US20030028226A1 - Medical management system integrated programming apparatus for communication with an implantable medical device - Google Patents
Medical management system integrated programming apparatus for communication with an implantable medical deviceDownload PDFInfo
- Publication number
- US20030028226A1 US20030028226A1US10/251,509US25150902AUS2003028226A1US 20030028226 A1US20030028226 A1US 20030028226A1US 25150902 AUS25150902 AUS 25150902AUS 2003028226 A1US2003028226 A1US 2003028226A1
- Authority
- US
- United States
- Prior art keywords
- interface
- module
- implantable medical
- medical device
- information management
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Abandoned
Links
- 238000004891communicationMethods0.000titleclaimsabstractdescription81
- 238000012545processingMethods0.000claimsabstractdescription36
- 238000000034methodMethods0.000claimsdescription31
- 230000005540biological transmissionEffects0.000claimsdescription11
- 210000004556brainAnatomy0.000claimsdescription8
- 239000003814drugSubstances0.000claimsdescription8
- 229940079593drugDrugs0.000claimsdescription8
- 230000002496gastric effectEffects0.000claimsdescription8
- 230000000004hemodynamic effectEffects0.000claimsdescription8
- 210000003205muscleAnatomy0.000claimsdescription8
- 230000008569processEffects0.000claimsdescription5
- 238000003780insertionMethods0.000claims1
- 230000037431insertionEffects0.000claims1
- 238000007726management methodMethods0.000description76
- 238000012544monitoring processMethods0.000description12
- 238000005516engineering processMethods0.000description6
- 230000008901benefitEffects0.000description5
- 238000010586diagramMethods0.000description5
- 230000006870functionEffects0.000description5
- 238000001514detection methodMethods0.000description4
- 230000000694effectsEffects0.000description4
- 238000012546transferMethods0.000description4
- 238000000605extractionMethods0.000description3
- 230000002093peripheral effectEffects0.000description3
- 206010011906DeathDiseases0.000description2
- 230000006978adaptationEffects0.000description2
- 230000003321amplificationEffects0.000description2
- 238000004458analytical methodMethods0.000description2
- 230000003139buffering effectEffects0.000description2
- 239000003990capacitorSubstances0.000description2
- 238000006243chemical reactionMethods0.000description2
- 230000003750conditioning effectEffects0.000description2
- 238000012937correctionMethods0.000description2
- 230000008878couplingEffects0.000description2
- 238000010168coupling processMethods0.000description2
- 238000005859coupling reactionMethods0.000description2
- 238000013144data compressionMethods0.000description2
- 238000013461designMethods0.000description2
- 238000011161developmentMethods0.000description2
- 238000003745diagnosisMethods0.000description2
- 239000007789gasSubstances0.000description2
- 239000007943implantSubstances0.000description2
- 238000002513implantationMethods0.000description2
- 238000005259measurementMethods0.000description2
- 230000028161membrane depolarizationEffects0.000description2
- 238000012806monitoring deviceMethods0.000description2
- 238000003199nucleic acid amplification methodMethods0.000description2
- 230000004044responseEffects0.000description2
- 230000004936stimulating effectEffects0.000description2
- 206010061216InfarctionDiseases0.000description1
- WHXSMMKQMYFTQS-UHFFFAOYSA-NLithiumChemical compound[Li]WHXSMMKQMYFTQS-UHFFFAOYSA-N0.000description1
- 230000003044adaptive effectEffects0.000description1
- QVGXLLKOCUKJST-UHFFFAOYSA-Natomic oxygenChemical compound[O]QVGXLLKOCUKJST-UHFFFAOYSA-N0.000description1
- 230000000747cardiac effectEffects0.000description1
- 239000004020conductorSubstances0.000description1
- 238000012790confirmationMethods0.000description1
- 238000013523data managementMethods0.000description1
- 238000013500data storageMethods0.000description1
- 210000005003heart tissueAnatomy0.000description1
- 238000010348incorporationMethods0.000description1
- 230000007574infarctionEffects0.000description1
- 229910052744lithiumInorganic materials0.000description1
- 229940127554medical productDrugs0.000description1
- 238000012986modificationMethods0.000description1
- 230000004048modificationEffects0.000description1
- 230000002107myocardial effectEffects0.000description1
- 210000005036nerveAnatomy0.000description1
- 230000001537neural effectEffects0.000description1
- 230000003287optical effectEffects0.000description1
- 239000001301oxygenSubstances0.000description1
- 229910052760oxygenInorganic materials0.000description1
- 238000007639printingMethods0.000description1
- 230000036279refractory periodEffects0.000description1
- 230000002336repolarizationEffects0.000description1
- 230000001020rhythmical effectEffects0.000description1
- 230000035945sensitivityEffects0.000description1
Images
Classifications
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61N—ELECTROTHERAPY; MAGNETOTHERAPY; RADIATION THERAPY; ULTRASOUND THERAPY
- A61N1/00—Electrotherapy; Circuits therefor
- A61N1/18—Applying electric currents by contact electrodes
- A61N1/32—Applying electric currents by contact electrodes alternating or intermittent currents
- A61N1/36—Applying electric currents by contact electrodes alternating or intermittent currents for stimulation
- A61N1/372—Arrangements in connection with the implantation of stimulators
- A61N1/37211—Means for communicating with stimulators
- A61N1/37217—Means for communicating with stimulators characterised by the communication link, e.g. acoustic or tactile
- A61N1/37223—Circuits for electromagnetic coupling
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61N—ELECTROTHERAPY; MAGNETOTHERAPY; RADIATION THERAPY; ULTRASOUND THERAPY
- A61N1/00—Electrotherapy; Circuits therefor
- A61N1/18—Applying electric currents by contact electrodes
- A61N1/32—Applying electric currents by contact electrodes alternating or intermittent currents
- A61N1/36—Applying electric currents by contact electrodes alternating or intermittent currents for stimulation
- A61N1/372—Arrangements in connection with the implantation of stimulators
- A61N1/37211—Means for communicating with stimulators
- A61N1/37217—Means for communicating with stimulators characterised by the communication link, e.g. acoustic or tactile
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61N—ELECTROTHERAPY; MAGNETOTHERAPY; RADIATION THERAPY; ULTRASOUND THERAPY
- A61N1/00—Electrotherapy; Circuits therefor
- A61N1/18—Applying electric currents by contact electrodes
- A61N1/32—Applying electric currents by contact electrodes alternating or intermittent currents
- A61N1/36—Applying electric currents by contact electrodes alternating or intermittent currents for stimulation
- A61N1/372—Arrangements in connection with the implantation of stimulators
- A61N1/37211—Means for communicating with stimulators
- A61N1/37235—Aspects of the external programmer
- G—PHYSICS
- G16—INFORMATION AND COMMUNICATION TECHNOLOGY [ICT] SPECIALLY ADAPTED FOR SPECIFIC APPLICATION FIELDS
- G16H—HEALTHCARE INFORMATICS, i.e. INFORMATION AND COMMUNICATION TECHNOLOGY [ICT] SPECIALLY ADAPTED FOR THE HANDLING OR PROCESSING OF MEDICAL OR HEALTHCARE DATA
- G16H40/00—ICT specially adapted for the management or administration of healthcare resources or facilities; ICT specially adapted for the management or operation of medical equipment or devices
- G16H40/40—ICT specially adapted for the management or administration of healthcare resources or facilities; ICT specially adapted for the management or operation of medical equipment or devices for the management of medical equipment or devices, e.g. scheduling maintenance or upgrades
- G—PHYSICS
- G16—INFORMATION AND COMMUNICATION TECHNOLOGY [ICT] SPECIALLY ADAPTED FOR SPECIFIC APPLICATION FIELDS
- G16H—HEALTHCARE INFORMATICS, i.e. INFORMATION AND COMMUNICATION TECHNOLOGY [ICT] SPECIALLY ADAPTED FOR THE HANDLING OR PROCESSING OF MEDICAL OR HEALTHCARE DATA
- G16H40/00—ICT specially adapted for the management or administration of healthcare resources or facilities; ICT specially adapted for the management or operation of medical equipment or devices
- G16H40/60—ICT specially adapted for the management or administration of healthcare resources or facilities; ICT specially adapted for the management or operation of medical equipment or devices for the operation of medical equipment or devices
- G16H40/67—ICT specially adapted for the management or administration of healthcare resources or facilities; ICT specially adapted for the management or operation of medical equipment or devices for the operation of medical equipment or devices for remote operation
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61N—ELECTROTHERAPY; MAGNETOTHERAPY; RADIATION THERAPY; ULTRASOUND THERAPY
- A61N1/00—Electrotherapy; Circuits therefor
- A61N1/18—Applying electric currents by contact electrodes
- A61N1/32—Applying electric currents by contact electrodes alternating or intermittent currents
- A61N1/36—Applying electric currents by contact electrodes alternating or intermittent currents for stimulation
- A61N1/372—Arrangements in connection with the implantation of stimulators
- A61N1/37211—Means for communicating with stimulators
- A61N1/37252—Details of algorithms or data aspects of communication system, e.g. handshaking, transmitting specific data or segmenting data
- A61N1/37282—Details of algorithms or data aspects of communication system, e.g. handshaking, transmitting specific data or segmenting data characterised by communication with experts in remote locations using a network
Definitions
- the present inventionrelates to implantable medical devices. More particularly, the present invention pertains to apparatus and methods for use in the communication of information to/from an implantable medical device, e.g., programming commands, diagnostic information, etc.
- RF communicationis used to establish “downlink” telemetry channels, in which operational data and commands are transmitted from an external programming unit transmitter to a receiver in an implanted medical device, and/or is used to establish “uplink” telemetry channels, in which information is transmitted from the implanted medical device's transmitter to a receiver in the external unit.
- a specific example of a particular component of a telemetry system for implantable medical devicesis the Medtronic Model 9790 programmer, commercially available from Medtronic, Inc., the assignee of the present invention.
- the Model 9790 programmerwith appropriate software modules, can be used to communicate (both uplink and downlink) with numerous body implanted devices manufactured by Medtronic, Inc.
- an antenna in the form of a multiple turn wire coilis disposed within the hermetic enclosure of the implanted medical device.
- Downlink RF signals transmitted to the implanted device from an external unitinduce a current in the coil antenna, and this current is amplified and applied to a receiver input for demodulation and extraction of the information content of the RF signal.
- electrical current applied directly to the implanted coil antennacause RF electromagnetic signals to be generated. Such signals can be received by a corresponding antenna associated with the external unit.
- the external unit, e.g., programmer, of an implantable medical device systemtypically includes a relatively small, hand-held programming head containing an external antenna, so that this programming head can be placed directly over the implant site of the implanted device. This minimizes the distance between the implanted antenna associated with the implanted device and the external antenna associated with the programmer.
- the headis typically connected to a larger base unit of a programmer via a multiple conductor cable.
- the aforementioned Model 9790is one example of an implantable device programmer having this configuration. The Model 9790 is described in further detail in U.S. Pat. No.
- implantable medical devicesinclude more advanced features, it is typically necessary to convey correspondingly more information to the implantable medical device relating to the selection and control of such advanced features.
- a pacemakerselectively operable in various pacing modes, it is desirable that the physician be able to non-invasively select a mode of operation.
- a pacemakeris capable of pacing at various rates or of delivering stimulating pulses of varying energy levels, it is desirable that the physician be able to select, on a patient-by-patient basis, appropriate values for such variable operational parameters.
- Various types of informationare conveyed to implanted medical devices by telemetry systems.
- information conveyed to pacemakersmay include, but is clearly not limited to, pacing modes, multiple rate response settings, electrode polarity, maximum and minimum pacing rates, output energy such as output pulse width and/or output current, sense amplifier sensitivity, refractory periods, and calibration information.
- implantable medical devicesNot only has the complexity of implantable medical devices led to the need to convey correspondingly more information to the implantable medical device, but it has also become desirable to enable the implanted medical device to communicate a large amount of information outside of the patient to an external communication device, e.g., programmer. For example, for diagnostic purposes, it is desirable for the implanted device to be able to provide information regarding its operational status to the physician. Further, various implantable medical devices are available which transmit information to an external communication device such as digitized physiological parameter signals, e.g., ECG, for display, storage, and/or analysis by the external communication unit. Generally, such information conveyed from the implanted medical device includes any type of diagnostic information and/or information relating to the physiological parameters of the patient in which the device is implanted.
- ECGdigitized physiological parameter signals
- a portable computer apparatustypically has at least a subset of the following components: a housing for containing the computer circuitry and other electronic components; a power source (e.g., a battery or a cable for connecting the apparatus to a source of power); a user input apparatus (e.g., an alphanumeric keyboard or a mouse); and an output means (e.g., a text and/or graphic display and/or a printer) for communicating information to the user.
- portable computer equipmentwill frequently be equipped with data storage devices, such as a floppy disk drive or a hard disk drive.
- Such conventional standalone programming apparatushave some shortcomings. For example, such standalone devices are generally time consuming to design and develop. Further, from a computer hardware perspective, such programming apparatus must continually be updated and upgraded to keep up with current hardware technology improvements, e.g., new processors, electrical circuitry, etc. As such, these programmers are generally relatively expensive.
- conventional programming apparatusgenerally require that an extensive amount of diagnostic data be generated which is reviewed and integrated into a patient's file.
- datais generally required to be manually entered by either or all of the following methods including handwritten entry, keyboard entry, and printing of forms and then assembly of them into the patient's file. This may lead to errors during the transfer of such data and limit the amount of data saved because of time constraints.
- the present inventionhas certain objects. That is, various embodiments of the present invention provide solutions to one or more problems existing in the prior art with respect to programming apparatus.
- One of such problemsinvolves the use of standalone programmers. For example, with the use of standalone programmers, it is generally required to continually update hardware with the development of new technology. Further, design and development of standalone programmers is relatively expensive. Yet further, data generated by standalone programmers must generally be reviewed and integrated into a patient's file. Due to the standalone nature of the programmers, it is sometimes necessary to manually enter such data into a patient's file. Generally, such manual entering leads to undesirable errors in the patient's file and limitation as to the amount of data saved.
- various embodiments of the present inventionmay provide one or more of the following advantages. For example, expensive hardware upgrading is reduced. Further, data generated by an implantable medical device may be integrated more readily and more comprehensively into a patient's file. As such, errors due to manual entry may be eliminated. Further, for example, the use of an already existing infrastructure into which a programming apparatus according to the present invention is integrated, allows the programming apparatus to “piggyback” on the natural progression of the infrastructure, e.g., hospital/clinic capital upgrades. Such a piggyback arrangement would reduce the need to continually upgrade operating systems, print options, database management, and connectivity requirements provided by the existing infrastructure. Further, it would reduce capital investment required by entities (e.g., hospitals, clinics, etc.) due to the already existing infrastructure to facilitate communication with the implantable medical device.
- entitiese.g., hospitals, clinics, etc.
- a module interface apparatusfor facilitating communication between an implantable medical device and a medical information management system (e.g., a currently existing infrastructure of a medical facility computer communication network); a module interface apparatus including at least one of an interface receiver and an interface transmitter coupled to an interface antenna to communicate with at least one of a device transmitter and a device receiver of an implantable medical device; a module interface apparatus that includes interface circuitry operable to adapt data received from the medical information management system for provision to the interface transmitter to communicate such information to the implantable medical device; a module interface apparatus that includes interface circuitry to adapt data received from the interface receiver to communicate such information to a medical information management system; an interface module that includes a module housing which encloses at least one of an interface receiver and an interface transmitter wherein a programming head contains an interface antenna and a cable electrically connects the programming head to the interface module; an interface module that includes a module housing, wherein at least one of the interface receiver and interface transmitter are enclosed within the module housing
- a medical information management systeme.g., a currently existing infrastructure
- FIG. 1is a diagram illustrating an implantable medical device communication system according to the present invention including an implantable medical device in a body for communication with a medical information management system.
- FIG. 2is a general block diagram of circuitry of one embodiment of the implantable medical device of FIG. 1 including receiver and transmitter circuitry for use in communication according to the present invention.
- FIG. 3is a general block diagram of the communication system of FIG. 1 according to the present invention using a module interface apparatus for communication between the implantable medical device and the medical information management system.
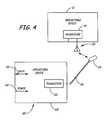
- FIG. 4is one embodiment of the module interface apparatus of FIG. 3 in communication with an implantable medical device according to the present invention.
- FIG. 5is an alternate embodiment of the module interface apparatus of FIG. 3 in communication with an implantable medical device according to the present invention.
- FIG. 6is one illustrative embodiment of a medical information management system for use in the system of FIG. 3 including a computer network according to the present invention.
- FIG. 7is an alternate medical information management system for use in the system of FIG. 3 wherein the medical information management system includes a portable battery operable computer processing system.
- FIG. 1is a simplified schematic view of an implantable medical device communication system 9 according to the present invention.
- the implantable medical device communication system 9includes an implantable medical device 12 for communication through a link 8 (including a module interface apparatus 15 shown in FIG. 3) with a medical information management system 13 .
- the present inventionleverages a medical information management system's infrastructure (e.g., computer subsystems, control stations, networks, print options, database management, operating systems, etc.) which are generally upgraded on a continuous basis with regard to hardware thereof.
- medical information management systemsas described further below, may be conventional monitoring systems such as a computer networked system in a hospital facility.
- such a medical information management system 13can be configured for acceptance of one or more interface modules, e.g., plug-in modules, for interfacing external devices to the medical information management system 13 .
- the present inventionuses the infrastructure of the medical information management system 13 to implement a programming apparatus for communication with an implantable medical device 12 .
- such communicationmay include both downlink communications, e.g., communication of programming commands to the implantable medical device 12 , and uplink communications, e.g., communication of diagnostic/physiological parameter data, to the medical information management system 13 .
- the implantable medical device 12is implanted in a body 10 near a human heart 16 .
- Implanted medical device 12is electrically connected to the heart by leads 14 .
- the leads 14are pacing and sensing leads connected to the heart 16 from the implanted medical device 12 .
- Such leadssense electrical signals attendant to the depolarization and repolarization of the heart 16 and provide pacing pulses for causing depolarization of cardiac tissue in the vicinity of the distal ends thereof.
- Implantable medical device 12may be any implantable cardiac pacemaker such as those disclosed in U.S. Pat. No. 5,158,078 to Bennett et al.; U.S. Pat. No. 5,312,453 to Shelton et al.; or U.S. Pat. No. 5,144,949 to Olson.
- Implantable medical device 12may also be a pacemaker-cardioverter-defibrillator (PCD) corresponding to any of the various commercially-available implantable PCDs.
- PCDpacemaker-cardioverter-defibrillator
- the present inventionmay be practiced in conjunction with PCDs such as those described in U.S. Pat. No. 5,545,186 to Olson et al.; U.S. Pat. No. 5,354,316 to Keimel; U.S. Pat. No. 5,314,430 to Bardy; U.S. Pat. No. 5,131,388 to Pless; or U.S. Pat. No. 4,821,723 to Baker, et al.
- implantable medical device 12may be an implantable nerve stimulator or muscle stimulator such as that disclosed in U.S. Pat. No. 5,199,428 to Obel et al.; U.S. Pat. No. 5,207,218 to Carpentier et al.; U.S. Pat. No. 5,330,507 to Schwartz; or an implantable monitoring device such as that disclosed in U.S. Pat. No. 5,331,966 issued to Bennett et al.
- the implanted medical device 12may be a defibrillator, a cardioverter-defibrillator, a brain stimulator, a gastric stimulator, a drug pump, a hemodynamic monitoring device, or any other implantable device that would benefit from a communication system according to the present invention as described herein. Therefore, the present invention is believed to find wide application for use with any form of implantable medical device. As such, a description herein making reference to any particular medical device is not to be taken as a limitation of the type of medical device which can benefit from and which can be employed with a communication system as described herein.
- the implantable medical device 12may include a hermetically sealed enclosure that may include various elements such as an electrochemical cell (e.g., a lithium battery), circuitry that controls device operations and records rhythmic EGM episodes, telemetry transceiver antenna and circuitry that communicates with the module interface apparatus as further described herein.
- an electrochemical celle.g., a lithium battery
- circuitrythat controls device operations and records rhythmic EGM episodes
- telemetry transceiver antennae.g., a telemetry transceiver antenna
- circuitrythat communicates with the module interface apparatus as further described herein.
- the implantable medical device 12is implemented with a microprocessor-based architecture.
- electronic features and operations of the implantable medical device 12may be implemented in discrete logic or as a microcomputer-based system, as would be readily apparent to one skilled in the art.
- FIG. 2shows a block diagram illustrating components of a pacemaker 11 in accordance with one embodiment of the present invention where pacemaker 11 has RF transmitter and receiver circuitry 54 for communication via the pacemaker antenna 56 with the module interface apparatus 15 according to the present invention as further described herein.
- the pacemaker 11is preferably programmable according to the present invention.
- Device antenna 56is connected to input/output circuit 32 to permit uplink/downlink communication through RF device transmitter and receiver circuitry 54 .
- Pacemaker 11illustratively shown in FIG. 2 is electrically coupled to the patient's heart 16 by lead 14 .
- Lead 14is coupled to a node 52 in the circuitry of pacemaker 11 through input capacitor 50 .
- an activity sensor 62provides a sensor output to an activity circuit 36 of input/output circuit 32 .
- Input/output circuit 32also contains circuits for interfacing to heart 16 , antenna 56 , and contains circuits 44 for application of stimulating pulses to heart 16 to control its rate under control of software-implemented algorithms in microcomputer unit 18 .
- Microcomputer unit 18preferably comprises on-board circuit 19 that includes microprocessor 20 , system clock 22 , and on-board random access memory (RAM) 24 and read-only memory (ROM) 26 .
- off-board circuit 28comprises a RAM/ROM unit.
- On-board circuit 19 and off-board circuit 28are each coupled by a communication bus 30 to digital controller/timer circuit 34 .
- FIG. 2The electrical components shown in FIG. 2 are powered by an appropriate implantable battery power source 64 in accordance with common practice in the art. For the sake of clarity, the coupling of battery power to the various components of pacemaker 11 is not shown in the figures.
- V REF and bias circuit 60generates a stable voltage reference and bias currents for circuits of input/output circuit 32 .
- Analog to digital converter (ADC) and multiplexer unit 58digitizes analog signals and voltages to provide “real-time” telemetry intracardiac and/or sensor signals and battery end-of-life (EOL) replacement function.
- ADCanalog to digital converter
- EOLbattery end-of-life
- Operating commands for controlling the timing of pacemaker 11are coupled by bus 30 to digital controller/timer circuit 34 , where digital timers and counters establish the overall escape interval of the pacemaker as well as various refractory, blanking, and other timing windows for controlling the operation of the peripheral components disposed within input/output circuit 32 .
- Digital controller/timer circuit 34is preferably coupled to sense circuitry 38 , including sense amplifier 42 , peak sense and threshold measurement unit 41 , and comparator/threshold detector 40 .
- Sense amplifier 42amplifies sensed electrocardiac signals and provides an amplified signal to peak sense and threshold measurement circuitry 41 .
- Circuitry 41in turn provides an indication of peak sensed voltages and measured sense amplifier threshold voltages on path 43 to digital controller/timer circuit 34 .
- An amplified sense amplifier signalis also provided to comparator/threshold detector 40 .
- Sense amplifier 42may, for example, correspond to that disclosed in U.S. Pat. No. 4,379,459 to Stein.
- Circuit 34is further preferably coupled to electrogram (EGM) amplifier 46 for receiving amplified process signals sensed by an electrode disposed on lead 14 .
- EGMelectrogram
- the electrogram signal provided by EGM amplifier 46is employed when the implanted device is being interrogated by an external programming apparatus to transmit by uplink telemetry a representation of an analog electrogram of the patient's electrical heart activity.
- EGMelectrogram
- Such functionalityis, for example, shown in U.S. Pat. No. 4,556,063 to Thompson et al.
- Output pulse generator 44provides pulsing stimuli to the patient's heart 16 through coupling capacitor 48 in response to a pacing trigger signal provided by digital controller/timer circuit 34 .
- Output amplifier 44may correspond generally to the output amplifier disclosed in U.S. Pat. No. 4,476,868 to Thompson.
- FIG. 3is a block diagram of the implantable medical device communication system 9 according to the present invention.
- the implantable medical device communication system 9includes the implantable medical device 12 , a medical information management system 13 , and a module interface apparatus 15 .
- the implantable medical device 12may include any implantable medical device such as those previously described herein.
- the implantable medical device 12includes transmitter and/or receiver circuitry 121 for use in communication of information between the implantable medical device 12 and the medical information management system 13 .
- the implantable medical device 12may include a receiver, a transmitter, or both depending upon whether uplink, downlink, or a combination of uplink and downlink channels for the communication of information between the implantable medical device 12 and the medical information management system 13 are to be established.
- implantable medical device 12may receive programming commands from an external device positioned external to the skin of the patient. Such programmer command instructions are referred to herein as downlink transmissions, i.e., transmissions to the implantable medical device 12 .
- received programming commandsmay include program instructions or steps for directing the operation of the implantable medical device 12 .
- the received command instructionsmay also include data such as program limits and timing data.
- the implantable medical device 12may transmit data external to the skin of the patient.
- Such transmissionsare referred to herein as uplink transmissions, i.e., transmissions from the implantable medical device 12 .
- the implantable medical devicetransmits data as well as receives data.
- implanted medical device 12includes receiver and transmitter circuitry 121 which cooperate with other circuitry of the implanted medical device 12 to receive information via a device antenna 130 and to transmit data via the device antenna 130 .
- Medical information management system 13refers generally to a system operable for receiving information from one or more external devices with the capability of processing such data.
- the medical information management system 13is operable with a plug-in module interface architecture.
- the plug-in module interface architectureincludes hardware for receiving a plurality of interface modules such that medical products which provide digital and/or analog outputs can be integrated, i.e., interfaced, to the medical information management system 13 .
- the medical information management system 13at a minimum includes a computer processing system for providing processing capabilities relative to information received from the implantable medical device 12 .
- the medical information management system 13includes a user interface (e.g., keyboard, mouse, touch screen, and associated software interface such as a Windows-based interface) associated with a display unit for use in providing programming for the implantable medical device 12 .
- a user interfacee.g., keyboard, mouse, touch screen, and associated software interface such as a Windows-based interface
- the medical information management system 13includes at least a processor, a communication network, storage media, a printer/plotter, and a display.
- FIGS. 6 and 7show two embodiments of illustrative medical information management systems 13 .
- FIG. 6shows a networked medical information management system 270 and
- FIG. 7shows a portable battery operable medical information management system 290 .
- the networked medical information management system 270 as shown in FIG. 6includes one or more computer processing subsystems (e.g., component monitoring system 274 ) connected to a computer network represented generally as controlled network 272 .
- a computer networkrepresented generally as controlled network 272 .
- such a system 270is utilized in larger medical facilities.
- other gateways to the controlled network 272are provided for various functionality of the networked medical information management system 270 .
- digital telemetry subsystem 288may be used to provide telemetry to the network 272 ; a component control manager subsystem 286 may provide for control of various components of the network 272 ; and various other subsystems 284 - 288 may provide for various other functions connected to the network, such as alarm systems, hospital patient archival records, laboratory information databases, remote access terminals, etc.
- a component control manager subsystem 286may provide for control of various components of the network 272 ;
- various other subsystems 284 - 288may provide for various other functions connected to the network, such as alarm systems, hospital patient archival records, laboratory information databases, remote access terminals, etc.
- FIG. 6is but one of an unlimited number of configurations possible for such a system 270 .
- the networked medical information management system 270includes at least one component monitoring system 274 (e.g., a computer processing subsystem) wherein the system interacts with a plurality of module interfaces 276 .
- Each of the module interfaces 276is operable for use in communication of information between an external device 277 corresponding to a module interface and the networked medical information management system 270 .
- the external devices 277may include devices such as a ventilator, a gas analyzer, a temperature sensor, or any other device external to the body that provides an analog and/or digital output which can be interfaced to the networked medical information management system 270 through the plurality of module interfaces 276 .
- such a networked medical information management system 270may include any number of different components, and a listing of the peripherals or components attached to the network 272 are not limited by any of those listed herein.
- the networked medical information management system 270may include various computer subsystems, various types of networks, remote modem connections, distributed hospital/clinic connections, database systems, various portable devices, battery back-ups, generators, etc.
- a networked medical information management system 270 or components thereofare described in U.S. Pat. No. 5,520,191 to Karlsson et al., entitled “Myocardial lschemia And Infarction Analysis And Monitoring Method And Apparatus”; U.S. Pat. No. 5,687,734 to Dempsey et al., entitled “Flexible Patient Monitoring System Featuring A Multiport Transmitter”; U.S. Pat. No. 5,417,222 to Dempsey et al., entitled “Patient Monitoring System”; and U.S. Pat. No.
- the networked medical information management system 270includes the utilization of the Hewlett Packard Component Monitoring System (CMS) available under the trade designation Viridia CMS which with its associated display unit (e.g., flat screen or CRT color monitor) can be networked to a computer network.
- CMSHewlett Packard Component Monitoring System
- the plurality of interface modules 276 for interfacing various external devices 277are provided through the use of a plug-in interface available from Hewlett Packard under the trade designation of HP VueLink Interface.
- a plug-in interfaceavailable from Hewlett Packard Company, Palo Alto, Calif.
- the Hewlett Packard CMSis an open architecture system which allows for use of specific functions to be integrated into a Hewlett Packard network and database system. For example, it is possible to provide software on the network for controlling external devices or for receiving information from external devices via the plug-in module open architecture. Such software according to the present invention would provide for the programming of the implantable medical device 12 and/or monitoring of the patient or device implanted therein.
- the Hewlett Packard CMSis a Windows NT-based system. As the above Hewlett Packard products are commercially available, further details with regard to the system shall not be provided herein for simplicity purposes.
- FIG. 7shows the portable battery operable medical information management system 290 for use according to the present invention.
- the portable medical information management system 290includes a display unit 291 and processing unit 292 which may be powered by a power source 296 that is either battery operated or has a connection to an AC power source, e.g., power cord connection.
- the battery operable system 290is operable with one or more interface modules 294 for interfacing various external devices 295 , such as those described above, to the processing unit 292 .
- Such a portable or smaller systemmay generally be used in a satellite office or in transport of a patient, or, further, for example, in a smaller medical facility.
- the portable, battery operable medical information management system 290is a system available from Hewlett Packard under the trade designation HP-M1275A Component Transport System (CTS).
- CTSComponent Transport System
- the Hewlett Packard CTSis a system 290 that is a flexible and transportable patient monitoring system based on a variety of components that enable customization to a hospital's requirements.
- the Hewlett Packard systemincludes a transport mainframe that contains the display unit and processing capabilities.
- the systemutilizes the same type of interface modules as the HP CMS, which is described in general above with regard to the networked medical information management system 270 .
- Both the HP CMS and CTS systemshave similar operator controls and waveform display.
- a computer subsystemis responsible for parameter processing, display control, and interfacing.
- the same types of interface modulesare receivable in plug-in slots of the component transport system, i.e., the HP VueLink Interface plug-in receiving unit is usable for both the HP CMS and CTS.
- Some patient-related settingsmay even be transferred between the CMS and the CTS via an interface module, and vital signs, may, for example, be transferred through such transfer modules.
- the interface modules 294are contained in a separable rack, but the rack is designed to dock with the monitor mainframe which eliminates extra cable requirements.
- This same type of HP VueLink slot rackcan be used for the Hewlett Packard CMS and the CTS.
- the medical information management system 13is preferably a system including the ability to implement software for providing program commands for communication to an implantable medical device and for receiving data and displaying data from the implantable medical device 12 , e.g., diagnostic data, physiological parameter data, histograms, counters, trend plots, etc., and preferably processing such data.
- the networked medical information management system 270monitoring or control of programming may be performed from any node on the network.
- the medical information management system 13is configured for receiving a plurality of interface modules for interfacing to corresponding external devices, e.g., ventilators, gas analyzers, etc.
- the Hewlett Packard CMS and the Hewlett Packard CTSsuch a configuration is provided with use of the Hewlett Packard VueLink Interface.
- such a configurationincludes a plug-in module rack.
- a plug-in rackmay not be necessary as in the case of information provided to the medical information management system 13 via a modem connection.
- the present inventionleverages the infrastructure of a medical information management system 13 , preferably, a commercially available system whose hardware is continually upgraded. Such leverage is attained by providing a module interface apparatus 15 , e.g., a plug-in module that is compatible with the Hewlett Packard VueLink Interface, to interface the implantable medical device 12 to the medical information management system 13 .
- a module interface apparatus 15e.g., a plug-in module that is compatible with the Hewlett Packard VueLink Interface
- communication of programming commands from the medical information management system 13 to the implantable medical device 12can be performed.
- the module interface apparatus 15provides for communication of device data from the implantable medical device 12 to the medical information management system 13 .
- This configuration for the implantable medical device communication system 9reduces the need for a standalone programming apparatus as previously described in the Background of the Invention section herein.
- the module interface apparatus 15provides the link 8 between the implantable medical device 12 and the medical information management system 13 and piggybacks on the natural progression of such a medical information management system 13 .
- the medical information management system 13is upgraded, such upgrades are passed through to the programming communication system according to the present invention without the necessary incorporation of technological advances in a conventional standalone programming apparatus.
- thisreduces the need to continually upgrade the operating system, print options, database management systems, connectivity requirements, etc. with respect to the integrated programming apparatus according to the present invention.
- the module interface apparatus 15 shown in FIG. 3includes at least transmitter/receiver circuitry 123 and an interface antenna 131 for transmitting and receiving electromagnetic energy.
- the module interface apparatus 15includes receiver circuitry that is compatible with the transmitter of the implantable medical device 12 and operable for receiving and extracting, e.g., demodulating, data from the transmitted signal from the implantable medical device 12 .
- the module interface apparatus 15includes transmitter circuitry that is compatible with the receiver of the implantable medical device 112 and operable for generating a modulated signal of which the receiver of the implantable medical device 112 is capable of receiving and extracting e.g., demodulating, information therefrom.
- the implantable medical device communication system 9may be used for communication of information to the implantable medical device 12 or from the implantable medical device 12 .
- data representative of programming commandsis received at the module interface apparatus 15 .
- the medical information management system 13includes the necessary operating system, user interface, database management, and any software necessary and compatible with the programming requirements of the implantable medical device 12 to provide the appropriate programming commands to the module interface apparatus 15 .
- the module interface apparatus 15includes interface circuitry 128 to adapt the programming data for transmission by the transmitter/receiver circuitry 123 to the implantable medical device 12 .
- the data representative of the programming commandsis adapted by the interface circuitry 128 and provided to the interface transmitter of the receiver/transmitter circuitry 123 .
- the adaptation of the data representative of the programming commands by the interface circuitry 128may include any number of operations, such as for example, amplification, processing, conditioning, parallel to serial conversion, data buffering, error detection and correction, data encryption/encoding, data compression, telemetry link management and channel management, operational status (e.g., module status lights, warnings indicators, etc.) and any other operation necessary as determined by the type of telemetry used between the transmitter of the module interface apparatus 15 and the receiver of the implantable medical device 12 .
- the adapted data representative of the programming commandsis then transmitted via the interface antenna 131 of the module interface apparatus 15 to the implantable medical device 12 where it is received at the device antenna 130 and detected by device receiver/transmitter circuitry 121 .
- Device datamay be provided by the implantable medical device 12 to the medical information management system 13 through the module interface apparatus 15 .
- Device datamay include any type of data provided by an implantable medical device including real-time and stored data, such as diagnostic data (e.g., data for patient diagnosis or implanted device function diagnosis), physiological parameter data (e.g., oxygen sensed levels, activity sensor data, ECG waveforms and data, etc.), operational data (e.g., lead impedance data, battery voltage data, etc., and analyzed data (e.g., variability plot data, trend data, etc.). Data is transmitted by the device transmitter of transmitter/receiver circuitry 121 via the device antenna 130 .
- diagnostic datae.g., data for patient diagnosis or implanted device function diagnosis
- physiological parameter datae.g., oxygen sensed levels, activity sensor data, ECG waveforms and data, etc.
- operational datae.g., lead impedance data, battery voltage data, etc.
- analyzed datae.g., variability
- the electromagnetic energyis received at the interface antenna 131 of the module interface apparatus 15 and a received signal is provided to the receiver of the receiver/transmitter circuitry 123 for detection and extraction of transmitted data from the signal.
- the interface circuitry 128 of the module interface apparatus 15adapts the data for provision to the medical information management system 13 .
- such adaptationmay include amplification, conditioning, processing, serial to parallel conversion of the stream of data, data management/formatting, data compression, data encryption, report generation, data buffering, error detection/correction, or any other any other operation necessary as determined by the type of telemetry used between the receiver of the module interface apparatus 15 and the transmitter of the implantable medical device 12 .
- the adapted data representative of the device data received via the interface antenna 131is then provided to the medical information management system 13 .
- processing circuitry 133may act upon the device data received from the implantable medical device 12 to provide a sampled amount of data, to provide data representative of a physiological parameter based upon multiple sensor outputs signals, to provide an alarm status upon operation of detection circuitry for detecting a warning event, and to provide operational status indication (e.g., status lights to indicate proper telemetry function, programming confirmation, etc.)
- operational status indicatione.g., status lights to indicate proper telemetry function, programming confirmation, etc.
- the communication of data from the module interface apparatus 15 to the medical information management system 13may be accomplished in a number of manners.
- the transfer of data between the module interface apparatus 15 and the medical information management system 13may be accomplished by a direct electrical line, e.g., Local Area Network (LAN) or Wide Area Network (WAN).
- LANLocal Area Network
- WANWide Area Network
- various other methods of transportmay be used as well, such as RF transmission, or any other wireless technologies, modem technologies, infrared technologies, optical technologies, etc.
- the module interface apparatus 15includes an interface antenna 131 for transmitting electromagnetic waves to the device antenna of the implantable medical device and an interface transmitter that is compatible with the device receiver of the implantable medical device 12 .
- the modulation and demodulation techniques and operating frequencies of the corresponding device receiver and module interface transmitterare compatible.
- the device transmitter of receiver/transmitter circuitry 121is compatible with the interface receiver of receiver/transmitter circuitry 123 of the module interface apparatus 15 .
- the modulation and demodulation techniques and operating frequencies of the corresponding device transmitter and the interface module receiverare compatible.
- FIGS. 4 and 5Two illustrative embodiments of the module interface apparatus 15 are illustrated in FIGS. 4 and 5.
- various systems for performing telemetry between the implantable medical device 12 and module interface apparatus 15may be used and are known.
- several of such systemsare described in U.S. Pat. No. 5,127,404 issued to Wyborney et al.; U.S. Pat. No. 4,556,063 issued to Thompson et al.; and U.S. Pat. No. 5,342,408 issued to De Coriolis et al.
- module interface apparatus 120includes an interface module 125 including a module housing which contains transceiver circuitry 122 and any other circuitry such as previously described herein.
- the module interface apparatus 120further includes an antenna positioned within a programmer head 124 connected to the transceiver circuitry 122 via a stretchable cable 126 and is powered by source 140 . Further, data 148 is communicated to the medical information management network 13 . Wireless communication and/or any other electrical connection may be possible between the programmer head and the interface module 121 .
- the programmer head 124 containing the antennamay then be positioned at the implant site relative to device antenna 130 and the implantable medical device 12 which contains the transceiver circuitry 121 .
- Such an interfacemay be used in conjunction with telemetry according to U.S. Pat. No. 5,527,348; a low frequency (175 kHz), near field (34 inches), and low data rate (4-50 k baud) telemetry system.
- FIG. 5provides an alternate module interface apparatus 150 .
- the module interface apparatus 150includes interface module 151 which has a module housing to contain the transceiver circuitry 152 and any other circuitry of the apparatus 150 such as described previously herein. Power is applied by power source 154 and data 156 is communicated to the medical information management system 13 .
- the transceiver circuitry 152is connected to one or more antennas 158 , represented by antenna elements 160 , 162 . Communication may then be performed via the device antenna 130 and the implantable medical device 12 which contains the transceiver circuitry 121 . In this case, a programmer head is unnecessary and telemetry is generally accomplished using a system such as described in U.S. Pat. No. 5,683,432; a high frequency (400 MHz), far field (to 30 feet), and high data rate (to 100 k baud) telemetry system.
Landscapes
- Health & Medical Sciences (AREA)
- Engineering & Computer Science (AREA)
- Biomedical Technology (AREA)
- General Health & Medical Sciences (AREA)
- Public Health (AREA)
- Life Sciences & Earth Sciences (AREA)
- Business, Economics & Management (AREA)
- Veterinary Medicine (AREA)
- Animal Behavior & Ethology (AREA)
- General Business, Economics & Management (AREA)
- Physics & Mathematics (AREA)
- Radiology & Medical Imaging (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Acoustics & Sound (AREA)
- Epidemiology (AREA)
- Primary Health Care (AREA)
- Medical Informatics (AREA)
- Electromagnetism (AREA)
- Electrotherapy Devices (AREA)
- Measuring And Recording Apparatus For Diagnosis (AREA)
Abstract
Description
- This application claims the benefit of U.S. Provisional Application No. 60/089,868, filed Jun. 19, 1998.[0001]
- The present invention relates to implantable medical devices. More particularly, the present invention pertains to apparatus and methods for use in the communication of information to/from an implantable medical device, e.g., programming commands, diagnostic information, etc.[0002]
- Communication systems employing radio frequency (RF) transmitters and receivers are common. One application of such communication systems is in the field of body implantable medical devices, such as pacemakers, defibrillators, neural stimulators, and the like. RF communication is used to establish “downlink” telemetry channels, in which operational data and commands are transmitted from an external programming unit transmitter to a receiver in an implanted medical device, and/or is used to establish “uplink” telemetry channels, in which information is transmitted from the implanted medical device's transmitter to a receiver in the external unit.[0003]
- A specific example of a particular component of a telemetry system for implantable medical devices is the Medtronic Model 9790 programmer, commercially available from Medtronic, Inc., the assignee of the present invention. The Model 9790 programmer, with appropriate software modules, can be used to communicate (both uplink and downlink) with numerous body implanted devices manufactured by Medtronic, Inc.[0004]
- Conventionally, as exemplified by the Model 9790 programmer in conjunction with a Medtronic implantable medical device (e.g., a pacemaker), an antenna in the form of a multiple turn wire coil is disposed within the hermetic enclosure of the implanted medical device. Downlink RF signals transmitted to the implanted device from an external unit induce a current in the coil antenna, and this current is amplified and applied to a receiver input for demodulation and extraction of the information content of the RF signal. Similarly, for uplink communication, electrical current applied directly to the implanted coil antenna, cause RF electromagnetic signals to be generated. Such signals can be received by a corresponding antenna associated with the external unit.[0005]
- For various reasons, including the desire to minimize the necessary strength of both uplink and downlink telemetry signals in implantable medical device systems, the external unit, e.g., programmer, of an implantable medical device system typically includes a relatively small, hand-held programming head containing an external antenna, so that this programming head can be placed directly over the implant site of the implanted device. This minimizes the distance between the implanted antenna associated with the implanted device and the external antenna associated with the programmer. For example, the head is typically connected to a larger base unit of a programmer via a multiple conductor cable. The aforementioned Model 9790 is one example of an implantable device programmer having this configuration. The Model 9790 is described in further detail in U.S. Pat. No. 5,345,362 to Winkler et al., entitled “Portable Computer Apparatus With Articulating Display Panel.” Further, a programming head and cable for use with such an implanted device programmer is described in U.S. Pat. No. 5,527,348 to Winkler et al., entitled “Magnetically Permeable E-Shield And A Method Of Connection Thereto.”[0006]
- Various communication systems provide the necessary uplink and downlink communication channels between an external unit, e.g., programmer and the implanted medical device. However, some communication systems do not require the use of a hand-held programming head containing the external antenna. For example, such a communication system is described in U.S. Pat. No. 5,683,432 to Goedeke et al., entitled, “Adaptive, Performance-Optimizing Communication System For Communicating With An Implanted Medical Device.”[0007]
- As the complexity of implantable medical devices increases over time, communication systems for enabling such implantable medical devices to communicate with external communication devices, e.g., programmers, has become more important. For example, it is desirable for a physician to non-invasively exercise some amount of control over the implanted medical device, e.g., to turn the device on or off after implantation, to adjust various parameters of the implantable medical device after implantation, etc.[0008]
- Further, as implantable medical devices include more advanced features, it is typically necessary to convey correspondingly more information to the implantable medical device relating to the selection and control of such advanced features. For example, not only is a pacemaker selectively operable in various pacing modes, it is desirable that the physician be able to non-invasively select a mode of operation. Further, for example, if a pacemaker is capable of pacing at various rates or of delivering stimulating pulses of varying energy levels, it is desirable that the physician be able to select, on a patient-by-patient basis, appropriate values for such variable operational parameters. Various types of information are conveyed to implanted medical devices by telemetry systems. For example, information conveyed to pacemakers may include, but is clearly not limited to, pacing modes, multiple rate response settings, electrode polarity, maximum and minimum pacing rates, output energy such as output pulse width and/or output current, sense amplifier sensitivity, refractory periods, and calibration information.[0009]
- Not only has the complexity of implantable medical devices led to the need to convey correspondingly more information to the implantable medical device, but it has also become desirable to enable the implanted medical device to communicate a large amount of information outside of the patient to an external communication device, e.g., programmer. For example, for diagnostic purposes, it is desirable for the implanted device to be able to provide information regarding its operational status to the physician. Further, various implantable medical devices are available which transmit information to an external communication device such as digitized physiological parameter signals, e.g., ECG, for display, storage, and/or analysis by the external communication unit. Generally, such information conveyed from the implanted medical device includes any type of diagnostic information and/or information relating to the physiological parameters of the patient in which the device is implanted.[0010]
- Substantial technological improvements in the field of electronics over the past years has enabled computer equipment manufacturers to provide powerful, fully-featured computers that are compact and portable. Such computers have proven to be extremely popular and a wide variety of such computers are known and commercially available. A portable computer apparatus typically has at least a subset of the following components: a housing for containing the computer circuitry and other electronic components; a power source (e.g., a battery or a cable for connecting the apparatus to a source of power); a user input apparatus (e.g., an alphanumeric keyboard or a mouse); and an output means (e.g., a text and/or graphic display and/or a printer) for communicating information to the user. In addition, portable computer equipment will frequently be equipped with data storage devices, such as a floppy disk drive or a hard disk drive.[0011]
- Conventional programmers have generally been “standalone” devices and portable like that of portable computer apparatus as described above. For example, several available programmers include the Medtronic Model 9710 for which some further detail is described in U.S. Pat. No. 5,168,871 to Grevious, entitled “Method And Apparatus For Processing Quasi-Transient Telemetry Signals In Noisy Environments”; Medtronic Model No. 9760 for which some further detail is described in U.S. Registration No. H1347 to Greeninger et al., entitled “Audio Feedback For Implantable Medical Device Instruments;” and Medtronic Model No. 9790 for which some further detail is described in U.S. Pat. No. 5,372,607 to Stone et al., entitled, “Method And Apparatus For Monitoring Pacemaker Intervals.” Generally, such standalone devices are portable, as described above, completely self-contained devices that require the use of paper, cable connections, diskettes, or an infrared (IR) link to generate archival data.[0012]
- Such conventional standalone programming apparatus have some shortcomings. For example, such standalone devices are generally time consuming to design and develop. Further, from a computer hardware perspective, such programming apparatus must continually be updated and upgraded to keep up with current hardware technology improvements, e.g., new processors, electrical circuitry, etc. As such, these programmers are generally relatively expensive.[0013]
- In addition, conventional programming apparatus generally require that an extensive amount of diagnostic data be generated which is reviewed and integrated into a patient's file. However, such data is generally required to be manually entered by either or all of the following methods including handwritten entry, keyboard entry, and printing of forms and then assembly of them into the patient's file. This may lead to errors during the transfer of such data and limit the amount of data saved because of time constraints.[0014]
- Table 1 below lists U.S. Patents relating to various components of implantable medical device communication systems.[0015]
TABLE 1 U.S. Pat. No. Inventor(s) Issue Date 5,527,348 Winkler et al. Jun. 18, 1996 5,372,607 Stone et al. Dec. 13, 1994 H1347 Greeninger et al. Aug. 2, 1994 5,683,432 Goedeke et at. Nov. 4, 1997 5,168,871 Grevious Dec. 8, 1998 - All references listed in Table[0016]1, and elsewhere herein, are incorporated by reference in their respective entireties. As those of ordinary skill in the art will appreciate readily upon reading the Summary of the Invention, Detailed Description of the Embodiments, and claims set forth below, at least some of the apparatus and methods disclosed in the references of Table1 and elsewhere herein may be modified advantageously by using the teachings of the present invention. However, the listing of any such references in Table 1, or elsewhere herein, is by no means an indication that such references are prior art to the present invention.
- The present invention has certain objects. That is, various embodiments of the present invention provide solutions to one or more problems existing in the prior art with respect to programming apparatus. One of such problems involves the use of standalone programmers. For example, with the use of standalone programmers, it is generally required to continually update hardware with the development of new technology. Further, design and development of standalone programmers is relatively expensive. Yet further, data generated by standalone programmers must generally be reviewed and integrated into a patient's file. Due to the standalone nature of the programmers, it is sometimes necessary to manually enter such data into a patient's file. Generally, such manual entering leads to undesirable errors in the patient's file and limitation as to the amount of data saved.[0017]
- In comparison to known programming apparatus, various embodiments of the present invention may provide one or more of the following advantages. For example, expensive hardware upgrading is reduced. Further, data generated by an implantable medical device may be integrated more readily and more comprehensively into a patient's file. As such, errors due to manual entry may be eliminated. Further, for example, the use of an already existing infrastructure into which a programming apparatus according to the present invention is integrated, allows the programming apparatus to “piggyback” on the natural progression of the infrastructure, e.g., hospital/clinic capital upgrades. Such a piggyback arrangement would reduce the need to continually upgrade operating systems, print options, database management, and connectivity requirements provided by the existing infrastructure. Further, it would reduce capital investment required by entities (e.g., hospitals, clinics, etc.) due to the already existing infrastructure to facilitate communication with the implantable medical device.[0018]
- Some embodiments of the present invention include one or more of the following features: a module interface apparatus for facilitating communication between an implantable medical device and a medical information management system (e.g., a currently existing infrastructure of a medical facility computer communication network); a module interface apparatus including at least one of an interface receiver and an interface transmitter coupled to an interface antenna to communicate with at least one of a device transmitter and a device receiver of an implantable medical device; a module interface apparatus that includes interface circuitry operable to adapt data received from the medical information management system for provision to the interface transmitter to communicate such information to the implantable medical device; a module interface apparatus that includes interface circuitry to adapt data received from the interface receiver to communicate such information to a medical information management system; an interface module that includes a module housing which encloses at least one of an interface receiver and an interface transmitter wherein a programming head contains an interface antenna and a cable electrically connects the programming head to the interface module; an interface module that includes a module housing, wherein at least one of the interface receiver and interface transmitter are enclosed within the module housing and further wherein the interface antenna is external to the module housing; an interface module that includes processing circuitry to receive and process information signals from an interface receiver received from a implantable medical device; a plurality of interface modules, wherein each interface module is operable for use in communication of information between an external device corresponding to the interface module and a medical information management system; a medical information management system that includes a battery operable processing unit; a medical information management system that includes a computer network; programming commands provided to the interface module from a medical information management system; diagnostic and/or physiological parameter data provided from an interface module to a medical information management system; a programming method wherein data representing programming commands is received at an interface module, the data is adapted for transmission to the implantable medical device, and the adapted data is transmitted by an interface antenna to the implantable medical device; an implantable medical device uplink communication method wherein data is received from an interface module from a device transmitter of an implantable medical device by an interface antenna, the data received is adapted for provision to a medical information management system, and the adapted data is provided to the medical information management system.[0019]
- The above summary of the present invention is not intended to describe each embodiment of every implementation of the present invention. Advantages, together with a more complete understanding of the invention, will become apparent and appreciated by referring to the following detailed description and claims taken in conjunction with the accompanying drawings.[0020]
- FIG. 1 is a diagram illustrating an implantable medical device communication system according to the present invention including an implantable medical device in a body for communication with a medical information management system.[0021]
- FIG. 2 is a general block diagram of circuitry of one embodiment of the implantable medical device of FIG. 1 including receiver and transmitter circuitry for use in communication according to the present invention.[0022]
- FIG. 3 is a general block diagram of the communication system of FIG. 1 according to the present invention using a module interface apparatus for communication between the implantable medical device and the medical information management system.[0023]
- FIG. 4 is one embodiment of the module interface apparatus of FIG. 3 in communication with an implantable medical device according to the present invention.[0024]
- FIG. 5 is an alternate embodiment of the module interface apparatus of FIG. 3 in communication with an implantable medical device according to the present invention.[0025]
- FIG. 6 is one illustrative embodiment of a medical information management system for use in the system of FIG. 3 including a computer network according to the present invention.[0026]
- FIG. 7 is an alternate medical information management system for use in the system of FIG. 3 wherein the medical information management system includes a portable battery operable computer processing system.[0027]
- FIG. 1 is a simplified schematic view of an implantable medical[0028]
device communication system 9 according to the present invention. The implantable medicaldevice communication system 9 includes an implantablemedical device 12 for communication through a link8 (including amodule interface apparatus 15 shown in FIG. 3) with a medicalinformation management system 13. The present invention leverages a medical information management system's infrastructure (e.g., computer subsystems, control stations, networks, print options, database management, operating systems, etc.) which are generally upgraded on a continuous basis with regard to hardware thereof. For example, such medical information management systems, as described further below, may be conventional monitoring systems such as a computer networked system in a hospital facility. Generally, for example, such a medicalinformation management system 13 can be configured for acceptance of one or more interface modules, e.g., plug-in modules, for interfacing external devices to the medicalinformation management system 13. The present invention uses the infrastructure of the medicalinformation management system 13 to implement a programming apparatus for communication with an implantablemedical device 12. For example, such communication may include both downlink communications, e.g., communication of programming commands to the implantablemedical device 12, and uplink communications, e.g., communication of diagnostic/physiological parameter data, to the medicalinformation management system 13. - As shown in FIG. 1, the implantable[0029]
medical device 12 is implanted in abody 10 near ahuman heart 16. Implantedmedical device 12 is electrically connected to the heart by leads14. In the case where the implantedmedical device 12 is a pacemaker, theleads 14 are pacing and sensing leads connected to theheart 16 from the implantedmedical device 12. Such leads sense electrical signals attendant to the depolarization and repolarization of theheart 16 and provide pacing pulses for causing depolarization of cardiac tissue in the vicinity of the distal ends thereof. Implantablemedical device 12 may be any implantable cardiac pacemaker such as those disclosed in U.S. Pat. No. 5,158,078 to Bennett et al.; U.S. Pat. No. 5,312,453 to Shelton et al.; or U.S. Pat. No. 5,144,949 to Olson. - Implantable[0030]
medical device 12 may also be a pacemaker-cardioverter-defibrillator (PCD) corresponding to any of the various commercially-available implantable PCDs. For example, the present invention may be practiced in conjunction with PCDs such as those described in U.S. Pat. No. 5,545,186 to Olson et al.; U.S. Pat. No. 5,354,316 to Keimel; U.S. Pat. No. 5,314,430 to Bardy; U.S. Pat. No. 5,131,388 to Pless; or U.S. Pat. No. 4,821,723 to Baker, et al. - Alternatively, implantable[0031]
medical device 12 may be an implantable nerve stimulator or muscle stimulator such as that disclosed in U.S. Pat. No. 5,199,428 to Obel et al.; U.S. Pat. No. 5,207,218 to Carpentier et al.; U.S. Pat. No. 5,330,507 to Schwartz; or an implantable monitoring device such as that disclosed in U.S. Pat. No. 5,331,966 issued to Bennett et al. - Further, for example, the implanted[0032]
medical device 12 may be a defibrillator, a cardioverter-defibrillator, a brain stimulator, a gastric stimulator, a drug pump, a hemodynamic monitoring device, or any other implantable device that would benefit from a communication system according to the present invention as described herein. Therefore, the present invention is believed to find wide application for use with any form of implantable medical device. As such, a description herein making reference to any particular medical device is not to be taken as a limitation of the type of medical device which can benefit from and which can be employed with a communication system as described herein. - In general, the implantable[0033]
medical device 12 may include a hermetically sealed enclosure that may include various elements such as an electrochemical cell (e.g., a lithium battery), circuitry that controls device operations and records rhythmic EGM episodes, telemetry transceiver antenna and circuitry that communicates with the module interface apparatus as further described herein. Generally, the implantablemedical device 12 is implemented with a microprocessor-based architecture. However, electronic features and operations of the implantablemedical device 12 may be implemented in discrete logic or as a microcomputer-based system, as would be readily apparent to one skilled in the art. - FIG. 2 shows a block diagram illustrating components of a[0034]
pacemaker 11 in accordance with one embodiment of the present invention wherepacemaker 11 has RF transmitter andreceiver circuitry 54 for communication via thepacemaker antenna 56 with themodule interface apparatus 15 according to the present invention as further described herein. In the illustrative embodiment shown in FIG. 2, thepacemaker 11 is preferably programmable according to the present invention.Device antenna 56 is connected to input/output circuit 32 to permit uplink/downlink communication through RF device transmitter andreceiver circuitry 54. - [0035]
Pacemaker 11 illustratively shown in FIG. 2 is electrically coupled to the patient'sheart 16 bylead 14.Lead 14 is coupled to anode 52 in the circuitry ofpacemaker 11 throughinput capacitor 50. In the presently disclosed embodiment, anactivity sensor 62 provides a sensor output to anactivity circuit 36 of input/output circuit 32. - Input/[0036]
output circuit 32 also contains circuits for interfacing toheart 16,antenna 56, and containscircuits 44 for application of stimulating pulses toheart 16 to control its rate under control of software-implemented algorithms inmicrocomputer unit 18. - [0037]
Microcomputer unit 18 preferably comprises on-board circuit 19 that includesmicroprocessor 20,system clock 22, and on-board random access memory (RAM)24 and read-only memory (ROM)26. In this illustrative embodiment, off-board circuit 28 comprises a RAM/ROM unit. On-board circuit 19 and off-board circuit 28 are each coupled by acommunication bus 30 to digital controller/timer circuit 34. - The electrical components shown in FIG. 2 are powered by an appropriate implantable[0038]
battery power source 64 in accordance with common practice in the art. For the sake of clarity, the coupling of battery power to the various components ofpacemaker 11 is not shown in the figures. - V[0039]REFand bias circuit60 generates a stable voltage reference and bias currents for circuits of input/
output circuit 32. Analog to digital converter (ADC) andmultiplexer unit 58 digitizes analog signals and voltages to provide “real-time” telemetry intracardiac and/or sensor signals and battery end-of-life (EOL) replacement function. - Operating commands for controlling the timing of[0040]
pacemaker 11 are coupled bybus 30 to digital controller/timer circuit 34, where digital timers and counters establish the overall escape interval of the pacemaker as well as various refractory, blanking, and other timing windows for controlling the operation of the peripheral components disposed within input/output circuit 32. Digital controller/timer circuit 34 is preferably coupled tosense circuitry 38, includingsense amplifier 42, peak sense andthreshold measurement unit 41, and comparator/threshold detector 40.Sense amplifier 42 amplifies sensed electrocardiac signals and provides an amplified signal to peak sense andthreshold measurement circuitry 41.Circuitry 41 in turn provides an indication of peak sensed voltages and measured sense amplifier threshold voltages onpath 43 to digital controller/timer circuit 34. An amplified sense amplifier signal is also provided to comparator/threshold detector 40.Sense amplifier 42 may, for example, correspond to that disclosed in U.S. Pat. No. 4,379,459 to Stein. - [0041]
Circuit 34 is further preferably coupled to electrogram (EGM)amplifier 46 for receiving amplified process signals sensed by an electrode disposed onlead 14. The electrogram signal provided byEGM amplifier 46 is employed when the implanted device is being interrogated by an external programming apparatus to transmit by uplink telemetry a representation of an analog electrogram of the patient's electrical heart activity. Such functionality is, for example, shown in U.S. Pat. No. 4,556,063 to Thompson et al.Output pulse generator 44 provides pulsing stimuli to the patient'sheart 16 throughcoupling capacitor 48 in response to a pacing trigger signal provided by digital controller/timer circuit 34.Output amplifier 44, for example, may correspond generally to the output amplifier disclosed in U.S. Pat. No. 4,476,868 to Thompson. - FIG. 3 is a block diagram of the implantable medical[0042]
device communication system 9 according to the present invention. The implantable medicaldevice communication system 9 includes the implantablemedical device 12, a medicalinformation management system 13, and amodule interface apparatus 15. The implantablemedical device 12 may include any implantable medical device such as those previously described herein. According to the present invention, the implantablemedical device 12 includes transmitter and/orreceiver circuitry 121 for use in communication of information between the implantablemedical device 12 and the medicalinformation management system 13. It will be recognized by one skilled in the art that the implantablemedical device 12 may include a receiver, a transmitter, or both depending upon whether uplink, downlink, or a combination of uplink and downlink channels for the communication of information between the implantablemedical device 12 and the medicalinformation management system 13 are to be established. - Generally, implantable[0043]
medical device 12 may receive programming commands from an external device positioned external to the skin of the patient. Such programmer command instructions are referred to herein as downlink transmissions, i.e., transmissions to the implantablemedical device 12. For example, received programming commands may include program instructions or steps for directing the operation of the implantablemedical device 12. Further, for example, the received command instructions may also include data such as program limits and timing data. Similarly, the implantablemedical device 12 may transmit data external to the skin of the patient. Such transmissions are referred to herein as uplink transmissions, i.e., transmissions from the implantablemedical device 12. In other words, the implantable medical device transmits data as well as receives data. Generally, implantedmedical device 12 includes receiver andtransmitter circuitry 121 which cooperate with other circuitry of the implantedmedical device 12 to receive information via adevice antenna 130 and to transmit data via thedevice antenna 130. - Medical[0044]
information management system 13 refers generally to a system operable for receiving information from one or more external devices with the capability of processing such data. Preferably, according to the present invention, the medicalinformation management system 13 is operable with a plug-in module interface architecture. Preferably, the plug-in module interface architecture includes hardware for receiving a plurality of interface modules such that medical products which provide digital and/or analog outputs can be integrated, i.e., interfaced, to the medicalinformation management system 13. Generally, the medicalinformation management system 13 at a minimum includes a computer processing system for providing processing capabilities relative to information received from the implantablemedical device 12. Further, preferably, the medicalinformation management system 13 includes a user interface (e.g., keyboard, mouse, touch screen, and associated software interface such as a Windows-based interface) associated with a display unit for use in providing programming for the implantablemedical device 12. One skilled in the art will recognize that any number of different peripherals may be components of the medicalinformation management system 13. Preferably, the medicalinformation management system 13 includes at least a processor, a communication network, storage media, a printer/plotter, and a display. - FIGS. 6 and 7 show two embodiments of illustrative medical[0045]
information management systems 13. FIG. 6 shows a networked medicalinformation management system 270 and FIG. 7 shows a portable battery operable medicalinformation management system 290. - The networked medical[0046]
information management system 270 as shown in FIG. 6 includes one or more computer processing subsystems (e.g., component monitoring system274) connected to a computer network represented generally as controllednetwork 272. Generally, such asystem 270 is utilized in larger medical facilities. Further, other gateways to the controllednetwork 272 are provided for various functionality of the networked medicalinformation management system 270. For example,digital telemetry subsystem 288 may be used to provide telemetry to thenetwork 272; a componentcontrol manager subsystem 286 may provide for control of various components of thenetwork 272; and various other subsystems284-288 may provide for various other functions connected to the network, such as alarm systems, hospital patient archival records, laboratory information databases, remote access terminals, etc. One skilled in the art will recognize that various configurations and components of thenetworked system 270 are possible and that the illustrative FIG. 6 is but one of an unlimited number of configurations possible for such asystem 270. - Preferably, the networked medical[0047]
information management system 270 includes at least one component monitoring system274 (e.g., a computer processing subsystem) wherein the system interacts with a plurality of module interfaces276. Each of the module interfaces276 is operable for use in communication of information between anexternal device 277 corresponding to a module interface and the networked medicalinformation management system 270. For example, theexternal devices 277 may include devices such as a ventilator, a gas analyzer, a temperature sensor, or any other device external to the body that provides an analog and/or digital output which can be interfaced to the networked medicalinformation management system 270 through the plurality of module interfaces276. - As described above, such a networked medical[0048]
information management system 270 may include any number of different components, and a listing of the peripherals or components attached to thenetwork 272 are not limited by any of those listed herein. For example, the networked medicalinformation management system 270 may include various computer subsystems, various types of networks, remote modem connections, distributed hospital/clinic connections, database systems, various portable devices, battery back-ups, generators, etc. - Various types of already existing infrastructures for networked medical[0049]
information management systems 270 are available. For example, a networked medicalinformation management system 270 or components thereof are described in U.S. Pat. No. 5,520,191 to Karlsson et al., entitled “Myocardial lschemia And Infarction Analysis And Monitoring Method And Apparatus”; U.S. Pat. No. 5,687,734 to Dempsey et al., entitled “Flexible Patient Monitoring System Featuring A Multiport Transmitter”; U.S. Pat. No. 5,417,222 to Dempsey et al., entitled “Patient Monitoring System”; and U.S. Pat. No. 5,579,775 to Dempsey et al., entitled, “Dynamic control Of A Patient Monitoring System.” Preferably, according to the present invention, the networked medicalinformation management system 270 includes the utilization of the Hewlett Packard Component Monitoring System (CMS) available under the trade designation Viridia CMS which with its associated display unit (e.g., flat screen or CRT color monitor) can be networked to a computer network. Further, preferably, the plurality ofinterface modules 276 for interfacing variousexternal devices 277 are provided through the use of a plug-in interface available from Hewlett Packard under the trade designation of HP VueLink Interface. Detailed information with regard to the Hewlett Packard CMS and Hewlett Packard VueLink Interface is available from Hewlett Packard Company, Palo Alto, Calif. - Generally, the Hewlett Packard CMS is an open architecture system which allows for use of specific functions to be integrated into a Hewlett Packard network and database system. For example, it is possible to provide software on the network for controlling external devices or for receiving information from external devices via the plug-in module open architecture. Such software according to the present invention would provide for the programming of the implantable[0050]
medical device 12 and/or monitoring of the patient or device implanted therein. The Hewlett Packard CMS is a Windows NT-based system. As the above Hewlett Packard products are commercially available, further details with regard to the system shall not be provided herein for simplicity purposes. - FIG. 7 shows the portable battery operable medical[0051]
information management system 290 for use according to the present invention. The portable medicalinformation management system 290 includes adisplay unit 291 andprocessing unit 292 which may be powered by apower source 296 that is either battery operated or has a connection to an AC power source, e.g., power cord connection. Further, the batteryoperable system 290 is operable with one ormore interface modules 294 for interfacing variousexternal devices 295, such as those described above, to theprocessing unit 292. Such a portable or smaller system, for example, may generally be used in a satellite office or in transport of a patient, or, further, for example, in a smaller medical facility. - Preferably, according to the present invention, the portable, battery operable medical[0052]
information management system 290 is a system available from Hewlett Packard under the trade designation HP-M1275A Component Transport System (CTS). Generally, the Hewlett Packard CTS is asystem 290 that is a flexible and transportable patient monitoring system based on a variety of components that enable customization to a hospital's requirements. For example, the Hewlett Packard system includes a transport mainframe that contains the display unit and processing capabilities. The system utilizes the same type of interface modules as the HP CMS, which is described in general above with regard to the networked medicalinformation management system 270. - Both the HP CMS and CTS systems have similar operator controls and waveform display. A computer subsystem is responsible for parameter processing, display control, and interfacing. The same types of interface modules are receivable in plug-in slots of the component transport system, i.e., the HP VueLink Interface plug-in receiving unit is usable for both the HP CMS and CTS. Some patient-related settings may even be transferred between the CMS and the CTS via an interface module, and vital signs, may, for example, be transferred through such transfer modules.[0053]
- Generally, the[0054]
interface modules 294, are contained in a separable rack, but the rack is designed to dock with the monitor mainframe which eliminates extra cable requirements. This same type of HP VueLink slot rack can be used for the Hewlett Packard CMS and the CTS. - As described above, one skilled in the art will recognize that the medical[0055]
information management system 13 according to the present invention is preferably a system including the ability to implement software for providing program commands for communication to an implantable medical device and for receiving data and displaying data from the implantablemedical device 12, e.g., diagnostic data, physiological parameter data, histograms, counters, trend plots, etc., and preferably processing such data. It will be recognized that in the case of the networked medicalinformation management system 270, monitoring or control of programming may be performed from any node on the network. Further, preferably, the medicalinformation management system 13 is configured for receiving a plurality of interface modules for interfacing to corresponding external devices, e.g., ventilators, gas analyzers, etc. For example, with regard to the Hewlett Packard CMS and the Hewlett Packard CTS, such a configuration is provided with use of the Hewlett Packard VueLink Interface. - Preferably, such a configuration includes a plug-in module rack. However, one skilled in the art will recognize that data may be transmitted over a variety of mediums. Therefore, a plug-in rack may not be necessary as in the case of information provided to the medical[0056]
information management system 13 via a modem connection. The present invention leverages the infrastructure of a medicalinformation management system 13, preferably, a commercially available system whose hardware is continually upgraded. Such leverage is attained by providing amodule interface apparatus 15, e.g., a plug-in module that is compatible with the Hewlett Packard VueLink Interface, to interface the implantablemedical device 12 to the medicalinformation management system 13. Through the use of themodule interface apparatus 15, communication of programming commands from the medicalinformation management system 13 to the implantablemedical device 12 can be performed. Further, themodule interface apparatus 15 provides for communication of device data from the implantablemedical device 12 to the medicalinformation management system 13. - This configuration for the implantable medical[0057]
device communication system 9 reduces the need for a standalone programming apparatus as previously described in the Background of the Invention section herein. In such a configuration of an implantable medicaldevice communication system 9, themodule interface apparatus 15 provides the link8 between the implantablemedical device 12 and the medicalinformation management system 13 and piggybacks on the natural progression of such a medicalinformation management system 13. For example, as the medicalinformation management system 13 is upgraded, such upgrades are passed through to the programming communication system according to the present invention without the necessary incorporation of technological advances in a conventional standalone programming apparatus. As such, this reduces the need to continually upgrade the operating system, print options, database management systems, connectivity requirements, etc. with respect to the integrated programming apparatus according to the present invention. This is unlike a standalone programmer apparatus which would require such continual upgrades. Further, data is available for input directly into a patient's record in the medicalinformation management system 13 without the need for transfer of such data from a standalone programmer which conventionally in some circumstances needed to be done manually with possibility of error. - Generally, the[0058]
module interface apparatus 15 shown in FIG. 3 includes at least transmitter/receiver circuitry 123 and aninterface antenna 131 for transmitting and receiving electromagnetic energy. Themodule interface apparatus 15 includes receiver circuitry that is compatible with the transmitter of the implantablemedical device 12 and operable for receiving and extracting, e.g., demodulating, data from the transmitted signal from the implantablemedical device 12. Further, themodule interface apparatus 15 includes transmitter circuitry that is compatible with the receiver of the implantable medical device112 and operable for generating a modulated signal of which the receiver of the implantable medical device112 is capable of receiving and extracting e.g., demodulating, information therefrom. - Generally, the implantable medical[0059]
device communication system 9 may be used for communication of information to the implantablemedical device 12 or from the implantablemedical device 12. In the case of providing programming commands to the implantablemedical device 12, data representative of programming commands is received at themodule interface apparatus 15. The medicalinformation management system 13 includes the necessary operating system, user interface, database management, and any software necessary and compatible with the programming requirements of the implantablemedical device 12 to provide the appropriate programming commands to themodule interface apparatus 15. Themodule interface apparatus 15 includesinterface circuitry 128 to adapt the programming data for transmission by the transmitter/receiver circuitry 123 to the implantablemedical device 12. The data representative of the programming commands is adapted by theinterface circuitry 128 and provided to the interface transmitter of the receiver/transmitter circuitry 123. - The adaptation of the data representative of the programming commands by the[0060]
interface circuitry 128 may include any number of operations, such as for example, amplification, processing, conditioning, parallel to serial conversion, data buffering, error detection and correction, data encryption/encoding, data compression, telemetry link management and channel management, operational status (e.g., module status lights, warnings indicators, etc.) and any other operation necessary as determined by the type of telemetry used between the transmitter of themodule interface apparatus 15 and the receiver of the implantablemedical device 12. The adapted data representative of the programming commands is then transmitted via theinterface antenna 131 of themodule interface apparatus 15 to the implantablemedical device 12 where it is received at thedevice antenna 130 and detected by device receiver/transmitter circuitry 121. Likewise, device data may be provided by the implantablemedical device 12 to the medicalinformation management system 13 through themodule interface apparatus 15. Device data may include any type of data provided by an implantable medical device including real-time and stored data, such as diagnostic data (e.g., data for patient diagnosis or implanted device function diagnosis), physiological parameter data (e.g., oxygen sensed levels, activity sensor data, ECG waveforms and data, etc.), operational data (e.g., lead impedance data, battery voltage data, etc., and analyzed data (e.g., variability plot data, trend data, etc.). Data is transmitted by the device transmitter of transmitter/receiver circuitry 121 via thedevice antenna 130. The electromagnetic energy is received at theinterface antenna 131 of themodule interface apparatus 15 and a received signal is provided to the receiver of the receiver/transmitter circuitry 123 for detection and extraction of transmitted data from the signal. Upon extraction of the data by the interface receiver of the transmitter/receiver circuitry 123, theinterface circuitry 128 of themodule interface apparatus 15 adapts the data for provision to the medicalinformation management system 13. For example, such adaptation may include amplification, conditioning, processing, serial to parallel conversion of the stream of data, data management/formatting, data compression, data encryption, report generation, data buffering, error detection/correction, or any other any other operation necessary as determined by the type of telemetry used between the receiver of themodule interface apparatus 15 and the transmitter of the implantablemedical device 12. The adapted data representative of the device data received via theinterface antenna 131 is then provided to the medicalinformation management system 13. - Further, more extensive processing may be performed in the[0061]
module interface apparatus 15 upon operation ofoptional processing circuitry 133. For example, theprocessing circuitry 133 may act upon the device data received from the implantablemedical device 12 to provide a sampled amount of data, to provide data representative of a physiological parameter based upon multiple sensor outputs signals, to provide an alarm status upon operation of detection circuitry for detecting a warning event, and to provide operational status indication (e.g., status lights to indicate proper telemetry function, programming confirmation, etc.) The adapted data is then provided to the medicalinformation management system 13. - The communication of data from the[0062]
module interface apparatus 15 to the medicalinformation management system 13 may be accomplished in a number of manners. For example, in conjunction with the use of a plug-in module such as used in the HP VueLink Interface for the HP CMS and CTS equipment, the transfer of data between themodule interface apparatus 15 and the medicalinformation management system 13 may be accomplished by a direct electrical line, e.g., Local Area Network (LAN) or Wide Area Network (WAN). However, various other methods of transport may be used as well, such as RF transmission, or any other wireless technologies, modem technologies, infrared technologies, optical technologies, etc. - In its simplest form for the transmission of programming information to the implantable[0063]
medical device 12, themodule interface apparatus 15 includes aninterface antenna 131 for transmitting electromagnetic waves to the device antenna of the implantable medical device and an interface transmitter that is compatible with the device receiver of the implantablemedical device 12. In other words, for example, the modulation and demodulation techniques and operating frequencies of the corresponding device receiver and module interface transmitter are compatible. Likewise, for transmission from the implantablemedical device 12 to themodule interface apparatus 15, the device transmitter of receiver/transmitter circuitry 121 is compatible with the interface receiver of receiver/transmitter circuitry 123 of themodule interface apparatus 15. In other words, for example, the modulation and demodulation techniques and operating frequencies of the corresponding device transmitter and the interface module receiver are compatible. - Two illustrative embodiments of the[0064]
module interface apparatus 15 are illustrated in FIGS. 4 and 5. However, various systems for performing telemetry between the implantablemedical device 12 andmodule interface apparatus 15 may be used and are known. For example, several of such systems are described in U.S. Pat. No. 5,127,404 issued to Wyborney et al.; U.S. Pat. No. 4,556,063 issued to Thompson et al.; and U.S. Pat. No. 5,342,408 issued to De Coriolis et al. - As shown in FIG. 4,[0065]
module interface apparatus 120 includes aninterface module 125 including a module housing which containstransceiver circuitry 122 and any other circuitry such as previously described herein. Themodule interface apparatus 120 further includes an antenna positioned within aprogrammer head 124 connected to thetransceiver circuitry 122 via astretchable cable 126 and is powered bysource 140. Further,data 148 is communicated to the medicalinformation management network 13. Wireless communication and/or any other electrical connection may be possible between the programmer head and theinterface module 121. Theprogrammer head 124 containing the antenna may then be positioned at the implant site relative todevice antenna 130 and the implantablemedical device 12 which contains thetransceiver circuitry 121. Such an interface may be used in conjunction with telemetry according to U.S. Pat. No. 5,527,348; a low frequency (175 kHz), near field (34 inches), and low data rate (4-50 k baud) telemetry system. - FIG. 5 provides an alternate[0066]
module interface apparatus 150. Themodule interface apparatus 150 includesinterface module 151 which has a module housing to contain thetransceiver circuitry 152 and any other circuitry of theapparatus 150 such as described previously herein. Power is applied bypower source 154 anddata 156 is communicated to the medicalinformation management system 13. Thetransceiver circuitry 152 is connected to one ormore antennas 158, represented byantenna elements device antenna 130 and the implantablemedical device 12 which contains thetransceiver circuitry 121. In this case, a programmer head is unnecessary and telemetry is generally accomplished using a system such as described in U.S. Pat. No. 5,683,432; a high frequency (400 MHz), far field (to 30 feet), and high data rate (to 100 k baud) telemetry system. - All patents and references cited herein are incorporated in their entirety as if each were incorporated separately. This invention has been described with reference to illustrative embodiments and is not meant to be construed in a limiting sense. As described previously, one skilled in the art will recognize that various other telemetry techniques for providing communication between an implantable medical device and module interface apparatus may be used in addition to those previously described herein. Further, various modifications of the illustrative embodiments, as well as additional embodiments of the invention, will be apparent to a person skilled in the art upon reference to this description.[0067]
Claims (59)
1. An implantable medical device communication system, the system comprising:
an implantable medical device, wherein the implantable medical device includes at least one of a device transmitter and device receiver coupled to a device antenna;
a medical information management system, wherein the medical information management system includes at least a computer processing unit and a display unit; and
at least one module interface apparatus, wherein the at least one module interface apparatus includes:
at least one of an interface receiver and an interface transmitter coupled to an interface antenna to communicate with the at least one of the device transmitter and the device receiver via the device antenna, and
interface circuitry operable to at least one of adapt data received from the medical information management system for provision to the interface transmitter to communicate such information to the implantable medical device and adapt data received from the interface receiver to communicate such information to the medical information management system.
2. The system ofclaim 1 , wherein the at least one module interface apparatus includes:
a module housing, wherein the interface circuitry and the at least one of the interface receiver and the interface transmitter are enclosed within the module housing;
a programming head containing the interface antenna; and
a cable electrically connecting the programming head to the at least one module housing.
3. The system ofclaim 1 , wherein the at least one module interface apparatus includes a module housing, wherein the interface circuitry and the at least one of the interface receiver and the interface transmitter are enclosed within the module housing, and further wherein the interface antenna includes one or more antennas external to the module housing.
4. The system ofclaim 1 , wherein the interface circuitry includes processing circuitry to receive and process information from the interface receiver received from the implantable medical device representative of device data for communication of such processed information to the medical information management system.
5. The system ofclaim 1 , wherein the at least one module interface apparatus comprises a plurality of interface modules, wherein each interface module is operable for use in communication of information between an external device corresponding to the interface module and the medical information management system, and further wherein at least one interface module of the plurality of interface modules is operable for use in communication of information between the implantable medical device and the medical information management system.
6. The system ofclaim 1 , wherein the medical information management system includes a battery operable computer processing unit.
7. The system ofclaim 6 , wherein the battery operable computer processing unit is associated with a user interface to allow a user to provide programming commands to the at least one module interface apparatus for communication to the implantable medical device.
8. The system ofclaim 6 , wherein the battery operable computer processing unit receives device data from the at least one module interface apparatus.
9. The system ofclaim 1 , wherein the medical information management system includes a computer network.
10. The system ofclaim 9 , wherein the computer network includes a computer subsystem including the computer processing unit having an associated user interface to allow a user to provide programming commands to the at least one module interface apparatus for communication to the implantable medical device.
11. The system ofclaim 9 , wherein the computer network receives device data from the at least one interface module.
12. The system ofclaim 1 , wherein the implantable medical device includes an implanted medical device selected from one of a pacemaker, a defibrillator, a pacemaker/cardioverter/defibrillator, a brain stimulator, a cardioverter/defibrillator, a neurostimulator, a muscle stimulator, a gastric stimulator, an implantable monitor, a hemodynamic monitor, and a drug pump.
13. A programming system for use in programming implantable medical devices, the system comprising:
an implantable medical device, wherein the implantable medical device includes at least a device receiver coupled to a device antenna; and
a medical information management system, wherein the medical information management system includes a computer network, at least one computer subsystem having a user interface associated with a display operable on the computer network, and a plurality of interface modules, wherein each of the interface modules is operable for use in communication of information between an external device corresponding to the interface module and the at least one computer subsystem, and further wherein at least one interface module of the plurality of interface modules is operable for use in communication of programming commands from the medical information management system to the implantable medical device.
14. The system ofclaim 13 , wherein the at least one interface module includes an interface transmitter coupled to an interface antenna to communicate with the device receiver via the device antenna.
15. The system ofclaim 14 , wherein the at least one interface module further includes interface circuitry operable to adapt data received from the medical information management system for provision to the interface transmitter to communicate programming commands to the implantable medical device.
16. The system ofclaim 14 , wherein the at least one interface module includes a module housing, wherein the interface transmitter is enclosed within the module housing and is operably associated with the interface antenna contained within a programming head, and further wherein a cable electrically connects the programming head to the interface transmitter.
17. The system ofclaim 14 , wherein the at least one interface module includes a module housing, wherein the interface transmitter is enclosed within the module housing and is operably associated with the interface antenna positioned external to the module housing.
18. The system ofclaim 13 , wherein the user interface allows a user to provide the programming commands to the at least one interface module for communication to the implantable medical device.
19. The system ofclaim 13 , wherein the implantable medical device includes an implanted medical device selected from one of a pacemaker, a defibrillator, a pacemaker/cardioverter/defibrillator, a brain stimulator, a cardioverter/defibrillator, a neurostimulator, a muscle stimulator, a gastric stimulator, an implantable monitor, a hemodynamic monitor, and a drug pump.
20. A system for uplink of data from implanted medical devices, the system comprising:
an implantable medical device, wherein the implantable medical device includes at least a device transmitter coupled to a device antenna; and
a medical information management system, wherein the medical information management system includes a computer network, at least one computer subsystem having a user interface associated with a display operable on the computer network, and a plurality of interface modules, wherein each of the interface modules is operable for use in communication of information between an external device corresponding to the interface module and the computer network, and further wherein at least one interface module of the plurality of interface modules is operable for use in communication of device data from the implantable medical device to the medical information management system.
21. The system ofclaim 20 , wherein the at least one interface module includes an interface receiver coupled to an interface antenna to communicate with the device transmitter via the device antenna.
22. The system ofclaim 21 , wherein the at least one interface module further includes interface circuitry operable to adapt device data received from the interface receiver to communicate such device data to the medical information management system.
23. The system ofclaim 22 , wherein the at least one interface module includes a module housing, wherein the interface receiver and interface circuitry is enclosed within the module housing and is operably associated with the interface antenna contained within a programming head, and further wherein a cable electrically connects the programming head to the interface receiver.
24. The system ofclaim 22 , wherein the at least one interface module includes a module, wherein the interface receiver and interface circuitry is enclosed within the module housing and is operably associated with the interface antenna positioned external to the module housing.
25. The system ofclaim 22 , wherein the interface circuitry includes processing circuitry to receive and process device data from the interface receiver received from the device transmitter of the implantable medical device.
26. The system ofclaim 25 , wherein the device data is one of diagnostic data, physiological parameter data, operational data, and analyzed data.
27. The system ofclaim 22 , wherein the interface circuitry includes at least a driver operable for communication of information to the medical information management system.
28. The system ofclaim 20 , wherein the implantable medical device includes an implanted medical device selected from one of a pacemaker, a defibrillator, a pacemaker/cardioverter/defibrillator, a brain stimulator, a cardioverter/defibrillator, a neurostimulator, a muscle stimulator, a gastric stimulator, an implantable monitor, a hemodynamic monitor, and a drug pump.
29. An interface apparatus for use in facilitating communication between an implantable medical device and a medical information management system, wherein the medical information management system includes at least a computer processing unit and a display unit, wherein the implantable medical device includes at least one of a device transmitter and device receiver coupled to a device antenna, the interface apparatus comprising:
an interface module, wherein the interface module includes:
at least one of an interface receiver and an interface transmitter coupled to an interface antenna operable to communicate with the at least one of the device transmitter and the device receiver via the device antenna, and
interface circuitry operable to at least one of adapt data received from the medical information management system for provision to the interface transmitter to communicate such information to the implantable medical device and adapt data received from the interface receiver to communicate such information to the medical information management system.
30. The interface apparatus ofclaim 29 , wherein the interface module includes:
a module housing, wherein the interface circuitry and the at least one of the interface receiver and the interface transmitter are enclosed within the module housing;
a programming head containing the interface antenna; and
a cable electrically connecting the programming head to the interface module.
31. The interface apparatus ofclaim 29 , wherein the medical information management system includes a plurality of plug-in slots for receiving a plurality of interface modules corresponding to a plurality of external devices, wherein a module housing enclosing the interface circuitry and the at least one of the interface receiver and the interface transmitter is configured for insertion in one of the plurality of plug-in slots of the medical information management system.
32. The interface apparatus ofclaim 29 , wherein the interface module includes a module housing, wherein the interface circuitry and the at least one of the interface receiver and the interface transmitter are enclosed within the module housing, and further wherein the interface antenna is external to the module housing.
33. The interface apparatus ofclaim 29 , wherein the interface circuitry includes processing circuitry to receive and process information from the interface receiver received from the implantable medical device representative of device data for communication of such processed information to the medical information management system.
34. The interface apparatus ofclaim 29 , wherein the medical information management system includes a user interface to allow a user to provide programming commands to the interface module for communication to the implantable medical device.
35. The interface apparatus ofclaim 29 , wherein the medical information management network receives device data from the interface module.
36. A programming system for use in programming implantable medical devices, the system comprising:
an implantable medical device, wherein the implantable medical device includes at least a device receiver coupled to a device antenna; and
a battery operable computer system having a user interface associated with a display unit and a plurality of interface modules connected thereto, wherein each of the interface modules is operable for use in communication of information between an external device corresponding to the interface module and the computer system, and further wherein at least one interface module of the plurality of interface modules is operable for use in providing programming information received from the computer system to the implantable medical device.
37. The system ofclaim 36 , wherein the at least one interface module includes an interface transmitter coupled to an interface antenna to communicate with the device receiver via the device antenna.
38. The system ofclaim 36 , wherein the at least one interface module further includes interface circuitry operable to adapt data received from the medical information management system for provision to the interface transmitter to communicate such information to the implantable medical device.
39. The system ofclaim 36 , wherein the at least one interface module includes a module housing, wherein the interface transmitter is enclosed within the module housing and is operably associated with the interface antenna contained within a programming head, and further wherein a cable electrically connects the programming head to the at least one interface module.
40. The system ofclaim 36 , wherein the at least one interface module includes a module housing, wherein the interface transmitter is enclosed within the module housing and is operably associated with the interface antenna positioned external to the module housing.
41. The system ofclaim 36 , wherein the implantable medical device includes an implanted medical device selected from one of a pacemaker, a defibrillator, a pacemaker/cardioverter/defibrillator, a brain stimulator, a cardioverter/defibrillator, a neurostimulator, a muscle stimulator, a gastric stimulator, an implantable monitor, a hemodynamic monitor, and a drug pump.
42. A system for uplink of information from implanted medical devices, the system comprising:
an implantable medical device, wherein the implantable medical device includes at least a device transmitter coupled to a device antenna; and
a battery operable computer system and a plurality of interface modules connected thereto, wherein each of the interface modules is operable for use in communication of information between an external device corresponding to the interface module and the computer system, and further wherein at least one interface module of the plurality of interface modules is operable for use in providing device data received from the implantable medical device to the computer system.
43. The system ofclaim 42 , wherein the at least one interface module includes an interface receiver coupled to an interface antenna to communicate with the device transmitter via the device antenna.
44. The system ofclaim 43 , wherein the at least one interface module further includes interface circuitry operable to adapt device data received from the interface receiver to communicate such device data to the battery operable computer system.
45. The system ofclaim 44 , wherein the at least one interface module includes a module housing, wherein the interface circuitry and the interface receiver are enclosed within the module housing and is operably associated with the interface antenna contained within a programming head, and further wherein a cable electrically connects the programming head to the at least one interface module.
46. The system ofclaim 44 , wherein the at least one interface module includes a module housing, wherein the interface receiver is enclosed within the module housing and is operably associated with the interface antenna positioned external to the module housing.
47. The system ofclaim 42 , wherein the device data is one of diagnostic data, physiological parameter data, operational data, and analyzed data.
48. The system ofclaim 42 , wherein the implantable medical device includes an implanted medical device selected from one of a pacemaker, a defibrillator, a pacemaker/cardioverter/defibrillator, a brain stimulator, a cardioverter/defibrillator, a neurostimulator, a muscle stimulator, a gastric stimulator, an implantable monitor, a hemodynamic monitor, and a drug pump.
49. An implantable medical device programming method, the method comprising steps of:
providing an implantable medical device, wherein the implantable medical device includes at least a device receiver coupled to a device antenna;
providing a medical information management system, wherein the medical information management system includes at least a processing unit and a display unit;
providing an interface module, wherein the at least one interface module includes at least an interface transmitter operable with the device receiver;
receiving data representative of programming commands at the interface module from the computer system;
adapting the data representative of programming commands for transmission to the implantable medical device; and
transmitting the adapted data by the interface transmitter via the interface antenna to the device antenna of the implantable medical device.
50. The method ofclaim 49 , wherein the medical information management system comprises a plurality of interface modules, wherein each interface module is operable for use in interfacing to an external device.
51. The method ofclaim 49 , wherein the medical information management system includes a battery operable processing unit.
52. The method ofclaim 49 , wherein the medical information management system includes a computer network, wherein the at least one processing unit is connected thereon.
53. The method ofclaim 49 , wherein the implantable medical device includes an implanted medical device selected from one of a pacemaker, a defibrillator, a pacemaker/cardioverter/defibrillator, a brain stimulator, a cardioverter/defibrillator, a neurostimulator, a muscle stimulator, a gastric stimulator, an implantable monitor, a hemodynamic monitor, and a drug pump.
54. An implantable medical device uplink communication method, the method comprising steps of:
providing an implantable medical device, wherein the implantable medical device includes at least a device transmitter coupled to a device antenna;
providing a medical information management system, wherein the medical management system includes at least a processing unit and a display unit;
providing an interface module, wherein the at least one interface module includes at least an interface receiver operable with the device transmitter; and
receiving device data at the interface module from the device transmitter of the implantable medical device via the interface antenna;
adapting the device data for provision to the medical
information management system; and
providing the adapted device data to the medical information management system.
55. The method ofclaim 54 , further comprising a step of processing data received by the interface receiver from the implantable medical device.
56. The method ofclaim 54 , wherein the medical information management system includes a battery operable processing unit.
57. The method ofclaim 54 , wherein the medical information management system includes a computer network, wherein the is processing unit is connected thereon.
58. The method ofclaim 54 , wherein the device data from the implantable medical device is one of physiological parameter data, diagnostic data, operational data, and analyzed data.
59. The method ofclaim 54 , wherein the implantable medical device includes an implanted medical device selected from one of a pacemaker, a defibrillator, a pacemaker/cardioverter/defibrillator, a brain stimulator, a cardioverter/defibrillator, a neurostimulator, a muscle stimulator, a gastric stimulator, an implantable monitor, a hemodynamic monitor, and a drug pump.
Priority Applications (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US10/251,509US20030028226A1 (en) | 1998-06-19 | 2002-09-20 | Medical management system integrated programming apparatus for communication with an implantable medical device |
Applications Claiming Priority (3)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US8986898P | 1998-06-19 | 1998-06-19 | |
| US09/322,560US6477424B1 (en) | 1998-06-19 | 1999-05-28 | Medical management system integrated programming apparatus for communication with an implantable medical device |
| US10/251,509US20030028226A1 (en) | 1998-06-19 | 2002-09-20 | Medical management system integrated programming apparatus for communication with an implantable medical device |
Related Parent Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US09/322,560DivisionUS6477424B1 (en) | 1998-06-19 | 1999-05-28 | Medical management system integrated programming apparatus for communication with an implantable medical device |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| US20030028226A1true US20030028226A1 (en) | 2003-02-06 |
Family
ID=26781021
Family Applications (2)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US09/322,560Expired - LifetimeUS6477424B1 (en) | 1998-06-19 | 1999-05-28 | Medical management system integrated programming apparatus for communication with an implantable medical device |
| US10/251,509AbandonedUS20030028226A1 (en) | 1998-06-19 | 2002-09-20 | Medical management system integrated programming apparatus for communication with an implantable medical device |
Family Applications Before (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US09/322,560Expired - LifetimeUS6477424B1 (en) | 1998-06-19 | 1999-05-28 | Medical management system integrated programming apparatus for communication with an implantable medical device |
Country Status (3)
| Country | Link |
|---|---|
| US (2) | US6477424B1 (en) |
| DE (1) | DE19927853B8 (en) |
| FR (1) | FR2780224B1 (en) |
Cited By (94)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US20050273170A1 (en)* | 2004-06-08 | 2005-12-08 | Navarro Richard R | Prosthetic intervertebral spinal disc with integral microprocessor |
| US7047076B1 (en) | 2001-08-03 | 2006-05-16 | Cardiac Pacemakers, Inc. | Inverted-F antenna configuration for an implantable medical device |
| US20060276857A1 (en)* | 2003-10-02 | 2006-12-07 | Medtronic, Inc. | Medical device programmer with infrared communication |
| WO2007058826A2 (en) | 2005-11-14 | 2007-05-24 | Edwards Lifesciences Corporation | Wireless communication system for pressure monitoring |
| US20070233192A1 (en)* | 2006-03-29 | 2007-10-04 | Catholic Healthcare West (D/B/A St. Joseph's Hospital And Medical Center) | Vagus nerve stimulation method |
| US20070255351A1 (en)* | 2006-04-28 | 2007-11-01 | Cyberonics, Inc. | Threshold optimization for tissue stimulation therapy |
| US20070288068A1 (en)* | 2003-10-02 | 2007-12-13 | Medtronic, Inc. | Medical device programmer with selective disablement of display during telemetry |
| US20080269839A1 (en)* | 2007-04-27 | 2008-10-30 | Armstrong Randolph K | Dosing Limitation for an Implantable Medical Device |
| US20080306360A1 (en)* | 2007-05-24 | 2008-12-11 | Robertson Timothy L | Low profile antenna for in body device |
| US20090076338A1 (en)* | 2006-05-02 | 2009-03-19 | Zdeblick Mark J | Patient customized therapeutic regimens |
| US20090082645A1 (en)* | 2007-09-25 | 2009-03-26 | Proteus Biomedical, Inc. | In-body device with virtual dipole signal amplification |
| US20090119067A1 (en)* | 2007-11-05 | 2009-05-07 | Wolfgang Stempfer | System and method to minimize downtimes of medical apparatuses |
| US20090135886A1 (en)* | 2007-11-27 | 2009-05-28 | Proteus Biomedical, Inc. | Transbody communication systems employing communication channels |
| US20090227204A1 (en)* | 2005-04-28 | 2009-09-10 | Timothy Robertson | Pharma-Informatics System |
| US20100022836A1 (en)* | 2007-03-09 | 2010-01-28 | Olivier Colliou | In-body device having a multi-directional transmitter |
| US7751894B1 (en) | 2004-03-04 | 2010-07-06 | Cardiac Pacemakers, Inc. | Systems and methods for indicating aberrant behavior detected by an implanted medical device |
| US20100185055A1 (en)* | 2007-02-01 | 2010-07-22 | Timothy Robertson | Ingestible event marker systems |
| US20100316158A1 (en)* | 2006-11-20 | 2010-12-16 | Lawrence Arne | Active signal processing personal health signal receivers |
| US7869867B2 (en) | 2006-10-27 | 2011-01-11 | Cyberonics, Inc. | Implantable neurostimulator with refractory stimulation |
| US20110054265A1 (en)* | 2009-04-28 | 2011-03-03 | Hooman Hafezi | Highly reliable ingestible event markers and methods for using the same |
| US20110065983A1 (en)* | 2008-08-13 | 2011-03-17 | Hooman Hafezi | Ingestible Circuitry |
| US20110196454A1 (en)* | 2008-11-18 | 2011-08-11 | Proteus Biomedical, Inc. | Sensing system, device, and method for therapy modulation |
| US20110212782A1 (en)* | 2008-10-14 | 2011-09-01 | Andrew Thompson | Method and System for Incorporating Physiologic Data in a Gaming Environment |
| US8036748B2 (en) | 2008-11-13 | 2011-10-11 | Proteus Biomedical, Inc. | Ingestible therapy activator system and method |
| US8055334B2 (en) | 2008-12-11 | 2011-11-08 | Proteus Biomedical, Inc. | Evaluation of gastrointestinal function using portable electroviscerography systems and methods of using the same |
| US8054140B2 (en) | 2006-10-17 | 2011-11-08 | Proteus Biomedical, Inc. | Low voltage oscillator for medical devices |
| US8114021B2 (en) | 2008-12-15 | 2012-02-14 | Proteus Biomedical, Inc. | Body-associated receiver and method |
| US8258962B2 (en) | 2008-03-05 | 2012-09-04 | Proteus Biomedical, Inc. | Multi-mode communication ingestible event markers and systems, and methods of using the same |
| US20120296397A1 (en)* | 2011-05-21 | 2012-11-22 | Boston Scientific Neuromodulation Corporation | System and method for programming neurostimulation devices using cached plug-in software drivers |
| US8540664B2 (en) | 2009-03-25 | 2013-09-24 | Proteus Digital Health, Inc. | Probablistic pharmacokinetic and pharmacodynamic modeling |
| US8547248B2 (en) | 2005-09-01 | 2013-10-01 | Proteus Digital Health, Inc. | Implantable zero-wire communications system |
| US8558563B2 (en) | 2009-08-21 | 2013-10-15 | Proteus Digital Health, Inc. | Apparatus and method for measuring biochemical parameters |
| US8565867B2 (en) | 2005-01-28 | 2013-10-22 | Cyberonics, Inc. | Changeable electrode polarity stimulation by an implantable medical device |
| US8597186B2 (en) | 2009-01-06 | 2013-12-03 | Proteus Digital Health, Inc. | Pharmaceutical dosages delivery system |
| US8730031B2 (en) | 2005-04-28 | 2014-05-20 | Proteus Digital Health, Inc. | Communication system using an implantable device |
| US20140188192A1 (en)* | 2004-06-10 | 2014-07-03 | Medtronic Urinary Solutions, Inc. | Systems and methods for clinician control of stimulation systems |
| US20140185107A1 (en)* | 2012-12-30 | 2014-07-03 | Shenyang Neusoft Medical Systems Co., Ltd. | Method and device for indicating scanning condition and scanning apparatus and scanning system related thereto |
| US8784308B2 (en) | 2009-12-02 | 2014-07-22 | Proteus Digital Health, Inc. | Integrated ingestible event marker system with pharmaceutical product |
| US8802183B2 (en) | 2005-04-28 | 2014-08-12 | Proteus Digital Health, Inc. | Communication system with enhanced partial power source and method of manufacturing same |
| US8836513B2 (en) | 2006-04-28 | 2014-09-16 | Proteus Digital Health, Inc. | Communication system incorporated in an ingestible product |
| WO2014144727A1 (en)* | 2008-07-08 | 2014-09-18 | Proteus Digital Health, Inc. | State characterization based on multi-variate data fusion techniques |
| US8868453B2 (en) | 2009-11-04 | 2014-10-21 | Proteus Digital Health, Inc. | System for supply chain management |
| US8912908B2 (en) | 2005-04-28 | 2014-12-16 | Proteus Digital Health, Inc. | Communication system with remote activation |
| US8945005B2 (en) | 2006-10-25 | 2015-02-03 | Proteus Digital Health, Inc. | Controlled activation ingestible identifier |
| US8956288B2 (en) | 2007-02-14 | 2015-02-17 | Proteus Digital Health, Inc. | In-body power source having high surface area electrode |
| US9014779B2 (en) | 2010-02-01 | 2015-04-21 | Proteus Digital Health, Inc. | Data gathering system |
| US9107806B2 (en) | 2010-11-22 | 2015-08-18 | Proteus Digital Health, Inc. | Ingestible device with pharmaceutical product |
| US9149423B2 (en) | 2009-05-12 | 2015-10-06 | Proteus Digital Health, Inc. | Ingestible event markers comprising an ingestible component |
| US9198608B2 (en) | 2005-04-28 | 2015-12-01 | Proteus Digital Health, Inc. | Communication system incorporated in a container |
| US9235683B2 (en) | 2011-11-09 | 2016-01-12 | Proteus Digital Health, Inc. | Apparatus, system, and method for managing adherence to a regimen |
| US9248299B2 (en) | 2003-10-02 | 2016-02-02 | Medtronic, Inc. | Medical device programmer |
| US9270503B2 (en) | 2013-09-20 | 2016-02-23 | Proteus Digital Health, Inc. | Methods, devices and systems for receiving and decoding a signal in the presence of noise using slices and warping |
| US9268909B2 (en) | 2012-10-18 | 2016-02-23 | Proteus Digital Health, Inc. | Apparatus, system, and method to adaptively optimize power dissipation and broadcast power in a power source for a communication device |
| US9270025B2 (en) | 2007-03-09 | 2016-02-23 | Proteus Digital Health, Inc. | In-body device having deployable antenna |
| US9271897B2 (en) | 2012-07-23 | 2016-03-01 | Proteus Digital Health, Inc. | Techniques for manufacturing ingestible event markers comprising an ingestible component |
| US9314633B2 (en) | 2008-01-25 | 2016-04-19 | Cyberonics, Inc. | Contingent cardio-protection for epilepsy patients |
| US9345879B2 (en) | 2006-10-09 | 2016-05-24 | Endostim, Inc. | Device and implantation system for electrical stimulation of biological systems |
| US9381344B2 (en) | 2010-03-05 | 2016-07-05 | Endostim, Inc. | Systems and methods for treating gastroesophageal reflux disease |
| US9439599B2 (en) | 2011-03-11 | 2016-09-13 | Proteus Digital Health, Inc. | Wearable personal body associated device with various physical configurations |
| US9439566B2 (en) | 2008-12-15 | 2016-09-13 | Proteus Digital Health, Inc. | Re-wearable wireless device |
| US9498619B2 (en) | 2013-02-26 | 2016-11-22 | Endostim, Inc. | Implantable electrical stimulation leads |
| US9577864B2 (en) | 2013-09-24 | 2017-02-21 | Proteus Digital Health, Inc. | Method and apparatus for use with received electromagnetic signal at a frequency not known exactly in advance |
| US9597487B2 (en) | 2010-04-07 | 2017-03-21 | Proteus Digital Health, Inc. | Miniature ingestible device |
| US9616225B2 (en) | 2006-05-18 | 2017-04-11 | Endostim, Inc. | Device and implantation system for electrical stimulation of biological systems |
| US9623238B2 (en) | 2012-08-23 | 2017-04-18 | Endostim, Inc. | Device and implantation system for electrical stimulation of biological systems |
| US9659423B2 (en) | 2008-12-15 | 2017-05-23 | Proteus Digital Health, Inc. | Personal authentication apparatus system and method |
| US9682234B2 (en) | 2014-11-17 | 2017-06-20 | Endostim, Inc. | Implantable electro-medical device programmable for improved operational life |
| US9724510B2 (en) | 2006-10-09 | 2017-08-08 | Endostim, Inc. | System and methods for electrical stimulation of biological systems |
| US9756874B2 (en) | 2011-07-11 | 2017-09-12 | Proteus Digital Health, Inc. | Masticable ingestible product and communication system therefor |
| US9789309B2 (en) | 2010-03-05 | 2017-10-17 | Endostim, Inc. | Device and implantation system for electrical stimulation of biological systems |
| US9796576B2 (en) | 2013-08-30 | 2017-10-24 | Proteus Digital Health, Inc. | Container with electronically controlled interlock |
| US9827425B2 (en) | 2013-09-03 | 2017-11-28 | Endostim, Inc. | Methods and systems of electrode polarity switching in electrical stimulation therapy |
| US9883819B2 (en) | 2009-01-06 | 2018-02-06 | Proteus Digital Health, Inc. | Ingestion-related biofeedback and personalized medical therapy method and system |
| CN107823794A (en)* | 2017-12-01 | 2018-03-23 | 苏州景昱医疗器械有限公司 | The program control transponder of touch screen pen type, program control device and Implanted medical system |
| US9925367B2 (en) | 2011-09-02 | 2018-03-27 | Endostim, Inc. | Laparoscopic lead implantation method |
| JPWO2017130646A1 (en)* | 2016-01-27 | 2018-07-05 | シャープ株式会社 | Biological signal processing device |
| US10084880B2 (en) | 2013-11-04 | 2018-09-25 | Proteus Digital Health, Inc. | Social media networking based on physiologic information |
| US10175376B2 (en) | 2013-03-15 | 2019-01-08 | Proteus Digital Health, Inc. | Metal detector apparatus, system, and method |
| US10187121B2 (en) | 2016-07-22 | 2019-01-22 | Proteus Digital Health, Inc. | Electromagnetic sensing and detection of ingestible event markers |
| US10223905B2 (en) | 2011-07-21 | 2019-03-05 | Proteus Digital Health, Inc. | Mobile device and system for detection and communication of information received from an ingestible device |
| US10398161B2 (en) | 2014-01-21 | 2019-09-03 | Proteus Digital Heal Th, Inc. | Masticable ingestible product and communication system therefor |
| US10426955B2 (en) | 2006-10-09 | 2019-10-01 | Endostim, Inc. | Methods for implanting electrodes and treating a patient with gastreosophageal reflux disease |
| US10529044B2 (en) | 2010-05-19 | 2020-01-07 | Proteus Digital Health, Inc. | Tracking and delivery confirmation of pharmaceutical products |
| US11051543B2 (en) | 2015-07-21 | 2021-07-06 | Otsuka Pharmaceutical Co. Ltd. | Alginate on adhesive bilayer laminate film |
| US11149123B2 (en) | 2013-01-29 | 2021-10-19 | Otsuka Pharmaceutical Co., Ltd. | Highly-swellable polymeric films and compositions comprising the same |
| US11158149B2 (en) | 2013-03-15 | 2021-10-26 | Otsuka Pharmaceutical Co., Ltd. | Personal authentication apparatus system and method |
| US11529071B2 (en) | 2016-10-26 | 2022-12-20 | Otsuka Pharmaceutical Co., Ltd. | Methods for manufacturing capsules with ingestible event markers |
| US11577077B2 (en) | 2006-10-09 | 2023-02-14 | Endostim, Inc. | Systems and methods for electrical stimulation of biological systems |
| US11672934B2 (en) | 2020-05-12 | 2023-06-13 | Covidien Lp | Remote ventilator adjustment |
| US11717681B2 (en) | 2010-03-05 | 2023-08-08 | Endostim, Inc. | Systems and methods for treating gastroesophageal reflux disease |
| US11744481B2 (en) | 2013-03-15 | 2023-09-05 | Otsuka Pharmaceutical Co., Ltd. | System, apparatus and methods for data collection and assessing outcomes |
| US11819683B2 (en) | 2016-11-17 | 2023-11-21 | Endostim, Inc. | Modular stimulation system for the treatment of gastrointestinal disorders |
| US12053626B2 (en) | 2017-04-06 | 2024-08-06 | Endostim, Inc. | Surface electrodes |
| US12343530B2 (en) | 2006-05-18 | 2025-07-01 | Paras Holdings, Llc | Device and implantation system for electrical stimulation of biological systems |
Families Citing this family (173)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US6325067B1 (en) | 1992-12-03 | 2001-12-04 | Wesley D. Sterman | Methods and systems for performing thoracoscopic coronary bypass and other procedures |
| US6036924A (en) | 1997-12-04 | 2000-03-14 | Hewlett-Packard Company | Cassette of lancet cartridges for sampling blood |
| US6391005B1 (en) | 1998-03-30 | 2002-05-21 | Agilent Technologies, Inc. | Apparatus and method for penetration with shaft having a sensor for sensing penetration depth |
| US6528856B1 (en)* | 1998-12-15 | 2003-03-04 | Intel Corporation | High dielectric constant metal oxide gate dielectrics |
| US6290646B1 (en) | 1999-04-16 | 2001-09-18 | Cardiocom | Apparatus and method for monitoring and communicating wellness parameters of ambulatory patients |
| US8419650B2 (en) | 1999-04-16 | 2013-04-16 | Cariocom, LLC | Downloadable datasets for a patient monitoring system |
| US6607485B2 (en) | 1999-06-03 | 2003-08-19 | Cardiac Intelligence Corporation | Computer readable storage medium containing code for automated collection and analysis of patient information retrieved from an implantable medical device for remote patient care |
| US6312378B1 (en)* | 1999-06-03 | 2001-11-06 | Cardiac Intelligence Corporation | System and method for automated collection and analysis of patient information retrieved from an implantable medical device for remote patient care |
| US7134996B2 (en) | 1999-06-03 | 2006-11-14 | Cardiac Intelligence Corporation | System and method for collection and analysis of patient information for automated remote patient care |
| US7429243B2 (en)* | 1999-06-03 | 2008-09-30 | Cardiac Intelligence Corporation | System and method for transacting an automated patient communications session |
| US6270457B1 (en)* | 1999-06-03 | 2001-08-07 | Cardiac Intelligence Corp. | System and method for automated collection and analysis of regularly retrieved patient information for remote patient care |
| US6221011B1 (en) | 1999-07-26 | 2001-04-24 | Cardiac Intelligence Corporation | System and method for determining a reference baseline of individual patient status for use in an automated collection and analysis patient care system |
| CA2314513A1 (en)* | 1999-07-26 | 2001-01-26 | Gust H. Bardy | System and method for providing normalized voice feedback from an individual patient in an automated collection and analysis patient care system |
| CA2314517A1 (en) | 1999-07-26 | 2001-01-26 | Gust H. Bardy | System and method for determining a reference baseline of individual patient status for use in an automated collection and analysis patient care system |
| US7260369B2 (en) | 2005-08-03 | 2007-08-21 | Kamilo Feher | Location finder, tracker, communication and remote control system |
| US6442433B1 (en)* | 1999-10-26 | 2002-08-27 | Medtronic, Inc. | Apparatus and method for remote troubleshooting, maintenance and upgrade of implantable device systems |
| US7039810B1 (en)* | 1999-11-02 | 2006-05-02 | Medtronic, Inc. | Method and apparatus to secure data transfer from medical device systems |
| US6411840B1 (en)* | 1999-11-16 | 2002-06-25 | Cardiac Intelligence Corporation | Automated collection and analysis patient care system and method for diagnosing and monitoring the outcomes of atrial fibrillation |
| US6368284B1 (en) | 1999-11-16 | 2002-04-09 | Cardiac Intelligence Corporation | Automated collection and analysis patient care system and method for diagnosing and monitoring myocardial ischemia and outcomes thereof |
| US6440066B1 (en)* | 1999-11-16 | 2002-08-27 | Cardiac Intelligence Corporation | Automated collection and analysis patient care system and method for ordering and prioritizing multiple health disorders to identify an index disorder |
| US6398728B1 (en) | 1999-11-16 | 2002-06-04 | Cardiac Intelligence Corporation | Automated collection and analysis patient care system and method for diagnosing and monitoring respiratory insufficiency and outcomes thereof |
| US8369937B2 (en) | 1999-11-16 | 2013-02-05 | Cardiac Pacemakers, Inc. | System and method for prioritizing medical conditions |
| US6336903B1 (en) | 1999-11-16 | 2002-01-08 | Cardiac Intelligence Corp. | Automated collection and analysis patient care system and method for diagnosing and monitoring congestive heart failure and outcomes thereof |
| US6790198B1 (en)* | 1999-12-01 | 2004-09-14 | B-Braun Medical, Inc. | Patient medication IV delivery pump with wireless communication to a hospital information management system |
| US7645258B2 (en) | 1999-12-01 | 2010-01-12 | B. Braun Medical, Inc. | Patient medication IV delivery pump with wireless communication to a hospital information management system |
| US20060189854A1 (en)* | 1999-12-17 | 2006-08-24 | Medtronic, Inc. | Method and apparatus for remotely programming implantable medical devices |
| US7060031B2 (en)* | 1999-12-17 | 2006-06-13 | Medtronic, Inc. | Method and apparatus for remotely programming implantable medical devices |
| DE19962915A1 (en)* | 1999-12-23 | 2001-09-06 | Intelligent Implants Gmbh | Device for the protected operation of neuroprostheses and method therefor |
| US6577899B2 (en) | 2000-01-21 | 2003-06-10 | Medtronic Minimed, Inc. | Microprocessor controlled ambulatory medical apparatus with hand held communication device |
| US7047553B1 (en)* | 2000-10-05 | 2006-05-16 | Arris International, Inc. | Method and apparatus for decreasing cable installation time and cable installation faults |
| US8641644B2 (en) | 2000-11-21 | 2014-02-04 | Sanofi-Aventis Deutschland Gmbh | Blood testing apparatus having a rotatable cartridge with multiple lancing elements and testing means |
| DE10057832C1 (en) | 2000-11-21 | 2002-02-21 | Hartmann Paul Ag | Blood analysis device has syringe mounted in casing, annular mounting carrying needles mounted behind test strip and being swiveled so that needle can be pushed through strip and aperture in casing to take blood sample |
| SE0004843D0 (en)* | 2000-12-22 | 2000-12-22 | St Jude Medical | Programming system for medical devices |
| AUPR247601A0 (en)* | 2001-01-10 | 2001-02-01 | Cochlear Limited | Cochlear implant communicator |
| EP1395185B1 (en) | 2001-06-12 | 2010-10-27 | Pelikan Technologies Inc. | Electric lancet actuator |
| US7041068B2 (en) | 2001-06-12 | 2006-05-09 | Pelikan Technologies, Inc. | Sampling module device and method |
| US9427532B2 (en) | 2001-06-12 | 2016-08-30 | Sanofi-Aventis Deutschland Gmbh | Tissue penetration device |
| JP4209767B2 (en) | 2001-06-12 | 2009-01-14 | ペリカン テクノロジーズ インコーポレイテッド | Self-optimized cutting instrument with adaptive means for temporary changes in skin properties |
| US9226699B2 (en) | 2002-04-19 | 2016-01-05 | Sanofi-Aventis Deutschland Gmbh | Body fluid sampling module with a continuous compression tissue interface surface |
| US7749174B2 (en) | 2001-06-12 | 2010-07-06 | Pelikan Technologies, Inc. | Method and apparatus for lancet launching device intergrated onto a blood-sampling cartridge |
| US9795747B2 (en) | 2010-06-02 | 2017-10-24 | Sanofi-Aventis Deutschland Gmbh | Methods and apparatus for lancet actuation |
| US7344507B2 (en) | 2002-04-19 | 2008-03-18 | Pelikan Technologies, Inc. | Method and apparatus for lancet actuation |
| US7981056B2 (en) | 2002-04-19 | 2011-07-19 | Pelikan Technologies, Inc. | Methods and apparatus for lancet actuation |
| US8337419B2 (en) | 2002-04-19 | 2012-12-25 | Sanofi-Aventis Deutschland Gmbh | Tissue penetration device |
| JP4272051B2 (en) | 2001-06-12 | 2009-06-03 | ペリカン テクノロジーズ インコーポレイテッド | Blood sampling apparatus and method |
| WO2002101359A2 (en) | 2001-06-12 | 2002-12-19 | Pelikan Technologies, Inc. | Integrated blood sampling analysis system with multi-use sampling module |
| AU2002344825A1 (en) | 2001-06-12 | 2002-12-23 | Pelikan Technologies, Inc. | Method and apparatus for improving success rate of blood yield from a fingerstick |
| US7344894B2 (en) | 2001-10-16 | 2008-03-18 | Agilent Technologies, Inc. | Thermal regulation of fluidic samples within a diagnostic cartridge |
| US7892183B2 (en) | 2002-04-19 | 2011-02-22 | Pelikan Technologies, Inc. | Method and apparatus for body fluid sampling and analyte sensing |
| US8221334B2 (en) | 2002-04-19 | 2012-07-17 | Sanofi-Aventis Deutschland Gmbh | Method and apparatus for penetrating tissue |
| US7909778B2 (en) | 2002-04-19 | 2011-03-22 | Pelikan Technologies, Inc. | Method and apparatus for penetrating tissue |
| US8702624B2 (en) | 2006-09-29 | 2014-04-22 | Sanofi-Aventis Deutschland Gmbh | Analyte measurement device with a single shot actuator |
| US7708701B2 (en) | 2002-04-19 | 2010-05-04 | Pelikan Technologies, Inc. | Method and apparatus for a multi-use body fluid sampling device |
| US9795334B2 (en) | 2002-04-19 | 2017-10-24 | Sanofi-Aventis Deutschland Gmbh | Method and apparatus for penetrating tissue |
| US7229458B2 (en) | 2002-04-19 | 2007-06-12 | Pelikan Technologies, Inc. | Method and apparatus for penetrating tissue |
| US8784335B2 (en) | 2002-04-19 | 2014-07-22 | Sanofi-Aventis Deutschland Gmbh | Body fluid sampling device with a capacitive sensor |
| US7648468B2 (en) | 2002-04-19 | 2010-01-19 | Pelikon Technologies, Inc. | Method and apparatus for penetrating tissue |
| US7481776B2 (en) | 2002-04-19 | 2009-01-27 | Pelikan Technologies, Inc. | Method and apparatus for penetrating tissue |
| US7297122B2 (en) | 2002-04-19 | 2007-11-20 | Pelikan Technologies, Inc. | Method and apparatus for penetrating tissue |
| US7491178B2 (en) | 2002-04-19 | 2009-02-17 | Pelikan Technologies, Inc. | Method and apparatus for penetrating tissue |
| US7291117B2 (en) | 2002-04-19 | 2007-11-06 | Pelikan Technologies, Inc. | Method and apparatus for penetrating tissue |
| US7374544B2 (en) | 2002-04-19 | 2008-05-20 | Pelikan Technologies, Inc. | Method and apparatus for penetrating tissue |
| US7976476B2 (en) | 2002-04-19 | 2011-07-12 | Pelikan Technologies, Inc. | Device and method for variable speed lancet |
| US7563232B2 (en) | 2002-04-19 | 2009-07-21 | Pelikan Technologies, Inc. | Method and apparatus for penetrating tissue |
| US7141058B2 (en) | 2002-04-19 | 2006-11-28 | Pelikan Technologies, Inc. | Method and apparatus for a body fluid sampling device using illumination |
| US7331931B2 (en) | 2002-04-19 | 2008-02-19 | Pelikan Technologies, Inc. | Method and apparatus for penetrating tissue |
| US7901362B2 (en) | 2002-04-19 | 2011-03-08 | Pelikan Technologies, Inc. | Method and apparatus for penetrating tissue |
| US7674232B2 (en) | 2002-04-19 | 2010-03-09 | Pelikan Technologies, Inc. | Method and apparatus for penetrating tissue |
| US8579831B2 (en) | 2002-04-19 | 2013-11-12 | Sanofi-Aventis Deutschland Gmbh | Method and apparatus for penetrating tissue |
| US8267870B2 (en) | 2002-04-19 | 2012-09-18 | Sanofi-Aventis Deutschland Gmbh | Method and apparatus for body fluid sampling with hybrid actuation |
| US9248267B2 (en) | 2002-04-19 | 2016-02-02 | Sanofi-Aventis Deustchland Gmbh | Tissue penetration device |
| US7582099B2 (en) | 2002-04-19 | 2009-09-01 | Pelikan Technologies, Inc | Method and apparatus for penetrating tissue |
| US7717863B2 (en) | 2002-04-19 | 2010-05-18 | Pelikan Technologies, Inc. | Method and apparatus for penetrating tissue |
| US7232451B2 (en) | 2002-04-19 | 2007-06-19 | Pelikan Technologies, Inc. | Method and apparatus for penetrating tissue |
| US7410468B2 (en) | 2002-04-19 | 2008-08-12 | Pelikan Technologies, Inc. | Method and apparatus for penetrating tissue |
| US7371247B2 (en) | 2002-04-19 | 2008-05-13 | Pelikan Technologies, Inc | Method and apparatus for penetrating tissue |
| US7547287B2 (en) | 2002-04-19 | 2009-06-16 | Pelikan Technologies, Inc. | Method and apparatus for penetrating tissue |
| US7524293B2 (en) | 2002-04-19 | 2009-04-28 | Pelikan Technologies, Inc. | Method and apparatus for penetrating tissue |
| US9314194B2 (en) | 2002-04-19 | 2016-04-19 | Sanofi-Aventis Deutschland Gmbh | Tissue penetration device |
| JP2004041587A (en)* | 2002-07-15 | 2004-02-12 | Morita Mfg Co Ltd | Connected body having communication function, medical treatment device using the same |
| US7035690B2 (en)* | 2002-11-15 | 2006-04-25 | Medtronic, Inc. | Human-implantable-neurostimulator user interface having multiple levels of abstraction |
| US7009511B2 (en) | 2002-12-17 | 2006-03-07 | Cardiac Pacemakers, Inc. | Repeater device for communications with an implantable medical device |
| US7127300B2 (en)* | 2002-12-23 | 2006-10-24 | Cardiac Pacemakers, Inc. | Method and apparatus for enabling data communication between an implantable medical device and a patient management system |
| US6978182B2 (en) | 2002-12-27 | 2005-12-20 | Cardiac Pacemakers, Inc. | Advanced patient management system including interrogator/transceiver unit |
| US8574895B2 (en) | 2002-12-30 | 2013-11-05 | Sanofi-Aventis Deutschland Gmbh | Method and apparatus using optical techniques to measure analyte levels |
| US7850621B2 (en) | 2003-06-06 | 2010-12-14 | Pelikan Technologies, Inc. | Method and apparatus for body fluid sampling and analyte sensing |
| US8460243B2 (en) | 2003-06-10 | 2013-06-11 | Abbott Diabetes Care Inc. | Glucose measuring module and insulin pump combination |
| WO2006001797A1 (en) | 2004-06-14 | 2006-01-05 | Pelikan Technologies, Inc. | Low pain penetrating |
| EP1635700B1 (en) | 2003-06-13 | 2016-03-09 | Sanofi-Aventis Deutschland GmbH | Apparatus for a point of care device |
| US7722536B2 (en) | 2003-07-15 | 2010-05-25 | Abbott Diabetes Care Inc. | Glucose measuring device integrated into a holster for a personal area network device |
| WO2005031632A2 (en)* | 2003-09-24 | 2005-04-07 | Medtronic, Inc. | Apparatus and method for serving medical device application content to a remote computing device |
| US8282576B2 (en) | 2003-09-29 | 2012-10-09 | Sanofi-Aventis Deutschland Gmbh | Method and apparatus for an improved sample capture device |
| US20050071199A1 (en)* | 2003-09-30 | 2005-03-31 | Riff Kenneth M. | Aggregating patient information for use in medical device programming |
| EP1680014A4 (en) | 2003-10-14 | 2009-01-21 | Pelikan Technologies Inc | METHOD AND DEVICE FOR A VARIABLE USER INTERFACE |
| US7822454B1 (en) | 2005-01-03 | 2010-10-26 | Pelikan Technologies, Inc. | Fluid sampling device with improved analyte detecting member configuration |
| US8668656B2 (en) | 2003-12-31 | 2014-03-11 | Sanofi-Aventis Deutschland Gmbh | Method and apparatus for improving fluidic flow and sample capture |
| US8025624B2 (en) | 2004-02-19 | 2011-09-27 | Cardiac Pacemakers, Inc. | System and method for assessing cardiac performance through cardiac vibration monitoring |
| US7488290B1 (en) | 2004-02-19 | 2009-02-10 | Cardiac Pacemakers, Inc. | System and method for assessing cardiac performance through transcardiac impedance monitoring |
| WO2006011062A2 (en) | 2004-05-20 | 2006-02-02 | Albatros Technologies Gmbh & Co. Kg | Printable hydrogel for biosensors |
| WO2005120365A1 (en) | 2004-06-03 | 2005-12-22 | Pelikan Technologies, Inc. | Method and apparatus for a fluid sampling device |
| EP1810185A4 (en) | 2004-06-04 | 2010-01-06 | Therasense Inc | Diabetes care host-client architecture and data management system |
| US7329226B1 (en) | 2004-07-06 | 2008-02-12 | Cardiac Pacemakers, Inc. | System and method for assessing pulmonary performance through transthoracic impedance monitoring |
| US8750983B2 (en) | 2004-09-20 | 2014-06-10 | P Tech, Llc | Therapeutic system |
| US8150509B2 (en) | 2004-10-21 | 2012-04-03 | Cardiac Pacemakers, Inc. | Systems and methods for drug therapy enhancement using expected pharmacodynamic models |
| US8388553B2 (en) | 2004-11-04 | 2013-03-05 | Smith & Nephew, Inc. | Cycle and load measurement device |
| US8652831B2 (en) | 2004-12-30 | 2014-02-18 | Sanofi-Aventis Deutschland Gmbh | Method and apparatus for analyte measurement test time |
| US20060173498A1 (en)* | 2005-01-31 | 2006-08-03 | Isabelle Banville | Communication between an external defibrillator and an implantable medical device |
| EP1880298B1 (en)* | 2005-02-17 | 2016-07-13 | Metacure Limited | Charger with data transfer capabilities |
| US7738960B2 (en)* | 2005-03-31 | 2010-06-15 | Medtronic, Inc. | System and method for remote pacing threshold assessment |
| US8781847B2 (en) | 2005-05-03 | 2014-07-15 | Cardiac Pacemakers, Inc. | System and method for managing alert notifications in an automated patient management system |
| US7752059B2 (en) | 2005-07-05 | 2010-07-06 | Cardiac Pacemakers, Inc. | Optimization of timing for data collection and analysis in advanced patient management system |
| AU2006282828B2 (en) | 2005-08-23 | 2013-01-31 | Smith & Nephew, Inc | Telemetric orthopaedic implant |
| US8027727B2 (en)* | 2005-08-29 | 2011-09-27 | Cardiac Pacemakers, Inc. | Pacemaker RF telemetry repeater and method |
| US8764654B2 (en) | 2008-03-19 | 2014-07-01 | Zin Technologies, Inc. | Data acquisition for modular biometric monitoring system |
| US8951190B2 (en) | 2005-09-28 | 2015-02-10 | Zin Technologies, Inc. | Transfer function control for biometric monitoring system |
| US20070078497A1 (en)* | 2005-10-03 | 2007-04-05 | Vandanacker John P | Remote programming of implantable medical devices |
| US9168383B2 (en) | 2005-10-14 | 2015-10-27 | Pacesetter, Inc. | Leadless cardiac pacemaker with conducted communication |
| EP1948296B2 (en) | 2005-10-14 | 2017-10-11 | Pacesetter, Inc. | Leadless cardiac pacemaker and system |
| US20070141106A1 (en)* | 2005-10-19 | 2007-06-21 | Bonutti Peter M | Drug eluting implant |
| US20080004663A1 (en) | 2005-12-22 | 2008-01-03 | Medtronic Emergency Response Systems, Inc. | Defibrillator with implantable medical device detection |
| US8612024B2 (en) | 2006-02-24 | 2013-12-17 | Medtronic, Inc. | User interface with 3D environment for configuring stimulation therapy |
| US8543217B2 (en) | 2006-02-24 | 2013-09-24 | Medtronic, Inc. | Stimulation templates for configuring stimulation therapy |
| US7676273B2 (en) | 2006-02-24 | 2010-03-09 | Medtronic, Inc. | Stimulation templates for programming a stimulation lead with complex electrode array geometry |
| US7657319B2 (en)* | 2006-02-24 | 2010-02-02 | Medtronic, Inc. | Programming interface with an unwrapped 2D view of a stimulation lead with complex electrode array geometry |
| US7822483B2 (en)* | 2006-02-24 | 2010-10-26 | Medtronic, Inc. | Electrical and activation field models for configuring stimulation therapy |
| US8452415B2 (en) | 2006-02-24 | 2013-05-28 | Medtronic, Inc. | Electrical and activation field models for programming a stimulation lead with complex electrode array geometry |
| US7848802B2 (en) | 2006-02-24 | 2010-12-07 | Medtronic, Inc. | Programming interface with a concentric axial view of a stimulation lead with complex electrode array geometry |
| US8380321B2 (en) | 2006-02-24 | 2013-02-19 | Medtronic, Inc. | Programming interface with a cross-sectional view of a stimulation lead with complex electrode array geometry |
| US7826902B2 (en) | 2006-02-24 | 2010-11-02 | Medtronic, Inc. | User interface with 2D views for configuring stimulation therapy |
| US20080076969A1 (en)* | 2006-08-29 | 2008-03-27 | Ulrich Kraft | Methods for modifying control software of electronic medical devices |
| AU2007294526B2 (en)* | 2006-09-08 | 2011-07-07 | Cardiomems, Inc. | Physiological data acquisition and management system for use with an implanted wireless sensor |
| DE102007001705A1 (en)* | 2007-01-11 | 2008-07-17 | Biotronik Crm Patent Ag | Patient device for wireless data communication with an implant |
| US9445720B2 (en) | 2007-02-23 | 2016-09-20 | Smith & Nephew, Inc. | Processing sensed accelerometer data for determination of bone healing |
| US20090063193A1 (en) | 2007-08-31 | 2009-03-05 | Mike Barton | Dashboard diagnostics for wireless patient communicator |
| US9848058B2 (en) | 2007-08-31 | 2017-12-19 | Cardiac Pacemakers, Inc. | Medical data transport over wireless life critical network employing dynamic communication link mapping |
| US8570187B2 (en) | 2007-09-06 | 2013-10-29 | Smith & Nephew, Inc. | System and method for communicating with a telemetric implant |
| US8915866B2 (en) | 2008-01-18 | 2014-12-23 | Warsaw Orthopedic, Inc. | Implantable sensor and associated methods |
| EP2265324B1 (en) | 2008-04-11 | 2015-01-28 | Sanofi-Aventis Deutschland GmbH | Integrated analyte measurement system |
| US20100036508A1 (en)* | 2008-08-06 | 2010-02-11 | Texas Instruments Incorporation | Enabling non-interoperability among transceivers of devices |
| WO2010025289A2 (en)* | 2008-08-28 | 2010-03-04 | Isense Corporation | Method and system for communication between wireless devices |
| US8635694B2 (en)* | 2009-01-10 | 2014-01-21 | Kaspersky Lab Zao | Systems and methods for malware classification |
| US8704124B2 (en) | 2009-01-29 | 2014-04-22 | Smith & Nephew, Inc. | Low temperature encapsulate welding |
| US9375169B2 (en) | 2009-01-30 | 2016-06-28 | Sanofi-Aventis Deutschland Gmbh | Cam drive for managing disposable penetrating member actions with a single motor and motor and control system |
| US8527068B2 (en) | 2009-02-02 | 2013-09-03 | Nanostim, Inc. | Leadless cardiac pacemaker with secondary fixation capability |
| US8812841B2 (en) | 2009-03-04 | 2014-08-19 | Cardiac Pacemakers, Inc. | Communications hub for use in life critical network |
| US8319631B2 (en) | 2009-03-04 | 2012-11-27 | Cardiac Pacemakers, Inc. | Modular patient portable communicator for use in life critical network |
| US8965476B2 (en) | 2010-04-16 | 2015-02-24 | Sanofi-Aventis Deutschland Gmbh | Tissue penetration device |
| US8543205B2 (en) | 2010-10-12 | 2013-09-24 | Nanostim, Inc. | Temperature sensor for a leadless cardiac pacemaker |
| US9060692B2 (en) | 2010-10-12 | 2015-06-23 | Pacesetter, Inc. | Temperature sensor for a leadless cardiac pacemaker |
| EP2627406A1 (en) | 2010-10-13 | 2013-08-21 | Nanostim, Inc. | Leadless cardiac pacemaker with anti-unscrewing feature |
| EP2651502B1 (en) | 2010-12-13 | 2016-11-09 | Pacesetter, Inc. | Pacemaker retrieval systems |
| US8615310B2 (en) | 2010-12-13 | 2013-12-24 | Pacesetter, Inc. | Delivery catheter systems and methods |
| WO2012088118A1 (en) | 2010-12-20 | 2012-06-28 | Nanostim, Inc. | Leadless pacemaker with radial fixation mechanism |
| US10136845B2 (en) | 2011-02-28 | 2018-11-27 | Abbott Diabetes Care Inc. | Devices, systems, and methods associated with analyte monitoring devices and devices incorporating the same |
| US9511236B2 (en) | 2011-11-04 | 2016-12-06 | Pacesetter, Inc. | Leadless cardiac pacemaker with integral battery and redundant welds |
| US9168006B2 (en) | 2012-01-05 | 2015-10-27 | General Electric Company | Systems and methods for wirelessly controlling medical devices |
| US10453573B2 (en) | 2012-06-05 | 2019-10-22 | Dexcom, Inc. | Dynamic report building |
| WO2014022661A1 (en) | 2012-08-01 | 2014-02-06 | Nanostim, Inc. | Biostimulator circuit with flying cell |
| US8761717B1 (en) | 2012-08-07 | 2014-06-24 | Brian K. Buchheit | Safety feature to disable an electronic device when a wireless implantable medical device (IMD) is proximate |
| US9258350B2 (en) | 2012-10-01 | 2016-02-09 | Dexcom, Inc. | Analyte data retriever |
| US9395234B2 (en) | 2012-12-05 | 2016-07-19 | Cardiocom, Llc | Stabilizing base for scale |
| US9730621B2 (en) | 2012-12-31 | 2017-08-15 | Dexcom, Inc. | Remote monitoring of analyte measurements |
| US9801541B2 (en) | 2012-12-31 | 2017-10-31 | Dexcom, Inc. | Remote monitoring of analyte measurements |
| US9878170B2 (en) | 2013-03-15 | 2018-01-30 | Globus Medical, Inc. | Spinal cord stimulator system |
| US9887574B2 (en) | 2013-03-15 | 2018-02-06 | Globus Medical, Inc. | Spinal cord stimulator system |
| US9440076B2 (en) | 2013-03-15 | 2016-09-13 | Globus Medical, Inc. | Spinal cord stimulator system |
| US9872997B2 (en) | 2013-03-15 | 2018-01-23 | Globus Medical, Inc. | Spinal cord stimulator system |
| US9821908B2 (en) | 2013-06-07 | 2017-11-21 | Bell Helicopter Textron Inc. | System and method for assisting in rotor speed control |
| WO2015103022A1 (en) | 2013-12-31 | 2015-07-09 | Senseonics, Incorporated | Continuous analyte monitoring system |
| EP4339825A1 (en) | 2015-05-12 | 2024-03-20 | Dexcom, Inc. | Distributed system architecture for continuous glucose monitoring |
| US10188866B2 (en) | 2015-06-24 | 2019-01-29 | Impulse Dynamics Nv | Simple control of complex bio-implants |
| WO2017116692A1 (en) | 2015-12-28 | 2017-07-06 | Dexcom, Inc. | Systems and methods for remote and host monitoring communications |
| US10667687B2 (en)* | 2016-05-31 | 2020-06-02 | Welch Allyn, Inc. | Monitoring system for physiological parameter sensing device |
Citations (30)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US4168871A (en)* | 1978-03-20 | 1979-09-25 | Firma Erwin Dierkes Kleinmobelfabrik | Shoe rack |
| US4379459A (en)* | 1981-04-09 | 1983-04-12 | Medtronic, Inc. | Cardiac pacemaker sense amplifier |
| US4476868A (en)* | 1978-11-06 | 1984-10-16 | Medtronic, Inc. | Body stimulator output circuit |
| US4556063A (en)* | 1980-10-07 | 1985-12-03 | Medtronic, Inc. | Telemetry system for a medical device |
| US4821723A (en)* | 1987-02-27 | 1989-04-18 | Intermedics Inc. | Biphasic waveforms for defibrillation |
| US5127404A (en)* | 1990-01-22 | 1992-07-07 | Medtronic, Inc. | Telemetry format for implanted medical device |
| US5131388A (en)* | 1991-03-14 | 1992-07-21 | Ventritex, Inc. | Implantable cardiac defibrillator with improved capacitors |
| US5144949A (en)* | 1991-03-15 | 1992-09-08 | Medtronic, Inc. | Dual chamber rate responsive pacemaker with automatic mode switching |
| US5158078A (en)* | 1990-08-14 | 1992-10-27 | Medtronic, Inc. | Rate responsive pacemaker and methods for optimizing its operation |
| US5199428A (en)* | 1991-03-22 | 1993-04-06 | Medtronic, Inc. | Implantable electrical nerve stimulator/pacemaker with ischemia for decreasing cardiac workload |
| US5207218A (en)* | 1991-02-27 | 1993-05-04 | Medtronic, Inc. | Implantable pulse generator |
| US5312453A (en)* | 1992-05-11 | 1994-05-17 | Medtronic, Inc. | Rate responsive cardiac pacemaker and method for work-modulating pacing rate deceleration |
| US5314430A (en)* | 1993-06-24 | 1994-05-24 | Medtronic, Inc. | Atrial defibrillator employing transvenous and subcutaneous electrodes and method of use |
| US5330507A (en)* | 1992-04-24 | 1994-07-19 | Medtronic, Inc. | Implantable electrical vagal stimulation for prevention or interruption of life threatening arrhythmias |
| US5331966A (en)* | 1991-04-05 | 1994-07-26 | Medtronic, Inc. | Subcutaneous multi-electrode sensing system, method and pacer |
| USH1347H (en)* | 1992-11-09 | 1994-08-02 | Medtronic Inc. | Audio feedback for implantable medical device instruments |
| US5342408A (en)* | 1993-01-07 | 1994-08-30 | Incontrol, Inc. | Telemetry system for an implantable cardiac device |
| US5345362A (en)* | 1993-04-29 | 1994-09-06 | Medtronic, Inc. | Portable computer apparatus with articulating display panel |
| US5354316A (en)* | 1993-01-29 | 1994-10-11 | Medtronic, Inc. | Method and apparatus for detection and treatment of tachycardia and fibrillation |
| US5372607A (en)* | 1993-06-23 | 1994-12-13 | Medtronic, Inc. | Method and apparatus for monitoring pacemaker intervals |
| US5417222A (en)* | 1994-01-21 | 1995-05-23 | Hewlett-Packard Company | Patient monitoring system |
| US5520191A (en)* | 1994-10-07 | 1996-05-28 | Ortivus Medical Ab | Myocardial ischemia and infarction analysis and monitoring method and apparatus |
| US5527348A (en)* | 1995-02-03 | 1996-06-18 | Medtronic, Inc. | Magnetically permeable E-shield and method of connection thereto |
| US5545186A (en)* | 1995-03-30 | 1996-08-13 | Medtronic, Inc. | Prioritized rule based method and apparatus for diagnosis and treatment of arrhythmias |
| US5579775A (en)* | 1994-10-20 | 1996-12-03 | Hewlett-Packard Company | Dynamic control of a patient monitoring system |
| US5626630A (en)* | 1994-10-13 | 1997-05-06 | Ael Industries, Inc. | Medical telemetry system using an implanted passive transponder |
| US5683432A (en)* | 1996-01-11 | 1997-11-04 | Medtronic, Inc. | Adaptive, performance-optimizing communication system for communicating with an implanted medical device |
| US5687734A (en)* | 1994-10-20 | 1997-11-18 | Hewlett-Packard Company | Flexible patient monitoring system featuring a multiport transmitter |
| US5752976A (en)* | 1995-06-23 | 1998-05-19 | Medtronic, Inc. | World wide patient location and data telemetry system for implantable medical devices |
| US6150951A (en)* | 1997-12-22 | 2000-11-21 | Hewlett-Packard | Medical telemetry system with wireless and physical communication channels |
Family Cites Families (6)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US4550370A (en)* | 1982-10-29 | 1985-10-29 | Medtronic, Inc. | Pacemaker programmer with telemetric functions |
| US5168871A (en) | 1990-11-09 | 1992-12-08 | Medtronic, Inc. | Method and apparatus for processing quasi-transient telemetry signals in noisy environments |
| US5383915A (en)* | 1991-04-10 | 1995-01-24 | Angeion Corporation | Wireless programmer/repeater system for an implanted medical device |
| US5782890A (en)* | 1992-07-01 | 1998-07-21 | Medtronic, Inc. | Method for heart transplant monitoring and analog telemetry calibration |
| US5630836A (en)* | 1995-01-19 | 1997-05-20 | Vascor, Inc. | Transcutaneous energy and information transmission apparatus |
| FR2749462B1 (en)* | 1996-06-04 | 1998-07-24 | Ela Medical Sa | AUTONOMOUS DEVICE, IN PARTICULAR ACTIVE IMPLANTABLE MEDICAL DEVICE, AND ITS EXTERNAL PROGRAMMER WITH SYNCHRONOUS TRANSMISSION |
- 1999
- 1999-05-28USUS09/322,560patent/US6477424B1/ennot_activeExpired - Lifetime
- 1999-06-18FRFR9907727Apatent/FR2780224B1/ennot_activeExpired - Fee Related
- 1999-06-18DEDE19927853Apatent/DE19927853B8/ennot_activeExpired - Fee Related
- 2002
- 2002-09-20USUS10/251,509patent/US20030028226A1/ennot_activeAbandoned
Patent Citations (30)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US4168871A (en)* | 1978-03-20 | 1979-09-25 | Firma Erwin Dierkes Kleinmobelfabrik | Shoe rack |
| US4476868A (en)* | 1978-11-06 | 1984-10-16 | Medtronic, Inc. | Body stimulator output circuit |
| US4556063A (en)* | 1980-10-07 | 1985-12-03 | Medtronic, Inc. | Telemetry system for a medical device |
| US4379459A (en)* | 1981-04-09 | 1983-04-12 | Medtronic, Inc. | Cardiac pacemaker sense amplifier |
| US4821723A (en)* | 1987-02-27 | 1989-04-18 | Intermedics Inc. | Biphasic waveforms for defibrillation |
| US5127404A (en)* | 1990-01-22 | 1992-07-07 | Medtronic, Inc. | Telemetry format for implanted medical device |
| US5158078A (en)* | 1990-08-14 | 1992-10-27 | Medtronic, Inc. | Rate responsive pacemaker and methods for optimizing its operation |
| US5207218A (en)* | 1991-02-27 | 1993-05-04 | Medtronic, Inc. | Implantable pulse generator |
| US5131388A (en)* | 1991-03-14 | 1992-07-21 | Ventritex, Inc. | Implantable cardiac defibrillator with improved capacitors |
| US5144949A (en)* | 1991-03-15 | 1992-09-08 | Medtronic, Inc. | Dual chamber rate responsive pacemaker with automatic mode switching |
| US5199428A (en)* | 1991-03-22 | 1993-04-06 | Medtronic, Inc. | Implantable electrical nerve stimulator/pacemaker with ischemia for decreasing cardiac workload |
| US5331966A (en)* | 1991-04-05 | 1994-07-26 | Medtronic, Inc. | Subcutaneous multi-electrode sensing system, method and pacer |
| US5330507A (en)* | 1992-04-24 | 1994-07-19 | Medtronic, Inc. | Implantable electrical vagal stimulation for prevention or interruption of life threatening arrhythmias |
| US5312453A (en)* | 1992-05-11 | 1994-05-17 | Medtronic, Inc. | Rate responsive cardiac pacemaker and method for work-modulating pacing rate deceleration |
| USH1347H (en)* | 1992-11-09 | 1994-08-02 | Medtronic Inc. | Audio feedback for implantable medical device instruments |
| US5342408A (en)* | 1993-01-07 | 1994-08-30 | Incontrol, Inc. | Telemetry system for an implantable cardiac device |
| US5354316A (en)* | 1993-01-29 | 1994-10-11 | Medtronic, Inc. | Method and apparatus for detection and treatment of tachycardia and fibrillation |
| US5345362A (en)* | 1993-04-29 | 1994-09-06 | Medtronic, Inc. | Portable computer apparatus with articulating display panel |
| US5372607A (en)* | 1993-06-23 | 1994-12-13 | Medtronic, Inc. | Method and apparatus for monitoring pacemaker intervals |
| US5314430A (en)* | 1993-06-24 | 1994-05-24 | Medtronic, Inc. | Atrial defibrillator employing transvenous and subcutaneous electrodes and method of use |
| US5417222A (en)* | 1994-01-21 | 1995-05-23 | Hewlett-Packard Company | Patient monitoring system |
| US5520191A (en)* | 1994-10-07 | 1996-05-28 | Ortivus Medical Ab | Myocardial ischemia and infarction analysis and monitoring method and apparatus |
| US5626630A (en)* | 1994-10-13 | 1997-05-06 | Ael Industries, Inc. | Medical telemetry system using an implanted passive transponder |
| US5579775A (en)* | 1994-10-20 | 1996-12-03 | Hewlett-Packard Company | Dynamic control of a patient monitoring system |
| US5687734A (en)* | 1994-10-20 | 1997-11-18 | Hewlett-Packard Company | Flexible patient monitoring system featuring a multiport transmitter |
| US5527348A (en)* | 1995-02-03 | 1996-06-18 | Medtronic, Inc. | Magnetically permeable E-shield and method of connection thereto |
| US5545186A (en)* | 1995-03-30 | 1996-08-13 | Medtronic, Inc. | Prioritized rule based method and apparatus for diagnosis and treatment of arrhythmias |
| US5752976A (en)* | 1995-06-23 | 1998-05-19 | Medtronic, Inc. | World wide patient location and data telemetry system for implantable medical devices |
| US5683432A (en)* | 1996-01-11 | 1997-11-04 | Medtronic, Inc. | Adaptive, performance-optimizing communication system for communicating with an implanted medical device |
| US6150951A (en)* | 1997-12-22 | 2000-11-21 | Hewlett-Packard | Medical telemetry system with wireless and physical communication channels |
Cited By (197)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US7047076B1 (en) | 2001-08-03 | 2006-05-16 | Cardiac Pacemakers, Inc. | Inverted-F antenna configuration for an implantable medical device |
| US20060276857A1 (en)* | 2003-10-02 | 2006-12-07 | Medtronic, Inc. | Medical device programmer with infrared communication |
| US9248299B2 (en) | 2003-10-02 | 2016-02-02 | Medtronic, Inc. | Medical device programmer |
| US9248298B2 (en) | 2003-10-02 | 2016-02-02 | Medtronic, Inc. | Medical device programmer with selective disablement of display during telemetry |
| US20070288068A1 (en)* | 2003-10-02 | 2007-12-13 | Medtronic, Inc. | Medical device programmer with selective disablement of display during telemetry |
| US7751894B1 (en) | 2004-03-04 | 2010-07-06 | Cardiac Pacemakers, Inc. | Systems and methods for indicating aberrant behavior detected by an implanted medical device |
| US20100262032A1 (en)* | 2004-03-04 | 2010-10-14 | Freeberg Scott M | Systems and Methods for Indicating Aberrant Behavior Detected by an Implanted Medical Device |
| US20050273170A1 (en)* | 2004-06-08 | 2005-12-08 | Navarro Richard R | Prosthetic intervertebral spinal disc with integral microprocessor |
| US7794499B2 (en) | 2004-06-08 | 2010-09-14 | Theken Disc, L.L.C. | Prosthetic intervertebral spinal disc with integral microprocessor |
| US20160129270A1 (en)* | 2004-06-10 | 2016-05-12 | Medtronic Urinary Solutions, Inc. | Systems and methods for clinician control of stimulation systems |
| US10293168B2 (en)* | 2004-06-10 | 2019-05-21 | Medtronic Urinary Solutions, Inc. | Systems and methods for clinician control of stimulation systems |
| US20140188192A1 (en)* | 2004-06-10 | 2014-07-03 | Medtronic Urinary Solutions, Inc. | Systems and methods for clinician control of stimulation systems |
| US9216294B2 (en)* | 2004-06-10 | 2015-12-22 | Medtronic Urinary Solutions, Inc. | Systems and methods for clinician control of stimulation systems |
| US8565867B2 (en) | 2005-01-28 | 2013-10-22 | Cyberonics, Inc. | Changeable electrode polarity stimulation by an implantable medical device |
| US9586047B2 (en) | 2005-01-28 | 2017-03-07 | Cyberonics, Inc. | Contingent cardio-protection for epilepsy patients |
| US9439582B2 (en) | 2005-04-28 | 2016-09-13 | Proteus Digital Health, Inc. | Communication system with remote activation |
| US9649066B2 (en) | 2005-04-28 | 2017-05-16 | Proteus Digital Health, Inc. | Communication system with partial power source |
| US9198608B2 (en) | 2005-04-28 | 2015-12-01 | Proteus Digital Health, Inc. | Communication system incorporated in a container |
| US20100081894A1 (en)* | 2005-04-28 | 2010-04-01 | Proteus Biomedical, Inc. | Communication system with partial power source |
| US9119554B2 (en) | 2005-04-28 | 2015-09-01 | Proteus Digital Health, Inc. | Pharma-informatics system |
| US8912908B2 (en) | 2005-04-28 | 2014-12-16 | Proteus Digital Health, Inc. | Communication system with remote activation |
| US8847766B2 (en) | 2005-04-28 | 2014-09-30 | Proteus Digital Health, Inc. | Pharma-informatics system |
| US8816847B2 (en) | 2005-04-28 | 2014-08-26 | Proteus Digital Health, Inc. | Communication system with partial power source |
| US9161707B2 (en) | 2005-04-28 | 2015-10-20 | Proteus Digital Health, Inc. | Communication system incorporated in an ingestible product |
| US8802183B2 (en) | 2005-04-28 | 2014-08-12 | Proteus Digital Health, Inc. | Communication system with enhanced partial power source and method of manufacturing same |
| US8730031B2 (en) | 2005-04-28 | 2014-05-20 | Proteus Digital Health, Inc. | Communication system using an implantable device |
| US8674825B2 (en) | 2005-04-28 | 2014-03-18 | Proteus Digital Health, Inc. | Pharma-informatics system |
| US11476952B2 (en) | 2005-04-28 | 2022-10-18 | Otsuka Pharmaceutical Co., Ltd. | Pharma-informatics system |
| US9597010B2 (en) | 2005-04-28 | 2017-03-21 | Proteus Digital Health, Inc. | Communication system using an implantable device |
| US7978064B2 (en) | 2005-04-28 | 2011-07-12 | Proteus Biomedical, Inc. | Communication system with partial power source |
| US20090227204A1 (en)* | 2005-04-28 | 2009-09-10 | Timothy Robertson | Pharma-Informatics System |
| US9681842B2 (en) | 2005-04-28 | 2017-06-20 | Proteus Digital Health, Inc. | Pharma-informatics system |
| US9962107B2 (en) | 2005-04-28 | 2018-05-08 | Proteus Digital Health, Inc. | Communication system with enhanced partial power source and method of manufacturing same |
| US10610128B2 (en) | 2005-04-28 | 2020-04-07 | Proteus Digital Health, Inc. | Pharma-informatics system |
| US10542909B2 (en) | 2005-04-28 | 2020-01-28 | Proteus Digital Health, Inc. | Communication system with partial power source |
| US10517507B2 (en) | 2005-04-28 | 2019-12-31 | Proteus Digital Health, Inc. | Communication system with enhanced partial power source and method of manufacturing same |
| US8547248B2 (en) | 2005-09-01 | 2013-10-01 | Proteus Digital Health, Inc. | Implantable zero-wire communications system |
| WO2007058826A2 (en) | 2005-11-14 | 2007-05-24 | Edwards Lifesciences Corporation | Wireless communication system for pressure monitoring |
| US20070233193A1 (en)* | 2006-03-29 | 2007-10-04 | Catholic Healthcare West (D/B/A St. Joseph's Hospital And Medical Center) | Microburst electrical stimulation of cranial nerves for the treatment of medical conditions |
| US9289599B2 (en) | 2006-03-29 | 2016-03-22 | Dignity Health | Vagus nerve stimulation method |
| US8219188B2 (en) | 2006-03-29 | 2012-07-10 | Catholic Healthcare West | Synchronization of vagus nerve stimulation with the cardiac cycle of a patient |
| US20070233192A1 (en)* | 2006-03-29 | 2007-10-04 | Catholic Healthcare West (D/B/A St. Joseph's Hospital And Medical Center) | Vagus nerve stimulation method |
| US8280505B2 (en) | 2006-03-29 | 2012-10-02 | Catholic Healthcare West | Vagus nerve stimulation method |
| US9108041B2 (en) | 2006-03-29 | 2015-08-18 | Dignity Health | Microburst electrical stimulation of cranial nerves for the treatment of medical conditions |
| US8615309B2 (en) | 2006-03-29 | 2013-12-24 | Catholic Healthcare West | Microburst electrical stimulation of cranial nerves for the treatment of medical conditions |
| US8150508B2 (en) | 2006-03-29 | 2012-04-03 | Catholic Healthcare West | Vagus nerve stimulation method |
| US20070233194A1 (en)* | 2006-03-29 | 2007-10-04 | Catholic Healthcare West (D/B/A St. Joseph's Hospital And Medical Center) | Synchronization of vagus nerve stimulation with the cardiac cycle of a patient |
| US8660666B2 (en) | 2006-03-29 | 2014-02-25 | Catholic Healthcare West | Microburst electrical stimulation of cranial nerves for the treatment of medical conditions |
| US9533151B2 (en) | 2006-03-29 | 2017-01-03 | Dignity Health | Microburst electrical stimulation of cranial nerves for the treatment of medical conditions |
| US20090177252A1 (en)* | 2006-03-29 | 2009-07-09 | Catholic Healthcare West (D/B/A St. Joseph's Hospital And Medical Center) | Synchronization of vagus nerve stimulation with the cardiac cycle of a patient |
| US8738126B2 (en) | 2006-03-29 | 2014-05-27 | Catholic Healthcare West | Synchronization of vagus nerve stimulation with the cardiac cycle of a patient |
| US20070255351A1 (en)* | 2006-04-28 | 2007-11-01 | Cyberonics, Inc. | Threshold optimization for tissue stimulation therapy |
| US7869885B2 (en) | 2006-04-28 | 2011-01-11 | Cyberonics, Inc | Threshold optimization for tissue stimulation therapy |
| US8836513B2 (en) | 2006-04-28 | 2014-09-16 | Proteus Digital Health, Inc. | Communication system incorporated in an ingestible product |
| US20090076338A1 (en)* | 2006-05-02 | 2009-03-19 | Zdeblick Mark J | Patient customized therapeutic regimens |
| US8956287B2 (en) | 2006-05-02 | 2015-02-17 | Proteus Digital Health, Inc. | Patient customized therapeutic regimens |
| US11928614B2 (en) | 2006-05-02 | 2024-03-12 | Otsuka Pharmaceutical Co., Ltd. | Patient customized therapeutic regimens |
| US9616225B2 (en) | 2006-05-18 | 2017-04-11 | Endostim, Inc. | Device and implantation system for electrical stimulation of biological systems |
| US12343530B2 (en) | 2006-05-18 | 2025-07-01 | Paras Holdings, Llc | Device and implantation system for electrical stimulation of biological systems |
| US11517750B2 (en) | 2006-05-18 | 2022-12-06 | Endostim, Inc. | Device and implantation system for electrical stimulation of biological systems |
| US10272242B2 (en) | 2006-05-18 | 2019-04-30 | Endostim, Inc. | Device and implantation system for electrical stimulation of biological systems |
| US9345879B2 (en) | 2006-10-09 | 2016-05-24 | Endostim, Inc. | Device and implantation system for electrical stimulation of biological systems |
| US9724510B2 (en) | 2006-10-09 | 2017-08-08 | Endostim, Inc. | System and methods for electrical stimulation of biological systems |
| US9561367B2 (en) | 2006-10-09 | 2017-02-07 | Endostim, Inc. | Device and implantation system for electrical stimulation of biological systems |
| US10426955B2 (en) | 2006-10-09 | 2019-10-01 | Endostim, Inc. | Methods for implanting electrodes and treating a patient with gastreosophageal reflux disease |
| US11786726B2 (en) | 2006-10-09 | 2023-10-17 | Endostim, Inc. | Device and implantation system for electrical stimulation of biological systems |
| US10406356B2 (en) | 2006-10-09 | 2019-09-10 | Endostim, Inc. | Systems and methods for electrical stimulation of biological systems |
| US11577077B2 (en) | 2006-10-09 | 2023-02-14 | Endostim, Inc. | Systems and methods for electrical stimulation of biological systems |
| US8054140B2 (en) | 2006-10-17 | 2011-11-08 | Proteus Biomedical, Inc. | Low voltage oscillator for medical devices |
| US10238604B2 (en) | 2006-10-25 | 2019-03-26 | Proteus Digital Health, Inc. | Controlled activation ingestible identifier |
| US11357730B2 (en) | 2006-10-25 | 2022-06-14 | Otsuka Pharmaceutical Co., Ltd. | Controlled activation ingestible identifier |
| US8945005B2 (en) | 2006-10-25 | 2015-02-03 | Proteus Digital Health, Inc. | Controlled activation ingestible identifier |
| US7869867B2 (en) | 2006-10-27 | 2011-01-11 | Cyberonics, Inc. | Implantable neurostimulator with refractory stimulation |
| US9444503B2 (en) | 2006-11-20 | 2016-09-13 | Proteus Digital Health, Inc. | Active signal processing personal health signal receivers |
| US20100316158A1 (en)* | 2006-11-20 | 2010-12-16 | Lawrence Arne | Active signal processing personal health signal receivers |
| US8718193B2 (en) | 2006-11-20 | 2014-05-06 | Proteus Digital Health, Inc. | Active signal processing personal health signal receivers |
| US9083589B2 (en) | 2006-11-20 | 2015-07-14 | Proteus Digital Health, Inc. | Active signal processing personal health signal receivers |
| US20100185055A1 (en)* | 2007-02-01 | 2010-07-22 | Timothy Robertson | Ingestible event marker systems |
| US8858432B2 (en) | 2007-02-01 | 2014-10-14 | Proteus Digital Health, Inc. | Ingestible event marker systems |
| US10441194B2 (en) | 2007-02-01 | 2019-10-15 | Proteus Digital Heal Th, Inc. | Ingestible event marker systems |
| US11464423B2 (en) | 2007-02-14 | 2022-10-11 | Otsuka Pharmaceutical Co., Ltd. | In-body power source having high surface area electrode |
| US8956288B2 (en) | 2007-02-14 | 2015-02-17 | Proteus Digital Health, Inc. | In-body power source having high surface area electrode |
| US8932221B2 (en) | 2007-03-09 | 2015-01-13 | Proteus Digital Health, Inc. | In-body device having a multi-directional transmitter |
| US20100022836A1 (en)* | 2007-03-09 | 2010-01-28 | Olivier Colliou | In-body device having a multi-directional transmitter |
| US9270025B2 (en) | 2007-03-09 | 2016-02-23 | Proteus Digital Health, Inc. | In-body device having deployable antenna |
| US7974701B2 (en) | 2007-04-27 | 2011-07-05 | Cyberonics, Inc. | Dosing limitation for an implantable medical device |
| US20110224758A1 (en)* | 2007-04-27 | 2011-09-15 | Cyberonics, Inc. | Dosing Limitation For An Implantable Medical Device |
| US8306627B2 (en) | 2007-04-27 | 2012-11-06 | Cyberonics, Inc. | Dosing limitation for an implantable medical device |
| US20080269839A1 (en)* | 2007-04-27 | 2008-10-30 | Armstrong Randolph K | Dosing Limitation for an Implantable Medical Device |
| US8540632B2 (en) | 2007-05-24 | 2013-09-24 | Proteus Digital Health, Inc. | Low profile antenna for in body device |
| US8115618B2 (en) | 2007-05-24 | 2012-02-14 | Proteus Biomedical, Inc. | RFID antenna for in-body device |
| US10517506B2 (en) | 2007-05-24 | 2019-12-31 | Proteus Digital Health, Inc. | Low profile antenna for in body device |
| US20080306360A1 (en)* | 2007-05-24 | 2008-12-11 | Robertson Timothy L | Low profile antenna for in body device |
| US20090082645A1 (en)* | 2007-09-25 | 2009-03-26 | Proteus Biomedical, Inc. | In-body device with virtual dipole signal amplification |
| US9433371B2 (en) | 2007-09-25 | 2016-09-06 | Proteus Digital Health, Inc. | In-body device with virtual dipole signal amplification |
| US8961412B2 (en) | 2007-09-25 | 2015-02-24 | Proteus Digital Health, Inc. | In-body device with virtual dipole signal amplification |
| US20090119067A1 (en)* | 2007-11-05 | 2009-05-07 | Wolfgang Stempfer | System and method to minimize downtimes of medical apparatuses |
| US8055476B2 (en)* | 2007-11-05 | 2011-11-08 | Siemens Aktiengesellschaft | System and method to minimize downtimes of medical apparatuses |
| US20090135886A1 (en)* | 2007-11-27 | 2009-05-28 | Proteus Biomedical, Inc. | Transbody communication systems employing communication channels |
| US9314633B2 (en) | 2008-01-25 | 2016-04-19 | Cyberonics, Inc. | Contingent cardio-protection for epilepsy patients |
| US8258962B2 (en) | 2008-03-05 | 2012-09-04 | Proteus Biomedical, Inc. | Multi-mode communication ingestible event markers and systems, and methods of using the same |
| US9258035B2 (en) | 2008-03-05 | 2016-02-09 | Proteus Digital Health, Inc. | Multi-mode communication ingestible event markers and systems, and methods of using the same |
| US9060708B2 (en) | 2008-03-05 | 2015-06-23 | Proteus Digital Health, Inc. | Multi-mode communication ingestible event markers and systems, and methods of using the same |
| US8542123B2 (en) | 2008-03-05 | 2013-09-24 | Proteus Digital Health, Inc. | Multi-mode communication ingestible event markers and systems, and methods of using the same |
| US8810409B2 (en) | 2008-03-05 | 2014-08-19 | Proteus Digital Health, Inc. | Multi-mode communication ingestible event markers and systems, and methods of using the same |
| US11217342B2 (en) | 2008-07-08 | 2022-01-04 | Otsuka Pharmaceutical Co., Ltd. | Ingestible event marker data framework |
| US10682071B2 (en) | 2008-07-08 | 2020-06-16 | Proteus Digital Health, Inc. | State characterization based on multi-variate data fusion techniques |
| WO2014144727A1 (en)* | 2008-07-08 | 2014-09-18 | Proteus Digital Health, Inc. | State characterization based on multi-variate data fusion techniques |
| US9603550B2 (en) | 2008-07-08 | 2017-03-28 | Proteus Digital Health, Inc. | State characterization based on multi-variate data fusion techniques |
| US8540633B2 (en) | 2008-08-13 | 2013-09-24 | Proteus Digital Health, Inc. | Identifier circuits for generating unique identifiable indicators and techniques for producing same |
| US20110065983A1 (en)* | 2008-08-13 | 2011-03-17 | Hooman Hafezi | Ingestible Circuitry |
| US9415010B2 (en) | 2008-08-13 | 2016-08-16 | Proteus Digital Health, Inc. | Ingestible circuitry |
| US8721540B2 (en) | 2008-08-13 | 2014-05-13 | Proteus Digital Health, Inc. | Ingestible circuitry |
| US20110212782A1 (en)* | 2008-10-14 | 2011-09-01 | Andrew Thompson | Method and System for Incorporating Physiologic Data in a Gaming Environment |
| US8036748B2 (en) | 2008-11-13 | 2011-10-11 | Proteus Biomedical, Inc. | Ingestible therapy activator system and method |
| US20110196454A1 (en)* | 2008-11-18 | 2011-08-11 | Proteus Biomedical, Inc. | Sensing system, device, and method for therapy modulation |
| US8583227B2 (en) | 2008-12-11 | 2013-11-12 | Proteus Digital Health, Inc. | Evaluation of gastrointestinal function using portable electroviscerography systems and methods of using the same |
| US8055334B2 (en) | 2008-12-11 | 2011-11-08 | Proteus Biomedical, Inc. | Evaluation of gastrointestinal function using portable electroviscerography systems and methods of using the same |
| US9439566B2 (en) | 2008-12-15 | 2016-09-13 | Proteus Digital Health, Inc. | Re-wearable wireless device |
| US9149577B2 (en) | 2008-12-15 | 2015-10-06 | Proteus Digital Health, Inc. | Body-associated receiver and method |
| US9659423B2 (en) | 2008-12-15 | 2017-05-23 | Proteus Digital Health, Inc. | Personal authentication apparatus system and method |
| US8545436B2 (en) | 2008-12-15 | 2013-10-01 | Proteus Digital Health, Inc. | Body-associated receiver and method |
| US8114021B2 (en) | 2008-12-15 | 2012-02-14 | Proteus Biomedical, Inc. | Body-associated receiver and method |
| US9883819B2 (en) | 2009-01-06 | 2018-02-06 | Proteus Digital Health, Inc. | Ingestion-related biofeedback and personalized medical therapy method and system |
| US8597186B2 (en) | 2009-01-06 | 2013-12-03 | Proteus Digital Health, Inc. | Pharmaceutical dosages delivery system |
| US8540664B2 (en) | 2009-03-25 | 2013-09-24 | Proteus Digital Health, Inc. | Probablistic pharmacokinetic and pharmacodynamic modeling |
| US9119918B2 (en) | 2009-03-25 | 2015-09-01 | Proteus Digital Health, Inc. | Probablistic pharmacokinetic and pharmacodynamic modeling |
| US9320455B2 (en) | 2009-04-28 | 2016-04-26 | Proteus Digital Health, Inc. | Highly reliable ingestible event markers and methods for using the same |
| US20110054265A1 (en)* | 2009-04-28 | 2011-03-03 | Hooman Hafezi | Highly reliable ingestible event markers and methods for using the same |
| US10588544B2 (en) | 2009-04-28 | 2020-03-17 | Proteus Digital Health, Inc. | Highly reliable ingestible event markers and methods for using the same |
| US8545402B2 (en) | 2009-04-28 | 2013-10-01 | Proteus Digital Health, Inc. | Highly reliable ingestible event markers and methods for using the same |
| US9149423B2 (en) | 2009-05-12 | 2015-10-06 | Proteus Digital Health, Inc. | Ingestible event markers comprising an ingestible component |
| US8558563B2 (en) | 2009-08-21 | 2013-10-15 | Proteus Digital Health, Inc. | Apparatus and method for measuring biochemical parameters |
| US10305544B2 (en) | 2009-11-04 | 2019-05-28 | Proteus Digital Health, Inc. | System for supply chain management |
| US8868453B2 (en) | 2009-11-04 | 2014-10-21 | Proteus Digital Health, Inc. | System for supply chain management |
| US9941931B2 (en) | 2009-11-04 | 2018-04-10 | Proteus Digital Health, Inc. | System for supply chain management |
| US8784308B2 (en) | 2009-12-02 | 2014-07-22 | Proteus Digital Health, Inc. | Integrated ingestible event marker system with pharmaceutical product |
| US9014779B2 (en) | 2010-02-01 | 2015-04-21 | Proteus Digital Health, Inc. | Data gathering system |
| US10376218B2 (en) | 2010-02-01 | 2019-08-13 | Proteus Digital Health, Inc. | Data gathering system |
| US11717681B2 (en) | 2010-03-05 | 2023-08-08 | Endostim, Inc. | Systems and methods for treating gastroesophageal reflux disease |
| US10058703B2 (en) | 2010-03-05 | 2018-08-28 | Endostim, Inc. | Methods of treating gastroesophageal reflux disease using an implanted device |
| US11058876B2 (en) | 2010-03-05 | 2021-07-13 | Endostim (Abc), Llc | Device and implantation system for electrical stimulation of biological systems |
| US10420934B2 (en) | 2010-03-05 | 2019-09-24 | Endostim, Inc. | Systems and methods for treating gastroesophageal reflux disease |
| US9789309B2 (en) | 2010-03-05 | 2017-10-17 | Endostim, Inc. | Device and implantation system for electrical stimulation of biological systems |
| US9381344B2 (en) | 2010-03-05 | 2016-07-05 | Endostim, Inc. | Systems and methods for treating gastroesophageal reflux disease |
| US9597487B2 (en) | 2010-04-07 | 2017-03-21 | Proteus Digital Health, Inc. | Miniature ingestible device |
| US11173290B2 (en) | 2010-04-07 | 2021-11-16 | Otsuka Pharmaceutical Co., Ltd. | Miniature ingestible device |
| US10207093B2 (en) | 2010-04-07 | 2019-02-19 | Proteus Digital Health, Inc. | Miniature ingestible device |
| US10529044B2 (en) | 2010-05-19 | 2020-01-07 | Proteus Digital Health, Inc. | Tracking and delivery confirmation of pharmaceutical products |
| US11504511B2 (en) | 2010-11-22 | 2022-11-22 | Otsuka Pharmaceutical Co., Ltd. | Ingestible device with pharmaceutical product |
| US9107806B2 (en) | 2010-11-22 | 2015-08-18 | Proteus Digital Health, Inc. | Ingestible device with pharmaceutical product |
| US9439599B2 (en) | 2011-03-11 | 2016-09-13 | Proteus Digital Health, Inc. | Wearable personal body associated device with various physical configurations |
| US20120296397A1 (en)* | 2011-05-21 | 2012-11-22 | Boston Scientific Neuromodulation Corporation | System and method for programming neurostimulation devices using cached plug-in software drivers |
| US9119969B2 (en) | 2011-05-21 | 2015-09-01 | Boston Scientific Neuromodulation Corporation | System and method for programming neurostimulation devices using cached plug-in software drivers |
| US8805506B2 (en)* | 2011-05-21 | 2014-08-12 | Boston Scientific Neuromodulation Corporation | System and method for programming neurostimulation devices using cached plug-in software drivers |
| US11229378B2 (en) | 2011-07-11 | 2022-01-25 | Otsuka Pharmaceutical Co., Ltd. | Communication system with enhanced partial power source and method of manufacturing same |
| US9756874B2 (en) | 2011-07-11 | 2017-09-12 | Proteus Digital Health, Inc. | Masticable ingestible product and communication system therefor |
| US10223905B2 (en) | 2011-07-21 | 2019-03-05 | Proteus Digital Health, Inc. | Mobile device and system for detection and communication of information received from an ingestible device |
| US9925367B2 (en) | 2011-09-02 | 2018-03-27 | Endostim, Inc. | Laparoscopic lead implantation method |
| US11052243B2 (en) | 2011-09-02 | 2021-07-06 | Endostim (Abc), Llc | Laparoscopic lead for esophageal sphincter implantation |
| US9235683B2 (en) | 2011-11-09 | 2016-01-12 | Proteus Digital Health, Inc. | Apparatus, system, and method for managing adherence to a regimen |
| US9271897B2 (en) | 2012-07-23 | 2016-03-01 | Proteus Digital Health, Inc. | Techniques for manufacturing ingestible event markers comprising an ingestible component |
| US11052248B2 (en) | 2012-08-23 | 2021-07-06 | Endostim (Abc), Llc | Device and implantation system for electrical stimulation of biological systems |
| US9623238B2 (en) | 2012-08-23 | 2017-04-18 | Endostim, Inc. | Device and implantation system for electrical stimulation of biological systems |
| US9268909B2 (en) | 2012-10-18 | 2016-02-23 | Proteus Digital Health, Inc. | Apparatus, system, and method to adaptively optimize power dissipation and broadcast power in a power source for a communication device |
| US20140185107A1 (en)* | 2012-12-30 | 2014-07-03 | Shenyang Neusoft Medical Systems Co., Ltd. | Method and device for indicating scanning condition and scanning apparatus and scanning system related thereto |
| US11149123B2 (en) | 2013-01-29 | 2021-10-19 | Otsuka Pharmaceutical Co., Ltd. | Highly-swellable polymeric films and compositions comprising the same |
| US9498619B2 (en) | 2013-02-26 | 2016-11-22 | Endostim, Inc. | Implantable electrical stimulation leads |
| US10175376B2 (en) | 2013-03-15 | 2019-01-08 | Proteus Digital Health, Inc. | Metal detector apparatus, system, and method |
| US11158149B2 (en) | 2013-03-15 | 2021-10-26 | Otsuka Pharmaceutical Co., Ltd. | Personal authentication apparatus system and method |
| US11741771B2 (en) | 2013-03-15 | 2023-08-29 | Otsuka Pharmaceutical Co., Ltd. | Personal authentication apparatus system and method |
| US11744481B2 (en) | 2013-03-15 | 2023-09-05 | Otsuka Pharmaceutical Co., Ltd. | System, apparatus and methods for data collection and assessing outcomes |
| US10421658B2 (en) | 2013-08-30 | 2019-09-24 | Proteus Digital Health, Inc. | Container with electronically controlled interlock |
| US9796576B2 (en) | 2013-08-30 | 2017-10-24 | Proteus Digital Health, Inc. | Container with electronically controlled interlock |
| US11052254B2 (en) | 2013-09-03 | 2021-07-06 | Endostim (Abc), Llc | Methods and systems of electrode polarity switching in electrical stimulation therapy |
| US9827425B2 (en) | 2013-09-03 | 2017-11-28 | Endostim, Inc. | Methods and systems of electrode polarity switching in electrical stimulation therapy |
| US10097388B2 (en) | 2013-09-20 | 2018-10-09 | Proteus Digital Health, Inc. | Methods, devices and systems for receiving and decoding a signal in the presence of noise using slices and warping |
| US11102038B2 (en) | 2013-09-20 | 2021-08-24 | Otsuka Pharmaceutical Co., Ltd. | Methods, devices and systems for receiving and decoding a signal in the presence of noise using slices and warping |
| US10498572B2 (en) | 2013-09-20 | 2019-12-03 | Proteus Digital Health, Inc. | Methods, devices and systems for receiving and decoding a signal in the presence of noise using slices and warping |
| US9787511B2 (en) | 2013-09-20 | 2017-10-10 | Proteus Digital Health, Inc. | Methods, devices and systems for receiving and decoding a signal in the presence of noise using slices and warping |
| US9270503B2 (en) | 2013-09-20 | 2016-02-23 | Proteus Digital Health, Inc. | Methods, devices and systems for receiving and decoding a signal in the presence of noise using slices and warping |
| US9577864B2 (en) | 2013-09-24 | 2017-02-21 | Proteus Digital Health, Inc. | Method and apparatus for use with received electromagnetic signal at a frequency not known exactly in advance |
| US10084880B2 (en) | 2013-11-04 | 2018-09-25 | Proteus Digital Health, Inc. | Social media networking based on physiologic information |
| US11950615B2 (en) | 2014-01-21 | 2024-04-09 | Otsuka Pharmaceutical Co., Ltd. | Masticable ingestible product and communication system therefor |
| US10398161B2 (en) | 2014-01-21 | 2019-09-03 | Proteus Digital Heal Th, Inc. | Masticable ingestible product and communication system therefor |
| US9682234B2 (en) | 2014-11-17 | 2017-06-20 | Endostim, Inc. | Implantable electro-medical device programmable for improved operational life |
| US11051543B2 (en) | 2015-07-21 | 2021-07-06 | Otsuka Pharmaceutical Co. Ltd. | Alginate on adhesive bilayer laminate film |
| JPWO2017130646A1 (en)* | 2016-01-27 | 2018-07-05 | シャープ株式会社 | Biological signal processing device |
| US10187121B2 (en) | 2016-07-22 | 2019-01-22 | Proteus Digital Health, Inc. | Electromagnetic sensing and detection of ingestible event markers |
| US10797758B2 (en) | 2016-07-22 | 2020-10-06 | Proteus Digital Health, Inc. | Electromagnetic sensing and detection of ingestible event markers |
| US11793419B2 (en) | 2016-10-26 | 2023-10-24 | Otsuka Pharmaceutical Co., Ltd. | Methods for manufacturing capsules with ingestible event markers |
| US11529071B2 (en) | 2016-10-26 | 2022-12-20 | Otsuka Pharmaceutical Co., Ltd. | Methods for manufacturing capsules with ingestible event markers |
| US11819683B2 (en) | 2016-11-17 | 2023-11-21 | Endostim, Inc. | Modular stimulation system for the treatment of gastrointestinal disorders |
| US12053626B2 (en) | 2017-04-06 | 2024-08-06 | Endostim, Inc. | Surface electrodes |
| CN107823794A (en)* | 2017-12-01 | 2018-03-23 | 苏州景昱医疗器械有限公司 | The program control transponder of touch screen pen type, program control device and Implanted medical system |
| US11672934B2 (en) | 2020-05-12 | 2023-06-13 | Covidien Lp | Remote ventilator adjustment |
| US12144925B2 (en) | 2020-05-12 | 2024-11-19 | Covidien Lp | Remote ventilator adjustment |
Also Published As
| Publication number | Publication date |
|---|---|
| DE19927853B8 (en) | 2008-07-24 |
| FR2780224B1 (en) | 2007-12-21 |
| DE19927853A1 (en) | 2000-02-03 |
| DE19927853B4 (en) | 2008-04-03 |
| FR2780224A1 (en) | 1999-12-24 |
| US6477424B1 (en) | 2002-11-05 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| US6477424B1 (en) | Medical management system integrated programming apparatus for communication with an implantable medical device | |
| US5683432A (en) | Adaptive, performance-optimizing communication system for communicating with an implanted medical device | |
| US6115636A (en) | Telemetry for implantable devices using the body as an antenna | |
| US7463930B2 (en) | Implantable medical device programmer module for use with existing clinical instrumentation | |
| US6250309B1 (en) | System and method for transferring information relating to an implantable medical device to a remote location | |
| US5759199A (en) | System and method for ambulatory monitoring and programming of an implantable medical device | |
| US6201993B1 (en) | Medical device telemetry receiver having improved noise discrimination | |
| US7660630B2 (en) | Variable implantable medical device power characteristics based upon implant depth | |
| US6473638B2 (en) | Medical device GUI for cardiac electrophysiology display and data communication | |
| US4543955A (en) | System for controlling body implantable action device | |
| US6754538B2 (en) | Apparatus and method for remote self-identification of components in medical device systems | |
| US5899928A (en) | Descriptive transtelephonic pacing intervals for use by an emplantable pacemaker | |
| US8170667B2 (en) | Implantable cardiac monitor upgradeable to pacemaker or cardiac resynchronization device | |
| EP1239764B1 (en) | System and method for remote therapy and diagnosis in medical devices | |
| EP1168960B1 (en) | Information remote monitor (irm) medical device | |
| US6249705B1 (en) | Distributed network system for use with implantable medical devices | |
| US6580948B2 (en) | Interface devices for instruments in communication with implantable medical devices | |
| US20020193846A1 (en) | Instrumentation and software for remote monitoring and programming of implantable medical devices (IMDs) | |
| US8838251B2 (en) | Variable implantable medical device power characteristics based upon data or device type | |
| US7092761B1 (en) | Telemetry wand with display and control for implantable medical devices | |
| US20010047314A1 (en) | Apparatus and method for automated invoicing of medical device systems | |
| JP2004524074A (en) | Leadless fully automatic pacemaker follow-up | |
| WO2005030326A2 (en) | System and method for real-time remote monitoring of implantable medical devices |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| STCB | Information on status: application discontinuation | Free format text:ABANDONED -- FAILURE TO RESPOND TO AN OFFICE ACTION |