US20020049498A1 - In situ bioprosthetic filler and methods, particularly for the in situ formation of vertebral disc bioprosthetics - Google Patents
In situ bioprosthetic filler and methods, particularly for the in situ formation of vertebral disc bioprostheticsDownload PDFInfo
- Publication number
- US20020049498A1 US20020049498A1US09/983,537US98353701AUS2002049498A1US 20020049498 A1US20020049498 A1US 20020049498A1US 98353701 AUS98353701 AUS 98353701AUS 2002049498 A1US2002049498 A1US 2002049498A1
- Authority
- US
- United States
- Prior art keywords
- cavity
- vertebral disc
- components
- bioprosthetic
- reactable
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Abandoned
Links
- 238000011065in-situ storageMethods0.000titleclaimsabstractdescription14
- 238000000034methodMethods0.000titleclaimsdescription32
- 239000000945fillerSubstances0.000titleclaimsdescription7
- 238000010952in-situ formationMethods0.000titleclaims2
- 239000000463materialSubstances0.000claimsabstractdescription47
- 229920001222biopolymerPolymers0.000claimsabstractdescription32
- 239000000203mixtureSubstances0.000claimsabstractdescription28
- 102000004169proteins and genesHuman genes0.000claimsabstractdescription18
- 108090000623proteins and genesProteins0.000claimsabstractdescription18
- 229920001744PolyaldehydePolymers0.000claimsabstractdescription13
- 239000007788liquidSubstances0.000claimsabstractdescription13
- 241001465754MetazoaSpecies0.000claimsabstractdescription8
- 230000009969flowable effectEffects0.000claimsabstractdescription6
- 239000007795chemical reaction productSubstances0.000claimsdescription7
- SXRSQZLOMIGNAQ-UHFFFAOYSA-NGlutaraldehydeChemical groupO=CCCCC=OSXRSQZLOMIGNAQ-UHFFFAOYSA-N0.000claimsdescription6
- 238000011049fillingMethods0.000claimsdescription6
- 125000004122cyclic groupChemical group0.000claimsdescription5
- 230000000269nucleophilic effectEffects0.000claimsdescription5
- 238000006243chemical reactionMethods0.000claimsdescription4
- 229920000642polymerPolymers0.000claimsdescription4
- 229920001184polypeptidePolymers0.000claimsdescription4
- 108090000765processed proteins & peptidesProteins0.000claimsdescription4
- 102000004196processed proteins & peptidesHuman genes0.000claimsdescription4
- 102000001554HemoglobinsHuman genes0.000claimsdescription3
- 108010054147HemoglobinsProteins0.000claimsdescription3
- 239000002202Polyethylene glycolSubstances0.000claimsdescription3
- 150000001412aminesChemical class0.000claimsdescription3
- 150000008064anhydridesChemical class0.000claimsdescription3
- 125000000524functional groupChemical group0.000claimsdescription3
- 229920001223polyethylene glycolPolymers0.000claimsdescription3
- -1succinimidylChemical group0.000claimsdescription3
- 230000015572biosynthetic processEffects0.000claimsdescription2
- 241000283690Bos taurusSpecies0.000claims2
- 108091003079Bovine Serum AlbuminProteins0.000claims2
- 108091006905Human Serum AlbuminProteins0.000claims2
- 102000008100Human Serum AlbuminHuman genes0.000claims2
- 150000001299aldehydesChemical class0.000claims2
- 125000003396thiol groupChemical group[H]S*0.000claims2
- 239000012620biological materialSubstances0.000description10
- 210000001519tissueAnatomy0.000description10
- 210000000988bone and boneAnatomy0.000description7
- 108010088751AlbuminsProteins0.000description5
- 102000009027AlbuminsHuman genes0.000description5
- 238000009472formulationMethods0.000description5
- 238000002156mixingMethods0.000description5
- 210000002966serumAnatomy0.000description5
- 238000012360testing methodMethods0.000description5
- 208000008035Back PainDiseases0.000description4
- 206010061246Intervertebral disc degenerationDiseases0.000description4
- 208000018180degenerative disc diseaseDiseases0.000description4
- 239000000017hydrogelSubstances0.000description4
- 239000004615ingredientSubstances0.000description4
- 208000021600intervertebral disc degenerative diseaseDiseases0.000description4
- 210000002381plasmaAnatomy0.000description4
- 239000011800void materialSubstances0.000description4
- 208000008930Low Back PainDiseases0.000description3
- 239000006096absorbing agentSubstances0.000description3
- 244000309466calfSpecies0.000description3
- 230000004927fusionEffects0.000description3
- LEQAOMBKQFMDFZ-UHFFFAOYSA-NglyoxalChemical compoundO=CC=OLEQAOMBKQFMDFZ-UHFFFAOYSA-N0.000description3
- 238000011068loading methodMethods0.000description3
- 239000011824nuclear materialSubstances0.000description3
- 239000012779reinforcing materialSubstances0.000description3
- 239000007787solidSubstances0.000description3
- 238000011282treatmentMethods0.000description3
- XLYOFNOQVPJJNP-UHFFFAOYSA-NwaterSubstancesOXLYOFNOQVPJJNP-UHFFFAOYSA-N0.000description3
- VTYYLEPIZMXCLO-UHFFFAOYSA-LCalcium carbonateChemical compound[Ca+2].[O-]C([O-])=OVTYYLEPIZMXCLO-UHFFFAOYSA-L0.000description2
- 239000004971Cross linkerSubstances0.000description2
- 102000009123FibrinHuman genes0.000description2
- 108010073385FibrinProteins0.000description2
- BWGVNKXGVNDBDI-UHFFFAOYSA-NFibrin monomerChemical compoundCNC(=O)CNC(=O)CNBWGVNKXGVNDBDI-UHFFFAOYSA-N0.000description2
- 208000003618Intervertebral Disc DisplacementDiseases0.000description2
- RTAQQCXQSZGOHL-UHFFFAOYSA-NTitaniumChemical compound[Ti]RTAQQCXQSZGOHL-UHFFFAOYSA-N0.000description2
- 239000007864aqueous solutionSubstances0.000description2
- 239000003139biocideSubstances0.000description2
- 239000000919ceramicSubstances0.000description2
- 238000007906compressionMethods0.000description2
- 230000006835compressionEffects0.000description2
- 229920001577copolymerPolymers0.000description2
- 230000007850degenerationEffects0.000description2
- 230000018044dehydrationEffects0.000description2
- 238000006297dehydration reactionMethods0.000description2
- 238000013461designMethods0.000description2
- 238000002224dissectionMethods0.000description2
- 239000000835fiberSubstances0.000description2
- 229950003499fibrinDrugs0.000description2
- 239000000834fixativeSubstances0.000description2
- 239000012530fluidSubstances0.000description2
- 239000011796hollow space materialSubstances0.000description2
- 239000007943implantSubstances0.000description2
- 238000003780insertionMethods0.000description2
- 230000037431insertionEffects0.000description2
- VNWKTOKETHGBQD-UHFFFAOYSA-NmethaneChemical compoundCVNWKTOKETHGBQD-UHFFFAOYSA-N0.000description2
- 210000005036nerveAnatomy0.000description2
- 239000002504physiological saline solutionSubstances0.000description2
- 230000002787reinforcementEffects0.000description2
- 230000035939shockEffects0.000description2
- 239000000243solutionSubstances0.000description2
- 239000010935stainless steelSubstances0.000description2
- 229910001220stainless steelInorganic materials0.000description2
- 238000001356surgical procedureMethods0.000description2
- 229910052719titaniumInorganic materials0.000description2
- 239000010936titaniumSubstances0.000description2
- 108010027529Bio-glueProteins0.000description1
- 102000004506Blood ProteinsHuman genes0.000description1
- 108010017384Blood ProteinsProteins0.000description1
- 229920000049Carbon (fiber)Polymers0.000description1
- KRKNYBCHXYNGOX-UHFFFAOYSA-KCitrateChemical compound[O-]C(=O)CC(O)(CC([O-])=O)C([O-])=OKRKNYBCHXYNGOX-UHFFFAOYSA-K0.000description1
- ZNZYKNKBJPZETN-WELNAUFTSA-NDialdehyde 11678Chemical compoundN1C2=CC=CC=C2C2=C1[C@H](C[C@H](/C(=C/O)C(=O)OC)[C@@H](C=C)C=O)NCC2ZNZYKNKBJPZETN-WELNAUFTSA-N0.000description1
- KCXVZYZYPLLWCC-UHFFFAOYSA-NEDTAChemical compoundOC(=O)CN(CC(O)=O)CCN(CC(O)=O)CC(O)=OKCXVZYZYPLLWCC-UHFFFAOYSA-N0.000description1
- 102000008946FibrinogenHuman genes0.000description1
- 108010049003FibrinogenProteins0.000description1
- 239000000899Gutta-PerchaSubstances0.000description1
- 208000031264Nerve root compressionDiseases0.000description1
- 108010058846OvalbuminProteins0.000description1
- CBENFWSGALASAD-UHFFFAOYSA-NOzoneChemical compound[O-][O+]=OCBENFWSGALASAD-UHFFFAOYSA-N0.000description1
- 208000002193PainDiseases0.000description1
- 240000000342Palaquium guttaSpecies0.000description1
- 239000004696Poly ether ether ketoneSubstances0.000description1
- 206010059604Radicular painDiseases0.000description1
- 206010037779RadiculopathyDiseases0.000description1
- 108010071390Serum AlbuminProteins0.000description1
- 102000007562Serum AlbuminHuman genes0.000description1
- 206010041549Spinal cord compressionDiseases0.000description1
- 229910001069Ti alloyInorganic materials0.000description1
- 230000004520agglutinationEffects0.000description1
- 238000013459approachMethods0.000description1
- 210000004369bloodAnatomy0.000description1
- 239000008280bloodSubstances0.000description1
- 239000002639bone cementSubstances0.000description1
- 230000008468bone growthEffects0.000description1
- 229910000019calcium carbonateInorganic materials0.000description1
- 239000001506calcium phosphateSubstances0.000description1
- 229910000389calcium phosphateInorganic materials0.000description1
- 235000011010calcium phosphatesNutrition0.000description1
- 150000001720carbohydratesChemical class0.000description1
- 235000014633carbohydratesNutrition0.000description1
- 239000004917carbon fiberSubstances0.000description1
- 238000005119centrifugationMethods0.000description1
- 239000003153chemical reaction reagentSubstances0.000description1
- 230000001684chronic effectEffects0.000description1
- 230000035602clottingEffects0.000description1
- 150000001875compoundsChemical class0.000description1
- 230000001054cortical effectEffects0.000description1
- 238000004132cross linkingMethods0.000description1
- 238000007907direct compressionMethods0.000description1
- 239000003814drugSubstances0.000description1
- 229940079593drugDrugs0.000description1
- 229920003247engineering thermoplasticPolymers0.000description1
- 238000002474experimental methodMethods0.000description1
- 238000001125extrusionMethods0.000description1
- 229940012952fibrinogenDrugs0.000description1
- 239000011521glassSubstances0.000description1
- 229940015043glyoxalDrugs0.000description1
- 229920000588gutta-perchaPolymers0.000description1
- 230000036571hydrationEffects0.000description1
- 238000006703hydration reactionMethods0.000description1
- 229910052588hydroxylapatiteInorganic materials0.000description1
- 230000002401inhibitory effectEffects0.000description1
- 239000007924injectionSubstances0.000description1
- 238000002347injectionMethods0.000description1
- 208000014674injuryDiseases0.000description1
- 230000007794irritationEffects0.000description1
- 230000000670limiting effectEffects0.000description1
- 229910001092metal group alloyInorganic materials0.000description1
- 230000004048modificationEffects0.000description1
- 238000012986modificationMethods0.000description1
- 210000000929nociceptorAnatomy0.000description1
- 239000004745nonwoven fabricSubstances0.000description1
- 229920001778nylonPolymers0.000description1
- 238000007248oxidative elimination reactionMethods0.000description1
- 239000002245particleSubstances0.000description1
- 239000013618particulate matterSubstances0.000description1
- XYJRXVWERLGGKC-UHFFFAOYSA-Dpentacalcium;hydroxide;triphosphateChemical compound[OH-].[Ca+2].[Ca+2].[Ca+2].[Ca+2].[Ca+2].[O-]P([O-])([O-])=O.[O-]P([O-])([O-])=O.[O-]P([O-])([O-])=OXYJRXVWERLGGKC-UHFFFAOYSA-D0.000description1
- KHIWWQKSHDUIBK-UHFFFAOYSA-Nperiodic acidChemical compoundOI(=O)(=O)=OKHIWWQKSHDUIBK-UHFFFAOYSA-N0.000description1
- 230000000704physical effectEffects0.000description1
- 238000000554physical therapyMethods0.000description1
- 239000004033plasticSubstances0.000description1
- 229920003023plasticPolymers0.000description1
- 229920000058polyacrylatePolymers0.000description1
- 229920000728polyesterPolymers0.000description1
- 229920002530polyetherether ketonePolymers0.000description1
- 238000006116polymerization reactionMethods0.000description1
- 229920000098polyolefinPolymers0.000description1
- 239000002244precipitateSubstances0.000description1
- 238000004321preservationMethods0.000description1
- 230000002265preventionEffects0.000description1
- 239000000047productSubstances0.000description1
- 230000000750progressive effectEffects0.000description1
- 239000012460protein solutionSubstances0.000description1
- 230000002829reductive effectEffects0.000description1
- 230000003014reinforcing effectEffects0.000description1
- 239000012783reinforcing fiberSubstances0.000description1
- 239000011347resinSubstances0.000description1
- 229920005989resinPolymers0.000description1
- 230000000717retained effectEffects0.000description1
- 150000003839saltsChemical class0.000description1
- 238000000926separation methodMethods0.000description1
- 210000004872soft tissueAnatomy0.000description1
- 239000008247solid mixtureSubstances0.000description1
- 125000006850spacer groupChemical group0.000description1
- 230000000087stabilizing effectEffects0.000description1
- 230000003068static effectEffects0.000description1
- 239000000126substanceSubstances0.000description1
- 238000011477surgical interventionMethods0.000description1
- 230000008961swellingEffects0.000description1
- 229920002994synthetic fiberPolymers0.000description1
- 239000012209synthetic fiberSubstances0.000description1
- 150000003573thiolsChemical group0.000description1
- 238000012546transferMethods0.000description1
- 230000008733traumaEffects0.000description1
- QORWJWZARLRLPR-UHFFFAOYSA-Htricalcium bis(phosphate)Chemical compound[Ca+2].[Ca+2].[Ca+2].[O-]P([O-])([O-])=O.[O-]P([O-])([O-])=OQORWJWZARLRLPR-UHFFFAOYSA-H0.000description1
- 230000001960triggered effectEffects0.000description1
- 239000002759woven fabricSubstances0.000description1
Images
Classifications
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61L—METHODS OR APPARATUS FOR STERILISING MATERIALS OR OBJECTS IN GENERAL; DISINFECTION, STERILISATION OR DEODORISATION OF AIR; CHEMICAL ASPECTS OF BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES; MATERIALS FOR BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES
- A61L27/00—Materials for grafts or prostheses or for coating grafts or prostheses
- A61L27/36—Materials for grafts or prostheses or for coating grafts or prostheses containing ingredients of undetermined constitution or reaction products thereof, e.g. transplant tissue, natural bone, extracellular matrix
- A61L27/3683—Materials for grafts or prostheses or for coating grafts or prostheses containing ingredients of undetermined constitution or reaction products thereof, e.g. transplant tissue, natural bone, extracellular matrix subjected to a specific treatment prior to implantation, e.g. decellularising, demineralising, grinding, cellular disruption/non-collagenous protein removal, anti-calcification, crosslinking, supercritical fluid extraction, enzyme treatment
- A61L27/3691—Materials for grafts or prostheses or for coating grafts or prostheses containing ingredients of undetermined constitution or reaction products thereof, e.g. transplant tissue, natural bone, extracellular matrix subjected to a specific treatment prior to implantation, e.g. decellularising, demineralising, grinding, cellular disruption/non-collagenous protein removal, anti-calcification, crosslinking, supercritical fluid extraction, enzyme treatment characterised by physical conditions of the treatment, e.g. applying a compressive force to the composition, pressure cycles, ultrasonic/sonication or microwave treatment, lyophilisation
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61L—METHODS OR APPARATUS FOR STERILISING MATERIALS OR OBJECTS IN GENERAL; DISINFECTION, STERILISATION OR DEODORISATION OF AIR; CHEMICAL ASPECTS OF BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES; MATERIALS FOR BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES
- A61L27/00—Materials for grafts or prostheses or for coating grafts or prostheses
- A61L27/14—Macromolecular materials
- A61L27/18—Macromolecular materials obtained otherwise than by reactions only involving carbon-to-carbon unsaturated bonds
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61L—METHODS OR APPARATUS FOR STERILISING MATERIALS OR OBJECTS IN GENERAL; DISINFECTION, STERILISATION OR DEODORISATION OF AIR; CHEMICAL ASPECTS OF BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES; MATERIALS FOR BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES
- A61L27/00—Materials for grafts or prostheses or for coating grafts or prostheses
- A61L27/14—Macromolecular materials
- A61L27/22—Polypeptides or derivatives thereof, e.g. degradation products
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61L—METHODS OR APPARATUS FOR STERILISING MATERIALS OR OBJECTS IN GENERAL; DISINFECTION, STERILISATION OR DEODORISATION OF AIR; CHEMICAL ASPECTS OF BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES; MATERIALS FOR BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES
- A61L27/00—Materials for grafts or prostheses or for coating grafts or prostheses
- A61L27/14—Macromolecular materials
- A61L27/22—Polypeptides or derivatives thereof, e.g. degradation products
- A61L27/227—Other specific proteins or polypeptides not covered by A61L27/222, A61L27/225 or A61L27/24
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61L—METHODS OR APPARATUS FOR STERILISING MATERIALS OR OBJECTS IN GENERAL; DISINFECTION, STERILISATION OR DEODORISATION OF AIR; CHEMICAL ASPECTS OF BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES; MATERIALS FOR BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES
- A61L27/00—Materials for grafts or prostheses or for coating grafts or prostheses
- A61L27/36—Materials for grafts or prostheses or for coating grafts or prostheses containing ingredients of undetermined constitution or reaction products thereof, e.g. transplant tissue, natural bone, extracellular matrix
- A61L27/3604—Materials for grafts or prostheses or for coating grafts or prostheses containing ingredients of undetermined constitution or reaction products thereof, e.g. transplant tissue, natural bone, extracellular matrix characterised by the human or animal origin of the biological material, e.g. hair, fascia, fish scales, silk, shellac, pericardium, pleura, renal tissue, amniotic membrane, parenchymal tissue, fetal tissue, muscle tissue, fat tissue, enamel
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61L—METHODS OR APPARATUS FOR STERILISING MATERIALS OR OBJECTS IN GENERAL; DISINFECTION, STERILISATION OR DEODORISATION OF AIR; CHEMICAL ASPECTS OF BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES; MATERIALS FOR BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES
- A61L27/00—Materials for grafts or prostheses or for coating grafts or prostheses
- A61L27/36—Materials for grafts or prostheses or for coating grafts or prostheses containing ingredients of undetermined constitution or reaction products thereof, e.g. transplant tissue, natural bone, extracellular matrix
- A61L27/3641—Materials for grafts or prostheses or for coating grafts or prostheses containing ingredients of undetermined constitution or reaction products thereof, e.g. transplant tissue, natural bone, extracellular matrix characterised by the site of application in the body
- A61L27/3645—Connective tissue
- A61L27/3654—Cartilage, e.g. meniscus
- A61L27/3658—Intervertebral discs
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61L—METHODS OR APPARATUS FOR STERILISING MATERIALS OR OBJECTS IN GENERAL; DISINFECTION, STERILISATION OR DEODORISATION OF AIR; CHEMICAL ASPECTS OF BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES; MATERIALS FOR BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES
- A61L27/00—Materials for grafts or prostheses or for coating grafts or prostheses
- A61L27/40—Composite materials, i.e. containing one material dispersed in a matrix of the same or different material
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F2/00—Filters implantable into blood vessels; Prostheses, i.e. artificial substitutes or replacements for parts of the body; Appliances for connecting them with the body; Devices providing patency to, or preventing collapsing of, tubular structures of the body, e.g. stents
- A61F2/02—Prostheses implantable into the body
- A61F2/30—Joints
- A61F2/44—Joints for the spine, e.g. vertebrae, spinal discs
- A61F2/442—Intervertebral or spinal discs, e.g. resilient
- A61F2002/4445—Means for culturing intervertebral disc tissue
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F2/00—Filters implantable into blood vessels; Prostheses, i.e. artificial substitutes or replacements for parts of the body; Appliances for connecting them with the body; Devices providing patency to, or preventing collapsing of, tubular structures of the body, e.g. stents
- A61F2/02—Prostheses implantable into the body
- A61F2/30—Joints
- A61F2/44—Joints for the spine, e.g. vertebrae, spinal discs
- A61F2/442—Intervertebral or spinal discs, e.g. resilient
- A61F2002/445—Intervertebral disc tissue harvest sites
Definitions
- the present inventionrelates generally to bioprosthetics.
- the present inventionis related to bioprosthetics formed in situ.
- the vertebral discis a collagenous spacer positioned between the vertebral bones of the spinal column.
- the discgenerically consists of a tough fibrillar outer annulus (annulus fibrosus) and a highly hydrated gelatinous core (nucleus pulposus).
- the vertebral discserves as a shock absorber to dissipate the energy of impact loading on the back, as well as a joint, allowing flexion and extension of the human torso.
- DDDDegenerative disc disease
- Disc herniationmay also precipitate the formation of fissures within the annulus that allows extrusion of the disc nucleus (disc herniation) resulting in a sudden collapse in the disc height and the potential for nerve root and/or spinal cord compression.
- Disc herniationmay also result due to trauma related over compression of the spine, such as a heavy sitting fall.
- tubular or other hollow devicesthat may, in addition, contain openings through their walls to allow bone growth through the device, enable the motion segment to be fused with the vertebral spacing maintained.
- These open or tubular devicesmay be constructed of metallic alloys traditional to implantable medical devices (e.g., stainless steel, titanium and titanium alloys), carbon fiber reinforced engineering thermoplastics (e.g., polyetheretherketones), or machined human cortical bone. These devices have been disclosed, for example, in U.S. Pat. No. 4,961,740 to Ray et al; U.S. Pat. No.
- Another general technique for the preservation of vertebral body separationis to replace the removed disc nuclear tissue with non-fusing, non-rigid materials.
- One prior proposalsuggests using a bladder that can be filled with liquid to restore disc height (see, U.S. Pat. No. 3,875,595 to Froning).
- One other prior proposalis disclosed in U.S. Pat. No. 5,534,028 to Bao et al, where a pre-cast pre-shaped hydrogel in placed into the void. Variations on the type of device disclosed in Bao et al '028 are likewise disclosed in U.S. Pat. No. 5,976,186, U.S. Pat. No. 5,192,326, and U.S. Pat. No. 5,047,055.
- 6,022,376is inserted into tunnels drilled into the disc as a dehydrated hydrogel resin, and is allowed to rehydrate and swell once it is inserted. The swelling holds the device in place while preventing the collapse of the denucleated disc. However, the device is neither chemically nor mechanically fixated in place.
- the present inventionrelates to bioprosthetic devices comprised of an exterior biological tissue member which at least partly defines a cavity, and a proteinaceous biopolymer which fills the cavity, and intercalates is chemically bound (linked) to the surrounding biological tissue member.
- the bioprosthetic deviceis a bioprosthetic vertebral disc having a fibrillar outer annulus which surrounds and defines an interior cavity and is formed by removal of at least a substantial portion of the natural gelatinous core therefrom.
- the cavity defined by the fibrillar outer annulusmay then be filled with a flowable biopolymeric material which is then allowed to at least partly solidify in situ (e.g., most preferably by in situ cross-linkage reaction) to form a proteinaceous biopolymer within the cavity.
- a flowable biopolymeric materialwhich is then allowed to at least partly solidify in situ (e.g., most preferably by in situ cross-linkage reaction) to form a proteinaceous biopolymer within the cavity.
- the flowable biopolymeric materialis most preferably a liquid mixture liquid mixture comprised of human or animal-derived protein material and a di- or polyaldehyde.
- the liquid mixturemay then react to form a cross-linked biopolymer in situ within the cavity thereby forming a bioprosthetic device therein.
- the liquid mixturemay be formed in advance of being introduced into the cavity, or may be formed simultaneously during introduction into the cavity.
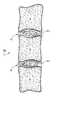
- FIGUREschematically depicts a portion of a patient's vertebral column showing a vertebral disc bioprosthetic in accordance with the present invention interposed between adjacent vertebrae.
- bioprosthetic deviceand like terms mean a combination comprised of a biological tissue member and a proteinaceous biopolymer which is chemically bound (linked) to the tissue of the tissue member.
- the accompanying drawing FIGUREshows a segment of a patient's vertebral column VC wherein vertebral disc bioprosthetics 10 in accordance with the present invention are interposed between adjacent ones of the individual vertebrae V.
- the vertebral disc bioprosthetics 10essentially include the fibrillar outer annulus 10 - 1 of the patient's natural vertebral disc following removal of the gelatinous core.
- the fibrillar outer annulus 10 - 1thus bounds and defines an inner cavity into which a proteinaceous biopolymer 10 - 2 is injected in situ.
- the proteinaceous biopolymer (usually referred to hereinafter more simply as the “biopolymer”) 10 - 2thus fills completely the void space left following removal of the natural gelatinous core of the patient's natural vertebral disc.
- the biopolymer 10 - 2thus acts as a shock-absorber of sorts similar to the natural functions attributable to the removed gelatinous core.
- proteinaceous biopolymerand like terms mean a polymeric or copolymeric material which contains one or more units in the polymer chain comprised of natural, synthetic or sequence-modified proteins or polypeptides, and mixtures and blends of such polymeric and/or copolymeric materials.
- biopolymer 10 - 2that may be employed in the practice of this invention is a cross-linked reaction product of a two part mixture initially comprised of:
- Part Aa water-soluble proteinaceous material of about 27-53% by weight of the mixture
- Part Bdi- or polyaldehydes present in a weight ratio of one part by weight to every 20-60 parts of protein present by weight in the mixture and water, optionally containing non-essential ingredients to make up the balance of the composition.
- Part A of the mixtureis most preferably substantially an aqueous solution of a proteinaceous material of human or animal origin.
- Albumins including ovalbuminsare preferred proteins, and serum albumins of human or animal origin are particularly preferred.
- the proteinaceous materialmay be a purified protein or a mixture in which the proteins such as serum albumins are the predominant ingredients.
- the solid mixtures obtained by dehydration of blood plasma or serum, or of commercial solutions of stabilized plasma proteinscan be used to prepare Part A.
- These mixturesgenerally referred to as plasma solids or serum solids, are known to contain albumins as their major ingredients, of the order of 50-90%.
- plasmarefers to whole blood from which the corpuscles have been removed by centrifugation.
- serumrefers to plasma which has additionally been treated to prevent agglutination by removal of its fibrinogen and/or fibrin, or by inhibiting the fibrin clot formation through addition of reagents, such as citrate or EDTA.
- reagentssuch as citrate or EDTA.
- the proteinaceous materialmay also contain an effective amount of hemoglobin.
- Part Bis substantially an aqueous solution of di- or polyaldehydes.
- di- or polyaldehydesA wide range of these substances exist, and their usefulness is restricted largely by availability and by their solubility in water.
- aqueous glyoxalethandial
- glutaraldehydepenentandial
- Water soluble mixtures of di- and polyaldehydes prepared by oxidative cleavage of appropriate carbohydrates with periodate, ozone or the likeare also useful.
- Glutaraldehydeis the preferred dialdehyde ingredient of Part B.
- Parts A and BWhen Parts A and B are brought together, the resultant product rapidly hardens to a strong, flexible, leathery or rubbery material within a short time of mixing, generally on the order of 15-30 seconds.
- the most preferred material for use in the present inventionis commercially available from CryoLife, Inc. of Kennesaw, Ga. under the registered trademark “BIOGLUE”. (See also, U.S. Pat. No. 5,385,606, the entire content of which is expressly incorporated hereinto by reference.)
- the two components A and B noted aboveare either premixed and then applied, or simultaneously mixed and delivered through an in-line mixing/dispensing tip during the filling of the tissue-defined cavity.
- the resulting biomaterialis a hydrogel that adheres to the surrounding tissue, intercalates into the voids of the surrounding tissues, is space filling, and is mechanically and biologically stable for some time.
- the materialmay be solid or sponge-like in appearance. Furthermore, it may contain organic or inorganic salts or other particulate matter to modify the physical properties of the resulting bioprosthetic device.
- the biopolymer 10 - 2will exhibit compressive strengths of at least 300 kPa (preferably between about 300 to about 600 kPa) and compressive moduli of 2.5 MPa, and creep moduli of 1.0 MPa.
- the ultimate compressive strength of the biopolymer 10 - 2can be adjusted by altering the composition of the protein and cross-linker components and/or through the addition of various fillers.
- the proteinaceous biopolymer that may be employed in the practice of the present inventionmay be include as on reactable component a natural, synthetic or sequence-modified (i.e., so-called “engineered”) polypeptides (e.g., as disclosed more fully in U.S. Pat. No. 6,018,030; U.S. Pat. No. 5,374,431; U.S. Pat. No. 5,606,019 or U.S. Pat. No. 5,817,303, incorporated fully by reference herein).
- engineereda natural, synthetic or sequence-modified polypeptides
- Reactable synthetic polymeric componentsnamely, those which contain functional groups to cause cross-linking (e.g. polyethylene-glycol polymers derivatized with electrophilic and nucleophilic groups such as amine, succinimidyl, anhydride, thiol) may also be employed in the practice of the present invention. See in this regard, U.S. Pat. No. 6,166,130; U.S. Pat. No. 6,051,648; or U.S. Pat. No. 5,900,245, the entirety of each being expressly incorporated hereinto by reference.
- functional groups to cause cross-linkinge.g. polyethylene-glycol polymers derivatized with electrophilic and nucleophilic groups such as amine, succinimidyl, anhydride, thiol
- bioprosthetic vertebral discs of the present inventionexhibit flexibility comparable to the biologically natural vertebral disc. More specifically, the bioprosthetic vertebral discs of the present invention exhibit flexibility comparable to the biologically natural vertebral disc after being subjected to at least about 5 million cycles of a cyclic load of about 0.85 MPa
- the particular properties of the biopolymer 10 - 2can be “engineered” to suit specific end uses.
- the biopolymermay include fibrous or particulate reinforcement (“filler”) material, provided it is biocompatible.
- the reinforcing fibersmay be used in the form of a continuous length of single fibers (i.e., monofilaments) or a yarn, roving or rope of multiple filaments.
- the reinforcing mediamay be in the form of staple fibers of predetermined lengths which are spun into yarns, rovings and/or ropes of desired denier and continuous length.
- the mono- or multifilamentary reinforcing materialsmay also be in the form of woven or non-woven fabric structures. Suffice it to say here, that virtually any physical form of fibrous reinforcing material may be satisfactorily employed in the practice of the present invention.
- the reinforcing materialmay also be in the form of particulates, such as synthetic or natural organic and inorganic particulate reinforcement materials.
- particulatessuch as synthetic or natural organic and inorganic particulate reinforcement materials.
- Some representative examples of such particulatesinclude calcium carbonate, calcium phosphate, hydroxyapatite bone chips, ceramic particles and the like.
- a formulation formed of a protein solution (serum albumin) and a cross linker (gluteraldehyde)was contained in the separate chambers of a delivery device.
- the two componentsare expelled from their respective chambers into a mixing tip that combines the two solutions and mixes them as they travel over the static mixing elements present in the tip.
- a medical needlewas attached to the mixing tip and the formulation injected into the distal space between the vertebra of an explanted pig spine.
- the tipcan be attached to a needle, catheter, or other hollow tubular device for delivery, for example. After 30 seconds, the needle was withdrawn from the injection site. The material that was injected had polymerized in place and did not exude out of the needle hole. After 2 minutes, the disc-vertebra plate was dissected and the presence of the biomaterial seen.
- Bovine calf spineswere obtained from a commercial slaughterhouse and cleaned by blunt and sharp dissection to expose the vertebral bodies and the discs. A 4 mm hole was made into the anterior face of the disc and the drill bit allowed to enter to the center of the nucleus. The nuclear material was removed using surgical forceps and curettes. The hollow space was filled with the formulation described in Example 1. The material that was injected polymerized in place and did not exude out of the hole. After 2 minutes the disc-vertebra plate was dissected and the presence of the biomaterial seen.
- Bovine calf spineswere obtained from a commercial slaughterhouse and cleaned by blunt and sharp dissection to expose the vertebral bodies and the discs. The top and bottom of the vertebral bodies were cut parallel to each other at mid-height using a miter box to yield a bone/disc/bone motion segment. A 4 mm hole was made into the anterior face of the disc and the drill bit allowed to enter into the center of the nucleus. The nuclear material was removed using surgical forceps and curettes. The hollow space was filled with the formulation described in Example 1. The material that was injected had polymerized in place and did not exude out of the hole.
- the constructcould be compressed by hand in the front-back and left-right axes, indicating flexibility was retained after repair of this segment. Then, the construct was placed in a biomaterials testing device (Instron electromechanical test station) and compressed repeatedly to a load of 700 N to condition the construct. Thereafter, a constant load of 700 N was applied to measure compressive creep. The load was held for 10 min. During this time, the polymerized material did not exit from the distal space or the hole. A force of 700 N is the published literature value for the load a lumbar spinal disc experiences when a person of average built is standing upright. The experiment was repeated on 5 separate samples.
- a biomaterials testing deviceInstron electromechanical test station
- the motion segment heightwas measured before removal of the nucleus, after removal of the nucleus, after filling with the biomaterial, and after loading and releasing the load. It was found that (1) the removal of the nucleus reduced the overall height of the material, as well as the compressibility, (2) the filling with the biomaterial restored the disc height and the compressibility.
- a disc of biomaterial formed by injecting a volume of material with the formulation described in Example 1 into a cavity moldwas compressed for 100 and 1000 cycles at a compression rate of 100 mm/min between a minimum stress of 200 kPa and a maximum stress of either 470 or 800 kPa (equivalent to a normal lumbar disc, cross sectional area of 1500 mm 2 , loaded between 300 N and 700 or 1200 N).
- the disc elementdid not exhibit fracture, permanent deformation, or demonstrate a loss of hydration (by mass loss analysis).
- a force of 1200 Nis the published literature value for the compressive load a lumbar spinal disc experiences when a person of average built flexes forward.
- Bovine calf spineswere obtained and prepared as described in Example 3.
- the nucleus pulposuswas accessed either from an anterior or a posterolateral direction.
- the constructswere then placed under a cyclic load of 0.85 MPa at 5 Hz and the load applied for >5 million cycles. During this time, the constructs were kept in physiological saline solution containing a non-fixative biocidal agent.
- the constructswere removed and the disc sliced parallel to the end plates to observe the status of the implants. The implant present in the cavity created by the removal of the nucleus pulposus, was intact and flexible.
Landscapes
- Health & Medical Sciences (AREA)
- Chemical & Material Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Transplantation (AREA)
- Public Health (AREA)
- Dermatology (AREA)
- Medicinal Chemistry (AREA)
- Oral & Maxillofacial Surgery (AREA)
- Veterinary Medicine (AREA)
- Epidemiology (AREA)
- Animal Behavior & Ethology (AREA)
- General Health & Medical Sciences (AREA)
- Engineering & Computer Science (AREA)
- Biomedical Technology (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Botany (AREA)
- Molecular Biology (AREA)
- Urology & Nephrology (AREA)
- Zoology (AREA)
- Vascular Medicine (AREA)
- Composite Materials (AREA)
- Materials Engineering (AREA)
- Prostheses (AREA)
- Materials For Medical Uses (AREA)
Abstract
Description
- The present application is based on, and claims domestic priority benefits under 35 USC §119(e) from, copending U.S. Provisional Application Ser. No. 60/242,457 filed on Oct. 24, 2000, the entire content of which is expressly incorporated hereinto by reference.[0001]
- The present invention relates generally to bioprosthetics. In especially preferred embodiments, the present invention is related to bioprosthetics formed in situ.[0002]
- The vertebral disc is a collagenous spacer positioned between the vertebral bones of the spinal column. The disc generically consists of a tough fibrillar outer annulus (annulus fibrosus) and a highly hydrated gelatinous core (nucleus pulposus). The vertebral disc serves as a shock absorber to dissipate the energy of impact loading on the back, as well as a joint, allowing flexion and extension of the human torso.[0003]
- Degeneration of vertebral disc function in the lumbar portion of the spine is the leading cause of debilitating low back pain in adults over the age of 35. Degenerative disc disease (DDD) is characterized by a gradual collapse of the vertebral disc due to dehydration of the nucleus pulposus, or by a bulging of the annulus fibrosus. DDD may also precipitate the formation of fissures within the annulus that allows extrusion of the disc nucleus (disc herniation) resulting in a sudden collapse in the disc height and the potential for nerve root and/or spinal cord compression. Disc herniation may also result due to trauma related over compression of the spine, such as a heavy sitting fall.[0004]
- Chronic diffuse low back pain results from irritation of pain receptors in the outer third of the disc annulus and surrounding soft tissues as the disc collapses. Radicular pain results from direct compression of the affected nerve root by extruded or bulging disc tissue. Aggressive and extensive physical therapy and drug treatments are the first line treatments for debilitating back pain. In the absence of acceptable pain resolution, surgical intervention is indicated.[0005]
- The traditional surgical procedures for treatment of intractable low back pain due to DDD call for either fusion of the vertebral bodies above and below the affected disc, or removal of the nuclear material thorough open surgical, micro-surgical or endoscopic procedures. Recently, novel procedures involving thermal shrinkage of the collagenous lamina with an electrothermal catheter or laser device have been applied. The removal of the nucleus leaves a void within the disc, and eliminates the viscoelastic fluid that acts as a shock absorber. This void and absence of the viscoelastic fluid creates an opportunity for the lamina to collapse inward and allows the disc space to collapse further. The collapse of the disc space can lead to loss of motion and morbidity, as during the collapse of the disc space the nerves radiating from the spinal column may be pinched.[0006]
- Many surgical techniques and specialized devices have been generated to combat the problem of progressive disc collapse resulting from disc denucleation. Harvested autologous bone has been placed within the denucleated disc space to afford a bony bridge or fusion between the two vertebral bodies. Pedicle screws and other spinal instruments, such as rods and plates, are mechanically affixed to the vertebral bodies, stabilizing the vertebra and preventing further collapse. The problem with these, and other fusion techniques, is the prevention of motion at the level of repair, and resultant transfer of stresses to the levels above and below. These additional loading stresses inevitably result in the degeneration of these disc levels as well.[0007]
- The patent literature discloses several apparati for the replacement of an entire disc (i.e., prosthetic vertebral disc), whereby the damaged disc is removed and a device is anchored to the vertebral bone below and above the damaged disc. The ultimate goal of such a design concept is to maintain or regain the mobility of the native vertebra-disc-vertebra motion segment. Varying degrees of mobility have been claimed for different types of mechanical disc replacements. The following is a non-exhaustive list of such U.S. Patent disclosures:[0008]1U.S. Pat. No. 4,309,777 to Patil; U.S. Pat. No. 5,865,845 to Thalgott; U.S. Pat. No. 5,827,328 to Buttermann; U.S. Pat. No. 5,865,846 to Bryan et al; U.S. Pat. No. 4,759,766 to Buettner-Jantz et al.; U.S. Pat. No. 5,071,437 to Steffe; U.S. Pat. No. 4,911,718 to Lee et al.; and U.S. Pat. No. 4,714,469 to Kenna. The utility of these prior design proposals has been principally limited by an inability to adequately anchor the flexible prosthetic disc to the bony vertebra.
- An alternate approach to the repair of damaged or diseased vertebral discs is to physically prevent disc collapse through the insertion of a rigid body into the disc space. The insertion of tubular or other hollow devices, that may, in addition, contain openings through their walls to allow bone growth through the device, enable the motion segment to be fused with the vertebral spacing maintained. These open or tubular devices may be constructed of metallic alloys traditional to implantable medical devices (e.g., stainless steel, titanium and titanium alloys), carbon fiber reinforced engineering thermoplastics (e.g., polyetheretherketones), or machined human cortical bone. These devices have been disclosed, for example, in U.S. Pat. No. 4,961,740 to Ray et al; U.S. Pat. No. 5,015,247 to Michelson; U.S. Pat. No. 5,766,253 to Brosnahan; U.S. Pat. No. 5,425,772 to Brantigan; and U.S. Pat. No. 5,814,084 to Grivas et al. While these devices may retain the proper spacing between the vertebra (i.e., the disc height), they are disadvantageous since, as the two vertebrae are fused, motion across the vertebra-disc-vertebra element is eliminated.[0009]
- Another general technique for the preservation of vertebral body separation is to replace the removed disc nuclear tissue with non-fusing, non-rigid materials. One prior proposal suggests using a bladder that can be filled with liquid to restore disc height (see, U.S. Pat. No. 3,875,595 to Froning). One other prior proposal is disclosed in U.S. Pat. No. 5,534,028 to Bao et al, where a pre-cast pre-shaped hydrogel in placed into the void. Variations on the type of device disclosed in Bao et al '028 are likewise disclosed in U.S. Pat. No. 5,976,186, U.S. Pat. No. 5,192,326, and U.S. Pat. No. 5,047,055. Preformed inserts made from a xerogel plastic as a nucleus pulposus replacement have also been disclosed in U.S. Pat. No. 6,264,695. A cylindrical hydrogel pillow that is contained within a non-expanding casing and assorted variations thereof are described in U.S. Pat. No. 4,772,287, U.S. Pat. No. 4,904,260, U.S. Pat. No. 5,674,295, U.S. Pat. No. 5,824,093, and U.S. Pat. No. 6,022,376. In this regard, the device shown in U.S. Pat. No. 6,022,376 is inserted into tunnels drilled into the disc as a dehydrated hydrogel resin, and is allowed to rehydrate and swell once it is inserted. The swelling holds the device in place while preventing the collapse of the denucleated disc. However, the device is neither chemically nor mechanically fixated in place.[0010]
- It has also been disclosed in U.S. Pat. No. 6,183,581, 6,206,921 and 6,264,659, that molten gutta percha and its compounds may be used as possible replacements of nucleus pulposus.[0011]
- Broadly, the present invention relates to bioprosthetic devices comprised of an exterior biological tissue member which at least partly defines a cavity, and a proteinaceous biopolymer which fills the cavity, and intercalates is chemically bound (linked) to the surrounding biological tissue member. In preferred forms, the bioprosthetic device is a bioprosthetic vertebral disc having a fibrillar outer annulus which surrounds and defines an interior cavity and is formed by removal of at least a substantial portion of the natural gelatinous core therefrom. The cavity defined by the fibrillar outer annulus may then be filled with a flowable biopolymeric material which is then allowed to at least partly solidify in situ (e.g., most preferably by in situ cross-linkage reaction) to form a proteinaceous biopolymer within the cavity.[0012]
- The flowable biopolymeric material is most preferably a liquid mixture liquid mixture comprised of human or animal-derived protein material and a di- or polyaldehyde. When introduced into the cavity of the tissue member, therefore, the liquid mixture may then react to form a cross-linked biopolymer in situ within the cavity thereby forming a bioprosthetic device therein. The liquid mixture may be formed in advance of being introduced into the cavity, or may be formed simultaneously during introduction into the cavity.[0013]
- These and other aspects and advantages will become more apparent after careful consideration is given to the following detailed description of the preferred exemplary embodiments thereof.[0014]
- Reference will hereinafter be made to the accompanying drawing FIGURE which schematically depicts a portion of a patient's vertebral column showing a vertebral disc bioprosthetic in accordance with the present invention interposed between adjacent vertebrae.[0015]
- As used herein and in the accompanying claims, the term “bioprosthetic device” and like terms mean a combination comprised of a biological tissue member and a proteinaceous biopolymer which is chemically bound (linked) to the tissue of the tissue member.[0016]
- The accompanying drawing FIGURE shows a segment of a patient's vertebral column VC wherein[0017]
vertebral disc bioprosthetics 10 in accordance with the present invention are interposed between adjacent ones of the individual vertebrae V. Thevertebral disc bioprosthetics 10 essentially include the fibrillar outer annulus10-1 of the patient's natural vertebral disc following removal of the gelatinous core. The fibrillar outer annulus10-1 thus bounds and defines an inner cavity into which a proteinaceous biopolymer10-2 is injected in situ. The proteinaceous biopolymer (usually referred to hereinafter more simply as the “biopolymer”)10-2 thus fills completely the void space left following removal of the natural gelatinous core of the patient's natural vertebral disc. The biopolymer10-2 thus acts as a shock-absorber of sorts similar to the natural functions attributable to the removed gelatinous core. - Virtually any suitable proteinaceous biopolymer may be employed in the practice of the present invention. In this regard, the term “proteinaceous biopolymer” and like terms mean a polymeric or copolymeric material which contains one or more units in the polymer chain comprised of natural, synthetic or sequence-modified proteins or polypeptides, and mixtures and blends of such polymeric and/or copolymeric materials.[0018]
- One especially preferred biopolymer[0019]10-2 that may be employed in the practice of this invention is a cross-linked reaction product of a two part mixture initially comprised of:
- Part A: a water-soluble proteinaceous material of about 27-53% by weight of the mixture, and[0020]
- Part B: di- or polyaldehydes present in a weight ratio of one part by weight to every 20-60 parts of protein present by weight in the mixture and water, optionally containing non-essential ingredients to make up the balance of the composition.[0021]
- Part A of the mixture is most preferably substantially an aqueous solution of a proteinaceous material of human or animal origin. Albumins including ovalbumins are preferred proteins, and serum albumins of human or animal origin are particularly preferred. The proteinaceous material may be a purified protein or a mixture in which the proteins such as serum albumins are the predominant ingredients. For example, the solid mixtures obtained by dehydration of blood plasma or serum, or of commercial solutions of stabilized plasma proteins, can be used to prepare Part A. These mixtures, generally referred to as plasma solids or serum solids, are known to contain albumins as their major ingredients, of the order of 50-90%. As used herein, the term “plasma” refers to whole blood from which the corpuscles have been removed by centrifugation. The term “serum” refers to plasma which has additionally been treated to prevent agglutination by removal of its fibrinogen and/or fibrin, or by inhibiting the fibrin clot formation through addition of reagents, such as citrate or EDTA. The proteinaceous material may also contain an effective amount of hemoglobin.[0022]
- Part B is substantially an aqueous solution of di- or polyaldehydes. A wide range of these substances exist, and their usefulness is restricted largely by availability and by their solubility in water. For example, aqueous glyoxal (ethandial) is useful, as is aqueous glutaraldehyde (pentandial). Water soluble mixtures of di- and polyaldehydes prepared by oxidative cleavage of appropriate carbohydrates with periodate, ozone or the like are also useful. Glutaraldehyde is the preferred dialdehyde ingredient of Part B. When Parts A and B are brought together, the resultant product rapidly hardens to a strong, flexible, leathery or rubbery material within a short time of mixing, generally on the order of 15-30 seconds. The most preferred material for use in the present invention is commercially available from CryoLife, Inc. of Kennesaw, Ga. under the registered trademark “BIOGLUE”. (See also, U.S. Pat. No. 5,385,606, the entire content of which is expressly incorporated hereinto by reference.)[0023]
- The two components A and B noted above are either premixed and then applied, or simultaneously mixed and delivered through an in-line mixing/dispensing tip during the filling of the tissue-defined cavity. Upon reaction of the two components, the resulting biomaterial is a hydrogel that adheres to the surrounding tissue, intercalates into the voids of the surrounding tissues, is space filling, and is mechanically and biologically stable for some time. The material may be solid or sponge-like in appearance. Furthermore, it may contain organic or inorganic salts or other particulate matter to modify the physical properties of the resulting bioprosthetic device. Preferably, the biopolymer[0024]10-2 will exhibit compressive strengths of at least 300 kPa (preferably between about 300 to about 600 kPa) and compressive moduli of 2.5 MPa, and creep moduli of 1.0 MPa. The ultimate compressive strength of the biopolymer10-2 can be adjusted by altering the composition of the protein and cross-linker components and/or through the addition of various fillers.
- As noted previously, the proteinaceous biopolymer that may be employed in the practice of the present invention may be include as on reactable component a natural, synthetic or sequence-modified (i.e., so-called “engineered”) polypeptides (e.g., as disclosed more fully in U.S. Pat. No. 6,018,030; U.S. Pat. No. 5,374,431; U.S. Pat. No. 5,606,019 or U.S. Pat. No. 5,817,303, incorporated fully by reference herein). Thus, although many of the following examples employ albumin, it will be understood by those in this art that other reactable components may be employed satisfactorily. Reactable synthetic polymeric components, namely, those which contain functional groups to cause cross-linking (e.g. polyethylene-glycol polymers derivatized with electrophilic and nucleophilic groups such as amine, succinimidyl, anhydride, thiol) may also be employed in the practice of the present invention. See in this regard, U.S. Pat. No. 6,166,130; U.S. Pat. No. 6,051,648; or U.S. Pat. No. 5,900,245, the entirety of each being expressly incorporated hereinto by reference.[0025]
- Nominal compressive mechanical properties that are obtained are similar to those of vertebral discs and lumbar vertebra. The compressive properties of the described biomaterial[0026]10-2 are very different from highly rigid materials traditionally used as implantable structural elements such as stainless steel, titanium, polyacrylate bone cements, ceramics or carbon fiber composites, and hence allow for better biomechanical compatibility in selected indications. For example, the bioprosthetic vertebral discs of the present invention exhibit flexibility comparable to the biologically natural vertebral disc. More specifically, the bioprosthetic vertebral discs of the present invention exhibit flexibility comparable to the biologically natural vertebral disc after being subjected to at least about 5 million cycles of a cyclic load of about 0.85 MPa
- The particular properties of the biopolymer[0027]10-2 can be “engineered” to suit specific end uses. For example, the biopolymer may include fibrous or particulate reinforcement (“filler”) material, provided it is biocompatible.
- Thus, natural or synthetic fibers, such as polyesters, nylons, polyolefins, glass and the like of virtually any desired denier may be employed. Furthermore, the reinforcing fibers may be used in the form of a continuous length of single fibers (i.e., monofilaments) or a yarn, roving or rope of multiple filaments. Moreover, the reinforcing media may be in the form of staple fibers of predetermined lengths which are spun into yarns, rovings and/or ropes of desired denier and continuous length. The mono- or multifilamentary reinforcing materials may also be in the form of woven or non-woven fabric structures. Suffice it to say here, that virtually any physical form of fibrous reinforcing material may be satisfactorily employed in the practice of the present invention.[0028]
- The reinforcing material may also be in the form of particulates, such as synthetic or natural organic and inorganic particulate reinforcement materials. Some representative examples of such particulates include calcium carbonate, calcium phosphate, hydroxyapatite bone chips, ceramic particles and the like.[0029]
- The present invention will be further described with reference to the following non-limiting Examples.[0030]
- A formulation formed of a protein solution (serum albumin) and a cross linker (gluteraldehyde) was contained in the separate chambers of a delivery device. When the device is triggered, the two components are expelled from their respective chambers into a mixing tip that combines the two solutions and mixes them as they travel over the static mixing elements present in the tip. A medical needle was attached to the mixing tip and the formulation injected into the distal space between the vertebra of an explanted pig spine. The tip can be attached to a needle, catheter, or other hollow tubular device for delivery, for example. After 30 seconds, the needle was withdrawn from the injection site. The material that was injected had polymerized in place and did not exude out of the needle hole. After 2 minutes, the disc-vertebra plate was dissected and the presence of the biomaterial seen.[0031]
- Bovine calf spines were obtained from a commercial slaughterhouse and cleaned by blunt and sharp dissection to expose the vertebral bodies and the discs. A 4 mm hole was made into the anterior face of the disc and the drill bit allowed to enter to the center of the nucleus. The nuclear material was removed using surgical forceps and curettes. The hollow space was filled with the formulation described in Example 1. The material that was injected polymerized in place and did not exude out of the hole. After 2 minutes the disc-vertebra plate was dissected and the presence of the biomaterial seen.[0032]
- Bovine calf spines were obtained from a commercial slaughterhouse and cleaned by blunt and sharp dissection to expose the vertebral bodies and the discs. The top and bottom of the vertebral bodies were cut parallel to each other at mid-height using a miter box to yield a bone/disc/bone motion segment. A 4 mm hole was made into the anterior face of the disc and the drill bit allowed to enter into the center of the nucleus. The nuclear material was removed using surgical forceps and curettes. The hollow space was filled with the formulation described in Example 1. The material that was injected had polymerized in place and did not exude out of the hole.[0033]
- Once polymerization had occurred, the construct could be compressed by hand in the front-back and left-right axes, indicating flexibility was retained after repair of this segment. Then, the construct was placed in a biomaterials testing device (Instron electromechanical test station) and compressed repeatedly to a load of 700 N to condition the construct. Thereafter, a constant load of 700 N was applied to measure compressive creep. The load was held for 10 min. During this time, the polymerized material did not exit from the distal space or the hole. A force of 700 N is the published literature value for the load a lumbar spinal disc experiences when a person of average built is standing upright. The experiment was repeated on 5 separate samples.[0034]
- In this example, the motion segment height was measured before removal of the nucleus, after removal of the nucleus, after filling with the biomaterial, and after loading and releasing the load. It was found that (1) the removal of the nucleus reduced the overall height of the material, as well as the compressibility, (2) the filling with the biomaterial restored the disc height and the compressibility.[0035]
- A disc of biomaterial formed by injecting a volume of material with the formulation described in Example 1 into a cavity mold was compressed for 100 and 1000 cycles at a compression rate of 100 mm/min between a minimum stress of 200 kPa and a maximum stress of either 470 or 800 kPa (equivalent to a normal lumbar disc, cross sectional area of 1500 mm[0036]2, loaded between 300 N and 700 or 1200 N). The disc element did not exhibit fracture, permanent deformation, or demonstrate a loss of hydration (by mass loss analysis). A force of 1200 N is the published literature value for the compressive load a lumbar spinal disc experiences when a person of average built flexes forward.
- Bovine calf spines were obtained and prepared as described in Example 3. In this example, the nucleus pulposus was accessed either from an anterior or a posterolateral direction. The constructs were then placed under a cyclic load of 0.85 MPa at 5 Hz and the load applied for >5 million cycles. During this time, the constructs were kept in physiological saline solution containing a non-fixative biocidal agent. At the end of the test period, the constructs were removed and the disc sliced parallel to the end plates to observe the status of the implants. The implant present in the cavity created by the removal of the nucleus pulposus, was intact and flexible.[0037]
- Samples of the biomaterial were formed as described in Example 4. The biomaterial was then placed under a cyclic load of 0.5 MPa at approximately 2 Hz and the load applied for either >5 million cycles or >10 million cycles. During this time, the constructs were kept in physiological saline solution containing a non-fixative biocidal agent. The test samples remained intact throughout the duration of the test, and demonstrated <10% loss in original height.[0038]
- While the invention has been described in connection with what is presently considered to be the most practical and preferred embodiment, it is to be understood that the invention is not to be limited to the disclosed embodiment, but on the contrary, is intended to cover various modifications and equivalent arrangements included within the spirit and scope of the appended claims.[0039]
Claims (42)
Priority Applications (4)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US09/983,537US20020049498A1 (en) | 2000-10-24 | 2001-10-24 | In situ bioprosthetic filler and methods, particularly for the in situ formation of vertebral disc bioprosthetics |
| US11/008,609US7621959B2 (en) | 2000-10-24 | 2004-12-10 | Methods for the in situ formation of a bioprosthetic device, particularly vertebral disc bioprosthetics |
| US11/635,928US7621954B2 (en) | 2000-10-24 | 2006-12-08 | In situ bioprosthetic filler and methods, particularly for in situ formation of vertebral disc bioprosthetics |
| US11/932,066US7896920B2 (en) | 2000-10-24 | 2007-10-31 | In situ bioprosthetic filler and method, particularly for the in situ formation of vertebral disc bioprosthetics |
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US24245700P | 2000-10-24 | 2000-10-24 | |
| US09/983,537US20020049498A1 (en) | 2000-10-24 | 2001-10-24 | In situ bioprosthetic filler and methods, particularly for the in situ formation of vertebral disc bioprosthetics |
Related Child Applications (3)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US11/008,609DivisionUS7621959B2 (en) | 2000-10-24 | 2004-12-10 | Methods for the in situ formation of a bioprosthetic device, particularly vertebral disc bioprosthetics |
| US11/635,928ContinuationUS7621954B2 (en) | 2000-10-24 | 2006-12-08 | In situ bioprosthetic filler and methods, particularly for in situ formation of vertebral disc bioprosthetics |
| US11/932,066ContinuationUS7896920B2 (en) | 2000-10-24 | 2007-10-31 | In situ bioprosthetic filler and method, particularly for the in situ formation of vertebral disc bioprosthetics |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| US20020049498A1true US20020049498A1 (en) | 2002-04-25 |
Family
ID=22914850
Family Applications (4)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US09/983,537AbandonedUS20020049498A1 (en) | 2000-10-24 | 2001-10-24 | In situ bioprosthetic filler and methods, particularly for the in situ formation of vertebral disc bioprosthetics |
| US11/008,609Expired - Fee RelatedUS7621959B2 (en) | 2000-10-24 | 2004-12-10 | Methods for the in situ formation of a bioprosthetic device, particularly vertebral disc bioprosthetics |
| US11/635,928Expired - Fee RelatedUS7621954B2 (en) | 2000-10-24 | 2006-12-08 | In situ bioprosthetic filler and methods, particularly for in situ formation of vertebral disc bioprosthetics |
| US11/932,066Expired - Fee RelatedUS7896920B2 (en) | 2000-10-24 | 2007-10-31 | In situ bioprosthetic filler and method, particularly for the in situ formation of vertebral disc bioprosthetics |
Family Applications After (3)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US11/008,609Expired - Fee RelatedUS7621959B2 (en) | 2000-10-24 | 2004-12-10 | Methods for the in situ formation of a bioprosthetic device, particularly vertebral disc bioprosthetics |
| US11/635,928Expired - Fee RelatedUS7621954B2 (en) | 2000-10-24 | 2006-12-08 | In situ bioprosthetic filler and methods, particularly for in situ formation of vertebral disc bioprosthetics |
| US11/932,066Expired - Fee RelatedUS7896920B2 (en) | 2000-10-24 | 2007-10-31 | In situ bioprosthetic filler and method, particularly for the in situ formation of vertebral disc bioprosthetics |
Country Status (9)
| Country | Link |
|---|---|
| US (4) | US20020049498A1 (en) |
| EP (1) | EP1328220B1 (en) |
| JP (1) | JP4202749B2 (en) |
| AT (1) | ATE494014T1 (en) |
| AU (2) | AU1538702A (en) |
| CA (1) | CA2422884C (en) |
| DE (1) | DE60143804D1 (en) |
| ES (1) | ES2358498T3 (en) |
| WO (1) | WO2002034111A2 (en) |
Cited By (72)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US20020151979A1 (en)* | 1999-08-18 | 2002-10-17 | Lambrecht Greg H. | Devices and method for nucleus pulposus augmentation and retention |
| US20030009227A1 (en)* | 1999-08-18 | 2003-01-09 | Lambrecht Gregory H. | Methods of reinforcing an annulus fibrosis |
| US20030153927A1 (en)* | 2001-05-15 | 2003-08-14 | Endius Incorporated | Structure for receiving surgical instruments |
| US20030181365A1 (en)* | 2002-03-19 | 2003-09-25 | Slivka Michael Andrew | Method for nonsurgical treatment of the intervertebral disc and kit therefor |
| US20030220691A1 (en)* | 2002-05-23 | 2003-11-27 | Pioneer Laboratories, Inc. | Artificial intervertebral disc device |
| US20040024465A1 (en)* | 1999-08-18 | 2004-02-05 | Gregory Lambrecht | Devices and method for augmenting a vertebral disc |
| US20040044412A1 (en)* | 1999-08-18 | 2004-03-04 | Gregory Lambrecht | Devices and method for augmenting a vertebral disc |
| WO2004028414A1 (en) | 2002-09-25 | 2004-04-08 | Medicinelodge, Inc. | Apparatus and method for the in-situ formation of a structural prosthesis |
| US20040133229A1 (en)* | 2000-08-18 | 2004-07-08 | Lambrecht Gregory H. | Minimally invasive system for manipulating intervertebral disc tissue |
| US20040186471A1 (en)* | 2002-12-07 | 2004-09-23 | Sdgi Holdings, Inc. | Method and apparatus for intervertebral disc expansion |
| US20040230305A1 (en)* | 2002-09-24 | 2004-11-18 | Bogomir Gorensek | Stabilizing device for intervertebral disc, and methods thereof |
| US20040228901A1 (en)* | 2002-09-18 | 2004-11-18 | Trieu Hai H. | Collagen-based materials and methods for treating synovial joints |
| US20040260300A1 (en)* | 2003-06-20 | 2004-12-23 | Bogomir Gorensek | Method of delivering an implant through an annular defect in an intervertebral disc |
| US20040260305A1 (en)* | 2003-06-20 | 2004-12-23 | Bogomir Gorensek | Device for delivering an implant through an annular defect in an intervertebral disc |
| US20050004578A1 (en)* | 1999-08-18 | 2005-01-06 | Lambrecht Gregory H. | Apparatus delivery in an intervertebral disc |
| US20050002909A1 (en)* | 2000-04-07 | 2005-01-06 | Centerpulse Biologics Inc | Methods and compositions for treating intervertebral disc degeneration |
| US20050071012A1 (en)* | 2003-09-30 | 2005-03-31 | Hassan Serhan | Methods and devices to replace spinal disc nucleus pulposus |
| US20050119754A1 (en)* | 2002-09-18 | 2005-06-02 | Trieu Hai H. | Compositions and methods for treating intervertebral discs with collagen-based materials |
| US20050116400A1 (en)* | 2003-11-14 | 2005-06-02 | White Moreno J. | Non-linear fiber/matrix architecture |
| US20050182414A1 (en)* | 2004-01-08 | 2005-08-18 | Richard Manzi | Apparatus and method for injecting fluent material at a distracted tissue site |
| US20050192671A1 (en)* | 2002-05-23 | 2005-09-01 | Pioneer Laboratories, Inc. | Artificial disc device |
| US20050209699A1 (en)* | 2002-03-19 | 2005-09-22 | Slivka Michael A | Method for nonsurgical treatment of the nucleus pulposus of the intervertebral disc using genipin or proanthrocyanidin, and kit therefor |
| US20050234557A1 (en)* | 1999-08-18 | 2005-10-20 | Lambrecht Gregory H | Stabilized intervertebral disc barrier |
| US20060188487A1 (en)* | 2005-02-23 | 2006-08-24 | Zimmer Technology, Inc. | Blend hydrogels and methods of making |
| US20060287727A1 (en)* | 2005-06-15 | 2006-12-21 | Jerome Segal | Mechanical apparatus and method for artificial disc replacement |
| US20070003525A1 (en)* | 2003-01-31 | 2007-01-04 | Moehlenbruck Jeffrey W | Hydrogel compositions comprising nucleus pulposus tissue |
| US20070100349A1 (en)* | 2005-10-27 | 2007-05-03 | O'neil Michael | Nucleus augmentation delivery device and technique |
| US20070134333A1 (en)* | 2005-12-07 | 2007-06-14 | Zimmer, Inc. | Methods of bonding or modifying hydrogels using irradiation |
| US20070134343A1 (en)* | 2002-11-15 | 2007-06-14 | Trieu Hai H | Collagen-based materials and methods for treating synovial joints |
| US20070173943A1 (en)* | 2003-01-17 | 2007-07-26 | Dulak Gary R | Artificial nucleus pulposus and method of injecting same |
| US20070203579A1 (en)* | 2006-02-27 | 2007-08-30 | Sdgi Holdings, Inc. | Prosthetic device for spinal arthroplasty |
| US20070233245A1 (en)* | 2006-03-31 | 2007-10-04 | Sdgi Holdings, Inc. | Methods and instruments for delivering intervertebral devices |
| US20070255286A1 (en)* | 2006-04-27 | 2007-11-01 | Sdgi Holdings, Inc. | Devices, apparatus, and methods for improved disc augmentation |
| US20070255406A1 (en)* | 2006-04-27 | 2007-11-01 | Sdgi Holdings, Inc. | Devices, apparatus, and methods for bilateral approach to disc augmentation |
| US20080004570A1 (en)* | 2006-06-30 | 2008-01-03 | Warsaw Orthopedic, Inc. | Collagen delivery device |
| US20080004703A1 (en)* | 2006-06-30 | 2008-01-03 | Warsaw Orthopedic, Inc. | Method of treating a patient using a collagen material |
| US20080004431A1 (en)* | 2006-06-30 | 2008-01-03 | Warsaw Orthopedic Inc | Method of manufacturing an injectable collagen material |
| US20080004214A1 (en)* | 2006-06-30 | 2008-01-03 | Warsaw Orthopedic, Inc | Injectable collagen material |
| US20080033575A1 (en)* | 2006-08-04 | 2008-02-07 | Christopher Walsh | Reversibly deformable implant |
| US20080268056A1 (en)* | 2007-04-26 | 2008-10-30 | Abhijeet Joshi | Injectable copolymer hydrogel useful for repairing vertebral compression fractures |
| US20080269897A1 (en)* | 2007-04-26 | 2008-10-30 | Abhijeet Joshi | Implantable device and methods for repairing articulating joints for using the same |
| US20090093852A1 (en)* | 2007-10-05 | 2009-04-09 | Hynes Richard A | Spinal stabilization treatment methods for maintaining axial spine height and sagital plane spine balance |
| US20090222096A1 (en)* | 2008-02-28 | 2009-09-03 | Warsaw Orthopedic, Inc. | Multi-compartment expandable devices and methods for intervertebral disc expansion and augmentation |
| US20090297603A1 (en)* | 2008-05-29 | 2009-12-03 | Abhijeet Joshi | Interspinous dynamic stabilization system with anisotropic hydrogels |
| US7655012B2 (en) | 2003-10-02 | 2010-02-02 | Zimmer Spine, Inc. | Methods and apparatuses for minimally invasive replacement of intervertebral discs |
| US7731988B2 (en) | 2007-08-03 | 2010-06-08 | Zimmer, Inc. | Multi-polymer hydrogels |
| US7753941B2 (en) | 2000-04-04 | 2010-07-13 | Anulex Technologies, Inc. | Devices and methods for annular repair of intervertebral discs |
| US20100184223A1 (en)* | 2007-07-13 | 2010-07-22 | Helmut Wurst | Biomaterial based on a hydrophilic polymeric carrier |
| US20100322993A1 (en)* | 2008-01-28 | 2010-12-23 | Nmi Naturwissenschaftliches Und Medizinisches Institut An Der Universitaet Tuebingen | Injectable biocompatible composition |
| US7947784B2 (en) | 2007-11-16 | 2011-05-24 | Zimmer, Inc. | Reactive compounding of hydrogels |
| US7959679B2 (en) | 1999-08-18 | 2011-06-14 | Intrinsic Therapeutics, Inc. | Intervertebral anulus and nucleus augmentation |
| US7972337B2 (en) | 2005-12-28 | 2011-07-05 | Intrinsic Therapeutics, Inc. | Devices and methods for bone anchoring |
| US7985781B2 (en) | 2004-10-12 | 2011-07-26 | Zimmer Gmbh | PVA hydrogel |
| US8017107B2 (en) | 2005-12-22 | 2011-09-13 | Zimmer, Inc. | Perfluorocyclobutane crosslinked hydrogels |
| US8034362B2 (en) | 2008-01-04 | 2011-10-11 | Zimmer, Inc. | Chemical composition of hydrogels for use as articulating surfaces |
| US20110270393A1 (en)* | 2008-06-04 | 2011-11-03 | James Marvel | Buffer for a human joint and method of arthroscopically inserting |
| US8062739B2 (en) | 2007-08-31 | 2011-11-22 | Zimmer, Inc. | Hydrogels with gradient |
| US8110242B2 (en) | 2006-03-24 | 2012-02-07 | Zimmer, Inc. | Methods of preparing hydrogel coatings |
| US8133279B2 (en) | 2006-04-27 | 2012-03-13 | Warsaw Orthopedic, Inc. | Methods for treating an annulus defect of an intervertebral disc |
| US8231678B2 (en) | 1999-08-18 | 2012-07-31 | Intrinsic Therapeutics, Inc. | Method of treating a herniated disc |
| US8323341B2 (en) | 2007-09-07 | 2012-12-04 | Intrinsic Therapeutics, Inc. | Impaction grafting for vertebral fusion |
| US8454612B2 (en) | 2007-09-07 | 2013-06-04 | Intrinsic Therapeutics, Inc. | Method for vertebral endplate reconstruction |
| US8518049B2 (en) | 2006-07-27 | 2013-08-27 | Lanx, Inc. | Methods and apparatuses for facilitating percutaneous fusion |
| WO2014074870A1 (en)* | 2012-11-08 | 2014-05-15 | Edwards Lifesciences Corporation | Methods for treating bioprosthetic tissue using a nucleophile/electrophile in a catalytic system |
| US9233011B2 (en) | 2006-09-15 | 2016-01-12 | Pioneer Surgical Technology, Inc. | Systems and apparatuses for inserting an implant in intervertebral space |
| US9241807B2 (en) | 2011-12-23 | 2016-01-26 | Pioneer Surgical Technology, Inc. | Systems and methods for inserting a spinal device |
| US9445916B2 (en) | 2003-10-22 | 2016-09-20 | Pioneer Surgical Technology, Inc. | Joint arthroplasty devices having articulating members |
| US9668875B2 (en) | 1999-03-07 | 2017-06-06 | Nuvasive, Inc. | Method and apparatus for computerized surgery |
| CN110680559A (en)* | 2019-09-27 | 2020-01-14 | 长沙晟天新材料有限公司 | Chest lock integrated piece and preparation method thereof |
| US20200108225A1 (en)* | 2018-10-04 | 2020-04-09 | Edwards Lifesciences Corporation | Stabilizer for a delivery system |
| USD907771S1 (en) | 2017-10-09 | 2021-01-12 | Pioneer Surgical Technology, Inc. | Intervertebral implant |
| US11147682B2 (en) | 2017-09-08 | 2021-10-19 | Pioneer Surgical Technology, Inc. | Intervertebral implants, instruments, and methods |
Families Citing this family (30)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| EP0873145A2 (en) | 1996-11-15 | 1998-10-28 | Advanced Bio Surfaces, Inc. | Biomaterial system for in situ tissue repair |
| EP1328220B1 (en)* | 2000-10-24 | 2011-01-05 | CryoLife, Inc. | Bioprosthetic filler and methods, particularly for the in situ formation of vertebral disc bioprosthetics |
| WO2002085284A2 (en) | 2000-11-07 | 2002-10-31 | Cryolife, Inc. | Expandable foam-like biomaterials and methods |
| DE60207902T2 (en)* | 2001-01-30 | 2006-06-14 | Nissan Chemical Ind Ltd | Isocyanurate compound and process for its preparation |
| AU2002350026A1 (en) | 2001-11-01 | 2003-05-12 | Lawrence M. Boyd | System and method for the pretreatment of the endplates of an intervertebral disc |
| CA2735324A1 (en)* | 2002-11-05 | 2004-05-21 | Spineology, Inc. | A semi-biological intervertebral disc replacement system |
| FR2854801B1 (en)* | 2003-05-12 | 2006-09-01 | Khorionyx | INJECTABLE GLOBINE INSOLUBLE IMPLANT |
| US7789913B2 (en)* | 2004-06-29 | 2010-09-07 | Spine Wave, Inc. | Methods for injecting a curable biomaterial into an intervertebral space |
| JP4601051B2 (en)* | 2004-12-20 | 2010-12-22 | 株式会社ユニバーサルエンターテインメント | Gaming chips |
| US7955339B2 (en)* | 2005-05-24 | 2011-06-07 | Kyphon Sarl | Low-compliance expandable medical device |
| US20070233244A1 (en)* | 2006-03-28 | 2007-10-04 | Depuy Spine, Inc. | Artificial Disc Replacement Using Posterior Approach |
| US8282641B2 (en) | 2006-03-28 | 2012-10-09 | Depuy Spine, Inc. | Methods and instrumentation for disc replacement |
| US8137404B2 (en)* | 2006-03-28 | 2012-03-20 | Depuy Spine, Inc. | Artificial disc replacement using posterior approach |
| US8092536B2 (en)* | 2006-05-24 | 2012-01-10 | Disc Dynamics, Inc. | Retention structure for in situ formation of an intervertebral prosthesis |
| US20070276491A1 (en)* | 2006-05-24 | 2007-11-29 | Disc Dynamics, Inc. | Mold assembly for intervertebral prosthesis |
| US8357168B2 (en)* | 2006-09-08 | 2013-01-22 | Spine Wave, Inc. | Modular injection needle and seal assembly |
| US8715352B2 (en)* | 2006-12-14 | 2014-05-06 | Depuy Spine, Inc. | Buckling disc replacement |
| US8864801B2 (en)* | 2007-04-30 | 2014-10-21 | Warsaw Orthopedic, Inc. | Method of deformity correction in a spine using injectable materials |
| NL1035724C2 (en)* | 2008-07-18 | 2010-01-22 | Univ Eindhoven Tech | Prosthesis comprising a core of a gel material with a woven envelope and a method for the manufacture thereof and the application thereof. |
| US9132207B2 (en) | 2009-10-27 | 2015-09-15 | Spine Wave, Inc. | Radiopaque injectable nucleus hydrogel compositions |
| JP2013520242A (en)* | 2010-02-23 | 2013-06-06 | クリオライフ,インコーポレーテッド | Applicator delivery tip extension |
| US9358122B2 (en) | 2011-01-07 | 2016-06-07 | K2M, Inc. | Interbody spacer |
| US9408624B2 (en) | 2011-03-31 | 2016-08-09 | Isis Innovation Limited | Intervertebral disc treatment apparatus |
| US20120276008A1 (en)* | 2011-04-26 | 2012-11-01 | Spine Wave, Inc. | Radiopaque injectable nucleus hydrogel compositions |
| US20140277467A1 (en) | 2013-03-14 | 2014-09-18 | Spinal Stabilization Technologies, Llc | Prosthetic Spinal Disk Nucleus |
| KR102464886B1 (en) | 2014-11-04 | 2022-11-08 | 스파이널 스태빌라이제이션 테크놀로지스, 엘엘씨 | Percutaneous implantable nuclear prosthesis |
| WO2016073587A1 (en) | 2014-11-04 | 2016-05-12 | Spinal Stabilization Technologies Llc | Percutaneous implantable nuclear prosthesis |
| JP6891176B2 (en) | 2015-09-01 | 2021-06-18 | スパイナル スタビライゼーション テクノロジーズ リミテッド ライアビリティ カンパニー | Implantable nucleus pulposus prosthesis |
| WO2017176973A1 (en)* | 2016-04-07 | 2017-10-12 | Rowan University | Methods and compositions for inducing multi-targeted healing of intervertebral disc defects |
| CA3111639A1 (en) | 2018-09-04 | 2020-05-28 | Spinal Stabilization Technologies, Llc | Implantable nuclear prosthesis, kits, and related methods |
Citations (12)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US5510418A (en)* | 1988-11-21 | 1996-04-23 | Collagen Corporation | Glycosaminoglycan-synthetic polymer conjugates |
| US5766584A (en)* | 1995-06-02 | 1998-06-16 | Massachusetts Institute Of Technology | Inhibition of vascular smooth muscle cell proliferation with implanted matrix containing vascular endothelial cells |
| US5824093A (en)* | 1994-10-17 | 1998-10-20 | Raymedica, Inc. | Prosthetic spinal disc nucleus |
| US5874500A (en)* | 1995-12-18 | 1999-02-23 | Cohesion Technologies, Inc. | Crosslinked polymer compositions and methods for their use |
| US6395032B1 (en)* | 1998-12-11 | 2002-05-28 | Dimso (Distribution Medicale Du Sud-Ouest) | Intervertebral disc prosthesis with liquid chamber |
| US6488952B1 (en)* | 2001-08-28 | 2002-12-03 | John P. Kennedy | Semisolid therapeutic delivery system and combination semisolid, multiparticulate, therapeutic delivery system |
| US20040091540A1 (en)* | 2000-11-15 | 2004-05-13 | Desrosiers Eric Andre | Method for restoring a damaged or degenerated intervertebral disc |
| US20040220296A1 (en)* | 2003-04-30 | 2004-11-04 | Lowman Anthony M. | Thermogelling polymer blends for biomaterial applications |
| US6936070B1 (en)* | 2001-01-17 | 2005-08-30 | Nabil L. Muhanna | Intervertebral disc prosthesis and methods of implantation |
| US7004945B2 (en)* | 2001-11-01 | 2006-02-28 | Spinewave, Inc. | Devices and methods for the restoration of a spinal disc |
| US20060089721A1 (en)* | 2001-01-17 | 2006-04-27 | Muhanna Nabil L | Intervertebral disc prosthesis and methods of implantation |
| US20070173943A1 (en)* | 2003-01-17 | 2007-07-26 | Dulak Gary R | Artificial nucleus pulposus and method of injecting same |
Family Cites Families (54)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US4309777A (en) | 1980-11-13 | 1982-01-12 | Patil Arun A | Artificial intervertebral disc |
| US4472840A (en)* | 1981-09-21 | 1984-09-25 | Jefferies Steven R | Method of inducing osseous formation by implanting bone graft material |
| US4394370A (en)* | 1981-09-21 | 1983-07-19 | Jefferies Steven R | Bone graft material for osseous defects and method of making same |
| EP0164483B1 (en)* | 1984-06-12 | 1989-08-16 | Oscobal Ag | Method of producing a bone replacement material |
| US4620327A (en)* | 1984-07-05 | 1986-11-04 | Caplan Arnold I | Process of adapting soluble bone protein for use in stimulating osteoinduction |
| EP0176728B1 (en) | 1984-09-04 | 1989-07-26 | Humboldt-Universität zu Berlin | Intervertebral-disc prosthesis |
| US4600533A (en) | 1984-12-24 | 1986-07-15 | Collagen Corporation | Collagen membranes for medical use |
| US6018030A (en) | 1986-11-04 | 2000-01-25 | Protein Polymer Technologies, Inc. | Peptides comprising repetitive units of amino acids and DNA sequences encoding the same |
| US4714469A (en) | 1987-02-26 | 1987-12-22 | Pfizer Hospital Products Group, Inc. | Spinal implant |
| DE3734923C1 (en) | 1987-10-15 | 1989-01-26 | Biotest Pharma Gmbh | Process for the preparation of a sterile plasma protein solution containing fibrinogen and coagulation factor XIII |
| US5606019A (en) | 1987-10-29 | 1997-02-25 | Protien Polymer Technologies, Inc. | Synthetic protein as implantables |
| WO1989004646A1 (en)* | 1987-11-13 | 1989-06-01 | Jefferies Steven R | Bone repair material and delayed drug delivery |
| US4911718A (en) | 1988-06-10 | 1990-03-27 | University Of Medicine & Dentistry Of N.J. | Functional and biocompatible intervertebral disc spacer |
| US5213580A (en) | 1988-08-24 | 1993-05-25 | Endoluminal Therapeutics, Inc. | Biodegradable polymeric endoluminal sealing process |
| US5258028A (en)* | 1988-12-12 | 1993-11-02 | Ersek Robert A | Textured micro implants |
| CA1318469C (en) | 1989-02-15 | 1993-06-01 | Acromed Corporation | Artificial disc |
| JP2817966B2 (en) | 1989-10-13 | 1998-10-30 | 旭光学工業株式会社 | Hard tissue replenishing kneaded material with reduced irritation to living body and method for producing the same |
| US5385606A (en)* | 1992-07-06 | 1995-01-31 | Kowanko; Nicholas | Adhesive composition and method |
| EP0621020A1 (en)* | 1993-04-21 | 1994-10-26 | SULZER Medizinaltechnik AG | Intervertebral prosthesis and method of implanting such a prosthesis |
| US5373431A (en) | 1993-08-31 | 1994-12-13 | Cooper Industries, Inc. | Ring/baffle element for a trim of a recessed lighting fixture |
| US5514180A (en) | 1994-01-14 | 1996-05-07 | Heggeness; Michael H. | Prosthetic intervertebral devices |
| JP3127709B2 (en) | 1994-04-25 | 2001-01-29 | ブラザー工業株式会社 | Recording device |
| US6140452A (en)* | 1994-05-06 | 2000-10-31 | Advanced Bio Surfaces, Inc. | Biomaterial for in situ tissue repair |
| AU2621295A (en)* | 1994-05-24 | 1995-12-18 | Smith & Nephew Plc | Intervertebral disc implant |
| US5674296A (en) | 1994-11-14 | 1997-10-07 | Spinal Dynamics Corporation | Human spinal disc prosthesis |
| US5900245A (en) | 1996-03-22 | 1999-05-04 | Focal, Inc. | Compliant tissue sealants |
| US5817303A (en) | 1995-05-05 | 1998-10-06 | Protein Polymer Technologies, Inc. | Bonding together tissue with adhesive containing polyfunctional crosslinking agent and protein polymer |
| US6099565A (en)* | 1995-06-07 | 2000-08-08 | Sakura, Jr.; Chester Y. | Prosthetic tissue implant and filler therefor |
| US5865845A (en) | 1996-03-05 | 1999-02-02 | Thalgott; John S. | Prosthetic intervertebral disc |
| US6066325A (en)* | 1996-08-27 | 2000-05-23 | Fusion Medical Technologies, Inc. | Fragmented polymeric compositions and methods for their use |
| US5895426A (en) | 1996-09-06 | 1999-04-20 | Osteotech, Inc. | Fusion implant device and method of use |
| US5827328A (en) | 1996-11-22 | 1998-10-27 | Buttermann; Glenn R. | Intervertebral prosthetic device |
| IT1291210B1 (en) | 1997-03-18 | 1998-12-29 | Bridgestone Firestone Tech | ANTI-STATIC TIRE. |
| US5800549A (en)* | 1997-04-30 | 1998-09-01 | Howmedica Inc. | Method and apparatus for injecting an elastic spinal implant |
| US5906997A (en)* | 1997-06-17 | 1999-05-25 | Fzio Med, Inc. | Bioresorbable compositions of carboxypolysaccharide polyether intermacromolecular complexes and methods for their use in reducing surgical adhesions |
| US6048346A (en)* | 1997-08-13 | 2000-04-11 | Kyphon Inc. | Systems and methods for injecting flowable materials into bones |
| JP3695511B2 (en) | 1998-07-03 | 2005-09-14 | ニプロ株式会社 | Bone regeneration material |
| KR100563476B1 (en) | 1998-07-03 | 2006-03-27 | 이진용 | Bone regeneration material |
| US6994686B2 (en)* | 1998-08-26 | 2006-02-07 | Neomend, Inc. | Systems for applying cross-linked mechanical barriers |
| DE60020526T2 (en) | 1999-02-04 | 2006-05-11 | SDGI Holdings, Inc., Wilmington | OSTEOGENIC PASTE COMPOSITIONS AND THEIR USE |
| GB9902976D0 (en) | 1999-02-11 | 1999-03-31 | Giltech Ltd | Composite |
| US6264659B1 (en)* | 1999-02-22 | 2001-07-24 | Anthony C. Ross | Method of treating an intervertebral disk |
| US6436143B1 (en)* | 1999-02-22 | 2002-08-20 | Anthony C. Ross | Method and apparatus for treating intervertebral disks |
| WO2000049978A1 (en)* | 1999-02-22 | 2000-08-31 | Guagliano Peter A | Method of treating an intervertebral disk |
| US6206921B1 (en) | 1999-02-22 | 2001-03-27 | Peter A. Guagliano | Method of replacing nucleus pulposus and repairing the intervertebral disk |
| US6428576B1 (en)* | 1999-04-16 | 2002-08-06 | Endospine, Ltd. | System for repairing inter-vertebral discs |
| US6805697B1 (en)* | 1999-05-07 | 2004-10-19 | University Of Virginia Patent Foundation | Method and system for fusing a spinal region |
| US6921412B1 (en)* | 1999-05-18 | 2005-07-26 | Cryolife, Inc. | Self-supporting, shaped, three-dimensional biopolymeric materials and methods |
| US6425919B1 (en) | 1999-08-18 | 2002-07-30 | Intrinsic Orthopedics, Inc. | Devices and methods of vertebral disc augmentation |
| US6264695B1 (en) | 1999-09-30 | 2001-07-24 | Replication Medical, Inc. | Spinal nucleus implant |
| US6332894B1 (en)* | 2000-03-07 | 2001-12-25 | Zimmer, Inc. | Polymer filled spinal fusion cage |
| US6723335B1 (en)* | 2000-04-07 | 2004-04-20 | Jeffrey William Moehlenbruck | Methods and compositions for treating intervertebral disc degeneration |
| EP1328220B1 (en)* | 2000-10-24 | 2011-01-05 | CryoLife, Inc. | Bioprosthetic filler and methods, particularly for the in situ formation of vertebral disc bioprosthetics |
| US7183369B1 (en) | 2003-02-14 | 2007-02-27 | Iowa State University Research Foundation, Inc. | Injectible bodily prosthetics employing methacrylic copolymer gels |
- 2001
- 2001-10-24EPEP01984004Apatent/EP1328220B1/ennot_activeExpired - Lifetime
- 2001-10-24AUAU1538702Apatent/AU1538702A/enactivePending
- 2001-10-24JPJP2002537171Apatent/JP4202749B2/ennot_activeExpired - Fee Related
- 2001-10-24WOPCT/US2001/032632patent/WO2002034111A2/enactiveIP Right Grant
- 2001-10-24AUAU2002215387Apatent/AU2002215387B2/ennot_activeCeased
- 2001-10-24USUS09/983,537patent/US20020049498A1/ennot_activeAbandoned
- 2001-10-24ATAT01984004Tpatent/ATE494014T1/enactive
- 2001-10-24ESES01984004Tpatent/ES2358498T3/ennot_activeExpired - Lifetime
- 2001-10-24CACA002422884Apatent/CA2422884C/ennot_activeExpired - Fee Related
- 2001-10-24DEDE60143804Tpatent/DE60143804D1/ennot_activeExpired - Lifetime
- 2004
- 2004-12-10USUS11/008,609patent/US7621959B2/ennot_activeExpired - Fee Related
- 2006
- 2006-12-08USUS11/635,928patent/US7621954B2/ennot_activeExpired - Fee Related
- 2007
- 2007-10-31USUS11/932,066patent/US7896920B2/ennot_activeExpired - Fee Related
Patent Citations (12)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US5510418A (en)* | 1988-11-21 | 1996-04-23 | Collagen Corporation | Glycosaminoglycan-synthetic polymer conjugates |
| US5824093A (en)* | 1994-10-17 | 1998-10-20 | Raymedica, Inc. | Prosthetic spinal disc nucleus |
| US5766584A (en)* | 1995-06-02 | 1998-06-16 | Massachusetts Institute Of Technology | Inhibition of vascular smooth muscle cell proliferation with implanted matrix containing vascular endothelial cells |
| US5874500A (en)* | 1995-12-18 | 1999-02-23 | Cohesion Technologies, Inc. | Crosslinked polymer compositions and methods for their use |
| US6395032B1 (en)* | 1998-12-11 | 2002-05-28 | Dimso (Distribution Medicale Du Sud-Ouest) | Intervertebral disc prosthesis with liquid chamber |
| US20040091540A1 (en)* | 2000-11-15 | 2004-05-13 | Desrosiers Eric Andre | Method for restoring a damaged or degenerated intervertebral disc |
| US6936070B1 (en)* | 2001-01-17 | 2005-08-30 | Nabil L. Muhanna | Intervertebral disc prosthesis and methods of implantation |
| US20060089721A1 (en)* | 2001-01-17 | 2006-04-27 | Muhanna Nabil L | Intervertebral disc prosthesis and methods of implantation |
| US6488952B1 (en)* | 2001-08-28 | 2002-12-03 | John P. Kennedy | Semisolid therapeutic delivery system and combination semisolid, multiparticulate, therapeutic delivery system |
| US7004945B2 (en)* | 2001-11-01 | 2006-02-28 | Spinewave, Inc. | Devices and methods for the restoration of a spinal disc |
| US20070173943A1 (en)* | 2003-01-17 | 2007-07-26 | Dulak Gary R | Artificial nucleus pulposus and method of injecting same |
| US20040220296A1 (en)* | 2003-04-30 | 2004-11-04 | Lowman Anthony M. | Thermogelling polymer blends for biomaterial applications |
Cited By (168)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US9668875B2 (en) | 1999-03-07 | 2017-06-06 | Nuvasive, Inc. | Method and apparatus for computerized surgery |
| US7124761B2 (en) | 1999-08-18 | 2006-10-24 | Intrinsic Therapeutics, Inc. | Deployment devices and methods for vertebral disc augmentation |
| US20060217812A1 (en)* | 1999-08-18 | 2006-09-28 | Lambrecht Greg H | Method of anchoring an implant in an intervertebral disc |
| US20030093155A1 (en)* | 1999-08-18 | 2003-05-15 | Lambrecht Gregory H. | Deployment devices and methods for vertebral disc augmentation |
| US8021425B2 (en) | 1999-08-18 | 2011-09-20 | Intrinsic Therapeutics, Inc. | Versatile method of repairing an intervertebral disc |
| US7258700B2 (en) | 1999-08-18 | 2007-08-21 | Intrinsic Therapeutics, Inc. | Devices and method for nucleus pulposus augmentation and retention |
| US7998213B2 (en) | 1999-08-18 | 2011-08-16 | Intrinsic Therapeutics, Inc. | Intervertebral disc herniation repair |
| US20040024465A1 (en)* | 1999-08-18 | 2004-02-05 | Gregory Lambrecht | Devices and method for augmenting a vertebral disc |
| US20040030392A1 (en)* | 1999-08-18 | 2004-02-12 | Lambrecht Greg. H. | Method of supporting nucleus pulposus |
| US20040034429A1 (en)* | 1999-08-18 | 2004-02-19 | Lambrecht Gregg H, | Anchored anulus method |
| US20040044412A1 (en)* | 1999-08-18 | 2004-03-04 | Gregory Lambrecht | Devices and method for augmenting a vertebral disc |
| US7553329B2 (en) | 1999-08-18 | 2009-06-30 | Intrinsic Therapeutics, Inc. | Stabilized intervertebral disc barrier |
| US20040097924A1 (en)* | 1999-08-18 | 2004-05-20 | Gregory Lambrecht | Devices and method for augmenting a vertebral disc |
| US7507243B2 (en) | 1999-08-18 | 2009-03-24 | Gregory Lambrecht | Devices and method for augmenting a vertebral disc |
| US7959679B2 (en) | 1999-08-18 | 2011-06-14 | Intrinsic Therapeutics, Inc. | Intervertebral anulus and nucleus augmentation |
| US7553330B2 (en) | 1999-08-18 | 2009-06-30 | Intrinsic Therapeutics, Inc. | Methods of reinforcing an intervertebral disc annulus |
| US8025698B2 (en) | 1999-08-18 | 2011-09-27 | Intrinsic Therapeutics, Inc. | Method of rehabilitating an anulus fibrosus |
| US7524333B2 (en) | 1999-08-18 | 2009-04-28 | Intrinsic Therapeutics, Inc. | Method of anchoring an implant in an intervertebral disc |
| US7658765B2 (en) | 1999-08-18 | 2010-02-09 | Intrinsic Therapeutics, Inc. | Resilient intervertebral disc implant |
| US9706947B2 (en) | 1999-08-18 | 2017-07-18 | Intrinsic Therapeutics, Inc. | Method of performing an anchor implantation procedure within a disc |
| US20030009227A1 (en)* | 1999-08-18 | 2003-01-09 | Lambrecht Gregory H. | Methods of reinforcing an annulus fibrosis |
| US20050004578A1 (en)* | 1999-08-18 | 2005-01-06 | Lambrecht Gregory H. | Apparatus delivery in an intervertebral disc |
| US7220281B2 (en) | 1999-08-18 | 2007-05-22 | Intrinsic Therapeutics, Inc. | Implant for reinforcing and annulus fibrosis |
| US8231678B2 (en) | 1999-08-18 | 2012-07-31 | Intrinsic Therapeutics, Inc. | Method of treating a herniated disc |
| US20050038519A1 (en)* | 1999-08-18 | 2005-02-17 | Lambrecht Gregory H. | Method of reducing spinal implant migration |
| US20030014118A1 (en)* | 1999-08-18 | 2003-01-16 | Lambrecht Gregory H. | Implant for reinforcing and annulus fibrosis |
| US7513911B2 (en) | 1999-08-18 | 2009-04-07 | Intrinsic Therapeutics, Inc. | Method of implanting dynamically stable spinal implant |
| US20020151979A1 (en)* | 1999-08-18 | 2002-10-17 | Lambrecht Greg H. | Devices and method for nucleus pulposus augmentation and retention |
| US9333087B2 (en) | 1999-08-18 | 2016-05-10 | Intrinsic Therapeutics, Inc. | Herniated disc repair |
| US8409284B2 (en) | 1999-08-18 | 2013-04-02 | Intrinsic Therapeutics, Inc. | Methods of repairing herniated segments in the disc |
| US7717961B2 (en) | 1999-08-18 | 2010-05-18 | Intrinsic Therapeutics, Inc. | Apparatus delivery in an intervertebral disc |
| US7749275B2 (en) | 1999-08-18 | 2010-07-06 | Intrinsic Therapeutics, Inc. | Method of reducing spinal implant migration |
| US8257437B2 (en) | 1999-08-18 | 2012-09-04 | Intrinsic Therapeutics, Inc. | Methods of intervertebral disc augmentation |
| US7879097B2 (en) | 1999-08-18 | 2011-02-01 | Intrinsic Therapeutics, Inc. | Method of performing a procedure within a disc |
| US20050234557A1 (en)* | 1999-08-18 | 2005-10-20 | Lambrecht Gregory H | Stabilized intervertebral disc barrier |
| US7563282B2 (en) | 1999-08-18 | 2009-07-21 | Intrinsic Therapeutics, Inc. | Method of supporting nucleus pulposus |
| US7094258B2 (en) | 1999-08-18 | 2006-08-22 | Intrinsic Therapeutics, Inc. | Methods of reinforcing an annulus fibrosis |
| US7867278B2 (en) | 1999-08-18 | 2011-01-11 | Intrinsic Therapeutics, Inc. | Intervertebral disc anulus implant |
| US20050060038A1 (en)* | 1999-08-18 | 2005-03-17 | Lambrecht Gregory E. | Flexible implant for intervertebral disc repair |
| US8002836B2 (en) | 1999-08-18 | 2011-08-23 | Intrinsic Therapeutics, Inc. | Method for the treatment of the intervertebral disc anulus |
| US7905923B2 (en) | 2000-04-04 | 2011-03-15 | Anulex Technologies, Inc. | Devices and methods for annular repair of intervertebral discs |
| US7753941B2 (en) | 2000-04-04 | 2010-07-13 | Anulex Technologies, Inc. | Devices and methods for annular repair of intervertebral discs |
| US7556649B2 (en) | 2000-04-07 | 2009-07-07 | Zimmer Orthobiologics, Inc. | Methods and compositions for treating intervertebral disc degeneration |
| US20050002909A1 (en)* | 2000-04-07 | 2005-01-06 | Centerpulse Biologics Inc | Methods and compositions for treating intervertebral disc degeneration |
| US20040138673A1 (en)* | 2000-08-18 | 2004-07-15 | Lambrecht Gregory H. | Lateral probe advancement in intervertebral disc tissue |
| US20040133229A1 (en)* | 2000-08-18 | 2004-07-08 | Lambrecht Gregory H. | Minimally invasive system for manipulating intervertebral disc tissue |
| US7144397B2 (en) | 2000-08-18 | 2006-12-05 | Intrinsic Therapeutics, Inc. | Minimally invasive system for manipulating intervertebral disc tissue |
| US20030153927A1 (en)* | 2001-05-15 | 2003-08-14 | Endius Incorporated | Structure for receiving surgical instruments |
| US20030181365A1 (en)* | 2002-03-19 | 2003-09-25 | Slivka Michael Andrew | Method for nonsurgical treatment of the intervertebral disc and kit therefor |
| US20050209699A1 (en)* | 2002-03-19 | 2005-09-22 | Slivka Michael A | Method for nonsurgical treatment of the nucleus pulposus of the intervertebral disc using genipin or proanthrocyanidin, and kit therefor |
| US6812211B2 (en)* | 2002-03-19 | 2004-11-02 | Michael Andrew Slivka | Method for nonsurgical treatment of the intervertebral disc and kit therefor |
| US7294617B2 (en) | 2002-03-19 | 2007-11-13 | Depuy Acromed, Inc. | Method for nonsurgical treatment of the intervertebral disc and kit therefor |
| US20050038518A1 (en)* | 2002-03-19 | 2005-02-17 | Depuy Spine, Inc. | Method for nonsurgical treatment of the intervertebral disc and kit therefor |
| US7001433B2 (en) | 2002-05-23 | 2006-02-21 | Pioneer Laboratories, Inc. | Artificial intervertebral disc device |
| US20050192671A1 (en)* | 2002-05-23 | 2005-09-01 | Pioneer Laboratories, Inc. | Artificial disc device |
| US20030220691A1 (en)* | 2002-05-23 | 2003-11-27 | Pioneer Laboratories, Inc. | Artificial intervertebral disc device |
| US9351852B2 (en) | 2002-05-23 | 2016-05-31 | Pioneer Surgical Technology, Inc. | Artificial disc device |
| US8241360B2 (en) | 2002-05-23 | 2012-08-14 | Pioneer Surgical Technology, Inc. | Artificial disc device |
| US8262731B2 (en) | 2002-05-23 | 2012-09-11 | Pioneer Surgical Technology, Inc. | Artificial disc device |
| US8388684B2 (en) | 2002-05-23 | 2013-03-05 | Pioneer Signal Technology, Inc. | Artificial disc device |
| US7744651B2 (en) | 2002-09-18 | 2010-06-29 | Warsaw Orthopedic, Inc | Compositions and methods for treating intervertebral discs with collagen-based materials |
| US20050197707A1 (en)* | 2002-09-18 | 2005-09-08 | Trieu Hai H. | Collagen-based materials and methods for augmenting intervertebral discs |
| US7713303B2 (en) | 2002-09-18 | 2010-05-11 | Warsaw Orthopedic, Inc. | Collagen-based materials and methods for augmenting intervertebral discs |
| US20050119754A1 (en)* | 2002-09-18 | 2005-06-02 | Trieu Hai H. | Compositions and methods for treating intervertebral discs with collagen-based materials |
| US20040228901A1 (en)* | 2002-09-18 | 2004-11-18 | Trieu Hai H. | Collagen-based materials and methods for treating synovial joints |
| US7201775B2 (en) | 2002-09-24 | 2007-04-10 | Bogomir Gorensek | Stabilizing device for intervertebral disc, and methods thereof |
| US20040230305A1 (en)* | 2002-09-24 | 2004-11-18 | Bogomir Gorensek | Stabilizing device for intervertebral disc, and methods thereof |
| US6932843B2 (en) | 2002-09-25 | 2005-08-23 | Medicinelodge, Inc. | Apparatus and method for the in-situ formation of a structural prosthesis |
| WO2004028414A1 (en) | 2002-09-25 | 2004-04-08 | Medicinelodge, Inc. | Apparatus and method for the in-situ formation of a structural prosthesis |
| US7731981B2 (en) | 2002-11-15 | 2010-06-08 | Warsaw Orthopedic, Inc. | Collagen-based materials and methods for treating synovial joints |
| US20070134343A1 (en)* | 2002-11-15 | 2007-06-14 | Trieu Hai H | Collagen-based materials and methods for treating synovial joints |
| US20040186471A1 (en)* | 2002-12-07 | 2004-09-23 | Sdgi Holdings, Inc. | Method and apparatus for intervertebral disc expansion |
| US20070173943A1 (en)* | 2003-01-17 | 2007-07-26 | Dulak Gary R | Artificial nucleus pulposus and method of injecting same |
| US20070003525A1 (en)* | 2003-01-31 | 2007-01-04 | Moehlenbruck Jeffrey W | Hydrogel compositions comprising nucleus pulposus tissue |
| US20040260300A1 (en)* | 2003-06-20 | 2004-12-23 | Bogomir Gorensek | Method of delivering an implant through an annular defect in an intervertebral disc |
| US20040260305A1 (en)* | 2003-06-20 | 2004-12-23 | Bogomir Gorensek | Device for delivering an implant through an annular defect in an intervertebral disc |
| US7500978B2 (en) | 2003-06-20 | 2009-03-10 | Intrinsic Therapeutics, Inc. | Method for delivering and positioning implants in the intervertebral disc environment |
| US7727241B2 (en) | 2003-06-20 | 2010-06-01 | Intrinsic Therapeutics, Inc. | Device for delivering an implant through an annular defect in an intervertebral disc |
| US20060247785A1 (en)* | 2003-06-20 | 2006-11-02 | Bogomir Gorensek | Method for delivering and positioning implants in the intervertebral disc environment |
| US20050071012A1 (en)* | 2003-09-30 | 2005-03-31 | Hassan Serhan | Methods and devices to replace spinal disc nucleus pulposus |
| US7655012B2 (en) | 2003-10-02 | 2010-02-02 | Zimmer Spine, Inc. | Methods and apparatuses for minimally invasive replacement of intervertebral discs |
| US9445916B2 (en) | 2003-10-22 | 2016-09-20 | Pioneer Surgical Technology, Inc. | Joint arthroplasty devices having articulating members |
| WO2005049308A3 (en)* | 2003-11-14 | 2007-04-05 | Moreno J White | Non-linear fiber/matrix architecture |
| US20050116400A1 (en)* | 2003-11-14 | 2005-06-02 | White Moreno J. | Non-linear fiber/matrix architecture |
| US8246630B2 (en) | 2004-01-08 | 2012-08-21 | Spine Wave, Inc. | Apparatus and method for injecting fluent material at a distracted tissue site |
| US8317802B1 (en) | 2004-01-08 | 2012-11-27 | Spine Wave, Inc. | System for distracting opposing vertebral bodies of a spine |
| US7789912B2 (en)* | 2004-01-08 | 2010-09-07 | Spine Wave, Inc. | Apparatus and method for injecting fluent material at a distracted tissue site |
| US8197544B1 (en) | 2004-01-08 | 2012-06-12 | Spine Wave, Inc. | Method for distracting opposing vertebral bodies of a spine |
| US20110004217A1 (en)* | 2004-01-08 | 2011-01-06 | Spine Wave, Inc. | Apparatus and Method for Injecting Fluent Material at a Distracted Tissue Site |
| US20050182414A1 (en)* | 2004-01-08 | 2005-08-18 | Richard Manzi | Apparatus and method for injecting fluent material at a distracted tissue site |
| US7985781B2 (en) | 2004-10-12 | 2011-07-26 | Zimmer Gmbh | PVA hydrogel |
| US20060188487A1 (en)* | 2005-02-23 | 2006-08-24 | Zimmer Technology, Inc. | Blend hydrogels and methods of making |
| US8017139B2 (en) | 2005-02-23 | 2011-09-13 | Zimmer Technology, Inc. | Blend hydrogels and methods of making |
| US7442210B2 (en)* | 2005-06-15 | 2008-10-28 | Jerome Segal | Mechanical apparatus and method for artificial disc replacement |
| US20060287727A1 (en)* | 2005-06-15 | 2006-12-21 | Jerome Segal | Mechanical apparatus and method for artificial disc replacement |
| US20070100349A1 (en)* | 2005-10-27 | 2007-05-03 | O'neil Michael | Nucleus augmentation delivery device and technique |
| US8357199B2 (en) | 2005-10-27 | 2013-01-22 | Depuy Spine, Inc. | Nucleus augmentation delivery device and technique |
| US9162041B2 (en) | 2005-10-27 | 2015-10-20 | DePuy Synthes Products, Inc. | Nucleus augmentation delivery device and technique |
| US8197545B2 (en) | 2005-10-27 | 2012-06-12 | Depuy Spine, Inc. | Nucleus augmentation delivery device and technique |
| US8262730B2 (en) | 2005-12-07 | 2012-09-11 | Zimmer, Inc. | Methods of bonding or modifying hydrogels using irradiation |
| US20070134333A1 (en)* | 2005-12-07 | 2007-06-14 | Zimmer, Inc. | Methods of bonding or modifying hydrogels using irradiation |
| US8017107B2 (en) | 2005-12-22 | 2011-09-13 | Zimmer, Inc. | Perfluorocyclobutane crosslinked hydrogels |
| US8114082B2 (en) | 2005-12-28 | 2012-02-14 | Intrinsic Therapeutics, Inc. | Anchoring system for disc repair |
| US11185354B2 (en) | 2005-12-28 | 2021-11-30 | Intrinsic Therapeutics, Inc. | Bone anchor delivery systems and methods |
| US9039741B2 (en) | 2005-12-28 | 2015-05-26 | Intrinsic Therapeutics, Inc. | Bone anchor systems |
| US8394146B2 (en) | 2005-12-28 | 2013-03-12 | Intrinsic Therapeutics, Inc. | Vertebral anchoring methods |
| US10470804B2 (en) | 2005-12-28 | 2019-11-12 | Intrinsic Therapeutics, Inc. | Bone anchor delivery systems and methods |
| US9610106B2 (en) | 2005-12-28 | 2017-04-04 | Intrinsic Therapeutics, Inc. | Bone anchor systems |
| US7972337B2 (en) | 2005-12-28 | 2011-07-05 | Intrinsic Therapeutics, Inc. | Devices and methods for bone anchoring |
| US20070203579A1 (en)* | 2006-02-27 | 2007-08-30 | Sdgi Holdings, Inc. | Prosthetic device for spinal arthroplasty |
| US7918889B2 (en) | 2006-02-27 | 2011-04-05 | Warsaw Orthopedic, Inc. | Expandable spinal prosthetic devices and associated methods |
| US8110242B2 (en) | 2006-03-24 | 2012-02-07 | Zimmer, Inc. | Methods of preparing hydrogel coatings |
| US20070233245A1 (en)* | 2006-03-31 | 2007-10-04 | Sdgi Holdings, Inc. | Methods and instruments for delivering intervertebral devices |
| US8157863B2 (en) | 2006-04-27 | 2012-04-17 | Warsaw Orthopedic, Inc. | Devices, apparatus, and methods for bilateral approach to disc augmentation |
| US20070255286A1 (en)* | 2006-04-27 | 2007-11-01 | Sdgi Holdings, Inc. | Devices, apparatus, and methods for improved disc augmentation |
| US20070255406A1 (en)* | 2006-04-27 | 2007-11-01 | Sdgi Holdings, Inc. | Devices, apparatus, and methods for bilateral approach to disc augmentation |
| US8133279B2 (en) | 2006-04-27 | 2012-03-13 | Warsaw Orthopedic, Inc. | Methods for treating an annulus defect of an intervertebral disc |
| US20090275913A1 (en)* | 2006-04-27 | 2009-11-05 | Warsaw Orthopedic, Inc. | Devices, apparatus, and methods for bilateral approach to disc augmentation |
| US8399619B2 (en) | 2006-06-30 | 2013-03-19 | Warsaw Orthopedic, Inc. | Injectable collagen material |
| US20080004214A1 (en)* | 2006-06-30 | 2008-01-03 | Warsaw Orthopedic, Inc | Injectable collagen material |
| US8118779B2 (en) | 2006-06-30 | 2012-02-21 | Warsaw Orthopedic, Inc. | Collagen delivery device |
| US20080004570A1 (en)* | 2006-06-30 | 2008-01-03 | Warsaw Orthopedic, Inc. | Collagen delivery device |
| US20080004703A1 (en)* | 2006-06-30 | 2008-01-03 | Warsaw Orthopedic, Inc. | Method of treating a patient using a collagen material |
| US20080004431A1 (en)* | 2006-06-30 | 2008-01-03 | Warsaw Orthopedic Inc | Method of manufacturing an injectable collagen material |
| US8518049B2 (en) | 2006-07-27 | 2013-08-27 | Lanx, Inc. | Methods and apparatuses for facilitating percutaneous fusion |
| US7758649B2 (en)* | 2006-08-04 | 2010-07-20 | Integrity Intellect Inc. | Reversibly deformable implant |
| US20080033575A1 (en)* | 2006-08-04 | 2008-02-07 | Christopher Walsh | Reversibly deformable implant |
| US9693872B2 (en) | 2006-09-15 | 2017-07-04 | Pioneer Surgical Technology, Inc. | Intervertebral disc implant |
| US9233011B2 (en) | 2006-09-15 | 2016-01-12 | Pioneer Surgical Technology, Inc. | Systems and apparatuses for inserting an implant in intervertebral space |
| US10080667B2 (en) | 2006-09-15 | 2018-09-25 | Pioneer Surgical Technology, Inc. | Intervertebral disc implant |
| US20080268056A1 (en)* | 2007-04-26 | 2008-10-30 | Abhijeet Joshi | Injectable copolymer hydrogel useful for repairing vertebral compression fractures |
| US20080269897A1 (en)* | 2007-04-26 | 2008-10-30 | Abhijeet Joshi | Implantable device and methods for repairing articulating joints for using the same |
| US20100184223A1 (en)* | 2007-07-13 | 2010-07-22 | Helmut Wurst | Biomaterial based on a hydrophilic polymeric carrier |
| US10196602B2 (en) | 2007-07-13 | 2019-02-05 | Nmi Naturwissenschaftliches Und Medizinisches Institut An Der Universitaet Tuebingen | Biomaterial based on a hydrophilic polymeric carrier |
| US20100204800A1 (en)* | 2007-08-03 | 2010-08-12 | Zimmer, Inc. | Multi-polymer hydrogels |
| US8236342B2 (en) | 2007-08-03 | 2012-08-07 | Zimmer, Inc. | Multi-polymer hydrogels |
| US7731988B2 (en) | 2007-08-03 | 2010-06-08 | Zimmer, Inc. | Multi-polymer hydrogels |
| US8062739B2 (en) | 2007-08-31 | 2011-11-22 | Zimmer, Inc. | Hydrogels with gradient |
| US10076424B2 (en) | 2007-09-07 | 2018-09-18 | Intrinsic Therapeutics, Inc. | Impaction systems |
| US9226832B2 (en) | 2007-09-07 | 2016-01-05 | Intrinsic Therapeutics, Inc. | Interbody fusion material retention methods |
| US10716685B2 (en) | 2007-09-07 | 2020-07-21 | Intrinsic Therapeutics, Inc. | Bone anchor delivery systems |
| US8454612B2 (en) | 2007-09-07 | 2013-06-04 | Intrinsic Therapeutics, Inc. | Method for vertebral endplate reconstruction |
| US8323341B2 (en) | 2007-09-07 | 2012-12-04 | Intrinsic Therapeutics, Inc. | Impaction grafting for vertebral fusion |
| US8361155B2 (en) | 2007-09-07 | 2013-01-29 | Intrinsic Therapeutics, Inc. | Soft tissue impaction methods |
| US20090093852A1 (en)* | 2007-10-05 | 2009-04-09 | Hynes Richard A | Spinal stabilization treatment methods for maintaining axial spine height and sagital plane spine balance |
| US7947784B2 (en) | 2007-11-16 | 2011-05-24 | Zimmer, Inc. | Reactive compounding of hydrogels |
| US8034362B2 (en) | 2008-01-04 | 2011-10-11 | Zimmer, Inc. | Chemical composition of hydrogels for use as articulating surfaces |
| US20100322993A1 (en)* | 2008-01-28 | 2010-12-23 | Nmi Naturwissenschaftliches Und Medizinisches Institut An Der Universitaet Tuebingen | Injectable biocompatible composition |
| US10500154B2 (en)* | 2008-01-28 | 2019-12-10 | Nmi Naturwissenschaftliches Und Medizinisches Institut An Der Universitaet Tuebingen | Injectable biocompatible composition |
| US20090222096A1 (en)* | 2008-02-28 | 2009-09-03 | Warsaw Orthopedic, Inc. | Multi-compartment expandable devices and methods for intervertebral disc expansion and augmentation |
| US20090297603A1 (en)* | 2008-05-29 | 2009-12-03 | Abhijeet Joshi | Interspinous dynamic stabilization system with anisotropic hydrogels |
| US8764829B2 (en)* | 2008-06-04 | 2014-07-01 | James Marvel | Buffer for a human joint and method of arthroscopically inserting |
| US20110270393A1 (en)* | 2008-06-04 | 2011-11-03 | James Marvel | Buffer for a human joint and method of arthroscopically inserting |
| US9241807B2 (en) | 2011-12-23 | 2016-01-26 | Pioneer Surgical Technology, Inc. | Systems and methods for inserting a spinal device |
| US10159514B2 (en) | 2011-12-23 | 2018-12-25 | Pioneer Surgical Technology, Inc. | Method of implanting a bone plate |
| US11696786B2 (en) | 2011-12-23 | 2023-07-11 | Pioneer Surgical Technology, Inc. | Instrument for inserting a spinal device |
| US10980575B2 (en) | 2011-12-23 | 2021-04-20 | Pioneer Surgical Technology, Inc. | Instrument for inserting a spinal device |
| US11590260B2 (en) | 2012-11-08 | 2023-02-28 | Edwards Lifesciences Corporation | Methods for treating bioprosthetic tissue using a nucleophile/electrophile in a catalytic system |
| WO2014074870A1 (en)* | 2012-11-08 | 2014-05-15 | Edwards Lifesciences Corporation | Methods for treating bioprosthetic tissue using a nucleophile/electrophile in a catalytic system |
| US10238771B2 (en) | 2012-11-08 | 2019-03-26 | Edwards Lifesciences Corporation | Methods for treating bioprosthetic tissue using a nucleophile/electrophile in a catalytic system |
| US11147682B2 (en) | 2017-09-08 | 2021-10-19 | Pioneer Surgical Technology, Inc. | Intervertebral implants, instruments, and methods |
| US12279965B2 (en) | 2017-09-08 | 2025-04-22 | Xtant Medical Holdings, Inc. | Intervertebral implants, instruments, and methods |
| USD907771S1 (en) | 2017-10-09 | 2021-01-12 | Pioneer Surgical Technology, Inc. | Intervertebral implant |
| USD968613S1 (en) | 2017-10-09 | 2022-11-01 | Pioneer Surgical Technology, Inc. | Intervertebral implant |
| US20200108225A1 (en)* | 2018-10-04 | 2020-04-09 | Edwards Lifesciences Corporation | Stabilizer for a delivery system |
| US11931525B2 (en)* | 2018-10-04 | 2024-03-19 | Edwards Lifesciences Corporation | Stabilizer for a delivery system |
| US12350446B2 (en) | 2018-10-04 | 2025-07-08 | Edwards Lifesciences Corporation | Method of using a stabilizer for a delivery system |
| CN110680559A (en)* | 2019-09-27 | 2020-01-14 | 长沙晟天新材料有限公司 | Chest lock integrated piece and preparation method thereof |
Also Published As
| Publication number | Publication date |
|---|---|
| DE60143804D1 (en) | 2011-02-17 |
| AU1538702A (en) | 2002-05-06 |
| US7621954B2 (en) | 2009-11-24 |
| EP1328220B1 (en) | 2011-01-05 |
| CA2422884A1 (en) | 2002-05-02 |
| US20080058942A1 (en) | 2008-03-06 |
| US7621959B2 (en) | 2009-11-24 |
| JP2004532656A (en) | 2004-10-28 |
| ES2358498T3 (en) | 2011-05-11 |
| CA2422884C (en) | 2009-05-19 |
| US7896920B2 (en) | 2011-03-01 |
| JP4202749B2 (en) | 2008-12-24 |
| US20070093902A1 (en) | 2007-04-26 |
| ATE494014T1 (en) | 2011-01-15 |
| EP1328220A2 (en) | 2003-07-23 |
| WO2002034111A3 (en) | 2003-01-16 |
| EP1328220A4 (en) | 2009-08-19 |
| AU2002215387B2 (en) | 2005-09-29 |
| WO2002034111A2 (en) | 2002-05-02 |
| US20050102030A1 (en) | 2005-05-12 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| US7621959B2 (en) | Methods for the in situ formation of a bioprosthetic device, particularly vertebral disc bioprosthetics | |
| AU2002215387A1 (en) | In situ bioprosthetic filler and methods, particularly for the in situ formation of vertebral disc bioprosthetics | |
| JP3007903B2 (en) | Artificial disc | |
| US6746485B1 (en) | Hair used as a biologic disk, replacement, and/or structure and method | |
| EP0353936B1 (en) | Prosthetic disc containing therapeutic material | |
| US7887598B2 (en) | Devices and methods for treating defects in the tissue of a living being | |
| US20090157194A1 (en) | Implant composite material | |
| US20070162131A1 (en) | Repair of spinal annular defects | |
| CN101616642A (en) | fusion device | |
| CN101400381A (en) | Composite implant material | |
| EP2600912B1 (en) | Self-expandable biopolymer-mineral composite | |
| WO2002098329A1 (en) | Biomedical graft for skeletal structures | |
| Turner et al. | Vertebroplasty using injectable calcium phosphate cement compared to polymethylmethacrylate in a unique canine vertebral body large defect model | |
| AU2011286008B9 (en) | Self-expandable biopolymer-mineral composite |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| AS | Assignment | Owner name:CRYOLIFE, INC., GEORGIA Free format text:ASSIGNMENT OF ASSIGNORS INTEREST;ASSIGNORS:YUKSEL, K. UMIT;WALSH, STEVEN P.;BLACK, KIRBY S.;REEL/FRAME:012347/0968;SIGNING DATES FROM 20011101 TO 20011107 | |
| AS | Assignment | Owner name:WELLS FARGO FOOTHILL, INC., GEORGIA Free format text:SECURITY AGREEMENT;ASSIGNORS:CRYOLIFE, INC.;CRYOLIFE TECHNOLOGY, INC.;REEL/FRAME:016256/0042 Effective date:20050208 | |
| AS | Assignment | Owner name:CRYOLIFE, INC., GEORGIA Free format text:RELEASE BY SECURED PARTY;ASSIGNOR:WELLS FARGO FOOTHILL, INC.;REEL/FRAME:020580/0036 Effective date:20080208 | |
| AS | Assignment | Owner name:GENERAL ELECTRIC CAPITAL CORPORATION, AS AGENT, MA Free format text:PATENT SECURITY AGREEMENT;ASSIGNORS:CRYOLIFE, INC.;CRYOLIFE ACQUISITION CORPORATION;REEL/FRAME:020723/0392 Effective date:20080327 | |
| STCB | Information on status: application discontinuation | Free format text:ABANDONED -- AFTER EXAMINER'S ANSWER OR BOARD OF APPEALS DECISION | |
| AS | Assignment | Owner name:HEALTHCARE FINANCIAL SOLUTIONS, LLC, AS SUCCESSOR AGENT, MARYLAND Free format text:ASSIGNMENT OF INTELLECTUAL PROPERTY SECURITY AGREEMENT;ASSIGNOR:GENERAL ELECTRIC CAPITAL CORPORATION, AS RETIRING AGENT;REEL/FRAME:037146/0466 Effective date:20151118 Owner name:HEALTHCARE FINANCIAL SOLUTIONS, LLC, AS SUCCESSOR Free format text:ASSIGNMENT OF INTELLECTUAL PROPERTY SECURITY AGREEMENT;ASSIGNOR:GENERAL ELECTRIC CAPITAL CORPORATION, AS RETIRING AGENT;REEL/FRAME:037146/0466 Effective date:20151118 | |
| AS | Assignment | Owner name:HEALTHCARE FINANCIAL SOLUTIONS, LLC, AS AGENT, MARYLAND Free format text:SECURITY INTEREST;ASSIGNORS:CRYOLIFE, INC., AS GRANTOR;VALVE SPECIAL PURPOSE CO., LLC, AS GRANTOR;ON-X LIFE TECHNOLOGIES, INC., AS GRANTOR;REEL/FRAME:037569/0212 Effective date:20160120 Owner name:HEALTHCARE FINANCIAL SOLUTIONS, LLC, AS AGENT, MAR Free format text:SECURITY INTEREST;ASSIGNORS:CRYOLIFE, INC., AS GRANTOR;VALVE SPECIAL PURPOSE CO., LLC, AS GRANTOR;ON-X LIFE TECHNOLOGIES, INC., AS GRANTOR;REEL/FRAME:037569/0212 Effective date:20160120 | |
| AS | Assignment | Owner name:ON-X LIFE TECHNOLOGIES, INC. (F/K/A MCRI, INC.), GEORGIA Free format text:RELEASE OF SECURITY INTEREST IN PATENTS;ASSIGNOR:HEALTHCARE FINANCIAL SOLUTIONS, LLC, AS ADMINISTRATIVE AGENT;REEL/FRAME:044621/0240 Effective date:20171201 Owner name:CARDIOGENESIS CORPORATION (N/K/A CRYOLIFE, INC.), GEORGIA Free format text:RELEASE OF SECURITY INTEREST IN PATENTS;ASSIGNOR:HEALTHCARE FINANCIAL SOLUTIONS, LLC, AS ADMINISTRATIVE AGENT;REEL/FRAME:044621/0240 Effective date:20171201 Owner name:CRYOLIFE ACQUISITION CORPORATION, GEORGIA Free format text:RELEASE OF SECURITY INTEREST IN PATENTS;ASSIGNOR:HEALTHCARE FINANCIAL SOLUTIONS, LLC, AS ADMINISTRATIVE AGENT;REEL/FRAME:044621/0240 Effective date:20171201 Owner name:CRYOLIFE, INC., GEORGIA Free format text:RELEASE OF SECURITY INTEREST IN PATENTS;ASSIGNOR:HEALTHCARE FINANCIAL SOLUTIONS, LLC, AS ADMINISTRATIVE AGENT;REEL/FRAME:044621/0240 Effective date:20171201 Owner name:ON-X LIFE TECHNOLOGIES, INC. (F/K/A MCRI, INC.), G Free format text:RELEASE OF SECURITY INTEREST IN PATENTS;ASSIGNOR:HEALTHCARE FINANCIAL SOLUTIONS, LLC, AS ADMINISTRATIVE AGENT;REEL/FRAME:044621/0240 Effective date:20171201 Owner name:CARDIOGENESIS CORPORATION (N/K/A CRYOLIFE, INC.), Free format text:RELEASE OF SECURITY INTEREST IN PATENTS;ASSIGNOR:HEALTHCARE FINANCIAL SOLUTIONS, LLC, AS ADMINISTRATIVE AGENT;REEL/FRAME:044621/0240 Effective date:20171201 Owner name:HEMOSPHERE, INC., GEORGIA Free format text:RELEASE OF SECURITY INTEREST IN PATENTS;ASSIGNOR:HEALTHCARE FINANCIAL SOLUTIONS, LLC, AS ADMINISTRATIVE AGENT;REEL/FRAME:044621/0240 Effective date:20171201 Owner name:VALVE SPECIAL PURPOSE CO., LLC, GEORGIA Free format text:RELEASE OF SECURITY INTEREST IN PATENTS;ASSIGNOR:HEALTHCARE FINANCIAL SOLUTIONS, LLC, AS ADMINISTRATIVE AGENT;REEL/FRAME:044621/0240 Effective date:20171201 |