CN1812776A - Inhibition of protein kinase c-mu (pkd) as a treatment for cardiac hypertrophy and heart failure - Google Patents
Inhibition of protein kinase c-mu (pkd) as a treatment for cardiac hypertrophy and heart failureDownload PDFInfo
- Publication number
- CN1812776A CN1812776ACNA2004800139983ACN200480013998ACN1812776ACN 1812776 ACN1812776 ACN 1812776ACN A2004800139983 ACNA2004800139983 ACN A2004800139983ACN 200480013998 ACN200480013998 ACN 200480013998ACN 1812776 ACN1812776 ACN 1812776A
- Authority
- CN
- China
- Prior art keywords
- pkd
- cell
- inhibitor
- cardiac hypertrophy
- promoter
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Pending
Links
Images
Classifications
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/045—Hydroxy compounds, e.g. alcohols; Salts thereof, e.g. alcoholates
- A61K31/05—Phenols
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/33—Heterocyclic compounds
- A61K31/395—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins
- A61K31/40—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins having five-membered rings with one nitrogen as the only ring hetero atom, e.g. sulpiride, succinimide, tolmetin, buflomedil
- A61K31/403—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins having five-membered rings with one nitrogen as the only ring hetero atom, e.g. sulpiride, succinimide, tolmetin, buflomedil condensed with carbocyclic rings, e.g. carbazole
- A61K31/404—Indoles, e.g. pindolol
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/33—Heterocyclic compounds
- A61K31/395—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins
- A61K31/40—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins having five-membered rings with one nitrogen as the only ring hetero atom, e.g. sulpiride, succinimide, tolmetin, buflomedil
- A61K31/407—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins having five-membered rings with one nitrogen as the only ring hetero atom, e.g. sulpiride, succinimide, tolmetin, buflomedil condensed with other heterocyclic ring systems, e.g. ketorolac, physostigmine
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/33—Heterocyclic compounds
- A61K31/395—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins
- A61K31/435—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins having six-membered rings with one nitrogen as the only ring hetero atom
- A61K31/44—Non condensed pyridines; Hydrogenated derivatives thereof
- A61K31/4425—Pyridinium derivatives, e.g. pralidoxime, pyridostigmine
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/33—Heterocyclic compounds
- A61K31/395—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins
- A61K31/55—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins having seven-membered rings, e.g. azelastine, pentylenetetrazole
- A61K31/553—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins having seven-membered rings, e.g. azelastine, pentylenetetrazole having at least one nitrogen and one oxygen as ring hetero atoms, e.g. loxapine, staurosporine
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/70—Carbohydrates; Sugars; Derivatives thereof
- A61K31/7028—Compounds having saccharide radicals attached to non-saccharide compounds by glycosidic linkages
- A61K31/7034—Compounds having saccharide radicals attached to non-saccharide compounds by glycosidic linkages attached to a carbocyclic compound, e.g. phloridzin
- A61K31/704—Compounds having saccharide radicals attached to non-saccharide compounds by glycosidic linkages attached to a carbocyclic compound, e.g. phloridzin attached to a condensed carbocyclic ring system, e.g. sennosides, thiocolchicosides, escin, daunorubicin
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K38/00—Medicinal preparations containing peptides
- A61K38/04—Peptides having up to 20 amino acids in a fully defined sequence; Derivatives thereof
- A61K38/10—Peptides having 12 to 20 amino acids
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K45/00—Medicinal preparations containing active ingredients not provided for in groups A61K31/00 - A61K41/00
- A61K45/06—Mixtures of active ingredients without chemical characterisation, e.g. antiphlogistics and cardiaca
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K49/00—Preparations for testing in vivo
- A61K49/0004—Screening or testing of compounds for diagnosis of disorders, assessment of conditions, e.g. renal clearance, gastric emptying, testing for diabetes, allergy, rheuma, pancreas functions
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P43/00—Drugs for specific purposes, not provided for in groups A61P1/00-A61P41/00
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P9/00—Drugs for disorders of the cardiovascular system
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P9/00—Drugs for disorders of the cardiovascular system
- A61P9/04—Inotropic agents, i.e. stimulants of cardiac contraction; Drugs for heart failure
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12Q—MEASURING OR TESTING PROCESSES INVOLVING ENZYMES, NUCLEIC ACIDS OR MICROORGANISMS; COMPOSITIONS OR TEST PAPERS THEREFOR; PROCESSES OF PREPARING SUCH COMPOSITIONS; CONDITION-RESPONSIVE CONTROL IN MICROBIOLOGICAL OR ENZYMOLOGICAL PROCESSES
- C12Q1/00—Measuring or testing processes involving enzymes, nucleic acids or microorganisms; Compositions therefor; Processes of preparing such compositions
- C12Q1/48—Measuring or testing processes involving enzymes, nucleic acids or microorganisms; Compositions therefor; Processes of preparing such compositions involving transferase
- C12Q1/485—Measuring or testing processes involving enzymes, nucleic acids or microorganisms; Compositions therefor; Processes of preparing such compositions involving transferase involving kinase
- G—PHYSICS
- G01—MEASURING; TESTING
- G01N—INVESTIGATING OR ANALYSING MATERIALS BY DETERMINING THEIR CHEMICAL OR PHYSICAL PROPERTIES
- G01N2500/00—Screening for compounds of potential therapeutic value
- G01N2500/04—Screening involving studying the effect of compounds C directly on molecule A (e.g. C are potential ligands for a receptor A, or potential substrates for an enzyme A)
Landscapes
- Health & Medical Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Chemical & Material Sciences (AREA)
- General Health & Medical Sciences (AREA)
- Veterinary Medicine (AREA)
- Public Health (AREA)
- Animal Behavior & Ethology (AREA)
- Pharmacology & Pharmacy (AREA)
- Medicinal Chemistry (AREA)
- Epidemiology (AREA)
- Engineering & Computer Science (AREA)
- Organic Chemistry (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Proteomics, Peptides & Aminoacids (AREA)
- Immunology (AREA)
- Gastroenterology & Hepatology (AREA)
- Zoology (AREA)
- Wood Science & Technology (AREA)
- Molecular Biology (AREA)
- Chemical Kinetics & Catalysis (AREA)
- General Chemical & Material Sciences (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Cardiology (AREA)
- Analytical Chemistry (AREA)
- General Engineering & Computer Science (AREA)
- Microbiology (AREA)
- Biophysics (AREA)
- Physics & Mathematics (AREA)
- Urology & Nephrology (AREA)
- Toxicology (AREA)
- Biochemistry (AREA)
- Rheumatology (AREA)
- Biotechnology (AREA)
- Genetics & Genomics (AREA)
- Pathology (AREA)
- Endocrinology (AREA)
- Diabetes (AREA)
- Biomedical Technology (AREA)
- Heart & Thoracic Surgery (AREA)
- Hospice & Palliative Care (AREA)
Abstract
Translated fromChineseDescription
Translated fromChinese发明背景Background of the Invention
本发明要求2003年5月21日提交的美国临时专利序列号60/472,298的优先权,将其整体内容引入本文作为参考。由于国立卫生研究院基金编号P01HL61544的资助,美国政府拥有本发明的权利。This application claims priority to US Provisional Patent Serial No. 60/472,298, filed May 21, 2003, the entire contents of which are incorporated herein by reference. The US Government owns rights in this invention due to support from National Institutes of Health Grant No. P01HL61544.
1.发明领域1. Field of invention
本发明总的涉及发育生物学和分子生物学领域。更具体说,它涉及心肌细胞中的基因调控和细胞生理学。具体地,本发明涉及采用蛋白激酶C-μ(PKD)抑制剂来阻断组蛋白脱乙酰酶的磷酸化。也涉及用PKD抑制剂治疗心脏肥大和心力衰竭。The present invention relates generally to the fields of developmental biology and molecular biology. More specifically, it concerns gene regulation and cellular physiology in cardiomyocytes. In particular, the invention relates to the use of protein kinase C-μ (PKD) inhibitors to block phosphorylation of histone deacetylases. It also relates to the treatment of cardiac hypertrophy and heart failure with PKD inhibitors.
2.相关领域描述2. Description of related fields
心脏肥大是对强加于心脏的工作负荷增加反应中的一种基础性适应机制。它反映了由于神经、内分泌或机械刺激之一或它们的组合作用使心脏细胞大小和质量(而不是细胞数量)增加的一种特定过程。高血压,另一种涉及心脏肥大的因素,经常是充血性心力衰竭的先兆。当发生心力衰竭时,左心室通常肥大并扩张,心脏收缩功能指数如心射血分数降低。已清楚,心脏肥大反应是一种复杂的综合征,阐明导致心脏肥大的途径将有益于各种刺激所致心脏病的治疗。Cardiac hypertrophy is a fundamental adaptive mechanism in response to increased workload imposed on the heart. It reflects a specific process by which cardiac cells increase in size and mass (rather than cell number) due to one or a combination of neural, endocrine, or mechanical stimuli. High blood pressure, another factor involved in an enlarged heart, is often a precursor to congestive heart failure. When heart failure occurs, the left ventricle is usually hypertrophied and dilated, and indices of systolic function such as cardiac ejection fraction are reduced. It is clear that the cardiac hypertrophic response is a complex syndrome, and elucidating the pathways leading to cardiac hypertrophy will benefit the treatment of heart disease caused by various stimuli.
转录因子亚家族,肌细胞增强子因子-2家族(MEF2)参与了心脏肥大的发生。例如,各种刺激可提高细胞内钙水平,导致细胞信号转导系统或途径,包括钙调磷酸酶、CAM激酶、PKC和MAP激酶的级联反应。所有这些信号都激活MEF2,导致心脏肥大。然而,人们仍然没有完全了解各种信号系统是如何行使它们对MEF2的作用并调节其肥大信号转导的。已知某些组蛋白脱乙酰酶蛋白(HDAC)涉及调节MEF2活性。A subfamily of transcription factors, the myocyte enhancer factor-2 family (MEF2), is involved in the development of cardiac hypertrophy. For example, various stimuli can increase intracellular calcium levels, leading to a cascade of cellular signaling systems or pathways, including calcineurin, CAM kinase, PKC, and MAP kinase. All of these signals activate MEF2, leading to cardiac hypertrophy. However, how various signaling systems exercise their effects on MEF2 and regulate its hypertrophic signaling is still not fully understood. Certain histone deacetylase proteins (HDACs) are known to be involved in regulating MEF2 activity.
已经克隆了脊椎生物的十一种不同的HDAC。所有HDAC的催化区域都有同源性。组蛋白乙酰化酶和脱乙酰酶在控制基因表达中起到重要作用。组蛋白乙酰化酶,通常称为乙酰基转移酶(HAT)和脱乙酰酶(HDAC)活性之间的平衡决定了组蛋白的乙酰化水平。因而,乙酰化的组蛋白引起染色质松弛和基因转录激活,而脱乙酰化的染色质通常无转录活性。在先前的报道中,本发明者的实验室证明了HDAC4和5与MEF2二聚化而抑制MEF2的转录活性,而且,该相互反应需要存在HDAC4和5蛋白的N末端(McKinsey等,2000a,b)。Eleven different HDACs from vertebrates have been cloned. The catalytic domains of all HDACs share homology. Histone acetylases and deacetylases play an important role in the control of gene expression. The balance between the activities of histone acetylases, commonly referred to as acetyltransferases (HATs) and deacetylases (HDACs), determines the level of acetylation of histones. Thus, acetylated histones cause chromatin relaxation and gene transcriptional activation, while deacetylated chromatin is usually inactive for transcription. In previous reports, the inventor's laboratory demonstrated that HDAC4 and 5 dimerize with MEF2 to inhibit the transcriptional activity of MEF2, and that this interaction requires the presence of the N-termini of the HDAC4 and 5 proteins (McKinsey et al., 2000a,b ).
近年来,已阐明和描述了HDAC与MEF2之间的联系(McKinsey等,2002)。表明HDAC和MEF2之间的结合受磷酸化控制,和一种未鉴定的激酶介导此结合。缺少磷酸化位点的突变HDAC对心肌细胞肥大起着信号转导抗性阻抑剂作用,使HDAC敲除小鼠对心力衰竭和肥大超敏感(Zhang等,2002)。也已证明,某些HDAC抑制剂是抗肥大的。在其他文章中,近年的研究也突出了HDAC在癌生物学中的重要作用。实际上,测试了各种HDAC抑制剂在诱导癌细胞分化和/或调亡的能力(Marks等,2000)。这些抑制剂包括N-辛二酰苯胺异羟肟酸(SAHA)(Butler等,2000;Marks等,2001)、间-羧基肉桂酸双-羟酰胺(Coffey等,2001)和焦酰胺(pyroxamide)(Butler等,2001)。In recent years, the link between HDACs and MEF2 has been elucidated and described (McKinsey et al., 2002). It was shown that the association between HDACs and MEF2 is controlled by phosphorylation, and an unidentified kinase mediates this association. Mutant HDACs lacking phosphorylation sites act as signal transduction resistance repressors of cardiomyocyte hypertrophy, rendering HDAC knockout mice hypersensitive to heart failure and hypertrophy (Zhang et al., 2002). Certain HDAC inhibitors have also been shown to be antihypertrophic. In other articles, recent studies have also highlighted the important role of HDACs in cancer biology. Indeed, various HDAC inhibitors were tested for their ability to induce differentiation and/or apoptosis in cancer cells (Marks et al., 2000). These inhibitors include N-suberoylanilide hydroxamic acid (SAHA) (Butler et al., 2000; Marks et al., 2001), m-carboxycinnamic acid bis-hydroxylamide (Coffey et al., 2001) and pyroxamide (Butler et al., 2001).
所有这些发现证明HDAC在疾病发展中的重要作用,具体数据证明HDAC-MEF2结合是心脏病的关键因素。因此,负责介导该结合的激酶是导致肥大的级联反应的焦点,是潜在的治疗靶。迄今,仍未鉴定到负责该结合的激酶。All of these findings demonstrate the important role of HDACs in disease development, with concrete data demonstrating that HDAC-MEF2 binding is a key factor in heart disease. The kinase responsible for mediating this binding is thus the focus of a cascade leading to hypertrophy and is a potential therapeutic target. To date, the kinase responsible for this binding has not been identified.
发明概述Summary of Invention
因此,本发明提供了一种治疗病理性心脏肥大和心力衰竭的方法,该方法包括(a)鉴定患有心脏肥大或心力衰竭的病人;和(b)给予病人PKD抑制剂。给药可包括静脉内、口服、透皮、缓释、延迟释放、控释、栓剂、舌下给药,或直接注入心脏组织给药。Accordingly, the present invention provides a method of treating pathological cardiac hypertrophy and heart failure comprising (a) identifying a patient with cardiac hypertrophy or heart failure; and (b) administering to the patient a PKD inhibitor. Administration may include intravenous, oral, transdermal, sustained release, delayed release, controlled release, suppositories, sublingual administration, or direct infusion into cardiac tissue.
该方法还可包括给予第二种治疗方案,如β阻断剂、变力药物(ionotrope)、利尿剂、ACE-I、AII拮抗剂、BNP、Ca++阻断剂或HDAC抑制剂。可以在给予PKD抑制剂的同时,或给予PKD抑制剂之前或之后给予第二治疗方案。该治疗可改善病理性心脏肥大或心力衰竭的一种或多种症状,如运动能力增强、心排出血量增加、左心室舒张末期压降低、肺毛细管嵌入压降低、心脏输出或心脏指数提高、肺动脉压降低、左心室收缩和舒张末期尺度降低、左右心室壁压力降低、壁张力和壁厚度降低、生活质量提高和疾病相关发病率和死亡率降低。The method may also include administering a second therapeutic regimen, such as a beta blocker, ionotrope, diuretic, ACE-I, AII antagonist, BNP, Ca++ blocker, or HDAC inhibitor. The second treatment regimen can be administered at the same time as, or before or after, the PKD inhibitor is administered. The treatment improves one or more symptoms of pathological cardiac hypertrophy or heart failure, such as increased exercise capacity, increased cardiac output, decreased left ventricular end-diastolic pressure, decreased pulmonary capillary insertion pressure, increased cardiac output or cardiac index, Reduced pulmonary artery pressure, reduced left ventricular systolic and end-diastolic dimensions, reduced left and right ventricular wall pressure, reduced wall tension and wall thickness, improved quality of life and reduced disease-related morbidity and mortality.
在另一实施方式中,提供了一种预防病理性心脏肥大或心力衰竭的方法,该方法包括(a)鉴定处于发生病理性心脏肥大或心力衰竭危险的病人;和(b)给予病人PKD抑制剂。给药可包括静脉内、口服、透皮、缓释、延迟释放、控释、栓剂、舌下给药,或直接注入心脏组织。危险病人可出现一系列危险因素的一种或多种,包括长时间未得到控制的高血压、未纠正的心瓣膜疾病、慢性心绞痛或近期心肌梗塞。危险病人也可能有先天性、家族性或遗传性易患心脏病、心力衰竭或心脏肥大的体质。心力衰竭或其症状可包括局部缺血、心肌病变、主动脉狭窄、或其它心肌疾病。In another embodiment, there is provided a method of preventing pathological cardiac hypertrophy or heart failure comprising (a) identifying a patient at risk of developing pathological cardiac hypertrophy or heart failure; and (b) administering to the patient a PKD inhibitory agent. Administration may include intravenous, oral, transdermal, sustained release, delayed release, controlled release, suppositories, sublingual administration, or infusion directly into cardiac tissue. At-risk patients may have one or more of a range of risk factors, including prolonged uncontrolled hypertension, uncorrected heart valve disease, chronic angina, or recent myocardial infarction. At-risk patients may also have a congenital, familial, or genetic predisposition to heart disease, heart failure, or an enlarged heart. Heart failure or symptoms thereof may include ischemia, cardiomyopathy, aortic stenosis, or other heart muscle disease.
根据前述的实施方式,PKD抑制剂可以是任何降低PKD活性或抑制II类HDAC PKD磷酸化的分子。这包括蛋白、肽、DNA分子(包括反义)、RNA分子(包括RNAi、反义和核酶)、抗体(包括单链抗体)、编码抗体的表达构建物和小分子。该小分子可包括但不限于白藜芦醇、吲哚咔唑、Godecke6976(G6976)、星孢素、K252a、包括[d-Arg(1)、d-Trp(5,7,9)、Leu(11)]SP的物质P(SP)类似物、PKC抑制剂109203X(GF-1)、PKC抑制剂Ro31-8220、GO7874、染料木黄酮、特异性Src抑制剂PP-1和PP-2、白屈菜季铵碱或粗糠柴毒素。According to the aforementioned embodiments, the PKD inhibitor can be any molecule that reduces PKD activity or inhibits PKD phosphorylation of class II HDACs. This includes proteins, peptides, DNA molecules (including antisense), RNA molecules (including RNAi, antisense and ribozymes), antibodies (including single chain antibodies), expression constructs encoding antibodies, and small molecules. The small molecules may include, but are not limited to, resveratrol, indolecarbazole, Godecke6976 (G6976), staurosporine, K252a, including [d-Arg(1), d-Trp(5,7,9) , substance P(SP) analogue of Leu(11)]SP, PKC inhibitor 109203X(GF-1), PKC inhibitor Ro31-8220, GO7874, genistein, specific Src inhibitors PP-1 and PP- 2. Celandine quaternary ammonium base or chaffin.
HDAC抑制剂可包括但不限于,曲古抑菌素A、trapoxin B、MS275-27、间-羧基肉桂酸双-羟酰胺、depudecin、oxamflatin、apicidin、N-辛二酰苯胺异羟肟酸、Scriptaid、焦酰胺、2-氨基-8-氧-9,10-环氧-癸酰酯、3-(4-芳酰基-1H吡咯-2-基)-N-羟基-2-亚克力酰胺和FR901228。此外,下面的参考文献描述了可选择用于本发明的组蛋白脱乙酰酶抑制剂:AU9,013,101;AU9,013,201;AU9,013,401;AU6,794,700;EP1,233,958;EP1,208,086;EP1,174,438;EP1,173,562;EP1,170,008;EP1,123,111;JP2001/348340;U.S.2002/103192;U.S.2002/65282;U.S.2002/61860;WO02/51842;WO02/50285;WO02/46144;WO02/46129;WO02/30879;WO02/26703;WO02/26696;WO01/70675;WO01/42437;WO01/38322;WO01/18045;WO01/14581;Furumai等(2002);Hinnebusch等(2002);Mai等(2002);Vigushin等(2002);Gottlicher等(2001);Jung(2001);Komatsu等(2001);Su等(2000)。HDAC inhibitors may include, but are not limited to, trichostatin A, trapoxin B, MS275-27, m-carboxycinnamic acid bis-hydroxylamide, depudecin, oxamflatin, apicidin, N-suberoylanilide hydroxamic acid, Scriptaid, pyroamide, 2-amino-8-oxo-9,10-epoxy-decanoyl ester, 3-(4-aroyl-1Hpyrrol-2-yl)-N-hydroxy-2-acrylamide and FR901228 . In addition, the following references describe histone deacetylase inhibitors that may be selected for use in the present invention: AU9,013,101; AU9,013,201; AU9,013,401; AU6,794,700; EP1,233,958; EP1,208,086; EP1,174,438 EP1,173,562; EP1,170,008; EP1,123,111; JP2001/348340; U.S.2002/103192; 30879; WO02/26703; WO02/26696; WO01/70675; WO01/42437; WO01/38322; (2002); Gottlicher et al. (2001); Jung (2001); Komatsu et al. (2001); Su et al. (2000).
在另一实施方式中,提供了评价PKD抑制剂治疗心脏肥大或心力衰竭的功效的方法,该方法包括(a)提供PKD抑制剂;(b)用PKD抑制剂处理细胞;和(c)测定一种或多种心脏肥大参数的表达,与没有用PKD抑制剂处理的细胞中一种或多种心脏肥大参数相比,其中若有一种或多种心脏肥大参数变化将鉴定该PKD抑制剂是心脏肥大或心力衰竭的抑制剂。In another embodiment, there is provided a method of evaluating the efficacy of a PKD inhibitor in the treatment of cardiac hypertrophy or heart failure, the method comprising (a) providing a PKD inhibitor; (b) treating cells with a PKD inhibitor; and (c) determining Expression of one or more parameters of cardiac hypertrophy as compared to one or more parameters of cardiac hypertrophy in cells not treated with a PKD inhibitor, wherein a change in one or more parameters of cardiac hypertrophy would identify that the PKD inhibitor is Inhibitors of cardiac hypertrophy or heart failure.
所述细胞可以是肌细胞,包含在分离的完整组织中的分离肌细胞如心肌细胞,初生大鼠心室肌细胞,H9C2细胞,体内位于完整心肌中,或转基因的非人哺乳动物的心肌细胞。可以刺激该肌细胞或完整心肌,以触发肥大反应的一种或多种心脏肥大参数。所述刺激可以是主动脉结扎,快速心脏起搏,诱导心肌梗塞,转基因表达,用化学品、药剂或药物处理。化学剂或药剂可包括但不限于血管紧张素II、异丙肾上腺素、苯肾上腺素、内皮缩血管肽-I、血管收缩药和抗利尿剂。治疗可以在体内或体外进行。The cells may be myocytes, isolated myocytes contained in isolated intact tissue such as cardiomyocytes, primary rat ventricular myocytes, H9C2 cells, located in intact myocardium in vivo, or transgenic non-human mammalian cardiomyocytes. The myocytes or intact myocardium can be stimulated to trigger one or more cardiac hypertrophy parameters of a hypertrophic response. The stimulus may be ligation of the aorta, rapid cardiac pacing, induction of myocardial infarction, expression of a transgene, treatment with chemicals, agents or drugs. Chemical agents or agents may include, but are not limited to, angiotensin II, isoproterenol, phenylephrine, endothelin-I, vasoconstrictors, and antidiuretics. Treatment can be performed in vivo or in vitro.
一种或多种心脏肥大参数可包括右心室射血分数、左心室射血分数、心室壁厚度、心脏重/体重比、右或左心室重/体重比或心脏重标准化度量。所述参数还可包括所述肌细胞或完整心肌中一种或多种靶基因的表达水平,其中所述一种或多种靶基因的表达水平是心脏肥大的指标。The one or more parameters of cardiac hypertrophy may include right ventricular ejection fraction, left ventricular ejection fraction, ventricular wall thickness, heart weight/body weight ratio, right or left ventricular weight/body weight ratio, or a normalized measure of heart weight. The parameters may also include the expression level of one or more target genes in the myocytes or intact myocardium, wherein the expression level of the one or more target genes is indicative of cardiac hypertrophy.
一种或多种靶基因可选自ANF、α-MyHC、β-MyHC、α-骨架肌动蛋白、心脏肌动蛋白、SERCA、细胞色素氧化酶亚基VIII、小鼠T-复合体蛋白、胰岛素生长因子结合蛋白、Tau-微管结合蛋白、泛素羧基端水解酶、Thy-1细胞表面糖蛋白或MyHCI型抗原。可用操作性连接于靶基因启动子的报道蛋白编码区,如荧光素酶,β-gal或绿色荧光蛋白来测定表达水平。可以用核酸探针与靶mRNA或扩增的核酸产物杂交来测定表达水平。The one or more target genes may be selected from ANF, α-MyHC, β-MyHC, α-skeletal actin, cardiac actin, SERCA, cytochrome oxidase subunit VIII, mouse T-complex protein, Insulin growth factor binding protein, Tau-microtubule binding protein, ubiquitin carboxy-terminal hydrolase, Thy-1 cell surface glycoprotein or MyHCI type antigen. Expression levels can be measured using a reporter protein coding region, such as luciferase, β-gal, or green fluorescent protein, operably linked to the target gene promoter. Expression levels can be determined using nucleic acid probes that hybridize to target mRNA or amplified nucleic acid products.
一种或多种心脏肥大参数也包括细胞形态的一个或多个方面,如肌节组装、细胞大小、细胞收缩性、总蛋白合成或细胞毒性。该细胞还可表达缺少一个或多个磷酸化位点的突变的II类HDAC蛋白,其中所述测定包括测定II类HDAC的磷酸化、II类HDAC的核输出或II类HDAC与MEF-2的结合。测定还可包括测定II类HDAC与MEF-2结合的增加或MEF-2依赖性转录的降低。The one or more parameters of cardiac hypertrophy also include one or more aspects of cell morphology, such as sarcomere assembly, cell size, cell contractility, total protein synthesis, or cytotoxicity. The cells may also express a mutated class II HDAC protein lacking one or more phosphorylation sites, wherein the assay comprises measuring phosphorylation of class II HDACs, nuclear export of class II HDACs, or association of class II HDACs with MEF-2 combined. Assaying can also include assaying for an increase in binding of class II HDACs to MEF-2 or a decrease in MEF-2-dependent transcription.
在另一实施方式中,提供了一种鉴定心脏肥大或心力衰竭抑制剂的方法,该方法包括(a)提供PKD;(b)使PKD与候选抑制剂物质接触;和(c)测定PKD的活性,其中PKD激酶活性的大大降低可将候选抑制剂物质鉴定为心脏肥大或心力衰竭抑制剂。可以从完整细胞或心脏细胞中纯化得到PKD,或PKD可位于完整细胞中。该细胞可以是肌细胞,如心肌细胞。In another embodiment, there is provided a method of identifying an inhibitor of cardiac hypertrophy or heart failure, the method comprising (a) providing a PKD; (b) contacting the PKD with a candidate inhibitor substance; and (c) measuring the PKD activity, wherein a substantial reduction in PKD kinase activity identifies a candidate inhibitor substance as an inhibitor of cardiac hypertrophy or heart failure. PKD can be purified from intact cells or cardiac cells, or PKD can be localized in intact cells. The cells may be muscle cells, such as cardiomyocytes.
可通过测定HDAC,更具体说是II类HDAC磷酸化的降低来测定激酶活性的降低。所述候选抑制剂物质可包括但不限于干扰RNA、抗体制剂、单链抗体、RNA或DNA反义构建物、酶、化学品、药物或药剂,或小分子。该候选抑制剂还可为白藜芦醇、吲哚咔唑、Godecke6976(G6976)、星孢素、K252a、包括[d-Arg(1)、d-Trp(5,7,9)、Leu(11)]SP的物质P(SP)类似物、PKC抑制剂109203X(GF-1)、PKC抑制剂Ro31-8220、GO7874、染料木黄酮、特异性Src抑制剂PP-1和PP-2、白屈菜季铵碱或粗糠柴毒素。A decrease in kinase activity can be measured by measuring a decrease in phosphorylation of HDACs, more specifically class II HDACs. Such candidate inhibitor substances may include, but are not limited to, interfering RNA, antibody preparations, single chain antibodies, RNA or DNA antisense constructs, enzymes, chemicals, drugs or agents, or small molecules. The candidate inhibitor can also be resveratrol, indolecarbazole, Godecke6976 (G6976), staurosporine, K252a, including [d-Arg (1), d-Trp (5, 7, 9), Substance P(SP) analogue of Leu(11)]SP, PKC inhibitor 109203X(GF-1), PKC inhibitor Ro31-8220, GO7874, genistein, specific Src inhibitors PP-1 and PP-2 , celandine quaternary ammonium or rough chai toxin.
测定激酶活性的降低还可通过免疫共沉淀测定PKD与II类HDAC结合的抑制,或通过测定II类HDAC磷酸化的阻断。PKD抑制剂可增强II类HDAC与MEF-2或其它II类HDAC调节的转录因子的结合。Determination of kinase activity reduction can also be determined by inhibition of PKD binding to class II HDACs by co-immunoprecipitation, or by blocking of class II HDAC phosphorylation. PKD inhibitors can enhance the binding of class II HDACs to MEF-2 or other class II HDAC-regulated transcription factors.
在本发明的另一实施方式中,提供了一种转基因的非人哺乳动物,其细胞含有在真核细胞中具有活性的启动子控制下的异源性PKD基因。在另一实施方式中,提供了一种转基因的非人哺乳动物,其细胞含有在所述非人哺乳动物细胞中具有活性的异源性启动子控制下的PKD基因。在另一实施方式中,提供了一种转基因的非人哺乳动物,其细胞缺少一个或两个天然PKD等位基因,还可通过同源重组敲除这些等位基因。该转基因的非人哺乳动物可以是小鼠。该启动子可以是组织特异性的,也可对肌肉组织具有特异性,还可对心肌组织具有特异性。In another embodiment of the present invention, there is provided a transgenic non-human mammal whose cells contain a heterologous PKD gene under the control of a promoter active in eukaryotic cells. In another embodiment, there is provided a transgenic non-human mammal whose cells contain a PKD gene under the control of a heterologous promoter active in said non-human mammal cell. In another embodiment, there is provided a transgenic non-human mammal whose cells lack one or both of the native PKD alleles and which can be knocked out by homologous recombination. The transgenic non-human mammal can be a mouse. The promoter may be tissue specific, muscle tissue specific, or cardiac muscle tissue specific.
PKD基因可以是人源的。该基因还可编码该蛋白的组成性活化形式或该蛋白的显性失活形式。该启动子可以是在真核细胞中具有活性的。肌肉特异性启动子可选自:肌球蛋白轻链-2启动子、α肌动蛋白启动子、肌钙蛋白1启动子、Na+/Ca2+交换蛋白启动子、肌营养不良蛋白启动子、肌酸激酶启动子、α7整合素启动子、脑钠尿肽启动子、肌球蛋白重链启动子、ANF启动子和αB-晶体蛋白/小热激蛋白启动子。The PKD gene may be of human origin. The gene may also encode a constitutively active form of the protein or a dominant negative form of the protein. The promoter may be active in eukaryotic cells. Muscle-specific promoters may be selected from: myosin light chain-2 promoter, alpha-actin promoter,
在本发明另一实施方式中,使患者心脏细胞的PKD活性降低,能治疗心肌梗塞、预防心脏肥大和扩张性心肌病变、抑制心脏肥大的发展、治疗心力衰竭、抑制心力衰竭的发展、提高心力衰竭或心脏肥大患者的运动耐量、减少心力衰竭或心脏肥大患者的住院治疗、改善心力衰竭或心脏肥大患者的生活质量和降低心力衰竭或心脏肥大患者的发病率和死亡率。In another embodiment of the present invention, the PKD activity of patient's heart cells can be reduced to treat myocardial infarction, prevent cardiac hypertrophy and dilated cardiomyopathy, inhibit the development of cardiac hypertrophy, treat heart failure, inhibit the development of heart failure, and improve heart function. exercise tolerance in patients with heart failure or hypertrophy, reduce hospitalizations in patients with heart failure or hypertrophy, improve quality of life in patients with heart failure or hypertrophy, and reduce morbidity and mortality in patients with heart failure or hypertrophy.
附图简要说明A brief description of the drawings
下面的附图构成本说明书的一部分,包括附图是为了进一步展示本发明的某些方面。通过参考这些附图中一幅或多幅,结合本文所述具体实施方式的详述,可以更好地理解本发明。The following drawings form a part of this specification and are included to further demonstrate certain aspects of the invention. The invention may be better understood by reference to one or more of these drawings in combination with the detailed description of specific embodiments presented herein.
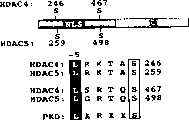
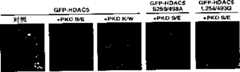
图1A-D-HDAC5的PKC依赖性核输出。图1A.在六孔板中培养COS细胞,用GFP-HDAC5表达载体(1微克)转染,用所示化合物刺激,如材料和方法中所述。加入化合物六十分钟后,用荧光显微镜测定GFP-HDAC5的分布。PMA刺激导致GFP-HDAC5从核中全部重定位至胞浆,而伊屋诺霉素引发了部分反应。图1B.用编码HDAC5或由丙氨酸替代丝氨酸259和498的HDAC5突变体(HDAC5S259/498A)的FLAG-标记的表达载体(各1微克)转染COS细胞。用PMA刺激细胞60分钟,用抗FLAG第一抗体和偶联荧光素的第二抗体作间接免疫荧光测定HDAC5分布。HDAC5259/498A能抵抗PMA的刺激。图1C.用编码GFPHDAC5的表达载体(1微克)转染COS细胞,并用PMA刺激所示时间。图1D.用编码HDAC5或HDAC5S259/498A的FLAG-标记的表达载体(各1微克)瞬时转染COS细胞。用PKC抑制剂双吲哚马来酰亚胺(Bis I;10μM)预处理细胞30分钟,用PMA刺激30分钟,如图所示。依次进行免疫沉淀和免疫印迹检测FLAG-HDAC5与内源性14-3-3的结合。Figure 1A-D - PKC-dependent nuclear export of HDAC5. Figure 1A. COS cells were cultured in six-well plates, transfected with GFP-HDAC5 expression vector (1 μg), and stimulated with the indicated compounds as described in Materials and Methods. Sixty minutes after compound addition, the distribution of GFP-HDAC5 was determined by fluorescence microscopy. PMA stimulation resulted in total relocalization of GFP-HDAC5 from the nucleus to the cytoplasm, whereas ionomycin elicited a partial response. Figure IB. COS cells were transfected with FLAG-tagged expression vectors (1 microgram each) encoding HDAC5 or a HDAC5 mutant (HDAC5S259/498A) with
图2A-D-PKC抑制阻断心肌细胞中PE-介导的HDAC5的核输出。图2A.定量测定HDAC5核输出的代表方案。在96孔板中培养NVRM,用编码GFP-HDAC5的腺病毒感染。血清饥饿细胞,用激动剂和抑制剂处理,固定并用Hoechst染料染色。用CellomicsHigh Content摄像系统定量核中与胞浆中GFP-HDAC5的相对丰度,根据Hoechst荧光区别细胞核,根据这些核的尺寸确定周围的胞浆。测得的值代表细胞核减去胞质荧光强度差的平均数。图2B.试验验证。用编码GFP-HDAC5的腺病毒感染NRVM,使之接触0.1至20μM浓度范围内的PE。刺激2小时后,准备细胞进行Cellomics分析。测定8孔/条件每孔至少50个细胞(总共400个细胞)的细胞核减去胞质荧光强度差的平均值。将未处理细胞的值设为100%。PE引发了剂量依赖性的HDAC5核输出。图2C.用腺病毒GFP-HDAC5感染NRVM,用激酶抑制剂(材料和方法中所述抑制剂的浓度)预处理NRVM。用PE(20μM)刺激2小时后定量测定HDAC5的亚细胞分布。测定8孔/条件每孔至少50个细胞(总共400个细胞)的细胞核减去胞质荧光强度差的平均值。较高值表明核中HDAC5丰度较高。显示了孔与孔间的标准差。只有星孢素和PKC抑制剂Bis I有效阻断了HDAC5的核输出。图2D中显示了代表性图片。Figure 2A-D - PKC inhibition blocks PE-mediated nuclear export of HDAC5 in cardiomyocytes. Figure 2A. Representative scheme for quantification of HDAC5 nuclear export. NVRMs were cultured in 96-well plates and infected with adenovirus encoding GFP-HDAC5. Serum starved cells, treated with agonists and inhibitors, fixed and stained with Hoechst dye. The relative abundance of GFP-HDAC5 in the nucleus and cytoplasm was quantified using the Cellomics High Content camera system, the nuclei were distinguished based on Hoechst fluorescence, and the surrounding cytoplasm was determined based on the size of these nuclei. Measured values represent the mean of the difference in nuclear minus cytoplasmic fluorescence intensity. Figure 2B. Experimental validation. NRVMs were infected with adenovirus encoding GFP-HDAC5 and exposed to PE at concentrations ranging from 0.1 to 20 [mu]M. After 2 hours of stimulation, cells were prepared for Cellomics analysis. The mean value of the difference in nuclear minus cytoplasmic fluorescence intensity was determined for at least 50 cells per well (400 cells in total) in 8 wells/condition. Set the value for untreated cells to 100%. PE triggered a dose-dependent nuclear export of HDAC5. Figure 2C. Infection of NRVMs with adenovirus GFP-HDAC5 and pretreatment of NRVMs with kinase inhibitors (inhibitor concentrations described in Materials and Methods). The subcellular distribution of HDAC5 was quantified after stimulation with PE (20 μM) for 2 hours. The mean value of the difference in nuclear minus cytoplasmic fluorescence intensity was determined for at least 50 cells per well (400 cells in total) in 8 wells/condition. Higher values indicate higher abundance of HDAC5 in the nucleus. The well-to-well standard deviation is shown. Only staurosporine and the PKC inhibitor Bis I effectively blocked HDAC5 nuclear export. Representative pictures are shown in Figure 2D.
图3A-C-通过信号抗性HDAC5抑制PKC-介导的心脏肥大。在6孔板中培养NRVM,用腺病毒(MOI=10)转染,该腺病毒编码LacZ对照(Ad-LacZ)或由丙氨酸替代丝氨酸259和498的FLAG-标记的HDAC5(Ad-HDAC5S/A),为14-3-3介导的核输出所必需。分析前,用PE(20μM)或PMA(100nM)处理细胞24小时。图3A.固定细胞,用特异性抗α-肌动蛋白的第一抗体和偶联荧光素的第二抗体作间接免疫荧光使肌节显色。图3B.用抗ANF第一抗体作间接免疫荧光检测ANF蛋白。图3C.从细胞中收集总RNA,用放射标记的所示转录物的特异性寡核苷酸进行斑点印迹分析。用磷光摄像仪定量测定RNA水平,表示为相对于感染Ad-LacZ而未受刺激细胞所含量的变化倍数。将数值标准化到GAPDH对照。Figure 3A-C - Inhibition of PKC-mediated cardiac hypertrophy by signaling resistant HDAC5. NRVMs were cultured in 6-well plates and transfected with adenovirus (MOI=10) encoding a LacZ control (Ad-LacZ) or a FLAG-tagged HDAC5 with
图4A-F-在激动剂-介导的HDAC5的核输出中对PKC的不同要求。图4A.在96孔板中培养NRVM,用腺病毒GFP-HDAC5感染,如上所述。血清饥饿细胞4小时后,用PE(20μM)、ET-1(50nM)或FBS(10%)刺激两小时。如图4A中所述地那样准备细胞。血清饥饿后,用Bis I(10μM)预处理感染的NRVM30分钟,用所示激动剂刺激2小时。如上所述,用Cellomics摄像系统定量测定HDAC5的核输出。较高值表明核中HDAC5丰度较高。图4C.如图4B所示进行实验,除了细胞在激动剂处理前接受G6983(10μM)。图4D.如图4B所示进行实验,除了细胞激动剂处理前接受增加剂量的G6976。图4E.用配有数字相机的荧光显微镜拍摄各治疗组的代表性图片。图4F.用编码GFP-HDAC5的腺病毒感染NRVM,在10厘米平皿中培养。感染后二十四小时,血清饥饿细胞四小时,用Bis I(10μM)或G6976(10μM)预处理一小时,然后用PE(20μM)或ET-1(50nM)刺激一小时。制备全细胞蛋白裂解物,如上所述依次进行免疫沉淀和免疫印迹。Figures 4A-F - Differential requirements for PKC in agonist-mediated nuclear export of HDAC5. Figure 4A. NRVM cultured in 96-well plates, infected with adenovirus GFP-HDAC5, as described above. After serum starving cells for 4 hours, they were stimulated with PE (20 μM), ET-1 (50 nM) or FBS (10%) for two hours. Cells were prepared as described in Figure 4A. After serum starvation, infected NRVMs were pretreated with Bis I (10 μM) for 30 min and stimulated with the indicated agonists for 2 h. Nuclear export of HDAC5 was quantified using the Cellomics camera system as described above. Higher values indicate higher abundance of HDAC5 in the nucleus. Figure 4C. Experiments were performed as in Figure 4B, except cells received G'6983 (10 [mu]M) prior to agonist treatment. Figure 4D. Experiments were performed as in Figure 4B, except that cells received increasing doses of G'6976 prior to agonist treatment. Figure 4E. Representative pictures of each treatment group taken with a fluorescence microscope equipped with a digital camera. Figure 4F. NRVM were infected with adenovirus encoding GFP-HDAC5 and cultured in 10 cm dishes. Twenty-four hours after infection, cells were serum starved for four hours, pretreated with Bis I (10 μM) or G6976 (10 μM) for one hour, and then stimulated with PE (20 μM) or ET-1 (50 nM) for one hour. Whole-cell protein lysates were prepared, followed by immunoprecipitation and immunoblotting sequentially as described above.
图5A-E-蛋白激酶D是一种HDAC5激酶。图5A.II类HDAC的调控性磷酸化位点周围的氨基酸序列。NLS:核定位信号;HDAC结构域:脱乙酰酶催化域。显示了PKD的共有序列靶位点。相对于磷酸化位点-5位置的亮氨酸是PKD-定向其它蛋白最佳磷酸化所必需的。图5B.用编码融合于由甘氨酸替代亮氨酸254和493的HDAC5(L254/493G)的GFP的表达载体COS转染细胞。转染后二十四小时,不处理细胞(对照)或用PMA刺激30分钟。图5C.用编码GFP-HDAC5或GFPHDAC5S/A的表达载体(各1微克)和PKD的组成性活化(S/E)或催化失活(K/W)形式共转染COS细胞。转染后24小时测定HDAC5的定位。图5D.COS细胞用编码FLAGHDAC5和野生型、组成性活化(S/E)或催化失活(K/W)的PKD的HA-标记的表达载体(各1微克)共转染。转染后二十四小时,用PMA或空载体对照处理细胞30分钟。如上所述,免疫沉淀全细胞蛋白裂解物中的FLAG-HDAC5,加入到体外激酶试验(IVK)中或通过SDS-PAGE分离作Western印迹分析,以测定结合的PKD。通过SDS-PAGE分离磷酸化的HDAC5,放射自显影检测。图5E.哺乳动物双杂交试验。将编码融合于HDAC5的GAL4DNA结合域(Gal4-HDAC5)的表达载体或所示的HDAC5丙氨酸取代的突变体,与编码融合于VP16转录激活域的14-3-3(14-3-3-VP16)的质粒、Gal4-依赖性荧光素酶报道物和编码组成性活化PKD(S/E)的载体共转染入COS细胞中。PKD激发HDAC5和14-3-3之间的结合,这取决于259和498位置的磷酸化受体。Figure 5A-E - Protein kinase D is an HDAC5 kinase. Figure 5A. Amino acid sequences surrounding regulatory phosphorylation sites of class II HDACs. NLS: nuclear localization signal; HDAC domain: deacetylase catalytic domain. Consensus target sites for PKD are shown. The leucine at position -5 relative to the phosphorylation site is required for PKD-directed optimal phosphorylation of other proteins. Figure 5B. Cells were transfected with the expression vector COS encoding GFP fused to HDAC5 (L254/493G) fused to glycine replacing leucines 254 and 493. Twenty-four hours after transfection, cells were left untreated (control) or stimulated with PMA for 30 minutes. Figure 5C. COS cells were co-transfected with expression vectors encoding GFP-HDAC5 or GFPHDAC5S/A (1 μg each) and constitutively active (S/E) or catalytically inactive (K/W) forms of PKD. The localization of HDAC5 was determined 24 hours after transfection. Figure 5D. COS cells were co-transfected with HA-tagged expression vectors encoding FLAGHDAC5 and wild-type, constitutively active (S/E) or catalytically inactive (K/W) PKD (1 microgram each). Twenty-four hours after transfection, cells were treated with PMA or empty vector control for 30 minutes. FLAG-HDAC5 was immunoprecipitated from whole-cell protein lysates, added to in vitro kinase assays (IVK) or separated by SDS-PAGE for Western blot analysis to determine bound PKD, as described above. Phosphorylated HDAC5 was resolved by SDS-PAGE and detected by autoradiography. Figure 5E. Mammalian two-hybrid assay. Expression vectors encoding the GAL4 DNA-binding domain fused to HDAC5 (Gal4-HDAC5) or the indicated HDAC5 alanine-substituted mutants were combined with 14-3-3 encoding the transcriptional activation domain fused to VP16 (14-3-3 - VP16), a Gal4-dependent luciferase reporter and a vector encoding constitutively active PKD (S/E) were co-transfected into COS cells. PKD triggers association between HDAC5 and 14-3-3, which is dependent on phosphorylated receptors at
图6A-B-心肌细胞中内源性PKD与HDAC5的结合。图6A.在10厘米平皿中培养NRVM,用编码FLAG-HDAC5的腺病毒感染。转染后二十四小时,用PMA刺激细胞30分钟,制备全细胞蛋白裂解物。在PMA刺激之前,用Bis I预处理一些细胞(pre-BisI;10μM)30分钟。如上所述,免疫沉淀FLAG-HDAC5,将其加入到含有Bis I(post-BisI;10μM)或G6976(post-G6976;10μM)的体外激酶反应中。当用Bis I(pre-BisI)预处理细胞时,HDAC5的磷酸化被阻断。当直接加入激酶反应混合物中时,G6976,而非Bis I阻断了磷酸基转移到HDAC5上。图6B.用编码GFP-HDAC5的腺病毒感染NRVM,在10厘米平皿中培养。感染后二十四小时,血清饥饿细胞4小时,用Bis I(10μM)预处理30分钟,然后用PE(20μM)刺激一小时。免疫沉淀全细胞裂解物的HDAC5,通过免疫印迹检测结合的总PKD或丝氨酸916(p-916)处的自磷酸化PKD。用GFP-特异性抗体再检测印迹,以测定免疫沉淀的HDAC5总量。Figure 6A-B - Binding of endogenous PKD to HDAC5 in cardiomyocytes. Figure 6A. NRVM cultured in 10 cm dishes infected with adenovirus encoding FLAG-HDAC5. Twenty-four hours after transfection, cells were stimulated with PMA for 30 minutes, and whole-cell protein lysates were prepared. Some cells were pretreated with BisI (pre-BisI; 10 μM) for 30 min before PMA stimulation. FLAG-HDAC5 was immunoprecipitated and added to in vitro kinase reactions containing Bis I (post-BisI; 10 μM) or G6976 (post-G6976; 10 μM) as described above. Phosphorylation of HDAC5 was blocked when cells were pretreated with Bis I (pre-BisI). When added directly to the kinase reaction mixture, G6976, but not Bis I, blocked the transfer of phosphate groups to HDAC5. Figure 6B. NRVM were infected with adenovirus encoding GFP-HDAC5 and cultured in 10 cm dishes. Twenty-four hours after infection, cells were serum starved for 4 hours, pretreated with Bis I (10 μM) for 30 minutes, and then stimulated with PE (20 μM) for one hour. HDAC5 was immunoprecipitated from whole cell lysates, and bound total PKD or autophosphorylated PKD at serine 916 (p-916) was detected by immunoblotting. The blot was reprobed with a GFP-specific antibody to determine the total amount of HDAC5 immunoprecipitated.
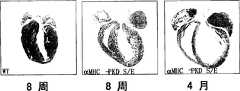
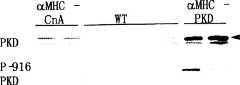
图7A-D-心脏表达激活的PKD导致扩张性心肌病。图7A.如材料和方法中所述产生在心脏特异性α-肌球蛋白重链(αMHC)启动子控制下表达组成性活化PKD的转基因小鼠。显示了四周龄的野生型和aMHC-PKD转基因(小鼠)心脏的H&E切片。也显示4个月的aMHC-PKD转基因心脏,出现扩张性心肌病。图7B.四周龄野生型和aMHC-PKD转基因小鼠的心脏重与体重之比。图7C.对心脏裂解物的野生型、aMHC-PKD和aMHC-钙调磷酸酶(CnA)作Western印迹分析。测定了总的和活化(P-916)PKD蛋白的水平。箭头标识出与PKD1相应的条带。图7D.对4周龄野生型、CnA或PKD转基因小鼠心脏的总RNA进行斑点印迹,分析胚胎基因标记。CnA和PKD印迹下方的数字代表标准化到GAPDH水平后相对于野生型样品的增加倍数。Figure 7A-D - Cardiac expression of activated PKD leads to dilated cardiomyopathy. Figure 7A. Transgenic mice expressing constitutively active PKD under the control of the cardiac-specific α-myosin heavy chain (αMHC) promoter were generated as described in Materials and Methods. H&E sections of four-week-old wild-type and aMHC-PKD transgenic (mouse) hearts are shown. Dilated cardiomyopathy was also shown in 4-month-old aMHC-PKD transgenic hearts. Figure 7B. Heart weight to body weight ratio of four-week-old wild-type and aMHC-PKD transgenic mice. Figure 7C. Western blot analysis of wild-type, aMHC-PKD and aMHC-calcineurin (CnA) of cardiac lysates. Levels of total and activated (P-916) PKD protein were determined. Arrows identify the band corresponding to PKD1. Figure 7D. Analysis of embryonic gene signatures by dot blot analysis of total RNA from hearts of 4-week-old wild-type, CnA, or PKD transgenic mice. Numbers below the CnA and PKD blots represent fold increases normalized to GAPDH levels relative to wild-type samples.
图8-调节II类HDAC的核输出和心脏肥大的激酶-依赖性信号转导途径的模型。用α-肾上腺素能激动剂苯肾上腺素(PE)或内皮缩血管肽-1(ET-1)刺激心肌细胞导致HDAC通过激活PKD而磷酸化和核输出。PE激活PKD是通过PKC依赖性途径,主要是钙非依赖性的新PKC(nPKC)途径而发生。然而,心肌细胞ET-1中激活PKD似乎是PKC非依赖性的。随后PKD使HDAC5磷酸化导致其通过与14-3-3结合和激活MEF2与肥大性遗传程序而核输出。Figure 8 - Model of the kinase-dependent signal transduction pathway regulating nuclear export of class II HDACs and cardiac hypertrophy. Stimulation of cardiomyocytes with the alpha-adrenergic agonists phenylephrine (PE) or endothelin-1 (ET-1) leads to phosphorylation and nuclear export of HDACs through activation of PKD. PE activates PKD through PKC-dependent pathways, mainly through the calcium-independent new PKC (nPKC) pathway. However, activation of PKD in cardiomyocyte ET-1 appears to be PKC-independent. Subsequent phosphorylation of HDAC5 by PKD leads to its nuclear export through association with 14-3-3 and activation of MEF2 and hypertrophic genetic programs.
示例性实施方式的详述Detailed Description of Exemplary Implementations
心力衰竭是全世界发病和致死的主要原因之一。仅在美国,估计显示三百万人目前有心肌病,另外每年还诊断到400,000病例。扩张性心肌病变(DCM),也称为“充血性心肌病”,是最常见的心肌病形式,估计发病率接近每100,000人中40人(Durand等,1995)。虽然引起DCM有其它原因,但是据显示,家族性扩张性心肌病占“特发性”DCM的约20%。大约一半的DCM病例是特发性,其余的病例与已知的疾病过程相关。例如,癌症化疗中使用的某些药物(例如阿霉素和柔红霉素)引起严重心肌损伤。此外,很多DCM病人是长期酗酒者。幸运的是,如果这些病人在病程早期减少或停止饮酒,心肌功能障碍的发展可以停止或逆转。分娩前后心肌病是另一类型的特发性DCM,与感染性后遗症有关。总之,包括DCM在内的心肌病是重要的公众健康问题。Heart failure is one of the leading causes of morbidity and mortality worldwide. In the United States alone, estimates indicate that three million people currently have cardiomyopathy, with an additional 400,000 cases diagnosed each year. Dilated cardiomyopathy (DCM), also known as "congestive cardiomyopathy", is the most common form of cardiomyopathy with an estimated incidence of nearly 40 per 100,000 (Durand et al., 1995). Although there are other causes of DCM, familial dilated cardiomyopathy has been shown to account for approximately 20% of "idiopathic" DCM. About half of DCM cases are idiopathic and the remainder are associated with a known disease process. For example, certain drugs used in cancer chemotherapy, such as doxorubicin and daunorubicin, cause severe heart muscle damage. In addition, many DCM patients are chronic alcoholics. Fortunately, if these patients reduce or stop drinking alcohol early in the course of the disease, the development of myocardial dysfunction can be halted or reversed. Peripartum cardiomyopathy is another type of idiopathic DCM associated with infectious sequelae. In conclusion, cardiomyopathy, including DCM, is an important public health problem.
心脏病及其表现,包括冠状动脉疾病、心肌梗塞、充血性心力衰竭和心脏肥大,无疑是当今美国的主要健康风险。诊断、治疗和支持这些疾病患者的费用达好几万美元。心脏病中两种尤其严重的表现是心肌梗塞和心脏肥大。就心肌梗塞而言,一般是冠状动脉动脉粥样硬化引起的急性血小板性冠状动脉闭塞,导致心肌细胞死亡。因为心肌细胞,即心脏肌肉细胞是终末分化细胞,通常不能细胞分裂,所以当它们在急性心肌梗塞过程中死亡时,常被疤痕组织代替。疤痕组织不具有收缩性,无助于心脏功能,但经常由于在心脏收缩期扩张或由于心室大小和有效半径增大,例如变肥大而对心脏功能起到有害作用。就心脏肥大而言,一种理论认为它类似于发育异常疾病,并由此提出了心脏中的发育信号可否促使肥大疾病发生的问题。心脏肥大是心脏对基本上所有心脏病,包括由高血压、机械载荷、心肌梗塞、心律失常、内分泌紊乱和心脏收缩蛋白基因中的遗传突变引起的心脏病的适应性反应。虽然肥大反应刚开始是增加心输出的补偿机制,但是持续性肥大可导致DCM、心力衰竭和猝死。在美国,每年将近五十万人被诊断为心力衰竭,死亡率接近50%。Heart disease and its manifestations, including coronary artery disease, myocardial infarction, congestive heart failure, and cardiac hypertrophy, are undoubtedly the leading health risks in the United States today. The cost of diagnosing, treating, and supporting patients with these diseases can run into the tens of thousands of dollars. Two particularly serious manifestations of heart disease are myocardial infarction and cardiac hypertrophy. In the case of myocardial infarction, it is generally acute platelet coronary artery occlusion caused by coronary atherosclerosis, resulting in myocardial cell death. Because cardiomyocytes, the heart muscle cells, are terminally differentiated cells that are generally incapable of cell division, when they die during an acute myocardial infarction, they are often replaced by scar tissue. Scar tissue is non-contractile and does not contribute to cardiac function, but often has a detrimental effect on cardiac function due to dilation during systole or due to an increase in the size and effective radius of the ventricles, eg, hypertrophy. In the case of cardiac hypertrophy, one theory is that it resembles dysplastic diseases, raising the question of whether developmental signals in the heart could contribute to hypertrophic diseases. Cardiac hypertrophy is the adaptive response of the heart to essentially all cardiac diseases, including those caused by hypertension, mechanical loading, myocardial infarction, cardiac arrhythmias, endocrine disturbances, and genetic mutations in the cardiac contractile protein gene. Although the hypertrophic response initially acts as a compensatory mechanism to increase cardiac output, persistent hypertrophy can lead to DCM, heart failure, and sudden death. In the United States, nearly half a million people are diagnosed with heart failure each year, with a mortality rate approaching 50%.
心脏肥大的原因和结果已有广泛记载,但还未阐明其背后的分子机制。对这些机制的理解主要关系到预防和治疗心脏病,并将成为设计特异性靶向心脏肥大和心力衰竭的药物中的主要治疗方式。因为病理性心脏肥大一般不产生任何症状,直到心脏损伤严重到足以产生心力衰竭,伴随心力衰竭的心肌病症状。这些症状包括呼吸急促、运动疲劳、不变成急促呼吸就不能躺平(端坐呼吸)、阵发性夜间呼吸困难、心脏尺寸增大和/或小腿水肿。病人经常伴有血压升高、额外心音、心脏杂音、肺和全身血栓、胸痛、肺充血和心悸。此外,DCM引起射血分数(即一种固有心脏收缩功能和重建的心脏收缩功能的测量方法)降低。该疾病的特征还有心室扩张和因心肌收缩力减弱而使心脏收缩功能严重受损,这在很多病人中导致扩张性心力衰竭。由于肌细胞/心肌功能障碍,患病的心脏也进行细胞/腔室重建,这导致“DCM表型”。随着疾病的发展,这些症状也不断发展。DCM病人发生威胁生命的心律不齐,包括心室心动过速和心室肌纤维震颤的发病率也大大增加。在这些病人中,昏厥发作(头昏)被认为是猝死的先兆。The causes and consequences of cardiac hypertrophy have been extensively documented, but the underlying molecular mechanisms have not been elucidated. Understanding these mechanisms is primarily relevant to the prevention and treatment of heart disease and will be a major therapeutic modality in the design of drugs specifically targeting cardiac hypertrophy and heart failure. Because pathological cardiac hypertrophy generally does not produce any symptoms until the damage to the heart is severe enough to produce heart failure, symptoms of cardiomyopathy accompany heart failure. These symptoms include shortness of breath, fatigue with exercise, inability to lie flat without becoming short of breath (orthopnea), paroxysmal nocturnal dyspnea, increased heart size, and/or calf edema. Patients often present with elevated blood pressure, extra heart sounds, heart murmurs, pulmonary and systemic thrombosis, chest pain, pulmonary congestion, and palpitations. In addition, DCM causes a decrease in ejection fraction, a measure of intrinsic and reconstituted systolic function of the heart. The disease is also characterized by ventricular dilatation and severe impairment of systolic function due to weakened myocardial contractility, leading to dilated heart failure in many patients. Diseased hearts also undergo cellular/chamber remodeling due to myocyte/cardiac dysfunction, which results in the "DCM phenotype". These symptoms evolve as the disease progresses. DCM patients also have a greatly increased incidence of life-threatening arrhythmias, including ventricular tachycardia and ventricular fibrillation. In these patients, fainting episodes (dizziness) are considered to be a precursor to sudden death.
扩张性心肌病的诊断一般依赖于证明心腔尺寸扩大,尤其是心室扩大。通常可以在胸部X光片上观察到扩大,但用超声心动图评价更准确。DCM常难以与急性心肌炎、瓣膜性心脏病、冠状动脉疾病和高血压性心脏病相区分。一旦作出扩张性心肌病的诊断,利用各种方法鉴定和治疗有可能逆转病原,并防止进一步心脏损伤。例如,必须排除冠状动脉疾病和瓣膜性心脏病。需要关注和控制贫血、异常心动过速、营养不良、酗酒、甲状腺病和/或其它问题。The diagnosis of dilated cardiomyopathy generally relies on evidence of enlarged cardiac chambers, especially ventricular enlargement. Enlargement is usually seen on chest x-ray but is more accurately evaluated with echocardiography. DCM is often indistinguishable from acute myocarditis, valvular heart disease, coronary artery disease, and hypertensive heart disease. Once dilated cardiomyopathy is diagnosed, identification and treatment using various methods has the potential to reverse the cause and prevent further cardiac damage. For example, coronary artery disease and valvular heart disease must be ruled out. Anemia, abnormal tachycardia, malnutrition, alcoholism, thyroid disease, and/or other problems require attention and management.
如上所述,用药物治疗仍然代表了减少或消除心力衰竭表现的主要机制。利尿剂构成轻度至中度心力衰竭的一线治疗药物。不幸的是,很多常用的利尿剂(如噻嗪类)具有很多副作用。例如,某些利尿剂可增加血清胆固醇和甘油三酯。而且,利尿剂对严重心力衰竭病人通常是无效的。As mentioned above, treatment with drugs still represents the main mechanism for reducing or eliminating the manifestations of heart failure. Diuretics constitute the first-line treatment for mild to moderate heart failure. Unfortunately, many commonly used diuretics (eg, thiazides) have many side effects. For example, certain diuretics can increase serum cholesterol and triglycerides. Also, diuretics are usually ineffective in patients with severe heart failure.
如果利尿剂无效,则可用血管舒张剂;血管紧张素转化(ACE)抑制剂(例如,依那普利和赖诺普利)不仅能使症状缓解,也有报道称它们能降低死亡率(Young等,1989)。然而,ACE抑制剂也伴有副作用,导致它们禁忌用于某些疾病(例如,肾动脉狭窄)的患者。类似地,变力剂药物治疗(即通过增加心肌肌肉收缩力而提高心脏输出的药物)伴有很多不良反应,包括胃肠道不良反应和中枢神经系统功能障碍。If diuretics are ineffective, vasodilators can be used; angiotensin-converting (ACE) inhibitors (eg, enalapril and lisinopril) not only provide symptom relief, they have also been reported to reduce mortality (Young et al., 1989). However, ACE inhibitors are also associated with side effects that make their use contraindicated in patients with certain diseases (eg, renal artery stenosis). Similarly, inotropic drug therapy (ie, drugs that increase cardiac output by increasing myocardial muscle contractility) is associated with a number of adverse effects, including gastrointestinal adverse effects and central nervous system dysfunction.
因此,当前使用的药物在特定病人群体中具有严重的缺点。取得新的安全有效的药物无疑将使不能使用现有药物治疗方式或不能从这些方式获得充分缓解的病人受益。DCM病人的预后是可变的,取决于心室功能障碍的程度,多数死亡发生在诊断后五年内。Therefore, currently used drugs have serious disadvantages in certain patient populations. Access to new safe and effective medicines will undoubtedly benefit patients who cannot use or obtain adequate relief from existing drug treatment modalities. The prognosis of patients with DCM is variable and depends on the degree of ventricular dysfunction, with most deaths occurring within five years of diagnosis.
I.本发明的内容I. Contents of the present invention
本发明者先前已经证明,通过MAP激酶磷酸化MEF2羧基端活化结构域中的三个保守位点可活化MEF2(参见,Katoh等1998)。CaMK信号也通过磷酸化II类HDAC而激活MEF2,而II类HDAC在成人心脏中高水平表达,它们可以抑制心脏的MEF2活性。一旦磷酸化,这些HDAC就与14-3-3结合,并与MEF2分离,导致移位到细胞核中,激活MEF2依赖性转录。不能被磷酸化的II类HDAC突变体不能从MEF2上解离,从而不可逆地阻断MEF2靶基因的表达。The present inventors have previously demonstrated that phosphorylation of three conserved sites in the carboxy-terminal activation domain of MEF2 by MAP kinase activates MEF2 (see, Katoh et al. 1998). CaMK signaling also activates MEF2 by phosphorylating class II HDACs, which are highly expressed in the adult heart, and they inhibit cardiac MEF2 activity. Once phosphorylated, these HDACs associate with 14-3-3 and dissociate from MEF2, leading to translocation into the nucleus and activation of MEF2-dependent transcription. Class II HDAC mutants that cannot be phosphorylated cannot dissociate from MEF2, thereby irreversibly blocking the expression of MEF2 target genes.
也证明,在体外对各种信号转导途径反应中,编码HDAC5的不可磷酸化突变体的腺病毒能够防止心肌细胞肥大(Lu等,2000)。这些发现提示II类HDAC中这些保守位点的磷酸化是引起心脏肥大的必需步骤。发现还提示通过隔离和靶向负责磷酸化II类HDAC的激酶而抑制II类HDAC的磷酸化,从而阻断肥大和后续心力衰竭的发展。Adenovirus encoding a non-phosphorylatable mutant of HDAC5 was also shown to prevent cardiomyocyte hypertrophy in response to various signal transduction pathways in vitro (Lu et al., 2000). These findings suggest that phosphorylation of these conserved sites in class II HDACs is an essential step in causing cardiac hypertrophy. The findings also suggest that inhibiting the phosphorylation of class II HDACs by sequestering and targeting the kinase responsible for their phosphorylation blocks hypertrophy and subsequent development of heart failure.
本发明者在本文中描述了PKD作为负责磷酸化II类HDAC、介导它们与MEF-2的相互作用和部分控制II类HDAC的核或胞浆定位的激酶的特征。本发明还提出通过抑制PKD治疗性干预心脏肥大和心力衰竭,以及筛选用于治疗心脏肥大和心力衰竭治疗药物的方法。The inventors describe herein the characterization of PKD as the kinase responsible for phosphorylating class II HDACs, mediating their interaction with MEF-2 and partially controlling the nuclear or cytoplasmic localization of class II HDACs. The invention also proposes therapeutically intervening cardiac hypertrophy and heart failure by inhibiting PKD, and screening methods for treating cardiac hypertrophy and heart failure.
II.蛋白激酶II. Protein Kinases
激酶通过将磷酸基团加入蛋白调节很多不同细胞的增殖、分化和信号转导过程。不受控制的信号转导与各种疾病,包括炎症、癌症、动脉粥样硬化、牛皮癣和心脏疾病和肥大有关。可逆性蛋白磷酸化是控制真核细胞活性的主要策略。据估计,在典型的哺乳动物细胞中,10,000个活性蛋白中有1000个以上是磷酸化的。通常,蛋白激酶驱动激活的高能磷酸根从三磷酸腺苷分子(ATP)转移到某特定蛋白上,而蛋白磷酸酶从该蛋白去除高能磷酸。在对胞外信号(激素、神经递质、生长和分化因子等)、细胞周期检查点、和环境或营养应激反应中发生的磷酸化,大概类似于打开分子开关。当开关打开时,合适的蛋白激酶即激活代谢酶、调节蛋白、受体、细胞骨架蛋白、离子通道或离子泵,或转录因子。Kinases regulate proliferation, differentiation and signal transduction processes in many different cells by adding phosphate groups to proteins. Uncontrolled signaling has been linked to a variety of diseases, including inflammation, cancer, atherosclerosis, psoriasis, and heart disease and hypertrophy. Reversible protein phosphorylation is a major strategy for controlling the activity of eukaryotic cells. It is estimated that more than 1000 out of 10,000 active proteins are phosphorylated in a typical mammalian cell. Typically, protein kinases drive the transfer of activated high-energy phosphates from the adenosine triphosphate molecule (ATP) to a specific protein, while protein phosphatases remove high-energy phosphates from the protein. Phosphorylation occurs in response to extracellular signals (hormones, neurotransmitters, growth and differentiation factors, etc.), cell cycle checkpoints, and environmental or nutritional stress, presumably analogous to turning on a molecular switch. When the switch is turned on, the appropriate protein kinase activates a metabolic enzyme, regulatory protein, receptor, cytoskeletal protein, ion channel or pump, or transcription factor.
激酶是最大的已知蛋白群体,是具有很大的不同功能和特异性的酶超家族。它们通常按它们的底物、它们调节的分子或突变表型的某些方面来命名。按照底物,可以将蛋白激酶粗略地分成两组;磷酸化酪氨酸残基的激酶(蛋白酪氨酸激酶,PTK)和磷酸化丝氨酸或苏氨酸残基的激酶(丝氨酸/苏氨酸激酶,STK)。一些蛋白激酶具有双重特异性,磷酸化苏氨酸和酪氨酸残基。几乎所有激酶都含有类似的250-300个氨基酸的催化域。含有亚域I-IV的N-末端域通常折叠成双叶结构,该结构能结合ATP(或GTP)供体分子,并确定其方向。含有亚域V IA-XI的较大C末端叶,能与蛋白底物结合,并将γ磷酸根从ATP转移到丝氨酸、苏氨酸或酪氨酸残基的羟基上。亚域V跨越两叶。Kinases are the largest known group of proteins and are a superfamily of enzymes with widely varying functions and specificities. They are often named after their substrates, the molecules they regulate, or some aspect of the mutant phenotype. According to their substrates, protein kinases can be roughly divided into two groups; kinases that phosphorylate tyrosine residues (protein tyrosine kinases, PTKs) and kinases that phosphorylate serine or threonine residues (serine/threonine Kinase, STK). Some protein kinases have dual specificity, phosphorylating both threonine and tyrosine residues. Almost all kinases contain a similar catalytic domain of 250-300 amino acids. The N-terminal domain containing subdomains I-IV usually folds into a bilobed structure that binds and orients the ATP (or GTP) donor molecule. The larger C-terminal lobe, containing subdomains VIA-XI, binds protein substrates and transfers the γ-phosphate from ATP to the hydroxyl groups of serine, threonine, or tyrosine residues. Subdomain V spans two lobes.
可根据位于激酶域任一侧或插入激酶域环中的不同氨基酸序列(通常5至100个残基)将激酶分类为家族。这些加入的氨基酸序列可以调节各激酶,因为它识别靶蛋白并与其相互作用。激酶域的一级结构保守,可以进一步再分成11个亚域。这11个亚域各自含有特异性残基和基序,或氨基酸模式,它们是该亚域的特征并高度保守(Hardie和Hanks,1995)。Kinases can be classified into families based on the distinct amino acid sequences (typically 5 to 100 residues) located on either side of the kinase domain or inserted in a loop of the kinase domain. These added amino acid sequences can regulate the respective kinase as it recognizes and interacts with the target protein. The primary structure of the kinase domain is conserved and can be further subdivided into 11 subdomains. Each of these 11 subdomains contains specific residues and motifs, or patterns of amino acids, that are characteristic of that subdomain and are highly conserved (Hardie and Hanks, 1995).
第二信使依赖性蛋白激酶主要介导第二信使如环化AMP(cAMP)、环化GMP、三磷酸肌醇、磷脂酰肌醇、3,4,5-三磷酸、环化ADP核糖、花生四烯酸、甘油二酯和钙-钙调蛋白的作用。环化AMP依赖性蛋白激酶(PKA)是STK家族的重要成员。在所有已经研究的原核和动物细胞中,环化AMP都是激素作用的胞内介质。这些激素诱导的细胞反应包括甲状腺激素分泌、皮质醇分泌、孕酮分泌、糖原降解、骨再吸收及心率和心肌收缩力的调节。在所有动物细胞中都发现了PKA,认为它是环化AMP在大多数这些细胞中起作用的原因。PKA表达的改变与各种不适和疾病,包括癌症、甲状腺机能障碍、糖尿病、动脉粥样硬化和心血管疾病相关(Isselbacher等,1994)。Second messenger-dependent protein kinases mainly mediate second messengers such as cyclic AMP (cAMP), cyclic GMP, inositol triphosphate, phosphatidylinositol, 3,4,5-triphosphate, cyclized ADP ribose, peanut Effects of tetraenoic acid, diglycerides, and calcium-calmodulin. Cyclic AMP-dependent protein kinase (PKA) is an important member of the STK family. In all prokaryotic and animal cells studied, cyclic AMP is an intracellular mediator of hormone action. These hormone-induced cellular responses include thyroid hormone secretion, cortisol secretion, progesterone secretion, glycogen degradation, bone resorption, and regulation of heart rate and myocardial contractility. PKA is found in all animal cells and is thought to be responsible for the action of cyclic AMP in most of these cells. Altered expression of PKA is associated with various disorders and diseases, including cancer, thyroid dysfunction, diabetes, atherosclerosis and cardiovascular disease (Isselbacher et al., 1994).
钙-钙调蛋白(CaM)依赖性蛋白激酶也是STK家族的成员。钙调蛋白是钙受体,它在对钙结合的反应中通过结合于靶蛋白而介导了很多钙调节过程。这些过程中的主要靶蛋白是CaM依赖性蛋白激酶。CaM-激酶参与平滑肌收缩(MLC激酶)、糖原降解(磷酸化酶激酶)和神经传递(CaM激酶I和CaM激酶II)的调节。CaM激酶I能磷酸化各种底物,包括神经递质相关的蛋白突触蛋白I和II、基因转录调节蛋白、CREB和囊性纤维化传导调节蛋白、CFTR(Haribabu等、1995)。CaMII激酶也在不同位点磷酸化突触蛋白,并通过磷酸化和激活酪氨酸羟化酶控制脑中儿茶酚胺的合成。除了结合于CAM以外,很多CaM激酶被磷酸化激活。激酶可自身磷酸化,或被另一激酶磷酸化,作为“激酶级联反应”的一部分。Calcium-calmodulin (CaM)-dependent protein kinases are also members of the STK family. Calmodulin is a calcium receptor that mediates many calcium regulatory processes by binding to target proteins in response to calcium binding. The main target proteins in these processes are CaM-dependent protein kinases. CaM-kinases are involved in the regulation of smooth muscle contraction (MLC kinase), glycogen degradation (phosphorylase kinase) and neurotransmission (CaM kinase I and CaM kinase II). CaM kinase I phosphorylates a variety of substrates, including the neurotransmitter-associated proteins synapsin I and II, gene transcription regulator protein, CREB and cystic fibrosis conduction regulator protein, CFTR (Haribabu et al., 1995). CaMII kinase also phosphorylates synaptic proteins at different sites and controls the synthesis of catecholamines in the brain by phosphorylating and activating tyrosine hydroxylase. In addition to binding to CAM, many CaM kinases are activated by phosphorylation. A kinase can be phosphorylated by itself, or by another kinase, as part of a "kinase cascade".
另一配体活化的蛋白激酶是5’-AMP-活化的蛋白激酶(AMPK)(Gao等,1996)。哺乳动物AMPK通过磷酸化乙酰辅酶A羧化酶和羟甲基戊二酰辅酶A还原酶调节脂肪酸和甾醇合成,并介导这些途径对细胞应激,如热激和葡萄糖和ATP消耗的反应。AMPK是一种异质三聚复合物,由催化α亚基和两个非催化性β和γ亚基构成,这两个非催化亚基被认为能调节α亚基的活性。AMPK的亚基比预期的更广泛分布于非生脂组织,如脑、心脏、脾和肺。此分布提示,它的作用可能不仅是调节脂类代谢。Another ligand-activated protein kinase is 5'-AMP-activated protein kinase (AMPK) (Gao et al., 1996). Mammalian AMPK regulates fatty acid and sterol synthesis by phosphorylating acetyl-CoA carboxylase and hydroxymethylglutaryl-CoA reductase and mediates the response of these pathways to cellular stresses such as heat shock and glucose and ATP depletion. AMPK is a heterotrimeric complex composed of a catalytic α subunit and two noncatalytic β and γ subunits, which are thought to regulate the activity of the α subunit. The subunits of AMPK are more widely distributed than expected in non-adipogenic tissues such as brain, heart, spleen and lung. This distribution suggests that its role may go beyond regulating lipid metabolism.
有丝分裂原-活化的蛋白激酶(MAP)也是STK家族的成员。MAP激酶也调节胞内信号转导途径。它们通过磷酸化级联反应介导信号从细胞表面转导到核中。已经鉴定到几个亚群,各自表现出不同的底物特异性和对不同胞外刺激的反应(Egan和Weinberg,1993)。MAP激酶的信号转导途径存在于哺乳动物细胞和酵母中。激活哺乳动物途径的胞外刺激物包括表皮生长因子(EGF)、紫外光、高渗透压培养基、热激效应、内毒性脂多糖(LPS)和促炎症细胞因子,如肿瘤坏死因子(TNF)和白介素-1(IL-1)。Mitogen-activated protein kinases (MAPs) are also members of the STK family. MAP kinases also regulate intracellular signal transduction pathways. They mediate signal transduction from the cell surface to the nucleus through a phosphorylation cascade. Several subpopulations have been identified, each exhibiting different substrate specificities and responses to different extracellular stimuli (Egan and Weinberg, 1993). Signal transduction pathways for MAP kinases exist in mammalian cells and yeast. Extracellular stimuli that activate mammalian pathways include epidermal growth factor (EGF), ultraviolet light, hyperosmolar media, heat shock effects, endotoxic lipopolysaccharide (LPS), and pro-inflammatory cytokines such as tumor necrosis factor (TNF) and interleukin-1 (IL-1).
PRK(增殖相关激酶)是一种血清/细胞因子可诱导的STK,参与人巨核细胞细胞周期和细胞增殖的调节(Li等,1996)。PRK涉及STK的polo(获自人polo基因)家族,与细胞分裂相关。PRK在肺肿瘤组织中下调,可能是在正常组织中表达下调导致癌转化的原癌基因。MAP激酶表达的改变与各种疾病,包括癌症、炎症、免疫失调和影响生长和发育的疾病相关。PRK (proliferation-associated kinase) is a serum/cytokine-inducible STK involved in the regulation of human megakaryocyte cell cycle and cell proliferation (Li et al., 1996). PRK is involved in the polo (derived from human polo gene) family of STKs and is involved in cell division. The down-regulation of PRK in lung tumor tissue may be the proto-oncogene whose down-regulation in normal tissue leads to cancer transformation. Altered expression of MAP kinases is associated with various diseases including cancer, inflammation, immune dysregulation and diseases affecting growth and development.
细胞周期蛋白-依赖性蛋白激酶(CDK)是另一组STK,它们通过细胞周期控制细胞发展。细胞周期蛋白是小调节蛋白,通过结合于或激活CDK起作用,然后CDK通过磷酸化和活化参与有丝分裂过程的选择蛋白而触发细胞周期各期。CDK的独特之处在于它们需要多次输入才能被激活。除了与细胞周期蛋白结合之外,CDK活化还需要特定苏氨酸残基的磷酸化和特定酪氨酸残基的去磷酸化。Cyclin-dependent protein kinases (CDKs) are another group of STKs that control cell development through the cell cycle. Cyclins are small regulatory proteins that act by binding to or activating CDKs, which then trigger the cell cycle phases by phosphorylating and activating select proteins involved in the mitotic process. CDKs are unique in that they require multiple inputs to be activated. In addition to binding to cyclins, phosphorylation of specific threonine residues and dephosphorylation of specific tyrosine residues are required for CDK activation.
蛋白酪氨酸激酶PTK,特异性磷酸化它们靶蛋白上的酪氨酸残基,它们可以分为跨膜的、受体PTK和非跨膜的、非受体PTK。跨膜的蛋白-酪氨酸激酶是大多数生长因子的受体。生长因子与该受体的结合激活磷酸基团从ATP转移到该受体和其它特异性蛋白所选酪氨酸侧链上。与受体PTK结合的生长因子(GF)包括;表皮GF、血小板衍生GF、纤维母细胞GF、肝细胞GF、胰岛素和胰岛素样GF、神经GF、血管内皮GF、和巨噬细胞集落刺激因子。Protein tyrosine kinases, PTKs, specifically phosphorylate tyrosine residues on their target proteins, and they can be divided into transmembrane, receptor PTKs and non-transmembrane, non-receptor PTKs. Transmembrane protein-tyrosine kinases are receptors for most growth factors. Binding of growth factors to the receptor activates the transfer of phosphate groups from ATP to selected tyrosine side chains of the receptor and other specific proteins. Growth factors (GFs) that bind receptor PTKs include; epidermal GF, platelet-derived GF, fibroblast GF, hepatocyte GF, insulin and insulin-like GF, neural GF, vascular endothelial GF, and macrophage colony-stimulating factor.
非受体PTK缺少跨膜区,而能与细胞表面受体的胞内区形成了复合物。此受体通过非受体PTK发挥作用,包括细胞因子、激素(人生长激素和促乳素)受体和T和B淋巴细胞上的抗原-特异性受体。Non-receptor PTKs lack transmembrane domains and form complexes with intracellular domains of cell surface receptors. This receptor acts through non-receptor PTKs, including cytokine, hormone (human growth hormone and prolactin) receptors, and antigen-specific receptors on T and B lymphocytes.
很多这些PTK都先鉴定为癌细胞突变癌基因的产物,在癌细胞中它们的活化不再受正常细胞的控制。实际上,大约三分之一的已知癌基因编码PTK,细胞转化(癌发生)经常伴有酪氨酸磷酸化活性升高(Carbonneau和Tonks,1992)是公知的。因此,在控制一些癌症类型中,调节PTK活性是一种重要的策略。Many of these PTKs were first identified as products of mutated oncogenes in cancer cells, where their activation is no longer under normal cell control. Indeed, approximately one-third of known oncogenes encode PTKs, and it is well known that cellular transformation (carcinogenesis) is often accompanied by increased tyrosine phosphorylation activity (Carbonneau and Tonks, 1992). Therefore, modulation of PTK activity is an important strategy in the control of some cancer types.
A.蛋白激酶C家族A. Protein kinase C family
蛋白激酶C(PKC)蛋白是STK家族的成员。蛋白激酶D(PKD)蛋白与佛波酯和甘油二酯结合,与PKC紧密相关(Valverde等,1994)。蛋白激酶C在调节多种胞外受体-介导的信号转导途径细胞反应中,和在调节多种细胞的分化和增殖中起着关键作用。Protein kinase C (PKC) proteins are members of the STK family. The protein kinase D (PKD) protein binds phorbol esters and diglycerides and is closely associated with PKC (Valverde et al., 1994). Protein kinase C plays a key role in regulating cellular responses in a variety of extracellular receptor-mediated signal transduction pathways, and in regulating the differentiation and proliferation of a variety of cells.
蛋白激酶C基因/蛋白可能在多种癌症中起重要作用,因此可以用于药物开发和筛检、诊断、预防和/或治疗各种癌症。例如,已在黑色素瘤细胞系的α-型蛋白激酶C基因内鉴定到了肿瘤特异性缺失(Linnenbach等,1988)。在某些肿瘤细胞系中观察到PKC表达水平升高,这提示PKC在涉及生长控制的信号转导途径中起重要作用(Johannes等,1994)。The protein kinase C gene/protein may play an important role in various cancers, and thus can be used for drug development and screening, diagnosis, prevention and/or treatment of various cancers. For example, tumor-specific deletions have been identified in the alpha-type protein kinase C gene in melanoma cell lines (Linnenbach et al., 1988). Elevated levels of PKC expression were observed in certain tumor cell lines, suggesting that PKC plays an important role in signal transduction pathways involved in growth control (Johannes et al., 1994).
激酶蛋白,特别是蛋白激酶C亚家族成员是药物作用和开发的主要靶。因此,对于药物开发领域,鉴定并表征该激酶蛋白亚家族先前未知的成员是有价值的。本发明通过提供与蛋白激酶C亚家族成员同源并与心血管疾病相关的人激酶蛋白提高了本领域技术水平。在下文中引入作为参考的以下参考文献都描述了可用于本发明的蛋白激酶C抑制剂:美国专利6,528,294;美国专利6,441,020;美国专利6,080,784;美国专利6,043,270;美国专利5,955,501。Kinase proteins, especially members of the protein kinase C subfamily, are major targets for drug action and development. Therefore, identifying and characterizing previously unknown members of this kinase protein subfamily would be valuable to the field of drug discovery. The present invention advances the state of the art by providing human kinase proteins homologous to members of the protein kinase C subfamily and associated with cardiovascular disease. The following references, incorporated by reference hereinafter, all describe protein kinase C inhibitors useful in the present invention: US Patent 6,528,294; US Patent 6,441,020; US Patent 6,080,784; US Patent 6,043,270; US Patent 5,955,501.
B.蛋白激酶DB. Protein Kinase D
PKD家族包括PKD1(小鼠PKD、人PKCμ)、PKD2和PKD3(也称为PKC)。它们是丝氨酸/苏氨酸激酶的AGC家族成员,但共有独特的分子架构,该架构与其它AGC家族成员不同。PKD1有多个结构域:含有高频率无极性氨基酸、主要是丙氨酸和脯氨酸的N-末端区;两个富含半胱氨酸的锌指区(也称为Cla和Clb)、一个富含带负电氨基酸的区域、一个血小板-白细胞C激酶底物-同源(PH)域和一个蛋白Ser/Thr激酶催化域(VanLint等,2002)。在PKD2和PKD3中发现了相似的模块结构。The PKD family includes PKD1 (mouse PKD, human PKCμ), PKD2 and PKD3 (also known as PKC). They are members of the AGC family of serine/threonine kinases, but share a unique molecular architecture that differs from other AGC family members. PKD1 has multiple domains: an N-terminal region containing a high frequency of nonpolar amino acids, mainly alanine and proline; two cysteine-rich zinc finger regions (also known as Cla and Clb), A region rich in negatively charged amino acids, a platelet-leukocyte C kinase substrate-homology (PH) domain, and a protein Ser/Thr kinase catalytic domain (VanLint et al., 2002). A similar modular structure was found in PKD2 and PKD3.
AGC家族包括各种亚类:环化核苷酸-调节的蛋白激酶、PKC、PKB/Akt、G-蛋白偶联的受体激酶(GRK)和核糖体蛋白S6激酶。可以将PKD归类于全新AGC亚类中,因为它看来组合了该家族不同亚类的特征,损害了它在现有亚类之一中的分类地位(VanLint等,2002)。例如,PKD家族的富含半胱氨酸结构域与经典和全新PKC的该结构域类似,而PH域更类似于PKB或GRK家族,而这并未在任何PKC酶中发现。该催化域在结构和功能上如几个标准所判断,都不同于PKC家族和其它AGC家族成员(Hayashi等,1999;Nishikawa等,1997;Sturany等,2001;Valverde等,1994)。首先,与所有其它已知蛋白激酶催化域相比,PKD催化域的氨基酸序列与网原虫(Dictyostelium)的肌球蛋白轻链激酶(MLCK)最相似(41%),与各PKC仅30-35%相似。第二,PKD1具有独特的底物特异性:它并不偏好含有PKC家族优选的基本残基的底物位点,而磷酸化相对于丝氨酸靶位点-5位置上具有亮氨酸的底物(VanLint等,2002)。第三,PKD1对PKC抑制剂GFI和Ro31-8220不敏感。第四,PKD1不具有自身抑制性假底物序列,而PKC家族的大多数成员中都可以发现该序列。因此,虽然历史上将PKD分类为PKC家族成员,但单独分类可能更加合适。The AGC family includes various subclasses: cyclic nucleotide-regulated protein kinase, PKC, PKB/Akt, G-protein coupled receptor kinase (GRK), and ribosomal protein S6 kinase. PKD can be placed in a completely new subclass of AGC because it appears to combine features of different subclasses of this family, compromising its taxonomic status in one of the existing subclasses (VanLint et al., 2002). For example, the cysteine-rich domain of the PKD family is similar to that of classical and novel PKC, whereas the PH domain is more similar to that of the PKB or GRK families, which are not found in any PKC enzymes. This catalytic domain is structurally and functionally distinct from the PKC family and other AGC family members as judged by several criteria (Hayashi et al., 1999; Nishikawa et al., 1997; Sturany et al., 2001; Valverde et al., 1994). First, compared with all other known protein kinase catalytic domains, the amino acid sequence of the PKD catalytic domain is most similar (41%) to myosin light chain kinase (MLCK) of Dictyostelium and only 30-35% similar to each PKC. %resemblance. Second, PKD1 has unique substrate specificity: it does not favor substrate sites containing essential residues preferred by the PKC family, while phosphorylating substrates with leucine at position -5 relative to the serine target site (Van Lint et al., 2002). Third, PKD1 is insensitive to the PKC inhibitors GFI and Ro31-8220. Fourth, PKD1 does not possess an autoinhibitory pseudosubstrate sequence, which can be found in most members of the PKC family. Therefore, although PKD has historically been classified as a member of the PKC family, a separate classification may be more appropriate.
有趣的是,已显示14-3-3蛋白能够调节PKD(VanLint等,2002),HDAC磷酸化后结合于14-3-3蛋白。而且,在成年大鼠心脏组织中发现了PKD(Haworth等,2000)。最后,如上所述,已显示PKD能够磷酸化相对于丝氨酸-5位置上具有亮氨酸的底物(VanLint等,2002),它正好与本发明者在II类HDAC中发现的磷酸化位点位置相对应(未发表)。可以在登录号NM002742处找到人PKD序列。Interestingly, 14-3-3 proteins have been shown to regulate PKD (VanLint et al., 2002), and HDACs bind to 14-3-3 proteins after phosphorylation. Furthermore, PKD has been found in adult rat heart tissue (Haworth et al., 2000). Finally, as mentioned above, PKD has been shown to be able to phosphorylate substrates with a leucine at the position relative to serine-5 (VanLint et al., 2002), which coincides with the phosphorylation site discovered by the inventors in class II HDACs. Positional correspondence (unpublished). The human PKD sequence can be found at accession number NM002742.
C.激酶抑制剂C. Kinase Inhibitors
如上所述,蛋白激酶是人类基因组的重要组成部分,是最基础的胞内信号转导机制之一。因此,控制很多类型细胞中的激酶活性(或缺乏激酶活性)是很多疾病的主要因素,尤其是涉及增生反应炎症的疾病。即使有各种各样的激酶靶(已知道大约500个激酶的序列),但是直到最近,特别为抑制激酶设计的药物才进入市场。即使认识到胞内激酶信号转导非常重要已经有很长时间,但是直到最近,才积累了关于激酶活性和相应的催化机制性质的足够知识,得以开发安全和选择性的激酶抑制药物。例如,GleevecTM(Novartis)和IressaTM(AstraZeneca)是新的和令人振奋的治疗剂类型的开拓性成员,它们现在已经成熟地用于临床。本领域技术人员会理解,有很多蛋白激酶C抑制剂,上述公司具有制造和筛选这些抑制剂的标准方法。因此,在下文中将这些方法和可用于这些系统的已知激酶或化合物引入,作为参考。As mentioned above, protein kinases are an important part of the human genome and one of the most basic intracellular signal transduction mechanisms. Thus, control of kinase activity (or lack thereof) in many types of cells is a major factor in many diseases, especially those involving inflammation of the proliferative response. Even though there is a wide variety of kinase targets (the sequences of about 500 kinases are known), until recently drugs specifically designed to inhibit kinases have entered the market. Even though the importance of intracellular kinase signaling has been recognized for a long time, only recently has sufficient knowledge of the nature of kinase activity and the corresponding catalytic mechanism accumulated to allow the development of safe and selective kinase-inhibiting drugs. For example, Gleevec™ (Novartis) and Iressa™ (AstraZeneca) are pioneering members of a new and exciting class of therapeutic agents that are now well established for clinical use. Those of skill in the art will appreciate that there are many protein kinase C inhibitors and the companies mentioned above have standard methods for making and screening for these inhibitors. Accordingly, these methods and known kinases or compounds useful in these systems are hereafter incorporated by reference.
D.PKD抑制剂D. PKD inhibitors
有报道说白藜芦醇可以抑制PKD(Haworth等,2001),因此可用于本发明的一个实施方式中。其它可能的抑制剂包括但不限于吲哚咔唑、Godecke6976(G6976)、星孢素、K252a、包括[d-Arg(1)、d-Trp(5,7,9)、Leu(11)]SP的物质P(SP)类似物、PKC抑制剂109203X(GF-1)、PKC抑制剂Ro31-8220、PKC抑制剂GO7874、染料木黄酮、特异性Src抑制剂PP-1和PP-2、白屈菜季铵碱、粗糠柴毒素。除了上述的之外,还有抑制基因的普通、非药理方法,下面进行讨论。Resveratrol has been reported to inhibit PKD (Haworth et al., 2001), and thus may be used in one embodiment of the present invention. Other possible inhibitors include, but are not limited to, indolecarbazole, Godecke6976 (G6976), staurosporine, K252a, including [d-Arg(1), d-Trp(5,7,9), Leu(11 )] Substance P(SP) analogs of SP, PKC inhibitor 109203X(GF-1), PKC inhibitor Ro31-8220, PKC inhibitor GO7874, genistein, specific Src inhibitors PP-1 and PP-2 , celandine quaternary ammonium, crude bran wood toxin. In addition to the above, there are common, non-pharmacological approaches to gene inhibition, which are discussed below.
I.核酸I. Nucleic acid
a.反义构建物a. Antisense constructs
反义方法利用了核酸倾向于与“互补”序列配对的优点。互补指那些能够按标准的Watson-Crick互补规则进行碱基配对的那些聚核苷酸。即,较大的嘌呤将与较小的嘧啶进行碱基配对,形成鸟嘌呤配对胞嘧啶(G:C)和在DNA情况下腺嘌啉配对胸腺嘧啶(A:T)、或在RNA情况下腺嘌啉配对尿嘧啶(A:U)的组合。杂交序列中包括的不常见碱基如次黄嘌呤核苷、5-甲基胞嘧啶、6-甲基腺嘌啉、次黄嘌呤和其它碱基不干扰配对。Antisense approaches take advantage of the tendency of nucleic acids to pair with "complementary" sequences. Complementary refers to those polynucleotides capable of base pairing according to the standard Watson-Crick complementarity rules. That is, a larger purine will base pair with a smaller pyrimidine, forming guanine pairing cytosine (G:C) and adenine pairing thymine (A:T) in the case of DNA, or adenine pairing thymine (A:T) in the case of RNA The combination of adenine paired with uracil (A:U). Uncommon bases such as inosine, 5-methylcytosine, 6-methyladenine, hypoxanthine, and others included in the hybridizing sequence do not interfere with pairing.
用聚核苷酸靶向双链(ds)DNA导致三螺旋形成;靶向RNA将导致双螺旋形成。当将反义聚核苷酸引入靶细胞中时,与它们的靶聚核苷酸特异性结合,并干扰转录、RNA加工、转运、翻译和/或稳定性。可采用反义RNA构建物,或编码反义RNA的DNA体内外抑制宿主细胞的基因转录或反义或二者,如在宿主动物,包括人中。Targeting double-stranded (ds) DNA with polynucleotides results in triple helix formation; targeting RNA will result in double helix formation. When introduced into target cells, antisense polynucleotides bind specifically to their target polynucleotides and interfere with transcription, RNA processing, transport, translation and/or stability. Antisense RNA constructs, or DNA encoding antisense RNA, can be used to inhibit gene transcription or antisense or both in host cells in vitro and in vivo, such as in host animals, including humans.
可以设计出能与启动子和其它控制区域、外显子、内含子或甚至基因的外显子-内含子边界结合的反义构建物。考虑到大多数有效的反义构建物将包括与内含子/外显子剪接点互补的区域。因此提议,优选实施方式包括与内含子-外显子剪接点50-200碱基内的区域互补的反义构建物。观察到可将一些外显子序列包括在该构建物中,而并不严重影响它的靶选择性。所包括的外显材料量将因所用具体外显子和内含子序列而不同。人们可以简单地通过体外测试这些构建物,来确定正常的细胞功能是否受到影响或具有互补序列的相关基因的表达是否受到影响,而容易地测试是否包括了过多外显子DNA。Antisense constructs can be designed to bind promoters and other control regions, exons, introns, or even exon-intron boundaries of genes. Consider that most effective antisense constructs will include regions complementary to intron/exon splice junctions. It is therefore proposed that preferred embodiments include antisense constructs complementary to regions within 50-200 bases of the intron-exon splice junction. It was observed that some exon sequences could be included in this construct without seriously affecting its target selectivity. The amount of exonic material included will vary depending on the particular exonic and intronic sequences used. One can easily test for the inclusion of excess exonic DNA by simply testing these constructs in vitro to determine whether normal cellular function is affected or whether the expression of related genes with complementary sequences is affected.
如上所述,“互补”或“反义”指基本上全长互补和只有极少数碱基错配的聚核苷酸序列。例如,当长十五个碱基的序列在十三个或十四个位置上是互补核苷酸时,可称为互补。在自然界,完全互补的序列是在其整个全长上完全互补且没有错配的序列。也考虑其它具有较低同源程度的序列。例如,可以设计具有有限的高度同源区,但也包含非同源区的反义构建物(如核酶;见下)。这些分子,虽然同源性小于50%,仍能在合适的条件下与靶序列结合。As noted above, "complementary" or "antisense" refers to polynucleotide sequences that are substantially complementary throughout their length and have only very few base mismatches. For example, a sequence of fifteen bases in length may be said to be complementary when it has complementary nucleotides at thirteen or fourteen positions. In nature, a perfectly complementary sequence is one that is completely complementary throughout its entire length and has no mismatches. Other sequences with lesser degrees of homology are also contemplated. For example, antisense constructs (such as ribozymes; see below) can be designed with limited regions of high homology, but also containing regions of non-homology. These molecules, although less than 50% homologous, are still able to bind to the target sequence under suitable conditions.
将部分基因组DNA与cDNA或合成序列组合以产生特异性构建物是有益的。例如,最终构建物中需要其内含子时,将需要采用基因组克隆。cDNA或合成的聚核苷酸可为此构建物的剩余部分提供更方便的限制性位点,因此,将用于剩余序列。It is beneficial to combine portions of genomic DNA with cDNA or synthetic sequences to generate specific constructs. For example, genomic cloning will be required when introns are required in the final construct. cDNA or synthetic polynucleotides may provide more convenient restriction sites for the remainder of the construct and, therefore, will be used for the remaining sequence.
ii.核酶ii. Ribozyme
虽然传统上将蛋白用于催化核酸,但发现了另一类可用于该用途的大分子。核酶是以位点特异性方式切割核酸的RNA-蛋白复合物。核酶具有特异性催化域,该域具有核酸内切酶活性(Kim和Cook,1987;Gerlach等,1987;Forster和Symons,1987)。例如,大量核酶能以高度特异性方式加速磷酯转移反应,通常切割寡核苷酸底物中的几种磷酯之一(Cook等,1981;Michel和Westhof,1990;Reinhold-Hurek和Shub,1992)。该特异性归因于底物在化学反应之前,需要通过碱基特异性配对相互作用与核酶的内部指导序列(“IGS”)结合。While proteins have traditionally been used to catalyze nucleic acids, another class of macromolecules has been discovered that can be used for this purpose. Ribozymes are RNA-protein complexes that cleave nucleic acids in a site-specific manner. Ribozymes possess a specific catalytic domain that possesses endonuclease activity (Kim and Cook, 1987; Gerlach et al., 1987; Forster and Symons, 1987). For example, a large number of ribozymes accelerate phospholipid transfer reactions in a highly specific manner, usually cleaving one of several phospholipids in an oligonucleotide substrate (Cook et al., 1981; Michel and Westhof, 1990; Reinhold-Hurek and Shub , 1992). This specificity is due to the need for the substrate to bind to the internal guide sequence ("IGS") of the ribozyme via base-specific pairing interactions prior to chemical reaction.
起初,核酶的催化作用被视为是涉及核酸的序列-特异性切割/连接反应的一部分(Joyce,1989;Cook等,1981)。例如,美国专利5,354,855报道,某些核酶可充当核酸内切酶,其序列特异性高于已知的核糖核酸酶,接近DNA限制性酶的特异性。因此,序列特异性核酶-介导的基因表达抑制可能尤其适合于治疗应用(Scanlon等,1991;Sarver等,1990)。最近报道,在加入核酶的某些细胞系中,核酶引起遗传改变;被改变的基因包括癌基因H-ras、c-fos和HIV基因。这些工作的大部分涉及基于特异性核酶切割的特异性突变密码子,所发生的靶mRNA修饰。Initially, catalysis by ribozymes was considered part of a sequence-specific cleavage/ligation reaction involving nucleic acids (Joyce, 1989; Cook et al., 1981). For example, US Patent No. 5,354,855 reports that certain ribozymes can act as endonucleases with sequence specificity higher than known ribonucleases and approaching that of DNA restriction enzymes. Therefore, sequence-specific ribozyme-mediated inhibition of gene expression may be particularly suitable for therapeutic applications (Scanlon et al., 1991; Sarver et al., 1990). It has recently been reported that ribozymes cause genetic changes in certain cell lines to which ribozymes have been added; the altered genes include the oncogenes H-ras, c-fos, and HIV genes. Most of these works involve the modification of target mRNAs based on specific mutated codons cleaved by specific ribozymes.
iii.RNAiiii. RNAi
RNA干扰(也称为“RNA-介导的干扰”或RNAi)是一种降低或消除基因表达的机制。据观察,双链RNA(dsRNA)介导此种降低,它是一个多步过程。dsRNA激活转录后基因表达监督机制,它的功能是保护细胞免受病毒感染和转位子活性(Fire等,1998;Grishok等,2000;Ketting等,1999;Lin和Avery等,1999;Montgomery等,1998;Sharp和Zamore,2000;Tabara等,1999)。这些机制的活化靶向成熟的dsRNA-互补mRNA,使其破坏。RNAi对研究基因功能提供了大量实验优点。这些优点包括非常高的特异性、容易通过细胞膜和靶基因下调时间长(Fire等,1998;Grishok等,2000;Ketting等,1999;Lin和Avery等,1999;Montgomery等,1998;Sharp等,1999;Sharp和Zamore,2000;Tabara等,1999)。而且,dsRNA显示能使广大系统,包括植物、原生生物、真菌、秀丽新小杆线虫、锥虫(Trypanasoma)、果蝇和哺乳动物中的基因沉默(Grishok等,2000;Sharp等,1999;Sharp和Zamore,2000;Elbashir等,2001)。广泛接受的是,RNAi在转录后起作用,靶向RNA转录物使其降解。看来核和胞浆RNA都可能是其靶(Bosher和Labouesse,2000)。RNA interference (also known as "RNA-mediated interference" or RNAi) is a mechanism that reduces or eliminates gene expression. This reduction was observed to be mediated by double-stranded RNA (dsRNA), which is a multi-step process. dsRNA activates post-transcriptional gene expression surveillance mechanisms that function to protect cells from viral infection and transposon activity (Fire et al., 1998; Grishok et al., 2000; Ketting et al., 1999; Lin and Avery et al., 1999; Montgomery et al., 1998 ; Sharp and Zamore, 2000; Tabara et al., 1999). Activation of these mechanisms targets the mature dsRNA-complementary mRNA for destruction. RNAi offers a number of experimental advantages for studying gene function. These advantages include very high specificity, easy passage through the cell membrane, and prolonged downregulation of target genes (Fire et al., 1998; Grishok et al., 2000; Ketting et al., 1999; Lin and Avery et al., 1999; Montgomery et al., 1998; Sharp et al., 1999 ; Sharp and Zamore, 2000; Tabara et al., 1999). Moreover, dsRNA has been shown to silence genes in a wide variety of systems, including plants, protists, fungi, C. elegans, Trypanasoma, Drosophila, and mammals (Grishok et al., 2000; Sharp et al., 1999; Sharp and Zamore, 2000; Elbashir et al., 2001). It is widely accepted that RNAi acts post-transcriptionally, targeting RNA transcripts for degradation. Both nuclear and cytoplasmic RNA appear to be possible targets (Bosher and Labouesse, 2000).
必须设计siRNA使其特异性和有效地抑制感兴趣基因的表达。选择靶序列的方法,即siRNA将降解机器导向感兴趣基因中存在的那些序列的方法,涉及避免可能干扰siRNA导向功能的序列同时包括对所述基因具有特异性的序列。一般,siRNA靶序列长约21至23核苷酸是最有效的。该长度反映了上述长得多的RNA加工产生的消化产物的长度(Montgomery等,1998)。siRNA must be designed to specifically and efficiently inhibit the expression of the gene of interest. The method of selecting target sequences, ie, the method by which the siRNA directs the degradation machinery to those sequences present in the gene of interest, involves avoiding sequences that might interfere with the targeting function of the siRNA while including sequences specific for the gene in question. Generally, siRNA target sequences that are about 21 to 23 nucleotides in length are most effective. This length reflects the length of the digestion product produced by the much longer RNA processing described above (Montgomery et al., 1998).
主要通过直接化学合成;通过与果蝇胚胎裂解物接触加工较长的双链RNA;或通过获自S2细胞的体外系统制备siRNA。细胞裂解物或体外加工的用途还可包括后来从裂解物中分离短的21-23个核苷酸siRNA,实施该过程有些麻烦和昂贵。化学合成过程是制备两种单链RNA-寡聚物,然后使这两种单链寡聚物退火成为双链RNA。化学合成方法是不同的。特意引入本文作为参考的美国专利5,889,136、4,415,723和4,458,066和Wincott等(1995)中提供了非限制性例子。Mainly by direct chemical synthesis; processing of longer double-stranded RNAs by contact with Drosophila embryo lysates; or preparation of siRNAs by in vitro systems obtained from S2 cells. The use of cell lysates or in vitro processing can also include subsequent isolation of short 21-23 nucleotide siRNAs from the lysates, which is somewhat cumbersome and expensive to perform. The process of chemical synthesis is to prepare two single-stranded RNA-oligos, which are then annealed to double-stranded RNA. The chemical synthesis methods are different. Non-limiting examples are provided in US Patent Nos. 5,889,136, 4,415,723 and 4,458,066 and Wincott et al. (1995), expressly incorporated herein by reference.
提出对siRNA序列进行几种进一步修饰,以改变其稳定性或提高其有效性。提出含有双-核苷酸突出端(即19个互补核苷酸+3’非互补二聚物)的合成性互补21-merRNA可提供最高的抑制水平。这些方法主要采用两个(2’-脱氧)胸腺嘧啶核苷酸的序列作为双-核苷酸突出端。这些双核苷酸突出端经常写作dTdT,以使其区别于掺入RNA中的典型核苷酸。文献表明,降低化学合成RNA成本的需要从根本上推动了dT突出端的使用。也提出dTdT突出端应该比UU突出端更稳定,虽然可得到的数据显示,与含有UU突出端的siRNA相比,dTdT突出端仅有轻微提高(<20%)。Several further modifications to the siRNA sequences were proposed to alter their stability or increase their effectiveness. It was suggested that a synthetic complementary 21-merRNA containing a two-nucleotide overhang (i.e. 19 complementary nucleotides + 3' non-complementary dimer) provided the highest level of inhibition. These methods mainly employ a sequence of two (2'-deoxy) thymidine nucleotides as a di-nucleotide overhang. These dinucleotide overhangs are often written dTdT to distinguish them from typical nucleotides incorporated into RNA. The literature suggests that the need to reduce the cost of chemically synthesized RNA fundamentally drives the use of dT overhangs. It was also suggested that dTdT overhangs should be more stable than UU overhangs, although available data showed only a slight increase (<20%) in dTdT overhangs compared to siRNAs containing UU overhangs.
据发现,当化学合成的siRNA在细胞培养中浓度为25-100nM时,它们工作得最好,但浓度为约100nM可达到有效抑制哺乳动物细胞哺乳动物的表达。在哺乳动物细胞培养中约100nM的siRNA最为有效。然而,在几种情况下,使用了较低浓度的化学合成siRNA(Caplen,等,2000;Elbashir等,2001)。Chemically synthesized siRNAs have been found to work best when they are present in cell culture at concentrations of 25-100 nM, but concentrations of about 100 nM can achieve effective inhibition of mammalian expression in mammalian cells. siRNA is most effective at about 100 nM in mammalian cell culture. However, in several cases, lower concentrations of chemically synthesized siRNAs were used (Caplen, et al., 2000; Elbashir et al., 2001).
WO99/32619和WO01/68836提出siRNA中所用的RNA可以化学或酶学合成。此二专利内容引入本文作为参考。这些参考文献中所述的酶学合成是,通过用细胞RNA聚合酶或噬菌体RNA聚合酶(例如,T3,T7,SP6)如本领域已知地使用和生产表达构建物。例如,参见美国专利5,795,715。所考虑的构建物提供了产生RNA的模板,该RNA含有与靶基因一部分相同的核苷酸序列。这些参考文献提供的相同序列的长度至少是25个碱基,可长达400个或更多个碱基。该参考文献的一个重要方面是作者考虑用体内的内源性核酸酶复合物将较长dsRNA消化成21-25mer长度,从而将长的dsRNA转变为siRNA。它们没有描述或提供体外合成和使用转录的21-25mer dsRNA的资料。在RNA干扰应用中,化学或酶学合成制备的dsRNA所期望的性能没有任何差别。WO99/32619 and WO01/68836 propose that the RNA used in siRNA can be synthesized chemically or enzymatically. The contents of these two patents are incorporated herein by reference. Enzymatic synthesis described in these references is by using and producing expression constructs with cellular RNA polymerase or phage RNA polymerase (eg, T3, T7, SP6) as known in the art. See, eg, US Patent 5,795,715. Contemplated constructs provide a template for the production of RNA containing a nucleotide sequence identical to a portion of the target gene. These references provide identical sequences that are at least 25 bases in length and can be as long as 400 or more bases. An important aspect of this reference is that the authors considered converting long dsRNAs to siRNAs by digesting the longer dsRNAs to 21-25mer lengths with endogenous nuclease complexes in vivo. They do not describe or provide data on in vitro synthesis and use of transcribed 21-25mer dsRNA. In RNA interference applications, there is no difference in the expected performance of dsRNA produced by chemical or enzymatic synthesis.
类似地,引入本文作为参考的WO00/44914提出,可通过酶学或通过部分/全有机合成产生RNA单链。宜从DNA模板的,优选克隆的cDNA模板的PCR产物酶学合成单链RNA,RNA产物是该cDNA的完整转录物,可包含几百个核苷酸。引入本文作为参考的WO01/36646对siRNA合成的方式没有设任何限制,提出RNA可在体外或体内用手动和/或自动步骤合成。该参考文献还提出体外合成可以是化学方法或酶学方法合成,例如用克隆的RNA聚合酶(例如,T3、T7、SP6)转录内源性DNA(或cDNA)模板,或二法的混合方法。对于RNA干扰应用来说,化学或酶学合成制备的siRNA之间在所需性能上没有差别。Similarly, WO 00/44914, incorporated herein by reference, proposes that RNA single strands can be produced enzymatically or by partial/total organic synthesis. Single-stranded RNA is advantageously synthesized enzymatically from the PCR product of a DNA template, preferably a cloned cDNA template, the RNA product being a complete transcript of the cDNA, which may contain several hundred nucleotides. WO 01/36646, incorporated herein by reference, does not place any restrictions on the manner in which siRNA may be synthesized, proposing that RNA may be synthesized in vitro or in vivo using manual and/or automated steps. This reference also suggests that in vitro synthesis can be chemical or enzymatic, e.g., using cloned RNA polymerases (e.g., T3, T7, SP6) to transcribe endogenous DNA (or cDNA) templates, or a hybrid of the two . For RNA interference applications, there is no difference in the desired properties between siRNAs prepared by chemical or enzymatic synthesis.
美国专利5,795,715报道了在一种反应混合物中同时转录两个互补DNA序列链,该反应中这两个转录物立刻发生杂交。所用模板优选40-100碱基对,两端都装配有启动子序列。该模板能优先附着到固体表面上。用RNA聚合酶转录后,可用产生的dsRNA片段检测和/或测定核酸靶序列。US Patent 5,795,715 reports the simultaneous transcription of two strands of complementary DNA sequences in a reaction mixture in which the two transcripts hybridize immediately. The template used is preferably 40-100 base pairs, and both ends are equipped with promoter sequences. The template is preferentially attached to solid surfaces. Following transcription with RNA polymerase, the resulting dsRNA fragments can be used to detect and/or determine nucleic acid target sequences.
III.组蛋白脱乙酰酶和抑制剂III. Histone deacetylases and inhibitors
核小体,染色质折叠的基本支架,是动态大分子结构,影响染色质溶液的构象(Workman和Kingston,1998)。核小体核心由组蛋白、H2A、HB、H3和H4组成。组蛋白乙酰化产生核小体和使核小体重排,表现为生物物理性质的改变。组蛋白乙酰基转移酶(HAT)和脱乙酰酶(HDAC)的活性之间的平衡决定组蛋白的乙酰化水平。乙酰化的组蛋白引起染色质松弛和激活基因转录,而脱乙酰化的染色质通常无转录活性。Nucleosomes, the basic scaffolds for chromatin folding, are dynamic macromolecular structures that affect the conformation of chromatin solutions (Workman and Kingston, 1998). The nucleosome core is composed of histones, H2A, HB, H3 and H4. Histone acetylation produces and rearranges nucleosomes, manifesting as changes in biophysical properties. The balance between the activities of histone acetyltransferases (HATs) and deacetylases (HDACs) determines the level of acetylation of histones. Acetylated histones cause chromatin relaxation and activate gene transcription, whereas deacetylated chromatin is usually transcriptionally inactive.
已克隆了脊椎生物的十一种不同HDAC。最先鉴定的三种人HDAC是HDAC1、HDAC2和HDAC3(称为I类人HDAC),HDAC8(Vanden Wyngaert等,2000)也加入此列。近年来,克隆并鉴定了(Grozinger等,1999;Zhou等2001;Tong等,2002)II类人HDAC:HDAC4、HDAC5、HDAC6、HDAC7、HDAC9和HDAC10(Kao等,2000)。此外,已经鉴定了HDAC11,但尚未将其归为I类或II类(Gao等,2002)。所有这些HDAC的催化区都有同源性。然而,HDAC4、5、7、9和10具有独特的氨基端延伸,这在其它HDAC中并未发现。该氨基端区域包含MEF2-结合域。已显示HDAC4、5和7参与心脏基因表达的调节,在具体实施方式中,抑制MEF2转录活性。还不完全了解II类HDAC抑制MEF2活性的确切机制。一种可能性是HDAC与MEF2的结合竞争性地或通过使天然的有转录活性的MEF2构象不稳定而抑制MEF2转录活性。也可能II类HDAC需要与MEF2二聚化,以将HDAC定位于或置于组蛋白附近,以进行脱乙酰化。Eleven different HDACs from vertebrates have been cloned. The first three human HDACs identified were HDAC1, HDAC2 and HDAC3 (referred to as class I human HDACs), to which HDAC8 (Vanden Wyngaert et al., 2000) was also added. In recent years, class II human HDACs were cloned and characterized (Grozinger et al., 1999; Zhou et al., 2001; Tong et al., 2002): HDAC4, HDAC5, HDAC6, HDAC7, HDAC9 and HDAC10 (Kao et al., 2000). Furthermore, HDAC11 has been identified but not yet classified as class I or II (Gao et al., 2002). All of these HDACs share homology in their catalytic domains. However, HDAC4, 5, 7, 9 and 10 have a unique amino-terminal extension that is not found in other HDACs. This amino-terminal region contains the MEF2-binding domain. HDAC4, 5 and 7 have been shown to be involved in the regulation of cardiac gene expression and, in a specific embodiment, inhibit MEF2 transcriptional activity. The exact mechanism by which class II HDACs inhibit MEF2 activity is not fully understood. One possibility is that HDAC binding to MEF2 inhibits MEF2 transcriptional activity competitively or by destabilizing the native transcriptionally active MEF2 conformation. It is also possible that class II HDACs are required to dimerize with MEF2 to localize HDACs on or near histones for deacetylation.
已经鉴定到各种组蛋白脱乙酰酶的抑制剂。建议广泛应用,但主要集中在癌症治疗上。Saunders等(1999);Jung等(1997);Jung等(1999);Vigushin等(1999);Kim等(1999);Kitazomo等(2001);Vigusin等(2001);Hoffmann等(2001);Kramer等(2001);Massa等(2001);Komatsu等(2001);Han等(2001)。这些疗法是NIH负责的I期临床试验的主题,用于实体瘤和非霍金奇氏淋巴瘤。HDAC也提高转基因的转录,因此构成了可能的基因治疗辅助方法。Yamano等(2000);Su等(2000)。Inhibitors of various histone deacetylases have been identified. It is recommended for broad application, but is mainly focused on cancer treatment. Saunders et al. (1999); Jung et al. (1997); Jung et al. (1999); Vigushin et al. (1999); Kim et al. (1999); Kitazomo et al. (2001); Vigusin et al. (2001); (2001); Massa et al. (2001); Komatsu et al. (2001); Han et al. (2001). These therapies are the subject of phase I clinical trials administered by the NIH in solid tumors and non-Hodgkin's lymphoma. HDACs also increase the transcription of transgenes and thus constitute a possible adjunct approach to gene therapy. Yamano et al. (2000); Su et al. (2000).
可通过各种不同机制-蛋白、肽和核酸(包括反义和RNAi分子)抑制HDAC。本领域技术人员广泛知晓克隆、转移和表达遗传构建物的方法,包括病毒和非病毒载体,以及脂质体。病毒载体包括腺病毒、腺相关病毒、逆转录病毒、痘苗病毒和疱疹病毒。HDACs can be inhibited by a variety of different mechanisms - proteins, peptides and nucleic acids including antisense and RNAi molecules. Methods for cloning, transferring and expressing genetic constructs, including viral and non-viral vectors, and liposomes, are widely known to those skilled in the art. Viral vectors include adenoviruses, adeno-associated viruses, retroviruses, vaccinia viruses, and herpes viruses.
也考虑到了小分子抑制剂。最广泛知晓的HDAC的小分子抑制剂也许是曲古抑菌素A,一种羟肟酸。据显示,它诱导过乙酰化并使ras转化的细胞逆转为正常形态(Taunton等,1996)和在小鼠模型中诱导免疫抑制(Takahashi等,1996)。它可从BIOMOL Research Labs,Inc.,Plymouth Meeting,PA购得。Small molecule inhibitors are also contemplated. Perhaps the most widely known small molecule inhibitor of HDACs is trichostatin A, a hydroxamic acid. It has been shown to induce hyperacetylation and reversion of ras-transformed cells to normal morphology (Taunton et al., 1996) and to induce immunosuppression in a mouse model (Takahashi et al., 1996). It is commercially available from BIOMOL Research Labs, Inc., Plymouth Meeting, PA.
以下引入本文作为参考的参考文献都描述了可用于本发明的HDAC抑制剂:AU9,013,101;AU9,013,201;AU9,013,401;AU6,794,700;EP1,233,958;EP1,208,086;EP1,174,438;EP1,173,562;EP1,170,008;EP1,123,111;JP2001/348340;美国申请2002/103192;美国申请2002/65282;美国申请2002/61860;WO02/51842;WO02/50285;WO02/46144;WO02/46129;WO02/30879;WO02/26703;WO02/26696;WO01/70675;WO01/42437;WO01/38322;WO01/18045;WO01/14581;Furumai等(2002);Hinnebusch等(2002);Mai等(2002);Vigushin等(2002);Gottlicher等(2001);Jung(2001);Komatsu等(2001);Su等(2000)。The following references, incorporated herein by reference, all describe HDAC inhibitors useful in the present invention: AU9,013,101; AU9,013,201; AU9,013,401; AU6,794,700; EP1,233,958; 173,562; EP1,170,008; EP1,123,111; JP2001/348340; US application 2002/103192; US application 2002/65282; US application 2002/61860; 30879; WO02/26703; WO02/26696; WO01/70675; WO01/42437; WO01/38322; (2002); Gottlicher et al. (2001); Jung (2001); Komatsu et al. (2001); Su et al. (2000).
IV.治疗心脏肥大的方法IV. Methods of Treating Cardiac Hypertrophy
A.治疗方案A. Treatment options
当前在处理心血管疾病中治疗心脏肥大的医学方法包括使用至少两类药物:肾素-血管紧张素系统的抑制剂和β-肾上腺素能阻断剂(Bristow,1999)。在处理心力衰竭中治疗病理性肥大的治疗剂包括血管紧张素II转化酶(ACE)抑制剂和β-肾上腺素能受体阻断剂(Eichhorn和Bristow,1996)。其它已公开用于治疗心脏肥大的药剂包括血管紧张素II受体拮抗剂(美国专利5,604,251)和神经肽Y拮抗剂(WO98/33791)。尽管当前有可用的药学化合物,但预防和治疗心脏肥大,以及随后的心力衰竭仍然是一种治疗挑战。Current medical approaches to treating cardiac hypertrophy in the management of cardiovascular disease include the use of at least two classes of drugs: inhibitors of the renin-angiotensin system and beta-adrenergic blockers (Bristow, 1999). Therapeutic agents for the treatment of pathological hypertrophy in the management of heart failure include angiotensin II converting enzyme (ACE) inhibitors and beta-adrenergic receptor blockers (Eichhorn and Bristow, 1996). Other agents disclosed for the treatment of cardiac hypertrophy include angiotensin II receptor antagonists (US Patent 5,604,251) and neuropeptide Y antagonists (WO98/33791). Despite currently available pharmaceutical compounds, preventing and treating cardiac hypertrophy, and consequently heart failure, remains a therapeutic challenge.
非药物治疗主要用作辅助药物治疗。非药物治疗的一种方法包括减少食物中的钠盐。此外,非药物治疗还需要清除某些沉淀性药物,包括负变力剂(例如,某些钙通道阻断剂和抗心律失常药如双异丙吡胺)、心脏毒素(如安非他明)和血浆容量扩充药(例如,非甾体抗炎药和糖皮质激素)。Non-drug treatments are mainly used as an adjunct to drug therapy. One approach to non-drug treatment involves reducing sodium in food. In addition, nonpharmacologic treatments require clearance of certain precipitating drugs, including negative inotropes (eg, certain calcium channel blockers and antiarrhythmics such as disopyramide), cardiotoxins (eg, amphetamine ) and plasma volume-expanding drugs (eg, nonsteroidal anti-inflammatory drugs and glucocorticoids).
在本发明的一个实施方式中,提供了利用PKD抑制剂治疗心脏肥大或心力衰竭的方法。对于本申请目的,治疗包括减少心脏肥大的一种或多种症状,如运动能力降低,心射血量降低,左心室舒张末期压增高,肺毛细管嵌入压增高,心脏输出、心脏指数降低,肺动脉压增高,左心室收缩和舒张末期尺度增大和左心室壁压力、壁张力和壁厚度升高-同样对于右心室。此外,PKD抑制剂可用于预防心脏肥大及其相关症状的发生。In one embodiment of the present invention, a method of treating cardiac hypertrophy or heart failure using a PKD inhibitor is provided. For the purposes of this application, treatment includes reduction of one or more symptoms of cardiac hypertrophy, such as decreased exercise capacity, decreased cardiac ejection volume, increased left ventricular end-diastolic pressure, increased pulmonary capillary insertion pressure, decreased cardiac output, decreased cardiac index, pulmonary arterial pressure, increased left ventricular systolic and end-diastolic dimensions and increased left ventricular wall pressure, wall tension and wall thickness - also for the right ventricle. In addition, PKD inhibitors can be used to prevent the development of cardiac hypertrophy and its associated symptoms.
治疗方案应根据临床情况而不同。然而,在大多数情况下,长期维持用药是合适的。也可能需要用PKD抑制剂间歇性治疗肥大,如在疾病进展期间短暂的治疗窗。Treatment options should vary according to the clinical situation. In most cases, however, long-term maintenance medication is appropriate. Intermittent treatment of hypertrophy with PKD inhibitors may also be required, such as a brief therapeutic window during disease progression.
B.联合疗法B. Combination therapy
在另一实施方式中,预想将PKD抑制剂与其它治疗方式联合使用。因此,除了上述疗法,也可以给病人提供更“标准的”心脏药物治疗。其它疗法的实例包括但不限于:所谓的“β阻断剂”、抗高血压药、强心剂、抗血栓剂、血管舒张剂、激素拮抗剂、变力药物、利尿剂、内皮缩血管肽拮抗剂、钙通道阻断剂、磷酸二酯酶抑制剂、ACE抑制剂、血管紧张素2型拮抗剂和细胞因子阻断剂/抑制剂、和HDAC抑制剂。In another embodiment, the use of PKD inhibitors in combination with other treatment modalities is envisioned. Thus, in addition to the aforementioned therapies, more "standard" cardiac medications may also be offered to the patient. Examples of other therapies include, but are not limited to: so-called "beta blockers", antihypertensives, cardiotonic agents, antithrombotics, vasodilators, hormone antagonists, inotropic drugs, diuretics, endothelin antagonists , calcium channel blockers, phosphodiesterase inhibitors, ACE inhibitors, angiotensin type 2 antagonists and cytokine blockers/inhibitors, and HDAC inhibitors.
可通过使心脏细胞与一种组合物或包括两种药物的药物制剂接触,或通过使细胞与两种不同组合物或制剂同时接触实现联合治疗,其中一种组合物包含表达构建物,另一组合物包含药物。或者,可在给予其它药剂之前或之后数分钟至数周间隔时,进行PKD抑制剂治疗。在其它药物和表达构建物分别应用于细胞的实施方式中,通常应保证每种药物递送之间有相当长的时间间隔,以使药物和表达构建物仍能对细胞施加有利的联合效应。在这种情况下通常考虑使细胞与两种方式的接触彼此相隔约12-24小时,更优选彼此相隔6-12小时,最优选延迟时间仅为约12小时。在一些情况下,可能需要显著延长治疗时间,然而,分别给药之间的间隔也可达数天(2、3、4、5、6或7)至数周(1、2、3、4、5、6、7或8)。Combination therapy can be achieved by contacting cardiac cells with a single composition or pharmaceutical formulation comprising both drugs, or by simultaneously contacting the cells with two different compositions or formulations, one comprising the expression construct and the other The composition contains a drug. Alternatively, PKD inhibitor therapy can be administered at intervals ranging from minutes to weeks before or after administration of the other agent. In other embodiments where the drug and the expression construct are applied separately to the cells, a substantial time interval between the delivery of each drug should generally be ensured so that the drug and the expression construct can still exert a beneficial combined effect on the cell. In this case it is generally contemplated that the contacting of the cells with the two means will be separated by about 12-24 hours from each other, more preferably 6-12 hours apart from each other, and most preferably the delay time will be only about 12 hours. In some cases, a significant prolongation of the treatment period may be required, however, the intervals between separate administrations may range from days (2, 3, 4, 5, 6 or 7) to weeks (1, 2, 3, 4 , 5, 6, 7 or 8).
也可想见,将需要给予PKD抑制剂或其它药物一次以上。在这点上,可采用各种组合。为了说明,PKD抑制剂是“A”,其它药剂是“B”,下面的排列是根据总共给药3和4次的范例:It is also conceivable that the PKD inhibitor or other drug will need to be administered more than once. In this regard, various combinations can be employed. To illustrate, the PKD inhibitor is "A" and the other agents are "B" and the following arrangement is based on an example of a total of 3 and 4 doses:
A/B/A B/A/B B/B/A A/A/B B/A/A A/B/B B/B/B/A B/B/A/BA/B/A B/A/B B/B/A A/A/B B/A/A A/B/B B/B/B/A B/B/A/B
A/A/B/B A/B/A/B A/B/B/A B/B/A/A B/A/B/A B/A/A/B B/B/B/AA/A/B/B A/B/A/B A/B/B/A B/B/A/A B/A/B/A B/A/A/B B/B/B/A
A/A/A/B B/A/A/A A/B/A/A A/A/B/A A/B/B/B B/A/B/B B/B/A/BA/A/A/B B/A/A/A A/B/A/A A/A/B/A A/B/B/B B/A/B/B B/B/A/B
同样考虑了其它组合。Other combinations are also contemplated.
C.药理治疗剂C. Pharmacological Therapeutics
药理治疗剂和给药方法、剂量等是本领域技术人员熟知的(参见例如,“医生桌面参考书(Physicians Desk Reference)”、Klaassend的“治疗的药理学基础”、“雷明顿药物科学(Remington’s Pharmaceutical Sciences)”和“莫克指数(TheMerck Index),第十一版”,本文引入它们的相关部分作为参考),可根据本文公开的内容结合本发明。必须待治疗患者的情况对剂量作出某些改变。负责给药的人必须为患者个体确定合适的剂量,确定患者个体的合适剂量是本领域普通技术人员的技术能力之内。Pharmacological therapeutic agents and methods of administration, dosage, etc. are well known to those skilled in the art (see, e.g., "Physicians Desk Reference", "Pharmacological Basis of Therapy" by Klaassend, "Remington's Pharmaceutical Sciences ( Remington's Pharmaceutical Sciences)" and "The Merck Index (The Merck Index), Eleventh Edition", the relevant parts of which are incorporated herein by reference), the present invention may be incorporated in light of the disclosure herein. The condition of the patient to be treated necessitates certain changes in dosage. Those responsible for the administration must determine the appropriate dosage for the individual patient, which is within the skill of one of ordinary skill in the art.
本发明中可使用的药物治疗剂的非限制性例子包括:抗高脂蛋白药、抗动脉硬化药、抗血栓/溶纤维蛋白药、血凝促进药、抗心律不齐药、抗高血压药、血管加压药、充血性心力衰竭治疗剂、防心绞痛药、抗菌药或它们的组合。Non-limiting examples of drug therapeutic agents that can be used in the present invention include: anti-hyperlipoprotein drugs, anti-arteriosclerotic drugs, anti-thrombotic/fibrinolytic drugs, coagulation-promoting drugs, anti-arrhythmic drugs, anti-hypertensive drugs , a vasopressor, a congestive heart failure treatment, an antiangina, an antibacterial, or a combination thereof.
此外,应该注意,可利用以下任何一种药物来发展新的心脏疾病治疗的靶基因,如本发明实施例中所用的β-阻断剂(见下)。虽然预计很多这些基因可能重叠,但很可能开发出新的靶基因。In addition, it should be noted that any of the following drugs can be used to develop new target genes for heart disease treatment, such as the beta-blockers used in the examples of the present invention (see below). While it is expected that many of these genes may overlap, it is likely that new target genes will be developed.
i.抗高脂蛋白药i. Anti-hyperlipoprotein drugs
在某些实施方式中,可将给予能降低一种或多种血脂和/或脂蛋白浓度的药物,本文中称为“抗高脂蛋白药”与本发明的心血管治疗药物,具体是治疗动脉粥样硬化和血管组织增厚或阻塞的药物相结合。在某些方面,抗高脂蛋白药可包括:芳氧基链烷酸/纤维酸衍生物、树脂/胆汁酸螯合剂、HMGCoA还原酶抑制剂、烟酸衍生物、甲状腺激素或甲状腺激素类似物、其它药物或它们的组合。In some embodiments, drugs that can reduce one or more blood lipids and/or lipoprotein concentrations, referred to herein as "anti-hyperlipoprotein drugs" can be combined with cardiovascular therapeutic drugs of the present invention, specifically for the treatment of A combination of drugs for atherosclerosis and thickening or blockage of blood vessel tissue. In certain aspects, anti-hyperlipoproteinagent agents may include: aryloxyalkanoic acid/fibric acid derivatives, resin/bile acid sequestrants, HMGCoA reductase inhibitors, niacin derivatives, thyroid hormones, or thyroid hormone analogs , other drugs, or combinations thereof.
a.芳氧基链烷酸/纤维酸衍生物a. Aryloxyalkanoic acid/fibric acid derivatives
芳氧基链烷/纤维酸衍生物的非限制性例子包括:苄氯贝特、enzafibrate、比尼贝特、环丙贝特、克利贝特、氯贝特(atromide-S)、氯贝酸、依托贝特、非诺贝特、吉非罗齐(lobid)、尼可贝特、吡贝特、氯烟贝特、双贝特和羟乙茶碱贝特。Non-limiting examples of aryloxyalkane/fibric acid derivatives include: benzofibrate, enzafibrate, binifibrate, ciprofibrate, clifibrate, atromide-S, clofibrate , Yitofibrate, fenofibrate, gemfibrozil (lobid), nicofibrate, piribibrate, clofibrate, bifibrate and ethynofibrate.
b.树脂/胆汁酸螯合剂b. Resins/Bile Acid Sequestrants
树脂/胆汁酸螯合剂的非限制性例子包括:消胆胺(消胆胺、降胆敏),降脂树脂(考来替泊)和降胆葡胺。Non-limiting examples of resins/bile acid sequestrants include: cholestyramines (cholestyramine, cholesterol), lipid-lowering resins (colestipol), and cholestipol.
c.HMG CoA还原酶抑制剂c. HMG CoA reductase inhibitors
HMG CoA还原酶抑制剂的非限制性例子包括:洛伐他汀(mevacor)、普伐他汀(普拉固)或辛伐他汀(舒降之)。Non-limiting examples of HMG CoA reductase inhibitors include: lovastatin (mevacor), pravastatin (pravastat), or simvastatin (Zocor).
d.烟酸衍生物d. Niacin derivatives
烟酸衍生物的非限制性例子包括:烟酸呋酯、阿西莫司、戊四烟酯、尼可氯酯、烟酸环己醇酯和氧烟酸。Non-limiting examples of niacin derivatives include: nicotinate furfurate, acipimox, penetronicotinate, nicochloride, cyclohexanol nicotinate, and oxynicotinate.
e.甲状腺激素和类似物e. Thyroid hormones and analogs
甲状腺激素及其类似物的非限制性例子包括:etoroxate、甲状丙酸和甲状腺素。Non-limiting examples of thyroid hormones and their analogs include etoroxate, thyropropionate, and thyroxine.
f.其它抗高脂蛋白药f. Other anti-hyperlipoprotein drugs
其它抗高脂蛋白药的非限制性例子包括:阿昔呋喃、阿扎胆醇、苯氟雷司、β-苄叉丁酰胺、卡尼汀、硫酸软骨素、氯雌酮甲醚、detaxtran、右旋糖酐硫酸钠、5,8,11,14,17-二十碳五烯酸、香菇嘌啉、夫拉扎勃、美格鲁托、甲亚油酰胺、双甲雌三醇、鸟氨酸、γ-谷维素、泛硫乙胺、四乙酸季戊四醇酯、α-苯基丁酰胺、吡扎地尔、丙丁酚(普罗布考片剂)、β-谷甾醇、磺托酸-哌嗪盐、硫癸二乙醇、三苯乙醇和联苯丁酸。Non-limiting examples of other anti-hyperlipoprotein drugs include: acifuran, azachol, benflurex, beta-benzylidene butyramide, carnitine, chondroitin sulfate, chlorestrone, detaxtran, Sodium Dextran Sulfate, 5,8,11,14,17-Eicosapentaenoic Acid, Mushroom Purine, Frazabo, Meglutol, A-Linoleamide, Dimethyl Estriol, Ornithine, γ-Oryzanol, Pantethine, Pentaerythritol Tetraacetate, α-Phenylbutyramide, Pizardil, Probucol (Probucol Tablets), β-Sitosterol, Sutropic Acid-Piperazine Salt, Sulfur Decanedethanol, triphenylethyl alcohol and biphenylbutyric acid.
ii.抗动脉硬化药ii. Anti-arteriosclerotic drugs
抗动脉硬化药的非限制性例子包括吡醇氨酯。Non-limiting examples of anti-arteriosclerotic drugs include pyroxcarbamate.
iii.抗血栓/溶纤维蛋白药iii. Antithrombotic/fibrinolytic agents
在某些实施方式中,可以将给予能帮助去除或防止血液凝块的药物与给予调节剂相结合,尤其是治疗动脉粥样硬化和和脉管系统(如动脉)阻塞。抗血栓和/或溶纤维蛋白药的非限制性例子包括:抗凝血药、抗凝血剂拮抗剂、抗血小板药、血栓溶解剂、血栓溶解剂拮抗剂或它们的组合。In certain embodiments, administration of a drug that helps remove or prevent blood clots may be combined with administration of a modulating agent, particularly to treat atherosclerosis and blockage of the vasculature, such as an artery. Non-limiting examples of antithrombotic and/or fibrinolytic agents include: anticoagulant agents, anticoagulant antagonists, antiplatelet agents, thrombolytic agents, thrombolytic agent antagonists, or combinations thereof.
在某些方面,可以口服给予抗血栓药,例如,优选阿司匹林和华法林(丙酮香豆素钠)。In certain aspects, antithrombotic agents may be administered orally, eg, preferably aspirin and warfarin (acetocoumarin sodium).
a.抗凝血药a. Anticoagulants
抗凝血药的非限制性例子包括:醋硝香豆酮、安克洛酶、茴茚二酮、溴茚二酮、氯茚二酮、库美香豆素、环香豆素、右旋糖酐硫酸钠、双羟香豆素、二苯茚酮、双香豆乙酯、亚乙基双羟香豆素、氟茚二酮、肝素、水蛭素、阿朴酸钠、奥沙二酮、木聚硫钠、苯茚二酮、苯丙香豆素、卵黄高磷蛋白、吡考酰胺、噻氯香豆素和华法林。Non-limiting examples of anticoagulant drugs include: acenocoumarone, ancrodase, anisindione, bromindione, chlorindione, coumecoumarol, cyclocoumarin, dextran sulfate sodium , Dihydroxycoumarin, Dipheninone, Dicoumaryl Ethyl Ester, Ethylenedihydroxycoumarin, Fluindione, Heparin, Hirudin, Sodium Apoate, Oxadione, Wood Polysulfide Sodium, phenindione, phenprocoumon, phosvitin, picocamide, ticlocoumarol, and warfarin.
b.抗血小板药b. Antiplatelet drugs
抗血小板药的非限制性例子包括:阿司匹林、右旋糖酐、双嘧哌胺醇(潘生丁)、肝素、苯磺唑酮(anturane)和噻氯匹定(ticlid)。Non-limiting examples of antiplatelet drugs include: aspirin, dextran, dipyridamole (dipyridamole), heparin, sulfazone (anturane), and ticlopidine (ticlid).
c.血栓溶解剂c. Thrombolytics
血栓溶解剂的非限制性例子包括:组织纤溶酶原激活剂(激活酶)、纤溶酶、尿激酶原、尿激酶(abbokinase)、链激酶(链酶)、阿尼普酶/APSAC(依米那酶)。Non-limiting examples of thrombolytic agents include: tissue plasminogen activator (activase), plasmin, prourokinase, urokinase (abbokinase), streptokinase (streptazyme), anistreplase/APSAC ( eminase).
iv.血凝促进药iv. Blood coagulation promoting drugs
在某些实施方式中,病人患有出血症(hemmorage)或出血的可能性增加,可采用能促进血液凝结的药物。血凝促进药的非限制性例子包括血栓溶解剂拮抗剂和抗凝血剂拮抗剂。In certain embodiments, a patient suffering from hemmorage or an increased likelihood of bleeding may be treated with a drug that promotes blood clotting. Non-limiting examples of coagulation promoting drugs include thrombolytic agent antagonists and anticoagulant antagonists.
a.抗凝血剂拮抗剂a. Anticoagulant antagonists
抗凝血剂拮抗剂的非限制性例子包括鱼精蛋白和维生素K1。Non-limiting examples of anticoagulant antagonists include protamine and vitamin K1.
b.血栓溶解剂拮抗剂和抗血栓剂b. Thrombolytic antagonists and antithrombotics
血栓溶解剂拮抗剂的非限制性例子包括氨基己酸(亮氨酸)和氨甲环酸(凝血酸)。抗血栓剂的非限制性例子包括:阿那格雷、阿加曲班、cilstazol、达曲班、去纤苷、依诺肝素、速避凝、吲哚布芬、lamoparan、奥扎格雷、吡考酰胺、普拉贝脲、替地肝素、噻氯匹定和三氟柳。Non-limiting examples of thrombolytic agent antagonists include aminocaproic acid (leucine) and tranexamic acid (tranexamic acid). Non-limiting examples of antithrombotic agents include: anagrelide, argatroban, cilstazol, datroban, defibrotide, enoxaparin, bolus, indobufen, lamoparan, ozagrel, picol amide, prabenamide, deteparin, ticlopidine, and triflusal.
v.抗心律不齐药v. Antiarrhythmic drugs
抗心律不齐药的非限制性例子包括I类抗心律不齐药(钠通道阻断剂),II类抗心律不齐药(β-肾上腺素能阻断剂);II类抗心律不齐药(复极化延长药物),IV类抗心律不齐药(钙通道阻断剂)和其它抗心律不齐药。Non-limiting examples of antiarrhythmics include class I antiarrhythmics (sodium channel blockers), class II antiarrhythmics (beta-adrenergic blockers); class II antiarrhythmics drugs (repolarization prolonging drugs), class IV antiarrhythmics (calcium channel blockers) and other antiarrhythmics.
a.钠通道阻断剂a. Sodium channel blockers
钠通道阻断剂的非限制性例子包括IA类、IB类和IC类抗心律不齐药。IA类抗心律不齐药的非限制性例子包括:双吡嗪酰胺(双异丙吡胺)、普鲁卡因胺(百浪斯剔)和奎尼丁(硫酸奎尼丁)。IB类抗心律不齐药的非限制性例子包括:利多卡因(赛罗卡因)、妥卡胺(tonocard)和脉律定(美西律)。IC类抗心律不齐药的非限制性例子包括恩卡胺(enkaid)和氟卡胺(tambocor)。Non-limiting examples of sodium channel blockers include Class IA, Class IB, and Class IC antiarrhythmics. Non-limiting examples of Class IA antiarrhythmic drugs include: disopyrazinamide (disopyramide), procainamide (Bronstat), and quinidine (quinidine sulfate). Non-limiting examples of Class IB antiarrhythmics include lidocaine (xylocaine), tocaine (tonocard), and mexiletine (mexiletine). Non-limiting examples of Class IC antiarrhythmics include enkaid and flecainide (tambocor).
b.β阻断剂b. Beta blockers
β阻断剂,也称为β-肾上腺素能阻断剂、β-肾上腺素能拮抗剂或II类抗心律不齐药的非限制性例子包括:醋丁洛尔(醋丁酰心安)、阿普洛尔、氨磺洛尔、arotinolol、阿替洛尔、苯呋洛尔、倍他索洛尔、贝凡洛尔、比索洛尔、波吲洛尔、布库洛尔、布非洛尔、丁呋洛尔、布尼洛尔、布拉洛尔、盐酸布替君、丁非洛尔、卡拉洛尔、卡替洛尔、卡维地洛、塞利洛尔、塞他洛尔、氯拉洛尔、地来洛尔、依泮洛尔、艾司洛尔(brevibloc)、茚诺洛尔、拉贝洛尔、左布诺洛尔、甲吲洛尔、美替洛尔、美托洛尔、莫普洛尔、纳多洛尔、萘肟洛尔、硝苯洛尔、尼普地洛、氧烯洛尔、喷布洛尔、吲哚洛尔、普拉洛尔、丙萘洛尔、丙诺洛尔(propanolol)(心得安)、索他洛尔(betapace)、硫氧洛尔、他林洛尔、特他洛尔、噻吗洛尔、托利洛尔和xibinolol。在某些方面,β阻断剂包括芳氧基丙醇胺衍生物。芳氧基丙醇胺衍生物的非限制性例子包括:醋丁洛尔、arotinolol、阿替洛尔、倍他索洛尔、贝凡洛尔、比索洛尔、波吲洛尔、布尼洛尔、丁非洛尔、卡拉洛尔、卡替洛尔、卡维地洛、塞利洛尔、塞他洛尔、依泮洛尔、茚诺洛尔、甲吲洛尔、美替洛尔、美托洛尔、莫普洛尔、纳多洛尔、尼普地洛、氧烯洛尔、喷布洛尔、吲哚洛尔、丙诺洛尔、他林洛尔、特他洛尔、噻吗洛尔和托利洛尔。Non-limiting examples of beta blockers, also known as beta-adrenergic blocking agents, beta-adrenergic antagonists, or class II antiarrhythmic drugs include: acebutolol (acebutyrolol), alprenolol, amisulolol, arotinolol, atenolol, benzfurolol, betasoprolol, bevanolol, bisoprolol, perinolol, bucurolol, bufemolol bufrolol, butyrolol, butyrolol, butidrine hydrochloride, buferolol, caralolol, carteolol, carvedilol, celipolol, cetalol , Chloralol, Dilevolol, Epanolol, Esmolol (brevibloc), Indenolol, Labetalol, Levobunolol, Metinolol, Metyrolol, Metoprolol, moprolol, nadolol, nadoximolol, nifevolol, nipredilol, oxprenolol, pembrolol, pindolol, pralolol, Propanolol, propanolol (propranolol), sotalol (betapace), thioxolol, talinolol, tetalol, timolol, tollyrolol, and Xibinolol. In certain aspects, beta blockers include aryloxypropanolamine derivatives. Non-limiting examples of aryloxypropanolamine derivatives include: acebutolol, arotinolol, atenolol, betasoprolol, bevanolol, bisoprolol, popinolol, bunilol Difenolol, caralolol, cartilol, carvedilol, celipolol, cetaxolol, epanolol, indenolol, metinolol, metyrolol , metoprolol, moprolol, nadolol, nipredilol, oxyprenolol, pembrolol, pindolol, propanolol, talinolol, tetalol , timolol and tollyrolol.
c.复极化延长药c. Repolarization prolonging drug
复极化延长药,也称为III类抗心律不齐药的非限制性例子包括胺碘酮(安律酮)和右索他洛尔(心得怡)。Non-limiting examples of repolarization-prolonging drugs, also known as Class III antiarrhythmic drugs, include amiodarone (Amrudin) and dexsotalol (Indigo).
d.钙通道阻断剂/拮抗剂d. Calcium channel blockers/antagonists
钙通道阻断剂,也称为IV类抗心律不齐药的非限制性例子包括:芳基烷基胺(如苄丙洛、地尔硫、芬地林、戈洛帕米、普尼拉明、特罗地林、维拉帕米)、二氢吡啶衍生物(非洛地平、伊拉地平、尼卡地平、硝苯地平、尼莫地平、尼索地平、尼群地平)、哌嗪衍生物(例如,桂利嗪,氟桂利嗪,利多氟嗪)或其它钙通道阻断剂如苄环烷,依他苯酮,镁,米贝拉地尔或米贝拉地尔。在某些实施方式中,钙通道阻断剂包括长效二氢吡啶(硝苯吡啶型)钙拮抗剂。Non-limiting examples of calcium channel blockers, also known as Class IV antiarrhythmics, include: arylalkylamines (eg, benzprorol, diltiazem, fendiline, golopamil, purney Lamin, Terodiline, Verapamil), dihydropyridine derivatives (felodipine, isradipine, nicardipine, nifedipine, nimodipine, nisoldipine, nitrendipine), piperidine Oxyzine derivatives (eg, cinnarizine, flunarizine, lidoflurazine) or other calcium channel blockers such as benzcyclanes, etabenzone, magnesium, miberadil or miberadil. In certain embodiments, calcium channel blockers include long-acting dihydropyridine (nifedipine-type) calcium antagonists.
e.其它抗心律不齐药e. Other antiarrhythmic drugs
其它抗心律不齐药的非限制性例子包括:阿糖腺苷(adenocard)、地高辛(拉诺辛)、乙酰卡尼、阿义马林、克冠吗啉、阿普林定、托西溴苄铵、丁萘夫汀、布托苯定、卡泊酸、西苯唑啉、双异丙吡胺、双氢奎尼丁、英地卡尼、溴化ipatropium、利多卡因、劳拉义明、劳卡尼、甲氧苯汀、莫雷西嗪、吡美诺、prajmaline、普罗帕酮、吡诺林、多聚半乳糖醛酸奎尼丁酯、硫酸奎尼丁酯和维喹地尔。Non-limiting examples of other antiarrhythmic drugs include: adenosine (adenocard), digoxin (lanoxin), acetylcarnitine, ayimarin, clomorpholine, aplinidine, Cetyl benzyl ammonium bromide, naftine, butorbendine, cappoic acid, cibenzoline, disopyramide, dihydroquinidine, indicarnide, ipatropium bromide, lidocaine, Laimin, locanide, methoxyphentine, morcizine, pimenol, prajmaline, propafenone, piranol, quinidine polygalacturonate, quinidine sulfate, and vitamin Quindil.
vi.抗高血压药vi. Antihypertensive drugs
抗高血压药的非限制性例子包括:交感神经阻滞药、α/β阻断剂、α阻断剂、抗血管紧张素II药、β阻断剂、钙通道阻断剂、血管舒张剂和其它抗高血压药。Non-limiting examples of antihypertensive drugs include: sympatholytics, alpha/beta blockers, alpha blockers, antiangiotensin II drugs, beta blockers, calcium channel blockers, vasodilators and other antihypertensive drugs.
a.α阻断剂a. Alpha blockers
α阻断剂,也称为α-肾上腺素能阻断剂或α-肾上腺素能拮抗剂的非限制性例子包括:氨磺洛尔、阿罗洛尔、达哌唑、多沙唑嗪、甲磺酸麦角固醇酯(ergoloidmesylates)、芬司匹利、吲哚拉明、拉贝洛尔、尼麦角林、哌唑嗪、特拉唑嗪、妥拉唑林、曲马唑嗪和育亨宾。在某些实施方式中,α阻断剂可包括喹唑啉衍生物。喹唑啉衍生物的非限制性例子包括:阿夫唑嗪、布那唑嗪、多沙唑嗪、哌唑嗪、特拉唑嗪和曲马唑嗪。Non-limiting examples of alpha blockers, also known as alpha-adrenergic blocking agents or alpha-adrenergic antagonists include: amisulolol, arololol, dapiprazole, doxazosin, Ergosterol mesylate (ergoloidmesylates), fenspiride, indolamin, labetalol, nicergoline, prazosin, terazosin, tolazoline, trimazosin, and ethanol Yohimbe. In certain embodiments, alpha blockers may include quinazoline derivatives. Non-limiting examples of quinazoline derivatives include: alfuzosin, bunazosin, doxazosin, prazosin, terazosin, and trimazosin.
b.α/β阻断剂b. Alpha/beta blockers
在某些实施方式中,抗高血压药是α和β肾上腺素能拮抗剂。α/β阻断剂的非限制性例子包括拉贝洛尔(柳胺心定,柳胺苄心定)。In certain embodiments, the antihypertensive agent is an alpha and beta adrenergic antagonist. A non-limiting example of an alpha/beta blocker includes labetalol (salatel, salatel).
c.抗血管紧张素II药c. Antiangiotensin II drugs
抗血管紧张素II药的非限制性例子包括血管紧张素转化酶抑制剂和血管紧张素II受体拮抗剂。血管紧张素转化酶抑制剂(ACE抑制剂)的非限制性例子包括:阿拉普利、依那普利(vasotec)、卡托普利、西拉普利、地拉普利、依那普利拉、福辛普利、赖诺普利、moveltopril、培哚普利、喹那普利和雷米普利。血管紧张素II受体阻断剂,也称为血管紧张素II受体拮抗剂、ANG受体阻断剂或ANG-II的1型受体阻断剂(ARBS)的非限制性例子包括:血管坎地沙坦、依普罗沙坦、厄贝沙坦、氯沙坦和缬沙坦。Non-limiting examples of anti-angiotensin II drugs include angiotensin converting enzyme inhibitors and angiotensin II receptor antagonists. Non-limiting examples of angiotensin converting enzyme inhibitors (ACE inhibitors) include: alapril, enalapril (vasotec), captopril, cilazapril, delapril, enalapril la, fosinopril, lisinopril, moveltopril, perindopril, quinapril, and ramipril. Non-limiting examples of angiotensin II receptor blockers, also known as angiotensin II receptor antagonists, ANG receptor blockers, or
d.交感神经阻滞药d. Sympathetic blockers
交感神经阻滞药的非限制性例子包括:作用于中枢的交感神经阻滞药或作用于外周的交感神经阻滞药。作用于中枢的交感神经阻滞药,也称为中枢神经系统(CNS)交感神经阻滞药的非限制性例子包括:可乐定(氯压定)、胍那苄(氯压胍)、胍法辛(盐酸胍法辛)和甲基多巴(爱道美)。作用于外周的交感神经阻滞药的非限制性例子包括:神经节阻断剂、肾上腺素能能神经元阻断剂、β-肾上腺素能阻断剂或αl-肾上腺素能阻断剂。神经节阻断剂的非限制性例子包括美卡拉明(美加明)和三甲噻方(阿方那特)。肾上腺素能能神经元阻断剂的非限制性例子包括胍乙啶(依斯米林)和利舍平(色巴息)。β-肾上腺素能阻断剂的非限制性例子包括:acenitolol(醋丁酰心安)、阿替洛尔(氨酰心安)、倍他洛尔(卡尔仑)、卡替洛尔(盐酸卡替洛尔)、拉贝洛尔(柳胺心定、柳胺羟胺)、美托洛尔(美多洛尔)、萘丁乐(康加尔多)、喷布洛尔(levatol)、吲哚洛尔(心得静)、普萘洛尔(萘心安)和噻吗洛尔(噻吗心安)。αl-肾上腺素能阻断剂的非限制性例子包括:哌唑嗪(盐酸哌唑嗪)、多沙唑嗪(doxazocin)(甲磺酸多沙唑嗪)和特拉唑嗪(高特灵)。Non-limiting examples of sympatholytics include: centrally acting sympatholytics or peripherally acting sympatholytics. Non-limiting examples of centrally acting sympatholytics, also known as central nervous system (CNS) sympatholytics include: Xin (guanfacine hydrochloride) and methyldopa (Adome). Non-limiting examples of peripherally acting sympatholytics include: ganglionic blocking agents, adrenergic neuron blocking agents, beta-adrenergic blocking agents or alphal-adrenergic blocking agents. Non-limiting examples of ganglionic blocking agents include mecamylamine (mecamylamine) and thiazine (alfonate). Non-limiting examples of adrenergic neuron blocking agents include guanethidine (exmirin) and reserpine (cepax). Non-limiting examples of beta-adrenergic blocking agents include: acenitolol (acenitolol), atenolol (acenolol), betaxolol (callen), carteolol (cateolol Lol), labetalol (salantol, salivaline), metoprolol (Metoprolol), nabuprol (Congaldo), pembrolol (levatol), pindolol Propranolol (propranolol), propranolol (propranolol), and timolol (timolol). Non-limiting examples of alpha 1-adrenergic blocking agents include: prazosin (prazosin hydrochloride), doxazocin (doxazosin mesylate) and terazosin (Gotamine ).
e.血管舒张剂e. Vasodilators
在某些实施方式中,心血管治疗剂可包括血管舒张剂(例如,脑血管舒张剂,冠状血管舒张剂或外周血管舒张剂)。在某些优选实施方式中,血管舒张剂是冠状血管舒张剂。冠状血管舒张剂的非限制性例子包括:胺氧三苯、阿苯达唑、琥珀苯呋地尔、苯碘达隆、氯吩嗪、卡波罗孟、氯苄呋醇、氯硝甘油、克冠二氮卓、双嘧达莫、氢普拉明、乙氧黄酮、四硝酸赤癣醇酯、依他苯酮、芬地林、夫洛地尔、更利芬、herestrol双(β-二乙氧基乙基醚)、海索苯定、硝乙醇胺、凯林、利多氟嗪、六硝酸己烷醇酯、美地巴嗪、烟酰甘油(nicorglycerin)、四硝酸季戊四醇酯、戊硝醇、哌克昔林、匹美茶碱、曲匹地尔、甲色酮、曲美他嗪、磷酸三硝乙醇胺和维司那定。In certain embodiments, cardiovascular therapeutic agents can include vasodilators (eg, cerebral vasodilators, coronary vasodilators, or peripheral vasodilators). In certain preferred embodiments, the vasodilator is a coronary vasodilator. Non-limiting examples of coronary vasodilators include: aminetriphenyl, albendazole, succinfuridil, pheniodarone, chlorphenazine, carbolomon, clobenzfuran, clonidine, Keguan diazepam, dipyridamole, hydropramin, ethoxylated flavone, erythrinol tetranitrate, etabenone, fendiline, frodil, ganlifen, herestrol bis(β- Diethoxyethyl ether), hexobendidine, nitethanolamine, kellyn, lidofluozine, hexanol hexanitrate, mediprazine, nicorglycerin, pentaerythritol tetranitrate, pentylnitrate Alcohol, perhexiline, pimefylline, tripidil, methroxone, trimetazidine, trinitrolamine phosphate and visnadine.
在某些方面,血管舒张剂可包括慢性疗法血管舒张剂或紧急高血压血管舒张剂。慢性疗法血管舒张剂的非限制性例子包括双肼屈嗪(肼苯哒嗪)和米诺地尔(降压定)。紧急高血压血管舒张剂的非限制性例子包括:硝普钠(亚硝基铁氰化钠)、二氮嗪(降压嗪IV)、双肼屈嗪(肼苯哒嗪)、米诺地尔(降压定)和维拉帕米。In certain aspects, vasodilators can include chronic therapy vasodilators or emergency hypertensive vasodilators. Non-limiting examples of chronic therapy vasodilators include dihydralazine (hydralazine) and minoxidil (vaperidin). Non-limiting examples of emergency hypertensive vasodilators include: sodium nitroprusside (sodium nitroferricyanide), diazoxide (hypertensin IV), dihydralazine (hydralazine), minoxidil Er (lower pressure) and verapamil.
f.其它抗高血压药f. Other antihypertensive drugs
其它抗高血压药的非限制性例子包括:阿义马林、γ-氨基丁酸、丁苯碘胺、西氯他宁、环西多明、丹宁酸绿藜安酯、非诺多泮、氟司喹南、酮色林、美布氨酯、美卡拉明、甲基多巴、甲基4-吡啶基酮缩氨基硫脲、莫唑胺、帕吉林、潘必啶、吡那地尔、哌罗克生、普立哌隆、原藜芦碱、萝巴新、瑞西美托、利美尼定、沙拉新、sodiumnitrorusside、替尼酸、右旋樟脑磺酸三甲噻方酯、酪氨酸酶和乌拉地尔。Non-limiting examples of other antihypertensive agents include: ajmaline, gamma-aminobutyric acid, fenfeniodine, cclotanine, cyclocidomine, ververol tannin, fenoldopam , flusquinan, ketanserin, mebutamate, mecamylamine, methyldopa, methyl 4-pyridyl ketone thiosemicarbazone, mozolomide, pagiline, pampidine, pinacidil, Peroxan, Propirerone, Proveratrine, Rabasin, Resimetol, Rilmenidine, Sarasin, Sodiumnitrorusside, Tinic Acid, D-Trimetyl Camphorsulfonate, Tyrosinase and uradil.
在某些方面,抗高血压药可包括:芳基乙醇胺衍生物、苯丙噻二嗪衍生物、N-羧基烷基(肽/内酰胺)衍生物、二氢吡啶衍生物、胍衍生物、肼/酞嗪、咪唑衍生物、季铵化合物、利血平衍生物或磺胺类衍生物。In certain aspects, antihypertensive agents may include: arylethanolamine derivatives, phenproterazine derivatives, N-carboxyalkyl (peptide/lactam) derivatives, dihydropyridine derivatives, guanidine derivatives, Hydrazine/phthalazine, imidazole derivatives, quaternary ammonium compounds, reserpine derivatives or sulfonamide derivatives.
芳基乙醇胺衍生物。芳基乙醇胺衍生物的非限制性例子包括:氨磺洛尔、丁呋洛尔、地来洛尔、拉贝洛尔、丙萘洛尔、索他洛尔和硫氧洛尔。Aryl ethanolamine derivatives. Non-limiting examples of arylethanolamine derivatives include: amisulfalol, bufurolol, delevolol, labetalol, propranolol, sotalol, and thioxolol.
苯丙噻二嗪衍生物。苯丙噻二嗪衍生物的非限制性例子包括:阿尔噻嗪、苄氟噻嗪、苄噻嗪、苄基氢氯噻嗪、布噻嗪、氯噻嗪、氯噻酮、环戊噻嗪、环噻嗪、二氮嗪、依匹噻嗪、乙噻嗪、芬喹唑、氢氯噻嗪、氢氟噻嗪、甲氯噻嗪、美替克仑、美托拉宗、对氟噻嗪、聚噻嗪、四氯噻嗪和三氯噻嗪。Diphenhydramine derivatives. Non-limiting examples of phenprophiazine derivatives include: Althiazine, Benzoflumethiazide, Benzothiazide, Benzylhydrochlorothiazide, Buthiazide, Chlorthiazide, Chlorthalidone, Cyclopenthiazine, Cyclothiazide Diazoxide, Epithiazide, Ethiazide, Fenquinazole, Hydrochlorothiazide, Hydrofluorothiazide, Methiazide, Meticlen, Metolazone, P-fluorothiazide, Polythiazide, tetrachlorothiazide and trichlorothiazide.
N-羧基烷基(肽/内酰胺)衍生物。N-羧基烷基(肽/内酰胺)衍生物的非限制性例子包括:阿拉普利、卡托普利、西拉普利、地拉普利、依那普利、依那普利拉、福辛普利、赖诺普利、莫维普利、培哚普利、喹那普利和雷米普利。N-carboxyalkyl (peptide/lactam) derivatives. Non-limiting examples of N-carboxyalkyl (peptide/lactam) derivatives include: alazapril, captopril, cilazapril, delapril, enalapril, enalaprilat, Fosinopril, lisinopril, movipril, perindopril, quinapril, and ramipril.
二氢吡啶衍生物。二氢吡啶衍生物的非限制性例子包括:氨氯地平、非洛地平、伊拉地平、尼卡地平、硝苯地平、尼伐地平、尼索地平和尼群地平。Dihydropyridine derivatives. Non-limiting examples of dihydropyridine derivatives include: amlodipine, felodipine, isradipine, nicardipine, nifedipine, nivadipine, nisoldipine, and nitrendipine.
胍衍生物。胍衍生物的非限制性例子包括:苄二甲胍、异喹胍、胍那苄、胍那克林、胍那决尔、胍那佐定、胍乙啶、胍法辛、胍氯、胍诺沙苄和胍生。Guanidine derivatives. Non-limiting examples of guanidine derivatives include: benzmetidine, isoquine, guanabenz, guanacrine, guanadol, guanazodine, guanethidine, guanfacine, guanidine chloride, guanidine Noxabenzyl and guanidine.
肼/酞嗪。肼/酞嗪的非限制性例子包括:布屈嗪、卡屈嗪、双肼屈嗪、恩屈嗪、肼卡巴嗪、肼屈嗪、苯异丙肼、匹尔屈嗪和托屈嗪。Hydrazine/phthalazine. Non-limiting examples of hydrazine/phthalazine include: butronazine, carbralazine, dihydralazine, endralazine, hydralazine, hydralazine, phenprohydrazine, pildralazine, and tolralazine.
咪唑衍生物。咪唑衍生物的非限制性例子包括:吡可乐定、右洛非西定、酚妥拉明、噻甲尼定和托洛尼定。imidazole derivatives. Non-limiting examples of imidazole derivatives include: piclonidine, dexlofexidine, phentolamine, timethadine, and tolonidine.
季铵化合物。季铵化合物的非限制性例子包括:阿扎溴铵、松达氯铵、六甲溴铵、喷他双甲硫铵、喷他溴铵、酒石酸喷托铵、芬托氯铵和甲硫曲美替定。Quaternary Ammonium Compounds. Non-limiting examples of quaternary ammonium compounds include: Azalonium Bromide, Sodatrimonium Chloride, Hexamethonium Bromide, Pentadimethonium, Pentatrimonium Bromide, Pentropium Tartrate, Fentolium Chloride, and Methiotrime Reserved.
利血平衍生物。利血平衍生物的非限制性例子包括:比他舍平、地舍平、瑞西那明、利舍平和昔洛舍平。Reserpine derivatives. Non-limiting examples of reserpine derivatives include: bitaserpine, deserpine, resinanamine, reserpine, and syrosingopine.
磺胺类衍生物。磺胺类衍生物的非限制性例子包括:氨丁磺酰胺、氯哌胺、利尿磺胺、茚磺苯酰胺、喹噻酮、曲帕胺和氯磺水杨胺。Sulfonamide derivatives. Non-limiting examples of sulfonamide derivatives include: sulfasulfonamide, loperamide, diuresulfonamide, indenesulfonamide, quethilon, tripamide, and salicylamine.
g。血管加压药g. Vasopressors
血管加压药通常用于在外科手术休克期间提高血压。血管加压药,也称为抗低血压药的非限制性例子包括:甲硫阿美铵、血管紧张素酰胺、二甲福林、多巴胺、双苯次甲丁胺、依替福林、氨苯福林、间羟胺、甲氧胺福林、去甲肾上腺素、福勒德林和脱氧肾上腺素。Vasopressors are commonly used to increase blood pressure during surgical shock. Non-limiting examples of vasopressors, also known as antihypertensives, include: Methiamethonium, Angiotensinamide, Metformin, Dopamine, Dibenzidine, Etiforine, Amphenes Forint, Metaril, Metamine, Norepinephrine, Foradrin, and Synephrine.
vii.充血性心力衰竭的治疗药vii. Drugs for the treatment of congestive heart failure
充血性心力衰竭的治疗药的非限制性例子包括:抗血管紧张素II药、后载-预载降低治疗、利尿剂和变力药物。Non-limiting examples of therapeutic agents for congestive heart failure include: anti-angiotensin II drugs, afterload-preload reducing therapy, diuretics, and inotropic drugs.
a.后载-预载降低a. Rear load - preload reduction
在某些实施方式中,可以用联合疗法治疗不能耐受血管紧张素拮抗剂的患病动物。该疗法可联合给予肼屈嗪(肼苯哒嗪)和二硝酸异山梨醇酯(硝异梨醇,消心痛)。In certain embodiments, a diseased animal intolerant to an angiotensin antagonist can be treated with combination therapy. This therapy may be given in combination with hydralazine (hydralazine) and isosorbide dinitrate (isosorbide dinitrate, nitrate).
b.利尿剂b. Diuretics
利尿剂的非限制性例子包括:噻嗪类或苯丙噻二嗪衍生物(如阿尔噻嗪、苯氟噻嗪、苄噻嗪、苄基氢氯噻嗪、异丁噻嗪、氯噻嗪、氯噻嗪、氯噻酮、环戊噻嗪、依匹噻嗪、乙噻嗪、乙噻嗪、芬喹唑、氢氯噻嗪、氢氟噻嗪、甲氯噻嗪、美替克仑、美托拉宗、对氟噻嗪、聚噻嗪、四氯噻嗪、三氯噻嗪)、有机汞制剂(如氯汞君、美拉鲁利、mercamphamide、硫汞林钠、mercumallylic acid、汞香豆林钠、氯化亚汞、汞撒利)、蝶啶(如呋氨蝶啶、氨苯蝶啶)、嘌呤(如茶碱、7-吗啉代甲基茶碱、pamobrom、丙可可碱、可可碱)、包括醛固酮拮抗剂的类固醇(如坎利酮、夹竹桃苷、螺内酯)、磺胺类衍生物(如乙酰唑胺、氨丁磺酰胺、阿佐寒米、布美他尼、布他唑胺、氯米非那胺、双氯非那胺、氯哌胺、氯索隆、二苯基甲烷-4,4’-二磺胺、二磺法胺、依索唑胺、利尿磺胺、茚磺苯酰胺、美夫西特、醋甲唑胺、吡咯他尼、喹噻酮、托拉塞米、曲帕胺、氯磺水杨胺)、尿嘧啶(如氨美啶、阿米美啶)、钾轻微(sparing)拮抗剂(如阿米洛利、氨苯蝶啶)或其它利尿剂,如aminozine、熊果苷、氯拉扎尼、依他尼酸、依托唑啉、肼卡巴嗪、异山梨醇、甘露醇、美托查酮、莫唑胺、哌克昔林、ticrnafen和脲。Non-limiting examples of diuretics include: thiazides or phenprophiazine derivatives (such as althiazide, fenfluthiazide, benzylthiazide, benzylhydrochlorothiazide, isobuthiazide, chlorothiazide, chlorothiazide Chlorthalidone, Cyclopenthiazine, Epithiazide, Ethiazide, Ethiazide, Fenquinazole, Hydrochlorothiazide, Hydrofluorothiazide, Methiazide, Meticlen, Metolazone, p-fluorothiazide, polythiazide, tetrachlorothiazide, trichlorothiazide), organic mercury preparations (such as chloromercuric, melaruril, mercamphamide, thimerosal sodium, mercumallylic acid, mercury coumarin sodium, mercurous chloride, mercurial), pteridines (eg, furamterene, triamterene), purines (eg, theophylline, 7-morpholinomethyl theophylline, pamobrom, propobromine, theobromine) , steroids including aldosterone antagonists (e.g. canrenone, oleandrin, spironolactone), sulfonamide derivatives (e.g. acetazolamide, sulfamethene, azolamide, bumetanide, butazolamide, Clomirphenamide, Diclofenamide, Cloperamide, Cloxolone, Diphenylmethane-4,4'-Disulfonamide, Disulfonamide, Esozolid, Diuretic Sulfonamide, Indenesulfonamide , mefucilide, methazolamide, piretanide, quethirone, torasemide, tripamide, salicylamine), uracil (such as aminomethidine, amimetidine), potassium Sparing antagonists (eg, amiloride, triamterene) or other diuretics, such as aminozine, arbutin, clorazani, ethacrynic acid, etzoline, hydrazine, isosorbide Alcohol, mannitol, metochalone, mozolomide, perhexiline, ticrnafen, and urea.
c.变力药物c. Inotropic drugs
正变力剂,也称为强心剂的非限制性例子包括:茶碱、乙酰基洋地黄毒苷、2-氨基-4-甲基吡啶、氨利酮、半琥珀酸琥珀呋酯、布拉地辛、黄花夹竹桃次苷B(cerberosine)、樟吡他胺、铃兰毒苷、磁麻苷、地诺帕明、去乙酰毛花苷、洋地黄苷、洋地黄、洋地黄毒苷、地高辛、多巴酚丁胺、多巴胺、多培沙明、依诺昔酮、格木碱、非那可明、吉他林、羟基洋地黄毒苷、胍基乙酸、辛胺醇、白毛莨分碱、异波帕胺、毛花苷、甲氧酚酰胺(metamivamn)、米力农、黄夹次苷乙、夹竹桃苷、毒毛花苷G、奥昔非君、普瑞特罗、次海葱苷、残余蟾蜍配基、海葱苷、海葱苷宁、毒毛旋花苷(strophanthin)、硫马唑、可可碱和扎莫特罗。Non-limiting examples of orthotropic agents, also known as cardiotonic agents, include: theophylline, acetyl digoxigenin, 2-amino-4-picoline, amrinone, succifuryl hemisuccinate, bradidine Xin, oleandrin B (cerberosine), fenpetamide, lily of the valley glycoside, magnetojamin, denopamine, deacetyllanatoside, digitalis, digitalis, digitalis, Digoxin, Dobutamine, Dopamine, Dopexamine, Enoximone, Geminidine, Phenacomine, Guitarin, Hydroxydigoxigenin, Guanidinoacetic Acid, Octyl Amol, Baimao Panicine, isobopamine, lanatoside, metamivamn, milrinone, xanthoside B, oleandrin, ouabain G, oxyfidrine, pretel Luo, hyposcaleonin, residual bufagenin, squallionin, squallionin, strophanthin, thimazole, theobromine, and zamoterol.
在具体方面,变力药物是强心苷、β-肾上腺素能激动剂或磷酸二酯酶抑制剂。强心苷的非限制性例子包括:地高辛(拉诺辛)和洋地黄毒苷(crystodigin)。β-肾上腺素能激动剂的非限制性例子包括:沙丁胺醇,班布特罗,比托特罗,卡布特罗,克仑特罗,氯丙那林,地诺帕明,双羟乙麻黄碱,多巴酚丁胺(杜丁胺),多巴胺(intropin),多培沙明,麻黄碱,乙非君,乙基去甲肾上腺素,非诺特罗,福莫特罗,海索那林,异波帕胺,新异丙肾上腺素,异丙肾上腺素,马布特罗,奥西那林,甲氧那明,奥昔非君,吡布特罗,丙卡特罗,普罗托醇,瑞普特罗,利米特罗,利托君,索特瑞醇,特布他林,曲托喹酚,妥洛特罗和扎莫特罗。In specific aspects, the inotropic drug is a cardiac glycoside, a beta-adrenergic agonist, or a phosphodiesterase inhibitor. Non-limiting examples of cardiac glycosides include: digoxin (lanoxin) and digitoxin (crystodigin). Non-limiting examples of beta-adrenergic agonists include: albuterol, bambuterol, bitolterol, caboterol, clenbuterol, chlorprenaline, denopramine, pedied Alkaline, Dobutamine (Durbutamine), Dopamine (Intropin), Dopexamine, Ephedrine, Efedrine, Ethyl Norepinephrine, Fenoterol, Formoterol, Hysona Lin, Ibopamide, Neoisoproterenol, Isoproterenol, Mabuterol, Oxinaline, Methoxyphenamine, Oxifadrine, Pibuterol, Procaterol, Protol, Riproterol, rimiterol, ritodrine, soteretol, terbutaline, tritoquinol, tulobuterol, and zamoterol.
磷酸二酯酶抑制剂的非限制性例子包括氨利酮(强心隆)。A non-limiting example of a phosphodiesterase inhibitor includes Amrinone (Qianxinlong).
d.防心绞痛药d. Anti-anginal drugs
防心绞痛药可包括有机硝酸盐、钙通道阻断剂、β阻断剂及它们的组合。Antianginal drugs may include organic nitrates, calcium channel blockers, beta blockers, and combinations thereof.
有机硝酸盐,也称为硝基血管舒张剂的非限制性例子包括:硝酸甘油(贴保宁,三硝酸甘油),二硝酸异山梨醇酯(硝异梨醇,消心痛)和硝酸戊酯(aspirol,亚硝酸异戊酯)。Non-limiting examples of organic nitrates, also known as nitro vasodilators, include: nitroglycerin (Tibolin, glyceryl trinitrate), isosorbide dinitrate (isosorbide nitrate, nitrate), and amyl nitrate (aspirol, isoamyl nitrite).
D.外科治疗D. surgical treatment
在某些方面,第二种治疗可包括一些类型的外科治疗,其包括,例如,预防、诊断或进程、治愈性和姑息性外科治疗。外科手术,具体说是治愈性外科手术,可与其它疗法,如本发明和一种或多种其它药物联合使用。In certain aspects, the second treatment can include some type of surgical treatment including, for example, prophylactic, diagnostic or procedural, curative and palliative surgical treatment. Surgery, particularly curative surgery, may be used in combination with other therapies, such as the present invention and one or more other drugs.
本领域技术人员熟知用于血管和心血管疾病和失调的外科治疗方法、这些治疗方法包括但不限于、对生物体进行外科手术、提供心血管机械修复假体、血管重建术、冠状动脉再灌注、导管摘除术、为患者提供可植入的心脏电复律器除颤器、机械性循环支持或它们的组合。可用于本发明的机械性循环支持的非限制性例子包括主动脉内气囊对抗搏动法、左心室辅助装置或它们的组合。Surgical treatments for vascular and cardiovascular diseases and disorders are well known to those skilled in the art, including, but not limited to, surgery on an organism, provision of a cardiovascular mechanical prosthesis, revascularization, coronary reperfusion , catheter removal, providing the patient with an implantable cardioverter-defibrillator, mechanical circulatory support, or a combination thereof. Non-limiting examples of mechanical circulatory support that may be used in the present invention include intra-aortic balloon anti-pulsation, left ventricular assist devices, or combinations thereof.
E.药物制剂和向病人给药的途径E. Pharmaceutical formulations and routes of administration to patients
考虑到临床应用,将药物组合物制备成适合于应用的形式。通常,这需要制备基本无热原质和其它对人或动物可能有害的杂质的组合物。In consideration of clinical application, the pharmaceutical composition is prepared in a form suitable for the application. Typically, this entails preparing the composition substantially free of pyrogens and other impurities that may be harmful to humans or animals.
通常,需要采用合适的盐和缓冲液来产生稳定的和可以被靶细胞摄取的递送载体。当将重组细胞引入病人时,也使用缓冲液。本发明的水相组合物包含有效量的溶解或分散于药学上可接受运载体或水相介质中的载体或细胞。术语“药学上或药理上可接受的”指当给予动物或人时,不产生不良的、过敏或其它不利反应的分子实体和组合物。本文所用的“药学上可接受的运载体”包括可用于配制药物,如适于给予人的药物的溶剂、缓冲液、溶液、分散介质、包衣剂、抗菌剂和抗真菌剂、等渗和吸收延迟剂等。用于药学活性物质的这些介质和制剂的用途是本领域公知的。除了迄今已知与本发明活性成分不相容的任何常规介质或制剂外,也考虑用于治疗组合物中。也可将补充的活性成分掺入该组合物中,只要它们不使组合物的载体或细胞失活。In general, appropriate salts and buffers are required to produce a delivery vehicle that is stable and can be taken up by target cells. Buffers are also used when introducing recombinant cells into a patient. The aqueous composition of the present invention comprises an effective amount of carriers or cells dissolved or dispersed in a pharmaceutically acceptable carrier or aqueous medium. The term "pharmaceutically or pharmacologically acceptable" refers to molecular entities and compositions that do not produce adverse, allergic or other adverse reactions when administered to animals or humans. As used herein, "pharmaceutically acceptable carrier" includes solvents, buffers, solutions, dispersion media, coatings, antibacterial and antifungal agents, isotonic and Absorption delaying agent, etc. The use of such media and formulations for pharmaceutically active substances is well known in the art. In addition to any conventional media or formulations which are heretofore known to be incompatible with the active ingredients of the invention, also contemplated for use in therapeutic compositions. Supplementary active ingredients can also be incorporated into the compositions provided they do not inactivate the vector or cells of the composition.
本发明的活性组合物可包括经典的药学制剂。可通过任何常规途径给予本发明的这些组合物,只要通过该途径可到达靶组织。这包括口服、鼻腔或含服。或者,可以皮内、皮下、肌肉内、腹腔内或静脉内注射,或直接注射入心脏组织给药。如上所述,通常以药学上可接受的组合物给予这些组合物。The active compositions of the present invention may include classic pharmaceutical preparations. These compositions of the present invention may be administered by any conventional route so long as the target tissue is accessible by that route. This includes oral, nasal or buccal. Alternatively, administration may be by intradermal, subcutaneous, intramuscular, intraperitoneal or intravenous injection, or injection directly into cardiac tissue. These compositions are generally administered as pharmaceutically acceptable compositions, as described above.
也可经胃肠道外或腹腔内给予该活性化合物。为了说明,可用适当混有表面活性剂,如羟丙基纤维素的水制备该活性化合物的游离碱或药理上可接受的盐溶液。也可用甘油、液体聚乙二醇和它们的混合物以及用油制备分散液。在普通的储存和使用条件下,这些制剂通常含有防腐剂,以防止微生物生长。The active compound can also be administered parenterally or intraperitoneally. For illustration, free base or pharmaceutically acceptable salt solutions of the active compounds can be prepared in water suitably mixed with a surfactant, such as hydroxypropylcellulose. Dispersions can also be prepared in glycerol, liquid polyethylene glycols, and mixtures thereof and in oils. Under ordinary conditions of storage and use, these preparations usually contain a preservative to prevent the growth of microorganisms.
适合注射用的药物形式包括,例如,无菌水溶液或分散液和可用于即时制备的无菌可注射溶液或分散液的无菌粉末。通常,这些制剂应无菌,其流动性易于注射。在制造和储存的条件下,制剂应稳定,应能抵抗微生物,如细菌和真菌的污染作用。合适的溶剂或分散介质可包括,例如水、乙醇、多元醇(如甘油、丙二醇和液体聚乙二醇等),它们的合适混合物和植物油。可通过,例如使用包衣剂,如卵磷脂维持合适的流动性,对于分散液可用表面活性剂维持所需粒度。可通过各种抗菌剂和抗真菌剂,如对羟基苯甲酸脂类、氯丁醇、苯酚、山梨酸、硫柳汞等防止微生物的作用。在很多情况下,将优选包括等渗剂,如糖或氯化钠。通过与延迟吸收剂,如单硬脂酸铝和明胶组合,可以实现可注射组合物的延长吸收。The pharmaceutical forms suitable for injectable use include, for example, sterile aqueous solutions or dispersions and sterile powders for the extemporaneous preparation of sterile injectable solutions or dispersion. In general, these preparations should be sterile and fluid for easy syringability. The preparations should be stable under the conditions of manufacture and storage and should be resistant to the contaminating action of microorganisms, such as bacteria and fungi. Suitable solvents or dispersion media can include, for example, water, ethanol, polyols (such as glycerol, propylene glycol, liquid polyethylene glycol, and the like), suitable mixtures thereof, and vegetable oils. Proper fluidity can be maintained, for example, by the use of coatings such as lecithin, and for dispersions surfactants can be used to maintain the desired particle size. Prevention of the action of microorganisms can be achieved by various antibacterial and antifungal agents, for example, parabens, chlorobutanol, phenol, sorbic acid, thimerosal, and the like. In many cases it will be preferable to include isotonic agents, such as sugars or sodium chloride. Prolonged absorption of the injectable compositions can be brought about by combination with agents which delay absorption, for example, aluminum monostearate and gelatin.
可通过将合适量的活性化合物与任何其它所需成分(例如,上述所列)一起掺入溶剂中,然后过滤除菌来制备无菌注射液。通常,通过将各种无菌活性成分掺入含有基本分散介质和所需其它成分(如上述所列)的无菌载体来制备分散液。在用于制备无菌注射液的无菌粉末时,优选的方法包括真空干燥和冻干技术,以此从之前过滤除菌的溶液中产生活性成分加任意附加所需成分的粉末。Sterile injectable solutions can be prepared by incorporating the active compound in an appropriate amount in a solvent along with any other desired ingredients (eg, from those listed above), followed by filtered sterilization. Generally, dispersions are prepared by incorporating the various sterilized active ingredients into a sterile vehicle which contains the basic dispersion medium and the required other ingredients from those enumerated above. In the case of sterile powders for the preparation of sterile injectable solutions, preferred methods include vacuum drying and the freeze-drying technique which yield a powder of the active ingredient plus any additional desired ingredient from a previously sterile-filtered solution thereof.
对于口服给药来说,通常可将本发明多肽与赋形剂掺和,以不可消化的漱口水和洁齿剂的形式使用。可通过将所需量的活性成分掺入合适的溶剂,如硼酸钠溶液(Dobell’s溶液)中制备漱口水。另外,可将活性成分掺入含有硼酸钠、甘油和碳酸氢钾的消毒水中。也可将活性成分分散到洁齿剂,包括:凝胶、膏、粉和浆中。可以将治疗有效量的活性成分加入牙膏中,牙膏可包括水、粘合剂、研磨剂、调味剂、发泡剂和湿润剂。For oral administration, the polypeptides of the present invention are usually mixed with excipients and used in the form of non-digestible mouthwashes and dentifrices. Mouthwashes can be prepared by incorporating the active ingredient in the required amount in a suitable solvent, such as sodium borate solution (Dobell's solution). Alternatively, the active ingredient may be incorporated into sterile water containing sodium borate, glycerin and potassium bicarbonate. The active ingredient can also be dispersed in dentifrices including: gels, pastes, powders and slurries. A therapeutically effective amount of the active ingredient can be incorporated into toothpaste which may include water, binders, abrasives, flavoring agents, foaming agents and humectants.
通常可将本发明组合物制成中性或盐形式。药学上可接受的盐包括,例如,获自无机酸(如盐酸或磷酸)或有机酸(如乙酸、草酸、酒石酸、扁桃酸等)的酸加成盐(与蛋白的游离氨基形成)。与蛋白的游离羧基形成的盐也可获自无机碱(如氢氧化钠、钾、铵、钙或铁)或有机碱(如异丙胺、三甲胺、组氨酸、普鲁卡因等。Generally, the compositions of the present invention can be prepared in neutral or salt form. Pharmaceutically acceptable salts include, for example, acid addition salts (formed with free amino groups of proteins) from inorganic acids such as hydrochloric acid or phosphoric acid or organic acids such as acetic acid, oxalic acid, tartaric acid, mandelic acid and the like. Salts formed with the free carboxyl groups of proteins can also be obtained from inorganic bases such as sodium, potassium, ammonium, calcium, or iron, or organic bases such as isopropylamine, trimethylamine, histidine, procaine, and the like.
关于剂型,优选以剂型相容形式和治疗有效量给予溶液。该剂型可以容易地以各种剂型,如注射液、药物释放胶囊等给予。对于水溶液的胃肠道外给药来说,例如,通常合适地缓冲该溶液,先使用,例如足够的盐水或葡萄糖使液体稀释剂等渗。可以将这些水溶液用于,例如,静脉内、肌内、皮下和腹腔内给药。优选地,如本领域技术人员所知,尤其是依照本发明公开的内容来使用无菌水相介质。为了说明,可将单剂溶解在1毫升等渗NaCl溶液中,然后加入到1000毫升皮下灌注液体中或在计划的注入位点注射,(参见例如,“雷明顿药物科学”第15版,第1035-1038和1570-1580页)。有必要根据受试者的情况对剂量作出某些改变。负责给药的人一定要为个体受试者确定合适的剂量。而且,对于人的给药,制剂的无菌性、致热性、总的安全性和纯度标准应该满足FDA生物制品标准办公室的要求。With regard to dosage form, solutions are preferably administered in a form compatible with the dosage form and in a therapeutically effective amount. The dosage form can be easily administered in various dosage forms, such as injection, drug release capsules and the like. For parenteral administration in aqueous solutions, for example, the solutions will usually be suitably buffered and the liquid diluent first rendered isotonic with, for example, sufficient saline or glucose. These aqueous solutions can be used, for example, for intravenous, intramuscular, subcutaneous and intraperitoneal administration. Preferably, sterile aqueous media are used as known to those skilled in the art, especially in light of the present disclosure. For illustration, a single dose can be dissolved in 1 mL of isotonic NaCl solution and added to 1000 mL of subcutaneous infusion fluid or injected at the planned infusion site, (see, e.g., "Remington Pharmaceutical Sciences," 15th ed., pp. 1035-1038 and 1570-1580). Some variation in dosage may be necessary depending on the condition of the subject. Those responsible for administering the drug will be sure to determine the appropriate dose for the individual subject. Furthermore, for human administration, the formulation's sterility, pyrogenicity, general safety, and purity standards should meet FDA's Office of Biologics Standards requirements.
V.筛选方法V. Screening method
本发明还包括鉴定用于预防或治疗或逆转心脏肥大或心力衰竭的PKD抑制剂的方法。这些测定可包括随机筛选候选物质的大文库;另外,该测定可用于集中在通过结构属性选择的具体类别的化合物上,据信该结构属性使它们更可能抑制PKD功能。The present invention also includes methods of identifying PKD inhibitors for preventing or treating or reversing cardiac hypertrophy or heart failure. These assays can involve random screening of large libraries of candidate substances; alternatively, the assay can be used to focus on specific classes of compounds selected by structural attributes believed to make them more likely to inhibit PKD function.
为鉴定PKD抑制剂,人们通常将确定在存在和不存在候选物质的情况下PKD的功能。例如,方法通常包括:To identify PKD inhibitors, one will typically determine the function of PKD in the presence and absence of a candidate substance. For example, methods often include:
(a)提供候选调节剂;(a) providing candidate modulators;
(b)将候选调节剂与PKD混合;(b) admixing the candidate modulator with PKD;
(c)测量PKD激酶活性;和(c) measuring PKD kinase activity; and
(d)比较步骤(c)中的活性和不存在候选调节剂的情况下的活性,(d) comparing the activity in step (c) to the activity in the absence of a candidate modulator,
其中测量到的活性差异说明候选调节剂实际上是该化合物、细胞或动物的调节剂。The difference in activity measured therein indicates that the candidate modulator is indeed a modulator of the compound, cell or animal.
也可在分离的细胞、器官或在活生物中进行测定。一般地,通过提供未磷酸化的II类HDAC和测量由PKD加入的标记量来测量PKD的激酶活性。Assays can also be performed in isolated cells, organs, or in living organisms. Generally, the kinase activity of PKD is measured by providing unphosphorylated class II HDACs and measuring the amount of label added by PKD.
当然应该理解,尽管可能没有找到有效的候选物,但本发明的所有筛选方法本身是有用的。本发明提供了筛选这些候选物的方法,而不仅是找到它们的方法。It will of course be understood that all screening methods of the invention are useful in themselves, although no effective candidate may be found. The present invention provides methods to screen for these candidates, not just to find them.
A.调节剂A. Regulator
本文使用的术语“候选物质”指可能抑制PKD的激酶活性或细胞功能的任何分子。候选物质可以是蛋白或其片段、小分子,或甚至是核酸。可以证明,药理上最有效的化合物是与已知PKC抑制剂结构相关的化合物,在本申请中的其它位置列出。用先导化合物帮助开发改进的化合物被称为“理性药物设计”,不仅包括与已知抑制剂和激活物比较,还包括靶分子结构的预测。The term "candidate substance" as used herein refers to any molecule that may inhibit the kinase activity or cellular function of PKD. Candidate substances can be proteins or fragments thereof, small molecules, or even nucleic acids. It can be shown that the most pharmacologically effective compounds are those structurally related to known PKC inhibitors, listed elsewhere in this application. Using lead compounds to help develop improved compounds is called "rational drug design" and includes not only comparisons with known inhibitors and activators, but also the prediction of target molecular structures.
理性药物设计的目标是产生生物活性多肽或靶化合物的结构类似物。通过建立这些类似物,就可能形成比天然分子活性更高或更稳定的药物,它对变化具有不同的易感性,或可能影响各种其它分子的功能。在一个方法中,人们将生成靶分子或其片段的三维结构。这可通过X射线晶体学、计算机建模或通过两种方法的组合来完成。The goal of rational drug design is to generate structural analogs of biologically active polypeptides or target compounds. By creating these analogs, it is possible to create drugs that are more active or more stable than the natural molecule, have different susceptibility to changes, or may affect the function of various other molecules. In one approach, one will generate a three-dimensional structure of a target molecule or fragment thereof. This can be done by X-ray crystallography, computer modeling or by a combination of both methods.
也可能用抗体确证靶化合物、激活物或抑制剂的结构。原则上,该方法产生一个药核心,后续的药物设计可以此为基础进行。可能通过生成功能、药理活性抗体的抗-独特型抗体而完全回避蛋白晶体学。作为镜像的镜像,预计抗独特型抗体的结合位点是原始抗原的类似物。然后,抗-独特型抗体可用于从化学或生物学生产的肽库中鉴定和分离肽。然后,选择的肽将用作药核心。可用本文中描述的生产抗体的方法,将抗体用作抗原,生成抗-独特型抗体。It is also possible to use antibodies to confirm the structure of a target compound, activator or inhibitor. In principle, this approach yields a drug core upon which subsequent drug design can be based. It is possible to sidestep protein crystallography altogether by generating anti-idiotypic antibodies of functional, pharmacologically active antibodies. As a mirror image of a mirror image, the binding site of an anti-idiotypic antibody is expected to be an analog of the original antigen. Anti-idiotypic antibodies can then be used to identify and isolate peptides from chemically or biologically produced peptide libraries. The selected peptides will then be used as drug cores. Anti-idiotypic antibodies can be produced using the antibody as an antigen using the methods for producing antibodies described herein.
另一方面,人们可以简单地从各种商业资源中购得据信满足有用药物基本标准的小分子文库,以“强力进行”有用化合物的鉴定。筛选这些文库,包括组合产生的文库(例如,肽文库),是快速有效地筛选大量相关(和不相关)化合物活性的方法。组合方法也通过建立第二、第三和第四代化合物帮助其自身快速发展出潜在药物,这些化合物模拟(modeled)具有活性、却不需要的化合物。On the other hand, one can "robust" the identification of useful compounds by simply purchasing libraries of small molecules from various commercial sources that are believed to meet the basic criteria for a useful drug. Screening of these libraries, including combinatorially generated libraries (eg, peptide libraries), is a method for rapidly and efficiently screening large numbers of related (and unrelated) compounds for activity. The combinatorial approach also aids in its own rapid development of potential drugs by creating second, third and fourth generation compounds that model active but unwanted compounds.
候选化合物可包括天然存在的化合物的片段或部分,或也可能是没有活性的已知化合物的活性组合。建议可将分离自天然来源,如动物、细菌、真菌、包括树叶和树皮的植物来源和海洋样品的化合物作为候选物测定可能有用药剂的存在。应理解,待筛选药剂也可获自或合成自化学组合物或人造化合物。因此,应理解本发明鉴定的候选物质可能是从已知抑制剂或刺激物通过理性药物设计的肽、多肽、聚核苷酸、小分子抑制剂或任何其他化合物。Candidate compounds may include fragments or portions of naturally occurring compounds, or may also be active combinations of known compounds that are inactive. It is suggested that compounds isolated from natural sources such as animals, bacteria, fungi, plant sources including leaves and bark, and marine samples can be tested as candidates for the presence of potentially useful agents. It is understood that agents to be screened may also be obtained or synthesized from chemical compositions or man-made compounds. Therefore, it should be understood that the candidate substances identified in the present invention may be peptides, polypeptides, polynucleotides, small molecule inhibitors or any other compounds designed by rational medicine from known inhibitors or stimuli.
其它合适的调节剂包括反义分子、核酶和抗体(包括单链抗体),各自对靶分子具有特异性。在本文中的其它地方更详细地描述了这些化合物。例如,结合到翻译或转录起始位点、或剪接接头的反义分子是理想的候选抑制剂。Other suitable modulators include antisense molecules, ribozymes, and antibodies (including single chain antibodies), each specific for the target molecule. These compounds are described in more detail elsewhere herein. For example, antisense molecules that bind to translational or transcriptional initiation sites, or splice junctions are ideal candidate inhibitors.
除了最初鉴定的调节化合物,本发明者也考虑到可能设计其它空间排列类似的化合物,以模仿调节剂结构核心部分。可包括肽调节剂的肽模拟物的这些化合物可以相同方式用作起始调节剂。In addition to the initially identified modulator compounds, the inventors also contemplated the possibility of designing other sterically similar compounds to mimic the structural core of the modulator. These compounds, which may include peptidomimetics of peptide modulators, may be used in the same manner as initial modulators.
B.体外测定B. In Vitro Assays
进行的快速、廉价和容易的测定是体外测定。这些测定通常使用分离的分子,可以迅速和大量地完成,从而提高在短时间内可获得的信息量。各种器皿均可用于进行这些测定,包括试管、板、皿和其它表面如标尺或珠子。A quick, cheap and easy assay to perform is the in vitro assay. These assays typically use isolated molecules and can be done rapidly and in large quantities, increasing the amount of information available in a short amount of time. A variety of vessels can be used to perform these assays, including test tubes, plates, dishes, and other surfaces such as rulers or beads.
WO 84/03564中描述了高通量筛选化合物的技术。在固体底材,如塑料杆或其它表面上合成大量小肽测试化合物。可以根据它们结合和抑制PKD的能力快速筛选这些肽。Techniques for high throughput screening of compounds are described in WO 84/03564. A large number of small peptide test compounds are synthesized on a solid substrate, such as a plastic rod or other surface. These peptides can be rapidly screened for their ability to bind and inhibit PKD.
C.细胞内测定C. Intracellular Assay
本发明也考虑到根据化合物在细胞内调节PKD的能力筛选化合物。各种细胞系均可用于这些筛选测定,包括专门为此目的设计的细胞。The present invention also contemplates screening compounds based on their ability to modulate PKD in cells. A variety of cell lines are available for these screening assays, including cells designed specifically for this purpose.
D.体内测定D. In vivo assays
体内测定包括使用各种心脏病动物模型,包括转基因动物,它们被设计具体特定的缺陷,或载有可用于测量候选化合物在生物体内到达和影响不同细胞的能力的标记。由于它们的大小、容易操作和它们生理和遗传构成信息,小鼠是优选的实施方式,尤其适于转基因。然而,其它动物也是合适的,包括大鼠、家兔、仓鼠、豚鼠、沙鼠、土拨鼠、猫、狗、绵羊、山羊、猪、牛、马和猴(包括黑猩猩、长臂猿和狒狒)。可以使用获自这些种的任一动物模型进行抑制剂的测定。In vivo assays include the use of various animal models of heart disease, including transgenic animals that have been engineered to have a specific defect, or loaded with markers that can be used to measure the ability of candidate compounds to reach and affect different cells within the organism. Due to their size, ease of handling and information about their physiological and genetic makeup, mice are a preferred embodiment, especially for transgenics. However, other animals are also suitable, including rats, rabbits, hamsters, guinea pigs, gerbils, woodchucks, cats, dogs, sheep, goats, pigs, cows, horses and monkeys (including chimpanzees, gibbons and baboons). Assays for inhibitors can be performed using any animal model obtained from these species.
用测试化合物处理动物将包括以合适形式将化合物给予动物。将通过可用于临床目的的任一途径给药。确定化合物在体内的有效性可包括各种不同标准,包括但不限于。也可以通过比体外或细胞内测定更有意义的方式在动物中进行毒性和剂量反应的测定。Treatment of an animal with a test compound will involve administering the compound to the animal in an appropriate form. Administration will be by any route available for clinical purposes. Determining the effectiveness of a compound in vivo can involve a variety of different criteria including, but not limited to. Toxicity and dose-response assays can also be performed in animals in a more meaningful manner than in vitro or intracellular assays.
VI.纯化蛋白VI. Purified Protein
需要纯化PKD。蛋白纯化技术是本领域技术人员熟知的。这些技术在一个水平上包括,将细胞整体粗分成多肽和非-多肽部分。将多肽与其它蛋白分离后,可用层析或电泳技术将感兴趣的多肽进一步纯化,以达到部分或完全纯化(或纯化至同质)。尤其适用于纯肽制备的分析方法是离子交换层析,排阻层析;聚丙烯酰胺凝胶电泳;等电点聚焦。尤其有效的纯化肽的方法是快速蛋白液相层析或甚至HPLC。PKD needs to be purified. Protein purification techniques are well known to those skilled in the art. These techniques involve, at one level, the coarse division of the cell body into polypeptide and non-polypeptide fractions. After separating the polypeptide from other proteins, the polypeptide of interest can be further purified by chromatography or electrophoretic techniques to achieve partial or complete purification (or purification to homogeneity). Analytical methods especially suitable for the preparation of pure peptides are ion exchange chromatography, size exclusion chromatography; polyacrylamide gel electrophoresis; isoelectric focusing. A particularly effective method of purifying peptides is fast protein liquid chromatography or even HPLC.
本发明某些方面涉及编码蛋白或肽的纯化,在具体实施方式中,是基本纯化。本文中使用的术语“纯化的蛋白或肽”意指可分离自其它组分的组合物,其中该蛋白或肽相对于其天然可获得状态纯化至任意程度。因此,纯化蛋白或肽也指从其天然存在的环境中游离出的蛋白或肽。Certain aspects of the invention relate to purification, in particular embodiments, substantial purification, of encoded proteins or peptides. The term "purified protein or peptide" as used herein means a composition isolatable from other components wherein the protein or peptide is purified to any degree relative to its naturally available state. Thus, a purified protein or peptide also refers to a protein or peptide that has been freed from its naturally occurring environment.
通常,“纯化的”将指已进行分级以去除各种其它组分的蛋白或肽组合物,组合物基本保持其表达的生物活性。其中使用术语“基本纯化”,该术语指一种组合物,其中蛋白或肽形成该组合物的主要组分,如占组合物中蛋白的约50%、约60%、约70%、约80%、约90%、约95%或更多。Generally, "purified" will refer to a protein or peptide composition that has been fractionated to remove various other components, the composition substantially retaining its expressed biological activity. Where the term "substantially purified" is used, the term refers to a composition in which the protein or peptide forms a major component of the composition, such as about 50%, about 60%, about 70%, about 80% of the protein in the composition %, about 90%, about 95% or more.
本领域技术人员根据本公开,将知晓定量蛋白或肽纯化程度的各种方法。这些方法包括,例如,确定活性部分的特异活性,或通过SDS/PAGE分析在某部分内评价多肽量。评价某部分纯度的优选方法是计算该部分的特异活性,将其与最初抽提物的特异活性比较,从而计算纯度,本文评价为“纯化倍数”。当然,用于代表活性量的实际单位将取决于根据纯化和是否表达的蛋白或肽表现出可检测的活性而选择的具体测定技术。Various methods for quantifying the degree of protein or peptide purification will be known to those skilled in the art from this disclosure. These methods include, for example, determining the specific activity of active fractions, or assessing the amount of polypeptide within a fraction by SDS/PAGE analysis. A preferred method of assessing the purity of a fraction is to calculate the specific activity of the fraction and compare it to the specific activity of the original extract to calculate the purity, evaluated herein as "fold purification". The actual units used to represent an amount of activity will, of course, depend on the particular assay technique chosen based on purification and whether the expressed protein or peptide exhibits detectable activity.
各种适用于蛋白纯化的技术是本领域技术人员所熟知的。这些技术包括,例如,用硫酸铵、PEG、抗体等或通过加热变性而沉淀,然后离心;层析步骤如离子交换、凝胶过滤、反向、羟磷灰石和亲核层析;等电点聚焦;凝胶电泳;和这些和其它技术的组合。如本领域所公知的,相信进行各种纯化步骤的顺序可以改变,或某些步骤可以省略,仍然产生用于制备基本纯化的蛋白或肽的合适方法。Various techniques suitable for protein purification are well known to those skilled in the art. These techniques include, for example, precipitation with ammonium sulfate, PEG, antibodies, etc. or denaturation by heat followed by centrifugation; chromatographic steps such as ion exchange, gel filtration, reverse, hydroxyapatite, and nucleophilic chromatography; isoelectric point focusing; gel electrophoresis; and combinations of these and other techniques. As is known in the art, it is believed that the order in which the various purification steps are performed may be altered, or that certain steps may be omitted, and still result in a suitable method for preparing a substantially purified protein or peptide.
并没有总的要求总是以最纯化状态提供蛋白或肽。实际上,考虑了在某些实施方式中可使用基本纯化的更少产物。可以通过在组合中使用更少的纯化步骤,或通过使用相同总纯化方案的不同形式来完成部分纯化。例如,应理解用HPLC设备进行的阳离子交换柱层析通常将导致比用低压层析系统的相同技术的纯化“倍数”更高。具有较低相关纯化程度的方法在总的蛋白产物回收方面,或在维持表达蛋白的活性方面会具有优势。There is no general requirement that a protein or peptide always be provided in its most purified state. Indeed, it is contemplated that substantially purified less product may be used in certain embodiments. Partial purification can be accomplished by using fewer purification steps in combination, or by using different versions of the same overall purification scheme. For example, it is understood that cation exchange column chromatography with HPLC equipment will generally result in a higher "fold" of purification than the same technique with a low pressure chromatography system. A method with a lower relative degree of purification may have advantages in terms of overall protein product recovery, or in maintaining the activity of the expressed protein.
已知在不同的SDS/PAGE条件下多肽的迁移可改变,有时还很明显(Capaldi等,1977)。因此应理解,在不同电泳条件下,显示出的纯化或部分纯化表达产物的分子量可能改变。It is known that the migration of polypeptides can vary, sometimes significantly, under different SDS/PAGE conditions (Capaldi et al., 1977). It is therefore to be understood that under different electrophoretic conditions, the molecular weights of purified or partially purified expression products shown may vary.
高效液相色谱(HPLC)以快速分离并具有出色的峰分辨率为特征。这通过使用非常精细的颗粒和高压,以维持足够流速来实现。大约几分钟、或最多一小时内就可完成分离。而且,仅需要很小体积的样品,因为该颗粒是很小且紧密封装的,使空隙体积占柱床体积的很小部分。样品浓度也不需要很高,因为其条带很窄,样品几乎没有稀释。High performance liquid chromatography (HPLC) is characterized by rapid separations with excellent peak resolution. This is achieved by using very fine particles and high pressure to maintain sufficient flow rates. Separation can be accomplished in a matter of minutes, or up to an hour. Also, only a small volume of sample is required because the particles are small and tightly packed, making the void volume a very small fraction of the bed volume. The sample concentration also does not need to be very high because the bands are very narrow and the sample is hardly diluted.
凝胶层析或分子筛层析是基于分子量的特殊分配层析类型。凝胶层析背后的理论是,用含有小孔的惰性物质的微小颗粒制备的柱子,在分子通过或传送到小孔时,根据它们的大小从较小分子中分离较大分子。只要制成颗粒的材料不吸收该分子,那么确定流速的唯一因素是大小。因此,只要其形状是相对不变的,分子就以大小降低的方式从柱中洗脱出来。对于分离不同大小的分子,凝胶层析是非常卓越的,因为分离不依赖于所有其它因素,如pH、离子强度、温度等。实质上也没有吸收,更小的区域扩展和洗脱体积简单地与分子量相关。Gel chromatography or molecular sieve chromatography is a special type of partition chromatography based on molecular weight. The theory behind gel chromatography is that columns made of tiny particles of an inert substance containing pores separate larger molecules from smaller molecules according to their size as the molecules pass or are transported through the pores. As long as the material from which the particles are made does not absorb the molecule, the only factor determining flow rate is size. Thus, as long as its shape is relatively unchanged, the molecule elutes from the column in a size-reducing manner. For separating molecules of different sizes, gel chromatography is excellent because the separation is not dependent on all other factors like pH, ionic strength, temperature, etc. There is also virtually no absorption, with less domain expansion and elution volumes simply related to molecular weight.
亲和层析是依赖待分离物质和它可特异性结合的分子之间特异性亲和力的层析过程。这是受体-配体类型的相互作用。通过将结合对的一方共价偶联到不溶性基质上合成该柱材料。然后,该柱材料能够从溶液中特异性吸收该物质。通过将条件改变为不会发生结合的条件(改变pH、离子强度、温度等)进行洗脱。Affinity chromatography is a chromatographic process that relies on the specific affinity between the substance to be separated and the molecules to which it can specifically bind. This is a receptor-ligand type of interaction. The column material is synthesized by covalently coupling one side of the binding pair to an insoluble matrix. The column material is then able to specifically absorb the substance from solution. Elution is performed by changing conditions to those where binding does not occur (changing pH, ionic strength, temperature, etc.).
用于纯化含有碳水化合物的亲和层析具体类型是凝集素亲和层析。凝集素是一类与各种多糖和糖蛋白结合的物质。凝集素通常通过溴化氰与琼脂糖偶联。与琼脂糖偶联的伴刀豆球蛋白(Conconavalin)A是第一个使用的这类材料,已经广泛用于分离多糖和糖蛋白。其它凝集素包括小扁豆凝集素、已用于纯化N-乙酰基葡糖胺残基的小麦胚芽凝集素和勃艮第蜗牛(Helix pomatia)凝集素。用使用碳水化合物配体的亲和层析纯化凝集素本身。用半乳糖从蓖麻子和花生中纯化凝集素;麦芽糖用于从小扁豆和刀豆中抽提凝集素;N-乙酰基-D葡糖胺用于从黄豆中纯化凝集素;N-乙酰葡糖胺基与来自小麦胚芽的凝集素结合;D-葡糖胺用于从蛤中获得凝集素,L-海藻糖将结合莲中的凝集素。A specific type of affinity chromatography used for purification of carbohydrate-containing is lectin affinity chromatography. Lectins are a class of substances that bind to various polysaccharides and glycoproteins. Lectins are usually coupled to agarose via cyanogen bromide. Concanavalin A coupled to agarose was the first material of its kind to be used and has been widely used for the separation of polysaccharides and glycoproteins. Other lectins include lentil agglutinin, wheat germ agglutinin and Burgundy snail (Helix pomatia) agglutinin which have been used to purify N-acetylglucosamine residues. The lectins themselves are purified by affinity chromatography using carbohydrate ligands. Galactose was used to purify lectins from castor beans and peanuts; maltose was used to extract lectins from lentils and beans; N-acetyl-D-glucosamine was used to purify lectins from soybeans; N-acetylglucose The amine groups bind to lectins from wheat germ; D-glucosamine is used to obtain lectins from clams, and L-trehalose will bind to lectins from lotus.
基质应该是本身不以任何明显程度吸收分子的物质,并且具有较宽的化学、物理和热稳定性范围。配体应该以不影响其结合性质的方式偶联。配体也应提供相对紧密的结合。应可能在不破坏样品或配体的情况下洗脱物质。最常见的亲和层析形式之一是免疫亲和层析。下面描述适用于本发明的抗体的产生。The matrix should be a substance that does not itself absorb molecules to any appreciable degree, and has a broad range of chemical, physical and thermal stability. Ligands should be coupled in a manner that does not affect their binding properties. The ligand should also provide relatively tight binding. It should be possible to elute the substance without destroying the sample or the ligand. One of the most common forms of affinity chromatography is immunoaffinity chromatography. The production of antibodies suitable for use in the present invention is described below.
VII.克隆载体、基因转移和表达VII. Cloning Vectors, Gene Transfer and Expression
在某些实施方式中,用表达载体表达PKD多肽产物,然后纯化该产物。在其它实施方式中,该表达载体可用于基因治疗。表达需要载体中提供合适的信号,包括各种调节元件,如来自病毒和哺乳动物来源的在宿主细胞中驱动感兴趣基因表达的增强子/启动子。也定义了设计用于使信使RNA稳定性和在宿主细胞中的可翻译性最优化的元件,使信使RNA稳定性最优化,也确定在宿主细胞中的可翻译性。也提供了为建立表达该产物的永久、稳定的细胞克隆,使用很多显性药物选择标记的条件,或者说是将药物选择标记的表达与多肽表达相联系的元件。In certain embodiments, an expression vector is used to express a PKD polypeptide product, and the product is then purified. In other embodiments, the expression vector can be used in gene therapy. Expression requires the provision of appropriate signals in the vector, including various regulatory elements such as enhancers/promoters from viral and mammalian sources that drive expression of the gene of interest in the host cell. Elements designed to optimize messenger RNA stability and translatability in host cells are also defined, optimizing messenger RNA stability and also determining translatability in host cells. Also provided are conditions for the use of a number of dominant drug-selectable markers for establishing permanent, stable cell clones expressing the product, or elements linking the expression of the drug-selectable marker to the expression of the polypeptide.
A.调节元件A. Adjustment element
该申请全文中的术语“表达构建物”意于包括含有编码基因产物的核酸的任意类型遗传构建物,其中编码序列的部分或全部核酸能够被转录。可将转录物翻译成蛋白,但不必如此。在某些实施方式中,表达包括基因转录和mRNA翻译成基因产物。在其它实施方式中,表达仅包括转录编码感兴趣基因的核酸。The term "expression construct" throughout this application is intended to include any type of genetic construct comprising nucleic acid encoding a gene product, wherein part or all of the nucleic acid encoding sequence is capable of being transcribed. Transcripts can be translated into protein, but need not be. In certain embodiments, expression includes gene transcription and translation of mRNA into a gene product. In other embodiments, expression only includes transcription of nucleic acid encoding the gene of interest.
在某些实施方式中,编码基因产物的核酸在启动子的转录控制下。“启动子”指细胞合成机器或引入的合成机器识别的DNA序列,需要它起始特异的基因转录。术语“在转录控制下”指相对于该核酸,启动子在正确位置和方向上,以控制RNA聚合酶起始和表达该基因。In certain embodiments, a nucleic acid encoding a gene product is under the transcriptional control of a promoter. "Promoter" refers to a DNA sequence recognized by the cellular synthetic machinery, or introduced synthetic machinery, that is required to initiate transcription of a specific gene. The term "under transcriptional control" means that the promoter is in the correct position and orientation relative to the nucleic acid to control RNA polymerase initiation and expression of the gene.
这里用术语启动子指簇集在RNA聚合酶II起始位点周围的转录控制模块群。关于启动子如何组织的很多想法得自对几个病毒启动子的分析,包括对HSV胸腺嘧啶激酶(tk)和SV40早期转录单位的分析。这些研究,加上最近的很多工作,显示出启动子由不连续的功能模块构成,这些功能模块各自由约7-20bp的DNA组成,并含有一个或多个转录激活或抑制蛋白的识别位点。The term promoter is used herein to refer to a group of transcriptional control modules clustered around the initiation site of RNA polymerase II. Much of the idea of how promoters are organized came from the analysis of several viral promoters, including analysis of the HSV thymidine kinase (tk) and SV40 early transcription units. These studies, together with much more recent work, have shown that promoters are composed of discrete functional modules each consisting of approximately 7-20 bp of DNA and containing recognition sites for one or more transcriptional activator or repressor proteins .
各启动子中至少一个模块的功能是为RNA合成定位起始位点。最为熟知的实例是TATA框,但是在一些缺少TATA框的启动子中,如哺乳动物末端脱氧核苷酸转移酶基因的启动子和SV40后期基因的启动子,位于起始位点本身上的不连续元件帮助固定起始的位置。The function of at least one module in each promoter is to position the initiation site for RNA synthesis. The best-known example is the TATA box, but in some promoters lacking the TATA box, such as the promoter of the mammalian terminal deoxynucleotidyl transferase gene and the promoter of the SV40 late gene, there is a difference in the start site itself. Continuous elements help fix the starting position.
附加启动子元件调节转录起始的频率。一般地,这些元件定位于起始位点上游30-110bp的区域,虽然最近很多启动子显示出在起始位点下游也含有功能元件。启动子元件之间的间隔经常是可变的,以致在元件被反转或互相移动时保持启动子功能。在tk启动子中,活性开始下降前,启动子元件之间的间隔可增加到50bp远。根据启动子来看,似乎单个元件可合作地或独立地用作激活转录。Additional promoter elements regulate the frequency of transcription initiation. Typically, these elements are located in a region 30-110 bp upstream of the initiation site, although recently many promoters have been shown to contain functional elements downstream of the initiation site as well. The spacing between promoter elements is often variable so that promoter function is maintained when elements are inverted or moved relative to one another. In the tk promoter, the spacing between promoter elements can be increased by up to 50 bp before activity begins to decline. Depending on the promoter, it appears that individual elements may act cooperatively or independently to activate transcription.
在某些实施方式中,将使用天然PKD启动子驱动相应的PKD基因、异源PKD基因、可筛选或可选择性的标记基因、或任何其它感兴趣基因的表达。In certain embodiments, a native PKD promoter will be used to drive the expression of the corresponding PKD gene, a heterologous PKD gene, a selectable or selectable marker gene, or any other gene of interest.
在其它实施方式中,可使用人巨细胞病毒(CMV)立即早期基因启动子、SV40早期启动子、劳氏肉瘤病毒长末端重复、大鼠胰岛素启动子和甘油醛-3-磷酸脱氢酶以获得感兴趣编码序列的高水平表达。也考虑了使用本领域公知的其它病毒或哺乳动物细胞或噬菌体启动子来完成感兴趣编码序列的表达,只要表达水平足够给定目的之需。In other embodiments, the human cytomegalovirus (CMV) immediate early gene promoter, SV40 early promoter, Rous sarcoma virus long terminal repeat, rat insulin promoter, and glyceraldehyde-3-phosphate dehydrogenase can be used to Obtain high-level expression of coding sequences of interest. The use of other viral or mammalian cell or phage promoters known in the art to accomplish expression of the coding sequence of interest is also contemplated, so long as expression levels are sufficient for a given purpose.
通过使用具有公知性质的启动子,可使转染或转化后感兴趣蛋白的表达水平和模式最优化。而且,选择响应于具体生理信号而进行调节的启动子,可允许基因产物的诱导表达。在本发明内容中,表1和2列出了几个可以使用的调节元件,以调节感兴趣基因的表达。该列表并未详尽地列举涉及启动基因表达的所有可能元件,而仅是它们的范例。By using promoters with known properties, the level and pattern of expression of the protein of interest following transfection or transformation can be optimized. Furthermore, selection of a promoter that is regulated in response to a particular physiological signal allows for the inducible expression of the gene product. In the context of the present invention, Tables 1 and 2 list several regulatory elements that can be used to regulate the expression of a gene of interest. This list is not exhaustive of all possible elements involved in initiating gene expression, but merely examples of them.
增强子是从位于相同DNA分子上较远位置的启动子增加转录的遗传元件。增强子的组织方式和启动子很相似。即,它们由很多单个元件组成,各自与一个或多个转录蛋白结合。Enhancers are genetic elements that increase transcription from a promoter located at a distant location on the same DNA molecule. Enhancers are organized in a manner similar to promoters. That is, they are composed of many individual elements, each of which binds to one or more transcriptional proteins.
增强子和启动子之间的基本差别是操作上的。增强子区,作为一个整体必须能够在远处促进转录;对于启动子区或其组成元件则不需如此。另一方面,启动子必须有一个或多个元件,将RNA合成的起始导向具体位点和具体方向,然而增强子缺少这些特性。启动子和增强子经常是重叠和邻接的,它们经常看上去具有非常类似的模块组织结构。The basic difference between enhancers and promoters is operational. An enhancer region, as a whole, must be capable of promoting transcription at a distance; this need not be the case for a promoter region or its constituent elements. Promoters, on the other hand, must have one or more elements that direct the initiation of RNA synthesis to a specific site and in a specific direction, whereas enhancers lack these properties. Promoters and enhancers are often overlapping and contiguous, and they often appear to have a very similar modular organization.
下面是病毒启动子、细胞启动子/增强子和诱导启动子/增强子的列表,可将它们与表达构建物中编码感兴趣基因的核酸结合使用(表1和表2)。此外,也可使用任何启动子/增强子组合(按照真核启动子数据库EPDB)来驱动基因表达。如果提供合适的细菌聚合酶,真核细胞可以支持从某些细菌启动子进行胞浆转录,作为部分输送复合物或作为附加遗传表达构建物。
具体感兴趣的是肌肉特异性启动子,更具体说是心脏特异性启动子。这些包括肌球蛋白轻链-2启动子(Franz等,1994;Kelly等,1995)、α肌动蛋白启动子(Moss等,1996)、肌钙蛋白1启动子(Bhavsar等,1996);Na+/Ca2+交换子启动子(Barnes等,1997)、抗肌萎缩蛋白启动子(Kimura等,1997)、α7整合素启动子(Ziober和Kramer,1996)、脑促尿钠排泄肽启动子(LaPointe等,1996)和αB-晶体蛋白/小热激蛋白启动子(Gopal-Srivastava,1995)、α肌球蛋白重链启动子(Yamauchi-Takihara等,1989)和ANF启动子(LaPointe等,1988)。Of particular interest are muscle specific promoters, more specifically cardiac specific promoters. These include the myosin light chain-2 promoter (Franz et al., 1994; Kelly et al., 1995), the α-actin promoter (Moss et al., 1996), the
在使用cDNA插入物的地方,一般会需要包括聚腺苷酸化信号,以影响基因转录物的合适聚腺苷酸化。相信对本发明实践来说,聚腺苷酸化信号的性质并不重要,可以使用任何这些序列,如人生长激素和和SV40聚腺苷酸化信号。也认为终止子是表达盒的元件。这些元件可用于增加信息水平并使从盒到其它序列的连读最小化。Where cDNA inserts are used, it will generally be desirable to include a polyadenylation signal to effect proper polyadenylation of the gene transcript. The nature of the polyadenylation signal is believed to be immaterial to the practice of the invention and any of these sequences can be used, eg, human growth hormone and SV40 polyadenylation signals. A terminator is also considered an element of the expression cassette. These elements can be used to increase message levels and minimize read-through from the cassette to other sequences.
B.可选择性标记B. Selectable markers
在本发明的某些实施方式中,细胞含有本发明的核酸构建物,可通过在表达构建物中包括标记以在体外或体内鉴定细胞。这些标记会赋予细胞可鉴定的改变,以允许容易地鉴定含有表达构建物的细胞。通常,包含药物选择标记有助于克隆和选择转化子,例如,赋予耐新霉素、嘌呤霉素、潮霉素、DHFR、GPT、零霉素和组氨醇的基因是有用的可选择性标记。另外,可使用酶,如单纯疱疹病毒胸腺嘧啶激酶(tk)或氯霉素乙酰基转移酶(CAT)。也可使用免疫标记。相信所用可选择性标记并不重要,只要它能够与编码基因产物的核酸同时表达。可选择性标记的其它实例是本领域技术人员所熟知的。In certain embodiments of the invention, cells containing a nucleic acid construct of the invention can be identified in vitro or in vivo by including a marker in the expression construct. These markers will confer identifiable changes on the cells to allow easy identification of cells containing the expression construct. Often, inclusion of a drug selectable marker facilitates cloning and selection of transformants, for example, genes conferring resistance to neomycin, puromycin, hygromycin, DHFR, GPT, zeocin and histidinol are useful selectable mark. Alternatively, enzymes such as herpes simplex virus thymidine kinase (tk) or chloramphenicol acetyltransferase (CAT) may be used. Immunolabeling can also be used. The selectable marker used is not believed to be critical so long as it can be expressed simultaneously with the nucleic acid encoding the gene product. Other examples of selectable markers are well known to those skilled in the art.
C.多基因构建物和IRESC. Multigene constructs and IRES
在本发明的某些实施方式中,用内部核糖体结合位点(IRES)元件建立多基因、或多顺反子信息。IRES元件能够绕过5’甲基化帽依赖性翻译的核糖体扫描模型,并在内部位点开始翻译(Pelletier和Sonenberg,1988)。已描述了来自picano病毒科中两个成员(脊髓灰质炎和脑心肌炎病毒)的IRES元件(Pelletier和Sonenberg,1988),以及来自哺乳动物信息的IRES(Macejak和Sarnow,1991)。IRES元件可以与异源开放阅读框连接。多开放阅读框可一起转录,各自被IRES分开,建立多顺反子信息。依靠IRES元件,核糖体可到达各开放阅读框,以有效翻译。可以用单个启动子/增强子有效表达多个基因,转录成单条信息。In certain embodiments of the invention, internal ribosome binding site (IRES) elements are used to create polygenic, or polycistronic messages. IRES elements are able to bypass the ribosomal scanning model of 5′ methylated cap-dependent translation and initiate translation at internal sites (Pelletier and Sonenberg, 1988). IRES elements from two members of the picanoviridae (poliomyelitis and encephalomyocarditis viruses) have been described (Pelletier and Sonenberg, 1988), as well as from mammalian information (Macejak and Sarnow, 1991). IRES elements can be linked to heterologous open reading frames. Multiple open reading frames can be transcribed together, each separated by an IRES, creating a polycistronic message. By virtue of the IRES element, ribosomes can access each open reading frame for efficient translation. Multiple genes can be efficiently expressed with a single promoter/enhancer, transcribed into a single message.
可将任何异源开放阅读框与IRES元件连接。这包括分泌蛋白,多亚基蛋白、独立基因编码的、胞内或膜结合蛋白和可选择性标记的基因。以这种方式,可用单个构建物和单个可选择性标记将几种蛋白的表达同时工程改造到一个细胞中。Any heterologous open reading frame can be linked to the IRES element. This includes secreted proteins, multi-subunit proteins, genes encoded by separate genes, intracellular or membrane-bound proteins, and selectable marker genes. In this way, the expression of several proteins can be simultaneously engineered into one cell with a single construct and a single selectable marker.
D.表达载体的递送D. Delivery of Expression Vectors
有多种方式可将表达载体引入细胞。在本发明的某些实施方式中,表达构建物包含病毒或获自病毒基因组的工程改造构建物。某些病毒通过受体介导的胞吞作用进入细胞,以掺入宿主细胞基因组并稳定和有效地表达病毒基因的能力使它们成为将外源基因转入哺乳动物细胞的诱人的候选物(Ridgeway,1988;Nicolas和Rubenstein,1988;Baichwal和Sugden,1986;Temin,1986)。首先用作基因载体的病毒是DNA病毒,包括乳头状多瘤空泡病毒(猿病毒40,牛乳头瘤病毒和多瘤病毒)(Ridgeway,1988;Baichwal和Sugden,1986)和腺病毒(Ridgeway,1988;Baichwal和Sugden,1986)。这些病毒对外源性DNA序列的容量较低,具有受限的宿主谱。而且,它们在感受态细胞中的致癌可能性和引起细胞病变的效果引起了人们对安全性的关注。它们仅可容纳长至8kB的外源性遗传物质,但可容易地引入各种细胞系和实验动物中(Nicolas和Rubenstein,1988;Temin,1986)。There are a variety of ways to introduce expression vectors into cells. In certain embodiments of the invention, the expression construct comprises a virus or an engineered construct obtained from a viral genome. The ability of certain viruses to enter cells via receptor-mediated endocytosis to incorporate into the host cell genome and to express viral genes stably and efficiently makes them attractive candidates for the transfer of foreign genes into mammalian cells ( Ridgeway, 1988; Nicolas and Rubenstein, 1988; Baichwal and Sugden, 1986; Temin, 1986). The first viruses used as gene vectors were DNA viruses, including papillary polyomaviruses (
用于体内递送的优选方法之一包括使用腺病毒表达载体。“腺病毒表达载体”意于包括含有足够(a)支持构建物包装和(b)表达其中克隆的反义聚核苷酸的腺病毒序列的构建物。在本文中,表达不需要合成基因产物。One of the preferred methods for in vivo delivery involves the use of adenoviral expression vectors. "Adenoviral expression vector" is intended to include a construct containing sufficient adenoviral sequences to (a) support packaging of the construct and (b) express an antisense polynucleotide cloned therein. In this context, expression does not require a synthetic gene product.
表达载体包含腺病毒的遗传工程改造形式。所知的腺病毒的遗传结构是36kB、线性、双链DNA病毒,这允许用长达7kB的外源性序列取代大段的腺病毒DNA(Grunhaus和Horwitz,1992)。与逆转录病毒相反,腺病毒感染宿主细胞并不产生染色体整合,因为腺病毒DNA可以游离基因(episomal)的方式复制,而没有潜在的基因毒性。腺病毒也是结构稳定的,在大量扩增后,并没有检测到基因组重排。腺病毒实质上可感染所有上皮细胞,不管其处于哪个细胞周期阶段。迄今,腺病毒感染似乎仅与轻微的疾病有关,如人类的急性呼吸道疾病。Expression vectors comprise genetically engineered forms of adenovirus. The known genetic structure of adenoviruses is a 36 kB, linear, double-stranded DNA virus, which allows the replacement of large stretches of adenoviral DNA with up to 7 kB of exogenous sequences (Grunhaus and Horwitz, 1992). In contrast to retroviruses, adenovirus infection of host cells does not result in chromosomal integration because adenovirus DNA can replicate episomally without potential genotoxicity. Adenoviruses are also structurally stable, and no genome rearrangements were detected after extensive amplification. Adenoviruses infect virtually all epithelial cells regardless of cell cycle stage. So far, adenovirus infection appears to be associated only with mild disease, such as acute respiratory disease in humans.
腺病毒因为其中等大小的基因组、容易操纵、高滴度、靶细胞范围宽和高感染活性,尤其适于用作基因转移载体。病毒基因组的两端都包含100-200个碱基对的反向重复序列(ITR),它是病毒DNA复制和包装必需的顺式元件。基因组的早期(E)和后期(L)区含有不同的转录单元,它们被病毒DNA复制的开始处分开。E1区(E1A和E1B)编码负责调节病毒基因组和几个细胞基因的转录的蛋白。E2区(E2A和E2B)的表达导致病毒DNA复制蛋白的合成。这些蛋白涉及DNA复制、后期基因表达和宿主细胞中断(shut-off)(Renan,1990)。包括大部分病毒衣壳蛋白的后期基因的产物仅在单个初级转录物明显加工之后表达,初级转录物由主要后期启动子(MLP)产生。MLP(位于16.8m.u.)在感染后期尤其有效,所有该启动子产生的mRNA都具有5’-三分先导(TPL)序列,这使它们成为进行翻译的优选mRNA。Adenoviruses are particularly suitable for use as gene transfer vectors because of their moderately sized genome, ease of manipulation, high titer, broad target cell range, and high infectious activity. Both ends of the viral genome contain 100-200 base pair inverted repeats (ITRs), which are cis elements necessary for viral DNA replication and packaging. The early (E) and late (L) regions of the genome contain distinct transcriptional units that are separated by the initiation of viral DNA replication. The El region (E1A and ElB) encodes proteins responsible for regulating the transcription of the viral genome and several cellular genes. Expression of the E2 region (E2A and E2B) results in the synthesis of viral DNA replication proteins. These proteins are involved in DNA replication, late gene expression and host cell shut-off (Renan, 1990). The products of late genes, including most of the viral capsid proteins, are expressed only after significant processing of a single primary transcript, which is produced by the major late promoter (MLP). MLP (located at 16.8 m.u.) is especially effective late in infection, and all mRNAs produced by this promoter have a 5'-triple leader (TPL) sequence, making them preferred mRNAs for translation.
在当前系统中,由穿梭载体和前病毒载体之间的同源重组产生重组腺病毒。由于两种前病毒载体之间可能重组,可通过该方法产生野生型腺病毒。因此,从单独的噬菌斑中分离单个病毒克隆并检查其基因组结构是重要的。In the current system, recombinant adenoviruses are generated by homologous recombination between the shuttle vector and the proviral vector. Due to possible recombination between the two proviral vectors, wild type adenovirus can be generated by this method. Therefore, it is important to isolate individual viral clones from individual plaques and examine their genome structure.
产生和增殖现有的复制缺陷的腺病毒载体,取决于独特的协助细胞系,称为293,它由Ad5DNA片段转化人胚肾细胞而得,连续表达E1蛋白(Graham等,1977)。因为E3区是腺病毒基因组中非必需的(Jones和Shenk,1978),所以现有的腺病毒载体,在293细胞的协助下,在E1、D3或这两个区域中携带外源性DNA(Graham和Prevec,1991)。实际上,腺病毒可包装约105%的野生型基因组(Ghosh-Choudhury等,1987),提供的DNA容量约多2kb。与E1和E3区中约5.5kb的可替代DNA结合时,现有腺病毒载体的最大容量低于7.5kb,或约占载体总长的15%。多于80%的腺病毒病毒基因组保留在载体骨架上,这是载体-产生的细胞毒性的来源。E1-缺失病毒的复制缺陷也是不完全的。Generation and propagation of existing replication-deficient adenoviral vectors depend on a unique helper cell line, termed 293, derived from human embryonic kidney cells transformed with Ad5 DNA fragments, which continuously express the E1 protein (Graham et al., 1977). Because the E3 region is dispensable in the adenoviral genome (Jones and Shenk, 1978), existing adenoviral vectors, with the assistance of 293 cells, carry exogenous DNA in E1, D3, or both regions ( Graham and Prevec, 1991). In fact, adenoviruses can package about 105% of the wild-type genome (Ghosh-Choudhury et al., 1987), providing about 2 kb more DNA capacity. When combined with about 5.5 kb of alternative DNA in the E1 and E3 regions, the maximum capacity of existing adenoviral vectors is less than 7.5 kb, or about 15% of the total length of the vector. More than 80% of the adenoviral viral genome remains on the vector backbone, which is the source of vector-generated cytotoxicity. The replication deficiency of E1-deleted viruses is also incomplete.
协助细胞系可获自人细胞,如人胚肾细胞、肌肉细胞、造血细胞或其它人胚间叶细胞或上皮细胞。另外,协助细胞可获自可感受人腺病毒的其它哺乳动物种的细胞。这些细胞包括,例如,Vero细胞或其它猴胚间叶细胞或上皮细胞。如上所述,优选的协助细胞系是293。Helper cell lines can be obtained from human cells, such as human embryonic kidney cells, muscle cells, hematopoietic cells, or other human embryonic mesenchymal or epithelial cells. Additionally, helper cells can be obtained from cells of other mammalian species that are competent for human adenoviruses. These cells include, for example, Vero cells or other monkey embryonic mesenchymal or epithelial cells. The preferred helper cell line is 293, as described above.
Racher等(1995)公开了培养293细胞和增殖腺病毒的改进方法。在一种形式中,通过将单个细胞接种到含有100-200毫升培养基的1升硅化旋转烧瓶中生长获得天然细胞集合(Techne,Cambridge,UK)。以40rpm搅拌后,用台盼蓝估计细胞活力。在另一种形式中,如下所述地使用Fibra-Cel微运载体(BibbySterlin,Stone,UK)(5克/升)。将重悬于5毫升培养基中的细胞接种体加入到250毫升锥形烧瓶中的运载体(50毫升)中并静置,偶尔搅拌,1至4小时。然后用50毫升新鲜培养基替换培养基,开始振荡。对于病毒生产来说,允许细胞长至约80%汇合,然后更换培养基(至最终体积的25%),以MOIO.05加入腺病毒。将培养物静置过夜,然后将体积增加到100%,开始再次振荡72小时。Racher et al. (1995) disclosed an improved method for culturing 293 cells and propagating adenovirus. In one format, native cell aggregates (Techne, Cambridge, UK) were obtained by inoculating single cells into 1 liter siliconized spinner flasks containing 100-200 ml of medium for growth. After agitation at 40 rpm, cell viability was estimated with trypan blue. In another format, Fibra-Cel microcarriers (BibbySterlin, Stone, UK) (5 g/L) were used as described below. The cell inoculum resuspended in 5 mL of culture medium was added to the vehicle (50 mL) in a 250 mL Erlenmeyer flask and allowed to stand, with occasional stirring, for 1 to 4 hours. Then replace the medium with 50 ml of fresh medium and start shaking. For virus production, cells were allowed to grow to approximately 80% confluency, then medium was changed (to 25% of final volume) and adenovirus was added at MO10.05. The culture was left overnight, then the volume was increased to 100%, and shaking was started again for 72 hours.
不同于要求腺病毒载体是复制缺陷的,或至少是条件缺陷的,相信腺病毒载体的性质对本发明的成功实践并不重要。腺病毒可以是42个不同的已知血清型或A-F亚组中任意一种。亚组C的5型腺病毒是优选的起始材料,以获得用于本发明的条件复制缺陷型腺病毒载体。这是因为5型腺病毒是人腺病毒,大量关于它的生化和遗传信息是已知的,它在历史上已用于腺病毒用作载体的大部分构建物。Unlike the requirement that the adenoviral vector be replication defective, or at least conditionally defective, it is believed that the nature of the adenoviral vector is not critical to the successful practice of the invention. Adenoviruses can be of any of the 42 different known serotypes or subgroups A-F.
如上所述,根据本发明的典型载体是复制缺陷型,不会有腺病毒E1区。因此,从去除了E1-编码序列的位置上引入编码感兴趣基因的聚核苷酸将是最方便的。然而,腺病毒序列内插入构建物的位置对于本发明来说并不重要。也可将编码感兴趣基因的聚核苷酸插入E3替代载体中缺失E3区的地方,如Karlsson等(1986)所述,或插入协助细胞系或协助病毒补足E4缺失的E4区中。As mentioned above, typical vectors according to the invention are replication deficient and will not have the adenovirus El region. Therefore, it will be most convenient to introduce the polynucleotide encoding the gene of interest from a position where the El-coding sequence has been removed. However, the position within the adenoviral sequence where the construct is inserted is not critical to the present invention. A polynucleotide encoding a gene of interest can also be inserted into an E3 replacement vector where the E3 region is deleted, as described by Karlsson et al. (1986), or into an E4 region that complements the E4 deletion in a helper cell line or helper virus.
在体外和体内,腺病毒容易培养和操纵,并显示出宽宿主范围。可以高滴度,例如,每毫升109-1012噬斑形成单位获得该群病毒,它们具有高感染性。腺病毒的生命周期不需要掺入宿主细胞基因组中。腺病毒载体递送的基因是游离的,因此对宿主细胞的基因毒性低。用野生型腺病毒接种疫苗的研究中未报道副作用(Couch等,1963;Top等,1971),证明其作为体内基因转移载体的安全性和治疗潜力。Adenoviruses are easy to grow and manipulate, and exhibit a broad host range, both in vitro and in vivo. This group of viruses can be obtained in high titers, for example, 109 -1012 plaque forming units per ml, and they are highly infective. The life cycle of adenoviruses does not require incorporation into the host cell genome. Genes delivered by adenoviral vectors are episomal and therefore have low genotoxicity to host cells. No side effects were reported in studies of vaccination with wild-type adenovirus (Couch et al., 1963; Top et al., 1971), demonstrating its safety and therapeutic potential as an in vivo gene transfer vector.
腺病毒载体已用于真核基因表达(Levrero等,1991;Gomez-Foix等,1992)和疫苗开发(Grunhaus和Horwitz,1992;Graham和Prevec,1991)。最近,动物研究提示重组腺病毒可用于基因治疗(Stratford-Perricaudet和Perricaudet,1991;Stratford-Perricaudet等,1990;Rich等,1993)。将重组腺病毒给予不同组织的研究包括气管滴注(Rosenfeld等,1991;Rosenfeld等,1992)、肌肉注射(Ragot等,1993)、外周静脉内注射(Herz和Gerard,1993)和定向接种到脑中(Le Gal La Salle等,1993)。Adenoviral vectors have been used for eukaryotic gene expression (Levrero et al., 1991; Gomez-Foix et al., 1992) and vaccine development (Grunhaus and Horwitz, 1992; Graham and Prevec, 1991). More recently, animal studies suggest that recombinant adenoviruses may be used in gene therapy (Stratford-Perricaudet and Perricaudet, 1991; Stratford-Perricaudet et al., 1990; Rich et al., 1993). Studies of administration of recombinant adenovirus to different tissues include tracheal instillation (Rosenfeld et al., 1991; Rosenfeld et al., 1992), intramuscular injection (Ragot et al., 1993), peripheral intravenous injection (Herz and Gerard, 1993) and directed inoculation into the brain. Medium (Le Gal La Salle et al., 1993).
逆转录病毒是一组单链RNA病毒,其特征为在感染细胞中能够通过逆转录过程将它们的RNA转化成双链(Coffin,1990)。然后,产生的DNA作为前病毒稳定地掺入细胞染色体,指导病毒蛋白的合成。整合导致病毒基因序列保留在受体细胞和它的后代中。逆转录病毒基因组含有三个基因,gag、pol和env,它们分别编码衣壳蛋白、聚合酶和包膜组件。在gag基因上游发现的序列包含将基因组包装到病毒粒子中的信号。在病毒基因组的5’和3’末端存在两个长末端重复(LTR)序列。它们包含强力启动子和增强子序列,也是掺入宿主细胞基因组所必需的(Coffin,1990)。Retroviruses are a group of single-stranded RNA viruses characterized by the ability to convert their RNA to double-stranded in infected cells by the process of reverse transcription (Coffin, 1990). The resulting DNA is then stably incorporated into cellular chromosomes as a provirus, directing the synthesis of viral proteins. Integration results in the retention of viral gene sequences in the recipient cell and its progeny. The retroviral genome contains three genes, gag, pol, and env, which encode capsid protein, polymerase, and envelope components, respectively. Sequences found upstream of the gag gene contain signals for packaging the genome into virions. There are two long terminal repeat (LTR) sequences at the 5' and 3' ends of the viral genome. They contain strong promoter and enhancer sequences and are also required for incorporation into the host cell genome (Coffin, 1990).
为了构建逆转录病毒载体,将编码感兴趣基因的核酸插入病毒基因组中,代替某些病毒序列,产生复制缺陷型病毒。为了产生病毒粒子,构建含有gag、pol和env基因但不含LTR的包装细胞系和包装组件(Mann等,1983)。当将含有cDNA与逆转录病毒LTR和包装序列的重组质粒引入该细胞系时(例如通过磷酸钙沉淀),包装序列允许将重组质粒的RNA转录物包装到病毒颗粒中,然后病毒颗粒被分泌到培养基中(Nicolas和Rubenstein,1988;Temin,1986;Mann等,1983)。然后收集含有重组逆转录病毒的培养基,任选地浓缩,并用于基因转移。逆转录病毒载体能够感染很多种细胞类型。然而,整合和稳定表达需要宿主细胞进行分裂(Paskind等,1975)。To construct a retroviral vector, a nucleic acid encoding a gene of interest is inserted into the viral genome, replacing certain viral sequences, resulting in a replication-defective virus. To produce virions, packaging cell lines and packaging components were constructed containing the gag, pol and env genes but no LTRs (Mann et al., 1983). When a recombinant plasmid containing the cDNA with the retroviral LTR and packaging sequence is introduced into this cell line (e.g. by calcium phosphate precipitation), the packaging sequence allows the RNA transcript of the recombinant plasmid to be packaged into virions, which are then secreted into culture medium (Nicolas and Rubenstein, 1988; Temin, 1986; Mann et al., 1983). The culture medium containing the recombinant retroviruses is then collected, optionally concentrated, and used for gene transfer. Retroviral vectors are capable of infecting a wide variety of cell types. However, integration and stable expression require host cell division (Paskind et al., 1975).
最近,根据通过将乳糖残基化学加成到病毒包膜上而化学修饰逆转录病毒,开发了设计用于特异性靶向逆转录病毒载体的全新方法。该修饰可允许通过唾液酸糖蛋白受体特异性感染肝细胞。Recently, a novel approach designed for specific targeting of retroviral vectors was developed based on the chemical modification of retroviruses by the chemical addition of lactose residues to the viral envelope. This modification may allow specific infection of hepatocytes via the sialoglycoprotein receptor.
设计了一个不同的靶向重组逆转录病毒的方法,该方法中使用抗逆转录病毒包膜蛋白和抗特异性细胞受体的生物素化抗体。通过生物素组件用抗生物素蛋白链菌素偶联这些抗体(Roux等,1989)。用抗主要组织相容性复合体I类和II类抗原的抗体,他们证明了亲嗜性病毒体外感染各种带有那些表面抗原的人细胞(Roux等,1989)。A different approach to targeting recombinant retroviruses was devised using biotinylated antibodies against retroviral envelope proteins and specific cellular receptors. These antibodies were coupled with streptavidin via a biotin module (Roux et al., 1989). Using antibodies against major histocompatibility complex class I and class II antigens, they demonstrated in vitro infection of a variety of human cells bearing those surface antigens by ecotropic viruses (Roux et al., 1989).
在本发明的所有方面,对逆转录病毒载体的用途有某些限制。例如,逆转录病毒载体通常整合入细胞基因组中的随机位点。这可导致通过中断宿主基因或通过插入可干扰侧翼基因功能的病毒调节序列进行插入诱变(Varmus等,1981)。使用缺陷型逆转录病毒载体中另一引人关注的问题是在包装细胞中可能出现野生型复制竞争型病毒。这可能是由重组事件引起,该事件中将来自重组病毒的完整序列插入到掺入宿主细胞基因组的gag、pol、env序列上游。然而,现在可用新包装细胞系,它应该能够大大降低重组的可能(Markowitz等,1988;Hersdorffer等,1990)。In all aspects of the invention, there are certain limitations to the use of retroviral vectors. For example, retroviral vectors typically integrate into random sites in the cellular genome. This can lead to insertional mutagenesis by disruption of host genes or by insertion of viral regulatory sequences that interfere with the function of flanking genes (Varmus et al., 1981). Another concern with the use of defective retroviral vectors is the possible emergence of wild-type replication-competitive virus in packaging cells. This may be caused by a recombination event in which the complete sequence from the recombinant virus is inserted upstream of the gag, pol, env sequences incorporated into the host cell genome. However, new packaging cell lines are now available which should greatly reduce the likelihood of recombination (Markowitz et al., 1988; Hersdorffer et al., 1990).
其它病毒载体可在本发明中用作表达构建物。可使用获自病毒,如牛痘病毒(Ridgeway,1988;Baichwal和Sugden,1986;Coupar等,1988)腺相关病毒(AAV)(Ridgeway,1988;Baichwal和Sugden,1986;Hermonat和Muzycska,1984)和疱疹病毒的载体。它们具有适用于各种哺乳动物细胞的几种诱人特征(Friedmann,1989;Ridgeway,1988;Baichwal和Sugden,1986;Coupar等,1988;Horwich等,1990)。通过识别缺陷型乙肝病毒,我们对不同病毒序列的结构-功能关系有了新的认识。体外研究显示,尽管病毒缺失了多达80%的基因组,它仍可保留依赖协助的包装和逆转录能力(Horwich等,1990)。这提示可用外源性遗传物质替换大部分基因组。对于肝脏-导向的基因转移来说,嗜肝性和持续性(整合)是尤其吸引人的特性。Chang等将氯霉素乙酰基转移酶(CAT)基因引入鸭乙肝病毒基因组代替聚合酶、表面和前表面编码序列。将该病毒与野生型病毒共转染到禽肝细胞系中。含有高滴度重组病毒的培养基用于感染初级小鸭肝细胞。转染后至少24天,检测到稳定的CAT基因表达(Chang等,1991)。为了实现正义或反义基因构建物的表达,必须将表达构建物递送到细胞中。可体外如在转染细胞系的实验室程序中,或体内或离体如在某些病症的治疗中完成递送。一种递送机制是通过病毒感染,其中将表达构建物包裹在感染性病毒颗粒中。Other viral vectors can be used as expression constructs in the present invention. Viruses obtained from, for example, vaccinia virus (Ridgeway, 1988; Baichwal and Sugden, 1986; Coupar et al., 1988) adeno-associated virus (AAV) (Ridgeway, 1988; Baichwal and Sugden, 1986; Hermonat and Muzycska, 1984) and herpes Virus vector. They have several attractive features applicable to various mammalian cells (Friedmann, 1989; Ridgeway, 1988; Baichwal and Sugden, 1986; Coupar et al., 1988; Horwich et al., 1990). By identifying defective HBV, we gained new insights into the structure-function relationships of different viral sequences. In vitro studies have shown that although the virus has lost up to 80% of its genome, it retains assistance-dependent packaging and reverse transcription capabilities (Horwich et al., 1990). This suggests that exogenous genetic material can be used to replace a large portion of the genome. Hepatotropism and persistence (integration) are particularly attractive properties for liver-directed gene transfer. Chang et al. introduced the chloramphenicol acetyltransferase (CAT) gene into the duck hepatitis B virus genome to replace the polymerase, surface and front surface coding sequences. The virus was co-transfected with wild-type virus into an avian liver cell line. Medium containing high titers of recombinant virus was used to infect primary duckling hepatocytes. Stable CAT gene expression was detected at least 24 days after transfection (Chang et al., 1991). In order to achieve expression of a sense or antisense gene construct, the expression construct must be delivered into the cell. Delivery can be accomplished in vitro, as in laboratory procedures to transfect cell lines, or in vivo or ex vivo, as in the treatment of certain disorders. One delivery mechanism is by viral infection, in which the expression construct is encapsulated in infectious viral particles.
本发明也考虑了几种将构建物转移到培养的哺乳动物细胞中的非病毒方法。这些方法包括磷酸钙沉淀(Graham和VanDerEb,1973;Chen和Okayama,1987;Rippe等,1990)DEAE-葡聚糖(Gopal,1985)、电穿孔(Tur-Kaspa等,1986;Potter等,1984)、直接显微注射(Harland和Weintraub,1985)、载有DNA的脂质体(Nicolau和Sene,1982;Fraley等,1979)和lipofectamine-DNA复合物、细胞超声波降解法(Fechheimer等,1987)、用高速微粒进行基因轰击(Yang等,1990)和受体介导的转染(Wu和Wu,1987;Wu和Wu,1988)。这些技术中的一部分可成功用于体内或离体用途。Several non-viral methods of transferring the constructs to cultured mammalian cells are also contemplated by the present invention. These methods include calcium phosphate precipitation (Graham and VanDerEb, 1973; Chen and Okayama, 1987; Rippe et al., 1990) DEAE-dextran (Gopal, 1985), electroporation (Tur-Kaspa et al., 1986; Potter et al., 1984) , direct microinjection (Harland and Weintraub, 1985), DNA-loaded liposomes (Nicolau and Sene, 1982; Fraley et al., 1979) and lipofectamine-DNA complexes, cell sonication (Fechheimer et al., 1987), Gene bombardment (Yang et al., 1990) and receptor-mediated transfection (Wu and Wu, 1987; Wu and Wu, 1988) were performed using high-speed microparticles. Some of these techniques can be successfully used in vivo or ex vivo.
一旦将表达构建物递送到细胞中,编码感兴趣基因的核酸在该细胞中可能位于和表达于不同位点。在某些实施方式中,可以将编码该基因的核酸稳定地整合入细胞基因组中。该掺入可能通过同源重组发生在关联位置和方向上(基因替换)或可能掺入随机、非特异性位置(基因增加)。在另一实施方式中,核酸可在细胞中稳定地维持为单独、游离节段的DNA。这些核酸节段或“游离基因”编码足够独立于或同步于宿主细胞周期进行维持和复制的序列。怎样将表达构建物递送到细胞中和核酸保留在细胞何处取决于所用表达构建物的类型。Once the expression construct is delivered into the cell, the nucleic acid encoding the gene of interest may be localized and expressed at different sites in the cell. In certain embodiments, the nucleic acid encoding the gene can be stably integrated into the genome of the cell. This incorporation may occur by homologous recombination in an associative position and orientation (gene replacement) or may incorporate at random, non-specific positions (gene gain). In another embodiment, a nucleic acid can be stably maintained in a cell as a single, episomal segment of DNA. These nucleic acid segments or "epigenes" encode sequences sufficient for maintenance and replication independently of or synchronously with the host cell cycle. How the expression construct is delivered into the cell and where the nucleic acid remains in the cell depends on the type of expression construct used.
在本发明另一实施方式中,表达构建物可简单地由裸重组DNA或质粒构成。可通过任何上述方法进行构建物的转移,该方法可以物理或化学地使细胞膜通透。这尤其可用于体外转移,但它也可应用于体内用途。Dubensky等(1984)成功地以磷酸钙沉淀的形式将多瘤病毒DNA注射到成年和新生小鼠的肝脏和脾脏中,证明了活性病毒复制和急性感染。Benvenisty和Neshif(1986)也证明直接腹腔内注射磷酸钙沉淀的质粒导致转染基因的表达。可以想像,也可将编码感兴趣基因的DNA以类似的方式向体内转移,并表达基因产物。In another embodiment of the invention, the expression construct may simply consist of naked recombinant DNA or plasmids. Transfer of the construct can be performed by any of the methods described above, which physically or chemically permeabilize the cell membrane. This is especially useful for in vitro transfer, but it can also be applied for in vivo use. Dubensky et al. (1984) successfully injected polyomavirus DNA into the liver and spleen of adult and neonatal mice in the form of calcium phosphate precipitates, demonstrating active viral replication and acute infection. Benvenisty and Neshif (1986) also demonstrated that direct intraperitoneal injection of calcium phosphate precipitated plasmids resulted in expression of transfected genes. It is conceivable that DNA encoding a gene of interest could be similarly transferred in vivo and the gene product expressed.
在本发明另一实施方式中,将裸DNA表达构建物转移到细胞中可包括颗粒轰击。该方法依赖于将包裹DNA的微粒加速到允许它们刺穿细胞膜并进入细胞而不会杀死细胞的高速(Klein等,1987)。已经开发了几种加速小颗粒的装置。该设备依赖于高压放电以产生电流,它反过来提供原动力(Yang等,1990)。所用微粒由生物学惰性物质,如钨或金珠组成。In another embodiment of the invention, transferring a naked DNA expression construct into a cell may comprise particle bombardment. This method relies on accelerating DNA-encapsulated microparticles to high speeds that allow them to pierce cell membranes and enter cells without killing them (Klein et al., 1987). Several devices for accelerating small particles have been developed. The device relies on a high voltage discharge to generate an electric current, which in turn provides the motive force (Yang et al., 1990). The microparticles used consist of biologically inert substances such as tungsten or gold beads.
体内轰击选择器官,包括大鼠和小鼠的肝脏、皮肤和肌肉组织(Yang等,1990;Zelenin等,1991)。这可能需要对组织或细胞进行外科处理,以消除枪和靶组织之间的任何介入组织,即离体处理。编码具体基因的DNA也可通过该方法递送,并仍通过本发明整合。In vivo bombardment selects organs including liver, skin and muscle tissue in rats and mice (Yang et al., 1990; Zelenin et al., 1991). This may require surgical manipulation of the tissue or cells to remove any intervening tissue between the gun and the target tissue, i.e. ex vivo. DNA encoding a particular gene can also be delivered by this method and still be integrated by the present invention.
在本发明的另一实施方式中,可将表达构建物包裹到脂质体中。脂质体是泡囊状结构,其特征为磷脂双层膜和内部水相介质。多层脂质体具有被水相介质分开的多个脂质层。当磷脂悬浮在过量水溶液中时,它们自发形成。在形成闭合结构之前,脂质组分进行自重排,将水和溶解的溶质包裹在脂质双层之间(Ghosh和Bachhawat,1991)。也考虑到lipofectamine-DNA复合物。In another embodiment of the invention, the expression construct can be encapsulated in liposomes. Liposomes are vesicular structures characterized by a phospholipid bilayer membrane and an inner aqueous medium. Multilamellar liposomes have multiple lipid layers separated by an aqueous medium. They form spontaneously when phospholipids are suspended in an excess of aqueous solution. Before forming a closed structure, lipid components undergo self-rearrangement, trapping water and dissolved solutes between lipid bilayers (Ghosh and Bachhawat, 1991). The lipofectamine-DNA complex is also considered.
脂质体介导的核酸递送和外源性DNA的体外表达已经是非常成功的。Wong等,(1980)证明了脂质体介导的递送和在培养的鸡胚、HeLa和肝细胞中表达外源性DNA的可行性。Nicolau等,(1987)在大鼠中完成了静脉注射后成功的脂质体介导的基因转移。Liposome-mediated nucleic acid delivery and in vitro expression of exogenous DNA has been very successful. Wong et al., (1980) demonstrated the feasibility of liposome-mediated delivery and expression of exogenous DNA in cultured chicken embryos, HeLa and hepatocytes. Nicolau et al., (1987) performed successful liposome-mediated gene transfer in rats following intravenous injection.
在本发明的某些实施方式中,可将脂质体与红细胞凝集病毒(HVJ)复合。结果显示它有助于与细胞膜的融合并促进脂质体-包裹的DNA进入细胞(Kaneda等,1989)。在其它实施方式中,脂质体可与核非组蛋白染色体蛋白(HMG-1)复合或与其联合使用(Kato等,1991)。在另外的实施方式中,该脂质体可与HVJ和HMG-1复合或与其联合使用。由于这些表达构建物已成功用于在体外和体内转移和表达核酸,所以它们适用于本发明。如果将细菌启动子用于DNA构建物,那么也将需要在脂质体中包括合适的细菌聚合酶。In certain embodiments of the invention, liposomes can be complexed with hemagglutinating virus (HVJ). It was shown to facilitate fusion with cell membranes and facilitate entry of liposome-encapsulated DNA into cells (Kaneda et al., 1989). In other embodiments, liposomes can be complexed with or used in conjunction with a nuclear non-histone chromosomal protein (HMG-1) (Kato et al., 1991). In additional embodiments, the liposomes may be complexed or used in conjunction with HVJ and HMG-1. Since these expression constructs have been successfully used to transfer and express nucleic acids in vitro and in vivo, they are suitable for use in the present invention. If a bacterial promoter is used in the DNA construct, it will also be necessary to include a suitable bacterial polymerase in the liposomes.
其它可用于将编码具体基因的核酸递送到细胞中的表达构建物是受体介导的递送载体。这些载体利用了在几乎所有真核细胞中通过受体介导的胞吞作用对大分子的选择性摄取。因为各种受体的细胞类型特异性分布,所以递送可以是高度特异性的(Wu和Wu,1993)。Other expression constructs that can be used to deliver a nucleic acid encoding a particular gene into a cell are receptor-mediated delivery vehicles. These vectors take advantage of the selective uptake of macromolecules by receptor-mediated endocytosis in virtually all eukaryotic cells. Because of the cell-type specific distribution of the various receptors, delivery can be highly specific (Wu and Wu, 1993).
受体介导的基因靶向载体通常由两个组件构成:细胞受体特异性配体和DNA结合剂。几种配体已用于受体介导的基因转移。最广泛表征的配体是脱唾液酸血清类粘蛋白(ASOR)(Wu和Wu,1987)和转铁蛋白(Wagner等,1990)。最近,已将与ASOR识别相同受体的合成新糖蛋白用作基因递送载体(Ferkol等,1993;Perales等,1994),也已将表皮生长因子(EGF)用于将基因递送到鳞状癌细胞中(Myers,EP00273085)。Receptor-mediated gene targeting vectors generally consist of two components: a cellular receptor-specific ligand and a DNA-binding agent. Several ligands have been used for receptor-mediated gene transfer. The most widely characterized ligands are asialosomucoid (ASOR) (Wu and Wu, 1987) and transferrin (Wagner et al., 1990). Recently, synthetic new glycoproteins that recognize the same receptor as ASOR have been used as gene delivery vehicles (Ferkol et al., 1993; Perales et al., 1994), and epidermal growth factor (EGF) has also been used to deliver genes to squamous carcinoma in cells (Myers, EP00273085).
在其它实施方式中,递送载体可包括配体和脂质体。例如,Nicolau等,(1987)用乳糖基-神经酰胺,一种末端为半乳糖的脱唾液酸神经节苷脂,掺入脂质体中,观察到肝细胞对胰岛素基因的摄取增加。因此,通过任意数量受体-配体系统用或不用脂质体将编码具体基因的核酸特异性递送到细胞类型中是可行的。例如,表皮生长因子(EGF)可用作将核酸介导递送到细胞中的受体,该类细胞显示出EGF受体上调。可将甘露糖用于靶向肝细胞上的甘露糖受体。抗CD5(CLL)、CD22(淋巴瘤)、CD25(T-细胞白血病)和MAA(黑色素瘤)的抗体也可类似地用作靶向部分。In other embodiments, delivery vehicles may include ligands and liposomes. For example, Nicolau et al., (1987) incorporated lactosyl-ceramide, a galactose-terminated asialoganglioside, into liposomes and observed increased uptake of the insulin gene by hepatocytes. Thus, the specific delivery of nucleic acids encoding particular genes into cell types via any number of receptor-ligand systems with or without liposomes is feasible. For example, epidermal growth factor (EGF) can be used as a receptor for mediated delivery of nucleic acids into cells that exhibit upregulation of the EGF receptor. Mannose can be used to target mannose receptors on liver cells. Antibodies against CD5 (CLL), CD22 (lymphoma), CD25 (T-cell leukemia) and MAA (melanoma) can similarly be used as targeting moieties.
在某些实施方式中,在离体条件下可能更容易进行基因转移。离体基因治疗指从动物分离细胞,将核酸体外递送到细胞中,然后将修饰的细胞送回动物体内。这可包括从动物或细胞和组织的原代培养物中手术去除组织/器官。In certain embodiments, gene transfer may be more readily performed under ex vivo conditions. Ex vivo gene therapy refers to the isolation of cells from an animal, the delivery of nucleic acids into the cells in vitro, and the return of the modified cells to the animal. This can include surgical removal of tissues/organs from animals or primary cultures of cells and tissues.
VIII.制造转基因小鼠的方法VIII. Methods of Making Transgenic Mice
本发明的具体实施方式提供了在启动子的控制下表达异源PKD基因的转基因动物。在诱导型或组成型启动子的控制下表达PKD编码核酸的转基因动物,获自该动物的重组细胞系和转基因胚胎可用于确定PKD在心肌细胞的发育和分化以及病理性心脏肥大和心力衰竭发展中起到的确切作用。而且,这些转基因动物可提供对心脏发育的认识。使用组成型表达的PKD编码核酸提供过表达或表达下调的模型。也考虑到“敲除”PKD的一个或两个等位基因的转基因动物。Particular embodiments of the present invention provide transgenic animals expressing heterologous PKD genes under the control of a promoter. Recombinant cell lines and transgenic embryos obtained from transgenic animals expressing a PKD-encoding nucleic acid under the control of an inducible or constitutive promoter can be used to determine the development and differentiation of PKD in cardiomyocytes and the development of pathological cardiac hypertrophy and heart failure the exact role played in it. Furthermore, these transgenic animals may provide insight into heart development. A model for overexpression or downregulation of expression is provided using constitutively expressed PKD-encoding nucleic acids. Transgenic animals that "knock out" one or both alleles of PKD are also contemplated.
在一个总的方面,通过给定转基因以允许转基因表达的方式整合入基因组中生产转基因动物。Wagner和Hoppe(美国专利4,873,191;引入本文作为参考)以及以整体引入本文作为参考的Brinster等,1985总地描述了生产转基因动物的方法。In one general aspect, transgenic animals are produced by integrating a given transgene into the genome in a manner that allows expression of the transgene. Methods for producing transgenic animals are generally described by Wagner and Hoppe (US Patent 4,873,191; incorporated herein by reference) and Brinster et al., 1985, which is incorporated herein by reference in its entirety.
通常,通过显微注射把侧翼连有基因组序列的基因转移到受精卵中。将该显微注射的卵移植到雌性宿主动物体内,筛选其后代中转基因的表达。可由许多动物,包括但不限于爬虫、两栖动物、鸟类、哺乳动物和鱼类的受精卵生产转基因动物。Typically, the gene flanked by the genomic sequence is transferred into fertilized eggs by microinjection. The microinjected eggs are transplanted into female host animals, and their progeny are screened for expression of the transgene. Transgenic animals can be produced from fertilized eggs of many animals including, but not limited to, reptiles, amphibians, birds, mammals, and fish.
可通过任何本领域已知手段制备用于显微注射的DNA克隆。例如,可用适用于去除细菌质粒序列的酶切割用于显微注射的DNA克隆,用标准技术在TBE缓冲液中在1%琼脂糖凝胶上电泳DNA片段。用溴化乙锭染色使DNA条带显色,切下含有表达序列的条带。然后将切下的条带置于含有0.3M乙酸钠、pH7.0的渗析袋中。将DNA电洗脱到透析袋中,用1∶1的苯酚∶氯仿溶液抽提DNA,并用两倍体积的乙醇沉淀。将DNA再溶解于1毫升低盐缓冲液(0.2M NaCl、20mM Tris、pH7.4和1mM EDTA)中,在Elutip-DTM柱上纯化。首先用3毫升高盐缓冲液(1M NaCl、20mM Tris、pH7.4和1mM EDTA)灌柱,然后用5毫升低盐缓冲液洗涤。将DNA溶液过柱三次,以使DNA结合到柱基质上。用3毫升低盐缓冲液洗涤一次后,用0.4毫升高盐缓冲液洗脱DNA,用两倍体积的乙醇沉淀。通过紫外分光光度计中260纳米处的吸光度测量DNA浓度。对于显微注射,在5mM Tris、pH7.4和0.1mM EDTA中将DNA浓度调整到3微克/毫升。Palmiter等(1982)和Sambrook等(2001)中描述了用于显微注射的其他DNA纯化方法。DNA clones for microinjection can be prepared by any means known in the art. For example, DNA clones for microinjection can be cut with enzymes suitable for removing bacterial plasmid sequences, and the DNA fragments run on 1% agarose gels in TBE buffer using standard techniques. The DNA bands were visualized by staining with ethidium bromide, and the bands containing the expressed sequences were excised. The excised bands were then placed in dialysis bags containing 0.3M sodium acetate, pH 7.0. The DNA was electroeluted into dialysis bags, extracted with a 1:1 phenol:chloroform solution, and precipitated with two volumes of ethanol. The DNA was redissolved in 1 mL of low-salt buffer (0.2M NaCl, 20 mM Tris, pH 7.4, and 1 mM EDTA) and purified on an Elutip-DTM column. First fill the column with 3 mL of high-salt buffer (1M NaCl, 20 mM Tris, pH 7.4, and 1 mM EDTA), and then wash with 5 mL of low-salt buffer. Pass the DNA solution through the column three times to allow the DNA to bind to the column matrix. After one wash with 3 mL of low-salt buffer, the DNA was eluted with 0.4 mL of high-salt buffer and precipitated with two volumes of ethanol. DNA concentration was measured by absorbance at 260 nm in a UV spectrophotometer. For microinjection, adjust the DNA concentration to 3 µg/mL in 5 mM Tris, pH 7.4, and 0.1 mM EDTA. Other DNA purification methods for microinjection are described in Palmiter et al. (1982) and Sambrook et al. (2001).
在示例性显微注射步骤中,通过注射5IU的怀孕马血清促性腺激素(PMSG;Sigma)(0.1毫升,腹腔内),48小时后再注射5IU的人绒毛膜促性腺激素(hCG;Sigma)(0.1毫升,腹腔内)来诱导六周龄的雌性小鼠排卵过度。hCG注射后立即将雌性小鼠与雄性放在一起。注射后二十一小时,用CO2窒息或断颈法处死交配过的雌性,从切下的输卵管中回收胚胎,置于含有0.5%牛血清白蛋白(BSA;Sigma)的Dulbecco氏磷酸盐缓冲液中。用透明质酸酶(1毫克/毫升)去除周围的堆积细胞。然后洗涤前核胚胎,并置于含有0.5%BSA的Earle氏平衡盐溶液(EBSS)中,在37.5℃的培养箱中,5%CO2、95%空气的潮湿环境下培养,直到注射。可以在二细胞期移植胚胎。In an exemplary microinjection procedure, by injecting 5 IU of pregnant horse serum gonadotropin (PMSG; Sigma) (0.1 mL, intraperitoneally) followed by 5 IU of human chorionic gonadotropin (hCG; Sigma) 48 hours later (0.1 ml, intraperitoneally) to induce hyperovulation in six-week-old female mice. Female mice were housed with males immediately after hCG injection. Twenty-one hours after injection, mated females were sacrificed byCO2 asphyxiation or neck dislocation, and embryos were recovered from excised oviducts and placed in Dulbecco's phosphate-buffered saline containing 0.5% bovine serum albumin (BSA; Sigma). in the liquid. Surrounding accumulated cells were removed with hyaluronidase (1 mg/ml). Pronuclear embryos were then washed and cultured in Earle's Balanced Salt Solution (EBSS) containing 0.5% BSA in a humidified environment of 5% CO2 and 95% air at 37.5°C until injection. Embryos can be transferred at the two-cell stage.
将成年雌性小鼠的生理周期随机化后,与切除输精管的雄性配对。可将C57BL/6或Swiss小鼠或其他相当品系用于此目的。受体雌性与供体雌性同时交配。在转移胚胎的同时,通过每克体重腹腔内注射0.015毫升2.5%阿佛丁麻醉受体雌性。通过背中线单切开暴露输卵管。然后在正好位于输卵管之上的体壁上作切口。然后用钟表(warchmaker)钳将卵巢囊撕碎。将待转染胚胎置于DPBS(Dulbecco氏磷酸盐缓冲液)中,并在移液管的顶端(约10至12个胚胎)。将移液管顶端插入输卵管漏斗,转移胚胎。转移后,缝合两针封闭切口。Adult female mice were paired with vasectomized males after randomizing their menstrual cycles. C57BL/6 or Swiss mice or other equivalent strains can be used for this purpose. Recipient females are mated with donor females at the same time. Simultaneously with embryo transfer, recipient females were anesthetized by intraperitoneal injection of 0.015 ml of 2.5% avertin per gram of body weight. The fallopian tubes are exposed through a single dorsal midline incision. An incision is then made in the body wall just above the fallopian tubes. Ovarian capsules were then shredded using warchmaker forceps. Place the embryos to be transfected in DPBS (Dulbecco's Phosphate Buffered Saline) on top of the pipette (approximately 10 to 12 embryos). Insert the tip of the pipette into the infundibulum of the oviduct to transfer the embryos. After the transfer, the incision was closed with two sutures.
IX.对PKD具有活性的抗体IX. Antibodies Active to PKD
在另一方面,本发明考虑了对本发明的PKD分子具有免疫活性的抗体,或其部分。抗体可以是多克隆或单克隆抗体。在优选实施方式中,抗体是单克隆抗体。本领域技术人员熟知制备和表征抗体的方法(参见,例如,Harlow和Lane,1988)。In another aspect, the invention contemplates antibodies, or portions thereof, that are immunologically active against the PKD molecules of the invention. Antibodies can be polyclonal or monoclonal. In a preferred embodiment, the antibody is a monoclonal antibody. Methods for preparing and characterizing antibodies are well known to those skilled in the art (see, eg, Harlow and Lane, 1988).
简要说,通过用含有本发明多肽的免疫原免疫动物,并从免疫的动物中收集抗血清来制备多克隆抗体。很多动物种都可用于生产抗血清。一般地,用于生产抗血清的动物是非人动物,包括家兔、小鼠、大鼠、仓鼠、猪或马。因为家兔的血液体积相对较大,所以家兔是生产多克隆抗体的优选选择。Briefly, polyclonal antibodies are prepared by immunizing animals with an immunogen containing a polypeptide of the invention, and collecting antisera from the immunized animals. A wide variety of animal species are available for the production of antisera. Typically, the animals used for the production of antisera are non-human animals, including rabbits, mice, rats, hamsters, pigs or horses. Because of their relatively large blood volume, rabbits are the preferred choice for the production of polyclonal antibodies.
可用常规免疫技术制备特异性抗抗原同种型的多克隆和单克隆抗体,如本领域技术人员通常所知。含本发明化合物的抗原表位的组合物可用于免疫一种或多种实验动物,如家兔或小鼠,然后进行抗本发明化合物的特异性抗体的生产。在经过产生抗体的时间后,简单地通过给动物放血和从全血中制备血清样品就可获得多克隆抗血清。Polyclonal and monoclonal antibodies specific for an antigen isotype can be prepared using conventional immunization techniques, as is generally known to those of skill in the art. The composition containing the epitope of the compound of the present invention can be used to immunize one or more experimental animals, such as rabbits or mice, and then produce specific antibodies against the compound of the present invention. Polyclonal antisera can be obtained simply by bleeding the animal and preparing a serum sample from the whole blood after the time for antibody production has elapsed.
据推断,将发现本发明单克隆抗体可应用于标准免疫化学方法,如ELISA和Western印迹法,和用于免疫组化方法,如组织染色,以及其他可能利用特异性抗PKD相关抗原表位的抗体的方法。Presumably, the monoclonal antibodies of the invention will find application in standard immunochemical methods, such as ELISA and Western blotting, and in immunohistochemical methods, such as tissue staining, and other methods that may utilize specific anti-PKD-associated epitopes. Antibody method.
总的来说,抗PKD的多克隆、单克隆和单链抗体都可用于各种实施方式。这些抗体的具体有用应用是在纯化天然或重组PKD中,例如,用抗体亲和柱。根据本公开,本领域技术人员将了解所有接受的免疫学技术的操作。In general, anti-PKD polyclonal, monoclonal, and single chain antibodies are useful in various embodiments. A particularly useful application of these antibodies is in the purification of native or recombinant PKD, for example, using antibody affinity columns. In light of the present disclosure, the skilled artisan will appreciate the manipulation of all accepted immunological techniques.
制备和表征抗体的方法是本领域中公知的(参见,例如,Harlow和Lane,1988;引入本文作为参考)。下面的实施例中给出了制备单克隆抗体的更具体的实例。Methods for preparing and characterizing antibodies are well known in the art (see, eg, Harlow and Lane, 1988; incorporated herein by reference). More specific examples of preparation of monoclonal antibodies are given in the following Examples.
正如本领域中所公知的,给定组合物的免疫原性可以改变。因此,经常有必要强化宿主免疫系统,如通过将肽或多肽免疫原偶联到运载体上所达到的那样。示例性和优选的运载体是钥孔血蓝素(KLH)和牛血清白蛋白(BSA)。其他白蛋白,如卵清蛋白,小鼠血清白蛋白或家兔血清白蛋白也可用作运载体。将多肽共轭到运载体蛋白上的方法是本领域中公知的,该方法包括戊二醛,间马来酰亚胺基苯甲酰基(m-maleimidobencoyl)-N-羟基琥珀酰亚胺酯,碳二亚胺和双二氮化(bis-biazotized)联苯胺。As is known in the art, the immunogenicity of a given composition can vary. Therefore, it is often necessary to boost the host immune system, as achieved by coupling the peptide or polypeptide immunogen to the carrier. Exemplary and preferred carriers are keyhole limpet hemocyanin (KLH) and bovine serum albumin (BSA). Other albumins such as ovalbumin, mouse serum albumin or rabbit serum albumin can also be used as carriers. Methods for conjugating polypeptides to carrier proteins are well known in the art and include glutaraldehyde, m-maleimidobencoyl-N-hydroxysuccinimide ester, Carbodiimides and bis-biazotized benzidines.
也如本领域中所公知的那样,可以通过使用称为佐剂的免疫反应非特异性刺激物,增强具体免疫原组合物的免疫原性。示例性和优选的佐剂包括完全弗氏佐剂(含杀死的结核分支杆菌的免疫反应非特异性刺激物)、不完全弗氏佐剂和氢氧化铝佐剂。As is also known in the art, the immunogenicity of particular immunogenic compositions can be enhanced through the use of non-specific stimulators of the immune response known as adjuvants. Exemplary and preferred adjuvants include complete Freund's adjuvant (a non-specific stimulator of immune response containing killed M. tuberculosis), incomplete Freund's adjuvant, and aluminum hydroxide adjuvant.
用于生产多克隆抗体的免疫原组合物的量因免疫原性质以及用于免疫的动物而改变。各种途径(皮下、肌内、皮内、静脉内和腹腔内)均可用于给予免疫原。可通过在免疫后不同时间点采集免疫动物的血样来监测多克隆抗体的生产。也可给予第二次加强注射。重复进行加强和测定滴度的过程,直到获得合适的滴度。当获得所需水平的免疫原性时,将免疫动物放血,分离血清并储存,和/或动物可用于产生mAb。The amount of immunogen composition used to produce polyclonal antibodies will vary with the nature of the immunogen and the animal used for immunization. Various routes (subcutaneous, intramuscular, intradermal, intravenous and intraperitoneal) can be used to administer the immunogen. Polyclonal antibody production can be monitored by taking blood samples from the immunized animal at various time points after immunization. A second booster injection may also be given. The process of boosting and titrating is repeated until a suitable titer is obtained. When the desired level of immunogenicity is achieved, the immunized animal is bled, the serum is isolated and stored, and/or the animal can be used for mAb production.
可以通过使用本领域公知技术,如引入本文作为参考的美国专利4,196,265中示例的技术容易地制备MAb。通常,该技术包括用选择的免疫原组合物,如纯化或部分纯化的PKD蛋白、多肽或肽或表达高水平PKD的细胞来免疫合适的动物。以有效刺激抗体生产细胞的方式给予该免疫组合物。啮齿类如小鼠和大鼠是优选的动物,然而,使用家兔、绵羊、蛙细胞也是可能的。使用大鼠可提供某些优点(Goding,1986),但优选小鼠,最优选BALB/c小鼠,因为这是最常规使用的动物,通常产生更高百分数的稳定融合。MAbs can be readily prepared using techniques known in the art, such as exemplified in US Patent 4,196,265, incorporated herein by reference. Generally, the technique involves immunizing a suitable animal with a selected immunogenic composition, such as a purified or partially purified PKD protein, polypeptide or peptide or a cell expressing high levels of PKD. The immunizing composition is administered in a manner effective to stimulate antibody-producing cells. Rodents such as mice and rats are preferred animals, however, it is also possible to use rabbit, sheep, frog cells. The use of rats may offer some advantages (Goding, 1986), but mice are preferred, most preferably BALB/c mice, as this is the most commonly used animal and generally produces a higher percentage of stable fusions.
免疫后,选择具有产生抗体的潜能的体细胞,具体是B淋巴细胞(B细胞)用于mAb产生方法。这些细胞可获自活组织检查的脾脏、扁桃体或淋巴结,或获自外周血样。优选脾细胞和外周血细胞,前者是因为它们是富含处于分裂成浆细胞阶段的抗体产生细胞的来源,后者是因为可容易地得到外周血。经常是免疫一系列动物,摘除抗体滴度最高的动物脾,通过用注射器匀浆脾脏获得脾淋巴细胞。一般地,来自免疫小鼠的脾脏含有大约5×107至2×108个淋巴细胞。After immunization, somatic cells, specifically B lymphocytes (B cells), with the potential to produce antibodies are selected for use in the mAb production method. These cells can be obtained from a biopsy of the spleen, tonsil or lymph nodes, or from a peripheral blood sample. Splenocytes and peripheral blood cells are preferred, the former because they are a rich source of antibody-producing cells at the stage of dividing into plasma cells, and the latter because peripheral blood is readily available. Often a series of animals are immunized, the spleen of the animal with the highest antibody titer is removed, and splenic lymphocytes are obtained by homogenizing the spleen with a syringe. Typically, spleens from immunized mice contain approximately 5 x107 to 2 x108 lymphocytes.
然后将来自免疫动物的产生抗体的B淋巴细胞与永生化的骨髓瘤细胞,通常是与免疫动物种相同的骨髓瘤细胞融合。适用于生产杂交瘤的融合方法的骨髓瘤细胞系优选不生产抗体,具有高融合效率和酶缺陷,酶缺陷使其后来不能在某些选择性培养基中生长,选择性培养基仅支持所需融合细胞(杂交瘤)生长。The antibody-producing B lymphocytes from the immunized animal are then fused with immortalized myeloma cells, usually of the same species as the immunized animal. Myeloma cell lines suitable for the fusion method of producing hybridomas preferably do not produce antibodies, have high fusion efficiencies and are enzyme deficient which render them incapable of subsequent growth in certain selective media which only support the desired Fused cells (hybridomas) are grown.
如本领域技术人员所知,可用许多种骨髓瘤细胞中的任意一种(Goding,1986;Campbell,1984)。例如,免疫动物是小鼠时,人们可使用P3-X63/Ag8、P3-X63-Ag8.653,NS1/1.Ag41、Sp210-Ag14、FO、NSO/U、MPC-11、MPC11-X45-GTG1.7和S194/5XXO Bul;对于大鼠来说,人们可使用R210.RCY3、Y3-Ag 1.2.3、IR983F和4B210;U-266、6M1500-GRG2、LICR-LON-HMy2和729-6都可用于细胞融合。Any of a number of myeloma cell types can be used, as known to those skilled in the art (Goding, 1986; Campbell, 1984). For example, when the immunized animal is a mouse, one can use P3-X63/Ag8, P3-X63-Ag8.653, NS1/1.Ag41, Sp210-Ag14, FO, NSO/U, MPC-11, MPC11-X45- GTG1.7 and S194/5XXO Bul; for rat one can use R210.RCY3, Y3-Ag 1.2.3, IR983F and 4B210; U-266, 6M1500-GRG2, LICR-LON-HMy2 and 729-6 can be used for cell fusion.
产生抗体生产脾脏或淋巴结细胞和骨髓瘤细胞的杂合子的方法通常包括,在一种或几种促进细胞膜融合药剂(化学或电学)的存在下,按2∶1的比例将体细胞与骨髓瘤细胞混合,但该比例可分别在约20∶1至约1∶1中变化。已经描述了使用仙台病毒的融合方法(Kohler和Milstein,1975;1976),Gefter等,(1977)也描述了使用聚乙二醇(PEG),如37%(v/v)PEG的方法。使用电诱导的融合方法也是合适的(Goding,1986)。The method for producing antibody-producing spleen or lymph node cells and heterozygotes of myeloma cells generally involves, in the presence of one or more agents (chemical or electrical) that promote cell membrane fusion, mixing somatic cells with myeloma cells in a 2:1 ratio. The cells are mixed, but the ratio can vary from about 20:1 to about 1:1, respectively. Fusion methods using Sendai virus have been described (Kohler and Milstein, 1975; 1976), and Gefter et al., (1977) have also described methods using polyethylene glycol (PEG), such as 37% (v/v) PEG. The use of electrically induced fusion methods is also suitable (Goding, 1986).
融合方法通常以低频率,大约1×10-6至1×10-8产生存活杂交子。然而,这并不造成问题,因为存活的融合杂交子是通过在选择性培养基中培养,从母代、未融合细胞(尤其是正常情况下会继续无限分裂的未融合骨髓瘤细胞)分化而来的。该选择性培养基通常在组织培养基中含有阻断核苷酸从头合成的药剂。示例性和优选的药剂是氨基蝶呤、氨甲蝶呤和重氮丝氨酸。氨基蝶呤和氨甲蝶呤阻断嘌呤和嘧啶的从头合成,而重氮丝氨酸仅阻断嘌呤合成。如果使用了氨基蝶呤或氨甲蝶呤,该培养基需补充有次黄嘌呤和胸腺嘧啶作为核苷酸来源(HAT培养基)。如果使用重氮丝氨酸,该培养基则需补充有次黄嘌呤。The fusion method usually produces viable hybrids at a low frequency, about 1 x 10-6 to 1 x 10-8 . However, this does not pose a problem because surviving fusion hybrids are differentiated from parental, unfused cells (especially unfused myeloma cells, which normally continue to divide indefinitely) by culturing in selective media. here. The selective medium typically contains an agent that blocks de novo nucleotide synthesis in the tissue culture medium. Exemplary and preferred agents are aminopterin, methotrexate and azaserine. Aminopterin and methotrexate block the de novo synthesis of purines and pyrimidines, whereas diazoserine blocks only purine synthesis. If aminopterin or methotrexate is used, the medium is supplemented with hypoxanthine and thymidine as nucleotide sources (HAT medium). If using azaserine, the medium should be supplemented with hypoxanthine.
优选的选择性培养基是HAT。只有能进行核苷酸补救途径的细胞才能够在HAT培养基中存活。骨髓瘤细胞缺少补救途径的关键酶,如次黄嘌呤磷酸核糖基转移酶(HPRT),因此它们不能存活。B细胞可进行该途径,但它们在培养条件中寿命有限,通常在约两星期内死亡。因此,在选择性培养基中可以存活的细胞只有骨髓瘤和B-细胞形成的杂合子。A preferred selective medium is HAT. Only cells capable of nucleotide salvage pathways are able to survive in HAT medium. Myeloma cells lack key enzymes of the salvage pathway, such as hypoxanthine phosphoribosyltransferase (HPRT), so they cannot survive. B cells can carry out this pathway, but they have a limited lifespan in culture conditions, typically dying within about two weeks. Thus, the only cells that can survive in selective media are myeloma and B-cell heterozygotes.
该培养提供了一群杂交瘤,从中选择特异性杂交瘤。一般地,通过在微量滴定板上稀释单个克隆以培养细胞进行杂交瘤的选择,然后测试单个克隆上清液(约两至三星期后)中所需的反应性。该测定应该是灵敏、简单和快速的,如放射性免疫测定、酶免疫测定、细胞毒性测定、噬菌斑测定,斑点免疫结合测定等。The culture provides a population of hybridomas from which specific hybridomas are selected. Typically, selection of hybridomas is performed by diluting individual clones on microtiter plates to grow cells and then testing the desired reactivity in individual clone supernatants (approximately two to three weeks later). The assay should be sensitive, simple and rapid, such as radioimmunoassay, enzyme immunoassay, cytotoxicity assay, plaque assay, dot immunobinding assay, etc.
然后将选择的杂交瘤连续稀释,并克隆到单个抗体生产细胞系中,然后这些克隆可无限传代以提供mAb,可以两种基本途径使该细胞系进行mAb生产。可将杂交瘤的样品注射(经常是腹腔内)到动物内,该动物与用于为最初融合提供体细胞和骨髓瘤细胞的动物类型组织相容。注射的动物发生肿瘤,该肿瘤分泌由融合细胞杂合子生产的特异性单克隆抗体。然后可放出该动物的体液,如血清或腹水,以提供高浓度mAb。也可在体外培养单个细胞系,mAb被自然地分泌到培养基中,可容易地从培养基中获得高浓度抗体。如果需要,两种方法生产的mAb均可进一步用过滤、离心和各种层析方法如HPLC或亲和层析来纯化。Selected hybridomas are then serially diluted and cloned into individual antibody-producing cell lines, which can then be passaged indefinitely to provide mAbs that can be subjected to mAb production in two basic ways. A sample of the hybridoma can be injected (often intraperitoneally) into an animal that is histocompatible with the type of animal used to provide the somatic and myeloma cells for the initial fusion. Injected animals develop tumors that secrete specific monoclonal antibodies produced heterozygously for the fusion cells. Body fluids of the animal, such as serum or ascites, can then be drained to provide high concentrations of mAb. Single cell lines can also be cultured in vitro, mAbs are naturally secreted into the medium, and high concentrations of antibodies can be easily obtained from the medium. mAbs produced by both methods can be further purified by filtration, centrifugation and various chromatographic methods such as HPLC or affinity chromatography, if desired.
X.定义X. Definition
本文使用的术语“心力衰竭”广泛用于指降低心脏泵血能力的任何病症。结果是组织内发生充血和水肿。最经常的情况是,心力衰竭由冠状血流减少产生的心肌收缩力降低引起;然而,很多其它因素可导致心力衰竭,包括心脏瓣膜的损伤、维生素缺乏和原发性心肌疾病。虽然我们还不完全了解心力衰竭的精确生理机制,但相信心力衰竭通常涉及几种心脏自律神经作用的紊乱,包括交感神经、副交感神经和压力感受器反应。将术语“心力衰竭表现”广泛用于包括所有与心力衰竭相关的后遗症,如呼吸急促、压痕性水肿、扩大的触痛肝、过饱的颈静脉、肺音等,包括有关心力衰竭的实验室发现。As used herein, the term "heart failure" is used broadly to refer to any condition that reduces the ability of the heart to pump blood. The result is hyperemia and edema within the tissue. Most often, heart failure is caused by reduced contractility of the heart muscle from reduced coronary blood flow; however, many other factors can lead to heart failure, including damage to the heart valves, vitamin deficiencies, and primary heart muscle disease. Although the precise physiology of heart failure is not fully understood, it is believed that heart failure often involves disturbances in several cardiac autonomic functions, including sympathetic, parasympathetic, and baroreceptor responses. The term "manifestations of heart failure" is used broadly to include all sequelae associated with heart failure, such as shortness of breath, indentation edema, enlarged tender liver, oversaturated jugular veins, lung sounds, etc., including experimental heart failure chamber found.
术语“治疗”或语法同等成分包括改善和/或逆转心力衰竭的症状(即心脏的泵血能力)。可用本文中描述的任意测量方法评价对心脏“生理功能的改善”(例如,测量射血分数、缩短分数、左心室内部尺寸、心率等),以及对动物存活的任何影响。在动物模型的使用中,用本文中描述的任一测定比较治疗的转基因动物和未治疗的转基因动物的反应(此外,可以包括治疗和未治疗的非转基因动物作为对照)。因此,可将引起本发明筛选方法中使用的任何心力衰竭相关参数的改善的化合物鉴定为治疗化合物。The term "treating" or grammatical equivalents includes improving and/or reversing the symptoms of heart failure (ie, the heart's ability to pump blood). "Improvement in physiological function" of the heart can be assessed using any of the measures described herein (eg, measuring ejection fraction, fractional shortening, left ventricular internal dimensions, heart rate, etc.), as well as any effect on animal survival. In the use of animal models, the responses of treated and untreated transgenic animals are compared using any of the assays described herein (in addition, treated and untreated non-transgenic animals can be included as controls). Thus, compounds that cause improvement in any of the heart failure-related parameters used in the screening methods of the invention can be identified as therapeutic compounds.
术语“扩张型心肌病”指一种心力衰竭的类型,其特征为出现对称性扩张的左心室,其心脏收缩功能差,此外,经常包括右心室。The term "dilated cardiomyopathy" refers to a type of heart failure characterized by a symmetrically dilated left ventricle with poor systolic function and, in addition, often including the right ventricle.
术语“化合物”指可用于治疗或预防身体功能的疾病或不适的任何化学实体、药剂、药物等。化合物包括已知和可能的治疗化合物。可通过用本发明的筛选方法筛选来确定化合物。“已知治疗化合物”指在这些治疗中已经显示有效(例如,通过动物试验或先前有给予人的经验)的治疗化合物。换句话说,已知治疗化合物并不限于在治疗心力衰竭中有效的化合物。The term "compound" refers to any chemical entity, agent, drug, etc. that is useful in the treatment or prevention of a disease or disorder in a bodily function. Compounds include known and potential therapeutic compounds. Compounds can be identified by screening with the screening methods of the present invention. "Known therapeutic compound" refers to a therapeutic compound that has been shown to be effective in such treatments (eg, by animal testing or prior experience in humans). In other words, known therapeutic compounds are not limited to those effective in the treatment of heart failure.
本文使用的术语“激动剂”指模拟“天然”或“自然”化合物作用的分子或化合物。激动剂可以与这些天然化合物在构象、电荷或其它特征上同源。因此,激动剂可被细胞表面表达的受体识别。该识别可导致细胞内的生理和/或生化改变,使细胞以相同方式与存在的激动剂发生反应,就像天然化合物存在一样。激动剂可包括与感兴趣分子、受体和/或通路相互作用的蛋白质、核酸、碳水化合物或任何其它分子。The term "agonist" as used herein refers to a molecule or compound that mimics the action of a "native" or "natural" compound. Agonists may be homologous in conformation, charge or other characteristics to these natural compounds. Thus, agonists are recognized by receptors expressed on the cell surface. This recognition can lead to physiological and/or biochemical changes within the cell, allowing the cell to respond to the presence of the agonist in the same manner as the natural compound is present. Agonists may include proteins, nucleic acids, carbohydrates or any other molecule that interacts with a molecule, receptor and/or pathway of interest.
本文使用的术语“心脏肥大”指成熟的心肌细胞通过肥大生长对应激作出反应的过程。这种生长的特征为,细胞大小增加而不进行细胞分裂,在细胞内组装附加肌节使产生的力最大化和激活胎心基因程序。心脏肥大经常与发病率和死亡率的风险升高相关,因此针对理解心脏肥大分子机制的研究可对人类健康具有重大影响。As used herein, the term "cardiac hypertrophy" refers to the process by which mature cardiomyocytes respond to stress by hypertrophic growth. This growth is characterized by an increase in cell size without cell division, intracellular assembly of additional sarcomeres to maximize force generation and activation of the fetal heart gene program. Cardiac hypertrophy is frequently associated with an increased risk of morbidity and mortality, so research aimed at understanding the molecular mechanisms of cardiac hypertrophy could have a major impact on human health.
本文使用的术语“拮抗剂”和“抑制剂”指抑制可能涉及心脏肥大的细胞因子作用的分子、化合物或核酸。拮抗剂与这些天然化合物在构象、电荷或其它特征上可以或可以不同源。因此,拮抗剂可被激动剂识别的相同或不同受体识别。拮抗剂可具有变构效应,它防止激动剂的作用。另外,拮抗剂可防止激动剂的功能。与激动剂不同的是,拮抗化合物不导致细胞内的病理性和/或生化改变,从而使细胞以与细胞因子存在时相同的方式与存在的拮抗剂发生反应。拮抗剂和抑制剂可包括与感兴趣受体、分子和/或通路相互作用的蛋白质、核酸、碳水化合物或任何其它分子。As used herein, the terms "antagonist" and "inhibitor" refer to molecules, compounds or nucleic acids that inhibit the action of cytokines that may be involved in cardiac hypertrophy. Antagonists may or may not be homologous to these natural compounds in conformation, charge or other characteristics. Thus, the antagonist may be recognized by the same or a different receptor that the agonist recognizes. Antagonists may have allosteric effects, which prevent the action of agonists. In addition, antagonists prevent the function of agonists. Unlike agonists, antagonistic compounds do not cause pathological and/or biochemical changes in the cell, thereby allowing the cell to respond to the presence of the antagonist in the same manner as in the presence of the cytokine. Antagonists and inhibitors may include proteins, nucleic acids, carbohydrates or any other molecule that interacts with a receptor, molecule and/or pathway of interest.
本文使用的术语“调节”指生物活性的改变或变化。调节可以是蛋白活性的升高或降低、激酶活性的改变、结合性质的改变或与感兴趣蛋白或其它结构相关的生物学、功能或免疫性质的任何其它改变。术语“调节剂”指能够改变或变化上述生物学活性的分子或化合物。As used herein, the term "modulation" refers to the alteration or variation of a biological activity. Modulation can be an increase or decrease in protein activity, a change in kinase activity, a change in binding properties, or any other change in biological, functional or immunological properties associated with a protein or other structure of interest. The term "modulator" refers to a molecule or compound capable of altering or altering the above-mentioned biological activity.
术语“β-肾上腺素能受体拮抗剂”指能够部分或完全阻断β型肾上腺素能受体(即响应于儿茶酚胺,尤其是去甲肾上腺素的肾上腺素能反应系统的受体)的化学化合物或实体。一些β-肾上腺素能受体拮抗剂对一个受体亚型(通常是β1)显示出一定程度的特异性;此类拮抗剂称为“β1-特异性肾上腺素能受体拮抗剂”和“β2-特异性肾上腺素能受体拮抗剂”。术语“β-肾上腺素能受体拮抗剂”指选择性和非选择性拮抗剂化学化合物。β-肾上腺素能受体拮抗剂的实例包括但不限于醋丁洛尔、阿替洛尔、布他沙明、卡替洛尔、艾司洛尔、拉贝洛尔、美托洛尔、纳多洛尔、喷布洛尔、丙诺洛尔和噻吗洛尔。本发明方法包括使用已知β-肾上腺素能受体拮抗剂的衍生物。实际上,本发明方法包括任何功能上相当于β-肾上腺素能受体拮抗剂的化合物。The term "beta-adrenergic receptor antagonist" refers to chemical agents capable of partially or completely blocking beta-adrenergic receptors (ie, receptors of the adrenergic response system that respond to catecholamines, especially norepinephrine). compound or entity. Some β-adrenergic receptor antagonists display some degree of specificity for one receptor subtype (usually β1 ); such antagonists are called "β1 -specific adrenergic receptor antagonists" and "β2 -Specific Adrenergic Receptor Antagonists". The term "beta-adrenergic receptor antagonist" refers to selective and non-selective antagonist chemical compounds. Examples of beta-adrenergic receptor antagonists include, but are not limited to, acebutolol, atenolol, butaxamine, carteolol, esmolol, labetalol, metoprolol, Nadolol, pembrolol, propanolol, and timolol. The methods of the present invention involve the use of derivatives of known beta-adrenergic receptor antagonists. Indeed, the methods of the present invention include any compound that is functionally equivalent to a beta-adrenergic receptor antagonist.
术语“血管紧张肽-转化酶抑制剂”或“ACE抑制剂”指能够部分或完全抑制涉及在凝乳酶-血管紧张肽系统中将相对失活的血管紧张肽I转化为活性血管紧张肽II的酶的化学化合物或实体。此外,ACE抑制剂也伴随地抑制缓激肽的降解,这很可能显著增强ACE抑制剂的抗高血压效果。ACE抑制剂的实例包括但不限于贝那普利、卡托普利、依那普利、福辛普利、赖诺普利、quiapril和雷米普利。本发明方法包括使用已知ACE抑制剂的衍生物。实际上,本发明方法包括任何功能上相当于ACE抑制剂的化合物。The term "angiotensin-converting enzyme inhibitor" or "ACE inhibitor" refers to a compound capable of partially or completely inhibiting the conversion of relatively inactive angiotensin I to active angiotensin II in the chymosin-angiotensin system. The chemical compound or entity of an enzyme. In addition, ACE inhibitors also concomitantly inhibit the degradation of bradykinin, which is likely to significantly enhance the antihypertensive effect of ACE inhibitors. Examples of ACE inhibitors include, but are not limited to, benazepril, captopril, enalapril, fosinopril, lisinopril, quiapril, and ramipril. The methods of the invention involve the use of derivatives of known ACE inhibitors. Indeed, the methods of the invention include any compound that is functionally equivalent to an ACE inhibitor.
本文使用的术语“基因型”指生物体的实际遗传构成,而“表型”指个体显示的物理特性。此外,“表型”是基因组选择表达的结果(即它是细胞历史和它对胞外环境反应的表达)。实际上,人类基因组估计含有30,000-35,000个基因。在各细胞类型中,只表达了这些基因的一小部分(即10-15%)。As used herein, the term "genotype" refers to the actual genetic makeup of an organism, while "phenotype" refers to the physical characteristics exhibited by an individual. Furthermore, a "phenotype" is the result of selective expression of the genome (ie it is the expression of the cell's history and its response to the extracellular environment). In fact, the human genome is estimated to contain 30,000-35,000 genes. In each cell type, only a small fraction (ie 10-15%) of these genes are expressed.
XI.实施例XI. Examples
包括了下面的实施例,以进一步说明本发明的各个方面。本领域技术人员应该理解,下面的实施例中公开的技术代表本发明者发现的在实践本发明中发挥良好作用的技术和/或组合物,因此可以认为这些技术构成其实践的优选模式。然而根据本公开,本领域技术人员应该了解,可对公开的具体实施方式作出很多改变并仍然获得同样或类似的结果,而不背离本发明精神和范围。The following examples are included to further illustrate various aspects of the invention. It should be appreciated by those of skill in the art that the techniques disclosed in the examples which follow represent techniques and/or compositions discovered by the inventors to function well in the practice of the invention, and thus can be considered to constitute preferred modes for its practice. However, those of skill in the art should, in light of the present disclosure, appreciate that many changes can be made in the specific embodiments which are disclosed and still obtain a like or a similar result without departing from the spirit and scope of the invention.
实施例1:材料和方法Example 1: Materials and methods
化学试剂和质粒。豆蔻酰12-佛波醇13-乙酯(PMA)、8-Br-cAMP、pCPT-cGMP、和茴香霉素购自Sigma Chemical(St.Louis,MO)。下面的激酶抑制剂购自所示公司:马来酰亚胺I和G6976(A.G.Scientific,San Diego,CA),KN93、SB216763和渥曼青霉素(BIOMOL,Plymouth Meeting,PA),G6983、星孢素、PD98059、渥曼青霉素、U1026、Y-27632、雷帕霉素和DAG激酶抑制剂II(Calbiochem)。以ImM使用KN93、渥曼青霉素和星孢素。以10mM使用U1026、HA1077、Y-27632、DAG激酶抑制剂II、SB216763和Bis I。以30纳克/毫升使用雷帕霉素。苯肾上腺素和内皮缩血管肽-1购自Sigma。Alex Toker善意地提供编码PKD同种型的哺乳动物表达载体,其它地方有该载体的描述(Storz和Toker,2003)。Chemical reagents and plasmids. Myristoyl 12-phorbol 13-ethyl ester (PMA), 8-Br-cAMP, pCPT-cGMP, and anisomycin were purchased from Sigma Chemical (St. Louis, MO). The following kinase inhibitors were purchased from the indicated companies: Maleimide I and G®6976 (A.G. Scientific, San Diego, CA), KN93, SB216763 and Wortmannin (BIOMOL, Plymouth Meeting, PA), G®6983 , staurosporine, PD98059, wortmannin, U1026, Y-27632, rapamycin and DAG kinase inhibitor II (Calbiochem). KN93, wortmannin and staurosporine were used at 1 mM. U1026, HA1077, Y-27632, DAG Kinase Inhibitor II, SB216763 and Bis I were used at 10 mM. Use rapamycin at 30 ng/ml. Phenylephrine and endothelin-1 were purchased from Sigma. A mammalian expression vector encoding a PKD isoform was kindly provided by Alex Toker and described elsewhere (Storz and Toker, 2003).
细胞培养和转染测定。在含有FBS(10%)、L-谷胺酰胺(2mM)和青霉素-链霉素的DMEM中培养COS细胞。根据生产商的说明书用Fugene 6(Roche MolecularBiochemicals)进行COS细胞的转染。对于HDAC定位实验,转染后用PMA(100nM)、伊屋诺霉素(1mM)、8-Br-cAMP(1mM)、pCPT-GMP(1mM)或茴香霉素(1mM)处理细胞16-24小时。注意,在加入任何化学刺激30分钟前,加入特异性蛋白激酶抑制剂。用标准荧光显微技术使GFP-HDAC5显色。对于FLAG-HDAC5的间接免疫荧光来说,将COS细胞接种在盖玻片上,转染,然后如上地处理。在特异性处理后,用福尔马林缓冲液(10%)固定细胞,在含有BSA(3%)和Nonidet P-40(0.1%)的PBS中染色。以1∶200的浓度使用Flag M2抗体(Sigma)。也以1∶200的浓度使用第二荧光素共轭抗体(Vector Laboratories)。通过上述间接免疫荧光检测,分别用抗肌节α-辅肌动蛋白(Sigma)和ANF(Peninsula Laboratories)的抗体对心肌细胞中的肌节和心钠素(ANF)进行染色。Cell culture and transfection assays. COS cells were cultured in DMEM containing FBS (10%), L-glutamine (2 mM) and penicillin-streptomycin. Transfection of COS cells was performed with Fugene 6 (Roche Molecular Biochemicals) according to the manufacturer's instructions. For HDAC localization experiments, cells were treated with PMA (100 nM), ionomycin (1 mM), 8-Br-cAMP (1 mM), pCPT-GMP (1 mM), or anisomycin (1 mM) after transfection for 16-24 Hour. Note that specific protein kinase inhibitors are added 30 min before the addition of any chemical stimulus. GFP-HDAC5 was visualized using standard fluorescence microscopy techniques. For indirect immunofluorescence of FLAG-HDAC5, COS cells were seeded on coverslips, transfected, and processed as above. After specific treatment, cells were fixed with buffered formalin (10%) and stained in PBS containing BSA (3%) and Nonidet P-40 (0.1%). Flag M2 antibody (Sigma) was used at a concentration of 1:200. A secondary fluorescein-conjugated antibody (Vector Laboratories) was also used at a concentration of 1:200. Sarcomeres and atrial natriuretic factor (ANF) in cardiomyocytes were stained with antibodies against sarcomere α-actinin (Sigma) and ANF (Peninsula Laboratories), respectively, by indirect immunofluorescence detection as described above.
心肌细胞培养和腺病毒感染。如之前所述地从1-2天龄的Sprague Dawley大鼠中分离新生大鼠心肌细胞(NRVM)(Antos等,2003)。对于腺病毒生产,将编码LacZ或FLAG-标记的HDAC5(S259/498A)的cDNA亚克隆化到pACCMV载体中,和pJM17共转染到293细胞中。将初级裂解物用于再感染293细胞,用琼脂覆盖法获得病毒噬菌斑。将全长人HDAC5(编码1122个氨基酸)的互补DNA与编码增强型绿色荧光蛋白(EGFP;Clontech)的序列在pcDNA3.1+(Invitrogen)内融合。产生的构建物编码框架内融合到HDAC5氨基末端的GFP。以相同方式产生编码GFP的构建物,该GFP融合到用丙氨酸代替丝氨酸259和498的HDAC5中。将GFP-HDAC5cDNA亚克隆化到pACCMV中,以进行腺病毒生产。扩增腺病毒的克隆群体并测定其滴度。Cardiomyocyte culture and adenovirus infection. Neonatal rat cardiomyocytes (NRVM) were isolated from 1-2 day old Sprague Dawley rats as previously described (Antos et al., 2003). For adenovirus production, cDNA encoding LacZ or FLAG-tagged HDAC5 (S259/498A) was subcloned into pACCMV vector and co-transfected with pJM17 into 293 cells. Primary lysates were used to reinfect 293 cells and viral plaques were obtained using the agar overlay method. The complementary DNA of full-length human HDAC5 (encoding 1122 amino acids) was fused within pcDNA3.1+ (Invitrogen) to the sequence encoding enhanced green fluorescent protein (EGFP; Clontech). The resulting construct encodes GFP fused in frame to the amino terminus of HDAC5. A construct encoding GFP fused to HDAC5 with
免疫共沉淀测定。将Flag-HDAC5表达质粒转染到如上述处理的COS细胞中。将处理细胞收集到Tris(50mM,pH7.4)、NaCl(150mM)、EDTA(1mM)和TritonX-100(1%)中。使细胞通过22号针头,以进一步瓦解细胞,通过离心去除细胞碎片。用M2-琼脂糖结合体(conjugate)(Sigma)免疫沉淀Flag-HDAC5,彻底洗涤。用SDS-PAGE解析结合蛋白,用Flag M2(Sigma)或14-3-3抗体(Santa CruzBiotechnology)进行Western印迹分析。对于用NRVM研究来说,用含有蛋白酶抑制剂混合物(完全;Roche)、PMSF(1mM)和磷酸酶抑制剂[焦磷酸钠(1mM)、氟化钠(2mM)、b-磷酸甘油(10mM)、钼酸钠(1mM)、原钒酸钠(1mM)]的相同缓冲液从表达GFP-HDAC5的细胞中制备全细胞蛋白抽提物。短时超声处理裂解物,离心以澄清。在免疫沉淀中,将蛋白裂解物与HDAC5特异性抗血清(19)和蛋白G琼脂糖珠子(Amersham Biosciences)接触。用裂解缓冲液洗涤免疫沉淀物5次,SDS-PAGE解析和用特异性抗GFP(BD Biosciences;1∶2,500稀释)或14-3-3(Santa Cruz[H-8];1∶1000稀释)的小鼠单克隆抗体进行免疫印迹。通过免疫印迹检测结合的PKD,所用抗体是抗PKD-1、丝氨酸744和748磷酸化的PKD-1或丝氨酸916磷酸化的PKD-1的兔多克隆抗体(Cell Signaling Technologies),1∶1000稀释。Co-immunoprecipitation assay. The Flag-HDAC5 expression plasmid was transfected into COS cells treated as above. Treated cells were collected into Tris (50 mM, pH 7.4), NaCl (150 mM), EDTA (1 mM) and TritonX-100 (1%). Cells were further disrupted by passing them through a 22-gauge needle, and cell debris was removed by centrifugation. Flag-HDAC5 was immunoprecipitated with M2-agarose conjugate (Sigma) and washed extensively. Bound proteins were resolved by SDS-PAGE, and Western blot analysis was performed with Flag M2 (Sigma) or 14-3-3 antibody (Santa Cruz Biotechnology). For studies with NRVM, a mixture containing protease inhibitor cocktail (complete; Roche), PMSF (1 mM) and phosphatase inhibitors [sodium pyrophosphate (1 mM), sodium fluoride (2 mM), b-glycerol phosphate (10 mM) , sodium molybdate (1 mM), sodium orthovanadate (1 mM)] in the same buffer to prepare whole-cell protein extracts from cells expressing GFP-HDAC5. Lysates were sonicated briefly and clarified by centrifugation. In immunoprecipitation, protein lysates were contacted with HDAC5-specific antiserum (19) and protein G agarose beads (Amersham Biosciences). Immunoprecipitates were washed 5 times with lysis buffer, resolved by SDS-PAGE and analyzed with specific anti-GFP (BD Biosciences; 1:2,500 dilution) or 14-3-3 (Santa Cruz[H-8]; 1:1000 dilution) mouse monoclonal antibody for western blotting. Bound PKD was detected by immunoblotting with rabbit polyclonal antibody against PKD-1, PKD-1 phosphorylated at serine 744 and 748, or PKD-1 phosphorylated at serine 916 (Cell Signaling Technologies), diluted 1:1000 .
体外激酶测定。用抗-Flag M2抗体免疫沉淀Flag-HDAC5,如上所述。用激酶缓冲液(Tris(25mM,pH7.4)、MgCl2(10mM)、DTT(1mM))洗涤和平衡结合的Flag-HDAC5。平衡后,加入激酶反应混合物(激酶缓冲液加ATP(0.1mM)和50mCi[g-32P]-ATP)。在30℃进行激酶反应30分钟,通过加入等体积的2xSDS-PAGE上样缓冲液终止反应。用SDS-PAGE解析磷酸化蛋白,通过放射自显影显色。In vitro kinase assay. Flag-HDAC5 was immunoprecipitated with anti-Flag M2 antibody as described above. Bound Flag-HDAC5 was washed and equilibrated with kinase buffer (Tris (25 mM, pH 7.4), MgCl2 (10 mM), DTT (1 mM)). After equilibration, the kinase reaction mixture (kinase buffer plus ATP (0.1 mM) and 50 mCi [g-32P]-ATP) was added. Kinase reactions were performed at 30°C for 30 minutes and terminated by adding an equal volume of 2xSDS-PAGE loading buffer. Phosphorylated proteins were resolved by SDS-PAGE and visualized by autoradiography.
GFP-HDAC5定位研究。为了在NRVM中分析GFP-HDAC5,在腺病毒存在下(感染复数=~50-100)将细胞铺板在明胶包被的96孔板(Costar;1×104细胞/孔)上,含有胎牛血清(FBS)(10%)、L-谷氨酰胺(2mM)和青霉素-链霉素的DMEM中。培养过夜后,用无血清培养基洗涤细胞,在含有Neutridoma-SP(0.1%;Roche Applied Science)的DMEM(100毫升)中培养,Neutridoma-SP含有白蛋白、胰岛素、转铁蛋白和其他明确的有机和无机化合物。在无血清培养基中培养(3小时)后,将细胞与激酶抑制剂接触(30分钟),然后用激动剂刺激2.5小时。用PBS洗涤细胞,并用含有Hoechst染料33342(H-3570,Molecular Probes)的10%福尔马林PBS溶液固定。用配有数码相机(Photometrics CoolSNAP HQ)的荧光显微镜(Nikon Eclipse TS100)40X放大和MetaMorph成像软件捕获图像。用High Content Imaging System(Cellomics,Inc.,Pittsburgh,PA)定量GFP-HDAC5在核中与胞浆中的相对丰度,该系统根据Hoechst荧光划分核界限,根据这些核尺度定义胞浆环。HDAC5定位值代表来自每个实验条件下最少200个细胞的平均值。GFP-HDAC5 localization study. For analysis of GFP-HDAC5 in NRVM, cells were plated on gelatin-coated 96-well plates (Costar; 1×104 cells/well) in the presence of adenovirus (MOI = ~50-100) containing fetal calf Serum (FBS) (10%), L-Glutamine (2mM) and Penicillin-Streptomycin in DMEM. After overnight culture, cells were washed with serum-free medium and cultured in DMEM (100 ml) containing Neutridoma-SP (0.1%; Roche Applied Science) containing albumin, insulin, transferrin and other defined organic and inorganic compounds. After incubation in serum-free medium (3 hours), cells were exposed to kinase inhibitors (30 minutes) and then stimulated with agonists for 2.5 hours. Cells were washed with PBS and fixed with 10% formalin in PBS containing Hoechst dye 33342 (H-3570, Molecular Probes). Images were captured with a fluorescence microscope (Nikon Eclipse TS100) equipped with a digital camera (Photometrics CoolSNAP HQ) at 40X magnification and MetaMorph imaging software. The relative abundance of GFP-HDAC5 in the nucleus versus the cytoplasm was quantified using the High Content Imaging System (Cellomics, Inc., Pittsburgh, PA), which demarcates nuclear boundaries based on Hoechst fluorescence and defines cytoplasmic rings based on these nuclear dimensions. HDAC5 localization values represent mean values from a minimum of 200 cells per experimental condition.
RNA分析。将NRVM铺于明胶包被的10厘米培养皿上(2×106细胞/皿)。按照指示的处理方法,用Trizol试剂(Gibco/BRL)从心肌细胞中分离RNA。用96-孔型斑点印迹仪(Bio-Rad)将总RNA(2微克)真空印迹到硝酸纤维素膜(Bio-Rad)上。在含SDS(1%)、5xDenhardt氏试剂、焦磷酸钠(0.05%)和100微克/毫升超声处理的鲑鱼精子DNA的4xSSC中封闭膜(500℃4小时),用32P-末端标记的寡核苷酸探针孵育(1×106cpm/毫升;500℃14小时)。寡核苷酸的序列如下:RNA analysis. NRVMs were plated on gelatin-coated 10 cm dishes (2×106 cells/dish). RNA was isolated from cardiomyocytes with Trizol reagent (Gibco/BRL) following the indicated treatments. Total RNA (2 micrograms) was vacuum blotted onto nitrocellulose membranes (Bio-Rad) using a 96-well dot blotter (Bio-Rad). Membranes were blocked (500°C for 4 hours) in 4xSSC containing SDS (1%), 5xDenhardt's reagent, sodium pyrophosphate (0.05%), and 100 μg/ml sonicated salmon sperm DNA, with 32P-terminally labeled oligonucleotides. Nucleotide probe incubation (1×106 cpm/ml; 500° C. for 14 hours). The sequence of the oligonucleotide is as follows:
ANF,5’-aatgtgaccaagctgcgtgacacaccacaagggcttaggatcttttgcgatctgctcaag-3’;ANF,5'-aatgtgaccaagctgcgtgacaccacaagggcttaggatcttttgcgatctgctcaag-3';
BNP,5’-tgaactatgtgccatcttggaatttcgaagtctctcct-3’;BNP, 5'-tgaactatgtgccatcttggaatttcgaagtctctcct-3';
α-SK-肌动蛋白5’-tggagcaaaacagaatggctggctttaatgcttcaagttttccatttcctttccacaggg-3’;α-SK-actin 5'-tggagcaaaacagaatggctggctttaatgcttcaagttttccatttcctttccacaggg-3';
GAPDH 5’-ggaacatgtagaccatgtagttgaggtcaatgaag-3’。GAPDH 5'-ggaacatgtagaccatgtagttgaggtcaatgaag-3'.
用含0.1%SDS的0.5xSSC洗涤印迹(500℃10分钟)两次,通过放射自显影分析。Blots were washed twice with 0.5xSSC containing 0.1% SDS (500°C for 10 minutes) and analyzed by autoradiography.
哺乳动物双杂交分析。在pM1表达载体(Sadowski)中产生编码融合到人HDAC5氨基端(氨基酸2-664)的GAL4DNA结合域的哺乳动物表达载体。以类似方式构建由丙氨酸代替丝氨酸259和/或498的GAL4-HDAC5融合蛋白。用pVP16(Clontech)产生编码融合到14-3-3σ的氨基末端的疱疹病毒VP16转录激活域的构建物。在存在或不存在组成性活化的PKD-1的构建物的情况下,在五个拷贝的GAL4DNA结合位点(5xUAS荧光素酶)的控制下,用GAL4-HDAC5、VP16-14-3-3和荧光素酶报道基因的载体瞬时转染COS细胞。转染四十八小时后,收集细胞,用荧光素酶测定试剂盒(Promega)定量荧光素酶的水平。Mammalian two-hybrid analysis. A mammalian expression vector encoding the GAL4 DNA binding domain fused to the amino terminus of human HDAC5 (amino acids 2-664) was produced in the pM1 expression vector (Sadowski). A GAL4-HDAC5 fusion protein with
转基因小鼠的生产。将编码PKD组成性活化形式的cDNA克隆到心脏特异性α-肌球蛋白重链启动子的下游。将该载体注射到B6C3F1小鼠卵母细胞中,并植入代用的雌性ICR小鼠。通过用转基因特异性引物进行PCR来鉴定转基因后代。结果显示在图7A-D中。Production of transgenic mice. The cDNA encoding the constitutively active form of PKD was cloned downstream of the cardiac-specific α-myosin heavy chain promoter. This vector was injected into B6C3F1 mouse oocytes and implanted into surrogate female ICR mice. Transgenic progeny were identified by PCR with transgene-specific primers. The results are shown in Figure 7A-D.
实施例2:结果Example 2: Results
PKC依赖途径刺激HDAC5的核输出。为了进一步确定导致II类HDAC磷酸化和核输出的信号转导途径,本发明者测试了各种蛋白激酶途径激活物在COS细胞中刺激HDAC5核输出的能力。HDAC5主要位于允许用方便系统评价核输出的COS细胞核中。测试了PKA(8-Br-cAMP)、PKG(pCPT-GMP)、PKC(PMA)、CaMK(伊屋诺霉素)和Jun-N-末端激酶(茴香霉素)的激活物激活与GFP融合的HDAC5的核输出能力。在这些化合物中,只有伊屋诺霉素和PMA刺激GFP-HDAC5的核输出(图1A)。在测试浓度下,PMA是比伊屋诺霉素更强效的输出刺激物。A PKC-dependent pathway stimulates nuclear export of HDAC5. To further define the signal transduction pathway leading to class II HDAC phosphorylation and nuclear export, the inventors tested the ability of various protein kinase pathway activators to stimulate HDAC5 nuclear export in COS cells. HDAC5 is predominantly localized in COS nuclei allowing assessment of nuclear export in a convenient system. Activator activation of PKA (8-Br-cAMP), PKG (pCPT-GMP), PKC (PMA), CaMK (Ionomycin) and Jun-N-terminal kinase (Anisomycin) fusions to GFP were tested Nuclear export capability of HDAC5. Of these compounds, only ionomycin and PMA stimulated nuclear export of GFP-HDAC5 (Fig. 1A). At the concentrations tested, PMA was a more potent export stimulator than ionomycin.
HDAC5和其他II类HDAC响应于CaMK信号转导的核输出需要位于HDAC蛋白的N-末端区的两个丝氨酸(Grozinger和Schreiber,2000;McKinsey等,2000)。由丙氨酸取代这些丝氨酸残基(残基249和498)的HDAC5突变体(HDAC5-S/A)不能响应于PMA处理而输出(图1B),验证了在对PKC激活的反应中这些位点的必需角色。加入PMA后15分钟内起始HDAC5的核输出,30分钟内完成(图1C)。Nuclear export of HDAC5 and other class II HDACs in response to CaMK signaling requires two serines located in the N-terminal region of HDAC proteins (Grozinger and Schreiber, 2000; McKinsey et al., 2000). A mutant of HDAC5 (HDAC5-S/A) in which these serine residues (residues 249 and 498) were substituted by alanine failed to export in response to PMA treatment (Fig. Point's required role. Nuclear export of HDAC5 was initiated within 15 minutes of PMA addition and completed within 30 minutes (Fig. 1C).
HDAC5中丝氨酸249和498的磷酸化为14-3-3蛋白建立了停靠位点,14-3-3蛋白护送HDAC5到胞浆中(Grozinger和Schreiber,2000;McKinsey等,2000)。为进一步确证PMA促进这些位点的磷酸化,本发明者在免疫共沉淀测定中分析了HDAC5与14-3-3的相互作用。如图1D所示,在PMA存在下,14-3-3与HDAC5的结合增强。相反,HDAC5-S/A突变体不能响应于PMA,不与14-3-3结合。因此,本发明者得出结论,PKC信号转导导致HDAC5丝氨酸249和498的磷酸化和随后通过14-3-3-依赖机制的核输出。Phosphorylation of
心肌细胞中HDAC5的PKC依赖性核输出。为了开始研究PKC信号转导在心脏肥大过程中控制HDAC5运输的作用,本发明人开发了定量测定,用于在原代心肌细胞中测量激动剂依赖性HDAC5核输出。该测定使用Cellomics High Content ImagingSystem,它快速定量核和胞浆的GFP荧光强度,并提供两个亚细胞室之间强度差异的读数(图2A)。为确认该测定,用表达GFP-HDAC5(Ad-GFP-HDAC5)的腺病毒感染新生大鼠心室心肌细胞(NRVM),并用递增剂量的α-1-肾上腺素能能激动剂苯肾上腺素(PE)(一种促进HDAC5核输出的肥大激动剂)刺激(Bush等,2004)。如图2B所示,PE以浓度依赖方式引发HDAC5的核输出。通过视觉检查这些细胞确证了这些结果(数据未显示)。PKC-dependent nuclear export of HDAC5 in cardiomyocytes. To begin investigating the role of PKC signaling in controlling HDAC5 trafficking during cardiac hypertrophy, the inventors developed a quantitative assay to measure agonist-dependent HDAC5 nuclear export in primary cardiomyocytes. The assay uses the Cellomics High Content Imaging System, which rapidly quantifies nuclear and cytoplasmic GFP fluorescence intensity and provides a readout of the difference in intensity between the two subcellular compartments (Figure 2A). To confirm this assay, neonatal rat ventricular cardiomyocytes (NRVM) were infected with adenovirus expressing GFP-HDAC5 (Ad-GFP-HDAC5) and treated with increasing doses of the α-1-adrenergic agonist phenylephrine (PE ) (a hypertrophic agonist that promotes HDAC5 nuclear export) stimulation (Bush et al., 2004). As shown in Figure 2B, PE triggered the nuclear export of HDAC5 in a concentration-dependent manner. These results were confirmed by visual inspection of these cells (data not shown).
确立定量核输出测定的有效性后,本发明人下一步测试一系列酶的抑制剂阻断PE-诱导的HDAC5易位出核的能力。普通的丝氨酸/苏氨酸蛋白激酶抑制剂星孢素和PKC抑制剂马来酰亚胺I(Bis I)有效阻断HDAC5的PE-依赖性输出(图2C和2D)。相反,CaMK(KN93)、MEK1(U1026)、ROCK(Y-27632)、甘油二酯激酶(DAGK抑制剂II)、PI3-激酶(渥曼青霉素)、S6激酶(雷帕霉素)、GSK(SB216763)的抑制剂或PKG、MLCK和PKA的抑制剂(HA1077)并不显著影响PE-诱导的HDAC5核输出。Having established the validity of the quantitative nuclear export assay, the inventors next tested a series of inhibitors of the enzyme for their ability to block PE-induced translocation of HDAC5 out of the nucleus. The common serine/threonine protein kinase inhibitor staurosporine and the PKC inhibitor maleimide I (Bis I) effectively blocked the PE-dependent export of HDAC5 (Fig. 2C and 2D). In contrast, CaMK (KN93), MEK1 (U1026), ROCK (Y-27632), Diacylglycerol Kinase (DAGK Inhibitor II), PI3-Kinase (Wortmannin), S6 Kinase (Rapamycin), GSK ( SB216763) or inhibitors of PKG, MLCK and PKA (HA1077) did not significantly affect PE-induced HDAC5 nuclear export.
PKC信号转导通过HDAC磷酸化诱导心脏肥大。已显示的激活足够引起心肌细胞肥大,在某些情况下,PKC激活对心肌细胞肥大是必要的(参见Antos,2003;Dunnmon等,1990)。上述结果使PKC-依赖性HDAC5核输出或其它II类HDAC牵连于心肌细胞肥大发展中。为证实该可能性,本发明人检测响应于PKC激活的肥大是否需要II类HDAC的磷酸化和核输出。用编码信号-抗性HDAC5-S/A突变蛋白的腺病毒感染NRVM,或用编码LacZ的腺病毒转染作为对照。如图3A所示,在原代心肌细胞中表达HDAC5-S/A突变体防止响应于PE或PMA的肌节装配和细胞增大。PKC signaling induces cardiac hypertrophy through HDAC phosphorylation. Activation has been shown to be sufficient to cause cardiomyocyte hypertrophy, and in some cases PKC activation is necessary for cardiomyocyte hypertrophy (see Antos, 2003; Dunnmon et al., 1990). The above results implicate PKC-dependent nuclear export of HDAC5 or other class II HDACs in the development of cardiomyocyte hypertrophy. To test this possibility, the inventors examined whether phosphorylation and nuclear export of class II HDACs are required for hypertrophy in response to PKC activation. NRVM were infected with adenovirus encoding the signal-resistant HDAC5-S/A mutant protein, or transfected with adenovirus encoding LacZ as a control. As shown in Figure 3A, expression of the HDAC5-S/A mutant in primary cardiomyocytes prevented sarcomere assembly and cell enlargement in response to PE or PMA.
心脏肥大与病理性“胎”基因程序的再活化相关,该基因程序包括编码心钠素(ANF)、脑钠尿肽(BNP)和α-骨架肌动蛋白的基因。也可通过用ANF特异性抗体免疫染色心肌细胞来检测ANF表达的激动剂依赖性升高。如图3B所示,在用PE或PMA处理的NRVM中观察到明显的核周ANF蛋白表达。ANF表达的激动剂依赖性诱导不受LacZ异位表达的影响,但在信号抗性HDAC5的存在下显著降低。此外,不可磷酸化的HDAC5阻断PE-和PMA-介导的ANF转录物诱导,以及BNP和α-骨架肌动蛋白的转录物诱导。总之,这些结果说明PKC信号转导部分地通过刺激II类HDAC的核输出而引发心脏肥大。Cardiac hypertrophy is associated with reactivation of a pathological "fetal" gene program, including genes encoding atrial natriuretic peptide (ANF), brain natriuretic peptide (BNP), and α-skeletal actin. Agonist-dependent increases in ANF expression can also be detected by immunostaining cardiomyocytes with ANF-specific antibodies. As shown in Fig. 3B, significant perinuclear ANF protein expression was observed in NRVM treated with PE or PMA. Agonist-dependent induction of ANF expression was not affected by ectopic expression of LacZ, but was significantly reduced in the presence of signaling-resistant HDAC5. Furthermore, nonphosphorylatable HDAC5 blocked PE- and PMA-mediated induction of ANF transcripts, as well as that of BNP and α-skeletal actin. Taken together, these results suggest that PKC signaling triggers cardiac hypertrophy in part by stimulating the nuclear export of class II HDACs.
HDAC5核输出对PKC抑制的差别敏感性。本发明者下一步检查响应于其它肥大信号的HDAC5核输出是否也依赖于PKC信号转导。刺激肥大的内皮缩血管肽-1(ET-1)和胎牛血清(FBS)也有效促进HDAC5核输出(数据未发表)。然而,与其对PE依赖性HDAC5核输出的抑制效果相反,Bis I对响应于ET-1或FBS的HDAC5核输出没有影响(图4A)。这些发现提示,PE引发不同于ET-1和FBS的激酶途径,以促进HDAC5的核输出。Differential sensitivity of HDAC5 nuclear export to PKC inhibition. The inventors next examined whether HDAC5 nuclear export in response to other hypertrophic signals is also dependent on PKC signaling. Hypertrophy-stimulating endothelin-1 (ET-1) and fetal bovine serum (FBS) were also effective in promoting HDAC5 nuclear export (unpublished data). However, in contrast to its inhibitory effect on PE-dependent HDAC5 nuclear export, Bis I had no effect on HDAC5 nuclear export in response to ET-1 or FBS (Fig. 4A). These findings suggest that PE triggers a different kinase pathway than ET-1 and FBS to promote nuclear export of HDAC5.
为了进一步检验上述肥大激动剂的蛋白激酶效应子的性质,本发明者测试了附加PKC抑制剂对HDAC5核输出的可能影响。G6983,另一普通PKC抑制剂的活性,与Bis I的活性类似,抑制响应于PE而非ET-1或FBS的HDAC5核输出(图4B和4D)。相反,G6976,钙依赖性PKCa和b同功酶的特异性抑制剂,则有效阻断PE、ET-1或FBS引发的HDAC5核输出(图4C和D)。上述抑制剂对HDAC5核输出的不同影响与它们对14-3-3与HDAC5结合(HDAC5磷酸化的指示)的影响类似。G6976而非Bis I阻断响应于PE和ET-1的HDAC5和14-3-3的结合(图4E)。To further examine the protein kinase effector properties of the hypertrophic agonists described above, the inventors tested the possible effect of additional PKC inhibitors on HDAC5 nuclear export. The activity of G.6983, another general PKC inhibitor, was similar to that of Bis I, inhibiting HDAC5 nuclear export in response to PE but not ET-1 or FBS (Fig. 4B and 4D). In contrast, G6976, a specific inhibitor of calcium-dependent PKCa and b isozymes, effectively blocked HDAC5 nuclear export triggered by PE, ET-1 or FBS (Fig. 4C and D). The differential effects of the above inhibitors on HDAC5 nuclear export are similar to their effects on 14-3-3 binding to HDAC5 (indicative of HDAC5 phosphorylation). G6976 but not Bis I blocked the binding of HDAC5 and 14-3-3 in response to PE and ET-1 (Fig. 4E).
G6976而非Bis I或G6983阻断响应于多个激动剂的HDAC5核输出的能力似乎是荒谬的,因为后者化合物与G6976同样有效地阻断PKCa和b。然而,该抑制剂性质与其他人用来区分PKCa或b和PKD/PKCm的作用的拟制剂类似(Zugaza等,1996),即对G6976而不是对Bis I或G6983敏感(Gschwendt等,1996)。The ability of G6976 but not Bis I or G6983 to block HDAC5 nuclear export in response to multiple agonists seems paradoxical, since the latter compound blocks PKCa and b equally effectively as G6976. However, the inhibitor properties were similar to those of mimics that others have used to differentiate the effects of PKCa or b from PKD/PKCm (Zugaza et al., 1996), being sensitive to G6976 but not to Bis I or G6983 (Gschwendt et al. , 1996).
蛋白激酶D刺激HDAC5的核输出。根据上述提示PKD可能参与激动剂依赖性HDAC5核输出的结果,本发明者检测了HDAC5中信号反应性丝氨酸周围氨基酸序列的潜在PKD共有磷酸化位点。PKD在相对于磷酸化丝氨酸-5位置上强烈优选亮氨酸残基(Nishikawa等,1997)。HDAC5在相对于两个信号反应性丝氨酸残基的这个位置上含有亮氨酸(图5A)。有趣的是,II类HDAC 4、7和9也在此位置上含有亮氨酸。Protein kinase D stimulates nuclear export of HDAC5. Based on the above results suggesting that PKD may be involved in agonist-dependent HDAC5 nuclear export, the present inventors examined potential PKD consensus phosphorylation sites in the amino acid sequence around signal-responsive serine in HDAC5. PKD strongly prefers leucine residues at positions relative to phosphorylated serine-5 (Nishikawa et al., 1997). HDAC5 contains a leucine at this position relative to the two signal-responsive serine residues (Fig. 5A). Interestingly, class II HDACs 4, 7 and 9 also contain leucine at this position.
为评价-5位置上亮氨酸的重要性,本发明者将HDAC5中的亮氨酸254和493突变成甘氨酸,未改变实际磷酸化位点。该HDAC5突变体(L254/493G)构成定位于核,完全不受PMA的控制(图5B)。转染测定进一步支持PKD参与HDAC5核输出,在该测定中PKD的激活形式(PKD S/E),而非催化失活的突变体(PKD K/W),有效刺激HDAC5的核输出(图5C)。信号反应性丝氨酸残基或HDAC5的254和493位亮氨酸的突变消除了响应于PKD的核输出(图5C)。To assess the importance of leucine at position -5, the inventors mutated leucine 254 and 493 in HDAC5 to glycine without changing the actual phosphorylation site. The HDAC5 mutant (L254/493G) constitutively localizes to the nucleus, completely independent of PMA control (Fig. 5B). The involvement of PKD in HDAC5 nuclear export was further supported by transfection assays in which the activated form of PKD (PKD S/E), but not the catalytically inactive mutant (PKD K/W), potently stimulated nuclear export of HDAC5 (Figure 5C ). Mutation of signaling-responsive serine residues or leucines 254 and 493 of HDAC5 abolished nuclear export in response to PKD (Fig. 5C).
为了进一步探索PKD作为HDAC5核输出激酶的可能作用,本发明者进行了免疫共沉淀和体外激酶测定。免疫共沉淀HDAC5和PKD后,体外激酶测定确证了PKD直接磷酸化HDAC5。如图5D所示,PKD的共转染几乎不导致HDAC5的磷酸化。用PMA处理细胞使与PKD结合同时发生的HDAC5磷酸化程度增加。虽然PMA处理增加HDAC5的磷酸化,但激活的PKD S/E结合和磷酸化HDAC5并不需要PMA,也许这是由于在免疫复合物中存在内源性PKD。用催化失活的突变PKD K/W没有观察到HDAC5的磷酸化。然而有趣的是,甚至在不存在PMA的情况下,PKD K/W也与HDAC5结合。To further explore the possible role of PKD as an HDAC5 nuclear export kinase, the inventors performed co-immunoprecipitation and in vitro kinase assays. After co-immunoprecipitation of HDAC5 and PKD, in vitro kinase assays confirmed that PKD directly phosphorylates HDAC5. As shown in Figure 5D, co-transfection of PKD hardly resulted in phosphorylation of HDAC5. Treatment of cells with PMA increased HDAC5 phosphorylation that coincided with PKD binding. Although PMA treatment increased phosphorylation of HDAC5, activated PKD S/E binding and phosphorylation of HDAC5 did not require PMA, perhaps due to the presence of endogenous PKD in immune complexes. Phosphorylation of HDAC5 was not observed with the catalytically inactive mutant PKD K/W. Interestingly, however, PKD K/W bound HDAC5 even in the absence of PMA.
PKD也增加HDAC5和14-3-3之间的相互作用,如哺乳动物双杂交测定所评价,该测定中将HDAC5与GAL4DNA结合域融合,并将14-3-3与VP16转录激活域融合(图5E)。HDAC5中任一信号反应性丝氨酸的突变使HDAC5和14-3-3之间的相互作用显著降低,同时突变两个信号反应性丝氨酸完全解除了HDAC5与14-3-3的结合。PKD also increases the interaction between HDAC5 and 14-3-3, as assessed in a mammalian two-hybrid assay in which HDAC5 is fused to the GAL4 DNA-binding domain and 14-3-3 is fused to the VP16 transcriptional activation domain ( Figure 5E). Mutation of any signaling-responsive serine in HDAC5 significantly reduced the interaction between HDAC5 and 14-3-3, while mutation of both signaling-responsive serines completely abolished the binding of HDAC5 and 14-3-3.
PKD是心脏HDAC5激酶。本发明者下一步检查了在心肌细胞中PKD是否可以用作HDAC激酶。用编码Flag-HDAC5的腺病毒感染细胞,用PMA处理细胞。用从PMA处理的细胞中免疫沉淀的FLAG-HDAC5进行体外激酶测定,在该测定中观察到HDAC5磷酸化增加(图6A)。加入PMA前,用BisI孵育细胞阻断HDAC5的磷酸化。然而当G6976阻断HDAC5的磷酸化时,将Bis I直接加入激酶反应没有效果。这些结果说明,在心肌细胞中PKD能够结合HDAC5,和Bis I阻断PMA诱导的激酶激活,而G6976能够直接抑制与HDAC5结合的PKD。PKD is the cardiac HDAC5 kinase. The inventors next examined whether PKD could act as an HDAC kinase in cardiomyocytes. Cells were infected with adenovirus encoding Flag-HDAC5 and treated with PMA. Increased phosphorylation of HDAC5 was observed in an in vitro kinase assay using FLAG-HDAC5 immunoprecipitated from PMA-treated cells (Fig. 6A). Phosphorylation of HDAC5 was blocked by incubating cells with BisI prior to the addition of PMA. However, adding Bis I directly to the kinase reaction had no effect when G6976 blocked the phosphorylation of HDAC5. These results suggest that PKD can bind HDAC5 in cardiomyocytes, and that Bis I blocks PMA-induced kinase activation, whereas G6976 can directly inhibit PKD bound to HDAC5.
通过连续的免疫沉淀和免疫印迹进一步证明心肌细胞中PKD与HDAC5相互作用的能力。用编码GFP-HDAC5的腺病毒感染NRVM,在不存在或存在Bis.I的情况下用PE处理NRVM。如图6A所示,内源性PKD有效地与HDAC5免疫共沉淀。在不存在激动剂的情况下,PKD与HDAC5结合,PE处理后以PKC依赖方式活化。该结果进一步说明PKD是心脏II类HDAC激酶,并支持图8中提出的模型。The ability of PKD to interact with HDAC5 in cardiomyocytes was further demonstrated by serial immunoprecipitation and immunoblotting. NRVMs were infected with adenovirus encoding GFP-HDAC5 and treated with PE in the absence or presence of Bis.I. As shown in Figure 6A, endogenous PKD was efficiently co-immunoprecipitated with HDAC5. In the absence of agonists, PKD binds HDAC5, which is activated in a PKC-dependent manner after PE treatment. This result further clarifies that PKD is a cardiac class II HDAC kinase and supports the model proposed in Figure 8.
根据本公开,不需过多的实验就可制成和实施本文中公开和要求权利的所有组合物和方法。虽然按照优选实施方式描述了本发明的组合物和方法,但本领域技术人员将明白,可以对这些组合物和方法,以及本文所述方法的步骤或步骤顺序加以改变,而不背离本发明的概念、精神和范围。更具体说,应明白,用某些化学和生理学相关试剂替代本文中描述的试剂也会获得相同或相似的结果。对本领域技术人员来说明显的所有这些类似替代物和修改都被认为是在如所附权利要求所限定的本发明的精神、范围和概念之内。All of the compositions and methods disclosed and claimed herein can be made and practiced without undue experimentation in light of the present disclosure. Although the compositions and methods of the present invention have been described in terms of preferred embodiments, those skilled in the art will appreciate that changes may be made to the compositions and methods, as well as to the steps or the order of the steps of the methods described herein, without departing from the principles of the present invention. Concept, spirit and scope. More specifically, it will be appreciated that substitution of certain chemically and physiologically related reagents for the reagents described herein will yield the same or similar results. All such similar substitutes and modifications apparent to those skilled in the art are deemed to be within the spirit, scope and concept of the invention as defined by the appended claims.
XII.参考文献XII. References
下面的参考文献,它们提供示例性程序和补充本文所述内容的其它细节,特别引入本文作为参考。The following references, which provide exemplary procedures and other details supplementing those described herein, are expressly incorporated herein by reference.
美国申请2002/103192US application 2002/103192
美国申请2002/65282US application 2002/65282
美国申请2002/61860US application 2002/61860
美国专利4,196,265US Patent 4,196,265
美国专利4,415,723US Patent 4,415,723
美国专利4,458,066US Patent 4,458,066
美国专利4,873,191US Patent 4,873,191
美国专利5,354,855US Patent 5,354,855
美国专利5,604,251US Patent 5,604,251
美国专利5,795,715US Patent 5,795,715
美国专利5,889,136US Patent 5,889,136
美国专利5,955,501US Patent 5,955,501
美国专利6,043,270US Patent 6,043,270
美国专利6,080,784US Patent 6,080,784
美国专利6,441,020US Patent 6,441,020
美国专利6,528,294US Patent 6,528,294
Angel等,Mol.Cell.Biol.,7:2256,1987。Angel et al., Mol. Cell. Biol., 7:2256,1987.
Angel等,Mol.Cell.Biol.,7:2256,1987a。Angel et al., Mol. Cell. Biol., 7:2256, 1987a.
Angel等,Cell,49:729,1987b。Angel et al., Cell, 49:729, 1987b.
Antos,等,2003.J.Biol.Chem.,278:28930-28937,2003。Antos, et al., 2003. J. Biol. Chem., 278:28930-28937, 2003.
Atchison和Perry,Cell,46:253,1986。Atchison and Perry, Cell, 46:253, 1986.
Atchison和Perry,Cell,48:121,1987。Atchison and Perry, Cell, 48:121, 1987.
AU 6,794,700AU6,794,700
AU 9,013,101AU 9,013,101
AU 9,013,201AU 9,013,201
AU 9,013,401AU 9,013,401
Baichwal和Sugden,基因转移(Gene Transfer),Kucherlapati(编),NY,PlenumPress,117-148,1986。Baichwal and Sugden, Gene Transfer, Kucherlapati (eds.), NY, Plenum Press, 117-148, 1986.
Banerji等,Cell,27(2Pt 1):299-308,1981。Banerji et al., Cell, 27(2Pt 1): 299-308, 1981.
Banerji等,Cell,33(3):729-740,1983。Banerji et al., Cell, 33(3):729-740,1983.
Barnes等,J.Biol.Chem.,272(17):11510-7,1997。Barnes et al., J. Biol. Chem., 272(17):11510-7, 1997.
Benvenisty和Neshif,Proc.Natl.Acad.Sci.USA,83:9551-9555,1986。Benvenisty and Neshif, Proc. Natl. Acad. Sci. USA, 83:9551-9555, 1986.
Berkhout等,Cell,59:273-282,1989。Berkhout et al., Cell, 59:273-282, 1989.
Bhavsar等,Genomics,35(1):11-23,1996。Bhavsar et al., Genomics, 35(1):11-23, 1996.
Blanar等,EMBO J.,8:1139,1989。Blanar et al., EMBO J., 8:1139,1989.
Bodine和Ley,EMBO J.,6:2997,1987。Bodine and Ley, EMBO J., 6:2997, 1987.
Boshart等,Cell,41:521,1985。Boshart et al., Cell, 41:521,1985.
Bosher和Labouesse,Nat.Cell.Biol.,2:E31-E36,2000。Bosher and Labouesse, Nat. Cell. Biol., 2: E31-E36, 2000.
Bosze等,EMBO J.,5(7):1615-1623,1986。Bosze et al., EMBO J., 5(7):1615-1623, 1986.
Braddock等,Cell,58:269,1989。Braddock et al., Cell, 58:269,1989.
Brinster等,Proc.Natl.Acad Sci.USA,82(13):4438-4442,1985。Brinster et al., Proc. Natl. Acad Sci. USA, 82(13):4438-4442,1985.
Bristow,Cardiology,92:3-6,1999。Bristow, Cardiology, 92:3-6, 1999.
Bulla和Siddiqui,J.Virol.,62:1437,1986。Bulla and Siddiqui, J. Virol., 62:1437, 1986.
Bush等,Proc.Natl.Acad.Sci.U.S.A.,101:2870-2875,2004。Bush et al., Proc. Natl. Acad. Sci. U.S.A., 101:2870-2875, 2004.
Butler等,Cancer Res.,60:5165-5170,2000。Butler et al., Cancer Res., 60:5165-5170, 2000.
Butler等,Clin.Cancer Res.,7:962-970,2001。Butler et al., Clin. Cancer Res., 7:962-970, 2001.
Campbell和Villarreal,Mol.Cell.Biol.,8:1993,1988。Campbell and Villarreal, Mol. Cell. Biol., 8:1993,1988.
Campbell等,J.Mol.Biol.,180:1-19,1984。Campbell et al., J. Mol. Biol., 180:1-19,1984.
Campere和Tilghman,Genes和Dev.,3:537,1989。Campere and Tilghman, Genes and Dev., 3:537, 1989.
Campo等,Nature,303:77,1983。Campo et al., Nature, 303:77,1983.
Capaldi等,Biochem.Biophys.Res.Comm.,76:425-433,1977。Capaldi et al., Biochem. Biophys. Res. Comm., 76:425-433,1977.
Caplen等,Gene,252(1-2):95-105,2000。Caplen et al., Gene, 252(1-2):95-105,2000.
Carbonneau和Tonks,Annu.Rev.Cell.Biol.,8:463-93,1992。Carbonneau and Tonks, Annu. Rev. Cell. Biol., 8:463-93,1992.
Celander和Haseltine,J.Virology,61:269,1987。Celander and Haseltine, J. Virology, 61:269, 1987.
Celander等,J.Virology,62:1314,1988。Celander et al., J. Virology, 62:1314,1988.
Chandler等,Cell,33:489,1983。Chandler et al., Cell, 33:489,1983.
Chang等,Hepatology,14:124A,1991。Chang et al., Hepatology, 14:124A, 1991.
Chang等,Mol.Cell.Biol.,9:2153,1989。Chang et al., Mol. Cell. Biol., 9:2153,1989.
Chatterjee等,Proc.Natl.Acad.Sci.USA,86:9114,1989。Chatterjee et al., Proc. Natl. Acad. Sci. USA, 86:9114,1989.
Chen和Okayama,Mol.Cell Biol.,7:2745-2752,1987。Chen and Okayama, Mol. Cell Biol., 7:2745-2752, 1987.
Choi等,Cell,53:519,1988。Choi et al., Cell, 53:519,1988.
Coffey等,Cancer Res.,61:3591-3594,2001。Coffey et al., Cancer Res., 61:3591-3594, 2001.
Coffin,病毒学(Virology),Fields等(编),Raven Press,NY,1437-1500,1990。Coffin, Virology, Fields et al. (eds.), Raven Press, NY, 1437-1500, 1990.
Cohen等,J.Cell.Pliysiol.,5:75,1987。Cohen et al., J. Cell. Pliysiol., 5:75,1987.
Cook等,Cell,27:487-496,1981。Cook et al., Cell, 27:487-496,1981.
Costa等,Mol.Cell.Biol.,8:81,1988。Costa et al., Mol. Cell. Biol., 8:81,1988.
Couch等,Am.Rev.Resp.Dis.,88:394-403,1963。Couch et al., Am. Rev. Resp. Dis., 88:394-403,1963.
Coupar等,Gene,68:1-10,1988。Coupar et al., Gene, 68:1-10,1988.
Cripe等,EMBO J.,6:3745,1987。Cripe et al., EMBO J., 6:3745,1987.
Culotta和Hamer,Mol.Cell.Biol.,9:1376,1989。Culotta and Hamer, Mol. Cell. Biol., 9:1376,1989.
Dandolo等,J.Virology,47:55-64,1983。Dandolo et al., J. Virology, 47:55-64, 1983.
De Villiers等,Nature,312(5991):242-246,1984。De Villiers et al., Nature, 312(5991): 242-246, 1984.
Deschamps等,Science,230:1174-1177,1985。Deschamps et al., Science, 230:1174-1177, 1985.
Dubensky等,Proc.Natl.Acad.Sci.USA,81:7529-7533,1984。Dubensky et al., Proc. Natl. Acad. Sci. USA, 81:7529-7533,1984.
Dunnmon等,J.Mol.Cell.Cardiol.,22:901-10,1990。Dunnmon et al., J. Mol. Cell. Cardiol., 22:901-10,1990.
Durand等,Ann.Med.,27:311-317,1995。Durand et al., Ann. Med., 27:311-317, 1995.
Edbrooke等,Mol.Cell.Biol.,9:1908,1989。Edbrooke et al., Mol. Cell. Biol., 9:1908,1989.
Edlund等,Science,230:912-916,1985。Edlund et al., Science, 230:912-916, 1985.
Egan和Weinberg,Nature,365:781-783,1993。Egan and Weinberg, Nature, 365:781-783, 1993.
Eichhorn和Bristow,Circulation,94:2285-2296,1996。Eichhorn and Bristow, Circulation, 94:2285-2296, 1996.
Elbashir等,Nature,411(6836):494-498,2001。Elbashir et al., Nature, 411(6836):494-498, 2001.
EP 0273085EP 0273085
EP 1,123,111EP 1,123,111
EP 1,170,008EP 1,170,008
EP 1,173,562EP 1,173,562
EP 1,174,438EP 1,174,438
EP 1,208,086EP 1,208,086
EP 1,233,958EP 1,233,958
Fechheimer等,Proc Natl.Acad.Sci.USA,84:8463-8467,1987。Fechheimer et al., Proc Natl. Acad. Sci. USA, 84:8463-8467,1987.
Feng和Holland,Nature,334:6178,1988。Feng and Holland, Nature, 334:6178, 1988.
Ferkol等,FASEB J.,7:1081-1091,1993。Ferkol et al., FASEB J., 7:1081-1091, 1993.
Firak和Subramanian,Mol.Cell.Biol.,6:3667,1986。Firak and Subramanian, Mol. Cell. Biol., 6:3667, 1986.
Fire等,Nature,391:806-811,1998。Fire et al., Nature, 391:806-811, 1998.
Foecking和Hofstetter,Gene,45(1):101-105,1986。Foecking and Hofstetter, Gene, 45(1):101-105, 1986.
Forster和Symons,Cell,49:211-220,1987。Forster and Symons, Cell, 49:211-220, 1987.
Fraley等,Proc Natl.Acad.Sci.USA,76:3348-3352,1979。Fraley et al., Proc Natl. Acad. Sci. USA, 76:3348-3352,1979.
Franz等,Cardoscience,5(4):235-43,1994。Franz et al., Cardoscience, 5(4):235-43,1994.
Friedmann,Science,244:1275-1281,1989。Friedmann, Science, 244:1275-1281, 1989.
Fujita等,Cell,49:357,1987。Fujita et al., Cell, 49:357,1987.
Furumai等,Cancer Res.,62:4916-21,2002。Furumai et al., Cancer Res., 62:4916-21, 2002.
Gao等,J.Biol.Chem.,277:25748-55,2002。Gao et al., J. Biol. Chem., 277:25748-55, 2002.
Gao,等,J.Biol Chem.,15:8675-81,1996。Gao, et al., J. Biol Chem., 15:8675-81, 1996.
Gefter,等,Somatic Cell Genet.,3:231-236,1977。Gefter, et al., Somatic Cell Genet., 3:231-236, 1977.
Gerlach等,Nature(London),328:802-805,1987。Gerlach et al., Nature (London), 328:802-805,1987.
Ghosh和Bachhawat,肝脏疾病、使用特异性受体和配体的靶向诊断和治疗(Liver Disease,targeted diagnosis and Therapy Using Specific Receptors andLigands),Wu等(编),Marcel Dekker,NY,87-104,1991。Ghosh and Bachhawat, Liver Disease, targeted diagnosis and Therapy Using Specific Receptors and Ligands, Wu et al. (eds.), Marcel Dekker, NY, 87-104, 1991.
Ghosh-Choudhury等,EMBO J.,6:1733-1739,1987。Ghosh-Choudhury et al., EMBO J., 6:1733-1739, 1987.
Gilles等,Cell,33:717,1983。Gilles et al., Cell, 33:717,1983.
Gloss等,EMBO J.,6:3735,1987。Gloss et al., EMBO J., 6:3735,1987.
Godbout等,Mol.Cell.Biol.,8:1169,1988。Godbout et al., Mol. Cell. Biol., 8:1169,1988.
Goding,单克隆抗体:原理和实践(Monoclonal Antibodies:Principles andPractice),第二版,Orlando,Fla.,Academic Press,60-61,65-66,71-74,1986。Goding, Monoclonal Antibodies: Principles and Practice, Second Edition, Orlando, Fla., Academic Press, 60-61, 65-66, 71-74, 1986.
Gomez-Foix等,J.Biol.Chem.,267:25129-25134,1992。Gomez-Foix et al., J. Biol. Chem., 267:25129-25134,1992.
Goodbourn和Maniatis,Proc.Natl.Acad.Sci.USA,85:1447,1988。Goodbourn and Maniatis, Proc. Natl. Acad. Sci. USA, 85:1447, 1988.
Goodbourn等,Cell,45:601,1986。Goodbourn et al., Cell, 45:601,1986.
Gopal,Mol.Cell Biol.,5:1188-1190,1985。Gopal, Mol. Cell Biol., 5:1188-1190, 1985.
Gopal-Srivastava等,J.Mol.Cell.Biol.,15(12):7081-90,1995。Gopal-Srivastava et al., J. Mol. Cell. Biol., 15(12):7081-90,1995.
Gottlicher等,EMBO J.,20:6969-78,2001。Gottlicher et al., EMBO J., 20:6969-78, 2001.
Graham和Prevec,分子生物学方法:基因转移和表达方法(Methods inMolecular Biology:Gene Transfer and Expression Protocol),Murray(编),Humana Press,Clifton,NJ,7:109-128,1991。Graham and Prevec, Methods in Molecular Biology: Gene Transfer and Expression Protocol, Murray (eds.), Humana Press, Clifton, NJ, 7:109-128, 1991.
Graham和van der Eb,Virology,52:456-467,1973。Graham and van der Eb, Virology, 52:456-467, 1973.
Graham等,J.Gen.Virol.,36:59-72,1977。Graham et al., J. Gen. Virol., 36:59-72, 1977.
Greene等,Immunology Today,10:272,1989Grishok等,Science,287:2494-2497,2000。Greene et al., Immunology Today, 10:272, 1989 Grishok et al., Science, 287:2494-2497, 2000.
Grosschedl和Baltimore,Cell,41:885,1985。Grosschedl and Baltimore, Cell, 41:885, 1985.
Grozinger和Schreiber,Proc.Nat’l.Acad.Sci.U.S.A.,97:7835-40,2000。Grozinger and Schreiber, Proc. Nat'l. Acad. Sci. U.S.A., 97:7835-40, 2000.
Grozinger等,Proc.Natl.Acad.Sci.USA,96:4868-4873,1999。Grozinger et al., Proc. Natl. Acad. Sci. USA, 96:4868-4873,1999.
Grunhaus和Horwitz,Seminar in Virology,3:237-252,1992。Grunhaus and Horwitz, Seminar in Virology, 3: 237-252, 1992.
Gschwendt等,FEBSLett.,392:77-80,1996。Gschwendt et al., FEBS Lett., 392:77-80,1996.
Han等,Cancer Research,60:6068-6074,2000。Han et al., Cancer Research, 60:6068-6074, 2000.
Hardie和Hanks(1995)蛋白激酶实际读本(The Protein Kinase Facts Books),第1卷:7-20Academic Press,San Diego,Calif.。Hardie and Hanks (1995) The Protein Kinase Facts Books, Vol. 1: 7-20 Academic Press, San Diego, Calif.
Haribabu等,EMBO J,14:3679-86,1995。Haribabu et al., EMBO J, 14:3679-86, 1995.
Harland和Weintraub,J.Cell Biol.,101:1094-1099,1985。Harland and Weintraub, J. Cell Biol., 101:1094-1099, 1985.
Harlow和Lane,抗体:实验室手册(Antibodies:A Laboratory manual),ColdSpring Harbor Laboratory,1988。Harlow and Lane, Antibodies: A Laboratory manual, Cold Spring Harbor Laboratory, 1988.
Haslinger和Karin,Proc.Natl.Acad.Sci.USA,82:8572,1985。Haslinger and Karin, Proc. Natl. Acad. Sci. USA, 82:8572, 1985.
Hauber和Cullen,J.Virology,62:673,1988。Hauber and Cullen, J. Virology, 62:673, 1988.
Haworth等,J.Mol.Cardiol.,32:1013-1023,2000。Haworth et al., J. Mol. Cardiol., 32:1013-1023, 2000.
Hayashi等,Biochim.Biophys.Acta,1450:99-106,1999。Hayashi et al., Biochim. Biophys. Acta, 1450:99-106, 1999.
Hen等,Nature,321:249,1986。Hen et al., Nature, 321:249,1986.
Hensel等,Lymphokine Res.,8:347,1989。Hensel et al., Lymphokine Res., 8:347, 1989.
Hermonat和Muzycska,Proc.Nat’Z Acad.Sci.USA,81:6466-6470,1984。Hermonat and Muzycska, Proc. Nat'Z Acad. Sci. USA, 81:6466-6470, 1984.
Herr和Clarke,Cell,45:461,1986。Herr and Clarke, Cell, 45:461, 1986.
Hersdorffer等,DNA Cell Biol.,9:713-723,1990。Hersdorffer et al., DNA Cell Biol., 9:713-723,1990.
Herz和Gerard,Proc.Nat’l.Acad.Sci.USA 90:2812-2816,1993。Herz and Gerard, Proc. Nat'l. Acad. Sci. USA 90:2812-2816, 1993.
Hinnebusch等,J.Nutr.,132:1012-7,2002。Hinnebusch et al., J. Nutr., 132:1012-7, 2002.
Hirochika等,J.Tirol.,61:2599,1987。Hirochika et al., J. Tirol., 61:2599,1987.
Hirsch等,Mol.Cell.Biol.,10:1959,1990。Hirsch et al., Mol. Cell. Biol., 10:1959,1990.
Hoffmann等,Bioconjugate Chem.,12:51-55,2001。Hoffmann et al., Bioconjugate Chem., 12:51-55, 2001.
Holbrook等,Virology,157:211,1987。Holbrook et al., Virology, 157:211,1987.
Horlick和Benfield,Mol.Cell.Biol.,9:2396,1989。Horlick and Benfield, Mol. Cell. Biol., 9:2396,1989.
Horwich等,J.Virol.,64:642-650,1990。Horwich et al., J. Virol., 64:642-650, 1990.
Huang等,Cell,27:245,1981。Huang et al., Cell, 27:245, 1981.
Hug等,Mol.Cell.Biol.,8:3065,1988。Hug et al., Mol. Cell. Biol., 8:3065,1988.
Hwang等,Mol.Cell.Biol.,10:585,1990。Hwang et al., Mol. Cell. Biol., 10:585,1990.
Imagawa等,Cell,51:251,1987。Imagawa et al., Cell, 51:251,1987.
Imbra和Karin,Nature,323:555,1986。Imbra and Karin, Nature, 323:555, 1986.
Imler等,Mol.Cell.Biol.,7:2558,1987。Imler et al., Mol. Cell. Biol., 7:2558,1987.
Imperial和Nevins,Mol.Cell.Biol.,4:875,1984。Imperial and Nevins, Mol. Cell. Biol., 4:875,1984.
Isselbacher等,Harrison氏内科学原理(Harrison’s Principles of InternalMedicine),McGraw-Hill,NY,416-431,1994。Isselbacher et al., Harrison's Principles of Internal Medicine, McGraw-Hill, NY, 416-431, 1994.
Jakobovits等,Mol.Cell.Biol.,8:2555,1988。Jakobovits et al., Mol. Cell. Biol., 8:2555,1988.
Jameel和Siddiqui,Mol.Cell.Biol.,6:710,1986。Jameel and Siddiqui, Mol. Cell. Biol., 6:710, 1986.
Japanese App.No.2001/348340。Japanese App. No. 2001/348340.
Jaynes等,Mol.Cell.Biol.,8:62,1988。Jaynes et al., Mol. Cell. Biol., 8:62,1988.
Johannes等,J.Biol.Chem.,269(8):6140-8,1994。Johannes et al., J. Biol. Chem., 269(8):6140-8, 1994.
Johnson等,Mol.Cell.Biol.,9:3393,1989。Johnson et al., Mol. Cell. Biol., 9:3393,1989.
Jones和Shenk,Cell,13:181-188,1978。Jones and Shenk, Cell, 13:181-188, 1978.
Joyce,Nature,338:217-244,1989。Joyce, Nature, 338:217-244, 1989.
Jung等,J.Med.Chem.,42:4669-4679,1999。Jung et al., J. Med. Chem., 42:4669-4679, 1999.
Jung等,Med.Chem.Lett.,7:1655-1658,1997。Jung et al., Med. Chem. Lett., 7:1655-1658, 1997.
Jung,Curr.Med.Chem.,8:1505-11,2001。Jung, Curr. Med. Chem., 8:1505-11, 2001.
Kadesch和Berg,Mol.Cell.Biol.,6:2593,1986。Kadesch and Berg, Mol. Cell. Biol., 6:2593,1986.
Kaneda等,Science,243:375-378,1989。Kaneda et al., Science, 243:375-378, 1989.
Kao等,Genes Dev.,14:55-66,2000。Kao et al., Genes Dev., 14:55-66, 2000.
Karin等,Mol.Cell.Biol.,7:606,1987。Karin et al., Mol. Cell. Biol., 7:606,1987.
Karin等,Mol.CelL Biol.,7:606,1987。Karin et al., Mol. Cell Biol., 7:606,1987.
Karlsson等,EMBO J.,5:2377-2385,1986。Karlsson et al., EMBO J., 5:2377-2385, 1986.
Katinka等,Cell,20:393,1980。Katinka et al., Cell, 20:393,1980.
Kato等,J.Biol Chem.,266(6):3361-3364,1991。Kato et al., J. Biol Chem., 266(6):3361-3364, 1991.
Katoh等,J.Biol.Chem.,273:1511-18,1998。Katoh et al., J. Biol. Chem., 273:1511-18, 1998.
Kawamoto等,Mol.Cell.Biol.,8:267,1988。Kawamoto et al., Mol. Cell. Biol., 8:267,1988.
Kelly等,J.Cell Biol.,129(2):383-96,1995。Kelly et al., J. Cell Biol., 129(2):383-96, 1995.
Ketting等,Cell,99:133-141,1999。Ketting et al., Cell, 99: 133-141, 1999.
Kiledjian等,Mol.Cell.Biol.,8:145,1988。Kiledjian et al., Mol. Cell. Biol., 8:145,1988.
Kim和Cook,Proc.Natl.Acad.Sci.USA,84:8788-8792,1987。Kim and Cook, Proc. Natl. Acad. Sci. USA, 84:8788-8792, 1987.
Kim等,J.Am.Chem.Soc.,121:2056,1999。Kim et al., J. Am. Chem. Soc., 121:2056, 1999.
Kimura等,Dev.Growth Differ.,39(3):257-65,1997。Kimura et al., Dev. Growth Differ., 39(3):257-65, 1997.
Kitazomo等,J.Clinical Endocr.Metabol.,86(7):3430-3435,2001。Kitazomo et al., J. Clinical Endocr. Metabol., 86(7):3430-3435, 2001.
Klamut等,Mol.Cell.Biol.,10:193,1990。Klamut et al., Mol. Cell. Biol., 10:193,1990.
Klassen,治疗的药理学基础(The Pharmacological Basis of Therapeutics),Goodman和Gilman编,Pergamon Press,第8版,49-61,1990。Klassen, The Pharmacological Basis of Therapeutics, eds. Goodman and Gilman, Pergamon Press, 8th ed., 49-61, 1990.
Klein等,Nature,327:70-73,1987。Klein et al., Nature, 327:70-73, 1987.
Koch等,Mol.Cell.Biol.,9:303,1989。Koch et al., Mol. Cell. Biol., 9:303,1989.
Kohler和Milstein,Eur.J.Immunol.,6:511-519,1976。Kohler and Milstein, Eur. J. Immunol., 6:511-519, 1976.
Kohler和Milstein,Nature,256:495-497,1975。Kohler and Milstein, Nature, 256:495-497, 1975.
Komastsu等,Cancer Res.,61:4459-4466,2001。Komastsu et al., Cancer Res., 61:4459-4466, 2001.
Kramer等,Trends in Etadoc.Metabolism,12)7):294-300,2001。Kramer et al., Trends in Etadoc. Metabolism, 12) 7): 294-300, 2001.
Kriegler和Botchan,真核病毒载体(Eukaryotic Viral Vectors),Gluzman(编),Cold Spring Harbor:Cold Spring Harbor Laboratory,NY,1982。Kriegler and Botchan, Eukaryotic Viral Vectors, Gluzman (eds), Cold Spring Harbor: Cold Spring Harbor Laboratory, NY, 1982.
Kriegler和Botchan,Mol.Cell.Biol.,3:325,1983。Kriegler and Botchan, Mol. Cell. Biol., 3:325,1983.
Kriegler等,Cell,38:483,1984。Kriegler et al., Cell, 38:483,1984.
Kriegler等,Cell,53:45,1988。Kriegler et al., Cell, 53:45,1988.
Kuhl等,Cell,50:1057,1987。Kuhl et al., Cell, 50:1057,1987.
Kunz等,Nucl.Acids Res.,17:1121,1989。Kunz et al., Nucl. Acids Res., 17:1121,1989.
LaPointe等,J.Biol.Chem.,263(19):9075-8,1988。LaPointe et al., J. Biol. Chem., 263(19):9075-8,1988.
Larsen等,Proc.Natl.Acad.Sci.USA.,83:8283,1986。Larsen et al., Proc. Natl. Acad. Sci. USA., 83:8283,1986.
Laspia等,Cell,59:283,1989。Laspia et al., Cell, 59:283,1989.
Latimer等,Mol.Cell.Biol.,10:760,1990。Latimer et al., Mol. Cell. Biol., 10:760,1990.
Le Gal La Salle等,Science,259:988-990,1993。Le Gal La Salle et al., Science, 259:988-990, 1993.
Lee等,Nature,294:228,1981。Lee et al., Nature, 294:228,1981.
Lee等,Nucleic Acids Res.,12:4191-206,1984。Lee et al., Nucleic Acids Res., 12:4191-206, 1984.
Levinson等,Nature,295:79,1982。Levinson et al., Nature, 295:79,1982.
Levrero等,Gene,101:195-202,1991。Levrero et al., Gene, 101:195-202, 1991.
Li等,J.Biol.Chem.,271:19402-8,1996。Li et al., J. Biol. Chem., 271:19402-8, 1996.
Lin和Avery,Nature,402:128-129,1999。Lin and Avery, Nature, 402:128-129, 1999.
Lin等,Mol.Cell.Biol.,10:850,1990。Lin et al., Mol. Cell. Biol., 10:850,1990.
Linnenbach等,Proc Natl.Acad.Sci.USA,85(1):74-8,1988。Linnenbach et al., Proc Natl. Acad. Sci. USA, 85(1):74-8, 1988.
Lu等,Proc.Natl.Acad.Sci.USA,97:4070-4075,2000。Lu et al., Proc. Natl. Acad. Sci. USA, 97:4070-4075, 2000.
Luria等,EMBO J.,6:3307,1987。Luria et al., EMBO J., 6:3307,1987.
Lusky和Botchan,Proc.Natl.Acad.Sci.USA,83:3609,1986。Lusky and Botchan, Proc. Natl. Acad. Sci. USA, 83:3609, 1986.
Lusky等,Mol.Cell.Biol.,3:1108,1983。Lusky et al., Mol. Cell. Biol., 3:1108,1983.
Macejak和Sarnow,Nature,353:90-94,1991。Macejak and Sarnow, Nature, 353:90-94, 1991.
Mai等,J.Med.Chem.,45:1778-1784,2002。Mai et al., J. Med. Chem., 45:1778-1784, 2002.
Majors和Varmus,Proc.Natl.Acad.Sci.USA,80:5866,1983。Majors and Varmus, Proc. Natl. Acad. Sci. USA, 80:5866, 1983.
Mann等,Cell,33:153-159,1983。Mann et al., Cell, 33:153-159,1983.
Markowitz等,J.Virol.,62:1120-1124,1988。Markowitz et al., J. Virol., 62: 1120-1124, 1988.
Marks等,Clin.Cancer Res.,7:759-760,2001。Marks et al., Clin. Cancer Res., 7:759-760, 2001.
Marks等,J.Natl.Cancer Inst.,92(15):1210-1216,2000。Marks et al., J. Natl. Cancer Inst., 92(15):1210-1216, 2000.
Massa等,J.Med.Chem.,44:2069-2072,2001。Massa et al., J. Med. Chem., 44:2069-2072, 2001.
McKinsey等,Nature,408:106-111,2000b。McKinsey et al., Nature, 408:106-111, 2000b.
McKinsey等,Proc.Natl.Acad.Sci.USA,97:14400-14405,2000a。McKinsey et al., Proc. Natl. Acad. Sci. USA, 97:14400-14405, 2000a.
McKinsey等,Trends Biochem.Sci.,27:40-47,2002。McKinsey et al., Trends Biochem. Sci., 27:40-47, 2002.
McNeall等,Gene,76:81,1989。McNeall et al., Gene, 76:81,1989.
Michel和Westhof,J.Mol.Biol.,216:585-610,1990。Michel and Westhof, J. Mol. Biol., 216:585-610, 1990.
Miksicek等,Cell,46:203,1986。Miksicek et al., Cell, 46:203,1986.
Montgomery等,Proc.Natl.Acad.Sci.USA,95:155-2-15507,1998。Montgomery et al., Proc. Natl. Acad. Sci. USA, 95:155-2-15507,1998.
Mordacq和Linzer,Genes and Dev.,3:760,1989。Mordacq and Linzer, Genes and Dev., 3:760, 1989.
Moreau等,Nucl.Acids Res.,9:6047,1981。Moreau et al., Nucl. Acids Res., 9:6047,1981.
Moss等,J.Gen.Physiol.,108(6):473-84,1996。Moss et al., J. Gen. Physiol., 108(6):473-84,1996.
Muesing等,Cell,48:691,1987。Muesing et al., Cell, 48:691,1987.
Ng等,Nuc.Acids Res.,17:601,1989。Ng et al., Nuc. Acids Res., 17:601,1989.
Nicolas和Rubinstein,载体:分子克隆载体及其用途概览(Vectors:A surveyof molecular cloning vectors and their uses),Rodriguez和Denhardt(编),Stoneham:Butterworth,494-513,1988。Nicolas and Rubinstein, Vectors: A survey of molecular cloning vectors and their uses (Vectors: A survey of molecular cloning vectors and their uses), Rodriguez and Denhardt (eds), Stoneham: Butterworth, 494-513, 1988.
Nicolau和Sene,Biochim.Biophys.Acta,721:185-190,1982。Nicolau and Sene, Biochim. Biophys. Acta, 721: 185-190, 1982.
Nicolau等,Methods Enzymol.,149:157-176,1987。Nicolau et al., Methods Enzymol., 149:157-176, 1987.
Nishikawa等,J.Biol.Chem.,276:3310-3318,1997。Nishikawa et al., J. Biol. Chem., 276:3310-3318, 1997.
Ondek等,EMBO J.,6:1017,1987。Ondek et al., EMBO J., 6:1017, 1987.
Ornitz等,Mol.Cell.Biol.,7:3466,1987。Ornitz et al., Mol. Cell. Biol., 7:3466,1987.
Palmiter等,Cell,29:701,1982。Palmiter et al., Cell, 29:701,1982.
Palmiter等,Nature,300:611,1982。Palmiter et al., Nature, 300:611,1982.
Paskind等,Virology,67:242-248,1975。Paskind et al., Virology, 67:242-248, 1975.
Pech等,Mol.Cell.Biol.,9:396,1989。Pech et al., Mol. Cell. Biol., 9:396,1989.
Pelletier和Sonenberg,Nature,334:320-325,1988。Pelletier and Sonenberg, Nature, 334:320-325, 1988.
Perales等,Proc.Natl.Acad.Sci.USA,91(9):4086-4090,1994。Perales et al., Proc. Natl. Acad. Sci. USA, 91(9):4086-4090,1994.
Perez-Stable和Constantini,Mol.Cell.Biol.,10:1116,1990。Perez-Stable and Constantini, Mol. Cell. Biol., 10:1116,1990.
医生桌面参考书(Physicians Desk Reference)。Physicians Desk Reference.
Picard和Schaffner,Nature,307:83,1984。Picard and Schaffner, Nature, 307:83, 1984.
Pinkert等,Genes和Dev.,1:268,1987。Pinkert et al., Genes and Dev., 1:268,1987.
Ponta等,Proc.Natl.Acad.Sci.USA,82:1020,1985。Ponta et al., Proc. Natl. Acad. Sci. USA, 82:1020,1985.
Porton等,Mol.Cell.Biol.,10:1076,1990。Porton et al., Mol. Cell. Biol., 10:1076,1990.
Potter等,Proc.Natl.Acad.Sci.USA,81:7161-7165,1984。Potter et al., Proc. Natl. Acad. Sci. USA, 81:7161-7165,1984.
Queen和Baltimore,Cell,35:741,1983。Queen and Baltimore, Cell, 35:741, 1983.
Quinn等,Mol.Cell.Biol.,9:4713,1989。Quinn et al., Mol. Cell. Biol., 9:4713,1989.
Racher等,Biotech.Techniques,9:169-174,1995。Racher et al., Biotech. Techniques, 9:169-174,1995.
Ragot等,Nature,361:647-650,1993。Ragot et al., Nature, 361:647-650,1993.
Redondo等,Science,247:1225,1990。Redondo et al., Science, 247:1225,1990.
Reinhold-Hurek和Shub,Nature,357:173-176,1992。Reinhold-Hurek and Shub, Nature, 357:173-176, 1992.
Reisman和Rotter,Mol.Cell.Biol.,9:3571,1989。Reisman and Rotter, Mol. Cell. Biol., 9:3571,1989.
雷明顿药物科学(Remington’s Pharmaceutical Sciences),第15版,1035-1038和1570-1580,Mack Publishing Company,PA,1980。Remington's Pharmaceutical Sciences, 15th Edition, 1035-1038 and 1570-1580, Mack Publishing Company, PA, 1980.
Renan,Radiother.Oncol.,19:197-218,1990。Renan, Radiother. Oncol., 19:197-218, 1990.
Resendez Jr.等,Mol.Cell.Biol.,8:4579,1988。Resendez Jr. et al., Mol. Cell. Biol., 8:4579,1988.
Rich等,Hum.Gene Ther.,4:461-476,1993。Rich et al., Hum. Gene Ther., 4:461-476, 1993.
Ridgeway,载体:分子克隆载体及其用途概览(Vectors:A survey of molecularcloning vectors and their uses),Stoneham:Butterworth,467-492,1988。Ridgeway, Vectors: A survey of molecular cloning vectors and their uses (Vectors: A survey of molecular cloning vectors and their uses), Stoneham: Butterworth, 467-492, 1988.
Ripe等,Mol.Cell.Biol.,9:2224,1989。Ripe et al., Mol. Cell. Biol., 9:2224,1989.
Rippe等,Mol.Cell Biol.,10:689-695,1990。Rippe et al., Mol. Cell Biol., 10:689-695,1990.
Rittling等,Nuc.Acids Res.,17:1619,1989。Rittling et al., Nuc. Acids Res., 17:1619,1989.
Rosen等,Cell,41:813,1988。Rosen et al., Cell, 41:813,1988.
Rosenfeld等,Cell,68:143-155,1992。Rosenfeld et al., Cell, 68:143-155, 1992.
Rosenfeld等,Science,252:431-434,1991。Rosenfeld et al., Science, 252:431-434, 1991.
Roux等,Proc.Natl.Acad.Sci.USA,86:9079-9083,1989。Roux et al., Proc. Natl. Acad. Sci. USA, 86:9079-9083,1989.
Sakai等,Genes and Dev.,2:1144,1988。Sakai et al., Genes and Dev., 2:1144, 1988.
Sambrook等,分子克隆:实验室手册(Molecular Cloning:A LaboratoryManual),第2版,Cold Spring Harbor Press,Cold Spring Harbor,NY,2001。Sambrook et al., Molecular Cloning: A Laboratory Manual, 2nd ed., Cold Spring Harbor Press, Cold Spring Harbor, NY, 2001.
Sarver等,Science,247:1222-1225,1990。Sarver et al., Science, 247:1222-1225, 1990.
Satake等,J.Virology,62:970,1988。Satake et al., J. Virology, 62:970,1988.
Saunders等,Cancer Res.,59-399-409,1999。Saunders et al., Cancer Res., 59-399-409, 1999.
Scanlon等,Proc.Natl.Acad.Sci.USA,88:10591-10595,1991。Scanlon et al., Proc. Natl. Acad. Sci. USA, 88:10591-10595,1991.
Schaffner等,J.Mol.Biol.,201:81,1988。Schaffner et al., J. Mol. Biol., 201:81,1988.
Searle等,Mol.Cell.Biol.,5:1480,1985。Searle et al., Mol. Cell. Biol., 5:1480,1985.
Sharp和Marciniak,Cell,59:229,1989。Sharp and Marciniak, Cell, 59:229, 1989.
Sharp和Zamore,Science,287:2431-2433,2000。Sharp and Zamore, Science, 287:2431-2433, 2000.
Sharp,Genes Dev.,13:139-141,1999。Sharp, Genes Dev., 13:139-141, 1999.
Shaul和Ben-Levy,EMBO J.,6:1913,1987。Shaul and Ben-Levy, EMBO J., 6:1913, 1987.
Sherman等,Mol.Cell.Biol.,9:50,1989。Sherman et al., Mol. Cell. Biol., 9:50,1989.
Sleigh和Lockett,J.EMBO,4:3831,1985。Sleigh and Lockett, J. EMBO, 4:3831, 1985.
Spalholz等,Cell,42:183,1985。Spalholz et al., Cell, 42:183,1985.
Spandau和Lee,J.Virology,62:427,1988。Spandau and Lee, J. Virology, 62:427, 1988.
Spandidos和Wilkie,EMBO J,2:1193,1983。Spandidos and Wilkie, EMBO J, 2:1193, 1983.
Stephens和Hentschel,Biochem.J,248:1,1987。Stephens and Hentschel, Biochem. J, 248:1, 1987.
Storz和Toker,Embo J.,22:109-20,2003。Storz and Toker, Embo J., 22:109-20, 2003.
Stratford-Perricaudet和Perricaudet,人类基因转移(Human GeneTransfer),Cohen-Haguenauer和Boiron(编),John Libbey Eurotext,法国,51-61,1991。Stratford-Perricaudet and Perricaudet, Human Gene Transfer, Cohen-Haguenauer and Boiron (eds), John Libbey Eurotext, France, 51-61, 1991.
Stratford-Perricaudet等,Hum.Gene.Ther.,1:241-256,1990。Stratford-Perricaudet et al., Hum. Gene. Ther., 1:241-256,1990.
Stuart等,Nature,317:828,1985。Stuart et al., Nature, 317:828,1985.
Sturany等,J.Biol.Chem.,276:3310-3318,2001。Sturany et al., J. Biol. Chem., 276:3310-3318, 2001.
Su等,Cancer Res.,60:3137-3142,2000。Su et al., Cancer Res., 60:3137-3142, 2000.
Sullivan和Peterlin,Mol.Cell.Biol.,7:3315,1987。Sullivan and Peterlin, Mol. Cell. Biol., 7:3315,1987.
Swartzendruber和Lehman,J.Cell.Physiology,85:179,1975。Swartzendruber and Lehman, J. Cell. Physiology, 85:179, 1975.
Tabara等,Cell,99:123-132,1999。Tabara et al., Cell, 99:123-132, 1999.
Takahashi等,Antibiotics,49:453,1996。Takahashi et al., Antibiotics, 49:453,1996.
Takebe等,Mol.Cell.Biol.,8:466,1988。Takebe et al., Mol. Cell. Biol., 8:466,1988.
Taunton等,Science,272:371,1996。Taunton et al., Science, 272:371, 1996.
Tavernier等,Nature,301:634,1983。Tavernier et al., Nature, 301:634,1983.
Taylor和Kingston,Mol.Cell.Biol.,10:165,1990a。Taylor and Kingston, Mol. Cell. Biol., 10:165, 1990a.
Taylor和Kingston,Mol.Cell.Biol.,10:176,1990b。Taylor and Kingston, Mol. Cell. Biol., 10:176, 1990b.
Taylor等,J BioL Chein.,264:15160,1989。Taylor et al., J BioL Chein., 264:15160, 1989.
Temin,基因转移(Gene Transfer),Kucherlapati(编),NY,Plenum Press,149-188,1986。Temin, Gene Transfer, Kucherlapati (ed.), NY, Plenum Press, 149-188, 1986.
莫克指数(The Merck Index),第11版。The Merck Index, 11th Edition.
Thiesen等,J.Virology,62:614,1988。Thiesen et al., J. Virology, 62:614,1988.
Tong等,Nucleic Acids Res.,30:1114-23,2002。Tong et al., Nucleic Acids Res., 30:1114-23, 2002.
Top等,J.Infact.Dis.,124:155-160,1971。Top et al., J. Infact. Dis., 124:155-160, 1971.
Treisman,Cell,42:889,1985。Treisman, Cell, 42:889, 1985.
Tronche等,Mol.Biol.Med.,7:173,1990。Tronche et al., Mol. Biol. Med., 7:173,1990.
Trudel和Constantini,Genes and Dev.6:954,1987。Trudel and Constantini, Genes and Dev. 6:954, 1987.
Tur-Kaspa等,Mol.Cell Biol.,6:716-718,1986。Tur-Kaspa et al., Mol. Cell Biol., 6:716-718,1986.
Tyndell等,Nuc.Acids.Res.,9:6231,1981。Tyndell et al., Nuc. Acids. Res., 9:6231,1981.
Valverde等,Proc.Natl.Acad.Sci.USA,91(18):8572-6,1994。Valverde et al., Proc. Natl. Acad. Sci. USA, 91(18):8572-6, 1994.
Van den Wyngaert等,FEBS Lett.,478:77-83,2000。Van den Wyngaert et al., FEBS Lett., 478:77-83, 2000.
Van Lint等,Trends in Cell.Biol.,12:193-200,2002。Van Lint et al., Trends in Cell. Biol., 12:193-200, 2002.
Vannice和Levinson,J.Virology,62:1305,1988。Vannice and Levinson, J. Virology, 62:1305, 1988.
Varmus等,Cell,25:23-36,1981。Varmus et al., Cell, 25:23-36,1981.
Vasseur等,Proc Natl.Acad.Sci.U.S.A.,77:1068,1980。Vasseur et al., Proc Natl. Acad. Sci. U.S.A., 77:1068,1980.
Vigushin等,Anticancer Drugs,13:1-13,2002。Vigushin et al., Anticancer Drugs, 13:1-13, 2002.
Vigushin等,Cancer Res.,5(Suppl),1999。Vigushin et al., Cancer Res., 5 (Suppl), 1999.
Wagner等,Proc.Natl.Acad.Sci.USA,87(9):3410-3414,1990。Wagner et al., Proc. Natl. Acad. Sci. USA, 87(9):3410-3414,1990.
Wang和Calame,Cell,47:241,1986。Wang and Calame, Cell, 47:241, 1986.
Weber等,Cell,36:983,1984。Weber et al., Cell, 36:983,1984.
Weinberger等Mol.Cell.Biol.,8:988,1984。Weinberger et al. Mol. Cell. Biol., 8:988,1984.
Wincott等,Nucleic Acids Res.,23(14):2677-2684,1995。Wincott et al., Nucleic Acids Res., 23(14):2677-2684, 1995.
Winoto和Baltimore,Cell,59:649,1989。Winoto and Baltimore, Cell, 59:649, 1989.
WO 00/44914WO 00/44914
WO 01/14581WO 01/14581
WO 01/18045WO 01/18045
WO 01/36646WO 01/36646
WO 01/38322WO 01/38322
WO 01/42437WO 01/42437
WO 01/68836WO 01/68836
WO 01/70675WO 01/70675
WO 02/26696WO 02/26696
WO 02/26703WO 02/26703
WO 02/30879WO 02/30879
WO 02/46129WO 02/46129
WO 02/46144WO 02/46144
WO 02/50285WO 02/50285
WO 02/51842WO 02/51842
WO 84/03564WO 84/03564
WO 98/33791WO 98/33791
WO 99/32619WO 99/32619
Wong等,Gene,10:87-94,1980。Wong et al., Gene, 10:87-94,1980.
Workman和Kingston,Annu.Rev.Biochem.,67:545-579,1998。Workman and Kingston, Annu. Rev. Biochem., 67:545-579, 1998.
Wu和Wu,Adv.Drug Delivery Rev.,12:159-167,1993。Wu and Wu, Adv. Drug Delivery Rev., 12:159-167, 1993.
Wu和Wu,Biochemistry,27:887-892,1988。Wu and Wu, Biochemistry, 27:887-892, 1988.
Wu和Wu,J.Biol.Chem.,262:4429-4432,1987。Wu and Wu, J. Biol. Chem., 262:4429-4432, 1987.
Yamano等,Amer.Soc.Gene Ther.,2000。Yamano et al., Amer. Soc. Gene Ther., 2000.
Yamauchi-Takihara等,Proc.Natl.Acad.Sci.USA,86(10):3504-3508,1989。Yamauchi-Takihara et al., Proc. Natl. Acad. Sci. USA, 86(10):3504-3508,1989.
Yang等,Proc.Natl.Acad.Sci.USA,87:9568-9572,1990。Yang et al., Proc. Natl. Acad. Sci. USA, 87:9568-9572,1990.
Young等,应用疗法手册(Handbook of Applied Therapeutics),7.1-7.12和9.1-9.10,1989。Young et al., Handbook of Applied Therapeutics, 7.1-7.12 and 9.1-9.10, 1989.
Yutzey等Mol.Cell.Biol.,9:1397,1989。Yutzey et al. Mol. Cell. Biol., 9:1397,1989.
Zelenin等,FEBSLett.,280:94-96,1991。Zelenin et al., FEBS Lett., 280:94-96,1991.
Zhang等,Cell,110:479-488,2002。Zhang et al., Cell, 110:479-488, 2002.
Zhou等,Proc.Natl.Acad.Sci.USA,98:10572-10577,2001。Zhou et al., Proc. Natl. Acad. Sci. USA, 98:10572-10577, 2001.
Ziober和Kramer,J.Bio.Clzem.,271(37):22915-22922,1996。Ziober and Kramer, J. Bio. Clzem., 271(37): 22915-22922, 1996.
Zugaza等,Embo J.,15:6220-30,1996。Zugaza et al., Embo J., 15:6220-30, 1996.
登录号NM002742。Accession number NM002742.
Claims (99)
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US47229803P | 2003-05-21 | 2003-05-21 | |
| US60/472,298 | 2003-05-21 |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| CN1812776Atrue CN1812776A (en) | 2006-08-02 |
Family
ID=33539040
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| CNA2004800139983APendingCN1812776A (en) | 2003-05-21 | 2004-05-19 | Inhibition of protein kinase c-mu (pkd) as a treatment for cardiac hypertrophy and heart failure |
Country Status (9)
| Country | Link |
|---|---|
| US (1) | US20050112128A1 (en) |
| EP (1) | EP1631268A2 (en) |
| JP (1) | JP2007505158A (en) |
| CN (1) | CN1812776A (en) |
| AU (1) | AU2004249114A1 (en) |
| BR (1) | BRPI0410787A (en) |
| CA (1) | CA2526423A1 (en) |
| MX (1) | MXPA05012619A (en) |
| WO (1) | WO2004112763A2 (en) |
Cited By (9)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN104558110A (en)* | 2013-10-21 | 2015-04-29 | 中国科学院大连化学物理研究所 | Polypeptide with ACE inhibitory activity and application thereof in antihypertensive drug |
| CN104558114A (en)* | 2013-10-21 | 2015-04-29 | 中国科学院大连化学物理研究所 | Polypeptide with ACE inhibiting activity in pollen as well as application of polypeptide |
| CN104872685A (en)* | 2014-02-27 | 2015-09-02 | 长庚医疗财团法人基隆长庚纪念医院 | Nutritional composition for improving heart failure symptoms |
| CN102046813B (en)* | 2008-06-02 | 2016-02-24 | 牛津生物动力有限公司 | Method for detecting long-range chromosome interactions |
| CN107811997A (en)* | 2017-11-10 | 2018-03-20 | 大连医科大学附属第医院 | Application of the proteasome inhibitor resveratrol in angiocardiopathy is prevented and treated |
| CN108379585A (en)* | 2018-04-16 | 2018-08-10 | 复旦大学附属中山医院 | Application of the HDAC4 inhibitor in the drug for preparing treatment heart failure |
| CN112451529A (en)* | 2020-12-18 | 2021-03-09 | 忻佑康医药科技(南京)有限公司 | New pharmaceutical application of Kb-NB 142-70 |
| CN112512546A (en)* | 2018-05-23 | 2021-03-16 | 匹兹堡大学联邦系统高等教育 | Heart-specific targeting peptides (CTPs), compositions and uses thereof |
| CN116942822A (en)* | 2023-06-12 | 2023-10-27 | 深圳大学 | HDAC2 in the treatment of ventricular arrhythmias |
Families Citing this family (26)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US7154002B1 (en) | 2002-10-08 | 2006-12-26 | Takeda San Diego, Inc. | Histone deacetylase inhibitors |
| KR20050122210A (en) | 2003-03-17 | 2005-12-28 | 다케다 샌디에고, 인코포레이티드 | Histone deacetylase inhibitors |
| WO2005018673A1 (en)* | 2003-08-21 | 2005-03-03 | Osaka University | Pharmaceutical composition for preventing or remedying cardiac hypertrophy and cardiocascular disease caused thereby |
| WO2006060196A2 (en)* | 2004-12-02 | 2006-06-08 | The Trustees Of Columbia University In The City Of New York | Use of rottlerin and its derivatives as activators of bk channel for therapy of hypertension and related disorders |
| JP2008524246A (en) | 2004-12-16 | 2008-07-10 | タケダ サン ディエゴ インコーポレイテッド | Histone deacetylase inhibitor |
| EP1896436A2 (en) | 2005-05-11 | 2008-03-12 | Takeda San Diego, Inc. | Histone deacetylase inhibitors |
| AU2006270322A1 (en) | 2005-07-14 | 2007-01-25 | Takeda San Diego, Inc. | Histone deacetylase inhibitors |
| EP1760092A1 (en)* | 2005-08-26 | 2007-03-07 | Applied Research Systems ARS Holding N.V. | System for screening cells for high expression of a protein of interest |
| WO2007084857A2 (en)* | 2006-01-13 | 2007-07-26 | President And Fellows Of Harvard College | Methods and compositions for treating cell proliferative disorders |
| AU2007234380A1 (en)* | 2006-04-06 | 2007-10-11 | Novartis Ag | Combination of organic compounds |
| US9580515B2 (en)* | 2006-08-21 | 2017-02-28 | Zensun (Shanghai) Science & Technology, Co., Ltd. | Neukinase, a downstream protein of neuregulin |
| EA201070572A1 (en)* | 2007-11-07 | 2010-12-30 | Фолдркс Фармасьютикалз, Инк. | MODULATION OF TRANSPORT OF PROTEINS |
| WO2013053076A1 (en) | 2011-10-10 | 2013-04-18 | Zensun (Shanghai)Science & Technology Limited | Compositions and methods for treating heart failure |
| WO2013074587A1 (en) | 2011-11-16 | 2013-05-23 | Duke University | Bishophonate compositions and methods for treating and/or reducing cardiac dysfunction |
| US9132174B2 (en) | 2013-03-15 | 2015-09-15 | Anchored Rsk3 Inhibitors, Llc | Treatment of heart disease by inhibition of the action of ribosomal S6 kinase 3 (RSK3) |
| EP3610905B1 (en) | 2013-05-22 | 2021-03-31 | Zensun (Shanghai) Science & Technology, Co., Ltd. | Extended release of neuregulin for treating heart failure |
| CA2932787A1 (en)* | 2013-12-06 | 2015-06-11 | Novimmune S.A. | Anti-tlr4 antibodies and methods of use thereof |
| CN110946993A (en) | 2014-01-03 | 2020-04-03 | 上海泽生科技开发股份有限公司 | Formula of neuregulin preparation |
| GB2527364A (en)* | 2014-06-20 | 2015-12-23 | Imp Innovations Ltd | Treatment |
| CN111407882A (en) | 2014-10-17 | 2020-07-14 | 上海泽生科技开发股份有限公司 | Methods and compositions of neuregulin for preventing, treating, or delaying heart failure with preserved ejection fraction |
| UY36714A (en)* | 2015-06-09 | 2016-12-30 | Bayer Pharma Akttiengesellschaft | POSITIVE ALLOSTERIC MODULATORS OF THE M2 MUSCARINIC RECEIVER |
| AU2018297274B2 (en) | 2017-07-06 | 2023-05-18 | Michael S. Kapiloff | Treatment of heart disease by inhibition of the action of muscle A-kinase anchoring protein (mAKAP) |
| EP3852763A1 (en)* | 2018-09-18 | 2021-07-28 | Société des Produits Nestlé S.A. | Src inhibitor compounds for skeletal muscle modulation, methods and uses thereof |
| CN109321602B (en)* | 2018-10-16 | 2021-11-30 | 汉恒生物科技(上海)有限公司 | PKD2 recombinant overexpression vector and construction method and application thereof |
| SG11202112499VA (en) | 2019-05-15 | 2021-12-30 | Univ Miami | Treatment of heart disease by disruption of the anchoring of pp2a |
| KR20240004887A (en)* | 2021-05-04 | 2024-01-11 | 에날레어 테라퓨틱스 인크. | High-conductivity potassium channel modulators, compositions thereof, methods of making the same, and methods of using the same |
Family Cites Families (20)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US4196265A (en)* | 1977-06-15 | 1980-04-01 | The Wistar Institute | Method of producing antibodies |
| US4458066A (en)* | 1980-02-29 | 1984-07-03 | University Patents, Inc. | Process for preparing polynucleotides |
| US4873191A (en)* | 1981-06-12 | 1989-10-10 | Ohio University | Genetic transformation of zygotes |
| US4415723A (en)* | 1982-03-19 | 1983-11-15 | General Electric Company | Randomly branched aromatic polycarbonate from triphenol |
| US6080784A (en)* | 1986-06-11 | 2000-06-27 | Procyon Pharmaceuticals, Inc. | Protein kinase C modulators N |
| US5955501A (en)* | 1986-06-11 | 1999-09-21 | Procyon Pharmaceuticals, Inc. | Protein kinase C modulators O |
| US6043270A (en)* | 1986-06-11 | 2000-03-28 | Procyon Pharmaceuticals, Inc. | Protein kinase C modulators V |
| US4987071A (en)* | 1986-12-03 | 1991-01-22 | University Patents, Inc. | RNA ribozyme polymerases, dephosphorylases, restriction endoribonucleases and methods |
| FR2685346B1 (en)* | 1991-12-18 | 1994-02-11 | Cis Bio International | PROCESS FOR THE PREPARATION OF DOUBLE-STRANDED RNA, AND ITS APPLICATIONS. |
| TW215434B (en)* | 1992-03-07 | 1993-11-01 | Hoechst Ag | |
| US5889136A (en)* | 1995-06-09 | 1999-03-30 | The Regents Of The University Of Colorado | Orthoester protecting groups in RNA synthesis |
| WO1997015575A1 (en)* | 1995-10-27 | 1997-05-01 | Procyon Pharmaceuticals, Inc. | Protein kinase c modulators. y. |
| US6228843B1 (en)* | 1999-04-23 | 2001-05-08 | University Technology Corporation | Method of using PKC inhibiting compounds to treat vascular disease |
| US6372468B1 (en)* | 2000-09-14 | 2002-04-16 | Pe Corporation (Ny) | Isolated human kinase proteins, nucleic acid molecules encoding human kinase proteins, and uses thereof |
| US20030134774A1 (en)* | 2001-06-15 | 2003-07-17 | Steinberg Susan F. | Methods for inhibiting cardiac disorders |
| US6706686B2 (en)* | 2001-09-27 | 2004-03-16 | The Regents Of The University Of Colorado | Inhibition of histone deacetylase as a treatment for cardiac hypertrophy |
| WO2003075910A1 (en)* | 2002-03-08 | 2003-09-18 | Protemix Corporation Limited | Preventing and/or treating vascular disease, cardiomyopathy and/or associated heart failure |
| US20030229030A1 (en)* | 2002-06-11 | 2003-12-11 | Theoharides Theoharis C. | Method of treating interleukin-6-mediated inflammatory diseases |
| WO2004014222A2 (en)* | 2002-08-12 | 2004-02-19 | The Regents Of The University Of Michigan | Diagnosis and treatment of tuberous sclerosis |
| WO2004017957A1 (en)* | 2002-08-20 | 2004-03-04 | Protemix Corporation Limited | Preventing and/or treating cardiovascular disease and/or associated heart failure |
- 2004
- 2004-05-19CNCNA2004800139983Apatent/CN1812776A/enactivePending
- 2004-05-19USUS10/848,820patent/US20050112128A1/ennot_activeAbandoned
- 2004-05-19EPEP04752691Apatent/EP1631268A2/ennot_activeWithdrawn
- 2004-05-19AUAU2004249114Apatent/AU2004249114A1/ennot_activeAbandoned
- 2004-05-19BRBRPI0410787-0Apatent/BRPI0410787A/ennot_activeIP Right Cessation
- 2004-05-19WOPCT/US2004/015715patent/WO2004112763A2/enactiveApplication Filing
- 2004-05-19MXMXPA05012619Apatent/MXPA05012619A/ennot_activeApplication Discontinuation
- 2004-05-19JPJP2006533221Apatent/JP2007505158A/enactivePending
- 2004-05-19CACA002526423Apatent/CA2526423A1/ennot_activeAbandoned
Cited By (12)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN102046813B (en)* | 2008-06-02 | 2016-02-24 | 牛津生物动力有限公司 | Method for detecting long-range chromosome interactions |
| CN104558110A (en)* | 2013-10-21 | 2015-04-29 | 中国科学院大连化学物理研究所 | Polypeptide with ACE inhibitory activity and application thereof in antihypertensive drug |
| CN104558114A (en)* | 2013-10-21 | 2015-04-29 | 中国科学院大连化学物理研究所 | Polypeptide with ACE inhibiting activity in pollen as well as application of polypeptide |
| CN104558110B (en)* | 2013-10-21 | 2018-06-08 | 中国科学院大连化学物理研究所 | Polypeptide with ACE inhibitory activity and its application in blood-pressure drug |
| CN104558114B (en)* | 2013-10-21 | 2018-06-08 | 中国科学院大连化学物理研究所 | Polypeptide with ACE inhibitory activity and its application in pollen |
| CN104872685A (en)* | 2014-02-27 | 2015-09-02 | 长庚医疗财团法人基隆长庚纪念医院 | Nutritional composition for improving heart failure symptoms |
| CN107811997A (en)* | 2017-11-10 | 2018-03-20 | 大连医科大学附属第医院 | Application of the proteasome inhibitor resveratrol in angiocardiopathy is prevented and treated |
| CN108379585A (en)* | 2018-04-16 | 2018-08-10 | 复旦大学附属中山医院 | Application of the HDAC4 inhibitor in the drug for preparing treatment heart failure |
| CN108379585B (en)* | 2018-04-16 | 2020-10-16 | 复旦大学附属中山医院 | Application of HDAC4 inhibitor in preparation of medicine for treating heart failure |
| CN112512546A (en)* | 2018-05-23 | 2021-03-16 | 匹兹堡大学联邦系统高等教育 | Heart-specific targeting peptides (CTPs), compositions and uses thereof |
| CN112451529A (en)* | 2020-12-18 | 2021-03-09 | 忻佑康医药科技(南京)有限公司 | New pharmaceutical application of Kb-NB 142-70 |
| CN116942822A (en)* | 2023-06-12 | 2023-10-27 | 深圳大学 | HDAC2 in the treatment of ventricular arrhythmias |
Also Published As
| Publication number | Publication date |
|---|---|
| US20050112128A1 (en) | 2005-05-26 |
| MXPA05012619A (en) | 2006-02-08 |
| AU2004249114A1 (en) | 2004-12-29 |
| WO2004112763A3 (en) | 2005-09-09 |
| EP1631268A2 (en) | 2006-03-08 |
| CA2526423A1 (en) | 2004-12-29 |
| JP2007505158A (en) | 2007-03-08 |
| WO2004112763A2 (en) | 2004-12-29 |
| BRPI0410787A (en) | 2006-06-20 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| CN1812776A (en) | Inhibition of protein kinase c-mu (pkd) as a treatment for cardiac hypertrophy and heart failure | |
| Tomasi et al. | S‐adenosyl methionine regulates ubiquitin‐conjugating enzyme 9 protein expression and sumoylation in murine liver and human cancers | |
| JP5798165B2 (en) | Identification of microRNAs that activate beta myosin heavy chain expression | |
| Jacobs et al. | IL-7 is essential for homeostatic control of T cell metabolism in vivo | |
| Murthy et al. | Ectodomain shedding of EGFR ligands and TNFR1 dictates hepatocyte apoptosis during fulminant hepatitis in mice | |
| CN1774243A (en) | therapeutic composition | |
| Su et al. | Akt phosphorylation at Thr308 and Ser473 is required for CHIP-mediated ubiquitination of the kinase | |
| US12351609B2 (en) | Use of a small native peptide activator of SERCA pump for treatment of heart failure and other disorders characterized by cytosolic calcium overload | |
| CN1829804A (en) | Targets for the treatment of cognitive impairment | |
| CN1537167A (en) | Van willebrand factor (vWF) -cleaving enzyme | |
| WO2007014029A2 (en) | Yin yang 1 as a treatment for cardiac hypertrophy and heart failure | |
| Nagaleekar et al. | Translational control of NKT cell cytokine production by p38 MAPK | |
| CN1671424A (en) | Decoy composition for treating and preventing inflammatory disease | |
| US20070275099A1 (en) | MST1 modulation of apoptosis in cardiac tissue and modulators of MST1 for treatment and prevention of cardiac disease | |
| CN1934256A (en) | Decoy nucleic acid targeting synoviolin gene promoter | |
| Humphrey et al. | Loss of TRB3 alters dynamics of MLK3-JNK signaling and inhibits cytokine-activated pancreatic beta cell death | |
| US20050283841A1 (en) | Inhibition of protein kinase C-related kinase (PRK) as a treatment for cardiac hypertrophy and heart failure | |
| WO2007131113A2 (en) | Modulation of calmodulin- binding transcription activator (camta) as a treatment for cardiac hypertrophy, heart failure, and heart injury | |
| Yang et al. | The E3 ubiquitin ligase c-Cbl mediates integrin β1 ubiquitination during dilated cardiomyopathy | |
| CN1599622A (en) | Myocardial dysfunction in structural heart disease treated with calmodulin kinase II inhibitors | |
| CN1739788A (en) | Modulation of TRIP-BR function and methods of treating proliferative disorders | |
| CN1738624A (en) | Methods of treating myocardial infarction | |
| EP1827458A2 (en) | Selective inhibition of rock 1 in cardiac therapy | |
| CN1875100A (en) | BACE455, an alternative splice variant of the human beta-secretase | |
| QUEZADA MEZA | PROTEIN KINASE CK2 IN HUMAN DISEASES: TOO MUCH OR TOO LITTLE? |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| C06 | Publication | ||
| PB01 | Publication | ||
| C10 | Entry into substantive examination | ||
| SE01 | Entry into force of request for substantive examination | ||
| C02 | Deemed withdrawal of patent application after publication (patent law 2001) | ||
| WD01 | Invention patent application deemed withdrawn after publication |