KR100629683B1 - Oxygen permeability measuring device of film and oxygen permeability measuring method using same - Google Patents
Oxygen permeability measuring device of film and oxygen permeability measuring method using sameDownload PDFInfo
- Publication number
- KR100629683B1 KR100629683B1KR1019990048100AKR19990048100AKR100629683B1KR 100629683 B1KR100629683 B1KR 100629683B1KR 1019990048100 AKR1019990048100 AKR 1019990048100AKR 19990048100 AKR19990048100 AKR 19990048100AKR 100629683 B1KR100629683 B1KR 100629683B1
- Authority
- KR
- South Korea
- Prior art keywords
- film
- oxygen permeability
- oxygen
- sensor
- measuring
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Expired - Fee Related
Links
Images
Classifications
- G—PHYSICS
- G01—MEASURING; TESTING
- G01N—INVESTIGATING OR ANALYSING MATERIALS BY DETERMINING THEIR CHEMICAL OR PHYSICAL PROPERTIES
- G01N27/00—Investigating or analysing materials by the use of electric, electrochemical, or magnetic means
- G01N27/26—Investigating or analysing materials by the use of electric, electrochemical, or magnetic means by investigating electrochemical variables; by using electrolysis or electrophoresis
- G01N27/28—Electrolytic cell components
- G01N27/30—Electrodes, e.g. test electrodes; Half-cells
- G01N27/304—Gas permeable electrodes
- G—PHYSICS
- G01—MEASURING; TESTING
- G01N—INVESTIGATING OR ANALYSING MATERIALS BY DETERMINING THEIR CHEMICAL OR PHYSICAL PROPERTIES
- G01N27/00—Investigating or analysing materials by the use of electric, electrochemical, or magnetic means
- G01N27/26—Investigating or analysing materials by the use of electric, electrochemical, or magnetic means by investigating electrochemical variables; by using electrolysis or electrophoresis
- G01N27/416—Systems
- G01N27/48—Systems using polarography, i.e. measuring changes in current under a slowly-varying voltage
Landscapes
- Chemical & Material Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Health & Medical Sciences (AREA)
- Physics & Mathematics (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Electrochemistry (AREA)
- Molecular Biology (AREA)
- Analytical Chemistry (AREA)
- Biochemistry (AREA)
- General Health & Medical Sciences (AREA)
- General Physics & Mathematics (AREA)
- Immunology (AREA)
- Pathology (AREA)
- Separation Using Semi-Permeable Membranes (AREA)
- Manufacture Of Macromolecular Shaped Articles (AREA)
Abstract
Translated fromKoreanDescription
Translated fromKorean도 1은 본 발명에 따른 필름의 산소 투과도 측정에 사용되는 전기화학적 센서의 종단면도이고,1 is a longitudinal sectional view of an electrochemical sensor used for measuring oxygen permeability of a film according to the present invention,
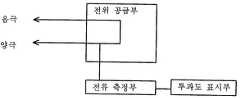
도 2는 센서에 일정한 전압을 공급하고 전류를 필름의 산소 투과도 값으로 변환하여 표시해주는 본 발명에 따른 산소 투과도 측정 장치의 개념도이고,2 is a conceptual diagram of an oxygen permeability measuring device according to the present invention for supplying a constant voltage to the sensor and converting the current into an oxygen permeability value of the film for display.
도 3은 본 발명에 따른 실시예에 있어서 센서에 장착된 폴리프로필렌 필름의 산소 투과도를 측정하기 위한 산소 분압에 따른 전류의 그래프이고,Figure 3 is a graph of the current according to the oxygen partial pressure for measuring the oxygen permeability of the polypropylene film mounted on the sensor in an embodiment according to the present invention,
도 4는 본 발명에 따른 실시예에 있어서 센서에 장착된 테프론 필름의 산소 투과도를 측정하기 위한 산소 분압에 따른 전류의 그래프이다.
Figure 4 is a graph of the current according to the oxygen partial pressure for measuring the oxygen permeability of the Teflon film mounted on the sensor in the embodiment according to the present invention.
본 발명은 필름의 산소 투과도를 측정하는 전기화학적 장치 및 이를 이용한 산소 투과도 측정 방법에 관한 것으로, 구체적으로는 양극과 음극으로 구성된 센서에 일정한 전압을 공급하고 전류를 필름의 산소 투과도 값으로 변환하여 표시해주는 산소 투과도 측정 장치 및 이를 이용하여 필름의 산소 투과도를 전기화학적으로 빠르고 간편하게 측정하는 방법에 관한 것이다.The present invention relates to an electrochemical device for measuring oxygen permeability of a film and a method for measuring oxygen permeability using the same, and specifically, a constant voltage is supplied to a sensor composed of an anode and a cathode and a current is converted into an oxygen permeability value of the film. The present invention relates to an oxygen permeability measuring device and a method for quickly and easily measuring the oxygen permeability of a film using the same.
이제까지는 필름의 산소 투과도를 측정하기 위해, 예를 들면 ASTM D 3985법에서와 같이, 필름의 양면에 산소 압력차를 두고 통과하는 산소의 양을 측정하는 방법을 사용하였다. 그러나, 이 방법은 반드시 진공조건하에서 수행되어야 하기 때문에 부가적인 진공설비를 필요로 하는 등 장치의 구성이 복잡해지는 단점이 있었다.Until now, in order to measure the oxygen permeability of the film, a method of measuring the amount of oxygen passing through the oxygen pressure difference on both sides of the film, as in the ASTM D 3985 method, for example, has been used. However, this method has a disadvantage in that the configuration of the apparatus is complicated, such as requiring an additional vacuum facility because it must be performed under vacuum conditions.
이에, 본 발명자들은 종래의 단점을 극복하고자 예의 연구를 계속한 결과, 양극과 음극으로 구성된 센서를 포함하는 특정의 장치를 이용함으로써 필름의 산소 투과도를 전기화학적으로 빠르고 간편하게 측정할 수 있음을 발견하고 본 발명을 완성하게 되었다.
Accordingly, the present inventors have intensively researched to overcome the shortcomings of the prior art, and found that the oxygen permeability of the film can be measured electrochemically and simply by using a specific device including a sensor composed of an anode and a cathode. The present invention has been completed.
본 발명의 목적은 부가적인 진공설비 필요없이 빠르고 간편하게 필름의 산소 투과도를 측정할 수 있는 장치 및 이를 이용한 산소 투과도 측정 방법을 제공하는 것이다.
It is an object of the present invention to provide an apparatus capable of measuring oxygen permeability of a film quickly and simply without the need for additional vacuum equipment and a method for measuring oxygen permeability using the same.
상기 목적을 달성하기 위하여 본 발명에서는, 양극과 음극으로 구성되고 두 전극간이 전해질로 채워진 폴라로그래픽형(polarographic) 또는 갈바닉형 (galvanic) 센서, 센서에 전압을 공급할 때 센서에 흐르는 전류를 측정하는 장치, 및 측정된 전류 값을 필름의 산소 투과도 값으로 변환시키는 장치를 포함하는 필름의 산소 투과도 측정 장치를 제공한다.In order to achieve the above object, in the present invention, a polarographic or galvanic sensor composed of an anode and a cathode and filled with an electrolyte between two electrodes, measuring a current flowing through the sensor when a voltage is supplied to the sensor. An apparatus for measuring oxygen permeability of a film is provided that includes a device, and a device for converting a measured current value into an oxygen permeability value of the film.
본 발명에서는 또한, 상기 장치를 이용하여 센서의 음극 표면에 필름을 밀착시킨 후 전압을 공급하여 필름의 산소 투과도를 측정하는 방법을 제공한다.The present invention also provides a method for measuring the oxygen permeability of the film by supplying a voltage after the film is in close contact with the cathode surface of the sensor using the device.
이하 본 발명에 대하여 보다 상세히 설명한다.Hereinafter, the present invention will be described in more detail.
도 1은 본 발명에 따른 필름의 산소 투과도 측정 장치에 주요부로 사용되는 전기화학적 센서의 종단면도로서, 본 발명에 사용되는 센서는 양극과 음극으로 구성되고 양 전극사이가 전해질로 채워지는 통상의 폴라로그래픽형(polarographic) 또는 갈바닉형(galvanic) 센서이다. 상기 양극 재료로는 은/염화은(Ag/AgCl), 납(Pb), 구리(Cu) 및 카드뮴(Cd) 등을 들 수 있고, 음극 재료로는 은(Ag), 금(Au), 백금(Pt), 아연(Zn) 및 탄소(C) 등을 들 수 있으며, 전해질로는 K2CO3, Na2CO3, KCl, NaCl, NaOH 및 KOH 등을 사용할 수 있다.1 is a longitudinal cross-sectional view of an electrochemical sensor used as a main part of an oxygen permeability measuring device of a film according to the present invention, wherein the sensor used in the present invention is composed of a positive electrode and a negative electrode and a common polar electrode filled with an electrolyte between both electrodes. It is a polarographic or galvanic sensor. The positive electrode material may be silver / silver chloride (Ag / AgCl), lead (Pb), copper (Cu), cadmium (Cd), and the like, and the negative electrode material may be silver (Ag), gold (Au), or platinum ( Pt), zinc (Zn) and carbon (C), and the like, and K2 CO3 , Na2 CO3 , KCl, NaCl, NaOH and KOH may be used as the electrolyte.
본 발명에 따르면, 상기 센서에 일정한 전압을 공급하였을 때 발생하는 전류를 측정하는 장치와 이 전류 값을 필름의 산소 투과도 값으로 변환하여 표시해주는 장치가 센서의 양극에 연결되어 있어, 이를 통해 필름의 산소 투과도를 빠르고 간 편하게 측정할 수 있다. 또한, 상기 센서에는 측정 필름을 음극에 밀착 고정시키기 위한 수단(예를 들면, O-링)이 갖추어져 있다. 도 2는 센서에 일정한 전압을 공급하여 음극과 양극 사이에 전위차를 유지시켜 주고 센서에 흐르는 전류를 필름의 산소 투과도 값으로 변환하여 표시해주는 본 발명에 따른 산소 투과도 측정 장치의 개념도를 나타낸다.According to the present invention, a device for measuring a current generated when a constant voltage is supplied to the sensor and a device for converting and displaying the current value into an oxygen permeability value of the film is connected to the anode of the sensor, thereby Oxygen permeability can be measured quickly and easily. Moreover, the said sensor is equipped with the means (for example, O-ring) for closely fixing a measuring film to a cathode. 2 is a conceptual diagram of an oxygen permeability measuring apparatus according to the present invention for maintaining a potential difference between a cathode and an anode by supplying a constant voltage to the sensor, and converting and displaying a current flowing through the sensor into an oxygen permeability value of the film.
본 발명의 필름 산소 투과도 측정 방법에 따르면, 이러한 센서의 음극 표면위에 산소 투과도를 측정하고자 하는 필름을 밀착시킨 후 전압을 인가하여 이때 센서에 흐르는 전류로부터 필름의 산소 투과도를 산출할 수 있다.According to the method of measuring the film oxygen permeability of the present invention, the film to measure the oxygen permeability on the cathode surface of the sensor is in close contact with a voltage and then the oxygen permeability of the film can be calculated from the current flowing through the sensor.
본 발명의 측정방법의 원리를 구체적으로 살펴보면, 예를 들면 염화칼륨과 같은 전해질을 포함하는 전도성 용액 중에서 은과 같은 귀금속을 음극으로 하고 표준전위가 상대적으로 높은 은/염화은과 같은 금속을 양극으로 하는 산소전극을 투과한 산소는 음극 표면에서 하기와 같이 전기화학적으로 반응한다:In detail, the principle of the measuring method of the present invention is, for example, in a conductive solution containing an electrolyte such as potassium chloride, a noble metal such as silver is used as a cathode, and a metal such as silver / silver chloride having a relatively high standard potential is used as an anode. Oxygen passing through the electrode reacts electrochemically at the cathode surface as follows:
본 발명의 방법에 따라 음극의 표면위에 위치된 필름을 투과한 산소는 상기 반응식 1에 나타낸 바와 같이 환원되며, 이때 투과한 산소량은 전극간의 전류값으로 하기 수학식 1에 의해 표시될 수 있다:According to the method of the present invention oxygen passed through the film located on the surface of the cathode is reduced as shown in
상기 식에서,Where
I는 전류값이고, n은 산소 1몰 당 소모되는 전자의 개수로서 4이며, A는 센서의 표면적이고, F는 파라데이(Faraday) 상수이며, Pm은 필름의 산소 투과도이고, dm은 필름의 두께이고, P0는 센서의 외부 산소 분압이다.I is the current value, n is the number of electrons consumed per mole of oxygen, 4 is A, the surface of the sensor, F is the Faraday constant, Pm is the oxygen permeability of the film, and dm is The thickness of the film, P0 is the external oxygen partial pressure of the sensor.
상기 수학식 1에 있어서 전류 I에 대하여 산소 분압 Po를 미분할 때의 그의 기울기는 하기 수학식 2와 같다:The slope of the derivative of oxygen partial pressure Po with respect to the current I in
상기 수학식 2에서 구한 기울기 B를 이용하고 주어진 산소 센서의 nFA와 dm은 측정가능하므로 필름의 산소 투과도는 하기 수학식 3과 같이 구할 수 있다:Using the slope B obtained in
본 발명에 따르면, 측정 장치에 -0.6 내지 -1.0 Volt의 전압을 공급하는 것이 바람직하며, 산소와 질소의 혼합가스를 이용하여 산소 분압을 조절하면서 필름의 산소투과에 따른 전류를 측정할 수 있다.According to the invention, it is preferable to supply a voltage of -0.6 to -1.0 Volt to the measuring device, it is possible to measure the current according to the oxygen transmission of the film while adjusting the oxygen partial pressure using a mixed gas of oxygen and nitrogen.
본 발명의 측정 장치를 이용하면, 부가적인 진공설비 없이도 빠르고 간편하게 필름의 산소 투과도를 측정할 수 있다.Using the measuring device of the present invention, the oxygen permeability of the film can be measured quickly and simply without additional vacuum equipment.
이하, 본 발명을 하기 실시예에 의거하여 좀더 상세하게 설명하고자 한다. 단, 하기 실시예는 본 발명을 예시하기 위한 것일 뿐, 본 발명의 범위가 이들만으로 제한되는 것은 아니다.
Hereinafter, the present invention will be described in more detail based on the following examples. However, the following examples are only for illustrating the present invention, and the scope of the present invention is not limited thereto.
실시예 1 : 폴리프로필렌 필름의 산소 투과도 측정Example 1 Oxygen Permeability Measurement of Polypropylene Film
전해질로서 염화칼륨을 포함하고 양극이 은/염화은이고 음극이 금으로 된 센서를 25℃로 유지되는 100ml 항온조에 설치하였다. 센서의 음극 표면위에 다양한 두께의 폴리프로필렌 필름을 밀착시킨 후, -0.8 Volt의 전압을 가하고 산소와 질소의 혼합가스를 이용하여 산소 분압을 0에서 700mmHg까지 100mmHg씩 증가시키면서 평형상태에 이를 때의 전류값을 기록하였다.A sensor containing potassium chloride as electrolyte and a silver / silver chloride positive electrode and gold negative electrode was installed in a 100 ml thermostat maintained at 25 ° C. After contacting the polypropylene film of various thicknesses on the cathode surface of the sensor, apply a voltage of -0.8 Volt and use a mixed gas of oxygen and nitrogen to increase the partial pressure of oxygen from 0 to 700mmHg in 100mmHg increments. The value was recorded.
산소 분압에 따른 전류의 변화를 도 3에 그래프로서 나타내었다. 도 3 및 상기 수학식 1로부터 알 수 있듯이, 산소 분압에 따른 전류의 거동은 비례관계를 가지며 이는 막을 투과하는 산소의 양에 비례하므로, 정상상태 전류(Is)와 산소 분압(Po)을 도시한 상기 수학식 1을 이용한 최소 자승법으로 필름의 산소 투과도를 구하여, 하기 표 1에 나타내었다.The change of the current according to the partial pressure of oxygen is shown graphically in FIG. As can be seen from FIG. 3 and
실시예 2 : 폴리테프론 필름의 산소 투과도 측정Example 2 Oxygen Permeability Measurement of Polyteflon Film
폴리프로필렌 필름 대신에 다양한 회사의 테프론 필름을 사용한 것을 제외하고는 상기 실시예 1과 동일한 방법으로 실험을 수행하고 필름의 산소 투과도를 구하였다.Except for using a polypropylene film Teflon film of various companies was carried out in the same manner as in Example 1 to obtain the oxygen permeability of the film.
산소 분압에 따른 전류의 변화를 도 4에 그래프로서 나타내었으며, 산출된 산소 투과도의 결과를 하기 표 2에 나타내었다.The change of the current according to the oxygen partial pressure is shown as a graph in FIG. 4, and the results of the calculated oxygen permeability are shown in Table 2 below.
실시예 3 : 각종 생활용품의 포장지로 사용되는 필름 분석에의 적용Example 3 Application to Film Analysis Used as Packaging Paper of Various Household Items
포장 제품의 공기에 의한 변질 또는 산화를 사전에 방지하기 위하여, 시판 전자부품 또는 생활용품 포장지로 사용된 12종류의 필름의 산소 투과도를 측정하였다. 산소 분압을 0, 350 및 700mmHg로 증가시킨 것을 제외하고는 상기 실시예 1과 동일한 방법으로 실험을 수행하여 각 필름의 산소 투과도를 구하였다. 그 결과를 하기 표 3에 나타내었으며, 이때 - 부호는 산소가 전혀 투과되지 않음을 의미한다.In order to prevent the deterioration or oxidation by the air of a packaged product in advance, the oxygen permeability of 12 kinds of films used as a commercial electronics or household packaging was measured. Except that the oxygen partial pressure was increased to 0, 350 and 700mmHg, the experiment was carried out in the same manner as in Example 1 to obtain the oxygen permeability of each film. The results are shown in Table 3 below, where-sign means that oxygen is not permeated at all.
본 발명의 전기화학적 산소 투과도 측정 장치는, 부가적인 진공설비 없이도 빠르고 간편하게 필름의 산소 투과도를 측정할 수 있어, 각종 산업 필름, 예를 들면 식품 포장용 및 전자부품 포장용 필름의 산소 투과도 측정에 유용하게 이용될 수 있다.The electrochemical oxygen permeability measuring device of the present invention can measure oxygen permeability of a film quickly and simply without additional vacuum equipment, and thus is useful for measuring oxygen permeability of various industrial films, for example, food packaging films and electronic component packaging films. Can be.
Claims (4)
Translated fromKoreanPriority Applications (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| KR1019990048100AKR100629683B1 (en) | 1999-11-02 | 1999-11-02 | Oxygen permeability measuring device of film and oxygen permeability measuring method using same |
Applications Claiming Priority (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| KR1019990048100AKR100629683B1 (en) | 1999-11-02 | 1999-11-02 | Oxygen permeability measuring device of film and oxygen permeability measuring method using same |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| KR20000006773A KR20000006773A (en) | 2000-02-07 |
| KR100629683B1true KR100629683B1 (en) | 2006-09-29 |
Family
ID=19618131
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| KR1019990048100AExpired - Fee RelatedKR100629683B1 (en) | 1999-11-02 | 1999-11-02 | Oxygen permeability measuring device of film and oxygen permeability measuring method using same |
Country Status (1)
| Country | Link |
|---|---|
| KR (1) | KR100629683B1 (en) |
Families Citing this family (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| KR100497463B1 (en)* | 2003-04-01 | 2005-07-01 | (주)바이오텔 | Oxygen sensor |
| JP6269821B2 (en)* | 2014-04-15 | 2018-01-31 | 日立化成株式会社 | Permeability evaluation method |
| WO2016122263A1 (en) | 2015-01-29 | 2016-08-04 | 주식회사 엘지화학 | Method for measuring metal ion permeability of polymer film, and apparatus for measuring metal ion permeability of polymer film |
| CN107076657B (en)* | 2015-01-29 | 2020-03-31 | 株式会社Lg化学 | Method and apparatus for measuring metal ion permeability of polymer membrane |
Citations (7)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JPS60105953A (en)* | 1983-11-14 | 1985-06-11 | Riken Keiki Kk | Electrochemical type gas sensor |
| JPS6280549A (en)* | 1985-10-03 | 1987-04-14 | Fujitsu Ltd | Carbon dioxide measurement method and measurement sensor |
| JPH03277959A (en)* | 1990-03-27 | 1991-12-09 | Chino Corp | gas concentration sensor |
| JPH04240560A (en)* | 1991-01-23 | 1992-08-27 | Nissan Motor Co Ltd | Method for measuring oxygen transmission speed and transmittance of film |
| JPH05180798A (en)* | 1991-02-28 | 1993-07-23 | Tokuyama Soda Co Ltd | Solid electrolyte gas sensor |
| KR19990014660A (en)* | 1998-11-30 | 1999-02-25 | 심윤보 | Dissolved oxygen measuring device using solid electrolyte |
| US5917924A (en)* | 1996-01-31 | 1999-06-29 | Neopost Limited | Postage metering system |
- 1999
- 1999-11-02KRKR1019990048100Apatent/KR100629683B1/ennot_activeExpired - Fee Related
Patent Citations (7)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JPS60105953A (en)* | 1983-11-14 | 1985-06-11 | Riken Keiki Kk | Electrochemical type gas sensor |
| JPS6280549A (en)* | 1985-10-03 | 1987-04-14 | Fujitsu Ltd | Carbon dioxide measurement method and measurement sensor |
| JPH03277959A (en)* | 1990-03-27 | 1991-12-09 | Chino Corp | gas concentration sensor |
| JPH04240560A (en)* | 1991-01-23 | 1992-08-27 | Nissan Motor Co Ltd | Method for measuring oxygen transmission speed and transmittance of film |
| JPH05180798A (en)* | 1991-02-28 | 1993-07-23 | Tokuyama Soda Co Ltd | Solid electrolyte gas sensor |
| US5917924A (en)* | 1996-01-31 | 1999-06-29 | Neopost Limited | Postage metering system |
| KR19990014660A (en)* | 1998-11-30 | 1999-02-25 | 심윤보 | Dissolved oxygen measuring device using solid electrolyte |
Also Published As
| Publication number | Publication date |
|---|---|
| KR20000006773A (en) | 2000-02-07 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| US4563249A (en) | Electroanalytical method and sensor for hydrogen determination | |
| US4568445A (en) | Electrode system for an electro-chemical sensor for measuring vapor concentrations | |
| US3454485A (en) | Oxygen sensor with scavenger means | |
| US4522690A (en) | Electrochemical sensing of carbon monoxide | |
| US3380905A (en) | Electrolytic sensor with anodic depolarization | |
| US10809223B2 (en) | Sensor | |
| EP0141282B1 (en) | Method of and system for real time differential pulse detection | |
| MXPA03000382A (en) | Electrochemical method for measuring chemical reaction rates. | |
| EP3227671A1 (en) | Contaminant detection device and method | |
| US4367133A (en) | Electrochemical gas analyzer | |
| US7208071B2 (en) | Amperometric sensor for low level dissolved oxygen with self-depleting sensor design | |
| EP0064337A1 (en) | Carbon dioxide measurement | |
| JP6275744B2 (en) | Electrochemical sensor for detecting nitrous oxide | |
| JPH06508432A (en) | Electrical analysis of liquids and detection elements used for it | |
| WO1999001757A1 (en) | Electrochemical sensor for the detection of hydrogen cyanide and method of use thereof | |
| KR100629683B1 (en) | Oxygen permeability measuring device of film and oxygen permeability measuring method using same | |
| JP2005530994A (en) | Electrochemical gas detection apparatus and method including a permeable membrane and an aqueous electrolyte | |
| US20070227908A1 (en) | Electrochemical cell sensor | |
| EP0103588A1 (en) | Device for determining hydrogen flux | |
| EP0418886B1 (en) | Apparatus and method for minimizing the effects of an electrolyte's dissolved oxygen content in low range oxygen analyzers | |
| JPH0755764A (en) | Constant potential electrolysis type acidic gas sensor | |
| CA2593815A1 (en) | Amperometric sensor comprising counter electrode isolated from liquid electrolyte | |
| US4370206A (en) | Method of operating an electrochemical gas measuring system | |
| US8496795B2 (en) | Electrochemical gas sensor with at least one punctiform measuring electrode | |
| JP2001289816A (en) | Constant potential electrolytic gas sensor |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| A201 | Request for examination | ||
| PA0109 | Patent application | St.27 status event code:A-0-1-A10-A12-nap-PA0109 | |
| PA0201 | Request for examination | St.27 status event code:A-1-2-D10-D11-exm-PA0201 | |
| P11-X000 | Amendment of application requested | St.27 status event code:A-2-2-P10-P11-nap-X000 | |
| P13-X000 | Application amended | St.27 status event code:A-2-2-P10-P13-nap-X000 | |
| R15-X000 | Change to inventor requested | St.27 status event code:A-3-3-R10-R15-oth-X000 | |
| R16-X000 | Change to inventor recorded | St.27 status event code:A-3-3-R10-R16-oth-X000 | |
| PN2301 | Change of applicant | St.27 status event code:A-3-3-R10-R13-asn-PN2301 St.27 status event code:A-3-3-R10-R11-asn-PN2301 | |
| R18-X000 | Changes to party contact information recorded | St.27 status event code:A-3-3-R10-R18-oth-X000 | |
| PG1501 | Laying open of application | St.27 status event code:A-1-1-Q10-Q12-nap-PG1501 | |
| PN2301 | Change of applicant | St.27 status event code:A-3-3-R10-R13-asn-PN2301 St.27 status event code:A-3-3-R10-R11-asn-PN2301 | |
| D13-X000 | Search requested | St.27 status event code:A-1-2-D10-D13-srh-X000 | |
| D14-X000 | Search report completed | St.27 status event code:A-1-2-D10-D14-srh-X000 | |
| R18-X000 | Changes to party contact information recorded | St.27 status event code:A-3-3-R10-R18-oth-X000 | |
| R18-X000 | Changes to party contact information recorded | St.27 status event code:A-3-3-R10-R18-oth-X000 | |
| E902 | Notification of reason for refusal | ||
| PE0902 | Notice of grounds for rejection | St.27 status event code:A-1-2-D10-D21-exm-PE0902 | |
| P11-X000 | Amendment of application requested | St.27 status event code:A-2-2-P10-P11-nap-X000 | |
| P13-X000 | Application amended | St.27 status event code:A-2-2-P10-P13-nap-X000 | |
| E902 | Notification of reason for refusal | ||
| PE0902 | Notice of grounds for rejection | St.27 status event code:A-1-2-D10-D21-exm-PE0902 | |
| E13-X000 | Pre-grant limitation requested | St.27 status event code:A-2-3-E10-E13-lim-X000 | |
| P11-X000 | Amendment of application requested | St.27 status event code:A-2-2-P10-P11-nap-X000 | |
| P13-X000 | Application amended | St.27 status event code:A-2-2-P10-P13-nap-X000 | |
| E701 | Decision to grant or registration of patent right | ||
| PE0701 | Decision of registration | St.27 status event code:A-1-2-D10-D22-exm-PE0701 | |
| GRNT | Written decision to grant | ||
| PR0701 | Registration of establishment | St.27 status event code:A-2-4-F10-F11-exm-PR0701 | |
| PR1002 | Payment of registration fee | St.27 status event code:A-2-2-U10-U11-oth-PR1002 Fee payment year number:1 | |
| PG1601 | Publication of registration | St.27 status event code:A-4-4-Q10-Q13-nap-PG1601 | |
| R18-X000 | Changes to party contact information recorded | St.27 status event code:A-5-5-R10-R18-oth-X000 | |
| LAPS | Lapse due to unpaid annual fee | ||
| PC1903 | Unpaid annual fee | St.27 status event code:A-4-4-U10-U13-oth-PC1903 Not in force date:20090923 Payment event data comment text:Termination Category : DEFAULT_OF_REGISTRATION_FEE | |
| PC1903 | Unpaid annual fee | St.27 status event code:N-4-6-H10-H13-oth-PC1903 Ip right cessation event data comment text:Termination Category : DEFAULT_OF_REGISTRATION_FEE Not in force date:20090923 | |
| P22-X000 | Classification modified | St.27 status event code:A-4-4-P10-P22-nap-X000 |