US20020120299A1 - Current waveforms for anti-tachycardia pacing for a subcutaneous implantable cardioverter- defibrillator - Google Patents
Current waveforms for anti-tachycardia pacing for a subcutaneous implantable cardioverter- defibrillatorDownload PDFInfo
- Publication number
- US20020120299A1 US20020120299A1US10/015,202US1520201AUS2002120299A1US 20020120299 A1US20020120299 A1US 20020120299A1US 1520201 AUS1520201 AUS 1520201AUS 2002120299 A1US2002120299 A1US 2002120299A1
- Authority
- US
- United States
- Prior art keywords
- approximately
- tachycardia pacing
- pacing energy
- energy comprises
- milliamps
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Granted
Links
- 208000001871TachycardiaDiseases0.000titleclaimsabstractdescription154
- 238000007920subcutaneous administrationMethods0.000titleclaimsabstractdescription81
- 239000003990capacitorSubstances0.000claimsabstractdescription27
- 210000004204blood vesselAnatomy0.000claimsabstractdescription6
- 230000002051biphasic effectEffects0.000claimsdescription89
- 238000000034methodMethods0.000claimsdescription55
- 210000001562sternumAnatomy0.000claimsdescription9
- 230000006794tachycardiaEffects0.000claimsdescription7
- 238000004146energy storageMethods0.000claims1
- 238000013194cardioversionMethods0.000description35
- 238000001514detection methodMethods0.000description23
- 210000000038chestAnatomy0.000description22
- 208000030990Impulse-control diseaseDiseases0.000description21
- 238000002560therapeutic procedureMethods0.000description18
- 230000033764rhythmic processEffects0.000description15
- 230000000747cardiac effectEffects0.000description14
- 208000003663ventricular fibrillationDiseases0.000description14
- 206010003658Atrial FibrillationDiseases0.000description13
- 238000002513implantationMethods0.000description9
- 230000037361pathwayEffects0.000description8
- 230000035939shockEffects0.000description8
- 230000002861ventricularEffects0.000description8
- 210000001991scapulaAnatomy0.000description6
- 208000010496Heart ArrestDiseases0.000description5
- 206010047281Ventricular arrhythmiaDiseases0.000description5
- 206010003119arrhythmiaDiseases0.000description5
- 230000006793arrhythmiaEffects0.000description5
- 208000006218bradycardiaDiseases0.000description5
- 239000002131composite materialSubstances0.000description5
- 238000013461designMethods0.000description5
- 238000003780insertionMethods0.000description5
- 230000037431insertionEffects0.000description5
- 238000012986modificationMethods0.000description5
- 230000004048modificationEffects0.000description5
- 230000008569processEffects0.000description5
- 206010003130Arrhythmia supraventricularDiseases0.000description4
- 229960003965antiepilepticsDrugs0.000description4
- 230000008859changeEffects0.000description4
- 230000001684chronic effectEffects0.000description4
- 230000012010growthEffects0.000description4
- 230000007774longtermEffects0.000description4
- 238000007726management methodMethods0.000description4
- 230000000069prophylactic effectEffects0.000description4
- 230000029058respiratory gaseous exchangeEffects0.000description4
- 238000012360testing methodMethods0.000description4
- 206010047302ventricular tachycardiaDiseases0.000description4
- 238000004873anchoringMethods0.000description3
- 230000036471bradycardiaEffects0.000description3
- 230000008878couplingEffects0.000description3
- 238000010168coupling processMethods0.000description3
- 238000005859coupling reactionMethods0.000description3
- 208000037265diseases, disorders, signs and symptomsDiseases0.000description3
- 208000035475disorderDiseases0.000description3
- 230000000694effectsEffects0.000description3
- 230000002600fibrillogenic effectEffects0.000description3
- 210000002837heart atriumAnatomy0.000description3
- 238000004519manufacturing processMethods0.000description3
- 239000000463materialSubstances0.000description3
- 238000012544monitoring processMethods0.000description3
- 239000004033plasticSubstances0.000description3
- 229920003023plasticPolymers0.000description3
- 206010040560shockDiseases0.000description3
- 210000001519tissueAnatomy0.000description3
- NNJVILVZKWQKPM-UHFFFAOYSA-NLidocaineChemical compoundCCN(CC)CC(=O)NC1=C(C)C=CC=C1CNNJVILVZKWQKPM-UHFFFAOYSA-N0.000description2
- 102100026827Protein associated with UVRAG as autophagy enhancerHuman genes0.000description2
- 101710102978Protein associated with UVRAG as autophagy enhancerProteins0.000description2
- 206010042600Supraventricular arrhythmiasDiseases0.000description2
- 229910001069Ti alloyInorganic materials0.000description2
- 230000001746atrial effectEffects0.000description2
- 230000008901benefitEffects0.000description2
- 230000000981bystanderEffects0.000description2
- 206010061592cardiac fibrillationDiseases0.000description2
- 238000002224dissectionMethods0.000description2
- 238000002695general anesthesiaMethods0.000description2
- 239000007943implantSubstances0.000description2
- 230000006698inductionEffects0.000description2
- 230000001788irregularEffects0.000description2
- 229960004194lidocaineDrugs0.000description2
- 239000003589local anesthetic agentSubstances0.000description2
- 210000003205muscleAnatomy0.000description2
- 210000004165myocardiumAnatomy0.000description2
- 230000035479physiological effects, processes and functionsEffects0.000description2
- 230000035945sensitivityEffects0.000description2
- 230000001360synchronised effectEffects0.000description2
- 230000007704transitionEffects0.000description2
- 229910001260Pt alloyInorganic materials0.000description1
- 206010040047SepsisDiseases0.000description1
- 208000003734Supraventricular TachycardiaDiseases0.000description1
- 206010065341Ventricular tachyarrhythmiaDiseases0.000description1
- 210000001015abdomenAnatomy0.000description1
- 230000003187abdominal effectEffects0.000description1
- 230000003213activating effectEffects0.000description1
- 230000002411adverseEffects0.000description1
- 229910045601alloyInorganic materials0.000description1
- 239000000956alloySubstances0.000description1
- 230000002763arrhythmic effectEffects0.000description1
- 230000004323axial lengthEffects0.000description1
- 238000005452bendingMethods0.000description1
- 210000005242cardiac chamberAnatomy0.000description1
- 230000005800cardiovascular problemEffects0.000description1
- 238000012790confirmationMethods0.000description1
- 230000006378damageEffects0.000description1
- 230000001862defibrillatory effectEffects0.000description1
- 230000003467diminishing effectEffects0.000description1
- 230000009977dual effectEffects0.000description1
- 230000002526effect on cardiovascular systemEffects0.000description1
- 238000013195electrical cardioversionMethods0.000description1
- 238000005516engineering processMethods0.000description1
- 238000011156evaluationMethods0.000description1
- 210000005003heart tissueAnatomy0.000description1
- 230000001939inductive effectEffects0.000description1
- 238000009413insulationMethods0.000description1
- 230000003601intercostal effectEffects0.000description1
- 238000001990intravenous administrationMethods0.000description1
- 230000007257malfunctionEffects0.000description1
- 230000003211malignant effectEffects0.000description1
- 229910052751metalInorganic materials0.000description1
- 239000002184metalSubstances0.000description1
- 150000002739metalsChemical class0.000description1
- 230000021332multicellular organism growthEffects0.000description1
- 239000000615nonconductorSubstances0.000description1
- 238000006213oxygenation reactionMethods0.000description1
- 230000002085persistent effectEffects0.000description1
- 229920001296polysiloxanePolymers0.000description1
- 229920002635polyurethanePolymers0.000description1
- 239000004814polyurethaneSubstances0.000description1
- 230000009862primary preventionEffects0.000description1
- 230000005180public healthEffects0.000description1
- 230000000241respiratory effectEffects0.000description1
- 230000036387respiratory rateEffects0.000description1
- 230000004044responseEffects0.000description1
- 210000005241right ventricleAnatomy0.000description1
- 230000037390scarringEffects0.000description1
- 238000002633shock therapyMethods0.000description1
- 210000004872soft tissueAnatomy0.000description1
- 238000010561standard procedureMethods0.000description1
- 238000001356surgical procedureMethods0.000description1
- 210000000115thoracic cavityAnatomy0.000description1
- 238000012549trainingMethods0.000description1
- 210000000591tricuspid valveAnatomy0.000description1
- 210000002620vena cava superiorAnatomy0.000description1
Images
Classifications
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61N—ELECTROTHERAPY; MAGNETOTHERAPY; RADIATION THERAPY; ULTRASOUND THERAPY
- A61N1/00—Electrotherapy; Circuits therefor
- A61N1/18—Applying electric currents by contact electrodes
- A61N1/32—Applying electric currents by contact electrodes alternating or intermittent currents
- A61N1/36—Applying electric currents by contact electrodes alternating or intermittent currents for stimulation
- A61N1/372—Arrangements in connection with the implantation of stimulators
- A61N1/375—Constructional arrangements, e.g. casings
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61N—ELECTROTHERAPY; MAGNETOTHERAPY; RADIATION THERAPY; ULTRASOUND THERAPY
- A61N1/00—Electrotherapy; Circuits therefor
- A61N1/02—Details
- A61N1/04—Electrodes
- A61N1/05—Electrodes for implantation or insertion into the body, e.g. heart electrode
- A61N1/0504—Subcutaneous electrodes
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61N—ELECTROTHERAPY; MAGNETOTHERAPY; RADIATION THERAPY; ULTRASOUND THERAPY
- A61N1/00—Electrotherapy; Circuits therefor
- A61N1/18—Applying electric currents by contact electrodes
- A61N1/32—Applying electric currents by contact electrodes alternating or intermittent currents
- A61N1/36—Applying electric currents by contact electrodes alternating or intermittent currents for stimulation
- A61N1/372—Arrangements in connection with the implantation of stimulators
- A61N1/375—Constructional arrangements, e.g. casings
- A61N1/37512—Pacemakers
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61N—ELECTROTHERAPY; MAGNETOTHERAPY; RADIATION THERAPY; ULTRASOUND THERAPY
- A61N1/00—Electrotherapy; Circuits therefor
- A61N1/18—Applying electric currents by contact electrodes
- A61N1/32—Applying electric currents by contact electrodes alternating or intermittent currents
- A61N1/38—Applying electric currents by contact electrodes alternating or intermittent currents for producing shock effects
- A61N1/39—Heart defibrillators
- A61N1/3906—Heart defibrillators characterised by the form of the shockwave
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61N—ELECTROTHERAPY; MAGNETOTHERAPY; RADIATION THERAPY; ULTRASOUND THERAPY
- A61N1/00—Electrotherapy; Circuits therefor
- A61N1/18—Applying electric currents by contact electrodes
- A61N1/32—Applying electric currents by contact electrodes alternating or intermittent currents
- A61N1/38—Applying electric currents by contact electrodes alternating or intermittent currents for producing shock effects
- A61N1/39—Heart defibrillators
- A61N1/3956—Implantable devices for applying electric shocks to the heart, e.g. for cardioversion
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61N—ELECTROTHERAPY; MAGNETOTHERAPY; RADIATION THERAPY; ULTRASOUND THERAPY
- A61N1/00—Electrotherapy; Circuits therefor
- A61N1/18—Applying electric currents by contact electrodes
- A61N1/32—Applying electric currents by contact electrodes alternating or intermittent currents
- A61N1/38—Applying electric currents by contact electrodes alternating or intermittent currents for producing shock effects
- A61N1/39—Heart defibrillators
- A61N1/3956—Implantable devices for applying electric shocks to the heart, e.g. for cardioversion
- A61N1/3962—Implantable devices for applying electric shocks to the heart, e.g. for cardioversion in combination with another heart therapy
- A61N1/39622—Pacing therapy
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61N—ELECTROTHERAPY; MAGNETOTHERAPY; RADIATION THERAPY; ULTRASOUND THERAPY
- A61N1/00—Electrotherapy; Circuits therefor
- A61N1/18—Applying electric currents by contact electrodes
- A61N1/32—Applying electric currents by contact electrodes alternating or intermittent currents
- A61N1/36—Applying electric currents by contact electrodes alternating or intermittent currents for stimulation
- A61N1/362—Heart stimulators
- A61N1/3621—Heart stimulators for treating or preventing abnormally high heart rate
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61N—ELECTROTHERAPY; MAGNETOTHERAPY; RADIATION THERAPY; ULTRASOUND THERAPY
- A61N1/00—Electrotherapy; Circuits therefor
- A61N1/18—Applying electric currents by contact electrodes
- A61N1/32—Applying electric currents by contact electrodes alternating or intermittent currents
- A61N1/36—Applying electric currents by contact electrodes alternating or intermittent currents for stimulation
- A61N1/372—Arrangements in connection with the implantation of stimulators
- A61N1/375—Constructional arrangements, e.g. casings
- A61N1/3756—Casings with electrodes thereon, e.g. leadless stimulators
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61N—ELECTROTHERAPY; MAGNETOTHERAPY; RADIATION THERAPY; ULTRASOUND THERAPY
- A61N1/00—Electrotherapy; Circuits therefor
- A61N1/18—Applying electric currents by contact electrodes
- A61N1/32—Applying electric currents by contact electrodes alternating or intermittent currents
- A61N1/38—Applying electric currents by contact electrodes alternating or intermittent currents for producing shock effects
- A61N1/39—Heart defibrillators
- A61N1/3968—Constructional arrangements, e.g. casings
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61N—ELECTROTHERAPY; MAGNETOTHERAPY; RADIATION THERAPY; ULTRASOUND THERAPY
- A61N1/00—Electrotherapy; Circuits therefor
- A61N1/18—Applying electric currents by contact electrodes
- A61N1/32—Applying electric currents by contact electrodes alternating or intermittent currents
- A61N1/38—Applying electric currents by contact electrodes alternating or intermittent currents for producing shock effects
- A61N1/39—Heart defibrillators
- A61N1/3975—Power supply
Definitions
- the present inventionrelates to an apparatus and method for performing electrical cardioversion/defibrillation and optional pacing of the heart via a totally subcutaneous non-transvenous system.
- Defibrillation/cardioversionis a technique employed to counter arrhythmic heart conditions including some tachycardias in the atria and/or ventricles.
- electrodesare employed to stimulate the heart with electrical impulses or shocks, of a magnitude substantially greater than pulses used in cardiac pacing.
- Shocks used for defibrillation therapycan comprise a biphasic truncated exponential waveform.
- pacinga constant current density is desired to reduce or eliminate variability due to the electrode/tissue interface
- ICDsimplantable cardioverter/defibrillators
- the electrodes used in ICDscan be in the form of patches applied directly to epicardial tissue, or, more commonly, are on the distal regions of small cylindrical insulated catheters that typically enter the subclavian venous system, pass through the superior vena cava and, into one or more endocardial areas of the heart.
- Such electrode systemsare called intravascular or transvenous electrodes.
- ICDsare now an established therapy for the management of life threatening cardiac rhythm disorders, primarily ventricular fibrillation (V-Fib). ICDs are very effective at treating V-Fib, but are therapies that still require significant surgery.
- V-Fibventricular fibrillation
- transvenous ICD systemsalso increase cost and require specialized interventional rooms and equipment as well as special skill for insertion. These systems are typically implanted by cardiac electrophysiologists who have had a great deal of extra training.
- AEDautomatic external defibrillator
- AEDsemploy the use of cutaneous patch electrodes, rather than implantable lead systems, to effect defibrillation under the direction of a bystander user who treats the patient suffering from V-Fib with a portable device containing the necessary electronics and power supply that allows defibrillation.
- AEDscan be nearly as effective as an ICD for defibrillation if applied to the victim of ventricular fibrillation promptly, i.e., within 2 to 3 minutes of the onset of the ventricular fibrillation.
- AED therapyhas great appeal as a tool for diminishing the risk of death in public venues such as in air flight.
- an AEDmust be used by another individual, not the person suffering from the potential fatal rhythm. It is more of a public health tool than a patient-specific tool like an ICD. Because >75% of cardiac arrests occur in the home, and over half occur in the bedroom, patients at risk of cardiac arrest are often alone or asleep and can not be helped in time with an AED. Moreover, its success depends to a reasonable degree on an acceptable level of skill and calm by the bystander user.
- a power supply for an implantable cardioverter-defibrillator for subcutaneous positioning between the third rib and the twelfth rib and using a lead system that does not directly contact a patient's heart or reside in the intrathorasic blood vessels and for providing anti-tachycardia pacing energy to the heartcomprising a capacitor subsystem for storing the anti-tachycardia pacing energy for delivery to the patient's heart; and a battery subsystem electrically coupled to the capacitor subsystem for providing the anti-tachycardia pacing energy to the capacitor subsystem.
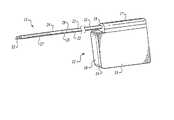
- FIG. 1is a schematic view of a Subcutaneous ICD (S-ICD) of the present invention
- FIG. 2is a schematic view of an alternate embodiment of a subcutaneous electrode of the present invention.
- FIG. 3is a schematic view of an alternate embodiment of a subcutaneous electrode of the present invention.
- FIG. 4is a schematic view of the S-ICD and lead of FIG. 1 subcutaneously implanted in the thorax of a patient;
- FIG. 5is a schematic view of the S-ICD and lead of FIG. 2 subcutaneously implanted in an alternate location within the thorax of a patient;
- FIG. 6is a schematic view of the S-ICD and lead of FIG. 3 subcutaneously implanted in the thorax of a patient;
- FIG. 7is a schematic view of the method of making a subcutaneous path from the preferred incision and housing implantation point to a termination point for locating a subcutaneous electrode of the present invention
- FIG. 8is a schematic view of an introducer set for performing the method of lead insertion of any of the described embodiments
- FIG. 9is a schematic view of an alternative S-ICD of the present invention illustrating a lead subcutaneously and serpiginously implanted in the thorax of a patient for use particularly in children;
- FIG. 10is a schematic view of an alternate embodiment of an S-ICD of the present invention.
- FIG. 11is a schematic view of the S-ICD of FIG. 10 subcutaneously implanted in the thorax of a patient;
- FIG. 12is a schematic view of yet a further embodiment where the canister of the S-ICD of the present invention is shaped to be particularly useful in placing subcutaneously adjacent and parallel to a rib of a patient;
- FIG. 13is a schematic of a different embodiment where the canister of the S-ICD of the present invention is shaped to be particularly useful in placing subcutaneously adjacent and parallel to a rib of a patient;
- FIG. 14is a schematic view of a Unitary Subcutaneous ICD (US-ICD) of the present invention.
- FIG. 15is a schematic view of the US-ICD subcutaneously implanted in the thorax of a patient
- FIG. 16is a schematic view of the method of making a subcutaneous path from the preferred incision for implanting the US-ICD;
- FIG. 17is a schematic view of an introducer for performing the method of US-ICD implantation
- FIG. 18is an exploded schematic view of an alternate embodiment of the present invention with a plug-in portion that contains operational circuitry and means for generating cardioversion/defibrillation shock waves;
- FIG. 19is a graph that shows an example of a biphasic waveform for use in anti-tachycardia pacing in an embodiment of the present invention.
- FIG. 20is a graph that shows an example of a monophonic waveform for use in anti-tachycardia pacing in an embodiment of the present invention.
- the S-ICDconsists of an electrically active canister 11 and a subcutaneous electrode 13 attached to the canister.
- the canisterhas an electrically active surface 15 that is electrically insulated from the electrode connector block 17 and the canister housing 16 via insulating area 14 .
- the canistercan be similar to numerous electrically active canisters commercially available in that the canister will contain a battery supply, capacitor and operational circuitry. Alternatively, the canister can be thin and elongated to conform to the intercostal space.
- the circuitrywill be able to monitor cardiac rhythms for tachycardia and fibrillation, and if detected, will initiate charging the capacitor and then delivering cardioversion/defibrillation energy through the active surface of the housing and to the subcutaneous electrode. Examples of such circuitry are described in U.S. Pat. Nos. 4,693,253 and 5,105,810, the entire disclosures of which are herein incorporated by reference.
- the canister circuitrycan provide cardioversion/defibrillation energy in different types of waveforms.
- a 100 uF biphasic waveformis used of approximately 10-20 ms total duration and with the initial phase containing approximately 2 ⁇ 3 of the energy, however, any type of waveform can be utilized such as monophasic, biphasic, multiphasic or alternative waveforms as is known in the art.
- the circuitrycan also provide transthoracic cardiac pacing energy.
- the optional circuitrywill be able to monitor the heart for bradycardia and/or tachycardia rhythms. Once a bradycardia or tachycardia rhythm is detected, the circuitry can then deliver appropriate pacing energy at appropriate intervals through the active surface and the subcutaneous electrode.
- Pacing stimulican be biphasic in one embodiment and similar in pulse amplitude to that used for conventional transthoracic pacing.
- This same circuitrycan also be used to deliver low amplitude shocks on the T-wave for induction of ventricular fibrillation for testing S-ICD performance in treating V-Fib as is described in U.S. Pat. No. 5,129,392, the entire disclosure of which is hereby incorporated by reference.
- the circuitrycan be provided with rapid induction of ventricular fibrillation or ventricular tachycardia using rapid ventricular pacing.
- Another optional way for inducing ventricular fibrillationwould be to provide a continuous low voltage, i.e., about 3 volts, across the heart during the entire cardiac cycle.
- the operational circuitrycan detect the presence of atrial fibrillation as described in Olson, W. et al. “Onset And Stability For Ventricular Tachyarrhythmia Detection in an Implantable Cardioverter and Defibrillator,” Computers in Cardiology (1986) pp. 167-170. Detection can be provided via R-R Cycle length instability detection algorithms. Once atrial fibrillation has been detected, the operational circuitry will then provide QRS synchronized atrial defibrillation/cardioversion using the same shock energy and waveshape characteristics used for ventricular defibrillation/cardioversion.
- the sensing circuitrywill utilize the electronic signals generated from the heart and will primarily detect QRS waves.
- the circuitrywill be programmed to detect only ventricular tachycardias or fibrillations.
- the detection circuitrywill utilize in its most direct form, a rate detection algorithm that triggers charging of the capacitor once the ventricular rate exceeds some predetermined level for a fixed period of time: for example, if the ventricular rate exceeds 240 bpm on average for more than 4 seconds. Once the capacitor is charged, a confirmatory rhythm check would ensure that the rate persists for at least another 1 second before discharge.
- termination algorithmscould be instituted that ensure that a rhythm less than 240 bpm persisting for at least 4 seconds before the capacitor charge is drained to an internal resistor.
- Detection, confirmation and termination algorithmsas are described above and in the art can be modulated to increase sensitivity and specificity by examining QRS beat-to-beat uniformity, QRS signal frequency content, R-R interval stability data, and signal amplitude characteristics all or part of which can be used to increase or decrease both sensitivity and specificity of S-ICD arrhythmia detection function.
- the sense circuitrycan check for the presence or the absence of respiration.
- the respiration ratecan be detected by monitoring the impedance across the thorax using subthreshold currents delivered across the active can and the high voltage subcutaneous lead electrode and monitoring the frequency in undulation in the waveform that results from the undulations of transthoracic impedance during the respiratory cycle. If there is no undulation, then the patent is not respiring and this lack of respiration can be used to confirm the QRS findings of cardiac arrest.
- the same techniquecan be used to provide information about the respiratory rate or estimate cardiac output as described in U.S. Pat. Nos. 6,095,987, 5,423,326, 4,450,527, the entire disclosures of which are incorporated herein by reference.
- the canister of the present inventioncan be made out of titanium alloy or other presently preferred electrically active canister designs. However, it is contemplated that a malleable canister that can conform to the curvature of the patient's chest will be preferred. In this way the patient can have a comfortable canister that conforms to the shape of the patient's rib cage. Examples of conforming canisters are provided in U.S. Pat. No. 5,645,586, the entire disclosure of which is herein incorporated by reference. Therefore, the canister can be made out of numerous materials such as medical grade plastics, metals, and alloys. In the preferred embodiment, the canister is smaller than 60 cc volume having a weight of less than 100 gms for long term wearability, especially in children.
- the canister and the lead of the S-ICDcan also use fractal or wrinkled surfaces to increase surface area to improve defibrillation capability. Because of the primary prevention role of the therapy and the likely need to reach energies over 40 Joules, a feature of one embodiment is that the charge time for the therapy, is intentionally left relatively long to allow capacitor charging within the limitations of device size. Examples of small ICD housings are disclosed in U.S. Pat. Nos. 5,597,956 and 5,405,363, the entire disclosures of which are herein incorporated by reference.
- FIGS. 1 - 3Different subcutaneous electrodes 13 of the present invention are illustrated in FIGS. 1 - 3 .
- the lead 21 for the subcutaneous electrodeis preferably composed of silicone or polyurethane insulation.
- the electrodeis connected to the canister at its proximal end via connection port 19 which is located on an electrically insulated area 17 of the canister.
- the electrode illustratedis a composite electrode with three different electrodes attached to the lead.
- an optional anchor segment 52is attached at the most distal end of the subcutaneous electrode for anchoring the electrode into soft tissue such that the electrode does not dislodge after implantation.
- the most distal electrode on the composite subcutaneous electrodeis a coil electrode 27 that is used for delivering the high voltage cardioversion/defibrillation energy across the heart.
- the coil cardioversion/defibrillation electrodeis about 5-10 cm in length.
- Proximal to the coil electrodeare two sense electrodes, a first sense electrode 25 is located proximally to the coil electrode and a second sense electrode 23 is located proximally to the first sense electrode.
- the sense electrodesare spaced far enough apart to be able to have good QRS detection. This spacing can range from 1 to 10 cm with 4 cm being presently preferred.
- the electrodesmay or may not be circumferential with the preferred embodiment.
- the sensing electrodesare electrically isolated from the cardioversion/defibrillation electrode via insulating areas 29 .
- Similar types of cardioversion/defibrillation electrodesare currently commercially available in a transvenous configuration.
- U.S. Pat. No. 5,534,022the entire disclosure of which is herein incorporated by reference, disclosures a composite electrode with a coil cardioversion/defibrillation electrode and sense electrodes. Modifications to this arrangement is contemplated within the scope of the invention. One such modification is illustrated in FIG.
- FIG. 3illustrates yet a further embodiment where the two sensing electrodes are located at the distal end to the composite electrode with the coil electrode located proximally thereto.
- the sensing of QRS wavescan be carried out via sense electrodes on the canister housing or in combination with the cardioversion/defibrillation coil electrode and/or the subcutaneous lead sensing electrode(s) In this way, sensing could be performed via the one coil electrode located on the subcutaneous electrode and the active surface on the canister housing. Another possibility would be to have only one sense electrode located on the subcutaneous electrode and the sensing would be performed by that one electrode and either the coil electrode on the subcutaneous electrode or by the active surface of the canister. The use of sensing electrodes on the canister would eliminate the need for sensing electrodes on the subcutaneous electrode.
- the subcutaneous electrodewould be provided with at least one sense electrode, the canister with at least one sense electrode, and if multiple sense electrodes are used on either the subcutaneous electrode and/or the canister, that the best QRS wave detection combination will be identified when the S-ICD is implanted and this combination can be selected, activating the best sensing arrangement from all the existing sensing possibilities.
- two sensing electrodes 26 and 28are located on the electrically active surface 15 with electrical insulator rings 30 placed between the sense electrodes and the active surface. These canister sense electrodes could be switched off and electrically insulated during and shortly after defibrillation/ cardioversion shock delivery.
- the canister sense electrodesmay also be placed on the electrically inactive surface of the canister.
- sensing electrodesthere are actually four sensing electrodes, two on the subcutaneous lead and two on the canister.
- the ability to change which electrodes are used for sensingwould be a programmable feature of the S-ICD to adapt to changes in the patient physiology and size (in the case of children) over time.
- the programmingcould be done via the use of physical switches on the canister, or as presently preferred, via the use of a programming wand or via a wireless connection to program the circuitry within the canister.
- the canistercould be employed as either a cathode or an anode of the S-ICD cardioversion/defibrillation system. If the canister is the cathode, then the subcutaneous coil electrode would be the anode. Likewise, if the canister is the anode, then the subcutaneous electrode would be the cathode.
- the active canister housingwill provide energy and voltage intermediate to that available with ICDs and most AEDs.
- the typical maximum voltage necessary for ICDs using most biphasic waveformsis approximately 750 Volts with an associated maximum energy of approximately 40 Joules.
- the typical maximum voltage necessary for AEDsis approximately 2000-5000 Volts with an associated maximum energy of approximately 200-360 Joules depending upon the model and waveform used.
- the S-ICD and the US-ICD of the present inventionuses maximum voltages in the range of about 50 to about 3500 Volts and is associated with energies of about 0.5 to about 350 Joules.
- the capacitance of the devicescan range from about 25 to about 200 micro farads.
- the S-ICD and US-ICD devicesprovide energy with a pulse width of approximately one millisecond to approximately 40 milliseconds.
- the devicescan provide pacing current of approximately one milliamp to approximately 250 milliamps.
- the sense circuitry contained within the canisteris highly sensitive and specific for the presence or absence of life threatening ventricular arrhythmias.
- Features of the detection algorithmare programmable and the algorithm is focused on the detection of V-FIB and high rate V-TACH (>240 bpm).
- V-FIB and high rate V-TACH>240 bpm.
- the S-ICD of the present inventionmay rarely be used for an actual life-threatening event, the simplicity of design and implementation allows it to be employed in large populations of patients at modest risk with modest cost by non-cardiac electrophysiologists. Consequently, the S-ICD of the present invention focuses mostly on the detection and therapy of the most malignant rhythm disorders.
- the upper rate rangeis programmable upward for use in children, known to have rapid supraventricular tachycardias and more rapid ventricular fibrillation. Energy levels also are programmable downward in order to allow treatment of neonates and infants.
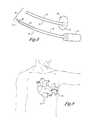
- FIG. 4the optimal subcutaneous placement of the S-ICD of the present invention is illustrated.
- the actual location of the S-ICDis in a subcutaneous space that is developed during the implantation process.
- the heartis not exposed during this process and the heart is schematically illustrated in the figures only for help in understanding where the canister and coil electrode are three dimensionally located in the left mid-clavicular line approximately at the level of the inframammary crease at approximately the 5th rib.
- the lead 21 of the subcutaneous electrodetraverses in a subcutaneous path around the thorax terminating with its distal electrode end at the posterior axillary line ideally just lateral to the left scapula. This way the canister and subcutaneous cardioversion/defibrillation electrode provide a reasonably good pathway for current delivery to the majority of the ventricular myocardium.
- FIG. 5illustrates a different placement of the present invention.
- the S-ICD canister with the active housingis located in the left posterior axillary line approximately lateral to the tip of the inferior portion of the scapula. This location is especially useful in children.
- the lead 21 of the subcutaneous electrodetraverses in a subcutaneous path around the thorax terminating with its distal electrode end at the anterior precordial region, ideally in the inframammary crease.
- FIG. 6illustrates the embodiment of FIG.
- proximal sense electrodes 23 and 25located at approximately the left axillary line with the cardioversion/defibrillation electrode just lateral to the tip of the inferior portion of the scapula.
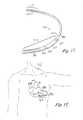
- FIG. 7schematically illustrates the method for implanting the S-ICD of the present invention.
- An incision 31is made in the left anterior axillary line approximately at the level of the cardiac apex. This incision location is distinct from that chosen for S-ICD placement and is selected specifically to allow both canister location more medially in the left inframammary crease and lead positioning more posteriorly via the introducer set (described below) around to the left posterior axillary line lateral to the left scapula. That said, the incision can be anywhere on the thorax deemed reasonably by the implanting physician although in the preferred embodiment, the S-ICD of the present invention will be applied in this region.
- a subcutaneous pathway 33is then created medially to the inframmary crease for the canister and posteriorly to the left posterior axillary line lateral to the left scapula for the lead.
- the S-ICD canister 11is then placed subcutaneously at the location of the incision or medially at the subcutaneous region at the left inframmary crease.
- the subcutaneous electrode 13is placed with a specially designed curved introducer set 40 (see FIG. 8).
- the introducer setcomprises a curved trocar 42 and a stiff curved peel away sheath 44 .
- the peel away sheathis curved to allow for placement around the rib cage of the patient in the subcutaneous space created by the trocar.
- the sheathhas to be stiff enough to allow for the placement of the electrodes without the sheath collapsing or bending.
- the sheathis made out of a biocompatible plastic material and is perforated along its axial length to allow for it to split apart into two sections.
- the trocarhas a proximal handle 41 and a curved shaft 43 .
- the distal end 45 of the trocaris tapered to allow for dissection of a subcutaneous path 33 in the patient.
- the trocaris cannulated having a central Lumen 46 and terminating in an opening 48 at the distal end.
- Local anestheticsuch as lidocaine can be delivered, if necessary, through the lumen or through a curved and elongated needle designed to anesthetize the path to be used for trocar insertion should general anesthesia not be employed.
- the curved peel away sheath 44has a proximal pull tab 49 for breaking the sheath into two halves along its axial shaft 47 .
- the sheathis placed over a guidewire inserted through the trocar after the subcutaneous path has been created.
- the subcutaneous pathwayis then developed until it terminates subcutaneously at a location that, if a straight line were drawn from the canister location to the path termination point the line would intersect a substantial portion of the left ventricular mass of the patient.
- the guidewireis then removed leaving the peel away sheath.
- the subcutaneous lead systemis then inserted through the sheath until it is in the proper location. Once the subcutaneous lead system is in the proper location, the sheath is split in half using the pull tab 49 and removed. If more than one subcutaneous electrode is being used, a new curved peel away sheath can be used for each subcutaneous electrode.
- the S-ICDwill have prophylactic use in adults where chronic transvenous/epicardial ICD lead systems pose excessive risk or have already resulted in difficulty, such as sepsis or lead fractures. It is also contemplated that a major use of the S-ICD system of the present invention will be for prophylactic use in children who are at risk for having fatal arrhythmias, where chronic transvenous lead systems pose significant management problems. Additionally, with the use of standard transvenous ICDs in children, problems develop during patient growth in that the lead system does not accommodate the growth. FIG. 9 illustrates the placement of the S-ICD subcutaneous lead system such that he problem that growth presents to the lead system is overcome.
- the distal end of the subcutaneous electrodeis placed in the same location as described above providing a good location for the coil cardioversion/defibrillation electrode 27 and the sensing electrodes 23 and 25 .
- the insulated lead 21is no longer placed in a taught configuration. Instead, the lead is serpiginously placed with a specially designed introducer trocar and sheath such that it has numerous waves or bends. As the child grows, the waves or bends will straighten out lengthening the lead system while maintaining proper electrode placement.
- a lead system with a distal tine or screw electrode anchoring system 52can also be incorporated into the distal tip of the lead to facilitate lead stability (see FIG. 1).
- Other anchoring systemscan also be used such as hooks, sutures, or the like.
- FIGS. 10 and 11illustrate another embodiment of the present S-ICD invention.
- the additional subcutaneous electrode 13 ′is essentially identical to the previously described electrode.
- the cardioversion/defibrillation energyis delivered between the active surface of the canister and the two coil electrodes 27 and 27 ′.
- provided in the canisteris means for selecting the optimum sensing arrangement between the four sense electrodes 23 , 23 ′, 25 , and 25 ′.
- the two electrodesare subcutaneously placed on the same side of the heart. As illustrated in FIG. 6, one subcutaneous electrode 13 is placed inferiorly and the other electrode 13 ′ is placed superiorly. It is also contemplated with this dual subcutaneous electrode system that the canister and one subcutaneous electrode are the same polarity and the other subcutaneous electrode is the opposite polarity.
- FIGS. 12 and 13further embodiments are illustrated where the canister 11 of the S-ICD of the present invention is shaped to be particularly useful in placing subcutaneously adjacent and parallel to a rib of a patient.
- the canisteris long, thin, and curved to conform to the shape of the patient's rib.
- the canisterhas a diameter ranging from about 0.5 cm to about 2 cm without 1 cm being presently preferred.
- the canistercould have a rectangular or square cross sectional area as illustrated in FIG. 13 without falling outside of the scope of the present invention.
- the length of the canistercan vary depending on the size of the patient's thorax.
- the canisteris about 5 cm to about 40 cm long.
- the canisteris curved to conform to the curvature of the ribs of the thorax.
- the radius of the curvaturewill vary depending on the size of the patient, with smaller radiuses for smaller patients and larger radiuses for larger patients.
- the radius of the curvaturecan range from about 5 cm to about 35 cm depending on the size of the patient. Additionally, the radius of the curvature need not be uniform throughout the canister such that it can be shaped closer to the shape of the ribs.
- the canisterhas an active surface, 15 that is located on the interior (concave) portion of the curvature and an inactive surface 16 that is located on the exterior (convex) portion of the curvature.
- the leads of these embodimentswhich are not illustrated except for the attachment port 19 and the proximal end of the lead 21 , can be any of the leads previously described above, with the lead illustrated in FIG. 1 being presently preferred.
- the circuitry of this canisteris similar to the circuitry described above. Additionally, the canister can optionally have at least one sense electrode located on either the active surface of the inactive surface and the circuitry within the canister can be programmable as described above to allow for the selection of the best sense electrodes. It is presently preferred that the canister have two sense electrodes 26 and 28 located on the inactive surface of the canisters as illustrated, where the electrodes are spaced from about 1 to about 10 cm apart with a spacing of about 3 cm being presently preferred. However, the sense electrodes can be located on the active surface as described above.
- FIG. 12It is envisioned that the embodiment of FIG. 12 will be subcutaneously implanted adjacent and parallel to the left anterior 5th rib, either between the 4th and 5th ribs or between the 5th and 6th ribs. However other locations can be used.
- Another component of the S-ICD of the present inventionis a cutaneous test electrode system designed to simulate the subcutaneous high voltage shock electrode system as well as the QRS cardiac rhythm detection system.
- This test electrode systemis comprised of a cutaneous patch electrode of similar surface area and impedance to that of the S-ICD canister itself together with a cutaneous strip electrode comprising a defibrillation strip as well as two button electrodes for sensing of the QRS.
- Several cutaneous strip electrodesare available to allow for testing various bipole spacings to optimize signal detection comparable to the implantable system.
- FIGS. 14 to 18depict particular US-ICD embodiments of the present invention.
- the various sensing, shocking and pacing circuitry, described in detail above with respect to the S-ICD embodiments,may additionally be incorporated into the following US-ICD embodiments.
- particular aspects of any individual S-ICD embodiment discussed above,may be incorporated, in whole or in part, into the US-ICD embodiments depicted in the following figures.
- the US-ICDconsists of a curved housing 1211 with a first and second end.
- the first end 1413is thicker than the second end 1215 .
- This thicker areahouses a battery supply, capacitor and operational circuitry for the US-ICD.
- the circuitrywill be able to monitor cardiac rhythms for tachycardia and fibrillation, and if detected, will initiate charging the capacitor and then delivering cardioversion/defibrillation energy through the two cardioversion/defibrillating electrodes 1417 and 1219 located on the outer surface of the two ends of the housing.
- the circuitrycan provide cardioversion/defibrillation energy in different types of waveforms.
- a 100 uF biphasic waveformis used of approximately 10-20 ms total duration and with the initial phase containing approximately 2 ⁇ 3 of the energy, however, any type of waveform can be utilized such as monophasic, biphasic, multiphasic or alternative waveforms as is known in the art.
- the housing of the present inventioncan be made out of titanium alloy or other presently preferred ICD designs. It is contemplated that the housing is also made out of biocompatible plastic materials that electronically insulate the electrodes from each other. However, it is contemplated that a malleable canister that can conform to the curvature of the patient's chest will be preferred. In this way the patient can have a comfortable canister that conforms to the unique shape of the patient's rib cage. Examples of conforming ICD housings are provided in U.S. Pat. No. 5,645,586, the entire disclosure of which is herein incorporated by reference. In the preferred embodiment, the housing is curved in the shape of a 5 th rib of a person.
- the housingwill come in different incremental sizes to allow a good match between the size of the rib cage and the size of the US-ICD.
- the length of the US-ICDwill range from about 15 to about 50 cm. Because of the primary preventative role of the therapy and the need to reach energies over 40 Joules, a feature of the preferred embodiment is that the charge time for the therapy, intentionally be relatively long to allow capacitor charging within the limitations of device size.
- the thick end of the housingis currently needed to allow for the placement of the battery supply, operational circuitry, and capacitors. It is contemplated that the thick end will be about 0.5 cm to about 2 cm wide with about 1 cm being presently preferred. As microtechnology advances, the thickness of the housing will become smaller.
- the two cardioversion/defibrillation electrodes on the housingare used for delivering the high voltage cardioversion/defibrillation energy across the heart.
- the cardioversion/defibrillation electrodesare coil electrodes, however, other cardioversion/defibrillation electrodes could be used such as having electrically isolated active surfaces or platinum alloy electrodes.
- the coil cardioversion/defibrillation electrodesare about 5-10 cm in length.
- Located on the housing between the two cardioversion/defibrillation electrodesare two sense electrodes 1425 and 1427 . The sense electrodes are spaced far enough apart to be able to have good QRS detection. This spacing can range from 1 to 10 cm with 4 cm being presently preferred.
- the electrodesmay or may not be circumferential with the preferred embodiment. Having the electrodes non-circumferential and positioned outward, toward the skin surface, is a means to minimize muscle artifact and enhance QRS signal quality.
- the sensing electrodesare electrically isolated from the cardioversion/defibrillation electrode via insulating areas 1423 .
- Analogous types of cardioversion/defibrillation electrodesare currently commercially available in a transvenous configuration.
- U.S. Pat. No. 5,534,022the entire disclosure of which is herein incorporated by reference, discloses a composite electrode with a coil cardioversion/defibrillation electrode and sense electrodes. Modifications to this arrangement is contemplated within the scope of the invention.
- One such modificationis to have the sense electrodes at the two ends of the housing and have the cardioversion/defibrillation electrodes located in between the sense electrodes.
- Another modificationis to have three or more sense electrodes spaced throughout the housing and allow for the selection of the two best sensing electrodes. If three or more sensing electrodes are used, then the ability to change which electrodes are used for sensing would be a programmable feature of the US-ICD to adapt to changes in the patient physiology and size over time.
- the programmingcould be done via the use of physical switches on the canister, or as presently preferred, via the use of a programming wand or via a wireless connection to program the circuitry within the canister.
- FIG. 15the optimal subcutaneous placement of the US-ICD of the present invention is illustrated.
- the actual location of the US-ICDis in a subcutaneous space that is developed during the implantation process.
- the heartis not exposed during this process and the heart is schematically illustrated in the figures only for help in understanding where the device and its various electrodes are three dimensionally located in the thorax of the patient.
- the US-ICDis located between the left mid-clavicular line approximately at the level of the inframammary crease at approximately the 5 th rib and the posterior axillary line, ideally just lateral to the left scapula. This way the US-ICD provides a reasonably good pathway for current delivery to the majority of the ventricular myocardium.
- FIG. 16schematically illustrates the method for implanting the US-ICD of the present invention.
- An incision 1631is made in the left anterior axillary line approximately at the level of the cardiac apex.
- a subcutaneous pathwayis then created that extends posteriorly to allow placement of the US-ICD.
- the incisioncan be anywhere on the thorax deemed reasonable by the implanting physician although in the preferred embodiment, the US-ICD of the present invention will be applied in this region.
- the subcutaneous pathwayis created medially to the inframammary crease and extends posteriorly to the left posterior axillary line.
- the pathwayis developed with a specially designed curved introducer 1742 (see FIG. 17).
- the trocarhas a proximal handle 1641 and a curved shaft 1643 .
- the distal end 1745 of the trocaris tapered to allow for dissection of a subcutaneous path in the patient.
- the trocaris cannulated having a central lumen 1746 and terminating in an opening 1748 at the distal end.
- Local anestheticsuch as lidocaine can be delivered, if necessary, through the lumen or through a curved and elongated needle designed to anesthetize the path to be used for trocar insertion should general anesthesia not be employed.
- the US-ICDs of the present inventionvary in length and curvature.
- the US-ICDsare provided in incremental sizes for subcutaneous implantation in different sized patients.
- FIG. 18a different embodiment is schematically illustrated in exploded view which provides different sized US-ICDs that are easier to manufacture.
- the different sized US-ICDswill all have the same sized and shaped thick end 1413 .
- the thick endis hollow inside allowing for the insertion of a core operational member 1853 .
- the core membercomprises a housing 1857 which contains the battery supply, capacitor and operational circuitry for the US-ICD.
- the proximal end of the core memberhas a plurality of electronic plug connectors.
- Plug connectors 1861 and 1863are electronically connected to the sense electrodes via pressure fit connectors (not illustrated) inside the thick end which are standard in the art.
- Plug connectors 1865 and 1867are also electronically connected to the cardioverter/defibrillator electrodes via pressure fit connectors inside the thick end.
- the distal end of the core membercomprises an end cap 1855 , and a ribbed fitting 1859 which creates a water-tight seal when the core member is inserted into opening 1851 of the thick end of the US-ICD.
- the S-ICD and US-ICDhave the ability to detect and treat atrial rhythm disorders, including atrial fibrillation.
- the S-ICD and US-ICDhave two or more electrodes that provide a far-field view of cardiac electrical activity that includes the ability to record the P-wave of the electrocardiogram as well as the QRS.
- One can detect the onset and offset of atrial fibrillationby referencing to the P-wave recorded during normal sinus rhythm and monitoring for its change in rate, morphology, amplitude and frequency content. For example, a well-defined P-wave that abruptly disappeared and was replaced by a low-amplitude, variable morphology signal would be a strong indication of the absence of sinus rhythm and the onset of atrial fibrillation.
- the ventricular detection ratecould be monitored for stability of the R-R coupling interval.
- atrial fibrillationcan be recognized by providing a near constant irregularly irregular coupling interval on a beat-by-beat basis.
- a R-R interval plot during AFappears “cloudlike” in appearance when several hundred or thousands of R-R intervals are plotted over time when compared to sinus rhythm or other supraventricular arrhythmias.
- a distinguishing feature compared to other rhythms that are irregularly irregularis that the QRS morphology is similar on a beat-by-beat basis despite the irregularity in the R-R coupling interval.
- Atrial fibrillationmay be detected by seeking to compare the timing and amplitude relationship of the detected P-wave of the electrocardiogram to the detected QRS (R-wave) of the electrocardiogram.
- Normal sinus rhythmhas a fixed relationship that can be placed into a template matching algorithm that can be used as a reference point should the relationship change.
- the arrhythmiacan be treated by delivery of a synchronized shock using energy levels up to the maximum output of the device therapy for terminating atrial fibrillation or for other supraventricular arrhythmias.
- the S-ICD or US-ICD electrode systemcan be used to treat both atrial and ventricular arrhythmias not only with shock therapy but also with pacing therapy.
- onemay be able to use different electrode systems than what is used to treat ventricular arrhythmias.
- Another embodimentwould be to allow for different types of therapies (amplitude, waveform, capacitance, etc.) for atrial arrhythmias compared to ventricular arrhythmias.
- the core member of the different sized and shaped US-ICDwill all be the same size and shape. That way, during an implantation procedures, multiple sized US-ICDs can be available for implantation, each one without a core member. Once the implantation procedure is being performed, then the correct sized US-ICD can be selected and the core member can be inserted into the US-ICD and then programmed as described above. Another advantage of this configuration is when the battery within the core member needs replacing it can be done without removing the entire US-ICD.
- the subcutaneous electrode systemespecially the anterior thoracic electrode system, that will be delivering the ATP stimuli should result in as high as a current density as possible in order to activate the cardiac tissues. This can be facilitated by using a small electrode as close to the sternum as possible in the tissues overlying the right ventricle, the cardiac chamber closest to the anterior subcutaneous space where the S-ICD of the present invention will lie.
- FIG. 19is a graph that shows an embodiment of the example of a biphasic waveform for use in antitachycardia pacing applications in subcutaneous implantable cardioverter-defibrillators (“S-ICD”) in an embodiment of the present invention. As shown in FIG. 19, the biphasic waveform is plotted as a function of current versus time.
- S-ICDsubcutaneous implantable cardioverter-defibrillators
- the biphasic waveform 1902comprises a positive portion 1904 , a negative portion 1906 and a transition portion 1908 .
- both the positive portion 1904 and the negative portion 1906are substantially rectangular in shape.
- the positive portion 1904 of the biphasic waveform 1902comprises an initial positive current 1910 , a positive fixed current 1912 and a final positive current 1914 .
- the negative portion 1906 of the biphasic waveform 1902comprises an initial negative current 1916 , a negative fixed current 1918 and a final negative current 1920 .
- the polarities of the biphasic waveform 1902can be reversed such that the negative portion 1906 precedes the positive portion 1904 in time.
- the biphasic waveform 1902is initially at zero current. Upon commencement of the anti-tachycardia pacing, a current of positive polarity is provided and the biphasic waveform 1902 rises to the initial positive current 1910 . Next, the current of the biphasic waveform 1902 remains at a constant level along the positive fixed current 1912 . The positive portion 1904 of the biphasic waveform 1902 is then truncated and a negative current is provided. The biphasic waveform 1902 then undergoes a relatively short transition portion 1908 where the current is approximately zero.
- the biphasic waveform 1902is increased (in absolute value) in the opposite (negative) polarity to the initial negative current 1916 .
- the current of the biphasic waveform 1902remains at a constant level along the negative fixed current 1918 .
- the biphasic waveform 1902returns to zero.
- the total amount of time that the biphasic waveform 1902 comprisesis known as the “pulse width.”
- the pulse width of the biphasic waveformcan range from approximately 1 millisecond to approximately 40 milliseconds.
- the total amount of energy deliveredis a function of the pulse width and the absolute value of the current.
- the amplitude of the initial positive current 1910can range from approximately one to approximately 250 milliamps.
- the amplitude of the initial negative current 1916can range from approximately one to approximately 250 milliamps.
- the pulse width of the biphasic waveform 1902can range from approximately 1 millisecond to approximately 40 milliseconds.
- the implantable cardioverter-defibrillatoremploys biphasic anti-tachycardia pacing at rates of approximately 20 to approximately 120 stimuli/minute for severe bradycardia episodes although programming of higher pacing rates up to 120 stimuli/minute is also possible.
- FIG. 20is a graph that shows an embodiment of the example of a monophasic waveform for use in antitachycardia pacing applications in subcutaneous implantable cardioverter-defibrillators (“S-ICD”) in an embodiment of the present invention. As shown in FIG. 20, the monophasic waveform is plotted as a function of current versus time.
- S-ICDsubcutaneous implantable cardioverter-defibrillators
- the monophasic waveform 2002comprises an initial positive current 2004 , a positive fixed current 2006 and a final positive current 2008 .
- the monophasic waveform 2002is substantially rectangular in shape.
- the polarities of the monophasic waveform 2002can be reversed such that the waveform 2002 is negative in polarity.
- the monophasic waveform 2002is initially at zero current. Upon commencement of the anti-tachycardia pacing, a current of positive polarity is provided and the monophasic waveform 2002 rises to the initial positive current 2004 . Next, the current of the monophasic waveform 2002 remains at a constant level along the positive fixed current 1906 . The monophasic waveform 2002 is then truncated.
- the amplitude of the initial positive current 2004can range from approximately one to approximately 250 milliamps.
- the pulse width of the biphasic waveform 2002can range from approximately 1 millisecond to approximately 40 milliseconds.
- the implantable cardioverter-defibrillatoremploys anti-tachycardia pacing at rates of approximately 100 to approximately 350 stimuli/minute for ventricular tachycardia episodes.
- up to 30 ATP stimuli for any single attemptcould be allowed and as many as 15 ATP attempts could be allowed for any effort to terminate a single episode of VT.
- the devicemay be allowed to auto-select the method of ATP to be used based upon the device's and/or the physician's experience with previous episodes of VT or with the patient's underlying cardiac condition.
- the power supplycontinues to operate to maintain a sufficient voltage to deliver a constant current.
- an S-ICDmay also be employed for the treatment of other arrhythmias such as atria tachyarrhythmias.
- the inventioncan provide ATP in response to a certain activity, respiration, pressure or oxygenation sensor as coupled to arrhythmia characteristics.
Landscapes
- Health & Medical Sciences (AREA)
- Cardiology (AREA)
- Life Sciences & Earth Sciences (AREA)
- Animal Behavior & Ethology (AREA)
- Radiology & Medical Imaging (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Biomedical Technology (AREA)
- General Health & Medical Sciences (AREA)
- Public Health (AREA)
- Veterinary Medicine (AREA)
- Engineering & Computer Science (AREA)
- Heart & Thoracic Surgery (AREA)
- Biophysics (AREA)
- Electrotherapy Devices (AREA)
Abstract
Description
- The present application is a continuation-in-part of U.S. patent application entitled “SUBCUTANEOUS ONLY IMPLANTABLE CARDIOVERTER-DEFIBRILLATOR AND OPTIONAL PACER,” having Serial No. 09/663,607, filed Sep. 18, 2000, pending, and U.S. patent application entitled “UNITARY SUBCUTANEOUS ONLY IMPLANTABLE CARDIOVERTER-DEFIBRILLATOR AND OPTIONAL PACER,” having Serial No. 09/663,606, filed Sep. 18, 2000, pending, of which both applications are assigned to the assignee of the present application, and the disclosures of both applications are hereby incorporated by reference.[0001]
- In addition, the present application is filed concurrently herewith U.S. patent application entitled “MONOPHASIC WAVEFORM FOR ANTI-BRADYCARDIA PACING FOR A SUBCUTANEOUS IMPLANTABLE CARDIOVERTER-DEFIBRILLATOR,” U.S. patent application entitled “MONOPHASIC WAVEFORM FOR ANTI-TACHYCARDIA PACING FOR A SUBCUTANEOUS IMPLANTABLE CARDIOVERTERDEFIBRILLATOR” and U.S. patent application entitled “CURRENT WAVEFORMS FOR ANTI-BRADYCARDIA PACING FOR A SUBCUTANEOUS CARDIOVERTER DEFIBRILLATOR,” the disclosures of which applications are hereby incorporated by reference.[0002]
- The present invention relates to an apparatus and method for performing electrical cardioversion/defibrillation and optional pacing of the heart via a totally subcutaneous non-transvenous system.[0003]
- Defibrillation/cardioversion is a technique employed to counter arrhythmic heart conditions including some tachycardias in the atria and/or ventricles. Typically, electrodes are employed to stimulate the heart with electrical impulses or shocks, of a magnitude substantially greater than pulses used in cardiac pacing. Shocks used for defibrillation therapy can comprise a biphasic truncated exponential waveform. As for pacing, a constant current density is desired to reduce or eliminate variability due to the electrode/tissue interface[0004]
- Defibrillation/cardioversion systems include body implantable electrodes that are connected to a hermetically sealed container housing the electronics, battery supply and capacitors. The entire system is referred to as implantable cardioverter/defibrillators (ICDs). The electrodes used in ICDs can be in the form of patches applied directly to epicardial tissue, or, more commonly, are on the distal regions of small cylindrical insulated catheters that typically enter the subclavian venous system, pass through the superior vena cava and, into one or more endocardial areas of the heart. Such electrode systems are called intravascular or transvenous electrodes. U.S. Pat. Nos. 4,603,705, 4,693,253, 4,944,300, 5,105,810, the disclosures of which are all incorporated herein by reference, disclose intravascular or transvenous electrodes, employed either alone, in combination with other intravascular or transvenous electrodes, or in combination with an epicardial patch or subcutaneous electrodes. Compliant epicardial defibrillator electrodes are disclosed in U.S. Pat. Nos. 4,567,900 and 5,618,287, the disclosures of which are incorporated herein by reference. A sensing epicardial electrode configuration is disclosed in U.S. Pat No. 5,476,503, the disclosure of which is incorporated herein by reference.[0005]
- In addition to epicardial and transvenous electrodes, subcutaneous electrode systems have also been developed. For example, U.S. Pat. Nos. 5,342,407 and 5,603,732, the disclosures of which are incorporated herein by reference, teach the use of a pulse monitor/generator surgically implanted into the abdomen and subcutaneous electrodes implanted in the thorax. This system is far more complicated to use than current ICD systems using transvenous lead systems together with an active can electrode and therefore it has no practical use. It has in fact never been used because of the surgical difficulty of applying such a device (3 incisions), the impractical abdominal location of the generator and the electrically poor sensing and defibrillation aspects of such a system.[0006]
- Recent efforts to improve the efficiency of ICDs have led manufacturers to produce ICDs which are small enough to be implanted in the pectoral region. In addition, advances in circuit design have enabled the housing of the ICD to form a subcutaneous electrode. Some examples of ICDs in which the housing of the ICD serves as an optional additional electrode are described in U.S. Pat. Nos. 5,133,353, 5,261,400, 5,620,477, and 5,658,321 the disclosures of which are incorporated herein by reference.[0007]
- ICDs are now an established therapy for the management of life threatening cardiac rhythm disorders, primarily ventricular fibrillation (V-Fib). ICDs are very effective at treating V-Fib, but are therapies that still require significant surgery.[0008]
- As ICD therapy becomes more prophylactic in nature and used in progressively less ill individuals, especially children at risk of cardiac arrest, the requirement of ICD therapy to use intravenous catheters and transvenous leads is an impediment to very long term management as most individuals will begin to develop complications related to lead system malfunction sometime in the 5-10 year time frame, often earlier. In addition, chronic transvenous lead systems, their reimplantation and removals, can damage major cardiovascular venous systems and the tricuspid valve, as well as result in life threatening perforations of the great vessels and heart. Consequently, use of transvenous lead systems, despite their many advantages, are not without their chronic patient management limitations in those with life expectancies of >5 years. The problem of lead complications is even greater in children where body growth can substantially alter transvenous lead function and lead to additional cardiovascular problems and revisions. Moreover, transvenous ICD systems also increase cost and require specialized interventional rooms and equipment as well as special skill for insertion. These systems are typically implanted by cardiac electrophysiologists who have had a great deal of extra training.[0009]
- In addition to the background related to ICD therapy, the present invention requires a brief understanding of a related therapy, the automatic external defibrillator (AED). AEDs employ the use of cutaneous patch electrodes, rather than implantable lead systems, to effect defibrillation under the direction of a bystander user who treats the patient suffering from V-Fib with a portable device containing the necessary electronics and power supply that allows defibrillation. AEDs can be nearly as effective as an ICD for defibrillation if applied to the victim of ventricular fibrillation promptly, i.e., within 2 to 3 minutes of the onset of the ventricular fibrillation.[0010]
- AED therapy has great appeal as a tool for diminishing the risk of death in public venues such as in air flight. However, an AED must be used by another individual, not the person suffering from the potential fatal rhythm. It is more of a public health tool than a patient-specific tool like an ICD. Because >75% of cardiac arrests occur in the home, and over half occur in the bedroom, patients at risk of cardiac arrest are often alone or asleep and can not be helped in time with an AED. Moreover, its success depends to a reasonable degree on an acceptable level of skill and calm by the bystander user.[0011]
- What is needed therefore, especially for children and for prophylactic long term use for those at risk of cardiac arrest, is a combination of the two forms of therapy which would provide prompt and near-certain defibrillation, like an ICD, but without the long-term adverse sequelae of a transvenous lead system while simultaneously using most of the simpler and lower cost technology of an AED. What is also needed is a cardioverter/defibrillator that is of simple design and can be comfortably implanted in a patient for many years.[0012]
- A power supply for an implantable cardioverter-defibrillator for subcutaneous positioning between the third rib and the twelfth rib and using a lead system that does not directly contact a patient's heart or reside in the intrathorasic blood vessels and for providing anti-tachycardia pacing energy to the heart, comprising a capacitor subsystem for storing the anti-tachycardia pacing energy for delivery to the patient's heart; and a battery subsystem electrically coupled to the capacitor subsystem for providing the anti-tachycardia pacing energy to the capacitor subsystem.[0013]
- For a better understanding of the invention, reference is now made to the drawings where like numerals represent similar objects throughout the figures where:[0014]
- FIG. 1 is a schematic view of a Subcutaneous ICD (S-ICD) of the present invention;[0015]
- FIG. 2 is a schematic view of an alternate embodiment of a subcutaneous electrode of the present invention;[0016]
- FIG. 3 is a schematic view of an alternate embodiment of a subcutaneous electrode of the present invention;[0017]
- FIG. 4 is a schematic view of the S-ICD and lead of FIG. 1 subcutaneously implanted in the thorax of a patient;[0018]
- FIG. 5 is a schematic view of the S-ICD and lead of FIG. 2 subcutaneously implanted in an alternate location within the thorax of a patient;[0019]
- FIG. 6 is a schematic view of the S-ICD and lead of FIG. 3 subcutaneously implanted in the thorax of a patient;[0020]
- FIG. 7 is a schematic view of the method of making a subcutaneous path from the preferred incision and housing implantation point to a termination point for locating a subcutaneous electrode of the present invention;[0021]
- FIG. 8 is a schematic view of an introducer set for performing the method of lead insertion of any of the described embodiments;[0022]
- FIG. 9 is a schematic view of an alternative S-ICD of the present invention illustrating a lead subcutaneously and serpiginously implanted in the thorax of a patient for use particularly in children;[0023]
- FIG. 10 is a schematic view of an alternate embodiment of an S-ICD of the present invention;[0024]
- FIG. 11 is a schematic view of the S-ICD of FIG. 10 subcutaneously implanted in the thorax of a patient;[0025]
- FIG. 12 is a schematic view of yet a further embodiment where the canister of the S-ICD of the present invention is shaped to be particularly useful in placing subcutaneously adjacent and parallel to a rib of a patient;[0026]
- FIG. 13 is a schematic of a different embodiment where the canister of the S-ICD of the present invention is shaped to be particularly useful in placing subcutaneously adjacent and parallel to a rib of a patient;[0027]
- FIG. 14 is a schematic view of a Unitary Subcutaneous ICD (US-ICD) of the present invention;[0028]
- FIG. 15 is a schematic view of the US-ICD subcutaneously implanted in the thorax of a patient;[0029]
- FIG. 16 is a schematic view of the method of making a subcutaneous path from the preferred incision for implanting the US-ICD;[0030]
- FIG. 17 is a schematic view of an introducer for performing the method of US-ICD implantation;[0031]
- FIG. 18 is an exploded schematic view of an alternate embodiment of the present invention with a plug-in portion that contains operational circuitry and means for generating cardioversion/defibrillation shock waves;[0032]
- FIG. 19 is a graph that shows an example of a biphasic waveform for use in anti-tachycardia pacing in an embodiment of the present invention; and[0033]
- FIG. 20 is a graph that shows an example of a monophonic waveform for use in anti-tachycardia pacing in an embodiment of the present invention.[0034]
- Turning now to FIG. 1, the S-ICD of the present invention is illustrated. The S-ICD consists of an electrically[0035]
active canister 11 and asubcutaneous electrode 13 attached to the canister. The canister has an electricallyactive surface 15 that is electrically insulated from theelectrode connector block 17 and thecanister housing 16 via insulatingarea 14. The canister can be similar to numerous electrically active canisters commercially available in that the canister will contain a battery supply, capacitor and operational circuitry. Alternatively, the canister can be thin and elongated to conform to the intercostal space. The circuitry will be able to monitor cardiac rhythms for tachycardia and fibrillation, and if detected, will initiate charging the capacitor and then delivering cardioversion/defibrillation energy through the active surface of the housing and to the subcutaneous electrode. Examples of such circuitry are described in U.S. Pat. Nos. 4,693,253 and 5,105,810, the entire disclosures of which are herein incorporated by reference. The canister circuitry can provide cardioversion/defibrillation energy in different types of waveforms. In one embodiment, a 100 uF biphasic waveform is used of approximately 10-20 ms total duration and with the initial phase containing approximately ⅔ of the energy, however, any type of waveform can be utilized such as monophasic, biphasic, multiphasic or alternative waveforms as is known in the art. - In addition to providing cardioversion/defibrillation energy, the circuitry can also provide transthoracic cardiac pacing energy. The optional circuitry will be able to monitor the heart for bradycardia and/or tachycardia rhythms. Once a bradycardia or tachycardia rhythm is detected, the circuitry can then deliver appropriate pacing energy at appropriate intervals through the active surface and the subcutaneous electrode. Pacing stimuli can be biphasic in one embodiment and similar in pulse amplitude to that used for conventional transthoracic pacing.[0036]
- This same circuitry can also be used to deliver low amplitude shocks on the T-wave for induction of ventricular fibrillation for testing S-ICD performance in treating V-Fib as is described in U.S. Pat. No. 5,129,392, the entire disclosure of which is hereby incorporated by reference. Also the circuitry can be provided with rapid induction of ventricular fibrillation or ventricular tachycardia using rapid ventricular pacing. Another optional way for inducing ventricular fibrillation would be to provide a continuous low voltage, i.e., about 3 volts, across the heart during the entire cardiac cycle.[0037]
- Another optional aspect of the present invention is that the operational circuitry can detect the presence of atrial fibrillation as described in Olson, W. et al. “Onset And Stability For Ventricular Tachyarrhythmia Detection in an Implantable Cardioverter and Defibrillator,” Computers in Cardiology (1986) pp. 167-170. Detection can be provided via R-R Cycle length instability detection algorithms. Once atrial fibrillation has been detected, the operational circuitry will then provide QRS synchronized atrial defibrillation/cardioversion using the same shock energy and waveshape characteristics used for ventricular defibrillation/cardioversion.[0038]
- The sensing circuitry will utilize the electronic signals generated from the heart and will primarily detect QRS waves. In one embodiment, the circuitry will be programmed to detect only ventricular tachycardias or fibrillations. The detection circuitry will utilize in its most direct form, a rate detection algorithm that triggers charging of the capacitor once the ventricular rate exceeds some predetermined level for a fixed period of time: for example, if the ventricular rate exceeds 240 bpm on average for more than[0039]4 seconds. Once the capacitor is charged, a confirmatory rhythm check would ensure that the rate persists for at least another 1 second before discharge. Similarly, termination algorithms could be instituted that ensure that a rhythm less than 240 bpm persisting for at least 4 seconds before the capacitor charge is drained to an internal resistor. Detection, confirmation and termination algorithms as are described above and in the art can be modulated to increase sensitivity and specificity by examining QRS beat-to-beat uniformity, QRS signal frequency content, R-R interval stability data, and signal amplitude characteristics all or part of which can be used to increase or decrease both sensitivity and specificity of S-ICD arrhythmia detection function.
- In addition to use of the sense circuitry for detection of V-Fib or V-Tach by examining the QRS waves, the sense circuitry can check for the presence or the absence of respiration. The respiration rate can be detected by monitoring the impedance across the thorax using subthreshold currents delivered across the active can and the high voltage subcutaneous lead electrode and monitoring the frequency in undulation in the waveform that results from the undulations of transthoracic impedance during the respiratory cycle. If there is no undulation, then the patent is not respiring and this lack of respiration can be used to confirm the QRS findings of cardiac arrest. The same technique can be used to provide information about the respiratory rate or estimate cardiac output as described in U.S. Pat. Nos. 6,095,987, 5,423,326, 4,450,527, the entire disclosures of which are incorporated herein by reference.[0040]
- The canister of the present invention can be made out of titanium alloy or other presently preferred electrically active canister designs. However, it is contemplated that a malleable canister that can conform to the curvature of the patient's chest will be preferred. In this way the patient can have a comfortable canister that conforms to the shape of the patient's rib cage. Examples of conforming canisters are provided in U.S. Pat. No. 5,645,586, the entire disclosure of which is herein incorporated by reference. Therefore, the canister can be made out of numerous materials such as medical grade plastics, metals, and alloys. In the preferred embodiment, the canister is smaller than 60 cc volume having a weight of less than 100 gms for long term wearability, especially in children. The canister and the lead of the S-ICD can also use fractal or wrinkled surfaces to increase surface area to improve defibrillation capability. Because of the primary prevention role of the therapy and the likely need to reach energies over 40 Joules, a feature of one embodiment is that the charge time for the therapy, is intentionally left relatively long to allow capacitor charging within the limitations of device size. Examples of small ICD housings are disclosed in U.S. Pat. Nos. 5,597,956 and 5,405,363, the entire disclosures of which are herein incorporated by reference.[0041]
- Different[0042]
subcutaneous electrodes 13 of the present invention are illustrated in FIGS.1-3. Turning to FIG. 1, thelead 21 for the subcutaneous electrode is preferably composed of silicone or polyurethane insulation. The electrode is connected to the canister at its proximal end viaconnection port 19 which is located on an electricallyinsulated area 17 of the canister. The electrode illustrated is a composite electrode with three different electrodes attached to the lead. In the embodiment illustrated, anoptional anchor segment 52 is attached at the most distal end of the subcutaneous electrode for anchoring the electrode into soft tissue such that the electrode does not dislodge after implantation. - The most distal electrode on the composite subcutaneous electrode is a[0043]
coil electrode 27 that is used for delivering the high voltage cardioversion/defibrillation energy across the heart. The coil cardioversion/defibrillation electrode is about 5-10 cm in length. Proximal to the coil electrode are two sense electrodes, afirst sense electrode 25 is located proximally to the coil electrode and asecond sense electrode 23 is located proximally to the first sense electrode. The sense electrodes are spaced far enough apart to be able to have good QRS detection. This spacing can range from 1 to 10 cm with 4 cm being presently preferred. The electrodes may or may not be circumferential with the preferred embodiment. Having the electrodes non-circumferential and positioned outward, toward the skin surface, is a means to minimize muscle artifact and enhance QRS signal quality. The sensing electrodes are electrically isolated from the cardioversion/defibrillation electrode via insulatingareas 29. Similar types of cardioversion/defibrillation electrodes are currently commercially available in a transvenous configuration. For example, U.S. Pat. No. 5,534,022, the entire disclosure of which is herein incorporated by reference, disclosures a composite electrode with a coil cardioversion/defibrillation electrode and sense electrodes. Modifications to this arrangement is contemplated within the scope of the invention. One such modification is illustrated in FIG. 2 where the twosensing electrodes - It is also contemplated within the scope of the invention that the sensing of QRS waves (and transthoracic impedance) can be carried out via sense electrodes on the canister housing or in combination with the cardioversion/defibrillation coil electrode and/or the subcutaneous lead sensing electrode(s) In this way, sensing could be performed via the one coil electrode located on the subcutaneous electrode and the active surface on the canister housing. Another possibility would be to have only one sense electrode located on the subcutaneous electrode and the sensing would be performed by that one electrode and either the coil electrode on the subcutaneous electrode or by the active surface of the canister. The use of sensing electrodes on the canister would eliminate the need for sensing electrodes on the subcutaneous electrode. It is also contemplated that the subcutaneous electrode would be provided with at least one sense electrode, the canister with at least one sense electrode, and if multiple sense electrodes are used on either the subcutaneous electrode and/or the canister, that the best QRS wave detection combination will be identified when the S-ICD is implanted and this combination can be selected, activating the best sensing arrangement from all the existing sensing possibilities. Turning again to FIG. 2, two[0044]
sensing electrodes active surface 15 with electrical insulator rings30 placed between the sense electrodes and the active surface. These canister sense electrodes could be switched off and electrically insulated during and shortly after defibrillation/ cardioversion shock delivery. The canister sense electrodes may also be placed on the electrically inactive surface of the canister. In the embodiment of FIG. 2, there are actually four sensing electrodes, two on the subcutaneous lead and two on the canister. In the preferred embodiment, the ability to change which electrodes are used for sensing would be a programmable feature of the S-ICD to adapt to changes in the patient physiology and size (in the case of children) over time. The programming could be done via the use of physical switches on the canister, or as presently preferred, via the use of a programming wand or via a wireless connection to program the circuitry within the canister. - The canister could be employed as either a cathode or an anode of the S-ICD cardioversion/defibrillation system. If the canister is the cathode, then the subcutaneous coil electrode would be the anode. Likewise, if the canister is the anode, then the subcutaneous electrode would be the cathode.[0045]
- The active canister housing will provide energy and voltage intermediate to that available with ICDs and most AEDs. The typical maximum voltage necessary for ICDs using most biphasic waveforms is approximately 750 Volts with an associated maximum energy of approximately 40 Joules. The typical maximum voltage necessary for AEDs is approximately 2000-5000 Volts with an associated maximum energy of approximately 200-360 Joules depending upon the model and waveform used. The S-ICD and the US-ICD of the present invention uses maximum voltages in the range of about 50 to about 3500 Volts and is associated with energies of about 0.5 to about 350 Joules. The capacitance of the devices can range from about 25 to about 200 micro farads.[0046]
- In another embodiment, the S-ICD and US-ICD devices provide energy with a pulse width of approximately one millisecond to approximately 40 milliseconds. The devices can provide pacing current of approximately one milliamp to approximately 250 milliamps.[0047]
- The sense circuitry contained within the canister is highly sensitive and specific for the presence or absence of life threatening ventricular arrhythmias. Features of the detection algorithm are programmable and the algorithm is focused on the detection of V-FIB and high rate V-TACH (>240 bpm). Although the S-ICD of the present invention may rarely be used for an actual life-threatening event, the simplicity of design and implementation allows it to be employed in large populations of patients at modest risk with modest cost by non-cardiac electrophysiologists. Consequently, the S-ICD of the present invention focuses mostly on the detection and therapy of the most malignant rhythm disorders. As part of the detection algorithm's applicability to children, the upper rate range is programmable upward for use in children, known to have rapid supraventricular tachycardias and more rapid ventricular fibrillation. Energy levels also are programmable downward in order to allow treatment of neonates and infants.[0048]
- Turning now to FIG. 4, the optimal subcutaneous placement of the S-ICD of the present invention is illustrated. As would be evidence to a person skilled in the art, the actual location of the S-ICD is in a subcutaneous space that is developed during the implantation process. The heart is not exposed during this process and the heart is schematically illustrated in the figures only for help in understanding where the canister and coil electrode are three dimensionally located in the left mid-clavicular line approximately at the level of the inframammary crease at approximately the 5th rib. The[0049]
lead 21 of the subcutaneous electrode traverses in a subcutaneous path around the thorax terminating with its distal electrode end at the posterior axillary line ideally just lateral to the left scapula. This way the canister and subcutaneous cardioversion/defibrillation electrode provide a reasonably good pathway for current delivery to the majority of the ventricular myocardium. - FIG. 5 illustrates a different placement of the present invention. The S-ICD canister with the active housing is located in the left posterior axillary line approximately lateral to the tip of the inferior portion of the scapula. This location is especially useful in children. The[0050]
lead 21 of the subcutaneous electrode traverses in a subcutaneous path around the thorax terminating with its distal electrode end at the anterior precordial region, ideally in the inframammary crease. FIG. 6 illustrates the embodiment of FIG. 1 subcutaneously implanted in the thorax with theproximal sense electrodes - FIG. 7 schematically illustrates the method for implanting the S-ICD of the present invention. An[0051]
incision 31 is made in the left anterior axillary line approximately at the level of the cardiac apex. This incision location is distinct from that chosen for S-ICD placement and is selected specifically to allow both canister location more medially in the left inframammary crease and lead positioning more posteriorly via the introducer set (described below) around to the left posterior axillary line lateral to the left scapula. That said, the incision can be anywhere on the thorax deemed reasonably by the implanting physician although in the preferred embodiment, the S-ICD of the present invention will be applied in this region. Asubcutaneous pathway 33 is then created medially to the inframmary crease for the canister and posteriorly to the left posterior axillary line lateral to the left scapula for the lead. - The S-[0052]
ICD canister 11 is then placed subcutaneously at the location of the incision or medially at the subcutaneous region at the left inframmary crease. Thesubcutaneous electrode 13 is placed with a specially designed curved introducer set40 (see FIG. 8). The introducer set comprises a curved trocar42 and a stiff curved peel awaysheath 44. The peel away sheath is curved to allow for placement around the rib cage of the patient in the subcutaneous space created by the trocar. The sheath has to be stiff enough to allow for the placement of the electrodes without the sheath collapsing or bending. Preferably the sheath is made out of a biocompatible plastic material and is perforated along its axial length to allow for it to split apart into two sections. The trocar has aproximal handle 41 and acurved shaft 43. Thedistal end 45 of the trocar is tapered to allow for dissection of asubcutaneous path 33 in the patient. Preferably, the trocar is cannulated having acentral Lumen 46 and terminating in anopening 48 at the distal end. Local anesthetic such as lidocaine can be delivered, if necessary, through the lumen or through a curved and elongated needle designed to anesthetize the path to be used for trocar insertion should general anesthesia not be employed. The curved peel awaysheath 44 has aproximal pull tab 49 for breaking the sheath into two halves along itsaxial shaft 47. The sheath is placed over a guidewire inserted through the trocar after the subcutaneous path has been created. The subcutaneous pathway is then developed until it terminates subcutaneously at a location that, if a straight line were drawn from the canister location to the path termination point the line would intersect a substantial portion of the left ventricular mass of the patient. The guidewire is then removed leaving the peel away sheath. The subcutaneous lead system is then inserted through the sheath until it is in the proper location. Once the subcutaneous lead system is in the proper location, the sheath is split in half using thepull tab 49 and removed. If more than one subcutaneous electrode is being used, a new curved peel away sheath can be used for each subcutaneous electrode. - The S-ICD will have prophylactic use in adults where chronic transvenous/epicardial ICD lead systems pose excessive risk or have already resulted in difficulty, such as sepsis or lead fractures. It is also contemplated that a major use of the S-ICD system of the present invention will be for prophylactic use in children who are at risk for having fatal arrhythmias, where chronic transvenous lead systems pose significant management problems. Additionally, with the use of standard transvenous ICDs in children, problems develop during patient growth in that the lead system does not accommodate the growth. FIG. 9 illustrates the placement of the S-ICD subcutaneous lead system such that he problem that growth presents to the lead system is overcome. The distal end of the subcutaneous electrode is placed in the same location as described above providing a good location for the coil cardioversion/[0053]
defibrillation electrode 27 and thesensing electrodes insulated lead 21, however is no longer placed in a taught configuration. Instead, the lead is serpiginously placed with a specially designed introducer trocar and sheath such that it has numerous waves or bends. As the child grows, the waves or bends will straighten out lengthening the lead system while maintaining proper electrode placement. Although it is expected that fibrous scarring especially around the defibrillation coil will help anchor it into position to maintain its posterior position during growth, a lead system with a distal tine or screwelectrode anchoring system 52 can also be incorporated into the distal tip of the lead to facilitate lead stability (see FIG. 1). Other anchoring systems can also be used such as hooks, sutures, or the like. - FIGS. 10 and 11 illustrate another embodiment of the present S-ICD invention. In this embodiment there are two[0054]
subcutaneous electrodes subcutaneous electrode 13′ is essentially identical to the previously described electrode. In this embodiment the cardioversion/defibrillation energy is delivered between the active surface of the canister and the twocoil electrodes sense electrodes subcutaneous electrode 13 is placed inferiorly and theother electrode 13′ is placed superiorly. It is also contemplated with this dual subcutaneous electrode system that the canister and one subcutaneous electrode are the same polarity and the other subcutaneous electrode is the opposite polarity. - Turning now to FIGS. 12 and 13, further embodiments are illustrated where the[0055]
canister 11 of the S-ICD of the present invention is shaped to be particularly useful in placing subcutaneously adjacent and parallel to a rib of a patient. The canister is long, thin, and curved to conform to the shape of the patient's rib. In the embodiment illustrated in FIG. 12, the canister has a diameter ranging from about 0.5 cm to about 2 cm without 1 cm being presently preferred. Alternatively, instead of having a circular cross sectional area, the canister could have a rectangular or square cross sectional area as illustrated in FIG. 13 without falling outside of the scope of the present invention. The length of the canister can vary depending on the size of the patient's thorax. In an embodiment, the canister is about 5 cm to about 40 cm long. The canister is curved to conform to the curvature of the ribs of the thorax. The radius of the curvature will vary depending on the size of the patient, with smaller radiuses for smaller patients and larger radiuses for larger patients. The radius of the curvature can range from about 5 cm to about 35 cm depending on the size of the patient. Additionally, the radius of the curvature need not be uniform throughout the canister such that it can be shaped closer to the shape of the ribs. The canister has an active surface,15 that is located on the interior (concave) portion of the curvature and aninactive surface 16 that is located on the exterior (convex) portion of the curvature. The leads of these embodiments, which are not illustrated except for theattachment port 19 and the proximal end of thelead 21, can be any of the leads previously described above, with the lead illustrated in FIG. 1 being presently preferred. - The circuitry of this canister is similar to the circuitry described above. Additionally, the canister can optionally have at least one sense electrode located on either the active surface of the inactive surface and the circuitry within the canister can be programmable as described above to allow for the selection of the best sense electrodes. It is presently preferred that the canister have two[0056]
sense electrodes - It is envisioned that the embodiment of FIG. 12 will be subcutaneously implanted adjacent and parallel to the left anterior 5th rib, either between the 4th and 5th ribs or between the 5th and 6th ribs. However other locations can be used.[0057]
- Another component of the S-ICD of the present invention is a cutaneous test electrode system designed to simulate the subcutaneous high voltage shock electrode system as well as the QRS cardiac rhythm detection system. This test electrode system is comprised of a cutaneous patch electrode of similar surface area and impedance to that of the S-ICD canister itself together with a cutaneous strip electrode comprising a defibrillation strip as well as two button electrodes for sensing of the QRS. Several cutaneous strip electrodes are available to allow for testing various bipole spacings to optimize signal detection comparable to the implantable system.[0058]
- FIGS.[0059]14 to18 depict particular US-ICD embodiments of the present invention. The various sensing, shocking and pacing circuitry, described in detail above with respect to the S-ICD embodiments, may additionally be incorporated into the following US-ICD embodiments. Furthermore, particular aspects of any individual S-ICD embodiment discussed above, may be incorporated, in whole or in part, into the US-ICD embodiments depicted in the following figures.
- Turning now to FIG. 14, the US-ICD of the present invention is illustrated. The US-ICD consists of a curved housing[0060]1211 with a first and second end. The
first end 1413 is thicker than thesecond end 1215. This thicker area houses a battery supply, capacitor and operational circuitry for the US-ICD. The circuitry will be able to monitor cardiac rhythms for tachycardia and fibrillation, and if detected, will initiate charging the capacitor and then delivering cardioversion/defibrillation energy through the two cardioversion/defibrillating electrodes - The housing of the present invention can be made out of titanium alloy or other presently preferred ICD designs. It is contemplated that the housing is also made out of biocompatible plastic materials that electronically insulate the electrodes from each other. However, it is contemplated that a malleable canister that can conform to the curvature of the patient's chest will be preferred. In this way the patient can have a comfortable canister that conforms to the unique shape of the patient's rib cage. Examples of conforming ICD housings are provided in U.S. Pat. No. 5,645,586, the entire disclosure of which is herein incorporated by reference. In the preferred embodiment, the housing is curved in the shape of a 5[0061]thrib of a person. Because there are many different sizes of people, the housing will come in different incremental sizes to allow a good match between the size of the rib cage and the size of the US-ICD. The length of the US-ICD will range from about 15 to about 50 cm. Because of the primary preventative role of the therapy and the need to reach energies over 40 Joules, a feature of the preferred embodiment is that the charge time for the therapy, intentionally be relatively long to allow capacitor charging within the limitations of device size.
- The thick end of the housing is currently needed to allow for the placement of the battery supply, operational circuitry, and capacitors. It is contemplated that the thick end will be about 0.5 cm to about 2 cm wide with about 1 cm being presently preferred. As microtechnology advances, the thickness of the housing will become smaller.[0062]
- The two cardioversion/defibrillation electrodes on the housing are used for delivering the high voltage cardioversion/defibrillation energy across the heart. In the preferred embodiment, the cardioversion/defibrillation electrodes are coil electrodes, however, other cardioversion/defibrillation electrodes could be used such as having electrically isolated active surfaces or platinum alloy electrodes. The coil cardioversion/defibrillation electrodes are about 5-10 cm in length. Located on the housing between the two cardioversion/defibrillation electrodes are two[0063]
sense electrodes areas 1423. Analogous types of cardioversion/defibrillation electrodes are currently commercially available in a transvenous configuration. For example, U.S. Pat. No. 5,534,022, the entire disclosure of which is herein incorporated by reference, discloses a composite electrode with a coil cardioversion/defibrillation electrode and sense electrodes. Modifications to this arrangement is contemplated within the scope of the invention. One such modification is to have the sense electrodes at the two ends of the housing and have the cardioversion/defibrillation electrodes located in between the sense electrodes. Another modification is to have three or more sense electrodes spaced throughout the housing and allow for the selection of the two best sensing electrodes. If three or more sensing electrodes are used, then the ability to change which electrodes are used for sensing would be a programmable feature of the US-ICD to adapt to changes in the patient physiology and size over time. The programming could be done via the use of physical switches on the canister, or as presently preferred, via the use of a programming wand or via a wireless connection to program the circuitry within the canister. - Turning now to FIG. 15, the optimal subcutaneous placement of the US-ICD of the present invention is illustrated. As would be evident to a person skilled in the art, the actual location of the US-ICD is in a subcutaneous space that is developed during the implantation process. The heart is not exposed during this process and the heart is schematically illustrated in the figures only for help in understanding where the device and its various electrodes are three dimensionally located in the thorax of the patient. The US-ICD is located between the left mid-clavicular line approximately at the level of the inframammary crease at approximately the 5[0064]thrib and the posterior axillary line, ideally just lateral to the left scapula. This way the US-ICD provides a reasonably good pathway for current delivery to the majority of the ventricular myocardium.
- FIG. 16 schematically illustrates the method for implanting the US-ICD of the present invention. An[0065]
incision 1631 is made in the left anterior axillary line approximately at the level of the cardiac apex. A subcutaneous pathway is then created that extends posteriorly to allow placement of the US-ICD. The incision can be anywhere on the thorax deemed reasonable by the implanting physician although in the preferred embodiment, the US-ICD of the present invention will be applied in this region. The subcutaneous pathway is created medially to the inframammary crease and extends posteriorly to the left posterior axillary line. The pathway is developed with a specially designed curved introducer1742 (see FIG. 17). The trocar has aproximal handle 1641 and acurved shaft 1643. The distal end1745 of the trocar is tapered to allow for dissection of a subcutaneous path in the patient. Preferably, the trocar is cannulated having acentral lumen 1746 and terminating in an opening1748 at the distal end. Local anesthetic such as lidocaine can be delivered, if necessary, through the lumen or through a curved and elongated needle designed to anesthetize the path to be used for trocar insertion should general anesthesia not be employed. Once the subcutaneous pathway is developed, the US-ICD is implanted in the subcutaneous space, the skin incision is closed using standard techniques. - As described previously, the US-ICDs of the present invention vary in length and curvature. The US-ICDs are provided in incremental sizes for subcutaneous implantation in different sized patients. Turning now to FIG. 18, a different embodiment is schematically illustrated in exploded view which provides different sized US-ICDs that are easier to manufacture. The different sized US-ICDs will all have the same sized and shaped[0066]
thick end 1413. The thick end is hollow inside allowing for the insertion of a coreoperational member 1853. The core member comprises ahousing 1857 which contains the battery supply, capacitor and operational circuitry for the US-ICD. The proximal end of the core member has a plurality of electronic plug connectors.Plug connectors Plug connectors opening 1851 of the thick end of the US-ICD. - The S-ICD and US-ICD, in alternative embodiments, have the ability to detect and treat atrial rhythm disorders, including atrial fibrillation. The S-ICD and US-ICD have two or more electrodes that provide a far-field view of cardiac electrical activity that includes the ability to record the P-wave of the electrocardiogram as well as the QRS. One can detect the onset and offset of atrial fibrillation by referencing to the P-wave recorded during normal sinus rhythm and monitoring for its change in rate, morphology, amplitude and frequency content. For example, a well-defined P-wave that abruptly disappeared and was replaced by a low-amplitude, variable morphology signal would be a strong indication of the absence of sinus rhythm and the onset of atrial fibrillation. In an alternative embodiment of a detection algorithm, the ventricular detection rate could be monitored for stability of the R-R coupling interval. In the examination of the R-R interval sequence, atrial fibrillation can be recognized by providing a near constant irregularly irregular coupling interval on a beat-by-beat basis. A R-R interval plot during AF appears “cloudlike” in appearance when several hundred or thousands of R-R intervals are plotted over time when compared to sinus rhythm or other supraventricular arrhythmias. Moreover, a distinguishing feature compared to other rhythms that are irregularly irregular, is that the QRS morphology is similar on a beat-by-beat basis despite the irregularity in the R-R coupling interval. This is a distinguishing feature of atrial fibrillation compared to ventricular fibrillation where the QRS morphology varies on a beat-by-beat basis. In yet another embodiment, atrial fibrillation may be detected by seeking to compare the timing and amplitude relationship of the detected P-wave of the electrocardiogram to the detected QRS (R-wave) of the electrocardiogram. Normal sinus rhythm has a fixed relationship that can be placed into a template matching algorithm that can be used as a reference point should the relationship change.[0067]
- In other aspects of the atrial fibrillation detection process, one may include alternative electrodes that may be brought to bear in the S-ICD or US-ICD systems either by placing them in the detection algorithm circuitry through a programming maneuver or by manually adding such additional electrode systems to the S-ICD or US-ICD at the time of implant or at the time of follow-up evaluation. One may also use electrodes for the detection of atrial fibrillation that may or may not also be used for the detection of ventricular arrhythmias given the different anatomic locations of the atria and ventricles with respect to the S-ICD or US-ICD housing and surgical implant sites.[0068]
- Once atrial fibrillation is detected, the arrhythmia can be treated by delivery of a synchronized shock using energy levels up to the maximum output of the device therapy for terminating atrial fibrillation or for other supraventricular arrhythmias. The S-ICD or US-ICD electrode system can be used to treat both atrial and ventricular arrhythmias not only with shock therapy but also with pacing therapy. In a further embodiment of the treatment of atrial fibrillation or other atrial arrhythmias, one may be able to use different electrode systems than what is used to treat ventricular arrhythmias. Another embodiment, would be to allow for different types of therapies (amplitude, waveform, capacitance, etc.) for atrial arrhythmias compared to ventricular arrhythmias.[0069]
- The core member of the different sized and shaped US-ICD will all be the same size and shape. That way, during an implantation procedures, multiple sized US-ICDs can be available for implantation, each one without a core member. Once the implantation procedure is being performed, then the correct sized US-ICD can be selected and the core member can be inserted into the US-ICD and then programmed as described above. Another advantage of this configuration is when the battery within the core member needs replacing it can be done without removing the entire US-ICD.[0070]
- To ensure adequate pacing capture of the heart through an S-ICD having a subcutaneous only lead system, pacing therapy needs to be considerably enhanced by using a biphasic rather than the conventional monophasic waveform for pacing. In addition, to further compensate for the lack of direct contact with the heart, the subcutaneous electrode system, especially the anterior thoracic electrode system, that will be delivering the ATP stimuli should result in as high as a current density as possible in order to activate the cardiac tissues. This can be facilitated by using a small electrode as close to the sternum as possible in the tissues overlying the right ventricle, the cardiac chamber closest to the anterior subcutaneous space where the S-ICD of the present invention will lie.[0071]
- FIG. 19 is a graph that shows an embodiment of the example of a biphasic waveform for use in antitachycardia pacing applications in subcutaneous implantable cardioverter-defibrillators (“S-ICD”) in an embodiment of the present invention. As shown in FIG. 19, the biphasic waveform is plotted as a function of current versus time.[0072]
- In an embodiment, the[0073]
biphasic waveform 1902 comprises apositive portion 1904, anegative portion 1906 and atransition portion 1908. In an embodiment, both thepositive portion 1904 and thenegative portion 1906 are substantially rectangular in shape. Thepositive portion 1904 of thebiphasic waveform 1902 comprises an initial positive current1910, a positive fixed current1912 and a final positive current1914. Thenegative portion 1906 of thebiphasic waveform 1902 comprises an initial negative current1916, a negative fixed current1918 and a final negative current1920. In an embodiment, the polarities of thebiphasic waveform 1902 can be reversed such that thenegative portion 1906 precedes thepositive portion 1904 in time. - As shown in FIG. 19, the[0074]
biphasic waveform 1902 is initially at zero current. Upon commencement of the anti-tachycardia pacing, a current of positive polarity is provided and thebiphasic waveform 1902 rises to the initial positive current1910. Next, the current of thebiphasic waveform 1902 remains at a constant level along the positive fixed current1912. Thepositive portion 1904 of thebiphasic waveform 1902 is then truncated and a negative current is provided. Thebiphasic waveform 1902 then undergoes a relativelyshort transition portion 1908 where the current is approximately zero. Next, thebiphasic waveform 1902 is increased (in absolute value) in the opposite (negative) polarity to the initial negative current1916. After reaching its maximum negative current (in absolute value), the current of thebiphasic waveform 1902 remains at a constant level along the negative fixed current1918. After thenegative portion 1906 of thebiphasic waveform 1902 is truncated at the final negative current1914, thebiphasic waveform 1902 returns to zero. - The total amount of time that the[0075]
biphasic waveform 1902 comprises is known as the “pulse width.” In an embodiment, the pulse width of the biphasic waveform can range from approximately 1 millisecond to approximately 40 milliseconds. The total amount of energy delivered is a function of the pulse width and the absolute value of the current. - An example of one embodiment of the[0076]
biphasic waveform 1902 will now be described. In this embodiment, the amplitude of the initial positive current1910 can range from approximately one to approximately 250 milliamps. Similarly, the amplitude of the initial negative current1916 can range from approximately one to approximately 250 milliamps. - In the example, the pulse width of the[0077]
biphasic waveform 1902 can range from approximately 1 millisecond to approximately 40 milliseconds. In addition, the implantable cardioverter-defibrillator employs biphasic anti-tachycardia pacing at rates of approximately 20 to approximately 120 stimuli/minute for severe bradycardia episodes although programming of higher pacing rates up to 120 stimuli/minute is also possible. - FIG. 20 is a graph that shows an embodiment of the example of a monophasic waveform for use in antitachycardia pacing applications in subcutaneous implantable cardioverter-defibrillators (“S-ICD”) in an embodiment of the present invention. As shown in FIG. 20, the monophasic waveform is plotted as a function of current versus time.[0078]
- In an embodiment, the[0079]
monophasic waveform 2002 comprises an initial positive current2004, a positive fixed current2006 and a final positive current2008. In an embodiment, themonophasic waveform 2002 is substantially rectangular in shape. In an embodiment, the polarities of themonophasic waveform 2002 can be reversed such that thewaveform 2002 is negative in polarity. - As shown in FIG. 20, the[0080]
monophasic waveform 2002 is initially at zero current. Upon commencement of the anti-tachycardia pacing, a current of positive polarity is provided and themonophasic waveform 2002 rises to the initial positive current2004. Next, the current of themonophasic waveform 2002 remains at a constant level along the positive fixed current1906. Themonophasic waveform 2002 is then truncated. - An example of one embodiment of the[0081]
monophasic waveform 2002 will now be described. In this embodiment, the amplitude of the initial positive current2004 can range from approximately one to approximately 250 milliamps. - In an embodiment, the pulse width of the[0082]
biphasic waveform 2002 can range from approximately 1 millisecond to approximately 40 milliseconds. In addition, the implantable cardioverter-defibrillator employs anti-tachycardia pacing at rates of approximately 100 to approximately 350 stimuli/minute for ventricular tachycardia episodes. In addition, up to 30 ATP stimuli for any single attempt could be allowed and as many as 15 ATP attempts could be allowed for any effort to terminate a single episode of VT. One might also allow for different ATP methods to be employed for VTs of different rates or ECG characteristics. Moreover, the device may be allowed to auto-select the method of ATP to be used based upon the device's and/or the physician's experience with previous episodes of VT or with the patient's underlying cardiac condition. In order to maintain these rates, in one embodiment of the invention, the power supply continues to operate to maintain a sufficient voltage to deliver a constant current. - Although it possible for the present invention to provide standard ATP at predetermined or preprogrammed rates for monomorphic VT, the use of an S-ICD may also be employed for the treatment of other arrhythmias such as atria tachyarrhythmias. In another embodiment, the invention can provide ATP in response to a certain activity, respiration, pressure or oxygenation sensor as coupled to arrhythmia characteristics.[0083]
- The S-ICD and US-ICD devices and methods of the present invention may be embodied in other specific forms without departing from the teachings or essential characteristics of the invention. The described embodiments are therefore to be considered in all respects as illustrative and not restrictive, the scope of the invention being indicated by the appended claims rather than by the foregoing description, and all changes which come within the meaning and range of equivalency of the claims are therefore to be embraced therein.[0084]
Claims (149)
Priority Applications (7)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US10/015,202US6952610B2 (en) | 2000-09-18 | 2001-11-05 | Current waveforms for anti-tachycardia pacing for a subcutaneous implantable cardioverter- defibrillator |
| US10/124,159US7194302B2 (en) | 2000-09-18 | 2002-04-17 | Subcutaneous cardiac stimulator with small contact surface electrodes |
| PCT/IB2002/004507WO2003039667A1 (en) | 2001-11-05 | 2002-10-28 | Current waveforms for anti-tachycardia pacing for a subcutaneous implantable cardioverter-defibrillator |
| US11/205,447US8412320B2 (en) | 2000-09-18 | 2005-08-17 | Nontransvenous and nonepicardial methods of cardiac treatment and stimulus |
| US11/680,107US8447398B2 (en) | 2000-09-18 | 2007-02-28 | Subcutaneous implantable cardioverter-defibrillator placement methods |
| US13/785,894US8838234B2 (en) | 2000-09-18 | 2013-03-05 | Methods for implanting a subcutaneous defibrillator |
| US13/887,652US8718760B2 (en) | 2000-09-18 | 2013-05-06 | Subcutaneous implantable cardioverter-defibrillator placement methods |
Applications Claiming Priority (3)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US09/663,606US6647292B1 (en) | 2000-09-18 | 2000-09-18 | Unitary subcutaneous only implantable cardioverter-defibrillator and optional pacer |
| US09/663,607US6721597B1 (en) | 2000-09-18 | 2000-09-18 | Subcutaneous only implantable cardioverter defibrillator and optional pacer |
| US10/015,202US6952610B2 (en) | 2000-09-18 | 2001-11-05 | Current waveforms for anti-tachycardia pacing for a subcutaneous implantable cardioverter- defibrillator |
Related Parent Applications (3)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US09/663,607Continuation-In-PartUS6721597B1 (en) | 2000-09-18 | 2000-09-18 | Subcutaneous only implantable cardioverter defibrillator and optional pacer |
| US09/663,606Continuation-In-PartUS6647292B1 (en) | 2000-09-18 | 2000-09-18 | Unitary subcutaneous only implantable cardioverter-defibrillator and optional pacer |
| US10/011,955Continuation-In-PartUS6952608B2 (en) | 2000-09-18 | 2001-11-05 | Defibrillation pacing circuitry |
Related Child Applications (3)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US10/011,506Continuation-In-PartUS20020107544A1 (en) | 2000-09-18 | 2001-11-05 | Current waveform for anti-bradycardia pacing for a subcutaneous implantable cardioverter-defibrillator |
| US10/124,159Continuation-In-PartUS7194302B2 (en) | 2000-09-18 | 2002-04-17 | Subcutaneous cardiac stimulator with small contact surface electrodes |
| US11/205,447ContinuationUS8412320B2 (en) | 2000-09-18 | 2005-08-17 | Nontransvenous and nonepicardial methods of cardiac treatment and stimulus |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| US20020120299A1true US20020120299A1 (en) | 2002-08-29 |
| US6952610B2 US6952610B2 (en) | 2005-10-04 |
Family
ID=21770073
Family Applications (3)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US10/015,202Expired - LifetimeUS6952610B2 (en) | 2000-09-18 | 2001-11-05 | Current waveforms for anti-tachycardia pacing for a subcutaneous implantable cardioverter- defibrillator |
| US11/205,447Expired - Fee RelatedUS8412320B2 (en) | 2000-09-18 | 2005-08-17 | Nontransvenous and nonepicardial methods of cardiac treatment and stimulus |
| US13/785,894Expired - Fee RelatedUS8838234B2 (en) | 2000-09-18 | 2013-03-05 | Methods for implanting a subcutaneous defibrillator |
Family Applications After (2)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US11/205,447Expired - Fee RelatedUS8412320B2 (en) | 2000-09-18 | 2005-08-17 | Nontransvenous and nonepicardial methods of cardiac treatment and stimulus |
| US13/785,894Expired - Fee RelatedUS8838234B2 (en) | 2000-09-18 | 2013-03-05 | Methods for implanting a subcutaneous defibrillator |
Country Status (2)
| Country | Link |
|---|---|
| US (3) | US6952610B2 (en) |
| WO (1) | WO2003039667A1 (en) |
Cited By (134)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US20030212436A1 (en)* | 2000-11-22 | 2003-11-13 | Brown Ward M. | Apparatus for detecting and treating ventricular arrhythmia |
| US20040204734A1 (en)* | 2003-04-11 | 2004-10-14 | Wagner Darrell Orvin | Tunneling tool with subcutaneous transdermal illumination |
| US20040220626A1 (en)* | 2003-04-11 | 2004-11-04 | Wagner Darrell Orvin | Distributed subcutaneous defibrillation system |
| US20040225329A1 (en)* | 2003-04-11 | 2004-11-11 | Wagner Darrell Orvin | Electrode placement determination for subcutaneous cardiac monitoring and therapy |
| US20040230281A1 (en)* | 2003-04-11 | 2004-11-18 | Ron Heil | Expandable fixation elements for subcutaneous electrodes |
| US20040230280A1 (en)* | 2003-04-11 | 2004-11-18 | Cates Adam W. | Helical fixation elements for subcutaneous electrodes |
| US20040230273A1 (en)* | 2003-04-11 | 2004-11-18 | Cates Adam W. | Subcutaneous electrode and lead with temporary pharmacological agents |
| US20040230274A1 (en)* | 2003-04-11 | 2004-11-18 | Ron Heil | Subcutaneous electrode and lead with phoresis based pharmacological agent delivery |
| US20040230129A1 (en)* | 2003-04-11 | 2004-11-18 | Paul Haefner | Multi-parameter arrhythmia discrimination |
| US20050107838A1 (en)* | 2003-09-18 | 2005-05-19 | Lovett Eric G. | Subcutaneous cardiac rhythm management with disordered breathing detection and treatment |
| US20050143778A1 (en)* | 2000-09-18 | 2005-06-30 | Cameron Health, Inc. | Current waveforms for anti-bradycardia pacing for a subcutaneous implantable cardioverter-defibrillator |
| US7047071B2 (en) | 2003-04-11 | 2006-05-16 | Cardiac Pacemakers, Inc. | Patient stratification for implantable subcutaneous cardiac monitoring and therapy |
| US7082336B2 (en) | 2003-06-04 | 2006-07-25 | Synecor, Llc | Implantable intravascular device for defibrillation and/or pacing |
| US7117035B2 (en) | 2003-04-11 | 2006-10-03 | Cardiac Pacemakers, Inc. | Subcutaneous cardiac stimulation system with patient activity sensing |
| US20060265018A1 (en)* | 2005-05-18 | 2006-11-23 | Cardiac Pacemakers, Inc. | Modular antitachyarrhythmia therapy system |
| US20070049975A1 (en)* | 2005-09-01 | 2007-03-01 | Cates Adam W | Active can with dedicated defibrillation and sensing electrodes |
| US20070142732A1 (en)* | 2005-12-20 | 2007-06-21 | Marina Brockway | Detection of heart failure decompensation based on cumulative changes in sensor signals |
| US7236819B2 (en) | 2003-04-11 | 2007-06-26 | Cardiac Pacemakers, Inc. | Separation of a subcutaneous cardiac signal from a plurality of composite signals |
| US7260433B1 (en) | 2004-09-08 | 2007-08-21 | Pacesetter, Inc. | Subcutaneous cardiac stimulation device providing anti-tachycardia pacing therapy and method |
| US7277755B1 (en) | 2004-09-08 | 2007-10-02 | Pacesetter, Inc. | Subcutaneous cardiac stimulation device providing anti-tachycardia pacing therapy and method |
| US7302294B2 (en) | 2003-04-11 | 2007-11-27 | Cardiac Pacemakers, Inc. | Subcutaneous cardiac sensing and stimulation system employing blood sensor |
| US7386342B1 (en) | 2004-09-08 | 2008-06-10 | Pacesetter, Inc. | Subcutaneous cardiac stimulation device providing anti-tachycardia pacing therapy and method |
| US7392081B2 (en) | 2003-02-28 | 2008-06-24 | Cardiac Pacemakers, Inc. | Subcutaneous cardiac stimulator employing post-shock transthoracic asystole prevention pacing |
| US7396333B2 (en) | 2003-08-18 | 2008-07-08 | Cardiac Pacemakers, Inc. | Prediction of disordered breathing |
| US7457664B2 (en) | 2005-05-09 | 2008-11-25 | Cardiac Pacemakers, Inc. | Closed loop cardiac resynchronization therapy using cardiac activation sequence information |
| US7493175B2 (en) | 2003-04-11 | 2009-02-17 | Cardiac Pacemakers, Inc. | Subcutaneous lead with tined fixation |
| US7499750B2 (en) | 2003-04-11 | 2009-03-03 | Cardiac Pacemakers, Inc. | Noise canceling cardiac electrodes |
| US7509170B2 (en) | 2005-05-09 | 2009-03-24 | Cardiac Pacemakers, Inc. | Automatic capture verification using electrocardiograms sensed from multiple implanted electrodes |
| US7529589B2 (en) | 2003-06-04 | 2009-05-05 | Synecor Llc | Intravascular electrophysiological system and methods |
| US7555335B2 (en) | 2003-04-11 | 2009-06-30 | Cardiac Pacemakers, Inc. | Biopotential signal source separation using source impedances |
| US7566318B2 (en) | 2003-04-11 | 2009-07-28 | Cardiac Pacemakers, Inc. | Ultrasonic subcutaneous dissection tool incorporating fluid delivery |
| US7570997B2 (en) | 2003-04-11 | 2009-08-04 | Cardiac Pacemakers, Inc. | Subcutaneous cardiac rhythm management with asystole prevention therapy |
| US7617007B2 (en) | 2003-06-04 | 2009-11-10 | Synecor Llc | Method and apparatus for retaining medical implants within body vessels |
| US7706866B2 (en) | 2004-06-24 | 2010-04-27 | Cardiac Pacemakers, Inc. | Automatic orientation determination for ECG measurements using multiple electrodes |
| US7747335B2 (en) | 2003-12-12 | 2010-06-29 | Synecor Llc | Implantable medical device having pre-implant exoskeleton |
| US7787946B2 (en) | 2003-08-18 | 2010-08-31 | Cardiac Pacemakers, Inc. | Patient monitoring, diagnosis, and/or therapy systems and methods |
| US7797036B2 (en) | 2004-11-30 | 2010-09-14 | Cardiac Pacemakers, Inc. | Cardiac activation sequence monitoring for ischemia detection |
| US7805185B2 (en) | 2005-05-09 | 2010-09-28 | Cardiac Pacemakers, In. | Posture monitoring using cardiac activation sequences |
| US7865233B2 (en) | 2003-04-11 | 2011-01-04 | Cardiac Pacemakers, Inc. | Subcutaneous cardiac signal discrimination employing non-electrophysiologic signal |
| US7887493B2 (en) | 2003-09-18 | 2011-02-15 | Cardiac Pacemakers, Inc. | Implantable device employing movement sensing for detecting sleep-related disorders |
| US7890159B2 (en) | 2004-09-30 | 2011-02-15 | Cardiac Pacemakers, Inc. | Cardiac activation sequence monitoring and tracking |
| US7917196B2 (en) | 2005-05-09 | 2011-03-29 | Cardiac Pacemakers, Inc. | Arrhythmia discrimination using electrocardiograms sensed from multiple implanted electrodes |
| US7979122B2 (en) | 2003-04-11 | 2011-07-12 | Cardiac Pacemakers, Inc. | Implantable sudden cardiac death prevention device with reduced programmable feature set |
| US8002553B2 (en) | 2003-08-18 | 2011-08-23 | Cardiac Pacemakers, Inc. | Sleep quality data collection and evaluation |
| US8116868B2 (en) | 2003-04-11 | 2012-02-14 | Cardiac Pacemakers, Inc. | Implantable device with cardiac event audio playback |
| US8239045B2 (en) | 2003-06-04 | 2012-08-07 | Synecor Llc | Device and method for retaining a medical device within a vessel |
| US8515535B2 (en) | 2005-02-28 | 2013-08-20 | Cardiac Pacemakers, Inc. | Implantable cardiac device with dyspnea measurement |
| US8527048B2 (en) | 2006-06-29 | 2013-09-03 | Cardiac Pacemakers, Inc. | Local and non-local sensing for cardiac pacing |
| US8535222B2 (en) | 2002-12-04 | 2013-09-17 | Cardiac Pacemakers, Inc. | Sleep detection using an adjustable threshold |
| US8606356B2 (en) | 2003-09-18 | 2013-12-10 | Cardiac Pacemakers, Inc. | Autonomic arousal detection system and method |
| US9144683B2 (en) | 2000-09-18 | 2015-09-29 | Cameron Health, Inc. | Post-shock treatment in a subcutaneous device |
| US20150360040A1 (en)* | 2001-11-21 | 2015-12-17 | Cameron Health, Inc. | Apparatus and method for identifying atrial arrhythmia by far-field sensing |
| US9526909B2 (en) | 2014-08-28 | 2016-12-27 | Cardiac Pacemakers, Inc. | Medical device with triggered blanking period |
| US9592391B2 (en) | 2014-01-10 | 2017-03-14 | Cardiac Pacemakers, Inc. | Systems and methods for detecting cardiac arrhythmias |
| US9669230B2 (en) | 2015-02-06 | 2017-06-06 | Cardiac Pacemakers, Inc. | Systems and methods for treating cardiac arrhythmias |
| US9853743B2 (en) | 2015-08-20 | 2017-12-26 | Cardiac Pacemakers, Inc. | Systems and methods for communication between medical devices |
| US9931074B2 (en) | 2005-11-18 | 2018-04-03 | Cardiac Pacemakers, Inc. | Cardiac resynchronization therapy for improved hemodynamics based on disordered breathing detection |
| US9956414B2 (en) | 2015-08-27 | 2018-05-01 | Cardiac Pacemakers, Inc. | Temporal configuration of a motion sensor in an implantable medical device |
| US9968787B2 (en) | 2015-08-27 | 2018-05-15 | Cardiac Pacemakers, Inc. | Spatial configuration of a motion sensor in an implantable medical device |
| US10029107B1 (en) | 2017-01-26 | 2018-07-24 | Cardiac Pacemakers, Inc. | Leadless device with overmolded components |
| US10046167B2 (en) | 2015-02-09 | 2018-08-14 | Cardiac Pacemakers, Inc. | Implantable medical device with radiopaque ID tag |
| US10050700B2 (en) | 2015-03-18 | 2018-08-14 | Cardiac Pacemakers, Inc. | Communications in a medical device system with temporal optimization |
| US10065041B2 (en) | 2015-10-08 | 2018-09-04 | Cardiac Pacemakers, Inc. | Devices and methods for adjusting pacing rates in an implantable medical device |
| US10092760B2 (en) | 2015-09-11 | 2018-10-09 | Cardiac Pacemakers, Inc. | Arrhythmia detection and confirmation |
| US10137305B2 (en) | 2015-08-28 | 2018-11-27 | Cardiac Pacemakers, Inc. | Systems and methods for behaviorally responsive signal detection and therapy delivery |
| US10159842B2 (en) | 2015-08-28 | 2018-12-25 | Cardiac Pacemakers, Inc. | System and method for detecting tamponade |
| US10183170B2 (en) | 2015-12-17 | 2019-01-22 | Cardiac Pacemakers, Inc. | Conducted communication in a medical device system |
| US10213610B2 (en) | 2015-03-18 | 2019-02-26 | Cardiac Pacemakers, Inc. | Communications in a medical device system with link quality assessment |
| US10220213B2 (en) | 2015-02-06 | 2019-03-05 | Cardiac Pacemakers, Inc. | Systems and methods for safe delivery of electrical stimulation therapy |
| US10226631B2 (en) | 2015-08-28 | 2019-03-12 | Cardiac Pacemakers, Inc. | Systems and methods for infarct detection |
| US10328272B2 (en) | 2016-05-10 | 2019-06-25 | Cardiac Pacemakers, Inc. | Retrievability for implantable medical devices |
| US10350423B2 (en) | 2016-02-04 | 2019-07-16 | Cardiac Pacemakers, Inc. | Delivery system with force sensor for leadless cardiac device |
| US10357159B2 (en) | 2015-08-20 | 2019-07-23 | Cardiac Pacemakers, Inc | Systems and methods for communication between medical devices |
| US10391319B2 (en) | 2016-08-19 | 2019-08-27 | Cardiac Pacemakers, Inc. | Trans septal implantable medical device |
| US10413733B2 (en) | 2016-10-27 | 2019-09-17 | Cardiac Pacemakers, Inc. | Implantable medical device with gyroscope |
| US10426962B2 (en) | 2016-07-07 | 2019-10-01 | Cardiac Pacemakers, Inc. | Leadless pacemaker using pressure measurements for pacing capture verification |
| US10434314B2 (en) | 2016-10-27 | 2019-10-08 | Cardiac Pacemakers, Inc. | Use of a separate device in managing the pace pulse energy of a cardiac pacemaker |
| US10434317B2 (en) | 2016-10-31 | 2019-10-08 | Cardiac Pacemakers, Inc. | Systems and methods for activity level pacing |
| US10463305B2 (en) | 2016-10-27 | 2019-11-05 | Cardiac Pacemakers, Inc. | Multi-device cardiac resynchronization therapy with timing enhancements |
| US10512784B2 (en) | 2016-06-27 | 2019-12-24 | Cardiac Pacemakers, Inc. | Cardiac therapy system using subcutaneously sensed P-waves for resynchronization pacing management |
| US10561330B2 (en) | 2016-10-27 | 2020-02-18 | Cardiac Pacemakers, Inc. | Implantable medical device having a sense channel with performance adjustment |
| US10583303B2 (en) | 2016-01-19 | 2020-03-10 | Cardiac Pacemakers, Inc. | Devices and methods for wirelessly recharging a rechargeable battery of an implantable medical device |
| US10583301B2 (en) | 2016-11-08 | 2020-03-10 | Cardiac Pacemakers, Inc. | Implantable medical device for atrial deployment |
| US10617874B2 (en) | 2016-10-31 | 2020-04-14 | Cardiac Pacemakers, Inc. | Systems and methods for activity level pacing |
| US10632313B2 (en) | 2016-11-09 | 2020-04-28 | Cardiac Pacemakers, Inc. | Systems, devices, and methods for setting cardiac pacing pulse parameters for a cardiac pacing device |
| US10639486B2 (en) | 2016-11-21 | 2020-05-05 | Cardiac Pacemakers, Inc. | Implantable medical device with recharge coil |
| US10668294B2 (en) | 2016-05-10 | 2020-06-02 | Cardiac Pacemakers, Inc. | Leadless cardiac pacemaker configured for over the wire delivery |
| US10688304B2 (en) | 2016-07-20 | 2020-06-23 | Cardiac Pacemakers, Inc. | Method and system for utilizing an atrial contraction timing fiducial in a leadless cardiac pacemaker system |
| US10722720B2 (en) | 2014-01-10 | 2020-07-28 | Cardiac Pacemakers, Inc. | Methods and systems for improved communication between medical devices |
| US10737102B2 (en) | 2017-01-26 | 2020-08-11 | Cardiac Pacemakers, Inc. | Leadless implantable device with detachable fixation |
| US10758724B2 (en) | 2016-10-27 | 2020-09-01 | Cardiac Pacemakers, Inc. | Implantable medical device delivery system with integrated sensor |
| US10758737B2 (en) | 2016-09-21 | 2020-09-01 | Cardiac Pacemakers, Inc. | Using sensor data from an intracardially implanted medical device to influence operation of an extracardially implantable cardioverter |
| US10765871B2 (en) | 2016-10-27 | 2020-09-08 | Cardiac Pacemakers, Inc. | Implantable medical device with pressure sensor |
| US10780278B2 (en) | 2016-08-24 | 2020-09-22 | Cardiac Pacemakers, Inc. | Integrated multi-device cardiac resynchronization therapy using P-wave to pace timing |
| US10821288B2 (en) | 2017-04-03 | 2020-11-03 | Cardiac Pacemakers, Inc. | Cardiac pacemaker with pacing pulse energy adjustment based on sensed heart rate |
| US10835753B2 (en) | 2017-01-26 | 2020-11-17 | Cardiac Pacemakers, Inc. | Intra-body device communication with redundant message transmission |
| US10870008B2 (en) | 2016-08-24 | 2020-12-22 | Cardiac Pacemakers, Inc. | Cardiac resynchronization using fusion promotion for timing management |
| US10874861B2 (en) | 2018-01-04 | 2020-12-29 | Cardiac Pacemakers, Inc. | Dual chamber pacing without beat-to-beat communication |
| US10881863B2 (en) | 2016-11-21 | 2021-01-05 | Cardiac Pacemakers, Inc. | Leadless cardiac pacemaker with multimode communication |
| US10881869B2 (en) | 2016-11-21 | 2021-01-05 | Cardiac Pacemakers, Inc. | Wireless re-charge of an implantable medical device |
| US10894163B2 (en) | 2016-11-21 | 2021-01-19 | Cardiac Pacemakers, Inc. | LCP based predictive timing for cardiac resynchronization |
| US10905872B2 (en) | 2017-04-03 | 2021-02-02 | Cardiac Pacemakers, Inc. | Implantable medical device with a movable electrode biased toward an extended position |
| US10905886B2 (en) | 2015-12-28 | 2021-02-02 | Cardiac Pacemakers, Inc. | Implantable medical device for deployment across the atrioventricular septum |
| US10905889B2 (en) | 2016-09-21 | 2021-02-02 | Cardiac Pacemakers, Inc. | Leadless stimulation device with a housing that houses internal components of the leadless stimulation device and functions as the battery case and a terminal of an internal battery |
| US10918875B2 (en) | 2017-08-18 | 2021-02-16 | Cardiac Pacemakers, Inc. | Implantable medical device with a flux concentrator and a receiving coil disposed about the flux concentrator |
| US10994145B2 (en) | 2016-09-21 | 2021-05-04 | Cardiac Pacemakers, Inc. | Implantable cardiac monitor |
| US11052258B2 (en) | 2017-12-01 | 2021-07-06 | Cardiac Pacemakers, Inc. | Methods and systems for detecting atrial contraction timing fiducials within a search window from a ventricularly implanted leadless cardiac pacemaker |
| US11058880B2 (en) | 2018-03-23 | 2021-07-13 | Medtronic, Inc. | VFA cardiac therapy for tachycardia |
| US11065459B2 (en) | 2017-08-18 | 2021-07-20 | Cardiac Pacemakers, Inc. | Implantable medical device with pressure sensor |
| US11071870B2 (en) | 2017-12-01 | 2021-07-27 | Cardiac Pacemakers, Inc. | Methods and systems for detecting atrial contraction timing fiducials and determining a cardiac interval from a ventricularly implanted leadless cardiac pacemaker |
| US11116988B2 (en) | 2016-03-31 | 2021-09-14 | Cardiac Pacemakers, Inc. | Implantable medical device with rechargeable battery |
| US11147979B2 (en) | 2016-11-21 | 2021-10-19 | Cardiac Pacemakers, Inc. | Implantable medical device with a magnetically permeable housing and an inductive coil disposed about the housing |
| US11185703B2 (en) | 2017-11-07 | 2021-11-30 | Cardiac Pacemakers, Inc. | Leadless cardiac pacemaker for bundle of his pacing |
| US11207527B2 (en) | 2016-07-06 | 2021-12-28 | Cardiac Pacemakers, Inc. | Method and system for determining an atrial contraction timing fiducial in a leadless cardiac pacemaker system |
| US11207532B2 (en) | 2017-01-04 | 2021-12-28 | Cardiac Pacemakers, Inc. | Dynamic sensing updates using postural input in a multiple device cardiac rhythm management system |
| US11213676B2 (en) | 2019-04-01 | 2022-01-04 | Medtronic, Inc. | Delivery systems for VfA cardiac therapy |
| US11235159B2 (en) | 2018-03-23 | 2022-02-01 | Medtronic, Inc. | VFA cardiac resynchronization therapy |
| US11235161B2 (en) | 2018-09-26 | 2022-02-01 | Medtronic, Inc. | Capture in ventricle-from-atrium cardiac therapy |
| US11235163B2 (en) | 2017-09-20 | 2022-02-01 | Cardiac Pacemakers, Inc. | Implantable medical device with multiple modes of operation |
| US11260216B2 (en) | 2017-12-01 | 2022-03-01 | Cardiac Pacemakers, Inc. | Methods and systems for detecting atrial contraction timing fiducials during ventricular filling from a ventricularly implanted leadless cardiac pacemaker |
| US11285326B2 (en) | 2015-03-04 | 2022-03-29 | Cardiac Pacemakers, Inc. | Systems and methods for treating cardiac arrhythmias |
| US11305127B2 (en) | 2019-08-26 | 2022-04-19 | Medtronic Inc. | VfA delivery and implant region detection |
| US11400296B2 (en) | 2018-03-23 | 2022-08-02 | Medtronic, Inc. | AV synchronous VfA cardiac therapy |
| US11524169B2 (en) | 2017-02-06 | 2022-12-13 | Medtronic, Inc. | Charge balanced cardiac pacing from high voltage circuitry of an extra-cardiovascular implantable cardioverter defibrillator system |
| US11529523B2 (en) | 2018-01-04 | 2022-12-20 | Cardiac Pacemakers, Inc. | Handheld bridge device for providing a communication bridge between an implanted medical device and a smartphone |
| US11679265B2 (en) | 2019-02-14 | 2023-06-20 | Medtronic, Inc. | Lead-in-lead systems and methods for cardiac therapy |
| US11697025B2 (en) | 2019-03-29 | 2023-07-11 | Medtronic, Inc. | Cardiac conduction system capture |
| US11712188B2 (en) | 2019-05-07 | 2023-08-01 | Medtronic, Inc. | Posterior left bundle branch engagement |
| US11813463B2 (en) | 2017-12-01 | 2023-11-14 | Cardiac Pacemakers, Inc. | Leadless cardiac pacemaker with reversionary behavior |
| US11813464B2 (en) | 2020-07-31 | 2023-11-14 | Medtronic, Inc. | Cardiac conduction system evaluation |
| US11813466B2 (en) | 2020-01-27 | 2023-11-14 | Medtronic, Inc. | Atrioventricular nodal stimulation |
| US11911168B2 (en) | 2020-04-03 | 2024-02-27 | Medtronic, Inc. | Cardiac conduction system therapy benefit determination |
| US11951313B2 (en) | 2018-11-17 | 2024-04-09 | Medtronic, Inc. | VFA delivery systems and methods |
| US12296177B2 (en) | 2018-12-21 | 2025-05-13 | Medtronic, Inc. | Delivery systems and methods for left ventricular pacing |
Families Citing this family (67)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US7751885B2 (en) | 2000-09-18 | 2010-07-06 | Cameron Health, Inc. | Bradycardia pacing in a subcutaneous device |
| US20020035381A1 (en) | 2000-09-18 | 2002-03-21 | Cameron Health, Inc. | Subcutaneous electrode with improved contact shape for transthoracic conduction |
| US7194302B2 (en) | 2000-09-18 | 2007-03-20 | Cameron Health, Inc. | Subcutaneous cardiac stimulator with small contact surface electrodes |
| US7149575B2 (en) | 2000-09-18 | 2006-12-12 | Cameron Health, Inc. | Subcutaneous cardiac stimulator device having an anteriorly positioned electrode |
| US6647292B1 (en)* | 2000-09-18 | 2003-11-11 | Cameron Health | Unitary subcutaneous only implantable cardioverter-defibrillator and optional pacer |
| US6865417B2 (en)* | 2001-11-05 | 2005-03-08 | Cameron Health, Inc. | H-bridge with sensing circuit |
| US20020095184A1 (en)* | 2000-09-18 | 2002-07-18 | Bardy Gust H. | Monophasic waveform for anti-tachycardia pacing for a subcutaneous implantable cardioverter-defibrillator |
| US7076296B2 (en)* | 2000-09-18 | 2006-07-11 | Cameron Health, Inc. | Method of supplying energy to subcutaneous cardioverter-defibrillator and pacer |
| US6721597B1 (en) | 2000-09-18 | 2004-04-13 | Cameron Health, Inc. | Subcutaneous only implantable cardioverter defibrillator and optional pacer |
| US6988003B2 (en)* | 2000-09-18 | 2006-01-17 | Cameron Health, Inc. | Implantable cardioverter-defibrillator having two spaced apart shocking electrodes on housing |
| US7069080B2 (en) | 2000-09-18 | 2006-06-27 | Cameron Health, Inc. | Active housing and subcutaneous electrode cardioversion/defibrillating system |
| US7321794B2 (en)* | 2002-11-15 | 2008-01-22 | Advanced Bionics Corporation | Method and system for treating atrial fibrillation |
| US7069075B2 (en)* | 2002-11-22 | 2006-06-27 | Medtronic, Inc. | Subcutaneous implantable cardioverter/defibrillator |
| US20060247693A1 (en) | 2005-04-28 | 2006-11-02 | Yanting Dong | Non-captured intrinsic discrimination in cardiac pacing response classification |
| US7774064B2 (en) | 2003-12-12 | 2010-08-10 | Cardiac Pacemakers, Inc. | Cardiac response classification using retriggerable classification windows |
| US8521284B2 (en) | 2003-12-12 | 2013-08-27 | Cardiac Pacemakers, Inc. | Cardiac response classification using multisite sensing and pacing |
| US7996072B2 (en) | 2004-12-21 | 2011-08-09 | Cardiac Pacemakers, Inc. | Positionally adaptable implantable cardiac device |
| US7392086B2 (en) | 2005-04-26 | 2008-06-24 | Cardiac Pacemakers, Inc. | Implantable cardiac device and method for reduced phrenic nerve stimulation |
| US8116867B2 (en) | 2005-08-04 | 2012-02-14 | Cameron Health, Inc. | Methods and devices for tachyarrhythmia sensing and high-pass filter bypass |
| US8255049B2 (en) | 2006-05-08 | 2012-08-28 | Cardiac Pacemakers, Inc. | Method and device for providing anti-tachyarrhythmia therapy |
| US8200341B2 (en) | 2007-02-07 | 2012-06-12 | Cameron Health, Inc. | Sensing vector selection in a cardiac stimulus device with postural assessment |
| US7783340B2 (en) | 2007-01-16 | 2010-08-24 | Cameron Health, Inc. | Systems and methods for sensing vector selection in an implantable medical device using a polynomial approach |
| US20070282376A1 (en) | 2006-06-06 | 2007-12-06 | Shuros Allan C | Method and apparatus for neural stimulation via the lymphatic system |
| US7623913B2 (en) | 2006-08-01 | 2009-11-24 | Cameron Health, Inc. | Implantable medical devices using heuristic filtering in cardiac event detection |
| US8718793B2 (en) | 2006-08-01 | 2014-05-06 | Cameron Health, Inc. | Electrode insertion tools, lead assemblies, kits and methods for placement of cardiac device electrodes |
| US7580741B2 (en) | 2006-08-18 | 2009-08-25 | Cardiac Pacemakers, Inc. | Method and device for determination of arrhythmia rate zone thresholds using a probability function |
| US8712507B2 (en) | 2006-09-14 | 2014-04-29 | Cardiac Pacemakers, Inc. | Systems and methods for arranging and labeling cardiac episodes |
| US8209013B2 (en) | 2006-09-14 | 2012-06-26 | Cardiac Pacemakers, Inc. | Therapeutic electrical stimulation that avoids undesirable activation |
| US7877139B2 (en) | 2006-09-22 | 2011-01-25 | Cameron Health, Inc. | Method and device for implantable cardiac stimulus device lead impedance measurement |
| US8014851B2 (en) | 2006-09-26 | 2011-09-06 | Cameron Health, Inc. | Signal analysis in implantable cardiac treatment devices |
| US8983598B2 (en)* | 2006-10-04 | 2015-03-17 | Cardiac Pacemakers, Inc. | System for neurally-mediated anti-arrhythmic therapy |
| US7941208B2 (en) | 2006-11-29 | 2011-05-10 | Cardiac Pacemakers, Inc. | Therapy delivery for identified tachyarrhythmia episode types |
| US7623916B2 (en) | 2006-12-20 | 2009-11-24 | Cameron Health, Inc. | Implantable cardiac stimulus devices and methods with input recharge circuitry |
| US9037239B2 (en) | 2007-08-07 | 2015-05-19 | Cardiac Pacemakers, Inc. | Method and apparatus to perform electrode combination selection |
| US8265736B2 (en) | 2007-08-07 | 2012-09-11 | Cardiac Pacemakers, Inc. | Method and apparatus to perform electrode combination selection |
| US8649866B2 (en) | 2008-02-14 | 2014-02-11 | Cardiac Pacemakers, Inc. | Method and apparatus for phrenic stimulation detection |
| US8412325B2 (en)* | 2008-04-08 | 2013-04-02 | Cardiac Pacemakers, Inc. | High-energy anti-tachycardia therapy |
| EP2349467B1 (en) | 2008-10-06 | 2017-08-23 | Cardiac Pacemakers, Inc. | Dynamic cardiac resynchronization therapy by tracking intrinsic conduction |
| US9849291B2 (en) | 2011-06-09 | 2017-12-26 | Cameron Health, Inc. | Antitachycardia pacing pulse from a subcutaneous defibrillator |
| US9579065B2 (en) | 2013-03-12 | 2017-02-28 | Cameron Health Inc. | Cardiac signal vector selection with monophasic and biphasic shape consideration |
| US10556117B2 (en) | 2013-05-06 | 2020-02-11 | Medtronic, Inc. | Implantable cardioverter-defibrillator (ICD) system including substernal pacing lead |
| US10532203B2 (en) | 2013-05-06 | 2020-01-14 | Medtronic, Inc. | Substernal electrical stimulation system |
| US10471267B2 (en) | 2013-05-06 | 2019-11-12 | Medtronic, Inc. | Implantable cardioverter-defibrillator (ICD) system including substernal lead |
| US9717923B2 (en) | 2013-05-06 | 2017-08-01 | Medtronic, Inc. | Implantable medical device system having implantable cardioverter-defibrillator (ICD) system and substernal leadless pacing device |
| US9789319B2 (en) | 2013-11-21 | 2017-10-17 | Medtronic, Inc. | Systems and methods for leadless cardiac resynchronization therapy |
| US10448855B2 (en) | 2014-04-25 | 2019-10-22 | Medtronic, Inc. | Implantable medical device (IMD) sensing modifications responsive to detected pacing pulses |
| US20150306375A1 (en)* | 2014-04-25 | 2015-10-29 | Medtronic, Inc. | Implantable extravascular electrical stimulation lead having improved sensing and pacing capability |
| US10154794B2 (en) | 2014-04-25 | 2018-12-18 | Medtronic, Inc. | Implantable cardioverter-defibrillator (ICD) tachyarrhythmia detection modifications responsive to detected pacing |
| US10226197B2 (en) | 2014-04-25 | 2019-03-12 | Medtronic, Inc. | Pace pulse detector for an implantable medical device |
| US9669224B2 (en) | 2014-05-06 | 2017-06-06 | Medtronic, Inc. | Triggered pacing system |
| US9492671B2 (en) | 2014-05-06 | 2016-11-15 | Medtronic, Inc. | Acoustically triggered therapy delivery |
| US10743960B2 (en) | 2014-09-04 | 2020-08-18 | AtaCor Medical, Inc. | Cardiac arrhythmia treatment devices and delivery |
| US10328268B2 (en) | 2014-09-04 | 2019-06-25 | AtaCor Medical, Inc. | Cardiac pacing |
| US9636512B2 (en) | 2014-11-05 | 2017-05-02 | Medtronic, Inc. | Implantable cardioverter-defibrillator (ICD) system having multiple common polarity extravascular defibrillation electrodes |
| US11097109B2 (en) | 2014-11-24 | 2021-08-24 | AtaCor Medical, Inc. | Cardiac pacing sensing and control |
| JP6854239B2 (en) | 2014-12-09 | 2021-04-07 | メドトロニック,インコーポレイテッド | Extravascular implantable electrical lead with wavy structure |
| US10449362B2 (en) | 2015-12-03 | 2019-10-22 | Medtronic, Inc. | Extra-cardiovascular cardiac pacing system for delivering composite pacing pulses |
| US10080891B2 (en) | 2015-12-03 | 2018-09-25 | Medtronic, Inc. | Extra-cardiovascular cardiac pacing system |
| EP3383488B1 (en) | 2015-12-03 | 2023-10-25 | Medtronic, Inc. | Tachyarrhythmia induction by an extra-cardiovascular implantable cardioverter defibrillator |
| WO2017096181A1 (en) | 2015-12-03 | 2017-06-08 | Medtronic, Inc. | Extra-cardiovascular pacing using high-voltage therapy circuitry of an implantable cardioverter defibrillator |
| US9731138B1 (en) | 2016-02-17 | 2017-08-15 | Medtronic, Inc. | System and method for cardiac pacing |
| US11027132B2 (en) | 2016-03-16 | 2021-06-08 | Medtronic, Inc. | Synchronization of anti-tachycardia pacing in an extra-cardiovascular implantable system |
| US9802055B2 (en) | 2016-04-04 | 2017-10-31 | Medtronic, Inc. | Ultrasound powered pulse delivery device |
| US11583685B2 (en) | 2017-12-21 | 2023-02-21 | Galvani Bioelectronics Limited | Systems and methods configured to insert an implant in an abdominal cavity |
| US10596383B2 (en) | 2018-04-03 | 2020-03-24 | Medtronic, Inc. | Feature based sensing for leadless pacing therapy |
| EP4527449A3 (en) | 2019-05-29 | 2025-04-30 | Atacor Medical, Inc. | Implantable electrical leads and associated delivery systems |
| US11666771B2 (en) | 2020-05-29 | 2023-06-06 | AtaCor Medical, Inc. | Implantable electrical leads and associated delivery systems |
Citations (93)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US3653387A (en)* | 1970-05-08 | 1972-04-04 | Cardiac Electronics Inc | Protector circuit for cardiac apparatus |
| US3707974A (en)* | 1970-12-11 | 1973-01-02 | W Raddi | Body organ stimulator with voltage converter |
| US3710374A (en)* | 1970-03-16 | 1973-01-09 | Wester Instr Inc | Dual-slope and analog-to-digital converter wherein two analog input signals are selectively integrated with respect to time |
| US3911925A (en)* | 1974-05-23 | 1975-10-14 | Jr Joe B Tillery | Ear trimming forceps |
| US4157720A (en)* | 1977-09-16 | 1979-06-12 | Greatbatch W | Cardiac pacemaker |
| US4191942A (en)* | 1978-06-08 | 1980-03-04 | National Semiconductor Corporation | Single slope A/D converter with sample and hold |
| US4223678A (en)* | 1978-05-03 | 1980-09-23 | Mieczyslaw Mirowski | Arrhythmia recorder for use with an implantable defibrillator |
| US4248237A (en)* | 1978-03-07 | 1981-02-03 | Needle Industries Limited | Cardiac pacemakers |
| US4290430A (en)* | 1980-06-05 | 1981-09-22 | Intermedics, Inc. | Pacer analyzer |
| US4291707A (en)* | 1979-04-30 | 1981-09-29 | Mieczyslaw Mirowski | Implantable cardiac defibrillating electrode |
| US4314095A (en)* | 1979-04-30 | 1982-02-02 | Mieczyslaw Mirowski | Device and method for making electrical contact |
| US4402322A (en)* | 1981-03-25 | 1983-09-06 | Medtronic, Inc. | Pacer output circuit |
| US4406286A (en)* | 1981-04-09 | 1983-09-27 | Medtronic, Inc. | Fast recharge output circuit |
| US4407288A (en)* | 1981-02-18 | 1983-10-04 | Mieczyslaw Mirowski | Implantable heart stimulator and stimulation method |
| US4424818A (en)* | 1982-02-18 | 1984-01-10 | Medtronic, Inc. | Electrical lead and insertion tool |
| US4450527A (en)* | 1982-06-29 | 1984-05-22 | Bomed Medical Mfg. Ltd. | Noninvasive continuous cardiac output monitor |
| US4543956A (en)* | 1984-05-24 | 1985-10-01 | Cordis Corporation | Biphasic cardiac pacer |
| US4567900A (en)* | 1984-06-04 | 1986-02-04 | Moore J Paul | Internal deployable defibrillator electrode |
| US4602637A (en)* | 1983-01-11 | 1986-07-29 | Siemens Aktiengesellschaft | Heart pacemaker system |
| US4603705A (en)* | 1984-05-04 | 1986-08-05 | Mieczyslaw Mirowski | Intravascular multiple electrode unitary catheter |
| US4693253A (en)* | 1981-03-23 | 1987-09-15 | Medtronic, Inc. | Automatic implantable defibrillator and pacer |
| US4765341A (en)* | 1981-06-22 | 1988-08-23 | Mieczyslaw Mirowski | Cardiac electrode with attachment fin |
| US4800883A (en)* | 1986-04-02 | 1989-01-31 | Intermedics, Inc. | Apparatus for generating multiphasic defibrillation pulse waveform |
| US4830005A (en)* | 1987-07-23 | 1989-05-16 | Siemens-Pacesetter, Inc. | Disposable in-package load test element for pacemakers |
| US4878497A (en)* | 1988-03-25 | 1989-11-07 | Telectronics N.V. | Pacemaker with improved automatic output regulation |
| US4944300A (en)* | 1987-04-28 | 1990-07-31 | Sanjeev Saksena | Method for high energy defibrillation of ventricular fibrillation in humans without a thoracotomy |
| US5105810A (en)* | 1990-07-24 | 1992-04-21 | Telectronics Pacing Systems, Inc. | Implantable automatic and haemodynamically responsive cardioverting/defibrillating pacemaker with means for minimizing bradycardia support pacing voltages |
| US5109842A (en)* | 1990-09-24 | 1992-05-05 | Siemens Pacesetter, Inc. | Implantable tachyarrhythmia control system having a patch electrode with an integrated cardiac activity system |
| US5133353A (en)* | 1990-04-25 | 1992-07-28 | Cardiac Pacemakers, Inc. | Implantable intravenous cardiac stimulation system with pulse generator housing serving as optional additional electrode |
| US5144946A (en)* | 1991-08-05 | 1992-09-08 | Siemens Pacesetter, Inc. | Combined pacemaker substrate and electrical interconnect and method of assembly |
| US5184616A (en)* | 1991-10-21 | 1993-02-09 | Telectronics Pacing Systems, Inc. | Apparatus and method for generation of varying waveforms in arrhythmia control system |
| US5191901A (en)* | 1991-08-29 | 1993-03-09 | Mieczyslaw Mirowski | Controlled discharge defibrillation electrode |
| US5192392A (en)* | 1991-02-28 | 1993-03-09 | The Bottling Room, Inc. | Container labeler |
| US5203348A (en)* | 1990-06-06 | 1993-04-20 | Cardiac Pacemakers, Inc. | Subcutaneous defibrillation electrodes |
| US5230337A (en)* | 1990-06-06 | 1993-07-27 | Cardiac Pacemakers, Inc. | Process for implanting subcutaneous defibrillation electrodes |
| US5255692A (en)* | 1992-09-04 | 1993-10-26 | Siemens Aktiengesellschaft | Subcostal patch electrode |
| US5261400A (en)* | 1992-02-12 | 1993-11-16 | Medtronic, Inc. | Defibrillator employing transvenous and subcutaneous electrodes and method of use |
| US5300106A (en)* | 1991-06-07 | 1994-04-05 | Cardiac Pacemakers, Inc. | Insertion and tunneling tool for a subcutaneous wire patch electrode |
| US5318591A (en)* | 1992-11-23 | 1994-06-07 | Siemens Pacesetter, Inc. | Implantable cardioverter-defibrillator having early charging capability |
| US5331966A (en)* | 1991-04-05 | 1994-07-26 | Medtronic, Inc. | Subcutaneous multi-electrode sensing system, method and pacer |
| US5366496A (en)* | 1993-04-01 | 1994-11-22 | Cardiac Pacemakers, Inc. | Subcutaneous shunted coil electrode |
| US5376104A (en)* | 1992-02-07 | 1994-12-27 | Nihon Kohden Corporation | Defibrillator with electrocardiogram monitor |
| US5376103A (en)* | 1992-03-19 | 1994-12-27 | Angeion Corporation | Electrode system for implantable defibrillator |
| US5391200A (en)* | 1992-09-30 | 1995-02-21 | Cardiac Pacemakers, Inc. | Defibrillation patch electrode having conductor-free resilient zone for minimally invasive deployment |
| US5391191A (en)* | 1990-04-24 | 1995-02-21 | Siemens Aktiengesellschaft | Device for tissue stimulation |
| US5405363A (en)* | 1991-03-15 | 1995-04-11 | Angelon Corporation | Implantable cardioverter defibrillator having a smaller displacement volume |
| US5411547A (en)* | 1993-08-09 | 1995-05-02 | Pacesetter, Inc. | Implantable cardioversion-defibrillation patch electrodes having means for passive multiplexing of discharge pulses |
| US5413591A (en)* | 1992-02-26 | 1995-05-09 | Angeion Corporation | Current truncated waveform defibrillator |
| US5423326A (en)* | 1991-09-12 | 1995-06-13 | Drexel University | Apparatus and method for measuring cardiac output |
| US5476503A (en)* | 1994-03-28 | 1995-12-19 | Pacesetter, Inc. | Sense array intelligent patch lead for an implantable defibrillator and method |
| US5509928A (en)* | 1995-03-02 | 1996-04-23 | Pacesetter, Inc. | Internally supported self-sealing septum |
| US5509923A (en)* | 1989-08-16 | 1996-04-23 | Raychem Corporation | Device for dissecting, grasping, or cutting an object |
| US5531766A (en)* | 1995-01-23 | 1996-07-02 | Angeion Corporation | Implantable cardioverter defibrillator pulse generator kite-tail electrode system |
| US5531765A (en)* | 1990-12-18 | 1996-07-02 | Ventritex, Inc. | Method and apparatus for producing configurable biphasic defibrillation waveforms |
| US5534019A (en)* | 1994-12-09 | 1996-07-09 | Ventritex, Inc. | Cardiac defibrillator with case that can be electrically active or inactive |
| US5534022A (en)* | 1994-11-22 | 1996-07-09 | Ventritex, Inc. | Lead having an integrated defibrillation/sensing electrode |
| US5597956A (en)* | 1994-08-24 | 1997-01-28 | Murata Manufacturing Co., Ltd. | Capacitor type acceleration sensor |
| US5601607A (en)* | 1992-03-19 | 1997-02-11 | Angeion Corporation | Implantable cardioverter defibrillator housing plated electrode |
| US5618287A (en)* | 1994-01-28 | 1997-04-08 | Thomas J. Fogarty | Methods of surgically implanting a defibrillator electrode within a patient |
| US5620477A (en)* | 1994-03-31 | 1997-04-15 | Ventritex, Inc. | Pulse generator with case that can be active or inactive |
| US5643328A (en)* | 1996-07-19 | 1997-07-01 | Sulzer Intermedics Inc. | Implantable cardiac stimulation device with warning system having elongated stimulation electrode |
| US5645586A (en)* | 1994-07-08 | 1997-07-08 | Ventritex, Inc. | Conforming implantable defibrillator |
| US5658321A (en)* | 1995-06-09 | 1997-08-19 | Ventritex, Inc. | Conductive housing for implantable cardiac device |
| US5658317A (en)* | 1995-08-14 | 1997-08-19 | Cardiac Pacemakers, Inc. | Threshold templating for digital AGC |
| US5674260A (en)* | 1996-02-23 | 1997-10-07 | Pacesetter, Inc. | Apparatus and method for mounting an activity sensor or other component within a pacemaker using a contoured hybrid lid |
| US5690683A (en)* | 1995-06-19 | 1997-11-25 | Cardiac Pacemakers, Inc. | After potential removal in cardiac rhythm management device |
| US5697953A (en)* | 1993-03-13 | 1997-12-16 | Angeion Corporation | Implantable cardioverter defibrillator having a smaller displacement volume |
| US5713926A (en)* | 1990-04-25 | 1998-02-03 | Cardiac Pacemakers, Inc. | Implantable intravenous cardiac stimulation system with pulse generator housing serving as optional additional electrode |
| US5766226A (en)* | 1996-12-09 | 1998-06-16 | Angeion Corporation | Switched discharge pathways for ICD having multiple output capacitors |
| US5776169A (en)* | 1997-04-28 | 1998-07-07 | Sulzer Intermedics Inc. | Implantable cardiac stimulator for minimally invasive implantation |
| US5800464A (en)* | 1996-10-03 | 1998-09-01 | Medtronic, Inc. | System for providing hyperpolarization of cardiac to enhance cardiac function |
| US5814090A (en)* | 1995-06-07 | 1998-09-29 | Angeion Corporation | Implantable medical device having heat-shrink conforming shield |
| US5836976A (en)* | 1997-04-30 | 1998-11-17 | Medtronic, Inc. | Cardioversion energy reduction system |
| US5895414A (en)* | 1996-04-19 | 1999-04-20 | Sanchez-Zambrano; Sergio | Pacemaker housing |
| US5919211A (en)* | 1996-06-27 | 1999-07-06 | Adams; Theodore P. | ICD power source using multiple single use batteries |
| US5919222A (en)* | 1998-01-06 | 1999-07-06 | Medtronic Inc. | Adjustable medical electrode lead |
| US5925069A (en)* | 1997-11-07 | 1999-07-20 | Sulzer Intermedics Inc. | Method for preparing a high definition window in a conformally coated medical device |
| US5935154A (en)* | 1997-01-24 | 1999-08-10 | Cardiac Pacemakers, Inc. | Implantable tissue stimulator incorporating deposited multilayer capacitor |
| US5941904A (en)* | 1997-09-12 | 1999-08-24 | Sulzer Intermedics Inc. | Electromagnetic acceleration transducer for implantable medical device |
| US5964787A (en)* | 1998-04-17 | 1999-10-12 | Vitatron Medical B.V. | Stimulus system with controllable switched capacitor output stage |
| US6014586A (en)* | 1995-11-20 | 2000-01-11 | Pacesetter, Inc. | Vertically integrated semiconductor package for an implantable medical device |
| US6026325A (en)* | 1998-06-18 | 2000-02-15 | Pacesetter, Inc. | Implantable medical device having an improved packaging system and method for making electrical connections |
| US6058328A (en)* | 1996-08-06 | 2000-05-02 | Pacesetter, Inc. | Implantable stimulation device having means for operating in a preemptive pacing mode to prevent tachyarrhythmias and method thereof |
| US6093173A (en)* | 1998-09-09 | 2000-07-25 | Embol-X, Inc. | Introducer/dilator with balloon protection and methods of use |
| US6095987A (en)* | 1996-04-17 | 2000-08-01 | Imagyn Medical Techonologies California, Inc. | Apparatus and methods of bioelectrical impedance analysis of blood flow |
| US6128531A (en)* | 1998-04-01 | 2000-10-03 | Pacesetter, Inc. | Delivery of ICD shock capacitor energy via a controlled current source |
| USH1905H (en)* | 1997-03-21 | 2000-10-03 | Medtronic, Inc. | Mechanism for adjusting the exposed surface area and position of an electrode along a lead body |
| US6144866A (en)* | 1998-10-30 | 2000-11-07 | Medtronic, Inc. | Multiple sensor assembly for medical electric lead |
| US6148230A (en)* | 1998-01-30 | 2000-11-14 | Uab Research Foundation | Method for the monitoring and treatment of spontaneous cardiac arrhythmias |
| US6185450B1 (en)* | 1998-01-26 | 2001-02-06 | Physio-Control Manufacturing Corporation | Digital sliding pole fast-restore for an electrocardiograph display |
| US6411844B1 (en)* | 1999-10-19 | 2002-06-25 | Pacesetter, Inc. | Fast recovery sensor amplifier circuit for implantable medical device |
| US6519493B1 (en)* | 1999-12-23 | 2003-02-11 | Pacesetter, Inc. | Methods and apparatus for overdrive pacing heart tissue using an implantable cardiac stimulation device |
| US6647292B1 (en)* | 2000-09-18 | 2003-11-11 | Cameron Health | Unitary subcutaneous only implantable cardioverter-defibrillator and optional pacer |
Family Cites Families (72)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US3593718A (en)* | 1967-07-13 | 1971-07-20 | Biocybernetics Inc | Physiologically controlled cardiac pacer |
| US3713449A (en)* | 1970-08-31 | 1973-01-30 | P Mulier | Cardiac pacer with externally controllable variable width output pulse |
| EP0095727A1 (en) | 1982-06-01 | 1983-12-07 | Purdue Research Foundation | Method and apparatus for inserting a defibrillator electrode and defibrillator electrode |
| ES2069540T3 (en) | 1987-11-19 | 1995-05-16 | Siemens Ag | ANALOG-DIGITAL CONVERTER. |
| FR2632865A1 (en) | 1988-06-15 | 1989-12-22 | Atesys Sa | HIGH PERFORMANCE DEFIBRILLATOR WITH SEVERAL ELECTRODES OUTSIDE THE HEART |
| US5170784A (en) | 1990-11-27 | 1992-12-15 | Ceon Ramon | Leadless magnetic cardiac pacemaker |
| US5129392A (en) | 1990-12-20 | 1992-07-14 | Medtronic, Inc. | Apparatus for automatically inducing fibrillation |
| DE4110402A1 (en)* | 1991-03-28 | 1992-10-01 | Siemens Ag | DEFIBRILLATOR / CONVERTER |
| US5292339A (en) | 1991-06-14 | 1994-03-08 | Telectronics Pacing Systems, Inc. | Implantable pacemaker/cardioverter/defibrillator device and method incorporating multiple bradycardia support pacing rates |
| US5243977A (en) | 1991-06-26 | 1993-09-14 | Trabucco Hector O | Pacemaker |
| US5215083A (en) | 1991-10-07 | 1993-06-01 | Telectronics Pacing Systems, Inc. | Apparatus and method for arrhythmia induction in arrhythmia control system |
| US5314448A (en) | 1991-10-28 | 1994-05-24 | Angeion Corporation | Process for defibrillation pretreatment of a heart |
| EP0642368B1 (en) | 1992-04-06 | 1999-01-27 | Angeion Corporation | Apparatus for treatment of ventricular tachycardia using a far-field pulse series |
| US5265623A (en)* | 1992-07-16 | 1993-11-30 | Angeion Corporation | Optimized field defibrillation catheter |
| US5441518A (en)* | 1993-07-22 | 1995-08-15 | Angeion Corporation | Implantable cardioverter defibrillator system having independently controllable electrode discharge pathway |
| US5522853A (en)* | 1992-10-27 | 1996-06-04 | Angeion Corporation | Method and apparatus for progressive recruitment of cardiac fibrillation |
| US5447518A (en) | 1993-08-31 | 1995-09-05 | Ventritex, Inc. | Method and apparatus for phase related cardiac defibrillation |
| US5411539A (en) | 1993-08-31 | 1995-05-02 | Medtronic, Inc. | Active can emulator and method of use |
| SE9401267D0 (en) | 1994-04-14 | 1994-04-14 | Siemens Elema Ab | The electrode device |
| US6051017A (en) | 1996-02-20 | 2000-04-18 | Advanced Bionics Corporation | Implantable microstimulator and systems employing the same |
| US6295470B1 (en) | 1996-08-19 | 2001-09-25 | The Mower Family Chf Treatment Irrevocable Trust | Antitachycardial pacing |
| WO1998025349A1 (en) | 1996-12-03 | 1998-06-11 | Microchip Technology Incorporated | Slope analog-to-digital converter with ramp initiated prior to counter |
| DK9700059U1 (en) | 1997-02-04 | 1998-05-04 | Ralph Mathar | Apparatus for use in by-pass operations and the use of such apparatus |
| JP4430744B2 (en)* | 1997-03-14 | 2010-03-10 | ユニヴァーシティ・オヴ・アラバマ・アト・バーミンガム・リサーチ・ファンデイション | Implantable system for patients in need of such treatment with cardiac cardioversion |
| EP0996483A1 (en) | 1997-07-17 | 2000-05-03 | CPR Medical, Inc. | Defibrillator/pacemaker |
| SE9704311D0 (en) | 1997-11-24 | 1997-11-24 | Pacesetter Ab | A cardiac event detecting system for a heart stimulator |
| FR2772516B1 (en) | 1997-12-12 | 2003-07-04 | Ela Medical Sa | ELECTRONIC CIRCUIT, IN PARTICULAR FOR AN ACTIVE IMPLANTABLE MEDICAL DEVICE SUCH AS A CARDIAC STIMULATOR OR DEFIBRILLATOR, AND ITS MANUFACTURING METHOD |
| CA2318907C (en) | 1998-01-27 | 2004-05-04 | Vitatron Medical, B.V. | System for inducing tachycardia utilizing near field t-wave sensing |
| US5968079A (en)* | 1998-03-18 | 1999-10-19 | Medtronic, Inc. | Method and apparatus for diagnosis and treatment of arrhythmias |
| MY128127A (en) | 1998-04-23 | 2007-01-31 | Alza Corp | Trocar for inserting implants |
| US6208895B1 (en) | 1998-10-13 | 2001-03-27 | Physio-Control Manufacturing Corporation | Circuit for performing external pacing and biphasic defibrillation |
| EP1000634A1 (en) | 1998-11-10 | 2000-05-17 | Sulzer Osypka GmbH | Stimulation electrode for both defibrillation and pacing |
| SE9900682D0 (en) | 1999-02-25 | 1999-02-25 | Pacesetter Ab | Implantable tissue stimulating device |
| US6272379B1 (en)* | 1999-03-17 | 2001-08-07 | Cathco, Inc. | Implantable electronic system with acute myocardial infarction detection and patient warning capabilities |
| US6263241B1 (en) | 1999-04-30 | 2001-07-17 | Intermedics, Inc. | Method and apparatus for treatment of cardiac electromechanical dissociation |
| WO2001043649A1 (en) | 1999-12-17 | 2001-06-21 | Fogarty Thomas J | Method and device for use in minimally invasive approximation of muscle and other tissue |
| WO2001056166A2 (en) | 2000-01-28 | 2001-08-02 | Infineon Technologies Ag | Method and analog-to-digital converter for converting an analog voltage into an arithmetical value |
| US6988003B2 (en) | 2000-09-18 | 2006-01-17 | Cameron Health, Inc. | Implantable cardioverter-defibrillator having two spaced apart shocking electrodes on housing |
| US6834204B2 (en) | 2001-11-05 | 2004-12-21 | Cameron Health, Inc. | Method and apparatus for inducing defibrillation in a patient using a T-shock waveform |
| US6721597B1 (en)* | 2000-09-18 | 2004-04-13 | Cameron Health, Inc. | Subcutaneous only implantable cardioverter defibrillator and optional pacer |
| US20020035381A1 (en) | 2000-09-18 | 2002-03-21 | Cameron Health, Inc. | Subcutaneous electrode with improved contact shape for transthoracic conduction |
| US7090682B2 (en) | 2000-09-18 | 2006-08-15 | Cameron Health, Inc. | Method and apparatus for extraction of a subcutaneous electrode |
| US7146212B2 (en) | 2000-09-18 | 2006-12-05 | Cameron Health, Inc. | Anti-bradycardia pacing for a subcutaneous implantable cardioverter-defibrillator |
| US6866044B2 (en)* | 2000-09-18 | 2005-03-15 | Cameron Health, Inc. | Method of insertion and implantation of implantable cardioverter-defibrillator canisters |
| US6778860B2 (en)* | 2001-11-05 | 2004-08-17 | Cameron Health, Inc. | Switched capacitor defibrillation circuit |
| US7043299B2 (en) | 2000-09-18 | 2006-05-09 | Cameron Health, Inc. | Subcutaneous implantable cardioverter-defibrillator employing a telescoping lead |
| US6856835B2 (en)* | 2000-09-18 | 2005-02-15 | Cameron Health, Inc. | Biphasic waveform for anti-tachycardia pacing for a subcutaneous implantable cardioverter-defibrillator |
| US7120495B2 (en) | 2000-09-18 | 2006-10-10 | Cameron Health, Inc. | Flexible subcutaneous implantable cardioverter-defibrillator |
| US6865417B2 (en)* | 2001-11-05 | 2005-03-08 | Cameron Health, Inc. | H-bridge with sensing circuit |
| US7149575B2 (en) | 2000-09-18 | 2006-12-12 | Cameron Health, Inc. | Subcutaneous cardiac stimulator device having an anteriorly positioned electrode |
| US7751885B2 (en) | 2000-09-18 | 2010-07-06 | Cameron Health, Inc. | Bradycardia pacing in a subcutaneous device |
| US6927721B2 (en)* | 2001-11-05 | 2005-08-09 | Cameron Health, Inc. | Low power A/D converter |
| US7069080B2 (en) | 2000-09-18 | 2006-06-27 | Cameron Health, Inc. | Active housing and subcutaneous electrode cardioversion/defibrillating system |
| US7194302B2 (en) | 2000-09-18 | 2007-03-20 | Cameron Health, Inc. | Subcutaneous cardiac stimulator with small contact surface electrodes |
| US6950705B2 (en) | 2000-09-18 | 2005-09-27 | Cameron Health, Inc. | Canister designs for implantable cardioverter-defibrillators |
| US20020107544A1 (en) | 2000-09-18 | 2002-08-08 | Cameron Health, Inc. | Current waveform for anti-bradycardia pacing for a subcutaneous implantable cardioverter-defibrillator |
| US7194309B2 (en) | 2000-09-18 | 2007-03-20 | Cameron Health, Inc. | Packaging technology for non-transvenous cardioverter/defibrillator devices |
| US6754528B2 (en)* | 2001-11-21 | 2004-06-22 | Cameraon Health, Inc. | Apparatus and method of arrhythmia detection in a subcutaneous implantable cardioverter/defibrillator |
| US7076296B2 (en)* | 2000-09-18 | 2006-07-11 | Cameron Health, Inc. | Method of supplying energy to subcutaneous cardioverter-defibrillator and pacer |
| US6788974B2 (en)* | 2000-09-18 | 2004-09-07 | Cameron Health, Inc. | Radian curve shaped implantable cardioverter-defibrillator canister |
| US20020095184A1 (en) | 2000-09-18 | 2002-07-18 | Bardy Gust H. | Monophasic waveform for anti-tachycardia pacing for a subcutaneous implantable cardioverter-defibrillator |
| US6804552B2 (en) | 2000-11-03 | 2004-10-12 | Medtronic, Inc. | MEMs switching circuit and method for an implantable medical device |
| CA2428873C (en) | 2000-11-22 | 2013-05-14 | Medtronic, Inc. | Apparatus for detecting and treating ventricular arrhythmia |
| US6721602B2 (en) | 2001-08-21 | 2004-04-13 | Medtronic, Inc. | Implantable medical device assembly and manufacturing method |
| US7062329B2 (en) | 2002-10-04 | 2006-06-13 | Cameron Health, Inc. | Implantable cardiac system with a selectable active housing |
| US7979122B2 (en) | 2003-04-11 | 2011-07-12 | Cardiac Pacemakers, Inc. | Implantable sudden cardiac death prevention device with reduced programmable feature set |
| WO2004112645A2 (en) | 2003-06-17 | 2004-12-29 | Brown Ward M | Subcutaneous lead system |
| US20050038476A1 (en) | 2003-08-14 | 2005-02-17 | Team Brown Enterprises, Llc | Coating/covering materials for the enhancement of defibrillation thresholds of implantable defibrillators/leads |
| US20050107838A1 (en) | 2003-09-18 | 2005-05-19 | Lovett Eric G. | Subcutaneous cardiac rhythm management with disordered breathing detection and treatment |
| US20060015163A1 (en) | 2004-07-19 | 2006-01-19 | Team Brown Enterprises, Llc | Lead extender for implantable device |
| US20060174898A1 (en) | 2005-02-10 | 2006-08-10 | Team Brown Enterprises, Llc | Defibrillator insertion device and method |
| US20070135847A1 (en) | 2005-12-12 | 2007-06-14 | Kenknight Bruce H | Subcutaneous defibrillation system and method using same |
- 2001
- 2001-11-05USUS10/015,202patent/US6952610B2/ennot_activeExpired - Lifetime
- 2002
- 2002-10-28WOPCT/IB2002/004507patent/WO2003039667A1/ennot_activeApplication Discontinuation
- 2005
- 2005-08-17USUS11/205,447patent/US8412320B2/ennot_activeExpired - Fee Related
- 2013
- 2013-03-05USUS13/785,894patent/US8838234B2/ennot_activeExpired - Fee Related
Patent Citations (99)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US3710374A (en)* | 1970-03-16 | 1973-01-09 | Wester Instr Inc | Dual-slope and analog-to-digital converter wherein two analog input signals are selectively integrated with respect to time |
| US3653387A (en)* | 1970-05-08 | 1972-04-04 | Cardiac Electronics Inc | Protector circuit for cardiac apparatus |
| US3707974A (en)* | 1970-12-11 | 1973-01-02 | W Raddi | Body organ stimulator with voltage converter |
| US3911925A (en)* | 1974-05-23 | 1975-10-14 | Jr Joe B Tillery | Ear trimming forceps |
| US4157720A (en)* | 1977-09-16 | 1979-06-12 | Greatbatch W | Cardiac pacemaker |
| US4248237A (en)* | 1978-03-07 | 1981-02-03 | Needle Industries Limited | Cardiac pacemakers |
| US4223678A (en)* | 1978-05-03 | 1980-09-23 | Mieczyslaw Mirowski | Arrhythmia recorder for use with an implantable defibrillator |
| US4191942A (en)* | 1978-06-08 | 1980-03-04 | National Semiconductor Corporation | Single slope A/D converter with sample and hold |
| US4291707A (en)* | 1979-04-30 | 1981-09-29 | Mieczyslaw Mirowski | Implantable cardiac defibrillating electrode |
| US4314095A (en)* | 1979-04-30 | 1982-02-02 | Mieczyslaw Mirowski | Device and method for making electrical contact |
| US4290430A (en)* | 1980-06-05 | 1981-09-22 | Intermedics, Inc. | Pacer analyzer |
| US4407288B1 (en)* | 1981-02-18 | 2000-09-19 | Mieczyslaw Mirowski | Implantable heart stimulator and stimulation method |
| US4407288A (en)* | 1981-02-18 | 1983-10-04 | Mieczyslaw Mirowski | Implantable heart stimulator and stimulation method |
| US4693253A (en)* | 1981-03-23 | 1987-09-15 | Medtronic, Inc. | Automatic implantable defibrillator and pacer |
| US4402322A (en)* | 1981-03-25 | 1983-09-06 | Medtronic, Inc. | Pacer output circuit |
| US4406286A (en)* | 1981-04-09 | 1983-09-27 | Medtronic, Inc. | Fast recharge output circuit |
| US4765341A (en)* | 1981-06-22 | 1988-08-23 | Mieczyslaw Mirowski | Cardiac electrode with attachment fin |
| US4424818A (en)* | 1982-02-18 | 1984-01-10 | Medtronic, Inc. | Electrical lead and insertion tool |
| US4450527A (en)* | 1982-06-29 | 1984-05-22 | Bomed Medical Mfg. Ltd. | Noninvasive continuous cardiac output monitor |
| US4602637A (en)* | 1983-01-11 | 1986-07-29 | Siemens Aktiengesellschaft | Heart pacemaker system |
| US4603705A (en)* | 1984-05-04 | 1986-08-05 | Mieczyslaw Mirowski | Intravascular multiple electrode unitary catheter |
| US4543956A (en)* | 1984-05-24 | 1985-10-01 | Cordis Corporation | Biphasic cardiac pacer |
| US4567900A (en)* | 1984-06-04 | 1986-02-04 | Moore J Paul | Internal deployable defibrillator electrode |
| US4800883A (en)* | 1986-04-02 | 1989-01-31 | Intermedics, Inc. | Apparatus for generating multiphasic defibrillation pulse waveform |
| US4944300A (en)* | 1987-04-28 | 1990-07-31 | Sanjeev Saksena | Method for high energy defibrillation of ventricular fibrillation in humans without a thoracotomy |
| US4830005A (en)* | 1987-07-23 | 1989-05-16 | Siemens-Pacesetter, Inc. | Disposable in-package load test element for pacemakers |
| US4878497A (en)* | 1988-03-25 | 1989-11-07 | Telectronics N.V. | Pacemaker with improved automatic output regulation |
| US5509923A (en)* | 1989-08-16 | 1996-04-23 | Raychem Corporation | Device for dissecting, grasping, or cutting an object |
| US5391191A (en)* | 1990-04-24 | 1995-02-21 | Siemens Aktiengesellschaft | Device for tissue stimulation |
| US5385574A (en)* | 1990-04-25 | 1995-01-31 | Cardiac Pacemakers, Inc. | Implantable intravenous cardiac stimulation system with pulse generator housing serving as optional additional electrode |
| US5133353A (en)* | 1990-04-25 | 1992-07-28 | Cardiac Pacemakers, Inc. | Implantable intravenous cardiac stimulation system with pulse generator housing serving as optional additional electrode |
| US5713926A (en)* | 1990-04-25 | 1998-02-03 | Cardiac Pacemakers, Inc. | Implantable intravenous cardiac stimulation system with pulse generator housing serving as optional additional electrode |
| US5203348A (en)* | 1990-06-06 | 1993-04-20 | Cardiac Pacemakers, Inc. | Subcutaneous defibrillation electrodes |
| US5230337A (en)* | 1990-06-06 | 1993-07-27 | Cardiac Pacemakers, Inc. | Process for implanting subcutaneous defibrillation electrodes |
| US5342407A (en)* | 1990-06-06 | 1994-08-30 | Cardiac Pacemakers, Inc. | Body implantable defibrillation system |
| US5603732A (en)* | 1990-06-06 | 1997-02-18 | Cardiac Pacemakers, Inc. | Subcutaneous defibrillation electrodes |
| US5105810A (en)* | 1990-07-24 | 1992-04-21 | Telectronics Pacing Systems, Inc. | Implantable automatic and haemodynamically responsive cardioverting/defibrillating pacemaker with means for minimizing bradycardia support pacing voltages |
| US5109842A (en)* | 1990-09-24 | 1992-05-05 | Siemens Pacesetter, Inc. | Implantable tachyarrhythmia control system having a patch electrode with an integrated cardiac activity system |
| US5531765A (en)* | 1990-12-18 | 1996-07-02 | Ventritex, Inc. | Method and apparatus for producing configurable biphasic defibrillation waveforms |
| US5192392A (en)* | 1991-02-28 | 1993-03-09 | The Bottling Room, Inc. | Container labeler |
| US5405363A (en)* | 1991-03-15 | 1995-04-11 | Angelon Corporation | Implantable cardioverter defibrillator having a smaller displacement volume |
| US5331966A (en)* | 1991-04-05 | 1994-07-26 | Medtronic, Inc. | Subcutaneous multi-electrode sensing system, method and pacer |
| US5300106A (en)* | 1991-06-07 | 1994-04-05 | Cardiac Pacemakers, Inc. | Insertion and tunneling tool for a subcutaneous wire patch electrode |
| US5144946A (en)* | 1991-08-05 | 1992-09-08 | Siemens Pacesetter, Inc. | Combined pacemaker substrate and electrical interconnect and method of assembly |
| US5191901A (en)* | 1991-08-29 | 1993-03-09 | Mieczyslaw Mirowski | Controlled discharge defibrillation electrode |
| US5423326A (en)* | 1991-09-12 | 1995-06-13 | Drexel University | Apparatus and method for measuring cardiac output |
| US5184616A (en)* | 1991-10-21 | 1993-02-09 | Telectronics Pacing Systems, Inc. | Apparatus and method for generation of varying waveforms in arrhythmia control system |
| US5376104A (en)* | 1992-02-07 | 1994-12-27 | Nihon Kohden Corporation | Defibrillator with electrocardiogram monitor |
| US5261400A (en)* | 1992-02-12 | 1993-11-16 | Medtronic, Inc. | Defibrillator employing transvenous and subcutaneous electrodes and method of use |
| US5413591A (en)* | 1992-02-26 | 1995-05-09 | Angeion Corporation | Current truncated waveform defibrillator |
| US5514160A (en)* | 1992-02-26 | 1996-05-07 | Angeion Corporation | Implantable defibrillator for producing a rectangular-shaped defibrillation waveform |
| US5601607A (en)* | 1992-03-19 | 1997-02-11 | Angeion Corporation | Implantable cardioverter defibrillator housing plated electrode |
| US5376103A (en)* | 1992-03-19 | 1994-12-27 | Angeion Corporation | Electrode system for implantable defibrillator |
| US5255692A (en)* | 1992-09-04 | 1993-10-26 | Siemens Aktiengesellschaft | Subcostal patch electrode |
| US5391200A (en)* | 1992-09-30 | 1995-02-21 | Cardiac Pacemakers, Inc. | Defibrillation patch electrode having conductor-free resilient zone for minimally invasive deployment |
| US5318591A (en)* | 1992-11-23 | 1994-06-07 | Siemens Pacesetter, Inc. | Implantable cardioverter-defibrillator having early charging capability |
| US5697953A (en)* | 1993-03-13 | 1997-12-16 | Angeion Corporation | Implantable cardioverter defibrillator having a smaller displacement volume |
| US5366496A (en)* | 1993-04-01 | 1994-11-22 | Cardiac Pacemakers, Inc. | Subcutaneous shunted coil electrode |
| US5411547A (en)* | 1993-08-09 | 1995-05-02 | Pacesetter, Inc. | Implantable cardioversion-defibrillation patch electrodes having means for passive multiplexing of discharge pulses |
| US5618287A (en)* | 1994-01-28 | 1997-04-08 | Thomas J. Fogarty | Methods of surgically implanting a defibrillator electrode within a patient |
| US5690648A (en)* | 1994-01-28 | 1997-11-25 | Thomas J. Fogarty | Methods and apparatus for rolling a defibrillator electrode |
| US5476503A (en)* | 1994-03-28 | 1995-12-19 | Pacesetter, Inc. | Sense array intelligent patch lead for an implantable defibrillator and method |
| US5620477A (en)* | 1994-03-31 | 1997-04-15 | Ventritex, Inc. | Pulse generator with case that can be active or inactive |
| US5645586A (en)* | 1994-07-08 | 1997-07-08 | Ventritex, Inc. | Conforming implantable defibrillator |
| US5597956A (en)* | 1994-08-24 | 1997-01-28 | Murata Manufacturing Co., Ltd. | Capacitor type acceleration sensor |
| US5534022A (en)* | 1994-11-22 | 1996-07-09 | Ventritex, Inc. | Lead having an integrated defibrillation/sensing electrode |
| US5534019A (en)* | 1994-12-09 | 1996-07-09 | Ventritex, Inc. | Cardiac defibrillator with case that can be electrically active or inactive |
| US5531766A (en)* | 1995-01-23 | 1996-07-02 | Angeion Corporation | Implantable cardioverter defibrillator pulse generator kite-tail electrode system |
| US5509928A (en)* | 1995-03-02 | 1996-04-23 | Pacesetter, Inc. | Internally supported self-sealing septum |
| US5814090A (en)* | 1995-06-07 | 1998-09-29 | Angeion Corporation | Implantable medical device having heat-shrink conforming shield |
| US5658321A (en)* | 1995-06-09 | 1997-08-19 | Ventritex, Inc. | Conductive housing for implantable cardiac device |
| US5690683A (en)* | 1995-06-19 | 1997-11-25 | Cardiac Pacemakers, Inc. | After potential removal in cardiac rhythm management device |
| US5658317A (en)* | 1995-08-14 | 1997-08-19 | Cardiac Pacemakers, Inc. | Threshold templating for digital AGC |
| US6014586A (en)* | 1995-11-20 | 2000-01-11 | Pacesetter, Inc. | Vertically integrated semiconductor package for an implantable medical device |
| US5674260A (en)* | 1996-02-23 | 1997-10-07 | Pacesetter, Inc. | Apparatus and method for mounting an activity sensor or other component within a pacemaker using a contoured hybrid lid |
| US6095987A (en)* | 1996-04-17 | 2000-08-01 | Imagyn Medical Techonologies California, Inc. | Apparatus and methods of bioelectrical impedance analysis of blood flow |
| US5895414A (en)* | 1996-04-19 | 1999-04-20 | Sanchez-Zambrano; Sergio | Pacemaker housing |
| US5919211A (en)* | 1996-06-27 | 1999-07-06 | Adams; Theodore P. | ICD power source using multiple single use batteries |
| US5643328A (en)* | 1996-07-19 | 1997-07-01 | Sulzer Intermedics Inc. | Implantable cardiac stimulation device with warning system having elongated stimulation electrode |
| US6058328A (en)* | 1996-08-06 | 2000-05-02 | Pacesetter, Inc. | Implantable stimulation device having means for operating in a preemptive pacing mode to prevent tachyarrhythmias and method thereof |
| US5800464A (en)* | 1996-10-03 | 1998-09-01 | Medtronic, Inc. | System for providing hyperpolarization of cardiac to enhance cardiac function |
| US5766226A (en)* | 1996-12-09 | 1998-06-16 | Angeion Corporation | Switched discharge pathways for ICD having multiple output capacitors |
| US5935154A (en)* | 1997-01-24 | 1999-08-10 | Cardiac Pacemakers, Inc. | Implantable tissue stimulator incorporating deposited multilayer capacitor |
| USH1905H (en)* | 1997-03-21 | 2000-10-03 | Medtronic, Inc. | Mechanism for adjusting the exposed surface area and position of an electrode along a lead body |
| US5776169A (en)* | 1997-04-28 | 1998-07-07 | Sulzer Intermedics Inc. | Implantable cardiac stimulator for minimally invasive implantation |
| US5836976A (en)* | 1997-04-30 | 1998-11-17 | Medtronic, Inc. | Cardioversion energy reduction system |
| US5941904A (en)* | 1997-09-12 | 1999-08-24 | Sulzer Intermedics Inc. | Electromagnetic acceleration transducer for implantable medical device |
| US5925069A (en)* | 1997-11-07 | 1999-07-20 | Sulzer Intermedics Inc. | Method for preparing a high definition window in a conformally coated medical device |
| US5919222A (en)* | 1998-01-06 | 1999-07-06 | Medtronic Inc. | Adjustable medical electrode lead |
| US6185450B1 (en)* | 1998-01-26 | 2001-02-06 | Physio-Control Manufacturing Corporation | Digital sliding pole fast-restore for an electrocardiograph display |
| US6148230A (en)* | 1998-01-30 | 2000-11-14 | Uab Research Foundation | Method for the monitoring and treatment of spontaneous cardiac arrhythmias |
| US6128531A (en)* | 1998-04-01 | 2000-10-03 | Pacesetter, Inc. | Delivery of ICD shock capacitor energy via a controlled current source |
| US5964787A (en)* | 1998-04-17 | 1999-10-12 | Vitatron Medical B.V. | Stimulus system with controllable switched capacitor output stage |
| US6026325A (en)* | 1998-06-18 | 2000-02-15 | Pacesetter, Inc. | Implantable medical device having an improved packaging system and method for making electrical connections |
| US6093173A (en)* | 1998-09-09 | 2000-07-25 | Embol-X, Inc. | Introducer/dilator with balloon protection and methods of use |
| US6144866A (en)* | 1998-10-30 | 2000-11-07 | Medtronic, Inc. | Multiple sensor assembly for medical electric lead |
| US6411844B1 (en)* | 1999-10-19 | 2002-06-25 | Pacesetter, Inc. | Fast recovery sensor amplifier circuit for implantable medical device |
| US6519493B1 (en)* | 1999-12-23 | 2003-02-11 | Pacesetter, Inc. | Methods and apparatus for overdrive pacing heart tissue using an implantable cardiac stimulation device |
| US6647292B1 (en)* | 2000-09-18 | 2003-11-11 | Cameron Health | Unitary subcutaneous only implantable cardioverter-defibrillator and optional pacer |
Cited By (190)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US7502645B2 (en) | 2000-09-18 | 2009-03-10 | Cameron Health, Inc. | Current waveforms for anti-bradycardia pacing for a subcutaneous implantable cardioverter-defibrillator |
| US9144683B2 (en) | 2000-09-18 | 2015-09-29 | Cameron Health, Inc. | Post-shock treatment in a subcutaneous device |
| US20050143778A1 (en)* | 2000-09-18 | 2005-06-30 | Cameron Health, Inc. | Current waveforms for anti-bradycardia pacing for a subcutaneous implantable cardioverter-defibrillator |
| US9022962B2 (en) | 2000-11-22 | 2015-05-05 | Boston Scientific Scimed, Inc. | Apparatus for detecting and treating ventricular arrhythmia |
| US20030212436A1 (en)* | 2000-11-22 | 2003-11-13 | Brown Ward M. | Apparatus for detecting and treating ventricular arrhythmia |
| US20080140139A1 (en)* | 2000-11-22 | 2008-06-12 | Heinrich Stephen D | Apparatus for detecting and treating ventricular arrhythmia |
| US9522283B2 (en)* | 2001-11-21 | 2016-12-20 | Cameron Health Inc. | Apparatus and method for identifying atrial arrhythmia by far-field sensing |
| US20170056681A1 (en)* | 2001-11-21 | 2017-03-02 | Cameron Health, Inc. | Apparatus and method for identifying atrial arrhythmia by far-field sensing |
| US9993653B2 (en)* | 2001-11-21 | 2018-06-12 | Cameron Health, Inc. | Apparatus and method for identifying atrial arrhythmia by far-field sensing |
| US20150360040A1 (en)* | 2001-11-21 | 2015-12-17 | Cameron Health, Inc. | Apparatus and method for identifying atrial arrhythmia by far-field sensing |
| US8535222B2 (en) | 2002-12-04 | 2013-09-17 | Cardiac Pacemakers, Inc. | Sleep detection using an adjustable threshold |
| US8956295B2 (en) | 2002-12-04 | 2015-02-17 | Cardiac Pacemakers, Inc. | Sleep detection using an adjustable threshold |
| US7392081B2 (en) | 2003-02-28 | 2008-06-24 | Cardiac Pacemakers, Inc. | Subcutaneous cardiac stimulator employing post-shock transthoracic asystole prevention pacing |
| US7996071B2 (en) | 2003-04-11 | 2011-08-09 | Cardiac Pacemakers, Inc. | Biopotential signal source separation using source impedances |
| US7117035B2 (en) | 2003-04-11 | 2006-10-03 | Cardiac Pacemakers, Inc. | Subcutaneous cardiac stimulation system with patient activity sensing |
| US20040204734A1 (en)* | 2003-04-11 | 2004-10-14 | Wagner Darrell Orvin | Tunneling tool with subcutaneous transdermal illumination |
| US7218966B2 (en) | 2003-04-11 | 2007-05-15 | Cardiac Pacemakers, Inc. | Multi-parameter arrhythmia discrimination |
| US9655540B2 (en) | 2003-04-11 | 2017-05-23 | Cardiac Pacemakers, Inc. | Subcutaneous cardiac signal discrimination employing non-electrophysiologic signal |
| US7236819B2 (en) | 2003-04-11 | 2007-06-26 | Cardiac Pacemakers, Inc. | Separation of a subcutaneous cardiac signal from a plurality of composite signals |
| US20040220626A1 (en)* | 2003-04-11 | 2004-11-04 | Wagner Darrell Orvin | Distributed subcutaneous defibrillation system |
| US20040225329A1 (en)* | 2003-04-11 | 2004-11-11 | Wagner Darrell Orvin | Electrode placement determination for subcutaneous cardiac monitoring and therapy |
| US7302294B2 (en) | 2003-04-11 | 2007-11-27 | Cardiac Pacemakers, Inc. | Subcutaneous cardiac sensing and stimulation system employing blood sensor |
| US7349742B2 (en) | 2003-04-11 | 2008-03-25 | Cardiac Pacemakers, Inc. | Expandable fixation elements for subcutaneous electrodes |
| US8843196B2 (en) | 2003-04-11 | 2014-09-23 | Cardiac Pacemakers, Inc. | Subcutaneous cardiac sensing and stimulation system |
| US7047071B2 (en) | 2003-04-11 | 2006-05-16 | Cardiac Pacemakers, Inc. | Patient stratification for implantable subcutaneous cardiac monitoring and therapy |
| US7389138B2 (en) | 2003-04-11 | 2008-06-17 | Cardiac Pacemakers, Inc. | Electrode placement determination for subcutaneous cardiac monitoring and therapy |
| US20040230281A1 (en)* | 2003-04-11 | 2004-11-18 | Ron Heil | Expandable fixation elements for subcutaneous electrodes |
| US20040230280A1 (en)* | 2003-04-11 | 2004-11-18 | Cates Adam W. | Helical fixation elements for subcutaneous electrodes |
| US20040230273A1 (en)* | 2003-04-11 | 2004-11-18 | Cates Adam W. | Subcutaneous electrode and lead with temporary pharmacological agents |
| US8116868B2 (en) | 2003-04-11 | 2012-02-14 | Cardiac Pacemakers, Inc. | Implantable device with cardiac event audio playback |
| US7493175B2 (en) | 2003-04-11 | 2009-02-17 | Cardiac Pacemakers, Inc. | Subcutaneous lead with tined fixation |
| US7499750B2 (en) | 2003-04-11 | 2009-03-03 | Cardiac Pacemakers, Inc. | Noise canceling cardiac electrodes |
| US7499758B2 (en) | 2003-04-11 | 2009-03-03 | Cardiac Pacemakers, Inc. | Helical fixation elements for subcutaneous electrodes |
| US20040230129A1 (en)* | 2003-04-11 | 2004-11-18 | Paul Haefner | Multi-parameter arrhythmia discrimination |
| US8024039B2 (en) | 2003-04-11 | 2011-09-20 | Cardiac Pacemakers, Inc. | Subcutaneous cardiac sensing and stimulation system employing blood sensor |
| US7979122B2 (en) | 2003-04-11 | 2011-07-12 | Cardiac Pacemakers, Inc. | Implantable sudden cardiac death prevention device with reduced programmable feature set |
| US7529592B2 (en) | 2003-04-11 | 2009-05-05 | Cardiac Pacemakers, Inc. | Subcutaneous electrode and lead with temporary pharmacological agents |
| US7555335B2 (en) | 2003-04-11 | 2009-06-30 | Cardiac Pacemakers, Inc. | Biopotential signal source separation using source impedances |
| US7566318B2 (en) | 2003-04-11 | 2009-07-28 | Cardiac Pacemakers, Inc. | Ultrasonic subcutaneous dissection tool incorporating fluid delivery |
| US7570997B2 (en) | 2003-04-11 | 2009-08-04 | Cardiac Pacemakers, Inc. | Subcutaneous cardiac rhythm management with asystole prevention therapy |
| US7865233B2 (en) | 2003-04-11 | 2011-01-04 | Cardiac Pacemakers, Inc. | Subcutaneous cardiac signal discrimination employing non-electrophysiologic signal |
| US7702399B2 (en) | 2003-04-11 | 2010-04-20 | Cardiac Pacemakers, Inc. | Subcutaneous electrode and lead with phoresis based pharmacological agent delivery |
| US20040230274A1 (en)* | 2003-04-11 | 2004-11-18 | Ron Heil | Subcutaneous electrode and lead with phoresis based pharmacological agent delivery |
| US7715916B2 (en) | 2003-04-11 | 2010-05-11 | Cardiac Pacemakers, Inc. | Multi-parameter arrhythmia discrimination |
| US7734343B2 (en) | 2003-06-04 | 2010-06-08 | Synecor, Llc | Implantable intravascular device for defibrillation and/or pacing |
| US7899554B2 (en) | 2003-06-04 | 2011-03-01 | Synecor Llc | Intravascular System and Method |
| US8239045B2 (en) | 2003-06-04 | 2012-08-07 | Synecor Llc | Device and method for retaining a medical device within a vessel |
| US7082336B2 (en) | 2003-06-04 | 2006-07-25 | Synecor, Llc | Implantable intravascular device for defibrillation and/or pacing |
| US7840282B2 (en) | 2003-06-04 | 2010-11-23 | Synecor Llc | Method and apparatus for retaining medical implants within body vessels |
| US7617007B2 (en) | 2003-06-04 | 2009-11-10 | Synecor Llc | Method and apparatus for retaining medical implants within body vessels |
| US7529589B2 (en) | 2003-06-04 | 2009-05-05 | Synecor Llc | Intravascular electrophysiological system and methods |
| US7787946B2 (en) | 2003-08-18 | 2010-08-31 | Cardiac Pacemakers, Inc. | Patient monitoring, diagnosis, and/or therapy systems and methods |
| US7396333B2 (en) | 2003-08-18 | 2008-07-08 | Cardiac Pacemakers, Inc. | Prediction of disordered breathing |
| US8002553B2 (en) | 2003-08-18 | 2011-08-23 | Cardiac Pacemakers, Inc. | Sleep quality data collection and evaluation |
| US20050107838A1 (en)* | 2003-09-18 | 2005-05-19 | Lovett Eric G. | Subcutaneous cardiac rhythm management with disordered breathing detection and treatment |
| US8606356B2 (en) | 2003-09-18 | 2013-12-10 | Cardiac Pacemakers, Inc. | Autonomic arousal detection system and method |
| US9014819B2 (en) | 2003-09-18 | 2015-04-21 | Cardiac Pacemakers, Inc. | Autonomic arousal detection system and method |
| US7887493B2 (en) | 2003-09-18 | 2011-02-15 | Cardiac Pacemakers, Inc. | Implantable device employing movement sensing for detecting sleep-related disorders |
| US8657756B2 (en) | 2003-09-18 | 2014-02-25 | Cardiac Pacemakers, Inc. | Implantable device employing movement sensing for detecting sleep-related disorders |
| US7747335B2 (en) | 2003-12-12 | 2010-06-29 | Synecor Llc | Implantable medical device having pre-implant exoskeleton |
| US7706866B2 (en) | 2004-06-24 | 2010-04-27 | Cardiac Pacemakers, Inc. | Automatic orientation determination for ECG measurements using multiple electrodes |
| US8121680B2 (en) | 2004-09-08 | 2012-02-21 | Pacesetter, Inc. | Subcutaneous cardiac stimulation device providing anti-tachycardia pacing therapy and method |
| US20080234769A1 (en)* | 2004-09-08 | 2008-09-25 | Pacesetter, Inc. | Subcutaneous cardiac stimulation device providing anti-tachycardia pacing therapy and method |
| US7260433B1 (en) | 2004-09-08 | 2007-08-21 | Pacesetter, Inc. | Subcutaneous cardiac stimulation device providing anti-tachycardia pacing therapy and method |
| US7277755B1 (en) | 2004-09-08 | 2007-10-02 | Pacesetter, Inc. | Subcutaneous cardiac stimulation device providing anti-tachycardia pacing therapy and method |
| US7386342B1 (en) | 2004-09-08 | 2008-06-10 | Pacesetter, Inc. | Subcutaneous cardiac stimulation device providing anti-tachycardia pacing therapy and method |
| US7890159B2 (en) | 2004-09-30 | 2011-02-15 | Cardiac Pacemakers, Inc. | Cardiac activation sequence monitoring and tracking |
| US7797036B2 (en) | 2004-11-30 | 2010-09-14 | Cardiac Pacemakers, Inc. | Cardiac activation sequence monitoring for ischemia detection |
| US8626276B2 (en) | 2004-11-30 | 2014-01-07 | Cardiac Pacemakers, Inc. | Cardiac activation sequence monitoring for ischemia detection |
| US9277885B2 (en) | 2005-02-28 | 2016-03-08 | Cardiac Pacemakers, Inc. | Implantable cardiac device with dyspnea measurement |
| US8750992B2 (en) | 2005-02-28 | 2014-06-10 | Cardiac Pacemakers, Inc. | Implantable cardiac device with dyspnea measurement |
| US8954146B2 (en) | 2005-02-28 | 2015-02-10 | Cardiac Pacemakers, Inc. | Implantable cardiac device with dyspnea measurement |
| US8515535B2 (en) | 2005-02-28 | 2013-08-20 | Cardiac Pacemakers, Inc. | Implantable cardiac device with dyspnea measurement |
| US7805185B2 (en) | 2005-05-09 | 2010-09-28 | Cardiac Pacemakers, In. | Posture monitoring using cardiac activation sequences |
| US7917196B2 (en) | 2005-05-09 | 2011-03-29 | Cardiac Pacemakers, Inc. | Arrhythmia discrimination using electrocardiograms sensed from multiple implanted electrodes |
| US7509170B2 (en) | 2005-05-09 | 2009-03-24 | Cardiac Pacemakers, Inc. | Automatic capture verification using electrocardiograms sensed from multiple implanted electrodes |
| US7457664B2 (en) | 2005-05-09 | 2008-11-25 | Cardiac Pacemakers, Inc. | Closed loop cardiac resynchronization therapy using cardiac activation sequence information |
| US9993654B2 (en) | 2005-05-18 | 2018-06-12 | Cardiac Pacemakers, Inc. | Modular antitachyarrhythmia therapy system |
| US8391990B2 (en) | 2005-05-18 | 2013-03-05 | Cardiac Pacemakers, Inc. | Modular antitachyarrhythmia therapy system |
| US8903500B2 (en) | 2005-05-18 | 2014-12-02 | Cardiac Pacemakers, Inc. | Modular antitachyarrhythmia therapy system |
| US8649859B2 (en) | 2005-05-18 | 2014-02-11 | Cardiac Pacemakers, Inc. | Modular antitachyarrhythmia therapy system |
| US9242113B2 (en) | 2005-05-18 | 2016-01-26 | Cardiac Pacemarkers, Inc. | Modular antitachyarrhythmia therapy system |
| US9002467B2 (en) | 2005-05-18 | 2015-04-07 | Cardiac Pacemakers, Inc. | Modular antitachyarrhythmia therapy system |
| US9352164B2 (en) | 2005-05-18 | 2016-05-31 | Cardiac Pacemakers, Inc. | Modular antitachyarrhythmia therapy system |
| US11083898B2 (en) | 2005-05-18 | 2021-08-10 | Cardiac Pacemakers, Inc. | Modular antitachyarrhythmia therapy system |
| US10363428B2 (en) | 2005-05-18 | 2019-07-30 | Cardiac Pacemakers, Inc. | Modular antitachyarrhythmia therapy system |
| US20060265018A1 (en)* | 2005-05-18 | 2006-11-23 | Cardiac Pacemakers, Inc. | Modular antitachyarrhythmia therapy system |
| US20070049975A1 (en)* | 2005-09-01 | 2007-03-01 | Cates Adam W | Active can with dedicated defibrillation and sensing electrodes |
| US9931074B2 (en) | 2005-11-18 | 2018-04-03 | Cardiac Pacemakers, Inc. | Cardiac resynchronization therapy for improved hemodynamics based on disordered breathing detection |
| US7761158B2 (en) | 2005-12-20 | 2010-07-20 | Cardiac Pacemakers, Inc. | Detection of heart failure decompensation based on cumulative changes in sensor signals |
| US20070142732A1 (en)* | 2005-12-20 | 2007-06-21 | Marina Brockway | Detection of heart failure decompensation based on cumulative changes in sensor signals |
| US8527048B2 (en) | 2006-06-29 | 2013-09-03 | Cardiac Pacemakers, Inc. | Local and non-local sensing for cardiac pacing |
| US10722720B2 (en) | 2014-01-10 | 2020-07-28 | Cardiac Pacemakers, Inc. | Methods and systems for improved communication between medical devices |
| US9592391B2 (en) | 2014-01-10 | 2017-03-14 | Cardiac Pacemakers, Inc. | Systems and methods for detecting cardiac arrhythmias |
| US9526909B2 (en) | 2014-08-28 | 2016-12-27 | Cardiac Pacemakers, Inc. | Medical device with triggered blanking period |
| US10238882B2 (en) | 2015-02-06 | 2019-03-26 | Cardiac Pacemakers | Systems and methods for treating cardiac arrhythmias |
| US10220213B2 (en) | 2015-02-06 | 2019-03-05 | Cardiac Pacemakers, Inc. | Systems and methods for safe delivery of electrical stimulation therapy |
| US11020595B2 (en) | 2015-02-06 | 2021-06-01 | Cardiac Pacemakers, Inc. | Systems and methods for treating cardiac arrhythmias |
| US9669230B2 (en) | 2015-02-06 | 2017-06-06 | Cardiac Pacemakers, Inc. | Systems and methods for treating cardiac arrhythmias |
| US11224751B2 (en) | 2015-02-06 | 2022-01-18 | Cardiac Pacemakers, Inc. | Systems and methods for safe delivery of electrical stimulation therapy |
| US10046167B2 (en) | 2015-02-09 | 2018-08-14 | Cardiac Pacemakers, Inc. | Implantable medical device with radiopaque ID tag |
| US11020600B2 (en) | 2015-02-09 | 2021-06-01 | Cardiac Pacemakers, Inc. | Implantable medical device with radiopaque ID tag |
| US11285326B2 (en) | 2015-03-04 | 2022-03-29 | Cardiac Pacemakers, Inc. | Systems and methods for treating cardiac arrhythmias |
| US10050700B2 (en) | 2015-03-18 | 2018-08-14 | Cardiac Pacemakers, Inc. | Communications in a medical device system with temporal optimization |
| US10213610B2 (en) | 2015-03-18 | 2019-02-26 | Cardiac Pacemakers, Inc. | Communications in a medical device system with link quality assessment |
| US10946202B2 (en) | 2015-03-18 | 2021-03-16 | Cardiac Pacemakers, Inc. | Communications in a medical device system with link quality assessment |
| US11476927B2 (en) | 2015-03-18 | 2022-10-18 | Cardiac Pacemakers, Inc. | Communications in a medical device system with temporal optimization |
| US9853743B2 (en) | 2015-08-20 | 2017-12-26 | Cardiac Pacemakers, Inc. | Systems and methods for communication between medical devices |
| US10357159B2 (en) | 2015-08-20 | 2019-07-23 | Cardiac Pacemakers, Inc | Systems and methods for communication between medical devices |
| US9956414B2 (en) | 2015-08-27 | 2018-05-01 | Cardiac Pacemakers, Inc. | Temporal configuration of a motion sensor in an implantable medical device |
| US10709892B2 (en) | 2015-08-27 | 2020-07-14 | Cardiac Pacemakers, Inc. | Temporal configuration of a motion sensor in an implantable medical device |
| US9968787B2 (en) | 2015-08-27 | 2018-05-15 | Cardiac Pacemakers, Inc. | Spatial configuration of a motion sensor in an implantable medical device |
| US10159842B2 (en) | 2015-08-28 | 2018-12-25 | Cardiac Pacemakers, Inc. | System and method for detecting tamponade |
| US10589101B2 (en) | 2015-08-28 | 2020-03-17 | Cardiac Pacemakers, Inc. | System and method for detecting tamponade |
| US10226631B2 (en) | 2015-08-28 | 2019-03-12 | Cardiac Pacemakers, Inc. | Systems and methods for infarct detection |
| US10137305B2 (en) | 2015-08-28 | 2018-11-27 | Cardiac Pacemakers, Inc. | Systems and methods for behaviorally responsive signal detection and therapy delivery |
| US10092760B2 (en) | 2015-09-11 | 2018-10-09 | Cardiac Pacemakers, Inc. | Arrhythmia detection and confirmation |
| US10065041B2 (en) | 2015-10-08 | 2018-09-04 | Cardiac Pacemakers, Inc. | Devices and methods for adjusting pacing rates in an implantable medical device |
| US10183170B2 (en) | 2015-12-17 | 2019-01-22 | Cardiac Pacemakers, Inc. | Conducted communication in a medical device system |
| US10933245B2 (en) | 2015-12-17 | 2021-03-02 | Cardiac Pacemakers, Inc. | Conducted communication in a medical device system |
| US10905886B2 (en) | 2015-12-28 | 2021-02-02 | Cardiac Pacemakers, Inc. | Implantable medical device for deployment across the atrioventricular septum |
| US10583303B2 (en) | 2016-01-19 | 2020-03-10 | Cardiac Pacemakers, Inc. | Devices and methods for wirelessly recharging a rechargeable battery of an implantable medical device |
| US10350423B2 (en) | 2016-02-04 | 2019-07-16 | Cardiac Pacemakers, Inc. | Delivery system with force sensor for leadless cardiac device |
| US11116988B2 (en) | 2016-03-31 | 2021-09-14 | Cardiac Pacemakers, Inc. | Implantable medical device with rechargeable battery |
| US10328272B2 (en) | 2016-05-10 | 2019-06-25 | Cardiac Pacemakers, Inc. | Retrievability for implantable medical devices |
| US10668294B2 (en) | 2016-05-10 | 2020-06-02 | Cardiac Pacemakers, Inc. | Leadless cardiac pacemaker configured for over the wire delivery |
| US10512784B2 (en) | 2016-06-27 | 2019-12-24 | Cardiac Pacemakers, Inc. | Cardiac therapy system using subcutaneously sensed P-waves for resynchronization pacing management |
| US11497921B2 (en) | 2016-06-27 | 2022-11-15 | Cardiac Pacemakers, Inc. | Cardiac therapy system using subcutaneously sensed p-waves for resynchronization pacing management |
| US11207527B2 (en) | 2016-07-06 | 2021-12-28 | Cardiac Pacemakers, Inc. | Method and system for determining an atrial contraction timing fiducial in a leadless cardiac pacemaker system |
| US10426962B2 (en) | 2016-07-07 | 2019-10-01 | Cardiac Pacemakers, Inc. | Leadless pacemaker using pressure measurements for pacing capture verification |
| US10688304B2 (en) | 2016-07-20 | 2020-06-23 | Cardiac Pacemakers, Inc. | Method and system for utilizing an atrial contraction timing fiducial in a leadless cardiac pacemaker system |
| US10391319B2 (en) | 2016-08-19 | 2019-08-27 | Cardiac Pacemakers, Inc. | Trans septal implantable medical device |
| US10780278B2 (en) | 2016-08-24 | 2020-09-22 | Cardiac Pacemakers, Inc. | Integrated multi-device cardiac resynchronization therapy using P-wave to pace timing |
| US11464982B2 (en) | 2016-08-24 | 2022-10-11 | Cardiac Pacemakers, Inc. | Integrated multi-device cardiac resynchronization therapy using p-wave to pace timing |
| US10870008B2 (en) | 2016-08-24 | 2020-12-22 | Cardiac Pacemakers, Inc. | Cardiac resynchronization using fusion promotion for timing management |
| US10994145B2 (en) | 2016-09-21 | 2021-05-04 | Cardiac Pacemakers, Inc. | Implantable cardiac monitor |
| US10758737B2 (en) | 2016-09-21 | 2020-09-01 | Cardiac Pacemakers, Inc. | Using sensor data from an intracardially implanted medical device to influence operation of an extracardially implantable cardioverter |
| US10905889B2 (en) | 2016-09-21 | 2021-02-02 | Cardiac Pacemakers, Inc. | Leadless stimulation device with a housing that houses internal components of the leadless stimulation device and functions as the battery case and a terminal of an internal battery |
| US10765871B2 (en) | 2016-10-27 | 2020-09-08 | Cardiac Pacemakers, Inc. | Implantable medical device with pressure sensor |
| US10758724B2 (en) | 2016-10-27 | 2020-09-01 | Cardiac Pacemakers, Inc. | Implantable medical device delivery system with integrated sensor |
| US10463305B2 (en) | 2016-10-27 | 2019-11-05 | Cardiac Pacemakers, Inc. | Multi-device cardiac resynchronization therapy with timing enhancements |
| US10561330B2 (en) | 2016-10-27 | 2020-02-18 | Cardiac Pacemakers, Inc. | Implantable medical device having a sense channel with performance adjustment |
| US11305125B2 (en) | 2016-10-27 | 2022-04-19 | Cardiac Pacemakers, Inc. | Implantable medical device with gyroscope |
| US10434314B2 (en) | 2016-10-27 | 2019-10-08 | Cardiac Pacemakers, Inc. | Use of a separate device in managing the pace pulse energy of a cardiac pacemaker |
| US10413733B2 (en) | 2016-10-27 | 2019-09-17 | Cardiac Pacemakers, Inc. | Implantable medical device with gyroscope |
| US10434317B2 (en) | 2016-10-31 | 2019-10-08 | Cardiac Pacemakers, Inc. | Systems and methods for activity level pacing |
| US10617874B2 (en) | 2016-10-31 | 2020-04-14 | Cardiac Pacemakers, Inc. | Systems and methods for activity level pacing |
| US10583301B2 (en) | 2016-11-08 | 2020-03-10 | Cardiac Pacemakers, Inc. | Implantable medical device for atrial deployment |
| US10632313B2 (en) | 2016-11-09 | 2020-04-28 | Cardiac Pacemakers, Inc. | Systems, devices, and methods for setting cardiac pacing pulse parameters for a cardiac pacing device |
| US10894163B2 (en) | 2016-11-21 | 2021-01-19 | Cardiac Pacemakers, Inc. | LCP based predictive timing for cardiac resynchronization |
| US10639486B2 (en) | 2016-11-21 | 2020-05-05 | Cardiac Pacemakers, Inc. | Implantable medical device with recharge coil |
| US10881863B2 (en) | 2016-11-21 | 2021-01-05 | Cardiac Pacemakers, Inc. | Leadless cardiac pacemaker with multimode communication |
| US10881869B2 (en) | 2016-11-21 | 2021-01-05 | Cardiac Pacemakers, Inc. | Wireless re-charge of an implantable medical device |
| US11147979B2 (en) | 2016-11-21 | 2021-10-19 | Cardiac Pacemakers, Inc. | Implantable medical device with a magnetically permeable housing and an inductive coil disposed about the housing |
| US11207532B2 (en) | 2017-01-04 | 2021-12-28 | Cardiac Pacemakers, Inc. | Dynamic sensing updates using postural input in a multiple device cardiac rhythm management system |
| US10835753B2 (en) | 2017-01-26 | 2020-11-17 | Cardiac Pacemakers, Inc. | Intra-body device communication with redundant message transmission |
| US11590353B2 (en) | 2017-01-26 | 2023-02-28 | Cardiac Pacemakers, Inc. | Intra-body device communication with redundant message transmission |
| US10029107B1 (en) | 2017-01-26 | 2018-07-24 | Cardiac Pacemakers, Inc. | Leadless device with overmolded components |
| US10737102B2 (en) | 2017-01-26 | 2020-08-11 | Cardiac Pacemakers, Inc. | Leadless implantable device with detachable fixation |
| US12005263B2 (en) | 2017-02-06 | 2024-06-11 | Medtronic, Inc. | Charge balanced cardiac pacing from high voltage circuitry of an extra-cardiovascular implantable cardioverter defibrillator system |
| US11524169B2 (en) | 2017-02-06 | 2022-12-13 | Medtronic, Inc. | Charge balanced cardiac pacing from high voltage circuitry of an extra-cardiovascular implantable cardioverter defibrillator system |
| US10821288B2 (en) | 2017-04-03 | 2020-11-03 | Cardiac Pacemakers, Inc. | Cardiac pacemaker with pacing pulse energy adjustment based on sensed heart rate |
| US10905872B2 (en) | 2017-04-03 | 2021-02-02 | Cardiac Pacemakers, Inc. | Implantable medical device with a movable electrode biased toward an extended position |
| US11065459B2 (en) | 2017-08-18 | 2021-07-20 | Cardiac Pacemakers, Inc. | Implantable medical device with pressure sensor |
| US10918875B2 (en) | 2017-08-18 | 2021-02-16 | Cardiac Pacemakers, Inc. | Implantable medical device with a flux concentrator and a receiving coil disposed about the flux concentrator |
| US12151116B2 (en) | 2017-08-18 | 2024-11-26 | Cardiac Pacemakers, Inc. | Implantable medical device with pressure sensor |
| US11235163B2 (en) | 2017-09-20 | 2022-02-01 | Cardiac Pacemakers, Inc. | Implantable medical device with multiple modes of operation |
| US11185703B2 (en) | 2017-11-07 | 2021-11-30 | Cardiac Pacemakers, Inc. | Leadless cardiac pacemaker for bundle of his pacing |
| US11052258B2 (en) | 2017-12-01 | 2021-07-06 | Cardiac Pacemakers, Inc. | Methods and systems for detecting atrial contraction timing fiducials within a search window from a ventricularly implanted leadless cardiac pacemaker |
| US11813463B2 (en) | 2017-12-01 | 2023-11-14 | Cardiac Pacemakers, Inc. | Leadless cardiac pacemaker with reversionary behavior |
| US11260216B2 (en) | 2017-12-01 | 2022-03-01 | Cardiac Pacemakers, Inc. | Methods and systems for detecting atrial contraction timing fiducials during ventricular filling from a ventricularly implanted leadless cardiac pacemaker |
| US11071870B2 (en) | 2017-12-01 | 2021-07-27 | Cardiac Pacemakers, Inc. | Methods and systems for detecting atrial contraction timing fiducials and determining a cardiac interval from a ventricularly implanted leadless cardiac pacemaker |
| US11529523B2 (en) | 2018-01-04 | 2022-12-20 | Cardiac Pacemakers, Inc. | Handheld bridge device for providing a communication bridge between an implanted medical device and a smartphone |
| US10874861B2 (en) | 2018-01-04 | 2020-12-29 | Cardiac Pacemakers, Inc. | Dual chamber pacing without beat-to-beat communication |
| US11400296B2 (en) | 2018-03-23 | 2022-08-02 | Medtronic, Inc. | AV synchronous VfA cardiac therapy |
| US11058880B2 (en) | 2018-03-23 | 2021-07-13 | Medtronic, Inc. | VFA cardiac therapy for tachycardia |
| US11235159B2 (en) | 2018-03-23 | 2022-02-01 | Medtronic, Inc. | VFA cardiac resynchronization therapy |
| US11819699B2 (en) | 2018-03-23 | 2023-11-21 | Medtronic, Inc. | VfA cardiac resynchronization therapy |
| US12172021B2 (en) | 2018-09-26 | 2024-12-24 | Medtronic, Inc. | Capture in ventricle-from-atrium cardiac therapy |
| US11235161B2 (en) | 2018-09-26 | 2022-02-01 | Medtronic, Inc. | Capture in ventricle-from-atrium cardiac therapy |
| US11951313B2 (en) | 2018-11-17 | 2024-04-09 | Medtronic, Inc. | VFA delivery systems and methods |
| US12296177B2 (en) | 2018-12-21 | 2025-05-13 | Medtronic, Inc. | Delivery systems and methods for left ventricular pacing |
| US11679265B2 (en) | 2019-02-14 | 2023-06-20 | Medtronic, Inc. | Lead-in-lead systems and methods for cardiac therapy |
| US11697025B2 (en) | 2019-03-29 | 2023-07-11 | Medtronic, Inc. | Cardiac conduction system capture |
| US11213676B2 (en) | 2019-04-01 | 2022-01-04 | Medtronic, Inc. | Delivery systems for VfA cardiac therapy |
| US11712188B2 (en) | 2019-05-07 | 2023-08-01 | Medtronic, Inc. | Posterior left bundle branch engagement |
| US11305127B2 (en) | 2019-08-26 | 2022-04-19 | Medtronic Inc. | VfA delivery and implant region detection |
| US11813466B2 (en) | 2020-01-27 | 2023-11-14 | Medtronic, Inc. | Atrioventricular nodal stimulation |
| US11911168B2 (en) | 2020-04-03 | 2024-02-27 | Medtronic, Inc. | Cardiac conduction system therapy benefit determination |
| US11813464B2 (en) | 2020-07-31 | 2023-11-14 | Medtronic, Inc. | Cardiac conduction system evaluation |
Also Published As
| Publication number | Publication date |
|---|---|
| US8838234B2 (en) | 2014-09-16 |
| US20050277990A1 (en) | 2005-12-15 |
| US6952610B2 (en) | 2005-10-04 |
| US20130184802A1 (en) | 2013-07-18 |
| US8412320B2 (en) | 2013-04-02 |
| WO2003039667A1 (en) | 2003-05-15 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| US8838234B2 (en) | Methods for implanting a subcutaneous defibrillator | |
| US7502645B2 (en) | Current waveforms for anti-bradycardia pacing for a subcutaneous implantable cardioverter-defibrillator | |
| US7536222B2 (en) | Nonvascular implantable defibrillator and method | |
| US8831720B2 (en) | Method of implanting and using a subcutaneous defibrillator | |
| US6856835B2 (en) | Biphasic waveform for anti-tachycardia pacing for a subcutaneous implantable cardioverter-defibrillator | |
| US7146212B2 (en) | Anti-bradycardia pacing for a subcutaneous implantable cardioverter-defibrillator | |
| US7090682B2 (en) | Method and apparatus for extraction of a subcutaneous electrode | |
| US7751885B2 (en) | Bradycardia pacing in a subcutaneous device | |
| US7720536B2 (en) | Power supply for an implantable subcutaneous cardioverter-defibrillator |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| AS | Assignment | Owner name:CAMERON HEALTH, INC., CALIFORNIA Free format text:ASSIGNMENT OF ASSIGNORS INTEREST;ASSIGNORS:OSTROFF, ALAN H.;RISSMANN, WILLIAM J.;MEZACK, GARY P.;REEL/FRAME:012674/0325 Effective date:20020219 | |
| AS | Assignment | Owner name:COMERICA BANK-CALIFORNIA, CALIFORNIA Free format text:SECURITY INTEREST;ASSIGNOR:CAMERON HEALTH, INC.;REEL/FRAME:013848/0266 Effective date:20030121 | |
| AS | Assignment | Owner name:CAMERON HEALTH, INC., CALIFORNIA Free format text:ASSIGNMENT OF ASSIGNORS INTEREST;ASSIGNOR:BARDY, GUST H;REEL/FRAME:015674/0165 Effective date:20050126 | |
| STCF | Information on status: patent grant | Free format text:PATENTED CASE | |
| CC | Certificate of correction | ||
| AS | Assignment | Owner name:CAMERON HEALTH INC., CALIFORNIA Free format text:RELEASE BY SECURED PARTY;ASSIGNOR:COMERICA BANK;REEL/FRAME:020758/0413 Effective date:20080401 | |
| FPAY | Fee payment | Year of fee payment:4 | |
| FEPP | Fee payment procedure | Free format text:PAT HOLDER NO LONGER CLAIMS SMALL ENTITY STATUS, ENTITY STATUS SET TO UNDISCOUNTED (ORIGINAL EVENT CODE: STOL); ENTITY STATUS OF PATENT OWNER: LARGE ENTITY | |
| FEPP | Fee payment procedure | Free format text:PAYER NUMBER DE-ASSIGNED (ORIGINAL EVENT CODE: RMPN); ENTITY STATUS OF PATENT OWNER: LARGE ENTITY Free format text:PAYOR NUMBER ASSIGNED (ORIGINAL EVENT CODE: ASPN); ENTITY STATUS OF PATENT OWNER: LARGE ENTITY | |
| FPAY | Fee payment | Year of fee payment:8 | |
| FPAY | Fee payment | Year of fee payment:12 |