CN116000308A - Synthesis method of submicron flake silver powder - Google Patents
Synthesis method of submicron flake silver powderDownload PDFInfo
- Publication number
- CN116000308A CN116000308ACN202211674653.9ACN202211674653ACN116000308ACN 116000308 ACN116000308 ACN 116000308ACN 202211674653 ACN202211674653 ACN 202211674653ACN 116000308 ACN116000308 ACN 116000308A
- Authority
- CN
- China
- Prior art keywords
- silver powder
- reaction
- submicron
- silver nitrate
- powder according
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Pending
Links
Images
Classifications
- Y—GENERAL TAGGING OF NEW TECHNOLOGICAL DEVELOPMENTS; GENERAL TAGGING OF CROSS-SECTIONAL TECHNOLOGIES SPANNING OVER SEVERAL SECTIONS OF THE IPC; TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y02—TECHNOLOGIES OR APPLICATIONS FOR MITIGATION OR ADAPTATION AGAINST CLIMATE CHANGE
- Y02E—REDUCTION OF GREENHOUSE GAS [GHG] EMISSIONS, RELATED TO ENERGY GENERATION, TRANSMISSION OR DISTRIBUTION
- Y02E10/00—Energy generation through renewable energy sources
- Y02E10/50—Photovoltaic [PV] energy
Landscapes
- Manufacture Of Metal Powder And Suspensions Thereof (AREA)
Abstract
Description
Translated fromChinese技术领域Technical Field
本发明涉及银粉的合成方法,具体涉及一种亚微米级片状银粉的合成方法。The invention relates to a method for synthesizing silver powder, in particular to a method for synthesizing submicron flaky silver powder.
背景技术Background Art
银粉由于其自身优异的导电性、导热性及抗氧化性,被广泛应用于电子相关行业,而根据用途的不同,对银粉形貌及尺寸的要求也不同。片状银粉堆积时相互之间是线接触和面接触,相对于块状或类球状超细银粉颗粒之间点接触的接触面积大,因此具有更低的堆积电阻率与更好的导电性。在银浆体系中,片状银粉又分为微米级与亚微米级,其中亚微米级(粒径100nm~1μm)片状银粉更适用于高精度、高效率的网板印刷。Silver powder is widely used in electronic related industries due to its excellent electrical conductivity, thermal conductivity and oxidation resistance. Depending on the purpose, the requirements for the morphology and size of silver powder are also different. When flaky silver powder is piled up, it is in line contact and surface contact with each other. Compared with the point contact between blocky or spherical ultrafine silver powder particles, the contact area is large, so it has lower stacking resistivity and better conductivity. In the silver paste system, flaky silver powder is divided into micron and submicron levels, among which submicron (particle size 100nm~1μm) flaky silver powder is more suitable for high-precision and high-efficiency screen printing.
目前片状银粉的制备方法主要有球磨法、光诱导法、模板法、化学还原法等,其中球磨法需要先使用还原法制备出合适粒径的微纳米银颗粒,再通过机械球磨的方法得到片状银粉,但机械球磨法变量较多,不同批次银粉成片效果难以控制,在球磨过程中容易引入杂质,而且粉体可能发生冷焊,产品粒径难以达标。At present, the main methods for preparing flaky silver powder include ball milling, photoinduction, template method, chemical reduction method, etc. The ball milling method requires first using a reduction method to prepare micro-nano silver particles of suitable particle size, and then obtaining flaky silver powder by mechanical ball milling. However, the mechanical ball milling method has many variables, and the flaking effect of different batches of silver powder is difficult to control. Impurities are easily introduced during the ball milling process, and cold welding may occur in the powder, making it difficult for the product particle size to meet the standard.
发明专利CN101947655A公布了一种三角形银纳米片的制备方法,其是以硼氢化钠与柠檬酸三钠为还原剂的双还原体系,聚乙烯吡咯烷酮作为表面活性剂与保护剂,最终加入双氧水调控晶体形貌得到粒径为50nm~120nm的三角形银纳米片。但该方法步骤繁杂,硼氢化钠作为还原剂反应速率过快,反应过程难以控制。Invention patent CN101947655A discloses a method for preparing triangular silver nanosheets, which uses a double reduction system with sodium borohydride and trisodium citrate as reducing agents, polyvinyl pyrrolidone as a surfactant and protective agent, and finally adds hydrogen peroxide to adjust the crystal morphology to obtain triangular silver nanosheets with a particle size of 50nm to 120nm. However, this method has complicated steps, the reaction rate of sodium borohydride as a reducing agent is too fast, and the reaction process is difficult to control.
发明内容Summary of the invention
为克服上述缺点,本发明的目的在于提供一种亚微米级片状银粉的合成方法,该方法制备过程外源杂质引入少,产物纯度高,形貌可控,工艺设备简单。In order to overcome the above-mentioned shortcomings, the purpose of the present invention is to provide a method for synthesizing submicron flaky silver powder, which has few exogenous impurities introduced during the preparation process, high product purity, controllable morphology, and simple process equipment.
为了达到以上目的,本发明采用的技术方案是:一种亚微米级片状银粉的合成方法,包括以下步骤:In order to achieve the above object, the technical solution adopted by the present invention is: a method for synthesizing submicron flaky silver powder, comprising the following steps:
S1、通过硝酸银和溶剂配制硝酸银溶液,溶剂选自乙二醇、丙三醇、N,N-二甲基甲酰胺、正辛醇、正庚醇中的一种或多种;S1, preparing a silver nitrate solution by using silver nitrate and a solvent, wherein the solvent is selected from one or more of ethylene glycol, glycerol, N,N-dimethylformamide, n-octanol, and n-heptanol;
S2、在硝酸银溶液中加入分散剂和晶片调控剂;S2. adding a dispersant and a wafer regulator to the silver nitrate solution;
S3、在密闭的反应釜中进行还原反应;S3, performing a reduction reaction in a closed reactor;
S4、后处理反应液,得到银粉。S4, post-treating the reaction solution to obtain silver powder.
进一步地,S1中,硝酸银溶液的浓度为40~500mmol/L。Furthermore, in S1, the concentration of the silver nitrate solution is 40 to 500 mmol/L.
进一步地,S2中,分散剂选自油酸、亚油酸、聚乙烯吡咯烷酮、油酸甲酯中的一种或多种。Furthermore, in S2, the dispersant is selected from one or more of oleic acid, linoleic acid, polyvinyl pyrrolidone, and methyl oleate.
进一步地,S2中,以硝酸银的质量100份为基准,分散剂为5~100份。Furthermore, in S2, the amount of the dispersant is 5 to 100 parts based on 100 parts by mass of silver nitrate.
进一步地,S2中,晶片调控剂为双氧水,以硝酸银的质量100份为基准,晶片调控剂为1~100份。Furthermore, in S2, the chip regulating agent is hydrogen peroxide, and the chip regulating agent is 1 to 100 parts by mass based on 100 parts by mass of silver nitrate.
进一步地,S2中,加入分散剂和晶片调控剂后搅拌1~5min。Furthermore, in S2, a dispersant and a wafer control agent are added and stirred for 1 to 5 minutes.
进一步地,S3中,反应液在反应釜中反应的温度为130~170℃。Furthermore, in S3, the temperature of the reaction liquid in the reaction kettle is 130-170°C.
进一步地,S3中,反应液在反应釜中反应的时间为8~16h。Furthermore, in S3, the reaction time of the reaction liquid in the reactor is 8 to 16 hours.
进一步地,S4中,后处理反应液包括以下步骤:Further, in S4, post-processing the reaction solution comprises the following steps:
S41、将反应液取出后自然沉降,弃去上清液,留下反应产物;S41, taking out the reaction solution and allowing it to settle naturally, discarding the supernatant, and leaving the reaction product;
S42、反应产物中加入去离子水超声搅拌清洗,弃去上清液;S42, adding deionized water to the reaction product for ultrasonic stirring and cleaning, and discarding the supernatant;
S43、反应产物中加入乙醇清洗,弃去上清液;S43, adding ethanol to the reaction product for washing, and discarding the supernatant;
S44、将反应产物置于真空烘箱中烘干,研磨粉碎制得银粉。S44, drying the reaction product in a vacuum oven, and grinding it to obtain silver powder.
本发明的有益效果是:The beneficial effects of the present invention are:
1)本发明采用一步溶剂热法,操作简单,溶剂同时作为还原剂,无需额外加入还原剂,降低了外源杂质引入量,产物纯度高,形貌规整,均为亚微米级片状银粉,粒径为200-600nm;1) The present invention adopts a one-step solvothermal method, which is simple to operate. The solvent also serves as a reducing agent, and no additional reducing agent needs to be added, thereby reducing the amount of exogenous impurities introduced. The product has high purity and regular morphology, and is all submicron flaky silver powder with a particle size of 200-600nm;
2)本发明银源利用率高,收率可达95%以上。2) The silver source utilization rate of the present invention is high, and the yield can reach more than 95%.
附图说明BRIEF DESCRIPTION OF THE DRAWINGS
构成本申请的一部分的附图用来提供对本发明的进一步理解,本发明的示意性实施例及其说明用于解释本发明,并不构成对本发明的不当限定。The drawings constituting a part of this application are used to provide a further understanding of the present invention. The illustrative embodiments of the present invention and their descriptions are used to explain the present invention and do not constitute improper limitations on the present invention.
为了更清楚地说明本发明实施例中的技术方案,下面将对实施例描述中所需要使用的附图作简单地介绍,显而易见地,下面描述中的附图仅仅是本发明的一些实施例,对于本领域普通技术人员来讲,在不付出创造性劳动的前提下,还可以根据这些附图获得其他的附图。In order to more clearly illustrate the technical solutions in the embodiments of the present invention, the drawings required for use in the description of the embodiments will be briefly introduced below. Obviously, the drawings described below are only some embodiments of the present invention. For ordinary technicians in this field, other drawings can be obtained based on these drawings without creative work.
图1为本发明实施例1制备的银粉的扫描电镜图;FIG1 is a scanning electron microscope image of the silver powder prepared in Example 1 of the present invention;
图2为本发明实施例2制备的银粉的扫描电镜图;FIG2 is a scanning electron microscope image of the silver powder prepared in Example 2 of the present invention;
图3为本发明实施例3制备的银粉的扫描电镜图;FIG3 is a scanning electron microscope image of the silver powder prepared in Example 3 of the present invention;
图4为本发明实施例4制备的银粉的扫描电镜图;FIG4 is a scanning electron microscope image of the silver powder prepared in Example 4 of the present invention;
图5为本发明对比例1制备的银粉的扫描电镜图;FIG5 is a scanning electron microscope image of the silver powder prepared in Comparative Example 1 of the present invention;
图6为本发明对比例2制备的银粉的扫描电镜图;FIG6 is a scanning electron microscope image of the silver powder prepared in Comparative Example 2 of the present invention;
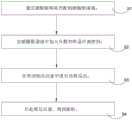
图7为本发明亚微米级片状银粉的合成方法的流程图。FIG. 7 is a flow chart of a method for synthesizing submicron flaky silver powder according to the present invention.
具体实施方式DETAILED DESCRIPTION
为使本发明的上述目的、特征和优点能够更加明显易懂,下面结合附图对本发明的具体实施方式做详细的说明。在下面的描述中阐述了很多具体细节以便于充分理解本发明。但是本发明能够以很多不同于在此描述的其它方式来实施,本领域技术人员可以在不违背本发明内涵的情况下做类似改进,因此本发明不受下面公开的具体实施例的限制。In order to make the above-mentioned objects, features and advantages of the present invention more obvious and easy to understand, the specific embodiments of the present invention are described in detail below in conjunction with the accompanying drawings. In the following description, many specific details are set forth to facilitate a full understanding of the present invention. However, the present invention can be implemented in many other ways different from those described herein, and those skilled in the art can make similar improvements without violating the connotation of the present invention, so the present invention is not limited by the specific embodiments disclosed below.
参见附图7所示,一种亚微米级片状银粉的合成方法,包括以下步骤:Referring to FIG. 7 , a method for synthesizing submicron flaky silver powder comprises the following steps:
S1、通过硝酸银和溶剂配制硝酸银溶液,溶剂选自乙二醇、丙三醇、N,N-二甲基甲酰胺、正辛醇、正庚醇中的一种或多种,硝酸银溶液的浓度为40~500mmol/L。示例性地,硝酸银溶液的浓度为40mmol/L、70mmol/L、100mmol/L、200mmol/L、300mmol/L、400mmol/L、500mmol/L。S1. Prepare a silver nitrate solution by using silver nitrate and a solvent, wherein the solvent is selected from one or more of ethylene glycol, glycerol, N,N-dimethylformamide, n-octanol, and n-heptanol, and the concentration of the silver nitrate solution is 40 to 500 mmol/L. Exemplarily, the concentration of the silver nitrate solution is 40 mmol/L, 70 mmol/L, 100 mmol/L, 200 mmol/L, 300 mmol/L, 400 mmol/L, and 500 mmol/L.
在本发明中,反应的溶剂也是还原剂,无需额外加入还原剂,制备过程引入杂质少,产品纯度高。溶剂还可以是常用有机溶剂如芳香烃类、脂肪烃类、脂环烃类、卤化烃类、醇类、醚类、酯类、酮类等。In the present invention, the solvent of the reaction is also a reducing agent, and no additional reducing agent is needed, so that less impurities are introduced during the preparation process and the product purity is high. The solvent can also be a commonly used organic solvent such as aromatic hydrocarbons, aliphatic hydrocarbons, alicyclic hydrocarbons, halogenated hydrocarbons, alcohols, ethers, esters, ketones, etc.
S2、在硝酸银溶液中加入分散剂和晶片调控剂,加入分散剂和晶片调控剂后搅拌1~5min,以确保分散剂和晶片调控剂充分溶解在硝酸银溶液中。S2. Add a dispersant and a chip regulator to the silver nitrate solution, and stir for 1 to 5 minutes after adding the dispersant and the chip regulator to ensure that the dispersant and the chip regulator are fully dissolved in the silver nitrate solution.
其中:分散剂选自油酸、亚油酸、聚乙烯吡咯烷酮、油酸甲酯中的一种或多种。以硝酸银的质量100份为基准,分散剂为5~100份。晶片调控剂为双氧水,以硝酸银的质量100份为基准,晶片调控剂为1~100份。示例性地,分散剂为5份、10份、30份、50份、80份、100份,晶片调控剂为1份、10份、30份、50份、80份、100份。Wherein: the dispersant is selected from one or more of oleic acid, linoleic acid, polyvinyl pyrrolidone, and methyl oleate. Based on 100 parts of silver nitrate, the dispersant is 5 to 100 parts. The chip control agent is hydrogen peroxide, and based on 100 parts of silver nitrate, the chip control agent is 1 to 100 parts. Exemplarily, the dispersant is 5 parts, 10 parts, 30 parts, 50 parts, 80 parts, and 100 parts, and the chip control agent is 1 part, 10 parts, 30 parts, 50 parts, 80 parts, and 100 parts.
S3、在反应釜中进行还原反应;反应液在反应釜中反应的温度为130~170℃,反应液在反应釜中反应的时间为8~16h。示例性地,反应液在反应釜中反应的温度为130℃、140℃、150℃、160℃、170℃;反应时间为8h、10h、12h、14h、16h。S3, performing a reduction reaction in a reactor; the temperature of the reaction liquid in the reactor is 130-170°C, and the reaction time of the reaction liquid in the reactor is 8-16 hours. Exemplarily, the temperature of the reaction liquid in the reactor is 130°C, 140°C, 150°C, 160°C, 170°C; the reaction time is 8h, 10h, 12h, 14h, 16h.
S4、后处理反应液,得到银粉。其中后处理反应液包括以下步骤:S4, post-processing the reaction liquid to obtain silver powder. The post-processing of the reaction liquid comprises the following steps:
S41、将反应液取出后自然沉降,弃去上清液,留下反应产物;S41, taking out the reaction solution and allowing it to settle naturally, discarding the supernatant, and leaving the reaction product;
S42、反应产物中加入去离子水超声搅拌清洗10~20min,弃去上清,反复清洗三次;S42, adding deionized water to the reaction product, stirring and washing with ultrasonic stirring for 10 to 20 minutes, discarding the supernatant, and repeating the washing three times;
S43、反应产物中加入乙醇清洗10~20min,弃去上清液,反复清洗三次;S43, adding ethanol to the reaction product for washing for 10 to 20 minutes, discarding the supernatant, and repeating the washing three times;
S44、将反应产物置于真空烘箱中烘干6~10h,最后将烘干后的样品进行粉碎处理制得银粉。S44, placing the reaction product in a vacuum oven for drying for 6 to 10 hours, and finally pulverizing the dried sample to obtain silver powder.
实施例Example
下述实施例更具体地描述了本发明公开的内容,这些实施例仅仅用于阐述性说明,因为在本发明公开内容的范围内进行各种修改和变化对本领域技术人员来说是明显的。除非另有声明,以下实施例中所报道的所有份、百分比、和比值都是基于重量计,而且实施例中使用的所有试剂都可商购获得或是按照常规方法进行合成获得,并且可直接使用而无需进一步处理,以及实施例中使用的仪器均可商购获得。The following examples describe the disclosure of the present invention in more detail, and these examples are intended for illustrative purposes only, as various modifications and variations within the scope of the disclosure of the present invention will be apparent to those skilled in the art. Unless otherwise stated, all parts, percentages, and ratios reported in the following examples are by weight, and all reagents used in the examples are commercially available or synthesized according to conventional methods and can be used directly without further processing, and the instruments used in the examples are commercially available.
实施例1Example 1
S1、反应体系溶液的配置:量取60mL的N,N-二甲基甲酰胺于100mL烧杯中,加入0.41g硝酸银,搅拌至完全溶解;S1. Preparation of reaction system solution: Measure 60 mL of N,N-dimethylformamide into a 100 mL beaker, add 0.41 g of silver nitrate, and stir until completely dissolved;
S2、称取0.14g聚乙烯吡咯烷酮加入上述硝酸银溶液,搅拌至完全溶解,最后加入0.1mL双氧水(30%),混合搅拌5min;S2, weigh 0.14g polyvinyl pyrrolidone and add it to the above silver nitrate solution, stir until it is completely dissolved, and finally add 0.1mL hydrogen peroxide (30%), mix and stir for 5min;
S3、将反应体系倒入反应釜中,设置温度为130℃,反应时间为8小时;S3, pour the reaction system into a reactor, set the temperature to 130°C, and the reaction time to 8 hours;
S4、反应结束后将反应釜冷却至40℃以下开釜,将反应液取出后自然沉降,弃去上清液,留下反应产物;将反应产物使用50mL去离子水超声搅拌15min,待沉降完全后弃去上清液,重复洗涤三次。再使用50mL无水乙醇超声搅拌15min,待沉降完全后弃去上清液,重复洗涤三次。洗涤结束后将产物放入45℃真空烘箱中干燥处理6小时,样品烘干后研磨破碎即可得到分散较好的亚微米级片状银粉0.25g,收率96.02%。参见附图1所示,用扫描电镜测试形貌,粒径主要分布在200~600nm范围内。S4. After the reaction is completed, the reactor is cooled to below 40°C and the reactor is opened. The reaction solution is taken out and naturally settled, and the supernatant is discarded to leave the reaction product; the reaction product is ultrasonically stirred for 15 minutes using 50mL deionized water, and the supernatant is discarded after the sedimentation is complete, and the washing is repeated three times. Then 50mL of anhydrous ethanol is ultrasonically stirred for 15 minutes, and the supernatant is discarded after the sedimentation is complete, and the washing is repeated three times. After washing, the product is placed in a 45°C vacuum oven for drying for 6 hours. After the sample is dried, it is ground and crushed to obtain 0.25g of well-dispersed submicron flaky silver powder, with a yield of 96.02%. As shown in Figure 1, the morphology is tested by scanning electron microscopy, and the particle size is mainly distributed in the range of 200 to 600nm.
实施例2Example 2
S1、反应体系溶液的配置:量取60mL乙二醇于100mL烧杯中,加入0.82g硝酸银,搅拌至完全溶解;S1. Preparation of reaction system solution: Measure 60 mL of ethylene glycol into a 100 mL beaker, add 0.82 g of silver nitrate, and stir until completely dissolved;
S2、称取0.28g油酸加入上述溶液,搅拌3min,最后加入0.2mL双氧水(30%),混合搅拌5min;S2, weigh 0.28g oleic acid and add it to the above solution, stir for 3min, and finally add 0.2mL hydrogen peroxide (30%), mix and stir for 5min;
S3、将反应体系倒入反应釜中,设置温度为140℃,反应时间为8小时;S3, pour the reaction system into a reactor, set the temperature to 140°C, and the reaction time to 8 hours;
S4、反应结束后将反应釜冷却至40℃以下开釜,将反应液取出后自然沉降,弃去上清液,留下反应产物;将产物用去50mL离子水超声搅拌15min,待沉降完全后弃去上清液,重复洗涤三次。再使用50mL无水乙醇超声搅拌15min,待沉降完全后弃去上清液,重复洗涤三次。洗涤结束后将产物放入45℃真空烘箱中干燥处理6小时,样品烘干后研磨破碎即可得到分散较好的亚微米级片状银粉0.50g,收率为96.02%。参见附图2所示,用扫描电镜测试形貌,粒径主要分布在200~600nm范围内。S4. After the reaction is completed, the reactor is cooled to below 40°C and the reactor is opened. The reaction solution is taken out and naturally settled, and the supernatant is discarded to leave the reaction product; the product is ultrasonically stirred with 50mL of ionized water for 15min, and the supernatant is discarded after the sedimentation is complete, and the washing is repeated three times. Then 50mL of anhydrous ethanol is ultrasonically stirred for 15min, and the supernatant is discarded after the sedimentation is complete, and the washing is repeated three times. After washing, the product is placed in a 45°C vacuum oven for drying for 6 hours. After the sample is dried, it is ground and crushed to obtain 0.50g of submicron flaky silver powder with good dispersion, and the yield is 96.02%. As shown in Figure 2, the morphology is tested by scanning electron microscopy, and the particle size is mainly distributed in the range of 200 to 600nm.
实施例3Example 3
S1、反应体系溶液的配置:量取60mL丙三醇于100mL烧杯中,加入1.64g硝酸银,搅拌至完全溶解;S1. Preparation of reaction system solution: Measure 60 mL of glycerol into a 100 mL beaker, add 1.64 g of silver nitrate, and stir until completely dissolved;
S2、称取0.56g油酸甲酯加入上述硝酸银溶液,搅拌3min,最后加入0.4mL双氧水(30%),混合搅拌5min;S2, weigh 0.56g of methyl oleate and add it to the above silver nitrate solution, stir for 3min, and finally add 0.4mL of hydrogen peroxide (30%), mix and stir for 5min;
S3、最后将反应体系倒入反应釜中,设置温度为150℃,反应时间为10小时;S3. Finally, the reaction system was poured into a reactor, the temperature was set to 150°C, and the reaction time was 10 hours;
S4、反应结束后将反应釜冷却至40℃以下开釜,将反应液取出后自然沉降,弃去上清液,留下反应产物;将产物使用50mL去离子水超声搅拌15min,待沉降完全后弃去上清液,重复洗涤三次。再使用50mL无水乙醇超声搅拌15min,待沉降完全后弃去上清液,重复洗涤三次。洗涤结束后将产物放入45℃真空烘箱中干燥处理6小时,样品烘干后研磨破碎即可得到分散较好的亚微米级片状银粉1.01g。收率96.98%。参见附图3所示,用扫描电镜测试形貌,粒径主要分布在200~600nm范围内。S4. After the reaction is completed, the reactor is cooled to below 40°C and opened. The reaction solution is taken out and naturally settled. The supernatant is discarded, leaving the reaction product; the product is ultrasonically stirred with 50mL deionized water for 15min, and the supernatant is discarded after the sedimentation is complete, and the washing is repeated three times. Then 50mL of anhydrous ethanol is ultrasonically stirred for 15min, and the supernatant is discarded after the sedimentation is complete, and the washing is repeated three times. After washing, the product is placed in a 45°C vacuum oven for drying for 6 hours. After the sample is dried, it is ground and crushed to obtain 1.01g of submicron flaky silver powder with good dispersion. The yield is 96.98%. As shown in Figure 3, the morphology is tested by scanning electron microscopy, and the particle size is mainly distributed in the range of 200 to 600nm.
实施例4Example 4
S1、反应体系溶液的配置:量取60mL正辛醇于100mL烧杯中,加入3.28g硝酸银,搅拌至完全溶解;S1. Preparation of reaction system solution: Measure 60 mL of n-octanol into a 100 mL beaker, add 3.28 g of silver nitrate, and stir until completely dissolved;
S2、称取1.12g亚油酸加入上述溶液,搅拌3min,最后加入0.8mL双氧水(30%),混合搅拌5min;S2, weigh 1.12g of linoleic acid and add it to the above solution, stir for 3min, and finally add 0.8mL of hydrogen peroxide (30%), mix and stir for 5min;
S3、将反应体系倒入反应釜中,设置温度为160℃,反应时间为12小时;S3, pour the reaction system into a reactor, set the temperature to 160°C, and the reaction time to 12 hours;
S4、反应结束后将反应釜冷却至40℃以下开釜,将反应液取出后自然沉降,弃去上清液,留下反应产物;将产物使用50mL去离子水超声搅拌15min,待沉降完全后弃去上清液,重复洗涤三次。再使用50mL无水乙醇超声搅拌15min,待沉降完全后弃去上清液,重复洗涤三次。洗涤结束后将产物放入45℃真空烘箱中干燥处理6小时,样品烘干后研磨破碎即可得到分散较好的亚微米级片状银粉1.99g,收率95.54%。参见附图4所示,用扫描电镜测试形貌,粒径主要分布在200~600nm范围内。S4. After the reaction is completed, the reactor is cooled to below 40°C and the reactor is opened. The reaction solution is taken out and naturally settled, and the supernatant is discarded to leave the reaction product; the product is ultrasonically stirred with 50mL deionized water for 15min, and the supernatant is discarded after the sedimentation is complete, and the washing is repeated three times. Then 50mL of anhydrous ethanol is ultrasonically stirred for 15min, and the supernatant is discarded after the sedimentation is complete, and the washing is repeated three times. After washing, the product is placed in a 45°C vacuum oven for drying for 6 hours. After the sample is dried, it is ground and crushed to obtain 1.99g of submicron flaky silver powder with good dispersion, and the yield is 95.54%. As shown in Figure 4, the morphology is tested by scanning electron microscopy, and the particle size is mainly distributed in the range of 200 to 600nm.
对比例1Comparative Example 1
S1、反应体系溶液的配置:量取60mL甲醇于100mL烧杯中,加入3.28g硝酸银,搅拌至完全溶解;S1. Preparation of reaction system solution: Measure 60 mL of methanol into a 100 mL beaker, add 3.28 g of silver nitrate, and stir until completely dissolved;
S2、加入7.31g果糖搅拌至完全溶解,称取1.12g亚油酸加入上述硝酸银溶液,混合搅拌5min至混合均匀;S2, add 7.31g fructose and stir until completely dissolved, weigh 1.12g linoleic acid and add it to the above silver nitrate solution, mix and stir for 5min until the mixture is uniform;
S3、将反应体系倒入反应釜中,设置温度为140℃,反应时间为6小时;S3, pour the reaction system into a reactor, set the temperature to 140°C, and the reaction time to 6 hours;
S4、反应结束后将反应釜冷却至40℃以下开釜,将反应液取出后自然沉降,弃去上清液,留下反应产物;将产物使用50mL去离子水超声搅拌15min,待沉降完全后弃去上清液,重复洗涤三次。再使用50mL无水乙醇超声搅拌15min,待沉降完全后弃去上清液,重复洗涤三次。洗涤结束后将产物放入45℃真空烘箱中干燥处理6小时,样品烘干后研磨破碎即可得纳米级银粉1.69g,收率为81.14%。参见附图5所示,用扫描电镜测试形貌,银粉粒径分布宽,分散较差。S4. After the reaction is completed, the reactor is cooled to below 40°C and the reactor is opened. The reaction solution is taken out and naturally settled, and the supernatant is discarded to leave the reaction product; the product is ultrasonically stirred with 50mL deionized water for 15min, and the supernatant is discarded after the sedimentation is complete, and the washing is repeated three times. Then 50mL of anhydrous ethanol is ultrasonically stirred for 15min, and the supernatant is discarded after the sedimentation is complete, and the washing is repeated three times. After washing, the product is placed in a 45°C vacuum oven for drying for 6 hours. After the sample is dried, it is ground and crushed to obtain 1.69g of nano-scale silver powder, and the yield is 81.14%. As shown in Figure 5, the morphology is tested by scanning electron microscopy. The silver powder particle size distribution is wide and the dispersion is poor.
对比例2Comparative Example 2
S1、反应体系溶液的配置:量取60mL水于100mL烧杯中,加入3.28g硝酸银,搅拌至完全溶解;S1. Preparation of reaction system solution: Measure 60 mL of water into a 100 mL beaker, add 3.28 g of silver nitrate, and stir until completely dissolved;
S2、加入7.31g葡萄糖搅拌至完全溶解,称取2.24g油酸乙酯加入上述溶液,搅拌3min,最后加入0.8mL双氧水(30%),混合搅拌5min至混合均匀;S2, add 7.31g glucose and stir until completely dissolved, weigh 2.24g ethyl oleate and add to the above solution, stir for 3min, finally add 0.8mL hydrogen peroxide (30%), mix and stir for 5min until mixed evenly;
S3、最后将反应体系倒入反应釜中,设置温度为160℃,反应时间为8小时;S3. Finally, the reaction system was poured into a reactor, the temperature was set to 160°C, and the reaction time was 8 hours;
S4、反应结束后将反应釜冷却至40℃以下开釜,将反应液取出后自然沉降,弃去上清液,留下反应产物;将产物使用50mL去离子水超声搅拌15min,待沉降完全后弃去上清液,重复洗涤三次。再使用50mL无水乙醇超声搅拌15min,待沉降完全后弃去上清液,重复洗涤三次。洗涤结束后将产物放入45℃真空烘箱中干燥处理6小时,样品烘干后研磨破碎即可得纳米级银粉1.89g,收率为90.74%。参见附图6所示,用扫描电镜测试形貌,银粉粒径分布宽,分散较差。S4. After the reaction is completed, the reactor is cooled to below 40°C and the reactor is opened. The reaction solution is taken out and naturally settled, and the supernatant is discarded to leave the reaction product; the product is ultrasonically stirred for 15 minutes with 50mL deionized water, and the supernatant is discarded after the sedimentation is complete, and the washing is repeated three times. Then 50mL of anhydrous ethanol is ultrasonically stirred for 15 minutes, and the supernatant is discarded after the sedimentation is complete, and the washing is repeated three times. After washing, the product is placed in a 45°C vacuum oven for drying for 6 hours. After the sample is dried, it is ground and crushed to obtain 1.89g of nano-grade silver powder, and the yield is 90.74%. As shown in Figure 6, the morphology is tested by scanning electron microscopy. The silver powder particle size distribution is wide and the dispersion is poor.
从实施例1-4和对比例1-2的实验数据以及扫描电镜图可得知:From the experimental data of Examples 1-4 and Comparative Examples 1-2 and the scanning electron microscope images, it can be seen that:
1)本发明制备过程引入杂质少,产品纯度高;1) The preparation process of the present invention introduces few impurities and the product purity is high;
2)本发明片状银粉粒径可控,粒径主要分布在200~600nm范围内;2) The particle size of the flaky silver powder of the present invention is controllable, and the particle size is mainly distributed in the range of 200 to 600 nm;
3)本发明银源利用率高,收率可达95%以上。3) The silver source utilization rate of the present invention is high, and the yield can reach more than 95%.
因此本发明提供一种制备过程外源杂质引入少,产物纯度高,形貌可控,工艺设备简单的亚微米级片状银粉的合成方法。Therefore, the present invention provides a method for synthesizing submicron flaky silver powder with less introduction of exogenous impurities in the preparation process, high product purity, controllable morphology, and simple process equipment.
另外本发明使用的试剂信息见下表:In addition, the reagent information used in the present invention is shown in the following table:
以上实施方式只为说明本发明的技术构思及特点,其目的在于让熟悉此项技术的人了解本发明的内容并加以实施,并不能以此限制本发明的保护范围,凡根据本发明精神实质所做的等效变化或修饰,都应涵盖在本发明的保护范围内。The above implementation modes are only for illustrating the technical concept and features of the present invention, and their purpose is to enable people familiar with this technology to understand the content of the present invention and implement it, and they cannot be used to limit the protection scope of the present invention. Any equivalent changes or modifications made according to the spirit of the present invention should be included in the protection scope of the present invention.
Claims (9)
Translated fromChinesePriority Applications (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| CN202211674653.9ACN116000308A (en) | 2022-12-26 | 2022-12-26 | Synthesis method of submicron flake silver powder |
Applications Claiming Priority (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| CN202211674653.9ACN116000308A (en) | 2022-12-26 | 2022-12-26 | Synthesis method of submicron flake silver powder |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| CN116000308Atrue CN116000308A (en) | 2023-04-25 |
Family
ID=86036656
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| CN202211674653.9APendingCN116000308A (en) | 2022-12-26 | 2022-12-26 | Synthesis method of submicron flake silver powder |
Country Status (1)
| Country | Link |
|---|---|
| CN (1) | CN116000308A (en) |
Cited By (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN116855787A (en)* | 2023-07-12 | 2023-10-10 | 安徽中航纳米技术发展有限公司 | Method for preparing metal simple substance and metal composite heat conduction material |
Citations (11)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US4539041A (en)* | 1982-12-21 | 1985-09-03 | Universite Paris Vii | Process for the reduction of metallic compounds by polyols, and metallic powders obtained by this process |
| US20040025635A1 (en)* | 2001-04-02 | 2004-02-12 | Kurihara Lynn K. | Processing of nanocrystalline metallic powders and coatings using the polyol process |
| CN101947655A (en)* | 2010-10-25 | 2011-01-19 | 江苏技术师范学院 | Preparation method of triangular silver nanosheet |
| CN104907582A (en)* | 2015-06-23 | 2015-09-16 | 南开大学 | Synthetic method of hydroxypropyl methyl cellulose-clad nanometer silver material |
| CN108421987A (en)* | 2018-03-16 | 2018-08-21 | 南京工业大学 | Preparation method of flaky elemental bismuth |
| CN110355380A (en)* | 2019-08-13 | 2019-10-22 | 山东建邦胶体材料有限公司 | A kind of preparation method of hexagonal flake micron crystalline substance silver powder |
| CN110899722A (en)* | 2019-12-26 | 2020-03-24 | 无锡晶睿光电新材料有限公司 | A kind of thin single-plate silver powder synthesized by chemical method and preparation method thereof |
| CN110947980A (en)* | 2019-12-24 | 2020-04-03 | 长沙新材料产业研究院有限公司 | Preparation method of micron/submicron silver powder |
| CN111168083A (en)* | 2020-02-24 | 2020-05-19 | 深圳先进技术研究院 | A kind of preparation method of nano silver powder |
| CN114131035A (en)* | 2021-11-30 | 2022-03-04 | 长沙新材料产业研究院有限公司 | Silver powder preparation method and silver powder |
| CN114309632A (en)* | 2021-11-19 | 2022-04-12 | 长沙新材料产业研究院有限公司 | Micron-sized silver powder and preparation method thereof |
- 2022
- 2022-12-26CNCN202211674653.9Apatent/CN116000308A/enactivePending
Patent Citations (11)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US4539041A (en)* | 1982-12-21 | 1985-09-03 | Universite Paris Vii | Process for the reduction of metallic compounds by polyols, and metallic powders obtained by this process |
| US20040025635A1 (en)* | 2001-04-02 | 2004-02-12 | Kurihara Lynn K. | Processing of nanocrystalline metallic powders and coatings using the polyol process |
| CN101947655A (en)* | 2010-10-25 | 2011-01-19 | 江苏技术师范学院 | Preparation method of triangular silver nanosheet |
| CN104907582A (en)* | 2015-06-23 | 2015-09-16 | 南开大学 | Synthetic method of hydroxypropyl methyl cellulose-clad nanometer silver material |
| CN108421987A (en)* | 2018-03-16 | 2018-08-21 | 南京工业大学 | Preparation method of flaky elemental bismuth |
| CN110355380A (en)* | 2019-08-13 | 2019-10-22 | 山东建邦胶体材料有限公司 | A kind of preparation method of hexagonal flake micron crystalline substance silver powder |
| CN110947980A (en)* | 2019-12-24 | 2020-04-03 | 长沙新材料产业研究院有限公司 | Preparation method of micron/submicron silver powder |
| CN110899722A (en)* | 2019-12-26 | 2020-03-24 | 无锡晶睿光电新材料有限公司 | A kind of thin single-plate silver powder synthesized by chemical method and preparation method thereof |
| CN111168083A (en)* | 2020-02-24 | 2020-05-19 | 深圳先进技术研究院 | A kind of preparation method of nano silver powder |
| CN114309632A (en)* | 2021-11-19 | 2022-04-12 | 长沙新材料产业研究院有限公司 | Micron-sized silver powder and preparation method thereof |
| CN114131035A (en)* | 2021-11-30 | 2022-03-04 | 长沙新材料产业研究院有限公司 | Silver powder preparation method and silver powder |
Cited By (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN116855787A (en)* | 2023-07-12 | 2023-10-10 | 安徽中航纳米技术发展有限公司 | Method for preparing metal simple substance and metal composite heat conduction material |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| CN110355380B (en) | Preparation method of hexagonal flaky micron-crystal silver powder | |
| TWI324953B (en) | ||
| CN107971502B (en) | Preparation method of high-dispersity spherical silver powder | |
| US11767443B2 (en) | Copper particle mixture and method for manufacturing same, copper particle mixture dispersion, ink containing copper particle mixture, method for storing copper particle mixture, and method for sintering copper particle mixture | |
| CN108213456B (en) | A kind of preparation method of cubic nanometer copper powder | |
| CN114188066B (en) | A high-crystallization silver powder and low-cost heterojunction silver paste and preparation method and application thereof | |
| CN116000308A (en) | Synthesis method of submicron flake silver powder | |
| CN107876795A (en) | A kind of preparation method of monocrystalline copper powder | |
| CN110340348B (en) | Nano silver powder, preparation method, silver paste and application | |
| TWI271240B (en) | Method for surface treatment of nickel nanoparticles, nickel nanoparticles prepared by the method, and conductive paste and multi-layer ceramic capacitor containing the nickel nanoparticles | |
| WO2024217073A1 (en) | Silver-copper composite conductive paste capable of being sintered at low temperature, method for preparing same and use thereof | |
| CN116571734B (en) | A kind of silver particle and preparation method and application thereof | |
| CN114749654A (en) | Blocky superfine silver powder and preparation method and application thereof | |
| WO2025130280A1 (en) | Graphene-coated silver powder and heterojunction low-temperature silver paste, and preparation methods therefor | |
| CN115722677A (en) | Preparation method of high-dispersity submicron spherical palladium powder | |
| CN116251961A (en) | A kind of ultrasonic-assisted method for preparing silver powder and the prepared silver powder and application | |
| CN117444227A (en) | Silver powder, conductive silver paste, and preparation method and application thereof | |
| CN107282940A (en) | A kind of method that utilization pseudo-ginseng extract solution prepares gold nano grain | |
| CN119319257B (en) | A kind of high tap density silver powder and its preparation method and application | |
| CN112453420A (en) | Preparation method and application of high-performance silver powder | |
| CN114734033A (en) | Flake silver powder suitable for heterojunction solar cell conductive adhesive and preparation method thereof | |
| CN109550933B (en) | A kind of ultra-thin silver and its chemical synthesis method | |
| CN111590086A (en) | Ultra-flake silver powder with smooth surface and preparation method thereof | |
| CN117733170A (en) | Nanometer silver powder and preparation method and application thereof | |
| CN118155906A (en) | Low-temperature curing conductive silver paste, preparation method and application |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PB01 | Publication | ||
| PB01 | Publication | ||
| SE01 | Entry into force of request for substantive examination | ||
| SE01 | Entry into force of request for substantive examination |