CN116396372A - Peptide-loaded nanoparticle and application thereof in treatment of eye infection of pets - Google Patents
Peptide-loaded nanoparticle and application thereof in treatment of eye infection of petsDownload PDFInfo
- Publication number
- CN116396372A CN116396372ACN202310135438.XACN202310135438ACN116396372ACN 116396372 ACN116396372 ACN 116396372ACN 202310135438 ACN202310135438 ACN 202310135438ACN 116396372 ACN116396372 ACN 116396372A
- Authority
- CN
- China
- Prior art keywords
- peptide
- plga
- cath20
- eye
- nanoparticle
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Granted
Links
- 108090000765processed proteins & peptidesProteins0.000titleclaimsabstractdescription43
- 239000002105nanoparticleSubstances0.000titleclaimsabstractdescription18
- 208000001860Eye InfectionsDiseases0.000titleabstractdescription4
- 208000011323eye infectious diseaseDiseases0.000titleabstractdescription4
- 229920001606poly(lactic acid-co-glycolic acid)Polymers0.000claimsabstractdescription39
- 230000000844anti-bacterial effectEffects0.000claimsabstractdescription19
- 125000003275alpha amino acid groupChemical group0.000claimsabstract2
- 102000004196processed proteins & peptidesHuman genes0.000claimsdescription14
- 229920001184polypeptidePolymers0.000claimsdescription11
- 241000894006BacteriaSpecies0.000claimsdescription8
- 238000002360preparation methodMethods0.000claimsdescription6
- 239000002539nanocarrierSubstances0.000claimsdescription5
- 229920001577copolymerPolymers0.000claimsdescription3
- 230000002265preventionEffects0.000claims3
- 238000003259recombinant expressionMethods0.000claims3
- 239000013604expression vectorSubstances0.000claims2
- 150000007523nucleic acidsChemical group0.000claims1
- 239000004005microsphereSubstances0.000abstractdescription36
- 241000272108Ophiophagus hannahSpecies0.000abstractdescription17
- 208000035143Bacterial infectionDiseases0.000abstractdescription4
- 208000022362bacterial infectious diseaseDiseases0.000abstractdescription4
- 210000001519tissueAnatomy0.000abstractdescription4
- 210000004400mucous membraneAnatomy0.000abstractdescription2
- 150000003384small moleculesChemical class0.000abstractdescription2
- 150000001413amino acidsChemical group0.000description14
- 102000044503Antimicrobial PeptidesHuman genes0.000description11
- 108700042778Antimicrobial PeptidesProteins0.000description11
- 239000003910polypeptide antibiotic agentSubstances0.000description9
- 239000000243solutionSubstances0.000description9
- 238000000338in vitroMethods0.000description7
- 230000007794irritationEffects0.000description7
- 241000283973Oryctolagus cuniculusSpecies0.000description6
- FAPWRFPIFSIZLT-UHFFFAOYSA-MSodium chlorideChemical compound[Na+].[Cl-]FAPWRFPIFSIZLT-UHFFFAOYSA-M0.000description6
- 229940024606amino acidDrugs0.000description6
- 238000000034methodMethods0.000description5
- 230000001580bacterial effectEffects0.000description4
- 231100000286mucous membrane, eye irritation or corrosion testingToxicity0.000description4
- 239000002245particleSubstances0.000description4
- 238000012360testing methodMethods0.000description4
- YMWUJEATGCHHMB-UHFFFAOYSA-NDichloromethaneChemical compoundClCClYMWUJEATGCHHMB-UHFFFAOYSA-N0.000description3
- 239000003814drugSubstances0.000description3
- 229940079593drugDrugs0.000description3
- 238000011156evaluationMethods0.000description3
- 230000002209hydrophobic effectEffects0.000description3
- 239000012071phaseSubstances0.000description3
- 239000000725suspensionSubstances0.000description3
- XLYOFNOQVPJJNP-UHFFFAOYSA-NwaterSubstancesOXLYOFNOQVPJJNP-UHFFFAOYSA-N0.000description3
- FWMNVWWHGCHHJJ-SKKKGAJSSA-N4-amino-1-[(2r)-6-amino-2-[[(2r)-2-[[(2r)-2-[[(2r)-2-amino-3-phenylpropanoyl]amino]-3-phenylpropanoyl]amino]-4-methylpentanoyl]amino]hexanoyl]piperidine-4-carboxylic acidChemical compoundC([C@H](C(=O)N[C@H](CC(C)C)C(=O)N[C@H](CCCCN)C(=O)N1CCC(N)(CC1)C(O)=O)NC(=O)[C@H](N)CC=1C=CC=CC=1)C1=CC=CC=C1FWMNVWWHGCHHJJ-SKKKGAJSSA-N0.000description2
- AGPKZVBTJJNPAG-WHFBIAKZSA-NL-isoleucineChemical compoundCC[C@H](C)[C@H](N)C(O)=OAGPKZVBTJJNPAG-WHFBIAKZSA-N0.000description2
- 241001465754MetazoaSpecies0.000description2
- 241000589517Pseudomonas aeruginosaSpecies0.000description2
- 241000191967Staphylococcus aureusSpecies0.000description2
- 210000003022colostrumAnatomy0.000description2
- 235000021277colostrumNutrition0.000description2
- 238000011161developmentMethods0.000description2
- 230000000694effectsEffects0.000description2
- 238000000635electron micrographMethods0.000description2
- 239000000839emulsionSubstances0.000description2
- 238000005538encapsulationMethods0.000description2
- 238000004128high performance liquid chromatographyMethods0.000description2
- 230000007246mechanismEffects0.000description2
- 239000012074organic phaseSubstances0.000description2
- 230000028327secretionEffects0.000description2
- 239000006228supernatantSubstances0.000description2
- 241000196324EmbryophytaSpecies0.000description1
- 241000588724Escherichia coliSpecies0.000description1
- 206010015946Eye irritationDiseases0.000description1
- 206010061218InflammationDiseases0.000description1
- 238000010171animal modelMethods0.000description1
- 239000003242anti bacterial agentSubstances0.000description1
- 229940124350antibacterial drugDrugs0.000description1
- 229940088710antibiotic agentDrugs0.000description1
- 230000003385bacteriostatic effectEffects0.000description1
- 230000015572biosynthetic processEffects0.000description1
- 210000004899c-terminal regionAnatomy0.000description1
- 238000005119centrifugationMethods0.000description1
- 238000012512characterization methodMethods0.000description1
- 210000000795conjunctivaAnatomy0.000description1
- 210000004087corneaAnatomy0.000description1
- 230000001186cumulative effectEffects0.000description1
- 239000008367deionised waterSubstances0.000description1
- 229910021641deionized waterInorganic materials0.000description1
- 238000001514detection methodMethods0.000description1
- 238000010586diagramMethods0.000description1
- 239000006185dispersionSubstances0.000description1
- 239000012154double-distilled waterSubstances0.000description1
- 238000002296dynamic light scatteringMethods0.000description1
- 231100000013eye irritationToxicity0.000description1
- 239000012634fragmentSubstances0.000description1
- 125000000524functional groupChemical group0.000description1
- 239000003292glueSubstances0.000description1
- ZDXPYRJPNDTMRX-UHFFFAOYSA-NglutamineNatural productsOC(=O)C(N)CCC(N)=OZDXPYRJPNDTMRX-UHFFFAOYSA-N0.000description1
- 230000007124immune defenseEffects0.000description1
- 238000001727in vivoMethods0.000description1
- 230000004054inflammatory processEffects0.000description1
- 229960000310isoleucineDrugs0.000description1
- AGPKZVBTJJNPAG-UHFFFAOYSA-NisoleucineNatural productsCCC(C)C(N)C(O)=OAGPKZVBTJJNPAG-UHFFFAOYSA-N0.000description1
- 239000002502liposomeSubstances0.000description1
- 239000007788liquidSubstances0.000description1
- 231100000053low toxicityToxicity0.000description1
- 230000014759maintenance of locationEffects0.000description1
- 229940127554medical productDrugs0.000description1
- 244000005700microbiomeSpecies0.000description1
- 239000011259mixed solutionSubstances0.000description1
- 210000004877mucosaAnatomy0.000description1
- 239000007908nanoemulsionSubstances0.000description1
- 239000002077nanosphereSubstances0.000description1
- 239000006070nanosuspensionSubstances0.000description1
- 229940023490ophthalmic productDrugs0.000description1
- 239000003960organic solventSubstances0.000description1
- 238000007747platingMethods0.000description1
- 229920000642polymerPolymers0.000description1
- 239000000843powderSubstances0.000description1
- 230000008569processEffects0.000description1
- 239000000047productSubstances0.000description1
- 230000002035prolonged effectEffects0.000description1
- 238000000746purificationMethods0.000description1
- 238000011160researchMethods0.000description1
- 238000005070samplingMethods0.000description1
- UQDJGEHQDNVPGU-UHFFFAOYSA-Nserine phosphoethanolamineChemical compound[NH3+]CCOP([O-])(=O)OCC([NH3+])C([O-])=OUQDJGEHQDNVPGU-UHFFFAOYSA-N0.000description1
- 238000003756stirringMethods0.000description1
- 238000013269sustained drug releaseMethods0.000description1
- 238000003786synthesis reactionMethods0.000description1
- 230000001225therapeutic effectEffects0.000description1
- 238000002560therapeutic procedureMethods0.000description1
- 230000009466transformationEffects0.000description1
- 238000002525ultrasonicationMethods0.000description1
- 230000035899viabilityEffects0.000description1
Images
Classifications
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K14/00—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof
- C07K14/435—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof from animals; from humans
- C07K14/46—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof from animals; from humans from vertebrates
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K38/00—Medicinal preparations containing peptides
- A61K38/16—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof
- A61K38/17—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof from animals; from humans
- A61K38/1703—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof from animals; from humans from vertebrates
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/48—Preparations in capsules, e.g. of gelatin, of chocolate
- A61K9/50—Microcapsules having a gas, liquid or semi-solid filling; Solid microparticles or pellets surrounded by a distinct coating layer, e.g. coated microspheres, coated drug crystals
- A61K9/51—Nanocapsules; Nanoparticles
- A61K9/5107—Excipients; Inactive ingredients
- A61K9/513—Organic macromolecular compounds; Dendrimers
- A61K9/5146—Organic macromolecular compounds; Dendrimers obtained otherwise than by reactions only involving carbon-to-carbon unsaturated bonds, e.g. polyethylene glycol, polyamines, polyanhydrides
- A61K9/5153—Polyesters, e.g. poly(lactide-co-glycolide)
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P31/00—Antiinfectives, i.e. antibiotics, antiseptics, chemotherapeutics
- A61P31/04—Antibacterial agents
- Y—GENERAL TAGGING OF NEW TECHNOLOGICAL DEVELOPMENTS; GENERAL TAGGING OF CROSS-SECTIONAL TECHNOLOGIES SPANNING OVER SEVERAL SECTIONS OF THE IPC; TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y02—TECHNOLOGIES OR APPLICATIONS FOR MITIGATION OR ADAPTATION AGAINST CLIMATE CHANGE
- Y02A—TECHNOLOGIES FOR ADAPTATION TO CLIMATE CHANGE
- Y02A50/00—TECHNOLOGIES FOR ADAPTATION TO CLIMATE CHANGE in human health protection, e.g. against extreme weather
- Y02A50/30—Against vector-borne diseases, e.g. mosquito-borne, fly-borne, tick-borne or waterborne diseases whose impact is exacerbated by climate change
Landscapes
- Health & Medical Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Chemical & Material Sciences (AREA)
- Medicinal Chemistry (AREA)
- General Health & Medical Sciences (AREA)
- Engineering & Computer Science (AREA)
- Animal Behavior & Ethology (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Veterinary Medicine (AREA)
- Public Health (AREA)
- Pharmacology & Pharmacy (AREA)
- Organic Chemistry (AREA)
- Proteomics, Peptides & Aminoacids (AREA)
- Epidemiology (AREA)
- Gastroenterology & Hepatology (AREA)
- Zoology (AREA)
- General Chemical & Material Sciences (AREA)
- Biophysics (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Oncology (AREA)
- Biochemistry (AREA)
- Genetics & Genomics (AREA)
- Communicable Diseases (AREA)
- Physics & Mathematics (AREA)
- Toxicology (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Biomedical Technology (AREA)
- Nanotechnology (AREA)
- Optics & Photonics (AREA)
- Molecular Biology (AREA)
- Marine Sciences & Fisheries (AREA)
- Immunology (AREA)
- Peptides Or Proteins (AREA)
- Medicines That Contain Protein Lipid Enzymes And Other Medicines (AREA)
Abstract
Translated fromChineseDescription
Translated fromChinese技术领域technical field
本发明属于宠物医疗制品技术领域,具体涉及一种载肽纳米颗粒及在宠物眼部感染治疗上的应用。The invention belongs to the technical field of pet medical products, and in particular relates to a peptide-loaded nanoparticle and its application in the treatment of pet eye infections.
背景技术Background technique
抗菌肽是生物体先天性免疫防御系统的重要组分,广泛存在于微生物、动物和植物等生命体中,是一类具有广谱抗菌活性的多肽分子。抗菌肽的抗菌机制不同于传统抗生素,其具有独特的膜靶向作用机制,因此这类分子在抗菌过程中不易产生耐受,被认为是极具开发潜力的新型抗耐药菌候选分子。Antimicrobial peptides are an important component of the innate immune defense system of organisms. They are widely found in living organisms such as microorganisms, animals, and plants. They are a class of polypeptide molecules with broad-spectrum antibacterial activity. The antibacterial mechanism of antimicrobial peptides is different from that of traditional antibiotics, and has a unique membrane-targeting mechanism. Therefore, such molecules are not easy to produce resistance during the antibacterial process, and are considered to be new candidate molecules for anti-drug-resistant bacteria with great potential for development.
国内外对于纳米载体包被抗菌药物的研究已经取得了很大的成果,积极探索研究了多种纳米载体递送系统,如脂质体、纳米乳、纳米粒、纳米混悬剂及纳米胶束等,在体外和体内动物模型中展现了优异的递送潜力,具有使其在眼部保留时间延长等特点。有研究表明,纳米粒在眼部的分布主要取决于其大小和表面性质,200~2000nm的纳米粒可在眼部组织停留至少2个月。纳米粒可以附着在黏膜上,并在角膜前组织中停留很长时间而不被快速清除,提示纳米载体递送系统在眼部用药上有良好的应用前景,具备进一步临床试验开发的潜力。At home and abroad, research on nanocarrier-coated antibacterial drugs has achieved great results, and actively explored and studied a variety of nanocarrier delivery systems, such as liposomes, nanoemulsions, nanoparticles, nanosuspensions and nanomicelles, etc. , exhibited excellent delivery potential in in vitro and in vivo animal models, with features such as prolonged retention in the eye. Studies have shown that the distribution of nanoparticles in the eye mainly depends on their size and surface properties, and nanoparticles of 200-2000nm can stay in the eye tissue for at least 2 months. Nanoparticles can attach to the mucosa and stay in the precorneal tissue for a long time without being quickly cleared, suggesting that the nanocarrier delivery system has a good application prospect in ophthalmic medicine and has the potential for further clinical trial development.
发明内容Contents of the invention
本发明的目的在于提供一种载肽纳米颗粒及在宠物眼部感染治疗上的应用,即将小分子眼镜王蛇衍生肽结合到纳米颗粒上,并用于治疗宠物眼部细菌感染。The purpose of the present invention is to provide a peptide-loaded nanoparticle and its application in the treatment of pet eye infections, that is, to bind the small molecule king cobra-derived peptide to the nanoparticle, and to treat the pet eye bacterial infection.
本发明首先提供一种眼镜王蛇衍生肽,是对氨基酸序列为KRFKKFFKKLKNSVKKRAKKFFKKPRVIGVSIPF(SEQ ID NO:1)的多肽进行改造,改造后的眼镜王蛇衍生肽命名为CATH20,其氨基酸序列为KKRAKKFFKKPRVIGVSQPF(SEQ ID NO:2)。The present invention firstly provides a king cobra-derived peptide, which is to modify the polypeptide whose amino acid sequence is KRFKKFFKKLKNSVKKRAKKFFKKPRVIGVSIPF (SEQ ID NO: 1). The modified king cobra-derived peptide is named CATH20, and its amino acid sequence is KKRAKKFFKKPRVIGVSQPF (SEQ ID NO: 2).
本发明再一个方面提供一种纳米颗粒,所述的纳米微球为携带有所述的眼镜王蛇衍生肽CATH20的聚乳酸-羟基乙酸共聚物PLGA;Another aspect of the present invention provides a nanoparticle, the nanosphere is polylactic acid-glycolic acid copolymer PLGA carrying the king cobra-derived peptide CATH20;
本发明所提供的纳米颗粒可用于制备用于预防治疗宠物眼部细菌的制品。The nano particles provided by the invention can be used to prepare products for preventing and treating eye bacteria in pets.
本发明制备的PLGA(CATH20)微球适合在宠物眼部细菌感染使用,有较好的杀菌作用,且可以附着在黏膜上,在角膜前组织中停留很长时间而不被快速清除,具有很好的生物相容性特点,在生物医用领域具有良好的实用价值。The PLGA (CATH20) microspheres prepared by the present invention are suitable for use in pet eye bacterial infections, have a good bactericidal effect, and can be attached to the mucous membrane, staying in the precorneal tissue for a long time without being quickly cleared, and have great It has good biocompatibility characteristics and has good practical value in the field of biomedicine.
附图说明Description of drawings
图1:眼镜王蛇肽与眼镜王蛇衍生肽测得的两亲性预测结果图;Figure 1: The results of amphiphilicity prediction measured by king cobra peptide and king cobra derived peptide;
图2:PLGA(CATH20)微球的扫描电镜电镜照片图;Figure 2: SEM photo of PLGA (CATH20) microspheres;
图3:抗菌肽PLGA(CATH20)微球的体外释放曲线图。Figure 3: The in vitro release curve of the antimicrobial peptide PLGA (CATH20) microspheres.
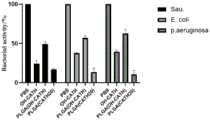
图4:抗菌肽PLGA(CATH20)微球的体外抑菌效果对比图。Figure 4: Comparison of antibacterial effect of antibacterial peptide PLGA (CATH20) microspheres in vitro.
图5:抗菌肽PLGA(CATH20)微球动物试验效果图。Figure 5: Effect diagram of antimicrobial peptide PLGA (CATH20) microspheres animal test.
具体实施方式Detailed ways
下面结合具体实施例对本发明作进一步描述,以助于更好地理解本发明,但本发明的保护范围并不仅限于这些实施例。The present invention will be further described below in conjunction with specific examples to help better understand the present invention, but the protection scope of the present invention is not limited to these examples.
实施例1:眼镜王蛇衍生肽的制备Example 1: Preparation of King Cobra Derived Peptides
眼镜王蛇肽氨基酸序列为KRFKKFFKKLKNSVKKRAKKFFKKP RVIGVSIPF,由34个氨基酸组成,分子量为4155.73道尔顿,等电点为12.34,所有氨基酸均为L型。由于眼镜王蛇肽的氨基酸序列中亲水性官能团较多,序列较长,申请人发现直接使用眼镜王蛇肽所制备的微球不稳定,容易发生突释,从而导致抗菌肽的生物利用度较低,抑菌效果降低,因此用眼镜王蛇肽氨基酸序列为模板进行改造。The amino acid sequence of king cobra peptide is KRFKKFFKKLKNSVKKRAKKFFKKP RVIGVSIPF, which consists of 34 amino acids, with a molecular weight of 4155.73 Daltons and an isoelectric point of 12.34. All amino acids are L-form. Since the amino acid sequence of the king cobra peptide has more hydrophilic functional groups and the sequence is longer, the applicant found that the microspheres prepared directly using the king cobra peptide are unstable and prone to burst release, which leads to the bioavailability of the antimicrobial peptide. Lower, the antibacterial effect is reduced, so the amino acid sequence of the king cobra peptide was used as a template for transformation.
具体就是截取该成熟肽C-端的20个氨基酸,并将该20个氨基酸序列中的第十八位非极性疏水氨基酸异亮氨酸(Ile)用极性疏水性氨基酸谷氨酰胺(Gln)替代,将该序列的N-端酰胺化,得到衍生肽CATH20,序列交由生物公司合成。Specifically, 20 amino acids at the C-terminal of the mature peptide are intercepted, and the eighteenth non-polar hydrophobic amino acid isoleucine (Ile) in the 20 amino acid sequence is replaced with a polar hydrophobic amino acid glutamine (Gln) Instead, the N-terminus of the sequence was amidated to obtain a derivative peptide CATH20, and the sequence was synthesized by a biological company.
改造后的衍生肽CATH20与眼镜王蛇肽OH-CATH的抗菌活性相似,低溶血性、且毒性低,保留了其两亲性的特征。如图1所示,眼镜王蛇衍生肽和眼镜王蛇肽的的总体疏水值分布都是从N-末端到C-末端不断增加,形成了明显的两亲性趋势,保证了CATH20与OH-CATH相似的抗菌活性。The modified derivative peptide CATH20 has similar antibacterial activity to king cobra peptide OH-CATH, has low hemolyticity and low toxicity, and retains its amphipathic characteristics. As shown in Figure 1, the overall hydrophobic value distribution of the king cobra-derived peptide and the king cobra peptide increases continuously from the N-terminus to the C-terminus, forming an obvious amphipathic trend, ensuring that CATH20 and OH- Antibacterial activity similar to CATH.
本发明对眼镜王蛇衍生肽进行改造,保留原肽的活性片段及两亲性的特征,缩短肽序列且对序列中的氨基酸进行替换,避免后期微球制备过程中发生突释,提高肽的生物利用度,从而改善所制备的微球的抑菌效果,且减小了微球包埋的难度,降低了肽的合成成本及纯化难度。The invention transforms the king cobra-derived peptide, retains the active fragment and amphipathic characteristics of the original peptide, shortens the peptide sequence and replaces the amino acids in the sequence, avoids sudden release in the later stage of microsphere preparation, and improves the peptide Bioavailability, thereby improving the antibacterial effect of the prepared microspheres, reducing the difficulty of embedding the microspheres, and reducing the synthesis cost and purification difficulty of the peptide.
实施例2:制备抗菌肽(CATH20)PLGA微球Embodiment 2: Preparation of antimicrobial peptide (CATH20) PLGA microspheres
(1)称取10mg抗菌肽(CATH20)和40mgPLGA于烧杯中备用,PLGA的分子量为29000;(1) Weigh 10 mg antimicrobial peptide (CATH20) and 40 mg PLGA in a beaker for standby use, the molecular weight of PLGA is 29000;
(2)配内水相:将称取好的CATH20用1ml ddH2O溶解,形成CATH20溶液,浓度为10mg/ml;(2) Prepare the inner water phase: dissolve the weighed CATH20 with 1ml ddH2O to form a CATH20 solution with a concentration of 10mg/ml;
(3)配有机相:将称取好的PLGA用5ml二氯甲烷溶解,得到PLGA溶液,浓度为8mg/ml;(3) Equipped with an organic phase: dissolve the weighed PLGA with 5ml of dichloromethane to obtain a PLGA solution with a concentration of 8mg/ml;
(4)用注射器将(2)内水相缓慢的注射到(3)有机相中,超声功率为150w,时间为90s,形成W/O初乳,;(4) Slowly inject (2) the inner water phase into (3) the organic phase with a syringe, the ultrasonic power is 150w, and the time is 90s to form W/O colostrum;
(5)将初乳用注射器注入15ml的1% PVA溶液中并进行超声,超声功率为100w,时间为60s,形成W/O/W复乳;(5) Inject the colostrum into 15ml of 1% PVA solution with a syringe and perform ultrasonication, the ultrasonic power is 100w, and the time is 60s to form W/O/W double emulsion;
(6)将(5)所得的复乳倾入20ml的0.75% PVA溶液中,在室温下进行搅拌,使微球固化并使有机溶剂完全挥发即得到PLGA(CATH20)微球混合溶液;15000rpm,4℃离心10min后,弃上清用PBS重悬反复冲洗三次,低温的冷冻干燥48h,即得抗菌肽PLGA(CATH20)微球,密封保存于4℃冰箱。(6) Pour the double emulsion obtained in (5) into 20ml of 0.75% PVA solution, stir at room temperature, make the microsphere solidify and make the organic solvent completely volatilize to obtain the PLGA (CATH20) microsphere mixed solution; 15000rpm, After centrifugation at 4°C for 10 minutes, the supernatant was discarded, resuspended in PBS, washed three times repeatedly, and freeze-dried at low temperature for 48 hours to obtain antimicrobial peptide PLGA (CATH20) microspheres, which were sealed and stored in a refrigerator at 4°C.
取2.0ml混悬液离心管中,15000r/min离心15min,取上清,过0.22um的滤器,用高效液相色谱法测定CATH20在PLGA中游离的CATH20含量;计算包封率,公式分别为包封率=[(m1-m2)/m1]×100%。m1:实际投入的CATH20量、m2:实游离的CATH20。结果表明制备的微球较高的包埋率可达到87.6%。Take 2.0ml of the suspension in a centrifuge tube, centrifuge at 15000r/min for 15min, take the supernatant, pass through a 0.22um filter, and measure the free CATH20 content of CATH20 in PLGA by high performance liquid chromatography; calculate the encapsulation efficiency, the formula is respectively Encapsulation efficiency=[(m1-m2)/m1]×100%. m1: the amount of CATH20 actually injected, m2: the actual free CATH20. The results showed that the high embedding efficiency of the prepared microspheres could reach 87.6%.
实施例3:PLGA(CATH20)微球表征的测定Example 3: Determination of PLGA (CATH20) Microsphere Characterization
(1)颗粒大小和zeta电位(1) Particle size and zeta potential
采用Zetasizer-nano-ZS动态光散射法测定了实施例2制备的三组纳米颗粒的平均直径、PDI和zeta电位(n=5),结果如表1所示,可以看出The average diameter, PDI and zeta potential (n=5) of three groups of nanoparticles prepared in Example 2 were measured by Zetasizer-nano-ZS dynamic light scattering method, and the results are shown in Table 1, as can be seen
实施例2所制备的微球在合适的PDI范围下测得的直径大约为(728±4.87)nm,zeta电位为(-26.36±1.81)mv。表明颗粒大小符合眼部给药的要求,且ζ电位较稳定,不易聚集。The diameter of the microspheres prepared in Example 2 is about (728±4.87) nm measured under a suitable PDI range, and the zeta potential is (-26.36±1.81) mv. It shows that the size of the particles meets the requirements of eye administration, and the ζ potential is relatively stable, and it is not easy to aggregate.
表1:PLGA(CATH20)微球所测得的ζ电位和粒径检测结果表Table 1: ζ potential and particle size detection results table measured by PLGA (CATH20) microspheres
(2)表面形貌的测定(2) Determination of surface morphology
将冻干完成的PLGA微球粉末用导电胶黏附在样品座上,镀金后,用扫描电镜在千伏下分析即获得包载抗菌肽PLGA(CATH20)微球的电镜图,图2为相应PLGA聚合物微球的电镜图片;Adhere the freeze-dried PLGA microsphere powder on the sample holder with conductive glue, and after gold-plating, use a scanning electron microscope to analyze at kilovolts to obtain the electron micrograph of PLGA (CATH20) microspheres loaded with antimicrobial peptides. Figure 2 shows the corresponding PLGA Electron microscope pictures of polymer microspheres;
根据图2,可以看出实施例2所制备的微球的电镜照片显示负载抗菌肽PLGA(CATH20)微球呈规则性球状,且微球尺寸均一,分散性好,未发生结块。According to Figure 2, it can be seen that the electron micrographs of the microspheres prepared in Example 2 show that the microspheres loaded with antimicrobial peptide PLGA (CATH20) are regular spherical, and the microspheres are uniform in size, good in dispersion, and do not agglomerate.
实施例4:体外药物释放曲线的测定Embodiment 4: the determination of drug release curve in vitro
按照实施例2的方法制备携带氨基酸序列为SEQ ID NO:1的多肽的微球PLGA(OH-CATH)、携带氨基酸序列为SEQ ID NO:2的多肽的PLGA(CATH20)微球。称取冻干纳米颗粒(10mg)重悬在1ml去离子水中。悬浮液在25℃下摇动(200rpm)。样品在预定的时间间隔内取样(0.5h、1h、2h、4h、6h、8h、12h,24h、48h),取样后以14 000rpm离心10分钟,并通过0.22μm过滤器过滤以除去剩余的颗粒。通过HPLC方法测定游离肽的含量,得到图3。According to the method of Example 2, microspheres PLGA (OH-CATH) carrying the polypeptide with the amino acid sequence of SEQ ID NO: 1 and PLGA (CATH20) microspheres carrying the polypeptide with the amino acid sequence of SEQ ID NO: 2 were prepared. Weigh lyophilized nanoparticles (10 mg) and resuspend in 1 ml deionized water. The suspension was shaken (200 rpm) at 25°C. Samples were taken at predetermined time intervals (0.5h, 1h, 2h, 4h, 6h, 8h, 12h, 24h, 48h), centrifuged at 14 000 rpm for 10 min after sampling, and filtered through a 0.22 μm filter to remove remaining particles . The content of free peptide was determined by HPLC method, and Figure 3 was obtained.
根据图3,可以看出所制得的PLGA (CATH20)载药微球主要表现为初级快速释放和后期缓慢释放两种状态,在第24小时累计释放量为(86.9±2.23)%,可以满足在较长时间内持续释放药物。According to Figure 3, it can be seen that the prepared PLGA (CATH20) drug-loaded microspheres mainly exhibit two states of primary rapid release and later slow release, and the cumulative release amount at 24 hours is (86.9±2.23)%, which can meet the requirements of Sustained drug release over an extended period of time.
而PLGA(OH-CATH)微球在2小时内快速释放,用药后可能会导致肽在眼部的浓度快速升高,眼睛无法快速吸收,不能达到预期的缓释杀菌效果。However, PLGA (OH-CATH) microspheres are released quickly within 2 hours, which may lead to a rapid increase in the concentration of peptides in the eyes after administration, and the eyes cannot be absorbed quickly, and the expected slow-release bactericidal effect cannot be achieved.
实施例5:体外抑菌试验的测定Embodiment 5: the mensuration of in vitro antibacterial test
将PLGA(OH-CATH)、PLGA(CATH20)微球用于体外大肠杆菌、铜绿假单胞菌、金黄色葡萄球菌评价其体外抑菌效果。将三种细菌于液体培养基中培养至其对数期,用PBS重悬制成105CFU/mL菌悬液。将菌液分别与PBS、OH-CATH(100μg/m)、PLGA(OH-CATH)(100μg/ml)、PLGA(CATH20)(100μg/ml)共同孵育24h,取20μl的菌液涂布平板,放入培养箱37℃培养24h,计算其细菌活力。PLGA (OH-CATH) and PLGA (CATH20) microspheres were used for Escherichia coli, Pseudomonas aeruginosa, and Staphylococcus aureus in vitro to evaluate their antibacterial effects in vitro. The three kinds of bacteria were cultured to their logarithmic phase in liquid medium, and resuspended with PBS to make 105 CFU/mL bacterial suspension. Incubate the bacterial solution with PBS, OH-CATH (100 μg/m), PLGA (OH-CATH) (100 μg/ml), and PLGA (CATH20) (100 μg/ml) for 24 hours, and take 20 μl of the bacterial solution to coat the plate. Put them into an incubator for 24 hours at 37°C, and calculate their bacterial viability.
从图4中可以看出PLGA(OH-CATH)微球的抑菌效果明显低于PLGA(CATH20)微球的抑菌效果,且改造得到的衍生肽所制得微球的抑菌活性高于单独使用眼镜王蛇肽的抑菌活性。It can be seen from Figure 4 that the antibacterial effect of PLGA (OH-CATH) microspheres is significantly lower than that of PLGA (CATH20) microspheres, and the antibacterial activity of the microspheres prepared by the modified derivative peptide is higher than that of Bacteriostatic activity of king cobra peptides alone.
实施例6:单次及多次给药兔眼刺激性试验Embodiment 6: Single and multiple administration rabbit eye irritation test
取9只健康家兔(雌雄兼用)随机分成3组,各组左眼滴加100μl的生理盐水作为对照,右眼滴加100μl的含有PLGA(CATH20)微球200μg/ml的生理盐水。给药后1、2、4、24、48和72h对眼部进行检查。依据“Draize”眼部刺激试验评分标准”对各样品进行刺激性评价。眼刺激性评价标准:无刺激性:0~3分;轻度刺激性:4~8分;中度刺激性:9~12分,;重度刺激性:13~16分。Take 9 healthy rabbits (both male and female) and divide them into 3 groups at random. The left eye of each group is dripped with 100 μl of normal saline as a control, and the right eye is dripped with 100 μl of normal saline containing 200 μg/ml of PLGA (CATH20) microspheres. The eyes were examined 1, 2, 4, 24, 48 and 72 hours after administration. According to the "Draize" Eye Irritation Test Scoring Standard", the irritation evaluation of each sample was carried out. Eye irritation evaluation standards: no irritation: 0-3 points; mild irritation: 4-8 points; moderate irritation: 9 ~12 points; severe irritation: 13~16 points.
选取9只健康家兔(雌雄兼用),随机分成3组,各组左眼滴加100μl的生理盐水作为对照,右眼滴加100μl的含有PLGA(CATH20)微球200μg/ml的生理盐水。每天3次,连续7天。每次给药前及给药后1、2、4、24、48和72h对眼部进行检查,根据“Draize眼部刺激试验评分标准”对各样品进行刺激性评价,结果如表2所示。Select 9 healthy rabbits (both male and female) and divide them into 3 groups at random. The left eye of each group is dripped with 100 μl of normal saline as a control, and the right eye is dripped with 100 μl of normal saline containing 200 μg/ml of PLGA (CATH20) microspheres. 3 times a day for 7 consecutive days. Before each administration and 1, 2, 4, 24, 48 and 72 hours after administration, the eyes were inspected, and the irritation evaluation of each sample was carried out according to the "Draize eye irritation test scoring standard", and the results are shown in Table 2 .
按评分标准评分,单次给药结果显示生理盐水组平均积分值为0分,PLGA(CATH20)溶液组为2分。According to the scoring standard, the results of a single administration showed that the average integral value of the normal saline group was 0, and that of the PLGA (CATH20) solution group was 2 points.
按评分标准评分,多次给药结果显示生理盐水组平均积分值为1分,PLGA(CATH20)溶液组为4分。According to the scoring standard, the results of multiple administrations showed that the average integral value of the normal saline group was 1 point, and that of the PLGA (CATH20) solution group was 4 points.
本试验结果显示,由实施例2制备的PLGA(CATH20)溶液单次给药和多次给药对眼均无明显刺激性。The results of this test show that the PLGA (CATH20) solution prepared in Example 2 has no obvious irritation to the eyes in a single administration and in multiple administrations.
表2:抗菌肽PLGA(CATH20)微球“Draize”眼部刺激试验评分表Table 2: Antimicrobial peptide PLGA (CATH20) microspheres "Draize" eye irritation test score table
实施例7:眼部治疗试验Example 7: Eye Therapy Test
选取6只健康试验家兔(雌雄兼用),随机分成3组,用金黄色葡萄球菌和绿脓杆菌进行攻毒,然后实例2所制备的微球进行治疗,观察治疗效果、副作用测定。治疗前后家兔眼部变化,如图5。Select 6 healthy experimental rabbits (both male and female), randomly divide them into 3 groups, challenge with Staphylococcus aureus and Pseudomonas aeruginosa, then treat with the microspheres prepared in Example 2, observe the therapeutic effect and measure the side effects. The eye changes of rabbits before and after treatment are shown in Figure 5.
由图5,可以看出经过治疗后,家兔眼部炎症明显减轻,在治疗后第三天脓性分泌液已经明显减少。第7天已无脓性分泌液,角膜透亮,结膜粉红色。From Figure 5, it can be seen that after treatment, the inflammation of the rabbit's eyes was significantly reduced, and the purulent secretion had been significantly reduced on the third day after treatment. On the 7th day, there was no purulent secretion, the cornea was clear, and the conjunctiva was pink.
Claims (10)
Translated fromChinesePriority Applications (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| CN202310135438.XACN116396372B (en) | 2023-02-18 | 2023-02-18 | A peptide-loaded nanoparticle and its application in treating pet eye infection |
Applications Claiming Priority (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| CN202310135438.XACN116396372B (en) | 2023-02-18 | 2023-02-18 | A peptide-loaded nanoparticle and its application in treating pet eye infection |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| CN116396372Atrue CN116396372A (en) | 2023-07-07 |
| CN116396372B CN116396372B (en) | 2024-05-24 |
Family
ID=87013063
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| CN202310135438.XAActiveCN116396372B (en) | 2023-02-18 | 2023-02-18 | A peptide-loaded nanoparticle and its application in treating pet eye infection |
Country Status (1)
| Country | Link |
|---|---|
| CN (1) | CN116396372B (en) |
Citations (7)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN102311492A (en)* | 2010-07-09 | 2012-01-11 | 中国科学院昆明动物研究所 | Non-natural fully D-type snake venom cathelicidin antibacterial peptide and derivative, preparation method as well as application thereof |
| CN102702333A (en)* | 2012-05-29 | 2012-10-03 | 中国药科大学 | Drug-resistant pathogen infection resistant polypeptide and uses thereof |
| WO2017176041A1 (en)* | 2016-04-04 | 2017-10-12 | 건국대학교 산학협력단 | Novel antimicrobial peptide isolated from python and method for discovering same |
| CN113754738A (en)* | 2021-08-30 | 2021-12-07 | 国家纳米科学中心 | A polypeptide monomer molecule and its self-assembled nanomaterials and applications |
| CN115536738A (en)* | 2022-04-13 | 2022-12-30 | 江苏亢钧生物科技有限公司 | Application of king cobra antimicrobial peptide OH-CATH30 in the treatment of fish enteritis |
| CN115536737A (en)* | 2022-04-13 | 2022-12-30 | 江苏亢钧生物科技有限公司 | Application of cobra antibacterial peptide OH-CATH30 in resisting aquatic animal pathogenic bacteria |
| CN116333163A (en)* | 2022-09-29 | 2023-06-27 | 国家纳米科学中心 | Recombinant fusion protein and application thereof |
- 2023
- 2023-02-18CNCN202310135438.XApatent/CN116396372B/enactiveActive
Patent Citations (7)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN102311492A (en)* | 2010-07-09 | 2012-01-11 | 中国科学院昆明动物研究所 | Non-natural fully D-type snake venom cathelicidin antibacterial peptide and derivative, preparation method as well as application thereof |
| CN102702333A (en)* | 2012-05-29 | 2012-10-03 | 中国药科大学 | Drug-resistant pathogen infection resistant polypeptide and uses thereof |
| WO2017176041A1 (en)* | 2016-04-04 | 2017-10-12 | 건국대학교 산학협력단 | Novel antimicrobial peptide isolated from python and method for discovering same |
| CN113754738A (en)* | 2021-08-30 | 2021-12-07 | 国家纳米科学中心 | A polypeptide monomer molecule and its self-assembled nanomaterials and applications |
| CN115536738A (en)* | 2022-04-13 | 2022-12-30 | 江苏亢钧生物科技有限公司 | Application of king cobra antimicrobial peptide OH-CATH30 in the treatment of fish enteritis |
| CN115536737A (en)* | 2022-04-13 | 2022-12-30 | 江苏亢钧生物科技有限公司 | Application of cobra antibacterial peptide OH-CATH30 in resisting aquatic animal pathogenic bacteria |
| CN116333163A (en)* | 2022-09-29 | 2023-06-27 | 国家纳米科学中心 | Recombinant fusion protein and application thereof |
Non-Patent Citations (3)
| Title |
|---|
| FENG ZHAO: "King cobra peptide OH-CATH30 as a potential candidate drug through clinic drug-resistant isolates", 《ZOOLOGICAL RESEARCH》, vol. 39, no. 2, 31 December 2018 (2018-12-31), pages 87 - 96* |
| SHENG-AN LI: "Therapeutic Potential of the Antimicrobial Peptide OH-CATH30 for Antibiotic-Resistant Pseudomonas aeruginosa Keratitis", 《ANTIMICROBIAL AGENTS AND CHEMOTHERAPY》, vol. 58, no. 6, 17 March 2014 (2014-03-17), pages 3144* |
| 李盛安: "眼镜王蛇抗菌肽OH-CATH30选择性地调节天然免疫反应抵御细菌感染", 《第十一届中国生物毒素研究及医药应用年会论文摘要集》, 17 October 2013 (2013-10-17)* |
Also Published As
| Publication number | Publication date |
|---|---|
| CN116396372B (en) | 2024-05-24 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| Biswaro et al. | Antimicrobial peptides and nanotechnology, recent advances and challenges | |
| Das et al. | Sericin based nanoformulations: a comprehensive review on molecular mechanisms of interaction with organisms to biological applications | |
| Li et al. | Antibacterial hydrogels | |
| Sun et al. | Carboxymethyl chitosan nanoparticles loaded with bioactive peptide OH-CATH30 benefit nonscar wound healing | |
| Borro et al. | Microgels and hydrogels as delivery systems for antimicrobial peptides | |
| Oyarzun-Ampuero et al. | Nanoparticles for the treatment of wounds | |
| Kłodzińska et al. | Hyaluronic acid-based nanogels improve in vivo compatibility of the anti-biofilm peptide DJK-5 | |
| Vijayan et al. | Multiple cargo deliveries of growth factors and antimicrobial peptide using biodegradable nanopolymer as a potential wound healing system | |
| Lu et al. | Imidazole-molecule-capped chitosan–gold nanocomposites with enhanced antimicrobial activity for treating biofilm-related infections | |
| Jin et al. | Urokinase-coated chitosan nanoparticles for thrombolytic therapy: preparation and pharmacodynamics in vivo | |
| CN101189020A (en) | Compositions for administration of RNA III-inhibitory peptides | |
| Peng et al. | Facile design of gemini surfactant-like peptide for hydrophobic drug delivery and antimicrobial activity | |
| Nordström et al. | Degradable dendritic nanogels as carriers for antimicrobial peptides | |
| Chen et al. | Biomimetic nucleation of Metal–Organic frameworks on silk fibroin nanoparticles for designing Core–Shell-Structured pH-Responsive anticancer drug carriers | |
| Ryan et al. | The development of a solid lipid nanoparticle (SLN)-based lacticin 3147 hydrogel for the treatment of wound infections | |
| Xu et al. | Antimicrobial peptide functionalized gold nanorods combining near-infrared photothermal therapy for effective wound healing | |
| Ong et al. | Nanoparticular and other carriers to deliver lactoferrin for antimicrobial, antibiofilm and bone-regenerating effects: a review | |
| Lin et al. | Advances in delivery systems for the therapeutic application of LL37 | |
| KR20070083927A (en) | Method for forming morphology aggregates of gel particles and uses thereof | |
| Zhu et al. | Antibacterial peptide encapsulation and sustained release from chitosan-based delivery system | |
| Bayat et al. | Evaluation of debridement effects of bromelain-loaded sodium alginate nanoparticles incorporated into chitosan hydrogel in animal models | |
| Alamdaran et al. | In-vitro study of the novel nanocarrier of chitosan-based nanoparticles conjugated HIV-1 P24 protein-derived peptides | |
| Xu et al. | A novel approach of curcumin loaded chitosan/dextran nanocomposite for the management of complicated abdominal wound dehiscence | |
| Srivastava et al. | In vitro coronal protein signatures and biological impact of silver nanoparticles synthesized with different natural polymers as capping agents | |
| CN110496229A (en) | Nanoparticle-coated antimicrobial peptide with sustained release and preparation method thereof |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PB01 | Publication | ||
| PB01 | Publication | ||
| SE01 | Entry into force of request for substantive examination | ||
| SE01 | Entry into force of request for substantive examination | ||
| GR01 | Patent grant | ||
| GR01 | Patent grant | ||
| CB03 | Change of inventor or designer information | Inventor after:Qin Zhihua Inventor after:Jiao Xiaoqian Inventor after:Dong Xufeng Inventor after:Yu Dezheng Inventor after:Hao Zhipeng Inventor after:Zhang Zhongtao Inventor before:Jiao Xiaoqian Inventor before:Dong Xufeng Inventor before:Yu Dezheng Inventor before:Hao Zhipeng Inventor before:Zhang Zhongtao Inventor before:Qin Zhihua | |
| CB03 | Change of inventor or designer information |