CN115927642A - Application of RGS18 in the treatment of tumor metastasis - Google Patents
Application of RGS18 in the treatment of tumor metastasisDownload PDFInfo
- Publication number
- CN115927642A CN115927642ACN202310061424.8ACN202310061424ACN115927642ACN 115927642 ACN115927642 ACN 115927642ACN 202310061424 ACN202310061424 ACN 202310061424ACN 115927642 ACN115927642 ACN 115927642A
- Authority
- CN
- China
- Prior art keywords
- rgs18
- tumor
- cells
- metastasis
- expression
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Granted
Links
Images
Classifications
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K45/00—Medicinal preparations containing active ingredients not provided for in groups A61K31/00 - A61K41/00
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P35/00—Antineoplastic agents
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P35/00—Antineoplastic agents
- A61P35/04—Antineoplastic agents specific for metastasis
- Y—GENERAL TAGGING OF NEW TECHNOLOGICAL DEVELOPMENTS; GENERAL TAGGING OF CROSS-SECTIONAL TECHNOLOGIES SPANNING OVER SEVERAL SECTIONS OF THE IPC; TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y02—TECHNOLOGIES OR APPLICATIONS FOR MITIGATION OR ADAPTATION AGAINST CLIMATE CHANGE
- Y02A—TECHNOLOGIES FOR ADAPTATION TO CLIMATE CHANGE
- Y02A50/00—TECHNOLOGIES FOR ADAPTATION TO CLIMATE CHANGE in human health protection, e.g. against extreme weather
- Y02A50/30—Against vector-borne diseases, e.g. mosquito-borne, fly-borne, tick-borne or waterborne diseases whose impact is exacerbated by climate change
Landscapes
- Health & Medical Sciences (AREA)
- Public Health (AREA)
- General Health & Medical Sciences (AREA)
- Veterinary Medicine (AREA)
- Pharmacology & Pharmacy (AREA)
- Medicinal Chemistry (AREA)
- Animal Behavior & Ethology (AREA)
- Chemical & Material Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Organic Chemistry (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- General Chemical & Material Sciences (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Oncology (AREA)
- Epidemiology (AREA)
- Measuring Or Testing Involving Enzymes Or Micro-Organisms (AREA)
Abstract
Description
Translated fromChinese技术领域technical field
本发明属于生物医药技术领域,具体涉及一种RGS18在治疗肿瘤转移中的应用。The invention belongs to the technical field of biomedicine, and in particular relates to an application of RGS18 in the treatment of tumor metastasis.
背景技术Background technique
肿瘤细胞的转移扩散是癌症相关死亡的主要原因。为了降低或消除肿瘤细胞的转移,本领域需要通过多种方式鉴定肿瘤细胞转移的关键基因靶点及其促进肿瘤转移的机制,并利用这些靶点构建肿瘤转移模型或者设计治疗肿瘤转移的药物。因此,了解肿瘤细胞播散的潜在机制,并针对播散的“种子”肿瘤细胞开发新的治疗策略,已成为癌症研究的热点。Metastatic spread of tumor cells is a major cause of cancer-related death. In order to reduce or eliminate the metastasis of tumor cells, the field needs to identify the key gene targets of tumor cell metastasis and the mechanism of promoting tumor metastasis in various ways, and use these targets to construct tumor metastasis models or design drugs for the treatment of tumor metastasis. Therefore, understanding the underlying mechanism of tumor cell dissemination and developing new therapeutic strategies against disseminated "seed" tumor cells have become a hotspot in cancer research.
从原发灶肿瘤脱落释放到血液循环中的循环肿瘤细胞(Circulating Tumorcells,CTCs)被认为是肿瘤转移的“种子”。在肿瘤形成和发展过程中,CTC脱离肿瘤原发灶或转移灶而进入外周血循环,通常以单个细胞与细胞簇(两到数十个细胞)的形式存在,被认为是导致肿瘤转移的关键。在转移性胰腺癌、乳腺癌、肺癌、结直肠癌、前列腺癌等肿瘤的血液中都有检测到CTC,其与肿瘤无进展生存期(PFS)和总生存期(OS)的降低密切有关。CTC形成的远端器官转移是癌症患者预后较差、癌症复发以及死亡的主要原因。因此,挖掘驱动CTCs转移的关键基因和靶点,对治疗肿瘤转移具有重要的意义。Circulating tumor cells (Circulating Tumor cells, CTCs) released from the primary tumor into the blood circulation are considered as the "seeds" of tumor metastasis. In the process of tumor formation and development, CTCs break away from the primary tumor or metastases and enter the peripheral blood circulation, usually in the form of single cells and cell clusters (two to dozens of cells), which is considered to be the key to tumor metastasis. CTCs are detected in the blood of metastatic pancreatic cancer, breast cancer, lung cancer, colorectal cancer, prostate cancer and other tumors, which are closely related to the reduction of tumor progression-free survival (PFS) and overall survival (OS). Distant organ metastasis formed by CTC is the main cause of poor prognosis, cancer recurrence and death in cancer patients. Therefore, mining the key genes and targets that drive the metastasis of CTCs is of great significance for the treatment of tumor metastasis.
RGS18是RGS蛋白家族成员,参与巨核细胞的生成、分化和趋化。目前尚未报到其在肿瘤中的作用。本发明中鉴定出RGS18在肿瘤转移中起重要作用,并验证了其作为靶点在治疗肿瘤转移中的应用,以及在快速构建小鼠肿瘤转移模型中的应用。RGS18 is a member of the RGS protein family and is involved in the generation, differentiation and chemotaxis of megakaryocytes. Its role in tumors has not yet been reported. In the present invention, it is identified that RGS18 plays an important role in tumor metastasis, and its application as a target in the treatment of tumor metastasis and its application in the rapid construction of a mouse tumor metastasis model are verified.
发明内容Contents of the invention
本发明旨在鉴定治疗肿瘤转移的新靶点,提供一种RGS18作为靶点在肿瘤转移中的应用。本发明通过结合微流控芯片技术和单细胞转录组测序技术,利用生物信息学在单细胞尺度上分析了CTCs的差异基因,筛选出以RGS18为代表的新靶点,并提供所述靶点在治疗肿瘤转移中的应用。The invention aims at identifying a new target for treating tumor metastasis, and provides an application of RGS18 as a target in tumor metastasis. The present invention combines microfluidic chip technology and single-cell transcriptome sequencing technology, uses bioinformatics to analyze the differential genes of CTCs on a single-cell scale, screens out new targets represented by RGS18, and provides the target Application in the treatment of tumor metastasis.
本发明通过研究发现,RGS18在CTCs中高表达,并通过抑制肿瘤细胞AKT-GSK3β-CREB通路促进免疫检查点分子HLA-E的表达,与NK细胞表面的配体分子CD94:NKG2A结合后逃避NK细胞的免疫监视;在此基础上,本发明通过干预肿瘤细胞中RGS18及下游调控基因HLA-E的表达及功能可抑制肿瘤的转移,达到治疗肿瘤转移的效果;同时,本发明通过上调肿瘤细胞RGS18的表达,可以促进肿瘤的转移,达到快速构建肿瘤转移模型的效果。Through research, the present invention finds that RGS18 is highly expressed in CTCs, and promotes the expression of immune checkpoint molecule HLA-E by inhibiting the AKT-GSK3β-CREB pathway of tumor cells, and evades NK cells after combining with the ligand molecule CD94:NKG2A on the surface of NK cells on this basis, the present invention can inhibit tumor metastasis by interfering with the expression and function of RGS18 and downstream regulatory gene HLA-E in tumor cells, and achieve the effect of treating tumor metastasis; at the same time, the present invention can up-regulate tumor cell RGS18 The expression of can promote tumor metastasis and achieve the effect of quickly constructing a tumor metastasis model.
本发明中,促进肿瘤转移的新靶点通过以下方案鉴定:In the present invention, new targets that promote tumor metastasis are identified through the following scheme:
(S1)捕获血液中CTCs,同时收取原发性和转移性的恶性实体肿瘤样本制备单细胞悬液;(S1) Capture CTCs in the blood, and simultaneously collect primary and metastatic malignant solid tumor samples to prepare single cell suspension;
(S2)将步骤(S1)获得的单细胞进行单细胞转录组测序,分析获得CTCs与原发性和转移性肿瘤细胞的差异基因(即鉴定新靶点);(S2) Perform single-cell transcriptome sequencing on the single cells obtained in step (S1), and analyze and obtain differential genes between CTCs and primary and metastatic tumor cells (i.e. identify new targets);
(S3)对步骤(S2)获得的差异基因进行功能验证。(S3) Perform functional verification on the differential genes obtained in step (S2).
本发明通过上述方案鉴定出驱动肿瘤转移的新靶点RGS18,并提供了其在肿瘤转移中的用途。The present invention identifies a new target RGS18 that drives tumor metastasis through the above scheme, and provides its use in tumor metastasis.
进一步的,所述RGS18在肿瘤转移中具有如下(1)-(4)中至少一种功能产品中的应用:Further, the application of the RGS18 in tumor metastasis has at least one function in the following (1)-(4):
(1)通过促进RGS18的表达构建肿瘤转移模型;(1) Construct a tumor metastasis model by promoting the expression of RGS18;
(2)通过促进RGS18的表达抑制肿瘤细胞AKT-GSK3β-CREB通路;(2) Inhibit the AKT-GSK3β-CREB pathway of tumor cells by promoting the expression of RGS18;
(3)通过促进RGS18的表达促进肿瘤细胞免疫检查点分子HLA-E的表达;(3) Promote the expression of tumor cell immune checkpoint molecule HLA-E by promoting the expression of RGS18;
(4)通过抑制RGS18的表达及下游功能抑制肿瘤转移。(4) Inhibit tumor metastasis by inhibiting the expression and downstream functions of RGS18.
上述应用中,所述促进RGS18表达的物质包括但不限于RGS18 DNA分子、RGS18慢病毒、血小板、RGS18可溶性蛋白中的一种或多种。In the above application, the substances that promote the expression of RGS18 include but are not limited to one or more of RGS18 DNA molecules, RGS18 lentiviruses, platelets, and RGS18 soluble proteins.
上述应用中,所述抑制RGS18的表达及下游功能的物质为如下任一种:干扰RGS18表达的RNA分子、干扰RGS18表达的小分子抑制剂、干扰HLA-E表达的RNA分子、干扰HLA-E表达的小分子抑制剂、抗HLA-E抗体、抗NKG2A抗体、干扰CREB1表达的RNA分子、干扰CREB1表达的小分子抑制剂、干扰AKT激活的小分子抑制剂。In the above application, the substance that inhibits the expression and downstream functions of RGS18 is any of the following: RNA molecules that interfere with the expression of RGS18, small molecule inhibitors that interfere with the expression of RGS18, RNA molecules that interfere with the expression of HLA-E, or RNA molecules that interfere with the expression of HLA-E Small molecule inhibitors of expression, anti-HLA-E antibodies, anti-NKG2A antibodies, RNA molecules that interfere with CREB1 expression, small molecule inhibitors that interfere with CREB1 expression, small molecule inhibitors that interfere with AKT activation.
进一步的,所述肿瘤包括但不限于胰腺癌、黑色素瘤、乳腺癌、肝癌、结直肠癌。Further, the tumor includes but not limited to pancreatic cancer, melanoma, breast cancer, liver cancer, and colorectal cancer.
本发明的有益效果为:The beneficial effects of the present invention are:
本发明通过实验揭示了RGS18基因的表达对肿瘤转移的影响,将RGS18作为治疗靶点,能抑制肿瘤的转移,达到治疗肿瘤转移的目的。同时,本发明还揭示了RGS18可通过抑制肿瘤细胞AKT-GSK3β-CREB通路促进免疫检查点分子HLA-E的表达,HLA-E与NK细胞表面的配体分子CD94:NKG2A结合后逃避NK细胞的免疫监视;并验证了通过沉默HLA-E表达或者抗体阻断肿瘤细胞表面HLA-E与NK细胞表面CD94:NKG2A结合后可抑制肿瘤转移,达到治疗肿瘤转移的目的,为抑制肿瘤转移提供了新的靶点。此外,本发明通过研究发现在肿瘤细胞中促进RGS18的表达可以诱导肿瘤肝转移和肺转移的形成,缩短了小鼠转移模型的建立时间,为快速构建小鼠肿瘤转移模型提供了新的方案。本发明鉴定的肿瘤转移新靶点RGS18在胰腺癌、黑色素瘤、肝癌、乳腺癌、结肠癌等多个癌种的CTCs中都存在,可广泛用于多种肿瘤远端转移的治疗和研究。因此,本发明鉴定的驱动肿瘤转移的新靶点RGS18不仅可以作为临床治疗肿瘤转移的新靶点,而且可以用于快速构建小鼠肿瘤转移模型,为研究肿瘤转移提供了新的建模方案。The present invention reveals the influence of RGS18 gene expression on tumor metastasis through experiments, uses RGS18 as a therapeutic target, can inhibit tumor metastasis, and achieves the purpose of treating tumor metastasis. At the same time, the present invention also reveals that RGS18 can promote the expression of immune checkpoint molecule HLA-E by inhibiting the AKT-GSK3β-CREB pathway of tumor cells. Immune surveillance; and verified that by silencing the expression of HLA-E or blocking the combination of HLA-E on the surface of tumor cells and CD94:NKG2A on the surface of NK cells by antibodies, tumor metastasis can be inhibited, and the purpose of treating tumor metastasis can be achieved, providing a new method for inhibiting tumor metastasis. target. In addition, the present invention found that promoting the expression of RGS18 in tumor cells can induce the formation of tumor liver metastasis and lung metastasis, which shortens the establishment time of the mouse metastasis model and provides a new solution for the rapid construction of the mouse tumor metastasis model. The new tumor metastasis target RGS18 identified in the present invention exists in CTCs of multiple cancer types such as pancreatic cancer, melanoma, liver cancer, breast cancer, and colon cancer, and can be widely used in the treatment and research of distant metastasis of various tumors. Therefore, the new target RGS18 identified in the present invention to drive tumor metastasis can not only be used as a new target for clinical treatment of tumor metastasis, but also can be used to quickly build a mouse tumor metastasis model, providing a new modeling scheme for the study of tumor metastasis.
附图说明Description of drawings
为了更清楚地说明本发明实施例或现有技术中的技术方案,下面将对实施例或现有技术描述中所需要使用的附图作简单地介绍,显而易见地,下面描述中的附图仅仅是本发明的一些实施例,对于本领域普通技术人员来讲,在不付出创造性劳动的前提下,还可以根据这些附图获得其他的附图。In order to more clearly illustrate the technical solutions in the embodiments of the present invention or the prior art, the following will briefly introduce the drawings that need to be used in the description of the embodiments or the prior art. Obviously, the accompanying drawings in the following description are only These are some embodiments of the present invention. Those skilled in the art can also obtain other drawings based on these drawings without creative work.
图1显示为CopyKAT分析原发灶和转移灶肿瘤中恶性的肿瘤细胞;Figure 1 shows the analysis of malignant tumor cells in primary and metastatic tumors for CopyKAT;
图2显示为基于单细胞测序技术对胰腺的的原发性/转移性肿瘤和门静脉血中肿瘤细胞聚类分析图;其中,图2A图为t-SNE降维聚类分析基于各细胞亚群的肿瘤细胞和上皮细胞,图2B图为测序细胞的组织来源的t-SNE降维聚类分析图,图2C图为测序细胞的患者来源的t-SNE降维聚类分析图,图2D图为CTCs的t-SNE放大图。Figure 2 shows the clustering analysis of primary/metastatic tumors of the pancreas and tumor cells in portal blood based on single-cell sequencing technology; among them, Figure 2A shows the t-SNE dimensionality reduction clustering analysis based on each cell subgroup Tumor cells and epithelial cells, Figure 2B is the t-SNE dimensionality reduction cluster analysis diagram of the sequenced cells derived from the tissue, Figure 2C is the t-SNE dimensionality reduction cluster analysis diagram of the sequenced cells derived from the patient, Figure 2D It is the t-SNE enlarged image of CTCs.
图3显示为热图展示了CTCs与原发灶和转移灶肿瘤细胞之间的差异基因;Figure 3 shows a heat map showing the differential genes between CTCs and primary and metastatic tumor cells;
图4显示为小提琴展示胰腺癌肝转移来源的CTCs、原发和转移灶肿瘤细胞中RGS18的表达;Figure 4 shows the expression of RGS18 in CTCs derived from pancreatic cancer liver metastases, primary and metastatic tumor cells as a violin;
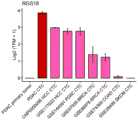
图5显示为柱状图展示肝细胞癌、乳腺癌、结肠腺癌、黑色素瘤和胰腺癌病人来源的CTCs中RGS18的表达水平;Figure 5 shows the expression level of RGS18 in CTCs derived from patients with hepatocellular carcinoma, breast cancer, colon adenocarcinoma, melanoma and pancreatic cancer as a histogram;
图6显示为柱状图展示胰腺癌小鼠来源的CTCs中HLA-E的表达水平;Figure 6 shows a histogram showing the expression level of HLA-E in CTCs derived from pancreatic cancer mice;
图7显示为多重免疫荧光染色检测CTC中RGS18的表达水平;Figure 7 shows the expression level of RGS18 in CTCs detected by multiple immunofluorescence staining;
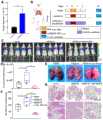
图8显示为过表达RGS18可促进胰腺癌肿瘤细胞的肝转移;其中,图8A为小鼠肝转移模型构建示意图;图8B和图8C分别为接种14天后小动物活体成像检测小鼠肝转移生长情况和定量统计(mean±SD,n=5,***p<0.001);图8D为各组小鼠肝脏解剖照片,图8E为H&E染色、hEpCAM和hRGS18免疫组化染色检测肿瘤细胞肝转移情况(比例尺,50μm),图8F为Kaplan–Meier曲线绘制肝转移小鼠生存期(n=6);Figure 8 shows that the overexpression of RGS18 can promote the liver metastasis of pancreatic cancer tumor cells; among them, Figure 8A is a schematic diagram of the establishment of a mouse liver metastasis model; Figure 8B and Figure 8C are respectively 14 days after inoculation in vivo imaging of small animals to detect the growth of liver metastasis in mice Situation and quantitative statistics (mean±SD, n=5, ***p<0.001); Figure 8D is an anatomical photo of the livers of mice in each group, and Figure 8E is H&E staining, hEpCAM and hRGS18 immunohistochemical staining to detect liver metastasis of tumor cells Situation (scale bar, 50 μm), Fig. 8F is the Kaplan–Meier curve plotting the survival period of mice with liver metastasis (n=6);
图9显示为CTCs中RGS18与HLA-E表达水平的相关性分析;Figure 9 shows the correlation analysis between RGS18 and HLA-E expression levels in CTCs;
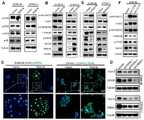
图10显示为RGS18通过调控AKT-GSK3β-CREB1信号轴促进肿瘤细胞HLA-E的表达;其中,图10A为SU86.86和CFPAC-1细胞过表达RGS18后,western blot检测下游信号通路的活性;图10B为SU86.86和CFPAC-1细胞过表达RGS18后,westernblot检测AKT-GSK3β-CREB通路相关蛋白的活性变化和HLA-E的表达水平;图10C为免疫荧光染色检测PDAC细胞过表达RGS18后p-CREB1(Ser133,绿色)的亚细胞分布。细胞核用DAPI标记(蓝色);比例尺,100μm;图10D为三条独立的shCREB1敲低SU86.86和CFPAC-1细胞中CREB1后,westernblot检测PDAC细胞中HLA-E和CREB1蛋白的表达水平;图10E为Western blot检测RGS18敲低的SU86.86和CFPAC-1细胞中AKT-GSK3β-CREB通路相关蛋白及HLA-E的表达水平;图10F为在SU86.86中过表达AKT激活性突变体AKT(E17K)和AKT(Q79K)后,westernblot检测AKT-GSK3β-CREB相关蛋白及HLA-E的表达水平;Figure 10 shows that RGS18 promotes the expression of tumor cell HLA-E by regulating the AKT-GSK3β-CREB1 signaling axis; among them, Figure 10A shows the activity of downstream signaling pathways detected by western blot after SU86.86 and CFPAC-1 cells overexpress RGS18; Figure 10B shows the changes in the activity of AKT-GSK3β-CREB pathway-related proteins and the expression level of HLA-E detected by western blot after the overexpression of RGS18 in SU86.86 and CFPAC-1 cells; Figure 10C shows the detection of RGS18 overexpression in PDAC cells by immunofluorescence staining Subcellular distribution of p-CREB1 (Ser133, green). Nuclei are marked with DAPI (blue); scale bar, 100 μm; Figure 10D shows the expression levels of HLA-E and CREB1 proteins in PDAC cells detected by western blot after three independent shCREB1 knockdowns of CREB1 in SU86.86 and CFPAC-1 cells; 10E is Western blot detection of the expression levels of AKT-GSK3β-CREB pathway-related proteins and HLA-E in RGS18 knockdown SU86.86 and CFPAC-1 cells; Figure 10F is the overexpression of the AKT activating mutant AKT in SU86.86 After (E17K) and AKT (Q79K), western blot was used to detect the expression levels of AKT-GSK3β-CREB related proteins and HLA-E;
图11显示为LDH释放实验检测在不同效靶比(E:T)条件下NK细胞对SU86.86肿瘤细胞的杀伤能力;Figure 11 shows the killing ability of NK cells to SU86.86 tumor cells under different effect-to-target ratio (E:T) conditions detected by LDH release experiment;
图12显示为RGS18通过促进肿瘤细胞逃避NK细胞的免疫监视进行转移;其中,图12A和图12B为在小鼠胰腺癌KPC细胞接种14天后,小动物活体成像检测小鼠肝转移生长情况(图12A)并进行定量统计;图12C为接种14天后,解剖小鼠取肝脏组拍照观察;图12D和图12E为在免疫缺陷小鼠B-NDG-hIL15中构建胰腺癌肝转移模型,小动物活体成像检测小鼠肝转移生长情况(图12D)并进行定量统计(图12E),ns表示无统计学差异;Figure 12 shows that RGS18 transfers by promoting tumor cells to evade the immune surveillance of NK cells; wherein, Figure 12A and Figure 12B show the growth of liver metastases in mice detected by live imaging of
图13显示为敲低RGS18的表达减少小鼠肺转移的体内研究结果图;其中,图13A为Balb/c裸鼠注射敲除RGS18的KPC-Luc细胞后肺转移生物发光图像;图13B图为图13A的定量图;Figure 13 shows the in vivo study results of knocking down the expression of RGS18 to reduce lung metastasis in mice; among them, Figure 13A is a bioluminescent image of lung metastasis after injection of KPC-Luc cells knocking out RGS18 in Balb/c nude mice; Figure 13B is Quantitative plot of Figure 13A;
图14显示为抑制RGS18下游HLA-E功能可治疗肿瘤转移;接种14天后,小动物活体成像检测小鼠肝转移生长情况(图14A)并进行定量统计(图14B);Figure 14 shows that inhibiting the function of HLA-E downstream of RGS18 can treat tumor metastasis; 14 days after inoculation, live imaging of small animals was used to detect the growth of liver metastases in mice (Figure 14A) and quantitative statistics were performed (Figure 14B);
图15显示为利用阻断抗体anti-NKG2A抑制RGS18下游HLA-E功能可治疗肿瘤转移;其中,图15A为在mRGS18过表达的KPC细胞中通过qPCR检测H2-T23(HLA-E)mRNA表达水平;图15B为小鼠肺转移实验方案设计图;Balb/c裸鼠5×104个KPC-Luc细胞或KPC-Luc-mRGS18细胞建立小鼠肺转移模型;其中anti-NKG2A治疗组,在接种前一天开始连续注射四剂anti-NKG2A抗体(10mg/kg)进行阻断治疗;图15C和图15D为静脉注射KPC-Luc细胞15天后,小动物活体成像检测小鼠肺转移生长情况(图15C)并进行定量统计(图15D);图15E和图15F为静脉注射KPC-Luc细胞15天后,解剖小鼠,取小鼠肺组织进行拍照(图15E)并统计肺转移结节数目(图15F);图15G为H&E染色检测肺组织病理变化。Figure 15 shows that using the blocking antibody anti-NKG2A to inhibit the function of HLA-E downstream of RGS18 can treat tumor metastasis; wherein, Figure 15A is the expression level of H2-T23 (HLA-E) mRNA detected by qPCR in mRGS18 overexpressed KPC cells ; Figure 15B is a design diagram of the mouse lung metastasis experiment scheme; Balb/
图16显示为血小板促进肿瘤细胞RGS18的表达;不同浓度肿瘤患者来源的血小板与SU86.86和CFPAC-1肿瘤细胞共孵育72小时后,westernblot肿瘤细胞中HLA-E、RGS18和CD41蛋白表达水平。Figure 16 shows that platelets promote the expression of tumor cell RGS18; after 72 hours of co-incubation of platelets derived from tumor patients with different concentrations of SU86.86 and CFPAC-1 tumor cells, the protein expression levels of HLA-E, RGS18 and CD41 in tumor cells by western blot.
具体实施方式Detailed ways
为使本发明的目的、技术方案和优点更加清楚,下面将对本发明的技术方案进行详细的描述。显然,所描述的实施例仅是本发明一部分实施例,而不是全部的实施例。基于本发明中的实施例,本领域普通技术人员在没有做出创造性劳动的前提下所得到的所有其它实施方式,都属于本发明所保护的范围。In order to make the purpose, technical solution and advantages of the present invention clearer, the technical solution of the present invention will be described in detail below. Apparently, the described embodiments are only some of the embodiments of the present invention, but not all of them. Based on the embodiments of the present invention, all other implementations obtained by persons of ordinary skill in the art without making creative efforts fall within the protection scope of the present invention.
本发明以胰腺癌肝转移为研究对象,利用单细胞转录组测序分析了胰腺原发灶肿瘤细胞、CTCs和肝转移灶肿瘤细胞的转录组特征,利用生物信息学在单细胞尺度上分析了CTCs的差异基因,鉴定出驱动CTCs转移的关键靶点。通过实验揭示了RGS18基因的表达对肿瘤转移的影响,将RGS18作为抑制靶点,能抑制肿瘤的转移,达到治疗肿瘤转移的目的。在体内肿瘤转移模型中,本发明发现RGS18的表达可以诱导肿瘤肝转移和肺转移的形成,缩短了小鼠肿瘤转移模型的建立时间,为研究肿瘤转移提供了新的建模方案。在体外实验中,本发明发现RGS18通过抑制肿瘤细胞AKT-GSK3β-CREB通路促进免疫检查点分子HLA-E的表达,与NK细胞表面的配体分子CD94:NKG2A结合后逃避NK细胞的免疫监视。在进一步的体内功能实验中,本发明发现直接沉默RGS18的表达、沉默HLA-E表达或者抗体阻断肿瘤细胞表面HLA-E与NK细胞表面CD94:NKG2A结合后可抑制肿瘤转移,达到治疗肿瘤转移的目的,为抑制肿瘤转移提供了新的靶点。The present invention takes pancreatic cancer liver metastasis as the research object, and analyzes the transcriptome characteristics of pancreatic primary tumor cells, CTCs and liver metastases tumor cells by single-cell transcriptome sequencing, and analyzes CTCs on a single-cell scale by using bioinformatics The differential genes identified key targets that drive the metastasis of CTCs. Through experiments, the effect of RGS18 gene expression on tumor metastasis was revealed, and RGS18 was used as an inhibitory target to inhibit tumor metastasis and achieve the purpose of treating tumor metastasis. In the tumor metastasis model in vivo, the present invention found that the expression of RGS18 can induce the formation of tumor liver metastasis and lung metastasis, shorten the establishment time of the mouse tumor metastasis model, and provide a new modeling scheme for the study of tumor metastasis. In the in vitro experiments, the present invention found that RGS18 promotes the expression of immune checkpoint molecule HLA-E by inhibiting the AKT-GSK3β-CREB pathway of tumor cells, and escapes the immune surveillance of NK cells after combining with the ligand molecule CD94:NKG2A on the surface of NK cells. In further functional experiments in vivo, the present invention found that directly silencing the expression of RGS18, silencing the expression of HLA-E, or blocking the combination of HLA-E on the surface of tumor cells and CD94:NKG2A on the surface of NK cells by antibodies can inhibit tumor metastasis and achieve the goal of treating tumor metastasis. It provides a new target for inhibiting tumor metastasis.
以下结合具体实施例,对上述技术方案详细说明。The above technical solutions will be described in detail below in conjunction with specific embodiments.
下述实施例中所使用的实验方法如无特殊说明,均为常规方法。下述实施例中用的材料、试剂等,如无特殊说明,均可从商业途径得到。The experimental methods used in the following examples are conventional methods unless otherwise specified. The materials and reagents used in the following examples can be obtained from commercial sources unless otherwise specified.
实施例1鉴定驱动CTCs转移的关键靶点RGS18Example 1 Identification of key target RGS18 driving CTCs metastasis
一、临床样本肿瘤细胞单细胞转录组测序1. Single-cell transcriptome sequencing of tumor cells in clinical samples
1.收集临床样本1. Collection of Clinical Samples
为了寻找驱动CTCs转移的关键靶点,以胰腺癌肝转移为例,收集同一个患者的原发灶肿瘤、CTCs和转移灶肿瘤进行单细胞转录组测序。In order to find the key targets driving the metastasis of CTCs, taking pancreatic cancer liver metastasis as an example, the primary tumor, CTCs and metastatic tumors of the same patient were collected for single-cell transcriptome sequencing.
2.临床样本单细胞悬液的制备2. Preparation of single cell suspension of clinical samples
将上述原发灶和转移灶肿瘤组织于冰上用剪刀剪成肉糜状。随后将组织块转移到50ml离心管中,并加入20ml消化液于37℃消化15min;其中消化液含有0.25%胰蛋白酶、0.4mg/ml I型胶原酶和0.4mg/mlⅣ型胶原酶。待肿瘤组织消化完全后,用等体积预冷的含10%胎牛血清的DMEM培养基终止反应,并用70μm细胞筛网去除大块碎片,收集单细胞悬液。接下来,将单细胞悬液于4℃,500g,离心5min。离心后的细胞沉淀加入红细胞裂解液冰上裂解5min;然后用HBSS(含0.1%BSA)洗涤两次,4℃,500g,离心5min收集细胞沉淀。然后,用含0.1%BSA的HBSS重悬细胞,并加入PI,流式细胞术分选状态良好的活细胞用于单细胞转录组测序。The above-mentioned primary tumor and metastatic tumor tissue were cut into minced meat with scissors on ice. Then the tissue pieces were transferred to a 50ml centrifuge tube, and 20ml of digestion solution was added to digest at 37°C for 15min; the digestion solution contained 0.25% trypsin, 0.4mg/ml type I collagenase and 0.4mg/ml type IV collagenase. After the tumor tissue was completely digested, the reaction was terminated with an equal volume of pre-cooled DMEM medium containing 10% fetal bovine serum, and large debris was removed with a 70 μm cell mesh to collect a single cell suspension. Next, the single cell suspension was centrifuged at 500 g for 5 min at 4°C. The centrifuged cell pellet was added to erythrocyte lysate and lysed on ice for 5 min; then washed twice with HBSS (containing 0.1% BSA), centrifuged at 500 g at 4°C for 5 min to collect the cell pellet. Then, the cells were resuspended in HBSS containing 0.1% BSA, and PI was added, and living cells in good condition were sorted by flow cytometry for single-cell transcriptome sequencing.
对于血液中的CTCs样本,采用微流控芯片捕获门静脉血中的CTCs。捕获流程包括如下步骤:(1)在CTCs捕获前将捕获抗体的anti-EpCAM和anti-CA19.9抗体注入微流控芯片的泳道内,4℃包被过夜,制备可用于捕获胰腺癌CTC的微流控捕获系统;(2)在捕获当天,收集患者肝门静脉血,并利用红细胞裂解液去除红细胞;(3)将细胞用HBSS重悬至密度为2×107个/ml,以5ml/h的流速注入微流控芯片;(4)用10ml HBSS以30ml/h的流速冲洗3次,去除非特异性结合细胞;(5)用15ml洗脱缓冲液以50ml/h的流速冲洗芯片,释放和收集CTC。收集的CTC可用于单细胞转录组测序。For the CTCs samples in blood, a microfluidic chip is used to capture CTCs in portal vein blood. The capture process includes the following steps: (1) before capturing CTCs, inject the anti-EpCAM and anti-CA19.9 antibodies of the capture antibodies into the swimming lanes of the microfluidic chip, and coat them overnight at 4°C to prepare the CTCs that can be used to capture pancreatic cancer CTCs. Microfluidic capture system; (2) On the day of capture, collect the patient’s hepatic portal vein blood, and use red blood cell lysate to remove red blood cells; (3) Resuspend the cells with HBSS to a density of 2×107 cells/ml, and use 5ml/ The flow rate of h is injected into the microfluidic chip; (4)
3.10×Genomics单细胞文库构建及测序3. Construction and sequencing of 10×Genomics single-cell library
本发明中利用10×Genomics Chromium 3'Gene Expression Kit V3试剂盒制备单细胞转录组文库进行测序,详细步骤严格执行10×Genomics单细胞操作规范。具体的,经过上述步骤获得单细胞悬液后,将目标细胞和对应的10×Genomics试剂加入Chromium芯片中生成含有单个细胞和单个凝胶珠的乳胶滴(Gel Bead in Emulsion,GEM),并进行后续的逆转录、双链DNA生成、文库构建和测序。实验室,我们设定每个样品的目标捕获细胞为6,000~8,000。最终构建的文库在Illumina HiSeq 4000平台上进行测序,每个细胞目标测序深度为100,000个reads。In the present invention, the 10×Genomics Chromium 3' Gene Expression Kit V3 kit is used to prepare a single-cell transcriptome library for sequencing, and the detailed steps strictly follow the 10×Genomics single-cell operation specification. Specifically, after obtaining the single cell suspension through the above steps, the target cells and corresponding 10×Genomics reagents were added to the Chromium chip to generate latex droplets (Gel Bead in Emulsion, GEM) containing single cells and single gel beads, and carried out Subsequent reverse transcription, double-stranded DNA generation, library construction and sequencing. In the laboratory, we set the target capture cells to be 6,000-8,000 per sample. The final constructed library was sequenced on the Illumina HiSeq 4000 platform, with a target sequencing depth of 100,000 reads per cell.
二、鉴定驱动CTC转移的关键基因2. Identification of key genes driving CTC metastasis
1.单细胞转录组数据预处理及质量控制1. Single-cell transcriptome data preprocessing and quality control
首先利用Illumina公司bcl2fastq软件将上述测序所得原始测序数据图像转换为双端150碱基对配对的Fastq格式的序列信息。随后利用Cell Ranger(v.3.0.0,10×Genomics)软件将所得序列与GRCh38版本的人体参考基因组进行比对,得到基因表达矩阵。能够专一比对到转录组基因的外显子区域的序列的UMI标签将用于后续统计计数。然后Seurat R软件包进行后续质量控制,我们通过以下三个标准对细胞和基因进行过滤:(1)排除掉基因表达数目超过7500和小于200个的细胞,因为低质量的细胞通常基因表达数量较少,而双细胞或者多细胞基因表达数通常较高;(2)去除线粒体基因数目超过25%的细胞,因为死亡细胞通常表现出高线粒体污染;(3)排除掉在少于三个细胞中表达的基因。此外,具有多种不同类型细胞标记基因的细胞被鉴定为多细胞。质量控制后,18个样本共测得74,206个细胞和26,808个基因。First, use the bcl2fastq software of Illumina Company to convert the original sequencing data image obtained from the above sequencing into sequence information in Fastq format with paired-
2.拷贝数变异分析和细胞类型的鉴定2. Copy Number Variation Analysis and Identification of Cell Types
首先通过上皮细胞的经典标记基因(PTPRC-、EPCAM+、KRT8+、KRT18+、KRT19+)鉴定出原发灶和转移肿瘤中的上皮细胞。然后,通过CopyKT算法从所有上皮细胞中定义出恶性的肿瘤细胞(图1)。CopyKAT使用综合贝叶斯分析方法(integrative Bayesian approaches)在5MB分辨率下发现全基因组非整倍体,具有大的全基因组非整倍体的细胞被鉴定为恶性肿瘤细胞。使用UMI计数矩阵作为输入,其他为默认参数。而来自门静脉血中的CTC通过标记基因(PTPRC-、PPBP+、PF4+、CD9+、KRT8+、TIMP1+)来确定定义,并使用CopyKT算法计算恶性指数CNV进行验证。Epithelial cells in primary and metastatic tumors were first identified by classical marker genes of epithelial cells (PTPRC− , EPCAM+ , KRT8+ , KRT18+ , KRT19+ ). Then, malignant tumor cells were defined from all epithelial cells by the CopyKT algorithm (Fig. 1). CopyKAT uses integrative Bayesian approaches to discover genome-wide aneuploidy at 5MB resolution, and cells with large genome-wide aneuploidy are identified as malignant cells. Use UMI count matrix as input, others are default parameters. The CTCs from portal vein blood were defined by marker genes (PTPRC- , PPBP+ , PF4+ , CD9+ , KRT8+ , TIMP1+ ), and the malignancy index CNV was calculated using the CopyKT algorithm for verification.
3.单细胞转录组数据降维和聚类分析3. Dimensionality reduction and cluster analysis of single-cell transcriptome data
在对细胞和基因进行质量控制后,采用Seurat(v.4.0.1)R软件包对单细胞转录组数据分别进行标准化处理,以消除各细胞数据间的差异,并进行后续的降维和无监督聚类分析。首先,我们使用全局缩放归一化方法“LogNormalize”对单细胞合集的基因表达矩阵进行全局标准化,将基因的表达值在除以相应单细胞的总表达值后乘以参数因子(使用默认标准参数10000)后取log值。接下来,利用FindVariableFeatures函数选取前2000个高变量基因,并用ScaleData函数将标准化数据缩放为z-scores。然后利用RunPCA函数对高变量的基因进行主成分分析(Principal ComponentsAnalysis,PCA),对高维数据进行降维处理。在完成降维后,利用FindNeighbors功能构建KNN图来定义两个细胞间的权重。然后应用Louvain算法,通过FindClusters函数对相似的细胞进行分组,分辨率(resolution)参数设置为1。随后使用非线性的t-SNE降维方法对聚类结果进行可视化(图2)。After the quality control of cells and genes, the Seurat (v.4.0.1) R software package was used to standardize the single-cell transcriptome data to eliminate the differences between the data of each cell, and to perform subsequent dimensionality reduction and unsupervised Cluster analysis. First, we use the global scaling normalization method "LogNormalize" to globally normalize the gene expression matrix of the single-cell collection, and multiply the expression value of the gene by the parameter factor after dividing by the total expression value of the corresponding single cell (using the default standard parameter 10000) to take the log value. Next, use the FindVariableFeatures function to select the top 2000 highly variable genes, and use the ScaleData function to scale the normalized data to z-scores. Then use the RunPCA function to perform principal component analysis (Principal Components Analysis, PCA) on high-variable genes, and perform dimensionality reduction processing on high-dimensional data. After completing the dimensionality reduction, use the FindNeighbors function to build a KNN graph to define the weights between two cells. The Louvain algorithm was then applied to group similar cells by the FindClusters function with the resolution parameter set to 1. The clustering results were then visualized using the non-linear t-SNE dimensionality reduction method (Fig. 2).
4.差异基因表达分析4. Differential gene expression analysis
为了寻找鉴定CTCs中差异表达基因,我们利用R studio通过FindMarkers函数进行差异表达分析,基于非参数Wilcoxon秩和检验评估基因的显著性。min.pct参数设置为0.25,logfc阈值参数设置为0.25。最终,我们鉴定出包括RGS18在内的差异基因(图3)。In order to find and identify differentially expressed genes in CTCs, we used R studio to perform differential expression analysis through the FindMarkers function, and evaluated the significance of genes based on the non-parametric Wilcoxon rank sum test. The min.pct parameter is set to 0.25 and the logfc threshold parameter is set to 0.25. Finally, we identified differential genes including RGS18 (Fig. 3).
实施例2验证多种临床样本的CTCs表达RGS18Example 2 Verification that CTCs of various clinical samples express RGS18
1.检测CTCs中RGS18的表达水平1. Detection of the expression level of RGS18 in CTCs
首先,我们利用上述CTCs转录组测序数据,并以原发灶肿瘤细胞和转移灶肿瘤细胞为对照,对基因表达值用Log2(TPM+1)归一化处理后,计算RGS18在原发灶肿瘤细胞、血液中CTCs和转移灶肿瘤细胞中的表达水平。结果如图4所示,与原发灶和转移灶来源的肿瘤细胞相比,RGS18在CTCs中高表达,表明其在CTCs转移过程中可能起潜在作用。First, we used the above CTCs transcriptome sequencing data, and compared the primary tumor cells and metastatic tumor cells, after normalizing the gene expression values with Log2(TPM+1), we calculated the role of RGS18 in the primary tumor. Cells, CTCs in blood and expression levels in metastatic tumor cells. The results are shown in Figure 4. Compared with tumor cells derived from primary tumors and metastases, RGS18 was highly expressed in CTCs, suggesting that it may play a potential role in the process of CTCs metastasis.
同时,还检测了RGS18在其他肿瘤来源CTCs中的表达水平。利用数据库中肝细胞癌(HCC;CNP0000095,GSE117623)、PDAC(GSE144561)、乳腺癌(BRCA;GSE67939、GSE86978)、结肠腺癌(COAD;GSE74369)和黑色素瘤(SKCM;GSE38495)来源CTCs的转录组数据,并对基因表达值用Log2(TPM+1)归一化处理后,计算HLA-E在不同肿瘤来源的CTCs中的表达水平。结果如图5所示,RGS18在肝细胞癌、胰腺癌、乳腺癌、结肠腺癌和黑色素瘤来源的CTCs中都高表达,表明该基因具有广谱应用性。At the same time, the expression level of RGS18 in other tumor-derived CTCs was also detected. Transcriptomes of CTCs derived from hepatocellular carcinoma (HCC; CNP0000095, GSE117623), PDAC (GSE144561), breast cancer (BRCA; GSE67939, GSE86978), colon adenocarcinoma (COAD; GSE74369) and melanoma (SKCM; GSE38495) were used in the database After normalizing the gene expression values with Log2(TPM+1), the expression levels of HLA-E in CTCs derived from different tumors were calculated. Results As shown in Figure 5, RGS18 was highly expressed in CTCs derived from hepatocellular carcinoma, pancreatic cancer, breast cancer, colon adenocarcinoma, and melanoma, indicating that the gene has broad-spectrum applicability.
此外,还检测了RGS18在小鼠肿瘤来源CTCs中的表达水平。利用数据库中小鼠胰腺癌(GSE51372)来源CTCs的转录组数据,并对基因表达值用Log2(TPM+1)归一化处理后,计算RGS18在小鼠肿瘤来源的CTCs中的表达水平。结果如图6所示,RGS18在小鼠肿瘤来源的CTCs中也高表达。In addition, the expression level of RGS18 in mouse tumor-derived CTCs was also detected. Using the transcriptome data of mouse pancreatic cancer (GSE51372)-derived CTCs in the database, and normalizing the gene expression values with Log2(TPM+1), the expression level of RGS18 in mouse tumor-derived CTCs was calculated. Results As shown in Figure 6, RGS18 was also highly expressed in mouse tumor-derived CTCs.
2.通过多重免疫荧光染色证明了RGS18在CTCs中高表达2. The high expression of RGS18 in CTCs was proved by multiple immunofluorescence staining
为了验证CTCs中高表达RGS18,利用微流控芯片捕获了PDAC肝转移患者门静脉血液中的CTC。洗脱收集CTC后,利用EpCAM和RGS18免疫荧光共染色检测CTCs是否表达RGS18。结果如图7所示,多个病人来源的CTCs中均表达RGS18。To verify the high expression of RGS18 in CTCs, a microfluidic chip was used to capture CTCs in portal vein blood of patients with liver metastases from PDAC. After elution and collection of CTCs, EpCAM and RGS18 immunofluorescence co-staining was used to detect whether CTCs expressed RGS18. The results are shown in Figure 7, RGS18 was expressed in CTCs derived from multiple patients.
实施例3RGS18过表达促进了肿瘤转移的形成Example 3 RGS18 overexpression promotes the formation of tumor metastasis
为了研究RGS18是否可以促进肿瘤转移,以小鼠胰腺癌肝转移为模型,研究RGS18对胰腺癌肝转移的影响。In order to study whether RGS18 can promote tumor metastasis, the effect of RGS18 on liver metastasis of pancreatic cancer was studied by using mouse pancreatic cancer liver metastasis as a model.
为了实现这一目的,利用Balb/c裸鼠将荧光素素标记SU86.86细胞(SU86.86-Luc)和RGS18过表达(通过慢病毒转染)的SU86.86-Luc细胞通过脾静脉注射构建胰腺癌肝转移模型(图8A)。具体的,脾静脉注射1×106个SU86.86-Luc细胞构建PDAC肝转移模型,细胞注射完成后用手术线结扎脾静脉和脾脏,并切掉注射部位防止脾坏死。接种14天后,小动物活体成像检测小鼠肝脏荧光信号的强弱用于监测肝转移生长情况。生物发光图像和信号定量显示,接种人RGS18(hRGS18)过表达的SU86.86细胞(SU86.86-hRGS18)的小鼠在第14天肝脏部位即可检测到极强的荧光信号;相反,接种空白载体SU86.86细胞(SU86.86-vector)的对照组小鼠肝脏部位未检测到荧光信号(图8B,C),表明肿瘤细胞过表达RGS18可以快速的形成肝转移灶。为了验证这一现象,我们解剖小鼠后检测肝脏组织中肿瘤转移灶,发现接种SU86.86-hRGS18小鼠的肝脏组织有严重的肿瘤转移灶,而对照组未观察到转移灶肿瘤(图8D)。为了进一步验证这一现象,接下来通过H&E染色检测了两组小鼠的肝脏组织。H&E染色结果显示,SU86.86-hRGS18组小鼠肝脏组织存在明显的肿瘤转移灶,而SU86.86-vector组未检测到肿瘤细胞(图8E)。人类EpCAM(hEpCAM)和人类hRGS18(hRGS18)免疫组化染色进一步验证了这一现象,SU86.86-hRGS18组小鼠肝脏组织存在hEpCAM和hRGS18阳性的肿瘤细胞,而对照组未检测到阳性细胞(图8E)。此外,我们在另一组独立的肝转移实验中研究了RGS18过表达对小鼠生存期的影响。结果显示,接种SU86.86-hRGS18组小鼠生存期显著低于对照组小鼠,小鼠在20天之内全部死亡;而SU86.86-vector对照组小鼠在接种35天后依旧存活(图8F)。以上结果表明,RGS18可以促进胰腺癌肝转移的形成,降低小鼠的生存期。To achieve this, fluorescein-labeled SU86.86 cells (SU86.86-Luc) and RGS18-overexpressed (by lentiviral transfection) SU86.86-Luc cells were injected via the splenic vein using Balb/c nude mice A pancreatic cancer liver metastasis model was constructed (Fig. 8A). Specifically, 1×106 SU86.86-Luc cells were injected into the splenic vein to construct a PDAC liver metastasis model. After the cell injection, the splenic vein and spleen were ligated with surgical thread, and the injection site was cut off to prevent spleen necrosis. Fourteen days after inoculation, small animal in vivo imaging was used to detect the intensity of the fluorescence signal of the mouse liver to monitor the growth of liver metastases. Bioluminescence images and signal quantification showed that mice inoculated with human RGS18 (hRGS18) overexpressed SU86. No fluorescence signal was detected in the liver of mice in the blank vector SU86.86 cells (SU86.86-vector) control group (Fig. 8B, C), indicating that tumor cells overexpressing RGS18 can rapidly form liver metastases. In order to verify this phenomenon, we dissected the mice and detected tumor metastases in the liver tissues, and found that the liver tissues of mice inoculated with SU86.86-hRGS18 had severe tumor metastases, while no metastatic tumors were observed in the control group (Fig. 8D ). To further verify this phenomenon, the liver tissues of the two groups of mice were next detected by H&E staining. The results of H&E staining showed that there were obvious tumor metastases in the liver tissues of mice in the SU86.86-hRGS18 group, but no tumor cells were detected in the SU86.86-vector group (Fig. 8E). Immunohistochemical staining of human EpCAM (hEpCAM) and human hRGS18 (hRGS18) further verified this phenomenon. There were hEpCAM and hRGS18-positive tumor cells in the liver tissues of mice in the SU86.86-hRGS18 group, while no positive cells were detected in the control group ( Figure 8E). Furthermore, we investigated the effect of RGS18 overexpression on the survival of mice in another independent set of liver metastasis experiments. The results showed that the survival period of the mice inoculated with SU86.86-hRGS18 group was significantly lower than that in the control group, and all the mice died within 20 days; while the mice in the SU86.86-vector control group still survived 35 days after inoculation (Fig. 8F). The above results indicated that RGS18 could promote the formation of pancreatic cancer liver metastases and reduce the survival time of mice.
实施例4RGS18通过AKT-GSK3β-CREB1信号轴促进HLA-E的表达,进而逃避NK细胞的免疫监视Example 4 RGS18 promotes the expression of HLA-E through the AKT-GSK3β-CREB1 signaling axis, thereby evading the immune surveillance of NK cells
1.RGS18通过AKT-GSK3β-CREB1信号轴促进HLA-E的表达1. RGS18 promotes the expression of HLA-E through the AKT-GSK3β-CREB1 signaling axis
接下来,我们研究了RGS18促进肿瘤转移的机制。首先通过相关性分析发现CTCs中RGS18的表达与免疫检查点分子HLA-E的表达高度正相关(图9)。然后在SU86.86和CFPAC-1肿瘤细胞中利用慢病毒介导的过表达技术过表达RGS18,并通过westernblot检测其对HLA-E的表达。结果如图10A所示,RGS18过表达显著促进了肿瘤细胞HLA-E的表达。进一步的,我们研究了RGS18促进HLA-E表的机制。我们通过westernblot检测了RGS18对下游激酶磷酸化水平的变化,发现过表达RGS18调控了AKT通路的活性,而对MAPK、p38、PKC和PKA通路无影响(图10A,B)。肿瘤细胞RGS18表达上调后,AKT磷酸化水平下调,并抑制其下游GSK3β丝氨酸残基9的磷酸化,增加了GSK3β的稳定性(图10B)。GSK3β进一步通过磷酸化丝氨酸残基129促进了CREB1的活性(图10B)。重要的是,我们发现激活的CREB1主要在细胞核中分布,表明其可能参与了HLA-E的转录调控(图10C)。为了研究CREB1是否直接调控HLA-E的表达,我们利用shRNA干扰技术在敲除肿瘤细胞CREB1的表达。结果显示,shCREB1在降低CREB1表达的同时显著抑制了HLA-E的表达(图10D)。Next, we investigated the mechanism by which RGS18 promotes tumor metastasis. First, through correlation analysis, it was found that the expression of RGS18 in CTCs was highly positively correlated with the expression of the immune checkpoint molecule HLA-E (Figure 9). Then, RGS18 was overexpressed in SU86.86 and CFPAC-1 tumor cells by lentivirus-mediated overexpression technology, and its expression to HLA-E was detected by western blot. Results As shown in Figure 10A, the overexpression of RGS18 significantly promoted the expression of HLA-E in tumor cells. Further, we investigated the mechanism by which RGS18 promotes HLA-E expression. We detected the changes of phosphorylation levels of downstream kinases by RGS18 by western blot, and found that overexpression of RGS18 regulated the activity of AKT pathway, but had no effect on MAPK, p38, PKC and PKA pathways (Fig. 10A, B). After the expression of RGS18 in tumor cells was up-regulated, the phosphorylation level of AKT was down-regulated, and the phosphorylation of its downstream GSK3β serine residue 9 was inhibited, which increased the stability of GSK3β (Fig. 10B). GSK3β further promoted the activity of CREB1 by phosphorylating serine residue 129 ( FIG. 10B ). Importantly, we found that activated CREB1 was mainly distributed in the nucleus, suggesting that it may be involved in the transcriptional regulation of HLA-E (Fig. 10C). In order to study whether CREB1 directly regulates the expression of HLA-E, we used shRNA interference technology to knock out the expression of CREB1 in tumor cells. The results showed that shCREB1 significantly inhibited the expression of HLA-E while reducing the expression of CREB1 ( FIG. 10D ).
相反,利用慢病毒介导的shRNA干扰技术降低肿瘤细胞RGS18的表达后,AKT磷酸化水平上调,促进了GSK3β(Ser9)的磷酸化导致其被降解,从而抑制了GSK3β的活性;降低的GSK3B使CREB1的磷酸化水平下降,并导致HLA-E的表达水平下降(图10E)。为了进一步证明AKT通路在调控HLA-E表达中起重要作用,我们再PDAC细胞中导入AKT激活性突变体AKT(E17K)和AKT(Q79K)激活肿瘤细胞AKT通路(图10F)。结果显示,两个AKT激活突变体均显著上调了SU86.86细胞AKT磷酸化水平;同时抑制了GSK3β的活性,导致CREB1的活性降低;进而引发HLA-E的表达水平显著降低。以上结果表明,RGS18通过调控AKT-GSK3β-CREB1信号通路促进HLA-E的表达。On the contrary, after reducing the expression of tumor cell RGS18 by using lentivirus-mediated shRNA interference technology, the phosphorylation level of AKT is up-regulated, which promotes the phosphorylation of GSK3β (Ser9) and leads to its degradation, thereby inhibiting the activity of GSK3β; the reduced GSK3B makes The phosphorylation level of CREB1 was decreased, which led to the decreased expression level of HLA-E (Fig. 10E). In order to further prove that the AKT pathway plays an important role in the regulation of HLA-E expression, we introduced AKT activating mutants AKT (E17K) and AKT (Q79K) into PDAC cells to activate the AKT pathway of tumor cells (Fig. 10F). The results showed that the two AKT activating mutants significantly up-regulated the phosphorylation level of AKT in SU86.86 cells; at the same time inhibited the activity of GSK3β, resulting in a decrease in the activity of CREB1; and then caused a significant decrease in the expression level of HLA-E. The above results indicated that RGS18 promotes the expression of HLA-E by regulating the AKT-GSK3β-CREB1 signaling pathway.
2.RGS18通过促进肿瘤细胞逃避NK细胞的免疫监视进行转移2. RGS18 promotes tumor cells to evade the immune surveillance of NK cells for metastasis
本实施例中,我们通过体外和体内实验评估了RGS18通过促进肿瘤细胞逃避NK细胞的免疫监视进行转移。In this example, we evaluated RGS18 by promoting tumor cell evasion of NK cell immune surveillance for metastasis through in vitro and in vivo experiments.
首先,我们在SU86.86肿瘤细胞中过表达RGS18,促进HLA-E的表达。然后将NK细胞与SU86.86肿瘤细胞按不同的效靶比(1:5、1:10和1:20)共孵育;共孵育24小时后,收集细胞上清通过LDH试剂盒检测NK细胞对肿瘤细胞的杀伤能力。结果如图11所示,过表达RGS18可使NK细胞对肿瘤细胞的杀伤能力减缩,表明RGS18可保护肿瘤细胞逃避NK细胞的免疫监视。First, we overexpressed RGS18 in SU86.86 tumor cells to promote HLA-E expression. Then NK cells were co-incubated with SU86.86 tumor cells at different effect-to-target ratios (1:5, 1:10 and 1:20); after 24 hours of co-incubation, the cell supernatant was collected to detect the effect of NK cells on the LDH kit. tumor cell killing ability. The results are shown in Figure 11, overexpression of RGS18 can reduce the ability of NK cells to kill tumor cells, indicating that RGS18 can protect tumor cells from evading the immune surveillance of NK cells.
此外,我们在体内实验中发现RGS18通过保护肿瘤细胞免受NK细胞的攻击,进行远端转移。实验中,我们在荧光素酶标记的小鼠胰腺癌细胞KPC(KPC-Luc)中利用慢病毒转染过表达小鼠RGS18(mRGS18),并通过小鼠脾静脉注射肿瘤细胞,构建胰腺癌肝转移模型。具体的,接种前一天在Balb/cl裸鼠(该品系小鼠含有NK细胞)体内尾静脉注射anti-Asialo-GM1抗体去除体内NK细胞;然后在接种当天脾静脉注射5×105个KPC-Luc细胞或过表达小鼠RGS18(mRGS18)的KPC-Luc细胞(KPC-Luc-mRGS18);接种第二天在NK去除组的小鼠中继续静脉注射一剂anti-Asialo-GM1抗体。接种14天后,小动物活体成像检测小鼠肝脏荧光信号的强弱用于监测肝转移生长情况。生物发光图像和信号定量显示,接种KPC-Luc细胞的小鼠肝脏部位未检测到荧光信号;而利用anti-Asialo-GM1抗体去除NK细胞的小鼠,接种KPC-Luc细胞后在肝脏部位检测到极强的荧光信号,且荧光信号与KPC-Luc-mRGS18组相当(图12A,B),表明RGS18可以肿瘤细胞逃避NK细胞的免疫监视。为了验证这一现象,我们解剖小鼠后检测肝脏组织中肿瘤转移灶,发现NK去除组和KPC-Luc-mRGS18组的肝脏组织存在严重的肿瘤转移灶,而对照组未观察到转移灶肿瘤(图12C)。此外,我们在完全免疫缺陷鼠(B-NDG-hIL15)中重复验证了NK细胞在抑制肿瘤转移中的重要作用。具体的,在未进行人NK免疫重构的B-NDG-hIL15小鼠中脾静脉注射1×106个SU86.86-Luc或SU86.86-Luc-hRGS18细胞构建胰腺癌肝转移模型。接种14天后,小动物活体成像检测小鼠肝转移生长情况。。结果显示,在无NK细胞的监视下,RGS18过表达的SU86.86细胞和对照组细胞具有相当的转移能力,两组小鼠的肝脏部位均检测到强烈的荧光信号,表明RGS18的促转移功能依赖于NK细胞(图12D,E)。In addition, we found in vivo experiments that RGS18 carries out distant metastasis by protecting tumor cells from NK cell attack. In the experiment, we used lentivirus transfection to overexpress mouse RGS18 (mRGS18) in luciferase-labeled mouse pancreatic cancer cell KPC (KPC-Luc), and injected tumor cells through mouse splenic vein to construct pancreatic cancer liver transfer model. Specifically, anti-Asialo-GM1 antibody was injected into the tail vein of Balb/cl nude mice (this strain contains NK cells) one day before inoculation to remove NK cells in the body; then 5×105 KPC- Luc cells or KPC-Luc cells (KPC-Luc-mRGS18) overexpressing mouse RGS18 (mRGS18); on the second day after inoculation, a dose of anti-Asialo-GM1 antibody was continued intravenously in mice in the NK depletion group. Fourteen days after inoculation, small animal in vivo imaging was used to detect the intensity of the fluorescence signal of the mouse liver to monitor the growth of liver metastases. Bioluminescent images and signal quantification showed that no fluorescent signal was detected in the liver of mice inoculated with KPC-Luc cells; however, in mice inoculated with KPC-Luc cells, the fluorescence signal was detected in the liver of mice depleted of NK cells by anti-Asialo-GM1 antibody The extremely strong fluorescent signal, which was comparable to that of the KPC-Luc-mRGS18 group (Fig. 12A, B), indicated that RGS18 can evade the immune surveillance of NK cells by tumor cells. In order to verify this phenomenon, we dissected the mice and detected tumor metastases in the liver tissues, and found that there were severe tumor metastases in the liver tissues of the NK removal group and the KPC-Luc-mRGS18 group, while no metastatic tumors were observed in the control group ( Figure 12C). In addition, we repeatedly verified the important role of NK cells in suppressing tumor metastasis in completely immunodeficient mice (B-NDG-hIL15). Specifically, 1×106 SU86.86-Luc or SU86.86-Luc-hRGS18 cells were injected into the splenic vein of B-NDG-hIL15 mice that had not undergone human NK immune reconstitution to establish a liver metastasis model of pancreatic cancer. Fourteen days after inoculation, small animal in vivo imaging was used to detect the growth of liver metastases in mice. . The results showed that under the supervision of NK cells, RGS18 overexpressed SU86.86 cells and control cells had comparable metastatic ability, and strong fluorescent signals were detected in the liver parts of both groups of mice, indicating the pro-metastasis function of RGS18 Dependence on NK cells (Fig. 12D, E).
实施例5抑制肿瘤细胞RGS18的表达可治疗肿瘤转移Example 5 Inhibiting the expression of tumor cell RGS18 can treat tumor metastasis
为了进一步验证RGS18作为靶点在抑制肿瘤转移中的作用,我们利用KPC-Luc细胞构建肺转移模型,利用慢病毒介导的shRNA干扰技术降低肿瘤细胞RGS18的表达,研究其对小鼠肺转移肿瘤形成的影响。我们首先在KPC-Luc细胞中转染shRGS18质粒,构建RGS18敲除的细胞系。然后将肿瘤细胞以5×104个/只的数目注射入Balb/c裸鼠尾静脉内,构建小鼠肺转移模型。接种15天后,利用小动物活体成像检测小鼠肺转移生长情况。结果如图13A和图13B所示,shRGS18显著抑制了肺转移灶的形成,表明降低肿瘤细胞RGS18的表达可抑制肿瘤转移。In order to further verify the role of RGS18 as a target in inhibiting tumor metastasis, we used KPC-Luc cells to construct a lung metastasis model, and used lentivirus-mediated shRNA interference technology to reduce the expression of RGS18 in tumor cells to study its effect on lung metastasis in mice. The impact of formation. We first transfected the shRGS18 plasmid in KPC-Luc cells to construct a RGS18 knockout cell line. Then, tumor cells were injected into the tail vein of Balb/c nude mice at a rate of 5×104 per mouse to construct a mouse model of lung metastasis. Fifteen days after inoculation, the growth of lung metastases in mice was detected by live imaging of small animals. Results As shown in Figure 13A and Figure 13B, shRGS18 significantly inhibited the formation of lung metastases, indicating that reducing the expression of RGS18 in tumor cells can inhibit tumor metastasis.
实施例6抑制RGS18下游调控基因HLA-E的功能可治疗肿瘤转移Example 6 Inhibiting the function of RGS18 downstream regulatory gene HLA-E can treat tumor metastasis
本实施例中,我们通过肿瘤肝转移和肺转移模型发现抑制RGS18下游调控基因HLA-E的功能可治疗肿瘤转移。In this example, we found that inhibiting the function of the downstream regulatory gene HLA-E of RGS18 can treat tumor metastasis through tumor liver metastasis and lung metastasis models.
首先,我们通过功能缺失实验在过表达RGS18的细胞中利用shRNA感染技术敲除HLA-E的表达,研究其抗肿瘤转移能力。我们以hIL15-B-NDG小鼠为模型,静脉注射病人来源经体外激活的NK细胞(5×106个细胞)进行免疫重构。NK细胞回输第二天,我们通过脾静脉分别注射空载的SU86.86-Luc细胞、SU86.86-Luc-hRGS18细胞以及shHLA-E敲除的SU86.86-Luc-hRGS18细胞(SU86.86-Luc-hRGS18-shHLA-E),建立SU86.86肝转移模型。接种第二天继续回输一次激活的NK细胞,用于维持血液中较高的NK细胞数目。接种14天后,小动物活体成像检测小鼠肝转移生长情况(图14A)。与裸鼠模型的结果一致,相比于对照组,hRGS18过表达显著促进了SU86.86细胞的肝转移,进一步验证了RGS18具有促进PDAC细胞肝转移的能力。相反,在SU86.86-Luc-hRGS18细胞中利用shHLA-E降低HLA-E的表达后显著抑制了肿瘤细胞的肝转移能力,肝脏部位未检测到明显的肝转移信号。荧光定量结果进一步验证了这一现象(图14B)。First, we used shRNA infection technology to knock down the expression of HLA-E in cells overexpressing RGS18 through loss-of-function experiments to study its anti-tumor metastasis ability. Using hIL15-B-NDG mice as a model, we injected NK cells (5×106 cells) activated in vitro from patients intravenously for immune reconstitution. On the second day after NK cell reinfusion, we injected empty SU86.86-Luc cells, SU86.86-Luc-hRGS18 cells and shHLA-E knockout SU86.86-Luc-hRGS18 cells (SU86.86. 86-Luc-hRGS18-shHLA-E), to establish SU86.86 liver metastasis model. On the second day of inoculation, the activated NK cells will be infused again to maintain a high number of NK cells in the blood. Fourteen days after inoculation, live imaging of small animals was performed to detect the growth of liver metastases in mice ( FIG. 14A ). Consistent with the results of the nude mouse model, compared with the control group, hRGS18 overexpression significantly promoted the liver metastasis of SU86.86 cells, which further verified the ability of RGS18 to promote liver metastasis of PDAC cells. On the contrary, using shHLA-E to reduce the expression of HLA-E in SU86.86-Luc-hRGS18 cells significantly inhibited the liver metastasis ability of tumor cells, and no obvious liver metastasis signal was detected in the liver. Fluorescence quantitative results further verified this phenomenon ( FIG. 14B ).
此外,我们在小鼠肺转移模型中进一步验证了抑制RGS18下游调控基因HLA-E的功能可治疗肿瘤转移。首先,我们在小鼠KPC细胞中过表达mRGS18基因,qPCR显示KPC细胞的H2-T23(HLA-E)基因表达显著上调(图15A)。然后将过表达mRGS18的KPC细胞(KPC-mRGS18)或转染空载载体的KPC细胞尾静脉注射入Balb/c裸鼠体内,构建小鼠肺转移模型。由于肿瘤细胞表面HLA-E主要通过与NK细胞表面CD94-NKG2A分子结合抑制NK细胞的活性,从而进行免疫逃逸。因此,我们通过静脉注射anti-NKG2A抗体(10mg/kg)用于阻断肿瘤细胞对NK细胞的免疫抑制(图15B)恢复NK细胞对肿瘤细胞的杀伤能力,从而抑制肿瘤转移。接种15天后,小动物活体成像检测小鼠肺部荧光信号的强弱用于监测肺转移生长情况(图15C)。结果表明,过表达RGS18显著促进了小鼠肺转移的形成;而利用anti-NKG2A抗体阻断HLA-E与CD94-NKG2A结合后,显著缓解了RGS18引起的肺转移(图15C,D)。实验结束后,我们收集小鼠肺部组织拍照,并统计肺表面的肿瘤结节数目。与荧光信号结果一致,RGS18过表达组肺部检测到大量的肿瘤结节,而anti-NKG2A几乎未检测到肿瘤结节(图15E,F)。此外,我们对肺组织进一步进行H&E病理分析,结果与荧光信号和肺转移结节计数一致(图15G)。以上结果表明,抑制RGS18下游调控基因HLA-E的功能可治疗肿瘤转移。In addition, we further verified that inhibiting the function of the downstream regulatory gene HLA-E of RGS18 can treat tumor metastasis in a mouse lung metastasis model. First, we overexpressed the mRGS18 gene in mouse KPC cells, and qPCR showed that the H2-T23 (HLA-E) gene expression in KPC cells was significantly up-regulated ( FIG. 15A ). Then KPC cells overexpressing mRGS18 (KPC-mRGS18) or KPC cells transfected with empty vector were injected into Balb/c nude mice through the tail vein to construct a mouse lung metastasis model. Because HLA-E on the surface of tumor cells mainly inhibits the activity of NK cells by binding to CD94-NKG2A molecules on the surface of NK cells, thereby performing immune escape. Therefore, we used anti-NKG2A antibody (10 mg/kg) intravenously to block the immunosuppression of NK cells by tumor cells (Figure 15B) to restore the ability of NK cells to kill tumor cells, thereby inhibiting tumor metastasis. After 15 days of inoculation, live imaging of small animals was used to detect the strength of the fluorescent signal in the lungs of the mice to monitor the growth of lung metastases ( FIG. 15C ). The results showed that overexpression of RGS18 significantly promoted the formation of lung metastases in mice; while blocking the combination of HLA-E and CD94-NKG2A with anti-NKG2A antibody significantly alleviated the lung metastases caused by RGS18 (Fig. 15C, D). After the experiment, we collected the lung tissues of the mice to take pictures, and counted the number of tumor nodules on the lung surface. Consistent with the fluorescent signal results, a large number of tumor nodules were detected in the lungs of the RGS18 overexpression group, while almost no tumor nodules were detected by anti-NKG2A (Fig. 15E, F). In addition, we further performed H&E pathological analysis on the lung tissue, and the results were consistent with the fluorescent signal and lung metastatic nodule count (Fig. 15G). The above results indicate that inhibiting the function of the downstream regulatory gene HLA-E of RGS18 can treat tumor metastasis.
实施例7血小板可促进肿瘤细胞RGS18的表达Example 7 Platelets can promote the expression of tumor cell RGS18
本实施例中,我们发现血小板可促进肿瘤细胞RGS18的表达。我们收集肿瘤病人来源的血小板,然后将肿瘤细胞与不同剂量的血小板(0.75×106血小板/ml、1.5×106血小板/ml和3×106血小板/ml)孵育72小时,western blot检测肿瘤细胞RGS18和HLA-E的表达水平。结果如图16所示,肿瘤细胞中RGS18和HLA-E的表达水平显著上调,表明血小板可以促进肿瘤细胞RGS18和HLA-E的表达。In this example, we found that platelets can promote the expression of RGS18 in tumor cells. We collected platelets from tumor patients, then incubated tumor cells with different doses of platelets (0.75×106 platelets/ml, 1.5×10 6 platelets/ml, and 3×106 platelets/ml) for 72 hours, and detected tumors by western blot Expression levels of RGS18 and HLA-E in cells. The results are shown in Figure 16, the expression levels of RGS18 and HLA-E in tumor cells were significantly up-regulated, indicating that platelets can promote the expression of RGS18 and HLA-E in tumor cells.
以上所述,仅为本发明的具体实施方式,但本发明的保护范围并不局限于此,任何熟悉本技术领域的技术人员在本发明揭露的技术范围内,可轻易想到变化或替换,都应涵盖在本发明的保护范围之内。因此,本发明的保护范围应以所述权利要求的保护范围为准。The above is only a specific embodiment of the present invention, but the scope of protection of the present invention is not limited thereto. Anyone skilled in the art can easily think of changes or substitutions within the technical scope disclosed in the present invention. Should be covered within the protection scope of the present invention. Therefore, the protection scope of the present invention should be determined by the protection scope of the claims.
Claims (8)
Translated fromChinesePriority Applications (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| CN202411237382.XACN119033938A (en) | 2023-01-21 | 2023-01-21 | Application of RGS18 in treatment of tumor metastasis |
| CN202310061424.8ACN115927642B (en) | 2023-01-21 | 2023-01-21 | Application of RGS18 in treatment of tumor metastasis |
Applications Claiming Priority (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| CN202310061424.8ACN115927642B (en) | 2023-01-21 | 2023-01-21 | Application of RGS18 in treatment of tumor metastasis |
Related Child Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| CN202411237382.XADivisionCN119033938A (en) | 2023-01-21 | 2023-01-21 | Application of RGS18 in treatment of tumor metastasis |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| CN115927642Atrue CN115927642A (en) | 2023-04-07 |
| CN115927642B CN115927642B (en) | 2024-08-13 |
Family
ID=86649432
Family Applications (2)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| CN202411237382.XAPendingCN119033938A (en) | 2023-01-21 | 2023-01-21 | Application of RGS18 in treatment of tumor metastasis |
| CN202310061424.8AActiveCN115927642B (en) | 2023-01-21 | 2023-01-21 | Application of RGS18 in treatment of tumor metastasis |
Family Applications Before (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| CN202411237382.XAPendingCN119033938A (en) | 2023-01-21 | 2023-01-21 | Application of RGS18 in treatment of tumor metastasis |
Country Status (1)
| Country | Link |
|---|---|
| CN (2) | CN119033938A (en) |
Cited By (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN118858637A (en)* | 2024-09-26 | 2024-10-29 | 四川大学华西医院 | A marker group, a kit and a device for capturing circulating tumor cells |
Citations (8)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2005005601A2 (en)* | 2003-06-09 | 2005-01-20 | The Regents Of The University Of Michigan | Compositions and methods for treating and diagnosing cancer |
| CN1852974A (en)* | 2003-06-09 | 2006-10-25 | 密歇根大学董事会 | Compositions and methods for treating and diagnosing cancer |
| WO2016049045A1 (en)* | 2014-09-24 | 2016-03-31 | Fred Hutchinson Cancer Research Center | Pancreatic cancer diagnostic |
| WO2016172710A2 (en)* | 2015-04-24 | 2016-10-27 | Cornell University | Methods and reagents for determination and treatment of organotropic metastasis |
| WO2022012420A1 (en)* | 2020-07-17 | 2022-01-20 | 信达生物制药(苏州)有限公司 | Nucleotide combination and use thereof |
| WO2022046576A1 (en)* | 2020-08-22 | 2022-03-03 | The Broad Institute, Inc. | Pancreatic ductal adenocarcinoma signatures and uses thereof |
| CN114292920A (en)* | 2021-12-10 | 2022-04-08 | 中国人民解放军军事科学院军事医学研究院 | Plasma RNA marker combination for gastric precancerous lesion and early gastric cancer diagnosis and application |
| CN116356018A (en)* | 2022-12-14 | 2023-06-30 | 四川大学华西医院 | A circulating tumor cell immune checkpoint and its application in inhibiting tumor metastasis |
Family Cites Families (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN111729090A (en)* | 2013-12-20 | 2020-10-02 | 通用医疗公司 | Methods and Assays Related to Circulating Tumor Cells |
| EP3209687A1 (en)* | 2014-10-23 | 2017-08-30 | Innate Pharma | Treatment of cancers using anti-nkg2a agents |
| EP3370742A1 (en)* | 2015-11-05 | 2018-09-12 | Glycostem Therapeutics B.V. | Composition for use in immunotherapy |
| JPWO2020116536A1 (en)* | 2018-12-05 | 2021-10-21 | 日東電工株式会社 | Combinations for treating cancer |
- 2023
- 2023-01-21CNCN202411237382.XApatent/CN119033938A/enactivePending
- 2023-01-21CNCN202310061424.8Apatent/CN115927642B/enactiveActive
Patent Citations (8)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2005005601A2 (en)* | 2003-06-09 | 2005-01-20 | The Regents Of The University Of Michigan | Compositions and methods for treating and diagnosing cancer |
| CN1852974A (en)* | 2003-06-09 | 2006-10-25 | 密歇根大学董事会 | Compositions and methods for treating and diagnosing cancer |
| WO2016049045A1 (en)* | 2014-09-24 | 2016-03-31 | Fred Hutchinson Cancer Research Center | Pancreatic cancer diagnostic |
| WO2016172710A2 (en)* | 2015-04-24 | 2016-10-27 | Cornell University | Methods and reagents for determination and treatment of organotropic metastasis |
| WO2022012420A1 (en)* | 2020-07-17 | 2022-01-20 | 信达生物制药(苏州)有限公司 | Nucleotide combination and use thereof |
| WO2022046576A1 (en)* | 2020-08-22 | 2022-03-03 | The Broad Institute, Inc. | Pancreatic ductal adenocarcinoma signatures and uses thereof |
| CN114292920A (en)* | 2021-12-10 | 2022-04-08 | 中国人民解放军军事科学院军事医学研究院 | Plasma RNA marker combination for gastric precancerous lesion and early gastric cancer diagnosis and application |
| CN116356018A (en)* | 2022-12-14 | 2023-06-30 | 四川大学华西医院 | A circulating tumor cell immune checkpoint and its application in inhibiting tumor metastasis |
Non-Patent Citations (2)
| Title |
|---|
| XIAOWEI LIU: "Immune checkpoint HLA-E:CD94-NKG2A mediates evasion of circulating tumor cells from NK cell surveillance", CANCER CELL, vol. 41, 13 February 2023 (2023-02-13), pages 272 - 287* |
| 刘小伟: "阻断循环肿瘤细胞免疫检查点HLA-E:CD94-NKG2A 抑制肿瘤转移", 科学通报, vol. 68, no. 15, 31 May 2023 (2023-05-31), pages 1864 - 1866* |
Cited By (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN118858637A (en)* | 2024-09-26 | 2024-10-29 | 四川大学华西医院 | A marker group, a kit and a device for capturing circulating tumor cells |
| CN118858637B (en)* | 2024-09-26 | 2024-12-17 | 四川大学华西医院 | Marker group for capturing circulating tumor cells, kit and device |
Also Published As
| Publication number | Publication date |
|---|---|
| CN115927642B (en) | 2024-08-13 |
| CN119033938A (en) | 2024-11-29 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| Zhang et al. | Dissecting transcriptional heterogeneity in primary gastric adenocarcinoma by single cell RNA sequencing | |
| Ma et al. | Clinical significance of PD-1/PD-Ls gene amplification and overexpression in patients with hepatocellular carcinoma | |
| Butler et al. | Prevention of human lymphoproliferative tumor formation in ovarian cancer patient-derived xenografts | |
| Gentles et al. | A human lung tumor microenvironment interactome identifies clinically relevant cell-type cross-talk | |
| Tan et al. | Identification of early diagnostic and prognostic biomarkers via WGCNA in stomach adenocarcinoma | |
| CN106414768A (en) | Gene fusions and gene variants associated with cancer | |
| Zheng et al. | Systematical analysis reveals a strong cancer relevance of CREB1-regulated genes | |
| Letouzé et al. | Analysis of the copy number profiles of several tumor samples from the same patient reveals the successive steps in tumorigenesis | |
| Jia et al. | Spatial immune scoring system predicts hepatocellular carcinoma recurrence | |
| Jiang et al. | Analysis of differentially expressed genes based on microarray data of glioma | |
| CN116356018B (en) | A circulating tumor cell immune checkpoint and its application in inhibiting tumor metastasis | |
| Lin et al. | Identification of colorectal cancer cell stemness from single-cell RNA sequencing | |
| Laise et al. | Pancreatic ductal adenocarcinoma comprises coexisting regulatory states with both common and distinct dependencies | |
| CN115927642B (en) | Application of RGS18 in treatment of tumor metastasis | |
| Perampalam et al. | Netrin signaling mediates survival of dormant epithelial ovarian cancer cells | |
| Vandenbon et al. | Murine breast cancers disorganize the liver transcriptome in a zonated manner | |
| Yen et al. | Genomic and molecular signatures of successful patient-derived xenografts for oral cavity squamous cell carcinoma | |
| Yu et al. | TBX2 identified as a potential predictor of bone metastasis in lung adenocarcinoma via integrated bioinformatics analyses and verification of functional assay | |
| Wang et al. | Single-cell transcriptome analysis identifies a novel tumor-associated macrophage subtype predicting better prognosis in pancreatic ductal adenocarcinoma | |
| Tan et al. | SMAD9-MYCN positive feedback loop represents a unique dependency for MYCN-amplified neuroblastoma | |
| Meng et al. | Combined analysis of RNA‐sequence and microarray data reveals effective metabolism‐based prognostic signature for neuroblastoma | |
| Huang et al. | N6-methyladenosine-related lncRNAs in combination with computational histopathology and radiomics predict the prognosis of bladder cancer | |
| Li et al. | An integrated analysis identifies six molecular subtypes of pancreatic ductal adenocarcinoma revealing cellular and molecular landscape | |
| Mittelbronn | Neurooncology: 2023 update | |
| CN103800918B (en) | The application in preparing anti-tumor drug of a kind of Microrna |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PB01 | Publication | ||
| PB01 | Publication | ||
| SE01 | Entry into force of request for substantive examination | ||
| SE01 | Entry into force of request for substantive examination | ||
| GR01 | Patent grant | ||
| GR01 | Patent grant |