US20030127318A1 - Method for sputtering TiNi shape-memory alloys - Google Patents
Method for sputtering TiNi shape-memory alloysDownload PDFInfo
- Publication number
- US20030127318A1 US20030127318A1US10/345,782US34578203AUS2003127318A1US 20030127318 A1US20030127318 A1US 20030127318A1US 34578203 AUS34578203 AUS 34578203AUS 2003127318 A1US2003127318 A1US 2003127318A1
- Authority
- US
- United States
- Prior art keywords
- mandrel
- film
- thin
- expanse
- sputtering
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Abandoned
Links
- 238000004544sputter depositionMethods0.000titleclaimsabstractdescription32
- 229910001285shape-memory alloyInorganic materials0.000titleclaimsabstractdescription17
- 238000000034methodMethods0.000titleclaimsdescription34
- 229910010380TiNiInorganic materials0.000titleclaimsdescription24
- 239000010409thin filmSubstances0.000claimsabstractdescription75
- 229910000734martensiteInorganic materials0.000claimsabstractdescription11
- 229910001000nickel titaniumInorganic materials0.000claimsabstractdescription8
- HLXZNVUGXRDIFK-UHFFFAOYSA-Nnickel titaniumChemical compound[Ti].[Ti].[Ti].[Ti].[Ti].[Ti].[Ti].[Ti].[Ti].[Ti].[Ti].[Ni].[Ni].[Ni].[Ni].[Ni].[Ni].[Ni].[Ni].[Ni].[Ni].[Ni].[Ni].[Ni].[Ni]HLXZNVUGXRDIFK-UHFFFAOYSA-N0.000claimsabstractdescription6
- 238000011084recoveryMethods0.000claimsabstractdescription5
- PXHVJJICTQNCMI-UHFFFAOYSA-NNickelChemical compound[Ni]PXHVJJICTQNCMI-UHFFFAOYSA-N0.000claimsdescription19
- 239000000463materialSubstances0.000claimsdescription16
- 239000010408filmSubstances0.000claimsdescription15
- 238000000151depositionMethods0.000claimsdescription14
- 229910045601alloyInorganic materials0.000claimsdescription13
- 239000000956alloySubstances0.000claimsdescription13
- 238000010438heat treatmentMethods0.000claimsdescription11
- VYZAMTAEIAYCRO-UHFFFAOYSA-NChromiumChemical compound[Cr]VYZAMTAEIAYCRO-UHFFFAOYSA-N0.000claimsdescription10
- KWYUFKZDYYNOTN-UHFFFAOYSA-MPotassium hydroxideChemical compound[OH-].[K+]KWYUFKZDYYNOTN-UHFFFAOYSA-M0.000claimsdescription9
- 229910052759nickelInorganic materials0.000claimsdescription9
- 239000001301oxygenSubstances0.000claimsdescription9
- 229910052760oxygenInorganic materials0.000claimsdescription9
- 239000010936titaniumSubstances0.000claimsdescription8
- RTAQQCXQSZGOHL-UHFFFAOYSA-NTitaniumChemical compound[Ti]RTAQQCXQSZGOHL-UHFFFAOYSA-N0.000claimsdescription7
- 229910052804chromiumInorganic materials0.000claimsdescription7
- 239000011651chromiumSubstances0.000claimsdescription7
- 239000000203mixtureSubstances0.000claimsdescription7
- 238000000137annealingMethods0.000claimsdescription6
- GRYLNZFGIOXLOG-UHFFFAOYSA-NNitric acidChemical compoundO[N+]([O-])=OGRYLNZFGIOXLOG-UHFFFAOYSA-N0.000claimsdescription5
- 239000004642PolyimideSubstances0.000claimsdescription5
- 229910052782aluminiumInorganic materials0.000claimsdescription5
- XAGFODPZIPBFFR-UHFFFAOYSA-NaluminiumChemical compound[Al]XAGFODPZIPBFFR-UHFFFAOYSA-N0.000claimsdescription5
- 229910017604nitric acidInorganic materials0.000claimsdescription5
- 229920001721polyimidePolymers0.000claimsdescription5
- 229910052719titaniumInorganic materials0.000claimsdescription5
- RYGMFSIKBFXOCR-UHFFFAOYSA-NCopperChemical compound[Cu]RYGMFSIKBFXOCR-UHFFFAOYSA-N0.000claimsdescription4
- 229910052802copperInorganic materials0.000claimsdescription4
- 239000010949copperSubstances0.000claimsdescription4
- 238000001755magnetron sputter depositionMethods0.000claimsdescription3
- 239000002904solventSubstances0.000claimsdescription2
- 229910002070thin film alloyInorganic materials0.000claimsdescription2
- 229910001566austeniteInorganic materials0.000abstractdescription5
- 239000010410layerSubstances0.000description25
- 239000011248coating agentSubstances0.000description7
- 238000000576coating methodMethods0.000description7
- 230000008021depositionEffects0.000description7
- QVGXLLKOCUKJST-UHFFFAOYSA-Natomic oxygenChemical compound[O]QVGXLLKOCUKJST-UHFFFAOYSA-N0.000description6
- 210000004204blood vesselAnatomy0.000description5
- 238000005530etchingMethods0.000description5
- 229910052751metalInorganic materials0.000description5
- 229920002120photoresistant polymerPolymers0.000description5
- 230000009466transformationEffects0.000description5
- XKRFYHLGVUSROY-UHFFFAOYSA-NArgonChemical compound[Ar]XKRFYHLGVUSROY-UHFFFAOYSA-N0.000description4
- 238000004519manufacturing processMethods0.000description4
- 239000002184metalSubstances0.000description4
- 239000000523sampleSubstances0.000description4
- HZEWFHLRYVTOIW-UHFFFAOYSA-N[Ti].[Ni]Chemical compound[Ti].[Ni]HZEWFHLRYVTOIW-UHFFFAOYSA-N0.000description3
- 239000003795chemical substances by applicationSubstances0.000description3
- 238000001816coolingMethods0.000description3
- 239000013078crystalSubstances0.000description3
- 238000005259measurementMethods0.000description3
- 150000002739metalsChemical class0.000description3
- 239000010703siliconSubstances0.000description3
- 229910052710siliconInorganic materials0.000description3
- 239000000758substrateSubstances0.000description3
- 230000007704transitionEffects0.000description3
- FAPWRFPIFSIZLT-UHFFFAOYSA-MSodium chlorideChemical compound[Na+].[Cl-]FAPWRFPIFSIZLT-UHFFFAOYSA-M0.000description2
- 229910001069Ti alloyInorganic materials0.000description2
- 229910052786argonInorganic materials0.000description2
- 230000006399behaviorEffects0.000description2
- 230000036760body temperatureEffects0.000description2
- XMPZTFVPEKAKFH-UHFFFAOYSA-Pceric ammonium nitrateChemical compound[NH4+].[NH4+].[Ce+4].[O-][N+]([O-])=O.[O-][N+]([O-])=O.[O-][N+]([O-])=O.[O-][N+]([O-])=O.[O-][N+]([O-])=O.[O-][N+]([O-])=OXMPZTFVPEKAKFH-UHFFFAOYSA-P0.000description2
- 239000007789gasSubstances0.000description2
- 239000007788liquidSubstances0.000description2
- BASFCYQUMIYNBI-UHFFFAOYSA-NplatinumChemical compound[Pt]BASFCYQUMIYNBI-UHFFFAOYSA-N0.000description2
- 229920000642polymerPolymers0.000description2
- 239000010935stainless steelSubstances0.000description2
- 229910001220stainless steelInorganic materials0.000description2
- XLYOFNOQVPJJNP-UHFFFAOYSA-NwaterSubstancesOXLYOFNOQVPJJNP-UHFFFAOYSA-N0.000description2
- 229910001868waterInorganic materials0.000description2
- 206010002329AneurysmDiseases0.000description1
- OKTJSMMVPCPJKN-UHFFFAOYSA-NCarbonChemical compound[C]OKTJSMMVPCPJKN-UHFFFAOYSA-N0.000description1
- 229920002160CelluloidPolymers0.000description1
- LFQSCWFLJHTTHZ-UHFFFAOYSA-NEthanolChemical compoundCCOLFQSCWFLJHTTHZ-UHFFFAOYSA-N0.000description1
- KRHYYFGTRYWZRS-UHFFFAOYSA-NFluoraneChemical compoundFKRHYYFGTRYWZRS-UHFFFAOYSA-N0.000description1
- 229910000990Ni alloyInorganic materials0.000description1
- 208000031481Pathologic ConstrictionDiseases0.000description1
- 229910000831SteelInorganic materials0.000description1
- 230000009471actionEffects0.000description1
- 238000013019agitationMethods0.000description1
- 238000002399angioplastyMethods0.000description1
- 239000007864aqueous solutionSubstances0.000description1
- 239000003125aqueous solventSubstances0.000description1
- 238000009529body temperature measurementMethods0.000description1
- 229910052799carbonInorganic materials0.000description1
- 210000001715carotid arteryAnatomy0.000description1
- 230000008859changeEffects0.000description1
- -1chromium or platinumChemical class0.000description1
- 239000000356contaminantSubstances0.000description1
- 238000013461designMethods0.000description1
- 239000003814drugSubstances0.000description1
- 229940079593drugDrugs0.000description1
- 230000000694effectsEffects0.000description1
- 238000009713electroplatingMethods0.000description1
- 238000011049fillingMethods0.000description1
- 239000012530fluidSubstances0.000description1
- 239000011521glassSubstances0.000description1
- 230000035876healingEffects0.000description1
- 238000007654immersionMethods0.000description1
- 239000007943implantSubstances0.000description1
- 239000012535impuritySubstances0.000description1
- 239000011261inert gasSubstances0.000description1
- 238000007917intracranial administrationMethods0.000description1
- 238000011068loading methodMethods0.000description1
- 238000003754machiningMethods0.000description1
- 230000008018meltingEffects0.000description1
- 238000002844meltingMethods0.000description1
- 239000007769metal materialSubstances0.000description1
- 238000012986modificationMethods0.000description1
- 230000004048modificationEffects0.000description1
- 238000012544monitoring processMethods0.000description1
- 229910052697platinumInorganic materials0.000description1
- 238000005498polishingMethods0.000description1
- 239000002861polymer materialSubstances0.000description1
- 239000002952polymeric resinSubstances0.000description1
- 230000008569processEffects0.000description1
- 230000002441reversible effectEffects0.000description1
- 150000003839saltsChemical class0.000description1
- 238000002791soakingMethods0.000description1
- 239000011780sodium chlorideSubstances0.000description1
- 238000009987spinningMethods0.000description1
- 239000010959steelSubstances0.000description1
- 208000037804stenosisDiseases0.000description1
- 230000036262stenosisEffects0.000description1
- 239000000126substanceSubstances0.000description1
- 239000002344surface layerSubstances0.000description1
- 229920003002synthetic resinPolymers0.000description1
- 239000013077target materialSubstances0.000description1
- 238000002207thermal evaporationMethods0.000description1
- 238000000427thin-film depositionMethods0.000description1
- 230000001131transforming effectEffects0.000description1
- 230000002792vascularEffects0.000description1
Images
Classifications
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F2/00—Filters implantable into blood vessels; Prostheses, i.e. artificial substitutes or replacements for parts of the body; Appliances for connecting them with the body; Devices providing patency to, or preventing collapsing of, tubular structures of the body, e.g. stents
- A61F2/82—Devices providing patency to, or preventing collapsing of, tubular structures of the body, e.g. stents
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F2/00—Filters implantable into blood vessels; Prostheses, i.e. artificial substitutes or replacements for parts of the body; Appliances for connecting them with the body; Devices providing patency to, or preventing collapsing of, tubular structures of the body, e.g. stents
- A61F2/82—Devices providing patency to, or preventing collapsing of, tubular structures of the body, e.g. stents
- A61F2/86—Stents in a form characterised by the wire-like elements; Stents in the form characterised by a net-like or mesh-like structure
- A61F2/90—Stents in a form characterised by the wire-like elements; Stents in the form characterised by a net-like or mesh-like structure characterised by a net-like or mesh-like structure
- A61F2/91—Stents in a form characterised by the wire-like elements; Stents in the form characterised by a net-like or mesh-like structure characterised by a net-like or mesh-like structure made from perforated sheets or tubes, e.g. perforated by laser cuts or etched holes
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F2/00—Filters implantable into blood vessels; Prostheses, i.e. artificial substitutes or replacements for parts of the body; Appliances for connecting them with the body; Devices providing patency to, or preventing collapsing of, tubular structures of the body, e.g. stents
- A61F2/82—Devices providing patency to, or preventing collapsing of, tubular structures of the body, e.g. stents
- A61F2/86—Stents in a form characterised by the wire-like elements; Stents in the form characterised by a net-like or mesh-like structure
- A61F2/90—Stents in a form characterised by the wire-like elements; Stents in the form characterised by a net-like or mesh-like structure characterised by a net-like or mesh-like structure
- A61F2/91—Stents in a form characterised by the wire-like elements; Stents in the form characterised by a net-like or mesh-like structure characterised by a net-like or mesh-like structure made from perforated sheets or tubes, e.g. perforated by laser cuts or etched holes
- A61F2/915—Stents in a form characterised by the wire-like elements; Stents in the form characterised by a net-like or mesh-like structure characterised by a net-like or mesh-like structure made from perforated sheets or tubes, e.g. perforated by laser cuts or etched holes with bands having a meander structure, adjacent bands being connected to each other
- C—CHEMISTRY; METALLURGY
- C22—METALLURGY; FERROUS OR NON-FERROUS ALLOYS; TREATMENT OF ALLOYS OR NON-FERROUS METALS
- C22F—CHANGING THE PHYSICAL STRUCTURE OF NON-FERROUS METALS AND NON-FERROUS ALLOYS
- C22F1/00—Changing the physical structure of non-ferrous metals or alloys by heat treatment or by hot or cold working
- C22F1/006—Resulting in heat recoverable alloys with a memory effect
- C—CHEMISTRY; METALLURGY
- C23—COATING METALLIC MATERIAL; COATING MATERIAL WITH METALLIC MATERIAL; CHEMICAL SURFACE TREATMENT; DIFFUSION TREATMENT OF METALLIC MATERIAL; COATING BY VACUUM EVAPORATION, BY SPUTTERING, BY ION IMPLANTATION OR BY CHEMICAL VAPOUR DEPOSITION, IN GENERAL; INHIBITING CORROSION OF METALLIC MATERIAL OR INCRUSTATION IN GENERAL
- C23C—COATING METALLIC MATERIAL; COATING MATERIAL WITH METALLIC MATERIAL; SURFACE TREATMENT OF METALLIC MATERIAL BY DIFFUSION INTO THE SURFACE, BY CHEMICAL CONVERSION OR SUBSTITUTION; COATING BY VACUUM EVAPORATION, BY SPUTTERING, BY ION IMPLANTATION OR BY CHEMICAL VAPOUR DEPOSITION, IN GENERAL
- C23C14/00—Coating by vacuum evaporation, by sputtering or by ion implantation of the coating forming material
- C23C14/0005—Separation of the coating from the substrate
- C—CHEMISTRY; METALLURGY
- C23—COATING METALLIC MATERIAL; COATING MATERIAL WITH METALLIC MATERIAL; CHEMICAL SURFACE TREATMENT; DIFFUSION TREATMENT OF METALLIC MATERIAL; COATING BY VACUUM EVAPORATION, BY SPUTTERING, BY ION IMPLANTATION OR BY CHEMICAL VAPOUR DEPOSITION, IN GENERAL; INHIBITING CORROSION OF METALLIC MATERIAL OR INCRUSTATION IN GENERAL
- C23C—COATING METALLIC MATERIAL; COATING MATERIAL WITH METALLIC MATERIAL; SURFACE TREATMENT OF METALLIC MATERIAL BY DIFFUSION INTO THE SURFACE, BY CHEMICAL CONVERSION OR SUBSTITUTION; COATING BY VACUUM EVAPORATION, BY SPUTTERING, BY ION IMPLANTATION OR BY CHEMICAL VAPOUR DEPOSITION, IN GENERAL
- C23C14/00—Coating by vacuum evaporation, by sputtering or by ion implantation of the coating forming material
- C23C14/06—Coating by vacuum evaporation, by sputtering or by ion implantation of the coating forming material characterised by the coating material
- C23C14/14—Metallic material, boron or silicon
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F2/00—Filters implantable into blood vessels; Prostheses, i.e. artificial substitutes or replacements for parts of the body; Appliances for connecting them with the body; Devices providing patency to, or preventing collapsing of, tubular structures of the body, e.g. stents
- A61F2/82—Devices providing patency to, or preventing collapsing of, tubular structures of the body, e.g. stents
- A61F2/86—Stents in a form characterised by the wire-like elements; Stents in the form characterised by a net-like or mesh-like structure
- A61F2/90—Stents in a form characterised by the wire-like elements; Stents in the form characterised by a net-like or mesh-like structure characterised by a net-like or mesh-like structure
- A61F2/91—Stents in a form characterised by the wire-like elements; Stents in the form characterised by a net-like or mesh-like structure characterised by a net-like or mesh-like structure made from perforated sheets or tubes, e.g. perforated by laser cuts or etched holes
- A61F2/915—Stents in a form characterised by the wire-like elements; Stents in the form characterised by a net-like or mesh-like structure characterised by a net-like or mesh-like structure made from perforated sheets or tubes, e.g. perforated by laser cuts or etched holes with bands having a meander structure, adjacent bands being connected to each other
- A61F2002/91533—Stents in a form characterised by the wire-like elements; Stents in the form characterised by a net-like or mesh-like structure characterised by a net-like or mesh-like structure made from perforated sheets or tubes, e.g. perforated by laser cuts or etched holes with bands having a meander structure, adjacent bands being connected to each other characterised by the phase between adjacent bands
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F2/00—Filters implantable into blood vessels; Prostheses, i.e. artificial substitutes or replacements for parts of the body; Appliances for connecting them with the body; Devices providing patency to, or preventing collapsing of, tubular structures of the body, e.g. stents
- A61F2/82—Devices providing patency to, or preventing collapsing of, tubular structures of the body, e.g. stents
- A61F2/86—Stents in a form characterised by the wire-like elements; Stents in the form characterised by a net-like or mesh-like structure
- A61F2/90—Stents in a form characterised by the wire-like elements; Stents in the form characterised by a net-like or mesh-like structure characterised by a net-like or mesh-like structure
- A61F2/91—Stents in a form characterised by the wire-like elements; Stents in the form characterised by a net-like or mesh-like structure characterised by a net-like or mesh-like structure made from perforated sheets or tubes, e.g. perforated by laser cuts or etched holes
- A61F2/915—Stents in a form characterised by the wire-like elements; Stents in the form characterised by a net-like or mesh-like structure characterised by a net-like or mesh-like structure made from perforated sheets or tubes, e.g. perforated by laser cuts or etched holes with bands having a meander structure, adjacent bands being connected to each other
- A61F2002/9155—Adjacent bands being connected to each other
Definitions
- the invention hereinis directed to a method of fabricating thin-film devices from a shape memory alloy, and to space-filling devices, e.g., intravascular devices, made by these methods.
- intravascular stentsare used to reinforce blood vessels to promote healing and to prevent stenosis (narrowing) of blood vessels following procedures such as angioplasty.
- Alloys of titanium nickel (TiNi or Nitinol shape memory alloy)are gaining popularity over more traditional metals such as stainless steel for use in medical implants because the properties of shape memory and superelasticity enable improvements in design and methods of deployment of these devices. Demonstrated biocompatibility and novel methods of fabrication have resulted in wide acceptance of orthodontic braces, catheter guidewires, surgical tools, and implantable coronary stents.
- Fabrication of stents from drawn TiNi tubesis practical only for a limited range of sizes. In particular, it has not been feasible to make stents having the flexibility and size required for delivery intravascularly through small catheters via the carotid arteries.
- Suitably flexible structurescan be fabricated of thin film (2-10 micrometers thick) shape memory alloys that are sputter deposited on a substrate and heat treated. Composition and heat treatment affect the phase transition temperature of the alloy, which in turn determines whether it exhibits shape memory or superelastic properties.
- an intracranial deviceshould be installed through a small diameter catheter, then changed to a pre-determined shape so as to fill a space and apply continuous outward pressure against the blood vessel wall.
- three-dimensional shapessuch as cylinders, cones, and hemispheres are required, and a shape-changing capability is highly advantageous.
- the inventionincludes, in one embodiment, a thin film device comprising a seamless thin-film expanse (i) formed of a sputtered Nitinol shape memory alloy; (ii) defining, in an austenitic state, an open, interior volume;(iii) having a thickness between 0.5-100, preferably 2-50 microns; (iv) having an austenite finish temperature A f below 37° C.; and (v) demonstrating a stress/strain recovery greater than 3% at 37° C.
- the expansecan be deformed into a substantially compacted configuration in a martensitic state, and assumes, in its austenitic state, a shape defining such open, interior volume.
- the expansemay have, for example, a cylindrical, hemispherical or sock-like shape.
- the devicemay include a skeletal member to which the expanse is attached, and these members may have a thickness greater than the thickness of the expanse.
- the expansemay be fenestrated with a selected pattern of openings in the thin film.
- the inventionincludes a method of forming the thin-film device.
- the methodincludes the steps of placing in a magnetron sputtering device, a mandrel having an exposed, etchable outer layer that corresponds to the open, interior volume of the device to be formed, providing the sputtering apparatus with a TiNi alloy target composed of between 45-55% each of titanium and nickel, and sputter depositing material from the target adjacent said mandrel under low-pressure, low-oxygen conditions.
- the mandrelis moved relative to said target, to achieve substantially uniform sputter deposition over the entire exposed surface of the mandrel, and the deposition is continued until a desired sputtered film thickness between 0.5 and 100 microns, preferably 2 and 50 microns, is formed on the mandrel.
- the thin film on the mandrelis heated under annealing conditions.
- the thin-film device so formedis then released from the mandrel, typically by exposing the mandrel and deposited thin film to an etchant, under conditions effective to dissolve the outer layer of the mandrel.
- the mandrel's outer layermay be a separate coating formed on the mandrel surface, or the surface of the mandrel itself.
- the mandrelmay be coated with a smooth surface such as polyimide before sputtering to ensure a continuous layer of deposited material.
- the targethas a preferred composition of between about 48 to 51 atomic percent nickel to 52 to 49 atomic percent titanium.
- the sacrificial layer materialis chromium, aluminum, or copper
- the etchantmay be a chrome etch, potassium hydroxide, and nitric acid.
- the mandrelis preferably rotated during the sputtering step to achieve substantially uniform sputter deposition over the entire exposed surface of the mandrel.
- the mandrelmay be cylindrical, e.g., for producing a thin-film stent, sock-like, e.g., for producing an intravascular filter, or hemispherical, e.g., for producing a vaso-occlusive device.
- the methodmay further include applying structural members to the mandrel, prior to depositing the thin film thereon, to form structural members in the formed device.
- the methodmay further include forming on the annealed thin film, an resist layer containing a pattern of openings, exposing the coated thin film with a solvent under conditions effective to create fenestrations in the thin film corresponding to the pattern of openings, and removing the resist layer.
- the fenestrationsmay have dimensions and interfenestration spacings in the 10-50 micron range.
- FIGS. 1 A- 1 Dillustrate steps in practicing the method of the invention
- FIG. 2shows a resistivity versus temperature curve of a TiNi thin-film expanse formed in accordance with the invention
- FIG. 3illustrates a stress-strain curve for TiNi thin film at 37° C.
- FIG. 4illustrates a thin-film solid-wall stent constructed in accordance with an embodiment of the invention
- FIGS. 5 and 6illustrate open and folded states of the stent of FIG. 4, respectively, as seen in front-on view;
- FIG. 7illustrates a sock-like thin-film device formed in accordance with another embodiment of the invention.
- FIG. 8illustrates a hemispherical device formed in accordance with still another embodiment of the invention.
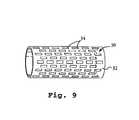
- FIG. 9illustrates a stent with a fenestrated thin-film expanse formed in accordance with the invention.
- a shape defining an open, interior volumerefers to an expanse that defines an open volume-containing space, e.g., a cylindrical, sock-like or hemispherical volume-containing space.
- “Seamless thin-film expanse”means an expanse that forms an open interior volume without edge-to-edge seams.
- NiNirefers to an alloy containing titanium and nickel, typically each between 45-55 atom percent, and optionally, other metals, such as chromium in relatively minor amount, e.g., 1-5 atom percent.
- shape memory alloyis an alloy that displays thermoelastic martensitic transformation defined as the ability to undergo a reversible transformation from an austentite to a martensite with a change in temperature such that an article made from such alloy has a heat stable configuration and is capable of being deformed to a heat unstable position.
- Austenitic staterefers to the stronger, higher temperature phase (crystal structure) present in a shape-memory alloy.
- Austentite finish temperature A frefers to the temperature at which a shape memory alloy finishes transforming martensite to austentite upon heating.
- Martensitic staterefers to the more deformable, lower temperature phase (crystal structure) present in a shape-memory alloy.
- Sputter alloyrefers to an alloy formed by sputter depositing a target-material alloy on a substrate, such as a mandrel.
- Low-pressure, low-oxygen conditionsrefers to sputtering conditions in which the pressure is preferably below 10 ⁇ 5 Torr, typically 10 ⁇ 6 to 10 ⁇ 8 Torr, and the predominant gas is an inert gas such as argon.
- the amount of oxygenis kept low, e.g., below 0.1 percent, by the low pressure and low oxygen content of the sputter target alloy.
- “Etchable” with reference to a mandrel surfacemeans refers to a surface layer, either part of the mandrel or a coating thereon, that can be removed by exposure to a dissolving agent, e.g., etchant.
- FIGS. 1 A- 1 Dillustrate steps in forming a thin-film device in accordance with one aspect of the invention.
- the basic components employed in the methodinclude a mandrel 10 having an outer exposed surface corresponding to the open, interior volume of the device to be formed.
- the mandrelmay have a variety of volume-defining surfaces, such as cylindrical, ellipsoidal, hemispherical, tapered cylindrical, and conical volume shapes, and may be constructed of a variety of materials, such as steel, glass, or silicon.
- the dimensions of the mandrelare dictated by the dimensions of the desired thin-film device to be formed.
- the mandrelis preferably polished, and may be coated with a material such as polyimide to produce a smooth, regular surface on which to deposit shape memory alloy.
- the mandrelis coated with a three micron thick layer of pyralin polymer resin liquid coating type PI2611 (also known as polyimide coating) obtained from HD Microsystems, by spinning the mandrel to create a thin uniform layer, curing the high-temperature polymer coating in successive steps of baking at 150° C., then curing at 250° C. and finally curing at 450-500° C. to cross-link the polymer.
- PI2611also known as polyimide coating
- the mandrelmay be formed of a material that itself has an etchable surface, such as such as one formed of silicon, that can be removed upon exposure to an etchant, or may be formed of a material, such as NaCl, KCl, NaF 2 , which are available in flat or cylindrical shapes, and which can be shaped by machining and polishing. These substrates can be dissolved directly, e.g., with an aqueous solvent, without the need of an etchable coating.
- the mandrelis formed of a material that is itself not easily etchable, it is preferably coated with an etchable outer sacrificial layer 14 , e.g., formed over a polyimide coating.
- Layer 14 on the mandrelis preferably metal such as chromium or other material having a highly specific etch rate relative to TiNi so that the sacrificial layer may be removed without damaging the TiNi thin film. It is preferred that the layer used not diffuse readily into TiNi during heat treatment.
- Alternative sacrificial layersinclude aluminum, copper, and photoresist such as SU8 and OCG825.
- An etchantsuch as potassium hydroxide used for etching aluminum, nitric acid used for etching copper, and Chrome Etch from Arch Chemicals Inc. containing ceric ammonium nitrate, nitric acid, and water.
- the etchantmay be an aqueous solution for water-soluble mandrels, such as the salt mandrels noted above.
- the sacrificial layermay be formed by conventional thin-film deposition means, such as vacuum thermal evaporation, electroplating, or sputtering, to form a sacrificial layer preferably less than 1 micron in thickness.
- a chromium layeris applied to a thickness of about of 0.1 micron by the sputter deposition method detailed below, but where the target is chromium rather than a TiNi alloy.
- the mandrelis mounted on mandrel holder 12 for rotation thereon in the direction of arrow 13 in FIG. 1B.
- the mandrel holderincludes a mandrel-support rod 12 a , and a motor 12 b for rotating the mandrel at a desired speed, e.g., 0.5 to 2 rpms, during sputter deposition.
- a sputter deposition target 18composed of a nickel-titanium shape-memory alloy, preferably nearly 50 atomic percent Ti, 50 atomic percent nickel, and containing minimal impurities especially carbon and oxygen. Composition control is critical to obtaining TiNi having appropriate shape memory and superelastic qualities. Increasing the content of nickel lowers the transition temperature. As little as 0.1% oxygen renders the film product brittle and unusable.
- the targetmay include minor amounts, e.g., 1-5 atomic percent, of other metals, e.g., chromium or platinum, known to effect the behavior of TiNi shape-memory alloys is specific ways.
- the targetis preferably selected to produce in the thin-film expanse, an austentite finish temperature A f less than 37° C. characterized by a four-point resistivity measurement in which the temperature is cycled to above 100° C. and below 0° C.; and a stress/strain recovery curve characterized by greater than 3% at 37° C.
- One exemplary targetis composed of a nickel-titanium shape-memory alloy, preferably 46.75 weight percent Ti and 53.25 weight percent Ni, and less than 200 parts per million oxygen; formed by vacuum arc melting.
- the alloy compositionmay be enriched in nickel by as much as 1-2 percent to lower the transition temperature, and heat treatment such as rapid thermal anneal followed by heat-soaking at a lowered temperature may be employed as a method of obtaining special stress-strain-temperature characteristics.
- the targethas exemplary diameter and thickness dimensions of 20 cm by 0.6 cm, respectively.
- the targetis placed in the sputtering apparatus approximately 3-5 cm from the mandrel and parallel to the axis of the mandrel, as shown in FIGS. 1A and 1B.
- the mandrel and targetare contained in a conventional high-vacuum sputtering apparatus, indicated at 16 in FIGS. 1A and 1B.
- the apparatusmay be any of a number of known sputtering systems that employ a direct current magnetron or radio frequency sputtering source.
- One exemplary apparatusis a Perkin-Elmer PE4400 series sputtering system.
- the coated mandrel and targetare placed in the vacuum chamber of the sputtering apparatus, and sputter deposition is carried out in a vacuum of low 10 ⁇ 7 Torr base pressure using a single TiNi DC magnetron target, argon gas, and a 5 kW DC power supply. High vacuum is necessary to minimize the oxygen (and other contaminants).
- the mandrelmay be placed in a cylindrical magnetron sputtering system for deposition of TiNi.
- Sputter depositionis carried out until a selected thickness of thin-film expanse of between 0.5-100, preferably 2-50 microns is achieved.
- film thicknessmay be determined by measuring the time of deposition and comparing to calibrated samples that are measured by a Tencor Alpha-Step profilometer. Alternatively, film thickness may be measured by placing a piezoelectric crystal adjacent to the target and monitoring its resonant frequency during deposition.
- deposition onto the rotating mandrelis effective to produce a substantially uniform thickness of deposited thin film on the exposed surface of the mandrel, that is, the surface region directly exposed to the target.
- the sputter deposition stepis terminated, and the thin-film expanse on the mandrel is then annealed under heating/cooling conditions to achieve desired shape-memory alloy properties in the device.
- the annealing stepmay be by thermal heating or by exposure to an infrared heater in vacuum. Use of infrared heating permits rapid heating and cooling so that sacrificial layers such as aluminum may be used, and solvent-removable sacrificial layers such as photoresist.
- the thin-film expanseis heated in vacuum at 500-550° C. for 20 minutes followed by gradual cooling to ambient temperature.
- the mandrelis enclosed in a stainless steel fixture to ensure uniform heating.
- the thin-film devicemust be released from the mandrel. This is done preferably by exposing the mandrel and thin-film device thereon to a dissolving agent, e.g., etchant, to remove the outer mandrel layer or the sacrificial layer formed thereon.
- a dissolving agente.g., etchant
- FIG. 1Cwhere sacrificial layer 14 (FIGS. 1A and 1B) is removed by the etchant.
- the mandrel with its two or more layers of depositsis immersed in liquid etchant at room temperature and allowed to soak until the TiNi layer is freed from the surface.
- the timemay vary from one to 24 hours depending on the degree of fenestration of the TiNi, the thickness of the sacrificial layer, and the degree of agitation applied to the mandrel.
- Ultrasonic powermay be used to accelerate action of the etchant.
- the coated mandrelis washed and the thin-film expanse is removed from the mandrel. This step is shown in FIG. 1D, showing a thin-film stent 22 formed in accordance with the method.
- the method of producing a thin-film devicecan be extended to produce micro-sized, precisely shaped and spaced openings or in the film.
- fenestration patternsare selectively etched in TiNi thin film, either before or after the above annealing step, to enhance mechanical flexibility of the film, to permit fluid to flow through, to increase the expansion rate of the device or achieve improved adhesion to vascular-wall structure.
- a positive photoresistis spun on the thin film/silicon mandrel.
- One preferred photoresist materialis Olin OCG825; other suitable materials are available from alternative vendors.
- the photoresist and thin film TiNiis then photo-lithographically patterned and etched, respectively.
- the etchantis, for example, a mixture of nitric acid and buffered oxide etch containing hydrogen flouride. Fenestrations as small as 25 microns ⁇ 25 microns in the film have been created. More generally, fenestrations and spacing between adjacent fenestrations may have dimensions in the 5-50 micron size range or larger.
- the methodis adapted to produce a thin-film device having internal ribs or struts.
- the coated mandrelis first provided with structural members or ribs applied or deposited on the coated surface, to serve as structural members in a thin-film device as illustrated below in FIG. 7.
- the structural membersare preferably deposited or placed circumferentially about the coated mandrel at one or more positions along the mandrel.
- the ribsmay be, for example, nickel/titanium wire or strips, or some other metal or polymer materials upon which a thin-film expanse can be deposited.
- the surfacemust be coated or polished to a sub-micron finish for TiNi to be successfully sputtered onto the mandrel after placement of rib structure.
- Narrow strips of thin film prepared as abovewere prepared for transformation temperature measurements and stress-strain measurements. To measure transformation temperature, each thin film sample was heated and cooled while changes in voltage were measured and recorded using a 4-probe constant-current technique to produce temperature versus resistivity data.
- FIG. 2shows a typical resistivity versus temperature curve of a TiNi film.
- the transformation temperatures of the alloyare as follows: Martensite start (M s ) ⁇ 30° C. Martensite finish (M f ) ⁇ 80° C. Austenite start (A s ) 0° C. Austenite finish (A f ) 12-15° C.
- the A f of this thin film alloyis below body temperature, it is well suited to medical devices that are actuated within the blood vessel.
- FIG. 3illustrates a stress-strain curve for TiNi thin film at 38° C.
- the loading plateau for the filmis at about 300 MPa ( ⁇ 45 kpsi) and unloading plateau is at about 150 MPa ( ⁇ 22 kpsi).
- the thin filmclearly exhibits superelastic behavior at body temperature that makes this an excellent material for medical devices, particular miniature implantable devices.
- the inventionincludes a device formed of a seamless thin-film expanse, in accordance with the method above.
- the “seamless” featurerefers to the fact that the expanse forms a continuous surface without interior edges.
- the devicesare formed of a sputtered nitinol shape memory alloy.
- the expanseis formed by sputtering a thin film from a titanium/nickel alloy target, as described above.
- the thin-film materialdefines, in an austenitic state, an open, interior volume.
- the open interior volumeis the volume of the space defined by the interior of the expanse.
- the expanse in the devicehas a film thickness of between 0.5 and 100 microns, preferably 2-50 microns, more preferably 5-50 microns, and a substantially uniform in thickness and composition throughout the expanse.
- the thin-film expansehas an austenite finish temperature A f below 37° C. and demonstrates a stress/strain recovery greater than 3% at 37° C., where the expanse can be deformed into a substantially compacted configuration in a martensitic state.
- FIGS. 4 - 6One exemplary device is shown in FIGS. 4 - 6 , where a cylindrical thin-film expanse forms an intraluminal a stent 22 .
- the stenthas typical dimensions of about 5-75 microns film thickness, 5-50 mm in length, and 1-10 mm in diameter, in its open conditions shown in FIGS. 4 and 5.
- a compacted or “folded” configurationis illustrated in FIG. 6, showing stent 22 in a front-on view.
- the dimensions of the stenthave been reduced considerably in the compacted configuration, allowing the stent, for example, to be delivered through a catheter to a vascular site in need of a stent, e.g., in a compacted, stress-induced martensite form.
- the stentUpon release of the stent from the catheter at the target site, the stent regains its fully austenitic, open form, with its outer surface impinging against the walls of the vessel.
- FIG. 7Shown in FIG. 7 is a sock-like device 24 formed of a thin-film expanse 25 and supported internally by structural members 28 .
- the structural ribsmay be disposed in a circumferential direction, as shown, or in a longitudinal direction, like the struts in an umbrella.
- This devicecan be formed as described above, on a cone-shaped mandrel, where the mandrel is first coated prior to sputtering the thin-film expanse, and the structural members are formed on the mandrel prior to sputtering.
- the devicemay be fenestrated, as illustrated in FIG. 12, and may have a wire or other guide structure attached, for example, for pulling the device through an internal vessel, where the device acts like a filter.
- the deviceis a hemispherical cap device 30 . e.g., for use as a vaso-occlusive agent for treating an aneurysm.
- the thin-film devicemay be further etched, e.g., by photolithographic etching, to produce a device, such as stent 32 in. FIG. 12, having defined-shape, size and position fenestrations. Fenestration patterns as small as 25 microns ⁇ 25 microns have been etched in the film using photolithographic technique.
- FIG. 12shows a TiNi thin film stent 30 formed of a thin-film expanse 32 having fenestrations or windows, such as windows 34 , formed therein.
- Windows 25 microns ⁇ 25 microns with spacing of about 10 microns between the adjacent openingshas been achieved. Since fenestrations are produced by using photolithographic technique, very precise, clean and sharp edges can be achieved. The ability to etch micrometer scale features with an excellent etching quality allows for the production of devices such as implantable filters, or drug-release devices.
Landscapes
- Health & Medical Sciences (AREA)
- Chemical & Material Sciences (AREA)
- Engineering & Computer Science (AREA)
- Biomedical Technology (AREA)
- General Health & Medical Sciences (AREA)
- Organic Chemistry (AREA)
- Oral & Maxillofacial Surgery (AREA)
- Transplantation (AREA)
- Heart & Thoracic Surgery (AREA)
- Vascular Medicine (AREA)
- Life Sciences & Earth Sciences (AREA)
- Animal Behavior & Ethology (AREA)
- Physics & Mathematics (AREA)
- Public Health (AREA)
- Veterinary Medicine (AREA)
- Cardiology (AREA)
- Metallurgy (AREA)
- Materials Engineering (AREA)
- Mechanical Engineering (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Optics & Photonics (AREA)
- Thermal Sciences (AREA)
- Crystallography & Structural Chemistry (AREA)
- Media Introduction/Drainage Providing Device (AREA)
- Materials For Medical Uses (AREA)
- Physical Vapour Deposition (AREA)
Abstract
Description
- This application claims priority of U.S. Provisional Application Serial No. 60/177,881 filed Jan. 24, 2000 and 60/211,352 filed Jun. 13, 2000, both of which are incorporated in their entirety herein by reference.[0001]
- The invention herein is directed to a method of fabricating thin-film devices from a shape memory alloy, and to space-filling devices, e.g., intravascular devices, made by these methods.[0002]
- J. D. Busch and A. D. Johnson, Shape-memory alloy microactuator, U.S. Pat. No. 5,061,914; Oct. 29, 1991.[0003]
- P. Krulevitch, A. P. Lee, P. B. Ramsey, J. C. Trevino, J. Hamilton, M. A. Northrup, Thin film Shape Memory Alloy Microactuators, J. Micromech. Microeng. Vol. 5, No. 4, December 1996.[0004]
- A. D. Johnson, Vacuum-Deposited TiNi Shape memory Film: Characterization and Applications in Micro-Devices, J. Micromech. Microeng. Vol. 1, (1991) 34-41.[0005]
- L. M. Schetky, Shape-Memory Alloys, Scientific American, November 1979, pages 74-82.[0006]
- Hiroyasu Funakubo, ed., Shape Memory Alloys, Gordon and Breach Publishers, 1984 Translated by J. B. Kennedy.[0007]
- P. Dario, M. C. Montesi, Shape memory alloy microactuators for minimally invasive surgery, Proceedings of SMST-94 conference, Asilomar Calif., March 1994.[0008]
- Medical implants are increasing in use in minimally invasive surgery because of the improved medical results attainable. In particular, intravascular stents are used to reinforce blood vessels to promote healing and to prevent stenosis (narrowing) of blood vessels following procedures such as angioplasty. Alloys of titanium nickel (TiNi or Nitinol shape memory alloy) are gaining popularity over more traditional metals such as stainless steel for use in medical implants because the properties of shape memory and superelasticity enable improvements in design and methods of deployment of these devices. Demonstrated biocompatibility and novel methods of fabrication have resulted in wide acceptance of orthodontic braces, catheter guidewires, surgical tools, and implantable coronary stents.[0009]
- Fabrication of stents from drawn TiNi tubes is practical only for a limited range of sizes. In particular, it has not been feasible to make stents having the flexibility and size required for delivery intravascularly through small catheters via the carotid arteries.[0010]
- There is a growing demand for smaller and thinner, more flexible stents that can be surgically implanted or delivered via catheter, into small diameter, highly tortuous blood vessels. Suitably flexible structures can be fabricated of thin film (2-10 micrometers thick) shape memory alloys that are sputter deposited on a substrate and heat treated. Composition and heat treatment affect the phase transition temperature of the alloy, which in turn determines whether it exhibits shape memory or superelastic properties.[0011]
- For maximum effectiveness, an intracranial device should be installed through a small diameter catheter, then changed to a pre-determined shape so as to fill a space and apply continuous outward pressure against the blood vessel wall. To accomplish this, three-dimensional shapes such as cylinders, cones, and hemispheres are required, and a shape-changing capability is highly advantageous.[0012]
- The invention includes, in one embodiment, a thin film device comprising a seamless thin-film expanse (i) formed of a sputtered Nitinol shape memory alloy; (ii) defining, in an austenitic state, an open, interior volume;(iii) having a thickness between 0.5-100, preferably 2-50 microns; (iv) having an austenite finish temperature A[0013]fbelow 37° C.; and (v) demonstrating a stress/strain recovery greater than 3% at 37° C. The expanse can be deformed into a substantially compacted configuration in a martensitic state, and assumes, in its austenitic state, a shape defining such open, interior volume. The expanse may have, for example, a cylindrical, hemispherical or sock-like shape.
- The device may include a skeletal member to which the expanse is attached, and these members may have a thickness greater than the thickness of the expanse. In addition, the expanse may be fenestrated with a selected pattern of openings in the thin film.[0014]
- In another aspect, the invention includes a method of forming the thin-film device. The method includes the steps of placing in a magnetron sputtering device, a mandrel having an exposed, etchable outer layer that corresponds to the open, interior volume of the device to be formed, providing the sputtering apparatus with a TiNi alloy target composed of between 45-55% each of titanium and nickel, and sputter depositing material from the target adjacent said mandrel under low-pressure, low-oxygen conditions. During the deposition, the mandrel is moved relative to said target, to achieve substantially uniform sputter deposition over the entire exposed surface of the mandrel, and the deposition is continued until a desired sputtered film thickness between 0.5 and 100 microns, preferably 2 and 50 microns, is formed on the mandrel.[0015]
- Following sputter deposition, the thin film on the mandrel is heated under annealing conditions. The thin-film device so formed is then released from the mandrel, typically by exposing the mandrel and deposited thin film to an etchant, under conditions effective to dissolve the outer layer of the mandrel. The mandrel's outer layer may be a separate coating formed on the mandrel surface, or the surface of the mandrel itself. The mandrel may be coated with a smooth surface such as polyimide before sputtering to ensure a continuous layer of deposited material.[0016]
- The target has a preferred composition of between about 48 to 51 atomic percent nickel to 52 to 49 atomic percent titanium. Where the sacrificial layer material is chromium, aluminum, or copper, and the etchant may be a chrome etch, potassium hydroxide, and nitric acid.[0017]
- The mandrel is preferably rotated during the sputtering step to achieve substantially uniform sputter deposition over the entire exposed surface of the mandrel.[0018]
- In various embodiments the mandrel may be cylindrical, e.g., for producing a thin-film stent, sock-like, e.g., for producing an intravascular filter, or hemispherical, e.g., for producing a vaso-occlusive device.[0019]
- The method may further include applying structural members to the mandrel, prior to depositing the thin film thereon, to form structural members in the formed device. For use in forming a fenestrated thin-film device, the method may further include forming on the annealed thin film, an resist layer containing a pattern of openings, exposing the coated thin film with a solvent under conditions effective to create fenestrations in the thin film corresponding to the pattern of openings, and removing the resist layer. The fenestrations may have dimensions and interfenestration spacings in the 10-50 micron range.[0020]
- These and other objects and features of the invention will be more fully appreciated when the following detailed description n of the invention is read in conjunction with the accompanying drawings.[0021]
- FIGS.[0022]1A-1D illustrate steps in practicing the method of the invention;
- FIG. 2 shows a resistivity versus temperature curve of a TiNi thin-film expanse formed in accordance with the invention;[0023]
- FIG. 3 illustrates a stress-strain curve for TiNi thin film at 37° C.;[0024]
- FIG. 4 illustrates a thin-film solid-wall stent constructed in accordance with an embodiment of the invention;[0025]
- FIGS. 5 and 6 illustrate open and folded states of the stent of FIG. 4, respectively, as seen in front-on view;[0026]
- FIG. 7 illustrates a sock-like thin-film device formed in accordance with another embodiment of the invention;[0027]
- FIG. 8 illustrates a hemispherical device formed in accordance with still another embodiment of the invention; and[0028]
- FIG. 9 illustrates a stent with a fenestrated thin-film expanse formed in accordance with the invention.[0029]
- I. Definitions[0030]
- Unless indicated otherwise, the terms below have the following meanings:[0031]
- “A shape defining an open, interior volume” refers to an expanse that defines an open volume-containing space, e.g., a cylindrical, sock-like or hemispherical volume-containing space. “Seamless thin-film expanse” means an expanse that forms an open interior volume without edge-to-edge seams.[0032]
- “Nitinol” or “TiNi” refers to an alloy containing titanium and nickel, typically each between 45-55 atom percent, and optionally, other metals, such as chromium in relatively minor amount, e.g., 1-5 atom percent. “Shape memory alloy” is an alloy that displays thermoelastic martensitic transformation defined as the ability to undergo a reversible transformation from an austentite to a martensite with a change in temperature such that an article made from such alloy has a heat stable configuration and is capable of being deformed to a heat unstable position.[0033]
- “Austenitic state” refers to the stronger, higher temperature phase (crystal structure) present in a shape-memory alloy.[0034]
- “Austentite finish temperature A[0035]f” refers to the temperature at which a shape memory alloy finishes transforming martensite to austentite upon heating.
- “Martensitic state” refers to the more deformable, lower temperature phase (crystal structure) present in a shape-memory alloy.[0036]
- “Sputtered alloy” refers to an alloy formed by sputter depositing a target-material alloy on a substrate, such as a mandrel.[0037]
- “Low-pressure, low-oxygen conditions” refers to sputtering conditions in which the pressure is preferably below 10[0038]−5Torr, typically 10−6to 10−8Torr, and the predominant gas is an inert gas such as argon. The amount of oxygen is kept low, e.g., below 0.1 percent, by the low pressure and low oxygen content of the sputter target alloy.
- “Etchable” with reference to a mandrel surface means refers to a surface layer, either part of the mandrel or a coating thereon, that can be removed by exposure to a dissolving agent, e.g., etchant.[0039]
- II. Method of Forming a Thin-Film Expanse[0040]
- FIGS.[0041]1A-1D illustrate steps in forming a thin-film device in accordance with one aspect of the invention. The basic components employed in the method include a
mandrel 10 having an outer exposed surface corresponding to the open, interior volume of the device to be formed. The mandrel may have a variety of volume-defining surfaces, such as cylindrical, ellipsoidal, hemispherical, tapered cylindrical, and conical volume shapes, and may be constructed of a variety of materials, such as steel, glass, or silicon. The dimensions of the mandrel are dictated by the dimensions of the desired thin-film device to be formed. - The mandrel is preferably polished, and may be coated with a material such as polyimide to produce a smooth, regular surface on which to deposit shape memory alloy. In one exemplary embodiment, the mandrel is coated with a three micron thick layer of pyralin polymer resin liquid coating type PI2611 (also known as polyimide coating) obtained from HD Microsystems, by spinning the mandrel to create a thin uniform layer, curing the high-temperature polymer coating in successive steps of baking at 150° C., then curing at 250° C. and finally curing at 450-500° C. to cross-link the polymer.[0042]
- The mandrel may be formed of a material that itself has an etchable surface, such as such as one formed of silicon, that can be removed upon exposure to an etchant, or may be formed of a material, such as NaCl, KCl, NaF[0043]2, which are available in flat or cylindrical shapes, and which can be shaped by machining and polishing. These substrates can be dissolved directly, e.g., with an aqueous solvent, without the need of an etchable coating.
- Where the mandrel is formed of a material that is itself not easily etchable, it is preferably coated with an etchable outer[0044]
sacrificial layer 14, e.g., formed over a polyimide coating.Layer 14 on the mandrel is preferably metal such as chromium or other material having a highly specific etch rate relative to TiNi so that the sacrificial layer may be removed without damaging the TiNi thin film. It is preferred that the layer used not diffuse readily into TiNi during heat treatment. Alternative sacrificial layers include aluminum, copper, and photoresist such as SU8 and OCG825. An etchant such as potassium hydroxide used for etching aluminum, nitric acid used for etching copper, and Chrome Etch from Arch Chemicals Inc. containing ceric ammonium nitrate, nitric acid, and water. The etchant may be an aqueous solution for water-soluble mandrels, such as the salt mandrels noted above. - The sacrificial layer may be formed by conventional thin-film deposition means, such as vacuum thermal evaporation, electroplating, or sputtering, to form a sacrificial layer preferably less than 1 micron in thickness. In one embodiment, a chromium layer is applied to a thickness of about of 0.1 micron by the sputter deposition method detailed below, but where the target is chromium rather than a TiNi alloy.[0045]
- The mandrel is mounted on[0046]
mandrel holder 12 for rotation thereon in the direction of arrow13 in FIG. 1B. The mandrel holder includes a mandrel-support rod 12a, and amotor 12bfor rotating the mandrel at a desired speed, e.g., 0.5 to 2 rpms, during sputter deposition. - Also shown in the figures is a[0047]
sputter deposition target 18 composed of a nickel-titanium shape-memory alloy, preferably nearly 50 atomic percent Ti, 50 atomic percent nickel, and containing minimal impurities especially carbon and oxygen. Composition control is critical to obtaining TiNi having appropriate shape memory and superelastic qualities. Increasing the content of nickel lowers the transition temperature. As little as 0.1% oxygen renders the film product brittle and unusable. The target may include minor amounts, e.g., 1-5 atomic percent, of other metals, e.g., chromium or platinum, known to effect the behavior of TiNi shape-memory alloys is specific ways. - In particular, the target is preferably selected to produce in the thin-film expanse, an austentite finish temperature A[0048]fless than 37° C. characterized by a four-point resistivity measurement in which the temperature is cycled to above 100° C. and below 0° C.; and a stress/strain recovery curve characterized by greater than 3% at 37° C. One exemplary target is composed of a nickel-titanium shape-memory alloy, preferably 46.75 weight percent Ti and 53.25 weight percent Ni, and less than 200 parts per million oxygen; formed by vacuum arc melting. The alloy composition may be enriched in nickel by as much as 1-2 percent to lower the transition temperature, and heat treatment such as rapid thermal anneal followed by heat-soaking at a lowered temperature may be employed as a method of obtaining special stress-strain-temperature characteristics.
- The target has exemplary diameter and thickness dimensions of 20 cm by 0.6 cm, respectively. The target is placed in the sputtering apparatus approximately 3-5 cm from the mandrel and parallel to the axis of the mandrel, as shown in FIGS. 1A and 1B.[0049]
- The mandrel and target are contained in a conventional high-vacuum sputtering apparatus, indicated at[0050]16 in FIGS. 1A and 1B. The apparatus may be any of a number of known sputtering systems that employ a direct current magnetron or radio frequency sputtering source. One exemplary apparatus is a Perkin-Elmer PE4400 series sputtering system.
- In operation, the coated mandrel and target are placed in the vacuum chamber of the sputtering apparatus, and sputter deposition is carried out in a vacuum of low 10[0051]−7Torr base pressure using a single TiNi DC magnetron target, argon gas, and a 5 kW DC power supply. High vacuum is necessary to minimize the oxygen (and other contaminants). Alternatively, the mandrel may be placed in a cylindrical magnetron sputtering system for deposition of TiNi.
- Sputter deposition is carried out until a selected thickness of thin-film expanse of between 0.5-100, preferably 2-50 microns is achieved. During deposition, film thickness may be determined by measuring the time of deposition and comparing to calibrated samples that are measured by a Tencor Alpha-Step profilometer. Alternatively, film thickness may be measured by placing a piezoelectric crystal adjacent to the target and monitoring its resonant frequency during deposition.[0052]
- As can be appreciated from the sputtering configuration shown in FIG. 1B, deposition onto the rotating mandrel is effective to produce a substantially uniform thickness of deposited thin film on the exposed surface of the mandrel, that is, the surface region directly exposed to the target.[0053]
- When a desired film thickness is reached, the sputter deposition step is terminated, and the thin-film expanse on the mandrel is then annealed under heating/cooling conditions to achieve desired shape-memory alloy properties in the device. The annealing step may be by thermal heating or by exposure to an infrared heater in vacuum. Use of infrared heating permits rapid heating and cooling so that sacrificial layers such as aluminum may be used, and solvent-removable sacrificial layers such as photoresist. In a typical annealing process, the thin-film expanse is heated in vacuum at 500-550° C. for 20 minutes followed by gradual cooling to ambient temperature. For heat treatment the mandrel is enclosed in a stainless steel fixture to ensure uniform heating.[0054]
- Following annealing, the thin-film device must be released from the mandrel. This is done preferably by exposing the mandrel and thin-film device thereon to a dissolving agent, e.g., etchant, to remove the outer mandrel layer or the sacrificial layer formed thereon. The step is shown in FIG. 1C, where sacrificial layer[0055]14 (FIGS. 1A and 1B) is removed by the etchant. The mandrel with its two or more layers of deposits is immersed in liquid etchant at room temperature and allowed to soak until the TiNi layer is freed from the surface. The time may vary from one to 24 hours depending on the degree of fenestration of the TiNi, the thickness of the sacrificial layer, and the degree of agitation applied to the mandrel. Ultrasonic power may be used to accelerate action of the etchant.
- At the end of the etching period, the coated mandrel is washed and the thin-film expanse is removed from the mandrel. This step is shown in FIG. 1D, showing a thin-[0056]
film stent 22 formed in accordance with the method. - For many applications it will be desirable to form a pattern of openings or fenestrations in the thin-film device, such as will be described below with respect to FIG. 12. According to an important feature of the invention, the method of producing a thin-film device can be extended to produce micro-sized, precisely shaped and spaced openings or in the film. In this embodiment, fenestration patterns are selectively etched in TiNi thin film, either before or after the above annealing step, to enhance mechanical flexibility of the film, to permit fluid to flow through, to increase the expansion rate of the device or achieve improved adhesion to vascular-wall structure. A positive photoresist is spun on the thin film/silicon mandrel. One preferred photoresist material is Olin OCG825; other suitable materials are available from alternative vendors. The photoresist and thin film TiNi is then photo-lithographically patterned and etched, respectively. The etchant is, for example, a mixture of nitric acid and buffered oxide etch containing hydrogen flouride. Fenestrations as small as 25 microns×25 microns in the film have been created. More generally, fenestrations and spacing between adjacent fenestrations may have dimensions in the 5-50 micron size range or larger.[0057]
- In another embodiment, the method is adapted to produce a thin-film device having internal ribs or struts. In this embodiment, the coated mandrel is first provided with structural members or ribs applied or deposited on the coated surface, to serve as structural members in a thin-film device as illustrated below in FIG. 7. The structural members are preferably deposited or placed circumferentially about the coated mandrel at one or more positions along the mandrel. The ribs may be, for example, nickel/titanium wire or strips, or some other metal or polymer materials upon which a thin-film expanse can be deposited. The surface must be coated or polished to a sub-micron finish for TiNi to be successfully sputtered onto the mandrel after placement of rib structure.[0058]
- III. Properties of the Thin-Film Expanse[0059]
- Narrow strips of thin film prepared as above were prepared for transformation temperature measurements and stress-strain measurements. To measure transformation temperature, each thin film sample was heated and cooled while changes in voltage were measured and recorded using a 4-probe constant-current technique to produce temperature versus resistivity data.[0060]
- FIG. 2 shows a typical resistivity versus temperature curve of a TiNi film. The transformation temperatures of the alloy are as follows:[0061]
Martensite start (Ms) −30° C. Martensite finish (Mf) −80° C. Austenite start (As) 0° C. Austenite finish (Af) 12-15° C. - Since the A[0062]fof this thin film alloy is below body temperature, it is well suited to medical devices that are actuated within the blood vessel.
- In stress-strain measurements,[0063]
film samples 20 mm×1 mm×5 microns in size were used. The deformation fixture used allowed the deformation at a constant temperature in the temperature range from −50° C. to +90° C. by immersion in an alcohol or water bath. The force applied to the sample and the sample elongation was measured respectively by a strain gage and an LVDT connected to a computer. LABVIEW™ software was used for collecting the data and plotting the stress-strain and resistivity graphs. - FIG. 3 illustrates a stress-strain curve for TiNi thin film at 38° C. The loading plateau for the film is at about 300 MPa (˜45 kpsi) and unloading plateau is at about 150 MPa (˜22 kpsi). The thin film clearly exhibits superelastic behavior at body temperature that makes this an excellent material for medical devices, particular miniature implantable devices.[0064]
- IV. Exemplary Devices[0065]
- In another aspect, the invention includes a device formed of a seamless thin-film expanse, in accordance with the method above. The “seamless” feature refers to the fact that the expanse forms a continuous surface without interior edges. The important features of devices formed in accordance with the above method are as follows:[0066]
- The devices are formed of a sputtered nitinol shape memory alloy. The expanse is formed by sputtering a thin film from a titanium/nickel alloy target, as described above. The thin-film material defines, in an austenitic state, an open, interior volume. The open interior volume is the volume of the space defined by the interior of the expanse. The expanse in the device has a film thickness of between 0.5 and 100 microns, preferably 2-50 microns, more preferably 5-50 microns, and a substantially uniform in thickness and composition throughout the expanse. The thin-film expanse has an austenite finish temperature A[0067]fbelow 37° C. and demonstrates a stress/strain recovery greater than 3% at 37° C., where the expanse can be deformed into a substantially compacted configuration in a martensitic state.
- One exemplary device is shown in FIGS.[0068]4-6, where a cylindrical thin-film expanse forms an intraluminal a
stent 22. The stent has typical dimensions of about 5-75 microns film thickness, 5-50 mm in length, and 1-10 mm in diameter, in its open conditions shown in FIGS. 4 and 5. A compacted or “folded” configuration is illustrated in FIG. 6, showingstent 22 in a front-on view. As seen, the dimensions of the stent have been reduced considerably in the compacted configuration, allowing the stent, for example, to be delivered through a catheter to a vascular site in need of a stent, e.g., in a compacted, stress-induced martensite form. Upon release of the stent from the catheter at the target site, the stent regains its fully austenitic, open form, with its outer surface impinging against the walls of the vessel. - A variety of other devices or articles are encompassed by the invention. Shown in FIG. 7 is a sock-[0069]
like device 24 formed of a thin-film expanse 25 and supported internally bystructural members 28. The structural ribs may be disposed in a circumferential direction, as shown, or in a longitudinal direction, like the struts in an umbrella. This device can be formed as described above, on a cone-shaped mandrel, where the mandrel is first coated prior to sputtering the thin-film expanse, and the structural members are formed on the mandrel prior to sputtering. The device may be fenestrated, as illustrated in FIG. 12, and may have a wire or other guide structure attached, for example, for pulling the device through an internal vessel, where the device acts like a filter. - In another embodiment, the device is a hemispherical cap device[0070]30. e.g., for use as a vaso-occlusive agent for treating an aneurysm. Also as discussed above, the thin-film device may be further etched, e.g., by photolithographic etching, to produce a device, such as
stent 32 in. FIG. 12, having defined-shape, size and position fenestrations. Fenestration patterns as small as 25 microns×25 microns have been etched in the film using photolithographic technique. - FIG. 12 shows a TiNi thin film stent[0071]30 formed of a thin-
film expanse 32 having fenestrations or windows, such aswindows 34, formed therein.Windows 25 microns×25 microns with spacing of about 10 microns between the adjacent openings has been achieved. Since fenestrations are produced by using photolithographic technique, very precise, clean and sharp edges can be achieved. The ability to etch micrometer scale features with an excellent etching quality allows for the production of devices such as implantable filters, or drug-release devices. - While the invention has been described with reference to particular embodiments and conditions, it will be appreciated that a variety of changes and modifications may be made without departing from the scope of the invention.[0072]
Claims (18)
Priority Applications (4)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US10/345,782US20030127318A1 (en) | 2000-01-24 | 2003-01-16 | Method for sputtering TiNi shape-memory alloys |
| US11/027,814US20050159808A1 (en) | 2000-01-24 | 2004-12-28 | Method for sputtering TiNi shape-memory alloys |
| US12/357,104US7981258B2 (en) | 2000-01-24 | 2009-01-21 | Thin-film shape memory alloy device and method |
| US13/171,243US8506767B2 (en) | 2000-01-24 | 2011-06-28 | Thin-film shape memory alloy device and method |
Applications Claiming Priority (4)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US17788100P | 2000-01-24 | 2000-01-24 | |
| US21135200P | 2000-06-13 | 2000-06-13 | |
| US09/768,700US6533905B2 (en) | 2000-01-24 | 2001-01-24 | Method for sputtering tini shape-memory alloys |
| US10/345,782US20030127318A1 (en) | 2000-01-24 | 2003-01-16 | Method for sputtering TiNi shape-memory alloys |
Related Parent Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US09/768,700DivisionUS6533905B2 (en) | 2000-01-24 | 2001-01-24 | Method for sputtering tini shape-memory alloys |
Related Child Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US11/027,814ContinuationUS20050159808A1 (en) | 2000-01-24 | 2004-12-28 | Method for sputtering TiNi shape-memory alloys |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| US20030127318A1true US20030127318A1 (en) | 2003-07-10 |
Family
ID=26873741
Family Applications (5)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US09/768,700Expired - LifetimeUS6533905B2 (en) | 2000-01-24 | 2001-01-24 | Method for sputtering tini shape-memory alloys |
| US10/345,782AbandonedUS20030127318A1 (en) | 2000-01-24 | 2003-01-16 | Method for sputtering TiNi shape-memory alloys |
| US11/027,814AbandonedUS20050159808A1 (en) | 2000-01-24 | 2004-12-28 | Method for sputtering TiNi shape-memory alloys |
| US12/357,104Expired - Fee RelatedUS7981258B2 (en) | 2000-01-24 | 2009-01-21 | Thin-film shape memory alloy device and method |
| US13/171,243Expired - Fee RelatedUS8506767B2 (en) | 2000-01-24 | 2011-06-28 | Thin-film shape memory alloy device and method |
Family Applications Before (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US09/768,700Expired - LifetimeUS6533905B2 (en) | 2000-01-24 | 2001-01-24 | Method for sputtering tini shape-memory alloys |
Family Applications After (3)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US11/027,814AbandonedUS20050159808A1 (en) | 2000-01-24 | 2004-12-28 | Method for sputtering TiNi shape-memory alloys |
| US12/357,104Expired - Fee RelatedUS7981258B2 (en) | 2000-01-24 | 2009-01-21 | Thin-film shape memory alloy device and method |
| US13/171,243Expired - Fee RelatedUS8506767B2 (en) | 2000-01-24 | 2011-06-28 | Thin-film shape memory alloy device and method |
Country Status (3)
| Country | Link |
|---|---|
| US (5) | US6533905B2 (en) |
| AU (1) | AU2001231099A1 (en) |
| WO (1) | WO2001053559A1 (en) |
Cited By (25)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US20050197689A1 (en)* | 2004-03-02 | 2005-09-08 | Masoud Molaei | Medical devices including metallic films and methods for making same |
| US20050197687A1 (en)* | 2004-03-02 | 2005-09-08 | Masoud Molaei | Medical devices including metallic films and methods for making same |
| US20050197690A1 (en)* | 2004-03-02 | 2005-09-08 | Masoud Molaei | Medical devices including metallic films and methods for making same |
| WO2005084585A1 (en)* | 2004-03-02 | 2005-09-15 | Boston Scientific Scimed, Inc. | Medical devices including metallic films and methods for making same |
| US20060113054A1 (en)* | 2004-12-01 | 2006-06-01 | Silvestrini Thomas A | Method of making an ocular implant |
| US20060118263A1 (en)* | 2004-12-01 | 2006-06-08 | Silvestrini Thomas A | Method of making an ocular implant |
| US20060142845A1 (en)* | 2004-12-29 | 2006-06-29 | Masoud Molaei | Medical devices including metallic films and methods for making same |
| US20060142851A1 (en)* | 2004-12-29 | 2006-06-29 | Masoud Molaei | Medical devices including metallic films and methods for making same |
| US20060142838A1 (en)* | 2004-12-29 | 2006-06-29 | Masoud Molaei | Medical devices including metallic films and methods for loading and deploying same |
| US20060142842A1 (en)* | 2004-12-29 | 2006-06-29 | Masoud Molaei | Medical devices including metallic films and methods for making same |
| WO2006071243A1 (en)* | 2004-12-29 | 2006-07-06 | Boston Scientific Limited | Medical devices including metallic films and methods for making same |
| WO2006071244A1 (en)* | 2004-12-29 | 2006-07-06 | Boston Scientific Limited | Medical devices including metallic films and methods for making the same |
| US20060259131A1 (en)* | 2005-05-16 | 2006-11-16 | Masoud Molaei | Medical devices including metallic films and methods for making same |
| US20060282115A1 (en)* | 2005-06-09 | 2006-12-14 | Abrams Robert M | Thin film vessel occlusion device |
| US20070048383A1 (en)* | 2005-08-25 | 2007-03-01 | Helmus Michael N | Self-assembled endovascular structures |
| US20070106374A1 (en)* | 2004-01-22 | 2007-05-10 | Isoflux, Inc. | Radiopaque coating for biomedical devices |
| US7628810B2 (en) | 2003-05-28 | 2009-12-08 | Acufocus, Inc. | Mask configured to maintain nutrient transport without producing visible diffraction patterns |
| US7976577B2 (en) | 2005-04-14 | 2011-07-12 | Acufocus, Inc. | Corneal optic formed of degradation resistant polymer |
| US8079706B2 (en) | 2003-06-17 | 2011-12-20 | Acufocus, Inc. | Method and apparatus for aligning a mask with the visual axis of an eye |
| USD656526S1 (en) | 2009-11-10 | 2012-03-27 | Acufocus, Inc. | Ocular mask |
| US8343215B2 (en) | 1999-03-01 | 2013-01-01 | Acufocus, Inc. | System and method for increasing the depth of focus of the human eye |
| US9005281B2 (en) | 2009-08-13 | 2015-04-14 | Acufocus, Inc. | Masked intraocular implants and lenses |
| US9204962B2 (en) | 2013-03-13 | 2015-12-08 | Acufocus, Inc. | In situ adjustable optical mask |
| US9427922B2 (en) | 2013-03-14 | 2016-08-30 | Acufocus, Inc. | Process for manufacturing an intraocular lens with an embedded mask |
| US9545303B2 (en) | 2011-12-02 | 2017-01-17 | Acufocus, Inc. | Ocular mask having selective spectral transmission |
Families Citing this family (163)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US6733513B2 (en) | 1999-11-04 | 2004-05-11 | Advanced Bioprosthetic Surfaces, Ltd. | Balloon catheter having metal balloon and method of making same |
| US10172730B2 (en) | 1999-11-19 | 2019-01-08 | Vactronix Scientific, Llc | Stents with metallic covers and methods of making same |
| US6379383B1 (en) | 1999-11-19 | 2002-04-30 | Advanced Bio Prosthetic Surfaces, Ltd. | Endoluminal device exhibiting improved endothelialization and method of manufacture thereof |
| US7736687B2 (en)* | 2006-01-31 | 2010-06-15 | Advance Bio Prosthetic Surfaces, Ltd. | Methods of making medical devices |
| US8458879B2 (en) | 2001-07-03 | 2013-06-11 | Advanced Bio Prosthetic Surfaces, Ltd., A Wholly Owned Subsidiary Of Palmaz Scientific, Inc. | Method of fabricating an implantable medical device |
| US7235092B2 (en) | 1999-11-19 | 2007-06-26 | Advanced Bio Prosthetic Surfaces, Ltd. | Guidewires and thin film catheter-sheaths and method of making same |
| US6849085B2 (en) | 1999-11-19 | 2005-02-01 | Advanced Bio Prosthetic Surfaces, Ltd. | Self-supporting laminated films, structural materials and medical devices manufactured therefrom and method of making same |
| US7300457B2 (en) | 1999-11-19 | 2007-11-27 | Advanced Bio Prosthetic Surfaces, Ltd. | Self-supporting metallic implantable grafts, compliant implantable medical devices and methods of making same |
| US7195641B2 (en) | 1999-11-19 | 2007-03-27 | Advanced Bio Prosthetic Surfaces, Ltd. | Valvular prostheses having metal or pseudometallic construction and methods of manufacture |
| US6537310B1 (en) | 1999-11-19 | 2003-03-25 | Advanced Bio Prosthetic Surfaces, Ltd. | Endoluminal implantable devices and method of making same |
| US7335426B2 (en) | 1999-11-19 | 2008-02-26 | Advanced Bio Prosthetic Surfaces, Ltd. | High strength vacuum deposited nitinol alloy films and method of making same |
| US6936066B2 (en) | 1999-11-19 | 2005-08-30 | Advanced Bio Prosthetic Surfaces, Ltd. | Complaint implantable medical devices and methods of making same |
| US6533905B2 (en)* | 2000-01-24 | 2003-03-18 | Tini Alloy Company | Method for sputtering tini shape-memory alloys |
| US6312463B1 (en)* | 2000-02-01 | 2001-11-06 | Endotex Interventional Systems, Inc. | Micro-porous mesh stent with hybrid structure |
| US6695865B2 (en) | 2000-03-20 | 2004-02-24 | Advanced Bio Prosthetic Surfaces, Ltd. | Embolic protection device |
| US9566148B2 (en) | 2000-05-12 | 2017-02-14 | Vactronix Scientific, Inc. | Self-supporting laminated films, structural materials and medical devices manufactured therefrom and methods of making same |
| US8632583B2 (en) | 2011-05-09 | 2014-01-21 | Palmaz Scientific, Inc. | Implantable medical device having enhanced endothelial migration features and methods of making the same |
| WO2002006567A1 (en)* | 2000-07-13 | 2002-01-24 | Discovery Commercial Enterprises Ltd. | Method and device for the manufacture of the medical expanding stents |
| US6602272B2 (en) | 2000-11-02 | 2003-08-05 | Advanced Cardiovascular Systems, Inc. | Devices configured from heat shaped, strain hardened nickel-titanium |
| US7976648B1 (en) | 2000-11-02 | 2011-07-12 | Abbott Cardiovascular Systems Inc. | Heat treatment for cold worked nitinol to impart a shape setting capability without eventually developing stress-induced martensite |
| WO2002038080A2 (en) | 2000-11-07 | 2002-05-16 | Advanced Bio Prosthetic Surfaces, Ltd. | Endoluminal stent, self-fupporting endoluminal graft and methods of making same |
| US6626937B1 (en) | 2000-11-14 | 2003-09-30 | Advanced Cardiovascular Systems, Inc. | Austenitic nitinol medical devices |
| US6855161B2 (en) | 2000-12-27 | 2005-02-15 | Advanced Cardiovascular Systems, Inc. | Radiopaque nitinol alloys for medical devices |
| US20010044650A1 (en)* | 2001-01-12 | 2001-11-22 | Simso Eric J. | Stent for in-stent restenosis |
| WO2003002243A2 (en)* | 2001-06-27 | 2003-01-09 | Remon Medical Technologies Ltd. | Method and device for electrochemical formation of therapeutic species in vivo |
| US20030060878A1 (en) | 2001-08-31 | 2003-03-27 | Shadduck John H. | Intraocular lens system and method for power adjustment |
| US6669795B2 (en)* | 2002-01-17 | 2003-12-30 | Tini Alloy Company | Methods of fabricating high transition temperature SMA, and SMA materials made by the methods |
| US8048155B2 (en) | 2002-02-02 | 2011-11-01 | Powervision, Inc. | Intraocular implant devices |
| US7063707B2 (en) | 2002-03-06 | 2006-06-20 | Scimed Life Systems, Inc. | Medical retrieval device |
| DE10226734A1 (en)* | 2002-05-10 | 2003-11-20 | Lothar Sellin | Stent consists of a material with shape memory activatable by temperature, with the stent diameter varying within given ranges at temperatures below and above a specified level |
| US6865810B2 (en)* | 2002-06-27 | 2005-03-15 | Scimed Life Systems, Inc. | Methods of making medical devices |
| US20040123510A1 (en)* | 2002-07-12 | 2004-07-01 | Larry Essad | Shape-retaining baits and leaders |
| US6746890B2 (en)* | 2002-07-17 | 2004-06-08 | Tini Alloy Company | Three dimensional thin film devices and methods of fabrication |
| US7040323B1 (en)* | 2002-08-08 | 2006-05-09 | Tini Alloy Company | Thin film intrauterine device |
| US8679517B2 (en) | 2002-09-26 | 2014-03-25 | Palmaz Scientific, Inc. | Implantable materials having engineered surfaces made by vacuum deposition and method of making same |
| AU2003270817B2 (en) | 2002-09-26 | 2009-09-17 | Vactronix Scientific, Llc | High strength vacuum deposited nitionol alloy films, medical thin film graft materials and method of making same |
| US8268340B2 (en) | 2002-09-26 | 2012-09-18 | Advanced Bio Prosthetic Surfaces, Ltd. | Implantable materials having engineered surfaces and method of making same |
| AU2003272710B2 (en) | 2002-09-26 | 2008-05-15 | Vactronix Scientific, Llc | Implantable materials having engineered surfaces and method of making same |
| US6923829B2 (en) | 2002-11-25 | 2005-08-02 | Advanced Bio Prosthetic Surfaces, Ltd. | Implantable expandable medical devices having regions of differential mechanical properties and methods of making same |
| US7217288B2 (en) | 2002-12-12 | 2007-05-15 | Powervision, Inc. | Accommodating intraocular lens having peripherally actuated deflectable surface and method |
| US8361145B2 (en) | 2002-12-12 | 2013-01-29 | Powervision, Inc. | Accommodating intraocular lens system having circumferential haptic support and method |
| US10835373B2 (en) | 2002-12-12 | 2020-11-17 | Alcon Inc. | Accommodating intraocular lenses and methods of use |
| US8328869B2 (en) | 2002-12-12 | 2012-12-11 | Powervision, Inc. | Accommodating intraocular lenses and methods of use |
| JP2006523130A (en) | 2003-03-06 | 2006-10-12 | ジョン エイチ. シャダック, | Compatible optical lens and manufacturing method |
| US7658709B2 (en)* | 2003-04-09 | 2010-02-09 | Medtronic, Inc. | Shape memory alloy actuators |
| US7690621B2 (en)* | 2003-04-15 | 2010-04-06 | Board Of Trustees Operating Michigan State University | Prestrained thin-film shape memory actuator using polymeric substrates |
| US7044461B2 (en)* | 2003-04-30 | 2006-05-16 | The Regents Of The University Of California | Method and apparatus for adjustably induced biaxial strain |
| US7942892B2 (en) | 2003-05-01 | 2011-05-17 | Abbott Cardiovascular Systems Inc. | Radiopaque nitinol embolic protection frame |
| EP1620047B1 (en) | 2003-05-07 | 2009-11-11 | Advanced Bio Prosthetic Surfaces, Ltd. | Metallic implantable grafts and method of making same |
| US20040236409A1 (en)* | 2003-05-20 | 2004-11-25 | Pelton Alan R. | Radiopacity intraluminal medical device |
| US7332475B2 (en)* | 2003-07-22 | 2008-02-19 | Kyowa Hakko Kogyo Co., Ltd. | Preventive or therapeutic composition for viral infectious disease |
| US20050021128A1 (en)* | 2003-07-24 | 2005-01-27 | Medtronic Vascular, Inc. | Compliant, porous, rolled stent |
| DE60310776T2 (en)* | 2003-08-08 | 2007-10-11 | Biorthex Inc., Blainville | BIOKOMPATIBLE POROUS TI-NI MATERIAL |
| US9579194B2 (en) | 2003-10-06 | 2017-02-28 | Medtronic ATS Medical, Inc. | Anchoring structure with concave landing zone |
| US20060259137A1 (en)* | 2003-10-06 | 2006-11-16 | Jason Artof | Minimally invasive valve replacement system |
| US7586828B1 (en) | 2003-10-23 | 2009-09-08 | Tini Alloy Company | Magnetic data storage system |
| US7422403B1 (en) | 2003-10-23 | 2008-09-09 | Tini Alloy Company | Non-explosive releasable coupling device |
| US20050090888A1 (en)* | 2003-10-28 | 2005-04-28 | Hines Richard A. | Pleated stent assembly |
| US8333798B2 (en)* | 2003-11-07 | 2012-12-18 | Merlin Md Pte Ltd. | Implantable medical devices with enhanced visibility, mechanical properties and biocompatability |
| US20080027531A1 (en)* | 2004-02-12 | 2008-01-31 | Reneker Darrell H | Stent for Use in Cardiac, Cranial, and Other Arteries |
| US20050203606A1 (en)* | 2004-03-09 | 2005-09-15 | Vancamp Daniel H. | Stent system for preventing restenosis |
| WO2005094725A1 (en)* | 2004-03-31 | 2005-10-13 | Merlin Md Pte Ltd | A method for treating aneurysms |
| US8500751B2 (en)* | 2004-03-31 | 2013-08-06 | Merlin Md Pte Ltd | Medical device |
| US8715340B2 (en)* | 2004-03-31 | 2014-05-06 | Merlin Md Pte Ltd. | Endovascular device with membrane |
| US7632361B2 (en)* | 2004-05-06 | 2009-12-15 | Tini Alloy Company | Single crystal shape memory alloy devices and methods |
| EP1871288B1 (en)* | 2004-06-08 | 2016-02-24 | Tini Alloy Company | Self-expandable and collapsible three-dimensional devices and methods |
| EP1621158A1 (en)* | 2004-07-28 | 2006-02-01 | Cordis Corporation | Reduced profile abdominal aortic aneurysm device |
| US20080004653A1 (en)* | 2004-09-17 | 2008-01-03 | Sherman Darren R | Thin Film Devices for Occlusion of a Vessel |
| WO2006034153A2 (en)* | 2004-09-17 | 2006-03-30 | Cordis Neurovascular, Inc. | Thin film metallic devices for plugging aneurysms or vessels |
| US20060118210A1 (en)* | 2004-10-04 | 2006-06-08 | Johnson A D | Portable energy storage devices and methods |
| US9872763B2 (en) | 2004-10-22 | 2018-01-23 | Powervision, Inc. | Accommodating intraocular lenses |
| US8070718B2 (en)* | 2004-12-13 | 2011-12-06 | Boston Scientific Scimed, Inc. | Medical devices formed with a sacrificial structure and processes of forming the same |
| EP1809202A4 (en)* | 2004-12-22 | 2011-04-27 | Merlin Md Pte Ltd | A medical device |
| WO2006071245A1 (en)* | 2004-12-29 | 2006-07-06 | Boston Scientific Limited | Medical devices including metallic films and methods for loading and deploying same |
| US20060155367A1 (en)* | 2005-01-07 | 2006-07-13 | Hines Richard A | Micro-pleated stent assembly |
| US8057543B2 (en)* | 2005-01-28 | 2011-11-15 | Greatbatch Ltd. | Stent coating for eluting medication |
| FR2881946B1 (en)* | 2005-02-17 | 2008-01-04 | Jacques Seguin | DEVICE FOR THE TREATMENT OF BODILY CONDUIT AT BIFURCATION LEVEL |
| US7763342B2 (en)* | 2005-03-31 | 2010-07-27 | Tini Alloy Company | Tear-resistant thin film methods of fabrication |
| US7441888B1 (en) | 2005-05-09 | 2008-10-28 | Tini Alloy Company | Eyeglass frame |
| US7540899B1 (en) | 2005-05-25 | 2009-06-02 | Tini Alloy Company | Shape memory alloy thin film, method of fabrication, and articles of manufacture |
| US8545530B2 (en)* | 2005-10-19 | 2013-10-01 | Pulsar Vascular, Inc. | Implantable aneurysm closure systems and methods |
| EP1951129B1 (en) | 2005-10-19 | 2012-11-21 | Pulsar Vascular, Inc. | Methods and systems for endovascularly clipping and repairing lumen and tissue defects |
| US7867176B2 (en)* | 2005-12-27 | 2011-01-11 | Cordis Corporation | Variable stiffness guidewire |
| US8840660B2 (en) | 2006-01-05 | 2014-09-23 | Boston Scientific Scimed, Inc. | Bioerodible endoprostheses and methods of making the same |
| US20090054966A1 (en)* | 2006-02-13 | 2009-02-26 | Merlin Md Pte Ltd. | Endovascular device with membrane |
| US7785317B2 (en) | 2006-03-29 | 2010-08-31 | Codman & Shurtleff, Inc. | Joined metal tubing and method of manufacture |
| US20070246233A1 (en)* | 2006-04-04 | 2007-10-25 | Johnson A D | Thermal actuator for fire protection sprinkler head |
| US20070293939A1 (en)* | 2006-05-15 | 2007-12-20 | Abbott Laboratories | Fatigue resistant endoprostheses |
| US8690938B2 (en)* | 2006-05-26 | 2014-04-08 | DePuy Synthes Products, LLC | Occlusion device combination of stent and mesh with diamond-shaped porosity |
| US8118859B2 (en)* | 2006-05-26 | 2012-02-21 | Codman & Shurtleff, Inc. | Occlusion device combination of stent and mesh having offset parallelogram porosity |
| US7670353B2 (en)* | 2006-06-12 | 2010-03-02 | Codman & Shurtleff, Inc. | Modified headpiece for hydraulic coil deployment system |
| US7766935B2 (en) | 2006-06-12 | 2010-08-03 | Codman & Shurtleff, Inc. | Modified headpiece for hydraulic coil deployment system |
| US8585732B2 (en)* | 2006-06-14 | 2013-11-19 | DePuy Synthes Products, LLC | Retrieval device with sidewall grippers |
| WO2008034007A2 (en)* | 2006-09-15 | 2008-03-20 | Boston Scientific Limited | Medical devices |
| JP2010503489A (en) | 2006-09-15 | 2010-02-04 | ボストン サイエンティフィック リミテッド | Biodegradable endoprosthesis and method for producing the same |
| US20100145436A1 (en)* | 2006-09-18 | 2010-06-10 | Boston Scientific Scimed, Inc. | Bio-erodible Stent |
| US20080213062A1 (en)* | 2006-09-22 | 2008-09-04 | Tini Alloy Company | Constant load fastener |
| US20080075557A1 (en)* | 2006-09-22 | 2008-03-27 | Johnson A David | Constant load bolt |
| WO2008133738A2 (en) | 2006-12-01 | 2008-11-06 | Tini Alloy Company | Method of alloying reactive components |
| ES2506144T3 (en) | 2006-12-28 | 2014-10-13 | Boston Scientific Limited | Bioerodible endoprosthesis and their manufacturing procedure |
| WO2008092028A1 (en)* | 2007-01-25 | 2008-07-31 | Tini Alloy Company | Frangible shape memory alloy fire sprinkler valve actuator |
| US8584767B2 (en)* | 2007-01-25 | 2013-11-19 | Tini Alloy Company | Sprinkler valve with active actuation |
| AU2008218313B2 (en) | 2007-02-21 | 2014-01-09 | Alcon Inc. | Polymeric materials suitable for ophthalmic devices and methods of manufacture |
| US20080249597A1 (en) | 2007-04-05 | 2008-10-09 | Russell Scott M | Thin film tissue repair matrix |
| CN101795642B (en) | 2007-07-23 | 2013-11-27 | 力景公司 | Post-implant lens power modification |
| US8668734B2 (en) | 2010-07-09 | 2014-03-11 | Powervision, Inc. | Intraocular lens delivery devices and methods of use |
| EP2671541B1 (en) | 2007-07-23 | 2019-04-17 | PowerVision, Inc. | Accommodating intraocular lenses |
| US8968396B2 (en) | 2007-07-23 | 2015-03-03 | Powervision, Inc. | Intraocular lens delivery systems and methods of use |
| US8314927B2 (en) | 2007-07-23 | 2012-11-20 | Powervision, Inc. | Systems and methods for testing intraocular lenses |
| US8956408B2 (en) | 2007-07-23 | 2015-02-17 | Powervision, Inc. | Lens delivery system |
| US8007674B2 (en) | 2007-07-30 | 2011-08-30 | Tini Alloy Company | Method and devices for preventing restenosis in cardiovascular stents |
| US8556969B2 (en) | 2007-11-30 | 2013-10-15 | Ormco Corporation | Biocompatible copper-based single-crystal shape memory alloys |
| US7842143B2 (en)* | 2007-12-03 | 2010-11-30 | Tini Alloy Company | Hyperelastic shape setting devices and fabrication methods |
| US8382917B2 (en)* | 2007-12-03 | 2013-02-26 | Ormco Corporation | Hyperelastic shape setting devices and fabrication methods |
| JP2009208467A (en)* | 2008-02-07 | 2009-09-17 | Seiko Epson Corp | Manufacturing method for liquid jet head, manufacturing method for piezoelectric element, liquid jet head, and liquid jet apparatus |
| US8236046B2 (en)* | 2008-06-10 | 2012-08-07 | Boston Scientific Scimed, Inc. | Bioerodible endoprosthesis |
| US9005274B2 (en)* | 2008-08-04 | 2015-04-14 | Stentys Sas | Method for treating a body lumen |
| US8262692B2 (en)* | 2008-09-05 | 2012-09-11 | Merlin Md Pte Ltd | Endovascular device |
| WO2010028314A1 (en) | 2008-09-05 | 2010-03-11 | Pulsar Vascular, Inc. | Systems and methods for supporting or occluding a physiological opening or cavity |
| US8382824B2 (en) | 2008-10-03 | 2013-02-26 | Boston Scientific Scimed, Inc. | Medical implant having NANO-crystal grains with barrier layers of metal nitrides or fluorides |
| JP5646492B2 (en) | 2008-10-07 | 2014-12-24 | エムシー10 インコーポレイテッドMc10,Inc. | Stretchable integrated circuit and device with sensor array |
| US8886334B2 (en) | 2008-10-07 | 2014-11-11 | Mc10, Inc. | Systems, methods, and devices using stretchable or flexible electronics for medical applications |
| US8097926B2 (en) | 2008-10-07 | 2012-01-17 | Mc10, Inc. | Systems, methods, and devices having stretchable integrated circuitry for sensing and delivering therapy |
| US10299913B2 (en) | 2009-01-09 | 2019-05-28 | Powervision, Inc. | Accommodating intraocular lenses and methods of use |
| CA2753853C (en)* | 2009-03-06 | 2017-04-25 | Daniel S. Levi | Thin film vascular stent and biocompatible surface treatment |
| EP2461900A4 (en) | 2009-08-07 | 2017-08-02 | Innovative Processing Technologies Inc. | Methods and systems for processing materials, including shape memory materials |
| EP2473138A4 (en) | 2009-08-31 | 2017-08-16 | PowerVision, Inc. | Lens capsule size estimation |
| EP2805680B1 (en) | 2009-09-04 | 2017-10-25 | Pulsar Vascular, Inc. | Systems for enclosing an anatomical opening |
| WO2011041727A1 (en) | 2009-10-01 | 2011-04-07 | Mc10, Inc. | Protective cases with integrated electronics |
| US20110160839A1 (en)* | 2009-12-29 | 2011-06-30 | Boston Scientific Scimed, Inc. | Endoprosthesis |
| US8900298B2 (en) | 2010-02-23 | 2014-12-02 | Powervision, Inc. | Fluid for accommodating intraocular lenses |
| WO2011119603A1 (en)* | 2010-03-23 | 2011-09-29 | Boston Scientific Scimed, Inc. | Bioerodible medical implants |
| US8668732B2 (en) | 2010-03-23 | 2014-03-11 | Boston Scientific Scimed, Inc. | Surface treated bioerodible metal endoprostheses |
| WO2011150118A2 (en) | 2010-05-25 | 2011-12-01 | The Regents Of The University Of California | Ultra-low fractional area coverage flow diverter for treating aneurysms and vascular diseases |
| DE102010024498B4 (en)* | 2010-06-21 | 2014-10-16 | Heraeus Precious Metals Gmbh & Co. Kg | Method for producing a three-dimensional structure for medical technology |
| JP5757518B2 (en)* | 2011-01-13 | 2015-07-29 | 国立研究開発法人物質・材料研究機構 | Method for manufacturing thin film actuator |
| EP2688515B1 (en) | 2011-03-24 | 2021-05-19 | Alcon Inc. | Intraocular lens loading systems and methods of use |
| US8728563B2 (en)* | 2011-05-03 | 2014-05-20 | Palmaz Scientific, Inc. | Endoluminal implantable surfaces, stents, and grafts and method of making same |
| JP2014523633A (en)* | 2011-05-27 | 2014-09-11 | エムシー10 インコーポレイテッド | Electronic, optical and / or mechanical devices and systems and methods of manufacturing these devices and systems |
| CN103582460B (en) | 2011-06-03 | 2019-03-19 | 帕尔萨维斯库勒公司 | Aneurysm devices and related systems and methods with additional anchoring mechanism |
| EP2713905B1 (en) | 2011-06-03 | 2022-03-16 | Pulsar Vascular, Inc. | Systems for enclosing an anatomical opening, including shock absorbing aneurysm devices |
| US9119625B2 (en) | 2011-10-05 | 2015-09-01 | Pulsar Vascular, Inc. | Devices, systems and methods for enclosing an anatomical opening |
| US20150004432A1 (en)* | 2011-10-28 | 2015-01-01 | Korea Institute Of Machinery & Materials | Titanium-nickel alloy thin film, and preparation method of titanium-nickel alloy thin film using multiple sputtering method |
| US10433949B2 (en) | 2011-11-08 | 2019-10-08 | Powervision, Inc. | Accommodating intraocular lenses |
| US10987208B2 (en) | 2012-04-06 | 2021-04-27 | Merlin Md Pte Ltd. | Devices and methods for treating an aneurysm |
| EP2846706A1 (en) | 2012-05-10 | 2015-03-18 | Pulsar Vascular, Inc. | Coil-tipped aneurysm devices |
| US9061371B2 (en)* | 2012-05-14 | 2015-06-23 | Megastir Technologies Llc | Disposable mandrel for friction stir joining |
| JP6338575B2 (en) | 2012-05-21 | 2018-06-06 | ユニバーシティ・オブ・シンシナティ | Method for making a magnesium biodegradable stent for medical implant applications |
| US10124197B2 (en) | 2012-08-31 | 2018-11-13 | TiNi Allot Company | Fire sprinkler valve actuator |
| US11040230B2 (en) | 2012-08-31 | 2021-06-22 | Tini Alloy Company | Fire sprinkler valve actuator |
| US9171794B2 (en) | 2012-10-09 | 2015-10-27 | Mc10, Inc. | Embedding thin chips in polymer |
| CA2908497A1 (en)* | 2013-02-25 | 2014-08-28 | The Regents Of The University Of California | Thin film vascular stent for arterial disease |
| EP3785668A1 (en) | 2013-03-15 | 2021-03-03 | Alcon Inc. | Intraocular lens storage and loading devices and methods of use |
| WO2016012236A1 (en)* | 2014-07-24 | 2016-01-28 | Nv Bekaert Sa | High fatigue resistant wire |
| CN104388903A (en)* | 2014-12-09 | 2015-03-04 | 哈尔滨工业大学 | Single-target low-cost preparation method for multi-component alloy film |
| CN104388904A (en)* | 2014-12-09 | 2015-03-04 | 哈尔滨工业大学 | Method for efficiently preparing Ti-Ta high-temperature memory alloy thin film |
| EP3307206B1 (en) | 2015-06-10 | 2023-09-20 | Alcon Inc. | Intraocular lens materials and components |
| WO2017079733A1 (en) | 2015-11-06 | 2017-05-11 | Powervision, Inc. | Accommodating intraocular lenses and methods of manufacturing |
| US12037672B2 (en) | 2016-10-21 | 2024-07-16 | Confluent Medical Technologies, Inc. | Materials having superelastic properties including related methods of fabrication and design for medical devices |
| DE102017222168B4 (en)* | 2017-12-07 | 2021-02-11 | Fraunhofer-Gesellschaft zur Förderung der angewandten Forschung e.V. | Method for manufacturing a stent and stent manufactured by the method |
| US11220735B2 (en)* | 2018-02-08 | 2022-01-11 | Medtronic Minimed, Inc. | Methods for controlling physical vapor deposition metal film adhesion to substrates and surfaces |
| US11471272B2 (en) | 2019-10-04 | 2022-10-18 | Alcon Inc. | Adjustable intraocular lenses and methods of post-operatively adjusting intraocular lenses |
| US20210254237A1 (en)* | 2020-02-19 | 2021-08-19 | Jagannathan Rajagopalan | Synthesis of single crystal films on amorphous substrates |
Citations (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US6358380B1 (en)* | 1999-09-22 | 2002-03-19 | Delphi Technologies, Inc. | Production of binary shape-memory alloy films by sputtering using a hot pressed target |
Family Cites Families (15)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US3668131A (en)* | 1968-08-09 | 1972-06-06 | Allied Chem | Dissolution of metal with acidified hydrogen peroxide solutions |
| US3991898A (en)* | 1975-09-16 | 1976-11-16 | The United States Of America As Represented By The United States Energy Research And Development Administration | Vacuum foil insulation system |
| US4151064A (en)* | 1977-12-27 | 1979-04-24 | Coulter Stork U.S.A., Inc. | Apparatus for sputtering cylinders |
| US5061914A (en) | 1989-06-27 | 1991-10-29 | Tini Alloy Company | Shape-memory alloy micro-actuator |
| US6107004A (en)* | 1991-09-05 | 2000-08-22 | Intra Therapeutics, Inc. | Method for making a tubular stent for use in medical applications |
| US5474563A (en)* | 1993-03-25 | 1995-12-12 | Myler; Richard | Cardiovascular stent and retrieval apparatus |
| DE69528216T2 (en)* | 1994-06-17 | 2003-04-17 | Terumo K.K., Tokio/Tokyo | Process for the production of a permanent stent |
| US5772864A (en)* | 1996-02-23 | 1998-06-30 | Meadox Medicals, Inc. | Method for manufacturing implantable medical devices |
| US6072154A (en)* | 1996-09-05 | 2000-06-06 | Medtronic, Inc. | Selectively activated shape memory device |
| US6224626B1 (en)* | 1998-02-17 | 2001-05-01 | Md3, Inc. | Ultra-thin expandable stent |
| AU756080B2 (en) | 1998-06-04 | 2003-01-02 | New York University | Endovascular thin film devices and methods for treating and preventing stroke |
| US6096175A (en)* | 1998-07-17 | 2000-08-01 | Micro Therapeutics, Inc. | Thin film stent |
| US6849085B2 (en)* | 1999-11-19 | 2005-02-01 | Advanced Bio Prosthetic Surfaces, Ltd. | Self-supporting laminated films, structural materials and medical devices manufactured therefrom and method of making same |
| US6379383B1 (en)* | 1999-11-19 | 2002-04-30 | Advanced Bio Prosthetic Surfaces, Ltd. | Endoluminal device exhibiting improved endothelialization and method of manufacture thereof |
| US6533905B2 (en)* | 2000-01-24 | 2003-03-18 | Tini Alloy Company | Method for sputtering tini shape-memory alloys |
- 2001
- 2001-01-24USUS09/768,700patent/US6533905B2/ennot_activeExpired - Lifetime
- 2001-01-24WOPCT/US2001/002253patent/WO2001053559A1/enactiveApplication Filing
- 2001-01-24AUAU2001231099Apatent/AU2001231099A1/ennot_activeAbandoned
- 2003
- 2003-01-16USUS10/345,782patent/US20030127318A1/ennot_activeAbandoned
- 2004
- 2004-12-28USUS11/027,814patent/US20050159808A1/ennot_activeAbandoned
- 2009
- 2009-01-21USUS12/357,104patent/US7981258B2/ennot_activeExpired - Fee Related
- 2011
- 2011-06-28USUS13/171,243patent/US8506767B2/ennot_activeExpired - Fee Related
Patent Citations (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US6358380B1 (en)* | 1999-09-22 | 2002-03-19 | Delphi Technologies, Inc. | Production of binary shape-memory alloy films by sputtering using a hot pressed target |
Cited By (49)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US8752958B2 (en) | 1999-03-01 | 2014-06-17 | Boston Innovative Optics, Inc. | System and method for increasing the depth of focus of the human eye |
| US8343215B2 (en) | 1999-03-01 | 2013-01-01 | Acufocus, Inc. | System and method for increasing the depth of focus of the human eye |
| US7628810B2 (en) | 2003-05-28 | 2009-12-08 | Acufocus, Inc. | Mask configured to maintain nutrient transport without producing visible diffraction patterns |
| US9138142B2 (en) | 2003-05-28 | 2015-09-22 | Acufocus, Inc. | Masked intraocular devices |
| US8858624B2 (en) | 2003-05-28 | 2014-10-14 | Acufocus, Inc. | Method for increasing the depth of focus of a patient |
| US8460374B2 (en) | 2003-05-28 | 2013-06-11 | Acufocus, Inc. | Mask configured to maintain nutrient transport without producing visible diffraction patterns |
| US8864824B2 (en) | 2003-06-17 | 2014-10-21 | Acufocus, Inc. | Method and apparatus for aligning a mask with the visual axis of an eye |
| US8079706B2 (en) | 2003-06-17 | 2011-12-20 | Acufocus, Inc. | Method and apparatus for aligning a mask with the visual axis of an eye |
| US20070106374A1 (en)* | 2004-01-22 | 2007-05-10 | Isoflux, Inc. | Radiopaque coating for biomedical devices |
| US8591568B2 (en) | 2004-03-02 | 2013-11-26 | Boston Scientific Scimed, Inc. | Medical devices including metallic films and methods for making same |
| US20050197687A1 (en)* | 2004-03-02 | 2005-09-08 | Masoud Molaei | Medical devices including metallic films and methods for making same |
| US8998973B2 (en) | 2004-03-02 | 2015-04-07 | Boston Scientific Scimed, Inc. | Medical devices including metallic films |
| US20050197689A1 (en)* | 2004-03-02 | 2005-09-08 | Masoud Molaei | Medical devices including metallic films and methods for making same |
| US20050197690A1 (en)* | 2004-03-02 | 2005-09-08 | Masoud Molaei | Medical devices including metallic films and methods for making same |
| WO2005084585A1 (en)* | 2004-03-02 | 2005-09-15 | Boston Scientific Scimed, Inc. | Medical devices including metallic films and methods for making same |
| US20060271185A1 (en)* | 2004-12-01 | 2006-11-30 | Silvestrini Thomas A | Method of making an ocular implant |
| US20060113054A1 (en)* | 2004-12-01 | 2006-06-01 | Silvestrini Thomas A | Method of making an ocular implant |
| US20060271184A1 (en)* | 2004-12-01 | 2006-11-30 | Silvestrini Thomas A | Method of making an ocular implant |
| US7491350B2 (en) | 2004-12-01 | 2009-02-17 | Acufocus, Inc. | Method of making an ocular implant |
| US20060118263A1 (en)* | 2004-12-01 | 2006-06-08 | Silvestrini Thomas A | Method of making an ocular implant |
| US8992592B2 (en) | 2004-12-29 | 2015-03-31 | Boston Scientific Scimed, Inc. | Medical devices including metallic films |
| US8864815B2 (en) | 2004-12-29 | 2014-10-21 | Boston Scientific Scimed, Inc. | Medical devices including metallic film and at least one filament |
| US7901447B2 (en) | 2004-12-29 | 2011-03-08 | Boston Scientific Scimed, Inc. | Medical devices including a metallic film and at least one filament |
| US20060142838A1 (en)* | 2004-12-29 | 2006-06-29 | Masoud Molaei | Medical devices including metallic films and methods for loading and deploying same |
| US20060142842A1 (en)* | 2004-12-29 | 2006-06-29 | Masoud Molaei | Medical devices including metallic films and methods for making same |
| US20060142851A1 (en)* | 2004-12-29 | 2006-06-29 | Masoud Molaei | Medical devices including metallic films and methods for making same |
| WO2006071243A1 (en)* | 2004-12-29 | 2006-07-06 | Boston Scientific Limited | Medical devices including metallic films and methods for making same |
| WO2006071244A1 (en)* | 2004-12-29 | 2006-07-06 | Boston Scientific Limited | Medical devices including metallic films and methods for making the same |
| US20060142845A1 (en)* | 2004-12-29 | 2006-06-29 | Masoud Molaei | Medical devices including metallic films and methods for making same |
| US8632580B2 (en) | 2004-12-29 | 2014-01-21 | Boston Scientific Scimed, Inc. | Flexible medical devices including metallic films |
| US8287592B2 (en) | 2005-04-14 | 2012-10-16 | Acufocus, Inc. | Ophthalmic devices having a degradation resistant polymer |
| US7976577B2 (en) | 2005-04-14 | 2011-07-12 | Acufocus, Inc. | Corneal optic formed of degradation resistant polymer |
| US20100204784A1 (en)* | 2005-05-16 | 2010-08-12 | Boston Scientific Scimed, Inc. | Medical devices including metallic films |
| US7854760B2 (en) | 2005-05-16 | 2010-12-21 | Boston Scientific Scimed, Inc. | Medical devices including metallic films |
| US8152841B2 (en) | 2005-05-16 | 2012-04-10 | Boston Scientific Scimed, Inc. | Medical devices including metallic films |
| US20060259131A1 (en)* | 2005-05-16 | 2006-11-16 | Masoud Molaei | Medical devices including metallic films and methods for making same |
| US20060282115A1 (en)* | 2005-06-09 | 2006-12-14 | Abrams Robert M | Thin film vessel occlusion device |
| US20070048383A1 (en)* | 2005-08-25 | 2007-03-01 | Helmus Michael N | Self-assembled endovascular structures |
| US9492272B2 (en) | 2009-08-13 | 2016-11-15 | Acufocus, Inc. | Masked intraocular implants and lenses |
| US9005281B2 (en) | 2009-08-13 | 2015-04-14 | Acufocus, Inc. | Masked intraocular implants and lenses |
| USD681086S1 (en) | 2009-11-10 | 2013-04-30 | Acufocus, Inc. | Ocular mask |
| USD656526S1 (en) | 2009-11-10 | 2012-03-27 | Acufocus, Inc. | Ocular mask |
| US9545303B2 (en) | 2011-12-02 | 2017-01-17 | Acufocus, Inc. | Ocular mask having selective spectral transmission |
| US9204962B2 (en) | 2013-03-13 | 2015-12-08 | Acufocus, Inc. | In situ adjustable optical mask |
| US9603704B2 (en) | 2013-03-13 | 2017-03-28 | Acufocus, Inc. | In situ adjustable optical mask |
| US10350058B2 (en) | 2013-03-13 | 2019-07-16 | Acufocus, Inc. | In situ adjustable optical mask |
| US10939995B2 (en) | 2013-03-13 | 2021-03-09 | Acufocus, Inc. | In situ adjustable optical mask |
| US11771552B2 (en) | 2013-03-13 | 2023-10-03 | Acufocus, Inc. | In situ adjustable optical mask |
| US9427922B2 (en) | 2013-03-14 | 2016-08-30 | Acufocus, Inc. | Process for manufacturing an intraocular lens with an embedded mask |
Also Published As
| Publication number | Publication date |
|---|---|
| US20050159808A1 (en) | 2005-07-21 |
| US8506767B2 (en) | 2013-08-13 |
| WO2001053559A1 (en) | 2001-07-26 |
| US20010039449A1 (en) | 2001-11-08 |
| US20090183986A1 (en) | 2009-07-23 |
| US20110253525A1 (en) | 2011-10-20 |
| US6533905B2 (en) | 2003-03-18 |
| AU2001231099A1 (en) | 2001-07-31 |
| US7981258B2 (en) | 2011-07-19 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| US7981258B2 (en) | Thin-film shape memory alloy device and method | |
| US6746890B2 (en) | Three dimensional thin film devices and methods of fabrication | |
| US11045338B2 (en) | Implantable expandable medical devices having regions of differential mechanical properties and methods of making same | |
| US6855161B2 (en) | Radiopaque nitinol alloys for medical devices | |
| US8702790B2 (en) | Thermoelastic and superelastic Ni—Ti—W alloy | |
| US8273117B2 (en) | Low texture, quasi-isotropic metallic stent | |
| US20050165472A1 (en) | Radiopaque coating for biomedical devices | |
| JP4620109B2 (en) | Radiopaque coatings for biomedical devices | |
| EP1871288B1 (en) | Self-expandable and collapsible three-dimensional devices and methods | |
| MX2008005406A (en) | A method for production of a coated endovascular device |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| STCB | Information on status: application discontinuation | Free format text:ABANDONED -- FAILURE TO RESPOND TO AN OFFICE ACTION | |
| AS | Assignment | Owner name:STRYKER MEDTECH LIMITED, MALTA Free format text:NUNC PRO TUNC ASSIGNMENT;ASSIGNOR:STRYKER NV OPERATIONS LIMITED;REEL/FRAME:037153/0034 Effective date:20151013 Owner name:STRYKER EUROPEAN HOLDINGS I, LLC, MICHIGAN Free format text:NUNC PRO TUNC ASSIGNMENT;ASSIGNOR:STRYKER MEDTECH LIMITED;REEL/FRAME:037153/0241 Effective date:20151013 | |
| AS | Assignment | Owner name:STRYKER EUROPEAN HOLDINGS I, LLC, MICHIGAN Free format text:CORRECTIVE ASSIGNMENT TO CORRECT THE INCORRECT LISTED SERIAL NOS. 09/905,670 AND 07/092,079 PREVIOUSLY RECORDED AT REEL: 037153 FRAME: 0241. ASSIGNOR(S) HEREBY CONFIRMS THE NUNC PRO TUNC ASSIGNMENT EFFECTIVE DATE 9/29/2014;ASSIGNOR:STRYKER MEDTECH LIMITED;REEL/FRAME:038043/0011 Effective date:20151013 Owner name:STRYKER MEDTECH LIMITED, MALTA Free format text:CORRECTIVE ASSIGNMENT TO CORRECT THE INCORRECT SERIAL # 09/905,670 AND 07/092,079 PREVIOUSLY RECORDED AT REEL: 037153 FRAME: 0034. ASSIGNOR(S) HEREBY CONFIRMS THE NUNC PRO TUNC ASSIGNMENT;ASSIGNOR:STRYKER NV OPERATIONS LIMITED;REEL/FRAME:038039/0001 Effective date:20151013 | |
| AS | Assignment | Owner name:TINI ALLOY COMPANY, CALIFORNIA Free format text:ASSIGNMENT OF ASSIGNORS INTEREST;ASSIGNORS:JOHNSON, A. D.;MARTYNOV, VALERY V.;GUPTA, VIKAS;REEL/FRAME:039177/0551 Effective date:20010523 Owner name:NSVASCULAR, INC., CALIFORNIA Free format text:ASSIGNMENT OF ASSIGNORS INTEREST;ASSIGNOR:TINI ALLOY COMPANY;REEL/FRAME:039177/0714 Effective date:20120514 | |
| AS | Assignment | Owner name:SMART THERAPEUTICS, INC., CALIFORNIA Free format text:ASSIGNMENT OF ASSIGNORS INTEREST;ASSIGNOR:BOSE, ARANI;REEL/FRAME:039521/0917 Effective date:20010523 | |
| AS | Assignment | Owner name:STRYKER EUROPEAN OPERATIONS HOLDINGS LLC, MICHIGAN Free format text:CHANGE OF NAME;ASSIGNOR:STRYKER EUROPEAN HOLDINGS III, LLC;REEL/FRAME:052860/0716 Effective date:20190226 Owner name:STRYKER EUROPEAN HOLDINGS III, LLC, DELAWARE Free format text:NUNC PRO TUNC ASSIGNMENT;ASSIGNOR:STRYKER EUROPEAN HOLDINGS I, LLC;REEL/FRAME:052861/0001 Effective date:20200519 |