US11033291B2 - Surgical device for use in endoscopic carpal tunnel release (ECTR), endoscopic cubital tunnel release (ECuTR), and endoscopic plantar fasciitis release (EPFR) - Google Patents
Surgical device for use in endoscopic carpal tunnel release (ECTR), endoscopic cubital tunnel release (ECuTR), and endoscopic plantar fasciitis release (EPFR)Download PDFInfo
- Publication number
- US11033291B2 US11033291B2US15/282,839US201615282839AUS11033291B2US 11033291 B2US11033291 B2US 11033291B2US 201615282839 AUS201615282839 AUS 201615282839AUS 11033291 B2US11033291 B2US 11033291B2
- Authority
- US
- United States
- Prior art keywords
- prongs
- surgical guide
- endoscopic
- tool
- release
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Active, expires
Links
Images
Classifications
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61B—DIAGNOSIS; SURGERY; IDENTIFICATION
- A61B17/00—Surgical instruments, devices or methods
- A61B17/32—Surgical cutting instruments
- A61B17/320016—Endoscopic cutting instruments, e.g. arthroscopes, resectoscopes
- A61B17/320036—Endoscopic cutting instruments, e.g. arthroscopes, resectoscopes adapted for use within the carpal tunnel
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61B—DIAGNOSIS; SURGERY; IDENTIFICATION
- A61B17/00—Surgical instruments, devices or methods
- A61B2017/0023—Surgical instruments, devices or methods disposable
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61B—DIAGNOSIS; SURGERY; IDENTIFICATION
- A61B17/00—Surgical instruments, devices or methods
- A61B17/32—Surgical cutting instruments
- A61B2017/320052—Guides for cutting instruments
Definitions
- Endoscopic Carpal Tunnel Release(ECTR), Endoscopic Cupital Tunnel Release (ECuTR), and Endoscopic Plantar Fasciitis Release (EPFR) are three surgical procedures used to relieve symptoms in the hand, elbow, and heel, respectively.
- the surgeonmakes a small incision and inserts a thin tube called an endoscope with a tiny camera attached to it to view the affected area.
- the surgeonthen inserts a cutting instrument through this same, single portal to perform the procedure.
- the benefit of endoscopic proceduresis that they require smaller incisions, leading to the diminution of early post-operative pain, decreasing the amount of recovery time, and expediting patients' return to regular activity.
- the device described hereinis a disposable, sterile guide constructed of medically-acceptable plastic used for compartmentalizing and therefore protecting the ligament or fascia during three different orthopedic surgical procedures: ECTR, ECuTR, and EPFR.
- This devicereduces the risk of damage to any other part of the surrounding anatomy.
- the fact that the device is disposable and packaged so as to be sterile and therefore readily usable by the surgeonmeans that it can reduce the risk of infection and is a less expensive alternative to traditional non-disposable, metal instruments that must be sterilized before each procedure.
- FIG. 1shows a dilator used to prepare a surgical area for the device.
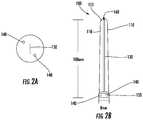
- FIGS. 2A and 2Bshow side and elevation views of the device.
- FIGS. 3A-3Dshow an alternate embodiment of the device.
- FIGS. 4A-4Cshow a further alternate embodiment of the device.
- FIG. 5shows another alternate embodiment of the device.
- FIGS. 6A and 6Bshow blade and tool variants used with the device.
- Endoscopic Carpal Tunnel ReleaseHand
- Endoscopic Cubital Tunnel ReleaseElbow
- Endoscopic Plantar Fasciitis ReleaseHeel
- the surgeonmay use a dilator 10 ( FIG. 1 ) to dilate the surgical area.
- the dilatormay be about 6 inches long and taper at a broader end 14 to a narrow end 12 from 6 mm to 4 mm.
- the dilatormay include hand grips 16 marked to minimize slipping.
- the surgeonmay insert the device 100 , 300 , 400 in order to compartmentalize the ligament or fascia.
- the surgeonmay then use the endoscope that has been inserted through a cameral passage or hole to visualize the ligament or fascia area to ensure that there are no other parts of the anatomy, such as nerves or tendons, obstructing the incision path.
- the surgeoncan either antegrade cut or retrograde cut the ligament or fascia in a safe environment by inserting the knife through the appropriate slot, because the device or guide has helped the surgeon to compartmentalize the ligament or fascia to be incised, isolating it from other parts of the anatomy that could otherwise be in jeopardy of being inadvertently cut.
- the endoscopic camera and the knifemay work independently of each other inside the guide, making it safer for the surgeon to look ahead of the knife when needed.
- FIG. 1is a drawing of the surgical device 100 for assistance in endoscopic surgical procedures, especially those discussed above but not necessarily limited thereto.
- the surgical device 100may be 100 mm in length, 8 mm wide, and constructed from ABS plastic.
- the device 100may be hollow and cylindrical with 3 mm-thick plastic prongs 110 separated by a 2 mm gap 130 .
- FIG. 2shows one end of the device 100 , which is closed with the exception of three holes: the upper and lower circular holes 140 may be each 2.5 mm in diameter, may be used for the endoscopic camera, and may be located on either side of the 5 mm long slot 150 used for the knife.
- the location of the upper and lower circular holes 140 on either side of the slot 150gives the surgeon the ability to use the endoscopic camera to visualize the surgical field more comprehensively from many angles before a cut is made to avoid damaging other portions of the anatomy.
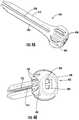
- FIGS. 6A and 6Bshow tools like the knife ( 600 ) and wire 605 for use with the device 100 , 300 , or 400 .
- a surgeonmay grasp the knife 600 or wire 605 by a handle 610 that may include finger cutouts for a thumb 612 and fingers 614 .
- the tool 600 , 605may comprise both a handle portion 610 and working portion 620 .
- the working portion 620is sized to fit within the slot 150 , 380 and includes a blade 630 for performing the incisions as the blade moves within the slot 150 , 380 .
- the wire tool 605operates similarly to the knife tool 600 except that its working end 620 includes a narrow wire end 635 for removing fine tissue or moving a nerve out of the way of a later incision by the knife tool 600 .
- the wire end 635may extend in any direction (upwards towards the viewer as shown in FIG. 6B being on alternative) but importantly fits within the knife slot.
- the guide device 100may include a wedge-shaped protrusion 160 at a terminal end of one (or both) of the prongs 110 that may help in clearing tissue from within the gap 130 .
- the wedgemay be sharp, extend only from the narrow terminal end 115 of the prongs 110 or extend across the width of the prong 110 .
- FIGS. 3A-3Eshow an alternate embodiment of the device from FIG. 2 .
- the device 300includes prongs 310 separated by a gap 330 similar to the geometry of FIGS. 1 and 2 .
- the device 300 's prongs 310have a narrow terminal end 315 opposite a head portion 320 having finger cutouts 340 that in combination help in grasping the device 300 .
- This head portion 320helps in device 300 insertion into the patient as well as removal, and also positioning the device 300 during surgery.
- the head portion end face 325has a tool opening 350 therein.
- the tool opening 350passes through the head portion 320 and is in fluid communication with the gap 330 .
- the tool openingmay include a camera opening 360 and a blade slot 380 separated by an open space 370 that allows for a small tool insertion to remove unwanted tissue or other waste from the scope or camera opening.
- the camera openingis for scope insertion, and allows the surgeon to inspect the incision, ensure the area to be incised is clear of nerves, and generally allow the surgeon to see the work to be performed.
- the cameramay travel within one of the prongs 310 within a camera groove 312 formed along and within each prong 310 .
- a second groove 314may also include room for the tools 600 , 605 .
- the guide device 400 in FIGS. 4A-Cis similar to the one in FIGS. 3A-3D except for finger grooves 490 .
- These finger grooves 490extend into the head portion 420 and serve two purposes: First, they act to help a surgeon grasp the guide 400 during insertion, when slipping tools can be a problem. Second the finger grooves 490 help with cooling the device 400 during manufacture, allowing for uniform cooling and thus, decrease defect formation.
- FIG. 5shows a further alternate design of the device 500 .
- a shelf 565extends to divide the camera opening halves 560 a, and 560 b. This helps support the scope when inserted into the camera openings.
- the device guide described hereinmay be shipped in sterile packaging to ensure sterility in use, which overcomes the issues with certain steel guides that must be sterilized on each use. Because it is plastic, the device may be discarded after use and easily replaced, thus making it less expensive than a stainless steel tool but also safer.
- the devicemay encompass the transverse ligament therefore avoiding the challenge of synovium and fat dropping into view when cutting the ligament. This improves visibility because the surgeon isn't cutting underneath the ligament but encapsulating the ligament and cutting either antegrade or retrograde and seeing the ligament with a top view as well as bottom view while cutting.
- top and bottom portions of the guideencompasses the ligament and that makes the guide safer for ECTR.
- the guideis as a unitary construction molded in plastic, although it is possible to 3D print the guide as well. Multi-piece construction is possible and may be advantageous in certain contexts.
- the deviceis also made to accommodate both left and right hand for same procedure by just turning it upside down to always cut on the ulnar safe side of the hand, which is the ulnar side of anatomy.
Landscapes
- Health & Medical Sciences (AREA)
- Surgery (AREA)
- Life Sciences & Earth Sciences (AREA)
- Biomedical Technology (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Engineering & Computer Science (AREA)
- Orthopedic Medicine & Surgery (AREA)
- Heart & Thoracic Surgery (AREA)
- Medical Informatics (AREA)
- Molecular Biology (AREA)
- Animal Behavior & Ethology (AREA)
- General Health & Medical Sciences (AREA)
- Public Health (AREA)
- Veterinary Medicine (AREA)
- Surgical Instruments (AREA)
Abstract
Description
Claims (12)
Priority Applications (4)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US15/282,839US11033291B2 (en) | 2015-10-02 | 2016-09-30 | Surgical device for use in endoscopic carpal tunnel release (ECTR), endoscopic cubital tunnel release (ECuTR), and endoscopic plantar fasciitis release (EPFR) |
| US16/001,887US10806481B2 (en) | 2015-10-02 | 2018-06-06 | Surgical device for use in endoscopic carpal tunnel release (ECTR), endoscopic cubital tunnel release (ECuTR), and endoscopic plantar fasciitis release (EPFR) |
| US17/067,710US12232759B2 (en) | 2015-10-02 | 2020-10-11 | Surgical device for use in endoscopic carpal tunnel release (ECTR), endoscopic cubital tunnel release (ECuTR), and endoscopic plantar fasciitis release (EPFR) |
| US17/823,549US12318106B2 (en) | 2015-10-02 | 2022-08-31 | Method for using a surgical device to transect a transverse carpal ligament |
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US201562236728P | 2015-10-02 | 2015-10-02 | |
| US15/282,839US11033291B2 (en) | 2015-10-02 | 2016-09-30 | Surgical device for use in endoscopic carpal tunnel release (ECTR), endoscopic cubital tunnel release (ECuTR), and endoscopic plantar fasciitis release (EPFR) |
Related Child Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US16/001,887Continuation-In-PartUS10806481B2 (en) | 2015-10-02 | 2018-06-06 | Surgical device for use in endoscopic carpal tunnel release (ECTR), endoscopic cubital tunnel release (ECuTR), and endoscopic plantar fasciitis release (EPFR) |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| US20170095266A1 US20170095266A1 (en) | 2017-04-06 |
| US11033291B2true US11033291B2 (en) | 2021-06-15 |
Family
ID=58446526
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US15/282,839Active2037-09-12US11033291B2 (en) | 2015-10-02 | 2016-09-30 | Surgical device for use in endoscopic carpal tunnel release (ECTR), endoscopic cubital tunnel release (ECuTR), and endoscopic plantar fasciitis release (EPFR) |
Country Status (1)
| Country | Link |
|---|---|
| US (1) | US11033291B2 (en) |
Cited By (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US12318106B2 (en) | 2015-10-02 | 2025-06-03 | Nanice Medical Solutions, LLC | Method for using a surgical device to transect a transverse carpal ligament |
| USD1086449S1 (en)* | 2015-03-25 | 2025-07-29 | Orthocision Inc. | Surgical cannula |
| USD1088224S1 (en) | 2013-03-15 | 2025-08-12 | Orthocision Inc. | Surgical cannula |
Citations (22)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US5507800A (en) | 1993-05-14 | 1996-04-16 | Strickland; James W. | Carpal tunnel tome and carpal tunnel release surgery |
| US5827311A (en) | 1997-05-08 | 1998-10-27 | Biomet Inc | Carpal tunnel tome |
| US5908431A (en) | 1995-12-15 | 1999-06-01 | Battenfield; Harold L. | Carpal tunnel surgery instruments |
| US6283948B1 (en) | 1999-07-13 | 2001-09-04 | Ethicon, Inc. | Trocar obturator having grooved passageway |
| US20050159730A1 (en)* | 2004-01-20 | 2005-07-21 | Kathrani Biten K. | Method for accessing an operative space |
| US20080200758A1 (en)* | 2007-02-09 | 2008-08-21 | Skeletal Dynamics, Inc. | Endo-surgical Device and Method |
| US20100280368A1 (en) | 2007-08-27 | 2010-11-04 | Technische Universitat Munchen | Trocar tube, Trocar, Obturator and/or Rectoscope for the Transluminal Endoscopic Surgery Via Natural Body Orifices |
| US8252011B1 (en)* | 2003-04-21 | 2012-08-28 | Osteomed Llc | Minimally invasive technique for performing plantar fasciotomies and surgical instrument for use in such a technique |
| US8523892B2 (en) | 2006-06-08 | 2013-09-03 | Recon Surgical, Inc. | Instruments and methods for minimally invasive carpal tunnel release |
| US20140066709A1 (en)* | 2012-09-04 | 2014-03-06 | A.M. Surgical, Inc. | Compact endoscopic surgical blade assembly and method of use thereof |
| US8672960B2 (en)* | 2010-07-14 | 2014-03-18 | SEG-Way Orthopaedics, Inc | Method and apparatus for endoscopic ligament release |
| US20140121456A1 (en) | 2009-08-07 | 2014-05-01 | Thayer Intellectual Property, Inc. | Systems and methods for treatment of compressed nerves |
| US8852191B2 (en) | 2011-06-20 | 2014-10-07 | Surgenco, Llc | Cutting guide and method for performing lateral retinacular release |
| US8882772B2 (en) | 2005-07-29 | 2014-11-11 | Vertos Medical, Inc. | Percutaneous tissue excision devices and methods |
| US20140371526A1 (en) | 2009-03-09 | 2014-12-18 | A.M. Surgical, Inc. | Slotted clear cannula |
| US8951273B1 (en) | 2009-09-25 | 2015-02-10 | Mike Fard | Surgical instrument for endoscopic surgical procedures |
| US20150272617A1 (en)* | 2014-03-17 | 2015-10-01 | Arthroptics, L.L.C. | Cannula assembly |
| WO2015171785A1 (en) | 2014-05-06 | 2015-11-12 | Agee, John M. And Karen K., Trustees Of The John M. Agee Trust | Method and apparatus for treatment of cts using endoscopic carpal tunnel release |
| US20160157881A1 (en) | 2010-07-14 | 2016-06-09 | Stuart Seymour | Method and Apparatus for Endoscopic Ligament Release |
| US20160192828A1 (en)* | 2015-01-07 | 2016-07-07 | Nicholes Sexton | Retractor cannula and method of use |
| US20160287322A1 (en)* | 2013-11-13 | 2016-10-06 | Thixos Llc | Devices, kits and methods relating to treatment of facet joints |
| US20160345998A1 (en)* | 2013-11-27 | 2016-12-01 | Segway Orthopaedics, Inc. | Surgical Guide |
- 2016
- 2016-09-30USUS15/282,839patent/US11033291B2/enactiveActive
Patent Citations (22)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US5507800A (en) | 1993-05-14 | 1996-04-16 | Strickland; James W. | Carpal tunnel tome and carpal tunnel release surgery |
| US5908431A (en) | 1995-12-15 | 1999-06-01 | Battenfield; Harold L. | Carpal tunnel surgery instruments |
| US5827311A (en) | 1997-05-08 | 1998-10-27 | Biomet Inc | Carpal tunnel tome |
| US6283948B1 (en) | 1999-07-13 | 2001-09-04 | Ethicon, Inc. | Trocar obturator having grooved passageway |
| US8252011B1 (en)* | 2003-04-21 | 2012-08-28 | Osteomed Llc | Minimally invasive technique for performing plantar fasciotomies and surgical instrument for use in such a technique |
| US20050159730A1 (en)* | 2004-01-20 | 2005-07-21 | Kathrani Biten K. | Method for accessing an operative space |
| US8882772B2 (en) | 2005-07-29 | 2014-11-11 | Vertos Medical, Inc. | Percutaneous tissue excision devices and methods |
| US8523892B2 (en) | 2006-06-08 | 2013-09-03 | Recon Surgical, Inc. | Instruments and methods for minimally invasive carpal tunnel release |
| US20080200758A1 (en)* | 2007-02-09 | 2008-08-21 | Skeletal Dynamics, Inc. | Endo-surgical Device and Method |
| US20100280368A1 (en) | 2007-08-27 | 2010-11-04 | Technische Universitat Munchen | Trocar tube, Trocar, Obturator and/or Rectoscope for the Transluminal Endoscopic Surgery Via Natural Body Orifices |
| US20140371526A1 (en) | 2009-03-09 | 2014-12-18 | A.M. Surgical, Inc. | Slotted clear cannula |
| US20140121456A1 (en) | 2009-08-07 | 2014-05-01 | Thayer Intellectual Property, Inc. | Systems and methods for treatment of compressed nerves |
| US8951273B1 (en) | 2009-09-25 | 2015-02-10 | Mike Fard | Surgical instrument for endoscopic surgical procedures |
| US8672960B2 (en)* | 2010-07-14 | 2014-03-18 | SEG-Way Orthopaedics, Inc | Method and apparatus for endoscopic ligament release |
| US20160157881A1 (en) | 2010-07-14 | 2016-06-09 | Stuart Seymour | Method and Apparatus for Endoscopic Ligament Release |
| US8852191B2 (en) | 2011-06-20 | 2014-10-07 | Surgenco, Llc | Cutting guide and method for performing lateral retinacular release |
| US20140066709A1 (en)* | 2012-09-04 | 2014-03-06 | A.M. Surgical, Inc. | Compact endoscopic surgical blade assembly and method of use thereof |
| US20160287322A1 (en)* | 2013-11-13 | 2016-10-06 | Thixos Llc | Devices, kits and methods relating to treatment of facet joints |
| US20160345998A1 (en)* | 2013-11-27 | 2016-12-01 | Segway Orthopaedics, Inc. | Surgical Guide |
| US20150272617A1 (en)* | 2014-03-17 | 2015-10-01 | Arthroptics, L.L.C. | Cannula assembly |
| WO2015171785A1 (en) | 2014-05-06 | 2015-11-12 | Agee, John M. And Karen K., Trustees Of The John M. Agee Trust | Method and apparatus for treatment of cts using endoscopic carpal tunnel release |
| US20160192828A1 (en)* | 2015-01-07 | 2016-07-07 | Nicholes Sexton | Retractor cannula and method of use |
Non-Patent Citations (7)
| Title |
|---|
| Arthrex Inc., Centerline Endoscopic Carpal Tunnel Release Surgical Technique, Centerline Carpal Tunnel Release, Entire Document, Brochure No. LT1-0412-EN_C, United States. |
| Einhorn, N., and Leddy, J.P., "Pitfalls of Endoscopic Carpal Tunnel Release," Orthopedic Clinic of North America, vol. 27, No. 2, pp. 373-380 (1996). |
| Final Office Action received for U.S. Appl. No. 16/001,887, dated Jan. 8, 2020, 19 pages. |
| Microaire Surgical Instruments, SmartRelease ECTR Endoscopic Carpal Tunnel Release Surgical Technique, Entire Document, Charlottesville, VA, United States. |
| Non Final Office Action received for U.S. Appl. No. 16/001,887, dated Aug. 22, 2019, 13 pages. |
| Notice of Allowance received for U.S. Appl. No. 16/001,887, dated Jun. 18, 2020, 9 pages. |
| Smith&Nephew, Ectra II Carpal Ligament System, Entire URL, United States. |
Cited By (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| USD1088224S1 (en) | 2013-03-15 | 2025-08-12 | Orthocision Inc. | Surgical cannula |
| USD1086449S1 (en)* | 2015-03-25 | 2025-07-29 | Orthocision Inc. | Surgical cannula |
| US12318106B2 (en) | 2015-10-02 | 2025-06-03 | Nanice Medical Solutions, LLC | Method for using a surgical device to transect a transverse carpal ligament |
Also Published As
| Publication number | Publication date |
|---|---|
| US20170095266A1 (en) | 2017-04-06 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| US10806481B2 (en) | Surgical device for use in endoscopic carpal tunnel release (ECTR), endoscopic cubital tunnel release (ECuTR), and endoscopic plantar fasciitis release (EPFR) | |
| US10945742B2 (en) | Anti-skid surgical instrument for use in preparing holes in bone tissue | |
| JP5595522B2 (en) | Surgical system | |
| US8252011B1 (en) | Minimally invasive technique for performing plantar fasciotomies and surgical instrument for use in such a technique | |
| US10869686B2 (en) | Method for ultrasonic tissue excision with tissue selectivity | |
| AU2019335236B2 (en) | Passage establishment device for posterior spinal fusion minimally invasive surgery | |
| JP2019505320A (en) | Minimally invasive cutting instrument with guided incision device for multiple applications | |
| EP2036506A1 (en) | Trocar cannula | |
| US11033291B2 (en) | Surgical device for use in endoscopic carpal tunnel release (ECTR), endoscopic cubital tunnel release (ECuTR), and endoscopic plantar fasciitis release (EPFR) | |
| US10617440B2 (en) | Systems and methods for performing endoscopic release procedures | |
| US10383609B2 (en) | Surgical instrument for making incisions | |
| US12232759B2 (en) | Surgical device for use in endoscopic carpal tunnel release (ECTR), endoscopic cubital tunnel release (ECuTR), and endoscopic plantar fasciitis release (EPFR) | |
| KR20150103583A (en) | Surgical method for fixing a screw to a pedicle and instruments for inserting a screw which is used for the same | |
| US12318106B2 (en) | Method for using a surgical device to transect a transverse carpal ligament | |
| KR101794273B1 (en) | Surgical instrument ist for treatment for osmidrosis axillae | |
| JP5804428B2 (en) | Incision surgery instrument | |
| US20040181248A1 (en) | Suction round knife | |
| JP6531231B1 (en) | Transplant tendon collector | |
| US11330974B2 (en) | Endomicroscopic device | |
| Velho et al. | Ultrasonic osteotome: A cutting edge technology, our experience in 96 patients | |
| CN213047167U (en) | Novel tendon cutting device | |
| US20090182367A1 (en) | Adjustable Width Trocar | |
| EP3533397B1 (en) | Guide for surgical purpose | |
| KR101580858B1 (en) | Skin insision blade for operation | |
| JP2013202400A (en) | Percutaneous endoscopic bone cutting chisel |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| AS | Assignment | Owner name:NANICE MEDICAL SOLUTIONS LLC, PENNSYLVANIA Free format text:ASSIGNMENT OF ASSIGNORS INTEREST;ASSIGNOR:BRIGHT, PAUL J.;REEL/FRAME:040100/0581 Effective date:20161024 | |
| STPP | Information on status: patent application and granting procedure in general | Free format text:RESPONSE TO NON-FINAL OFFICE ACTION ENTERED AND FORWARDED TO EXAMINER | |
| STPP | Information on status: patent application and granting procedure in general | Free format text:FINAL REJECTION MAILED | |
| STPP | Information on status: patent application and granting procedure in general | Free format text:DOCKETED NEW CASE - READY FOR EXAMINATION | |
| STPP | Information on status: patent application and granting procedure in general | Free format text:NON FINAL ACTION MAILED | |
| AS | Assignment | Owner name:NANICE MEDICAL SOLUTIONS LLC, PENNSYLVANIA Free format text:ASSIGNMENT OF ASSIGNORS INTEREST;ASSIGNOR:BRIGHT, PAUL J.;REEL/FRAME:053183/0995 Effective date:20200629 | |
| FEPP | Fee payment procedure | Free format text:ENTITY STATUS SET TO MICRO (ORIGINAL EVENT CODE: MICR); ENTITY STATUS OF PATENT OWNER: MICROENTITY | |
| STPP | Information on status: patent application and granting procedure in general | Free format text:NOTICE OF ALLOWANCE MAILED -- APPLICATION RECEIVED IN OFFICE OF PUBLICATIONS | |
| STPP | Information on status: patent application and granting procedure in general | Free format text:PUBLICATIONS -- ISSUE FEE PAYMENT RECEIVED | |
| STPP | Information on status: patent application and granting procedure in general | Free format text:PUBLICATIONS -- ISSUE FEE PAYMENT VERIFIED | |
| STCF | Information on status: patent grant | Free format text:PATENTED CASE | |
| FEPP | Fee payment procedure | Free format text:MAINTENANCE FEE REMINDER MAILED (ORIGINAL EVENT CODE: REM.); ENTITY STATUS OF PATENT OWNER: MICROENTITY | |
| FEPP | Fee payment procedure | Free format text:SURCHARGE FOR LATE PAYMENT, MICRO ENTITY (ORIGINAL EVENT CODE: M3554); ENTITY STATUS OF PATENT OWNER: MICROENTITY | |
| MAFP | Maintenance fee payment | Free format text:PAYMENT OF MAINTENANCE FEE, 4TH YEAR, MICRO ENTITY (ORIGINAL EVENT CODE: M3551); ENTITY STATUS OF PATENT OWNER: MICROENTITY Year of fee payment:4 |