CN116371441A - A kind of sulfur-containing carbon nitride material and its preparation method and its application in photocatalytic hydrogen production - Google Patents
A kind of sulfur-containing carbon nitride material and its preparation method and its application in photocatalytic hydrogen productionDownload PDFInfo
- Publication number
- CN116371441A CN116371441ACN202310168130.5ACN202310168130ACN116371441ACN 116371441 ACN116371441 ACN 116371441ACN 202310168130 ACN202310168130 ACN 202310168130ACN 116371441 ACN116371441 ACN 116371441A
- Authority
- CN
- China
- Prior art keywords
- sulfur
- containing carbon
- carbon nitride
- nitride material
- porcelain boat
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Granted
Links
- 239000000463materialSubstances0.000titleclaimsabstractdescription82
- JMANVNJQNLATNU-UHFFFAOYSA-NoxalonitrileChemical compoundN#CC#NJMANVNJQNLATNU-UHFFFAOYSA-N0.000titleclaimsabstractdescription79
- NINIDFKCEFEMDL-UHFFFAOYSA-NSulfurChemical compound[S]NINIDFKCEFEMDL-UHFFFAOYSA-N0.000titleclaimsabstractdescription77
- 229910052717sulfurInorganic materials0.000titleclaimsabstractdescription77
- 239000011593sulfurSubstances0.000titleclaimsabstractdescription77
- 238000004519manufacturing processMethods0.000titleclaimsabstractdescription38
- 229910052739hydrogenInorganic materials0.000titleclaimsabstractdescription37
- UFHFLCQGNIYNRP-UHFFFAOYSA-NHydrogenChemical compound[H][H]UFHFLCQGNIYNRP-UHFFFAOYSA-N0.000titleclaimsabstractdescription36
- 239000001257hydrogenSubstances0.000titleclaimsabstractdescription36
- 230000001699photocatalysisEffects0.000titleclaimsabstractdescription30
- 238000002360preparation methodMethods0.000titleabstractdescription15
- 238000001354calcinationMethods0.000claimsabstractdescription13
- 229910052573porcelainInorganic materials0.000claimsdescription24
- IJGRMHOSHXDMSA-UHFFFAOYSA-NAtomic nitrogenChemical compoundN#NIJGRMHOSHXDMSA-UHFFFAOYSA-N0.000claimsdescription22
- 229920000877Melamine resinPolymers0.000claimsdescription20
- JDSHMPZPIAZGSV-UHFFFAOYSA-NmelamineChemical compoundNC1=NC(N)=NC(N)=N1JDSHMPZPIAZGSV-UHFFFAOYSA-N0.000claimsdescription20
- 229910052757nitrogenInorganic materials0.000claimsdescription9
- ZFSLODLOARCGLH-UHFFFAOYSA-Nisocyanuric acidChemical compoundOC1=NC(O)=NC(O)=N1ZFSLODLOARCGLH-UHFFFAOYSA-N0.000claimsdescription6
- 238000000034methodMethods0.000claimsdescription6
- XKRFYHLGVUSROY-UHFFFAOYSA-NArgonChemical compound[Ar]XKRFYHLGVUSROY-UHFFFAOYSA-N0.000claimsdescription4
- 239000007789gasSubstances0.000claimsdescription4
- 229910052786argonInorganic materials0.000claimsdescription2
- 238000001816coolingMethods0.000claimsdescription2
- 238000010438heat treatmentMethods0.000claimsdescription2
- 239000011261inert gasSubstances0.000claimsdescription2
- 238000005303weighingMethods0.000claimsdescription2
- 238000003892spreadingMethods0.000claims2
- 230000000694effectsEffects0.000abstractdescription9
- 239000002994raw materialSubstances0.000abstractdescription6
- 239000000919ceramicSubstances0.000abstractdescription3
- 230000031700light absorptionEffects0.000abstractdescription2
- ZMZDMBWJUHKJPS-UHFFFAOYSA-Nthiocyanic acidChemical compoundSC#NZMZDMBWJUHKJPS-UHFFFAOYSA-N0.000description30
- 230000000052comparative effectEffects0.000description27
- 238000010586diagramMethods0.000description15
- 238000012360testing methodMethods0.000description12
- 239000000243solutionSubstances0.000description10
- 239000011941photocatalystSubstances0.000description9
- 238000009826distributionMethods0.000description5
- 239000011148porous materialSubstances0.000description5
- QGZKDVFQNNGYKY-UHFFFAOYSA-NAmmoniaChemical compoundNQGZKDVFQNNGYKY-UHFFFAOYSA-N0.000description4
- 238000011161developmentMethods0.000description4
- 229910001873dinitrogenInorganic materials0.000description4
- 238000005516engineering processMethods0.000description4
- 238000013508migrationMethods0.000description4
- 230000005012migrationEffects0.000description4
- XLYOFNOQVPJJNP-UHFFFAOYSA-NwaterSubstancesOXLYOFNOQVPJJNP-UHFFFAOYSA-N0.000description4
- WZRRRFSJFQTGGB-UHFFFAOYSA-N1,3,5-triazinane-2,4,6-trithioneChemical compoundS=C1NC(=S)NC(=S)N1WZRRRFSJFQTGGB-UHFFFAOYSA-N0.000description3
- GSEJCLTVZPLZKY-UHFFFAOYSA-NTriethanolamineChemical compoundOCCN(CCO)CCOGSEJCLTVZPLZKY-UHFFFAOYSA-N0.000description3
- 239000007864aqueous solutionSubstances0.000description3
- 239000004065semiconductorSubstances0.000description3
- 238000002336sorption--desorption measurementMethods0.000description3
- 238000013112stability testMethods0.000description3
- 229910052724xenonInorganic materials0.000description3
- FHNFHKCVQCLJFQ-UHFFFAOYSA-Nxenon atomChemical compound[Xe]FHNFHKCVQCLJFQ-UHFFFAOYSA-N0.000description3
- OKTJSMMVPCPJKN-UHFFFAOYSA-NCarbonChemical compound[C]OKTJSMMVPCPJKN-UHFFFAOYSA-N0.000description2
- 229910052799carbonInorganic materials0.000description2
- 239000003575carbonaceous materialSubstances0.000description2
- 230000003197catalytic effectEffects0.000description2
- 239000003426co-catalystSubstances0.000description2
- 230000007547defectEffects0.000description2
- 239000006185dispersionSubstances0.000description2
- 238000005265energy consumptionMethods0.000description2
- 229910002804graphiteInorganic materials0.000description2
- 239000010439graphiteSubstances0.000description2
- 238000001027hydrothermal synthesisMethods0.000description2
- 239000011229interlayerSubstances0.000description2
- 238000011068loading methodMethods0.000description2
- 239000011259mixed solutionSubstances0.000description2
- 238000002156mixingMethods0.000description2
- 239000000203mixtureSubstances0.000description2
- 238000012986modificationMethods0.000description2
- 230000004048modificationEffects0.000description2
- 239000000843powderSubstances0.000description2
- 238000000926separation methodMethods0.000description2
- 239000007787solidSubstances0.000description2
- 238000001228spectrumMethods0.000description2
- ZMZDMBWJUHKJPS-UHFFFAOYSA-MThiocyanate anionChemical compound[S-]C#NZMZDMBWJUHKJPS-UHFFFAOYSA-M0.000description1
- XSQUKJJJFZCRTK-UHFFFAOYSA-NUreaChemical compoundNC(N)=OXSQUKJJJFZCRTK-UHFFFAOYSA-N0.000description1
- 238000002441X-ray diffractionMethods0.000description1
- PFRUBEOIWWEFOL-UHFFFAOYSA-N[N].[S]Chemical class[N].[S]PFRUBEOIWWEFOL-UHFFFAOYSA-N0.000description1
- 229910021529ammoniaInorganic materials0.000description1
- 125000003118aryl groupChemical group0.000description1
- 230000009286beneficial effectEffects0.000description1
- 239000004202carbamideSubstances0.000description1
- 125000004432carbon atomChemical groupC*0.000description1
- 239000000969carrierSubstances0.000description1
- 239000003054catalystSubstances0.000description1
- 238000006555catalytic reactionMethods0.000description1
- 238000006243chemical reactionMethods0.000description1
- 239000003153chemical reaction reagentSubstances0.000description1
- 239000002131composite materialSubstances0.000description1
- 150000001875compoundsChemical class0.000description1
- 239000013078crystalSubstances0.000description1
- 125000004122cyclic groupChemical group0.000description1
- 230000007812deficiencyEffects0.000description1
- 238000003795desorptionMethods0.000description1
- 230000002708enhancing effectEffects0.000description1
- 238000002474experimental methodMethods0.000description1
- 229910021397glassy carbonInorganic materials0.000description1
- 229910052751metalInorganic materials0.000description1
- 239000002184metalSubstances0.000description1
- 150000002739metalsChemical class0.000description1
- 238000000696nitrogen adsorption--desorption isothermMethods0.000description1
- 125000004433nitrogen atomChemical groupN*0.000description1
- 229910000510noble metalInorganic materials0.000description1
- 229910052755nonmetalInorganic materials0.000description1
- 150000002843nonmetalsChemical class0.000description1
- 238000011056performance testMethods0.000description1
- 238000006116polymerization reactionMethods0.000description1
- 238000012805post-processingMethods0.000description1
- 239000002243precursorSubstances0.000description1
- 238000005215recombinationMethods0.000description1
- 230000006798recombinationEffects0.000description1
- 238000011160researchMethods0.000description1
- 239000002904solventSubstances0.000description1
- 238000001179sorption measurementMethods0.000description1
- 239000000126substanceSubstances0.000description1
- 238000001132ultrasonic dispersionMethods0.000description1
Images
Classifications
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01J—CHEMICAL OR PHYSICAL PROCESSES, e.g. CATALYSIS OR COLLOID CHEMISTRY; THEIR RELEVANT APPARATUS
- B01J27/00—Catalysts comprising the elements or compounds of halogens, sulfur, selenium, tellurium, phosphorus or nitrogen; Catalysts comprising carbon compounds
- B01J27/24—Nitrogen compounds
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01J—CHEMICAL OR PHYSICAL PROCESSES, e.g. CATALYSIS OR COLLOID CHEMISTRY; THEIR RELEVANT APPARATUS
- B01J27/00—Catalysts comprising the elements or compounds of halogens, sulfur, selenium, tellurium, phosphorus or nitrogen; Catalysts comprising carbon compounds
- B01J27/02—Sulfur, selenium or tellurium; Compounds thereof
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01J—CHEMICAL OR PHYSICAL PROCESSES, e.g. CATALYSIS OR COLLOID CHEMISTRY; THEIR RELEVANT APPARATUS
- B01J35/00—Catalysts, in general, characterised by their form or physical properties
- B01J35/30—Catalysts, in general, characterised by their form or physical properties characterised by their physical properties
- B01J35/39—Photocatalytic properties
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01J—CHEMICAL OR PHYSICAL PROCESSES, e.g. CATALYSIS OR COLLOID CHEMISTRY; THEIR RELEVANT APPARATUS
- B01J37/00—Processes, in general, for preparing catalysts; Processes, in general, for activation of catalysts
- B01J37/08—Heat treatment
- B01J37/082—Decomposition and pyrolysis
- C—CHEMISTRY; METALLURGY
- C01—INORGANIC CHEMISTRY
- C01B—NON-METALLIC ELEMENTS; COMPOUNDS THEREOF; METALLOIDS OR COMPOUNDS THEREOF NOT COVERED BY SUBCLASS C01C
- C01B3/00—Hydrogen; Gaseous mixtures containing hydrogen; Separation of hydrogen from mixtures containing it; Purification of hydrogen
- C01B3/02—Production of hydrogen or of gaseous mixtures containing a substantial proportion of hydrogen
- Y—GENERAL TAGGING OF NEW TECHNOLOGICAL DEVELOPMENTS; GENERAL TAGGING OF CROSS-SECTIONAL TECHNOLOGIES SPANNING OVER SEVERAL SECTIONS OF THE IPC; TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y02—TECHNOLOGIES OR APPLICATIONS FOR MITIGATION OR ADAPTATION AGAINST CLIMATE CHANGE
- Y02E—REDUCTION OF GREENHOUSE GAS [GHG] EMISSIONS, RELATED TO ENERGY GENERATION, TRANSMISSION OR DISTRIBUTION
- Y02E60/00—Enabling technologies; Technologies with a potential or indirect contribution to GHG emissions mitigation
- Y02E60/30—Hydrogen technology
- Y02E60/36—Hydrogen production from non-carbon containing sources, e.g. by water electrolysis
Landscapes
- Chemical & Material Sciences (AREA)
- Organic Chemistry (AREA)
- Engineering & Computer Science (AREA)
- Materials Engineering (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Physics & Mathematics (AREA)
- Thermal Sciences (AREA)
- Combustion & Propulsion (AREA)
- Inorganic Chemistry (AREA)
- Catalysts (AREA)
Abstract
Translated fromChineseDescription
Translated fromChinese技术领域technical field
本发明属于包含硫元素的氮的化合物的催化剂技术领域,具体涉及一种含硫氮化碳材料及其制备方法与在光催化产氢方面的应用。The invention belongs to the technical field of catalysts containing sulfur-nitrogen compounds, and specifically relates to a sulfur-containing carbon nitride material, a preparation method thereof, and an application in photocatalytic hydrogen production.
背景技术Background technique
合理开发资源尤其重要。地球上的水资源丰富,且太阳能取之不竭,用之不尽,所以利用太阳能分解水制氢是解决能源问题的关键途径之一。Rational development of resources is particularly important. The earth is rich in water resources, and solar energy is inexhaustible and inexhaustible, so using solar energy to split water to produce hydrogen is one of the key ways to solve energy problems.
光催化产氢技术将太阳能转化为氢能,是一种低成本、环保无污染的技术,反应条件温和,适用范围广,具有非常广阔的发展前景。光催化产氢技术需要光催化剂的催化作用,近几十年来,研究人员主要集中于氧化物的半导体光催化材料的研究,该类材料普遍存在易溶于酸碱试剂因而难以改性的特点,从而限制了其应用发展。Photocatalytic hydrogen production technology converts solar energy into hydrogen energy. It is a low-cost, environmentally friendly and pollution-free technology with mild reaction conditions and a wide range of applications. It has very broad development prospects. Photocatalytic hydrogen production technology requires the catalysis of photocatalysts. In recent decades, researchers have mainly focused on the research of oxide semiconductor photocatalytic materials. Such materials are generally soluble in acid-base reagents and thus difficult to modify. Thereby limiting its application development.
近年来,非金属类半导体光催化剂石墨相氮化碳(g-C3N4)材料进入人们视野,由于g-C3N4结构中sp2杂化的C和N原子形成了一定的共轭体系,使其具有优良的热稳定性、化学稳定性等性质,除此以外,g-C3N4还能够吸收可见光,制备工艺简单且成本低廉,在可见光照射和三乙醇胺作为空穴清除剂的情况下能够催化分解水制氢,在光催化产氢领域有大力发展的空间。然而,g-C3N4比表面积低,禁带宽度较宽约为2.72eV,光生电子-空穴易复合以及导电性低等缺点,导致催化活性有限,因而需要对其进行改性。目前可以通过掺杂(金属、非金属、贵金属等),In recent years, non-metallic semiconductor photocatalyst graphite phase carbon nitride (gC3 N4 ) materials have entered people's field of vision, because the sp2 hybridized C and N atoms in the gC3 N4 structure form a certain conjugated system, making It has excellent thermal stability, chemical stability and other properties. In addition, gC3 N4 can also absorb visible light. The preparation process is simple and low cost. It can catalyze There is room for great development in the field of photocatalytic hydrogen production by splitting water to produce hydrogen. However, gC3 N4 has disadvantages such as low specific surface area, wide band gap of about 2.72eV, easy recombination of photogenerated electron-holes, and low conductivity, resulting in limited catalytic activity, so it needs to be modified. At present, through doping (metals, non-metals, noble metals, etc.),
形貌调控,半导体复合改性以及负载助催化剂等手段对g-C3N4进行改性,调控材料组成或改变比表面积等性质来改善上述缺点,从而提高光催化活性。当对g-C3N4进行硫掺杂时,通过调控g-C3N4组成、电子结构和片层厚薄,可增大比表面积的同时使其产生一定的缺陷,暴露出更多的活性位点,提高光催化产氢的性能。专利CN111185216A公开了将三聚氰胺与三聚硫氰酸混合于溶剂中,再进行水热反应,得到实心管状的复合物,再与尿素煅烧得到中空管状硫掺杂氮化碳/石墨相氮化碳同质结光催化剂的方案。专利CN107930667A公开了一种通过三聚氰胺和三聚硫氰酸分别溶于热水后混合均匀,水热反应后煅烧制备得到硫掺杂的氮化碳的制备方法。这几种公开的制备方法步骤繁琐复杂,需要后处理,成本相对较高。Modification of gC3 N4 by means of morphology control, semiconductor composite modification and loading co-catalysts, etc. can improve the above shortcomings by adjusting the material composition or changing the specific surface area and other properties, thereby improving the photocatalytic activity. When gC3 N4 is doped with sulfur, by adjusting the composition, electronic structure and sheet thickness of gC3 N4 , the specific surface area can be increased and certain defects can be generated to expose more active sites. Improve the performance of photocatalytic hydrogen production. Patent CN111185216A discloses mixing melamine and cyanuric acid in a solvent, then carrying out a hydrothermal reaction to obtain a solid tubular compound, and then calcining with urea to obtain a hollow tubular sulfur-doped carbon nitride/graphite phase carbon nitride. Scheme of mass-junction photocatalyst. Patent CN107930667A discloses a method for preparing sulfur-doped carbon nitride by dissolving melamine and thiocyanic acid in hot water, mixing them uniformly, and calcining after hydrothermal reaction. The steps of these several disclosed preparation methods are cumbersome and complicated, require post-processing, and the cost is relatively high.
由此看来,还需发展一些操作更加简便,能耗成本更低,效果更好的实验方法,从而应用于光催化产氢领域。本发明只采用了三聚硫氰酸和三聚氰胺两种原料,分开称量后不进行直接接触,放入管式炉内直接煅烧即得到含硫氮化碳材料,操作简便,能耗低,且制备的含硫氮化碳材料光电流响应性好,产氢速率达165.4μmol/h,并且有良好的循环产氢稳定性。From this point of view, it is necessary to develop some experimental methods with simpler operation, lower energy consumption cost and better effect, so as to be applied in the field of photocatalytic hydrogen production. The present invention only adopts two raw materials of thiocyanic acid and melamine, which are weighed separately without direct contact, put into a tube furnace and directly calcined to obtain sulfur-containing carbon nitride material, which is easy to operate, low in energy consumption, and The prepared sulfur-containing carbon nitride material has good photocurrent response, a hydrogen production rate of 165.4 μmol/h, and good cycle hydrogen production stability.
发明内容Contents of the invention
本发明所要解决的技术问题是针对现有技术中存在的上述不足,提供一种含硫氮化碳材料及其制备方法与在光催化产氢方面的应用,所述含硫氮化碳材料比表面积大,对可见光响应敏感,光催化产氢活性高,制备方法简单易操作,重复性好。The technical problem to be solved by the present invention is to provide a sulfur-containing carbon nitride material and its preparation method and its application in photocatalytic hydrogen production in view of the above-mentioned deficiencies in the prior art. The surface area is large, the response to visible light is sensitive, the photocatalytic hydrogen production activity is high, the preparation method is simple and easy to operate, and the repeatability is good.
为解决上述技术问题,本发明提供的技术方案是:In order to solve the problems of the technologies described above, the technical solution provided by the invention is:
提供一种含硫氮化碳材料,其由三聚硫氰酸在与三聚氰胺非直接接触下于惰性气氛下煅烧得到。Provided is a sulfur-containing carbon nitride material, which is obtained by calcining thiocyanic acid in an inert atmosphere under non-direct contact with melamine.
按上述方案,所述含硫氮化碳材料比表面积为50~150m2/g。According to the above solution, the specific surface area of the sulfur-containing carbon nitride material is 50-150 m2 /g.
本发明还提供上述含硫氮化碳材料的制备方法,具体步骤如下:The present invention also provides a preparation method for the above sulfur-containing carbon nitride material, the specific steps are as follows:
1)按比例称取三聚硫氰酸、三聚氰胺,备用;1) Take thiocyanic acid and melamine in proportion, for subsequent use;
2)将三聚硫氰酸均匀平铺于1号瓷舟表面,将三聚氰胺均匀平铺于2号瓷舟表面,然后将1号瓷舟放入管式炉恒温区域正中间,2号瓷舟放于管式炉内靠近进气口处,经进气口向管式炉内通入惰性气体,加热进行煅烧,随炉冷却至室温,收集1号瓷舟内样品研磨得到含硫氮化碳材料。2) Evenly spread thiocyanic acid on the surface of No. 1 porcelain boat, evenly spread melamine on the surface of No. 2 porcelain boat, then put No. 1 porcelain boat into the middle of the constant temperature area of the tube furnace, and No. 2 porcelain boat Put it in the tube furnace close to the air inlet, pass inert gas into the tube furnace through the air inlet, heat for calcination, cool down to room temperature with the furnace, collect the samples in No. 1 porcelain boat and grind to obtain sulfur-containing carbon nitride Material.
按上述方案,步骤1)所述三聚硫氰酸纯度≥95wt%,所述三聚氰胺纯度≥According to the above-mentioned scheme, step 1) described thiocyanate purity ≥ 95wt%, described melamine purity ≥
99wt%。99% by weight.
按上述方案,步骤1)所述三聚硫氰酸与三聚氰胺的质量比为0.5~2:1。According to the above scheme, the mass ratio of thiocyanic acid to melamine in step 1) is 0.5-2:1.
按上述方案,步骤2)所述1号瓷舟与2号瓷舟中心距离为10~20cm。According to the above scheme, the distance between the centers of No. 1 porcelain boat and No. 2 porcelain boat described in step 2) is 10-20 cm.
按上述方案,步骤2)所述惰性气氛为氮气或氩气,气体纯度为99.99vol%以上,气体流量为60~100mL/min。According to the above scheme, the inert atmosphere in step 2) is nitrogen or argon, the gas purity is above 99.99 vol%, and the gas flow rate is 60-100 mL/min.
按上述方案,步骤2)所述煅烧工艺条件为:室温下以5~15℃/min的升温速率升温至500~600℃,保温3~5h。According to the above scheme, the calcination process conditions in step 2) are as follows: at room temperature, the temperature is raised to 500-600° C. at a heating rate of 5-15° C./min, and the temperature is kept for 3-5 hours.
本发明还包括上述含硫氮化碳材料在光催化产氢方面的应用。The present invention also includes the application of the above-mentioned sulfur-containing carbon nitride material in photocatalytic hydrogen production.
本发明将三聚硫氰酸与三聚氰胺在非直接接触的状态下于惰性气氛下煅烧得到含硫氮化碳材料,三聚硫氰酸为前驱体,煅烧过程中三聚氰胺产生氨气流动于三聚硫氰酸表面,在三聚硫氰酸聚合产生含硫氮化碳过程中有一定的剥离作用,生成结晶度高并具有一定缺陷的含硫氮化碳材料,测试结果表明该含硫氮化碳材料光催化产氢性能优异。In the present invention, thiocyanic acid and melamine are calcined under an inert atmosphere in a non-direct contact state to obtain a sulfur-containing carbon nitride material, and thiocyanic acid is used as a precursor. The surface of thiocyanic acid has a certain peeling effect during the polymerization of thiocyanic acid to produce sulfur-containing carbon nitride, and a sulfur-containing carbon nitride material with high crystallinity and certain defects is formed. The test results show that the sulfur-containing carbon nitride Carbon materials have excellent photocatalytic hydrogen production performance.
本发明的有益效果在于:1、本发明提供的含硫氮化碳材料比表面积大,光吸收能力强,光催化产氢活性高,且循环稳定性优异,具有良好的光催化产氢应用前景。2、本发明只采用三聚硫氰酸和三聚氰胺两种原料,间接利用三聚氰胺产生氨气进行剥离,不直接利用大量氨气气体进行直接接触,危险性降低,安全性好,合成原料简单,且操作步骤少,将按比例称取的两种原料分别放入置于管式炉内且保持一定距离的两瓷舟中一步煅烧即可得到含硫氮化碳材料,成本低。The beneficial effects of the present invention are: 1. The sulfur-containing carbon nitride material provided by the present invention has a large specific surface area, strong light absorption capacity, high photocatalytic hydrogen production activity, and excellent cycle stability, and has good application prospects for photocatalytic hydrogen production . 2. The present invention only adopts two kinds of raw materials, melamine and melamine, and indirectly utilizes melamine to generate ammonia gas for peeling, without directly using a large amount of ammonia gas for direct contact, thus reducing risk, good safety, simple synthetic raw materials, and The operation steps are few, and the two raw materials weighed in proportion are put into two ceramic boats placed in the tube furnace and kept at a certain distance and calcined in one step to obtain the sulfur-containing carbon nitride material, and the cost is low.
附图说明Description of drawings
图1为本发明对比例1及实施例1-5所制备的含硫氮化碳材料的光催化产氢效果对比图;Fig. 1 is the comparison diagram of the photocatalytic hydrogen production effect of the sulfur-containing carbon nitride material prepared in Comparative Example 1 and Examples 1-5 of the present invention;
图2为对比例1及实施例1-3所制备的含硫氮化碳材料的光电流测试图;Fig. 2 is the photocurrent test chart of the sulfur-containing carbon nitride material prepared by Comparative Example 1 and Examples 1-3;
图3为对比例1及实施例1、4、5所制备的含硫氮化碳材料的光电流测试图;Fig. 3 is the photocurrent test chart of the sulfur-containing carbon nitride material prepared by Comparative Example 1 and Examples 1, 4, and 5;
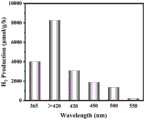
图4为实施例4所制备的含硫氮化碳材料在不同波长情况下的产氢对比图;Fig. 4 is the comparison diagram of the hydrogen production of the sulfur-containing carbon nitride material prepared in Example 4 at different wavelengths;
图5为实施例4所制备的含硫氮化碳材料连续20h光催化产氢循环稳定性测试图;Fig. 5 is the continuous 20h photocatalytic hydrogen production cycle stability test chart of the sulfur-containing carbon nitride material prepared in embodiment 4;
图6为对比例1及实施例1-3所制备的含硫氮化碳材料的XRD谱图;Fig. 6 is the XRD spectrogram of the sulfur-containing carbon nitride material prepared in Comparative Example 1 and Examples 1-3;
图7为对比例1及实施例1、4、5所制备的含硫氮化碳材料的XRD谱图;Fig. 7 is the XRD spectrogram of the sulfur-containing carbon nitride material prepared by Comparative Example 1 and Examples 1, 4, and 5;
图8为对比例1及实施例1-3所制备的含硫氮化碳材料的氮气等温吸附脱附曲线图;Fig. 8 is the nitrogen isothermal adsorption-desorption curve diagram of the sulfur-containing carbon nitride material prepared in Comparative Example 1 and Examples 1-3;
图9为对比例1及实施例1、4、5所制备的含硫氮化碳材料的氮气等温吸附脱附曲线图;Fig. 9 is the nitrogen isothermal adsorption-desorption curve diagram of the sulfur-containing carbon nitride material prepared in Comparative Example 1 and Examples 1, 4, and 5;
图10为对比例1及实施例1-3所制备的含硫氮化碳材料的孔径分布图;Fig. 10 is the pore size distribution diagram of the sulfur-containing carbon nitride material prepared in Comparative Example 1 and Examples 1-3;
图11为对比例1及实施例1、4、5所制备的含硫氮化碳材料的孔径分布图;Fig. 11 is the pore size distribution diagram of the sulfur-containing carbon nitride material prepared in Comparative Example 1 and Examples 1, 4, and 5;
图12为对比例1及实施例1-3所制备的含硫氮化碳材料的阻抗图;FIG. 12 is an impedance diagram of sulfur-containing carbon nitride materials prepared in Comparative Example 1 and Examples 1-3;
图13为对比例1及实施例1、4、5所制备的含硫氮化碳材料的阻抗图。FIG. 13 is an impedance diagram of sulfur-containing carbon nitride materials prepared in Comparative Example 1 and Examples 1, 4, and 5.
具体实施方式Detailed ways
为使本领域技术人员更好地理解本发明的技术方案,下面结合附图对本发明作进一步详细描述。In order to enable those skilled in the art to better understand the technical solutions of the present invention, the present invention will be further described in detail below in conjunction with the accompanying drawings.
对比例1Comparative example 1
一种含硫氮化碳材料,具体制备方法如下:A sulfur-containing carbon nitride material, the specific preparation method is as follows:
1)称取2g三聚硫氰酸(纯度95wt%),备用;1) take by weighing 2g thiocyanuric acid (purity 95wt%), for subsequent use;
2)将三聚硫氰酸固体平铺于瓷舟内,放入管式炉恒温区域正中间,向管式炉内通入氮气(99.99vol%),设置氮气气流量为80mL/min,然后室温下以10℃/min的升温速率升温至550℃进行煅烧,煅烧时间为3h,自然冷却至室温后研磨成粉末得到含硫氮化碳材料(记为SCN)。2) Spread the solid thiocyanuric acid in the porcelain boat, put it in the middle of the constant temperature area of the tube furnace, feed nitrogen (99.99vol%) into the tube furnace, set the nitrogen gas flow rate to 80mL/min, and then At room temperature, the temperature was raised to 550°C at a rate of 10°C/min for calcination, and the calcination time was 3 hours. After natural cooling to room temperature, it was ground into powder to obtain a sulfur-containing carbon nitride material (referred to as SCN).
实施例1Example 1
一种含硫氮化碳材料,具体制备方法如下:A sulfur-containing carbon nitride material, the specific preparation method is as follows:
1)称取2g三聚硫氰酸(纯度95wt%)和3g三聚氰胺(纯度99wt%),备用;1) Weigh 2g thiocyanuric acid (purity 95wt%) and 3g melamine (purity 99wt%) for subsequent use;
2)将三聚硫氰酸平铺于1号瓷舟,放入管式炉恒温区域正中间,将三聚氰胺平铺于2号瓷舟,放入管式炉内靠近进气口处,1号瓷舟与2号瓷舟中心距离为15cm,向管式炉内通入氮气(99.99vol%),设置氮气气流量为80mL/min,然后室温下以10℃/min的升温速率升温至550℃进行煅烧,煅烧时间为3h,自然冷却至室温后研磨成粉末得到含硫氮化碳材料(记为A)。2) Spread thiocyanic acid on the No. 1 porcelain boat, put it in the middle of the constant temperature area of the tube furnace, spread the melamine on the No. 2 porcelain boat, put it into the tube furnace near the air inlet, and put it in the middle of the constant temperature area of the tube furnace. The distance between the porcelain boat and the No. 2 porcelain boat center is 15cm, and nitrogen (99.99vol%) is passed into the tube furnace, and the nitrogen gas flow rate is set to 80mL/min, and then the temperature is raised to 550°C at a rate of 10°C/min at room temperature Carry out calcination, the calcination time is 3h, cool naturally to room temperature and then grind into powder to obtain sulfur-containing carbon nitride material (denoted as A).
实施例2Example 2
一种含硫氮化碳材料,其制备方法与实施例1相似,不同之处在于步骤2)设置氮气气流量为100mL/min,所得产物记为B。A sulfur-containing carbon nitride material, the preparation method of which is similar to that of Example 1, except that in step 2) the flow rate of nitrogen gas is set to 100mL/min, and the obtained product is denoted as B.
实施例3Example 3
一种含硫氮化碳材料,其制备方法与实施例1相似,不同之处在于步骤2)设置氮气气流量为60mL/min,所得产物记为C。A sulfur-containing carbon nitride material, the preparation method of which is similar to that of Example 1, except that in step 2) the flow rate of nitrogen gas is set to 60mL/min, and the obtained product is denoted as C.
实施例4Example 4
一种含硫氮化碳材料,其制备方法与实施例1相似,不同之处在于步骤2)1号瓷舟与2号瓷舟中心距离为10cm,所得产物记为D。A sulfur-containing carbon nitride material, the preparation method of which is similar to that of Example 1, except that step 2) the distance between the centers of No. 1 porcelain boat and No. 2 porcelain boat is 10 cm, and the obtained product is marked as D.
实施例5Example 5
一种含硫氮化碳材料,其制备方法与实施例1相似,不同之处在于步骤2)1号瓷舟与2号瓷舟中心距离为20cm,所得产物记为E。A sulfur-containing carbon nitride material, the preparation method of which is similar to that of Example 1, except that step 2) the distance between the centers of No. 1 porcelain boat and No. 2 porcelain boat is 20 cm, and the obtained product is marked as E.
光催化产氢性能测试:将20mg的对比例1及实施例1-5所制备的含硫氮化碳材料分别加入到6份含有10wt%三乙醇胺的100mL去离子水溶液中,每份溶液中加入0.33mL的H2PtCl4溶液(基于20mg的光催化剂负载Pt的质量分数为3wt%,H2PtCl4在光催化体系中起到助催化剂的作用),混合溶液超声分散30min后,在光反应器(Labsolar-6A,光源为插有420nm截止滤光片的300W氙灯)中光照4h进行测试,对比例1及实施例1-5所制备的含硫氮化碳材料的可见光光催化产氢效果对比图如图1所示,可以看出对比例1及实施例1-5所制备的含硫氮化碳材料产H2速率分别为:SCN 62.9μmol/h,A 144.9μmol/h,B 127.1μmol/h,C108.1μmol/h,D 165.4μmol/h,E 119.9μmol/h,其中A至E的光催化产氢速率高于SCN,D的光催化产氢速率最高。光电流测试:将对比例1及实施例1-5所制备的含硫氮化碳材料分别配制为1.0g/L的水分散液,每份水分散液吸取8μL滴涂到玻碳电极(直径3mm)表面,采用辰华电化学工作站,在>420nm的光照条件下,放入0.5mol/L的Na2SO4溶液中进行光电流测试。光电流能够反映材料的光催化性能,光电流越高,越能促进光生载流子的分离,材料的光催化效果越好,对比例1及实施例1-3所制备的含硫氮化碳材料的光电流测试图如图2所示,对比例1及实施例1、4、5所制备的含硫氮化碳材料的光电流测试图如图3所示,测试结果表明SCN光电流最小,对比实施例1-3可看出,保持瓷舟距离为15cm不变,调整气流量时实施例1所制备的A光电流最大。对比实施例1、4、5可看出,保持气流量为80mL/min不变,调整瓷舟距离时实施例4所制备的D光电流最大。Photocatalytic hydrogen production performance test: Add 20 mg of the sulfur-containing carbon nitride materials prepared in Comparative Example 1 and Examples 1-5 to 6 parts of 100 mL deionized aqueous solution containing 10 wt % triethanolamine, and add 0.33mL of H2 PtCl4 solution (based on 20mg of photocatalyst-loaded Pt mass fraction is 3wt%, H2 PtCl4 plays the role of co-catalyst in the photocatalytic system), after the mixed solution was ultrasonically dispersed for 30min, after the photoreaction Light source (Labsolar-6A, the light source is a 300W xenon lamp with a 420nm cut-off filter) for 4 hours to test the visible light photocatalytic hydrogen production effect of sulfur-containing carbon nitride materials prepared in Comparative Example 1 and Examples 1-5 The comparison chart is shown in Figure 1. It can be seen that theH2 production rates of the sulfur-containing carbon nitride materials prepared in Comparative Example 1 and Examples 1-5 are: SCN 62.9 μmol/h, A 144.9 μmol/h, B 127.1 μmol/h, C 108.1 μmol/h, D 165.4 μmol/h, E 119.9 μmol/h, among which the photocatalytic hydrogen production rate of A to E is higher than that of SCN, and the photocatalytic hydrogen production rate of D is the highest. Photocurrent test: The sulfur-containing carbon nitride materials prepared in Comparative Example 1 and Examples 1-5 were respectively prepared into 1.0 g/L aqueous dispersions, and each aqueous dispersion absorbed 8 μL and was drip-coated on the glassy carbon electrode (diameter 3mm) surface, using Chenhua Electrochemical Workstation, under the condition of >420nm light, put it into 0.5mol/L Na2 SO4 solution for photocurrent test. The photocurrent can reflect the photocatalytic performance of the material. The higher the photocurrent, the more the separation of photogenerated carriers can be promoted, and the photocatalytic effect of the material is better. The sulfur-containing carbon nitride prepared in Comparative Example 1 and Examples 1-3 The photocurrent test diagram of the material is shown in Figure 2, and the photocurrent test diagram of the sulfur-containing carbon nitride material prepared in Comparative Example 1 and Examples 1, 4, and 5 is shown in Figure 3. The test results show that the SCN photocurrent is the smallest , Comparing Examples 1-3, it can be seen that the photocurrent of A prepared in Example 1 is the largest when the distance between the porcelain boats is kept constant at 15 cm and the air flow is adjusted. Comparing Examples 1, 4, and 5, it can be seen that the D photocurrent prepared in Example 4 is the largest when the air flow rate is kept constant at 80 mL/min and the distance between the ceramic boats is adjusted.
图4为实施例4所制备的含硫氮化碳材料在不同波长情况下的产氢对比图,其中,配制数份光催化剂溶液,每份光催化剂溶液的制备方法为:将20mg实施例4制备的含硫氮化碳材料D加入到含有10wt%三乙醇胺的100mL去离子水溶液中,并加入0.33mL的H2PtCl4溶液(基于20mg的光催化剂负载Pt的质量分数为3wt%),混合溶液超声分散30min后得到。将配制的光催化剂溶液置于光反应器(Labsolar-6A,光源为300W氙灯)中光照4h进行产氢测试,光的波长分别为,365nm、420nm、450nm、500nm、550nm以及截止420nm(即>420nm),从图4可以看出,在各波长光照情况下均有一定的产氢效果。Fig. 4 is the comparison chart of the hydrogen production of the sulfur-containing carbon nitride material prepared in Example 4 under different wavelength conditions, wherein several parts of photocatalyst solutions are prepared, and the preparation method of each part of photocatalyst solution is as follows: 20 mg of Example 4 The prepared sulfur-containing carbon nitride material D was added to 100mL deionized aqueous solution containing 10wt% triethanolamine, and 0.33mL of H2 PtCl4 solution (based on the mass fraction of 20mg of photocatalyst loaded Pt was 3wt%), mixed The solution was obtained after ultrasonic dispersion for 30 min. Put the prepared photocatalyst solution in a photoreactor (Labsolar-6A, the light source is a 300W xenon lamp) for 4 hours to test the hydrogen production. 420nm), it can be seen from Figure 4 that there is a certain hydrogen production effect under the light conditions of various wavelengths.
测试含硫氮化碳材料催化产氢循环稳定性:将20mg实施例4制备的含硫氮化碳材料D加入到含有10wt%三乙醇胺的100mL去离子水溶液中,并加入0.33mL的H2PtCl4(基于20mg的光催化剂负载Pt的质量分数为3wt%),混合溶液超声分散30min后,在光反应器(Labsolar-6A,光源为插有420nm截止滤光片的300W氙灯)中进行循环产氢稳定性测试,每4h停一次,测试5个周期共20h,实施例4所制备的含硫氮化碳材料连续20h光催化产氢循环稳定性测试图如图5所示,从图发现该含硫氮化碳材料循环稳定性良好。Test the cycle stability of sulfur-containing carbon nitride materials for catalytic hydrogen production: add 20 mg of the sulfur-containing carbon nitride material D prepared in Example 4 to 100 mL of deionized aqueous solution containing 10 wt % triethanolamine, and add 0.33 mL of H2 PtCl4 (Based on 20mg of photocatalyst-loaded Pt mass fraction is 3wt%), after the mixed solution was ultrasonically dispersed for 30min, the photoreactor (Labsolar-6A, the light source was a 300W xenon lamp inserted with a 420nm cut-off filter) was used for cyclic production. Hydrogen stability test, stop once every 4h, test 5 cycles for a total of 20h, the continuous 20h photocatalytic hydrogen production cycle stability test diagram of the sulfur-containing carbon nitride material prepared in Example 4 is shown in Figure 5, it is found from the figure that the The sulfur-containing carbon nitride material has good cycle stability.
图6为对比例1及实施例1-3所制备的含硫氮化碳材料的XRD谱图,图7为对比例1及实施例1、4、5所制备的含硫氮化碳材料的XRD谱图。由图6,图7可知,在样品的XRD图谱中有两个主峰,13°左右对应的(100)平面的峰代表材料单元环的层内结构堆叠。大约27.1°处的另一个峰对应于共轭芳族单元的层间堆叠,其归属为(002)平面。比较相应峰的位置和强度,SCN的相应(002)面峰强度最低,实施例1-5制备的材料峰强有所提高,表明引入三聚氰胺作为氨气来源合成的含硫氮化碳材料结构更加有序,具有更高的结晶度,实施例1-5制备的材料的(002)晶面均发生了一定的移动,导致层间距变小,载流子的迁移路径变少,从而提高了载流子的分离效率。Fig. 6 is the XRD spectrogram of the sulfur-containing carbon nitride material prepared by Comparative Example 1 and Examples 1-3, and Fig. 7 is the XRD spectrum of the sulfur-containing carbon nitride material prepared by Comparative Example 1 and Examples 1, 4, and 5. XRD spectrum. It can be seen from Figure 6 and Figure 7 that there are two main peaks in the XRD pattern of the sample, and the peak corresponding to the (100) plane at about 13° represents the intralayer structure stacking of the material unit rings. Another peak at about 27.1° corresponds to the interlayer stacking of conjugated aromatic units, which is assigned to the (002) plane. Comparing the positions and intensities of the corresponding peaks, the corresponding (002) surface peak intensity of SCN is the lowest, and the peak intensity of the materials prepared in Examples 1-5 is increased, indicating that the structure of the sulfur-containing carbon nitride material synthesized by introducing melamine as the source of ammonia is more Orderly, with higher crystallinity, the (002) crystal planes of the materials prepared in Examples 1-5 all moved to a certain extent, resulting in smaller interlayer spacing and fewer carrier migration paths, thereby improving the carrying capacity. flow separation efficiency.
图8为对比例1及实施例1-3所制备的含硫氮化碳材料的氮气等温吸附脱附曲线图,图9为对比例1及实施例1、4、5所制备的含硫氮化碳材料的氮气等温吸附脱附曲线图,图10为对比例1及实施例1-3所制备的含硫氮化碳材料的孔径分布图,图11为对比例1及实施例1、4、5所制备的含硫氮化碳材料的孔径分布图。由图8,图9可知,典型的N2吸附脱附等温线表明该类含硫氮化碳材料样品具有中孔结构,其中样品D的比表面积最大,达到了135.75m2/g,比表面积大可以暴露更多的活性位点,从而提升光催化产氢活性。由图10,图11孔径分布图表明类含硫氮化碳材料样品中含有介孔。Fig. 8 is the nitrogen isothermal adsorption-desorption curve diagram of the sulfur-containing carbon nitride material prepared in Comparative Example 1 and Examples 1-3, and Fig. 9 is the sulfur-containing nitrogen prepared in Comparative Example 1 and Examples 1, 4, and 5 Nitrogen isothermal adsorption and desorption curves of carbonized carbon materials, Figure 10 is the pore size distribution figure of the sulfur-containing carbon nitride materials prepared in Comparative Example 1 and Examples 1-3, and Figure 11 is Comparative Example 1 and Examples 1 and 4 , 5 The pore size distribution diagram of the prepared sulfur-containing carbon nitride material. It can be seen from Figure 8 and Figure 9 that the typical N2 adsorption-desorption isotherms indicate that this type of sulfur-containing carbon nitride material sample has a mesopore structure, and the specific surface area of sample D is the largest, reaching 135.75m2 /g, and the specific surface area Larger can expose more active sites, thereby enhancing the photocatalytic hydrogen production activity. The pore size distribution diagrams in Figure 10 and Figure 11 show that the samples of sulfur-containing carbon nitride materials contain mesopores.
图12为对比例1及实施例1-3所制备的含硫氮化碳材料的阻抗图,图13为对比例1及实施例1、4、5所制备的含硫氮化碳材料的阻抗图。由图12,图13可知,与对比例SCN相比,实施例1-5制备的含硫氮化碳材料具有更小的半圆弧,样品D半圆弧最小,这表明SCN具有最高的载流子迁移阻力,D的载流子迁移阻力相对最小,迁移效率高,电子传输能力更强。Figure 12 is an impedance diagram of the sulfur-containing carbon nitride material prepared in Comparative Example 1 and Examples 1-3, and Figure 13 is the impedance of the sulfur-containing carbon nitride material prepared in Comparative Example 1 and Examples 1, 4, and 5 picture. It can be seen from Fig. 12 and Fig. 13 that compared with the comparative example SCN, the sulfur-containing carbon nitride materials prepared in Examples 1-5 have smaller semicircle arcs, and the semicircle arc of sample D is the smallest, which indicates that SCN has the highest loading capacity. Carrier migration resistance, the carrier migration resistance of D is relatively the smallest, the migration efficiency is high, and the electron transport ability is stronger.
Claims (9)
Priority Applications (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| CN202310168130.5ACN116371441B (en) | 2023-02-22 | 2023-02-22 | A sulfur-containing carbon nitride material and its preparation method and application in photocatalytic hydrogen production |
Applications Claiming Priority (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| CN202310168130.5ACN116371441B (en) | 2023-02-22 | 2023-02-22 | A sulfur-containing carbon nitride material and its preparation method and application in photocatalytic hydrogen production |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| CN116371441Atrue CN116371441A (en) | 2023-07-04 |
| CN116371441B CN116371441B (en) | 2024-11-05 |
Family
ID=86962405
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| CN202310168130.5AActiveCN116371441B (en) | 2023-02-22 | 2023-02-22 | A sulfur-containing carbon nitride material and its preparation method and application in photocatalytic hydrogen production |
Country Status (1)
| Country | Link |
|---|---|
| CN (1) | CN116371441B (en) |
Cited By (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN117380195A (en)* | 2023-09-25 | 2024-01-12 | 武汉工程大学 | Iron cobaltate/carbon thiosulfate heterojunction material, preparation method thereof and application thereof in photocatalytic hydrogen production |
| CN117534479A (en)* | 2023-11-23 | 2024-02-09 | 西安稀有金属材料研究院有限公司 | Preparation method of aluminum nitride nanopowder based on continuous gas phase activation |
Citations (9)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN103861632A (en)* | 2014-04-07 | 2014-06-18 | 吉林大学 | Preparation method for multi-hole carbon nitride photocatalytic material doped with sulphur |
| CN103920518A (en)* | 2014-04-14 | 2014-07-16 | 哈尔滨工业大学 | High-visible-light-activity sulfur-modified carbon nitride photocatalyst as well as synthetic method and application of photocatalyst |
| CN107930667A (en)* | 2017-11-16 | 2018-04-20 | 山东大学 | A kind of g C of sulfur doping3N4/TiO2Heterojunction photocatalyst and preparation method and application |
| CN108579785A (en)* | 2018-04-20 | 2018-09-28 | 武汉工程大学 | Efficient visible light decomposes aquatic products H2Sulfur doping carbonitride preparation method |
| CN108940348A (en)* | 2018-09-07 | 2018-12-07 | 湖南大学 | Siliver chromate/sulphur mixes carbonitride Z-type photochemical catalyst and preparation method thereof |
| CN110064429A (en)* | 2019-05-31 | 2019-07-30 | 上海纳米技术及应用国家工程研究中心有限公司 | Preparation method of sulfur doping azotized carbon nano piece and products thereof and application |
| CN112023971A (en)* | 2020-08-26 | 2020-12-04 | 中国科学院山西煤炭化学研究所 | Application of cyano group-modified carbon nitride in the field of phenol photomineralization |
| CN112473713A (en)* | 2020-11-26 | 2021-03-12 | 南开大学 | Sulfur-doped crystalline carbon nitride for producing hydrogen by photocatalytic decomposition of water and preparation method and application thereof |
| CN115138382A (en)* | 2022-05-13 | 2022-10-04 | 宁波工程学院 | Flower-ball-shaped sulfur-doped carbon nitride catalyst for hydrogen production by visible light decomposition of water and preparation method thereof |
- 2023
- 2023-02-22CNCN202310168130.5Apatent/CN116371441B/enactiveActive
Patent Citations (9)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN103861632A (en)* | 2014-04-07 | 2014-06-18 | 吉林大学 | Preparation method for multi-hole carbon nitride photocatalytic material doped with sulphur |
| CN103920518A (en)* | 2014-04-14 | 2014-07-16 | 哈尔滨工业大学 | High-visible-light-activity sulfur-modified carbon nitride photocatalyst as well as synthetic method and application of photocatalyst |
| CN107930667A (en)* | 2017-11-16 | 2018-04-20 | 山东大学 | A kind of g C of sulfur doping3N4/TiO2Heterojunction photocatalyst and preparation method and application |
| CN108579785A (en)* | 2018-04-20 | 2018-09-28 | 武汉工程大学 | Efficient visible light decomposes aquatic products H2Sulfur doping carbonitride preparation method |
| CN108940348A (en)* | 2018-09-07 | 2018-12-07 | 湖南大学 | Siliver chromate/sulphur mixes carbonitride Z-type photochemical catalyst and preparation method thereof |
| CN110064429A (en)* | 2019-05-31 | 2019-07-30 | 上海纳米技术及应用国家工程研究中心有限公司 | Preparation method of sulfur doping azotized carbon nano piece and products thereof and application |
| CN112023971A (en)* | 2020-08-26 | 2020-12-04 | 中国科学院山西煤炭化学研究所 | Application of cyano group-modified carbon nitride in the field of phenol photomineralization |
| CN112473713A (en)* | 2020-11-26 | 2021-03-12 | 南开大学 | Sulfur-doped crystalline carbon nitride for producing hydrogen by photocatalytic decomposition of water and preparation method and application thereof |
| CN115138382A (en)* | 2022-05-13 | 2022-10-04 | 宁波工程学院 | Flower-ball-shaped sulfur-doped carbon nitride catalyst for hydrogen production by visible light decomposition of water and preparation method thereof |
Non-Patent Citations (5)
| Title |
|---|
| CONGHAO KU等: "One-step construction of mesoporous cyano and sulfur co-modified carbon nitride for photocatalytic valorization of lignin to functionalized aromatics", 《APPLIED SURFACE SCIENCE》, 4 April 2022 (2022-04-04), pages 1 - 13* |
| HAITAO WANG: "Strategically designing and fabricating nitrogen and sulfur Co-doped g-C3 N4 for accelerating photocatalytic H2 evolution", 《JOURNAL OF MATERIALS SCIENCE & TECHNOLOGY》, 17 May 2024 (2024-05-17), pages 2* |
| JINGLING CHEN等: "One-step synthesis of sulfur-doped and nitrogen-deficient g-C3N4 photocatalyst for enhanced hydrogen evolution under visible light", 《MATERIALS LETTERS》, 28 January 2015 (2015-01-28), pages 129 - 132* |
| 漆小鹏等: "钼基化合物复合材料的设计及其电解水催化性能》", 31 December 2022, 冶金工业出版社, pages: 86* |
| 王海涛等: "硫取代氮增强g-C3N4光催化产氢性能", 《物理化学学报》, 30 May 2024 (2024-05-30), pages 1 - 9* |
Cited By (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN117380195A (en)* | 2023-09-25 | 2024-01-12 | 武汉工程大学 | Iron cobaltate/carbon thiosulfate heterojunction material, preparation method thereof and application thereof in photocatalytic hydrogen production |
| CN117534479A (en)* | 2023-11-23 | 2024-02-09 | 西安稀有金属材料研究院有限公司 | Preparation method of aluminum nitride nanopowder based on continuous gas phase activation |
Also Published As
| Publication number | Publication date |
|---|---|
| CN116371441B (en) | 2024-11-05 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| CN112973750B (en) | Carbon quantum dot coated metal monoatomic-carbon nitride composite material and preparation method thereof | |
| CN107376944B (en) | Application of Transition Metal Sulfide Supported Mn-Cd-S Solid Solution in Photocatalytic Hydrogen Production | |
| CN116371441A (en) | A kind of sulfur-containing carbon nitride material and its preparation method and its application in photocatalytic hydrogen production | |
| CN113134392B (en) | perovskite-MOFs composite photocatalyst and preparation method and application thereof | |
| CN114367299A (en) | Graphite carbon nitride photocatalyst for photocatalytic hydrogen production and preparation method thereof | |
| CN110813280A (en) | High-dispersion platinum-loaded surface-modified black titanium dioxide photocatalyst, and preparation method and application thereof | |
| CN111215118B (en) | Sodium-boron double-doped nano-layered graphite-like phase carbon nitride and preparation method and application thereof | |
| CN114177940B (en) | Preparation and application of a single-atom Cu-anchored covalent organic framework material | |
| CN115007194A (en) | A kind of preparation method and application of amorphous boron doped carbon nitride | |
| CN112675894A (en) | Hollow annular carbon nitride photocatalyst and preparation method thereof | |
| Yang et al. | 3D mesoporous ultra-thin g-C3N4 coupled with monoclinic β-AgVO3 as pn heterojunction for photocatalytic hydrogen evolution | |
| CN114534783B (en) | Method for preparing single-atom Pt-embedded covalent organic framework photocatalyst and application thereof | |
| CN106694016A (en) | A kind of g-C3N4/Bi2O3 composite powder and its preparation method and application | |
| CN111437869A (en) | g-C3N4-ZnIn2S4Heterojunction photocatalytic hydrogen production material and preparation method thereof | |
| CN109985653A (en) | A kind of carbon nitride-based material for photocatalytic total water splitting and its preparation and application | |
| CN113546631A (en) | A kind of La-modified Ni/Al2O3 catalyst, preparation method and application | |
| CN111790431A (en) | A kind of preparation method of g-C3N4 photocatalytic material modified with Al2O3 | |
| CN114602450B (en) | Co/Zn-g-C 3 N 4 Photocatalytic material, preparation and application thereof | |
| CN116212926A (en) | A kind of preparation method and application of brown carbon nitride photocatalyst | |
| CN115888788A (en) | Preparation method and product of a three-dimensional honeycomb graphite phase carbon nitride composite photothermal catalyst and its application | |
| CN102489329B (en) | Catalysis system for hydrogen generation by catalytic reduction of water with visible light, and preparation method thereof | |
| CN115090318A (en) | A kind of preparation method and application of high specific surface area intermolecular heterojunction carbon nitride photocatalyst | |
| CN103276474B (en) | Method for preparing (Ga1-xZnx)(N1-xOx) nanofibers by electrospinning | |
| CN111790369A (en) | A silver-supported black indium-based composite photothermal catalytic material for methane coupling and its preparation method and application | |
| CN118663301A (en) | Schottky junction photo-thermal catalytic carbon dioxide reduction catalyst based on induced hot electron transport and preparation method thereof |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PB01 | Publication | ||
| PB01 | Publication | ||
| SE01 | Entry into force of request for substantive examination | ||
| SE01 | Entry into force of request for substantive examination | ||
| GR01 | Patent grant | ||
| GR01 | Patent grant |