TWI730307B - Intracanalicular dissolvable punctum plug inserter device, kit comprising the same and its assembling method - Google Patents
Intracanalicular dissolvable punctum plug inserter device, kit comprising the same and its assembling methodDownload PDFInfo
- Publication number
- TWI730307B TWI730307BTW108109792ATW108109792ATWI730307BTW I730307 BTWI730307 BTW I730307BTW 108109792 ATW108109792 ATW 108109792ATW 108109792 ATW108109792 ATW 108109792ATW I730307 BTWI730307 BTW I730307B
- Authority
- TW
- Taiwan
- Prior art keywords
- plug
- insert
- button
- main body
- opening
- Prior art date
Links
- 238000000034methodMethods0.000titleclaimsabstractdescription33
- 241000083513PunctumSpecies0.000titleclaimsdescription23
- 208000003556Dry Eye SyndromesDiseases0.000claimsabstractdescription12
- 206010013774Dry eyeDiseases0.000claimsabstractdescription12
- 238000011282treatmentMethods0.000claimsabstractdescription6
- 210000005239tubuleAnatomy0.000claimsdescription8
- 238000003825pressingMethods0.000claimsdescription6
- 230000004888barrier functionEffects0.000claimsdescription4
- 230000001954sterilising effectEffects0.000claimsdescription4
- 238000007789sealingMethods0.000claimsdescription2
- 230000008569processEffects0.000description7
- 239000000463materialSubstances0.000description6
- IAYPIBMASNFSPL-UHFFFAOYSA-NEthylene oxideChemical compoundC1CO1IAYPIBMASNFSPL-UHFFFAOYSA-N0.000description4
- 238000003780insertionMethods0.000description4
- 230000037431insertionEffects0.000description4
- 230000036316preloadEffects0.000description4
- 230000008901benefitEffects0.000description3
- 239000000853adhesiveSubstances0.000description2
- 230000001070adhesive effectEffects0.000description2
- 239000000560biocompatible materialSubstances0.000description2
- 230000000916dilatatory effectEffects0.000description2
- 230000010339dilationEffects0.000description2
- 210000003811fingerAnatomy0.000description2
- 238000004806packaging method and processMethods0.000description2
- 239000004033plasticSubstances0.000description2
- 229920003023plasticPolymers0.000description2
- 229920002463poly(p-dioxanone) polymerPolymers0.000description2
- 229920000515polycarbonatePolymers0.000description2
- 239000004417polycarbonateSubstances0.000description2
- 239000000622polydioxanoneSubstances0.000description2
- 239000002195soluble materialSubstances0.000description2
- 238000004659sterilization and disinfectionMethods0.000description2
- 102000008186CollagenHuman genes0.000description1
- 108010035532CollagenProteins0.000description1
- 239000004593EpoxySubstances0.000description1
- 208000023715Ocular surface diseaseDiseases0.000description1
- 230000003213activating effectEffects0.000description1
- 239000003795chemical substances by applicationSubstances0.000description1
- 229920001436collagenPolymers0.000description1
- 239000003086colorantSubstances0.000description1
- 230000006835compressionEffects0.000description1
- 238000007906compressionMethods0.000description1
- 230000000881depressing effectEffects0.000description1
- 230000000694effectsEffects0.000description1
- 210000000744eyelidAnatomy0.000description1
- 239000012530fluidSubstances0.000description1
- 239000011521glassSubstances0.000description1
- 230000036541healthEffects0.000description1
- 239000007943implantSubstances0.000description1
- 230000003993interactionEffects0.000description1
- 206010023332keratitisDiseases0.000description1
- 238000011866long-term treatmentMethods0.000description1
- 238000004519manufacturing processMethods0.000description1
- 230000007246mechanismEffects0.000description1
- 239000002184metalSubstances0.000description1
- 239000000203mixtureSubstances0.000description1
- 238000012986modificationMethods0.000description1
- 230000004048modificationEffects0.000description1
- 210000003928nasal cavityAnatomy0.000description1
- 229920000642polymerPolymers0.000description1
- 230000001681protective effectEffects0.000description1
- 229910001220stainless steelInorganic materials0.000description1
- 239000010935stainless steelSubstances0.000description1
- 239000000829suppositorySubstances0.000description1
- 210000003813thumbAnatomy0.000description1
- 238000009736wettingMethods0.000description1
Images
Classifications
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F9/00—Methods or devices for treatment of the eyes; Devices for putting in contact-lenses; Devices to correct squinting; Apparatus to guide the blind; Protective devices for the eyes, carried on the body or in the hand
- A61F9/007—Methods or devices for eye surgery
- A61F9/00772—Apparatus for restoration of tear ducts
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F9/00—Methods or devices for treatment of the eyes; Devices for putting in contact-lenses; Devices to correct squinting; Apparatus to guide the blind; Protective devices for the eyes, carried on the body or in the hand
- A61F9/0008—Introducing ophthalmic products into the ocular cavity or retaining products therein
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F9/00—Methods or devices for treatment of the eyes; Devices for putting in contact-lenses; Devices to correct squinting; Apparatus to guide the blind; Protective devices for the eyes, carried on the body or in the hand
- A61F9/0008—Introducing ophthalmic products into the ocular cavity or retaining products therein
- A61F9/0017—Introducing ophthalmic products into the ocular cavity or retaining products therein implantable in, or in contact with, the eye, e.g. ocular inserts
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61B—DIAGNOSIS; SURGERY; IDENTIFICATION
- A61B17/00—Surgical instruments, devices or methods
- A61B17/0057—Implements for plugging an opening in the wall of a hollow or tubular organ, e.g. for sealing a vessel puncture or closing a cardiac septal defect
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F2230/00—Geometry of prostheses classified in groups A61F2/00 - A61F2/26 or A61F2/82 or A61F9/00 or A61F11/00 or subgroups thereof
- A61F2230/0063—Three-dimensional shapes
- A61F2230/0067—Three-dimensional shapes conical
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F2230/00—Geometry of prostheses classified in groups A61F2/00 - A61F2/26 or A61F2/82 or A61F9/00 or A61F11/00 or subgroups thereof
- A61F2230/0063—Three-dimensional shapes
- A61F2230/0069—Three-dimensional shapes cylindrical
Landscapes
- Health & Medical Sciences (AREA)
- Ophthalmology & Optometry (AREA)
- Life Sciences & Earth Sciences (AREA)
- Animal Behavior & Ethology (AREA)
- Veterinary Medicine (AREA)
- Public Health (AREA)
- General Health & Medical Sciences (AREA)
- Engineering & Computer Science (AREA)
- Biomedical Technology (AREA)
- Heart & Thoracic Surgery (AREA)
- Vascular Medicine (AREA)
- Surgery (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Plastic & Reconstructive Surgery (AREA)
- Prostheses (AREA)
- Media Introduction/Drainage Providing Device (AREA)
- Cardiology (AREA)
- Medical Informatics (AREA)
- Molecular Biology (AREA)
Abstract
Description
Translated fromChinese本發明大體上係關於用於治療乾眼之裝置及方法。The present invention generally relates to devices and methods for treating dry eye.
乾眼症每年影響成千上萬的人,致使人感到不舒服、眼睛變紅、角膜發炎及接觸眼鏡不耐受。眼淚通常會因為通過每個眼瞼之近中面上的兩個淚孔或淚點(上及下)、接著流過垂直及水平之小管進入鼻腔中而排出。可藉由使用淚點阻塞器阻塞淚點或藉由將植入物放置於小管中來治療乾眼症。Dry eye affects thousands of people every year, causing people to feel uncomfortable, red eyes, corneal inflammation and intolerance to contact with glasses. Tears are usually discharged by passing through two tear holes or puncta (upper and lower) on the mesial surface of each eyelid, and then flowing through vertical and horizontal small tubes into the nasal cavity. Dry eye can be treated by using a punctum occluder to block the punctum or by placing an implant in a small tube.
栓為可用於治療乾眼的極細小之生物相容性裝置。視栓之位置而定,有兩種常見類型之栓。表面栓置於淚管之表面處且其僅可在淚管外部可見。另一方面,小管栓或管內栓置於小管(垂直或水平小管)之內部/內深處。Suppository is a very small biocompatible device that can be used to treat dry eye. Depending on the location of the peg, there are two common types of pegs. The surface plug is placed on the surface of the tear duct and it can only be seen outside the tear duct. On the other hand, the small tube plug or the tube plug is placed inside/deep inside the small tube (vertical or horizontal small tube).
目前,使用一對鑷子將管內栓推入或插入小管中。此過程非常繁瑣-例如,眼科醫師(諸如,驗光師、眼科醫生或另一類眼睛護理專業人員)在進行裂隙燈檢查時必須首先打開含有小管栓之包裝。之後要使用鑷子拾取該栓且將該栓插入小管中。由於栓為細小裝置,因此很可能會出現之場景係眼科醫師在栓插入小管中之前無意中使栓掉落。一些眼科醫師亦可能會缺少進行該過程之經驗且在嘗試將栓插入小管中時由於其大小較小而可能會感到害怕。Currently, a pair of tweezers is used to push or insert the tube plug into the small tube. This process is very cumbersome-for example, an ophthalmologist (such as an optometrist, an ophthalmologist or another type of eye care professional) must first open the package containing the small tube plug when performing a slit lamp examination. Then use forceps to pick up the plug and insert the plug into the small tube. Since the plug is a small device, it is likely that the ophthalmologist accidentally drops the plug before it is inserted into the small tube. Some ophthalmologyThe physician may also lack experience in performing this procedure and may feel scared when trying to insert the plug into the tubule due to its small size.
美國專利第8,591,484號揭示了用於插入表面栓之裝置。該裝置包括能夠撓曲之金屬或塑膠線或任何其他類型之接線。該栓位於該線之尖端處。該線嵌入於一槽內,該槽具有用於將該線固持在適當位置之托板。將黏合劑施加至該線與該槽之間的接觸區域以將該線固持在適當位置。當按下按鈕時,其對在托板近側之線施加向下力。因為該線之一端在托板處固持在適當位置,故藉由該按鈕施加之向下力會對該線產生張力,將其向內拉動。完全按下栓噴射器會使線之尖端自栓撤出,藉此釋放該栓。該裝置之缺點為其涉及使用用於固持該栓之線。已申請專利之裝置係針對表面栓之放置而組態。由於該裝置未組態成進入小管之開口,因此其將不會將栓推入小管中。U.S. Patent No. 8,591,484 discloses a device for inserting surface plugs. The device includes a flexible metal or plastic wire or any other type of wiring. The pin is located at the tip of the thread. The wire is embedded in a slot with a support plate for holding the wire in place. An adhesive is applied to the contact area between the wire and the groove to hold the wire in place. When the button is pressed, it exerts a downward force on the line near the pallet. Because one end of the wire is held in place at the pallet, the downward force exerted by the button will generate tension on the wire and pull it inward. Depressing the plug ejector fully will cause the tip of the thread to withdraw from the plug, thereby releasing the plug. The disadvantage of this device is that it involves the use of a wire for holding the bolt. The patented device is configured for the placement of surface bolts. Since the device is not configured to enter the opening of the small tube, it will not push the plug into the small tube.
因此,需要一種便利之裝置,該裝置可有助於在不使用鑷子或不使用包括用於固持栓之線的插入裝置的情況下將管內栓放置於小管中。理想地,該裝置具有預載栓且不依賴於眼科醫師來將其裝載至該裝置上。Therefore, there is a need for a convenient device that can facilitate the placement of the intratubular plug in the small tube without the use of forceps or an insertion device including a wire for holding the plug. Ideally, the device has a preload plug and does not rely on the ophthalmologist to load it onto the device.
本發明涉及一種用於將管內栓插入於小管中之裝置及用於治療乾眼之方法。該栓可由合適之生物相容性材料製成,諸如聚二氧六環酮或任何其他合適材料。方便地,該栓可預載(或預安裝)於該裝置上。The invention relates to a device for inserting a tube plug into a small tube and a method for treating dry eye. The plug can be made of a suitable biocompatible material, such as polydioxanone or any other suitable material. Conveniently, the bolt can be pre-loaded (or pre-installed) on the device.
本發明有助於用於藉由使用單個裝置將栓插入於小管中以暫時限制自然潤濕之眼淚排出眼睛的一步治療過程,該單個裝置預載有可溶性管內栓。如此避免需要眼科醫師將栓自單獨包裝移除且消除了使用鑷子固持該栓且將其插入小管或淚點中的繁瑣過程。有利地,甚至很少經驗或無經驗之眼科醫師亦能夠容易地將可溶性淚點栓插入小管中。該治療可用於通常被稱作乾眼症之某些眼睛病症以及眼表疾病之乾眼部分及淚液不足之其他病症的長期治療。The present invention facilitates a one-step treatment process for temporarily restricting the drainage of naturally wetted tears from the eye by inserting a plug into a small tube using a single device preloaded with a soluble intratubular plug. This avoids the need for the ophthalmologist to remove the plug from the separate packaging and eliminates the use of forcepsThe cumbersome process of holding the plug and inserting it into the tubule or punctum. Advantageously, even an ophthalmologist with little or no experience can easily insert the soluble punctal plug into the tubule. The treatment can be used for long-term treatment of certain ocular conditions commonly referred to as dry eye, as well as dry eye parts of ocular surface diseases and other conditions of insufficient tear fluid.
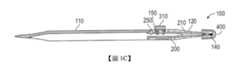
在一個實施例中,一種管內栓插入物裝置包括:(a)細長的一主體,細長的該主體具有一縱軸,該主體具有:一插入物端,其中該插入物端在其中具有一開口;及一遠端,其中該遠端與該插入物端在縱向上相對;及(b)一栓噴射器,其中該栓噴射器包括:一滑件;及一棒,該棒耦接至該滑件之一第一端,其中該栓噴射器被組態成可在與該插入物端中之一開口相鄰的一第一位置與離該開口較遠之一第二位置之間移動。該栓安裝於該主體內且鄰接與該插入物端中之該開口相鄰的、該棒之第一端。該棒經組態以在該滑件之該第一端朝向該插入物端移動時將該栓自該插入物端中之該開口噴射。該栓噴射器進一步包括一可按下按鈕,該可按下按鈕具有位於該主體外部之一第一(上)側及安裝於該滑件上之一第二側。該按鈕側面為一第一側壁及一第二側壁。該按鈕之該第二(基底)側包括一對支腳,該對支腳卡在該滑件之一第一臂上。該按鈕藉由一鎖定構件而實質上鎖定於一第一位置,該鎖定構件包括在該滑件之該第一臂上的一突起及一凹口。該突起與該第一側壁相鄰。該按鈕經組態以藉由按下該按鈕以將其自該凹口釋放且接著將其沿著該滑件朝向與該第二側壁相鄰之一第二位置滑動而沿著該主體之該縱軸自其第一位置移動至該第二位置。該裝置進一步包括一可移除罩,該可移除罩被裝配而遮蓋安裝於該主體之該插入物端處的栓。該裝置亦包括安置於該主體之該遠端處的用於擴張一淚點之整體構件。用於擴張該淚點之該構件包括細尖端。In one embodiment, an intratubular plug insert device includes: (a) an elongated body, the elongated body has a longitudinal axis, the body has: an insert end, wherein the insert end has an insert end therein Opening; and a distal end, wherein the distal end is longitudinally opposed to the insert end; and (b) a bolt ejector, wherein the bolt ejector includes: a slider; and a rod coupled to A first end of the slider, wherein the plug ejector is configured to be movable between a first position adjacent to an opening in the insert end and a second position farther from the opening . The bolt is installed in the main body and adjacent to the first end of the rod adjacent to the opening in the insert end. The rod is configured to eject the plug from the opening in the insert end when the first end of the slider moves toward the insert end. The bolt ejector further includes a depressible button having a first (upper) side located outside the main body and a second side mounted on the sliding member. The side of the button is a first side wall and a second side wall. The second (base) side of the button includes a pair of supporting legs, which are clamped on a first arm of the sliding member. The button is substantially locked in a first position by a locking member, and the locking member includes a protrusion and a notch on the first arm of the slider. The protrusion is adjacent to the first side wall. The button is configured to release it from the recess by pressing the button and then slide it along the slider toward a second position adjacent to the second side wall along the main body The longitudinal axis moves from its first position to the second position. The device further includes a removable cover that is assembled to cover the plug installed at the end of the insert of the main body. The device also includes an integral member for dilating a punctum placed at the distal end of the main body. The member for expanding the punctum includes a thin tip.
在另一個實施例中,提供一種包括該管內栓插入物裝置之套件。該套件進一步包括用於收納該裝置之一托盤及用於使用該裝置之說明書。In another embodiment, a kit including the plug insert device is provided. The kit further includes a tray for storing the device and instructions for using the device.
在另一個實施例中,揭示一種組裝一管內栓插入物裝置之方法。該方法涉及提供一管內栓;將該管內栓安裝於該管內栓插入物裝置內;及將一罩壓在該裝置之該插入物端上以將該管內栓穩固地固持在該裝置上之適當位置。該方法亦涉及藉由一無菌障壁蓋將該裝置密封於一預模製托盤中。該方法進一步包括對該托盤進行殺菌。In another embodiment, a method of assembling a plug insert device is disclosed. The method involves providing a tube plug; installing the tube plug in the tube plug insert device; and pressing a cover on the insert end of the device to firmly hold the tube plug in the tube Appropriate location on the device. The method also involves sealing the device in a pre-molded tray by a sterile barrier cover. The method further includes sterilizing the tray.
在另一個實施例中,一種治療乾眼之方法涉及提供本文中揭示之管內栓插入物裝置;將該裝置之該插入物端插入一患者之小管中;及致動該裝置之該栓噴射器以使該栓自該插入物端處之該開口噴射出。該方法進一步包括在插入該栓之前擴張該患者之淚點。可使用該裝置之該遠端來將該栓進一步推動至該小管中。In another embodiment, a method of treating dry eye involves providing the intratubular plug insert device disclosed herein; inserting the insert end of the device into a small tube of a patient; and activating the plug ejection of the device To eject the plug from the opening at the end of the insert. The method further includes dilating the patient's punctum before inserting the plug. The distal end of the device can be used to push the plug further into the tubule.
100:裝置100: device
110:裝置主體110: Device body
110B:通道110B: Channel
110B’:側壁110B’: side wall
110B”:側壁110B": side wall
115:凹槽115: Groove
120:主體之第一端120: The first end of the main body
125:插入物尖端125: Insert tip
125A:圓筒形或圓錐形部分125A: cylindrical or conical part
125B:通道125B: Channel
127:開口127: open
130:主體之第二端130: The second end of the main body
135:尖端/尖頭135: tip/pointed
140:栓140: bolt
150:凹口150: Notch
200:栓噴射器200: Bolt ejector
210:滑件210: Slider
210A:第一滑件臂210A: First slider arm
210B:第二滑件臂210B: second slider arm
210C:第二端210C: second end
210D:開口210D: opening
215:狹槽215: Slot
220:棒220: great
220A:棒之尖端220A: the tip of the stick
230:棒之第一端230: The first end of the stick
240:棒之相對端240: The opposite end of the stick
250:突起250: protrusion
300:噴射器構件/按鈕300: Injector component/button
310:按鈕310: Button
320:按鈕之基底320: Button Base
330:按鈕之凸起部分330: The raised part of the button
400:蓋或罩400: cover or hood
410:殼體410: Shell
420:開口420: open
510:托盤510: Pallet
圖1A至圖1C示出根據本發明之一或多個實施例的管內栓插入物裝置之各種視圖。Figures 1A to 1C show various views of an intratubular plug insert device according to one or more embodiments of the present invention.
圖2A至圖2E示出根據本發明之一或多個實施例的該裝置及其組件之各種視圖。2A to 2E show various views of the device and its components according to one or more embodiments of the present invention.
圖3示出根據本發明之一或多個實施例之滑件。Figure 3 shows a slider according to one or more embodiments of the present invention.
圖4示出根據本發明之一或多個實施例之按鈕。Figure 4 shows a button according to one or more embodiments of the present invention.
圖5示出根據本發明之一實施例的具有該按鈕之該裝置的另一視圖。Fig. 5 shows another view of the device with the button according to an embodiment of the present invention.
圖6A至圖6B示出根據本發明之一實施例的套件之視圖。6A to 6B show views of a kit according to an embodiment of the present invention.
相關申請案之交叉參考Cross reference of related applications
本申請案主張2018年3月22日申請且標題為「INTRACANALICULAR DISSOLVABLE PUNCTUM PLUG INSERTER」之臨時美國專利申請案第62/646538號之利益,該案之全文及揭示內容(明確的與隱含的)以引用方式併入本文中。This application claims the benefits of provisional U.S. Patent Application No. 62/646538 filed on March 22, 2018 and titled "INTRACANALICULAR DISSOLVABLE PUNCTUM PLUG INSERTER", the full text of the case and its disclosure (explicit and implicit) Incorporated into this article by reference.
術語及片語「發明」、「本發明」、「當前發明」及類似術語及片語如本文中所使用係非限制性的且不欲將本主題限於任何單個實施例,而是包括如所描述之所有可能實施例。The terms and phrases "invention", "present invention", "current invention" and similar terms and phrases as used herein are non-limiting and are not intended to limit the subject matter to any single embodiment, but include such All possible embodiments described.
本文中描述之裝置及方法可「包含」在本說明書全文中揭示之任何特徵或步驟、「基本上由在本說明書全文中揭示之任何特徵或步驟組成」或「由在本說明書全文中揭示之任何特徵或步驟組成」。如本說明書及申請專利範圍中所使用,詞語「包含」(及任何形式之包含,諸如「包含了」及「包含有」)、「具有」(及任何形式之具有,諸如「具有著」及「具有了」)、「包括」(及任何形式之包括,諸如「包括了」及「包括有」)或「含有」(及任何形式之含有,諸如「含有著」及「含有了」)係包括性或開放性的且可包括本發明之特徵及步驟且不排除本文中描述之其他特徵或步驟。詞語「一」或「一個」在與術語「包含」結合使用時在申請專利範圍及/或說明書中使用可表示「一個」,但其亦與「一或多個」、「至少一個」及「一個或一個以上」之意義一致。如本文中所使用,「基本上由......組成」表示本發明可包括除了申請專利範圍中所述之特徵或步驟之外的特徵或步驟,但額外特徵或步驟不能實質上更改要求權利保護之發明的基本及新穎特性。The devices and methods described herein can "include" any feature or step disclosed in the full text of this specification, "essentially consist of any feature or step disclosed in the full text of this specification", or "comprise from any feature or step disclosed in the full text of this specification". Any feature or step composition". As used in this specification and the scope of the patent application, the words "include" (and include in any form, such as "includes" and "includes"), "have" (and have in any form, such as "have" and "Have"), "include" (and any form of inclusion, such as "included" and "included") or "contained" (and any form of inclusion, such as "included" and "included") are It is inclusive or open and can include the features and steps of the present invention and does not exclude other features or steps described herein. The term "a" or "an" when used in combination with the term "including" can mean "a" when used in the scope of patent application and/or specification, but it is also used with "one or more", "at least one" and " "One or more than one" has the same meaning. As used herein, "essentially consisting of" means that the present invention may include features or steps other than those described in the scope of the patent application, but the additional features or steps cannot be substantially altered The basic and novel characteristics of the claimed invention.
本文中所述之所有範圍包括端點,包括敍述兩個值「之間」之範圍的彼等端點。術語諸如「約」、「大體上」、「實質上」及其類似者將被理解為修飾術語或值,使得其並非絕對值。此類術語將受在熟習此項技術者理解彼等術語時之情景及其修飾之術語限定。術語「實質上」及其變體被限定為很大程度上但不一定全部為被指定為熟習此項技術者所理解的事物,且在一個非限制性實施例中實質上係指誤差在0.5%至5%內之範圍。All ranges described herein include end points, including those end points that describe the range "between" two values. Terms such as "about", "substantially", "substantially" and the like will be understood to modify the term or value so that it is not an absolute value. Such terms will be limited by the context and modified terms when those familiar with the art understand the terms. The term "substantially" and its variants are limited to a large extent, but not necessarily all, that is understood by those who are designated as familiar with the art, and in a non-limiting embodiment essentially means that the error is within 0.5 Within the range of% to 5%.
圖1A至圖1C示出用於插入預載管內栓(在下文可互換地被稱作「栓」)以治療乾眼的裝置100之實施例的不同視圖。裝置100被組態為栓140之一次性插入物工具。栓140經組態以暫時限制自然潤濕之眼淚自眼睛排出。裝置100亦具有整體擴張器構件130。裝置100具有人體工學設計且如鉛筆般大小而便於操縱。在某些實施例中,裝置100之長度可在5cm至25cm之間。此外,與鉛筆類似,裝置100可被使用者拿在一隻手之拇指、食指及中指之一側之間並進行操縱。輕質、手持式裝置100便於使用且其促進效率及成本節省,此最終可利及患者。1A to 1C show different views of an embodiment of a
裝置100可由不鏽鋼、聚碳酸酯、塑膠、此等材料之任何組合或另一種合適材料製成。裝置100包括:細長殼體或主體110,其中該主體具有插入物端120及與該插入物端在縱向上相對的遠端130;及安裝於該主體內部之栓噴射器200。栓噴射器200被組態有整體滑件210及用於將預載管內栓140噴射至小管中之按鈕300。裝置100進一步包括用於將栓140保護於插入物端120內之蓋或罩400。The
細長的主體110具有實質上縱向之軸線。主體110之第一端終止於栓插入物端120中,而該主體之第二端終止於擴張器尖端135中。主體110可具有凸起之脊狀物、擦痕或粗糙表面以有助於穩固地握在眼科醫師之手中。在一或多個實施例中,主體110可具有六邊形橫截面。在其他實施例中,主體110可具有圓形或多邊形橫截面。視情況地,主體110之底部部分可包括細長凹槽115。凹槽115可實質上自該主體之第一端120實質上延伸至該主體之第二端130。凹槽115確保裝置100為輕質的且其亦可有助於在製造/組裝過程期間插入栓噴射器200及栓140。主體110可朝向其中間具有較大直徑或其可具有均一直徑。The
栓140可由生物相容性材料製成。較佳地,該栓可由可溶於水之可溶性材料(諸如膠原)或聚二氧六環酮栓形成,然而,其亦可包括為醫學相容的且由合適材料製成的其他類型之可溶性栓。在一些實施例中,栓140為不透明的且形狀為圓柱形。栓140被設計成緊貼地配合在小管內部以阻止眼淚之流動。由於大多數患者之淚點直徑的直徑為約0.4mm至0.5mm,因此典型栓之直徑亦將在約0.4mm至約0.5mm之間。因此,一旦已插入栓,則眼淚可在較長之持續時間內停留在眼睛表面上,此轉而確保眼睛之自然潤濕。結果,眼睛保持濕潤及舒適。The
該主體之第一端120經組態用於將栓插入小管中。栓插入物端120包括插入物尖端125。插入物尖端125經組態以穩固地夾持栓140。在一個非限制性實施例中,如圖2A至圖2D中所示,插入物尖端125具有「摺疊」型設計。舉例而言,插入物尖端125涉及實質上圓筒形或圓錐形部分125A、向內變細之通道125B及開口127。通道125B提供與栓140之外表面的緊密配合,且其經組態以可摩擦地固持栓140,直至其自開口127噴射出為止。可調整開口127之直徑以實質上匹配栓140之直徑。The
如圖1A及圖1C中所示,裝置110進一步包括蓋或罩400。罩400在插入物尖端125處保護栓140。罩400包括圓頂形殼體410及用於收納栓及插入物尖端125之開口420。罩400可由聚碳酸酯聚合物或任何其他合適材料製成。罩400經組態以扣搭在插入物尖端125上。在某些實施例中,該罩包括用於將栓140壓緊約千分之一吋的壓緊構件。As shown in FIGS. 1A and 1C, the
栓噴射器200之截面圖示出於圖1C中。栓噴射器200包括整體滑件210及噴射器構件300。滑件210安裝於主體110之縱向凹槽115內。滑件210具有藉由第一端處之開口210D隔開的第一臂210A及第二臂210B。臂210A、臂210B在變細之第二端210C處耦接在一起。滑件210被組態成撓曲的(亦即,其並非由剛性材料製成)。當在裝置110之組裝期間將滑件插入於主體110中時,滑件之兩個臂210A、210B可朝向滑件之中線撓曲。臂210A、210B經組態以在滑件插入於主體110內後往回撓曲(遠離中線)至其原始位置。The cross-sectional view of the
栓噴射器200進一步包括柱塞或棒220。棒220之第一端230附著至滑件之第二端210C。棒220之相對端240經組態以鄰接通道125B內部之栓140(如圖1C中所示)的第一端。有利地,棒220係精密模製的,使得其可配合於通道125B內部。栓140之第二端經組態以自插入物尖端之開口127稍稍延伸出。The
栓噴射器200進一步包括噴射器構件300。如圖4及圖5中進一步所示,噴射器構件300可包括按鈕310或任何其他合適機構(諸如槓桿)。按鈕310設於主體110之上部部分上且其可經組態以具有任何合適形狀。在一個實施例中,該按鈕之基底包括一對支腳320。支腳320經由狹槽215卡到滑件之第一臂210A上。該按鈕之凸起部分330自主體之上部部分向外突出。凸起部分330可包括用於有助於穩定夾持之脊狀物或溝槽。按鈕310可位於主體110之上部部分上的通道110B內。通道110B側面為側壁110B'及110B"。按鈕310藉由側壁110B"藉由鎖定構件(諸如突起250)與第一臂210A之凹口150之相互作用而鎖定於第一位置。按鈕310最初被壓縮於適當位置。為了釋放按鈕310,可按下該按鈕以將突起250自凹口150移除,且該按鈕可接著自第一位置移動至在相對側壁110B'近側之第二位置或移動至通道110B內的其間之任何位置。The
在使用中,眼科醫師可藉由輕輕地按下該按鈕之頂部/凸起部分330以將其自其鎖定位置釋放而致動栓噴射器200。按壓按鈕330之頂部迫使該按鈕之基底320將第一滑件臂210B朝向第二滑件臂210B推動。按鈕300接著可沿著主體110之軸線自沿著側壁110B"之第一位置移動至沿著側壁110B'之第二位置。此導致滑件臂210A及210B與棒220朝向插入物端處之開口127向前滑動。棒220使栓140移動通過通道125B且將其自開口127噴出至小管中。棒之尖端220A具有與開口127相比稍小之直徑。如圖5中所示,在栓140已噴出之後,棒之尖端220A的小部分可能會穿過開口127突出。In use, the ophthalmologist can actuate the
棒220被組態成可縮回的且在向後推動按鈕300時(亦即,在按鈕移動遠離插入物尖端時)可在主體110內往回滑動。該插入物端亦可包括卡圈。該卡圈可加襯墊以促進穩固夾持。The
主體之遠端130與插入物端120在縱向上對置。如圖1A及圖2E中所示,遠端130可能會變細為極小且細之尖端/尖頭135。尖端135可相對於主體110之縱軸成角度。舉例而言,尖端135可經組態以指向上或指向下。在某些實施例中,尖端亦可具有與主體110之縱軸實質上重合之縱軸。The
在一些情況中,在插入栓之前,可能必須擴張患者之淚點。擴張可能會涉及鑷子或其他專業擴張工具之使用。此增加了治療過程之複雜性/繁瑣性及費用。In some cases, it may be necessary to dilate the patient's punctum before inserting the plug. Dilation may involve the use of tweezers or other professional dilation tools. This increases the complexity/cumbersomeness and cost of the treatment process.
尖端135可方便地用於擴張淚點。可定製擴張器尖端135之大小及形狀。舉例而言,擴張器尖端135之大小可被定製為接近於淚點直徑。因此,裝置100將用於將預載栓插入於淚點中之構件與用於擴張淚點之構件結合。方便地,尖端135亦可經組態以在其已藉由該裝置噴射至小管中之後將所插入之栓進一步推動至小管中。The
然而,應理解,雖然擴張器與插入物之組合消除了在治療過程期間多種裝置之浪費使用,但不包括擴張器尖端之裝置亦屬於本發明之範疇內。However, it should be understood that although the combination of the dilator and the insert eliminates the wasteful use of multiple devices during the treatment process, devices that do not include the dilator tip also fall within the scope of the present invention.
本發明不限於圖中所示之裝置100的特定設計,且形狀、大小及組態之變化屬於本發明之範疇內。The present invention is not limited tothe specific design of the
在一或多個實施例中,裝置100可製造成多種顏色。每種顏色可與預載栓之特定大小或直徑相關聯。在另一個實施例中,裝置100可包括在栓插入物端120近側之栓的直徑資訊。在其他實施例中,該直徑資訊可與顏色編碼系統關聯。In one or more embodiments, the
裝置100可按單獨包裝之無菌或非無菌包裝來售賣。在作為無菌包裝售賣時,裝置100可作為具有兩個密封托盤之無菌套件來售賣,該等托盤中之每一者穩固地固持具有預載栓140之單個裝置100。該托盤可具有障壁蓋。可使用合適之劑(諸如環氧乙烷)來對托盤及裝置100進行預殺菌。環氧乙烷殺菌涉及在密封腔室中在真空下使托盤及裝置100暴露於環氧乙烷氣體中。殺菌可確保向眼睛護理專業人員提供安全且無菌之裝置100。The
在一個特定實施例中,如圖6A及圖6B中所示,套件500包括:一或多個無菌托盤510,每個托盤具有預載有栓之裝置100;及使用說明書(「IFU」)。可將該一或多個托盤及該IFU真空密封於袋中。可接著將該袋置於容器(諸如盒子)內部。該一或多個托盤及該盒子可在其表面上包括產品標記及其他必要資訊,諸如栓大小。In a specific embodiment, as shown in FIGS. 6A and 6B, the kit500 includes: one or more
根據一實施例,揭示一種組裝該裝置之方法。該方法涉及提供一管內栓(諸如栓140)。該栓可為無菌或非無菌栓,該栓可提供於密封袋中。該方法進一步涉及提供本文中揭示之裝置100的主體110。該方法涉及將該栓自該袋移除且將其安裝於插入物尖端125之通道125B中。此後可接著將栓噴射器200扣搭至主體110,使得棒之尖端鄰接栓之一端,且該栓之另一端突出至插入物尖端之開口外。該方法接著涉及將罩壓在插入物尖端上以將栓穩固地保持在裝置100上之適當位置。接著用無菌障壁蓋將裝置100密封於預模製之托盤/容器中。可使用環氧乙烷對托盤及裝置100進行殺菌。According to an embodiment, a method of assembling the device is disclosed. The method involves providing a plug (such as plug140 ). The plug can be a sterile or non-sterile plug, and the plug can be provided in a sealed bag. The method further involves providing the
根據另一個實施例,在本文中揭示一種治療乾眼之方法。該方法涉及提供如本文中所揭示的預載有管內栓之裝置100。該方法涉及將保護罩自插入物尖端移除。眼科醫師接著可定位該裝置,使得插入物尖端面向患者之淚點。接著將插入物尖端插入於小管中。此後輕輕地按下按鈕且接著朝向插入物尖端向前移動按鈕。此導致棒將栓噴射出插入物尖端處之開口且進入小管中。眼科醫師可使用裝置100之擴張器端來將栓進一步推動至小管內部之所要位置中。According to another embodiment, a method of treating dry eye is disclosed herein.The method involves providing a
0.5mm之淚點直徑常見於許多患者。在一些實施例中,眼科醫師可在將插入物尖端插入於小管中之前使用裝置100之擴張器端來擴張淚點。此允許更容易地插入栓。然而,在某些實施例中,擴張步驟可為任選的。在擴張了淚點之後,醫師可翻轉該裝置180°,使得插入物尖端面向患者之淚點以將預載栓插入於小管中。A punctum diameter of 0.5mm is common in many patients. In some embodiments, the ophthalmologist may use the dilator end of the
本發明之實施例涉及單步插入過程,因為栓已預載於該裝置中。裝置100方便地不涉及使用任何線來釋放該栓。另外,插入物之組裝不涉及任何黏合劑或環氧樹脂。各種組件(諸如栓及罩)可扣搭在一起以使其亦成為功能齊全之插入物。方便地,裝置100可經組態以適應不同大小之栓。The embodiment of the present invention involves a single-step insertion process because the pin is pre-loaded in the device. The
裝置100可由眼睛護理醫師及專業人員(諸如眼科醫生、驗光師及其他健康護理專業人員)使用。裝置100為可能需要處方之醫療裝置。本發明之一或多個實施例允許眼科醫師進行擴張淚點及利用單式或單個裝置來插入淚點栓的功能。該裝置可為手持式的且可能會能夠由眼科醫師單手操縱,藉此確保方便及效率。單個設備之利用亦可能會導致成本節省,此可最終利及患者。The
雖然已結合較佳實施例描述了本發明,但不欲將本發明之範疇限於所陳述之特定形式,而相反地,意欲涵蓋可能屬於本發明之精神及範疇內的此類替代、修改及等效物。Although the present invention has been described in conjunction with the preferred embodiments, it is not intended to limit the scope of the present invention to the specific forms stated, but on the contrary, it is intended to cover such alternatives, modifications and the like that may fall within the spirit and scope of the present invention. Effect.
100:裝置100: device
110:裝置主體110: Device body
110B:通道110B: Channel
110B’:側壁110B’: side wall
110B”:側壁110B": side wall
120:主體之第一端120: The first end of the main body
130:主體之第二端130: The second end of the main body
135:尖端/尖頭135: tip/pointed
300:噴射器構件/按鈕無300: No injector component/button
400:蓋或罩400: cover or hood
410:殼體410: Shell
420:開口420: open
Claims (14)
Translated fromChineseApplications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US201862646538P | 2018-03-22 | 2018-03-22 | |
| US62/646,538 | 2018-03-22 |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| TW201940141A TW201940141A (en) | 2019-10-16 |
| TWI730307Btrue TWI730307B (en) | 2021-06-11 |
Family
ID=67984493
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| TW108109792ATWI730307B (en) | 2018-03-22 | 2019-03-21 | Intracanalicular dissolvable punctum plug inserter device, kit comprising the same and its assembling method |
Country Status (17)
| Country | Link |
|---|---|
| US (1) | US11497649B2 (en) |
| EP (1) | EP3768206B1 (en) |
| JP (1) | JP7296446B2 (en) |
| KR (1) | KR102765111B1 (en) |
| CN (1) | CN111936093A (en) |
| AR (1) | AR115010A1 (en) |
| AU (1) | AU2019240207B2 (en) |
| CA (1) | CA3094359C (en) |
| ES (1) | ES2989955T3 (en) |
| GB (1) | GB2587132B (en) |
| MX (1) | MX2020009754A (en) |
| MY (1) | MY204381A (en) |
| NZ (1) | NZ769069A (en) |
| SG (1) | SG11202009056PA (en) |
| TW (1) | TWI730307B (en) |
| UY (1) | UY38150A (en) |
| WO (1) | WO2019183322A1 (en) |
Families Citing this family (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US12023276B2 (en) | 2021-02-24 | 2024-07-02 | Ocular Therapeutix, Inc. | Intracanalicular depot inserter device |
Citations (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US20090281520A1 (en)* | 2007-11-08 | 2009-11-12 | Brian Highley | Ocular Implantation Device |
| US20110009874A1 (en)* | 2009-07-09 | 2011-01-13 | John Wardle | Single Operator Device for Delivering an Ocular Implant |
| WO2013013207A1 (en)* | 2011-07-20 | 2013-01-24 | Becker Bruce B | Punctal plug inserter and method |
| US20140243763A1 (en)* | 2013-02-25 | 2014-08-28 | Sara Heikali | Devices for the Placement of Medical Compounds in Natural Orifices of a Body |
Family Cites Families (16)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US5669501A (en)* | 1996-06-05 | 1997-09-23 | Xomed Surgical Products, Inc. | Package and method for delivering a medical implant |
| US5921990A (en)* | 1997-08-06 | 1999-07-13 | Eagle Vision | Collagen forceps |
| US6344047B1 (en)* | 2000-02-02 | 2002-02-05 | Eagle Vision | Instrument for inserting a punctum plug and method for manufacturing the instrument |
| US20040068235A1 (en)* | 2002-10-07 | 2004-04-08 | Hallam Clive T. | Packaging system for a medical device and particularly a punctum plug insertion device |
| US20040068286A1 (en)* | 2002-10-07 | 2004-04-08 | Mendius Richard W. | Punctum dilating and punctum plug insertion instrument |
| TWI369970B (en)* | 2004-10-20 | 2012-08-11 | Beaver Visitec Int Us Inc | Surgical knife safety handle having user operable lock |
| NZ572193A (en)* | 2006-03-31 | 2011-10-28 | Quadra Logic Tech Inc | Nasolacrimal drainage system implants for drug therapy with non-fluid swellable retention structure around drug core |
| WO2009035567A2 (en) | 2007-09-07 | 2009-03-19 | Qlt Plug Delivery, Inc | Insertion and extraction tools for lacrimal implants |
| US8591484B2 (en) | 2010-09-15 | 2013-11-26 | AlphaMed, Inc. | Lacrimal punctum measurement and occlusion |
| CN201890013U (en)* | 2010-10-25 | 2011-07-06 | 周新潮 | Pressing type pen |
| TW201242582A (en)* | 2011-04-21 | 2012-11-01 | Johnson & Johnson Vision Care | Implantation instruments, system, and kit for punctal implants |
| US20130013207A1 (en) | 2011-07-08 | 2013-01-10 | Artic Ice Management Ab | Support system for use when managing ice |
| CN102848803B (en)* | 2012-10-15 | 2014-08-20 | 青岛点石文具用品有限公司 | Slidable type pen |
| US9592151B2 (en)* | 2013-03-15 | 2017-03-14 | Glaukos Corporation | Systems and methods for delivering an ocular implant to the suprachoroidal space within an eye |
| EP3068354B1 (en)* | 2013-11-14 | 2023-06-28 | Aquesys, Inc. | Intraocular shunt inserter |
| KR102381775B1 (en) | 2016-03-16 | 2022-04-04 | 옥슬러 리미티드 | Ophthalmic delivery device and ophthalmic drug composition |
- 2019
- 2019-03-21ESES19771603Tpatent/ES2989955T3/enactiveActive
- 2019-03-21SGSG11202009056PApatent/SG11202009056PA/enunknown
- 2019-03-21MYMYPI2020004846Apatent/MY204381A/enunknown
- 2019-03-21AUAU2019240207Apatent/AU2019240207B2/enactiveActive
- 2019-03-21WOPCT/US2019/023323patent/WO2019183322A1/ennot_activeCeased
- 2019-03-21KRKR1020207030281Apatent/KR102765111B1/enactiveActive
- 2019-03-21CNCN201980020943.1Apatent/CN111936093A/enactivePending
- 2019-03-21ARARP190100719Apatent/AR115010A1/enactiveIP Right Grant
- 2019-03-21GBGB2016681.5Apatent/GB2587132B/enactiveActive
- 2019-03-21USUS16/360,287patent/US11497649B2/enactiveActive
- 2019-03-21NZNZ769069Apatent/NZ769069A/enunknown
- 2019-03-21JPJP2021500491Apatent/JP7296446B2/enactiveActive
- 2019-03-21MXMX2020009754Apatent/MX2020009754A/enunknown
- 2019-03-21TWTW108109792Apatent/TWI730307B/enactive
- 2019-03-21UYUY38150Apatent/UY38150A/enactiveIP Right Grant
- 2019-03-21CACA3094359Apatent/CA3094359C/enactiveActive
- 2019-03-21EPEP19771603.8Apatent/EP3768206B1/enactiveActive
Patent Citations (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US20090281520A1 (en)* | 2007-11-08 | 2009-11-12 | Brian Highley | Ocular Implantation Device |
| US20110009874A1 (en)* | 2009-07-09 | 2011-01-13 | John Wardle | Single Operator Device for Delivering an Ocular Implant |
| WO2013013207A1 (en)* | 2011-07-20 | 2013-01-24 | Becker Bruce B | Punctal plug inserter and method |
| US20140243763A1 (en)* | 2013-02-25 | 2014-08-28 | Sara Heikali | Devices for the Placement of Medical Compounds in Natural Orifices of a Body |
Also Published As
| Publication number | Publication date |
|---|---|
| WO2019183322A1 (en) | 2019-09-26 |
| ES2989955T3 (en) | 2024-11-28 |
| NZ769069A (en) | 2024-03-22 |
| TW201940141A (en) | 2019-10-16 |
| SG11202009056PA (en) | 2020-10-29 |
| CA3094359A1 (en) | 2019-09-26 |
| EP3768206B1 (en) | 2024-09-04 |
| AU2019240207B2 (en) | 2023-05-18 |
| JP7296446B2 (en) | 2023-06-22 |
| UY38150A (en) | 2019-10-01 |
| US20190290488A1 (en) | 2019-09-26 |
| AR115010A1 (en) | 2020-11-18 |
| MY204381A (en) | 2024-08-27 |
| CA3094359C (en) | 2023-07-25 |
| GB2587132A (en) | 2021-03-17 |
| GB202016681D0 (en) | 2020-12-02 |
| US11497649B2 (en) | 2022-11-15 |
| CN111936093A (en) | 2020-11-13 |
| GB2587132B (en) | 2022-04-13 |
| EP3768206A1 (en) | 2021-01-27 |
| EP3768206A4 (en) | 2022-01-05 |
| KR102765111B1 (en) | 2025-02-07 |
| AU2019240207A1 (en) | 2020-11-12 |
| JP2021518250A (en) | 2021-08-02 |
| MX2020009754A (en) | 2020-10-08 |
| EP3768206C0 (en) | 2024-09-04 |
| KR20200134280A (en) | 2020-12-01 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| US4763650A (en) | Instrument for inserting a deformable lens into the eye | |
| US6344047B1 (en) | Instrument for inserting a punctum plug and method for manufacturing the instrument | |
| JP3688287B1 (en) | Device for delivering an ophthalmic implant | |
| US5921990A (en) | Collagen forceps | |
| US7429263B2 (en) | Preloaded IOL injector | |
| JP5041322B2 (en) | Intraocular lens insertion device | |
| EP3389576B1 (en) | Patch for sealing retinal breaks and associated devices and systems. | |
| WO2007080868A1 (en) | Instrument for inserting intraocular lens | |
| US20130178822A1 (en) | Delivery and extraction devices | |
| US20100228260A1 (en) | Injector for intraocular lens | |
| WO2001028475A9 (en) | Deformable intraocular lens injecting apparatus and method | |
| US9265655B2 (en) | Punctum plug insertion device and device packaging | |
| US9192514B2 (en) | Medical instrument | |
| KR20190058868A (en) | hair implanter | |
| ES2244886T3 (en) | SURGICAL INSTRUMENTAL SUPPORT. | |
| US20040068286A1 (en) | Punctum dilating and punctum plug insertion instrument | |
| TWI730307B (en) | Intracanalicular dissolvable punctum plug inserter device, kit comprising the same and its assembling method | |
| US20040068235A1 (en) | Packaging system for a medical device and particularly a punctum plug insertion device | |
| US20120065601A1 (en) | Lacrimal Punctum Measurement and Occlusion | |
| HK40038421A (en) | Intracanalicular dissolvable punctum plug inserter | |
| US20140243763A1 (en) | Devices for the Placement of Medical Compounds in Natural Orifices of a Body | |
| JP6084408B2 (en) | Surgical instruments | |
| JP6822628B2 (en) | Intraocular lens insertion system, intraocular lens insertion device and tube | |
| JP2025526573A (en) | Ophthalmic Surgical Probes | |
| PL227414B1 (en) | Equipment for implanting an ophthalmological implant |