US20030051733A1 - Method and apparatus for endobronchial diagnosis - Google Patents
Method and apparatus for endobronchial diagnosisDownload PDFInfo
- Publication number
- US20030051733A1 US20030051733A1US10/241,733US24173302AUS2003051733A1US 20030051733 A1US20030051733 A1US 20030051733A1US 24173302 AUS24173302 AUS 24173302AUS 2003051733 A1US2003051733 A1US 2003051733A1
- Authority
- US
- United States
- Prior art keywords
- lung
- compartment
- catheter
- measurement data
- pulmonary
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Abandoned
Links
- 238000000034methodMethods0.000titleclaimsabstractdescription59
- 238000003745diagnosisMethods0.000titledescription6
- 210000004072lungAnatomy0.000claimsabstractdescription263
- 230000002685pulmonary effectEffects0.000claimsabstractdescription130
- 238000005259measurementMethods0.000claimsabstractdescription99
- 238000011282treatmentMethods0.000claimsabstractdescription59
- 238000012360testing methodMethods0.000claimsabstractdescription48
- 230000000007visual effectEffects0.000claimsabstractdescription42
- 239000007789gasSubstances0.000claimsdescription83
- 230000007246mechanismEffects0.000claimsdescription72
- 238000003384imaging methodMethods0.000claimsdescription57
- 239000012530fluidSubstances0.000claimsdescription36
- 238000012545processingMethods0.000claimsdescription22
- CURLTUGMZLYLDI-UHFFFAOYSA-NCarbon dioxideChemical compoundO=C=OCURLTUGMZLYLDI-UHFFFAOYSA-N0.000claimsdescription16
- 238000013507mappingMethods0.000claimsdescription15
- 238000010790dilutionMethods0.000claimsdescription13
- 239000012895dilutionSubstances0.000claimsdescription13
- QVGXLLKOCUKJST-UHFFFAOYSA-Natomic oxygenChemical compound[O]QVGXLLKOCUKJST-UHFFFAOYSA-N0.000claimsdescription12
- 229910052756noble gasInorganic materials0.000claimsdescription12
- 239000001301oxygenSubstances0.000claimsdescription12
- 229910052760oxygenInorganic materials0.000claimsdescription12
- 238000011038discontinuous diafiltration by volume reductionMethods0.000claimsdescription10
- 229910002092carbon dioxideInorganic materials0.000claimsdescription9
- 239000001569carbon dioxideSubstances0.000claimsdescription7
- 230000033001locomotionEffects0.000claimsdescription6
- 230000003287optical effectEffects0.000claimsdescription6
- 238000012546transferMethods0.000claimsdescription6
- 2390000111653D compositeSubstances0.000claimsdescription4
- 238000002594fluoroscopyMethods0.000claimsdescription4
- 230000000241respiratory effectEffects0.000claimsdescription4
- 238000013125spirometryMethods0.000claimsdescription4
- 238000002604ultrasonographyMethods0.000claimsdescription4
- 239000000126substanceSubstances0.000claimsdescription3
- 239000002131composite materialSubstances0.000claimsdescription2
- 239000012528membraneSubstances0.000claimsdescription2
- 238000006552photochemical reactionMethods0.000claimsdescription2
- 238000002834transmittanceMethods0.000claimsdescription2
- 239000003570airSubstances0.000claims2
- UBAZGMLMVVQSCD-UHFFFAOYSA-Ncarbon dioxide;molecular oxygenChemical compoundO=O.O=C=OUBAZGMLMVVQSCD-UHFFFAOYSA-N0.000claims2
- 239000000203mixtureSubstances0.000claims2
- 239000000243solutionSubstances0.000claims2
- XLYOFNOQVPJJNP-UHFFFAOYSA-NwaterSubstancesOXLYOFNOQVPJJNP-UHFFFAOYSA-N0.000claims2
- 238000009530blood pressure measurementMethods0.000claims1
- 238000002595magnetic resonance imagingMethods0.000claims1
- 201000010099diseaseDiseases0.000abstractdescription40
- 208000037265diseases, disorders, signs and symptomsDiseases0.000abstractdescription40
- 238000002955isolationMethods0.000abstractdescription7
- 208000019693Lung diseaseDiseases0.000abstractdescription3
- 230000009286beneficial effectEffects0.000abstractdescription2
- 230000000694effectsEffects0.000description15
- 230000006870functionEffects0.000description12
- 210000003484anatomyAnatomy0.000description10
- 238000002405diagnostic procedureMethods0.000description9
- 239000000835fiberSubstances0.000description9
- 210000000621bronchiAnatomy0.000description8
- 238000002591computed tomographyMethods0.000description6
- 239000007788liquidSubstances0.000description6
- 230000009467reductionEffects0.000description6
- 230000007423decreaseEffects0.000description5
- 230000029058respiratory gaseous exchangeEffects0.000description5
- 238000002001electrophysiologyMethods0.000description4
- 230000007831electrophysiologyEffects0.000description4
- 238000011156evaluationMethods0.000description4
- 230000003434inspiratory effectEffects0.000description4
- 239000000463materialSubstances0.000description4
- 238000002156mixingMethods0.000description4
- 230000010412perfusionEffects0.000description4
- 210000003437tracheaAnatomy0.000description4
- 238000009423ventilationMethods0.000description4
- 208000002352blisterDiseases0.000description3
- 238000001125extrusionMethods0.000description3
- 230000036541healthEffects0.000description3
- 239000001307heliumSubstances0.000description3
- 229910052734heliumInorganic materials0.000description3
- SWQJXJOGLNCZEY-UHFFFAOYSA-Nhelium atomChemical compound[He]SWQJXJOGLNCZEY-UHFFFAOYSA-N0.000description3
- 238000012634optical imagingMethods0.000description3
- 230000009325pulmonary functionEffects0.000description3
- 238000012800visualizationMethods0.000description3
- 208000006545Chronic Obstructive Pulmonary DiseaseDiseases0.000description2
- 206010014561EmphysemaDiseases0.000description2
- 230000008901benefitEffects0.000description2
- 239000008280bloodSubstances0.000description2
- 210000004369bloodAnatomy0.000description2
- 206010006451bronchitisDiseases0.000description2
- 230000015556catabolic processEffects0.000description2
- 230000008859changeEffects0.000description2
- 238000006243chemical reactionMethods0.000description2
- 238000013170computed tomography imagingMethods0.000description2
- 238000010276constructionMethods0.000description2
- 238000002059diagnostic imagingMethods0.000description2
- 230000026058directional locomotionEffects0.000description2
- 238000005286illuminationMethods0.000description2
- 229920001684low density polyethylenePolymers0.000description2
- 238000012544monitoring processMethods0.000description2
- 238000004806packaging method and processMethods0.000description2
- 230000035479physiological effects, processes and functionsEffects0.000description2
- 238000002600positron emission tomographyMethods0.000description2
- 230000008569processEffects0.000description2
- 230000004044responseEffects0.000description2
- 230000000717retained effectEffects0.000description2
- 238000007789sealingMethods0.000description2
- 238000001356surgical procedureMethods0.000description2
- 238000002560therapeutic procedureMethods0.000description2
- 206010001052Acute respiratory distress syndromeDiseases0.000description1
- 206010003598AtelectasisDiseases0.000description1
- 206010006458Bronchitis chronicDiseases0.000description1
- 102000008186CollagenHuman genes0.000description1
- 108010035532CollagenProteins0.000description1
- 206010061218InflammationDiseases0.000description1
- 206010030113OedemaDiseases0.000description1
- 239000004696Poly ether ether ketoneSubstances0.000description1
- 239000004952PolyamideSubstances0.000description1
- 208000007123Pulmonary AtelectasisDiseases0.000description1
- 208000013616Respiratory Distress SyndromeDiseases0.000description1
- 238000013473artificial intelligenceMethods0.000description1
- 208000006673asthmaDiseases0.000description1
- JUPQTSLXMOCDHR-UHFFFAOYSA-Nbenzene-1,4-diol;bis(4-fluorophenyl)methanoneChemical compoundOC1=CC=C(O)C=C1.C1=CC(F)=CC=C1C(=O)C1=CC=C(F)C=C1JUPQTSLXMOCDHR-UHFFFAOYSA-N0.000description1
- 238000004364calculation methodMethods0.000description1
- 208000007451chronic bronchitisDiseases0.000description1
- 229920001436collagenPolymers0.000description1
- 238000004891communicationMethods0.000description1
- 238000004590computer programMethods0.000description1
- 239000002872contrast mediaSubstances0.000description1
- 229940039231contrast mediaDrugs0.000description1
- 230000001419dependent effectEffects0.000description1
- 238000013461designMethods0.000description1
- 238000011161developmentMethods0.000description1
- 238000007435diagnostic evaluationMethods0.000description1
- 239000003172expectorant agentSubstances0.000description1
- 238000011990functional testingMethods0.000description1
- 230000004927fusionEffects0.000description1
- 238000010438heat treatmentMethods0.000description1
- 229920001903high density polyethylenePolymers0.000description1
- 208000015181infectious diseaseDiseases0.000description1
- 230000004054inflammatory processEffects0.000description1
- 230000002262irrigationEffects0.000description1
- 238000003973irrigationMethods0.000description1
- 229920000126latexPolymers0.000description1
- 239000004816latexSubstances0.000description1
- 239000004702low-density polyethyleneSubstances0.000description1
- 230000004199lung functionEffects0.000description1
- 238000000691measurement methodMethods0.000description1
- 238000000968medical method and processMethods0.000description1
- 238000012986modificationMethods0.000description1
- 230000004048modificationEffects0.000description1
- 229940066491mucolyticsDrugs0.000description1
- 229920001778nylonPolymers0.000description1
- 239000013307optical fiberSubstances0.000description1
- 230000003534oscillatory effectEffects0.000description1
- 230000036284oxygen consumptionEffects0.000description1
- 238000006213oxygenation reactionMethods0.000description1
- 229920002647polyamidePolymers0.000description1
- 229920002530polyetherether ketonePolymers0.000description1
- 229920001343polytetrafluoroethylenePolymers0.000description1
- 239000004810polytetrafluoroethyleneSubstances0.000description1
- 229920002635polyurethanePolymers0.000description1
- 239000004814polyurethaneSubstances0.000description1
- 229920000915polyvinyl chloridePolymers0.000description1
- 239000004800polyvinyl chlorideSubstances0.000description1
- 238000011160researchMethods0.000description1
- 238000002271resectionMethods0.000description1
- 229920002379silicone rubberPolymers0.000description1
- 239000004945silicone rubberSubstances0.000description1
- 238000002603single-photon emission computed tomographyMethods0.000description1
- 238000009662stress testingMethods0.000description1
- 239000004094surface-active agentSubstances0.000description1
- 230000008685targetingEffects0.000description1
- 239000003106tissue adhesiveSubstances0.000description1
- 229940075469tissue adhesivesDrugs0.000description1
- 230000000472traumatic effectEffects0.000description1
Images
Classifications
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61B—DIAGNOSIS; SURGERY; IDENTIFICATION
- A61B5/00—Measuring for diagnostic purposes; Identification of persons
- A61B5/68—Arrangements of detecting, measuring or recording means, e.g. sensors, in relation to patient
- A61B5/6846—Arrangements of detecting, measuring or recording means, e.g. sensors, in relation to patient specially adapted to be brought in contact with an internal body part, i.e. invasive
- A61B5/6847—Arrangements of detecting, measuring or recording means, e.g. sensors, in relation to patient specially adapted to be brought in contact with an internal body part, i.e. invasive mounted on an invasive device
- A61B5/6852—Catheters
- A61B5/6853—Catheters with a balloon
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61B—DIAGNOSIS; SURGERY; IDENTIFICATION
- A61B5/00—Measuring for diagnostic purposes; Identification of persons
- A61B5/05—Detecting, measuring or recording for diagnosis by means of electric currents or magnetic fields; Measuring using microwaves or radio waves
- A61B5/055—Detecting, measuring or recording for diagnosis by means of electric currents or magnetic fields; Measuring using microwaves or radio waves involving electronic [EMR] or nuclear [NMR] magnetic resonance, e.g. magnetic resonance imaging
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61B—DIAGNOSIS; SURGERY; IDENTIFICATION
- A61B5/00—Measuring for diagnostic purposes; Identification of persons
- A61B5/08—Measuring devices for evaluating the respiratory organs
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61B—DIAGNOSIS; SURGERY; IDENTIFICATION
- A61B5/00—Measuring for diagnostic purposes; Identification of persons
- A61B5/08—Measuring devices for evaluating the respiratory organs
- A61B5/0813—Measurement of pulmonary parameters by tracers, e.g. radioactive tracers
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61B—DIAGNOSIS; SURGERY; IDENTIFICATION
- A61B5/00—Measuring for diagnostic purposes; Identification of persons
- A61B5/08—Measuring devices for evaluating the respiratory organs
- A61B5/082—Evaluation by breath analysis, e.g. determination of the chemical composition of exhaled breath
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61B—DIAGNOSIS; SURGERY; IDENTIFICATION
- A61B5/00—Measuring for diagnostic purposes; Identification of persons
- A61B5/08—Measuring devices for evaluating the respiratory organs
- A61B5/083—Measuring rate of metabolism by using breath test, e.g. measuring rate of oxygen consumption
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61B—DIAGNOSIS; SURGERY; IDENTIFICATION
- A61B5/00—Measuring for diagnostic purposes; Identification of persons
- A61B5/08—Measuring devices for evaluating the respiratory organs
- A61B5/085—Measuring impedance of respiratory organs or lung elasticity
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61B—DIAGNOSIS; SURGERY; IDENTIFICATION
- A61B5/00—Measuring for diagnostic purposes; Identification of persons
- A61B5/08—Measuring devices for evaluating the respiratory organs
- A61B5/087—Measuring breath flow
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61B—DIAGNOSIS; SURGERY; IDENTIFICATION
- A61B5/00—Measuring for diagnostic purposes; Identification of persons
- A61B5/72—Signal processing specially adapted for physiological signals or for diagnostic purposes
- A61B5/7271—Specific aspects of physiological measurement analysis
- A61B5/7278—Artificial waveform generation or derivation, e.g. synthesizing signals from measured signals
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61B—DIAGNOSIS; SURGERY; IDENTIFICATION
- A61B5/00—Measuring for diagnostic purposes; Identification of persons
- A61B5/74—Details of notification to user or communication with user or patient; User input means
- A61B5/742—Details of notification to user or communication with user or patient; User input means using visual displays
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61B—DIAGNOSIS; SURGERY; IDENTIFICATION
- A61B6/00—Apparatus or devices for radiation diagnosis; Apparatus or devices for radiation diagnosis combined with radiation therapy equipment
- A61B6/48—Diagnostic techniques
- A61B6/481—Diagnostic techniques involving the use of contrast agents
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61B—DIAGNOSIS; SURGERY; IDENTIFICATION
- A61B6/00—Apparatus or devices for radiation diagnosis; Apparatus or devices for radiation diagnosis combined with radiation therapy equipment
- A61B6/48—Diagnostic techniques
- A61B6/485—Diagnostic techniques involving fluorescence X-ray imaging
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61B—DIAGNOSIS; SURGERY; IDENTIFICATION
- A61B8/00—Diagnosis using ultrasonic, sonic or infrasonic waves
- A61B8/12—Diagnosis using ultrasonic, sonic or infrasonic waves in body cavities or body tracts, e.g. by using catheters
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M16/00—Devices for influencing the respiratory system of patients by gas treatment, e.g. ventilators; Tracheal tubes
- A61M16/04—Tracheal tubes
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M16/00—Devices for influencing the respiratory system of patients by gas treatment, e.g. ventilators; Tracheal tubes
- A61M16/04—Tracheal tubes
- A61M16/0402—Special features for tracheal tubes not otherwise provided for
- A61M16/0404—Special features for tracheal tubes not otherwise provided for with means for selective or partial lung respiration
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M16/00—Devices for influencing the respiratory system of patients by gas treatment, e.g. ventilators; Tracheal tubes
- A61M16/04—Tracheal tubes
- A61M16/0434—Cuffs
- A61M16/0454—Redundant cuffs
- A61M16/0459—Redundant cuffs one cuff behind another
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M16/00—Devices for influencing the respiratory system of patients by gas treatment, e.g. ventilators; Tracheal tubes
- A61M16/04—Tracheal tubes
- A61M16/0486—Multi-lumen tracheal tubes
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M25/00—Catheters; Hollow probes
- A61M25/0021—Catheters; Hollow probes characterised by the form of the tubing
- A61M25/0023—Catheters; Hollow probes characterised by the form of the tubing by the form of the lumen, e.g. cross-section, variable diameter
- A61M25/0026—Multi-lumen catheters with stationary elements
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M25/00—Catheters; Hollow probes
- A61M25/10—Balloon catheters
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61B—DIAGNOSIS; SURGERY; IDENTIFICATION
- A61B2562/00—Details of sensors; Constructional details of sensor housings or probes; Accessories for sensors
- A61B2562/02—Details of sensors specially adapted for in-vivo measurements
- A61B2562/0247—Pressure sensors
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M16/00—Devices for influencing the respiratory system of patients by gas treatment, e.g. ventilators; Tracheal tubes
- A61M16/04—Tracheal tubes
- A61M16/0434—Cuffs
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M16/00—Devices for influencing the respiratory system of patients by gas treatment, e.g. ventilators; Tracheal tubes
- A61M16/04—Tracheal tubes
- A61M16/0402—Special features for tracheal tubes not otherwise provided for
- A61M16/0411—Special features for tracheal tubes not otherwise provided for with means for differentiating between oesophageal and tracheal intubation
- A61M2016/0413—Special features for tracheal tubes not otherwise provided for with means for differentiating between oesophageal and tracheal intubation with detectors of CO2 in exhaled gases
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M25/00—Catheters; Hollow probes
- A61M25/10—Balloon catheters
- A61M2025/1043—Balloon catheters with special features or adapted for special applications
- A61M2025/1052—Balloon catheters with special features or adapted for special applications for temporarily occluding a vessel for isolating a sector
Definitions
- the present inventionrelates generally to medical methods, systems, and kits. Particularly, the present invention relates to methods and apparatus for performing diagnostic testing on individual subsections or segments of a lung. Further, the present invention provides methods and apparatus for more accurate evaluation of the extent and severity of pulmonary disease in the subsections and segments and the effectiveness of various treatment options.
- COPDchronic obstructive pulmonary disease
- imaging testssuch as chest x-rays, CT scans, MRI, perfusion scans, and bronchograms, provide a good indicator of the location, homogeneity and progression of the diseased tissue.
- functional testingsuch as spirometry, plethysmography, oxygen saturation, and oxygen consumption stress testing, to name a few.
- Treatment for emphysemamay include a variety of options, one such option is Lung Volume Reduction which typically involves resecting diseased portions of the lung. Resection of diseased portions of the lungs both promotes expansion of the non-diseased regions of the lung and decreases the portion of inhaled air which goes into the lungs but is unable to transfer oxygen to the blood. Lung reduction is conventionally performed in open chest or thoracoscopic procedures where the lung is resected, typically using stapling devices having integral cutting blades. While effective in many cases, conventional lung reduction surgery is significantly traumatic to the patient, even when thoracoscopic procedures are employed.
- diagnostic testsare limited in the amount and type of information that may be generated.
- diagnostic imagingmay provide information to the physician regarding which lung segments “appear” more diseased, but in fact a segment that appears more diseased may actually function better than one that appears less diseased.
- Functional testingis performed on the lungs as a whole.
- the information provided to the physicianis generalized to the whole lung and does not provide information about functionality of individual lung segments.
- physiciansmay find difficulty targeting interventional treatments to the segments most in need and to avoid unnecessarily treating segments that are not in need of treatment or less in need.
- the diseased segmentscannot be differentiated, prioritized for treatment or assessed after treatment for level of response to therapy.
- Patents and applications relating to lung access, diagnosis, and/or treatmentinclude U.S. Pat. Nos. 6,174,323, 6,083,255, 5,972,026, 5,752,921; 5,707,352; 5,682,880; 5,660,175; 5,653,231; 5,645,519; 5,642,730; 5,598,840; 5,499,625; 5,477,851; 5,361,753; 5,331,947; 5,309,903; 5,285,778; 5,146,916; 5,143,062; 5,056,529; 4,976,710; 4,955,375; 4,961,738; 4,958,932; 4,949,716; 4,896,941; 4,862,874; 4,850,371; 4,846,153; 4,819,664; 4,784,133; 4,742,819; 4,716,896; 4,567,882; 4,453,545; 4,468,216; 4,327,721; 4,327
- WO 99/01076describes devices and methods for reducing the size of lung tissue by applying heat energy to shrink collagen in the tissue.
- airmay be removed from a bleb in the lung to reduce its size. Air passages to the bleb may then be sealed, e.g., by heating, to fix the size of the bleb.
- WO 98/49191describes a plug-like device for placement in a lung air passage to isolate a region of lung tissue, where air is not removed from the tissue prior to plugging.
- WO 98/48706describes the use of surfactants in lung lavage for treating respiratory distress syndrome.
- the present inventionprovides systems, methods, devices and kits for assessing the level of pulmonary disease in individual lung compartments.
- a lung compartmentcomprises a subportion of a lung, such as a lobe or a segment, for example.
- the level of disease of the pulmonary systemmay be more precisely defined.
- compartmentsmay be separately imaged to provide further diagnostic information.
- Once individual compartments are characterized, theymay be compared and ranked based on a number of variables reflecting, for example, level of disease or need for treatment. Such comparison may be aided by simultaneous display of such variables or images on a visual display.
- the same diagnostic testsmay be performed on the lung as a whole or on both lungs and to determine the effect of the diseased lung compartments on the overall lung performance.
- the diseased lung compartmentsmay be temporarily isolated and the diagnostic tests performed on the remainder of the lung to determine the affect of the isolation on lung performance. As a result, the most beneficial treatment options may be selected.
- a pulmonary diagnostic systemcomprising an Endobronchial Pulmonary Diagnostic (EPD) device.
- the EPD deviceis connectable with a pulmonary catheter configured for introduction into a compartment of a lung.
- the pulmonary cathetermay take a variety of forms, each suitable for acquiring measurement data to characterize the lung compartment or to perform a treatment on the lung compartment. In some cases, such measurement is aided by one or more sensors positioned on the catheter, often near the catheter tip.
- the pulmonary cathetercomprises an access catheter.
- Typical access catheterscomprise a catheter body having a relatively large inner diameter to allow sufficient flow of gas or air through the catheter to and/or from the lung compartment.
- access cathetersoften include an occlusion member, such as an inflatable occlusion balloon, near its distal end to seal off the lung passageway around the access catheter leading to the compartment. This provides direct communication with the lung compartment, isolated from the remainder of the lung.
- the access cathetermay have a number of additional features, such as a guidewire lumen, optical imaging capability and steering capability, to name a few. Additional embodiments of the pulmonary catheter will be described later in conjunction with their use.
- a sensormay be disposed on the catheter for generating measurement data reflecting a respiratory feature of the lung compartment.
- the EPD devicetypically comprises mechanisms for transferring fluid or gas to or from the lung compartment through the pulmonary catheter. This may be performed to pressurize the lung compartment, a state desired during many testing or measurement procedures.
- this mechanisms for transferringmay comprise a pump or other driving mechanisms and appropriate tubing or conduits for passage of the fluid or gas.
- a pump or other driving mechanismsmay be disposed outside of the EPD device. In this case, the mechanisms for transferring the fluid or gas of the EPD device may simply comprise a conduit between the driving mechanisms and the pulmonary catheter.
- the sensorsgather measurement data or information which is transmitted to the EPD device.
- the EPD devicehas a mechanisms for receiving the measurement data.
- the EPD devicealso comprises mechanisms for processing the measurement data. Processing may comprise converting the measurement data into a form which may be visually displayed, such as in graphs, charts, tables, numbers, images or figures. Or, processing may comprise analyzing the data wherein the data is used to determine or calculate secondary information or data such as an average pressure value, a volume value, a compliance value, an average tidal volume value and/or a resistance value, to name a few. Alternatively, processing may comprise converting the measurement data into a computer readable format. Such conversion may be of the measurement data itself or of secondary data derived from the measurement data.
- the processed datais then received by a data receiving component.
- the receiving componentoften comprises a visual display.
- the componentmay alternatively take the form of a computer readable medium, a printer, or a chart recorder, to name a few.
- the computer readable mediummay comprise, for example, disks, diskettes, CD ROMs and tapes.
- a variety of measuring componentsmay be used in connection with or disposed within the EPD device.
- the componentsinclude mechanical, electrical, chemical or other means to generate measurement data which characterizes the compartment of the lung which is being measured.
- a componentmay include a gas source and a pump which are used to fill the compartment with the gas for pressure or volume measurement.
- a componentworks in conjunction with one or more sensors which are located at any location within the pulmonary diagnostic system. The component may collect data from the sensor and utilize the data in further calculations and measurement functions. Or, the component may simply display the data on a visual display or readout.
- the EPD deviceserves as a central feature of the measurement system, providing user input to control the measurement procedures, coordinating the activities of the measuring components, and transmitting the measurement data between the sensors, for example.
- the measuring componentcomprises a pulmonary mechanics unit.
- the pulmonary mechanics unitis used for measuring a number of variables related to the pulmonary mechanics of the lung compartment.
- the pulmonary mechanics unitincludes mechanisms for generating pressure and volume data of the lung compartment. Pressure is measured by a pressure sensor and volume is derived from measurement by a flow sensor.
- the sensorsmay be located near the distal end of the catheter or at any other locations throughout the pulmonary diagnostic system.
- the pressure and volume datamay be plotted on a graph, the pressure data plotted along an x-axis and the volume plotted along a y-axis.
- the resulting pressure-volume (PV) curveprovides information regarding physical characteristics and corresponding level of disease of the lung compartment which is being measured.
- the pulmonary mechanics unit or the EPD devicemay be used to calculate a variety of data values related to the physical characteristics of the lung compartment.
- the unit or devicemay include mechanisms for calculating a compliance value for the lung compartment, mechanisms for calculating an average tidal volume value, and mechanisms for calculating a resistance value corresponding to the lung compartment.
- the measuring componentcomprises a physiological testing unit.
- the physiological testing unitis used for measuring a number of variables related to the physiology of the lung compartment.
- the physiological testing unitmay include mechanisms for measuring ventilation or air flow movement in and out of the lung compartment.
- the pulmonary cathetermay comprise a microcatheter having a velocity sensor mounted on its distal end. After the microcatheter is positioned such that the sensor is located in the passageway entering the compartment to be measured, the velocity sensor measures the movement of airflow into and out of the compartment. Comparison of these values to standard values or values from other compartments in the lung gives an indication of the degree of air trapping or bulk gas exchange in the compartment.
- the physiological testing unitmay include mechanisms to measure CO 2 and/or O 2 concentration in the compartment in real time during a breathing cycle to provide an indication of gas exchange.
- the physiological testing unitmay include mechanisms for measuring electrophysiology characteristics of the lung compartment.
- the mechanismsincludes mechanisms for measuring the electrical resistance of the tissue in the compartment and in another embodiment mechanisms includes mechanisms for measuring the electrical activity of the musculature of the tissue in the compartment. Graphical or numerical representation of these values generated by the physiological testing unit or the EPD device may be stored for later use or displayed on the visual display.
- the measuring componentcomprises a gas dilution unit.
- the gas dilution unitincludes mechanisms for performing Functional Residual Capacity (FRC) testing.
- FRC testingtypically involves introducing a known volume of a noble gas, such as helium, to the lung compartment through, for example, an access catheter.
- the known volume of noble gasis allowed to mix with the unknown volume of air in the compartment.
- a sensormeasures the concentration of one of the gases in the system and the volume of air that was initially in the compartment is then calculated. Determining the volume of air initially in the compartment may be useful information used during later treatment.
- the measuring componentcomprises an imaging unit.
- the imaging unitmay include mechanisms for generating at least one image of a lung compartment.
- the imageincludes an X-ray image, a fluoroscopic image, a computed tomography (CT) image, a positron emission tomography (PET) image, a single-photon emission computed tomography (SPECT) image, magnetic resonance image (MRI), or an ultrasonic image.
- CTcomputed tomography
- PETpositron emission tomography
- SPECTsingle-photon emission computed tomography
- MRImagnetic resonance image
- ultrasonic imageOften traditional external imaging equipment is used while the imaging unit provides, for example, mechanisms for transferring various gases to the lung compartment, including a gas having radiopaque properties, a polarized gas as in the case of MRI, or a liquid as in the case of ultrasonic imaging.
- Such transfer of gas or liquidmay be accomplished with the use of any pulmonary catheter.
- the resulting imagesmay be individual views of the lung compartment or the views may be combined to generate a composite three-dimensional image of the lung compartment.
- the viewsmay be of the entire lung minus an isolated compartment or compartments.
- the measuring componentcomprises a mapping unit.
- the mapping unitis used for determining the position of the pulmonary catheter as it is introduced to the lung and advanced through the bronchial passageways. Due to the multiple branchings of the bronchial anatomy, the position of the catheter within the passageways may be difficult to determine. Thus, the mapping unit can be used to locate the catheter at any time.
- a sensoris mounted on the catheter tip and the unit may include mechanisms for locating the sensor and imaging the position of the sensor within the passageways, reflecting the real time position of the catheter in the lung passageways
- the sensormay track directional movements. The positioning images may be shown on the visual display for user ease.
- the EPD devicemay be connected with a data receiving component comprising a visual display that is suitable for displaying various acquired data and graphical outputs.
- a data receiving componentcomprising a visual display that is suitable for displaying various acquired data and graphical outputs.
- the information provided by the visual displaymay be presented in a number of formats and may include a limitless number and type of measurement information. For example, information collected and generated from one or many measuring components may be compiled and displayed on the visual display. Such combination of data may allow the operator or physician to more readily compare information related to various compartments in the lung anatomy, compare data related to an individual patient's lung compartments to other patient's data, compare current measurement data to baseline or previous values, and compare individual compartments to whole lung data.
- Such displaymay be graphical, numerical or any other type.
- the multiple sets of informationmay be displayed simultaneously or individually, wherein viewing is controlled by the user. Such display may more easily allow the user to rank the compartments in order of level of disease or in order of need for treatment. Likewise, it may be appreciated that images generated from the imaging unit may also be displayed on the visual display.
- lung volume reductionmay be prescribed as the desired treatment protocol.
- the lung passageway which leads to the lung compartment to be reducedmay be temporarily occluded with a blockage catheter.
- the blockage cathetercomprises a catheter body having an occlusion member mounted near its distal end. The blockage catheter is advanced through the lung passageways to the compartment that is to be reduced. At this point the lung passageway is occluded by the occlusion member and the lung compartment is effectively isolated from the remainder of the lung. Testing, imaging and evaluation of the overall lung performance may be undertaken to measure the effects of such isolation.
- This technique of temporary occlusion with a blockage cathetermay also be employed as a stand alone diagnostic tool wherein a compartment or compartments are isolated and the remainder of the lung is functionally measured or imaged to assess level of disease.
- the measuring componentmay comprise a treatment unit.
- the treatment unitis used to perform a lung volume reduction procedure on a lung compartment or any other treatment option.
- Minimally invasive lung volume reductiontypically involves aspirating the contents of the compartment after isolating the compartment from the remainder of the anatomy. This is typically achieved with the use of the an access catheter introduced endotracheally to the target compartment.
- the compartmentOnce in position, the compartment is isolated by occluding the air passageway, typically by inflating an occlusion balloon mounted on the access catheter.
- the target compartmentis then collapsed by aspirating air and any other gases or liquids that may have been introduced, from the compartment, typically through a lumen in the access catheter.
- the passagewaymay then be sealed, for example by deploying a plug within the air passageway.
- a pulmonary catheteris connected to the EPD device for introduction into the lung anatomy of the patient.
- the distal end of the catheteris introduced through the bronchial passageways of the lung to the compartment of the lung to be measured.
- Measurement datais generated characterizing the compartment of the lung with the use of the pulmonary diagnostic system. Any of the above described measuring components and/or pulmonary catheters may be used to generate such measurement data. As previously described in relation to each of the components, the generated information and images may be displayed on the visual display.
- the pulmonary cathetermay then be repositioned to another compartment of the lung and measurement data characterizing the other compartment of the lung may then be generated using the pulmonary diagnostic system.
- the data and/or imagesmay be displayed on the visual display unit.
- data characterizing the compartment and the other compartmentsmay be simultaneously displayed on the visual display.
- a blockage cathetermay be introduced to the compartment or compartments targeted for possible treatment.
- the compartmentis then isolated from the remainder of the lung by occluding the lung passageway leading to the compartment with an occlusion member on the blockage catheter.
- a pulmonary cathetermay then be positioned or repositioned in a lung passageway leading to the whole lung or a portion of the lung having the isolated compartment within.
- the pulmonary cathetermay then be used to generate measurement data characterizing the whole lung (or portion having the isolated compartment therein) with the use of the pulmonary diagnostic system.
- kitsmay include a pulmonary diagnostic system comprising an EPD device and optionally at least one measuring component connectable with the device.
- the kitshall include instructions for use, setting forth methods according to the present invention. For example, such methods may include connecting a pulmonary catheter to the EPD device, introducing the distal end of the catheter to a compartment of a lung and generating measurement data characterizing the compartment of the lung with the use of the pulmonary diagnostic system.
- kitsmay further include any of the other system components described in relation to the present invention, any of the other materials or items relevant to the present invention.
- FIG. 1is a schematic illustration of an EPD device, wherein the measuring components are housed within the EPD device, having a typical pulmonary catheter removably attached.
- FIG. 2is a schematic illustration of the EPD device, wherein the measuring components are external and removably attached to the EPD device, having a typical pulmonary catheter removably attached.
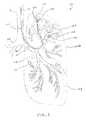
- FIG. 3illustrates a patient's lung wherein the pulmonary catheter is introduced to various locations in the bronchial tree isolating a variety of different sized lung compartments for diagnosis and/or treatment.
- FIGS. 4 - 5illustrate a preferred embodiment of an access catheter.
- FIGS. 6 A- 6 Fare schematic cross sectional views of a catheter body of an embodiment of an access catheter.
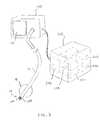
- FIG. 7illustrates a pulmonary catheter introduced through a visualizing endotracheal tube.
- FIG. 8illustrates an EPD device and a measuring component comprising a pulmonary mechanics unit, wherein an access catheter is attached to the EPD device.
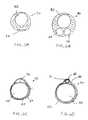
- FIGS. 9 A- 9 Dillustrate a number of different types of pressure sensors located on an access catheter.
- FIG. 10Ashows example PV curves plotted on a graph.
- FIG. 10Billustrates regions of various lung compliances along a PV curve.
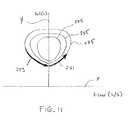
- FIG. 11shows example flow-volume loops plotted on a graph.
- FIG. 12is a schematic illustration of an EPD device and a measuring component comprising a physiological testing unit. In addition, a microcatheter is shown removably attached to the EPD device.
- FIG. 12Ashows example velocity traces plotted on a graph.
- FIG. 13is a schematic illustration of an EPD device and measuring components comprising physiological testing units which include mechanisms for measuring electrophysiology characteristics of a lung compartment.
- FIG. 14is a schematic illustration of an EPD device and a measuring component comprising a gas dilution unit, wherein a pulmonary catheter is shown attached to the EPD device.
- FIG. 15is a schematic illustration of an EPD device and a measuring component comprising an imaging unit, wherein a pulmonary catheter is shown attached to the EPD device.
- FIG. 16is a schematic illustration of an EPD device and a measuring component comprising a mapping unit, wherein a pulmonary catheter is shown attached to the EPD device and advanced through the bronchial tree to a location in the patient's lungs.
- FIGS. 17 A- 17 Ddepict embodiments of a blockage catheter.
- FIG. 18is a schematic illustration of an EPD device and a measuring component comprising a treatment unit, wherein a treatment catheter is shown attached to the EPD device.
- FIG. 19illustrates a kit constructed in accordance with the principles of the present invention.
- the present inventionprovides for a pulmonary diagnostic system for measuring one or more of a number of parameters related to pulmonary function and/or appearance which may be used in diagnosis, treatment and monitoring or occasional assessment of a patient's disease level.
- a pulmonary diagnostic systemfor measuring one or more of a number of parameters related to pulmonary function and/or appearance which may be used in diagnosis, treatment and monitoring or occasional assessment of a patient's disease level.
- EPDEndobronchial Pulmonary Diagnostic
- the EPD device 102may include a variety of mechanisms and features, which will be described hereinafter, depending on its intended use.
- the deviceis connectable with a pulmonary catheter 120 , as shown, which is configured for accessing a lung compartment through one or more lung passageways.
- the catheter 120is shown as having a proximal end 122 , distal end 124 , an optional lumen 126 therethrough and an optional occlusion member 128 , the lumen 126 and occlusion member 128 shown in dashed-line.
- pulmonary catheters 120may be used, a few embodiments of which will be discussed in later sections.
- the sensorsmay include pressure sensors, temperature sensors, air flow sensors, CO 2 sensors, O 2 sensors, infrared Doppler devices, current or resistivity sensors, laser diode sensors, pulse emitting diode sensors, and/or frequency emitting diodes, to name a few.
- the sensors 140may be located near the distal end 124 of the catheter 120 .
- the sensors 140may be located at any point along the catheter 120 or within the EPD device 102 or one or more measuring components 104 .
- Measuring components 104may take many forms and may perform a variety of functions.
- the components 104may include a pulmonary mechanics unit 107 , a physiological testing unit 109 , a gas dilution unit 106 , an imaging unit 108 , a mapping unit 112 or a treatment unit 113 , to name a few. Embodiments of such components 104 will be discussed in detail in later sections.
- the components 104may be integral with or disposed within the EPD device 102 .
- FIG. Ithe components 104 may take many forms and may perform a variety of functions.
- the components 104may include a pulmonary mechanics unit 107 , a physiological testing unit 109 , a gas dilution unit 106 , an imaging unit 108 , a mapping unit 112 or a treatment unit 113 , to name a few. Embodiments of such components 104 will be discussed in detail in later sections.
- the components 104may be integral with or disposed within the EPD device
- some or all of the components 104may be external to and/or removably connectable with the EPD device 102 .
- a data receiving component 115may be integral with, disposed within or removably connectable with the EPD device 102 .
- the data receiving component 115is shown as a visual display 110 .
- the component 115may alternatively take the form of a computer readable medium, a printer, or a chart recorder, to name a few.
- the catheter 120is configured for introduction into the pulmonary anatomy 150 , particularly into a bronchial passageway.
- the catheter 120may be introduced into the bronchial passageways of a lung LNG to various depths.
- the catheter 120may be introduced so that it's distal end 124 is positioned within a distant lung segment 152 of the branching passageways.
- the catheter 120can optionally isolate and measure an individual compartment 154 of the lung LNG, illustrated by a shaded dashed-lined circle.
- the distal end 124may be positioned in a larger lung segment 156 of the branching passageways.
- the catheter 120can measure another individual compartment 154 of the lung LNG, illustrated by a larger shaded dashed-lined circle.
- the distal end 124may be positioned in an even larger lung segment 158 to measure an even larger compartment 154 , such as one or more lobes.
- the lung LNGitself can be measured for comparison to the individual compartments.
- the distal end 124 in the trachea T, above the takeoff branches 160the both of the lungs can be measured.
- any testing, imaging or other functions described in relation to a compartment 154may also be performed on an entire lung LNG or both lungs.
- the isolated compartment 154can be assessed.
- fluid or gasis transferred to or from the lung compartment through the pulmonary catheter. This may be performed to pressurize the lung compartment, a state desired during many testing or measurement procedures.
- the EPD device 102comprises mechanisms for transferring such fluid or gas. In some instances, this mechanisms for transferring may comprise a pump or other driving mechanisms and appropriate tubing or conduits for passage of the fluid or gas. In other instances, a pump or other driving mechanisms may be disposed outside of the EPD device 102 . In this case, the mechanisms for transferring the fluid or gas of the EPD device 102 may simply comprise a conduit between the driving mechanisms and the pulmonary catheter.
- the sensors 140gather measurement data or information which is transmitted to the EPD device 102 .
- the EPD device 102has a mechanisms for receiving the measurement data.
- the EPD device 102also comprises mechanisms for processing the measurement data. Processing may comprise converting the measurement data into a form which may be visually displayed, such as in graphs, charts, tables, numbers, images or figures. Or, processing may comprise analyzing the data wherein the data is used to determine or calculate secondary information or data such as an average pressure value, a volume value, a compliance value, an average tidal volume value and/or a resistance value, to name a few. Alternatively, processing may comprise converting the measurement data into a computer readable format. Such conversion may be of the measurement data itself or of secondary data derived from the measurement data.
- the processed datais then received by a data receiving component 115 .
- the receiving component 115often comprises a visual display 110 .
- the component 115may alternatively take the form of a computer readable medium, a printer, or a chart recorder, to name a few.
- the computer readable mediummay comprise, for example, disks, diskettes, CD-ROMs and tapes.
- one or more measuring components 104receive the processed data.
- the processed datamay then be used in conjunction other mechanisms within the components. For example, a component may perform a testing function while maintaining the lung compartment at a specific level of pressurization. Thus, the component may utilize measurement data from a pressure sensor while performing testing functions.
- the EPD device 102comprises mechanisms for coordinating the functioning of the measuring components, such as the transfer of gas or fluid between the components and the lung compartment, the passage of information or measurement data between the measuring components, between the sensors and the measuring components or between the measuring components and the data receiving components, to name a few. Such control of activities may result from pre-programming, user input or both.
- measurementsimply involves one or more measuring components without the use of a sensor.
- pulmonary imagingin which a component 104 infuses an isolated compartment 154 with an imaging fluid or gas.
- the lung compartment 154may be visualized externally, with the use of a fluoroscopy, nuclear, MRI or CT imaging system, or may be visualized with the use of another component 104 within or attached to the EPD device 102 .
- Thismay also be the case in measuring perfusion parameters.
- the measurement informationis then processed by the EPD device 102 and received by a receiving component 115 .
- Measurement information for a given lung compartment 154may be compared with measurement information from one or more other lung compartments 154 .
- information from a distant lung segmentmay be compared to information from another distant lung segment.
- the segmentscan be ranked in terms of level of disease, for example.
- information from a lobecan be compared with information from a distant lung segment within the lobe. In this way, the affect of the lung segment on overall performance of the lobe can be compared.
- a lung compartment 154may be treated, such as by reduction and/or isolation, and remaining areas of the lung or lungs can be measured to determine the effect of the treatment.
- a blockage cathetermay be used which is introduced to a target compartment, the compartment which has been targeted for treatment. With the blockage catheter in place, such treatment is simulated and the effect of the treatment may be determined by measuring the untreated areas, such as a larger compartment which encompasses or contains the target compartment, using, for example, CT imaging or plethysmography. Thus, more effective treatments may be achieved by pinpointing the most efficient compartments to treat.
- the above described measurement data or informationmay be provided to the user in various formats. Typically, such information will be displayed on the visual display 110 in visual form. This may include graphs, charts, tables, number images or figures, to name a few. Alternatively, the data can be recorded in a computer readable format onto computer readable medium, such as diskettes, CD-ROMs, tapes, etc. The data may then be utilized by a computer, a printer, a visual display or any other accessory. In any case, measurement data or information from a number of compartments may be directly compared by simultaneous display of the information from each compartment . Or, multiple imaging views of a compartment may be obtained to establish a three-dimensional composite view of the compartment. In addition, other types of displays may be provided.
- the EPD device 102performs a variety of functions which depend on the elements included in the pulmonary diagnostic system 100 and the functions in which the system 100 is designed to perform. Descriptive embodiments of possible elements comprising the pulmonary diagnostic system 100 are presented below.
- the pulmonary catheter 120comprises an access catheter 10 .
- An exemplary access catheter 10is illustrated in FIG. 4 and comprises a catheter body 12 having a distal end 14 , a proximal end 16 , an inflatable occlusion balloon 18 near its distal end, and at least one lumen therethrough.
- catheter 10will have at least two lumens, and catheter 10 includes both a central lumen 20 and an annular lumen 22 defined by inner body member 24 and outer body member 26 which is coaxially disposed about the inner body member.
- the annular lumen 22opens to port 30 on a proximal hub 31 and provides for inflation of balloon 18 .
- the central lumen 20opens to port 36 on hub 31 and provides for multiple functions, including optional introduction over a guidewire, aspiration, introduction of secondary catheters, such as sealing catheters, measurement catheters and the like.
- the access catheter 10may be modified in a number of ways, some of which are illustrated in FIGS. 6 A- 6 F.
- the cathetercan be a single extrusion having a catheter body 30 with a circular main lumen 32 and a crescent-shaped inflation lumen 34 , as illustrated in FIG. 6A.
- the catheter body 40may be formed as a single extrusion having three lumens, i.e., a primary lumen 42 for receiving a guidewire, applying aspiration, delivering secondary catheters, and/or other functions.
- a second lumen 44can be provided for inflating the occlusion balloon, and a third lumen 46 can be provided as an alternative guidewire or functional lumen.
- Catheter body 50comprising a main tubular body 52 having an outer layer 54 fused thereover to define a lumen 56 suitable for balloon inflation as shown in FIG. 6C.
- a primary lumen 58is formed within the main tubular member 52 .
- catheter body 60can be formed from a primary tubular member 62 , and a secondary tubular member 64 , where the tubular members are held together by an outer member 66 , such as a layer which is applied by heat shrinking.
- the primary tubular member 62provides the main lumen 68 while secondary tube 64 provides a secondary lumen 70 .
- the secondary lumen 70will typically be used for balloon inflation, while the primary lumen 68 can be used for all other functions of the access catheter.
- the dimensions and materials of access catheter 10are selected to permit endotracheal introduction and intraluminal advancement through the lung bronchus, optionally over a guidewire, and/or through a primary tracheal tube structure and/or inside the working channel of a bronchoscope.
- Suitable materialsinclude low and high density polyethylenes, polyamides, nylons, PTFE, PEEK, and the like, particularly for the inner tubular member 24 .
- the outer member, including the occlusion balloon,can be made from elastomeric materials, such as polyurethane, low density polyethylene, polyvinylchloride, silicone rubber, latex, and the like.
- portions of the outer tubular member 26 proximal to the inflatable ballooncan be made thicker and/or reinforced so that they do not dilate upon pressurization of the balloon.
- Exemplary dimensions for the access catheter 10are dependent on its use.
- a multi-purpose access catheter 10should have a working lumen, such as a central lumen 20 , main lumen 32 , primary lumen 42 or similar such lumen, adequately sized for a number of procedures. If the catheter 10 is to be used in procedures such as functional residual capacity testing or the generation of pressure vs. volume curves, the working lumen should be approximately 1.5-3.5 mm ID, assuming a catheter 10 length of approximately 45-80 cm. In other situations, however, the working lumen may be smaller.
- the access catheter in the present inventioncan be provided with optical imaging capability.
- catheter body 80can be formed to include four lumens, typically by conventional extrusion processes.
- Lumen 82is suitable for passage over a guidewire.
- Lumens 84 and 86both contain light fibers 88 for illumination.
- Lumen 90carries an optical wave guide or image fiber 92 .
- Lumen 82can be used for irrigation, aspiration or other functions, typically after the guidewire is withdrawn.
- Balloon inflationcan be effected through the space remaining and lumens 84 and 86 surrounding the light fibers 88 .
- an alternative embodiment of the catheter body 71is formed as a coaxial arrangement of a number separate tubes.
- Outer tube 72contains a separate guidewire tube 74 defining lumen 76 which permits introduction over a guidewire as well as perfusion and aspiration after the guidewire is removed.
- Second inner tubular member 75will carry an optical image fiber 77 and a plurality of light fibers 78 are passed within the remaining space 79 within the outer tubular member.
- forward imagingcan be effected by illuminating through the light fibers and detecting an image through a lens at the distal end of the catheter. The image can be displayed on conventional cathode-ray or other types of imaging screens.
- forward imagingpermits a user to selectively place the guidewire for advancing the catheters through a desired route through the branching bronchus.
- an alternative cross-sectional designwill be implemented to provide the necessary dimensions.
- the catheter 10can be advanced to a compartment within a lung through a patient's trachea. Advancement through the trachea T is relatively simple and will optionally employ a guidewire to select the advancement route through the branching bronchus. As described above, steering can be effected under real time imaging using the imaging access catheters illustrated in FIGS. 6 E- 6 F.
- the cathetermay be inserted through the working channel of a bronchoscope, using the bronchoscope vision for navigation.
- the access catheter 10may be introduced through a visualizing tracheal tube, such as that described in U.S. Pat. No. 5,285,778, licensed to the assignee of the present application and incorporated by reference for all purposes. As shown in FIG.
- the visualizing endotracheal tube 130includes an occlusion cuff 132 which may be inflated within the trachea just above the branch of the left bronchus and right bronchus LB and RB, respectively.
- the visualizing endotracheal tube 130includes a forward-viewing optical system, typically including both illumination fibers and an image fiber to permit direct viewing of the main branch between the left bronchus LB and right bronchus RB.
- initial placement of access cathetercan be made under visualization of the visualizing endotracheal tube 130 and optionally the access catheter 10 itself.
- the access catheter 10is advanced until its distal end 14 reaches a region in the bronchus which leads directly into the lung compartment.
- the access catheter 10may have elements or accessories for steering and sufficient torque response and pushability to make advancement and navigation through the bronchial tree possible.
- the catheter 10may include positioning sensors so as to determine the location of the catheter with respect to the complete lung anatomy. This will be described in detail in a later section.
- the access catheter 10can be a modular system or a multi-component system.
- the access catheter 10may comprise a viewing scope and a sheath for use with the viewing scope as described in co-pending Application No. 09/699313 (Attorney Docket No. 17534-001300), the full disclosure of which is incorporated herein by reference.
- the viewing scopeincludes or consists essentially of a flexible elongated body, an optical viewing fiber or video chip, and a light transmitting bundle.
- the viewing scopemay be in the form of conventional bronchoscope or a conventional articulated flexible scope having dimensions suitable for introduction in and through the lung passageways.
- the sheathcomprises a flexible tubular body having a proximal end, a distal end, and at least a first lumen therethrough.
- the sheathwill further comprise an inflatable cuff disposed near its distal end, where the inflatable cuff may be inflated through a lumen which is present in the tubular body itself or formed in a separate inflation tube.
- the viewing scopeis introduced into the lumen of the flexible tubular body of the sheath to form an assembly where a viewing end of the viewing scope is located at the distal end of the sheath.
- the assembly of the viewing scope and sheathmay then be introduced to a lung passageway so that the inflatable cuff lies adjacent to a target location in the passageway.
- the cuffmay then be inflated to temporarily occlude the target location.
- the sheathmay also have additional working channels in order to perform aspects of the diagnostic testing, such as carbon dioxide sensing or polarized gas delivery.
- the access catheter 10may comprise one or more sensors to measure a variety of variables related to pulmonary function. Such sensors will typically be located near the distal end 14 of the catheter 10 , however they may be located at any location along the length of the catheter body 12 . Individual sensor types will be described in relation to each type of measurement described below.
- a measuring component 104 of the pulmonary diagnostic system 100comprises a pulmonary mechanics unit 200 , as shown in FIG. 8.
- the pulmonary mechanics unit 200is illustrated as a separate attachable unit, however it may be appreciated that the unit 200 may be internal to the EPD device 102 .
- the pulmonary mechanics unit 200is used for measuring a number of variables related to the pulmonary mechanics of a compartment 154 of a lung LNG.
- the pulmonary mechanics unit 200may include mechanisms 202 for generating pressure and volume data of the lung compartment 154 . Generation of such data is achieved by slowly inflating the lung compartment 154 and measuring volume delivered and real-time pressure. The inflation process is performed slowly to minimize the affect of any system resistance on the pressure readings.
- the inflation mediumis delivered to the compartment 154 through an access catheter 10 which is removably attached to the EPD device 102 . In this case, the distal end 14 of the catheter 10 is inserted into the lung passageway leading to the compartment 154 to be measured and the balloon 18 is inflated to occlude the passageway. In this way, all inflation medium is delivered to the compartment 154 and cannot escape to other areas of the lung.
- Pressureis measured by a pressure sensor 204 and volume is derived from measurement by a flow sensor 206 .
- the sensors 204 , 206may be disposed near the distal end 14 of the catheter 10 or at other locations, including within the EPD device 102 and/or the pulmonary mechanics unit 200 .
- a number of different types of pressure sensors 204are shown in FIGS. 9 A- 9 D.
- one embodiment of the pressure sensor 204comprises a secondary cuff 210 disposed at the distal end 14 of the access catheter 10 , distal to the occlusion balloon 18 .
- FIG. 9Bcomprises a Wheatstone bridge, microbellows pressure transducer, or optical fiber 212 imbedded in the wall of the distal end 14 .
- the embodiment of a pressure sensor 204 in FIG. 9Ccomprises ultrasonic or fiberoptic pressure transducers with a send element 214 and a receive element 216 .
- the embodiment shown in FIG. 9Dcomprises a bellows or Wheatstone bridge 218 protruding from a channel 219 in the catheter 10 .
- Pressurization of the compartment 154can be performed while the rest of the lung is at an expiratory hold to truly isolate the target compartment and eliminate extraneous events.
- pressurizationcan be performed during an inspiratory hold, during regular ventilation or during a pressure hold that is in between end-expiratory pressure and peak inspiratory pressure.
- the pressure and volume datais plotted on a graph wherein the pressure data is plotted along an x-axis X and the volume is plotted along a y-axis Y, as illustrated in FIG. 10A.
- the resulting PV curve 220provides information regarding the health and level of disease of the compartment 154 .
- FIG. 10Ashows three such PV curves 220 , the difference in the curves are due to various disease states as stated.
- Compliancerefers to the distensibility of an elastic structure (such as a lung compartment 154 ) and is defined as the change in volume of that structure produced by a change in pressure across the structure. In other words, compliance can be defined as the slope of a PV curve 220 at a given point along the curve. As shown in Fig. 10B, in a normal healthy lung compartment at low volume, relatively little positive pressure needs to be applied to increase the volume of the lung quite a bit, as shown by the high compliance area 222 . Lung compliance decreases with increasing volume so as the lung compartment is further inflated, more pressure must be applied to get the same increase in volume.
- the compliancewill be calculated at an upper inflection point or peak inspiratory pressure PIP, identified in FIG. 10A.
- Mechanisms 226 for calculating a compliance value from the pressure and volume datais depicted within the pulmonary mechanics unit 200 in FIG. 8, however such mechanisms 226 may alternatively be disposed within the EPD device 102 ..
- the PV curves 220 and compliance valueswill typically be displayed on the visual display 110 .
- volume and flow datamay be plotted on a graph as in FIG. 11.
- flow datais plotted along an x-axis X and volume is plotted along a y-axis Y.
- volumeincreases as flow increases and then declines, as indicated by arrow 221 .
- volumedecreases as flow increases and then declines, as indicated by arrow 223 .
- the resulting traceis a loop 225 indicative of the flow volume characteristics of the compartment 154 accessed.
- the loop 225provides information regarding the health and level of disease of the compartment 154 .
- FIG. 11shows three such loops 225 , each corresponding to a different compartment 154 or to the same compartment 154 over time as disease progresses.
- Additional respiratory parametersmay also be derived from pressure and volume data.
- the average tidal volumecan be measured for a given lung compartment 154 . Tidal volume may be described as the volume of air inhaled and exhaled with each breath.
- the pulmonary mechanics unit 200 or the EPD device 102may comprise mechanisms 228 for calculating an average tidal volume value.
- pressureis set to the PIP and the compartment is ventilated at that pressure. This may be performed while the rest of the lung is in an expiratory hold. Volume is typically measured for three to five breaths over approximately 30 seconds and an average is taken of these values to determine the average tidal volume.
- the resistance of a compartmentcan be derived from pressure and volume data.
- Resistancemay be described as the pressure divided by the volumetric flow rate.
- the EPD device 102 or the pulmonary mechanics unit 200may comprise mechanisms 230 for calculating a resistance value.
- work of breathing of a compartmentcan be derived from pressure and volume data. This is done by converting pressure and volume into Joules/liter.
- the EPD device 102 or the pulmonary mechanics unit 200may also comprise mechanisms 234 for calculating an average work of breathing value. Graphical or numerical representation of these values may be received by a data receiving component 115 for visual display.
- a measuring component 104comprises a physiological testing unit 300 , as shown in FIGS. 12 - 15 .
- the physiological testing unit 300is illustrated as a separate attachable unit, however it may be appreciated that the unit 300 may be integral or internal to the EPD device 102 .
- the physiological testing unit 300is used for measuring a number of variables related to the physiology of a compartment 154 of a lung LNG.
- the physiological testing unit 300may include mechanisms 400 for measuring ventilation or velocity of air movement in and out of a compartment 154 .
- the pulmonary catheter 120comprises a microcatheter 402 having a proximal end 404 , a distal end 406 , a lumen 408 therethrough and at least one sensor 410 mounted on its distal end 406 .
- the sensor 410may be a velocity sensor.
- the microcatheter 402is positioned such that its distal end 406 is entering a compartment 154 to be measured.
- the microcatheter 402is sized so that the compartment 154 is not isolated and air movement is not retarded.
- the velocity sensor 410measures the velocity of airflow into and out of the compartment 154 . Comparison of these values to other compartments gives an indication of the degree of disease of the compartment. For example, as shown in FIG. 12A, velocity versus time data is plotted wherein time is plotted along an x-axis X and velocity is plotted along a y-axis Y. During inspiration, velocity increases to an inspiratory peak 421 and then decreases over time. During expiration, velocity increases to an expiratory peak 423 and then decreases over time. The resulting trace 425 is indicative of the characteristics of the compartment 154 accessed. The trace 425 provides information regarding the health and level of disease of the compartment 154 . FIG. 12A shows two such traces 425 , each corresponding to a different compartment 154 or to the same compartment 154 over time as disease progresses.
- the senor 410may be an oxygen and/or carbon dioxide sensor.
- the sensor 410can measure the amount of, for example, carbon dioxide retained in the compartment. Carbon dioxide is indicative of trapped air. Therefore, data derived from such a sensor may provide information as to the level of disease in the compartment.
- a sensor that measures the amount of oxygen retained in the compartmentmay indicate the level of disease affecting gas transfer through the alveolar sacs.
- Oxygen sensorscan also be used in the performance of oxygen wash-out tests. Here, the air in a lung compartment is replaced as much as possible with 100% oxygen. Then, the decay of oxygen concentration is measured over time using sensor 410 . Such decay indicates how well a compartment contributes to ventilation. Further, the ratio of carbon dioxide to oxygen can be determined which is also indicative of disease state.
- the physiological testing unit 300may also include mechanisms 450 for measuring electrophysiology characteristics of a lung compartment 154 .
- the mechanisms 450includes measuring the resistance of the tissue in the compartment 154 .
- a pulse emitting sensor 452is mounted on the distal end 124 of the pulmonary catheter 120 .
- the proximal end 122 of the catheter 120is removably attached to the EPD device 102 and the distal end 124 is inserted into a lung compartment 154 .
- a receiver 454is positioned at a second location, for example on the outside of the patient P, and is connected to the EPD device 102 .
- a pulseis emitted from the sensor 452 and a signal is measured by the receiver 454 .
- the signaldetermines the resistance of the tissue and therefore the state of the disease. For example, diseased tissue will have a different conductivity because of the breakdown of elasticity and/or because of edema/inflammation of the tissue.
- the mechanisms 450 for measuring electrophysiology characteristics of a lung compartment 154includes measuring the electrical activity of the musculature of the tissue in the compartment 154 .
- two or more leadsare mounted on the pulmonary catheter.
- the leadsmeasure a characteristic voltage signal of the tissue which determines the state of the disease. For example, diseased tissue will have weaker signals due to the breakdown of elasticity.
- the senor 410is an infrared sensor which is positioned against the bronchial tissue and a venous oxygen saturation measurement is made. Because blood perfusing diseased lung compartments will have lower oxygenation, disease level can be determined.
- a measuring component 104 of the pulmonary diagnostic system 100comprises a gas dilution unit 500 , as shown in FIG. 14.
- the gas dilution unit 500is illustrated as a separate attachable unit, however it may be appreciated that the unit 500 may be internal to the EPD device 102 .
- the gas dilution unit 500is used primarily for Functional Residual Capacity (FRC) testing and/or residual volume (RV) testing of a compartment 154 of a lung LNG, since these parameters reflect level of disease.
- FRCFunctional Residual Capacity
- RVresidual volume
- the access catheter 10is used as the pulmonary catheter 120 attached to the EPD device 102 , as shown.
- the compartment 154is inflated to the PIP, as previously determined by the mechanisms 202 for generating pressure and volume data. This can be achieved by the pulmonary mechanics unit 200 , if available, or it may be achieved by mechanisms 504 for generating pressure and volume data within the gas dilution unit 500 or the EPD device 102 .
- a known volume of a noble gassuch as helium, is introduced from a source of noble gas 506 to the compartment 154 through the access catheter 10 .
- the known volume of noble gasis allowed to mix with the unknown volume of air in the compartment 154 (at PIP). Thorough mixing is accomplished by using a pump 508 that moves gas back and forth through the access catheter 10 in an oscillatory motion. Due to the low volume of the access catheter 10 compared to that of the compartment 154 , complete mixing should be accomplished in approximately 1-5 minutes, depending on the mixing efficiency of the incoming noble gas.
- a sensor 502measures the concentration of one of the gases in the system.
- the sensor 502is mounted on the distal end 14 of the catheter 10 as shown.
- the sensor 502may be any of the following: a membrane chemical transfer sensor, a photochemical reaction sensor, an electropotential sensor, a microchip, a laser diode, an optical transmittance sensor, or a piezoelectric sensor.
- the gas dilution unit 500 or EPD device 102may include mechanisms 510 for determining the concentration of a gas, such as helium, in the system and mechanisms 512 for calculating the initial volume of air in a lung compartment. Determining the volume of air initially in the compartment may be useful information used during later treatment. For example, the compartment may be treated by aspirating trapped air in the compartment. By comparing the measured volume of air aspirated with the calculated initial volume of air in the compartment, the effectiveness of the treatment may be determined.
- a gassuch as helium
- a measuring component 104 of the pulmonary diagnostic systemcomprises an imaging unit 600 , as shown in FIG. 15.
- the imaging unit 600is illustrated as a separate attachable unit, however it may be appreciated that the unit 600 may be integral or internal to the EPD device 102 .
- the imaging unit 600is used for generating or assisting in the generation of two-dimensional or three-dimensional images of a compartment 154 of a lung LNG. Often traditional external imaging equipment is used to generate the images while the imaging unit 600 assists in the visualization of individual compartments, as will be described below. In this case, the imaging unit 600 may also serve to transmit and optionally manipulate the images for display in the visual display 110 .
- the access catheter 10is typically used as the pulmonary catheter 120 attached to the EPD device 102 , as shown.
- the compartmentmay be visualized by imaging. Such imaging may be enhanced by occluding the lung passageway with the balloon 18 prior to visualization.
- the imaging unit 600may include mechanisms 602 for transferring a fluid or gas having radiopaque properties to the lung compartment 154 .
- a fluid or gasmay be radiopaque or be labeled with radiopaque markers, for example.
- the compartment 154is inflated with the fluid or gas to the PIP, as previously determined by the mechanisms 202 for generating pressure and volume data.
- the imaging unit 600may further include mechanisms 606 for generating an ultrasound or MRI image of the lung compartment 154 . Or, an image may be taken with equipment external to the pulmonary diagnostic system 100 using CT, MRI, PET, x-ray, ultrasound, fluoroscopy or a perfusion scan. While the compartment is filled with an imaging gas or fluid as described above, the remainder of the lung may be filled with a gas or fluid having a stronger or weaker imaging capability.
- the lung compartmentmay be filled with a radiopaque gas and the remainder of the lung filled with a weaker concentration of radiopaque gas.
- the complete lungwill be visible under fluoroscopy with the lung compartment “highlighted” by the stronger concentration of radiopaque gas.
- concentrationsmay be reversed wherein the lung is filled with a gas or fluid having a higher concentration than the lung compartment and the lung compartment is highlighted by the weaker concentration of radiopaque gas.
- an individual lung compartmentmay be blocked or isolated and the remainder of the lung imaged by the methods described above. In any case, the individual compartments may be evaluated which may provide information as to its level of disease.
- Multiple images of the lung compartment 154may be generated, each image having a different view. This may be achieved externally or by mechanisms 608 for generating multiple images. Mechanisms 610 for generating a three-dimensional composite image of the compartment 154 from the individual views may also be included in the imaging unit 600 or the EPD device 102 . Alternatively, multiple images of the lung compartment 154 may be generated so that a three-dimensional image is obtained by combining image “slices” of the compartment. This may provide even more diagnostic information regarding the status of the compartment and its level of disease.
- the imaging unit 600may include mechanisms 612 for transferring a polarized gas to the lung compartment 154 .
- the compartment 154is inflated with the gas to the PIP, either with the mechanisms 202 or the mechanisms 604 for generating pressure and volume data.
- the imaging unit 600may further include mechanisms 614 for generating at least one magnetic resonance image (MRI) of the lung compartment 154 .
- MRImagnetic resonance image
- an imagemay be taken with external MRI equipment.
- the anatomy of the compartmentmay be visualized which may provide information as to its level of disease.
- the imaging unit 600may further include mechanisms 616 for generating multiple magnetic resonance images of the lung compartment 154 and mechanisms 618 for generating a three-dimensional composite image of the compartment 154 . It may be appreciated that some of these mechanisms may be included in the EPD device 102 . These images may provide even more diagnostic information regarding the status the compartment and its level of disease.
- the imaging unit 600may include mechanisms 620 for transferring a liquid such as perfluroban to the lung compartment 154 .
- the imaging unit 600may further include mechanisms 622 for generating an ultrasonic image of the lung compartment 154 .
- an imagemay be taken with external ultrasound equipment. In either case, the anatomy of the compartment may be visualized which may provide information as to its level of disease.
- imagingmay be undertaken with the use of any contrast media appropriate for the imaging technique.
- imagingmay be performed at different points in the breathing cycle, such as at the end of normal inspiration and exhalation and/or at the end of forced inspiration and exhalation.
- These imagescan then be used to calculate lung volumes relevant to disease, such as residual volume (RV), total lung capacity (TLC) and RV/TLC.
- imagingmay be performed on any number of lung compartments and imaging results may be compared for diagnostic or other purposes.
- a measuring component 104 of the pulmonary diagnostic system 100comprises an mapping unit 700 , as shown in FIG. 16.
- the mapping unit 700is illustrated as a separate attachable unit, however it may be appreciated that the unit 700 may be integral or internal to the EPD device 102 .
- the mapping unit 700is used for determining the position of the pulmonary catheter 120 as it is introduced and advanced through the bronchial passageways. Due to the multiple branchings of the bronchial anatomy, the position of the catheter 120 within the passageways may be difficult to determine and thus the lung compartment 154 to be measured may also be difficult to determine. With the use of the mapping unit 700 , the position of the catheter 120 may be more readily visualized.
- a sensor 702is used to track the position of the catheter 120 in the bronchial passageways.
- the sensor 702is mounted on the catheter 120 , typically near its distal end 124 .
- the sensor 702can be a simple magnetic type device or a frequency emitting device, to name a few.
- the sensor 702could be an optical imaging element and coupled with artificial intelligence that tracks successive directional movements of the catheter tip, hence knowing its position at any given time.
- the mapping unit 700may include mechanisms 704 for receiving the signal from the sensor and mechanisms 706 for processing the signal.
- Mechanisms for processing the signalinclude mechanisms for generating positioning data of the sensor 702 within the passageways and mechanisms for generating an image of the sensor positioned within the passageways reflecting the positioning data of the sensor 702 .
- the catheter 120is attached to the EPD device 102 and its distal end 124 is introduced to a lung passageway of a patient P.
- a computer generated pulmonary anatomy image 708is shown on the visual display 110 .
- a catheter positioning image 710is shown within the anatomy image 708 reflecting the real-time position of the catheter 120 within the actual patient's anatomy. In this way, the target lung compartment may be more easily located and identified for later access if desired.
- the data receiving component 115receives processed data from the EPD device 102 or any of the components 104 for output to the user.
- the component 115is the visual display 110
- the processed datais presented in visual form.
- the component 115may be a computer readable medium, such as disks, diskettes, CD-ROMs, tapes or the like.
- the computer readable mediummay then be transported to another device, such as a computer, workstation or even another EPD device 102 for use.
- the processed datais typically displayed in visual form. It may be appreciated that the possibilities of displaying measurement information in visual form are limitless. A few embodiments are presented as examples.
- the pulmonary diagnostic systemis used to measure compartments of a lung, wherein a compartment could be an entire lobe, a segment or a subsegment and beyond.
- information generated from a compartmentmay be used in determining the level of disease of the compartment itself, comparison of the generated information to other information is also useful in diagnosis and assessment of disease.
- information generated from a compartmentmay be compared with baseline information from the patient or to information from a healthy patient.
- both or multiple sets of informationmay be displayed in the visual display 110 simultaneously. Such display may be graphical, numerical or any other type.
- information generated from one compartmentmay be compared with information from another similar compartment within the same patient. This concept may be extended to numerous compartments.
- multiple sets of informationmay be displayed in the visual display 110 simultaneously. This in turn may allow the physician to rank the compartments in order of level of disease or in order of need for treatment. Similarly, information generated from one compartment may be compared with information from other types or sizes of compartments to determine the affect of each compartment on the others. Again, multiple sets of information may be displayed in the visual display 110 simultaneously for this purpose.
- visual images of the lung anatomycan be displayed on the visual display 110 as noted above. This may be useful in both diagnosis of disease, positioning of the pulmonary catheter 120 and determining the affect of treatment.
- a treatment protocolmay be determined.
- lung volume reductionmay be prescribed.
- the lung passageway which leads to the lung compartment to be reducedmay be temporarily occluded with a blockage catheter.
- this temporary occlusion with a blockage cathetermay itself be the diagnostic test.
- FIG. 17Aillustrates the blockage catheter 750 as comprising a catheter body 752 having a proximal end 756 , a distal end 754 , and an inflatable occlusion balloon 758 near its distal end 754 .
- the blockage catheter 750may have a smaller outer diameter than the access catheter 10 or other pulmonary catheters 120 which have lumens of significant diameter for various testing purposes.
- lumensmay simply be sized for inflation of the occlusion balloon 758 or passage of a guidewire.
- FIGS. 17 B-Dillustrates the blockage catheter 750 as comprising a catheter body 770 having a proximal end 772 and a multiplicity of distal ends 774 , each distal end 774 having an inflatable occlusion balloon 776 mounted thereon.
- a separate guidewire lumen 780 and balloon inflation lumen 782 within the catheter body 770are present from the proximal end 772 to each distal end 774 .
- End connectors 784may be present near the proximal end 772 for access to each lumen 780 , 782 .
- an outer sleeve 786 encasing the catheter body 770unites the distal ends 774 for introduction purposes.
- FIG. 17Cshows a cross-section of the distal ends 774 of the catheter body 770 .
- the catheter body 770may comprise separate catheters, each having a guidewire lumen 780 , inflation lumen 782 and occlusion balloon 776 , which are held together by the outer sleeve 786 or other means.
- placement of the blockage catheter 750involves positioning each distal end 774 into a different lung passageway.
- Each distal end 774may be independently positioned with the use of a guidewire.
- a number of lung compartments 154may be simultaneously isolated by the blockage catheter 750 .
- the blockage catheter 750When the blockage catheter 750 is in place and the lung passageway(s) occluded by the balloon(s), the affected lung compartment 154 will be isolated from the remainder of the lung. At this point, testing, imaging and evaluation of the overall lung performance may be undertaken to measure the effects of the isolation. Such techniques would include, for example, CT scanning, spirometry, or plethysmography to obtain images, spirometry data or plethysmography data, respectively. This in turn reflects the effect of reduction of that isolated compartment. Similarly, such testing and evaluation may be performed on specific segments of the lung for assessing particular regions of the lung. Referring back to FIG. 17A, such testing with a pulmonary catheter 120 while the blockage catheter 750 is in place is illustrated.
- the blockage catheter 750may be introduced through a lumen in the pulmonary catheter 120 or the blockage catheter 750 may simply lie in parallel with the pulmonary catheter 120 . If the pulmonary catheter 120 utilizes an occlusion member 128 , the member 128 may seal against the lumen and the catheter body 752 of the blockage catheter 750 . Both the blockage catheter 750 and pulmonary catheter 120 , or any additional catheters, may be simultaneously connected to the EPD device 100 if desired. The results of such assessment may determine the most effective course of treatment.
- a measuring component 104 of the pulmonary diagnostic system 100may comprise a treatment unit 800 , as shown in FIG. 18.
- the treatment unit 800is illustrated as a separate attachable unit, however it may be appreciated that the unit 800 may be integral or internal to the EPD device 102 .
- the treatment unit 800may be used to perform a lung volume reduction procedure on a lung compartment 154 .
- the unit 800would include mechanisms 802 for performing lung volume reduction. This typically involves aspirating the contents of the compartment after isolating the compartment from the remainder of the anatomy. This is typically achieved by introducing the distal end 14 of the access catheter 10 endotracheally to the target compartment 154 .
- the compartmentmay be isolated by occluding the air passageway, such as by inflating the occlusion balloon 18 within the passageway.
- the target compartmentmay then be collapsed by aspirating air, and any other gases or liquids that may have been introduced, from the compartment, such as through a lumen in the access catheter 10 .
- the passagewaymay then be sealed, for example by deploying a plug within the air passageway.
- Kits 900comprise any number of items related to the pulmonary diagnostic system described and instructions for use IFU. As shown in FIG. 19, such kits 900 typically include the EPD device 102 and instructions for use IFU setting forth methods according to the present invention.
- the EPD device 102may have one or more measuring components disposed internally, however the kit 900 may also include one or more measuring components 104 as shown.
- the kits 900may further include any of the other system components described above, such as one or more pulmonary catheters 120 , guidewires 902 , or a variety of accessories, such as a receiver 454 .
- Some or all kit componentswill usually be packaged together in a pouch 905 or other conventional medical device packaging.
- kit componentssuch as a pulmonary catheter 120
- pulmonary catheter 120which will be used in performing the procedure on the patient will be sterilized and maintained within the kit.
- separate pouches, bags, trays or other packagingmay be provided within a larger package, where the smaller packs may be opened separately to separately maintain the components in a sterile fashion.
Landscapes
- Health & Medical Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Pulmonology (AREA)
- Engineering & Computer Science (AREA)
- Heart & Thoracic Surgery (AREA)
- General Health & Medical Sciences (AREA)
- Biomedical Technology (AREA)
- Animal Behavior & Ethology (AREA)
- Public Health (AREA)
- Veterinary Medicine (AREA)
- Biophysics (AREA)
- Medical Informatics (AREA)
- Physics & Mathematics (AREA)
- Surgery (AREA)
- Molecular Biology (AREA)
- Pathology (AREA)
- Physiology (AREA)
- Hematology (AREA)
- Anesthesiology (AREA)
- Emergency Medicine (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Radiology & Medical Imaging (AREA)
- High Energy & Nuclear Physics (AREA)
- Optics & Photonics (AREA)
- Obesity (AREA)
- Computer Vision & Pattern Recognition (AREA)
- Signal Processing (AREA)
- Child & Adolescent Psychology (AREA)
- Psychiatry (AREA)
- Artificial Intelligence (AREA)
- Apparatus For Radiation Diagnosis (AREA)
- Measuring And Recording Apparatus For Diagnosis (AREA)
- Measurement Of The Respiration, Hearing Ability, Form, And Blood Characteristics Of Living Organisms (AREA)
- Magnetic Resonance Imaging Apparatus (AREA)
- Ultra Sonic Daignosis Equipment (AREA)
Abstract
Description
- The present application claims benefit, under 37 C.F.R. §1.78, to U.S. Provisional Patent Application No. 60/318,539, filed Sep. 10, 2001, the complete disclosure of which is incorporated herein by reference.[0001]
- NOT APPLICABLE[0002]
- NOT APPLICABLE[0003]
- 1. Field of the Invention[0004]
- The present invention relates generally to medical methods, systems, and kits. Particularly, the present invention relates to methods and apparatus for performing diagnostic testing on individual subsections or segments of a lung. Further, the present invention provides methods and apparatus for more accurate evaluation of the extent and severity of pulmonary disease in the subsections and segments and the effectiveness of various treatment options.[0005]
- Chronic obstructive pulmonary disease (COPD) is a significant medical problem affecting 16 million people or about 6% of the U.S. population. Specific diseases in this group include chronic bronchitis, asthmatic bronchitis, and emphysema. In general, two types of diagnostic tests are performed on a patient to determine the extent and severity of COPD: 1) imaging tests and 2) functional tests. Imaging tests, such as chest x-rays, CT scans, MRI, perfusion scans, and bronchograms, provide a good indicator of the location, homogeneity and progression of the diseased tissue. However, these test do not give a direct indication of how the disease is affecting the patient's overall lung function and respiration capabilities. This can be measured with functional testing, such as spirometry, plethysmography, oxygen saturation, and oxygen consumption stress testing, to name a few. Together, these diagnostic tests are used to determine the course of treatment for the patient.[0006]
- Treatment for emphysema may include a variety of options, one such option is Lung Volume Reduction which typically involves resecting diseased portions of the lung. Resection of diseased portions of the lungs both promotes expansion of the non-diseased regions of the lung and decreases the portion of inhaled air which goes into the lungs but is unable to transfer oxygen to the blood. Lung reduction is conventionally performed in open chest or thoracoscopic procedures where the lung is resected, typically using stapling devices having integral cutting blades. While effective in many cases, conventional lung reduction surgery is significantly traumatic to the patient, even when thoracoscopic procedures are employed. Such procedures often result in the unintentional removal of relatively healthy lung tissue or leaving behind of relatively diseased tissue, and frequently result in air leakage or infection. Consequently, alternative therapies have been developed which utilize minimally invasive techniques to isolate target lung tissue segments from other regions of the lung. Isolation is usually achieved by introducing an access catheter endotracheally or thorascopically to the target air passage of the lung. The target lung tissue segment is then collapsed by aspirating air (and any other gases or liquids that may have been introduced) from the segment and optionally sealed off. Exemplary methods and systems to perform such isolation procedures are described U.S. patent application 09/606320 (Attorney Docket No. 017534-000710), incorporated herein by reference.[0007]
- Currently, the diagnostic tests are limited in the amount and type of information that may be generated. For example, diagnostic imaging may provide information to the physician regarding which lung segments “appear” more diseased, but in fact a segment that appears more diseased may actually function better than one that appears less diseased. Functional testing is performed on the lungs as a whole. Thus, the information provided to the physician is generalized to the whole lung and does not provide information about functionality of individual lung segments. Thus, physicians may find difficulty targeting interventional treatments to the segments most in need and to avoid unnecessarily treating segments that are not in need of treatment or less in need. In general, the diseased segments cannot be differentiated, prioritized for treatment or assessed after treatment for level of response to therapy.[0008]
- For these reasons, it would be desirable to provide systems, methods, devices and kits which would overcome at least some of the shortcomings discussed above. In particular, it would be desirable to provide systems and methods for monitoring, assessing or measuring the functional state of individual lung compartments; such compartments could be an entire lobe, a segment or a subsegment and beyond, hereinafter subsegments and beyond will be referred to simply as segments. It would be further desirable to provide systems and methods of comparing measured data of individual lung compartments to other individual lung compartments and/or to measured data of the lung as a whole. In addition, it would be desirable to provide systems and methods of estimating or predicting the outcome of treatment options prior to actual treatment and also to assess the state of disease and functionality post-treatment. At least some of these objectives will be met by the inventions described hereinafter.[0009]
- 2. Description of the Background Art[0010]
- Patents and applications relating to lung access, diagnosis, and/or treatment include U.S. Pat. Nos. 6,174,323, 6,083,255, 5,972,026, 5,752,921; 5,707,352; 5,682,880; 5,660,175; 5,653,231; 5,645,519; 5,642,730; 5,598,840; 5,499,625; 5,477,851; 5,361,753; 5,331,947; 5,309,903; 5,285,778; 5,146,916; 5,143,062; 5,056,529; 4,976,710; 4,955,375; 4,961,738; 4,958,932; 4,949,716; 4,896,941; 4,862,874; 4,850,371; 4,846,153; 4,819,664; 4,784,133; 4,742,819; 4,716,896; 4,567,882; 4,453,545; 4,468,216; 4,327,721; 4,327,720; 4,041,936; 3,913,568 3,866,599; 3,776,222; 3,677,262; 3,669,098; 3,498,286; 3,322,126; EP 1078601, WO 01/13908, WO 01/13839, WO 01/10314, WO 00/62699, WO 00/51510, WO 00/03642, WO 99/64109, WO 99/34741, WO 99/01076, WO 98/44854, WO 95/33506, and WO 92/10971.[0011]
- WO 99/01076 describes devices and methods for reducing the size of lung tissue by applying heat energy to shrink collagen in the tissue. In one embodiment, air may be removed from a bleb in the lung to reduce its size. Air passages to the bleb may then be sealed, e.g., by heating, to fix the size of the bleb. WO 98/49191 describes a plug-like device for placement in a lung air passage to isolate a region of lung tissue, where air is not removed from the tissue prior to plugging. WO 98/48706 describes the use of surfactants in lung lavage for treating respiratory distress syndrome.[0012]
- Lung volume reduction surgery is described in many publications, including Becker et al. (1998) Am. J. Respir. Crit. Care Med. 157:1593-1599; Criner et al. (1998) Am. J. Respir. Crit. Care Med. 157:1578-1585; Kotloff et al. (1998) Chest 113:890-895; and Ojo et al. (1997) Chest 112:1494-1500.[0013]
- The use of mucolytic agents for clearing lung obstructions is described in Sclafani (1999) AARC Times, January, 69-97. Use of a balloon-cuffed bronchofiberscope to reinflate a lung segment suffering from refractory atelectasis is described in Harada et al. (1983) Chest 84:725-728.[0014]
- The present invention provides systems, methods, devices and kits for assessing the level of pulmonary disease in individual lung compartments. A lung compartment comprises a subportion of a lung, such as a lobe or a segment, for example. By testing individual lung compartments and determining values of disease parameters reflective of individual subportions or compartments of a lung, the level of disease of the pulmonary system may be more precisely defined. Likewise, compartments may be separately imaged to provide further diagnostic information. Once individual compartments are characterized, they may be compared and ranked based on a number of variables reflecting, for example, level of disease or need for treatment. Such comparison may be aided by simultaneous display of such variables or images on a visual display. Further, the same diagnostic tests may be performed on the lung as a whole or on both lungs and to determine the effect of the diseased lung compartments on the overall lung performance. In addition, the diseased lung compartments may be temporarily isolated and the diagnostic tests performed on the remainder of the lung to determine the affect of the isolation on lung performance. As a result, the most beneficial treatment options may be selected.[0015]
- In a first aspect of the present invention, a pulmonary diagnostic system is provided comprising an Endobronchial Pulmonary Diagnostic (EPD) device. The EPD device is connectable with a pulmonary catheter configured for introduction into a compartment of a lung. The pulmonary catheter may take a variety of forms, each suitable for acquiring measurement data to characterize the lung compartment or to perform a treatment on the lung compartment. In some cases, such measurement is aided by one or more sensors positioned on the catheter, often near the catheter tip. In a first embodiment, the pulmonary catheter comprises an access catheter. Typical access catheters comprise a catheter body having a relatively large inner diameter to allow sufficient flow of gas or air through the catheter to and/or from the lung compartment. In addition, access catheters often include an occlusion member, such as an inflatable occlusion balloon, near its distal end to seal off the lung passageway around the access catheter leading to the compartment. This provides direct communication with the lung compartment, isolated from the remainder of the lung. In addition, the access catheter may have a number of additional features, such as a guidewire lumen, optical imaging capability and steering capability, to name a few. Additional embodiments of the pulmonary catheter will be described later in conjunction with their use.[0016]
- As mentioned, a sensor may be disposed on the catheter for generating measurement data reflecting a respiratory feature of the lung compartment. However, such a sensor may be disposed anywhere in the system, including with the EPD device or within connected devices or components. The EPD device typically comprises mechanisms for transferring fluid or gas to or from the lung compartment through the pulmonary catheter. This may be performed to pressurize the lung compartment, a state desired during many testing or measurement procedures. In some embodiments, this mechanisms for transferring may comprise a pump or other driving mechanisms and appropriate tubing or conduits for passage of the fluid or gas. In other embodiments, a pump or other driving mechanisms may be disposed outside of the EPD device. In this case, the mechanisms for transferring the fluid or gas of the EPD device may simply comprise a conduit between the driving mechanisms and the pulmonary catheter.[0017]
- Generally, the sensors gather measurement data or information which is transmitted to the EPD device. In this case, the EPD device has a mechanisms for receiving the measurement data. Often, the EPD device also comprises mechanisms for processing the measurement data. Processing may comprise converting the measurement data into a form which may be visually displayed, such as in graphs, charts, tables, numbers, images or figures. Or, processing may comprise analyzing the data wherein the data is used to determine or calculate secondary information or data such as an average pressure value, a volume value, a compliance value, an average tidal volume value and/or a resistance value, to name a few. Alternatively, processing may comprise converting the measurement data into a computer readable format. Such conversion may be of the measurement data itself or of secondary data derived from the measurement data.[0018]
- The processed data is then received by a data receiving component. The receiving component often comprises a visual display. However, the component may alternatively take the form of a computer readable medium, a printer, or a chart recorder, to name a few. The computer readable medium may comprise, for example, disks, diskettes, CD ROMs and tapes.[0019]
- A variety of measuring components may be used in connection with or disposed within the EPD device. The components include mechanical, electrical, chemical or other means to generate measurement data which characterizes the compartment of the lung which is being measured. For example, a component may include a gas source and a pump which are used to fill the compartment with the gas for pressure or volume measurement. Typically, a component works in conjunction with one or more sensors which are located at any location within the pulmonary diagnostic system. The component may collect data from the sensor and utilize the data in further calculations and measurement functions. Or, the component may simply display the data on a visual display or readout. In any case, the EPD device serves as a central feature of the measurement system, providing user input to control the measurement procedures, coordinating the activities of the measuring components, and transmitting the measurement data between the sensors, for example.[0020]
- A number of embodiments of the measuring components will be presented. In a first embodiment, the measuring component comprises a pulmonary mechanics unit. The pulmonary mechanics unit is used for measuring a number of variables related to the pulmonary mechanics of the lung compartment. Typically the pulmonary mechanics unit includes mechanisms for generating pressure and volume data of the lung compartment. Pressure is measured by a pressure sensor and volume is derived from measurement by a flow sensor. As mentioned, the sensors may be located near the distal end of the catheter or at any other locations throughout the pulmonary diagnostic system. The pressure and volume data may be plotted on a graph, the pressure data plotted along an x-axis and the volume plotted along a y-axis. The resulting pressure-volume (PV) curve provides information regarding physical characteristics and corresponding level of disease of the lung compartment which is being measured. Based on information provided by the PV curve, the pulmonary mechanics unit or the EPD device may be used to calculate a variety of data values related to the physical characteristics of the lung compartment. For example, the unit or device may include mechanisms for calculating a compliance value for the lung compartment, mechanisms for calculating an average tidal volume value, and mechanisms for calculating a resistance value corresponding to the lung compartment.[0021]
- In another embodiment the measuring component comprises a physiological testing unit. The physiological testing unit is used for measuring a number of variables related to the physiology of the lung compartment. For example, the physiological testing unit may include mechanisms for measuring ventilation or air flow movement in and out of the lung compartment. In this case the pulmonary catheter may comprise a microcatheter having a velocity sensor mounted on its distal end. After the microcatheter is positioned such that the sensor is located in the passageway entering the compartment to be measured, the velocity sensor measures the movement of airflow into and out of the compartment. Comparison of these values to standard values or values from other compartments in the lung gives an indication of the degree of air trapping or bulk gas exchange in the compartment. Alternatively or in addition, the physiological testing unit may include mechanisms to measure CO[0022]2and/or O2concentration in the compartment in real time during a breathing cycle to provide an indication of gas exchange. The physiological testing unit may include mechanisms for measuring electrophysiology characteristics of the lung compartment. In one embodiment the mechanisms includes mechanisms for measuring the electrical resistance of the tissue in the compartment and in another embodiment mechanisms includes mechanisms for measuring the electrical activity of the musculature of the tissue in the compartment. Graphical or numerical representation of these values generated by the physiological testing unit or the EPD device may be stored for later use or displayed on the visual display.
- In another embodiment, the measuring component comprises a gas dilution unit. The gas dilution unit includes mechanisms for performing Functional Residual Capacity (FRC) testing. FRC testing typically involves introducing a known volume of a noble gas, such as helium, to the lung compartment through, for example, an access catheter. The known volume of noble gas is allowed to mix with the unknown volume of air in the compartment. A sensor then measures the concentration of one of the gases in the system and the volume of air that was initially in the compartment is then calculated. Determining the volume of air initially in the compartment may be useful information used during later treatment.[0023]
- In some embodiments, the measuring component comprises an imaging unit. The imaging unit may include mechanisms for generating at least one image of a lung compartment. Typically the image includes an X-ray image, a fluoroscopic image, a computed tomography (CT) image, a positron emission tomography (PET) image, a single-photon emission computed tomography (SPECT) image, magnetic resonance image (MRI), or an ultrasonic image. Often traditional external imaging equipment is used while the imaging unit provides, for example, mechanisms for transferring various gases to the lung compartment, including a gas having radiopaque properties, a polarized gas as in the case of MRI, or a liquid as in the case of ultrasonic imaging. Such transfer of gas or liquid may be accomplished with the use of any pulmonary catheter. The resulting images may be individual views of the lung compartment or the views may be combined to generate a composite three-dimensional image of the lung compartment. Alternatively, the views may be of the entire lung minus an isolated compartment or compartments.[0024]
- In yet another embodiment, the measuring component comprises a mapping unit. The mapping unit is used for determining the position of the pulmonary catheter as it is introduced to the lung and advanced through the bronchial passageways. Due to the multiple branchings of the bronchial anatomy, the position of the catheter within the passageways may be difficult to determine. Thus, the mapping unit can be used to locate the catheter at any time. Often a sensor is mounted on the catheter tip and the unit may include mechanisms for locating the sensor and imaging the position of the sensor within the passageways, reflecting the real time position of the catheter in the lung passageways Optionally, the sensor may track directional movements. The positioning images may be shown on the visual display for user ease.[0025]
- As mentioned, the EPD device may be connected with a data receiving component comprising a visual display that is suitable for displaying various acquired data and graphical outputs. It may be appreciated that the information provided by the visual display may be presented in a number of formats and may include a limitless number and type of measurement information. For example, information collected and generated from one or many measuring components may be compiled and displayed on the visual display. Such combination of data may allow the operator or physician to more readily compare information related to various compartments in the lung anatomy, compare data related to an individual patient's lung compartments to other patient's data, compare current measurement data to baseline or previous values, and compare individual compartments to whole lung data. Such display may be graphical, numerical or any other type. The multiple sets of information may be displayed simultaneously or individually, wherein viewing is controlled by the user. Such display may more easily allow the user to rank the compartments in order of level of disease or in order of need for treatment. Likewise, it may be appreciated that images generated from the imaging unit may also be displayed on the visual display.[0026]
- Once the lung compartments have been sufficiently assessed to determine level of disease, treatment options for the patient may be determined. In some cases, lung volume reduction may be prescribed as the desired treatment protocol. To test the effects of such reduction prior to actual treatment, the lung passageway which leads to the lung compartment to be reduced may be temporarily occluded with a blockage catheter. Typically, the blockage catheter comprises a catheter body having an occlusion member mounted near its distal end. The blockage catheter is advanced through the lung passageways to the compartment that is to be reduced. At this point the lung passageway is occluded by the occlusion member and the lung compartment is effectively isolated from the remainder of the lung. Testing, imaging and evaluation of the overall lung performance may be undertaken to measure the effects of such isolation. This can be performed with multiple permutations of compartments being isolated, either by repositioning the blockage catheter in various passageways or introducing a blockage catheter configured to block numerous passageways at once. If such effects are satisfactory, the physician may choose to reduce the targeted compartment(s) as the treatment option. This technique of temporary occlusion with a blockage catheter may also be employed as a stand alone diagnostic tool wherein a compartment or compartments are isolated and the remainder of the lung is functionally measured or imaged to assess level of disease.[0027]
- Finally, the measuring component may comprise a treatment unit. The treatment unit is used to perform a lung volume reduction procedure on a lung compartment or any other treatment option. Minimally invasive lung volume reduction typically involves aspirating the contents of the compartment after isolating the compartment from the remainder of the anatomy. This is typically achieved with the use of the an access catheter introduced endotracheally to the target compartment. Once in position, the compartment is isolated by occluding the air passageway, typically by inflating an occlusion balloon mounted on the access catheter. The target compartment is then collapsed by aspirating air and any other gases or liquids that may have been introduced, from the compartment, typically through a lumen in the access catheter. The passageway may then be sealed, for example by deploying a plug within the air passageway.[0028]
- In a third aspect of the present invention, methods are provided for assessing a lung compartment. Providing a pulmonary diagnostic system as described above, including an EPD device and at least one measuring component connected with the device, a pulmonary catheter is connected to the EPD device for introduction into the lung anatomy of the patient. The distal end of the catheter is introduced through the bronchial passageways of the lung to the compartment of the lung to be measured. Measurement data is generated characterizing the compartment of the lung with the use of the pulmonary diagnostic system. Any of the above described measuring components and/or pulmonary catheters may be used to generate such measurement data. As previously described in relation to each of the components, the generated information and images may be displayed on the visual display. The pulmonary catheter may then be repositioned to another compartment of the lung and measurement data characterizing the other compartment of the lung may then be generated using the pulmonary diagnostic system. As before, the data and/or images may be displayed on the visual display unit. Further, data characterizing the compartment and the other compartments may be simultaneously displayed on the visual display. These steps may be repeated for any number of compartments in the patient's lung and the results may be simultaneously or individually displayed for comparison purposes. Methods may further include ranking the compartments based on level of disease or need for treatment.[0029]
- If treatment is desired at one or more locations, the effects of treatment may be determined prior to actual treatment. To accomplish this, a blockage catheter may be introduced to the compartment or compartments targeted for possible treatment. The compartment is then isolated from the remainder of the lung by occluding the lung passageway leading to the compartment with an occlusion member on the blockage catheter. A pulmonary catheter may then be positioned or repositioned in a lung passageway leading to the whole lung or a portion of the lung having the isolated compartment within. The pulmonary catheter may then be used to generate measurement data characterizing the whole lung (or portion having the isolated compartment therein) with the use of the pulmonary diagnostic system. It may be appreciated that conventional measurement systems, such as CT or plethysmography, can alternatively be used with the blockage catheter in place to generate such data. If the generated measurement data reflects improved pulmonary function, the isolated compartment may then be reduced by any method. Such treatment may be performed with the use of the pulmonary diagnostic system, specifically with the use of the treatment unit.[0030]
- In a fourth aspect of the present invention, the methods and devices may be provided in one or more kits. The kits may include a pulmonary diagnostic system comprising an EPD device and optionally at least one measuring component connectable with the device. In addition, the kit shall include instructions for use, setting forth methods according to the present invention. For example, such methods may include connecting a pulmonary catheter to the EPD device, introducing the distal end of the catheter to a compartment of a lung and generating measurement data characterizing the compartment of the lung with the use of the pulmonary diagnostic system. Such kits may further include any of the other system components described in relation to the present invention, any of the other materials or items relevant to the present invention.[0031]
- Other objects and advantages of the present invention will become apparent from the detailed description to follow, together with the accompanying drawings.[0032]
- FIG. 1 is a schematic illustration of an EPD device, wherein the measuring components are housed within the EPD device, having a typical pulmonary catheter removably attached.[0033]
- FIG. 2 is a schematic illustration of the EPD device, wherein the measuring components are external and removably attached to the EPD device, having a typical pulmonary catheter removably attached.[0034]
- FIG. 3 illustrates a patient's lung wherein the pulmonary catheter is introduced to various locations in the bronchial tree isolating a variety of different sized lung compartments for diagnosis and/or treatment.[0035]
- FIGS.[0036]4-5 illustrate a preferred embodiment of an access catheter.
- FIGS.[0037]6A-6F are schematic cross sectional views of a catheter body of an embodiment of an access catheter.
- FIG. 7 illustrates a pulmonary catheter introduced through a visualizing endotracheal tube.[0038]
- FIG. 8 illustrates an EPD device and a measuring component comprising a pulmonary mechanics unit, wherein an access catheter is attached to the EPD device.[0039]
- FIGS.[0040]9A-9D illustrate a number of different types of pressure sensors located on an access catheter.
- Fig. 10A shows example PV curves plotted on a graph.[0041]
- FIG. 10B illustrates regions of various lung compliances along a PV curve.[0042]
- FIG. 11 shows example flow-volume loops plotted on a graph.[0043]
- FIG. 12 is a schematic illustration of an EPD device and a measuring component comprising a physiological testing unit. In addition, a microcatheter is shown removably attached to the EPD device.[0044]
- FIG. 12A shows example velocity traces plotted on a graph.[0045]
- FIG. 13 is a schematic illustration of an EPD device and measuring components comprising physiological testing units which include mechanisms for measuring electrophysiology characteristics of a lung compartment.[0046]
- FIG. 14 is a schematic illustration of an EPD device and a measuring component comprising a gas dilution unit, wherein a pulmonary catheter is shown attached to the EPD device.[0047]
- FIG. 15 is a schematic illustration of an EPD device and a measuring component comprising an imaging unit, wherein a pulmonary catheter is shown attached to the EPD device.[0048]
- FIG. 16 is a schematic illustration of an EPD device and a measuring component comprising a mapping unit, wherein a pulmonary catheter is shown attached to the EPD device and advanced through the bronchial tree to a location in the patient's lungs.[0049]
- FIGS.[0050]17A-17D depict embodiments of a blockage catheter.
- FIG. 18 is a schematic illustration of an EPD device and a measuring component comprising a treatment unit, wherein a treatment catheter is shown attached to the EPD device.[0051]
- FIG. 19 illustrates a kit constructed in accordance with the principles of the present invention.[0052]
- The present invention provides for a pulmonary diagnostic system for measuring one or more of a number of parameters related to pulmonary function and/or appearance which may be used in diagnosis, treatment and monitoring or occasional assessment of a patient's disease level. Central to such a[0053]
system 100 is an Endobronchial Pulmonary Diagnostic (EPD)device 102, as shown in FIGS.1-2. TheEPD device 102 may include a variety of mechanisms and features, which will be described hereinafter, depending on its intended use. The device is connectable with apulmonary catheter 120, as shown, which is configured for accessing a lung compartment through one or more lung passageways. Here, thecatheter 120 is shown as having aproximal end 122,distal end 124, anoptional lumen 126 therethrough and anoptional occlusion member 128, thelumen 126 andocclusion member 128 shown in dashed-line. A variety of different types ofpulmonary catheters 120 may be used, a few embodiments of which will be discussed in later sections. - With the[0054]
pulmonary catheter 120 positioned in the desired lung passageway, measurement information can be obtained regarding the accessedcompartment 154. Typically, this involves the use of at least one sensor. The sensors may include pressure sensors, temperature sensors, air flow sensors, CO2sensors, O2sensors, infrared Doppler devices, current or resistivity sensors, laser diode sensors, pulse emitting diode sensors, and/or frequency emitting diodes, to name a few. As shown in FIG. 1, thesensors 140 may be located near thedistal end 124 of thecatheter 120. Alternatively, thesensors 140 may be located at any point along thecatheter 120 or within theEPD device 102 or one ormore measuring components 104. - Measuring[0055]
components 104, shown schematically in FIG. I as dashed-lined boxes within theEPD device 102, may take many forms and may perform a variety of functions. For example, thecomponents 104 may include a pulmonary mechanics unit107, a physiological testing unit109, agas dilution unit 106, animaging unit 108, amapping unit 112 or a treatment unit113, to name a few. Embodiments ofsuch components 104 will be discussed in detail in later sections. As illustrated, thecomponents 104 may be integral with or disposed within theEPD device 102. Alternatively, as shown in FIG. 2, some or all of thecomponents 104 may be external to and/or removably connectable with theEPD device 102. In addition, adata receiving component 115 may be integral with, disposed within or removably connectable with theEPD device 102. Here, thedata receiving component 115 is shown as avisual display 110. However, thecomponent 115 may alternatively take the form of a computer readable medium, a printer, or a chart recorder, to name a few. - As illustrated in FIG. 3, the[0056]
catheter 120 is configured for introduction into thepulmonary anatomy 150, particularly into a bronchial passageway. As shown, thecatheter 120 may be introduced into the bronchial passageways of a lung LNG to various depths. For example, as shown in solid line, thecatheter 120 may be introduced so that it'sdistal end 124 is positioned within adistant lung segment 152 of the branching passageways. In this position, thecatheter 120 can optionally isolate and measure anindividual compartment 154 of the lung LNG, illustrated by a shaded dashed-lined circle. Alternatively, as shown in dashed-line, thedistal end 124 may be positioned in alarger lung segment 156 of the branching passageways. In this position, thecatheter 120 can measure anotherindividual compartment 154 of the lung LNG, illustrated by a larger shaded dashed-lined circle. Similarly, thedistal end 124 may be positioned in an evenlarger lung segment 158 to measure an evenlarger compartment 154, such as one or more lobes. By positioning thedistal end 124 in amajor takeoff branch 160 to the lung LNG, the lung LNG itself can be measured for comparison to the individual compartments. And, by positioning thedistal end 124 in the trachea T, above thetakeoff branches 160, the both of the lungs can be measured. Thus, it may be appreciated that any testing, imaging or other functions described in relation to acompartment 154 may also be performed on an entire lung LNG or both lungs. - With the[0057]
pulmonary catheter 120 positioned in the desired lung passageway as described, theisolated compartment 154 can be assessed. In some instances, fluid or gas is transferred to or from the lung compartment through the pulmonary catheter. This may be performed to pressurize the lung compartment, a state desired during many testing or measurement procedures. In some embodiments, theEPD device 102 comprises mechanisms for transferring such fluid or gas. In some instances, this mechanisms for transferring may comprise a pump or other driving mechanisms and appropriate tubing or conduits for passage of the fluid or gas. In other instances, a pump or other driving mechanisms may be disposed outside of theEPD device 102. In this case, the mechanisms for transferring the fluid or gas of theEPD device 102 may simply comprise a conduit between the driving mechanisms and the pulmonary catheter. - Generally, the[0058]
sensors 140 gather measurement data or information which is transmitted to theEPD device 102. In this case, theEPD device 102 has a mechanisms for receiving the measurement data. Often, theEPD device 102 also comprises mechanisms for processing the measurement data. Processing may comprise converting the measurement data into a form which may be visually displayed, such as in graphs, charts, tables, numbers, images or figures. Or, processing may comprise analyzing the data wherein the data is used to determine or calculate secondary information or data such as an average pressure value, a volume value, a compliance value, an average tidal volume value and/or a resistance value, to name a few. Alternatively, processing may comprise converting the measurement data into a computer readable format. Such conversion may be of the measurement data itself or of secondary data derived from the measurement data. - The processed data is then received by a[0059]
data receiving component 115. As mentioned, the receivingcomponent 115 often comprises avisual display 110. However, thecomponent 115 may alternatively take the form of a computer readable medium, a printer, or a chart recorder, to name a few. The computer readable medium may comprise, for example, disks, diskettes, CD-ROMs and tapes. In other cases, one ormore measuring components 104 receive the processed data. The processed data may then be used in conjunction other mechanisms within the components. For example, a component may perform a testing function while maintaining the lung compartment at a specific level of pressurization. Thus, the component may utilize measurement data from a pressure sensor while performing testing functions. - When more than one measuring component or a[0060]
measuring component 104 and adata receiving component 115 are included in the pulmonarydiagnostic system 100, theEPD device 102 comprises mechanisms for coordinating the functioning of the measuring components, such as the transfer of gas or fluid between the components and the lung compartment, the passage of information or measurement data between the measuring components, between the sensors and the measuring components or between the measuring components and the data receiving components, to name a few. Such control of activities may result from pre-programming, user input or both. - In other embodiments, measurement simply involves one or more measuring components without the use of a sensor. This may be the case in pulmonary imaging in which a[0061]
component 104 infuses anisolated compartment 154 with an imaging fluid or gas. Thelung compartment 154 may be visualized externally, with the use of a fluoroscopy, nuclear, MRI or CT imaging system, or may be visualized with the use of anothercomponent 104 within or attached to theEPD device 102. This may also be the case in measuring perfusion parameters. The measurement information is then processed by theEPD device 102 and received by a receivingcomponent 115. - Measurement information for a given[0062]
lung compartment 154 may be compared with measurement information from one or more other lung compartments154. For example, information from a distant lung segment may be compared to information from another distant lung segment. By comparing a number of lung segments, the segments can be ranked in terms of level of disease, for example. Or, information from a lobe can be compared with information from a distant lung segment within the lobe. In this way, the affect of the lung segment on overall performance of the lobe can be compared. Further, alung compartment 154 may be treated, such as by reduction and/or isolation, and remaining areas of the lung or lungs can be measured to determine the effect of the treatment. To determine such effects prior to actual treatment, a blockage catheter may be used which is introduced to a target compartment, the compartment which has been targeted for treatment. With the blockage catheter in place, such treatment is simulated and the effect of the treatment may be determined by measuring the untreated areas, such as a larger compartment which encompasses or contains the target compartment, using, for example, CT imaging or plethysmography. Thus, more effective treatments may be achieved by pinpointing the most efficient compartments to treat. - As mentioned, the above described measurement data or information may be provided to the user in various formats. Typically, such information will be displayed on the[0063]
visual display 110 in visual form. This may include graphs, charts, tables, number images or figures, to name a few. Alternatively, the data can be recorded in a computer readable format onto computer readable medium, such as diskettes, CD-ROMs, tapes, etc. The data may then be utilized by a computer, a printer, a visual display or any other accessory. In any case, measurement data or information from a number of compartments may be directly compared by simultaneous display of the information from each compartment . Or, multiple imaging views of a compartment may be obtained to establish a three-dimensional composite view of the compartment. In addition, other types of displays may be provided. - As generally described above, the[0064]
EPD device 102 performs a variety of functions which depend on the elements included in the pulmonarydiagnostic system 100 and the functions in which thesystem 100 is designed to perform.. Descriptive embodiments of possible elements comprising the pulmonarydiagnostic system 100 are presented below. - I. ACCESS CATHETER[0065]
- In a number of embodiments, the[0066]
pulmonary catheter 120 comprises anaccess catheter 10. Anexemplary access catheter 10 is illustrated in FIG. 4 and comprises acatheter body 12 having adistal end 14, aproximal end 16, aninflatable occlusion balloon 18 near its distal end, and at least one lumen therethrough. Usually, the catheter will have at least two lumens, andcatheter 10 includes both acentral lumen 20 and anannular lumen 22 defined byinner body member 24 andouter body member 26 which is coaxially disposed about the inner body member. Theannular lumen 22 opens to port30 on aproximal hub 31 and provides for inflation ofballoon 18. Thecentral lumen 20 opens to port36 onhub 31 and provides for multiple functions, including optional introduction over a guidewire, aspiration, introduction of secondary catheters, such as sealing catheters, measurement catheters and the like. - The[0067]
access catheter 10 may be modified in a number of ways, some of which are illustrated in FIGS.6A-6F. For example, instead of a inner and outer coaxial tube construction, the catheter can be a single extrusion having acatheter body 30 with a circularmain lumen 32 and a crescent-shapedinflation lumen 34, as illustrated in FIG. 6A. Alternatively, shown in FIG. 6B, thecatheter body 40 may be formed as a single extrusion having three lumens, i.e., aprimary lumen 42 for receiving a guidewire, applying aspiration, delivering secondary catheters, and/or other functions. Asecond lumen 44 can be provided for inflating the occlusion balloon, and athird lumen 46 can be provided as an alternative guidewire or functional lumen.Catheter body 50 comprising a maintubular body 52 having anouter layer 54 fused thereover to define a lumen56 suitable for balloon inflation as shown in FIG. 6C. Aprimary lumen 58 is formed within the maintubular member 52. As a slight alternative,catheter body 60 can be formed from aprimary tubular member 62, and asecondary tubular member 64, where the tubular members are held together by anouter member 66, such as a layer which is applied by heat shrinking. Theprimary tubular member 62 provides themain lumen 68 whilesecondary tube 64 provides asecondary lumen 70. Thesecondary lumen 70 will typically be used for balloon inflation, while theprimary lumen 68 can be used for all other functions of the access catheter. - The dimensions and materials of[0068]
access catheter 10 are selected to permit endotracheal introduction and intraluminal advancement through the lung bronchus, optionally over a guidewire, and/or through a primary tracheal tube structure and/or inside the working channel of a bronchoscope. Suitable materials include low and high density polyethylenes, polyamides, nylons, PTFE, PEEK, and the like, particularly for theinner tubular member 24. The outer member, including the occlusion balloon, can be made from elastomeric materials, such as polyurethane, low density polyethylene, polyvinylchloride, silicone rubber, latex, and the like. Optionally, portions of the outertubular member 26 proximal to the inflatable balloon can be made thicker and/or reinforced so that they do not dilate upon pressurization of the balloon. Exemplary dimensions for theaccess catheter 10 are dependent on its use. Amulti-purpose access catheter 10 should have a working lumen, such as acentral lumen 20,main lumen 32,primary lumen 42 or similar such lumen, adequately sized for a number of procedures. If thecatheter 10 is to be used in procedures such as functional residual capacity testing or the generation of pressure vs. volume curves, the working lumen should be approximately 1.5-3.5 mm ID, assuming acatheter 10 length of approximately 45-80 cm. In other situations, however, the working lumen may be smaller. - Optionally, the access catheter in the present invention can be provided with optical imaging capability. As shown in FIG. 6E,[0069]
catheter body 80 can be formed to include four lumens, typically by conventional extrusion processes.Lumen 82 is suitable for passage over a guidewire.Lumens light fibers 88 for illumination.Lumen 90 carries an optical wave guide orimage fiber 92.Lumen 82 can be used for irrigation, aspiration or other functions, typically after the guidewire is withdrawn. Balloon inflation can be effected through the space remaining andlumens light fibers 88. Referring to FIG. 6F, an alternative embodiment of thecatheter body 71 is formed as a coaxial arrangement of a number separate tubes.Outer tube 72 contains aseparate guidewire tube 74 defininglumen 76 which permits introduction over a guidewire as well as perfusion and aspiration after the guidewire is removed. Second innertubular member 75 will carry anoptical image fiber 77 and a plurality oflight fibers 78 are passed within the remainingspace 79 within the outer tubular member. In bothcatheter constructions - As previously described, the[0070]
catheter 10 can be advanced to a compartment within a lung through a patient's trachea. Advancement through the trachea T is relatively simple and will optionally employ a guidewire to select the advancement route through the branching bronchus. As described above, steering can be effected under real time imaging using the imaging access catheters illustrated in FIGS.6E-6F. Optionally, the catheter may be inserted through the working channel of a bronchoscope, using the bronchoscope vision for navigation. Or theaccess catheter 10 may be introduced through a visualizing tracheal tube, such as that described in U.S. Pat. No. 5,285,778, licensed to the assignee of the present application and incorporated by reference for all purposes. As shown in FIG. 7, the visualizingendotracheal tube 130 includes anocclusion cuff 132 which may be inflated within the trachea just above the branch of the left bronchus and right bronchus LB and RB, respectively. The visualizingendotracheal tube 130 includes a forward-viewing optical system, typically including both illumination fibers and an image fiber to permit direct viewing of the main branch between the left bronchus LB and right bronchus RB. Thus, initial placement of access catheter can be made under visualization of the visualizingendotracheal tube 130 and optionally theaccess catheter 10 itself. Theaccess catheter 10 is advanced until itsdistal end 14 reaches a region in the bronchus which leads directly into the lung compartment. Theaccess catheter 10 may have elements or accessories for steering and sufficient torque response and pushability to make advancement and navigation through the bronchial tree possible. In addition, thecatheter 10 may include positioning sensors so as to determine the location of the catheter with respect to the complete lung anatomy. This will be described in detail in a later section. - In addition, it may be appreciated that the[0071]
access catheter 10 can be a modular system or a multi-component system. For instance, theaccess catheter 10 may comprise a viewing scope and a sheath for use with the viewing scope as described in co-pending Application No. 09/699313 (Attorney Docket No. 17534-001300), the full disclosure of which is incorporated herein by reference. The viewing scope includes or consists essentially of a flexible elongated body, an optical viewing fiber or video chip, and a light transmitting bundle. The viewing scope may be in the form of conventional bronchoscope or a conventional articulated flexible scope having dimensions suitable for introduction in and through the lung passageways. The sheath comprises a flexible tubular body having a proximal end, a distal end, and at least a first lumen therethrough. The sheath will further comprise an inflatable cuff disposed near its distal end, where the inflatable cuff may be inflated through a lumen which is present in the tubular body itself or formed in a separate inflation tube. The viewing scope is introduced into the lumen of the flexible tubular body of the sheath to form an assembly where a viewing end of the viewing scope is located at the distal end of the sheath. The assembly of the viewing scope and sheath may then be introduced to a lung passageway so that the inflatable cuff lies adjacent to a target location in the passageway. The cuff may then be inflated to temporarily occlude the target location. The sheath may also have additional working channels in order to perform aspects of the diagnostic testing, such as carbon dioxide sensing or polarized gas delivery. - Further, the[0072]
access catheter 10 may comprise one or more sensors to measure a variety of variables related to pulmonary function. Such sensors will typically be located near thedistal end 14 of thecatheter 10, however they may be located at any location along the length of thecatheter body 12. Individual sensor types will be described in relation to each type of measurement described below. - I. PULMONARY MECHANICS UNIT[0073]
- In some embodiments, a[0074]
measuring component 104 of the pulmonarydiagnostic system 100 comprises apulmonary mechanics unit 200, as shown in FIG. 8. For clarity, thepulmonary mechanics unit 200 is illustrated as a separate attachable unit, however it may be appreciated that theunit 200 may be internal to theEPD device 102. Thepulmonary mechanics unit 200 is used for measuring a number of variables related to the pulmonary mechanics of acompartment 154 of a lung LNG. - For example, the[0075]
pulmonary mechanics unit 200 may includemechanisms 202 for generating pressure and volume data of thelung compartment 154. Generation of such data is achieved by slowly inflating thelung compartment 154 and measuring volume delivered and real-time pressure. The inflation process is performed slowly to minimize the affect of any system resistance on the pressure readings. The inflation medium is delivered to thecompartment 154 through anaccess catheter 10 which is removably attached to theEPD device 102. In this case, thedistal end 14 of thecatheter 10 is inserted into the lung passageway leading to thecompartment 154 to be measured and theballoon 18 is inflated to occlude the passageway. In this way, all inflation medium is delivered to thecompartment 154 and cannot escape to other areas of the lung. - Pressure is measured by a[0076]
pressure sensor 204 and volume is derived from measurement by aflow sensor 206. As mentioned previously, thesensors distal end 14 of thecatheter 10 or at other locations, including within theEPD device 102 and/or thepulmonary mechanics unit 200. A number of different types ofpressure sensors 204 are shown in FIGS.9A-9D. Referring to FIG. 9A, one embodiment of thepressure sensor 204 comprises asecondary cuff 210 disposed at thedistal end 14 of theaccess catheter 10, distal to theocclusion balloon 18. Another embodiment, shown in FIG. 9B, comprises a Wheatstone bridge, microbellows pressure transducer, oroptical fiber 212 imbedded in the wall of thedistal end 14. The embodiment of apressure sensor 204 in FIG. 9C comprises ultrasonic or fiberoptic pressure transducers with asend element 214 and a receiveelement 216. And the embodiment shown in FIG. 9D comprises a bellows orWheatstone bridge 218 protruding from achannel 219 in thecatheter 10. - Pressurization of the[0077]
compartment 154 can be performed while the rest of the lung is at an expiratory hold to truly isolate the target compartment and eliminate extraneous events. Alternatively, pressurization can be performed during an inspiratory hold, during regular ventilation or during a pressure hold that is in between end-expiratory pressure and peak inspiratory pressure. These options may provide useful information as to the pulmonary mechanics of the compartment. - Typically, the pressure and volume data is plotted on a graph wherein the pressure data is plotted along an x-axis X and the volume is plotted along a y-axis Y, as illustrated in FIG. 10A. The resulting[0078]
PV curve 220 provides information regarding the health and level of disease of thecompartment 154. FIG. 10A shows three such PV curves220, the difference in the curves are due to various disease states as stated. - One type of information which can be derived from a[0079]
PV curve 220 is compliance. Compliance refers to the distensibility of an elastic structure (such as a lung compartment154) and is defined as the change in volume of that structure produced by a change in pressure across the structure. In other words, compliance can be defined as the slope of aPV curve 220 at a given point along the curve. As shown in Fig. 10B, in a normal healthy lung compartment at low volume, relatively little positive pressure needs to be applied to increase the volume of the lung quite a bit, as shown by thehigh compliance area 222. Lung compliance decreases with increasing volume so as the lung compartment is further inflated, more pressure must be applied to get the same increase in volume. This corresponds to the low-compliance area 224. Typically, the compliance will be calculated at an upper inflection point or peak inspiratory pressure PIP, identified in FIG. 10A.Mechanisms 226 for calculating a compliance value from the pressure and volume data is depicted within thepulmonary mechanics unit 200 in FIG. 8, howeversuch mechanisms 226 may alternatively be disposed within theEPD device 102.. The PV curves220 and compliance values will typically be displayed on thevisual display 110. - Alternatively, volume and flow data may be plotted on a graph as in FIG. 11. Here, flow data is plotted along an x-axis X and volume is plotted along a y-axis Y. During inspiration, volume increases as flow increases and then declines, as indicated by[0080]
arrow 221 . During expiration, volume decreases as flow increases and then declines, as indicated byarrow 223. The resulting trace is aloop 225 indicative of the flow volume characteristics of thecompartment 154 accessed. Theloop 225 provides information regarding the health and level of disease of thecompartment 154. FIG. 11 shows threesuch loops 225, each corresponding to adifferent compartment 154 or to thesame compartment 154 over time as disease progresses. - Additional respiratory parameters may also be derived from pressure and volume data. For example, the average tidal volume can be measured for a given[0081]
lung compartment 154. Tidal volume may be described as the volume of air inhaled and exhaled with each breath. Thus, thepulmonary mechanics unit 200 or theEPD device 102 may comprisemechanisms 228 for calculating an average tidal volume value. Here, pressure is set to the PIP and the compartment is ventilated at that pressure. This may be performed while the rest of the lung is in an expiratory hold. Volume is typically measured for three to five breaths over approximately 30 seconds and an average is taken of these values to determine the average tidal volume. In addition, the resistance of a compartment can be derived from pressure and volume data. Resistance may be described as the pressure divided by the volumetric flow rate. In this case, theEPD device 102 or thepulmonary mechanics unit 200 may comprisemechanisms 230 for calculating a resistance value. Further, work of breathing of a compartment can be derived from pressure and volume data. This is done by converting pressure and volume into Joules/liter. TheEPD device 102 or thepulmonary mechanics unit 200 may also comprisemechanisms 234 for calculating an average work of breathing value. Graphical or numerical representation of these values may be received by adata receiving component 115 for visual display. - II. PHYSIOLOGICAL TESTING UNIT[0082]
- In some embodiments, a[0083]
measuring component 104 comprises aphysiological testing unit 300, as shown in FIGS.12-15. Again, for clarity, thephysiological testing unit 300 is illustrated as a separate attachable unit, however it may be appreciated that theunit 300 may be integral or internal to theEPD device 102. Thephysiological testing unit 300 is used for measuring a number of variables related to the physiology of acompartment 154 of a lung LNG. - Referring to FIG. 12, the[0084]
physiological testing unit 300 may includemechanisms 400 for measuring ventilation or velocity of air movement in and out of acompartment 154. Here, thepulmonary catheter 120 comprises amicrocatheter 402 having aproximal end 404, adistal end 406, alumen 408 therethrough and at least onesensor 410 mounted on itsdistal end 406. Thesensor 410 may be a velocity sensor. In this instance, themicrocatheter 402 is positioned such that itsdistal end 406 is entering acompartment 154 to be measured. Themicrocatheter 402 is sized so that thecompartment 154 is not isolated and air movement is not retarded. Thevelocity sensor 410 measures the velocity of airflow into and out of thecompartment 154. Comparison of these values to other compartments gives an indication of the degree of disease of the compartment. For example, as shown in FIG. 12A, velocity versus time data is plotted wherein time is plotted along an x-axis X and velocity is plotted along a y-axis Y. During inspiration, velocity increases to aninspiratory peak 421 and then decreases over time. During expiration, velocity increases to anexpiratory peak 423 and then decreases over time. The resultingtrace 425 is indicative of the characteristics of thecompartment 154 accessed. Thetrace 425 provides information regarding the health and level of disease of thecompartment 154. FIG. 12A shows twosuch traces 425, each corresponding to adifferent compartment 154 or to thesame compartment 154 over time as disease progresses. - In other embodiments, the[0085]
sensor 410 may be an oxygen and/or carbon dioxide sensor. When thedistal end 406 of themicrocatheter 402 is introduced to a lung compartment, thesensor 410 can measure the amount of, for example, carbon dioxide retained in the compartment. Carbon dioxide is indicative of trapped air. Therefore, data derived from such a sensor may provide information as to the level of disease in the compartment. Similarly, a sensor that measures the amount of oxygen retained in the compartment may indicate the level of disease affecting gas transfer through the alveolar sacs. Oxygen sensors can also be used in the performance of oxygen wash-out tests. Here, the air in a lung compartment is replaced as much as possible with 100% oxygen. Then, the decay of oxygen concentration is measured overtime using sensor 410. Such decay indicates how well a compartment contributes to ventilation. Further, the ratio of carbon dioxide to oxygen can be determined which is also indicative of disease state. - Referring to FIG. 13, the[0086]
physiological testing unit 300 may also includemechanisms 450 for measuring electrophysiology characteristics of alung compartment 154. In one embodiment, themechanisms 450 includes measuring the resistance of the tissue in thecompartment 154. Here, apulse emitting sensor 452 is mounted on thedistal end 124 of thepulmonary catheter 120. Theproximal end 122 of thecatheter 120 is removably attached to theEPD device 102 and thedistal end 124 is inserted into alung compartment 154. Areceiver 454 is positioned at a second location, for example on the outside of the patient P, and is connected to theEPD device 102. A pulse is emitted from thesensor 452 and a signal is measured by thereceiver 454. The signal determines the resistance of the tissue and therefore the state of the disease. For example, diseased tissue will have a different conductivity because of the breakdown of elasticity and/or because of edema/inflammation of the tissue. - In another embodiment, the[0087]
mechanisms 450 for measuring electrophysiology characteristics of alung compartment 154 includes measuring the electrical activity of the musculature of the tissue in thecompartment 154. Here, two or more leads are mounted on the pulmonary catheter. The leads measure a characteristic voltage signal of the tissue which determines the state of the disease. For example, diseased tissue will have weaker signals due to the breakdown of elasticity. - In another embodiment, the[0088]
sensor 410 is an infrared sensor which is positioned against the bronchial tissue and a venous oxygen saturation measurement is made. Because blood perfusing diseased lung compartments will have lower oxygenation, disease level can be determined. - Graphical or numerical representation of these values generated by the[0089]
EPD device 102 orphysiological testing unit 300 may be displayed on thevisual display 110. - III. GAS DILUTION UNIT[0090]
- In some embodiments, a[0091]
measuring component 104 of the pulmonarydiagnostic system 100 comprises agas dilution unit 500, as shown in FIG. 14. Again, for clarity, thegas dilution unit 500 is illustrated as a separate attachable unit, however it may be appreciated that theunit 500 may be internal to theEPD device 102. Thegas dilution unit 500 is used primarily for Functional Residual Capacity (FRC) testing and/or residual volume (RV) testing of acompartment 154 of a lung LNG, since these parameters reflect level of disease. - Typically, the[0092]
access catheter 10 is used as thepulmonary catheter 120 attached to theEPD device 102, as shown. After thedistal end 14 of thecatheter 10 is inserted in alung compartment 154 and the lung passageway is occluded by theballoon 18, thecompartment 154 is inflated to the PIP, as previously determined by themechanisms 202 for generating pressure and volume data. This can be achieved by thepulmonary mechanics unit 200, if available, or it may be achieved bymechanisms 504 for generating pressure and volume data within thegas dilution unit 500 or theEPD device 102. Then, a known volume of a noble gas, such as helium, is introduced from a source ofnoble gas 506 to thecompartment 154 through theaccess catheter 10. The known volume of noble gas is allowed to mix with the unknown volume of air in the compartment154 (at PIP). Thorough mixing is accomplished by using apump 508 that moves gas back and forth through theaccess catheter 10 in an oscillatory motion. Due to the low volume of theaccess catheter 10 compared to that of thecompartment 154, complete mixing should be accomplished in approximately 1-5 minutes, depending on the mixing efficiency of the incoming noble gas. - A[0093]
sensor 502 measures the concentration of one of the gases in the system. In some embodiments, thesensor 502 is mounted on thedistal end 14 of thecatheter 10 as shown. Thesensor 502 may be any of the following: a membrane chemical transfer sensor, a photochemical reaction sensor, an electropotential sensor, a microchip, a laser diode, an optical transmittance sensor, or a piezoelectric sensor. When the concentration of this measured gas equilibrates, simple volume mixing laws are used to calculate the volume of air that was initially in the compartment. Thus, thegas dilution unit 500 orEPD device 102 may includemechanisms 510 for determining the concentration of a gas, such as helium, in the system andmechanisms 512 for calculating the initial volume of air in a lung compartment. Determining the volume of air initially in the compartment may be useful information used during later treatment. For example, the compartment may be treated by aspirating trapped air in the compartment. By comparing the measured volume of air aspirated with the calculated initial volume of air in the compartment, the effectiveness of the treatment may be determined. - IV. IMAGING UNIT[0094]
- In some embodiments, a[0095]
measuring component 104 of the pulmonary diagnostic system comprises animaging unit 600, as shown in FIG. 15. Again, for clarity, theimaging unit 600 is illustrated as a separate attachable unit, however it may be appreciated that theunit 600 may be integral or internal to theEPD device 102. Theimaging unit 600 is used for generating or assisting in the generation of two-dimensional or three-dimensional images of acompartment 154 of a lung LNG. Often traditional external imaging equipment is used to generate the images while theimaging unit 600 assists in the visualization of individual compartments, as will be described below. In this case, theimaging unit 600 may also serve to transmit and optionally manipulate the images for display in thevisual display 110. - Referring to FIG. 15, the[0096]
access catheter 10 is typically used as thepulmonary catheter 120 attached to theEPD device 102, as shown. After thedistal end 14 of thecatheter 10 is inserted in alung compartment 154 the compartment may be visualized by imaging. Such imaging may be enhanced by occluding the lung passageway with theballoon 18 prior to visualization. Theimaging unit 600 may includemechanisms 602 for transferring a fluid or gas having radiopaque properties to thelung compartment 154. Such a fluid or gas may be radiopaque or be labeled with radiopaque markers, for example. Thecompartment 154 is inflated with the fluid or gas to the PIP, as previously determined by themechanisms 202 for generating pressure and volume data. This can be achieved by thepulmonary mechanics unit 200 orEPD device 102, if available, or it may be achieved bymechanisms 604 for generating pressure and volume data within theimaging unit 600. Theimaging unit 600 may further includemechanisms 606 for generating an ultrasound or MRI image of thelung compartment 154. Or, an image may be taken with equipment external to the pulmonarydiagnostic system 100 using CT, MRI, PET, x-ray, ultrasound, fluoroscopy or a perfusion scan. While the compartment is filled with an imaging gas or fluid as described above, the remainder of the lung may be filled with a gas or fluid having a stronger or weaker imaging capability. For example, the lung compartment may be filled with a radiopaque gas and the remainder of the lung filled with a weaker concentration of radiopaque gas. As a result, the complete lung will be visible under fluoroscopy with the lung compartment “highlighted” by the stronger concentration of radiopaque gas. It may be appreciated that the concentrations may be reversed wherein the lung is filled with a gas or fluid having a higher concentration than the lung compartment and the lung compartment is highlighted by the weaker concentration of radiopaque gas. Similarly, an individual lung compartment may be blocked or isolated and the remainder of the lung imaged by the methods described above. In any case, the individual compartments may be evaluated which may provide information as to its level of disease. - Multiple images of the[0097]
lung compartment 154 may be generated, each image having a different view. This may be achieved externally or by mechanisms608 for generating multiple images.Mechanisms 610 for generating a three-dimensional composite image of thecompartment 154 from the individual views may also be included in theimaging unit 600 or theEPD device 102. Alternatively, multiple images of thelung compartment 154 may be generated so that a three-dimensional image is obtained by combining image “slices” of the compartment. This may provide even more diagnostic information regarding the status of the compartment and its level of disease. - Alternatively or in addition, the[0098]
imaging unit 600 may includemechanisms 612 for transferring a polarized gas to thelung compartment 154. Again, thecompartment 154 is inflated with the gas to the PIP, either with themechanisms 202 or themechanisms 604 for generating pressure and volume data. Theimaging unit 600 may further includemechanisms 614 for generating at least one magnetic resonance image (MRI) of thelung compartment 154. Or, an image may be taken with external MRI equipment. In either case, the anatomy of the compartment may be visualized which may provide information as to its level of disease. Additionally, theimaging unit 600 may further includemechanisms 616 for generating multiple magnetic resonance images of thelung compartment 154 and mechanisms618 for generating a three-dimensional composite image of thecompartment 154. It may be appreciated that some of these mechanisms may be included in theEPD device 102. These images may provide even more diagnostic information regarding the status the compartment and its level of disease. - Alternatively or in addition, the[0099]
imaging unit 600 may includemechanisms 620 for transferring a liquid such as perfluroban to thelung compartment 154. Theimaging unit 600 may further includemechanisms 622 for generating an ultrasonic image of thelung compartment 154. Or, an image may be taken with external ultrasound equipment. In either case, the anatomy of the compartment may be visualized which may provide information as to its level of disease. - It may be appreciated that imaging may be undertaken with the use of any contrast media appropriate for the imaging technique. In addition, such imaging may be performed at different points in the breathing cycle, such as at the end of normal inspiration and exhalation and/or at the end of forced inspiration and exhalation. These images can then be used to calculate lung volumes relevant to disease, such as residual volume (RV), total lung capacity (TLC) and RV/TLC. In addition, imaging may be performed on any number of lung compartments and imaging results may be compared for diagnostic or other purposes.[0100]
- V. MAPPING UNIT[0101]
- In some embodiments, a[0102]
measuring component 104 of the pulmonarydiagnostic system 100 comprises anmapping unit 700, as shown in FIG. 16. Again, for clarity, themapping unit 700 is illustrated as a separate attachable unit, however it may be appreciated that theunit 700 may be integral or internal to theEPD device 102. Themapping unit 700 is used for determining the position of thepulmonary catheter 120 as it is introduced and advanced through the bronchial passageways. Due to the multiple branchings of the bronchial anatomy, the position of thecatheter 120 within the passageways may be difficult to determine and thus thelung compartment 154 to be measured may also be difficult to determine. With the use of themapping unit 700, the position of thecatheter 120 may be more readily visualized. - In one embodiment, a[0103]
sensor 702 is used to track the position of thecatheter 120 in the bronchial passageways. Thesensor 702 is mounted on thecatheter 120, typically near itsdistal end 124. Thesensor 702 can be a simple magnetic type device or a frequency emitting device, to name a few. In another example, thesensor 702 could be an optical imaging element and coupled with artificial intelligence that tracks successive directional movements of the catheter tip, hence knowing its position at any given time. Themapping unit 700 may includemechanisms 704 for receiving the signal from the sensor andmechanisms 706 for processing the signal. Mechanisms for processing the signal include mechanisms for generating positioning data of thesensor 702 within the passageways and mechanisms for generating an image of the sensor positioned within the passageways reflecting the positioning data of thesensor 702. This is illustrated in FIG. 16. Here, thecatheter 120 is attached to theEPD device 102 and itsdistal end 124 is introduced to a lung passageway of a patient P. A computer generatedpulmonary anatomy image 708 is shown on thevisual display 110. Further, acatheter positioning image 710 is shown within theanatomy image 708 reflecting the real-time position of thecatheter 120 within the actual patient's anatomy. In this way, the target lung compartment may be more easily located and identified for later access if desired. - VI. DATA RECEIVING COMPONENTS[0104]
- Certain aspects of the[0105]
data receiving components 115 have been presented above. Thedata receiving component 115 receives processed data from theEPD device 102 or any of thecomponents 104 for output to the user. When thecomponent 115 is thevisual display 110, the processed data is presented in visual form. Alternatively, thecomponent 115 may be a computer readable medium, such as disks, diskettes, CD-ROMs, tapes or the like. The computer readable medium may then be transported to another device, such as a computer, workstation or even anotherEPD device 102 for use. In any case, at some point the processed data is typically displayed in visual form. It may be appreciated that the possibilities of displaying measurement information in visual form are limitless. A few embodiments are presented as examples. - As previously described, the pulmonary diagnostic system is used to measure compartments of a lung, wherein a compartment could be an entire lobe, a segment or a subsegment and beyond. Although information generated from a compartment may be used in determining the level of disease of the compartment itself, comparison of the generated information to other information is also useful in diagnosis and assessment of disease. For example, information generated from a compartment may be compared with baseline information from the patient or to information from a healthy patient. For ease in comparison, both or multiple sets of information may be displayed in the[0106]
visual display 110 simultaneously. Such display may be graphical, numerical or any other type. Further, information generated from one compartment may be compared with information from another similar compartment within the same patient. This concept may be extended to numerous compartments. Again, for ease in comparison, multiple sets of information may be displayed in thevisual display 110 simultaneously. This in turn may allow the physician to rank the compartments in order of level of disease or in order of need for treatment. Similarly, information generated from one compartment may be compared with information from other types or sizes of compartments to determine the affect of each compartment on the others. Again, multiple sets of information may be displayed in thevisual display 110 simultaneously for this purpose. - In addition, as described in relation to the[0107]
imaging unit 600 andmapping unit 700, visual images of the lung anatomy can be displayed on thevisual display 110 as noted above. This may be useful in both diagnosis of disease, positioning of thepulmonary catheter 120 and determining the affect of treatment. - VII. BLOCKAGE CATHETER[0108]
- Once sufficient diagnostic testing, imaging and evaluation has been performed on the lung compartments[0109]154, a treatment protocol may be determined. In some cases, lung volume reduction may be prescribed. To test the effects of such reduction prior to actual treatment, the lung passageway which leads to the lung compartment to be reduced may be temporarily occluded with a blockage catheter. Optionally this temporary occlusion with a blockage catheter may itself be the diagnostic test. One embodiment, shown in FIG. 17A, illustrates the
blockage catheter 750 as comprising acatheter body 752 having a proximal end756, adistal end 754, and aninflatable occlusion balloon 758 near itsdistal end 754. Theblockage catheter 750 may have a smaller outer diameter than theaccess catheter 10 or otherpulmonary catheters 120 which have lumens of significant diameter for various testing purposes. Here, lumens may simply be sized for inflation of theocclusion balloon 758 or passage of a guidewire. Another embodiment, shown in FIGS.17B-D, illustrates theblockage catheter 750 as comprising acatheter body 770 having aproximal end 772 and a multiplicity ofdistal ends 774, eachdistal end 774 having aninflatable occlusion balloon 776 mounted thereon. Typically, aseparate guidewire lumen 780 andballoon inflation lumen 782 within thecatheter body 770 are present from theproximal end 772 to eachdistal end 774.End connectors 784 may be present near theproximal end 772 for access to eachlumen outer sleeve 786 encasing thecatheter body 770 unites the distal ends774 for introduction purposes. FIG. 17C shows a cross-section of the distal ends774 of thecatheter body 770. It may be appreciated that thecatheter body 770 may comprise separate catheters, each having aguidewire lumen 780,inflation lumen 782 andocclusion balloon 776, which are held together by theouter sleeve 786 or other means. Referring to FIG. 17D, placement of theblockage catheter 750 involves positioning eachdistal end 774 into a different lung passageway. Eachdistal end 774 may be independently positioned with the use of a guidewire. Thus, a number oflung compartments 154 may be simultaneously isolated by theblockage catheter 750. - When the[0110]
blockage catheter 750 is in place and the lung passageway(s) occluded by the balloon(s), the affectedlung compartment 154 will be isolated from the remainder of the lung. At this point, testing, imaging and evaluation of the overall lung performance may be undertaken to measure the effects of the isolation. Such techniques would include, for example, CT scanning, spirometry, or plethysmography to obtain images, spirometry data or plethysmography data, respectively. This in turn reflects the effect of reduction of that isolated compartment. Similarly, such testing and evaluation may be performed on specific segments of the lung for assessing particular regions of the lung. Referring back to FIG. 17A, such testing with apulmonary catheter 120 while theblockage catheter 750 is in place is illustrated. Theblockage catheter 750 may be introduced through a lumen in thepulmonary catheter 120 or theblockage catheter 750 may simply lie in parallel with thepulmonary catheter 120. If thepulmonary catheter 120 utilizes anocclusion member 128, themember 128 may seal against the lumen and thecatheter body 752 of theblockage catheter 750. Both theblockage catheter 750 andpulmonary catheter 120, or any additional catheters, may be simultaneously connected to theEPD device 100 if desired. The results of such assessment may determine the most effective course of treatment. - VIII. TREATMENT UNIT[0111]
- In some embodiments, a[0112]
measuring component 104 of the pulmonarydiagnostic system 100 may comprise atreatment unit 800, as shown in FIG. 18. Again, for clarity, thetreatment unit 800 is illustrated as a separate attachable unit, however it may be appreciated that theunit 800 may be integral or internal to theEPD device 102. Thetreatment unit 800 may be used to perform a lung volume reduction procedure on alung compartment 154. In this case, theunit 800 would includemechanisms 802 for performing lung volume reduction. This typically involves aspirating the contents of the compartment after isolating the compartment from the remainder of the anatomy. This is typically achieved by introducing thedistal end 14 of theaccess catheter 10 endotracheally to thetarget compartment 154. Once in position, the compartment may be isolated by occluding the air passageway, such as by inflating theocclusion balloon 18 within the passageway. The target compartment may then be collapsed by aspirating air, and any other gases or liquids that may have been introduced, from the compartment, such as through a lumen in theaccess catheter 10. Optionally, the passageway may then be sealed, for example by deploying a plug within the air passageway. Some sealing methods include the use of tissue adhesives, occlusive balloons, expanding occlusive structures, the use of energy-induced tissue fusion and the like. Preferred embodiments of various types of treatments are described in copending U.S. patent application Ser. Nos. 09/425,272 (Attorney Docket No. 017534-000600), 09/347,032 (Attorney Docket No. 017534-000700), 09/606,320 (Attorney Docket No. 017534-000710), 09/523,016 (Attorney Docket No. 017534-001000), 09/699,302 (Attorney Docket No. 017534-001200), all of which are incorporated as a reference herein for all purposes. After treatment, the affect of treatment may be evaluated by some of the measurement methods described above. - [0113]
Kits 900 according to the present invention comprise any number of items related to the pulmonary diagnostic system described and instructions for use IFU. As shown in FIG. 19,such kits 900 typically include theEPD device 102 and instructions for use IFU setting forth methods according to the present invention. TheEPD device 102 may have one or more measuring components disposed internally, however thekit 900 may also include one ormore measuring components 104 as shown. Optionally, thekits 900 may further include any of the other system components described above, such as one or morepulmonary catheters 120,guidewires 902, or a variety of accessories, such as areceiver 454. Some or all kit components will usually be packaged together in apouch 905 or other conventional medical device packaging. Usually, those kit components, such as apulmonary catheter 120, which will be used in performing the procedure on the patient will be sterilized and maintained within the kit. Optionally, separate pouches, bags, trays or other packaging may be provided within a larger package, where the smaller packs may be opened separately to separately maintain the components in a sterile fashion. - Although the foregoing invention has been described in some detail by way of illustration and example, for purposes of clarity of understanding, it will be obvious that various alternatives, modifications and equivalents may be used and the above description should not be taken as limiting in scope of the invention which is defined by the appended claims.[0114]
Claims (68)
1. A pulmonary diagnostic system comprising:
at least one sensor which generates measurement data reflecting a respiratory feature of a lung compartment;
an endobronchial pulmonary diagnostic device connectable with a pulmonary catheter which is configured for accessing the lung compartment, the device comprising
means for transferring fluid or gas to or from the lung compartment through the pulmonary catheter,
means for receiving the measurement data from the sensor, and
means for processing the measurement data; and at least one data receiving component which receives the processed data.
2. A system as inclaim 1 , wherein the respiratory feature comprises pressure, flow rate, velocity, oxygen concentration, carbon dioxide concentration, or noble gas concentration.
3. A system as inclaim 1 , wherein the means for processing the measurement data comprises an analyzing means which analyzes the measurement data.
4. A system as inclaim 3 , wherein the measuring component comprises a visual display which displays the processed data in visual form.
5. A system as inclaim 4 , wherein the visual form includes graphs, charts, tables, numbers, images, or figures.
6. A system as inclaim 3 , wherein the analyzing means calculates an average pressure value, a volume value, compliance value, an average tidal volume value, and a resistance value.
7. A system as inclaim 1 , wherein the means for processing the measurement data converts the measurement data into a computer readable format.
8. A system as inclaim 7 , wherein the data receiving component comprises a recording means which records the measurement data onto computer readable medium.
9. A system as inclaim 8 , wherein the computer readable medium comprises disks, diskettes, CD-ROMs and tapes.
10. A system as inclaim 1 , further comprising at least one measuring component comprising a source of the fluid or gas and a means for generating flow of the fluid or gas.
11. A system as inclaim 10 , wherein the means for transferring fluid or gas comprises a conduit between the means for generating flow and the catheter.
12. A system as inclaim 10 , wherein the gas comprises air, oxygen, carbon dioxide, noble gas, radiopaque gas, polarized gas or a mixture of any of these.
13. A system as inclaim 10 , wherein the fluid comprises radiopaque contrast solution, water, or ultrasonic imaging fluid.
14. A system as inclaim 1 , wherein the means for transferring fluid or gas comprises a source of the fluid or gas and a means for generating flow of the fluid or gas.
15. A system as inclaim 14 , wherein the gas comprises air, oxygen, carbon dioxide, noble gas, radiopaque gas, polarized gas or a mixture of any of these.
16. A system as inclaim 14 , wherein the fluid comprises radiopaque contrast solution, water, or ultrasonic imaging fluid.
17. A system as inclaim 1 , further comprising the pulmonary catheter.
18. A system as inclaim 17 , wherein the sensor is disposed on or in the pulmonary catheter.
19. A system as inclaim 18 , wherein pulmonary catheter has a proximal end connectable with the device and a distal tip and wherein the sensor is disposed near the distal tip.
20. A system as inclaim 1 , wherein the sensor is disposed within the device.
21. A system as inclaim 1 , further comprising at least one measuring component and wherein the sensor is disposed within the measuring component.
22. A pulmonary diagnostic system comprising:
at least one pressure sensor which generates pressure measurement data reflecting the pressure within a lung compartment;
at least one flow sensor which generates flow measurement data reflecting flow to or from the lung compartment;
a physiological testing unit comprising
a source of fluid or gas,
means for generating flow of the fluid or gas,
means for receiving the pressure and flow measurement data from the sensors, and
means for processing the pressure and flow measurement data;
a data receiving component which receives the processed pressure and flow measurement data; and
an endobronchial pulmonary diagnostic device connectable with the physiological testing unit, the measuring component and a pulmonary catheter which is configured for accessing the lung compartment, the device comprising
means for transferring the fluid or gas to or from the lung compartment, and
means for controlling the generation of flow and the receiving of pressure and flow measurement data by the physiological testing unit and the receiving of processed pressure and flow measurement data by the measuring component.
23. A system as inclaim 22 , wherein the means for processing the pressure and flow measurement data comprises an analyzing means comprising means for calculating volume measurement data from the flow measurement data and means for generating a pressure-volume curve from the pressure and volume measurement data.
24. A system as inclaim 23 , wherein the data receiving component comprises a visual display which displays the pressure-volume curve.
25. A system as inclaim 23 , wherein the analyzing means further comprises means for calculating a compliance value.
26. A system as inclaim 23 , wherein the analyzing means further comprises means for calculating a compliance value, average tidal volume value or resistance value with the use of the pressure and volume measurement data.
27. A system as inclaim 22 , further comprising
a sensor which measures the concentration of a gas,
a gas dilution unit connectable with the device comprising a source of noble gas,
means for generating flow of the noble gas,
means for receiving the gas concentration measurement data from the sensor, and
means for processing the gas concentration measurement data.
28. A system as inclaim 27 , wherein the measuring component further receives the processed gas concentration measurement data, and the device further comprises means for controlling the generation of flow of the noble gas and the receiving of gas concentration measurement data by the gas dilution unit and the receiving of processed gas concentration measurement data by the measuring component.
29. A system as inclaim 27 , wherein the sensor which measures the concentration of gas comprises a membrane chemical transfer sensor, a photochemical reaction sensor, an electropotential sensor, a microchip, a laser diode, an optical transmittance sensor or a piezoelectric sensor.
30. A system as inclaim 27 , wherein the means for processing the gas concentration measurement data comprises an analyzing means comprising means for calculating the initial volume of air in the compartment using the gas concentration measurement data.
31. A system as inclaim 22 , further comprising an imaging unit connectable with the device comprising
a source of imaging fluid or gas, and
means for generating flow of the imaging fluid or gas.
32. A system as inclaim 31 , wherein the imaging unit or the device is connectable with a mechanism for generating at least one image of the compartment.
33. A system as inclaim 32 , wherein the measuring component further comprises means for receiving the image and the device further comprises means for controlling the generation of flow of the imaging fluid or gas and the receiving of the image by the measuring component.
34. A system as inclaim 32 , wherein the fluid or gas is radiopaque and the image is generated with the use of fluoroscopy.
35. A system as inclaim 32 , wherein the fluid or gas is polarized and the image is generated with the use of magnetic resonance imaging.
36. A system as inclaim 32 , wherein the fluid is ultrasonic imaging fluid and the image is generated with the use of ultrasound.
37. A system as inclaim 32 , wherein the mechanism generates more than one image of the compartment and the measuring component further comprises means for generating a three dimensional composite image of the compartment from the images.
38. A pulmonary diagnostic system comprising:
a pulmonary catheter configured for accessing a lung compartment through a lung passageway;
at least one velocity sensor disposed near the tip of the catheter which generates air velocity measurement data characterizing the lung compartment;
an endobronchial pulmonary diagnostic device connectable with the catheter comprising
means for receiving the measurement data from the sensor, and
means for processing the measurement data; and
at least one data receiving component which receives the processed data.
39. A pulmonary diagnostic system comprising:
a pulmonary catheter configured for accessing a lung compartment through a lung passageway;
at least one signal emitting sensor disposed on the catheter;
at least one receiver positionable on the exterior of a body which receives the signal emitted from the sensor to generate resistivity measurement data;
an endobronchial pulmonary diagnostic device connectable with the catheter and the receiver comprising
means for receiving the measurement data from the receiver, and
means for processing the measurement data; and
at least one data receiving component which receives the processed data.
40. A system as inclaim 39 , wherein the means for processing the measurement data comprises an analyzing means which analyzes the measurement data.
41. A system as inclaim 40 , wherein the compartment comprises lung tissue and the means for analyzing means calculates the resistance of the lung tissue of the compartment.
42. A pulmonary diagnostic system comprising:
a pulmonary catheter configured for accessing a lung compartment through a lung passageway;
at least one signal emitting sensor disposed on the catheter;
a mapping unit connectable with the device comprising
means for receiving the signal from the sensor, and
means for processing the signal;
at least one data receiving component which receives the processed signal; and
an endobronchial pulmonary diagnostic device connectable with the catheter, the mapping unit and the data receiving component comprising means for controlling the receiving of the processed signal by the data receiving component.
43. A system as inclaim 42 , wherein the means for processing the signal comprises analyzing means which analyzes the signal to determine the location of the sensor when within the lung passageway.
44. A system as inclaim 43 , wherein the data receiving component comprises a visual display which displays the location of the sensor in visual form.
45. A method for assessment of a lung compartment comprising the steps of:
providing a pulmonary diagnostic system comprising an endobronchial pulmonary diagnostic device and at least one measuring component connected with the device;
connecting a pulmonary catheter to the device, said catheter having a proximal end and a distal end;
introducing the distal end of the catheter to a compartment of a lung; and
generating measurement data characterizing the compartment of the lung with the pulmonary diagnostic system.
46. A method as inclaim 45 , wherein the compartment comprises a lung segment and introducing comprises advancing the distal end through a bronchial passageway to the lung segment.
47. A method as inclaim 45 , wherein the compartment comprises a lung lobe and introducing comprises advancing the distal end through a bronchial passageway to the lung lobe.
48. A method as inclaim 45 , wherein the measuring component comprises a pulmonary mechanics unit and the step of generating measurement data comprises generating pressure data or volume data characterizing the compartment.
49. A method as inclaim 45 , wherein the measuring component comprises a physiological testing unit and the step of generating measurement data comprises generating airflow movement data characterizing airflow into or out of the compartment, generating oxygen saturation data, generating carbon dioxide saturation data, or generating electrophysiological measurements characterizing the lung compartment.
50. A method as inclaim 45 , wherein the measuring component comprises a gas dilution unit and the step of gathering measurement data comprises performing functional residual capacity testing.
51. A method as inclaim 45 , wherein the measuring component comprises an imaging unit and the step of gathering measurement data comprises generating at least one image of the lung compartment.
52. A method as inclaim 51 , wherein the step of gathering measurement data further comprises combining images to generate a composite three-dimensional image of the lung compartment.
53. A method as inclaim 45 , wherein the measuring component comprises a mapping unit and the step of generating measurement data comprises generating positioning data reflecting the position of the catheter in the compartment.
54. A method as inclaim 45 , wherein the pulmonary diagnostic system further comprises a data receiving component comprising a visual display and the step of generating measurement data comprises displaying the measurement data characterizing the compartment on the visual display.
55. A method as inclaim 45 , further comprising:
repositioning the catheter to another compartment of the lung; and
generating measurement data characterizing the another compartment of the lung with the use of the pulmonary diagnostic system.
56. A method as inclaim 55 , wherein the pulmonary diagnostic system further comprises a data receiving component comprising a visual display, the method further comprising displaying the data characterizing the compartment and the other compartment on the visual display.
57. A method as inclaim 56 , wherein the step of displaying the data comprises simultaneously displaying the data characterizing the compartment and the other compartment.
58. A method as inclaim 55 , further comprising comparing the data characterizing the compartment and the other compartment to each other.
59. A method as inclaim 58 , further comprising ranking the compartments.
60. A method as inclaim 45 , further comprising performing lung volume reduction treatment to reduce the compartment.
61. A method for assessment of a lung comprising the steps of:
providing a pulmonary diagnostic system comprising an endobronchial pulmonary diagnostic device and at least one measuring component connected with the device;
connecting a blockage catheter to the device, said catheter having a proximal end and a distal end;
introducing the distal end of the blockage catheter to a target compartment of the lung and isolating the target compartment; and
generating measurement data characterizing the lung having the isolated target compartment with the pulmonary diagnostic system.
62. A method as inclaim 61 , wherein the blockage catheter has multiple distal ends and the introducing step comprises introducing each distal end to a different target compartment of the lung and isolating the target compartments.
63. A method as inclaim 61 , wherein measurement data comprises images, spirometry data, or plethysmography data.
64. A method as inclaim 61 , further comprising introducing an access catheter to a larger compartment of the lung which includes the target compartment and performing the generating step with the access catheter.
65. A method as inclaim 61 , further comprising performing lung volume reduction treatment to reduce the isolated target compartment.
66. A kit comprising:
a pulmonary diagnostic system comprising an endobronchial pulmonary diagnostic device and at least one measuring component connected with the device; and
instructions for use setting forth methods including the steps of:
connecting a pulmonary catheter to the device, said catheter having a proximal end and a distal end;
introducing the distal end of the catheter to a compartment of a lung; and
gathering measurement data characterizing the compartment of the lung with the use of the pulmonary diagnostic system.
67. A kit as inclaim 66 , further comprising a pulmonary catheter.
68. A kit as inclaim 67 , wherein the pulmonary catheter comprises an access catheter, microcatheter, or blockage catheter.
Priority Applications (8)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US10/241,733US20030051733A1 (en) | 2001-09-10 | 2002-09-10 | Method and apparatus for endobronchial diagnosis |
| US11/296,951US7883471B2 (en) | 2001-09-10 | 2005-12-07 | Minimally invasive determination of collateral ventilation in lungs |
| US12/108,035US20080200797A1 (en) | 2001-09-10 | 2008-04-23 | Method and apparatus for endobronchial diagnosis |
| US12/972,225US8454527B2 (en) | 2001-09-10 | 2010-12-17 | Minimally invasive determination of collateral ventilation in lungs |
| US13/830,642US20140107396A1 (en) | 2002-09-10 | 2013-03-14 | Systems and methods for delivering a therapeutic agent |
| US13/891,719US20130245484A1 (en) | 2001-09-10 | 2013-05-10 | Minimally invasive determination of collateral ventilation in lungs |
| US14/919,480US10413244B2 (en) | 2001-09-10 | 2015-10-21 | Method and apparatus for endobronchial diagnosis |
| US16/562,158US20190388034A1 (en) | 2001-09-10 | 2019-09-05 | Method and apparatus for endobronchial diagnosis |
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US31853901P | 2001-09-10 | 2001-09-10 | |
| US10/241,733US20030051733A1 (en) | 2001-09-10 | 2002-09-10 | Method and apparatus for endobronchial diagnosis |
Related Parent Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US12/474,169Continuation-In-PartUS20100158795A1 (en) | 2002-09-10 | 2009-05-28 | Methods and systems for assessing lung function and delivering therapeutic agents |
Related Child Applications (4)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US11/296,951Continuation-In-PartUS7883471B2 (en) | 2001-09-10 | 2005-12-07 | Minimally invasive determination of collateral ventilation in lungs |
| US12/108,035DivisionUS20080200797A1 (en) | 2001-09-10 | 2008-04-23 | Method and apparatus for endobronchial diagnosis |
| US13/830,642Continuation-In-PartUS20140107396A1 (en) | 2002-09-10 | 2013-03-14 | Systems and methods for delivering a therapeutic agent |
| US14/919,480ContinuationUS10413244B2 (en) | 2001-09-10 | 2015-10-21 | Method and apparatus for endobronchial diagnosis |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| US20030051733A1true US20030051733A1 (en) | 2003-03-20 |
Family
ID=23238603
Family Applications (5)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US10/241,733AbandonedUS20030051733A1 (en) | 2001-09-10 | 2002-09-10 | Method and apparatus for endobronchial diagnosis |
| US12/108,035AbandonedUS20080200797A1 (en) | 2001-09-10 | 2008-04-23 | Method and apparatus for endobronchial diagnosis |
| US14/919,480Expired - Fee RelatedUS10413244B2 (en) | 2001-09-10 | 2015-10-21 | Method and apparatus for endobronchial diagnosis |
| US15/150,324AbandonedUS20160249860A1 (en) | 2001-09-10 | 2016-05-09 | Method and apparatus for endobronchial diagnosis |
| US16/562,158AbandonedUS20190388034A1 (en) | 2001-09-10 | 2019-09-05 | Method and apparatus for endobronchial diagnosis |
Family Applications After (4)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US12/108,035AbandonedUS20080200797A1 (en) | 2001-09-10 | 2008-04-23 | Method and apparatus for endobronchial diagnosis |
| US14/919,480Expired - Fee RelatedUS10413244B2 (en) | 2001-09-10 | 2015-10-21 | Method and apparatus for endobronchial diagnosis |
| US15/150,324AbandonedUS20160249860A1 (en) | 2001-09-10 | 2016-05-09 | Method and apparatus for endobronchial diagnosis |
| US16/562,158AbandonedUS20190388034A1 (en) | 2001-09-10 | 2019-09-05 | Method and apparatus for endobronchial diagnosis |
Country Status (5)
| Country | Link |
|---|---|
| US (5) | US20030051733A1 (en) |
| EP (1) | EP1435833B1 (en) |
| JP (1) | JP4301945B2 (en) |
| AU (1) | AU2002331842A1 (en) |
| WO (1) | WO2003022221A2 (en) |
Cited By (152)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US20020112729A1 (en)* | 2001-02-21 | 2002-08-22 | Spiration, Inc. | Intra-bronchial obstructing device that controls biological interaction with the patient |
| US20040092811A1 (en)* | 2002-11-12 | 2004-05-13 | Hill David Guy | System and method for measurement of local lung function using electron beam CT |
| US20040143282A1 (en)* | 2002-05-17 | 2004-07-22 | Dillard David H. | Methods of achieving lung volume reduction with removable anchored devices |
| US20040206349A1 (en)* | 2001-09-11 | 2004-10-21 | Alferness Clifton A. | Removable lung reduction devices, systems, and methods |
| US20040225254A1 (en)* | 2003-05-07 | 2004-11-11 | Don Tanaka | Localized pleurodesis chemical delivery |
| US20040237966A1 (en)* | 2003-05-29 | 2004-12-02 | Don Tanaka | Methods and devices to assist pulmonary decompression |
| US20040244803A1 (en)* | 2003-06-05 | 2004-12-09 | Don Tanaka | Intra-thoracic collateral ventilation bypass system |
| US20050016530A1 (en)* | 2003-07-09 | 2005-01-27 | Mccutcheon John | Treatment planning with implantable bronchial isolation devices |
| US20050025816A1 (en)* | 2003-07-15 | 2005-02-03 | Don Tanaka | Methods and devices to accelerate wound healing in thoracic anastomosis applications |
| US6929637B2 (en) | 2002-02-21 | 2005-08-16 | Spiration, Inc. | Device and method for intra-bronchial provision of a therapeutic agent |
| US20050178389A1 (en)* | 2004-01-27 | 2005-08-18 | Shaw David P. | Disease indications for selective endobronchial lung region isolation |
| US20050288702A1 (en)* | 2004-06-16 | 2005-12-29 | Mcgurk Erin | Intra-bronchial lung volume reduction system |
| US20050288549A1 (en)* | 2004-06-14 | 2005-12-29 | Pneumrx, Inc. | Guided access to lung tissues |
| US20060009801A1 (en)* | 2004-07-08 | 2006-01-12 | Mcgurk Erin | Pleural effusion treatment device, method and material |
| US20060025815A1 (en)* | 2004-07-08 | 2006-02-02 | Mcgurk Erin | Lung device with sealing features |
| US20060025650A1 (en)* | 2002-10-03 | 2006-02-02 | Oren Gavriely | Tube for inspecting internal organs of a body |
| US20060107961A1 (en)* | 2004-11-19 | 2006-05-25 | Don Tanaka | Localized pleurodesis evacuation device |
| US20060118126A1 (en)* | 2004-11-19 | 2006-06-08 | Don Tanaka | Methods and devices for controlling collateral ventilation |
| US20060118125A1 (en)* | 2004-11-19 | 2006-06-08 | Don Tanaka | Pulmonary drug delivery |
| US20060124126A1 (en)* | 2004-12-10 | 2006-06-15 | Don Tanaka | Collateral ventilation device with chest tube/evacuation features |
| US20060155217A1 (en)* | 2002-09-24 | 2006-07-13 | Devore Lauri J | Device and method for measuring the diameter of an air passageway |
| US20060167416A1 (en)* | 2004-11-23 | 2006-07-27 | Mark Mathis | Steerable device for accessing a target site and methods |
| US20060184016A1 (en)* | 2005-01-18 | 2006-08-17 | Glossop Neil D | Method and apparatus for guiding an instrument to a target in the lung |
| US7100616B2 (en) | 2003-04-08 | 2006-09-05 | Spiration, Inc. | Bronchoscopic lung volume reduction method |
| US20060206147A1 (en)* | 2002-02-21 | 2006-09-14 | Devore Lauri J | Intra-bronchial obstruction device that provides a medicant intra-bronchially to the patient |
| US20060235432A1 (en)* | 2002-02-21 | 2006-10-19 | Devore Lauri J | Intra-bronchial obstructing device that controls biological interaction with the patient |
| US20060235467A1 (en)* | 2002-04-16 | 2006-10-19 | Devore Lauri J | Removable anchored lung volume reduction device and methods |
| JP2006525080A (en)* | 2003-04-22 | 2006-11-09 | メディ−フィジックス・インコーポレイテッド | One-period volume control and measurement system, method, and device for respiratory hyperpolarized gas transmission for MRI / NMR |
| US20060254600A1 (en)* | 2000-03-27 | 2006-11-16 | Asthmatx, Inc. | Methods for treating airways |
| US20060264772A1 (en)* | 2001-09-10 | 2006-11-23 | Pulmonx | Minimally invasive determination of collateral ventilation in lungs |
| US20070043350A1 (en)* | 2005-08-17 | 2007-02-22 | Pulmonx | Selective lung tissue ablation |
| US20070051372A1 (en)* | 2005-08-23 | 2007-03-08 | Don Tanaka | Collateral ventilation bypass system with retention features |
| US20070133894A1 (en)* | 2005-12-07 | 2007-06-14 | Siemens Corporate Research, Inc. | Fissure Detection Methods For Lung Lobe Segmentation |
| US20070142742A1 (en)* | 2005-07-13 | 2007-06-21 | Pulmonx | Methods and systems for segmental lung diagnostics |
| US20070167698A1 (en)* | 2005-12-14 | 2007-07-19 | General Electric Company | Method and apparatus for alignment of a mobile fluoroscopic imaging system |
| US20070167683A1 (en)* | 2006-01-19 | 2007-07-19 | Boston Scientific Scimed, Inc. | Endoscopic system with integrated patient respiratory status indicator |
| US20070163598A1 (en)* | 2006-01-17 | 2007-07-19 | Asia Chang | Variable resistance pulmonary ventilation bypass valve |
| US20070185531A1 (en)* | 2001-10-25 | 2007-08-09 | Spiration, Inc. | Apparatus and method for deployment of a bronchial obstruction device |
| US20070186932A1 (en)* | 2006-01-06 | 2007-08-16 | Pulmonx | Collateral pathway treatment using agent entrained by aspiration flow current |
| US20070221230A1 (en)* | 2006-03-13 | 2007-09-27 | David Thompson | Minimally invasive lung volume reduction device and method |
| US20070232992A1 (en)* | 2006-03-31 | 2007-10-04 | James Kutsko | Articulable anchor |
| US20070235846A1 (en)* | 2006-04-01 | 2007-10-11 | Stats Chippac Ltd. | Integrated circuit package system with net spacer |
| WO2006078451A3 (en)* | 2005-01-20 | 2007-10-18 | Pulmonx | Minimally invasive determination of collateral ventilation in lungs |
| US20070270776A1 (en)* | 2003-06-03 | 2007-11-22 | Respira, Inc. | Lung reduction system |
| US20080072914A1 (en)* | 2006-08-25 | 2008-03-27 | Hendricksen Michael J | Bronchial Isolation Devices for Placement in Short Lumens |
| US20080077038A1 (en)* | 2004-11-02 | 2008-03-27 | Children's Hospital Of Philadelphia | Respiratory Volume/Flow Gating, Monitoring, and Spirometry System for Mri |
| US20080119866A1 (en)* | 2002-03-20 | 2008-05-22 | Alferness Clifton A | Removable anchored lung volume reduction devices and methods |
| US20080132757A1 (en)* | 2006-12-01 | 2008-06-05 | General Electric Company | System and Method for Performing Minimally Invasive Surgery Using a Multi-Channel Catheter |
| US20080200797A1 (en)* | 2001-09-10 | 2008-08-21 | Pulmonx | Method and apparatus for endobronchial diagnosis |
| US7426929B2 (en) | 2003-05-20 | 2008-09-23 | Portaero, Inc. | Intra/extra-thoracic collateral ventilation bypass system and method |
| US20080281151A1 (en)* | 2007-05-11 | 2008-11-13 | Portaero, Inc. | Pulmonary pleural stabilizer |
| US20080281433A1 (en)* | 2007-05-11 | 2008-11-13 | Portaero, Inc. | Methods and devices to create a chemically and/or mechanically localized pleurodesis |
| US20080287878A1 (en)* | 2007-05-15 | 2008-11-20 | Portaero, Inc. | Pulmonary visceral pleura anastomosis reinforcement |
| US20080283065A1 (en)* | 2007-05-15 | 2008-11-20 | Portaero, Inc. | Methods and devices to maintain patency of a lumen in parenchymal tissue of the lung |
| US20080295829A1 (en)* | 2007-05-30 | 2008-12-04 | Portaero, Inc. | Bridge element for lung implant |
| US20090013995A1 (en)* | 2006-10-13 | 2009-01-15 | Williams Andrea R | Oral airway for endoscopic and intubating procedures |
| US20090038620A1 (en)* | 2005-12-05 | 2009-02-12 | Shai Efrati | Endotracheal Tube and Intubation System Including Same |
| US20090099530A1 (en)* | 2007-10-12 | 2009-04-16 | Martin Neal Adams | Valve loader method, system, and apparatus |
| US20090125007A1 (en)* | 2007-11-09 | 2009-05-14 | Spectranetics | Intra-Vascular Device With Pressure Detection Capabilities Using Pressure Sensitive Material |
| US7533671B2 (en) | 2003-08-08 | 2009-05-19 | Spiration, Inc. | Bronchoscopic repair of air leaks in a lung |
| US20090205648A1 (en)* | 2008-02-19 | 2009-08-20 | Portaero, Inc. | Pneumostoma management system with secretion management features for treatment of chronic obstructive pulmonary disease |
| US20090205641A1 (en)* | 2008-02-19 | 2009-08-20 | Portaero, Inc. | Pneumostoma management device and method for treatment of chronic obstructive pulmonary disease |
| US20090229605A1 (en)* | 2005-08-24 | 2009-09-17 | Hospitech Respiration Ltd. | Ajustment of endotracheal tube cuff filling |
| WO2009135070A1 (en)* | 2008-05-01 | 2009-11-05 | Spiration, Inc. | Direct lung sensor systems, methods, and apparatuses |
| US20090292262A1 (en)* | 2007-10-12 | 2009-11-26 | Martin Neal Adams | Valve loader method, system, and apparatus |
| WO2009152013A1 (en) | 2008-06-12 | 2009-12-17 | Pulmonx | Methods and systems for assessing lung function and delivering therapeutic agents |
| WO2010030691A1 (en)* | 2008-09-09 | 2010-03-18 | Pulmonx Corporation | Systems and methods for inhibiting secretion flow into a functional assessment catheter |
| US20100070050A1 (en)* | 2008-09-12 | 2010-03-18 | Pneumrx, Inc. | Enhanced Efficacy Lung Volume Reduction Devices, Methods, and Systems |
| US20100170507A1 (en)* | 2009-01-08 | 2010-07-08 | Portaero, Inc. | Pneumostoma management device with integrated patency sensor and method |
| US20100204707A1 (en)* | 2009-02-11 | 2010-08-12 | Portaero, Inc. | Surgical instruments for creating a pneumostoma and treating chronic obstructive pulmonary disease |
| US20100286544A1 (en)* | 2008-02-19 | 2010-11-11 | Portaero, Inc. | Methods and devices for assessment of pneumostoma function |
| US20100305715A1 (en)* | 2009-05-18 | 2010-12-02 | Pneumrx, Inc. | Cross-Sectional Modification During Deployment of an Elongate Lung Volume Reduction Device |
| US7931641B2 (en) | 2007-05-11 | 2011-04-26 | Portaero, Inc. | Visceral pleura ring connector |
| US20110152678A1 (en)* | 2005-01-20 | 2011-06-23 | Pulmonx Corporation | Methods and devices for passive residual lung volume reduction and functional lung volume expansion |
| US20110209702A1 (en)* | 2010-02-26 | 2011-09-01 | Nellcor Puritan Bennett Llc | Proportional Solenoid Valve For Low Molecular Weight Gas Mixtures |
| US8062315B2 (en)* | 2007-05-17 | 2011-11-22 | Portaero, Inc. | Variable parietal/visceral pleural coupling |
| US20120150027A1 (en)* | 2010-07-01 | 2012-06-14 | Pulmonx Corporation | Methods and systems for endobronchial diagnosis and treatment |
| US20120149995A1 (en)* | 2010-07-01 | 2012-06-14 | Pulmonx Corporation | Methods and systems for endobronchial diagnostics |
| US20120150057A1 (en)* | 2010-07-01 | 2012-06-14 | Pulmonx Corporation | Methods and systems for endobronchial diagnosis |
| US20120172748A1 (en)* | 2010-12-29 | 2012-07-05 | Dunn Lisa A | Temperature measuring device |
| US20120172749A1 (en)* | 2009-12-10 | 2012-07-05 | He Kongyuan | Laryngeal Mask Airway (LMA) with Integrated Core Temperature Monitor and Display |
| US8523782B2 (en) | 2005-12-07 | 2013-09-03 | Pulmonx Corporation | Minimally invasive determination of collateral ventilation in lungs |
| US20130338524A1 (en)* | 2006-08-28 | 2013-12-19 | Pulmonx, Inc. | Functional assessment and treatment catheters and methods for their use in the lung |
| US20140097847A1 (en)* | 2011-06-15 | 2014-04-10 | Koninklijke Philips N.V. | Optical angular momentum induced hyperpolarisation in interventional applications |
| US8740921B2 (en) | 2006-03-13 | 2014-06-03 | Pneumrx, Inc. | Lung volume reduction devices, methods, and systems |
| US8795241B2 (en) | 2011-05-13 | 2014-08-05 | Spiration, Inc. | Deployment catheter |
| US8876791B2 (en) | 2005-02-25 | 2014-11-04 | Pulmonx Corporation | Collateral pathway treatment using agent entrained by aspiration flow current |
| CN104173033A (en)* | 2013-05-24 | 2014-12-03 | 株式会社电装 | Apparatus for testing respiratory function |
| US8950398B2 (en) | 2008-09-30 | 2015-02-10 | Covidien Lp | Supplemental gas safety system for a breathing assistance system |
| CN104491976A (en)* | 2014-09-05 | 2015-04-08 | 广州中医药大学第一附属医院 | Visible tracheal catheter and bronchus blocking tube connecting tube |
| US9050094B2 (en) | 2007-03-12 | 2015-06-09 | Pulmonx Corporation | Methods and devices for passive residual lung volume reduction and functional lung volume expansion |
| WO2015127355A1 (en)* | 2014-02-24 | 2015-08-27 | Vida Diagnostics, Inc. | Treatment outcome prediction for lung volume reduction procedures |
| US9138165B2 (en) | 2012-02-22 | 2015-09-22 | Veran Medical Technologies, Inc. | Systems, methods and devices for forming respiratory-gated point cloud for four dimensional soft tissue navigation |
| US20150327836A1 (en)* | 2014-05-16 | 2015-11-19 | University Of Virginia Patent Foundation | Endovascular occlusion device and method of use |
| US9218663B2 (en) | 2005-09-13 | 2015-12-22 | Veran Medical Technologies, Inc. | Apparatus and method for automatic image guided accuracy verification |
| US9339337B2 (en) | 2008-07-21 | 2016-05-17 | The Spectranetics Corporation | Tapered liquid light guide |
| US9402633B2 (en) | 2006-03-13 | 2016-08-02 | Pneumrx, Inc. | Torque alleviating intra-airway lung volume reduction compressive implant structures |
| US9717468B2 (en) | 2006-01-10 | 2017-08-01 | Mediguide Ltd. | System and method for positioning an artificial heart valve at the position of a malfunctioning valve of a heart through a percutaneous route |
| US9826999B2 (en)* | 2004-04-21 | 2017-11-28 | Acclarent, Inc. | Methods and apparatus for treating disorders of the ear nose and throat |
| US9855100B2 (en) | 2008-04-02 | 2018-01-02 | The Spectranetics Corporation | Liquid light-guide catheter with optically diverging tip |
| US20180078119A1 (en)* | 2016-09-19 | 2018-03-22 | Covidien Lp | System and method for cleansing segments of a luminal network |
| US10076380B2 (en) | 2004-11-05 | 2018-09-18 | Boston Scientific Scimed, Inc. | Energy delivery devices and methods |
| US10149602B2 (en) | 2011-07-11 | 2018-12-11 | Ambu A/S | Endobronchial tube with integrated image sensor and a cleaning nozzle arrangement |
| US10195404B2 (en) | 2015-05-13 | 2019-02-05 | Atrium Medical Corporation | Chest drainage system |
| US10245402B2 (en) | 2011-07-11 | 2019-04-02 | Ambu A/S | Endobronchial tube with integrated image sensor |
| US10376665B2 (en) | 2016-05-05 | 2019-08-13 | Covidien Lp | Fluid dispensing catheter |
| US10390838B1 (en) | 2014-08-20 | 2019-08-27 | Pneumrx, Inc. | Tuned strength chronic obstructive pulmonary disease treatment |
| US10413300B2 (en) | 2015-06-22 | 2019-09-17 | Pulmonx Corporation | Collateral flow channel sealant delivery methods and systems |
| US10456562B2 (en) | 2015-01-20 | 2019-10-29 | Pulmonx Corporation | Bronchial sealant delivery methods and systems |
| US20190350443A1 (en)* | 2018-05-21 | 2019-11-21 | Mark D. Noar | Method For Monitoring A Property Of Tissue Of An Internal Bodily Organ And Adjusting The Tissue Property |
| US10500380B2 (en) | 2004-04-21 | 2019-12-10 | Acclarent, Inc. | Devices, systems and methods useable for treating sinusitis |
| US10537334B2 (en) | 2013-10-25 | 2020-01-21 | Pneumrx, Inc. | Genetically-associated chronic obstructive pulmonary disease treatment |
| US20200037958A1 (en)* | 2008-09-09 | 2020-02-06 | Pulmonx Corporation | Systems and methods for flushing an assessment catheter |
| US10617324B2 (en) | 2014-04-23 | 2020-04-14 | Veran Medical Technologies, Inc | Apparatuses and methods for endobronchial navigation to and confirmation of the location of a target tissue and percutaneous interception of the target tissue |
| US10624733B2 (en) | 2015-03-24 | 2020-04-21 | Spiration, Inc. | Airway stent |
| US10624701B2 (en) | 2014-04-23 | 2020-04-21 | Veran Medical Technologies, Inc. | Apparatuses and methods for registering a real-time image feed from an imaging device to a steerable catheter |
| US10695080B2 (en) | 2004-04-21 | 2020-06-30 | Acclarent, Inc. | Devices, systems and methods for diagnosing and treating sinusitis and other disorders of the ears, nose and/or throat |
| US10743978B2 (en) | 2010-07-01 | 2020-08-18 | Pulmonx Corporation | Devices and systems for lung treatment |
| US10770182B2 (en) | 2017-05-19 | 2020-09-08 | Boston Scientific Scimed, Inc. | Systems and methods for assessing the health status of a patient |
| US10779752B2 (en) | 2004-04-21 | 2020-09-22 | Acclarent, Inc. | Guidewires for performing image guided procedures |
| US10806477B2 (en) | 2004-04-21 | 2020-10-20 | Acclarent, Inc. | Systems and methods for transnasal dilation of passageways in the ear, nose or throat |
| US10842978B2 (en) | 2005-06-10 | 2020-11-24 | Acclarent, Inc. | Catheters with non-removable guide members useable for treatment of sinusitis |
| US10842368B2 (en) | 2016-06-10 | 2020-11-24 | Ambu A/S | Suction catheter with brush and method of use for lens cleaning |
| US10852264B2 (en) | 2017-07-18 | 2020-12-01 | Boston Scientific Scimed, Inc. | Systems and methods for analyte sensing in physiological gas samples |
| US10856727B2 (en) | 2004-04-21 | 2020-12-08 | Acclarent, Inc. | Endoscopic methods and devices for transnasal procedures |
| US10874838B2 (en) | 2004-04-21 | 2020-12-29 | Acclarent, Inc. | Systems and methods for transnasal dilation of passageways in the ear, nose or throat |
| US10952799B2 (en) | 2017-05-31 | 2021-03-23 | Covidien Lp | Systems and methods for navigational bronchoscopy and selective drug delivery |
| US11019989B2 (en) | 2004-04-21 | 2021-06-01 | Acclarent, Inc. | Methods and apparatus for treating disorders of the ear nose and throat |
| US11020136B2 (en) | 2004-04-21 | 2021-06-01 | Acclarent, Inc. | Deflectable guide catheters and related methods |
| US11097084B2 (en)* | 2014-09-19 | 2021-08-24 | Acclarent, Inc. | Balloon catheter assembly |
| US11166636B2 (en) | 2018-02-20 | 2021-11-09 | Boston Scientific Scimed, Inc. | Breath sampling mask and system |
| US11172846B2 (en) | 2016-10-21 | 2021-11-16 | Boston Scientific Scimed, Inc. | Gas sampling device |
| US11191457B2 (en) | 2016-06-15 | 2021-12-07 | Boston Scientific Scimed, Inc. | Gas sampling catheters, systems and methods |
| WO2021244690A1 (en)* | 2020-06-05 | 2021-12-09 | W.O.M. World Of Medicine Gmbh | Method for determining compliance of a cavity in minimally invasive surgery |
| WO2022010720A1 (en) | 2020-07-10 | 2022-01-13 | Pulmonx Corporation | Systems and methods for endobronchial diagnostics |
| US11262354B2 (en) | 2014-10-20 | 2022-03-01 | Boston Scientific Scimed, Inc. | Disposable sensor elements, systems, and related methods |
| US11304629B2 (en) | 2005-09-13 | 2022-04-19 | Veran Medical Technologies, Inc. | Apparatus and method for image guided accuracy verification |
| US11442056B2 (en) | 2018-10-19 | 2022-09-13 | Regents Of The University Of Minnesota | Systems and methods for detecting a brain condition |
| US11529502B2 (en) | 2004-04-21 | 2022-12-20 | Acclarent, Inc. | Apparatus and methods for dilating and modifying ostia of paranasal sinuses and other intranasal or paranasal structures |
| US20230000497A1 (en)* | 2005-01-20 | 2023-01-05 | Pulmonx Corporation | Methods and devices for passive residual lung volume reduction and functional lung volume expansion |
| CN115836194A (en)* | 2020-06-05 | 2023-03-21 | Wom医药世界公司 | Method of determining cavity compliance of an elastomeric medical product for leak testing |
| WO2023049799A1 (en)* | 2021-09-24 | 2023-03-30 | Gyrus Acmi, Inc. D/B/A Olympus Surgical Technologies America | Occlusion device, systems, and method of use |
| US11662325B2 (en) | 2018-12-18 | 2023-05-30 | Regents Of The University Of Minnesota | Systems and methods for measuring kinetic response of chemical sensor elements |
| CN116211281A (en)* | 2023-04-17 | 2023-06-06 | 安徽肺畅医疗科技有限公司 | A device for detecting pulmonary bypass ventilation |
| CN116919687A (en)* | 2022-04-02 | 2023-10-24 | 苏州市美新迪斯医疗科技有限公司 | A stent conveyor |
| US11835435B2 (en) | 2018-11-27 | 2023-12-05 | Regents Of The University Of Minnesota | Systems and methods for detecting a health condition |
| US11875459B2 (en) | 2020-04-07 | 2024-01-16 | Vida Diagnostics, Inc. | Subject specific coordinatization and virtual navigation systems and methods |
| US11911145B2 (en) | 2020-07-10 | 2024-02-27 | Pulmonx Corporation | Methods and systems for determining collateral ventilation |
| US11921096B2 (en) | 2019-09-10 | 2024-03-05 | Regents Of The University Of Minnesota | Fluid analysis system |
| US11937913B2 (en) | 2010-01-08 | 2024-03-26 | Pulmonx Corporation | Measuring lung function and lung disease progression at a lobar/segmental level |
| US20240299686A1 (en)* | 2012-05-18 | 2024-09-12 | Fisher & Paykel Healthcare Limited | Control of flow and/or pressure provided by breathing apparatus |
| US12266111B2 (en) | 2020-11-27 | 2025-04-01 | Vida Diagnostics, Inc. | Visualization of sub-pleural regions |
Families Citing this family (15)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CA2458595C (en)* | 2001-10-11 | 2007-12-04 | Peter M. Wilson | Bronchial flow control devices and methods of use |
| JP4276948B2 (en)* | 2001-10-30 | 2009-06-10 | ハミルトン メディカル アーゲー | Pressure-volume curve monitoring device |
| EP1901653A4 (en)* | 2005-07-13 | 2009-12-30 | Pulmonx | Methods and systems for segmental lung diagnostics |
| JP5149018B2 (en)* | 2008-01-11 | 2013-02-20 | 国立大学法人名古屋大学 | Flow sensor |
| JP5132427B2 (en)* | 2008-05-27 | 2013-01-30 | オリンパスメディカルシステムズ株式会社 | Endoscope |
| CN101422363B (en)* | 2008-12-04 | 2011-10-26 | 中国科学院电工研究所 | Micro flux-gate lung magnetic signal detection device |
| US8400149B2 (en)* | 2009-09-25 | 2013-03-19 | Nellcor Puritan Bennett Ireland | Systems and methods for gating an imaging device |
| US20110295141A1 (en)* | 2009-12-23 | 2011-12-01 | Pulmonx Corporation | Methods and systems for endobronchial diagnostics |
| WO2012082715A2 (en)* | 2010-12-13 | 2012-06-21 | Case Western Reserve University | Device with external pressure sensors for enhancing patient care and methods of using same |
| US20140081110A1 (en)* | 2011-06-03 | 2014-03-20 | University Of Liverpool | Electrophysiological diagnosis and treatment for asthma |
| JP6604856B2 (en)* | 2016-01-12 | 2019-11-13 | 株式会社コスモスウェブ | Airway information display device |
| US20180049808A1 (en)* | 2016-08-17 | 2018-02-22 | Covidien Lp | Method of using soft point features to predict breathing cycles and improve end registration |
| CA3100063A1 (en) | 2018-04-17 | 2019-10-24 | The Board Of Trustees Of The Leland Stanford Junior University | Airway visualization system |
| JP7445569B2 (en)* | 2020-09-15 | 2024-03-07 | キヤノンメディカルシステムズ株式会社 | Image processing device |
| US20230263980A1 (en)* | 2022-02-23 | 2023-08-24 | Stargard Technologies, Inc. | Pulmonary isolation, ventilation and treatment apparatus and methods for use |
Citations (72)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US3322126A (en)* | 1963-04-19 | 1967-05-30 | Willy Rusch Fa | Endotracheal catheter |
| US3498286A (en)* | 1966-09-21 | 1970-03-03 | American Optical Corp | Catheters |
| US3669098A (en)* | 1968-10-05 | 1972-06-13 | Olympus Optical Co | Endotracheal tube |
| US3677262A (en)* | 1970-07-23 | 1972-07-18 | Henry J Zukowski | Surgical instrument illuminating endotracheal tube inserter |
| US3776222A (en)* | 1971-12-23 | 1973-12-04 | Lurosso A | Fiber optic entubator and method of entubation of the trachea through the nasopharynx |
| US3866599A (en)* | 1972-01-21 | 1975-02-18 | Univ Washington | Fiberoptic catheter |
| US3913568A (en)* | 1973-01-22 | 1975-10-21 | American Optical Corp | Nasopharyngoscope |
| US4031885A (en)* | 1975-10-15 | 1977-06-28 | Puritan-Bennett Corporation | Method and apparatus for determining patient lung pressure, compliance and resistance |
| US4036222A (en)* | 1974-05-08 | 1977-07-19 | Soram S.A. | Automatic electronic apparatus for measuring the bronchial resistance and the elastance of the pulmonary tissue |
| US4041936A (en)* | 1975-04-23 | 1977-08-16 | Medical Engineering Corporation | Bronchoscopy tube |
| US4121583A (en)* | 1976-07-13 | 1978-10-24 | Wen Yuan Chen | Method and apparatus for alleviating asthma attacks |
| US4327721A (en)* | 1978-07-07 | 1982-05-04 | George Hanover | Endotracheal tube with topical agent delivery system and method of using the same |
| US4327720A (en)* | 1979-01-22 | 1982-05-04 | Bronson Paul A | Esophageal-endotracheal airway |
| US4413632A (en)* | 1979-10-09 | 1983-11-08 | Critikon, Inc. | Pulmonary monitor |
| US4453545A (en)* | 1981-05-07 | 1984-06-12 | Hiroshi Inoue | Endotracheal tube with movable endobronchial blocker for one-lung anesthesia |
| US4468216A (en)* | 1982-05-20 | 1984-08-28 | Rudolph Muto | Irrigation suction catheter |
| US4567882A (en)* | 1982-12-06 | 1986-02-04 | Vanderbilt University | Method for locating the illuminated tip of an endotracheal tube |
| US4716896A (en)* | 1986-08-01 | 1988-01-05 | Ackrad Laboratories | Bronchial catheter |
| US4742819A (en)* | 1987-03-23 | 1988-05-10 | George Gordon P | Intubating scope with camera and screen |
| US4784133A (en)* | 1987-01-28 | 1988-11-15 | Mackin Robert A | Working well balloon angioscope and method |
| US4796639A (en)* | 1987-11-05 | 1989-01-10 | Medical Graphics Corporation | Pulmonary diagnostic system |
| US4819664A (en)* | 1984-11-15 | 1989-04-11 | Stefano Nazari | Device for selective bronchial intubation and separate lung ventilation, particularly during anesthesia, intensive therapy and reanimation |
| US4846153A (en)* | 1988-06-10 | 1989-07-11 | George Berci | Intubating video endoscope |
| US4850371A (en)* | 1988-06-13 | 1989-07-25 | Broadhurst John H | Novel endotracheal tube and mass spectrometer |
| US4862874A (en)* | 1987-06-10 | 1989-09-05 | Kellner Hans Joerg | Endoscope for removal of thrombi from pulmonary arterial vessels |
| US4896941A (en)* | 1985-04-27 | 1990-01-30 | Doryokuro Kakunenryo Kaihatsu Jigyodan | Image-transmitting fiber |
| US4949716A (en)* | 1988-10-31 | 1990-08-21 | Medical Devices, Inc. | Nasal intubation adjunct |
| US4955375A (en)* | 1989-01-23 | 1990-09-11 | Ricardo Martinez | Endotracheal tube with channel for delivering drugs |
| US4958932A (en)* | 1988-08-18 | 1990-09-25 | Mcdonnell Douglas Corporation | Optical measuring apparatus |
| US4961738A (en)* | 1987-01-28 | 1990-10-09 | Mackin Robert A | Angioplasty catheter with illumination and visualization within angioplasty balloon |
| US4972842A (en)* | 1988-06-09 | 1990-11-27 | Vital Signals, Inc. | Method and apparatus for precision monitoring of infants on assisted ventilation |
| US4976710A (en)* | 1987-01-28 | 1990-12-11 | Mackin Robert A | Working well balloon method |
| US5056529A (en)* | 1990-04-03 | 1991-10-15 | Groot William J De | Apparatus and method for performing a transbroncheal biopsy |
| US5143062A (en)* | 1990-10-26 | 1992-09-01 | Mallinckrodt Medical, Inc. | Endotracheal tube having irrigation means |
| US5146916A (en)* | 1990-01-05 | 1992-09-15 | Catalani Angelo S | Endotracheal tube incorporating a drug-irrigation device |
| US5187579A (en)* | 1990-01-19 | 1993-02-16 | Olympus Optical Co., Ltd. | Medical image displaying method and apparatus |
| US5285778A (en)* | 1991-04-19 | 1994-02-15 | Mackin Robert A | Endotracheal tube wih fibers optic illumination and viewing and auxiliary tube |
| US5309903A (en)* | 1989-12-12 | 1994-05-10 | Burroughs Wellcome Co. | Method for administering surfactant to the lungs while concurrently providing one-lung ventilation |
| US5331947A (en)* | 1992-05-01 | 1994-07-26 | Shturman Cardiology Systems, Inc. | Inflatable sheath for introduction of ultrasonic catheter through the lumen of a fiber optic endoscope |
| US5361753A (en)* | 1992-07-07 | 1994-11-08 | Deutsche Aerospace Ag | Method of measuring and regulating the pressure in the sealing cuff of a tracheal tube and apparatus for implementing the method |
| US5447165A (en)* | 1991-09-27 | 1995-09-05 | Gustafsson; Lars E. | Method for ascertaining prevailing lung condition and a device |
| US5477851A (en)* | 1995-01-26 | 1995-12-26 | Callaghan; Eric B. | Laryngeal mask assembly and method for removing same |
| US5499625A (en)* | 1994-01-27 | 1996-03-19 | The Kendall Company | Esophageal-tracheal double lumen airway |
| US5598840A (en)* | 1995-03-17 | 1997-02-04 | Sorenson Critical Care, Inc. | Apparatus and method for ventilation and aspiration |
| US5642730A (en)* | 1994-06-17 | 1997-07-01 | Trudell Medical Limited | Catheter system for delivery of aerosolized medicine for use with pressurized propellant canister |
| US5645519A (en)* | 1994-03-18 | 1997-07-08 | Jai S. Lee | Endoscopic instrument for controlled introduction of tubular members in the body and methods therefor |
| US5653231A (en)* | 1995-11-28 | 1997-08-05 | Medcare Medical Group, Inc. | Tracheostomy length single use suction catheter |
| US5660175A (en)* | 1995-08-21 | 1997-08-26 | Dayal; Bimal | Endotracheal device |
| US5682880A (en)* | 1996-07-26 | 1997-11-04 | Brain; Archibald Ian Jeremy | Laryngeal-mask airway with guide element, stiffener, and fiberoptic access |
| US5707352A (en)* | 1989-08-28 | 1998-01-13 | Alliance Pharmaceutical Corp. | Pulmonary delivery of therapeutic agent |
| US5738090A (en)* | 1995-06-02 | 1998-04-14 | Burkhard Lachmann | Respiratory system for determining an opening pressure of a long system and maintaining the lung system open |
| US5752921A (en)* | 1996-01-11 | 1998-05-19 | Korr Medical Technologies, Inc. | Method and apparatus for determining tracheal pressure |
| US5810741A (en)* | 1992-11-05 | 1998-09-22 | Synectics Medical Ab | Method of measuring respiration and respiratory effort using plural catheters |
| US5972026A (en)* | 1997-04-07 | 1999-10-26 | Broncus Technologies, Inc. | Bronchial stenter having diametrically adjustable electrodes |
| US6068602A (en)* | 1997-09-26 | 2000-05-30 | Ohmeda Inc. | Method and apparatus for determining airway resistance and lung compliance |
| US6083255A (en)* | 1997-04-07 | 2000-07-04 | Broncus Technologies, Inc. | Bronchial stenter |
| US6083162A (en)* | 1994-10-27 | 2000-07-04 | Wake Forest University | Method and system for producing interactive, three-dimensional renderings of selected body organs having hollow lumens to enable simulated movement through the lumen |
| US6155252A (en)* | 1995-03-17 | 2000-12-05 | Board Of Regents, The University Of Texas System | Method and apparatus for directing air flow within an intubated patient |
| US6174323B1 (en)* | 1998-06-05 | 2001-01-16 | Broncus Technologies, Inc. | Method and assembly for lung volume reduction |
| US6287290B1 (en)* | 1999-07-02 | 2001-09-11 | Pulmonx | Methods, systems, and kits for lung volume reduction |
| US6370415B1 (en)* | 1998-04-10 | 2002-04-09 | Medi-Physics Inc. | Magnetic resonance imaging method |
| US20020049370A1 (en)* | 1999-08-05 | 2002-04-25 | Laufer Michael D. | Devices for creating collateral channels in the lungs |
| US20020198449A1 (en)* | 2001-02-08 | 2002-12-26 | The Trustees Of The University Of Pennsylvania | Quantitative pulmonary imaging |
| US6712812B2 (en)* | 1999-08-05 | 2004-03-30 | Broncus Technologies, Inc. | Devices for creating collateral channels |
| US20040073130A1 (en)* | 1998-12-10 | 2004-04-15 | Stephan Bohm | Method and apparatus for determining alveolar opening and closing |
| US6749606B2 (en)* | 1999-08-05 | 2004-06-15 | Thomas Keast | Devices for creating collateral channels |
| US6886558B2 (en)* | 2002-08-28 | 2005-05-03 | Cordis Corporation | Collateral ventilation bypass trap system |
| US6904909B2 (en)* | 2000-03-04 | 2005-06-14 | Emphasys Medical, Inc. | Methods and devices for use in performing pulmonary procedures |
| US6997189B2 (en)* | 1998-06-05 | 2006-02-14 | Broncus Technologies, Inc. | Method for lung volume reduction |
| US7022088B2 (en)* | 1999-08-05 | 2006-04-04 | Broncus Technologies, Inc. | Devices for applying energy to tissue |
| US7086398B2 (en)* | 2002-07-31 | 2006-08-08 | Cordis Corporation | Long term oxygen therapy system |
| US20080200797A1 (en)* | 2001-09-10 | 2008-08-21 | Pulmonx | Method and apparatus for endobronchial diagnosis |
Family Cites Families (22)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US4448192A (en)* | 1982-03-05 | 1984-05-15 | Hewlett Packard Company | Medical ventilator device parametrically controlled for patient ventilation |
| US6013619A (en) | 1988-01-06 | 2000-01-11 | The Scripps Research Institute | Pulmonary surfactants and therapeutic uses, including pulmonary lavage |
| US5246012A (en) | 1990-12-21 | 1993-09-21 | Ballard Medical Products | Bronchoalveolar lavage catheter |
| US5540229A (en)* | 1993-09-29 | 1996-07-30 | U.S. Philips Cororation | System and method for viewing three-dimensional echographic data |
| GB9411215D0 (en) | 1994-06-04 | 1994-07-27 | Brain Archibald Ian Jeremy | A fibreoptic intubating laryngeal mask airway |
| NZ272354A (en)* | 1994-06-17 | 1997-10-24 | Trudell Medical Ltd | Catheter system; method and apparatus for delivering an aerosol form of medication to the lungs, details of method and of catheter apparatus |
| US5692497A (en)* | 1996-05-16 | 1997-12-02 | Children's Medical Center Corporation | Microprocessor-controlled ventilator system and methods |
| US6283988B1 (en) | 1997-04-07 | 2001-09-04 | Broncus Technologies, Inc. | Bronchial stenter having expandable electrodes |
| US6273907B1 (en) | 1997-04-07 | 2001-08-14 | Broncus Technologies, Inc. | Bronchial stenter |
| US6634363B1 (en) | 1997-04-07 | 2003-10-21 | Broncus Technologies, Inc. | Methods of treating lungs having reversible obstructive pulmonary disease |
| US6411852B1 (en) | 1997-04-07 | 2002-06-25 | Broncus Technologies, Inc. | Modification of airways by application of energy |
| US6488673B1 (en) | 1997-04-07 | 2002-12-03 | Broncus Technologies, Inc. | Method of increasing gas exchange of a lung |
| GB2324729B (en) | 1997-04-30 | 2002-01-02 | Bradford Hospitals Nhs Trust | Lung treatment device |
| US5957919A (en) | 1997-07-02 | 1999-09-28 | Laufer; Michael D. | Bleb reducer |
| SE513980C2 (en)* | 1997-11-13 | 2000-12-04 | Mincor Ab | Method and apparatus for determining effective lung volume |
| US6117073A (en)* | 1998-03-02 | 2000-09-12 | Jones; Scott J. | Integrated emergency medical transportation database system |
| US6071237A (en) | 1999-02-19 | 2000-06-06 | Institute Of Critical Care Medicine | Device and method for assessing perfusion failure in a patient during endotracheal intubation |
| US6610043B1 (en) | 1999-08-23 | 2003-08-26 | Bistech, Inc. | Tissue volume reduction |
| US6293951B1 (en) | 1999-08-24 | 2001-09-25 | Spiration, Inc. | Lung reduction device, system, and method |
| DE60031052T2 (en) | 1999-08-24 | 2007-04-05 | Spiration, Inc., Redmond | Set for reducing the lung volume |
| US7130457B2 (en)* | 2001-07-17 | 2006-10-31 | Accuimage Diagnostics Corp. | Systems and graphical user interface for analyzing body images |
| US7068398B2 (en)* | 2001-11-07 | 2006-06-27 | International Business Machines Corporation | System and method for efficient tile generation from complex raster data |
- 2002
- 2002-09-10USUS10/241,733patent/US20030051733A1/ennot_activeAbandoned
- 2002-09-10EPEP02768834.0Apatent/EP1435833B1/ennot_activeExpired - Lifetime
- 2002-09-10AUAU2002331842Apatent/AU2002331842A1/ennot_activeAbandoned
- 2002-09-10WOPCT/US2002/028863patent/WO2003022221A2/enactiveApplication Filing
- 2002-09-10JPJP2003526351Apatent/JP4301945B2/ennot_activeExpired - Lifetime
- 2008
- 2008-04-23USUS12/108,035patent/US20080200797A1/ennot_activeAbandoned
- 2015
- 2015-10-21USUS14/919,480patent/US10413244B2/ennot_activeExpired - Fee Related
- 2016
- 2016-05-09USUS15/150,324patent/US20160249860A1/ennot_activeAbandoned
- 2019
- 2019-09-05USUS16/562,158patent/US20190388034A1/ennot_activeAbandoned
Patent Citations (75)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US3322126A (en)* | 1963-04-19 | 1967-05-30 | Willy Rusch Fa | Endotracheal catheter |
| US3498286A (en)* | 1966-09-21 | 1970-03-03 | American Optical Corp | Catheters |
| US3669098A (en)* | 1968-10-05 | 1972-06-13 | Olympus Optical Co | Endotracheal tube |
| US3677262A (en)* | 1970-07-23 | 1972-07-18 | Henry J Zukowski | Surgical instrument illuminating endotracheal tube inserter |
| US3776222A (en)* | 1971-12-23 | 1973-12-04 | Lurosso A | Fiber optic entubator and method of entubation of the trachea through the nasopharynx |
| US3866599A (en)* | 1972-01-21 | 1975-02-18 | Univ Washington | Fiberoptic catheter |
| US3913568A (en)* | 1973-01-22 | 1975-10-21 | American Optical Corp | Nasopharyngoscope |
| US4036222A (en)* | 1974-05-08 | 1977-07-19 | Soram S.A. | Automatic electronic apparatus for measuring the bronchial resistance and the elastance of the pulmonary tissue |
| US4041936A (en)* | 1975-04-23 | 1977-08-16 | Medical Engineering Corporation | Bronchoscopy tube |
| US4031885A (en)* | 1975-10-15 | 1977-06-28 | Puritan-Bennett Corporation | Method and apparatus for determining patient lung pressure, compliance and resistance |
| US4121583A (en)* | 1976-07-13 | 1978-10-24 | Wen Yuan Chen | Method and apparatus for alleviating asthma attacks |
| US4327721A (en)* | 1978-07-07 | 1982-05-04 | George Hanover | Endotracheal tube with topical agent delivery system and method of using the same |
| US4327720A (en)* | 1979-01-22 | 1982-05-04 | Bronson Paul A | Esophageal-endotracheal airway |
| US4413632A (en)* | 1979-10-09 | 1983-11-08 | Critikon, Inc. | Pulmonary monitor |
| US4453545A (en)* | 1981-05-07 | 1984-06-12 | Hiroshi Inoue | Endotracheal tube with movable endobronchial blocker for one-lung anesthesia |
| US4468216A (en)* | 1982-05-20 | 1984-08-28 | Rudolph Muto | Irrigation suction catheter |
| US4567882A (en)* | 1982-12-06 | 1986-02-04 | Vanderbilt University | Method for locating the illuminated tip of an endotracheal tube |
| US4819664A (en)* | 1984-11-15 | 1989-04-11 | Stefano Nazari | Device for selective bronchial intubation and separate lung ventilation, particularly during anesthesia, intensive therapy and reanimation |
| US4896941A (en)* | 1985-04-27 | 1990-01-30 | Doryokuro Kakunenryo Kaihatsu Jigyodan | Image-transmitting fiber |
| US4716896A (en)* | 1986-08-01 | 1988-01-05 | Ackrad Laboratories | Bronchial catheter |
| US4961738A (en)* | 1987-01-28 | 1990-10-09 | Mackin Robert A | Angioplasty catheter with illumination and visualization within angioplasty balloon |
| US4976710A (en)* | 1987-01-28 | 1990-12-11 | Mackin Robert A | Working well balloon method |
| US4784133A (en)* | 1987-01-28 | 1988-11-15 | Mackin Robert A | Working well balloon angioscope and method |
| US4742819A (en)* | 1987-03-23 | 1988-05-10 | George Gordon P | Intubating scope with camera and screen |
| US4862874A (en)* | 1987-06-10 | 1989-09-05 | Kellner Hans Joerg | Endoscope for removal of thrombi from pulmonary arterial vessels |
| US4796639A (en)* | 1987-11-05 | 1989-01-10 | Medical Graphics Corporation | Pulmonary diagnostic system |
| US4972842A (en)* | 1988-06-09 | 1990-11-27 | Vital Signals, Inc. | Method and apparatus for precision monitoring of infants on assisted ventilation |
| US4846153A (en)* | 1988-06-10 | 1989-07-11 | George Berci | Intubating video endoscope |
| US4850371A (en)* | 1988-06-13 | 1989-07-25 | Broadhurst John H | Novel endotracheal tube and mass spectrometer |
| US4958932A (en)* | 1988-08-18 | 1990-09-25 | Mcdonnell Douglas Corporation | Optical measuring apparatus |
| US4949716A (en)* | 1988-10-31 | 1990-08-21 | Medical Devices, Inc. | Nasal intubation adjunct |
| US4955375A (en)* | 1989-01-23 | 1990-09-11 | Ricardo Martinez | Endotracheal tube with channel for delivering drugs |
| US5707352A (en)* | 1989-08-28 | 1998-01-13 | Alliance Pharmaceutical Corp. | Pulmonary delivery of therapeutic agent |
| US5309903A (en)* | 1989-12-12 | 1994-05-10 | Burroughs Wellcome Co. | Method for administering surfactant to the lungs while concurrently providing one-lung ventilation |
| US5146916A (en)* | 1990-01-05 | 1992-09-15 | Catalani Angelo S | Endotracheal tube incorporating a drug-irrigation device |
| US5187579A (en)* | 1990-01-19 | 1993-02-16 | Olympus Optical Co., Ltd. | Medical image displaying method and apparatus |
| US5056529A (en)* | 1990-04-03 | 1991-10-15 | Groot William J De | Apparatus and method for performing a transbroncheal biopsy |
| US5143062A (en)* | 1990-10-26 | 1992-09-01 | Mallinckrodt Medical, Inc. | Endotracheal tube having irrigation means |
| US5285778A (en)* | 1991-04-19 | 1994-02-15 | Mackin Robert A | Endotracheal tube wih fibers optic illumination and viewing and auxiliary tube |
| US5447165A (en)* | 1991-09-27 | 1995-09-05 | Gustafsson; Lars E. | Method for ascertaining prevailing lung condition and a device |
| US5331947A (en)* | 1992-05-01 | 1994-07-26 | Shturman Cardiology Systems, Inc. | Inflatable sheath for introduction of ultrasonic catheter through the lumen of a fiber optic endoscope |
| US5361753A (en)* | 1992-07-07 | 1994-11-08 | Deutsche Aerospace Ag | Method of measuring and regulating the pressure in the sealing cuff of a tracheal tube and apparatus for implementing the method |
| US5810741A (en)* | 1992-11-05 | 1998-09-22 | Synectics Medical Ab | Method of measuring respiration and respiratory effort using plural catheters |
| US5499625A (en)* | 1994-01-27 | 1996-03-19 | The Kendall Company | Esophageal-tracheal double lumen airway |
| US5645519A (en)* | 1994-03-18 | 1997-07-08 | Jai S. Lee | Endoscopic instrument for controlled introduction of tubular members in the body and methods therefor |
| US5642730A (en)* | 1994-06-17 | 1997-07-01 | Trudell Medical Limited | Catheter system for delivery of aerosolized medicine for use with pressurized propellant canister |
| US6083162A (en)* | 1994-10-27 | 2000-07-04 | Wake Forest University | Method and system for producing interactive, three-dimensional renderings of selected body organs having hollow lumens to enable simulated movement through the lumen |
| US5477851A (en)* | 1995-01-26 | 1995-12-26 | Callaghan; Eric B. | Laryngeal mask assembly and method for removing same |
| US6155252A (en)* | 1995-03-17 | 2000-12-05 | Board Of Regents, The University Of Texas System | Method and apparatus for directing air flow within an intubated patient |
| US5598840A (en)* | 1995-03-17 | 1997-02-04 | Sorenson Critical Care, Inc. | Apparatus and method for ventilation and aspiration |
| US5738090A (en)* | 1995-06-02 | 1998-04-14 | Burkhard Lachmann | Respiratory system for determining an opening pressure of a long system and maintaining the lung system open |
| US5660175A (en)* | 1995-08-21 | 1997-08-26 | Dayal; Bimal | Endotracheal device |
| US5653231A (en)* | 1995-11-28 | 1997-08-05 | Medcare Medical Group, Inc. | Tracheostomy length single use suction catheter |
| US5752921A (en)* | 1996-01-11 | 1998-05-19 | Korr Medical Technologies, Inc. | Method and apparatus for determining tracheal pressure |
| US5682880A (en)* | 1996-07-26 | 1997-11-04 | Brain; Archibald Ian Jeremy | Laryngeal-mask airway with guide element, stiffener, and fiberoptic access |
| US5972026A (en)* | 1997-04-07 | 1999-10-26 | Broncus Technologies, Inc. | Bronchial stenter having diametrically adjustable electrodes |
| US6083255A (en)* | 1997-04-07 | 2000-07-04 | Broncus Technologies, Inc. | Bronchial stenter |
| US6068602A (en)* | 1997-09-26 | 2000-05-30 | Ohmeda Inc. | Method and apparatus for determining airway resistance and lung compliance |
| US6370415B1 (en)* | 1998-04-10 | 2002-04-09 | Medi-Physics Inc. | Magnetic resonance imaging method |
| US6174323B1 (en)* | 1998-06-05 | 2001-01-16 | Broncus Technologies, Inc. | Method and assembly for lung volume reduction |
| US6997189B2 (en)* | 1998-06-05 | 2006-02-14 | Broncus Technologies, Inc. | Method for lung volume reduction |
| US20040073130A1 (en)* | 1998-12-10 | 2004-04-15 | Stephan Bohm | Method and apparatus for determining alveolar opening and closing |
| US6287290B1 (en)* | 1999-07-02 | 2001-09-11 | Pulmonx | Methods, systems, and kits for lung volume reduction |
| US6709401B2 (en)* | 1999-07-02 | 2004-03-23 | Pulmonx | Methods, systems, and kits for lung volume reduction |
| US20020049370A1 (en)* | 1999-08-05 | 2002-04-25 | Laufer Michael D. | Devices for creating collateral channels in the lungs |
| US7022088B2 (en)* | 1999-08-05 | 2006-04-04 | Broncus Technologies, Inc. | Devices for applying energy to tissue |
| US6629951B2 (en)* | 1999-08-05 | 2003-10-07 | Broncus Technologies, Inc. | Devices for creating collateral in the lungs |
| US6692494B1 (en)* | 1999-08-05 | 2004-02-17 | Broncus Technologies, Inc. | Methods and devices for creating collateral channels in the lungs |
| US6712812B2 (en)* | 1999-08-05 | 2004-03-30 | Broncus Technologies, Inc. | Devices for creating collateral channels |
| US6749606B2 (en)* | 1999-08-05 | 2004-06-15 | Thomas Keast | Devices for creating collateral channels |
| US6904909B2 (en)* | 2000-03-04 | 2005-06-14 | Emphasys Medical, Inc. | Methods and devices for use in performing pulmonary procedures |
| US20020198449A1 (en)* | 2001-02-08 | 2002-12-26 | The Trustees Of The University Of Pennsylvania | Quantitative pulmonary imaging |
| US20080200797A1 (en)* | 2001-09-10 | 2008-08-21 | Pulmonx | Method and apparatus for endobronchial diagnosis |
| US7086398B2 (en)* | 2002-07-31 | 2006-08-08 | Cordis Corporation | Long term oxygen therapy system |
| US6886558B2 (en)* | 2002-08-28 | 2005-05-03 | Cordis Corporation | Collateral ventilation bypass trap system |
Non-Patent Citations (1)
| Title |
|---|
| Airflow resistance in lower airway of the dog, Robert G. Rossing, Journal of Applied PhysiologyNov 1962,17(6)877-884* |
Cited By (381)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US20060254600A1 (en)* | 2000-03-27 | 2006-11-16 | Asthmatx, Inc. | Methods for treating airways |
| US10278766B2 (en) | 2000-03-27 | 2019-05-07 | Boston Scientific Scimed, Inc. | Methods for treating airways |
| US10561458B2 (en) | 2000-03-27 | 2020-02-18 | Boston Scientific Scimed, Inc. | Methods for treating airways |
| US8251070B2 (en)* | 2000-03-27 | 2012-08-28 | Asthmatx, Inc. | Methods for treating airways |
| US20020112729A1 (en)* | 2001-02-21 | 2002-08-22 | Spiration, Inc. | Intra-bronchial obstructing device that controls biological interaction with the patient |
| US20080200797A1 (en)* | 2001-09-10 | 2008-08-21 | Pulmonx | Method and apparatus for endobronchial diagnosis |
| US8454527B2 (en) | 2001-09-10 | 2013-06-04 | Pulmonx Corporation | Minimally invasive determination of collateral ventilation in lungs |
| US7883471B2 (en) | 2001-09-10 | 2011-02-08 | Pulmonx Corporation | Minimally invasive determination of collateral ventilation in lungs |
| US20060264772A1 (en)* | 2001-09-10 | 2006-11-23 | Pulmonx | Minimally invasive determination of collateral ventilation in lungs |
| US10413244B2 (en) | 2001-09-10 | 2019-09-17 | Pulmonx Corporation | Method and apparatus for endobronchial diagnosis |
| US20110087122A1 (en)* | 2001-09-10 | 2011-04-14 | Pulmonx Corporation | Minimally invasive determination of collateral ventilation in lungs |
| US20110054632A1 (en)* | 2001-09-11 | 2011-03-03 | Spiration, Inc. | Removable lung reduction devices, systems, and methods |
| US20040206349A1 (en)* | 2001-09-11 | 2004-10-21 | Alferness Clifton A. | Removable lung reduction devices, systems, and methods |
| US8414655B2 (en) | 2001-09-11 | 2013-04-09 | Spiration, Inc. | Removable lung reduction devices, systems, and methods |
| US20050033310A1 (en)* | 2001-09-11 | 2005-02-10 | Alferness Clifton A. | Intra-bronchial valve devices |
| US20040211412A1 (en)* | 2001-09-11 | 2004-10-28 | Alferness Clifton A. | Removable lung reduction devices, systems, and method |
| US8974484B2 (en) | 2001-09-11 | 2015-03-10 | Spiration, Inc. | Removable lung reduction devices, systems, and methods |
| US7757692B2 (en) | 2001-09-11 | 2010-07-20 | Spiration, Inc. | Removable lung reduction devices, systems, and methods |
| US20090205667A1 (en)* | 2001-09-11 | 2009-08-20 | Spiration, Inc. | Removable lung reduction devices, systems, and methods |
| US8986336B2 (en) | 2001-10-25 | 2015-03-24 | Spiration, Inc. | Apparatus and method for deployment of a bronchial obstruction device |
| US7896887B2 (en) | 2001-10-25 | 2011-03-01 | Spiration, Inc. | Apparatus and method for deployment of a bronchial obstruction device |
| US20070185531A1 (en)* | 2001-10-25 | 2007-08-09 | Spiration, Inc. | Apparatus and method for deployment of a bronchial obstruction device |
| US20060074382A1 (en)* | 2002-02-21 | 2006-04-06 | Gonzalez Hugo X | Device and method for intra-bronchial provision of a therapeutic agent |
| US20060200076A1 (en)* | 2002-02-21 | 2006-09-07 | Gonzalez Hugo X | Device and method for intra-bronchial provision of a therapeutic agent |
| US6929637B2 (en) | 2002-02-21 | 2005-08-16 | Spiration, Inc. | Device and method for intra-bronchial provision of a therapeutic agent |
| US20060235432A1 (en)* | 2002-02-21 | 2006-10-19 | Devore Lauri J | Intra-bronchial obstructing device that controls biological interaction with the patient |
| US7942931B2 (en) | 2002-02-21 | 2011-05-17 | Spiration, Inc. | Device and method for intra-bronchial provision of a therapeutic agent |
| US20060206147A1 (en)* | 2002-02-21 | 2006-09-14 | Devore Lauri J | Intra-bronchial obstruction device that provides a medicant intra-bronchially to the patient |
| US8177805B2 (en) | 2002-03-20 | 2012-05-15 | Spiration, Inc. | Removable anchored lung volume reduction devices and methods |
| US8926647B2 (en) | 2002-03-20 | 2015-01-06 | Spiration, Inc. | Removable anchored lung volume reduction devices and methods |
| US8021385B2 (en) | 2002-03-20 | 2011-09-20 | Spiration, Inc. | Removable anchored lung volume reduction devices and methods |
| US20080119866A1 (en)* | 2002-03-20 | 2008-05-22 | Alferness Clifton A | Removable anchored lung volume reduction devices and methods |
| US20060235467A1 (en)* | 2002-04-16 | 2006-10-19 | Devore Lauri J | Removable anchored lung volume reduction device and methods |
| US20040143282A1 (en)* | 2002-05-17 | 2004-07-22 | Dillard David H. | Methods of achieving lung volume reduction with removable anchored devices |
| US8257381B2 (en) | 2002-05-17 | 2012-09-04 | Spiration, Inc. | One-way valve devices for anchored implantation in a lung |
| US7434578B2 (en) | 2002-05-17 | 2008-10-14 | Spiration, Inc. | Methods of achieving lung volume reduction with removable anchored devices |
| US8956319B2 (en) | 2002-05-17 | 2015-02-17 | Spiration, Inc. | One-way valve devices for anchored implantation in a lung |
| US20050033344A1 (en)* | 2002-05-17 | 2005-02-10 | Dillard David H. | One-way valve devices for anchored implantation in a lung |
| US7842061B2 (en) | 2002-05-17 | 2010-11-30 | Spiration, Inc. | Methods of achieving lung volume reduction with removable anchored devices |
| US7875048B2 (en) | 2002-05-17 | 2011-01-25 | Spiration, Inc. | One-way valve devices for anchored implantation in a lung |
| US20110079221A1 (en)* | 2002-05-17 | 2011-04-07 | Spiration, Inc. | One-way valve devices for anchored implantation in a lung |
| US7476203B2 (en) | 2002-09-24 | 2009-01-13 | Spiration, Inc. | Device and method for measuring the diameter of an air passageway |
| US20060155217A1 (en)* | 2002-09-24 | 2006-07-13 | Devore Lauri J | Device and method for measuring the diameter of an air passageway |
| US20060025650A1 (en)* | 2002-10-03 | 2006-02-02 | Oren Gavriely | Tube for inspecting internal organs of a body |
| US7447536B2 (en)* | 2002-11-12 | 2008-11-04 | G.E. Medical Systems Global Technology Company, Llc | System and method for measurement of local lung function using electron beam CT |
| US20040092811A1 (en)* | 2002-11-12 | 2004-05-13 | Hill David Guy | System and method for measurement of local lung function using electron beam CT |
| US20100256714A1 (en)* | 2003-04-08 | 2010-10-07 | Springmeyer Steven C | Bronchoscopic lung volume reduction method |
| US7100616B2 (en) | 2003-04-08 | 2006-09-05 | Spiration, Inc. | Bronchoscopic lung volume reduction method |
| US8079368B2 (en) | 2003-04-08 | 2011-12-20 | Spiration, Inc. | Bronchoscopic lung volume reduction method |
| US8667973B2 (en) | 2003-04-08 | 2014-03-11 | Spiration, Inc. | Bronchoscopic lung volume reduction method |
| US20060249164A1 (en)* | 2003-04-08 | 2006-11-09 | Springmeyer Steven C | Bronchoscopic lung volume reduction method |
| JP2006525080A (en)* | 2003-04-22 | 2006-11-09 | メディ−フィジックス・インコーポレイテッド | One-period volume control and measurement system, method, and device for respiratory hyperpolarized gas transmission for MRI / NMR |
| US7828789B2 (en) | 2003-05-07 | 2010-11-09 | Portaero, Inc. | Device and method for creating a localized pleurodesis and treating a lung through the localized pleurodesis |
| US20040225254A1 (en)* | 2003-05-07 | 2004-11-11 | Don Tanaka | Localized pleurodesis chemical delivery |
| US8029492B2 (en) | 2003-05-07 | 2011-10-04 | Portaero, Inc. | Method for treating chronic obstructive pulmonary disease |
| US7811274B2 (en) | 2003-05-07 | 2010-10-12 | Portaero, Inc. | Method for treating chronic obstructive pulmonary disease |
| US20080188809A1 (en)* | 2003-05-07 | 2008-08-07 | Portaero, Inc. | Device and method for creating a localized pleurodesis and treating a lung through the localized pleurodesis |
| US7426929B2 (en) | 2003-05-20 | 2008-09-23 | Portaero, Inc. | Intra/extra-thoracic collateral ventilation bypass system and method |
| US7789083B2 (en) | 2003-05-20 | 2010-09-07 | Portaero, Inc. | Intra/extra thoracic system for ameliorating a symptom of chronic obstructive pulmonary disease |
| US7533667B2 (en) | 2003-05-29 | 2009-05-19 | Portaero, Inc. | Methods and devices to assist pulmonary decompression |
| US20040237966A1 (en)* | 2003-05-29 | 2004-12-02 | Don Tanaka | Methods and devices to assist pulmonary decompression |
| US7896008B2 (en) | 2003-06-03 | 2011-03-01 | Portaero, Inc. | Lung reduction system |
| US20070270776A1 (en)* | 2003-06-03 | 2007-11-22 | Respira, Inc. | Lung reduction system |
| US7377278B2 (en) | 2003-06-05 | 2008-05-27 | Portaero, Inc. | Intra-thoracic collateral ventilation bypass system and method |
| US7753052B2 (en) | 2003-06-05 | 2010-07-13 | Portaero, Inc. | Intra-thoracic collateral ventilation bypass system |
| US20040244803A1 (en)* | 2003-06-05 | 2004-12-09 | Don Tanaka | Intra-thoracic collateral ventilation bypass system |
| US20050016530A1 (en)* | 2003-07-09 | 2005-01-27 | Mccutcheon John | Treatment planning with implantable bronchial isolation devices |
| US20050025816A1 (en)* | 2003-07-15 | 2005-02-03 | Don Tanaka | Methods and devices to accelerate wound healing in thoracic anastomosis applications |
| US8323230B2 (en) | 2003-07-15 | 2012-12-04 | Portaero, Inc. | Methods and devices to accelerate wound healing in thoracic anastomosis applications |
| US20100129420A1 (en)* | 2003-07-15 | 2010-05-27 | Portaero, Inc. | Methods and devices to accelerate wound healing in thoracic anastomosis applications |
| US7682332B2 (en) | 2003-07-15 | 2010-03-23 | Portaero, Inc. | Methods to accelerate wound healing in thoracic anastomosis applications |
| US20090182369A1 (en)* | 2003-08-08 | 2009-07-16 | Spiration, Inc. | Bronchoscopic repair of air leaks in a lung |
| US8444690B2 (en) | 2003-08-08 | 2013-05-21 | Spiration, Inc. | Bronchoscopic repair of air leaks in a lung |
| US20110208228A1 (en)* | 2003-08-08 | 2011-08-25 | Spiration, Inc. | Bronchoscopic repair of air leaks in a lung |
| US9622752B2 (en) | 2003-08-08 | 2017-04-18 | Spiration, Inc. | Bronchoscopic repair of air leaks in a lung |
| US8974527B2 (en) | 2003-08-08 | 2015-03-10 | Spiration, Inc. | Bronchoscopic repair of air leaks in a lung |
| US7887585B2 (en) | 2003-08-08 | 2011-02-15 | Spiration, Inc. | Bronchoscopic repair of air leaks in a lung |
| US7533671B2 (en) | 2003-08-08 | 2009-05-19 | Spiration, Inc. | Bronchoscopic repair of air leaks in a lung |
| US20090255537A1 (en)* | 2004-01-27 | 2009-10-15 | Pulmonx | Disease indications for selective endobronchial lung region isolation |
| US20050178389A1 (en)* | 2004-01-27 | 2005-08-18 | Shaw David P. | Disease indications for selective endobronchial lung region isolation |
| US10702295B2 (en) | 2004-04-21 | 2020-07-07 | Acclarent, Inc. | Methods and apparatus for treating disorders of the ear nose and throat |
| US10874838B2 (en) | 2004-04-21 | 2020-12-29 | Acclarent, Inc. | Systems and methods for transnasal dilation of passageways in the ear, nose or throat |
| US11019989B2 (en) | 2004-04-21 | 2021-06-01 | Acclarent, Inc. | Methods and apparatus for treating disorders of the ear nose and throat |
| US11589742B2 (en) | 2004-04-21 | 2023-02-28 | Acclarent, Inc. | Methods and apparatus for treating disorders of the ear nose and throat |
| US9826999B2 (en)* | 2004-04-21 | 2017-11-28 | Acclarent, Inc. | Methods and apparatus for treating disorders of the ear nose and throat |
| US11864725B2 (en) | 2004-04-21 | 2024-01-09 | Acclarent, Inc. | Devices, systems and methods for diagnosing and treating sinusitis and other disorders of the ears, nose and/or throat |
| US11020136B2 (en) | 2004-04-21 | 2021-06-01 | Acclarent, Inc. | Deflectable guide catheters and related methods |
| US10779752B2 (en) | 2004-04-21 | 2020-09-22 | Acclarent, Inc. | Guidewires for performing image guided procedures |
| US11957318B2 (en) | 2004-04-21 | 2024-04-16 | Acclarent, Inc. | Methods and apparatus for treating disorders of the ear nose and throat |
| US10856727B2 (en) | 2004-04-21 | 2020-12-08 | Acclarent, Inc. | Endoscopic methods and devices for transnasal procedures |
| US10806477B2 (en) | 2004-04-21 | 2020-10-20 | Acclarent, Inc. | Systems and methods for transnasal dilation of passageways in the ear, nose or throat |
| US11529502B2 (en) | 2004-04-21 | 2022-12-20 | Acclarent, Inc. | Apparatus and methods for dilating and modifying ostia of paranasal sinuses and other intranasal or paranasal structures |
| US10500380B2 (en) | 2004-04-21 | 2019-12-10 | Acclarent, Inc. | Devices, systems and methods useable for treating sinusitis |
| US10695080B2 (en) | 2004-04-21 | 2020-06-30 | Acclarent, Inc. | Devices, systems and methods for diagnosing and treating sinusitis and other disorders of the ears, nose and/or throat |
| US11511090B2 (en) | 2004-04-21 | 2022-11-29 | Acclarent, Inc. | Devices, systems and methods useable for treating sinusitis |
| US7670282B2 (en) | 2004-06-14 | 2010-03-02 | Pneumrx, Inc. | Lung access device |
| US20050288549A1 (en)* | 2004-06-14 | 2005-12-29 | Pneumrx, Inc. | Guided access to lung tissues |
| US7775968B2 (en) | 2004-06-14 | 2010-08-17 | Pneumrx, Inc. | Guided access to lung tissues |
| US7549984B2 (en) | 2004-06-16 | 2009-06-23 | Pneumrx, Inc. | Method of compressing a portion of a lung |
| EP3542736A1 (en)* | 2004-06-16 | 2019-09-25 | PneumRx, Inc. | Intra-bronchial lung volume reduction system |
| US20050288702A1 (en)* | 2004-06-16 | 2005-12-29 | Mcgurk Erin | Intra-bronchial lung volume reduction system |
| US20050288684A1 (en)* | 2004-06-16 | 2005-12-29 | Aronson Nathan A | Method of reducing collateral flow in a portion of a lung |
| US7766938B2 (en) | 2004-07-08 | 2010-08-03 | Pneumrx, Inc. | Pleural effusion treatment device, method and material |
| US20060025815A1 (en)* | 2004-07-08 | 2006-02-02 | Mcgurk Erin | Lung device with sealing features |
| US20060009801A1 (en)* | 2004-07-08 | 2006-01-12 | Mcgurk Erin | Pleural effusion treatment device, method and material |
| US7766891B2 (en) | 2004-07-08 | 2010-08-03 | Pneumrx, Inc. | Lung device with sealing features |
| US20080077038A1 (en)* | 2004-11-02 | 2008-03-27 | Children's Hospital Of Philadelphia | Respiratory Volume/Flow Gating, Monitoring, and Spirometry System for Mri |
| US10398502B2 (en) | 2004-11-05 | 2019-09-03 | Boston Scientific Scimed, Inc. | Energy delivery devices and methods |
| US10076380B2 (en) | 2004-11-05 | 2018-09-18 | Boston Scientific Scimed, Inc. | Energy delivery devices and methods |
| US20060118126A1 (en)* | 2004-11-19 | 2006-06-08 | Don Tanaka | Methods and devices for controlling collateral ventilation |
| US7398782B2 (en) | 2004-11-19 | 2008-07-15 | Portaero, Inc. | Method for pulmonary drug delivery |
| US8220460B2 (en) | 2004-11-19 | 2012-07-17 | Portaero, Inc. | Evacuation device and method for creating a localized pleurodesis |
| US20060118125A1 (en)* | 2004-11-19 | 2006-06-08 | Don Tanaka | Pulmonary drug delivery |
| US20060107961A1 (en)* | 2004-11-19 | 2006-05-25 | Don Tanaka | Localized pleurodesis evacuation device |
| US20060167416A1 (en)* | 2004-11-23 | 2006-07-27 | Mark Mathis | Steerable device for accessing a target site and methods |
| US9125639B2 (en) | 2004-11-23 | 2015-09-08 | Pneumrx, Inc. | Steerable device for accessing a target site and methods |
| US7824366B2 (en) | 2004-12-10 | 2010-11-02 | Portaero, Inc. | Collateral ventilation device with chest tube/evacuation features and method |
| US20060124126A1 (en)* | 2004-12-10 | 2006-06-15 | Don Tanaka | Collateral ventilation device with chest tube/evacuation features |
| US8611983B2 (en)* | 2005-01-18 | 2013-12-17 | Philips Electronics Ltd | Method and apparatus for guiding an instrument to a target in the lung |
| US20060184016A1 (en)* | 2005-01-18 | 2006-08-17 | Glossop Neil D | Method and apparatus for guiding an instrument to a target in the lung |
| US10758239B2 (en)* | 2005-01-20 | 2020-09-01 | Pulmonx Corporation | Methods and devices for passive residual lung volume reduction and functional lung volume expansion |
| US20110152678A1 (en)* | 2005-01-20 | 2011-06-23 | Pulmonx Corporation | Methods and devices for passive residual lung volume reduction and functional lung volume expansion |
| US11883029B2 (en)* | 2005-01-20 | 2024-01-30 | Pulmonx Corporation | Methods and devices for passive residual lung volume reduction and functional lung volume expansion |
| WO2006078451A3 (en)* | 2005-01-20 | 2007-10-18 | Pulmonx | Minimally invasive determination of collateral ventilation in lungs |
| US8496006B2 (en) | 2005-01-20 | 2013-07-30 | Pulmonx Corporation | Methods and devices for passive residual lung volume reduction and functional lung volume expansion |
| US20170071606A1 (en)* | 2005-01-20 | 2017-03-16 | Pulmonx Corporation | Methods and devices for passive residual lung volume reduction and functional lung volume expansion |
| US9533116B2 (en) | 2005-01-20 | 2017-01-03 | Pulmonx Corporation | Methods and devices for passive residual lung volume reduction and functional lung volume expansion |
| EP2614853B1 (en)* | 2005-01-20 | 2016-12-28 | Pulmonx | Minimally invasive determination of collateral ventilation in lungs |
| US20230000497A1 (en)* | 2005-01-20 | 2023-01-05 | Pulmonx Corporation | Methods and devices for passive residual lung volume reduction and functional lung volume expansion |
| US11413045B2 (en)* | 2005-01-20 | 2022-08-16 | Pulmonx Corporation | Methods and devices for passive residual lung volume reduction and functional lung volume expansion |
| US8876791B2 (en) | 2005-02-25 | 2014-11-04 | Pulmonx Corporation | Collateral pathway treatment using agent entrained by aspiration flow current |
| US10842978B2 (en) | 2005-06-10 | 2020-11-24 | Acclarent, Inc. | Catheters with non-removable guide members useable for treatment of sinusitis |
| US20070142742A1 (en)* | 2005-07-13 | 2007-06-21 | Pulmonx | Methods and systems for segmental lung diagnostics |
| US20100042089A1 (en)* | 2005-08-17 | 2010-02-18 | Pulmonx Corporation | Selective lung tissue ablation |
| US7628789B2 (en) | 2005-08-17 | 2009-12-08 | Pulmonx Corporation | Selective lung tissue ablation |
| US11304742B2 (en) | 2005-08-17 | 2022-04-19 | Pulmonx Corporation | Selective lung tissue ablation |
| US9486266B2 (en) | 2005-08-17 | 2016-11-08 | Pulmonx Corporation | Selective lung tissue ablation |
| US20070043350A1 (en)* | 2005-08-17 | 2007-02-22 | Pulmonx | Selective lung tissue ablation |
| US10470811B2 (en) | 2005-08-17 | 2019-11-12 | Pulmonx Corporation | Selective lung tissue ablation |
| US8568403B2 (en) | 2005-08-17 | 2013-10-29 | Pulmonx Corporation | Selective lung tissue ablation |
| US20070051372A1 (en)* | 2005-08-23 | 2007-03-08 | Don Tanaka | Collateral ventilation bypass system with retention features |
| US8104474B2 (en) | 2005-08-23 | 2012-01-31 | Portaero, Inc. | Collateral ventilation bypass system with retention features |
| US20090229605A1 (en)* | 2005-08-24 | 2009-09-17 | Hospitech Respiration Ltd. | Ajustment of endotracheal tube cuff filling |
| US10780238B2 (en) | 2005-08-24 | 2020-09-22 | Hospitech Respiration Ltd. | Method of detecting endotracheal tube misplacement |
| US20110100373A1 (en)* | 2005-08-24 | 2011-05-05 | Hospitech Respiration Ltd. | Method of detecting endotracheal tube misplacement |
| US9004069B2 (en) | 2005-08-24 | 2015-04-14 | Hospitech Respiration Ltd. | Method of detecting endotracheal tube misplacement |
| US8424529B2 (en)* | 2005-08-24 | 2013-04-23 | Hospitech Respiration Ltd. | Adjustment of endotracheal tube cuff filling |
| US9218663B2 (en) | 2005-09-13 | 2015-12-22 | Veran Medical Technologies, Inc. | Apparatus and method for automatic image guided accuracy verification |
| US9218664B2 (en) | 2005-09-13 | 2015-12-22 | Veran Medical Technologies, Inc. | Apparatus and method for image guided accuracy verification |
| US11304629B2 (en) | 2005-09-13 | 2022-04-19 | Veran Medical Technologies, Inc. | Apparatus and method for image guided accuracy verification |
| US11304630B2 (en) | 2005-09-13 | 2022-04-19 | Veran Medical Technologies, Inc. | Apparatus and method for image guided accuracy verification |
| US10617332B2 (en) | 2005-09-13 | 2020-04-14 | Veran Medical Technologies, Inc. | Apparatus and method for image guided accuracy verification |
| US11938270B2 (en) | 2005-12-05 | 2024-03-26 | Hospitech Respiration Ltd. | Endotracheal tube and intubation system including same |
| US9555205B2 (en) | 2005-12-05 | 2017-01-31 | Hospitech Respiration Ltd. | Endotracheal tube and intubation system including same |
| US20090038620A1 (en)* | 2005-12-05 | 2009-02-12 | Shai Efrati | Endotracheal Tube and Intubation System Including Same |
| US20070133894A1 (en)* | 2005-12-07 | 2007-06-14 | Siemens Corporate Research, Inc. | Fissure Detection Methods For Lung Lobe Segmentation |
| US7711167B2 (en)* | 2005-12-07 | 2010-05-04 | Siemens Medical Solutions Usa, Inc. | Fissure detection methods for lung lobe segmentation |
| US8523782B2 (en) | 2005-12-07 | 2013-09-03 | Pulmonx Corporation | Minimally invasive determination of collateral ventilation in lungs |
| US20070167698A1 (en)* | 2005-12-14 | 2007-07-19 | General Electric Company | Method and apparatus for alignment of a mobile fluoroscopic imaging system |
| US9402639B2 (en)* | 2005-12-14 | 2016-08-02 | General Electric Company | Method and apparatus for alignment of a mobile fluoroscopic imaging system |
| US20070186932A1 (en)* | 2006-01-06 | 2007-08-16 | Pulmonx | Collateral pathway treatment using agent entrained by aspiration flow current |
| US9717468B2 (en) | 2006-01-10 | 2017-08-01 | Mediguide Ltd. | System and method for positioning an artificial heart valve at the position of a malfunctioning valve of a heart through a percutaneous route |
| EP1971289B1 (en)* | 2006-01-10 | 2017-08-09 | Mediguide Ltd. | System for positioning an artificial heart valve at the position of a malfunctioning valve of a heart through a percutaneous route |
| US7726305B2 (en) | 2006-01-17 | 2010-06-01 | Portaero, Inc. | Variable resistance pulmonary ventilation bypass valve |
| US7406963B2 (en) | 2006-01-17 | 2008-08-05 | Portaero, Inc. | Variable resistance pulmonary ventilation bypass valve and method |
| US7686013B2 (en) | 2006-01-17 | 2010-03-30 | Portaero, Inc. | Variable resistance pulmonary ventilation bypass valve |
| US20070163598A1 (en)* | 2006-01-17 | 2007-07-19 | Asia Chang | Variable resistance pulmonary ventilation bypass valve |
| US7967759B2 (en) | 2006-01-19 | 2011-06-28 | Boston Scientific Scimed, Inc. | Endoscopic system with integrated patient respiratory status indicator |
| US20070167683A1 (en)* | 2006-01-19 | 2007-07-19 | Boston Scientific Scimed, Inc. | Endoscopic system with integrated patient respiratory status indicator |
| WO2007084189A1 (en)* | 2006-01-19 | 2007-07-26 | Boston Scientific Scimed, Inc. | Endoscopic system with integrated patient respiratory status indicator |
| US9402632B2 (en) | 2006-03-13 | 2016-08-02 | Pneumrx, Inc. | Lung volume reduction devices, methods, and systems |
| US10226257B2 (en) | 2006-03-13 | 2019-03-12 | Pneumrx, Inc. | Lung volume reduction devices, methods, and systems |
| US9782558B2 (en) | 2006-03-13 | 2017-10-10 | Pneumrx, Inc. | Minimally invasive lung volume reduction devices, methods, and systems |
| US20090076622A1 (en)* | 2006-03-13 | 2009-03-19 | Pneumrx, Inc. | Delivery of Minimally Invasive Lung Volume Reduction Devices |
| US8668707B2 (en) | 2006-03-13 | 2014-03-11 | Pneumrx, Inc. | Minimally invasive lung volume reduction devices, methods, and systems |
| US8282660B2 (en) | 2006-03-13 | 2012-10-09 | Pneumrx, Inc. | Minimally invasive lung volume reduction devices, methods, and systems |
| US8157823B2 (en) | 2006-03-13 | 2012-04-17 | Pneumrx, Inc. | Lung volume reduction devices, methods, and systems |
| US10188398B2 (en) | 2006-03-13 | 2019-01-29 | Pneumrx, Inc. | Cross-sectional modification during deployment of an elongate lung volume reduction device |
| US10188397B2 (en) | 2006-03-13 | 2019-01-29 | Pneumrx, Inc. | Torque alleviating intra-airway lung volume reduction compressive implant structures |
| US8932310B2 (en) | 2006-03-13 | 2015-01-13 | Pneumrx, Inc. | Minimally invasive lung volume reduction devices, methods, and systems |
| US20070221230A1 (en)* | 2006-03-13 | 2007-09-27 | David Thompson | Minimally invasive lung volume reduction device and method |
| US9402633B2 (en) | 2006-03-13 | 2016-08-02 | Pneumrx, Inc. | Torque alleviating intra-airway lung volume reduction compressive implant structures |
| US8888800B2 (en) | 2006-03-13 | 2014-11-18 | Pneumrx, Inc. | Lung volume reduction devices, methods, and systems |
| US9402971B2 (en) | 2006-03-13 | 2016-08-02 | Pneumrx, Inc. | Minimally invasive lung volume reduction devices, methods, and systems |
| US8157837B2 (en) | 2006-03-13 | 2012-04-17 | Pneumrx, Inc. | Minimally invasive lung volume reduction device and method |
| US8740921B2 (en) | 2006-03-13 | 2014-06-03 | Pneumrx, Inc. | Lung volume reduction devices, methods, and systems |
| US8142455B2 (en) | 2006-03-13 | 2012-03-27 | Pneumrx, Inc. | Delivery of minimally invasive lung volume reduction devices |
| US9474533B2 (en) | 2006-03-13 | 2016-10-25 | Pneumrx, Inc. | Cross-sectional modification during deployment of an elongate lung volume reduction device |
| US20100262071A1 (en)* | 2006-03-31 | 2010-10-14 | James Kutsko | Articulable anchor |
| US8454708B2 (en) | 2006-03-31 | 2013-06-04 | Spiration, Inc. | Articulable anchor |
| US8647392B2 (en) | 2006-03-31 | 2014-02-11 | Spiration, Inc. | Articulable anchor |
| US20070232992A1 (en)* | 2006-03-31 | 2007-10-04 | James Kutsko | Articulable anchor |
| US9198669B2 (en) | 2006-03-31 | 2015-12-01 | Spiration, Inc. | Articulable anchor |
| US7691151B2 (en) | 2006-03-31 | 2010-04-06 | Spiration, Inc. | Articulable Anchor |
| US20070235846A1 (en)* | 2006-04-01 | 2007-10-11 | Stats Chippac Ltd. | Integrated circuit package system with net spacer |
| US20080072914A1 (en)* | 2006-08-25 | 2008-03-27 | Hendricksen Michael J | Bronchial Isolation Devices for Placement in Short Lumens |
| US9439583B2 (en)* | 2006-08-28 | 2016-09-13 | Pulmonx Corporation | Functional assessment and treatment catheters and methods for their use in the lung |
| US10517529B2 (en) | 2006-08-28 | 2019-12-31 | Pulmonx Corporation | Functional assessment and treatment catheters and methods for their use in the lung |
| US20130338524A1 (en)* | 2006-08-28 | 2013-12-19 | Pulmonx, Inc. | Functional assessment and treatment catheters and methods for their use in the lung |
| US20200107776A1 (en)* | 2006-08-28 | 2020-04-09 | Pulmonx Corporation | Functional assessment and treatment catheters and methods for their use in the lung |
| US11931170B2 (en)* | 2006-08-28 | 2024-03-19 | Pulmonx Corporation | Functional assessment and treatment catheters and methods for their use in the lung |
| US20090013995A1 (en)* | 2006-10-13 | 2009-01-15 | Williams Andrea R | Oral airway for endoscopic and intubating procedures |
| US8413658B2 (en)* | 2006-10-13 | 2013-04-09 | Andrea R. Williams | Oral airway for endoscopic and intubating procedures |
| US20080132757A1 (en)* | 2006-12-01 | 2008-06-05 | General Electric Company | System and Method for Performing Minimally Invasive Surgery Using a Multi-Channel Catheter |
| NL1034787C2 (en)* | 2006-12-01 | 2009-03-30 | Gen Electric | System and method for performing minimally invasive surgery using multi-channel catheter. |
| US20150231353A1 (en)* | 2007-03-12 | 2015-08-20 | Pulmonx Corporation | Methods and devices for passive residual lung volume reduction and functional lung volume expansion |
| US9050094B2 (en) | 2007-03-12 | 2015-06-09 | Pulmonx Corporation | Methods and devices for passive residual lung volume reduction and functional lung volume expansion |
| US11298489B2 (en) | 2007-03-12 | 2022-04-12 | Pulmonx Corporation | Methods and devices for passive residual lung volume reduction and functional lung volume expansion |
| US10314992B2 (en)* | 2007-03-12 | 2019-06-11 | Pulmonx Corporation | Methods and devices for passive residual lung volume reduction and functional lung volume expansion |
| US12350428B2 (en) | 2007-03-12 | 2025-07-08 | Pulmonx Corporation | Methods and devices for passive residual lung volume reduction and functional lung volume expansion |
| US20080281433A1 (en)* | 2007-05-11 | 2008-11-13 | Portaero, Inc. | Methods and devices to create a chemically and/or mechanically localized pleurodesis |
| US20080281151A1 (en)* | 2007-05-11 | 2008-11-13 | Portaero, Inc. | Pulmonary pleural stabilizer |
| US8163034B2 (en) | 2007-05-11 | 2012-04-24 | Portaero, Inc. | Methods and devices to create a chemically and/or mechanically localized pleurodesis |
| US7931641B2 (en) | 2007-05-11 | 2011-04-26 | Portaero, Inc. | Visceral pleura ring connector |
| US20100147295A1 (en)* | 2007-05-15 | 2010-06-17 | Portaero, Inc. | Devices and methods to create and maintain the patency of an opening relative to parenchymal tissue of the lung |
| US20080283065A1 (en)* | 2007-05-15 | 2008-11-20 | Portaero, Inc. | Methods and devices to maintain patency of a lumen in parenchymal tissue of the lung |
| US20100147294A1 (en)* | 2007-05-15 | 2010-06-17 | Portaero, Inc. | Devices and methods to maintain the patency of an opening relative to parenchymal tissue of the lung |
| US20080287878A1 (en)* | 2007-05-15 | 2008-11-20 | Portaero, Inc. | Pulmonary visceral pleura anastomosis reinforcement |
| US8062315B2 (en)* | 2007-05-17 | 2011-11-22 | Portaero, Inc. | Variable parietal/visceral pleural coupling |
| US20080295829A1 (en)* | 2007-05-30 | 2008-12-04 | Portaero, Inc. | Bridge element for lung implant |
| US9326873B2 (en) | 2007-10-12 | 2016-05-03 | Spiration, Inc. | Valve loader method, system, and apparatus |
| US8136230B2 (en) | 2007-10-12 | 2012-03-20 | Spiration, Inc. | Valve loader method, system, and apparatus |
| US8043301B2 (en) | 2007-10-12 | 2011-10-25 | Spiration, Inc. | Valve loader method, system, and apparatus |
| US20090099530A1 (en)* | 2007-10-12 | 2009-04-16 | Martin Neal Adams | Valve loader method, system, and apparatus |
| US20090292262A1 (en)* | 2007-10-12 | 2009-11-26 | Martin Neal Adams | Valve loader method, system, and apparatus |
| US9289173B2 (en) | 2007-11-09 | 2016-03-22 | The Spectranetics Corporation | Intra-vascular device with pressure detection capabilities using pressure sensitive material |
| US11166647B2 (en) | 2007-11-09 | 2021-11-09 | The Spectranetics Corporation | Intra-vascular device with pressure detection capabilities using pressure sensitive material |
| US20090125007A1 (en)* | 2007-11-09 | 2009-05-14 | Spectranetics | Intra-Vascular Device With Pressure Detection Capabilities Using Pressure Sensitive Material |
| US9066742B2 (en)* | 2007-11-09 | 2015-06-30 | The Spectranetics Corporation | Intra-vascular device with pressure detection capabilities using pressure sensitive material |
| US20090209856A1 (en)* | 2008-02-19 | 2009-08-20 | Portaero, Inc. | Two-phase surgical procedure for creating a pneumostoma to treat chronic obstructive pulmonary disease |
| US20090205650A1 (en)* | 2008-02-19 | 2009-08-20 | Portaero, Inc. | Variable length pneumostoma management system for treatment of chronic obstructive pulmonary disease |
| US20090209971A1 (en)* | 2008-02-19 | 2009-08-20 | Portaero, Inc. | Surgical instruments for creating a pneumostoma and treating chronic obstructive pulmonary disease |
| US8475389B2 (en) | 2008-02-19 | 2013-07-02 | Portaero, Inc. | Methods and devices for assessment of pneumostoma function |
| US7927324B2 (en) | 2008-02-19 | 2011-04-19 | Portaero, Inc. | Aspirator and method for pneumostoma management |
| US20090205641A1 (en)* | 2008-02-19 | 2009-08-20 | Portaero, Inc. | Pneumostoma management device and method for treatment of chronic obstructive pulmonary disease |
| US20090205645A1 (en)* | 2008-02-19 | 2009-08-20 | Portaero, Inc. | Pneumostoma management method for the treatment of chronic obstructive pulmonary disease |
| US20090205643A1 (en)* | 2008-02-19 | 2009-08-20 | Portaero, Inc. | Accelerated two-phase surgical procedure for creating a pneumostoma to treat chronic obstructive pulmonary disease |
| US8453637B2 (en) | 2008-02-19 | 2013-06-04 | Portaero, Inc. | Pneumostoma management system for treatment of chronic obstructive pulmonary disease |
| US8021320B2 (en) | 2008-02-19 | 2011-09-20 | Portaero, Inc. | Self-sealing device and method for delivery of a therapeutic agent through a pneumostoma |
| US8474449B2 (en) | 2008-02-19 | 2013-07-02 | Portaero, Inc. | Variable length pneumostoma management system for treatment of chronic obstructive pulmonary disease |
| US7909803B2 (en) | 2008-02-19 | 2011-03-22 | Portaero, Inc. | Enhanced pneumostoma management device and methods for treatment of chronic obstructive pulmonary disease |
| US20100286544A1 (en)* | 2008-02-19 | 2010-11-11 | Portaero, Inc. | Methods and devices for assessment of pneumostoma function |
| US8491602B2 (en) | 2008-02-19 | 2013-07-23 | Portaero, Inc. | Single-phase surgical procedure for creating a pneumostoma to treat chronic obstructive pulmonary disease |
| US8453638B2 (en) | 2008-02-19 | 2013-06-04 | Portaero, Inc. | One-piece pneumostoma management system and methods for treatment of chronic obstructive pulmonary disease |
| US8231581B2 (en) | 2008-02-19 | 2012-07-31 | Portaero, Inc. | Enhanced pneumostoma management device and methods for treatment of chronic obstructive pulmonary disease |
| US20090209936A1 (en)* | 2008-02-19 | 2009-08-20 | Portaero, Inc. | Aspirator and method for pneumostoma management |
| US8506577B2 (en) | 2008-02-19 | 2013-08-13 | Portaero, Inc. | Two-phase surgical procedure for creating a pneumostoma to treat chronic obstructive pulmonary disease |
| US20090209924A1 (en)* | 2008-02-19 | 2009-08-20 | Portaero, Inc. | Enhanced pneumostoma management device and methods for treatment of chronic obstructive pulmonary disease |
| US8252003B2 (en) | 2008-02-19 | 2012-08-28 | Portaero, Inc. | Surgical instruments for creating a pneumostoma and treating chronic obstructive pulmonary disease |
| US20090205646A1 (en)* | 2008-02-19 | 2009-08-20 | Portaero, Inc. | Flexible pneumostoma management system and methods for treatment of chronic obstructive pulmonary disease |
| US20090209909A1 (en)* | 2008-02-19 | 2009-08-20 | Portaero, Inc. | Percutaneous single-phase surgical procedure for creating a pneumostoma to treat chronic obstructive pulmonary disease |
| US8430094B2 (en) | 2008-02-19 | 2013-04-30 | Portaero, Inc. | Flexible pneumostoma management system and methods for treatment of chronic obstructive pulmonary disease |
| US8464708B2 (en) | 2008-02-19 | 2013-06-18 | Portaero, Inc. | Pneumostoma management system having a cosmetic and/or protective cover |
| US20090205644A1 (en)* | 2008-02-19 | 2009-08-20 | Portaero, Inc. | Pneumostoma management system for treatment of chronic obstructive pulmonary disease |
| US8336540B2 (en) | 2008-02-19 | 2012-12-25 | Portaero, Inc. | Pneumostoma management device and method for treatment of chronic obstructive pulmonary disease |
| US20090205649A1 (en)* | 2008-02-19 | 2009-08-20 | Portaero, Inc. | Multi-layer pneumostoma management system and methods for treatment of chronic obstructive pulmonary disease |
| US8348906B2 (en) | 2008-02-19 | 2013-01-08 | Portaero, Inc. | Aspirator for pneumostoma management |
| US20090205665A1 (en)* | 2008-02-19 | 2009-08-20 | Portaero, Inc. | Methods and devices for follow-up care and treatment of a pneumostoma |
| US20090205648A1 (en)* | 2008-02-19 | 2009-08-20 | Portaero, Inc. | Pneumostoma management system with secretion management features for treatment of chronic obstructive pulmonary disease |
| US8347880B2 (en) | 2008-02-19 | 2013-01-08 | Potaero, Inc. | Pneumostoma management system with secretion management features for treatment of chronic obstructive pulmonary disease |
| US8365722B2 (en) | 2008-02-19 | 2013-02-05 | Portaero, Inc. | Multi-layer pneumostoma management system and methods for treatment of chronic obstructive pulmonary disease |
| US20110118669A1 (en)* | 2008-02-19 | 2011-05-19 | Portaero, Inc. | Enhanced pneumostoma management device and methods for treatment of chronic obstructive pulmonary disease |
| US9855100B2 (en) | 2008-04-02 | 2018-01-02 | The Spectranetics Corporation | Liquid light-guide catheter with optically diverging tip |
| US10716625B2 (en) | 2008-04-02 | 2020-07-21 | The Spectranetics Corporation | Liquid light-guide catheter with optically diverging tip |
| CN102083354B (en)* | 2008-05-01 | 2014-02-26 | 斯波瑞申有限公司 | Direct Lung Sensor Systems and Devices |
| CN103892836A (en)* | 2008-05-01 | 2014-07-02 | 斯波瑞申有限公司 | Direct lung sensor systems, methods, and apparatuses |
| WO2009135070A1 (en)* | 2008-05-01 | 2009-11-05 | Spiration, Inc. | Direct lung sensor systems, methods, and apparatuses |
| US20110201956A1 (en)* | 2008-05-01 | 2011-08-18 | Alferness Clifton A | Direct lung sensor systems, methods, and apparatuses |
| CN102083354A (en)* | 2008-05-01 | 2011-06-01 | 斯波瑞申有限公司 | Direct lung sensor systems, methods and devices |
| US20100158795A1 (en)* | 2008-06-12 | 2010-06-24 | Pulmonx | Methods and systems for assessing lung function and delivering therapeutic agents |
| EP2285442A4 (en)* | 2008-06-12 | 2012-08-15 | Pulmonx Corp | METHOD AND SYSTEMS FOR TESTING THE LUNGS FUNCTION AND FOR SUBMITTING REMEDIES |
| WO2009152013A1 (en) | 2008-06-12 | 2009-12-17 | Pulmonx | Methods and systems for assessing lung function and delivering therapeutic agents |
| US10092357B2 (en) | 2008-07-21 | 2018-10-09 | The Spectranetics Corporation | Tapered liquid light guide |
| US9339337B2 (en) | 2008-07-21 | 2016-05-17 | The Spectranetics Corporation | Tapered liquid light guide |
| US11813085B2 (en)* | 2008-09-09 | 2023-11-14 | Pulmonx Corporation | Systems and methods for flushing an assessment catheter |
| CN102149421A (en)* | 2008-09-09 | 2011-08-10 | 普尔蒙克斯股份有限公司 | Systems and methods for inhibiting secretion flow into a functional assessment catheter |
| US20200037958A1 (en)* | 2008-09-09 | 2020-02-06 | Pulmonx Corporation | Systems and methods for flushing an assessment catheter |
| WO2010030691A1 (en)* | 2008-09-09 | 2010-03-18 | Pulmonx Corporation | Systems and methods for inhibiting secretion flow into a functional assessment catheter |
| US10058331B2 (en) | 2008-09-12 | 2018-08-28 | Pneumrx, Inc. | Enhanced efficacy lung volume reduction devices, methods, and systems |
| US9173669B2 (en)* | 2008-09-12 | 2015-11-03 | Pneumrx, Inc. | Enhanced efficacy lung volume reduction devices, methods, and systems |
| US10285707B2 (en) | 2008-09-12 | 2019-05-14 | Pneumrx, Inc. | Enhanced efficacy lung volume reduction devices, methods, and systems |
| US9192403B2 (en) | 2008-09-12 | 2015-11-24 | Pneumrx, Inc. | Elongated lung volume reduction devices, methods, and systems |
| US8632605B2 (en) | 2008-09-12 | 2014-01-21 | Pneumrx, Inc. | Elongated lung volume reduction devices, methods, and systems |
| US20100070050A1 (en)* | 2008-09-12 | 2010-03-18 | Pneumrx, Inc. | Enhanced Efficacy Lung Volume Reduction Devices, Methods, and Systems |
| US8950398B2 (en) | 2008-09-30 | 2015-02-10 | Covidien Lp | Supplemental gas safety system for a breathing assistance system |
| US20100170507A1 (en)* | 2009-01-08 | 2010-07-08 | Portaero, Inc. | Pneumostoma management device with integrated patency sensor and method |
| US8347881B2 (en) | 2009-01-08 | 2013-01-08 | Portaero, Inc. | Pneumostoma management device with integrated patency sensor and method |
| US20100204707A1 (en)* | 2009-02-11 | 2010-08-12 | Portaero, Inc. | Surgical instruments for creating a pneumostoma and treating chronic obstructive pulmonary disease |
| US8518053B2 (en) | 2009-02-11 | 2013-08-27 | Portaero, Inc. | Surgical instruments for creating a pneumostoma and treating chronic obstructive pulmonary disease |
| US20100305715A1 (en)* | 2009-05-18 | 2010-12-02 | Pneumrx, Inc. | Cross-Sectional Modification During Deployment of an Elongate Lung Volume Reduction Device |
| US8721734B2 (en) | 2009-05-18 | 2014-05-13 | Pneumrx, Inc. | Cross-sectional modification during deployment of an elongate lung volume reduction device |
| US20120172749A1 (en)* | 2009-12-10 | 2012-07-05 | He Kongyuan | Laryngeal Mask Airway (LMA) with Integrated Core Temperature Monitor and Display |
| US11937913B2 (en) | 2010-01-08 | 2024-03-26 | Pulmonx Corporation | Measuring lung function and lung disease progression at a lobar/segmental level |
| US20110209702A1 (en)* | 2010-02-26 | 2011-09-01 | Nellcor Puritan Bennett Llc | Proportional Solenoid Valve For Low Molecular Weight Gas Mixtures |
| US11317825B2 (en) | 2010-07-01 | 2022-05-03 | Pulmonx Corporation | Methods and systems for endobronchial diagnosis |
| US20230000387A1 (en)* | 2010-07-01 | 2023-01-05 | Pulmonx Corporation | Methods and systems for endobronchial diagnosis |
| US20180353116A1 (en)* | 2010-07-01 | 2018-12-13 | Pulmonx Corporation | Methods and systems for endobronchial diagnostics |
| US10932706B2 (en) | 2010-07-01 | 2021-03-02 | Pulmonx Corporation | Methods and systems for endobronchial diagnostics |
| US11819328B2 (en) | 2010-07-01 | 2023-11-21 | Pulmonx Corporation | Methods and systems for endobronchial diagnostics |
| US12029637B2 (en) | 2010-07-01 | 2024-07-09 | Pulmonx Corporation | Devices and systems for lung treatment |
| US10076271B2 (en) | 2010-07-01 | 2018-09-18 | Pulmonx Corporation | Methods and systems for endobronchial diagnostics |
| US10314516B2 (en)* | 2010-07-01 | 2019-06-11 | Pulmonx Corporation | Methods and systems for endobronchial diagnosis |
| US20120150057A1 (en)* | 2010-07-01 | 2012-06-14 | Pulmonx Corporation | Methods and systems for endobronchial diagnosis |
| US8808194B2 (en)* | 2010-07-01 | 2014-08-19 | Pulmonx Corporation | Methods and systems for endobronchial diagnostics |
| US10743978B2 (en) | 2010-07-01 | 2020-08-18 | Pulmonx Corporation | Devices and systems for lung treatment |
| US20120149995A1 (en)* | 2010-07-01 | 2012-06-14 | Pulmonx Corporation | Methods and systems for endobronchial diagnostics |
| US20120150027A1 (en)* | 2010-07-01 | 2012-06-14 | Pulmonx Corporation | Methods and systems for endobronchial diagnosis and treatment |
| US12364412B2 (en)* | 2010-07-01 | 2025-07-22 | Pulmonx Corporation | Methods and systems for endobronchial diagnosis |
| US11471069B2 (en) | 2010-07-01 | 2022-10-18 | Pulmonx Corporation | Methods and systems for endobronchial diagnosis |
| US9364168B2 (en)* | 2010-07-01 | 2016-06-14 | Pulmonx Corporation | Methods and systems for endobronchial diagnosis |
| US20120172748A1 (en)* | 2010-12-29 | 2012-07-05 | Dunn Lisa A | Temperature measuring device |
| US8795241B2 (en) | 2011-05-13 | 2014-08-05 | Spiration, Inc. | Deployment catheter |
| US20140097847A1 (en)* | 2011-06-15 | 2014-04-10 | Koninklijke Philips N.V. | Optical angular momentum induced hyperpolarisation in interventional applications |
| US10245402B2 (en) | 2011-07-11 | 2019-04-02 | Ambu A/S | Endobronchial tube with integrated image sensor |
| US10406309B2 (en) | 2011-07-11 | 2019-09-10 | Ambu A/S | Endobronchial tube with integrated image sensor and a cleaning nozzle arrangement |
| US10149602B2 (en) | 2011-07-11 | 2018-12-11 | Ambu A/S | Endobronchial tube with integrated image sensor and a cleaning nozzle arrangement |
| US10888679B2 (en) | 2011-07-11 | 2021-01-12 | Ambu A/S | Endobronchial tube with integrated image sensor |
| US9972082B2 (en) | 2012-02-22 | 2018-05-15 | Veran Medical Technologies, Inc. | Steerable surgical catheter having biopsy devices and related systems and methods for four dimensional soft tissue navigation |
| US11830198B2 (en) | 2012-02-22 | 2023-11-28 | Veran Medical Technologies, Inc. | Systems, methods and devices for forming respiratory-gated point cloud for four dimensional soft tissue navigation |
| US10977789B2 (en) | 2012-02-22 | 2021-04-13 | Veran Medical Technologies, Inc. | Systems, methods and devices for forming respiratory-gated point cloud for four dimensional soft tissue navigation |
| US10460437B2 (en) | 2012-02-22 | 2019-10-29 | Veran Medical Technologies, Inc. | Method for placing a localization element in an organ of a patient for four dimensional soft tissue navigation |
| US11551359B2 (en) | 2012-02-22 | 2023-01-10 | Veran Medical Technologies, Inc | Systems, methods and devices for forming respiratory-gated point cloud for four dimensional soft tissue navigation |
| US11403753B2 (en) | 2012-02-22 | 2022-08-02 | Veran Medical Technologies, Inc. | Surgical catheter having side exiting medical instrument and related systems and methods for four dimensional soft tissue navigation |
| US9138165B2 (en) | 2012-02-22 | 2015-09-22 | Veran Medical Technologies, Inc. | Systems, methods and devices for forming respiratory-gated point cloud for four dimensional soft tissue navigation |
| US10249036B2 (en) | 2012-02-22 | 2019-04-02 | Veran Medical Technologies, Inc. | Surgical catheter having side exiting medical instrument and related systems and methods for four dimensional soft tissue navigation |
| US10140704B2 (en) | 2012-02-22 | 2018-11-27 | Veran Medical Technologies, Inc. | Systems, methods and devices for forming respiratory-gated point cloud for four dimensional soft tissue navigation |
| US20240299686A1 (en)* | 2012-05-18 | 2024-09-12 | Fisher & Paykel Healthcare Limited | Control of flow and/or pressure provided by breathing apparatus |
| CN104173033A (en)* | 2013-05-24 | 2014-12-03 | 株式会社电装 | Apparatus for testing respiratory function |
| US10537334B2 (en) | 2013-10-25 | 2020-01-21 | Pneumrx, Inc. | Genetically-associated chronic obstructive pulmonary disease treatment |
| US10226299B2 (en) | 2014-02-24 | 2019-03-12 | Vida Diagnostics, Inc. | Treatment outcome prediction for lung volume reduction procedures |
| US9504529B2 (en) | 2014-02-24 | 2016-11-29 | Vida Diagnostics, Inc. | Treatment outcome prediction for lung volume reduction procedures |
| US11304756B2 (en) | 2014-02-24 | 2022-04-19 | Vida Diagnostics, Inc. | Treatment outcome prediction for lung volume reduction procedures |
| WO2015127355A1 (en)* | 2014-02-24 | 2015-08-27 | Vida Diagnostics, Inc. | Treatment outcome prediction for lung volume reduction procedures |
| US10624701B2 (en) | 2014-04-23 | 2020-04-21 | Veran Medical Technologies, Inc. | Apparatuses and methods for registering a real-time image feed from an imaging device to a steerable catheter |
| US11553968B2 (en) | 2014-04-23 | 2023-01-17 | Veran Medical Technologies, Inc. | Apparatuses and methods for registering a real-time image feed from an imaging device to a steerable catheter |
| US10617324B2 (en) | 2014-04-23 | 2020-04-14 | Veran Medical Technologies, Inc | Apparatuses and methods for endobronchial navigation to and confirmation of the location of a target tissue and percutaneous interception of the target tissue |
| US20150327836A1 (en)* | 2014-05-16 | 2015-11-19 | University Of Virginia Patent Foundation | Endovascular occlusion device and method of use |
| US10390838B1 (en) | 2014-08-20 | 2019-08-27 | Pneumrx, Inc. | Tuned strength chronic obstructive pulmonary disease treatment |
| CN104491976A (en)* | 2014-09-05 | 2015-04-08 | 广州中医药大学第一附属医院 | Visible tracheal catheter and bronchus blocking tube connecting tube |
| US11097084B2 (en)* | 2014-09-19 | 2021-08-24 | Acclarent, Inc. | Balloon catheter assembly |
| US11262354B2 (en) | 2014-10-20 | 2022-03-01 | Boston Scientific Scimed, Inc. | Disposable sensor elements, systems, and related methods |
| US10456562B2 (en) | 2015-01-20 | 2019-10-29 | Pulmonx Corporation | Bronchial sealant delivery methods and systems |
| US10624733B2 (en) | 2015-03-24 | 2020-04-21 | Spiration, Inc. | Airway stent |
| US10195404B2 (en) | 2015-05-13 | 2019-02-05 | Atrium Medical Corporation | Chest drainage system |
| US11129971B2 (en) | 2015-05-13 | 2021-09-28 | Atrium Medical Corporation | Chest drainage system |
| US12310594B2 (en) | 2015-06-22 | 2025-05-27 | Pulmonx Corporation | Collateral flow channel sealant delivery methods and systems |
| US10413300B2 (en) | 2015-06-22 | 2019-09-17 | Pulmonx Corporation | Collateral flow channel sealant delivery methods and systems |
| US11696763B2 (en) | 2015-06-22 | 2023-07-11 | Pulmonx Corporation | Collateral flow channel sealant delivery methods and systems |
| US11471630B2 (en) | 2016-05-05 | 2022-10-18 | Covidien Lp | Fluid dispensing catheter |
| US10376665B2 (en) | 2016-05-05 | 2019-08-13 | Covidien Lp | Fluid dispensing catheter |
| US10842368B2 (en) | 2016-06-10 | 2020-11-24 | Ambu A/S | Suction catheter with brush and method of use for lens cleaning |
| US11191457B2 (en) | 2016-06-15 | 2021-12-07 | Boston Scientific Scimed, Inc. | Gas sampling catheters, systems and methods |
| US20180078119A1 (en)* | 2016-09-19 | 2018-03-22 | Covidien Lp | System and method for cleansing segments of a luminal network |
| US12185909B2 (en)* | 2016-09-19 | 2025-01-07 | Covidien Lp | System and method for cleansing segments of a luminal network |
| EP3299058A1 (en)* | 2016-09-19 | 2018-03-28 | Covidien LP | System for cleansing segments of a luminal network |
| US20210000330A1 (en)* | 2016-09-19 | 2021-01-07 | Covidien Lp | System and method for cleansing segments of a luminal network |
| US10799092B2 (en)* | 2016-09-19 | 2020-10-13 | Covidien Lp | System and method for cleansing segments of a luminal network |
| US11172846B2 (en) | 2016-10-21 | 2021-11-16 | Boston Scientific Scimed, Inc. | Gas sampling device |
| US10770182B2 (en) | 2017-05-19 | 2020-09-08 | Boston Scientific Scimed, Inc. | Systems and methods for assessing the health status of a patient |
| US10952799B2 (en) | 2017-05-31 | 2021-03-23 | Covidien Lp | Systems and methods for navigational bronchoscopy and selective drug delivery |
| US11707331B2 (en) | 2017-05-31 | 2023-07-25 | Covidien Lp | Systems and methods for navigational bronchoscopy and selective drug delivery |
| US12102396B2 (en) | 2017-05-31 | 2024-10-01 | Covidien Lp | Systems and methods for navigational bronchoscopy and selective drug delivery |
| US11714058B2 (en) | 2017-07-18 | 2023-08-01 | Regents Of The University Of Minnesota | Systems and methods for analyte sensing in physiological gas samples |
| US10852264B2 (en) | 2017-07-18 | 2020-12-01 | Boston Scientific Scimed, Inc. | Systems and methods for analyte sensing in physiological gas samples |
| US11166636B2 (en) | 2018-02-20 | 2021-11-09 | Boston Scientific Scimed, Inc. | Breath sampling mask and system |
| US20190350443A1 (en)* | 2018-05-21 | 2019-11-21 | Mark D. Noar | Method For Monitoring A Property Of Tissue Of An Internal Bodily Organ And Adjusting The Tissue Property |
| US12007385B2 (en) | 2018-10-19 | 2024-06-11 | Regents Of The University Of Minnesota | Systems and methods for detecting a brain condition |
| US11442056B2 (en) | 2018-10-19 | 2022-09-13 | Regents Of The University Of Minnesota | Systems and methods for detecting a brain condition |
| US11835435B2 (en) | 2018-11-27 | 2023-12-05 | Regents Of The University Of Minnesota | Systems and methods for detecting a health condition |
| US11662325B2 (en) | 2018-12-18 | 2023-05-30 | Regents Of The University Of Minnesota | Systems and methods for measuring kinetic response of chemical sensor elements |
| US11921096B2 (en) | 2019-09-10 | 2024-03-05 | Regents Of The University Of Minnesota | Fluid analysis system |
| US11875459B2 (en) | 2020-04-07 | 2024-01-16 | Vida Diagnostics, Inc. | Subject specific coordinatization and virtual navigation systems and methods |
| WO2021244690A1 (en)* | 2020-06-05 | 2021-12-09 | W.O.M. World Of Medicine Gmbh | Method for determining compliance of a cavity in minimally invasive surgery |
| CN115836194A (en)* | 2020-06-05 | 2023-03-21 | Wom医药世界公司 | Method of determining cavity compliance of an elastomeric medical product for leak testing |
| CN115811956A (en)* | 2020-06-05 | 2023-03-17 | Wom医药世界公司 | Method for Determining Cavity Compliance in Minimally Invasive Surgery |
| US11911145B2 (en) | 2020-07-10 | 2024-02-27 | Pulmonx Corporation | Methods and systems for determining collateral ventilation |
| WO2022010720A1 (en) | 2020-07-10 | 2022-01-13 | Pulmonx Corporation | Systems and methods for endobronchial diagnostics |
| US12266111B2 (en) | 2020-11-27 | 2025-04-01 | Vida Diagnostics, Inc. | Visualization of sub-pleural regions |
| WO2023049799A1 (en)* | 2021-09-24 | 2023-03-30 | Gyrus Acmi, Inc. D/B/A Olympus Surgical Technologies America | Occlusion device, systems, and method of use |
| CN116919687A (en)* | 2022-04-02 | 2023-10-24 | 苏州市美新迪斯医疗科技有限公司 | A stent conveyor |
| CN116211281A (en)* | 2023-04-17 | 2023-06-06 | 安徽肺畅医疗科技有限公司 | A device for detecting pulmonary bypass ventilation |
Also Published As
| Publication number | Publication date |
|---|---|
| WO2003022221A2 (en) | 2003-03-20 |
| WO2003022221A9 (en) | 2003-09-18 |
| WO2003022221A3 (en) | 2003-07-03 |
| JP4301945B2 (en) | 2009-07-22 |
| EP1435833A2 (en) | 2004-07-14 |
| AU2002331842A1 (en) | 2003-03-24 |
| EP1435833B1 (en) | 2014-05-21 |
| US20160249860A1 (en) | 2016-09-01 |
| US20160038058A1 (en) | 2016-02-11 |
| US20080200797A1 (en) | 2008-08-21 |
| EP1435833A4 (en) | 2008-06-18 |
| JP2005514081A (en) | 2005-05-19 |
| US10413244B2 (en) | 2019-09-17 |
| US20190388034A1 (en) | 2019-12-26 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| US20190388034A1 (en) | Method and apparatus for endobronchial diagnosis | |
| US11413045B2 (en) | Methods and devices for passive residual lung volume reduction and functional lung volume expansion | |
| US7883471B2 (en) | Minimally invasive determination of collateral ventilation in lungs | |
| US20030055331A1 (en) | Methods of endobronchial diagnosis using imaging | |
| US11819328B2 (en) | Methods and systems for endobronchial diagnostics | |
| US8523782B2 (en) | Minimally invasive determination of collateral ventilation in lungs | |
| US20200329996A1 (en) | Measuring lung function and lung disease progression at a lobar/segmental level | |
| EP1838217B1 (en) | Minimally invasive determination of collateral ventilation in lungs | |
| US20140142455A1 (en) | Minimally invasive determination of collateral ventilation in lungs | |
| US11883029B2 (en) | Methods and devices for passive residual lung volume reduction and functional lung volume expansion | |
| EP2903514B1 (en) | Systems for pulmonary diagnosis | |
| US20120150027A1 (en) | Methods and systems for endobronchial diagnosis and treatment |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| AS | Assignment | Owner name:PULMONX, CALIFORNIA Free format text:ASSIGNMENT OF ASSIGNORS INTEREST;ASSIGNORS:KOTMEL, ROBERT;SOLTESZ, PETER;WONDKA, ANTHONY;AND OTHERS;REEL/FRAME:013495/0244;SIGNING DATES FROM 20021008 TO 20021011 | |
| STCB | Information on status: application discontinuation | Free format text:ABANDONED -- FAILURE TO RESPOND TO AN OFFICE ACTION |