CN115959984A - A kind of degradation method of polyester polymer - Google Patents
A kind of degradation method of polyester polymerDownload PDFInfo
- Publication number
- CN115959984A CN115959984ACN202111190964.3ACN202111190964ACN115959984ACN 115959984 ACN115959984 ACN 115959984ACN 202111190964 ACN202111190964 ACN 202111190964ACN 115959984 ACN115959984 ACN 115959984A
- Authority
- CN
- China
- Prior art keywords
- pet
- polyester
- depolymerization
- reaction
- degradation method
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Pending
Links
Images
Classifications
- Y—GENERAL TAGGING OF NEW TECHNOLOGICAL DEVELOPMENTS; GENERAL TAGGING OF CROSS-SECTIONAL TECHNOLOGIES SPANNING OVER SEVERAL SECTIONS OF THE IPC; TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y02—TECHNOLOGIES OR APPLICATIONS FOR MITIGATION OR ADAPTATION AGAINST CLIMATE CHANGE
- Y02W—CLIMATE CHANGE MITIGATION TECHNOLOGIES RELATED TO WASTEWATER TREATMENT OR WASTE MANAGEMENT
- Y02W30/00—Technologies for solid waste management
- Y02W30/50—Reuse, recycling or recovery technologies
- Y02W30/62—Plastics recycling; Rubber recycling
Landscapes
- Organic Low-Molecular-Weight Compounds And Preparation Thereof (AREA)
Abstract
Description
Translated fromChinese技术领域technical field
本发明涉及一种聚酯类聚合物的降解方法,通过本方法可以得到高纯度的二羧酸聚合单体和二元醇二羧酸酯,属于有机化学领域。The invention relates to a method for degrading polyester polymers. The method can obtain high-purity dicarboxylic acid polymer monomers and glycol dicarboxylates, and belongs to the field of organic chemistry.
背景技术Background technique
聚酯是由多元醇和多元酸缩聚而得的聚合物总称,主要指聚对苯二甲酸乙二醇酯(PET),广义上也包括聚对苯二甲酸丁二酯(PBT)和聚芳酯等线型热塑性树脂,是一类性能优异、用途广泛的工程塑料。聚酯的具体品种有:聚对苯二甲酸乙二酯(PET),聚对苯二甲酸丁二酯(PBT),聚对苯二甲酸丙二酯(PTT),聚-2,6-萘二酸乙二酯(PEN),聚呋喃二甲酸乙二醇酯(PEF)以及多种改性的聚酯基纤维,其中使用最为广泛的是PET聚酯。Polyester is a general term for polymers obtained by polycondensation of polyols and polybasic acids, mainly referring to polyethylene terephthalate (PET), and broadly including polybutylene terephthalate (PBT) and polyarylate Isolinear thermoplastic resin is a kind of engineering plastic with excellent performance and wide application. The specific varieties of polyester are: polyethylene terephthalate (PET), polybutylene terephthalate (PBT), polytrimethylene terephthalate (PTT), poly-2,6-naphthalene Ethylene dicarboxylate (PEN), polyethylene furandicarboxylate (PEF) and various modified polyester-based fibers, among which PET polyester is the most widely used.
PET聚酯是一种在世界塑料中具有重要地位的聚合物,广泛应用于各类容器、包装材料、薄膜、工程塑料等领域,并且正在越来越多的取代其他合成材料。废弃PET材料本身并无毒害作用,但其在自然环境下的降解周期太过漫长,由于PET聚酯产量和消费量的迅速增长,废弃的PET也造成了巨大的环境污染和资源浪费。因此实现对PET的降解回收不仅可以减少其对环境的污染还可以有效利用废塑料中的能量。PET polyester is a polymer with an important position in the world's plastics. It is widely used in various containers, packaging materials, films, engineering plastics and other fields, and is increasingly replacing other synthetic materials. The waste PET material itself has no toxic effect, but its degradation cycle in the natural environment is too long. Due to the rapid increase in the production and consumption of PET polyester, the waste PET also causes huge environmental pollution and waste of resources. Therefore, realizing the degradation and recycling of PET can not only reduce its pollution to the environment but also effectively utilize the energy in waste plastics.
目前废弃PET的商业化回收利用主要以物理回收法为主,但该方法会导致材料的结构恶化,性能下降,因此,它只能被加工成低价值的产品,如纺织纤维或低品位的树脂,并很快彻底成为废物。PET超过80%的用途是用作食品包装材料,而只有在化学回收中通过纯化单体或低聚物才可以除去键合在聚合物链上的污染物,也只有这种材料才可以被重新加工成用于食品接触类的PET制品。目前废PET聚酯化学循环方法主要有醇解法、糖酵解法、水解法等。At present, the commercial recycling of waste PET is mainly based on the physical recycling method, but this method will lead to the deterioration of the material's structure and performance, so it can only be processed into low-value products, such as textile fibers or low-grade resins , and soon become completely useless. More than 80% of the uses of PET are used as food packaging materials, and only by purifying monomers or oligomers in chemical recycling can the contaminants bonded to the polymer chain be removed, and only this material can be regenerated. Processed into PET products for food contact. At present, the chemical recycling methods of waste PET polyester mainly include alcoholysis, glycolysis, and hydrolysis.
醇解法主要解聚产物为对苯二甲酸二甲酯(DMT),醇解通常需要在较为苛刻的反应条件下进行降解(温度200~350℃,压力10~20MPa,氮气保护),并且PET的轻微污染也是不能被容忍的。糖酵解主要解聚产物为对苯二甲酸乙二醇酯(BHET)及一些齐聚物,糖酵解虽然反应条件较为温和,但由于降解不彻底,使得一些低聚物的产物,导致分离纯化的的成本大大增加。在所有的化学回收中,只有通过水解法才可以得到对苯二甲酸(TPA)。目前世界PET总生产能力中75%以上均采用高纯度的对苯二甲酸或中纯度对苯二甲酸为聚合原料与乙二醇(EG)直接酯化,连续缩聚成PET聚酯。因此PET水解回收法日益受到重视。The main depolymerization product of the alcoholysis method is dimethyl terephthalate (DMT), and the alcoholysis usually needs to be degraded under relatively harsh reaction conditions (temperature 200-350°C, pressure 10-20MPa, nitrogen protection), and PET Slight contamination is also not tolerated. The main depolymerization products of glycolysis are ethylene terephthalate (BHET) and some oligomers. Although the reaction conditions of glycolysis are relatively mild, due to incomplete degradation, some oligomers are produced, resulting in separation The cost of purification is greatly increased. Of all chemical recycling, terephthalic acid (TPA) is obtained only by hydrolysis. At present, more than 75% of the world's total PET production capacity uses high-purity terephthalic acid or medium-purity terephthalic acid as the polymerization raw material to directly esterify with ethylene glycol (EG), and continuously polycondensate into PET polyester. Therefore, the PET hydrolysis recovery method has been paid more and more attention.
水解法按照pH可以分为碱性水解、中性水解和酸性水解。Hydrolysis can be divided into alkaline hydrolysis, neutral hydrolysis and acidic hydrolysis according to pH.
中性水解是PET在水或水蒸气条件下采用醋酸锌、醋酸锰等作为催化剂进行反应,水解产物为EG和TPA。中性水解需要在高温高压下进行,对设备材料要求高,很难连续化操作,且中性水解法得到的产品纯度不高,后期提纯工艺复杂、成本高。Neutral hydrolysis is the reaction of PET under water or steam conditions using zinc acetate, manganese acetate, etc. as catalysts, and the hydrolysis products are EG and TPA. Neutral hydrolysis needs to be carried out under high temperature and high pressure, which requires high equipment and materials, and it is difficult to operate continuously. Moreover, the purity of the product obtained by neutral hydrolysis is not high, and the subsequent purification process is complicated and costly.
碱性水解一般在浓度为4%-20%NaOH水溶液中进行,但该法反应较慢,产品TPA纯度不高,反应完成后用酸洗涤才能得到对苯二甲酸,在此过程中也会产生大量的废盐和废液。Alkaline hydrolysis is generally carried out in an aqueous NaOH solution with a concentration of 4%-20%, but the reaction of this method is slow, and the purity of the product TPA is not high. After the reaction is completed, terephthalic acid can only be obtained by washing with acid. In the process, it will also produce A large amount of waste salt and waste liquid.
酸性水解一般用浓硫酸为催化剂。Pusztaszeri于1982年发表了用酸催化水解进行PET循环利用的专利,采用浓硫酸(>14.5mol/L)作催化剂,水解完成后,用冷水稀释产物,然后加NaOH溶液至pH=11。此时体系由乙二醇、TPA的钠盐和Na2SO4水溶液及不溶性杂质组成。过滤,将滤液酸化至pH=1-3,析出固态TPA,再过滤、洗涤得纯度>99%的TPA。该法的不足之处在于由于化学平衡的存在PET未能完全解聚,存在一些PET及其低聚物,使得TPA纯化过程复杂,反应消耗的大量的浓酸和强碱难以循环使用,易造成环境污染产生大量废盐,且生成的乙二醇亦较难回收,原子经济性不高。且该方法中PET底物的浓度不到2wt%,难以实现工业化生产。Acidic hydrolysis generally uses concentrated sulfuric acid as a catalyst. In 1982, Pusztaszeri published a patent on recycling PET by acid-catalyzed hydrolysis. Concentrated sulfuric acid (>14.5mol/L) was used as a catalyst. After the hydrolysis was completed, the product was diluted with cold water, and then NaOH solution was added to pH=11. At this time, the system is composed of ethylene glycol, sodium salt of TPA, Na2 SO4 aqueous solution and insoluble impurities. Filtrate, acidify the filtrate to pH=1-3, precipitate solid TPA, then filter and wash to obtain TPA with a purity >99%. The disadvantage of this method is that due to the existence of chemical balance, PET cannot be completely depolymerized, and some PET and its oligomers exist, which makes the TPA purification process complicated, and the large amount of concentrated acid and strong alkali consumed by the reaction is difficult to recycle, which is easy to cause Environmental pollution produces a large amount of waste salt, and the produced ethylene glycol is difficult to recycle, and the atom economy is not high. And the concentration of PET substrate in this method is less than 2wt%, it is difficult to realize industrial production.
因此发展一种解聚彻底,后期纯化简单,对苯二甲酸的产率高、纯度高、原子经济的新型废弃PET的降解方法十分重要。Therefore, it is very important to develop a novel waste PET degradation method with thorough depolymerization, simple purification in the later stage, high yield of terephthalic acid, high purity, and atomic economy.
发明内容Contents of the invention
本发明旨在提供一种聚酯类聚合物的降解方法,以有机酸作为溶剂对废弃PET进行化学解聚,降解获得的二羧酸单体可以用于聚酯再聚合的高纯单体,得到的小分子二元醇二羧酸酯为高附加值化学品,可以通过分离纯化用于其他用途,有利于实现聚合物降解的经济效益最大化。The purpose of the present invention is to provide a degradation method for polyester polymers, which uses organic acids as solvents to chemically depolymerize waste PET, and the dicarboxylic acid monomers obtained through degradation can be used as high-purity monomers for polyester repolymerization. The obtained small-molecule diol dicarboxylate is a high value-added chemical, which can be used for other purposes through separation and purification, which is conducive to maximizing the economic benefits of polymer degradation.
本发明聚酯类聚合物的降解方法,利用有机酸作为溶剂,将有机酸、催化剂与废旧聚酯聚合物混合,即可实现聚合物的解聚。所述废旧聚酯聚合物包含二元羧酸与二元醇共聚形成的聚酯重复单元;解聚产物包含二元羧酸单体和二元醇羧酸酯;通过固液分离得到二元羧酸单体,通过蒸馏回收溶剂得到二元醇羧酸酯。The method for degrading polyester polymers of the present invention uses organic acids as solvents, and mixes organic acids, catalysts and waste polyester polymers to realize depolymerization of polymers. The waste polyester polymer comprises polyester repeating units formed by the copolymerization of dibasic carboxylic acid and dibasic alcohol; the depolymerization product contains dibasic carboxylic acid monomer and dibasic alcohol carboxylate; Acid monomers, the solvent is recovered by distillation to obtain glycol carboxylic acid esters.
所述废旧聚酯聚合物包括下述重复结构单元片段。The waste polyester polymer includes the following repeating structural unit fragments.
其中片段A为芳香区片段,片段B为脂肪区片段。聚合物降解后芳香区片段可以分离得到二羧酸单体的固体产物,脂肪区可以分离得到二元醇羧酸酯的液体产物。Fragment A is an aromatic region fragment, and fragment B is an aliphatic region fragment. After the degradation of the polymer, the aromatic region fragments can be separated to obtain solid products of dicarboxylic acid monomers, and the fatty regions can be separated to obtain liquid products of diol carboxylates.
其中n=2~6,优选n=2~4。Wherein n=2-6, preferably n=2-4.
Ar为芳香环结构,优选为Ar is an aromatic ring structure, preferably
所述聚合物除含有聚酯结构外,可以存在其他任何形式的聚合物,均不会影响聚酯降解。In addition to the polyester structure, the polymer may be in any other form, which will not affect the degradation of the polyester.
优选地,所述废旧聚酯聚合物包括商购的PET粉末或颗粒,目前市场上主流消费后的PET材质的饮料瓶,包括但不限于:哇哈哈等透明矿泉水瓶、可乐瓶、雪碧瓶、脉动瓶、格瓦斯瓶等无色或有色的PET材质的饮料瓶;无色或有色的PET材质的塑料托盘、果盒和餐盒等以及无法再回收的各类PET材质的无纺布。Preferably, the waste polyester polymer includes commercially available PET powder or granules, and beverage bottles made of PET after mainstream consumption on the market include but are not limited to: Wahaha and other transparent mineral water bottles, Coke bottles, Sprite bottles, Colorless or colored PET beverage bottles such as pulsation bottles and kvass bottles; colorless or colored PET plastic trays, fruit boxes and lunch boxes, and non-woven fabrics made of PET that cannot be recycled.
所述有机酸的结构如下所示:The structure of the organic acid is as follows:
其中R为氢,或者为C1-C6的直链、支链或环状的烷基。Wherein R is hydrogen, or C1-C6 linear, branched or cyclic alkyl.
优选地,所述有机酸的R为H或链状烷基,最优选为甲基。乙酸效果好,且价廉易得。Preferably, R of the organic acid is H or a chain alkyl group, most preferably methyl. Acetic acid works well and is cheap and readily available.
溶剂有机酸的体积与废旧聚酯聚合物的质量比为100-1mL:1g,优选为20~10。The volume ratio of solvent organic acid to waste polyester polymer is 100-1 mL:1 g, preferably 20-10.
所述催化剂包括:H2O、HBr、HCl、H2SO4、HOTf、CF3COOH、MeSO3H、TsOH中的一种或几种的组合。优选的,所述催化剂为H2O和CF3COOH,最优选为不加。The catalyst includes: one or a combination of H2 O, HBr, HCl, H2 SO4 , HOTf, CF3 COOH, MeSO3 H, and TsOH. Preferably, the catalyst is H2 O and CF3 COOH, most preferably not added.
所述催化剂和废旧聚酯聚合物的质量比为1:250至1:2,优选为1:100至1:50,最优选为不加。The mass ratio of the catalyst to the waste polyester polymer is 1:250 to 1:2, preferably 1:100 to 1:50, most preferably not added.
H2O可以作为催化剂加入,能够提高TPA的收率,但对乙二醇二乙酸酯的产量有影响;优选地,水的含量为0~20wt%,最优选为2~5wt%。H2 O can be added as a catalyst, which can increase the yield of TPA, but has an impact on the yield of ethylene glycol diacetate; preferably, the content of water is 0-20wt%, most preferably 2-5wt%.
所述解聚反应在法兰式水热反应釜中进行,反应温度为180-300℃,反应时间为1-24小时。The depolymerization reaction is carried out in a flanged hydrothermal reactor, the reaction temperature is 180-300°C, and the reaction time is 1-24 hours.
本发明的解聚方法简单,聚酯转化率高,产物纯度高,且所得产物分离简单。此外,通过本方法降解聚酯,经过简单处理可以得到重新用于聚合的二羧酸单体,还可以得到高价值的化学原料二元醇二羧酸酯,更具经济效益。The depolymerization method of the present invention is simple, the polyester conversion rate is high, the product purity is high, and the separation of the obtained product is simple. In addition, by degrading polyester through the method, dicarboxylic acid monomers reused for polymerization can be obtained through simple treatment, and high-value chemical raw material diol dicarboxylates can also be obtained, which is more economical.
本发明操作简单:将聚合物、溶剂、催化剂加入高压反应釜后加热搅拌至反应完全。所得产物分离简单:反应完毕后产物二羧酸单体会从溶剂中结晶析出,过滤即可达到高纯的二羧酸产物,产品为层状晶体,晶型通过SEM和XRD证实;二元醇二羧酸酯存在于有机酸溶液中,通过减压蒸馏,即可得到二元醇二羧酸酯和有机酸溶剂。The operation of the invention is simple: add polymer, solvent and catalyst into a high-pressure reactor, heat and stir until the reaction is complete. The separation of the obtained product is simple: after the reaction is completed, the product dicarboxylic acid monomer will crystallize out from the solvent, and the high-purity dicarboxylic acid product can be obtained by filtration. The product is a layered crystal, and the crystal form is confirmed by SEM and XRD; The dicarboxylate exists in the organic acid solution, and the glycol dicarboxylate and the organic acid solvent can be obtained by distillation under reduced pressure.
本发明可将含有聚酯重复单元的聚合物降解转化为二羧酸和二元醇二羧酸酯,转化率高,产物单一,且分离简单。二羧酸可直接用于聚酯的再合成,二元醇二羧酸酯可作为化学品直接用于其他化学转化中,有利于实现聚酯降解的经济效益最大化。The invention can degrade and convert the polymer containing polyester repeating unit into dicarboxylic acid and glycol dicarboxylate, with high conversion rate, single product and simple separation. Dicarboxylic acid can be directly used in the resynthesis of polyester, and glycol dicarboxylate can be directly used in other chemical transformations as chemicals, which is beneficial to maximize the economic benefits of polyester degradation.
附图说明Description of drawings
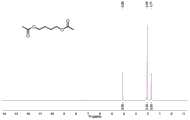
图1是根据本发明方法获得的对苯二甲酸的1H NMR谱图。Fig. 1 is a1 H NMR spectrum of terephthalic acid obtained according to the method of the present invention.
图2是根据本发明方法获得的2,5-呋喃二甲酸的1H NMR谱图。Fig. 2 is the1 H NMR spectrum of 2,5-furandicarboxylic acid obtained according to the method of the present invention.
图3是根据本发明方法获得的2,6-萘二甲酸的1H NMR谱图。Fig. 3 is the1 H NMR spectrum of 2,6-naphthalene dicarboxylic acid obtained according to the method of the present invention.
图4是根据本发明方法获得的乙二醇二乙酸酯的1H NMR谱图。Fig. 4 is the1 H NMR spectrum of ethylene glycol diacetate obtained according to the method of the present invention.
图5是根据本发明方法获得的乙二醇单乙酸酯的1H NMR谱图。Fig. 5 is the1 H NMR spectrum of ethylene glycol monoacetate obtained according to the method of the present invention.
图6是根据本发明方法获得的丁二醇二乙酸酯的1H NMR谱图。Fig. 6 is a1 H NMR spectrum of butanediol diacetate obtained according to the method of the present invention.
图7是根据本发明方法获得的1,4-环己烷二甲醇二乙酸酯的1H NMR谱图。Fig. 7 is the 1H NMR spectrogram of 1,4-cyclohexanedimethanol diacetate obtained according to the method of the present invention.
图1-图7是在环境温度下在Bruker Advance 400Spectrometer上获得的谱图。Figures 1-7 are spectra obtained on a Bruker Advance 400 Spectrometer at ambient temperature.
图8是商购的TPA粉末的SEM图。Figure 8 is a SEM image of commercially available TPA powder.
图9是降解PET碎片得到的TPA的SEM图(150X)。Figure 9 is a SEM image (150X) of TPA obtained by degrading PET fragments.
图10是降解PET碎片得到的TPA的SEM图(500X)。Figure 10 is a SEM image (500X) of TPA obtained by degrading PET fragments.
图11是商购的PET粉末(50μm)的SEM图。Fig. 11 is a SEM image of commercially available PET powder (50 μm).
图12是降解PET粉末得到的TPA的SEM图(200X)。Figure 12 is a SEM image (200X) of TPA obtained by degrading PET powder.
图13是降解PET粉末得到的TPA的SEM图(500X)。Figure 13 is a SEM image (500X) of TPA obtained by degrading PET powder.
图14是TPA的XRD图案。a)通过本发明方法获得的TPA;b)通过碱水解得到的TPA;c)TPA的标准PDF卡片。Figure 14 is the XRD pattern of TPA. a) TPA obtained by the method of the present invention; b) TPA obtained by alkaline hydrolysis; c) a standard PDF card of TPA.
图15是实施例1-8中采用不同催化剂得到的对苯二甲酸的照片。Figure 15 is a photograph of terephthalic acid obtained using different catalysts in Examples 1-8.
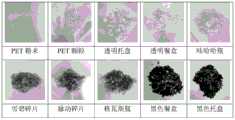
图16是不同来源的PET照片。Figure 16 is a PET photograph from a different source.
图17是通过不同来源的PET降解得到的TPA的照片。Figure 17 is a photograph of TPA obtained by degradation of PET from different sources.
图18是实施例38中以PEF聚酯降解获得2,5-呋喃二甲酸的照片。Figure 18 is a photo of 2,5-furandicarboxylic acid obtained by degradation of PEF polyester in Example 38.
图19是实施例39中以PEN聚酯均为原料降解获得2,6-萘二甲酸的照片。Fig. 19 is a photo of 2,6-naphthalene dicarboxylic acid obtained by degrading PEN polyester as a raw material in Example 39.
图20是实施例41中以PET聚酯和PP聚合物作为混合原料降解获得对苯二甲酸的照片。Fig. 20 is a photo of terephthalic acid obtained by degrading PET polyester and PP polymer as mixed raw materials in Example 41.
图21是实施例42中以PET和聚乙烯混纺而成的无纺布为原料降解获得对苯二甲酸的照片。Fig. 21 is a photo of terephthalic acid obtained by degrading a non-woven fabric made of PET and polyethylene blended as a raw material in Example 42.
图22是实施例44中以聚对苯二甲酸乙二醇酯-1,4-环己烷二甲醇酯(PETG)为原料降解获得对苯二甲酸的照片。Fig. 22 is a photo of terephthalic acid obtained by degrading polyethylene terephthalate-1,4-cyclohexanedimethanol (PETG) as a raw material in Example 44.
具体实施方式Detailed ways
下面结合具体实施例,进一步阐述本发明。应理解,这些实施例仅用于说明本发明而不用于限制本发明的范围。此外应理解,在阅读了本发明讲授的内容之后,本领域技术人员可以对本发明作各种改动或修改,这些等价形式同样落于本申请所附权利要求书所限定的范围。Below in conjunction with specific embodiment, further illustrate the present invention. It should be understood that these examples are only used to illustrate the present invention and are not intended to limit the scope of the present invention. In addition, it should be understood that after reading the teachings of the present invention, those skilled in the art can make various changes or modifications to the present invention, and these equivalent forms also fall within the scope defined by the appended claims of the present application.
实施例1~8为以PET聚酯为模板原料,考察其不同催化剂对解聚产物TPA和EGDA收率的影响。表9~14为以PET聚酯为模板原料,考察其不同水含量对解聚产物TPA和EGDA以及EGMA收率的影响。实施例15~19为以PET聚酯为原料,考察不同PET浓度下解聚反应的结果。实施例20~29为考察不同来源PET对解聚反应的结果。实施例30和31为降解PET聚酯的放大实验。实施例32和33为考察不同有机酸溶剂对PET解聚的结果。实施例34~37为考察PET碎片在不同温度下的降解结果。实施例38~44为考察其他非PET的聚酯的乙酸解结果。Examples 1-8 use PET polyester as the template raw material to investigate the influence of different catalysts on the yield of depolymerization products TPA and EGDA. Tables 9-14 use PET polyester as the template raw material to investigate the influence of different water contents on the yield of depolymerization products TPA, EGDA and EGMA. Examples 15-19 are the results of investigating the depolymerization reaction at different PET concentrations using PET polyester as the raw material. Examples 20-29 are the results of investigating the depolymerization reaction of PET from different sources. Examples 30 and 31 are scale-up experiments for degrading PET polyester. Examples 32 and 33 are the results of investigating the depolymerization of PET by different organic acid solvents. Examples 34-37 are the results of investigating the degradation of PET chips at different temperatures. Examples 38-44 are for investigating the results of acetic acid hydrolysis of other non-PET polyesters.
解聚过程中固体产物通过核磁共振表征其结构,所得收率均为分离收率,通过“中华人民共和国国家标准—工业用精对苯二甲酸(PTA)实验方法第5部分:酸值的测定(GB/T30921.5-2016)”确定其纯度,液体产物通过核磁共振确认结构,气相色谱确定其收率。During the depolymerization process, the structure of the solid product was characterized by nuclear magnetic resonance, and the obtained yields were all isolated yields, which passed the "National Standard of the People's Republic of China-Industrial Purified Terephthalic Acid (PTA) Experimental Method Part 5: Determination of Acid Value (GB/T30921.5-2016)" to determine its purity, the structure of the liquid product was confirmed by nuclear magnetic resonance, and the yield was determined by gas chromatography.
本发明是一种新型的废旧PET的降解方法,其核心技术在于乙酸作为溶剂和酸催化剂进行反应,解聚产物为对苯二甲酸(TPA),乙二醇二乙酸酯(EGDA)和乙二醇单乙酸酯(EGMA)。我们选用消费后的哇哈哈空瓶,将其剪成碎片,考察PET碎片降解的反应参数。经多次实验验证,该解聚反应中的解聚产物主要为TPA和EGDA,而EGMA以及未完全解聚的中间产物较少。反应路线如下所示:The present invention is a novel degradation method of waste PET. Its core technology is that acetic acid is used as a solvent to react with an acid catalyst, and the depolymerization products are terephthalic acid (TPA), ethylene glycol diacetate (EGDA) and ethylene glycol diacetate (EGDA). Glycol monoacetate (EGMA). We selected post-consumer Wahaha empty bottles and cut them into fragments to investigate the reaction parameters of PET fragment degradation. It has been verified by many experiments that the depolymerization products in this depolymerization reaction are mainly TPA and EGDA, while EGMA and incompletely depolymerized intermediate products are less. The reaction scheme is as follows:
首先考察不同的布朗斯特酸作为催化剂对PET降解的影响。Firstly, the effects of different Bronsted acids as catalysts on the degradation of PET were investigated.
实施例1~8:
在30mL法兰式水热反应釜中依次加入2.5g PET碎片,25ml冰醋酸,表1中不同的酸催化剂(表1),将水热反应釜密封后置于均相合成仪中,均相合成仪转速为10Hz,加热至220℃,在恒定温度为220℃的条件下,进行解聚反应10小时。反应完毕过滤,所得固体即为对苯二甲酸。滤液中含有EGDA和乙酸,以丁二醇二乙酸酯为内标,通过气相色谱进行定量。In the 30mL flange type hydrothermal reaction kettle, add 2.5g PET chips successively, 25ml glacial acetic acid, different acid catalysts (table 1) in table 1, place in the homogeneous synthesizer after the hydrothermal reaction kettle is sealed, homogeneous The rotating speed of the synthesizer was 10 Hz, heated to 220° C., and depolymerization reaction was carried out for 10 hours under the condition of a constant temperature of 220° C. After the reaction is completed and filtered, the resulting solid is terephthalic acid. The filtrate contained EGDA and acetic acid, and was quantified by gas chromatography with butanediol diacetate as internal standard.
对于实施例1~8,使用不同催化剂,聚酯的降解率都大于99.9%,以三氟甲磺酸和甲基磺酸作为催化剂时,反应所得产物TPA和EGDA的收率最高,但有部分碳化导致产物色泽不好(图15)。因此相对来说,在保证产物色泽前提下,不外加催化剂就有较高的分离收率。For
表1不同酸作为催化剂的实验参数Table 1 Experimental parameters of different acids as catalysts
以下实验以冰醋酸为溶剂在不外加催化剂的情况下,考察不同水含量对单体收率的影响。In the following experiment, glacial acetic acid was used as a solvent without adding a catalyst to investigate the influence of different water contents on the monomer yield.
实施例9~14:
在30mL法兰式水热反应釜中依次加入5.0g PET碎片,25ml乙酸水溶液,水含量见表2,将水热釜密封后置于均相合成仪中,均相合成仪转速为10Hz,加热至220℃,在恒定温度为220℃的条件下,进行解聚反应10小时。反应完毕过滤,所得固体即为对苯二甲酸。滤液中含有EGDA、EGMA和乙酸,以丁二醇二乙酸酯为内标,通过气相色谱进行定量。Add 5.0g of PET fragments and 25ml of acetic acid aqueous solution to the 30mL flanged hydrothermal reaction kettle successively. To 220° C., under the condition of a constant temperature of 220° C., the depolymerization reaction was carried out for 10 hours. After the reaction is completed and filtered, the resulting solid is terephthalic acid. The filtrate contained EGDA, EGMA and acetic acid, and was quantified by gas chromatography with butanediol diacetate as internal standard.
表2不同水含量对PET聚酯降解的影响The influence of table 2 different water contents on the degradation of PET polyester
对于实施例9~14,以冰醋酸为溶剂在不外加催化剂的情况下,不同水含量聚酯的降解率都大于99.9%,可以发现,随着水分的增加EGDA的产率逐渐下降,EGMA的产率逐渐升高,总产率在一定范围内保持稳定,在含水量0~20wt%时,TPA和乙二醇羧酸酯的收率均很高。说明在该条件下,聚酯的降解反应对水具有很强的耐受性。For Examples 9 to 14, with glacial acetic acid as a solvent without adding a catalyst, the degradation rates of polyesters with different water contents are all greater than 99.9%. It can be found that the yield of EGDA decreases gradually along with the increase of moisture, and the yield of EGMA The yield increases gradually, and the total yield remains stable within a certain range. When the water content is 0-20 wt%, the yields of TPA and ethylene glycol carboxylate are both high. It shows that under this condition, the degradation reaction of polyester has a strong tolerance to water.
以下实验以含水量5wt%的乙酸水溶液为溶剂在不外加催化剂的情况下,考察不同PET浓度对单体收率的影响。In the following experiment, acetic acid aqueous solution with a water content of 5 wt% was used as a solvent without adding a catalyst to investigate the influence of different PET concentrations on the monomer yield.
实施例15~19:
在30mL法兰式水热反应釜中依次加入一定量的PET碎片(表3),25ml含水量为5wt%的乙酸水溶液,将水热釜密封后置于均相合成仪中,均相合成仪转速为10Hz,加热至220℃,在恒定温度为220℃的条件下,进行解聚反应10小时。反应完毕过滤,所得固体即为对苯二甲酸。滤液中含有EGDA、EGMA和乙酸,以丁二醇二乙酸酯为内标,通过气相色谱进行定量。In the 30mL flange type hydrothermal reaction kettle, add a certain amount of PET chips (Table 3) successively, 25ml water content is the acetic acid aqueous solution of 5wt%, place in the homogeneous synthesizer after the hydrothermal kettle is sealed, homogeneous synthesizer The rotational speed is 10 Hz, heated to 220° C., and depolymerization reaction is performed for 10 hours under the condition of a constant temperature of 220° C. After the reaction is completed and filtered, the resulting solid is terephthalic acid. The filtrate contained EGDA, EGMA and acetic acid, and was quantified by gas chromatography with butanediol diacetate as internal standard.
表3不同PET底物浓度对聚酯降解的影响Table 3 Effects of different PET substrate concentrations on polyester degradation
对于实施例14~19,以含水量5wt%的乙酸水溶液为溶剂在不外加催化剂的情况下,可以发现PET浓度对解聚有较大影响,当PET与乙酸水溶液质量比为1/2时,对苯二甲酸和酯收率明显降低,但当PET与乙酸水溶液质量比为1/5时,解聚效果最好。For Examples 14 to 19, the acetic acid aqueous solution with a water content of 5wt% is used as a solvent without adding a catalyst, and it can be found that the PET concentration has a greater impact on depolymerization. When the mass ratio of PET to acetic acid aqueous solution was 1/2, The yield of terephthalic acid and ester decreased obviously, but when the mass ratio of PET to acetic acid aqueous solution was 1/5, the depolymerization effect was the best.
以下实验以含水量5wt%的乙酸水溶液为溶剂在不外加催化剂的情况下,考察不同来源PET的解聚情况。In the following experiments, the depolymerization of PET from different sources was investigated using acetic acid aqueous solution with a water content of 5 wt% as a solvent without adding a catalyst.
实施例20~29:
在30mL法兰式水热反应釜中依次加入5.0g不同来源的PET碎片(表4),25ml含水量为5wt%的乙酸水溶液,将水热釜密封后置于均相合成仪中,均相合成仪转速为10Hz,加热至220℃,在恒定温度为220℃的条件下,进行解聚反应10小时。反应完毕过滤,所得固体即为对苯二甲酸。滤液中含有EGDA、EGMA和乙酸,以丁二醇二乙酸酯为内标,通过气相色谱进行定量。In the 30mL flanged hydrothermal reaction kettle, add 5.0g of different sources of PET fragments (Table 4) successively, 25ml water content is the acetic acid aqueous solution of 5wt%, place in the homogeneous synthesizer after the hydrothermal kettle is sealed, homogeneous The rotating speed of the synthesizer was 10 Hz, heated to 220° C., and depolymerization reaction was carried out for 10 hours under the condition of a constant temperature of 220° C. After the reaction is completed and filtered, the resulting solid is terephthalic acid. The filtrate contained EGDA, EGMA and acetic acid, and was quantified by gas chromatography with butanediol diacetate as internal standard.
表4不同来源的PET对聚酯降解的影响The impact of different sources of PET on the degradation of polyester in table 4
对于实施例20~29,可以看出对于不同来源、不同颜色的PET(图16),通过本发明的方法解聚效果都很好(图17),说明本发明对于不同来源的PET耐受性很好,对于实现PET的闭环循环十分有利。下图为不同来源的PET照片。For Examples 20-29, it can be seen that for different sources and different colors of PET (Figure 16), the depolymerization effect by the method of the present invention is very good (Figure 17), indicating that the present invention is resistant to PET from different sources Very good, it is very beneficial to realize the closed loop circulation of PET. Below are PET photos from different sources.
实施例30:Example 30:
在500mL反应釜中依次加入60g PET碎片,300ml含水量为5wt%的乙酸水溶液,将反应釜密封后,开启机械搅拌,在恒定温度为220℃的条件下,进行解聚反应10小时。反应完毕过滤,过滤得45.86g对苯二甲酸固体,分离收率88.40%。滤液中含有EGDA、EGMA和乙酸,以丁二醇二乙酸酯为内标,通过气相色谱进行定量,气相收率88.2%(乙二醇二乙酸酯:乙二醇单乙酸酯=77%:23%)。通过减压蒸馏回收溶剂乙酸,并获得35.7g无色液体,其中EGDA,29.41g(100Pa,62℃),EGMA6.29g(100Pa,68℃),分离收率共计83.81%。Add 60g of PET fragments and 300ml of acetic acid aqueous solution with a water content of 5wt% to a 500mL reactor in sequence. After sealing the reactor, turn on the mechanical stirring, and carry out the depolymerization reaction at a constant temperature of 220°C for 10 hours. After the reaction was completed and filtered, 45.86 g of terephthalic acid solids were obtained by filtration, and the separation yield was 88.40%. Contain EGDA, EGMA and acetic acid in the filtrate, take butanediol diacetate as internal standard, carry out quantification by gas chromatography, gas phase yield 88.2% (ethylene glycol diacetate: ethylene glycol monoacetate=77 %:twenty three%). The solvent acetic acid was recovered by distillation under reduced pressure, and 35.7g of colorless liquid was obtained, of which EGDA, 29.41g (100Pa, 62°C), EGMA6.29g (100Pa, 68°C), the total separation yield was 83.81%.
实施例31:Example 31:
在500mL反应釜中依次加入60g PET碎片,300ml冰醋酸,将反应釜密封后,开启机械搅拌,加热至220℃,在恒定温度为220℃的条件下,进行解聚反应10小时。反应完毕过滤,过滤得42.91g对苯二甲酸固体,分离收率76.20%。滤液中含有EGDA和乙酸,以丁二醇二乙酸酯为内标,通过气相色谱进行定量,乙二醇二乙酸酯的气相收率77.3%。通过减压蒸馏回收溶剂并得到EGDA33.7g(100Pa,62℃),分离收率共计73.81%。Add 60g of PET fragments and 300ml of glacial acetic acid to the 500mL reactor in sequence. After sealing the reactor, turn on the mechanical stirring, heat to 220°C, and carry out the depolymerization reaction at a constant temperature of 220°C for 10 hours. After the reaction was completed, 42.91 g of terephthalic acid solids were obtained by filtration, and the separation yield was 76.20%. Containing EGDA and acetic acid in the filtrate, using butanediol diacetate as an internal standard, quantified by gas chromatography, the gas phase yield of ethylene glycol diacetate was 77.3%. The solvent was recovered by distillation under reduced pressure and 33.7 g (100 Pa, 62° C.) of EGDA were obtained, and the isolated yield was 73.81% in total.
实施例32:Example 32:
在30mL带磁力搅拌子的耐压管中依次加入1.0gPET碎片,5ml正戊酸,将耐压管密封后置于高温导热硅油中,磁力搅拌速度为700rpm,加热至220℃,在恒定温度为220℃的条件下,进行解聚反应5小时。反应完毕过滤,所得固体即为对苯二甲酸(产率78%,纯度99.30%)。Add 1.0g of PET fragments and 5ml of n-valeric acid to a 30mL pressure-resistant tube with a magnetic stirrer, seal the pressure-resistant tube and place it in high-temperature heat-conducting silicone oil with a magnetic stirring speed of 700rpm, heat to 220°C, and Under the condition of 220°C, the depolymerization reaction was carried out for 5 hours. After the reaction was completed and filtered, the obtained solid was terephthalic acid (yield 78%, purity 99.30%).
实施例33:Example 33:
在30mL带磁力搅拌子的耐压管中依次加入1.0gPET碎片,5ml正己酸,将耐压管密封后置于高温导热硅油中,磁力搅拌速度为700rpm,加热至250℃,在恒定温度为250℃的条件下,进行解聚反应3小时。反应完毕过滤,所得固体即为对苯二甲酸(产率82%,纯度99.18%)。Add 1.0g of PET fragments and 5ml of n-hexanoic acid to a 30mL pressure-resistant tube with a magnetic stirrer in turn, seal the pressure-resistant tube and place it in high-temperature heat-conducting silicone oil with a magnetic stirring speed of 700rpm, heat to 250°C, and Under the condition of ℃, the depolymerization reaction was carried out for 3 hours. After the reaction was completed and filtered, the obtained solid was terephthalic acid (yield 82%, purity 99.18%).
实施例34:Example 34:
在30mL法兰式水热反应釜中依次加入5.0gPET碎片,25ml含水量为5wt%的乙酸水溶液,将水热釜密封后置于均相合成仪中,均相合成仪转速为10Hz,在恒定温度为180℃的条件下,进行解聚反应10小时。反应完毕过滤,所得固体即为对苯二甲酸(产率30.2%,纯度79.68%)。以丁二醇二乙酸酯为内标,通过气相色谱检测滤液中含有的EGDA(23.7%)、EGMA(5.9%)。In the 30mL flange type hydrothermal reaction kettle, add 5.0g PET fragments successively, 25ml water content is the acetic acid aqueous solution of 5wt%, after sealing the hydrothermal kettle, place it in the homogeneous synthesizer, the homogeneous synthesizer rotating speed is 10Hz, at constant The depolymerization reaction was carried out for 10 hours at a temperature of 180°C. After the reaction was completed and filtered, the obtained solid was terephthalic acid (yield 30.2%, purity 79.68%). Using butanediol diacetate as an internal standard, EGDA (23.7%) and EGMA (5.9%) contained in the filtrate were detected by gas chromatography.
实施例35:Example 35:
在30mL法兰式水热反应釜中依次加入5.0gPET碎片,25ml含水量为5wt%的乙酸水溶液,将水热釜密封后置于均相合成仪中,均相合成仪转速为10Hz,在恒定温度为250℃的条件下,进行解聚反应10小时。反应完毕过滤,所得固体即为对苯二甲酸(产率93.8%,纯度99.25%)。以丁二醇二乙酸酯为内标,通过气相色谱检测滤液中含有的EGDA(70.4%)、EGMA(17.7%)。In the 30mL flange type hydrothermal reaction kettle, add 5.0g PET fragments successively, 25ml water content is the acetic acid aqueous solution of 5wt%, after sealing the hydrothermal kettle, place it in the homogeneous synthesizer, the homogeneous synthesizer rotating speed is 10Hz, at constant The depolymerization reaction was carried out for 10 hours at a temperature of 250°C. After the reaction was completed and filtered, the obtained solid was terephthalic acid (yield 93.8%, purity 99.25%). Using butanediol diacetate as an internal standard, EGDA (70.4%) and EGMA (17.7%) contained in the filtrate were detected by gas chromatography.
实施例36:Example 36:
在30mL法兰式水热反应釜中依次加入5.0gPET碎片,25ml含水量为5wt%的乙酸水溶液,将水热釜密封后置于均相合成仪中,均相合成仪转速为10Hz,在恒定温度为280℃的条件下,进行解聚反应2小时。反应完毕过滤,所得固体即为对苯二甲酸(产率96.9%,纯度99.68%)。以丁二醇二乙酸酯为内标,通过气相色谱检测滤液中含有的EGDA(75.6%)、EGMA(18.9%)。In the 30mL flange type hydrothermal reaction kettle, add 5.0g PET fragments successively, 25ml water content is the acetic acid aqueous solution of 5wt%, after sealing the hydrothermal kettle, place it in the homogeneous synthesizer, the homogeneous synthesizer rotating speed is 10Hz, at constant Under the condition of temperature of 280° C., the depolymerization reaction was carried out for 2 hours. After the reaction was completed and filtered, the obtained solid was terephthalic acid (96.9% yield, 99.68% purity). Using butanediol diacetate as an internal standard, EGDA (75.6%) and EGMA (18.9%) contained in the filtrate were detected by gas chromatography.
实施例37:Example 37:
在30mL法兰式水热反应釜中依次加入5.0gPET碎片,25ml冰醋酸,将水热釜密封后置于均相合成仪中,均相合成仪转速为10Hz,在恒定温度为280℃的条件下,进行解聚反应2小时。反应完毕过滤,所得固体即为对苯二甲酸(产率95.8%,纯度99.72%)。以丁二醇二乙酸酯为内标,通过气相色谱检测滤液中含有的EGDA(95.3%)。Add 5.0g of PET fragments and 25ml of glacial acetic acid to a 30mL flanged hydrothermal reaction kettle in sequence, seal the hydrothermal kettle and place it in a homogeneous synthesizer with a speed of 10Hz at a constant temperature of 280°C Next, the depolymerization reaction was carried out for 2 hours. After the reaction was completed and filtered, the obtained solid was terephthalic acid (95.8% yield, 99.72% purity). Using butanediol diacetate as an internal standard, EGDA (95.3%) contained in the filtrate was detected by gas chromatography.
实施例38:Example 38:
一种PEF聚酯的降解方法,具体步骤为:A kind of degradation method of PEF polyester, concrete steps are:
在30mL法兰式水热反应釜中加入5.0gPEF聚酯(分子量>30000),25ml冰醋酸,将水热釜密封后置于均相合成仪中,均相合成仪转速为10Hz,加热至220℃,在恒定温度为220℃的条件下,进行解聚反应10小时。反应完毕过滤,所得固体即为2,5-呋喃二甲酸(产率84.3%,纯度99.32%)。以丁二醇二乙酸酯为内标,通过气相色谱进行检测滤液中的EGDA(85.1%)。Add 5.0g of PEF polyester (molecular weight>30000) and 25ml of glacial acetic acid into a 30mL flanged hydrothermal reactor, seal the hydrothermal reactor and place it in a homogeneous synthesizer with a speed of 10Hz and heat to 220 °C, under the condition of a constant temperature of 220 °C, the depolymerization reaction was carried out for 10 hours. After the reaction was completed and filtered, the obtained solid was 2,5-furandicarboxylic acid (yield 84.3%, purity 99.32%). EGDA (85.1%) in the filtrate was detected by gas chromatography with butanediol diacetate as internal standard.
实施例39:Example 39:
一种PEN聚酯的降解方法,具体步骤为:A kind of degradation method of PEN polyester, concrete steps are:
在30mL法兰式水热反应釜中依次加入5.0gPEN聚酯,25ml冰醋酸,将水热釜密封后置于均相合成仪中,均相合成仪转速为10Hz,加热至220℃,在恒定温度为220℃的条件下,进行解聚反应10小时。反应完毕过滤,所得固体即为2,6-萘二甲酸(产率80.9%,纯度99.18%)。以乙二醇二乙酸酯为内标,通过气相色谱进行检测滤液中的EGDA(77.2%)。Add 5.0g of PEN polyester and 25ml of glacial acetic acid to a 30mL flange-type hydrothermal reaction kettle in sequence, seal the hydrothermal kettle and place it in a homogeneous synthesizer with a speed of 10Hz, heat to 220°C, and Under the condition that the temperature is 220°C, the depolymerization reaction is carried out for 10 hours. After the reaction was completed and filtered, the obtained solid was 2,6-naphthalene dicarboxylic acid (yield 80.9%, purity 99.18%). EGDA (77.2%) in the filtrate was detected by gas chromatography with ethylene glycol diacetate as the internal standard.
实施例40:Example 40:
一种PBT聚酯的降解方法,具体步骤为:A kind of degradation method of PBT polyester, concrete steps are:
在30mL法兰式水热反应釜中依次加入5.0gPBT聚酯粉末,25ml冰醋酸,将水热釜密封后置于均相合成仪中,均相合成仪转速为10Hz,加热至220℃,在恒定温度为220℃的条件下,进行解聚反应10小时。反应完毕过滤,所得固体即为对苯二甲酸(产率83.5%,纯度99.74%)。以乙二醇二乙酸酯为内标,通过气相色谱进行检测滤液中的BGDA(83.6%)。Add 5.0g of PBT polyester powder and 25ml of glacial acetic acid into a 30mL flanged hydrothermal reaction kettle in sequence, seal the hydrothermal kettle and place it in a homogeneous synthesizer with a speed of 10Hz, heat to 220°C, and Under the condition of a constant temperature of 220° C., the depolymerization reaction was carried out for 10 hours. After the reaction was completed and filtered, the obtained solid was terephthalic acid (yield 83.5%, purity 99.74%). Using ethylene glycol diacetate as an internal standard, BGDA (83.6%) in the filtrate was detected by gas chromatography.
实施例41:Example 41:
一种PET和聚丙烯(PP)混合物的降解方法,具体步骤为:A kind of degradation method of PET and polypropylene (PP) mixture, concrete steps are:
在30mL法兰式水热反应釜中依次加入4.5gPET聚酯和0.5g PP聚合物碎片,25ml冰醋酸,将水热釜密封后置于均相合成仪中,均相合成仪转速为10Hz,加热至220℃,在恒定温度为220℃的条件下,进行解聚反应10小时。反应完毕后固体颗粒沉于底部,过滤所得固体即为对苯二甲酸(产率94.1%,纯度99.34%)。以乙二醇二乙酸酯为内标,通过气相色谱进行检测滤液中的EGDA(99.8%)。其中PP部分重新凝固成球状固体漂浮于体系上方。Add 4.5g of PET polyester and 0.5g of PP polymer fragments, 25ml of glacial acetic acid in the 30mL flange type hydrothermal reaction kettle successively, seal the hydrothermal kettle and place it in a homogeneous synthesizer, the speed of the homogeneous synthesizer is 10Hz, Heating to 220°C, under the condition of a constant temperature of 220°C, depolymerization reaction was carried out for 10 hours. After the reaction, the solid particles settled at the bottom, and the solid obtained by filtration was terephthalic acid (94.1% yield, 99.34% purity). EGDA (99.8%) in the filtrate was detected by gas chromatography with ethylene glycol diacetate as the internal standard. Among them, the PP part is re-solidified into a spherical solid floating above the system.
实施例42:Example 42:
一种PET和聚乙烯(PE)混纺而成的无纺布的降解方法,具体步骤为:A kind of degradation method of the non-woven fabric that PET and polyethylene (PE) are blended into, concrete steps are:
在30mL法兰式水热反应釜中加入5g无纺布碎片,25ml冰醋酸,将水热釜密封后置于均相合成仪中,均相合成仪转速为10Hz,在恒定温度为220℃的条件下,进行解聚反应10小时。反应完毕后固体颗粒沉于底部,过滤所得固体即为对苯二甲酸(产率94.2%,纯度99.75%)。以乙二醇二乙酸酯为内标,通过气相色谱进行检测滤液中的EGDA(96.6%)。其中PE部分从无纺布中脱离出来重新凝固成球状固体漂浮于体系上方。Add 5g of non-woven fabric fragments and 25ml of glacial acetic acid into a 30mL flange-type hydrothermal reaction kettle, seal the hydrothermal kettle and place it in a homogeneous synthesizer. The homogeneous synthesizer rotates at a speed of 10Hz. Under these conditions, the depolymerization reaction was carried out for 10 hours. After the reaction, the solid particles settled at the bottom, and the solid obtained by filtration was terephthalic acid (94.2% yield, 99.75% purity). EGDA (96.6%) in the filtrate was detected by gas chromatography with ethylene glycol diacetate as the internal standard. The PE part is detached from the non-woven fabric and re-solidified into a spherical solid floating above the system.
实施例43:Example 43:
一种涤纶布料(主要成分为PET)的降解方法,具体步骤为:A kind of degradation method of polyester cloth (main component is PET), concrete steps are:
在30mL法兰式水热反应釜中加入5g涤纶布碎片,25ml冰醋酸,将水热釜密封后置于均相合成仪中,均相合成仪转速为10Hz,在恒定温度为220℃的条件下,进行解聚反应10小时。反应完毕后过滤所得固体即为对苯二甲酸(产率89.4%,纯度97.38%)。以乙二醇二乙酸酯为内标,通过气相色谱进行检测滤液中的EGDA(85.1%)。Add 5g of polyester cloth fragments and 25ml of glacial acetic acid into a 30mL flange-type hydrothermal reaction kettle, seal the hydrothermal kettle and place it in a homogeneous synthesizer with a speed of 10Hz at a constant temperature of 220°C Next, the depolymerization reaction was carried out for 10 hours. After the reaction was completed, the solid obtained by filtration was terephthalic acid (yield 89.4%, purity 97.38%). EGDA (85.1%) in the filtrate was detected by gas chromatography with ethylene glycol diacetate as the internal standard.
实施例44:Example 44:
一种聚对苯二甲酸乙二醇酯-1,4-环己烷二甲醇酯(PETG)的降解方法,具体步骤为:A kind of degradation method of polyethylene terephthalate-1,4-cyclohexane dimethanol (PETG), concrete steps are:
在30mL法兰式水热反应釜中加入5gPETG颗粒,25ml冰醋酸,将水热釜密封后置于均相合成仪中,均相合成仪转速为10Hz,在恒定温度为220℃的条件下,进行解聚反应10小时。反应完毕后固体颗粒沉于底部,过滤所得固体即为对苯二甲酸(产率87.3%,纯度97.20%)。以乙二醇二乙酸酯为内标,通过气相色谱对EGDA、CHDMDA进行检测。Add 5g of PETG particles and 25ml of glacial acetic acid into a 30mL flange-type hydrothermal reaction kettle, seal the hydrothermal kettle and place it in a homogeneous synthesizer with a speed of 10Hz at a constant temperature of 220°C. The depolymerization reaction was carried out for 10 hours. After the reaction, the solid particles settled at the bottom, and the solid obtained by filtration was terephthalic acid (yield 87.3%, purity 97.20%). EGDA and CHDMDA were detected by gas chromatography with ethylene glycol diacetate as internal standard.
Claims (7)
Translated fromChinesePriority Applications (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| CN202111190964.3ACN115959984A (en) | 2021-10-13 | 2021-10-13 | A kind of degradation method of polyester polymer |
Applications Claiming Priority (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| CN202111190964.3ACN115959984A (en) | 2021-10-13 | 2021-10-13 | A kind of degradation method of polyester polymer |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| CN115959984Atrue CN115959984A (en) | 2023-04-14 |
Family
ID=87353444
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| CN202111190964.3APendingCN115959984A (en) | 2021-10-13 | 2021-10-13 | A kind of degradation method of polyester polymer |
Country Status (1)
| Country | Link |
|---|---|
| CN (1) | CN115959984A (en) |
Citations (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| KR20090024799A (en)* | 2009-01-20 | 2009-03-09 | 코바르 에스.피.에이. | Method for Recovering Aromatic Dicarboxylic Acid from Waste Polyester Resin |
| CN101456809A (en)* | 2009-01-08 | 2009-06-17 | 中国科学院嘉兴材料与化工技术工程中心 | Method for disaggregation of waste and old PET |
| CN111777489A (en)* | 2020-07-08 | 2020-10-16 | 中国科学院山西煤炭化学研究所 | A kind of degradation method of catalyzed polyethylene terephthalate waste |
| CN112961045A (en)* | 2018-05-30 | 2021-06-15 | 上海科技大学 | Method for degrading polymer |
- 2021
- 2021-10-13CNCN202111190964.3Apatent/CN115959984A/enactivePending
Patent Citations (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN101456809A (en)* | 2009-01-08 | 2009-06-17 | 中国科学院嘉兴材料与化工技术工程中心 | Method for disaggregation of waste and old PET |
| KR20090024799A (en)* | 2009-01-20 | 2009-03-09 | 코바르 에스.피.에이. | Method for Recovering Aromatic Dicarboxylic Acid from Waste Polyester Resin |
| CN112961045A (en)* | 2018-05-30 | 2021-06-15 | 上海科技大学 | Method for degrading polymer |
| CN111777489A (en)* | 2020-07-08 | 2020-10-16 | 中国科学院山西煤炭化学研究所 | A kind of degradation method of catalyzed polyethylene terephthalate waste |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| CN112851502B (en) | A kind of choline and terephthalic acid non-metallic ionic liquid catalyze the method of methanolysis of waste PET polyester | |
| Al-Sabagh et al. | Greener routes for recycling of polyethylene terephthalate | |
| Park et al. | Poly (ethylene terephthalate) recycling for high value added textiles | |
| CA2934544C (en) | Method for forming an aromatic diacid and/or an aromatic diacid precursor from a polyester-containing feedstock | |
| US6136869A (en) | Depolymerization process for recycling polyesters | |
| US6472557B1 (en) | Process for recycling polyesters | |
| CN109134244A (en) | A kind of biodegrading process of waste and old polyester | |
| CN105658611A (en) | Methods and materials for depolymerizing polyesters | |
| MXPA02003928A (en) | Method for separating and recovering dimethyl terephthalate and ethylene glycol from polyester waste. | |
| JP5343628B2 (en) | Process for producing biomaterial-derived glycol having excellent light transmittance and process for producing polyester obtained therefrom | |
| CN104774153A (en) | Recycling method for catalytic degradation of waste PET | |
| CN108947798A (en) | A method of degrading polymers | |
| CN110818886A (en) | Method for preparing regenerated food-grade PET polyester from waste PET polyester | |
| CN106866413A (en) | A kind of method that efficient cryogenic reclaims Waste Polyester PET | |
| CN102153443B (en) | Method for degrading polyethylene terephthalate | |
| CN118159514A (en) | Process for recovering dialkyl terephthalate from polyester composition | |
| CN102532591B (en) | Method for depolymerizing waste polyester bottle | |
| CN113735705A (en) | Method for catalyzing waste PET (polyethylene terephthalate) polyester to carry out methanol alcoholysis by polyion liquid | |
| CN111217700A (en) | Method for catalyzing alcoholysis of polyethylene terephthalate by using non-metal choline ionic liquid | |
| CN117279987A (en) | Method for depolymerizing polyethylene terephthalate by glycol hydrolysis | |
| CN115959984A (en) | A kind of degradation method of polyester polymer | |
| CN110172140B (en) | A method for preparing unsaturated polyester resin using microwave hydrolysis of waste polyester textiles | |
| CN114736358B (en) | Preparation method and recovery method of recyclable PETG material | |
| CN116623310A (en) | Polyester regeneration method with low ethylene glycol use ratio | |
| CN114907211A (en) | A kind of degradation method of polyester |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PB01 | Publication | ||
| PB01 | Publication | ||
| SE01 | Entry into force of request for substantive examination | ||
| SE01 | Entry into force of request for substantive examination |