CN116178408A - Criborole salt and its crystal form, preparation method and application - Google Patents
Criborole salt and its crystal form, preparation method and applicationDownload PDFInfo
- Publication number
- CN116178408A CN116178408ACN202310033870.8ACN202310033870ACN116178408ACN 116178408 ACN116178408 ACN 116178408ACN 202310033870 ACN202310033870 ACN 202310033870ACN 116178408 ACN116178408 ACN 116178408A
- Authority
- CN
- China
- Prior art keywords
- salt
- crystalline form
- solvent
- piperazine
- clenbuterol
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Pending
Links
Images
Classifications
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07F—ACYCLIC, CARBOCYCLIC OR HETEROCYCLIC COMPOUNDS CONTAINING ELEMENTS OTHER THAN CARBON, HYDROGEN, HALOGEN, OXYGEN, NITROGEN, SULFUR, SELENIUM OR TELLURIUM
- C07F5/00—Compounds containing elements of Groups 3 or 13 of the Periodic Table
- C07F5/02—Boron compounds
- C07F5/025—Boronic and borinic acid compounds
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P17/00—Drugs for dermatological disorders
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P17/00—Drugs for dermatological disorders
- A61P17/06—Antipsoriatics
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P29/00—Non-central analgesic, antipyretic or antiinflammatory agents, e.g. antirheumatic agents; Non-steroidal antiinflammatory drugs [NSAID]
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D295/00—Heterocyclic compounds containing polymethylene-imine rings with at least five ring members, 3-azabicyclo [3.2.2] nonane, piperazine, morpholine or thiomorpholine rings, having only hydrogen atoms directly attached to the ring carbon atoms
- C07D295/02—Heterocyclic compounds containing polymethylene-imine rings with at least five ring members, 3-azabicyclo [3.2.2] nonane, piperazine, morpholine or thiomorpholine rings, having only hydrogen atoms directly attached to the ring carbon atoms containing only hydrogen and carbon atoms in addition to the ring hetero elements
- C07D295/023—Preparation; Separation; Stabilisation; Use of additives
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D295/00—Heterocyclic compounds containing polymethylene-imine rings with at least five ring members, 3-azabicyclo [3.2.2] nonane, piperazine, morpholine or thiomorpholine rings, having only hydrogen atoms directly attached to the ring carbon atoms
- C07D295/02—Heterocyclic compounds containing polymethylene-imine rings with at least five ring members, 3-azabicyclo [3.2.2] nonane, piperazine, morpholine or thiomorpholine rings, having only hydrogen atoms directly attached to the ring carbon atoms containing only hydrogen and carbon atoms in addition to the ring hetero elements
- C07D295/027—Heterocyclic compounds containing polymethylene-imine rings with at least five ring members, 3-azabicyclo [3.2.2] nonane, piperazine, morpholine or thiomorpholine rings, having only hydrogen atoms directly attached to the ring carbon atoms containing only hydrogen and carbon atoms in addition to the ring hetero elements containing only one hetero ring
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07B—GENERAL METHODS OF ORGANIC CHEMISTRY; APPARATUS THEREFOR
- C07B2200/00—Indexing scheme relating to specific properties of organic compounds
- C07B2200/13—Crystalline forms, e.g. polymorphs
- Y—GENERAL TAGGING OF NEW TECHNOLOGICAL DEVELOPMENTS; GENERAL TAGGING OF CROSS-SECTIONAL TECHNOLOGIES SPANNING OVER SEVERAL SECTIONS OF THE IPC; TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y02—TECHNOLOGIES OR APPLICATIONS FOR MITIGATION OR ADAPTATION AGAINST CLIMATE CHANGE
- Y02P—CLIMATE CHANGE MITIGATION TECHNOLOGIES IN THE PRODUCTION OR PROCESSING OF GOODS
- Y02P20/00—Technologies relating to chemical industry
- Y02P20/50—Improvements relating to the production of bulk chemicals
- Y02P20/55—Design of synthesis routes, e.g. reducing the use of auxiliary or protecting groups
Landscapes
- Chemical & Material Sciences (AREA)
- Organic Chemistry (AREA)
- Health & Medical Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- General Health & Medical Sciences (AREA)
- Chemical Kinetics & Catalysis (AREA)
- General Chemical & Material Sciences (AREA)
- Medicinal Chemistry (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Pharmacology & Pharmacy (AREA)
- Veterinary Medicine (AREA)
- Animal Behavior & Ethology (AREA)
- Public Health (AREA)
- Engineering & Computer Science (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Dermatology (AREA)
- Rheumatology (AREA)
- Pain & Pain Management (AREA)
- Medicinal Preparation (AREA)
- Pharmaceuticals Containing Other Organic And Inorganic Compounds (AREA)
Abstract
Description
Translated fromChinese本申请是申请日为2022年12月30日,申请号为CN202211732252.4,发明名称为克立硼罗盐及其晶型、制备方法和用途的分案申请。This application is a divisional application with the application date of December 30, 2022, application number CN202211732252.4, and the invention name is "Criborole salt and its crystal form, preparation method and use".
技术领域Technical Field
本发明涉及药物化学技术领域,具体涉及克立硼罗哌嗪盐及其晶型、制备方法和用途。The present invention relates to the technical field of pharmaceutical chemistry, and in particular to a clebor ropiperazine salt and a crystal form thereof, a preparation method and use thereof.
背景技术Background Art
2016年12月,美国食品药品管理局(FDA)批准Anacor公司的Crisaborole(商品名为Eucrisa)软膏上市,该药是一种非甾体PDE-4抑制剂,可用于治疗特应性皮炎,其结构式如下:In December 2016, the U.S. Food and Drug Administration (FDA) approved Anacor's Crisaborole (trade name Eucrisa) ointment for marketing. The drug is a non-steroidal PDE-4 inhibitor that can be used to treat atopic dermatitis. Its structural formula is as follows:
发明内容Summary of the invention
本发明提供一种克立硼罗哌嗪盐及其晶型,具有至少以下一种优势:水中溶解度好、稳定性良好、溶出度好、纯度高、吸湿性低流动性好,符合药用要求,能稳定储存,制备方式简便,生物利用率度高,适合于工业化生产等。The present invention provides a clebor-ropizine salt and a crystal form thereof, which has at least one of the following advantages: good solubility in water, good stability, good dissolution, high purity, low hygroscopicity, good fluidity, meets pharmaceutical requirements, can be stably stored, has a simple preparation method, has high bioavailability, is suitable for industrial production, etc.
本发明的第一方面,提供结构式如下式(I)所示的克立硼罗哌嗪盐,In a first aspect of the present invention, a clebor-ropizine salt having a structural formula as shown in the following formula (I) is provided:
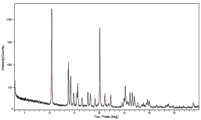
本发明第一方面所述的克立硼罗哌嗪盐的晶型,所述晶型为Form I,在以衍射角2θ表示的X射线粉末衍射图谱(XRPD)中,具有选自10.42±0.2°、13.77±0.2°、14.22±0.2°和20.07±0.2°中的至少一个特征衍射峰;优选为至少三个特征衍射峰。The crystalline form of the cleboridine salt described in the first aspect of the present invention is Form I, and in the X-ray powder diffraction pattern (XRPD) represented by the diffraction angle 2θ, it has at least one characteristic diffraction peak selected from 10.42±0.2°, 13.77±0.2°, 14.22±0.2° and 20.07±0.2°; preferably at least three characteristic diffraction peaks.
优选地,所述晶型Form I,在以衍射角2θ表示的X射线粉末衍射图谱中,进一步具有选自15.65±0.2°、17.67±0.2°、18.19±0.2°和25.15±0.2°中的至少一个特征衍射峰。Preferably, the crystalline form Form I further has at least one characteristic diffraction peak selected from 15.65±0.2°, 17.67±0.2°, 18.19±0.2° and 25.15±0.2° in the X-ray powder diffraction pattern represented by the diffraction angle 2θ.
优选地,所述晶型Form I,在以衍射角2θ表示的X射线粉末衍射图谱中,进一步具有选自16.47±0.2°、22.25±0.2°、26.09±0.2°和26.55±0.2°中的至少一个特征衍射峰。Preferably, the crystalline form Form I further has at least one characteristic diffraction peak selected from 16.47±0.2°, 22.25±0.2°, 26.09±0.2° and 26.55±0.2° in the X-ray powder diffraction pattern represented by the diffraction angle 2θ.
优选地,所述晶型Form I的X射线粉末衍射数据如表1所示。Preferably, the X-ray powder diffraction data of the crystalline form Form I is shown in Table 1.
优选地,所述晶型Form I,具有基本上如图1所示的X射线粉末衍射图谱。Preferably, the crystalline form Form I has an X-ray powder diffraction pattern substantially as shown in FIG1 .
优选地,所述晶型Form I,在傅里叶红外光谱中,在选自3255.96cm-1±2cm-1、3045.12cm-1±2cm-1、2847.14cm-1±2cm-1、2718.09cm-1±2cm-1、1470.43cm-1±2cm-1、1415.23cm-1±2cm-1、1388.20cm-1±2cm-1、1108.24cm-1±2cm-1、1061.31cm-1±2cm-1、1036.14cm-1±2cm-1、833.31cm-1±2cm-1、783.53cm-1±2cm-1、710.01cm-1±2cm-1中的至少之一处具有特征峰。Preferably, the crystalline form Form I has a characteristic peak at at least one selected from 3255.96cm-1 ±2cm-1 , 3045.12cm-1 ±2cm-1 , 2847.14cm-1 ±2cm-1 , 2718.09cm-1 ±2cm-1 , 1470.43cm-1 ±2cm-1 , 1415.23cm-1 ±2cm-1 , 1388.20cm-1 ±2cm-1 , 1108.24cm-1 ±2cm-1 , 1061.31cm-1 ±2cm-1 , 1036.14cm-1 ±2cm-1 , 833.31cm-1 ±2cm-1 , 783.53cm-1 ±2cm-1 , and 710.01cm-1 ±2cm-1 in Fourier infrared spectrum.
优选地,所述晶型Form I,具有如图6所示的傅里叶红外光谱。Preferably, the crystal form Form I has a Fourier transform infrared spectrum as shown in FIG6 .
优选地,所述晶型Form I为无水物。Preferably, the crystalline form Form I is an anhydrate.
优选地,所述晶型Form I具有基本上如图2所示的TGA图谱。Preferably, the crystalline form Form I has a TGA spectrum substantially as shown in Figure 2.
优选地,所述晶型Form I具有基本上如图3所示的DSC图谱。Preferably, the crystalline form Form I has a DSC spectrum substantially as shown in Figure 3.
优选地,所述晶型Form I具有基本上如图4所示的DVS图谱。Preferably, the crystalline form Form I has a DVS spectrum substantially as shown in Figure 4.
本发明的第二方面,提供第一方面所述的克立硼罗哌嗪盐的制备方法,其包括以下步骤:将克立硼罗和哌嗪加入溶剂中得到混悬液,搅拌至析出固体,分离,干燥后即得;优选地,所述溶剂为有机溶剂。The second aspect of the present invention provides a method for preparing the crisaborole piperazine salt described in the first aspect, which comprises the following steps: adding crisaborole and piperazine to a solvent to obtain a suspension, stirring until a solid precipitates, separating, and drying to obtain the suspension; preferably, the solvent is an organic solvent.
本发明进一步提供第一方面所述的克立硼罗哌嗪盐的晶型Form I的制备方法,其包括以下步骤:The present invention further provides a method for preparing the crystalline form Form I of the cleborine salt of the first aspect, which comprises the following steps:
将克立硼罗和哌嗪加入有机溶剂中得到混悬液,晶浆,分离,干燥后得到所述晶型。The crystalline form is obtained by adding crisaborole and piperazine into an organic solvent to obtain a suspension, a slurry, separating, and drying.
优选地,克立硼罗哌嗪盐的晶型Form I,其包括以下步骤:将克立硼罗和哌嗪加入有机溶剂中得到混悬液,晶浆≥1h,分离固体,干燥后得到所述晶型。Preferably, the crystalline form Form I of crisaborole piperazine salt comprises the following steps: adding crisaborole and piperazine to an organic solvent to obtain a suspension, slurrying for ≥1h, separating the solid, and drying to obtain the crystalline form.
优选地,所述晶浆≥6h,更优选地,所述晶浆≥1天;最优选为2-5天。Preferably, the slurry is ≥6h, more preferably, the slurry is ≥1 day; most preferably, it is 2-5 days.
优选地,所述晶浆为室温晶浆。Preferably, the slurry is room temperature slurry.
优选地,所述晶浆过程中可补加溶剂。Preferably, a solvent may be added during the slurry process.
优选地,上述制备方法中,所述有机溶剂为醚类和酯类中的一种或混合溶剂;更优选地,所述溶剂选自四氢呋喃和乙酸乙酯或二者的混合溶剂。Preferably, in the above preparation method, the organic solvent is one of ethers and esters or a mixed solvent; more preferably, the solvent is selected from tetrahydrofuran and ethyl acetate or a mixed solvent of the two.
优选地,上述制备方法中,优选地,克立硼罗和溶剂的质量体积比(mg/mL)≥50:1,更优选为60-120。Preferably, in the above preparation method, preferably, the mass volume ratio (mg/mL) of crisaborole to the solvent is ≥50:1, more preferably 60-120.
优选地,上述制备方法中,克立硼罗和哌嗪的摩尔比为1-5:1,还优选为1.5-3:1,进一步优选为1.9-2.2:1;Preferably, in the above preparation method, the molar ratio of crisaborole to piperazine is 1-5:1, preferably 1.5-3:1, and further preferably 1.9-2.2:1;
优选地,上述制备方法中,所述干燥的温度为40-70℃,还优选45-55℃,进一步优选为48-52℃。Preferably, in the above preparation method, the drying temperature is 40-70°C, preferably 45-55°C, and further preferably 48-52°C.
所述克立硼罗哌嗪盐及其晶型具有以下有益效果:The clebor ropizine salt and its crystal form have the following beneficial effects:
1)本发明盐及其晶型的稳定性好。其中,晶型Form I分别在长期、加速条件下放置21天晶型保持不变,说明Form I原料药具有较好的晶型稳定性和化学稳定性。1) The salt of the present invention and its crystal form have good stability. Among them, the crystal form Form I remains unchanged after being placed under long-term and accelerated conditions for 21 days, indicating that the Form I raw material has good crystal stability and chemical stability.
2)本发明盐及其晶型具有良好的水中溶解度,有助于提高克立硼罗药物的生物利用度,从而提高药物的成药性及药效;在保证药物疗效的同时,降低药品的剂量,从而降低药品的副作用并提高药品的安全性。2) The salt and its crystal form of the present invention have good solubility in water, which helps to improve the bioavailability of the crisaborole drug, thereby improving the drugability and efficacy of the drug; while ensuring the efficacy of the drug, the dosage of the drug is reduced, thereby reducing the side effects of the drug and improving the safety of the drug.
3)本发明盐及其晶型几乎不吸湿,无需严格控制湿度,更适合工业化生产。3) The salt and its crystal form of the present invention are almost non-hygroscopic and do not require strict humidity control, which makes them more suitable for industrial production.
4)本发明盐及其晶型几乎没有溶剂残留,符合药用要求。4) The salt and its crystal form of the present invention have almost no solvent residue and meet the requirements for pharmaceutical use.
本发明的第三方面,提供一种药物组合物,其包含根据第一方面所述的克立硼罗哌嗪盐或/和及其晶型以及至少一种药学上可接受的载体。The third aspect of the present invention provides a pharmaceutical composition comprising the crisaborole ropizide salt or/and its crystal form according to the first aspect and at least one pharmaceutically acceptable carrier.
本发明中,药学上可接受的载体包括稀释剂或赋形剂或其他添加剂,其实例包括但不限于,例如润湿剂、崩解剂、润滑剂、粘结剂、表面活性剂等。其他添加剂的实例包括但不限于,例如紫胶、阿拉伯树胶、滑石、氧化钛、糖(例如,蔗糖)、明胶、水、多糖诸如乳糖或葡萄糖、石蜡(例如,石油馏分)、植物油(例如,花生油或芝麻油)、以及药学上可接受的有机溶剂如醇(例如,乙醇或甘油)、天然矿物粉末(例如,高岭土、粘土、滑石和白垩)、合成矿物粉末(例如,高度分散的硅酸和硅酸盐)、乳化剂(例如,木素、亚硫酸盐溶液、甲基纤维素、淀粉和聚乙烯基吡咯烷酮)、硬脂酸镁、硬脂酸、月桂基硫酸钠等。In the present invention, pharmaceutically acceptable carriers include diluents or excipients or other additives, examples of which include, but are not limited to, wetting agents, disintegrants, lubricants, binders, surfactants, etc. Examples of other additives include, but are not limited to, shellac, gum arabic, talc, titanium oxide, sugar (e.g., sucrose), gelatin, water, polysaccharides such as lactose or glucose, paraffin (e.g., petroleum fractions), vegetable oils (e.g., peanut oil or sesame oil), and pharmaceutically acceptable organic solvents such as alcohols (e.g., ethanol or glycerol), natural mineral powders (e.g., kaolin, clay, talc and chalk), synthetic mineral powders (e.g., highly dispersed silicic acid and silicates), emulsifiers (e.g., lignin, sulfite solutions, methylcellulose, starch and polyvinyl pyrrolidone), magnesium stearate, stearic acid, sodium lauryl sulfate, etc.
本发明的药物组合物可制备为各种剂型,包括但不限于,例如适用于局部皮肤给药的半固体药物制剂,例如软膏剂、凝胶剂和乳膏剂等,或适于口服给药的药物制剂,例如固体口服制剂包括片剂、包衣剂、胶囊剂、颗粒剂、散剂、丸剂、粉剂等,或液体口服制剂包括溶液剂、糖浆剂、混悬剂、乳液剂等;适于肠胃外给药的药物制剂,例如静脉滴注制剂,肌肉或皮下注射制剂,经直肠给药的栓剂,经鼻内给药的吸入制剂,或另外的局部给药的透皮贴剂形式。在制剂生产中,还可包括另外的辅料,优选辅料的加入不引起晶型的转变。The pharmaceutical composition of the present invention can be prepared into various dosage forms, including but not limited to, for example, semi-solid pharmaceutical preparations suitable for topical skin administration, such as ointments, gels and creams, etc., or pharmaceutical preparations suitable for oral administration, such as solid oral preparations including tablets, coatings, capsules, granules, powders, pills, powders, etc., or liquid oral preparations including solutions, syrups, suspensions, emulsions, etc.; pharmaceutical preparations suitable for parenteral administration, such as intravenous drip preparations, intramuscular or subcutaneous injection preparations, suppositories for rectal administration, inhalation preparations for intranasal administration, or other transdermal patch forms for topical administration. In the production of the preparation, additional excipients may also be included, and it is preferred that the addition of the excipients does not cause the transformation of the crystal form.
优选地,本发明的药物组合物为软膏剂。Preferably, the pharmaceutical composition of the present invention is an ointment.
本发明的第四方面,提供第一方面所述的克立硼罗哌嗪盐及其晶型在制备用于治疗炎症相关疾病或病症的局部药物制剂中的用途,其中,所述炎症相关疾病或病症包括牛皮癣和特应性皮炎。The fourth aspect of the present invention provides the use of the cleborine salt and its crystal form described in the first aspect in the preparation of a topical pharmaceutical preparation for treating inflammation-related diseases or conditions, wherein the inflammation-related diseases or conditions include psoriasis and atopic dermatitis.
优选地,本发明提供第一方面所述克立硼罗哌嗪盐及其晶型在制备用于预防或治疗磷酸二酯酶4(PDE-4)相关疾病的药物中的用途。Preferably, the present invention provides use of the clebor-ropizine salt and its crystal form described in the first aspect in the preparation of a medicament for preventing or treating phosphodiesterase 4 (PDE-4) related diseases.
本发明的第五方面,提供一种用于预防或治疗疾病的方法,所述方法包括向有需要的受试者给予第一方面所述的克立硼罗哌嗪盐及其晶型或第三方面所述的药物组合物的步骤,其中所述疾病为磷酸二酯酶4相关疾病,其包括牛皮癣和特应性皮炎的炎症相关疾病。优选地,所述药物组合物包含预防和/或治疗有效量的克立硼罗哌嗪盐及其晶型。The fifth aspect of the present invention provides a method for preventing or treating a disease, the method comprising the step of administering the cristabor ropizine salt and its crystal form described in the first aspect or the pharmaceutical composition described in the third aspect to a subject in need, wherein the disease is a phosphodiesterase 4-related disease, which includes inflammatory-related diseases such as psoriasis and atopic dermatitis. Preferably, the pharmaceutical composition comprises a preventive and/or therapeutically effective amount of the cristabor ropizine salt and its crystal form.
本发明的第六方面,提供根据第一方面所述的克立硼罗哌嗪盐及其晶型或第三方面所述的药物组合物与其他药物活性成分的联合应用。The sixth aspect of the present invention provides the combined use of the clebor-ropizine salt and its crystal form according to the first aspect or the pharmaceutical composition according to the third aspect and other active pharmaceutical ingredients.
附图说明BRIEF DESCRIPTION OF THE DRAWINGS
图1为根据实施例1制备得到的克立硼罗哌嗪盐晶型Form I的XRPD图谱。FIG. 1 is an XRPD spectrum of the crystalline form Form I of the cleborine salt prepared according to Example 1.
图2为根据实施例1制备得到的克立硼罗哌嗪盐晶型Form I的TGA图谱。FIG. 2 is a TGA spectrum of the crystal form Form I of the clebor ropizine salt prepared according to Example 1.
图3为根据实施例1制备得到的克立硼罗哌嗪盐晶型Form I的DSC图谱。FIG3 is a DSC spectrum of the crystalline form Form I of the cleboridine salt prepared according to Example 1.
图4为根据实施例1制备得到的克立硼罗哌嗪盐晶型Form I的DVS图谱。FIG4 is a DVS spectrum of the crystal form Form I of the cleborine salt prepared according to Example 1.
图5为根据实施例1制备得到的克立硼罗哌嗪盐晶型Form I的PLM图谱。FIG5 is a PLM spectrum of the crystal form Form I of the clebor-ropizine salt prepared according to Example 1.
图6为根据实施例1制备得到的克立硼罗哌嗪盐晶型Form I的傅里叶红外光谱。FIG6 is a Fourier transform infrared spectrum of the crystalline form I of the cleboridine salt prepared according to Example 1.
图7为克立硼罗哌嗪盐晶型Form I的单晶PLM图谱。FIG. 7 is a single crystal PLM spectrum of Form I of clebor-ropizine salt.
图8为克立硼罗哌嗪盐晶型Form I的在加速和长期条件下放置前后的XRPD图。FIG8 is an XRPD diagram of the crystal form I of the cleborine salt of ropizine before and after being placed under accelerated and long-term conditions.
具体实施方式DETAILED DESCRIPTION
现详细说明本发明的多种示例性实施方式,该详细说明不应认为是对本发明的限制,而应理解为是对本发明的某些方面、特性和实施方案的更详细的描述。Various exemplary embodiments of the present invention will now be described in detail. This detailed description should not be considered as limiting the present invention, but should be understood as a more detailed description of certain aspects, features, and embodiments of the present invention.
应理解本发明中所述的术语仅仅是为描述特别的实施方式,并非用于限制本发明。另外,对于本发明中的数值范围,应理解为具体公开了该范围的上限和下限以及它们之间的每个中间值。在任何陈述值或陈述范围内的中间值以及任何其他陈述值或在所述范围内的中间值之间的每个较小的范围也包括在本发明内。这些较小范围的上限和下限可独立地包括或排除在范围内。It should be understood that the terms described in the present invention are only for describing special embodiments and are not intended to limit the present invention. In addition, for the numerical range in the present invention, it should be understood that the upper and lower limits of the scope and each intermediate value therebetween are specifically disclosed. Each smaller range between the intermediate value in any stated value or stated range and any other stated value or intermediate value in the described range is also included in the present invention. The upper and lower limits of these smaller ranges can be independently included or excluded in the scope.
除非另有说明,否则本文使用的所有技术和科学术语具有本发明所述领域的常规技术人员通常理解的相同含义。虽然本发明仅描述了优选的方法和材料,但是在本发明的实施或测试中也可以使用与本文所述相似或等同的任何方法和材料。本说明书中提到的所有文献通过引用并入,用以公开和描述与所述文献相关的方法和/或材料。在与任何并入的文献冲突时,以本说明书的内容为准。除非另有说明,否则“%”为基于重量的百分数。Unless otherwise specified, all technical and scientific terms used herein have the same meanings as commonly understood by those skilled in the art to which the invention relates. Although the present invention describes only preferred methods and materials, any methods and materials similar or equivalent to those described herein may also be used in the practice or testing of the present invention. All documents mentioned in this specification are incorporated by reference to disclose and describe the methods and/or materials related to the documents. In the event of a conflict with any incorporated document, the content of this specification shall prevail. Unless otherwise specified, "%" is a percentage based on weight.
本发明的方法中,一般以Crisaborole(克立硼罗)作为起始物料制备本发明的盐及其晶型,作为起始物料的Crisaborole可通过市售产品获得,也可通过AnacorPharmaceutical公司的专利申请WO2006/089067中描述的方法制备得到,作为起始物料的哌嗪可通过市售产品获得。In the method of the present invention, Crisaborole is generally used as a starting material to prepare the salt of the present invention and its crystalline form. Crisaborole as a starting material can be obtained through commercially available products, and can also be prepared by the method described in patent application WO2006/089067 of Anacor Pharmaceutical Company. Piperazine as a starting material can be obtained from commercially available products.
本文所用术语“受试者”包括哺乳动物。哺乳动物可为例如任何哺乳动物,例如,人、灵长类动物、鸟、小鼠、大鼠、家禽、狗、猫、牛、马、山羊、骆驼、绵羊或猪。优选哺乳动物为人。The term "subject" as used herein includes mammals. The mammal can be, for example, any mammal, for example, a human, a primate, a bird, a mouse, a rat, poultry, a dog, a cat, a cow, a horse, a goat, a camel, a sheep or a pig. Preferably, the mammal is a human.
本文所用术语“室温”一般指10-30℃,较佳地指20±5℃。The term "room temperature" as used herein generally refers to 10-30°C, preferably 20±5°C.
本发明中,术语“晶型”是指晶体物质的某种晶格构型。本领域已知的是,晶型在制药中和稳定性、溶出性和机械性有关。相同物质的不同晶型通常具有其特有的不同物理性质的不同的晶格(例如晶胞)。不同的晶型可通过本领域已知的方法进行表征。例如,可通过固态表征方法例如通过X射线粉末衍射(XRPD)来鉴定。其它表征方法包括示差扫描量热法(DSC)、热解重量分析(TGA)、动态蒸汽吸附(DVS)、固态NMR等。可以使用上述任一种方法对晶型进行表征,或者组合使用两种以上的方法进行表征。In the present invention, the term "crystal form" refers to a certain lattice configuration of a crystalline substance. It is known in the art that crystal form is related to stability, dissolution and mechanical properties in pharmaceutical preparation. Different crystal forms of the same substance usually have different lattices (e.g., unit cells) with different physical properties. Different crystal forms can be characterized by methods known in the art. For example, it can be identified by solid-state characterization methods such as X-ray powder diffraction (XRPD). Other characterization methods include differential scanning calorimetry (DSC), thermogravimetric analysis (TGA), dynamic vapor sorption (DVS), solid-state NMR, etc. The crystal form can be characterized using any of the above methods, or a combination of two or more methods can be used for characterization.
除本发明特别说明外,其他实验方法均为本领域公知的方法,这些方法可参考例如《中华人民共和国药典》等。其中,进行检测的参数设置如下:Except for special instructions in the present invention, other experimental methods are well-known methods in the art, and these methods can be referred to, for example, in the Pharmacopoeia of the People's Republic of China. Among them, the parameters for the detection are set as follows:
XRPD(X-射线粉末衍射)测的具体条件为:Bruker D8,Cu-Kα辐射,检测范围3°-40°2θ,步长0.02°2θ,扫描速率0.2s.step-1。The specific conditions of XRPD (X-ray powder diffraction) measurement are: Bruker D8, Cu-Kα radiation, detection range 3°-40°2θ, step size 0.02°2θ, scanning rate 0.2 s.step-1.
热重分析(TGA)数据采自于TA Instruments Q500TGA。参数如下:Thermogravimetric analysis (TGA) data were collected from TA Instruments Q500TGA. The parameters are as follows:
扫描速率:以10℃/min;保护气体:N2。Scanning rate: 10°C/min; protective gas: N2 .
差热分析(DSC)数据采自于TA Instruments Q200 DSC。参数如下:Differential thermal analysis (DSC) data were collected from TA Instruments Q200 DSC. The parameters are as follows:
扫描速率:以10℃/min;保护气体:N2。Scanning rate: 10°C/min; protective gas: N2 .
动态水份吸附分析(DVS)数据和等温吸附分析数据采自于TA InstrumentsQ5000SA。参数如下:Dynamic moisture sorption analysis (DVS) data and isothermal sorption analysis data were collected from TA Instruments Q5000SA. The parameters are as follows:
温度:25℃;相对湿度范围:0%RH-80%RH。Temperature: 25℃; Relative humidity range: 0%RH-80%RH.
偏振光显微镜(PLM)图谱采自于XP-500E偏振光显微镜。取少量粉末样品置于载玻片上,滴加少量矿物油分散样品,盖上盖玻片,放置在载物台上进行观测并拍照。Polarized light microscopy (PLM) images were obtained from an XP-500E polarized light microscope. A small amount of powder sample was placed on a glass slide, a small amount of mineral oil was added to disperse the sample, a cover glass was placed, and the sample was placed on the stage for observation and photography.
傅里叶红外光谱的测试条件为:Bruker tensor 27,ATR法,分辨率4cm-1。The test conditions of Fourier transform infrared spectroscopy are: Bruker tensor 27, ATR method, resolution 4 cm-1 .
本发明中,术语“性能”或“晶型性能”或“性质”或“药学性质”包括其物理和化学性质,评价指标包括但不限于,例如熔点、水溶性、溶出度、机械特性、稳定性、药动学或药效学、引湿性、流动性、可压性、竞争性等。In the present invention, the term "performance" or "crystal performance" or "property" or "pharmaceutical property" includes its physical and chemical properties, and the evaluation indicators include but are not limited to, for example, melting point, water solubility, solubility, mechanical properties, stability, pharmacokinetics or pharmacodynamics, hygroscopicity, fluidity, compressibility, competitiveness, etc.
实施例中所用的各种试剂如无特别说明均为商购获得。除非另有说明,实施例均在室温下操作。All reagents used in the examples were commercially available unless otherwise specified. Unless otherwise specified, the examples were operated at room temperature.
实施例1Example 1
取约60.67mg克立硼罗和10.76mg的哌嗪,加入0.7mL四氢呋喃,室温搅拌,得到白色混悬液,室温搅拌2天后,有大量固体析出,向其中补加2mL四氢呋喃溶剂,继续室温搅拌1天,离心过滤,所得固体50℃下真空干燥1小时,得到克立硼罗哌嗪盐的晶型Form I。Take about 60.67 mg of crisaborole and 10.76 mg of piperazine, add 0.7 mL of tetrahydrofuran, stir at room temperature to obtain a white suspension. After stirring at room temperature for 2 days, a large amount of solid precipitates. Add 2 mL of tetrahydrofuran solvent, continue stirring at room temperature for 1 day, centrifuge and filter. The obtained solid is vacuum dried at 50°C for 1 hour to obtain the crystal form I of crisaborole piperazine salt.
取实施例1样品进行表征,具体如下。The sample of Example 1 was taken for characterization, as follows.
1、XRPD图谱图谱分析1. XRPD spectrum analysis
图1为Form I的XRPD图谱,表1示出了Form I的X射线粉末衍射数据。FIG1 is an XRPD pattern of Form I, and Table 1 shows the X-ray powder diffraction data of Form I.
表1:Form I的X射线粉末衍射数据Table 1: X-ray powder diffraction data of Form I
2、TGA图谱分析2. TGA spectrum analysis
TGA结果如图2所示。在94℃前具有失重台阶,失重率为0.5%,在94-150℃之间具有失重台阶,失重率为10.2%。The TGA results are shown in Figure 2. There is a weight loss step before 94°C, with a weight loss rate of 0.5%, and a weight loss step between 94-150°C, with a weight loss rate of 10.2%.
3、DSC图谱分析3.DSC spectrum analysis
差示量热扫描分析结果如图3所示。晶型Form I具有在102℃的哌嗪解离温度,在213℃熔融并伴随着分解。The results of differential scanning calorimetry analysis are shown in Figure 3. Crystalline Form I has a piperazine dissociation temperature of 102°C and melts at 213°C accompanied by decomposition.
4、DVS图谱分析4. DVS graph analysis
DVS分析结果如图4所示。晶型Form I在80%RH湿度下吸湿约0.3%,表现为略有引湿性。The DVS analysis results are shown in Figure 4. Crystalline Form I absorbs about 0.3% moisture at 80% RH, showing slight hygroscopicity.
5、PLM分析5. PLM Analysis
偏振光显微镜(PLM)结果如图5所示,其显示为细小颗粒。The polarized light microscopy (PLM) results are shown in FIG5 , which show fine particles.
6、FT-IR分析6. FT-IR analysis
傅里叶红外光谱结果如图6所示。The Fourier transform infrared spectrum results are shown in Figure 6.
7、单晶PLM图谱7. Single crystal PLM map
单晶培养方法如下:取实施例1制备的样品10mg放于玻璃小瓶中,用3.4mL乙酸乙酯超声溶解,使用注射器,用0.45μm的滤头过滤。得到的澄清溶液放置室温下小孔挥发,得到块状晶体,对得到的单晶进行结构表征。单晶图谱如图7所示,克立硼罗:哌嗪=2:1,且以配位共价键的方式结合(N→B),其具有表2的单晶结构数据。The single crystal cultivation method is as follows: 10 mg of the sample prepared in Example 1 is placed in a glass vial, ultrasonically dissolved with 3.4 mL of ethyl acetate, and filtered with a 0.45 μm filter head using a syringe. The obtained clear solution is placed at room temperature for small pore volatilization to obtain block crystals, and the obtained single crystals are structurally characterized. The single crystal spectrum is shown in Figure 7, and the ratio of cresborole to piperazine is 2:1, and they are combined in a coordinated covalent bond (N→B), which has the single crystal structure data of Table 2.
表2晶型Form I的单晶结构信息表Table 2 Single crystal structure information of Form I
实施例2Example 2
取60.38mg克立硼罗和10.59mg的哌嗪,加入0.9mL乙酸乙酯,室温搅拌,得到白色混悬液,室温搅拌2天后,有大量固体析出,往其中补加2mL乙酸乙酯溶剂,继续室温搅拌1天,离心过滤,所得固体50℃下真空干燥1小时,得到克立硼罗哌嗪盐Form I。Take 60.38 mg of crisaborole and 10.59 mg of piperazine, add 0.9 mL of ethyl acetate, stir at room temperature to obtain a white suspension. After stirring at room temperature for 2 days, a large amount of solid precipitated. Add 2 mL of ethyl acetate solvent, continue stirring at room temperature for 1 day, centrifuge and filter. The obtained solid is vacuum dried at 50°C for 1 hour to obtain crisaborole piperazine salt Form I.
实施例3Example 3
取实施例1制备得到的Form I样品5mg,分别在加速(40℃,75%RH,敞口避光)和长期(25℃,60%RH,敞口避光)条件下放置,结果显示:Form I在加速和长期条件下保持21天晶型不变,说明Form I稳定性好,取样稳定性测试的XRPD图谱如图8所示。5 mg of the Form I sample prepared in Example 1 was placed under accelerated (40° C., 75% RH, open to light) and long-term (25° C., 60% RH, open to light) conditions, respectively. The results showed that Form I maintained its crystal form unchanged for 21 days under accelerated and long-term conditions, indicating that Form I had good stability. The XRPD spectrum of the sampling stability test is shown in FIG8 .
尽管本发明已经参考示例性实施方案进行了描述,但应理解本发明不限于公开的示例性实施方案。在不背离本发明的范围或精神的情况下,可对本发明说明书的示例性实施方案做多种调整或变化。权利要求的范围应基于最宽的解释以涵盖所有修改和等同结构与功能。Although the present invention has been described with reference to exemplary embodiments, it should be understood that the present invention is not limited to the disclosed exemplary embodiments. Various adjustments or changes may be made to the exemplary embodiments of the present specification without departing from the scope or spirit of the present invention. The scope of the claims should be based on the broadest interpretation to cover all modifications and equivalent structures and functions.
Claims (10)
Applications Claiming Priority (3)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| CN202111677660 | 2021-12-31 | ||
| CN202111677660X | 2021-12-31 | ||
| CN202211732252.4 | 2022-12-30 |
Related Parent Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| CN202211732252.4Division | 2021-12-31 | 2022-12-30 |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| CN116178408Atrue CN116178408A (en) | 2023-05-30 |
Family
ID=86469457
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| CN202310033870.8APendingCN116178408A (en) | 2021-12-31 | 2022-12-30 | Criborole salt and its crystal form, preparation method and application |
Country Status (1)
| Country | Link |
|---|---|
| CN (1) | CN116178408A (en) |
- 2022
- 2022-12-30CNCN202310033870.8Apatent/CN116178408A/enactivePending
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| US9090598B2 (en) | Nilotinib salts and crystalline forms thereof | |
| TWI597277B (en) | Form i crystal of dimaleate salt of tyrosine kinase inhibitor and preparation method thereof | |
| JP2013018789A (en) | Crystalline forms of 4-methyl-n-[3-(4-methyl-imidazol-1-yl)-5-trifluoromethyl-phenyl]-3-(4-pyridin-3-yl-pyrimidin-2-ylamino)-benzamide | |
| TWI519532B (en) | A crystalline form of (r)-7-chloro-n-(quinuclidin-3-yl)benzo(b)thiophene-2-carboxamide hydrochloride monohydrate | |
| WO2016184436A1 (en) | New crystal form of lenvatinib methanesulfonate salt and preparation method thereof | |
| US20240083914A1 (en) | Crystalline Polymorphs of a Muscarinic Acetylcholine Receptor Agonist | |
| JP2017137330A (en) | 4-tert-Butyl-N- [4-chloro-2- (1-oxy-pyridine-4-carbonyl) -phenyl] -benzenesulfonamide sodium salt polymorph | |
| KR102657147B1 (en) | Crystalline form of bilastin and method for producing the same | |
| WO2017025045A1 (en) | Novel crystal form of 3-(6-(1-(2,2-difluorobenzo[d][1,3]dioxole-5-yl)cyclopropane formamido)-3-methylpyridine-2-yl)benzoic acid and preparation method thereof | |
| CN116178408A (en) | Criborole salt and its crystal form, preparation method and application | |
| CN108570045B (en) | Crystal form of anisodamine hydrobromide, preparation method and pharmaceutical composition thereof | |
| JP2022508945A (en) | Crystal morphology of potassium channel modulators | |
| US11512078B2 (en) | Addition salt of S1P1 receptor agonist and crystal form thereof, and pharmaceutical composition | |
| TWI836379B (en) | Polymorphic forms of a compound and preparation methods and applications thereof | |
| TWI680983B (en) | The l-proline complex, monohydrate and crystal of a sodium-glucose contransporter 2 inhibitor | |
| WO2018214877A1 (en) | Crystal form of dezocine and preparation method therefor | |
| CN110845442A (en) | Levocetirizine hydrochloride compound and preparation method thereof | |
| CN113620982A (en) | Crystal form of Crisaborole solvate and its preparation and use | |
| JP2022540275A (en) | Crystal form, preparation method and use of tetramethylpyrazine nitrone | |
| WO2017076358A1 (en) | New crystal form of imidazolyl biphenyl compound salt and preparation method thereof | |
| JP2015057445A (en) | Solid form of chemokine receptor antagonist and application method thereof | |
| CN101775005B (en) | Cadrofloxacin hydrochloride-I crystal and preparation method thereof | |
| CN120208778A (en) | A tilatracol crystal form and preparation method thereof | |
| BR112019005450B1 (en) | Crystalline polymorphs of a muscarinic acetylcholine receptor agonist, processes for preparing such polymorphs, process for maintaining a stable Form II of the polymorph, pharmaceutical composition, process for preparing medicament and use of such polymorphs | |
| HK40042284A (en) | Addition salt of s1p1 receptor agonist and crystal form thereof, and pharmaceutical composition |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PB01 | Publication | ||
| PB01 | Publication | ||
| WD01 | Invention patent application deemed withdrawn after publication | Application publication date:20230530 | |
| WD01 | Invention patent application deemed withdrawn after publication |