US20020072739A1 - Methods and devices for radiofrequency electrosurgery - Google Patents
Methods and devices for radiofrequency electrosurgeryDownload PDFInfo
- Publication number
- US20020072739A1 US20020072739A1US09/732,848US73284800AUS2002072739A1US 20020072739 A1US20020072739 A1US 20020072739A1US 73284800 AUS73284800 AUS 73284800AUS 2002072739 A1US2002072739 A1US 2002072739A1
- Authority
- US
- United States
- Prior art keywords
- active element
- tissue
- insulating
- window
- patient
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Abandoned
Links
- 238000000034methodMethods0.000titleclaimsdescription28
- 239000000523sampleSubstances0.000claimsabstractdescription81
- 238000003780insertionMethods0.000claimsabstractdescription5
- 230000037431insertionEffects0.000claimsabstractdescription5
- 206010020843HyperthermiaDiseases0.000claimsdescription9
- 230000036031hyperthermiaEffects0.000claimsdescription9
- 230000002631hypothermal effectEffects0.000claimsdescription9
- 239000012530fluidSubstances0.000claimsdescription6
- 238000002679ablationMethods0.000claimsdescription5
- 238000002224dissectionMethods0.000claimsdescription5
- 230000017074necrotic cell deathEffects0.000claimsdescription5
- 230000001939inductive effectEffects0.000claimsdescription3
- 230000003247decreasing effectEffects0.000abstract1
- 210000001519tissueAnatomy0.000description136
- 210000001124body fluidAnatomy0.000description4
- 210000003813thumbAnatomy0.000description4
- 238000005520cutting processMethods0.000description3
- 230000000694effectsEffects0.000description3
- 239000012811non-conductive materialSubstances0.000description3
- 210000000577adipose tissueAnatomy0.000description2
- 239000008280bloodSubstances0.000description2
- 210000004369bloodAnatomy0.000description2
- 239000003795chemical substances by applicationSubstances0.000description2
- 230000015271coagulationEffects0.000description2
- 238000005345coagulationMethods0.000description2
- 230000007423decreaseEffects0.000description2
- 239000008177pharmaceutical agentSubstances0.000description2
- 239000000779smokeSubstances0.000description2
- 238000002604ultrasonographyMethods0.000description2
- 239000000730antalgic agentSubstances0.000description1
- 230000003115biocidal effectEffects0.000description1
- 239000010839body fluidSubstances0.000description1
- 239000000919ceramicSubstances0.000description1
- 230000001112coagulating effectEffects0.000description1
- 235000013870dimethyl polysiloxaneNutrition0.000description1
- 239000004205dimethyl polysiloxaneSubstances0.000description1
- 238000010292electrical insulationMethods0.000description1
- 238000010438heat treatmentMethods0.000description1
- 238000002955isolationMethods0.000description1
- 230000003902lesionEffects0.000description1
- 239000000463materialSubstances0.000description1
- 239000012528membraneSubstances0.000description1
- 239000000615nonconductorSubstances0.000description1
- 230000035515penetrationEffects0.000description1
- 229920000435poly(dimethylsiloxane)Polymers0.000description1
- 238000009877renderingMethods0.000description1
- 150000003839saltsChemical class0.000description1
- 229920002379silicone rubberPolymers0.000description1
- 230000002792vascularEffects0.000description1
Images
Classifications
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61B—DIAGNOSIS; SURGERY; IDENTIFICATION
- A61B18/00—Surgical instruments, devices or methods for transferring non-mechanical forms of energy to or from the body
- A61B18/04—Surgical instruments, devices or methods for transferring non-mechanical forms of energy to or from the body by heating
- A61B18/12—Surgical instruments, devices or methods for transferring non-mechanical forms of energy to or from the body by heating by passing a current through the tissue to be heated, e.g. high-frequency current
- A61B18/14—Probes or electrodes therefor
- A61B18/1482—Probes or electrodes therefor having a long rigid shaft for accessing the inner body transcutaneously in minimal invasive surgery, e.g. laparoscopy
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61B—DIAGNOSIS; SURGERY; IDENTIFICATION
- A61B18/00—Surgical instruments, devices or methods for transferring non-mechanical forms of energy to or from the body
- A61B2018/00005—Cooling or heating of the probe or tissue immediately surrounding the probe
- A61B2018/00011—Cooling or heating of the probe or tissue immediately surrounding the probe with fluids
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61B—DIAGNOSIS; SURGERY; IDENTIFICATION
- A61B18/00—Surgical instruments, devices or methods for transferring non-mechanical forms of energy to or from the body
- A61B2018/00053—Mechanical features of the instrument of device
- A61B2018/00059—Material properties
- A61B2018/00071—Electrical conductivity
- A61B2018/00083—Electrical conductivity low, i.e. electrically insulating
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61B—DIAGNOSIS; SURGERY; IDENTIFICATION
- A61B18/00—Surgical instruments, devices or methods for transferring non-mechanical forms of energy to or from the body
- A61B2018/00053—Mechanical features of the instrument of device
- A61B2018/00184—Moving parts
- A61B2018/00196—Moving parts reciprocating lengthwise
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61B—DIAGNOSIS; SURGERY; IDENTIFICATION
- A61B18/00—Surgical instruments, devices or methods for transferring non-mechanical forms of energy to or from the body
- A61B2018/00053—Mechanical features of the instrument of device
- A61B2018/00214—Expandable means emitting energy, e.g. by elements carried thereon
- A61B2018/0022—Balloons
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61B—DIAGNOSIS; SURGERY; IDENTIFICATION
- A61B18/00—Surgical instruments, devices or methods for transferring non-mechanical forms of energy to or from the body
- A61B18/04—Surgical instruments, devices or methods for transferring non-mechanical forms of energy to or from the body by heating
- A61B18/12—Surgical instruments, devices or methods for transferring non-mechanical forms of energy to or from the body by heating by passing a current through the tissue to be heated, e.g. high-frequency current
- A61B18/14—Probes or electrodes therefor
- A61B2018/1475—Electrodes retractable in or deployable from a housing
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61B—DIAGNOSIS; SURGERY; IDENTIFICATION
- A61B18/00—Surgical instruments, devices or methods for transferring non-mechanical forms of energy to or from the body
- A61B18/04—Surgical instruments, devices or methods for transferring non-mechanical forms of energy to or from the body by heating
- A61B18/12—Surgical instruments, devices or methods for transferring non-mechanical forms of energy to or from the body by heating by passing a current through the tissue to be heated, e.g. high-frequency current
- A61B18/14—Probes or electrodes therefor
- A61B2018/1497—Electrodes covering only part of the probe circumference
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61B—DIAGNOSIS; SURGERY; IDENTIFICATION
- A61B2218/00—Details of surgical instruments, devices or methods for transferring non-mechanical forms of energy to or from the body
- A61B2218/001—Details of surgical instruments, devices or methods for transferring non-mechanical forms of energy to or from the body having means for irrigation and/or aspiration of substances to and/or from the surgical site
- A61B2218/002—Irrigation
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61B—DIAGNOSIS; SURGERY; IDENTIFICATION
- A61B2218/00—Details of surgical instruments, devices or methods for transferring non-mechanical forms of energy to or from the body
- A61B2218/001—Details of surgical instruments, devices or methods for transferring non-mechanical forms of energy to or from the body having means for irrigation and/or aspiration of substances to and/or from the surgical site
- A61B2218/007—Aspiration
- A61B2218/008—Aspiration for smoke evacuation
Definitions
- the present inventionrelates primarily to the field of electrosurgery.
- the present inventionrelates to the field of radiofrequency (RF) electrosurgery, including RF cautery, RF dissection, RF fulguration or coagulation, RF desiccation or any other forms of RF-assisted electrosurgery.
- RFradiofrequency
- Electrosurgerygenerally refers to the use of electrically energized surgical devices to operate upon a patient.
- Common electrosurgical devicesinclude electrocautery devices in which a direct current (DC) is caused to flow through a wire loop. The resistance of the wire causes the loop to heat up, thereby enabling the cauterization of the tissue.
- DCdirect current
- electrosurgical devicesutilize alternating current (AC) in the range of about 200 kHz to about 3.3 MHz (hereafter “RF”) that is applied to the patient via a RF electrode.
- RF electrosurgical instrumentsare typically used to dissect (cut), fulgurate (coagulate) or desiccate (dry out) tissue by selectively varying the power and duty cycle of the RF signal applied to the electrode of the electrosurgical instrument.
- a large low impedance return (dispersive) electrodeis affixed to the patient to provide the current return path to ground.
- a bipolar RF electrosurgical instrumenthas two electrodes, the active electrode and the return electrode. The flow of current occurs between these two electrodes, thus obviating the need for a large low impedance return electrode.
- An insulated RF generatoris typically used to energize the RF electrode(s) of the RF electrosurgical instrument.
- the density of the current delivered to the active electrodemay be affected by the impedance (a vector quantity consisting of the tissue's resistance and its reactance) of the tissue.
- the impedance of the tissue to which the electrodes are exposedhas an important effect upon the functioning of RF electrosurgical devices. This is because the tissue becomes an integral part of the circuit comprising the RF generator, the active electrode, the patient's tissue, the return electrode and back to the RF generator to ground or a reference voltage. Therefore, a voltage controlled or limited RF generator (generally providing power between about 10 W and 200 W) is generally used, so that when the impedance of tissue rises, the current delivered to the active electrode safely decreases.
- the density and impedance of tissueaffects the manner in which it is affected by the active electrode.
- Tissues with higher salt content and body fluids such as bloodhave a high conductance and therefore, lower impedance.
- Fatty tissueshave a lower fluid content and generally exhibit relatively higher impedance than leaner tissues. Thus, for a given voltage, fatty tissue will dissipate less current than leaner tissue.
- Highly vascularized tissueshave very low impedance and tend to conduct current more efficiently than less vascularized tissues. Therefore, to achieve the same tissue effect, current must be applied to a RF electrode in contact with highly vascularized tissue for a longer period of time than would be the case if the RF electrode were in contact with comparatively less vascularized tissue.
- the initial power applied to the RF electrodemay need to be momentarily increased to achieve the same tissue effect in a fixed period of time.
- FIGS. 1 - 3show a RF excisional device 100 that includes a probe 102 , the distal end 108 of which includes a conductive ribbon or wire loop 106 configured to bow out of and back into a window 104 defined within the probe 102 .
- the loop 106is connected to a RF power supply (not shown).
- the loop 106is energized with RF energy and the probe 102 is rotated as shown at 112 with the loop 106 in an extended or deployed configuration, as shown in FIG. 3.
- a volume of revolution of tissueis cut by the RF-energized loop 106 .
- One solution to this problemis to initially increase or surge the power delivered to the loop 106 to decrease the time required for the current density therein to reach the desired level.
- thisis a less than optimal solution, as such an initial power surge may cause pain, excessive charring of the tissue 114 at the initial cut site, and may present a safety risk to the patient, who is better served by maintaining power levels as low as practicable to achieve the objective of the procedure.
- Such initial power surgesmoreover, require specialized RF generators that are configured to deliver such surges safely and on demand.
- an electrosurgical deviceincludes a body, the body defining an outer surface, a proximal end, a distal end and a window defined within the outer surface; an electrically insulating layer and an active element, the active element being adapted to electrically connect to a power source and configured to selectively assume a non-deployed configuration in which the insulating layer electrically insulates the active element from a patient's tissue when the device is in use and a variable deployed configuration in which the active element at least partially emerges from the window out of the body to make contact with the patient's tissue.
- the active elementmay include one of a ribbon and wire, said one of ribbon and wire being adapted to selectively bow out of the window when transitioning from the non-deployed configuration to the deployed configuration.
- a tissue collection meansmay be attached thereto or trail behind the active element, as disclosed in co-pending and commonly assigned U.S. patent application Ser. No. 09/565,611 filed on May 4, 2000 and/or commonly assigned U.S. Pat. No. 6,022,362 filed on Sept. 3, 1998, the disclosure of each being incorporated herein by reference in its entirety.
- the active elementmay be configured to carry tissue ablation, dissection, fulguration, cautery, desiccation, hyperthermia or hypothermia, for example.
- the thickness of the insulating layermay be selected within a range of about 0.1 mm to about 5 mm, for example.
- the power sourcemay include a RF power source.
- the windowmay be disposed near the distal end of the body or at the distal end thereof.
- the active elementmay be ring-shaped and may include a trailing tissue collection means attached thereto adapted to collect tissue cut by the ring-shaped active element.
- the insulating layermay include an electrically insulating sliding door mounted within the body, the sliding door being configured to selectively cover at least a portion of the window.
- the sliding doormay be configured to slide between a variable first position in which at least a portion of the active element is exposed to the patient's tissue through the window and a second position in which the sliding door electrically insulates the active element within the body from the patient's tissue.
- the insulating layermay include a layer of air and the window may be configured to maintain the layer in the non-deployed configuration.
- the insulating layermay include an electrically insulating outer sleeve configured to selectively slide over the outer surface of the body, the outer sleeve being configured to at least partially cover the active element to electrically insulate the active element from the patient's tissue and uncover the active element to expose the active element to the patient's tissue.
- the insulating layerfurther may include an electrically insulating sheath disposed around at least a portion of the device body, the insulating sheath defining a cutaway portion aligned with the window and defining one or more raised portions configured to prevent the patient's tissue from prolapsing into the window when the active element is in its non-deployed configuration.
- the insulating layermay include an insulating sleeve disposed at least partially around the active element, the insulating sleeve being configured to controllably slide over the active element to selectively electrically insulate the active element from the patient's tissue and expose the active element thereto when the device is in use.
- the bodymay be shaped as a non-coring needle.
- the insulating layermay include a layer of air and the device body may define one or more raised portions adjacent the window, the raised portion(s) maintaining the layer of air between the active element and the patient's tissue when the active element is in its non-deployed state.
- Suction meansmay be included within the body for controllably removing fluids from the operative site within the patient's tissue.
- the present inventionmay also be viewed as a method of treating tissue using a probe, the probe including an active element that may be extendable out of and retractable back into a window defined in the probe, the active element being electrically connected to a power source, comprising the steps of: inserting the probe into the tissue; electrically insulating the active element from the tissue; energizing the active element using power from the power source, and exposing the energized active element to the tissue.
- the power sourcemay be adapted to supply RF power to the active element.
- the insulating stepmay include a step of maintaining an electrically insulating layer between the active element and the tissue.
- the probefurther may include an electrically insulating sliding door and the insulating step may include a step of sliding the door across the window.
- the methodmay further comprise the step of inducing hyperthermia or hypothermia and necrosis in the tissue exposed to the active element.
- the probefurther may include an electrically insulating outer sleeve adapted to slide over an outer surface of the probe and the insulating step may include a step of sliding the outer sleeve over the window.
- the probefurther may include an insulating sheath around at least a portion of the probe body, the insulating sheath including at least one raised portion adjacent the window and the insulating step may include a step of maintaining the active element retracted within the window.
- the insulating stepmay include a step of maintaining the active element retracted within the window.
- the probefurther may include an insulating sleeve adapted to slide over the active element and the insulating step may include a step of sliding the insulating sleeve over the active element.
- the insulating stepmay include a step of creating or maintaining a layer of air between the active element and the tissue.
- the probefurther may include an inflatable balloon disposed adjacent the window, and the insulating step further may include a step of inflating the balloon to create a layer of air between the tissue and the active element.
- the present inventionis also an electrosurgical device, comprising a device body, the body defining a proximal end and a distal end; an active element coupled to the body, the active element being adapted to be controllably energized from a power source; and means for selectively electrically insulating and exposing the active element from and to a patient's tissue when the device is in use.
- the active elementmay be configured to selectively assume a non-deployed configuration in which the active element is recessed within the body and a variable deployed configuration in which the active element at least partially emerges from the body.
- the active elementmay be configured to carry out tissue ablation, dissection, fulguration, cautery, desiccation, hyperthermia and/or hypothermia, for example.
- the active elementmay be configured to be energized by a RF power source, for example.
- the device bodymay define a window disposed near the distal end of the body, and the active element may be configured to selectively retract into and extend from the window.
- a windowmay be defined within the body and the insulating means may include an electrically insulating sliding door mounted within the body, the sliding door being configured to selectively cover at least a portion of the window.
- the sliding doormay be configured to slide between a variable first position in which at least a portion of the active element is exposed through the window and a second position in which the sliding door electrically insulates the active element from the patient's tissue.
- the insulating meansmay be integral with the window and the window may be configured to prevent tissue from prolapsing therein and making physical and/or electrical contact with the active element when the active element is in a non-deployed configuration.
- the insulating meansmay include an electrically insulating outer sleeve configured to selectively slide over the outer surface of the body, the outer sleeve being configured to at least partially cover the active element and electrically insulate the active element from the patient's tissue when the device is in use and the active element is in a non-deployed configuration.
- the insulating meansmay include an electrically insulating sleeve disposed at least partially around the active element, the insulating sleeve being configured to controllably slide over the active element to selectively electrically insulate the active element from the patient's tissue and expose the active element thereto when the device is in use.
- the insulating meansmay be integral with the body and may define one or more raised portions, the raised portion(s) being configured to prevent tissue from making physical and/or electrical contact with the active element until the active element is deployed.
- the insulating meansmay include an electrically insulating sheath disposed over the body, the insulating sheath defining at least one raised portion configured to prevent the patient's tissue from coming into contact with the active element until the active element is deployed.
- the active elementmay be shaped as a ring and the insulating means may be integral with the distal end of the body, the ring-shaped active element being configured to be recessed away from the distal end of the body until fully energized.
- the present inventionis also an electrosurgical device adapted for insertion into tissue, comprising: a device body; a RF energizable active element disposed within the device body, and an insulating structure.
- the device body and/or the insulating structuremay be configured to insulate the active element from the tissue until the active element is sufficiently energized to be therapeutically effective when the active element is brought into contact with the tissue.
- FIG. 1is a top view of the distal end of an expandable loop excisional device.
- FIG. 2is a side view of the distal end of the excisional device of FIG. 1, in which the loop is in its non-extended (non-deployed) configuration.
- FIG. 3is a side view of the distal end of the excisional device of FIG. 1, in which the loop is in its extended (deployed) configuration.
- FIG. 4is a cross-sectional view of the excisional device of FIG. 2, taken along cross-sectional lines AA′.
- FIG. 5shows the excisional device of FIG. 4 in which the loop is in contact with tissue, to illustrate the current dissipation occurring at the loop-tissue interface.
- FIG. 6shows an embodiment of a RF electrosurgical device, according to an embodiment of the present invention.
- FIG. 7shows the distal end of the RF electrosurgical device of FIG. 6, in which the active element thereof is in its non-extended (non-deployed) configuration.
- FIG. 8shows the distal end of the RF electrosurgical device of FIG. 7, in which the active element thereof is in its extended (deployed) configuration.
- FIG. 9is a cross-sectional view of the excisional device of FIG. 7, taken along cross-sectional lines BB′.
- FIG. 10shows the excisional device of FIG. 9 and illustrates the absence of active element-tissue interface when the RF electrosurgical device of the present invention is initially inserted in tissue and RF power applied to the active element.
- FIG. 11shows another embodiment of a RF electrosurgical device, according to the present invention.
- FIG. 12shows the distal end of the RF electrosurgical device of FIG. 11, in which the active element thereof is in a non-extended (non-deployed configuration).
- FIG. 13Ashows the distal end of the RF electrosurgical device of FIG. 11, in which the active element thereof is in a first illustrative extended (deployed configuration).
- FIG. 13Bshows the distal end of the RF electrosurgical device of FIG. 11, in which the active element thereof is in a second illustrative extended (deployed configuration).
- FIG. 14is a cross-sectional view of the excisional device of FIG. 12, taken along cross-sectional lines CC′.
- FIG. 15shows the excisional device of FIG. 14 and illustrates the absence of active element-tissue interface when the RF electrosurgical device of the present invention is initially inserted in tissue and RF power is applied to the active element.
- FIG. 16is a top view of another embodiment of an electrosurgical device according to the present invention.
- FIG. 17shows a side view of the distal end of the electrosurgical device of FIG. 16, in which the active element thereof is in its non-deployed configuration.
- FIG. 18shows the electrosurgical device of FIG. 17, in which the active element thereof is in its deployed configuration.
- FIG. 19shows a side view of another embodiment of an electrosurgical device according to the present invention, in which the active element thereof is in its non-deployed configuration.
- FIG. 20shows the electrosurgical device of FIG. 19, in which the active element thereof is in its deployed configuration.
- FIG. 21is a top view of the distal end of an electrosurgical device according to a still further embodiment of the present invention.
- FIG. 22Ais a side view of the device of FIG. 21, in which the inflatable balloon(s) thereof has (have) been inflated to create an insulating layer between the active element and the patient's tissue.
- FIG. 22Bis a front view of the device of FIG. 22A, in which the balloons are in their inflated configuration.
- FIG. 23is a side view of the device of FIG. 21, after deflation of the balloon(s) and deployment of the active element.
- FIG. 24shows a top view of the distal end of an electrosurgical device according to another embodiment of the present invention.
- FIG. 25shows a side view of the device of FIG. 24.
- FIG. 26shows the device of FIG. 25, after the outer sleeve thereof has been slid away from the window from which the active element is deployed.
- FIG. 27is a flowchart of a method for carrying out an electrosurgical procedure according to an embodiment of the present invention.
- FIG. 28is a top view of an electrosurgical device, according to another embodiment of the present invention.
- FIG. 29is a side view of the distal portion of the device of FIG. 28, showing the active element thereof in its non-extended and sheathed state.
- FIG. 30is a side view of the distal portion of the device of claim 28, showing the active element thereof in its extended and unsheathed state.
- FIG. 31is a cross-sectional view of the electrosurgical device of FIG. 29, taken along cross-sectional lines DD′.
- FIG. 32is a top view of an electrosurgical device, according to another embodiment of the present invention.
- FIG. 33is a side view of the distal portion of the device of FIG. 32.
- FIG. 34is a side view of the distal portion of the device of FIG. 32, in which the active element is in its extended state.
- FIG. 35is a cross-sectional view of the electrosurgical device of FIG. 33, taken along cross-sectional lines EE′.
- FIG. 36shows a cross-sectional view of another embodiment of the electrosurgical device of FIG. 33, taken along cross sectional lines EE′
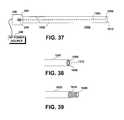
- FIG. 37shows another electrosurgical device, according to yet another embodiment of the present invention.
- FIG. 38is a perspective side view of the distal portion of the electrosurgical device of FIG. 37, in which the electrosurgical ring is in its non-extended state.
- FIG. 39is a perspective side view of the distal portion of the electrosurgical device of FIG. 37, in which the electrosurgical ring is in its extended state.
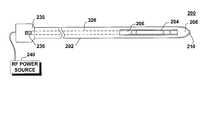
- FIGS. 6 - 10show an embodiment of a RF electrosurgical device 200 , according to the present invention.
- the device 200includes a probe body 202 , the probe body 202 defining an outer surface, a proximal end 230 , a distal end 208 and a window 204 (shown in dashed lines in FIGS. 7 and 8) defined within the outer surface near the distal end 208 .
- the probe 200includes an active element 206 .
- the active element 206may include, for example one or more wires and/or loops, or any other therapeutically effective RF element effective to cut, dissect, fulgurate and/or desiccate tissue (for example) using RF power.
- the active element 206may be electrically connected to a power source, such as the RF power source 240 .
- a distal RF element 210may also be mounted to the distal end 208 of the probe body 202 , to facilitate penetration of the probe 200 into the patient's tissue.
- the distal RF element 210may also be independently electrically connected to the RF power source 240 .
- the active element 206may be configured to selectively assume a non-deployed configuration (FIG. 7) and a variable deployed configuration (FIG. 8). In the variable deployed configuration (FIG. 8), the active element 206 at least partially emerges from the window 204 out of the probe body 202 .
- the deployment of the active element 206may be controlled by the physician by means of an actuator 236 , such as the thumb wheel shown in FIG. 6 or other actuation means.
- an actuator 236such as the thumb wheel shown in FIG. 6 or other actuation means.
- the physicianBy turning the actuator 236 with his or her thumb, the physician causes the active element 206 to slide within a guide 326 defined within the probe 202 .
- the active element 206bows out of the window 204 , the degree of bowing and deployment out of the window 204 being controlled by the actuator 236 .
- the active element 206is recessed within the probe body 202 to define an insulating layer 212 (a layer of air, for example) with respect to the outer surface of the probe body 202 .
- the recessed active element 206 and/or the presence of the insulating layer 212enables the active element 206 to be energized without contacting the patient's tissue. Indeed, by avoiding contact with the patient's tissue during the energizing of the active element 206 , the current delivered to the active element 206 is not dissipated by the patient's tissue that would otherwise be in contact therewith but for the presence of the insulating layer 212 .
- the active element 206may be deployed to (for example) cut, ablate or coagulate (depending upon the application) the patient's tissue without the physician having to wait for the tissue impedance to rise sufficiently (while the patient's tissue heats up and/or chars) to enable the current density within the active element 206 to reach the desired level for the procedure to be carried out.
- FIGS. 9 and 10show a cross-sectional view of the probe 200 , taken along the cross-sectional lines BB′of FIG. 7. As shown therein, to electrically insulate the active element 206 from the patient's tissue (reference 214 in FIG.
- the window 204 defined within the probe body 202may be shaped and/or dimensioned so as to prevent the tissue 214 through which the probe 200 is inserted from prolapsing therein.
- the window 204is configured to define an electrically insulating layer 212 between the active element 206 and the patient's tissue 214 when the active element 206 is in its non-deployed (retracted) configuration.
- the window 204may define a steep aspect ratio, in which the depth of the window 204 is greater than its width.
- FIGS. 11 - 15show a probe 300 according to another embodiment of the present invention.
- Reference numerals that are identical to those shown in FIGS. 6 - 10denote identical structures. For the sake of brevity, the description of those identical structures will not be explicitly repeated here.
- the insulating layer that electrically insulates the active element 206 from the patient's tissue 214includes a sliding door 216 disposed within the probe body 202 .
- the sliding door 216may be made of an electrical insulator or other substantially non-conductive material or materials.
- the sliding door 216is configured to selectively cover at least a portion of the window 204 to electrically insulate the active element 206 of the device 300 from the patient's tissue 214 until the active element 206 is fully energized and/or until the physician chooses to expose the active element 206 to the patient's tissue 214 .
- the physicianmay cause the sliding door 216 to slide and expose the active element 206 by means of a sliding door actuator such as a manual thumb wheel, as shown at 356 . It is understood that other means of acting upon the sliding door 216 and/or active element 206 may be employed, as the present invention is not limited to thumb wheel actuators, as shown at 356 and 236 .
- the sliding door 216is configured to slide between a variable first position in which at least a portion of the active element is exposed through the window (as shown in FIGS. 13A and 13B) and a second position (shown in FIGS. 11, 12, 14 and 15 ) in which the sliding door 216 insulates the active element 206 within the probe body 202 .
- the sliding door 216may be caused to expose a selectable portion of the active element 206 to the patient's tissue 214 . In so doing, the sliding door 216 may affect the shape of the deployed active element: if the sliding door 216 is fully or near fully retracted as shown in FIG.
- FIGS. 14 and 15show the sliding door 216 as it covers the window 204 , thereby electrically insulating the active element 206 from the patient's tissue 214 .
- the sliding door 216may be guided within the probe body 202 by means of, for example, internal guides or rails 218 .
- the physicianmay apply RF power to the active element 206 and begin the intended RF procedure (cutting, ablating, coagulating, etc.) sooner than would otherwise be the case if the de-energized active element were energized while in contact with the patient's tissue 214 .
- the layer of air 213 between the active element 206 and the sliding door 216may be reduced or eliminated altogether, as the sliding door 216 electrically insulates and physically isolates the active element 206 from the patient's tissue 214 .
- the sliding door 216is preferably formed of non-conductive material, such as a non-conductive plastic or ceramic, for example.
- FIGS. 16 - 18show another embodiment of the present invention.
- the electrosurgical device 400includes a proximal end 260 , a distal end 208 , a probe body 202 , an active element actuator 236 and a sliding door actuator 356 , as described above.
- the distal tip of the probe bodydefines an opening 290 that is aligned with an internal lumen 282 defined within the probe body 202 .
- the exemplary active element 218 shown in FIGS. 16 - 18includes an elongated conductive stem that is distally terminated by a conductive bulb.
- any electrically active elementmay be used in conjunction with or in place of the active element depicted in FIGS. 16 - 18 .
- the active element 218may be electrically coupled to the RF power source 240 .
- the bulbmay be used, for example, to induce hyperthermia and/or necrosis in the tissue 214 with which it comes into contact.
- the duty cycle of the RF current applied to the active element 218may be adjusted to be, for example, 50%-25%, so as to induce substantial heating in the tissue 214 in contact with the active element 218 .
- the insulating layer separating the active element 218 from the patient's tissue 214 when the active element 206 is in its non-deployed configurationmay include a sliding and electrically insulating door 220 mounted within the probe body 202 .
- the sliding door 220may be configured to slide axially in the distal and proximal directions (away from and toward the physician, respectively, when the device 400 is in use) when the sliding door actuator 356 is actuated.
- the distal end of the sliding door 220may be advantageously configured to cover (see FIG. 17) the opening 290 during insertion of the device 400 into the patient's tissue 214 and during the energizing of the active element 218 .
- the physicianmay actuate the sliding door actuator 356 to retract the sliding door 220 away from the opening 290 , in the proximal direction 294 .
- the energized active element 218may be advanced in the distal direction 296 into the target area of the patient's tissue 214 to begin treatment thereof, as shown in FIG. 18.
- the sliding door 220may be retracted before the active element 218 is energized.
- the active element 218may be energized without dissipating current into the surrounding tissue 214 , as the active element 218 is still recessed and electrically insulated from the patient's tissue by an air layer 215 .
- the shape and/or size of the opening 290 and/or the thickness of the air layer 215may also prevent the patient's tissue 214 from prolapsing into the opening 290 , thereby further insulating the active element 218 . As shown in FIGS.
- the active element 218is fully insulated from the patient's tissue 214 by the sliding door 220 until the active element 218 is appropriately energized, thereby preventing any disadvantageous dissipation of RF current into the tissue 214 upon the application of RF current to the active element 218 .
- FIGS. 19 - 20show a still further embodiment of the present invention.
- the electrosurgical device 500includes a probe body 202 defining a proximal end 230 , a distal end 208 and an internal lumen 217 .
- the proximal end 230includes an active element actuator 236 , as described above.
- An active element 218is configured to slide within the internal lumen 217 , as controlled by the active element actuator 236 .
- Most any RF active elementmay be used within the electrosurgical device 500 .
- the stem and bulb active element 218 of FIGS. 16 - 18is again used here, it being understood that this embodiment is not limited thereby.
- the distal end 208 of the probe body 202may be shaped as a non-coring needle.
- the active element 218may be recessed within the internal lumen 217 of the probe body to define an electrically insulating layer 219 .
- the layer 219may also physically isolate the active element 218 from the patient's tissue when the active element is in its non-deployed state, as shown in FIG. 19.
- the active element actuator 236may be used to advance the energized active element 218 in the distal direction 296 until the active element 218 emerges from the distal opening 292 and extends to the desired length, as shown in FIG. 20.
- the insulating layer 219(FIG. 19) between the active element 218 and the opening 294 defined in the distal end of the probe body 202 (and/or the tissue 214 ) prevents any of the RF current applied to the active element 218 from dissipating into the patient's tissue 214 during the energizing of the active element 218 .
- Some or all of the through holes 938may be utilized for the delivery of a fluid to the patient during the procedure, such as antibiotic agents, analgesic agents or most any pharmaceutical agent. Such agents may be administered to the patient from the port 942 .
- the port 942may be coupled to suction and some or all of the through holes 938 may be utilized to suction out the operative site of smoke, blood or other bodily fluids during or after the operative procedure.
- more than one port 942may be provided in the proximal portion 902 and more than one internal lumen 940 may be defined along the length of the probe 500 .
- the additional lumenmay be in fluid communication with selected through holes 938 .
- both delivery of a pharmaceutical agent and suctioningmay be provided within a single probe 500 .
- any of the devices according to the present inventionmay incorporate all or a portion of the structures referenced by 938 , 940 and 942 or like structures to provide suctioning and/or fluid delivery functionality.
- FIGS. 21 - 23shows the distal portion of a still further embodiment of the present invention.
- the device 600defines a distal end 208 , a window 204 and an active element 206 , as described above.
- the device 600also includes one or more inflatable balloons 602 .
- at least one such balloon 602may advantageously be disposed in close proximity to and may be co-extensive with the window 204 from which the active element 206 emerges and deploys.
- FIG. 22Ashows the device 600 in a configuration wherein the active element 206 is in its non-deployed configuration and the balloons 602 are in their inflated configuration.
- the balloons 602may be inflated with CO 2 (for example) from a CO 2 reservoir (not shown) located within or without the device 600 .
- FIG. 22Bshows a front view of the device of FIG. 22A and illustrates the insulating layer 212 between the active element 206 and the tissue 214 created by the inflation of the balloons 602 .
- FIG. 23shows a side view of the device 600 in a configuration wherein the active element 206 is deployed and the balloons 602 deflated, rendering them (preferably) flush or substantially flush with the outer surface of the device 600 .
- the device 600may be advanced through the patient's tissue 214 until the target area is reached.
- the balloon or balloons 602may then be inflated, thereby creating the insulating layer 212 between the active element 206 and the tissue 214 .
- the active element 206may then be energized with RF current from a RF power supply, such as shown at 240 in FIG. 16, for example.
- the balloon 602 or balloons 602may be deflated and the active element deployed, as described above.
- initially electrically insulating the active element 206 from the tissue 214advantageously prevents current from dissipating into the tissue 214 during the energizing of the active element 206 .
- the active element 206may be brought into contact with the patient's tissue 214 when the active element 206 has been energized to a therapeutically effective level.
- FIGS. 24 - 26show the distal portion of a still further embodiment of the present invention.
- the electrosurgical device 700includes a probe body 202 , a window 204 defined within the probe body 202 near the distal end 208 thereof, and an active element 206 within the window 204 , as previously described.
- an electrically insulating outer sleeve 702is fitted over the probe body 202 .
- the outer sleeve 702is configured to slide over and/or against the outer surface of the probe body 202 in the proximal and axial directions.
- the outer sleeve 702may advantageously be maintained in a position wherein it prevents tissue 214 from coming into contact with the active element 206 .
- the physicianmay retract the outer sleeve 702 by sliding it in the proximal direction 294 .
- the physicianmay expose the entire active element 206 to the tissue 214 .
- the physicianmay advantageously change the extent of deployment and/or shape of the active element, as shown at 206 A.
- the active element in FIGS. 6 - 26may also include a device to cause hypothermia and subsequent necrosis in the patient's tissue; i.e., to freeze the patient's tissue in contact with the active element. Indeed, by energizing such an active device while it is electrically insulated and/or physically isolated from the patient's tissue 214 , the temperature of the active element will drop to the intended low temperature faster than if the active element were initially in contact with the patient's tissue 214 , especially if the tissue is highly vascular (and thus able to transport heat efficiently).
- FIG. 27is a flowchart of a method of carrying out an electrosurgical procedure, according to the present invention.
- Step SIcalls for the insertion of a RF electrosurgical device, such as shown in FIGS. 6 - 26 , into the patient's tissue.
- ultrasound (or any other radiological) guidancemay be employed to guide the device to the targeted site within the patient's tissue.
- Step S 2calls for electrically insulating (and/or physically isolating) the active element (such as shown at 206 , 218 ) of the RF device from the surrounding tissue.
- the insulating step S 2may be carried out either before, during or after step S 1 , but should be carried out before step S 3 that is, before energizing the active element.
- Insulating and/or electrically isolating the active elementmay entail ensuring the presence of an insulating layer (preferably about 0.1 mm to about 5 mm in thickness) by maintaining the active element recessed within the probe body, in its retracted, non-deployed configuration and/or sliding a sliding door ( 216 , 220 ) over the active element, inflating a balloon or balloons 602 and/or maintaining an outer sleeve 702 over the active element.
- an insulating layer 212 and/or insulating the active element from the surrounding tissue during the energizing thereofmay be employed within the scope of the present invention.
- Step S 4calls for exposing at least a portion of the energized active element to the patient's tissue and step S 5 calls for carrying out the intended procedure, such as cutting, ablation, coagulation, etc.
- FIGS. 28 - 31represent different views of an electrosurgical device 800 , according to another embodiment of the present invention.
- the device 800includes a proximal end 260 , a distal end 208 and may include a distal RF element 210 , as described above.
- the device 800includes an active element 206 , which may include one or more wires and/or loops, or any other therapeutically effective RF element effective to cut, dissect, fulgurate, induce hyper or hypothermia (from a different power source) and/or to desiccate tissue using RF power.
- the active element 206may be electrically coupled to a power source, such as the RF power source 240 .
- FIGS. 28 - 31is configured to selectively electrically insulate and/or physically isolate the active element 206 from the patient's tissue 214 .
- the active element 206may be selectively insulated and/or isolated from the patient's tissue 214 by means of an insulating sleeve 802 that is configured to at least partially surround and at least partially cover the active element 206 , as most clearly shown in the cross-sectional view of FIG. 31.
- the insulating sleeve 802is further configured to slide over the active element 206 and to loosely cover the element 206 , as shown in FIG. 29.
- the sleeve 802When the active element 206 is fully energized, the sleeve 802 may be retracted in the proximal direction (toward the physician) using an appropriate actuator such as the insulating sleeve actuator 357 for example, thereby exposing the active element 206 to the patient's tissue and enabling deployment of the active element 206 , as shown in FIG. 30.
- the insulating and retractable sleeve 802may include or be formed of a flexible material, such as a silicone elastomer (such as polydimethyl siloxane, for example).
- the active element 206may be retracted within the window 204 defined within the probe body and the insulating sleeve 802 advanced in the distal direction (i.e., toward the distal end 208 ). This may enable the operator to remove any char that may have adhered to the active element 206 without, however, removing the probe 800 from the patient.
- FIGS. 32 - 36show a still further embodiment of the present invention, in which the active element 206 of the electrosurgical device 900 is initially electrically insulated from the patient's tissue 214 not by an insulating sleeve, but by an external sheath 902 (FIGS. 32 - 35 ) or by the configuration or topology of the probe body 200 (FIG. 36).
- this embodiment of the electrosurgical device 900 of the present inventionincludes a probe body 202 defining a proximal end 260 and a distal end 208 .
- the device 900includes an active element 206 , which may include one or more wires and/or loops, or any other therapeutically effective element effective to cut, dissect, fulgurate, or induce hyper or hypothermia.
- the active element 206may be electrically coupled to a power source, such as the RF power source 240 .
- the device 900may also include a distal RF element 210 .
- the device 900may also include an insulating sheath 902 disposed over at least a portion of the probe body 202 .
- the sheath 902may include a cutaway portion 910 that may be aligned with and expose the underlying window 204 defined within the probe body 202 . As shown in FIG.
- the insulating sheath 902electrically insulates the active element 206 from the patient's tissue 214 , whereas the sheath 902 does not interfere with the active element 206 when the active element 206 is in its extended configuration, as shown in FIG. 34. Suction may be advantageously employed here to clear the operative site from electrically conductive bodily fluids and/or smoke. As shown most clearly in the cross-sectional view of FIG. 35, the sheath 902 may have a generally circular cross section, and may include one or more raised portions 906 adjacent to the window 204 and substantially aligned therewith.
- the raised portion(s) 906enable the active element 206 , in its retracted position, to be electrically insulated from the patient's tissue 214 by an insulating layer 904 measuring about 0.1 mm to about 5 mm in thickness, for example. In this manner, the active element 206 may be fully energized by the RF power source 240 before being brought into contact with the patient's tissue, with the accompanying advantages discussed above.
- the sheath 902may cover all or only a portion of the probe body 202 .
- FIG. 36shows another embodiment of the present invention, wherein the probe body alone is shaped so as to insulate the active element 206 from the patient's tissue 214 until the active element 206 is fully energized.
- the probe body 202may include one or more locally raised portions 907 adjacent to the window 204 from which the active element 206 extends.
- the raised portion(s) 907may be defined along the long sides of the window 204 or around the entire perimeter thereof.
- the raised portion(s) 907need not be continuous, as long as they serve to effectively electrically insulate the tissue 214 from the active element 206 until the active element 206 is fully energized. As shown in FIG. 36, the tissue 214 is separated from the non-extended active element 206 by the insulating (air) layer 904 by the raised portions 907 of the probe body 202 which prevent the tissue 214 from prolapsing into the window 204 and coming into contact with the active element 206 until the physician chooses to extend the active element 206 and begin cutting the patient's tissue 214 .
- any geometry of the sheath 902 and/or probe body 202 and/or active element that forms an insulating layer between the active element and the patient's tissue when the active element is in its non-deployed statefalls into the scope of the present invention.
- any structure that prevents the tissue 214 from prolapsing into the window 204 or that generally prevents the active element 206 from coming into contact with the tissue until the active element 206 is fully energizedalso falls within the scope of the present invention.
- the present inventionis not limited to any particular active element. Indeed, although the embodiments of the present invention are herein illustrated with an extendible loop active element, the present invention is not so limited.
- An important aspect of the present inventionis the electrical insulation and/or physical isolation of the active element of an electrosurgical device from the patient's tissue until the active element is fully energized. This aspect is again illustrated in FIGS. 37 - 39 , in which the active element is shown as having a ring shape and the probe body little more than a hollow cannula.
- the electrosurgical device 1000 shown in FIGS. 37 - 39includes a probe body 1002 that defines a proximal end 260 and a distal end 1012 .
- An active element actuator 236is configured to extend and retract a ring-shaped RF element 1006 from and back into the distal end 1012 of the probe body 1002 .
- the ring-shaped active element 1006is electrically coupled to the RF power source 240 and mechanically coupled to the actuator 236 by means of rod 1004 or by other means known to those of skill in this art.
- FIG. 38shows the distal end 1012 of the probe body 1002 and the ring-shaped active element 1006 in its retracted position.
- the active element 1006is electrically insulated from the patient's tissue (not shown) by virtue of its recessed position (recessed by a distance of about 0.1 mm to about 5 mm, for example) within the probe body 1002 .
- Suctionmay be employed to maintain the recess clear of bodily fluids. This enables the physician to insure that the active element 1006 is fully energized before the active element 1006 is deployed or extended (see FIG. 39) out of the distal end 1012 of the probe body 1002 and comes into contact with the patient's tissue.
- a mesh or other membrane 1010may be attached to and trail the active element 1006 to collect tissue cut by the active RF element 1006 .
- This meshmay be expandable, to enable the collection of a relatively large mass of tissue.
- the ring-shaped active element 1006may itself be expandable in diameter or deformed in shape so as to enable collection of large or irregularly shaped lesions or specimens.
Landscapes
- Health & Medical Sciences (AREA)
- Surgery (AREA)
- Engineering & Computer Science (AREA)
- Life Sciences & Earth Sciences (AREA)
- Biomedical Technology (AREA)
- Otolaryngology (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Plasma & Fusion (AREA)
- Physics & Mathematics (AREA)
- Heart & Thoracic Surgery (AREA)
- Medical Informatics (AREA)
- Molecular Biology (AREA)
- Animal Behavior & Ethology (AREA)
- General Health & Medical Sciences (AREA)
- Public Health (AREA)
- Veterinary Medicine (AREA)
- Surgical Instruments (AREA)
Abstract
Description
- 1. Field of the Invention[0001]
- The present invention relates primarily to the field of electrosurgery. In particular, the present invention relates to the field of radiofrequency (RF) electrosurgery, including RF cautery, RF dissection, RF fulguration or coagulation, RF desiccation or any other forms of RF-assisted electrosurgery.[0002]
- 2. Description of the Related Art[0003]
- Electrosurgery generally refers to the use of electrically energized surgical devices to operate upon a patient. Common electrosurgical devices include electrocautery devices in which a direct current (DC) is caused to flow through a wire loop. The resistance of the wire causes the loop to heat up, thereby enabling the cauterization of the tissue.[0004]
- More commonly, electrosurgical devices (whether monopolar or bipolar) utilize alternating current (AC) in the range of about 200 kHz to about 3.3 MHz (hereafter “RF”) that is applied to the patient via a RF electrode. Such RF electrosurgical instruments are typically used to dissect (cut), fulgurate (coagulate) or desiccate (dry out) tissue by selectively varying the power and duty cycle of the RF signal applied to the electrode of the electrosurgical instrument. When the RF electrosurgical instrument is monopolar, a large low impedance return (dispersive) electrode is affixed to the patient to provide the current return path to ground. A bipolar RF electrosurgical instrument has two electrodes, the active electrode and the return electrode. The flow of current occurs between these two electrodes, thus obviating the need for a large low impedance return electrode. An insulated RF generator is typically used to energize the RF electrode(s) of the RF electrosurgical instrument.[0005]
- The density of the current delivered to the active electrode may be affected by the impedance (a vector quantity consisting of the tissue's resistance and its reactance) of the tissue. The impedance of the tissue to which the electrodes are exposed has an important effect upon the functioning of RF electrosurgical devices. This is because the tissue becomes an integral part of the circuit comprising the RF generator, the active electrode, the patient's tissue, the return electrode and back to the RF generator to ground or a reference voltage. Therefore, a voltage controlled or limited RF generator (generally providing power between about 10 W and 200 W) is generally used, so that when the impedance of tissue rises, the current delivered to the active electrode safely decreases. The density and impedance of tissue affects the manner in which it is affected by the active electrode. Tissues with higher salt content and body fluids such as blood have a high conductance and therefore, lower impedance. Fatty tissues have a lower fluid content and generally exhibit relatively higher impedance than leaner tissues. Thus, for a given voltage, fatty tissue will dissipate less current than leaner tissue. Highly vascularized tissues, on the other hand, have very low impedance and tend to conduct current more efficiently than less vascularized tissues. Therefore, to achieve the same tissue effect, current must be applied to a RF electrode in contact with highly vascularized tissue for a longer period of time than would be the case if the RF electrode were in contact with comparatively less vascularized tissue. Alternatively, the initial power applied to the RF electrode may need to be momentarily increased to achieve the same tissue effect in a fixed period of time.[0006]
- FIGS.[0007]1-3 show a RF
excisional device 100 that includes aprobe 102, thedistal end 108 of which includes a conductive ribbon orwire loop 106 configured to bow out of and back into awindow 104 defined within theprobe 102. Theloop 106 is connected to a RF power supply (not shown). Theloop 106 is energized with RF energy and theprobe 102 is rotated as shown at112 with theloop 106 in an extended or deployed configuration, as shown in FIG. 3. As theprobe 102 is rotated, a volume of revolution of tissue is cut by the RF-energizedloop 106. - However, when the[0008]
loop 106 is in contact with the tissue to be cut, the physician often must wait a relatively long period of time (several seconds or longer) between initial application of the RF power to theloop 106 and the time at which the current density within theloop 106 is high enough to cut (vaporize) the tissue. This is because the relatively low impedance of the interface between theloop 106 and the patient's tissue shown at114 in FIG. 5 dissipates a substantial amount of the current delivered to theloop 106. Highly vascularized tissue may also increase the current conduction (symbolized by the arrows emanating from theloop 106 in FIG. 5) away from theloop 106 and further delay the time at which theRF loop 106 is able to cut through thetissue 114. Indeed, until thetissue 114 chars and the impedance thereof increases, the current density within theloop 106 may not quickly reach that level at which theenergized loop 106 is therapeutically effective. - One solution to this problem is to initially increase or surge the power delivered to the[0009]
loop 106 to decrease the time required for the current density therein to reach the desired level. However, this is a less than optimal solution, as such an initial power surge may cause pain, excessive charring of thetissue 114 at the initial cut site, and may present a safety risk to the patient, who is better served by maintaining power levels as low as practicable to achieve the objective of the procedure. Such initial power surges, moreover, require specialized RF generators that are configured to deliver such surges safely and on demand. - What are needed, therefore, are improved methods and RF electrosurgical devices that do not suffer from the aforementioned disadvantages. More particularly, what are needed are RF electrosurgical devices that energize quickly, cut more efficiently and less painfully, reduce tissue charring at the initial cut site, and do not require an initial power surge from a specialized RF generator.[0010]
- It is, therefore, an object of the present invention to provide methods and RF electrosurgical instruments that do not suffer from the aforementioned disadvantages. It is also an object of the present invention to provide RF electrosurgical instruments that energize quickly, are safer, cut more efficiently and less painfully, reduce tissue charring at the initial cut site and do not require an initial power surge from a specialized RF generator.[0011]
- In accordance with the above-described objects and those that will be mentioned and will become apparent below, an electrosurgical device according to an embodiment of the present invention, includes a body, the body defining an outer surface, a proximal end, a distal end and a window defined within the outer surface; an electrically insulating layer and an active element, the active element being adapted to electrically connect to a power source and configured to selectively assume a non-deployed configuration in which the insulating layer electrically insulates the active element from a patient's tissue when the device is in use and a variable deployed configuration in which the active element at least partially emerges from the window out of the body to make contact with the patient's tissue.[0012]
- The active element may include one of a ribbon and wire, said one of ribbon and wire being adapted to selectively bow out of the window when transitioning from the non-deployed configuration to the deployed configuration. A tissue collection means may be attached thereto or trail behind the active element, as disclosed in co-pending and commonly assigned U.S. patent application Ser. No. 09/565,611 filed on May 4, 2000 and/or commonly assigned U.S. Pat. No. 6,022,362 filed on Sept. 3, 1998, the disclosure of each being incorporated herein by reference in its entirety.[0013]
- The active element may be configured to carry tissue ablation, dissection, fulguration, cautery, desiccation, hyperthermia or hypothermia, for example. The thickness of the insulating layer may be selected within a range of about 0.1 mm to about 5 mm, for example. The power source may include a RF power source. The window may be disposed near the distal end of the body or at the distal end thereof. The active element may be ring-shaped and may include a trailing tissue collection means attached thereto adapted to collect tissue cut by the ring-shaped active element. The insulating layer may include an electrically insulating sliding door mounted within the body, the sliding door being configured to selectively cover at least a portion of the window. The sliding door may be configured to slide between a variable first position in which at least a portion of the active element is exposed to the patient's tissue through the window and a second position in which the sliding door electrically insulates the active element within the body from the patient's tissue. The insulating layer may include a layer of air and the window may be configured to maintain the layer in the non-deployed configuration. The insulating layer may include an electrically insulating outer sleeve configured to selectively slide over the outer surface of the body, the outer sleeve being configured to at least partially cover the active element to electrically insulate the active element from the patient's tissue and uncover the active element to expose the active element to the patient's tissue. The insulating layer further may include an electrically insulating sheath disposed around at least a portion of the device body, the insulating sheath defining a cutaway portion aligned with the window and defining one or more raised portions configured to prevent the patient's tissue from prolapsing into the window when the active element is in its non-deployed configuration. The insulating layer may include an insulating sleeve disposed at least partially around the active element, the insulating sleeve being configured to controllably slide over the active element to selectively electrically insulate the active element from the patient's tissue and expose the active element thereto when the device is in use. The body may be shaped as a non-coring needle. The insulating layer may include a layer of air and the device body may define one or more raised portions adjacent the window, the raised portion(s) maintaining the layer of air between the active element and the patient's tissue when the active element is in its non-deployed state. Suction means may be included within the body for controllably removing fluids from the operative site within the patient's tissue.[0014]
- The present invention may also be viewed as a method of treating tissue using a probe, the probe including an active element that may be extendable out of and retractable back into a window defined in the probe, the active element being electrically connected to a power source, comprising the steps of: inserting the probe into the tissue; electrically insulating the active element from the tissue; energizing the active element using power from the power source, and exposing the energized active element to the tissue.[0015]
- The power source may be adapted to supply RF power to the active element. The insulating step may include a step of maintaining an electrically insulating layer between the active element and the tissue. The probe further may include an electrically insulating sliding door and the insulating step may include a step of sliding the door across the window. The method may further comprise the step of inducing hyperthermia or hypothermia and necrosis in the tissue exposed to the active element. The probe further may include an electrically insulating outer sleeve adapted to slide over an outer surface of the probe and the insulating step may include a step of sliding the outer sleeve over the window. The probe further may include an insulating sheath around at least a portion of the probe body, the insulating sheath including at least one raised portion adjacent the window and the insulating step may include a step of maintaining the active element retracted within the window. The insulating step may include a step of maintaining the active element retracted within the window. The probe further may include an insulating sleeve adapted to slide over the active element and the insulating step may include a step of sliding the insulating sleeve over the active element. The insulating step may include a step of creating or maintaining a layer of air between the active element and the tissue. The probe further may include an inflatable balloon disposed adjacent the window, and the insulating step further may include a step of inflating the balloon to create a layer of air between the tissue and the active element.[0016]
- The present invention is also an electrosurgical device, comprising a device body, the body defining a proximal end and a distal end; an active element coupled to the body, the active element being adapted to be controllably energized from a power source; and means for selectively electrically insulating and exposing the active element from and to a patient's tissue when the device is in use.[0017]
- The active element may be configured to selectively assume a non-deployed configuration in which the active element is recessed within the body and a variable deployed configuration in which the active element at least partially emerges from the body. The active element may be configured to carry out tissue ablation, dissection, fulguration, cautery, desiccation, hyperthermia and/or hypothermia, for example. The active element may be configured to be energized by a RF power source, for example. The device body may define a window disposed near the distal end of the body, and the active element may be configured to selectively retract into and extend from the window. A window may be defined within the body and the insulating means may include an electrically insulating sliding door mounted within the body, the sliding door being configured to selectively cover at least a portion of the window. The sliding door may be configured to slide between a variable first position in which at least a portion of the active element is exposed through the window and a second position in which the sliding door electrically insulates the active element from the patient's tissue. The insulating means may be integral with the window and the window may be configured to prevent tissue from prolapsing therein and making physical and/or electrical contact with the active element when the active element is in a non-deployed configuration. The insulating means may include an electrically insulating outer sleeve configured to selectively slide over the outer surface of the body, the outer sleeve being configured to at least partially cover the active element and electrically insulate the active element from the patient's tissue when the device is in use and the active element is in a non-deployed configuration. The insulating means may include an electrically insulating sleeve disposed at least partially around the active element, the insulating sleeve being configured to controllably slide over the active element to selectively electrically insulate the active element from the patient's tissue and expose the active element thereto when the device is in use. The insulating means may be integral with the body and may define one or more raised portions, the raised portion(s) being configured to prevent tissue from making physical and/or electrical contact with the active element until the active element is deployed. The insulating means may include an electrically insulating sheath disposed over the body, the insulating sheath defining at least one raised portion configured to prevent the patient's tissue from coming into contact with the active element until the active element is deployed. The active element may be shaped as a ring and the insulating means may be integral with the distal end of the body, the ring-shaped active element being configured to be recessed away from the distal end of the body until fully energized.[0018]
- The present invention is also an electrosurgical device adapted for insertion into tissue, comprising: a device body; a RF energizable active element disposed within the device body, and an insulating structure. The device body and/or the insulating structure may be configured to insulate the active element from the tissue until the active element is sufficiently energized to be therapeutically effective when the active element is brought into contact with the tissue.[0019]
- FIG. 1 is a top view of the distal end of an expandable loop excisional device.[0020]
- FIG. 2 is a side view of the distal end of the excisional device of FIG. 1, in which the loop is in its non-extended (non-deployed) configuration.[0021]
- FIG. 3 is a side view of the distal end of the excisional device of FIG. 1, in which the loop is in its extended (deployed) configuration.[0022]
- FIG. 4 is a cross-sectional view of the excisional device of FIG. 2, taken along cross-sectional lines AA′.[0023]
- FIG. 5 shows the excisional device of FIG. 4 in which the loop is in contact with tissue, to illustrate the current dissipation occurring at the loop-tissue interface.[0024]
- FIG. 6 shows an embodiment of a RF electrosurgical device, according to an embodiment of the present invention.[0025]
- FIG. 7 shows the distal end of the RF electrosurgical device of FIG. 6, in which the active element thereof is in its non-extended (non-deployed) configuration.[0026]
- FIG. 8 shows the distal end of the RF electrosurgical device of FIG. 7, in which the active element thereof is in its extended (deployed) configuration.[0027]
- FIG. 9 is a cross-sectional view of the excisional device of FIG. 7, taken along cross-sectional lines BB′.[0028]
- FIG. 10 shows the excisional device of FIG. 9 and illustrates the absence of active element-tissue interface when the RF electrosurgical device of the present invention is initially inserted in tissue and RF power applied to the active element.[0029]
- FIG. 11 shows another embodiment of a RF electrosurgical device, according to the present invention.[0030]
- FIG. 12 shows the distal end of the RF electrosurgical device of FIG. 11, in which the active element thereof is in a non-extended (non-deployed configuration).[0031]
- FIG. 13A shows the distal end of the RF electrosurgical device of FIG. 11, in which the active element thereof is in a first illustrative extended (deployed configuration).[0032]
- FIG. 13B shows the distal end of the RF electrosurgical device of FIG. 11, in which the active element thereof is in a second illustrative extended (deployed configuration).[0033]
- FIG. 14 is a cross-sectional view of the excisional device of FIG. 12, taken along cross-sectional lines CC′. FIG. 15 shows the excisional device of FIG. 14 and illustrates the absence of active element-tissue interface when the RF electrosurgical device of the present invention is initially inserted in tissue and RF power is applied to the active element.[0034]
- FIG. 16 is a top view of another embodiment of an electrosurgical device according to the present invention.[0035]
- FIG. 17 shows a side view of the distal end of the electrosurgical device of FIG. 16, in which the active element thereof is in its non-deployed configuration.[0036]
- FIG. 18 shows the electrosurgical device of FIG. 17, in which the active element thereof is in its deployed configuration.[0037]
- FIG. 19 shows a side view of another embodiment of an electrosurgical device according to the present invention, in which the active element thereof is in its non-deployed configuration.[0038]
- FIG. 20 shows the electrosurgical device of FIG. 19, in which the active element thereof is in its deployed configuration.[0039]
- FIG. 21 is a top view of the distal end of an electrosurgical device according to a still further embodiment of the present invention.[0040]
- FIG. 22A is a side view of the device of FIG. 21, in which the inflatable balloon(s) thereof has (have) been inflated to create an insulating layer between the active element and the patient's tissue.[0041]
- FIG. 22B is a front view of the device of FIG. 22A, in which the balloons are in their inflated configuration.[0042]
- FIG. 23 is a side view of the device of FIG. 21, after deflation of the balloon(s) and deployment of the active element.[0043]
- FIG. 24 shows a top view of the distal end of an electrosurgical device according to another embodiment of the present invention.[0044]
- FIG. 25 shows a side view of the device of FIG. 24.[0045]
- FIG. 26 shows the device of FIG. 25, after the outer sleeve thereof has been slid away from the window from which the active element is deployed.[0046]
- FIG. 27 is a flowchart of a method for carrying out an electrosurgical procedure according to an embodiment of the present invention.[0047]
- FIG. 28 is a top view of an electrosurgical device, according to another embodiment of the present invention.[0048]
- FIG. 29 is a side view of the distal portion of the device of FIG. 28, showing the active element thereof in its non-extended and sheathed state.[0049]
- FIG. 30 is a side view of the distal portion of the device of claim 28, showing the active element thereof in its extended and unsheathed state.[0050]
- FIG. 31 is a cross-sectional view of the electrosurgical device of FIG. 29, taken along cross-sectional lines DD′.[0051]
- FIG. 32 is a top view of an electrosurgical device, according to another embodiment of the present invention.[0052]
- FIG. 33 is a side view of the distal portion of the device of FIG. 32.[0053]
- FIG. 34 is a side view of the distal portion of the device of FIG. 32, in which the active element is in its extended state.[0054]
- FIG. 35 is a cross-sectional view of the electrosurgical device of FIG. 33, taken along cross-sectional lines EE′.[0055]
- FIG. 36 shows a cross-sectional view of another embodiment of the electrosurgical device of FIG. 33, taken along cross sectional lines EE′[0056]
- FIG. 37 shows another electrosurgical device, according to yet another embodiment of the present invention.[0057]
- FIG. 38 is a perspective side view of the distal portion of the electrosurgical device of FIG. 37, in which the electrosurgical ring is in its non-extended state.[0058]
- FIG. 39 is a perspective side view of the distal portion of the electrosurgical device of FIG. 37, in which the electrosurgical ring is in its extended state.[0059]
- FIGS.[0060]6-10 show an embodiment of a RF
electrosurgical device 200, according to the present invention. As shown therein, thedevice 200 includes aprobe body 202, theprobe body 202 defining an outer surface, aproximal end 230, adistal end 208 and a window204 (shown in dashed lines in FIGS. 7 and 8) defined within the outer surface near thedistal end 208. Theprobe 200 includes anactive element 206. Theactive element 206 may include, for example one or more wires and/or loops, or any other therapeutically effective RF element effective to cut, dissect, fulgurate and/or desiccate tissue (for example) using RF power. Theactive element 206 may be electrically connected to a power source, such as theRF power source 240. Adistal RF element 210 may also be mounted to thedistal end 208 of theprobe body 202, to facilitate penetration of theprobe 200 into the patient's tissue. Thedistal RF element 210 may also be independently electrically connected to theRF power source 240. According to the present invention, theactive element 206 may be configured to selectively assume a non-deployed configuration (FIG. 7) and a variable deployed configuration (FIG. 8). In the variable deployed configuration (FIG. 8), theactive element 206 at least partially emerges from thewindow 204 out of theprobe body 202. The deployment of theactive element 206 may be controlled by the physician by means of anactuator 236, such as the thumb wheel shown in FIG. 6 or other actuation means. By turning theactuator 236 with his or her thumb, the physician causes theactive element 206 to slide within aguide 326 defined within theprobe 202. As the distal end of theactive element 206 is attached to thedistal end 208 of theprobe 200, theactive element 206 bows out of thewindow 204, the degree of bowing and deployment out of thewindow 204 being controlled by theactuator 236. - In the non-deployed configuration, the[0061]
active element 206 is recessed within theprobe body 202 to define an insulating layer212 (a layer of air, for example) with respect to the outer surface of theprobe body 202. The recessedactive element 206 and/or the presence of the insulatinglayer 212 enables theactive element 206 to be energized without contacting the patient's tissue. Indeed, by avoiding contact with the patient's tissue during the energizing of theactive element 206, the current delivered to theactive element 206 is not dissipated by the patient's tissue that would otherwise be in contact therewith but for the presence of the insulatinglayer 212. According to the present invention, after theactive element 206 is energized, theactive element 206 may be deployed to (for example) cut, ablate or coagulate (depending upon the application) the patient's tissue without the physician having to wait for the tissue impedance to rise sufficiently (while the patient's tissue heats up and/or chars) to enable the current density within theactive element 206 to reach the desired level for the procedure to be carried out. FIGS. 9 and 10 show a cross-sectional view of theprobe 200, taken along the cross-sectional lines BB′of FIG. 7. As shown therein, to electrically insulate theactive element 206 from the patient's tissue (reference 214 in FIG. 10), thewindow 204 defined within theprobe body 202 may be shaped and/or dimensioned so as to prevent thetissue 214 through which theprobe 200 is inserted from prolapsing therein. As shown, thewindow 204 is configured to define an electrically insulatinglayer 212 between theactive element 206 and the patient'stissue 214 when theactive element 206 is in its non-deployed (retracted) configuration. Toward that end, thewindow 204 may define a steep aspect ratio, in which the depth of thewindow 204 is greater than its width. - FIGS.[0062]11-15 show a
probe 300 according to another embodiment of the present invention. Reference numerals that are identical to those shown in FIGS.6-10 denote identical structures. For the sake of brevity, the description of those identical structures will not be explicitly repeated here. In FIGS.11-15, the insulating layer that electrically insulates theactive element 206 from the patient'stissue 214 includes a slidingdoor 216 disposed within theprobe body 202. The slidingdoor 216 may be made of an electrical insulator or other substantially non-conductive material or materials. The slidingdoor 216 is configured to selectively cover at least a portion of thewindow 204 to electrically insulate theactive element 206 of thedevice 300 from the patient'stissue 214 until theactive element 206 is fully energized and/or until the physician chooses to expose theactive element 206 to the patient'stissue 214. The physician, according to the present invention, may cause the slidingdoor 216 to slide and expose theactive element 206 by means of a sliding door actuator such as a manual thumb wheel, as shown at356. It is understood that other means of acting upon the slidingdoor 216 and/oractive element 206 may be employed, as the present invention is not limited to thumb wheel actuators, as shown at356 and236. According to the present invention, the slidingdoor 216 is configured to slide between a variable first position in which at least a portion of the active element is exposed through the window (as shown in FIGS. 13A and 13B) and a second position (shown in FIGS. 11, 12,14 and15) in which the slidingdoor 216 insulates theactive element 206 within theprobe body 202. As shown in FIGS. 13A and 13B, the slidingdoor 216 may be caused to expose a selectable portion of theactive element 206 to the patient'stissue 214. In so doing, the slidingdoor 216 may affect the shape of the deployed active element: if the slidingdoor 216 is fully or near fully retracted as shown in FIG. 13A, a greater portion of theactive element 206 may emerge from thewindow 204 than is the case wherein the slidingdoor 216 is only partially retracted and only exposes a portion of thewindow 204, as shown in FIG. 13B. This may enable the physician to control the extent of deployment and the shape of the extendedactive element 206. FIGS. 14 and 15 show the slidingdoor 216 as it covers thewindow 204, thereby electrically insulating theactive element 206 from the patient'stissue 214. The slidingdoor 216 may be guided within theprobe body 202 by means of, for example, internal guides or rails218. Using this configuration, the physician may apply RF power to theactive element 206 and begin the intended RF procedure (cutting, ablating, coagulating, etc.) sooner than would otherwise be the case if the de-energized active element were energized while in contact with the patient'stissue 214. The layer ofair 213 between theactive element 206 and the slidingdoor 216 may be reduced or eliminated altogether, as the slidingdoor 216 electrically insulates and physically isolates theactive element 206 from the patient'stissue 214. The slidingdoor 216, for that purpose, is preferably formed of non-conductive material, such as a non-conductive plastic or ceramic, for example. - FIGS.[0063]16-18 show another embodiment of the present invention. As shown therein, the
electrosurgical device 400 includes aproximal end 260, adistal end 208, aprobe body 202, anactive element actuator 236 and a slidingdoor actuator 356, as described above. The distal tip of the probe body defines anopening 290 that is aligned with aninternal lumen 282 defined within theprobe body 202. The exemplaryactive element 218 shown in FIGS.16-18 includes an elongated conductive stem that is distally terminated by a conductive bulb. However, any electrically active element may be used in conjunction with or in place of the active element depicted in FIGS.16-18. Theactive element 218 may be electrically coupled to theRF power source 240. The bulb may be used, for example, to induce hyperthermia and/or necrosis in thetissue 214 with which it comes into contact. In that case, the duty cycle of the RF current applied to theactive element 218 may be adjusted to be, for example, 50%-25%, so as to induce substantial heating in thetissue 214 in contact with theactive element 218. In this embodiment, the insulating layer separating theactive element 218 from the patient'stissue 214 when theactive element 206 is in its non-deployed configuration may include a sliding and electrically insulatingdoor 220 mounted within theprobe body 202. The slidingdoor 220 may be configured to slide axially in the distal and proximal directions (away from and toward the physician, respectively, when thedevice 400 is in use) when the slidingdoor actuator 356 is actuated. The distal end of the slidingdoor 220 may be advantageously configured to cover (see FIG. 17) theopening 290 during insertion of thedevice 400 into the patient'stissue 214 and during the energizing of theactive element 218. After thedevice 400 has been inserted up to the targeted site within the patient'stissue 214 and theactive element 218 has been energized, the physician may actuate the slidingdoor actuator 356 to retract the slidingdoor 220 away from theopening 290, in theproximal direction 294. Thereafter, the energizedactive element 218 may be advanced in thedistal direction 296 into the target area of the patient'stissue 214 to begin treatment thereof, as shown in FIG. 18. Alternatively, the slidingdoor 220 may be retracted before theactive element 218 is energized. Thereafter, theactive element 218 may be energized without dissipating current into the surroundingtissue 214, as theactive element 218 is still recessed and electrically insulated from the patient's tissue by anair layer 215. The shape and/or size of theopening 290 and/or the thickness of theair layer 215 may also prevent the patient'stissue 214 from prolapsing into theopening 290, thereby further insulating theactive element 218. As shown in FIGS. 16 and 17, theactive element 218 is fully insulated from the patient'stissue 214 by the slidingdoor 220 until theactive element 218 is appropriately energized, thereby preventing any disadvantageous dissipation of RF current into thetissue 214 upon the application of RF current to theactive element 218. - FIGS.[0064]19-20 show a still further embodiment of the present invention. As shown therein, the
electrosurgical device 500 includes aprobe body 202 defining aproximal end 230, adistal end 208 and aninternal lumen 217. Theproximal end 230 includes anactive element actuator 236, as described above. Anactive element 218 is configured to slide within theinternal lumen 217, as controlled by theactive element actuator 236. Most any RF active element may be used within theelectrosurgical device 500. For simplicity's sake, however, the stem and bulbactive element 218 of FIGS.16-18 is again used here, it being understood that this embodiment is not limited thereby. As shown in FIGS.19-20, thedistal end 208 of theprobe body 202 may be shaped as a non-coring needle. As shown in FIG. 19, theactive element 218 may be recessed within theinternal lumen 217 of the probe body to define an electrically insulatinglayer 219. Thelayer 219 may also physically isolate theactive element 218 from the patient's tissue when the active element is in its non-deployed state, as shown in FIG. 19. When thedevice 500 is properly positioned within the patient (as verified, for example, through the use of ultrasound) and theactive element 218 energized, theactive element actuator 236 may be used to advance the energizedactive element 218 in thedistal direction 296 until theactive element 218 emerges from thedistal opening 292 and extends to the desired length, as shown in FIG. 20. The insulating layer219 (FIG. 19) between theactive element 218 and theopening 294 defined in the distal end of the probe body202 (and/or the tissue214) prevents any of the RF current applied to theactive element 218 from dissipating into the patient'stissue 214 during the energizing of theactive element 218. - Some or all of the through[0065]
holes 938 may be utilized for the delivery of a fluid to the patient during the procedure, such as antibiotic agents, analgesic agents or most any pharmaceutical agent. Such agents may be administered to the patient from theport 942. Alternatively, theport 942 may be coupled to suction and some or all of the throughholes 938 may be utilized to suction out the operative site of smoke, blood or other bodily fluids during or after the operative procedure. Alternatively still, more than oneport 942 may be provided in theproximal portion 902 and more than oneinternal lumen 940 may be defined along the length of theprobe 500. The additional lumen may be in fluid communication with selected throughholes 938. By this structure, both delivery of a pharmaceutical agent and suctioning may be provided within asingle probe 500. It is to be understood that any of the devices according to the present invention may incorporate all or a portion of the structures referenced by938,940 and942 or like structures to provide suctioning and/or fluid delivery functionality. - FIGS.[0066]21-23 shows the distal portion of a still further embodiment of the present invention. As shown therein, the
device 600 defines adistal end 208, awindow 204 and anactive element 206, as described above. Thedevice 600 also includes one or moreinflatable balloons 602. As best shown in FIG. 21, at least onesuch balloon 602 may advantageously be disposed in close proximity to and may be co-extensive with thewindow 204 from which theactive element 206 emerges and deploys. FIG. 22A shows thedevice 600 in a configuration wherein theactive element 206 is in its non-deployed configuration and theballoons 602 are in their inflated configuration. For example, theballoons 602 may be inflated with CO2(for example) from a CO2reservoir (not shown) located within or without thedevice 600. FIG. 22B shows a front view of the device of FIG. 22A and illustrates the insulatinglayer 212 between theactive element 206 and thetissue 214 created by the inflation of theballoons 602. Finally, FIG. 23 shows a side view of thedevice 600 in a configuration wherein theactive element 206 is deployed and theballoons 602 deflated, rendering them (preferably) flush or substantially flush with the outer surface of thedevice 600. In operation, thedevice 600 may be advanced through the patient'stissue 214 until the target area is reached. The balloon or balloons602 may then be inflated, thereby creating the insulatinglayer 212 between theactive element 206 and thetissue 214. Theactive element 206 may then be energized with RF current from a RF power supply, such as shown at240 in FIG. 16, for example. After energizing theactive element 206, theballoon 602 orballoons 602 may be deflated and the active element deployed, as described above. Once again, initially electrically insulating theactive element 206 from thetissue 214 advantageously prevents current from dissipating into thetissue 214 during the energizing of theactive element 206. Thereafter, theactive element 206 may be brought into contact with the patient'stissue 214 when theactive element 206 has been energized to a therapeutically effective level. - FIGS.[0067]24-26 show the distal portion of a still further embodiment of the present invention. As shown therein, the
electrosurgical device 700 includes aprobe body 202, awindow 204 defined within theprobe body 202 near thedistal end 208 thereof, and anactive element 206 within thewindow 204, as previously described. To electrically insulate theactive element 206 during the energizing thereof using a RF power source (such as shown at240 in FIG. 16), an electrically insulatingouter sleeve 702 is fitted over theprobe body 202. Theouter sleeve 702 is configured to slide over and/or against the outer surface of theprobe body 202 in the proximal and axial directions. Upon energizing theactive element 206, theouter sleeve 702 may advantageously be maintained in a position wherein it preventstissue 214 from coming into contact with theactive element 206. After theactive element 206 is energized, the physician may retract theouter sleeve 702 by sliding it in theproximal direction 294. By fully retracting theouter sleeve 702, the physician may expose the entireactive element 206 to thetissue 214. However, by only partially retracting theouter sleeve 702, as shown at702A, the physician may advantageously change the extent of deployment and/or shape of the active element, as shown at206A. - It should also be noted that the active element in FIGS.[0068]6-26 may also include a device to cause hypothermia and subsequent necrosis in the patient's tissue; i.e., to freeze the patient's tissue in contact with the active element. Indeed, by energizing such an active device while it is electrically insulated and/or physically isolated from the patient's
tissue 214, the temperature of the active element will drop to the intended low temperature faster than if the active element were initially in contact with the patient'stissue 214, especially if the tissue is highly vascular (and thus able to transport heat efficiently). - FIG. 27 is a flowchart of a method of carrying out an electrosurgical procedure, according to the present invention. Step SI calls for the insertion of a RF electrosurgical device, such as shown in FIGS.[0069]6-26, into the patient's tissue. Advantageously, ultrasound (or any other radiological) guidance may be employed to guide the device to the targeted site within the patient's tissue. Step S2 calls for electrically insulating (and/or physically isolating) the active element (such as shown at206,218) of the RF device from the surrounding tissue. The insulating step S2 may be carried out either before, during or after step S1, but should be carried out before step S3 that is, before energizing the active element. Insulating and/or electrically isolating the active element may entail ensuring the presence of an insulating layer (preferably about 0.1 mm to about 5 mm in thickness) by maintaining the active element recessed within the probe body, in its retracted, non-deployed configuration and/or sliding a sliding door (216,220) over the active element, inflating a balloon or balloons602 and/or maintaining an
outer sleeve 702 over the active element. Alternatively, any means of creating an insulatinglayer 212 and/or insulating the active element from the surrounding tissue during the energizing thereof may be employed within the scope of the present invention. Step S4 calls for exposing at least a portion of the energized active element to the patient's tissue and step S5 calls for carrying out the intended procedure, such as cutting, ablation, coagulation, etc. - FIGS.[0070]28-31 represent different views of an
electrosurgical device 800, according to another embodiment of the present invention. Considering now FIGS.28-31 collectively, thedevice 800 includes aproximal end 260, adistal end 208 and may include adistal RF element 210, as described above. Thedevice 800 includes anactive element 206, which may include one or more wires and/or loops, or any other therapeutically effective RF element effective to cut, dissect, fulgurate, induce hyper or hypothermia (from a different power source) and/or to desiccate tissue using RF power. Theactive element 206 may be electrically coupled to a power source, such as theRF power source 240. The embodiment illustrated in FIGS.28-31 is configured to selectively electrically insulate and/or physically isolate theactive element 206 from the patient'stissue 214. Indeed, theactive element 206 may be selectively insulated and/or isolated from the patient'stissue 214 by means of aninsulating sleeve 802 that is configured to at least partially surround and at least partially cover theactive element 206, as most clearly shown in the cross-sectional view of FIG. 31. The insulatingsleeve 802 is further configured to slide over theactive element 206 and to loosely cover theelement 206, as shown in FIG. 29. When theactive element 206 is fully energized, thesleeve 802 may be retracted in the proximal direction (toward the physician) using an appropriate actuator such as the insulatingsleeve actuator 357 for example, thereby exposing theactive element 206 to the patient's tissue and enabling deployment of theactive element 206, as shown in FIG. 30. The insulating andretractable sleeve 802 may include or be formed of a flexible material, such as a silicone elastomer (such as polydimethyl siloxane, for example). During the procedure, theactive element 206 may be retracted within thewindow 204 defined within the probe body and the insulatingsleeve 802 advanced in the distal direction (i.e., toward the distal end208). This may enable the operator to remove any char that may have adhered to theactive element 206 without, however, removing theprobe 800 from the patient. - FIGS.[0071]32-36 show a still further embodiment of the present invention, in which the
active element 206 of theelectrosurgical device 900 is initially electrically insulated from the patient'stissue 214 not by an insulating sleeve, but by an external sheath902 (FIGS.32-35) or by the configuration or topology of the probe body200 (FIG. 36). Considering FIGS.32-35 collectively, this embodiment of theelectrosurgical device 900 of the present invention includes aprobe body 202 defining aproximal end 260 and adistal end 208. Thedevice 900 includes anactive element 206, which may include one or more wires and/or loops, or any other therapeutically effective element effective to cut, dissect, fulgurate, or induce hyper or hypothermia. Theactive element 206 may be electrically coupled to a power source, such as theRF power source 240. Thedevice 900 may also include adistal RF element 210. As shown, thedevice 900 may also include an insulatingsheath 902 disposed over at least a portion of theprobe body 202. According to the present invention, thesheath 902 may include acutaway portion 910 that may be aligned with and expose theunderlying window 204 defined within theprobe body 202. As shown in FIG. 33, when theactive element 206 is in its retracted position, the insulatingsheath 902 electrically insulates theactive element 206 from the patient'stissue 214, whereas thesheath 902 does not interfere with theactive element 206 when theactive element 206 is in its extended configuration, as shown in FIG. 34. Suction may be advantageously employed here to clear the operative site from electrically conductive bodily fluids and/or smoke. As shown most clearly in the cross-sectional view of FIG. 35, thesheath 902 may have a generally circular cross section, and may include one or more raisedportions 906 adjacent to thewindow 204 and substantially aligned therewith. The raised portion(s)906 enable theactive element 206, in its retracted position, to be electrically insulated from the patient'stissue 214 by an insulatinglayer 904 measuring about 0.1 mm to about 5 mm in thickness, for example. In this manner, theactive element 206 may be fully energized by theRF power source 240 before being brought into contact with the patient's tissue, with the accompanying advantages discussed above. Thesheath 902 may cover all or only a portion of theprobe body 202. - FIG. 36 shows another embodiment of the present invention, wherein the probe body alone is shaped so as to insulate the[0072]
active element 206 from the patient'stissue 214 until theactive element 206 is fully energized. As shown therein, theprobe body 202 may include one or more locally raisedportions 907 adjacent to thewindow 204 from which theactive element 206 extends. The raised portion(s)907 (raised about 0.5 mm to about 5 mm away from the outer surface of the probe body, for example) may be defined along the long sides of thewindow 204 or around the entire perimeter thereof. The raised portion(s)907 need not be continuous, as long as they serve to effectively electrically insulate thetissue 214 from theactive element 206 until theactive element 206 is fully energized. As shown in FIG. 36, thetissue 214 is separated from the non-extendedactive element 206 by the insulating (air)layer 904 by the raisedportions 907 of theprobe body 202 which prevent thetissue 214 from prolapsing into thewindow 204 and coming into contact with theactive element 206 until the physician chooses to extend theactive element 206 and begin cutting the patient'stissue 214. - Any geometry of the[0073]
sheath 902 and/or probebody 202 and/or active element that forms an insulating layer between the active element and the patient's tissue when the active element is in its non-deployed state falls into the scope of the present invention. Likewise, any structure that prevents thetissue 214 from prolapsing into thewindow 204 or that generally prevents theactive element 206 from coming into contact with the tissue until theactive element 206 is fully energized also falls within the scope of the present invention. - The present invention, moreover, is not limited to any particular active element. Indeed, although the embodiments of the present invention are herein illustrated with an extendible loop active element, the present invention is not so limited. An important aspect of the present invention is the electrical insulation and/or physical isolation of the active element of an electrosurgical device from the patient's tissue until the active element is fully energized. This aspect is again illustrated in FIGS.[0074]37-39, in which the active element is shown as having a ring shape and the probe body little more than a hollow cannula. The
electrosurgical device 1000 shown in FIGS.37-39 includes aprobe body 1002 that defines aproximal end 260 and adistal end 1012. Anactive element actuator 236 is configured to extend and retract a ring-shapedRF element 1006 from and back into thedistal end 1012 of theprobe body 1002. The ring-shapedactive element 1006 is electrically coupled to theRF power source 240 and mechanically coupled to theactuator 236 by means ofrod 1004 or by other means known to those of skill in this art. FIG. 38 shows thedistal end 1012 of theprobe body 1002 and the ring-shapedactive element 1006 in its retracted position. In accordance with the present invention, theactive element 1006 is electrically insulated from the patient's tissue (not shown) by virtue of its recessed position (recessed by a distance of about 0.1 mm to about 5 mm, for example) within theprobe body 1002. Suction may be employed to maintain the recess clear of bodily fluids. This enables the physician to insure that theactive element 1006 is fully energized before theactive element 1006 is deployed or extended (see FIG. 39) out of thedistal end 1012 of theprobe body 1002 and comes into contact with the patient's tissue. As shown in FIG. 39, a mesh orother membrane 1010 may be attached to and trail theactive element 1006 to collect tissue cut by theactive RF element 1006. This mesh may be expandable, to enable the collection of a relatively large mass of tissue. Likewise, the ring-shapedactive element 1006 may itself be expandable in diameter or deformed in shape so as to enable collection of large or irregularly shaped lesions or specimens. - While the foregoing detailed description has described preferred embodiments of the present invention, it is to be understood that the above description is illustrative only and not limiting of the disclosed invention. Those of skill in this art will recognize other alternative embodiments and all such embodiments are deemed to fall within the scope of the present invention. Thus, the present invention should be limited only by the claims as set forth below.[0075]
Claims (42)
Priority Applications (9)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US09/732,848US20020072739A1 (en) | 2000-12-07 | 2000-12-07 | Methods and devices for radiofrequency electrosurgery |
| CA002436844ACA2436844A1 (en) | 2000-12-07 | 2001-12-03 | Methods and devices for radiofrequency electrosurgery |
| JP2002547396AJP3806088B2 (en) | 2000-12-07 | 2001-12-03 | Equipment for high frequency electrosurgery |
| NZ526314ANZ526314A (en) | 2000-12-07 | 2001-12-03 | Methods and devices for radiofrequency electrosurgery |
| PCT/US2001/046288WO2002045609A1 (en) | 2000-12-07 | 2001-12-03 | Methods and devices for radiofrequency electrosurgery |
| AU2002225886AAU2002225886B2 (en) | 2000-12-07 | 2001-12-03 | Methods and devices for radiofrequency electrosurgery |
| AU2588602AAU2588602A (en) | 2000-12-07 | 2001-12-03 | Methods and devices for radiofrequency electrosurgery |
| EP01995335AEP1339339A4 (en) | 2000-12-07 | 2001-12-03 | Methods and devices for radiofrequency electrosurgery |
| US10/349,659US7198626B2 (en) | 2000-12-07 | 2003-01-23 | Methods and devices for radiofrequency electrosurgery |
Applications Claiming Priority (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US09/732,848US20020072739A1 (en) | 2000-12-07 | 2000-12-07 | Methods and devices for radiofrequency electrosurgery |
Related Child Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US10/349,659DivisionUS7198626B2 (en) | 2000-12-07 | 2003-01-23 | Methods and devices for radiofrequency electrosurgery |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| US20020072739A1true US20020072739A1 (en) | 2002-06-13 |
Family
ID=24945182
Family Applications (2)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US09/732,848AbandonedUS20020072739A1 (en) | 2000-12-07 | 2000-12-07 | Methods and devices for radiofrequency electrosurgery |
| US10/349,659Expired - Fee RelatedUS7198626B2 (en) | 2000-12-07 | 2003-01-23 | Methods and devices for radiofrequency electrosurgery |
Family Applications After (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US10/349,659Expired - Fee RelatedUS7198626B2 (en) | 2000-12-07 | 2003-01-23 | Methods and devices for radiofrequency electrosurgery |
Country Status (7)
| Country | Link |
|---|---|
| US (2) | US20020072739A1 (en) |
| EP (1) | EP1339339A4 (en) |
| JP (1) | JP3806088B2 (en) |
| AU (2) | AU2002225886B2 (en) |
| CA (1) | CA2436844A1 (en) |
| NZ (1) | NZ526314A (en) |
| WO (1) | WO2002045609A1 (en) |
Cited By (55)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2004002293A2 (en) | 2002-06-27 | 2004-01-08 | Arthrocare Corporation | Systems and methods for electrosurgical tissue treatment |
| US20040024396A1 (en)* | 1999-12-27 | 2004-02-05 | Eggers Philip E. | Electrosurgical accessing of tissue with controlled collateral thermal phenomena |
| US20060189971A1 (en)* | 1995-11-22 | 2006-08-24 | Arthrocare Corporation | Systems and methods for electrosurgical treatment of fasciitis |
| US20060200124A1 (en)* | 2002-06-14 | 2006-09-07 | Ncontact Surgical, Inc. | Vacuum coagulation probes |
| US20060217705A1 (en)* | 2005-02-17 | 2006-09-28 | Baylis Medical Company Inc. | Electrosurgical device with discontinuous flow density |
| US20060293646A1 (en)* | 2002-06-14 | 2006-12-28 | Whayne James G | Vacuum coagulation & dissection probes |
| US20070027449A1 (en)* | 2002-03-05 | 2007-02-01 | Baylis Medical Company Inc. | Electrosurgical device and methods |
| US20070156136A1 (en)* | 2002-03-05 | 2007-07-05 | Neil Godara | Methods of treating the sacroiliac region of a patient's body |
| US20070203402A1 (en)* | 2002-03-05 | 2007-08-30 | Baylis Medical | Elongate member providing a variation in radiopacity |
| US20070250058A1 (en)* | 2002-06-14 | 2007-10-25 | Ncontact Surgical, Inc. | Vacuum coagulation probes |
| US20080114354A1 (en)* | 2003-04-29 | 2008-05-15 | Ncontact Surgical, Inc. | Vacuum coagulation probes |
| US20080114355A1 (en)* | 2006-11-09 | 2008-05-15 | Ncontact Surgical, Inc. | Vacuum coagulation probes |
| US7429260B2 (en) | 1996-07-16 | 2008-09-30 | Arthrocare Corporation | Systems and methods for electrosurgical tissue contraction within the spine |
| US20080243119A1 (en)* | 2002-06-14 | 2008-10-02 | Ncontact Surgical, Inc. | Vacuum coagulation probe for atrial fibrillation treatment |
| US7445618B2 (en) | 1993-05-10 | 2008-11-04 | Arthrocare Corporation | Methods for tissue ablation using pulsed energy |
| US20090024124A1 (en)* | 2005-07-14 | 2009-01-22 | Lefler Amy | Methods for treating the thoracic region of a patient's body |
| US20090054962A1 (en)* | 2002-03-05 | 2009-02-26 | Baylis Medical Company Inc. | Methods for treating the thoracic region of a patient's body |
| US7507236B2 (en) | 1992-01-07 | 2009-03-24 | Arthrocare Corporation | System and method for electrosurgical cutting and ablation |
| US20100249499A1 (en)* | 2002-01-30 | 2010-09-30 | Power Medical Interventions, Llc | Surgical imaging device |
| US20100298820A1 (en)* | 2005-04-13 | 2010-11-25 | Joshua Ben-Nun | Thermal capsulotomy tool and system |
| US20100305562A1 (en)* | 2007-12-21 | 2010-12-02 | Winkler Matthew J | Ablation Device With Cooled Electrodes And Methods of Use |
| US8012153B2 (en) | 2003-07-16 | 2011-09-06 | Arthrocare Corporation | Rotary electrosurgical apparatus and methods thereof |
| US8298243B2 (en) | 2007-07-30 | 2012-10-30 | Tyco Healthcare Group Lp | Combination wire electrode and tube electrode polypectomy device |
| US8317786B2 (en) | 2009-09-25 | 2012-11-27 | AthroCare Corporation | System, method and apparatus for electrosurgical instrument with movable suction sheath |
| US20120303018A1 (en)* | 2011-05-23 | 2012-11-29 | Tyco Healthcare Group Lp | Tissue Dissectors |
| US8323279B2 (en) | 2009-09-25 | 2012-12-04 | Arthocare Corporation | System, method and apparatus for electrosurgical instrument with movable fluid delivery sheath |
| US8328803B2 (en) | 2008-01-31 | 2012-12-11 | Covidien Lp | Polyp removal device and method of use |
| US8355799B2 (en) | 2008-12-12 | 2013-01-15 | Arthrocare Corporation | Systems and methods for limiting joint temperature |
| US8663216B2 (en) | 1998-08-11 | 2014-03-04 | Paul O. Davison | Instrument for electrosurgical tissue treatment |
| US8696659B2 (en) | 2010-04-30 | 2014-04-15 | Arthrocare Corporation | Electrosurgical system and method having enhanced temperature measurement |
| US8747400B2 (en) | 2008-08-13 | 2014-06-10 | Arthrocare Corporation | Systems and methods for screen electrode securement |
| US20150126994A1 (en)* | 2012-12-12 | 2015-05-07 | Olympus Medical Systems Corp. | Puncture tool and ultrasound endoscope |
| US9526556B2 (en) | 2014-02-28 | 2016-12-27 | Arthrocare Corporation | Systems and methods systems related to electrosurgical wands with screen electrodes |
| US9597142B2 (en) | 2014-07-24 | 2017-03-21 | Arthrocare Corporation | Method and system related to electrosurgical procedures |
| US9649148B2 (en) | 2014-07-24 | 2017-05-16 | Arthrocare Corporation | Electrosurgical system and method having enhanced arc prevention |
| US20170202616A1 (en)* | 2016-01-15 | 2017-07-20 | Tva Medical, Inc. | Devices and methods for forming a fistula |
| US9949789B2 (en) | 2002-03-05 | 2018-04-24 | Avent, Inc. | Methods of treating the sacroiliac region of a patient's body |
| CN108348288A (en)* | 2015-10-28 | 2018-07-31 | 波士顿科学国际有限公司 | retractable tissue cutting device |
| US10363079B2 (en) | 2015-08-13 | 2019-07-30 | Medtronic Inc. | Electrosurgical method and apparatus with varying stiffness capture components |
| CN110585588A (en)* | 2014-05-16 | 2019-12-20 | 阿莱瓦神经治疗股份有限公司 | Implantable microelectrode device |
| US10603040B1 (en) | 2015-02-09 | 2020-03-31 | Tva Medical, Inc. | Methods for treating hypertension and reducing blood pressure with formation of fistula |
| US10646666B2 (en) | 2014-08-27 | 2020-05-12 | Tva Medical, Inc. | Cryolipolysis devices and methods therefor |
| US10695534B2 (en) | 2014-03-14 | 2020-06-30 | Tva Medical, Inc. | Fistula formation devices and methods therefor |
| US10786300B2 (en) | 2015-04-13 | 2020-09-29 | Carlos Fernando Bazoberry | Radiofrequency denervation needle and method |
| US10813685B2 (en) | 2014-09-25 | 2020-10-27 | Covidien Lp | Single-handed operable surgical instrument including loop electrode with integrated pad electrode |
| US10821217B2 (en) | 2013-03-14 | 2020-11-03 | Tva Medical, Inc. | Fistula formation devices and methods therefor |
| US10869717B2 (en) | 2012-10-11 | 2020-12-22 | Tva Medical, Inc. | Devices and methods for fistula formation |
| US10874422B2 (en) | 2016-01-15 | 2020-12-29 | Tva Medical, Inc. | Systems and methods for increasing blood flow |
| US11051880B2 (en) | 2010-11-16 | 2021-07-06 | Tva Medical, Inc. | Devices and methods for forming a fistula |
| US11285028B2 (en) | 2016-09-25 | 2022-03-29 | Tva Medical, Inc. | Vascular stent devices and methods |
| US11291496B2 (en) | 2002-03-05 | 2022-04-05 | Avent, Inc. | Methods of treating the sacroiliac region of a patient's body |
| US11590322B2 (en) | 2016-01-15 | 2023-02-28 | Tva Medical, Inc. | Devices and methods for advancing a wire |
| US11766560B2 (en) | 2010-04-01 | 2023-09-26 | Ecole Polytechnique Federale De Lausanne | Device for interacting with neurological tissue and methods of making and using the same |
| US12035961B2 (en) | 2015-04-13 | 2024-07-16 | Carlos Fernando Bazoberry | Radiofrequency denervation needle and method |
| US12295645B2 (en) | 2016-01-15 | 2025-05-13 | Tva Medical, Inc. | Systems and methods for adhering vessels |
Families Citing this family (64)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US6626903B2 (en) | 1997-07-24 | 2003-09-30 | Rex Medical, L.P. | Surgical biopsy device |
| US6331166B1 (en)* | 1998-03-03 | 2001-12-18 | Senorx, Inc. | Breast biopsy system and method |
| US6540693B2 (en) | 1998-03-03 | 2003-04-01 | Senorx, Inc. | Methods and apparatus for securing medical instruments to desired locations in a patients body |
| US7517348B2 (en)* | 1998-09-03 | 2009-04-14 | Rubicor Medical, Inc. | Devices and methods for performing procedures on a breast |
| US7534242B2 (en)* | 2003-02-25 | 2009-05-19 | Artemis Medical, Inc. | Tissue separating catheter assembly and method |
| US20060259026A1 (en)* | 2005-05-05 | 2006-11-16 | Baylis Medical Company Inc. | Electrosurgical treatment method and device |
| GB0314863D0 (en)* | 2003-06-26 | 2003-07-30 | Univ Dundee | Medical apparatus and method |
| US7833187B2 (en)* | 2004-04-16 | 2010-11-16 | Nuvue Therapeutics, Inc. | Systems and methods for improving image-guided tissue ablation |
| US9101386B2 (en) | 2004-10-15 | 2015-08-11 | Amendia, Inc. | Devices and methods for treating tissue |
| US7963915B2 (en) | 2004-10-15 | 2011-06-21 | Baxano, Inc. | Devices and methods for tissue access |
| US7887538B2 (en)* | 2005-10-15 | 2011-02-15 | Baxano, Inc. | Methods and apparatus for tissue modification |
| US20070213734A1 (en)* | 2006-03-13 | 2007-09-13 | Bleich Jeffery L | Tissue modification barrier devices and methods |
| US8048080B2 (en) | 2004-10-15 | 2011-11-01 | Baxano, Inc. | Flexible tissue rasp |
| US7857813B2 (en) | 2006-08-29 | 2010-12-28 | Baxano, Inc. | Tissue access guidewire system and method |
| US9247952B2 (en) | 2004-10-15 | 2016-02-02 | Amendia, Inc. | Devices and methods for tissue access |
| US8062300B2 (en) | 2006-05-04 | 2011-11-22 | Baxano, Inc. | Tissue removal with at least partially flexible devices |
| US8613745B2 (en) | 2004-10-15 | 2013-12-24 | Baxano Surgical, Inc. | Methods, systems and devices for carpal tunnel release |
| US7959577B2 (en) | 2007-09-06 | 2011-06-14 | Baxano, Inc. | Method, system, and apparatus for neural localization |
| US8221397B2 (en) | 2004-10-15 | 2012-07-17 | Baxano, Inc. | Devices and methods for tissue modification |
| US7578819B2 (en) | 2005-05-16 | 2009-08-25 | Baxano, Inc. | Spinal access and neural localization |
| US8257356B2 (en) | 2004-10-15 | 2012-09-04 | Baxano, Inc. | Guidewire exchange systems to treat spinal stenosis |
| US20110190772A1 (en) | 2004-10-15 | 2011-08-04 | Vahid Saadat | Powered tissue modification devices and methods |
| US20080103504A1 (en)* | 2006-10-30 | 2008-05-01 | Schmitz Gregory P | Percutaneous spinal stenosis treatment |
| US20100331883A1 (en) | 2004-10-15 | 2010-12-30 | Schmitz Gregory P | Access and tissue modification systems and methods |
| US7938830B2 (en) | 2004-10-15 | 2011-05-10 | Baxano, Inc. | Powered tissue modification devices and methods |
| US8430881B2 (en) | 2004-10-15 | 2013-04-30 | Baxano, Inc. | Mechanical tissue modification devices and methods |
| JP5243034B2 (en) | 2004-10-15 | 2013-07-24 | バクサノ,インク. | Tissue removal device |
| US7738969B2 (en) | 2004-10-15 | 2010-06-15 | Baxano, Inc. | Devices and methods for selective surgical removal of tissue |
| US8211104B2 (en)* | 2005-01-06 | 2012-07-03 | Boston Scientific Scimed, Inc. | Co-access bipolar ablation probe |
| CA2541108A1 (en)* | 2005-03-31 | 2006-09-30 | Sherwood Services Ag | Electrosurgical cannulas, systems and method |
| US10555775B2 (en)* | 2005-05-16 | 2020-02-11 | Intuitive Surgical Operations, Inc. | Methods and system for performing 3-D tool tracking by fusion of sensor and/or camera derived data during minimally invasive robotic surgery |
| US8366712B2 (en) | 2005-10-15 | 2013-02-05 | Baxano, Inc. | Multiple pathways for spinal nerve root decompression from a single access point |
| US8062298B2 (en) | 2005-10-15 | 2011-11-22 | Baxano, Inc. | Flexible tissue removal devices and methods |
| US8092456B2 (en) | 2005-10-15 | 2012-01-10 | Baxano, Inc. | Multiple pathways for spinal nerve root decompression from a single access point |
| JP4744284B2 (en)* | 2005-12-19 | 2011-08-10 | 株式会社デージーエス・コンピュータ | Treatment child |
| US7896874B2 (en)* | 2005-12-29 | 2011-03-01 | Boston Scientific Scimed, Inc. | RF ablation probes with tine valves |
| CA2661594A1 (en)* | 2006-06-16 | 2007-12-27 | Wilson-Cook Medical Inc. | Wire guide sphincterotome |
| BRPI0814101A2 (en)* | 2007-07-26 | 2015-02-03 | Alpha Scient Corp | APPARATUS AND METHOD FOR REMOVAL AND SUBCUTANTIALLY POSITIONING A FABRIC, LIFTING, AND, SUTURE |
| US9226748B2 (en)* | 2007-07-26 | 2016-01-05 | Alpha Scientific Corporation | Surgical suturing device, method and tools used therewith |
| US8192436B2 (en) | 2007-12-07 | 2012-06-05 | Baxano, Inc. | Tissue modification devices |
| US9314253B2 (en) | 2008-07-01 | 2016-04-19 | Amendia, Inc. | Tissue modification devices and methods |
| US8409206B2 (en) | 2008-07-01 | 2013-04-02 | Baxano, Inc. | Tissue modification devices and methods |
| US8398641B2 (en) | 2008-07-01 | 2013-03-19 | Baxano, Inc. | Tissue modification devices and methods |
| AU2009271047B2 (en) | 2008-07-14 | 2014-04-17 | Baxano Surgical, Inc. | Tissue modification devices |
| CA2732309C (en) | 2008-07-30 | 2018-04-10 | Ecole Polytechnique Federale De Lausanne (Epfl) | Apparatus and method for optimized stimulation of a neurological target |
| JP5667987B2 (en) | 2008-11-12 | 2015-02-12 | エコーレ ポリテクニーク フェデラーレ デ ローザンヌ (イーピーエフエル) | Micromachined nerve stimulation device |
| US20100160906A1 (en)* | 2008-12-23 | 2010-06-24 | Asthmatx, Inc. | Expandable energy delivery devices having flexible conductive elements and associated systems and methods |
| EP2405823A4 (en) | 2009-03-13 | 2012-07-04 | Baxano Inc | Flexible neural localization devices and methods |
| US8394102B2 (en) | 2009-06-25 | 2013-03-12 | Baxano, Inc. | Surgical tools for treatment of spinal stenosis |
| US9216054B2 (en) | 2010-11-04 | 2015-12-22 | Mayo Foundation For Medical Education And Research | Esophageal mucosectomy systems, devices and methods |
| US9636110B2 (en) | 2013-03-13 | 2017-05-02 | Alpha Scientific Corporation | Structural support incorporating multiple strands |
| US9375253B2 (en) | 2013-03-14 | 2016-06-28 | Megadyne Medical Products, Inc. | Electrosurgical instrument |
| US9259260B2 (en) | 2013-03-14 | 2016-02-16 | Megadyne Medical Products, Inc. | Fluid evacuation device |
| BR112015023550A2 (en) | 2013-03-15 | 2017-07-18 | Alpha Scient Corporation | coupling tool for capturing a suture or surgical thread, and tissue manipulation system |
| US11311718B2 (en)* | 2014-05-16 | 2022-04-26 | Aleva Neurotherapeutics Sa | Device for interacting with neurological tissue and methods of making and using the same |
| US9474894B2 (en) | 2014-08-27 | 2016-10-25 | Aleva Neurotherapeutics | Deep brain stimulation lead |
| US10080571B2 (en) | 2015-03-06 | 2018-09-25 | Warsaw Orthopedic, Inc. | Surgical instrument and method |
| JP6495123B2 (en)* | 2015-07-02 | 2019-04-03 | オリンパス株式会社 | Ablation probe |
| US11039876B2 (en) | 2017-05-16 | 2021-06-22 | Megadyne Medical Products, Inc. | Hand-held instrument with extendable shaft locking mechanism |
| US11992261B2 (en) | 2017-05-16 | 2024-05-28 | Megadyne Medical Products, Inc. | Locking mechanism and sliding conductor for extendable shaft |
| US10765472B2 (en) | 2017-05-16 | 2020-09-08 | Megadyne Medical Products, Inc. | Electrosurgical instrument extension attachment |
| US10702692B2 (en) | 2018-03-02 | 2020-07-07 | Aleva Neurotherapeutics | Neurostimulation device |
| WO2024190496A1 (en)* | 2023-03-10 | 2024-09-19 | 株式会社カネカ | Incision device |
| WO2025158375A1 (en)* | 2024-01-26 | 2025-07-31 | Medtronic, Inc. | Transcatheter septal myotomy devices to improve lvot patency and methods of use |
Family Cites Families (89)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US2816552A (en) | 1954-06-29 | 1957-12-17 | Roy D Hoffman | Teat bistoury with improved cutter blade adjusting means |
| US3320957A (en) | 1964-05-21 | 1967-05-23 | Sokolik Edward | Surgical instrument |
| US3732858A (en) | 1968-09-16 | 1973-05-15 | Surgical Design Corp | Apparatus for removing blood clots, cataracts and other objects from the eye |
| US3749085A (en) | 1970-06-26 | 1973-07-31 | J Willson | Vascular tissue removing device |
| JPS5727445Y2 (en)* | 1973-06-20 | 1982-06-15 | ||
| US3939839A (en)* | 1974-06-26 | 1976-02-24 | American Cystoscope Makers, Inc. | Resectoscope and electrode therefor |
| JPS5486991A (en) | 1977-12-21 | 1979-07-10 | Olympus Optical Co | Device for incising in body cavity |
| US4245653A (en) | 1979-01-02 | 1981-01-20 | Kenneth Weaver | Method and apparatus for obtaining specimens of endometrial tissue |
| IT1126526B (en) | 1979-12-07 | 1986-05-21 | Enrico Dormia | SURGICAL EXTRACTOR TO REMOVE FOREIGN BODIES THAT ARE FOUND IN THE NATURAL ROUTES OF THE HUMAN BODY, AS CALCULATIONS AND SIMILAR |
| NZ202965A (en) | 1982-01-07 | 1986-05-09 | Technicare Corp | Exploratory needle with ultrasonic conducting coaxial stylet |
| US4611594A (en) | 1984-04-11 | 1986-09-16 | Northwestern University | Medical instrument for containment and removal of calculi |
| US4650466A (en) | 1985-11-01 | 1987-03-17 | Angiobrade Partners | Angioplasty device |
| GB2204496B (en) | 1987-05-15 | 1991-09-11 | Syntex Inc | Device for collecting biological material |
| US4890611A (en) | 1988-04-05 | 1990-01-02 | Thomas J. Fogarty | Endarterectomy apparatus and method |
| US5147355A (en) | 1988-09-23 | 1992-09-15 | Brigham And Womens Hospital | Cryoablation catheter and method of performing cryoablation |
| US4903696A (en) | 1988-10-06 | 1990-02-27 | Everest Medical Corporation | Electrosurgical generator |
| US4966604A (en) | 1989-01-23 | 1990-10-30 | Interventional Technologies Inc. | Expandable atherectomy cutter with flexibly bowed blades |
| US5211651A (en) | 1989-08-18 | 1993-05-18 | Evi Corporation | Catheter atherotome |
| US5071424A (en) | 1989-08-18 | 1991-12-10 | Evi Corporation | Catheter atherotome |
| US5156610A (en) | 1989-08-18 | 1992-10-20 | Evi Corporation | Catheter atherotome |
| US5282484A (en) | 1989-08-18 | 1994-02-01 | Endovascular Instruments, Inc. | Method for performing a partial atherectomy |
| US5797907A (en)* | 1989-11-06 | 1998-08-25 | Mectra Labs, Inc. | Electrocautery cutter |
| JPH03280939A (en) | 1990-03-29 | 1991-12-11 | Fujitsu Ltd | Ultrasonic probe |
| US5083570A (en) | 1990-06-18 | 1992-01-28 | Mosby Richard A | Volumetric localization/biopsy/surgical device |
| US5100423A (en) | 1990-08-21 | 1992-03-31 | Medical Engineering & Development Institute, Inc. | Ablation catheter |
| US5217479A (en) | 1991-02-14 | 1993-06-08 | Linvatec Corporation | Surgical cutting instrument |
| US5279309A (en)* | 1991-06-13 | 1994-01-18 | International Business Machines Corporation | Signaling device and method for monitoring positions in a surgical operation |
| US5152293A (en) | 1991-07-01 | 1992-10-06 | Northwestern University | Finger-mounted intraoperative imaging device |
| US5176688A (en) | 1991-07-17 | 1993-01-05 | Perinchery Narayan | Stone extractor and method |
| US5741271A (en)* | 1991-11-05 | 1998-04-21 | Nakao; Naomi L. | Surgical retrieval assembly and associated method |
| US5486182A (en)* | 1991-11-05 | 1996-01-23 | Wilk & Nakao Medical Technology Inc. | Polyp retrieval assembly with separable web member |
| US5325860A (en) | 1991-11-08 | 1994-07-05 | Mayo Foundation For Medical Education And Research | Ultrasonic and interventional catheter and method |
| US5192291A (en) | 1992-01-13 | 1993-03-09 | Interventional Technologies, Inc. | Rotationally expandable atherectomy cutter assembly |
| US5224945A (en) | 1992-01-13 | 1993-07-06 | Interventional Technologies, Inc. | Compressible/expandable atherectomy cutter |
| WO1993019679A1 (en) | 1992-04-07 | 1993-10-14 | The Johns Hopkins University | A percutaneous mechanical fragmentation catheter system |
| US5308321A (en) | 1992-05-05 | 1994-05-03 | Castro Donna J | Retainer assisted by vacuum expansion system |
| US5224488A (en) | 1992-08-31 | 1993-07-06 | Neuffer Francis H | Biopsy needle with extendable cutting means |
| US5318576A (en) | 1992-12-16 | 1994-06-07 | Plassche Jr Walter M | Endovascular surgery systems |
| US5527326A (en) | 1992-12-29 | 1996-06-18 | Thomas J. Fogarty | Vessel deposit shearing apparatus |
| US5403311A (en)* | 1993-03-29 | 1995-04-04 | Boston Scientific Corporation | Electro-coagulation and ablation and other electrotherapeutic treatments of body tissue |
| GB9314641D0 (en) | 1993-07-15 | 1993-08-25 | Salim Aws S M | Tunnelling umbrella |
| GB9314640D0 (en) | 1993-07-15 | 1993-08-25 | Salim Aws S M | Tunnellimg catheter |
| US5441510A (en) | 1993-09-01 | 1995-08-15 | Technology Development Center | Bi-axial cutter apparatus for catheter |
| US5415656A (en) | 1993-09-28 | 1995-05-16 | American Medical Systems, Inc. | Electrosurgical apparatus |
| US5558091A (en)* | 1993-10-06 | 1996-09-24 | Biosense, Inc. | Magnetic determination of position and orientation |
| US5683384A (en)* | 1993-11-08 | 1997-11-04 | Zomed | Multiple antenna ablation apparatus |
| US5458597A (en)* | 1993-11-08 | 1995-10-17 | Zomed International | Device for treating cancer and non-malignant tumors and methods |
| US5526822A (en) | 1994-03-24 | 1996-06-18 | Biopsys Medical, Inc. | Method and apparatus for automated biopsy and collection of soft tissue |
| JPH07265329A (en)* | 1994-03-31 | 1995-10-17 | Fuji Photo Optical Co Ltd | Puncture high frequency treatment device |
| US5672172A (en) | 1994-06-23 | 1997-09-30 | Vros Corporation | Surgical instrument with ultrasound pulse generator |
| US5794626A (en) | 1994-08-18 | 1998-08-18 | Kieturakis; Maciej J. | Excisional stereotactic apparatus |
| US5954670A (en) | 1994-10-05 | 1999-09-21 | Baker; Gary H. | Mandrel-guided tandem multiple channel biopsy guide device and method of use |
| US5632754A (en) | 1994-12-23 | 1997-05-27 | Devices For Vascular Intervention | Universal catheter with interchangeable work element |
| US5947964A (en) | 1995-03-03 | 1999-09-07 | Neothermia Corporation | Methods and apparatus for therapeutic cauterization of predetermined volumes of biological tissue |
| US5630426A (en) | 1995-03-03 | 1997-05-20 | Neovision Corporation | Apparatus and method for characterization and treatment of tumors |
| US6106524A (en) | 1995-03-03 | 2000-08-22 | Neothermia Corporation | Methods and apparatus for therapeutic cauterization of predetermined volumes of biological tissue |
| US5782771A (en) | 1995-04-17 | 1998-07-21 | Hussman; Karl L. | Dual, fused, and grooved optical localization fibers |
| US5554163A (en) | 1995-04-27 | 1996-09-10 | Shturman Cardiology Systems, Inc. | Atherectomy device |
| DE19528440C2 (en) | 1995-08-02 | 1998-09-10 | Harald Dr Med Kuebler | Surgical cutting instrument |
| US5913855A (en)* | 1995-08-15 | 1999-06-22 | Rita Medical Systems, Inc. | Multiple antenna ablation apparatus and method |
| US5800484A (en)* | 1995-08-15 | 1998-09-01 | Rita Medical Systems, Inc. | Multiple antenna ablation apparatus with expanded electrodes |
| US5709697A (en) | 1995-11-22 | 1998-01-20 | United States Surgical Corporation | Apparatus and method for removing tissue |
| DE19706751A1 (en) | 1996-03-27 | 1997-10-02 | Valleylab Inc | Electrosurgical device for removing tissue in body areas |
| US5863291A (en)* | 1996-04-08 | 1999-01-26 | Cardima, Inc. | Linear ablation assembly |
| US6096053A (en) | 1996-05-03 | 2000-08-01 | Scimed Life Systems, Inc. | Medical retrieval basket |
| US5662671A (en) | 1996-07-17 | 1997-09-02 | Embol-X, Inc. | Atherectomy device having trapping and excising means for removal of plaque from the aorta and other arteries |
| US5810806A (en)* | 1996-08-29 | 1998-09-22 | Ethicon Endo-Surgery | Methods and devices for collection of soft tissue |
| JPH1099342A (en) | 1996-09-26 | 1998-04-21 | Sumitomo Bakelite Co Ltd | High-frequency snare |
| US6080151A (en) | 1997-07-21 | 2000-06-27 | Daig Corporation | Ablation catheter |
| US6238389B1 (en) | 1997-09-30 | 2001-05-29 | Boston Scientific Corporation | Deflectable interstitial ablation device |
| US6099534A (en) | 1997-10-01 | 2000-08-08 | Scimed Life Systems, Inc. | Releasable basket |
| US6063082A (en) | 1997-11-04 | 2000-05-16 | Scimed Life Systems, Inc. | Percutaneous myocardial revascularization basket delivery system and radiofrequency therapeutic device |
| US6080149A (en) | 1998-01-09 | 2000-06-27 | Radiotherapeutics, Corporation | Method and apparatus for monitoring solid tissue heating |
| WO1999039648A1 (en) | 1998-02-10 | 1999-08-12 | Dubrul William R | Entrapping apparatus and method for use |
| US6344026B1 (en) | 1998-04-08 | 2002-02-05 | Senorx, Inc. | Tissue specimen encapsulation device and method thereof |
| US6261241B1 (en) | 1998-03-03 | 2001-07-17 | Senorx, Inc. | Electrosurgical biopsy device and method |
| US6331166B1 (en) | 1998-03-03 | 2001-12-18 | Senorx, Inc. | Breast biopsy system and method |
| US6540693B2 (en) | 1998-03-03 | 2003-04-01 | Senorx, Inc. | Methods and apparatus for securing medical instruments to desired locations in a patients body |
| US6659105B2 (en) | 1998-02-26 | 2003-12-09 | Senorx, Inc. | Tissue specimen isolating and damaging device and method |
| US6454727B1 (en)* | 1998-03-03 | 2002-09-24 | Senorx, Inc. | Tissue acquisition system and method of use |
| US6161034A (en) | 1999-02-02 | 2000-12-12 | Senorx, Inc. | Methods and chemical preparations for time-limited marking of biopsy sites |
| US6015390A (en) | 1998-06-12 | 2000-01-18 | D. Krag Llc | System and method for stabilizing and removing tissue |
| US6036708A (en) | 1998-08-13 | 2000-03-14 | Advanced Cardiovascular Systems, Inc. | Cutting stent with flexible tissue extractor |
| US6179860B1 (en) | 1998-08-19 | 2001-01-30 | Artemis Medical, Inc. | Target tissue localization device and method |
| US6022362A (en) | 1998-09-03 | 2000-02-08 | Rubicor Medical, Inc. | Excisional biopsy devices and methods |
| US6325797B1 (en)* | 1999-04-05 | 2001-12-04 | Medtronic, Inc. | Ablation catheter and method for isolating a pulmonary vein |
| WO2000074561A1 (en) | 1999-06-04 | 2000-12-14 | Artemis Medical, Inc. | Tissue removal methods and apparatus |
| US6514248B1 (en) | 1999-10-15 | 2003-02-04 | Neothermia Corporation | Accurate cutting about and into tissue volumes with electrosurgically deployed electrodes |
| US6287304B1 (en)* | 1999-10-15 | 2001-09-11 | Neothermia Corporation | Interstitial cauterization of tissue volumes with electrosurgically deployed electrodes |
- 2000
- 2000-12-07USUS09/732,848patent/US20020072739A1/ennot_activeAbandoned
- 2001
- 2001-12-03WOPCT/US2001/046288patent/WO2002045609A1/enactiveApplication Filing
- 2001-12-03EPEP01995335Apatent/EP1339339A4/ennot_activeWithdrawn
- 2001-12-03AUAU2002225886Apatent/AU2002225886B2/ennot_activeCeased
- 2001-12-03CACA002436844Apatent/CA2436844A1/ennot_activeAbandoned
- 2001-12-03AUAU2588602Apatent/AU2588602A/enactivePending
- 2001-12-03JPJP2002547396Apatent/JP3806088B2/ennot_activeExpired - Fee Related
- 2001-12-03NZNZ526314Apatent/NZ526314A/enunknown
- 2003
- 2003-01-23USUS10/349,659patent/US7198626B2/ennot_activeExpired - Fee Related
Cited By (109)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US7507236B2 (en) | 1992-01-07 | 2009-03-24 | Arthrocare Corporation | System and method for electrosurgical cutting and ablation |
| US7819863B2 (en) | 1992-01-07 | 2010-10-26 | Arthrocare Corporation | System and method for electrosurgical cutting and ablation |
| US7445618B2 (en) | 1993-05-10 | 2008-11-04 | Arthrocare Corporation | Methods for tissue ablation using pulsed energy |
| US20060189971A1 (en)* | 1995-11-22 | 2006-08-24 | Arthrocare Corporation | Systems and methods for electrosurgical treatment of fasciitis |
| US7429260B2 (en) | 1996-07-16 | 2008-09-30 | Arthrocare Corporation | Systems and methods for electrosurgical tissue contraction within the spine |
| US8663216B2 (en) | 1998-08-11 | 2014-03-04 | Paul O. Davison | Instrument for electrosurgical tissue treatment |
| US7828797B2 (en) | 1999-12-27 | 2010-11-09 | Intact Medical Corporation | Electrosurgical accessing of tissue with controlled collateral thermal phenomena |
| US7041101B2 (en) | 1999-12-27 | 2006-05-09 | Neothermia Corporation | Electrosurgical accessing of tissue with controlled collateral thermal phenomena |
| US20060095027A1 (en)* | 1999-12-27 | 2006-05-04 | Eggers Philip E | Electrosurgical accessing of tissue with controlled collateral thermal phenomena |
| US20040024396A1 (en)* | 1999-12-27 | 2004-02-05 | Eggers Philip E. | Electrosurgical accessing of tissue with controlled collateral thermal phenomena |
| US20140066702A1 (en)* | 2002-01-30 | 2014-03-06 | Covidien Lp | Surgical imaging device |
| US9867523B2 (en)* | 2002-01-30 | 2018-01-16 | Covidien Lp | Surgical imaging device |
| US8812086B2 (en)* | 2002-01-30 | 2014-08-19 | Covidien Lp | Surgical imaging device |
| US20100249499A1 (en)* | 2002-01-30 | 2010-09-30 | Power Medical Interventions, Llc | Surgical imaging device |
| US9949789B2 (en) | 2002-03-05 | 2018-04-24 | Avent, Inc. | Methods of treating the sacroiliac region of a patient's body |
| US20070027449A1 (en)* | 2002-03-05 | 2007-02-01 | Baylis Medical Company Inc. | Electrosurgical device and methods |
| US9364281B2 (en) | 2002-03-05 | 2016-06-14 | Avent, Inc. | Methods for treating the thoracic region of a patient's body |
| US11291496B2 (en) | 2002-03-05 | 2022-04-05 | Avent, Inc. | Methods of treating the sacroiliac region of a patient's body |
| US20070203402A1 (en)* | 2002-03-05 | 2007-08-30 | Baylis Medical | Elongate member providing a variation in radiopacity |
| US9820808B2 (en) | 2002-03-05 | 2017-11-21 | Avent, Inc. | Method for treating the thoracic region of a patient's body |
| US20070156136A1 (en)* | 2002-03-05 | 2007-07-05 | Neil Godara | Methods of treating the sacroiliac region of a patient's body |
| US9216053B2 (en) | 2002-03-05 | 2015-12-22 | Avent, Inc. | Elongate member providing a variation in radiopacity |
| US20090054962A1 (en)* | 2002-03-05 | 2009-02-26 | Baylis Medical Company Inc. | Methods for treating the thoracic region of a patient's body |
| US10206739B2 (en) | 2002-03-05 | 2019-02-19 | Avent, Inc. | Electrosurgical device and methods |
| US8454598B2 (en) | 2002-06-14 | 2013-06-04 | Ncontact Surgical, Inc. | Vacuum coagulation probes |
| US10932849B2 (en) | 2002-06-14 | 2021-03-02 | Atricure, Inc. | Vacuum coagulation probe for atrial fibrillation treatment |
| US7780661B2 (en) | 2002-06-14 | 2010-08-24 | nContact Surgical, Inc | Vacuum coagulation probes |
| US7803155B2 (en) | 2002-06-14 | 2010-09-28 | Ncontact Surgical, Inc. | Vacuum coagulation probes |
| US7572257B2 (en)* | 2002-06-14 | 2009-08-11 | Ncontact Surgical, Inc. | Vacuum coagulation and dissection probes |
| US20100262138A1 (en)* | 2002-06-14 | 2010-10-14 | Ncontact Surgical, Inc. | Methods of coagulating tissue |
| US20060293646A1 (en)* | 2002-06-14 | 2006-12-28 | Whayne James G | Vacuum coagulation & dissection probes |
| US20060235381A1 (en)* | 2002-06-14 | 2006-10-19 | Ncontact Surgical, Inc. | Vacuum coagulation probes |
| US10758305B2 (en) | 2002-06-14 | 2020-09-01 | Atricure, Inc. | Vacuum coagulation probes |
| US10702330B2 (en) | 2002-06-14 | 2020-07-07 | Atricure, Inc. | Vacuum coagulation probes |
| US9308042B2 (en) | 2002-06-14 | 2016-04-12 | Ncontact Surgical, Inc. | Vacuum coagulation probes |
| US8858552B2 (en) | 2002-06-14 | 2014-10-14 | Ncontact Surgical, Inc. | Vacuum coagulation probes |
| US8034053B2 (en) | 2002-06-14 | 2011-10-11 | Ncontact Surgical, Inc. | Vacuum coagulation and dissection probes |
| US9603658B2 (en) | 2002-06-14 | 2017-03-28 | Atricure, Inc. | Methods of coagulating tissue |
| US8235990B2 (en) | 2002-06-14 | 2012-08-07 | Ncontact Surgical, Inc. | Vacuum coagulation probes |
| US10219859B2 (en) | 2002-06-14 | 2019-03-05 | Atricure, Inc. | Vacuum coagulation probes |
| US20060200124A1 (en)* | 2002-06-14 | 2006-09-07 | Ncontact Surgical, Inc. | Vacuum coagulation probes |
| US7758578B2 (en) | 2002-06-14 | 2010-07-20 | Ncontact Surgical, Inc. | Methods of coagulating tissue |
| US20060206113A1 (en)* | 2002-06-14 | 2006-09-14 | Ncontact Surgical, Inc. | Methods of coagulating tissue |
| US20080243119A1 (en)* | 2002-06-14 | 2008-10-02 | Ncontact Surgical, Inc. | Vacuum coagulation probe for atrial fibrillation treatment |
| US9603657B2 (en) | 2002-06-14 | 2017-03-28 | Atricure, Inc. | Vacuum coagulation probe for atrial fibrillation treatment |
| US20070250058A1 (en)* | 2002-06-14 | 2007-10-25 | Ncontact Surgical, Inc. | Vacuum coagulation probes |
| EP1571969A4 (en)* | 2002-06-27 | 2006-07-05 | Arthrocare Corp | Systems and methods for electrosurgical tissue treatment |
| WO2004002293A2 (en) | 2002-06-27 | 2004-01-08 | Arthrocare Corporation | Systems and methods for electrosurgical tissue treatment |
| US11364074B2 (en) | 2003-04-29 | 2022-06-21 | Atricure, Inc. | Vacuum coagulation probes |
| US20070043351A1 (en)* | 2003-04-29 | 2007-02-22 | Ncontact Surgical, Inc. | Vacuum coagulation probes |
| US10342610B2 (en) | 2003-04-29 | 2019-07-09 | Atricure, Inc. | Vacuum coagulation probes |
| US9439714B2 (en) | 2003-04-29 | 2016-09-13 | Atricure, Inc. | Vacuum coagulation probes |
| US8998900B2 (en) | 2003-04-29 | 2015-04-07 | Ncontact Surgical, Inc. | Vacuum coagulation probes |
| US20080114354A1 (en)* | 2003-04-29 | 2008-05-15 | Ncontact Surgical, Inc. | Vacuum coagulation probes |
| US8012153B2 (en) | 2003-07-16 | 2011-09-06 | Arthrocare Corporation | Rotary electrosurgical apparatus and methods thereof |
| US8864759B2 (en) | 2004-11-15 | 2014-10-21 | Kimberly-Clark Inc. | Methods of treating the sacroiliac region of a patient's body |
| US20110040362A1 (en)* | 2004-11-15 | 2011-02-17 | Neil Godara | Methods of Treating the Sacroiliac Region of a Patient's Body |
| US8951249B2 (en) | 2005-02-17 | 2015-02-10 | Avent Inc. | Electrosurgical device with discontinuous flow density |
| US20060217705A1 (en)* | 2005-02-17 | 2006-09-28 | Baylis Medical Company Inc. | Electrosurgical device with discontinuous flow density |
| US20100298820A1 (en)* | 2005-04-13 | 2010-11-25 | Joshua Ben-Nun | Thermal capsulotomy tool and system |
| US8162931B2 (en)* | 2005-04-13 | 2012-04-24 | Valens Associated Inc. | Thermal capsulotomy tool and system |
| US20090024124A1 (en)* | 2005-07-14 | 2009-01-22 | Lefler Amy | Methods for treating the thoracic region of a patient's body |
| US20080114355A1 (en)* | 2006-11-09 | 2008-05-15 | Ncontact Surgical, Inc. | Vacuum coagulation probes |
| US8298243B2 (en) | 2007-07-30 | 2012-10-30 | Tyco Healthcare Group Lp | Combination wire electrode and tube electrode polypectomy device |
| US8998892B2 (en)* | 2007-12-21 | 2015-04-07 | Atricure, Inc. | Ablation device with cooled electrodes and methods of use |
| US20100305562A1 (en)* | 2007-12-21 | 2010-12-02 | Winkler Matthew J | Ablation Device With Cooled Electrodes And Methods of Use |
| US9918771B2 (en) | 2008-01-31 | 2018-03-20 | Covidien Lp | Polyp removal device and method of use |
| US10959767B2 (en) | 2008-01-31 | 2021-03-30 | Covidien Lp | Polyp removal device and method of use |
| US8328803B2 (en) | 2008-01-31 | 2012-12-11 | Covidien Lp | Polyp removal device and method of use |
| USRE46063E1 (en) | 2008-01-31 | 2016-07-12 | Covidien Lp | Polyp removal device and method of use |
| US8747400B2 (en) | 2008-08-13 | 2014-06-10 | Arthrocare Corporation | Systems and methods for screen electrode securement |
| US8355799B2 (en) | 2008-12-12 | 2013-01-15 | Arthrocare Corporation | Systems and methods for limiting joint temperature |
| US9452008B2 (en) | 2008-12-12 | 2016-09-27 | Arthrocare Corporation | Systems and methods for limiting joint temperature |
| US8323279B2 (en) | 2009-09-25 | 2012-12-04 | Arthocare Corporation | System, method and apparatus for electrosurgical instrument with movable fluid delivery sheath |
| US8317786B2 (en) | 2009-09-25 | 2012-11-27 | AthroCare Corporation | System, method and apparatus for electrosurgical instrument with movable suction sheath |
| US11766560B2 (en) | 2010-04-01 | 2023-09-26 | Ecole Polytechnique Federale De Lausanne | Device for interacting with neurological tissue and methods of making and using the same |
| US8696659B2 (en) | 2010-04-30 | 2014-04-15 | Arthrocare Corporation | Electrosurgical system and method having enhanced temperature measurement |
| US11986236B2 (en) | 2010-11-16 | 2024-05-21 | Tva Medical, Inc. | Devices and methods for forming a fistula |
| US11051880B2 (en) | 2010-11-16 | 2021-07-06 | Tva Medical, Inc. | Devices and methods for forming a fistula |
| US12178501B2 (en) | 2010-11-16 | 2024-12-31 | Tva Medical, Inc. | Devices and methods for forming a fistula |
| US20120303018A1 (en)* | 2011-05-23 | 2012-11-29 | Tyco Healthcare Group Lp | Tissue Dissectors |
| US10869717B2 (en) | 2012-10-11 | 2020-12-22 | Tva Medical, Inc. | Devices and methods for fistula formation |
| US20150126994A1 (en)* | 2012-12-12 | 2015-05-07 | Olympus Medical Systems Corp. | Puncture tool and ultrasound endoscope |
| US10821217B2 (en) | 2013-03-14 | 2020-11-03 | Tva Medical, Inc. | Fistula formation devices and methods therefor |
| US11707562B2 (en) | 2013-03-14 | 2023-07-25 | Tva Medical, Inc. | Fistula formation devices and methods therefor |
| US9526556B2 (en) | 2014-02-28 | 2016-12-27 | Arthrocare Corporation | Systems and methods systems related to electrosurgical wands with screen electrodes |
| US10695534B2 (en) | 2014-03-14 | 2020-06-30 | Tva Medical, Inc. | Fistula formation devices and methods therefor |
| US11219745B2 (en) | 2014-03-14 | 2022-01-11 | Tva Medical, Inc. | Fistula formation devices and methods therefor |
| CN110585588A (en)* | 2014-05-16 | 2019-12-20 | 阿莱瓦神经治疗股份有限公司 | Implantable microelectrode device |
| US9597142B2 (en) | 2014-07-24 | 2017-03-21 | Arthrocare Corporation | Method and system related to electrosurgical procedures |
| US9649148B2 (en) | 2014-07-24 | 2017-05-16 | Arthrocare Corporation | Electrosurgical system and method having enhanced arc prevention |
| US10646666B2 (en) | 2014-08-27 | 2020-05-12 | Tva Medical, Inc. | Cryolipolysis devices and methods therefor |
| US10813685B2 (en) | 2014-09-25 | 2020-10-27 | Covidien Lp | Single-handed operable surgical instrument including loop electrode with integrated pad electrode |
| US11207070B2 (en) | 2015-02-09 | 2021-12-28 | Tva Medical, Inc. | Methods for treating hypertension and reducing blood pressure with formation of fistula |
| US10603040B1 (en) | 2015-02-09 | 2020-03-31 | Tva Medical, Inc. | Methods for treating hypertension and reducing blood pressure with formation of fistula |
| US12369916B2 (en) | 2015-02-09 | 2025-07-29 | Tva Medical, Inc. | Methods for treating hypertension and reducing blood pressure with formation of fistula |
| US12035961B2 (en) | 2015-04-13 | 2024-07-16 | Carlos Fernando Bazoberry | Radiofrequency denervation needle and method |
| US10786300B2 (en) | 2015-04-13 | 2020-09-29 | Carlos Fernando Bazoberry | Radiofrequency denervation needle and method |
| US11129660B2 (en) | 2015-08-13 | 2021-09-28 | Covidien Ag | Electrosurgical generator and methods |
| US10363079B2 (en) | 2015-08-13 | 2019-07-30 | Medtronic Inc. | Electrosurgical method and apparatus with varying stiffness capture components |
| CN108348288A (en)* | 2015-10-28 | 2018-07-31 | 波士顿科学国际有限公司 | retractable tissue cutting device |
| US11026743B2 (en)* | 2016-01-15 | 2021-06-08 | Tva Medical, Inc. | Devices and methods for forming a fistula |
| US11826093B2 (en) | 2016-01-15 | 2023-11-28 | Tva Medical, Inc. | Devices and methods for forming a fistula |
| US10874422B2 (en) | 2016-01-15 | 2020-12-29 | Tva Medical, Inc. | Systems and methods for increasing blood flow |
| US11590322B2 (en) | 2016-01-15 | 2023-02-28 | Tva Medical, Inc. | Devices and methods for advancing a wire |
| US20170202616A1 (en)* | 2016-01-15 | 2017-07-20 | Tva Medical, Inc. | Devices and methods for forming a fistula |
| US12295645B2 (en) | 2016-01-15 | 2025-05-13 | Tva Medical, Inc. | Systems and methods for adhering vessels |
| US11285028B2 (en) | 2016-09-25 | 2022-03-29 | Tva Medical, Inc. | Vascular stent devices and methods |
| US12427044B2 (en) | 2016-09-25 | 2025-09-30 | Tva Medical, Inc. | Vascular stent devices and methods |
Also Published As
| Publication number | Publication date |
|---|---|
| AU2002225886B2 (en) | 2006-11-02 |
| EP1339339A4 (en) | 2008-05-28 |
| CA2436844A1 (en) | 2002-06-13 |
| AU2588602A (en) | 2002-06-18 |
| US20030109870A1 (en) | 2003-06-12 |
| WO2002045609A1 (en) | 2002-06-13 |
| JP3806088B2 (en) | 2006-08-09 |
| US7198626B2 (en) | 2007-04-03 |
| EP1339339A1 (en) | 2003-09-03 |
| JP2004531290A (en) | 2004-10-14 |
| NZ526314A (en) | 2006-11-30 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| US7198626B2 (en) | Methods and devices for radiofrequency electrosurgery | |
| AU2002225886A1 (en) | Methods and devices for radiofrequency electrosurgery | |
| JP4108928B2 (en) | Device for electrosurgical tissue removal | |
| EP0527848B1 (en) | Electrosurgical electrode | |
| US7147635B2 (en) | Bipolar electrosurgical snare | |
| CA2741453C (en) | Tissue ablation systems | |
| AU2005244649B2 (en) | A surgical instrument | |
| EP0898465B1 (en) | A moisture transport system for contact electrocoagulation | |
| US5906615A (en) | Serpentine ablation/coagulation electrode | |
| CA2226484C (en) | Medical probe device | |
| US8197477B2 (en) | Tissue ablation methods | |
| US6852111B1 (en) | Laparoscopic electrotome | |
| CN113677282A (en) | Diathermy internal treatment device | |
| EP3236869B1 (en) | Variable thickness electrosurgical snare |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| AS | Assignment | Owner name:RUBICOR MEDICAL, INC., A CORPORATION ORGANIZED UND Free format text:ASSIGNMENT OF ASSIGNORS INTEREST;ASSIGNORS:LEE, ROBERTA;CHERNOMORSKY, ARY S.;CLIFFORD, MARK J.;REEL/FRAME:011361/0670 Effective date:20001207 | |
| STCB | Information on status: application discontinuation | Free format text:ABANDONED -- FAILURE TO RESPOND TO AN OFFICE ACTION | |
| AS | Assignment | Owner name:DEMA, JOHN K., MARYLAND Free format text:INTELLECTUAL PROPERTY SECURITY AGREEMENT;ASSIGNOR:RUBICOR MEDICAL, INC.;REEL/FRAME:015653/0052 Effective date:20050106 | |
| AS | Assignment | Owner name:RUBICOR MEDICAL, INC., CALIFORNIA Free format text:RELEASE BY SECURED PARTY;ASSIGNOR:DEMA, JOHN K;REEL/FRAME:018711/0587 Effective date:20060824 | |
| AS | Assignment | Owner name:COMERICA BANK, CALIFORNIA Free format text:SECURITY AGREEMENT;ASSIGNOR:RUBICOR MEDICAL, INC.;REEL/FRAME:020828/0811 Effective date:20080421 Owner name:OXFORD FINANCE CORPORATION, VIRGINIA Free format text:SECURITY AGREEMENT;ASSIGNOR:RUBICOR MEDICAL, INC.;REEL/FRAME:020828/0811 Effective date:20080421 Owner name:COMERICA BANK,CALIFORNIA Free format text:SECURITY AGREEMENT;ASSIGNOR:RUBICOR MEDICAL, INC.;REEL/FRAME:020828/0811 Effective date:20080421 Owner name:OXFORD FINANCE CORPORATION,VIRGINIA Free format text:SECURITY AGREEMENT;ASSIGNOR:RUBICOR MEDICAL, INC.;REEL/FRAME:020828/0811 Effective date:20080421 | |
| AS | Assignment | Owner name:HOLOGIC, INC., MASSACHUSETTS Free format text:SECURITY AGREEMENT;ASSIGNOR:RUBICOR MEDICAL, INC.;REEL/FRAME:020845/0034 Effective date:20080421 Owner name:HOLOGIC, INC.,MASSACHUSETTS Free format text:SECURITY AGREEMENT;ASSIGNOR:RUBICOR MEDICAL, INC.;REEL/FRAME:020845/0034 Effective date:20080421 | |
| AS | Assignment | Owner name:ENCAPSULE MEDICAL, LLC, CALIFORNIA Free format text:MERGER;ASSIGNOR:RUBICOR MEDICAL, LLC;REEL/FRAME:029655/0927 Effective date:20120822 |