JP5499509B2 - Manufacturing method of needle-like object - Google Patents
Manufacturing method of needle-like objectDownload PDFInfo
- Publication number
- JP5499509B2 JP5499509B2JP2009089938AJP2009089938AJP5499509B2JP 5499509 B2JP5499509 B2JP 5499509B2JP 2009089938 AJP2009089938 AJP 2009089938AJP 2009089938 AJP2009089938 AJP 2009089938AJP 5499509 B2JP5499509 B2JP 5499509B2
- Authority
- JP
- Japan
- Prior art keywords
- needle
- polysaccharide
- water
- desalting
- producing
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Expired - Fee Related
Links
Images
Landscapes
- Medicinal Preparation (AREA)
- Media Introduction/Drainage Providing Device (AREA)
Description
Translated fromJapanese本発明は、分子構造内にカルボキシル基やアミノ基を持つ天然物由来の多糖類を含む針状物体、およびその製造方法に関するものであり、成形後の吸湿による変形や変質を抑制することが可能な針状物体、およびその製造方法に関する。The present invention relates to a needle-like object containing a polysaccharide derived from a natural product having a carboxyl group or an amino group in the molecular structure, and a method for producing the same, and can suppress deformation and alteration due to moisture absorption after molding. And a manufacturing method thereof.
針状物体とは痛みを伴わない微細な注射針であり、これまでの経皮吸収では難しかった薬剤の投与も可能になり、治療の幅が大きく広がるため医療分野等において非常に注目されている。また、針の先端部が毛細血管や神経まで到達しないように設計することで、使用時に出血や痛みを伴わないようにすることが提案されている(特許文献1)。A needle-like object is a fine needle that does not cause pain, and can be used to administer drugs that have been difficult to absorb by transdermal absorption so far. . In addition, it has been proposed to prevent bleeding and pain during use by designing the tip of the needle so as not to reach capillaries and nerves (Patent Document 1).
剛性に優れる金属やシリコン等、また靭性に優れる合成高分子等を用いた針状物体が開発されているが、表皮に直接穿刺するため、万が一皮膚内に残存した場合には危険性を伴う。そのため皮膚内に残存した際に、速やかに分解されるよう、針や基材の部分には生分解性材料の利用が好まれる。Needle-like objects using metals, silicon, etc., which are excellent in rigidity, and synthetic polymers, etc., which are excellent in toughness have been developed. However, since they puncture directly into the epidermis, there is a danger if they remain in the skin. For this reason, the use of biodegradable materials is preferred for the needle and base material parts so that they can be quickly decomposed when they remain in the skin.
分子構造内にカルボキシル基やアミノ基を有する天然物由来の多糖類は、地球上に豊富に存在する生分解性材料であり、酵素や生化学、薬学、健康食品、医療材料、化粧品など多岐に渡って研究されている。水に可溶であり、乾燥させるだけで容易に成形することが可能である。Polysaccharides derived from natural products that have a carboxyl group or amino group in the molecular structure are biodegradable materials that exist abundantly on the earth, and are widely used in enzymes, biochemistry, pharmaceuticals, health foods, medical materials, cosmetics, etc. It has been studied across the board. It is soluble in water and can be easily molded simply by drying.
しかしながら、分子構造内にカルボキシル基やアミノ基を有する天然物由来の多糖類はもともと水溶性であるため、針状物体を成形した際に、吸湿による変形、長期保存時の劣化、水性薬剤が塗布出来ない、におい等の問題があった。However, since polysaccharides derived from natural products having a carboxyl group or amino group in the molecular structure are inherently water-soluble, deformation due to moisture absorption, deterioration during long-term storage, and application of aqueous drugs are applied when molding needle-like objects. There were problems such as odors that could not be made.
分子構造内にカルボキシル基やアミノ基の塩を有する多糖類を乾燥固化した針状物体は、水に溶解または膨潤しやすく、大気中でも吸湿してしまい、変形や変質の原因となってしまう。特許文献2に記載の技術のように、キトサンフィルムを水含有親水性有機溶剤に浸漬することで有機酸塩を除去する方法が知られている。しかし、キトサンのみならず分子構造内にカルボキシル基やアミノ基を有する多糖類成形体を脱塩する際には、劣化やにおいの発生を抑えて水性薬剤塗布を可能とするためには、90%以上の脱塩が必要であるため、特許文献2に記載の技術では力不足であった。A needle-like object obtained by drying and solidifying a polysaccharide having a carboxyl group or amino group salt in the molecular structure easily dissolves or swells in water, absorbs moisture in the air, and causes deformation or alteration. As in the technique described in Patent Document 2, a method for removing an organic acid salt by immersing a chitosan film in a water-containing hydrophilic organic solvent is known. However, when desalting not only chitosan but also a polysaccharide molded product having a carboxyl group or amino group in the molecular structure, 90% Since the above desalting is necessary, the technique described in Patent Document 2 is insufficient.
本発明は上記の問題点に鑑みてなされたものであり、耐水性が良好であり、分子構造内にカルボキシル基やアミノ基を有する多糖類を含む針状物体、およびその製造方法を提供することを目的とする。The present invention has been made in view of the above-mentioned problems, and provides a needle-like object having a good water resistance and containing a polysaccharide having a carboxyl group or an amino group in the molecular structure, and a method for producing the needle-like object. With the goal.
上記の課題を解決するため本発明の請求項1においては、微細な針で皮膚を穿刺する針状物体の製造方法であって、前記針状物体は、基板と、前記基板上に複数配列された針状部分と、を備え、分子構造内にカルボキシル基および/またはアミノ基を持つ多糖類を、水を含む溶媒に溶解させて多糖類溶液を作製する多糖類溶解段階と、前記針状部分と同形状の凹部を有する凹型に前記多糖類溶液を流し込み、さらに乾燥固化する成形段階と、前記多糖類溶液が固化した多糖類成形体を前記凹型から剥離する剥離段階と、剥離した多糖類成形体を水含有親水性有機溶媒で脱塩する脱塩段階と、を有することを特徴とする針状物体の製造方法、としたものである。
In order to solve the above-mentioned problems, according to a first aspect of the present invention, there is provided a method of manufacturing a needle-like object for puncturing the skin with afine needle, the needle-like object being arranged in a plurality on the substrate. A polysaccharide dissolving step of preparing a polysaccharide solution by dissolving a polysaccharidehaving acarboxyl group and / or an amino group in the molecular structure in a solvent containing water, and the needle-like portion The polysaccharide solution is poured into a concave mold having the same shape as the concave shape, and further dried and solidified, and the polysaccharide molded body solidified with the polysaccharide solution is peeled off from the concave mold. And a desalting step in which the body is desalted with a water-containing hydrophilic organic solvent.
また、請求項2の発明は、微細な針で皮膚を穿刺する針状物体の製造方法であって、前記針状物体は、基板と、前記基板上に複数配列された針状部分と、を備え、分子構造内にカルボキシル基および/またはアミノ基を持つ多糖類を、水を含む溶媒に溶解させて多糖類溶液を作製する多糖類溶解段階と、所定形状の容器に所定量の前記多糖類溶液を注入する多糖類溶液注入段階と前記容器に注入された前記多糖類溶液の表面に、複数配列された針状突起を有する凸型をいったん押し当てた後に垂直に引き上げた状態で乾燥固化する成形段階と、前記多糖類溶液が固化した多糖類成形体の針状部分の先端部分を切断して、前記凸型の針状突起から分離する分離段階と、分離した多糖類成形体を水含有親水性有機溶媒で脱塩する脱塩段階と、を有することを特徴とする針状物体の製造方法、としたものである。
The invention of claim 2 is amethod of manufacturing a needle-like object for puncturing the skin with afine needle, wherein the needle-like object comprises a substrate and a plurality of needle-like portions arranged on the substrate. A polysaccharide dissolving step of preparing a polysaccharide solution by dissolving a polysaccharide having a carboxyl group and / or an amino group in the molecular structure in a solvent containing water; and a predetermined amount of the polysaccharide in a container of a predetermined shape A polysaccharide solution injecting step for injecting the solution and a convex mold having a plurality of needle-like projections once pressed against the surface of the polysaccharide solution injected into the container and then dried and solidified in a state of being pulled up vertically A forming step, a separation step of cutting the tip of the needle-shaped portion of the polysaccharide molded body in which the polysaccharide solution is solidified, and separating from the convex needle-shaped protrusion, and the separated polysaccharide molded body containing water A desalting step of desalting with a hydrophilic organic solvent. A method of manufacturing a needle-shaped object, characterized in Rukoto is obtained by a,.
また、請求項3の発明は、前記多糖類は、キトサン、アルギン酸、ヒアルロン酸、ペクチン、ヒドロキシメチルセルロースから選択されることを特徴とする請求項1または請求項2記載の針状物体の製造方法、としたものである。
The invention according to claim 3 is characterized in that thepolysaccharide is selected from chitosan, alginic acid, hyaluronic acid, pectin, and hydroxymethylcellulose. The method for producing a needle-like object according to claim 1 or 2 , It is what.
また、請求項4の発明は、前記針状物体は、生理食塩水に1時間浸漬した際の溶解性が不溶であることを特徴とする請求項1乃至3のいずれかに記載の針状物体の製造方法、としたものである。
The invention according to claim 4 is characterized in that theacicular object is insoluble when immersed in physiological saline for 1 hour. Manufacturing method .
本発明によれば、医療分野あるいは化粧品分野において有用で、天然由来の生体材料であるため人体への安全性が高く、水不溶性の多糖類針状物体を簡便な方法で安価に得ることができ、吸湿による変形や長期保存の際の劣化がなく、水性薬剤の塗布が可能で、におい等の問題を改善することができる。
INDUSTRIAL APPLICABILITY According to the present invention, it is useful in the medical field or cosmetic field, and since it is a naturally derived biomaterial, it is highly safe for the human body, and a water-insoluble polysaccharide needle can be obtained at a low cost by a simple method. In addition, there is no deformation due to moisture absorption or deterioration during long-term storage, and an aqueous drug can be applied, and problems such as odor can be improved.
以下、本発明の詳細を説明する。Details of the present invention will be described below.
本発明の針状物体は、分子構造内にカルボキシル基やアミノ基を持つ多糖類からなり、この針状物体を脱塩して耐水性を付与していることを特徴とする。分子構造内にカルボキシル基やアミノ基を持つ多糖類からなる針状物体は、穿刺時に万が一皮膚内に残存した際にも、生分解性を持つ材料であるため速やかに分解されるため、安全である。The needle-shaped object of the present invention is made of a polysaccharide having a carboxyl group or an amino group in the molecular structure, and is water-resistant by desalting the needle-shaped object. Needle-like objects consisting of polysaccharides with carboxyl groups or amino groups in the molecular structure are biodegradable even if they remain in the skin at the time of puncture. is there.
天然多糖類の種類、由来などは特に限定されず、例えばキトサン、アルギン酸、ヒアルロン酸、ペクチン、ヒドロキシメチルセルロース等を含む群より選ばれた少なくとも一つ以上の物質を含む針状物体である。The type and origin of the natural polysaccharide are not particularly limited, and for example, it is a needle-like object containing at least one substance selected from the group containing chitosan, alginic acid, hyaluronic acid, pectin, hydroxymethylcellulose and the like.
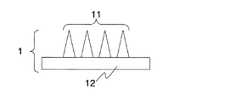
ここで、針状物体の形状について説明する。図1は針状物体1の形状を模式的に表した側面図である。平らな基板12の片面側に微小な針状部分11が複数配列されている。針状部分11の形状は、円錐形状、多角錐形状、その他の適宜の形状を選択することが可能である。針状部分11の配列は適宜選択可能で、例えば、矩形格子配列、三角格子配列、六角格子配列、同心円状の配列、1次元的な配列、その他の適宜の配列を選択すればよい。また、基板12の形状も矩形板状、円板状、楕円板状、その他の適宜の形状を選択可能である。Here, the shape of the needle-like object will be described. FIG. 1 is a side view schematically showing the shape of the needle-
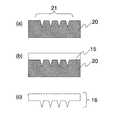
次に、本発明の針状物体の製造方法の第1実施形態を、図2を参照しながら説明する。図2(a)に示したように、凹型20は、片面に複数配列された凹部21を有している。凹型20を構成する物質は、例えば非水溶性高分子、金属、シリコン、ガラスなど、適宜のものを選択可能である。Next, a first embodiment of the method for manufacturing a needle-like object of the present invention will be described with reference to FIG. As shown in FIG. 2A, the
針状物体の針状部分の型となる凹部21の形状は、円錐形状、多角錐形状、その他の適宜の形状を選択することが可能である。また、ヒトの表皮の厚さは70μm〜200μmの範囲内にあるため、凹部21の深さは300μm以上とする。太さ200μm以下の針には痛みを感じないことが知られているので、凹部21の最大径は200μm以下とする。また、凹部21の配列は適宜選択可能で、例えば、矩形格子配列、三角格子配列、六角格子配列、同心円状の配列、1次元的な配列、その他の適宜の配列を選択すればよい。As the shape of the
前述の多糖類を水(酸性、アルカリ性、中性のいずれでもよい)を含む溶媒に、固形分濃度が20%以下になるように溶解させた多糖類溶液15を、この凹型20上に流し込む(図2(b)参照)。その後、周囲を真空引きすることにより凹部21から脱気(脱泡)を行ってもよい。多糖類溶液15の固形分濃度を20%以下とするのは、凹部21への流入時や、真空引きにより脱泡を行う際に、多糖類溶液15にある程度の流動性があったほうがよいからである。A
次に、凹型20上に多糖類溶液15を載せた状態で乾燥固化を行う。このときの乾燥温度は前記多糖類の分解温度よりも低温度に設定する。乾燥による収縮や多糖類の劣化等を考慮し、水の沸点である100度以下が好ましい。乾燥時間は設定された乾燥温度において、溶媒中の水の蒸発による質量変化がなくなる時間より適宜決定する。この乾燥中に乾燥炉内の水蒸気量を一定にするためにエアーを供給するなどしてもよい。Next, drying and solidification are performed with the
乾燥が完了すると多糖類溶液15は固化して、多糖類成形体16となるので、凹型20から剥離する(図2(c)参照)。When the drying is completed, the
本発明の脱塩工程において使用する親水性有機溶媒は特に限定はなく、例えばメチルアルコール、エチルアルコール、プロピルアルコール、ブチルアルコール、アセトン、メチルエチルケトン、酢酸メチル、酢酸エチル、ピリジン等を含む群より選ばれた少なくとも一つの物質を含むものである。The hydrophilic organic solvent used in the desalting step of the present invention is not particularly limited, and is selected from the group including, for example, methyl alcohol, ethyl alcohol, propyl alcohol, butyl alcohol, acetone, methyl ethyl ketone, methyl acetate, ethyl acetate, pyridine and the like. Including at least one substance.
また、親水性有機溶媒と水を混合して水含有親水性有機溶媒を作製する際に、親水性有機溶媒に対し水の割合が多くなってしまうと脱塩時に多糖類成形体が溶解もしくは歪んでしまう問題があるため、親水性有機溶剤に対する水の重量濃度は1〜20%以下が好ましい。In addition, when a water-containing hydrophilic organic solvent is prepared by mixing a hydrophilic organic solvent and water, if the proportion of water increases with respect to the hydrophilic organic solvent, the polysaccharide molded product will be dissolved or distorted during desalting. The weight concentration of water with respect to the hydrophilic organic solvent is preferably 1 to 20% or less.
さらに、この水含有親水性有機溶媒に酸を添加する場合、酸の種類には特に限定はなく、例えば塩酸や硫酸、硝酸等、適宜の酸を少なくとも一つ選択すればよい。Further, when an acid is added to the water-containing hydrophilic organic solvent, the type of acid is not particularly limited, and at least one appropriate acid such as hydrochloric acid, sulfuric acid, nitric acid, etc. may be selected.
または、この水含有親水性有機溶媒にアルカリを添加する場合、アルカリの種類には特に限定はなく、例えば水酸化ナトリウムやアンモニア等、適宜のアルカリを少なくとも一つ選択すればよい。Or when adding an alkali to this water containing hydrophilic organic solvent, there is no limitation in particular in the kind of alkali, For example, what is necessary is just to select at least one suitable alkalis, such as sodium hydroxide and ammonia.
酸またはアルカリを混合した水含有親水性有機溶媒を使用する場合、前記多糖類針状物体に含まれるカルボキシル基やアミノ基に対して、水含有親水性有機溶媒中の水素イオンまたは水酸化物イオンのモル量が1:1になるように混合することが好ましい。When water-containing hydrophilic organic solvent mixed with acid or alkali is used, hydrogen ion or hydroxide ion in water-containing hydrophilic organic solvent with respect to carboxyl group or amino group contained in the polysaccharide needle-shaped object It is preferable to mix so that the molar amount of this may become 1: 1.
酸またはアルカリを混合した水含有親水性有機溶媒を作製する際、まず酸またはアルカリを水に添加し、それを親水性有機溶剤に対して、重量濃度が1〜20%になるように加え、水含有親水性有機溶剤とする。When preparing a water-containing hydrophilic organic solvent mixed with an acid or an alkali, first an acid or an alkali is added to water, and it is added so that the weight concentration is 1 to 20% with respect to the hydrophilic organic solvent, A water-containing hydrophilic organic solvent is used.
脱塩工程においては、乾燥固化して得られた多糖類成形体16を、水含有親水性有機溶剤に浸漬することにより脱塩する。浸漬時間は、多糖類針状物体の脱塩が90%以上になるまでとする。5時間未満の浸漬時間では脱塩の効果が確認できないため、5時間以上の浸漬時間が好ましい。また、脱塩の際には水含有親水性有機溶剤の攪拌を行うことが好ましい。In the desalting step, the polysaccharide molded
所定の浸漬時間が終了したら、水含有親水性有機溶剤から多糖類針状物体を取り出す。エアブローを施すなどして水含有親水性有機溶剤を大まかに除去して自然乾燥させてもよいし、必要に応じて適宜の洗浄液で洗浄を行ってから、エアブローを施すなどして洗浄液を大まかに除去して自然乾燥させてもよい。このようにして、本発明の第1実施形態の製造方法により針状物体を得ることができる。When the predetermined immersion time is completed, the polysaccharide needle-shaped object is taken out from the water-containing hydrophilic organic solvent. The water-containing hydrophilic organic solvent may be roughly removed by air blowing or the like, and may be naturally dried, or after cleaning with an appropriate cleaning liquid as necessary, the cleaning liquid may be roughly removed by air blowing or the like. It may be removed and air dried. Thus, a needle-like object can be obtained by the manufacturing method according to the first embodiment of the present invention.
次に、本発明の針状物体の製造方法の第2実施形態を、図3を参照しながら説明する。図3(a)に示したように、凸型30は、片面に複数配列された凸部31を有している。凸型30を構成する物質は、例えば非水溶性高分子、金属、シリコン、ガラスなど、適宜のものを選択可能である。Next, 2nd Embodiment of the manufacturing method of the acicular object of this invention is described, referring FIG. As shown in FIG. 3A, the
凸部31の形状は、円錐形状、多角錐形状、その他の適宜の形状を選択することが可能である。また、凸部31の配列は適宜選択可能で、例えば、矩形格子配列、三角格子配列、六角格子配列、同心円状の配列、1次元的な配列、その他の適宜の配列を選択すればよい。As the shape of the
多糖類溶液17は第1の実施形態と同様の手順で作製し、所定形状の容器40に注入する。容器40に注入された多糖類溶液17の表面に、真上から凸型30を押し当てて少なくとも凸部31の先端を接触させた後(図3(b)参照)、垂直に引き上げそのまま停止する。The
このとき、凸部31それぞれの先端には多糖類溶液17が付着しておりそのまま垂直に引き上げられているので、多糖類溶液17表面からツノ状の突起18が複数形成された状態となる(図3(c)参照)。この状態のまま、多糖類溶液17を乾燥固化させる。At this time, since the
このツノ状の突起18が、後に針状物体1の針状部分11となる。ヒトの表皮の厚さは70μm〜200μmの範囲内にあるため、このツノ状の突起18の高さが300μm以上となるように凸型30の停止位置を調製する。また、太さ200μm以下の針には痛みを感じないことが知られているので、ツノ状の突起18の最大径は200μm以下となるようにする。This horn-
本実施形態においては、凸型30を垂直に引き上げてツノ状の突起18の形状を保持しながら乾燥固化させるため、多糖類溶液17は、固形分濃度は20%以上としておくことが好ましい。In the present embodiment, the
乾燥固化の際の条件と手順は、第1実施形態と同様である。
乾燥が完了すると多糖類溶液17は固化して多糖類成形体19となるので、凸型30の凸部31先端とツノ状の突起18の先端を切り離し、さらに容器40を外す。(図3(d)参照)。The conditions and procedures for drying and solidifying are the same as in the first embodiment.
When the drying is completed, the
脱塩工程の条件と手順は、第1実施形態と同様である。このようにして、本発明の第2実施形態の製造方法により針状物体を得ることができる。The conditions and procedure of the desalting step are the same as in the first embodiment. Thus, a needle-like object can be obtained by the manufacturing method according to the second embodiment of the present invention.
こうして得られる本発明の多糖類の針状物体は、脱塩処理によって耐水性を持ち、吸湿による変形や長期保存する際の劣化が少なく、水性薬剤の塗布が可能で、におい等の問題が改善された針状物体である。The polysaccharide needle-like object of the present invention thus obtained has water resistance by desalting treatment, is less susceptible to deformation due to moisture absorption and deterioration during long-term storage, can be applied with aqueous chemicals, and improves problems such as odor. Needle-like object.
以下、本発明を実施例に基づいて具体的に説明する。
実施例1では、アミノ基を持つ多糖類としてキトサンを用い、凹部を有する凹型により針状物体を作製した。Hereinafter, the present invention will be specifically described based on examples.
In Example 1, chitosan was used as a polysaccharide having an amino group, and a needle-like object was produced by a concave mold having a concave portion.
0.16gのキトサンに0.06gの酢酸および1.78gの精製水を加え、スターラーを用いて攪拌し、溶解させた。不溶物はアスピレーターを用いて濾過し除去した。この溶液を、針状部分の型となる凹部を有する凹型に流入し、アスピレーターと栓をした濾過鐘を用いて脱気を行った。その後エアーを流しながら、80℃のオーブン中で3時間加熱乾燥したのち、凹型から剥離した。0.06 g of acetic acid and 1.78 g of purified water were added to 0.16 g of chitosan, and the mixture was stirred and dissolved using a stirrer. Insoluble matter was removed by filtration using an aspirator. This solution was introduced into a concave mold having a concave portion that became a mold of a needle-shaped portion, and degassed by using a filtration bell with an aspirator and a stopper. Then, while flowing air, it was heated and dried in an oven at 80 ° C. for 3 hours, and then peeled off from the concave mold.
重量濃度が10%になるように水をアセトンに添加し、水含有アセトンとした。この水含有アセトンに乾燥固化したキトサン酢酸塩成形体を浸漬させ15時間放置し、その後水含有アセトンから取り出し乾燥させることにより、キトサン針状物体を作製した。Water was added to acetone so that the weight concentration was 10% to obtain water-containing acetone. A chitosan needle-like object was prepared by immersing the dried and solidified chitosan acetate molded body in this water-containing acetone and allowing it to stand for 15 hours.
KBr錠剤法により測定したキトサン原料のFT−IRスペクトルを図4に、脱塩前のFT−IRスペクトルを図5に、脱塩後のFT−IRスペクトルを図6に示す。またキトサン原料、脱塩前、脱塩後の1H−NMRスペクトルを図7にそれぞれ示す。FIG. 4 shows the FT-IR spectrum of the chitosan raw material measured by the KBr tablet method, FIG. 5 shows the FT-IR spectrum before desalting, and FIG. 6 shows the FT-IR spectrum after desalting. In addition, FIG. 7 shows1 H-NMR spectra of the chitosan raw material, before desalting, and after desalting.
図4〜図6のFT−IRスペクトルから、15時間脱塩することにより酢酸塩が減少していることが確認できた。また、図7の1H−NMRスペクトルからも3.2ppmのC4位のプロトン量を一定にした際に2.1ppmのメチル基のプロトン量が減少し、99.7%が原料と同じ状態に戻っていることが確認できた。From the FT-IR spectra of FIGS. 4 to 6, it was confirmed that acetate was reduced by desalting for 15 hours. In addition, from the1 H-NMR spectrum of FIG. 7, when the amount of proton at the C4 position of 3.2 ppm was made constant, the amount of proton of the methyl group at 2.1 ppm decreased, and 99.7% was in the same state as the raw material. I was able to confirm that I was back.
実施例2では、アミノ基を持つ多糖類としてキトサンを用い、凸部を有する凸型により針状物体を作製した。In Example 2, chitosan was used as a polysaccharide having an amino group, and a needle-like object was produced with a convex shape having a convex portion.
0.4gのキトサンに0.15gの酢酸および1.45gの精製水を加え、スターラー上にて攪拌して溶解させた。不溶物はアスピレーターを用いて濾過し除去した。この溶液を平面部を有する容器内に展延した後、真上から凸部を有する凸型を押し当て、垂直に引き上げてそのまま固定した。その後エアーを流しながら、80度オーブン中で3時間加熱乾燥したのち、ツノ状部分の先端部分を断裁した。To 0.4 g of chitosan, 0.15 g of acetic acid and 1.45 g of purified water were added and dissolved by stirring on a stirrer. Insoluble matter was removed by filtration using an aspirator. This solution was spread in a container having a flat portion, and then a convex mold having a convex portion was pressed from directly above, and pulled up vertically and fixed as it was. Then, after flowing and drying in an oven at 80 ° C. for 3 hours while flowing air, the tip portion of the horn-shaped portion was cut.
重量濃度が10%になるように水をアセトンに添加し、水含有アセトンとした。この水含有アセトンに乾燥固化したキトサン酢酸塩成形体を浸漬させ15時間放置し、その後水含有アセトンから取り出し乾燥させることにより、キトサン針状物体を作製した。Water was added to acetone so that the weight concentration was 10% to obtain water-containing acetone. A chitosan needle-like object was prepared by immersing the dried and solidified chitosan acetate molded body in this water-containing acetone and allowing it to stand for 15 hours.
実施例3では、カルボン酸を持つ多糖類としてアルギン酸を用い、凹部を有する凹型により針状物体を作製した。In Example 3, alginic acid was used as a polysaccharide having a carboxylic acid, and a needle-like object was produced with a concave mold having a concave portion.
0.16gのアルギン酸ナトリウムに1.84gの精製水を加え、スターラーを用いて攪拌し、溶解させた。不溶物はアスピレーターを用いて濾過し除去した。この溶液を針状部分の型となる凹部を有する凹型に流入し、アスピレーターと栓をした濾過鐘を用いて脱気を行った。その後エアーを流しながら、80度オーブン中で3時間加熱乾燥し、パターンから剥離した。1.84 g of purified water was added to 0.16 g of sodium alginate, and the mixture was stirred and dissolved using a stirrer. Insoluble matter was removed by filtration using an aspirator. This solution was poured into a concave mold having a concave portion that became a needle-shaped mold, and degassed using a filtration bell with an aspirator and a stopper. Then, while flowing air, it was dried by heating in an oven at 80 ° C. for 3 hours, and peeled off from the pattern.
水に塩酸を0.9m mol加え、その後、重量濃度が10%になるようにアセトンに添加し、塩酸含有アセトンとした。この塩酸含有アセトンに乾燥固化したアルギン酸ナトリウム成形体を浸漬させ15時間放置し、その後塩酸含有アセトンから取り出し乾燥させることにより、アルギン酸針状物体を作製した。Hydrochloric acid (0.9 mmol) was added to water, and then added to acetone so that the weight concentration became 10% to obtain hydrochloric acid-containing acetone. The sodium alginate molded body dried and solidified in this hydrochloric acid-containing acetone was immersed and allowed to stand for 15 hours, and then taken out from the hydrochloric acid-containing acetone and dried to prepare an alginate needle-shaped body.
実施例4では、カルボン酸を持つ多糖類としてアルギン酸を用い、凸部を有する凸型により針状物体を作製した。In Example 4, alginic acid was used as a polysaccharide having a carboxylic acid, and a needle-like object was produced by a convex mold having a convex portion.
0.4gのアルギン酸ナトリウムに1.6gの精製水を加え、スターラー上にて攪拌して溶解させた。不溶物はアスピレーターを用いて濾過し除去した。この溶液を平面部を有する容器内に展延した後、真上から凸部を有する凸型を押し当て、垂直に引き上げてそのまま固定した。その後エアーを流しながら、80度オーブン中で3時間加熱乾燥したのち、ツノ状部分の先端部分を断裁した。To 0.4 g of sodium alginate, 1.6 g of purified water was added and stirred on a stirrer to dissolve. Insoluble matter was removed by filtration using an aspirator. This solution was spread in a container having a flat portion, and then a convex mold having a convex portion was pressed from directly above, and pulled up vertically and fixed as it was. Then, after flowing and drying in an oven at 80 ° C. for 3 hours while flowing air, the tip portion of the horn-shaped portion was cut.
水に塩酸を2m mol加え、その後、重量濃度が10%になるようにアセトンに添加し、塩酸含有アセトンとした。この塩酸含有アセトンに乾燥固化したアルギン酸ナトリウム成形体を浸漬させ15時間放置し、その後塩酸含有アセトンから取り出し乾燥させることにより、アルギン酸針状物体を作製した。2 mmol of hydrochloric acid was added to water, and then added to acetone so that the weight concentration was 10% to obtain hydrochloric acid-containing acetone. The sodium alginate molded body dried and solidified in this hydrochloric acid-containing acetone was immersed and allowed to stand for 15 hours, and then taken out from the hydrochloric acid-containing acetone and dried to prepare an alginate needle-shaped body.
実施例5では、カルボン酸を持つ多糖類としてセルロースのC6位一級水酸基のみを選択的に酸化したセロウロン酸を用い、凹部を有する凹型により針状物体を作製した。In Example 5, a cellulosic acid obtained by selectively oxidizing only the C6-position primary hydroxyl group of cellulose as a polysaccharide having a carboxylic acid was used, and a needle-like object was produced with a concave mold having a concave part.
0.16gのセロウロン酸ナトリウムに1.84gの精製水を加え、スターラーを用いて攪拌し、溶解させた。不溶物はアスピレーターを用いて濾過し除去した。この溶液を針状部分の型となる凹部を有する凹型に流入し、アスピレーターと栓をした濾過鐘を用いて脱気を行った。その後エアーを流しながら、80度オーブン中で3時間加熱乾燥し、パターンから剥離した。1.84 g of purified water was added to 0.16 g of sodium cellulonate, and the mixture was stirred and dissolved using a stirrer. Insoluble matter was removed by filtration using an aspirator. This solution was poured into a concave mold having a concave portion that became a needle-shaped mold, and degassed using a filtration bell with an aspirator and a stopper. Then, while flowing air, it was dried by heating in an oven at 80 ° C. for 3 hours, and peeled off from the pattern.
水に塩酸を0.8m mol加え、その後、重量濃度が10%になるようにアセトンに添加し、塩酸含有アセトンとした。この塩酸含有アセトンに乾燥固化したセロウロン酸ナトリウム成形体を浸漬させ15時間放置し、その後塩酸含有アセトンから取り出し乾燥させることにより、セロウロン酸針状物体を作製した。Hydrochloric acid was added to water in an amount of 0.8 mmol, and then added to acetone so that the weight concentration was 10% to obtain hydrochloric acid-containing acetone. The molded product of sodium seurouronate dried and solidified in hydrochloric acid-containing acetone was dipped and allowed to stand for 15 hours, and then taken out from the hydrochloric acid-containing acetone and dried to prepare a seuroronic acid needle-like body.
本実施例では、カルボン酸を持つ多糖類としてセルロースのC6位一級水酸基のみを選択的に酸化したセロウロン酸を用い、凸部を有する凸型により針状物体を作製した。In this example, a cellulosic acid obtained by selectively oxidizing only the C6-position primary hydroxyl group of cellulose as a polysaccharide having a carboxylic acid was used to produce a needle-like object with a convex shape having a convex portion.
0.4gのセロウロン酸ナトリウムに1.6gの精製水を加え、スターラー上にて攪拌して溶解させた。不溶物はアスピレーターを用いて濾過し除去した。この溶液を平面部を有する容器内に展延した後、真上から凸部を有する凸型を押し当て、垂直に引き上げてそのまま固定した。その後エアーを流しながら、80度オーブン中で3時間加熱乾燥したのち、ツノ状部分の先端部分を断裁した。1.6 g of purified water was added to 0.4 g of sodium cellulonate, and dissolved by stirring on a stirrer. Insoluble matter was removed by filtration using an aspirator. This solution was spread in a container having a flat portion, and then a convex mold having a convex portion was pressed from directly above, and pulled up vertically and fixed as it was. Then, after flowing and drying in an oven at 80 ° C. for 3 hours while flowing air, the tip portion of the horn-shaped portion was cut.
水に塩酸を2m mol加え、その後、重量濃度が10%になるようにアセトンに添加し、塩酸含有アセトンとした。この塩酸含有アセトンに乾燥固化したセロウロン酸ナトリウム成形体を浸漬させ15時間放置し、その後塩酸含有アセトンから取り出し乾燥させることにより、セロウロン酸針状物体を作製した。2 mmol of hydrochloric acid was added to water, and then added to acetone so that the weight concentration was 10% to obtain hydrochloric acid-containing acetone. The molded product of sodium seurouronate dried and solidified in hydrochloric acid-containing acetone was dipped and allowed to stand for 15 hours, and then taken out from the hydrochloric acid-containing acetone and dried to prepare a seuroronic acid needle-like body.
以上の実施例1〜6において作製した多糖類針状物体(脱塩後)と、その脱塩前の多糖類成形体を、生理食塩水に1週間浸漬して溶解性を調べた結果を表1に示す。
実施例1および2で作製したキトサン酢酸塩成形体(脱塩前)と、キトサン針状物体(脱塩後)を生理食塩水に一週間浸漬したところ、キトサン酢酸塩成形体はいずれも溶解したが、キトサン針状物体はいずれも不溶であり、脱塩により耐水性が向上していることが確認できた。When the chitosan acetate molded body (before desalting) prepared in Examples 1 and 2 and the chitosan acicular body (after desalting) were immersed in physiological saline for one week, both the chitosan acetate molded bodies were dissolved. However, all the chitosan needle-like objects were insoluble, and it was confirmed that the water resistance was improved by desalting.
実施例3および4で作製したアルギン酸ナトリウム成形体(脱塩前)と、アルギン酸針状物体(脱塩後)を生理食塩水に一週間浸漬したところ、アルギン酸ナトリウム成形体はいずれも溶解したが、アルギン酸針状物体はいずれも不溶であり、脱塩により耐水性が向上していることが確認できた。When the sodium alginate molded body (before desalting) prepared in Examples 3 and 4 and the alginate needle-shaped body (after desalting) were immersed in physiological saline for one week, both the sodium alginate molded bodies were dissolved. All the alginate needles were insoluble, and it was confirmed that the water resistance was improved by desalting.
実施例5および6で作製したセロウロン酸ナトリウム成形体(脱塩前)と、セロウロン酸針状物体(脱塩後)を生理食塩水に一週間浸漬したところ、セロウロン酸ナトリウム成形体はいずれも溶解したが、セロウロン酸針状物体はいずれも不溶であり、脱塩により耐水性が向上していることが確認できた。
When the sodium seurouronate molded product (before desalting) prepared in Examples 5 and 6 and the needles of seuroronic acid (after desalting) were immersed in physiological saline for one week, both sodium seuroronate molded products were dissolved. However, it was confirmed that all of the needles of the cellulonic acid were insoluble and the water resistance was improved by desalting.
1・・・針状物体
11・・針状部分
12・・基板
15、17・・多糖類溶液
16、19・・多糖類成形体
18・・ツノ状の突起
20・・凹型
21・・凹部
30・・凸型
31・・凸部
40・・多糖類溶液の容器DESCRIPTION OF
Claims (4)
Translated fromJapanese前記針状物体は、基板と、前記基板上に複数配列された針状部分と、を備え、
分子構造内にカルボキシル基および/またはアミノ基を持つ多糖類を、水を含む溶媒に溶解させて多糖類溶液を作製する多糖類溶解段階と、
前記針状部分と同形状の凹部を有する凹型に前記多糖類溶液を流し込み、さらに乾燥固化する成形段階と、
前記多糖類溶液が固化した多糖類成形体を前記凹型から剥離する剥離段階と、
剥離した多糖類成形体を水含有親水性有機溶媒で脱塩する脱塩段階と、
を有することを特徴とする針状物体の製造方法。A method for producing a needle-like object for puncturing the skin with a fine needle,
The acicular object includes a substrate and a plurality of acicular portions arranged on the substrate,
A polysaccharide dissolving step of preparing a polysaccharide solution by dissolving a polysaccharidehaving acarboxyl group and / or an amino group in the molecular structure in a solvent containing water;
A molding step of pouring the polysaccharide solution into a concave mold having a concave portion having the same shape as the needle-shaped portion, and further drying and solidifying;
A peeling step of peeling the polysaccharide molded body solidified by the polysaccharide solution from the concave mold;
A desalting step of desalting the exfoliated polysaccharide molded body with a water-containing hydrophilic organic solvent;
A method for producing a needle-like object comprising:
前記針状物体は、基板と、前記基板上に複数配列された針状部分と、を備え、
分子構造内にカルボキシル基および/またはアミノ基を持つ多糖類を、水を含む溶媒に溶解させて多糖類溶液を作製する多糖類溶解段階と、
所定形状の容器に所定量の前記多糖類溶液を注入する多糖類溶液注入段階と
前記容器に注入された前記多糖類溶液の表面に、複数配列された針状突起を有する凸型をいったん押し当てた後に垂直に引き上げた状態で乾燥固化する成形段階と、
前記多糖類溶液が固化した多糖類成形体の針状部分の先端部分を切断して、前記凸型の針状突起から分離する分離段階と、
分離した多糖類成形体を水含有親水性有機溶媒で脱塩する脱塩段階と、
を有することを特徴とする針状物体の製造方法。A method for producing a needle-like object for puncturing the skin with a fine needle,
The acicular object includes a substrate and a plurality of acicular portions arranged on the substrate,
A polysaccharide dissolving step of preparing a polysaccharide solution by dissolving a polysaccharidehaving acarboxyl group and / or an amino group in the molecular structure in a solvent containing water;
A polysaccharide solution injecting step of injecting a predetermined amount of the polysaccharide solution into a container of a predetermined shape, and a convex mold having a plurality of needle-like protrusions once pressed against the surface of the polysaccharide solution injected into the container A molding stage that dries and solidifies in a state of being pulled up vertically after
Separating the tip portion of the needle-shaped portion of the polysaccharide molded body in which the polysaccharide solution is solidified, and separating it from the convex needle-shaped protrusion;
A desalting step of desalting the separated polysaccharide molded body with a water-containing hydrophilic organic solvent;
A method for producing a needle-like object comprising:
Priority Applications (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| JP2009089938AJP5499509B2 (en) | 2009-04-02 | 2009-04-02 | Manufacturing method of needle-like object |
Applications Claiming Priority (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| JP2009089938AJP5499509B2 (en) | 2009-04-02 | 2009-04-02 | Manufacturing method of needle-like object |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| JP2010241702A JP2010241702A (en) | 2010-10-28 |
| JP5499509B2true JP5499509B2 (en) | 2014-05-21 |
Family
ID=43095150
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2009089938AExpired - Fee RelatedJP5499509B2 (en) | 2009-04-02 | 2009-04-02 | Manufacturing method of needle-like object |
Country Status (1)
| Country | Link |
|---|---|
| JP (1) | JP5499509B2 (en) |
Families Citing this family (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP5874303B2 (en)* | 2011-10-19 | 2016-03-02 | 凸版印刷株式会社 | Needle-like body and method for producing needle-like body |
| JP6098059B2 (en)* | 2012-07-20 | 2017-03-22 | 凸版印刷株式会社 | Acicular body manufacturing method and acicular body |
| JP2017145226A (en)* | 2016-02-19 | 2017-08-24 | 日本写真印刷株式会社 | Microneedle sheet |
| JP2018088995A (en)* | 2016-11-30 | 2018-06-14 | 富士フイルム株式会社 | Method of manufacturing medical transdermal absorption sheet |
Family Cites Families (7)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JPS5428369A (en)* | 1977-08-03 | 1979-03-02 | Yamakawa Tsuneko | Method of forming needleelike projection of thermoplastic resin on sheet |
| JPH0721062B2 (en)* | 1992-04-30 | 1995-03-08 | アイセロ化学株式会社 | Chitosan molded article having good water resistance and method for producing the same |
| JP5882556B2 (en)* | 2004-12-28 | 2016-03-09 | ナブテスコ株式会社 | Skin needle, skin needle manufacturing apparatus, and skin needle manufacturing method |
| WO2008020633A1 (en)* | 2006-08-18 | 2008-02-21 | Toppan Printing Co., Ltd. | Microneedle and microneedle patch |
| JP2008142183A (en)* | 2006-12-07 | 2008-06-26 | Fujifilm Corp | Microneedle sheet and manufacturing method thereof |
| JP2008284318A (en)* | 2007-05-15 | 2008-11-27 | Kosumedei Seiyaku Kk | Microneedle for dosing, including living body origin matter |
| JP5031483B2 (en)* | 2007-08-15 | 2012-09-19 | 凸版印刷株式会社 | Needle-like body, needle-like body manufacturing method, needle-like body manufacturing apparatus |
- 2009
- 2009-04-02JPJP2009089938Apatent/JP5499509B2/ennot_activeExpired - Fee Related
Also Published As
| Publication number | Publication date |
|---|---|
| JP2010241702A (en) | 2010-10-28 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| Capanema et al. | Superabsorbent crosslinked carboxymethyl cellulose-PEG hydrogels for potential wound dressing applications | |
| KR102372049B1 (en) | Cosmetic gel sheet and production method therefor | |
| Yan et al. | Snakegourd root/Astragalus polysaccharide hydrogel preparation and application in 3D printing | |
| JP5499509B2 (en) | Manufacturing method of needle-like object | |
| CN103893018A (en) | Soluble hyaluronic acid micro-needle patch | |
| Sarkar et al. | Physico-chemical/biological properties of tripolyphosphate cross-linked chitosan based nanofibers | |
| CN101821294B (en) | Swellable cross-linked hyaluronic acid powder and manufacturing method thereof | |
| KR20100107419A (en) | Gel sheet for cosmetics pack | |
| Pillai et al. | Novel combination of bioactive agents in bilayered dermal patches provides superior wound healing | |
| CN110812688A (en) | A kind of microneedle for transdermal administration and preparation method thereof | |
| Zhao et al. | Preparation and characterization of a novel pH-response dietary fiber: Chitosan-coated konjac glucomannan | |
| KR20210081657A (en) | Composition for conductive micro needle patch, conductive micro needle patch and method for preparing the same | |
| JPWO2017018086A1 (en) | Micro needle seat | |
| CN111068098A (en) | A kind of preparation method of high-strength polyvinyl alcohol hydrogel film | |
| Badhe et al. | Development of polylactic acid and bovine serum albumin-layered-coated chitosan microneedles using novel bees wax mould | |
| Stolz et al. | Cryo‐3D Printing of Hierarchically Porous Polyhydroxymethylene Scaffolds for Hard Tissue Regeneration | |
| CN105194734A (en) | Chitosan-extracellular matrix tissue repairing membrane and preparation method thereof | |
| Gao et al. | Synthesis of hydrogels based on nanocellulose from garlic straw and regulating the release of allicin and its cytotoxicity | |
| Sabbagh et al. | Transdermal delivery of catechin using dissolving poly (vinyl alcohol)-based microneedles: Effect of microneedle composition on drug release | |
| TW477802B (en) | Method for preparing hydrophilic porous polymeric materials | |
| KR20140006172A (en) | Skin temperature-sensitive hydrogel composition and a preparing method thereof | |
| Abualsoud et al. | Pectin-based composites: a promising approach for tissue engineering and wound healing | |
| CN114129503A (en) | Preparation method of porous microneedle with adjustable pore size | |
| JPWO2013129028A1 (en) | Acicular body and method for producing acicular body | |
| Wu et al. | Interlocked chitosan/poly (dl-lactide) blends |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| A621 | Written request for application examination | Free format text:JAPANESE INTERMEDIATE CODE: A621 Effective date:20120316 | |
| A977 | Report on retrieval | Free format text:JAPANESE INTERMEDIATE CODE: A971007 Effective date:20130809 | |
| A131 | Notification of reasons for refusal | Free format text:JAPANESE INTERMEDIATE CODE: A131 Effective date:20130813 | |
| A521 | Written amendment | Free format text:JAPANESE INTERMEDIATE CODE: A523 Effective date:20131010 | |
| TRDD | Decision of grant or rejection written | ||
| A01 | Written decision to grant a patent or to grant a registration (utility model) | Free format text:JAPANESE INTERMEDIATE CODE: A01 Effective date:20140212 | |
| A61 | First payment of annual fees (during grant procedure) | Free format text:JAPANESE INTERMEDIATE CODE: A61 Effective date:20140225 | |
| R150 | Certificate of patent or registration of utility model | Ref document number:5499509 Country of ref document:JP Free format text:JAPANESE INTERMEDIATE CODE: R150 | |
| LAPS | Cancellation because of no payment of annual fees |