanium (NiTi) wires.
- NiTiNickel-Titanium
- vaso-occlusive coilsit may still be desirable to deploy vaso-occlusive coils with such a device, but the bulbous body of the vaso-occlusive device does not provide much space within the aneurysm to allow for insertion and deployment of the coils.
- the present inventionis directed to systems and methods for occluding an aneurysm having an aneurysmal neck and an aneurysmal inner wall.
- a device in accordance with the present inventionincludes a mesh-like structure that may be integrally composed of a shape-memory alloy such as NiTi.
- the devicemay be deployed within the aneurysm through the aneurysmal neck.
- the devicemay be configured to be in a deployed state and an undeployed state, and may be configured to transition from the undeployed state to the deployed state by exposure to a higher temperature and/or by being released from a compressive force.
- the devicemay function to retain finer vaso-occlusive devices such as vaso-occlusive coils and/or embolic liquids. Furthermore, the device itself may function as a vaso-occlusive device. The device may be delivered to the aneurysm by, e.g., a catheter.
- At least one of the proximal and distal ends of the mesh-like structureis configured to be inserted through the aneurysmal neck when in an undeployed state, and configured to flare open and expand against at least a portion of the aneurysmal inner wall when in a deployed state. Having a flared open end may advantageously allow the device to conform to the shape of the aneurysm.
- the distal end of the mesh-like structuremay be configured to be inserted through the aneurysmal neck when in an undeployed state and configured to expand into a retainer against at least a portion of the aneurysmal inner wall when in a deployed state.
- the proximal endmay be configured to expand into an anchor just outside of the aneurysm to secure the mesh-like structure in position.
- FIG. 1is an illustration of a vaso-occlusive device constructed in accordance with a preferred embodiment of the present inventions, wherein the vaso-occlusive device is shown fully deployed within an aneurysm;
- FIGS. 2 ( a ) and 2 ( b )illustrate one preferred method of manufacturing the vaso-occlusive device of FIG. 1 in accordance with the present inventions
- FIG. 3is an illustration of another vaso-occlusive device constructed in accordance with a preferred embodiment of the present inventions, wherein the vaso-occlusive device is shown fully deployed within an aneurysm;
- FIG. 4illustrates one preferred method of manufacturing the vaso-occlusive device of FIG. 3 in accordance with the present inventions
- FIG. 5is an illustration of still another vaso-occlusive device constructed in accordance with a preferred embodiment of the present inventions, wherein the vaso-occlusive device is shown fully deployed within an aneurysm;
- FIG. 6illustrates one preferred method of manufacturing the vaso-occlusive device of FIG. 5 in accordance with the present inventions
- FIG. 7is an illustration of still another vaso-occlusive device constructed in accordance with a preferred embodiment of the present inventions, wherein the vaso-occlusive device is shown fully deployed within an aneurysm;
- FIG. 8illustrates one preferred method of manufacturing the vaso-occlusive device of FIG. 7 in accordance with the present inventions
- FIGS. 9 ( a )-( c )illustrate a preferred method of delivering and deploying the vaso-occlusive device of FIG. 1 into the aneurysm;
- FIGS. 10 ( a )-( d )illustrate a preferred method of delivering and deploying the vaso-occlusive device of FIG. 7 into the aneurysm
- FIGS. 11 ( a )-( c )illustrate a preferred method of delivering and deploying the vaso-occlusive device of FIG. 5 into the aneurysm.
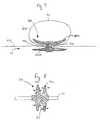
- a vaso-occlusive element 10 constructed in accordance with a preferred embodiment of the present inventionis shown completely deployed within an aneurysm 40 of a blood vessel 60 .
- the aneurysm 40is shown with an oppositely disposed neck 70 and dome 45 .
- the vaso-occlusive element 10When deployed in its secondary shape, i.e., its deployed shape, the vaso-occlusive element 10 generally includes a central tubular element 15 and proximal 20 and distal 30 ends that are flared open into “umbrella” shapes. As illustrated, the flared opened ends 20 / 30 advantageously conform to the shape of the aneurysm 40 .
- the diameter of the proximal end 20 of the vaso-occlusive element 10is larger than the neck 70 , and thus the proximal end 20 may completely cover the neck 70 .
- the vaso-occlusive element 10is manufactured from a relatively dense braid.
- the proximal end 20may densely cover the neck 70 and function as a retainer.
- finer vaso-occlusive devicessuch as vaso-occlusive coils or embolic liquids, may effectively be retained within the aneurysm 40 , or at the least, the likelihood of these devices migrating out from the aneurysm 40 is minimized.
- the relatively dense coverage of the proximal end 20allows the vaso-occlusive element 10 itself to function as a vaso-occlusive device.
- the flared open distal end 30is placed adjacent the dome 45 of the aneurysm 40 and allows the element 10 to safely conform to the shape of the dome 45 .
- the element 10may conform to a large variety of shapes and sizes of aneurysms 40 since the element 10 need only be secured within the aneurysm 40 by conforming the flared open distal end 30 to the dome 45 .
- a braided tubular element 1is constructed by braiding multiple fine wires 2 together.
- the tubular element 1may also be formed of wires with varying diameters braided together. It may be desirable to use a larger number of wires 2 when forming the tubular element 1 , resulting in a relatively dense braid, which provides denser coverage within the aneurysm 40 .
- the tubular element 1may be radiopaque.
- One methodinvolves braiding a radiopaque fiber, such as platinum, Pt, or gold, Au, into the tubular element 1 .
- Another methodinvolves plating or coating the tubular element 1 with radiopaque material. Additionally, one or more radiopaque markers may be added to the tubular element 1 .
- the wires 2are made from a shape memory alloy, which can be of any type, but preferably is a “one way” trainable shape memory alloy.
- a preferable shape memory alloy for forming the braided tubular element 1is Nickel-Titanium (NiTi)—e.g., 144 strands of 0.001′′ NiTi wires.
- NiTiNickel-Titanium
- Shape memory alloyscomprise a unique class of metal alloys that, once trained, are configured to “remember” a preselected shape, i.e., deployed shape, and can return to the preselected shape even if subsequently reshaped.
- the shape memory alloyis molded and heated at or above a training, or austenite, temperature to place the shape memory alloy in an austenite phase.
- the shape memory alloyis formed in the first preselected shape and then, once formed, is permitted to cool to a martensite finish temperature, whereupon the shape memory alloy enters a martensite phase.
- the martensite finish temperaturecan be any temperature that is less than the training temperature.
- the shape memory alloyhas been trained to “remember” the first preselected shape. While in the martensite phase, the alloy is in a soft state and is formed into a second preselected shape, e.g., an undeployed shape.
- the shape memory alloy in the martensite phaseis configured to maintain the second preselected shape and, if subsequently reheated to an activation temperature, automatically returns to the first preselected shape.
- the activation temperaturecan comprise any temperature that is greater than the martensite finish temperature and generally approximately equals the training temperature.
- the shape memory alloyis configured to maintain the first preselected shape irrespective of temperature.
- the training, martensite finishing, and activation temperatures for a shape memory alloyare adjustable, depending on the composition. For example, a slight extra amount of Nickel added to a NiTi alloy composition can change the training temperature from 0° C. to 100° C.
- the vaso-occlusive device 10 described aboveis delivered to the aneurysm 40 via a delivery catheter.
- the catheterdelivers the device 10 to the aneurysm 40 and during the delivery, the device 10 maintains an undeployed shape, e.g., the original tubular shape—methods of delivery will be described in more detail below.
- the device 10When the device 10 is deployed, the device 10 expands into its deployed shape, e.g., having flared open proximal and distal ends 20 / 30 , as shown in FIG. 1.
- Using a shape memory alloy to form the device 10provides for alternative methods of expanding the device 10 into its deployed shape within the aneurysm 40 .
- One methodinvolves configuring the device 10 to self-expand within the aneurysm 40 when deployed and exposed to the temperature of the aneurysm 40 .
- the training and activation temperaturesare adjusted to be at, or just below, the temperature of the aneurysm, which is approximately 37° Celsius—the human body temperature.
- the martensite temperatureis adjusted to be at a lower temperature. With these temperatures set, the device 10 is heated to, or above the training temperature—austenite phase—and shaped into its desired deployed shape, as described above.
- the temperatureis lowered to, or below, the martensite finish temperature—martensite phase—and shaped into its desired undeployed shape.
- the device 10is then placed inside the catheter as will be described below.
- the cathetercan be constructed of a material that insulates the device 10 from the outside environment and maintains the temperature of the device 10 below the activation temperature.
- the device 10does not expand into its deployed shape within the catheter.
- the temperature of the devicewill rise at or above the activation temperature, and the device 10 will expand into its deployed shape. Because the alloy is preferably “one way” trained, the device 10 will then be configured to maintain the deployed shape irrespective of the temperature, as described above.
- An alternative method for expanding the device 10 into its deployed shape within the aneurysmutilizes the super-elastic characteristic of shape memory alloys. After the device 10 is configured to maintain its deployed shape irrespective of the temperature, as described above, the device 10 is then maintained in its austenite phase. When the device 10 is in its austenite phase, the device 10 is super-elastic, i.e., the device 10 may be deformed to a certain degree and still be able to return to its deployed shape. The device 10 is then placed within the catheter as will be described below, with the inner wall of the catheter compressing the device 10 into its undeployed shape. When the device 10 is released out of the catheter into the aneurysm 40 , the uncompressed device 10 automatically expands into its deployed shape because of its super-elasticity.
- Forming the deployed shape shown in FIG. 1is achieved by using two mandrels 85 , each having a cylindrical portion 90 and an umbrella dome-shaped mold 85 , as illustrated in FIG. 2( a ).
- two mandrels 85each having a cylindrical portion 90 and an umbrella dome-shaped mold 85 , as illustrated in FIG. 2( a ).
- each endis placed over the cylindrical portion 90 of a mandrel 85 and compressed against the dome 85 , causing the ends to conform to the shape of the dome 85 and flare open.
- the assembly of the two mandrels 80 and the element 1is then heated at or above the training temperature to place the element 1 in the austenite phase. The assembly is maintained at that temperature until the deployed shape has been formed.
- the assemblyis cooled to, or below, the martensite finishing temperature to place the element 1 in the martensite phase.
- the mandrels 80are removed from the element 1 , and the element 1 is formed into an undeployed shape, e.g., the original tubular shape. This may be achieved by placing the element 1 over a cylindrical mandrel 95 and compressing the flared ends to conform to the shape of the cylindrical mandrel 95 , as shown in FIG. 2 b .
- the element 1may be reheated to the activation temperature, causing the element 1 to return to the deployed shape. Once the deployed shape has been recovered, the element 1 is configured to maintain the deployed shape irrespective of temperature.
- FIG. 3another vaso-occlusive element 110 constructed in accordance with a preferred embodiment of the present invention is shown completely deployed within the aneurysm 40 .
- the vaso-occlusive element 110when deployed, generally includes a central tubular element 115 and a flared distal end 130 .
- the vaso-occlusive element 110further includes a proximal end 120 shaped as a flattened disk, which functions as a retainer.
- the proximal end 130has a diameter larger than the diameter of the neck 70 , and thus completely covers the entrance zone 150 .
- the flattened disk 130has a layer of braided wires on both sides, and thus may advantageously provide two layers of braided wires to cover the entrance zone 150 instead of one layer with the flared open proximal end 20 shown in FIG. 1.
- the flared open distal end 130is placed adjacent the dome 45 of the aneurysm 40 and allows the element 110 to safely conform to the shape of the dome 45 .
- FIG. 4one preferred method of manufacturing the vaso-occlusive device 110 shown in FIG. 3 will now be described.
- a braided tubular element 1is first constructed. Forming the deployed shape is achieved by using one of the mandrels 85 shown in FIG. 2( a ) and the cylindrical mandrel shown in FIG. 2( b ), as illustrated in FIG. 4.
- the distal endis placed over the cylindrical portion 90 of the mandrel 85 with the dome 80 and compressed against the dome 80 , causing the distal end to flare open.
- the proximal endis placed on the cylindrical mandrel 95 .
- the proximal tip and a portion of the central tubular portion 115are then compressed together along the axis of the cylindrical mandrel 95 . This causes the proximal end to expand into a flattened disk 120 .
- the assembly of the two mandrels 80 / 95 and the element 1is then heated at or above the training temperature to place the element 1 in the austenite phase.
- the assemblyis maintained at that temperature until the deployed shape 110 has been formed.
- the assemblyis cooled to, or below, the martensite finishing temperature to place the element 110 in the martensite phase.
- the mandrels 80 / 95are removed from the element 110 , and the element 110 is formed into an undeployed shape, e.g., the original tubular shape 1 . This is achieved by placing the element 110 over the cylindrical mandrel 95 and compressing the flared open end and flattened disk to conform to the shape of the cylindrical mandrel 95 , as shown in FIG.
- the element 1is configured to expand into its deployed shape upon exposure to a higher temperature, then the element 1 may be placed within a delivery catheter in preparation for deployment, as described above. Alternatively, if the element 110 is configured to expand upon being released by a compressive force, then the element 1 is reheated to, or above, the activation temperature, causing the element 1 to return to the deployed shape 110 and to be configured to maintain the deployed shape 110 irrespective of temperature.
- FIG. 5another vaso-occlusive element 210 constructed in accordance with a preferred embodiment of the present invention is shown deployed within the aneurysm 40 .
- the element 210When in a deployed shape, the element 210 generally includes a central portion 215 and a distal end flared open into a “cup” shape 230 . Alternatively, the distal end may be flared open into a “cone” shape.
- the vaso-occlusive element 210further includes a proximal end 220 shaped as a flattened disk. The proximal end 220 has a diameter larger than the diameter of the neck 70 .
- the cup shaped distal end 230may retain fine vaso-occlusive devices such as vaso-occlusive coils and embolic liquids.
- a braided tubular element 1is first constructed. Forming the deployed shape is achieved by using a mandrel 280 having a cup shaped dome 285 facing outward coupled to a cylindrical portion 290 and a cylindrical mandrel 95 . To form the cup shape, the distal end of the element 1 is placed over the cylindrical portion 290 of the mandrel 280 having the dome 285 and compressed against the dome 285 , causing the distal end to flare open into a cup shape. To form the flattened disk, the proximal end is placed over the cylindrical mandrel 95 . The proximal tip and a portion of the central tubular portion 215 are then compressed together along the axis of the cylindrical mandrel 95 . This causes the proximal end to expand into a flattened disk 220 .
- the assembly of the two mandrels 280 / 95 and the element 1is then heated at or above the training temperature to place the element 1 in the austenite phase.
- the assemblyis maintained at that temperature until the deployed shape 210 has been formed.
- the assemblyis cooled to, or below, the martensite finishing temperature to place the element 210 in the martensite phase.
- the mandrels 280 / 95are removed from the element 210 , and the element 210 is formed into an undeployed shape, e.g., the original tubular shape 1 .
- FIG. 7another vaso-occlusive element 310 constructed in accordance with a preferred embodiment of the present invention is shown deployed within the aneurysm 40 .
- the element 310When in a deployed shape, the element 310 generally includes a central portion 315 and first and second flattened disks 330 / 320 formed at the distal and proximal ends respectively. Only the first flattened disk 330 at the distal end is deployed within the aneurysm 40 whereas the second flattened disk 320 is deployed within the lumen 75 of the blood vessel 60 just outside of the aneurysm 40 , functioning as an anchor to secure the element 310 in its position.
- the first flattened disk 330provides a wider coverage than the cup shape 230 shown in FIG. 5.
- this device 310may also retain fine vaso-occlusive devices such as vaso-occlusive coils and embolic liquids.
- the central portion 315may be conventionally sealed to prevent the fine vaso-occlusive devices from migrating out.
- a braided tubular element 1is first constructed. Forming the deployed shape is achieved by using a cylindrical mandrel 95 , as illustrated in FIG. 8. To form the first flattened disk 330 , the distal end is placed on the cylindrical mandrel 95 . The distal tip and a portion of the central tubular portion 315 are then compressed together along the axis of the cylindrical mandrel 95 . This causes the distal end to expand into a flattened disk 330 . To form the second flattened disk 320 , the proximal end is placed on the cylindrical mandrel 95 . The proximal tip and a portion of the central tubular portion 315 are then compressed together along the axis of the cylindrical mandrel 95 . This causes the proximal end to expand into a flattened disk 320 .
- the assembly of the mandrel 95 and the element 1is then heated at or above the training temperature to place the element 1 in the austenite phase.
- the assemblyis maintained at that temperature until the deployed shape 310 has been formed.
- the assemblyis cooled to, or below, the martensite finishing temperature to place the element 310 in the martensite phase.
- the mandrel 95is removed from the element 310 , and the element 310 is formed into an undeployed shape, e.g., the original tubular shape 1 . This may be achieved by placing the element 310 over the cylindrical mandrel 95 and compressing the flattened disks 320 / 330 to conform to the shape of the cylindrical mandrel 95 , as shown in FIG.
- the element 1is configured to expand into its deployed shape upon exposure to a higher temperature, then the element 1 may be placed within a delivery catheter in preparation for deployment, as described above. Alternatively, if the element 110 is configured to expand upon being released by a compressive force, then the element 1 is reheated to, or above, the activation temperature, causing the element 1 to return to the deployed shape 110 and to be configured to maintain the deployed shape 110 irrespective of temperature.
- the vaso-occlusive devices described aboveare delivered to an aneurysm within a blood vessel via a delivery catheter.
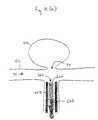
- FIGS. 9 ( a )-( c )a method of deploying a vaso-occlusive device, and in this case the vaso-occlusive device 10 , to the aneurysm 40 via a delivery catheter 450 is illustrated.
- the catheter 450is steered to just within the neck 70 of the aneurysm 40 .
- the vaso-occlusive device 10is in its undeployed shape, and is coupled to an inner guidewire 465 via an electrolytically severable joint 455 .
- the assembly of the vaso-occlusive device 10 and the guidewire 465extends through the lumen of the delivery catheter 450 such that the device 10 is positioned at the distal end of the catheter 450 .
- the guidewire 465is then pushed toward the distal end of the catheter 450 causing the vaso-occlusive device 10 to extend out of the distal end of the catheter 450 , through the neck 70 , and into the aneurysm 40 .
- the portion of the device 10 that is free from the constraints of the catheter 450expands into its deployed shape, e.g., the distal end 30 flares open.
- the device 10may be configured to be in its martensite phase when delivered to the aneurysm 40 and expand upon exposure to the temperature of the aneurysm 40 .
- the temperature of the device 10rises and the device 10 enters the austenite phase and expands into its deployed shape.
- the device 10may be configured to be super-elastic and automatically expand upon being-unconstrained by the catheter 450 .
- the guidewire 465continues to push the device 10 out of the catheter until the proximal end 20 of the device 10 is deployed within the aneurysm 40 , expanding into its deployed shape, e.g., the proximal end 20 flares open, as described above with the distal end 30 .
- a currentis applied to the guidewire 465 , which causes the joint 455 to sever from the proximal end 20 of the catheter 10 .
- vaso-occlusive device 110can be delivered within the aneurysm 40 in the manner described immediately above.
- FIGS. 10 ( a )-( c )a retractable sheath method of delivering a vaso-occlusive device, and in this case vaso-occlusive device 310 , to the aneurysm 40 is illustrated.
- the catheter 550is steered to just within the neck 70 of the aneurysm 40 .
- the vaso-occlusive device 10is in its undeployed shape, and is placed over an inner guidewire 565 that extends through the lumen 580 of the delivery catheter 565 .
- the outer wall 555 of the catheter 565 that defines the lumen 580functions as a sheath.
- the assembly of the vaso-occlusive device 310 and the guidewire 565extends through the lumen 580 of the delivery catheter 450 such that the device 10 is positioned at the distal end of the catheter 550 .
- the guidewire 565is then pushed so that the distal end of vaso-occlusive device 310 extends out of the catheter 550 , through the neck 70 , and into the aneurysm 40 .
- the distal end 330 of the device 310is allowed to expand into a first flattened disk adjacent the inner wall near the neck 70 within the aneurysm 40 .
- the device 310may be configured to be in its martensite phase when delivered to the aneurysm 40 and expand upon exposure to the temperature of the aneurysm 40 .
- the temperature of the device 310rises and the device 310 enters the austenite phase and expands into its deployed shape.
- the device 310may be configured to be super-elastic and expand upon being unconstrained by the catheter 550 .
- the guidewire 565continues to push the device 310 out of the catheter until the proximal end 320 of the device 310 is deployed just outside of the aneurysm 40 , expanding into its deployed shape, e.g., a second flattened disk, as described above with the distal end 330 .
- the guidewire 565is pulled out of the device 310 and the catheter 550 is pulled out of the aneurysm 40 and blood vessel 60 .
- the expanded 310 deviceis secured in position within the aneurysm 40 and will not be pulled out with the catheter 550 .
- other types of vaso-occlusive devicessuch as vaso-occlusive device 310 , can be delivered within the aneurysm 40 in the manner described immediately above.
- a vaso-occlusive devicesuch as vaso-occlusive device 310
- finer vaso-occlusive devices 730may be delivered into the aneurysm 40 to be retained by the device 310 , as illustrated in FIG. 10( d ). This is achieved by maintaining an axial lumen within a vaso-occlusive device to allow a catheter 740 to fit through and insert materials such as vaso-occlusive coils 730 or embolic liquids to further improve thrombogenecity within the aneurysm 700 .
- FIGS. 11 ( a )-( c )a push method of delivering a vaso-occlusive device, and in this case vaso-occlusive device 210 , to the aneurysm 40 is illustrated.
- the catheter 650is steered to just within the neck 70 of the aneurysm 40 .
- the vaso-occlusive device 210is in its undeployed shape, and is placed within the lumen 680 of a delivery catheter 650 near the distal tip of the catheter 650 , which is steered to aneurysm 40 , just outside of the neck 70 .
- a guidewire 665extending through the lumen 655 is positioned such that it is capable of pushing the device 210 out of the catheter 650 and into the aneurysm 40 , where the device 230 may expand into its deployed shape, as described above.
- the guidewire 665is then pushed so that the distal end of vaso-occlusive device 210 extends out of the catheter 650 , through the neck 70 , and into the aneurysm 40 .
- the distal end 230 of the device 210is allowed to expand into a flared open cup shape adjacent the inner wall near the neck 70 within the aneurysm 40 .
- the device 210may be configured to be in its martensite phase when delivered to the aneurysm 40 and expand upon exposure to the temperature of the aneurysm 40 .
- the temperature of the device 210rises and the device 210 enters the austenite phase and expands into its deployed shape.
- the device 210may be configured to be super-elastic and expand upon being unconstrained by the catheter 650 .
- the guidewire 665continues to push the device 210 out of the catheter until the proximal end 220 of the device 210 is deployed just outside of the aneurysm 40 , expanding into its deployed shape, e.g., a flattened disk, as described above with the distal end 230 .
- the catheter 650is then pulled out of the aneurysm 40 and blood vessel 60 to separate itself from the device 210 .
- the expanded 210 deviceis secured in position within the aneurysm 40 and will not be pulled out with the catheter 650 .
- other types of vaso-occlusive devicessuch as vaso-occlusive device 210 , can be delivered within the aneurysm 40 in the manner described immediately above.
Landscapes
- Health & Medical Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Surgery (AREA)
- Engineering & Computer Science (AREA)
- Veterinary Medicine (AREA)
- Public Health (AREA)
- General Health & Medical Sciences (AREA)
- Biomedical Technology (AREA)
- Heart & Thoracic Surgery (AREA)
- Vascular Medicine (AREA)
- Animal Behavior & Ethology (AREA)
- Molecular Biology (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Reproductive Health (AREA)
- Medical Informatics (AREA)
- Transplantation (AREA)
- Oral & Maxillofacial Surgery (AREA)
- Neurosurgery (AREA)
- Gastroenterology & Hepatology (AREA)
- Cardiology (AREA)
- Pulmonology (AREA)
- Surgical Instruments (AREA)
- Pharmaceuticals Containing Other Organic And Inorganic Compounds (AREA)
- Medicines Containing Material From Animals Or Micro-Organisms (AREA)
- Medicines Containing Antibodies Or Antigens For Use As Internal Diagnostic Agents (AREA)
Abstract
Description
- The present invention relates generally to vaso-occlusion, and more particularly to systems and methods for retaining vaso-occlusive devices within an aneurysm.[0001]
- Different implantable medical devices have been developed for treating a number of ailments associated with body lumens. In particular, occlusive devices are useful in filling vascular or other body spaces. Some body spaces, such as vascular aneurysms, are formed due to a weakening in the wall of an artery. Often these aneurysms are the site of internal bleeding and stroke. A variety of different embolic agents are known to be, at least arguably, suitable for treatment of these anomalies. These treatments are commonly known as “artificial vasoocclusion.”[0002]
- Over the past few years, advancements in the artificial occlusion of vessels and aneurysms have included the delivery and implantation of metal coils as vaso-occlusive devices. Implantable metal coils that are useful as artificial occlusion devices in vasculature lumens or aneurysms are herein referred to as “vaso-occlusive coils.” Vaso-occlusive coils are generally constructed of a wire, usually made of a metal or metal alloy that is wound to a helix. The vaso-occlusive coil assumes an irregular shape upon discharge of the device from the distal end of the catheter. A variety of vaso-occlusive coils are known. For instance, U.S. Pat. No. 4,994,069, issued to Ritchart et al., shows a flexible, preferably coiled, wire for use in small vessel vaso-occlusion. Unlike vaso-occlusive coils used prior to that time, Ritchart et al. teach a coil that is fairly soft and is delivered to the site using a pusher within a catheter lumen. Upon discharge from the delivery catheter, the coil may undertake any number of random or regular configurations used to fill the site.[0003]
- The coils may be used for small vessel sites, e.g., 0.5-6 mm in diameter. The coils themselves are described as being between 0.010 and 0.030 inches in diameter. The length of the coil wire is typically 15 to 20 times the diameter of the vessel to be occluded. The wire used to make up the coils may be, for instance, 0.002 to 0.006 inches in diameter. Tungsten, platinum, and gold threads or wires are said to be preferred. These coils have a variety of benefits including the fact that they are relatively permanent, they may be easily imaged radiographically, they may be located at a well defined vessel site, and they can be retrieved.[0004]
- In addition to the various types of space filling mechanisms and geometries of vaso-occlusive coils, other particularized features of coil designs, such as mechanisms for delivering vaso-occlusive coils through delivery catheters and implanting them in a desired occlusion site, have also been described. The examples of categories of vaso-occlusive coils based upon their delivery mechanisms include pushable coils, mechanically detachable coils, and electrolytically detachable coils.[0005]
- One example of the type of vaso-occlusive coil referred to above as the “pushable coil” is disclosed in Ritchart et al., discussed above. Pushable coils are commonly provided in a cartridge and are pushed or “plunged” from the cartridge into a delivery catheter lumen. A pusher advances the pushable coil through and out of the delivery catheter lumen and into the site for occlusion.[0006]
- Mechanically detachable vaso-occlusive coils are typically integrated with a pusher rod and are mechanically detached from the distal end of that pusher after exiting a delivery catheter. Examples of such mechanically detachable vaso-occlusive coils are found in U.S. Pat. No. 5,261,916 to Engelson or U.S. Pat. No. 5,250,071 to Palermo.[0007]
- Finally, examples of electrolytically detachable vaso-occlusive coils may be found in U.S. Pat. Nos. 5,122,136 and 5,354,295, each issued to Guglielmi et al. In these devices, the vaso-occlusive portion of the assembly is attached to a pusher via a small, electrolytically severable joint. The electrolytically severable joint is severed by the placement of an appropriate voltage on the core wire. The joint erodes in preference either to the vaso-occlusive coil itself or to the pusher core wire.[0008]
- As noted above, aneurysms present a particularly acute medical risk due to the dangers of potential rupture of the thin wall inherent in such aneurysms. Occlusion of aneurysms by use of vaso-occlusive coils without occluding the adjacent artery is a special challenge and is a desirable method of reducing such risk of rupture. These vaso-occlusive devices are typically placed in an aneurysm in a manner described in U.S. Pat. No. 4,739,768 issued to Engelson. In particular, a microcatheter is initially steered into or adjacent to the entrance of an aneurysm, typically aided by the use of a steerable guidewire. The wire is then withdrawn from the microcatheter lumen and replaced by the vaso-occlusive coil. The vaso-occlusive coil is advanced through and out of the microcatheter, desirably being completely delivered into the aneurysm.[0009]
- However, after, or perhaps during, delivery of the coil into the aneurysm, there is a specific risk that a portion of the coil might migrate out of the aneurysm entrance zone and into the feeding vessel. The presence of the coil in that feeding vessel may cause a highly undesirable occlusion there. Also, there is a quantifiable risk that the blood flow in the vessel and aneurysm may induce movement of the coil farther out of the aneurysm, resulting in a more developed embolus in the feeding vessel.[0010]
- One type of aneurysm, commonly known as a “wide neck aneurysm,” is known to present particular difficulty in the placement and retention of vaso-occlusive coils, because vaso-occlusive coils lacking substantial secondary shape strength may be difficult to maintain in position within an aneurysm no matter how skillfully they are placed. Wide neck aneurysms are herein referred to as aneurysms of vessel walls having a neck or “entrance zone” from the adjacent vessel, wherein the entrance zone has a diameter that either: (1) is at least 80% of the largest diameter of the aneurysm; or (2) is clinically observed to be too wide effectively to retain vaso-occlusive coils that are deployed using the techniques discussed above.[0011]
- One approach to occlude such an aneurysm is described in U.S. Pat. No. 6,168,622, which describes a vaso-occlusive device with a secondary shape having a bulbous body portion and an anchor. The bulbous body portion is deployed within the aneurysm while the anchor is set just outside of the aneurysm, covering the aneurysm's neck or entrance zone. The device is integrally formed from a tube—clamped at both ends—of braided Nickel-Titanium (NiTi) wires. The bulbous body functions to occlude the aneurysm, while the anchor covers the entrance zone. In some cases, it may still be desirable to deploy vaso-occlusive coils with such a device, but the bulbous body of the vaso-occlusive device does not provide much space within the aneurysm to allow for insertion and deployment of the coils.[0012]
- Accordingly, an improved system and method for occluding an aneurysm neck would be desirable.[0013]
- The present invention is directed to systems and methods for occluding an aneurysm having an aneurysmal neck and an aneurysmal inner wall. Generally, a device in accordance with the present invention includes a mesh-like structure that may be integrally composed of a shape-memory alloy such as NiTi. The device may be deployed within the aneurysm through the aneurysmal neck. The device may be configured to be in a deployed state and an undeployed state, and may be configured to transition from the undeployed state to the deployed state by exposure to a higher temperature and/or by being released from a compressive force. The device may function to retain finer vaso-occlusive devices such as vaso-occlusive coils and/or embolic liquids. Furthermore, the device itself may function as a vaso-occlusive device. The device may be delivered to the aneurysm by, e.g., a catheter.[0014]
- In accordance with a first aspect of the present invention, at least one of the proximal and distal ends of the mesh-like structure is configured to be inserted through the aneurysmal neck when in an undeployed state, and configured to flare open and expand against at least a portion of the aneurysmal inner wall when in a deployed state. Having a flared open end may advantageously allow the device to conform to the shape of the aneurysm.[0015]
- In accordance with another aspect of the present invention, the distal end of the mesh-like structure may be configured to be inserted through the aneurysmal neck when in an undeployed state and configured to expand into a retainer against at least a portion of the aneurysmal inner wall when in a deployed state. The proximal end may be configured to expand into an anchor just outside of the aneurysm to secure the mesh-like structure in position.[0016]
- Other objects and features of the present invention will become apparent from consideration of the following description taken in conjunction with the accompanying drawings.[0017]
- In order to better appreciate how the above-recited and other advantages and objects of the present inventions are obtained, a more particular description of the present inventions briefly described above will be rendered by reference to specific embodiments thereof, which are illustrated in the accompanying drawings. Understanding that these drawings depict only typical embodiments of the invention and are not therefore to be considered limiting of its scope, the invention will be described and explained with additional specificity and detail through the use of the accompanying drawings in which:[0018]
- FIG. 1 is an illustration of a vaso-occlusive device constructed in accordance with a preferred embodiment of the present inventions, wherein the vaso-occlusive device is shown fully deployed within an aneurysm;[0019]
- FIGS.[0020]2(a) and2(b) illustrate one preferred method of manufacturing the vaso-occlusive device of FIG. 1 in accordance with the present inventions;
- FIG. 3 is an illustration of another vaso-occlusive device constructed in accordance with a preferred embodiment of the present inventions, wherein the vaso-occlusive device is shown fully deployed within an aneurysm;[0021]
- FIG. 4 illustrates one preferred method of manufacturing the vaso-occlusive device of FIG. 3 in accordance with the present inventions;[0022]
- FIG. 5 is an illustration of still another vaso-occlusive device constructed in accordance with a preferred embodiment of the present inventions, wherein the vaso-occlusive device is shown fully deployed within an aneurysm;[0023]
- FIG. 6 illustrates one preferred method of manufacturing the vaso-occlusive device of FIG. 5 in accordance with the present inventions;[0024]
- FIG. 7 is an illustration of still another vaso-occlusive device constructed in accordance with a preferred embodiment of the present inventions, wherein the vaso-occlusive device is shown fully deployed within an aneurysm;[0025]
- FIG. 8 illustrates one preferred method of manufacturing the vaso-occlusive device of FIG. 7 in accordance with the present inventions;[0026]
- FIGS.[0027]9(a)-(c) illustrate a preferred method of delivering and deploying the vaso-occlusive device of FIG. 1 into the aneurysm;
- FIGS.[0028]10(a)-(d) illustrate a preferred method of delivering and deploying the vaso-occlusive device of FIG. 7 into the aneurysm; and
- FIGS.[0029]11(a)-(c) illustrate a preferred method of delivering and deploying the vaso-occlusive device of FIG. 5 into the aneurysm.
- Turning to FIG. 1, a vaso-[0030]
occlusive element 10 constructed in accordance with a preferred embodiment of the present invention is shown completely deployed within ananeurysm 40 of ablood vessel 60. Theaneurysm 40 is shown with an oppositelydisposed neck 70 anddome 45. When deployed in its secondary shape, i.e., its deployed shape, the vaso-occlusive element 10 generally includes a centraltubular element 15 and proximal20 and distal30 ends that are flared open into “umbrella” shapes. As illustrated, the flared opened ends20/30 advantageously conform to the shape of theaneurysm 40. The diameter of theproximal end 20 of the vaso-occlusive element 10 is larger than theneck 70, and thus theproximal end 20 may completely cover theneck 70. - As will be described in further detail below, the vaso-[0031]
occlusive element 10 is manufactured from a relatively dense braid. In this manner, theproximal end 20 may densely cover theneck 70 and function as a retainer. Thus, finer vaso-occlusive devices, such as vaso-occlusive coils or embolic liquids, may effectively be retained within theaneurysm 40, or at the least, the likelihood of these devices migrating out from theaneurysm 40 is minimized. Further, as can be appreciated by one of ordinary skill in the art, the relatively dense coverage of theproximal end 20 allows the vaso-occlusive element 10 itself to function as a vaso-occlusive device. The flared opendistal end 30 is placed adjacent thedome 45 of theaneurysm 40 and allows theelement 10 to safely conform to the shape of thedome 45. This allows for thedistal end 30 to function as an anchor and secure itself within theaneurysm 40 without the need to conform to the entire shape of theaneurysm 40. Thus, theelement 10 may conform to a large variety of shapes and sizes ofaneurysms 40 since theelement 10 need only be secured within theaneurysm 40 by conforming the flared opendistal end 30 to thedome 45. - Turning to FIGS.[0032]2(a)-(b), one preferred method of manufacturing the vaso-
occlusive device 10 will now be described. First, a braided tubular element1 is constructed by braiding multiplefine wires 2 together. The tubular element1 may also be formed of wires with varying diameters braided together. It may be desirable to use a larger number ofwires 2 when forming the tubular element1, resulting in a relatively dense braid, which provides denser coverage within theaneurysm 40. Optionally, the tubular element1 may be radiopaque. One method involves braiding a radiopaque fiber, such as platinum, Pt, or gold, Au, into the tubular element1. Another method involves plating or coating the tubular element1 with radiopaque material. Additionally, one or more radiopaque markers may be added to the tubular element1. - Preferably, the[0033]
wires 2 are made from a shape memory alloy, which can be of any type, but preferably is a “one way” trainable shape memory alloy. A preferable shape memory alloy for forming the braided tubular element1 is Nickel-Titanium (NiTi)—e.g., 144 strands of 0.001″ NiTi wires. Shape memory alloys comprise a unique class of metal alloys that, once trained, are configured to “remember” a preselected shape, i.e., deployed shape, and can return to the preselected shape even if subsequently reshaped. To be trained to “remember” a first preselected shape, the shape memory alloy is molded and heated at or above a training, or austenite, temperature to place the shape memory alloy in an austenite phase. In the austenite phase, the shape memory alloy is formed in the first preselected shape and then, once formed, is permitted to cool to a martensite finish temperature, whereupon the shape memory alloy enters a martensite phase. The martensite finish temperature can be any temperature that is less than the training temperature. Upon entering the martensite phase, the shape memory alloy has been trained to “remember” the first preselected shape. While in the martensite phase, the alloy is in a soft state and is formed into a second preselected shape, e.g., an undeployed shape. The shape memory alloy in the martensite phase is configured to maintain the second preselected shape and, if subsequently reheated to an activation temperature, automatically returns to the first preselected shape. The activation temperature can comprise any temperature that is greater than the martensite finish temperature and generally approximately equals the training temperature. Once the first preselected shape has been recovered, the shape memory alloy is configured to maintain the first preselected shape irrespective of temperature. Generally, as can be appreciated by one of ordinary skill in the art, the training, martensite finishing, and activation temperatures for a shape memory alloy are adjustable, depending on the composition. For example, a slight extra amount of Nickel added to a NiTi alloy composition can change the training temperature from 0° C. to 100° C. - Generally, the vaso-[0034]
occlusive device 10 described above is delivered to theaneurysm 40 via a delivery catheter. The catheter delivers thedevice 10 to theaneurysm 40 and during the delivery, thedevice 10 maintains an undeployed shape, e.g., the original tubular shape—methods of delivery will be described in more detail below. When thedevice 10 is deployed, thedevice 10 expands into its deployed shape, e.g., having flared open proximal and distal ends20/30, as shown in FIG. 1. Using a shape memory alloy to form thedevice 10 provides for alternative methods of expanding thedevice 10 into its deployed shape within theaneurysm 40. - One method involves configuring the[0035]
device 10 to self-expand within theaneurysm 40 when deployed and exposed to the temperature of theaneurysm 40. To configure thedevice 10 to self-expand within theaneurysm 40 when deployed and exposed to the temperature of theaneurysm 40, the training and activation temperatures are adjusted to be at, or just below, the temperature of the aneurysm, which is approximately 37° Celsius—the human body temperature. The martensite temperature is adjusted to be at a lower temperature. With these temperatures set, thedevice 10 is heated to, or above the training temperature—austenite phase—and shaped into its desired deployed shape, as described above. Then, the temperature is lowered to, or below, the martensite finish temperature—martensite phase—and shaped into its desired undeployed shape. Subsequently, thedevice 10 is then placed inside the catheter as will be described below. The catheter can be constructed of a material that insulates thedevice 10 from the outside environment and maintains the temperature of thedevice 10 below the activation temperature. Thus, when the catheter is inserted into thelumen 75 of theblood vessel 60, thedevice 10 does not expand into its deployed shape within the catheter. When thedevice 10 is deployed into theaneurysm 40 and exposed to the aneurysm temperature, the temperature of the device will rise at or above the activation temperature, and thedevice 10 will expand into its deployed shape. Because the alloy is preferably “one way” trained, thedevice 10 will then be configured to maintain the deployed shape irrespective of the temperature, as described above. - An alternative method for expanding the[0036]
device 10 into its deployed shape within the aneurysm utilizes the super-elastic characteristic of shape memory alloys. After thedevice 10 is configured to maintain its deployed shape irrespective of the temperature, as described above, thedevice 10 is then maintained in its austenite phase. When thedevice 10 is in its austenite phase, thedevice 10 is super-elastic, i.e., thedevice 10 may be deformed to a certain degree and still be able to return to its deployed shape. Thedevice 10 is then placed within the catheter as will be described below, with the inner wall of the catheter compressing thedevice 10 into its undeployed shape. When thedevice 10 is released out of the catheter into theaneurysm 40, theuncompressed device 10 automatically expands into its deployed shape because of its super-elasticity. - Further details on the structure and manufacturing process of braided tubular elements[0037]1 are disclosed in U.S. Pat. No. 6,168,622 issued to Mazzochhi, column 4, line 33 to column 6, line 24, and FIGS. 1A and 1B, of which are hereby incorporated by reference.
- Forming the deployed shape shown in FIG. 1 is achieved by using two[0038]
mandrels 85, each having acylindrical portion 90 and an umbrella dome-shapedmold 85, as illustrated in FIG. 2(a). To flare open the ends of the element1, each end is placed over thecylindrical portion 90 of amandrel 85 and compressed against thedome 85, causing the ends to conform to the shape of thedome 85 and flare open. The assembly of the twomandrels 80 and the element1 is then heated at or above the training temperature to place the element1 in the austenite phase. The assembly is maintained at that temperature until the deployed shape has been formed. - Then, the assembly is cooled to, or below, the martensite finishing temperature to place the element[0039]1 in the martensite phase. In the martensite phase, the
mandrels 80 are removed from the element1, and the element1 is formed into an undeployed shape, e.g., the original tubular shape. This may be achieved by placing the element1 over acylindrical mandrel 95 and compressing the flared ends to conform to the shape of thecylindrical mandrel 95, as shown in FIG. 2b. Subsequently, the element1 may be reheated to the activation temperature, causing the element1 to return to the deployed shape. Once the deployed shape has been recovered, the element1 is configured to maintain the deployed shape irrespective of temperature. - Turning to FIG. 3, another vaso-[0040]
occlusive element 110 constructed in accordance with a preferred embodiment of the present invention is shown completely deployed within theaneurysm 40. As with the embodiment in FIG. 1, the vaso-occlusive element 110, when deployed, generally includes a centraltubular element 115 and a flareddistal end 130. The vaso-occlusive element 110 further includes aproximal end 120 shaped as a flattened disk, which functions as a retainer. Theproximal end 130 has a diameter larger than the diameter of theneck 70, and thus completely covers the entrance zone150. The flatteneddisk 130 has a layer of braided wires on both sides, and thus may advantageously provide two layers of braided wires to cover the entrance zone150 instead of one layer with the flared openproximal end 20 shown in FIG. 1. As with the flared opendistal end 30 of the vaso-occlusive element 10, shown in FIG. 1, the flared opendistal end 130 is placed adjacent thedome 45 of theaneurysm 40 and allows theelement 110 to safely conform to the shape of thedome 45. - Turning to FIG. 4, one preferred method of manufacturing the vaso-[0041]
occlusive device 110 shown in FIG. 3 will now be described. As described above, a braided tubular element1 is first constructed. Forming the deployed shape is achieved by using one of themandrels 85 shown in FIG. 2(a) and the cylindrical mandrel shown in FIG. 2(b), as illustrated in FIG. 4. As described above, to flare open the distal end of the element1, the distal end is placed over thecylindrical portion 90 of themandrel 85 with thedome 80 and compressed against thedome 80, causing the distal end to flare open. To form the flatteneddisk 120, the proximal end is placed on thecylindrical mandrel 95. The proximal tip and a portion of the centraltubular portion 115 are then compressed together along the axis of thecylindrical mandrel 95. This causes the proximal end to expand into a flatteneddisk 120. - The assembly of the two[0042]
mandrels 80/95 and the element1 is then heated at or above the training temperature to place the element1 in the austenite phase. The assembly is maintained at that temperature until the deployedshape 110 has been formed. Then, the assembly is cooled to, or below, the martensite finishing temperature to place theelement 110 in the martensite phase. In the martensite phase, themandrels 80/95 are removed from theelement 110, and theelement 110 is formed into an undeployed shape, e.g., the original tubular shape1. This is achieved by placing theelement 110 over thecylindrical mandrel 95 and compressing the flared open end and flattened disk to conform to the shape of thecylindrical mandrel 95, as shown in FIG. 2(b). If the element1 is configured to expand into its deployed shape upon exposure to a higher temperature, then the element1 may be placed within a delivery catheter in preparation for deployment, as described above. Alternatively, if theelement 110 is configured to expand upon being released by a compressive force, then the element1 is reheated to, or above, the activation temperature, causing the element1 to return to the deployedshape 110 and to be configured to maintain the deployedshape 110 irrespective of temperature. - Turning to FIG. 5, another vaso-[0043]
occlusive element 210 constructed in accordance with a preferred embodiment of the present invention is shown deployed within theaneurysm 40. When in a deployed shape, theelement 210 generally includes acentral portion 215 and a distal end flared open into a “cup”shape 230. Alternatively, the distal end may be flared open into a “cone” shape. The vaso-occlusive element 210 further includes aproximal end 220 shaped as a flattened disk. Theproximal end 220 has a diameter larger than the diameter of theneck 70. Only the flaredopen cup shape 230 is deployed within theaneurysm 40, whereas the flatteneddisk 220 is deployed in thelumen 75 of theblood vessel 60 just outside of theaneurysm 40, functioning as an anchor for theelement 210. The cup shapeddistal end 230 may retain fine vaso-occlusive devices such as vaso-occlusive coils and embolic liquids. - Turning to FIG. 6, one preferred method of manufacturing the vaso-[0044]
occlusive device 310 shown in FIG. 5 will now be described. As described above, a braided tubular element1 is first constructed. Forming the deployed shape is achieved by using amandrel 280 having a cup shapeddome 285 facing outward coupled to acylindrical portion 290 and acylindrical mandrel 95. To form the cup shape, the distal end of the element1 is placed over thecylindrical portion 290 of themandrel 280 having thedome 285 and compressed against thedome 285, causing the distal end to flare open into a cup shape. To form the flattened disk, the proximal end is placed over thecylindrical mandrel 95. The proximal tip and a portion of the centraltubular portion 215 are then compressed together along the axis of thecylindrical mandrel 95. This causes the proximal end to expand into a flatteneddisk 220. - The assembly of the two[0045]
mandrels 280/95 and the element1 is then heated at or above the training temperature to place the element1 in the austenite phase. The assembly is maintained at that temperature until the deployedshape 210 has been formed. Then, the assembly is cooled to, or below, the martensite finishing temperature to place theelement 210 in the martensite phase. In the martensite phase, themandrels 280/95 are removed from theelement 210, and theelement 210 is formed into an undeployed shape, e.g., the original tubular shape1. This may be achieved by placing theelement 210 over thecylindrical mandrel 95 and compressing the flared open end and flattened disk to conform to the shape of thecylindrical mandrel 95. If the element1 is configured to expand into its deployed shape upon exposure to a higher temperature, then the element1 may be placed within a delivery catheter in preparation for deployment, as described above. Alternatively, if theelement 110 is configured to expand upon being released by a compressive force, then the element1 is reheated to, or above, the activation temperature, causing the element1 to return to the deployedshape 110 and to be configured to maintain the deployedshape 110 irrespective of temperature. - Turning to FIG. 7, another vaso-[0046]
occlusive element 310 constructed in accordance with a preferred embodiment of the present invention is shown deployed within theaneurysm 40. When in a deployed shape, theelement 310 generally includes acentral portion 315 and first and second flatteneddisks 330/320 formed at the distal and proximal ends respectively. Only the first flatteneddisk 330 at the distal end is deployed within theaneurysm 40 whereas the second flatteneddisk 320 is deployed within thelumen 75 of theblood vessel 60 just outside of theaneurysm 40, functioning as an anchor to secure theelement 310 in its position. The first flatteneddisk 330 provides a wider coverage than thecup shape 230 shown in FIG. 5. As with the other devices described above, thisdevice 310 may also retain fine vaso-occlusive devices such as vaso-occlusive coils and embolic liquids. Thecentral portion 315 may be conventionally sealed to prevent the fine vaso-occlusive devices from migrating out. - Turning to FIG. 8, one preferred method of manufacturing the vaso-[0047]
occlusive device 310 shown in FIG. 7 will now be described. As described above, a braided tubular element1 is first constructed. Forming the deployed shape is achieved by using acylindrical mandrel 95, as illustrated in FIG. 8. To form the first flatteneddisk 330, the distal end is placed on thecylindrical mandrel 95. The distal tip and a portion of the centraltubular portion 315 are then compressed together along the axis of thecylindrical mandrel 95. This causes the distal end to expand into a flatteneddisk 330. To form the second flatteneddisk 320, the proximal end is placed on thecylindrical mandrel 95. The proximal tip and a portion of the centraltubular portion 315 are then compressed together along the axis of thecylindrical mandrel 95. This causes the proximal end to expand into a flatteneddisk 320. - The assembly of the[0048]
mandrel 95 and the element1 is then heated at or above the training temperature to place the element1 in the austenite phase. The assembly is maintained at that temperature until the deployedshape 310 has been formed. Then, the assembly is cooled to, or below, the martensite finishing temperature to place theelement 310 in the martensite phase. In the martensite phase, themandrel 95 is removed from theelement 310, and theelement 310 is formed into an undeployed shape, e.g., the original tubular shape1. This may be achieved by placing theelement 310 over thecylindrical mandrel 95 and compressing the flatteneddisks 320/330 to conform to the shape of thecylindrical mandrel 95, as shown in FIG. 2(b). If the element1 is configured to expand into its deployed shape upon exposure to a higher temperature, then the element1 may be placed within a delivery catheter in preparation for deployment, as described above. Alternatively, if theelement 110 is configured to expand upon being released by a compressive force, then the element1 is reheated to, or above, the activation temperature, causing the element1 to return to the deployedshape 110 and to be configured to maintain the deployedshape 110 irrespective of temperature. - Generally, as mentioned above, the vaso-occlusive devices described above are delivered to an aneurysm within a blood vessel via a delivery catheter. Referring to FIGS.[0049]9(a)-(c), a method of deploying a vaso-occlusive device, and in this case the vaso-
occlusive device 10, to theaneurysm 40 via adelivery catheter 450 is illustrated. Turning first to FIG. 9(a), thecatheter 450 is steered to just within theneck 70 of theaneurysm 40. At this point, the vaso-occlusive device 10 is in its undeployed shape, and is coupled to aninner guidewire 465 via an electrolytically severable joint455. The assembly of the vaso-occlusive device 10 and theguidewire 465 extends through the lumen of thedelivery catheter 450 such that thedevice 10 is positioned at the distal end of thecatheter 450. - Turning to FIG. 9([0050]b), the
guidewire 465 is then pushed toward the distal end of thecatheter 450 causing the vaso-occlusive device 10 to extend out of the distal end of thecatheter 450, through theneck 70, and into theaneurysm 40. As the vaso-occlusive device 10 is pushed out of thecatheter 450, the portion of thedevice 10 that is free from the constraints of thecatheter 450 expands into its deployed shape, e.g., thedistal end 30 flares open. - As described above, the[0051]
device 10 may be configured to be in its martensite phase when delivered to theaneurysm 40 and expand upon exposure to the temperature of theaneurysm 40. Thus, when outside of thecatheter 450, the temperature of thedevice 10 rises and thedevice 10 enters the austenite phase and expands into its deployed shape. Alternatively, thedevice 10 may be configured to be super-elastic and automatically expand upon being-unconstrained by thecatheter 450. - Turning now to FIG. 9([0052]c), the
guidewire 465 continues to push thedevice 10 out of the catheter until theproximal end 20 of thedevice 10 is deployed within theaneurysm 40, expanding into its deployed shape, e.g., theproximal end 20 flares open, as described above with thedistal end 30. To release thedevice 10 from the catheter450 a current is applied to theguidewire 465, which causes the joint455 to sever from theproximal end 20 of thecatheter 10. Further discussion of the construction of, placement of, and other physical details of such electrolytically severable joints may be found in U.S. Pat. No. 5,122,136 to Guglielmi et al.; U.S. Pat. No. 5,354,295 to Guglielmi et al.; and U.S. Pat. No. 5,624,449 to Pham et al; and others. It should be noted that other types of vaso-occlusive devices, such as vaso-occlusive device 110, can be delivered within theaneurysm 40 in the manner described immediately above. - Alternatively, referring to FIGS.[0053]10(a)-(c), a retractable sheath method of delivering a vaso-occlusive device, and in this case vaso-
occlusive device 310, to theaneurysm 40 is illustrated. Turning to FIG. 10(a), thecatheter 550 is steered to just within theneck 70 of theaneurysm 40. At this point, the vaso-occlusive device 10 is in its undeployed shape, and is placed over aninner guidewire 565 that extends through thelumen 580 of thedelivery catheter 565. Theouter wall 555 of thecatheter 565 that defines thelumen 580 functions as a sheath. The assembly of the vaso-occlusive device 310 and theguidewire 565 extends through thelumen 580 of thedelivery catheter 450 such that thedevice 10 is positioned at the distal end of thecatheter 550. - Turning to FIG. 10([0054]b), the
guidewire 565 is then pushed so that the distal end of vaso-occlusive device 310 extends out of thecatheter 550, through theneck 70, and into theaneurysm 40. Once unconstrained from thecatheter 550, thedistal end 330 of thedevice 310 is allowed to expand into a first flattened disk adjacent the inner wall near theneck 70 within theaneurysm 40. - As described above, the[0055]
device 310 may be configured to be in its martensite phase when delivered to theaneurysm 40 and expand upon exposure to the temperature of theaneurysm 40. Thus, when outside of thecatheter 550, the temperature of thedevice 310 rises and thedevice 310 enters the austenite phase and expands into its deployed shape. Alternatively, thedevice 310 may be configured to be super-elastic and expand upon being unconstrained by thecatheter 550. - Turning now to FIG. 10([0056]c), the
guidewire 565 continues to push thedevice 310 out of the catheter until theproximal end 320 of thedevice 310 is deployed just outside of theaneurysm 40, expanding into its deployed shape, e.g., a second flattened disk, as described above with thedistal end 330. To release thedevice 310 from thecatheter 450, theguidewire 565 is pulled out of thedevice 310 and thecatheter 550 is pulled out of theaneurysm 40 andblood vessel 60. The expanded310 device is secured in position within theaneurysm 40 and will not be pulled out with thecatheter 550. It should be noted that other types of vaso-occlusive devices, such as vaso-occlusive device 310, can be delivered within theaneurysm 40 in the manner described immediately above. - As mentioned above, a vaso-occlusive device, such as vaso-[0057]
occlusive device 310, may function as a vaso-occlusive device and/or function as a vaso-occlusive device retainer. In this regard, after deployment, finer vaso-occlusive devices 730 may be delivered into theaneurysm 40 to be retained by thedevice 310, as illustrated in FIG. 10(d). This is achieved by maintaining an axial lumen within a vaso-occlusive device to allow acatheter 740 to fit through and insert materials such as vaso-occlusive coils 730 or embolic liquids to further improve thrombogenecity within the aneurysm700. - In another alternative, turning to FIGS.[0058]11(a)-(c), a push method of delivering a vaso-occlusive device, and in this case vaso-
occlusive device 210, to theaneurysm 40 is illustrated. Turning to FIG. 11(a), thecatheter 650 is steered to just within theneck 70 of theaneurysm 40. At this point, the vaso-occlusive device 210 is in its undeployed shape, and is placed within thelumen 680 of adelivery catheter 650 near the distal tip of thecatheter 650, which is steered toaneurysm 40, just outside of theneck 70. Aguidewire 665, extending through the lumen655 is positioned such that it is capable of pushing thedevice 210 out of thecatheter 650 and into theaneurysm 40, where thedevice 230 may expand into its deployed shape, as described above. - Turning to FIG. 11([0059]b), the
guidewire 665 is then pushed so that the distal end of vaso-occlusive device 210 extends out of thecatheter 650, through theneck 70, and into theaneurysm 40. Once unconstrained from thecatheter 550, thedistal end 230 of thedevice 210 is allowed to expand into a flared open cup shape adjacent the inner wall near theneck 70 within theaneurysm 40. - As described above, the[0060]
device 210 may be configured to be in its martensite phase when delivered to theaneurysm 40 and expand upon exposure to the temperature of theaneurysm 40. Thus, when outside of thecatheter 650, the temperature of thedevice 210 rises and thedevice 210 enters the austenite phase and expands into its deployed shape. Alternatively, thedevice 210 may be configured to be super-elastic and expand upon being unconstrained by thecatheter 650. - Turning now to FIG. 11([0061]c), the
guidewire 665 continues to push thedevice 210 out of the catheter until theproximal end 220 of thedevice 210 is deployed just outside of theaneurysm 40, expanding into its deployed shape, e.g., a flattened disk, as described above with thedistal end 230. Thecatheter 650 is then pulled out of theaneurysm 40 andblood vessel 60 to separate itself from thedevice 210. The expanded210 device is secured in position within theaneurysm 40 and will not be pulled out with thecatheter 650. It should be noted that other types of vaso-occlusive devices, such as vaso-occlusive device 210, can be delivered within theaneurysm 40 in the manner described immediately above. - While embodiments of the present invention have been shown and described, various modifications may be made without departing from the spirit and scope of the present invention, and all such modifications and equivalents are intended to be covered.[0062]
Claims (72)
Priority Applications (11)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US10/121,980US20030195553A1 (en) | 2002-04-12 | 2002-04-12 | System and method for retaining vaso-occlusive devices within an aneurysm |
| CA2481224ACA2481224C (en) | 2002-04-12 | 2003-04-10 | Devices for retaining vaso-occlussive devices within an aneurysm |
| ES03721607TES2331567T3 (en) | 2002-04-12 | 2003-04-10 | DEVICES FOR THE RETENTION OF GLASS-OCLUSIVE DEVICES WITHIN AN ANEURISM. |
| PCT/US2003/010997WO2003086240A1 (en) | 2002-04-12 | 2003-04-10 | Devices for retaining vaso-occlussive devices within an aneurysm |
| JP2003583267AJP4414767B2 (en) | 2002-04-12 | 2003-04-10 | Fixation device for vascular occlusion device in aneurysm |
| AU2003224913AAU2003224913A1 (en) | 2002-04-12 | 2003-04-10 | Devices for retaining vaso-occlussive devices within an aneurysm |
| EP03721607AEP1494619B1 (en) | 2002-04-12 | 2003-04-10 | Devices for retaining vaso-occlussive devices within an aneurysm |
| DE60328765TDE60328765D1 (en) | 2002-04-12 | 2003-04-10 | DEVICES FOR HOLDING VASOOKCLUSIVE DEVICES IN ANEURYSMA |
| AT03721607TATE439092T1 (en) | 2002-04-12 | 2003-04-10 | DEVICES FOR RETAINING VAS-OCCLUSIVE DEVICES IN AN ANEURYSMA |
| US11/617,293US20070106311A1 (en) | 2002-04-12 | 2006-12-28 | System and method for retaining vaso-occlusive devices within an aneurysm |
| US13/091,608US9314326B2 (en) | 2002-04-12 | 2011-04-21 | System and method for retaining vaso-occlusive devices within an aneurysm |
Applications Claiming Priority (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US10/121,980US20030195553A1 (en) | 2002-04-12 | 2002-04-12 | System and method for retaining vaso-occlusive devices within an aneurysm |
Related Child Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US11/617,293ContinuationUS20070106311A1 (en) | 2002-04-12 | 2006-12-28 | System and method for retaining vaso-occlusive devices within an aneurysm |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| US20030195553A1true US20030195553A1 (en) | 2003-10-16 |
Family
ID=28790456
Family Applications (3)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US10/121,980AbandonedUS20030195553A1 (en) | 2002-04-12 | 2002-04-12 | System and method for retaining vaso-occlusive devices within an aneurysm |
| US11/617,293AbandonedUS20070106311A1 (en) | 2002-04-12 | 2006-12-28 | System and method for retaining vaso-occlusive devices within an aneurysm |
| US13/091,608Expired - LifetimeUS9314326B2 (en) | 2002-04-12 | 2011-04-21 | System and method for retaining vaso-occlusive devices within an aneurysm |
Family Applications After (2)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US11/617,293AbandonedUS20070106311A1 (en) | 2002-04-12 | 2006-12-28 | System and method for retaining vaso-occlusive devices within an aneurysm |
| US13/091,608Expired - LifetimeUS9314326B2 (en) | 2002-04-12 | 2011-04-21 | System and method for retaining vaso-occlusive devices within an aneurysm |
Country Status (9)
| Country | Link |
|---|---|
| US (3) | US20030195553A1 (en) |
| EP (1) | EP1494619B1 (en) |
| JP (1) | JP4414767B2 (en) |
| AT (1) | ATE439092T1 (en) |
| AU (1) | AU2003224913A1 (en) |
| CA (1) | CA2481224C (en) |
| DE (1) | DE60328765D1 (en) |
| ES (1) | ES2331567T3 (en) |
| WO (1) | WO2003086240A1 (en) |
Cited By (114)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US20050049681A1 (en)* | 2003-05-19 | 2005-03-03 | Secant Medical, Llc | Tissue distention device and related methods for therapeutic intervention |
| US20050283183A1 (en)* | 2004-06-21 | 2005-12-22 | Tri Tran | Expanding vaso-occlusive device |
| US20060064151A1 (en)* | 2004-09-22 | 2006-03-23 | Guterman Lee R | Cranial aneurysm treatment arrangement |
| US20060155323A1 (en)* | 2005-01-07 | 2006-07-13 | Porter Stephen C | Intra-aneurysm devices |
| US20060247680A1 (en)* | 2004-04-08 | 2006-11-02 | Aga Medical Corporation | Flanged occlusion devices and methods |
| US20070088387A1 (en)* | 2005-10-19 | 2007-04-19 | Pulsar Vascular, Inc. | Implantable aneurysm closure systems and methods |
| US20070233174A1 (en)* | 2005-04-01 | 2007-10-04 | Gordon Hocking | Trapping Filter for Blood Vessel |
| US20070270902A1 (en)* | 2004-09-17 | 2007-11-22 | Slazas Robert R | Thin Film Metallic Devices for Plugging Aneurysms or Vessels |
| WO2007140797A1 (en)* | 2006-06-02 | 2007-12-13 | Occlutech Gmbh | Occlusion instrument for closing a cardiac auricle |
| US20080200945A1 (en)* | 2004-03-19 | 2008-08-21 | Aga Medical Corporation | Device for occluding vascular defects |
| US20080221600A1 (en)* | 2006-08-17 | 2008-09-11 | Dieck Martin S | Isolation devices for the treatment of aneurysms |
| US20090062841A1 (en)* | 2004-03-19 | 2009-03-05 | Aga Medical Corporation | Device for occluding vascular defects |
| US20110046719A1 (en)* | 2004-06-03 | 2011-02-24 | Noureddine Frid | Luminal Endoprosthesis For the Occlusion of an Aneurysm and Method of Manufacturing Such an Endoprosthesis |
| US8142456B2 (en) | 2008-04-21 | 2012-03-27 | Nfocus Neuromedical, Inc. | Braid-ball embolic devices |
| US20120271337A1 (en)* | 2007-04-16 | 2012-10-25 | Hans-Reiner Figulla | Occluder For Occluding an Atrial Appendage and Production Process Therefor |
| WO2012163880A1 (en)* | 2011-05-31 | 2012-12-06 | Acandis Gmbh & Co. Kg | Medical implant for arrangement within a hollow body, in particular an aneurysm, and method for producing a medical implant |
| US8388650B2 (en) | 2008-09-05 | 2013-03-05 | Pulsar Vascular, Inc. | Systems and methods for supporting or occluding a physiological opening or cavity |
| US8398670B2 (en) | 2004-03-19 | 2013-03-19 | Aga Medical Corporation | Multi-layer braided structures for occluding vascular defects and for occluding fluid flow through portions of the vasculature of the body |
| US8425548B2 (en) | 2010-07-01 | 2013-04-23 | Aneaclose LLC | Occluding member expansion and then stent expansion for aneurysm treatment |
| AU2010249161B2 (en)* | 2004-01-22 | 2013-05-16 | Microvention, Inc. | Aneurysm treatment device and method of use |
| US8551132B2 (en) | 2005-10-19 | 2013-10-08 | Pulsar Vascular, Inc. | Methods and systems for endovascularly clipping and repairing lumen and tissue defects |
| EP1901665A4 (en)* | 2005-07-11 | 2014-01-08 | Usgi Medical Inc | Compressible tissue anchor assemblies |
| US8636760B2 (en) | 2009-04-20 | 2014-01-28 | Covidien Lp | System and method for delivering and deploying an occluding device within a vessel |
| US8747453B2 (en) | 2008-02-18 | 2014-06-10 | Aga Medical Corporation | Stent/stent graft for reinforcement of vascular abnormalities and associated method |
| US8777974B2 (en)* | 2004-03-19 | 2014-07-15 | Aga Medical Corporation | Multi-layer braided structures for occluding vascular defects |
| WO2014128313A1 (en)* | 2013-02-21 | 2014-08-28 | Castaño Duque Carlos | Device for supporting and positioning occlusive elements |
| WO2014137467A1 (en)* | 2013-03-05 | 2014-09-12 | Aga Medical Corporation | Medical device for treating a target site |
| US20140350588A1 (en)* | 2011-11-09 | 2014-11-27 | Easynotes Ltd. | Obstruction device |
| US8906057B2 (en) | 2010-01-04 | 2014-12-09 | Aneuclose Llc | Aneurysm embolization by rotational accumulation of mass |
| US8926681B2 (en) | 2010-01-28 | 2015-01-06 | Covidien Lp | Vascular remodeling device |
| US8974487B2 (en) | 2008-05-01 | 2015-03-10 | Aneuclose Llc | Aneurysm occlusion device |
| JP2015083139A (en)* | 2005-10-19 | 2015-04-30 | パルサー バスキュラー インコーポレイテッド | Method and system for endovascularly clipping and repairing lumen and tissue defect |
| US9023094B2 (en) | 2007-06-25 | 2015-05-05 | Microvention, Inc. | Self-expanding prosthesis |
| US9060886B2 (en) | 2011-09-29 | 2015-06-23 | Covidien Lp | Vascular remodeling device |
| US9089332B2 (en) | 2011-03-25 | 2015-07-28 | Covidien Lp | Vascular remodeling device |
| US9095342B2 (en) | 2009-11-09 | 2015-08-04 | Covidien Lp | Braid ball embolic device features |
| US9095343B2 (en) | 2005-05-25 | 2015-08-04 | Covidien Lp | System and method for delivering and deploying an occluding device within a vessel |
| US9119625B2 (en) | 2011-10-05 | 2015-09-01 | Pulsar Vascular, Inc. | Devices, systems and methods for enclosing an anatomical opening |
| US9138232B2 (en) | 2011-05-24 | 2015-09-22 | Aneuclose Llc | Aneurysm occlusion by rotational dispensation of mass |
| US9155647B2 (en) | 2012-07-18 | 2015-10-13 | Covidien Lp | Methods and apparatus for luminal stenting |
| WO2015166013A1 (en) | 2014-04-30 | 2015-11-05 | Cerus Endovascular Limited | Occlusion device |
| US9179918B2 (en) | 2008-07-22 | 2015-11-10 | Covidien Lp | Vascular remodeling device |
| US9186267B2 (en) | 2012-10-31 | 2015-11-17 | Covidien Lp | Wing bifurcation reconstruction device |
| CN105105812A (en)* | 2015-09-18 | 2015-12-02 | 王奎重 | Intracranial aneurysm interventional embolization treatment device |
| US9204983B2 (en) | 2005-05-25 | 2015-12-08 | Covidien Lp | System and method for delivering and deploying an occluding device within a vessel |
| US9259229B2 (en) | 2012-05-10 | 2016-02-16 | Pulsar Vascular, Inc. | Systems and methods for enclosing an anatomical opening, including coil-tipped aneurysm devices |
| US9277924B2 (en) | 2009-09-04 | 2016-03-08 | Pulsar Vascular, Inc. | Systems and methods for enclosing an anatomical opening |
| US9295571B2 (en) | 2013-01-17 | 2016-03-29 | Covidien Lp | Methods and apparatus for luminal stenting |
| US9314248B2 (en) | 2012-11-06 | 2016-04-19 | Covidien Lp | Multi-pivot thrombectomy device |
| US9351859B2 (en) | 2010-12-06 | 2016-05-31 | Covidien Lp | Vascular remodeling device |
| US9358140B1 (en) | 2009-11-18 | 2016-06-07 | Aneuclose Llc | Stent with outer member to embolize an aneurysm |
| US9393022B2 (en) | 2011-02-11 | 2016-07-19 | Covidien Lp | Two-stage deployment aneurysm embolization devices |
| WO2016161344A1 (en)* | 2015-04-01 | 2016-10-06 | Boston Scientific Scimed, Inc. | Systems and methods for delivery of gel embolics |
| US9463105B2 (en) | 2013-03-14 | 2016-10-11 | Covidien Lp | Methods and apparatus for luminal stenting |
| US9468442B2 (en) | 2010-01-28 | 2016-10-18 | Covidien Lp | Vascular remodeling device |
| US9675482B2 (en) | 2008-05-13 | 2017-06-13 | Covidien Lp | Braid implant delivery systems |
| US20170252045A1 (en)* | 2013-03-13 | 2017-09-07 | Lawrence Livermore National Security, Llc | Shape-memory polymer foam device for treating aneurysms |
| WO2017153603A1 (en) | 2016-03-11 | 2017-09-14 | Cerus Endovascular Limited | Occlusion device |
| WO2017201316A1 (en)* | 2016-05-18 | 2017-11-23 | Microvention, Inc. | Embolic containment |
| US9901435B2 (en)* | 2013-03-15 | 2018-02-27 | Insera Therapeutics, Inc. | Longitudinally variable vascular treatment devices |
| US10004510B2 (en) | 2011-06-03 | 2018-06-26 | Pulsar Vascular, Inc. | Systems and methods for enclosing an anatomical opening, including shock absorbing aneurysm devices |
| US10028747B2 (en) | 2008-05-01 | 2018-07-24 | Aneuclose Llc | Coils with a series of proximally-and-distally-connected loops for occluding a cerebral aneurysm |
| US10028745B2 (en) | 2011-03-30 | 2018-07-24 | Noha, Llc | Advanced endovascular clip and method of using same |
| US20180242979A1 (en)* | 2017-02-23 | 2018-08-30 | DePuy Synthes Products, Inc. | Aneurysm device and delivery system |
| US20180333158A1 (en)* | 2015-02-25 | 2018-11-22 | Galaxy Therapeutics, Llc | System for and method of treating aneurysms |
| US10327781B2 (en) | 2012-11-13 | 2019-06-25 | Covidien Lp | Occlusive devices |
| US20190223876A1 (en)* | 2018-01-19 | 2019-07-25 | Galaxy Therapeutics, Llc | System for and method of treating aneurysms |
| US10390926B2 (en) | 2013-07-29 | 2019-08-27 | Insera Therapeutics, Inc. | Aspiration devices and methods |
| US10478194B2 (en) | 2015-09-23 | 2019-11-19 | Covidien Lp | Occlusive devices |
| US10555738B2 (en) | 2016-05-18 | 2020-02-11 | Microvention, Inc. | Embolic containment |
| EP2228020B2 (en)† | 2004-03-19 | 2020-03-18 | AGA Medical Corporation | Multi-layer braided structures for occluding vascular defects |
| US20200100795A1 (en)* | 2008-05-01 | 2020-04-02 | Aneuclose Llc | "Bowl-of-Spaghetti" Type Intrasacular Aneurysm Occlusion Device |
| CN111031938A (en)* | 2017-08-22 | 2020-04-17 | 柯惠有限合伙公司 | Devices, systems, and methods for treating vascular defects |
| US10624647B2 (en) | 2011-06-03 | 2020-04-21 | Pulsar Vascular, Inc. | Aneurysm devices with additional anchoring mechanisms and associated systems and methods |
| US10653425B1 (en) | 2019-05-21 | 2020-05-19 | DePuy Synthes Products, Inc. | Layered braided aneurysm treatment device |
| US10716573B2 (en) | 2008-05-01 | 2020-07-21 | Aneuclose | Janjua aneurysm net with a resilient neck-bridging portion for occluding a cerebral aneurysm |
| US10736758B2 (en) | 2013-03-15 | 2020-08-11 | Covidien | Occlusive device |
| US10856880B1 (en) | 2019-05-25 | 2020-12-08 | Galaxy Therapeutics, Inc. | Systems and methods for treating aneurysms |
| US10905430B2 (en) | 2018-01-24 | 2021-02-02 | DePuy Synthes Products, Inc. | Aneurysm device and delivery system |
| US10939915B2 (en) | 2018-05-31 | 2021-03-09 | DePuy Synthes Products, Inc. | Aneurysm device and delivery system |
| US11058430B2 (en)* | 2018-05-25 | 2021-07-13 | DePuy Synthes Products, Inc. | Aneurysm device and delivery system |
| CN113180882A (en)* | 2016-06-21 | 2021-07-30 | 内流医疗有限公司 | Device and kit for treating vascular malformed portions |
| US11076861B2 (en)* | 2018-10-12 | 2021-08-03 | DePuy Synthes Products, Inc. | Folded aneurysm treatment device and delivery method |
| US11076860B2 (en) | 2014-03-31 | 2021-08-03 | DePuy Synthes Products, Inc. | Aneurysm occlusion device |
| WO2021130064A3 (en)* | 2019-12-23 | 2021-08-19 | Phenox Gmbh | Implant for treating aneurysms |
| US11123077B2 (en) | 2018-09-25 | 2021-09-21 | DePuy Synthes Products, Inc. | Intrasaccular device positioning and deployment system |
| US11134953B2 (en) | 2019-02-06 | 2021-10-05 | DePuy Synthes Products, Inc. | Adhesive cover occluding device for aneurysm treatment |
| US11154302B2 (en) | 2014-03-31 | 2021-10-26 | DePuy Synthes Products, Inc. | Aneurysm occlusion device |
| US20210378681A1 (en)* | 2010-09-10 | 2021-12-09 | Covidien Lp | Devices, systems, and methods for the treatment of vascular defects |
| US11272939B2 (en)* | 2018-12-18 | 2022-03-15 | DePuy Synthes Products, Inc. | Intrasaccular flow diverter for treating cerebral aneurysms |
| US11278292B2 (en) | 2019-05-21 | 2022-03-22 | DePuy Synthes Products, Inc. | Inverting braided aneurysm treatment system and method |
| US11284903B2 (en) | 2011-03-30 | 2022-03-29 | Hesham Morsi | Advanced endovascular clip and method of using same |
| CN114391910A (en)* | 2014-12-31 | 2022-04-26 | 先健科技(深圳)有限公司 | Left atrial appendage occluder |
| US11337706B2 (en) | 2019-03-27 | 2022-05-24 | DePuy Synthes Products, Inc. | Aneurysm treatment device |
| US11406392B2 (en) | 2018-12-12 | 2022-08-09 | DePuy Synthes Products, Inc. | Aneurysm occluding device for use with coagulating agents |
| US11406404B2 (en) | 2020-02-20 | 2022-08-09 | Cerus Endovascular Limited | Clot removal distal protection methods |
| US11413046B2 (en) | 2019-05-21 | 2022-08-16 | DePuy Synthes Products, Inc. | Layered braided aneurysm treatment device |
| US11457926B2 (en) | 2019-12-18 | 2022-10-04 | DePuy Synthes Products, Inc. | Implant having an intrasaccular section and intravascular section |
| US11471162B2 (en) | 2015-12-07 | 2022-10-18 | Cerus Endovascular Limited | Occlusion device |
| US11497504B2 (en) | 2019-05-21 | 2022-11-15 | DePuy Synthes Products, Inc. | Aneurysm treatment with pushable implanted braid |
| US11583288B2 (en) | 2018-08-08 | 2023-02-21 | DePuy Synthes Products, Inc. | Delivery of embolic braid |
| CN115721366A (en)* | 2022-11-16 | 2023-03-03 | 广西医科大学第二附属医院(广西医科大学第二临床医学院) | A hollow-structure embolism coil assembly and a push system |
| US11596412B2 (en) | 2018-05-25 | 2023-03-07 | DePuy Synthes Products, Inc. | Aneurysm device and delivery system |
| US11602350B2 (en) | 2019-12-05 | 2023-03-14 | DePuy Synthes Products, Inc. | Intrasaccular inverting braid with highly flexible fill material |
| US11607226B2 (en) | 2019-05-21 | 2023-03-21 | DePuy Synthes Products, Inc. | Layered braided aneurysm treatment device with corrugations |
| US11633818B2 (en) | 2019-11-04 | 2023-04-25 | Covidien Lp | Devices, systems, and methods for treatment of intracranial aneurysms |
| US11672542B2 (en) | 2019-05-21 | 2023-06-13 | DePuy Synthes Products, Inc. | Aneurysm treatment with pushable ball segment |
| CN116407191A (en)* | 2021-12-29 | 2023-07-11 | 赛诺神畅医疗科技有限公司 | Aneurysm occlusion system |
| CN116687496A (en)* | 2023-08-04 | 2023-09-05 | 北京久事神康医疗科技有限公司 | Plug device with net disc |
| US11812971B2 (en) | 2017-08-21 | 2023-11-14 | Cerus Endovascular Limited | Occlusion device |
| US11986189B2 (en) | 2021-01-27 | 2024-05-21 | Galaxy Therapeutics, Inc. | Systems and methods for treating aneurysms |
| US12102327B2 (en) | 2019-05-25 | 2024-10-01 | Galaxy Therapeutics, Inc. | Systems and methods for treating aneurysms |
| US12318294B2 (en) | 2017-07-06 | 2025-06-03 | Raghuveer Basude | Tissue grasping devices and related methods |
| US12364708B2 (en) | 2016-10-21 | 2025-07-22 | Covidien Lp | Injectable scaffold for treatment of intracranial aneurysms and related technology |
Families Citing this family (65)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| DE502004008712D1 (en) | 2004-09-22 | 2009-01-29 | Dendron Gmbh | MEDICAL IMPLANT |
| ATE448737T1 (en) | 2004-09-22 | 2009-12-15 | Dendron Gmbh | DEVICE FOR IMPLANTING MICROWL COILS |
| WO2007006139A1 (en)* | 2005-07-12 | 2007-01-18 | Smart Biotech Inc. | Aneurysm occlusion device |
| JP5230602B2 (en) | 2006-04-17 | 2013-07-10 | タイコ ヘルスケア グループ リミテッド パートナーシップ | System and method for mechanically positioning an endovascular implant |
| US8372114B2 (en)* | 2006-11-13 | 2013-02-12 | Electroformed Stents, Inc. | Over-the-wire exclusion device and system for delivery |
| CA2680607C (en) | 2007-03-13 | 2015-07-14 | Microtherapeutics, Inc. | An implant including a coil and a stretch-resistant member |
| US20090227938A1 (en)* | 2008-03-05 | 2009-09-10 | Insitu Therapeutics, Inc. | Wound Closure Devices, Methods of Use, and Kits |
| US11484322B2 (en) | 2018-01-03 | 2022-11-01 | Aneuclose Llc | Aneurysm neck bridge with a closeable opening or lumen through which embolic material is inserted into the aneurysm sac |
| US11464518B2 (en) | 2008-05-01 | 2022-10-11 | Aneuclose Llc | Proximal concave neck bridge with central lumen and distal net for occluding cerebral aneurysms |
| US11471163B2 (en) | 2008-05-01 | 2022-10-18 | Aneuclose Llc | Intrasaccular aneurysm occlusion device with net or mesh expanded by string-of-pearls embolies |
| US20100131002A1 (en)* | 2008-11-24 | 2010-05-27 | Connor Robert A | Stent with a net layer to embolize and aneurysm |
| US20110034941A1 (en)* | 2009-08-10 | 2011-02-10 | Joseph Iraci | Surgical Instrument for Hernia Repair and Method |
| WO2011098633A1 (en)* | 2010-02-12 | 2011-08-18 | Vasuclip, S.L. | Device for aneurysm occlusion and a treatment method |
| US9579104B2 (en) | 2011-11-30 | 2017-02-28 | Covidien Lp | Positioning and detaching implants |
| US9011480B2 (en) | 2012-01-20 | 2015-04-21 | Covidien Lp | Aneurysm treatment coils |
| US9687245B2 (en) | 2012-03-23 | 2017-06-27 | Covidien Lp | Occlusive devices and methods of use |
| WO2013159065A1 (en)* | 2012-04-20 | 2013-10-24 | Paul Lubock | Expandable occlusion devices and methods of use |
| US8690907B1 (en) | 2013-03-15 | 2014-04-08 | Insera Therapeutics, Inc. | Vascular treatment methods |
| US8679150B1 (en) | 2013-03-15 | 2014-03-25 | Insera Therapeutics, Inc. | Shape-set textile structure based mechanical thrombectomy methods |
| US8715314B1 (en) | 2013-03-15 | 2014-05-06 | Insera Therapeutics, Inc. | Vascular treatment measurement methods |
| US9259237B2 (en) | 2013-07-12 | 2016-02-16 | Inceptus Medical, Llc | Methods and apparatus for treating pulmonary embolism |
| US9955976B2 (en) | 2013-08-16 | 2018-05-01 | Sequent Medical, Inc. | Filamentary devices for treatment of vascular defects |
| US9629635B2 (en)* | 2014-04-14 | 2017-04-25 | Sequent Medical, Inc. | Devices for therapeutic vascular procedures |
| US9713475B2 (en) | 2014-04-18 | 2017-07-25 | Covidien Lp | Embolic medical devices |
| US9918718B2 (en) | 2014-08-08 | 2018-03-20 | DePuy Synthes Products, Inc. | Embolic coil delivery system with retractable mechanical release mechanism |
| CA2972620C (en) | 2015-01-20 | 2023-08-01 | Neurogami Medical, Inc. | Micrograft for the treatment of intracranial aneurysms and method for use |
| US10925611B2 (en) | 2015-01-20 | 2021-02-23 | Neurogami Medical, Inc. | Packaging for surgical implant |
| US10857012B2 (en) | 2015-01-20 | 2020-12-08 | Neurogami Medical, Inc. | Vascular implant |
| US11484319B2 (en) | 2015-01-20 | 2022-11-01 | Neurogami Medical, Inc. | Delivery system for micrograft for treating intracranial aneurysms |
| US10736730B2 (en) | 2015-01-20 | 2020-08-11 | Neurogami Medical, Inc. | Vascular implant |
| DE102015104785A1 (en)* | 2015-03-27 | 2016-09-29 | Pfm Medical Ag | Device for closing a cardiac ear |
| WO2017106567A1 (en)* | 2015-12-15 | 2017-06-22 | Nsvascular, Inc. | Intrasaccular thin-film flow diverters and related methods |
| AU2017218115B2 (en) | 2016-02-10 | 2020-03-05 | Microvention, Inc. | Devices for vascular occlusion |
| US10420563B2 (en) | 2016-07-08 | 2019-09-24 | Neurogami Medical, Inc. | Delivery system insertable through body lumen |
| US20180049859A1 (en)* | 2016-08-16 | 2018-02-22 | Spartan Micro, Inc. | Intravascular flow diversion devices |
| EP3526379B1 (en) | 2016-10-14 | 2021-08-11 | Inceptus Medical, LLC | Braiding machine and methods of use |
| EP4331536A3 (en)* | 2016-10-21 | 2024-05-22 | Javelin Medical Ltd. | Systems, methods and devices for embolic protection |
| EP3554391A4 (en) | 2017-02-24 | 2020-09-16 | Inceptus Medical LLC | VESSEL LOCKING DEVICES AND METHODS |
| US11812968B2 (en)* | 2017-05-10 | 2023-11-14 | Lifetech Scientific (Shenzhen) Co. Ltd. | Left atrial appendage occluder |
| US11885051B2 (en) | 2017-10-14 | 2024-01-30 | Inceptus Medical, Llc | Braiding machine and methods of use |
| US10806462B2 (en) | 2017-12-21 | 2020-10-20 | DePuy Synthes Products, Inc. | Implantable medical device detachment system with split tube and cylindrical coupling |
| US10716574B2 (en) | 2017-12-22 | 2020-07-21 | DePuy Synthes Products, Inc. | Aneurysm device and delivery method |
| US10751065B2 (en) | 2017-12-22 | 2020-08-25 | DePuy Synthes Products, Inc. | Aneurysm device and delivery system |
| US11147562B2 (en) | 2018-12-12 | 2021-10-19 | DePuy Synthes Products, Inc. | Systems and methods for embolic implant detachment |
| US11253265B2 (en) | 2019-06-18 | 2022-02-22 | DePuy Synthes Products, Inc. | Pull wire detachment for intravascular devices |
| US11426174B2 (en) | 2019-10-03 | 2022-08-30 | DePuy Synthes Products, Inc. | Medical device delivery member with flexible stretch resistant mechanical release |
| US11207494B2 (en) | 2019-07-03 | 2021-12-28 | DePuy Synthes Products, Inc. | Medical device delivery member with flexible stretch resistant distal portion |
| US11439403B2 (en) | 2019-09-17 | 2022-09-13 | DePuy Synthes Products, Inc. | Embolic coil proximal connecting element and stretch resistant fiber |
| US12376859B2 (en) | 2019-09-17 | 2025-08-05 | DePuy Synthes Products, Inc. | Embolic coil proximal connecting element and stretch resistant fiber |
| US11376013B2 (en) | 2019-11-18 | 2022-07-05 | DePuy Synthes Products, Inc. | Implant delivery system with braid cup formation |
| US11457922B2 (en) | 2020-01-22 | 2022-10-04 | DePuy Synthes Products, Inc. | Medical device delivery member with flexible stretch resistant distal portion |
| US11432822B2 (en) | 2020-02-14 | 2022-09-06 | DePuy Synthes Products, Inc. | Intravascular implant deployment system |
| US20210282789A1 (en) | 2020-03-11 | 2021-09-16 | Microvention, Inc. | Multiple layer devices for treatment of vascular defects |
| US11951026B2 (en) | 2020-06-30 | 2024-04-09 | DePuy Synthes Products, Inc. | Implantable medical device detachment system with flexible braid section |
| US12127743B2 (en) | 2020-09-23 | 2024-10-29 | DePuy Synthes Products, Inc. | Inverting braided aneurysm implant with dome feature |
| JP7427202B2 (en)* | 2021-05-28 | 2024-02-05 | 有限会社ウサミナノテクノロジー | Aneurysm neck embolization member and method for manufacturing the aneurysm neck embolization member |
| US11998213B2 (en) | 2021-07-14 | 2024-06-04 | DePuy Synthes Products, Inc. | Implant delivery with modified detachment feature and pull wire engagement |
| US11844490B2 (en) | 2021-12-30 | 2023-12-19 | DePuy Synthes Products, Inc. | Suture linkage for inhibiting premature embolic implant deployment |
| US11937824B2 (en) | 2021-12-30 | 2024-03-26 | DePuy Synthes Products, Inc. | Implant detachment systems with a modified pull wire |
| US12011171B2 (en) | 2022-01-06 | 2024-06-18 | DePuy Synthes Products, Inc. | Systems and methods for inhibiting premature embolic implant deployment |
| US11937825B2 (en) | 2022-03-02 | 2024-03-26 | DePuy Synthes Products, Inc. | Hook wire for preventing premature embolic implant detachment |
| US12137915B2 (en) | 2022-03-03 | 2024-11-12 | DePuy Synthes Products, Inc. | Elongating wires for inhibiting premature implant detachment |
| US11937826B2 (en) | 2022-03-14 | 2024-03-26 | DePuy Synthes Products, Inc. | Proximal link wire for preventing premature implant detachment |
| US12402886B2 (en) | 2022-06-23 | 2025-09-02 | DePuy Synthes Products, Inc. | Detachment indicator for implant deployment |
| US12396730B2 (en) | 2022-09-28 | 2025-08-26 | DePuy Synthes Products, Inc. | Braided implant with detachment mechanism |
Citations (15)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US4739768A (en)* | 1986-06-02 | 1988-04-26 | Target Therapeutics | Catheter for guide-wire tracking |
| US4994069A (en)* | 1988-11-02 | 1991-02-19 | Target Therapeutics | Vaso-occlusion coil and method |
| US5122136A (en)* | 1990-03-13 | 1992-06-16 | The Regents Of The University Of California | Endovascular electrolytically detachable guidewire tip for the electroformation of thrombus in arteries, veins, aneurysms, vascular malformations and arteriovenous fistulas |
| US5250071A (en)* | 1992-09-22 | 1993-10-05 | Target Therapeutics, Inc. | Detachable embolic coil assembly using interlocking clasps and method of use |
| US5261916A (en)* | 1991-12-12 | 1993-11-16 | Target Therapeutics | Detachable pusher-vasoocclusive coil assembly with interlocking ball and keyway coupling |
| US5354295A (en)* | 1990-03-13 | 1994-10-11 | Target Therapeutics, Inc. | In an endovascular electrolytically detachable wire and tip for the formation of thrombus in arteries, veins, aneurysms, vascular malformations and arteriovenous fistulas |
| US5928260A (en)* | 1997-07-10 | 1999-07-27 | Scimed Life Systems, Inc. | Removable occlusion system for aneurysm neck |
| US5944750A (en)* | 1997-06-30 | 1999-08-31 | Eva Corporation | Method and apparatus for the surgical repair of aneurysms |
| US6063070A (en)* | 1997-08-05 | 2000-05-16 | Target Therapeutics, Inc. | Detachable aneurysm neck bridge (II) |
| US6077281A (en)* | 1992-01-21 | 2000-06-20 | Regents Of The University Of Minnesota | Septal defect closure device |
| US6080182A (en)* | 1996-12-20 | 2000-06-27 | Gore Enterprise Holdings, Inc. | Self-expanding defect closure device and method of making and using |
| US6086577A (en)* | 1997-08-13 | 2000-07-11 | Scimed Life Systems, Inc. | Detachable aneurysm neck bridge (III) |
| US6168622B1 (en)* | 1996-01-24 | 2001-01-02 | Microvena Corporation | Method and apparatus for occluding aneurysms |
| US6346117B1 (en)* | 2000-03-02 | 2002-02-12 | Prodesco, Inc. | Bag for use in the intravascular treatment of saccular aneurysms |
| US6375668B1 (en)* | 1999-06-02 | 2002-04-23 | Hanson S. Gifford | Devices and methods for treating vascular malformations |
Family Cites Families (52)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US634617A (en)* | 1898-09-06 | 1899-10-10 | Charles H Hansen | Brush. |
| US4830003A (en)* | 1988-06-17 | 1989-05-16 | Wolff Rodney G | Compressive stent and delivery system |
| US4991602A (en)* | 1989-06-27 | 1991-02-12 | Flexmedics Corporation | Flexible guide wire with safety tip |
| US5527338A (en)* | 1992-09-02 | 1996-06-18 | Board Of Regents, The University Of Texas System | Intravascular device |
| US5725552A (en) | 1994-07-08 | 1998-03-10 | Aga Medical Corporation | Percutaneous catheter directed intravascular occlusion devices |
| ES2340142T3 (en)* | 1994-07-08 | 2010-05-31 | Ev3 Inc. | SYSTEM TO CARRY OUT AN INTRAVASCULAR PROCEDURE. |
| US6123715A (en) | 1994-07-08 | 2000-09-26 | Amplatz; Curtis | Method of forming medical devices; intravascular occlusion devices |
| US8790363B2 (en) | 1995-04-20 | 2014-07-29 | DePuy Synthes Products, LLC | Three dimensional, low friction vasoocclusive coil, and method of manufacture |
| US5944730A (en)* | 1997-05-19 | 1999-08-31 | Cardio Medical Solutions, Inc. | Device and method for assisting end-to-side anastomosis |
| US5951599A (en) | 1997-07-09 | 1999-09-14 | Scimed Life Systems, Inc. | Occlusion system for endovascular treatment of an aneurysm |
| GB9715241D0 (en) | 1997-07-18 | 1997-09-24 | Jeffree Martin A | Device for treating aneurysms |
| EP1006890B1 (en) | 1997-08-04 | 2006-09-20 | Boston Scientific Limited | Occlusion system for aneurysm repair |
| US6511468B1 (en) | 1997-10-17 | 2003-01-28 | Micro Therapeutics, Inc. | Device and method for controlling injection of liquid embolic composition |
| US6159165A (en) | 1997-12-05 | 2000-12-12 | Micrus Corporation | Three dimensional spherical micro-coils manufactured from radiopaque nickel-titanium microstrand |
| US5944738A (en)* | 1998-02-06 | 1999-08-31 | Aga Medical Corporation | Percutaneous catheter directed constricting occlusion device |
| JP2003522550A (en) | 1998-02-10 | 2003-07-29 | アーテミス・メディカル・インコーポレイテッド | Occlusion, fixation, tensioning, and diverting devices and methods of use |
| US6168615B1 (en) | 1998-05-04 | 2001-01-02 | Micrus Corporation | Method and apparatus for occlusion and reinforcement of aneurysms |
| US6093199A (en) | 1998-08-05 | 2000-07-25 | Endovascular Technologies, Inc. | Intra-luminal device for treatment of body cavities and lumens and method of use |
| EP1109499B1 (en) | 1998-09-04 | 2007-08-15 | Boston Scientific Limited | Detachable aneurysm neck closure patch |
| US7044134B2 (en)* | 1999-11-08 | 2006-05-16 | Ev3 Sunnyvale, Inc | Method of implanting a device in the left atrial appendage |
| US6613074B1 (en) | 1999-03-10 | 2003-09-02 | Cordis Corporation | Endovascular aneurysm embolization device |
| US6238403B1 (en) | 1999-10-04 | 2001-05-29 | Microvention, Inc. | Filamentous embolic device with expansible elements |
| US6551303B1 (en)* | 1999-10-27 | 2003-04-22 | Atritech, Inc. | Barrier device for ostium of left atrial appendage |
| US6391037B1 (en) | 2000-03-02 | 2002-05-21 | Prodesco, Inc. | Bag for use in the intravascular treatment of saccular aneurysms |
| US6454780B1 (en) | 2001-06-21 | 2002-09-24 | Scimed Life Systems, Inc. | Aneurysm neck obstruction device |
| US6811560B2 (en) | 2001-09-20 | 2004-11-02 | Cordis Neurovascular, Inc. | Stent aneurysm embolization method and device |
| US6638257B2 (en) | 2002-03-01 | 2003-10-28 | Aga Medical Corporation | Intravascular flow restrictor |
| US8398670B2 (en) | 2004-03-19 | 2013-03-19 | Aga Medical Corporation | Multi-layer braided structures for occluding vascular defects and for occluding fluid flow through portions of the vasculature of the body |
| US8777974B2 (en) | 2004-03-19 | 2014-07-15 | Aga Medical Corporation | Multi-layer braided structures for occluding vascular defects |
| US8313505B2 (en) | 2004-03-19 | 2012-11-20 | Aga Medical Corporation | Device for occluding vascular defects |
| JP4395519B2 (en) | 2004-04-08 | 2010-01-13 | エイジーエイ メディカル コーポレイション | Flange closure device and method |
| WO2006034153A2 (en)* | 2004-09-17 | 2006-03-30 | Cordis Neurovascular, Inc. | Thin film metallic devices for plugging aneurysms or vessels |
| CA2580752C (en) | 2004-09-17 | 2014-11-25 | Cordis Neurovascular, Inc. | Vascular occlusion device with an embolic mesh ribbon |
| JP5230602B2 (en) | 2006-04-17 | 2013-07-10 | タイコ ヘルスケア グループ リミテッド パートナーシップ | System and method for mechanically positioning an endovascular implant |
| US8034061B2 (en) | 2007-07-12 | 2011-10-11 | Aga Medical Corporation | Percutaneous catheter directed intravascular occlusion devices |
| US8361138B2 (en) | 2007-07-25 | 2013-01-29 | Aga Medical Corporation | Braided occlusion device having repeating expanded volume segments separated by articulation segments |
| US20090112251A1 (en) | 2007-07-25 | 2009-04-30 | Aga Medical Corporation | Braided occlusion device having repeating expanded volume segments separated by articulation segments |
| EP2190380A1 (en) | 2007-09-11 | 2010-06-02 | NFocus Neuromedical, Inc. | Aneurysm cover device for embolic delivery and retention |
| US9743918B2 (en) | 2008-01-18 | 2017-08-29 | St. Jude Medical, Cardiology Division, Inc. | Percutaneous catheter directed intravascular occlusion device |
| EP2633823B1 (en) | 2008-04-21 | 2016-06-01 | Covidien LP | Braid-ball embolic devices and delivery systems |
| US9232992B2 (en) | 2008-07-24 | 2016-01-12 | Aga Medical Corporation | Multi-layered medical device for treating a target site and associated method |
| US9427304B2 (en) | 2008-10-27 | 2016-08-30 | St. Jude Medical, Cardiology Division, Inc. | Multi-layer device with gap for treating a target site and associated method |
| US8940015B2 (en) | 2008-11-11 | 2015-01-27 | Aga Medical Corporation | Asymmetrical medical devices for treating a target site and associated method |
| US8906057B2 (en)* | 2010-01-04 | 2014-12-09 | Aneuclose Llc | Aneurysm embolization by rotational accumulation of mass |
| US8821529B2 (en) | 2011-03-25 | 2014-09-02 | Aga Medical Corporation | Device and method for occluding a septal defect |
| US8511214B2 (en) | 2011-04-21 | 2013-08-20 | Aga Medical Corporation | Tubular structure and method for making the same |
| US8764787B2 (en) | 2011-06-17 | 2014-07-01 | Aga Medical Corporation | Occlusion device and associated deployment method |
| US8758389B2 (en) | 2011-11-18 | 2014-06-24 | Aga Medical Corporation | Devices and methods for occluding abnormal openings in a patient's vasculature |
| US9113890B2 (en) | 2012-02-06 | 2015-08-25 | Aga Medical Corporation | Devices and methods for occluding vascular abnormalities |
| US9687245B2 (en) | 2012-03-23 | 2017-06-27 | Covidien Lp | Occlusive devices and methods of use |
| US10327781B2 (en) | 2012-11-13 | 2019-06-25 | Covidien Lp | Occlusive devices |
| US10973523B2 (en) | 2013-03-08 | 2021-04-13 | Aga Medical Corporation | Medical device for treating a target site |
- 2002
- 2002-04-12USUS10/121,980patent/US20030195553A1/ennot_activeAbandoned
- 2003
- 2003-04-10AUAU2003224913Apatent/AU2003224913A1/ennot_activeAbandoned
- 2003-04-10ATAT03721607Tpatent/ATE439092T1/ennot_activeIP Right Cessation
- 2003-04-10DEDE60328765Tpatent/DE60328765D1/ennot_activeExpired - Lifetime
- 2003-04-10WOPCT/US2003/010997patent/WO2003086240A1/enactiveApplication Filing
- 2003-04-10CACA2481224Apatent/CA2481224C/ennot_activeExpired - Fee Related
- 2003-04-10ESES03721607Tpatent/ES2331567T3/ennot_activeExpired - Lifetime
- 2003-04-10EPEP03721607Apatent/EP1494619B1/ennot_activeExpired - Lifetime
- 2003-04-10JPJP2003583267Apatent/JP4414767B2/ennot_activeExpired - Lifetime
- 2006
- 2006-12-28USUS11/617,293patent/US20070106311A1/ennot_activeAbandoned
- 2011
- 2011-04-21USUS13/091,608patent/US9314326B2/ennot_activeExpired - Lifetime
Patent Citations (17)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US4739768B1 (en)* | 1986-06-02 | 1994-11-15 | Target Therapeutics Inc | Catheter for guide-wire tracking |
| US4739768B2 (en)* | 1986-06-02 | 1995-10-24 | Target Therapeutics Inc | Catheter for guide-wire tracking |
| US4739768A (en)* | 1986-06-02 | 1988-04-26 | Target Therapeutics | Catheter for guide-wire tracking |
| US4994069A (en)* | 1988-11-02 | 1991-02-19 | Target Therapeutics | Vaso-occlusion coil and method |
| US5122136A (en)* | 1990-03-13 | 1992-06-16 | The Regents Of The University Of California | Endovascular electrolytically detachable guidewire tip for the electroformation of thrombus in arteries, veins, aneurysms, vascular malformations and arteriovenous fistulas |
| US5354295A (en)* | 1990-03-13 | 1994-10-11 | Target Therapeutics, Inc. | In an endovascular electrolytically detachable wire and tip for the formation of thrombus in arteries, veins, aneurysms, vascular malformations and arteriovenous fistulas |
| US5261916A (en)* | 1991-12-12 | 1993-11-16 | Target Therapeutics | Detachable pusher-vasoocclusive coil assembly with interlocking ball and keyway coupling |
| US6077281A (en)* | 1992-01-21 | 2000-06-20 | Regents Of The University Of Minnesota | Septal defect closure device |
| US5250071A (en)* | 1992-09-22 | 1993-10-05 | Target Therapeutics, Inc. | Detachable embolic coil assembly using interlocking clasps and method of use |
| US6168622B1 (en)* | 1996-01-24 | 2001-01-02 | Microvena Corporation | Method and apparatus for occluding aneurysms |
| US6080182A (en)* | 1996-12-20 | 2000-06-27 | Gore Enterprise Holdings, Inc. | Self-expanding defect closure device and method of making and using |
| US5944750A (en)* | 1997-06-30 | 1999-08-31 | Eva Corporation | Method and apparatus for the surgical repair of aneurysms |
| US5928260A (en)* | 1997-07-10 | 1999-07-27 | Scimed Life Systems, Inc. | Removable occlusion system for aneurysm neck |
| US6063070A (en)* | 1997-08-05 | 2000-05-16 | Target Therapeutics, Inc. | Detachable aneurysm neck bridge (II) |
| US6086577A (en)* | 1997-08-13 | 2000-07-11 | Scimed Life Systems, Inc. | Detachable aneurysm neck bridge (III) |
| US6375668B1 (en)* | 1999-06-02 | 2002-04-23 | Hanson S. Gifford | Devices and methods for treating vascular malformations |
| US6346117B1 (en)* | 2000-03-02 | 2002-02-12 | Prodesco, Inc. | Bag for use in the intravascular treatment of saccular aneurysms |
Cited By (255)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US20050049681A1 (en)* | 2003-05-19 | 2005-03-03 | Secant Medical, Llc | Tissue distention device and related methods for therapeutic intervention |
| US8758395B2 (en) | 2003-05-19 | 2014-06-24 | Septrx, Inc. | Embolic filtering method and apparatus |
| US7648532B2 (en) | 2003-05-19 | 2010-01-19 | Septrx, Inc. | Tissue distention device and related methods for therapeutic intervention |
| US20060178694A1 (en)* | 2003-05-19 | 2006-08-10 | Secant Medical, Llc | Tissue distention device and related methods for therapeutic intervention |
| US7122043B2 (en) | 2003-05-19 | 2006-10-17 | Stout Medical Group, L.P. | Tissue distention device and related methods for therapeutic intervention |
| AU2010249161B2 (en)* | 2004-01-22 | 2013-05-16 | Microvention, Inc. | Aneurysm treatment device and method of use |
| US11134933B2 (en) | 2004-03-19 | 2021-10-05 | St. Jude Medical, Cardiology Division, Inc. | Multi-layer braided structures for occluding vascular defects |
| EP2228020B2 (en)† | 2004-03-19 | 2020-03-18 | AGA Medical Corporation | Multi-layer braided structures for occluding vascular defects |
| US9445799B2 (en) | 2004-03-19 | 2016-09-20 | St. Jude Medical, Cardiology Division, Inc. | Multi-layer braided structures for occluding vascular defects |
| US8777974B2 (en)* | 2004-03-19 | 2014-07-15 | Aga Medical Corporation | Multi-layer braided structures for occluding vascular defects |
| US9877710B2 (en)* | 2004-03-19 | 2018-01-30 | St. Jude Medical, Cardiology Division, Inc. | Multi-layer braided structures for occluding vascular defects and for occluding fluid flow through portions of the vasculature of the body |
| US20080200945A1 (en)* | 2004-03-19 | 2008-08-21 | Aga Medical Corporation | Device for occluding vascular defects |
| US9039724B2 (en)* | 2004-03-19 | 2015-05-26 | Aga Medical Corporation | Device for occluding vascular defects |
| US20090062841A1 (en)* | 2004-03-19 | 2009-03-05 | Aga Medical Corporation | Device for occluding vascular defects |
| US8398670B2 (en) | 2004-03-19 | 2013-03-19 | Aga Medical Corporation | Multi-layer braided structures for occluding vascular defects and for occluding fluid flow through portions of the vasculature of the body |
| US20150133994A1 (en)* | 2004-03-19 | 2015-05-14 | Aga Medical Corporation | Multi-layer braided structures for occluding vascular defects |
| US9445798B2 (en) | 2004-03-19 | 2016-09-20 | St. Jude Medical, Cardiology Division, Inc. | Multi-layer braided structures for occluding vascular defects |
| US10624619B2 (en) | 2004-03-19 | 2020-04-21 | St. Jude Medical, Cardiology Division, Inc. | Multi-layer braided structures for occluding vascular defects and for occluding fluid flow through portions of the vasculature of the body |
| US8313505B2 (en) | 2004-03-19 | 2012-11-20 | Aga Medical Corporation | Device for occluding vascular defects |
| US9743932B2 (en)* | 2004-04-08 | 2017-08-29 | St. Jude Medical, Cardiology Division, Inc. | Flanged occlusion devices and methods |
| US20060247680A1 (en)* | 2004-04-08 | 2006-11-02 | Aga Medical Corporation | Flanged occlusion devices and methods |
| US10231737B2 (en) | 2004-04-08 | 2019-03-19 | St. Jude Medical, Cardiology Division, Inc. | Flanged occlusion devices and methods |
| US20210275183A1 (en)* | 2004-04-08 | 2021-09-09 | St. Jude Medical, Cardiology Division, Inc. | Flanged occlusion devices and methods |
| US11839379B2 (en)* | 2004-04-08 | 2023-12-12 | St. Jude Medical, Cardiology Division, Inc. | Flanged occlusion devices and methods |
| US11045202B2 (en)* | 2004-04-08 | 2021-06-29 | St. Jude Medical, Cardiology Division, Inc. | Flanged occlusion devices and methods |
| US12290263B2 (en) | 2004-04-08 | 2025-05-06 | St. Jude Medical, Cardiology Division, Inc. | Flanged occlusion devices and methods |
| EP2856949B2 (en)† | 2004-04-08 | 2019-09-11 | Aga Medical Corporation | Flanged occlusion devices |
| EP2856949B1 (en) | 2004-04-08 | 2015-10-07 | Aga Medical Corporation | Flanged occlusion devices |
| US20110046719A1 (en)* | 2004-06-03 | 2011-02-24 | Noureddine Frid | Luminal Endoprosthesis For the Occlusion of an Aneurysm and Method of Manufacturing Such an Endoprosthesis |
| US8715338B2 (en)* | 2004-06-03 | 2014-05-06 | Noureddine Frid | Luminal endoprosthesis for the occlusion of an aneurysm and method of manufacturing such an endoprosthesis |
| US20050283183A1 (en)* | 2004-06-21 | 2005-12-22 | Tri Tran | Expanding vaso-occlusive device |
| US8486101B2 (en) | 2004-06-21 | 2013-07-16 | Stryker Corporation | Expanding vaso-occlusive device |
| US7749242B2 (en)* | 2004-06-21 | 2010-07-06 | Boston Scientific Scimed, Inc. | Expanding vaso-occlusive device |
| US20100228278A1 (en)* | 2004-06-21 | 2010-09-09 | Boston Scientific Scimed, Inc. | Expanding vaso-occlusive device |
| US20070270902A1 (en)* | 2004-09-17 | 2007-11-22 | Slazas Robert R | Thin Film Metallic Devices for Plugging Aneurysms or Vessels |
| US20060064151A1 (en)* | 2004-09-22 | 2006-03-23 | Guterman Lee R | Cranial aneurysm treatment arrangement |
| WO2006052322A3 (en)* | 2004-09-22 | 2009-04-09 | Lee R Guterman | Cranial aneurysm treatment arrangement |
| US12059157B2 (en)* | 2005-01-07 | 2024-08-13 | Stryker Corporation | Intra-aneurysm devices |
| US10265075B2 (en)* | 2005-01-07 | 2019-04-23 | Stryker Corporation | Intra-aneurysm devices |
| US12042151B2 (en)* | 2005-01-07 | 2024-07-23 | Stryker Corporation and Stryker European Operations Holdings LLC | Intra-aneurysm devices |
| US20130325053A1 (en)* | 2005-01-07 | 2013-12-05 | Stryker Nv Operations Limited | Intra-aneurysm devices |
| US20060155323A1 (en)* | 2005-01-07 | 2006-07-13 | Porter Stephen C | Intra-aneurysm devices |
| US20230015848A1 (en)* | 2005-01-07 | 2023-01-19 | Stryker Corporation | Intra-Aneurysm Devices |
| US12016568B2 (en)* | 2005-01-07 | 2024-06-25 | Stryker Corporation | Intra-aneurysm devices |
| US20070233174A1 (en)* | 2005-04-01 | 2007-10-04 | Gordon Hocking | Trapping Filter for Blood Vessel |
| US10064747B2 (en) | 2005-05-25 | 2018-09-04 | Covidien Lp | System and method for delivering and deploying an occluding device within a vessel |
| US9204983B2 (en) | 2005-05-25 | 2015-12-08 | Covidien Lp | System and method for delivering and deploying an occluding device within a vessel |
| US10322018B2 (en) | 2005-05-25 | 2019-06-18 | Covidien Lp | System and method for delivering and deploying an occluding device within a vessel |
| US9095343B2 (en) | 2005-05-25 | 2015-08-04 | Covidien Lp | System and method for delivering and deploying an occluding device within a vessel |
| US9198666B2 (en) | 2005-05-25 | 2015-12-01 | Covidien Lp | System and method for delivering and deploying an occluding device within a vessel |
| US9381104B2 (en) | 2005-05-25 | 2016-07-05 | Covidien Lp | System and method for delivering and deploying an occluding device within a vessel |
| EP1901665A4 (en)* | 2005-07-11 | 2014-01-08 | Usgi Medical Inc | Compressible tissue anchor assemblies |
| JP2015083139A (en)* | 2005-10-19 | 2015-04-30 | パルサー バスキュラー インコーポレイテッド | Method and system for endovascularly clipping and repairing lumen and tissue defect |
| US9510835B2 (en) | 2005-10-19 | 2016-12-06 | Pulsar Vascular, Inc. | Methods and systems for endovascularly clipping and repairing lumen and tissue defects |
| US8551132B2 (en) | 2005-10-19 | 2013-10-08 | Pulsar Vascular, Inc. | Methods and systems for endovascularly clipping and repairing lumen and tissue defects |
| US8545530B2 (en)* | 2005-10-19 | 2013-10-01 | Pulsar Vascular, Inc. | Implantable aneurysm closure systems and methods |
| US20070088387A1 (en)* | 2005-10-19 | 2007-04-19 | Pulsar Vascular, Inc. | Implantable aneurysm closure systems and methods |
| US10499927B2 (en) | 2005-10-19 | 2019-12-10 | Pulsar Vascular, Inc. | Methods and systems for endovascularly clipping and repairing lumen and tissue defects |
| WO2007140797A1 (en)* | 2006-06-02 | 2007-12-13 | Occlutech Gmbh | Occlusion instrument for closing a cardiac auricle |
| US20080221600A1 (en)* | 2006-08-17 | 2008-09-11 | Dieck Martin S | Isolation devices for the treatment of aneurysms |
| US9161758B2 (en)* | 2007-04-16 | 2015-10-20 | Occlutech Holding Ag | Occluder for occluding an atrial appendage and production process therefor |
| US20120271337A1 (en)* | 2007-04-16 | 2012-10-25 | Hans-Reiner Figulla | Occluder For Occluding an Atrial Appendage and Production Process Therefor |
| US9826980B2 (en)* | 2007-04-16 | 2017-11-28 | Occlutech Holding Ag | Occluder for occluding an atrial appendage and production process therefor |
| US9023094B2 (en) | 2007-06-25 | 2015-05-05 | Microvention, Inc. | Self-expanding prosthesis |
| US8747453B2 (en) | 2008-02-18 | 2014-06-10 | Aga Medical Corporation | Stent/stent graft for reinforcement of vascular abnormalities and associated method |
| US8696701B2 (en) | 2008-04-21 | 2014-04-15 | Covidien Lp | Braid-ball embolic devices |
| US9585669B2 (en) | 2008-04-21 | 2017-03-07 | Covidien Lp | Multiple layer filamentary devices for treatment of vascular defects |
| US11844528B2 (en)* | 2008-04-21 | 2023-12-19 | Covidien Lp | Multiple layer filamentary devices for treatment of vascular defects |
| US8747597B2 (en) | 2008-04-21 | 2014-06-10 | Covidien Lp | Methods for making braid-ball occlusion devices |
| US20220022886A1 (en)* | 2008-04-21 | 2022-01-27 | Covidien Lp | Multiple layer filamentary devices for treatment of vascular defects |
| US9039726B2 (en) | 2008-04-21 | 2015-05-26 | Covidien Lp | Filamentary devices for treatment of vascular defects |
| US8142456B2 (en) | 2008-04-21 | 2012-03-27 | Nfocus Neuromedical, Inc. | Braid-ball embolic devices |
| US10716573B2 (en) | 2008-05-01 | 2020-07-21 | Aneuclose | Janjua aneurysm net with a resilient neck-bridging portion for occluding a cerebral aneurysm |
| US10028747B2 (en) | 2008-05-01 | 2018-07-24 | Aneuclose Llc | Coils with a series of proximally-and-distally-connected loops for occluding a cerebral aneurysm |
| US20200100795A1 (en)* | 2008-05-01 | 2020-04-02 | Aneuclose Llc | "Bowl-of-Spaghetti" Type Intrasacular Aneurysm Occlusion Device |
| US8974487B2 (en) | 2008-05-01 | 2015-03-10 | Aneuclose Llc | Aneurysm occlusion device |
| US11707371B2 (en) | 2008-05-13 | 2023-07-25 | Covidien Lp | Braid implant delivery systems |
| US10610389B2 (en) | 2008-05-13 | 2020-04-07 | Covidien Lp | Braid implant delivery systems |
| US9675482B2 (en) | 2008-05-13 | 2017-06-13 | Covidien Lp | Braid implant delivery systems |
| US9179918B2 (en) | 2008-07-22 | 2015-11-10 | Covidien Lp | Vascular remodeling device |
| US8388650B2 (en) | 2008-09-05 | 2013-03-05 | Pulsar Vascular, Inc. | Systems and methods for supporting or occluding a physiological opening or cavity |
| US11185333B2 (en) | 2008-09-05 | 2021-11-30 | Pulsar Vascular, Inc. | Systems and methods for supporting or occluding a physiological opening or cavity |
| US8979893B2 (en) | 2008-09-05 | 2015-03-17 | Pulsar Vascular, Inc. | Systems and methods for supporting or occluding a physiological opening or cavity |
| US9615831B2 (en) | 2008-09-05 | 2017-04-11 | Pulsar Vascular, Inc. | Systems and methods for supporting or occluding a physiological opening or cavity |
| US10285709B2 (en) | 2008-09-05 | 2019-05-14 | Pulsar Vascular, Inc. | Systems and methods for supporting or occluding a physiological opening or cavity |
| US8636760B2 (en) | 2009-04-20 | 2014-01-28 | Covidien Lp | System and method for delivering and deploying an occluding device within a vessel |
| US10335153B2 (en) | 2009-09-04 | 2019-07-02 | Pulsar Vascular, Inc. | Systems and methods for enclosing an anatomical opening |
| US11633189B2 (en) | 2009-09-04 | 2023-04-25 | Pulsar Vascular, Inc. | Systems and methods for enclosing an anatomical opening |
| US9277924B2 (en) | 2009-09-04 | 2016-03-08 | Pulsar Vascular, Inc. | Systems and methods for enclosing an anatomical opening |
| US9095342B2 (en) | 2009-11-09 | 2015-08-04 | Covidien Lp | Braid ball embolic device features |
| US9358140B1 (en) | 2009-11-18 | 2016-06-07 | Aneuclose Llc | Stent with outer member to embolize an aneurysm |
| US8906057B2 (en) | 2010-01-04 | 2014-12-09 | Aneuclose Llc | Aneurysm embolization by rotational accumulation of mass |
| US9468442B2 (en) | 2010-01-28 | 2016-10-18 | Covidien Lp | Vascular remodeling device |
| US8926681B2 (en) | 2010-01-28 | 2015-01-06 | Covidien Lp | Vascular remodeling device |
| US8425548B2 (en) | 2010-07-01 | 2013-04-23 | Aneaclose LLC | Occluding member expansion and then stent expansion for aneurysm treatment |
| US20210378681A1 (en)* | 2010-09-10 | 2021-12-09 | Covidien Lp | Devices, systems, and methods for the treatment of vascular defects |
| US9610180B2 (en) | 2010-12-06 | 2017-04-04 | Covidien Lp | Vascular remodeling device |
| US9351859B2 (en) | 2010-12-06 | 2016-05-31 | Covidien Lp | Vascular remodeling device |
| US9393022B2 (en) | 2011-02-11 | 2016-07-19 | Covidien Lp | Two-stage deployment aneurysm embolization devices |
| US10004511B2 (en) | 2011-03-25 | 2018-06-26 | Covidien Lp | Vascular remodeling device |
| US11147563B2 (en) | 2011-03-25 | 2021-10-19 | Covidien Lp | Vascular remodeling device |
| US9089332B2 (en) | 2011-03-25 | 2015-07-28 | Covidien Lp | Vascular remodeling device |
| US10028745B2 (en) | 2011-03-30 | 2018-07-24 | Noha, Llc | Advanced endovascular clip and method of using same |
| US11284903B2 (en) | 2011-03-30 | 2022-03-29 | Hesham Morsi | Advanced endovascular clip and method of using same |
| US10959735B2 (en) | 2011-03-30 | 2021-03-30 | Noha, Llc | Advanced endovascular clip and method of using same |
| US9138232B2 (en) | 2011-05-24 | 2015-09-22 | Aneuclose Llc | Aneurysm occlusion by rotational dispensation of mass |
| WO2012163880A1 (en)* | 2011-05-31 | 2012-12-06 | Acandis Gmbh & Co. Kg | Medical implant for arrangement within a hollow body, in particular an aneurysm, and method for producing a medical implant |
| US10004510B2 (en) | 2011-06-03 | 2018-06-26 | Pulsar Vascular, Inc. | Systems and methods for enclosing an anatomical opening, including shock absorbing aneurysm devices |
| US11344311B2 (en) | 2011-06-03 | 2022-05-31 | Pulsar Vascular, Inc. | Aneurysm devices with additional anchoring mechanisms and associated systems and methods |
| US10624647B2 (en) | 2011-06-03 | 2020-04-21 | Pulsar Vascular, Inc. | Aneurysm devices with additional anchoring mechanisms and associated systems and methods |
| US9060886B2 (en) | 2011-09-29 | 2015-06-23 | Covidien Lp | Vascular remodeling device |
| US11654037B2 (en) | 2011-09-29 | 2023-05-23 | Covidien Lp | Vascular remodeling device |
| US10828182B2 (en) | 2011-09-29 | 2020-11-10 | Covidien Lp | Vascular remodeling device |
| US9636117B2 (en) | 2011-10-05 | 2017-05-02 | Pulsar Vascular, Inc. | Devices, systems and methods for enclosing an anatomical opening |
| US11457923B2 (en) | 2011-10-05 | 2022-10-04 | Pulsar Vascular, Inc. | Devices, systems and methods for enclosing an anatomical opening |
| US9119625B2 (en) | 2011-10-05 | 2015-09-01 | Pulsar Vascular, Inc. | Devices, systems and methods for enclosing an anatomical opening |
| US10426487B2 (en) | 2011-10-05 | 2019-10-01 | Pulsar Vascular, Inc. | Devices, systems and methods for enclosing an anatomical opening |
| US20140350588A1 (en)* | 2011-11-09 | 2014-11-27 | Easynotes Ltd. | Obstruction device |
| US10219931B2 (en)* | 2011-11-09 | 2019-03-05 | Easynotes Ltd. | Obstruction device |
| US9259229B2 (en) | 2012-05-10 | 2016-02-16 | Pulsar Vascular, Inc. | Systems and methods for enclosing an anatomical opening, including coil-tipped aneurysm devices |
| US9877856B2 (en) | 2012-07-18 | 2018-01-30 | Covidien Lp | Methods and apparatus for luminal stenting |
| US9155647B2 (en) | 2012-07-18 | 2015-10-13 | Covidien Lp | Methods and apparatus for luminal stenting |
| US9186267B2 (en) | 2012-10-31 | 2015-11-17 | Covidien Lp | Wing bifurcation reconstruction device |
| US9962164B2 (en) | 2012-10-31 | 2018-05-08 | Covidien Lp | Wing bifurcation reconstruction device |
| US11406405B2 (en) | 2012-11-06 | 2022-08-09 | Covidien Lp | Multi-pivot thrombectomy device |
| US9924959B2 (en) | 2012-11-06 | 2018-03-27 | Covidien Lp | Multi-pivot thrombectomy device |
| US9314248B2 (en) | 2012-11-06 | 2016-04-19 | Covidien Lp | Multi-pivot thrombectomy device |
| US12089863B2 (en) | 2012-11-06 | 2024-09-17 | Covidien Lp | Multi-pivot thrombectomy device |
| US11690628B2 (en) | 2012-11-13 | 2023-07-04 | Covidien Lp | Occlusive devices |
| US12193675B2 (en)* | 2012-11-13 | 2025-01-14 | Covidien Lp | Occlusive devices |
| US10327781B2 (en) | 2012-11-13 | 2019-06-25 | Covidien Lp | Occlusive devices |
| US11786253B2 (en)* | 2012-11-13 | 2023-10-17 | Covidien Lp | Occlusive devices |
| US20190343532A1 (en)* | 2012-11-13 | 2019-11-14 | Covidien Lp | Occlusive devices |
| US9901472B2 (en) | 2013-01-17 | 2018-02-27 | Covidien Lp | Methods and apparatus for luminal stenting |
| US9295571B2 (en) | 2013-01-17 | 2016-03-29 | Covidien Lp | Methods and apparatus for luminal stenting |
| WO2014128313A1 (en)* | 2013-02-21 | 2014-08-28 | Castaño Duque Carlos | Device for supporting and positioning occlusive elements |
| US10716549B2 (en) | 2013-03-05 | 2020-07-21 | St. Jude Medical, Cardiology Division, Inc. | Medical device for treating a target site |
| WO2014137467A1 (en)* | 2013-03-05 | 2014-09-12 | Aga Medical Corporation | Medical device for treating a target site |
| US12268393B2 (en) | 2013-03-13 | 2025-04-08 | Lawrence Livermore National Security, Inc. | Shape-memory polymer foam device for treating aneurysms |
| US11141164B2 (en)* | 2013-03-13 | 2021-10-12 | Lawrence Livermore National Security, Llc | Shape-memory polymer foam device for treating aneurysms |
| US20170252045A1 (en)* | 2013-03-13 | 2017-09-07 | Lawrence Livermore National Security, Llc | Shape-memory polymer foam device for treating aneurysms |
| US9463105B2 (en) | 2013-03-14 | 2016-10-11 | Covidien Lp | Methods and apparatus for luminal stenting |
| US11298144B2 (en) | 2013-03-15 | 2022-04-12 | Insera Therapeutics, Inc. | Thrombus aspiration facilitation systems |
| US10463468B2 (en) | 2013-03-15 | 2019-11-05 | Insera Therapeutics, Inc. | Thrombus aspiration with different intensity levels |
| US10736758B2 (en) | 2013-03-15 | 2020-08-11 | Covidien | Occlusive device |
| US10342655B2 (en) | 2013-03-15 | 2019-07-09 | Insera Therapeutics, Inc. | Methods of treating a thrombus in an artery using cyclical aspiration patterns |
| US12150871B2 (en) | 2013-03-15 | 2024-11-26 | Covidien Lp | Occlusive device |
| US9901435B2 (en)* | 2013-03-15 | 2018-02-27 | Insera Therapeutics, Inc. | Longitudinally variable vascular treatment devices |
| US10335260B2 (en) | 2013-03-15 | 2019-07-02 | Insera Therapeutics, Inc. | Methods of treating a thrombus in a vein using cyclical aspiration patterns |
| US10251739B2 (en) | 2013-03-15 | 2019-04-09 | Insera Therapeutics, Inc. | Thrombus aspiration using an operator-selectable suction pattern |
| US11389309B2 (en) | 2013-03-15 | 2022-07-19 | Covidien Lp | Occlusive device |
| US10751159B2 (en) | 2013-07-29 | 2020-08-25 | Insera Therapeutics, Inc. | Systems for aspirating thrombus during neurosurgical procedures |
| US10390926B2 (en) | 2013-07-29 | 2019-08-27 | Insera Therapeutics, Inc. | Aspiration devices and methods |
| US11076860B2 (en) | 2014-03-31 | 2021-08-03 | DePuy Synthes Products, Inc. | Aneurysm occlusion device |
| US11154302B2 (en) | 2014-03-31 | 2021-10-26 | DePuy Synthes Products, Inc. | Aneurysm occlusion device |
| WO2015166013A1 (en) | 2014-04-30 | 2015-11-05 | Cerus Endovascular Limited | Occlusion device |
| US11389174B2 (en) | 2014-04-30 | 2022-07-19 | Cerus Endovascular Limited | Occlusion device |
| US11284901B2 (en) | 2014-04-30 | 2022-03-29 | Cerus Endovascular Limited | Occlusion device |
| US12029431B2 (en) | 2014-04-30 | 2024-07-09 | Stryker Ireland Technology, Ltd. | Occlusion device |
| US10130372B2 (en) | 2014-04-30 | 2018-11-20 | Cerus Endovascular Limited | Occlusion Device |
| EP3510945A1 (en) | 2014-04-30 | 2019-07-17 | Cerus Endovascular Limited | Occlusion device |
| EP3970635A1 (en) | 2014-04-30 | 2022-03-23 | Cerus Endovascular Limited | Occlusion device |
| US12414775B1 (en) | 2014-04-30 | 2025-09-16 | Stryker Ireland Technology LTD | Occlusion device |
| CN114391910A (en)* | 2014-12-31 | 2022-04-26 | 先健科技(深圳)有限公司 | Left atrial appendage occluder |
| US10856879B2 (en)* | 2015-02-25 | 2020-12-08 | Galaxy Therapeutics Inc. | System for and method of treating aneurysms |
| US20180333158A1 (en)* | 2015-02-25 | 2018-11-22 | Galaxy Therapeutics, Llc | System for and method of treating aneurysms |
| US11883032B2 (en) | 2015-02-25 | 2024-01-30 | Galaxy Therapeutics, Inc. | System for and method of treating aneurysms |
| EP4299019A3 (en)* | 2015-04-01 | 2024-03-06 | Boston Scientific Scimed, Inc. | Systems for delivery of gel embolics |
| US10265078B2 (en) | 2015-04-01 | 2019-04-23 | Boston Scientific Scimed, Inc. | Systems and methods for delivery of gel embolics |
| WO2016161344A1 (en)* | 2015-04-01 | 2016-10-06 | Boston Scientific Scimed, Inc. | Systems and methods for delivery of gel embolics |
| US11877753B2 (en) | 2015-04-01 | 2024-01-23 | Boston Scientific Scimed, Inc. | Systems and methods for delivery of gel embolics |
| CN105105812A (en)* | 2015-09-18 | 2015-12-02 | 王奎重 | Intracranial aneurysm interventional embolization treatment device |
| US11357510B2 (en) | 2015-09-23 | 2022-06-14 | Covidien Lp | Occlusive devices |
| US10478194B2 (en) | 2015-09-23 | 2019-11-19 | Covidien Lp | Occlusive devices |
| US11471162B2 (en) | 2015-12-07 | 2022-10-18 | Cerus Endovascular Limited | Occlusion device |
| US12076022B2 (en) | 2015-12-07 | 2024-09-03 | Stryker Ireland Technology Ltd. | Occlusion device |
| US12285175B2 (en) | 2016-03-11 | 2025-04-29 | Stryker Ireland Technology Ltd. | Occlusion device |
| US10869672B2 (en) | 2016-03-11 | 2020-12-22 | Cents Endovascular Limited | Occlusion device |
| EP3782576A1 (en) | 2016-03-11 | 2021-02-24 | Cerus Endovascular Limited | Occlusion device |
| US11648013B2 (en) | 2016-03-11 | 2023-05-16 | Cerus Endovascular Limited | Occlusion device |
| WO2017153603A1 (en) | 2016-03-11 | 2017-09-14 | Cerus Endovascular Limited | Occlusion device |
| US10898203B2 (en) | 2016-05-18 | 2021-01-26 | Microvention, Inc. | Embolic containment |
| US10555738B2 (en) | 2016-05-18 | 2020-02-11 | Microvention, Inc. | Embolic containment |
| US12383277B2 (en) | 2016-05-18 | 2025-08-12 | Microvention, Inc. | Embolic containment |
| US11484323B2 (en) | 2016-05-18 | 2022-11-01 | Microvention, Inc. | Embolic containment |
| WO2017201316A1 (en)* | 2016-05-18 | 2017-11-23 | Microvention, Inc. | Embolic containment |
| CN113180882A (en)* | 2016-06-21 | 2021-07-30 | 内流医疗有限公司 | Device and kit for treating vascular malformed portions |
| US12396733B2 (en) | 2016-06-21 | 2025-08-26 | Endostream Medical Ltd. | Device for restricting blood flow to aneurysms |
| US12364708B2 (en) | 2016-10-21 | 2025-07-22 | Covidien Lp | Injectable scaffold for treatment of intracranial aneurysms and related technology |
| US10751066B2 (en)* | 2017-02-23 | 2020-08-25 | DePuy Synthes Products, Inc. | Aneurysm device and delivery system |
| US20190374232A1 (en)* | 2017-02-23 | 2019-12-12 | DePuy Synthes Products, Inc. | Aneurysm device and delivery system |
| US11890020B2 (en) | 2017-02-23 | 2024-02-06 | DePuy Synthes Products, Inc. | Intrasaccular aneurysm treatment device with varying coatings |
| US11672543B2 (en) | 2017-02-23 | 2023-06-13 | DePuy Synthes Products, Inc. | Aneurysm method and system |
| US20180242979A1 (en)* | 2017-02-23 | 2018-08-30 | DePuy Synthes Products, Inc. | Aneurysm device and delivery system |
| US10743884B2 (en)* | 2017-02-23 | 2020-08-18 | DePuy Synthes Products, Inc. | Aneurysm device and delivery system |
| US12318294B2 (en) | 2017-07-06 | 2025-06-03 | Raghuveer Basude | Tissue grasping devices and related methods |
| US12251112B2 (en) | 2017-08-21 | 2025-03-18 | Stryker Ireland Technology Ltd. | Occlusion device |
| US11812971B2 (en) | 2017-08-21 | 2023-11-14 | Cerus Endovascular Limited | Occlusion device |
| CN111031938A (en)* | 2017-08-22 | 2020-04-17 | 柯惠有限合伙公司 | Devices, systems, and methods for treating vascular defects |
| CN112770682A (en)* | 2018-01-19 | 2021-05-07 | 星系疗法有限公司 | Systems and methods for treating aneurysms |
| US11185335B2 (en)* | 2018-01-19 | 2021-11-30 | Galaxy Therapeutics Inc. | System for and method of treating aneurysms |
| AU2019209409B2 (en)* | 2018-01-19 | 2024-11-07 | Galaxy Therapeutics Inc. | System for and method of treating aneurysms |
| US12114862B2 (en) | 2018-01-19 | 2024-10-15 | Galaxy Therapeutics, Inc. | System for and method of treating aneurysms |
| US20190223876A1 (en)* | 2018-01-19 | 2019-07-25 | Galaxy Therapeutics, Llc | System for and method of treating aneurysms |
| US10905430B2 (en) | 2018-01-24 | 2021-02-02 | DePuy Synthes Products, Inc. | Aneurysm device and delivery system |
| US11672540B2 (en) | 2018-01-24 | 2023-06-13 | DePuy Synthes Products, Inc. | Aneurysm device and delivery system |
| US11058430B2 (en)* | 2018-05-25 | 2021-07-13 | DePuy Synthes Products, Inc. | Aneurysm device and delivery system |
| US11596412B2 (en) | 2018-05-25 | 2023-03-07 | DePuy Synthes Products, Inc. | Aneurysm device and delivery system |
| US10939915B2 (en) | 2018-05-31 | 2021-03-09 | DePuy Synthes Products, Inc. | Aneurysm device and delivery system |
| US11583288B2 (en) | 2018-08-08 | 2023-02-21 | DePuy Synthes Products, Inc. | Delivery of embolic braid |
| US12150650B2 (en) | 2018-08-08 | 2024-11-26 | DePuy Synthes Products, Inc. | Delivery of embolic braid |
| US11123077B2 (en) | 2018-09-25 | 2021-09-21 | DePuy Synthes Products, Inc. | Intrasaccular device positioning and deployment system |
| US11076861B2 (en)* | 2018-10-12 | 2021-08-03 | DePuy Synthes Products, Inc. | Folded aneurysm treatment device and delivery method |
| US11633191B2 (en) | 2018-10-12 | 2023-04-25 | DePuy Synthes Products, Inc. | Folded aneurysm treatment device and delivery method |
| US11406392B2 (en) | 2018-12-12 | 2022-08-09 | DePuy Synthes Products, Inc. | Aneurysm occluding device for use with coagulating agents |
| US11272939B2 (en)* | 2018-12-18 | 2022-03-15 | DePuy Synthes Products, Inc. | Intrasaccular flow diverter for treating cerebral aneurysms |
| US11134953B2 (en) | 2019-02-06 | 2021-10-05 | DePuy Synthes Products, Inc. | Adhesive cover occluding device for aneurysm treatment |
| US11337706B2 (en) | 2019-03-27 | 2022-05-24 | DePuy Synthes Products, Inc. | Aneurysm treatment device |
| US11672542B2 (en) | 2019-05-21 | 2023-06-13 | DePuy Synthes Products, Inc. | Aneurysm treatment with pushable ball segment |
| US11607226B2 (en) | 2019-05-21 | 2023-03-21 | DePuy Synthes Products, Inc. | Layered braided aneurysm treatment device with corrugations |
| US11497504B2 (en) | 2019-05-21 | 2022-11-15 | DePuy Synthes Products, Inc. | Aneurysm treatment with pushable implanted braid |
| US11413046B2 (en) | 2019-05-21 | 2022-08-16 | DePuy Synthes Products, Inc. | Layered braided aneurysm treatment device |
| US11278292B2 (en) | 2019-05-21 | 2022-03-22 | DePuy Synthes Products, Inc. | Inverting braided aneurysm treatment system and method |
| US11583282B2 (en) | 2019-05-21 | 2023-02-21 | DePuy Synthes Products, Inc. | Layered braided aneurysm treatment device |
| US10653425B1 (en) | 2019-05-21 | 2020-05-19 | DePuy Synthes Products, Inc. | Layered braided aneurysm treatment device |
| US11974754B2 (en) | 2019-05-25 | 2024-05-07 | Galaxy Therapeutics, Inc. | Systems and methods for treating aneurysms |
| US12310595B2 (en) | 2019-05-25 | 2025-05-27 | Galaxy Therapeutics, Inc. | Systems and methods for treating aneurysms |
| US11166731B2 (en) | 2019-05-25 | 2021-11-09 | Galaxy Therapeutics Inc. | Systems and methods for treating aneurysms |
| US12256937B2 (en) | 2019-05-25 | 2025-03-25 | Galaxy Therapeutics, Inc. | Systems and methods for treating aneurysms |
| US10856880B1 (en) | 2019-05-25 | 2020-12-08 | Galaxy Therapeutics, Inc. | Systems and methods for treating aneurysms |
| US11202636B2 (en) | 2019-05-25 | 2021-12-21 | Galaxy Therapeutics Inc. | Systems and methods for treating aneurysms |
| US11969171B2 (en) | 2019-05-25 | 2024-04-30 | Galaxy Therapeutics, Inc. | Systems and methods for treating aneurysms |
| US11033277B2 (en) | 2019-05-25 | 2021-06-15 | Galaxy Therapeutics, Inc. | Systems and methods for treating aneurysms |
| US12102327B2 (en) | 2019-05-25 | 2024-10-01 | Galaxy Therapeutics, Inc. | Systems and methods for treating aneurysms |
| US11622771B2 (en) | 2019-05-25 | 2023-04-11 | Galaxy Therapeutics, Inc. | Systems and methods for treating aneurysms |
| US11058431B2 (en) | 2019-05-25 | 2021-07-13 | Galaxy Therapeutics, Inc. | Systems and methods for treating aneurysms |
| US11679458B2 (en) | 2019-11-04 | 2023-06-20 | Covidien Lp | Devices, systems, and methods for treating aneurysms |
| US11633818B2 (en) | 2019-11-04 | 2023-04-25 | Covidien Lp | Devices, systems, and methods for treatment of intracranial aneurysms |
| US11717924B2 (en) | 2019-11-04 | 2023-08-08 | Covidien Lp | Devices, systems, and methods for treatment of intracranial aneurysms |
| US11685007B2 (en) | 2019-11-04 | 2023-06-27 | Covidien Lp | Devices, systems, and methods for treatment of intracranial aneurysms |
| US12251110B2 (en) | 2019-11-04 | 2025-03-18 | Covidien Lp | Systems and methods for treating aneurysms |
| US12295582B2 (en) | 2019-11-04 | 2025-05-13 | Covidien Lp | Devices, systems, and methods for treating aneurysms |
| US12256936B2 (en) | 2019-11-04 | 2025-03-25 | Covidien Lp | Devices, systems, and methods for treatment of intracranial aneurysms |
| US11602350B2 (en) | 2019-12-05 | 2023-03-14 | DePuy Synthes Products, Inc. | Intrasaccular inverting braid with highly flexible fill material |
| US11457926B2 (en) | 2019-12-18 | 2022-10-04 | DePuy Synthes Products, Inc. | Implant having an intrasaccular section and intravascular section |
| WO2021130064A3 (en)* | 2019-12-23 | 2021-08-19 | Phenox Gmbh | Implant for treating aneurysms |
| US20230039773A1 (en)* | 2019-12-23 | 2023-02-09 | Femtos Gmbh | Implant for treating aneurysms |
| CN115052535A (en)* | 2019-12-23 | 2022-09-13 | 菲姆托斯股份有限公司 | Implants for the treatment of aneurysms |
| US12303153B2 (en) | 2020-02-20 | 2025-05-20 | Stryker Ireland Technology Ltd. | Clot removal distal protection methods |
| US11406404B2 (en) | 2020-02-20 | 2022-08-09 | Cerus Endovascular Limited | Clot removal distal protection methods |
| US11986189B2 (en) | 2021-01-27 | 2024-05-21 | Galaxy Therapeutics, Inc. | Systems and methods for treating aneurysms |
| US12201303B2 (en) | 2021-01-27 | 2025-01-21 | Galaxy Therapeutics, Inc. | Systems and methods for treating aneurysms |
| CN116407191A (en)* | 2021-12-29 | 2023-07-11 | 赛诺神畅医疗科技有限公司 | Aneurysm occlusion system |
| CN115721366A (en)* | 2022-11-16 | 2023-03-03 | 广西医科大学第二附属医院(广西医科大学第二临床医学院) | A hollow-structure embolism coil assembly and a push system |
| CN116687496A (en)* | 2023-08-04 | 2023-09-05 | 北京久事神康医疗科技有限公司 | Plug device with net disc |
Also Published As
| Publication number | Publication date |
|---|---|
| US9314326B2 (en) | 2016-04-19 |
| AU2003224913A1 (en) | 2003-10-27 |
| JP4414767B2 (en) | 2010-02-10 |
| JP2005522266A (en) | 2005-07-28 |
| US20110196413A1 (en) | 2011-08-11 |
| EP1494619A1 (en) | 2005-01-12 |
| ES2331567T3 (en) | 2010-01-08 |
| EP1494619B1 (en) | 2009-08-12 |
| CA2481224A1 (en) | 2003-10-23 |
| ATE439092T1 (en) | 2009-08-15 |
| CA2481224C (en) | 2010-12-21 |
| DE60328765D1 (en) | 2009-09-24 |
| WO2003086240A1 (en) | 2003-10-23 |
| US20070106311A1 (en) | 2007-05-10 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| US9314326B2 (en) | System and method for retaining vaso-occlusive devices within an aneurysm | |
| US20200000477A1 (en) | Embolization Plug | |
| US11076860B2 (en) | Aneurysm occlusion device | |
| US6036720A (en) | Sheet metal aneurysm neck bridge | |
| EP1109499B1 (en) | Detachable aneurysm neck closure patch | |
| JP6719963B2 (en) | Inflatable vasoocclusive device with leadframe coil | |
| US11154302B2 (en) | Aneurysm occlusion device | |
| US6193708B1 (en) | Detachable aneurysm neck bridge (I) | |
| US20170035430A1 (en) | Devices and Methods for Treatment of Endovascular and Non-Endovascular Defects in Humans Using Occlusion Implants | |
| US20180317933A1 (en) | Devices and Methods for Treatment of Endovascular and Non-Endovascular Defects in Humans | |
| EP3622901A1 (en) | Aneurysm occlusion device | |
| US20180221030A1 (en) | Devices and Methods for Treatment of Endovascular and Non-Endovascular Defects in Humans Using Tandem Embolization Devices | |
| JPH1071154A (en) | Aneurysm closure device assembly | |
| JP2003524433A (en) | Quickly detachable electrically insulated implant | |
| KR102359744B1 (en) | Vascular Implantation System and Vascular Implantation Process Using Flexible Separation Area | |
| WO2020139544A2 (en) | Shape adaptable multi-layered intra-saccular flow reduction device and methods of manufacturing same | |
| JP2023179476A (en) | Vaso-occlusive device | |
| WO2024035592A1 (en) | Delivery devices for treatment of vascular defects |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| AS | Assignment | Owner name:TARGET THERAPEUTICS, INC., CALIFORNIA Free format text:ASSIGNMENT OF ASSIGNORS INTEREST;ASSIGNORS:WALLACE, MICHAEL;EDER, JOSEPH;GERBERDING, BRENT;REEL/FRAME:012812/0175 Effective date:20020411 | |
| AS | Assignment | Owner name:BOSTON SCIENTIFIC SCIMED, INC. (FORMERLY SCIMED LI Free format text:CORRECTIVE ASSIGNMENT TO CORRECT THE ASSIGNEE PREVIOUSLY RECORDED ON REEL 012812 FRAME 0175;ASSIGNORS:WALLACE, MICHAEL;EDER, JOSEPH;GERBERDING, BRENT;REEL/FRAME:018863/0279 Effective date:20020411 | |
| STCB | Information on status: application discontinuation | Free format text:ABANDONED -- FAILURE TO RESPOND TO AN OFFICE ACTION | |
| AS | Assignment | Owner name:STRYKER NV OPERATIONS LIMITED, IRELAND Free format text:ASSIGNMENT OF ASSIGNORS INTEREST;ASSIGNOR:BOSTON SCIENTIFIC SCIMED, INC.;REEL/FRAME:026162/0967 Effective date:20110103 Owner name:STRYKER CORPORATION, MICHIGAN Free format text:ASSIGNMENT OF ASSIGNORS INTEREST;ASSIGNOR:BOSTON SCIENTIFIC SCIMED, INC.;REEL/FRAME:026162/0967 Effective date:20110103 | |
| AS | Assignment | Owner name:STRYKER MEDTECH LIMITED, MALTA Free format text:NUNC PRO TUNC ASSIGNMENT;ASSIGNOR:STRYKER NV OPERATIONS LIMITED;REEL/FRAME:037153/0034 Effective date:20151013 Owner name:STRYKER EUROPEAN HOLDINGS I, LLC, MICHIGAN Free format text:NUNC PRO TUNC ASSIGNMENT;ASSIGNOR:STRYKER MEDTECH LIMITED;REEL/FRAME:037153/0241 Effective date:20151013 | |
| AS | Assignment | Owner name:STRYKER CORPORATION, MICHIGAN Free format text:ASSIGNMENT OF ASSIGNORS INTEREST;ASSIGNOR:BOSTON SCIENTIFIC SCIMED, INC.;REEL/FRAME:037156/0607 Effective date:20110103 Owner name:STRYKER NV OPERATIONS LIMITED, IRELAND Free format text:ASSIGNMENT OF ASSIGNORS INTEREST;ASSIGNOR:BOSTON SCIENTIFIC SCIMED, INC.;REEL/FRAME:037156/0607 Effective date:20110103 | |
| AS | Assignment | Owner name:STRYKER EUROPEAN HOLDINGS I, LLC, MICHIGAN Free format text:CORRECTIVE ASSIGNMENT TO CORRECT THE INCORRECT LISTED SERIAL NOS. 09/905,670 AND 07/092,079 PREVIOUSLY RECORDED AT REEL: 037153 FRAME: 0241. ASSIGNOR(S) HEREBY CONFIRMS THE NUNC PRO TUNC ASSIGNMENT EFFECTIVE DATE 9/29/2014;ASSIGNOR:STRYKER MEDTECH LIMITED;REEL/FRAME:038043/0011 Effective date:20151013 Owner name:STRYKER MEDTECH LIMITED, MALTA Free format text:CORRECTIVE ASSIGNMENT TO CORRECT THE INCORRECT SERIAL # 09/905,670 AND 07/092,079 PREVIOUSLY RECORDED AT REEL: 037153 FRAME: 0034. ASSIGNOR(S) HEREBY CONFIRMS THE NUNC PRO TUNC ASSIGNMENT;ASSIGNOR:STRYKER NV OPERATIONS LIMITED;REEL/FRAME:038039/0001 Effective date:20151013 | |
| AS | Assignment | Owner name:STRYKER EUROPEAN OPERATIONS HOLDINGS LLC, MICHIGAN Free format text:CHANGE OF NAME;ASSIGNOR:STRYKER EUROPEAN HOLDINGS III, LLC;REEL/FRAME:052860/0716 Effective date:20190226 Owner name:STRYKER EUROPEAN HOLDINGS III, LLC, DELAWARE Free format text:NUNC PRO TUNC ASSIGNMENT;ASSIGNOR:STRYKER EUROPEAN HOLDINGS I, LLC;REEL/FRAME:052861/0001 Effective date:20200519 |