TWI717530B - Method manufacturing thin film containing metal carbide - Google Patents
Method manufacturing thin film containing metal carbideDownload PDFInfo
- Publication number
- TWI717530B TWI717530BTW106121626ATW106121626ATWI717530BTW I717530 BTWI717530 BTW I717530BTW 106121626 ATW106121626 ATW 106121626ATW 106121626 ATW106121626 ATW 106121626ATW I717530 BTWI717530 BTW I717530B
- Authority
- TW
- Taiwan
- Prior art keywords
- film
- metal carbide
- raw material
- thin film
- general formula
- Prior art date
Links
- 229910052751metalInorganic materials0.000titleclaimsabstractdescription72
- 239000002184metalSubstances0.000titleclaimsabstractdescription71
- 239000010409thin filmSubstances0.000titleclaimsdescription56
- 238000004519manufacturing processMethods0.000titleclaimsdescription35
- 238000000034methodMethods0.000titledescription57
- 239000002994raw materialSubstances0.000claimsabstractdescription67
- 150000001875compoundsChemical class0.000claimsabstractdescription58
- 125000000217alkyl groupChemical group0.000claimsabstractdescription14
- RTAQQCXQSZGOHL-UHFFFAOYSA-NTitaniumChemical group[Ti]RTAQQCXQSZGOHL-UHFFFAOYSA-N0.000claimsabstractdescription12
- 229910052719titaniumInorganic materials0.000claimsabstractdescription12
- 239000010936titaniumSubstances0.000claimsabstractdescription12
- 125000004432carbon atomChemical groupC*0.000claimsabstractdescription9
- QCWXUUIWCKQGHC-UHFFFAOYSA-NZirconiumChemical group[Zr]QCWXUUIWCKQGHC-UHFFFAOYSA-N0.000claimsabstractdescription7
- 229910052735hafniumChemical group0.000claimsabstractdescription7
- VBJZVLUMGGDVMO-UHFFFAOYSA-Nhafnium atomChemical group[Hf]VBJZVLUMGGDVMO-UHFFFAOYSA-N0.000claimsabstractdescription7
- 229910052726zirconiumInorganic materials0.000claimsabstractdescription7
- 125000004435hydrogen atomChemical group[H]*0.000claimsabstractdescription5
- 239000010408filmSubstances0.000claimsdescription96
- 239000007789gasSubstances0.000claimsdescription36
- 239000002243precursorSubstances0.000claimsdescription35
- 239000000758substrateSubstances0.000claimsdescription21
- 230000008016vaporizationEffects0.000claimsdescription11
- IJGRMHOSHXDMSA-UHFFFAOYSA-NAtomic nitrogenChemical compoundN#NIJGRMHOSHXDMSA-UHFFFAOYSA-N0.000claimsdescription8
- QGZKDVFQNNGYKY-UHFFFAOYSA-NAmmoniaChemical compoundNQGZKDVFQNNGYKY-UHFFFAOYSA-N0.000claimsdescription4
- OAKJQQAXSVQMHS-UHFFFAOYSA-NHydrazineChemical compoundNNOAKJQQAXSVQMHS-UHFFFAOYSA-N0.000claimsdescription4
- 229910052757nitrogenInorganic materials0.000claimsdescription4
- 239000006227byproductSubstances0.000claimsdescription3
- 239000001257hydrogenSubstances0.000claimsdescription3
- 229910052739hydrogenInorganic materials0.000claimsdescription3
- UFHFLCQGNIYNRP-UHFFFAOYSA-NHydrogenChemical compound[H][H]UFHFLCQGNIYNRP-UHFFFAOYSA-N0.000claimsdescription2
- 125000005263alkylenediamine groupChemical group0.000claimsdescription2
- 229910021529ammoniaInorganic materials0.000claimsdescription2
- 125000005265dialkylamine groupChemical group0.000claimsdescription2
- 125000005270trialkylamine groupChemical group0.000claimsdescription2
- 125000002496methyl groupChemical group[H]C([H])([H])*0.000abstractdescription4
- -1alkyl lithiumChemical compound0.000description22
- 239000000126substanceSubstances0.000description19
- 238000000231atomic layer depositionMethods0.000description15
- MTPVUVINMAGMJL-UHFFFAOYSA-Ntrimethyl(1,1,2,2,2-pentafluoroethyl)silaneChemical compoundC[Si](C)(C)C(F)(F)C(F)(F)FMTPVUVINMAGMJL-UHFFFAOYSA-N0.000description15
- HQABUPZFAYXKJW-UHFFFAOYSA-Nbutan-1-amineChemical compoundCCCCNHQABUPZFAYXKJW-UHFFFAOYSA-N0.000description12
- 230000015572biosynthetic processEffects0.000description11
- 238000006243chemical reactionMethods0.000description11
- 239000000203mixtureSubstances0.000description11
- 239000003960organic solventSubstances0.000description11
- OKTJSMMVPCPJKN-UHFFFAOYSA-NCarbonChemical compound[C]OKTJSMMVPCPJKN-UHFFFAOYSA-N0.000description10
- 229910052799carbonInorganic materials0.000description10
- 239000012434nucleophilic reagentSubstances0.000description10
- LFQSCWFLJHTTHZ-UHFFFAOYSA-NEthanolChemical compoundCCOLFQSCWFLJHTTHZ-UHFFFAOYSA-N0.000description9
- 239000002245particleSubstances0.000description9
- XKRFYHLGVUSROY-UHFFFAOYSA-NArgonChemical compound[Ar]XKRFYHLGVUSROY-UHFFFAOYSA-N0.000description8
- LRHPLDYGYMQRHN-UHFFFAOYSA-NN-ButanolChemical compoundCCCCOLRHPLDYGYMQRHN-UHFFFAOYSA-N0.000description8
- 235000019441ethanolNutrition0.000description8
- 239000012535impuritySubstances0.000description7
- 238000009834vaporizationMethods0.000description7
- XEKOWRVHYACXOJ-UHFFFAOYSA-NEthyl acetateChemical compoundCCOC(C)=OXEKOWRVHYACXOJ-UHFFFAOYSA-N0.000description6
- OKKJLVBELUTLKV-UHFFFAOYSA-NMethanolChemical compoundOCOKKJLVBELUTLKV-UHFFFAOYSA-N0.000description6
- UAOMVDZJSHZZME-UHFFFAOYSA-NdiisopropylamineChemical compoundCC(C)NC(C)CUAOMVDZJSHZZME-UHFFFAOYSA-N0.000description6
- 239000000463materialSubstances0.000description6
- 239000013110organic ligandSubstances0.000description6
- 238000005229chemical vapour depositionMethods0.000description5
- 238000000151depositionMethods0.000description5
- 239000007788liquidSubstances0.000description5
- KDSNLYIMUZNERS-UHFFFAOYSA-N2-methylpropanamineChemical compoundCC(C)CNKDSNLYIMUZNERS-UHFFFAOYSA-N0.000description4
- CSCPPACGZOOCGX-UHFFFAOYSA-NAcetoneChemical groupCC(C)=OCSCPPACGZOOCGX-UHFFFAOYSA-N0.000description4
- QUSNBJAOOMFDIB-UHFFFAOYSA-NEthylamineChemical compoundCCNQUSNBJAOOMFDIB-UHFFFAOYSA-N0.000description4
- LYCAIKOWRPUZTN-UHFFFAOYSA-NEthylene glycolChemical classOCCOLYCAIKOWRPUZTN-UHFFFAOYSA-N0.000description4
- KFZMGEQAYNKOFK-UHFFFAOYSA-NIsopropanolChemical compoundCC(C)OKFZMGEQAYNKOFK-UHFFFAOYSA-N0.000description4
- BAVYZALUXZFZLV-UHFFFAOYSA-NMethylamineChemical compoundNCBAVYZALUXZFZLV-UHFFFAOYSA-N0.000description4
- PXHVJJICTQNCMI-UHFFFAOYSA-NNickelChemical compound[Ni]PXHVJJICTQNCMI-UHFFFAOYSA-N0.000description4
- JUJWROOIHBZHMG-UHFFFAOYSA-NPyridineChemical groupC1=CC=NC=C1JUJWROOIHBZHMG-UHFFFAOYSA-N0.000description4
- XUIMIQQOPSSXEZ-UHFFFAOYSA-NSiliconChemical compound[Si]XUIMIQQOPSSXEZ-UHFFFAOYSA-N0.000description4
- WYURNTSHIVDZCO-UHFFFAOYSA-NTetrahydrofuranChemical compoundC1CCOC1WYURNTSHIVDZCO-UHFFFAOYSA-N0.000description4
- 229910052786argonInorganic materials0.000description4
- ZSWFCLXCOIISFI-UHFFFAOYSA-NcyclopentadieneChemical classC1C=CC=C1ZSWFCLXCOIISFI-UHFFFAOYSA-N0.000description4
- 230000006837decompressionEffects0.000description4
- 230000008021depositionEffects0.000description4
- 238000010586diagramMethods0.000description4
- 238000010438heat treatmentMethods0.000description4
- 239000007791liquid phaseSubstances0.000description4
- 150000001247metal acetylidesChemical class0.000description4
- 239000005416organic matterSubstances0.000description4
- WGYKZJWCGVVSQN-UHFFFAOYSA-NpropylamineChemical compoundCCCNWGYKZJWCGVVSQN-UHFFFAOYSA-N0.000description4
- 229910052710siliconInorganic materials0.000description4
- 239000010703siliconSubstances0.000description4
- 238000005979thermal decomposition reactionMethods0.000description4
- PUPZLCDOIYMWBV-UHFFFAOYSA-N(+/-)-1,3-ButanediolChemical compoundCC(O)CCOPUPZLCDOIYMWBV-UHFFFAOYSA-N0.000description3
- FTFYDDRPCCMKBT-UHFFFAOYSA-N1-butylcyclopenta-1,3-dieneChemical compoundCCCCC1=CC=CC1FTFYDDRPCCMKBT-UHFFFAOYSA-N0.000description3
- XLLIQLLCWZCATF-UHFFFAOYSA-N2-methoxyethyl acetateChemical compoundCOCCOC(C)=OXLLIQLLCWZCATF-UHFFFAOYSA-N0.000description3
- TWZMAMFYBNNBQK-UHFFFAOYSA-NCC(C)(C)C[Ti]Chemical compoundCC(C)(C)C[Ti]TWZMAMFYBNNBQK-UHFFFAOYSA-N0.000description3
- XTHFKEDIFFGKHM-UHFFFAOYSA-NDimethoxyethaneChemical compoundCOCCOCXTHFKEDIFFGKHM-UHFFFAOYSA-N0.000description3
- ROSDSFDQCJNGOL-UHFFFAOYSA-NDimethylamineChemical compoundCNCROSDSFDQCJNGOL-UHFFFAOYSA-N0.000description3
- SJRJJKPEHAURKC-UHFFFAOYSA-NN-MethylmorpholineChemical compoundCN1CCOCC1SJRJJKPEHAURKC-UHFFFAOYSA-N0.000description3
- RWRDLPDLKQPQOW-UHFFFAOYSA-NPyrrolidineChemical compoundC1CCNC1RWRDLPDLKQPQOW-UHFFFAOYSA-N0.000description3
- YXFVVABEGXRONW-UHFFFAOYSA-NTolueneChemical compoundCC1=CC=CC=C1YXFVVABEGXRONW-UHFFFAOYSA-N0.000description3
- 150000001298alcoholsChemical class0.000description3
- 229910052783alkali metalInorganic materials0.000description3
- 229910052782aluminiumInorganic materials0.000description3
- XAGFODPZIPBFFR-UHFFFAOYSA-NaluminiumChemical compound[Al]XAGFODPZIPBFFR-UHFFFAOYSA-N0.000description3
- 239000000919ceramicSubstances0.000description3
- 238000002485combustion reactionMethods0.000description3
- VLKZOEOYAKHREP-UHFFFAOYSA-Nn-HexaneChemical compoundCCCCCCVLKZOEOYAKHREP-UHFFFAOYSA-N0.000description3
- 230000008569processEffects0.000description3
- 239000000243solutionSubstances0.000description3
- 230000002269spontaneous effectEffects0.000description3
- RYHBNJHYFVUHQT-UHFFFAOYSA-N1,4-DioxaneChemical compoundC1COCCO1RYHBNJHYFVUHQT-UHFFFAOYSA-N0.000description2
- PAMIQIKDUOTOBW-UHFFFAOYSA-N1-methylpiperidineChemical compoundCN1CCCCC1PAMIQIKDUOTOBW-UHFFFAOYSA-N0.000description2
- XGGCYHBYGFNQIP-UHFFFAOYSA-N2,2,6-trimethyloctane-3,5-dioneChemical groupCCC(C)C(=O)CC(=O)C(C)(C)CXGGCYHBYGFNQIP-UHFFFAOYSA-N0.000description2
- OISVCGZHLKNMSJ-UHFFFAOYSA-N2,6-dimethylpyridineChemical groupCC1=CC=CC(C)=N1OISVCGZHLKNMSJ-UHFFFAOYSA-N0.000description2
- SBASXUCJHJRPEV-UHFFFAOYSA-N2-(2-methoxyethoxy)ethanolChemical compoundCOCCOCCOSBASXUCJHJRPEV-UHFFFAOYSA-N0.000description2
- SVTBMSDMJJWYQN-UHFFFAOYSA-N2-methylpentane-2,4-diolChemical compoundCC(O)CC(C)(C)OSVTBMSDMJJWYQN-UHFFFAOYSA-N0.000description2
- HCFAJYNVAYBARA-UHFFFAOYSA-N4-heptanoneChemical compoundCCCC(=O)CCCHCFAJYNVAYBARA-UHFFFAOYSA-N0.000description2
- RYGMFSIKBFXOCR-UHFFFAOYSA-NCopperChemical compound[Cu]RYGMFSIKBFXOCR-UHFFFAOYSA-N0.000description2
- XDTMQSROBMDMFD-UHFFFAOYSA-NCyclohexaneChemical compoundC1CCCCC1XDTMQSROBMDMFD-UHFFFAOYSA-N0.000description2
- XEEYBQQBJWHFJM-UHFFFAOYSA-NIronChemical compound[Fe]XEEYBQQBJWHFJM-UHFFFAOYSA-N0.000description2
- ZOKXTWBITQBERF-UHFFFAOYSA-NMolybdenumChemical compound[Mo]ZOKXTWBITQBERF-UHFFFAOYSA-N0.000description2
- YNAVUWVOSKDBBP-UHFFFAOYSA-NMorpholineChemical compoundC1COCCN1YNAVUWVOSKDBBP-UHFFFAOYSA-N0.000description2
- IMNFDUFMRHMDMM-UHFFFAOYSA-NN-HeptaneChemical compoundCCCCCCCIMNFDUFMRHMDMM-UHFFFAOYSA-N0.000description2
- AMQJEAYHLZJPGS-UHFFFAOYSA-NN-PentanolChemical compoundCCCCCOAMQJEAYHLZJPGS-UHFFFAOYSA-N0.000description2
- KDLHZDBZIXYQEI-UHFFFAOYSA-NPalladiumChemical compound[Pd]KDLHZDBZIXYQEI-UHFFFAOYSA-N0.000description2
- NQRYJNQNLNOLGT-UHFFFAOYSA-NPiperidineChemical compoundC1CCNCC1NQRYJNQNLNOLGT-UHFFFAOYSA-N0.000description2
- KJTLSVCANCCWHF-UHFFFAOYSA-NRutheniumChemical compound[Ru]KJTLSVCANCCWHF-UHFFFAOYSA-N0.000description2
- 229910052772SamariumInorganic materials0.000description2
- VYPSYNLAJGMNEJ-UHFFFAOYSA-NSilicium dioxideChemical compoundO=[Si]=OVYPSYNLAJGMNEJ-UHFFFAOYSA-N0.000description2
- DHXVGJBLRPWPCS-UHFFFAOYSA-NTetrahydropyranChemical compoundC1CCOCC1DHXVGJBLRPWPCS-UHFFFAOYSA-N0.000description2
- NRTOMJZYCJJWKI-UHFFFAOYSA-NTitanium nitrideChemical compound[Ti]#NNRTOMJZYCJJWKI-UHFFFAOYSA-N0.000description2
- 238000002441X-ray diffractionMethods0.000description2
- 238000004833X-ray photoelectron spectroscopyMethods0.000description2
- 229910026551ZrCInorganic materials0.000description2
- OTCHGXYCWNXDOA-UHFFFAOYSA-N[C].[Zr]Chemical compound[C].[Zr]OTCHGXYCWNXDOA-UHFFFAOYSA-N0.000description2
- 238000009825accumulationMethods0.000description2
- 238000007664blowingMethods0.000description2
- 125000000484butyl groupChemical group[H]C([*])([H])C([H])([H])C([H])([H])C([H])([H])[H]0.000description2
- 238000003763carbonizationMethods0.000description2
- 229910017052cobaltInorganic materials0.000description2
- 239000010941cobaltSubstances0.000description2
- GUTLYIVDDKVIGB-UHFFFAOYSA-Ncobalt atomChemical compound[Co]GUTLYIVDDKVIGB-UHFFFAOYSA-N0.000description2
- 230000000052comparative effectEffects0.000description2
- 229910052802copperInorganic materials0.000description2
- 239000010949copperSubstances0.000description2
- 238000005520cutting processMethods0.000description2
- JHIVVAPYMSGYDF-UHFFFAOYSA-NcyclohexanoneChemical compoundO=C1CCCCC1JHIVVAPYMSGYDF-UHFFFAOYSA-N0.000description2
- 238000002716delivery methodMethods0.000description2
- HPNMFZURTQLUMO-UHFFFAOYSA-NdiethylamineChemical compoundCCNCCHPNMFZURTQLUMO-UHFFFAOYSA-N0.000description2
- SBZXBUIDTXKZTM-UHFFFAOYSA-NdiglymeChemical compoundCOCCOCCOCSBZXBUIDTXKZTM-UHFFFAOYSA-N0.000description2
- 229940043279diisopropylamineDrugs0.000description2
- WEHWNAOGRSTTBQ-UHFFFAOYSA-NdipropylamineChemical compoundCCCNCCCWEHWNAOGRSTTBQ-UHFFFAOYSA-N0.000description2
- 239000012776electronic materialSubstances0.000description2
- RTZKZFJDLAIYFH-UHFFFAOYSA-NetherSubstancesCCOCCRTZKZFJDLAIYFH-UHFFFAOYSA-N0.000description2
- 125000001495ethyl groupChemical group[H]C([H])([H])C([H])([H])*0.000description2
- IIEWJVIFRVWJOD-UHFFFAOYSA-NethylcyclohexaneChemical compoundCCC1CCCCC1IIEWJVIFRVWJOD-UHFFFAOYSA-N0.000description2
- LIWAQLJGPBVORC-UHFFFAOYSA-NethylmethylamineChemical compoundCCNCLIWAQLJGPBVORC-UHFFFAOYSA-N0.000description2
- 238000011156evaluationMethods0.000description2
- WHJFNYXPKGDKBB-UHFFFAOYSA-Nhafnium;methaneChemical compoundC.[Hf]WHJFNYXPKGDKBB-UHFFFAOYSA-N0.000description2
- 229910052736halogenInorganic materials0.000description2
- 150000002367halogensChemical class0.000description2
- 239000001307heliumSubstances0.000description2
- 229910052734heliumInorganic materials0.000description2
- SWQJXJOGLNCZEY-UHFFFAOYSA-Nhelium atomChemical compound[He]SWQJXJOGLNCZEY-UHFFFAOYSA-N0.000description2
- ILPNRWUGFSPGAA-UHFFFAOYSA-Nheptane-2,4-dioneChemical groupCCCC(=O)CC(C)=OILPNRWUGFSPGAA-UHFFFAOYSA-N0.000description2
- FFUAGWLWBBFQJT-UHFFFAOYSA-NhexamethyldisilazaneChemical compoundC[Si](C)(C)N[Si](C)(C)CFFUAGWLWBBFQJT-UHFFFAOYSA-N0.000description2
- NDOGLIPWGGRQCO-UHFFFAOYSA-Nhexane-2,4-dioneChemical groupCCC(=O)CC(C)=ONDOGLIPWGGRQCO-UHFFFAOYSA-N0.000description2
- 229930195733hydrocarbonNatural products0.000description2
- 229910017053inorganic saltInorganic materials0.000description2
- PHTQWCKDNZKARW-UHFFFAOYSA-NisoamylolChemical compoundCC(C)CCOPHTQWCKDNZKARW-UHFFFAOYSA-N0.000description2
- ZXEKIIBDNHEJCQ-UHFFFAOYSA-NisobutanolChemical compoundCC(C)COZXEKIIBDNHEJCQ-UHFFFAOYSA-N0.000description2
- JJWLVOIRVHMVIS-UHFFFAOYSA-NisopropylamineChemical compoundCC(C)NJJWLVOIRVHMVIS-UHFFFAOYSA-N0.000description2
- 150000002576ketonesChemical class0.000description2
- MRELNEQAGSRDBK-UHFFFAOYSA-Nlanthanum(3+);oxygen(2-)Chemical compound[O-2].[O-2].[O-2].[La+3].[La+3]MRELNEQAGSRDBK-UHFFFAOYSA-N0.000description2
- 229910052744lithiumInorganic materials0.000description2
- 150000002736metal compoundsChemical class0.000description2
- 238000002488metal-organic chemical vapour depositionMethods0.000description2
- 150000002739metalsChemical class0.000description2
- UAEPNZWRGJTJPN-UHFFFAOYSA-NmethylcyclohexaneChemical compoundCC1CCCCC1UAEPNZWRGJTJPN-UHFFFAOYSA-N0.000description2
- 239000011259mixed solutionSubstances0.000description2
- 238000002156mixingMethods0.000description2
- 229910052750molybdenumInorganic materials0.000description2
- 239000011733molybdenumSubstances0.000description2
- GVWISOJSERXQBM-UHFFFAOYSA-Nn-methylpropan-1-amineChemical compoundCCCNCGVWISOJSERXQBM-UHFFFAOYSA-N0.000description2
- XHFGWHUWQXTGAT-UHFFFAOYSA-Nn-methylpropan-2-amineChemical compoundCNC(C)CXHFGWHUWQXTGAT-UHFFFAOYSA-N0.000description2
- 229910052759nickelInorganic materials0.000description2
- 125000004433nitrogen atomChemical groupN*0.000description2
- 238000006864oxidative decomposition reactionMethods0.000description2
- 125000004430oxygen atomChemical groupO*0.000description2
- AQIXEPGDORPWBJ-UHFFFAOYSA-Npentan-3-olChemical compoundCCC(O)CCAQIXEPGDORPWBJ-UHFFFAOYSA-N0.000description2
- 238000005268plasma chemical vapour depositionMethods0.000description2
- 238000009832plasma treatmentMethods0.000description2
- BASFCYQUMIYNBI-UHFFFAOYSA-NplatinumChemical compound[Pt]BASFCYQUMIYNBI-UHFFFAOYSA-N0.000description2
- 229920000768polyaminePolymers0.000description2
- UMJSCPRVCHMLSP-UHFFFAOYSA-NpyridineChemical groupCOC1=CC=CN=C1UMJSCPRVCHMLSP-UHFFFAOYSA-N0.000description2
- 229910052707rutheniumInorganic materials0.000description2
- KZUNJOHGWZRPMI-UHFFFAOYSA-Nsamarium atomChemical compound[Sm]KZUNJOHGWZRPMI-UHFFFAOYSA-N0.000description2
- 239000004065semiconductorSubstances0.000description2
- YLQBMQCUIZJEEH-UHFFFAOYSA-NtetrahydrofuranNatural productsC=1C=COC=1YLQBMQCUIZJEEH-UHFFFAOYSA-N0.000description2
- 238000002230thermal chemical vapour depositionMethods0.000description2
- YFNKIDBQEZZDLK-UHFFFAOYSA-NtriglymeChemical compoundCOCCOCCOCCOCYFNKIDBQEZZDLK-UHFFFAOYSA-N0.000description2
- DNIAPMSPPWPWGF-VKHMYHEASA-N(+)-propylene glycolChemical compoundC[C@H](O)CODNIAPMSPPWPWGF-VKHMYHEASA-N0.000description1
- DNIAPMSPPWPWGF-GSVOUGTGSA-N(R)-(-)-Propylene glycolChemical compoundC[C@@H](O)CODNIAPMSPPWPWGF-GSVOUGTGSA-N0.000description1
- BVPKYBMUQDZTJH-UHFFFAOYSA-N1,1,1-trifluoro-5,5-dimethylhexane-2,4-dioneChemical compoundCC(C)(C)C(=O)CC(=O)C(F)(F)FBVPKYBMUQDZTJH-UHFFFAOYSA-N0.000description1
- SHXHPUAKLCCLDV-UHFFFAOYSA-N1,1,1-trifluoropentane-2,4-dioneChemical compoundCC(=O)CC(=O)C(F)(F)FSHXHPUAKLCCLDV-UHFFFAOYSA-N0.000description1
- VNPQQEYMXYCAEZ-UHFFFAOYSA-N1,2,3,4-tetramethylcyclopenta-1,3-dieneChemical compoundCC1=C(C)C(C)=C(C)C1VNPQQEYMXYCAEZ-UHFFFAOYSA-N0.000description1
- YPFDHNVEDLHUCE-UHFFFAOYSA-N1,3-propanediolSubstancesOCCCOYPFDHNVEDLHUCE-UHFFFAOYSA-N0.000description1
- QBPPRVHXOZRESW-UHFFFAOYSA-N1,4,7,10-tetraazacyclododecaneChemical compoundC1CNCCNCCNCCN1QBPPRVHXOZRESW-UHFFFAOYSA-N0.000description1
- MDAXKAUIABOHTD-UHFFFAOYSA-N1,4,8,11-tetraazacyclotetradecaneChemical compoundC1CNCCNCCCNCCNC1MDAXKAUIABOHTD-UHFFFAOYSA-N0.000description1
- SIUWDFVMEASCRP-UHFFFAOYSA-N1-(2-methoxyethoxy)-2-methylpropan-2-olChemical compoundCOCCOCC(C)(C)OSIUWDFVMEASCRP-UHFFFAOYSA-N0.000description1
- GHJATKVLNMETBA-UHFFFAOYSA-N1-(2-methylpropyl)cyclopenta-1,3-dieneChemical compoundCC(C)CC1=CC=CC1GHJATKVLNMETBA-UHFFFAOYSA-N0.000description1
- JCALRHVFTLBTOZ-UHFFFAOYSA-N1-butoxy-2-methylpropan-2-olChemical compoundCCCCOCC(C)(C)OJCALRHVFTLBTOZ-UHFFFAOYSA-N0.000description1
- DURPTKYDGMDSBL-UHFFFAOYSA-N1-butoxybutaneChemical compoundCCCCOCCCCDURPTKYDGMDSBL-UHFFFAOYSA-N0.000description1
- BCYNAHGOLUTMDM-UHFFFAOYSA-N1-ethoxy-2-methylpropan-2-olChemical compoundCCOCC(C)(C)OBCYNAHGOLUTMDM-UHFFFAOYSA-N0.000description1
- IQSUNBLELDRPEY-UHFFFAOYSA-N1-ethylcyclopenta-1,3-dieneChemical compoundCCC1=CC=CC1IQSUNBLELDRPEY-UHFFFAOYSA-N0.000description1
- QNLXXWOBNIYLGO-UHFFFAOYSA-N1-methoxy-2,2,6,6-tetramethylheptane-3,5-dioneChemical compoundCOCC(C)(C)C(=O)CC(=O)C(C)(C)CQNLXXWOBNIYLGO-UHFFFAOYSA-N0.000description1
- MXUXZWFVAPTPAG-UHFFFAOYSA-N1-methoxy-2-methylpropan-2-olChemical compoundCOCC(C)(C)OMXUXZWFVAPTPAG-UHFFFAOYSA-N0.000description1
- AVFZOVWCLRSYKC-UHFFFAOYSA-N1-methylpyrrolidineChemical compoundCN1CCCC1AVFZOVWCLRSYKC-UHFFFAOYSA-N0.000description1
- MWQKURVBJZAOSC-UHFFFAOYSA-N1-propan-2-ylcyclopenta-1,3-dieneChemical compoundCC(C)C1=CC=CC1MWQKURVBJZAOSC-UHFFFAOYSA-N0.000description1
- RZPAXISDLOEXPI-UHFFFAOYSA-N1-propylcyclopenta-1,3-dieneChemical compoundCCCC1=CC=CC1RZPAXISDLOEXPI-UHFFFAOYSA-N0.000description1
- XEZNGIUYQVAUSS-UHFFFAOYSA-N18-crown-6Chemical compoundC1COCCOCCOCCOCCOCCO1XEZNGIUYQVAUSS-UHFFFAOYSA-N0.000description1
- VILCJCGEZXAXTO-UHFFFAOYSA-N2,2,2-tetramineChemical compoundNCCNCCNCCNVILCJCGEZXAXTO-UHFFFAOYSA-N0.000description1
- YRAJNWYBUCUFBD-UHFFFAOYSA-N2,2,6,6-tetramethylheptane-3,5-dioneChemical groupCC(C)(C)C(=O)CC(=O)C(C)(C)CYRAJNWYBUCUFBD-UHFFFAOYSA-N0.000description1
- KLKRGCUPZROPPO-UHFFFAOYSA-N2,2,6-trimethylheptane-3,5-dioneChemical groupCC(C)C(=O)CC(=O)C(C)(C)CKLKRGCUPZROPPO-UHFFFAOYSA-N0.000description1
- AKPLTHZVVWBOPT-UHFFFAOYSA-N2,2-diethylbutane-1,3-diolChemical compoundCCC(CC)(CO)C(C)OAKPLTHZVVWBOPT-UHFFFAOYSA-N0.000description1
- LHQYNVWJWUCTSS-UHFFFAOYSA-N2,2-dimethylheptane-3,5-dioneChemical groupCCC(=O)CC(=O)C(C)(C)CLHQYNVWJWUCTSS-UHFFFAOYSA-N0.000description1
- DBTGFWMBFZBBEF-UHFFFAOYSA-N2,4-dimethylpentane-2,4-diolChemical compoundCC(C)(O)CC(C)(C)ODBTGFWMBFZBBEF-UHFFFAOYSA-N0.000description1
- CEGGECULKVTYMM-UHFFFAOYSA-N2,6-dimethylheptane-3,5-dioneChemical groupCC(C)C(=O)CC(=O)C(C)CCEGGECULKVTYMM-UHFFFAOYSA-N0.000description1
- XNWFRZJHXBZDAG-UHFFFAOYSA-N2-METHOXYETHANOLChemical compoundCOCCOXNWFRZJHXBZDAG-UHFFFAOYSA-N0.000description1
- PTTPXKJBFFKCEK-UHFFFAOYSA-N2-Methyl-4-heptanoneChemical compoundCC(C)CC(=O)CC(C)CPTTPXKJBFFKCEK-UHFFFAOYSA-N0.000description1
- QQZOPKMRPOGIEB-UHFFFAOYSA-N2-OxohexaneChemical compoundCCCCC(C)=OQQZOPKMRPOGIEB-UHFFFAOYSA-N0.000description1
- POAOYUHQDCAZBD-UHFFFAOYSA-N2-butoxyethanolChemical compoundCCCCOCCOPOAOYUHQDCAZBD-UHFFFAOYSA-N0.000description1
- DSKYSDCYIODJPC-UHFFFAOYSA-N2-butyl-2-ethylpropane-1,3-diolChemical compoundCCCCC(CC)(CO)CODSKYSDCYIODJPC-UHFFFAOYSA-N0.000description1
- ZNQVEEAIQZEUHB-UHFFFAOYSA-N2-ethoxyethanolChemical compoundCCOCCOZNQVEEAIQZEUHB-UHFFFAOYSA-N0.000description1
- 2299400934752-ethoxyethanolDrugs0.000description1
- VJTFBKZTQWRDKE-UHFFFAOYSA-N2-methanidyl-2-methylpropane;titanium(4+)Chemical compound[Ti+4].CC(C)(C)[CH2-].CC(C)(C)[CH2-].CC(C)(C)[CH2-].CC(C)(C)[CH2-]VJTFBKZTQWRDKE-UHFFFAOYSA-N0.000description1
- RBPYZXJJLCKEHL-UHFFFAOYSA-N2-methoxyethanol;propan-2-olChemical compoundCC(C)O.COCCORBPYZXJJLCKEHL-UHFFFAOYSA-N0.000description1
- IHGXRBJFCJZJSC-UHFFFAOYSA-N2-methyl-1-propan-2-yloxypropan-2-olChemical compoundCC(C)OCC(C)(C)OIHGXRBJFCJZJSC-UHFFFAOYSA-N0.000description1
- JPYKRAVOZNWRKH-UHFFFAOYSA-N2-methyldecane-4,6-dioneChemical groupCCCCC(=O)CC(=O)CC(C)CJPYKRAVOZNWRKH-UHFFFAOYSA-N0.000description1
- VVALZQWOQKHDIM-UHFFFAOYSA-N2-methylheptane-3,5-dioneChemical groupCCC(=O)CC(=O)C(C)CVVALZQWOQKHDIM-UHFFFAOYSA-N0.000description1
- QWGRWMMWNDWRQN-UHFFFAOYSA-N2-methylpropane-1,3-diolChemical compoundOCC(C)COQWGRWMMWNDWRQN-UHFFFAOYSA-N0.000description1
- BYEAPGRMZGQWND-UHFFFAOYSA-N3-(butan-2-yloxymethyl)pentan-3-olChemical compoundCCC(C)OCC(O)(CC)CCBYEAPGRMZGQWND-UHFFFAOYSA-N0.000description1
- FNFKMJVRDHWAOR-UHFFFAOYSA-N3-(propoxymethyl)pentan-3-olChemical compoundCCCOCC(O)(CC)CCFNFKMJVRDHWAOR-UHFFFAOYSA-N0.000description1
- LHUPIZXVJQEKCY-UHFFFAOYSA-N4-methoxy-2-methylbutan-2-olChemical compoundCOCCC(C)(C)OLHUPIZXVJQEKCY-UHFFFAOYSA-N0.000description1
- VGVHNLRUAMRIEW-UHFFFAOYSA-N4-methylcyclohexan-1-oneChemical compoundCC1CCC(=O)CC1VGVHNLRUAMRIEW-UHFFFAOYSA-N0.000description1
- QWJWPDHACGGABF-UHFFFAOYSA-N5,5-dimethylcyclopenta-1,3-dieneChemical compoundCC1(C)C=CC=C1QWJWPDHACGGABF-UHFFFAOYSA-N0.000description1
- VMPZHUZUESBODJ-UHFFFAOYSA-N5-methylheptane-2,4-dioneChemical groupCCC(C)C(=O)CC(C)=OVMPZHUZUESBODJ-UHFFFAOYSA-N0.000description1
- KHZGUWAFFHXZLC-UHFFFAOYSA-N5-methylhexane-2,4-dioneChemical groupCC(C)C(=O)CC(C)=OKHZGUWAFFHXZLC-UHFFFAOYSA-N0.000description1
- IGMOYJSFRIASIE-UHFFFAOYSA-N6-Methylheptan-2,4-dioneChemical groupCC(C)CC(=O)CC(C)=OIGMOYJSFRIASIE-UHFFFAOYSA-N0.000description1
- NZJWGSQIJIIXQK-UHFFFAOYSA-N6-ethyl-2,2-dimethyldecane-3,5-dioneChemical groupCCCCC(CC)C(=O)CC(=O)C(C)(C)CNZJWGSQIJIIXQK-UHFFFAOYSA-N0.000description1
- GALSRMFPHSGNRR-UHFFFAOYSA-N6-ethyl-2-methyldecane-3,5-dioneChemical groupCCCCC(CC)C(=O)CC(=O)C(C)CGALSRMFPHSGNRR-UHFFFAOYSA-N0.000description1
- DKPFZGUDAPQIHT-UHFFFAOYSA-NButyl acetateNatural productsCCCCOC(C)=ODKPFZGUDAPQIHT-UHFFFAOYSA-N0.000description1
- OYPRJOBELJOOCE-UHFFFAOYSA-NCalciumChemical compound[Ca]OYPRJOBELJOOCE-UHFFFAOYSA-N0.000description1
- 229910052684CeriumInorganic materials0.000description1
- ZAMOUSCENKQFHK-UHFFFAOYSA-NChlorine atomChemical compound[Cl]ZAMOUSCENKQFHK-UHFFFAOYSA-N0.000description1
- RPNUMPOLZDHAAY-UHFFFAOYSA-NDiethylenetriamineChemical compoundNCCNCCNRPNUMPOLZDHAAY-UHFFFAOYSA-N0.000description1
- 229910052692DysprosiumInorganic materials0.000description1
- 229910052691ErbiumInorganic materials0.000description1
- OTMSDBZUPAUEDD-UHFFFAOYSA-NEthaneChemical compoundCCOTMSDBZUPAUEDD-UHFFFAOYSA-N0.000description1
- PIICEJLVQHRZGT-UHFFFAOYSA-NEthylenediamineChemical compoundNCCNPIICEJLVQHRZGT-UHFFFAOYSA-N0.000description1
- 229910052693EuropiumInorganic materials0.000description1
- 229910002601GaNInorganic materials0.000description1
- GYHNNYVSQQEPJS-UHFFFAOYSA-NGalliumChemical compound[Ga]GYHNNYVSQQEPJS-UHFFFAOYSA-N0.000description1
- 229910000530Gallium indium arsenideInorganic materials0.000description1
- JMASRVWKEDWRBT-UHFFFAOYSA-NGallium nitrideChemical compound[Ga]#NJMASRVWKEDWRBT-UHFFFAOYSA-N0.000description1
- 229910000673Indium arsenideInorganic materials0.000description1
- FYYHWMGAXLPEAU-UHFFFAOYSA-NMagnesiumChemical compound[Mg]FYYHWMGAXLPEAU-UHFFFAOYSA-N0.000description1
- NTIZESTWPVYFNL-UHFFFAOYSA-NMethyl isobutyl ketoneChemical compoundCC(C)CC(C)=ONTIZESTWPVYFNL-UHFFFAOYSA-N0.000description1
- UIHCLUNTQKBZGK-UHFFFAOYSA-NMethyl isobutyl ketoneNatural productsCCC(C)C(C)=OUIHCLUNTQKBZGK-UHFFFAOYSA-N0.000description1
- WRQNANDWMGAFTP-UHFFFAOYSA-NMethylacetoacetic acidChemical compoundCOC(=O)CC(C)=OWRQNANDWMGAFTP-UHFFFAOYSA-N0.000description1
- OHJRDMBJSIHZMT-UHFFFAOYSA-NN'-[2-(2-aminoethylamino)ethyl]-N,N,1-triethoxyethane-1,2-diamineChemical compoundC(C)OC(N(OCC)OCC)CNCCNCCNOHJRDMBJSIHZMT-UHFFFAOYSA-N0.000description1
- KWYHDKDOAIKMQN-UHFFFAOYSA-NN,N,N',N'-tetramethylethylenediamineChemical compoundCN(C)CCN(C)CKWYHDKDOAIKMQN-UHFFFAOYSA-N0.000description1
- AHVYPIQETPWLSZ-UHFFFAOYSA-NN-methyl-pyrrolidineNatural productsCN1CC=CC1AHVYPIQETPWLSZ-UHFFFAOYSA-N0.000description1
- 229910002651NO3Inorganic materials0.000description1
- 229910052779NeodymiumInorganic materials0.000description1
- NHNBFGGVMKEFGY-UHFFFAOYSA-NNitrateChemical compound[O-][N+]([O-])=ONHNBFGGVMKEFGY-UHFFFAOYSA-N0.000description1
- CTQNGGLPUBDAKN-UHFFFAOYSA-NO-XyleneChemical compoundCC1=CC=CC=C1CCTQNGGLPUBDAKN-UHFFFAOYSA-N0.000description1
- QQYLKEWQTYXRPM-UHFFFAOYSA-NO1SCCC1.O1SCCC1Chemical compoundO1SCCC1.O1SCCC1QQYLKEWQTYXRPM-UHFFFAOYSA-N0.000description1
- ZCQWOFVYLHDMMC-UHFFFAOYSA-NOxazoleChemical compoundC1=COC=N1ZCQWOFVYLHDMMC-UHFFFAOYSA-N0.000description1
- RFFFKMOABOFIDF-UHFFFAOYSA-NPentanenitrileChemical compoundCCCCC#NRFFFKMOABOFIDF-UHFFFAOYSA-N0.000description1
- 229910052581Si3N4Inorganic materials0.000description1
- BQCADISMDOOEFD-UHFFFAOYSA-NSilverChemical compound[Ag]BQCADISMDOOEFD-UHFFFAOYSA-N0.000description1
- FZWLAAWBMGSTSO-UHFFFAOYSA-NThiazoleChemical compoundC1=CSC=N1FZWLAAWBMGSTSO-UHFFFAOYSA-N0.000description1
- ATJFFYVFTNAWJD-UHFFFAOYSA-NTinChemical compound[Sn]ATJFFYVFTNAWJD-UHFFFAOYSA-N0.000description1
- GWEVSGVZZGPLCZ-UHFFFAOYSA-NTitan oxideChemical compoundO=[Ti]=OGWEVSGVZZGPLCZ-UHFFFAOYSA-N0.000description1
- HCHKCACWOHOZIP-UHFFFAOYSA-NZincChemical compound[Zn]HCHKCACWOHOZIP-UHFFFAOYSA-N0.000description1
- LCRTXDONVQDQEH-UHFFFAOYSA-K[Cl-].[Cl-].[Cl-].CC=CC=C[Ti+3]Chemical compound[Cl-].[Cl-].[Cl-].CC=CC=C[Ti+3]LCRTXDONVQDQEH-UHFFFAOYSA-K0.000description1
- KXNLCSXBJCPWGL-UHFFFAOYSA-N[Ga].[As].[In]Chemical compound[Ga].[As].[In]KXNLCSXBJCPWGL-UHFFFAOYSA-N0.000description1
- 150000001242acetic acid derivativesChemical class0.000description1
- 230000009471actionEffects0.000description1
- BTGRAWJCKBQKAO-UHFFFAOYSA-NadiponitrileChemical compoundN#CCCCCC#NBTGRAWJCKBQKAO-UHFFFAOYSA-N0.000description1
- 229910052784alkaline earth metalInorganic materials0.000description1
- PNEYBMLMFCGWSK-UHFFFAOYSA-Naluminium oxideInorganic materials[O-2].[O-2].[O-2].[Al+3].[Al+3]PNEYBMLMFCGWSK-UHFFFAOYSA-N0.000description1
- 238000000137annealingMethods0.000description1
- 229910052787antimonyInorganic materials0.000description1
- WATWJIUSRGPENY-UHFFFAOYSA-Nantimony atomChemical compound[Sb]WATWJIUSRGPENY-UHFFFAOYSA-N0.000description1
- 238000000149argon plasma sinteringMethods0.000description1
- QVGXLLKOCUKJST-UHFFFAOYSA-Natomic oxygenChemical compound[O]QVGXLLKOCUKJST-UHFFFAOYSA-N0.000description1
- 229910052788bariumInorganic materials0.000description1
- DSAJWYNOEDNPEQ-UHFFFAOYSA-Nbarium atomChemical compound[Ba]DSAJWYNOEDNPEQ-UHFFFAOYSA-N0.000description1
- 230000004888barrier functionEffects0.000description1
- JFDZBHWFFUWGJE-UHFFFAOYSA-NbenzonitrileChemical compoundN#CC1=CC=CC=C1JFDZBHWFFUWGJE-UHFFFAOYSA-N0.000description1
- 229910052797bismuthInorganic materials0.000description1
- JCXGWMGPZLAOME-UHFFFAOYSA-Nbismuth atomChemical compound[Bi]JCXGWMGPZLAOME-UHFFFAOYSA-N0.000description1
- 238000009835boilingMethods0.000description1
- 230000005587bubblingEffects0.000description1
- KVNRLNFWIYMESJ-UHFFFAOYSA-NbutyronitrileChemical compoundCCCC#NKVNRLNFWIYMESJ-UHFFFAOYSA-N0.000description1
- 229910052791calciumInorganic materials0.000description1
- 239000011575calciumSubstances0.000description1
- 239000012159carrier gasSubstances0.000description1
- 239000003054catalystSubstances0.000description1
- ZMIGMASIKSOYAM-UHFFFAOYSA-NceriumChemical compound[Ce][Ce][Ce][Ce][Ce][Ce][Ce][Ce][Ce][Ce][Ce][Ce][Ce][Ce][Ce][Ce][Ce][Ce][Ce][Ce][Ce][Ce][Ce][Ce][Ce][Ce][Ce][Ce][Ce][Ce][Ce][Ce][Ce][Ce][Ce][Ce][Ce][Ce]ZMIGMASIKSOYAM-UHFFFAOYSA-N0.000description1
- 239000000460chlorineSubstances0.000description1
- 229910052801chlorineInorganic materials0.000description1
- 239000011248coating agentSubstances0.000description1
- 238000000576coating methodMethods0.000description1
- 239000000470constituentSubstances0.000description1
- 238000011109contaminationMethods0.000description1
- 150000003983crown ethersChemical class0.000description1
- 125000004093cyano groupChemical group*C#N0.000description1
- 125000004122cyclic groupChemical group0.000description1
- MGWYSXZGBRHJNE-UHFFFAOYSA-Ncyclohexane-1,4-dicarbonitrileChemical compoundN#CC1CCC(C#N)CC1MGWYSXZGBRHJNE-UHFFFAOYSA-N0.000description1
- AENCLWKVWIIOQH-UHFFFAOYSA-Kcyclopentane;trichlorotitaniumChemical compoundCl[Ti](Cl)Cl.[CH]1C=CC=C1AENCLWKVWIIOQH-UHFFFAOYSA-K0.000description1
- 230000006866deteriorationEffects0.000description1
- HPYNZHMRTTWQTB-UHFFFAOYSA-NdimethylpyridineChemical groupCC1=CC=CN=C1CHPYNZHMRTTWQTB-UHFFFAOYSA-N0.000description1
- KBQHZAAAGSGFKK-UHFFFAOYSA-Ndysprosium atomChemical compound[Dy]KBQHZAAAGSGFKK-UHFFFAOYSA-N0.000description1
- UYAHIZSMUZPPFV-UHFFFAOYSA-NerbiumChemical compound[Er]UYAHIZSMUZPPFV-UHFFFAOYSA-N0.000description1
- 125000001033ether groupChemical group0.000description1
- 150000002170ethersChemical class0.000description1
- XYIBRDXRRQCHLP-UHFFFAOYSA-Nethyl acetoacetateChemical compoundCCOC(=O)CC(C)=OXYIBRDXRRQCHLP-UHFFFAOYSA-N0.000description1
- OGPBJKLSAFTDLK-UHFFFAOYSA-Neuropium atomChemical compound[Eu]OGPBJKLSAFTDLK-UHFFFAOYSA-N0.000description1
- 229910052733galliumInorganic materials0.000description1
- 238000002309gasificationMethods0.000description1
- 229910052732germaniumInorganic materials0.000description1
- GNPVGFCGXDBREM-UHFFFAOYSA-Ngermanium atomChemical compound[Ge]GNPVGFCGXDBREM-UHFFFAOYSA-N0.000description1
- 239000011521glassSubstances0.000description1
- ZTOMUSMDRMJOTH-UHFFFAOYSA-NglutaronitrileChemical compoundN#CCCCC#NZTOMUSMDRMJOTH-UHFFFAOYSA-N0.000description1
- PCHJSUWPFVWCPO-UHFFFAOYSA-NgoldChemical compound[Au]PCHJSUWPFVWCPO-UHFFFAOYSA-N0.000description1
- 229910052737goldInorganic materials0.000description1
- 239000010931goldSubstances0.000description1
- 229910000449hafnium oxideInorganic materials0.000description1
- WIHZLLGSGQNAGK-UHFFFAOYSA-Nhafnium(4+);oxygen(2-)Chemical compound[O-2].[O-2].[Hf+4]WIHZLLGSGQNAGK-UHFFFAOYSA-N0.000description1
- CATSNJVOTSVZJV-UHFFFAOYSA-Nheptan-2-oneChemical compoundCCCCCC(C)=OCATSNJVOTSVZJV-UHFFFAOYSA-N0.000description1
- NGAZZOYFWWSOGK-UHFFFAOYSA-Nheptan-3-oneChemical compoundCCCCC(=O)CCNGAZZOYFWWSOGK-UHFFFAOYSA-N0.000description1
- DGCTVLNZTFDPDJ-UHFFFAOYSA-Nheptane-3,5-dioneChemical compoundCCC(=O)CC(=O)CCDGCTVLNZTFDPDJ-UHFFFAOYSA-N0.000description1
- SDAXRHHPNYTELL-UHFFFAOYSA-NheptanenitrileChemical compoundCCCCCCC#NSDAXRHHPNYTELL-UHFFFAOYSA-N0.000description1
- 150000002391heterocyclic compoundsChemical class0.000description1
- QAMFBRUWYYMMGJ-UHFFFAOYSA-NhexafluoroacetylacetoneChemical compoundFC(F)(F)C(=O)CC(=O)C(F)(F)FQAMFBRUWYYMMGJ-UHFFFAOYSA-N0.000description1
- TXGJTWACJNYNOJ-UHFFFAOYSA-Nhexane-2,4-diolChemical compoundCCC(O)CC(C)OTXGJTWACJNYNOJ-UHFFFAOYSA-N0.000description1
- FUZZWVXGSFPDMH-UHFFFAOYSA-MhexanoateChemical compoundCCCCCC([O-])=OFUZZWVXGSFPDMH-UHFFFAOYSA-M0.000description1
- WGCNASOHLSPBMP-UHFFFAOYSA-NhydroxyacetaldehydeNatural productsOCC=OWGCNASOHLSPBMP-UHFFFAOYSA-N0.000description1
- 229910052738indiumInorganic materials0.000description1
- RPQDHPTXJYYUPQ-UHFFFAOYSA-Nindium arsenideChemical compound[In]#[As]RPQDHPTXJYYUPQ-UHFFFAOYSA-N0.000description1
- APFVFJFRJDLVQX-UHFFFAOYSA-Nindium atomChemical compound[In]APFVFJFRJDLVQX-UHFFFAOYSA-N0.000description1
- 239000011261inert gasSubstances0.000description1
- 238000007733ion platingMethods0.000description1
- 229910052741iridiumInorganic materials0.000description1
- GKOZUEZYRPOHIO-UHFFFAOYSA-Niridium atomChemical compound[Ir]GKOZUEZYRPOHIO-UHFFFAOYSA-N0.000description1
- 229910052742ironInorganic materials0.000description1
- 125000000959isobutyl groupChemical group[H]C([H])([H])C([H])(C([H])([H])[H])C([H])([H])*0.000description1
- 125000001972isopentyl groupChemical group[H]C([H])([H])C([H])(C([H])([H])[H])C([H])([H])C([H])([H])*0.000description1
- 125000001449isopropyl groupChemical group[H]C([H])([H])C([H])(*)C([H])([H])[H]0.000description1
- 229910052746lanthanumInorganic materials0.000description1
- FZLIPJUXYLNCLC-UHFFFAOYSA-Nlanthanum atomChemical compound[La]FZLIPJUXYLNCLC-UHFFFAOYSA-N0.000description1
- 239000003446ligandSubstances0.000description1
- 229910052749magnesiumInorganic materials0.000description1
- 239000011777magnesiumSubstances0.000description1
- WPBNNNQJVZRUHP-UHFFFAOYSA-Lmanganese(2+);methyl n-[[2-(methoxycarbonylcarbamothioylamino)phenyl]carbamothioyl]carbamate;n-[2-(sulfidocarbothioylamino)ethyl]carbamodithioateChemical compound[Mn+2].[S-]C(=S)NCCNC([S-])=S.COC(=O)NC(=S)NC1=CC=CC=C1NC(=S)NC(=O)OCWPBNNNQJVZRUHP-UHFFFAOYSA-L0.000description1
- 238000005259measurementMethods0.000description1
- 229910001507metal halideInorganic materials0.000description1
- 150000005309metal halidesChemical class0.000description1
- GYNNXHKOJHMOHS-UHFFFAOYSA-Nmethyl-cycloheptaneNatural productsCC1CCCCCC1GYNNXHKOJHMOHS-UHFFFAOYSA-N0.000description1
- NFWSQSCIDYBUOU-UHFFFAOYSA-NmethylcyclopentadieneChemical compoundCC1=CC=CC1NFWSQSCIDYBUOU-UHFFFAOYSA-N0.000description1
- DNIAPMSPPWPWGF-UHFFFAOYSA-Nmonopropylene glycolNatural productsCC(O)CODNIAPMSPPWPWGF-UHFFFAOYSA-N0.000description1
- DWFKOMDBEKIATP-UHFFFAOYSA-Nn'-[2-[2-(dimethylamino)ethyl-methylamino]ethyl]-n,n,n'-trimethylethane-1,2-diamineChemical compoundCN(C)CCN(C)CCN(C)CCN(C)CDWFKOMDBEKIATP-UHFFFAOYSA-N0.000description1
- LSHROXHEILXKHM-UHFFFAOYSA-Nn'-[2-[2-[2-(2-aminoethylamino)ethylamino]ethylamino]ethyl]ethane-1,2-diamineChemical compoundNCCNCCNCCNCCNCCNLSHROXHEILXKHM-UHFFFAOYSA-N0.000description1
- QEFYFXOXNSNQGX-UHFFFAOYSA-Nneodymium atomChemical compound[Nd]QEFYFXOXNSNQGX-UHFFFAOYSA-N0.000description1
- SLCVBVWXLSEKPL-UHFFFAOYSA-Nneopentyl glycolChemical compoundOCC(C)(C)COSLCVBVWXLSEKPL-UHFFFAOYSA-N0.000description1
- 229910052758niobiumInorganic materials0.000description1
- 239000010955niobiumSubstances0.000description1
- GUCVJGMIXFAOAE-UHFFFAOYSA-Nniobium atomChemical compound[Nb]GUCVJGMIXFAOAE-UHFFFAOYSA-N0.000description1
- 150000004767nitridesChemical class0.000description1
- TVMXDCGIABBOFY-UHFFFAOYSA-NoctaneChemical compoundCCCCCCCCTVMXDCGIABBOFY-UHFFFAOYSA-N0.000description1
- GJYXGIIWJFZCLN-UHFFFAOYSA-Noctane-2,4-dioneChemical groupCCCCC(=O)CC(C)=OGJYXGIIWJFZCLN-UHFFFAOYSA-N0.000description1
- BTNXBLUGMAMSSH-UHFFFAOYSA-NoctanedinitrileChemical compoundN#CCCCCCCC#NBTNXBLUGMAMSSH-UHFFFAOYSA-N0.000description1
- 230000003287optical effectEffects0.000description1
- 150000002894organic compoundsChemical class0.000description1
- 239000001301oxygenSubstances0.000description1
- 229910052760oxygenInorganic materials0.000description1
- BPUBBGLMJRNUCC-UHFFFAOYSA-Noxygen(2-);tantalum(5+)Chemical compound[O-2].[O-2].[O-2].[O-2].[O-2].[Ta+5].[Ta+5]BPUBBGLMJRNUCC-UHFFFAOYSA-N0.000description1
- RVTZCBVAJQQJTK-UHFFFAOYSA-Noxygen(2-);zirconium(4+)Chemical compound[O-2].[O-2].[Zr+4]RVTZCBVAJQQJTK-UHFFFAOYSA-N0.000description1
- 229910052763palladiumInorganic materials0.000description1
- GTCCGKPBSJZVRZ-UHFFFAOYSA-Npentane-2,4-diolChemical compoundCC(O)CC(C)OGTCCGKPBSJZVRZ-UHFFFAOYSA-N0.000description1
- 125000001147pentyl groupChemical groupC(CCCC)*0.000description1
- XQZYPMVTSDWCCE-UHFFFAOYSA-NphthalonitrileChemical groupN#CC1=CC=CC=C1C#NXQZYPMVTSDWCCE-UHFFFAOYSA-N0.000description1
- 230000000704physical effectEffects0.000description1
- 229910052697platinumInorganic materials0.000description1
- 229920000166polytrimethylene carbonatePolymers0.000description1
- 229910052700potassiumInorganic materials0.000description1
- 239000011591potassiumSubstances0.000description1
- XRVCFZPJAHWYTB-UHFFFAOYSA-NprenderolChemical compoundCCC(CC)(CO)COXRVCFZPJAHWYTB-UHFFFAOYSA-N0.000description1
- 229950006800prenderolDrugs0.000description1
- BDERNNFJNOPAEC-UHFFFAOYSA-Npropan-1-olChemical compoundCCCOBDERNNFJNOPAEC-UHFFFAOYSA-N0.000description1
- 125000001436propyl groupChemical group[H]C([*])([H])C([H])([H])C([H])([H])[H]0.000description1
- 235000013772propylene glycolNutrition0.000description1
- 238000010926purgeMethods0.000description1
- 230000009257reactivityEffects0.000description1
- 229910052703rhodiumInorganic materials0.000description1
- 239000010948rhodiumSubstances0.000description1
- MHOVAHRLVXNVSD-UHFFFAOYSA-Nrhodium atomChemical compound[Rh]MHOVAHRLVXNVSD-UHFFFAOYSA-N0.000description1
- 229910001925ruthenium oxideInorganic materials0.000description1
- WOCIAKWEIIZHES-UHFFFAOYSA-Nruthenium(iv) oxideChemical compoundO=[Ru]=OWOCIAKWEIIZHES-UHFFFAOYSA-N0.000description1
- 229910052706scandiumInorganic materials0.000description1
- SIXSYDAISGFNSX-UHFFFAOYSA-Nscandium atomChemical compound[Sc]SIXSYDAISGFNSX-UHFFFAOYSA-N0.000description1
- HBMJWWWQQXIZIP-UHFFFAOYSA-Nsilicon carbideChemical compound[Si+]#[C-]HBMJWWWQQXIZIP-UHFFFAOYSA-N0.000description1
- 229910010271silicon carbideInorganic materials0.000description1
- 235000012239silicon dioxideNutrition0.000description1
- 239000000377silicon dioxideSubstances0.000description1
- HQVNEWCFYHHQES-UHFFFAOYSA-Nsilicon nitrideChemical compoundN12[Si]34N5[Si]62N3[Si]51N64HQVNEWCFYHHQES-UHFFFAOYSA-N0.000description1
- 229910052709silverInorganic materials0.000description1
- 239000004332silverSubstances0.000description1
- 229910052708sodiumInorganic materials0.000description1
- 239000011734sodiumSubstances0.000description1
- 238000004544sputter depositionMethods0.000description1
- 229910052712strontiumInorganic materials0.000description1
- CIOAGBVUUVVLOB-UHFFFAOYSA-Nstrontium atomChemical compound[Sr]CIOAGBVUUVVLOB-UHFFFAOYSA-N0.000description1
- 238000004381surface treatmentMethods0.000description1
- 229910052715tantalumInorganic materials0.000description1
- GUVRBAGPIYLISA-UHFFFAOYSA-Ntantalum atomChemical compound[Ta]GUVRBAGPIYLISA-UHFFFAOYSA-N0.000description1
- MZLGASXMSKOWSE-UHFFFAOYSA-Ntantalum nitrideChemical compound[Ta]#NMZLGASXMSKOWSE-UHFFFAOYSA-N0.000description1
- 229910001936tantalum oxideInorganic materials0.000description1
- FAGUFWYHJQFNRV-UHFFFAOYSA-NtetraethylenepentamineChemical compoundNCCNCCNCCNCCNFAGUFWYHJQFNRV-UHFFFAOYSA-N0.000description1
- ZUHZGEOKBKGPSW-UHFFFAOYSA-NtetraglymeChemical compoundCOCCOCCOCCOCCOCZUHZGEOKBKGPSW-UHFFFAOYSA-N0.000description1
- 229910052718tinInorganic materials0.000description1
- 150000003609titanium compoundsChemical class0.000description1
- OGIDPMRJRNCKJF-UHFFFAOYSA-Ntitanium oxideInorganic materials[Ti]=OOGIDPMRJRNCKJF-UHFFFAOYSA-N0.000description1
- WFKWXMTUELFFGS-UHFFFAOYSA-NtungstenChemical compound[W]WFKWXMTUELFFGS-UHFFFAOYSA-N0.000description1
- 229910052721tungstenInorganic materials0.000description1
- 239000010937tungstenSubstances0.000description1
- 229910052720vanadiumInorganic materials0.000description1
- GPPXJZIENCGNKB-UHFFFAOYSA-NvanadiumChemical compound[V]#[V]GPPXJZIENCGNKB-UHFFFAOYSA-N0.000description1
- XLYOFNOQVPJJNP-UHFFFAOYSA-NwaterSubstancesOXLYOFNOQVPJJNP-UHFFFAOYSA-N0.000description1
- 239000008096xyleneSubstances0.000description1
- 229910052727yttriumInorganic materials0.000description1
- VWQVUPCCIRVNHF-UHFFFAOYSA-Nyttrium atomChemical compound[Y]VWQVUPCCIRVNHF-UHFFFAOYSA-N0.000description1
- 229910052725zincInorganic materials0.000description1
- 239000011701zincSubstances0.000description1
- 229910001928zirconium oxideInorganic materials0.000description1
Images
Classifications
- C—CHEMISTRY; METALLURGY
- C23—COATING METALLIC MATERIAL; COATING MATERIAL WITH METALLIC MATERIAL; CHEMICAL SURFACE TREATMENT; DIFFUSION TREATMENT OF METALLIC MATERIAL; COATING BY VACUUM EVAPORATION, BY SPUTTERING, BY ION IMPLANTATION OR BY CHEMICAL VAPOUR DEPOSITION, IN GENERAL; INHIBITING CORROSION OF METALLIC MATERIAL OR INCRUSTATION IN GENERAL
- C23C—COATING METALLIC MATERIAL; COATING MATERIAL WITH METALLIC MATERIAL; SURFACE TREATMENT OF METALLIC MATERIAL BY DIFFUSION INTO THE SURFACE, BY CHEMICAL CONVERSION OR SUBSTITUTION; COATING BY VACUUM EVAPORATION, BY SPUTTERING, BY ION IMPLANTATION OR BY CHEMICAL VAPOUR DEPOSITION, IN GENERAL
- C23C16/00—Chemical coating by decomposition of gaseous compounds, without leaving reaction products of surface material in the coating, i.e. chemical vapour deposition [CVD] processes
- C23C16/22—Chemical coating by decomposition of gaseous compounds, without leaving reaction products of surface material in the coating, i.e. chemical vapour deposition [CVD] processes characterised by the deposition of inorganic material, other than metallic material
- C23C16/30—Deposition of compounds, mixtures or solid solutions, e.g. borides, carbides, nitrides
- C23C16/32—Carbides
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01L—SEMICONDUCTOR DEVICES NOT COVERED BY CLASS H10
- H01L21/00—Processes or apparatus adapted for the manufacture or treatment of semiconductor or solid state devices or of parts thereof
- H01L21/02—Manufacture or treatment of semiconductor devices or of parts thereof
- H01L21/04—Manufacture or treatment of semiconductor devices or of parts thereof the devices having potential barriers, e.g. a PN junction, depletion layer or carrier concentration layer
- H01L21/18—Manufacture or treatment of semiconductor devices or of parts thereof the devices having potential barriers, e.g. a PN junction, depletion layer or carrier concentration layer the devices having semiconductor bodies comprising elements of Group IV of the Periodic Table or AIIIBV compounds with or without impurities, e.g. doping materials
- H01L21/28—Manufacture of electrodes on semiconductor bodies using processes or apparatus not provided for in groups H01L21/20 - H01L21/268
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01L—SEMICONDUCTOR DEVICES NOT COVERED BY CLASS H10
- H01L21/00—Processes or apparatus adapted for the manufacture or treatment of semiconductor or solid state devices or of parts thereof
- H01L21/02—Manufacture or treatment of semiconductor devices or of parts thereof
- H01L21/04—Manufacture or treatment of semiconductor devices or of parts thereof the devices having potential barriers, e.g. a PN junction, depletion layer or carrier concentration layer
- H01L21/18—Manufacture or treatment of semiconductor devices or of parts thereof the devices having potential barriers, e.g. a PN junction, depletion layer or carrier concentration layer the devices having semiconductor bodies comprising elements of Group IV of the Periodic Table or AIIIBV compounds with or without impurities, e.g. doping materials
- H01L21/28—Manufacture of electrodes on semiconductor bodies using processes or apparatus not provided for in groups H01L21/20 - H01L21/268
- H01L21/283—Deposition of conductive or insulating materials for electrodes conducting electric current
- H01L21/285—Deposition of conductive or insulating materials for electrodes conducting electric current from a gas or vapour, e.g. condensation
Landscapes
- Chemical & Material Sciences (AREA)
- Engineering & Computer Science (AREA)
- Manufacturing & Machinery (AREA)
- General Physics & Mathematics (AREA)
- Physics & Mathematics (AREA)
- Computer Hardware Design (AREA)
- Microelectronics & Electronic Packaging (AREA)
- Power Engineering (AREA)
- Condensed Matter Physics & Semiconductors (AREA)
- Inorganic Chemistry (AREA)
- General Chemical & Material Sciences (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Materials Engineering (AREA)
- Mechanical Engineering (AREA)
- Metallurgy (AREA)
- Organic Chemistry (AREA)
- Chemical Vapour Deposition (AREA)
- Electrodes Of Semiconductors (AREA)
Abstract
Translated fromChineseDescription
Translated fromChinese本發明係關於一種含有特定之化合物而成之含金屬碳化物之薄膜形成用原料及使用該原料之含金屬碳化物之薄膜之製造方法。本發明之含金屬碳化物之薄膜形成用原料係可使用於形成含金屬碳化物之薄膜之原料。The present invention relates to a raw material for forming a metal carbide thin film containing a specific compound and a method for manufacturing a metal carbide thin film using the raw material. The raw material for forming a metal carbide-containing thin film of the present invention can be used as a raw material for forming a metal carbide-containing thin film.
含有碳化鋯、碳化鉿、碳化鈦等的金屬碳化物之薄膜可使用於切削工具、電子材料用之配線或電極,例如亦被檢討對半導體記憶體材料或鋰空氣電池用的電極等之應用。Films containing metal carbides such as zirconium carbide, hafnium carbide, titanium carbide, etc. can be used for wiring or electrodes for cutting tools and electronic materials. For example, applications to semiconductor memory materials or electrodes for lithium-air batteries have also been reviewed.
作為上述薄膜之製造法,舉例為濺鍍法、離子鍍敷法、塗佈熱分解法或溶凝膠法等之MOD法、化學氣相成長法等,但包含ALD(Atomic Layer Deposition,原子層成長)法之化學氣相成長法(以下有時僅記載為「CVD」)法,由於具有組成控制性、階差被覆性優異,適於量產化,且可混成積體等之多種優點,故為最適之製造製程。Examples of the above-mentioned thin film manufacturing methods include sputtering, ion plating, MOD methods such as coating thermal decomposition or solvogel methods, chemical vapor growth methods, etc., but include ALD (Atomic Layer Deposition, atomic layer Growth) method of chemical vapor growth method (hereinafter sometimes only described as "CVD")The method is the most suitable manufacturing process because it has many advantages such as composition controllability, excellent step coverage, suitable for mass production, and can be mixed into an integrated body.
在非專利文獻1及非專利文獻2中,揭示作為使用於將碳化鈦薄膜以MOCVD法製造時之鈦源之肆新戊基鈦(Tetraneopentyltitanium)。然而,使用肆新戊基鈦藉由MOCVD法,製造碳化鈦薄膜時,碳化鈦中之碳成分濃度成為比理論值少之狀態,不能製造品質佳的碳化鈦薄膜。為了使品質安定,於高溫成膜時,肆新戊基鈦之熱穩定性不佳,故在薄膜中混入作為有機物之殘留碳成分,具有不易形成高品質的碳化鈦薄膜之問題。Non-Patent Document 1 and Non-Patent Document 2 disclose Tetraneopentyltitanium as a titanium source used when the titanium carbide thin film is produced by the MOCVD method. However, when titanium carbide thin films are produced by MOCVD using neopentyl titanium, the concentration of carbon in the titanium carbide becomes less than the theoretical value, and high-quality titanium carbide thin films cannot be produced. In order to make the quality stable, the thermal stability of neopentyl titanium is not good when the film is formed at high temperature. Therefore, the residual carbon component as an organic substance is mixed into the film, which has the problem that it is difficult to form a high-quality titanium carbide film.
[非專利文獻1]Journal of American Chemical Society. 1987, 109, 1579-1580[Non-Patent Document 1] Journal of American Chemical Society. 1987, 109, 1579-1580
[非專利文獻2]Journal of American Ceramic Society. 2013, 96, 4, 1060-1062[Non-Patent Literature 2] Journal of American Ceramic Society. 2013, 96, 4, 1060-1062
使用化學氣相成長法之含金屬碳化物之薄膜之製造方法中所要求的事係在所使用之薄膜形成用原料中無自燃性,可安全地形成薄膜,且該薄膜形成用原料之熱分解性及/或反應性氣體之反應性佳、生產性優良。又,在所得之含金屬碳化物之薄膜中混入作為有機物之殘留碳成分為少,亦要求高品質者。以往,藉由該等之點,並無可充份地滿足之薄膜形成用原料及製造方法。What is required in the manufacturing method of the metal carbide-containing film using the chemical vapor growth method is that there is no spontaneous combustion in the film forming raw material used, the film can be formed safely, and the thermal decomposition of the film forming raw material The reactivity and productivity of the reactive gas and/or reactive gas are good. In addition, in the obtained metal carbide-containing film, the residual carbon component as an organic matter is low and high quality is required. In the past, due to these points, there were no sufficiently satisfactory raw materials and manufacturing methods for thin film formation.
本發明人等重複檢討之結果,發現含有特定之化合物而成之含金屬碳化物之薄膜形成用原料及使用該含金屬碳化物之薄膜形成用原料之含金屬碳化物之薄膜之製造方法可解決上述課題,因而完成本發明。The inventors of the present invention have repeatedly reviewed the results and found that a material for forming a metal carbide film containing a specific compound and a method for manufacturing a metal carbide film using the raw material for forming a metal carbide film can be solved The above-mentioned problems have led to completion of the present invention.
本發明係提供一種含金屬碳化物之薄膜形成用原料,其係含有以下述通式(I)表示之化合物而成:
(式中,R1~R5係表示可相同或相異之氫原子或碳原子數1~5之烷基,R6~R8係表示可相同或相異之碳原子數1~5之烷基,M係表示鈦、鋯或鉿)。(In the formula, R1 ~R5 represent the same or different hydrogen atoms or alkyl groups with 1 to 5 carbon atoms, and R6 ~R8 represent the same or different carbon atoms with 1 to 5 Alkyl group, M represents titanium, zirconium or hafnium).
本發明係提供一種含金屬碳化物之薄膜之製 造方法,其係將含有使上述之薄膜形成用原料氣化而得之以通式(I)表示之化合物的蒸氣,導入設置有基體的成膜腔室內,使該化合物分解及/或使其進行化學反應而於該基體之表面形成含金屬碳化物之薄膜之含金屬碳化物之薄膜之製造方法。The present invention provides a method for producing a metal carbide-containing thin film, which is to introduce a vapor containing a compound represented by the general formula (I) obtained by vaporizing the above-mentioned thin film forming raw material into a film formed with a substrate In the chamber, the compound is decomposed and/or chemically reacted to form a metal carbide-containing film on the surface of the substrate.
依據本發明,可提供一種薄膜形成用原料,其適合於用以形成含有金屬碳化物之薄膜所使用的化學氣相成長用之薄膜形成用原料,故再進一步安全地製造生產性優良、品質佳之含有金屬碳化物的薄膜。According to the present invention, it is possible to provide a raw material for forming a thin film, which is suitable for forming a raw material for chemical vapor growth used for forming a thin film containing metal carbides, so that it can be further safely manufactured with high productivity and high quality Films containing metal carbides.
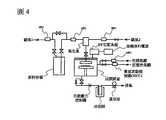
[圖1]圖1係顯示本發明之含金屬碳化物之薄膜之製造方法中可使用之化學氣相成長用裝置之一例之概要圖。[FIG. 1] FIG. 1 is a schematic diagram showing an example of an apparatus for chemical vapor growth that can be used in the method of manufacturing a metal carbide-containing thin film of the present invention.
[圖2]圖2係顯示本發明之含金屬碳化物之薄膜之製造方法中可使用之化學氣相成長用裝置之另一例之概要圖。[FIG. 2] FIG. 2 is a schematic diagram showing another example of a chemical vapor growth apparatus that can be used in the method of manufacturing a metal carbide-containing thin film of the present invention.
[圖3]圖3係顯示本發明之含金屬碳化物之薄膜之製造方法中可使用之化學氣相成長用裝置之一例之概要圖。[FIG. 3] FIG. 3 is a schematic diagram showing an example of an apparatus for chemical vapor growth that can be used in the method of manufacturing a metal carbide-containing thin film of the present invention.
[圖4]圖4係顯示本發明之含金屬碳化物之薄膜之製造方法中可使用之化學氣相成長用裝置之一例之概要圖。[FIG. 4] FIG. 4 is a schematic diagram showing an example of an apparatus for chemical vapor growth that can be used in the method of manufacturing a metal carbide-containing thin film of the present invention.
本發明中含金屬碳化物之薄膜係不特別限定,例如含有碳化鋯、碳化鉿、碳化鈦、碳氮化鋯、碳氮化鉿、碳氮化鈦等之薄膜即可,進一步可舉例此等與鉬、鋁(alumina)、氮化物、硼化物等之陶瓷等。In the present invention, the metal carbide-containing film is not particularly limited. For example, a film containing zirconium carbide, hafnium carbide, titanium carbide, zirconium carbonitride, hafnium carbonitride, titanium carbonitride, etc. may be used. Examples of these With molybdenum, aluminum (alumina), nitride, boride and other ceramics.
上述通式(I)中,R1~R5係表示可相同或相異之氫原子或碳原子數1~5之烷基,R6~R8係表示可相同或相異之碳原子數1~5之烷基,M係表示鈦、鋯或鉿。In the above general formula (I), R1 to R5 represent hydrogen atoms or alkyl groups with 1 to 5 carbon atoms that may be the same or different, and R6 to R8 represent the same or different carbon atoms 1~5 alkyl group, M represents titanium, zirconium or hafnium.
作為以上述R1~R5及以R6~R8表示之碳原子數1~5之烷基係可舉例甲基、乙基、丙基、異丙基、丁基、異丁基、第二丁基、第三丁基、戊基、異戊基等。Examples of the alkyl system having 1 to 5 carbon atoms represented by R1 to R5 and R6 to R8 include methyl, ethyl, propyl, isopropyl, butyl, isobutyl, and Dibutyl, tertiary butyl, pentyl, isopentyl, etc.
上述通式(I)中,R1~R5之至少1種為1級烷基之時或R6~R8之至少1種為1級烷基之時,以通式(I)表示之化合物的蒸氣壓為高,故可製造生產性佳之含金屬碳化物之薄膜,故為佳。其中,R1~R5之全部為1級烷基之時或、R6~R8之全部為1級烷基之時,以通式(I)表示之化合物之蒸氣壓特別高,故可製造生產性佳之含金屬碳化物之薄膜,故為佳。再者,R1~R5及R6~R8之全部為甲基之時,可適用於稱為ALD視窗之ALD法的溫度範圍寬廣,可製造高品質之含金屬碳化物的薄膜,故為佳。In the above general formula (I), when at least one of R1 to R5 is a primary alkyl group or when at least one of R6 to R8 is a primary alkyl group, it is represented by the general formula (I) The vapor pressure of the compound is high, so a metal carbide-containing film with good productivity can be produced, so it is preferable. Among them, when all of R1 to R5 are first-level alkyl groups or when all of R6 to R8 are first-level alkyl groups, the vapor pressure of the compound represented by general formula (I) is particularly high, so it can It is better to produce metal carbide-containing films with good productivity. Furthermore, when all of R1 to R5 and R6 to R8 are methyl groups, the ALD method called ALD window can be applied to a wide temperature range and can produce high-quality metal carbide-containing films. Better.
以上述通式(I)表示之化合物係依據其製造方法並不特別限制,應用周知之反應而製造。作為製造方法係例如M為鈦之時,在單環戊二烯基三氯鈦或單烷基戊二烯基三氯鈦中,於-20℃~50℃下,較佳於0℃~30℃下, 藉由使其反應得到烷基鋰。M為鋯或鉿之時亦可用相同之方法製造。The compound represented by the above general formula (I) is not particularly limited depending on the production method thereof, and is produced by applying a well-known reaction. As a manufacturing method, for example, when M is titanium, in monocyclopentadienyl titanium trichloride or monoalkyl pentadienyl titanium trichloride, the temperature is -20℃~50℃, preferably 0℃~30 At °C, alkyl lithium is obtained by making it react. When M is zirconium or hafnium, it can also be manufactured by the same method.
作為以通式(I)表示之化合物之較佳具體例以例如舉例為以下述化合物No.1~No.18表示之化合物。尚,下述化合物No.1~No.18中,「Me」表示甲基,「Et」表示乙基。As a preferable specific example of the compound represented by general formula (I), for example, the compound represented by the following compound No.1~No.18 is illustrated. In addition, in the following compounds No. 1 to No. 18, "Me" represents a methyl group, and "Et" represents an ethyl group.
所謂本發明之含金屬碳化物之薄膜形成用原料係將以通式(I)表示之化合物,作為用以形成含金屬碳化物之薄膜的化學氣相成長法用前驅物者,且該形態可依據該薄膜形成用原料所適用之製造製程而相異。例如,製造碳化鈦之薄膜時,本發明之含金屬碳化物之薄膜形成用原料不含上述通式(I)中之M為鈦之化合物以外之金屬化合物及半金屬化合物。另一方面,製造含有鈦以外之金屬及/或半金屬與碳化鈦之薄膜時,除了上述通式(I)中之M為鈦之化合物以外,本發明之含金屬碳化物之薄膜形成用原料含有包含鈦以外之金屬的化合物及/或包含半金屬之化合物(以下亦稱為「其他前驅物」)。本發明之含金屬碳化物之薄膜形成用原料如後述,亦可進一步含有有機溶劑及/或親核性試藥。本發明之含金屬碳化物之薄膜形成用原料如上述說明,由於適用於以前驅物之通式(I)表示之化合物的物性為CVD法、ALD法,故特別作為化學氣相成長用原料(以下亦稱為「CVD用原料」)有用。The raw material for forming a metal carbide-containing thin film of the present invention is a compound represented by the general formula (I) as a precursor for the chemical vapor growth method for forming a metal carbide-containing thin film, and the form can be It varies according to the applicable manufacturing process of the film forming raw material. For example, when manufacturing a thin film of titanium carbide, the raw material for forming a thin film containing metal carbide of the present invention does not contain metal compounds and semimetal compounds other than the compound in which M is titanium in the general formula (I). On the other hand, when manufacturing a thin film containing a metal and/or semimetal other than titanium and titanium carbide, in addition to the compound in which M in the above general formula (I) is a titanium compound, the raw material for forming a metal carbide-containing thin film of the present invention Contains compounds containing metals other than titanium and/or compounds containing semimetals (hereinafter also referred to as "other precursors"). The raw material for forming a metal carbide-containing thin film of the present invention may further contain an organic solvent and/or a nucleophilic reagent as described later. The raw material for forming a metal carbide-containing thin film of the present invention is as described above. Since the physical properties of the compound represented by the general formula (I) suitable for the precursor are the CVD method and the ALD method, it is particularly used as a raw material for chemical vapor growth ( Hereinafter, it is also referred to as "CVD raw material") is useful.
本發明之含金屬碳化物薄膜形成用原料之形態可依據所使用的CVD法之輸送供給方法等方法而適當選擇。The form of the raw material for forming the metal-containing carbide thin film of the present invention can be appropriately selected depending on the method of transportation and supply of the CVD method used.
作為上述之輸送供給方法,有將CVD用原料於儲存該原料之容器(以下有時亦簡單記載為「原料容器」)中加熱及/或減壓而氣化為蒸氣,將該蒸氣與根據需要使用之氬、氮、氦等之載氣一起導入設置基體之成膜腔室內(以下有時亦記載為「堆積反應部」)之氣體輸送法, 將CVD用原料以液體或溶液狀態輸送至氣化室,於氣化室藉由加熱及/或減壓而氣化為蒸氣,將該蒸氣導入成膜腔室內之液體輸送法。氣體輸送法時,以上述通式(I)表示之化合物本身可使用作為CVD用原料。液體輸送法之情況,可將以上述通式(I)表示之化合物本身或該化合物溶於有機溶劑中之溶液作為CVD用原料。該等之CVD用原料亦可進一部包含其他前驅物或親核性試藥。As the above-mentioned transportation and supply method, there is heating and/or decompression of the CVD raw material in a container storing the raw material (hereinafter sometimes simply referred to as "raw material container") to vaporize it into steam, and the steam is combined with it as needed. Carrier gases such as argon, nitrogen, helium, etc. used are introduced together into the film forming chamber where the substrate is installed (hereinafter sometimes referred to as the "deposition reaction part"). The CVD raw material is transported to the gas in a liquid or solution state. In the vaporization chamber, it is vaporized into vapor by heating and/or decompression in the vaporization chamber, and the vapor is introduced into the liquid transportation method in the film forming chamber. In the gas delivery method, the compound represented by the general formula (I) itself can be used as a raw material for CVD. In the case of the liquid delivery method, the compound represented by the above general formula (I) or a solution in which the compound is dissolved in an organic solvent can be used as a raw material for CVD. The raw materials for CVD can also further contain other precursors or nucleophilic reagents.
又,於多成分系之CVD法中,有使CVD用原料各成分獨立氣化並供給之方法(以下有時亦記載為「單一源法」)與使多成分原料預先以期望組成混合之混合原料氣化並供給之方法(以下有時亦記載為「混合源法」)。混合源法之情況,可將以通式(I)表示之化合物與其他前驅物之混合物或者將該混合物溶於有機溶劑之混合溶液作為CVD用原料。該混合物或混合溶液亦可進一步包含親核性試藥等。In addition, in the multi-component system CVD method, there are a method of separately vaporizing and supplying each component of the CVD raw material (hereinafter sometimes referred to as the "single source method") and mixing of the multi-component raw material in a desired composition in advance A method of gasifying and supplying raw materials (hereinafter sometimes referred to as "mixed source method"). In the case of the mixed source method, a mixture of a compound represented by the general formula (I) and other precursors or a mixed solution in which the mixture is dissolved in an organic solvent can be used as a raw material for CVD. The mixture or mixed solution may further include a nucleophilic reagent and the like.
作為上述有機溶劑並未特別限制,可使用周知之一般有機溶劑。作為有機溶劑可舉例為例如甲醇、乙醇、異丙醇、正丁醇等之醇類;乙酸乙酯、乙酸丁酯、乙酸甲氧基乙酯等之乙酸酯類;四氫呋喃、四氫吡喃、乙二醇二甲醚、二乙二醇二甲醚、三乙二醇二甲醚、二丁醚、二噁烷等之醚類;甲基丁基酮、甲基異丁基酮、乙基丁基酮、二丙基酮、二異丁基酮、甲基戊基酮、環己酮、甲基環己酮等之酮類;己烷、環己烷、甲基環己烷、二甲基環己烷、乙基環己烷、庚烷、辛烷、甲苯、二甲苯等之烴 類;1-氰基丙烷、1-氰基丁烷、1-氰基己烷、氰基環己烷、氰基苯、1,3-二氰基丙烷、1,4-二氰基丁烷、1,6-二氰基己烷、1,4-二氰基環己烷、1,4-二氰基苯等之具有氰基之烴類;吡啶、二甲基吡啶(lutidine)等。該等之有機溶劑可根據溶質之溶解性、使用溫度與沸點、起火點之關係等而單獨或混合兩種類以上之使用。使用該等之有機溶劑時,將前驅物溶於有機溶劑之溶液的CVD用原料中之前驅物全體量0.01~2.0莫耳/升為佳,特佳為0.05~1.0莫耳/升。所謂前驅物全體量,於本發明之含金屬碳化物之薄膜形成用原料不含以上述通式(I)表示之化合物以外之金屬化合物及半金屬化合物時,為以上述通式(I)表示之化合物的量,於本發明之含金屬碳化物之薄膜形成用原料含有除了包含以上述通式(I)表示之化合物以外之金屬的化合物及或包含半金屬的化合物(其他前驅物)時,為以上述通式(I)表示之化合物及其他前驅物之合計量。The organic solvent is not particularly limited, and well-known general organic solvents can be used. Examples of organic solvents include alcohols such as methanol, ethanol, isopropanol, and n-butanol; acetates such as ethyl acetate, butyl acetate, and methoxyethyl acetate; tetrahydrofuran, tetrahydropyran, Ethylene glycol dimethyl ether, diethylene glycol dimethyl ether, triethylene glycol dimethyl ether, dibutyl ether, dioxane and other ethers; methyl butyl ketone, methyl isobutyl ketone, ethyl Butyl ketone, dipropyl ketone, diisobutyl ketone, methyl amyl ketone, cyclohexanone, methyl cyclohexanone and other ketones; hexane, cyclohexane, methyl cyclohexane, dimethyl Hydrocarbons such as cyclohexane, ethylcyclohexane, heptane, octane, toluene, xylene, etc.; 1-cyanopropane, 1-cyanobutane, 1-cyanohexane, cyanocyclohexane Alkyl, cyanobenzene, 1,3-dicyanopropane, 1,4-dicyanobutane, 1,6-dicyanohexane, 1,4-dicyanocyclohexane, 1,4- Hydrocarbons with cyano groups such as dicyanobenzene; pyridine, lutidine, etc. These organic solvents can be used alone or in a mixture of two or more types according to the solubility of the solute, the relationship between the use temperature and the boiling point, and the ignition point. When these organic solvents are used, the total amount of the precursor in the CVD raw material of the organic solvent solution is preferably 0.01 to 2.0 mol/liter, particularly preferably 0.05 to 1.0 mol/liter. The total amount of the precursor is expressed by the above general formula (I) when the raw material for forming a metal carbide-containing thin film of the present invention does not contain metal compounds and semimetal compounds other than the compound represented by the above general formula (I) The amount of the compound, when the raw material for forming a metal carbide-containing thin film of the present invention contains a compound containing a metal other than the compound represented by the above general formula (I) and or a compound containing a semimetal (other precursors), It is the total amount of the compound represented by the above general formula (I) and other precursors.
且,於多成分系之CVD法時,作為與以上述通式(I)表示之化合物一起使用之其他前驅物並未特別限制,可使用於CVD原料中所用之周知一般前驅物。該前驅物所使用之配位基係於構造中不含氧原子,但可減少所得之含金屬碳化物之薄膜中之氧的混入量,故特別佳。In addition, in the case of a multi-component CVD method, other precursors used with the compound represented by the above general formula (I) are not particularly limited, and well-known general precursors used in CVD raw materials can be used. The ligand used in the precursor does not contain oxygen atoms in the structure, but can reduce the amount of oxygen mixed in the obtained metal carbide-containing film, which is particularly preferred.
作為上述之其他的前驅物係可舉例選擇自醇化合物、乙二醇化合物、β-二酮化合物、環戊二烯化合物、作為有機胺化合物等之有機配位基可使用之化合物所成之群中一種類或二種類以上與矽或金屬(但是,除了 鈦、鋯及鉿之外)之化合物。又,作為其他前驅物之金屬種,可舉例為鎂、鈣、鍶、鋇、釩、鈮、鉭、鋁、錳、鐵、釕、鈷、銠、銥、鎳、鈀、鉑、銅、銀、金、鋅、鎵、銦、鍺、錫、鉛、銻、鉍、鈧、釔、鑭、鈰、鐠、釹、鉅、釤、銪、釓、鋱、鏑、鈥、鉺、銩、鐿、鎦。As the above-mentioned other precursor systems, examples can be selected from a group of compounds that can be used as organic ligands such as alcohol compounds, ethylene glycol compounds, β-diketone compounds, cyclopentadiene compounds, and organic amine compounds. One kind or two or more kinds of compounds with silicon or metals (but, except for titanium, zirconium and hafnium). Also, as the metal species of other precursors, magnesium, calcium, strontium, barium, vanadium, niobium, tantalum, aluminum, manganese, iron, ruthenium, cobalt, rhodium, iridium, nickel, palladium, platinum, copper, silver can be exemplified , Gold, zinc, gallium, indium, germanium, tin, lead, antimony, bismuth, scandium, yttrium, lanthanum, cerium, samarium, neodymium, giant, samarium, europium, dimmer, porcium, dysprosium, 鈥, erbium, thu, ytte , 镏.
作為上述之有機配位基可使用之醇化合物係可舉例甲醇、乙醇、丙醇、異丙醇、丁醇、第2丁醇、異丁醇、第3丁醇、戊醇、異戊醇、第3戊醇等之烷醇類;2-甲氧基乙醇、2-乙氧基乙醇、2-丁氧基乙醇、2-(2-甲氧基乙氧基)乙醇、2-甲氧基-1-甲基乙醇、2-甲氧基-1,1-二甲基乙醇、2-乙氧基-1,1-二甲基乙醇、2-異丙氧基-1,1-二甲基乙醇、2-丁氧基-1,1-二甲基乙醇、2-(2-甲氧基乙氧基)-1,1-二甲基乙醇、2-丙氧基-1,1-二乙基乙醇、2-仲丁氧基-1,1-二乙基乙醇、3-甲氧基-1,1-二甲基丙醇等之醚醇類等。Alcohol compounds that can be used as the above-mentioned organic ligands include methanol, ethanol, propanol, isopropanol, butanol, 2nd butanol, isobutanol, 3rd butanol, pentanol, isoamyl alcohol, Alkanols such as 3-pentanol; 2-methoxyethanol, 2-ethoxyethanol, 2-butoxyethanol, 2-(2-methoxyethoxy)ethanol, 2-methoxyethanol -1-methylethanol, 2-methoxy-1,1-dimethylethanol, 2-ethoxy-1,1-dimethylethanol, 2-isopropoxy-1,1-dimethylethanol Ethyl alcohol, 2-butoxy-1,1-dimethylethanol, 2-(2-methoxyethoxy)-1,1-dimethylethanol, 2-propoxy-1,1- Diethyl ethanol, 2-sec-butoxy-1,1-diethylethanol, 3-methoxy-1,1-dimethylpropanol and other ether alcohols.
作為上述之其他前驅物之有機配位基可使用之有機配位基二醇化合物係可舉例1,2-乙二醇、1,2-丙二醇、1,3-丙二醇、2,4-己二醇、2,2-二甲基-1,3-丙二醇、2,2-二乙基-1,3-丙二醇、1,3-丁二醇、2,4-丁二醇、2,2-二乙基-1,3-丁二醇、2-乙基-2-丁基-1,3-丙二醇、2,4-戊二醇、2-甲基-1,3-丙二醇、2-甲基-2,4-戊二醇、2,4-己二醇、2,4-二甲基-2,4-戊二醇等。Examples of organic ligand diol compounds that can be used as organic ligands of the other precursors mentioned above include 1,2-ethylene glycol, 1,2-propanediol, 1,3-propanediol, and 2,4-hexanedi Alcohol, 2,2-dimethyl-1,3-propanediol, 2,2-diethyl-1,3-propanediol, 1,3-butanediol, 2,4-butanediol, 2,2- Diethyl-1,3-butanediol, 2-ethyl-2-butyl-1,3-propanediol, 2,4-pentanediol, 2-methyl-1,3-propanediol, 2-methyl 2,4-pentanediol, 2,4-hexanediol, 2,4-dimethyl-2,4-pentanediol, etc.
又,作為β-二酮化合物係可舉例乙醯丙酮、己烷-2,4-二酮、5-甲基己烷-2,4-二酮、庚烷-2,4-二酮、2- 甲基庚烷-3,5-二酮、5-甲基庚烷-2,4-二酮、6-甲基庚烷-2,4-二酮、2,2-二甲基庚烷-3,5-二酮、2,6-二甲基庚烷-3,5-二酮、2,2,6-三甲基庚烷-3,5-二酮、2,2,6,6-四甲基庚烷-3,5-二酮、辛烷-2,4-二酮、2,2,6-三甲基辛烷-3,5-二酮、2,6-二甲基辛烷-3,5-二酮、2,9-二甲基壬烷-4,6-二酮、2-甲基-6-乙基癸烷-3,5-二酮、2,2-二甲基-6-乙基癸烷-3,5-二酮等之烷基取代β-二酮類;1,1,1-三氟戊烷-2,4-二酮、1,1,1-三氟-5,5-二甲基己烷-2,4-二酮、1,1,1,5,5,5-六氟戊烷-2,4-二酮、1,3-二全氟己基丙烷-1,3-二酮等之氟取代烷基β-二酮類;1,1,5,5-四甲基-1-甲氧基己烷-2,4-二酮、2,2,6,6-四甲基-1-甲氧基庚烷-3,5-二酮、2,2,6,6-四甲基-1-(2-甲氧基乙氧基)庚烷-3,5-二酮等之醚取代β-二酮類等。In addition, as the β-diketone compound system, acetone, hexane-2,4-dione, 5-methylhexane-2,4-dione, heptane-2,4-dione, 2 -Methylheptane-3,5-dione, 5-methylheptane-2,4-dione, 6-methylheptane-2,4-dione, 2,2-dimethylheptane -3,5-dione, 2,6-dimethylheptane-3,5-dione, 2,2,6-trimethylheptane-3,5-dione, 2,2,6, 6-Tetramethylheptane-3,5-dione, octane-2,4-dione, 2,2,6-trimethyloctane-3,5-dione, 2,6-dimethyl 2-methyloctane-3,5-dione, 2,9-dimethylnonane-4,6-dione, 2-methyl-6-ethyldecane-3,5-dione, 2,2 -Dimethyl-6-ethyldecane-3,5-dione and other alkyl substituted β-diketones; 1,1,1-trifluoropentane-2,4-dione, 1,1 ,1-Trifluoro-5,5-dimethylhexane-2,4-dione, 1,1,1,5,5,5-hexafluoropentane-2,4-dione, 1,3 -Diperfluorohexylpropane-1,3-dione and other fluoro-substituted alkyl β-diketones; 1,1,5,5-tetramethyl-1-methoxyhexane-2,4-di Ketone, 2,2,6,6-tetramethyl-1-methoxyheptane-3,5-dione, 2,2,6,6-tetramethyl-1-(2-methoxyethyl (Oxy)heptane-3,5-dione and other ether substituted β-diketones, etc.
又,作為環戊二烯化合物係可舉例環戊二烯、甲基環戊二烯、乙基環戊二烯、丙基環戊二烯、異丙基環戊二烯、丁基環戊二烯、第2丁基環戊二烯、異丁基環戊二烯、第3丁基環戊二烯、二甲基環戊二烯、四甲基環戊二烯等;作為上述之有機配位基可使用之有機胺化合物係可舉例甲基胺、乙基胺、丙基胺、異丙基胺、丁基胺、第2丁基胺、第3丁基胺、異丁基胺、二甲基胺、二乙基胺、二丙基胺、二異丙基胺、乙基甲基胺、丙基甲基胺、異丙基甲基胺等。In addition, examples of the cyclopentadiene compound system include cyclopentadiene, methylcyclopentadiene, ethylcyclopentadiene, propylcyclopentadiene, isopropylcyclopentadiene, and butylcyclopentadiene. Ene, 2nd butylcyclopentadiene, isobutylcyclopentadiene, 3rd butylcyclopentadiene, dimethylcyclopentadiene, tetramethylcyclopentadiene, etc.; as the above organic compound The organic amine compounds that can be used in the position group can be exemplified by methylamine, ethylamine, propylamine, isopropylamine, butylamine, 2nd butylamine, 3rd butylamine, isobutylamine, di Methylamine, diethylamine, dipropylamine, diisopropylamine, ethylmethylamine, propylmethylamine, isopropylmethylamine, etc.
作為上述之有機配位基可使用之有機胺化合物係可舉例酮亞胺化合物、脒基化合物(amidinate compound)、甲基胺、乙基胺、丙基胺、異丙基胺、丁基 胺、第2丁基胺、第3丁基胺、異丁基胺、二甲基胺、二乙基胺、二丙基胺、二異丙基胺、乙基甲基胺、丙基甲基胺、異丙基甲基胺、雙(三甲基矽烷基)胺等。Examples of organic amine compounds that can be used as the above-mentioned organic ligands include ketimine compounds, amidinate compounds, methylamine, ethylamine, propylamine, isopropylamine, butylamine, 2nd butylamine, 3rd butylamine, isobutylamine, dimethylamine, diethylamine, dipropylamine, diisopropylamine, ethylmethylamine, propylmethylamine, Isopropylmethylamine, bis(trimethylsilyl)amine, etc.
其他前驅物為本技術領域中習知者,其製造方法亦為習知。若舉製造方法之一例,在例如使用醇化合物作為有機配位基時,藉由使如前述之金屬無機鹽或其水合物與該醇化合物之鹼金屬烷氧化物反應,可製造前驅物。於此,金屬之無機鹽或其水合物可舉例為金屬之鹵化物、硝酸鹽等,作為鹼金屬烷氧化物可舉例為烷氧化鈉、烷氧化鋰、烷氧化鉀等。Other precursors are known in the technical field, and their manufacturing methods are also known. To cite an example of the production method, when an alcohol compound is used as an organic ligand, a precursor can be produced by reacting the aforementioned metal inorganic salt or its hydrate with the alkali metal alkoxide of the alcohol compound. Here, the inorganic salt of metal or its hydrate can be exemplified by metal halide, nitrate, etc., as the alkali metal alkoxide, sodium alkoxide, lithium alkoxide, potassium alkoxide, etc. can be exemplified.
其他前驅物,於單一源法時,以通式(I)表示之化合物與熱及/或氧化分解行為類似之化合物為佳,於混合源法時,除了熱及/或氧化分解行為類似以外,混合時不引起化學反應等變質者為較佳。For other precursors, in the single source method, the compound represented by general formula (I) is preferably a compound with similar thermal and/or oxidative decomposition behavior. In the mixed source method, except for the similar thermal and/or oxidative decomposition behavior, It is preferable that it does not cause deterioration such as chemical reaction during mixing.
又,本發明之含金屬碳化物之薄膜形成用材料,根據需要,為了賦予該原料之安定性,亦可含有親核性試藥。作為該親核性試藥舉例為甘醇二甲醚、二甘醇二甲醚、三甘醇二甲醚、四甘醇二甲醚等之乙二醇醚類,18-冠狀醚-6、二環己基-18-冠狀醚-6、24-冠狀醚-8、二環己基-24-冠狀醚-8、二苯并-24-冠狀醚-8等之冠狀醚類,乙二胺、N,N’-四甲基乙二胺、二伸乙三胺、三伸乙四胺、四伸乙五胺、五伸乙六胺、1,1,4,7,7-五甲基二伸乙三胺、1,1,4,7,10,10-六甲基三伸乙四胺、三乙氧基三伸乙胺等之聚胺類,四氮雜環十四烷(cyclam)、四氮雜環十二烷 (cyclen)等之環狀聚胺類,吡啶、吡咯啶、哌啶、嗎啉、N-甲基吡咯啶、N-甲基哌啶、N-甲基嗎啉、四氫呋喃、四氫吡喃、1,4-二噁烷、噁唑、噻唑、氧雜硫雜環戊烷(oxathiolane)等之雜環化合物類,乙醯乙酸甲酯、乙醯乙酸乙酯、乙醯乙酸-2-甲氧基乙酯等之β-酮酯類或乙醯丙酮、2,4-己二酮、2,4-庚二酮、3,5-庚二酮、二特戊醯基甲烷等之β-二酮類。該等親核性試藥之使用量,對於上述通式(I)表示之化合物1莫耳,0.1莫耳~10莫耳之範圍為佳,1~4莫耳為更佳。又,使用該等之親核性試藥時,於該親核性試藥之構造中不包含氧原子為佳,進一步於構造中包含氮原子為特別佳。In addition, the metal carbide-containing thin film forming material of the present invention may contain a nucleophilic reagent as needed in order to impart stability to the raw material. Examples of the nucleophilic reagent include glycol ethers such as glyme, diglyme, triglyme, and tetraglyme, 18-crown-6, Dicyclohexyl-18-Crown Ether-6, 24-Crown Ether-8, Dicyclohexyl-24-Crown Ether-8, Dibenzo-24-Crown Ether-8 and other crown ethers, ethylenediamine, N ,N'-Tetramethylethylenediamine, Diethylenetriamine, Triethylenetetramine, Tetraethylenepentamine, Pentaethylenehexamine, 1,1,4,7,7-Pentamethylethylenediamine Polyamines such as ethylenetriamine, 1,1,4,7,10,10-hexamethyltriethylenetetramine, triethoxytriethylenetetramine, tetraazacyclotetradecane (cyclam), Cyclic polyamines such as tetraazacyclododecane (cyclen), pyridine, pyrrolidine, piperidine, morpholine, N-methylpyrrolidine, N-methylpiperidine, N-methylmorpholine, Tetrahydrofuran, tetrahydropyran, 1,4-dioxane, oxazole, thiazole, oxathiolane (oxathiolane) and other heterocyclic compounds, methyl acetylacetate, ethyl acetylacetate, ethyl acetate Β-ketoesters such as 2-methoxyethyl acetate or acetone, 2,4-hexanedione, 2,4-heptanedione, 3,5-heptanedione, ditepentanone Β-diketones such as methyl methane. The usage amount of these nucleophilic reagents is preferably in the range of 0.1 mol to 10 mol, and more preferably 1 to 4 mol, for 1 mol of the compound represented by the general formula (I). In addition, when these nucleophilic reagents are used, it is preferable that the structure of the nucleophilic reagent does not contain oxygen atoms, and it is particularly preferable to include nitrogen atoms in the structure.
在本發明之含金屬碳化物之薄膜形成用原料中極力不包含構成其之成分以外之雜質金屬元素分、雜質氯等之雜質鹵素分及雜質有機分。雜質金屬元素分之每原素100ppb以下為佳,10ppb以下為更佳,總量計係1ppm以下為佳,100ppb以下為更佳。尤其,使用作為LSI之閘極絕緣膜、閘極膜、障壁層時,必須減少對所得薄膜之電特性有影響之鹼金屬元素、鹼土類金屬元素及同族元素之含量。雜質鹵素分100ppm以下為佳,10ppm以下為更佳,1ppm以下為最佳。雜質有機分總量計係500ppm以下為佳,50ppm以下為更佳,10ppm以下為最佳。又,由於水分係化學氣相成長用原料中之顆粒發生或薄膜形成中之顆粒發生之原因,故前驅物、有機溶劑及親核性試藥中,為了減低各自之水分,使用時較佳預先儘可能去除水分。前 驅物、有機溶劑及親核性試藥各水分量10ppm以下為佳,1ppm以下為更佳。The raw material for forming a metal carbide-containing thin film of the present invention does not contain impurity metal elements, impurity halogens such as impurity chlorine, and impurity organic elements other than the constituent components. The impurity metal element is preferably 100 ppb or less per element, more preferably 10 ppb or less, preferably 1 ppm or less in total, and more preferably 100 ppb or less. In particular, when using gate insulating films, gate films, and barrier layers as LSIs, it is necessary to reduce the content of alkali metal elements, alkaline earth metal elements, and elements of the same group that affect the electrical properties of the resulting film. The impurity halogen content is preferably 100 ppm or less, more preferably 10 ppm or less, and most preferably 1 ppm or less. The total impurity organic content is preferably 500ppm or less, 50ppm or less is more preferable, and 10ppm or less is the best. In addition, since moisture is the cause of particle generation in the raw material for chemical vapor growth or particle generation in film formation, in the precursor, organic solvent, and nucleophilic reagent, in order to reduce the respective moisture, it is better to use it beforehand. Remove as much water as possible. The moisture content of each precursor, organic solvent and nucleophilic reagent is preferably 10 ppm or less, and more preferably 1 ppm or less.
又,本發明之含金屬碳化物之薄膜形成用原料,為了減低或防止所形成之薄膜的顆粒污染,較好極力不包含顆粒。具體而言,於液相之藉由光散射式液中粒子檢測器之顆粒測定中,大於0.3μm之粒子數於液相1ml中100個以下為佳,大於0.2μm之粒子數於液相1ml中1000個以下為更佳,大於0.2μm之粒子數於液相1ml中100個以下為最佳。In addition, in order to reduce or prevent particle contamination of the formed film, the raw material for forming a metal carbide-containing film of the present invention preferably does not contain particles as much as possible. Specifically, in liquid phase particle measurement by light scattering type liquid particle detector, the number of particles larger than 0.3μm should be less than 100 in 1ml of liquid phase, and the number of particles larger than 0.2μm should be less than 100 in 1ml of liquid phase. 1000 or less is more preferable, and the number of particles larger than 0.2μm is 100 or less in 1 ml of liquid phase.
作為本發明之含金屬碳化物之薄膜之製造方法,係將以上述通式(I)表示之化合物氣化之蒸氣及根據需要使用之反應性氣體導入設置有基體之成膜腔室內,接者,使前驅物於基體上及/或成膜腔室內及/或氣體導入口附近分解及/或化學反應,於基體表面成長並堆積含金屬碳化物之薄膜的CVD法者。原料之輸送供給方法、堆積方法、製造條件、製造裝置等並未特別受到限制,可使用周知之一般條件及方法。As the method of manufacturing the metal carbide-containing thin film of the present invention, the vapor of the compound represented by the above general formula (I) and the reactive gas used as needed are introduced into the film forming chamber provided with the substrate, and then CVD method in which precursors are decomposed and/or chemically reacted on the substrate and/or in the film forming chamber and/or near the gas inlet to grow and deposit metal carbide-containing films on the surface of the substrate. The raw material transportation and supply method, stacking method, manufacturing conditions, manufacturing equipment, etc. are not particularly limited, and well-known general conditions and methods can be used.
作為上述根據需要使用之反應性氣體,例如舉例於單烷胺、二烷胺、三烷胺、伸烷二胺等之有機胺化合物、聯胺、氨、氮等之構造中具有氮原子之化合物之氣體或氫氣,該等可使用一種類或二種類以上。又,使上述反應性氣體與前驅物反應之前,亦可預先進行電漿表面處理。As the above-mentioned reactive gas used as required, for example, organic amine compounds such as monoalkylamine, dialkylamine, trialkylamine, and alkylene diamine, and compounds having nitrogen atoms in the structure of hydrazine, ammonia, nitrogen, etc. For the gas or hydrogen, one type or two or more types can be used. In addition, before reacting the reactive gas with the precursor, plasma surface treatment may be performed in advance.
且,作為上述輸送供給方法,舉例為前述之 氣體輸送法、液體輸送法、單一源法、混合源法等。Also, as the above-mentioned transportation and supply method, the aforementioned gas transportation method, liquid transportation method, single source method, mixed source method, etc. are exemplified.
又,作為上述之堆積方法,舉例為使原料氣體或使原料氣體與反應性氣體僅藉由熱而反應並堆積薄膜之熱CVD,使用熱與電漿之電漿CVD,使用熱與光之光CVD,使用熱、光及電漿之光電漿CVD,將CVD之堆積反應分為基本過程,以分子等級進行階段性堆積之ALD。In addition, as the above-mentioned deposition method, for example, there are thermal CVD in which a raw material gas or raw gas and a reactive gas react with only heat to deposit a thin film, plasma CVD using heat and plasma, and light using heat and light. CVD, photo-plasma CVD using heat, light, and plasma, divides the accumulation reaction of CVD into a basic process, and performs ALD for staged accumulation at the molecular level.
上述基體之材質舉例為例如矽;砷化銦、砷化銦鎵、二氧化矽、氮化矽、碳化矽、氮化鈦、氧化鉭、氮化鉭、氧化鈦、氮化鈦、氧化釕、氧化鋯、氧化鉿、氧化鑭、氮化鎵等之陶瓷;玻璃;鉑-釕、鋁、銅、鎳、鈷、鎢、鉬等之金屬。基體之形狀舉例為板狀、球狀、纖維狀、鱗片狀,基體表面可為平面,亦可成為溝槽構造等之三次元構造。Examples of the material of the substrate are silicon; indium arsenide, indium gallium arsenide, silicon dioxide, silicon nitride, silicon carbide, titanium nitride, tantalum oxide, tantalum nitride, titanium oxide, titanium nitride, ruthenium oxide, Ceramics such as zirconium oxide, hafnium oxide, lanthanum oxide, and gallium nitride; glass; platinum-metals such as ruthenium, aluminum, copper, nickel, cobalt, tungsten, molybdenum, etc. Examples of the shape of the substrate are plate, spherical, fibrous, and scaly. The surface of the substrate can be flat or a three-dimensional structure such as a grooved structure.
且,作為上述製造條件,舉例為反應溫度(基體溫度)、反應壓力、堆積速度等。關於反應溫度,使之以上述通式(I)表示之化合物充分地反應之溫度的100℃以上為佳,150℃~400℃為更佳。又,反應壓力於熱CVD、光CVD時,大氣壓~10Pa為佳,使用電漿時,2000Pa~10Pa為佳。又,堆積速度可藉由原料之供給條件(氣化溫度、氣化壓力)、反應溫度、反應壓力而控制。堆積速度若太大則有所得薄膜特性惡化之情況,太小則有生產性產生問題之情況,因此0.01~100nm/分鐘為佳,1~50nm/分鐘為更佳。且,ALD法時,以循環次數進行控制以獲得所期望膜厚。In addition, as the above-mentioned production conditions, the reaction temperature (substrate temperature), the reaction pressure, the deposition rate, etc. are exemplified. Regarding the reaction temperature, the temperature at which the compound represented by the above general formula (I) is fully reacted is preferably 100°C or higher, and more preferably 150°C to 400°C. In addition, when the reaction pressure is thermal CVD or optical CVD, atmospheric pressure~10Pa is better, and when plasma is used, 2000Pa~10Pa is better. In addition, the deposition rate can be controlled by the supply conditions of the raw materials (vaporization temperature, vaporization pressure), reaction temperature, and reaction pressure. If the deposition rate is too high, the properties of the obtained film may deteriorate, and if it is too small, there may be problems with productivity. Therefore, 0.01-100nm/min is better, and 1-50nm/min is more preferable. In the ALD method, the number of cycles is controlled to obtain the desired film thickness.
作為上述製造條件進一步舉例為使薄膜形成用原料氣化成為蒸氣時之溫度或壓力。使薄膜形成用原料氣化成為蒸氣之步驟可在原料容器內進行,亦可在氣化室內進行。任一情況下,本發明薄膜之製造方法中使用之薄膜形成用原料在0~150℃蒸發為佳。又,於原料容器內或氣化室內使薄膜形成用原料氣化成為蒸氣時,原料容器內或氣化室內之壓力均佳為1~10000Pa。The above-mentioned production conditions are further exemplified by the temperature or pressure when the raw material for forming a thin film is vaporized into steam. The step of vaporizing the raw material for film formation into vapor may be performed in the raw material container or in the vaporization chamber. In either case, it is preferable that the raw material for film formation used in the method of manufacturing the film of the present invention evaporate at 0 to 150°C. In addition, when the film-forming raw material is vaporized into vapor in the raw material container or the vaporization chamber, the pressure in the raw material container or the vaporization chamber is preferably 1 to 10,000 Pa.
本發明之薄膜之製造方法除了採用ALD法,藉由上述輸送供給方法使薄膜形成用原料氣化成為蒸氣,將該蒸氣導入成膜腔室內之原料導入步驟以外,亦可具有藉由該蒸氣中之以上述通式(I)表示之化合物於上述基體表面形成前驅物薄膜之前驅物薄膜成膜步驟,將未反應之以上述通式(I)表示之化合物排氣之排氣步驟,及使該前驅物薄膜與反應性氣體化學反應,於該基體表面形成含金屬碳化物之含金屬碳化物之薄膜形成步驟。The thin film manufacturing method of the present invention uses the ALD method to vaporize the raw material for thin film formation into vapor by the above-mentioned transport and supply method, and introduce the vapor into the film forming chamber. The compound represented by the above general formula (I) forms a precursor film on the surface of the substrate, the precursor film forming step, the exhaust step of exhausting the unreacted compound represented by the above general formula (I), and The precursor film chemically reacts with the reactive gas to form a metal carbide-containing metal carbide film forming step on the surface of the substrate.
以下,針對上述各步驟,以形成將含金屬碳化物的薄膜藉由ALD法之情況為例詳細說明。藉由ALD法形成含金屬碳化物之薄膜時,首先進行前述說明之原料導入步驟。使薄膜形成用原料成為蒸氣時之較佳溫度或壓力與上述說明者相同。接者,藉由導入成膜腔室部之以上述通式(I)表示之化合物,於基體表面成膜前驅物薄膜(前驅物薄膜成膜步驟)。此時,亦可加熱基體、或加熱成膜腔室部而施加熱。Hereinafter, for the above-mentioned steps, a case where the metal carbide-containing film is formed by the ALD method is taken as an example for detailed description. When forming a metal carbide-containing thin film by the ALD method, first, the aforementioned raw material introduction step is performed. The preferable temperature or pressure when the raw material for film formation becomes vapor is the same as described above. Next, by introducing the compound represented by the above general formula (I) into the film forming chamber, a precursor film is formed on the surface of the substrate (precursor film forming step). At this time, the substrate may be heated or the film forming chamber may be heated to apply heat.
以該步驟成膜之前驅物薄膜為以上述通式(I) 表示之化合物吸著於基體表面者或該化合物或是該化合物之一部分分解及/或反應而生成之薄膜,具有與目的之金屬氧化物薄膜不同之組成。進行本步驟時之基體溫度室溫~600℃為佳,150~400℃為更佳。進行本步驟時之系(成膜腔室內)之壓力1~10000Pa為佳,10~1000Pa為更佳。The precursor film formed by this step is the compound represented by the above general formula (I) adsorbed on the surface of the substrate, or the compound or a part of the compound decomposes and/or reacts to form a thin film, and has a target metal Different composition of oxide film. The substrate temperature during this step is preferably from room temperature to 600°C, more preferably from 150 to 400°C. The pressure of the system (in the film forming chamber) during this step is preferably 1~10000Pa, more preferably 10~1000Pa.
接者,自成膜腔室部排出未反應之以上述通式(I)表示之化合物氣體或副生之氣體(排氣步驟)。未反應之以上述通式(I)表示之化合物氣體或副生之氣體理想上係自成膜腔室部完全排氣,但並無必要必定完全排氣。排氣方法有藉由氮氣、氦氣、氬氣等之惰性氣體吹拂系內之方法,藉由使系內減壓而排氣之方法,組合該等之方法等。減壓時之減壓度0.01~300Pa為佳,0.01~100Pa為更佳。Next, the unreacted compound gas or by-product gas represented by the above general formula (I) is discharged from the film forming chamber (exhaust step). The unreacted compound gas or by-product gas represented by the above general formula (I) is ideally completely exhausted from the film forming chamber, but it is not necessarily completely exhausted. The exhaust method includes a method of blowing an inert gas such as nitrogen, helium, argon, etc. into the system, a method of depressurizing the system to exhaust, and a combination of these methods. The decompression degree during decompression is preferably 0.01~300Pa, more preferably 0.01~100Pa.
接者,於成膜腔室部導入反應性氣體,藉由該反應性氣體或反應性氣體及熱之作用,自以先前之前驅物薄膜成膜步驟所得之前驅物薄膜形成含有金屬碳化物之薄膜(含金屬碳化物之薄膜形成步驟)。本步驟中,使熱作用時之溫度的室溫~600℃為佳,150~400℃為更佳。進行本步驟時之系(成膜腔室內)之壓力1~10000Pa為佳,10~1000Pa為更佳。Next, a reactive gas is introduced into the film forming chamber, and by the action of the reactive gas or reactive gas and heat, a precursor film containing metal carbides is formed from the precursor film obtained by the previous precursor film forming step Thin film (a step of forming a thin film containing metal carbide). In this step, the temperature at the time of heating is preferably from room temperature to 600°C, and more preferably from 150 to 400°C. The pressure of the system (in the film forming chamber) during this step is preferably 1~10000Pa, more preferably 10~1000Pa.
本發明的薄膜之製造方法,採用如上述之ALD法時,將由上述之原料導入步驟、前驅物薄膜成膜步驟、排氣步驟及含金屬碳化物之薄膜形成步驟所成之一連串操作之薄膜堆積設為1循環,可重複複數次該循環直至獲得必要膜厚之薄膜。該情況下,進行1循環後,與上述 排氣步驟同樣地,自成膜腔室部排出未反應之以上述通式(I)表示之化合物氣體及反應性氣體進一部排出副生之氣體後,進行下1循環為佳。When the method for manufacturing a thin film of the present invention adopts the ALD method as described above, the thin film is deposited in a series of operations including the above-mentioned raw material introduction step, precursor thin film formation step, exhaust step, and metal carbide-containing thin film formation step Set as 1 cycle, and this cycle can be repeated several times until a film with the necessary film thickness is obtained. In this case, after performing one cycle, the unreacted compound gas and reactive gas represented by the above general formula (I) are discharged from the film-forming chamber in the same manner as the above-mentioned exhausting step. , The next cycle is better.
又,本發明之薄膜之製造方法中,採用上述之ALD法之情況,亦可施加電漿、光、電壓等之能量,亦可使用觸媒。施加該等之能量之時期並未特別限定,宜為例如於原料導入步驟中之化合物氣體導入時,前驅物薄膜成膜步驟或含金屬碳化物之薄膜形成步驟中之加溫,排氣步驟之系內排氣時,含金屬碳化物之薄膜形成步驟中之反應性氣體導入時,亦可為上述各步驟之間。又,於反應性氣體導入前,亦可對於反應性氣體施加該等之能量。In addition, in the method of manufacturing the thin film of the present invention, when the above-mentioned ALD method is used, energy such as plasma, light, and voltage may be applied, and a catalyst may also be used. The time period for applying the energy is not particularly limited. For example, during the introduction of the compound gas in the raw material introduction step, the heating in the precursor film formation step or the metal carbide-containing film formation step, the exhaust step When the system is exhausted, when the reactive gas is introduced in the metal carbide-containing film formation step, it can also be between the above steps. In addition, before the introduction of the reactive gas, such energy may be applied to the reactive gas.
又,在本發明的薄膜之製造方法中,採用如上述之上述的電漿ALD法時,反應性氣體可於製造方法中全部步驟之間對成膜腔室內持續流入,並僅於含金屬碳化物之薄膜形成步驟時,將對於反應性氣體進行電漿處理者導入成膜腔室即可。高頻(以下,有時亦稱為RF)輸出係過低,則不易成為良好的金屬碳化物之膜,過高則對基板之破損為大,故0~1500W為佳,50~600W為更佳。在本發明之製造方法中,採用電漿ALD法時,可含有非常高品質的含金屬碳化物之薄膜,故為佳。Furthermore, in the thin film manufacturing method of the present invention, when the above-mentioned plasma ALD method is used, the reactive gas can continuously flow into the film forming chamber between all steps in the manufacturing method, and only the metal-containing carbonization In the step of forming the thin film of the object, the plasma treatment of the reactive gas may be introduced into the film forming chamber. If the high frequency (hereafter, sometimes referred to as RF) output is too low, it will not easily become a good metal carbide film. If it is too high, the damage to the substrate will be greater, so 0~1500W is better, 50~600W is more good. In the manufacturing method of the present invention, when the plasma ALD method is used, it can contain a very high-quality metal carbide-containing film, so it is preferred.
又,本發明之薄膜之製造方法中,薄膜堆積後,為了獲得更良好之電特性,亦可在惰性環境下進行退火處理,於必須埋入階差時,亦可設回焊步驟。該情況之溫度為200~1000℃,250~500℃為佳。Furthermore, in the method of manufacturing a thin film of the present invention, after the thin film is deposited, in order to obtain better electrical characteristics, an annealing process may be performed in an inert environment, and a reflow step may be provided when the step must be embedded. The temperature in this case is 200~1000°C, preferably 250~500°C.
使用本發明之含金屬碳化物之薄膜形成用原料製造含金屬碳化物之薄膜之裝置可使用周知的化學氣相成長法用裝置。作為具體的裝置之一例舉例為例如圖1所示之可藉冒泡供給前驅物而進行之裝置,或如圖2所示之具有氣化室之裝置。且,舉例為如圖3及圖4之可對反應性氣體進行電漿處理之裝置。不限於如圖1、圖2、圖3、圖4之單片式裝置,亦可使用利用批式爐可同時處理多數片之裝置。An apparatus for producing a metal carbide-containing thin film using the raw material for forming a metal carbide-containing thin film of the present invention can use a well-known chemical vapor growth method. As an example of a specific device, for example, the device shown in FIG. 1 that can be used to supply the precursor by bubbling, or the device having a gasification chamber as shown in FIG. 2. And, for example, as shown in FIG. 3 and FIG. 4, the device that can perform plasma treatment on the reactive gas. It is not limited to the single-chip devices shown in Figure 1, Figure 2, Figure 3, and Figure 4, and a batch furnace that can process multiple chips at the same time can also be used.
使用本發明之含金屬碳化物之薄膜形成用原料而製造的含金屬碳化物之薄膜可使用於切削工具、電子材料用之配線或電極,例如可使用於半導體記憶體材料或鋰空氣電池用之電極等。The metal carbide-containing film produced by using the raw material for forming a metal carbide-containing film of the present invention can be used for cutting tools, wiring or electrodes for electronic materials, for example, for semiconductor memory materials or lithium-air batteries Electrodes etc.
以下列舉實施例及評估例更詳細說明本發明。然而,本發明不受以下實施例之任何限制。The following examples and evaluation examples illustrate the present invention in more detail. However, the present invention is not limited in any way by the following examples.
藉由將上述化合物No.1放置於大氣中,來確認自燃性之有無。將結果表示於表1。By placing the above compound No. 1 in the atmosphere, the presence or absence of spontaneous combustion was confirmed. The results are shown in Table 1.
依據表1之結果,化合物No.1係無法顯示自燃性,可了解即使於大氣中可安全地使用作為化學氣相成長用原料。According to the results in Table 1, the compound No. 1 system cannot show spontaneous combustion, and it can be understood that it can be safely used as a raw material for chemical vapor growth even in the atmosphere.
將化合物No.1作為化學氣相成長用原料,使用圖3所示之裝置,藉由以下之條件的ALD法,於矽晶圓上製造碳化鈦薄膜。對於所得到之薄膜,利用X射線反射率法測定膜厚、利用X射線繞射法及X射線光電子光譜法(X-ray Photoelectron Spectroscopy)進行薄膜構造及薄膜組成之確認時,膜厚係7.5nm,膜組成係碳化鈦,含碳量係58atom%(理論值60atom%)。無法檢測作為有機物之殘留碳成分。每1循環所得之膜厚為0.15nm。Using compound No. 1 as a raw material for chemical vapor growth, using the apparatus shown in FIG. 3, a titanium carbide thin film was produced on a silicon wafer by the ALD method under the following conditions. For the obtained film, the film thickness is measured by X-ray reflectance method, and the film structure and film composition are confirmed by X-ray diffraction method and X-ray Photoelectron Spectroscopy. The film thickness is 7.5nm , The film composition is titanium carbide, and the carbon content is 58atom% (theoretical value 60atom%). Unable to detect residual carbon content as organic matter. The film thickness obtained per cycle is 0.15 nm.
反應溫度(基板溫度):200℃,反應性氣體:氫Reaction temperature (substrate temperature): 200°C, reactive gas: hydrogen
將由下述(1)~(4)所成之一連串步驟設為1循環,重複50次循環:(1)於原料容器溫度:70℃、原料容器壓力:0.8Torr(106Pa)之條件下,將氣化之化學氣相成長用原料之蒸氣導入於成膜腔室內,於系壓力0.6Torr(80Pa)下,於矽晶圓堆積10秒;(2)藉由20秒之氬吹拂,去除未反應原料; (3)導入反應性氣體,以系壓力0.6Pa(80Pa)反應10秒;此時於反應氣體中,藉由施加13.56MHz、100W之高頻輸出電漿化。Set a series of steps consisting of the following (1) to (4) as 1 cycle and repeat 50 cycles: (1) Under the conditions of raw material container temperature: 70℃ and raw material container pressure: 0.8 Torr (106 Pa), The vaporized chemical vapor growth material is introduced into the film forming chamber and deposited on the silicon wafer for 10 seconds at a system pressure of 0.6 Torr (80 Pa); (2) Unreacted is removed by blowing argon for 20 seconds Raw materials; (3) Introduce reactive gas and react at a pressure of 0.6Pa (80Pa) for 10 seconds; at this time, plasma is formed by applying 13.56MHz, 100W high-frequency output in the reactive gas.
(4)藉由15秒之氬吹拂,去除未反應原料。(4) Purge with argon for 15 seconds to remove unreacted raw materials.
除了使用作為化學氣相成長法原料之肆新戊基鈦以外,於與實施例1相同條件下進行碳化鈦薄膜之製造。The titanium carbide thin film was manufactured under the same conditions as in Example 1, except that neopentyl titanium used as the raw material of the chemical vapor growth method was used.
對於所得到之薄膜,利用X射線反射率法測定膜厚、利用X射線繞射法及X射線光電子光譜法進行薄膜構造及薄膜組成之確認時,膜厚係6nm,膜組成係碳化鈦,含碳量係40atom%(理論值60atom%)。檢測出作為有機物之殘留碳成分係10atom%以上。每1循環所得之膜厚為0.12nm。For the obtained film, when the film thickness is measured by X-ray reflectance method, the film structure and film composition are confirmed by X-ray diffraction method and X-ray photoelectron spectroscopy, the film thickness is 6nm, and the film composition is titanium carbide, containing The carbon content is 40atom% (theoretical value 60atom%). The residual carbon content detected as organic matter is 10atom% or more. The film thickness obtained per cycle is 0.12 nm.
由實施例1之結果得知,使用本發明之含金屬碳化物之薄膜形成用原料之時,作為有機物之殘留碳成分之含量非常少,可形成含有接近理論值的碳成分之高品質的碳化鈦薄膜。另一方面,在比較例1中,可了解大量混入作為有機物之殘留碳成分於薄膜中,所得到之薄膜係得到不含有理論值之品質不佳之碳化鈦薄膜。From the results of Example 1, it is known that when the metal carbide-containing film forming raw material of the present invention is used, the content of residual carbon components as organic matter is very small, and high-quality carbonization containing carbon components close to the theoretical value can be formed. Titanium film. On the other hand, in Comparative Example 1, it can be understood that a large amount of residual carbon components, which are organic substances, are mixed in the film, and the resulting film is a titanium carbide film of poor quality that does not contain theoretical values.
尚,本國際申請係基於2016年7月5日申請之日本國專利申請第2016-133137號主張優先權,且將該日本國際申請之全內容納入本國際申請。Still, this international application claims priority based on Japanese Patent Application No. 2016-133137 filed on July 5, 2016, and incorporates the entire content of the Japanese international application into this international application.
Claims (1)
Translated fromChineseApplications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| JP2016133137AJP6691009B2 (en) | 2016-07-05 | 2016-07-05 | Raw material for forming metal carbide-containing thin film and method for producing metal carbide-containing thin film |
| JP2016-133137 | 2016-07-05 |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| TW201815802A TW201815802A (en) | 2018-05-01 |
| TWI717530Btrue TWI717530B (en) | 2021-02-01 |
Family
ID=60901473
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| TW106121626ATWI717530B (en) | 2016-07-05 | 2017-06-28 | Method manufacturing thin film containing metal carbide |
Country Status (3)
| Country | Link |
|---|---|
| JP (1) | JP6691009B2 (en) |
| TW (1) | TWI717530B (en) |
| WO (1) | WO2018008351A1 (en) |
Families Citing this family (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| KR102802670B1 (en)* | 2022-05-10 | 2025-05-07 | 주식회사 이지티엠 | Aluminum precursor and method of forming a thin layer using the same, method of manufacturing the same |
| JP7587873B2 (en) | 2022-05-10 | 2024-11-21 | イージーティーエム カンパニー リミテッド | Thin film forming method and memory device manufacturing method including the same |
Citations (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US20010034097A1 (en)* | 1997-09-29 | 2001-10-25 | Hyun-Seok Lim | Method of forming metal nitride film by chemical vapor deposition and method of forming metal contact and capacitor of semiconductor device using the same |
| US20100036144A1 (en)* | 2006-07-20 | 2010-02-11 | Ce Ma | Methods for atomic layer deposition |
Family Cites Families (5)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US6197683B1 (en)* | 1997-09-29 | 2001-03-06 | Samsung Electronics Co., Ltd. | Method of forming metal nitride film by chemical vapor deposition and method of forming metal contact of semiconductor device using the same |
| JP2004189690A (en)* | 2002-12-13 | 2004-07-08 | Sumitomo Chem Co Ltd | Transition metal complex, catalyst for olefin polymerization, and method for producing olefin polymer using the same |
| EP1764376A1 (en)* | 2005-09-16 | 2007-03-21 | DSM IP Assets B.V. | Process for the preparation of a metal-organic compound comprising a spectator ligand |
| EP2029790A1 (en)* | 2006-06-02 | 2009-03-04 | L'AIR LIQUIDE, Société Anonyme pour l'Etude et l'Exploitation des Procédés Georges Claude | Method of forming high-k dielectric films based on novel titanium, zirconium, and hafnium precursors and their use for semiconductor manufacturing |
| WO2013150533A2 (en)* | 2012-04-03 | 2013-10-10 | Yissum Research Development Company Of The Hebrew University Of Jerusalem Ltd. | Hybrid metal and metal oxide layers with enhanced activity |
- 2016
- 2016-07-05JPJP2016133137Apatent/JP6691009B2/enactiveActive
- 2017
- 2017-06-15WOPCT/JP2017/022099patent/WO2018008351A1/ennot_activeCeased
- 2017-06-28TWTW106121626Apatent/TWI717530B/enactive
Patent Citations (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US20010034097A1 (en)* | 1997-09-29 | 2001-10-25 | Hyun-Seok Lim | Method of forming metal nitride film by chemical vapor deposition and method of forming metal contact and capacitor of semiconductor device using the same |
| US20100036144A1 (en)* | 2006-07-20 | 2010-02-11 | Ce Ma | Methods for atomic layer deposition |
Also Published As
| Publication number | Publication date |
|---|---|
| JP2018003110A (en) | 2018-01-11 |
| TW201815802A (en) | 2018-05-01 |
| JP6691009B2 (en) | 2020-04-28 |
| WO2018008351A1 (en) | 2018-01-11 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| JP5843318B2 (en) | Raw material for forming aluminum nitride thin film for ALD method and method for producing the thin film | |
| TWI795553B (en) | Method for manufacturing thin film with the use of a raw material for forming thin film by atomic layer deposition method | |
| KR102775932B1 (en) | Raw material for forming thin film for atomic layer deposition, raw material for forming thin film, method for producing thin film and compound | |
| JP6735163B2 (en) | Vanadium compound, thin film forming raw material, and thin film manufacturing method | |
| WO2020170853A1 (en) | Starting material for forming gallium nitride-containing thin film for atomic layer deposition method, and method for producing gallium nitride-containing thin film | |
| JP7717051B2 (en) | Raw materials for forming thin films by atomic layer deposition method and method for manufacturing thin films | |
| TWI717530B (en) | Method manufacturing thin film containing metal carbide | |
| EP3643700A1 (en) | Metal alkoxide compound, thin-film-forming raw material, and method for producing thin film | |
| JP5912911B2 (en) | Method for producing thin film by ALD method using aluminum compound | |
| KR20220161372A (en) | Zinc compound, raw material for thin film formation, thin film and manufacturing method thereof | |
| JP6116007B2 (en) | Thin film forming raw material and thin film manufacturing method | |
| KR102822492B1 (en) | Compounds, raw materials for forming thin films and methods for producing thin films | |
| WO2023171489A1 (en) | Starting material for thin film formation by atomic layer deposition, thin film, and method for producing thin film | |
| JP7636336B2 (en) | Method for producing yttrium oxide-containing film | |
| WO2013105310A1 (en) | Aluminum compound, starting material for forming thin film, and method for producing thin film | |
| TWI715787B (en) | Raw material for forming thin film and method for manufacturing thin film | |
| TWI805584B (en) | Raw material for thin film formation, method for manufacturing thin film, and novel compound | |
| JP7641235B2 (en) | Method for producing copper-containing layer | |
| WO2023090179A1 (en) | Thin film-forming material for use in atomic layer deposition, thin film, method for producing thin film, and ruthenium compound | |
| WO2022107769A1 (en) | Method for manufacturing thin film | |
| WO2023276716A1 (en) | Starting material for forming thin film, thin film and method for producing thin film | |
| KR20230107612A (en) | Thin film manufacturing method | |
| WO2022220153A1 (en) | Thin film-forming feedstock for use in atomic layer deposition, thin film, method for producing thin film, and ruthenium compound |