TWI769410B - New induced pluripotent stem cells (ipscs) and applications thereof - Google Patents
New induced pluripotent stem cells (ipscs) and applications thereofDownload PDFInfo
- Publication number
- TWI769410B TWI769410BTW108138904ATW108138904ATWI769410BTW I769410 BTWI769410 BTW I769410BTW 108138904 ATW108138904 ATW 108138904ATW 108138904 ATW108138904 ATW 108138904ATW I769410 BTWI769410 BTW I769410B
- Authority
- TW
- Taiwan
- Prior art keywords
- cells
- family gene
- vector
- ipscs
- disease
- Prior art date
Links
Images
Landscapes
- Medicines Containing Material From Animals Or Micro-Organisms (AREA)
- Micro-Organisms Or Cultivation Processes Thereof (AREA)
Abstract
Description
Translated fromChinese本發明係關於誘導性多能幹細胞(iPSC)之領域。詳言之,本發明提供一種iPSC細胞及其應用,該iPSC細胞衍生自包含Cbx家族基因之體細胞。The present invention relates to the field of induced pluripotent stem cells (iPSCs). Specifically, the present invention provides iPSC cells derived from somatic cells comprising Cbx family genes and applications thereof.
誘導性多能幹細胞(iPSC)最早係Shinya Yamanaka及其同事於2006年在日本之京都大學(Kyoto University)產生。iPSC為多能細胞,能夠分化為全部三個胚層,且因此在用於再生醫學之臨床應用中擁有較高的潛能。Induced pluripotent stem cells (iPSCs) were first produced by Shinya Yamanaka and colleagues at Kyoto University in Japan in 2006. iPSCs are pluripotent cells capable of differentiating into all three germ layers, and thus have a high potential for clinical application in regenerative medicine.
Yamanaka等人先前已鑑別出24種因子為胚胎幹細胞中之關鍵因子,該等因子在胚胎幹細胞而非體細胞中表現(Cell. 2006年8月25日; 126(4):663-76)。在24種因子當中,Yamanaka及同事進一步證明,四種基因Oct4、Sox2、c-Myc及KLF4為藉由用反轉錄病毒引入24種因子中之23種將小鼠纖維母細胞重新編程為多能細胞所必需。其後,四種基因Oct4、Sox2、c-Myc及KLF4已被視為習知重新編程因子。隨後針對其促成胚胎發育(亦即嵌合小鼠中之嵌合)之能力來測試細胞之多能性(Cell. 2006年8月25日; 126(4):663-76)。Yamanaka et al. have previously identified 24 factors as key factors in embryonic stem cells that are expressed in embryonic stem cells but not in somatic cells (Cell. 2006 Aug 25; 126(4):663-76). Among the 24 factors, Yamanaka and colleagues further demonstrated that four genes, Oct4, Sox2, c-Myc and KLF4, were used to reprogram mouse fibroblasts to pluripotency by introducing 23 of the 24 factors with retrovirus necessary for cells. Since then, four genes, Oct4, Sox2, c-Myc and KLF4, have been regarded as known reprogramming factors. The cells were then tested for their ability to contribute to embryonic development (ie, chimerism in chimeric mice) for pluripotency (Cell. 2006 Aug 25; 126(4):663-76).
四種習知基因中之兩種c-Myc及KLF4為致癌的,進而導致20%嵌合小鼠之癌症發展。然而,在無c-Myc及KLF4之情況下,iPSC產生之效率極低。因此,需要研發能夠將體細胞重新編程為iPS之新穎基因/其因子。Two of the four known genes, c-Myc and KLF4, are oncogenic, leading to cancer development in 20% of chimeric mice. However, in the absence of c-Myc and KLF4, iPSC generation was extremely inefficient. Therefore, there is a need to develop novel genes/factors thereof capable of reprogramming somatic cells into iPS.
本發明出人意料地發現,使用Cbx家族基因序列作為重新編程因子而非cMyc及Klf4可產生維持多能性及分化能力同時無致癌特性之iPSC。The present inventors have unexpectedly found that the use of Cbx family gene sequences as reprogramming factors rather than cMyc and Klf4 results in iPSCs that maintain pluripotency and differentiation capacity without oncogenic properties.
在一個態樣中,本發明提供一種iPSC群體,其中經基因修飾之體細胞包含Cbx家族基因序列及除cMyc家族基因序列及Klf4家族基因序列以外的一或多個重新編程因子序列。In one aspect, the invention provides a population of iPSCs wherein the genetically modified somatic cells comprise Cbx family gene sequences and one or more reprogramming factor sequences other than cMyc family gene sequences and Klf4 family gene sequences.
在一些實施例中,該體細胞來自外胚層(例如角質細胞)、中胚層(例如纖維母細胞)、內胚層(例如胰臟細胞)或神經脊譜系(例如黑色素細胞)。在一些實施例中,該體細胞為纖維母細胞、角質細胞、胰臟β細胞、神經元、寡樹突神經膠質細胞、星形膠質細胞、肝細胞、肝幹細胞、心肌細胞、骨胳肌細胞、平滑肌細胞、造血細胞、蝕骨細胞、成骨細胞、外被細胞、血管內皮細胞或神經鞘細胞。在一個實施例中,該體細胞為纖維母細胞。In some embodiments, the somatic cells are from ectoderm (eg, keratinocytes), mesoderm (eg, fibroblasts), endoderm (eg, pancreatic cells), or neural crest lineages (eg, melanocytes). In some embodiments, the somatic cells are fibroblasts, keratinocytes, pancreatic beta cells, neurons, oligodendritic glial cells, astrocytes, hepatocytes, hepatic stem cells, cardiomyocytes, skeletal muscle cells , smooth muscle cells, hematopoietic cells, osteoblasts, osteoblasts, coat cells, vascular endothelial cells or nerve sheath cells. In one embodiment, the somatic cells are fibroblasts.
在一個實施例中,該Cbx家族基因序列為Cbx7。In one embodiment, the Cbx family gene sequence is Cbx7.
在一些實施例中,該重新編程因子序列包括(但不限於) Oct家族基因序列、Sox家族基因序列、c-myc家族基因序列、Klf家族基因序列、Nanog家族基因序列、Lin28家族基因序列及Glis1家族基因序列。在其他實施例中,該重新編程因子序列為Oct家族基因序列、Sox家族基因序列或包含Oct家族基因序列及Sox家族基因序列之序列。較佳地,該Oct家族基因序列為Oct3或Oct4,且該Sox家族基因序列為Sox2。在另一實施例中,該重新編程因子序列包含Oct4及Sox2之序列。In some embodiments, the reprogramming factor sequences include, but are not limited to, Oct family gene sequences, Sox family gene sequences, c-myc family gene sequences, Klf family gene sequences, Nanog family gene sequences, Lin28 family gene sequences, and Glis1 family gene sequence. In other embodiments, the reprogramming factor sequence is an Oct family gene sequence, a Sox family gene sequence, or a sequence comprising an Oct family gene sequence and a Sox family gene sequence. Preferably, the Oct family gene sequence is Oct3 or Oct4, and the Sox family gene sequence is Sox2. In another embodiment, the reprogramming factor sequence comprises the sequences of Oct4 and Sox2.
在一些實施例中,該體細胞來自哺乳動物細胞。在一個實施例中,該哺乳動物細胞為人類細胞。In some embodiments, the somatic cells are derived from mammalian cells. In one embodiment, the mammalian cell is a human cell.
在一個態樣中,本發明提供一種醫藥組合物,其包含本發明之iPSC群體、自如技術方案1之iPSC分化的多能幹細胞或由該等多能幹細胞產生之經分化細胞。In one aspect, the present invention provides a pharmaceutical composition comprising the iPSC population of the present invention, pluripotent stem cells differentiated from the iPSCs of
在另一態樣中,本發明提供一種產生誘導性多能幹細胞(iPSC)之方法,其包含(a)用表現Cbx家族基因序列之載體及表現一或多個重新編程因子多肽而非cMyc家族多肽及Klf4家族多肽之一或多個載體來引入體細胞;及(b)在對(a)之所得體細胞重新編程以產生該等iPSC之條件下培養(a)之所得體細胞。In another aspect, the invention provides a method of generating induced pluripotent stem cells (iPSCs) comprising (a) using a vector expressing a Cbx family gene sequence and expressing one or more reprogramming factor polypeptides other than the cMyc family one or more vectors of the polypeptide and the Klf4 family polypeptide to introduce into somatic cells; and (b) culturing the resulting somatic cells of (a) under conditions that reprogram the resulting somatic cells of (a) to generate the iPSCs.
在一些實施例中,該方法進一步包含在飼養細胞之存在下在幹細胞培育培養基中擴增該等iPSC的步驟。In some embodiments, the method further comprises the step of expanding the iPSCs in stem cell culture medium in the presence of feeder cells.
在一些實施例中,該載體為慢病毒載體、反轉錄病毒載體、腺病毒載體、腺病毒相關病毒載體(AAV)、痘病毒載體、疱疹病毒載體、麻疹病毒載體、泡沫病毒載體、α病毒載體、水泡性口炎病毒載體、轉染載體或轉位子載體。In some embodiments, the vector is a lentiviral vector, a retroviral vector, an adenoviral vector, an adeno-associated viral vector (AAV), a poxvirus vector, a herpes virus vector, a measles virus vector, a foamy virus vector, an alpha virus vector , vesicular stomatitis virus vector, transfection vector or transposon vector.
本發明之另一態樣為一種醫藥組合物,其包含本發明之iPSC群體、自本發明之iPSC分化之多能幹細胞或由該等多能幹細胞產生之經分化細胞。Another aspect of the present invention is a pharmaceutical composition comprising the iPSC population of the present invention, pluripotent stem cells differentiated from the iPSCs of the present invention, or differentiated cells generated from the pluripotent stem cells.
在一個態樣中,本發明提供一種產生誘導性多能幹細胞(iPSC)之方法,其包含(a)用表現Cbx7及一或多個重新編程因子序列而非cMyc家族基因序列及Klf4家族基因序列之載體來引入體細胞的步驟,及(b)在飼養細胞之存在下在幹細胞培育培養基中培養及擴增該iPSC之步驟。在一個實施例中,該重新編程因子序列包含Oct4序列、Sox2序列或Oct4及Sox2之序列。在另一實施例中,該重新編程因子序列包含Oct4序列及Sox2序列。In one aspect, the invention provides a method of generating induced pluripotent stem cells (iPSCs) comprising (a) expressing Cbx7 and one or more reprogramming factor sequences instead of cMyc family gene sequences and Klf4 family gene sequences and (b) the steps of culturing and expanding the iPSCs in stem cell culture medium in the presence of feeder cells. In one embodiment, the reprogramming factor sequence comprises an Oct4 sequence, a Sox2 sequence, or a sequence of Oct4 and Sox2. In another embodiment, the reprogramming factor sequence comprises an Oct4 sequence and a Sox2 sequence.
本發明之另一態樣提供一種治療及/或緩解有需要之患有疾病或病症之個體的方法,該方法包含向該個體投與有效量的自該等經重新編程之iPSC分化之細胞。在一個實施例中,自該等經重新編程之iPSC分化之該等細胞為多能幹細胞。Another aspect of the present invention provides a method of treating and/or alleviating a disease or disorder in an individual in need thereof, the method comprising administering to the individual an effective amount of cells differentiated from the reprogrammed iPSCs. In one embodiment, the cells differentiated from the reprogrammed iPSCs are pluripotent stem cells.
在一個實施例中,自該等經重新編程之iPSC分化之該等細胞為神經元幹細胞。在一個實施例中,該疾病或病症為神經退化性病狀。在一個實施例中,該神經退化性病狀為帕金森氏病(Parkinson's disease)、多發性硬化症(MS)、肌肉萎縮性側索硬化(ALS)、脊髓性肌萎縮(SMA)、亨廷頓氏病(Huntington's disease)或阿茲海默病(Alzheimer disease)。在一較佳實施例中,該疾病或病症為帕金森氏病。在一個實施例中,該等經分化神經元幹細胞以範圍介於約1 × 105個細胞/劑量/天至約1 × 108個細胞/劑量/天之劑量投與。在一個實施例中,該等經分化神經元幹細胞以約1 × 106個細胞/劑量/天之劑量投與。In one embodiment, the cells differentiated from the reprogrammed iPSCs are neuronal stem cells. In one embodiment, the disease or disorder is a neurodegenerative condition. In one embodiment, the neurodegenerative condition is Parkinson's disease, multiple sclerosis (MS), amyotrophic lateral sclerosis (ALS), spinal muscular atrophy (SMA), Huntington's disease (Huntington's disease) or Alzheimer's disease. In a preferred embodiment, the disease or disorder is Parkinson's disease. In one embodiment, the differentiated neuronal stem cells are administered at a dose ranging from about1 x 105 cells/dose/day to about 1 x108 cells/dose/day. In one embodiment, the differentiated neuronal stem cells are administered at a dose of about1 x 106 cells/dose/day.
提供一種誘導性多能幹(iPS)細胞(iPSC)群體,其衍生自具有增強的變為無致癌特性之潛能的經基因修飾之體細胞。亦提供用於產生具有變為誘導性多能幹(iPS)細胞(iPS細胞)之潛能而無致癌特性之體細胞的方法,及用於自細胞群體產生iPS細胞之方法,該細胞群體可隨後用於移植以及用於細胞分化及相互作用。在閱讀如下文更充分描述之主題方法及組合物的細節時,本發明之此等及其他目標、優點及特徵將對熟習此項技術者變得顯而易見。定義Provided is a population of induced pluripotent stem (iPS) cells (iPSCs) derived from genetically modified somatic cells with enhanced potential to become non-oncogenic. Also provided are methods for generating somatic cells with the potential to become induced pluripotent stem (iPS) cells (iPS cells) without oncogenic properties, and methods for generating iPS cells from cell populations that can subsequently be used for transplantation and for cell differentiation and interaction. These and other objects, advantages and features of the present invention will become apparent to those skilled in the art upon reading the details of the subject methods and compositions as more fully described below.definition
除非以其他方式定義,否則本文中所使用之所有科學或技術術語具有與一般熟習本發明所屬技術者所理解相同的含義。一般熟習此項技術者可理解及使用類似或等效於本文中所描述之彼等的任何方法及材料來實踐本發明。Unless otherwise defined, all scientific or technical terms used herein have the same meaning as understood by one of ordinary skill in the art to which this invention belongs. One of ordinary skill in the art could understand and use any methods and materials similar or equivalent to those described herein to practice the present invention.
除非另有指示,否則本說明書及申請專利範圍中所使用之表述成分數量、反應條件等之所有數字應理解為在所有情況下皆經術語「約」修飾。因此,除非有相反指示,否則本發明之說明書及申請專利範圍中所闡述的數值參數大約可視設法藉由本發明所獲得之所要特性而變化。Unless otherwise indicated, all numbers used in this specification and in the claims for ingredient quantities, reaction conditions, etc., are to be understood as being modified by the term "about" in all cases. Accordingly, unless indicated to the contrary, the numerical parameters set forth in the specification and claims of the present invention may vary approximately depending upon the desired properties sought to be obtained by the present invention.
術語「一(a/an)」意謂本發明中所描述之目標中之一者或多於一者。術語「及/或」意謂替代方案中之一者或兩者。術語「細胞(a cell)」或「細胞(the cell)」可包括複數個細胞。The term "a/an" means one or more than one of the objects described in this disclosure. The term "and/or" means one or both of the alternatives. The term "a cell" or "the cell" can include a plurality of cells.
如本文中所使用之術語「體細胞」係指促成生殖系(亦稱為性細胞)外部之多細胞有機體的充分成型體且區別於構成早期胚胎之未分化幹細胞的細胞。The term "somatic cell" as used herein refers to the fully formed body of a multicellular organism that contributes to the outside of the germline (also known as sex cells) and is distinct from the cells that make up the undifferentiated stem cells of the early embryo.
如本文中所使用,術語「重新編程(reprogram)」、「經重新編程」及「重新編程(reprogramming)」係指經分化體細胞基於來自轉基因載體之重新編程因子的異位表現而去分化為多能幹細胞之過程,且一般更廣泛地係指經技術上誘導之細胞譜系轉化。As used herein, the terms "reprogram", "reprogramming" and "reprogramming" refer to the dedifferentiation of differentiated somatic cells based on ectopic expression of reprogramming factors from transgenic vectors into The process of pluripotent stem cells, and generally more broadly, refers to technically induced transformation of cell lineages.
如本文中所使用,術語「重新編程因子」係指用以促進細胞重新編程之轉基因,通常(而非必定)係指轉錄因子或微RNA。As used herein, the term "reprogramming factor" refers to a transgene used to promote cell reprogramming, usually (but not necessarily) to a transcription factor or microRNA.
術語「活體內」一般意謂活有機體內部。術語「活體外」一般意謂活有機體外部,諸如在形成於有機體外部之人工環境中進行的實驗。The term "in vivo" generally means inside a living organism. The term "in vitro" generally means outside of a living organism, such as experiments performed in an artificial environment formed outside the organism.
術語「三胚層」係指在脊椎動物之原腸胚形成期間的三個層:外胚層、中胚層及內胚層,其衍生為所有體細胞。The term "three germ layers" refers to the three layers during gastrulation in vertebrates: ectoderm, mesoderm and endoderm, from which all somatic cells are derived.
本文中之術語細胞之「多能性」及「幹性」係指細胞(通常幹細胞)產生有機體中之所有類型之細胞的能力。在發育生物學之態樣中,多能細胞在胚胎發育期間可產生全部三種生殖譜系。The terms "pluripotency" and "stemness" of a cell herein refer to the ability of a cell (usually a stem cell) to give rise to all types of cells in an organism. In terms of developmental biology, pluripotent cells give rise to all three germline lineages during embryonic development.
術語「胚胎幹細胞」係指發現於胚胎囊胚之內部細胞塊(ICM)中之天然存在的多能細胞。術語「誘導性多能幹細胞」(「iPSC」或「iPS」可互換使用)意謂在重新編程之人工過程中獲得多能性的自成年或胚胎有機體分離之經分化細胞。「iPSCs」為複數個iPSC。與胚胎幹細胞相比,iPSC不為自然界中發現之多能細胞。The term "embryonic stem cells" refers to naturally occurring pluripotent cells found in the inner cell mass (ICM) of embryonic blastocysts. The term "induced pluripotent stem cell" ("iPSC" or "iPS" used interchangeably) means a differentiated cell isolated from an adult or embryonic organism that acquires pluripotency in an artificial process of reprogramming. "iPSCs" are a plurality of iPSCs. In contrast to embryonic stem cells, iPSCs are not the pluripotent cells found in nature.
術語細胞之「分化(differentiate/ differentiation)」係指細胞在細胞分裂期間損失其多能性之過程,其中子細胞中之至少一者失去多能性。當細胞經歷分化時,其處於向三種生殖譜系中之一者的譜系特異性分化之過程中。The term "differentiate/differentiation" of a cell refers to the process by which a cell loses its pluripotency during cell division, wherein at least one of the daughter cells loses pluripotency. When a cell undergoes differentiation, it is in the process of lineage-specific differentiation into one of the three germline lineages.
術語「引入」或「轉導」係指經由編碼所關注基因以改變其表現水平之載體將基因遞送至細胞中之過程。轉基因為天然地或藉由任何基因工程改造技術轉移至有機體或細胞中之基因或遺傳物質,諸如DNA或RNA。遞送轉基因之方法可經由病毒載體或非病毒載體來進行。在本發明之一個實施例中,病毒載體用以將重新編程因子引入至細胞中。病毒載體可為整合或非整合病毒。本發明中所使用之整合病毒可為慢病毒或反轉錄病毒。整合病毒允許將其編碼基因與經病毒粒子感染之經轉導細胞整合。本發明中所使用之非整合病毒可為腺病毒或仙台(Sendai)病毒。本發明中亦可使用非病毒方法,諸如藉由將DNA或RNA材料轉染至細胞中。DNA材料可呈PiggyBac、微型環載體或游離型質體之形式。RNA材料可呈mRNA或miRNA之形式。The term "introduction" or "transduction" refers to the process of delivering a gene into a cell via a vector encoding the gene of interest to alter its level of expression. A transgene is a gene or genetic material, such as DNA or RNA, that has been transferred into an organism or cell, either naturally or by any genetic engineering technique. Methods of delivering transgenes can be via viral or non-viral vectors. In one embodiment of the invention, viral vectors are used to introduce reprogramming factors into cells. Viral vectors can be integrating or non-integrating viruses. The integrating virus used in the present invention can be a lentivirus or a retrovirus. Integrating viruses allow integration of their encoding genes into transduced cells infected with virions. The non-integrating virus used in the present invention can be adenovirus or Sendai virus. Non-viral methods can also be used in the present invention, such as by transfection of DNA or RNA material into cells. The DNA material can be in the form of PiggyBac, minicircular vectors or episomal plastids. The RNA material can be in the form of mRNA or miRNA.
術語「細胞培養」或「培養」係指在受控環境下通常在有機體外部(活體外)或其自然環境外部使細胞生長之過程。在細胞培養中之術語「解離(dissociate/dissociation)」意謂使用力或酶來破壞向單細胞之細胞聚集。術語「胰蛋白酶消化」係指使用胰蛋白酶來消化胞外分子之過程,細胞利用該等胞外分子來附著至其生長環境。在胰蛋白酶消化之後,細胞自其生長表面剝離,且可用生長培養基收集。術語「集結粒」係指由允許細胞與其生長培養基分離之離心過程造成的細胞聚集。術語「再懸浮(resuspend/resuspension)」係指添加新液體以製造細胞懸浮液之過程。The term "cell culture" or "cultivation" refers to the process of growing cells under a controlled environment, usually outside the organism (in vitro) or outside its natural environment. The term "dissociate/dissociation" in cell culture means the use of force or enzymes to disrupt cell aggregation into single cells. The term "trypsinization" refers to the process of using trypsin to digest extracellular molecules that cells use to attach to their growth environment. Following trypsinization, cells are detached from their growth surfaces and can be harvested with growth medium. The term "agglomerate" refers to aggregation of cells caused by a centrifugation process that allows cells to separate from their growth medium. The term "resuspend/resuspension" refers to the process of adding new liquid to make a cell suspension.
除非指定,否則本發明中之培養基為習知實驗室用途之培養基。用於飼養細胞及MEF細胞之培養基為具有10%胎牛血清、1%青黴素及鏈黴素及1%非必需胺基酸(NEAA)之DMEM。幹細胞培育培養基為含有1% L-麩醯胺酸、7.5ml Hyclone FBS、0.5ml NEAA、91μl β-巰基乙醇及5μl白血病抑制因子之DMEM。一般熟習此項技術者將能夠使用類似或等效於本文中所描述之培養基來為iPSC提供相同或類似生長效果。Unless specified, the culture medium in the present invention is that of conventional laboratory use. The medium used for feeder cells and MEF cells was DMEM with 10% fetal bovine serum, 1% penicillin and streptomycin, and 1% non-essential amino acids (NEAA). Stem cell culture medium was DMEM containing 1% L-glutamic acid, 7.5 ml Hyclone FBS, 0.5 ml NEAA, 91 μl β-mercaptoethanol and 5 μl leukemia inhibitory factor. One of ordinary skill in the art will be able to use media similar or equivalent to those described herein to provide iPSCs with the same or similar growth effects.
術語「群落」係指由一個單細胞之生長造成的細胞培養物中之細胞的聚集物。術語細胞之「後代」係指子細胞及在母細胞之細胞分裂/增殖後衍生的細胞。術語後代可用以定義細胞之「譜系」,其中某些細胞之相同譜系可經由細胞分裂之歷程及細胞之後代來追蹤。The term "colony" refers to an aggregate of cells in a cell culture resulting from the growth of a single cell. The term "progeny" of a cell refers to daughter cells and cells derived after cell division/proliferation of the parent cell. The term progeny can be used to define a "lineage" of cells, where the same lineage of certain cells can be traced through the course of cell division and the progeny of cells.
術語「產生之效率」係指自體細胞重新編程為iPS細胞之效率。存在用以自體細胞產生iPS細胞之許多方法,包括反轉錄病毒、慢病毒、腺病毒、質體轉染、轉位子….等。藉由各方法產生iPS細胞之效率不同(Cell Transplant. 2011;20(1):15-9.)。(請提供更多資訊:)The term "efficiency of generation" refers to the efficiency of reprogramming of autologous cells into iPS cells. There are many methods to generate iPS cells from autologous cells, including retroviruses, lentiviruses, adenoviruses, plastid transfection, transposon...etc. The efficiency of generating iPS cells by each method varies (Cell Transplant. 2011;20(1):15-9.). (Please provide more information:)
術語「胚狀體(embryoid body/embryoid bodies)」或「EB」係指細胞培養物中之多能幹細胞之三維聚集物。胚狀體內之胚胎幹細胞或誘導性多能幹細胞經歷沿三種生殖細胞譜系之分化及細胞特異性分化。The term "embryoid body/embryoid bodies" or "EB" refers to a three-dimensional aggregate of pluripotent stem cells in cell culture. Embryonic stem cells or induced pluripotent stem cells within the embryoid body undergo differentiation along three germ cell lineages and cell-specific differentiation.
術語「飼養細胞」或「飼養者」係指與第二類型之細胞共培養以提供該第二類型之細胞可生長於其中之環境的一種類型之細胞,此係因為飼養細胞提供生長因子及營養物質以支援第二細胞類型。飼養細胞視情況來自與其所支援之細胞不同的物種。當藉由輻照或用諸如絲裂黴素之抗有絲分裂藥劑處理來與其他細胞共培養時,飼養細胞可典型地「失活」以防止其生長超過其所支援之細胞。飼養細胞可包括內皮細胞、基質細胞(例如上皮細胞或纖維母細胞)及白血病細胞。在不限制前述內容之情況下,一種特異性飼養細胞類型可為人類飼養者,諸如人類皮膚纖維母細胞。另一飼養細胞類型可為鼠胚胎纖維母細胞(MEF)。一般而言,多種飼養細胞可部分地用以維持多能性,引導朝向某一譜系分化且促進成熟為特殊細胞類型。The term "feeder cell" or "feeder" refers to a type of cell that is co-cultured with cells of a second type to provide an environment in which the cells of the second type can grow because feeder cells provide growth factors and nutrients Substances to support the second cell type. Feeder cells are optionally from a different species than the cells they support. When co-cultured with other cells by irradiation or treatment with anti-mitotic agents such as mitomycin, feeder cells can typically be "inactivated" to prevent them from growing beyond the cells they support. Feeder cells can include endothelial cells, stromal cells (eg, epithelial cells or fibroblasts), and leukemia cells. Without limiting the foregoing, one specific feeder cell type can be a human feeder, such as human dermal fibroblasts. Another feeder cell type may be murine embryonic fibroblasts (MEFs). In general, a variety of feeder cells can be used in part to maintain pluripotency, direct differentiation towards a lineage and promote maturation into a particular cell type.
術語「神經幹細胞」係指能夠在發育或再生過程期間產生神經系統中之神經元及膠細胞的先驅細胞。The term "neural stem cells" refers to precursor cells capable of generating neurons and glial cells in the nervous system during developmental or regenerative processes.
術語「退化性病症」及「退化性疾病」在本文中互換使用,係指退化性細胞變化、影響器官或組織以及導致惡化之連續過程的結果。術語「神經退化」在本文中係指因中樞神經細胞喪失或功能障礙所造成的退化性病症。在本發明之一些實施例中,神經退化可藉由引入藥劑來破壞個體或動物模型之中樞神經系統以進行人工方式地誘導。術語「動物模型」係指患有與人類中疾病相同或相似疾病的動物。在本發明之一些實施例中,動物模型之疾病係經人工方式誘導,且熟習此項技術者應理解,此模型可轉譯成特定人類疾病之進展。術語「治療(treatment/treating/treat)」一般係指獲得所要藥理學及/或生理學效果。就完全或部分預防疾病、病症或其症狀而言,該效果可為預防性的,且就部分或完全治癒疾病、病症及/或其所帶來之症狀而言,該效果可為治療性的。本文中所使用之「治療」包括對哺乳動物(較佳地,人類)中疾病的任何治療,且包括(1)遏制個體中疾病、病症或其症狀之發展,或(2)緩解或減輕個體中疾病、病症或其症狀。The terms "degenerative disorder" and "degenerative disease" are used interchangeably herein to refer to degenerative cellular changes, the result of a continuum of processes affecting organs or tissues, and leading to deterioration. The term "neurodegeneration" as used herein refers to degenerative disorders resulting from loss or dysfunction of central nervous cells. In some embodiments of the invention, neurodegeneration can be artificially induced by introducing an agent to disrupt the central nervous system of an individual or animal model. The term "animal model" refers to an animal suffering from the same or similar disease as in humans. In some embodiments of the invention, the disease in the animal model is artificially induced, and it will be understood by those skilled in the art that this model can be translated into the progression of a specific human disease. The terms "treatment/treating/treat" generally refer to obtaining a desired pharmacological and/or physiological effect. The effect may be prophylactic in terms of complete or partial prevention of the disease, disorder or symptoms thereof, and therapeutic in terms of partial or complete cure of the disease, disorder and/or symptoms thereof . "Treatment" as used herein includes any treatment of a disease in a mammal (preferably, a human) and includes (1) arresting the development of a disease, disorder or symptom thereof in an individual, or (2) alleviating or alleviating the individual disease, disorder, or symptoms thereof.
術語「個體(individual/subject)」及「患者」在本文中互換使用,且係指需要對其診斷、治療(treatment)或治療(therapy)之任何哺乳動物個體。The terms "individual/subject" and "patient" are used interchangeably herein and refer to any mammalian subject for whom diagnosis, treatment or therapy is required.
術語「治療有效量」係指當向需要治療疾病或病症之患者或個體投與時,足以對該疾病或病症具有有益效果之細胞或其衍生後代的量。治療有效量將視疾病或病症之病狀及其嚴重程度而變化。其不限於本說明書中所陳述之範圍。確定給定細胞或其衍生後代之治療量在此項技術之一般技能內,且僅需要常規實驗。The term "therapeutically effective amount" refers to an amount of a cell or its derived progeny sufficient to have a beneficial effect on a disease or disorder when administered to a patient or individual in need of treatment of the disease or disorder. A therapeutically effective amount will vary depending on the condition and severity of the disease or disorder. It is not limited to the scope stated in this specification. Determining the therapeutic amount of a given cell or its derived progeny is within the ordinary skill of the art and requires only routine experimentation.
本發明之術語「醫藥組合物」包括用以治療退化性病狀之有效量的活細胞。細胞組分可為培養細胞或經分離之細胞群體之混合物,諸如經分化組織細胞、先驅細胞及/或幹細胞。本發明之醫藥組合物呈液體形式或為細胞懸浮緩衝液,且可含有穩定液體懸浮液且有助於細胞生存力的醫藥學上可接受之賦形劑。本發明之iPSC及其產生The term "pharmaceutical composition" of the present invention includes an effective amount of viable cells for the treatment of degenerative conditions. The cellular component can be a mixture of cultured cells or isolated cell populations, such as differentiated tissue cells, precursor cells, and/or stem cells. The pharmaceutical compositions of the present invention are in liquid form or cell suspension buffers, and may contain pharmaceutically acceptable excipients that stabilize the liquid suspension and aid cell viability.iPSCsof the present inventionand their generation
此項技術中已知,Oct4、Sox2、c-Myc及Klf4為產生iPSC所必需。然而,四種基因中之兩者(c-Myc及Klf4)為致癌的,且可能導致癌症。若不使用c-Myc及Klf4,則產生iPSC之效率極低。Cbx7在ESC分化期間下調,且Cbx7決不用以產生iPS細胞世代。本發明出人意料地發現,將Cbx家族基因序列而非c-Myc家族基因序列及Klf4家族基因序列引入至體細胞可將經分化細胞有效地重新編程為不具有致癌特性之iPSC。It is known in the art that Oct4, Sox2, c-Myc and Klf4 are required for the generation of iPSCs. However, two of the four genes (c-Myc and Klf4) are oncogenic and may cause cancer. Without the use of c-Myc and Klf4, the generation of iPSCs is extremely inefficient. Cbx7 is down-regulated during ESC differentiation, and Cbx7 is never used to generate iPS cell generations. The present inventors have surprisingly found that the introduction of Cbx family gene sequences but not c-Myc family gene sequences and Klf4 family gene sequences into somatic cells efficiently reprograms differentiated cells into iPSCs without oncogenic properties.
因此,本發明提供一種iPSC群體,其中經基因修飾之體細胞包含Cbx家族基因序列及除cMyc家族基因序列及Klf4家族基因序列以外的一或多個重新編程因子序列。Accordingly, the present invention provides a population of iPSCs wherein the genetically modified somatic cells comprise Cbx family gene sequences and one or more reprogramming factor sequences other than the cMyc family gene sequences and the Klf4 family gene sequences.
體細胞為已充分分化之細胞,其將不會以天然地方是產生身體之全部三個胚層(亦即外胚層、中胚層及內胚層)的細胞。該等細胞可分化至其能夠產生特定譜系之細胞的點,例如成年非多能性多能幹細胞,例如間葉幹細胞、神經幹細胞、心臟幹細胞、肝幹細胞及其類似者。體細胞之實例包括來自外胚層譜系(例如角質細胞)、中胚層譜系(例如纖維母細胞)、內胚層譜系(例如胰臟細胞)或神經脊譜系(例如黑色素細胞)之細胞。某些實施例包括纖維母細胞、角質細胞、胰臟β細胞、神經元、寡樹突神經膠質細胞、星形膠質細胞、肝細胞、肝幹細胞、心肌細胞、骨骼肌細胞、平滑肌細胞、造血細胞、蝕骨細胞、成骨細胞、外被細胞、血管內皮細胞、神經鞘細胞及其類似者。在一個非限制性實例中,體細胞可為纖維母細胞譜系之細胞。Somatic cells are fully differentiated cells that will not naturally be the cells that give rise to all three germ layers of the body (ie, ectoderm, mesoderm, and endoderm). These cells can differentiate to the point where they can give rise to cells of a particular lineage, such as adult non-pluripotent pluripotent stem cells, such as mesenchymal stem cells, neural stem cells, cardiac stem cells, hepatic stem cells, and the like. Examples of somatic cells include cells from ectodermal lineages (eg, keratinocytes), mesodermal lineages (eg, fibroblasts), endodermal lineages (eg, pancreatic cells), or neural crest lineages (eg, melanocytes). Certain embodiments include fibroblasts, keratinocytes, pancreatic beta cells, neurons, oligodendritic glial cells, astrocytes, hepatocytes, hepatic stem cells, cardiomyocytes, skeletal muscle cells, smooth muscle cells, hematopoietic cells , Osteoblasts, osteoblasts, coat cells, vascular endothelial cells, nerve sheath cells and the like. In one non-limiting example, the somatic cells can be cells of the fibroblast lineage.
使用Cbx家族基因序列及一或多個重新編程因子序列(除cMyc家族基因序列及Klf4家族基因序列以外)對體細胞重新編程。較佳地,Cbx家族基因序列為與Cbx7之序列具有至少70%一致性的核酸序列。更佳地,Cbx家族基因序列為Cbx7。Somatic cells are reprogrammed using Cbx family gene sequences and one or more reprogramming factor sequences (in addition to the cMyc family gene sequences and the Klf4 family gene sequences). Preferably, the Cbx family gene sequence is a nucleic acid sequence with at least 70% identity to the sequence of Cbx7. More preferably, the Cbx family gene sequence is Cbx7.
一或多個重新編程因子序列較佳地為Oct家族基因序列、Sox家族基因序列、Nanog家族基因序列、Lin28家族基因序列。在一較佳實施例中,一或多個重新編程因子序列包括Oct家族基因序列及Sox家族基因序列。The one or more reprogramming factor sequences are preferably Oct family gene sequences, Sox family gene sequences, Nanog family gene sequences, and Lin28 family gene sequences. In a preferred embodiment, the one or more reprogramming factor sequences include Oct family gene sequences and Sox family gene sequences.
較佳地,Oct家族基因序列為與Oct 3/4之胺基酸序列具有至少70%一致性的核酸序列。較佳地,Sox家族基因序列為與Sox2之胺基酸序列具有至少70%一致性的核酸序列。較佳地,Nanog家族基因序列為與Nanog之胺基酸序列具有至少70%一致性的核酸序列。較佳地,Lin28家族基因序列為與Lin28之胺基酸序列具有至少70%一致性的核酸序列。Preferably, the Oct family gene sequence is a nucleic acid sequence with at least 70% identity to the amino acid sequence of
本發明之iPSC細胞藉由包含以下步驟之方法來產生:(a)用表現Cbx家族基因之載體及表現一或多個重新編程因子基因而非cMyc家族基因及Klf4家族基因之一或多個載體來引入體細胞;及(b)在對(a)之所得體細胞重新編程以產生iPSC之條件下培養(a)之所得體細胞。The iPSC cells of the present invention are generated by a method comprising the following steps: (a) using a vector expressing a Cbx family gene and a vector expressing one or more reprogramming factor genes instead of one or more of the cMyc family gene and the Klf4 family gene and (b) culturing the resulting somatic cells of (a) under conditions that reprogram the resulting somatic cells of (a) to generate iPSCs.
可使用表現本文中所描述之重新編程因子的任何適當載體來將轉基因引入至體細胞中。合適的載體尤其包括質體載體及病毒載體。病毒載體可為複製勝任型或複製選擇性的(例如經工程改造以在特定宿主細胞中較佳或選擇性複製),或可為基因失能的以便成為複製缺陷型或複製受損的。典型地,此類載體為可商購的(例如在Invitrogen、Stratagene、Amersham Biosciences、Promega等中)或可購自寄存機構,諸如American Type Culture Collection (ATCC, Rockville, Md.),或已成為眾多公開案之主題,該等公開案描述其序列、組織及生產之方法,從而允許技術人員將其應用。Transgenes can be introduced into somatic cells using any suitable vector expressing the reprogramming factors described herein. Suitable vectors include, inter alia, plastid vectors and viral vectors. Viral vectors can be replication-competent or replication-selective (eg, engineered to replicate preferentially or selectively in a particular host cell), or can be genetically disabled so as to be replication-deficient or replication-impaired. Typically, such vectors are commercially available (eg, in Invitrogen, Stratagene, Amersham Biosciences, Promega, etc.) or from depository institutions such as the American Type Culture Collection (ATCC, Rockville, Md.), or have become numerous The subject matter of publications that describe its sequence, organization, and methods of production, allowing skilled artisans to apply it.
合適的病毒載體之代表性實例由多種不同病毒(例如反轉錄病毒、腺病毒、腺病毒相關病毒(AAV)、痘病毒、疱疹病毒、麻疹病毒、泡沫病毒、α病毒、水泡性口炎病毒、慢病毒等)產生。如上文所描述,術語「病毒載體」涵蓋載體DNA、基因組DNA以及由其產生之病毒粒子,且尤其感染性病毒粒子。在一較佳實施例中,反轉錄病毒載體或慢病毒載體。在本發明之一較佳實施例中,慢病毒用以將轉基因引入至經分化細胞中。Representative examples of suitable viral vectors are derived from a variety of different viruses (eg, retroviruses, adenoviruses, adeno-associated viruses (AAV), poxviruses, herpesviruses, measles viruses, foamy viruses, alphaviruses, vesicular stomatitis virus, lentivirus, etc.). As described above, the term "viral vector" encompasses vector DNA, genomic DNA, and virions produced therefrom, and especially infectious virions. In a preferred embodiment, a retroviral vector or a lentiviral vector. In a preferred embodiment of the present invention, lentiviruses are used to introduce transgenes into differentiated cells.
合適的質體載體之代表性實例包括(但不限於) pREP4、pCEP4 (Invitrogen)、pCI (Promega)、pVAX (Invitrogen)及pGWiz (Gene Therapy System Inc.)。Representative examples of suitable plastid vectors include, but are not limited to, pREP4, pCEP4 (Invitrogen), pCI (Promega), pVAX (Invitrogen), and pGWiz (Gene Therapy System Inc.).
用於向主題細胞提供重新編程因子作為核酸之載體將典型地包含用於驅動重新編程因子核酸之表現(亦即轉錄活化)之合適啟動子。此可包括廣泛起作用的啟動子,例如CMV-b-肌蛋白啟動子或誘導性啟動子,諸如在特定細胞群體中有效或對諸如四環素之藥物的存在有反應之啟動子。A vector used to provide a reprogramming factor as a nucleic acid to a subject cell will typically comprise a suitable promoter for driving the expression (ie, transcriptional activation) of the reprogramming factor nucleic acid. This can include broadly acting promoters such as the CMV-b-myosin promoter or inducible promoters such as those that are efficient in a particular cell population or responsive to the presence of drugs such as tetracycline.
隨後,可藉由在飼養細胞之存在下在重新編程所得體細胞以產生iPSC之條件下培養及擴增所得體細胞而將如本文中所描述的含有Cbx家族基因序列及重新編程因子序列之經基因修飾之體細胞轉變為iPSC。本發明之iPSC的組合物及應用Subsequently, a recombinant protein containing Cbx family gene sequences and reprogramming factor sequences as described herein can be grown by culturing and expanding the resulting somatic cells in the presence of feeder cells under conditions that reprogram the resulting somatic cells to generate iPSCs. Genetically modified somatic cells are transformed into iPSCs.Composition and application ofiPSCof thepresent invention
在與將不干擾其預期用途的諸如培養基之載劑或稀釋劑混合時,iPSC為實質上分離的。可替代地,本發明之iPSC可存在於生長基質中或經固定化於表面上。iPSCs are substantially isolated when mixed with a carrier or diluent, such as a culture medium, that will not interfere with their intended use. Alternatively, the iPSCs of the present invention may be present in a growth matrix or immobilized on a surface.
由以上方法產生之iPSC可用於經由植入來復原或補充分化或分化接受者中之細胞。接受者中之誘導性細胞可分化為各種譜系之細胞類型。經分化細胞之實例包括來自外胚層(例如神經元及纖維母細胞)、中胚層(例如心肌細胞)或內胚層(例如胰臟細胞)譜系之任何經分化細胞。經分化細胞可為一或多個:胰臟β細胞、神經幹細胞、神經元(例如多巴胺激導性神經元)、寡樹突神經膠質細胞、寡樹突神經膠質細胞先驅細胞、肝細胞、肝幹細胞、星形膠質細胞、肌細胞、造血細胞或心肌細胞。iPSCs generated by the above methods can be used to restore or replenish cells in differentiated or differentiated recipients via engraftment. Induced cells in recipients can differentiate into cell types of various lineages. Examples of differentiated cells include any differentiated cell from the ectoderm (eg, neurons and fibroblasts), mesoderm (eg, cardiomyocytes), or endoderm (eg, pancreatic cells) lineages. Differentiated cells can be one or more of: pancreatic beta cells, neural stem cells, neurons (eg, dopamine stimulating neurons), oligodendritic glial cells, oligodendritic glial precursor cells, hepatocytes, liver Stem cells, astrocytes, muscle cells, hematopoietic cells or cardiomyocytes.
衍生自誘導性細胞之經分化細胞可為終末分化細胞,或其可能夠產生特定譜系之細胞。舉例而言,誘導性細胞可分化為多種多能細胞類型,例如神經幹細胞、心臟幹細胞或肝幹細胞。幹細胞可隨後進一步分化為新穎細胞類型,例如神經幹細胞可分化為神經元;心臟幹細胞可分化為心肌細胞;且肝幹細胞可分化為肝細胞。Differentiated cells derived from inducible cells can be terminally differentiated cells, or they can be capable of giving rise to cells of a particular lineage. For example, induced cells can differentiate into various pluripotent cell types, such as neural stem cells, cardiac stem cells, or hepatic stem cells. Stem cells can then be further differentiated into novel cell types, eg, neural stem cells can be differentiated into neurons; cardiac stem cells can be differentiated into cardiomyocytes; and liver stem cells can be differentiated into hepatocytes.
在本發明之一些實施例中,藉由檢查其生長為含有全部三種胚細胞之畸胎瘤的能力來活體內測試細胞之多能性。在另一實施例中,藉由某些標記物在活體外培養細胞中的表現來測試多能性。在又另一實施例中,藉由其對胚胎發育為活有機體之貢獻度來測試細胞之多能性。將多能幹細胞注射至胚胎囊胚之內部細胞塊(ICM)中,該胚胎囊胚隨後經植入至雌性有機體之子宮中且發育為胎兒。術語「嵌合」係指幹細胞及其後代對產生活有機體中之各種組織的全部三種胚層之貢獻度。In some embodiments of the invention, cells are tested for pluripotency in vivo by examining their ability to grow into teratomas containing all three types of blast cells. In another embodiment, pluripotency is tested by the expression of certain markers in cultured cells in vitro. In yet another embodiment, a cell is tested for pluripotency by its contribution to the development of an embryo into a living organism. Pluripotent stem cells are injected into the inner cell mass (ICM) of embryonic blastocysts, which are then implanted into the uterus of a female organism and develop into a fetus. The term "chimerism" refers to the degree to which stem cells and their progeny contribute to all three germ layers that produce various tissues in a living organism.
存在使誘導性細胞分化為更特殊的細胞類型之眾多方法。分化誘導性細胞之方法可類似於用以分化幹細胞,詳言之,ES細胞、MSC、MAPC、MIAMI、造血幹細胞(HSC)的彼等方法。在一些情況下,分化在活體外發生;在一些情況下,分化在活體內發生。Numerous methods exist for differentiating induced cells into more specialized cell types. Methods of differentiation-inducing cells can be similar to those used to differentiate stem cells, in particular, ES cells, MSCs, MAPCs, MIAMIs, hematopoietic stem cells (HSCs). In some cases, differentiation occurs in vitro; in some cases, differentiation occurs in vivo.
在一個實施例中,神經幹細胞可藉由在頭蛋白或其他骨形態生成蛋白質拮抗劑之存在下將誘導性細胞培養為浮置聚集物來產生。在另一實例中,神經幹細胞可藉由在生長因子之存在下在懸浮液中培養誘導性細胞以形成聚集物來產生。In one embodiment, neural stem cells can be generated by culturing induced cells as floating aggregates in the presence of noggin or other bone morphogenic protein antagonists. In another example, neural stem cells can be generated by culturing induced cells in suspension in the presence of growth factors to form aggregates.
衍生自誘導性細胞之神經幹細胞可分化為神經元、寡樹突神經膠質細胞或星形膠質細胞。通常,用以產生神經幹細胞之條件亦可用以產生神經元、寡樹突神經膠質細胞或星形膠質細胞。Neural stem cells derived from induced cells can differentiate into neurons, oligodendritic glial cells, or astrocytes. Typically, the conditions used to generate neural stem cells can also be used to generate neurons, oligodendritic glial cells, or astrocytes.
誘導性細胞或自誘導性細胞分化之細胞可用作療法以治療疾病(例如遺傳缺陷)。該療法可針對治療疾病之病因;或可替代地,該療法可用以治療疾病或病狀之影響。誘導性細胞可經轉移至或接近於個體中之受損部位;或可以允許細胞遷移或回歸至受損部位之方式將該等細胞引入至個體中。經轉移細胞可有利地替換損壞或受損細胞,且允許個體之總體病狀的改善。在一些情況下,經轉移細胞可刺激組織再生或修復。Induced cells or cells differentiated from induced cells can be used as therapy to treat diseases (eg, genetic defects). The therapy can be directed towards treating the cause of the disease; or alternatively, the therapy can be used to treat the effects of the disease or condition. The inducible cells can be transferred to or near the site of damage in the subject; or the cells can be introduced into the subject in a manner that allows the cells to migrate or return to the site of damage. The transferred cells can advantageously replace damaged or damaged cells and allow for an improvement in the overall condition of the individual. In some instances, the transferred cells can stimulate tissue regeneration or repair.
經轉移細胞可為自誘導性細胞分化之細胞。經轉移細胞亦可為自誘導性細胞分化之多能幹細胞。在一些情況下,經轉移細胞可為尚未分化之誘導性細胞。The transferred cells can be cells differentiated from induced cells. The transferred cells can also be pluripotent stem cells differentiated from induced cells. In some cases, the transferred cells can be induced cells that have not yet differentiated.
誘導性細胞可分化為細胞,且隨後經轉移至罹患廣泛範圍之疾病或病症的個體。罹患神經疾病或病症之個體可尤其得益於幹細胞療法。在一些方法中,誘導性細胞可分化為神經幹細胞或神經細胞,且隨後經移植至受損部位以治療神經病狀,例如阿茲海默症、帕金森氏病、多發性硬化症、腦梗塞、脊髓損傷或其他中樞神經系統病症。因此,本發明之另一態樣提供一種使用本發明之iPSC來治療神經退化性疾病的方法。在一些實施例中,神經退化性疾病包括(但不限於)帕金森氏病、多發性硬化症(MS)、肌肉萎縮性側索硬化(ALS)、脊髓性肌萎縮(SMA)、亨廷頓氏病或阿茲海默病。在一個實施例中,iPSC以範圍介於約1 × 105個細胞/劑量/天至約1 × 108個細胞/劑量/天之劑量投與。在一個實施例中,經分化神經元幹細胞以約1 × 106個細胞/劑量/天之劑量投與。Induced cells can be differentiated into cells and subsequently transferred to individuals suffering from a wide range of diseases or disorders. Individuals suffering from neurological diseases or disorders may especially benefit from stem cell therapy. In some methods, the induced cells can be differentiated into neural stem cells or neural cells, and subsequently transplanted into the damaged site to treat neurological conditions, such as Alzheimer's disease, Parkinson's disease, multiple sclerosis, cerebral infarction, Spinal cord injury or other central nervous system disorders. Accordingly, another aspect of the present invention provides a method of treating neurodegenerative diseases using the iPSCs of the present invention. In some embodiments, neurodegenerative diseases include, but are not limited to, Parkinson's disease, multiple sclerosis (MS), amyotrophic lateral sclerosis (ALS), spinal muscular atrophy (SMA), Huntington's disease or Alzheimer's disease. In one embodiment, iPSCs are administered at a dose ranging from about1 x 105 cells/dose/day to about 1 x108 cells/dose/day. In one embodiment, the differentiated neuronal stem cells are administered at a dose of about1 x 106 cells/dose/day.
在本發明之一個實施例中,iPSC分化為隨後經投與至有需要之個體中的神經幹細胞及/或含有神經元幹細胞之先驅細胞。一般熟習此項技術者將能夠使用類似或等效於本文中所描述之方法及材料自iPSC培養物衍生神經幹細胞及/或含有神經幹細胞之先驅細胞混合物。In one embodiment of the invention, iPSCs differentiate into neural stem cells and/or precursor cells containing neuronal stem cells that are subsequently administered to an individual in need. One of ordinary skill in the art will be able to derive neural stem cells and/or precursor cell mixtures containing neural stem cells from iPSC cultures using methods and materials similar or equivalent to those described herein.
在一較佳實施例中,將自iPSC分化之神經元幹細胞投與至需要治療或減輕帕金森氏病之個體中。一般熟習此項技術者將能夠富集或分離疾病治療所需要之神經元幹細胞,且使用類似或等效於本文中所描述之方法及材料以將細胞投與至需要所要治療效果之個體中。In a preferred embodiment, neuronal stem cells differentiated from iPSCs are administered to an individual in need of treatment or alleviation of Parkinson's disease. One of ordinary skill in the art will be able to enrich or isolate neuronal stem cells needed for disease treatment, and use methods and materials similar or equivalent to those described herein to administer the cells to individuals in need of the desired therapeutic effect.
對於多發性硬化症之治療,神經幹細胞可分化為隨後經轉移至罹患MS之個體中的寡樹突神經膠質細胞或寡樹突神經膠質細胞之先驅細胞。For the treatment of multiple sclerosis, neural stem cells can differentiate into oligodendritic glial cells or precursor cells of oligodendritic glial cells that are subsequently transferred into individuals suffering from MS.
除神經病症以外之疾病亦可藉由使用自誘導性細胞分化之細胞的幹細胞療法來治療,該等誘導性細胞例如誘導性多能或多能性幹細胞。諸如缺血性心肌症之退化性心臟疾病、傳導疾病及先天性缺陷可得益於幹細胞療法。Diseases other than neurological disorders can also be treated by stem cell therapy using cells differentiated from induced cells, such as induced pluripotent or pluripotent stem cells. Degenerative heart diseases, conduction diseases, and birth defects such as ischemic cardiomyopathy can benefit from stem cell therapy.
可經由以下途徑中之任一者將細胞引入至個體中:非經腸、靜脈內、動脈內、肌內、皮下、經皮、氣管內、腹膜內或向脊髓液中。The cells can be introduced into the individual via any of the following routes: parenteral, intravenous, intraarterial, intramuscular, subcutaneous, transdermal, intratracheal, intraperitoneal, or into spinal fluid.
本發明亦提供一種醫藥組合物,其包含本發明之iPSC群體、自本發明之iPSC分化之多能幹細胞或由多能幹細胞產生之經分化細胞。The present invention also provides a pharmaceutical composition comprising the iPSC population of the present invention, pluripotent stem cells differentiated from the iPSCs of the present invention, or differentiated cells generated from pluripotent stem cells.
根據一些實施例,本發明之組合物可用包括(但不限於)溶劑之賦形劑、載劑或媒劑來調配。醫藥學上可接受之載劑必須具有充分高的純度且具有足夠低的毒性以使其適用於向所治療之哺乳動物投與。該醫藥學上可接受之載劑進一步應維持活性劑之穩定性及生物可用性。醫藥學上可接受之載劑可為液體或固體,且經選擇(考慮計劃投與方式)以在與活性劑及給定組合物之其他組分組合時提供所要體積、稠度等。用於本發明之組合物之合適的醫藥學上可接受之載劑包括(但不限於)水、鹽溶液、醇、聚乙二醇、明膠、直鏈澱粉、硬脂酸鎂、滑石、矽酸、黏稠石蠟、羥甲基纖維素、聚乙烯吡咯啶酮及其類似者。此類載劑溶液亦可含有緩衝劑、稀釋劑及其他合適的添加劑。如本文中所使用,術語「緩衝劑」係指其化學組成中和酸或鹼而無pH之顯著變化的溶液或液體。本發明所設想之緩衝劑之實例包括(但不限於)杜爾貝科氏(Dulbecco's)磷酸鹽緩衝鹽水(PBS)、林格氏溶液(Ringer's solution)、含5%右旋糖之水(D5W)、生理(normal/physiologic)鹽水(0.9% NaCl)。根據一些實施例,輸注溶液與個體組織等張。根據一些實施例,輸注溶液對於個體組織為高張的。用於非經腸投與之本發明之組合物可包括在諸如醇之常見溶劑中的醫藥學上可接受之載劑,諸如無菌水溶液、非水溶液,或在液體油基中之溶液。According to some embodiments, the compositions of the present invention may be formulated with excipients, carriers or vehicles including, but not limited to, solvents. A pharmaceutically acceptable carrier must be of sufficiently high purity and sufficiently low toxicity to be suitable for administration to the mammal being treated. The pharmaceutically acceptable carrier should further maintain the stability and bioavailability of the active agent. Pharmaceutically acceptable carriers can be liquid or solid, and are selected (considering the intended mode of administration) to provide the desired volume, consistency, etc. when combined with the active agent and other components of a given composition. Suitable pharmaceutically acceptable carriers for the compositions of the present invention include, but are not limited to, water, saline solutions, alcohols, polyethylene glycols, gelatin, amylose, magnesium stearate, talc, silicon Acid, viscous paraffin, hydroxymethylcellulose, polyvinylpyrrolidone and the like. Such carrier solutions may also contain buffers, diluents and other suitable additives. As used herein, the term "buffer" refers to a solution or liquid whose chemical composition neutralizes acid or base without significant changes in pH. Examples of buffers contemplated by the present invention include, but are not limited to, Dulbecco's Phosphate Buffered Saline (PBS), Ringer's solution, 5% dextrose in water (D5W ), normal/physiologic saline (0.9% NaCl). According to some embodiments, the infusion solution is isotonic with the individual tissue. According to some embodiments, the infusion solution is hypertonic to the individual tissue. Compositions of the present invention for parenteral administration may include pharmaceutically acceptable carriers in common solvents such as alcohols, such as sterile aqueous solutions, non-aqueous solutions, or solutions in liquid oil bases.
本發明之組合物可以無菌可注射水性或油性懸浮液之形式非經腸投與。如本文中所使用,術語「非經腸」或「非經腸地」係指藉助於注射(亦即藉由注射投與)引入至身體中,該注射包括(但不限於)輸注技術。根據一些實施例,非經腸投與包括(但不限於)血管內遞送(意指向血管中)、靜脈內遞送(意指向靜脈中)、動脈內遞送(意指向動脈中)、骨內遞送(意指向骨髓中)、肌內遞送(意指向肌肉中)、皮下遞送(意指在皮膚下)、心臟遞送(意指向心臟、心肌中)等。The compositions of the present invention can be administered parenterally in the form of sterile injectable aqueous or oily suspensions. As used herein, the term "parenteral" or "parenterally" refers to introduction into the body by means of injection (ie, administration by injection), including but not limited to infusion techniques. According to some embodiments, parenteral administration includes, but is not limited to, intravascular delivery (meaning into a blood vessel), intravenous delivery (meaning into a vein), intraarterial delivery (meaning into an artery), intraosseous delivery ( Intramuscular delivery (meaning into the bone marrow), intramuscular delivery (meaning into the muscle), subcutaneous delivery (meaning under the skin), cardiac delivery (meaning into the heart, into the myocardium), and the like.
遞送途徑可變化,且視退化性疾病之起源而定。在一較佳實施例中,用於治療中樞神經系統中之退化性病狀之遞送途徑為顱內注射。The route of delivery can vary and depends on the origin of the degenerative disease. In a preferred embodiment, the route of delivery for the treatment of degenerative conditions in the central nervous system is intracranial injection.
在本發明之一個實施例中,藉由個體在諸如旋轉桿中跑步、在平衡木上行走或在開放空間中之認知運動的各種運動中維持其平衡的能力來檢測個體之認知及運動行為。實施例不限於類似或等效於本發明在本文中所描述之彼等。對測試個體之認知及運動行為的方法進行修改在此項技術之一般技能內,且僅需要常規實驗。In one embodiment of the invention, an individual's cognitive and motor behavior is detected by the individual's ability to maintain their balance in various movements such as running in a spin bar, walking on a balance beam, or cognitive movement in an open space. Embodiments are not limited to those similar or equivalent to the invention described herein. Modifications to methods of testing an individual's cognitive and motor behavior are within the general skill of the art and require only routine experimentation.
以下實例說明本發明。The following examples illustrate the invention.
實例1 自鼠胚胎纖維母細胞產生誘導性多能幹細胞Example 1 Generation of induced pluripotent stem cells from murine embryonic fibroblasts
製備病毒virus preparation
單獨製備三種病毒載體:在一個實施例中,製備慢病毒載體pAS3-Oct4、pAS3-Sox2及PAS3-Cbx7,其中各基因之cDNA為可商購的(Thermos Scientific),其中限制位點EcoRI允許選殖至pAS3載體中。反轉錄病毒或慢病毒藉由使用293T細胞株作為宿主細胞來製備。針對各反轉錄病毒或慢病毒收集且濃縮經轉染293T細胞之上清液,該上清液隨後用於製備誘導性多能幹細胞(iPSC) (圖1)。Three viral vectors were prepared separately: In one example, lentiviral vectors pAS3-Oct4, pAS3-Sox2, and PAS3-Cbx7 were prepared, wherein the cDNA for each gene was commercially available (Thermos Scientific), and the restriction site EcoRI allowed selection. into the pAS3 vector. Retroviruses or lentiviruses were prepared by using the 293T cell line as host cells. Transfected 293T cell supernatants were collected and concentrated for each retrovirus or lentivirus, which were then used to prepare induced pluripotent stem cells (iPSCs) (Figure 1).
製備鼠胚胎纖維母細胞Preparation of mouse embryonic fibroblasts
鼠胚胎纖維母細胞(MEF)用於製備iPSC。自懷孕C57BL6小鼠取出處於E13.5之胎兒,且用磷酸鹽緩衝鹽水(PBS)使胎兒暴露於培養皿中。藉由自胎兒解剖出四肢、頭部、尾部及器官而獲取含有MEF之組織,且在用PBS洗滌三次之後在培養皿中絞碎該等組織。隨後將0.1mM胰蛋白酶/1mM EDTA溶液(GIBCO BRL)添加至培養皿中以用於胰蛋白酶消化。隨後將已絞碎組織轉移至錐形管中且在振盪器上搖盪20分鐘。隨後將組織培養基(具有10%胎牛血清、1%青黴素及鏈黴素及1%非必需胺基酸(NEAA)之DMEM)添加至該管中。隨後將上清液轉移至新錐形管中且在離心機中旋轉。將細胞集結粒再懸浮於組織培養基中且轉移至培養皿以用於培育(5% CO2及37℃)。Murine embryonic fibroblasts (MEFs) were used to prepare iPSCs. Fetuses at E13.5 were removed from pregnant C57BL6 mice and exposed to petri dishes with phosphate buffered saline (PBS). Tissues containing MEFs were obtained by dissecting limbs, heads, tails and organs from fetuses and minced in petri dishes after washing three times with PBS. A 0.1 mM trypsin/1 mM EDTA solution (GIBCO BRL) was then added to the dishes for trypsinization. The minced tissue was then transferred to a conical tube and shaken on a shaker for 20 minutes. Tissue culture medium (DMEM with 10% fetal bovine serum, 1% penicillin and streptomycin, and 1% non-essential amino acids (NEAA)) was then added to the tube. The supernatant was then transferred to a new conical tube and spun in a centrifuge. Cell pellets were resuspended in tissue culture medium and transferred to petri dishes for incubation (5%CO2 and 37°C).
飼養細胞用以養育纖維母細胞培養物,且為稍後發育之iPSC提供生長因子,從而預防幹細胞之底層分化。飼養細胞衍生自在37℃下用含有絲裂黴素C (購自Roche)之細胞培養基培育2.5小時的MEF。絲裂黴素C阻止MEF之增殖,其隨後經歷與鼠胚胎纖維母細胞之共培養。Feeder cells are used to feed fibroblast cultures and to provide growth factors for later developing iPSCs, preventing basal differentiation of stem cells. Feeder cells were derived from MEFs incubated with cell culture medium containing mitomycin C (purchased from Roche) for 2.5 hours at 37°C. Mitomycin C prevented the proliferation of MEFs, which were subsequently subjected to co-culture with murine embryonic fibroblasts.
iPSCiPSC細胞培養cell culture
藉由在37℃培育箱(5% CO2)中將6孔盤培育30分鐘而用明膠塗佈該盤,其中各孔中具有1ml之0.2%明膠。將2ml之溫熱DMEM培養基轉移至各孔中以在37℃下以5%CO2將新鮮解凍之飼養細胞培育隔夜。用幹細胞培育培養基(含有1% L-麩醯胺酸、7.5ml Hyclone FBS、0.5ml NEAA、91μlβ-巰基乙醇及5μl白血病抑制因子之42.5ml DMEM)替換DMEM培養基,且隨後將該等細胞培養1小時。隨後在37℃下以5% CO2用飼養細胞培養誘導性多能幹細胞。在細胞培養後兩天更新培養基,且之後每日更新以確保對幹細胞生長之營養物質的適當供應。The 6-well plate was coated with gelatin by incubating it in a 37°C incubator (5% CO2 ) for 30 minutes with 1 ml of 0.2% gelatin in each well. 2 ml of warm DMEM medium was transferred to each well to incubate freshly thawed feeder cells overnight at 37°C with 5% CO2 . DMEM medium was replaced with stem cell culture medium (42.5 ml DMEM containing 1% L-glutamic acid, 7.5 ml Hyclone FBS, 0.5 ml NEAA, 91 μl β-mercaptoethanol, and 5 μl leukemia inhibitory factor), and the cells were then cultured for 1 Hour. Induced pluripotent stem cells were subsequently cultured with feeder cells at 37 °C with 5%CO . The medium was refreshed two days after cell culture and daily thereafter to ensure proper supply of nutrients for stem cell growth.
將Cbx-7引入至MEF細胞(經分化細胞)導致iPSC群落產生。Cbx-7與Sox-2及Oct-4之組合導致iPSC產生增加,其中產生之效率大於或為約0.01%。Introduction of Cbx-7 into MEF cells (differentiated cells) resulted in the generation of iPSC colonies. The combination of Cbx-7 with Sox-2 and Oct-4 resulted in increased iPSC production with an efficiency of production greater than or about 0.01%.
鹼性磷酸酶染色alkaline phosphatase staining
鹼性磷酸酶染色係用以測試所得iPSC或ES細胞之幹性。在用幹細胞培育培養基培育48小時之後,用各種濃度之新鮮N-丁烯基苯酞緩衝液替換iPSC或ES細胞培養物。在某一培養時長之後,將細胞用PBS洗滌兩次,且在4℃下用1ml之80%乙醇固定2至24小時。隨後替換細胞且將其浸沒於蒸餾水中以洗掉乙醇。用蒸餾水施加額外洗滌及浸沒2至3分鐘。在移除蒸餾水之後,隨後替換細胞且在室溫下將其浸沒於100mM Tris-HCl緩衝液(pH 8.2至8.5)中5分鐘。移除Tris緩衝液且用白細胞鹼性磷酸酶套組替換以用於染色20至30分鐘。在移除鹼性磷酸酶基質工作溶液之後,將細胞準備好針對鹼性磷酸酶之活性進行觀測及攝影,且用100mM Tris-HCl緩衝液(pH 8.2至8.5)洗滌。鹼性磷酸酶之活性在幹細胞中較高,以基質染色展示為紅色,而在經分化幹細胞中較低,以淡紅色或無顏色活性展示(圖3)。Alkaline phosphatase staining was used to test the stemness of the resulting iPSCs or ES cells. After 48 hours of incubation with stem cell culture medium, iPSC or ES cell cultures were replaced with fresh N-butenylphthalide buffer at various concentrations. After a certain incubation period, cells were washed twice with PBS and fixed with 1 ml of 80% ethanol for 2 to 24 hours at 4°C. The cells were then replaced and submerged in distilled water to wash off the ethanol. An additional wash and immersion was applied with distilled water for 2 to 3 minutes. After removal of distilled water, cells were then replaced and immersed in 100 mM Tris-HCl buffer (pH 8.2 to 8.5) for 5 minutes at room temperature. Tris buffer was removed and replaced with leukocyte alkaline phosphatase kit for staining for 20 to 30 minutes. After removal of the alkaline phosphatase substrate working solution, cells were prepared for observation and photography for alkaline phosphatase activity, and washed with 100 mM Tris-HCl buffer (pH 8.2 to 8.5). Alkaline phosphatase activity was higher in stem cells, shown as red by stromal staining, and lower in differentiated stem cells, shown as reddish or no color activity (Figure 3).
免疫螢光染色Immunofluorescence staining
諸如Nanog、Oct4及Sox2之某些標記物之表現可用以研究幹細胞培養物之幹性(圖3)。為測定此等標記物之表現,利用此等標記物之免疫螢光染色。在染色之前,iPSC或ES細胞經歷幹細胞培養基48小時。各種濃度之新鮮N-丁烯基苯酞緩衝劑用以培育細胞持續某一時長,且將細胞用PBS洗滌並在室溫下用4%多聚甲醛固定10分鐘。使用0.1% Tween-20/1X PBS洗滌細胞三次,每次浸沒10分鐘。隨後在室溫下藉由0.3% Triton-X 100/1× PBS滲透細胞30分鐘,且在室溫下用5% FBS/1× PBS使該等細胞經歷阻斷持續2小時。在室溫下在1:100稀釋下使用所關注標記物之初級抗體來染色隔夜。隨後將細胞浸沒且用0.1% Tween-20/1× PBS洗滌5分鐘。在室溫下在1:500稀釋下使用螢光結合二級抗體來染色1小時。隨後將細胞浸沒且用0.1% Tween-20/1× PBS洗滌10分鐘。使用含有DAPI之封固劑(用於DNA染色)來封固及密封細胞以在倒置顯微鏡下觀測。The expression of certain markers such as Nanog, Oct4 and Sox2 can be used to study the stemness of stem cell cultures (Figure 3). To determine the performance of these markers, immunofluorescent staining of these markers was used. Before staining, iPSCs or ES cells were subjected to stem cell medium for 48 hours. Various concentrations of fresh N-butenylphthalide buffer were used to incubate cells for a certain length of time, and cells were washed with PBS and fixed with 4% paraformaldehyde for 10 minutes at room temperature. Cells were washed three times with 0.1% Tween-20/1X PBS by immersion for 10 minutes each. Cells were then permeabilized with 0.3% Triton-
用Cbx7引入MEF細胞(經分化細胞)意外導致iPSC群落形成。另外,用三種因子Oct-4、Sox-2及Cbx-7引入MEF細胞導致高效率之iPSC產生,其中經重新編程之iPSC群落表現幹細胞標記物AP、Oct4、Sox2、SSEA1及SSEA4 (圖3)。Introduction of MEF cells (differentiated cells) with Cbx7 unexpectedly resulted in the formation of iPSC colonies. In addition, introduction of MEF cells with the three factors Oct-4, Sox-2 and Cbx-7 resulted in high-efficiency iPSC generation, with the reprogrammed iPSC population expressing stem cell markers AP, Oct4, Sox2, SSEA1 and SSEA4 (Figure 3) .
畸胎瘤形成Teratoma formation
畸胎瘤形成為用以活體內研究iPSC或ES細胞培養物之幹性的標準方法。為測試iPSC培養物之幹性,在SCID小鼠之背部皮下注射iPSC,且在6至8週之後形成畸胎瘤。將腫瘤/畸胎瘤細胞以手術方式移除且浸沒於4%多聚甲醛中。畸胎瘤細胞隨後經冷凍且經歷組織之剖切。隨後用蘇木精及曙紅對腫瘤切片染色,且觀測到三個生殖細胞層:外胚層、中胚層及內胚層之完全發育(圖4)。Teratoma formation is a standard method to study the stemness of iPSC or ES cell cultures in vivo. To test the dryness of iPSC cultures, iPSCs were injected subcutaneously on the back of SCID mice and teratomas formed after 6 to 8 weeks. Tumor/teratoma cells were surgically removed and submerged in 4% paraformaldehyde. Teratoma cells were then frozen and subjected to dissection of the tissue. Tumor sections were subsequently stained with hematoxylin and eosin, and the complete development of the three germ cell layers: ectoderm, mesoderm and endoderm was observed (Figure 4).
Cbx7Cbx7、,Oct4Oct4及andSox2Sox2之組合導致高效率之The combination leads to high efficiencyiPSCiPSC產生produce
引入Sox-2或Oct-4一般不會導致iPSC產生,而將單個Cbx-7基因引入至經分化細胞中導致低效率之iPSC產生(圖2)。一次引入兩種基因(諸如Sox2及Cbx7或Oct4及Cbx7)增加iPSC產生之發生率。iPSC產生之最佳效率發現於用三種基因(Oct4、Sox2及Cbx7)引入之經分化細胞中(圖2),藉由該三種基因,iPSC產生之效率為約0.01%。Introduction of Sox-2 or Oct-4 generally did not result in iPSC production, whereas introduction of a single Cbx-7 gene into differentiated cells resulted in inefficient iPSC production (Figure 2). Introduction of two genes at once, such as Sox2 and Cbx7 or Oct4 and Cbx7, increases the incidence of iPSC generation. The best efficiency of iPSC generation was found in differentiated cells introduced with three genes (Oct4, Sox2 and Cbx7) (Figure 2), with which the efficiency of iPSC generation was about 0.01%.
實例Example2 iPSC2 iPSCs分化為神經元幹細胞Differentiate into neuronal stem cells
首先在無LIF之幹細胞培育培養基:含有1% L-麩醯胺酸、15% Hyclone FBS、1% NEAA、1mM β-巰基乙醇、100U/ml青黴素及100μg/ml鏈黴素之85% DMEM中培養iPSC。將液體懸浮液中之2 × 106個細胞接種於各10cm2培養皿中,且隔日用無LIF之新鮮幹細胞培育培養基替換細胞培養物。在接種細胞之後四天,將iPS衍生之胚狀體(EB)轉移至新培養皿,且使其接種24小時。隨後用ITN-FN培養基:含有2mM L-麩醯胺酸、100U/ml青黴素、100 μg/ml鏈黴素、1% ITS-G培養基補充劑及5 μg/ml纖維結合蛋白之DMEM/F12供應細胞。在4天培育之後,隨後將細胞用PBS洗滌兩次,且用0.05%胰蛋白酶-EDTA胰蛋白酶化(以使細胞自培養皿剝離)5分鐘。用無LIF之等體積的幹細胞培育培養基將經剝離細胞中和且轉移至15ml錐形管。使該等管靜置5分鐘以沈澱較大細胞塊(未分化之胚狀體)。將上清液轉移至新錐形管,且在800 rpm下離心5分鐘。使細胞集結粒再懸浮,且以1.5 × 105個細胞/毫升在N-2培養基:具有2mM L-麩醯胺酸、100U/ml青黴素、100 μg/ml鏈黴素、1% N-2培養基補充劑及20ng/ml bFGF之DMEM/F12中培養。隨後將細胞接種至經聚-L-鳥胺酸/纖維結合蛋白預處理之培養皿上。經聚-L-鳥胺酸/纖維結合蛋白預處理之培養皿藉由以下方法製備:在37℃下用15 μg/ml聚-L-鳥胺酸/PBS溶液培育培養皿24小時。移除聚-L-鳥胺酸/PBS溶液,且用PBS緩衝液將培養皿洗滌三次,且在37℃下用PBS培育24小時。自培養皿移除PBS,且用新鮮製備之PBS洗滌。在37℃下使用1 μg/ml纖維結合蛋白/PBS來處理培養皿6小時。在移除纖維結合蛋白溶液且用PBS洗滌培養皿之後,將培養皿準備好使用。First in LIF-free stem cell culture medium: 85% DMEM containing 1% L-glutamic acid, 15% Hyclone FBS, 1% NEAA, 1 mM β-mercaptoethanol, 100 U/ml penicillin and 100 μg/ml streptomycin Culture iPSCs.2 x 106 cells in liquid suspension were seeded in each 10cm2 petri dish, and the cell culture was replaced every other day with fresh stem cell culture medium without LIF. Four days after seeding the cells, iPS-derived embryoid bodies (EBs) were transferred to new dishes and allowed to seed for 24 hours. Subsequent use of ITN-FN medium: DMEM/F12 containing 2 mM L-glutamic acid, 100 U/ml penicillin, 100 μg/ml streptomycin, 1% ITS-G medium supplement and 5 μg/ml fibronectin was supplied cell. After 4 days of incubation, cells were then washed twice with PBS and trypsinized with 0.05% trypsin-EDTA (to detach cells from the dish) for 5 minutes. The stripped cells were neutralized with an equal volume of stem cell growth medium without LIF and transferred to a 15 ml conical tube. The tubes were allowed to stand for 5 minutes to pellet larger cell clumps (undifferentiated embryoid bodies). The supernatant was transferred to a new conical tube and centrifuged at 800 rpm for 5 minutes. Cell pellets were resuspended at1.5 x 10 cells/ml in N-2 medium: with 2 mM L-glutamic acid, 100 U/ml penicillin, 100 μg/ml streptomycin, 1% N-2 Medium supplements and 20ng/ml bFGF in DMEM/F12. Cells were then seeded onto poly-L-ornithine/fibronectin pretreated dishes. The dishes pretreated with poly-L-ornithine/fibronectin were prepared by incubating the dishes with 15 μg/ml poly-L-ornithine/PBS solution for 24 hours at 37°C. The poly-L-ornithine/PBS solution was removed, and the dishes were washed three times with PBS buffer and incubated with PBS for 24 hours at 37°C. PBS was removed from the dish and washed with freshly prepared PBS. The dishes were treated with 1 μg/ml fibronectin/PBS for 6 hours at 37°C. After removing the fibronectin solution and washing the dishes with PBS, the dishes were ready for use.
細胞培養物每日用20 ng/mL bFGF補給,且隔日用新鮮N-2培養基補給。每4至5天將細胞繼代至經聚-L-鳥胺酸/纖維結合蛋白預處理之新培養皿,且可使其繼代高達五倍。在分化之後,藉由免疫螢光染色針對iPS-NSC測定神經元幹細胞製造者巢蛋白及Tuj1之表現(圖5)。Cell cultures were supplemented with 20 ng/mL bFGF daily and fresh N-2 medium every other day. Cells were passaged every 4 to 5 days to new dishes pretreated with poly-L-ornithine/fibronectin and could be passaged up to five-fold. After differentiation, the expression of neuronal stem cell maker Nestin and Tuj1 was determined by immunofluorescence staining against iPS-NSCs (Figure 5).
實例Example33使用經use theiPSCiPSC分化之神經元幹細胞來治療帕金森氏病Differentiated neuronal stem cells to treat Parkinson's disease
MPTPMPTP誘導性帕金森氏病模型Induced Parkinson's Disease Model
以7 mg/ml之濃度在溶液中製備MPTP之工作溶液。以20 mg/kg之劑量經由腹膜內注射一天4次且各劑量之間2小時來給與各小鼠MPTP。熟習此項技術者已知,以此劑量及程序,小鼠隨時間發展帕金森氏病。A working solution of MPTP was prepared in solution at a concentration of 7 mg/ml. Each mouse was administered MPTP at a dose of 20 mg/kg via
移植神經元幹細胞Transplanted neuronal stem cells
在右頂骨及額骨處鑽三個點(A、B及C)以用於細胞移植。點A定位於前囟門與λ之間的中點之右側0.1mm處;點B定位於冠狀縫之頂部處;點C定位於點A與點B之間的中點處。Three points (A, B and C) were drilled at the right parietal and frontal bones for cell transplantation. Point A is located 0.1 mm to the right of the midpoint between bregma and λ; point B is located at the top of the coronal suture; point C is located at the midpoint between point A and point B.
出於標記目的,用1 μl/ml之Hoechst 33342對神經元幹細胞染色持續1小時。隨後,將神經元幹細胞離心且再懸浮於鹽水中。將再懸浮之細胞自點C至點A且隨後點B依序注射至小鼠顱中。對點A及點C之注射深度為3.5mm,且對點B為3 mm。在各點處以總計1 × 106個細胞/鼠注射8 ml體積之細胞。For labeling purposes, neuronal stem cells were stained with 1 μl/ml of Hoechst 33342 for 1 hour. Subsequently, neuronal stem cells were centrifuged and resuspended in saline. The resuspended cells were injected into the mouse skull sequentially from point C to point A and then point B. The injection depth was 3.5 mm for points A and C, and 3 mm for point B. Cells in a volume of 8 ml were injected at each point at a total of1 x 106 cells/mouse.
動物行為之分析Analysis of animal behavior
為評定動物自帕金森氏病症之恢復情況,使用三種工具來量測及分析動物行為:旋轉桿、運動機及平衡木。To assess the recovery of animals from Parkinson's disease, three tools were used to measure and analyze animal behavior: spin bar, exercise machine, and balance beam.
旋轉桿量測動物平衡及鍛煉之能力。旋轉桿在內側處具有用於嚙齒動物攀爬之旋轉軸及用以分隔通道之壁。旋轉軸之大小為直徑3 cm且長度5 cm。分隔壁為直徑25 cm且長度1cm。先前已確立,初始嚙齒動物(未經任何治療)可在5rpm之速度下停留於旋轉軸處超過3分鐘。每7天執行測試,總計3次。The rotating rod measures the ability of the animal to balance and exercise. The rotating rod has a rotating shaft at the inner side for rodents to climb and walls to separate the passages. The size of the rotating shaft is 3 cm in diameter and 5 cm in length. The dividing wall is 25 cm in diameter and 1 cm in length. It was previously established that naive rodents (without any treatment) could stay on the axis of rotation for more than 3 minutes at a speed of 5 rpm. Tests were performed every 7 days for a total of 3 times.
運動機為一種允許記錄動物之活動而不受外部之任何干擾的自動系統。An exercise machine is an automatic system that allows the movement of animals to be recorded without any external interference.
平衡木行走為一種用以測定嚙齒動物在高且窄的橋上之協調性的方法。平衡木之大小為寬度0.6 cm且長度120 cm。存在暗盒(13 cm3)以用於嚙齒動物在一側休息。將嚙齒動物置放於暗盒中3分鐘,且隨後置放於與暗盒相距15 cm之平衡木上。一旦嚙齒動物可輕易步行回至暗盒,則將與暗盒之距離增加至80cm。一旦達至80cm之距離,則收集兩種類型之量測值:(1)嚙齒動物自80cm點返回步行至暗盒之時長,及(2)嚙齒動物四肢滑出平衡木之頻率。每7天收集量測值3次。Balance beam walking is a method used to determine the coordination of rodents on tall and narrow bridges. The size of the balance beam is 0.6 cm in width and 120 cm in length. A cassette (13 cm3 ) was present for the rodents to rest on one side. Rodents were placed in the cassette for 3 minutes and then placed on a
在MPTP誘導性帕金森氏症候群模型中,衍生自經重新編程之iPSC的經分化神經元幹細胞(iPS-NSC)顯著改善平衡木行走及旋轉桿運動中之動物行為(*p<0.05. **p<0.01)。平衡木行走行為分析用以測試小鼠之平衡能力。相較於在用鹽水移植之小鼠之平衡能力,用iPS衍生之神經元幹細胞(iPS-NSC)移植顯著改善平衡能力(包括滑倒(圖6A)及通過時間(圖6B))。另外,旋轉桿分析用以測試小鼠之協調能力。相較於用鹽水移植之小鼠組中的協調能力,經iPS-NSC治療之小鼠改善協調能力(圖6C)。Differentiated neuronal stem cells (iPS-NSCs) derived from reprogrammed iPSCs significantly improved animal behavior during balance beam walking and rotarod exercise in the MPTP-induced Parkinson's syndrome model (*p < 0.05. **p <0.01). Balance beam walking behavior analysis was used to test the balance ability of mice. Transplantation with iPS-derived neuronal stem cells (iPS-NSCs) significantly improved balance ability, including slip (FIG. 6A) and transit time (FIG. 6B), compared to balance ability in mice transplanted with saline. In addition, rotarod analysis was used to test the coordination ability of the mice. Coordination was improved in iPS-NSC-treated mice compared to coordination in the group of mice transplanted with saline (FIG. 6C).
iPSCiPSC分化之神經元幹細胞能夠治療帕金森氏病症Differentiated neuronal stem cells could treat Parkinson's disease
吾等亦藉由免疫螢光染色而獲得黑質中之TH陽性細胞(多巴胺神經元)數目。結果顯示,在與鹽水治療組比較時,iPS-NSC治療組中之TH陽性細胞之數目增加。此資料指示,iPSC衍生之神經元幹細胞(iPS-NSC)治療之治療挽救黑質中之多巴胺神經元的數目(圖7)。另外,在iPS-NSC治療組中發現顯著減少之Iba1陽性細胞(作為炎症之指示的免疫細胞)之數目,從而指示在MPTP誘導性帕金森氏病模型中之免疫反應減小(圖8)。We also obtained the number of TH positive cells (dopamine neurons) in the substantia nigra by immunofluorescence staining. The results showed that the number of TH positive cells increased in the iPS-NSC treated group when compared to the saline treated group. This data indicates that treatment with iPSC-derived neuronal stem cell (iPS-NSC) treatment rescued the number of dopamine neurons in the substantia nigra (Figure 7). In addition, a significantly reduced number of Iba1-positive cells (immune cells indicative of inflammation) was found in the iPS-NSC treated group, indicating a reduced immune response in the MPTP-induced Parkinson's disease model (Figure 8).
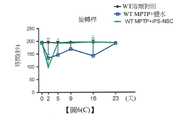
在existMPTPMPTP誘導性帕金森氏病中induced Parkinson's disease,,血清多巴胺之含量藉由Serum dopamine levels areiPS-NSCiPS-NSC治療增加Treatment increases
用ELISA套組來分析多巴胺含量-在行為評定之前一天藉由後眼眶出血自小鼠收集500 μl之血液。將收集之血液在室溫下靜置30分鐘,且在4℃下以1,000 rpm之速度旋轉10分鐘。收集血清/上清液,且在-20℃下儲存以用於後續分析。用購自Uson的多巴胺之酶免疫吸附劑分析套組來執行ELISA實驗。Dopamine levels were analyzed with an ELISA kit - 500 μl of blood was collected from mice by retro-orbital hemorrhage one day prior to behavioral assessment. The collected blood was left to stand at room temperature for 30 minutes and spun at 1,000 rpm for 10 minutes at 4°C. Serum/supernatant was collected and stored at -20°C for subsequent analysis. ELISA experiments were performed using the Dopamine Enzyme Immunosorbent Assay Kit from Uson.
在MPTP誘導性帕金森氏病模型中量測多巴胺之含量。發現iPS-NSC將血清中之多巴胺含量挽救至與未受損對照組相當的含量,從而指示iPS-NSC細胞修復神經元之能力(圖9)。Dopamine levels were measured in an MPTP-induced Parkinson's disease model. It was found that iPS-NSCs rescued dopamine levels in serum to levels comparable to those of undamaged controls, indicating the ability of iPS-NSC cells to repair neurons (Figure 9).
免疫螢光染色及分析Immunofluorescence staining and analysis
在行為評定之後,殺死小鼠並解剖以收集大腦組織。用4%多聚甲醛固定大腦組織且用OCT冷凍。將腦切片置於載片上且用TBST/PBST洗滌5分鐘以自大腦組織之邊緣清除剩餘OCT。藉由將載片浸沒於抗原獲取溶液(237.5ml之ddH2O中之12.5 ml Trilogy)中且在灶具中將其汽蒸3分鐘來執行抗原獲取。隨後用TBST/PBST將載片洗滌10分鐘。藉由在室溫下用0.3% Triton-X100將其培育30分鐘且用TBST/PBST洗滌10分鐘來滲透組織細胞。隨後在室溫下經1小時用含有5% FBS之阻斷緩衝液來阻斷載片。將所關注標記物之初級抗體添加至阻斷緩衝液中且在4℃下培育隔夜。用TBST/PBST將載片洗滌10分鐘,且在室溫下用二級抗體培育1小時。在用TBST/PBST洗滌10分鐘之後,在室溫下用PI對載片染色15分鐘。隨後再次用TBST/PBST將載片洗滌10分鐘,且用封固劑密封。Following behavioral assessment, mice were sacrificed and dissected to collect brain tissue. Brain tissue was fixed with 4% paraformaldehyde and frozen with OCT. Brain sections were placed on slides and washed with TBST/PBST for 5 minutes to remove residual OCT from the edges of the brain tissue. Antigen retrieval was performed by immersing the slides in antigen retrieval solution (12.5 ml Trilogy in 237.5 ml of ddH2O) and steaming it in the cooktop for3 minutes. The slides were then washed with TBST/PBST for 10 minutes. Tissue cells were infiltrated by incubating with 0.3% Triton-X100 for 30 minutes at room temperature and washing with TBST/PBST for 10 minutes. The slides were then blocked with blocking buffer containing 5% FBS for 1 hour at room temperature. Primary antibodies to the marker of interest were added to blocking buffer and incubated overnight at 4°C. Slides were washed with TBST/PBST for 10 minutes and incubated with secondary antibody for 1 hour at room temperature. After washing with TBST/PBST for 10 minutes, the slides were stained with PI for 15 minutes at room temperature. The slides were then washed again with TBST/PBST for 10 minutes and sealed with mounting medium.
MPTPMPTP誘導性帕金森氏病模型之model of induced Parkinson's diseaseTUNELTUNEL檢定check
為量測自小鼠收集之大腦組織內之細胞死亡,對載片上之大腦切片執行TUNEL檢定。使用可商購套組「DeadEndTMFluorometric TUNEL System」執行實驗。一般熟習此項技術者將能夠理解且藉由類似於或等效於本文中所描述之反應劑來執行此實驗。To measure cell death in brain tissue collected from mice, a TUNEL assay was performed on brain sections on slides. Experiments were performed using the commercially available kit "DeadEnd™ Fluorometric TUNEL System". One of ordinary skill in the art will be able to understand and perform this experiment with reagents similar or equivalent to those described herein.
用PBS將載片洗滌5分鐘,且隨後轉移至0.2% Triton-100/PBS進行5分鐘培育以滲透細胞膜。隨後,將載片洗滌兩次,每次5分鐘。隨後在室溫下在具有100 μl之平衡緩衝液的暗染色盒中將載片培育10分鐘,且將50 μl之TdT Mix添加至各樣本中。在37℃下,在暗處將載片進一步培育1小時。隨後,藉由用2× SSC緩衝液將載片培育15分鐘來阻止反應。藉由用PBS將其各自培育5分鐘而將載片洗滌三次,用DAPI染色,且隨後用封固劑密封。Slides were washed with PBS for 5 minutes and then transferred to 0.2% Triton-100/PBS for 5 minutes incubation to permeate cell membranes. Subsequently, the slides were washed twice for 5 minutes each. The slides were then incubated for 10 minutes at room temperature in a dark staining box with 100 μl of equilibration buffer, and 50 μl of TdT Mix was added to each sample. The slides were further incubated for 1 hour at 37°C in the dark. Subsequently, the reaction was stopped by incubating the slides with 2X SSC buffer for 15 minutes. The slides were washed three times by incubating them with PBS for 5 minutes each, stained with DAPI, and then sealed with mounting medium.
因此,在與MPTP受損之對照組比較時,發現經iPS-NSC治療之小鼠在黑質中呈現顯著低水準的細胞死亡。細胞死亡之水準與未受損之溶劑對照組相當,指示iPS-NSC細胞在神經元再生中之挽救能力(圖10)。Thus, iPS-NSC-treated mice were found to exhibit significantly lower levels of cell death in the substantia nigra when compared to MPTP-impaired controls. The level of cell death was comparable to the undamaged solvent control, indicating the rescue ability of iPS-NSC cells in neuronal regeneration (Figure 10).
圖1(A)及圖1(B):用293T細胞產生慢病毒以用於轉導鼠胚胎纖維母細胞(MEF) (A)。在轉導Cbx7、Oct4及Sox2之慢病毒攜載轉基因之後,可發現在MEF細胞中表現之蛋白質(B)。Figure 1(A) and Figure 1(B): 293T cells were used to generate lentivirus for transduction of murine embryonic fibroblasts (MEFs) (A). After transduction of the lentivirus-carrying transgenes of Cbx7, Oct4 and Sox2, the proteins expressed in MEF cells can be found (B).
圖2(A)及圖2(B):使用轉基因之不同組合之iPSC產生的效率。iPSC產生之效率藉由經轉導之分化細胞產生iPSC群落之能力來量測(A)。條形圖展示在此特定實驗中產生之群落數目(SC:Sox2及Cbx7;OC:Oct4及Cbx7;SOC:Sox2、Oct4及Cbx7) (B)。Figures 2(A) and 2(B): Efficiency of iPSC generation using different combinations of transgenes. The efficiency of iPSC generation was measured by the ability of the transduced differentiated cells to generate iPSC colonies (A). Bar graphs show the number of colonies generated in this particular experiment (SC: Sox2 and Cbx7; OC: Oct4 and Cbx7; SOC: Sox2, Oct4 and Cbx7) (B).
圖3:iPSC細胞之免疫組織化學染色表明,經重新編程之iPSC表現幹細胞標記物,諸如鹼性磷酸酶(AP)、Oct4、Sox2、SSEA1及SSEA4。Figure 3: Immunohistochemical staining of iPSC cells shows that reprogrammed iPSCs express stem cell markers such as alkaline phosphatase (AP), Oct4, Sox2, SSEA1 and SSEA4.
圖4:經iPSC注射之裸鼠中的畸胎瘤形成。全部三個胚層內胚層、中胚層及外胚層存在於iPSC衍生之畸胎瘤中證實iPSC活體內之多能性。Figure 4: Teratoma formation in iPSC-injected nude mice. The presence of all three germ layers, endoderm, mesoderm and ectoderm, in iPSC-derived teratomas confirms the pluripotency of iPSCs in vivo.
圖5:經重新編程之iPSC能夠產生神經元幹細胞,指示iPSC活體外之分化能力。Figure 5: Reprogrammed iPSCs are capable of generating neuronal stem cells, indicating the differentiation capacity of iPSCs in vitro.
圖6(A)至圖6(C):在MPTP誘導性帕金森氏症候群模型中,衍生自經重新編程之iPSC的經分化神經元幹細胞(iPS-NSC)在平衡木行走及旋轉桿運動中顯著改善動物行為(*p<0.05. **p<0.01)。(A及B)平衡木行走行為分析用以測試小鼠之平衡能力。相較於用鹽水移植之小鼠的平衡能力,使用iPS衍生之神經元幹細胞(iPS-NSC)的移植顯著改善平衡能力(包括滑倒(A)及通過時間(B))。(C)藉由旋轉桿分析,相較於用鹽水移植之小鼠的協調能力,經iPS-NSC治療之小鼠改善協調能力。Figures 6(A) to 6(C): Differentiated Neuronal Stem Cells (iPS-NSCs) Derived from Reprogrammed iPSCs Are Significant in Balance Beam Walking and Spinning Bar Movement in MPTP-Induced Parkinson's Syndrome Model Improved animal behavior (*p < 0.05. **p < 0.01). (A and B) Balance beam walking behavior analysis to test the balance ability of mice. Transplantation with iPS-derived neuronal stem cells (iPS-NSCs) significantly improved balance ability (including slip (A) and transit time (B)) compared to the balance ability of mice transplanted with saline. (C) iPS-NSC-treated mice have improved coordination compared to saline-transplanted mice by rotarod analysis.
圖7(A)至圖7(D):小鼠中之酪胺酸羥化酶(TH)表現細胞之數目:(A)溶劑對照;(B)對誘導性帕金森病之MPTP治療;(C)對誘導性帕金森病之MPTP治療且用iPS-NSC移植。(D)對於TH為陽性之細胞經計數,且在條形圖中呈現為與MPTP組之百分比。誤差杠表示平均+SD。在iPS-NSC治療之後,黑質中之多巴胺分泌神經元之數目增加。發現多巴胺分泌神經元(TH陽性細胞)在MPTP受損組中減少,其中iPS-NSC之治療挽救TH陽性細胞之數目。Figures 7(A) to 7(D): Number of tyrosine hydroxylase (TH) expressing cells in mice: (A) solvent control; (B) MPTP treatment for induced Parkinson's disease; ( C) MPTP treatment for induced Parkinson's disease and transplantation with iPS-NSCs. (D) Cells positive for TH were counted and presented in a bar graph as a percentage of the MPTP group. Error bars represent mean + SD. The number of dopamine-secreting neurons in the substantia nigra increased following iPS-NSC treatment. Dopamine-secreting neurons (TH-positive cells) were found to be reduced in the MPTP-impaired group, where iPS-NSC treatment rescued the number of TH-positive cells.
圖8(A)至圖8(D):小鼠中之Iba1表現細胞之數目:(A)溶劑對照;(B)對誘導性帕金森病之MPTP治療;(C)對誘導性帕金森病之MPTP治療且用iPS-NSC移植。(D)對於Iba1為陽性之細胞經計數,且在條形圖中呈現為與MPTP組之百分比。誤差杠表示平均+SD。在iPS-NSC治療之後,紋狀體中之免疫反應減小。發現Iba1陽性細胞在MPTP受損組中增加,其中iPS-NSC之治療顯著減少Iba1陽性細胞,從而指示免疫反應減小。(***p<0.001)Figures 8(A) to 8(D): Number of Iba1 expressing cells in mice: (A) vehicle control; (B) MPTP treatment for induced Parkinson's disease; (C) for induced Parkinson's disease treated with MPTP and transplanted with iPS-NSCs. (D) Cells positive for Iba1 were counted and presented in bar graphs as a percentage of the MPTP group. Error bars represent mean + SD. The immune response in the striatum was reduced following iPS-NSC treatment. Iba1-positive cells were found to be increased in the MPTP-impaired group, where treatment of iPS-NSCs significantly reduced Iba1-positive cells, indicating a reduced immune response. (***p < 0.001)
圖9:在MPTP誘導之帕金森氏病模型中,血清多巴胺之含量隨iPS-NSC治療而增加(***p<0.001)。Figure 9: Serum dopamine levels increased with iPS-NSC treatment in MPTP-induced Parkinson's disease model (***p < 0.001).
圖10(A)至圖10(D):藉由TUNEL檢定之小鼠中之凋亡細胞的數目:(A)溶劑對照;(B)對誘導性帕金森病之MPTP治療;(C)對誘導性帕金森病之MPTP治療且用iPS-NSC移植。(D)對於TUNEL檢定為陽性之細胞經計數,且在條形圖中呈現為與MPTP組之百分比。誤差杠表示平均+SD。在iPS-NSC治療之後,黑質中之神經元細胞死亡減少。當相較於MPTP受損對照組時,iPS-NSC治療顯著減少細胞死亡(**p<0.01)。Figures 10(A) to 10(D): Number of apoptotic cells in mice assayed by TUNEL: (A) vehicle control; (B) MPTP treatment for induced Parkinson's disease; (C) pair MPTP treatment of induced Parkinson's disease and transplantation with iPS-NSCs. (D) Cells positive for the TUNEL assay were counted and presented in bar graphs as a percentage of the MPTP group. Error bars represent mean + SD. Neuronal cell death in the substantia nigra was reduced following iPS-NSC treatment. iPS-NSC treatment significantly reduced cell death when compared to MPTP-impaired controls (**p < 0.01).
Claims (18)
Translated fromChinesePriority Applications (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| TW108138904ATWI769410B (en) | 2019-10-28 | 2019-10-28 | New induced pluripotent stem cells (ipscs) and applications thereof |
Applications Claiming Priority (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| TW108138904ATWI769410B (en) | 2019-10-28 | 2019-10-28 | New induced pluripotent stem cells (ipscs) and applications thereof |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| TW202117005A TW202117005A (en) | 2021-05-01 |
| TWI769410Btrue TWI769410B (en) | 2022-07-01 |
Family
ID=77020617
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| TW108138904ATWI769410B (en) | 2019-10-28 | 2019-10-28 | New induced pluripotent stem cells (ipscs) and applications thereof |
Country Status (1)
| Country | Link |
|---|---|
| TW (1) | TWI769410B (en) |
Citations (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2009006930A1 (en)* | 2007-06-15 | 2009-01-15 | Izumi Bio, Inc. | Human pluripotent stem cells induced from undifferentiated stem cells derived from a human postnatal tissue |
- 2019
- 2019-10-28TWTW108138904Apatent/TWI769410B/enactive
Patent Citations (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2009006930A1 (en)* | 2007-06-15 | 2009-01-15 | Izumi Bio, Inc. | Human pluripotent stem cells induced from undifferentiated stem cells derived from a human postnatal tissue |
Non-Patent Citations (4)
| Title |
|---|
| 專書 Banito, Ana Cristina Tome Moita. Senescence impairs successful reprogramming to pluripotent stem cells . Imperial College London. 2011 Mar.;* |
| 期刊 Bo Ning et al. Nat Commun. 8 (1). 2017 Aug 24.349.* |
| 期刊 Junying Yu et al. Science. 318 (5858). Epub 2007 Nov 20. 1917-1920.;* |
| 期刊 Luigi Aloia et al. Development. 140(12). The Company of Biologists. 2013 Jun. 2525-2534.;* |
Also Published As
| Publication number | Publication date |
|---|---|
| TW202117005A (en) | 2021-05-01 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| JP7134317B2 (en) | Pluripotent stem cells that can be isolated from living tissue | |
| US11261426B2 (en) | Pluripotent stem cell that can be isolated from body tissue | |
| JP6603529B2 (en) | Generation of reprogrammed pluripotent cells | |
| JPWO2014057997A1 (en) | Initializing peptide and use thereof | |
| US9549954B2 (en) | Method and composition for inducing human pluripotent stem cells | |
| WO2021081721A1 (en) | New induced pluripotent stem cells (ipscs) and applications thereof | |
| TWI769410B (en) | New induced pluripotent stem cells (ipscs) and applications thereof | |
| TW202340454A (en) | A method of differentiating an induced pluripotent stem cell into a retinal pigment epithelial cell, a retinal pigment epithelial cell and methods of using the retinal pigment epithelial cell | |
| US11760980B2 (en) | Induced pluripotent stem cells (IPSCS) and applications thereof | |
| Huang et al. | Induced pluripotent stem cell technologies for tissue engineering | |
| Xu et al. | Road to future: iPSC clinical application in Parkinson’s disease treatment | |
| Henning | Identification and characterisation of a novel, multi-potent, skeletal muscle-derived stem cell with broad developmental plasticity |