CN1690142A - Preparation method of fluorine-containing nano-SiO2 composite non-stick coating material - Google Patents
Preparation method of fluorine-containing nano-SiO2 composite non-stick coating materialDownload PDFInfo
- Publication number
- CN1690142A CN1690142ACN 200410010214CN200410010214ACN1690142ACN 1690142 ACN1690142 ACN 1690142ACN 200410010214CN200410010214CN 200410010214CN 200410010214 ACN200410010214 ACN 200410010214ACN 1690142 ACN1690142 ACN 1690142A
- Authority
- CN
- China
- Prior art keywords
- fluorine
- coating
- silicate
- acrylate
- coupling agent
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Pending
Links
Images
Landscapes
- Paints Or Removers (AREA)
Abstract
Description
Translated fromChinese技术领域technical field
本发明属于纳米材料技术领域,特别涉及一种含氟纳米SiO2复合不粘性涂层材料制备方法。The invention belongs to the technical field of nanometer materials, in particular to a method for preparing a fluorine-containing nanometerSiO2 composite non-stick coating material.
背景技术Background technique
含氟聚合物具有很低的表面能、双疏性(既疏水又疏油)、摩擦系数小、易滑动等特点,因此具有广泛的应用。中国专利CN1097447公开了一种制备无毒海洋防污涂料的方法,将PTFE、石蜡油和氧化物(氧化锌、氧化钛)混合研磨,然后加入氟碳表面活性剂等助剂与研磨好的固体料球磨。将聚二甲基硅氧烷和环氧树脂溶解于有机溶剂中与球磨好的物料充分搅拌,再球磨,制得无毒海洋防污涂料。欧洲专利EP0635535A1公开了制备不粘性涂层的方法,将1,1二氟乙烯(VDF)/六氟丙烯(HFP)/四氟乙烯(TFE)的合成橡胶、PTFE和无机组分溶于丙烯酸丁酯和丁酮的混合溶剂中,作为A组分;以胺类交联剂作为B组分。A组分和B组分在24℃共混6小时,然后在4个大气压下喷涂于钢板上得到300μm厚的涂层,室温下干燥8小时即得到不粘性涂层。欧洲专利EP0408917A1指出以含氟羰基的(甲基)丙烯酸酯与含羟基的(甲基)丙烯酸酯反应也可制得双疏性共聚物。由于上述方法制得的涂层与基材的不相容性,导致涂层存在很多缺陷,使得涂层的疏水性较低,分散稳定性较差。Fluoropolymers have the characteristics of very low surface energy, amphobia (both hydrophobic and oleophobic), low coefficient of friction, and easy sliding, so they have a wide range of applications. Chinese patent CN1097447 discloses a method for preparing non-toxic marine antifouling coatings, mixing and grinding PTFE, paraffin oil and oxides (zinc oxide, titanium oxide), and then adding additives such as fluorocarbon surfactants and ground solids Material ball mill. Dissolving polydimethylsiloxane and epoxy resin in an organic solvent, fully stirring with the ball-milled material, and then ball-milling to prepare a non-toxic marine antifouling paint. European patent EP0635535A1 discloses a method for preparing a non-stick coating, dissolving synthetic rubber, PTFE and inorganic components of 1,1 difluoroethylene (VDF)/hexafluoropropylene (HFP)/tetrafluoroethylene (TFE) in butyl acrylate In the mixed solvent of ester and butanone, it is used as component A; with amine crosslinking agent as component B. Components A and B were blended at 24°C for 6 hours, then sprayed on the steel plate at 4 atmospheres to obtain a 300 μm thick coating, and dried at room temperature for 8 hours to obtain a non-stick coating. European Patent EP0408917A1 pointed out that an amphiphobic copolymer can also be prepared by reacting a fluorocarbonyl-containing (meth)acrylate with a hydroxyl-containing (meth)acrylate. Due to the incompatibility between the coating prepared by the above method and the base material, there are many defects in the coating, so that the hydrophobicity of the coating is low and the dispersion stability is poor.
发明内容Contents of the invention
本发明目的在于提供一种含氟纳米SiO2复合不粘性涂层材料制备方法,该方法工艺简单、合成的涂层材料分散稳定性好。The purpose of the present invention is to provide a preparation method of fluorine-containing nano-SiO2 composite non-stick coating material, which has simple process and good dispersion stability of the synthesized coating material.
为达上述目的,本发明采用如下技术方案:含氟纳米SiO2复合不粘性涂层材料制备方法,包括如下步骤:(1)在反应介质存在下以AIBN或BPO为引发剂将含氟丙烯酸酯与硅烷偶联剂和(甲基)丙烯酸酯共聚,(2)向聚合物溶液中加入硅酸酯类物质水解得到均匀透明的纳米SiO2复合材料。In order to achieve the above object, the present invention adopts the following technical scheme: the preparation method of fluorine-containing nano-SiO2 composite non-stick coating material, comprising the following steps: (1) in the presence of a reaction medium, use AIBN or BPO as an initiator to convert fluorine-containing acrylate Copolymerize with silane coupling agent and (meth)acrylic acid ester, (2) Add silicate substances to polymer solution for hydrolysis to obtain uniform and transparent nano-SiO2 composite material.
步骤(1)中含氟丙烯酸酯与(甲基)丙烯酸酯摩尔比为0-0.16∶1,含氟丙烯酸酯与偶联剂的摩尔比为0-0.5∶1,反应介质选自四氢呋喃、异丙醇、丁酮、二甲苯单独或混合溶剂,反应温度60-130℃、反应时间4-12小时;步骤(2)中聚合物与硅酸酯的摩尔比为0-0.2∶1,硅酸酯与水的摩尔比为1∶2-8,PH=6-9,水解反应温度70℃-90℃,时间为6小时。In step (1), the molar ratio of fluorine-containing acrylate to (meth)acrylate is 0-0.16:1, the molar ratio of fluorine-containing acrylate to coupling agent is 0-0.5:1, and the reaction medium is selected from tetrahydrofuran, iso Propanol, butanone, xylene alone or mixed solvents, reaction temperature 60-130 ° C, reaction time 4-12 hours; the molar ratio of polymer to silicate in step (2) is 0-0.2:1, silicic acid The molar ratio of ester to water is 1:2-8, pH=6-9, the hydrolysis reaction temperature is 70°C-90°C, and the time is 6 hours.
硅烷偶联剂选用KH-570,反应介质选用四氢呋喃和异丙醇体积比为1∶2的混合溶剂,聚合反应温度70-90℃,反应时间5-7小时;硅酸酯选择硅酸乙酯或者硅酸丁酯,水解的反应温度为75-80℃。The silane coupling agent is KH-570, the reaction medium is a mixed solvent of tetrahydrofuran and isopropanol with a volume ratio of 1:2, the polymerization reaction temperature is 70-90°C, and the reaction time is 5-7 hours; the silicate is ethyl silicate Or butyl silicate, the reaction temperature of hydrolysis is 75-80°C.
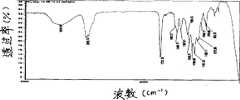
本发明的不粘性涂层材料的制备方法包括由含氟丙烯酸酯与硅烷偶联剂和(甲基)丙烯酸酯共聚,然后由硅酸酯与硅烷偶联剂进行共水解制得有机-无机纳米复合材料。由涂层材料的红外光谱图可知,1638cm-1处的C=C双键特征吸收峰以及3095-3010cm-1处的C=C键上的C-H吸收峰均消失。同时,1733.19cm-1的吸收峰为C=O特征峰,与单体的C=O峰相比发生蓝移,其原因在于C=C双键被打开、结构由共扼变成非共扼所致。上述结果表明所加入的单体发生了聚合反应。曲线中1480.51-953.78cm-1处为多氟代烷特征峰。1387cm-1和1151cm-1处为磺酰胺的峰,它们和多氟代烷特征峰重叠。2972-2843cm-1为CH3-和-CH2-的C-H伸缩振动峰。3436.84cm-1处为-OH峰,也可能为含氟单体的杂质峰,说明目的产物中含有氟基团。1120-1000cm-1为Si-O-R键的伸缩振动峰,水解反应发生后,Si-O-R键的伸缩振动峰明显减弱,说明硅氧烷基发生了水解,然而由于它们和多氟代烷特征峰重叠,所以较难分辨。红外光谱分析结果表明,在所选择的试验条件下,合成了含氟聚合物,并且硅氧烷基发生了水解反应。由反应产物纳米微粒的透射电镜图可知,纳米微粒分散均匀,粒径约50nm。将聚合物稀释,将用于聚合的溶剂做分散剂、聚合物溶液与分散剂体积比例为1∶1,将干净的载玻片浸入聚合物稀释液匀速提拉2次,提拉速度为5cm/min,可得到含氟纳米SiO2复合不粘性涂层。由涂层的原子力形貌图可发现,复合聚合物涂层表面均匀,其表面粗糙度仅为28nm。涂层中具有细小均匀的岛状物,在100-300nm之间。纳米SiO2微粒在聚合物中所占的质量百分比为5-15%。The preparation method of the non-stick coating material of the present invention comprises the copolymerization of fluorine-containing acrylate and silane coupling agent and (meth)acrylate, and then co-hydrolysis of silicate and silane coupling agent to prepare organic-inorganic nano composite material. It can be seen from the infrared spectrogram of the coating material that the characteristic absorption peak of the C=C double bond at 1638cm-1 and the CH absorption peak on the C=C bond at 3095-3010cm-1 all disappear. At the same time, the absorption peak at 1733.19 cm-1 is the characteristic peak of C=O, which is blue-shifted compared with the C=O peak of the monomer. The reason is that the C=C double bond is opened and the structure changes from conjugated to non-conjugated due to. The above results indicated that the polymerization reaction of the added monomer had occurred. The 1480.51-953.78cm-1 in the curve is the characteristic peak of polyfluoroalkane. The peaks of sulfonamide at 1387cm-1 and 1151cm-1 overlap with the characteristic peaks of polyfluoroalkanes. 2972-2843cm-1 is the CH stretching vibration peak of CH3 - and -CH2 -. The -OH peak at 3436.84cm-1 may also be the impurity peak of fluorine-containing monomers, indicating that the target product contains fluorine groups. 1120-1000cm-1 is the stretching vibration peak of the Si-OR bond. After the hydrolysis reaction, the stretching vibration peak of the Si-OR bond is obviously weakened, indicating that the siloxane group has been hydrolyzed. However, due to their and polyfluoroalkane characteristic peaks overlap, so it is difficult to distinguish. The results of infrared spectrum analysis showed that under the selected test conditions, the fluoropolymer was synthesized and the siloxane group was hydrolyzed. It can be seen from the transmission electron microscope image of the nanoparticle of the reaction product that the nanoparticle is uniformly dispersed and the particle size is about 50nm. Dilute the polymer, use the solvent used for polymerization as a dispersant, the volume ratio of the polymer solution to the dispersant is 1:1, immerse the clean glass slide in the polymer diluent and pull it at a constant speed for 2 times, the pulling speed is 5cm /min, fluorine-containing nano-SiO2 composite non-stick coating can be obtained. It can be found from the atomic force topography of the coating that the surface of the composite polymer coating is uniform, and its surface roughness is only 28nm. There are fine and uniform islands in the coating, between 100-300nm. The mass percent of nanometer SiO2 particles in the polymer is 5-15%.
本发明在含氟聚合物中原位形成有机-无机纳米复合材料,即纳米微粒的形成以及纳米微粒被有机组分的包覆在体系中一次完成,并实现纳米微粒表面和有机组分分子之间的化学键合,从而有效解决了纳米微粒在非极性溶剂中长期分散稳定性问题,该产品可以长期稳定存在3个月以上。通过调节pH值的大小、水解时间及聚合物单体的比例可以控制纳米微粒的粒径大小和涂层的双疏效果。含氟单体价格昂贵,本发明通过将含氟单体与(甲基)丙烯酸酯共聚,在不影响涂层性能的情况下有效降低了涂层材料的成本。纳米SiO2的加入很好地弥补涂层的缺陷,提高了涂层的双疏性和抗摩耐磨性,涂层与水的接触角可达130°以上。本发明中产品原料为普通工业产品,制备方法采用化学合成方法,易于工业化。利用本发明制成的涂层材料在非极性溶剂中分散性好,可以长期稳定存在。可采用浸涂法、提拉法、喷涂法、刷涂法、抹涂法、擦涂法等涂敷方法,涂敷工艺简便易行。制备的涂层材料可用于涂料油漆、织物整理、造纸业。The present invention forms the organic-inorganic nanocomposite material in situ in the fluorine-containing polymer, that is, the formation of nanoparticles and the coating of nanoparticles by organic components are completed in the system at one time, and the connection between the surface of nanoparticles and the molecules of organic components is realized. The chemical bonding of nanoparticles effectively solves the problem of long-term dispersion stability of nanoparticles in non-polar solvents, and the product can exist stably for more than 3 months. By adjusting the pH value, hydrolysis time and the ratio of polymer monomers, the particle size of nanoparticles and the amphiphobic effect of the coating can be controlled. The fluorine-containing monomer is expensive, and the invention effectively reduces the cost of the coating material without affecting the coating performance by copolymerizing the fluorine-containing monomer and (meth)acrylate. The addition of nano-SiO2 can make up for the defects of the coating, improve the coating's amphobia and anti-friction and wear resistance, and the contact angle between the coating and water can reach more than 130°. The raw materials of the product in the present invention are common industrial products, and the preparation method adopts a chemical synthesis method, which is easy for industrialization. The coating material prepared by the invention has good dispersibility in nonpolar solvents and can exist stably for a long time. Dip coating, pulling method, spray coating method, brush coating method, wipe coating method, wiping coating method and other coating methods can be used, and the coating process is simple and easy. The prepared coating material can be used in coating paint, fabric finishing and paper making.
附图说明Description of drawings
图1为涂层材料的红外光谱图;Fig. 1 is the infrared spectrogram of coating material;
图2为涂层的透射电镜图;Fig. 2 is the transmission electron microscope picture of coating;
图3为涂层的原子力形貌图。Figure 3 is the atomic force topography of the coating.
具体实施方式Detailed ways
实施例1、取25ml四氢呋喃和10ml异丙醇置于100ml三颈瓶中,加入4g甲基丙烯酸甲酯,2g含氟丙烯酸酯(F-814)和1g硅烷偶联剂(KH-570),搅拌充N2。加入0.084gAIBN。将溶液升温至回流温度(约70℃),回流6小时。将溶液冷却至室温,加入25ml四氢呋喃和10ml异丙醇,然后加入0.7g硅酸乙酯,加入0.16ml水,回流水解8小时,得到有机一无机复合的含氟纳米SiO2复合不粘性涂层材料。将干净的载玻片浸入溶液,匀速降落液面,将涂层晾干然后重复一次即可。Example 1. Put 25ml tetrahydrofuran and 10ml isopropanol in a 100ml three-necked flask, add 4g methyl methacrylate, 2g fluorine-containing acrylate (F-814) and 1g silane coupling agent (KH-570), Stir and fill withN2 . Add 0.084g AIBN. The solution was warmed to reflux temperature (about 70°C) and refluxed for 6 hours. Cool the solution to room temperature, add 25ml tetrahydrofuran and 10ml isopropanol, then add 0.7g ethyl silicate, add 0.16ml water, reflux and hydrolyze for 8 hours to obtain an organic-inorganic composite fluorine-containing nano-SiO2 composite non-stick coating Material. Dip a clean glass slide into the solution, drop the liquid level at a constant speed, let the coating dry and repeat once.
实施例2、取25ml丁酮和10ml二甲苯置于100ml三颈瓶中,加入4g丙烯酸丁酯,1g含氟丙烯酸酯(F-814)和1g硅烷偶联剂(KH-570),搅拌充N2。加入0.072gAIBN。将溶液升温至回流温度,回流6小时。将溶液冷却,加入25ml丁酮和10ml二甲苯,然后加入0.6g硅酸丁酯,加入0.15ml水,回流水解8小时。得到有机-无机复合含氟纳米SiO2复合不粘性涂层材料。将干净的载玻片浸入溶液,匀速降落液面,将涂层晾干然后重复一次即可。Embodiment 2, get 25ml methyl ethyl ketone and 10ml xylene and place in 100ml three-neck flask, add 4g butyl acrylate, 1g fluorine-containing acrylate (F-814) and 1g silane coupling agent (KH-570), stir and fill N2 . Add 0.072g AIBN. The solution was warmed to reflux temperature and refluxed for 6 hours. Cool the solution, add 25ml butanone and 10ml xylene, then add 0.6g butyl silicate, add 0.15ml water, and reflux for 8 hours for hydrolysis. An organic-inorganic composite fluorine-containing nano-SiO2 composite non-stick coating material is obtained. Dip a clean glass slide into the solution, drop the liquid level at a constant speed, let the coating dry and repeat once.
实施例3、取25ml四氢呋喃和10ml异丙醇置于100ml三颈瓶中,加入4g甲基丙烯酸-β-羟丙酯,5g含氟丙烯酸酯(F-814)和1g硅烷偶联剂(KH-570),搅拌充N2。加入0.12gAIBN。将溶液升温至70℃,回流6小时。将溶液冷却,加入25ml四氢呋喃和10ml异丙醇,然后加入1g硅酸乙酯,加入0.24ml水,回流水解8小时。得到有机-无机纳米复合材料。将干净的载玻片浸入溶液,匀速降落液面,将涂层晾干然后重复一次即可。Embodiment 3, get 25ml tetrahydrofuran and 10ml isopropanol and place in 100ml three-necked flask, add 4g methacrylic acid-beta-hydroxypropyl ester, 5g fluorine-containing acrylate (F-814) and 1g silane coupling agent (KH -570), stirred and filled with N2 . Add 0.12g AIBN. The solution was warmed to 70°C and refluxed for 6 hours. Cool the solution, add 25ml of tetrahydrofuran and 10ml of isopropanol, then add 1g of ethyl silicate, add 0.24ml of water, and reflux for 8 hours for hydrolysis. An organic-inorganic nanocomposite material is obtained. Dip a clean glass slide into the solution, drop the liquid level at a constant speed, let the coating dry and repeat once.
Claims (3)
Translated fromChinesePriority Applications (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| CN 200410010214CN1690142A (en) | 2004-04-19 | 2004-04-19 | Preparation method of fluorine-containing nano-SiO2 composite non-stick coating material |
Applications Claiming Priority (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| CN 200410010214CN1690142A (en) | 2004-04-19 | 2004-04-19 | Preparation method of fluorine-containing nano-SiO2 composite non-stick coating material |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| CN1690142Atrue CN1690142A (en) | 2005-11-02 |
Family
ID=35345907
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| CN 200410010214PendingCN1690142A (en) | 2004-04-19 | 2004-04-19 | Preparation method of fluorine-containing nano-SiO2 composite non-stick coating material |
Country Status (1)
| Country | Link |
|---|---|
| CN (1) | CN1690142A (en) |
Cited By (8)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN101177485B (en)* | 2007-10-31 | 2010-06-23 | 大连理工大学 | Low surface-energy fluorosiloxane containing double parallel main-chain structure and method for synthesizing the same |
| CN101045833B (en)* | 2007-04-11 | 2010-09-08 | 江苏工业学院 | Preparation method of UV-cured wood coating modified by in-situ method |
| CN102086333A (en)* | 2010-11-29 | 2011-06-08 | 江苏足迹涂料有限公司 | Organic-inorganic composite coating in chrysanthemum-shaped micro-nano structure and preparation method thereof |
| CN101842445B (en)* | 2008-10-14 | 2012-05-30 | 金泰雄 | Non-adhesive coating composition and preparation method thereof |
| US8314037B2 (en) | 2007-12-07 | 2012-11-20 | E. I. Du Pont De Nemours And Company | Fluoropolymer emulsions |
| US8329822B2 (en) | 2009-11-09 | 2012-12-11 | E.I. Du Pont De Nemours And Company | Fluoropolymer emulsions |
| US8507601B2 (en) | 2009-11-09 | 2013-08-13 | E. I. Du Pont De Nemours And Company | Method using fluoropolymer emulsions |
| CN103819995A (en)* | 2014-01-20 | 2014-05-28 | 浙江大学 | Nano-composite superhydrophobic icing-proof coating material and preparation method thereof |
- 2004
- 2004-04-19CNCN 200410010214patent/CN1690142A/enactivePending
Cited By (10)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN101045833B (en)* | 2007-04-11 | 2010-09-08 | 江苏工业学院 | Preparation method of UV-cured wood coating modified by in-situ method |
| CN101177485B (en)* | 2007-10-31 | 2010-06-23 | 大连理工大学 | Low surface-energy fluorosiloxane containing double parallel main-chain structure and method for synthesizing the same |
| US8314037B2 (en) | 2007-12-07 | 2012-11-20 | E. I. Du Pont De Nemours And Company | Fluoropolymer emulsions |
| CN101842445B (en)* | 2008-10-14 | 2012-05-30 | 金泰雄 | Non-adhesive coating composition and preparation method thereof |
| US8329822B2 (en) | 2009-11-09 | 2012-12-11 | E.I. Du Pont De Nemours And Company | Fluoropolymer emulsions |
| US8507601B2 (en) | 2009-11-09 | 2013-08-13 | E. I. Du Pont De Nemours And Company | Method using fluoropolymer emulsions |
| CN102086333A (en)* | 2010-11-29 | 2011-06-08 | 江苏足迹涂料有限公司 | Organic-inorganic composite coating in chrysanthemum-shaped micro-nano structure and preparation method thereof |
| CN102086333B (en)* | 2010-11-29 | 2013-04-03 | 江苏足迹涂料有限公司 | Organic-inorganic composite coating in chrysanthemum-shaped micro-nano structure and preparation method thereof |
| CN103819995A (en)* | 2014-01-20 | 2014-05-28 | 浙江大学 | Nano-composite superhydrophobic icing-proof coating material and preparation method thereof |
| CN103819995B (en)* | 2014-01-20 | 2015-12-02 | 浙江大学 | A kind of nano composite super-hydrophobic ice-covering-proof functional coating material and preparation method thereof |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| CN109868026B (en) | Organic silicon modified acrylate resin, preparation method thereof and hydrophobic weather-resistant slow-release modified acrylic resin coating | |
| CN103788802B (en) | A kind of preparation method of superhydrophobic coating | |
| CN1824699A (en) | A method for reducing the surface energy of acrylate materials | |
| CN101372527B (en) | Nano-zinc oxide/acrylic ester grafting composite emulsion and preparation thereof | |
| CN104073116B (en) | Preparation method of super-amphiphobic coating | |
| CN103408990B (en) | Nano-particle super-hydrophobic self-cleaning wall paint and preparation method thereof | |
| JP5072820B2 (en) | Silicone resin composition | |
| CN113088123A (en) | Micro-nano composite SiO2Particle, micro-nano composite structure super-hydrophobic coating, preparation method and application thereof | |
| CN106085070A (en) | A kind of low-surface-energy micro nano-coatings material and preparation method thereof | |
| CN1380369A (en) | Water high-weatherability nano external wall paint and its preparation method | |
| CN109294382B (en) | Super-weather-resistant acid-rain-resistant stain-resistant water-based inorganic nano ceramic coating and preparation method thereof | |
| CN108517161A (en) | Super-hydrophobic oleophobic coating and preparation method thereof, super-hydrophobic oleophobic film | |
| CN104744639A (en) | Preparation method of organic silicon modified normal-temperature multiple self-crosslinked epoxy resin emulsion | |
| Wang et al. | Synthesis and characterization of nanosilica/polyacrylate composite latex | |
| CN1690142A (en) | Preparation method of fluorine-containing nano-SiO2 composite non-stick coating material | |
| CN106752424A (en) | A kind of wear-resistant integrated super-hydrophobic coat of polystyrene and preparation method thereof | |
| CN109575732A (en) | A kind of self-cleaning nona Ti02-Si02/ fluorine-containing ester paint | |
| CN111393592A (en) | Nano SiO2Super-hydrophobic material of toughened and modified epoxy resin and preparation method thereof | |
| CN1632015A (en) | Nano self-crosslinked acrylate paint for external wall and method for making same | |
| CN116769344A (en) | Fluorinated graphene/SiO2/fluorosilane composite material and preparation method thereof, superhydrophobic coating | |
| CN112126350B (en) | Recyclable super-amphiphobic composite coating and preparation and application thereof | |
| CN111073510B (en) | A kind of hydrophobic and oleophobic self-cleaning material and preparation method and application thereof | |
| KR20150023868A (en) | Silsesquioxane-like particles | |
| CN109970977B (en) | A kind of titanium dioxide doped silicone resin micropowder and its preparation method and application | |
| CN110804136B (en) | Preparation method of core-shell type fluorine-containing emulsion microsphere and application of core-shell type fluorine-containing emulsion microsphere in super-hydrophobic coating |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| C06 | Publication | ||
| PB01 | Publication | ||
| C10 | Entry into substantive examination | ||
| SE01 | Entry into force of request for substantive examination | ||
| C02 | Deemed withdrawal of patent application after publication (patent law 2001) | ||
| WD01 | Invention patent application deemed withdrawn after publication |