US20030118664A1 - Encapsulated inorganic antimicrobial additive for controlled release - Google Patents
Encapsulated inorganic antimicrobial additive for controlled releaseDownload PDFInfo
- Publication number
- US20030118664A1 US20030118664A1US10/032,372US3237201AUS2003118664A1US 20030118664 A1US20030118664 A1US 20030118664A1US 3237201 AUS3237201 AUS 3237201AUS 2003118664 A1US2003118664 A1US 2003118664A1
- Authority
- US
- United States
- Prior art keywords
- polymer
- microcapsule
- antimicrobial
- antimicrobial agent
- hydrophilic
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Granted
Links
Images
Classifications
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08K—Use of inorganic or non-macromolecular organic substances as compounding ingredients
- C08K9/00—Use of pretreated ingredients
- C08K9/10—Encapsulated ingredients
- A—HUMAN NECESSITIES
- A01—AGRICULTURE; FORESTRY; ANIMAL HUSBANDRY; HUNTING; TRAPPING; FISHING
- A01N—PRESERVATION OF BODIES OF HUMANS OR ANIMALS OR PLANTS OR PARTS THEREOF; BIOCIDES, e.g. AS DISINFECTANTS, AS PESTICIDES OR AS HERBICIDES; PEST REPELLANTS OR ATTRACTANTS; PLANT GROWTH REGULATORS
- A01N25/00—Biocides, pest repellants or attractants, or plant growth regulators, characterised by their forms, or by their non-active ingredients or by their methods of application, e.g. seed treatment or sequential application; Substances for reducing the noxious effect of the active ingredients to organisms other than pests
- A01N25/26—Biocides, pest repellants or attractants, or plant growth regulators, characterised by their forms, or by their non-active ingredients or by their methods of application, e.g. seed treatment or sequential application; Substances for reducing the noxious effect of the active ingredients to organisms other than pests in coated particulate form
- A—HUMAN NECESSITIES
- A01—AGRICULTURE; FORESTRY; ANIMAL HUSBANDRY; HUNTING; TRAPPING; FISHING
- A01N—PRESERVATION OF BODIES OF HUMANS OR ANIMALS OR PLANTS OR PARTS THEREOF; BIOCIDES, e.g. AS DISINFECTANTS, AS PESTICIDES OR AS HERBICIDES; PEST REPELLANTS OR ATTRACTANTS; PLANT GROWTH REGULATORS
- A01N59/00—Biocides, pest repellants or attractants, or plant growth regulators containing elements or inorganic compounds
- A01N59/16—Heavy metals; Compounds thereof
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08K—Use of inorganic or non-macromolecular organic substances as compounding ingredients
- C08K9/00—Use of pretreated ingredients
- C08K9/08—Ingredients agglomerated by treatment with a binding agent
Definitions
- This inventionrelates to antimicrobial agents that are encapsulated with a hydrophilic coating.
- a number of inorganic materialshave been shown to possess antimicrobial activity. They include metal ions such as silver, copper, zinc, mercury, tin, lead, bismuth, cadmium, chromium and thallium ions. It is theorized that these antimicrobial metal ions exert their effects by disrupting respiration and electron transport systems upon absorption into bacterial or fungal cells. Antimicrobial metal ions of silver, copper, zinc, and gold, in particular, are considered safe for in vivo use. Antimicrobial silver ions are particularly useful for in vivo uses due to the fact that they have the highest ratio of efficacy to toxicity.
- Antimicrobial zeolitescan be prepared by replacing all or part of the ion-exchangeable ions in zeolite with antimicrobial metal ions, as described in U.S. Pat. Nos. 4,911,898; 4,911,899; 4,938,955; 4,906,464; and 4,775,585.
- Zirconium compoundssuch as zirconium phosphates
- have also been modified to provide antimicrobial characteristicsas described in U.S. Pat. Nos. 4,025,608 and 4,059,679. J. Antibact. Antifung. Agents Vol. 22, No. 10, pp. 595-601, 1994 and references therein describe the antimicrobial characteristics of zirconium phosphate ceramics.
- Antimicrobial water soluble glasseshave been used and are described in U.S. Pat. No. 5,470,585.
- Antimicrobial hydroxyapatite powdershave been prepared and are described in U.S. Pat. Nos. 5,009,898 and 5,268,174.
- U.S. Pat. No. 4,775,585discloses incorporating metal-zeolite into a polymer to obtain a polymer with bactericidal activity.
- U.S. Pat. No. 4,923,450discloses incorporating zeolite in bulk materials for production of medical tubes.
- Dependent upon material selection and processing conditionswhen zeolite is conventionally compounded into polymers, the zeolite often agglomerates, causing poor dispersion of the zeolite in the polymer. When such material is molded or extruded, the surface of the polymer is frequently beaded instead of flat. Poor dispersion of the zeolite also can cause changes in the bulk properties of the polymer, such as a reduction in tensile strength.
- U.S. Pat. No. 5,094,847recognizes that in order to get the desired antibacterial activity, a large amount of zeolite powder must be added to the polyolefin resin and that this is accompanied by poorer appearance, lower physical properties and roughened surface appearance. They disclose using low levels of antimicrobial agent followed by a corona discharge treatment. This requires additional equipment and an extra processing step.
- U.S. Pat. No. 5,614,568discloses an antibacterial resin composition comprising a styrene resin, an antibacterial agent and a compound or polymer having at least one functional group and a molecular weight of 300 to 10,000. In order to obtain good antimicrobial activity, they require the use of low molecular weight compounds or low molecular weight polymers, which can have deleterious effects on properties.
- U.S. Pat. No. 6,013,275discloses a copolymer of an antibacterial agent and a hydrophilic substance. Copolymerization is an extra step, which adds to complexity and cost and limits the choice of both the hydrophilic substance and the antibacterial agent.
- the hydrophilic substancemust have functional groups capable of reacting with and copolymerizing with the antibacterial agent.
- the antibacterial agentmust have reactive groups capable of forming a copolymer with the antibacterial agent.
- WO 00/30697discloses an antimicrobial coated substrate comprising an antimicrobial coating composition coated on a substrate.
- the antimicrobial coating compositioncomprises a hydrophilic polymer having antimicrobial ceramic particles dispersed therein. They require a coating process. This requires additional equipment and an extra processing step.
- One of the problems in the prior artis the unavailability, for the most part, of that quantity of the antimicrobial agent which lies beneath the surface of the article or coating into which it is incorporated. Unless the antimicrobial agent migrates from the polymer matrix, a characteristic not common to inorganic, especially ion-exchange type, antimicrobial agents, the entombed antimicrobial agent is without utility or efficacy. This requires the use of a larger quantity of antimicrobial agents so as to provide a higher concentration at the surface, which is more costly and often imparts deleterious properties. There remains a need to provide an antimicrobial agent in a form that is suitable to impart antimicrobial properties without the accompanying problems of the prior art.
- hydrophilic coatings and polymer matricesmay mitigate, at least in part, the foregoing problem, this beneficial improvement is limited to utility in the narrow class of hydrophilic coatings and polymers and, more importantly, the very limited end use applications for which such hydrophilic coatings and polymers are appropriate.
- microcapsulescomprise an antimicrobial agent, typically in the form of a particle or particles, encapsulated within a hydrophilic polymer.
- the hydrophilic polymeris able to absorb sufficient water as to enable the action of the encapsulated antimicrobial agent.
- microcapsulesare useful to impart antimicrobial activity and can be used in polymer compositions, sprays and coatings.
- a method for preparation of the microcapsuleis provided.
- the antimicrobial agentis dispersed in a solution of the hydrophilic polymer and the mixture is added to an antisolvent to precipitate the hydrophilic polymer in the form of a microcapsule.
- the microcapsuleis prepared by reaction of monomers containing a dispersion of the antimicrobial agent.
- Another embodiment of the inventionprovides for the preparation of the microcapsules by compounding of the antimicrobial agent with the hydrophilic polymer and subsequent grinding of the compounded product to the desired particle size.
- Yet another embodiment of the inventionprovides for antimicrobial polymer compositions prepared from the microcapsules. These compositions are suitable for use as, for example, molding compositions or in coatings. Articles are prepared from or treated with, respectively, these compositions.
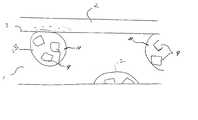
- FIGS. 1 - 3depicts a cross-sectional view of a piece of cut polymer sheet. Each depiction presumes a similar concentration and, in the case of FIGS. 1 and 2, dispersion of the antimicrobial particles.
- FIG. 1represents the prior art wherein the antimicrobial particles are dispersed throughout a non-hydrophilic polymer matrix.
- FIG. 2represents one embodiment of the present invention wherein the individual antimicrobial particles are encapsulated.
- FIG. 3represents one embodiment of the present invention wherein multiple particles of the antimicrobial agent are encapsulated in the same microcapsule.
- an antimicrobial agentis encapsulated with a hydrophilic polymer to form a microcapsule.
- the encapsulating polymerenables good dispersion of the particle and prevents agglomeration of the antimicrobial agent. Lack of good dispersion necessitates higher loading to impart antimicrobial activity and causes deleterious effects such as poor appearance or decreased physical properties.
- the hydrophilic polymercan absorb sufficient water so as to allow slow transport of the metal ion through the encapsulating polymer layer. Consequently, essentially all of the encapsulated antimicrobial agent in microcapsules touching the surface is available to be transported through the hydrophilic polymer to impart antimicrobial activity. Without this encapsulation, except in the case of hydrophilic coatings and polymers, only the antimicrobial particles at the surface are available to impart activity.
- the use of encapsulated antimicrobial agents according to the present inventionallows one to regulate, to a large extent, the rate of release of the antimicrobial agent. Generally, the rate of release increases as the water absorption of the hydrophilic polymer increases.
- the use of antimicrobial agents, especially encapsulated antimicrobial agentsallows one to regulate the overall release rate through the polymer coating or matrix. Thus, while a highly hydrophilic polymer matrix would allow for fast water transmission, a lesser hydrophilic polymer used to encapsulate the antimicrobial agent would slow the release of the antimicrobial agent.
- FIG. 1represents the prior art system.
- the non-hydrophilic polymer matrix ( 1 )has dispersed therein multiple particles of the antimicrobial agent ( 4 , 5 and 6 ). Only those particles ( 4 ) which touch or protrude through the surface ( 3 ) are able to release the antimicrobial agent or active ( 7 ) into the water film ( 2 ). Antimicrobial particles entombed within the polymer matrix ( 5 and 6 ), including those close to the surface ( 5 ), are incapable of releasing the antimicrobial active since there is no diffusion path to the surface.
- the source of efficacious antimicrobial agentis essentially limited to the surface layer of particles having a depth defined by the diameter of the particles.
- the diffusion kinetics out of those particlesis defined by the particle structure, which cannot be altered.
- the prior art systemhas no flexibility with regard to source or kinetics, the only parameters we might control.
- FIG. 2depicts the same matrix and particle dispersion as in FIG. 1 except here one embodiment of the present invention is illustrated wherein the individual antimicrobial particles are coated or encased within a hydrophilic polymer ( 8 ) to form microcapsules ( 9 and 10 ).
- the hydrophilic polymer coatingessentially increases the effective diameter of the antimicrobial particles.
- particles near the surface ( 5 ) in FIG. 1were not capable of releasing antimicrobial agent, like encapsulated particles ( 10 ) in FIG. 2 are.
- the amount and rate of release of the antimicrobial agent from the defined surface areawould be about twice that of FIG. 1, depending upon the diffusion rate through the hydrophilic coating.
- FIG. 3depicts yet another embodiment of the present invention wherein multiple antimicrobial particles ( 4 ) are contained in a single hydrophilic capsule ( 11 , 12 ) resulting in particle clusters within hydrophilic zones ( 13 ).
- particle cluster capsuleswhich do not touch the surface ( 3 ) are not available to provide antimicrobial efficacy.
- the same or more antimicrobial activesare available for providing antimicrobial activity than depicted in either of FIG. 1 or 2 . All of the particles ( 4 ) contained within the microcapsules ( 11 ) touching the surface ( 3 ) are available as a source of antimicrobial actives.
- hydrophilicwe mean water absorbing, water vapor adsorbing and wettable.
- microcapsulewe mean the antimicrobial agent encapsulated with a hydrophilic polymer.
- individual particles of antimicrobial agentcan be encapsulated (FIG. 2) or aggregates of particles of antimicrobial agent can be encapsulated (FIG. 3) to form microcapsules.
- encapsulate or the phrase encapsulated withincontemplates that the antimicrobial agent is completely surrounded or encased by hydrophilic polymer as well as that it is substantially surrounded or encased by the hydrophilic polymer.
- the antimicrobial agentin the form of a particle, may touch or protrude from the surface of the microcapsule or a surface of the antimicrobial agent, in the form of a particle, may form part of the surface of the microcapsule.
- the flakein the case of a flake, the flake may lie in the surface of the microcapsule, i.e., in the surface layer of the microcapsule, so that one entire face of the flake is in direct contact with the matrix polymer and not the encapsulating polymer.
- the rate of releasecan be tailored by proper selection of the hydrophilic polymer used as encapsulant in combination with the choice of the matrix polymer.
- the microcapsuleshould be dispersed in the matrix polymer, but remain as a second phase.
- the greater the water absorption of the hydrophilic polymer encapsulantthe greater the rate of release.
- the rate of releasecan be slowed by choice of a hydrophilic polymer encapsulant with a lower water absorption than the matrix polymer.
- any discoloration due to the antimicrobialis primarily, if not completely, limited to the microcapsule and is not throughout the entire polymer matrix. Also, since less antimicrobial agent is necessary with the microcapsule, the extent of any discoloration is less and, thus, less of a problem.
- the antimicrobial agent to be encapsulatedcontains a metal or metal ion that can impart antimicrobial activity.
- metal ionsinclude silver, copper, zinc, tin, gold, mercury, lead, iron, cobalt, nickel, manganese, arsenic, antimony, bismuth, barium, cadmium, chromium and thallium ions.
- Metal ions of silver, copper, zinc, and goldare preferred because they are considered safe for in vivo use. Silver ions are more preferred due to the fact that they have the highest ratio of efficacy to toxicity, i.e., high efficacy to low toxicity.
- the antimicrobial agentmay include or be used in conjunction with discoloration inhibiting agents and/or dopants.
- Preferred discoloration inhibiting agentsinclude, but are not limited to inorganic discoloration inhibitors such as those of various ammonium salts.
- Preferred dopantsinclude, but are not limited to inorganic salts of sodium such as sodium nitrate.
- sodium nitrateif sodium nitrate is used with a silver containing ion-exchange type antimicrobial agent, the sodium nitrate dissociates providing sodium ions which exchange with the antimicrobial silver ions, thereby releasing the silver ion for transport to the surface.
- the sodium nitrateexpedites the release of the silver from the antimicrobial agent.
- the antimicrobial agentcan be in the form of a simple salt of the antimicrobial metal such as the oxide, sulfide, chloride, bromide, carbonate, nitrate, phosphate, dihydrogen phosphate, sulfate, oxalate, acetate, benzoate, thiosulfate and the like.
- a simple salt of the antimicrobial metalsuch as the oxide, sulfide, chloride, bromide, carbonate, nitrate, phosphate, dihydrogen phosphate, sulfate, oxalate, acetate, benzoate, thiosulfate and the like.
- Specific examplesinclude silver nitrate, cupric oxide, zinc acetate and zinc oxide.
- the antimicrobial agentmay be in the form of a water soluble glass containing the antimicrobial agent or compound.
- Suitable antimicrobial water soluble glassesinclude those disclose in U.S. Pat. No. 5,470,585.
- the dissolution rates in watercan be controlled. Since their effectiveness requires dissolution in water, they are effective only at the surface where water may be present.
- the microcapsules of this inventionalleviates this problem since the hydrophilic polymer coating can enable water transport to the glass.
- the antimicrobial agentwill be in the form of an ion-exchange type ceramic particle wherein antimicrobial metal ions have been exchanged for (replaced) other non-antimicrobially effective ions in the ceramic particles or a combination of the foregoing with an antimicrobial metal salt.
- Antimicrobial ceramic particlesinclude, but are not limited to zeolites, hydroxyapatite, zirconium phosphates and other ion-exchange ceramics. Hydroxyapatite particles containing antimicrobial metals are described, e.g., in U.S. Pat. No. 5,009,898. Zirconium phosphates containing antimicrobial metals are described, e.g., in U.S. Pat.
- zirconium phosphate type particlethe antimicrobial actives are present between sheets of the zirconium phosphate and the passage of the antimicrobial metal ions is limited to the x and y directions defining the space between the sheet, not in the z direction through the sheets themselves.
- the zirconium phosphate particleis like an Oreo cookie where the antimicrobial agent is present in the filling. The point of release is at the edges open to the filling, not through the cookies.
- microcapsules of this inventionalleviates this problem since the metal can be transported through the hydrophilic encapsulant to the surface of the matrix. More preferably, antimicrobial zeolite is employed containing ion-exchanged antimicrobial metal ions.
- antimicrobial zeolite particles used in the preferred embodiment of the present inventionion-exchangeable ions present in zeolite, such as sodium ions, calcium ions, potassium ions and iron ions are partially replaced with antimicrobial metal ions.
- antimicrobial metal ionssuch as sodium ions, calcium ions, potassium ions and iron ions are partially replaced with antimicrobial metal ions.
- other ionsmay also be exchanged for better efficacy and/or color stability, including ammonium ions.
- Such ionsmay co-exist in the antimicrobial zeolite particle since they do not prevent the bactericidal effect.
- antimicrobial metal ionsinclude, but are not limited to, ions of silver, copper, zinc, gold, mercury, tin, lead, bismuth, cadmium, chromium and thallium.
- the antimicrobial metal ionsare silver, copper or zinc ions, and most preferably silver is employed. These antimicrobial metal ions may be incorporated into the zeolite by themselves or in a mixture, for example mixtures of silver and zinc ions or mixtures of silver and copper ions.
- the antimicrobial metal ionis present in the range of from about 0.1 to about 25 wt % of the zeolite based upon 100% total weight of zeolite.
- the antimicrobial metal ionis present in the range of from about 0.3 to about 20 wt % of the zeolite based upon 100% total weight of zeolite.
- the antimicrobial metal ionis present in the range of from about 2 to about 10 wt % of the zeolite based upon 100% total weight of zeolite.
- the zeolitecontains from about 0.1 to about 15 wt % of silver ions and from about 0.1 to about 15 wt % of copper and/or zinc ions.
- ammonium ionmay be contained in the zeolite at a concentration as high as about 20 wt % of the zeolite, it is desirable to limit the content of ammonium ions to about 0.5 to about 2.5 wt % of the zeolite, more preferably from about 0.5 to about 2.0 wt %, and most preferably, from 0.5 to about 1.5 wt %.
- Antimicrobial zeolitesincluding the antimicrobial zeolites disclosed in U.S. Pat. No. 4,911,898; 4,911,899 and 4,938,958, are well known and may be prepared for use in the present invention using known methods.
- Zeoliteis an aluminosilicate having a three dimensional skeletal structure that is represented by the formula: XM 2 /nO—Al 2 O 3 —YSiO 2 -ZH 2 O.
- Mrepresents an ion-exchangeable ion, generally a monovalent or divalent metal ion;
- nrepresents the atomic valency of the (metal) ion;
- X and Yrepresent coefficients of metal oxide and silica, respectively; and Z represents the number of water of crystallization.
- zeolitesexamples include A-type zeolites, X-type zeolites, Y-type zeolites, T-type zeolites, high-silica zeolites, sodalite, mordenite, analcite, clinoptilolite, chabazite and erionite.
- the present inventionis not restricted to use of these specific zeolites.
- the specific surface area of preferred zeolite particlesis preferably at least 150 m 2 /g (anhydrous zeolite as standard) and the SiO 2 /Al 2 O 3 mole ratio in the zeolite composition is preferably less than 14 and more preferably less than 11.
- the antimicrobial metal ions used in the antimicrobial zeolitesshould be retained on the zeolite particles through an ion-exchange reaction.
- Antimicrobial zeolites in which the antimicrobial metal ions are adsorbed or attached without an ion-exchange reactiontypically exhibit an overall decreased bactericidal effect and their antimicrobial effect is not long-lasting. Nevertheless, it can be advantageous for imparting quick antimicrobial action to maintain a sufficient amount of surface adsorbed metal ion in addition to the ion-exchanged metal ion.
- the antimicrobial zeolites, as well as other antimicrobial ceramic particles,may also contain a discoloration agent.
- the discoloration agentis biocompatible.
- Preferred discoloration agentsinclude, but are not limited to, inorganic discoloration inhibitors such as ammonium. More preferably, the inorganic discoloration inhibitor is an ion-exchanged ammonium ion in the zeolite.
- a preferred antimicrobial zeolite for use in the inventionis type A zeolite containing a combination of ion-exchanged silver, zinc, copper, and ammonium; silver copper and ammonium or silver and ammonium.
- One such zeoliteis distributed by AgION Technologies, L.L.C. under the product number AW-10N and consists of 0.6% by weight of silver ion-exchanged in Type A zeolite particles having a mean average diameter of about 3 p.
- Another grade, AJ-10Nconsists of about 2% by weight of silver ion-exchanged in Type A zeolite particles having a mean average diameter of about 3 p.
- AW-80contains 0.6% by weight of silver ion-exchanged in Type A zeolite particles having a mean average diameter of about 2 p.
- Another grade, AJ-80Nconsists of about 2% by weight of silver ion-exchanged in Type A zeolite particles having a mean average diameter of about 1 p.
- These specific zeolitestypically contain about 14% by weight zinc in combination with between about 0.5% and 2.5% by weight of ion-exchanged ammonium as a discoloration inhibiting agent.
- the hydrophilic polymer used to encapsulate the antimicrobial agentis a polymer that can absorb sufficient water to enable the encapsulated particle to exhibit good antimicrobial behavior, i.e., to allow for the migration and release of the antimicrobial active agent.
- the polymerwill be characterized by having water absorption at equilibrium of at least about 2% by weight measured by ASTM D570.
- the polymerwill have water absorption at equilibrium of at least about 5 % by weight. More preferably, the polymer will have water absorption at equilibrium of at least about 20% by weight.
- Especially suitable hydrophilic polymersinclude those having water contents of from about 50 and to about 150% by weight.
- Polymeric compositions for use as the encapsulant in the present inventioninclude polymers, which are comprised of substantial quantities of monomers having polar groups associated with them, such that the overall polymeric composition is rendered hydrophilic.
- the polar groupscan be incorporated into the polymer main chain as in for example polyesters, polyurethanes, polyethers or polyamides.
- the polar groupscan be pendant to the main chain as in for example, polyvinyl alcohol, polyacrylic acids or as in ionomers such as Surlyn®.
- Surlyn®is available from Dupont and is the random copolymer poly(ethylene-co-methacrylic acid) wherein some or all of the methacrylic acid units are neutralized with a suitable cation, commonly Na + or Zn +2 . While not being limited by way of theory, it is believed that the inclusion of polar groups allows water to more readily permeate the polymer and consequently, to allow slow transport of the metal ion through the encapsulating polymer layer.
- hydrophilic polymersmay be used in the present invention and include, for example, (poly)hydroxyethyl methacrylate, (poly)hydroxypropyl methacrylate, (poly)glycerol methacrylate, copolymers of hydroxyethyl methacrylate and methacrylic acid, polyacrylamide, hyaluronan, polysaccharides, polylactic acid, copolymers of lactic acid, (poly)vinyl pyrrolidone, polyamides such as Nylon 6,6 or Nylon 4,6 or Nylon 6,12, cellulosics, polyureas, polyurethanes and certain polyesters containing a high percentage (at least about 10% by weight, preferably at least about 25% by weight or more) of polyalkylene oxide.
- the hydrophilic polymermay be a copolymer containing at least a substantial amount of at least one or more of the above-mentioned hydrophilic monomers, including, for example, styrene/methacrylic acid/hydroxyethyl methacrylate copolymers, styrene/methacrylic acid/hydroxypropyl methacrylate copolymers, methylmethacrylate/methacrylic acid copolymers, ethyl methacrylate/styrene/methacrylic acid copolymers and ethyl methacrylate/methyl methacrylate/styrene/methacrylic acid copolymers, copolymers based upon the cellulosics, and copolymers which utilize vinylpyrrolidone monomers, among numerous others.
- hydrophilic polymersthat may be used in the present invention include polyvinyl acetate, polyvinyl alcohol, and copolymers of polyvinyl alcohol and polyvinylacetate, polyvinylchloride, copolymers of polyvinylacetate and polyvinylchloride and hydroxyl-modified vinyl chloride/vinyl acetate copolymers.
- Polyurethanes containing a high percentage (at least about 10% by weight, preferably at least about 25% by weight or more) of polyalkylene oxideare especially useful in this invention.
- the hydrophilic polymeris chosen from polyhydroxyethyl methacrylate, polyacrylamide, polyvinylpyrrolidinone, polyurea, polysaccharides, polylactic acid and polyurethane. More preferably, the hydrophilic polymer is hydrophilic polyurethane, such as the TECOPHILIC® polyurethane sold by Thermedics of Woburn, Mass.
- the microcapsulehas a coating thickness of up to 15 ⁇ of hydrophilic polymer on the antimicrobial agent, preferably a coating thickness of 1 to 10 ⁇ . Thicker coatings could be used efficaciously; however, concern must then be given to the impact, if any, of the presence of the hydrophilic polymer on the properties of the matrix resin into which the encapsulated particles are to be incorporated.
- the microcapsulesmay have a mean average diameter of up to and over 2000 ⁇ , but not likely over 3000 ⁇ . Generally the microcapsules will have a mean average diameter of from about 15 to about 1000 ⁇ , preferably from about 50 to about 300 ⁇ , most preferably from about 90 to about 200 ⁇ . Of course smaller or larger microcapsules can be used. However, the smaller the microcapsule the more closely one approaches a system of individually encapsulated particles.
- microcapsule size and shapeis somewhat dependent upon the size and shape of the antimicrobial particles to be incorporated therein as well as the process by which the microcapsules are made.
- the microcapsules made in accordance with the present inventionwill be of a generally spherical or elliptical shape, although other shapes such as flakes and fibers are also contemplated.
- the aspect ratio of these microcapsuleswill be from 1 to about 4, preferably from 1 to about 2, most preferably from 1 to about 1.5. Depending upon the method of manufacture, in most instances the aspect ration will be about 1.
- the term aspect ratiorefers to the quotient of the largest dimension divided by the smallest dimension of the particle: the largest dimension being the mean average diameter of the particle in the case of spherical/-elliptical particles and, vice-versa, the mean average diameter being the largest dimension in the case of non-spherical and non-elliptical particles.
- high aspect ratio microcapsulesthose up to 100 or more, but typically up to 30, are also useful and provide certain added benefits, in certain applications, as compared to the lower aspect ratios within the ranges set forth above.
- the microcapsulemay be prepared by dissolving the hydrophilic polymer in a suitable solvent. Preferably this is done to make a solution that contains from about 1% to about 75% by weight of the hydrophilic polymer. More preferably, the solution contains from about 2% to about 40% by weight of the hydrophilic polymer and most preferably, the solution contains from about 5% to about 30% by weight of the hydrophilic polymer. Solutions that are more dilute than 1%, while useful in this invention, require handling larger levels of solvent. Solutions that are more concentrated than about 75% often are quite viscous and present some difficulties in handling.
- solventThe choice of solvent will vary based upon the particular hydrophilic polymer as well as the end use application for the antimicrobial composition. It should be a solvent or solvent mixture that dissolves the hydrophilic polymer and one in which the antibacterial agent is mainly insoluble. For those applications in which residual solvents can pose problems, efforts should be taken to select more compatible or appropriate solvents and/or ensure that the solvent is completely driven from the microcapsules.
- Preferred solventsinclude tetrahydrofuran, dimethylacetamide, methylethylketone, and mixtures thereof.
- the antimicrobial agentis added to the solution of the hydrophilic polymer, preferably with agitation.
- the amount of antimicrobial agentcan be varied.
- the antimicrobial agentis added at 1 to 1000 parts by weight per 100 parts by weight of the hydrophilic polymer. More preferably, the antimicrobial agent is added at 10 to 200 parts by weight per 100 parts by weight of the hydrophilic polymer. Most preferably, the antimicrobial agent is added at 20 to 100 parts by weight per 100 parts by weight of the hydrophilic polymer. If the amount of antimicrobial agent added is too low, then the resultant microcapsules may have lower and shorter lived antimicrobial activity.
- the level of incorporationis dependent upon whether the individual antimicrobial particles are to be encapsulated or microcapsules containing multiple antimicrobial particles are to be prepared. Additional factors affecting the level of incorporation include the particle size of the antimicrobial agent and the “loading capacity” of the hydrophilic polymer encapsulant. By loading capacity is meant the ability of the polymer to incorporate the antimicrobial particles without compromising too much the integrity of the microcapsule, i.e., there must be sufficient hydrophilic polymer to create a microcapsule. Generally speaking, these same factors and considerations pertain to all methods for the preparation of the microcapsules according to the present invention.
- This mixture of the antimicrobial agent in the hydrophilic polymer solutionis then added to an antisolvent, preferably with agitation, to precipitate the microcapsules.
- the antisolventis a solvent or solvent mixture that is miscible with the solvent chosen to dissolve the polymer. Further the antisolvent is chosen such that the hydrophilic polymer is substantially insoluble in the antisolvent. It is preferable that the antisolvent has a boiling point below 150° C. so that residual amounts can be easily removed from the microcapsule by drying.
- the amount of antisolventcan vary, but should be enough to precipitate the microcapsules. Preferably the amount of antisolvent used is from 50 to 2000 parts based upon 100 parts of solvent. Smaller amounts are often insufficient. Larger amounts are not necessary and add to the cost.
- the microcapsulesare separated from the liquid and dried.
- the drying temperatureis chosen such that it is low enough to have little affect on the hydrophilic polymer and high enough to remove residual solvent.
- the particular conditionswill vary based upon the polymer, but temperatures from 20-100° C. are common.
- vacuummay be employed in the drying step.
- precipitation conditionssuch as stirring speed, rate of precipitation, choice of antisolvent and amount of antisolvent, individually encapsulated particles as well as microcapsules having a varied amount of individual particles and/or agglomerates of particles within the encapsulant can be prepared.
- Optimum conditionswill vary dependent upon the particular antimicrobial agent used, the hydrophilic polymer and the desired end use.
- the selection of solvent and antisolventmust be such as not to adversely affect the efficacy of the antimicrobial agent.
- the microcapsulesmay be prepared by treating the antimicrobial agent with a polymer precursor and then subsequent polymerization to prepare the hydrophilic polymer.
- the antimicrobial agentis mixed with a polymer precursor, optionally with a solvent, to obtain an antimicrobial agent coated with the polymer precursor.
- the polymer precursorcan be a simple polymer precursor such as ethylene glycol, hydroxyethylmethacrylate or 1,6-diaminohexane.
- the polymer precursoris oligomeric or polymeric such as a polyol, polyamine, polyether diol, polyether diamine or polyalcohol amine.
- the subsequent polymerizationcan be effected by treatment with a reactive ingredient such as a diisocyanate.
- the subsequent polymerizationcan be done by different techniques.
- One preferred methodinvolves suspension of the antimicrobial agent coated with the polymer precursor in a fluidized bed and addition of a reactive ingredient such as a diisocyanate.
- a reactive ingredientsuch as a diisocyanate.
- reaction conditionssuch as stirring speed, temperature, and choice of polymer precursors, individually encapsulated particles as well as microcapsules having a varied amount of individual particles and/or agglomerates of particles within the encapsulant can be prepared.
- Optimum conditionswill vary dependent upon the particular antimicrobial agent used, the hydrophilic polymer and the desired end use.
- Another method for preparation of the microcapsulesis the compounding of the antimicrobial agent with the hydrophilic polymer and subsequent grinding of the compounded product to the desired particle size. This can be done by mixing the antimicrobial agent with the hydrophilic polymer to obtain a blend. The blend is then melt compounded to obtain solid particles, which can then be ground to give the microcapsules. The melt compounding can be done by several methods such as with an extruder or kneader to yield a compounded product, preferably in the form of pellets. The optimum conditions will vary based upon the choice of the hydrophilic polymer. The compounded product can be ground to give the microcapsules of the desired particle size.
- the microcapsulehas a mean average particle size of 15 to 1000 ⁇ , more preferably an average particle size of 50-300 ⁇ , most preferably an average particle size of 90-200 ⁇ .
- This methodtends to give agglomerates of antimicrobial agent encapsulated with the hydrophilic polymer as the microcapsules.
- Dependent upon the hydrophilic polymerit may be preferable to cryogenically grind the compounded product.
- the amount of antimicrobial agent encapsulated with the hydrophilic polymeris an amount that is effective to form a microcapsule with good antimicrobial activity.
- the microcapsulespreferably contains from about 1 to about 1000 parts by weight of antimicrobial agent per 100 parts by weight of hydrophilic polymer. More preferably, the microcapsules contain from about 10 to about 200 parts by weight of antimicrobial agent per 100 parts by weight of hydrophilic polymer and, most preferably, from about 20 to about 100 parts by weight of antimicrobial agent per 100 parts by weight of hydrophilic polymer.
- the microcapsulescan be used to impart antimicrobial properties to a variety of compositions. They may be blended into various formulations to provide compositions useful for molding, coatings or films with antimicrobial activity. They are particularly useful when blended with polymer formulations to provide compositions useful for molding articles with antimicrobial properties.
- the combination of hydrophilic polymer encapsulant and matrix polymershould be such that they have a different level of hydrophilicity and such that the microcapsule remains as a second phase in the matrix polymer.
- the matrix polymer formulationsmay be based upon either hydrophilic or non-hydrophilic polymers including, but not limited to: polypropylene, polyethylene, polystyrene, ABS, SAN, polybutylene terephthalate, polyethylene terephthalate, nylon 6, nylon 6,6, nylon 4,6, nylon 12, phenolic resins, urea resins, epoxy resins, polyvinylchloride, polyurethanes, silicone polymers, polycarbonates, polyphenylene ethers, polyamides, polyethylene vinylacetate, polyethylene ethyl acrylate, polylactic acid, polysaccharides, polytetrafluoroethylene, polyimides, polysulfones, and a variety of other polymers and copolymers.
- hydrophilic or non-hydrophilic polymersincluding, but not limited to: polypropylene, polyethylene, polystyrene, ABS, SAN, polybutylene terephthalate, polyethylene terephthalate, nylon 6, nylon 6,6, nylon 4,
- the antimicrobial polymer and polymer coating compositions made in accordance with the practice of the present inventionmay be used in any applications where antimicrobial properties are desirable.
- Exemplary applicationsinclude cutting boards, catheters and other medical devices, pipes, containers, toothbrushes, diapers, air filters, appliances, conveying belts, bottles, liquid dispensers, faucets, humidifiers, air conditioners, mats, razors, and bandages.
- Coatings comprising the microcapsulemay be in liquid or solution form or may be in the form of a powder coating. In the latter instance, the microcapsule may be dry blended with another polymer resin powder which is then used to powder coat a substrate.
- the antimicrobial properties of the articles made from the microcapsules of the inventionshow efficacy for the end use applications.
- the degree of efficacymay be determined by any of several tests such as the Dow shaker test, direct inoculation and several others known to those skilled in the art and chosen based upon the end use application.
- a polymer blendwas made by first compounding an antimicrobial agent with a nonhydrophilic polymer and then using that mixture in a polymer formulation.
- Twenty parts by weight of AgION AJ10D(about 2.5% by weight of silver ion-exchanged in Type A zeolite particles having a mean average diameter of about 3.) was gravimetrically fed with 80 parts by weight of LDPE resin, extruded, pelletized and ground to give particles.
- a mixture of 25 parts by weight of these particles combined with 75 parts by weight of LDPEwas blended by shaking and injection molded into 5 cm by 5 cm by 0.16 cm test parts.
- the injection molding temperature conditionswere 385° F. rear zone; 390° F. front zone and 395° F. nozzle temperature. Analysis of the test parts was done in duplicate by ashing the sample and showed a measured loading of AgION powder of 4.8% and 4.5% by weight. No other inorganic materials were present in the sample.
- a polymer blendwas made by first compounding an antimicrobial agent with a hydrophilic polymer and then using the microcapsule in a polymer formulation. Fifty parts by weight of AgION AJ10D (about 2.5% by weight of silver ion-exchanged in Type A zeolite particles having a mean average diameter of about 3.) was gravimetrically fed with 50 parts by weight of Tecophilic® 60 resin, a hydrophilic polyurethane available from Thermedics Inc. having a moisture absorption of about 60%. The blend was extruded using a Leistritz 27 mm twin screw extruder, pelletized, dried and cryogenically ground. Sieving was performed with a 100 mesh screen to remove large particles.
- Extraction experimentswere performed on the test parts from examples 1-3 to determine the total amount of silver accessible from the samples. This was accomplished using the basic approach of serial extraction. The method involves placing the samples in a test solution and changing it periodically, measuring the volume and Ag concentration each time to determine the amount of silver removed with each cycle. The extraction experiment was performed in duplicate so six samples total were tested. The samples were placed in plastic beakers containing 40 ml of extraction solution (0.8% NaNO3). To keep the samples from floating and only contacting the solution on one side, the beakers were crimped from the sides, pinning the coupons to the bottom of the beaker. To ensure even release, the beakers were placed on a rocking table to agitate during the release time. The samples soaked for at least 24 hours between solution changes.
- Example 2delivers more silver (12.1 micrograms on average) than the formulation of Comparative Example 1 (8.9 micrograms on average). This is true despite the fact that the formulation of Example 2 has about half of the loading of antimicrobial agent.
- Example 3delivers remarkably more (25.5 micrograms) silver than the formulation of Comparative Example 1 (8.9 micrograms). This is true despite the fact that they have similar loadings of antimicrobial agent. That half the loading gives slightly more silver and therefore slightly better antimicrobial activity and that similar loadings give remarkably greater silver and therefore remarkably greater antimicrobial activity is unexpected and shows the advantage of using microcapsules.
- a test partwas prepared as in Comparative Example 1. Analysis by ashing the sample showed a powder loading of 5.0% by weight. No other inorganic materials were present in the sample. The surface was analyzed for surface exposure on the SEM using image analysis. The voltage used was 10 keV, chosen to detect only the immediate surface particles. Ten measurements were done and averaged to give a value of 0.9% by area of powder at the surface.
- a test partwas prepared as in Example 3. Analysis by ashing the sample showed a powder loading of 4.8%. No other inorganic materials were present in the sample. The surface was analyzed as in Comparative Example 4 and an average value of 1.3% by area of powder at the surface was obtained. This result shows that the article has a higher level of antimicrobial agent at the surface, where it is needed, than the prior art formulation of Comparative Example 4, despite the fact that the overall loading is the same.
- Example 5also demonstrates another beneficial and surprising phenomenon of the present invention which is manifested by an enhancement in the amount of antimicrobial agent at the surface of parts molded from polymer compositions containing the microcapsules made in accordance with the teaching of the present invention. Specifically, by controlled selection of the hydrophilic polymer encapsulant and matrix polymer as well as the processing conditions for molding parts from such a formulation, one is able to generate a smearing of the microcapsule along the surface of the molded part.
- microcapsules of the inventionare exceptionally suitable for use in various compositions and provide compositions without the deleterious effects of the prior art.
Landscapes
- Life Sciences & Earth Sciences (AREA)
- Health & Medical Sciences (AREA)
- Chemical & Material Sciences (AREA)
- General Health & Medical Sciences (AREA)
- Wood Science & Technology (AREA)
- Organic Chemistry (AREA)
- Pest Control & Pesticides (AREA)
- Engineering & Computer Science (AREA)
- Dentistry (AREA)
- Plant Pathology (AREA)
- Agronomy & Crop Science (AREA)
- Zoology (AREA)
- Environmental Sciences (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Medicinal Chemistry (AREA)
- Polymers & Plastics (AREA)
- Inorganic Chemistry (AREA)
- Toxicology (AREA)
- Agricultural Chemicals And Associated Chemicals (AREA)
- Materials For Medical Uses (AREA)
- Manufacturing Of Micro-Capsules (AREA)
Abstract
Description
- This invention relates to antimicrobial agents that are encapsulated with a hydrophilic coating.[0001]
- A number of inorganic materials have been shown to possess antimicrobial activity. They include metal ions such as silver, copper, zinc, mercury, tin, lead, bismuth, cadmium, chromium and thallium ions. It is theorized that these antimicrobial metal ions exert their effects by disrupting respiration and electron transport systems upon absorption into bacterial or fungal cells. Antimicrobial metal ions of silver, copper, zinc, and gold, in particular, are considered safe for in vivo use. Antimicrobial silver ions are particularly useful for in vivo uses due to the fact that they have the highest ratio of efficacy to toxicity.[0002]
- Antimicrobial zeolites can be prepared by replacing all or part of the ion-exchangeable ions in zeolite with antimicrobial metal ions, as described in U.S. Pat. Nos. 4,911,898; 4,911,899; 4,938,955; 4,906,464; and 4,775,585.[0003]
- Zirconium compounds, such as zirconium phosphates, have also been modified to provide antimicrobial characteristics, as described in U.S. Pat. Nos. 4,025,608 and 4,059,679. J. Antibact. Antifung. Agents Vol. 22, No. 10, pp. 595-601, 1994 and references therein describe the antimicrobial characteristics of zirconium phosphate ceramics.[0004]
- Antimicrobial water soluble glasses have been used and are described in U.S. Pat. No. 5,470,585.[0005]
- Antimicrobial hydroxyapatite powders have been prepared and are described in U.S. Pat. Nos. 5,009,898 and 5,268,174.[0006]
- U.S. Pat. No. 4,775,585 discloses incorporating metal-zeolite into a polymer to obtain a polymer with bactericidal activity. U.S. Pat. No. 4,923,450 discloses incorporating zeolite in bulk materials for production of medical tubes. Dependent upon material selection and processing conditions, when zeolite is conventionally compounded into polymers, the zeolite often agglomerates, causing poor dispersion of the zeolite in the polymer. When such material is molded or extruded, the surface of the polymer is frequently beaded instead of flat. Poor dispersion of the zeolite also can cause changes in the bulk properties of the polymer, such as a reduction in tensile strength.[0007]
- Furthermore, it has been found by the present inventors that conventionally compounding antimicrobial zeolites in many polymeric materials, dependent upon processing conditions and the particular polymer, can result in discoloration. This appears also to result from inadequate dispersion of the zeolite, i.e., the formation of zeolite aggregates in the material as well as from chemical reactions involving the antimicrobial metal ions and the polymer itself, e.g., additives, contaminants, residual catalysts, moisture, etc. in the polymer, and/or any air or water introduced during the compounding process.[0008]
- In certain instances, these problems can be avoided by use of antimicrobial coatings. However, this requires an extra processing step and raises additional problems such as adherence and permanence of the coating.[0009]
- U.S. Pat. No. 5,094,847 recognizes that in order to get the desired antibacterial activity, a large amount of zeolite powder must be added to the polyolefin resin and that this is accompanied by poorer appearance, lower physical properties and roughened surface appearance. They disclose using low levels of antimicrobial agent followed by a corona discharge treatment. This requires additional equipment and an extra processing step.[0010]
- U.S. Pat. No. 5,614,568 discloses an antibacterial resin composition comprising a styrene resin, an antibacterial agent and a compound or polymer having at least one functional group and a molecular weight of 300 to 10,000. In order to obtain good antimicrobial activity, they require the use of low molecular weight compounds or low molecular weight polymers, which can have deleterious effects on properties.[0011]
- U.S. Pat. No. 6,013,275 discloses a copolymer of an antibacterial agent and a hydrophilic substance. Copolymerization is an extra step, which adds to complexity and cost and limits the choice of both the hydrophilic substance and the antibacterial agent. The hydrophilic substance must have functional groups capable of reacting with and copolymerizing with the antibacterial agent. Similarly, the antibacterial agent must have reactive groups capable of forming a copolymer with the antibacterial agent.[0012]
- WO 00/30697 discloses an antimicrobial coated substrate comprising an antimicrobial coating composition coated on a substrate. The antimicrobial coating composition comprises a hydrophilic polymer having antimicrobial ceramic particles dispersed therein. They require a coating process. This requires additional equipment and an extra processing step.[0013]
- One of the problems in the prior art is the unavailability, for the most part, of that quantity of the antimicrobial agent which lies beneath the surface of the article or coating into which it is incorporated. Unless the antimicrobial agent migrates from the polymer matrix, a characteristic not common to inorganic, especially ion-exchange type, antimicrobial agents, the entombed antimicrobial agent is without utility or efficacy. This requires the use of a larger quantity of antimicrobial agents so as to provide a higher concentration at the surface, which is more costly and often imparts deleterious properties. There remains a need to provide an antimicrobial agent in a form that is suitable to impart antimicrobial properties without the accompanying problems of the prior art.[0014]
- Another problem in the prior art is that while the use of hydrophilic coatings and polymer matrices may mitigate, at least in part, the foregoing problem, this beneficial improvement is limited to utility in the narrow class of hydrophilic coatings and polymers and, more importantly, the very limited end use applications for which such hydrophilic coatings and polymers are appropriate. Thus, there is also a need in the art to find a means by which antimicrobial agent entombed within a polymer coating or matrix can be accessed or available for providing antimicrobial efficacy regardless of polymer comprising the coating or matrix.[0015]
- Yet another problem in the prior art is that the rate of release of the antimicrobial agent is determined by solubility of the antimicrobial agent or, in the case of the ion-exchange type antimicrobial agents, the ion-exchange rate of the ceramic carrier and the water exposure or flow across the surface containing the antimicrobial agent. In high moisture, especially high flow environments, for example, a dishwasher interior, a high solubility or ion-exchange rate may lead to a premature depletion of the antimicrobial agent; thus, greatly reducing the life time of the antimicrobial efficacy. Thus, there is also a need in the art to be able to control the release rate of the antimicrobial agent.[0016]
- This invention provides for microcapsules. These microcapsules comprise an antimicrobial agent, typically in the form of a particle or particles, encapsulated within a hydrophilic polymer. The hydrophilic polymer is able to absorb sufficient water as to enable the action of the encapsulated antimicrobial agent. These microcapsules are useful to impart antimicrobial activity and can be used in polymer compositions, sprays and coatings.[0017]
- Also a method is provided for preparation of the microcapsule. The antimicrobial agent is dispersed in a solution of the hydrophilic polymer and the mixture is added to an antisolvent to precipitate the hydrophilic polymer in the form of a microcapsule.[0018]
- In another embodiment, the microcapsule is prepared by reaction of monomers containing a dispersion of the antimicrobial agent.[0019]
- Another embodiment of the invention provides for the preparation of the microcapsules by compounding of the antimicrobial agent with the hydrophilic polymer and subsequent grinding of the compounded product to the desired particle size.[0020]
- Yet another embodiment of the invention provides for antimicrobial polymer compositions prepared from the microcapsules. These compositions are suitable for use as, for example, molding compositions or in coatings. Articles are prepared from or treated with, respectively, these compositions.[0021]
- Each of FIGS.[0022]1-3 depicts a cross-sectional view of a piece of cut polymer sheet. Each depiction presumes a similar concentration and, in the case of FIGS. 1 and 2, dispersion of the antimicrobial particles.
- FIG. 1 represents the prior art wherein the antimicrobial particles are dispersed throughout a non-hydrophilic polymer matrix.[0023]
- FIG. 2 represents one embodiment of the present invention wherein the individual antimicrobial particles are encapsulated.[0024]
- FIG. 3 represents one embodiment of the present invention wherein multiple particles of the antimicrobial agent are encapsulated in the same microcapsule.[0025]
- All patent applications, patents, patent publications, and literature references cited in this specification are hereby incorporated by reference in their entirety. In the case of inconsistencies, the present description, including definitions, is intended to control.[0026]
- According to the present invention, an antimicrobial agent is encapsulated with a hydrophilic polymer to form a microcapsule. While not wanting to be bound by theory, we believe that the encapsulating polymer enables good dispersion of the particle and prevents agglomeration of the antimicrobial agent. Lack of good dispersion necessitates higher loading to impart antimicrobial activity and causes deleterious effects such as poor appearance or decreased physical properties. We believe that the hydrophilic polymer can absorb sufficient water so as to allow slow transport of the metal ion through the encapsulating polymer layer. Consequently, essentially all of the encapsulated antimicrobial agent in microcapsules touching the surface is available to be transported through the hydrophilic polymer to impart antimicrobial activity. Without this encapsulation, except in the case of hydrophilic coatings and polymers, only the antimicrobial particles at the surface are available to impart activity.[0027]
- Additionally, the use of encapsulated antimicrobial agents according to the present invention allows one to regulate, to a large extent, the rate of release of the antimicrobial agent. Generally, the rate of release increases as the water absorption of the hydrophilic polymer increases. When used in hydrophilic coatings and polymers, the use of antimicrobial agents, especially encapsulated antimicrobial agents, allows one to regulate the overall release rate through the polymer coating or matrix. Thus, while a highly hydrophilic polymer matrix would allow for fast water transmission, a lesser hydrophilic polymer used to encapsulate the antimicrobial agent would slow the release of the antimicrobial agent.[0028]
- Again, while not wanting to be bound by theory, our understanding can be illustrated by reference to FIGS.[0029]1-3. FIG. 1 represents the prior art system. The non-hydrophilic polymer matrix (1) has dispersed therein multiple particles of the antimicrobial agent (4,5 and6). Only those particles (4) which touch or protrude through the surface (3) are able to release the antimicrobial agent or active (7) into the water film (2). Antimicrobial particles entombed within the polymer matrix (5 and6), including those close to the surface (5), are incapable of releasing the antimicrobial active since there is no diffusion path to the surface. If water could diffuse through the polymer matrix, it could reach the subsurface particles and access the antimicrobial there. Thus, despite the large deposit of antimicrobial agent in the polymer matrix, the source of efficacious antimicrobial agent is essentially limited to the surface layer of particles having a depth defined by the diameter of the particles. The diffusion kinetics out of those particles is defined by the particle structure, which cannot be altered. The prior art system has no flexibility with regard to source or kinetics, the only parameters we might control.
- FIG. 2 depicts the same matrix and particle dispersion as in FIG. 1 except here one embodiment of the present invention is illustrated wherein the individual antimicrobial particles are coated or encased within a hydrophilic polymer ([0030]8) to form microcapsules (9 and10). The hydrophilic polymer coating essentially increases the effective diameter of the antimicrobial particles. Thus, whereas particles near the surface (5) in FIG. 1 were not capable of releasing antimicrobial agent, like encapsulated particles (10) in FIG. 2 are. In the depiction shown in FIG. 2, the amount and rate of release of the antimicrobial agent from the defined surface area would be about twice that of FIG. 1, depending upon the diffusion rate through the hydrophilic coating.
- FIG. 3 depicts yet another embodiment of the present invention wherein multiple antimicrobial particles ([0031]4) are contained in a single hydrophilic capsule (11,12) resulting in particle clusters within hydrophilic zones (13). Here, as with the prior art systems as well as the systems shown in the first embodiment, particle cluster capsules which do not touch the surface (3) are not available to provide antimicrobial efficacy. On the other hand, despite the lower concentration of antimicrobial particles depicted in FIG. 3, the same or more antimicrobial actives are available for providing antimicrobial activity than depicted in either of FIG. 1 or2. All of the particles (4) contained within the microcapsules (11) touching the surface (3) are available as a source of antimicrobial actives.
- By the term hydrophilic, we mean water absorbing, water vapor adsorbing and wettable. By the term microcapsule, we mean the antimicrobial agent encapsulated with a hydrophilic polymer. Dependent upon the method used and the desired end use, individual particles of antimicrobial agent can be encapsulated (FIG. 2) or aggregates of particles of antimicrobial agent can be encapsulated (FIG. 3) to form microcapsules. Furthermore, the term encapsulate or the phrase encapsulated within contemplates that the antimicrobial agent is completely surrounded or encased by hydrophilic polymer as well as that it is substantially surrounded or encased by the hydrophilic polymer. As such, in the latter instance, the antimicrobial agent, in the form of a particle, may touch or protrude from the surface of the microcapsule or a surface of the antimicrobial agent, in the form of a particle, may form part of the surface of the microcapsule. For example, in the case of a flake, the flake may lie in the surface of the microcapsule, i.e., in the surface layer of the microcapsule, so that one entire face of the flake is in direct contact with the matrix polymer and not the encapsulating polymer.[0032]
- Dependent upon the application, the rate of release can be tailored by proper selection of the hydrophilic polymer used as encapsulant in combination with the choice of the matrix polymer. The microcapsule should be dispersed in the matrix polymer, but remain as a second phase. Generally, the greater the water absorption of the hydrophilic polymer encapsulant, the greater the rate of release. For a polymer matrix that is highly hydrophilic, the rate of release can be slowed by choice of a hydrophilic polymer encapsulant with a lower water absorption than the matrix polymer.[0033]
- Dependent upon the polymer and the processing conditions, prior art antimicrobial agents can cause discolorations. By use of the microcapsules of this invention, any discoloration due to the antimicrobial is primarily, if not completely, limited to the microcapsule and is not throughout the entire polymer matrix. Also, since less antimicrobial agent is necessary with the microcapsule, the extent of any discoloration is less and, thus, less of a problem.[0034]
- The antimicrobial agent to be encapsulated contains a metal or metal ion that can impart antimicrobial activity. Examples of such metal ions include silver, copper, zinc, tin, gold, mercury, lead, iron, cobalt, nickel, manganese, arsenic, antimony, bismuth, barium, cadmium, chromium and thallium ions. Metal ions of silver, copper, zinc, and gold are preferred because they are considered safe for in vivo use. Silver ions are more preferred due to the fact that they have the highest ratio of efficacy to toxicity, i.e., high efficacy to low toxicity.[0035]
- In addition to the metal or metal ion that imparts antimicrobial activity, optionally the antimicrobial agent may include or be used in conjunction with discoloration inhibiting agents and/or dopants. Preferred discoloration inhibiting agents include, but are not limited to inorganic discoloration inhibitors such as those of various ammonium salts. Dopants, which are particularly of use with the ion-exchange type antimicrobial agents, aid in the transport of the antimicrobial metal ion. These dopants provide a ready source of cations which exchange with and replace the antimicrobial silver metal ions in the ion-exchange ceramic particles, thereby facilitating release and transport of the silver ion. Preferred dopants include, but are not limited to inorganic salts of sodium such as sodium nitrate. For example, if sodium nitrate is used with a silver containing ion-exchange type antimicrobial agent, the sodium nitrate dissociates providing sodium ions which exchange with the antimicrobial silver ions, thereby releasing the silver ion for transport to the surface. In this example, the sodium nitrate expedites the release of the silver from the antimicrobial agent.[0036]
- The antimicrobial agent can be in the form of a simple salt of the antimicrobial metal such as the oxide, sulfide, chloride, bromide, carbonate, nitrate, phosphate, dihydrogen phosphate, sulfate, oxalate, acetate, benzoate, thiosulfate and the like. Specific examples include silver nitrate, cupric oxide, zinc acetate and zinc oxide.[0037]
- Alternatively, the antimicrobial agent may be in the form of a water soluble glass containing the antimicrobial agent or compound. Suitable antimicrobial water soluble glasses include those disclose in U.S. Pat. No. 5,470,585. By suitable adjustment of the glass composition, the dissolution rates in water can be controlled. Since their effectiveness requires dissolution in water, they are effective only at the surface where water may be present. The microcapsules of this invention, alleviates this problem since the hydrophilic polymer coating can enable water transport to the glass.[0038]
- Preferably, the antimicrobial agent will be in the form of an ion-exchange type ceramic particle wherein antimicrobial metal ions have been exchanged for (replaced) other non-antimicrobially effective ions in the ceramic particles or a combination of the foregoing with an antimicrobial metal salt. Antimicrobial ceramic particles include, but are not limited to zeolites, hydroxyapatite, zirconium phosphates and other ion-exchange ceramics. Hydroxyapatite particles containing antimicrobial metals are described, e.g., in U.S. Pat. No. 5,009,898. Zirconium phosphates containing antimicrobial metals are described, e.g., in U.S. Pat. Nos. 5,296,238; 5,441,717 and 5,405,644. Because of the two dimensional network structure of zirconium phosphates, delivery of the antimicrobial metal to the surface can be especially difficult. In a zirconium phosphate type particle, the antimicrobial actives are present between sheets of the zirconium phosphate and the passage of the antimicrobial metal ions is limited to the x and y directions defining the space between the sheet, not in the z direction through the sheets themselves. In essence, the zirconium phosphate particle is like an Oreo cookie where the antimicrobial agent is present in the filling. The point of release is at the edges open to the filling, not through the cookies. It is believed that this phenomenon results in somewhat lower efficacy for the zirconium phosphates versus those ion-exchange type antimicrobial particles which release three dimensionally. In this case even zirconium phosphate particles which touch the surface but do not have an open edge touching the surface would not be available to release antimicrobial metal ions. Thus, the use of microcapsules of this invention alleviates this problem since the metal can be transported through the hydrophilic encapsulant to the surface of the matrix. More preferably, antimicrobial zeolite is employed containing ion-exchanged antimicrobial metal ions.[0039]
- In antimicrobial zeolite particles used in the preferred embodiment of the present invention, ion-exchangeable ions present in zeolite, such as sodium ions, calcium ions, potassium ions and iron ions are partially replaced with antimicrobial metal ions. Optionally, other ions may also be exchanged for better efficacy and/or color stability, including ammonium ions. Such ions may co-exist in the antimicrobial zeolite particle since they do not prevent the bactericidal effect. Examples of antimicrobial metal ions include, but are not limited to, ions of silver, copper, zinc, gold, mercury, tin, lead, bismuth, cadmium, chromium and thallium. Preferably, the antimicrobial metal ions are silver, copper or zinc ions, and most preferably silver is employed. These antimicrobial metal ions may be incorporated into the zeolite by themselves or in a mixture, for example mixtures of silver and zinc ions or mixtures of silver and copper ions.[0040]
- The antimicrobial metal ion is present in the range of from about 0.1 to about 25 wt % of the zeolite based upon 100% total weight of zeolite. Preferably, the antimicrobial metal ion is present in the range of from about 0.3 to about 20 wt % of the zeolite based upon 100% total weight of zeolite. Most preferably, the antimicrobial metal ion is present in the range of from about 2 to about 10 wt % of the zeolite based upon 100% total weight of zeolite. In one embodiment, the zeolite contains from about 0.1 to about 15 wt % of silver ions and from about 0.1 to about 15 wt % of copper and/or zinc ions. Although ammonium ion may be contained in the zeolite at a concentration as high as about 20 wt % of the zeolite, it is desirable to limit the content of ammonium ions to about 0.5 to about 2.5 wt % of the zeolite, more preferably from about 0.5 to about 2.0 wt %, and most preferably, from 0.5 to about 1.5 wt %.[0041]
- Antimicrobial zeolites, including the antimicrobial zeolites disclosed in U.S. Pat. No. 4,911,898; 4,911,899 and 4,938,958, are well known and may be prepared for use in the present invention using known methods.[0042]
- Either natural zeolites or synthetic zeolites may be used to prepare the antimicrobial zeolites used in the present invention. “Zeolite” is an aluminosilicate having a three dimensional skeletal structure that is represented by the formula: XM[0043]2/nO—Al2O3—YSiO2-ZH2O. M represents an ion-exchangeable ion, generally a monovalent or divalent metal ion; n represents the atomic valency of the (metal) ion; X and Y represent coefficients of metal oxide and silica, respectively; and Z represents the number of water of crystallization. Examples of such zeolites include A-type zeolites, X-type zeolites, Y-type zeolites, T-type zeolites, high-silica zeolites, sodalite, mordenite, analcite, clinoptilolite, chabazite and erionite. The present invention is not restricted to use of these specific zeolites.
- The ion-exchange capacities of these zeolites are as follows: A-type zeolite=7 meq/g; X-type zeolite=6.4 meq/g; Y-type zeolite=5 meq/g; T-type zeolite=3.4 meq/g; sodalite=11.5 meq/g; mordenite=2.6 meq/g; analcite =[0044]5 meq/g; clinoptilolite=2.6 meq/g; chabazite=5 meq/g; and erionite=3.8 meq/g. These ion-exchange capacities are sufficient for the zeolites to undergo ion-exchange with ammonium and antimicrobial metal ions.
- The specific surface area of preferred zeolite particles is preferably at least 150 m[0045]2/g (anhydrous zeolite as standard) and the SiO2/Al2O3mole ratio in the zeolite composition is preferably less than 14 and more preferably less than 11.
- The antimicrobial metal ions used in the antimicrobial zeolites should be retained on the zeolite particles through an ion-exchange reaction. Antimicrobial zeolites in which the antimicrobial metal ions are adsorbed or attached without an ion-exchange reaction typically exhibit an overall decreased bactericidal effect and their antimicrobial effect is not long-lasting. Nevertheless, it can be advantageous for imparting quick antimicrobial action to maintain a sufficient amount of surface adsorbed metal ion in addition to the ion-exchanged metal ion.[0046]
- The antimicrobial zeolites, as well as other antimicrobial ceramic particles, may also contain a discoloration agent. Preferably, the discoloration agent is biocompatible. Preferred discoloration agents include, but are not limited to, inorganic discoloration inhibitors such as ammonium. More preferably, the inorganic discoloration inhibitor is an ion-exchanged ammonium ion in the zeolite.[0047]
- A preferred antimicrobial zeolite for use in the invention is type A zeolite containing a combination of ion-exchanged silver, zinc, copper, and ammonium; silver copper and ammonium or silver and ammonium. One such zeolite is distributed by AgION Technologies, L.L.C. under the product number AW-10N and consists of 0.6% by weight of silver ion-exchanged in Type A zeolite particles having a mean average diameter of about 3 p. Another grade, AJ-10N, consists of about 2% by weight of silver ion-exchanged in Type A zeolite particles having a mean average diameter of about 3 p. Yet another grade, AW-80, contains 0.6% by weight of silver ion-exchanged in Type A zeolite particles having a mean average diameter of about 2 p. Another grade, AJ-80N, consists of about 2% by weight of silver ion-exchanged in Type A zeolite particles having a mean average diameter of about 1 p. These specific zeolites typically contain about 14% by weight zinc in combination with between about 0.5% and 2.5% by weight of ion-exchanged ammonium as a discoloration inhibiting agent.[0048]
- The hydrophilic polymer used to encapsulate the antimicrobial agent is a polymer that can absorb sufficient water to enable the encapsulated particle to exhibit good antimicrobial behavior, i.e., to allow for the migration and release of the antimicrobial active agent. The polymer will be characterized by having water absorption at equilibrium of at least about 2% by weight measured by ASTM D570. Preferably, the polymer will have water absorption at equilibrium of at least about 5 % by weight. More preferably, the polymer will have water absorption at equilibrium of at least about 20% by weight. Especially suitable hydrophilic polymers include those having water contents of from about 50 and to about 150% by weight.[0049]
- Polymeric compositions for use as the encapsulant in the present invention include polymers, which are comprised of substantial quantities of monomers having polar groups associated with them, such that the overall polymeric composition is rendered hydrophilic. The polar groups can be incorporated into the polymer main chain as in for example polyesters, polyurethanes, polyethers or polyamides. Optionally the polar groups can be pendant to the main chain as in for example, polyvinyl alcohol, polyacrylic acids or as in ionomers such as Surlyn®. Surlyn®) is available from Dupont and is the random copolymer poly(ethylene-co-methacrylic acid) wherein some or all of the methacrylic acid units are neutralized with a suitable cation, commonly Na[0050]+ or Zn+2. While not being limited by way of theory, it is believed that the inclusion of polar groups allows water to more readily permeate the polymer and consequently, to allow slow transport of the metal ion through the encapsulating polymer layer.
- A number of hydrophilic polymers may be used in the present invention and include, for example, (poly)hydroxyethyl methacrylate, (poly)hydroxypropyl methacrylate, (poly)glycerol methacrylate, copolymers of hydroxyethyl methacrylate and methacrylic acid, polyacrylamide, hyaluronan, polysaccharides, polylactic acid, copolymers of lactic acid, (poly)vinyl pyrrolidone, polyamides such as Nylon 6,6 or[0051]
Nylon 4,6 or Nylon 6,12, cellulosics, polyureas, polyurethanes and certain polyesters containing a high percentage (at least about 10% by weight, preferably at least about 25% by weight or more) of polyalkylene oxide. - The hydrophilic polymer may be a copolymer containing at least a substantial amount of at least one or more of the above-mentioned hydrophilic monomers, including, for example, styrene/methacrylic acid/hydroxyethyl methacrylate copolymers, styrene/methacrylic acid/hydroxypropyl methacrylate copolymers, methylmethacrylate/methacrylic acid copolymers, ethyl methacrylate/styrene/methacrylic acid copolymers and ethyl methacrylate/methyl methacrylate/styrene/methacrylic acid copolymers, copolymers based upon the cellulosics, and copolymers which utilize vinylpyrrolidone monomers, among numerous others.[0052]
- Other hydrophilic polymers that may be used in the present invention include polyvinyl acetate, polyvinyl alcohol, and copolymers of polyvinyl alcohol and polyvinylacetate, polyvinylchloride, copolymers of polyvinylacetate and polyvinylchloride and hydroxyl-modified vinyl chloride/vinyl acetate copolymers.[0053]
- Polyurethanes containing a high percentage (at least about 10% by weight, preferably at least about 25% by weight or more) of polyalkylene oxide are especially useful in this invention.[0054]
- Preferably the hydrophilic polymer is chosen from polyhydroxyethyl methacrylate, polyacrylamide, polyvinylpyrrolidinone, polyurea, polysaccharides, polylactic acid and polyurethane. More preferably, the hydrophilic polymer is hydrophilic polyurethane, such as the TECOPHILIC® polyurethane sold by Thermedics of Woburn, Mass.[0055]
- In the case of individually encapsulated antimicrobial particles, the microcapsule has a coating thickness of up to 15 μ of hydrophilic polymer on the antimicrobial agent, preferably a coating thickness of 1 to 10 μ. Thicker coatings could be used efficaciously; however, concern must then be given to the impact, if any, of the presence of the hydrophilic polymer on the properties of the matrix resin into which the encapsulated particles are to be incorporated.[0056]
- In the case of microcapsules containing multiple antimicrobial particles, especially of the ion-exchange type, the microcapsules may have a mean average diameter of up to and over 2000 μ, but not likely over 3000 μ. Generally the microcapsules will have a mean average diameter of from about 15 to about 1000 μ, preferably from about 50 to about 300 μ, most preferably from about 90 to about 200 μ. Of course smaller or larger microcapsules can be used. However, the smaller the microcapsule the more closely one approaches a system of individually encapsulated particles. Similarly, the larger the particles, the more potential there is for a deleterious effect on the physical properties of the polymer matrix and the greater the distance between particles at the surface, in the case of equivalent weights of larger versus small particle size microcapsules. Of course, microcapsule size and shape is somewhat dependent upon the size and shape of the antimicrobial particles to be incorporated therein as well as the process by which the microcapsules are made.[0057]
- Typically the microcapsules made in accordance with the present invention will be of a generally spherical or elliptical shape, although other shapes such as flakes and fibers are also contemplated. Generally the aspect ratio of these microcapsules will be from 1 to about 4, preferably from 1 to about 2, most preferably from 1 to about 1.5. Depending upon the method of manufacture, in most instances the aspect ration will be about 1. As used herein, the term aspect ratio refers to the quotient of the largest dimension divided by the smallest dimension of the particle: the largest dimension being the mean average diameter of the particle in the case of spherical/-elliptical particles and, vice-versa, the mean average diameter being the largest dimension in the case of non-spherical and non-elliptical particles. As disclosed in applicants co-filed application entitled “High Aspect Ratio Encapsulated Inorganic Antimicrobial Additive for Controlled Release”, high aspect ratio microcapsules, those up to 100 or more, but typically up to 30, are also useful and provide certain added benefits, in certain applications, as compared to the lower aspect ratios within the ranges set forth above.[0058]
- The microcapsule may be prepared by dissolving the hydrophilic polymer in a suitable solvent. Preferably this is done to make a solution that contains from about 1% to about 75% by weight of the hydrophilic polymer. More preferably, the solution contains from about 2% to about 40% by weight of the hydrophilic polymer and most preferably, the solution contains from about 5% to about 30% by weight of the hydrophilic polymer. Solutions that are more dilute than 1%, while useful in this invention, require handling larger levels of solvent. Solutions that are more concentrated than about 75% often are quite viscous and present some difficulties in handling.[0059]
- The choice of solvent will vary based upon the particular hydrophilic polymer as well as the end use application for the antimicrobial composition. It should be a solvent or solvent mixture that dissolves the hydrophilic polymer and one in which the antibacterial agent is mainly insoluble. For those applications in which residual solvents can pose problems, efforts should be taken to select more compatible or appropriate solvents and/or ensure that the solvent is completely driven from the microcapsules. Preferred solvents include tetrahydrofuran, dimethylacetamide, methylethylketone, and mixtures thereof.[0060]
- The antimicrobial agent is added to the solution of the hydrophilic polymer, preferably with agitation. The amount of antimicrobial agent can be varied. Preferably the antimicrobial agent is added at 1 to 1000 parts by weight per 100 parts by weight of the hydrophilic polymer. More preferably, the antimicrobial agent is added at 10 to 200 parts by weight per 100 parts by weight of the hydrophilic polymer. Most preferably, the antimicrobial agent is added at 20 to 100 parts by weight per 100 parts by weight of the hydrophilic polymer. If the amount of antimicrobial agent added is too low, then the resultant microcapsules may have lower and shorter lived antimicrobial activity. If the amount of antimicrobial agent added is too high, then the resultant microcapsules will not have good dispersion capabilities. Of course the level of incorporation is dependent upon whether the individual antimicrobial particles are to be encapsulated or microcapsules containing multiple antimicrobial particles are to be prepared. Additional factors affecting the level of incorporation include the particle size of the antimicrobial agent and the “loading capacity” of the hydrophilic polymer encapsulant. By loading capacity is meant the ability of the polymer to incorporate the antimicrobial particles without compromising too much the integrity of the microcapsule, i.e., there must be sufficient hydrophilic polymer to create a microcapsule. Generally speaking, these same factors and considerations pertain to all methods for the preparation of the microcapsules according to the present invention.[0061]
- This mixture of the antimicrobial agent in the hydrophilic polymer solution is then added to an antisolvent, preferably with agitation, to precipitate the microcapsules. The antisolvent is a solvent or solvent mixture that is miscible with the solvent chosen to dissolve the polymer. Further the antisolvent is chosen such that the hydrophilic polymer is substantially insoluble in the antisolvent. It is preferable that the antisolvent has a boiling point below 150° C. so that residual amounts can be easily removed from the microcapsule by drying. The amount of antisolvent can vary, but should be enough to precipitate the microcapsules. Preferably the amount of antisolvent used is from 50 to 2000 parts based upon 100 parts of solvent. Smaller amounts are often insufficient. Larger amounts are not necessary and add to the cost.[0062]
- The microcapsules are separated from the liquid and dried. The drying temperature is chosen such that it is low enough to have little affect on the hydrophilic polymer and high enough to remove residual solvent. The particular conditions will vary based upon the polymer, but temperatures from 20-100° C. are common. Optionally, vacuum may be employed in the drying step.[0063]
- By varying the precipitation conditions such as stirring speed, rate of precipitation, choice of antisolvent and amount of antisolvent, individually encapsulated particles as well as microcapsules having a varied amount of individual particles and/or agglomerates of particles within the encapsulant can be prepared. Optimum conditions will vary dependent upon the particular antimicrobial agent used, the hydrophilic polymer and the desired end use. Of course, in the solvent processing methodology, the selection of solvent and antisolvent must be such as not to adversely affect the efficacy of the antimicrobial agent.[0064]
- Alternatively, the microcapsules may be prepared by treating the antimicrobial agent with a polymer precursor and then subsequent polymerization to prepare the hydrophilic polymer. In this embodiment, the antimicrobial agent is mixed with a polymer precursor, optionally with a solvent, to obtain an antimicrobial agent coated with the polymer precursor. The polymer precursor can be a simple polymer precursor such as ethylene glycol, hydroxyethylmethacrylate or 1,6-diaminohexane. Preferably the polymer precursor is oligomeric or polymeric such as a polyol, polyamine, polyether diol, polyether diamine or polyalcohol amine. The subsequent polymerization can be effected by treatment with a reactive ingredient such as a diisocyanate. The subsequent polymerization can be done by different techniques. One preferred method involves suspension of the antimicrobial agent coated with the polymer precursor in a fluidized bed and addition of a reactive ingredient such as a diisocyanate. By varying the reaction conditions such as stirring speed, temperature, and choice of polymer precursors, individually encapsulated particles as well as microcapsules having a varied amount of individual particles and/or agglomerates of particles within the encapsulant can be prepared. Optimum conditions will vary dependent upon the particular antimicrobial agent used, the hydrophilic polymer and the desired end use.[0065]
- Another method for preparation of the microcapsules is the compounding of the antimicrobial agent with the hydrophilic polymer and subsequent grinding of the compounded product to the desired particle size. This can be done by mixing the antimicrobial agent with the hydrophilic polymer to obtain a blend. The blend is then melt compounded to obtain solid particles, which can then be ground to give the microcapsules. The melt compounding can be done by several methods such as with an extruder or kneader to yield a compounded product, preferably in the form of pellets. The optimum conditions will vary based upon the choice of the hydrophilic polymer. The compounded product can be ground to give the microcapsules of the desired particle size. Preferably, the microcapsule has a mean average particle size of 15 to 1000 μ, more preferably an average particle size of 50-300 μ, most preferably an average particle size of 90-200 μ. This method tends to give agglomerates of antimicrobial agent encapsulated with the hydrophilic polymer as the microcapsules. Dependent upon the hydrophilic polymer, it may be preferable to cryogenically grind the compounded product.[0066]
- The amount of antimicrobial agent encapsulated with the hydrophilic polymer is an amount that is effective to form a microcapsule with good antimicrobial activity. The microcapsules preferably contains from about 1 to about 1000 parts by weight of antimicrobial agent per 100 parts by weight of hydrophilic polymer. More preferably, the microcapsules contain from about 10 to about 200 parts by weight of antimicrobial agent per 100 parts by weight of hydrophilic polymer and, most preferably, from about 20 to about 100 parts by weight of antimicrobial agent per 100 parts by weight of hydrophilic polymer.[0067]
- The microcapsules can be used to impart antimicrobial properties to a variety of compositions. They may be blended into various formulations to provide compositions useful for molding, coatings or films with antimicrobial activity. They are particularly useful when blended with polymer formulations to provide compositions useful for molding articles with antimicrobial properties. The combination of hydrophilic polymer encapsulant and matrix polymer should be such that they have a different level of hydrophilicity and such that the microcapsule remains as a second phase in the matrix polymer. The matrix polymer formulations may be based upon either hydrophilic or non-hydrophilic polymers including, but not limited to: polypropylene, polyethylene, polystyrene, ABS, SAN, polybutylene terephthalate, polyethylene terephthalate, nylon 6, nylon 6,6,[0068]
nylon 4,6, nylon 12, phenolic resins, urea resins, epoxy resins, polyvinylchloride, polyurethanes, silicone polymers, polycarbonates, polyphenylene ethers, polyamides, polyethylene vinylacetate, polyethylene ethyl acrylate, polylactic acid, polysaccharides, polytetrafluoroethylene, polyimides, polysulfones, and a variety of other polymers and copolymers. - The antimicrobial polymer and polymer coating compositions made in accordance with the practice of the present invention may be used in any applications where antimicrobial properties are desirable. Exemplary applications include cutting boards, catheters and other medical devices, pipes, containers, toothbrushes, diapers, air filters, appliances, conveying belts, bottles, liquid dispensers, faucets, humidifiers, air conditioners, mats, razors, and bandages. Coatings comprising the microcapsule may be in liquid or solution form or may be in the form of a powder coating. In the latter instance, the microcapsule may be dry blended with another polymer resin powder which is then used to powder coat a substrate.[0069]
- The antimicrobial properties of the articles made from the microcapsules of the invention show efficacy for the end use applications. The degree of efficacy may be determined by any of several tests such as the Dow shaker test, direct inoculation and several others known to those skilled in the art and chosen based upon the end use application.[0070]
- The present invention will hereunder be explained in more detail with reference to the following non-limiting working examples.[0071]
- For comparative purposes, a polymer blend was made by first compounding an antimicrobial agent with a nonhydrophilic polymer and then using that mixture in a polymer formulation. Twenty parts by weight of AgION AJ10D (about 2.5% by weight of silver ion-exchanged in Type A zeolite particles having a mean average diameter of about 3.) was gravimetrically fed with 80 parts by weight of LDPE resin, extruded, pelletized and ground to give particles. A mixture of 25 parts by weight of these particles combined with 75 parts by weight of LDPE was blended by shaking and injection molded into 5 cm by 5 cm by 0.16 cm test parts. The injection molding temperature conditions were 385° F. rear zone; 390° F. front zone and 395° F. nozzle temperature. Analysis of the test parts was done in duplicate by ashing the sample and showed a measured loading of AgION powder of 4.8% and 4.5% by weight. No other inorganic materials were present in the sample.[0072]
- A polymer blend was made by first compounding an antimicrobial agent with a hydrophilic polymer and then using the microcapsule in a polymer formulation. Fifty parts by weight of AgION AJ10D (about 2.5% by weight of silver ion-exchanged in Type A zeolite particles having a mean average diameter of about 3.) was gravimetrically fed with 50 parts by weight of Tecophilic® 60 resin, a hydrophilic polyurethane available from Thermedics Inc. having a moisture absorption of about 60%. The blend was extruded using a Leistritz 27 mm twin screw extruder, pelletized, dried and cryogenically ground. Sieving was performed with a 100 mesh screen to remove large particles. SEM analysis of the resultant microcapsules showed particles that were roughly equiaxed and below about 150 μ in size. A mixture of 4 parts by weight of microcapsule was combined with 96 parts by weight of LDPE was blended by shaking and injection molded into 5 cm by 5 cm by 0.16 test parts. The injection molding temperature conditions were 385° F. rear zone; 390° F. front zone and 395° F. nozzle temperature. Analysis of the test parts was done in duplicate by ashing the sample and showed a measured loading of AgION powder of 2.3% and 2.3% by weight. No other inorganic materials were present in the sample.[0073]
- The procedure of example 2 was repeated, but with a mixture of 10 parts by weight of microcapsule and 90 parts by weight of LDPE. Analysis of the test parts was done in duplicate and showed a measured loading of AgION powder of 5.4% and 5.2% by weight. No other inorganic materials were present in the sample.[0074]
- Extraction experiments were performed on the test parts from examples 1-3 to determine the total amount of silver accessible from the samples. This was accomplished using the basic approach of serial extraction. The method involves placing the samples in a test solution and changing it periodically, measuring the volume and Ag concentration each time to determine the amount of silver removed with each cycle. The extraction experiment was performed in duplicate so six samples total were tested. The samples were placed in plastic beakers containing 40 ml of extraction solution (0.8% NaNO3). To keep the samples from floating and only contacting the solution on one side, the beakers were crimped from the sides, pinning the coupons to the bottom of the beaker. To ensure even release, the beakers were placed on a rocking table to agitate during the release time. The samples soaked for at least 24 hours between solution changes.[0075]
- The results are in Table 1.[0076]
TABLE 1 Extraction of Test Parts. Comparative Example 1, Example 2 and Example 3 in duplicate. Measurements Expressed in micrograms of silver extracted. Extraction C1 C1d Mean E2 E2d Mean E3 E3d Mean 1 8.5 7.4 7.9 7.5 7.0 7.2 14.4 11.8 13.1 2 0.7 0.7 0.7 1.2 1.4 1.3 4.0 3.1 3.6 3 0.2 0.2 0.2 1.2 1.4 1.3 3.6 2.5 3.1 4 0.1 0.1 0.1 0.3 0.4 0.4 2.0 1.4 1.7 5 0.0 0.0 0.0 0.2 0.3 0.2 0.7 0.5 0.6 6 0.0 0.0 0.0 0.2 0.2 0.2 0.4 0.4 0.4 7 0.0 0.0 0.0 0.8 0.4 0.6 1.1 0.4 0.7 8 0.0 0.0 0.0 0.2 0.2 0.2 0.5 0.4 0.5 9 0.0 0.0 0.0 0.2 0.2 0.2 1.0 0.6 0.8 10 0.0 0.0 0.0 0.2 0.3 0.2 0.7 0.4 0.6 11 0.0 0.0 0.0 0.1 0.1 0.1 0.3 0.2 0.3 12 0.0 0.0 0.0 0.1 0.1 0.1 0.2 0.2 0.2 Total 9.4 8.4 8.9 12.3 12.0 12.1 28.9 22.1 25.5 - As can be seen by inspection of Table 1, the formulation of Example 2 delivers more silver (12.1 micrograms on average) than the formulation of Comparative Example 1 (8.9 micrograms on average). This is true despite the fact that the formulation of Example 2 has about half of the loading of antimicrobial agent. Example 3 delivers remarkably more (25.5 micrograms) silver than the formulation of Comparative Example 1 (8.9 micrograms). This is true despite the fact that they have similar loadings of antimicrobial agent. That half the loading gives slightly more silver and therefore slightly better antimicrobial activity and that similar loadings give remarkably greater silver and therefore remarkably greater antimicrobial activity is unexpected and shows the advantage of using microcapsules.[0077]
- Note also that after the fourth extraction, no appreciable amount of silver is available from Comparative Example 1, whereas the formulations of the invention continue to deliver silver and therefore antimicrobial activity even after eleven extractions. This shows the ability of formulations of the invention to impart sustained antimicrobial activity long after prior art inventions are inactive.[0078]
- A test part was prepared as in Comparative Example 1. Analysis by ashing the sample showed a powder loading of 5.0% by weight. No other inorganic materials were present in the sample. The surface was analyzed for surface exposure on the SEM using image analysis. The voltage used was 10 keV, chosen to detect only the immediate surface particles. Ten measurements were done and averaged to give a value of 0.9% by area of powder at the surface.[0079]
- A test part was prepared as in Example 3. Analysis by ashing the sample showed a powder loading of 4.8%. No other inorganic materials were present in the sample. The surface was analyzed as in Comparative Example 4 and an average value of 1.3% by area of powder at the surface was obtained. This result shows that the article has a higher level of antimicrobial agent at the surface, where it is needed, than the prior art formulation of Comparative Example 4, despite the fact that the overall loading is the same.[0080]
- Example 5 also demonstrates another beneficial and surprising phenomenon of the present invention which is manifested by an enhancement in the amount of antimicrobial agent at the surface of parts molded from polymer compositions containing the microcapsules made in accordance with the teaching of the present invention. Specifically, by controlled selection of the hydrophilic polymer encapsulant and matrix polymer as well as the processing conditions for molding parts from such a formulation, one is able to generate a smearing of the microcapsule along the surface of the molded part. It is believed that by selecting a polymer matrix resin whose processing temperature is near, at or above, preferably at or above, the temperature at which the hydrophilic polymer begins to manifest tackiness or plastic deformation, microcapsules which, upon injection into the mold, touch the surface of the mold, adhere to the surface and, as the flow of matrix resin continues to fill the mold, is pulled along the surface of the mold, smearing the microcapsule at the part surface. This same phenomenon is also believed to manifest itself when the mold surface temperatures are near, at or above, preferably at or above, the temperature at which the hydrophilic polymer begins to manifest tackiness or plastic deformation.[0081]
- Thus the microcapsules of the invention are exceptionally suitable for use in various compositions and provide compositions without the deleterious effects of the prior art.[0082]
- While preferred embodiments of the invention have been described in the foregoing examples, it will be understood by those skilled in the art that various changes and modifications may be made therein without departing from the spirit and the scope of the invention. Accordingly, the above description should be construed as illustrating and not limiting the scope of the invention.[0083]
Claims (50)
Priority Applications (8)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US10/032,372US7357949B2 (en) | 2001-12-21 | 2001-12-21 | Encapsulated inorganic antimicrobial additive for controlled release |
| JP2003556466AJP2005514402A (en) | 2001-12-21 | 2002-12-11 | Inorganic antimicrobial additive encapsulated for controlled release |
| PCT/US2002/039709WO2003055941A1 (en) | 2001-12-21 | 2002-12-11 | Encapsulated inorganic antimicrobial additive for controlled release |
| EP02787023AEP1456291A1 (en) | 2001-12-21 | 2002-12-11 | Encapsulated inorganic antimicrobial additive for controlled release |
| CA2470122ACA2470122C (en) | 2001-12-21 | 2002-12-11 | Encapsulated inorganic antimicrobial additive for controlled release |
| AU2002351366AAU2002351366B2 (en) | 2001-12-21 | 2002-12-11 | Encapsulated inorganic antimicrobial additive for controlled release |
| MXPA04006185AMXPA04006185A (en) | 2001-12-21 | 2002-12-11 | Encapsulated inorganic antimicrobial additive for controlled release. |
| US11/336,699US7354605B2 (en) | 2001-12-21 | 2006-01-20 | Antimicrobial medical device |
Applications Claiming Priority (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US10/032,372US7357949B2 (en) | 2001-12-21 | 2001-12-21 | Encapsulated inorganic antimicrobial additive for controlled release |
Related Parent Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US09/844,225Continuation-In-PartUS6517536B2 (en) | 2000-04-27 | 2001-04-27 | Transmural ablation device and method |
Related Child Applications (3)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US10/038,506Continuation-In-PartUS20020107514A1 (en) | 2000-04-27 | 2001-11-09 | Transmural ablation device with parallel jaws |
| US11/336,699DivisionUS7354605B2 (en) | 2001-12-21 | 2006-01-20 | Antimicrobial medical device |
| US11/336,699ContinuationUS7354605B2 (en) | 2001-12-21 | 2006-01-20 | Antimicrobial medical device |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| US20030118664A1true US20030118664A1 (en) | 2003-06-26 |
| US7357949B2 US7357949B2 (en) | 2008-04-15 |
Family
ID=21864610
Family Applications (2)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US10/032,372Expired - Fee RelatedUS7357949B2 (en) | 2001-12-21 | 2001-12-21 | Encapsulated inorganic antimicrobial additive for controlled release |
| US11/336,699Expired - Fee RelatedUS7354605B2 (en) | 2001-12-21 | 2006-01-20 | Antimicrobial medical device |
Family Applications After (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US11/336,699Expired - Fee RelatedUS7354605B2 (en) | 2001-12-21 | 2006-01-20 | Antimicrobial medical device |
Country Status (7)
| Country | Link |
|---|---|
| US (2) | US7357949B2 (en) |
| EP (1) | EP1456291A1 (en) |
| JP (1) | JP2005514402A (en) |
| AU (1) | AU2002351366B2 (en) |
| CA (1) | CA2470122C (en) |
| MX (1) | MXPA04006185A (en) |
| WO (1) | WO2003055941A1 (en) |
Cited By (32)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US20040215164A1 (en)* | 2002-02-20 | 2004-10-28 | Abbott Chun Lim | Methods of treating abnormal biological conditions using metal oxides |
| WO2005011768A1 (en)* | 2003-07-31 | 2005-02-10 | Scimed Life Systems, Inc. | Latex medical articles for release of antimicrobial agents |
| WO2005048708A1 (en)* | 2003-11-17 | 2005-06-02 | Bio-Gate Ag | Antimicrobial composite material |
| US20050191355A1 (en)* | 1999-05-27 | 2005-09-01 | Foss Manufacturing Co., Inc. | Anti-microbial and antifungal fluid conduits and methods of manufacture thereof |
| US20050271746A1 (en)* | 2004-05-18 | 2005-12-08 | Abbott Chun L | Topical treatments for abnormal biological conditions and method of topically treating such conditions |
| US20060008646A1 (en)* | 2002-06-12 | 2006-01-12 | Traptek Llc. | Encapsulated active particles and methods for making and using the same |
| US20060034423A1 (en)* | 1998-08-07 | 2006-02-16 | Juergen Pensel | Surgical microscope |
| US20060156948A1 (en)* | 2004-06-24 | 2006-07-20 | Hendriks Eugene P | Color stable antimicrobial coatings |
| US7276056B2 (en) | 2002-02-20 | 2007-10-02 | Abbott Research Group, Inc. | Methods of treating abnormal biological conditions by vaginal douching |
| US20070243263A1 (en)* | 2006-04-14 | 2007-10-18 | Agion Technologies, Inc. | Antiviral Methods |
| US20070260202A1 (en)* | 2002-02-20 | 2007-11-08 | Dominic Abbott | Deodorizer devices, systems and methods for controlling perspiration-related body odor |
| US20070264203A1 (en)* | 2006-05-09 | 2007-11-15 | Traptek Llc | Active particle-enhanced membrane and methods for making and using the same |
| US20070269499A1 (en)* | 2006-04-28 | 2007-11-22 | John Hen | Materials and methods for wound treatment |
| US20080044474A1 (en)* | 2006-08-18 | 2008-02-21 | Bayer Materialscience Ag | Dispersions of nanoureas comprising biologically active compounds |
| US20080121141A1 (en)* | 2006-11-16 | 2008-05-29 | Haggquist Gregory W | Exothermic-enhanced articles and methods for making the same |
| US20080131606A1 (en)* | 2004-06-24 | 2008-06-05 | Trogolo Jeffrey A | Antimicrobial coating for erosive environments |
| WO2009036862A2 (en) | 2007-09-12 | 2009-03-26 | Trovotech Gmbh | Compositions with an antimicrobial action |
| US20090098373A1 (en)* | 2001-10-02 | 2009-04-16 | Henkelstrasse 67 | Anodized coating over aluminum and aluminum alloy coated substrates and coated articles |
| US7641912B1 (en)* | 2003-01-13 | 2010-01-05 | EnviroCare Corporation | Antimicrobial coatings for treatment of surfaces in a building setting and method of applying same |
| US20100050197A1 (en)* | 2008-07-25 | 2010-02-25 | Disctekk, Llc | Optical card |
| WO2010090594A1 (en)* | 2009-02-09 | 2010-08-12 | Swetree Technologies Ab | Polymer shells |
| US20100252241A1 (en)* | 2009-04-02 | 2010-10-07 | Mcdermott Chris | Ceramic coated automotive heat exchanger components |
| US20110104477A1 (en)* | 2008-04-04 | 2011-05-05 | Michael Wagener | Coating material |
| US20110152843A1 (en)* | 2009-12-18 | 2011-06-23 | Wedlin Charlotte | Medical device for short time use with quickly releasable antibacterial agent |
| US8282951B2 (en) | 2003-01-13 | 2012-10-09 | EnviroCare Corporation | Antimicrobial coatings for treatment of surfaces in a building setting and method of applying same |
| US8663807B2 (en) | 2001-10-02 | 2014-03-04 | Henkel Ag & Co. Kgaa | Article of manufacture and process for anodically coating aluminum and/or titanium with ceramic oxides |
| WO2014143695A1 (en)* | 2013-03-15 | 2014-09-18 | Arch Chemicals, Inc. | Encapsulation of active ingredients and method of making |
| US9243141B1 (en)* | 2014-11-03 | 2016-01-26 | Xerox Corporation | Coated silver nanoparticle composites comprising a sulfonated polyester matrix and methods of making the same |
| CN107847637A (en)* | 2015-07-24 | 2018-03-27 | 墨尼克医疗用品有限公司 | The antimicrobial wound dressing of absorbability |
| WO2018090152A1 (en)* | 2016-11-15 | 2018-05-24 | Roberto Felipe Moser Rossel | Particles with antimicrobial properties, completely coated by at least one polymer, and which maintain the particle state thereof |
| US20220112029A1 (en)* | 2015-11-10 | 2022-04-14 | Simplehuman, Llc | Household goods with antimicrobial coatings and methods of making thereof |
| US11622557B2 (en)* | 2016-10-31 | 2023-04-11 | Applied Silver, Inc. | Dispensing of metal ions into batch laundry washers and dryers |
Families Citing this family (92)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| IL135487A (en) | 2000-04-05 | 2005-07-25 | Cupron Corp | Antimicrobial and antiviral polymeric materials and a process for preparing the same |
| US20060291756A1 (en)* | 2002-02-27 | 2006-12-28 | Thomas Toby R | Web materials with active agent for use in forming reclosable packages |
| US20050220375A1 (en)* | 2002-02-27 | 2005-10-06 | Thomas Toby R | Pakages with active agents |
| US20060110080A1 (en)* | 2002-02-27 | 2006-05-25 | Thomas Toby R | Packages and structures with selective dosing of active agent |
| US9474524B2 (en) | 2002-10-04 | 2016-10-25 | Ethicon, Inc. | Packaged antimicrobial medical device having improved shelf life and method of preparing same |
| US9597067B2 (en) | 2002-10-04 | 2017-03-21 | Ethicon, Inc. | Packaged antimicrobial medical device and method of preparing same |
| US7513093B2 (en) | 2002-10-04 | 2009-04-07 | Ethicon, Inc. | Method of preparing a packaged antimicrobial medical device |
| US7364756B2 (en)* | 2003-08-28 | 2008-04-29 | The Cuprin Corporation | Anti-virus hydrophilic polymeric material |
| CA2558200A1 (en)* | 2004-03-02 | 2005-09-15 | Nanotherapeutics, Inc. | Compositions for repairing bone and methods for preparing and using such compositions |
| US8414547B2 (en) | 2004-04-29 | 2013-04-09 | C. R. Bard, Inc. | Modulating agents for antimicrobial coatings |
| JP5411431B2 (en) | 2004-11-09 | 2014-02-12 | カプロン インコーポレイテッド | Methods and materials for skin care |
| MX2007011572A (en)* | 2005-03-21 | 2007-12-06 | Cupron Corp | Antimicrobial and antiviral polymeric master batch, processes for producing polymeric material therefrom and products produced therefrom. |
| JP2008546832A (en) | 2005-06-27 | 2008-12-25 | スミス アンド ネフュー ピーエルシー | Antibacterial biguanide metal complex |
| CN101252905A (en)* | 2005-07-15 | 2008-08-27 | 泰科保健集团有限合伙公司 | Wound dressing and methods of making and using the same |
| JP2007159727A (en)* | 2005-12-12 | 2007-06-28 | Risen Kim | Antibacterial toothbrush which has bristles evenly impregnated with nanometer-sized antibacterial metal particle which are coated with silicone oxide, and method for manufacturing the same |
| GB0601687D0 (en)* | 2006-01-27 | 2006-03-08 | Smith & Nephew | Antimicrobial materials |
| US8512294B2 (en)* | 2006-07-28 | 2013-08-20 | Becton, Dickinson And Company | Vascular access device antimicrobial materials and solutions |
| US7998498B2 (en) | 2008-01-22 | 2011-08-16 | Michael Szycher | Antimicrobial material and method for making the same |
| US8367094B2 (en) | 2008-01-22 | 2013-02-05 | Michael Szycher | Antimicrobial material and method for making the same |
| US20090214658A1 (en)* | 2008-02-22 | 2009-08-27 | Grigor Evich Gritsenko Anatoly | Inorganic sorbent copolymer |
| EP2342248A1 (en) | 2008-09-26 | 2011-07-13 | Nesrin Hasirci | Process for preparation of medical grade polyurethane composites containing antibacterial zeolite |
| US8349346B2 (en)* | 2008-11-18 | 2013-01-08 | Koken Ltd. | Antimicrobial composition, process for preparing the same, and utilization thereof |
| US8551517B2 (en)* | 2008-12-16 | 2013-10-08 | Kimberly-Clark Worldwide, Inc. | Substrates providing multiple releases of active agents |
| US8741325B2 (en)* | 2008-12-18 | 2014-06-03 | The Hong Kong University Of Science And Technology | Material for forming a multi-level antimicrobial surface coating and its preparation |
| US20100175627A1 (en)* | 2009-01-09 | 2010-07-15 | Carpenter James R | Wild animal care devices having an antimicrobial agent |
| EP2206427A1 (en) | 2009-01-09 | 2010-07-14 | Wild Birds Unlimited, Inc. | Wild animal care devices having an antimicrobial agent |
| US20100183739A1 (en)* | 2009-01-21 | 2010-07-22 | Karel Newman | Treatment and prevention of systemic bacterial infections in plants using antimicrobial metal compositions |
| US20090202444A1 (en)* | 2009-01-21 | 2009-08-13 | Karel Newman | Treatment and prevention of systemic Xylella fastidiosa infections of plants using antimicrobial metal compositions |
| DE102009030121A1 (en) | 2009-06-22 | 2010-12-30 | Rent-A-Scientist Gmbh | Additive, useful e.g. for improving the growth of photosynthetic microorganisms, and as feedstock for the producing agar, carrageenan, alginic acid and alginates, comprises nanoparticulate metals comprising silver, gold, copper or zinc |
| IN2012DN04965A (en) | 2009-11-25 | 2015-09-25 | Difusion Technologies Inc | |
| BR112012016027B1 (en) | 2009-12-11 | 2019-01-15 | Difusion Technologies, Inc. | production method of polyetheretherketone antimicrobial implants |
| US8673018B2 (en)* | 2010-02-05 | 2014-03-18 | AMx Tek LLC | Methods of using water-soluble inorganic compounds for implants |
| CA2795836C (en) | 2010-05-07 | 2015-11-17 | Difusion Technologies, Inc. | Medical implant comprising a thermoplastic resin having aluminosilicate particles incorporated therein |
| US9211175B2 (en) | 2010-07-08 | 2015-12-15 | Covidien Lp | Self-detachable medical devices |
| FR2962646B1 (en) | 2010-07-16 | 2012-06-22 | Sofradim Production | PROSTHETIC WITH RADIO OPAQUE ELEMENT |
| US9572907B2 (en) | 2010-10-01 | 2017-02-21 | Covidien Lp | Implantable polymeric films |
| US9861590B2 (en) | 2010-10-19 | 2018-01-09 | Covidien Lp | Self-supporting films for delivery of therapeutic agents |
| US8632839B2 (en) | 2010-10-19 | 2014-01-21 | Covidien Lp | Methods of forming self-supporting films for delivery of therapeutic agents |
| US8920867B2 (en) | 2010-10-19 | 2014-12-30 | Covidien Lp | Methods of forming self-supporting films for delivery of therapeutic agents |
| US8927480B2 (en) | 2010-12-14 | 2015-01-06 | Bissell Homecare, Inc. | Cleaning cloth with encapsulated formulation, steam mop and method |
| US9144634B2 (en) | 2011-01-14 | 2015-09-29 | Covidien Lp | Medical device with intrapore films |
| US20140008324A1 (en)* | 2011-03-31 | 2014-01-09 | 3M Innovative Properties Company | Method for making plastic articles having an antimicrobial surface |
| EP2713747B1 (en) | 2011-05-24 | 2023-02-22 | Agienic, Inc. | Compositions and methods for antimicrobial metal nanoparticles |
| US9155310B2 (en) | 2011-05-24 | 2015-10-13 | Agienic, Inc. | Antimicrobial compositions for use in products for petroleum extraction, personal care, wound care and other applications |
| FR2977790B1 (en) | 2011-07-13 | 2013-07-19 | Sofradim Production | PROSTHETIC FOR UMBILIC HERNIA |
| US8579924B2 (en) | 2011-07-26 | 2013-11-12 | Covidien Lp | Implantable devices including a mesh and a pivotable film |
| CN102964898B (en) | 2011-08-05 | 2016-05-25 | 罗门哈斯公司 | There is the water-based paint compositions of improved hydrophilic spot repellency |
| US9782957B2 (en) | 2011-08-24 | 2017-10-10 | Covidien Lp | Medical device films |
| US8932621B2 (en) | 2011-10-25 | 2015-01-13 | Covidien Lp | Implantable film/mesh composite |
| US9005308B2 (en) | 2011-10-25 | 2015-04-14 | Covidien Lp | Implantable film/mesh composite for passage of tissue therebetween |
| US9179994B2 (en) | 2011-10-25 | 2015-11-10 | Covidien Lp | Implantable film/mesh composite |
| JP6114758B2 (en)* | 2011-12-29 | 2017-04-12 | ローム アンド ハース カンパニーRohm And Haas Company | Encapsulated active substance |
| US10206769B2 (en) | 2012-03-30 | 2019-02-19 | Covidien Lp | Implantable devices including a film providing folding characteristics |
| US10245025B2 (en)* | 2012-04-06 | 2019-04-02 | Ethicon, Inc. | Packaged antimicrobial medical device having improved shelf life and method of preparing same |
| EP2838653A4 (en)* | 2012-04-18 | 2015-11-25 | Cerulean Pharma Inc | METHODS AND SYSTEMS FOR POLYMER PRECIPITATION AND PARTICLE GENERATION |
| CA2874951C (en) | 2012-06-05 | 2020-04-28 | Dow Global Technologies Llc | Aqueous coating composition with improved stability |
| FR2992662B1 (en) | 2012-06-28 | 2014-08-08 | Sofradim Production | KNIT WITH PICOTS |
| FR2992547B1 (en) | 2012-06-29 | 2015-04-24 | Sofradim Production | PROSTHETIC FOR HERNIA |
| US20140097660A1 (en)* | 2012-10-10 | 2014-04-10 | Regency Seating, Inc. | Antimicrobial furniture and method of making |
| CA2887157C (en) | 2012-10-12 | 2020-01-14 | Dow Global Technologies Llc | Aqueous coating composition with improved viscosity stability |
| US20140220331A1 (en) | 2013-02-02 | 2014-08-07 | Cosilion LLC | Antimicrobial compositions |
| US20150351851A1 (en) | 2013-02-22 | 2015-12-10 | Eastern Maine Healthcare Services | Blood Pressure Cuff Shield Incorporating Antimicrobial Technology |
| EP3055373B1 (en) | 2013-10-10 | 2020-03-11 | Rohm and Haas Company | Binder composition and coating composition made thereof |
| EP3055368B1 (en) | 2013-10-10 | 2019-08-14 | Rohm and Haas Company | Coating composition with improved liquid stain repellency and process for making the same |
| CN105579535B (en) | 2013-10-10 | 2018-10-26 | 罗门哈斯公司 | Coating composition with improved liquid stain repellency |
| US9622483B2 (en) | 2014-02-19 | 2017-04-18 | Corning Incorporated | Antimicrobial glass compositions, glasses and polymeric articles incorporating the same |
| US11039620B2 (en) | 2014-02-19 | 2021-06-22 | Corning Incorporated | Antimicrobial glass compositions, glasses and polymeric articles incorporating the same |
| US11039621B2 (en) | 2014-02-19 | 2021-06-22 | Corning Incorporated | Antimicrobial glass compositions, glasses and polymeric articles incorporating the same |
| AU2014391028B2 (en) | 2014-04-17 | 2018-11-08 | Rohm And Haas Company | Polymer dispersion and its application in high pigment volume concentration coatings |
| AU2014402271B2 (en) | 2014-07-28 | 2018-11-29 | Dow Global Technologies Llc | Poly(vinyl acetate) dispersion, and paint formulation comprising thereof |
| CN107075032B (en) | 2014-11-13 | 2019-12-10 | 罗门哈斯公司 | Polymer dispersions and their use in paints with high pigment volume concentration |
| GB201423348D0 (en) | 2014-12-30 | 2015-02-11 | Imerys Minerals Ltd | Substates having a functional Capability |
| US10076123B1 (en) | 2015-02-19 | 2018-09-18 | Zeco, Inc. | Method for reduction in microbial activity in red meat |
| EP4062868A3 (en) | 2015-03-30 | 2022-12-21 | C. R. Bard, Inc. | Application of antimicrobial agents to medical devices |
| CN104801248B (en)* | 2015-04-23 | 2016-06-01 | 北京宇田相变储能科技有限公司 | Water-soluble inorganic salt microcapsule and preparation method |
| US10918110B2 (en) | 2015-07-08 | 2021-02-16 | Corning Incorporated | Antimicrobial phase-separating glass and glass ceramic articles and laminates |
| US10064273B2 (en) | 2015-10-20 | 2018-08-28 | MR Label Company | Antimicrobial copper sheet overlays and related methods for making and using |
| EP3170847A1 (en) | 2015-11-20 | 2017-05-24 | OMG Borchers GmbH | Encapsulated accelerators for coatings |
| IT201600119819A1 (en)* | 2016-11-25 | 2018-05-25 | Thales Alenia Space Italia Spa Con Unico Socio | POLYMERIC MATERIAL ANTIBACTERIAL, ITS COMPOSITE AND THEIR USES |
| CN110494488B (en) | 2017-05-19 | 2022-04-19 | 大金美国股份有限公司 | Composition and method for producing the same |
| KR102626745B1 (en) | 2017-05-26 | 2024-01-17 | 인피니트 머티리얼 솔루션즈, 엘엘씨 | Water-soluble polymer composition |
| GB201711181D0 (en) | 2017-07-12 | 2017-08-23 | Smith & Nephew | Polymer foam material, device and use |
| GB201711183D0 (en) | 2017-07-12 | 2017-08-23 | Smith & Nephew | Antimicrobial or wound care materials, devices and uses |
| GB201711179D0 (en) | 2017-07-12 | 2017-08-23 | Smith & Nephew | Wound care materials, devices and uses |
| EP3694327A4 (en)* | 2017-10-13 | 2021-06-23 | Corning Incorporated | Method for encapsulating hydrophobic materials in stabilized yeast cells suitable for processing with polymers |
| US20200323217A1 (en)* | 2017-11-01 | 2020-10-15 | Corning Incorporated | Antimicrobial floor coatings and formulations |
| WO2019104288A1 (en) | 2017-11-27 | 2019-05-31 | Colgate-Palmolive Company | Oral care implement |
| WO2020010152A1 (en) | 2018-07-02 | 2020-01-09 | C.R. Bard, Inc. | Antimicrobial catheter assemblies and methods thereof |
| WO2020112332A1 (en) | 2018-11-27 | 2020-06-04 | Colgate-Palmolive Company | Oral care implement having a release component |
| US12371581B2 (en) | 2020-03-25 | 2025-07-29 | Infinite Material Solutions, Llc | High performance water soluble polymer compositions |
| US20210402052A1 (en) | 2020-06-30 | 2021-12-30 | Difusion, Inc. | Medical Implants And Methods Of Manufacture |
| US20240416074A1 (en)* | 2023-06-13 | 2024-12-19 | Bard Access Systems, Inc. | Antimicrobial Catheters and Methods |
Citations (21)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US3975350A (en)* | 1972-08-02 | 1976-08-17 | Princeton Polymer Laboratories, Incorporated | Hydrophilic or hydrogel carrier systems such as coatings, body implants and other articles |
| US4403083A (en)* | 1979-06-01 | 1983-09-06 | W. R. Grace & Co. | Preparation of solid polyurethane particles |
| US4657835A (en)* | 1984-05-31 | 1987-04-14 | Canon Kabushiki Kaisha | Electrophotographic photosensitive member having an intermediate layer of conductive powder and resin or oligimer |
| US5180585A (en)* | 1991-08-09 | 1993-01-19 | E. I. Du Pont De Nemours And Company | Antimicrobial compositions, process for preparing the same and use |
| US5238749A (en)* | 1986-03-27 | 1993-08-24 | Clinitex Corporation | Antimicrobial coating process and product |
| US5382424A (en)* | 1991-12-11 | 1995-01-17 | The Procter & Gamble Company | Breath protection microcapsules |
| US5599583A (en)* | 1994-05-27 | 1997-02-04 | Micro Flo Company | Encapsulation with water soluble polymer |
| US5980620A (en)* | 1996-06-05 | 1999-11-09 | Brodie; Harold | Inhibition of bacterial growth |
| US6046243A (en)* | 1993-02-12 | 2000-04-04 | Bernard Technologies, Inc. | Compositions for sustained release of a gas |
| US6093407A (en)* | 1997-10-03 | 2000-07-25 | Dupont Powder Coatings Usa, Inc. | Anti-microbial powder coatings |
| US6113936A (en)* | 1998-05-18 | 2000-09-05 | Sumitomo Chemical Company, Limited | Method for microencapsulating a solid substance |
| US6123925A (en)* | 1998-07-27 | 2000-09-26 | Healthshield Technologies L.L.C. | Antibiotic toothpaste |
| US6156245A (en)* | 1998-03-05 | 2000-12-05 | Sumitomo Chemical Company, Limited | Method for microencapsulating of a solid substance |
| US20010009831A1 (en)* | 1999-12-03 | 2001-07-26 | Michael Schink | Antimicrobial wound coverings |
| US6287285B1 (en)* | 1998-01-30 | 2001-09-11 | Advanced Cardiovascular Systems, Inc. | Therapeutic, diagnostic, or hydrophilic coating for an intracorporeal medical device |
| US6290962B1 (en)* | 1992-11-03 | 2001-09-18 | Oravax, Inc. | Urease-based vaccine and treatment for helicobacter infection |
| US6296834B1 (en)* | 1999-04-19 | 2001-10-02 | Unilever Home & Personal Care Usa, Division Of Conopco, Inc. | Oral care composition |
| US6399735B1 (en)* | 1992-12-17 | 2002-06-04 | Henkel Kommanditgesellschaft Auf Aktien | Hydrophilic polyurethanes |
| US6413536B1 (en)* | 1995-06-07 | 2002-07-02 | Southern Biosystems, Inc. | High viscosity liquid controlled delivery system and medical or surgical device |
| US6432416B1 (en)* | 1997-10-03 | 2002-08-13 | Dupont Powder Coatings Usa, Inc. | Anti-microbial power coating |
| US20030043341A1 (en)* | 2001-08-02 | 2003-03-06 | Turner David C. | Antimicrobial lenses and methods of their use |
Family Cites Families (41)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US3981970A (en)* | 1968-03-20 | 1976-09-21 | Takeda Chemical Industries, Ltd. | Method for the production of a zeolite material |
| JPS59133235A (en)* | 1983-01-21 | 1984-07-31 | Kanebo Ltd | Zeolite particle-containing polymer and its production |
| GB8616294D0 (en) | 1986-07-03 | 1986-08-13 | Johnson Matthey Plc | Antimicrobial compositions |
| JPS6323960A (en)* | 1986-07-16 | 1988-02-01 | Zenji Hagiwara | High molecular material containing amorphous aluminosilicate particle and production thereof |
| JPH0618899B2 (en)* | 1987-06-30 | 1994-03-16 | 品川燃料株式会社 | Film containing antibacterial zeolite |
| JPH0741061B2 (en) | 1987-07-09 | 1995-05-10 | 華郎 前田 | Medical dressing |
| JPH0372562A (en) | 1989-08-11 | 1991-03-27 | Nippon Paint Co Ltd | Hydrophilic surface treatment, hydrophilic surface treatment bath and hydrophilic surface treatment process |
| JPH0466512A (en) | 1990-06-29 | 1992-03-02 | Sintokogio Ltd | Inorganic antimicrobial agent surface-coated with polyurethane resin, its production and antimicrobial resin composition |
| JP2939036B2 (en) | 1991-12-04 | 1999-08-25 | 鐘紡株式会社 | Non-woven bandage for cast bottom winding |
| US5305827A (en) | 1992-03-04 | 1994-04-26 | United Technologies Corporation | Antimicrobial hydrophilic coating |
| JPH0825850B2 (en)* | 1992-05-13 | 1996-03-13 | 中村物産株式会社 | Antibacterial composition and method for producing the same |
| JPH06117797A (en) | 1992-10-01 | 1994-04-28 | Zexel Corp | Treating method for anti-fungus of heat exchanger |
| US5650446A (en) | 1993-02-12 | 1997-07-22 | Southwest Research Institute | Sustained release biocidal composition |
| JP3400489B2 (en) | 1993-05-20 | 2003-04-28 | 東京電測株式会社 | Composite discharge lamp |
| JP2918771B2 (en) | 1993-10-06 | 1999-07-12 | 日本ペイント株式会社 | Hydrophilic surface treatment aqueous solution, hydrophilic surface treatment method and hydrophilic surface treatment film |
| JPH08165210A (en) | 1994-12-14 | 1996-06-25 | Taki Chem Co Ltd | Production of antimicrobial agent and antimicrobial agent |
| JPH08199089A (en) | 1995-01-25 | 1996-08-06 | Tomoegawa Paper Co Ltd | Powder paint |
| GB2297552B (en) | 1995-02-03 | 1998-12-16 | Rainer Clover | Powder coating |
| JPH08217998A (en) | 1995-02-15 | 1996-08-27 | Kansai Paint Co Ltd | Antibacterial powder coating composition and production thereof |
| DE69632996T2 (en) | 1995-12-26 | 2005-07-21 | Toyo Boseki K.K. | Organic solvent-soluble mucopolysaccharides, antibacterial antithrombogenic agent and medical material |
| WO1997042824A1 (en)* | 1996-05-10 | 1997-11-20 | Toyo Boseki Kabushiki Kaisha | Antimicrobial composition and antimicrobial laminate |
| JPH1023425A (en) | 1996-07-01 | 1998-01-23 | Sony Corp | Device and method for encoding picture, device and method for decoding picture and picture recording medium |
| WO1998008884A1 (en)* | 1996-08-26 | 1998-03-05 | Tyndale Plains-Hunter, Ltd. | Hydrophilic and hydrophobic polyether polyurethanes and uses therefor |
| JPH10316899A (en) | 1997-05-19 | 1998-12-02 | Makoto Sangyo:Kk | Construction material having antifungal and antibacterial function |
| JP4005184B2 (en) | 1997-09-01 | 2007-11-07 | インターメタリックス株式会社 | Powder coating and film formation method using the powder coating |
| JPH11169724A (en)* | 1997-12-17 | 1999-06-29 | San Techno:Kk | Composite particle material having anti-bacterial function |
| JPH11222402A (en)* | 1998-02-04 | 1999-08-17 | Osaka Gas Co Ltd | Antimicrobial polymer particle and its production |
| JP4874452B2 (en)* | 1998-03-05 | 2012-02-15 | 住友化学株式会社 | Method for microencapsulation of solid substance and microcapsule composition |
| US7354596B1 (en)* | 1998-05-01 | 2008-04-08 | 3M Innovative Properties Company | Anti-microbial agent delivery system |
| JP4066512B2 (en) | 1998-05-20 | 2008-03-26 | マツダ株式会社 | Engine oil pump structure |
| JP2000028286A (en) | 1998-07-08 | 2000-01-28 | Toyobo Co Ltd | Fin for heat exchanger with excellent antibacterial, mildew protective and hydrophilic properties |
| JP2000159614A (en)* | 1998-09-24 | 2000-06-13 | Sumitomo Chem Co Ltd | Termite composition |
| US6585767B1 (en) | 1998-11-23 | 2003-07-01 | Agion Technologies, Inc. | Antimicrobial suturing ring for heart valve |
| US6436422B1 (en) | 1998-11-23 | 2002-08-20 | Agion Technologies L.L.C. | Antibiotic hydrophilic polymer coating |
| US6296863B1 (en) | 1998-11-23 | 2001-10-02 | Agion Technologies, Llc | Antimicrobial fabric and medical graft of the fabric |
| EP1175236A1 (en) | 1999-04-23 | 2002-01-30 | Agion Technologies, L.L.C. | Stent having antimicrobial agent |
| US6582715B1 (en) | 1999-04-27 | 2003-06-24 | Agion Technologies, Inc. | Antimicrobial orthopedic implants |
| IL135487A (en) | 2000-04-05 | 2005-07-25 | Cupron Corp | Antimicrobial and antiviral polymeric materials and a process for preparing the same |
| US6866859B2 (en) | 2000-08-30 | 2005-03-15 | Biocoat Incorporated | Bi-laminar, hyaluronan coatings with silver-based anti-microbial properties |
| US6479144B2 (en) | 2000-12-04 | 2002-11-12 | Milliken & Company | Anti-tack spandex fibers containing antimicrobial agents therein and fabrics made therefrom |
| US7906148B2 (en) | 2003-07-31 | 2011-03-15 | Boston Scientific Scimed, Inc. | Latex medical articles for release of antimicrobial agents |
- 2001
- 2001-12-21USUS10/032,372patent/US7357949B2/ennot_activeExpired - Fee Related
- 2002
- 2002-12-11MXMXPA04006185Apatent/MXPA04006185A/enactiveIP Right Grant
- 2002-12-11AUAU2002351366Apatent/AU2002351366B2/ennot_activeCeased
- 2002-12-11JPJP2003556466Apatent/JP2005514402A/enactivePending
- 2002-12-11CACA2470122Apatent/CA2470122C/ennot_activeExpired - Fee Related
- 2002-12-11WOPCT/US2002/039709patent/WO2003055941A1/enactiveApplication Filing
- 2002-12-11EPEP02787023Apatent/EP1456291A1/ennot_activeWithdrawn
- 2006
- 2006-01-20USUS11/336,699patent/US7354605B2/ennot_activeExpired - Fee Related
Patent Citations (22)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US3975350A (en)* | 1972-08-02 | 1976-08-17 | Princeton Polymer Laboratories, Incorporated | Hydrophilic or hydrogel carrier systems such as coatings, body implants and other articles |
| US4403083A (en)* | 1979-06-01 | 1983-09-06 | W. R. Grace & Co. | Preparation of solid polyurethane particles |
| US4657835A (en)* | 1984-05-31 | 1987-04-14 | Canon Kabushiki Kaisha | Electrophotographic photosensitive member having an intermediate layer of conductive powder and resin or oligimer |
| US5238749A (en)* | 1986-03-27 | 1993-08-24 | Clinitex Corporation | Antimicrobial coating process and product |
| US5180585A (en)* | 1991-08-09 | 1993-01-19 | E. I. Du Pont De Nemours And Company | Antimicrobial compositions, process for preparing the same and use |
| US5382424A (en)* | 1991-12-11 | 1995-01-17 | The Procter & Gamble Company | Breath protection microcapsules |
| US6290962B1 (en)* | 1992-11-03 | 2001-09-18 | Oravax, Inc. | Urease-based vaccine and treatment for helicobacter infection |
| US6399735B1 (en)* | 1992-12-17 | 2002-06-04 | Henkel Kommanditgesellschaft Auf Aktien | Hydrophilic polyurethanes |
| US6046243A (en)* | 1993-02-12 | 2000-04-04 | Bernard Technologies, Inc. | Compositions for sustained release of a gas |
| US5599583A (en)* | 1994-05-27 | 1997-02-04 | Micro Flo Company | Encapsulation with water soluble polymer |
| US6413536B1 (en)* | 1995-06-07 | 2002-07-02 | Southern Biosystems, Inc. | High viscosity liquid controlled delivery system and medical or surgical device |
| US6129782A (en)* | 1996-06-05 | 2000-10-10 | Brodie; Harold | Inhibition of bacterial growth |
| US5980620A (en)* | 1996-06-05 | 1999-11-09 | Brodie; Harold | Inhibition of bacterial growth |
| US6432416B1 (en)* | 1997-10-03 | 2002-08-13 | Dupont Powder Coatings Usa, Inc. | Anti-microbial power coating |
| US6093407A (en)* | 1997-10-03 | 2000-07-25 | Dupont Powder Coatings Usa, Inc. | Anti-microbial powder coatings |
| US6287285B1 (en)* | 1998-01-30 | 2001-09-11 | Advanced Cardiovascular Systems, Inc. | Therapeutic, diagnostic, or hydrophilic coating for an intracorporeal medical device |
| US6156245A (en)* | 1998-03-05 | 2000-12-05 | Sumitomo Chemical Company, Limited | Method for microencapsulating of a solid substance |
| US6113936A (en)* | 1998-05-18 | 2000-09-05 | Sumitomo Chemical Company, Limited | Method for microencapsulating a solid substance |
| US6123925A (en)* | 1998-07-27 | 2000-09-26 | Healthshield Technologies L.L.C. | Antibiotic toothpaste |
| US6296834B1 (en)* | 1999-04-19 | 2001-10-02 | Unilever Home & Personal Care Usa, Division Of Conopco, Inc. | Oral care composition |
| US20010009831A1 (en)* | 1999-12-03 | 2001-07-26 | Michael Schink | Antimicrobial wound coverings |
| US20030043341A1 (en)* | 2001-08-02 | 2003-03-06 | Turner David C. | Antimicrobial lenses and methods of their use |
Cited By (58)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US20060034423A1 (en)* | 1998-08-07 | 2006-02-16 | Juergen Pensel | Surgical microscope |
| US20050191355A1 (en)* | 1999-05-27 | 2005-09-01 | Foss Manufacturing Co., Inc. | Anti-microbial and antifungal fluid conduits and methods of manufacture thereof |
| US20090098373A1 (en)* | 2001-10-02 | 2009-04-16 | Henkelstrasse 67 | Anodized coating over aluminum and aluminum alloy coated substrates and coated articles |
| US8663807B2 (en) | 2001-10-02 | 2014-03-04 | Henkel Ag & Co. Kgaa | Article of manufacture and process for anodically coating aluminum and/or titanium with ceramic oxides |
| US9023481B2 (en) | 2001-10-02 | 2015-05-05 | Henkel Ag & Co. Kgaa | Anodized coating over aluminum and aluminum alloy coated substrates and coated articles |
| US7276056B2 (en) | 2002-02-20 | 2007-10-02 | Abbott Research Group, Inc. | Methods of treating abnormal biological conditions by vaginal douching |
| US20040215164A1 (en)* | 2002-02-20 | 2004-10-28 | Abbott Chun Lim | Methods of treating abnormal biological conditions using metal oxides |
| US8118789B2 (en) | 2002-02-20 | 2012-02-21 | Abbott Research Group, Inc. | Deodorizer devices and systems for controlling perspiration-related body odor |
| US20070260202A1 (en)* | 2002-02-20 | 2007-11-08 | Dominic Abbott | Deodorizer devices, systems and methods for controlling perspiration-related body odor |
| US7270653B2 (en) | 2002-02-20 | 2007-09-18 | Abbott Research Group | Methods of treating abnormal biological conditions using metal oxides |
| US20060008646A1 (en)* | 2002-06-12 | 2006-01-12 | Traptek Llc. | Encapsulated active particles and methods for making and using the same |
| US7641912B1 (en)* | 2003-01-13 | 2010-01-05 | EnviroCare Corporation | Antimicrobial coatings for treatment of surfaces in a building setting and method of applying same |
| US8282951B2 (en) | 2003-01-13 | 2012-10-09 | EnviroCare Corporation | Antimicrobial coatings for treatment of surfaces in a building setting and method of applying same |
| US20110125093A1 (en)* | 2003-07-31 | 2011-05-26 | Boston Scientific Scimed, Inc. | Latex medical articles for release of antimicrobial agents |
| US7906148B2 (en) | 2003-07-31 | 2011-03-15 | Boston Scientific Scimed, Inc. | Latex medical articles for release of antimicrobial agents |
| WO2005011768A1 (en)* | 2003-07-31 | 2005-02-10 | Scimed Life Systems, Inc. | Latex medical articles for release of antimicrobial agents |
| EP1790224A1 (en)* | 2003-11-17 | 2007-05-30 | Bio-Gate AG | Antimicrobial layered material |
| US10299472B2 (en) | 2003-11-17 | 2019-05-28 | Bio-Gate Ag | Coating material |
| US9622471B2 (en) | 2003-11-17 | 2017-04-18 | Bio-Gate Ag | Coating material |
| US20090035341A1 (en)* | 2003-11-17 | 2009-02-05 | Michael Wagener | Coating material |
| WO2005048708A1 (en)* | 2003-11-17 | 2005-06-02 | Bio-Gate Ag | Antimicrobial composite material |
| US20050271746A1 (en)* | 2004-05-18 | 2005-12-08 | Abbott Chun L | Topical treatments for abnormal biological conditions and method of topically treating such conditions |
| US20060156948A1 (en)* | 2004-06-24 | 2006-07-20 | Hendriks Eugene P | Color stable antimicrobial coatings |
| US7595355B2 (en) | 2004-06-24 | 2009-09-29 | Agion Technologies, Inc. | Antimicrobial coating for erosive environments |
| US7598300B2 (en) | 2004-06-24 | 2009-10-06 | Agion Technologies | Antimicrobial coating for erosive environments |
| US7585902B2 (en) | 2004-06-24 | 2009-09-08 | Agion Technologies | Antimicrobial coating for erosive environments |
| US7645824B2 (en) | 2004-06-24 | 2010-01-12 | Agion Technologies, Inc | Color stable antimicrobial coatings |
| US20080131606A1 (en)* | 2004-06-24 | 2008-06-05 | Trogolo Jeffrey A | Antimicrobial coating for erosive environments |
| US20070243263A1 (en)* | 2006-04-14 | 2007-10-18 | Agion Technologies, Inc. | Antiviral Methods |
| US20070269499A1 (en)* | 2006-04-28 | 2007-11-22 | John Hen | Materials and methods for wound treatment |
| US20110020425A1 (en)* | 2006-04-28 | 2011-01-27 | Biolife, Llc | Materials and methods for wound treament |
| US8945287B2 (en)* | 2006-05-09 | 2015-02-03 | Cocona, Inc. | Active particle-enhanced membrane and methods for making and using the same |
| TWI449566B (en)* | 2006-05-09 | 2014-08-21 | Cocona Inc | Active particle-enhanced membrane and methods for making and using the same |
| US20070264203A1 (en)* | 2006-05-09 | 2007-11-15 | Traptek Llc | Active particle-enhanced membrane and methods for making and using the same |
| WO2007133640A1 (en) | 2006-05-09 | 2007-11-22 | Traptek Llc | Active particle-enhanced membrane and methods for making and using the same |
| US20080044474A1 (en)* | 2006-08-18 | 2008-02-21 | Bayer Materialscience Ag | Dispersions of nanoureas comprising biologically active compounds |
| US20080121141A1 (en)* | 2006-11-16 | 2008-05-29 | Haggquist Gregory W | Exothermic-enhanced articles and methods for making the same |
| US20100196487A1 (en)* | 2007-09-12 | 2010-08-05 | Trovotech Gmbh | Composition with antimicrobial effect |
| WO2009036862A3 (en)* | 2007-09-12 | 2010-02-18 | Trovotech Gmbh | Compositions with an antimicrobial action |
| WO2009036862A2 (en) | 2007-09-12 | 2009-03-26 | Trovotech Gmbh | Compositions with an antimicrobial action |
| US9173406B2 (en)* | 2008-04-04 | 2015-11-03 | Fraunhofer-Gesellschaft zur Förderung der angewandten Forschung e.V. | Coating material |
| US20110104477A1 (en)* | 2008-04-04 | 2011-05-05 | Michael Wagener | Coating material |
| US20100050197A1 (en)* | 2008-07-25 | 2010-02-25 | Disctekk, Llc | Optical card |
| US10099192B2 (en) | 2009-02-09 | 2018-10-16 | Cellutech Ab | Polymer shells |
| WO2010090594A1 (en)* | 2009-02-09 | 2010-08-12 | Swetree Technologies Ab | Polymer shells |
| US20100252241A1 (en)* | 2009-04-02 | 2010-10-07 | Mcdermott Chris | Ceramic coated automotive heat exchanger components |
| US9701177B2 (en)* | 2009-04-02 | 2017-07-11 | Henkel Ag & Co. Kgaa | Ceramic coated automotive heat exchanger components |
| US20110152843A1 (en)* | 2009-12-18 | 2011-06-23 | Wedlin Charlotte | Medical device for short time use with quickly releasable antibacterial agent |
| US10172978B2 (en)* | 2009-12-18 | 2019-01-08 | Astra Tech Ab | Medical device for short time use with quickly releasable antibacterial agent |
| CN105120660A (en)* | 2013-03-15 | 2015-12-02 | 奥麒化工股份有限公司 | Active ingredient encapsulation and method of manufacture |
| US9820481B2 (en) | 2013-03-15 | 2017-11-21 | Arch Chemicals, Inc. | Encapsulation of active ingredients and method of making |
| WO2014143695A1 (en)* | 2013-03-15 | 2014-09-18 | Arch Chemicals, Inc. | Encapsulation of active ingredients and method of making |
| US9243141B1 (en)* | 2014-11-03 | 2016-01-26 | Xerox Corporation | Coated silver nanoparticle composites comprising a sulfonated polyester matrix and methods of making the same |
| CN107847637A (en)* | 2015-07-24 | 2018-03-27 | 墨尼克医疗用品有限公司 | The antimicrobial wound dressing of absorbability |
| US20220112029A1 (en)* | 2015-11-10 | 2022-04-14 | Simplehuman, Llc | Household goods with antimicrobial coatings and methods of making thereof |
| US20220204258A1 (en)* | 2015-11-10 | 2022-06-30 | Simplehuman, Llc | Antimicrobial coatings for household goods |
| US11622557B2 (en)* | 2016-10-31 | 2023-04-11 | Applied Silver, Inc. | Dispensing of metal ions into batch laundry washers and dryers |
| WO2018090152A1 (en)* | 2016-11-15 | 2018-05-24 | Roberto Felipe Moser Rossel | Particles with antimicrobial properties, completely coated by at least one polymer, and which maintain the particle state thereof |
Also Published As
| Publication number | Publication date |
|---|---|
| CA2470122A1 (en) | 2003-07-10 |
| AU2002351366B2 (en) | 2007-12-06 |
| US7357949B2 (en) | 2008-04-15 |
| WO2003055941A1 (en) | 2003-07-10 |
| CA2470122C (en) | 2012-02-14 |
| MXPA04006185A (en) | 2005-06-20 |
| EP1456291A1 (en) | 2004-09-15 |
| US7354605B2 (en) | 2008-04-08 |
| AU2002351366A1 (en) | 2003-07-15 |
| JP2005514402A (en) | 2005-05-19 |
| US20060121078A1 (en) | 2006-06-08 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| US7354605B2 (en) | Antimicrobial medical device | |
| US20030118658A1 (en) | High aspect ratio encapsulated inorganic antimicrobial additive for controlled release | |
| EP1133327B1 (en) | Antibiotic hydrophilic polymer coating | |
| US7595355B2 (en) | Antimicrobial coating for erosive environments | |
| JP2005514402A5 (en) | ||
| AU604724B2 (en) | Polymer containing amorphous aluminosilicate particles and process for producing the same | |
| KR950013687B1 (en) | Antimicrobial Zeolite-Containing Film | |
| US8063116B2 (en) | Antimicrobial powder coatings and method | |
| US20070199890A1 (en) | Antimicrobial activated carbon and use thereof | |
| WO2000006208A1 (en) | Antibiotic toothpaste | |
| KR100587465B1 (en) | Silver-supported inorganic antimicrobial agent and thermoplastic resin masterbatch containing same | |
| JP3705920B2 (en) | Antibacterial zeolite | |
| CN113736200A (en) | Antibacterial super absorbent resin composite material and preparation method thereof | |
| JPH04126152A (en) | Antimicrobial composition | |
| US7179530B2 (en) | Antimicrobial composite material | |
| JP3333791B2 (en) | Antibacterial resin composition | |
| JPH04231063A (en) | Antimicrobial composition | |
| JP3571373B2 (en) | Antibacterial resin composition for stationery and antibacterial stationery | |
| JP2826944B2 (en) | Antimicrobial polyolefin resin composition and molded article thereof | |
| KR0152315B1 (en) | Manufacturing method of antimicrobial resin | |
| JPH0816178B2 (en) | Polymer | |
| JPH04142349A (en) | Antibacterial and fungicidal polyolefin rein molding and preparation thereof | |
| JPH0525012A (en) | Antibacterial composition | |
| JPS63270756A (en) | Silver ion-containing bactericidal halogenated polymer composition and its production | |
| PL378952A1 (en) | Silver preparations for imparting bactericidal and fungicidal properties as well as method for imparting bactericidal and fungicidal properties to materials |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| AS | Assignment | Owner name:AGION TECHNOLOGIES, INC., MASSACHUSETTS Free format text:ASSIGNMENT OF ASSIGNORS INTEREST;ASSIGNORS:TROGOLO, JEFFREY A.;ROSSITTO, FRANK C.;WELCH, EDWARD K. II;REEL/FRAME:013925/0405 Effective date:20030116 | |
| AS | Assignment | Owner name:PALADIN CAPITAL MANAGEMENT II, LLC, GEORGIA Free format text:SECURITY INTEREST;ASSIGNOR:AGION TECHNOLOGIES, INC.;REEL/FRAME:014830/0935 Effective date:20031219 | |
| AS | Assignment | Owner name:AGION TECHNOLOGIES, INC., MASSACHUSETTS Free format text:TERMINATION OF PATENT SECURITY AGREEMENT;ASSIGNOR:PALADIN CAPITAL MANAGEMENT II, LLC;REEL/FRAME:015494/0070 Effective date:20041223 | |
| AS | Assignment | Owner name:PALADIN CAPITAL MANAGEMENT II, LLC, AS AGENT, DIST Free format text:PATENT SECURITY AGREEMENT;ASSIGNOR:AGION TECHNOLOGIES, INC.;REEL/FRAME:020690/0251 Effective date:20080325 | |
| AS | Assignment | Owner name:PALADIN CAPITAL MANAGEMENT II, LLC, DISTRICT OF CO Free format text:SECURITY AGREEMENT;ASSIGNOR:AGION TECHNOLOGIES, INC.;REEL/FRAME:021763/0538 Effective date:20081029 Owner name:PALADIN CAPITAL MANAGEMENT II, LLC, DISTRICT OF CO Free format text:SECURITY AGREEMENT;ASSIGNOR:AGION TECHNOLOGIES, INC.;REEL/FRAME:021763/0730 Effective date:20081029 | |
| AS | Assignment | Owner name:SCIESSENT LLC, MASSACHUSETTS Free format text:ASSIGNMENT OF ASSIGNORS INTEREST;ASSIGNOR:AGION TECHNOLOGIES, INC.;REEL/FRAME:026323/0601 Effective date:20110222 | |
| FPAY | Fee payment | Year of fee payment:4 | |
| REMI | Maintenance fee reminder mailed | ||
| LAPS | Lapse for failure to pay maintenance fees | ||
| STCH | Information on status: patent discontinuation | Free format text:PATENT EXPIRED DUE TO NONPAYMENT OF MAINTENANCE FEES UNDER 37 CFR 1.362 | |
| FP | Lapsed due to failure to pay maintenance fee | Effective date:20160415 |