JP7673944B2 - Preventive or therapeutic agent for osteoclast proliferative disorders - Google Patents
Preventive or therapeutic agent for osteoclast proliferative disordersDownload PDFInfo
- Publication number
- JP7673944B2 JP7673944B2JP2020213717AJP2020213717AJP7673944B2JP 7673944 B2JP7673944 B2JP 7673944B2JP 2020213717 AJP2020213717 AJP 2020213717AJP 2020213717 AJP2020213717 AJP 2020213717AJP 7673944 B2JP7673944 B2JP 7673944B2
- Authority
- JP
- Japan
- Prior art keywords
- heparin
- therapeutic agent
- bone
- preventive
- molecular weight
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Active
Links
Images
Landscapes
- Pharmaceuticals Containing Other Organic And Inorganic Compounds (AREA)
Description
Translated fromJapanese本発明は、破骨細胞増殖性疾患の予防又は治療剤等に関する。The present invention relates to a preventive or therapeutic agent for osteoclast proliferative disorders.
骨は、破骨細胞と骨芽細胞がバランスを保つことで正常なリモデリングが行われる。破骨細胞は、骨を破壊(吸収)する役割を担う細胞であり、一方の骨芽細胞は、骨形成担う細胞である。両者のバランスが崩れ、破骨細胞による骨吸収が、骨芽細胞による骨形成を上回ると、骨量が減少したり、骨がもろくなり骨折しやすくなったりする。Normal bone remodeling is achieved by maintaining a balance between osteoclasts and osteoblasts. Osteoclasts are cells that break down (resorb) bone, while osteoblasts are cells that form bone. If the balance between the two is disrupted and bone resorption by osteoclasts exceeds bone formation by osteoblasts, bone mass decreases and bones become brittle and more susceptible to fracture.
このような破骨細胞の働きが優位な状態の疾患に対しては、一般的に、デノスマブ等の抗RANKL抗体やビスホスホネート製剤等による投薬治療が行われる。しかし、抗RANKL抗体やビスホスホネート製剤は、破骨細胞の活動を抑制することで骨量を増加させるものの、長期投与すると適切な骨吸収が行われなくなること等により、顎骨壊死、大腿骨骨折等の副作用が生じることが問題となっている。このような問題を解決するために、新たな治療薬の開発が行われている(特許文献1)。For such diseases in which the activity of osteoclasts is dominant, drug treatment is generally performed using anti-RANKL antibodies such as denosumab, bisphosphonate preparations, etc. However, although anti-RANKL antibodies and bisphosphonate preparations increase bone mass by suppressing the activity of osteoclasts, there is a problem in that side effects such as osteonecrosis of the jaw and femoral fractures occur due to inappropriate bone resorption when administered for a long period of time. In order to solve such problems, new therapeutic drugs are being developed (Patent Document 1).
本発明は、破骨細胞増殖性疾患の予防又は治療剤を提供することを課題とする。The objective of the present invention is to provide a preventive or therapeutic agent for osteoclast proliferative disorders.
本発明者らは、上記課題を解決すべく、鋭意検討を重ねていたところ、ヘパリンが破骨細胞への分化を抑制する作用を有することを見出した。本発明者はこれらの知見に基づいてさらに研究を進めた結果、本発明を完成させた。The inventors of the present invention conducted intensive research to solve the above problems and discovered that heparin has the effect of suppressing differentiation into osteoclasts. Based on these findings, the inventors of the present invention conducted further research and completed the present invention.
本発明は、例えば以下の項に記載の主題を包含する。
項1.
高分子ヘパリンを含む未分画ヘパリンを有効成分とする、破骨細胞増殖性疾患の予防又は治療剤。
項2.
高分子ヘパリンを含む未分画ヘパリンがヘパリンのアルカリ土類金属塩である、項1に記載の予防又は治療剤。
項3.
高分子ヘパリンを含む未分画ヘパリンがヘパリンカルシウムである、項2に記載の予防又は治療剤。
項4.
前記破骨細胞増殖性疾患が、骨巨細胞腫を含む良悪性骨軟部原発性腫瘍、がんの骨転移、骨粗鬆症、関節リウマチに伴う骨びらん、骨Paget病、SAPHO症候群、及び慢性再発性多発性骨髄炎からなる群より選択される少なくとも1種である、項1から3のいずれかに記載の予防又は治療剤。
項5.
高分子ヘパリンを含む未分画ヘパリンを含む、破骨細胞分化抑制剤。 The present invention encompasses, for example, the subject matter described in the following paragraphs.
Item 1.
A preventive or therapeutic agent for osteoclast proliferative disorders, comprising unfractionated heparin including high molecular weight heparin as an active ingredient.
Item 3.
Item 3. The preventive or therapeutic agent according to
Item 4.
Item 4. The preventive or therapeutic agent according to any one of Items 1 to 3, wherein the osteoclast proliferative disorder is at least one selected from the group consisting of benign or malignant primary bone and soft tissue tumors including giant cell tumor of bone, bone metastasis of cancer, osteoporosis, bone erosion associated with rheumatoid arthritis, Paget's disease of bone, SAPHO syndrome, and chronic recurrent multiple osteomyelitis.
Item 5.
An osteoclast differentiation inhibitor comprising unfractionated heparin including high molecular weight heparin.
破骨細胞増殖性疾患の予防又は治療剤が提供される。A preventive or therapeutic agent for osteoclast proliferative disorders is provided.
以下、本発明に包含される各実施形態について、さらに詳細に説明する。Each embodiment of the present invention will be described in more detail below.
本発明は、高分子ヘパリンを含む未分画ヘパリンを有効成分とする、破骨細胞増殖性疾患の予防又は治療剤を包含する。本明細書において、当該破骨細胞増殖性疾患の予防又は治療剤を、「本発明の予防又は治療剤」と表記することがある。The present invention includes a preventive or therapeutic agent for osteoclast proliferative disorders, which contains unfractionated heparin, including high molecular weight heparin, as an active ingredient. In this specification, the preventive or therapeutic agent for osteoclast proliferative disorders may be referred to as the "preventive or therapeutic agent of the present invention."
ヘパリンとは、ウロン酸とグルコサミンの繰り返し構造を有する酸性ムコ多糖類である。
ヘパリンとしては、特に限定されないが、天然物から精製したものや商業的に入手可能なものなどが例示される。ヘパリンとしては、例えば、健康な豚、牛等の肝、肺、腸粘膜等から分離したもの等が挙げられる。 Heparin is an acidic mucopolysaccharide having a repeating structure of uronic acid and glucosamine.
Examples of heparin include, but are not limited to, those purified from natural products, commercially available products, etc. Examples of heparin include those isolated from the liver, lung, intestinal mucosa, etc. of healthy pigs, cows, etc.
本明細書において、「未分画ヘパリン」とは、分子量が、例えば、6000~20000程度の広い分子量分布を有するヘパリンを意味する。分子量の上限又は下限は、例えば、7000、8000、9000、10000、12000、14000、16000、又は18000程度であってもよい。より具体的には、7000~18000程度であってもよい。未分画ヘパリンとしては、酵素処理や化学的処理により低分子化されていないヘパリン等が例示される。In this specification, "unfractionated heparin" means heparin having a wide molecular weight distribution, for example, from about 6,000 to 20,000. The upper or lower limit of the molecular weight may be, for example, about 7,000, 8,000, 9,000, 10,000, 12,000, 14,000, 16,000, or 18,000. More specifically, it may be about 7,000 to 18,000. Examples of unfractionated heparin include heparin that has not been degraded by enzymatic or chemical treatment.
本発明に用いられる未分画ヘパリンは、数平均分子量が、例えば、6000~20000程度のヘパリンが挙げられる。数平均分子量の上限又は下限は、例えば、7000、8000、9000、10000、12000、14000、16000、又は18000程度であってもよい。より具体的には、7000~18000程度であってもよい。The unfractionated heparin used in the present invention may have a number average molecular weight of, for example, about 6,000 to 20,000. The upper or lower limit of the number average molecular weight may be, for example, about 7,000, 8,000, 9,000, 10,000, 12,000, 14,000, 16,000, or 18,000. More specifically, it may be about 7,000 to 18,000.
本明細書において、「高分子ヘパリン」とは、分子量が、例えば、6000以上程度のヘパリンを意味する。分子量の上限又は下限は、例えば、7000、8000、9000、10000、12000、14000、16000、18000、又は20000程度であってもよい。In this specification, "high molecular weight heparin" means heparin having a molecular weight of, for example, about 6000 or more. The upper or lower limit of the molecular weight may be, for example, about 7000, 8000, 9000, 10000, 12000, 14000, 16000, 18000, or 20000.
本発明に用いられる高分子ヘパリンを含む未分画ヘパリンとしては、例えば、分子量が、6000以上程度の高分子ヘパリンを含み、分子量が、例えば、6000~20000程度の未分画ヘパリン等が挙げられる。The unfractionated heparin containing high molecular weight heparin used in the present invention includes, for example, unfractionated heparin containing high molecular weight heparin having a molecular weight of about 6,000 or more, for example, about 6,000 to 20,000.
本明細書において、「ヘパリン」は、ヘパリンの塩をも包含する。
本発明に用いられる高分子ヘパリンを含む未分画ヘパリンは、例えば、塩の形態であってもよい。具体的には、アルカリ金属塩、アルカリ土類金属塩などが例示される。アルカリ金属塩としては、例えば、ヘパリンナトリウム等が挙げられ、アルカリ土類金属塩としては、例えば、ヘパリンカルシウム等が挙げられる。中でも、ヘパリンのアルカリ土類金属塩が好ましく、とりわけヘパリンカルシウムが特に好ましい。
なお、ヘパリンは1種単独で又は2種以上を組み合わせて用いることができる。 As used herein, "heparin" also encompasses salts of heparin.
The unfractionated heparin including the high molecular weight heparin used in the present invention may be in the form of a salt, for example. Specific examples include alkali metal salts and alkaline earth metal salts. Examples of the alkali metal salts include sodium heparin, and examples of the alkaline earth metal salts include calcium heparin. Among these, alkaline earth metal salts of heparin are preferred, and calcium heparin is particularly preferred.
In addition, heparin can be used alone or in combination of two or more types.
本発明の予防又は治療剤中、高分子ヘパリンを含む未分画ヘパリンの含有量は、特に限定されず、100質量%を限度として適宜設定することができる。The content of unfractionated heparin, including high molecular weight heparin, in the preventive or therapeutic agent of the present invention is not particularly limited and can be set appropriately up to a maximum of 100% by mass.
本発明の予防又は治療剤は、上述した有効成分を含み、さらに他の成分を含むことができる。当該他の成分としては、薬学的に許容される基剤、担体、及び/又は添加剤(例えば溶剤、分散剤、乳化剤、緩衝剤、安定剤、賦形剤、結合剤、崩壊剤、滑沢剤、抗酸化剤、保存剤、コーティング剤、着色料、胃粘膜保護剤などのその他の薬剤等)等が例示される。The preventive or therapeutic agent of the present invention contains the above-mentioned active ingredient, and may further contain other ingredients. Examples of such other ingredients include pharma-ceutically acceptable bases, carriers, and/or additives (e.g., solvents, dispersants, emulsifiers, buffers, stabilizers, excipients, binders, disintegrants, lubricants, antioxidants, preservatives, coating agents, colorants, other drugs such as gastric mucosa protectants, etc.).
本発明の予防又は治療剤の形態は、特に限定されず、錠剤、丸剤、カプセル剤、散剤、細粒剤、顆粒剤、液剤、トローチ剤、ゼリー剤、注射剤、硬膏剤、エキス剤、坐剤、懸濁剤、チンキ剤、軟膏剤、パップ剤、点鼻剤、吸入剤、リニメント剤、ローション剤、エアゾール剤等が例示される。中でも注射剤が好ましい。The form of the preventive or therapeutic agent of the present invention is not particularly limited, and examples include tablets, pills, capsules, powders, fine granules, granules, liquids, troches, jellies, injections, plasters, extracts, suppositories, suspensions, tinctures, ointments, poultices, nasal drops, inhalants, liniments, lotions, aerosols, etc. Among these, injections are preferred.
本発明の予防又は治療剤は、上述した有効成分と、必要に応じて他の成分とを組み合わせて常法により調製することができる。The preventive or therapeutic agent of the present invention can be prepared by a conventional method by combining the above-mentioned active ingredient and, if necessary, other ingredients.
破骨細胞増殖性疾患としては、骨巨細胞腫を含む良悪性骨軟部原発性腫瘍、がんの骨転移、骨粗鬆症、関節リウマチに伴う骨びらん、骨Paget病、SAPHO症候群、慢性再発性多発性骨髄炎等が例示される。Examples of osteoclast proliferative disorders include benign and malignant primary bone and soft tissue tumors including giant cell tumor of bone, bone metastasis from cancer, osteoporosis, bone erosion associated with rheumatoid arthritis, Paget's disease of bone, SAPHO syndrome, and chronic recurrent multiple osteomyelitis.
本発明の予防又は治療剤は、破骨細胞への分化を抑制することができるため、骨巨細胞腫を含む良悪性骨軟部原発性腫瘍、がんの骨転移、骨粗鬆症、関節リウマチに伴う骨びらん、骨Paget病、SAPHO症候群、慢性再発性多発性骨髄炎等の破骨細胞増殖性疾患の予防又は治療のために用いることができる。破骨細胞増殖性疾患は、1種単独であってもよく又は2種以上の組み合わせてであってもよい。The preventive or therapeutic agent of the present invention can suppress differentiation into osteoclasts, and therefore can be used to prevent or treat osteoclast proliferative diseases such as benign and malignant primary bone and soft tissue tumors including giant cell tumor of bone, bone metastasis of cancer, osteoporosis, bone erosion associated with rheumatoid arthritis, Paget's disease of bone, SAPHO syndrome, and chronic recurrent multiple osteomyelitis. The osteoclast proliferative disease may be one type alone or two or more types in combination.
本発明の予防又は治療剤を投与される対象としては、哺乳動物が好ましい。ヒトのみならず、非ヒト哺乳動物であってもよい。対象となるヒトとしては、例えば、骨巨細胞腫を含む良悪性骨軟部原発性腫瘍患者、骨巨細胞腫を含む良悪性骨軟部原発性腫瘍が疑われるヒト;がんの骨転移が認められるヒト、がんの骨転移が疑われるヒト;癌患者;原発性骨粗鬆症患者、薬剤、生活習慣病、リウマチ、腎症等に由来する続発性骨粗鬆症患者、骨粗鬆症が疑われるヒト;関節リウマチ患者、関節リウマチに伴う骨びらんが認められるヒト、関節リウマチが疑われるヒト;骨Paget病患者、骨Paget病が疑われるヒト;SAPHO症候群患者、SAPHO症候群が疑われるヒト;慢性再発性多発性骨髄炎患者、慢性再発性多発性骨髄炎患者が疑われるヒト等が挙げられる。
また、非ヒト哺乳動物としては、例えばペット、家畜、実験動物等として飼育される哺乳動物などが例示される。このような非ヒト哺乳動物としては、例えば、イヌ、ネコ、サル、ウシ、ウマ、ヒツジ、ヤギ、ブタ、ウサギ、マウス、ラット、ラクダ、リャマ等が挙げられる。 The subject to which the preventive or therapeutic agent of the present invention is administered is preferably a mammal. It may be not only a human but also a non-human mammal. Examples of the subject include patients with benign or malignant primary bone and soft tissue tumors including giant cell tumor of bone, and humans suspected of benign or malignant primary bone and soft tissue tumors including giant cell tumor of bone; humans with bone metastasis of cancer, and humans suspected of bone metastasis of cancer; cancer patients; patients with primary osteoporosis, patients with secondary osteoporosis caused by drugs, lifestyle-related diseases, rheumatism, nephropathy, and the like, and humans suspected of osteoporosis; patients with rheumatoid arthritis, humans with bone erosion associated with rheumatoid arthritis, and humans suspected of rheumatoid arthritis; patients with Paget's disease of bone, and humans suspected of Paget's disease of bone; patients with SAPHO syndrome, and humans suspected of SAPHO syndrome; patients with chronic recurrent multiple osteomyelitis, and humans suspected of chronic recurrent multiple osteomyelitis.
Examples of non-human mammals include mammals kept as pets, livestock, laboratory animals, etc. Examples of such non-human mammals include dogs, cats, monkeys, cows, horses, sheep, goats, pigs, rabbits, mice, rats, camels, llamas, etc.
投与方法としては、例えば、経口投与、非経口(例えば静脈、動脈、筋肉、皮下、腹腔、直腸、経皮、局所など)投与等が挙げられる。中でも、非経口投与が好ましく、皮下投与、静脈内注射がより好ましい。Examples of administration methods include oral administration and parenteral (e.g., intravenous, arterial, intramuscular, subcutaneous, peritoneal, rectal, transdermal, topical, etc.) administration. Among these, parenteral administration is preferred, and subcutaneous administration and intravenous injection are more preferred.
本発明の予防又は治療剤の投与(摂取)量は、特に限定されず、投与する対象の年齢、性別、症状の程度、投与方法等により決定される。例えば、有効成分の投与量として、1日あたり50~1000IU/kg体重程度とすることができる。The dosage (intake) of the preventive or therapeutic agent of the present invention is not particularly limited and is determined based on the age, sex, severity of symptoms, administration method, etc. of the subject to be administered. For example, the dosage of the active ingredient can be about 50 to 1000 IU/kg body weight per day.
本発明の予防又は治療剤は、1日1回投与するものであってもよく、1日2~3回に分けて投与するものであってもよい。The preventive or therapeutic agent of the present invention may be administered once a day, or may be administered in divided doses two to three times a day.
また、本発明は、高分子ヘパリンを含む未分画ヘパリンを含む、破骨細胞分化抑制剤をも包含する。なお、破骨細胞分化抑制作用とは、前駆細胞の破骨細胞への分化を抑制する作用を意味する。前駆細胞の破骨細胞への分化は、後述する実施例に示すように、破骨細胞をTRAP染色することにより確認することができる。The present invention also includes an osteoclast differentiation inhibitor that contains unfractionated heparin, including high molecular weight heparin. The osteoclast differentiation inhibitory effect means the effect of inhibiting the differentiation of precursor cells into osteoclasts. The differentiation of precursor cells into osteoclasts can be confirmed by TRAP staining of osteoclasts, as shown in the examples described below.
なお、本明細書において「含む」とは、「本質的にからなる」と、「からなる」をも包含する(The term "comprising" includes "consisting essentially of” and "consisting of.")。また、本発明は、本明細書に説明した構成要件を任意の組み合わせを全て包含する。In this specification, the term "comprising" includes "consisting essentially of" and "consisting of." In addition, the present invention includes any combination of the constituent elements described in this specification.
また、上述した本発明の各実施形態について説明した各種特性(性質、構造、機能等)は、本発明に包含される主題を特定するにあたり、どのように組み合わせられてもよい。すなわち、本発明には、本明細書に記載される組み合わせ可能な各特性のあらゆる組み合わせからなる主題が全て包含される。Furthermore, the various characteristics (properties, structures, functions, etc.) described for each embodiment of the present invention above may be combined in any way to identify the subject matter encompassed by the present invention. In other words, the present invention encompasses all subject matter consisting of any combination of the combinable characteristics described in this specification.
本発明の内容を以下の実験例を用いて具体的に説明する。しかし、本発明はこれらに何ら限定されるものではない。下記において、特に言及する場合を除いて、実験は大気圧及び常温条件下で行っている。また特に言及する場合を除いて、「%」は「体積%」を意味する。The present invention will be specifically explained using the following experimental examples. However, the present invention is in no way limited to these. In the following, unless otherwise specified, the experiments were performed under atmospheric pressure and room temperature conditions. Also, unless otherwise specified, "%" means "volume %."
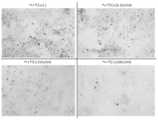
(1)in vitroにおける破骨細胞分化抑制実験
マウスの破骨細胞前駆細胞様細胞株RAW264.7を96穴プレートに5x103 cells/wellの濃度で播種し、α-Minimum Essential Medium (a-MEM)、10%ウシ胎仔血清(FBS)を使用して、破骨細胞への分化因子としてマウス可溶性RANKL(100ng/ml)を添加し、37℃、5%CO2気相下で培養した。同時にヘパリンカルシウム分子量6000~20000を各濃度(0, 0.1, 0.5, 1, 10 IU/ml)にて加えた。培養3日目に同じ条件のヘパリンカルシウムを含む溶液に交換し、培養7日目にTRAP染色を実施した。TRAP染色陽性細胞を分化した破骨細胞と評価して、染色性を写真で評価した。また1wellあたりの破骨細胞数を計測した。ヘパリン各濃度における4wellの平均破骨細胞数を計測した。結果を図1及び2に示す。(1) In vitro osteoclast differentiation inhibition experiment Mouse osteoclast precursor-like cell line RAW264.7 was seeded at a concentration of5x103 cells/well in a 96-well plate, and cultured in α-Minimum Essential Medium (a-MEM) with 10% fetal bovine serum (FBS) at 37°C under 5%CO2 gas phase with the addition of mouse soluble RANKL (100ng/ml) as a differentiation factor for osteoclasts. At the same time, heparin calcium molecular weight 6000-20000 was added at various concentrations (0, 0.1, 0.5, 1, 10 IU/ml). On the third day of culture, the solution was replaced with that containing heparin calcium under the same conditions, and TRAP staining was performed on the seventh day of culture. TRAP-stained positive cells were evaluated as differentiated osteoclasts, and staining was evaluated by photograph. The number of osteoclasts per well was also counted. The average number of osteoclasts in 4 wells at each heparin concentration was counted. The results are shown in Figures 1 and 2.
ヘパリン濃度に比例して、TRAP染色陽性細胞(破骨細胞)が減少したことが確認された。よって、ヘパリンを添加することによって、破骨細胞への分化が抑制されることが分かった。It was confirmed that the number of TRAP-positive cells (osteoclasts) decreased in proportion to the heparin concentration. This indicates that the addition of heparin inhibits differentiation into osteoclasts.
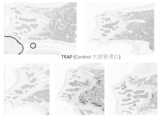
(2) in vivoにおけるマウスにおける破骨細胞の発現抑制実験
12週齢のC57BL/6マウスにビタミンD欠乏食を20週齢時まで8週間投与し、20週齢時から3群に分け、分子量6000~20000のヘパリンカルシウム0(5匹), 100(4匹), 500(4匹)IU/kgを皮下注で1日1回の頻度で32週齢時まで毎日投与し、その時点で大腿骨を摘出した。ビタミンD欠乏食は32週齢時まで継続して与えた。ホルマリン固定後、TRAP染色を実施して、大腿骨遠位骨幹端から骨端までに発現する破骨細胞を評価した。結果を図3に示す。(2) In vivo experiment to inhibit osteoclast expression in mice
C57BL/6 mice aged 12 weeks were fed a vitamin D deficient diet for 8 weeks until they reached 20 weeks of age. From 20 weeks of age, they were divided into three groups and administered heparin calcium with molecular weights of 6,000-20,000 at 0 (5 mice), 100 (4 mice), or 500 (4 mice) IU/kg subcutaneously once a day until they reached 32 weeks of age, at which point their femurs were removed. The vitamin D deficient diet was continued until the mice reached 32 weeks of age. After formalin fixation, TRAP staining was performed to evaluate osteoclasts expressed in the distal femoral metaphysis to epiphysis. The results are shown in Figure 3.
ヘパリン濃度に比例して、TRAP染色陽性細胞(破骨細胞)が減少したことが確認された。よって、ヘパリンを添加することによって、破骨細胞への分化が抑制されることが分かった。It was confirmed that the number of TRAP-positive cells (osteoclasts) decreased in proportion to the heparin concentration. This indicates that the addition of heparin inhibits differentiation into osteoclasts.
(3) 骨巨細胞腫局所再発+肺転移患者に対する、ヘパリンカルシウム投与の治療効果
骨巨細胞腫局所再発+肺転移患者に対して、分子量6000~20000のヘパリンカルシウム1日40000IUを皮下注射で投与したところ病変は著明に縮小した。しかしヘパリンカルシウムを経口ワーファリンに変更したところ病変は増大し、その後、分子量6000~20000のヘパリンカルシウム1日40000IU皮下注射に戻したところ再度腫瘍は縮小した。ヘパリンカルシウムを1週に2回、1回20000IU皮下注射投与まで減量したが、腫瘍は縮小したままで維持されている。ヘパリンカルシウム投与前(左上)、ヘパリンカルシウム投与後(右上)、ワーファリン変更後(左下)、及び再度ヘパリンカルシウム投与後(右下)の腫瘍写真を図4に示す。(3) Therapeutic effect of heparin calcium administration in patients with local recurrence and lung metastasis of giant cell tumor of bone When a patient with local recurrence and lung metastasis of giant cell tumor of bone was administered 40,000 IU of heparin calcium with a molecular weight of 6,000 to 20,000 by subcutaneous injection per day, the lesions significantly shrank. However, when heparin calcium was changed to oral warfarin, the lesions increased. After that, when heparin calcium with a molecular weight of 6,000 to 20,000 was administered by subcutaneous injection per day at 40,000 IU, the tumor shrank again. The dose of heparin calcium was reduced to 20,000 IU subcutaneous injection twice a week, but the tumor remained shrunken. Photographs of the tumor before heparin calcium administration (upper left), after heparin calcium administration (upper right), after changing warfarin (lower left), and after heparin calcium administration again (lower right) are shown in Figure 4.
Claims (3)
Translated fromJapanesePriority Applications (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| JP2020213717AJP7673944B2 (en) | 2020-12-23 | 2020-12-23 | Preventive or therapeutic agent for osteoclast proliferative disorders |
Applications Claiming Priority (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| JP2020213717AJP7673944B2 (en) | 2020-12-23 | 2020-12-23 | Preventive or therapeutic agent for osteoclast proliferative disorders |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| JP2022099744A JP2022099744A (en) | 2022-07-05 |
| JP7673944B2true JP7673944B2 (en) | 2025-05-09 |
Family
ID=82269751
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2020213717AActiveJP7673944B2 (en) | 2020-12-23 | 2020-12-23 | Preventive or therapeutic agent for osteoclast proliferative disorders |
Country Status (1)
| Country | Link |
|---|---|
| JP (1) | JP7673944B2 (en) |
Citations (9)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2003534265A (en) | 2000-05-26 | 2003-11-18 | イタルファルマコ ソシエタ ペル アチオニ | Sustained release pharmaceutical composition for parenterally administering a biologically active hydrophilic compound |
| JP2004210715A (en) | 2002-12-27 | 2004-07-29 | Seikagaku Kogyo Co Ltd | Inhibitor of formation of osteoclast |
| US20060083711A1 (en) | 2004-04-15 | 2006-04-20 | Massachusetts Institute Of Technology | Methods and products related to the intracellular delivery of polysaccharides |
| JP2008543870A (en) | 2005-06-17 | 2008-12-04 | ザ ボード オブ リージェンツ オブ ザ ユニバーシティー オブ テキサス システム | Inhibition of osteolytic lesions by Src kinase inhibitors |
| US20120295871A1 (en) | 2011-05-19 | 2012-11-22 | Oliva Eugene J | Heparin-based compositions and methods for the inhibition of metastasis |
| CN103720658A (en) | 2014-01-07 | 2014-04-16 | 中国药科大学 | Heparin-modified adriamycin liposome preparation and preparation method thereof |
| WO2015046388A1 (en) | 2013-09-25 | 2015-04-02 | 日本ケミファ株式会社 | Drug for preventing, treating or preventing metastasis of giant cell tumor that occurs in bone or soft parts, chondrosarcoma, or osteosarcoma, local injection for arterial embolization, and artificial bone |
| CN110124053A (en) | 2019-06-13 | 2019-08-16 | 扬州大学 | A kind of synthetic method and its drug of heparin Allan sodium phosphate conjugate |
| CN111001011A (en) | 2019-11-05 | 2020-04-14 | 中国药科大学 | Low-molecular-weight heparin-modified bone targeting liposome and preparation method thereof |
- 2020
- 2020-12-23JPJP2020213717Apatent/JP7673944B2/enactiveActive
Patent Citations (9)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2003534265A (en) | 2000-05-26 | 2003-11-18 | イタルファルマコ ソシエタ ペル アチオニ | Sustained release pharmaceutical composition for parenterally administering a biologically active hydrophilic compound |
| JP2004210715A (en) | 2002-12-27 | 2004-07-29 | Seikagaku Kogyo Co Ltd | Inhibitor of formation of osteoclast |
| US20060083711A1 (en) | 2004-04-15 | 2006-04-20 | Massachusetts Institute Of Technology | Methods and products related to the intracellular delivery of polysaccharides |
| JP2008543870A (en) | 2005-06-17 | 2008-12-04 | ザ ボード オブ リージェンツ オブ ザ ユニバーシティー オブ テキサス システム | Inhibition of osteolytic lesions by Src kinase inhibitors |
| US20120295871A1 (en) | 2011-05-19 | 2012-11-22 | Oliva Eugene J | Heparin-based compositions and methods for the inhibition of metastasis |
| WO2015046388A1 (en) | 2013-09-25 | 2015-04-02 | 日本ケミファ株式会社 | Drug for preventing, treating or preventing metastasis of giant cell tumor that occurs in bone or soft parts, chondrosarcoma, or osteosarcoma, local injection for arterial embolization, and artificial bone |
| CN103720658A (en) | 2014-01-07 | 2014-04-16 | 中国药科大学 | Heparin-modified adriamycin liposome preparation and preparation method thereof |
| CN110124053A (en) | 2019-06-13 | 2019-08-16 | 扬州大学 | A kind of synthetic method and its drug of heparin Allan sodium phosphate conjugate |
| CN111001011A (en) | 2019-11-05 | 2020-04-14 | 中国药科大学 | Low-molecular-weight heparin-modified bone targeting liposome and preparation method thereof |
Non-Patent Citations (7)
| Title |
|---|
| Annals of Oncology,2015年,Vol. 26,pp. 2149-2154,doi:10.1093/annonc/mdv307 |
| Clin Exp Metastasis,2008年,Vol. 25,pp. 903-911,DOI 10.1007/s10585-008-9212-0 |
| International Journal of Biological Macromolecules,2020年08月11日,Vol. 164,pp. 2583-2597,<URL: https://doi.org/10.1016/j.ijbiomac.2020.08.068> |
| Joumal of Hard Tissue Biology,2020年07月17日,Vol. 29, No. 3,pp. 137-146 |
| Journal of Oral and Maxillofacial Surgery,2014年,Vol. 72, No. 12,pp. 2469-2484,DOI: 10.1016/j.joms.2014.06.456 |
| Molecules,2018年,Vol. 23, No. 3121,pp. 1-15,doi:10.3390/molecules23123121 |
| Pathol. Oncol. Res.,2019年,Vol. 25,pp. 409-419,<URL: https://doi.org/10.1007/s12253-017-0362-8> |
Also Published As
| Publication number | Publication date |
|---|---|
| JP2022099744A (en) | 2022-07-05 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| EP3429614B1 (en) | Method of treating triple negative breast cancer | |
| RU2671488C2 (en) | Pharmaceutical combination comprising metformin and dihydroquercetin and its use for treatment of cancer | |
| EP0567613B1 (en) | Compositions for therapy and prevention of cancer | |
| US20110077198A1 (en) | Compositions and methods for inhibiting the activation of dsrna-dependent protein kinase and tumor growth inhibition | |
| JP2020055884A (en) | Glufosfamide combination therapies for cancer | |
| EP3042669B1 (en) | Antitumor agent and antitumor effect enhancer | |
| EP1484058A2 (en) | Pharmaceutical composition for inhibiting altering gene expression and for inducing differentiation in non-malignant cells | |
| JP2023533485A (en) | How to treat severe pulmonary hypertension | |
| EP4424316A1 (en) | Antitumor pharmaceutical composition comprising azvudine and chemotherapeutic agent | |
| US20220339233A1 (en) | Compositions and methods for preventing recurrence of cancer | |
| RU2730998C2 (en) | Phorbol ester compositions and methods of using them for treating or reducing duration of cytopenia | |
| US20250134953A1 (en) | Compositions for treating autoimmune arthritis | |
| WO2019016928A1 (en) | New anti-malignant tumor agent based on specificity of cancer cell metabolism | |
| JP7673944B2 (en) | Preventive or therapeutic agent for osteoclast proliferative disorders | |
| CN113577070B (en) | A combined pharmaceutical composition for treating acute myeloid leukemia and its application | |
| CN115551501A (en) | Application of LSD1 inhibitor | |
| JP2008540585A (en) | Compounds for treating metastatic melanoma and other cancers, compositions containing such compounds, and methods of treatment | |
| EP1506778A1 (en) | TGF-a EXPRESSION INHIBITORS | |
| KR20090125246A (en) | Prevention and / or treatment of systemic erythematodes | |
| JP5792322B2 (en) | Vitamin D and metformin-containing pharmaceutical composition | |
| CN109528731B (en) | Pharmaceutical composition with synergistic effect for treating multiple myeloma and application thereof | |
| KR20170045751A (en) | Pharmaceutical composition for treatment or palliation of elderly or end-stage cancer patient | |
| KR101828997B1 (en) | A pharmaceutical composition for prevention or treatment of cancer and use thereof | |
| KR101824205B1 (en) | A pharmaceutical composition for prevention or treatment of cancer and use thereof | |
| EP3969118A1 (en) | Combination therapy for proliferative conditions |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| A621 | Written request for application examination | Free format text:JAPANESE INTERMEDIATE CODE: A621 Effective date:20231020 | |
| A131 | Notification of reasons for refusal | Free format text:JAPANESE INTERMEDIATE CODE: A131 Effective date:20240716 | |
| A977 | Report on retrieval | Free format text:JAPANESE INTERMEDIATE CODE: A971007 Effective date:20240724 | |
| A601 | Written request for extension of time | Free format text:JAPANESE INTERMEDIATE CODE: A601 Effective date:20240826 | |
| A521 | Request for written amendment filed | Free format text:JAPANESE INTERMEDIATE CODE: A523 Effective date:20241108 | |
| A131 | Notification of reasons for refusal | Free format text:JAPANESE INTERMEDIATE CODE: A131 Effective date:20250121 | |
| A521 | Request for written amendment filed | Free format text:JAPANESE INTERMEDIATE CODE: A523 Effective date:20250318 | |
| TRDD | Decision of grant or rejection written | ||
| A521 | Request for written amendment filed | Free format text:JAPANESE INTERMEDIATE CODE: A821 Effective date:20250318 | |
| A01 | Written decision to grant a patent or to grant a registration (utility model) | Free format text:JAPANESE INTERMEDIATE CODE: A01 Effective date:20250408 | |
| A61 | First payment of annual fees (during grant procedure) | Free format text:JAPANESE INTERMEDIATE CODE: A61 Effective date:20250417 | |
| R150 | Certificate of patent or registration of utility model | Ref document number:7673944 Country of ref document:JP Free format text:JAPANESE INTERMEDIATE CODE: R150 |