EP3192491B1 - Composition comprising polyglycerol esters and hydroxy-alkyl modified guar - Google Patents
Composition comprising polyglycerol esters and hydroxy-alkyl modified guarDownload PDFInfo
- Publication number
- EP3192491B1 EP3192491B1EP16151443.5AEP16151443AEP3192491B1EP 3192491 B1EP3192491 B1EP 3192491B1EP 16151443 AEP16151443 AEP 16151443AEP 3192491 B1EP3192491 B1EP 3192491B1
- Authority
- EP
- European Patent Office
- Prior art keywords
- mol
- acid
- carboxylic acid
- aluminum
- composition according
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Active
Links
- 239000000203mixtureSubstances0.000titleclaimsdescription127
- 229920000223polyglycerolPolymers0.000titleclaimsdescription69
- 150000002148estersChemical class0.000titleclaimsdescription34
- 125000002768hydroxyalkyl groupChemical group0.000titleclaimsdescription5
- 244000007835Cyamopsis tetragonolobaSpecies0.000titleclaims5
- PEDCQBHIVMGVHV-UHFFFAOYSA-NGlycerineChemical compoundOCC(O)COPEDCQBHIVMGVHV-UHFFFAOYSA-N0.000claimsdescription111
- 239000000839emulsionSubstances0.000claimsdescription27
- 150000001735carboxylic acidsChemical class0.000claimsdescription21
- IPCSVZSSVZVIGE-UHFFFAOYSA-Nhexadecanoic acidChemical compoundCCCCCCCCCCCCCCCC(O)=OIPCSVZSSVZVIGE-UHFFFAOYSA-N0.000claimsdescription18
- WWZKQHOCKIZLMA-UHFFFAOYSA-Noctanoic acidChemical compoundCCCCCCCC(O)=OWWZKQHOCKIZLMA-UHFFFAOYSA-N0.000claimsdescription18
- 238000006116polymerization reactionMethods0.000claimsdescription18
- 239000002781deodorant agentSubstances0.000claimsdescription16
- 230000007062hydrolysisEffects0.000claimsdescription16
- 238000006460hydrolysis reactionMethods0.000claimsdescription16
- 239000004480active ingredientSubstances0.000claimsdescription12
- GHVNFZFCNZKVNT-UHFFFAOYSA-Ndecanoic acidChemical compoundCCCCCCCCCC(O)=OGHVNFZFCNZKVNT-UHFFFAOYSA-N0.000claimsdescription12
- 125000004432carbon atomChemical groupC*0.000claimsdescription11
- QIQXTHQIDYTFRH-UHFFFAOYSA-Noctadecanoic acidChemical compoundCCCCCCCCCCCCCCCCCC(O)=OQIQXTHQIDYTFRH-UHFFFAOYSA-N0.000claimsdescription10
- 235000021314Palmitic acidNutrition0.000claimsdescription9
- 235000021355Stearic acidNutrition0.000claimsdescription9
- WQEPLUUGTLDZJY-UHFFFAOYSA-Nn-Pentadecanoic acidNatural productsCCCCCCCCCCCCCCC(O)=OWQEPLUUGTLDZJY-UHFFFAOYSA-N0.000claimsdescription9
- OQCDKBAXFALNLD-UHFFFAOYSA-Noctadecanoic acidNatural productsCCCCCCCC(C)CCCCCCCCC(O)=OOQCDKBAXFALNLD-UHFFFAOYSA-N0.000claimsdescription9
- 239000008117stearic acidSubstances0.000claimsdescription9
- 239000005635Caprylic acid (CAS 124-07-2)Substances0.000claimsdescription7
- 150000001875compoundsChemical class0.000claimsdescription7
- 229960002446octanoic acidDrugs0.000claimsdescription7
- 239000005632Capric acid (CAS 334-48-5)Substances0.000claimsdescription6
- GPLRAVKSCUXZTP-UHFFFAOYSA-NdiglycerolChemical compoundOCC(O)COCC(O)COGPLRAVKSCUXZTP-UHFFFAOYSA-N0.000claimsdescription6
- UKMSUNONTOPOIO-UHFFFAOYSA-Ndocosanoic acidChemical compoundCCCCCCCCCCCCCCCCCCCCCC(O)=OUKMSUNONTOPOIO-UHFFFAOYSA-N0.000claimsdescription6
- 230000001166anti-perspirative effectEffects0.000claimsdescription5
- 239000003213antiperspirantSubstances0.000claimsdescription5
- LVYZJEPLMYTTGH-UHFFFAOYSA-Hdialuminum chloride pentahydroxide dihydrateChemical compound[Cl-].[Al+3].[OH-].[OH-].[Al+3].[OH-].[OH-].[OH-].O.OLVYZJEPLMYTTGH-UHFFFAOYSA-H0.000claimsdescription4
- 239000007764o/w emulsionSubstances0.000claimsdescription4
- 239000010695polyglycolSubstances0.000claimsdescription4
- 229920000151polyglycolPolymers0.000claimsdescription4
- 150000003751zincChemical class0.000claimsdescription4
- 235000021357Behenic acidNutrition0.000claimsdescription3
- 229940116226behenic acidDrugs0.000claimsdescription3
- 229920006395saturated elastomerPolymers0.000claimsdescription3
- 150000003754zirconiumChemical class0.000claimsdescription3
- GAWWVVGZMLGEIW-GNNYBVKZSA-Lzinc ricinoleateChemical compound[Zn+2].CCCCCC[C@@H](O)C\C=C/CCCCCCCC([O-])=O.CCCCCC[C@@H](O)C\C=C/CCCCCCCC([O-])=OGAWWVVGZMLGEIW-GNNYBVKZSA-L0.000claimsdescription2
- 229940100530zinc ricinoleateDrugs0.000claimsdescription2
- 229960001422aluminium chlorohydrateDrugs0.000claims1
- 159000000013aluminium saltsChemical class0.000claims1
- 150000002170ethersChemical class0.000claims1
- XLYOFNOQVPJJNP-UHFFFAOYSA-NwaterSubstancesOXLYOFNOQVPJJNP-UHFFFAOYSA-N0.000description50
- 238000003786synthesis reactionMethods0.000description47
- 235000011187glycerolNutrition0.000description40
- 230000015572biosynthetic processEffects0.000description38
- KWYUFKZDYYNOTN-UHFFFAOYSA-MPotassium hydroxideChemical compound[OH-].[K+]KWYUFKZDYYNOTN-UHFFFAOYSA-M0.000description26
- 229910052782aluminiumInorganic materials0.000description23
- 239000003995emulsifying agentSubstances0.000description23
- 238000009472formulationMethods0.000description23
- IJGRMHOSHXDMSA-UHFFFAOYSA-NAtomic nitrogenChemical compoundN#NIJGRMHOSHXDMSA-UHFFFAOYSA-N0.000description22
- 244000303965Cyamopsis psoralioidesSpecies0.000description21
- 150000001732carboxylic acid derivativesChemical class0.000description21
- XAGFODPZIPBFFR-UHFFFAOYSA-NaluminiumChemical compound[Al]XAGFODPZIPBFFR-UHFFFAOYSA-N0.000description17
- -1Aluminum DibenzoateChemical compound0.000description16
- 239000002253acidSubstances0.000description14
- 238000000926separation methodMethods0.000description13
- 239000003795chemical substances by applicationSubstances0.000description12
- 229910052757nitrogenInorganic materials0.000description11
- RTZKZFJDLAIYFH-UHFFFAOYSA-NDiethyl etherChemical compoundCCOCCRTZKZFJDLAIYFH-UHFFFAOYSA-N0.000description10
- HEMHJVSKTPXQMS-UHFFFAOYSA-MSodium hydroxideChemical compound[OH-].[Na+]HEMHJVSKTPXQMS-UHFFFAOYSA-M0.000description9
- 238000000034methodMethods0.000description9
- 239000003921oilSubstances0.000description9
- POULHZVOKOAJMA-UHFFFAOYSA-MdodecanoateChemical compoundCCCCCCCCCCCC([O-])=OPOULHZVOKOAJMA-UHFFFAOYSA-M0.000description8
- 229940070765laurateDrugs0.000description8
- LXCFILQKKLGQFO-UHFFFAOYSA-NmethylparabenChemical compoundCOC(=O)C1=CC=C(O)C=C1LXCFILQKKLGQFO-UHFFFAOYSA-N0.000description8
- 238000003860storageMethods0.000description8
- 125000002887hydroxy groupChemical group[H]O*0.000description7
- 239000011541reaction mixtureSubstances0.000description7
- XEEYBQQBJWHFJM-UHFFFAOYSA-NIronChemical compound[Fe]XEEYBQQBJWHFJM-UHFFFAOYSA-N0.000description6
- MUBZPKHOEPUJKR-UHFFFAOYSA-NOxalic acidChemical compoundOC(=O)C(O)=OMUBZPKHOEPUJKR-UHFFFAOYSA-N0.000description6
- WPYMKLBDIGXBTP-UHFFFAOYSA-Nbenzoic acidChemical compoundOC(=O)C1=CC=CC=C1WPYMKLBDIGXBTP-UHFFFAOYSA-N0.000description6
- KRKNYBCHXYNGOX-UHFFFAOYSA-Ncitric acidChemical compoundOC(=O)CC(O)(C(O)=O)CC(O)=OKRKNYBCHXYNGOX-UHFFFAOYSA-N0.000description6
- 235000014113dietary fatty acidsNutrition0.000description6
- 239000000194fatty acidSubstances0.000description6
- 229930195729fatty acidNatural products0.000description6
- 150000004665fatty acidsChemical class0.000description6
- NIXOWILDQLNWCW-UHFFFAOYSA-NAcrylic acidChemical compoundOC(=O)C=CNIXOWILDQLNWCW-UHFFFAOYSA-N0.000description5
- 229920002125Sokalan®Polymers0.000description5
- JPNZKPRONVOMLL-UHFFFAOYSA-Nazane;octadecanoic acidChemical class[NH4+].CCCCCCCCCCCCCCCCCC([O-])=OJPNZKPRONVOMLL-UHFFFAOYSA-N0.000description5
- 229960001631carbomerDrugs0.000description5
- 235000019447hydroxyethyl celluloseNutrition0.000description5
- 239000000243solutionSubstances0.000description5
- 241000871495Heeria argenteaSpecies0.000description4
- 229920000663Hydroxyethyl cellulosePolymers0.000description4
- ZGUQGPFMMTZGBQ-UHFFFAOYSA-N[Al].[Al].[Zr]Chemical compound[Al].[Al].[Zr]ZGUQGPFMMTZGBQ-UHFFFAOYSA-N0.000description4
- AZDRQVAHHNSJOQ-UHFFFAOYSA-NalumaneChemical class[AlH3]AZDRQVAHHNSJOQ-UHFFFAOYSA-N0.000description4
- 238000004458analytical methodMethods0.000description4
- SZXQTJUDPRGNJN-UHFFFAOYSA-Ndipropylene glycolChemical compoundOCCCOCCCOSZXQTJUDPRGNJN-UHFFFAOYSA-N0.000description4
- 229960001617ethyl hydroxybenzoateDrugs0.000description4
- 239000004403ethyl p-hydroxybenzoateSubstances0.000description4
- 235000010228ethyl p-hydroxybenzoateNutrition0.000description4
- NUVBSKCKDOMJSU-UHFFFAOYSA-NethylparabenChemical compoundCCOC(=O)C1=CC=C(O)C=C1NUVBSKCKDOMJSU-UHFFFAOYSA-N0.000description4
- 239000007789gasSubstances0.000description4
- 239000004615ingredientSubstances0.000description4
- 235000013980iron oxideNutrition0.000description4
- UQSXHKLRYXJYBZ-UHFFFAOYSA-Niron oxideInorganic materials[Fe]=OUQSXHKLRYXJYBZ-UHFFFAOYSA-N0.000description4
- 235000010213iron oxides and hydroxidesNutrition0.000description4
- 239000004407iron oxides and hydroxidesSubstances0.000description4
- VBMVTYDPPZVILR-UHFFFAOYSA-Niron(2+);oxygen(2-)Chemical class[O-2].[Fe+2]VBMVTYDPPZVILR-UHFFFAOYSA-N0.000description4
- 238000004519manufacturing processMethods0.000description4
- 239000004292methyl p-hydroxybenzoateSubstances0.000description4
- 235000010270methyl p-hydroxybenzoateNutrition0.000description4
- 229960002216methylparabenDrugs0.000description4
- 238000005191phase separationMethods0.000description4
- 239000000049pigmentSubstances0.000description4
- 239000000126substanceSubstances0.000description4
- KDYFGRWQOYBRFD-UHFFFAOYSA-Lsuccinate(2-)Chemical compound[O-]C(=O)CCC([O-])=OKDYFGRWQOYBRFD-UHFFFAOYSA-L0.000description4
- 150000003626triacylglycerolsChemical class0.000description4
- DLICJXPMMTZITN-UHFFFAOYSA-N3-methyl-1,2-thiazol-4-oneChemical classCC1=NSCC1=ODLICJXPMMTZITN-UHFFFAOYSA-N0.000description3
- QTBSBXVTEAMEQO-UHFFFAOYSA-NAcetic acidChemical classCC(O)=OQTBSBXVTEAMEQO-UHFFFAOYSA-N0.000description3
- 239000005711Benzoic acidSubstances0.000description3
- LFQSCWFLJHTTHZ-UHFFFAOYSA-NEthanolChemical compoundCCOLFQSCWFLJHTTHZ-UHFFFAOYSA-N0.000description3
- 239000004354Hydroxyethyl celluloseSubstances0.000description3
- DGAQECJNVWCQMB-PUAWFVPOSA-MIlexoside XXIXChemical compoundC[C@@H]1CC[C@@]2(CC[C@@]3(C(=CC[C@H]4[C@]3(CC[C@@H]5[C@@]4(CC[C@@H](C5(C)C)OS(=O)(=O)[O-])C)C)[C@@H]2[C@]1(C)O)C)C(=O)O[C@H]6[C@@H]([C@H]([C@@H]([C@H](O6)CO)O)O)O.[Na+]DGAQECJNVWCQMB-PUAWFVPOSA-M0.000description3
- OKKJLVBELUTLKV-UHFFFAOYSA-NMethanolChemical compoundOCOKKJLVBELUTLKV-UHFFFAOYSA-N0.000description3
- QAOWNCQODCNURD-UHFFFAOYSA-LSulfateChemical compound[O-]S([O-])(=O)=OQAOWNCQODCNURD-UHFFFAOYSA-L0.000description3
- GWEVSGVZZGPLCZ-UHFFFAOYSA-NTitan oxideChemical compoundO=[Ti]=OGWEVSGVZZGPLCZ-UHFFFAOYSA-N0.000description3
- WYANSMZYIOPJFV-UHFFFAOYSA-Laluminum;2-aminoacetic acid;zirconium(4+);chloride;hydroxide;hydrateChemical compoundO.[OH-].[Al+3].[Cl-].[Zr+4].NCC(O)=OWYANSMZYIOPJFV-UHFFFAOYSA-L0.000description3
- HAMGNFFXQJOFRZ-UHFFFAOYSA-Laluminum;zirconium(4+);chloride;hydroxide;hydrateChemical compoundO.[OH-].[Al+3].[Cl-].[Zr+4]HAMGNFFXQJOFRZ-UHFFFAOYSA-L0.000description3
- 229940116224behenateDrugs0.000description3
- UKMSUNONTOPOIO-UHFFFAOYSA-MbehenateChemical compoundCCCCCCCCCCCCCCCCCCCCCC([O-])=OUKMSUNONTOPOIO-UHFFFAOYSA-M0.000description3
- 235000010233benzoic acidNutrition0.000description3
- 238000007664blowingMethods0.000description3
- 239000011575calciumSubstances0.000description3
- 239000002537cosmeticSubstances0.000description3
- BEFDCLMNVWHSGT-UHFFFAOYSA-NethenylcyclopentaneChemical compoundC=CC1CCCC1BEFDCLMNVWHSGT-UHFFFAOYSA-N0.000description3
- XLYOFNOQVPJJNP-UHFFFAOYSA-MhydroxideChemical compound[OH-]XLYOFNOQVPJJNP-UHFFFAOYSA-M0.000description3
- 238000002347injectionMethods0.000description3
- 239000007924injectionSubstances0.000description3
- 229910052742ironInorganic materials0.000description3
- XUGNVMKQXJXZCD-UHFFFAOYSA-Nisopropyl palmitateChemical compoundCCCCCCCCCCCCCCCC(=O)OC(C)CXUGNVMKQXJXZCD-UHFFFAOYSA-N0.000description3
- 150000002942palmitic acid derivativesChemical class0.000description3
- 239000003208petroleumSubstances0.000description3
- WVDDGKGOMKODPV-ZQBYOMGUSA-Nphenyl(114C)methanolChemical compoundO[14CH2]C1=CC=CC=C1WVDDGKGOMKODPV-ZQBYOMGUSA-N0.000description3
- 239000011734sodiumSubstances0.000description3
- 229910052708sodiumInorganic materials0.000description3
- 239000004334sorbic acidSubstances0.000description3
- 235000010199sorbic acidNutrition0.000description3
- 229940075582sorbic acidDrugs0.000description3
- LENZDBCJOHFCAS-UHFFFAOYSA-NtrisChemical compoundOCC(N)(CO)COLENZDBCJOHFCAS-UHFFFAOYSA-N0.000description3
- CQRIWHZHWKQGQD-JWXFUTCRSA-N(2r,3s,4r,5r)-2,3,4,5,6-pentahydroxy-2-methylhexanalChemical compoundO=C[C@@](O)(C)[C@@H](O)[C@H](O)[C@H](O)COCQRIWHZHWKQGQD-JWXFUTCRSA-N0.000description2
- CUVSTAMIHSSVKL-UWVGGRQHSA-N(4s)-4-[(2-aminoacetyl)amino]-5-[[(2s)-6-amino-1-(carboxymethylamino)-1-oxohexan-2-yl]amino]-5-oxopentanoic acidChemical compoundNCCCC[C@@H](C(=O)NCC(O)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)CNCUVSTAMIHSSVKL-UWVGGRQHSA-N0.000description2
- VBICKXHEKHSIBG-UHFFFAOYSA-N1-monostearoylglycerolChemical compoundCCCCCCCCCCCCCCCCCC(=O)OCC(O)COVBICKXHEKHSIBG-UHFFFAOYSA-N0.000description2
- OPJWPPVYCOPDCM-UHFFFAOYSA-N2-ethylhexyl octadecanoateChemical compoundCCCCCCCCCCCCCCCCCC(=O)OCC(CC)CCCCOPJWPPVYCOPDCM-UHFFFAOYSA-N0.000description2
- FTLLYZOWBWEERE-UHFFFAOYSA-N2-phenoxyethyl octanoateChemical compoundCCCCCCCC(=O)OCCOC1=CC=CC=C1FTLLYZOWBWEERE-UHFFFAOYSA-N0.000description2
- BVKZGUZCCUSVTD-UHFFFAOYSA-LCarbonateChemical compound[O-]C([O-])=OBVKZGUZCCUSVTD-UHFFFAOYSA-L0.000description2
- HEDRZPFGACZZDS-UHFFFAOYSA-NChloroformChemical compoundClC(Cl)ClHEDRZPFGACZZDS-UHFFFAOYSA-N0.000description2
- VZCYOOQTPOCHFL-OWOJBTEDSA-NFumaric acidChemical compoundOC(=O)\C=C\C(O)=OVZCYOOQTPOCHFL-OWOJBTEDSA-N0.000description2
- FYYHWMGAXLPEAU-UHFFFAOYSA-NMagnesiumChemical compound[Mg]FYYHWMGAXLPEAU-UHFFFAOYSA-N0.000description2
- MSPCIZMDDUQPGJ-UHFFFAOYSA-NN-methyl-N-(trimethylsilyl)trifluoroacetamideChemical compoundC[Si](C)(C)N(C)C(=O)C(F)(F)FMSPCIZMDDUQPGJ-UHFFFAOYSA-N0.000description2
- JUJWROOIHBZHMG-UHFFFAOYSA-NPyridineChemical compoundC1=CC=NC=C1JUJWROOIHBZHMG-UHFFFAOYSA-N0.000description2
- PMZURENOXWZQFD-UHFFFAOYSA-LSodium SulfateChemical compound[Na+].[Na+].[O-]S([O-])(=O)=OPMZURENOXWZQFD-UHFFFAOYSA-L0.000description2
- 229920002385Sodium hyaluronatePolymers0.000description2
- QAOWNCQODCNURD-UHFFFAOYSA-NSulfuric acidChemical compoundOS(O)(=O)=OQAOWNCQODCNURD-UHFFFAOYSA-N0.000description2
- HCHKCACWOHOZIP-UHFFFAOYSA-NZincChemical compound[Zn]HCHKCACWOHOZIP-UHFFFAOYSA-N0.000description2
- 235000011126aluminium potassium sulphateNutrition0.000description2
- DIZPMCHEQGEION-UHFFFAOYSA-Haluminium sulfate (anhydrous)Chemical compound[Al+3].[Al+3].[O-]S([O-])(=O)=O.[O-]S([O-])(=O)=O.[O-]S([O-])(=O)=ODIZPMCHEQGEION-UHFFFAOYSA-H0.000description2
- VSCWAEJMTAWNJL-UHFFFAOYSA-Kaluminium trichlorideChemical compoundCl[Al](Cl)ClVSCWAEJMTAWNJL-UHFFFAOYSA-K0.000description2
- CEGOLXSVJUTHNZ-UHFFFAOYSA-Kaluminium tristearateChemical compound[Al+3].CCCCCCCCCCCCCCCCCC([O-])=O.CCCCCCCCCCCCCCCCCC([O-])=O.CCCCCCCCCCCCCCCCCC([O-])=OCEGOLXSVJUTHNZ-UHFFFAOYSA-K0.000description2
- SNAAJJQQZSMGQD-UHFFFAOYSA-Naluminum magnesiumChemical compound[Mg].[Al]SNAAJJQQZSMGQD-UHFFFAOYSA-N0.000description2
- YXZZLAMCXFHTTE-UHFFFAOYSA-Naluminum;propane-1,2-diol;trihypochlorite;hydrateChemical compoundO.[Al+3].Cl[O-].Cl[O-].Cl[O-].CC(O)COYXZZLAMCXFHTTE-UHFFFAOYSA-N0.000description2
- HSMXEPWDIJUMSS-UHFFFAOYSA-Kaluminum;tetradecanoateChemical compound[Al+3].CCCCCCCCCCCCCC([O-])=O.CCCCCCCCCCCCCC([O-])=O.CCCCCCCCCCCCCC([O-])=OHSMXEPWDIJUMSS-UHFFFAOYSA-K0.000description2
- TZCXTZWJZNENPQ-UHFFFAOYSA-Lbarium sulfateChemical compound[Ba+2].[O-]S([O-])(=O)=OTZCXTZWJZNENPQ-UHFFFAOYSA-L0.000description2
- XVAMCHGMPYWHNL-UHFFFAOYSA-NbemotrizinolChemical compoundOC1=CC(OCC(CC)CCCC)=CC=C1C1=NC(C=2C=CC(OC)=CC=2)=NC(C=2C(=CC(OCC(CC)CCCC)=CC=2)O)=N1XVAMCHGMPYWHNL-UHFFFAOYSA-N0.000description2
- 239000012159carrier gasSubstances0.000description2
- 239000001913celluloseSubstances0.000description2
- 229920002678cellulosePolymers0.000description2
- 238000006243chemical reactionMethods0.000description2
- 238000001816coolingMethods0.000description2
- CVSVTCORWBXHQV-UHFFFAOYSA-NcreatineChemical compoundNC(=[NH2+])N(C)CC([O-])=OCVSVTCORWBXHQV-UHFFFAOYSA-N0.000description2
- 229920006037cross link polymerPolymers0.000description2
- 125000004122cyclic groupChemical group0.000description2
- JAONJTDQXUSBGG-UHFFFAOYSA-Ndialuminum;dizinc;oxygen(2-)Chemical compound[O-2].[O-2].[O-2].[O-2].[O-2].[Al+3].[Al+3].[Zn+2].[Zn+2]JAONJTDQXUSBGG-UHFFFAOYSA-N0.000description2
- 229940105990diglycerinDrugs0.000description2
- POULHZVOKOAJMA-UHFFFAOYSA-Ndodecanoic acidChemical classCCCCCCCCCCCC(O)=OPOULHZVOKOAJMA-UHFFFAOYSA-N0.000description2
- 235000019387fatty acid methyl esterNutrition0.000description2
- 239000001307heliumSubstances0.000description2
- 229910052734heliumInorganic materials0.000description2
- SWQJXJOGLNCZEY-UHFFFAOYSA-Nhelium atomChemical compound[He]SWQJXJOGLNCZEY-UHFFFAOYSA-N0.000description2
- 239000001257hydrogenSubstances0.000description2
- 229910052739hydrogenInorganic materials0.000description2
- 125000004435hydrogen atomChemical class[H]*0.000description2
- 150000004679hydroxidesChemical class0.000description2
- 238000010813internal standard methodMethods0.000description2
- 229940039346isoamyl cocoateDrugs0.000description2
- JVTAAEKCZFNVCJ-UHFFFAOYSA-Nlactic acidChemical classCC(O)C(O)=OJVTAAEKCZFNVCJ-UHFFFAOYSA-N0.000description2
- 239000011777magnesiumSubstances0.000description2
- 229910052749magnesiumInorganic materials0.000description2
- WWZKQHOCKIZLMA-UHFFFAOYSA-MoctanoateChemical compoundCCCCCCCC([O-])=OWWZKQHOCKIZLMA-UHFFFAOYSA-M0.000description2
- 229920000058polyacrylatePolymers0.000description2
- GRLPQNLYRHEGIJ-UHFFFAOYSA-Jpotassium aluminium sulfateChemical compound[Al+3].[K+].[O-]S([O-])(=O)=O.[O-]S([O-])(=O)=OGRLPQNLYRHEGIJ-UHFFFAOYSA-J0.000description2
- 239000003755preservative agentSubstances0.000description2
- 229940010747sodium hyaluronateDrugs0.000description2
- YWIVKILSMZOHHF-QJZPQSOGSA-Nsodium;(2s,3s,4s,5r,6r)-6-[(2s,3r,4r,5s,6r)-3-acetamido-2-[(2s,3s,4r,5r,6r)-6-[(2r,3r,4r,5s,6r)-3-acetamido-2,5-dihydroxy-6-(hydroxymethyl)oxan-4-yl]oxy-2-carboxy-4,5-dihydroxyoxan-3-yl]oxy-5-hydroxy-6-(hydroxymethyl)oxan-4-yl]oxy-3,4,5-trihydroxyoxane-2-Chemical compound[Na+].CC(=O)N[C@H]1[C@H](O)O[C@H](CO)[C@@H](O)[C@@H]1O[C@H]1[C@H](O)[C@@H](O)[C@H](O[C@H]2[C@@H]([C@@H](O[C@H]3[C@@H]([C@@H](O)[C@H](O)[C@H](O3)C(O)=O)O)[C@H](O)[C@@H](CO)O2)NC(C)=O)[C@@H](C(O)=O)O1YWIVKILSMZOHHF-QJZPQSOGSA-N0.000description2
- 239000006228supernatantSubstances0.000description2
- 239000000725suspensionSubstances0.000description2
- 238000012360testing methodMethods0.000description2
- VZCYOOQTPOCHFL-UHFFFAOYSA-Ntrans-butenedioic acidNatural productsOC(=O)C=CC(O)=OVZCYOOQTPOCHFL-UHFFFAOYSA-N0.000description2
- 229910052725zincInorganic materials0.000description2
- 239000011701zincSubstances0.000description2
- PUPZLCDOIYMWBV-UHFFFAOYSA-N(+/-)-1,3-ButanediolChemical compoundCC(O)CCOPUPZLCDOIYMWBV-UHFFFAOYSA-N0.000description1
- KIUKXJAPPMFGSW-DNGZLQJQSA-N(2S,3S,4S,5R,6R)-6-[(2S,3R,4R,5S,6R)-3-Acetamido-2-[(2S,3S,4R,5R,6R)-6-[(2R,3R,4R,5S,6R)-3-acetamido-2,5-dihydroxy-6-(hydroxymethyl)oxan-4-yl]oxy-2-carboxy-4,5-dihydroxyoxan-3-yl]oxy-5-hydroxy-6-(hydroxymethyl)oxan-4-yl]oxy-3,4,5-trihydroxyoxane-2-carboxylic acidChemical compoundCC(=O)N[C@H]1[C@H](O)O[C@H](CO)[C@@H](O)[C@@H]1O[C@H]1[C@H](O)[C@@H](O)[C@H](O[C@H]2[C@@H]([C@@H](O[C@H]3[C@@H]([C@@H](O)[C@H](O)[C@H](O3)C(O)=O)O)[C@H](O)[C@@H](CO)O2)NC(C)=O)[C@@H](C(O)=O)O1KIUKXJAPPMFGSW-DNGZLQJQSA-N0.000description1
- OMDQUFIYNPYJFM-XKDAHURESA-N(2r,3r,4s,5r,6s)-2-(hydroxymethyl)-6-[[(2r,3s,4r,5s,6r)-4,5,6-trihydroxy-3-[(2s,3s,4s,5s,6r)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxyoxan-2-yl]methoxy]oxane-3,4,5-triolChemical groupO[C@@H]1[C@@H](O)[C@@H](O)[C@@H](CO)O[C@@H]1OC[C@@H]1[C@@H](O[C@H]2[C@H]([C@@H](O)[C@H](O)[C@@H](CO)O2)O)[C@H](O)[C@H](O)[C@H](O)O1OMDQUFIYNPYJFM-XKDAHURESA-N0.000description1
- UMPNFVHHMOSNAC-WLDMJGECSA-N(3r,4s,5s,6r)-6-(hydroxymethyl)-3-methoxyoxane-2,4,5-triolChemical compoundCO[C@H]1C(O)O[C@H](CO)[C@@H](O)[C@@H]1OUMPNFVHHMOSNAC-WLDMJGECSA-N0.000description1
- OKMWKBLSFKFYGZ-UHFFFAOYSA-N1-behenoylglycerolChemical compoundCCCCCCCCCCCCCCCCCCCCCC(=O)OCC(O)COOKMWKBLSFKFYGZ-UHFFFAOYSA-N0.000description1
- PHSXXZQAGYUASL-UHFFFAOYSA-N2,3-dihydroxypropyl 16-methylheptadecyl hydrogen phosphateChemical compoundCC(C)CCCCCCCCCCCCCCCOP(O)(=O)OCC(O)COPHSXXZQAGYUASL-UHFFFAOYSA-N0.000description1
- TYYHDKOVFSVWON-UHFFFAOYSA-N2-butyl-2-methoxy-1,3-diphenylpropane-1,3-dioneChemical compoundC=1C=CC=CC=1C(=O)C(OC)(CCCC)C(=O)C1=CC=CC=C1TYYHDKOVFSVWON-UHFFFAOYSA-N0.000description1
- OSCJHTSDLYVCQC-UHFFFAOYSA-N2-ethylhexyl 4-[[4-[4-(tert-butylcarbamoyl)anilino]-6-[4-(2-ethylhexoxycarbonyl)anilino]-1,3,5-triazin-2-yl]amino]benzoateChemical compoundC1=CC(C(=O)OCC(CC)CCCC)=CC=C1NC1=NC(NC=2C=CC(=CC=2)C(=O)NC(C)(C)C)=NC(NC=2C=CC(=CC=2)C(=O)OCC(CC)CCCC)=N1OSCJHTSDLYVCQC-UHFFFAOYSA-N0.000description1
- 2299401005552-methyl-4-isothiazolin-3-oneDrugs0.000description1
- QCDWFXQBSFUVSP-UHFFFAOYSA-N2-phenoxyethanolChemical compoundOCCOC1=CC=CC=C1QCDWFXQBSFUVSP-UHFFFAOYSA-N0.000description1
- ANZUDYZHSVGBRF-UHFFFAOYSA-N3-ethylnonane-1,2,3-triolChemical compoundCCCCCCC(O)(CC)C(O)COANZUDYZHSVGBRF-UHFFFAOYSA-N0.000description1
- HIQIXEFWDLTDED-UHFFFAOYSA-N4-hydroxy-1-piperidin-4-ylpyrrolidin-2-oneChemical compoundO=C1CC(O)CN1C1CCNCC1HIQIXEFWDLTDED-UHFFFAOYSA-N0.000description1
- YCOKXGSMJCJMQZ-AMAPTOPXSA-K5-O-bis[[(4S)-4-amino-5-octadecanoyloxy-5-oxopentanoyl]oxy]alumanyl 1-O-octadecanoyl (2S)-2-aminopentanedioateChemical compoundN[C@@H](CCC(=O)[O-])C(=O)OC(CCCCCCCCCCCCCCCCC)=O.[Al+3].C(CCCCCCCCCCCCCCCCC)(=O)OC([C@@H](N)CCC(=O)[O-])=O.C(CCCCCCCCCCCCCCCCC)(=O)OC([C@@H](N)CCC(=O)[O-])=OYCOKXGSMJCJMQZ-AMAPTOPXSA-K0.000description1
- CWSZBVAUYPTXTG-UHFFFAOYSA-N5-[6-[[3,4-dihydroxy-6-(hydroxymethyl)-5-methoxyoxan-2-yl]oxymethyl]-3,4-dihydroxy-5-[4-hydroxy-3-(2-hydroxyethoxy)-6-(hydroxymethyl)-5-methoxyoxan-2-yl]oxyoxan-2-yl]oxy-6-(hydroxymethyl)-2-methyloxane-3,4-diolChemical compoundO1C(CO)C(OC)C(O)C(O)C1OCC1C(OC2C(C(O)C(OC)C(CO)O2)OCCO)C(O)C(O)C(OC2C(OC(C)C(O)C2O)CO)O1CWSZBVAUYPTXTG-UHFFFAOYSA-N0.000description1
- ZUGAOYSWHHGDJY-UHFFFAOYSA-K5-hydroxy-2,8,9-trioxa-1-aluminabicyclo[3.3.2]decane-3,7,10-trioneChemical compound[Al+3].[O-]C(=O)CC(O)(CC([O-])=O)C([O-])=OZUGAOYSWHHGDJY-UHFFFAOYSA-K0.000description1
- WZUKKIPWIPZMAS-UHFFFAOYSA-KAmmonium alumChemical compound[NH4+].O.O.O.O.O.O.O.O.O.O.O.O.[Al+3].[O-]S([O-])(=O)=O.[O-]S([O-])(=O)=OWZUKKIPWIPZMAS-UHFFFAOYSA-K0.000description1
- OYPRJOBELJOOCE-UHFFFAOYSA-NCalciumChemical compound[Ca]OYPRJOBELJOOCE-UHFFFAOYSA-N0.000description1
- OKTJSMMVPCPJKN-UHFFFAOYSA-NCarbonChemical group[C]OKTJSMMVPCPJKN-UHFFFAOYSA-N0.000description1
- KRKNYBCHXYNGOX-UHFFFAOYSA-KCitrateChemical compound[O-]C(=O)CC(O)(CC([O-])=O)C([O-])=OKRKNYBCHXYNGOX-UHFFFAOYSA-K0.000description1
- 102000008186CollagenHuman genes0.000description1
- 108010035532CollagenProteins0.000description1
- FEWJPZIEWOKRBE-JCYAYHJZSA-NDextrotartaric acidChemical compoundOC(=O)[C@H](O)[C@@H](O)C(O)=OFEWJPZIEWOKRBE-JCYAYHJZSA-N0.000description1
- 240000002989Euphorbia neriifoliaSpecies0.000description1
- 229920000926GalactomannanPolymers0.000description1
- DHMQDGOQFOQNFH-UHFFFAOYSA-NGlycineChemical compoundNCC(O)=ODHMQDGOQFOQNFH-UHFFFAOYSA-N0.000description1
- NHTMVDHEPJAVLT-UHFFFAOYSA-NIsooctaneChemical compoundCC(C)CC(C)(C)CNHTMVDHEPJAVLT-UHFFFAOYSA-N0.000description1
- WHUUTDBJXJRKMK-VKHMYHEASA-NL-glutamic acidChemical compoundOC(=O)[C@@H](N)CCC(O)=OWHUUTDBJXJRKMK-VKHMYHEASA-N0.000description1
- 235000017011Mandorlo dulceNutrition0.000description1
- 244000076313Mandorlo dulceSpecies0.000description1
- KTHIZMKNWOPUAW-UHFFFAOYSA-NN.[Al+3].[Zn+2].[Ag+]Chemical compoundN.[Al+3].[Zn+2].[Ag+]KTHIZMKNWOPUAW-UHFFFAOYSA-N0.000description1
- WXOMTJVVIMOXJL-BOBFKVMVSA-AO.O.O.O.O.O.O.O.O.O.O.O.O.O.O.O.O.O.O.O.O.O.O[Al](O)O.O[Al](O)O.O[Al](O)O.O[Al](O)O.O[Al](O)O.O[Al](O)O.O[Al](O)O.O[Al](O)O.O[Al](O)OS(=O)(=O)OC[C@H]1O[C@@H](O[C@]2(COS(=O)(=O)O[Al](O)O)O[C@H](OS(=O)(=O)O[Al](O)O)[C@@H](OS(=O)(=O)O[Al](O)O)[C@@H]2OS(=O)(=O)O[Al](O)O)[C@H](OS(=O)(=O)O[Al](O)O)[C@@H](OS(=O)(=O)O[Al](O)O)[C@@H]1OS(=O)(=O)O[Al](O)OChemical compoundO.O.O.O.O.O.O.O.O.O.O.O.O.O.O.O.O.O.O.O.O.O.O[Al](O)O.O[Al](O)O.O[Al](O)O.O[Al](O)O.O[Al](O)O.O[Al](O)O.O[Al](O)O.O[Al](O)O.O[Al](O)OS(=O)(=O)OC[C@H]1O[C@@H](O[C@]2(COS(=O)(=O)O[Al](O)O)O[C@H](OS(=O)(=O)O[Al](O)O)[C@@H](OS(=O)(=O)O[Al](O)O)[C@@H]2OS(=O)(=O)O[Al](O)O)[C@H](OS(=O)(=O)O[Al](O)O)[C@@H](OS(=O)(=O)O[Al](O)O)[C@@H]1OS(=O)(=O)O[Al](O)OWXOMTJVVIMOXJL-BOBFKVMVSA-A0.000description1
- OFOBLEOULBTSOW-UHFFFAOYSA-NPropanedioic acidNatural productsOC(=O)CC(O)=OOFOBLEOULBTSOW-UHFFFAOYSA-N0.000description1
- KDYFGRWQOYBRFD-UHFFFAOYSA-NSuccinic acidChemical classOC(=O)CCC(O)=OKDYFGRWQOYBRFD-UHFFFAOYSA-N0.000description1
- 239000004904UV filterSubstances0.000description1
- QCWXUUIWCKQGHC-UHFFFAOYSA-NZirconiumChemical compound[Zr]QCWXUUIWCKQGHC-UHFFFAOYSA-N0.000description1
- ULGYAEQHFNJYML-UHFFFAOYSA-N[AlH3].[Ca]Chemical compound[AlH3].[Ca]ULGYAEQHFNJYML-UHFFFAOYSA-N0.000description1
- ZXRRHFSTAFVGOC-UHFFFAOYSA-N[AlH3].[K]Chemical compound[AlH3].[K]ZXRRHFSTAFVGOC-UHFFFAOYSA-N0.000description1
- HEAFLBOWLRRIHV-UHFFFAOYSA-N[Na].[P]Chemical compound[Na].[P]HEAFLBOWLRRIHV-UHFFFAOYSA-N0.000description1
- 235000011054acetic acidNutrition0.000description1
- WETWJCDKMRHUPV-UHFFFAOYSA-Nacetyl chlorideChemical compoundCC(Cl)=OWETWJCDKMRHUPV-UHFFFAOYSA-N0.000description1
- 239000012346acetyl chlorideSubstances0.000description1
- HDYRYUINDGQKMC-UHFFFAOYSA-Macetyloxyaluminum;dihydrateChemical compoundO.O.CC(=O)O[Al]HDYRYUINDGQKMC-UHFFFAOYSA-M0.000description1
- 150000007513acidsChemical class0.000description1
- 150000001252acrylic acid derivativesChemical class0.000description1
- 239000013543active substanceSubstances0.000description1
- 150000001298alcoholsChemical class0.000description1
- 238000005904alkaline hydrolysis reactionMethods0.000description1
- 125000005250alkyl acrylate groupChemical group0.000description1
- 125000000217alkyl groupChemical group0.000description1
- 229940037003alumDrugs0.000description1
- 235000011124aluminium ammonium sulphateNutrition0.000description1
- MJWPFSQVORELDX-UHFFFAOYSA-Kaluminium formateChemical compound[Al+3].[O-]C=O.[O-]C=O.[O-]C=OMJWPFSQVORELDX-UHFFFAOYSA-K0.000description1
- BWZOPYPOZJBVLQ-UHFFFAOYSA-Kaluminium glycinateChemical compoundO[Al+]O.NCC([O-])=OBWZOPYPOZJBVLQ-UHFFFAOYSA-K0.000description1
- WNROFYMDJYEPJX-UHFFFAOYSA-Kaluminium hydroxideChemical compound[OH-].[OH-].[OH-].[Al+3]WNROFYMDJYEPJX-UHFFFAOYSA-K0.000description1
- SMZOGRDCAXLAAR-UHFFFAOYSA-Naluminium isopropoxideChemical compound[Al+3].CC(C)[O-].CC(C)[O-].CC(C)[O-]SMZOGRDCAXLAAR-UHFFFAOYSA-N0.000description1
- 229940009827aluminum acetateDrugs0.000description1
- YCLAMANSVUJYPT-UHFFFAOYSA-Laluminum chloride hydroxide hydrateChemical compoundO.[OH-].[Al+3].[Cl-]YCLAMANSVUJYPT-UHFFFAOYSA-L0.000description1
- 229940051182aluminum dimyristateDrugs0.000description1
- 229940083916aluminum distearateDrugs0.000description1
- 229940024548aluminum oxideDrugs0.000description1
- 229940053431aluminum sesquichlorohydrateDrugs0.000description1
- 229940099583aluminum starch octenylsuccinateDrugs0.000description1
- 229940063655aluminum stearateDrugs0.000description1
- 229940071540aluminum zirconium octachlorohydrex glyDrugs0.000description1
- 229940048506aluminum zirconium tetrachlorohydrex glyDrugs0.000description1
- 229940072028aluminum zirconium trichlorohydrex glyDrugs0.000description1
- JECUDTNJDOAEOR-UHFFFAOYSA-Kaluminum;16-methylheptadecanoateChemical compound[Al+3].CC(C)CCCCCCCCCCCCCCC([O-])=O.CC(C)CCCCCCCCCCCCCCC([O-])=O.CC(C)CCCCCCCCCCCCCCC([O-])=OJECUDTNJDOAEOR-UHFFFAOYSA-K0.000description1
- OYGSFRHDRNJHBP-UHFFFAOYSA-Kaluminum;5-oxopyrrolidine-2-carboxylateChemical compound[Al+3].[O-]C(=O)C1CCC(=O)N1.[O-]C(=O)C1CCC(=O)N1.[O-]C(=O)C1CCC(=O)N1OYGSFRHDRNJHBP-UHFFFAOYSA-K0.000description1
- QPLNUHHRGZVCLQ-UHFFFAOYSA-Kaluminum;[oxido(phosphonooxy)phosphoryl] phosphateChemical compound[Al+3].OP([O-])(=O)OP([O-])(=O)OP(O)([O-])=OQPLNUHHRGZVCLQ-UHFFFAOYSA-K0.000description1
- GCVCIVDNHNBFMS-UHFFFAOYSA-Kaluminum;benzenesulfonic acid;trihydroxideChemical compound[OH-].[OH-].[OH-].[Al+3].OS(=O)(=O)C1=CC=CC=C1.OS(=O)(=O)C1=CC=CC=C1.OS(=O)(=O)C1=CC=CC=C1GCVCIVDNHNBFMS-UHFFFAOYSA-K0.000description1
- IDZYHGDLWGVHQM-UHFFFAOYSA-Naluminum;calcium;sodium;silicateChemical compound[Na+].[Al+3].[Ca+2].[O-][Si]([O-])([O-])[O-]IDZYHGDLWGVHQM-UHFFFAOYSA-N0.000description1
- HQQUTGFAWJNQIP-UHFFFAOYSA-Kaluminum;diacetate;hydroxideChemical compoundCC(=O)O[Al](O)OC(C)=OHQQUTGFAWJNQIP-UHFFFAOYSA-K0.000description1
- IIALMOLUUQESHG-UHFFFAOYSA-Kaluminum;dihexadecyl phosphateChemical compound[Al+3].CCCCCCCCCCCCCCCCOP([O-])(=O)OCCCCCCCCCCCCCCCC.CCCCCCCCCCCCCCCCOP([O-])(=O)OCCCCCCCCCCCCCCCC.CCCCCCCCCCCCCCCCOP([O-])(=O)OCCCCCCCCCCCCCCCCIIALMOLUUQESHG-UHFFFAOYSA-K0.000description1
- BBMXVTPBLPQMAE-UHFFFAOYSA-Kaluminum;docosanoateChemical compound[Al+3].CCCCCCCCCCCCCCCCCCCCCC([O-])=O.CCCCCCCCCCCCCCCCCCCCCC([O-])=O.CCCCCCCCCCCCCCCCCCCCCC([O-])=OBBMXVTPBLPQMAE-UHFFFAOYSA-K0.000description1
- XNLYYQDZUNCJRA-UHFFFAOYSA-Naluminum;hypobromous acidChemical compound[Al].BrOXNLYYQDZUNCJRA-UHFFFAOYSA-N0.000description1
- UAYUMUJFJMGOAL-UHFFFAOYSA-Haluminum;magnesium;silver;diphosphateChemical compound[Mg+2].[Al+3].[Ag+].[O-]P([O-])([O-])=O.[O-]P([O-])([O-])=OUAYUMUJFJMGOAL-UHFFFAOYSA-H0.000description1
- RDIVANOKKPKCTO-UHFFFAOYSA-Kaluminum;octadecanoate;hydroxideChemical compound[OH-].[Al+3].CCCCCCCCCCCCCCCCCC([O-])=O.CCCCCCCCCCCCCCCCCC([O-])=ORDIVANOKKPKCTO-UHFFFAOYSA-K0.000description1
- SJXYSRSHDPPYIU-UHFFFAOYSA-Laluminum;propane-1,2-diol;chloride;hydroxide;hydrateChemical compoundO.[OH-].[Al+3].[Cl-].CC(O)COSJXYSRSHDPPYIU-UHFFFAOYSA-L0.000description1
- YPPRKFXMPDSDJV-UHFFFAOYSA-Jaluminum;sodium;2-hydroxypropanoateChemical compound[Na+].[Al+3].CC(O)C([O-])=O.CC(O)C([O-])=O.CC(O)C([O-])=O.CC(O)C([O-])=OYPPRKFXMPDSDJV-UHFFFAOYSA-J0.000description1
- YAKZEVHORUHNLS-UHFFFAOYSA-Kaluminum;sodium;2-hydroxypropanoate;chloride;hydroxide;hydrateChemical compoundO.[OH-].[Na+].[Al+3].[Cl-].CC(O)C([O-])=OYAKZEVHORUHNLS-UHFFFAOYSA-K0.000description1
- ZEMWIYASLJTEHQ-UHFFFAOYSA-Jaluminum;sodium;disulfate;dodecahydrateChemical compoundO.O.O.O.O.O.O.O.O.O.O.O.[Na+].[Al+3].[O-]S([O-])(=O)=O.[O-]S([O-])(=O)=OZEMWIYASLJTEHQ-UHFFFAOYSA-J0.000description1
- YNQRLDQNSBVJGU-UHFFFAOYSA-Kaluminum;tetradecanoate;hydroxideChemical compound[OH-].[Al+3].CCCCCCCCCCCCCC([O-])=O.CCCCCCCCCCCCCC([O-])=OYNQRLDQNSBVJGU-UHFFFAOYSA-K0.000description1
- CSJKPFQJIDMSGF-UHFFFAOYSA-Kaluminum;tribenzoateChemical compound[Al+3].[O-]C(=O)C1=CC=CC=C1.[O-]C(=O)C1=CC=CC=C1.[O-]C(=O)C1=CC=CC=C1CSJKPFQJIDMSGF-UHFFFAOYSA-K0.000description1
- 239000004599antimicrobialSubstances0.000description1
- 239000007900aqueous suspensionSubstances0.000description1
- 229960005193avobenzoneDrugs0.000description1
- RQPZNWPYLFFXCP-UHFFFAOYSA-Lbarium dihydroxideChemical compound[OH-].[OH-].[Ba+2]RQPZNWPYLFFXCP-UHFFFAOYSA-L0.000description1
- 229910001863barium hydroxideInorganic materials0.000description1
- 229960004101bemotrizinolDrugs0.000description1
- 238000009835boilingMethods0.000description1
- KDYFGRWQOYBRFD-NUQCWPJISA-Nbutanedioic acidChemical classO[14C](=O)CC[14C](O)=OKDYFGRWQOYBRFD-NUQCWPJISA-N0.000description1
- 229910052791calciumInorganic materials0.000description1
- 238000005119centrifugationMethods0.000description1
- 229940048851cetyl ricinoleateDrugs0.000description1
- 229940071160cocoateDrugs0.000description1
- 229920001436collagenPolymers0.000description1
- 230000000052comparative effectEffects0.000description1
- 239000006071creamSubstances0.000description1
- 229960003624creatineDrugs0.000description1
- 239000006046creatineSubstances0.000description1
- 239000013078crystalSubstances0.000description1
- GHVNFZFCNZKVNT-UHFFFAOYSA-MdecanoateChemical compoundCCCCCCCCCC([O-])=OGHVNFZFCNZKVNT-UHFFFAOYSA-M0.000description1
- 125000002704decyl groupChemical group[H]C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])*0.000description1
- VAROLYSFQDGFMV-UHFFFAOYSA-Kdi(octanoyloxy)alumanyl octanoateChemical compound[Al+3].CCCCCCCC([O-])=O.CCCCCCCC([O-])=O.CCCCCCCC([O-])=OVAROLYSFQDGFMV-UHFFFAOYSA-K0.000description1
- AHEUTCASMSZYAZ-UHFFFAOYSA-Hdialuminum 10-[2-(7-carboxylatoheptyl)-5,6-dihexylcyclohex-3-en-1-yl]dec-9-enoateChemical compound[Al+3].[Al+3].CCCCCCC1C=CC(CCCCCCCC([O-])=O)C(C=CCCCCCCCC([O-])=O)C1CCCCCC.CCCCCCC1C=CC(CCCCCCCC([O-])=O)C(C=CCCCCCCCC([O-])=O)C1CCCCCC.CCCCCCC1C=CC(CCCCCCCC([O-])=O)C(C=CCCCCCCCC([O-])=O)C1CCCCCCAHEUTCASMSZYAZ-UHFFFAOYSA-H0.000description1
- LTXHKPDRHPMBKA-UHFFFAOYSA-Ndialuminum;cobalt(2+);oxygen(2-)Chemical compound[O-2].[O-2].[O-2].[O-2].[Al+3].[Al+3].[Co+2]LTXHKPDRHPMBKA-UHFFFAOYSA-N0.000description1
- XILPPDQAWPSZIL-UHFFFAOYSA-Hdialuminum;dichloride;tetrahydroxideChemical compound[OH-].[OH-].[OH-].[OH-].[Al+3].[Al+3].[Cl-].[Cl-]XILPPDQAWPSZIL-UHFFFAOYSA-H0.000description1
- JYIMWRSJCRRYNK-UHFFFAOYSA-Ndialuminum;disodium;oxygen(2-);silicon(4+);hydrateChemical compoundO.[O-2].[O-2].[O-2].[O-2].[O-2].[O-2].[Na+].[Na+].[Al+3].[Al+3].[Si+4]JYIMWRSJCRRYNK-UHFFFAOYSA-N0.000description1
- UAMZXLIURMNTHD-UHFFFAOYSA-Ndialuminum;magnesium;oxygen(2-)Chemical compound[O-2].[O-2].[O-2].[O-2].[Mg+2].[Al+3].[Al+3]UAMZXLIURMNTHD-UHFFFAOYSA-N0.000description1
- NMTQHSJCFMAVNA-UHFFFAOYSA-Hdialuminum;methanedisulfonateChemical compoundO1S(=O)(=O)CS(=O)(=O)O[Al]2OS(=O)(=O)CS(=O)(=O)O[Al]1OS(=O)(=O)CS(=O)(=O)O2NMTQHSJCFMAVNA-UHFFFAOYSA-H0.000description1
- KNXDJTLIRRQLBE-UHFFFAOYSA-Hdialuminum;propane-1,2-diol;chloride;pentahydroxide;hydrateChemical compoundO.[OH-].[OH-].[OH-].[OH-].[OH-].[Al+3].[Al+3].[Cl-].CC(O)COKNXDJTLIRRQLBE-UHFFFAOYSA-H0.000description1
- 230000004069differentiationEffects0.000description1
- JVSWJIKNEAIKJW-UHFFFAOYSA-Ndimethyl-hexaneNatural productsCCCCCC(C)CJVSWJIKNEAIKJW-UHFFFAOYSA-N0.000description1
- 238000009826distributionMethods0.000description1
- 230000000694effectsEffects0.000description1
- UVCJGUGAGLDPAA-UHFFFAOYSA-NensulizoleChemical compoundN1C2=CC(S(=O)(=O)O)=CC=C2N=C1C1=CC=CC=C1UVCJGUGAGLDPAA-UHFFFAOYSA-N0.000description1
- 229960000655ensulizoleDrugs0.000description1
- 239000012259ether extractSubstances0.000description1
- 238000001704evaporationMethods0.000description1
- 230000008020evaporationEffects0.000description1
- 230000007717exclusionEffects0.000description1
- 239000000284extractSubstances0.000description1
- 238000000605extractionMethods0.000description1
- 239000001530fumaric acidSubstances0.000description1
- 235000011087fumaric acidNutrition0.000description1
- 238000004817gas chromatographyMethods0.000description1
- 238000000769gas chromatography-flame ionisation detectionMethods0.000description1
- 229930195712glutamateNatural products0.000description1
- 229940049906glutamateDrugs0.000description1
- 150000002314glycerolsChemical class0.000description1
- 229940081618glyceryl monobehenateDrugs0.000description1
- 229940075507glyceryl monostearateDrugs0.000description1
- 238000010438heat treatmentMethods0.000description1
- XAMHKORMKJIEFW-AYTKPMRMSA-Nhexadecyl (z,12r)-12-hydroxyoctadec-9-enoateChemical compoundCCCCCCCCCCCCCCCCOC(=O)CCCCCCC\C=C/C[C@H](O)CCCCCCXAMHKORMKJIEFW-AYTKPMRMSA-N0.000description1
- UQEAIHBTYFGYIE-UHFFFAOYSA-NhexamethyldisiloxaneChemical compoundC[Si](C)(C)O[Si](C)(C)CUQEAIHBTYFGYIE-UHFFFAOYSA-N0.000description1
- 238000000265homogenisationMethods0.000description1
- 229920002674hyaluronanPolymers0.000description1
- 229960003160hyaluronic acidDrugs0.000description1
- 235000014413iron hydroxideNutrition0.000description1
- NCNCGGDMXMBVIA-UHFFFAOYSA-Liron(ii) hydroxideChemical compound[OH-].[OH-].[Fe+2]NCNCGGDMXMBVIA-UHFFFAOYSA-L0.000description1
- 239000004310lactic acidChemical class0.000description1
- 235000014655lactic acidNutrition0.000description1
- 230000007774longtermEffects0.000description1
- 239000006210lotionSubstances0.000description1
- LKKQEPWPIQAZPX-UHFFFAOYSA-Lmagnesium;octadecanoate;hydroxideChemical compound[OH-].[Mg+2].CCCCCCCCCCCCCCCCCC([O-])=OLKKQEPWPIQAZPX-UHFFFAOYSA-L0.000description1
- VZCYOOQTPOCHFL-UPHRSURJSA-Nmaleic acidChemical compoundOC(=O)\C=C/C(O)=OVZCYOOQTPOCHFL-UPHRSURJSA-N0.000description1
- 239000011976maleic acidSubstances0.000description1
- 238000005259measurementMethods0.000description1
- 150000004702methyl estersChemical class0.000description1
- BEGLCMHJXHIJLR-UHFFFAOYSA-NmethylisothiazolinoneChemical compoundCN1SC=CC1=OBEGLCMHJXHIJLR-UHFFFAOYSA-N0.000description1
- 239000004530micro-emulsionSubstances0.000description1
- 238000012986modificationMethods0.000description1
- 230000004048modificationEffects0.000description1
- 230000003020moisturizing effectEffects0.000description1
- 239000001788mono and diglycerides of fatty acidsSubstances0.000description1
- 229940078812myristyl myristateDrugs0.000description1
- LNCDZGSESVBWGF-UHFFFAOYSA-Noctadecanoic acid;hydrateChemical compoundO.CCCCCCCCCCCCCCCCCC(O)=OLNCDZGSESVBWGF-UHFFFAOYSA-N0.000description1
- FMJSMJQBSVNSBF-UHFFFAOYSA-NoctocryleneChemical groupC=1C=CC=CC=1C(=C(C#N)C(=O)OCC(CC)CCCC)C1=CC=CC=C1FMJSMJQBSVNSBF-UHFFFAOYSA-N0.000description1
- 229960000601octocryleneDrugs0.000description1
- 150000007524organic acidsChemical class0.000description1
- 235000005985organic acidsNutrition0.000description1
- 235000006408oxalic acidNutrition0.000description1
- OTCVAHKKMMUFAY-UHFFFAOYSA-NoxosilverChemical class[Ag]=OOTCVAHKKMMUFAY-UHFFFAOYSA-N0.000description1
- 229960005323phenoxyethanolDrugs0.000description1
- 229940061570polyglyceryl-10 stearateDrugs0.000description1
- 229920000642polymerPolymers0.000description1
- 229940050271potassium alumDrugs0.000description1
- 238000002360preparation methodMethods0.000description1
- UMJSCPRVCHMLSP-UHFFFAOYSA-NpyridineNatural productsCOC1=CC=CN=C1UMJSCPRVCHMLSP-UHFFFAOYSA-N0.000description1
- 239000002994raw materialSubstances0.000description1
- 238000010992refluxMethods0.000description1
- WBHHMMIMDMUBKC-XLNAKTSKSA-Nricinelaidic acidChemical compoundCCCCCC[C@@H](O)C\C=C\CCCCCCCC(O)=OWBHHMMIMDMUBKC-XLNAKTSKSA-N0.000description1
- 229960003656ricinoleic acidDrugs0.000description1
- FEUQNCSVHBHROZ-UHFFFAOYSA-Nricinoleic acidNatural productsCCCCCCC(O[Si](C)(C)C)CC=CCCCCCCCC(=O)OCFEUQNCSVHBHROZ-UHFFFAOYSA-N0.000description1
- 150000003839saltsChemical class0.000description1
- 229940116351sebacateDrugs0.000description1
- CXMXRPHRNRROMY-UHFFFAOYSA-Lsebacate(2-)Chemical compound[O-]C(=O)CCCCCCCCC([O-])=OCXMXRPHRNRROMY-UHFFFAOYSA-L0.000description1
- 229910052710siliconInorganic materials0.000description1
- 239000010703siliconSubstances0.000description1
- 229910001923silver oxideInorganic materials0.000description1
- 229910001388sodium aluminateInorganic materials0.000description1
- 235000011127sodium aluminium sulphateNutrition0.000description1
- FRHNXUKHAUWMOQ-UHFFFAOYSA-Msodium;16-methylheptadecanoateChemical compound[Na+].CC(C)CCCCCCCCCCCCCCC([O-])=OFRHNXUKHAUWMOQ-UHFFFAOYSA-M0.000description1
- 230000000087stabilizing effectEffects0.000description1
- 238000003756stirringMethods0.000description1
- 239000004094surface-active agentSubstances0.000description1
- 239000003760tallowSubstances0.000description1
- TUNFSRHWOTWDNC-UHFFFAOYSA-Ntetradecanoic acidChemical classCCCCCCCCCCCCCC(O)=OTUNFSRHWOTWDNC-UHFFFAOYSA-N0.000description1
- DZKXJUASMGQEMA-UHFFFAOYSA-Ntetradecyl tetradecanoateChemical compoundCCCCCCCCCCCCCCOC(=O)CCCCCCCCCCCCCDZKXJUASMGQEMA-UHFFFAOYSA-N0.000description1
- 229940094879tetrapeptide-21Drugs0.000description1
- 239000002562thickening agentSubstances0.000description1
- 239000004408titanium dioxideSubstances0.000description1
- WOZZOSDBXABUFO-UHFFFAOYSA-Ntri(butan-2-yloxy)alumaneChemical compound[Al+3].CCC(C)[O-].CCC(C)[O-].CCC(C)[O-]WOZZOSDBXABUFO-UHFFFAOYSA-N0.000description1
- VXYADVIJALMOEQ-UHFFFAOYSA-Ktris(lactato)aluminiumChemical compoundCC(O)C(=O)O[Al](OC(=O)C(C)O)OC(=O)C(C)OVXYADVIJALMOEQ-UHFFFAOYSA-K0.000description1
- 229960000281trometamolDrugs0.000description1
- 229910052726zirconiumInorganic materials0.000description1
Images
Classifications
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K8/00—Cosmetics or similar toiletry preparations
- A61K8/18—Cosmetics or similar toiletry preparations characterised by the composition
- A61K8/72—Cosmetics or similar toiletry preparations characterised by the composition containing organic macromolecular compounds
- A61K8/84—Cosmetics or similar toiletry preparations characterised by the composition containing organic macromolecular compounds obtained by reactions otherwise than those involving only carbon-carbon unsaturated bonds
- A61K8/85—Polyesters
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K8/00—Cosmetics or similar toiletry preparations
- A61K8/02—Cosmetics or similar toiletry preparations characterised by special physical form
- A61K8/04—Dispersions; Emulsions
- A61K8/06—Emulsions
- A61K8/062—Oil-in-water emulsions
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K8/00—Cosmetics or similar toiletry preparations
- A61K8/18—Cosmetics or similar toiletry preparations characterised by the composition
- A61K8/19—Cosmetics or similar toiletry preparations characterised by the composition containing inorganic ingredients
- A61K8/26—Aluminium; Compounds thereof
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K8/00—Cosmetics or similar toiletry preparations
- A61K8/18—Cosmetics or similar toiletry preparations characterised by the composition
- A61K8/19—Cosmetics or similar toiletry preparations characterised by the composition containing inorganic ingredients
- A61K8/27—Zinc; Compounds thereof
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K8/00—Cosmetics or similar toiletry preparations
- A61K8/18—Cosmetics or similar toiletry preparations characterised by the composition
- A61K8/19—Cosmetics or similar toiletry preparations characterised by the composition containing inorganic ingredients
- A61K8/28—Zirconium; Compounds thereof
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K8/00—Cosmetics or similar toiletry preparations
- A61K8/18—Cosmetics or similar toiletry preparations characterised by the composition
- A61K8/30—Cosmetics or similar toiletry preparations characterised by the composition containing organic compounds
- A61K8/33—Cosmetics or similar toiletry preparations characterised by the composition containing organic compounds containing oxygen
- A61K8/36—Carboxylic acids; Salts or anhydrides thereof
- A61K8/361—Carboxylic acids having more than seven carbon atoms in an unbroken chain; Salts or anhydrides thereof
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K8/00—Cosmetics or similar toiletry preparations
- A61K8/18—Cosmetics or similar toiletry preparations characterised by the composition
- A61K8/30—Cosmetics or similar toiletry preparations characterised by the composition containing organic compounds
- A61K8/33—Cosmetics or similar toiletry preparations characterised by the composition containing organic compounds containing oxygen
- A61K8/36—Carboxylic acids; Salts or anhydrides thereof
- A61K8/365—Hydroxycarboxylic acids; Ketocarboxylic acids
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K8/00—Cosmetics or similar toiletry preparations
- A61K8/18—Cosmetics or similar toiletry preparations characterised by the composition
- A61K8/30—Cosmetics or similar toiletry preparations characterised by the composition containing organic compounds
- A61K8/33—Cosmetics or similar toiletry preparations characterised by the composition containing organic compounds containing oxygen
- A61K8/37—Esters of carboxylic acids
- A61K8/375—Esters of carboxylic acids the alcohol moiety containing more than one hydroxy group
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K8/00—Cosmetics or similar toiletry preparations
- A61K8/18—Cosmetics or similar toiletry preparations characterised by the composition
- A61K8/30—Cosmetics or similar toiletry preparations characterised by the composition containing organic compounds
- A61K8/33—Cosmetics or similar toiletry preparations characterised by the composition containing organic compounds containing oxygen
- A61K8/39—Derivatives containing from 2 to 10 oxyalkylene groups
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K8/00—Cosmetics or similar toiletry preparations
- A61K8/18—Cosmetics or similar toiletry preparations characterised by the composition
- A61K8/72—Cosmetics or similar toiletry preparations characterised by the composition containing organic macromolecular compounds
- A61K8/73—Polysaccharides
- A61K8/737—Galactomannans, e.g. guar; Derivatives thereof
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K8/00—Cosmetics or similar toiletry preparations
- A61K8/18—Cosmetics or similar toiletry preparations characterised by the composition
- A61K8/92—Oils, fats or waxes; Derivatives thereof, e.g. hydrogenation products thereof
- A61K8/922—Oils, fats or waxes; Derivatives thereof, e.g. hydrogenation products thereof of vegetable origin
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61Q—SPECIFIC USE OF COSMETICS OR SIMILAR TOILETRY PREPARATIONS
- A61Q1/00—Make-up preparations; Body powders; Preparations for removing make-up
- A61Q1/02—Preparations containing skin colorants, e.g. pigments
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61Q—SPECIFIC USE OF COSMETICS OR SIMILAR TOILETRY PREPARATIONS
- A61Q15/00—Anti-perspirants or body deodorants
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61Q—SPECIFIC USE OF COSMETICS OR SIMILAR TOILETRY PREPARATIONS
- A61Q17/00—Barrier preparations; Preparations brought into direct contact with the skin for affording protection against external influences, e.g. sunlight, X-rays or other harmful rays, corrosive materials, bacteria or insect stings
- A61Q17/04—Topical preparations for affording protection against sunlight or other radiation; Topical sun tanning preparations
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61Q—SPECIFIC USE OF COSMETICS OR SIMILAR TOILETRY PREPARATIONS
- A61Q19/00—Preparations for care of the skin
- A61Q19/007—Preparations for dry skin
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61Q—SPECIFIC USE OF COSMETICS OR SIMILAR TOILETRY PREPARATIONS
- A61Q19/00—Preparations for care of the skin
- A61Q19/04—Preparations for care of the skin for chemically tanning the skin
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61Q—SPECIFIC USE OF COSMETICS OR SIMILAR TOILETRY PREPARATIONS
- A61Q19/00—Preparations for care of the skin
- A61Q19/08—Anti-ageing preparations
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K2800/00—Properties of cosmetic compositions or active ingredients thereof or formulation aids used therein and process related aspects
- A61K2800/20—Chemical, physico-chemical or functional or structural properties of the composition as a whole
- A61K2800/30—Characterized by the absence of a particular group of ingredients
Definitions
- the inventionrelates to compositions containing polyglycerol esters and long-chain hydroxy-alkyl-modified guar.
- WO2015132053discloses a deodorant formulation comprising esters of a polyglycerol with stearic acid, palmitic acid and caprylic acid, and also hydroxyethyl cellulose.
- the object of the inventionwas to provide compositions which enable a low-viscosity and stable formulation of emulsion-stressing ingredients, such as AP / deodorant active ingredients.
- compositions described beloware able to achieve the object of the invention.
- the present inventiontherefore relates to compositions comprising certain polyglycerol esters and hydroxyalkyl-modified guar.
- An advantage of the present inventionis that the polyglycerol ester contained in the compositions according to the invention is based entirely on renewable raw materials.
- composition according to the inventionis suitable for the formulation of PEG-free emulsions, in particular low-viscosity PEG-free emulsions.
- composition according to the inventionis suitable for the formulation of emulsions which have a yield point.
- Another advantage of the present inventionis that the composition according to the invention is easy to handle due to its consistency.
- composition according to the inventionproduces a light skin feeling in formulations.
- Another advantage of the present inventionis that the use of the composition according to the invention imparts moisturizing properties to the formulations.
- polyglycerinin the context of the present invention is to be understood as meaning a polyglycerin which contains glycerin.
- the glycerol contentmust also be taken into account when calculating amounts, masses and the like.
- polyglycerolsare therefore mixtures of glycerol with at least one glycerol oligomer.
- Glycerol oligomersinclude all corresponding structures, e.g. to understand linear and cyclic connections.
- Suitable determination methods for determining the hydroxyl numberare in particular those according to DGF C-V 17 a (53), Ph. Eur. 2.5.3 Method A and DIN 53240.
- compositions according to the inventionare preferred in which the complete hydrolysis of the polyglycerol ester released polyglycerol has a mass ratio of glycerol to diglycerol of greater than 1, preferably greater than 1.2, particularly preferably greater than 1.4.
- the mass fraction of glycerin, diglycerin, triglycerin, tetraglycerin and the fatty acidscan be determined in the context of the present invention by two GC methods; these methods include the alkaline hydrolysis of the polyglycerol ester according to the invention, separation of the polyglycerol from the released acids and analysis of the fatty acids and of the glycerol oligomers (linear and cyclic).
- 0.5 g of the polyglycerol ester according to the inventionis boiled in 25 ml of an ethanolic 0.5 M KOH solution under reflux for 4 hours. Then 10 ml of water are added and the pH is adjusted to pH 2-3 with sulfuric acid. The carboxylic acids formed are separated off by extraction three times, each with a volume (20 ml) of petroleum ether.
- the combined extractsare concentrated to about 1 ml by evaporation.
- Suitable determination methods for determining the fatty acid distributionare in particular those according to DGF C VI 11a, DGF C-VI 10 a and GAT - ring test 7/99.
- a 0.5 ml aliquot of the petroleum ether extract obtained as described aboveis treated in a vessel with 1 ml of a mixture of acetyl chloride: methanol (1: 4) with the exclusion of atmospheric moisture at the boiling point for 30 minutes.
- the resulting fatty acid methyl estersare extracted twice with 5 ml each of iso-octane and analyzed by GC.
- the carboxylic acidsare separated as their methyl esters according to their carbon chain length and their mass fraction is determined using an internal standard method.
- the GC systemis calibrated by measuring fatty acid methyl ester mixtures of the fatty acids to be examined with a known composition. With this method, the total mass and the mass fractions of carboxylic acid (s) are obtained, which allows the amount of substance (s) to be determined by using the respective molecular weights.
- the mass of polyglycerol containedfor example, in 0.5 g of polyglycerol ester can also be determined by subtraction. The amount of substance of the polyglycerol can be determined therefrom by means of the molecular weight of the polyglycerol.
- m pmass of polyglycerol in 1 g of polyglycerol ester [g]
- M pmolecular weight of polyglycerol [g / mol]
- Glycerol, diglycerols, triglycerols and tetraglycerolsare separated and their mass fraction determined according to an internal standard method.
- the GC systemis calibrated by measuring mixtures of the glycerols to be examined and the internal standard with a known composition.
- the mass ratio of glycerin to diglycerolcan be determined, and by subtracting 100%, the content of polyglycerols with a degree of polymerization of 2 and greater (100% minus mass fraction of glycerol), the content of polyglycerols with a degree of polymerization of 3 and greater (100% minus mass fractions of glycerin and diglycerols), the content of polyglycerols with a degree of polymerization of 4 and greater (100% minus mass fractions of glycerin, diglycerols and triglycerols) and the content of polyglycerols with a degree of polymerization of 5 and greater ( 100% minus mass fractions of glycerol, diglycerols, triglycerols and tetraglycerols). If there is no detectable amount of diglycerin in a polyglycerol under consideration, but glycerol is present, this corresponds to a mass ratio of
- a composition preferred according to the inventionis characterized in that after its complete hydrolysis, the polyglycerol ester averages (number average) per mole of polyglycerol ester from 0.01 to 0.07 mol, preferably from 0.01 to 0.50 mol, particularly preferably from 0.01 to 0.30 mol, of at least one carboxylic acid a) from 0.10 to 1.70 mol, preferably from 0.30 to 1.50 mol, particularly preferably from 0.40 to 1.40 mol, of at least one carboxylic acid b) from 0.01 to 0.80 mol, preferably from 0.01 to 0.60 mol, particularly preferably from 0.05 to 0.40 mol, of at least one carboxylic acid c) releases.
- the molar ratio of the carboxylic acid a) to carboxylic acid b) to carboxylic acid c)obtained after complete hydrolysis of the polyglycerol ester 0.6 to 1.4: 16.5 to 20.5: 3.0 to 4.8, preferred 0.8 to 1.2: 17.5 to 19.5: 3.5 to 4.3, particularly preferred 0.9 to 1.1: 18.0 to 19.0: 3.7 to 4.1.
- the method described abovecan be used as a method to determine the molar ratios.
- the carboxylic acids a), b) and c)are selected from fatty acids; those are in particular selected from linear, saturated, unsubstituted carboxylic acids.
- compositions according to the inventionare preferred which are characterized in that the carboxylic acid a) is selected from caprylic acid and capric acid, the carboxylic acid b) is selected from stearic acid and palmitic acid and the carboxylic acid c) is behenic acid.
- Hydroxy-alkyl-modified guarhas been widely described and is commercially available, for example, as Esaflor HM 22. Guar is a galactomannan, into which long-term hydroxy alkyl modifications can be inserted simply by reaction with epoxyalkanes.
- Compositions preferred according to the inventioncontain hydroxy-alkyl-modified guar which is modified with alkyl groups having 16 to 24, preferably 18 to 22, carbon atoms. It is preferred according to the invention that the hydroxy-alkyl-modified guar is additionally hydroxypropyl-modified.
- the AP active ingredientis selected from the group comprising, preferably consisting of, aluminum salts and zirconium salts.
- Compositions according to the inventionare particularly preferred which are characterized in that the aluminum salt is selected from the group comprising, preferably consisting of: Aluminum Acetate, Aluminum Behenate, Aluminum Benzoate, Aluminum Bromohydrate, Aluminum Butoxide, Aluminum Calcium Sodium Silicate, Aluminum Caprylate, Aluminum Capryloyl Hydrolyzed Collagen, Aluminum Chloride, Aluminum Chlorohydrate, Aluminum Chlorohydrex, Aluminum Chlorohydrex PEG, Aluminum Chlorohydrex PG, Aluminum Citrate, Aluminum Diacetate, Aluminum Dibenzoate / Stearate Hydroxide, Aluminum Dicetyl Phosphate, Aluminum Dichlorohydrate, Aluminum Dichlorohydrex PEG, Aluminum Dichlorohydrex PG, Aluminum Dilinoleate, Aluminum Dimyristate, Aluminum Distearate, Aluminum Glycinate, Aluminum Hydrogenated Tallow Glutamate, Aluminum Hydroxy
- Suitable deodorant active ingredientsare selected from the group comprising, preferably consisting of the in EP2421614 deodorants and antiseptic agents and zinc salts.
- the zinc saltis preferably selected from the group of the zinc salts of acetic acid, lactic acid, oxalic acid, succinic acid, fumaric acid, maleic acid, ricinoleic acid and / or citric acid.
- compositions according to the inventionin particular stabilize emulsions, so that a composition preferred according to the invention is characterized in that it is an emulsion, in particular an oil-in-water emulsion.
- Emulsions preferred according to the inventioncontain, based on the total emulsion, from 0.1% by weight to 60.0% by weight, preferably from 4.0% by weight to 30.0% by weight, particularly preferably from 5.0% by weight .-% to 20.0 wt .-%, at least one oil, in particular a cosmetic oil. Suitable cosmetic oils are for example in DE10361202 listed.
- the emulsions according to the inventionbased on the total emulsion, from 0.1% by weight to 15.0% by weight, preferably from 0.5% by weight to 10.0% by weight, particularly preferably from 1.0% by weight to 7.0% by weight, contain at least one emulsifier.
- Emulsifiers preferably containedare in particular oil-in-water emulsifiers, in particular in EP2421614 listed emulsifiers.
- compositions according to the inventionalso stabilize otherwise difficult formulations which have a low viscosity, so a composition preferred according to the invention is characterized in that it has a viscosity in a range from 500 to 20,000, preferably from 1,500 to 10,000 mPas, the viscosity at 25 ° C with Brookfield RVT, spindle 4, 5 rpm.
- compositionsare preferred which are essentially free of polyglycol ether and essentially free of alkoxylated compounds.
- the term “essentially free of alkoxylated compounds” and “essentially free of polyglycol ether” in connection with the present inventionis to be understood to mean that the compositions, with the exception of component B) according to the invention, as an exception, do not contain any appreciable amounts of alkoxylated or polyglycol ether Has compounds that have a surface-active effect. In particular, this means that these compounds in amounts of less than 1% by weight, preferably less than 0.1% by weight, particularly preferably less than 0.01% by weight, based on the total composition, in particular no detectable amounts , are included.
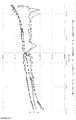
- illustration 1shows storage modulus and loss modulus for the emulsions produced according to formulation 1-2 according to the invention or formulation 1-9 not according to the invention.
- a mixture of the polyglycerol thus obtained (252.2 g, 0.8 mol) and stearic acid and palmitic acid in a ratio of 1: 1 (97.8 g, 0.36 mol)was brought up to 240 ° C. in the course of 3 h with nitrogen blowing heated and the mixture was then stirred at this temperature and the water formed was continuously removed until an acid number of ⁇ 1.0 was reached and the mixture was clear and homogeneous at 240 ° C.
- a mixture of the polyglycerol thus obtained (1008.7 g, 2.2 mol) and stearic acid and palmitic acid in a ratio of 1: 1 (391.3 g, 1.5 mol)was brought up to 240 ° C. in the course of 3 h with nitrogen blowing heated and the mixture was then stirred at this temperature and the water formed was continuously removed until an acid number of ⁇ 1.0 was reached and the mixture was clear and homogeneous at 240 ° C.
- a mixture of the polyglycerol thus obtained (252.2 g, 0.33 mol) and stearic acid and palmitic acid in a ratio of 1: 1 (97.8 g, 0.36 mol)was brought up to 240 ° C. in the course of 3 h with nitrogen blowing heated and the mixture was then stirred at this temperature and the water formed was continuously removed until an acid number of ⁇ 1.0 was reached and the mixture was clear and homogeneous at 240 ° C.
- a polyglycerol obtained as described in Example 5a(240 g; 0.32 mol) and caprylic acid and capric acid in a ratio of 60:40 (78.8 g, 0.5 mol) were heated to 240 ° C. in the course of 3 h while introducing nitrogen and then the mixture stirred at this temperature and the water formed was continuously removed until an acid number of ⁇ 1.0 was reached.
- a mixture of the polyglycerol thus obtained (231.9 g, 0.296 mol) and stearic acid and palmitic acid in a ratio of 1: 1 (85.42 g, 0.32 mol) and caprylic acid and capric acid in a ratio of 60:40 (3.82 g , 0.025 mol)was heated to 240 ° C. in the course of 3 hours with nitrogen injection and the mixture was then stirred at this temperature and the water formed was continuously removed until an acid number of ⁇ 1.0 was reached and the mixture was clear at 240 ° C. and was homogeneous.
- a mixture of the polyglycerol thus obtained (243.5 g, 0.32 mol) and stearic acid and palmitic acid in a ratio of 1: 1 (85.42 g, 0.32 mol) and caprylic acid and capric acid in a ratio of 60:40 (3, 82 g, 0.025 mol)was heated to 240 ° C. in the course of 3 hours while introducing nitrogen and the mixture was then stirred at this temperature and the water formed was continuously removed until an acid number of ⁇ 1.0 was reached and the mixture at 240 ° C was clear and homogeneous.
- compositions according to the inventionvs. Compositions not according to the invention:
- Emulsifier according to synthesis example 13.2 Invention.
- Emulsifier according to synthesis example 23.2 Invention.
- Emulsifier according to synthesis example 33.2 3.2 Methyl glucose sesquistearate 1) 2.8 2.8 Polyglyceryl-4 laurate 2) 0.4 0.4 Polyglyceryl-4 Laurate / Succinate (and) Aqua 3) 3.2 3.2 Polyglyceryl-6 stearates; Polyglyceryl-6 behenate 29) 3.2 C18-C22 hydroxyalkyl hydroxypropyl guar 4) 0.15 0.15 0.15 0.15 0.15 0.15 Hydroxyethyl cellulose 5) 0.15 0.15 Hydroxypropyl guar 30) 0.15 Polyglyceryl-3 caprylates 9) 0.5 0.5 0.5 0.5 0.5 0.5 0.5 Zinc Ricinoleate 10) 1.0 1.0 1.0 1.0 1.0 1.0 1.0 1.0 1.0 1.0 1.0 1.0 Caprylic / Capric triglycerides 5.65 5.65 5.65 5.65 5.65 5.65 5.65 5.65 5.65 5.65 5.65 5.65 5.65 5.65 5.65 5.65 5.65
- Emulsifier according to synthesis example 14.8 Invention.
- Emulsifier according to synthesis example 24.8 4.8 Invention.
- Emulsifier according to synthesis example 34.8 Methyl glucose sesquistearate 1) 4.2 4.2 Polyglyceryl-4 laurate 2) 0.6 0.6 Polyglyceryl-4 Laurate / Succinate (and) Aqua 3) 4.8 4.8 Polyglyceryl-6 stearates; Polyglyceryl-6 behenate 29) 4.8 C18-C22 hydroxyalkyl hydroxypropyl guar 4) 0.25 0.25 0.25 0.25 0.25 0.25 Hydroxyethyl cellulose 5) 0.25 0.25 Hydroxypropyl guar 30) 0.25 Isopropyl palmitate 5.0 5.0 5.0 5.0 5.0 5.0 5.0 water Ad 100 Ad 100 Ad 100 Ad 100 Ad 100 Ad 100 Ad 100 Ad 100 Ad 100 Ad 100 Ad 100 Ad 100 Ad 100 Ad 100 Ad 100 Ad 100 Ad 100 Ad 100 Ad 100 Ad 100 Ad 100 Ad 100 Ad 100 Ad 100 Ad 100 Ad 100 Ad 100 Ad 100 Ad 100 Ad 100 Ad 100 Ad 100 Ad 100 Ad 100 Ad 100 Ad 100 Ad 100 Ad 100 Ad 100 Ad 100 Ad 100 Ad
- the emulsifiers according to the inventionallow the formulation of a stable emulsion, whereas the emulsifier not according to the invention and the representatives of the prior art do not enable a stable emulsion.
- the use of guar not modified according to the inventionlikewise leads to emulsions which are not stable in storage.
- Formulations 1-2 and 1-9were examined for their ability to develop a yield point.
- the measurementswere carried out with a rheometer from Anton Paar, model MCR 301, plate-plate (40 mm) geometry with a gap of 1 mm at a temperature of 25 ° C. and 1 bar pressure.
- the storage module and loss modulewere determined for the emulsions produced according to formulation 1-2 according to the invention or formulation 1-9 not according to the invention.
- the sampleswere measured at a constant load of 0.20 Pa over the frequency range from 0.005 to 90 Hz.
- the rheological yield pointis defined by the fact that in the measured frequency range the memory module (G ') always has higher values than the loss module (G ”) (see Fig. 1 ).
- compositions according to the inventioncan be used in a large number of cosmetic formulations.
- Sun Care Spray SPF 30⁇ /i> recipe 4 Emulsifier according to the invention according to synthesis example 4 4.0 Phenoxyethyl caprylate 12) 3.2 Isopropyl palmitate 2.0 Bis-ethylhexyloxyphenol methoxyphenyl triazine 13) 3.0 Butyl methoxydibenzoylmethane 2.0 EHC 2.0 ethylhexyl 4.0 Octocrylene 4.0 glycerin 3.0 water ad 100 Carbomer suspension 1 14) 1.0 Tris (hydroxymethyl) aminomethane (30% aq.) 0.6 UV filter solution 15) 10.0 Methylisothiazolinones, methylparaben, ethylparaben; Dipropylene Glycol 8) 0.8 11) TEGOSOFT XC
Landscapes
- Health & Medical Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Public Health (AREA)
- Veterinary Medicine (AREA)
- Animal Behavior & Ethology (AREA)
- General Health & Medical Sciences (AREA)
- Birds (AREA)
- Epidemiology (AREA)
- Chemical & Material Sciences (AREA)
- Inorganic Chemistry (AREA)
- Dermatology (AREA)
- Emergency Medicine (AREA)
- Oil, Petroleum & Natural Gas (AREA)
- Gerontology & Geriatric Medicine (AREA)
- Dispersion Chemistry (AREA)
- Cosmetics (AREA)
Description
Translated fromGermanGegenstand der Erfindung sind Zusammensetzungen enthaltend Polyglycerinester und langkettig hydroxy-alkylmodifiziertes Guar.The invention relates to compositions containing polyglycerol esters and long-chain hydroxy-alkyl-modified guar.
- a) mindestens ein schweißhemmendes Aluminiumsalz in einer Gesamtmenge von 2 - 40 Gew%, wobei sich die Gew.-%-Angaben auf das Gesamtgewicht der kristallwasserfreien und ligandenfreien Aktivsubstanz (USP) in dem Mittel beziehen, und zusätzlich dazu
- b) in einer Gesamtmenge von 0, 1 - 2 Gew.-% mindestens eine oberflächenaktive Verbindung mit einem HLB-Wert im Bereich von 9 bis 15, ausgewählt aus den Partialestern eines Polyglycerins, das 3, 4 oder 5 Glycerin-Einheiten umfasst, mit einer linearen oder verzweigten, gesättigten oder ungesättigten Carbonsäure mit 8 bis 22 C-Atomen und mit einer organischen Genusssäure,
und zusätzlich dazu - c) in einer Gesamtmenge von 0,1 - 2,0 Gew.-% mindestens ein bestimmtes N-Acyl-L-glutaminsäure-Natriumsalz.
- a) at least one antiperspirant aluminum salt in a total amount of 2 - 40% by weight, the% by weight data relating to the total weight of the crystal water-free and ligand-free active substance (USP) in the composition, and in addition to this
- b) in a total amount of 0.1-2% by weight, at least one surface-active compound with an HLB value in the range from 9 to 15, selected from the partial esters of a polyglycerol which comprises 3, 4 or 5 glycerol units a linear or branched, saturated or unsaturated carboxylic acid with 8 to 22 carbon atoms and with an organic edible acid,
and in addition to that - c) in a total amount of 0.1 - 2.0 wt .-% at least one specific N-acyl-L-glutamic acid sodium salt.
Überraschenderweise wurde gefunden, dass die im Folgenden beschriebenen Zusammensetzungen die der Erfindung gestellte Aufgabe zu lösen vermögen.Surprisingly, it was found that the compositions described below are able to achieve the object of the invention.
Gegenstand der vorliegenden Erfindung sind daher Zusammensetzungen enthaltend bestimmte Polyglycerinester und hydroxy-alkylmodifiziertes Guar.The present invention therefore relates to compositions comprising certain polyglycerol esters and hydroxyalkyl-modified guar.
Ein Vorteil der vorliegenden Erfindung besteht darin, dass der in den erfindungsgemäßen Zusammensetzungen enthaltene Polyglycerinester vollkommen auf nachwachsenden Rohstoffen basiert.An advantage of the present invention is that the polyglycerol ester contained in the compositions according to the invention is based entirely on renewable raw materials.
Noch ein Vorteil der vorliegenden Erfindung ist es, dass die erfindungsgemäße Zusammensetzung sich zur Formulierung von O/W Emulsionen (Cremes, Lotionen) mit hervorragender Lagerstabilität eignet.Another advantage of the present invention is that the composition according to the invention is suitable for the formulation of O / W emulsions (creams, lotions) with excellent storage stability.
Ein weiterer Vorteil der vorliegenden Erfindung ist es, dass die erfindungsgemäße Zusammensetzung sich zur Formulierung PEG-freier Emulsionen, insbesondere dünnflüssiger PEG-freier Emulsionen eignet.Another advantage of the present invention is that the composition according to the invention is suitable for the formulation of PEG-free emulsions, in particular low-viscosity PEG-free emulsions.
Ein weiterer Vorteil der vorliegenden Erfindung ist es, dass die erfindungsgemäße Zusammensetzung sich zur Formulierung PEG-freier AP/Deo oder Deo-Emulsionen, insbesondere Roll-on-Emulsionen eignet.Another advantage of the present invention is that the composition according to the invention is suitable for the formulation of PEG-free AP / deodorant or deodorant emulsions, in particular roll-on emulsions.
Ein weiterer Vorteil der vorliegenden Erfindung ist es, dass die erfindungsgemäße Zusammensetzung sich zur Formulierung von Emulsionen eignet, welche eine Fließgrenze aufweisen.Another advantage of the present invention is that the composition according to the invention is suitable for the formulation of emulsions which have a yield point.
Emulsionen und Formulierungen enthaltend solchen Emulgator auf Basis der erfindungsgemäßen Zusammensetzung weisen überdies ein gutes Hautgefühl auf. Vorteilhafterweise benötigen Emulsionen und Formulierungen enthaltend die erfindungsgemäße Zusammensetzung keine parabenhaltigen Konservierungsmittel. Ein weiterer Vorteil der vorliegenden Erfindung ist es, dass die erfindungsgemäße Zusammensetzung sich zur Formulierung von Emulsionen ohne polyacrylat-basierte Verdicker eignet.Emulsions and formulations containing such an emulsifier based on the composition according to the invention also have a good feeling on the skin. Emulsions and formulations containing the composition according to the invention advantageously do not require any preservatives containing parabens. A further advantage of the present invention is that the composition according to the invention is suitable for the formulation of emulsions without polyacrylate-based thickeners.
Ein weiterer Vorteil der vorliegenden Erfindung ist es, dass die erfindungsgemäße Zusammensetzung aufgrund ihrer Konsistenz gut handhabbar ist.Another advantage of the present invention is that the composition according to the invention is easy to handle due to its consistency.
Ein weiterer Vorteil der vorliegenden Erfindung ist es, dass die erfindungsgemäße Zusammensetzung in Formulierungen ein leichtes Hautgefühl erzeugt.Another advantage of the present invention is that the composition according to the invention produces a light skin feeling in formulations.
Ein weiterer Vorteil der vorliegenden Erfindung ist es, dass die Verwendung der erfindungsgemäßen Zusammensetzung den Formulierungen feuchtigkeitsspendende Eigenschaften verleiht.Another advantage of the present invention is that the use of the composition according to the invention imparts moisturizing properties to the formulations.
Beansprucht wird daher eine Zusammensetzung enthaltend
- A) Polyglycerinester, der nach seiner vollständiger Hydrolyse
- a) mindestens eine Carbonsäure mit 6 bis 14, bevorzugt 8 bis 12, Kohlenstoffatomen
- b) mindestens eine Carbonsäure mit 16 bis 20, bevorzugt 18 bis 20, Kohlenstoffatomen
- c) mindestens eine Carbonsäure mit 22 bis 28, bevorzugt 22 bis 24, Kohlenstoffatomen freisetzt und
der nach seiner vollständiger Hydrolyse ein Polyglycerin freisetzt, das einen mittleren Polymerisationsgrad von 3,0 bis 5,0, bevorzugt von 3,3 bis 4,7, besonders bevorzugt von 3,6 bis 4,5, aufweist,
und
- B) mindestens ein hydroxy-alkylmodifziertes Guar,
dadurch gekennzeichnet, dass das hydroxy-alkylmodifzierte Guar mit HydroxyAlkylgruppen mit 12 bis 26, bevorzugt 16 bis 24, besonders bevorzugt 18 bis 22, Kohlenstoffatomen modifiziert ist.
- A) Polyglycerol ester, which after its complete hydrolysis
- a) at least one carboxylic acid having 6 to 14, preferably 8 to 12, carbon atoms
- b) at least one carboxylic acid having 16 to 20, preferably 18 to 20, carbon atoms
- c) releases at least one carboxylic acid with 22 to 28, preferably 22 to 24, carbon atoms and
which after its complete hydrolysis releases a polyglycerol which has an average degree of polymerization from 3.0 to 5.0, preferably from 3.3 to 4.7, particularly preferably from 3.6 to 4.5,
and
- B) at least one hydroxy-alkyl-modified guar,
characterized in that the hydroxy-alkyl-modified guar is modified with hydroxyalkyl groups having 12 to 26, preferably 16 to 24, particularly preferably 18 to 22, carbon atoms.
Unter dem Begriff "Polyglycerin" im Sinne der vorliegenden Erfindung ist ein Polyglycerin zu verstehen, welches Glycerin enthält. Somit ist zur Berechnung von Mengen, Massen und dergleichen der Glycerinanteil mit zu berücksichtigen. Polyglycerine im Sinne der vorliegenden Erfindung sind somit Mischungen von Glycerin mit mindestens einem Glycerin-Oligomer. Unter Glycerin-Oligomeren sind jeweils alle entsprechenden Strukturen, z.B. also lineare und zyklische Verbindungen zu verstehen.The term “polyglycerin” in the context of the present invention is to be understood as meaning a polyglycerin which contains glycerin. Thus, the glycerol content must also be taken into account when calculating amounts, masses and the like. For the purposes of the present invention, polyglycerols are therefore mixtures of glycerol with at least one glycerol oligomer. Glycerol oligomers include all corresponding structures, e.g. to understand linear and cyclic connections.
Analoges gilt für den Begriff "Polyglycerinester" im Zusammenhang mit der vorliegenden Erfindung.The same applies to the term "polyglycerol ester" in connection with the present invention.
Das angegebene Zahlenmittel der Säurereste bezieht sich bei mehr als einer der Carbonsäure a), b) oder c) jeweils auf die akkumulierte Summe aller Carbonsäuren a), b) oder c).In the case of more than one of the carboxylic acids a), b) or c), the number average of the acid residues relates in each case to the accumulated sum of all carboxylic acids a), b) or c).
Der mittlere Polymerisationsgrad des PolyglycerinsN wird über dessen Hydroxylzahl OHV, in mg KOH/g) gemäß folgender Formel berechnet:
Geeignete Bestimmungsmethoden zur Ermittlung der Hydroxylzahl sind insbesondere solche gemäß DGF C-V 17 a (53), Ph. Eur. 2.5.3 Method A und DIN 53240.Suitable determination methods for determining the hydroxyl number are in particular those according to DGF C-V 17 a (53), Ph. Eur. 2.5.3 Method A and DIN 53240.
Alle angegebenen Prozent (%) sind, wenn nicht anders angegeben, Massenprozent.Unless stated otherwise, all percentages (%) are percentages by mass.
Es werden erfindungsgemäß Zusammensetzungen bevorzugt, bei denen das nachvollständiger Hydrolyse des Polyglycerinesters freigesetzte Polyglycerin ein Massenverhältnis von Glycerin zu Diglycerin von größer 1, bevorzugt größer 1,2, besonders bevorzugt größer 1,4 aufweist.Compositions according to the invention are preferred in which the complete hydrolysis of the polyglycerol ester released polyglycerol has a mass ratio of glycerol to diglycerol of greater than 1, preferably greater than 1.2, particularly preferably greater than 1.4.
Es werden erfindungsgemäß Zusammensetzungen besonders bevorzugt, bei denen das nach vollständiger Hydrolyse des Polyglycerinesters freigesetzte Polyglycerin 5 Gew.-% bis 30 Gew.-%, bevorzugt 7 Gew.-% bis 25 Gew.-%, besonders bevorzugt
10 Gew.-% bis 22 Gew.-%, mono-Glycerin,
1 Gew.-% bis 25 Gew.-%, bevorzugt 3 Gew.-% bis 18 Gew.-%, besonders bevorzugt 5 Gew.-% bis 15 Gew.-%, Diglycerine,
1 Gew.-% bis 25 Gew.-%, bevorzugt 1 Gew.-% bis 20 Gew.-%, besonders bevorzugt 3 Gew.-% bis 17 Gew.-%, Triglycerine und
1 Gew.-% bis 20 Gew.-%, bevorzugt 2 Gew.-% bis 15 Gew.-%, besonders bevorzugt 4 Gew.-% bis 10 Gew.-%, Tetraglycerine
enthält, wobei sich die Gewichtsprozente auf das gesamte Polyglycerin beziehen.Compositions are particularly preferred according to the invention in which the polyglycerol released after complete hydrolysis of the polyglycerol ester is particularly preferably 5% by weight to 30% by weight, preferably 7% by weight to 25% by weight
10% by weight to 22% by weight, mono-glycerol,
1% by weight to 25% by weight, preferably 3% by weight to 18% by weight, particularly preferably 5% by weight to 15% by weight, diglycerols,
1% by weight to 25% by weight, preferably 1% by weight to 20% by weight, particularly preferably 3% by weight to 17% by weight, triglycerols and
1% by weight to 20% by weight, preferably 2% by weight to 15% by weight, particularly preferably 4% by weight to 10% by weight, tetraglycerols
contains, the weight percentages refer to the total polyglycerol.
In diesem Zusammenhang weist das freigesetzte Polyglycerin bevorzugt
≥70 Gew.-%, bevorzugt ≥75 Gew.-%, besonders bevorzugt ≥80 Gew.-%, Polyglycerine mit einem Polymerisationsgrad von ≥2,
≥60 Gew.-%, bevorzugt ≥65 Gew.-%, besonders bevorzugt ≥70 Gew.-%, Polyglycerine mit einem Polymerisationsgrad von ≥3,
≥50 Gew.-%, bevorzugt ≥55 Gew.-%, besonders bevorzugt ≥60 Gew.-%, Polyglycerine mit einem Polymerisationsgrad von ≥4 und
≥40 Gew.-%, bevorzugt ≥45 Gew.-%, besonders bevorzugt ≥50 Gew.-%, Polyglycerine mit einem Polymerisationsgrad von ≥5
auf, wobei sich die Gewichtsprozent auf das gesamte Polyglycerin beziehen.In this connection, the released polyglycerol is preferred
≥70% by weight, preferably ≥75% by weight, particularly preferably ≥80% by weight, polyglycerols with a degree of polymerization of ≥2,
≥60% by weight, preferably ≥65% by weight, particularly preferably ≥70% by weight, polyglycerols with a degree of polymerization of ≥3,
≥50% by weight, preferably ≥55% by weight, particularly preferably ≥60% by weight, polyglycerols with a degree of polymerization of ≥4 and
≥40% by weight, preferably ≥45% by weight, particularly preferably ≥50% by weight, polyglycerols with a degree of polymerization of ≥5
on, the weight percent based on the total polyglycerol.
Der Massenanteil von Glycerin, Diglycerin, Triglycerin, Tetraglycerin sowie der Fettsäuren lässt sich im Rahmen der vorliegenden Erfindung durch zwei GC-Methoden bestimmen; diese Methoden beinhalten die alkalische Hydrolyse des erfindungsgemäßen Polyglycerinesters, Trennung des Polyglycerins von den freigesetzten Säuren und Analyse der Fettsäuren sowie der Glycerin-Oligomere (lineare und zyklische).The mass fraction of glycerin, diglycerin, triglycerin, tetraglycerin and the fatty acids can be determined in the context of the present invention by two GC methods; these methods include the alkaline hydrolysis of the polyglycerol ester according to the invention, separation of the polyglycerol from the released acids and analysis of the fatty acids and of the glycerol oligomers (linear and cyclic).
Hierzu werden 0,5 g des erfindungsgemäßen Polyglycerinesters in 25 ml einer ethanolischen 0,5 M KOH Lösung unter Rückfluss für 4 Stunden gekocht. Dann werden 10 ml Wasser zugegeben und der pH mit Schwefelsäure auf pH 2-3 eingestellt. Die entstandenen Carbonsäuren werden durch dreimalige Extraktion mit jeweils einem Volumen (20 ml) Petrolether abgetrennt.For this purpose, 0.5 g of the polyglycerol ester according to the invention is boiled in 25 ml of an ethanolic 0.5 M KOH solution under reflux for 4 hours. Then 10 ml of water are added and the pH is adjusted to pH 2-3 with sulfuric acid. The carboxylic acids formed are separated off by extraction three times, each with a volume (20 ml) of petroleum ether.
Die vereinigten Extrakte werden durch Evaporation auf ca. 1 ml eingeengt. Geeignete Bestimmungsmethoden zur Ermittlung der Fettsäureverteilung sind insbesondere solche gemäß DGF C VI 11a, DGF C-VI 10 a und GAT - Ringtest 7/99. Ein 0,5 ml Aliquot des wie oben beschriebenen erhaltenen Petroletherextraktes wird in einem Gefäß mit 1 ml einer Mischung von Acetylchlorid: Methanol (1:4) unter Ausschluss von Luftfeuchtigkeit 30 min in der Siedehitze behandelt. Die entstehenden Fettsäuremethylester werden 2 mal mit jeweils 5 ml iso-Octan extrahiert und mittels GC analysiert. Dieses wird in einem Gaschromatograph, welcher mit einem split/splitless Injektor, einer Kapillarsäule und einem Flammenionisationdetektor ausgestattet ist, unter folgenden Bedingungen durchgeführt:
Die Carbonsäuren werden als ihre Methylester nach ihrer Kohlenstoffkettenlänge getrennt und ihr Massenanteil nach einer Methode des internen Standards bestimmt. Hierzu wird das GC System durch Vermessen von Fettsäuremethylester-Mischungen der zu untersuchenden Fettsäuren mit bekannter Zusammensetzung kalibriert. Mit diesem Verfahren wird die Gesamtmasse und die Massenanteile an Carbonsäure(n) erhalten, die über die Verwendung der jeweiligen Molekulargewichte eine Bestimmung der Stoffemenge(n) erlaubt. Aus der Gesamtmasse an Carbonsäure(n) lässt sich außerdem durch Subtraktion die z.B. in 0.5 g Polyglycerinester enthaltene Masse an Polyglycerin bestimmen.
Mittels des Molekulargewichts des Polyglycerins lässt sich daraus die Stoffmenge des Polyglycerins bestimmen.
The amount of substance of the polyglycerol can be determined therefrom by means of the molecular weight of the polyglycerol.
Zusammen lassen sich aus diesen Werten die Molverhältnisse von Polyglycerin zu Carbonsäuren bestimmen.Together, the molar ratios of polyglycerol to carboxylic acids can be determined from these values.
Analyse von Glycerin, Diglycerinen, Triglycerinen und Tetraglycerinen:
Der mit Petrolether extrahierte Rückstand wird mit Bariumhydroxid auf pH 7 bis 8 eingestellt. Das ausgefallene Bariumsulfat wird durch Zentrifugation abgetrennt.
Der Überstand wird abgenommen und der Rückstand wird dreimal mit 20 ml Ethanol extrahiert.
Die vereinigten Überstände werden für 30 min bei 80 °C und 50 mbar eingeengt und getrocknet.Analysis of glycerol, diglycerols, triglycerols and tetraglycerols:
The residue extracted with petroleum ether is adjusted to pH 7 to 8 with barium hydroxide. The precipitated barium sulfate is separated off by centrifugation.
The supernatant is removed and the residue is extracted three times with 20 ml of ethanol.
The combined supernatants are concentrated at 80 ° C. and 50 mbar for 30 min and dried.
Für die Analyse von Glycerin, Diglycerinen, Triglycerinen und Tetraglycerinen mittels GC wird der Rückstand in 2 ml Pyridin:Chloroform (4:1) gelöst. 0,5 ml dieser Lösung werden mit 1 ml MSTFA [N-Methyl-N-(trimethylsilyl) trifluoroacetamide] versetzt. Die Alkohole werden durch Reaktion bei 80°C (30 Minuten) quantitativ in ihre Trimethylsilyether überführt und anschließend mittels GC/FID untersucht.
Dieses wird in einem Gaschromatograph, welcher mit einem split/splitless Injektor, einer Kapillarsäule und einem Flammenionisationdetektor ausgestattet ist, unter folgenden Bedingungen durchgeführt:
This is carried out in a gas chromatograph equipped with a split / splitless injector, a capillary column and a flame ionization detector under the following conditions:
Glycerin, Diglycerine, Triglycerine und Tetraglycerine werden aufgetrennt und ihr Massenanteil nach einer Methode des internen Standards bestimmt. Hierzu wird das GC System durch Vermessen von Mischungen der zu untersuchenden Glycerine und des internen Standards mit bekannter Zusammensetzung kalibriert.
Aus den Massenanteilen lässt sich das Massenverhältnis von Glycerin zu Diglycerin bestimmen sowie durch Subtraktion von 100% auch der Gehalt an Polyglycerinen mit einem Polymerisationsgrad von 2 und größer (100% minus Massenanteil des Glycerins), der Gehalt an Polyglycerinen mit einem Polymerisationsgrad von 3 und größer (100% minus Massenanteile des Glycerins und der Diglycerine), der Gehalt an Polyglycerinen mit einem Polymerisationsgrad von 4 und größer (100% minus Massenanteile des Glycerins, der Diglycerine und der Triglycerine) und der Gehalt an Polyglycerinen mit einem Polymerisationsgrad von 5 und größer (100% minus Massenanteile des Glycerins, der Diglycerine, der Triglycerine und der Tetraglycerine). Sollte in einem betrachteten Polyglycerin keine nachweisbare Menge an Diglycerin, jedoch Glycerin enthalten sein, so entspricht dies einem Massenverhältnis von Glycerin zu Diglycerin größer 1,4.Glycerol, diglycerols, triglycerols and tetraglycerols are separated and their mass fraction determined according to an internal standard method. For this purpose, the GC system is calibrated by measuring mixtures of the glycerols to be examined and the internal standard with a known composition.
From the mass fractions, the mass ratio of glycerin to diglycerol can be determined, and by subtracting 100%, the content of polyglycerols with a degree of polymerization of 2 and greater (100% minus mass fraction of glycerol), the content of polyglycerols with a degree of polymerization of 3 and greater (100% minus mass fractions of glycerin and diglycerols), the content of polyglycerols with a degree of polymerization of 4 and greater (100% minus mass fractions of glycerin, diglycerols and triglycerols) and the content of polyglycerols with a degree of polymerization of 5 and greater ( 100% minus mass fractions of glycerol, diglycerols, triglycerols and tetraglycerols). If there is no detectable amount of diglycerin in a polyglycerol under consideration, but glycerol is present, this corresponds to a mass ratio of glycerol to diglycerol greater than 1.4.
Eine erfindungsgemäß bevorzugte Zusammensetzung ist dadurch gekennzeichnet, dass der Polyglycerinester nach seiner vollständiger Hydrolyse im Mittel (Zahlenmittel) pro Mol Polyglycerinester
von 0,01 bis 0,07 Mol, bevorzugt von 0,01 bis 0,50 Mol, besonders bevorzugt von 0,01 bis 0,30 Mol, mindestens einer Carbonsäure a)
von 0,10 bis 1,70 Mol, bevorzugt von 0,30 bis 1,50 Mol, besonders bevorzugt von 0,40 bis 1,40 Mol, mindestens einer Carbonsäure b)
von 0,01 bis 0,80 Mol, bevorzugt von 0,01 bis 0,60 Mol, besonders bevorzugt von 0,05 bis 0,40 Mol, mindestens einer Carbonsäure c)
freisetzt.
Insbesondere ist sie dadurch gekennzeichnet, dass das Molverhältnis der nach vollständiger Hydrolyse des Polyglycerinesters erhaltenen Carbonsäure a) zu Carbonsäure b) zu Carbonsäure c)
0,6 bis 1,4 : 16,5 bis 20,5 : 3,0 bis 4,8, bevorzugt
0,8 bis 1,2 : 17,5 bis 19,5 : 3,5 bis 4,3, besonders bevorzugt
0,9 bis 1,1 : 18,0 bis 19,0 : 3,7 bis 4,1, beträgt.
Zur Bestimmung der Molverhältnisse lässt sich als Methode die oben beschriebene Methode einsetzen.
Es ist erfindungsgemäß bevorzugt, dass die Carbonsäuren a), b) und c) ausgewählt ist aus Fettsäuren, solche sind insbesondere ausgewählt aus linearen, gesättigten, unsubstituierten Carbonsäuren.
Insbesondere sind erfindungsgemäß Zusammensetzungen bevorzugt, die dadurch gekennzeichnet sind, dass die Carbonsäure a) ausgewählt ist aus Caprylsäure und Caprinsäure, die Carbonsäure b) ausgewählt ist aus Stearinsäure und Palmitinsäure und die Carbonsäure c) Behensäure ist.A composition preferred according to the invention is characterized in that after its complete hydrolysis, the polyglycerol ester averages (number average) per mole of polyglycerol ester
from 0.01 to 0.07 mol, preferably from 0.01 to 0.50 mol, particularly preferably from 0.01 to 0.30 mol, of at least one carboxylic acid a)
from 0.10 to 1.70 mol, preferably from 0.30 to 1.50 mol, particularly preferably from 0.40 to 1.40 mol, of at least one carboxylic acid b)
from 0.01 to 0.80 mol, preferably from 0.01 to 0.60 mol, particularly preferably from 0.05 to 0.40 mol, of at least one carboxylic acid c)
releases.
In particular, it is characterized in that the molar ratio of the carboxylic acid a) to carboxylic acid b) to carboxylic acid c) obtained after complete hydrolysis of the polyglycerol ester
0.6 to 1.4: 16.5 to 20.5: 3.0 to 4.8, preferred
0.8 to 1.2: 17.5 to 19.5: 3.5 to 4.3, particularly preferred
0.9 to 1.1: 18.0 to 19.0: 3.7 to 4.1.
The method described above can be used as a method to determine the molar ratios.
It is preferred according to the invention that the carboxylic acids a), b) and c) are selected from fatty acids; those are in particular selected from linear, saturated, unsubstituted carboxylic acids.
In particular, compositions according to the invention are preferred which are characterized in that the carboxylic acid a) is selected from caprylic acid and capric acid, the carboxylic acid b) is selected from stearic acid and palmitic acid and the carboxylic acid c) is behenic acid.
Hydroxy-alkylmodifiziertes Guar ist vielfach beschrieben und beispielsweise als Esaflor HM 22 kommerziell erhältlich. Guar ist ein Galaktomannan, in welches langekttige Hydroxy-Alkylmodifikationen einfach durch Umsetzung mit Epoxyalkanen eingefügt werden können.
Erfindungsgemäß bevorzugte Zusammensetzungen enthalten hydroxy-alkylmodifziertes Guar, welches mit Alkylgruppen mit 16 bis 24, bevorzugt 18 bis 22, Kohlenstoffatomen modifiziert ist.
Es ist erfindungsgemäß bevorzugt, dass das hydroxy-alkylmodifzierte Guar zusätzlich hydroxypropyl modifiziert, ist. Ein erfindungsgemäß besonders bevorzugt enthaltenes Guar ist der nach INCI benannte Stoff C18-C22 Hydroxyalkyl Hydroxypropyl Guar. Hydroxy-alkylmodifiziertes Guar und solches, welches zusätzlich hydroxypropyl modifiziert ist, sowie Verfahren zur Herstellung dieser Verbindungen sind beispielsweise in der
Compositions preferred according to the invention contain hydroxy-alkyl-modified guar which is modified with alkyl groups having 16 to 24, preferably 18 to 22, carbon atoms.
It is preferred according to the invention that the hydroxy-alkyl-modified guar is additionally hydroxypropyl-modified. A guar which is particularly preferably contained according to the invention is the substance C18-C22 hydroxyalkyl hydroxypropyl guar named after INCI. Hydroxy-alkyl-modified guar and those which are additionally hydroxypropyl-modified, and processes for the preparation of these compounds are, for example, in US Pat
Die erfindungsgemäßen Zusammensetzungen sind hervorragend zur Formulierung von Deo oder AP-Deo-Zusammensetzungen (AP=Antiperspirant) geeignet, daher enthalten sie bevorzugt zusätzlich C) mindestens einen ausgewählt aus Deo-Wirkstoff und AP-Wirkstoff, insbesondere mindestens einen Deo-Wirkstoff und mindestens einen AP-Wirkstoff.The compositions according to the invention are outstandingly suitable for the formulation of deodorant or AP deodorant compositions (AP = antiperspirant), therefore they preferably additionally contain C) at least one selected from deodorant active ingredient and AP active ingredient, in particular at least one deodorant active ingredient and at least one AP agent.
Es ist erfindungsgemäß bevorzugt, dass der AP-Wirkstoff ausgewählt ist aus der Gruppe umfassen, bevorzugt bestehend aus, Aluminium-Salzen und Zirkonium-Salzen. Es sind insbesondere erfindungsgemäße Zusammensetzungen bevorzugt, die dadurch gekennzeichnet sind, dass das Aluminium-Salz ausgewählt ist aus der Gruppe umfassend, bevorzugt bestehend aus:
Aluminum Acetate, Aluminum Behenate, Aluminum Benzoate, Aluminum Bromohydrate, Aluminum Butoxide, Aluminum Calcium Sodium Silicate, Aluminum Caprylate, Aluminum Capryloyl Hydrolyzed Collagen, Aluminum Chloride, Aluminiumchlorohydrat, Aluminum Chlorohydrex, Aluminum Chlorohydrex PEG, Aluminum Chlorohydrex PG, Aluminum Citrate, Aluminum Diacetate, Aluminum Dibenzoate/Stearate Hydroxide, Aluminum Dicetyl Phosphate, Aluminum Dichlorohydrate, Aluminum Dichlorohydrex PEG, Aluminum Dichlorohydrex PG, Aluminum Dilinoleate, Aluminum Dimyristate, Aluminum Distearate, Aluminum Glycinate, Aluminum Hydrogenated Tallow Glutamate, Aluminum Hydroxy Bis-Methylene Bis-Di-t-Butylphenyl Phosphate, Aluminum Iron Calcium Magnesium Germanium Silicates, Aluminum Iron Calcium Magnesium Zirconium Silicates, Aluminum Iron Silicates, Aluminum Isopropoxide, Aluminum Isostearate, Aluminum Isostearates/Laurates/Palmitates, Aluminum Isostearates/Laurates/Stearates, Aluminum Isostearates/Myristates, Aluminum Isostearates/Palmitates, Aluminum Isostearates/Stearates, Aluminum Isostearyl Glyceryl Phosphate, Aluminum Laccate, Aluminum Lactate, Aluminum Lanolate, Aluminum/Magnesium Hydroxide Stearate, Aluminum Magnesium Oxide, Aluminum Methionate, Aluminum Myristate, Aluminum Myristates/Palmitates, Aluminum PCA, Aluminum Phenolsulfonate, Aluminum Sesquichlorohydrate, Aluminum Sesquichlorohydrex PEG, Aluminum Sesquichlorohydrex PG, Aluminum Starch Octenylsuccinate, Aluminum Stearate, Aluminum Stearates, Aluminum Stearoyl Glutamate, Aluminum Sucrose Octasulfate, Aluminum Sulfate, Aluminum Triformate, Aluminum Triphosphate, Aluminum Tristearate, Aluminum Undecylenoyl Collagen Amino Acids, Aluminum Zinc Oxide, Aluminum Zirconium Trichlorohydrate, Aluminum Zirconium Trichlorohydrex GLY, Aluminum Zirconium Tetrachlorohydrate, Aluminium Zirconium Tetrachlorohydrex GLY, Aluminum Zirconium Tetrachlorohydrex PEG, Aluminum Zirconium Tetrachlorohydrex PG, Aluminium Zirconium Pentachlorohydrat, Aluminum Zirconium Pentachlorohydrex GLY, Aluminum Zirconium Octachlorohydrate, Aluminum Zirconium Octachlorohydrex GLY, Ammonium Alum, Ammonium Silver Zinc Aluminum Silicate, Calcium Aluminum Borosilicate, Cobalt Aluminum Oxide, Magnesium/Aluminum/Hydroxide/Carbonate, Magnesium Aluminum Silicate, Magnesium/Aluminum/Zinc/Hydroxide/Carbonate, Potassium Alum, Potassium Aluminum Polyacrylate, Silver Magnesium Aluminum Phosphate, Sodium Alum, Sodium Aluminate, Sodium Aluminum Chlorohydroxy Lactate, Sodium/Aluminum Hydroxide/Oxalate/Sulfate, Sodium/Aluminum/Iron Hydroxide/Oxalate/Sulfate, Sodium/Aluminum/Iron/Sulfate/Citrate/Hydroxide, Sodium/Aluminum/Iron/Sulfate/Oxalate/Hydroxide,
Sodium/Aluminum/Iron/Sulfate/Tartarate/Hydroxide, Sodium Aluminum Lactate, Sodium Phosphorus/Zinc/Calcium/Silicon/Aluminum/Silver Oxides, Sodium Potassium Aluminum Silicate, Sodium Silicoaluminate, Sodium Silver Aluminum Silicate, Tromethamine Magnesium Aluminum Silicate und Alaun, insbesondere Aluminiumchlorohydrat.
Unter dem Begriff "Aluminiumchlorohydrat" werden im Zusammenhang mit der vorliegenden Erfindung die Salze der allgemeinen Summenformel AlnCl(3n-m)(OH)m, insbesondere Al2Cl(OH)5, verstanden.
Geeignete Zirkonium-Salze sind ausgewählt aus den in
Aluminum Acetate, Aluminum Behenate, Aluminum Benzoate, Aluminum Bromohydrate, Aluminum Butoxide, Aluminum Calcium Sodium Silicate, Aluminum Caprylate, Aluminum Capryloyl Hydrolyzed Collagen, Aluminum Chloride, Aluminum Chlorohydrate, Aluminum Chlorohydrex, Aluminum Chlorohydrex PEG, Aluminum Chlorohydrex PG, Aluminum Citrate, Aluminum Diacetate, Aluminum Dibenzoate / Stearate Hydroxide, Aluminum Dicetyl Phosphate, Aluminum Dichlorohydrate, Aluminum Dichlorohydrex PEG, Aluminum Dichlorohydrex PG, Aluminum Dilinoleate, Aluminum Dimyristate, Aluminum Distearate, Aluminum Glycinate, Aluminum Hydrogenated Tallow Glutamate, Aluminum Hydroxy Bis-Methylene Bis-Di-t-Butylphenyl Phosphate, Aluminum Iron Calcium Magnesium Germanium Silicates, Aluminum Iron Calcium Magnesium Zirconium Silicates, Aluminum Iron Silicates, Aluminum Isopropoxide, Aluminum Isostearate, Aluminum Isostearates / Laurates / Palmitates, Aluminum Isostearates / Laurates / Stearates, Aluminum Isostearates / Myristates, Aluminum Isosteara tes / Palmitates, Aluminum Isostearates / Stearates, Aluminum Isostearyl Glyceryl Phosphate, Aluminum Laccate, Aluminum Lactate, Aluminum Lanolate, Aluminum / Magnesium Hydroxide Stearate, Aluminum Magnesium Oxide, Aluminum Methionate, Aluminum Myristate, Aluminum Myristates / Palmitates, Aluminum PCA, Aluminum Phenolsulfonate, Aluminum Sesquichlorohydrate, Aluminum Sesquichlorohydrex PEG, Aluminum Sesquichlorohydrex PG, Aluminum Starch Octenylsuccinate, Aluminum Stearate, Aluminum Stearates, Aluminum Stearoyl Glutamate, Aluminum Sucrose Octasulfate, Aluminum Sulfate, Aluminum Triformate, Aluminum Triphosphate, Aluminum Tristearids, Aluminum Zinc Oxide Acid, Aluminum Zinc Oxide Acid , Aluminum Zirconium Trichlorohydrate, Aluminum Zirconium Trichlorohydrex GLY, Aluminum Zirconium Tetrachlorohydrate, Aluminum Zirconium Tetrachlorohydrex GLY, Aluminum Zirconium Tetrachlorohydrex PEG, Aluminum Zirconium Tetrachlorohydrex PG, Aluminum Zirconium Pentachlorohydrat, Aluminum Zirconium Pe ntachlorohydrex GLY, Aluminum Zirconium Octachlorohydrate, Aluminum Zirconium Octachlorohydrex GLY, Ammonium Alum, Ammonium Silver Zinc Aluminum Silicate, Calcium Aluminum Borosilicate, Cobalt Aluminum Oxide, Magnesium / Aluminum / Hydroxide / Carbonate, Magnesium Aluminum Silicate, Magnesium / Aluminum / Zinc / Hydroxide / Carbonate , Potassium Alum, Potassium Aluminum Polyacrylate, Silver Magnesium Aluminum Phosphate, Sodium Alum, Sodium Aluminate, Sodium Aluminum Chlorohydroxy Lactate, Sodium / Aluminum Hydroxide / Oxalate / Sulfate, Sodium / Aluminum / Iron Hydroxide / Oxalate / Sulfate, Sodium / Aluminum / Iron / Sulfate / Citrate / hydroxides, Sodium / Aluminum / Iron / Sulfate / oxalate / hydroxides,
Sodium / Aluminum / Iron / Sulfate / Tartarate / Hydroxide, Sodium Aluminum Lactate, Sodium Phosphorus / Zinc / Calcium / Silicon / Aluminum / Silver Oxides, Sodium Potassium Aluminum Silicate, Sodium Silicoaluminate, Sodium Silver Aluminum Silicate, Tromethamine Magnesium Aluminum Silicate and Alum, especially aluminum chlorohydrate.
In connection with the present invention, the term “aluminum chlorohydrate” means the salts of the general empirical formula Aln Cl(3n-m ) (OH)m , in particular Al2 Cl (OH)5 .
Suitable zirconium salts are selected from the in
Geeignete Deo-Wirkstoffe sind ausgewählt aus der Gruppe umfassend, bevorzugt bestehend aus den in
Erfindungsgemäß bevorzugt ist das Zink-Salz ausgewählt aus der Gruppe der Zink-Salze der Essigsäure, Milchsäure, Oxalsäure, Bernsteinsäure, Fumarsäure, Maleinsäure, Ricinolsäure und/oder Citronensäure gewählt werden.Suitable deodorant active ingredients are selected from the group comprising, preferably consisting of the in
According to the invention, the zinc salt is preferably selected from the group of the zinc salts of acetic acid, lactic acid, oxalic acid, succinic acid, fumaric acid, maleic acid, ricinoleic acid and / or citric acid.
Die erfindungsgemäßen Zusammensetzungen stabilisieren insbesondere Emulsionen, so dass eine erfindungsgemäß bevorzugte Zusammensetzung dadurch gekennzeichnet ist, dass sie eine Emulsion, insbesondere eine Öl-in-Wasser-Emulsion ist.
Erfindungsgemäß bevorzugte Emulsion enthalten bezogen auf die Gesamtemulsion von 0,1 Gew.-% bis 60,0 Gew.-%, bevorzugt von 4,0 Gew.-% bis 30,0 Gew.-%, besonders bevorzugt von 5,0 Gew.-% bis 20,0 Gew.-%, mindestens ein Öl, insbesondere ein kosmetisches Öl.
Geeignete kosmetische Öle sind beispielsweise in
Zur guten Emulsionsstabilität ist es bevorzugt, dass die erfindungsgemäßen Emulsionen bezogen auf die Gesamtemulsion von 0,1 Gew.-% bis 15,0 Gew.-%, bevorzugt von 0,5 Gew.-% bis 10,0 Gew.-%, besonders bevorzugt von 1,0 Gew.-% bis 7,0 Gew.-%, mindestens eines Emulgators enthalten.
Bevorzugt enthaltene Emulgatoren sind insbesondere Öl-in-Wasser-Emulgatoren, insbesondere in
Emulsions preferred according to the invention contain, based on the total emulsion, from 0.1% by weight to 60.0% by weight, preferably from 4.0% by weight to 30.0% by weight, particularly preferably from 5.0% by weight .-% to 20.0 wt .-%, at least one oil, in particular a cosmetic oil.
Suitable cosmetic oils are for example in
For good emulsion stability, it is preferred that the emulsions according to the invention, based on the total emulsion, from 0.1% by weight to 15.0% by weight, preferably from 0.5% by weight to 10.0% by weight, particularly preferably from 1.0% by weight to 7.0% by weight, contain at least one emulsifier.
Emulsifiers preferably contained are in particular oil-in-water emulsifiers, in particular in
Die erfindungsgemäßen Zusammensetzungen stabilisieren auch sonst schwierige Formulierungen, die eine niedrige Viskosität aufweisen, somit ist eine erfindungsgemäß bevorzugte Zusammensetzung dadurch gekennzeichnet, dass sie eine Viskosität in einem Bereich von 500 bis 20000, bevorzugt von 1500 bis 10000 mPas, aufweist, wobei die Viskosität bei 25 °C mit Brookfield RVT, Spindel 4, 5 UpM, gemessen wird.The compositions according to the invention also stabilize otherwise difficult formulations which have a low viscosity, so a composition preferred according to the invention is characterized in that it has a viscosity in a range from 500 to 20,000, preferably from 1,500 to 10,000 mPas, the viscosity at 25 ° C with Brookfield RVT, spindle 4, 5 rpm.
Insbesondere sind Zusammensetzungen bevorzugt, die im Wesentlichen polyglycoletherfrei und im Wesentlichen frei von alkoxylierten Verbindungen sind. Unter dem Begriff "im Wesentlichen frei von alkoxylierten Verbindungen" und "im Wesentlichen polyglycoletherfrei" im Zusammenhang mit der vorliegenden Erfindung ist zu verstehen, dass die Zusammensetzungen, mit gegebenenfalls der erfindungsgemäß enthaltenen Komponente B) als Ausnahme, keine nennenswerten Mengen an alkoxylierten oder polyglycolether enthaltende Verbindungen aufweist, die eine oberflächenaktive Wirkung ausüben. Insbesondere ist hierunter zu verstehen, dass diese Verbindungen in Mengen von kleiner 1 Gew.-%, bevorzugt von kleiner 0,1 Gew.-%, besonders bevorzugt von kleiner 0,01 Gew.-% bezogen auf die Gesamtzusammensetzung, insbesondere keine nachweisbaren Mengen, enthalten sind.In particular, compositions are preferred which are essentially free of polyglycol ether and essentially free of alkoxylated compounds. The term “essentially free of alkoxylated compounds” and “essentially free of polyglycol ether” in connection with the present invention is to be understood to mean that the compositions, with the exception of component B) according to the invention, as an exception, do not contain any appreciable amounts of alkoxylated or polyglycol ether Has compounds that have a surface-active effect. In particular, this means that these compounds in amounts of less than 1% by weight, preferably less than 0.1% by weight, particularly preferably less than 0.01% by weight, based on the total composition, in particular no detectable amounts , are included.
In den nachfolgend aufgeführten Beispielen wird die vorliegende Erfindung beispielhaft beschrieben, ohne dass die Erfindung, deren Anwendungsbreite sich aus der gesamten Beschreibung und den Ansprüchen ergibt, auf die in den Beispielen genannten Ausführungsformen beschränkt sein soll.In the examples listed below, the present invention is described by way of example, without the invention, the scope of which results from the entire description and the claims, being restricted to the embodiments mentioned in the examples.
Ein Gemisch aus Glycerin (92 g, 1.0 mol), Behensäure (234.6 g, 0.69 mol) und Ca(OH)2 (0.06 g) wurde innerhalb von 3 h unter Stickstoffeinleitung bis auf 240 °C erhitzt und das Gemisch anschließend so lange bei dieser Temperatur gerührt und das entstehende Wasser kontinuierlich entfernt, bis eine Säurezahl von ≤1.5 erreicht war. Nach Abkühlen auf 90 °C wurde anschließend eine 5%ige Lösung von H3PO4 in Glycerin (2 g) zugegeben und das Reaktionsgemisch wieder auf 240 °C erhitzt. Ab einer Temperatur von ≥100 °C wurde der Druck bei gleichzeitiger Stickstoffeinleitung auf 10 mbar gesenkt und so lange destilliert, bis kein Destillat mehr anfiel. Nach Zugabe eines Filterhilfsmittels wird über einen Filter filtriert.A mixture of glycerol (92 g, 1.0 mol), behenic acid (234.6 g, 0.69 mol) and Ca (OH)2 (0.06 g) was brought up to 240 ° C. in the course of 3 h while introducing nitrogen heated and the mixture was then stirred at this temperature and the water formed was continuously removed until an acid number of ≤1.5 was reached. After cooling to 90 ° C., a 5% solution of H3 PO4 in glycerol (2 g) was then added and the reaction mixture was heated to 240 ° C. again. From a temperature of ≥100 ° C, the pressure was reduced to 10 mbar while simultaneously introducing nitrogen and distilled until no more distillate was obtained. After adding a filter aid, it is filtered through a filter.
Ein Gemisch aus Glycerin (198,3 g, 2,15 mol), Stearinsäure und Palmitinsäure im Verhältnis 1:1 (401.8 g, 1.49 mol) und Ca(OH)2 (0.13 g) wurde innerhalb von 3 h unter Stickstoffeinleitung bis auf 240 °C erhitzt und das Gemisch anschließend so lange bei dieser Temperatur gerührt und das entstehende Wasser kontinuierlich entfernt, bis eine Säurezahl von ≤1.5 erreicht war. Nach Abkühlen auf 90 °C wurde anschließend eine 5%ige Lösung von H3PO4 in Glycerin (4.3 g) zugegeben und das Reaktionsgemisch wieder auf 240 °C erhitzt. Ab einer Temperatur von ≥100 °C wurde der Druck bei gleichzeitiger Stickstoffeinleitung auf 10 mbar gesenkt und so lange destilliert, bis kein Destillat mehr anfiel. Nach Zugabe eines Filterhilfsmittels wird über einen Filter filtriert.A mixture of glycerol (198.3 g, 2.15 mol), stearic acid and palmitic acid in a ratio of 1: 1 (401.8 g, 1.49 mol) and Ca (OH)2 (0.13 g) was stirred up to within 3 h with nitrogen Heated 240 ° C and the mixture was then stirred at this temperature and the water formed was continuously removed until an acid number of ≤1.5 was reached. After cooling to 90 ° C., a 5% solution of H3 PO4 in glycerol (4.3 g) was then added and the reaction mixture was heated again to 240 ° C. From a temperature of ≥100 ° C, the pressure was reduced to 10 mbar while simultaneously introducing nitrogen and distilled until no more distillate was obtained. After adding a filter aid, it is filtered through a filter.
Ein Gemisch aus Glycerin (2102 g, 22,8 mol) und 45%iger wässriger Kalilauge (24,2 g) wurde bei 400 mbar innerhalb von 1 Stunde auf 240 °C erhitzt und das entstehende Wasser kontinuierlich abdestilliert. Sobald das Reaktionsgemisch einen Brechungsindex von ≥1.4830 erreicht hatte, wurde der Druck langsam auf 50 mbar gesenkt und weiter Wasser sowie überschüssiges Glycerin bei 240 °C abdestilliert, bis das verbleibende Gemisch eine Hydroxylzahl von 990 mg KOH/g aufwies.
Ein Gemisch aus dem so erhaltenen Polyglycerin (252,2 g, 0,8 mol) und Stearinsäure und Palmitinsäure im Verhältnis 1:1 (97,8 g, 0,36 mol) wurde innerhalb von 3 h unter Stickstoffeinleitung bis auf 240 °C erhitzt und das Gemisch anschließend so lange bei dieser Temperatur gerührt und das entstehende Wasser kontinuierlich entfernt, bis eine Säurezahl von ≤1.0 erreicht war und das Gemisch bei 240 °C klar und homogen war.A mixture of glycerol (2102 g, 22.8 mol) and 45% aqueous potassium hydroxide solution (24.2 g) was heated at 400 mbar to 240 ° C. within 1 hour and the water formed was distilled off continuously. As soon as the reaction mixture had reached a refractive index of ≥1.4830, the pressure was slowly reduced to 50 mbar and further water and excess glycerol were distilled off at 240 ° C. until the remaining mixture had a hydroxyl number of 990 mg KOH / g.
A mixture of the polyglycerol thus obtained (252.2 g, 0.8 mol) and stearic acid and palmitic acid in a ratio of 1: 1 (97.8 g, 0.36 mol) was brought up to 240 ° C. in the course of 3 h with nitrogen blowing heated and the mixture was then stirred at this temperature and the water formed was continuously removed until an acid number of ≤1.0 was reached and the mixture was clear and homogeneous at 240 ° C.
Ein Gemisch aus kommerziell erhältlichem Polyglycerin-3 (Solvay; 240 g, 1 mol) und Caprylsäure und Caprinsäure im Verhältnis 60:40 (78,8 g, 0,5 mol) wurde innerhalb von 3 h unter Stickstoffeinleitung bis auf 240 °C erhitzt und das Gemisch anschließend so lange bei dieser Temperatur gerührt und das entstehende Wasser kontinuierlich entfernt, bis eine Säurezahl von ≤0.5 erreicht war.A mixture of commercially available polyglycerol-3 (Solvay; 240 g, 1 mol) and caprylic acid and capric acid in a ratio of 60:40 (78.8 g, 0.5 mol) was heated to 240 ° C. in the course of 3 h while introducing nitrogen and the mixture was then stirred at this temperature and the water formed was continuously removed until an acid number of ≤0.5 was reached.
Ein Gemisch aus Estern erhalten wie beschrieben in Vorsynthesebeispiel 1 (22,5 g), in Vorsynthesebeispiel 2 (34,5 g), in Vorsynthesebeispiel 3(88,5 g) und in Vorsynthesebeispiel 4 (4,5 g) wurde auf 80 °C erhitzt und das Gemisch anschließend für 3 h bei dieser Temperatur gerührt..
Der so erhaltene Polyglycerinester weist nach seiner vollständigen Hydrolyse einen Polymerisationsgrad des Polyglycerins von <3 auf.A mixture of esters was obtained as described in pre-synthesis example 1 (22.5 g), in pre-synthesis example 2 (34.5 g), in pre-synthesis example 3 (88.5 g) and in pre-synthesis example 4 (4.5 g) at 80 ° C heated and the mixture was then stirred at this temperature for 3 h.
After complete hydrolysis, the polyglycerol ester thus obtained has a degree of polymerization of the polyglycerol of <3.
Ein Gemisch aus Glycerin (2102 g, 22,8 mol) und 45%iger wässriger Kalilauge (24,2 g) wurde bei 400 mbar innerhalb von 1 Stunde auf 240 °C erhitzt und das entstehende Wasser kontinuierlich abdestilliert. Sobald das Reaktionsgemisch einen Brechungsindex von ≥1.4830 erreicht hatte, wurde der Druck langsam auf 50 mbar gesenkt und weiter Wasser sowie überschüssiges Glycerin bei 240 °C abdestilliert, bis das verbleibende Gemisch eine Hydroxylzahl von 960 mg KOH/g aufwies.
Ein Gemisch aus dem so erhaltenen Polyglycerin (1008,7 g, 2,2 mol) und Stearinsäure und Palmitinsäure im Verhältnis 1:1 (391,3 g, 1,5 mol) wurde innerhalb von 3 h unter Stickstoffeinleitung bis auf 240 °C erhitzt und das Gemisch anschließend so lange bei dieser Temperatur gerührt und das entstehende Wasser kontinuierlich entfernt, bis eine Säurezahl von ≤1.0 erreicht war und das Gemisch bei 240 °C klar und homogen war.A mixture of glycerol (2102 g, 22.8 mol) and 45% aqueous potassium hydroxide solution (24.2 g) was heated at 400 mbar to 240 ° C. within 1 hour and the water formed was distilled off continuously. As soon as the reaction mixture had a refractive index of ≥1.4830, the pressure was slowly reduced to 50 mbar and further water and excess glycerol were distilled off at 240 ° C. until the remaining mixture had a hydroxyl number of 960 mg KOH / g.
A mixture of the polyglycerol thus obtained (1008.7 g, 2.2 mol) and stearic acid and palmitic acid in a ratio of 1: 1 (391.3 g, 1.5 mol) was brought up to 240 ° C. in the course of 3 h with nitrogen blowing heated and the mixture was then stirred at this temperature and the water formed was continuously removed until an acid number of ≤1.0 was reached and the mixture was clear and homogeneous at 240 ° C.
Ein Gemisch aus Estern erhalten wie beschrieben in Vorsynthesebeispiel 1 (18,75 g), in Vorsynthesebeispiel 2 (32,25 g), in Vorsynthesebeispiel 5(94,5 g) und in Vorsynthesebeispiel 4 (4,5 g) wurde auf 80 °C erhitzt und das Gemisch anschließend für 3 h bei dieser Temperatur gerührt..
Der so erhaltene Polyglycerinester weist nach seiner vollständigen Hydrolyse einen Polymerisationsgrad des Polyglycerins von ca. 3,9 auf.A mixture of esters was obtained as described in pre-synthesis example 1 (18.75 g), in pre-synthesis example 2 (32.25 g), in pre-synthesis example 5 (94.5 g) and in pre-synthesis example 4 (4.5 g) at 80 ° C heated and the mixture was then stirred at this temperature for 3 h.
After complete hydrolysis, the polyglycerol ester thus obtained has a degree of polymerization of the polyglycerol of approximately 3.9.
Ein Gemisch aus Glycerin (2102 g, 22,8 mol) und 45%iger wässriger Kalilauge (24,2 g) wurde bei 400 mbar innerhalb von 1 Stunde auf 240 °C erhitzt und das entstehende Wasser kontinuierlich abdestilliert. Sobald das Reaktionsgemisch einen Brechungsindex von ≥1.4830 erreicht hatte, wurde der Druck langsam auf 50 mbar gesenkt und weiter Wasser sowie überschüssiges Glycerin bei 240 °C abdestilliert, bis das verbleibende Gemisch eine Hydroxylzahl von 875 mg KOH/g aufwies.
Ein Gemisch aus dem so erhaltenen Polyglycerin (252,2 g, 0,33 mol) und Stearinsäure und Palmitinsäure im Verhältnis 1:1 (97,8 g, 0,36 mol) wurde innerhalb von 3 h unter Stickstoffeinleitung bis auf 240 °C erhitzt und das Gemisch anschließend so lange bei dieser Temperatur gerührt und das entstehende Wasser kontinuierlich entfernt, bis eine Säurezahl von ≤1.0 erreicht war und das Gemisch bei 240 °C klar und homogen war.A mixture of glycerol (2102 g, 22.8 mol) and 45% aqueous potassium hydroxide solution (24.2 g) was heated at 400 mbar to 240 ° C. within 1 hour and the water formed was distilled off continuously. As soon as the reaction mixture had reached a refractive index of ≥ 1.4830, the pressure was slowly reduced to 50 mbar and water and excess glycerol were further distilled off at 240 ° C. until the remaining mixture had a hydroxyl number of 875 mg KOH / g.
A mixture of the polyglycerol thus obtained (252.2 g, 0.33 mol) and stearic acid and palmitic acid in a ratio of 1: 1 (97.8 g, 0.36 mol) was brought up to 240 ° C. in the course of 3 h with nitrogen blowing heated and the mixture was then stirred at this temperature and the water formed was continuously removed until an acid number of ≤1.0 was reached and the mixture was clear and homogeneous at 240 ° C.
Ein Polyglycerin erhalten wie beschrieben in Beispiel 5a (240 g; 0,32 mol) und Caprylsäure und Caprinsäure im Verhältnis 60:40 (78,8 g, 0,5 mol) wurde innerhalb von 3 h unter Stickstoffeinleitung bis auf 240 °C erhitzt und das Gemisch anschließend so lange bei dieser Temperatur gerührt und das entstehende Wasser kontinuierlich entfernt, bis eine Säurezahl von ≤1.0 erreicht war.A polyglycerol obtained as described in Example 5a (240 g; 0.32 mol) and caprylic acid and capric acid in a ratio of 60:40 (78.8 g, 0.5 mol) were heated to 240 ° C. in the course of 3 h while introducing nitrogen and then the mixture stirred at this temperature and the water formed was continuously removed until an acid number of ≤1.0 was reached.
Ein Gemisch aus Estern erhalten wie beschrieben in Vorsynthesebeispiel 1 (18,8 g), in Vorsynthesebeispiel 2 (32,7 g), in Vorsynthesebeispiel 6 (93,8 g) und in Vorsynthesebeispiel 7(4,7 g) wurde auf 80 °C erhitzt und das Gemisch anschließend für 3 h bei dieser Temperatur gerührt.
Der so erhaltene Polyglycerinester weist nach seiner vollständigen Hydrolyse einen Polymerisationsgrad des Polyglycerins von ca. 4,5 auf.A mixture of esters was obtained as described in pre-synthesis example 1 (18.8 g), in pre-synthesis example 2 (32.7 g), in pre-synthesis example 6 (93.8 g) and in pre-synthesis example 7 (4.7 g) C heated and the mixture was then stirred at this temperature for 3 h.
After complete hydrolysis, the polyglycerol ester thus obtained has a degree of polymerization of the polyglycerol of approximately 4.5.
Ein Gemisch aus Glycerin (2102 g, 22,8 mol) und 45%iger wässriger Kalilauge (24,2 g) wurde bei 400 mbar innerhalb von 1 Stunde auf 240 °C erhitzt und das entstehende Wasser kontinuierlich abdestilliert. Sobald das Reaktionsgemisch einen Brechungsindex von ≥1.4830 erreicht hatte, wurde der Druck langsam auf 50 mbar gesenkt und weiter Wasser sowie überschüssiges Glycerin bei 240 °C abdestilliert, bis das verbleibende Gemisch eine Hydroxylzahl von 924 mg KOH/g aufwies.
Ein Gemisch aus dem so erhaltenen Polyglycerin (231,9 g, 0,296 mol) und Stearinsäure und Palmitinsäure im Verhältnis 1:1 (85,42 g, 0,32 mol) und Caprylsäure und Caprinsäure im Verhältnis 60:40 (3,82 g, 0,025 mol) wurde innerhalb von 3 h unter Stickstoffeinleitung bis auf 240 °C erhitzt und das Gemisch anschließend so lange bei dieser Temperatur gerührt und das entstehende Wasser kontinuierlich entfernt, bis eine Säurezahl von ≤1.0 erreicht war und das Gemisch bei 240 °C klar und homogen war.A mixture of glycerol (2102 g, 22.8 mol) and 45% aqueous potassium hydroxide solution (24.2 g) was heated at 400 mbar to 240 ° C. within 1 hour and the water formed was distilled off continuously. As soon as the reaction mixture had reached a refractive index of ≥1.4830, the pressure was slowly reduced to 50 mbar and water and excess glycerol were further distilled off at 240 ° C. until the remaining mixture had a hydroxyl number of 924 mg KOH / g.
A mixture of the polyglycerol thus obtained (231.9 g, 0.296 mol) and stearic acid and palmitic acid in a ratio of 1: 1 (85.42 g, 0.32 mol) and caprylic acid and capric acid in a ratio of 60:40 (3.82 g , 0.025 mol) was heated to 240 ° C. in the course of 3 hours with nitrogen injection and the mixture was then stirred at this temperature and the water formed was continuously removed until an acid number of ≤1.0 was reached and the mixture was clear at 240 ° C. and was homogeneous.
Ein Gemisch aus Estern erhalten wie beschrieben in Vorsynthesebeispiel 1 (18,75 g), in Vorsynthesebeispiel 2 (33,75 g) und in Vorsynthesebeispiel 8(97,5 g) wurde auf 80 °C erhitzt und das Gemisch anschließend für 3 h bei dieser Temperatur gerührt. Der so erhaltene Polyglycerinester weist nach seiner vollständigen Hydrolyse einen Polymerisationsgrad des Polyglycerins von ca. 3,6 auf.A mixture of esters obtained as described in pre-synthesis example 1 (18.75 g), in pre-synthesis example 2 (33.75 g) and in pre-synthesis example 8 (97.5 g) was heated to 80 ° C. and the mixture was then stirred for 3 hours this temperature stirred. After complete hydrolysis, the polyglycerol ester thus obtained has a degree of polymerization of the polyglycerol of approximately 3.6.
Ein Gemisch aus Glycerin (2102 g, 22,8 mol) und 45%iger wässriger Kalilauge (24,2 g) wurde bei 400 mbar innerhalb von 1 Stunde auf 240 °C erhitzt und das entstehende Wasser kontinuierlich abdestilliert. Sobald das Reaktionsgemisch einen Brechungsindex von ≥1.4830 erreicht hatte, wurde der Druck langsam auf 50 mbar gesenkt und weiter Wasser sowie überschüssiges Glycerin bei 240 °C abdestilliert, bis das verbleibende Gemisch eine Hydroxylzahl von 884 mg KOH/g aufwies.
Ein Gemisch aus dem so erhaltenen Polyglycerin (243,5 g, 0,32 mol) und Stearinsäure und Palmitinsäure im Verhältnis 1:1 (85,42 g, 0,32 mol) und Caprylsäure und Caprinsäure im Verhältnis 60:40 (3,82 g, 0,025 mol) wurde innerhalb von 3 h unter Stickstoffeinleitung bis auf 240 °C erhitzt und das Gemisch anschließend so lange bei dieser Temperatur gerührt und das entstehende Wasser kontinuierlich entfernt, bis eine Säurezahl von ≤1.0 erreicht war und das Gemisch bei 240 °C klar und homogen war.A mixture of glycerol (2102 g, 22.8 mol) and 45% aqueous potassium hydroxide solution (24.2 g) was heated at 400 mbar to 240 ° C. within 1 hour and the water formed was distilled off continuously. As soon as the reaction mixture had reached a refractive index of ≥1.4830, the pressure was slowly reduced to 50 mbar and further water and excess glycerol were distilled off at 240 ° C. until the remaining mixture had a hydroxyl number of 884 mg KOH / g.
A mixture of the polyglycerol thus obtained (243.5 g, 0.32 mol) and stearic acid and palmitic acid in a ratio of 1: 1 (85.42 g, 0.32 mol) and caprylic acid and capric acid in a ratio of 60:40 (3, 82 g, 0.025 mol) was heated to 240 ° C. in the course of 3 hours while introducing nitrogen and the mixture was then stirred at this temperature and the water formed was continuously removed until an acid number of ≤1.0 was reached and the mixture at 240 ° C was clear and homogeneous.
Ein Gemisch aus Estern erhalten wie beschrieben in Vorsynthesebeispiel 1 (18,75 g), in Vorsynthesebeispiel 2 (33,75 g) und in Vorsynthesebeispiel 8(97,5 g) wurde auf 80 °C erhitzt und das Gemisch anschließend für 3 h bei dieser Temperatur gerührt. Der so erhaltene Polyglycerinester weist nach seiner vollständigen Hydrolyse einen Polymerisationsgrad des Polyglycerins von ca. 3,8 auf.A mixture of esters obtained as described in pre-synthesis example 1 (18.75 g), in pre-synthesis example 2 (33.75 g) and in pre-synthesis example 8 (97.5 g) was heated to 80 ° C. and the mixture was then stirred for 3 hours this temperature stirred. After complete hydrolysis, the polyglycerol ester thus obtained has a degree of polymerization of the polyglycerol of approximately 3.8.
Alle Konzentrationen in den Anwendungsbeispielen sind in Gewichtsprozent angegeben. Zur Herstellung der Emulsionen wurden dem Fachmann bekannte übliche Homogenisierverfahren eingesetzt.
Die Herstellung der Emulsionen erfolgte daher typischerweise so, dass Öl- und Wasserphase auf 70 - 75°C erwärmt wurden. Anschließend wurde entweder die Ölphase in die Wasserphase eingerührt oder Öl- und Wasserphase ohne Rühren zusammengegeben. Anschließend wurde mit einem geeigneten Homogenisator (z.B. Ultra Turrax) für ca. 1-2 Minuten homogenisiert.
Stabilisierende Polymere wurden entweder als Bestandteil der Ölphase (z.B. Guarderivate) oder als wässrige Suspension (z.B. Cellulosederivate) bei Temperaturen von 50 - 60°C in die Emulsion eingerührt. Anschließend wurde kurz homogenisiert. Zugabe weiterer Inhaltsstoffe (z.B. Konservierungsmittel, Wirkstoffe) erfolgte bevorzugt bei 40°C. Falls die Rezepturen mit organischen Säuren konserviert wurden, wurde der pH-Wert der Emulsionen auf ca. 5 eingestellt.All concentrations in the application examples are given in percent by weight. Conventional homogenization processes known to those skilled in the art were used to prepare the emulsions.
The emulsions were therefore typically prepared by heating the oil and water phases to 70-75 ° C. The oil phase was then either stirred into the water phase or the oil and water phases were combined without stirring. The mixture was then homogenized with a suitable homogenizer (eg Ultra Turrax) for approx. 1-2 minutes.
Stabilizing polymers were either stirred into the emulsion as a component of the oil phase (eg guar derivatives) or as an aqueous suspension (eg cellulose derivatives) at temperatures of 50 - 60 ° C. The mixture was then briefly homogenized. Additional ingredients (eg preservatives, active ingredients) were preferably added at 40 ° C. If the formulations were preserved with organic acids, the pH of the emulsions was adjusted to approx. 5.
Diese Versuche zeigen, dass die erfindungsgemäßen Emulgatoren Vorteile in Bezug auf Emulsionsstabilität aufweisen. Als Repräsentanten für PEG-freie O/W Emulgator wurden dabei die Kombination aus Methyglucose Sesquistearat / Polyglyceryl-4 Laurat sowie Polyglyceryl-4 Laurate/Succinate (and) Aqua gewählt.
Zur Überprüfung der Lagerstabilität der Emulsionen wurden diese drei Monate bei Raumtemperatur und 40°C gelagert. Zur Überprüfung der Kältestabilität wurde außerdem ein Monat lang bei -5°C gelagert und drei Gefrier-Tau-Zyklen von 25°C/- 15°C/25°C durchgeführt. Deutliche Veränderungen im Erscheinungsbild oder der Konsistenz sowie insbesondere Öl- oder Wasserabscheidungen wurden als Kriterien für Instabilität gewichtet.
2) TEGO Care PL 4 (Evonik Industries AG)
29) Polyglycerinester entsprechend Synthesebeispiel 1 in
30) ESAFLOR HDR (Lamberti S.p.A.)
3) NatraGem E145 (Croda Int. Plc)
4) ESAFLOR HM 22 (Lamberti S.p.A.)
5) Natrosol 250 HHR (Ashland Specialty Ingredients)
6) TEGOSOFT AC (Evonik Industries AG)
7) Locron LIC (Clariant AG)
8) Microcare MEM (Thor)
10) TEGODEO PY 88 G (Evonik Industries AG)
11) Rokonsal BSB-N (Ashland Specialty Ingredients)
To check the storage stability of the emulsions, they were stored at room temperature and 40 ° C. for three months. To check the cold stability, storage was also carried out at -5 ° C for one month and three freeze-thaw cycles of 25 ° C / - 15 ° C / 25 ° C were carried out. Significant changes in appearance or consistency, and in particular oil or water deposits, were weighted as criteria for instability.
2) TEGO Care PL 4 (Evonik Industries AG)
29) polyglycerol esters according to synthesis example 1 in
30) ESAFLOR HDR (Lamberti SpA)
3) NatraGem E145 (Croda Int.Plc)
4) ESAFLOR HM 22 (Lamberti SpA)
5) Natrosol 250 HHR (Ashland Specialty Ingredients)
6) TEGOSOFT AC (Evonik Industries AG)
7) Locron LIC (Clariant AG)
8) Microcare MEM (Thor)
10) TEGODEO PY 88 G (Evonik Industries AG)
11) Rokonsal BSB-N (Ashland Specialty Ingredients)
In allen drei Vergleichsformulierungen aus dem Anwendungsbereich AP/Deo- bzw. Deo-Roll-on, welche verschiedene Wirkstoffe enthalten, erlauben die erfindungsgemäßen Emulgatoren die Formulierung einer stabilen Emulsion, wohingegen der nicht erfindungsgemäße Emulgator und die Repräsentanten des Stand der Technik keine stabile Emulsion ermöglichen. Die Verwendung von nicht erfindungsgemäß modifiziertem Guar führt ebenfalls zu nicht lagerstabilen Emulsionen.In all three comparative formulations from the AP / deodorant or deodorant roll-on area of application, which contain different active ingredients, the emulsifiers according to the invention allow the formulation of a stable emulsion, whereas the emulsifier not according to the invention and the representatives of the prior art do not enable a stable emulsion. The use of guar not modified according to the invention likewise leads to emulsions which are not stable in storage.
Formulierungen 1-2 und 1-9 wurden hinsichtlich ihrer Ausbildungsfähigkeit einer Fließgrenze untersucht. Die Messungen wurden mit einem Rheometer von Anton Paar, Modell MCR 301, Platte - Platte (40 mm) Geometrie mit einem Spalt von 1 mm bei einer Temperatur von 25°C und 1 bar Druck durchgeführt. Bestimmt wurden Speichermodul und Verlustmodul für die nach erfindungsgemäßer Rezeptur 1-2 bzw. nicht erfindungsgemäßer Rezeptur 1-9 hergestellten Emulsionen. Die Proben wurden bei konstanter Belastung von 0,20 Pa über den Frequenzbereich von 0,005 bis 90 Hz vermessen. Die rheologische Fließgrenze ist dadurch definiert, dass im vermessenen Frequenzbereich das Speichermodul (G') stets höhere Werte aufweist als das Verlustmodul (G") (siehe
Weitere Anwendungsbeispiele außerhalb des AP/Deo Bereichs.
Diese Beispiele zeigen, dass die erfindungsgemäßen Zusammensetzungen in einer Vielzahl kosmetischer Formulierungen eingesetzt werden können.
13) Tinosorb S (BASF SE)
14) Acrylates/C10-30 Alkyl Acrylate Crosspolymer (TEGO Carbomer 341ER, Evonik Industries AG) 20%ig in Phenoxyethyl Caprylate
15) 20 % Phenylbenzimidazole Sulfonic Acid, 8,8% Tris (hydroxymethyl)-aminomethan, demineralisiertes Wasser ad 100%
17) Carbomer (TEGO Carbomer 141, Evonik Industries AG) 20%ig in Ethylhexyl Stearate
18) Euxyl PE 9010 (Schülke & Mayr GmbH)
20) HyaCare (Evonik Industries)
21) HyaCare 50 (Evonik Industries)
22) HyaCare Filler CL (Evonik Industries)
24) Sicovit Gelb 10 E 172 (Rockwood Pigments)
25) Sicovit Rot 30 E 172 (Rockwood Pigments)
26) Sicovit Braun 70 E 172 (Rockwood Pigments)
27) Sicovit Schwarz 80 E 172 (Rockwood Pigments)
28) TEGO Feel Green (Evonik Industries AG)
These examples show that the compositions according to the invention can be used in a large number of cosmetic formulations.
13) Tinosorb S (BASF SE)
14) Acrylates / C10-30 Alkyl Acrylate Crosspolymer (TEGO Carbomer 341ER, Evonik Industries AG) 20% in phenoxyethyl caprylate
15) 20% phenylbenzimidazole sulfonic acid, 8.8% tris (hydroxymethyl) aminomethane, demineralized water ad 100%
17) Carbomer (TEGO Carbomer 141, Evonik Industries AG) 20% in ethylhexyl stearate
18) Euxyl PE 9010 (Schülke & Mayr GmbH)
20) HyaCare (Evonik Industries)
21) HyaCare 50 (Evonik Industries)
22) HyaCare Filler CL (Evonik Industries)
24) Sicovit Yellow 10 E 172 (Rockwood Pigments)
25) Sicovit Red 30 E 172 (Rockwood Pigments)
26) Sicovit Braun 70 E 172 (Rockwood Pigments)
27) Sicovit Black 80 E 172 (Rockwood Pigments)
28) TEGO Feel Green (Evonik Industries AG)
Claims (13)
- Composition comprisingA) polyglycerol ester,
which, after its complete hydrolysis, releasesa) at least one carboxylic acid having 6 to 14, preferably 8 to 12, carbon atoms,b) at least one carboxylic acid having 16 to 20, preferably 18 to 20, carbon atoms,c) at least one carboxylic acid having 22 to 28, preferably 22 to 24, carbon atoms,
and
which, after its complete hydrolysis, releases a polyglycerol having an average degree of polymerization of from 3.0 to 5.0, preferably from 3.3 to 4.7, particularly preferably from 3.6 to 4.5,
andB) at least one hydroxyalkyl-modified guar,characterized in that the hydroxyalkyl-modified guar has been modified with hydroxyalkyl groups having 12 to 26 carbon atoms. - Composition according to Claim 1,characterized in that the polyglycerol ester, after its complete hydrolysis, releases an average (number average) per mole of polyglycerol ester of
from 0.01 to 0.07 mol, preferably from 0.01 to 0.50 mol, particularly preferably from 0.01 to 0.30 mol, of at least one carboxylic acid a)
from 0.10 to 1.70 mol, preferably from 0.30 to 1.50 mol, particularly preferably from 0.40 to 1.40 mol, of at least one carboxylic acid b)
from 0.01 to 0.80 mol, preferably from 0.01 to 0.60 mol, particularly preferably from 0.05 to 0.40 mol, of at least one carboxylic acid c). - Composition according to Claim 1 or 2,characterized in that the molar ratio of carboxylic acid a) to carboxylic acid b) to carboxylic acid c) obtained after complete hydrolysis of the polyglycerol ester is
0.6 to 1.4:16.5 to 20.5:3.0 to 4.8, preferably
0.8 to 1.2:17.5 to 19.5:3.5 to 4.3, particularly preferably 0.9 to 1.1:18.0 to 19.0:3.7 to 4.1. - Composition according to at least one of the preceding claims,characterized in that the carboxylic acids a), b) and c) are selected from linear, saturated, unsubstituted carboxylic acids.
- Composition according to at least one of the preceding claims,characterized in that carboxylic acid a) is selected from caprylic acid and capric acid, carboxylic acid b) is selected from stearic acid and palmitic acid and carboxylic acid c) is behenic acid.
- Composition according to at least one of the preceding claims,characterized in that the polyglycerol released after complete hydrolysis of the polyglycerol ester has a mass ratio of glycerol to diglycerol of greater than 1, preferably greater than 1.2, particularly preferably greater than 1.4.
- Composition according to at least one of the preceding claims,characterized in that the hydroxyalkyl-modified guar is modified with hydroxyalkyl groups having 18 to 24, preferably 20 to 22, carbon atoms.
- Composition according to at least one of the preceding claims,characterized in that the hydroxyalkyl-modified guar has additionally been hydroxypropyl-modified.
- Composition according to at least one of the preceding claims,characterized in that said composition additionally comprises
C) at least one selected from deodorant active ingredient and antiperspirant active ingredient. - Composition according to at least one of the preceding claims,characterized in that the antiperspirant active ingredient is selected from the group comprising, preferably consisting of, aluminium salts and zirconium salts, and the deodorant active ingredient from the group of zinc salts, in particular aluminium chlorohydrate and zinc ricinoleate.
- Composition according to at least one of the preceding claims,characterized in that said composition is an emulsion, particularly an oil-in-water emulsion.
- Composition according to at least one of the preceding claims,characterized in that said composition has a viscosity in a range from 500 to 20 000, preferably 1500 to 10 000 mPas.
- Composition according to at least one of the preceding claims,characterized in that said composition is free from polyglycol ethers and free from alkoxylated compounds.
Priority Applications (4)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| EP16151443.5AEP3192491B1 (en) | 2016-01-15 | 2016-01-15 | Composition comprising polyglycerol esters and hydroxy-alkyl modified guar |
| US15/380,137US20170202770A1 (en) | 2016-01-15 | 2016-12-15 | Composition comprising polyglycerol esters and hydroxyalkyl-modified guar |
| BR102017000660-3ABR102017000660B1 (en) | 2016-01-15 | 2017-01-12 | COMPOSITION INCLUDING POLYGLYCEROL ESTERS AND GUAR GUM MODIFIED BY HYDROXYALKYL GROUPS |
| CN201710032429.2ACN107028795B (en) | 2016-01-15 | 2017-01-16 | Composition comprising polyglycerol ester and hydroxyalkyl modified guar gum |
Applications Claiming Priority (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| EP16151443.5AEP3192491B1 (en) | 2016-01-15 | 2016-01-15 | Composition comprising polyglycerol esters and hydroxy-alkyl modified guar |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| EP3192491A1 EP3192491A1 (en) | 2017-07-19 |
| EP3192491B1true EP3192491B1 (en) | 2020-01-08 |
Family
ID=55177758
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| EP16151443.5AActiveEP3192491B1 (en) | 2016-01-15 | 2016-01-15 | Composition comprising polyglycerol esters and hydroxy-alkyl modified guar |
Country Status (4)
| Country | Link |
|---|---|
| US (1) | US20170202770A1 (en) |
| EP (1) | EP3192491B1 (en) |
| CN (1) | CN107028795B (en) |
| BR (1) | BR102017000660B1 (en) |
Families Citing this family (400)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US20070084897A1 (en) | 2003-05-20 | 2007-04-19 | Shelton Frederick E Iv | Articulating surgical stapling instrument incorporating a two-piece e-beam firing mechanism |
| US9060770B2 (en) | 2003-05-20 | 2015-06-23 | Ethicon Endo-Surgery, Inc. | Robotically-driven surgical instrument with E-beam driver |
| US8215531B2 (en) | 2004-07-28 | 2012-07-10 | Ethicon Endo-Surgery, Inc. | Surgical stapling instrument having a medical substance dispenser |
| US11890012B2 (en) | 2004-07-28 | 2024-02-06 | Cilag Gmbh International | Staple cartridge comprising cartridge body and attached support |
| US9072535B2 (en) | 2011-05-27 | 2015-07-07 | Ethicon Endo-Surgery, Inc. | Surgical stapling instruments with rotatable staple deployment arrangements |
| US11998198B2 (en) | 2004-07-28 | 2024-06-04 | Cilag Gmbh International | Surgical stapling instrument incorporating a two-piece E-beam firing mechanism |
| US7934630B2 (en) | 2005-08-31 | 2011-05-03 | Ethicon Endo-Surgery, Inc. | Staple cartridges for forming staples having differing formed staple heights |
| US11484312B2 (en) | 2005-08-31 | 2022-11-01 | Cilag Gmbh International | Staple cartridge comprising a staple driver arrangement |
| US9237891B2 (en) | 2005-08-31 | 2016-01-19 | Ethicon Endo-Surgery, Inc. | Robotically-controlled surgical stapling devices that produce formed staples having different lengths |
| US10159482B2 (en) | 2005-08-31 | 2018-12-25 | Ethicon Llc | Fastener cartridge assembly comprising a fixed anvil and different staple heights |
| US11246590B2 (en) | 2005-08-31 | 2022-02-15 | Cilag Gmbh International | Staple cartridge including staple drivers having different unfired heights |
| US7669746B2 (en) | 2005-08-31 | 2010-03-02 | Ethicon Endo-Surgery, Inc. | Staple cartridges for forming staples having differing formed staple heights |
| US20070106317A1 (en) | 2005-11-09 | 2007-05-10 | Shelton Frederick E Iv | Hydraulically and electrically actuated articulation joints for surgical instruments |
| US8186555B2 (en) | 2006-01-31 | 2012-05-29 | Ethicon Endo-Surgery, Inc. | Motor-driven surgical cutting and fastening instrument with mechanical closure system |
| US8820603B2 (en) | 2006-01-31 | 2014-09-02 | Ethicon Endo-Surgery, Inc. | Accessing data stored in a memory of a surgical instrument |
| US20120292367A1 (en) | 2006-01-31 | 2012-11-22 | Ethicon Endo-Surgery, Inc. | Robotically-controlled end effector |
| US7845537B2 (en) | 2006-01-31 | 2010-12-07 | Ethicon Endo-Surgery, Inc. | Surgical instrument having recording capabilities |
| US11224427B2 (en) | 2006-01-31 | 2022-01-18 | Cilag Gmbh International | Surgical stapling system including a console and retraction assembly |
| US20110024477A1 (en) | 2009-02-06 | 2011-02-03 | Hall Steven G | Driven Surgical Stapler Improvements |
| US11278279B2 (en) | 2006-01-31 | 2022-03-22 | Cilag Gmbh International | Surgical instrument assembly |
| US7753904B2 (en) | 2006-01-31 | 2010-07-13 | Ethicon Endo-Surgery, Inc. | Endoscopic surgical instrument with a handle that can articulate with respect to the shaft |
| US8708213B2 (en) | 2006-01-31 | 2014-04-29 | Ethicon Endo-Surgery, Inc. | Surgical instrument having a feedback system |
| US20110295295A1 (en) | 2006-01-31 | 2011-12-01 | Ethicon Endo-Surgery, Inc. | Robotically-controlled surgical instrument having recording capabilities |
| US11793518B2 (en) | 2006-01-31 | 2023-10-24 | Cilag Gmbh International | Powered surgical instruments with firing system lockout arrangements |
| US8992422B2 (en) | 2006-03-23 | 2015-03-31 | Ethicon Endo-Surgery, Inc. | Robotically-controlled endoscopic accessory channel |
| US8322455B2 (en) | 2006-06-27 | 2012-12-04 | Ethicon Endo-Surgery, Inc. | Manually driven surgical cutting and fastening instrument |
| US10568652B2 (en) | 2006-09-29 | 2020-02-25 | Ethicon Llc | Surgical staples having attached drivers of different heights and stapling instruments for deploying the same |
| US11980366B2 (en) | 2006-10-03 | 2024-05-14 | Cilag Gmbh International | Surgical instrument |
| US11291441B2 (en) | 2007-01-10 | 2022-04-05 | Cilag Gmbh International | Surgical instrument with wireless communication between control unit and remote sensor |
| US8632535B2 (en) | 2007-01-10 | 2014-01-21 | Ethicon Endo-Surgery, Inc. | Interlock and surgical instrument including same |
| US8684253B2 (en) | 2007-01-10 | 2014-04-01 | Ethicon Endo-Surgery, Inc. | Surgical instrument with wireless communication between a control unit of a robotic system and remote sensor |
| US20080169333A1 (en) | 2007-01-11 | 2008-07-17 | Shelton Frederick E | Surgical stapler end effector with tapered distal end |
| US11039836B2 (en) | 2007-01-11 | 2021-06-22 | Cilag Gmbh International | Staple cartridge for use with a surgical stapling instrument |
| US7673782B2 (en) | 2007-03-15 | 2010-03-09 | Ethicon Endo-Surgery, Inc. | Surgical stapling instrument having a releasable buttress material |
| US8931682B2 (en) | 2007-06-04 | 2015-01-13 | Ethicon Endo-Surgery, Inc. | Robotically-controlled shaft based rotary drive systems for surgical instruments |
| US11564682B2 (en) | 2007-06-04 | 2023-01-31 | Cilag Gmbh International | Surgical stapler device |
| US7753245B2 (en) | 2007-06-22 | 2010-07-13 | Ethicon Endo-Surgery, Inc. | Surgical stapling instruments |
| US11849941B2 (en) | 2007-06-29 | 2023-12-26 | Cilag Gmbh International | Staple cartridge having staple cavities extending at a transverse angle relative to a longitudinal cartridge axis |
| US8573465B2 (en) | 2008-02-14 | 2013-11-05 | Ethicon Endo-Surgery, Inc. | Robotically-controlled surgical end effector system with rotary actuated closure systems |
| US11986183B2 (en) | 2008-02-14 | 2024-05-21 | Cilag Gmbh International | Surgical cutting and fastening instrument comprising a plurality of sensors to measure an electrical parameter |
| US8636736B2 (en) | 2008-02-14 | 2014-01-28 | Ethicon Endo-Surgery, Inc. | Motorized surgical cutting and fastening instrument |
| US9179912B2 (en) | 2008-02-14 | 2015-11-10 | Ethicon Endo-Surgery, Inc. | Robotically-controlled motorized surgical cutting and fastening instrument |
| US7819298B2 (en) | 2008-02-14 | 2010-10-26 | Ethicon Endo-Surgery, Inc. | Surgical stapling apparatus with control features operable with one hand |
| JP5410110B2 (en) | 2008-02-14 | 2014-02-05 | エシコン・エンド−サージェリィ・インコーポレイテッド | Surgical cutting / fixing instrument with RF electrode |
| US8758391B2 (en) | 2008-02-14 | 2014-06-24 | Ethicon Endo-Surgery, Inc. | Interchangeable tools for surgical instruments |
| US7866527B2 (en) | 2008-02-14 | 2011-01-11 | Ethicon Endo-Surgery, Inc. | Surgical stapling apparatus with interlockable firing system |
| US9585657B2 (en) | 2008-02-15 | 2017-03-07 | Ethicon Endo-Surgery, Llc | Actuator for releasing a layer of material from a surgical end effector |
| US11648005B2 (en) | 2008-09-23 | 2023-05-16 | Cilag Gmbh International | Robotically-controlled motorized surgical instrument with an end effector |
| US8210411B2 (en) | 2008-09-23 | 2012-07-03 | Ethicon Endo-Surgery, Inc. | Motor-driven surgical cutting instrument |
| US9005230B2 (en) | 2008-09-23 | 2015-04-14 | Ethicon Endo-Surgery, Inc. | Motorized surgical instrument |
| US9386983B2 (en) | 2008-09-23 | 2016-07-12 | Ethicon Endo-Surgery, Llc | Robotically-controlled motorized surgical instrument |
| US8608045B2 (en) | 2008-10-10 | 2013-12-17 | Ethicon Endo-Sugery, Inc. | Powered surgical cutting and stapling apparatus with manually retractable firing system |
| US8517239B2 (en) | 2009-02-05 | 2013-08-27 | Ethicon Endo-Surgery, Inc. | Surgical stapling instrument comprising a magnetic element driver |
| RU2525225C2 (en) | 2009-02-06 | 2014-08-10 | Этикон Эндо-Серджери, Инк. | Improvement of drive surgical suturing instrument |
| US8220688B2 (en) | 2009-12-24 | 2012-07-17 | Ethicon Endo-Surgery, Inc. | Motor-driven surgical cutting instrument with electric actuator directional control assembly |
| US8851354B2 (en) | 2009-12-24 | 2014-10-07 | Ethicon Endo-Surgery, Inc. | Surgical cutting instrument that analyzes tissue thickness |
| US8783543B2 (en) | 2010-07-30 | 2014-07-22 | Ethicon Endo-Surgery, Inc. | Tissue acquisition arrangements and methods for surgical stapling devices |
| US11925354B2 (en) | 2010-09-30 | 2024-03-12 | Cilag Gmbh International | Staple cartridge comprising staples positioned within a compressible portion thereof |
| US11812965B2 (en) | 2010-09-30 | 2023-11-14 | Cilag Gmbh International | Layer of material for a surgical end effector |
| US9351730B2 (en) | 2011-04-29 | 2016-05-31 | Ethicon Endo-Surgery, Llc | Tissue thickness compensator comprising channels |
| US12213666B2 (en) | 2010-09-30 | 2025-02-04 | Cilag Gmbh International | Tissue thickness compensator comprising layers |
| US11298125B2 (en) | 2010-09-30 | 2022-04-12 | Cilag Gmbh International | Tissue stapler having a thickness compensator |
| US9629814B2 (en) | 2010-09-30 | 2017-04-25 | Ethicon Endo-Surgery, Llc | Tissue thickness compensator configured to redistribute compressive forces |
| US10945731B2 (en) | 2010-09-30 | 2021-03-16 | Ethicon Llc | Tissue thickness compensator comprising controlled release and expansion |
| US9788834B2 (en) | 2010-09-30 | 2017-10-17 | Ethicon Llc | Layer comprising deployable attachment members |
| US9386988B2 (en) | 2010-09-30 | 2016-07-12 | Ethicon End-Surgery, LLC | Retainer assembly including a tissue thickness compensator |
| US9016542B2 (en) | 2010-09-30 | 2015-04-28 | Ethicon Endo-Surgery, Inc. | Staple cartridge comprising compressible distortion resistant components |
| US8695866B2 (en) | 2010-10-01 | 2014-04-15 | Ethicon Endo-Surgery, Inc. | Surgical instrument having a power control circuit |
| AU2012250197B2 (en) | 2011-04-29 | 2017-08-10 | Ethicon Endo-Surgery, Inc. | Staple cartridge comprising staples positioned within a compressible portion thereof |
| US11207064B2 (en) | 2011-05-27 | 2021-12-28 | Cilag Gmbh International | Automated end effector component reloading system for use with a robotic system |
| US9044230B2 (en) | 2012-02-13 | 2015-06-02 | Ethicon Endo-Surgery, Inc. | Surgical cutting and fastening instrument with apparatus for determining cartridge and firing motion status |
| JP6224070B2 (en) | 2012-03-28 | 2017-11-01 | エシコン・エンド−サージェリィ・インコーポレイテッドEthicon Endo−Surgery,Inc. | Retainer assembly including tissue thickness compensator |
| BR112014024098B1 (en) | 2012-03-28 | 2021-05-25 | Ethicon Endo-Surgery, Inc. | staple cartridge |
| MX358135B (en) | 2012-03-28 | 2018-08-06 | Ethicon Endo Surgery Inc | Tissue thickness compensator comprising a plurality of layers. |
| US9101358B2 (en) | 2012-06-15 | 2015-08-11 | Ethicon Endo-Surgery, Inc. | Articulatable surgical instrument comprising a firing drive |
| US9282974B2 (en) | 2012-06-28 | 2016-03-15 | Ethicon Endo-Surgery, Llc | Empty clip cartridge lockout |
| US9289256B2 (en) | 2012-06-28 | 2016-03-22 | Ethicon Endo-Surgery, Llc | Surgical end effectors having angled tissue-contacting surfaces |
| BR112014032776B1 (en) | 2012-06-28 | 2021-09-08 | Ethicon Endo-Surgery, Inc | SURGICAL INSTRUMENT SYSTEM AND SURGICAL KIT FOR USE WITH A SURGICAL INSTRUMENT SYSTEM |
| US9408606B2 (en) | 2012-06-28 | 2016-08-09 | Ethicon Endo-Surgery, Llc | Robotically powered surgical device with manually-actuatable reversing system |
| US11278284B2 (en) | 2012-06-28 | 2022-03-22 | Cilag Gmbh International | Rotary drive arrangements for surgical instruments |
| US20140001231A1 (en) | 2012-06-28 | 2014-01-02 | Ethicon Endo-Surgery, Inc. | Firing system lockout arrangements for surgical instruments |
| US12383267B2 (en) | 2012-06-28 | 2025-08-12 | Cilag Gmbh International | Robotically powered surgical device with manually-actuatable reversing system |
| JP6290201B2 (en) | 2012-06-28 | 2018-03-07 | エシコン・エンド−サージェリィ・インコーポレイテッドEthicon Endo−Surgery,Inc. | Lockout for empty clip cartridge |
| RU2672520C2 (en) | 2013-03-01 | 2018-11-15 | Этикон Эндо-Серджери, Инк. | Hingedly turnable surgical instruments with conducting ways for signal transfer |
| BR112015021082B1 (en) | 2013-03-01 | 2022-05-10 | Ethicon Endo-Surgery, Inc | surgical instrument |
| US9629629B2 (en) | 2013-03-14 | 2017-04-25 | Ethicon Endo-Surgey, LLC | Control systems for surgical instruments |
| US9808244B2 (en) | 2013-03-14 | 2017-11-07 | Ethicon Llc | Sensor arrangements for absolute positioning system for surgical instruments |
| BR112015026109B1 (en) | 2013-04-16 | 2022-02-22 | Ethicon Endo-Surgery, Inc | surgical instrument |
| US9826976B2 (en) | 2013-04-16 | 2017-11-28 | Ethicon Llc | Motor driven surgical instruments with lockable dual drive shafts |
| MX369362B (en) | 2013-08-23 | 2019-11-06 | Ethicon Endo Surgery Llc | Firing member retraction devices for powered surgical instruments. |
| US9775609B2 (en) | 2013-08-23 | 2017-10-03 | Ethicon Llc | Tamper proof circuit for surgical instrument battery pack |
| US9962161B2 (en) | 2014-02-12 | 2018-05-08 | Ethicon Llc | Deliverable surgical instrument |
| US12232723B2 (en) | 2014-03-26 | 2025-02-25 | Cilag Gmbh International | Systems and methods for controlling a segmented circuit |
| BR112016021943B1 (en) | 2014-03-26 | 2022-06-14 | Ethicon Endo-Surgery, Llc | SURGICAL INSTRUMENT FOR USE BY AN OPERATOR IN A SURGICAL PROCEDURE |
| US10004497B2 (en) | 2014-03-26 | 2018-06-26 | Ethicon Llc | Interface systems for use with surgical instruments |
| US10013049B2 (en) | 2014-03-26 | 2018-07-03 | Ethicon Llc | Power management through sleep options of segmented circuit and wake up control |
| US20150272580A1 (en) | 2014-03-26 | 2015-10-01 | Ethicon Endo-Surgery, Inc. | Verification of number of battery exchanges/procedure count |
| US20150297225A1 (en) | 2014-04-16 | 2015-10-22 | Ethicon Endo-Surgery, Inc. | Fastener cartridges including extensions having different configurations |
| BR112016023825B1 (en) | 2014-04-16 | 2022-08-02 | Ethicon Endo-Surgery, Llc | STAPLE CARTRIDGE FOR USE WITH A SURGICAL STAPLER AND STAPLE CARTRIDGE FOR USE WITH A SURGICAL INSTRUMENT |
| CN106456176B (en) | 2014-04-16 | 2019-06-28 | 伊西康内外科有限责任公司 | Fastener Cartridge Including Extensions With Different Configurations |
| CN106456159B (en) | 2014-04-16 | 2019-03-08 | 伊西康内外科有限责任公司 | Fastener Cartridge Assembly and Nail Retainer Cover Arrangement |
| US10327764B2 (en) | 2014-09-26 | 2019-06-25 | Ethicon Llc | Method for creating a flexible staple line |
| US10470768B2 (en) | 2014-04-16 | 2019-11-12 | Ethicon Llc | Fastener cartridge including a layer attached thereto |
| US11311294B2 (en) | 2014-09-05 | 2022-04-26 | Cilag Gmbh International | Powered medical device including measurement of closure state of jaws |
| BR112017004361B1 (en) | 2014-09-05 | 2023-04-11 | Ethicon Llc | ELECTRONIC SYSTEM FOR A SURGICAL INSTRUMENT |
| US10135242B2 (en) | 2014-09-05 | 2018-11-20 | Ethicon Llc | Smart cartridge wake up operation and data retention |
| US10105142B2 (en) | 2014-09-18 | 2018-10-23 | Ethicon Llc | Surgical stapler with plurality of cutting elements |
| US11523821B2 (en) | 2014-09-26 | 2022-12-13 | Cilag Gmbh International | Method for creating a flexible staple line |
| CN107427300B (en) | 2014-09-26 | 2020-12-04 | 伊西康有限责任公司 | Surgical suture buttresses and auxiliary materials |
| US10076325B2 (en) | 2014-10-13 | 2018-09-18 | Ethicon Llc | Surgical stapling apparatus comprising a tissue stop |
| US9924944B2 (en) | 2014-10-16 | 2018-03-27 | Ethicon Llc | Staple cartridge comprising an adjunct material |
| US10517594B2 (en) | 2014-10-29 | 2019-12-31 | Ethicon Llc | Cartridge assemblies for surgical staplers |
| US11141153B2 (en) | 2014-10-29 | 2021-10-12 | Cilag Gmbh International | Staple cartridges comprising driver arrangements |
| US9844376B2 (en) | 2014-11-06 | 2017-12-19 | Ethicon Llc | Staple cartridge comprising a releasable adjunct material |
| US10736636B2 (en) | 2014-12-10 | 2020-08-11 | Ethicon Llc | Articulatable surgical instrument system |
| US9844374B2 (en) | 2014-12-18 | 2017-12-19 | Ethicon Llc | Surgical instrument systems comprising an articulatable end effector and means for adjusting the firing stroke of a firing member |
| US9844375B2 (en) | 2014-12-18 | 2017-12-19 | Ethicon Llc | Drive arrangements for articulatable surgical instruments |
| US10085748B2 (en) | 2014-12-18 | 2018-10-02 | Ethicon Llc | Locking arrangements for detachable shaft assemblies with articulatable surgical end effectors |
| US9987000B2 (en) | 2014-12-18 | 2018-06-05 | Ethicon Llc | Surgical instrument assembly comprising a flexible articulation system |
| US9943309B2 (en) | 2014-12-18 | 2018-04-17 | Ethicon Llc | Surgical instruments with articulatable end effectors and movable firing beam support arrangements |
| MX389118B (en) | 2014-12-18 | 2025-03-20 | Ethicon Llc | SURGICAL INSTRUMENT WITH AN ANVIL THAT CAN BE SELECTIVELY MOVED ON A DISCRETE, NON-MOBILE AXIS RELATIVE TO A STAPLE CARTRIDGE. |
| EP3061442A1 (en) | 2015-02-27 | 2016-08-31 | Evonik Degussa GmbH | Composition comprising rhamnolipid and siloxane |
| US11154301B2 (en) | 2015-02-27 | 2021-10-26 | Cilag Gmbh International | Modular stapling assembly |
| US10617412B2 (en) | 2015-03-06 | 2020-04-14 | Ethicon Llc | System for detecting the mis-insertion of a staple cartridge into a surgical stapler |
| US9993248B2 (en) | 2015-03-06 | 2018-06-12 | Ethicon Endo-Surgery, Llc | Smart sensors with local signal processing |
| JP2020121162A (en) | 2015-03-06 | 2020-08-13 | エシコン エルエルシーEthicon LLC | Time dependent evaluation of sensor data to determine stability element, creep element and viscoelastic element of measurement |
| US10687806B2 (en) | 2015-03-06 | 2020-06-23 | Ethicon Llc | Adaptive tissue compression techniques to adjust closure rates for multiple tissue types |
| US10245033B2 (en) | 2015-03-06 | 2019-04-02 | Ethicon Llc | Surgical instrument comprising a lockable battery housing |
| US9901342B2 (en) | 2015-03-06 | 2018-02-27 | Ethicon Endo-Surgery, Llc | Signal and power communication system positioned on a rotatable shaft |
| US10441279B2 (en) | 2015-03-06 | 2019-10-15 | Ethicon Llc | Multiple level thresholds to modify operation of powered surgical instruments |
| US10548504B2 (en) | 2015-03-06 | 2020-02-04 | Ethicon Llc | Overlaid multi sensor radio frequency (RF) electrode system to measure tissue compression |
| WO2016145561A1 (en) | 2015-03-13 | 2016-09-22 | Evonik Specialty Chemicals (Shanghai) Co., Ltd. | Peg free stable low viscosity oil-in-water emulsion and use thereof |
| US10433844B2 (en) | 2015-03-31 | 2019-10-08 | Ethicon Llc | Surgical instrument with selectively disengageable threaded drive systems |
| US10835249B2 (en) | 2015-08-17 | 2020-11-17 | Ethicon Llc | Implantable layers for a surgical instrument |
| US10238386B2 (en) | 2015-09-23 | 2019-03-26 | Ethicon Llc | Surgical stapler having motor control based on an electrical parameter related to a motor current |
| US10105139B2 (en) | 2015-09-23 | 2018-10-23 | Ethicon Llc | Surgical stapler having downstream current-based motor control |
| US10299878B2 (en) | 2015-09-25 | 2019-05-28 | Ethicon Llc | Implantable adjunct systems for determining adjunct skew |
| US10980539B2 (en) | 2015-09-30 | 2021-04-20 | Ethicon Llc | Implantable adjunct comprising bonded layers |
| US11890015B2 (en) | 2015-09-30 | 2024-02-06 | Cilag Gmbh International | Compressible adjunct with crossing spacer fibers |
| US10478188B2 (en) | 2015-09-30 | 2019-11-19 | Ethicon Llc | Implantable layer comprising a constricted configuration |
| US10433846B2 (en) | 2015-09-30 | 2019-10-08 | Ethicon Llc | Compressible adjunct with crossing spacer fibers |
| US10368865B2 (en) | 2015-12-30 | 2019-08-06 | Ethicon Llc | Mechanisms for compensating for drivetrain failure in powered surgical instruments |
| US10292704B2 (en) | 2015-12-30 | 2019-05-21 | Ethicon Llc | Mechanisms for compensating for battery pack failure in powered surgical instruments |
| US10265068B2 (en) | 2015-12-30 | 2019-04-23 | Ethicon Llc | Surgical instruments with separable motors and motor control circuits |
| US11213293B2 (en) | 2016-02-09 | 2022-01-04 | Cilag Gmbh International | Articulatable surgical instruments with single articulation link arrangements |
| BR112018016098B1 (en) | 2016-02-09 | 2023-02-23 | Ethicon Llc | SURGICAL INSTRUMENT |
| US11224426B2 (en) | 2016-02-12 | 2022-01-18 | Cilag Gmbh International | Mechanisms for compensating for drivetrain failure in powered surgical instruments |
| US10448948B2 (en) | 2016-02-12 | 2019-10-22 | Ethicon Llc | Mechanisms for compensating for drivetrain failure in powered surgical instruments |
| US10357247B2 (en) | 2016-04-15 | 2019-07-23 | Ethicon Llc | Surgical instrument with multiple program responses during a firing motion |
| US10335145B2 (en) | 2016-04-15 | 2019-07-02 | Ethicon Llc | Modular surgical instrument with configurable operating mode |
| US11607239B2 (en) | 2016-04-15 | 2023-03-21 | Cilag Gmbh International | Systems and methods for controlling a surgical stapling and cutting instrument |
| US10426467B2 (en) | 2016-04-15 | 2019-10-01 | Ethicon Llc | Surgical instrument with detection sensors |
| US10828028B2 (en) | 2016-04-15 | 2020-11-10 | Ethicon Llc | Surgical instrument with multiple program responses during a firing motion |
| US11179150B2 (en) | 2016-04-15 | 2021-11-23 | Cilag Gmbh International | Systems and methods for controlling a surgical stapling and cutting instrument |
| US10492783B2 (en) | 2016-04-15 | 2019-12-03 | Ethicon, Llc | Surgical instrument with improved stop/start control during a firing motion |
| US10456137B2 (en) | 2016-04-15 | 2019-10-29 | Ethicon Llc | Staple formation detection mechanisms |
| US11317917B2 (en) | 2016-04-18 | 2022-05-03 | Cilag Gmbh International | Surgical stapling system comprising a lockable firing assembly |
| US10363037B2 (en) | 2016-04-18 | 2019-07-30 | Ethicon Llc | Surgical instrument system comprising a magnetic lockout |
| US20170296173A1 (en) | 2016-04-18 | 2017-10-19 | Ethicon Endo-Surgery, Llc | Method for operating a surgical instrument |
| EP3478655B1 (en) | 2016-06-29 | 2020-09-30 | Evonik Operations GmbH | Method for producing surfactants |
| CN109476949B (en) | 2016-07-19 | 2021-06-08 | 赢创运营有限公司 | Use of polyol esters for the production of porous plastic coatings |
| US10500000B2 (en) | 2016-08-16 | 2019-12-10 | Ethicon Llc | Surgical tool with manual control of end effector jaws |
| CN109563021B (en) | 2016-08-18 | 2021-11-26 | 赢创运营有限公司 | Cross-linked polyglycerol esters |
| JP2020501815A (en) | 2016-12-21 | 2020-01-23 | エシコン エルエルシーEthicon LLC | Surgical stapling system |
| US11090048B2 (en) | 2016-12-21 | 2021-08-17 | Cilag Gmbh International | Method for resetting a fuse of a surgical instrument shaft |
| MX2019007295A (en) | 2016-12-21 | 2019-10-15 | Ethicon Llc | Surgical instrument system comprising an end effector lockout and a firing assembly lockout. |
| US10813638B2 (en) | 2016-12-21 | 2020-10-27 | Ethicon Llc | Surgical end effectors with expandable tissue stop arrangements |
| JP7010956B2 (en) | 2016-12-21 | 2022-01-26 | エシコン エルエルシー | How to staple tissue |
| US20180168615A1 (en) | 2016-12-21 | 2018-06-21 | Ethicon Endo-Surgery, Llc | Method of deforming staples from two different types of staple cartridges with the same surgical stapling instrument |
| US10898186B2 (en) | 2016-12-21 | 2021-01-26 | Ethicon Llc | Staple forming pocket arrangements comprising primary sidewalls and pocket sidewalls |
| US10542982B2 (en) | 2016-12-21 | 2020-01-28 | Ethicon Llc | Shaft assembly comprising first and second articulation lockouts |
| US10485543B2 (en) | 2016-12-21 | 2019-11-26 | Ethicon Llc | Anvil having a knife slot width |
| US10758229B2 (en) | 2016-12-21 | 2020-09-01 | Ethicon Llc | Surgical instrument comprising improved jaw control |
| US10582928B2 (en) | 2016-12-21 | 2020-03-10 | Ethicon Llc | Articulation lock arrangements for locking an end effector in an articulated position in response to actuation of a jaw closure system |
| US10695055B2 (en) | 2016-12-21 | 2020-06-30 | Ethicon Llc | Firing assembly comprising a lockout |
| US10973516B2 (en) | 2016-12-21 | 2021-04-13 | Ethicon Llc | Surgical end effectors and adaptable firing members therefor |
| US10980536B2 (en) | 2016-12-21 | 2021-04-20 | Ethicon Llc | No-cartridge and spent cartridge lockout arrangements for surgical staplers |
| JP7010957B2 (en) | 2016-12-21 | 2022-01-26 | エシコン エルエルシー | Shaft assembly with lockout |
| US10568625B2 (en) | 2016-12-21 | 2020-02-25 | Ethicon Llc | Staple cartridges and arrangements of staples and staple cavities therein |
| US11419606B2 (en) | 2016-12-21 | 2022-08-23 | Cilag Gmbh International | Shaft assembly comprising a clutch configured to adapt the output of a rotary firing member to two different systems |
| JP6983893B2 (en) | 2016-12-21 | 2021-12-17 | エシコン エルエルシーEthicon LLC | Lockout configuration for surgical end effectors and replaceable tool assemblies |
| CN110087565A (en) | 2016-12-21 | 2019-08-02 | 爱惜康有限责任公司 | Surgical stapling system |
| US20180168625A1 (en) | 2016-12-21 | 2018-06-21 | Ethicon Endo-Surgery, Llc | Surgical stapling instruments with smart staple cartridges |
| US11134942B2 (en) | 2016-12-21 | 2021-10-05 | Cilag Gmbh International | Surgical stapling instruments and staple-forming anvils |
| JP7271431B2 (en) | 2017-02-10 | 2023-05-11 | エボニック オペレーションズ ゲーエムベーハー | Oral care composition containing at least one biosurfactant and fluoride |
| US11071554B2 (en) | 2017-06-20 | 2021-07-27 | Cilag Gmbh International | Closed loop feedback control of motor velocity of a surgical stapling and cutting instrument based on magnitude of velocity error measurements |
| US11382638B2 (en) | 2017-06-20 | 2022-07-12 | Cilag Gmbh International | Closed loop feedback control of motor velocity of a surgical stapling and cutting instrument based on measured time over a specified displacement distance |
| US11517325B2 (en) | 2017-06-20 | 2022-12-06 | Cilag Gmbh International | Closed loop feedback control of motor velocity of a surgical stapling and cutting instrument based on measured displacement distance traveled over a specified time interval |
| US10307170B2 (en) | 2017-06-20 | 2019-06-04 | Ethicon Llc | Method for closed loop control of motor velocity of a surgical stapling and cutting instrument |
| US10624633B2 (en) | 2017-06-20 | 2020-04-21 | Ethicon Llc | Systems and methods for controlling motor velocity of a surgical stapling and cutting instrument |
| US10881396B2 (en) | 2017-06-20 | 2021-01-05 | Ethicon Llc | Surgical instrument with variable duration trigger arrangement |
| US10813639B2 (en) | 2017-06-20 | 2020-10-27 | Ethicon Llc | Closed loop feedback control of motor velocity of a surgical stapling and cutting instrument based on system conditions |
| US10881399B2 (en) | 2017-06-20 | 2021-01-05 | Ethicon Llc | Techniques for adaptive control of motor velocity of a surgical stapling and cutting instrument |
| US11090046B2 (en) | 2017-06-20 | 2021-08-17 | Cilag Gmbh International | Systems and methods for controlling displacement member motion of a surgical stapling and cutting instrument |
| US11653914B2 (en) | 2017-06-20 | 2023-05-23 | Cilag Gmbh International | Systems and methods for controlling motor velocity of a surgical stapling and cutting instrument according to articulation angle of end effector |
| US10646220B2 (en) | 2017-06-20 | 2020-05-12 | Ethicon Llc | Systems and methods for controlling displacement member velocity for a surgical instrument |
| US10888321B2 (en) | 2017-06-20 | 2021-01-12 | Ethicon Llc | Systems and methods for controlling velocity of a displacement member of a surgical stapling and cutting instrument |
| USD879808S1 (en) | 2017-06-20 | 2020-03-31 | Ethicon Llc | Display panel with graphical user interface |
| USD879809S1 (en) | 2017-06-20 | 2020-03-31 | Ethicon Llc | Display panel with changeable graphical user interface |
| US10779820B2 (en) | 2017-06-20 | 2020-09-22 | Ethicon Llc | Systems and methods for controlling motor speed according to user input for a surgical instrument |
| USD890784S1 (en) | 2017-06-20 | 2020-07-21 | Ethicon Llc | Display panel with changeable graphical user interface |
| US10980537B2 (en) | 2017-06-20 | 2021-04-20 | Ethicon Llc | Closed loop feedback control of motor velocity of a surgical stapling and cutting instrument based on measured time over a specified number of shaft rotations |
| US11324503B2 (en) | 2017-06-27 | 2022-05-10 | Cilag Gmbh International | Surgical firing member arrangements |
| US11090049B2 (en) | 2017-06-27 | 2021-08-17 | Cilag Gmbh International | Staple forming pocket arrangements |
| US11266405B2 (en) | 2017-06-27 | 2022-03-08 | Cilag Gmbh International | Surgical anvil manufacturing methods |
| US10993716B2 (en) | 2017-06-27 | 2021-05-04 | Ethicon Llc | Surgical anvil arrangements |
| US10856869B2 (en) | 2017-06-27 | 2020-12-08 | Ethicon Llc | Surgical anvil arrangements |
| US10772629B2 (en) | 2017-06-27 | 2020-09-15 | Ethicon Llc | Surgical anvil arrangements |
| US10758232B2 (en) | 2017-06-28 | 2020-09-01 | Ethicon Llc | Surgical instrument with positive jaw opening features |
| EP3420947B1 (en) | 2017-06-28 | 2022-05-25 | Cilag GmbH International | Surgical instrument comprising selectively actuatable rotatable couplers |
| US11246592B2 (en) | 2017-06-28 | 2022-02-15 | Cilag Gmbh International | Surgical instrument comprising an articulation system lockable to a frame |
| US10903685B2 (en) | 2017-06-28 | 2021-01-26 | Ethicon Llc | Surgical shaft assemblies with slip ring assemblies forming capacitive channels |
| USD906355S1 (en) | 2017-06-28 | 2020-12-29 | Ethicon Llc | Display screen or portion thereof with a graphical user interface for a surgical instrument |
| US10765427B2 (en) | 2017-06-28 | 2020-09-08 | Ethicon Llc | Method for articulating a surgical instrument |
| US10716614B2 (en) | 2017-06-28 | 2020-07-21 | Ethicon Llc | Surgical shaft assemblies with slip ring assemblies with increased contact pressure |
| US11259805B2 (en) | 2017-06-28 | 2022-03-01 | Cilag Gmbh International | Surgical instrument comprising firing member supports |
| US11564686B2 (en) | 2017-06-28 | 2023-01-31 | Cilag Gmbh International | Surgical shaft assemblies with flexible interfaces |
| US11484310B2 (en) | 2017-06-28 | 2022-11-01 | Cilag Gmbh International | Surgical instrument comprising a shaft including a closure tube profile |
| US11007022B2 (en) | 2017-06-29 | 2021-05-18 | Ethicon Llc | Closed loop velocity control techniques based on sensed tissue parameters for robotic surgical instrument |
| US10932772B2 (en) | 2017-06-29 | 2021-03-02 | Ethicon Llc | Methods for closed loop velocity control for robotic surgical instrument |
| US10898183B2 (en) | 2017-06-29 | 2021-01-26 | Ethicon Llc | Robotic surgical instrument with closed loop feedback techniques for advancement of closure member during firing |
| US11944300B2 (en) | 2017-08-03 | 2024-04-02 | Cilag Gmbh International | Method for operating a surgical system bailout |
| US11304695B2 (en) | 2017-08-03 | 2022-04-19 | Cilag Gmbh International | Surgical system shaft interconnection |
| US11974742B2 (en) | 2017-08-03 | 2024-05-07 | Cilag Gmbh International | Surgical system comprising an articulation bailout |
| US11471155B2 (en) | 2017-08-03 | 2022-10-18 | Cilag Gmbh International | Surgical system bailout |
| WO2019038125A1 (en) | 2017-08-24 | 2019-02-28 | Evonik Degussa Gmbh | RHAMNOLIPID DERIVATIVES AS EMULSIFIERS AND DISPERSION TOOLS |
| JP7428637B2 (en) | 2017-08-30 | 2024-02-06 | エボニック オペレーションズ ゲーエムベーハー | Use of polyol ethers to produce porous plastic coatings |
| JP7049868B2 (en)* | 2017-09-21 | 2022-04-07 | ライオン株式会社 | Antiperspirant composition |
| WO2019058904A1 (en)* | 2017-09-21 | 2019-03-28 | ライオン株式会社 | Antiperspirant composition |
| US10765429B2 (en) | 2017-09-29 | 2020-09-08 | Ethicon Llc | Systems and methods for providing alerts according to the operational state of a surgical instrument |
| US10743872B2 (en) | 2017-09-29 | 2020-08-18 | Ethicon Llc | System and methods for controlling a display of a surgical instrument |
| USD907648S1 (en) | 2017-09-29 | 2021-01-12 | Ethicon Llc | Display screen or portion thereof with animated graphical user interface |
| USD907647S1 (en) | 2017-09-29 | 2021-01-12 | Ethicon Llc | Display screen or portion thereof with animated graphical user interface |
| US11399829B2 (en) | 2017-09-29 | 2022-08-02 | Cilag Gmbh International | Systems and methods of initiating a power shutdown mode for a surgical instrument |
| USD917500S1 (en) | 2017-09-29 | 2021-04-27 | Ethicon Llc | Display screen or portion thereof with graphical user interface |
| US11134944B2 (en) | 2017-10-30 | 2021-10-05 | Cilag Gmbh International | Surgical stapler knife motion controls |
| US11090075B2 (en) | 2017-10-30 | 2021-08-17 | Cilag Gmbh International | Articulation features for surgical end effector |
| US10842490B2 (en) | 2017-10-31 | 2020-11-24 | Ethicon Llc | Cartridge body design with force reduction based on firing completion |
| US10779903B2 (en) | 2017-10-31 | 2020-09-22 | Ethicon Llc | Positive shaft rotation lock activated by jaw closure |
| EP3710503B1 (en) | 2017-11-15 | 2022-01-05 | Evonik Operations GmbH | Functionalized polymers |
| US10743875B2 (en) | 2017-12-15 | 2020-08-18 | Ethicon Llc | Surgical end effectors with jaw stiffener arrangements configured to permit monitoring of firing member |
| US11071543B2 (en) | 2017-12-15 | 2021-07-27 | Cilag Gmbh International | Surgical end effectors with clamping assemblies configured to increase jaw aperture ranges |
| US11033267B2 (en) | 2017-12-15 | 2021-06-15 | Ethicon Llc | Systems and methods of controlling a clamping member firing rate of a surgical instrument |
| US10828033B2 (en) | 2017-12-15 | 2020-11-10 | Ethicon Llc | Handheld electromechanical surgical instruments with improved motor control arrangements for positioning components of an adapter coupled thereto |
| US11006955B2 (en) | 2017-12-15 | 2021-05-18 | Ethicon Llc | End effectors with positive jaw opening features for use with adapters for electromechanical surgical instruments |
| US11197670B2 (en) | 2017-12-15 | 2021-12-14 | Cilag Gmbh International | Surgical end effectors with pivotal jaws configured to touch at their respective distal ends when fully closed |
| US10779825B2 (en) | 2017-12-15 | 2020-09-22 | Ethicon Llc | Adapters with end effector position sensing and control arrangements for use in connection with electromechanical surgical instruments |
| US10743874B2 (en)* | 2017-12-15 | 2020-08-18 | Ethicon Llc | Sealed adapters for use with electromechanical surgical instruments |
| US10779826B2 (en) | 2017-12-15 | 2020-09-22 | Ethicon Llc | Methods of operating surgical end effectors |
| US10966718B2 (en) | 2017-12-15 | 2021-04-06 | Ethicon Llc | Dynamic clamping assemblies with improved wear characteristics for use in connection with electromechanical surgical instruments |
| US10869666B2 (en) | 2017-12-15 | 2020-12-22 | Ethicon Llc | Adapters with control systems for controlling multiple motors of an electromechanical surgical instrument |
| US10687813B2 (en) | 2017-12-15 | 2020-06-23 | Ethicon Llc | Adapters with firing stroke sensing arrangements for use in connection with electromechanical surgical instruments |
| US10835330B2 (en) | 2017-12-19 | 2020-11-17 | Ethicon Llc | Method for determining the position of a rotatable jaw of a surgical instrument attachment assembly |
| US10729509B2 (en) | 2017-12-19 | 2020-08-04 | Ethicon Llc | Surgical instrument comprising closure and firing locking mechanism |
| US10716565B2 (en) | 2017-12-19 | 2020-07-21 | Ethicon Llc | Surgical instruments with dual articulation drivers |
| US11045270B2 (en) | 2017-12-19 | 2021-06-29 | Cilag Gmbh International | Robotic attachment comprising exterior drive actuator |
| US11020112B2 (en) | 2017-12-19 | 2021-06-01 | Ethicon Llc | Surgical tools configured for interchangeable use with different controller interfaces |
| USD910847S1 (en) | 2017-12-19 | 2021-02-16 | Ethicon Llc | Surgical instrument assembly |
| US11076853B2 (en) | 2017-12-21 | 2021-08-03 | Cilag Gmbh International | Systems and methods of displaying a knife position during transection for a surgical instrument |
| US11129680B2 (en) | 2017-12-21 | 2021-09-28 | Cilag Gmbh International | Surgical instrument comprising a projector |
| US12336705B2 (en) | 2017-12-21 | 2025-06-24 | Cilag Gmbh International | Continuous use self-propelled stapling instrument |
| US11311290B2 (en) | 2017-12-21 | 2022-04-26 | Cilag Gmbh International | Surgical instrument comprising an end effector dampener |
| US11179151B2 (en) | 2017-12-21 | 2021-11-23 | Cilag Gmbh International | Surgical instrument comprising a display |
| US11291440B2 (en) | 2018-08-20 | 2022-04-05 | Cilag Gmbh International | Method for operating a powered articulatable surgical instrument |
| US20200054321A1 (en) | 2018-08-20 | 2020-02-20 | Ethicon Llc | Surgical instruments with progressive jaw closure arrangements |
| US11253256B2 (en) | 2018-08-20 | 2022-02-22 | Cilag Gmbh International | Articulatable motor powered surgical instruments with dedicated articulation motor arrangements |
| US11083458B2 (en) | 2018-08-20 | 2021-08-10 | Cilag Gmbh International | Powered surgical instruments with clutching arrangements to convert linear drive motions to rotary drive motions |
| USD914878S1 (en) | 2018-08-20 | 2021-03-30 | Ethicon Llc | Surgical instrument anvil |
| US11039834B2 (en) | 2018-08-20 | 2021-06-22 | Cilag Gmbh International | Surgical stapler anvils with staple directing protrusions and tissue stability features |
| US11324501B2 (en) | 2018-08-20 | 2022-05-10 | Cilag Gmbh International | Surgical stapling devices with improved closure members |
| US11207065B2 (en) | 2018-08-20 | 2021-12-28 | Cilag Gmbh International | Method for fabricating surgical stapler anvils |
| US10912559B2 (en) | 2018-08-20 | 2021-02-09 | Ethicon Llc | Reinforced deformable anvil tip for surgical stapler anvil |
| US10856870B2 (en) | 2018-08-20 | 2020-12-08 | Ethicon Llc | Switching arrangements for motor powered articulatable surgical instruments |
| US11045192B2 (en) | 2018-08-20 | 2021-06-29 | Cilag Gmbh International | Fabricating techniques for surgical stapler anvils |
| US10779821B2 (en) | 2018-08-20 | 2020-09-22 | Ethicon Llc | Surgical stapler anvils with tissue stop features configured to avoid tissue pinch |
| US10842492B2 (en) | 2018-08-20 | 2020-11-24 | Ethicon Llc | Powered articulatable surgical instruments with clutching and locking arrangements for linking an articulation drive system to a firing drive system |
| US11147551B2 (en) | 2019-03-25 | 2021-10-19 | Cilag Gmbh International | Firing drive arrangements for surgical systems |
| US11172929B2 (en) | 2019-03-25 | 2021-11-16 | Cilag Gmbh International | Articulation drive arrangements for surgical systems |
| US11696761B2 (en) | 2019-03-25 | 2023-07-11 | Cilag Gmbh International | Firing drive arrangements for surgical systems |
| US11147553B2 (en) | 2019-03-25 | 2021-10-19 | Cilag Gmbh International | Firing drive arrangements for surgical systems |
| US11432816B2 (en) | 2019-04-30 | 2022-09-06 | Cilag Gmbh International | Articulation pin for a surgical instrument |
| US11471157B2 (en) | 2019-04-30 | 2022-10-18 | Cilag Gmbh International | Articulation control mapping for a surgical instrument |
| US11426251B2 (en) | 2019-04-30 | 2022-08-30 | Cilag Gmbh International | Articulation directional lights on a surgical instrument |
| US11253254B2 (en) | 2019-04-30 | 2022-02-22 | Cilag Gmbh International | Shaft rotation actuator on a surgical instrument |
| US11903581B2 (en) | 2019-04-30 | 2024-02-20 | Cilag Gmbh International | Methods for stapling tissue using a surgical instrument |
| US11452528B2 (en) | 2019-04-30 | 2022-09-27 | Cilag Gmbh International | Articulation actuators for a surgical instrument |
| US11648009B2 (en) | 2019-04-30 | 2023-05-16 | Cilag Gmbh International | Rotatable jaw tip for a surgical instrument |
| US11478241B2 (en) | 2019-06-28 | 2022-10-25 | Cilag Gmbh International | Staple cartridge including projections |
| US11464601B2 (en) | 2019-06-28 | 2022-10-11 | Cilag Gmbh International | Surgical instrument comprising an RFID system for tracking a movable component |
| US11771419B2 (en) | 2019-06-28 | 2023-10-03 | Cilag Gmbh International | Packaging for a replaceable component of a surgical stapling system |
| US11497492B2 (en) | 2019-06-28 | 2022-11-15 | Cilag Gmbh International | Surgical instrument including an articulation lock |
| US11553971B2 (en) | 2019-06-28 | 2023-01-17 | Cilag Gmbh International | Surgical RFID assemblies for display and communication |
| US11298132B2 (en) | 2019-06-28 | 2022-04-12 | Cilag GmbH Inlernational | Staple cartridge including a honeycomb extension |
| US11399837B2 (en) | 2019-06-28 | 2022-08-02 | Cilag Gmbh International | Mechanisms for motor control adjustments of a motorized surgical instrument |
| US11523822B2 (en) | 2019-06-28 | 2022-12-13 | Cilag Gmbh International | Battery pack including a circuit interrupter |
| US11219455B2 (en) | 2019-06-28 | 2022-01-11 | Cilag Gmbh International | Surgical instrument including a lockout key |
| US12004740B2 (en) | 2019-06-28 | 2024-06-11 | Cilag Gmbh International | Surgical stapling system having an information decryption protocol |
| US11376098B2 (en) | 2019-06-28 | 2022-07-05 | Cilag Gmbh International | Surgical instrument system comprising an RFID system |
| US11627959B2 (en) | 2019-06-28 | 2023-04-18 | Cilag Gmbh International | Surgical instruments including manual and powered system lockouts |
| US11241235B2 (en) | 2019-06-28 | 2022-02-08 | Cilag Gmbh International | Method of using multiple RFID chips with a surgical assembly |
| US11426167B2 (en) | 2019-06-28 | 2022-08-30 | Cilag Gmbh International | Mechanisms for proper anvil attachment surgical stapling head assembly |
| US11224497B2 (en) | 2019-06-28 | 2022-01-18 | Cilag Gmbh International | Surgical systems with multiple RFID tags |
| US11291451B2 (en) | 2019-06-28 | 2022-04-05 | Cilag Gmbh International | Surgical instrument with battery compatibility verification functionality |
| US11051807B2 (en) | 2019-06-28 | 2021-07-06 | Cilag Gmbh International | Packaging assembly including a particulate trap |
| US11298127B2 (en) | 2019-06-28 | 2022-04-12 | Cilag GmbH Interational | Surgical stapling system having a lockout mechanism for an incompatible cartridge |
| US11660163B2 (en) | 2019-06-28 | 2023-05-30 | Cilag Gmbh International | Surgical system with RFID tags for updating motor assembly parameters |
| US11246678B2 (en) | 2019-06-28 | 2022-02-15 | Cilag Gmbh International | Surgical stapling system having a frangible RFID tag |
| US11638587B2 (en) | 2019-06-28 | 2023-05-02 | Cilag Gmbh International | RFID identification systems for surgical instruments |
| US11259803B2 (en) | 2019-06-28 | 2022-03-01 | Cilag Gmbh International | Surgical stapling system having an information encryption protocol |
| US11684434B2 (en) | 2019-06-28 | 2023-06-27 | Cilag Gmbh International | Surgical RFID assemblies for instrument operational setting control |
| US11607219B2 (en) | 2019-12-19 | 2023-03-21 | Cilag Gmbh International | Staple cartridge comprising a detachable tissue cutting knife |
| US11701111B2 (en) | 2019-12-19 | 2023-07-18 | Cilag Gmbh International | Method for operating a surgical stapling instrument |
| US11504122B2 (en) | 2019-12-19 | 2022-11-22 | Cilag Gmbh International | Surgical instrument comprising a nested firing member |
| US11464512B2 (en) | 2019-12-19 | 2022-10-11 | Cilag Gmbh International | Staple cartridge comprising a curved deck surface |
| US11529139B2 (en) | 2019-12-19 | 2022-12-20 | Cilag Gmbh International | Motor driven surgical instrument |
| US11304696B2 (en) | 2019-12-19 | 2022-04-19 | Cilag Gmbh International | Surgical instrument comprising a powered articulation system |
| US12035913B2 (en) | 2019-12-19 | 2024-07-16 | Cilag Gmbh International | Staple cartridge comprising a deployable knife |
| US11234698B2 (en) | 2019-12-19 | 2022-02-01 | Cilag Gmbh International | Stapling system comprising a clamp lockout and a firing lockout |
| US11529137B2 (en) | 2019-12-19 | 2022-12-20 | Cilag Gmbh International | Staple cartridge comprising driver retention members |
| US11559304B2 (en) | 2019-12-19 | 2023-01-24 | Cilag Gmbh International | Surgical instrument comprising a rapid closure mechanism |
| US11844520B2 (en) | 2019-12-19 | 2023-12-19 | Cilag Gmbh International | Staple cartridge comprising driver retention members |
| US11446029B2 (en) | 2019-12-19 | 2022-09-20 | Cilag Gmbh International | Staple cartridge comprising projections extending from a curved deck surface |
| US11576672B2 (en) | 2019-12-19 | 2023-02-14 | Cilag Gmbh International | Surgical instrument comprising a closure system including a closure member and an opening member driven by a drive screw |
| US11911032B2 (en) | 2019-12-19 | 2024-02-27 | Cilag Gmbh International | Staple cartridge comprising a seating cam |
| US11931033B2 (en) | 2019-12-19 | 2024-03-19 | Cilag Gmbh International | Staple cartridge comprising a latch lockout |
| US11291447B2 (en) | 2019-12-19 | 2022-04-05 | Cilag Gmbh International | Stapling instrument comprising independent jaw closing and staple firing systems |
| US11905492B2 (en)* | 2020-02-21 | 2024-02-20 | Illinois Tool Works Inc. | Odor removing composition comprising zinc ricinoleate and fluorosurfactant and methods of making thereof |
| FR3108034B1 (en) | 2020-03-11 | 2022-09-09 | Hyteck | Deodorant composition |
| USD975851S1 (en) | 2020-06-02 | 2023-01-17 | Cilag Gmbh International | Staple cartridge |
| USD974560S1 (en) | 2020-06-02 | 2023-01-03 | Cilag Gmbh International | Staple cartridge |
| USD976401S1 (en) | 2020-06-02 | 2023-01-24 | Cilag Gmbh International | Staple cartridge |
| USD975278S1 (en) | 2020-06-02 | 2023-01-10 | Cilag Gmbh International | Staple cartridge |
| USD967421S1 (en) | 2020-06-02 | 2022-10-18 | Cilag Gmbh International | Staple cartridge |
| USD966512S1 (en) | 2020-06-02 | 2022-10-11 | Cilag Gmbh International | Staple cartridge |
| USD975850S1 (en) | 2020-06-02 | 2023-01-17 | Cilag Gmbh International | Staple cartridge |
| US11871925B2 (en) | 2020-07-28 | 2024-01-16 | Cilag Gmbh International | Surgical instruments with dual spherical articulation joint arrangements |
| US11452526B2 (en) | 2020-10-29 | 2022-09-27 | Cilag Gmbh International | Surgical instrument comprising a staged voltage regulation start-up system |
| US11517390B2 (en) | 2020-10-29 | 2022-12-06 | Cilag Gmbh International | Surgical instrument comprising a limited travel switch |
| US11717289B2 (en) | 2020-10-29 | 2023-08-08 | Cilag Gmbh International | Surgical instrument comprising an indicator which indicates that an articulation drive is actuatable |
| US11896217B2 (en) | 2020-10-29 | 2024-02-13 | Cilag Gmbh International | Surgical instrument comprising an articulation lock |
| US11534259B2 (en) | 2020-10-29 | 2022-12-27 | Cilag Gmbh International | Surgical instrument comprising an articulation indicator |
| US11844518B2 (en) | 2020-10-29 | 2023-12-19 | Cilag Gmbh International | Method for operating a surgical instrument |
| US11617577B2 (en) | 2020-10-29 | 2023-04-04 | Cilag Gmbh International | Surgical instrument comprising a sensor configured to sense whether an articulation drive of the surgical instrument is actuatable |
| USD1013170S1 (en) | 2020-10-29 | 2024-01-30 | Cilag Gmbh International | Surgical instrument assembly |
| US12053175B2 (en) | 2020-10-29 | 2024-08-06 | Cilag Gmbh International | Surgical instrument comprising a stowed closure actuator stop |
| USD980425S1 (en) | 2020-10-29 | 2023-03-07 | Cilag Gmbh International | Surgical instrument assembly |
| US11779330B2 (en) | 2020-10-29 | 2023-10-10 | Cilag Gmbh International | Surgical instrument comprising a jaw alignment system |
| US11931025B2 (en) | 2020-10-29 | 2024-03-19 | Cilag Gmbh International | Surgical instrument comprising a releasable closure drive lock |
| US11944296B2 (en) | 2020-12-02 | 2024-04-02 | Cilag Gmbh International | Powered surgical instruments with external connectors |
| US11653915B2 (en) | 2020-12-02 | 2023-05-23 | Cilag Gmbh International | Surgical instruments with sled location detection and adjustment features |
| US11678882B2 (en) | 2020-12-02 | 2023-06-20 | Cilag Gmbh International | Surgical instruments with interactive features to remedy incidental sled movements |
| US11849943B2 (en) | 2020-12-02 | 2023-12-26 | Cilag Gmbh International | Surgical instrument with cartridge release mechanisms |
| US11627960B2 (en) | 2020-12-02 | 2023-04-18 | Cilag Gmbh International | Powered surgical instruments with smart reload with separately attachable exteriorly mounted wiring connections |
| US11653920B2 (en) | 2020-12-02 | 2023-05-23 | Cilag Gmbh International | Powered surgical instruments with communication interfaces through sterile barrier |
| US11890010B2 (en) | 2020-12-02 | 2024-02-06 | Cllag GmbH International | Dual-sided reinforced reload for surgical instruments |
| US11737751B2 (en) | 2020-12-02 | 2023-08-29 | Cilag Gmbh International | Devices and methods of managing energy dissipated within sterile barriers of surgical instrument housings |
| US11744581B2 (en) | 2020-12-02 | 2023-09-05 | Cilag Gmbh International | Powered surgical instruments with multi-phase tissue treatment |
| US12324580B2 (en) | 2021-02-26 | 2025-06-10 | Cilag Gmbh International | Method of powering and communicating with a staple cartridge |
| US12108951B2 (en) | 2021-02-26 | 2024-10-08 | Cilag Gmbh International | Staple cartridge comprising a sensing array and a temperature control system |
| US11793514B2 (en) | 2021-02-26 | 2023-10-24 | Cilag Gmbh International | Staple cartridge comprising sensor array which may be embedded in cartridge body |
| US11980362B2 (en) | 2021-02-26 | 2024-05-14 | Cilag Gmbh International | Surgical instrument system comprising a power transfer coil |
| US11723657B2 (en) | 2021-02-26 | 2023-08-15 | Cilag Gmbh International | Adjustable communication based on available bandwidth and power capacity |
| US11812964B2 (en) | 2021-02-26 | 2023-11-14 | Cilag Gmbh International | Staple cartridge comprising a power management circuit |
| US11744583B2 (en) | 2021-02-26 | 2023-09-05 | Cilag Gmbh International | Distal communication array to tune frequency of RF systems |
| US11751869B2 (en) | 2021-02-26 | 2023-09-12 | Cilag Gmbh International | Monitoring of multiple sensors over time to detect moving characteristics of tissue |
| US11925349B2 (en) | 2021-02-26 | 2024-03-12 | Cilag Gmbh International | Adjustment to transfer parameters to improve available power |
| US11730473B2 (en) | 2021-02-26 | 2023-08-22 | Cilag Gmbh International | Monitoring of manufacturing life-cycle |
| US11950777B2 (en) | 2021-02-26 | 2024-04-09 | Cilag Gmbh International | Staple cartridge comprising an information access control system |
| US11696757B2 (en) | 2021-02-26 | 2023-07-11 | Cilag Gmbh International | Monitoring of internal systems to detect and track cartridge motion status |
| US11701113B2 (en) | 2021-02-26 | 2023-07-18 | Cilag Gmbh International | Stapling instrument comprising a separate power antenna and a data transfer antenna |
| US11950779B2 (en) | 2021-02-26 | 2024-04-09 | Cilag Gmbh International | Method of powering and communicating with a staple cartridge |
| US11749877B2 (en) | 2021-02-26 | 2023-09-05 | Cilag Gmbh International | Stapling instrument comprising a signal antenna |
| US11723658B2 (en) | 2021-03-22 | 2023-08-15 | Cilag Gmbh International | Staple cartridge comprising a firing lockout |
| US11806011B2 (en) | 2021-03-22 | 2023-11-07 | Cilag Gmbh International | Stapling instrument comprising tissue compression systems |
| US11717291B2 (en) | 2021-03-22 | 2023-08-08 | Cilag Gmbh International | Staple cartridge comprising staples configured to apply different tissue compression |
| US11826042B2 (en) | 2021-03-22 | 2023-11-28 | Cilag Gmbh International | Surgical instrument comprising a firing drive including a selectable leverage mechanism |
| US11759202B2 (en) | 2021-03-22 | 2023-09-19 | Cilag Gmbh International | Staple cartridge comprising an implantable layer |
| US11826012B2 (en) | 2021-03-22 | 2023-11-28 | Cilag Gmbh International | Stapling instrument comprising a pulsed motor-driven firing rack |
| US11737749B2 (en) | 2021-03-22 | 2023-08-29 | Cilag Gmbh International | Surgical stapling instrument comprising a retraction system |
| US11857183B2 (en) | 2021-03-24 | 2024-01-02 | Cilag Gmbh International | Stapling assembly components having metal substrates and plastic bodies |
| US11744603B2 (en) | 2021-03-24 | 2023-09-05 | Cilag Gmbh International | Multi-axis pivot joints for surgical instruments and methods for manufacturing same |
| US11786239B2 (en) | 2021-03-24 | 2023-10-17 | Cilag Gmbh International | Surgical instrument articulation joint arrangements comprising multiple moving linkage features |
| US11849945B2 (en) | 2021-03-24 | 2023-12-26 | Cilag Gmbh International | Rotary-driven surgical stapling assembly comprising eccentrically driven firing member |
| US11896219B2 (en) | 2021-03-24 | 2024-02-13 | Cilag Gmbh International | Mating features between drivers and underside of a cartridge deck |
| US11849944B2 (en) | 2021-03-24 | 2023-12-26 | Cilag Gmbh International | Drivers for fastener cartridge assemblies having rotary drive screws |
| US11832816B2 (en) | 2021-03-24 | 2023-12-05 | Cilag Gmbh International | Surgical stapling assembly comprising nonplanar staples and planar staples |
| US11903582B2 (en) | 2021-03-24 | 2024-02-20 | Cilag Gmbh International | Leveraging surfaces for cartridge installation |
| US11786243B2 (en) | 2021-03-24 | 2023-10-17 | Cilag Gmbh International | Firing members having flexible portions for adapting to a load during a surgical firing stroke |
| US11896218B2 (en) | 2021-03-24 | 2024-02-13 | Cilag Gmbh International | Method of using a powered stapling device |
| US12102323B2 (en) | 2021-03-24 | 2024-10-01 | Cilag Gmbh International | Rotary-driven surgical stapling assembly comprising a floatable component |
| US11944336B2 (en) | 2021-03-24 | 2024-04-02 | Cilag Gmbh International | Joint arrangements for multi-planar alignment and support of operational drive shafts in articulatable surgical instruments |
| US11793516B2 (en) | 2021-03-24 | 2023-10-24 | Cilag Gmbh International | Surgical staple cartridge comprising longitudinal support beam |
| US11826047B2 (en) | 2021-05-28 | 2023-11-28 | Cilag Gmbh International | Stapling instrument comprising jaw mounts |
| US11957337B2 (en) | 2021-10-18 | 2024-04-16 | Cilag Gmbh International | Surgical stapling assembly with offset ramped drive surfaces |
| US11980363B2 (en) | 2021-10-18 | 2024-05-14 | Cilag Gmbh International | Row-to-row staple array variations |
| US11877745B2 (en) | 2021-10-18 | 2024-01-23 | Cilag Gmbh International | Surgical stapling assembly having longitudinally-repeating staple leg clusters |
| US12239317B2 (en) | 2021-10-18 | 2025-03-04 | Cilag Gmbh International | Anvil comprising an arrangement of forming pockets proximal to tissue stop |
| US11937816B2 (en) | 2021-10-28 | 2024-03-26 | Cilag Gmbh International | Electrical lead arrangements for surgical instruments |
| US12089841B2 (en) | 2021-10-28 | 2024-09-17 | Cilag CmbH International | Staple cartridge identification systems |
| US12432790B2 (en) | 2021-10-28 | 2025-09-30 | Cilag Gmbh International | Method and device for transmitting UART communications over a security short range wireless communication |
Family Cites Families (12)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US2854382A (en) | 1957-06-25 | 1958-09-30 | Procter & Gamble | Zirconyl hydroxy chloride antiperspirant compositions |
| US3792068A (en) | 1971-04-02 | 1974-02-12 | Procter & Gamble | Dry powder aerosol antiperspirant composition incorporating dry powder antiperspirant active complex and process for its preparation |
| DE3041073C2 (en)* | 1980-10-31 | 1986-01-02 | Dynamit Nobel Ag, 5210 Troisdorf | Wool wax substitutes |
| IT1224421B (en) | 1987-12-29 | 1990-10-04 | Lamberti Flli Spa | MODIFIED GALATTOMANNANS AND REALIVE PREPARATION PROCEDURE |
| DE19922229A1 (en)* | 1999-05-14 | 2000-11-16 | Cognis Deutschland Gmbh | Cosmetic and / or pharmaceutical preparations |
| GB0304508D0 (en) | 2003-02-27 | 2003-04-02 | Unilever Plc | Antiperspirant actives and compositions |
| DE10361202A1 (en) | 2003-12-24 | 2005-07-28 | Beiersdorf Ag | Cosmetic emulsion |
| EP2243517A1 (en) | 2009-04-20 | 2010-10-27 | Dr. Straetmans GmbH | Cosmetic or dermatological preparation |
| BR112013019103B1 (en)* | 2011-02-03 | 2018-02-14 | Akzo Nobel Chemicals International B.V. | FORMULATION FOR PERSONAL CARE, METHOD FOR MANUFACTURING FORMULATION FOR PERSONAL CARE AND USE OF A MODIFIED STARCH WITH A SILICONATE AND A COSMETICALLY ACCEPTABLE VEHICLE |
| DE102011089340A1 (en)* | 2011-12-21 | 2013-06-27 | Henkel Ag & Co. Kgaa | PEG-free antiperspirant oil-in-water emulsions with improved feel |
| DE102012215707A1 (en)* | 2012-09-05 | 2014-03-06 | Evonik Industries Ag | Polyglycerol ester with particular oligomer distribution of polyglycerol |
| DE102014203868A1 (en)* | 2014-03-04 | 2015-09-10 | Evonik Degussa Gmbh | Polyglycerol ester with particular oligomer distribution of polyglycerol |
- 2016
- 2016-01-15EPEP16151443.5Apatent/EP3192491B1/enactiveActive
- 2016-12-15USUS15/380,137patent/US20170202770A1/ennot_activeAbandoned
- 2017
- 2017-01-12BRBR102017000660-3Apatent/BR102017000660B1/enactiveIP Right Grant
- 2017-01-16CNCN201710032429.2Apatent/CN107028795B/enactiveActive
Non-Patent Citations (1)
| Title |
|---|
| None* |
Also Published As
| Publication number | Publication date |
|---|---|
| CN107028795A (en) | 2017-08-11 |
| CN107028795B (en) | 2021-09-28 |
| US20170202770A1 (en) | 2017-07-20 |
| BR102017000660B1 (en) | 2021-11-09 |
| EP3192491A1 (en) | 2017-07-19 |
| BR102017000660A2 (en) | 2017-07-25 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| EP3192491B1 (en) | Composition comprising polyglycerol esters and hydroxy-alkyl modified guar | |
| EP3113846B1 (en) | Polyglycerol esters featuring a particular oligomer distribution in the polyglycerol | |
| DE69403082T2 (en) | AQUEOUS ALCOHOLIC COSMETIC MICROEMULSIONS | |
| EP2705832B1 (en) | Polyglycerol esters with special oligomer distribution of polyglycerol | |
| EP2782645B1 (en) | Use of isosorbide caprylates/caprates in deodorants and antiperspirants | |
| EP3500550A1 (en) | Cross-linked polyglycerol esters | |
| EP2275078B1 (en) | Cosmetic and dermatological compositions comprising (2-hydroxyethyl)urea | |
| EP2739137B1 (en) | Use of isosorbide monoesters as thickeners | |
| EP1753513B1 (en) | Cosmetic microemulsion-based formulation containing mandelic acid | |
| WO2014060150A1 (en) | Cosmetic or dermatological preparation for prophylaxis and/or treatment of atopic dermatitis | |
| WO2015024567A1 (en) | Antiperspirants with reduced itching effect | |
| EP1092415A2 (en) | Cosmetic and dermatologic photoprotective O/W-macroemulsions or O/W-microemulsions containing dihydroxyacetone | |
| EP2033617A2 (en) | Cosmetic care cream comprising a protein | |
| DE602005001478T2 (en) | Oil-in-water emulsified composition | |
| EP1071466A1 (en) | Glycolipid creams | |
| EP2603194B1 (en) | Stabilized w/o emulsions | |
| WO2013182667A2 (en) | Thickened antiperspirant roll-ons having an improved residue behavior | |
| EP2086497B1 (en) | Cosmetic or dermatological formulation with mandelic acid and auxiliaries | |
| WO1996032923A2 (en) | Anti-transpirants | |
| EP2654698A2 (en) | Low-viscosity w/o emulsions having a content of one or more glucosyl glycerides and one or more electrolytes | |
| DE102016210037A1 (en) | "Highly effective antiperspirants with improved skin tolerance" | |
| DE202010011395U1 (en) | Stabilized W / O emulsions | |
| DE4421208A1 (en) | Cosmetic and / or pharmaceutical O / W emulsions | |
| DE102011077042A1 (en) | Hair cosmetic preparations comprising polyglyceryl-10-stearate | |
| JP2022110911A (en) | external composition |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PUAI | Public reference made under article 153(3) epc to a published international application that has entered the european phase | Free format text:ORIGINAL CODE: 0009012 | |
| STAA | Information on the status of an ep patent application or granted ep patent | Free format text:STATUS: THE APPLICATION HAS BEEN PUBLISHED | |
| AK | Designated contracting states | Kind code of ref document:A1 Designated state(s):AL AT BE BG CH CY CZ DE DK EE ES FI FR GB GR HR HU IE IS IT LI LT LU LV MC MK MT NL NO PL PT RO RS SE SI SK SM TR | |
| AX | Request for extension of the european patent | Extension state:BA ME | |
| STAA | Information on the status of an ep patent application or granted ep patent | Free format text:STATUS: REQUEST FOR EXAMINATION WAS MADE | |
| 17P | Request for examination filed | Effective date:20180111 | |
| RBV | Designated contracting states (corrected) | Designated state(s):AL AT BE BG CH CY CZ DE DK EE ES FI FR GB GR HR HU IE IS IT LI LT LU LV MC MK MT NL NO PL PT RO RS SE SI SK SM TR | |
| GRAP | Despatch of communication of intention to grant a patent | Free format text:ORIGINAL CODE: EPIDOSNIGR1 | |
| STAA | Information on the status of an ep patent application or granted ep patent | Free format text:STATUS: GRANT OF PATENT IS INTENDED | |
| INTG | Intention to grant announced | Effective date:20190613 | |
| GRAJ | Information related to disapproval of communication of intention to grant by the applicant or resumption of examination proceedings by the epo deleted | Free format text:ORIGINAL CODE: EPIDOSDIGR1 | |
| STAA | Information on the status of an ep patent application or granted ep patent | Free format text:STATUS: REQUEST FOR EXAMINATION WAS MADE | |
| GRAP | Despatch of communication of intention to grant a patent | Free format text:ORIGINAL CODE: EPIDOSNIGR1 | |
| STAA | Information on the status of an ep patent application or granted ep patent | Free format text:STATUS: GRANT OF PATENT IS INTENDED | |
| INTC | Intention to grant announced (deleted) | ||
| INTG | Intention to grant announced | Effective date:20190917 | |
| GRAS | Grant fee paid | Free format text:ORIGINAL CODE: EPIDOSNIGR3 | |
| GRAA | (expected) grant | Free format text:ORIGINAL CODE: 0009210 | |
| STAA | Information on the status of an ep patent application or granted ep patent | Free format text:STATUS: THE PATENT HAS BEEN GRANTED | |
| RAP1 | Party data changed (applicant data changed or rights of an application transferred) | Owner name:EVONIK OPERATIONS GMBH | |
| AK | Designated contracting states | Kind code of ref document:B1 Designated state(s):AL AT BE BG CH CY CZ DE DK EE ES FI FR GB GR HR HU IE IS IT LI LT LU LV MC MK MT NL NO PL PT RO RS SE SI SK SM TR | |
| REG | Reference to a national code | Ref country code:GB Ref legal event code:FG4D Free format text:NOT ENGLISH | |
| REG | Reference to a national code | Ref country code:CH Ref legal event code:EP | |
| REG | Reference to a national code | Ref country code:DE Ref legal event code:R096 Ref document number:502016008297 Country of ref document:DE | |
| REG | Reference to a national code | Ref country code:IE Ref legal event code:FG4D Free format text:LANGUAGE OF EP DOCUMENT: GERMAN | |
| REG | Reference to a national code | Ref country code:AT Ref legal event code:REF Ref document number:1221796 Country of ref document:AT Kind code of ref document:T Effective date:20200215 | |
| REG | Reference to a national code | Ref country code:NL Ref legal event code:MP Effective date:20200108 | |
| REG | Reference to a national code | Ref country code:LT Ref legal event code:MG4D | |
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] | Ref country code:LT Free format text:LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date:20200108 Ref country code:NO Free format text:LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date:20200408 Ref country code:NL Free format text:LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date:20200108 Ref country code:FI Free format text:LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date:20200108 Ref country code:RS Free format text:LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date:20200108 Ref country code:PT Free format text:LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date:20200531 | |
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] | Ref country code:IS Free format text:LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date:20200508 Ref country code:GR Free format text:LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date:20200409 Ref country code:HR Free format text:LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date:20200108 Ref country code:LV Free format text:LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date:20200108 Ref country code:SE Free format text:LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date:20200108 Ref country code:BG Free format text:LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date:20200408 | |
| REG | Reference to a national code | Ref country code:CH Ref legal event code:PL | |
| REG | Reference to a national code | Ref country code:DE Ref legal event code:R097 Ref document number:502016008297 Country of ref document:DE | |
| REG | Reference to a national code | Ref country code:BE Ref legal event code:MM Effective date:20200131 | |
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] | Ref country code:DK Free format text:LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date:20200108 Ref country code:EE Free format text:LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date:20200108 Ref country code:SM Free format text:LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date:20200108 Ref country code:ES Free format text:LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date:20200108 Ref country code:RO Free format text:LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date:20200108 Ref country code:CZ Free format text:LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date:20200108 Ref country code:LU Free format text:LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date:20200115 Ref country code:MC Free format text:LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date:20200108 Ref country code:SK Free format text:LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date:20200108 | |
| PLBE | No opposition filed within time limit | Free format text:ORIGINAL CODE: 0009261 | |
| STAA | Information on the status of an ep patent application or granted ep patent | Free format text:STATUS: NO OPPOSITION FILED WITHIN TIME LIMIT | |
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] | Ref country code:BE Free format text:LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date:20200131 Ref country code:LI Free format text:LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date:20200131 Ref country code:CH Free format text:LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date:20200131 | |
| 26N | No opposition filed | Effective date:20201009 | |
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] | Ref country code:IT Free format text:LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date:20200108 Ref country code:IE Free format text:LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date:20200115 | |
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] | Ref country code:SI Free format text:LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date:20200108 Ref country code:PL Free format text:LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date:20200108 | |
| GBPC | Gb: european patent ceased through non-payment of renewal fee | Effective date:20200408 | |
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] | Ref country code:GB Free format text:LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date:20200408 | |
| REG | Reference to a national code | Ref country code:AT Ref legal event code:MM01 Ref document number:1221796 Country of ref document:AT Kind code of ref document:T Effective date:20210115 | |
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] | Ref country code:AT Free format text:LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date:20210115 | |
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] | Ref country code:TR Free format text:LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date:20200108 Ref country code:MT Free format text:LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date:20200108 Ref country code:CY Free format text:LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date:20200108 | |
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] | Ref country code:MK Free format text:LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date:20200108 Ref country code:AL Free format text:LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date:20200108 | |
| P01 | Opt-out of the competence of the unified patent court (upc) registered | Effective date:20230524 | |
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] | Ref country code:DE Payment date:20250121 Year of fee payment:10 | |
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] | Ref country code:FR Payment date:20250127 Year of fee payment:10 |