CN116106380A - Enhanced electrode for determining dopamine and preparation method and application method thereof - Google Patents
Enhanced electrode for determining dopamine and preparation method and application method thereofDownload PDFInfo
- Publication number
- CN116106380A CN116106380ACN202211698498.4ACN202211698498ACN116106380ACN 116106380 ACN116106380 ACN 116106380ACN 202211698498 ACN202211698498 ACN 202211698498ACN 116106380 ACN116106380 ACN 116106380A
- Authority
- CN
- China
- Prior art keywords
- electrode
- carbon fiber
- dopamine
- enhanced
- cfe
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Pending
Links
Images
Classifications
- G—PHYSICS
- G01—MEASURING; TESTING
- G01N—INVESTIGATING OR ANALYSING MATERIALS BY DETERMINING THEIR CHEMICAL OR PHYSICAL PROPERTIES
- G01N27/00—Investigating or analysing materials by the use of electric, electrochemical, or magnetic means
- G01N27/26—Investigating or analysing materials by the use of electric, electrochemical, or magnetic means by investigating electrochemical variables; by using electrolysis or electrophoresis
- G01N27/28—Electrolytic cell components
- G01N27/30—Electrodes, e.g. test electrodes; Half-cells
- G01N27/308—Electrodes, e.g. test electrodes; Half-cells at least partially made of carbon
- G—PHYSICS
- G01—MEASURING; TESTING
- G01N—INVESTIGATING OR ANALYSING MATERIALS BY DETERMINING THEIR CHEMICAL OR PHYSICAL PROPERTIES
- G01N27/00—Investigating or analysing materials by the use of electric, electrochemical, or magnetic means
- G01N27/26—Investigating or analysing materials by the use of electric, electrochemical, or magnetic means by investigating electrochemical variables; by using electrolysis or electrophoresis
- G01N27/28—Electrolytic cell components
- G01N27/30—Electrodes, e.g. test electrodes; Half-cells
- G01N27/327—Biochemical electrodes, e.g. electrical or mechanical details for in vitro measurements
- G01N27/3275—Sensing specific biomolecules, e.g. nucleic acid strands, based on an electrode surface reaction
Landscapes
- Life Sciences & Earth Sciences (AREA)
- Health & Medical Sciences (AREA)
- Chemical & Material Sciences (AREA)
- Physics & Mathematics (AREA)
- Molecular Biology (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Electrochemistry (AREA)
- Analytical Chemistry (AREA)
- Biochemistry (AREA)
- General Health & Medical Sciences (AREA)
- General Physics & Mathematics (AREA)
- Immunology (AREA)
- Pathology (AREA)
- Spectroscopy & Molecular Physics (AREA)
- Measuring Or Testing Involving Enzymes Or Micro-Organisms (AREA)
Abstract
Description
Translated fromChinese技术领域technical field
本发明涉及生物检测技术领域,特别是涉及一种用于测定多巴胺的增强电极及其制备方法和使用方法。The invention relates to the technical field of biological detection, in particular to an enhanced electrode for measuring dopamine, a preparation method and a use method thereof.
背景技术Background technique
活体动物中枢神经系统中的神经递质动力学是理解脑发育和疾病诊断的分子基础的重要工具。在活体脑细胞的相关神经标志物检测方面取得了良好的结果。多巴胺(Dopamine,DA)作为一种在多巴胺能系统中起重要作用的儿茶酚胺类神经递质,参与了激励机制、成瘾机制和强化学习机制。多巴胺能系统受损,导致多巴胺水平异常和神经病理学改变,与帕金森病和精神分裂症等神经疾病有关。脂多糖(Lipopolysaccharide,LPS)激活脑内小胶质细胞和星形胶质细胞,引起神经炎症,损伤多巴胺能神经元。LPS摄入引发的神经炎症和多巴胺能神经元损伤,稳定、实时特异性检测DA波动,将对多巴胺能神经疾病的研究和治疗具有重要的临床和科学价值。Neurotransmitter dynamics in the central nervous system of living animals is an important tool for understanding the molecular basis of brain development and disease diagnosis. Good results have been achieved in the detection of relevant neural markers in living brain cells. Dopamine (Dopamine, DA) is a catecholamine neurotransmitter that plays an important role in the dopaminergic system, and is involved in motivational mechanisms, addiction mechanisms and reinforcement learning mechanisms. Impairment of the dopaminergic system, leading to abnormal dopamine levels and neuropathological changes, has been associated with neurological disorders such as Parkinson's disease and schizophrenia. Lipopolysaccharide (LPS) activates microglia and astrocytes in the brain, causes neuroinflammation, and damages dopaminergic neurons. Neuroinflammation and dopaminergic neuron damage induced by LPS intake, stable and real-time specific detection of DA fluctuations will have important clinical and scientific value for the research and treatment of dopaminergic neurological diseases.
准确、高稳定性的多巴胺(dopamine,DA)监测对于研究脑功能和病理的化学基础至关重要。组织可植入碳纤维电极(CFE)由于其电化学原理,在亚秒时间尺度内显示出巨大的潜力,但在抗污染、选择性和稳定性方面面临着巨大的挑战。Accurate and highly stable monitoring of dopamine (DA) is essential for studying the chemical basis of brain function and pathology. Tissue-implantable carbon fiber electrodes (CFEs) have shown great potential in the subsecond time scale due to their electrochemical principles, but face great challenges in terms of anti-fouling, selectivity, and stability.
发明内容Contents of the invention
为了解决上述技术问题,本发明采用以下技术方案:In order to solve the above technical problems, the present invention adopts the following technical solutions:
根据本发明的第一方面,提供一种用于测定多巴胺的增强电极,包括碳纤维电极,在所述碳纤维电极上依次修饰壳聚糖、脑细胞膜和特异性适配子。According to the first aspect of the present invention, there is provided an enhanced electrode for measuring dopamine, comprising a carbon fiber electrode, on which chitosan, brain cell membrane and specific aptamers are sequentially modified.
进一步地,所述特异性适配子为胆固醇两亲性适配子。Further, the specific aptamer is a cholesterol amphipathic aptamer.
根据本发明的第二方面,提供一种如上所述的用于测定多巴胺的增强电极的制备方法,所述制备方法包括:According to a second aspect of the present invention, there is provided a method for preparing an enhanced electrode for measuring dopamine as described above, the preparation method comprising:
制备碳纤维电极;Preparation of carbon fiber electrodes;
将所述碳纤维电极浸泡在壳聚糖溶液中,并在预设时间内对其电极施加相对于溶液中对电极的负电位,得到被壳聚糖修饰后的碳纤维电极;Soaking the carbon fiber electrode in a chitosan solution, and applying a negative potential to the electrode relative to the counter electrode in the solution within a preset time, to obtain a carbon fiber electrode modified by chitosan;
在所述被壳聚糖修饰后的碳纤维电极上包被脑细胞膜;Coating the brain cell membrane on the carbon fiber electrode modified by chitosan;
将特异性适配子加入到被所述脑细胞膜包被后的碳纤维电极中得到用于测定多巴胺的增强电极。The specific aptamer is added to the carbon fiber electrode coated with the brain cell membrane to obtain an enhanced electrode for measuring dopamine.
进一步地,所述制备碳纤维电极,包括:Further, the preparation of carbon fiber electrodes includes:
将一个玻璃毛细血管拉入两条细毛细血管中,作为碳纤维电极的护套;A glass capillary is pulled into two thin capillaries to serve as a sheath for the carbon fiber electrode;
将一根碳纤维连接到一根带有银导电膏的铜丝上,并将铜丝插入所述护套中;A carbon fiber is attached to a copper wire with silver conductive paste and the copper wire is inserted into said sheath;
将碳纤维微的两端用融化的石蜡密封,并在空气中室温固化;Seal both ends of the carbon fiber micro with melted paraffin and cure in air at room temperature;
将从玻璃上延伸出的纤维长度固定在玻璃密封件处。Secure the length of fiber extending from the glass at the glass seal.
进一步地,将所述碳纤维电极浸泡在壳聚糖溶液中,并在300-500s内对其电极施加-0.5V的电压,以增加抗AA干扰效果。Further, soak the carbon fiber electrode in the chitosan solution, and apply a voltage of -0.5V to the electrode within 300-500s to increase the anti-AA interference effect.
进一步地,在所述被壳聚糖修饰后的碳纤维电极上包被脑细胞膜,包括:Further, coating the brain cell membrane on the carbon fiber electrode modified by chitosan, including:
在包被之前,用超声将囊泡溶液匀浆,选择0.5-3.0h的任意时间作为细胞膜的修饰时间,最后将包被脑细胞膜的碳纤维电极干燥。Before coating, the vesicle solution was homogenized by ultrasound, any time from 0.5 to 3.0 h was selected as the modification time of the cell membrane, and finally the carbon fiber electrode coated with the brain cell membrane was dried.
进一步地,将特异性适配子加入到被所述脑细胞膜包被后的碳纤维电极中得到用于测定多巴胺的增强电极,包括:Further, specific aptamers are added to the carbon fiber electrodes coated with the brain cell membrane to obtain enhanced electrodes for measuring dopamine, including:
基于胆固醇与细胞膜的亲和性,将胆固醇两亲性适配子加入到所述脑细胞膜包被后的碳纤维电极中;Based on the affinity between cholesterol and the cell membrane, the cholesterol amphiphilic aptamer is added to the carbon fiber electrode coated with the brain cell membrane;
将3端经过胆固醇基团修饰的适配子溶解于Mg2+溶液中;Dissolve the aptamer modified with cholesterol group at the 3 end in Mg2+ solution;
随后浸入适配体溶液中;Subsequent immersion in the aptamer solution;
冲洗电极并储存在磷酸盐缓冲液中,制备得到用于测定多巴胺的增强电极。Electrodes were rinsed and stored in phosphate buffered saline to prepare enhanced electrodes for the measurement of dopamine.
进一步地,将3端经过胆固醇基团修饰的适配子溶解于5mM Mg2+溶液中。Further, the aptamer modified with cholesterol group at the 3 end was dissolved in 5mM Mg2+ solution.
进一步地,浸入1uM适配体溶液中12小时。Further, immersed in 1uM aptamer solution for 12 hours.
根据本发明的第三方面,提供一种如上所述的用于测定多巴胺的增强电极的使用方法,所述使用方法包括:将所述增强电极插入黑质纹状体(SNc),并加入适量氯化钾,根据所述增强电极探测到的电流响应确定多巴胺浓度或确定是否含有多巴胺。According to a third aspect of the present invention, there is provided a method for using the enhanced electrode for measuring dopamine as described above, the method includes: inserting the enhanced electrode into the nigrostriatum (SNc), and adding an appropriate amount of Potassium chloride is used to determine the concentration of dopamine or determine whether it contains dopamine according to the current response detected by the enhanced electrode.
根据本发明的第四方面,提供一种如上所述的用于测定多巴胺的增强电极的使用方法,其特征在于,所述使用方法包括:将所述增强电极浸入PC12细胞群中,利用K+去极化细胞,根据述增强电极探测到的电流响应确定多巴胺浓度或确定是否含有多巴胺。According to a fourth aspect of the present invention, there is provided a method for using the enhanced electrode for measuring dopamine as described above, wherein the method includes: immersing the enhanced electrode in the PC12 cell population, using K+ to remove Polarize the cells, and determine the concentration of dopamine or whether it contains dopamine according to the current response detected by the enhanced electrodes.
与现有技术相比,本发明至少具备以下有益效果:Compared with the prior art, the present invention at least has the following beneficial effects:
本发明提出了一种利用壳聚糖(CS)膜和自体脑细胞膜(M)通过修饰适体胆固醇两亲分子(DNA-cho)来增强电极生物相容性和稳定性的新策略。采用扫描电镜、荧光显微镜、zeta电位分析仪和水接触角仪对其表面形貌进行了研究。结果表明,CFE改性成功,并被类蝉膜均匀覆盖。电化学表征表明,DNA-cho-M-CS-CFE具有较宽的DA浓度线性范围,具有较高的灵敏度、特异性和稳定性。电极还表现出良好的抗污性和生物相容性。此外,该生物传感器还可用于检测钾离子(K+)处理的脑片和神经细胞中的DA,具有良好的稳定性和灵敏度。此外,对脂多糖(LPS)处理的脑片和PC12细胞的检测也证明了电极在实际应用中的价值,证明了LPS诱导DA释放延迟和减少。适配子功能化细胞膜修饰的DNA-cho-M-CS-CFE不仅具有优异的电化学性能,而且在体内和活细胞的长期传感方面具有必要的优势,为研究神经化学动力学和脑部疾病提供了一种新的可行方案。The present invention proposes a new strategy to enhance electrode biocompatibility and stability by modifying aptamer cholesterol amphiphile (DNA-cho) using chitosan (CS) membrane and autologous brain cell membrane (M). The surface morphology was studied by scanning electron microscope, fluorescence microscope, zeta potential analyzer and water contact angle meter. The results showed that the CFE was successfully modified and covered evenly by cicada-like membranes. Electrochemical characterization showed that DNA-cho-M-CS-CFE had a wide linear range of DA concentration, and had high sensitivity, specificity and stability. The electrodes also exhibited good fouling resistance and biocompatibility. In addition, this biosensor can also be used to detect DA in potassium ion (K+)-treated brain slices and neurons with good stability and sensitivity. In addition, the detection of lipopolysaccharide (LPS)-treated brain slices and PC12 cells also demonstrated the value of the electrodes for practical applications, demonstrating that LPS induced delayed and reduced DA release. The aptamer-functionalized cell membrane-modified DNA-cho-M-CS-CFE not only possesses excellent electrochemical performance, but also has the necessary advantages in long-term sensing in vivo and in living cells, providing a useful tool for the study of neurochemical dynamics and brain Disease offers a new possibility.
附图说明Description of drawings
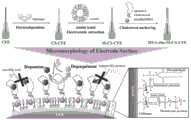
图1为本发明实施例中的DNA-cho-M-CS-CFE的制备原理和电极表面微形态多巴胺的测量示意图;1 is a schematic diagram of the preparation principle of DNA-cho-M-CS-CFE and the measurement of micromorphic dopamine on the electrode surface in the embodiment of the present invention;
图2为200uMAA和10uM DA的安培响应强度的比例(n=3),不同CS沉积时间所占比例,0、50、200、300、400、500s(A);不同细胞膜孵育时间所占比例(M),0、0.5、1.0、2.0、3.0h(B);不同干燥时间的影响(C);Figure 2 is the ratio (n=3) of the ampere response intensity of 200uMAA and 10uM DA, the ratio of different CS deposition times, 0, 50, 200, 300, 400, 500s (A); the ratio of different cell membrane incubation times ( M), 0, 0.5, 1.0, 2.0, 3.0h (B); the effect of different drying time (C);
图3为CS碳布、M-CS碳布和DNA-cho-M-CS碳布薄膜的zeta电位(n=3);Fig. 3 is the zeta potential of CS carbon cloth, M-CS carbon cloth and DNA-cho-M-CS carbon cloth film (n=3);
图4为裸-CFE(A&E)、CS-CFE(B&F)、M-CS-CF(C&G)和DNA-cho-M-CS-CF(D&H)的扫描电镜图像;Figure 4 is the SEM images of bare-CFE (A&E), CS-CFE (B&F), M-CS-CF (C&G) and DNA-cho-M-CS-CF (D&H);
图5为裸碳纤维、CS改性碳纤维和细胞膜-CS改性碳纤维的EIS Nyquist图;Figure 5 is the EIS Nyquist diagram of bare carbon fiber, CS modified carbon fiber and cell membrane-CS modified carbon fiber;
图6为采用循环伏安法(CV)和安培法(n=3)检测电化学特性。DNA-cho-M-CS-CFE的CV曲线分别为0、10、20、50uM DA(A)。对DNA-cho-M-CS-CFE进行电势优化,电势分别为+0.08V、+0.12V、+0.16V、+0.18V vs.Ag/AgCl(B)。200uMAA在CS和细胞膜修饰的玻碳电极(GCE)和裸GCE上的CV分别为(C)。在DNA-cho-M-CS-CFE(D)上,分别有10uM DA、10uM DOPAC、100uMAA、10uM UA、10uM NE和10uM DA的典型安培响应,所有检测均在PH=7.4的aCSF中实现。DNA-cho-M-CS-CFE对DA从5nM到100nM(E)和0.2uM到10.0uM(G)连续添加的安培电流响应。所有检测均在aCSF(PH=7.4)中实现。相应的校准曲线(F)。应用电位:+0.16V(对Ag/AgCl);Figure 6 shows the electrochemical characteristics detected by cyclic voltammetry (CV) and amperometry (n=3). The CV curves of DNA-cho-M-CS-CFE were 0, 10, 20, 50uM DA (A). The potentials were optimized for DNA-cho-M-CS-CFE, and the potentials were +0.08V, +0.12V, +0.16V, +0.18V vs. Ag/AgCl(B). The CVs of 200uMAA on CS and membrane-modified glassy carbon electrode (GCE) and bare GCE are (C), respectively. On DNA-cho-M-CS-CFE (D), there were typical amperometric responses of 10uM DA, 10uM DOPAC, 100uMAA, 10uM UA, 10uM NE and 10uM DA, respectively, all assays were realized in aCSF at pH=7.4. DNA-cho-M-CS-CFE responds to amperometric current additions of DA from 5nM to 100nM (E) and 0.2uM to 10.0uM (G) sequentially. All assays were performed in aCSF (PH=7.4). Corresponding calibration curve (F). Applied potential: +0.16V (for Ag/AgCl);
图7为研究DNA-cho-M-CS-CFE的稳定性、抗污性和机械稳定性的示意图:裸碳布(A)和CS与细胞膜改性碳布(B)的水接触角。用DNA-cho-M-CS-CFE(C)记录aCSF对10uM DA的安培电流响应。在加入10mg mL-1BSA(D)后,用裸CFE(黑色)和DNA-cho-M-CS-CFE(红色)记录aCSF中对10uM DA的典型安培响应。I0和I分别为起始时间和给定时间的电流值。DNA-cho-M-CS-CFE在DMEM中浸泡1h(PH=7.40)前(黑色)和后(红色)对DA的安培响应(E)。在脑片植入前(黑色)和植入后(红色)连续添加DA的aCSF中,DNA-cho-M-CS-CFE的安培电流反应(F);Figure 7 is a schematic diagram for studying the stability, stain resistance and mechanical stability of DNA-cho-M-CS-CFE: water contact angles of bare carbon cloth (A) and CS with cell membrane-modified carbon cloth (B). The amperometric response of aCSF to 10uM DA was recorded with DNA-cho-M-CS-CFE (C). Typical amperometric responses to 10uM DA in aCSF were recorded with bare CFE (black) and DNA-cho-M-CS-CFE (red) after addition of 10mg mL-1BSA (D). I0 and I are the starting time and the current value at a given time, respectively. Amperometric response (E) of DNA-cho-M-CS-CFE to DA before (black) and after (red) immersion in DMEM for 1 h (pH = 7.40). Amperometric response of DNA-cho-M-CS-CFE in aCSF with continuous DA supplementation before (black) and after (red) implantation of brain slices (F);
图8为材料的生物相容性(n=3)。CCK-8法检测L929对照组、裸碳布和DNA-cho-M-CS改性碳布(A)。裸碳布和改性碳布培养的Hela-RFP细胞荧光(B);Figure 8 shows the biocompatibility of materials (n=3). CCK-8 detection of L929 control group, bare carbon cloth and DNA-cho-M-CS modified carbon cloth (A). Fluorescence of Hela-RFP cells cultured on bare carbon cloth and modified carbon cloth (B);
图9为.bare-CFE依次对添加的0.04M K+和50nM DA进行监测的结果;Figure 9 is the result of monitoring the added 0.04M K+ and 50nM DA sequentially by .bare-CFE;
图10为DNA-cho-M-CS-CFE在脑切片和PC12细胞中的当前监测和应用(每个n=3)。DNA-cho-M-CS-CFE在0.04M K+高浓度(A)刺激下,有(红色)和无(黑色)脑片的安培响应。0.04K+添加连续三次监测DNA-cho-M-CS-CFE(B)。在LPS浸泡前(黑色)和浸泡后(红色)分别加入0.04M K+,用DNA-cho-M-CS-CFE(C)监测。DNA-cho-M-CS-CFE在0.4M K+(红色),0.4M K+LPS预处理(蓝色)和培养液(黑色)刺激PC12细胞时的安培反应(D)。Figure 10 presents the current monitoring and application of DNA-cho-M-CS-CFE in brain slices and PC12 cells (n=3 each). Amperometric responses of DNA-cho-M-CS-CFE with (red) and without (black) brain slices stimulated by a high concentration of 0.04M K+ (A). 0.04K+ addition of three consecutive monitoring DNA-cho-M-CS-CFE (B). 0.04M K+ was added before (black) and after (red) immersion in LPS, monitored by DNA-cho-M-CS-CFE (C). Amperometric responses of DNA-cho-M-CS-CFE to PC12 cells stimulated by 0.4M K+ (red), 0.4M K+LPS pretreatment (blue) and culture medium (black) (D).
具体实施方式Detailed ways
以下列举的部分实施例仅仅是为了更好地对本发明进行说明,但本发明的内容并不局限在应用于所举的实施例中。所以熟悉本领域的技术人员根据上述发明内容对实施方案进行非本质的改进和调整而应用于其他实施例中,仍在本发明的保护范围之内。Some of the examples listed below are just to better illustrate the present invention, but the content of the present invention is not limited to be applied to the examples cited. Therefore, it is still within the protection scope of the present invention that those skilled in the art make non-essential improvements and adjustments to the embodiments based on the above-mentioned content of the invention and apply them to other embodiments.
实施例1:用于测定多巴胺的增强电极Example 1: Enhanced electrode for measuring dopamine
本发明实施例提供一种用于测定多巴胺的增强电极,包括碳纤维电极,在所述碳纤维电极上依次修饰壳聚糖、脑细胞膜和特异性适配子。An embodiment of the present invention provides an enhanced electrode for measuring dopamine, including a carbon fiber electrode, on which chitosan, brain cell membrane and specific aptamer are sequentially modified.
需要说明的是,碳纤维电极(CFE)是一种具有高生物相容性、无毒、高灵敏度的电化学基底材料,几十年来广泛应用于电化学多巴胺检测领域。直径小于10μm的CFEs可植入活体脑组织或单细胞内进行局部组织监测或单细胞检测。然而,由于DA与抗坏血酸(ascorbic acid,AA)和尿酸(uric acid,UA)的分子相似性,DA的电化学检测经常受到干扰。同时,CFEs检测活体脑组织时,多巴胺常非特异性吸附蛋白质,造成污染,降低电极的检测性能。用抗污染材料修饰电极是常见的解决方案,但多次修饰后电极的灵敏度和时空分辨率降低,混合修饰材料会增加体内检测炎症等不良反应的风险,导致生物安全风险。因此,迫切需要开发一种生物相容性策略,在保证优异的特异性、敏感性等电化学性能的同时,赋予电极抗污性。It should be noted that carbon fiber electrode (CFE) is an electrochemical substrate material with high biocompatibility, non-toxicity and high sensitivity, and has been widely used in the field of electrochemical dopamine detection for decades. CFEs with a diameter of less than 10 μm can be implanted into living brain tissue or single cells for local tissue monitoring or single cell detection. However, the electrochemical detection of DA is often disturbed due to the molecular similarity of DA to ascorbic acid (AA) and uric acid (UA). At the same time, when CFEs detect living brain tissue, dopamine is very non-specifically adsorbed to proteins, causing contamination and reducing the detection performance of the electrodes. Modification of electrodes with anti-pollution materials is a common solution, but the sensitivity and spatiotemporal resolution of electrodes decrease after multiple modifications, and mixing modified materials will increase the risk of in vivo detection of adverse reactions such as inflammation, leading to biosafety risks. Therefore, there is an urgent need to develop a biocompatibility strategy to endow electrodes with antifouling properties while ensuring excellent electrochemical properties such as specificity and sensitivity.
壳聚糖以其生物活性在医药和生物医学材料中得到了广泛的应用。壳聚糖具有特殊的分子结构和理化性质,可分解为氨基糖等易吸收的安全化合物,具有良好的生物可降解性。此外,壳聚糖提供了带有正电荷的氨基,可以排斥AA。其无毒、修饰简单,具有良好的生物相容性,已成为一种理想的生物安全基质。研究人员利用脑细胞膜(M)覆盖电极,解决了蛋白质污染的问题。由于磷脂双分子层的亲水部分,可在电极表面上形成亲水膜。它可以通过氢键或离子相互作用与水分子结合,形成对非特异性蛋白吸附具有高度抗性的水合层,达到防污染的目的。最后,引入了特异性适配子以提高对多巴胺的选择性和敏感性。是一种含有胆固醇的单链DNA或RNA,与DA具有高亲和力。它可以通过胆固醇连接到电极表面,而不是静电作用,增加了电极的稳定性。通过施加的电压,DA被氧化,电子通过细胞膜和壳聚糖,CFE检测。从而实现DA的高选择性、高灵敏度和高生物相容性检测目标,提高电极的综合性能。Chitosan has been widely used in medicine and biomedical materials because of its biological activity. Chitosan has a special molecular structure and physical and chemical properties, can be decomposed into easily absorbed safe compounds such as amino sugar, and has good biodegradability. In addition, chitosan provides positively charged amino groups that repel AA. It is non-toxic, easy to modify, and has good biocompatibility, and has become an ideal biosafety matrix. The researchers solved the problem of protein contamination by covering the electrodes with brain cell membranes (M). Due to the hydrophilic part of the phospholipid bilayer, a hydrophilic film can be formed on the electrode surface. It can combine with water molecules through hydrogen bonds or ionic interactions to form a hydration layer that is highly resistant to non-specific protein adsorption and achieve the purpose of anti-pollution. Finally, specific aptamers were introduced to increase selectivity and sensitivity to dopamine. It is a single-stranded DNA or RNA containing cholesterol and has a high affinity with DA. It can be attached to the electrode surface through cholesterol instead of electrostatic interaction, which increases the stability of the electrode. By the applied voltage, DA is oxidized, and electrons pass through the cell membrane and chitosan, which are detected by CFE. In this way, the detection targets of high selectivity, high sensitivity and high biocompatibility of DA can be achieved, and the comprehensive performance of the electrode can be improved.
如图1所示,本发明提出了一种将壳聚糖、M和特异性适配子依次修饰在CFE上的策略,成功研制出一种由多种生物材料组成的高生物安全性电极(DNA-cho-M-CS-CFE)。我们构建了钾离子诱导的PC12细胞和LPS诱导的成年大鼠作为帕金森病模型。将DNA-cho-M-CS-CFE植入细胞群或脑片中。结果显示,K+刺激和LPS抑制DA释放均可产生显著的DA信号,表明该复合修饰策略具有检测稳定性。DNA-cho-M-CS-CFE在神经细胞和脑片中的性能证明了其在各种生物环境中进行长期、稳定、原位电化学监测和灵敏反应的可行性。它为揭示生物分子在大脑神经过程中的作用和相互关系提供了一种新的传感方法。此外,它为神经递质动力学和神经病理学提供了一种新的稳定和敏感的分析方法,为人类大脑计划做出了贡献。As shown in Figure 1, the present invention proposes a strategy of sequentially modifying chitosan, M and specific aptamers on CFE, successfully developing a high biosafety electrode composed of a variety of biological materials ( DNA-cho-M-CS-CFE). We constructed potassium ion-induced PC12 cells and LPS-induced adult rats as Parkinson's disease models. Implant DNA-cho-M-CS-CFE into cell populations or brain slices. The results showed that both K+ stimulation and LPS inhibition of DA release could generate significant DA signals, indicating that the composite modification strategy has detection stability. The performance of DNA-cho-M-CS-CFE in neural cells and brain slices demonstrates its feasibility for long-term, stable, in situ electrochemical monitoring and sensitive responses in various biological settings. It provides a new sensing method for revealing the roles and interrelationships of biomolecules in the neural processes of the brain. Furthermore, it provides a new robust and sensitive analytical method for neurotransmitter dynamics and neuropathology, contributing to the Human Brain Project.
实施例2:电极的制备Embodiment 2: the preparation of electrode
试剂和溶液说明:Reagent and Solution Description:
壳聚糖、Tris、乙二胺四乙酸(EDTA)、不含EDTA的微型蛋白酶抑制剂表、细胞膜红荧光探针(DiI)和胰蛋白酶购自BBI Life since(上海)。从BBI Life since(上海,中国)合成了胆固醇两亲性适配子(DNA-cho,5'-ggacgacgccagtttgaaggttcgttcgcaggtgggagtgacgtcgtcctttttt-teg-cho-3')。盐酸多巴胺(DA)购自Mackin(上海,中国)。抗坏血酸(AA)、二羟苯乙酸(DOPAC)、去甲肾上腺素(NE)、尿酸(UA)和牛血清白蛋白(BSA)从Sigma-Aldrich(St.Louis,MO,USA)获得。所有其他化学物质都是分析用的。将NaCl(126mM)、KCl(2.4mM)、KH2PO4(0.50mM)、MgCl2(0.85mM)、NaHCO3(27.5mM)、Na2SO4(0.50mM)、CaCl2(1.10mM)混合到milliq水中,然后调节PH值至7.4,制备人工脑脊液(aCSF)为魏欢等(Wei et al.2020)。将NaH2PO4(0.1M)和Na2HPO4(0.1M)混合,调整PH为7.4,制备磷酸盐缓冲液(PBS)。Chitosan, Tris, ethylenediaminetetraacetic acid (EDTA), EDTA-free miniature protease inhibitor sheets, cell membrane red fluorescent probe (DiI) and trypsin were purchased from BBI Life since (Shanghai). A cholesterol amphipathic aptamer (DNA-cho,5'-ggacgacgccagtttgaaggttcgttcgcaggtgggagtgacgtcgtcctttttt-teg-cho-3') was synthesized from BBI Life since (Shanghai, China). Dopamine hydrochloride (DA) was purchased from Mackin (Shanghai, China). Ascorbic acid (AA), dihydroxyphenylacetic acid (DOPAC), norepinephrine (NE), uric acid (UA) and bovine serum albumin (BSA) were obtained from Sigma-Aldrich (St. Louis, MO, USA). All other chemicals are analytical. Mix NaCl(126mM), KCl(2.4mM), KH2PO4(0.50mM), MgCl2(0.85mM), NaHCO3(27.5mM), Na2SO4(0.50mM), CaCl2(1.10mM) into milliq water, then adjust the pH To 7.4, prepare artificial cerebrospinal fluid (aCSF) as Wei Huan et al. (Wei et al.2020). Mix NaH2PO4 (0.1M) and Na2HPO4 (0.1M) to adjust the pH to 7.4 to prepare phosphate buffered saline (PBS).
脑细胞膜制备:Brain cell membrane preparation:
简单地说,用手术刀分离大脑,并用2mM的乙二胺四乙酸在磷酸盐缓冲盐水(PBS,PH=7.4)中洗涤。随后,将500uL的胰蛋白酶注入大脑,将其溶解成碎片,消化10分钟。为了停止消化,在PBS中使用2mM的乙二胺四乙酸。细胞悬液应搅拌、过滤并收集。将细胞悬液在500×g处离心3次,收集颗粒,然后悬浮于低渗溶解缓冲液(pH=7.5)中,每10mL溶液含20mMTris-HCl、10mM KCl、2mM MgCl2和1片不含EDTA的微型蛋白酶抑制剂片。为制备囊泡溶液,将细胞悬液置于10000×g中离心,4℃冰箱保存备用。Briefly, brains were dissected with a scalpel and washed with 2 mM EDTA in phosphate-buffered saline (PBS, pH=7.4). Subsequently, 500 uL of trypsin was injected into the brain to dissolve it into pieces and digested for 10 minutes. To stop digestion, 2 mM EDTA in PBS was used. The cell suspension should be stirred, filtered and collected. Centrifuge the
电极制造:Electrode Manufacturing:
简单地说,用微电极拉丝器(RWD MP-500微电机拉丝机,中国深圳)将一个玻璃毛细血管(径1.5mm,长1.0cm)拉入两条细毛细血管中,作为CFEs的护套。将一根直径7μm的碳纤维(深圳东利电子有限公司,中国深圳)连接到一根带有银导电膏的铜丝上。然后,将铜丝小心地插入毛细管中。将碳纤维微电极的两端用融化的石蜡密封,并在空气中室温固化。用手术刀将从玻璃上延伸出的纤维长度固定在距离玻璃密封件约0.5mm处。Briefly, a glass capillary (1.5 mm in diameter and 1.0 cm in length) was pulled into two thin capillaries with a microelectrode wire puller (RWD MP-500 micro motor wire drawing machine, Shenzhen, China) as a sheath for CFEs . A carbon fiber with a diameter of 7 μm (Shenzhen Dongli Electronics Co., Ltd., Shenzhen, China) was connected to a copper wire with silver conductive paste. Then, carefully insert the copper wire into the capillary. Both ends of the carbon fiber microelectrodes were sealed with melted paraffin and cured in air at room temperature. Fix the length of fiber extending from the glass about 0.5 mm from the glass seal with a scalpel.
简而言之,CS在0.1M HCl溶液中溶解后使用。然后,将CFE浸入壳聚糖溶液中,并对其电极施加相对于溶液中对电极的负电位。为了使壳聚糖的厚度与细胞膜之间建立紧密的联系,我们优化了沉积条件。因此,为了获得最佳的抗AA干扰效果,我们尝试了电压为-0.5V,施加0、50、200、300、400、500s,以获得最佳的抗AA干扰效果。最后选择400s作为壳聚糖电沉积的时间。Briefly, CS was used after being dissolved in 0.1M HCl solution. Then, the CFE was immersed in the chitosan solution and a negative potential was applied to its electrode relative to the counter electrode in solution. In order to establish a tight connection between the thickness of chitosan and the cell membrane, we optimized the deposition conditions. Therefore, in order to obtain the best anti-AA interference effect, we tried a voltage of -0.5V and applied 0, 50, 200, 300, 400, 500s to obtain the best anti-AA interference effect. Finally choose 400s as the time of chitosan electrodeposition.
脑细胞膜(Brain cell membrane,BCM)采用浸泡法包被。CS-CFE浸泡前,用超声将囊泡溶液匀浆15min。在50℃条件下,对0、0.5、1.0、2.0、3.0h孵育时间进行评估,以获得最佳孵育时间。我们选择1h作为细胞膜的修饰时间。将包被膜的CS-CFE分别在35℃下干燥0、3、24、36h,寻找最佳干燥时间。我们最终会让电极整夜彻底干燥。我们将细胞膜包被的CS-CFEs命名为M-CS-CFEs。将细胞膜包被的CS-CFEs命名为M-CS-CFEs。然后,通过胆固醇与细胞膜的亲和性,将该适配子加入到M-CS-CFEs中。将3端经过胆固醇基团修饰的适配子溶解于5mM Mg2+溶液中。然后,我们将M-CS-CFEs浸入1uM适配体溶液中12小时。之后,用milliq水冲洗电极并储存在磷酸盐缓冲液中。成功制备DNA-cho-M-CS-CFEs。Brain cell membrane (BCM) was coated by soaking method. Before soaking CS-CFE, the vesicle solution was homogenized for 15 min by ultrasound. Under the condition of 50°C, evaluate the incubation time of 0, 0.5, 1.0, 2.0, and 3.0 h to obtain the optimal incubation time. We choose 1h as the modification time of the cell membrane. The membrane-coated CS-CFE was dried at 35°C for 0, 3, 24, and 36 hours, respectively, to find the best drying time. We end up letting the electrodes dry completely overnight. We named the cell membrane-coated CS-CFEs as M-CS-CFEs. The membrane-coated CS-CFEs were named M-CS-CFEs. Then, this aptamer was incorporated into M-CS-CFEs through the affinity of cholesterol to the cell membrane. The aptamers modified with cholesterol groups at the 3 ends were dissolved in 5mM Mg2+ solution. Then, we immersed the M-CS-CFEs in 1uM aptamer solution for 12 hours. Afterwards, the electrodes were rinsed with milliq water and stored in phosphate buffered saline. Successfully prepared DNA-cho-M-CS-CFEs.
实施例3:电极的优化Embodiment 3: Optimization of electrodes
脑内存在多种小分子干扰DA的检测,其中以抗坏血酸(AA)为主要干扰物。为此,200uMAA和10uM DA依次加入系统中,通过安培分析以优化电极(每n=3)。反应强度的比例代表了不同优化条件下AA对DA的干扰程度。There are a variety of small molecules in the brain that interfere with the detection of DA, among which ascorbic acid (AA) is the main interferer. To this end, 200uMAA and 10uM DA were sequentially added to the system, and the electrodes were optimized by amperometric analysis (every n=3). The ratio of response intensity represents the interference degree of AA on DA under different optimized conditions.
CS不同沉积时间、浸泡连接细胞膜1h CFE的比例如图2中A所示。与空白组相比,CS组的DA比例增加,但沉积时间无明显差异。然后,不同的M孵育时间呈现不同的影响,如图2中B所示。未孵育的CS表现出更高的AA反应。推测是因为CS膜的正电荷吸引了携带负电荷的AA。对细胞膜的高级抗AA干扰解释如下:a)细胞膜具有负电荷,表现为AA的静电排斥和DA的静电吸附;b)水溶性维生素AA难以穿透细胞膜,而DA容易渗透。初步实验证明,M的干燥时间对结果有显著影响。其结果示于图2中C中。随着时间的延长,电极完全干燥,覆盖更加均匀,表现出更高的效果。CS和M优化完成后,在电极表面对适配子进行修饰,用于后续实验。The ratio of CS to CFE at different deposition times and soaking the junctional cell membrane for 1h is shown in Figure 2A. Compared with the blank group, the proportion of DA was increased in the CS group, but the deposition time was not significantly different. Then, different M incubation times presented different effects, as shown in B in Fig. 2. Unincubated CS showed a higher AA response. It is speculated that the positive charge of the CS membrane attracts the negatively charged AA. The advanced anti-AA interference on the cell membrane is explained as follows: a) the cell membrane has a negative charge, which is manifested by the electrostatic repulsion of AA and the electrostatic adsorption of DA; b) the water-soluble vitamin AA is difficult to penetrate the cell membrane, while DA is easily permeable. Preliminary experiments demonstrated that the drying time of M had a significant effect on the results. The results are shown in Figure 2, C. As time goes on, the electrode dries completely, and the coverage is more uniform, showing a higher effect. After the optimization of CS and M, the aptamers were modified on the electrode surface for subsequent experiments.
实施例4:表面特征Example 4: Surface Features
使用SEM SU8010(Hitachi,Tokyo,Japan)获得Bare-CFE、CS-CFE和M-CS-CFE的扫描电子显微镜(SEM)图像。碳布被修改为与我们处理CFE相同的方式。采用静态水接触角测量分析仪ThetaFlex(Biolin,哥德堡,瑞典)测量裸碳布和改性碳布的水接触角。然后,使用zeta电位分析仪(美国布鲁克海文仪器公司)在碳布上进行zeta电位测定。同时,采用DiI亲脂性膜染料标记BCM。然后,用六氯荧光素(hexlorofluorescein,HEX)和胆固醇修饰的适配子标记CFE。荧光显微镜(日本尼康公司)观察特殊荧光。结果如图3和图4所示。Scanning electron microscope (SEM) images of Bare-CFE, CS-CFE and M-CS-CFE were obtained using a SEM SU8010 (Hitachi, Tokyo, Japan). The carbon cloth was modified the same way we did the CFE. The water contact angles of bare carbon cloth and modified carbon cloth were measured by static water contact angle measurement analyzer ThetaFlex (Biolin, Gothenburg, Sweden). Then, the zeta potential was measured on the carbon cloth using a zeta potential analyzer (Brookhaven Instruments, USA). Simultaneously, BCMs were labeled with DiI lipophilic membrane dye. Then, CFE was labeled with hexlorofluorescein (HEX) and cholesterol-modified aptamers. A fluorescence microscope (Nikon, Japan) was used to observe special fluorescence. The results are shown in Figure 3 and Figure 4.
裸CFE、CS-CFE、M-CS-CFE和DNA-cho-M-CS-CFE的SEM图像如图4所示。在图4的A中可以清楚地看到光滑的表面和一些线纹。不显眼的薄膜覆盖CS-CFE显示在图4的B中。在CFE上,一些小山状的沉积物沉积,条纹变浅。大量CS-CFE的交点面如图4的F所示,在两个CF之间的间隙可以更清晰地观察到CS膜,均匀地覆盖在CF上。但是bare-CFE的交点没有覆盖,如图4的E所示。山丘样沉积物被脑细胞膜修饰后消失(图4的C)。随后对样本进行切割,并拍摄横断面图像,以更清楚地看到复合膜。M-CS-CF电极的横切面显示了一层蝉状膜,完全包裹了CF。DNA-cho-M-CS-CFE的SEM图像显示在图4的D和H中。修饰适配子后,电极表面变粗,显示适配子附着。The SEM images of bare CFE, CS-CFE, M-CS-CFE and DNA-cho-M-CS-CFE are shown in Fig. 4. The smooth surface and some lines can be clearly seen in A of Fig. 4. The unobtrusive film-covered CS-CFE is shown in Figure 4B. On the CFE, some hill-like sediments were deposited and the streaks became lighter. The intersection surface of a large number of CS-CFEs is shown in F of Fig. 4, and the CS film can be observed more clearly in the gap between the two CFs, uniformly covering the CFs. But the intersection of bare-CFE is not covered, as shown in E of Figure 4. The hillock-like deposits disappeared after being modified by brain cell membranes (Fig. 4C). The samples were then cut and cross-sectional images were taken to see the composite membrane more clearly. The cross-section of the M-CS-CF electrode revealed a cicada-like membrane that completely encapsulated the CF. The SEM images of DNA-cho-M-CS-CFE are shown in D and H of Fig. 4. After modification of the aptamers, the surface of the electrode becomes rough, showing aptamer attachment.
DiI和HEX分别标记了细胞膜和适配子。DiI是一种用于标记细胞膜的特殊荧光染料,在549nm波长激发下,其穿透细胞膜后仅呈现弱荧光,而呈现强荧光。从图4的I可以看出,DiI浸泡20min的裸CFs几乎没有荧光。此外,CS-CFE没有经过细胞膜的修饰,因此荧光也可以忽略不计(图4的J)。另一方面,DiI标记的M-CS-CFE显示出更强的明显荧光,如图4的K所示。M-CS-CFE的强荧光表明细胞膜修饰成功。用HEX标记DNA-cho的5-末端,在539nm波长下产生粉红色荧光。由HEX-DNA-cho标记的簇M-CS-CFE呈图4的L所示的强烈荧光,表明该特殊适配子修饰成功。此外,zeta电位分析也表明,在CS膜沉积时,膜修饰成功,如图3所示。使用zeta电位分析仪来执行薄膜(n=3)的zeta电位。CS膜携带正电荷,为(6.27±2.44)mV。M的负电荷为(-3.60±0.76)mV,适配子修饰前后无明显变化。因此,DA的浸润和检测是可行的。DiI and HEX labeled cell membranes and aptamers, respectively. DiI is a special fluorescent dye used to label cell membranes. Under the excitation of 549nm wavelength, it only shows weak fluorescence after penetrating the cell membrane, but strong fluorescence. It can be seen from I of Fig. 4 that bare CFs soaked in DiI for 20 min have almost no fluorescence. In addition, CS-CFE was not modified by the cell membrane, so the fluorescence was also negligible (Fig. 4J). On the other hand, DiI-labeled M-CS-CFE showed stronger apparent fluorescence, as shown in K of Fig. 4. The strong fluorescence of M-CS-CFE indicates successful cell membrane modification. The 5-terminus of DNA-cho was labeled with HEX, which produced pink fluorescence at a wavelength of 539 nm. The cluster M-CS-CFE marked by HEX-DNA-cho exhibited strong fluorescence as shown in L in Figure 4, indicating that the special aptamer was successfully modified. In addition, the zeta potential analysis also showed that the film modification was successful when the CS film was deposited, as shown in Figure 3. The zeta potential of thin films (n=3) was performed using a zeta potential analyzer. The CS membrane carries a positive charge of (6.27±2.44) mV. The negative charge of M was (-3.60±0.76) mV, and there was no significant change before and after aptamer modification. Therefore, infiltration and detection of DA is feasible.
实施例5:电化学性能Embodiment 5: electrochemical performance
电化学实验在CHI630E电化学工作站(上海晨华仪器有限公司)耦合三电极系统上进行。采用裸或改性的CFEs和玻碳电极(GCE)作为工作电极,Ag/AgCl电极作为参比电极,铂丝电极作为辅助电极。电势优化采用安培法。因此,在搅拌aCSF中使用安培法进行后期的DA检测,电位设置为0.16V。在含0.1MKCl的5.0mM[Fe(CN)6]3-/4-溶液中,进行电化学阻抗谱(EIS)测量。在室温下实施所有电化学测量。Electrochemical experiments were performed on a CHI630E electrochemical workstation (Shanghai Chenhua Instrument Co., Ltd.) coupled with a three-electrode system. Bare or modified CFEs and glassy carbon electrodes (GCE) were used as working electrodes, Ag/AgCl electrodes were used as reference electrodes, and platinum wire electrodes were used as auxiliary electrodes. Potential optimization was performed using the amperometric method. Therefore, late detection of DA was performed using amperometry in stirred aCSF with the potential set at 0.16 V. Electrochemical impedance spectroscopy (EIS) measurements were performed in 5.0 mM [Fe(CN)6]3-/4- solution containing 0.1M KCl. All electrochemical measurements were performed at room temperature.
在[Fe(CN)6]3-/4溶液中,利用电化学阻抗谱(EIS)对传感器的制备过程进行了表征。典型的奈奎斯特图由高频半圆和低频径向线组成,分别代表CFE上的界面电荷转移受限过程和扩散过程。半圆直径的增加与电荷转移电阻(Rct)的增加有关。图5展示了不同修饰电极的奈奎斯特图。如图5的A所示,使用Randles等效电路模型分析数据,包括Warburg阻抗(W)、电荷转移电阻(Rct)、双层电容(CDL)和电解质欧姆电阻(Rs)。Rs分别为bare-CFE、CS-CFE、M-CS-CFE的0.0021Ω、0.0008Ω、0.005Ω。其中,bare-CFE、CS-CFE和M-CS-CFE的Rct值分别为2072Ω、2227Ω和3971Ω。从图5的B可以看出,半圆部分即Rct反映了氧化还原探针在多层体系中的限制性扩散。裸CFE显示出非常小的半圆直径,这意味着电化学过程的扩散限制步骤。而在CS(红色)沉积和细胞膜(蓝色)逐渐孵育后,半圆直径逐渐增大,说明阻抗增加,这可能是由于生物膜削弱了电导率。此外,加入适配子后未观察到明显变化。以上结果表明,每一步改造都实现了成功的固定化。The fabrication process of the sensor was characterized by electrochemical impedance spectroscopy (EIS) in [Fe(CN)6]3-/4 solution. A typical Nyquist plot consists of high-frequency semicircles and low-frequency radial lines, representing interfacial charge-transfer-limited and diffusion processes on the CFE, respectively. An increase in the diameter of the semicircle is associated with an increase in the charge transfer resistance (Rct). Figure 5 shows the Nyquist plots of different modified electrodes. As shown in A of Figure 5, the data were analyzed using the Randles equivalent circuit model, including Warburg impedance (W), charge transfer resistance (Rct), double layer capacitance (CDL), and electrolyte ohmic resistance (Rs). Rs are 0.0021Ω, 0.0008Ω, and 0.005Ω for bare-CFE, CS-CFE, and M-CS-CFE, respectively. Among them, the Rct values of bare-CFE, CS-CFE and M-CS-CFE are 2072Ω, 2227Ω and 3971Ω, respectively. It can be seen from B of Fig. 5 that the semicircle part, namely Rct, reflects the restricted diffusion of the redox probe in the multilayer system. Bare CFE shows a very small semicircle diameter, implying a diffusion-limited step of the electrochemical process. Whereas, after CS (red) deposition and cell membrane (blue) gradual incubation, the diameter of the semicircle gradually increased, indicating an increase in impedance, which may be due to the weakened conductivity by the biofilm. Furthermore, no significant changes were observed upon addition of aptamers. The above results indicated that each step of transformation achieved successful immobilization.
CV研究了DNA-cho-M-CS-CFE对DA浓度的电催化响应,如图6的A所示。结果表明,多巴胺氧化峰电流随多巴胺浓度的增加而增加。为了获得更好的催化性能,研究了电压对催化性能的影响。当工作电位从0.08V增加到0.18V时,记录DNA-cho-M-CS-CFE的电流-时间(i-t)响应曲线。从图6的B可以看出,电流响应逐渐增大,在0.16V时达到最大值。图6的C给出了AA在裸和M-CS修饰的玻碳电极(GCE)上氧化还原反应的典型CV。与裸GCE相比,在M-CS修饰的GCE上,AA的氧化电子传递明显延迟,有利于区分AA和DA。随后,包括AA、NE、UA和DOPAC在内的多种生理相关物质依次添加到aCSF(pH=7.40)中(图6的D)。结果显示DNA-cho-M-CS-CFE对干扰物具有显著的选择性,为DA检测提供了保证(图6的D)。在最佳条件下,采用恒电位安培电流-时间法DNA-cho-M-CS-CFE在较低浓度范围内的灵敏度。图6中的E、F、G显示了DNA-cho-M-CS-CFE在0.16V时,在PH=7.40的aCSF中加入DA后的典型安培曲线。该传感器具有良好的快速响应性,线性范围为5nM~18.7μM(y=0.2404x+0.0076,R2=0.999)。DNA-cho-M-CS-CFE的检测限(LOD)是由5nM多巴胺的峰值电流获得的信噪比(S/N=3),包括纹状体中多巴胺的正常水平,范围为2.5~15nM。CV investigated the electrocatalytic response of DNA-cho-M-CS-CFE to DA concentration, as shown in A of Fig. 6. The results showed that the dopamine oxidation peak current increased with the increase of dopamine concentration. In order to obtain better catalytic performance, the effect of voltage on catalytic performance was investigated. When the working potential was increased from 0.08V to 0.18V, the current-time (i-t) response curve of DNA-cho-M-CS-CFE was recorded. It can be seen from B of Figure 6 that the current response increases gradually and reaches the maximum value at 0.16V. Figure 6C presents typical CVs of the redox reactions of AA on bare and M-CS-modified glassy carbon electrodes (GCEs). Compared with bare GCE, the oxidative electron transport of AA was significantly delayed on M-CS modified GCE, which was beneficial to distinguish AA from DA. Subsequently, various physiologically relevant substances including AA, NE, UA and DOPAC were sequentially added to aCSF (pH=7.40) (Fig. 6D). The results showed that DNA-cho-M-CS-CFE had remarkable selectivity to interfering substances, providing assurance for DA detection (Fig. 6D). Sensitivity of DNA-cho-M-CS-CFE in the lower concentration range by potentiostatic amperometric-time method under optimal conditions. E, F, G in Figure 6 show typical amperometric curves of DNA-cho-M-CS-CFE at 0.16 V after adding DA to aCSF at pH = 7.40. The sensor has good fast response, and the linear range is 5nM~18.7μM (y=0.2404x+0.0076, R2=0.999). The limit of detection (LOD) of DNA-cho-M-CS-CFE is the signal-to-noise ratio (S/N=3) obtained from a peak current of 5 nM dopamine, including normal levels of dopamine in the striatum, ranging from 2.5 to 15 nM .
表1汇总了DNA-cho-M-CS-CFE和其他电极的电分析性能,DNA-cho-M-CS-CFE显示出优越的线性范围和LOD。其出色的分析性能可能归功于CFs和适配子。CFs实现了高选择性和时空分辨率。此外,由于适配子的存在,我们绘制了被检测对象与电极表面之间的距离,揭示了DNA-cho-M-CS-CFE在DA传感分析应用中的前景。Table 1 summarizes the electroanalytical performance of DNA-cho-M-CS-CFE and other electrodes, DNA-cho-M-CS-CFE showed superior linear range and LOD. Its excellent analytical performance may be attributed to CFs and aptamers. CFs achieve high selectivity and spatiotemporal resolution. Furthermore, due to the presence of the aptamer, we mapped the distance between the detected object and the electrode surface, revealing the promise of DNA-cho-M-CS-CFE for DA sensing assay applications.
表1.不同修饰电极测定多巴胺的电分析性能比较。Table 1. Comparison of electroanalytical performance of different modified electrodes for the determination of dopamine.
实施例6:稳定性和抗污性Example 6: Stability and stain resistance
测定碰撞前DNA-Cho-M-CS-CFEs对10uM DA响应的循环伏安曲线。将载玻片固定在倒置显微镜上,电极固定在MM-500三轴电动显微机械手(RWDLifeScienceCo。中国,深圳)。然后使电极与载玻片边缘在同一平面并进入视场,使电极以45度角撞击玻璃边缘,然后取下电极测量循环伏安曲线。重复上述步骤三次,计算0.16V时的电流值的平均值和标准差。The cyclic voltammetry curve of DNA-Cho-M-CS-CFEs response to 10uM DA before the collision was determined. The glass slide was fixed on an inverted microscope, and the electrodes were fixed on a MM-500 three-axis motorized micromanipulator (RWD LifeScienceCo. China, Shenzhen). Then make the electrode on the same plane as the edge of the glass slide and enter the field of view, make the electrode hit the edge of the glass at an angle of 45 degrees, and then remove the electrode to measure the cyclic voltammetry curve. Repeat the above steps three times to calculate the average value and standard deviation of the current value at 0.16V.
检测DNA-cho-M-CS-CFE与裸电极的水接触角。如图7的A和B所示,DNA-cho-M-CS-CFE具有较小的水接触角,表现出较强的亲水性和较强的抵抗非特异性蛋白结合的能力。The water contact angle between DNA-cho-M-CS-CFE and bare electrode was detected. As shown in A and B of Figure 7, DNA-cho-M-CS-CFE has a smaller water contact angle, exhibits stronger hydrophilicity and stronger ability to resist non-specific protein binding.
利用i-t进一步评估DNA-cho-M-CS-CFE电氧化DA的稳定性。分别在添加10uM DA前和添加10uM DA后监测电流响应1h。正如预期的那样,加入DNA-cho-M-CS-CFE后显示出良好的稳定性,信号略有衰减(0.38%)(图7的C)。为进一步研究DNA-cho-M-CS-CFE的抗污性能,在aCSF中加入10mg mL-1BSA,模拟大鼠脑内微电极的表面生物污染。如图7中D所示,在裸CFE记录下,bsa诱导的快速电流响应降低了约60%。相反,DNA-cho-M-CS-CFE的当前应答仅下降3%。这一结果揭示了非凡的抵抗蛋白质吸附的能力,直接证实了我们之前的推测,这可能是由于细胞膜与蛋白质的非极性区域的疏水性相互作用。The stability of DA electrooxidized by DNA-cho-M-CS-CFE was further evaluated by using i-t. The current response was monitored for 1 h before and after adding 10uM DA, respectively. As expected, the addition of DNA-cho-M-CS-CFE showed good stability with a slight attenuation of the signal (0.38%) (Fig. 7C). To further study the antifouling performance of DNA-cho-M-CS-CFE, 10 mg mL-1BSA was added to aCSF to simulate the surface biofouling of microelectrodes in rat brains. As shown in Fig. 7D, the BSA-induced fast current response was reduced by about 60% under bare CFE recordings. In contrast, the current response for DNA-cho-M-CS-CFE decreased by only 3%. This result revealed an extraordinary ability to resist protein adsorption, directly confirming our previous speculation that it might be due to the hydrophobic interaction of the cell membrane with the nonpolar regions of the protein.
为了研究DNA-cho-M-CS-CFE在生物流体中的性能,比较DNA-cho-M-CS-CFE在DMEM培养基中浸泡1h前后的安培响应。从图7中E可以看出,DNA-cho-M-CS-CFE被DMEM浸泡后,电流响应基本保持不变。类似地,将传感器植入大鼠活体脑组织2h后,记录离体DA电流反应。如图7中F所示,DNA-cho-M-CS-CFE对DA的电流反应在植入脑组织后(红色)与植入前(黑色)相比略有变化。综上所述,DNA-cho-M-CS-CFE具有良好的监测稳定性和抗污性,可以满足体内长期监测的要求。To investigate the performance of DNA-cho-M-CS-CFE in biological fluids, the amperometric response of DNA-cho-M-CS-CFE was compared before and after soaking in DMEM medium for 1 h. From Fig. 7E, it can be seen that the current response of DNA-cho-M-CS-CFE remains basically unchanged after being soaked in DMEM. Similarly, after the sensor was implanted in the living rat brain tissue for 2 hours, the isolated DA current response was recorded. As shown in F in Fig. 7, the current response of DNA-cho-M-CS-CFE to DA was slightly changed after implantation in brain tissue (red) compared with that before implantation (black). In summary, DNA-cho-M-CS-CFE has good monitoring stability and anti-fouling properties, which can meet the requirements of long-term monitoring in vivo.
为了评估显著的机械力对电极变形的电化学响应的影响,在电极边缘碰撞3次,使其在45°左右弯曲,观察0.16V时(相对于Ag/AgCl)CV图像和电流的变化。从图8的I和J可以看出,经过3次碰撞后,电极的循环伏安图没有明显变化,电流值仅衰减了6.7%,证明DNA-cho-M-CS-CFE具有良好的机械稳定性。因此,植入过程中的力不会明显影响电极的性能。In order to evaluate the effect of significant mechanical force on the electrochemical response of electrode deformation, the electrode edge was bumped 3 times to make it bend around 45°, and the changes of CV image and current at 0.16V (vs. Ag/AgCl) were observed. From I and J of Figure 8, it can be seen that after 3 collisions, the cyclic voltammogram of the electrode does not change significantly, and the current value only decays by 6.7%, which proves that the DNA-cho-M-CS-CFE has good mechanical stability sex. Therefore, the force during implantation does not significantly affect the performance of the electrode.
实施例7:在aCSF和DMEM中对DA测量Example 7: DA measurement in aCSF and DMEM
为了验证其在实际应用中的可行性,采用标准添加法在aCSF(A)和DMEM(B)中进行了回收试验,如表2所示。在aCSF中以4.6uM检测回收率为92.0%(n=3),在培养基中以5.0uM检测回收率为100.0%(n=3),显示了新型电极在复杂环境中准确检测DA的巨大潜力。In order to verify its feasibility in practical application, recovery experiments were carried out in aCSF (A) and DMEM (B) by the standard addition method, as shown in Table 2. The detection recovery rate was 92.0% (n=3) at 4.6uM in aCSF and 100.0% (n=3) at 5.0uM in culture medium, showing the great potential of the new electrode for accurate detection of DA in complex environments potential.
表2.CSF和DMEM中DA检测(n=3)。Table 2. DA detection in CSF and DMEM (n=3).
实施例8:材料生物相容性Example 8: Material biocompatibility
将L929和Hela-RFP细胞置于含1%青霉素-链霉素(P/S)和10%胎牛血清(FBS)的DMEM培养基中,于37℃、5%CO2条件下培养。CCK-8法检测传感器表面的细胞毒性。在碳布上进行与之前提到的碳纤维相同的合成程序。将改性碳布和裸碳布分别修剪至0.3*0.3cm,置于96壁板中接种L929细胞。同时,将Hela-RFP细胞接种在改性后的裸碳布上,培养6h,进行下一步的生物相容性探索。采用荧光成像技术记录Hela-RFP细胞的增殖情况。L929 and Hela-RFP cells were cultured in DMEM medium containing 1% penicillin-streptomycin (P/S) and 10% fetal bovine serum (FBS) at 37°C and 5% CO2. The CCK-8 assay detects cytotoxicity on the sensor surface. The same synthesis procedure as previously mentioned for carbon fibers was carried out on carbon cloth. The modified carbon cloth and bare carbon cloth were trimmed to 0.3*0.3cm respectively, and placed in 96 wall plates to inoculate L929 cells. At the same time, Hela-RFP cells were seeded on the modified bare carbon cloth and cultured for 6 hours to explore the biocompatibility in the next step. The proliferation of Hela-RFP cells was recorded by fluorescence imaging technique.
通过细胞毒性实验(n=3)进一步考察DNA-cho-M-CS-CFE的生物相容性。L929的CCK-8检测结果如图8的A所示,对照组、裸碳布和DNA-cho-M-CS修饰碳布之间无显著差异,表明我们获得的传感器没有细胞毒性。同样,将Hela-RFP细胞分别在裸碳布(B)和改性碳布(C)上培养6h,荧光显微镜下观察,详情见图8的B。细胞在裸碳布和改性碳布上均顺利生长,生长状态良好。总体而言,本研究结果表明电极具有较高的生物相容性,显示了在活体脑组织中长期感知多巴胺的潜力。The biocompatibility of DNA-cho-M-CS-CFE was further investigated by cytotoxicity experiment (n=3). The CCK-8 detection results of L929 are shown in A of Fig. 8, there is no significant difference between the control group, bare carbon cloth and DNA-cho-M-CS modified carbon cloth, indicating that the sensor we obtained has no cytotoxicity. Similarly, the Hela-RFP cells were cultured on the bare carbon cloth (B) and the modified carbon cloth (C) for 6 hours respectively, and observed under a fluorescence microscope, see Figure 8B for details. The cells grew smoothly on both the bare carbon cloth and the modified carbon cloth, and the growth state was good. Overall, the results of this study demonstrate the high biocompatibility of the electrodes, showing the potential for long-term sensing of dopamine in living brain tissue.
实施例9:大鼠脑片DA检测Example 9: Detection of DA in rat brain slices
所有动物实验均经重庆医科大学伦理委员会批准。雄性SD大鼠(6~8周龄)饲养在饲养室内,随机给予食物和水。通过气体泵R520(RWD生命科学有限公司,中国深圳)对大鼠进行异氟烷麻醉(4%诱导,2%维持),切开头皮后立即处死大鼠。在1min内取出脑组织,立即置于含有(mM,PH=7.0)NaCl(119)、KCl(2.5)、NaH2PO4(1.25)、NaHCO3(26)、葡萄糖(10)、MgCl2(7)的冰冻切片液(0~4℃)中。采用KD-400振动切片机(浙江金华科帝仪器设备有限公司)将脑组织切成含尾状核的冠状脑片。所有手术均在冰冻切片液中进行。将脑片置于34℃的孵育液中(mM,PH=7.4):NaCl(119)、KCl(2.5)、NaH2PO4(1.25)、NaHCO3(26)、葡萄糖(10)、MgCl2(2)、CaCl2(2)。用5uL 40mM KCl溶液激发DA释放,用三电极系统监测反应。所有溶液均预先用95%氧气饱和,以保证足够的氧含量。All animal experiments were approved by the Ethics Committee of Chongqing Medical University. Male SD rats (6-8 weeks old) were kept in a breeding room and randomly given food and water. Rats were anesthetized with isoflurane (4% induction, 2% maintenance) by gas pump R520 (RWD Life Science Co., Ltd., Shenzhen, China), and the rats were sacrificed immediately after scalp incision. Remove the brain tissue within 1 min, and immediately place it in a frozen section containing (mM, PH=7.0) NaCl (119), KCl (2.5), NaH2PO4 (1.25), NaHCO3 (26), glucose (10), MgCl2 (7) liquid (0 ~ 4 ℃). Brain tissue was sliced into coronal slices containing the caudate nucleus using a KD-400 vibrating microtome (Zhejiang Jinhua Kedi Instrument Equipment Co., Ltd.). All operations were performed in frozen section fluid. Place the brain slices in 34°C incubation solution (mM, PH=7.4): NaCl (119), KCl (2.5), NaH2PO4 (1.25), NaHCO3 (26), glucose (10), MgCl2 (2), CaCl2 (2). DA release was stimulated with 5uL 40mM KCl solution, and the reaction was monitored with a three-electrode system. All solutions were pre-saturated with 95% oxygen to ensure sufficient oxygen content.
由于DNA-cho-M-CS-CFE具有良好的稳定性、敏感性、抗污性和高生物相容性,我们进一步将DNA-cho-M-CS-CFE应用于活体大鼠脑片(n=3)。电极被插入黑质纹状体(SNc)以监测电流反应,如图10中A所示。在钾离子诱导的DA胞吐过程中,SNc(红色)的DAergic神经元在0.16V时记录到典型的电流反应。同时采用DNA-cho-M-CS-CFE监测电流响应,以不含脑组织的孵育缓冲液作为空白对照,排除高K+(黑色)的电流信号。在加入0.04M KCl后,电流响应稳定上升,这源于DA氧化。K+刺激引起细胞膜去极化,诱导Ca2+内流,导致DAergic神经元去极化。这是DAergic神经元的动作电位。然后触发预先储存在囊泡中的DA的释放。在DNA-cho-M-CS-CFE上,通过积分记录电流检测和定量突触间隙DA氧化的稳定上升。由突触前自身受体调控的DA释放和摄取是决定大脑细胞外神经递质水平的关键机制。当多巴胺摄取大于释放时,信号逐渐减弱,直至基线。Due to the good stability, sensitivity, stain resistance and high biocompatibility of DNA-cho-M-CS-CFE, we further applied DNA-cho-M-CS-CFE to living rat brain slices (n =3). Electrodes were inserted into the nigrostriatum (SNc) to monitor the amperometric response, as shown in A in Figure 10. During potassium ion-induced DA exocytosis, DAergic neurons of SNc (red) recorded a typical current response at 0.16V. At the same time, DNA-cho-M-CS-CFE was used to monitor the current response, and the incubation buffer without brain tissue was used as a blank control to exclude high K+ (black) current signals. After the addition of 0.04M KCl, the current response increased steadily, which was attributed to DA oxidation. K+ stimulation causes cell membrane depolarization, induces Ca2+ influx, and leads to depolarization of DAergic neurons. This is the action potential of a DAergic neuron. The release of DA previously stored in the vesicle is then triggered. On DNA-cho-M-CS-CFE, a steady rise in DA oxidation in the synaptic cleft was detected and quantified by integrating the recording current. DA release and uptake regulated by presynaptic autoreceptors is a key mechanism that determines extracellular neurotransmitter levels in the brain. When dopamine uptake is greater than release, the signal tapers off until baseline.
令人惊奇的是,DNA-cho-M-CS-CFE在脑切片中表现出了显著的传感稳定性。在高K+刺激的脑片上连续3次进行DA的长时程感知。图10中B显示了三种类似的电流响应,表明了活体大鼠脑组织的高级稳定性感知。原因如上所述。随时间延长,上升速率逐渐降低,但反应强度差异无统计学意义。这可能是由于脑组织的失活。与DNA-cho-M-CS-CFE相比,即使加入50nM的DA,裸CFE也不能监测到稳定性响应。(图9)结果支持这种高生物相容性传感器提供的组织内DA的长期稳定性传感的潜力。Surprisingly, DNA-cho-M-CS-CFE exhibited remarkable sensing stability in brain slices. The long-term perception of DA was performed on the brain slices stimulated by high K+ for 3 consecutive times. Figure 10B shows three similar current responses, indicating a high-level stability perception of living rat brain tissue. The reason is as mentioned above. As time went on, the rate of ascent gradually decreased, but the difference in response intensity was not statistically significant. This may be due to inactivation of brain tissue. In contrast to DNA-cho-M-CS-CFE, no stability response could be detected in bare CFE even with the addition of 50 nM DA. (FIG. 9) The results support the potential of this highly biocompatible sensor for long-term stable sensing of DA in tissues.
已知炎症反应的激活在帕金森病中起重要作用,但其机制尚不清楚。然而,SNc中DAergic神经元的损伤和体内DA水平的降低是临床症状的起源。采用革兰阴性菌致炎成分LPS作为PD模型诱导。随着脑组织中LPS的增加,小胶质细胞、持续的小胶质细胞过度激活和神经炎症失调导致多巴胺能神经元死亡,这将表现出脑组织活性低下和多巴胺水平紊乱。因此,我们用LPS处理大鼠脑片。LPS处理的大鼠脑片在2ug/L LPS中浸泡1h后,再用相同量的KCl刺激,但只产生非常微弱的电流反应。治疗后,增加期明显延长,标准差为0.66时增加约113.24%(n=8),反应强度降低,标准差为0.22时减少约34.22%(n=8)。总之,DNA-cho-M-CS-CFE用于监测lps诱导的大鼠脑切片模型中DA的释放,具有较高的空间和时间分辨率。该方法成功实现了DA的长期感知,同时避免了细胞损伤,是炎症机制研究的一大突破。在LPS处理的脑切片上的应用表明,本研究提出的长期感知多巴胺的新策略显示出在神经生理学和病理学中探索炎症机制的潜力。Activation of the inflammatory response is known to play an important role in Parkinson's disease, but the mechanism is unclear. However, damage of DAergic neurons in SNc and decreased DA levels in vivo are the origin of clinical symptoms. LPS, an inflammatory component of Gram-negative bacteria, was used as a PD model to induce. With increased LPS in brain tissue, microglia, persistent microglial hyperactivation, and dysregulation of neuroinflammation lead to dopaminergic neuron death, which will manifest as hypoactive brain tissue and disturbed dopamine levels. Therefore, we treated rat brain slices with LPS. The brain slices of LPS-treated rats were soaked in 2ug/L LPS for 1h, and then stimulated with the same amount of KCl, but only a very weak current response was produced. After treatment, the increase period was significantly prolonged, and the increase was about 113.24% (n=8) when the standard deviation was 0.66, and the response intensity decreased, and the decrease was about 34.22% (n=8) when the standard deviation was 0.22. In conclusion, DNA-cho-M-CS-CFE was used to monitor LPS-induced DA release in a rat brain slice model with high spatial and temporal resolution. This method successfully achieves long-term sensing of DA while avoiding cell damage, which is a major breakthrough in the study of inflammatory mechanisms. Application on LPS-treated brain slices demonstrates that the novel strategy for long-term sensing of dopamine proposed in this study shows potential to explore mechanisms of inflammation in neurophysiology and pathology.
实施例10:神经细胞群中DA的检测Example 10: Detection of DA in neural cell populations
将细胞接种于35*10mm培养板上24h后,废弃原培养基,更换为2mL新鲜培养基。然后,将三电极体系浸入溶液中。待信号稳定后,将新鲜培养基更换为含0.4M K+的2mL培养基,对照组更换为空白培养基。After the cells were seeded on a 35*10mm culture plate for 24 hours, the original medium was discarded and replaced with 2 mL of fresh medium. Then, the three-electrode system was immersed in the solution. After the signal was stable, the fresh medium was replaced with 2mL medium containing 0.4M K+, and the control group was replaced with blank medium.
基于DNA-Cho-M-CS-CFE在脑片上优异的检测性能,我们尝试对均匀增殖的PC12神经元群体进行DA检测(n=3),进一步探索该传感器的潜在应用价值(图4D)。将DNA-cho-M-CS-CFE作为工作电极浸入PC12细胞群中,利用K+快速去极化细胞。如图10中D所示,加入空白培养基(黑色)的细胞群的电化学信号没有响应,而加入含0.4M K+的培养基(红色)的细胞群的电化学信号明显增加,且增长迅速,大约在10s内保持稳定。2μg/L LPS孵育30min后,电流下降约43.3%,标准差为0.94。这些结果与在脑切片中观察到的结果相似。这一发现证明了DNA-cho-M-CS-CFE电极在细胞群体中的灵敏和稳定的电化学响应,从而实现了实时检测单细胞囊泡释放多巴胺的潜力。Based on the excellent detection performance of DNA-Cho-M-CS-CFE on brain slices, we attempted to perform DA detection on uniformly proliferating PC12 neuron populations (n=3) to further explore the potential application value of this sensor (Fig. 4D). The DNA-cho-M-CS-CFE was immersed in the PC12 cell population as the working electrode, and K+ was used to rapidly depolarize the cells. As shown in D in Figure 10, the electrochemical signal of the cell population added with blank medium (black) did not respond, while the electrochemical signal of the cell population added with 0.4M K+-containing medium (red) increased significantly and grew rapidly , and remains stable within about 10s. After incubation with 2μg/L LPS for 30min, the current decreased by about 43.3%, with a standard deviation of 0.94. These results are similar to those observed in brain slices. This finding demonstrates the sensitive and stable electrochemical response of the DNA-cho-M-CS-CFE electrode in cell populations, thereby realizing the potential for real-time detection of dopamine release from single-cell vesicles.
综上所述,本发明提供了一种用于DA检测的新型CFE。采用壳聚糖、细胞膜和适配子等全生物相容性材料对其进行修饰。通过电沉积,壳聚糖薄膜均匀地沉积在纤维上。在CS覆盖的CFE上,浸泡法可以有效地掩盖从脑细胞中分离出的细胞膜,提供一种薄而温和的膜。胆固醇和细胞膜之间的亲和力允许适配子胆固醇两亲性被插入。基于这一设计,细胞膜被适配子功能化。这些生物材料极大地提高了CFE的抗污能力和稳定性。同样,基于细胞膜和适配子的仿生伪装增强了CFE的生物相容性,从而避免了免疫应答。在aCSF中采用CV法和i-t法进行DA传感,具有更宽的线性范围和更大的LOD。该电极具有良好的选择性、稳定性、抗污性和再生性等特点,在体内检测中具有广阔的应用前景。此外,DNA-cho-M-CS-CFE具有良好的稳定性,可在活体大鼠脑组织中进行3h以上的DA检测。同时,新型传感器可用于实时检测K+处理的神经细胞群释放的多巴胺。此外,对LPS处理的脑组织和PC12细胞的检测也证明了电极的敏感性和稳定性。值得期待的是,这种新型电极修饰策略将为探索DA在神经化学物质和脑部疾病中的作用提供一种在体和单细胞的长期传感方法。In summary, the present invention provides a novel CFE for DA detection. It is modified with fully biocompatible materials such as chitosan, cell membrane and aptamers. By electrodeposition, chitosan films were uniformly deposited on the fibers. On CS-covered CFE, the soaking method can effectively mask the cell membranes isolated from brain cells, providing a thin and gentle membrane. The affinity between cholesterol and cell membranes allows the aptamer cholesterol to be inserted amphipathically. Based on this design, the cell membrane is functionalized with aptamers. These biomaterials greatly enhance the antifouling ability and stability of CFE. Likewise, biomimetic camouflage based on cell membranes and aptamers enhanced the biocompatibility of CFE to avoid immune responses. The CV method and the i-t method were used for DA sensing in aCSF with a wider linear range and larger LOD. The electrode has the characteristics of good selectivity, stability, anti-fouling and reproducibility, and has broad application prospects in in vivo detection. In addition, DNA-cho-M-CS-CFE has good stability and can be used for DA detection in living rat brain tissue for more than 3 h. Meanwhile, the novel sensor can be used to detect dopamine released from K+-treated neuron populations in real time. In addition, the detection of LPS-treated brain tissue and PC12 cells also demonstrated the sensitivity and stability of the electrodes. It is worth looking forward to that this novel electrode modification strategy will provide an in vivo and single-cell long-term sensing method for exploring the role of DA in neurochemical substances and brain diseases.
以上描述旨在是说明性的而不是限制性的。例如,上述示例(或其一个或更多方案)可以彼此组合使用。例如本领域普通技术人员在阅读上述描述时可以使用其它实施例。另外,在上述具体实施方式中,各种特征可以被分组在一起以简单化本公开。这不应解释为一种不要求保护的公开的特征对于任一权利要求是必要的意图。相反,本发明的主题可以少于特定的公开的实施例的全部特征。从而,以下权利要求书作为示例或实施例在此并入具体实施方式中,其中每个权利要求独立地作为单独的实施例,并且考虑这些实施例可以以各种组合或排列彼此组合。本发明的范围应参照所附权利要求以及这些权利要求赋权的等同形式的全部范围来确定。The above description is intended to be illustrative rather than restrictive. For example, the above examples (or one or more aspects thereof) may be used in combination with each other. For example, other embodiments may be used by those of ordinary skill in the art upon reading the above description. Additionally, in the above Detailed Description, various features may be grouped together in order to simplify the disclosure. This is not to be interpreted as intending that an unclaimed disclosed feature is essential to any claim. Rather, inventive subject matter may lie in less than all features of a particular disclosed embodiment. Thus, the following claims are hereby incorporated into the detailed description as examples or embodiments, where each claim stands on its own as a separate embodiment, and it is contemplated that these embodiments may be combined with each other in various combinations or permutations. The scope of the invention should be determined with reference to the appended claims, along with the full scope of equivalents to which such claims are entitled.
Claims (10)
Priority Applications (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| CN202211698498.4ACN116106380A (en) | 2022-12-28 | 2022-12-28 | Enhanced electrode for determining dopamine and preparation method and application method thereof |
Applications Claiming Priority (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| CN202211698498.4ACN116106380A (en) | 2022-12-28 | 2022-12-28 | Enhanced electrode for determining dopamine and preparation method and application method thereof |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| CN116106380Atrue CN116106380A (en) | 2023-05-12 |
Family
ID=86257397
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| CN202211698498.4APendingCN116106380A (en) | 2022-12-28 | 2022-12-28 | Enhanced electrode for determining dopamine and preparation method and application method thereof |
Country Status (1)
| Country | Link |
|---|---|
| CN (1) | CN116106380A (en) |
Citations (14)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US4849343A (en)* | 1984-08-03 | 1989-07-18 | Krull Ulrich J | Process for analysis using a lipid membrane |
| US20070026440A1 (en)* | 2001-04-06 | 2007-02-01 | Broderick Patricia A | Identification, diagnosis, and treatment of neuropathologies, neurotoxicities, tumors, and brain and spinal cord injuries using electrodes with microvoltammetry |
| CN101285793A (en)* | 2008-04-21 | 2008-10-15 | 上海大学 | Method for Simultaneous Determination of Dopamine and Ascorbic Acid Using Novel Modified Glassy Carbon Electrode |
| WO2012007931A2 (en)* | 2010-07-16 | 2012-01-19 | Dublin City University | Silver electrode catalyst |
| CN108845014A (en)* | 2018-07-12 | 2018-11-20 | 福建师范大学 | A kind of molecular engram amphiphilic chitosan derivatives LB film modified electrode and its construction method and application |
| CN110031524A (en)* | 2019-02-21 | 2019-07-19 | 中国科学院化学研究所 | The method for measuring dopamine |
| CN110172438A (en)* | 2019-06-11 | 2019-08-27 | 湖南大学 | A kind of functional modification method of cell membrane |
| CN111333642A (en)* | 2018-12-18 | 2020-06-26 | 中国科学院大连化学物理研究所 | A class of cell membrane fluorescent probes with high brightness, high stability, and environmental insensitivity |
| CN113073131A (en)* | 2021-03-25 | 2021-07-06 | 苏州健雄职业技术学院 | Hepatocellular carcinoma nucleic acid labeled electrochemical biosensor based on nano-silver and anchored phospholipid double-layer membrane |
| CN113433190A (en)* | 2021-05-18 | 2021-09-24 | 重庆医科大学 | Biological conductive composite membrane and application thereof in detecting glucose |
| CN113484385A (en)* | 2021-03-11 | 2021-10-08 | 桂林理工大学 | Biosensor for immobilizing cholesterol oxidase based on MXene material and detection method thereof |
| CN114371203A (en)* | 2021-11-23 | 2022-04-19 | 北京师范大学 | Sensing electrode suitable for in-situ detection of living body and preparation method and application thereof |
| CN115040665A (en)* | 2022-06-16 | 2022-09-13 | 东南大学 | Cholesterol-modified acid-sensitive PEG magnetic lipid nanoparticle and preparation method and application thereof |
| CN116794133A (en)* | 2022-12-28 | 2023-09-22 | 重庆医科大学 | Composite membrane modified carbon fiber electrode and preparation method and application method thereof |
- 2022
- 2022-12-28CNCN202211698498.4Apatent/CN116106380A/enactivePending
Patent Citations (14)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US4849343A (en)* | 1984-08-03 | 1989-07-18 | Krull Ulrich J | Process for analysis using a lipid membrane |
| US20070026440A1 (en)* | 2001-04-06 | 2007-02-01 | Broderick Patricia A | Identification, diagnosis, and treatment of neuropathologies, neurotoxicities, tumors, and brain and spinal cord injuries using electrodes with microvoltammetry |
| CN101285793A (en)* | 2008-04-21 | 2008-10-15 | 上海大学 | Method for Simultaneous Determination of Dopamine and Ascorbic Acid Using Novel Modified Glassy Carbon Electrode |
| WO2012007931A2 (en)* | 2010-07-16 | 2012-01-19 | Dublin City University | Silver electrode catalyst |
| CN108845014A (en)* | 2018-07-12 | 2018-11-20 | 福建师范大学 | A kind of molecular engram amphiphilic chitosan derivatives LB film modified electrode and its construction method and application |
| CN111333642A (en)* | 2018-12-18 | 2020-06-26 | 中国科学院大连化学物理研究所 | A class of cell membrane fluorescent probes with high brightness, high stability, and environmental insensitivity |
| CN110031524A (en)* | 2019-02-21 | 2019-07-19 | 中国科学院化学研究所 | The method for measuring dopamine |
| CN110172438A (en)* | 2019-06-11 | 2019-08-27 | 湖南大学 | A kind of functional modification method of cell membrane |
| CN113484385A (en)* | 2021-03-11 | 2021-10-08 | 桂林理工大学 | Biosensor for immobilizing cholesterol oxidase based on MXene material and detection method thereof |
| CN113073131A (en)* | 2021-03-25 | 2021-07-06 | 苏州健雄职业技术学院 | Hepatocellular carcinoma nucleic acid labeled electrochemical biosensor based on nano-silver and anchored phospholipid double-layer membrane |
| CN113433190A (en)* | 2021-05-18 | 2021-09-24 | 重庆医科大学 | Biological conductive composite membrane and application thereof in detecting glucose |
| CN114371203A (en)* | 2021-11-23 | 2022-04-19 | 北京师范大学 | Sensing electrode suitable for in-situ detection of living body and preparation method and application thereof |
| CN115040665A (en)* | 2022-06-16 | 2022-09-13 | 东南大学 | Cholesterol-modified acid-sensitive PEG magnetic lipid nanoparticle and preparation method and application thereof |
| CN116794133A (en)* | 2022-12-28 | 2023-09-22 | 重庆医科大学 | Composite membrane modified carbon fiber electrode and preparation method and application method thereof |
Non-Patent Citations (1)
| Title |
|---|
| WEI, HUAN等: "Natural Leukocyte Membrane-Masked Microelectrodes with an Enhanced Antifouling Ability and Biocompatibility for In Vivo Electrochemical Sensing", ANALYTICAL CHEMISTRY, vol. 92, no. 16, 18 August 2020 (2020-08-18), pages 11374 - 11379* |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| Mir et al. | An amperometric nanobiosensor for the selective detection of K+-induced dopamine released from living cells | |
| Senel et al. | Electrochemical micropyramid array-based sensor for in situ monitoring of dopamine released from neuroblastoma cells | |
| He et al. | Nanoscale neuro-integrative coatings for neural implants | |
| Woeppel et al. | Nanoparticle and biomolecule surface modification synergistically increases neural electrode recording yield and minimizes inflammatory host response | |
| Wang et al. | Improvement of the biocompatibility and potential stability of chronically implanted electrodes incorporating coating cell membranes | |
| Moussa et al. | microelectrodes: an overview of probe development and bioelectrochemistry applications from 2013 to 2018 | |
| US20210355418A1 (en) | Electrode having nano structure at tip | |
| KR100987375B1 (en) | Multi-walled carbon nanotube-based biosensor and its manufacturing method | |
| Cao et al. | An integrated flexible implantable micro-probe for sensing neurotransmitters | |
| CN103336043A (en) | Preparation method of hydrogen peroxide biosensor | |
| Taskin et al. | Combined cell culture-biosensing platform using vertically aligned patterned peptide nanofibers for cellular studies | |
| CN108802148B (en) | A microfluidic paper chip for online monitoring of intracellular dopamine based on nano-gold modified screen-printed electrodes | |
| Sikder et al. | Laminin coated diamond electrodes for neural stimulation | |
| Lavanya et al. | Detection of catecholamine neurotransmitters by nanostructured SnO2-based electrochemical sensors: a review of recent progress | |
| Ali et al. | Intracellular K $^+ $ Determination With a Potentiometric Microelectrode Based on ZnO Nanowires | |
| Gabi et al. | Influence of applied currents on the viability of cells close to microelectrodes | |
| CN116106380A (en) | Enhanced electrode for determining dopamine and preparation method and application method thereof | |
| CN116794133A (en) | Composite membrane modified carbon fiber electrode and preparation method and application method thereof | |
| CN113504277A (en) | Carbon fiber/gold-plated carbon fiber electrode wrapped with avidin-polluted graphene oxide microstrip as well as preparation method and application of carbon fiber/gold-plated carbon fiber electrode | |
| Zhong et al. | Construction of a biocompatible MWCNTs–chitosan composite interface and its application to impedance cytosensing of osteoblastic MC3T3-E1 cells | |
| US20130122531A1 (en) | Nanostructured composite material and biosensor comprising it | |
| Wang | A carbon nanotube microelectrode array for neural stimulation | |
| Wang et al. | A glucose sensor based on glucose oxidase immobilized by electrospinning nanofibrous polymer membranes modified with carbon nanotubes | |
| Henderson | Development and Application of Electrochemical Sensors for Studying Neuromuscular Signaling in the Gastrointestinal Tract | |
| CN112798671B (en) | A lysing agent for mildly lysing cells and its application |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PB01 | Publication | ||
| PB01 | Publication | ||
| SE01 | Entry into force of request for substantive examination | ||
| SE01 | Entry into force of request for substantive examination |