US20010031254A1 - Assembled implant - Google Patents
Assembled implantDownload PDFInfo
- Publication number
- US20010031254A1 US20010031254A1US09/782,594US78259401AUS2001031254A1US 20010031254 A1US20010031254 A1US 20010031254A1US 78259401 AUS78259401 AUS 78259401AUS 2001031254 A1US2001031254 A1US 2001031254A1
- Authority
- US
- United States
- Prior art keywords
- bone
- graft
- unit
- implant
- portions
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Abandoned
Links
Images
Classifications
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F2/00—Filters implantable into blood vessels; Prostheses, i.e. artificial substitutes or replacements for parts of the body; Appliances for connecting them with the body; Devices providing patency to, or preventing collapsing of, tubular structures of the body, e.g. stents
- A61F2/02—Prostheses implantable into the body
- A61F2/28—Bones
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F2/00—Filters implantable into blood vessels; Prostheses, i.e. artificial substitutes or replacements for parts of the body; Appliances for connecting them with the body; Devices providing patency to, or preventing collapsing of, tubular structures of the body, e.g. stents
- A61F2/02—Prostheses implantable into the body
- A61F2/30—Joints
- A61F2/44—Joints for the spine, e.g. vertebrae, spinal discs
- A61F2/4455—Joints for the spine, e.g. vertebrae, spinal discs for the fusion of spinal bodies, e.g. intervertebral fusion of adjacent spinal bodies, e.g. fusion cages
- A61F2/446—Joints for the spine, e.g. vertebrae, spinal discs for the fusion of spinal bodies, e.g. intervertebral fusion of adjacent spinal bodies, e.g. fusion cages having a circular or elliptical cross-section substantially parallel to the axis of the spine, e.g. cylinders or frustocones
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F2/00—Filters implantable into blood vessels; Prostheses, i.e. artificial substitutes or replacements for parts of the body; Appliances for connecting them with the body; Devices providing patency to, or preventing collapsing of, tubular structures of the body, e.g. stents
- A61F2/02—Prostheses implantable into the body
- A61F2/30—Joints
- A61F2/44—Joints for the spine, e.g. vertebrae, spinal discs
- A61F2/4455—Joints for the spine, e.g. vertebrae, spinal discs for the fusion of spinal bodies, e.g. intervertebral fusion of adjacent spinal bodies, e.g. fusion cages
- A61F2/447—Joints for the spine, e.g. vertebrae, spinal discs for the fusion of spinal bodies, e.g. intervertebral fusion of adjacent spinal bodies, e.g. fusion cages substantially parallelepipedal, e.g. having a rectangular or trapezoidal cross-section
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61L—METHODS OR APPARATUS FOR STERILISING MATERIALS OR OBJECTS IN GENERAL; DISINFECTION, STERILISATION OR DEODORISATION OF AIR; CHEMICAL ASPECTS OF BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES; MATERIALS FOR BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES
- A61L2/00—Methods or apparatus for disinfecting or sterilising materials or objects other than foodstuffs or contact lenses; Accessories therefor
- A61L2/0005—Methods or apparatus for disinfecting or sterilising materials or objects other than foodstuffs or contact lenses; Accessories therefor for pharmaceuticals, biologicals or living parts
- A61L2/0082—Methods or apparatus for disinfecting or sterilising materials or objects other than foodstuffs or contact lenses; Accessories therefor for pharmaceuticals, biologicals or living parts using chemical substances
- A61L2/0088—Liquid substances
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61L—METHODS OR APPARATUS FOR STERILISING MATERIALS OR OBJECTS IN GENERAL; DISINFECTION, STERILISATION OR DEODORISATION OF AIR; CHEMICAL ASPECTS OF BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES; MATERIALS FOR BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES
- A61L2/00—Methods or apparatus for disinfecting or sterilising materials or objects other than foodstuffs or contact lenses; Accessories therefor
- A61L2/02—Methods or apparatus for disinfecting or sterilising materials or objects other than foodstuffs or contact lenses; Accessories therefor using physical phenomena
- A61L2/025—Ultrasonics
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F2/00—Filters implantable into blood vessels; Prostheses, i.e. artificial substitutes or replacements for parts of the body; Appliances for connecting them with the body; Devices providing patency to, or preventing collapsing of, tubular structures of the body, e.g. stents
- A61F2/02—Prostheses implantable into the body
- A61F2/08—Muscles; Tendons; Ligaments
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F2/00—Filters implantable into blood vessels; Prostheses, i.e. artificial substitutes or replacements for parts of the body; Appliances for connecting them with the body; Devices providing patency to, or preventing collapsing of, tubular structures of the body, e.g. stents
- A61F2/02—Prostheses implantable into the body
- A61F2/08—Muscles; Tendons; Ligaments
- A61F2/0811—Fixation devices for tendons or ligaments
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F2/00—Filters implantable into blood vessels; Prostheses, i.e. artificial substitutes or replacements for parts of the body; Appliances for connecting them with the body; Devices providing patency to, or preventing collapsing of, tubular structures of the body, e.g. stents
- A61F2/02—Prostheses implantable into the body
- A61F2/30—Joints
- A61F2/3094—Designing or manufacturing processes
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F2/00—Filters implantable into blood vessels; Prostheses, i.e. artificial substitutes or replacements for parts of the body; Appliances for connecting them with the body; Devices providing patency to, or preventing collapsing of, tubular structures of the body, e.g. stents
- A61F2/02—Prostheses implantable into the body
- A61F2/30—Joints
- A61F2/44—Joints for the spine, e.g. vertebrae, spinal discs
- A61F2/442—Intervertebral or spinal discs, e.g. resilient
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F2/00—Filters implantable into blood vessels; Prostheses, i.e. artificial substitutes or replacements for parts of the body; Appliances for connecting them with the body; Devices providing patency to, or preventing collapsing of, tubular structures of the body, e.g. stents
- A61F2/02—Prostheses implantable into the body
- A61F2/30—Joints
- A61F2/46—Special tools for implanting artificial joints
- A61F2/4644—Preparation of bone graft, bone plugs or bone dowels, e.g. grinding or milling bone material
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F2/00—Filters implantable into blood vessels; Prostheses, i.e. artificial substitutes or replacements for parts of the body; Appliances for connecting them with the body; Devices providing patency to, or preventing collapsing of, tubular structures of the body, e.g. stents
- A61F2/02—Prostheses implantable into the body
- A61F2/28—Bones
- A61F2002/2817—Bone stimulation by chemical reactions or by osteogenic or biological products for enhancing ossification, e.g. by bone morphogenetic or morphogenic proteins [BMP] or by transforming growth factors [TGF]
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F2/00—Filters implantable into blood vessels; Prostheses, i.e. artificial substitutes or replacements for parts of the body; Appliances for connecting them with the body; Devices providing patency to, or preventing collapsing of, tubular structures of the body, e.g. stents
- A61F2/02—Prostheses implantable into the body
- A61F2/28—Bones
- A61F2002/2835—Bone graft implants for filling a bony defect or an endoprosthesis cavity, e.g. by synthetic material or biological material
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F2/00—Filters implantable into blood vessels; Prostheses, i.e. artificial substitutes or replacements for parts of the body; Appliances for connecting them with the body; Devices providing patency to, or preventing collapsing of, tubular structures of the body, e.g. stents
- A61F2/02—Prostheses implantable into the body
- A61F2/28—Bones
- A61F2002/2835—Bone graft implants for filling a bony defect or an endoprosthesis cavity, e.g. by synthetic material or biological material
- A61F2002/2839—Bone plugs or bone graft dowels
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F2/00—Filters implantable into blood vessels; Prostheses, i.e. artificial substitutes or replacements for parts of the body; Appliances for connecting them with the body; Devices providing patency to, or preventing collapsing of, tubular structures of the body, e.g. stents
- A61F2/02—Prostheses implantable into the body
- A61F2/30—Joints
- A61F2002/30001—Additional features of subject-matter classified in A61F2/28, A61F2/30 and subgroups thereof
- A61F2002/30003—Material related properties of the prosthesis or of a coating on the prosthesis
- A61F2002/30004—Material related properties of the prosthesis or of a coating on the prosthesis the prosthesis being made from materials having different values of a given property at different locations within the same prosthesis
- A61F2002/30057—Material related properties of the prosthesis or of a coating on the prosthesis the prosthesis being made from materials having different values of a given property at different locations within the same prosthesis made from both cortical and cancellous adjacent parts
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F2/00—Filters implantable into blood vessels; Prostheses, i.e. artificial substitutes or replacements for parts of the body; Appliances for connecting them with the body; Devices providing patency to, or preventing collapsing of, tubular structures of the body, e.g. stents
- A61F2/02—Prostheses implantable into the body
- A61F2/30—Joints
- A61F2002/30001—Additional features of subject-matter classified in A61F2/28, A61F2/30 and subgroups thereof
- A61F2002/30003—Material related properties of the prosthesis or of a coating on the prosthesis
- A61F2002/30004—Material related properties of the prosthesis or of a coating on the prosthesis the prosthesis being made from materials having different values of a given property at different locations within the same prosthesis
- A61F2002/30059—Material related properties of the prosthesis or of a coating on the prosthesis the prosthesis being made from materials having different values of a given property at different locations within the same prosthesis differing in bone mineralization, e.g. made from both mineralized and demineralized adjacent parts
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F2/00—Filters implantable into blood vessels; Prostheses, i.e. artificial substitutes or replacements for parts of the body; Appliances for connecting them with the body; Devices providing patency to, or preventing collapsing of, tubular structures of the body, e.g. stents
- A61F2/02—Prostheses implantable into the body
- A61F2/30—Joints
- A61F2002/30001—Additional features of subject-matter classified in A61F2/28, A61F2/30 and subgroups thereof
- A61F2002/30003—Material related properties of the prosthesis or of a coating on the prosthesis
- A61F2002/3006—Properties of materials and coating materials
- A61F2002/30062—(bio)absorbable, biodegradable, bioerodable, (bio)resorbable, resorptive
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F2/00—Filters implantable into blood vessels; Prostheses, i.e. artificial substitutes or replacements for parts of the body; Appliances for connecting them with the body; Devices providing patency to, or preventing collapsing of, tubular structures of the body, e.g. stents
- A61F2/02—Prostheses implantable into the body
- A61F2/30—Joints
- A61F2002/30001—Additional features of subject-matter classified in A61F2/28, A61F2/30 and subgroups thereof
- A61F2002/30108—Shapes
- A61F2002/3011—Cross-sections or two-dimensional shapes
- A61F2002/30112—Rounded shapes, e.g. with rounded corners
- A61F2002/30113—Rounded shapes, e.g. with rounded corners circular
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F2/00—Filters implantable into blood vessels; Prostheses, i.e. artificial substitutes or replacements for parts of the body; Appliances for connecting them with the body; Devices providing patency to, or preventing collapsing of, tubular structures of the body, e.g. stents
- A61F2/02—Prostheses implantable into the body
- A61F2/30—Joints
- A61F2002/30001—Additional features of subject-matter classified in A61F2/28, A61F2/30 and subgroups thereof
- A61F2002/30108—Shapes
- A61F2002/3011—Cross-sections or two-dimensional shapes
- A61F2002/30138—Convex polygonal shapes
- A61F2002/30153—Convex polygonal shapes rectangular
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F2/00—Filters implantable into blood vessels; Prostheses, i.e. artificial substitutes or replacements for parts of the body; Appliances for connecting them with the body; Devices providing patency to, or preventing collapsing of, tubular structures of the body, e.g. stents
- A61F2/02—Prostheses implantable into the body
- A61F2/30—Joints
- A61F2002/30001—Additional features of subject-matter classified in A61F2/28, A61F2/30 and subgroups thereof
- A61F2002/30108—Shapes
- A61F2002/3011—Cross-sections or two-dimensional shapes
- A61F2002/30159—Concave polygonal shapes
- A61F2002/30179—X-shaped
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F2/00—Filters implantable into blood vessels; Prostheses, i.e. artificial substitutes or replacements for parts of the body; Appliances for connecting them with the body; Devices providing patency to, or preventing collapsing of, tubular structures of the body, e.g. stents
- A61F2/02—Prostheses implantable into the body
- A61F2/30—Joints
- A61F2002/30001—Additional features of subject-matter classified in A61F2/28, A61F2/30 and subgroups thereof
- A61F2002/30108—Shapes
- A61F2002/30199—Three-dimensional shapes
- A61F2002/30224—Three-dimensional shapes cylindrical
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F2/00—Filters implantable into blood vessels; Prostheses, i.e. artificial substitutes or replacements for parts of the body; Appliances for connecting them with the body; Devices providing patency to, or preventing collapsing of, tubular structures of the body, e.g. stents
- A61F2/02—Prostheses implantable into the body
- A61F2/30—Joints
- A61F2002/30001—Additional features of subject-matter classified in A61F2/28, A61F2/30 and subgroups thereof
- A61F2002/30108—Shapes
- A61F2002/30199—Three-dimensional shapes
- A61F2002/30224—Three-dimensional shapes cylindrical
- A61F2002/30225—Flat cylinders, i.e. discs
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F2/00—Filters implantable into blood vessels; Prostheses, i.e. artificial substitutes or replacements for parts of the body; Appliances for connecting them with the body; Devices providing patency to, or preventing collapsing of, tubular structures of the body, e.g. stents
- A61F2/02—Prostheses implantable into the body
- A61F2/30—Joints
- A61F2002/30001—Additional features of subject-matter classified in A61F2/28, A61F2/30 and subgroups thereof
- A61F2002/30108—Shapes
- A61F2002/30199—Three-dimensional shapes
- A61F2002/30224—Three-dimensional shapes cylindrical
- A61F2002/30235—Three-dimensional shapes cylindrical tubular, e.g. sleeves
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F2/00—Filters implantable into blood vessels; Prostheses, i.e. artificial substitutes or replacements for parts of the body; Appliances for connecting them with the body; Devices providing patency to, or preventing collapsing of, tubular structures of the body, e.g. stents
- A61F2/02—Prostheses implantable into the body
- A61F2/30—Joints
- A61F2002/30001—Additional features of subject-matter classified in A61F2/28, A61F2/30 and subgroups thereof
- A61F2002/30108—Shapes
- A61F2002/30199—Three-dimensional shapes
- A61F2002/30261—Three-dimensional shapes parallelepipedal
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F2/00—Filters implantable into blood vessels; Prostheses, i.e. artificial substitutes or replacements for parts of the body; Appliances for connecting them with the body; Devices providing patency to, or preventing collapsing of, tubular structures of the body, e.g. stents
- A61F2/02—Prostheses implantable into the body
- A61F2/30—Joints
- A61F2002/30001—Additional features of subject-matter classified in A61F2/28, A61F2/30 and subgroups thereof
- A61F2002/30108—Shapes
- A61F2002/30199—Three-dimensional shapes
- A61F2002/30261—Three-dimensional shapes parallelepipedal
- A61F2002/30266—Three-dimensional shapes parallelepipedal wedge-shaped parallelepipeds
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F2/00—Filters implantable into blood vessels; Prostheses, i.e. artificial substitutes or replacements for parts of the body; Appliances for connecting them with the body; Devices providing patency to, or preventing collapsing of, tubular structures of the body, e.g. stents
- A61F2/02—Prostheses implantable into the body
- A61F2/30—Joints
- A61F2002/30001—Additional features of subject-matter classified in A61F2/28, A61F2/30 and subgroups thereof
- A61F2002/30108—Shapes
- A61F2002/30199—Three-dimensional shapes
- A61F2002/3028—Three-dimensional shapes polyhedral different from parallelepipedal and pyramidal
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F2/00—Filters implantable into blood vessels; Prostheses, i.e. artificial substitutes or replacements for parts of the body; Appliances for connecting them with the body; Devices providing patency to, or preventing collapsing of, tubular structures of the body, e.g. stents
- A61F2/02—Prostheses implantable into the body
- A61F2/30—Joints
- A61F2002/30001—Additional features of subject-matter classified in A61F2/28, A61F2/30 and subgroups thereof
- A61F2002/30316—The prosthesis having different structural features at different locations within the same prosthesis; Connections between prosthetic parts; Special structural features of bone or joint prostheses not otherwise provided for
- A61F2002/30329—Connections or couplings between prosthetic parts, e.g. between modular parts; Connecting elements
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F2/00—Filters implantable into blood vessels; Prostheses, i.e. artificial substitutes or replacements for parts of the body; Appliances for connecting them with the body; Devices providing patency to, or preventing collapsing of, tubular structures of the body, e.g. stents
- A61F2/02—Prostheses implantable into the body
- A61F2/30—Joints
- A61F2002/30001—Additional features of subject-matter classified in A61F2/28, A61F2/30 and subgroups thereof
- A61F2002/30316—The prosthesis having different structural features at different locations within the same prosthesis; Connections between prosthetic parts; Special structural features of bone or joint prostheses not otherwise provided for
- A61F2002/30329—Connections or couplings between prosthetic parts, e.g. between modular parts; Connecting elements
- A61F2002/30331—Connections or couplings between prosthetic parts, e.g. between modular parts; Connecting elements made by longitudinally pushing a protrusion into a complementarily-shaped recess, e.g. held by friction fit
- A61F2002/30354—Cylindrically-shaped protrusion and recess, e.g. cylinder of circular basis
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F2/00—Filters implantable into blood vessels; Prostheses, i.e. artificial substitutes or replacements for parts of the body; Appliances for connecting them with the body; Devices providing patency to, or preventing collapsing of, tubular structures of the body, e.g. stents
- A61F2/02—Prostheses implantable into the body
- A61F2/30—Joints
- A61F2002/30001—Additional features of subject-matter classified in A61F2/28, A61F2/30 and subgroups thereof
- A61F2002/30316—The prosthesis having different structural features at different locations within the same prosthesis; Connections between prosthetic parts; Special structural features of bone or joint prostheses not otherwise provided for
- A61F2002/30329—Connections or couplings between prosthetic parts, e.g. between modular parts; Connecting elements
- A61F2002/30383—Connections or couplings between prosthetic parts, e.g. between modular parts; Connecting elements made by laterally inserting a protrusion, e.g. a rib into a complementarily-shaped groove
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F2/00—Filters implantable into blood vessels; Prostheses, i.e. artificial substitutes or replacements for parts of the body; Appliances for connecting them with the body; Devices providing patency to, or preventing collapsing of, tubular structures of the body, e.g. stents
- A61F2/02—Prostheses implantable into the body
- A61F2/30—Joints
- A61F2002/30001—Additional features of subject-matter classified in A61F2/28, A61F2/30 and subgroups thereof
- A61F2002/30316—The prosthesis having different structural features at different locations within the same prosthesis; Connections between prosthetic parts; Special structural features of bone or joint prostheses not otherwise provided for
- A61F2002/30329—Connections or couplings between prosthetic parts, e.g. between modular parts; Connecting elements
- A61F2002/30383—Connections or couplings between prosthetic parts, e.g. between modular parts; Connecting elements made by laterally inserting a protrusion, e.g. a rib into a complementarily-shaped groove
- A61F2002/30387—Dovetail connection
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F2/00—Filters implantable into blood vessels; Prostheses, i.e. artificial substitutes or replacements for parts of the body; Appliances for connecting them with the body; Devices providing patency to, or preventing collapsing of, tubular structures of the body, e.g. stents
- A61F2/02—Prostheses implantable into the body
- A61F2/30—Joints
- A61F2002/30001—Additional features of subject-matter classified in A61F2/28, A61F2/30 and subgroups thereof
- A61F2002/30316—The prosthesis having different structural features at different locations within the same prosthesis; Connections between prosthetic parts; Special structural features of bone or joint prostheses not otherwise provided for
- A61F2002/30329—Connections or couplings between prosthetic parts, e.g. between modular parts; Connecting elements
- A61F2002/30448—Connections or couplings between prosthetic parts, e.g. between modular parts; Connecting elements using adhesives
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F2/00—Filters implantable into blood vessels; Prostheses, i.e. artificial substitutes or replacements for parts of the body; Appliances for connecting them with the body; Devices providing patency to, or preventing collapsing of, tubular structures of the body, e.g. stents
- A61F2/02—Prostheses implantable into the body
- A61F2/30—Joints
- A61F2002/30001—Additional features of subject-matter classified in A61F2/28, A61F2/30 and subgroups thereof
- A61F2002/30316—The prosthesis having different structural features at different locations within the same prosthesis; Connections between prosthetic parts; Special structural features of bone or joint prostheses not otherwise provided for
- A61F2002/30329—Connections or couplings between prosthetic parts, e.g. between modular parts; Connecting elements
- A61F2002/30476—Connections or couplings between prosthetic parts, e.g. between modular parts; Connecting elements locked by an additional locking mechanism
- A61F2002/30492—Connections or couplings between prosthetic parts, e.g. between modular parts; Connecting elements locked by an additional locking mechanism using a locking pin
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F2/00—Filters implantable into blood vessels; Prostheses, i.e. artificial substitutes or replacements for parts of the body; Appliances for connecting them with the body; Devices providing patency to, or preventing collapsing of, tubular structures of the body, e.g. stents
- A61F2/02—Prostheses implantable into the body
- A61F2/30—Joints
- A61F2002/30001—Additional features of subject-matter classified in A61F2/28, A61F2/30 and subgroups thereof
- A61F2002/30316—The prosthesis having different structural features at different locations within the same prosthesis; Connections between prosthetic parts; Special structural features of bone or joint prostheses not otherwise provided for
- A61F2002/30535—Special structural features of bone or joint prostheses not otherwise provided for
- A61F2002/30563—Special structural features of bone or joint prostheses not otherwise provided for having elastic means or damping means, different from springs, e.g. including an elastomeric core or shock absorbers
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F2/00—Filters implantable into blood vessels; Prostheses, i.e. artificial substitutes or replacements for parts of the body; Appliances for connecting them with the body; Devices providing patency to, or preventing collapsing of, tubular structures of the body, e.g. stents
- A61F2/02—Prostheses implantable into the body
- A61F2/30—Joints
- A61F2002/30001—Additional features of subject-matter classified in A61F2/28, A61F2/30 and subgroups thereof
- A61F2002/30316—The prosthesis having different structural features at different locations within the same prosthesis; Connections between prosthetic parts; Special structural features of bone or joint prostheses not otherwise provided for
- A61F2002/30535—Special structural features of bone or joint prostheses not otherwise provided for
- A61F2002/30599—Special structural features of bone or joint prostheses not otherwise provided for stackable
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F2/00—Filters implantable into blood vessels; Prostheses, i.e. artificial substitutes or replacements for parts of the body; Appliances for connecting them with the body; Devices providing patency to, or preventing collapsing of, tubular structures of the body, e.g. stents
- A61F2/02—Prostheses implantable into the body
- A61F2/30—Joints
- A61F2002/30001—Additional features of subject-matter classified in A61F2/28, A61F2/30 and subgroups thereof
- A61F2002/30667—Features concerning an interaction with the environment or a particular use of the prosthesis
- A61F2002/30677—Means for introducing or releasing pharmaceutical products, e.g. antibiotics, into the body
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F2/00—Filters implantable into blood vessels; Prostheses, i.e. artificial substitutes or replacements for parts of the body; Appliances for connecting them with the body; Devices providing patency to, or preventing collapsing of, tubular structures of the body, e.g. stents
- A61F2/02—Prostheses implantable into the body
- A61F2/30—Joints
- A61F2/30767—Special external or bone-contacting surface, e.g. coating for improving bone ingrowth
- A61F2/30771—Special external or bone-contacting surface, e.g. coating for improving bone ingrowth applied in original prostheses, e.g. holes or grooves
- A61F2002/30772—Apertures or holes, e.g. of circular cross section
- A61F2002/30784—Plurality of holes
- A61F2002/30785—Plurality of holes parallel
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F2/00—Filters implantable into blood vessels; Prostheses, i.e. artificial substitutes or replacements for parts of the body; Appliances for connecting them with the body; Devices providing patency to, or preventing collapsing of, tubular structures of the body, e.g. stents
- A61F2/02—Prostheses implantable into the body
- A61F2/30—Joints
- A61F2/30767—Special external or bone-contacting surface, e.g. coating for improving bone ingrowth
- A61F2/30771—Special external or bone-contacting surface, e.g. coating for improving bone ingrowth applied in original prostheses, e.g. holes or grooves
- A61F2002/30795—Blind bores, e.g. of circular cross-section
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F2/00—Filters implantable into blood vessels; Prostheses, i.e. artificial substitutes or replacements for parts of the body; Appliances for connecting them with the body; Devices providing patency to, or preventing collapsing of, tubular structures of the body, e.g. stents
- A61F2/02—Prostheses implantable into the body
- A61F2/30—Joints
- A61F2/30767—Special external or bone-contacting surface, e.g. coating for improving bone ingrowth
- A61F2/30771—Special external or bone-contacting surface, e.g. coating for improving bone ingrowth applied in original prostheses, e.g. holes or grooves
- A61F2002/3085—Special external or bone-contacting surface, e.g. coating for improving bone ingrowth applied in original prostheses, e.g. holes or grooves with a threaded, e.g. self-tapping, bone-engaging surface, e.g. external surface
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F2/00—Filters implantable into blood vessels; Prostheses, i.e. artificial substitutes or replacements for parts of the body; Appliances for connecting them with the body; Devices providing patency to, or preventing collapsing of, tubular structures of the body, e.g. stents
- A61F2/02—Prostheses implantable into the body
- A61F2/30—Joints
- A61F2/30767—Special external or bone-contacting surface, e.g. coating for improving bone ingrowth
- A61F2/30771—Special external or bone-contacting surface, e.g. coating for improving bone ingrowth applied in original prostheses, e.g. holes or grooves
- A61F2002/30904—Special external or bone-contacting surface, e.g. coating for improving bone ingrowth applied in original prostheses, e.g. holes or grooves serrated profile, i.e. saw-toothed
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F2/00—Filters implantable into blood vessels; Prostheses, i.e. artificial substitutes or replacements for parts of the body; Appliances for connecting them with the body; Devices providing patency to, or preventing collapsing of, tubular structures of the body, e.g. stents
- A61F2/02—Prostheses implantable into the body
- A61F2/30—Joints
- A61F2/3094—Designing or manufacturing processes
- A61F2/30942—Designing or manufacturing processes for designing or making customized prostheses, e.g. using templates, CT or NMR scans, finite-element analysis or CAD-CAM techniques
- A61F2002/30957—Designing or manufacturing processes for designing or making customized prostheses, e.g. using templates, CT or NMR scans, finite-element analysis or CAD-CAM techniques using a positive or a negative model, e.g. moulds
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F2/00—Filters implantable into blood vessels; Prostheses, i.e. artificial substitutes or replacements for parts of the body; Appliances for connecting them with the body; Devices providing patency to, or preventing collapsing of, tubular structures of the body, e.g. stents
- A61F2/02—Prostheses implantable into the body
- A61F2/30—Joints
- A61F2/3094—Designing or manufacturing processes
- A61F2002/30975—Designing or manufacturing processes made of two halves
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F2/00—Filters implantable into blood vessels; Prostheses, i.e. artificial substitutes or replacements for parts of the body; Appliances for connecting them with the body; Devices providing patency to, or preventing collapsing of, tubular structures of the body, e.g. stents
- A61F2/02—Prostheses implantable into the body
- A61F2/30—Joints
- A61F2/46—Special tools for implanting artificial joints
- A61F2/4644—Preparation of bone graft, bone plugs or bone dowels, e.g. grinding or milling bone material
- A61F2002/4649—Bone graft or bone dowel harvest sites
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F2210/00—Particular material properties of prostheses classified in groups A61F2/00 - A61F2/26 or A61F2/82 or A61F9/00 or A61F11/00 or subgroups thereof
- A61F2210/0004—Particular material properties of prostheses classified in groups A61F2/00 - A61F2/26 or A61F2/82 or A61F9/00 or A61F11/00 or subgroups thereof bioabsorbable
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F2220/00—Fixations or connections for prostheses classified in groups A61F2/00 - A61F2/26 or A61F2/82 or A61F9/00 or A61F11/00 or subgroups thereof
- A61F2220/0025—Connections or couplings between prosthetic parts, e.g. between modular parts; Connecting elements
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F2220/00—Fixations or connections for prostheses classified in groups A61F2/00 - A61F2/26 or A61F2/82 or A61F9/00 or A61F11/00 or subgroups thereof
- A61F2220/0025—Connections or couplings between prosthetic parts, e.g. between modular parts; Connecting elements
- A61F2220/0033—Connections or couplings between prosthetic parts, e.g. between modular parts; Connecting elements made by longitudinally pushing a protrusion into a complementary-shaped recess, e.g. held by friction fit
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F2220/00—Fixations or connections for prostheses classified in groups A61F2/00 - A61F2/26 or A61F2/82 or A61F9/00 or A61F11/00 or subgroups thereof
- A61F2220/0025—Connections or couplings between prosthetic parts, e.g. between modular parts; Connecting elements
- A61F2220/005—Connections or couplings between prosthetic parts, e.g. between modular parts; Connecting elements using adhesives
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F2230/00—Geometry of prostheses classified in groups A61F2/00 - A61F2/26 or A61F2/82 or A61F9/00 or A61F11/00 or subgroups thereof
- A61F2230/0002—Two-dimensional shapes, e.g. cross-sections
- A61F2230/0004—Rounded shapes, e.g. with rounded corners
- A61F2230/0006—Rounded shapes, e.g. with rounded corners circular
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F2230/00—Geometry of prostheses classified in groups A61F2/00 - A61F2/26 or A61F2/82 or A61F9/00 or A61F11/00 or subgroups thereof
- A61F2230/0002—Two-dimensional shapes, e.g. cross-sections
- A61F2230/0017—Angular shapes
- A61F2230/0019—Angular shapes rectangular
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F2230/00—Geometry of prostheses classified in groups A61F2/00 - A61F2/26 or A61F2/82 or A61F9/00 or A61F11/00 or subgroups thereof
- A61F2230/0002—Two-dimensional shapes, e.g. cross-sections
- A61F2230/0028—Shapes in the form of latin or greek characters
- A61F2230/0058—X-shaped
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F2230/00—Geometry of prostheses classified in groups A61F2/00 - A61F2/26 or A61F2/82 or A61F9/00 or A61F11/00 or subgroups thereof
- A61F2230/0063—Three-dimensional shapes
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F2230/00—Geometry of prostheses classified in groups A61F2/00 - A61F2/26 or A61F2/82 or A61F9/00 or A61F11/00 or subgroups thereof
- A61F2230/0063—Three-dimensional shapes
- A61F2230/0069—Three-dimensional shapes cylindrical
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F2230/00—Geometry of prostheses classified in groups A61F2/00 - A61F2/26 or A61F2/82 or A61F9/00 or A61F11/00 or subgroups thereof
- A61F2230/0063—Three-dimensional shapes
- A61F2230/0082—Three-dimensional shapes parallelepipedal
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F2250/00—Special features of prostheses classified in groups A61F2/00 - A61F2/26 or A61F2/82 or A61F9/00 or A61F11/00 or subgroups thereof
- A61F2250/0058—Additional features; Implant or prostheses properties not otherwise provided for
- A61F2250/006—Additional features; Implant or prostheses properties not otherwise provided for modular
- A61F2250/0063—Nested prosthetic parts
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F2310/00—Prostheses classified in A61F2/28 or A61F2/30 - A61F2/44 being constructed from or coated with a particular material
- A61F2310/00005—The prosthesis being constructed from a particular material
- A61F2310/00365—Proteins; Polypeptides; Degradation products thereof
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F2310/00—Prostheses classified in A61F2/28 or A61F2/30 - A61F2/44 being constructed from or coated with a particular material
- A61F2310/00005—The prosthesis being constructed from a particular material
- A61F2310/00365—Proteins; Polypeptides; Degradation products thereof
- A61F2310/00383—Gelatin
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F2310/00—Prostheses classified in A61F2/28 or A61F2/30 - A61F2/44 being constructed from or coated with a particular material
- A61F2310/00389—The prosthesis being coated or covered with a particular material
- A61F2310/0097—Coating or prosthesis-covering structure made of pharmaceutical products, e.g. antibiotics
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F2310/00—Prostheses classified in A61F2/28 or A61F2/30 - A61F2/44 being constructed from or coated with a particular material
- A61F2310/00389—The prosthesis being coated or covered with a particular material
- A61F2310/00976—Coating or prosthesis-covering structure made of proteins or of polypeptides, e.g. of bone morphogenic proteins BMP or of transforming growth factors TGF
Definitions
- This inventionrelates to implants and methods for their preparation wherein components of the implant are assembled from constituent pieces to produce a complete implant.
- This inventionprovides a method for manufacture of autograft, allograft and xenograft implants which comprises assembling such implants from smaller pieces of graft materials to form a larger graft implant product.
- Another object of this inventionis to provide assembled bone implants.
- Another object of this inventionis to provide a method whereby otherwise wasted tissue may be used in the production of useful orthopedic implants.
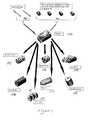
- FIG. 1is a flow chart showing the formation of various sub-component parts of an assembled implant according to this invention, from which assembled implants and a kit comprising these parts may be formed according to the disclosure of this invention.
- FIG. 2provides a schematic of an assembled implant according to this invention.
- FIG. 3provides a schematic of an assembled implant according to this invention.
- FIGS. 4 - 7provides a schematic of an assembled implant according to this invention.
- FIGS. 8 - 9provides a schematic of an assembled implant according to this invention.
- FIGS. 10 - 14provides a schematic of an assembled implant according to this invention.
- FIGS. 15 - 18provides a schematic of an assembled implant according to this invention.
- FIG. 19provides a schematic of an assembled implant according to this invention.
- FIG. 20provides a schematic of an assembled implant according to this invention.
- FIG. 21provides a schematic of an assembled implant according to this invention.
- FIG. 22provides a schematic of an assembled implant according to this invention.
- FIG. 23shows the assembly of a dowel from component pieces.
- FIG. 24shows the reinforcement of an implant using a cortical bone pin.
- FIG. 25shows the reinforcement of an implant using a cortical bone pin and a cortical bone disk.
- FIG. 26shows the reinforcement of cancellous bone implants using a plurality of cortical bone pins.
- FIG. 27shows the formation of an assembled implant comprising soft and hard tissues.
- autograft, allograft and xenograft productsare produced as solid, continuous materials.
- bone dowelssee U.S. Pat. No. 5,814,084, hereby incorporated by reference
- Smith-Robinson cervical spine implants, iliac crest grafts, and the likeare harvested and machined from single, continuous pieces of bone.
- the present inventionprovides methods for manufacture of autograft, allograft and xenograft implants by assembling such implants from smaller pieces of graft materials to form a larger graft implant product. As a result, increased utilization of valuable implant materials is achieved, thereby more effectively meeting the ever-increasing demands for graft implant materials.
- any implant piece that may be requiredmay be formed according to the present invention, and orthopedic surgeons may be provided with kits of assemblable parts which may be formed in the course of a surgical procedure to precisely meet the needs of a given patient or procedure.
- existing graft productsmay be strengthened or reinforced by assembly of different types of graft materials into an assembled product.
- a reinforced productis a cancellous wedge, block, dowel or the like into which is inserted reinforcing pins of cortical bone.
- this inventionprovides for the product of assembled implants comprising any one or combinations of allograft materials, autograft materials, xenograft materials, synthetic materials, metallic materials and the like.
- the assembled implants or the component pieces which are combined to form the assembled implantmay be pretreated or treated after assembly to incorporate any desired biologically active or inert materials.
- the assembled bone dowelcomprises segments of cortical bone pinned to each other by means of cortical bone pins.
- the graft materialsPrior to assembly or after assembly, the graft materials are soaked, infused, impregnated, coated or otherwise treated with bone morphogenetic proteins (BMP's), antibiotics, growth factors, nucleic acids, peptides, and the like.
- BMP'sbone morphogenetic proteins
- variously shaped wafers, blocks, rings, washer-shaped bone pieces and the likemay be affixed to each other in any secure and biologically acceptable manner.
- the assembled pieces of boneare affixed to each other by means of pins, screws, rods, interference fit, threaded fits, key-way fit, and the like made from cortical bone.
- fixation piecesare machined in a CNC lathe or the like to appropriate dimensions and are then threaded into mating holes tapped in the pieces to be assembled, or are pressed into drilled holes through adjacent pieces to be assembled by a pneumatic press or the like. In this fashion, very strong and tightly fitted pieces of implant materials may be joined and implanted.
- the assembled piecesmay first be machined to desired dimensions and shapes, prior to assembly, the assembled implant may be machined, or both.
- the implant according to this inventionmay comprise an assembled cancellous block, dowel or the like, harvested from the iliac crest or another suitable site.
- cancellous blockAs is known in the art, due to the wafer-like structure of cancellous bone, such grafts have low load-bearing characteristics.
- a Cloward Dowel, iliac crest wedge, or cancellous bone block, dowel or the likeis reinforced by insertion therein of cortical bone pins.
- cortical implantsmay also be reinforced by insertion therein of cortical bone pins, including when an assembled implant is prepared comprising different segments of cortical bone, cancerous bone or both. Insertion of the reinforcing pins provides an implant with multiple load-bearing pillars. The pins may be made to protrude from the surface of the implant to engage with inferior, superior or both surfaces of bone between which the implant is inserted.
- pin protrusionsmay be employed to created contact between the implant and the vertebral bodies, thus preventing extrusion and reinforcing a secure fit of the implant between adjacent vertebrae.
- cortical pins of about 4.5 mm in diametermay each support a load of up about 2700 newtons (160 Mpa).
- multiple pinsmay be inserted into an implant to produce a load-bearing capacity of known proportions (e.g. 10,000 newtons by insertion of five pins).
- a further advantage of this inventionis that it permits use of tissues that are not currently amenable to standard autograft, allograft or xenograft harvesting and processing procedures, such as ribs, metatarsal bone and the like.
- usefull implant materialsmay be harvested and produced from otherwise un-useable donor tissues.
- various shaping methods aside from CNC lathe or other known proceduresmay be applied to different segments of the implant.
- a cancellous portion of bone implantmay be compression molded, and then affixed to other portions of cortical or cancellous bone machined according to different or similar principles.
- implants of unusual sizes and dimensionsmay be prepared and machined.
- implants of 100 mm in sizecould be machined, for example, for corpectomies, when otherwise bone stock for manufacture of such implant dimensions would not be available.
- dowel shaped implantscomprising assembled dowel segments, between about two to about ten segments, pinned together by one or more cortical bone pins.
- the assembled segmentsmay closely abut each other or may be spread apart from each other.
- Such implantsmay be prepared by harvesting disks of cortical bone, drilling and optionally tapping holes therein, and inserting shafts of cortical pins therethrough, or therein, optionally by threading portions thereof for torquing into optionally tapped holes.
- the thus produced dowelsmay be tapered or have parallel sides.
- dowels which are harvested as a cross-section across the intramedullary canal of a long bonemay be completed by insertion therein of a cortical pin.
- a “doughnut” of bonemay be affixed to the sidewall by means of a cortical pin.
- a longer dowelmay be prepared by affixing two dowels to each other.
- a posterior longitudinal interbody fusion implantmay be machined from a single piece of cortical bone, or be assembled from two pieces of bone which are affixed to each other by means of a cortical pin.
- a bone screwmay also be prepared according to the method of this invention by affixing multiple pieces of cortical bone to each other with a cortical bone pin, and then machining a thread on the exterior of the assembled bone pieces. It will further be appreciated from this disclosure that different portions of the assembled implant may be demineralized, to achieve a level of elasticity or compressibility not otherwise present in cortical or cancellous bone. Different portions of bone may also be retained on a shaft by means of a cotter-pin type device.
- instrumentsmay be conveniently prepared according to the methods of this invention which may be utilized for insertion of other implants.
- an implant driveris produced wherein the driving mechanism itself is formed from assembled cortical pins which protrude into mating recesses in an implant device.
- the instrumentmay be torqued to adequate loads to induce implantation of spinal implants and the like.
- one technical issue of meritis the need to develop a process whereby donor tissue, whether hard or soft tissue, allograft or xenograft tissue, may be treated in such a fashion as to eliminate the possibility of cross contamination between tissue segments obtained from different sources. While it is possible to practice the present invention to advantage using tissue obtained from a single screened donor, the real economies of scale and commercially viable application of the present technology is best realized by implementation of an efficient and reliable tissue decontamination process. Ideally, the process is one which permits multiple segments of soft or hard tissue to be treated simultaneously so that a stock of materials for assemblage of implants according to the present invention is facilitated.
- an assembled allograft or xenograft tissue implantis prepared by treating the tissue in a closed container in which different cleaning solutions are contacted with the implant segments, either before or after assembly and machining into the final implant form, either in the presence or absence of sonication, with rapid oscillation of pressure in the closed container, to achieve deep cleaning and interpenetration of cleaning solvents into the interstices of porous implants or tissues.
- Solutionsincluding, but not limited to detergent solutions, peroxide solutions and the like are used in such procedure, and terminal sterilization with gamma irradiation, gaseous sterilants known in the art or other terminal sterilization procedures known in the art are employed to ensure safe implantation of the assembled implants according to this invention.
- Cortical bone pins 100are used to assemble a series of bone disks 101 into a pre-part 102 which is then machined into a series of final products: Threaded dowels, 103 ; small blocks 104 ; unique shapes, 105 such as a “wedding-cake” like shape wherein disks bearing threads are spaced apart from each other leaving voids 105 ′ into which additional materials may be inserted, with the disks retained in fixed relation to each other by means of the through pins 100 ; tapered dowels 106 ; screws 107 ; smooth cylinders 108 ; or large blocks 109 .
- a central concept relevant to the present inventionis the ability to machine smaller parts of tissue, specifically bone tissue, such as cortical bone, cancellous bone, cortical-cancellous bone, portions of which may be demineralized (see, for example, U.S. Pat. No. 6,090,998, hereby incorporated herein by reference for this purpose), and assemble these portions of tissue using, preferably, cortical bone pins.
- the assembled tissue piecesmay be machined prior to assembly, and then, upon assembly, a complete implant is ready for implantation. Alternatively, the tissue pieces may first be assembled, and the assembled pieces may then be machined into any desired final form. The order of assembly and machining will be determined by the specific forms of implant required for a particular application.
- FIG. 1a series of pre-machined tissue forms are disclosed, which may conveniently be included in a kit for use as needed by an orthopedic surgeon.
- a kit for usefor example, where a particular implant of specific dimensions is required, the surgeon is able to select pre-shaped implant segments to fill a particular geometric space and shape in the spine of an implant recipient.
- Numerous permutations and combinations of implant pieces for assemblyare possible, based on the pre-machined assemblable implant pieces included in such a kit, and those skilled in the art will appreciate that the skilled orthopedic surgeon will be able to create implants as needed when supplied with such a kit.
- a preferred kitincludes disks of bone, cortical bone, cancellous bone, allograft or xenograft, also referred to herein as “washers” or “doughnuts” such that a center hole is provided for press-fitting or screwing on of the disks to a cortical bone or synthetic or metallic shaft or pin.
- the disksmay be demineralized, mineralized, or partially demineralized.
- plugs of cortical bone, cancellous bone, or cortical-cancellous boneincluding at least one through hole, and optionally more than one such through hole, for insertion of pins therethrough. Ovals, squares, rectangles and irregular shapes may also be provided in certain kits for specific applications.
- a bone pastesuch as that disclosed in WO99/38543, hereby incorporated by reference, may be beneficial for filling any voids that remain, and to implant with the assembled implant, osteogenic material, (i.e. osteoconductive material, Osteoinductive material, or both, as well as material that assists in adhering the implant to the site of implantation).
- osteogenic materiali.e. osteoconductive material, Osteoinductive material, or both
- a molded implantmay be combined with the assembled implant of this invention.
- a preferred molded implant for orthopedic applicationsis disclosed in PCT publication WO 00/54821, the disclosure of which is hereby incorporated by reference.
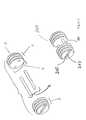
- the assembled graft 200comprises a void, 201 into which osteogenic material may be inserted prior to or after implantation.
- the pins Ymay be metal pins, but preferably are pins machined from cortical bone. This enables the entire implant to remodel into autogenous tissue over time, such as vertebral bone, when the implant 200 is inserted into the intervertebral space.
- the graft 201is also shown with a groove, 202 in which a driver may be inserted to provide rotational torque for insertion of the implant.
- An instrument attachment hole, 203is also provided, to ensure that the implant remains securely on the head of the driver means in the process of surgical implantation.
- the segments Z and Tmay be brought into close abutment with each other, thereby eliminating the space 201 .
- the length of the pins Awould be modified to prevent unnecessary protrusion, although in some applications, protrusion may be useful when driving the implant 200 into place.
- the number of pins usedwhile represented as two in this figure, may be fewer or more in number, depending on the particular application, the extent of torsional or compressive loads, and the like anticipated to be experienced by the implant once in situ.
- FIG. 3shows an implant assembled from three principal segments F, D, and E, which are held together by pins 300 .
- the waffle-shaped structure of implant segment Dis intended to represent the use of cancellous bone, which is abutted on either side by cortical bone, which forms segments F and E.
- the fully assembled implantis shown in FIG. 4, while FIGS. 5, 6 and 7 show end-on views, and cross sectional views A-A and B-B, respectively.
- segment F, segment D, or segment Emay be demineralized according to methods known in the art. Likewise, all of these segments may be demineralized. Where a flexible implant is required, the implant may be assembled, and the entire implant may be demineralized.
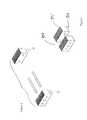
- FIG. 8shows an embodiment of this invention wherein rectangular bone segments N and G are assembled into implant 900 , shown in FIG. 9.
- Features 901 and 902which comprises ridges, teeth, or other external features are machined into the superior and inferior faces of the implants in order to assist in retention of the implants once placed in situ.
- FIGS. 10 - 14show the assembly of elements J, H, and I into implant 1100 , shown end-on, in cross-section A-A and B-B, in FIGS. 12 - 14 , respectively.
- bone element His shown with a waffle-like structure, to represent that this element may be cancellous bone, demineralized bone, a polymer composite, such as poly-L-Lactic acid, polyglycolic acid, or the like.
- Features 1101 and 1102represent external grooves or teeth machined into the superior and inferior surfaces of the implant to assist in retention of the implant once placed in situ.
- FIGS. 15 - 18show the assembly of elements M, K, and L, each of which is a substantially cubic bone element, using pins 1500 .
- FIG. 17is a top view, showing cross section A-A, represented in FIG. 18, with the final assembled implant 1600 shown in FIG. 16.
- FIG. 19shows a “Wedding-Cake” design of an implant 1900 assembled from units A-C, pinned together by pins a-c. Void area 1901 is available for filling with osteogenic materials.
- FIG. 20shows implant 2000 which is an assembled Cervical Smith Robinson implant similar to that shown in PCT publication WO99/09914, hereby incorporated by reference, except that this implant is fashioned from a series of assembled bone pieces 2001 and machined into the desired final shape.
- FIG. 21shows implant 2100 assembled from two cortical bone pieces and one cancellous bone piece, and pinned together.
- the implanthas an anterior height H 1 which is smaller than posterior height H 2 , which permits retention of correct spinal lordosis upon implantation, for example, in a posterior lumbar intervertebral implant fixation procedure.
- Superior and inferior features 2101 , 2102prevent expulsion of the implant once place in situ.
- FIG. 22shows an implant 2200 assembled from a series of sub-implant pieces 2201 .
- the implantmay contain cancellous bone 2202 segments, as well as cortical bone 2203 segments and cortical bone pins 2204 .
- FIG. 23shows the formation of a tapered dowel 2300 by assembling “doughnut” or “disk” or “washer” shaped bone pieces 2301 on a cortical bone shaft 2302 by using washer pieces of differing diameter.
- This figureonly shows two disks, but a continuous dowel is formed by using disks of a graded diameter between each end of the cortical bone shaft 2302 .
- FIG. 24Ashows a bone dowel in which one sidewall of a bone dowel 2400 such as that disclosed and claimed in U.S. Pat. No. 5,814,084, hereby incorporated by reference, is “out of specifications” due to being too narrow or absent. This is repaired in FIG.
- FIG. 25a similar procedure for salvaging a dowel 2500 is shown whereby a pin 2501 is driven through the center of the dowel 2500 to reinforce the dowel longitudinally. Furthermore, where an endcap 2503 of the dowel is “out of spec” for being too narrow, the endcap is reinforced by press-fitting a cortical bone disk 2502 onto the end of the pin 2501 .
- a series of cancellous bone implants 2600are reinforced by inclusion therein of a series of cortical pins 100 .
- Each cortical pin of a 2 mm diameterhas been found to support approximately 2000 newtons of axial compressive load. Accordingly, cancellous bone implants of essentially any desired height and compressive strength may be assembled in this manner by affixing several layers of cancellous bone with cortical bone pins.
- other materialsmay be included in such a “sandwich” of bone materials.
- the cancellous bonemay be soaked in a solution containing growth factors, such as, but not limited to, bone morphogenetic proteins, fibroblast growth factors, platelet derived growth factor, cartilage derived morphogenetic proteins, stem cells, such as mesenchymal stem cells, osteoprogenitor cells, antibiotics, antuinflammatory compounds, anti-neoplastic compounds, nucleic acids, peptides, and the like.
- growth factorssuch as, but not limited to, bone morphogenetic proteins, fibroblast growth factors, platelet derived growth factor, cartilage derived morphogenetic proteins, stem cells, such as mesenchymal stem cells, osteoprogenitor cells, antibiotics, antuinflammatory compounds, anti-neoplastic compounds, nucleic acids, peptides, and the like.
- growth factorssuch as, but not limited to, bone morphogenetic proteins, fibroblast growth factors, platelet derived growth factor, cartilage derived morphogenetic proteins, stem cells, such as mesenchymal stem cells
- the assembled implantis driven by cortical pins to seat in an implant site, using a driver that engages cortical bone pins with purchase sites on the implant.
- the drivermay comprise a handle with projecting cortical pins which engage with holes in the assembled allograft, thereby providing a site for torquing the implant into position.
- assembled cortical bone blocks, or cortical cancellous bone blocksare assembled in combination with wedged or pinned soft tissue, such as tendon, ligament, skin, collagen sheets, or the like, to create grafts similar to naturally occurring tissue sites, such as the bone-tendon interface found at the patella.
- wedged or pinned soft tissuesuch as tendon, ligament, skin, collagen sheets, or the like
- Such combination implantspermit reconstruction of sites such as the Anterior Cruciate Ligament (ACL) or Posterior Cruciate Ligament (PCL).
- ACLAnterior Cruciate Ligament
- PCLPosterior Cruciate Ligament
- a ligament or tendon or skin or collagen sheet membraneis pinned between adjacent blocks of cortical bone.
- an implant 2700is assembled from a superior bone block 2701 , an inferior bone block 2702 and a wedged flexible tissue, such as a ligament or tendon or portion of demineralized bone 2704 , all of which are pinned together with cortical bone pins 2703 or other fixation means.
- the cortical bone pins disclosed hereinmay have features defined thereon for various applications.
- the shaftsmay contain stops, such that other pieces of bone inserted thereon can only travel a certain distance down the shaft before encountering the stop.
- the shaftmay also contain through holes, to permit insertion of cotter pins or the like.
- the cortical bone shaftmay be demineralized, mineralized, or partially demineralized.
- the end of the cortical shaftcontains a tapped cannulation a short distance into the longitudinal end of the shaft. In this way, a screw may be driven into the cannulation to retain elements inserted over the shaft in association with the shaft.
- the screw end bearing the cannulationmay be partially demineralized, such that upon insertion of the retention screw, the shaft end does not shatter, but expands to accommodate the increasing diameter of the screw as it is driven into the shaft.
- the cortical pinsmay be cannulated throughout the longitudinal length thereof. However, care should be taken that this does not unduly weaken the overall compressive or torsional strength of the assembled implant. This may be addressed by including pins that are not cannulated, along with pins that are cannulated.
- the cannulated pinsmay be used in combination with sutures or the like, in order to hold an implant in a specific orientation, until fusion with adjacent bone has proceeded to a sufficient extent for the implant to become stable without the sutures.
- implantsthat have classically been fabricated from metals may be fabricated by assembling bone pieces.
- a benefit of the assembled graft according to this inventionis that the components of the assembled graft can be derived from various anatomical structures, thus circumventing limitations normally resulting from having to obtain a graft from a particular anatomical source of a particular donor. Not only can the components be sourced from different anatomies, but also different donors may yield various components for assembly into a unitary implant. The end result is maximization of the gift of donation and the preservation of precious tissue resources.
- a further benefit of the present inventionis that different implants with height or width limitations due to the anatomical structures from which the implant has been derived may be pinned together to form implants of essentially any desired dimensions. In this fashion, an inventory of building blocks in combination with the appropriate assembly pins, threaded or unthreaded, is useful to provide implants of essentially any dimensions in the course of given surgical procedure.
- a cervical Smith-Robinson (CSR) t of any desired heightmay be produced by attaching two or more existing CSR implants together with cortical bone pins. This is accomplished preferably using two machined CSR's of known height such that when added together, the desired overall height is achieved.
- the two CSR'sare stacked and drill holes are machined through the CSR bodies, following which the cortical bone pins are press-fit through the thus machined holes.
- the diameter of the pinsis slightly greater than the diameter of the drilled holes, such that a tight press-fit is achieved.
- implants according to this inventionmay be assembled in the operating room by a surgeon, using pre-formed implant pieces, from a kit. It will further be appreciated that the assembled implant pieces may be adhered to each other using any of a number of biologically acceptable glues, pastes and the like. In one such embodiment, the assembled implant pieces are assembled using a polymethyl-methacrylate glue, a cyanoacrylate glue, or any other adhesive known in the art, so long as the use of such an adhesive is confirmed to be nontoxic. It will further be appreciated that in forming the assembled grafts according to the present invention, it is acceptable, although not required, for interlocking features to be included on abutting faces of implant segments to be assembled together.
- the adjacent featuresare complementary, such that a protrusion on a first surface is met by a compatible indentation in the abutting surface.
- abutting featuresassist to provide torsional and structural strength to the assembled implant, and to relieve a measure of stress on the cortical bone pins used to assemble the implant.
Landscapes
- Health & Medical Sciences (AREA)
- Biomedical Technology (AREA)
- Engineering & Computer Science (AREA)
- Life Sciences & Earth Sciences (AREA)
- Public Health (AREA)
- Orthopedic Medicine & Surgery (AREA)
- Veterinary Medicine (AREA)
- General Health & Medical Sciences (AREA)
- Animal Behavior & Ethology (AREA)
- Neurology (AREA)
- Cardiology (AREA)
- Oral & Maxillofacial Surgery (AREA)
- Transplantation (AREA)
- Heart & Thoracic Surgery (AREA)
- Vascular Medicine (AREA)
- Epidemiology (AREA)
- Chemical & Material Sciences (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Medicinal Chemistry (AREA)
- Molecular Biology (AREA)
- Prostheses (AREA)
Abstract
Description
- This application is a continuation-in-part of pending provisional application serial No. 60/181,622, filed Feb. 10, 2000, pending, and of application serial Nos. 09/191,132, filed on Nov. 13, 1998, pending; and of 09/378,527, filed on Aug. 20, 1999, pending; and of 09/370,194, filed on Sep. 7, 1999, pending; 29/123,227, filed May 12, 2000, pending; the priority of all of which is claimed herein under 35 U.S.C. Section 120.[0001]
- This invention relates to implants and methods for their preparation wherein components of the implant are assembled from constituent pieces to produce a complete implant.[0002]
- In the field of medicine, there has been an increasing need to develop implant materials for correction of biological defects. Particularly in the field of orthopedic medicine, there has been the need to replace or correct bone, ligament and tendon defects or injuries. As a result, there have emerged a number of synthetic implant materials, including but not limited to metallic implant materials and devices, devices composed in whole or in part from polymeric substances, as well as allograft, autograft, and xenograft implants. It is generally recognized that for implant materials to be acceptable, they must be pathogenfree, and must be biologically acceptable. Generally, it is preferable if the implant materials may be remodeled over time such that autogenous bone replaces the implant materials. This goal is best achieved by utilizing autograft bone from a first site for implantation into a second site. However, use of autograft materials is attended by the significant disadvantage that a second site of morbidity must be created to harvest autograft for implantation into a first diseased or injured site. As a result, allograft and xenograft implants have been given increasing attention in recent years. However, use of such materials has the disadvantage that human allograft materials are frequently low in availability and are high in cost of recovery, treatment and preparation for implantation. By contrast, while xenograft implant materials, such as bovine bone, may be of ready availability, immunological and disease transmission considerations imply significant constraints on the ready use of such materials.[0003]
- In view of the foregoing considerations, it remains the case that there has been a long felt need for unlimited supplies of biologically acceptable implant materials for repair of bone and other defects or injuries. This invention provides a significant advance in the art, and largely meets this need, by providing materials and methods for production of essentially any form of implant from component parts to produce assembled implants.[0004]
- In recent months, there have appeared several patents and patent publications which address similar or identical considerations to those to which the present invention disclosure is directed. Specifically, reference is made to PCT publication WO00/40177, which published on Jul. 13, 2000, the disclosure of which is hereby incorporated by reference as if fully set forth herein.[0005]
- In addition, reference is made herein to U.S. Pat. No. 5,899,939 to Boyce, which issued on May 4, 1999, the disclosure of which is hereby incorporated by reference as if fully set forth herein.[0006]
- Finally, reference is made herein to U.S. Pat. No. 6,025,538 to Yaccarino, which issued on Feb. 15, 2000, the disclosure of which is hereby incorporated by reference as if fully set forth herein.[0007]
- This invention provides a method for manufacture of autograft, allograft and xenograft implants which comprises assembling such implants from smaller pieces of graft materials to form a larger graft implant product.[0008]
- Accordingly, it is one object of this invention to provide a method for assembly of multiple bone implant shapes from smaller bone implant pieces.[0009]
- Another object of this invention is to provide assembled bone implants.[0010]
- Another object of this invention is to provide a method whereby otherwise wasted tissue may be used in the production of useful orthopedic implants.[0011]
- Further objects and advantages of this invention will be appreciated from a review of the complete disclosure and the appended claims.[0012]
- Attached to this invention disclosure are a large number of sketches which demonstrate a wide variety of assembled implants which may be prepared and used according to this invention.[0013]
- FIG. 1 is a flow chart showing the formation of various sub-component parts of an assembled implant according to this invention, from which assembled implants and a kit comprising these parts may be formed according to the disclosure of this invention.[0014]
- FIG. 2 provides a schematic of an assembled implant according to this invention.[0015]
- FIG. 3 provides a schematic of an assembled implant according to this invention.[0016]
- FIGS.[0017]4-7 provides a schematic of an assembled implant according to this invention.
- FIGS.[0018]8-9 provides a schematic of an assembled implant according to this invention.
- FIGS.[0019]10-14 provides a schematic of an assembled implant according to this invention.
- FIGS.[0020]15-18 provides a schematic of an assembled implant according to this invention.
- FIG. 19 provides a schematic of an assembled implant according to this invention.[0021]
- FIG. 20 provides a schematic of an assembled implant according to this invention.[0022]
- FIG. 21 provides a schematic of an assembled implant according to this invention.[0023]
- FIG. 22 provides a schematic of an assembled implant according to this invention.[0024]
- FIG. 23 shows the assembly of a dowel from component pieces.[0025]
- FIG. 24 shows the reinforcement of an implant using a cortical bone pin.[0026]
- FIG. 25 shows the reinforcement of an implant using a cortical bone pin and a cortical bone disk.[0027]
- FIG. 26 shows the reinforcement of cancellous bone implants using a plurality of cortical bone pins.[0028]
- FIG. 27 shows the formation of an assembled implant comprising soft and hard tissues.[0029]
- Currently, autograft, allograft and xenograft products are produced as solid, continuous materials. For example, bone dowels (see U.S. Pat. No. 5,814,084, hereby incorporated by reference), Smith-Robinson cervical spine implants, iliac crest grafts, and the like are harvested and machined from single, continuous pieces of bone. The present invention provides methods for manufacture of autograft, allograft and xenograft implants by assembling such implants from smaller pieces of graft materials to form a larger graft implant product. As a result, increased utilization of valuable implant materials is achieved, thereby more effectively meeting the ever-increasing demands for graft implant materials. In addition, greater flexibility is achieved in the types and shapes of implant materials is achieved. Essentially, any implant piece that may be required may be formed according to the present invention, and orthopedic surgeons may be provided with kits of assemblable parts which may be formed in the course of a surgical procedure to precisely meet the needs of a given patient or procedure. In yet another aspect of this invention, existing graft products may be strengthened or reinforced by assembly of different types of graft materials into an assembled product. One example of such a reinforced product is a cancellous wedge, block, dowel or the like into which is inserted reinforcing pins of cortical bone. As a result, those skilled in the art will understand from this disclosure that different sections of tissue may be assembled to make a complete graft implant. Furthermore, this invention provides for the product of assembled implants comprising any one or combinations of allograft materials, autograft materials, xenograft materials, synthetic materials, metallic materials and the like. Furthermore, the assembled implants or the component pieces which are combined to form the assembled implant may be pretreated or treated after assembly to incorporate any desired biologically active or inert materials. Thus, for example, in an assembled bone dowel implant according to this invention, the assembled bone dowel comprises segments of cortical bone pinned to each other by means of cortical bone pins. Prior to assembly or after assembly, the graft materials are soaked, infused, impregnated, coated or otherwise treated with bone morphogenetic proteins (BMP's), antibiotics, growth factors, nucleic acids, peptides, and the like.[0030]
- It will be appreciated that variously shaped wafers, blocks, rings, washer-shaped bone pieces and the like may be affixed to each other in any secure and biologically acceptable manner. Preferably, the assembled pieces of bone are affixed to each other by means of pins, screws, rods, interference fit, threaded fits, key-way fit, and the like made from cortical bone. These fixation pieces are machined in a CNC lathe or the like to appropriate dimensions and are then threaded into mating holes tapped in the pieces to be assembled, or are pressed into drilled holes through adjacent pieces to be assembled by a pneumatic press or the like. In this fashion, very strong and tightly fitted pieces of implant materials may be joined and implanted. The assembled pieces may first be machined to desired dimensions and shapes, prior to assembly, the assembled implant may be machined, or both.[0031]
- As noted above, the implant according to this invention may comprise an assembled cancellous block, dowel or the like, harvested from the iliac crest or another suitable site. As is known in the art, due to the wafer-like structure of cancellous bone, such grafts have low load-bearing characteristics. There exist reports in the literature of instances of extrusion, expulsion or collapse of iliac crest wedges, Cloward Dowels, and the like when utilized, for example, in spinal fusions. Nonetheless, use of cancellous bone is preferable over use of cortical bone implants, since cancellous bone is more osteoconductive than cortical bone. According to this invention, a Cloward Dowel, iliac crest wedge, or cancellous bone block, dowel or the like is reinforced by insertion therein of cortical bone pins. According to the method of this invention, cortical implants may also be reinforced by insertion therein of cortical bone pins, including when an assembled implant is prepared comprising different segments of cortical bone, cancerous bone or both. Insertion of the reinforcing pins provides an implant with multiple load-bearing pillars. The pins may be made to protrude from the surface of the implant to engage with inferior, superior or both surfaces of bone between which the implant is inserted. Thus, in a spinal implant, pin protrusions may be employed to created contact between the implant and the vertebral bodies, thus preventing extrusion and reinforcing a secure fit of the implant between adjacent vertebrae. We have, surprisingly, found that cortical pins of about 4.5 mm in diameter may each support a load of up about 2700 newtons (160 Mpa). Thus, according to the method of this invention, multiple pins may be inserted into an implant to produce a load-bearing capacity of known proportions (e.g. 10,000 newtons by insertion of five pins).[0032]
- A further advantage of this invention is that it permits use of tissues that are not currently amenable to standard autograft, allograft or xenograft harvesting and processing procedures, such as ribs, metatarsal bone and the like. In addition, usefull implant materials may be harvested and produced from otherwise un-useable donor tissues. In addition, due to the different nature of various segments of bone that are incorporated into the assembled, reinforced implants of this invention, various shaping methods aside from CNC lathe or other known procedures may be applied to different segments of the implant. Thus, a cancellous portion of bone implant may be compression molded, and then affixed to other portions of cortical or cancellous bone machined according to different or similar principles. In addition, due to the ability provided by this invention to assemble implant pieces, implants of unusual sizes and dimensions may be prepared and machined. Thus, implants of 100 mm in size could be machined, for example, for corpectomies, when otherwise bone stock for manufacture of such implant dimensions would not be available.[0033]
- In view of the present disclosure, it will be appreciated that this invention provides a wide variety of assembled implants and implant parts: dowel shaped implants comprising assembled dowel segments, between about two to about ten segments, pinned together by one or more cortical bone pins. The assembled segments may closely abut each other or may be spread apart from each other. Such implants may be prepared by harvesting disks of cortical bone, drilling and optionally tapping holes therein, and inserting shafts of cortical pins therethrough, or therein, optionally by threading portions thereof for torquing into optionally tapped holes. The thus produced dowels may be tapered or have parallel sides. In addition, dowels which are harvested as a cross-section across the intramedullary canal of a long bone, as in U.S. Pat. No. 5,814,084, which might otherwise not pass production specifications, due to penetration of one outside wall into the intramedullary canal, may be completed by insertion therein of a cortical pin. Likewise, where a sidewall is otherwise considered to be too narrow, a “doughnut” of bone may be affixed to the sidewall by means of a cortical pin. A longer dowel may be prepared by affixing two dowels to each other. A posterior longitudinal interbody fusion implant (PLIF) may be machined from a single piece of cortical bone, or be assembled from two pieces of bone which are affixed to each other by means of a cortical pin. A bone screw may also be prepared according to the method of this invention by affixing multiple pieces of cortical bone to each other with a cortical bone pin, and then machining a thread on the exterior of the assembled bone pieces. It will further be appreciated from this disclosure that different portions of the assembled implant may be demineralized, to achieve a level of elasticity or compressibility not otherwise present in cortical or cancellous bone. Different portions of bone may also be retained on a shaft by means of a cotter-pin type device.[0034]
- In addition to assembled implants, instruments may be conveniently prepared according to the methods of this invention which may be utilized for insertion of other implants. In one embodiment of this invention, therefore, an implant driver is produced wherein the driving mechanism itself is formed from assembled cortical pins which protrude into mating recesses in an implant device. The instrument may be torqued to adequate loads to induce implantation of spinal implants and the like.[0035]
- In developing the various embodiments of the present invention, one technical issue of merit is the need to develop a process whereby donor tissue, whether hard or soft tissue, allograft or xenograft tissue, may be treated in such a fashion as to eliminate the possibility of cross contamination between tissue segments obtained from different sources. While it is possible to practice the present invention to advantage using tissue obtained from a single screened donor, the real economies of scale and commercially viable application of the present technology is best realized by implementation of an efficient and reliable tissue decontamination process. Ideally, the process is one which permits multiple segments of soft or hard tissue to be treated simultaneously so that a stock of materials for assemblage of implants according to the present invention is facilitated. Accordingly, on preferred method for treatment of tissue, disclosed in PCT publication WO 00/29037, the disclosure of which is hereby incorporated herein by reference as if fully set forth herein (and priority of the US Patent filings which gave rise to this application is hereby claimed for that purpose). Accordingly, in this aspect of the invention, a process is claimed whereby an assembled allograft or xenograft tissue implant is prepared by treating the tissue in a closed container in which different cleaning solutions are contacted with the implant segments, either before or after assembly and machining into the final implant form, either in the presence or absence of sonication, with rapid oscillation of pressure in the closed container, to achieve deep cleaning and interpenetration of cleaning solvents into the interstices of porous implants or tissues. Solutions including, but not limited to detergent solutions, peroxide solutions and the like are used in such procedure, and terminal sterilization with gamma irradiation, gaseous sterilants known in the art or other terminal sterilization procedures known in the art are employed to ensure safe implantation of the assembled implants according to this invention.[0036]
- Referring now to FIG. 1, there is shown a flow-chart representing various elements that may be processed and assembled according to this invention. Cortical bone pins[0037]100 are used to assemble a series of
bone disks 101 into a pre-part102 which is then machined into a series of final products: Threaded dowels,103; small blocks104; unique shapes,105 such as a “wedding-cake” like shape wherein disks bearing threads are spaced apart from each other leavingvoids 105′ into which additional materials may be inserted, with the disks retained in fixed relation to each other by means of the throughpins 100; tapereddowels 106;screws 107;smooth cylinders 108; orlarge blocks 109. From this figure, it will be appreciated that a central concept relevant to the present invention is the ability to machine smaller parts of tissue, specifically bone tissue, such as cortical bone, cancellous bone, cortical-cancellous bone, portions of which may be demineralized (see, for example, U.S. Pat. No. 6,090,998, hereby incorporated herein by reference for this purpose), and assemble these portions of tissue using, preferably, cortical bone pins. The assembled tissue pieces may be machined prior to assembly, and then, upon assembly, a complete implant is ready for implantation. Alternatively, the tissue pieces may first be assembled, and the assembled pieces may then be machined into any desired final form. The order of assembly and machining will be determined by the specific forms of implant required for a particular application. In FIG. 1, a series of pre-machined tissue forms are disclosed, which may conveniently be included in a kit for use as needed by an orthopedic surgeon. Thus, for example, where a particular implant of specific dimensions is required, the surgeon is able to select pre-shaped implant segments to fill a particular geometric space and shape in the spine of an implant recipient. Numerous permutations and combinations of implant pieces for assembly are possible, based on the pre-machined assemblable implant pieces included in such a kit, and those skilled in the art will appreciate that the skilled orthopedic surgeon will be able to create implants as needed when supplied with such a kit. Thus, a preferred kit includes disks of bone, cortical bone, cancellous bone, allograft or xenograft, also referred to herein as “washers” or “doughnuts” such that a center hole is provided for press-fitting or screwing on of the disks to a cortical bone or synthetic or metallic shaft or pin. The disks may be demineralized, mineralized, or partially demineralized. Also desirable in such a kit are plugs of cortical bone, cancellous bone, or cortical-cancellous bone, including at least one through hole, and optionally more than one such through hole, for insertion of pins therethrough. Ovals, squares, rectangles and irregular shapes may also be provided in certain kits for specific applications. It will further be appreciated, based on the present disclosure, that inclusion of a bone paste, such as that disclosed in WO99/38543, hereby incorporated by reference, may be beneficial for filling any voids that remain, and to implant with the assembled implant, osteogenic material, (i.e. osteoconductive material, Osteoinductive material, or both, as well as material that assists in adhering the implant to the site of implantation). Further, a molded implant may be combined with the assembled implant of this invention. A preferred molded implant for orthopedic applications is disclosed in PCT publication WO 00/54821, the disclosure of which is hereby incorporated by reference. - With reference to FIG. 2, there is shown two machined bone pieces, T and Z each of which bear external threading X and holes Y into which pins A are inserted to form the assembled[0038]
graft 200. As can be seen, the assembledgraft 200 comprises a void,201 into which osteogenic material may be inserted prior to or after implantation. The pins Y may be metal pins, but preferably are pins machined from cortical bone. This enables the entire implant to remodel into autogenous tissue over time, such as vertebral bone, when theimplant 200 is inserted into the intervertebral space. Thegraft 201 is also shown with a groove,202 in which a driver may be inserted to provide rotational torque for insertion of the implant. An instrument attachment hole,203, is also provided, to ensure that the implant remains securely on the head of the driver means in the process of surgical implantation. Naturally, those skilled in the art will appreciate that the segments Z and T may be brought into close abutment with each other, thereby eliminating thespace 201. In that event, the length of the pins A would be modified to prevent unnecessary protrusion, although in some applications, protrusion may be useful when driving theimplant 200 into place. It will also be appreciated that the number of pins used, while represented as two in this figure, may be fewer or more in number, depending on the particular application, the extent of torsional or compressive loads, and the like anticipated to be experienced by the implant once in situ. - FIG. 3 shows an implant assembled from three principal segments F, D, and E, which are held together by[0039]
pins 300. In this implant, the waffle-shaped structure of implant segment D is intended to represent the use of cancellous bone, which is abutted on either side by cortical bone, which forms segments F and E. The fully assembled implant is shown in FIG. 4, while FIGS. 5, 6 and7 show end-on views, and cross sectional views A-A and B-B, respectively. Those skilled in the art will appreciate from this disclosure that segment F, segment D, or segment E may be demineralized according to methods known in the art. Likewise, all of these segments may be demineralized. Where a flexible implant is required, the implant may be assembled, and the entire implant may be demineralized. - FIG. 8 shows an embodiment of this invention wherein rectangular bone segments N and G are assembled into[0040]
implant 900, shown in FIG. 9.Features - FIGS.[0041]10-14 show the assembly of elements J, H, and I into
implant 1100, shown end-on, in cross-section A-A and B-B, in FIGS.12-14, respectively. As can be seen, bone element H is shown with a waffle-like structure, to represent that this element may be cancellous bone, demineralized bone, a polymer composite, such as poly-L-Lactic acid, polyglycolic acid, or the like.Features - FIGS.[0042]15-18 show the assembly of elements M, K, and L, each of which is a substantially cubic bone element, using pins1500. FIG. 17 is a top view, showing cross section A-A, represented in FIG. 18, with the final assembled
implant 1600 shown in FIG. 16. - FIG. 19 shows a “Wedding-Cake” design of an[0043]
implant 1900 assembled from units A-C, pinned together by pins a-c.Void area 1901 is available for filling with osteogenic materials. - FIG. 20 shows implant[0044]2000 which is an assembled Cervical Smith Robinson implant similar to that shown in PCT publication WO99/09914, hereby incorporated by reference, except that this implant is fashioned from a series of assembled
bone pieces 2001 and machined into the desired final shape. - FIG. 21 shows implant[0045]2100 assembled from two cortical bone pieces and one cancellous bone piece, and pinned together. The implant has an anterior height H1 which is smaller than posterior height H2, which permits retention of correct spinal lordosis upon implantation, for example, in a posterior lumbar intervertebral implant fixation procedure. Superior and
inferior features - FIG. 22 shows an[0046]
implant 2200 assembled from a series ofsub-implant pieces 2201. The implant may containcancellous bone 2202 segments, as well ascortical bone 2203 segments and cortical bone pins2204. - FIG. 23 shows the formation of a tapered[0047]
dowel 2300 by assembling “doughnut” or “disk” or “washer” shapedbone pieces 2301 on acortical bone shaft 2302 by using washer pieces of differing diameter. This figure only shows two disks, but a continuous dowel is formed by using disks of a graded diameter between each end of thecortical bone shaft 2302. In FIG. 24, FIG. 24A shows a bone dowel in which one sidewall of abone dowel 2400 such as that disclosed and claimed in U.S. Pat. No. 5,814,084, hereby incorporated by reference, is “out of specifications” due to being too narrow or absent. This is repaired in FIG. 24B according to this embodiment of the invention by incorporation of an allograft or xenograftcortical bone pin 2401, to form a complete bone dowel. In this manner, valuable biological material which might otherwise be unusable for a particular application may be salvaged for use by employing the methodology of this invention. - In FIG. 25, a similar procedure for salvaging a[0048]
dowel 2500 is shown whereby apin 2501 is driven through the center of thedowel 2500 to reinforce the dowel longitudinally. Furthermore, where anendcap 2503 of the dowel is “out of spec” for being too narrow, the endcap is reinforced by press-fitting acortical bone disk 2502 onto the end of thepin 2501. - In FIG. 26, a series of cancellous bone implants[0049]2600 are reinforced by inclusion therein of a series of
cortical pins 100. Each cortical pin of a 2 mm diameter has been found to support approximately 2000 newtons of axial compressive load. Accordingly, cancellous bone implants of essentially any desired height and compressive strength may be assembled in this manner by affixing several layers of cancellous bone with cortical bone pins. Naturally, based on this disclosure, those skilled in the art will appreciate that other materials may be included in such a “sandwich” of bone materials. The cancellous bone may be soaked in a solution containing growth factors, such as, but not limited to, bone morphogenetic proteins, fibroblast growth factors, platelet derived growth factor, cartilage derived morphogenetic proteins, stem cells, such as mesenchymal stem cells, osteoprogenitor cells, antibiotics, antuinflammatory compounds, anti-neoplastic compounds, nucleic acids, peptides, and the like. Those skilled in the art will also appreciate that layers of cortical bone may be included, layers of biocompatible synthetic polymers and the like may also be included in the stacked bone implant. Various shapes may also be built upon, using for example, circles, ellipses, squares, and the like, as necessary for a given application. - In a further aspect of the present invention, the assembled implant is driven by cortical pins to seat in an implant site, using a driver that engages cortical bone pins with purchase sites on the implant. Thus, for example, not meant to be limiting, the driver may comprise a handle with projecting cortical pins which engage with holes in the assembled allograft, thereby providing a site for torquing the implant into position.[0050]
- In a further embodiment according to this invention, assembled cortical bone blocks, or cortical cancellous bone blocks are assembled in combination with wedged or pinned soft tissue, such as tendon, ligament, skin, collagen sheets, or the like, to create grafts similar to naturally occurring tissue sites, such as the bone-tendon interface found at the patella. Such combination implants permit reconstruction of sites such as the Anterior Cruciate Ligament (ACL) or Posterior Cruciate Ligament (PCL). According to this embodiment of the invention, a ligament or tendon or skin or collagen sheet membrane is pinned between adjacent blocks of cortical bone. Accordingly, various implants, such as known bone-tendon-bone implants which are in short supply may be supplanted by assemblage of an implant comprising assembled bone blocks, between which is fixed a ligamentous tissue, including but not limited to ligament, tendon, demineralized bone, and the like. Referring to FIG. 27, there is shown one example of this embodiment of the present invention in which an[0051]
implant 2700 is assembled from asuperior bone block 2701, aninferior bone block 2702 and a wedged flexible tissue, such as a ligament or tendon or portion ofdemineralized bone 2704, all of which are pinned together with cortical bone pins2703 or other fixation means. Naturally, those skilled in the art will appreciate, based on this disclosure, that other shapes of bone blocks, such as rounded bone blocks, and other types of combinations of soft and hard tissues may be assembled according to this disclosure. However, the example of such animplant 2700 may be used instead of having to harvest a bone-tendon-bone implant from cadaveric knees, which tissue is in short supply. - Based on the present disclosure, those skilled in the art will further appreciate that the cortical bone pins disclosed herein may have features defined thereon for various applications. For example, not meant to be limiting, the shafts may contain stops, such that other pieces of bone inserted thereon can only travel a certain distance down the shaft before encountering the stop. The shaft may also contain through holes, to permit insertion of cotter pins or the like. Furthermore, the cortical bone shaft may be demineralized, mineralized, or partially demineralized. In one specific embodiment, the end of the cortical shaft contains a tapped cannulation a short distance into the longitudinal end of the shaft. In this way, a screw may be driven into the cannulation to retain elements inserted over the shaft in association with the shaft. To accommodate the screw, the screw end bearing the cannulation may be partially demineralized, such that upon insertion of the retention screw, the shaft end does not shatter, but expands to accommodate the increasing diameter of the screw as it is driven into the shaft. Naturally, in certain applications, it may be desirable for the cortical pins to be cannulated throughout the longitudinal length thereof. However, care should be taken that this does not unduly weaken the overall compressive or torsional strength of the assembled implant. This may be addressed by including pins that are not cannulated, along with pins that are cannulated. The cannulated pins may be used in combination with sutures or the like, in order to hold an implant in a specific orientation, until fusion with adjacent bone has proceeded to a sufficient extent for the implant to become stable without the sutures.[0052]
- It will be appreciated from the present disclosure that implants that have classically been fabricated from metals may be fabricated by assembling bone pieces. In addition, a benefit of the assembled graft according to this invention is that the components of the assembled graft can be derived from various anatomical structures, thus circumventing limitations normally resulting from having to obtain a graft from a particular anatomical source of a particular donor. Not only can the components be sourced from different anatomies, but also different donors may yield various components for assembly into a unitary implant. The end result is maximization of the gift of donation and the preservation of precious tissue resources. As noted above, being able to pool tissues from different sources depends, to some significant extent, on the ability to treat portions of tissue harvested from different anatomies or donors so as to prevent any contamination of a recipient with pathological or antigenic agents. A further benefit of the present invention is that different implants with height or width limitations due to the anatomical structures from which the implant has been derived may be pinned together to form implants of essentially any desired dimensions. In this fashion, an inventory of building blocks in combination with the appropriate assembly pins, threaded or unthreaded, is useful to provide implants of essentially any dimensions in the course of given surgical procedure. According to this embodiment of the invention, for example, a cervical Smith-Robinson (CSR) t of any desired height may be produced by attaching two or more existing CSR implants together with cortical bone pins. This is accomplished preferably using two machined CSR's of known height such that when added together, the desired overall height is achieved. The two CSR's are stacked and drill holes are machined through the CSR bodies, following which the cortical bone pins are press-fit through the thus machined holes. Preferably, the diameter of the pins is slightly greater than the diameter of the drilled holes, such that a tight press-fit is achieved.[0053]
- From the present disclosure, it will further be appreciated that implants according to this invention may be assembled in the operating room by a surgeon, using pre-formed implant pieces, from a kit. It will further be appreciated that the assembled implant pieces may be adhered to each other using any of a number of biologically acceptable glues, pastes and the like. In one such embodiment, the assembled implant pieces are assembled using a polymethyl-methacrylate glue, a cyanoacrylate glue, or any other adhesive known in the art, so long as the use of such an adhesive is confirmed to be nontoxic. It will further be appreciated that in forming the assembled grafts according to the present invention, it is acceptable, although not required, for interlocking features to be included on abutting faces of implant segments to be assembled together. Where such features are included, it is preferred for the adjacent features to be complementary, such that a protrusion on a first surface is met by a compatible indentation in the abutting surface. Such abutting features assist to provide torsional and structural strength to the assembled implant, and to relieve a measure of stress on the cortical bone pins used to assemble the implant.[0054]
- According to U.S. Pat. No. 6,025,538, an elaborate system is disclosed for ensuring that a bore is provided in mating surfaces of a composite implant such that the bore is angularly aligned with respect to mating surfaces so as to be oblique to the plane of each mating surface. This is not required according to the present invention.[0055]
- According to U.S. Pat. No. 5,899,939, layers of bone are juxtaposed, but no mechanical fixation of the various layers to each other is provided for, such as the cortical bone pins disclosed herein.[0056]
- With respect to PCT Publication WO 00/40177 and the priority US Patent filings, Ser. Nos. 09/225,299, filed Jan. 5, 1999, 09/286,975, filed Apr. 6, 1999, and 09/368,263, filed Aug. 3, 1999, it is believed that there exists interfering subject matter claimed in the present and in those applications. As to the interfering subject matter, claims are presented herein which are believed to constitute the basis for initiation of an interference proceeding in the United States, and initiation of such a proceeding is hereby specifically elicited, in which it is believed that the present applicants are entitled to priority. As to the non-interfering subject matter disclosed and claimed herein, the right to file one or more continuation or divisional applications free of interfering subject matter is reserved.[0057]
- Having generally described this invention, including the methods of manufacture and use thereof, including the best mode thereof, those skilled in the art will appreciate that a large number of variations on the principles described herein may be accomplished. Thus, the specifics of this description and the attached drawings should not be interpreted to limit the scope of this invention to the specifics thereof. Rather, the scope of this invention should be evaluated with reference to the claims appended hereto.[0058]
Claims (60)
Priority Applications (16)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| PCT/US2001/004510WO2001078798A1 (en) | 2000-02-10 | 2001-02-12 | Assembled implant |
| US09/782,594US20010031254A1 (en) | 1998-11-13 | 2001-02-12 | Assembled implant |
| US09/941,154US20020106393A1 (en) | 2000-02-10 | 2001-08-27 | Assembled implant, including mixed-composition segment |
| US09/942,537US6893462B2 (en) | 2000-01-11 | 2001-08-29 | Soft and calcified tissue implants |
| EP01968600AEP1359950A1 (en) | 2000-02-10 | 2001-09-07 | Assembled implant, including mixed-composition segment |
| PCT/US2001/027683WO2002064180A1 (en) | 2000-02-10 | 2001-09-07 | Assembled implant, including mixed-composition segment |
| CA2437763ACA2437763C (en) | 2001-02-12 | 2001-09-07 | Assembled implant, including mixed-composition segment |
| JP2002563972AJP2005510258A (en) | 2001-02-12 | 2001-09-07 | An assembled implant comprising a mixed composition segment |
| AU2001288840AAU2001288840B2 (en) | 2000-02-10 | 2001-09-07 | Assembled implant, including mixed-composition segment |
| US10/387,322US20040115172A1 (en) | 1998-11-13 | 2002-12-23 | Assembled implant, including mixed-composition segment |
| US11/007,679US20050119744A1 (en) | 2000-01-11 | 2004-12-08 | Soft and calcified tissue implants |
| US11/007,525US7513910B2 (en) | 2000-01-11 | 2004-12-08 | Soft and calcified tissue implants |
| US12/260,898US20110301707A1 (en) | 2000-01-11 | 2008-10-29 | Soft and Calcified Tissue Implants |
| US12/690,074US9763787B2 (en) | 1997-08-27 | 2010-01-19 | Assembled implant |
| US13/593,218US20120323324A1 (en) | 2000-01-11 | 2012-08-23 | Soft and calcified tissue implants |
| US15/709,456US20180071102A1 (en) | 1998-11-13 | 2017-09-19 | Assembled implant |
Applications Claiming Priority (5)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US09/191,232US6482584B1 (en) | 1998-11-13 | 1998-11-13 | Cyclic implant perfusion cleaning and passivation process |
| US09/378,527US6652818B1 (en) | 1998-11-13 | 1999-08-20 | Implant sterilization apparatus |
| US09/390,174US6613278B1 (en) | 1998-11-13 | 1999-09-07 | Tissue pooling process |
| US18162200P | 2000-02-10 | 2000-02-10 | |
| US09/782,594US20010031254A1 (en) | 1998-11-13 | 2001-02-12 | Assembled implant |
Related Parent Applications (7)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US09/191,232Continuation-In-PartUS6482584B1 (en) | 1997-08-27 | 1998-11-13 | Cyclic implant perfusion cleaning and passivation process |
| US09/191,132Continuation-In-PartUS6947398B1 (en) | 1998-11-13 | 1998-11-13 | Addressing scheme for a multimedia mobile network |
| US09/370,194Continuation-In-PartUS6223534B1 (en) | 1998-08-13 | 1999-08-09 | Engine-braking arrangement for an internal combustion engine with an exhaust-gas turbocharger |
| US09/378,527Continuation-In-PartUS6652818B1 (en) | 1997-08-27 | 1999-08-20 | Implant sterilization apparatus |
| US09/390,174Continuation-In-PartUS6613278B1 (en) | 1997-08-27 | 1999-09-07 | Tissue pooling process |
| US09/750,192Continuation-In-PartUS20010018614A1 (en) | 1999-03-16 | 2000-12-28 | Implants for orthopedic applications |
| US09/750,192ContinuationUS20010018614A1 (en) | 1999-03-16 | 2000-12-28 | Implants for orthopedic applications |
Related Child Applications (7)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US09/481,319Continuation-In-PartUS6497726B1 (en) | 1998-11-13 | 2000-01-11 | Materials and methods for improved bone tendon bone transplantation |
| US09/941,154Continuation-In-PartUS20020106393A1 (en) | 1998-11-13 | 2001-08-27 | Assembled implant, including mixed-composition segment |
| US09/941,154ContinuationUS20020106393A1 (en) | 1998-11-13 | 2001-08-27 | Assembled implant, including mixed-composition segment |
| US09/942,537Continuation-In-PartUS6893462B2 (en) | 2000-01-11 | 2001-08-29 | Soft and calcified tissue implants |
| US09/942,537ContinuationUS6893462B2 (en) | 2000-01-11 | 2001-08-29 | Soft and calcified tissue implants |
| US11/007,525ContinuationUS7513910B2 (en) | 2000-01-11 | 2004-12-08 | Soft and calcified tissue implants |
| US12/690,074ContinuationUS9763787B2 (en) | 1997-08-27 | 2010-01-19 | Assembled implant |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| US20010031254A1true US20010031254A1 (en) | 2001-10-18 |
Family
ID=46257505
Family Applications (3)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US09/782,594AbandonedUS20010031254A1 (en) | 1997-08-27 | 2001-02-12 | Assembled implant |
| US12/690,074Expired - Fee RelatedUS9763787B2 (en) | 1997-08-27 | 2010-01-19 | Assembled implant |
| US15/709,456AbandonedUS20180071102A1 (en) | 1998-11-13 | 2017-09-19 | Assembled implant |
Family Applications After (2)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US12/690,074Expired - Fee RelatedUS9763787B2 (en) | 1997-08-27 | 2010-01-19 | Assembled implant |
| US15/709,456AbandonedUS20180071102A1 (en) | 1998-11-13 | 2017-09-19 | Assembled implant |
Country Status (1)
| Country | Link |
|---|---|
| US (3) | US20010031254A1 (en) |
Cited By (81)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US20010041941A1 (en)* | 2000-03-22 | 2001-11-15 | Boyer Michael L. | Multipiece implants formed of bone material |
| US20030004575A1 (en)* | 1999-06-23 | 2003-01-02 | Sulzer Spine-Tech Inc. | Expandable fusion device and method |
| US20030028197A1 (en)* | 2000-07-06 | 2003-02-06 | Hanson David A. | Bone implants and methods |
| US20030078661A1 (en)* | 2001-09-27 | 2003-04-24 | Houfburg Rodney L. | Modular spinal fusion device |
| US20030093153A1 (en)* | 2001-09-28 | 2003-05-15 | Banick Christopher M. | Skeletal stabilization implant |
| US20040088055A1 (en)* | 2001-02-16 | 2004-05-06 | Hanson David A | Bone implants and methods |
| US6740091B2 (en) | 1997-03-06 | 2004-05-25 | Sulzer Spine-Tech Inc. | Lordotic spinal implant |
| US6749636B2 (en) | 2001-04-02 | 2004-06-15 | Gary K. Michelson | Contoured spinal fusion implants made of bone or a bone composite material |
| US20040249463A1 (en)* | 2003-06-05 | 2004-12-09 | Bindseil James J. | Bone strip implants and method of making same |
| US20040249464A1 (en)* | 2003-06-05 | 2004-12-09 | Bindseil James J. | Bone implants and methods of making same |
| US20040249471A1 (en)* | 2003-06-05 | 2004-12-09 | Bindseil James J. | Fusion implant and method of making same |
| US20040258732A1 (en)* | 2001-11-27 | 2004-12-23 | Yasuo Shikinami | Implant material and process for producing the same |
| US20050065607A1 (en)* | 2003-09-24 | 2005-03-24 | Gross Jeffrey M. | Assembled fusion implant |
| US20050065613A1 (en)* | 2003-09-24 | 2005-03-24 | Gross Jeffrey M. | Reinforced fusion implant |
| US6890355B2 (en) | 2001-04-02 | 2005-05-10 | Gary K. Michelson | Artificial contoured spinal fusion implants made of a material other than bone |
| US20050229323A1 (en)* | 2004-04-20 | 2005-10-20 | Mills C R | Process and apparatus for treating implants comprising soft tissue |
| US20050234460A1 (en)* | 2004-02-13 | 2005-10-20 | Drew Miller | Soft tissue repair apparatus and method |
| US6989031B2 (en) | 2001-04-02 | 2006-01-24 | Sdgi Holdings, Inc. | Hemi-interbody spinal implant manufactured from a major long bone ring or a bone composite |
| US20060200235A1 (en)* | 2005-03-04 | 2006-09-07 | Regeneration Technologies, Inc. | Assembled bone-tendon-bone grafts |
| US20060212036A1 (en)* | 2005-03-04 | 2006-09-21 | Regeneration Technologies, Inc. | Bone block assemblies and their use in assembled bone-tendon-bone grafts |
| US20060228252A1 (en)* | 2004-04-20 | 2006-10-12 | Mills C R | Process and apparatus for treating implants comprising soft tissue |
| US20060229722A1 (en)* | 2005-03-04 | 2006-10-12 | Bianchi John R | Adjustable and fixed assembled bone-tendon-bone graft |
| US20060271192A1 (en)* | 2005-03-04 | 2006-11-30 | Olsen Raymond E | Self Fixing Assembled Bone-Tendon-Bone Graft |
| US7226482B2 (en) | 2003-09-02 | 2007-06-05 | Synthes (U.S.A.) | Multipiece allograft implant |
| US7232464B2 (en) | 2002-02-19 | 2007-06-19 | Synthes (Usa) | Intervertebral implant |
| US20080058953A1 (en)* | 2006-08-31 | 2008-03-06 | Scarborough Nelson L | Demineralized cancellous strip DBM graft |
| USD583053S1 (en) | 2005-12-19 | 2008-12-16 | Rti Biologics, Inc. | Orthopedic bone block |
| USD583055S1 (en) | 2005-03-04 | 2008-12-16 | Rti Biologics, Inc. | Orthopedic bone block assembly |
| US7488348B2 (en) | 2003-05-16 | 2009-02-10 | Musculoskeletal Transplant Foundation | Cartilage allograft plug |
| US20090075005A1 (en)* | 2005-02-10 | 2009-03-19 | Hideki Nagareo | Transfer-Type Pressure Sensitive Adhesive Tape |
| WO2009048314A1 (en)* | 2007-10-08 | 2009-04-16 | Sureshan Sivananthan | A scalable matrix for the in vivo cultivation of bone and cartilage |
| US20090312842A1 (en)* | 2008-06-16 | 2009-12-17 | Predrag Bursac | Assembled Cartilage Repair Graft |
| US7682392B2 (en) | 2002-10-30 | 2010-03-23 | Depuy Spine, Inc. | Regenerative implants for stabilizing the spine and devices for attachment of said implants |
| US20100172954A1 (en)* | 2004-04-28 | 2010-07-08 | Biomet Manufacturing Corp. | Irradiated implantable bone material |
| WO2010093955A1 (en)* | 2009-02-12 | 2010-08-19 | Osteotech,Inc. | Segmented delivery system |
| US7815926B2 (en) | 2005-07-11 | 2010-10-19 | Musculoskeletal Transplant Foundation | Implant for articular cartilage repair |
| US7837740B2 (en) | 2007-01-24 | 2010-11-23 | Musculoskeletal Transplant Foundation | Two piece cancellous construct for cartilage repair |
| US7846207B2 (en) | 2003-02-06 | 2010-12-07 | Synthes Usa, Llc | Intervertebral implant |
| USD630329S1 (en) | 2007-02-12 | 2011-01-04 | Rti Biologics, Inc. | Orthopedic bone block assembly |
| US7879103B2 (en) | 2005-04-15 | 2011-02-01 | Musculoskeletal Transplant Foundation | Vertebral disc repair |
| USRE42208E1 (en) | 2003-04-29 | 2011-03-08 | Musculoskeletal Transplant Foundation | Glue for cartilage repair |
| US7901457B2 (en)* | 2003-05-16 | 2011-03-08 | Musculoskeletal Transplant Foundation | Cartilage allograft plug |
| US7959683B2 (en) | 2006-07-25 | 2011-06-14 | Musculoskeletal Transplant Foundation | Packed demineralized cancellous tissue forms for disc nucleus augmentation, restoration, or replacement and methods of implantation |
| US8202539B2 (en) | 2007-10-19 | 2012-06-19 | Warsaw Orthopedic, Inc. | Demineralized bone matrix compositions and methods |
| US8292968B2 (en) | 2004-10-12 | 2012-10-23 | Musculoskeletal Transplant Foundation | Cancellous constructs, cartilage particles and combinations of cancellous constructs and cartilage particles |
| US8292957B2 (en) | 2000-04-19 | 2012-10-23 | Warsaw Orthopedic, Inc. | Bone hemi-lumbar arcuate interbody spinal fusion implant having an asymmetrical leading end |
| US8323340B2 (en) | 2000-04-19 | 2012-12-04 | Warsaw Orthopedic, Inc. | Artificial hemi-lumbar interbody spinal implant having an asymmetrical leading end |
| US8328876B2 (en) | 2003-12-31 | 2012-12-11 | Warsaw Orthopedic, Inc. | Bone matrix compositions and methods |
| US8337537B2 (en)* | 2001-07-16 | 2012-12-25 | Depuy Products, Inc. | Device from naturally occurring biologically derived materials |
| US8343220B2 (en) | 1999-05-05 | 2013-01-01 | Warsaw Orthopedic, Inc. | Nested interbody spinal fusion implants |
| US8343219B2 (en) | 2007-06-08 | 2013-01-01 | Ldr Medical | Intersomatic cage, intervertebral prosthesis, anchoring device and implantation instruments |
| US8357384B2 (en) | 2007-06-15 | 2013-01-22 | Warsaw Orthopedic, Inc. | Bone matrix compositions and methods |
| US8409288B2 (en) | 2006-02-15 | 2013-04-02 | Ldr Medical | Transforaminal intersomatic cage for an intervertebral fusion graft and an instrument for implanting the cage |
| US8435551B2 (en) | 2007-03-06 | 2013-05-07 | Musculoskeletal Transplant Foundation | Cancellous construct with support ring for repair of osteochondral defects |
| US20130173013A1 (en)* | 1999-01-05 | 2013-07-04 | Lifenet Health | Composite Bone Graft, Method of Making and Using the Same |
| US8540774B2 (en) | 2007-11-16 | 2013-09-24 | DePuy Synthes Products, LLC | Low profile intervertebral implant |
| US8642061B2 (en) | 2007-06-15 | 2014-02-04 | Warsaw Orthopedic, Inc. | Method of treating bone tissue |
| US8697139B2 (en) | 2004-09-21 | 2014-04-15 | Frank M. Phillips | Method of intervertebral disc treatment using articular chondrocyte cells |
| US8734525B2 (en) | 2003-12-31 | 2014-05-27 | Warsaw Orthopedic, Inc. | Osteoinductive demineralized cancellous bone |
| US8901078B2 (en) | 2011-07-28 | 2014-12-02 | Harbor Medtech, Inc. | Crosslinked human or animal tissue products and their methods of manufacture and use |
| US8911759B2 (en) | 2005-11-01 | 2014-12-16 | Warsaw Orthopedic, Inc. | Bone matrix compositions and methods |
| KR101479812B1 (en)* | 2011-12-30 | 2015-01-08 | 고려대학교 산학협력단 | Washer type connector for spinal fusion and pedicle screw using the same |
| US9039775B2 (en) | 2003-03-31 | 2015-05-26 | DePuy Synthes Products, Inc. | Spinal fixation plates |
| US9039774B2 (en) | 2012-02-24 | 2015-05-26 | Ldr Medical | Anchoring device and system for an intervertebral implant, intervertebral implant and implantation instrument |
| US9044337B2 (en) | 2009-12-31 | 2015-06-02 | Ldr Medical | Anchoring device and system for an intervertebral implant, intervertebral implant and implantation instrument |
| US9078765B2 (en) | 2001-07-13 | 2015-07-14 | Ldr Medical | Vertebral cage device with modular fixation |
| US9192419B2 (en) | 2008-11-07 | 2015-11-24 | DePuy Synthes Products, Inc. | Zero-profile interbody spacer and coupled plate assembly |
| US9220604B2 (en) | 2010-12-21 | 2015-12-29 | DePuy Synthes Products, Inc. | Intervertebral implants, systems, and methods of use |
| US9241809B2 (en) | 2010-12-21 | 2016-01-26 | DePuy Synthes Products, Inc. | Intervertebral implants, systems, and methods of use |
| CN105266929A (en)* | 2015-11-05 | 2016-01-27 | 同济大学 | Mountable allogenous cortical bone axial fusion device |
| US9333082B2 (en) | 2007-07-10 | 2016-05-10 | Warsaw Orthopedic, Inc. | Delivery system attachment |
| US9463091B2 (en) | 2009-09-17 | 2016-10-11 | Ldr Medical | Intervertebral implant having extendable bone fixation members |
| US9554920B2 (en) | 2007-06-15 | 2017-01-31 | Warsaw Orthopedic, Inc. | Bone matrix compositions having nanoscale textured surfaces |
| US9597194B2 (en) | 2005-09-23 | 2017-03-21 | Ldr Medical | Intervertebral disc prosthesis |
| US9701940B2 (en) | 2005-09-19 | 2017-07-11 | Histogenics Corporation | Cell-support matrix having narrowly defined uniformly vertically and non-randomly organized porosity and pore density and a method for preparation thereof |
| US9867718B2 (en) | 2014-10-22 | 2018-01-16 | DePuy Synthes Products, Inc. | Intervertebral implants, systems, and methods of use |
| US10077420B2 (en) | 2014-12-02 | 2018-09-18 | Histogenics Corporation | Cell and tissue culture container |
| US10512548B2 (en) | 2006-02-27 | 2019-12-24 | DePuy Synthes Products, Inc. | Intervertebral implant with fixation geometry |
| US11957598B2 (en) | 2004-02-04 | 2024-04-16 | Ldr Medical | Intervertebral disc prosthesis |
| US12102731B2 (en) | 2020-05-01 | 2024-10-01 | Harbor Medtech, Inc. | Port-accessible multidirectional reinforced minimally invasive collagen device for soft tissue repair |
| US20250082377A1 (en)* | 2023-12-12 | 2025-03-13 | The Fourth Medical Center Of The Chinese People's Liberation Army General Hospital | Proximal-femur intraosseous release device |
Families Citing this family (12)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US12171904B2 (en) | 2006-10-30 | 2024-12-24 | Trs Holdings Llc | Mineral coated scaffolds |
| US9943410B2 (en) | 2011-02-28 | 2018-04-17 | DePuy Synthes Products, Inc. | Modular tissue scaffolds |
| US9468536B1 (en) | 2011-11-02 | 2016-10-18 | Nuvasive, Inc. | Spinal fusion implants and related methods |
| EP2883511B1 (en) | 2012-07-03 | 2018-08-22 | KUKA Deutschland GmbH | Surgical instrument arrangement |
| USD731061S1 (en) | 2012-11-28 | 2015-06-02 | Nuvasive, Inc. | Intervertebral implant |
| US10194964B2 (en)* | 2014-11-14 | 2019-02-05 | Warsaw Orthopedic, Inc. | Shaped bone graft materials and methods of use |
| US11052175B2 (en) | 2015-08-19 | 2021-07-06 | Musculoskeletal Transplant Foundation | Cartilage-derived implants and methods of making and using same |
| US10736752B1 (en) | 2017-10-24 | 2020-08-11 | Omnia Medical, LLC | Multi-material multi-component spinal implant |
| US11766339B1 (en) | 2017-10-24 | 2023-09-26 | Omnia Medical, LLC | Multi-material multi-component spinal implant |
| CA3146093A1 (en)* | 2019-08-21 | 2021-02-25 | Mark Evans | Transforaminal posterior atraumatic lumbar bio-implant |
| KR102370651B1 (en)* | 2020-02-06 | 2022-03-04 | 주식회사 지비에스커먼웰스 | Structure of porous spinal implant |
| EP4193967B1 (en)* | 2021-12-08 | 2025-09-17 | PR Spine GbR | Modular support cage |
Citations (28)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US4950296A (en)* | 1988-04-07 | 1990-08-21 | Mcintyre Jonathan L | Bone grafting units |
| US4969909A (en)* | 1987-10-27 | 1990-11-13 | Barouk Louis S | Articular prosthetic implant with temporary fixing means |
| US5084051A (en)* | 1986-11-03 | 1992-01-28 | Toermaelae Pertti | Layered surgical biocomposite material |
| US5112354A (en)* | 1989-11-16 | 1992-05-12 | Northwestern University | Bone allograft material and method |
| US5147367A (en)* | 1991-02-22 | 1992-09-15 | Ellis Alfred B | Drill pin guide and method for orthopedic surgery |
| US5180388A (en)* | 1990-06-28 | 1993-01-19 | American Cyanamid Company | Bone pinning system |
| US5192327A (en)* | 1991-03-22 | 1993-03-09 | Brantigan John W | Surgical prosthetic implant for vertebrae |
| US5571190A (en)* | 1993-08-20 | 1996-11-05 | Heinrich Ulrich | Implant for the replacement of vertebrae and/or stabilization and fixing of the spinal column |
| US5676700A (en)* | 1994-10-25 | 1997-10-14 | Osteonics Corp. | Interlocking structural elements and method for bone repair, augmentation and replacement |
| US5716358A (en)* | 1994-12-02 | 1998-02-10 | Johnson & Johnson Professional, Inc. | Directional bone fixation device |
| US5814084A (en)* | 1996-01-16 | 1998-09-29 | University Of Florida Tissue Bank, Inc. | Diaphysial cortical dowel |
| US5865848A (en)* | 1997-09-12 | 1999-02-02 | Artifex, Ltd. | Dynamic intervertebral spacer and method of use |
| US5899939A (en)* | 1998-01-21 | 1999-05-04 | Osteotech, Inc. | Bone-derived implant for load-supporting applications |
| US5989289A (en)* | 1995-10-16 | 1999-11-23 | Sdgi Holdings, Inc. | Bone grafts |
| US6025538A (en)* | 1998-11-20 | 2000-02-15 | Musculoskeletal Transplant Foundation | Compound bone structure fabricated from allograft tissue |
| US6090998A (en)* | 1997-10-27 | 2000-07-18 | University Of Florida | Segmentally demineralized bone implant |
| US6146420A (en)* | 1997-12-10 | 2000-11-14 | Sdgi Holdings, Inc. | Osteogenic fusion device |
| US6200347B1 (en)* | 1999-01-05 | 2001-03-13 | Lifenet | Composite bone graft, method of making and using same |
| US20010039458A1 (en)* | 2000-03-22 | 2001-11-08 | Boyer Michael L. | Implants formed of coupled bone |
| US6398786B1 (en)* | 1997-10-09 | 2002-06-04 | Nenad Sesic | Strain-inducing conical screw for stimulating bone transplant growth |
| US6494883B1 (en)* | 2000-05-26 | 2002-12-17 | Bret A. Ferree | Bone reinforcers |
| US20030036800A1 (en)* | 2000-07-13 | 2003-02-20 | Meredith Thomas L. | Composite bone material implant and method |
| US20030077825A1 (en)* | 1994-07-22 | 2003-04-24 | Bhatnagar Rajendra S. | Structures useful for bone engineering and methods |
| US6554863B2 (en)* | 1998-08-03 | 2003-04-29 | Synthes | Intervertebral allograft spacer |
| US6986788B2 (en)* | 1998-01-30 | 2006-01-17 | Synthes (U.S.A.) | Intervertebral allograft spacer |
| US20060106460A1 (en)* | 2001-05-03 | 2006-05-18 | Synthes (Usa) | Intervertebral implant for transforaminal posterior lumbar interbody fusion procedure |
| US20060241763A1 (en)* | 1998-08-03 | 2006-10-26 | Synthes (Usa) | Multipiece bone implant |
| US20070016295A1 (en)* | 2000-09-19 | 2007-01-18 | Boyd Lawrence M | Reinforced molded implant formed of cortical bone |
Family Cites Families (69)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US3291640A (en)* | 1963-05-27 | 1966-12-13 | Chemclean Corp | Ultrasonic cleaning process |
| US4193818A (en)* | 1978-05-05 | 1980-03-18 | American Sterilizer Company | Combined ultrasonic cleaning and biocidal treatment in a single pressure vessel |
| US4294753A (en)* | 1980-08-04 | 1981-10-13 | The Regents Of The University Of California | Bone morphogenetic protein process |
| DE3521684A1 (en)* | 1985-06-18 | 1986-12-18 | Dr. Müller-Lierheim KG, Biologische Laboratorien, 8033 Planegg | METHOD FOR COATING POLYMERS |
| US4872840A (en)* | 1987-07-15 | 1989-10-10 | Team Incorporated | Dental implant and method |
| WO1991001367A1 (en)* | 1989-07-20 | 1991-02-07 | Bioeng, Inc. | Supercritical fluid disruption of and extraction from microbial cells |
| US5298222A (en)* | 1989-08-09 | 1994-03-29 | Osteotech, Inc. | Process for disinfecting musculoskeletal tissue and tissues prepared thereby |
| US5213619A (en)* | 1989-11-30 | 1993-05-25 | Jackson David P | Processes for cleaning, sterilizing, and implanting materials using high energy dense fluids |
| US5037437B1 (en)* | 1990-01-18 | 1998-04-14 | Univ Washington | Method of bone preparation for prosthetic fixation |
| CA2060635A1 (en)* | 1991-02-12 | 1992-08-13 | Keith D'alessio | Bioabsorbable medical implants |
| DE4215137A1 (en) | 1991-06-04 | 1992-12-10 | Man Ceramics Gmbh | IMPLANT FOR SPINE PILLARS |
| US5329846A (en)* | 1991-08-12 | 1994-07-19 | Bonutti Peter M | Tissue press and system |
| US6503277B2 (en)* | 1991-08-12 | 2003-01-07 | Peter M. Bonutti | Method of transplanting human body tissue |
| US5281422A (en)* | 1991-09-24 | 1994-01-25 | Purdue Research Foundation | Graft for promoting autogenous tissue growth |
| US5513662A (en)* | 1991-12-31 | 1996-05-07 | Osteotech, Inc. | Preparation of bone for transplantation |
| US5333626A (en)* | 1991-12-31 | 1994-08-02 | Cryolife, Inc. | Preparation of bone for transplantation |
| US5288462A (en)* | 1992-05-18 | 1994-02-22 | Stephen D. Carter | Sterilization apparatus and method |
| US5437287A (en)* | 1992-08-17 | 1995-08-01 | Carbomedics, Inc. | Sterilization of tissue implants using iodine |
| DE4227830C1 (en)* | 1992-08-21 | 1994-03-31 | Tulaszewski Olaf | Method and device for disinfecting a bone graft, in particular a human cancellous bone graft |
| FR2699408B1 (en)* | 1992-12-21 | 1995-03-24 | Bioland | Method for treating bone tissue and corresponding implantable biomaterials. |
| CA2176942C (en)* | 1993-12-07 | 2011-11-01 | Anthony J. Celeste | Bmp-12, bmp-13 and tendon-inducing compositions thereof |
| US5723012A (en)* | 1993-12-09 | 1998-03-03 | Bioland | Uses for a current of supercritical carbon dioxide as an antiviral agent |
| US5507813A (en)* | 1993-12-09 | 1996-04-16 | Osteotech, Inc. | Shaped materials derived from elongate bone particles |
| US5460962A (en)* | 1994-01-04 | 1995-10-24 | Organogenesis Inc. | Peracetic acid sterilization of collagen or collagenous tissue |
| US5716454A (en)* | 1994-02-03 | 1998-02-10 | New York Society For The Ruptured And Crippled Maintaining The Hospital For Special Surgery | Decontamination of devices and instruments contacted with body tissues |
| US5509968A (en)* | 1994-02-03 | 1996-04-23 | New York Society For The Ruptured And Crippled Maintaining The Hospital For Special Surgery | Decontamination of orthopaedic implants |
| US5626861A (en)* | 1994-04-01 | 1997-05-06 | Massachusetts Institute Of Technology | Polymeric-hydroxyapatite bone composite |
| US5906827A (en)* | 1994-06-03 | 1999-05-25 | Creative Biomolecules, Inc. | Matrix for the manufacture of autogenous replacement body parts |
| US5785966A (en)* | 1994-06-15 | 1998-07-28 | Coles; John G. | Inhibition of human xenogenic or allogenic antibodies to reduce xenograft or allograft rejection in human recipients |
| US5797871A (en)* | 1994-08-19 | 1998-08-25 | Lifenet Research Foundation | Ultrasonic cleaning of allograft bone |
| US5556379A (en)* | 1994-08-19 | 1996-09-17 | Lifenet Research Foundation | Process for cleaning large bone grafts and bone grafts produced thereby |
| US5674292A (en)* | 1995-06-07 | 1997-10-07 | Stryker Corporation | Terminally sterilized osteogenic devices and preparation thereof |
| US5944755A (en)* | 1995-09-15 | 1999-08-31 | Crosscart, Inc. | Articular cartilage xenografts |
| US6110206A (en)* | 1995-09-15 | 2000-08-29 | Crosscart, Inc. | Anterior cruciate ligament xenografts |
| US6423095B1 (en)* | 1995-10-16 | 2002-07-23 | Sdgi Holdings, Inc. | Intervertebral spacers |
| US5753195A (en)* | 1996-01-02 | 1998-05-19 | Kew Import/Export Inc. | Cleaning and sterilizing mechanism |
| US5711921A (en)* | 1996-01-02 | 1998-01-27 | Kew Import/Export Inc. | Medical cleaning and sterilizing apparatus |
| US6024735A (en)* | 1996-03-20 | 2000-02-15 | Lifenet Research Foundation | Process and composition for cleaning soft tissue grafts optionally attached to bone and soft tissue and bone grafts produced thereby |
| PL331765A1 (en)* | 1996-08-23 | 1999-08-02 | Cook Biotech Inc | Trnsplant prosthesis, materials and methods |
| WO1998017209A2 (en)* | 1996-10-23 | 1998-04-30 | Sdgi Holdings, Inc. | Spinal spacer |
| US5846484A (en)* | 1997-03-20 | 1998-12-08 | Osteotech, Inc. | Pressure flow system and method for treating a fluid permeable workpiece such as a bone |
| US5861041A (en)* | 1997-04-07 | 1999-01-19 | Arthit Sitiso | Intervertebral disk prosthesis and method of making the same |
| US5993844A (en)* | 1997-05-08 | 1999-11-30 | Organogenesis, Inc. | Chemical treatment, without detergents or enzymes, of tissue to form an acellular, collagenous matrix |
| US6482584B1 (en)* | 1998-11-13 | 2002-11-19 | Regeneration Technologies, Inc. | Cyclic implant perfusion cleaning and passivation process |
| US6613278B1 (en) | 1998-11-13 | 2003-09-02 | Regeneration Technologies, Inc. | Tissue pooling process |
| US6652818B1 (en)* | 1998-11-13 | 2003-11-25 | Regeneration Technologies, Inc. | Implant sterilization apparatus |
| WO1999009914A1 (en) | 1997-08-27 | 1999-03-04 | University Of Florida Tissue Bank, Inc. | Cortical bone cervical smith-robinson fusion implant |
| US20020076429A1 (en) | 1998-01-28 | 2002-06-20 | John F. Wironen | Bone paste subjected to irradiative and thermal treatment |
| US6123731A (en)* | 1998-02-06 | 2000-09-26 | Osteotech, Inc. | Osteoimplant and method for its manufacture |
| US6641593B1 (en)* | 1998-06-03 | 2003-11-04 | Coalescent Surgical, Inc. | Tissue connector apparatus and methods |
| US6149864A (en)* | 1998-06-25 | 2000-11-21 | Massachusetts Institute Of Technology | Supercritical fluid sterilization method |
| US6102056A (en)* | 1998-08-18 | 2000-08-15 | Kotsopey; Omelan | Cleaning apparatus |
| US6497726B1 (en)* | 2000-01-11 | 2002-12-24 | Regeneration Technologies, Inc. | Materials and methods for improved bone tendon bone transplantation |
| WO2000054821A1 (en) | 1999-03-16 | 2000-09-21 | Regeneration Technologies, Inc. | Molded implants for orthopedic applications |
| AU6499700A (en) | 1999-07-28 | 2001-02-19 | Regeneration Technologies, Inc. | Reduced antigenicity tissue (rat) implants |
| US6379385B1 (en)* | 2000-01-06 | 2002-04-30 | Tutogen Medical Gmbh | Implant of bone matter |
| US20030023304A1 (en)* | 2000-01-11 | 2003-01-30 | Carter Kevin C. | Materials and methods for improved bone tendon bone transplantation |
| US6893462B2 (en)* | 2000-01-11 | 2005-05-17 | Regeneration Technologies, Inc. | Soft and calcified tissue implants |
| US20030097179A1 (en)* | 2000-01-11 | 2003-05-22 | Carter Kevin C. | Materials and methods for improved bone tendon bone transplantation |
| WO2001078798A1 (en) | 2000-02-10 | 2001-10-25 | Regeneration Technologies, Inc. | Assembled implant |
| US6719794B2 (en)* | 2001-05-03 | 2004-04-13 | Synthes (U.S.A.) | Intervertebral implant for transforaminal posterior lumbar interbody fusion procedure |
| US6855167B2 (en)* | 2001-12-05 | 2005-02-15 | Osteotech, Inc. | Spinal intervertebral implant, interconnections for such implant and processes for making |
| US6730124B2 (en) | 2002-03-08 | 2004-05-04 | Musculoskeletal Transplant Foundation | Bone-tendon-bone assembly with cancellous allograft bone block |
| US6761739B2 (en)* | 2002-11-25 | 2004-07-13 | Musculoskeletal Transplant Foundation | Cortical and cancellous allograft spacer |
| US7108832B2 (en)* | 2003-06-23 | 2006-09-19 | Novasterilis Inc. | Sterialization methods and apparatus which employ additive-containing supercritical carbon dioxide sterilant |
| US20050065607A1 (en)* | 2003-09-24 | 2005-03-24 | Gross Jeffrey M. | Assembled fusion implant |
| US7300464B2 (en) | 2004-09-30 | 2007-11-27 | Alcon, Inc. | Intraocular lens |
| US7776089B2 (en)* | 2005-03-04 | 2010-08-17 | Rti Biologics, Inc. | Assembled bone-tendon-bone grafts |
| US7763071B2 (en) | 2005-03-04 | 2010-07-27 | Rti Biologics, Inc. | Bone block assemblies and their use in assembled bone-tendon-bone grafts |
- 2001
- 2001-02-12USUS09/782,594patent/US20010031254A1/ennot_activeAbandoned
- 2010
- 2010-01-19USUS12/690,074patent/US9763787B2/ennot_activeExpired - Fee Related
- 2017
- 2017-09-19USUS15/709,456patent/US20180071102A1/ennot_activeAbandoned
Patent Citations (30)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US5084051A (en)* | 1986-11-03 | 1992-01-28 | Toermaelae Pertti | Layered surgical biocomposite material |
| US4969909A (en)* | 1987-10-27 | 1990-11-13 | Barouk Louis S | Articular prosthetic implant with temporary fixing means |
| US4950296A (en)* | 1988-04-07 | 1990-08-21 | Mcintyre Jonathan L | Bone grafting units |
| US5112354A (en)* | 1989-11-16 | 1992-05-12 | Northwestern University | Bone allograft material and method |
| US5180388A (en)* | 1990-06-28 | 1993-01-19 | American Cyanamid Company | Bone pinning system |
| US5147367A (en)* | 1991-02-22 | 1992-09-15 | Ellis Alfred B | Drill pin guide and method for orthopedic surgery |
| US5192327A (en)* | 1991-03-22 | 1993-03-09 | Brantigan John W | Surgical prosthetic implant for vertebrae |
| US5571190A (en)* | 1993-08-20 | 1996-11-05 | Heinrich Ulrich | Implant for the replacement of vertebrae and/or stabilization and fixing of the spinal column |
| US20030077825A1 (en)* | 1994-07-22 | 2003-04-24 | Bhatnagar Rajendra S. | Structures useful for bone engineering and methods |
| US5676700A (en)* | 1994-10-25 | 1997-10-14 | Osteonics Corp. | Interlocking structural elements and method for bone repair, augmentation and replacement |
| US5716358A (en)* | 1994-12-02 | 1998-02-10 | Johnson & Johnson Professional, Inc. | Directional bone fixation device |
| US5989289A (en)* | 1995-10-16 | 1999-11-23 | Sdgi Holdings, Inc. | Bone grafts |
| US5814084A (en)* | 1996-01-16 | 1998-09-29 | University Of Florida Tissue Bank, Inc. | Diaphysial cortical dowel |
| US5865848A (en)* | 1997-09-12 | 1999-02-02 | Artifex, Ltd. | Dynamic intervertebral spacer and method of use |
| US6398786B1 (en)* | 1997-10-09 | 2002-06-04 | Nenad Sesic | Strain-inducing conical screw for stimulating bone transplant growth |
| US6090998A (en)* | 1997-10-27 | 2000-07-18 | University Of Florida | Segmentally demineralized bone implant |
| US6146420A (en)* | 1997-12-10 | 2000-11-14 | Sdgi Holdings, Inc. | Osteogenic fusion device |
| US5899939A (en)* | 1998-01-21 | 1999-05-04 | Osteotech, Inc. | Bone-derived implant for load-supporting applications |
| US7347873B2 (en)* | 1998-01-30 | 2008-03-25 | Synthes (U.S.A.) | Intervertebral allograft spacer |
| US7300465B2 (en)* | 1998-01-30 | 2007-11-27 | Synthes (U.S.A.) | Intervertebral allograft spacer |
| US6986788B2 (en)* | 1998-01-30 | 2006-01-17 | Synthes (U.S.A.) | Intervertebral allograft spacer |
| US20060241763A1 (en)* | 1998-08-03 | 2006-10-26 | Synthes (Usa) | Multipiece bone implant |
| US6554863B2 (en)* | 1998-08-03 | 2003-04-29 | Synthes | Intervertebral allograft spacer |
| US6025538A (en)* | 1998-11-20 | 2000-02-15 | Musculoskeletal Transplant Foundation | Compound bone structure fabricated from allograft tissue |
| US6200347B1 (en)* | 1999-01-05 | 2001-03-13 | Lifenet | Composite bone graft, method of making and using same |
| US20010039458A1 (en)* | 2000-03-22 | 2001-11-08 | Boyer Michael L. | Implants formed of coupled bone |
| US6494883B1 (en)* | 2000-05-26 | 2002-12-17 | Bret A. Ferree | Bone reinforcers |
| US20030036800A1 (en)* | 2000-07-13 | 2003-02-20 | Meredith Thomas L. | Composite bone material implant and method |
| US20070016295A1 (en)* | 2000-09-19 | 2007-01-18 | Boyd Lawrence M | Reinforced molded implant formed of cortical bone |
| US20060106460A1 (en)* | 2001-05-03 | 2006-05-18 | Synthes (Usa) | Intervertebral implant for transforaminal posterior lumbar interbody fusion procedure |
Cited By (193)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US20040249460A1 (en)* | 1997-03-06 | 2004-12-09 | Sulzer Spine-Tech Inc. | Lordotic spinal implant |
| US7267689B2 (en) | 1997-03-06 | 2007-09-11 | Zimmer Spine, Inc. | Lordotic spinal implant |
| US6740091B2 (en) | 1997-03-06 | 2004-05-25 | Sulzer Spine-Tech Inc. | Lordotic spinal implant |
| US20130173013A1 (en)* | 1999-01-05 | 2013-07-04 | Lifenet Health | Composite Bone Graft, Method of Making and Using the Same |
| US8343220B2 (en) | 1999-05-05 | 2013-01-01 | Warsaw Orthopedic, Inc. | Nested interbody spinal fusion implants |
| US20030004575A1 (en)* | 1999-06-23 | 2003-01-02 | Sulzer Spine-Tech Inc. | Expandable fusion device and method |
| US6830589B2 (en) | 1999-06-23 | 2004-12-14 | Zimmer Spine, Inc. | Expandable fusion device and method |
| US20010041941A1 (en)* | 2000-03-22 | 2001-11-15 | Boyer Michael L. | Multipiece implants formed of bone material |
| US7115146B2 (en)* | 2000-03-22 | 2006-10-03 | Boyer Ii Michael L | Multipiece implants formed of bone material |
| US8834569B2 (en) | 2000-04-19 | 2014-09-16 | Warsaw Orthopedic, Inc. | Artificial hemi-lumbar interbody spinal fusion cage having an asymmetrical leading end |
| US8292957B2 (en) | 2000-04-19 | 2012-10-23 | Warsaw Orthopedic, Inc. | Bone hemi-lumbar arcuate interbody spinal fusion implant having an asymmetrical leading end |
| US8323340B2 (en) | 2000-04-19 | 2012-12-04 | Warsaw Orthopedic, Inc. | Artificial hemi-lumbar interbody spinal implant having an asymmetrical leading end |
| US7018416B2 (en) | 2000-07-06 | 2006-03-28 | Zimmer Spine, Inc. | Bone implants and methods |
| US20030028197A1 (en)* | 2000-07-06 | 2003-02-06 | Hanson David A. | Bone implants and methods |
| US20070055377A1 (en)* | 2001-02-16 | 2007-03-08 | Zimmer Spine, Inc. | Bone implants and methods |
| US20040088055A1 (en)* | 2001-02-16 | 2004-05-06 | Hanson David A | Bone implants and methods |
| US7935149B2 (en) | 2001-04-02 | 2011-05-03 | Warsaw Orthopedic, Inc. | Spinal fusion implant with bone screws |
| US6989031B2 (en) | 2001-04-02 | 2006-01-24 | Sdgi Holdings, Inc. | Hemi-interbody spinal implant manufactured from a major long bone ring or a bone composite |
| US7611536B2 (en) | 2001-04-02 | 2009-11-03 | Warsaw Orthopedic, Inc. | Hemi-interbody spinal fusion implants manufactured from a major long bone ring |
| US7455692B2 (en) | 2001-04-02 | 2008-11-25 | Warsaw Orthopedic, Inc. | Hemi-artificial contoured spinal fusion implants made of a material other than bone |
| US7435262B2 (en) | 2001-04-02 | 2008-10-14 | Warsaw Orthopedic, Inc. | Contoured cortical bone implants |
| US7540882B2 (en) | 2001-04-02 | 2009-06-02 | Warsaw Orthopedic, Inc. | Artificial spinal fusion implant with asymmetrical leading end |
| US6749636B2 (en) | 2001-04-02 | 2004-06-15 | Gary K. Michelson | Contoured spinal fusion implants made of bone or a bone composite material |
| US6890355B2 (en) | 2001-04-02 | 2005-05-10 | Gary K. Michelson | Artificial contoured spinal fusion implants made of a material other than bone |
| US8137403B2 (en) | 2001-04-02 | 2012-03-20 | Warsaw Orthopedic, Inc. | Hemi-interbody spinal fusion implants manufactured from a major long bone ring |
| US9463098B2 (en) | 2001-04-02 | 2016-10-11 | Warsaw Orthopedic, Inc. | Spinal fusion implant with bone screws and a bone screw lock |
| US8926703B2 (en) | 2001-04-02 | 2015-01-06 | Warsaw Orthopedic, Inc. | Spinal fusion implant with bone screws and a bone screw lock |
| US9078765B2 (en) | 2001-07-13 | 2015-07-14 | Ldr Medical | Vertebral cage device with modular fixation |
| US8337537B2 (en)* | 2001-07-16 | 2012-12-25 | Depuy Products, Inc. | Device from naturally occurring biologically derived materials |
| US20030078661A1 (en)* | 2001-09-27 | 2003-04-24 | Houfburg Rodney L. | Modular spinal fusion device |
| US7037339B2 (en) | 2001-09-27 | 2006-05-02 | Zimmer Spine, Inc. | Modular spinal fusion device |
| US20030093153A1 (en)* | 2001-09-28 | 2003-05-15 | Banick Christopher M. | Skeletal stabilization implant |
| US7125424B2 (en) | 2001-09-28 | 2006-10-24 | Zimmer Spine, Inc. | Skeletal stabilization implant |
| US20070010886A1 (en)* | 2001-09-28 | 2007-01-11 | Zimmer Spine, Inc. | Skeletal Stabilization Implant |
| US20040258732A1 (en)* | 2001-11-27 | 2004-12-23 | Yasuo Shikinami | Implant material and process for producing the same |
| US8119152B2 (en) | 2001-11-27 | 2012-02-21 | Takiron Co., Ltd. | Implant material and process for producing the same |
| US7232464B2 (en) | 2002-02-19 | 2007-06-19 | Synthes (Usa) | Intervertebral implant |
| US7618456B2 (en) | 2002-02-19 | 2009-11-17 | Synthes Usa, Llc | Intervertebral implant |
| US9572681B2 (en) | 2002-02-19 | 2017-02-21 | DePuy Synthes Products, Inc. | Intervertebral implant |
| US10492922B2 (en) | 2002-02-19 | 2019-12-03 | DePuy Synthes Products, Inc. | Intervertebral implant |
| US7682392B2 (en) | 2002-10-30 | 2010-03-23 | Depuy Spine, Inc. | Regenerative implants for stabilizing the spine and devices for attachment of said implants |
| US7862616B2 (en) | 2003-02-06 | 2011-01-04 | Synthes Usa, Llc | Intervertebral implant |
| US8764831B2 (en) | 2003-02-06 | 2014-07-01 | DePuy Synthes Products, LLC | Intervertebral implant |
| US10064740B2 (en) | 2003-02-06 | 2018-09-04 | DePuy Synthes Products, LLC | Intervertebral implant |
| US7846207B2 (en) | 2003-02-06 | 2010-12-07 | Synthes Usa, Llc | Intervertebral implant |
| US9463097B2 (en) | 2003-02-06 | 2016-10-11 | DePuy Synthes Products, Inc. | Intervertebral implant |
| US8715354B2 (en) | 2003-02-06 | 2014-05-06 | DePuy Synthes Products, LLC | Intervertebral implant |
| US10660765B2 (en) | 2003-02-06 | 2020-05-26 | DePuy Synthes Products, Inc. | Intervertebral implant |
| US8709085B2 (en) | 2003-02-06 | 2014-04-29 | DePuy Synthes Products, LLC | Intervertebral implant |
| US9320549B2 (en) | 2003-03-31 | 2016-04-26 | DePuy Synthes Products, Inc. | Spinal fixation plates |
| US9039775B2 (en) | 2003-03-31 | 2015-05-26 | DePuy Synthes Products, Inc. | Spinal fixation plates |
| USRE43258E1 (en) | 2003-04-29 | 2012-03-20 | Musculoskeletal Transplant Foundation | Glue for cartilage repair |
| USRE42208E1 (en) | 2003-04-29 | 2011-03-08 | Musculoskeletal Transplant Foundation | Glue for cartilage repair |
| US8221500B2 (en) | 2003-05-16 | 2012-07-17 | Musculoskeletal Transplant Foundation | Cartilage allograft plug |
| US7901457B2 (en)* | 2003-05-16 | 2011-03-08 | Musculoskeletal Transplant Foundation | Cartilage allograft plug |
| US7488348B2 (en) | 2003-05-16 | 2009-02-10 | Musculoskeletal Transplant Foundation | Cartilage allograft plug |
| US7252685B2 (en) | 2003-06-05 | 2007-08-07 | Sdgi Holdings, Inc. | Fusion implant and method of making same |
| US7537617B2 (en) | 2003-06-05 | 2009-05-26 | Warsaw Orthopedic, Inc. | Bone strip implants and method of making same |
| US20040249463A1 (en)* | 2003-06-05 | 2004-12-09 | Bindseil James J. | Bone strip implants and method of making same |
| US7351262B2 (en)* | 2003-06-05 | 2008-04-01 | Warsaw Orthopedic, Inc. | Bone implants and methods of making same |
| US20040249464A1 (en)* | 2003-06-05 | 2004-12-09 | Bindseil James J. | Bone implants and methods of making same |
| US20040249471A1 (en)* | 2003-06-05 | 2004-12-09 | Bindseil James J. | Fusion implant and method of making same |
| US7601173B2 (en) | 2003-09-02 | 2009-10-13 | Synthes Usa, Llc | Multipiece allograft implant |
| US7226482B2 (en) | 2003-09-02 | 2007-06-05 | Synthes (U.S.A.) | Multipiece allograft implant |
| US20070208424A1 (en)* | 2003-09-02 | 2007-09-06 | Dominique Messerli | Multipiece allograft implant |
| US20050065607A1 (en)* | 2003-09-24 | 2005-03-24 | Gross Jeffrey M. | Assembled fusion implant |
| US20050065613A1 (en)* | 2003-09-24 | 2005-03-24 | Gross Jeffrey M. | Reinforced fusion implant |
| US8734525B2 (en) | 2003-12-31 | 2014-05-27 | Warsaw Orthopedic, Inc. | Osteoinductive demineralized cancellous bone |
| US9034358B2 (en) | 2003-12-31 | 2015-05-19 | Warsaw Orthopedic, Inc. | Bone matrix compositions and methods |
| US9415136B2 (en) | 2003-12-31 | 2016-08-16 | Warsaw Orthopedic, Inc. | Osteoinductive demineralized cancellous bone |
| US8328876B2 (en) | 2003-12-31 | 2012-12-11 | Warsaw Orthopedic, Inc. | Bone matrix compositions and methods |
| US11957598B2 (en) | 2004-02-04 | 2024-04-16 | Ldr Medical | Intervertebral disc prosthesis |
| US20050234460A1 (en)* | 2004-02-13 | 2005-10-20 | Drew Miller | Soft tissue repair apparatus and method |
| US20060228252A1 (en)* | 2004-04-20 | 2006-10-12 | Mills C R | Process and apparatus for treating implants comprising soft tissue |
| US20050229323A1 (en)* | 2004-04-20 | 2005-10-20 | Mills C R | Process and apparatus for treating implants comprising soft tissue |
| US7648676B2 (en) | 2004-04-20 | 2010-01-19 | Rti Biologics, Inc. | Process and apparatus for treating implants comprising soft tissue |
| US20100172954A1 (en)* | 2004-04-28 | 2010-07-08 | Biomet Manufacturing Corp. | Irradiated implantable bone material |
| US7976861B2 (en) | 2004-04-28 | 2011-07-12 | Biomet Manufacturing Corp. | Irradiated implantable bone material |
| US8697139B2 (en) | 2004-09-21 | 2014-04-15 | Frank M. Phillips | Method of intervertebral disc treatment using articular chondrocyte cells |
| US8292968B2 (en) | 2004-10-12 | 2012-10-23 | Musculoskeletal Transplant Foundation | Cancellous constructs, cartilage particles and combinations of cancellous constructs and cartilage particles |
| US20090075005A1 (en)* | 2005-02-10 | 2009-03-19 | Hideki Nagareo | Transfer-Type Pressure Sensitive Adhesive Tape |
| USD583473S1 (en) | 2005-03-04 | 2008-12-23 | Rti Biologics, Inc. | Set of orthopedic bone block assemblies |
| US7763072B2 (en) | 2005-03-04 | 2010-07-27 | Rti Biologics, Inc. | Intermediate bone block and its use in bone block assemblies and assembled bone-tendon-bone grafts |
| WO2006096513A3 (en)* | 2005-03-04 | 2007-10-25 | Regeneration Tech Inc | Assembled bone-tendon-bone grafts |
| USD604850S1 (en) | 2005-03-04 | 2009-11-24 | Rti Biologics, Inc. | Orthopedic bone block assembly |
| USD608891S1 (en) | 2005-03-04 | 2010-01-26 | Rti Biologics, Inc. | Set of orthopedic bone block assemblies |
| US9717586B2 (en) | 2005-03-04 | 2017-08-01 | Rti Surgical, Inc. | Adjustable and fixed assembled bone-tendon-bone graft |
| WO2006096511A3 (en)* | 2005-03-04 | 2007-10-18 | Regeneration Tech Inc | Intermediate bone block and its use in bone block assemblies and assembled bone-tendon-bone grafts |
| US7727278B2 (en)* | 2005-03-04 | 2010-06-01 | Rti Biologics, Inc. | Self fixing assembled bone-tendon-bone graft |
| USD583055S1 (en) | 2005-03-04 | 2008-12-16 | Rti Biologics, Inc. | Orthopedic bone block assembly |
| USD583056S1 (en) | 2005-03-04 | 2008-12-16 | Rti Biologics, Inc. | Orthopedic bone block assembly |
| US7763071B2 (en)* | 2005-03-04 | 2010-07-27 | Rti Biologics, Inc. | Bone block assemblies and their use in assembled bone-tendon-bone grafts |
| US7776089B2 (en)* | 2005-03-04 | 2010-08-17 | Rti Biologics, Inc. | Assembled bone-tendon-bone grafts |
| US20060271192A1 (en)* | 2005-03-04 | 2006-11-30 | Olsen Raymond E | Self Fixing Assembled Bone-Tendon-Bone Graft |
| US20060229722A1 (en)* | 2005-03-04 | 2006-10-12 | Bianchi John R | Adjustable and fixed assembled bone-tendon-bone graft |
| USD583054S1 (en) | 2005-03-04 | 2008-12-16 | Rti Biologics, Inc. | Set of orthopedic bone block assembly |
| USD625822S1 (en) | 2005-03-04 | 2010-10-19 | Rti Biologics, Inc. | Orthopedic bone block assembly |
| US8470038B2 (en)* | 2005-03-04 | 2013-06-25 | Rti Biologics, Inc. | Adjustable and fixed assembled bone-tendon-bone graft |
| US20060212036A1 (en)* | 2005-03-04 | 2006-09-21 | Regeneration Technologies, Inc. | Bone block assemblies and their use in assembled bone-tendon-bone grafts |
| US20060200235A1 (en)* | 2005-03-04 | 2006-09-07 | Regeneration Technologies, Inc. | Assembled bone-tendon-bone grafts |
| US7879103B2 (en) | 2005-04-15 | 2011-02-01 | Musculoskeletal Transplant Foundation | Vertebral disc repair |
| US7815926B2 (en) | 2005-07-11 | 2010-10-19 | Musculoskeletal Transplant Foundation | Implant for articular cartilage repair |
| US9701940B2 (en) | 2005-09-19 | 2017-07-11 | Histogenics Corporation | Cell-support matrix having narrowly defined uniformly vertically and non-randomly organized porosity and pore density and a method for preparation thereof |
| US10492919B2 (en) | 2005-09-23 | 2019-12-03 | Ldr Medical | Intervertebral disc prosthesis |
| US9597194B2 (en) | 2005-09-23 | 2017-03-21 | Ldr Medical | Intervertebral disc prosthesis |
| US11872138B2 (en) | 2005-09-23 | 2024-01-16 | Ldr Medical | Intervertebral disc prosthesis |
| US8992965B2 (en) | 2005-11-01 | 2015-03-31 | Warsaw Orthopedic, Inc. | Bone matrix compositions and methods |
| US10328179B2 (en) | 2005-11-01 | 2019-06-25 | Warsaw Orthopedic, Inc. | Bone matrix compositions and methods |
| US8911759B2 (en) | 2005-11-01 | 2014-12-16 | Warsaw Orthopedic, Inc. | Bone matrix compositions and methods |
| USD583053S1 (en) | 2005-12-19 | 2008-12-16 | Rti Biologics, Inc. | Orthopedic bone block |
| USD605768S1 (en) | 2005-12-19 | 2009-12-08 | Rti Biologics, Inc. | Orthopedic bone block |
| US9713535B2 (en) | 2006-02-15 | 2017-07-25 | Ldr Medical | Transforaminal intersomatic cage for an intervertebral fusion graft and an instrument for implanting the cage |
| US10758363B2 (en) | 2006-02-15 | 2020-09-01 | Ldr Medical | Transforaminal intersomatic cage for an intervertebral fusion graft and an instrument for implanting the cage |
| US8409288B2 (en) | 2006-02-15 | 2013-04-02 | Ldr Medical | Transforaminal intersomatic cage for an intervertebral fusion graft and an instrument for implanting the cage |
| US10512548B2 (en) | 2006-02-27 | 2019-12-24 | DePuy Synthes Products, Inc. | Intervertebral implant with fixation geometry |
| US11696837B2 (en) | 2006-02-27 | 2023-07-11 | DePuy Synthes Products, Inc. | Intervertebral implant with fixation geometry |
| US7959683B2 (en) | 2006-07-25 | 2011-06-14 | Musculoskeletal Transplant Foundation | Packed demineralized cancellous tissue forms for disc nucleus augmentation, restoration, or replacement and methods of implantation |
| US20080058953A1 (en)* | 2006-08-31 | 2008-03-06 | Scarborough Nelson L | Demineralized cancellous strip DBM graft |
| US9066994B2 (en)* | 2006-08-31 | 2015-06-30 | Warsaw Orthopedic, Inc. | Demineralized cancellous strip DBM graft |
| US7837740B2 (en) | 2007-01-24 | 2010-11-23 | Musculoskeletal Transplant Foundation | Two piece cancellous construct for cartilage repair |
| US8906110B2 (en) | 2007-01-24 | 2014-12-09 | Musculoskeletal Transplant Foundation | Two piece cancellous construct for cartilage repair |
| USD630329S1 (en) | 2007-02-12 | 2011-01-04 | Rti Biologics, Inc. | Orthopedic bone block assembly |
| USD630748S1 (en) | 2007-02-12 | 2011-01-11 | Rti Biologics, Inc. | Orthopedic bone block |
| US8435551B2 (en) | 2007-03-06 | 2013-05-07 | Musculoskeletal Transplant Foundation | Cancellous construct with support ring for repair of osteochondral defects |
| US10751187B2 (en) | 2007-06-08 | 2020-08-25 | Ldr Medical | Intersomatic cage, intervertebral prosthesis, anchoring device and implantation instruments |
| US8343219B2 (en) | 2007-06-08 | 2013-01-01 | Ldr Medical | Intersomatic cage, intervertebral prosthesis, anchoring device and implantation instruments |
| US8642061B2 (en) | 2007-06-15 | 2014-02-04 | Warsaw Orthopedic, Inc. | Method of treating bone tissue |
| US8357384B2 (en) | 2007-06-15 | 2013-01-22 | Warsaw Orthopedic, Inc. | Bone matrix compositions and methods |
| US10357511B2 (en) | 2007-06-15 | 2019-07-23 | Warsaw Orthopedic, Inc. | Bone matrix compositions and methods |
| US10220115B2 (en) | 2007-06-15 | 2019-03-05 | Warsaw Orthopedic, Inc. | Bone matrix compositions having nanoscale textured surfaces |
| US9554920B2 (en) | 2007-06-15 | 2017-01-31 | Warsaw Orthopedic, Inc. | Bone matrix compositions having nanoscale textured surfaces |
| US9717822B2 (en) | 2007-06-15 | 2017-08-01 | Warsaw Orthopedic, Inc. | Bone matrix compositions and methods |
| US9358113B2 (en) | 2007-07-10 | 2016-06-07 | Warsaw Orthopedic, Inc. | Delivery system |
| US9333082B2 (en) | 2007-07-10 | 2016-05-10 | Warsaw Orthopedic, Inc. | Delivery system attachment |
| US9492278B2 (en) | 2007-07-10 | 2016-11-15 | Warsaw Orthopedic, Inc. | Delivery system |
| US10028837B2 (en) | 2007-07-10 | 2018-07-24 | Warsaw Orthopedic, Inc. | Delivery system attachment |
| WO2009048314A1 (en)* | 2007-10-08 | 2009-04-16 | Sureshan Sivananthan | A scalable matrix for the in vivo cultivation of bone and cartilage |
| US20110076316A1 (en)* | 2007-10-08 | 2011-03-31 | Sureshan Sivananthan | Scalable matrix for the in vivo cultivation of bone and cartilage |
| US8435566B2 (en) | 2007-10-19 | 2013-05-07 | Warsaw Orthopedic, Inc. | Demineralized bone matrix compositions and methods |
| US8202539B2 (en) | 2007-10-19 | 2012-06-19 | Warsaw Orthopedic, Inc. | Demineralized bone matrix compositions and methods |
| US10137003B2 (en) | 2007-11-16 | 2018-11-27 | DePuy Synthes Products, Inc. | Low profile intervertebral implant |
| US8540774B2 (en) | 2007-11-16 | 2013-09-24 | DePuy Synthes Products, LLC | Low profile intervertebral implant |
| US9744049B2 (en) | 2007-11-16 | 2017-08-29 | DePuy Synthes Products, Inc. | Low profile intervertebral implant |
| US10543102B2 (en) | 2007-11-16 | 2020-01-28 | DePuy Synthes Products, Inc. | Low profile intervertebral implant |
| US9005295B2 (en) | 2007-11-16 | 2015-04-14 | DePuy Synthes Products, LLC | Low profile intervertebral implant |
| US9707082B2 (en) | 2008-06-16 | 2017-07-18 | Rti Surgical, Inc. | Assembled cartilage repair graft |
| US20090312842A1 (en)* | 2008-06-16 | 2009-12-17 | Predrag Bursac | Assembled Cartilage Repair Graft |
| US10531960B2 (en) | 2008-11-07 | 2020-01-14 | DePuy Synthes Products, Inc. | Zero-profile interbody spacer and coupled plate assembly |
| US9414935B2 (en) | 2008-11-07 | 2016-08-16 | DePuy Synthes Products, Inc. | Zero-profile interbody spacer and coupled plate assembly |
| US12263096B2 (en) | 2008-11-07 | 2025-04-01 | DePuy Synthes Products, Inc. | Zero-profile interbody spacer and coupled plate assembly |
| US9402735B2 (en) | 2008-11-07 | 2016-08-02 | DePuy Synthes Products, Inc. | Zero-profile interbody spacer and coupled plate assembly |
| US11612492B2 (en) | 2008-11-07 | 2023-03-28 | DePuy Synthes Products, Inc. | Zero-profile interbody spacer and coupled plate assembly |
| US11517444B2 (en) | 2008-11-07 | 2022-12-06 | DePuy Synthes Products, Inc. | Zero-profile interbody spacer and coupled plate assembly |
| US9192419B2 (en) | 2008-11-07 | 2015-11-24 | DePuy Synthes Products, Inc. | Zero-profile interbody spacer and coupled plate assembly |
| US10433976B2 (en) | 2008-11-07 | 2019-10-08 | DePuy Synthes Products, Inc. | Zero-profile interbody spacer and coupled plate assembly |
| US9101475B2 (en) | 2009-02-12 | 2015-08-11 | Warsaw Orthopedic, Inc. | Segmented delivery system |
| US10098681B2 (en) | 2009-02-12 | 2018-10-16 | Warsaw Orthopedic, Inc. | Segmented delivery system |
| US9011537B2 (en) | 2009-02-12 | 2015-04-21 | Warsaw Orthopedic, Inc. | Delivery system cartridge |
| US9220598B2 (en) | 2009-02-12 | 2015-12-29 | Warsaw Orthopedic, Inc. | Delivery systems, tools, and methods of use |
| WO2010093955A1 (en)* | 2009-02-12 | 2010-08-19 | Osteotech,Inc. | Segmented delivery system |
| US9463091B2 (en) | 2009-09-17 | 2016-10-11 | Ldr Medical | Intervertebral implant having extendable bone fixation members |
| US10195046B2 (en) | 2009-12-31 | 2019-02-05 | Ldr Medical | Instruments and methods for removing fixation devices from intervertebral implants |
| US10531961B2 (en) | 2009-12-31 | 2020-01-14 | Ldr Medical | Anchoring device and system for an intervertebral implant, intervertebral implant and implantation instrument |
| US11246715B2 (en) | 2009-12-31 | 2022-02-15 | Ldr Medical | Anchoring device and system for an intervertebral implant, intervertebral implant and implantation instrument |
| US9044337B2 (en) | 2009-12-31 | 2015-06-02 | Ldr Medical | Anchoring device and system for an intervertebral implant, intervertebral implant and implantation instrument |
| US9833331B2 (en) | 2009-12-31 | 2017-12-05 | Ldr Medical | Anchoring device and system for an intervertebral implant, intervertebral implant and implantation instrument |
| US11458027B2 (en) | 2010-12-21 | 2022-10-04 | DePuy Synthes Products, Inc. | Intervertebral implants, systems, and methods of use |
| US9241809B2 (en) | 2010-12-21 | 2016-01-26 | DePuy Synthes Products, Inc. | Intervertebral implants, systems, and methods of use |
| US10507117B2 (en) | 2010-12-21 | 2019-12-17 | DePuy Synthes Products, Inc. | Intervertebral implants, systems, and methods of use |
| US9848992B2 (en) | 2010-12-21 | 2017-12-26 | DePuy Synthes Products, Inc. | Intervertebral implants, systems, and methods of use |
| US9220604B2 (en) | 2010-12-21 | 2015-12-29 | DePuy Synthes Products, Inc. | Intervertebral implants, systems, and methods of use |
| US9220808B2 (en) | 2011-07-28 | 2015-12-29 | Harbor Medtech, Inc. | Crosslinked human or animal tissue products and their methods of manufacture and use |
| US10611822B2 (en) | 2011-07-28 | 2020-04-07 | Harbor Medtech, Inc. | Crosslinked human or animal tissue products and their methods of manufacture and use |
| US8901078B2 (en) | 2011-07-28 | 2014-12-02 | Harbor Medtech, Inc. | Crosslinked human or animal tissue products and their methods of manufacture and use |
| US12065480B2 (en) | 2011-07-28 | 2024-08-20 | Harbor Medtech, Inc. | Crosslinked human or animal tissue products and their methods of manufacture and use |
| US9592320B2 (en) | 2011-07-28 | 2017-03-14 | Harbor Medtech, Inc. | Crosslinked human or animal tissue products and their methods of manufacture and use |
| US9399084B2 (en) | 2011-07-28 | 2016-07-26 | Harbor Medtech, Inc. | Crosslinked human or animal tissue products and their methods of manufacture and use |
| KR101479812B1 (en)* | 2011-12-30 | 2015-01-08 | 고려대학교 산학협력단 | Washer type connector for spinal fusion and pedicle screw using the same |
| US11273056B2 (en) | 2012-02-24 | 2022-03-15 | Ldr Medical | Anchoring device and system for an intervertebral implant, intervertebral implant and implantation instrument |
| US10350083B2 (en) | 2012-02-24 | 2019-07-16 | Ldr Medical | Anchoring device and system for an intervertebral implant, intervertebral implant and implantation instrument |
| US10245156B2 (en) | 2012-02-24 | 2019-04-02 | Ldr Medical | Anchoring device and system for an intervertebral implant, intervertebral implant and implantation instrument |
| US9039774B2 (en) | 2012-02-24 | 2015-05-26 | Ldr Medical | Anchoring device and system for an intervertebral implant, intervertebral implant and implantation instrument |
| US10010432B2 (en) | 2014-10-22 | 2018-07-03 | DePuy Synthes Products, Inc. | Intervertebral implants, systems, and methods of use |
| US11540927B2 (en) | 2014-10-22 | 2023-01-03 | DePuy Synthes Products, Inc. | Intervertebral implants, systems, and methods of use |
| US10702394B2 (en) | 2014-10-22 | 2020-07-07 | DePuy Synthes Products, Inc. | Intervertebral implants, systems, and methods of use |
| US9867718B2 (en) | 2014-10-22 | 2018-01-16 | DePuy Synthes Products, Inc. | Intervertebral implants, systems, and methods of use |
| US10130492B2 (en) | 2014-10-22 | 2018-11-20 | DePuy Synthes Products, Inc. | Intervertebral implants, systems, and methods of use |
| US10077420B2 (en) | 2014-12-02 | 2018-09-18 | Histogenics Corporation | Cell and tissue culture container |
| US11555172B2 (en) | 2014-12-02 | 2023-01-17 | Ocugen, Inc. | Cell and tissue culture container |
| CN105266929A (en)* | 2015-11-05 | 2016-01-27 | 同济大学 | Mountable allogenous cortical bone axial fusion device |
| US12102731B2 (en) | 2020-05-01 | 2024-10-01 | Harbor Medtech, Inc. | Port-accessible multidirectional reinforced minimally invasive collagen device for soft tissue repair |
| US20250082377A1 (en)* | 2023-12-12 | 2025-03-13 | The Fourth Medical Center Of The Chinese People's Liberation Army General Hospital | Proximal-femur intraosseous release device |
| US12290291B2 (en)* | 2023-12-12 | 2025-05-06 | The Fourth Medical Center Of The Chinese People's Liberation Army General Hospital | Proximal-femur intraosseous release device |
Also Published As
| Publication number | Publication date |
|---|---|
| US9763787B2 (en) | 2017-09-19 |
| US20180071102A1 (en) | 2018-03-15 |
| US20100268349A1 (en) | 2010-10-21 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| US9763787B2 (en) | Assembled implant | |
| CA2399762C (en) | Assembled implant | |
| WO2001078798A1 (en) | Assembled implant | |
| EP2431007B1 (en) | Composite bone graft, method of making and using same | |
| US7323011B2 (en) | Cortical and cancellous allograft cervical fusion block | |
| US6761739B2 (en) | Cortical and cancellous allograft spacer | |
| US20080154379A1 (en) | Interbody fusion hybrid graft | |
| EP1883377B1 (en) | Synthetic loadbearing collagen-mineral composites for spinal implants | |
| US20010020186A1 (en) | Keyed intervertebral dowel | |
| US20040107003A1 (en) | Demineralized bone implants | |
| CA2437763C (en) | Assembled implant, including mixed-composition segment | |
| AU2001288840B2 (en) | Assembled implant, including mixed-composition segment | |
| AU2008202235A1 (en) | Assembled implant, including mixed-composition segment | |
| AU2001288840A1 (en) | Assembled implant, including mixed-composition segment | |
| WO2005063151A1 (en) | Hybrid surgical implants | |
| AU2007203182A1 (en) | Cortical and cancellous allograft cervical fusion block implant |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| AS | Assignment | Owner name:REGENERATION TECHNOLOGIES, INC., FLORIDA Free format text:ASSIGNMENT OF ASSIGNORS INTEREST;ASSIGNORS:BIANCHI, JOHN R.;MILLS, C. RANDAL;GORHAM, P.J.;AND OTHERS;REEL/FRAME:011814/0455;SIGNING DATES FROM 20010220 TO 20010313 | |
| AS | Assignment | Owner name:MERRILL LYNCH BUSINESS FINANCIAL SERVICES, INC., T Free format text:SECURITY AGREEMENT;ASSIGNORS:REGENERATION TECHNOLOGIES, INC.;ALABAMA TISSUE CENTER, INC.;RTI SERVICES, INC.;AND OTHERS;REEL/FRAME:015116/0841 Effective date:20040323 | |
| AS | Assignment | Owner name:REGENERATION TECHNOLOGIES, INC., FLORIDA Free format text:ASSIGNMENT OF ASSIGNORS INTEREST;ASSIGNORS:SOUTHEAST TISSUE ALLIANCE, INC.;UNIVERSITY OF FLORIDA ORTHOPAEDIC TISSUE BANK, INC.;UNIVERSITY OF FLORIDA TISSUE BANK, INC.;REEL/FRAME:015796/0186 Effective date:20050121 Owner name:REGENERATION TECHNOLOGIES, INC.,FLORIDA Free format text:ASSIGNMENT OF ASSIGNORS INTEREST;ASSIGNORS:SOUTHEAST TISSUE ALLIANCE, INC.;UNIVERSITY OF FLORIDA ORTHOPAEDIC TISSUE BANK, INC.;UNIVERSITY OF FLORIDA TISSUE BANK, INC.;REEL/FRAME:015796/0186 Effective date:20050121 | |
| AS | Assignment | Owner name:REGENERATION TECHNOLOGIES, INC., FLORIDA Free format text:ASSIGNMENT OF ASSIGNORS INTEREST;ASSIGNOR:REGENERATION TECHNOLOGIES, INC.;REEL/FRAME:016646/0918 Effective date:20050805 | |
| AS | Assignment | Owner name:RTI BIOLOGICS, INC., FLORIDA Free format text:CHANGE OF NAME;ASSIGNOR:REGENERATION TECHNOLOGIES, INC.;REEL/FRAME:020690/0942 Effective date:20080227 Owner name:RTI BIOLOGICS, INC.,FLORIDA Free format text:CHANGE OF NAME;ASSIGNOR:REGENERATION TECHNOLOGIES, INC.;REEL/FRAME:020690/0942 Effective date:20080227 | |
| AS | Assignment | Owner name:REGENERATION TECHNOLOGIES, INC.-CARDIOVASCULAR (F/ Free format text:RECORD OF RELEASE OF SECURITY INTEREST;ASSIGNOR:GE BUSINESS FINANCIAL SERVICES INC.;REEL/FRAME:022151/0633 Effective date:20081230 Owner name:BIOLOGICAL RECOVERY GROUP, INC., FLORIDA Free format text:RECORD OF RELEASE OF SECURITY INTEREST;ASSIGNOR:GE BUSINESS FINANCIAL SERVICES INC.;REEL/FRAME:022151/0633 Effective date:20081230 Owner name:RTI SERVICES, INC., FLORIDA Free format text:RECORD OF RELEASE OF SECURITY INTEREST;ASSIGNOR:GE BUSINESS FINANCIAL SERVICES INC.;REEL/FRAME:022151/0633 Effective date:20081230 Owner name:RTI BIOLOGICS, INC. (F/K/A) REGENERATION TECHNOLOG Free format text:RECORD OF RELEASE OF SECURITY INTEREST;ASSIGNOR:GE BUSINESS FINANCIAL SERVICES INC.;REEL/FRAME:022151/0633 Effective date:20081230 | |
| STCB | Information on status: application discontinuation | Free format text:ABANDONED -- AFTER EXAMINER'S ANSWER OR BOARD OF APPEALS DECISION |