US11083886B2 - Wearable defibrillator with audio input/output - Google Patents
Wearable defibrillator with audio input/outputDownload PDFInfo
- Publication number
- US11083886B2 US11083886B2US16/542,343US201916542343AUS11083886B2US 11083886 B2US11083886 B2US 11083886B2US 201916542343 AUS201916542343 AUS 201916542343AUS 11083886 B2US11083886 B2US 11083886B2
- Authority
- US
- United States
- Prior art keywords
- patient
- wearable defibrillator
- audio
- processor
- audio output
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Active, expires
Links
- 238000002560therapeutic procedureMethods0.000claimsabstractdescription16
- 230000004043responsivenessEffects0.000claimsdescription15
- 230000004044responseEffects0.000claimsdescription12
- 206010003119arrhythmiaDiseases0.000claimsdescription11
- 230000006793arrhythmiaEffects0.000claimsdescription11
- 230000036541healthEffects0.000claimsdescription6
- 238000003745diagnosisMethods0.000claimsdescription4
- 208000024891symptomDiseases0.000claimsdescription4
- 230000000747cardiac effectEffects0.000claimsdescription3
- 238000012552reviewMethods0.000claimsdescription3
- 238000012545processingMethods0.000description78
- 238000012360testing methodMethods0.000description17
- 238000004891communicationMethods0.000description11
- 238000000034methodMethods0.000description10
- 230000007246mechanismEffects0.000description7
- 230000006855networkingEffects0.000description6
- 238000002405diagnostic procedureMethods0.000description5
- 238000002513implantationMethods0.000description5
- 230000001755vocal effectEffects0.000description5
- 230000004913activationEffects0.000description4
- 238000001514detection methodMethods0.000description4
- 206010011086Coronary artery occlusionDiseases0.000description2
- 206010049447TachyarrhythmiaDiseases0.000description2
- 208000001871TachycardiaDiseases0.000description2
- 230000001413cellular effectEffects0.000description2
- 230000009849deactivationEffects0.000description2
- 230000001934delayEffects0.000description2
- 238000002565electrocardiographyMethods0.000description2
- 238000012544monitoring processMethods0.000description2
- 230000000737periodic effectEffects0.000description2
- 230000029058respiratory gaseous exchangeEffects0.000description2
- 230000000007visual effectEffects0.000description2
- 101100215340Solanum tuberosum AC97 geneProteins0.000description1
- 206010042434Sudden deathDiseases0.000description1
- 208000003443UnconsciousnessDiseases0.000description1
- 230000005540biological transmissionEffects0.000description1
- 230000000981bystanderEffects0.000description1
- 230000008859changeEffects0.000description1
- 230000003111delayed effectEffects0.000description1
- 238000010586diagramMethods0.000description1
- 230000007613environmental effectEffects0.000description1
- 230000006870functionEffects0.000description1
- 238000005259measurementMethods0.000description1
- 208000010125myocardial infarctionDiseases0.000description1
- 238000001356surgical procedureMethods0.000description1
- 230000008961swellingEffects0.000description1
- 238000012795verificationMethods0.000description1
Images
Classifications
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61N—ELECTROTHERAPY; MAGNETOTHERAPY; RADIATION THERAPY; ULTRASOUND THERAPY
- A61N1/00—Electrotherapy; Circuits therefor
- A61N1/02—Details
- A61N1/04—Electrodes
- A61N1/0404—Electrodes for external use
- A61N1/0472—Structure-related aspects
- A61N1/0484—Garment electrodes worn by the patient
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61B—DIAGNOSIS; SURGERY; IDENTIFICATION
- A61B5/00—Measuring for diagnostic purposes; Identification of persons
- A61B5/0002—Remote monitoring of patients using telemetry, e.g. transmission of vital signals via a communication network
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61B—DIAGNOSIS; SURGERY; IDENTIFICATION
- A61B5/00—Measuring for diagnostic purposes; Identification of persons
- A61B5/24—Detecting, measuring or recording bioelectric or biomagnetic signals of the body or parts thereof
- A61B5/25—Bioelectric electrodes therefor
- A61B5/279—Bioelectric electrodes therefor specially adapted for particular uses
- A61B5/28—Bioelectric electrodes therefor specially adapted for particular uses for electrocardiography [ECG]
- A61B5/282—Holders for multiple electrodes
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61B—DIAGNOSIS; SURGERY; IDENTIFICATION
- A61B5/00—Measuring for diagnostic purposes; Identification of persons
- A61B5/24—Detecting, measuring or recording bioelectric or biomagnetic signals of the body or parts thereof
- A61B5/316—Modalities, i.e. specific diagnostic methods
- A61B5/318—Heart-related electrical modalities, e.g. electrocardiography [ECG]
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61B—DIAGNOSIS; SURGERY; IDENTIFICATION
- A61B5/00—Measuring for diagnostic purposes; Identification of persons
- A61B5/68—Arrangements of detecting, measuring or recording means, e.g. sensors, in relation to patient
- A61B5/6801—Arrangements of detecting, measuring or recording means, e.g. sensors, in relation to patient specially adapted to be attached to or worn on the body surface
- A61B5/6802—Sensor mounted on worn items
- A61B5/6804—Garments; Clothes
- A61B5/6805—Vests, e.g. shirts or gowns
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61B—DIAGNOSIS; SURGERY; IDENTIFICATION
- A61B5/00—Measuring for diagnostic purposes; Identification of persons
- A61B5/74—Details of notification to user or communication with user or patient; User input means
- A61B5/7405—Details of notification to user or communication with user or patient; User input means using sound
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61N—ELECTROTHERAPY; MAGNETOTHERAPY; RADIATION THERAPY; ULTRASOUND THERAPY
- A61N1/00—Electrotherapy; Circuits therefor
- A61N1/02—Details
- A61N1/04—Electrodes
- A61N1/0404—Electrodes for external use
- A61N1/0408—Use-related aspects
- A61N1/046—Specially adapted for shock therapy, e.g. defibrillation
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61N—ELECTROTHERAPY; MAGNETOTHERAPY; RADIATION THERAPY; ULTRASOUND THERAPY
- A61N1/00—Electrotherapy; Circuits therefor
- A61N1/18—Applying electric currents by contact electrodes
- A61N1/32—Applying electric currents by contact electrodes alternating or intermittent currents
- A61N1/36—Applying electric currents by contact electrodes alternating or intermittent currents for stimulation
- A61N1/372—Arrangements in connection with the implantation of stimulators
- A61N1/37211—Means for communicating with stimulators
- A61N1/37217—Means for communicating with stimulators characterised by the communication link, e.g. acoustic or tactile
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61N—ELECTROTHERAPY; MAGNETOTHERAPY; RADIATION THERAPY; ULTRASOUND THERAPY
- A61N1/00—Electrotherapy; Circuits therefor
- A61N1/18—Applying electric currents by contact electrodes
- A61N1/32—Applying electric currents by contact electrodes alternating or intermittent currents
- A61N1/38—Applying electric currents by contact electrodes alternating or intermittent currents for producing shock effects
- A61N1/39—Heart defibrillators
- A61N1/3904—External heart defibrillators [EHD]
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61N—ELECTROTHERAPY; MAGNETOTHERAPY; RADIATION THERAPY; ULTRASOUND THERAPY
- A61N1/00—Electrotherapy; Circuits therefor
- A61N1/18—Applying electric currents by contact electrodes
- A61N1/32—Applying electric currents by contact electrodes alternating or intermittent currents
- A61N1/38—Applying electric currents by contact electrodes alternating or intermittent currents for producing shock effects
- A61N1/39—Heart defibrillators
- A61N1/3925—Monitoring; Protecting
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61N—ELECTROTHERAPY; MAGNETOTHERAPY; RADIATION THERAPY; ULTRASOUND THERAPY
- A61N1/00—Electrotherapy; Circuits therefor
- A61N1/18—Applying electric currents by contact electrodes
- A61N1/32—Applying electric currents by contact electrodes alternating or intermittent currents
- A61N1/38—Applying electric currents by contact electrodes alternating or intermittent currents for producing shock effects
- A61N1/39—Heart defibrillators
- A61N1/3987—Heart defibrillators characterised by the timing or triggering of the shock
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61N—ELECTROTHERAPY; MAGNETOTHERAPY; RADIATION THERAPY; ULTRASOUND THERAPY
- A61N1/00—Electrotherapy; Circuits therefor
- A61N1/18—Applying electric currents by contact electrodes
- A61N1/32—Applying electric currents by contact electrodes alternating or intermittent currents
- A61N1/38—Applying electric currents by contact electrodes alternating or intermittent currents for producing shock effects
- A61N1/39—Heart defibrillators
- A61N1/3993—User interfaces for automatic external defibrillators
Definitions
- This inventionrelates to wearable defibrillators.
- Wearable defibrillatorsare often used to help people who have an increased risk of experiencing a life threatening arrhythmia due to specific heart conditions. Such wearable defibrillators are typically configured to provide treatment if a life threatening arrhythmia is detected.
- U.S. Pat. Nos. 4,928,690, 5,078,134, 5,741,306, 5,944,669, 6,065,154, 6,097,987, 6,253,099, 6,280,461 and 6,681,003disclose wearable defibrillators.
- the entirety of U.S. Pat. Nos. 4,928,690, 5,078,134, 5,741,306, 5,944,669, 6,065,154, 6,097,987, 6,253,099, 6,280,461 and 6,681,003,are hereby incorporated herein by reference.
- Wearable defibrillatorsare typically used to help patients that either cannot risk an implantation of a defibrillator or are awaiting such an implantation. Occasionally, analysis performed by the wearable defibrillator may falsely indicate that the patient is experiencing an arrhythmia that requires treatment. In such circumstances, the wearable defibrillator generates an audible alarm that is configured to stop if a patient provides a required response, such as, for example, pressing one or more response buttons. If a patient fails to press such buttons or otherwise provide a required response, the device may assume the patient is unconscious and is experiencing a condition requiring treatment. Occasionally, a bystander who is unfamiliar with a wearable defibrillator may interfere with the device by intentionally pressing the response buttons or otherwise providing a response which delays or inhibits patient treatment.
- the present inventionis directed toward overcoming one or more of the above-mentioned problems.
- a wearable defibrillatorincludes one or more therapy pads one or more sensors, one or more processing units operatively connected to the one or more therapy pads and one or more sensors and one or more audio devices operatively connected to the one or more processing units.
- the one or more audio devicesare configured to receive audio input from a patient.
- One embodiment of the wearable defibrillatormay include one or more audio devices that are one or more microphones, one or more speakers or a combination of one or more speakers and one or more microphones.
- Another embodiment of the wearable defibrillatormay include one or more processing units that include one or more processors and at least one memory connected to the one or more processors.
- the one or more processing unitscan be configured to record the patient name using a microphone and store the audio recording in non volatile memory.
- a recording of the patient's name made during setup by either an operator or patientcan be played back during startup to uniquely identify who the wearable defibrillator belongs to.
- the one or more processing unitscan be configured to cause at least one patient responsiveness test to be run upon detection of an arrhythmia condition by the one or more sensors.
- one of the one or more responsiveness testscan include at least one voice recognition responsiveness test, at least one button responsiveness test, or any combination thereof.
- the one or more processing unitscan be configured to cause a response button responsiveness test to be run only after a voice recognition responsiveness test resulted in a response that indicated the patient is not conscious.
- other embodiments of the wearable defibrillatormay include one or more processing units that are configured to run other patient responsiveness tests or sequences of such patient responsiveness tests.
- the audio inputcan include one or more sounds, such as, for example, at least one spoken word, made by the patient and the one or more processing units can be configured to recognize the audio input from the patient.
- the one or more processing unitsmay be configured such that the wearable defibrillator either delays or does not provide a treatment to the patient when the one or more sounds is received by the one or more audio devices and recognized by the one or more processing units.
- the wearable defibrillatormay include one or more processing units that are configured to recognize audio input provided by a base unit configured to operatively connect to the wearable defibrillator to verify that the base unit is functioning properly.
- the base unitcan include a modem and the audio input provided by the base unit can include sound made by the modem.
- the wearable defibrillatorcan include one or more mechanisms connected to the one or more processing units and the one or more processing units are configured to recognize input provided by the one or more mechanisms to verify that the one or more mechanisms are operating correctly.
- the one or more mechanismscan include relays, switches or any combination thereof that produce an audible sound upon activation or deactivation.
- the processing unit of a wearable defibrillatorcan be configured to cause an alarm to be emitted to verify that the wearable defibrillator can properly emit the alarm.
- the one or more processing unitscan be configured to cause one or more speakers to produce audio output and be configured to adjust at least one of the frequency and the amplitude characteristics of the audio output based upon audio input received from one or more microphones.
- the one or more processing unitscan be configured to cause data obtained by the one or more sensors to be recorded in memory connected to the one or more processing units.
- the audio inputcan include a command and the one or more processing units can be configured to cause data obtained by the one or more sensors to be recorded in at least one memory after the command is received by the one or more audio devices.
- the one or more processing unitscan be configured to cause a self-diagnostic test to be run if the audio input includes a high amplitude and short duration noise that may be indicative of device abuse.
- a systemconfigured to monitor a patient.
- the systemincludes a central location, and a wearable defibrillator configured to operatively connect to the central location.
- the wearable defibrillatorincludes one or more therapy pads, one or more sensors, one or more processing units operatively connected to the one or more therapy pads and one or more sensors and one or more audio devices operatively connected to the one or more processing units.
- the one or more audio devicesare configured to receive audio input from a patient.
- the systemmay further include a base station configured to operatively connect the wearable defibrillator to the central location.
- the one or more processing units of the wearable defibrillatormay include at least one communication device configured to connect the wearable defibrillator to the central location.
- the one or more communication devicesinclude a modem, a network card, one or more networking programs, other networking mechanisms or any combination thereof.
- the one or more audio devicescan be a microphone.
- the base stationcan be configured to communicate with the central location and the wearable defibrillator may be configured to transmit data from the wearable defibrillator to the central location.
- the central locationcan include one or more memory and be configured to store data transmitted from the wearable defibrillator in the one or more memory.
- a method of providing treatment to a patientis also provided.
- the methodcan include providing a wearable defibrillator to the patient that includes one or more audio devices, monitoring the condition of the patient, providing audio output from the one or more audio devices to the patient to verify a monitored arrhythmia condition exists, receiving audio input from the patient with the one or more audio devices and providing treatment to the patient based on the audio input received from the patient.
- the audio input received from the patientmay include silence or may include audible responses.
- the methodmay also include recording the condition of the patient and communicating the recorded condition of the patient to a central location.
- the methodmay include utilizing one or more of the audio devices to conduct at least one diagnostic test of the wearable defibrillator, recording the results from the one or more diagnostic tests and evaluating the results of the test or tests.
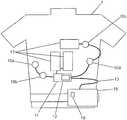
- FIG. 1is a schematic view of a first embodiment of the present invention, which illustrates an embodiment of a wearable defibrillator.
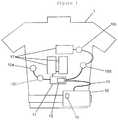
- FIG. 2is a block diagram of an embodiment of the present invention that illustrates a system that includes an embodiment of a wearable defibrillator configured to interact with a base station and a central location.
- a wearable defibrillatormay be worn by a patient and may include a belt or harness or other apparel configured to permit the patient to wear the defibrillator.
- Sensorssuch as electrodes 10 a , 10 b , 10 c and 10 d are removably attached to the patient when the wearable defibrillator is worn by the patient.
- the electrodes 10 a , 10 b , 10 c and 10 dform part of electrode assembly 11 and are operatively connected to a processing unit 15 via a trunk cable 13 .
- the processing unit 15may include, without limitation, one or more processors, one or more controllers and/or one or more programs or other software stored in memory operatively connected to one or more processors.
- the processing unit 15is operatively connected to therapy pads 17 , at least one tactile stimulator 12 , electrode assembly 11 , and one or more audio devices 16 .
- the audio devices 16may include, for example, a microphone and a speaker.
- the therapy pads 17are removably connected to the patient when the defibrillator is worn.
- the processing unit 15may include a visual read out and a speaker for communicating with the patient or others around the patient.
- a trunk cable 13may be used to connect the electrode assembly 11 to the processing unit 15 and audio devices 16 .
- other types of cables or other connection devices used to operatively connect the electrode assembly 11 to the processing unit 15 , speakers, microphones or other audio devices 16may also be used.
- Wiring or other connection devicesmay also be used to connect at least one portion of the electrode assembly 11 to the electrodes 10 a , 10 b , 10 c , and 10 d .
- the processing unit 15may alternatively be operatively connected to one or more of the electrodes 10 a , 10 b , 10 c , 10 d , therapy pads 17 , electrode assembly 11 , audio devices 16 and stimulator 12 by a wireless connection or a combination of wireless and wired connections.
- the audio devices 16preferably include a Knowles Acoustics WP-23502 microphone, a speaker and audio circuitry that include an audio CODEC and an audio amplifier.
- the audio CODECmay contain an interpolation filter and a noise shaper.
- An AC97 interfacemay be used to operatively connect the processing unit 15 and the one or more audio devices 16 .
- other interfaces or other connection mechanisms known to those skilled in the artmay also be used to operatively connect the processing unit 15 to the one or more audio devices 16 .
- At least one of the electrode assembly 11 and processing unit 15have at least one processor that is configured to evaluate the cardiac condition of the patient and cause delivery of the appropriate treatment to the patient.
- the therapy pads 17are configured to provide treatment, such as, for example, electric defibrillation, to the wearer after the processing unit 15 determines that such treatment should be delivered to the patient.
- the therapy pads 17may include the application devices disclosed in U.S. Pat. No. 5,078,134, or other devices configured to provide treatment to the patient.
- the processing unit 15may display one or more visuals identifying the patient's condition or conditions based at least in part on one or more conditions sensed by the electrodes 10 a , 10 b , 10 c , and 10 d .
- a speakermay be an audio device 16 that is used to communicate with the patient or others located near the patient.
- the speaker or other audio device 16may be housed within the processing unit 15 or be attached to the processing unit 15 or another portion of the wearable defibrillator such as, for example, the electrode assembly 11 .
- a microphonemay also be an audio device 16 attached to the processing unit 15 .
- the microphonemay be configured to detect patient and environmental noise.
- One or more portions of the processing unit 15can be operatively connected to, or otherwise incorporate, voice recognition software so the processing unit 15 can determine, based on whether or not it recognizes the voice of the wearer, whether an arrhythmia condition exists that warrants delivery of treatment or if such delivery should be delayed.
- the processing unit 15may also be operatively connected to memory or another storage device that stores information such as, for example, the patient's voice signature so the processing unit 15 may identify a speaker's voice and determine when the patient is providing audio input.
- the processing unit 15may include one or more processors that are configured to use a confidence algorithm to determine when treatment should be delivered.
- the confidence algorithmmay base arrhythmia detection on one or more inputs such as, but not limited to, data obtained from sensing electrodes 10 a , 10 b , 10 c and 10 d , one or more electrocardiograms. (“ECGs”), response button responsiveness test results, voice recognition responsiveness test results, etc.
- ECGselectrocardiograms.
- the processing unit 15is configured so that treatment is not delivered unless the confidence algorithm determines that there is a 100% confidence that the patient is experiencing a life threatening arrhythmia.
- the one or more audio devices 16may be configured to identify whether background noise exists. In the event no or little background noise is sensed, the confidence in the detection can be increased.
- the processing unit 15may also be configured to permit the delivery of treatment to be accelerated if no background noise or little background noise is detected by the one or more audio devices 16 .
- the processing unit 15may be configured to delay a delivery of treatment if a high level of background noise is identified because significant background noise may reduce the quality of the data obtained by the electrodes 10 a , 10 b , 10 c , or 10 d .
- the quality of ECG sensing electrodesmay be reduced with significant background noise possibly caused by patient motion and can result in false detection of a condition requiring treatment.
- the processing unit 15can be configured to delay treatment so other tests or data can be obtained to verify that the patient requires treatment or to increase the audio output level of alarms used to warn any people surrounding the patient that treatment is about to be provided to the patient so that no one touches the patient during the application of the treatment.
- the audio devices 16may also be configured so that speaker volumes are increased whenever certain background noise levels are detected. Such increased volumes permit audio outputs to be heard by the patient or people near the patient when the patient is in a high noise environment. Such audio outputs may include alarms, instructions or communication related to patient responsiveness tests, which are discussed more fully below.
- the processing unit 15may be configured to cause a voice recognition responsiveness test to be run as part of determining that the patient is experiencing a condition requiring treatment.
- the voice responsiveness testmay include an audio device 16 , such as, for example, a speaker, to verbally ask the patient if the patient is conscious.
- a microphone or other audio device 16senses that the patient responds with a positive verbal comment, such as, for example, “yes”, the processing unit 15 can be configured to delay treatment.
- the processing unit 15can be configured to cause a speaker to provide a verbal message asking the patient to press one or more buttons or activate one or more actuators connected to the defibrillator to verify the patient is conscious.
- the one or more buttonsmay be located on, in, or adjacent the belt, harness, vest, or monitor of the defibrillator.
- the processing unit 15can be configured to cause the speaker to ask the patient certain questions in the event a possible condition is identified that may require delivery of a treatment.
- the speakermay be configured to ask the patient “are you conscious?” or “if you are conscious, please state your name.”
- the processing unit 15can be operatively connected to memory or another storage device that contains the patient's voice signature to verify the patient is answering the questions. Such verification prevents a passerby from preventing treatment of the patient by improperly responding to the questions.
- audio devices 16such as, for example, a microphone and a speaker, permit the patient to have real-time input provided to the defibrillator.
- the processing unit 15may also be configured to record the audio input in the proximity of the wearable device for later review by emergency personnel. Such information may help care providers determine a diagnosis for the patient or treat the patient.
- the one or more audio devicesmay also be operatively connected to the processing unit 15 such that the processing unit 15 can cause the patient's conditions being sensed by electrodes 10 a , 10 b , 10 c , or 10 d to be recorded and stored.
- Such recording and storagecan be actuated by verbal commands issued by the patient that are received by the audio device 16 , such as, for example, a microphone, or by the actuation of an actuator such as, for example, a button operatively connected to the processing unit 15 .
- the processing unit 15can also be configured so the audio device 16 , such as, for example, a microphone, records a message provided by the patient that explains how he feels and why he initiated the recording of the conditions being sensed by one or more of the electrodes 10 a , 10 b , 10 c , and 10 d .
- the recorded audio and sensed informationmay be stored in memory operatively connected to the processing unit 15 or be transmitted to a central location and/or to health care providers. Transmissions to a central location are discussed more fully below. Such recordings may permit a health care provider or doctor to formulate a diagnosis based on the sensed conditions or otherwise act on such information to provide services the recorded conditions indicate the patient needs.
- the processing unit 15can be configured so a speaker or other audio device provides the patient with certain verbal instructions.
- the instructionsmay also be provided in specific situations where the patient has difficulty understanding instructions or the processing unit 15 is not receiving any expected input from the patient.
- Special messaging instructionscan be recorded during patient setup to support the personal communication needs of the patient or may be operatively connected to the processing unit 15 such that the instructions may be provided to the patient during the setup of the defibrillator in the event the patient is having difficulty with the setup.
- a special messagemay include contact information for customer support or a voice activated menu of different languages the instructions may be given in that the patient may select from.
- Standard voice messages or alarms delivered prior to or during the delivery of treatmentmay be customized for a patient.
- the standard alarmsmay also be modified so that a speaker or other audio device provides audio output in a language the patient understands (e.g., Spanish, English, French, German, etc.). Additionally, the messages may be customized to include the name of the patient to personalize the instructions.
- the audio input and outputs provided by the defibrillatormay be created or modified during a setup phase conducted during an initial use of the defibrillator by a patient.
- a setup phasemay be used to determine the language all audio output should be spoken in and permit the name of the patient to be learned by the processing unit or stored in memory connected to the processing unit.
- the processing unit 15may also be configured so that the patient's voice signature is identified and saved in a storage device, such as, for example, memory in the processing unit 15 or memory operatively connected to the processing unit 15 .
- the processing unit 15can also be configured to generate a unique identifier that is related to the patient. Such an identifier may be used to determine who the patient is or the patient that is assigned to wear the defibrillator. Features from the patient's voice signature or saved voice recordings can be used to create the identifier. Such an identifier may be created as part of the setup phase.
- Facilities that have multiple patients that are required to wear defibrillatorsmay need a method of determining which patient is assigned to wear a particular defibrillator so the facility can ensure the proper defibrillator is worn by the proper patient.
- a recording of the patient's namemay be stored in memory contained within the processing unit 15 , operatively connected to the processing unit 15 or otherwise stored by the processing unit 15 .
- the name of the patientmay then be identified by an audio message sent by a speaker or other audio device 16 to identify the intended user of the device.
- the processing unit 15may be configured so the audio device 16 provides such output whenever the device is activated or upon the activation of an actuator operatively connected to the processing unit 15 , such as, for example, a button on the monitor 15 or an actuator operatively connected to the processing unit 15 .
- the processing unit 15may also be configured so a verbal command received from an audio device 16 , such as, for example, a microphone, may cause the patient's name to be output provided by an audio device 16 , such as, for example, a speaker.
- a wearable defibrillator 41is typically incorporated into a system to provide treatment to a patient.
- the systemmay include a wearable defibrillator 41 that has a processor 33 operatively connected to memory 34 , a microphone 25 , a speaker 24 , one or more therapy pads 17 , one or more electrodes 10 and a base station 28 that includes one or more networking or communication devices such as, for example, a modem or other networking device configured to connect the processor 33 or defibrillator 41 to other devices such as, for example, a computer or server or other central location 18 .
- networking or communication devicessuch as, for example, a modem or other networking device configured to connect the processor 33 or defibrillator 41 to other devices such as, for example, a computer or server or other central location 18 .
- the processor 33 , memory 34 , speaker 24 and microphone 25may be housed within a processing unit 26 along with audio circuitry that includes, for example, a CODEC 23 .
- one or more communication devicessuch as, for example, a modem, network card, networking programs, other networking mechanisms or any combination thereof may be connected to or incorporated into the processing unit 26 such that the processor 33 of the processing unit 26 is configured to cause one or more of the communication devices to connect the wearable defibrillator 41 to the central location 18 .
- the central location 18may include an apparatus operated by a hospital or other care monitoring entity that is configured to store patient related data transmitted to the central location 18 .
- the central location 18may also be configured to oversee or manage at least a portion of the operation of one or more wearable defibrillators 41 .
- the processor 33may be configured so the patient may communicate with customer support personnel that are able to operatively connect to the central location 18 .
- the patientmay communicate with such personnel using the microphone 25 and speaker 24 .
- the base station 28is operatively connected with the processor 33 by a connection 20 , which may be a wireless connection or a wired connection such as, for example, a USB connection.
- the base station 28may include a wireless or wired modem or other transceiver that is preferably configured to establish a link 19 to the central location 18 that permits the microphone 25 to send input received from the patient to the central location 18 so that service or support representatives may receive the information.
- the processor 33 , base station 28 and central location 18are also configured so the speaker 24 may relay output obtained from the central location 18 .
- Such communicationsmay be transmitted to and from the central location 18 by transceivers, modems or other devices operatively connected to the central location 18 , base station 28 and/or processor 33 .
- the base station 28 or processing unit 26may be configured to encrypt data transmitted to the central location 18 so that any unsecured network (e.g., cellular, wireless, POTS, etc.) available to a patient can be used.
- any unsecured networke.g., cellular, wireless, POTS, etc.
- the communications obtained from the central location 18may include feedback from personnel connected to the central location 18 .
- the base station 28 and processor 33may also be configured so the patient may communicate with emergency medical support personnel attempting to help the patient or to report problems with the defibrillator to medical support staff or the manufacturer of the defibrillator.
- Such staffmay be connected to the central location 18 or may be available for communication through other means such as, for example, cellular phone connections or other communication apparatuses.
- the central location 18may include one or more computers, servers, and programs or other software that are configured to send survey questions or other queries to one or more patients. Such queries can include questions regarding a patient's health or the condition of the defibrillator.
- the processor 33may also be configured so the speaker 24 asks survey questions that are stored in the memory 34 so that periodic responses from the patient can be recorded and stored in the memory 34 , in the central location 18 or a device connected to the central location 18 .
- Such saved responsesmay be periodically updated and tracked to verify the patient is not experiencing any symptoms indicating increased risk of experiencing a condition that may require treatment. Changes in the patient's voice may also be stored and tracked to determine changes in breathing characteristics as an additional symptom that may be pertinent to a change in the patient's condition or diagnosis.
- Examples of survey questions or periodic condition status questionsmay include: Are your legs swelling?; Are you having breathing difficulties?; Have you experienced a gain in weight?; and Are you sitting up to sleep?
- various other questions relating to symptoms of health conditionsmay also be used in addition to or in place of such survey questions.
- the microphone 25 and processor 33can be configured to verify that the base station 28 is connected to the central location 18 by recording a modem speaker audio or other audio that may be produced by the portion or device of the base station 28 that is configured to connect the defibrillator 41 to the central location 18 .
- the tones of the audio produced by the base station 28 when trying to connect the defibrillator 41 to the central location 18can be analyzed by the processor 33 to determine if the modem is attempting a connection. For instance, such an analysis may be performed by comparing the audio input received by the microphone 25 with tone data stored in the memory 34 .
- the microphone 25may also be used to verify that certain internal components are operating correctly by analyzing noise associated with activation or deactivation of mechanical components of the wearable defibrillator such as, for example, relays or switches. Correlation of the activation of the components with recorded audio may verify the functionality of the components.
- the processor 33can be configured to use amplitude and other audio input provided by the microphone 25 and time measurements to verify that the correct relays are activated at the correct times.
- the processor 33may be configured to cause a self-diagnostic test to be run whenever audio input is received that indicates the wearable defibrillator 41 may have been damaged.
- audio inputmay include high amplitude and short duration noise.
- noisemay be equivalent or similar to noise produced from an object such as, for example, a wall, a floor, a chair or a door banging against a portion of the wearable defibrillator or when the defibrillator is dropped onto a hard surface.
- the processor 33may be configured to cause a system test to be run to verify that the wearable defibrillator 41 is functioning properly. For example, the processor 33 may cause an alarm to be emitted by the speaker 24 at different frequencies and volumes and verify the alarm is being emitted at the different volumes and frequencies by comparing the expected audio output of the speaker with the audio input received by the microphone 25 . If an alarm is found to not function properly, the processor 33 may be configured to operatively connect to the central location 18 and report the problem to the central location 18 or to schedule servicing of the wearable defibrillator 41 . The processor 33 may operatively connect to the central location 18 by interacting with the base station 28 , as discussed above.
Landscapes
- Health & Medical Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Engineering & Computer Science (AREA)
- Veterinary Medicine (AREA)
- Animal Behavior & Ethology (AREA)
- Public Health (AREA)
- General Health & Medical Sciences (AREA)
- Biomedical Technology (AREA)
- Heart & Thoracic Surgery (AREA)
- Radiology & Medical Imaging (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Cardiology (AREA)
- Physics & Mathematics (AREA)
- Biophysics (AREA)
- Surgery (AREA)
- Molecular Biology (AREA)
- Medical Informatics (AREA)
- Pathology (AREA)
- Human Computer Interaction (AREA)
- Computer Networks & Wireless Communication (AREA)
- Acoustics & Sound (AREA)
- Electrotherapy Devices (AREA)
Abstract
Description
Claims (17)
Priority Applications (3)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US16/542,343US11083886B2 (en) | 2007-06-06 | 2019-08-16 | Wearable defibrillator with audio input/output |
| US17/305,283US12138444B2 (en) | 2007-06-06 | 2021-07-02 | Wearable defibrillator with audio input/output |
| US18/906,254US20250090841A1 (en) | 2007-06-06 | 2024-10-04 | Wearable defibrillator with audio input/output |
Applications Claiming Priority (8)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US93331007P | 2007-06-06 | 2007-06-06 | |
| US12/082,168US8369944B2 (en) | 2007-06-06 | 2008-04-09 | Wearable defibrillator with audio input/output |
| US13/757,105US8774917B2 (en) | 2007-06-06 | 2013-02-01 | Wearable defibrillator with audio input/output |
| US14/325,041US8965500B2 (en) | 2007-06-06 | 2014-07-07 | Wearable defibrillator with audio input/output |
| US14/607,843US9492676B2 (en) | 2007-06-06 | 2015-01-28 | Wearable defibrillator with audio input/output |
| US15/285,295US10029110B2 (en) | 2007-06-06 | 2016-10-04 | Wearable defibrillator with audio input/output |
| US16/014,365US10426946B2 (en) | 2007-06-06 | 2018-06-21 | Wearable defibrillator with audio input/output |
| US16/542,343US11083886B2 (en) | 2007-06-06 | 2019-08-16 | Wearable defibrillator with audio input/output |
Related Parent Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US16/014,365ContinuationUS10426946B2 (en) | 2007-06-06 | 2018-06-21 | Wearable defibrillator with audio input/output |
Related Child Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US17/305,283ContinuationUS12138444B2 (en) | 2007-06-06 | 2021-07-02 | Wearable defibrillator with audio input/output |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| US20190374767A1 US20190374767A1 (en) | 2019-12-12 |
| US11083886B2true US11083886B2 (en) | 2021-08-10 |
Family
ID=39942355
Family Applications (10)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US12/082,168Expired - Fee RelatedUS8369944B2 (en) | 2007-06-06 | 2008-04-09 | Wearable defibrillator with audio input/output |
| US13/757,105ActiveUS8774917B2 (en) | 2007-06-06 | 2013-02-01 | Wearable defibrillator with audio input/output |
| US14/325,041Expired - Fee RelatedUS8965500B2 (en) | 2007-06-06 | 2014-07-07 | Wearable defibrillator with audio input/output |
| US14/607,843ActiveUS9492676B2 (en) | 2007-06-06 | 2015-01-28 | Wearable defibrillator with audio input/output |
| US15/285,295ActiveUS10029110B2 (en) | 2007-06-06 | 2016-10-04 | Wearable defibrillator with audio input/output |
| US15/468,196ActiveUS10004893B2 (en) | 2007-06-06 | 2017-03-24 | Wearable defibrillator with audio input/output |
| US16/014,365ActiveUS10426946B2 (en) | 2007-06-06 | 2018-06-21 | Wearable defibrillator with audio input/output |
| US16/542,343Active2028-06-03US11083886B2 (en) | 2007-06-06 | 2019-08-16 | Wearable defibrillator with audio input/output |
| US17/305,283Active2029-04-25US12138444B2 (en) | 2007-06-06 | 2021-07-02 | Wearable defibrillator with audio input/output |
| US18/906,254PendingUS20250090841A1 (en) | 2007-06-06 | 2024-10-04 | Wearable defibrillator with audio input/output |
Family Applications Before (7)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US12/082,168Expired - Fee RelatedUS8369944B2 (en) | 2007-06-06 | 2008-04-09 | Wearable defibrillator with audio input/output |
| US13/757,105ActiveUS8774917B2 (en) | 2007-06-06 | 2013-02-01 | Wearable defibrillator with audio input/output |
| US14/325,041Expired - Fee RelatedUS8965500B2 (en) | 2007-06-06 | 2014-07-07 | Wearable defibrillator with audio input/output |
| US14/607,843ActiveUS9492676B2 (en) | 2007-06-06 | 2015-01-28 | Wearable defibrillator with audio input/output |
| US15/285,295ActiveUS10029110B2 (en) | 2007-06-06 | 2016-10-04 | Wearable defibrillator with audio input/output |
| US15/468,196ActiveUS10004893B2 (en) | 2007-06-06 | 2017-03-24 | Wearable defibrillator with audio input/output |
| US16/014,365ActiveUS10426946B2 (en) | 2007-06-06 | 2018-06-21 | Wearable defibrillator with audio input/output |
Family Applications After (2)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US17/305,283Active2029-04-25US12138444B2 (en) | 2007-06-06 | 2021-07-02 | Wearable defibrillator with audio input/output |
| US18/906,254PendingUS20250090841A1 (en) | 2007-06-06 | 2024-10-04 | Wearable defibrillator with audio input/output |
Country Status (4)
| Country | Link |
|---|---|
| US (10) | US8369944B2 (en) |
| JP (3) | JP5467733B2 (en) |
| DE (1) | DE102008026871B4 (en) |
| FR (1) | FR2916981B1 (en) |
Families Citing this family (193)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US20040116969A1 (en) | 2002-08-26 | 2004-06-17 | Owen James M. | Pulse detection using patient physiological signals |
| DE102007014136B4 (en)* | 2007-03-23 | 2011-08-25 | Dr. Oestreich + Partner GmbH, 50670 | Device for medical care of a patient in an emergency |
| US8369944B2 (en) | 2007-06-06 | 2013-02-05 | Zoll Medical Corporation | Wearable defibrillator with audio input/output |
| US8271082B2 (en) | 2007-06-07 | 2012-09-18 | Zoll Medical Corporation | Medical device configured to test for user responsiveness |
| US7974689B2 (en)* | 2007-06-13 | 2011-07-05 | Zoll Medical Corporation | Wearable medical treatment device with motion/position detection |
| US8140154B2 (en) | 2007-06-13 | 2012-03-20 | Zoll Medical Corporation | Wearable medical treatment device |
| US9008801B2 (en) | 2010-05-18 | 2015-04-14 | Zoll Medical Corporation | Wearable therapeutic device |
| US8706215B2 (en) | 2010-05-18 | 2014-04-22 | Zoll Medical Corporation | Wearable ambulatory medical device with multiple sensing electrodes |
| US10493289B2 (en) | 2010-07-09 | 2019-12-03 | Zoll Medical Corporation | System and method for conserving power in a medical device |
| US9937355B2 (en)* | 2010-11-08 | 2018-04-10 | Zoll Medical Corporation | Remote medical device alarm |
| JP5963767B2 (en) | 2010-12-09 | 2016-08-03 | ゾール メディカル コーポレイションZOLL Medical Corporation | Electrode assembly |
| JP5988991B2 (en) | 2010-12-10 | 2016-09-07 | ゾール メディカル コーポレイションZOLL Medical Corporation | Wearable treatment device |
| US9427564B2 (en) | 2010-12-16 | 2016-08-30 | Zoll Medical Corporation | Water resistant wearable medical device |
| US9237858B2 (en) | 2011-02-09 | 2016-01-19 | West Affum Holdings Corp. | Detecting loss of full skin contact in patient electrodes |
| US9317729B2 (en) | 2011-02-09 | 2016-04-19 | West Affum Holdings Corp. | RFID-based sensing of changed condition |
| US9684767B2 (en) | 2011-03-25 | 2017-06-20 | Zoll Medical Corporation | System and method for adapting alarms in a wearable medical device |
| US9135398B2 (en) | 2011-03-25 | 2015-09-15 | Zoll Medical Corporation | System and method for adapting alarms in a wearable medical device |
| WO2012135028A1 (en) | 2011-03-25 | 2012-10-04 | Zoll Medical Corporation | Method of detecting signal clipping in a wearable ambulatory medical device |
| WO2012135062A1 (en)* | 2011-03-25 | 2012-10-04 | Zoll Medical Corporation | Selection of optimal channel for rate determination |
| JP2014516654A (en) | 2011-05-02 | 2014-07-17 | ゾール メディカル コーポレイション | Patient-worn energy supply device and technology for adjusting its size |
| CN103764222B (en) | 2011-09-01 | 2016-02-10 | 佐尔医药公司 | Wearable monitoring and treatment equipment |
| US20130123719A1 (en)* | 2011-11-12 | 2013-05-16 | Robert Bosch Gmbh | Medication compliance patch and control unit |
| CN104428034A (en) | 2012-05-31 | 2015-03-18 | 佐尔医药公司 | Systems and methods for detecting health disorders |
| US20140025131A1 (en)* | 2012-07-20 | 2014-01-23 | Physio-Control, Inc. | Wearable defibrillator with voice prompts and voice recognition |
| US10155110B2 (en) | 2012-08-10 | 2018-12-18 | West Affum Holdings Corp. | Controlling functions of wearable cardiac defibrillation system |
| US20140043149A1 (en) | 2012-08-10 | 2014-02-13 | Physio-Control, Inc | Mobile communication device & app for wearable defibrillator system |
| US9155903B2 (en) | 2012-09-24 | 2015-10-13 | West Affum Holdings Corp. | Wearable cardiac defibrillator receiving inputs by being deliberately tapped and methods |
| US9604070B2 (en) | 2012-10-10 | 2017-03-28 | West Affum Holdings Corp. | External defibrillation with automatic post-shock anti-tachycardia (APSAT) pacing |
| US9087402B2 (en) | 2013-03-13 | 2015-07-21 | Microsoft Technology Licensing, Llc | Augmenting images with higher resolution data |
| US9345898B2 (en) | 2013-01-23 | 2016-05-24 | West Affum Holdings Corp. | Wearable cardiac defibrillator system controlling conductive fluid deployment |
| US9895548B2 (en) | 2013-01-23 | 2018-02-20 | West Affum Holdings Corp. | Wearable cardiac defibrillator (WCD) system controlling conductive fluid deployment per impedance settling at terminal value |
| US9999393B2 (en) | 2013-01-29 | 2018-06-19 | Zoll Medical Corporation | Delivery of electrode gel using CPR puck |
| US12097379B2 (en) | 2013-02-25 | 2024-09-24 | West Affum Holdings Dac | Wearable cardioverter defibrillator (WCD) system making shock/no shock determinations from multiple patient parameters |
| US10543377B2 (en) | 2013-02-25 | 2020-01-28 | West Affum Holdings Corp. | Wearable cardioverter defibrillator (WCD) system making shock/no shock determinations by aggregating aspects of patient parameters |
| US9757579B2 (en) | 2013-02-25 | 2017-09-12 | West Affum Holdings Corp. | Wearable cardioverter defibrillator (WCD) system informing patient that it is validating just-detected cardiac arrhythmia |
| US20150328472A1 (en) | 2014-05-13 | 2015-11-19 | Physio-Control, Inc. | Wearable cardioverter defibrillator components discarding ecg signals prior to making shock/no shock determination |
| US9592403B2 (en) | 2013-02-25 | 2017-03-14 | West Affum Holdings Corp. | Wearable cardioverter defibrillator (WCD) system making shock/no shock determinations from multiple patient parameters |
| US10500403B2 (en)* | 2013-02-25 | 2019-12-10 | West Affum Holdings Corp. | WCD system validating detected cardiac arrhythmias thoroughly so as to not sound loudly due to some quickly self-terminating cardiac arrhythmias |
| US8880196B2 (en) | 2013-03-04 | 2014-11-04 | Zoll Medical Corporation | Flexible therapy electrode |
| JP2016513496A (en)* | 2013-03-07 | 2016-05-16 | ゾール メディカル コーポレイションZOLL Medical Corporation | Reduced defibrillation pad contact detection |
| US10016613B2 (en) | 2013-04-02 | 2018-07-10 | West Affum Holdings Corp. | Wearable cardiac defibrillator system long-term monitoring alternating patient parameters other than ECG |
| US9827431B2 (en) | 2013-04-02 | 2017-11-28 | West Affum Holdings Corp. | Wearable defibrillator with no long-term ECG monitoring |
| WO2014210510A1 (en)* | 2013-06-28 | 2014-12-31 | Zoll Medical Corporation | Systems and methods of delivering therapy using an ambulatory medical device |
| JP2016531663A (en) | 2013-08-01 | 2016-10-13 | ゾール メディカル コーポレイションZOLL Medical Corporation | System and method for utilizing an identification device in a wearable medical treatment device |
| IN2015DN02541A (en)* | 2013-10-18 | 2015-09-11 | Healthwatch Ltd | |
| WO2015123198A1 (en) | 2014-02-12 | 2015-08-20 | Zoll Medical Corporation | System and method for adapting alarms in a wearable medical device |
| US9757576B2 (en) | 2014-03-18 | 2017-09-12 | West Affum Holdings Corp. | Reliable readiness indication for a wearable defibrillator |
| US9352166B2 (en) | 2014-03-19 | 2016-05-31 | West Affum Holdings Corp. | Wearable cardiac defibrillator system sounding to bystanders in patient's own voice |
| US9393437B2 (en) | 2014-04-02 | 2016-07-19 | West Affum Holdings Corp. | Pressure resistant conductive fluid containment |
| US9402988B2 (en)* | 2014-05-06 | 2016-08-02 | West Affum Holdings Corp. | Wearable medical system with stretch-cable assembly |
| US10449370B2 (en) | 2014-05-13 | 2019-10-22 | West Affum Holdings Corp. | Network-accessible data about patient with wearable cardiac defibrillator system |
| USD764670S1 (en) | 2014-12-22 | 2016-08-23 | West Affum Holdings Corp. | Portable defibrillator |
| US12191030B2 (en) | 2014-07-07 | 2025-01-07 | Zoll Medical Corporation | Medical device with natural language processor |
| US9724008B2 (en) | 2014-07-07 | 2017-08-08 | Zoll Medical Corporation | System and method for distinguishing a cardiac event from noise in an electrocardiogram (ECG) signal |
| US20180116537A1 (en) | 2014-07-07 | 2018-05-03 | Zoll Medical Corporation | System and Method for Distinguishing a Cardiac Event From Noise in an Electrocardiogram (ECG) Signal |
| US9833607B2 (en) | 2014-10-30 | 2017-12-05 | West Affum Holdings Corp. | Wearable cardiac defibrillation system with flexible electrodes |
| US11540762B2 (en) | 2014-10-30 | 2023-01-03 | West Affum Holdings Dac | Wearable cardioverter defibrtillator with improved ECG electrodes |
| EP3892198B1 (en) | 2014-11-14 | 2024-03-06 | ZOLL Medical Corporation | Medical premonitory event estimation |
| US9902028B2 (en) | 2014-11-20 | 2018-02-27 | Zoll Medical Corporation | External case for a wearable medical device |
| US10201711B2 (en) | 2014-12-18 | 2019-02-12 | Zoll Medical Corporation | Pacing device with acoustic sensor |
| EP4218551A3 (en) | 2015-02-27 | 2023-08-09 | Zoll Medical Corporation | Downloading and booting method and system for a wearable medical device |
| US10321877B2 (en) | 2015-03-18 | 2019-06-18 | Zoll Medical Corporation | Medical device with acoustic sensor |
| WO2016160369A1 (en) | 2015-03-20 | 2016-10-06 | Zoll Medical Corporation | Systems for self-testing an ambulatory medical device |
| US10835449B2 (en) | 2015-03-30 | 2020-11-17 | Zoll Medical Corporation | Modular components for medical devices |
| US9901741B2 (en) | 2015-05-11 | 2018-02-27 | Physio-Control, Inc. | Wearable cardioverter defibrillator (WCD) system using sensor modules with reassurance code for confirmation before shock |
| US10127357B2 (en) | 2015-05-18 | 2018-11-13 | Zoll Medical Corporation | Mobile monitoring and patient management system |
| US10578677B2 (en) | 2015-06-30 | 2020-03-03 | Zoll Medical Corporation | Systems and methods for monitoring battery life status |
| US9839356B2 (en) | 2015-07-07 | 2017-12-12 | Zoll Medical Corporation | Systems and methods for communicating data |
| US10252070B2 (en) | 2015-09-08 | 2019-04-09 | Zoll Medical Corporation | Secure limited components for use with medical devices |
| EP3355770B1 (en) | 2015-09-30 | 2024-07-24 | ZOLL Medical Corporation | Medical device operational modes |
| US11364389B2 (en) | 2015-10-01 | 2022-06-21 | Zoll Medical Corporation | Training modules for an external medical device |
| US10105547B2 (en) | 2015-11-02 | 2018-10-23 | West Affum Holdings Corp. | Wearable cardioverter defibrillator (WCD) causing patient's QRS width to be plotted against the heart rate |
| PL3693057T3 (en) | 2015-11-23 | 2023-02-20 | Zoll Medical Corporation | Garments for wearable medical devices |
| US10179246B2 (en) | 2015-12-04 | 2019-01-15 | West Affum Holdings Corp. | Wearable cardioverter defibrillator (WCD) system using security NFC tag for uploading configuration data |
| US10322291B2 (en) | 2015-12-04 | 2019-06-18 | West Affum Holdings Corp. | Wearable cardioverter defibrillator (WCD) system with isolated patient parameter component |
| US11116426B2 (en)* | 2015-12-09 | 2021-09-14 | Zoll Medical Corporation | Device administered tests and adaptive interactions |
| US11064952B2 (en) | 2015-12-30 | 2021-07-20 | Zoll Medical Corporation | External medical device that identifies a response activity |
| US11709747B2 (en) | 2016-01-08 | 2023-07-25 | Zoll Medical Corporation | Patient assurance system and method |
| US11617538B2 (en) | 2016-03-14 | 2023-04-04 | Zoll Medical Corporation | Proximity based processing systems and methods |
| US10674911B2 (en) | 2016-03-30 | 2020-06-09 | Zoll Medical Corporation | Systems and methods of integrating ambulatory medical devices |
| US10426342B2 (en) | 2016-03-31 | 2019-10-01 | Zoll Medical Corporation | Remote access for ambulatory medical device |
| US10149981B2 (en)* | 2016-05-03 | 2018-12-11 | Cardiac Pacemakers, Inc. | Authentication of shock therapy deferral |
| US10632301B2 (en) | 2016-09-30 | 2020-04-28 | Zoll Medical Corporation | Medical device connector for coupling electrodes |
| US10940323B2 (en) | 2016-10-04 | 2021-03-09 | West Affum Holdings Corp. | Wearable cardioverter defibrillator (WCD) with power-saving function |
| US11077310B1 (en) | 2016-10-04 | 2021-08-03 | West Affum Holdings Corp. | Wearable cardioverter defibrillator (WCD) system detecting QRS complexes in ECG signal by matched difference filter |
| RU2019114363A (en)* | 2016-10-12 | 2020-11-13 | Конинклейке Филипс Н.В. | SUPPRESSING AN AUTOMATED EXTERNAL DEFIBRILLATOR (AED) TEST WARNING |
| US11052241B2 (en) | 2016-11-03 | 2021-07-06 | West Affum Holdings Corp. | Wearable cardioverter defibrillator (WCD) system measuring patient's respiration |
| US11154230B2 (en) | 2017-01-05 | 2021-10-26 | West Affum Holdings Corp. | Wearable cardioverter defibrillator having reduced noise prompts |
| US11938333B2 (en) | 2017-01-05 | 2024-03-26 | West Affum Holdings Dac | Detecting walking in a wearable cardioverter defibrillator system |
| US11400303B2 (en) | 2018-01-05 | 2022-08-02 | West Affum Holdings Corp. | Detecting walking in a wearable cardioverter defibrillator system |
| US11083906B2 (en) | 2017-01-05 | 2021-08-10 | West Affum Holdings Corp. | Wearable cardioverter defibrillator having adjustable alarm time |
| US10926080B2 (en) | 2017-01-07 | 2021-02-23 | West Affum Holdings Corp. | Wearable cardioverter defibrillator with breast support |
| US11235143B2 (en) | 2017-02-03 | 2022-02-01 | West Affum Holdings Corp. | Wearable cardiac defibrillator systems and methods and software for contacting non-witnessing responders |
| US10967193B2 (en) | 2017-02-03 | 2021-04-06 | West Affum Holdings Corp. | WCD with pacing analgesia |
| US10470702B2 (en) | 2017-02-21 | 2019-11-12 | Zoll Medical Corporation | Assigning zone-based rankings and actions |
| US20180243549A1 (en) | 2017-02-27 | 2018-08-30 | Zoll Medical Corporation | Support Garment for a Wearable Medical Device |
| US11213691B2 (en) | 2017-02-27 | 2022-01-04 | Zoll Medical Corporation | Ambulatory medical device interaction |
| US10960220B2 (en) | 2017-03-16 | 2021-03-30 | West Affum Holdings Corp. | Wearable cardioverter defibrillator (WCD) system evaluating its ECG signals for noise according to tall peak counts |
| US10589109B2 (en) | 2017-04-10 | 2020-03-17 | West Affum Holdings Corp. | Wearable cardioverter defibrillator (WCD) system computing patient heart rate by multiplying ECG signals from different channels |
| US10940324B2 (en) | 2017-05-03 | 2021-03-09 | West Affum Holdings Corp. | Wearable cardioverter defibrillator (WCD) system computing heart rate from noisy ECG signal |
| US10946207B2 (en) | 2017-05-27 | 2021-03-16 | West Affum Holdings Corp. | Defibrillation waveforms for a wearable cardiac defibrillator |
| US11009870B2 (en) | 2017-06-06 | 2021-05-18 | Zoll Medical Corporation | Vehicle compatible ambulatory defibrillator |
| US11103717B2 (en)* | 2017-07-28 | 2021-08-31 | West Affum Holdings Corp. | Wearable cardioverter defibrillator (WCD) system reacting to high-frequency ECG noise |
| US10918879B2 (en) | 2017-07-28 | 2021-02-16 | West Affum Holdings Corp. | Wearable cardioverter defibrillator (WCD) system reacting to high-amplitude ECG noise |
| US10737104B2 (en) | 2017-07-28 | 2020-08-11 | West Affum Holdings Corp. | WCD system outputting human-visible indication and proximate programming device with screen reproducing the human-visible indication in real time |
| US11364387B2 (en) | 2017-07-28 | 2022-06-21 | West Affum Holdings Corp. | Heart rate calculator with reduced overcounting |
| US11207538B2 (en) | 2017-09-12 | 2021-12-28 | West Affum Holdings Corp. | Wearable cardioverter defibrillator (WCD) system warning ambulatory patient by weak alerting shock |
| KR102506857B1 (en)* | 2017-09-18 | 2023-03-08 | 현대자동차주식회사 | Vehicle AED System and Method for operating thereof |
| WO2019075035A1 (en) | 2017-10-12 | 2019-04-18 | Zoll Medical Corporation | System and method for distinguishing a cardiac event from noise in an electrocardiogram (ecg) signal |
| US11185708B2 (en) | 2017-10-18 | 2021-11-30 | Rescuestat Llc | Heartstation remote monitor system |
| US10959674B2 (en) | 2017-10-23 | 2021-03-30 | Datafeel Inc. | Communication devices, methods, and systems |
| US11844954B2 (en) | 2017-11-09 | 2023-12-19 | West Affum Holdings Dac | WCD monitor supporting serviceability and reprocessing |
| US11260237B1 (en) | 2017-11-09 | 2022-03-01 | West Affum Holdings Corp. | Wearable defibrillator with output stage having diverting resistance |
| US11065463B2 (en) | 2017-11-10 | 2021-07-20 | West Affum Holdings Corp. | Wearable cardioverter defibrillator (WCD) system having WCD mode and also AED mode |
| US11058885B2 (en) | 2017-11-29 | 2021-07-13 | West Affum Holdings Corp. | Wearable cardioverter defibrillator (WCD) system detecting ventricular tachycardia and/or ventricular fibrillation using variable heart rate decision threshold |
| US10646707B2 (en) | 2017-11-30 | 2020-05-12 | Zoll Medical Corporation | Medical devices with rapid sensor recovery |
| US11278730B2 (en) | 2017-12-04 | 2022-03-22 | West Affum Holdings Corp. | Wearable cardioverter defibrillator (WCD) system making shock/no shock determinations from patient's rotational motion |
| US11160990B1 (en) | 2018-02-14 | 2021-11-02 | West Affum Holdings Corp. | Wearable cardioverter defibrillator (WCD) alarms |
| US11865354B1 (en) | 2018-02-14 | 2024-01-09 | West Affum Holdings Dac | Methods and systems for distinguishing VT from VF |
| US11471693B1 (en) | 2018-02-14 | 2022-10-18 | West Affum Holdings Dac | Wearable cardioverter defibrillator (WCD) system choosing to consider ECG signals from different channels per QRS complex widths of the ECG signals |
| US12179032B2 (en) | 2018-02-14 | 2024-12-31 | West Affum Holdings Dac | Wearable cardioverter defibrillator (WCD) segment based episode opening and confirmation periods |
| USD911527S1 (en) | 2018-02-15 | 2021-02-23 | West Affum Holdings Corp. | Wearable cardioverter defibrillator connector |
| US11724116B2 (en) | 2018-02-15 | 2023-08-15 | West Affum Holdings Dac | Wearable cardioverter defibrillator latching connector |
| DE102018001251A1 (en) | 2018-02-16 | 2019-08-22 | Arnulf Deinzer | Juxtacorporeal defibrillator |
| US11040214B2 (en) | 2018-03-01 | 2021-06-22 | West Affum Holdings Corp. | Wearable cardioverter defibrillator (WCD) system having main UI that conveys message and peripheral device that amplifies the message |
| US10960213B2 (en) | 2018-03-12 | 2021-03-30 | Zoll Medical Corporation | Verification of cardiac arrhythmia prior to therapeutic stimulation |
| US10602945B2 (en) | 2018-03-13 | 2020-03-31 | Zoll Medical Corporation | Telemetry of wearable medical device information to secondary medical device or system |
| WO2019178524A1 (en) | 2018-03-16 | 2019-09-19 | Zoll Medical Corporation | Monitoring physiological status based on bio-vibrational and radio frequency data analysis |
| US11160972B2 (en) | 2018-03-30 | 2021-11-02 | Zoll Medical Corporation | Garments for wearable cardiac monitoring and treatment devices |
| US11000691B2 (en) | 2018-04-24 | 2021-05-11 | West Affum Holdings Corp. | Substantially-median-based determination of long-term heart rates from ECG data of wearable cardioverter defibrillator (WCD) system |
| US11298556B2 (en) | 2018-04-25 | 2022-04-12 | West Affum Holdings Corp. | WCD user interface response to a change in device orientation |
| US11331508B1 (en) | 2018-04-25 | 2022-05-17 | West Affum Holdings Corp. | Wearable cardioverter defibrillator with a non-invasive blood pressure monitor |
| US11260238B2 (en) | 2018-04-26 | 2022-03-01 | West Affum Holdings Corp. | Wearable medical device (WMD) implementing adaptive techniques to save power |
| US11198015B2 (en) | 2018-04-26 | 2021-12-14 | West Affum Holdings Corp. | Multi-sensory alarm for a wearable cardiac defibrillator |
| US11058884B2 (en) | 2018-04-26 | 2021-07-13 | West Affum Holding Corp | Wearable medical (WM) system monitoring ECG signal of ambulatory patient for heart condition |
| US11324960B2 (en) | 2018-04-26 | 2022-05-10 | West Affum Holdings Corp. | Permission-based control of interfacing components with a medical device |
| US11534615B2 (en) | 2018-04-26 | 2022-12-27 | West Affum Holdings Dac | Wearable Cardioverter Defibrillator (WCD) system logging events and broadcasting state changes and system status information to external clients |
| US11833360B2 (en) | 2018-05-29 | 2023-12-05 | West Affum Holdings Dac | Carry pack for a wearable cardioverter defibrillator |
| US11942222B2 (en) | 2018-06-18 | 2024-03-26 | Zoll Medical Corporation | Medical device for estimating risk of patient deterioration |
| US11623102B2 (en) | 2018-07-31 | 2023-04-11 | Medtronic, Inc. | Wearable defibrillation apparatus configured to apply a machine learning algorithm |
| US11247041B2 (en) | 2018-08-10 | 2022-02-15 | West Affum Holdings Corp. | Wearable cardioverter defibrillator (WCD) with ECG preamp having active input capacitance balancing |
| WO2020069308A1 (en) | 2018-09-28 | 2020-04-02 | Zoll Medical Corporation | Adhesively coupled wearable medical device |
| US11568984B2 (en) | 2018-09-28 | 2023-01-31 | Zoll Medical Corporation | Systems and methods for device inventory management and tracking |
| US10918877B2 (en) | 2018-09-28 | 2021-02-16 | Zoll Medical Corporation | Battery lock for ambulatory medical device |
| EP3902598B1 (en) | 2018-12-28 | 2024-12-04 | ZOLL Medical Corporation | Wearable medical device response mechanisms |
| US11334826B2 (en) | 2019-01-18 | 2022-05-17 | West Affum Holdings Corp. | WCD system prioritization of alerts based on severity and/or required timeliness of user response |
| US11191971B2 (en) | 2019-03-07 | 2021-12-07 | West Affum Holdings Corp. | Wearable cardioverter defibrillator (WCD) system with active ECG cable shielding |
| US11063378B2 (en) | 2019-03-07 | 2021-07-13 | West Affum Holdings Corp. | Printed circuit board cable clip for signal sensitive applications |
| US12300368B1 (en) | 2019-03-07 | 2025-05-13 | West Affum Holdings Dac | Analysis and presentation of aggregated patient and device data within a system that includes a medical device |
| US12121329B2 (en) | 2019-03-08 | 2024-10-22 | West Affum Holdings Dac | Wearable vital signs monitor with selective signal acquisition |
| FR3096584B1 (en)* | 2019-05-29 | 2022-06-10 | Lifeaz | System comprising a measuring device and a defibrillation kit |
| US11672996B2 (en) | 2019-06-24 | 2023-06-13 | West Affum Holdings Dac | Wearable cardioverter defibrillator with AI-based features |
| US11793440B2 (en) | 2019-08-09 | 2023-10-24 | West Affum Holdings Dac | Method to detect noise in a wearable cardioverter defibrillator |
| US10957453B2 (en) | 2019-08-15 | 2021-03-23 | West Affum Holdings Corp. | WCD system alert issuance and resolution |
| US11484271B2 (en) | 2019-08-20 | 2022-11-01 | West Affum Holdings Dac | Alert presentation based on ancillary device conditions |
| US11771360B2 (en) | 2019-08-22 | 2023-10-03 | West Affum Holdings Dac | Cardiac monitoring system with normally conducted QRS complex identification |
| US11730418B2 (en) | 2019-08-22 | 2023-08-22 | West Affum Holdings Dac | Cardiac monitoring system with supraventricular tachycardia (SVT) classifications |
| CN112642061A (en) | 2019-10-09 | 2021-04-13 | Zoll医疗公司 | Modular electrotherapy device |
| JP7280641B2 (en)* | 2019-10-24 | 2023-05-24 | 一般社団法人メディカル・イノベーション・コンソーシアム | automated external defibrillator |
| US11344718B2 (en) | 2019-12-12 | 2022-05-31 | West Affum Holdings Corp. | Multichannel posture dependent template based rhythm discrimination in a wearable cardioverter defibrillator |
| US10964195B1 (en)* | 2020-01-05 | 2021-03-30 | Lina Huang | Method and system of alerting patient with sleep disorder |
| US11717687B2 (en) | 2020-01-06 | 2023-08-08 | West Affum Holdings Dac | Asystole and complete heart block detection |
| US11904176B1 (en) | 2020-01-27 | 2024-02-20 | West Affum Holdings Dac | Wearable defibrillator system forwarding patient information based on recipient profile and/or event type |
| US11679253B2 (en) | 2020-02-16 | 2023-06-20 | West Affum Holdings Dac | Wearable medical device with integrated blood oxygen saturation level device |
| US12337185B1 (en) | 2020-03-14 | 2025-06-24 | West Affum Holdings Designated Activity Company | Detecting shockable polymorphic ventricular tachycardia |
| US11819704B2 (en) | 2020-08-21 | 2023-11-21 | West Affum Holdings Dac | Positive system alerts |
| US12226219B1 (en) | 2020-08-21 | 2025-02-18 | West Affum Holdings Dac | Detecting nonsustained ventricular tachycardia in a wearable cardioverter defibrillator |
| US12220256B2 (en) | 2020-08-24 | 2025-02-11 | West Affum Holdings Dac | Autonomous event assistant device |
| US12011607B2 (en) | 2020-08-24 | 2024-06-18 | West Affum Holdings Dac | Assistant for garment and wearable device fitting |
| US11819703B2 (en) | 2020-09-17 | 2023-11-21 | West Affum Holdings Dac | Electrocardiogram (ECG) electrode with deposited ink resistive element |
| US12121737B2 (en) | 2020-09-23 | 2024-10-22 | West Affum Holdings Dac | Wearable cardioverter defibrillator system with remote alerts based on proximity |
| US12357838B2 (en) | 2020-09-30 | 2025-07-15 | West Affum Holdings Dac | Wearable cardioverter defibrillator (WCD) system selecting previously identified preferred channel for attempting to detect pacing artifacts |
| US11934583B2 (en) | 2020-10-30 | 2024-03-19 | Datafeel Inc. | Wearable data communication apparatus, kits, methods, and systems |
| US12151117B2 (en) | 2020-11-04 | 2024-11-26 | West Affum Holdings Dac | Wearable cardioverter defibrillator system with electrode moisture sensing |
| US11974855B2 (en) | 2020-11-04 | 2024-05-07 | West Affum Holdings Dac | Method for detecting noise levels in ECG signals using a channel consistency threshold |
| US12036416B2 (en) | 2020-11-09 | 2024-07-16 | West Affum Holdings Dac | Wearable cardioverter defibrillator (WCD) system with wireless battery charging |
| US12233253B2 (en) | 2020-11-10 | 2025-02-25 | West Affum Holdings Dac | Wearable cardioverter defibrillator (WCD) with bystander voice interaction |
| US11698385B2 (en) | 2020-11-11 | 2023-07-11 | West Affum Holdings Dac | Walking intensity detection and trending in a wearable cardioverter defibrillator |
| US11793469B2 (en) | 2020-11-17 | 2023-10-24 | West Affum Holdings Dac | Identifying reliable vectors |
| US11950174B2 (en) | 2020-12-02 | 2024-04-02 | West Affum Holdings Dac | Detailed alarm messages and support |
| US11730968B2 (en) | 2020-12-14 | 2023-08-22 | West Affum Holdings Dac | Wearable medical device with temperature managed electrodes |
| US11712573B2 (en) | 2020-12-16 | 2023-08-01 | West Affum Holdings Dac | Managing alerts in a WCD system |
| US12329973B2 (en) | 2020-12-23 | 2025-06-17 | West Affum Holdings Dac | Rhythm sensing during external pacing |
| US12127860B2 (en) | 2021-01-21 | 2024-10-29 | West Affum Holdings Dac | Wearable device network system |
| US12403322B2 (en) | 2021-02-08 | 2025-09-02 | Zoll Medical Corporation | Modular ingress protected electrode system for a wearable defibrillator |
| US12409331B2 (en) | 2021-02-12 | 2025-09-09 | West Affum Holdings Dac | Wearable Cardioverter Defibrillator (WCD) with artificial intelligence features |
| US12172023B2 (en) | 2021-03-05 | 2024-12-24 | West Affum Holdings Dac | Data channel selection and timeline navigation in a cardiac monitoring system |
| US12427302B2 (en) | 2021-03-08 | 2025-09-30 | West Affum Holdings Dac | On-demand remote data acquisition and programming in a wearable medical device |
| US12232879B2 (en) | 2021-06-29 | 2025-02-25 | West Affum Holdings Dac | Atrial fibrillation detection |
| US12311188B2 (en) | 2021-09-22 | 2025-05-27 | West Affum Holdings Dac | Power in a wearable cardioverter defibrillator (WCD) |
| US12296184B2 (en) | 2021-09-22 | 2025-05-13 | West Affum Holdings Dac | Providing wearable device information to rescuers |
| US12362065B2 (en) | 2021-09-23 | 2025-07-15 | West Affum Holdings Dac | Health data management and display |
| US12354750B2 (en) | 2021-12-22 | 2025-07-08 | West Affum Holdings Dac | Selection of a wearable article for a medical device |
Citations (269)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US2688752A (en) | 1953-01-30 | 1954-09-14 | Dominic G Sbarra | Undergarment with attached article carrying harness |
| US3241556A (en) | 1962-05-17 | 1966-03-22 | Cotelec Soc Fr D Etudes Et De | Cardiac stimulators |
| US3409007A (en) | 1965-11-26 | 1968-11-05 | Lockheed Aircraft Corp | Body electrode support garment |
| US3460542A (en) | 1966-02-09 | 1969-08-12 | Hellige & Co Gmbh F | Instrument for electrically stimulating the activity of the heart |
| US3553651A (en) | 1967-12-06 | 1971-01-05 | Singer General Precision | Memory storage system |
| US3664560A (en) | 1970-01-16 | 1972-05-23 | Safariland Ltd Inc | Belt |
| US3702613A (en) | 1971-03-04 | 1972-11-14 | Health Systems Inc | Electrolyte envelope for use on the active surface of a defibrillator paddle |
| US3706313A (en) | 1971-02-04 | 1972-12-19 | Medical Research Lab | Trapezoidal waveshape defibrillator |
| US3724455A (en) | 1970-06-02 | 1973-04-03 | P Unger | Cardiac warning device |
| US3744482A (en) | 1971-06-29 | 1973-07-10 | Hittman Ass Inc | Dry contact electrode with amplifier for physiological signals |
| US3826245A (en) | 1973-02-09 | 1974-07-30 | Statham Instrument Inc | Electrodes employing disposable electropods for cardiac instruments |
| US3862636A (en) | 1972-01-20 | 1975-01-28 | Health Technology Labs Inc | Computer controlled defibrillator |
| US3886950A (en) | 1973-10-01 | 1975-06-03 | Spacelabs Inc | Defibrillator |
| US3897785A (en) | 1973-10-24 | 1975-08-05 | Jr Homer D Barto | Harness for a disposable urinal |
| US3942533A (en) | 1974-10-17 | 1976-03-09 | Cannon Robert L Iii | Cardiac defibrillator depolarizing paddle arrangement |
| JPS5115450B1 (en) | 1970-06-06 | 1976-05-17 | ||
| US3961623A (en) | 1975-01-17 | 1976-06-08 | Medical Research Laboratories, Inc. | Method of using a disposable electrode pad |
| US4002239A (en) | 1973-11-15 | 1977-01-11 | Gilbert Buchalter | Cardiac defibrillator cup |
| US4058127A (en) | 1973-11-15 | 1977-11-15 | Gilbert Buchalter | Method of applying viscous fluid to a surface |
| US4088138A (en) | 1974-01-02 | 1978-05-09 | Cardiac Resuscitator Corp. | Cardiac resuscitator and monitoring apparatus |
| US4094310A (en) | 1976-10-04 | 1978-06-13 | American Optical Corporation | Apparatus for enhanced display of physiological waveforms and for defibrillation |
| US4136690A (en) | 1977-10-31 | 1979-01-30 | Del Mar Avionics | Method and apparatus for vector analysis of ECG arrhythmias |
| US4576170A (en) | 1980-07-09 | 1986-03-18 | Micro-Circuits Company | Heart monitor and defibrillator device |
| US4580572A (en) | 1983-06-01 | 1986-04-08 | Bio-Stimu Trend Corp. | Garment apparatus for delivering or receiving electric impulses |
| US4583547A (en) | 1983-06-01 | 1986-04-22 | Bio-Stimu Trend Corp. | Garment apparatus for delivering or receiving electric impulses |
| US4608987A (en) | 1982-12-03 | 1986-09-02 | Physioventures, Inc. | Apparatus for transmitting ECG data |
| US4619265A (en) | 1984-03-08 | 1986-10-28 | Physio-Control Corporation | Interactive portable defibrillator including ECG detection circuit |
| US4632122A (en) | 1985-04-24 | 1986-12-30 | Johansson Nils E | Method and apparatus for conducting brain function diagnostic test |
| US4679572A (en) | 1986-03-11 | 1987-07-14 | Intermedics, Inc. | Low threshold cardiac pacing electrodes |
| US4698848A (en) | 1986-09-26 | 1987-10-13 | Buckley Mary C | Blouse for cardiac patients |
| US4729377A (en) | 1983-06-01 | 1988-03-08 | Bio-Stimu Trend Corporation | Garment apparatus for delivering or receiving electric impulses |
| EP0295497A2 (en) | 1987-06-18 | 1988-12-21 | STMicroelectronics S.r.l. | High-dynamics amplifier stage with distortion detection |
| US4823796A (en) | 1987-04-03 | 1989-04-25 | Laerdal Manufacturing Corp. | Defibrillator circuit for producing a trapezoidal defibrillation pulse |
| EP0335356A2 (en) | 1988-03-30 | 1989-10-04 | Nellcor Incorporated | Method and apparatus for compensating for distortion in a pulse oximetry device |
| US4889131A (en) | 1987-12-03 | 1989-12-26 | American Health Products, Inc. | Portable belt monitor of physiological functions and sensors therefor |
| US4926879A (en) | 1988-06-13 | 1990-05-22 | Sevrain-Tech, Inc. | Electro-tactile stimulator |
| US4928690A (en)* | 1988-04-25 | 1990-05-29 | Lifecor, Inc. | Portable device for sensing cardiac function and automatically delivering electrical therapy |
| US4938231A (en) | 1985-10-22 | 1990-07-03 | Telectronics N.V. | Defibrillator electrode |
| EP0396048A1 (en) | 1989-05-01 | 1990-11-07 | Medard Limited Company | Electrocardiographic electrode assembly |
| US4978926A (en) | 1989-12-21 | 1990-12-18 | Ford Motor Company | Audio limiter using voltage multiplexed feedback |
| US4991217A (en) | 1984-11-30 | 1991-02-05 | Ibm Corporation | Dual processor speech recognition system with dedicated data acquisition bus |
| US5000189A (en) | 1989-11-15 | 1991-03-19 | Regents Of The University Of Michigan | Method and system for monitoring electrocardiographic signals and detecting a pathological cardiac arrhythmia such as ventricular tachycardia |
| US5007427A (en) | 1987-05-07 | 1991-04-16 | Capintec, Inc. | Ambulatory physiological evaluation system including cardiac monitoring |
| US5062834A (en) | 1989-02-24 | 1991-11-05 | Product Development (S.G.Z.) Ltd | Device for dispensing a liquid particularly useful for delivering medicaments at a predetermined rate |
| US5078134A (en) | 1988-04-25 | 1992-01-07 | Lifecor, Inc. | Portable device for sensing cardiac function and automatically delivering electrical therapy |
| US5097830A (en) | 1990-03-30 | 1992-03-24 | Laerdal Manufacturing Corporation | Defibrillator with reliability verification |
| US5224479A (en) | 1991-06-21 | 1993-07-06 | Topy Enterprises Limited | ECG diagnostic pad |
| US5225763A (en) | 1991-03-20 | 1993-07-06 | Sherwood Medical Company | Battery charging circuit and method for an ambulatory feeding pump |
| US5306956A (en) | 1990-12-29 | 1994-04-26 | Sony Corporation | Connecting system for supplying electrical power to a device in place of a battery along with the transmission of data signals from the device to monitoring apparatus, or supplying electrical power to a battery for the charging thereof |
| US5333616A (en) | 1991-12-26 | 1994-08-02 | Instromedix, Inc. | Wrist-worn ECG monitor |
| US5342404A (en) | 1992-04-03 | 1994-08-30 | Intermedics, Inc. | Implantable medical interventional device |
| US5348008A (en) | 1991-11-25 | 1994-09-20 | Somnus Corporation | Cardiorespiratory alert system |
| US5357696A (en) | 1992-05-01 | 1994-10-25 | Gray Frank B | Device for measuring force applied to a wearer's foot |
| US5361412A (en) | 1993-04-19 | 1994-11-08 | Perry Betty J | Emergency preparedness vest apparatus |
| US5365932A (en) | 1993-09-02 | 1994-11-22 | Telectronics Pacing System, Inc. | Cardiac signal sensing device having sensitivity automatically controlled in response to metabolic demand |
| US5371692A (en) | 1990-05-21 | 1994-12-06 | Hewlett-Packard Company | Activating circuit for modifying or adding a new program to an electronic device |
| US5381798A (en) | 1993-11-02 | 1995-01-17 | Quinton Instrument Company | Spread spectrum telemetry of physiological signals |
| US5405361A (en) | 1993-03-15 | 1995-04-11 | Surviva Link Corporation | External defibrillator circuit |
| US5413262A (en) | 1993-05-07 | 1995-05-09 | Sears Roebuck & Co. | Lumbar supporting belt |
| US5433737A (en) | 1990-03-30 | 1995-07-18 | Medisan S.R.L. | Method for the electrical stimulation of a group of muscles in order to improve their appearance, and apparatus for carrying out the method |
| US5443494A (en) | 1992-12-23 | 1995-08-22 | Vupiesse Italia S.A.S. Di Valentine E Paolizzi E.C. | Support for bearing and positionally adjusting electrodes of portable belt devices for passive gymnastics |
| US5470341A (en) | 1993-12-10 | 1995-11-28 | Medtronic, Inc. | High voltage switch drive for implantable cardioverter/defibrillator |
| EP0707825A2 (en) | 1994-10-20 | 1996-04-24 | Hewlett-Packard Company | Flexible patient monitoring system |
| US5544661A (en) | 1994-01-13 | 1996-08-13 | Charles L. Davis | Real time ambulatory patient monitor |
| US5558640A (en) | 1994-03-17 | 1996-09-24 | Siemens Aktiengesellschaft | System for infusion of medicine into the body of a patient |
| US5601612A (en) | 1993-08-06 | 1997-02-11 | Heartstream, Inc. | Method for applying a multiphasic waveform |
| US5606242A (en) | 1994-10-04 | 1997-02-25 | Duracell, Inc. | Smart battery algorithm for reporting battery parameters to an external device |
| US5607454A (en) | 1993-08-06 | 1997-03-04 | Heartstream, Inc. | Electrotherapy method and apparatus |
| EP0761255A1 (en) | 1995-08-02 | 1997-03-12 | Pacesetter, Inc. | A system and method for storing and displaying historical medical data measured by an implantable medical device |
| US5611085A (en) | 1992-11-02 | 1997-03-18 | Rasmussen; Verner | Garment for holding an electrocardiographic monitoring unit and cables |
| US5619117A (en) | 1982-06-07 | 1997-04-08 | Norand Corporation | Battery pack having memory |
| US5625291A (en) | 1995-05-16 | 1997-04-29 | Brink; Gregory D. | System for exchanging information in a battery mailbox |
| WO1997022297A1 (en) | 1995-12-20 | 1997-06-26 | Life Alert, Ltd. | Medical information record system |
| US5662689A (en) | 1995-09-08 | 1997-09-02 | Medtronic, Inc. | Method and apparatus for alleviating cardioversion shock pain |
| US5701894A (en) | 1995-11-09 | 1997-12-30 | Del Mar Avionics | Modular physiological computer-recorder |
| US5708978A (en) | 1994-08-17 | 1998-01-20 | Johnsrud; Anna C. | Medical vest |
| US5718242A (en) | 1992-12-01 | 1998-02-17 | Pacesetter, Inc. | Cardiac arrhythmia detection system for an implantable stimulation device and method |
| US5721482A (en) | 1996-01-16 | 1998-02-24 | Hewlett-Packard Company | Intelligent battery and method for providing an advance low battery warning for a battery powered device such as a defibrillator |
| US5724025A (en) | 1993-10-21 | 1998-03-03 | Tavori; Itzchak | Portable vital signs monitor |
| US5730143A (en) | 1996-05-03 | 1998-03-24 | Ralin Medical, Inc. | Electrocardiographic monitoring and recording device |
| US5738102A (en) | 1994-03-31 | 1998-04-14 | Lemelson; Jerome H. | Patient monitoring system |
| US5741306A (en) | 1996-05-23 | 1998-04-21 | Lifecor, Inc. | Patient-worn energy delivery apparatus |
| US5758443A (en) | 1993-08-03 | 1998-06-02 | Healtech S.A. | Patient Identification Device |
| US5758366A (en) | 1997-01-09 | 1998-06-02 | Wilson; Barry E. | Garment belt |
| US5772604A (en) | 1997-03-14 | 1998-06-30 | Emory University | Method, system and apparatus for determining prognosis in atrial fibrillation |
| US5792190A (en) | 1995-08-01 | 1998-08-11 | Survivalink Corporation | Automated external defibrillator operator interface |
| WO1998039061A2 (en) | 1997-03-07 | 1998-09-11 | Cadent Medical Corporation | Wearable defibrillation system |
| WO1998043537A1 (en) | 1997-03-31 | 1998-10-08 | Telecom Medical, Inc. | Patient monitoring apparatus |
| US5824017A (en) | 1997-03-05 | 1998-10-20 | Physio-Control Corporation | H-bridge circuit for generating a high-energy biphasic waveform in an external defibrillator |
| US5827196A (en) | 1997-07-15 | 1998-10-27 | Hewlett-Packard Company | Method and system for providing characterizations of waveform representations of heart function |
| US5830236A (en) | 1996-10-29 | 1998-11-03 | Pacesetter, Inc. | System for delivering low pain therapeutic electrical waveforms to the heart |
| US5833714A (en) | 1996-01-18 | 1998-11-10 | Loeb; Gerald E. | Cochlear electrode array employing tantalum metal |
| US5887978A (en) | 1996-08-23 | 1999-03-30 | Accutru International Corporation | Self-verifying temperature sensor |
| US5924979A (en) | 1996-02-09 | 1999-07-20 | Nellcor Puritan Bennett Incorporated | Medical diagnostic apparatus with sleep mode |
| US5929601A (en) | 1997-12-22 | 1999-07-27 | Lifecor, Inc. | Battery management apparatus for portable electronic devices |
| US5944669A (en) | 1997-11-20 | 1999-08-31 | Lifecor, Inc. | Apparatus and method for sensing cardiac function |
| WO1999059465A1 (en) | 1998-05-21 | 1999-11-25 | Telecom Medical, Inc. | Patient monitoring apparatus |
| US6016445A (en) | 1996-04-16 | 2000-01-18 | Cardiotronics | Method and apparatus for electrode and transthoracic impedance estimation |
| WO2000002484A1 (en) | 1998-07-10 | 2000-01-20 | Physiometrix, Inc. | Self adjusting headgear appliance using reservoir electrodes |
| US6047203A (en) | 1997-03-17 | 2000-04-04 | Nims, Inc. | Physiologic signs feedback system |
| US6045503A (en) | 1999-01-20 | 2000-04-04 | Kamilllo Eisner-Stiftung | Method of and apparatus for determining the topology of a cornea |
| US6065154A (en) | 1998-04-07 | 2000-05-23 | Lifecor, Inc. | Support garments for patient-worn energy delivery apparatus |
| WO2000030529A1 (en) | 1998-11-24 | 2000-06-02 | Medtronic, Inc. | World wide patient location and data telemetry system for implantable medical devices |
| US6097987A (en) | 1998-12-30 | 2000-08-01 | Medical Research Laboratories, Inc. | External defibrillator electrode apparatus |
| US6169397B1 (en) | 1997-08-13 | 2001-01-02 | University Technology Corp. | Damped superconducting coil system having a multiturn, planar geometry superconducting coil and shunt resistors electrically connecting successive coil turns |
| US6169387B1 (en) | 1997-12-22 | 2001-01-02 | Lifecor, Inc. | Battery management apparatus for portable electronic devices |
| US6208896B1 (en) | 1998-11-13 | 2001-03-27 | Agilent Technologies, Inc. | Method and apparatus for providing variable defibrillation waveforms using switch-mode amplification |
| US6253099B1 (en) | 1999-08-19 | 2001-06-26 | Lifecor, Inc. | Cardiac monitoring electrode apparatus and method |
| US6280461B1 (en) | 1996-05-23 | 2001-08-28 | Lifecor, Inc. | Patient-worn energy delivery apparatus |
| US20010031991A1 (en) | 1999-12-14 | 2001-10-18 | Joseph Russial | Circuit for producing an arbitrary defibrillation waveform |
| US6336900B1 (en) | 1999-04-12 | 2002-01-08 | Agilent Technologies, Inc. | Home hub for reporting patient health parameters |
| US6390996B1 (en) | 1998-11-09 | 2002-05-21 | The Johns Hopkins University | CPR chest compression monitor |
| US6406426B1 (en) | 1999-11-03 | 2002-06-18 | Criticare Systems | Medical monitoring and alert system for use with therapeutic devices |
| US20020077689A1 (en) | 2000-12-15 | 2002-06-20 | Kirkland Thomas Christopher | Electrode positioning bodysuit |
| US6418346B1 (en) | 1999-12-14 | 2002-07-09 | Medtronic, Inc. | Apparatus and method for remote therapy and diagnosis in medical devices via interface systems |
| JP2002200059A (en) | 2000-12-28 | 2002-07-16 | Terumo Corp | Body movement detector and medical instrument using the same and method of detecting body movement |
| US6442433B1 (en) | 1999-10-26 | 2002-08-27 | Medtronic, Inc. | Apparatus and method for remote troubleshooting, maintenance and upgrade of implantable device systems |
| US20030095648A1 (en) | 1999-10-05 | 2003-05-22 | Lifecor, Inc. | Fault-tolerant remote reprogramming for a patient-worn medical device |
| US20030109904A1 (en) | 2001-12-10 | 2003-06-12 | Medtronic Physio-Control Manufacturing Corp. | Enhanced interface for a medical device and a terminal |
| US20030149462A1 (en) | 2002-02-04 | 2003-08-07 | White Sheldon S. | Medical electrodes |
| US20030158593A1 (en) | 2002-02-19 | 2003-08-21 | Heilman Marlin S. | Cardiac garment |
| US20030174049A1 (en) | 2002-03-18 | 2003-09-18 | Precision Dynamics Corporation | Wearable identification appliance that communicates with a wireless communications network such as bluetooth |
| US20030195567A1 (en) | 2002-04-10 | 2003-10-16 | Medtronic Physio-Control Corp. | Automated external defibrillator with user interface for adult and pediatric applications |
| US20030212311A1 (en) | 2002-05-07 | 2003-11-13 | Medtronic Physio-Control Manufacturing Corp. | Therapy-delivering portable medical device capable of triggering and communicating with an alarm system |
| US20040007970A1 (en) | 2000-07-18 | 2004-01-15 | Kelvin Ma | Micro electro mechanical system controlled organic LED and pixel arrays and method of using and of manufacturing same |
| US6681003B2 (en) | 1999-10-05 | 2004-01-20 | Lifecor, Inc. | Data collection and system management for patient-worn medical devices |
| US6687523B1 (en) | 1997-09-22 | 2004-02-03 | Georgia Tech Research Corp. | Fabric or garment with integrated flexible information infrastructure for monitoring vital signs of infants |
| US6690969B2 (en) | 1999-03-05 | 2004-02-10 | Revivant Corporation | Public access CPR and AED device |
| US6694191B2 (en) | 2000-01-21 | 2004-02-17 | Medtronic Minimed, Inc. | Ambulatory medical apparatus and method having telemetry modifiable control software |
| US20040049233A1 (en) | 2002-09-11 | 2004-03-11 | Edwards D. Craig | Medical device status information system |
| US6736759B1 (en)* | 1999-11-09 | 2004-05-18 | Paragon Solutions, Llc | Exercise monitoring system and methods |
| WO2004054656A1 (en) | 2002-12-13 | 2004-07-01 | Koninklijke Philips Electronics N.V. | External defibrillator with shock activated by cessation of precordial compressions |
| US20040143297A1 (en) | 2003-01-21 | 2004-07-22 | Maynard Ramsey | Advanced automatic external defibrillator powered by alternative and optionally multiple electrical power sources and a new business method for single use AED distribution and refurbishment |
| WO2004067083A2 (en) | 2003-01-27 | 2004-08-12 | Cardiac Telecom, Corporation | Defibrillation system for non-medical environments |
| US20040162510A1 (en) | 2003-02-14 | 2004-08-19 | Medtronic Physio-Control Corp | Integrated external chest compression and defibrillation devices and methods of operation |
| EP1455640A2 (en) | 2001-10-26 | 2004-09-15 | Koninklijke Philips Electronics N.V. | Garment comprising medical sensors |
| WO2004078259A1 (en) | 2003-02-28 | 2004-09-16 | Medtronic Emergency Response Systems, Inc. | Recording information for emergency call by defibrillator apparatus |
| US6804554B2 (en) | 2001-10-19 | 2004-10-12 | Medtronic, Inc. | Arrangement and system for enabling patient control of electrical therapies |
| US6827695B2 (en) | 2002-10-25 | 2004-12-07 | Revivant Corporation | Method of determining depth of compressions during cardio-pulmonary resuscitation |
| US20040249419A1 (en) | 2003-04-02 | 2004-12-09 | Chapman Fred William | Defibrillators customized for anticipated patients |
| US20050049515A1 (en) | 2003-07-31 | 2005-03-03 | Dale Julian Misczynski | Electrode belt for acquisition, processing and transmission of cardiac (ECG) signals |
| US6889078B2 (en) | 2001-04-26 | 2005-05-03 | Medtronic, Inc. | Hysteresis activation of accelerated pacing |
| US6889079B2 (en) | 2002-04-12 | 2005-05-03 | Cardiac Pacemakers, Inc. | Method and system for characterizing supraventricular rhythm during cardiac pacing |
| US20050131465A1 (en) | 2000-02-04 | 2005-06-16 | Freeman Gary A. | Integrated resuscitation |
| US6908437B2 (en) | 1999-11-16 | 2005-06-21 | Cardiac Intelligence Corporation | System and method for diagnosing and monitoring congestive heart failure for automated remote patient care |
| US20050144043A1 (en) | 2003-10-07 | 2005-06-30 | Holland Geoffrey N. | Medication management system |
| WO2005082454A1 (en) | 2004-02-19 | 2005-09-09 | Koninklijke Philips Electronics, N.V. | Method and apparatus for broadcasting audible information prompts from an external defibrillator |
| US6961612B2 (en) | 2003-02-19 | 2005-11-01 | Zoll Medical Corporation | CPR sensitive ECG analysis in an automatic external defibrillator |
| US20050246199A1 (en) | 2004-05-03 | 2005-11-03 | Tom Futch | Health and wellness station |
| US20050283198A1 (en) | 2004-06-18 | 2005-12-22 | Haubrich Gregory J | Conditional requirements for remote medical device programming |
| US20060036292A1 (en) | 2004-08-16 | 2006-02-16 | Mitchell Smith | Risk of death indicator |
| US20060085049A1 (en) | 2004-10-20 | 2006-04-20 | Nervonix, Inc. | Active electrode, bio-impedance based, tissue discrimination system and methods of use |
| US20060095091A1 (en) | 2004-11-02 | 2006-05-04 | Medtronic, Inc. | Apparatus for data retention in an implantable medical device |
| WO2006050325A2 (en) | 2004-10-29 | 2006-05-11 | Worcester Polytechnic Institute | System and method for multi-channel electrophysiologic signal data acquisition |
| US20060167505A1 (en) | 2005-01-26 | 2006-07-27 | Medtronic Emergency Response Systems, Inc. | Defibrillator with overridable CPR-first protocol |
| US20060178706A1 (en) | 2005-02-10 | 2006-08-10 | Lisogurski Daniel M | Monitoring physiological signals during external electrical stimulation |
| US20060211934A1 (en) | 2005-03-16 | 2006-09-21 | Textronics, Inc. | Textile-based electrode |
| US20060220809A1 (en) | 2005-03-21 | 2006-10-05 | Rf Monolithics, Inc. | System and method for monitoring use of vehicles such as golf carts |
| US7130690B2 (en) | 2003-07-23 | 2006-10-31 | Medtronic, Inc. | Atrial capture management during atrial and ventricular pacing |
| EP1720446A1 (en) | 2004-02-27 | 2006-11-15 | Koninklijke Philips Electronics N.V. | Wearable wireless device for monitoring, analyzing and communicating physiological status |
| US20060270952A1 (en) | 2005-03-25 | 2006-11-30 | Freeman Gary A | Integrated resuscitation |
| US7149579B1 (en) | 2002-12-23 | 2006-12-12 | Pacesetter, Inc. | System and method for determining patient posture based on 3-D trajectory using an implantable medical device |
| WO2007019325A2 (en) | 2005-08-04 | 2007-02-15 | Access Cardiosystems, Inc. | Automatic external defibrillator (aed) with wireless communications |
| US20070073120A1 (en) | 2005-09-29 | 2007-03-29 | Li Li | System and method for pre-processing waveforms |
| US7220235B2 (en) | 2003-06-27 | 2007-05-22 | Zoll Medical Corporation | Method and apparatus for enhancement of chest compressions during CPR |
| WO2007057169A1 (en) | 2005-11-15 | 2007-05-24 | Metrax Gmbh | External automatic defibrillator |
| US20070118056A1 (en) | 2005-11-18 | 2007-05-24 | Hua Wang | Posture detector calibration and use |
| US20070129769A1 (en) | 2005-12-02 | 2007-06-07 | Medtronic, Inc. | Wearable ambulatory data recorder |
| US20070143864A1 (en) | 2005-12-15 | 2007-06-21 | Symbol Technologies, Inc. | Methods and apparatus for power source authentication |
| US20070162390A1 (en) | 2005-12-22 | 2007-07-12 | Macrovision Corporation | Techniques for distributing and monitoring content |
| US20070161913A1 (en) | 2004-03-31 | 2007-07-12 | Michael Farrell | Methods and apparatus for monitoring the cardiovascular condition of patients with sleep disordered breathing |
| US20070169364A1 (en) | 2001-02-23 | 2007-07-26 | Microstrain, Inc. | Posture and body movement measuring system |
| US20070197878A1 (en) | 2004-07-09 | 2007-08-23 | Dror Shklarski | Wearable device, system and method for monitoring physiological and/or environmental parameters |
| US20070239214A1 (en) | 2006-03-29 | 2007-10-11 | Can Cinbis | Method and system for aborting cardiac treatments |
| US20070239220A1 (en) | 2006-03-29 | 2007-10-11 | Greenhut Saul E | Implantable medical device system and method with signal quality monitoring and response |
| US20070265671A1 (en) | 2006-04-27 | 2007-11-15 | Roberts Jonathan P | Selectable switching of implantable sensors to provide fault toleance for implantable medical devices |
| US20080004536A1 (en) | 2006-06-30 | 2008-01-03 | Baxi Amit S | Method and apparatus for amplifying multiple signals using a single multiplexed amplifier channel with software controlled AC response |
| US20080021532A1 (en) | 2006-07-21 | 2008-01-24 | Kveen Graig L | Delivery of cardiac stimulation devices |
| US20080033495A1 (en) | 2006-08-01 | 2008-02-07 | Kumar Uday N | External Defibrillator |
| US20080030656A1 (en) | 2006-08-01 | 2008-02-07 | 3M Innovative Properties Company | Transflective lc display with internal reflector and reflective polarizer |
| US20080045815A1 (en) | 2006-06-20 | 2008-02-21 | Derchak P A | Automatic and ambulatory monitoring of congestive heart failure patients |
| US7340296B2 (en) | 2005-05-18 | 2008-03-04 | Cardiac Pacemakers, Inc. | Detection of pleural effusion using transthoracic impedance |
| US20080058884A1 (en) | 2006-08-29 | 2008-03-06 | Matos Jeffrey A | Control of a defibrillator and/or pacemaker |
| US20080097793A1 (en) | 2006-10-24 | 2008-04-24 | Kent Dicks | Systems and methods for remote patient monitoring and user interface |
| US20080103402A1 (en) | 2006-10-30 | 2008-05-01 | Medtronic Emergency Response Systems, Inc. | Motion detection system for an external defibrillator |
| US20080167535A1 (en) | 2002-08-22 | 2008-07-10 | Stivoric John M | Devices and systems for contextual and physiological-based reporting, entertainment, control of other devices, health assessment and therapy |
| US20080177341A1 (en) | 2006-10-27 | 2008-07-24 | Bowers Kyle R | Automated external defibrillator (AED) system with multiple patient wireless monitoring capability for use in mass casualty incidents |
| US20080183090A1 (en) | 2003-09-12 | 2008-07-31 | Jonathan Farringdon | Method and apparatus for measuring heart-related parameters and deriving human status parameters from sensed physiological and contextual parameters |
| US20080249591A1 (en) | 2007-04-06 | 2008-10-09 | Northstar Neuroscience, Inc. | Controllers for implantable medical devices, and associated methods |
| US7453354B2 (en) | 2003-10-17 | 2008-11-18 | Koninklijke Philips Electronics, N.V. | Device arranged for carrying out a bioelectrical interaction with an individual and a method for on-demand lead-off detection |
| US20080287749A1 (en) | 2005-11-23 | 2008-11-20 | Koninklijke Philips Electronics, N.V. | Method and Apparatus for Remote Patient Monitoring |
| US20080294019A1 (en) | 2007-05-24 | 2008-11-27 | Bao Tran | Wireless stroke monitoring |
| US20080306560A1 (en) | 2007-06-06 | 2008-12-11 | Macho John D | Wearable defibrillator with audio input/output |
| US20080312522A1 (en) | 2007-06-15 | 2008-12-18 | Gordon Ian Rowlandson | System and apparatus for collecting physiological signals from a plurality of electrodes |
| US20080312520A1 (en) | 2007-06-15 | 2008-12-18 | Gordon Ian Rowlandson | Method and apparatus for acquiring physiological data |
| US20080312709A1 (en) | 2007-06-13 | 2008-12-18 | Volpe Shane S | Wearable medical treatment device with motion/position detection |
| US20090018428A1 (en) | 2003-05-19 | 2009-01-15 | Umist Ventures Limited | Knitted transducer devices |
| US7488293B2 (en) | 2003-04-23 | 2009-02-10 | Zoll Medical Corporation | Processing pulse signal in conjunction with accelerometer signal in cardiac resuscitation |
| US20090066366A1 (en) | 2007-09-12 | 2009-03-12 | Solomon Research Llc | Reprogrammable three dimensional intelligent system on a chip |
| US20090076397A1 (en) | 2007-09-14 | 2009-03-19 | Corventis, Inc. | Adherent Emergency Patient Monitor |
| WO2009034506A1 (en) | 2007-09-12 | 2009-03-19 | Koninklijke Philips Electronics, N.V. | Remote status indicator for a defibrillator |
| US20090076345A1 (en) | 2007-09-14 | 2009-03-19 | Corventis, Inc. | Adherent Device with Multiple Physiological Sensors |
| US20090076346A1 (en) | 2007-09-14 | 2009-03-19 | Corventis, Inc. | Tracking and Security for Adherent Patient Monitor |
| US20090076410A1 (en) | 2007-09-14 | 2009-03-19 | Corventis, Inc. | System and Methods for Wireless Body Fluid Monitoring |
| US20090076348A1 (en) | 2007-09-14 | 2009-03-19 | Corventis, Inc. | Injectable Device for Physiological Monitoring |
| US20090076342A1 (en) | 2007-09-14 | 2009-03-19 | Corventis, Inc. | Adherent Multi-Sensor Device with Empathic Monitoring |
| US20090076559A1 (en) | 2007-09-14 | 2009-03-19 | Corventis, Inc. | Adherent Device for Cardiac Rhythm Management |
| US20090076340A1 (en) | 2007-09-14 | 2009-03-19 | Corventis, Inc. | Adherent Cardiac Monitor with Advanced Sensing Capabilities |
| US20090076336A1 (en) | 2007-09-14 | 2009-03-19 | Corventis, Inc. | Medical Device Automatic Start-up Upon Contact to Patient Tissue |
| US20090076349A1 (en) | 2007-09-14 | 2009-03-19 | Corventis, Inc. | Adherent Multi-Sensor Device with Implantable Device Communication Capabilities |
| US20090076341A1 (en) | 2007-09-14 | 2009-03-19 | Corventis, Inc. | Adherent Athletic Monitor |
| US20090076364A1 (en) | 2007-09-14 | 2009-03-19 | Corventis, Inc. | Adherent Device for Sleep Disordered Breathing |
| US20090093687A1 (en) | 2007-03-08 | 2009-04-09 | Telfort Valery G | Systems and methods for determining a physiological condition using an acoustic monitor |
| US20090118808A1 (en) | 2004-09-23 | 2009-05-07 | Medtronic, Inc. | Implantable Medical Lead |
| US20090138059A1 (en) | 2004-12-08 | 2009-05-28 | Koninklijke Philips Electronics N.V. | Heart Defibrillator With Contactless ECG Sensor For Diagnostics/Effectivity Feedback |
| US20090146822A1 (en) | 2007-11-13 | 2009-06-11 | Elevate Technologies Pty Ltd. | Telemedicine Application for Remote Monitoring, Viewing and Updating of Patient Records |
| US20090212984A1 (en) | 2007-06-15 | 2009-08-27 | Micron Technology, Inc. | Quantizing circuits with variable parameters |
| US20090231124A1 (en) | 2004-11-12 | 2009-09-17 | Koninklijke Philips Electronics, N.V. | Method for automatic association devices to a patient and concurrent creation of a patient record |
| US20090234410A1 (en) | 2008-03-12 | 2009-09-17 | Corventis, Inc. | Heart Failure Decompensation Prediction Based on Cardiac Rhythm |
| US20090232286A1 (en) | 2008-03-14 | 2009-09-17 | Gigle Semiconductor, Ltd. | Coupling signal processing circuitry with a wireline communications medium |
| US20090264792A1 (en) | 2008-04-18 | 2009-10-22 | Corventis, Inc. | Method and Apparatus to Measure Bioelectric Impedance of Patient Tissue |
| US20090275848A1 (en) | 2008-04-30 | 2009-11-05 | Transoma Medical, Inc. | Cardiac Risk Assessment |
| US20090281394A1 (en) | 2006-09-25 | 2009-11-12 | Brian Keith Russell | Bio-mechanical sensor system |
| US20090287120A1 (en) | 2007-12-18 | 2009-11-19 | Searete Llc, A Limited Liability Corporation Of The State Of Delaware | Circulatory monitoring systems and methods |
| US20090292194A1 (en) | 2008-05-23 | 2009-11-26 | Corventis, Inc. | Chiropractic Care Management Systems and Methods |
| US20090295326A1 (en) | 2008-06-02 | 2009-12-03 | Physio-Control, Inc. | Defibrillator Battery Authentication System |
| US20090307266A1 (en) | 2008-06-06 | 2009-12-10 | Apple Inc. | Processing a page |
| US20090318779A1 (en) | 2006-05-24 | 2009-12-24 | Bao Tran | Mesh network stroke monitoring appliance |
| US20100010559A1 (en) | 2008-07-09 | 2010-01-14 | Cheng Zhang | Event-based battery monitor for implantable devices |
| WO2010025432A1 (en) | 2008-08-31 | 2010-03-04 | Abbott Diabetes Care Inc. | Closed loop control with improved alarm functions |
| US20100052892A1 (en) | 2008-08-27 | 2010-03-04 | Searete Llc, A Limited Liability Corporation Of The State Of Delaware | Health-related signaling via wearable items |
| US20100052897A1 (en) | 2008-08-27 | 2010-03-04 | Allen Paul G | Health-related signaling via wearable items |
| US20100056881A1 (en) | 2008-08-29 | 2010-03-04 | Corventis, Inc. | Method and Apparatus For Acute Cardiac Monitoring |
| US20100069735A1 (en) | 2006-07-29 | 2010-03-18 | Lior Berkner | Device for mobile electrocardiogram recording |
| US20100076533A1 (en) | 2007-08-23 | 2010-03-25 | Amit Dar | System for transmitting electrical current to a bodily tissue |
| US20100076513A1 (en) | 2001-11-21 | 2010-03-25 | Cameron Health, Inc. | Multiple Electrode Vectors for Implantable Cardiac Treatment Devices |
| US20100081962A1 (en) | 2007-03-19 | 2010-04-01 | Omron Healthcare Co., Ltd | Visceral fat measurement device |
| US20100114243A1 (en) | 2008-10-30 | 2010-05-06 | Medtronic, Inc. | Preselector interference rejection and dynamic range extension |
| US7712373B2 (en) | 2006-03-03 | 2010-05-11 | Nagle H Troy | Sensor device for real-time monitoring or relative movement using capacitive fabric sensors |
| US20100171611A1 (en) | 2006-04-28 | 2010-07-08 | Tia Gao | Sensor-Based Adaptive Methods for Wearable Devices |
| WO2010077997A2 (en) | 2008-12-16 | 2010-07-08 | Bodymedia, Inc. | Method and apparatus for determining heart rate variability using wavelet transformation |
| US20100234716A1 (en) | 2009-03-12 | 2010-09-16 | Corventis, Inc. | Method and Apparatus for Monitoring Fluid Content within Body Tissues |
| US20100241181A1 (en) | 2009-03-17 | 2010-09-23 | Walter T. Savage | External defibrillator |
| US7831303B2 (en) | 2003-06-17 | 2010-11-09 | Medtronic, Inc. | Cardiac pacing apparatus and method for continuous capture management |
| US20100298899A1 (en) | 2007-06-13 | 2010-11-25 | Donnelly Edward J | Wearable medical treatment device |
| US20100295674A1 (en) | 2009-05-21 | 2010-11-25 | Silverplus, Inc. | Integrated health management console |
| US20110015533A1 (en) | 2007-11-26 | 2011-01-20 | C.R. Bard, Inc. | Stylets for use with apparatus for intravascular placement of a catheter |
| US20110093840A1 (en) | 2009-10-21 | 2011-04-21 | Micro Power Electronics, Inc. | Patches for battery-interfacing devices and associated systems and methods |
| US20110098765A1 (en) | 2009-10-27 | 2011-04-28 | Medtronic, Inc. | Detecting lead related condition during delivery of therapeutic electrical signals |
| US20110172550A1 (en) | 2009-07-21 | 2011-07-14 | Michael Scott Martin | Uspa: systems and methods for ems device communication interface |
| US20110170692A1 (en) | 2009-11-06 | 2011-07-14 | Roche Diagnostics International Ltd. | Method And System For Establishing Cryptographic Communications Between A Remote Device And A Medical Device |
| US7991460B2 (en) | 2002-09-20 | 2011-08-02 | Angel Medical Systems, Inc. | Methods and apparatus for detecting cardiac events based on heart rate sensitive parameters |
| US20110288605A1 (en) | 2010-05-18 | 2011-11-24 | Zoll Medical Corporation | Wearable ambulatory medical device with multiple sensing electrodes |
| US20110288604A1 (en) | 2010-05-18 | 2011-11-24 | Kaib Thomas E | Wearable therapeutic device |
| US20120011382A1 (en) | 2010-07-09 | 2012-01-12 | Shane Volpe | System and method for conserving power in a medical device |
| US20120016361A1 (en) | 2010-07-13 | 2012-01-19 | White Sheldon S | Deposit ablation within and external to circulatory systems |
| US8102842B2 (en) | 2006-08-04 | 2012-01-24 | Broadcom Corporation | Integrated switch |
| US20120053479A1 (en) | 2010-08-25 | 2012-03-01 | Angel Medical Systems, Inc. | Acute ischemia detection based on parameter value range analysis |
| US20120112903A1 (en) | 2010-11-08 | 2012-05-10 | Zoll Medical Corporation | Remote medical device alarm |
| US20120146797A1 (en) | 2010-12-10 | 2012-06-14 | Emil Oskin | Wearable therapeutic device |
| US20120150008A1 (en) | 2010-12-09 | 2012-06-14 | Kaib Thomas E | Electrode with redundant impedance reduction |
| US20120158075A1 (en) | 2010-12-16 | 2012-06-21 | Kaib Thomas E | Water resistant wearable medical device |
| US8271082B2 (en) | 2007-06-07 | 2012-09-18 | Zoll Medical Corporation | Medical device configured to test for user responsiveness |
| US20120283794A1 (en) | 2011-05-02 | 2012-11-08 | Kaib Thomas E | Patient-worn energy delivery apparatus and techniques for sizing same |
| US20120289809A1 (en) | 2011-03-25 | 2012-11-15 | Zoll Medical Corporation | Method of detecting signal clipping in a wearable ambulatory medical device |
| US20120293323A1 (en) | 2011-03-25 | 2012-11-22 | Zoll Medical Corporation | System and method for adapting alarms in a wearable medical device |
| JP5115450B2 (en) | 2008-11-04 | 2013-01-09 | 沖電気工業株式会社 | Optical code division multiplexing signal generator |
| US20130085538A1 (en) | 2011-09-01 | 2013-04-04 | Zoll Medical Corporation | Wearable monitoring and treatment device |
| US20130231711A1 (en) | 2012-03-02 | 2013-09-05 | Thomas E. Kaib | Systems and methods for configuring a wearable medical monitoring and/or treatment device |
Family Cites Families (6)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JPS5115450A (en) | 1974-07-29 | 1976-02-06 | Ritsuo Hasumi | HIKARIDENSOFUAIBAANO HANSHAFUIRUTA |
| DE2644236C3 (en) | 1976-09-30 | 1981-04-02 | Siemens AG, 1000 Berlin und 8000 München | Electrode for taking physiological signals |
| JP2765602B2 (en) | 1991-10-30 | 1998-06-18 | 日本電気株式会社 | Auto drift cancellation circuit of ECG analyzer |
| CA2487255C (en)* | 2002-06-11 | 2014-05-06 | Jeffrey A. Matos | System for cardiac resuscitation |
| AU2003303382A1 (en)* | 2002-12-20 | 2004-07-22 | Axon Medical, Inc. | System providing emergency medical care with real-time instructions and associated methods |
| US20060111750A1 (en)* | 2004-11-24 | 2006-05-25 | Bowers Kyle R | Automated external defibrillator (AED) with discrete sensing pulse for use in configuring a therapeutic biphasic waveform |
- 2008
- 2008-04-09USUS12/082,168patent/US8369944B2/ennot_activeExpired - Fee Related
- 2008-06-05DEDE102008026871.2Apatent/DE102008026871B4/enactiveActive
- 2008-06-05JPJP2008147733Apatent/JP5467733B2/ennot_activeExpired - Fee Related
- 2008-06-06FRFR0853740Apatent/FR2916981B1/enactiveActive
- 2013
- 2013-02-01USUS13/757,105patent/US8774917B2/enactiveActive
- 2014
- 2014-01-28JPJP2014013304Apatent/JP5932856B2/ennot_activeExpired - Fee Related
- 2014-07-07USUS14/325,041patent/US8965500B2/ennot_activeExpired - Fee Related
- 2015
- 2015-01-28USUS14/607,843patent/US9492676B2/enactiveActive
- 2016
- 2016-04-28JPJP2016092087Apatent/JP2016137321A/enactivePending
- 2016-10-04USUS15/285,295patent/US10029110B2/enactiveActive
- 2017
- 2017-03-24USUS15/468,196patent/US10004893B2/enactiveActive
- 2018
- 2018-06-21USUS16/014,365patent/US10426946B2/enactiveActive
- 2019
- 2019-08-16USUS16/542,343patent/US11083886B2/enactiveActive
- 2021
- 2021-07-02USUS17/305,283patent/US12138444B2/enactiveActive
- 2024
- 2024-10-04USUS18/906,254patent/US20250090841A1/enactivePending
Patent Citations (306)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US2688752A (en) | 1953-01-30 | 1954-09-14 | Dominic G Sbarra | Undergarment with attached article carrying harness |
| US3241556A (en) | 1962-05-17 | 1966-03-22 | Cotelec Soc Fr D Etudes Et De | Cardiac stimulators |
| US3409007A (en) | 1965-11-26 | 1968-11-05 | Lockheed Aircraft Corp | Body electrode support garment |
| US3460542A (en) | 1966-02-09 | 1969-08-12 | Hellige & Co Gmbh F | Instrument for electrically stimulating the activity of the heart |
| US3553651A (en) | 1967-12-06 | 1971-01-05 | Singer General Precision | Memory storage system |
| US3664560A (en) | 1970-01-16 | 1972-05-23 | Safariland Ltd Inc | Belt |
| US3724455A (en) | 1970-06-02 | 1973-04-03 | P Unger | Cardiac warning device |
| JPS5115450B1 (en) | 1970-06-06 | 1976-05-17 | ||
| US3706313A (en) | 1971-02-04 | 1972-12-19 | Medical Research Lab | Trapezoidal waveshape defibrillator |
| US3702613A (en) | 1971-03-04 | 1972-11-14 | Health Systems Inc | Electrolyte envelope for use on the active surface of a defibrillator paddle |
| US3744482A (en) | 1971-06-29 | 1973-07-10 | Hittman Ass Inc | Dry contact electrode with amplifier for physiological signals |
| US3862636A (en) | 1972-01-20 | 1975-01-28 | Health Technology Labs Inc | Computer controlled defibrillator |
| US3826245A (en) | 1973-02-09 | 1974-07-30 | Statham Instrument Inc | Electrodes employing disposable electropods for cardiac instruments |
| US3886950A (en) | 1973-10-01 | 1975-06-03 | Spacelabs Inc | Defibrillator |
| US3897785A (en) | 1973-10-24 | 1975-08-05 | Jr Homer D Barto | Harness for a disposable urinal |
| US4058127A (en) | 1973-11-15 | 1977-11-15 | Gilbert Buchalter | Method of applying viscous fluid to a surface |
| US4002239A (en) | 1973-11-15 | 1977-01-11 | Gilbert Buchalter | Cardiac defibrillator cup |
| US4088138A (en) | 1974-01-02 | 1978-05-09 | Cardiac Resuscitator Corp. | Cardiac resuscitator and monitoring apparatus |
| US3942533A (en) | 1974-10-17 | 1976-03-09 | Cannon Robert L Iii | Cardiac defibrillator depolarizing paddle arrangement |
| US3961623A (en) | 1975-01-17 | 1976-06-08 | Medical Research Laboratories, Inc. | Method of using a disposable electrode pad |
| US4094310A (en) | 1976-10-04 | 1978-06-13 | American Optical Corporation | Apparatus for enhanced display of physiological waveforms and for defibrillation |
| US4136690A (en) | 1977-10-31 | 1979-01-30 | Del Mar Avionics | Method and apparatus for vector analysis of ECG arrhythmias |
| US4576170A (en) | 1980-07-09 | 1986-03-18 | Micro-Circuits Company | Heart monitor and defibrillator device |
| US5619117A (en) | 1982-06-07 | 1997-04-08 | Norand Corporation | Battery pack having memory |
| US4608987A (en) | 1982-12-03 | 1986-09-02 | Physioventures, Inc. | Apparatus for transmitting ECG data |
| US4729377A (en) | 1983-06-01 | 1988-03-08 | Bio-Stimu Trend Corporation | Garment apparatus for delivering or receiving electric impulses |
| US4580572A (en) | 1983-06-01 | 1986-04-08 | Bio-Stimu Trend Corp. | Garment apparatus for delivering or receiving electric impulses |
| US4583547A (en) | 1983-06-01 | 1986-04-22 | Bio-Stimu Trend Corp. | Garment apparatus for delivering or receiving electric impulses |
| US4619265A (en) | 1984-03-08 | 1986-10-28 | Physio-Control Corporation | Interactive portable defibrillator including ECG detection circuit |
| US4991217A (en) | 1984-11-30 | 1991-02-05 | Ibm Corporation | Dual processor speech recognition system with dedicated data acquisition bus |
| US4632122A (en) | 1985-04-24 | 1986-12-30 | Johansson Nils E | Method and apparatus for conducting brain function diagnostic test |
| US4938231A (en) | 1985-10-22 | 1990-07-03 | Telectronics N.V. | Defibrillator electrode |
| US4679572A (en) | 1986-03-11 | 1987-07-14 | Intermedics, Inc. | Low threshold cardiac pacing electrodes |
| US4698848A (en) | 1986-09-26 | 1987-10-13 | Buckley Mary C | Blouse for cardiac patients |
| US4823796A (en) | 1987-04-03 | 1989-04-25 | Laerdal Manufacturing Corp. | Defibrillator circuit for producing a trapezoidal defibrillation pulse |
| US5007427A (en) | 1987-05-07 | 1991-04-16 | Capintec, Inc. | Ambulatory physiological evaluation system including cardiac monitoring |
| EP0295497A2 (en) | 1987-06-18 | 1988-12-21 | STMicroelectronics S.r.l. | High-dynamics amplifier stage with distortion detection |
| US4889131A (en) | 1987-12-03 | 1989-12-26 | American Health Products, Inc. | Portable belt monitor of physiological functions and sensors therefor |
| EP0335356A2 (en) | 1988-03-30 | 1989-10-04 | Nellcor Incorporated | Method and apparatus for compensating for distortion in a pulse oximetry device |
| US4928690A (en)* | 1988-04-25 | 1990-05-29 | Lifecor, Inc. | Portable device for sensing cardiac function and automatically delivering electrical therapy |
| US5078134A (en) | 1988-04-25 | 1992-01-07 | Lifecor, Inc. | Portable device for sensing cardiac function and automatically delivering electrical therapy |
| US4926879A (en) | 1988-06-13 | 1990-05-22 | Sevrain-Tech, Inc. | Electro-tactile stimulator |
| US5062834A (en) | 1989-02-24 | 1991-11-05 | Product Development (S.G.Z.) Ltd | Device for dispensing a liquid particularly useful for delivering medicaments at a predetermined rate |
| EP0396048A1 (en) | 1989-05-01 | 1990-11-07 | Medard Limited Company | Electrocardiographic electrode assembly |
| US5000189A (en) | 1989-11-15 | 1991-03-19 | Regents Of The University Of Michigan | Method and system for monitoring electrocardiographic signals and detecting a pathological cardiac arrhythmia such as ventricular tachycardia |
| US4978926A (en) | 1989-12-21 | 1990-12-18 | Ford Motor Company | Audio limiter using voltage multiplexed feedback |
| US5097830A (en) | 1990-03-30 | 1992-03-24 | Laerdal Manufacturing Corporation | Defibrillator with reliability verification |
| US5433737A (en) | 1990-03-30 | 1995-07-18 | Medisan S.R.L. | Method for the electrical stimulation of a group of muscles in order to improve their appearance, and apparatus for carrying out the method |
| US5371692A (en) | 1990-05-21 | 1994-12-06 | Hewlett-Packard Company | Activating circuit for modifying or adding a new program to an electronic device |
| US5306956A (en) | 1990-12-29 | 1994-04-26 | Sony Corporation | Connecting system for supplying electrical power to a device in place of a battery along with the transmission of data signals from the device to monitoring apparatus, or supplying electrical power to a battery for the charging thereof |
| US5225763A (en) | 1991-03-20 | 1993-07-06 | Sherwood Medical Company | Battery charging circuit and method for an ambulatory feeding pump |
| US5224479A (en) | 1991-06-21 | 1993-07-06 | Topy Enterprises Limited | ECG diagnostic pad |
| US5348008A (en) | 1991-11-25 | 1994-09-20 | Somnus Corporation | Cardiorespiratory alert system |
| US5333616A (en) | 1991-12-26 | 1994-08-02 | Instromedix, Inc. | Wrist-worn ECG monitor |
| US5342404A (en) | 1992-04-03 | 1994-08-30 | Intermedics, Inc. | Implantable medical interventional device |
| US5472453A (en) | 1992-04-03 | 1995-12-05 | Intermedics, Inc. | Medical interventional device with accelerometer for providing cardiac therapeutic functions |
| US5357696A (en) | 1992-05-01 | 1994-10-25 | Gray Frank B | Device for measuring force applied to a wearer's foot |
| US5611085A (en) | 1992-11-02 | 1997-03-18 | Rasmussen; Verner | Garment for holding an electrocardiographic monitoring unit and cables |
| US5718242A (en) | 1992-12-01 | 1998-02-17 | Pacesetter, Inc. | Cardiac arrhythmia detection system for an implantable stimulation device and method |
| US5443494A (en) | 1992-12-23 | 1995-08-22 | Vupiesse Italia S.A.S. Di Valentine E Paolizzi E.C. | Support for bearing and positionally adjusting electrodes of portable belt devices for passive gymnastics |
| US5405361A (en) | 1993-03-15 | 1995-04-11 | Surviva Link Corporation | External defibrillator circuit |
| US5361412A (en) | 1993-04-19 | 1994-11-08 | Perry Betty J | Emergency preparedness vest apparatus |
| US5413262A (en) | 1993-05-07 | 1995-05-09 | Sears Roebuck & Co. | Lumbar supporting belt |
| US5758443A (en) | 1993-08-03 | 1998-06-02 | Healtech S.A. | Patient Identification Device |
| US5620470A (en) | 1993-08-06 | 1997-04-15 | Heartstream, Inc. | Electrotherapy method |
| US5601612A (en) | 1993-08-06 | 1997-02-11 | Heartstream, Inc. | Method for applying a multiphasic waveform |
| US5607454A (en) | 1993-08-06 | 1997-03-04 | Heartstream, Inc. | Electrotherapy method and apparatus |
| US5365932A (en) | 1993-09-02 | 1994-11-22 | Telectronics Pacing System, Inc. | Cardiac signal sensing device having sensitivity automatically controlled in response to metabolic demand |
| US5724025A (en) | 1993-10-21 | 1998-03-03 | Tavori; Itzchak | Portable vital signs monitor |
| US5381798A (en) | 1993-11-02 | 1995-01-17 | Quinton Instrument Company | Spread spectrum telemetry of physiological signals |
| US5470341A (en) | 1993-12-10 | 1995-11-28 | Medtronic, Inc. | High voltage switch drive for implantable cardioverter/defibrillator |
| US5544661A (en) | 1994-01-13 | 1996-08-13 | Charles L. Davis | Real time ambulatory patient monitor |
| US5558640A (en) | 1994-03-17 | 1996-09-24 | Siemens Aktiengesellschaft | System for infusion of medicine into the body of a patient |
| US5738102A (en) | 1994-03-31 | 1998-04-14 | Lemelson; Jerome H. | Patient monitoring system |
| US5708978A (en) | 1994-08-17 | 1998-01-20 | Johnsrud; Anna C. | Medical vest |
| US5606242A (en) | 1994-10-04 | 1997-02-25 | Duracell, Inc. | Smart battery algorithm for reporting battery parameters to an external device |
| EP0707825A2 (en) | 1994-10-20 | 1996-04-24 | Hewlett-Packard Company | Flexible patient monitoring system |
| US5625291A (en) | 1995-05-16 | 1997-04-29 | Brink; Gregory D. | System for exchanging information in a battery mailbox |
| US5792190A (en) | 1995-08-01 | 1998-08-11 | Survivalink Corporation | Automated external defibrillator operator interface |
| EP0761255A1 (en) | 1995-08-02 | 1997-03-12 | Pacesetter, Inc. | A system and method for storing and displaying historical medical data measured by an implantable medical device |
| US5662689A (en) | 1995-09-08 | 1997-09-02 | Medtronic, Inc. | Method and apparatus for alleviating cardioversion shock pain |
| US5701894A (en) | 1995-11-09 | 1997-12-30 | Del Mar Avionics | Modular physiological computer-recorder |
| WO1997022297A1 (en) | 1995-12-20 | 1997-06-26 | Life Alert, Ltd. | Medical information record system |
| US5721482A (en) | 1996-01-16 | 1998-02-24 | Hewlett-Packard Company | Intelligent battery and method for providing an advance low battery warning for a battery powered device such as a defibrillator |
| US5833714A (en) | 1996-01-18 | 1998-11-10 | Loeb; Gerald E. | Cochlear electrode array employing tantalum metal |
| US5924979A (en) | 1996-02-09 | 1999-07-20 | Nellcor Puritan Bennett Incorporated | Medical diagnostic apparatus with sleep mode |
| US6016445A (en) | 1996-04-16 | 2000-01-18 | Cardiotronics | Method and apparatus for electrode and transthoracic impedance estimation |
| US5730143A (en) | 1996-05-03 | 1998-03-24 | Ralin Medical, Inc. | Electrocardiographic monitoring and recording device |
| US5741306A (en) | 1996-05-23 | 1998-04-21 | Lifecor, Inc. | Patient-worn energy delivery apparatus |
| US6097982A (en) | 1996-05-23 | 2000-08-01 | Lifecor, Inc. | Patient-worn energy delivery apparatus |
| US6280461B1 (en) | 1996-05-23 | 2001-08-28 | Lifecor, Inc. | Patient-worn energy delivery apparatus |
| US5887978A (en) | 1996-08-23 | 1999-03-30 | Accutru International Corporation | Self-verifying temperature sensor |
| US5830236A (en) | 1996-10-29 | 1998-11-03 | Pacesetter, Inc. | System for delivering low pain therapeutic electrical waveforms to the heart |
| US5758366A (en) | 1997-01-09 | 1998-06-02 | Wilson; Barry E. | Garment belt |
| US5824017A (en) | 1997-03-05 | 1998-10-20 | Physio-Control Corporation | H-bridge circuit for generating a high-energy biphasic waveform in an external defibrillator |
| US6148233A (en) | 1997-03-07 | 2000-11-14 | Cardiac Science, Inc. | Defibrillation system having segmented electrodes |
| WO1998039061A2 (en) | 1997-03-07 | 1998-09-11 | Cadent Medical Corporation | Wearable defibrillation system |
| US20030032988A1 (en) | 1997-03-07 | 2003-02-13 | Fincke Randall W. | Defibrillator with controller operating in a low power mode |
| US20030055460A1 (en) | 1997-03-07 | 2003-03-20 | Owen James M. | Defibrillator with configurable capacitor arrangement |
| US20110022105A9 (en) | 1997-03-07 | 2011-01-27 | Owen James M | Defibrillation system |
| US20030004547A1 (en) | 1997-03-07 | 2003-01-02 | Owen James M. | Defibrillation system |
| US6944498B2 (en) | 1997-03-07 | 2005-09-13 | Cardiac Science, Inc. | Method of utilizing an external defibrillator by replacing its electrodes |
| US5772604A (en) | 1997-03-14 | 1998-06-30 | Emory University | Method, system and apparatus for determining prognosis in atrial fibrillation |
| US6047203A (en) | 1997-03-17 | 2000-04-04 | Nims, Inc. | Physiologic signs feedback system |
| WO1998043537A1 (en) | 1997-03-31 | 1998-10-08 | Telecom Medical, Inc. | Patient monitoring apparatus |
| US5827196A (en) | 1997-07-15 | 1998-10-27 | Hewlett-Packard Company | Method and system for providing characterizations of waveform representations of heart function |
| US6169397B1 (en) | 1997-08-13 | 2001-01-02 | University Technology Corp. | Damped superconducting coil system having a multiturn, planar geometry superconducting coil and shunt resistors electrically connecting successive coil turns |
| US6687523B1 (en) | 1997-09-22 | 2004-02-03 | Georgia Tech Research Corp. | Fabric or garment with integrated flexible information infrastructure for monitoring vital signs of infants |
| US5944669A (en) | 1997-11-20 | 1999-08-31 | Lifecor, Inc. | Apparatus and method for sensing cardiac function |
| US6169387B1 (en) | 1997-12-22 | 2001-01-02 | Lifecor, Inc. | Battery management apparatus for portable electronic devices |
| US5929601A (en) | 1997-12-22 | 1999-07-27 | Lifecor, Inc. | Battery management apparatus for portable electronic devices |
| US6065154A (en) | 1998-04-07 | 2000-05-23 | Lifecor, Inc. | Support garments for patient-worn energy delivery apparatus |
| WO1999059465A1 (en) | 1998-05-21 | 1999-11-25 | Telecom Medical, Inc. | Patient monitoring apparatus |
| WO2000002484A1 (en) | 1998-07-10 | 2000-01-20 | Physiometrix, Inc. | Self adjusting headgear appliance using reservoir electrodes |
| US6865413B2 (en) | 1998-11-09 | 2005-03-08 | Revivant Corporation | ECG signal processor and method |
| US6390996B1 (en) | 1998-11-09 | 2002-05-21 | The Johns Hopkins University | CPR chest compression monitor |
| US7108665B2 (en) | 1998-11-09 | 2006-09-19 | Zoll Circulation, Inc. | CPR chest compression monitor |
| US7074199B2 (en) | 1998-11-09 | 2006-07-11 | Revivant Corporation | CPR chest compression monitor and method of use |
| US7295871B2 (en) | 1998-11-09 | 2007-11-13 | Zoll Circulation, Inc. | ECG signal processor and method |
| US6208896B1 (en) | 1998-11-13 | 2001-03-27 | Agilent Technologies, Inc. | Method and apparatus for providing variable defibrillation waveforms using switch-mode amplification |
| WO2000030529A1 (en) | 1998-11-24 | 2000-06-02 | Medtronic, Inc. | World wide patient location and data telemetry system for implantable medical devices |
| US6097987A (en) | 1998-12-30 | 2000-08-01 | Medical Research Laboratories, Inc. | External defibrillator electrode apparatus |
| US6045503A (en) | 1999-01-20 | 2000-04-04 | Kamilllo Eisner-Stiftung | Method of and apparatus for determining the topology of a cornea |
| US6690969B2 (en) | 1999-03-05 | 2004-02-10 | Revivant Corporation | Public access CPR and AED device |
| US6336900B1 (en) | 1999-04-12 | 2002-01-08 | Agilent Technologies, Inc. | Home hub for reporting patient health parameters |
| US6253099B1 (en) | 1999-08-19 | 2001-06-26 | Lifecor, Inc. | Cardiac monitoring electrode apparatus and method |
| US20030095648A1 (en) | 1999-10-05 | 2003-05-22 | Lifecor, Inc. | Fault-tolerant remote reprogramming for a patient-worn medical device |
| US6681003B2 (en) | 1999-10-05 | 2004-01-20 | Lifecor, Inc. | Data collection and system management for patient-worn medical devices |
| US6442433B1 (en) | 1999-10-26 | 2002-08-27 | Medtronic, Inc. | Apparatus and method for remote troubleshooting, maintenance and upgrade of implantable device systems |
| US6406426B1 (en) | 1999-11-03 | 2002-06-18 | Criticare Systems | Medical monitoring and alert system for use with therapeutic devices |
| US6736759B1 (en)* | 1999-11-09 | 2004-05-18 | Paragon Solutions, Llc | Exercise monitoring system and methods |
| US6908437B2 (en) | 1999-11-16 | 2005-06-21 | Cardiac Intelligence Corporation | System and method for diagnosing and monitoring congestive heart failure for automated remote patient care |
| US20030216786A1 (en) | 1999-12-14 | 2003-11-20 | Joseph Russial | Circuit for producing an arbitrary defibrillation waveform |
| US20010031991A1 (en) | 1999-12-14 | 2001-10-18 | Joseph Russial | Circuit for producing an arbitrary defibrillation waveform |
| US6418346B1 (en) | 1999-12-14 | 2002-07-09 | Medtronic, Inc. | Apparatus and method for remote therapy and diagnosis in medical devices via interface systems |
| US6694191B2 (en) | 2000-01-21 | 2004-02-17 | Medtronic Minimed, Inc. | Ambulatory medical apparatus and method having telemetry modifiable control software |
| US20050131465A1 (en) | 2000-02-04 | 2005-06-16 | Freeman Gary A. | Integrated resuscitation |
| US20040007970A1 (en) | 2000-07-18 | 2004-01-15 | Kelvin Ma | Micro electro mechanical system controlled organic LED and pixel arrays and method of using and of manufacturing same |
| US20020077689A1 (en) | 2000-12-15 | 2002-06-20 | Kirkland Thomas Christopher | Electrode positioning bodysuit |
| JP2002200059A (en) | 2000-12-28 | 2002-07-16 | Terumo Corp | Body movement detector and medical instrument using the same and method of detecting body movement |
| US20070169364A1 (en) | 2001-02-23 | 2007-07-26 | Microstrain, Inc. | Posture and body movement measuring system |
| US6889078B2 (en) | 2001-04-26 | 2005-05-03 | Medtronic, Inc. | Hysteresis activation of accelerated pacing |
| US6804554B2 (en) | 2001-10-19 | 2004-10-12 | Medtronic, Inc. | Arrangement and system for enabling patient control of electrical therapies |
| EP1455640A2 (en) | 2001-10-26 | 2004-09-15 | Koninklijke Philips Electronics N.V. | Garment comprising medical sensors |
| US20100076513A1 (en) | 2001-11-21 | 2010-03-25 | Cameron Health, Inc. | Multiple Electrode Vectors for Implantable Cardiac Treatment Devices |
| US20030109904A1 (en) | 2001-12-10 | 2003-06-12 | Medtronic Physio-Control Manufacturing Corp. | Enhanced interface for a medical device and a terminal |
| US20030149462A1 (en) | 2002-02-04 | 2003-08-07 | White Sheldon S. | Medical electrodes |
| US20030158593A1 (en) | 2002-02-19 | 2003-08-21 | Heilman Marlin S. | Cardiac garment |
| US20030174049A1 (en) | 2002-03-18 | 2003-09-18 | Precision Dynamics Corporation | Wearable identification appliance that communicates with a wireless communications network such as bluetooth |
| US20030195567A1 (en) | 2002-04-10 | 2003-10-16 | Medtronic Physio-Control Corp. | Automated external defibrillator with user interface for adult and pediatric applications |
| US6990373B2 (en) | 2002-04-10 | 2006-01-24 | Medtronic Emergency Response Systems, Inc. | Automated external defibrillator with user interface for adult and pediatric applications |
| US6889079B2 (en) | 2002-04-12 | 2005-05-03 | Cardiac Pacemakers, Inc. | Method and system for characterizing supraventricular rhythm during cardiac pacing |
| US20030212311A1 (en) | 2002-05-07 | 2003-11-13 | Medtronic Physio-Control Manufacturing Corp. | Therapy-delivering portable medical device capable of triggering and communicating with an alarm system |
| US20080167535A1 (en) | 2002-08-22 | 2008-07-10 | Stivoric John M | Devices and systems for contextual and physiological-based reporting, entertainment, control of other devices, health assessment and therapy |
| US20040049233A1 (en) | 2002-09-11 | 2004-03-11 | Edwards D. Craig | Medical device status information system |
| US7991460B2 (en) | 2002-09-20 | 2011-08-02 | Angel Medical Systems, Inc. | Methods and apparatus for detecting cardiac events based on heart rate sensitive parameters |
| US7118542B2 (en) | 2002-10-25 | 2006-10-10 | Zoll Circulation, Inc. | Devices for determining depth of chest compressions during CPR |
| US6827695B2 (en) | 2002-10-25 | 2004-12-07 | Revivant Corporation | Method of determining depth of compressions during cardio-pulmonary resuscitation |
| US7476206B2 (en) | 2002-10-25 | 2009-01-13 | Zoll Circulation, Inc. | System for estimating the actual ECG of a patient during CPR |
| US7122014B2 (en) | 2002-10-25 | 2006-10-17 | Zoll Circulation, Inc. | Method of determining depth of chest compressions during CPR |
| WO2004054656A1 (en) | 2002-12-13 | 2004-07-01 | Koninklijke Philips Electronics N.V. | External defibrillator with shock activated by cessation of precordial compressions |
| US7149579B1 (en) | 2002-12-23 | 2006-12-12 | Pacesetter, Inc. | System and method for determining patient posture based on 3-D trajectory using an implantable medical device |
| US20040143297A1 (en) | 2003-01-21 | 2004-07-22 | Maynard Ramsey | Advanced automatic external defibrillator powered by alternative and optionally multiple electrical power sources and a new business method for single use AED distribution and refurbishment |
| WO2004067083A2 (en) | 2003-01-27 | 2004-08-12 | Cardiac Telecom, Corporation | Defibrillation system for non-medical environments |
| US20040162510A1 (en) | 2003-02-14 | 2004-08-19 | Medtronic Physio-Control Corp | Integrated external chest compression and defibrillation devices and methods of operation |
| US6961612B2 (en) | 2003-02-19 | 2005-11-01 | Zoll Medical Corporation | CPR sensitive ECG analysis in an automatic external defibrillator |
| WO2004078259A1 (en) | 2003-02-28 | 2004-09-16 | Medtronic Emergency Response Systems, Inc. | Recording information for emergency call by defibrillator apparatus |
| US20040249419A1 (en) | 2003-04-02 | 2004-12-09 | Chapman Fred William | Defibrillators customized for anticipated patients |
| US7488293B2 (en) | 2003-04-23 | 2009-02-10 | Zoll Medical Corporation | Processing pulse signal in conjunction with accelerometer signal in cardiac resuscitation |
| US20090018428A1 (en) | 2003-05-19 | 2009-01-15 | Umist Ventures Limited | Knitted transducer devices |
| US7831303B2 (en) | 2003-06-17 | 2010-11-09 | Medtronic, Inc. | Cardiac pacing apparatus and method for continuous capture management |
| US7220235B2 (en) | 2003-06-27 | 2007-05-22 | Zoll Medical Corporation | Method and apparatus for enhancement of chest compressions during CPR |
| US7130690B2 (en) | 2003-07-23 | 2006-10-31 | Medtronic, Inc. | Atrial capture management during atrial and ventricular pacing |
| US20050049515A1 (en) | 2003-07-31 | 2005-03-03 | Dale Julian Misczynski | Electrode belt for acquisition, processing and transmission of cardiac (ECG) signals |
| US20080183090A1 (en) | 2003-09-12 | 2008-07-31 | Jonathan Farringdon | Method and apparatus for measuring heart-related parameters and deriving human status parameters from sensed physiological and contextual parameters |
| US20050144043A1 (en) | 2003-10-07 | 2005-06-30 | Holland Geoffrey N. | Medication management system |
| US7453354B2 (en) | 2003-10-17 | 2008-11-18 | Koninklijke Philips Electronics, N.V. | Device arranged for carrying out a bioelectrical interaction with an individual and a method for on-demand lead-off detection |
| WO2005082454A1 (en) | 2004-02-19 | 2005-09-09 | Koninklijke Philips Electronics, N.V. | Method and apparatus for broadcasting audible information prompts from an external defibrillator |
| EP1720446A1 (en) | 2004-02-27 | 2006-11-15 | Koninklijke Philips Electronics N.V. | Wearable wireless device for monitoring, analyzing and communicating physiological status |
| US20070161913A1 (en) | 2004-03-31 | 2007-07-12 | Michael Farrell | Methods and apparatus for monitoring the cardiovascular condition of patients with sleep disordered breathing |
| US20050246199A1 (en) | 2004-05-03 | 2005-11-03 | Tom Futch | Health and wellness station |
| US20050283198A1 (en) | 2004-06-18 | 2005-12-22 | Haubrich Gregory J | Conditional requirements for remote medical device programming |
| US20070197878A1 (en) | 2004-07-09 | 2007-08-23 | Dror Shklarski | Wearable device, system and method for monitoring physiological and/or environmental parameters |
| US20060036292A1 (en) | 2004-08-16 | 2006-02-16 | Mitchell Smith | Risk of death indicator |
| US20090118808A1 (en) | 2004-09-23 | 2009-05-07 | Medtronic, Inc. | Implantable Medical Lead |
| US20080046015A1 (en) | 2004-09-30 | 2008-02-21 | Zoll Medical Corporation | Integrated Resuscitation |
| US20060085049A1 (en) | 2004-10-20 | 2006-04-20 | Nervonix, Inc. | Active electrode, bio-impedance based, tissue discrimination system and methods of use |
| WO2006050325A2 (en) | 2004-10-29 | 2006-05-11 | Worcester Polytechnic Institute | System and method for multi-channel electrophysiologic signal data acquisition |
| US20060095091A1 (en) | 2004-11-02 | 2006-05-04 | Medtronic, Inc. | Apparatus for data retention in an implantable medical device |
| US20090231124A1 (en) | 2004-11-12 | 2009-09-17 | Koninklijke Philips Electronics, N.V. | Method for automatic association devices to a patient and concurrent creation of a patient record |
| US20090138059A1 (en) | 2004-12-08 | 2009-05-28 | Koninklijke Philips Electronics N.V. | Heart Defibrillator With Contactless ECG Sensor For Diagnostics/Effectivity Feedback |
| US20060167505A1 (en) | 2005-01-26 | 2006-07-27 | Medtronic Emergency Response Systems, Inc. | Defibrillator with overridable CPR-first protocol |
| US20060178706A1 (en) | 2005-02-10 | 2006-08-10 | Lisogurski Daniel M | Monitoring physiological signals during external electrical stimulation |
| US20060211934A1 (en) | 2005-03-16 | 2006-09-21 | Textronics, Inc. | Textile-based electrode |
| US20060220809A1 (en) | 2005-03-21 | 2006-10-05 | Rf Monolithics, Inc. | System and method for monitoring use of vehicles such as golf carts |
| US20060270952A1 (en) | 2005-03-25 | 2006-11-30 | Freeman Gary A | Integrated resuscitation |
| US7340296B2 (en) | 2005-05-18 | 2008-03-04 | Cardiac Pacemakers, Inc. | Detection of pleural effusion using transthoracic impedance |
| WO2007019325A2 (en) | 2005-08-04 | 2007-02-15 | Access Cardiosystems, Inc. | Automatic external defibrillator (aed) with wireless communications |
| US20070073120A1 (en) | 2005-09-29 | 2007-03-29 | Li Li | System and method for pre-processing waveforms |
| US8121683B2 (en) | 2005-11-15 | 2012-02-21 | Metrax Gmbh | External automatic defibrillator |
| WO2007057169A1 (en) | 2005-11-15 | 2007-05-24 | Metrax Gmbh | External automatic defibrillator |
| US20070118056A1 (en) | 2005-11-18 | 2007-05-24 | Hua Wang | Posture detector calibration and use |
| US20080287749A1 (en) | 2005-11-23 | 2008-11-20 | Koninklijke Philips Electronics, N.V. | Method and Apparatus for Remote Patient Monitoring |
| US20070129769A1 (en) | 2005-12-02 | 2007-06-07 | Medtronic, Inc. | Wearable ambulatory data recorder |
| US20070143864A1 (en) | 2005-12-15 | 2007-06-21 | Symbol Technologies, Inc. | Methods and apparatus for power source authentication |
| US20070162390A1 (en) | 2005-12-22 | 2007-07-12 | Macrovision Corporation | Techniques for distributing and monitoring content |
| US7712373B2 (en) | 2006-03-03 | 2010-05-11 | Nagle H Troy | Sensor device for real-time monitoring or relative movement using capacitive fabric sensors |
| US20070239220A1 (en) | 2006-03-29 | 2007-10-11 | Greenhut Saul E | Implantable medical device system and method with signal quality monitoring and response |
| US20070239214A1 (en) | 2006-03-29 | 2007-10-11 | Can Cinbis | Method and system for aborting cardiac treatments |
| US20070265671A1 (en) | 2006-04-27 | 2007-11-15 | Roberts Jonathan P | Selectable switching of implantable sensors to provide fault toleance for implantable medical devices |
| US20100171611A1 (en) | 2006-04-28 | 2010-07-08 | Tia Gao | Sensor-Based Adaptive Methods for Wearable Devices |
| US20090318779A1 (en) | 2006-05-24 | 2009-12-24 | Bao Tran | Mesh network stroke monitoring appliance |
| US20080045815A1 (en) | 2006-06-20 | 2008-02-21 | Derchak P A | Automatic and ambulatory monitoring of congestive heart failure patients |
| US20080004536A1 (en) | 2006-06-30 | 2008-01-03 | Baxi Amit S | Method and apparatus for amplifying multiple signals using a single multiplexed amplifier channel with software controlled AC response |
| US20080021532A1 (en) | 2006-07-21 | 2008-01-24 | Kveen Graig L | Delivery of cardiac stimulation devices |
| US20100069735A1 (en) | 2006-07-29 | 2010-03-18 | Lior Berkner | Device for mobile electrocardiogram recording |
| US20080030656A1 (en) | 2006-08-01 | 2008-02-07 | 3M Innovative Properties Company | Transflective lc display with internal reflector and reflective polarizer |
| US20080033495A1 (en) | 2006-08-01 | 2008-02-07 | Kumar Uday N | External Defibrillator |
| US8102842B2 (en) | 2006-08-04 | 2012-01-24 | Broadcom Corporation | Integrated switch |
| US20080058884A1 (en) | 2006-08-29 | 2008-03-06 | Matos Jeffrey A | Control of a defibrillator and/or pacemaker |
| US20090281394A1 (en) | 2006-09-25 | 2009-11-12 | Brian Keith Russell | Bio-mechanical sensor system |
| US20080097793A1 (en) | 2006-10-24 | 2008-04-24 | Kent Dicks | Systems and methods for remote patient monitoring and user interface |
| US20080177341A1 (en) | 2006-10-27 | 2008-07-24 | Bowers Kyle R | Automated external defibrillator (AED) system with multiple patient wireless monitoring capability for use in mass casualty incidents |
| US20080103402A1 (en) | 2006-10-30 | 2008-05-01 | Medtronic Emergency Response Systems, Inc. | Motion detection system for an external defibrillator |
| US20090093687A1 (en) | 2007-03-08 | 2009-04-09 | Telfort Valery G | Systems and methods for determining a physiological condition using an acoustic monitor |
| US20100081962A1 (en) | 2007-03-19 | 2010-04-01 | Omron Healthcare Co., Ltd | Visceral fat measurement device |
| US20080249591A1 (en) | 2007-04-06 | 2008-10-09 | Northstar Neuroscience, Inc. | Controllers for implantable medical devices, and associated methods |
| US20080294019A1 (en) | 2007-05-24 | 2008-11-27 | Bao Tran | Wireless stroke monitoring |
| US8774917B2 (en) | 2007-06-06 | 2014-07-08 | Zoll Medical Corporation | Wearable defibrillator with audio input/output |
| US20130144355A1 (en) | 2007-06-06 | 2013-06-06 | Zoll Medical Corporation | Wearable defibrillator with audio input/output |
| US20080306560A1 (en) | 2007-06-06 | 2008-12-11 | Macho John D | Wearable defibrillator with audio input/output |
| US8965500B2 (en) | 2007-06-06 | 2015-02-24 | Zoll Medical Corporation | Wearable defibrillator with audio input/output |
| US20150148857A1 (en) | 2007-06-06 | 2015-05-28 | Zoll Medical Corporation | Wearable defibrillator with audio input/output |
| US8369944B2 (en) | 2007-06-06 | 2013-02-05 | Zoll Medical Corporation | Wearable defibrillator with audio input/output |
| US8271082B2 (en) | 2007-06-07 | 2012-09-18 | Zoll Medical Corporation | Medical device configured to test for user responsiveness |
| US20130013014A1 (en) | 2007-06-07 | 2013-01-10 | Zoll Medical Corporation | Medical device configured to test for user responsiveness |
| US20100298899A1 (en) | 2007-06-13 | 2010-11-25 | Donnelly Edward J | Wearable medical treatment device |
| US8140154B2 (en) | 2007-06-13 | 2012-03-20 | Zoll Medical Corporation | Wearable medical treatment device |
| US20120197353A1 (en) | 2007-06-13 | 2012-08-02 | Zoll Medical Corporation | Wearable medical treatment device |
| US20100312297A1 (en) | 2007-06-13 | 2010-12-09 | Zoll Medical Corporation | Wearable medical treatment device with motion/position detection |
| US7974689B2 (en) | 2007-06-13 | 2011-07-05 | Zoll Medical Corporation | Wearable medical treatment device with motion/position detection |
| US20080312709A1 (en) | 2007-06-13 | 2008-12-18 | Volpe Shane S | Wearable medical treatment device with motion/position detection |
| US20080312520A1 (en) | 2007-06-15 | 2008-12-18 | Gordon Ian Rowlandson | Method and apparatus for acquiring physiological data |
| US20090212984A1 (en) | 2007-06-15 | 2009-08-27 | Micron Technology, Inc. | Quantizing circuits with variable parameters |
| US20080312522A1 (en) | 2007-06-15 | 2008-12-18 | Gordon Ian Rowlandson | System and apparatus for collecting physiological signals from a plurality of electrodes |
| US20100076533A1 (en) | 2007-08-23 | 2010-03-25 | Amit Dar | System for transmitting electrical current to a bodily tissue |
| WO2009034506A1 (en) | 2007-09-12 | 2009-03-19 | Koninklijke Philips Electronics, N.V. | Remote status indicator for a defibrillator |
| US20090066366A1 (en) | 2007-09-12 | 2009-03-12 | Solomon Research Llc | Reprogrammable three dimensional intelligent system on a chip |
| US20090076364A1 (en) | 2007-09-14 | 2009-03-19 | Corventis, Inc. | Adherent Device for Sleep Disordered Breathing |
| US20090076340A1 (en) | 2007-09-14 | 2009-03-19 | Corventis, Inc. | Adherent Cardiac Monitor with Advanced Sensing Capabilities |
| US20090076397A1 (en) | 2007-09-14 | 2009-03-19 | Corventis, Inc. | Adherent Emergency Patient Monitor |
| US20090076345A1 (en) | 2007-09-14 | 2009-03-19 | Corventis, Inc. | Adherent Device with Multiple Physiological Sensors |
| US20090076346A1 (en) | 2007-09-14 | 2009-03-19 | Corventis, Inc. | Tracking and Security for Adherent Patient Monitor |
| US20090076410A1 (en) | 2007-09-14 | 2009-03-19 | Corventis, Inc. | System and Methods for Wireless Body Fluid Monitoring |
| US20090076348A1 (en) | 2007-09-14 | 2009-03-19 | Corventis, Inc. | Injectable Device for Physiological Monitoring |
| US20090076342A1 (en) | 2007-09-14 | 2009-03-19 | Corventis, Inc. | Adherent Multi-Sensor Device with Empathic Monitoring |
| US20090076559A1 (en) | 2007-09-14 | 2009-03-19 | Corventis, Inc. | Adherent Device for Cardiac Rhythm Management |
| US20090076350A1 (en) | 2007-09-14 | 2009-03-19 | Corventis, Inc. | Data Collection in a Multi-Sensor Patient Monitor |
| US20090076343A1 (en) | 2007-09-14 | 2009-03-19 | Corventis, Inc. | Energy Management for Adherent Patient Monitor |
| US20090076344A1 (en) | 2007-09-14 | 2009-03-19 | Corventis, Inc. | Multi-Sensor Patient Monitor to Detect Impending Cardiac Decompensation |
| US20090076336A1 (en) | 2007-09-14 | 2009-03-19 | Corventis, Inc. | Medical Device Automatic Start-up Upon Contact to Patient Tissue |
| US20090076349A1 (en) | 2007-09-14 | 2009-03-19 | Corventis, Inc. | Adherent Multi-Sensor Device with Implantable Device Communication Capabilities |
| US20090076363A1 (en) | 2007-09-14 | 2009-03-19 | Corventis, Inc. | Adherent Device with Multiple Physiological Sensors |
| US20090076405A1 (en) | 2007-09-14 | 2009-03-19 | Corventis, Inc. | Adherent Device for Respiratory Monitoring |
| US20090076341A1 (en) | 2007-09-14 | 2009-03-19 | Corventis, Inc. | Adherent Athletic Monitor |
| US20090073991A1 (en) | 2007-09-14 | 2009-03-19 | Corventis, Inc. | Dynamic Pairing of Patients to Data Collection Gateways |
| US20090146822A1 (en) | 2007-11-13 | 2009-06-11 | Elevate Technologies Pty Ltd. | Telemedicine Application for Remote Monitoring, Viewing and Updating of Patient Records |
| US20110015533A1 (en) | 2007-11-26 | 2011-01-20 | C.R. Bard, Inc. | Stylets for use with apparatus for intravascular placement of a catheter |
| US20090287120A1 (en) | 2007-12-18 | 2009-11-19 | Searete Llc, A Limited Liability Corporation Of The State Of Delaware | Circulatory monitoring systems and methods |
| US20090234410A1 (en) | 2008-03-12 | 2009-09-17 | Corventis, Inc. | Heart Failure Decompensation Prediction Based on Cardiac Rhythm |
| US20090232286A1 (en) | 2008-03-14 | 2009-09-17 | Gigle Semiconductor, Ltd. | Coupling signal processing circuitry with a wireline communications medium |
| US20090264792A1 (en) | 2008-04-18 | 2009-10-22 | Corventis, Inc. | Method and Apparatus to Measure Bioelectric Impedance of Patient Tissue |
| US20090275848A1 (en) | 2008-04-30 | 2009-11-05 | Transoma Medical, Inc. | Cardiac Risk Assessment |
| US20090292194A1 (en) | 2008-05-23 | 2009-11-26 | Corventis, Inc. | Chiropractic Care Management Systems and Methods |
| US20090295326A1 (en) | 2008-06-02 | 2009-12-03 | Physio-Control, Inc. | Defibrillator Battery Authentication System |
| US20090307266A1 (en) | 2008-06-06 | 2009-12-10 | Apple Inc. | Processing a page |
| US20100010559A1 (en) | 2008-07-09 | 2010-01-14 | Cheng Zhang | Event-based battery monitor for implantable devices |
| US20100052897A1 (en) | 2008-08-27 | 2010-03-04 | Allen Paul G | Health-related signaling via wearable items |
| US20100052892A1 (en) | 2008-08-27 | 2010-03-04 | Searete Llc, A Limited Liability Corporation Of The State Of Delaware | Health-related signaling via wearable items |
| US20100056881A1 (en) | 2008-08-29 | 2010-03-04 | Corventis, Inc. | Method and Apparatus For Acute Cardiac Monitoring |
| WO2010025432A1 (en) | 2008-08-31 | 2010-03-04 | Abbott Diabetes Care Inc. | Closed loop control with improved alarm functions |
| US20100114243A1 (en) | 2008-10-30 | 2010-05-06 | Medtronic, Inc. | Preselector interference rejection and dynamic range extension |
| JP5115450B2 (en) | 2008-11-04 | 2013-01-09 | 沖電気工業株式会社 | Optical code division multiplexing signal generator |
| WO2010077997A2 (en) | 2008-12-16 | 2010-07-08 | Bodymedia, Inc. | Method and apparatus for determining heart rate variability using wavelet transformation |
| US20100234716A1 (en) | 2009-03-12 | 2010-09-16 | Corventis, Inc. | Method and Apparatus for Monitoring Fluid Content within Body Tissues |
| US20100241181A1 (en) | 2009-03-17 | 2010-09-23 | Walter T. Savage | External defibrillator |
| US20100295674A1 (en) | 2009-05-21 | 2010-11-25 | Silverplus, Inc. | Integrated health management console |
| US20110172550A1 (en) | 2009-07-21 | 2011-07-14 | Michael Scott Martin | Uspa: systems and methods for ems device communication interface |
| US20110093840A1 (en) | 2009-10-21 | 2011-04-21 | Micro Power Electronics, Inc. | Patches for battery-interfacing devices and associated systems and methods |
| US20110098765A1 (en) | 2009-10-27 | 2011-04-28 | Medtronic, Inc. | Detecting lead related condition during delivery of therapeutic electrical signals |
| US20110170692A1 (en) | 2009-11-06 | 2011-07-14 | Roche Diagnostics International Ltd. | Method And System For Establishing Cryptographic Communications Between A Remote Device And A Medical Device |
| US20110288605A1 (en) | 2010-05-18 | 2011-11-24 | Zoll Medical Corporation | Wearable ambulatory medical device with multiple sensing electrodes |
| US20110288604A1 (en) | 2010-05-18 | 2011-11-24 | Kaib Thomas E | Wearable therapeutic device |
| US20120011382A1 (en) | 2010-07-09 | 2012-01-12 | Shane Volpe | System and method for conserving power in a medical device |
| US20120016361A1 (en) | 2010-07-13 | 2012-01-19 | White Sheldon S | Deposit ablation within and external to circulatory systems |
| US20120053479A1 (en) | 2010-08-25 | 2012-03-01 | Angel Medical Systems, Inc. | Acute ischemia detection based on parameter value range analysis |
| US20120112903A1 (en) | 2010-11-08 | 2012-05-10 | Zoll Medical Corporation | Remote medical device alarm |
| US8406842B2 (en) | 2010-12-09 | 2013-03-26 | Zoll Medical Corporation | Electrode with redundant impedance reduction |
| US20120150008A1 (en) | 2010-12-09 | 2012-06-14 | Kaib Thomas E | Electrode with redundant impedance reduction |
| US20120146797A1 (en) | 2010-12-10 | 2012-06-14 | Emil Oskin | Wearable therapeutic device |
| US20120158075A1 (en) | 2010-12-16 | 2012-06-21 | Kaib Thomas E | Water resistant wearable medical device |
| US20120293323A1 (en) | 2011-03-25 | 2012-11-22 | Zoll Medical Corporation | System and method for adapting alarms in a wearable medical device |
| US20120289809A1 (en) | 2011-03-25 | 2012-11-15 | Zoll Medical Corporation | Method of detecting signal clipping in a wearable ambulatory medical device |
| US20120283794A1 (en) | 2011-05-02 | 2012-11-08 | Kaib Thomas E | Patient-worn energy delivery apparatus and techniques for sizing same |
| US20130085538A1 (en) | 2011-09-01 | 2013-04-04 | Zoll Medical Corporation | Wearable monitoring and treatment device |
| US20130231711A1 (en) | 2012-03-02 | 2013-09-05 | Thomas E. Kaib | Systems and methods for configuring a wearable medical monitoring and/or treatment device |
Non-Patent Citations (6)
| Title |
|---|
| American Journal of Respiratory and Critical Care Medicine, vol. 166, pp. 111-117 (2002), American Thoracic Society, ATS Statement: Guidelines for the Six-Minute Walk Test, available at http://ajrccm.atsjournals.org/cgi/content/full/166/1/111. |
| DeBock, et al., "Captopril treatment of chronic heart failure in the very old," J. Gerontol. (1994) 49: M148-M152. |
| Herlihy et al. The Art of Multiple Processor Programming. Chapter 1, p. 1. Mar. 3, 2008. |
| http://web.archive.org/web/20030427001846/http:/www.lifecor.com/imagelib/i- mageproduct.asp. Published by LifeCor, Inc., 2002, on a webpage owned by LifeCor, Inc. |
| O'Keeffe et al., "Reproducability and responsiveness of quality of life assessment and six minute walk test in elderly heart failure patients," Heart (1998) 80: 377-382. |
| Wikipedia , Multi-core processor, Dec. 11, 2009, http://web.archive.org/web/20091211134408/http://en.wikipedia.org/wiki/Mu- lticore_processor#Hardware. |
Also Published As
| Publication number | Publication date |
|---|---|
| US8369944B2 (en) | 2013-02-05 |
| JP2016137321A (en) | 2016-08-04 |
| US20130144355A1 (en) | 2013-06-06 |
| US20250090841A1 (en) | 2025-03-20 |
| US20190374767A1 (en) | 2019-12-12 |
| US10029110B2 (en) | 2018-07-24 |
| JP5467733B2 (en) | 2014-04-09 |
| US20140324112A1 (en) | 2014-10-30 |
| US9492676B2 (en) | 2016-11-15 |
| US10004893B2 (en) | 2018-06-26 |
| US20080306560A1 (en) | 2008-12-11 |
| US20170021186A1 (en) | 2017-01-26 |
| US20150148857A1 (en) | 2015-05-28 |
| US10426946B2 (en) | 2019-10-01 |
| DE102008026871B4 (en) | 2019-04-25 |
| US12138444B2 (en) | 2024-11-12 |
| DE102008026871A1 (en) | 2008-12-11 |
| JP5932856B2 (en) | 2016-06-08 |
| US20180304068A1 (en) | 2018-10-25 |
| US20170189672A1 (en) | 2017-07-06 |
| FR2916981B1 (en) | 2013-07-05 |
| US8774917B2 (en) | 2014-07-08 |
| US8965500B2 (en) | 2015-02-24 |
| JP2014111158A (en) | 2014-06-19 |
| JP2008302225A (en) | 2008-12-18 |
| US20210322761A1 (en) | 2021-10-21 |
| FR2916981A1 (en) | 2008-12-12 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| US12138444B2 (en) | Wearable defibrillator with audio input/output | |
| US12109004B2 (en) | Remote access for ambulatory medical device | |
| EP2475426B1 (en) | Automated external defibrillator device with integrated mobile station modem | |
| US20040073094A1 (en) | Fetal monitoring systems with ambulatory patient units and telemetric links for improved uses | |
| JP2011519583A (en) | Mobile phone terminal with cover for ECG monitoring system | |
| JP2008302228A (en) | A medical device configured to verify user responsiveness | |
| JP2011516110A (en) | Continuous outpatient ECG monitoring system | |
| EP4057295A1 (en) | Method for establishing a communication path between a subject and a medical system | |
| Palmisano et al. | Effectiveness of Implantable DEfibrillators Alert Systems: comparison between audible and vibratory alert: IDEAS study | |
| US20250316187A1 (en) | Alert button training system for a wearable medical device and method thereof | |
| US20240024194A1 (en) | Cardiopulmonary resuscitation decision support | |
| US20250316354A1 (en) | Systems and Methods for Voice Activation and Annotation of Medical Records | |
| KR20100042676A (en) | Mobile terminal performing automatic defibrillation and emergency saving system using the same |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| FEPP | Fee payment procedure | Free format text:ENTITY STATUS SET TO UNDISCOUNTED (ORIGINAL EVENT CODE: BIG.); ENTITY STATUS OF PATENT OWNER: LARGE ENTITY | |
| AS | Assignment | Owner name:ZOLL MEDICAL CORPORATION, MASSACHUSETTS Free format text:ASSIGNMENT OF ASSIGNORS INTEREST;ASSIGNORS:MACHO, JOHN D;VOLPE, SHANE S;RATTANNI, RICHARD A;AND OTHERS;REEL/FRAME:050085/0056 Effective date:20080407 | |
| STPP | Information on status: patent application and granting procedure in general | Free format text:APPLICATION DISPATCHED FROM PREEXAM, NOT YET DOCKETED | |
| STPP | Information on status: patent application and granting procedure in general | Free format text:DOCKETED NEW CASE - READY FOR EXAMINATION | |
| STPP | Information on status: patent application and granting procedure in general | Free format text:NON FINAL ACTION MAILED | |
| STPP | Information on status: patent application and granting procedure in general | Free format text:RESPONSE TO NON-FINAL OFFICE ACTION ENTERED AND FORWARDED TO EXAMINER | |
| STPP | Information on status: patent application and granting procedure in general | Free format text:NOTICE OF ALLOWANCE MAILED -- APPLICATION RECEIVED IN OFFICE OF PUBLICATIONS | |
| STPP | Information on status: patent application and granting procedure in general | Free format text:PUBLICATIONS -- ISSUE FEE PAYMENT RECEIVED | |
| STPP | Information on status: patent application and granting procedure in general | Free format text:PUBLICATIONS -- ISSUE FEE PAYMENT VERIFIED | |
| STCF | Information on status: patent grant | Free format text:PATENTED CASE | |
| MAFP | Maintenance fee payment | Free format text:PAYMENT OF MAINTENANCE FEE, 4TH YEAR, LARGE ENTITY (ORIGINAL EVENT CODE: M1551); ENTITY STATUS OF PATENT OWNER: LARGE ENTITY Year of fee payment:4 |