CN115869416A - Cell membrane, drug carrier and composition for ocular targeted drug delivery, and preparation method and application thereof - Google Patents
Cell membrane, drug carrier and composition for ocular targeted drug delivery, and preparation method and application thereofDownload PDFInfo
- Publication number
- CN115869416A CN115869416ACN202111144615.8ACN202111144615ACN115869416ACN 115869416 ACN115869416 ACN 115869416ACN 202111144615 ACN202111144615 ACN 202111144615ACN 115869416 ACN115869416 ACN 115869416A
- Authority
- CN
- China
- Prior art keywords
- cell membrane
- snps
- ocular
- dex
- drug
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Pending
Links
- 210000000170cell membraneAnatomy0.000titleclaimsabstractdescription45
- 239000003937drug carrierSubstances0.000titleclaimsabstractdescription30
- 238000012377drug deliveryMethods0.000titleclaimsabstractdescription30
- 238000002360preparation methodMethods0.000titleclaimsabstractdescription12
- 239000000203mixtureSubstances0.000titleabstractdescription22
- 239000003814drugSubstances0.000claimsabstractdescription75
- 229940079593drugDrugs0.000claimsabstractdescription64
- 210000004027cellAnatomy0.000claimsabstractdescription44
- 229920000642polymerPolymers0.000claimsabstractdescription36
- 102000012355Integrin beta1Human genes0.000claimsabstractdescription30
- 108010022222Integrin beta1Proteins0.000claimsabstractdescription30
- 210000002919epithelial cellAnatomy0.000claimsabstractdescription24
- 210000001732sebaceous glandAnatomy0.000claimsabstractdescription8
- 238000000034methodMethods0.000claimsdescription12
- 239000008194pharmaceutical compositionSubstances0.000claimsdescription10
- 239000002773nucleotideSubstances0.000claimsdescription7
- 125000003729nucleotide groupChemical group0.000claimsdescription7
- 239000002105nanoparticleSubstances0.000claimsdescription6
- 239000004697PolyetherimideSubstances0.000claimsdescription5
- 229920000954PolyglycolidePolymers0.000claimsdescription5
- 229920001610polycaprolactonePolymers0.000claimsdescription5
- 239000004632polycaprolactoneSubstances0.000claimsdescription5
- 229920001601polyetherimidePolymers0.000claimsdescription5
- 239000004633polyglycolic acidSubstances0.000claimsdescription5
- 229920001577copolymerPolymers0.000claimsdescription3
- 239000000412dendrimerSubstances0.000claimsdescription3
- 229920000736dendritic polymerPolymers0.000claimsdescription3
- 239000003862glucocorticoidSubstances0.000claimsdescription3
- PCHJSUWPFVWCPO-UHFFFAOYSA-NgoldChemical compound[Au]PCHJSUWPFVWCPO-UHFFFAOYSA-N0.000claimsdescription3
- 239000010931goldSubstances0.000claimsdescription3
- 229910052737goldInorganic materials0.000claimsdescription3
- 239000003120macrolide antibiotic agentSubstances0.000claimsdescription3
- 238000002156mixingMethods0.000claimsdescription3
- 239000004626polylactic acidSubstances0.000claimsdescription3
- 229920001184polypeptidePolymers0.000claimsdescription3
- 102000004196processed proteins & peptidesHuman genes0.000claimsdescription3
- 108090000765processed proteins & peptidesProteins0.000claimsdescription3
- LISFMEBWQUVKPJ-UHFFFAOYSA-Nquinolin-2-olChemical compoundC1=CC=C2NC(=O)C=CC2=C1LISFMEBWQUVKPJ-UHFFFAOYSA-N0.000claimsdescription2
- 125000003275alpha amino acid groupChemical group0.000claims1
- 230000000840anti-viral effectEffects0.000claims1
- 238000002955isolationMethods0.000claims1
- 229920000747poly(lactic acid)Polymers0.000claims1
- 210000004087corneaAnatomy0.000abstractdescription21
- 210000000981epitheliumAnatomy0.000abstractdescription9
- 239000012528membraneSubstances0.000abstractdescription8
- IYMAXBFPHPZYIK-BQBZGAKWSA-NArg-Gly-AspChemical groupNC(N)=NCCC[C@H](N)C(=O)NCC(=O)N[C@@H](CC(O)=O)C(O)=OIYMAXBFPHPZYIK-BQBZGAKWSA-N0.000abstractdescription6
- 102000016359FibronectinsHuman genes0.000abstractdescription6
- 108010067306FibronectinsProteins0.000abstractdescription6
- 239000002103nanocoatingSubstances0.000abstractdescription6
- 241000283973Oryctolagus cuniculusSpecies0.000description46
- 238000011282treatmentMethods0.000description36
- 210000001508eyeAnatomy0.000description24
- 239000000243solutionSubstances0.000description22
- 241000699670Mus sp.Species0.000description21
- 150000002632lipidsChemical class0.000description20
- 238000010186stainingMethods0.000description19
- 238000010586diagramMethods0.000description17
- 241000699666Mus <mouse, genus>Species0.000description14
- 239000010410layerSubstances0.000description14
- UREBDLICKHMUKA-CXSFZGCWSA-NdexamethasoneChemical compoundC1CC2=CC(=O)C=C[C@]2(C)[C@]2(F)[C@@H]1[C@@H]1C[C@@H](C)[C@@](C(=O)CO)(O)[C@@]1(C)C[C@@H]2OUREBDLICKHMUKA-CXSFZGCWSA-N0.000description12
- 208000003556Dry Eye SyndromesDiseases0.000description11
- 210000005252bulbus oculiAnatomy0.000description10
- 229960003957dexamethasoneDrugs0.000description10
- 210000003560epithelium cornealAnatomy0.000description10
- 230000001965increasing effectEffects0.000description10
- 238000002296dynamic light scatteringMethods0.000description9
- 239000003889eye dropSubstances0.000description9
- 230000014509gene expressionEffects0.000description9
- 239000000725suspensionSubstances0.000description9
- 229920002385Sodium hyaluronatePolymers0.000description8
- 229940012356eye dropsDrugs0.000description8
- 210000002175goblet cellAnatomy0.000description8
- 229940010747sodium hyaluronateDrugs0.000description8
- YWIVKILSMZOHHF-QJZPQSOGSA-Nsodium;(2s,3s,4s,5r,6r)-6-[(2s,3r,4r,5s,6r)-3-acetamido-2-[(2s,3s,4r,5r,6r)-6-[(2r,3r,4r,5s,6r)-3-acetamido-2,5-dihydroxy-6-(hydroxymethyl)oxan-4-yl]oxy-2-carboxy-4,5-dihydroxyoxan-3-yl]oxy-5-hydroxy-6-(hydroxymethyl)oxan-4-yl]oxy-3,4,5-trihydroxyoxane-2-Chemical compound[Na+].CC(=O)N[C@H]1[C@H](O)O[C@H](CO)[C@@H](O)[C@@H]1O[C@H]1[C@H](O)[C@@H](O)[C@H](O[C@H]2[C@@H]([C@@H](O[C@H]3[C@@H]([C@@H](O)[C@H](O)[C@H](O3)C(O)=O)O)[C@H](O)[C@@H](CO)O2)NC(C)=O)[C@@H](C(O)=O)O1YWIVKILSMZOHHF-QJZPQSOGSA-N0.000description8
- 230000001225therapeutic effectEffects0.000description8
- 230000006907apoptotic processEffects0.000description7
- 229960000686benzalkonium chlorideDrugs0.000description7
- CADWTSSKOVRVJC-UHFFFAOYSA-Nbenzyl(dimethyl)azanium;chlorideChemical compound[Cl-].C[NH+](C)CC1=CC=CC=C1CADWTSSKOVRVJC-UHFFFAOYSA-N0.000description7
- 230000000694effectsEffects0.000description7
- GNBHRKFJIUUOQI-UHFFFAOYSA-NfluoresceinChemical compoundO1C(=O)C2=CC=CC=C2C21C1=CC=C(O)C=C1OC1=CC(O)=CC=C21GNBHRKFJIUUOQI-UHFFFAOYSA-N0.000description7
- 210000004379membraneAnatomy0.000description7
- 108090000623proteins and genesProteins0.000description7
- 102000004169proteins and genesHuman genes0.000description7
- YMWUJEATGCHHMB-UHFFFAOYSA-NDichloromethaneChemical compoundClCClYMWUJEATGCHHMB-UHFFFAOYSA-N0.000description6
- 238000000576coating methodMethods0.000description6
- 238000000684flow cytometryMethods0.000description6
- 239000002502liposomeSubstances0.000description6
- 239000007788liquidSubstances0.000description6
- 239000006166lysateSubstances0.000description6
- 239000011259mixed solutionSubstances0.000description6
- 230000028327secretionEffects0.000description6
- 238000001890transfectionMethods0.000description6
- 206010052129Ciliary hyperaemiaDiseases0.000description5
- 238000001157Fourier transform infrared spectrumMethods0.000description5
- 108060008682Tumor Necrosis FactorProteins0.000description5
- 102000000852Tumor Necrosis Factor-alphaHuman genes0.000description5
- 239000011248coating agentSubstances0.000description5
- 239000006196dropSubstances0.000description5
- 239000002245particleSubstances0.000description5
- 239000004417polycarbonateSubstances0.000description5
- XLYOFNOQVPJJNP-UHFFFAOYSA-NwaterChemical compoundOXLYOFNOQVPJJNP-UHFFFAOYSA-N0.000description5
- MZOFCQQQCNRIBI-VMXHOPILSA-N(3s)-4-[[(2s)-1-[[(2s)-1-[[(1s)-1-carboxy-2-hydroxyethyl]amino]-4-methyl-1-oxopentan-2-yl]amino]-5-(diaminomethylideneamino)-1-oxopentan-2-yl]amino]-3-[[2-[[(2s)-2,6-diaminohexanoyl]amino]acetyl]amino]-4-oxobutanoic acidChemical compoundOC[C@@H](C(O)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@H](CC(O)=O)NC(=O)CNC(=O)[C@@H](N)CCCCNMZOFCQQQCNRIBI-VMXHOPILSA-N0.000description4
- FWBHETKCLVMNFS-UHFFFAOYSA-N4',6-Diamino-2-phenylindolChemical compoundC1=CC(C(=N)N)=CC=C1C1=CC2=CC=C(C(N)=N)C=C2N1FWBHETKCLVMNFS-UHFFFAOYSA-N0.000description4
- CSCPPACGZOOCGX-UHFFFAOYSA-NAcetoneChemical compoundCC(C)=OCSCPPACGZOOCGX-UHFFFAOYSA-N0.000description4
- 206010061218InflammationDiseases0.000description4
- 238000003917TEM imageMethods0.000description4
- 102000004142TrypsinHuman genes0.000description4
- 108090000631TrypsinProteins0.000description4
- 238000010609cell counting kit-8 assayMethods0.000description4
- 230000001186cumulative effectEffects0.000description4
- 230000003247decreasing effectEffects0.000description4
- 238000001514detection methodMethods0.000description4
- 238000000338in vitroMethods0.000description4
- 230000004054inflammatory processEffects0.000description4
- 238000011835investigationMethods0.000description4
- 238000011068loading methodMethods0.000description4
- 238000005259measurementMethods0.000description4
- 210000001747pupilAnatomy0.000description4
- 238000004445quantitative analysisMethods0.000description4
- 238000013268sustained releaseMethods0.000description4
- 239000012730sustained-release formSubstances0.000description4
- 238000004627transmission electron microscopyMethods0.000description4
- 239000012588trypsinSubstances0.000description4
- WEVYAHXRMPXWCK-UHFFFAOYSA-NAcetonitrileChemical compoundCC#NWEVYAHXRMPXWCK-UHFFFAOYSA-N0.000description3
- 208000006069Corneal OpacityDiseases0.000description3
- 206010013774Dry eyeDiseases0.000description3
- 238000002835absorbanceMethods0.000description3
- BQRGNLJZBFXNCZ-UHFFFAOYSA-Ncalcein amChemical compoundO1C(=O)C2=CC=CC=C2C21C1=CC(CN(CC(=O)OCOC(C)=O)CC(=O)OCOC(C)=O)=C(OC(C)=O)C=C1OC1=C2C=C(CN(CC(=O)OCOC(C)=O)CC(=O)OCOC(=O)C)C(OC(C)=O)=C1BQRGNLJZBFXNCZ-UHFFFAOYSA-N0.000description3
- 231100000269corneal opacityToxicity0.000description3
- 238000005538encapsulationMethods0.000description3
- 208000030533eye diseaseDiseases0.000description3
- 230000006870functionEffects0.000description3
- 238000004128high performance liquid chromatographyMethods0.000description3
- 238000003125immunofluorescent labelingMethods0.000description3
- 238000011534incubationMethods0.000description3
- 230000008595infiltrationEffects0.000description3
- 238000001764infiltrationMethods0.000description3
- 238000002347injectionMethods0.000description3
- 239000007924injectionSubstances0.000description3
- 239000000463materialSubstances0.000description3
- 210000000440neutrophilAnatomy0.000description3
- 229940023490ophthalmic productDrugs0.000description3
- KHIWWQKSHDUIBK-UHFFFAOYSA-Nperiodic acidChemical compoundOI(=O)(=O)=OKHIWWQKSHDUIBK-UHFFFAOYSA-N0.000description3
- 239000013612plasmidSubstances0.000description3
- 229920000515polycarbonatePolymers0.000description3
- 239000011148porous materialSubstances0.000description3
- 230000001681protective effectEffects0.000description3
- 230000000699topical effectEffects0.000description3
- 238000013042tunel stainingMethods0.000description3
- 238000005406washingMethods0.000description3
- 238000001262western blotMethods0.000description3
- QKNYBSVHEMOAJP-UHFFFAOYSA-N2-amino-2-(hydroxymethyl)propane-1,3-diol;hydron;chlorideChemical groupCl.OCC(N)(CO)COQKNYBSVHEMOAJP-UHFFFAOYSA-N0.000description2
- 108091003079Bovine Serum AlbuminProteins0.000description2
- 206010055665Corneal neovascularisationDiseases0.000description2
- 206010016654FibrosisDiseases0.000description2
- WZUVPPKBWHMQCE-UHFFFAOYSA-NHaematoxylinChemical compoundC12=CC(O)=C(O)C=C2CC2(O)C1C1=CC=C(O)C(O)=C1OC2WZUVPPKBWHMQCE-UHFFFAOYSA-N0.000description2
- SIKJAQJRHWYJAI-UHFFFAOYSA-NIndoleChemical compoundC1=CC=C2NC=CC2=C1SIKJAQJRHWYJAI-UHFFFAOYSA-N0.000description2
- 206010052143Ocular discomfortDiseases0.000description2
- 208000022873Ocular diseaseDiseases0.000description2
- 108010087230SincalideProteins0.000description2
- FAPWRFPIFSIZLT-UHFFFAOYSA-MSodium chlorideChemical compound[Na+].[Cl-]FAPWRFPIFSIZLT-UHFFFAOYSA-M0.000description2
- 206010047513Vision blurredDiseases0.000description2
- 230000001154acute effectEffects0.000description2
- 150000001413amino acidsChemical group0.000description2
- 239000003443antiviral agentSubstances0.000description2
- 108010072041arginyl-glycyl-aspartic acidProteins0.000description2
- 230000008901benefitEffects0.000description2
- 230000005540biological transmissionEffects0.000description2
- 239000000872bufferSubstances0.000description2
- 125000002915carbonyl groupChemical group[*:2]C([*:1])=O0.000description2
- 229910017052cobaltInorganic materials0.000description2
- 239000010941cobaltSubstances0.000description2
- GUTLYIVDDKVIGB-UHFFFAOYSA-Ncobalt atomChemical compound[Co]GUTLYIVDDKVIGB-UHFFFAOYSA-N0.000description2
- 210000000795conjunctivaAnatomy0.000description2
- 201000000159corneal neovascularizationDiseases0.000description2
- 238000012258culturingMethods0.000description2
- 230000006378damageEffects0.000description2
- 230000034994deathEffects0.000description2
- 238000000502dialysisMethods0.000description2
- 230000029087digestionEffects0.000description2
- NJDNXYGOVLYJHP-UHFFFAOYSA-Ldisodium;2-(3-oxido-6-oxoxanthen-9-yl)benzoateChemical compound[Na+].[Na+].[O-]C(=O)C1=CC=CC=C1C1=C2C=CC(=O)C=C2OC2=CC([O-])=CC=C21NJDNXYGOVLYJHP-UHFFFAOYSA-L0.000description2
- 238000001962electrophoresisMethods0.000description2
- 230000003203everyday effectEffects0.000description2
- 238000002474experimental methodMethods0.000description2
- 239000012091fetal bovine serumSubstances0.000description2
- 230000004761fibrosisEffects0.000description2
- 238000009472formulationMethods0.000description2
- 238000007490hematoxylin and eosin (H&E) stainingMethods0.000description2
- 238000001727in vivoMethods0.000description2
- 230000001788irregularEffects0.000description2
- 206010023332keratitisDiseases0.000description2
- 230000002934lysing effectEffects0.000description2
- 230000014759maintenance of locationEffects0.000description2
- 230000004879molecular functionEffects0.000description2
- 230000002035prolonged effectEffects0.000description2
- 238000011555rabbit modelMethods0.000description2
- 238000011084recoveryMethods0.000description2
- 210000004378sebocyteAnatomy0.000description2
- IZTQOLKUZKXIRV-YRVFCXMDSA-NsincalideChemical compoundC([C@@H](C(=O)N[C@@H](CCSC)C(=O)NCC(=O)N[C@@H](CC=1C2=CC=CC=C2NC=1)C(=O)N[C@@H](CCSC)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CC=1C=CC=CC=1)C(N)=O)NC(=O)[C@@H](N)CC(O)=O)C1=CC=C(OS(O)(=O)=O)C=C1IZTQOLKUZKXIRV-YRVFCXMDSA-N0.000description2
- 239000006228supernatantSubstances0.000description2
- 238000012360testing methodMethods0.000description2
- 229940126585therapeutic drugDrugs0.000description2
- 210000001519tissueAnatomy0.000description2
- LZDKZFUFMNSQCJ-UHFFFAOYSA-N1,2-diethoxyethaneChemical compoundCCOCCOCCLZDKZFUFMNSQCJ-UHFFFAOYSA-N0.000description1
- TZCPCKNHXULUIY-RGULYWFUSA-N1,2-distearoyl-sn-glycero-3-phosphoserineChemical compoundCCCCCCCCCCCCCCCCCC(=O)OC[C@H](COP(O)(=O)OC[C@H](N)C(O)=O)OC(=O)CCCCCCCCCCCCCCCCCTZCPCKNHXULUIY-RGULYWFUSA-N0.000description1
- 201000004569BlindnessDiseases0.000description1
- 238000011740C57BL/6 mouseMethods0.000description1
- 102000008186CollagenHuman genes0.000description1
- 108010035532CollagenProteins0.000description1
- 206010010741ConjunctivitisDiseases0.000description1
- 102000004127CytokinesHuman genes0.000description1
- 108090000695CytokinesProteins0.000description1
- 102400001368Epidermal growth factorHuman genes0.000description1
- 101800003838Epidermal growth factorProteins0.000description1
- 241000283074Equus asinusSpecies0.000description1
- 206010015548EuthanasiaDiseases0.000description1
- 102000020897ForminsHuman genes0.000description1
- 108091022623ForminsProteins0.000description1
- 238000005033Fourier transform infrared spectroscopyMethods0.000description1
- 102100031181Glyceraldehyde-3-phosphate dehydrogenaseHuman genes0.000description1
- JZNWSCPGTDBMEW-UHFFFAOYSA-NGlycerophosphorylethanolaminNatural productsNCCOP(O)(=O)OCC(O)COJZNWSCPGTDBMEW-UHFFFAOYSA-N0.000description1
- ZWZWYGMENQVNFU-UHFFFAOYSA-NGlycerophosphorylserinNatural productsOC(=O)C(N)COP(O)(=O)OCC(O)COZWZWYGMENQVNFU-UHFFFAOYSA-N0.000description1
- 108010043121Green Fluorescent ProteinsProteins0.000description1
- 101500025419Homo sapiens Epidermal growth factorProteins0.000description1
- 238000012404In vitro experimentMethods0.000description1
- ZDXPYRJPNDTMRX-VKHMYHEASA-NL-glutamineChemical compoundOC(=O)[C@@H](N)CCC(N)=OZDXPYRJPNDTMRX-VKHMYHEASA-N0.000description1
- 229930182816L-glutamineNatural products0.000description1
- GSDSWSVVBLHKDQ-JTQLQIEISA-NLevofloxacinChemical compoundC([C@@H](N1C2=C(C(C(C(O)=O)=C1)=O)C=C1F)C)OC2=C1N1CCN(C)CC1GSDSWSVVBLHKDQ-JTQLQIEISA-N0.000description1
- 206010027146MelanodermaDiseases0.000description1
- 102000010750MetalloproteinsHuman genes0.000description1
- 108010063312MetalloproteinsProteins0.000description1
- 241001465754MetazoaSpecies0.000description1
- 241000283977OryctolagusSpecies0.000description1
- 108091005804PeptidasesProteins0.000description1
- 102000004160Phosphoric Monoester HydrolasesHuman genes0.000description1
- 108090000608Phosphoric Monoester HydrolasesProteins0.000description1
- 239000004365ProteaseSubstances0.000description1
- 102100037486Reverse transcriptase/ribonuclease HHuman genes0.000description1
- 229930006000SucroseNatural products0.000description1
- CZMRCDWAGMRECN-UGDNZRGBSA-NSucroseChemical compoundO[C@H]1[C@H](O)[C@@H](CO)O[C@@]1(CO)O[C@@H]1[C@H](O)[C@@H](O)[C@H](O)[C@@H](CO)O1CZMRCDWAGMRECN-UGDNZRGBSA-N0.000description1
- 229920004890Triton X-100Polymers0.000description1
- 239000013504Triton X-100Substances0.000description1
- 206010046851UveitisDiseases0.000description1
- 206010047571Visual impairmentDiseases0.000description1
- 208000027418Wounds and injuryDiseases0.000description1
- ATBOMIWRCZXYSZ-XZBBILGWSA-N[1-[2,3-dihydroxypropoxy(hydroxy)phosphoryl]oxy-3-hexadecanoyloxypropan-2-yl] (9e,12e)-octadeca-9,12-dienoateChemical compoundCCCCCCCCCCCCCCCC(=O)OCC(COP(O)(=O)OCC(O)CO)OC(=O)CCCCCCC\C=C\C\C=C\CCCCCATBOMIWRCZXYSZ-XZBBILGWSA-N0.000description1
- 238000010521absorption reactionMethods0.000description1
- VYTBPJNGNGMRFH-UHFFFAOYSA-Nacetic acid;azaneChemical compoundN.N.CC(O)=O.CC(O)=O.CC(O)=O.CC(O)=OVYTBPJNGNGMRFH-UHFFFAOYSA-N0.000description1
- 229960004150aciclovirDrugs0.000description1
- MKUXAQIIEYXACX-UHFFFAOYSA-NaciclovirChemical compoundN1C(N)=NC(=O)C2=C1N(COCCO)C=N2MKUXAQIIEYXACX-UHFFFAOYSA-N0.000description1
- 239000003732agents acting on the eyeSubstances0.000description1
- AWUCVROLDVIAJX-UHFFFAOYSA-Nalpha-glycerophosphateNatural productsOCC(O)COP(O)(O)=OAWUCVROLDVIAJX-UHFFFAOYSA-N0.000description1
- 239000007864aqueous solutionSubstances0.000description1
- 238000003556assayMethods0.000description1
- 210000004082barrier epithelial cellAnatomy0.000description1
- 230000009286beneficial effectEffects0.000description1
- UHOVQNZJYSORNB-UHFFFAOYSA-NbenzeneSubstancesC1=CC=CC=C1UHOVQNZJYSORNB-UHFFFAOYSA-N0.000description1
- 102000023732binding proteinsHuman genes0.000description1
- 108091008324binding proteinsProteins0.000description1
- 230000003592biomimetic effectEffects0.000description1
- 230000004397blinkingEffects0.000description1
- 210000004204blood vesselAnatomy0.000description1
- 239000007975buffered salineSubstances0.000description1
- 230000003197catalytic effectEffects0.000description1
- 230000021164cell adhesionEffects0.000description1
- 238000004113cell cultureMethods0.000description1
- 230000003833cell viabilityEffects0.000description1
- 238000005119centrifugationMethods0.000description1
- 230000008859changeEffects0.000description1
- 230000001684chronic effectEffects0.000description1
- 229940121657clinical drugDrugs0.000description1
- 229920001436collagenPolymers0.000description1
- 238000010276constructionMethods0.000description1
- 239000011258core-shell materialSubstances0.000description1
- 210000003683corneal stromaAnatomy0.000description1
- 230000004453corneal transparencyEffects0.000description1
- 231100000135cytotoxicityToxicity0.000description1
- 230000003013cytotoxicityEffects0.000description1
- 230000002354daily effectEffects0.000description1
- 230000008021depositionEffects0.000description1
- ZGSPNIOCEDOHGS-UHFFFAOYSA-Ldisodium [3-[2,3-di(octadeca-9,12-dienoyloxy)propoxy-oxidophosphoryl]oxy-2-hydroxypropyl] 2,3-di(octadeca-9,12-dienoyloxy)propyl phosphateChemical compound[Na+].[Na+].CCCCCC=CCC=CCCCCCCCC(=O)OCC(OC(=O)CCCCCCCC=CCC=CCCCCC)COP([O-])(=O)OCC(O)COP([O-])(=O)OCC(OC(=O)CCCCCCCC=CCC=CCCCCC)COC(=O)CCCCCCCC=CCC=CCCCCCZGSPNIOCEDOHGS-UHFFFAOYSA-L0.000description1
- SZGZILRQIYNODJ-UHFFFAOYSA-Ldisodium;7,12-dihydroquinoxalino[3,2-b]phenazine-2,9-disulfonateChemical compound[Na+].[Na+].[O-]S(=O)(=O)C1=CC=C2N=C(C=C3C(NC4=CC=C(C=C4N3)S(=O)(=O)[O-])=C3)C3=NC2=C1SZGZILRQIYNODJ-UHFFFAOYSA-L0.000description1
- 239000012153distilled waterSubstances0.000description1
- 239000002552dosage formSubstances0.000description1
- 230000003828downregulationEffects0.000description1
- 238000001647drug administrationMethods0.000description1
- 210000003038endotheliumAnatomy0.000description1
- 238000005516engineering processMethods0.000description1
- 230000002708enhancing effectEffects0.000description1
- YQGOJNYOYNNSMM-UHFFFAOYSA-NeosinChemical compound[Na+].OC(=O)C1=CC=CC=C1C1=C2C=C(Br)C(=O)C(Br)=C2OC2=C(Br)C(O)=C(Br)C=C21YQGOJNYOYNNSMM-UHFFFAOYSA-N0.000description1
- 229940116977epidermal growth factorDrugs0.000description1
- 230000004890epithelial barrier functionEffects0.000description1
- GTSMOYLSFUBTMV-UHFFFAOYSA-Nethidium homodimerChemical compound[H+].[H+].[Cl-].[Cl-].[Cl-].[Cl-].C12=CC(N)=CC=C2C2=CC=C(N)C=C2C(C)=[N+]1CCCNCCNCCC[N+](C1=CC(N)=CC=C1C1=CC=C(N)C=C11)=C1C1=CC=CC=C1GTSMOYLSFUBTMV-UHFFFAOYSA-N0.000description1
- DEFVIWRASFVYLL-UHFFFAOYSA-Nethylene glycol bis(2-aminoethyl)tetraacetic acidChemical compoundOC(=O)CN(CC(O)=O)CCOCCOCCN(CC(O)=O)CC(O)=ODEFVIWRASFVYLL-UHFFFAOYSA-N0.000description1
- 238000001125extrusionMethods0.000description1
- 210000000744eyelidAnatomy0.000description1
- HJUFTIJOISQSKQ-UHFFFAOYSA-NfenoxycarbChemical compoundC1=CC(OCCNC(=O)OCC)=CC=C1OC1=CC=CC=C1HJUFTIJOISQSKQ-UHFFFAOYSA-N0.000description1
- 239000007850fluorescent dyeSubstances0.000description1
- 238000001215fluorescent labellingMethods0.000description1
- 229960001048fluorometholoneDrugs0.000description1
- FAOZLTXFLGPHNG-KNAQIMQKSA-NfluorometholoneChemical compoundC([C@@]12C)=CC(=O)C=C1[C@@H](C)C[C@@H]1[C@]2(F)[C@@H](O)C[C@]2(C)[C@@](O)(C(C)=O)CC[C@H]21FAOZLTXFLGPHNG-KNAQIMQKSA-N0.000description1
- 229960002963ganciclovirDrugs0.000description1
- IRSCQMHQWWYFCW-UHFFFAOYSA-NganciclovirChemical compoundO=C1NC(N)=NC2=C1N=CN2COC(CO)COIRSCQMHQWWYFCW-UHFFFAOYSA-N0.000description1
- 238000010353genetic engineeringMethods0.000description1
- 210000004907glandAnatomy0.000description1
- 108020004445glyceraldehyde-3-phosphate dehydrogenaseProteins0.000description1
- 230000036732histological changeEffects0.000description1
- 238000010562histological examinationMethods0.000description1
- 229940116978human epidermal growth factorDrugs0.000description1
- 230000002209hydrophobic effectEffects0.000description1
- 230000006872improvementEffects0.000description1
- 238000011065in-situ storageMethods0.000description1
- PZOUSPYUWWUPPK-UHFFFAOYSA-NindoleNatural productsCC1=CC=CC2=C1C=CN2PZOUSPYUWWUPPK-UHFFFAOYSA-N0.000description1
- RKJUIXBNRJVNHR-UHFFFAOYSA-NindolenineNatural productsC1=CC=C2CC=NC2=C1RKJUIXBNRJVNHR-UHFFFAOYSA-N0.000description1
- 230000006698inductionEffects0.000description1
- 208000014674injuryDiseases0.000description1
- 230000003993interactionEffects0.000description1
- 230000007794irritationEffects0.000description1
- 150000002596lactonesChemical class0.000description1
- 229960003376levofloxacinDrugs0.000description1
- 230000007774longtermEffects0.000description1
- 239000011159matrix materialSubstances0.000description1
- 230000001404mediated effectEffects0.000description1
- 239000000693micelleSubstances0.000description1
- 238000012986modificationMethods0.000description1
- 230000004048modificationEffects0.000description1
- 238000010172mouse modelMethods0.000description1
- 210000003205muscleAnatomy0.000description1
- NCXMLFZGDNKEPB-FFPOYIOWSA-NnatamycinChemical compoundO[C@H]1[C@@H](N)[C@H](O)[C@@H](C)O[C@H]1O[C@H]1/C=C/C=C/C=C/C=C/C[C@@H](C)OC(=O)/C=C/[C@H]2O[C@@H]2C[C@H](O)C[C@](O)(C[C@H](O)[C@H]2C(O)=O)O[C@H]2C1NCXMLFZGDNKEPB-FFPOYIOWSA-N0.000description1
- 229960003255natamycinDrugs0.000description1
- 235000010298natamycinNutrition0.000description1
- 239000004311natamycinSubstances0.000description1
- GVUGOAYIVIDWIO-UFWWTJHBSA-NnepiderminChemical compoundC([C@@H](C(=O)N[C@@H]([C@@H](C)CC)C(=O)NCC(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CS)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CC=1C=CC(O)=CC=1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CC=1C2=CC=CC=C2NC=1)C(=O)N[C@@H](CC=1C2=CC=CC=C2NC=1)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCCNC(N)=N)C(O)=O)NC(=O)CNC(=O)[C@@H](NC(=O)[C@@H](NC(=O)[C@H](CS)NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CS)NC(=O)[C@H](C)NC(=O)[C@H](CC=1C=CC(O)=CC=1)NC(=O)[C@H](CCCCN)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](C)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@@H](NC(=O)[C@H](CC=1C=CC(O)=CC=1)NC(=O)[C@H](CCSC)NC(=O)[C@H](CS)NC(=O)[C@@H](NC(=O)CNC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CC=1NC=NC=1)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CS)NC(=O)[C@H](CC=1C=CC(O)=CC=1)NC(=O)CNC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CC=1NC=NC=1)NC(=O)[C@H](CO)NC(=O)[C@H](CC(C)C)NC(=O)[C@H]1N(CCC1)C(=O)[C@H](CS)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CO)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CO)NC(=O)[C@@H](N)CC(N)=O)C(C)C)[C@@H](C)CC)C(C)C)C(C)C)C1=CC=C(O)C=C1GVUGOAYIVIDWIO-UFWWTJHBSA-N0.000description1
- 239000003960organic solventSubstances0.000description1
- 230000003204osmotic effectEffects0.000description1
- 239000012188paraffin waxSubstances0.000description1
- 239000008188pelletSubstances0.000description1
- VLTRZXGMWDSKGL-UHFFFAOYSA-MperchlorateInorganic materials[O-]Cl(=O)(=O)=OVLTRZXGMWDSKGL-UHFFFAOYSA-M0.000description1
- VLTRZXGMWDSKGL-UHFFFAOYSA-Nperchloric acidChemical compoundOCl(=O)(=O)=OVLTRZXGMWDSKGL-UHFFFAOYSA-N0.000description1
- 239000008363phosphate bufferSubstances0.000description1
- 239000008055phosphate buffer solutionSubstances0.000description1
- WTJKGGKOPKCXLL-RRHRGVEJSA-NphosphatidylcholineChemical compoundCCCCCCCCCCCCCCCC(=O)OC[C@H](COP([O-])(=O)OCC[N+](C)(C)C)OC(=O)CCCCCCCC=CCCCCCCCCWTJKGGKOPKCXLL-RRHRGVEJSA-N0.000description1
- 150000008104phosphatidylethanolaminesChemical class0.000description1
- 150000003905phosphatidylinositolsChemical class0.000description1
- 238000012805post-processingMethods0.000description1
- 239000002244precipitateSubstances0.000description1
- 230000035755proliferationEffects0.000description1
- 239000011251protective drugSubstances0.000description1
- 150000007660quinolonesChemical class0.000description1
- 230000008439repair processEffects0.000description1
- 230000000717retained effectEffects0.000description1
- 238000000926separation methodMethods0.000description1
- 210000002966serumAnatomy0.000description1
- 239000002356single layerSubstances0.000description1
- 239000011734sodiumSubstances0.000description1
- 239000011780sodium chlorideSubstances0.000description1
- 238000002415sodium dodecyl sulfate polyacrylamide gel electrophoresisMethods0.000description1
- 239000007787solidSubstances0.000description1
- 230000009870specific bindingEffects0.000description1
- 239000005720sucroseSubstances0.000description1
- 239000013589supplementSubstances0.000description1
- 238000004347surface barrierMethods0.000description1
- 238000013269sustained drug releaseMethods0.000description1
- 208000024891symptomDiseases0.000description1
- 231100000901systemic toxic effectToxicity0.000description1
- 230000002123temporal effectEffects0.000description1
- 238000012349terminal deoxynucleotidyl transferase dUTP nick-end labelingMethods0.000description1
- 238000010998test methodMethods0.000description1
- 238000011200topical administrationMethods0.000description1
- 229940126702topical medicationDrugs0.000description1
- 102000040811transporter activityHuman genes0.000description1
- 108091092194transporter activityProteins0.000description1
- 230000035899viabilityEffects0.000description1
- 208000029257vision diseaseDiseases0.000description1
- 230000004393visual impairmentEffects0.000description1
- 238000012800visualizationMethods0.000description1
Images
Landscapes
- Medicinal Preparation (AREA)
Abstract
Description
Translated fromChinese技术领域technical field
本发明涉及生物医药技术领域,特别是涉及用于眼部靶向递药的细胞膜、药物载体、组合物及其制备方法和用途。The invention relates to the technical field of biomedicine, in particular to a cell membrane, a drug carrier, a composition and a preparation method and use thereof for eye-targeted drug delivery.
背景技术Background technique
眼疾是失明的主要原因,在世界范围内患病率日益上升,直接影响着人类的视力和生活质量。目前,估计来自39个国家的2.85亿人正遭受视觉障碍。使用滴眼液或眼内注射局部给药是治疗各种眼部疾病的最容易的治疗途径。但是,常规眼药水的治疗效果在很大程度上受到眼表屏障的限制,例如泪液的循环,持续眨眼,紧密的上皮屏障和泪液的引流,这些因素都会导致眼部药物的生物利用度低,通常会低于5%。同时,一些眼内注射这类的有创治疗手段会引起患者的抗拒以及潜在的副作用和并发症。此外,常规局部药物递送总是需要高频词给药,例如眼药水需要每天点数次,长此以往会导致眼表炎症和暂时性视力模糊。更重要的是,长期频繁使用局部用药可能会引起眼部不适,并损害眼表,从而降低患者的依从性。因此,建立一种新的更安全,更有效并且持续时间更长的眼部药物递送途径是十分必要的。Eye disease is the main cause of blindness, and its prevalence is increasing worldwide, directly affecting human vision and quality of life. Currently, an estimated 285 million people in 39 countries are suffering from visual impairment. Topical administration using eye drops or intraocular injections is the easiest route of treatment for various eye conditions. However, the therapeutic effect of conventional eye drops is largely limited by the ocular surface barrier, such as tear circulation, continuous blinking, tight epithelial barrier, and tear drainage, which all lead to low bioavailability of ocular drugs, Usually it will be less than 5%. At the same time, some invasive treatments such as intraocular injections can cause patient resistance and potential side effects and complications. In addition, conventional topical drug delivery always requires high-frequency word administration, such as eye drops, which need to be dispensed several times a day, which can lead to ocular surface inflammation and temporary blurred vision in the long run. More importantly, long-term frequent use of topical medications may cause ocular discomfort and damage the ocular surface, thereby reducing patient compliance. Therefore, it is necessary to establish a new safer, more effective and longer-lasting ocular drug delivery route.
为了改善眼部药物的吸收和停留时间,目前已开发出多种纳米颗粒,例如脂质体和胶束等用于局部药物的递送。与传统治疗方式相比,纳米颗粒介导的眼部药物递送具有封装疏水性药物、药物持续释放和增强进入细胞的能力等优势。尽管药物的生物利用度和释放曲线有所改善,但由于与普通眼部给药相似的给药途径,其增量仍然受到限制。可替代地,目前已经探索了包含低渗,增粘和原位胶凝聚合物的多种聚合物溶液用于局部眼部药物的递送。尽管通过减少泪液引流可以提高药物的利用率并延长药物停留时间,但这些药物递送系统通常会导致涂层的不均匀,从而造成多个部位的黏附,并可能导致视力模糊。同时,眼表和滴加的聚合物溶液之间存在的渗透压差也可能引发潜在的风险,例如眼部不适,眼表刺激和全身毒性作用。另外,既往的药物递送系统很少直接对眼表提供保护作用。因此,一种温和的既安全又能保护眼表,且还能延长局部药物递送的给药途径对于治疗眼部疾病是非常必要的。To improve the absorption and residence time of ocular drugs, a variety of nanoparticles, such as liposomes and micelles, have been developed for topical drug delivery. Compared with traditional therapeutic modalities, nanoparticle-mediated ocular drug delivery has the advantages of encapsulating hydrophobic drugs, sustained release of drugs, and enhanced ability to enter cells. Although the bioavailability and release profile of the drug have improved, its incrementality is still limited due to the similar route of administration to common ocular administration. Alternatively, various polymer solutions containing hypotonic, viscosifying and in situ gelling polymers have currently been explored for topical ocular drug delivery. Although improved drug availability and longer drug residence time can be achieved by reducing tear drainage, these drug delivery systems often result in uneven coatings, causing adhesions at multiple sites, and can lead to blurred vision. At the same time, the osmotic pressure difference between the ocular surface and the dripped polymer solution may also trigger potential risks, such as ocular discomfort, ocular surface irritation, and systemic toxic effects. In addition, few previous drug delivery systems provided protection directly to the ocular surface. Therefore, a mild drug administration route that is safe, protects the ocular surface, and prolongs local drug delivery is very necessary for the treatment of ocular diseases.
发明内容Contents of the invention
鉴于以上所述现有技术的缺点,本发明的目的在于提供用于眼部靶向递药的细胞膜、药物载体、组合物及其制备方法和用途,用于解决现有技术中的问题。In view of the above-mentioned shortcomings of the prior art, the purpose of the present invention is to provide cell membranes, drug carriers, compositions and their preparation methods and uses for ocular targeted drug delivery, so as to solve the problems in the prior art.
为实现上述目的及其他相关目的,本发明是通过以下技术方案获得的。In order to achieve the above purpose and other related purposes, the present invention is achieved through the following technical solutions.
本发明的目的之一在于提供用于眼部靶向递药的细胞膜,所述细胞膜由细胞分离获得,所述细胞为过表达整合素β1的细胞。One of the objectives of the present invention is to provide a cell membrane for ocular targeted drug delivery, the cell membrane is obtained by separating cells, and the cells are cells overexpressing integrin β1.
根据本申请的技术方案,所述细胞为皮脂腺上皮细胞。According to the technical solution of the present application, the cells are sebaceous gland epithelial cells.
根据本申请的技术方案,所述整合素β1的氨基酸序列包括SEQ ID NO:1所示核苷酸序列编码的多肽。According to the technical solution of the present application, the amino acid sequence of integrin β1 includes the polypeptide encoded by the nucleotide sequence shown in SEQ ID NO:1.
根据本申请的技术方案,所述细胞膜的直径为100~300nm。According to the technical solution of the present application, the diameter of the cell membrane is 100-300 nm.
根据本申请的技术方案,所述细胞膜的ζ电势为-5~-10mV。According to the technical solution of the present application, the zeta potential of the cell membrane is -5~-10mV.
本发明的目的之二在于提供上述所述的细胞膜的制备方法:包括:在载体的基因组中整合编码整合素β1的核苷酸,转染细胞,培养后,分离细胞膜。The second object of the present invention is to provide the above-mentioned cell membrane preparation method: comprising: integrating the nucleotide encoding integrin β1 into the genome of the vector, transfecting the cells, and separating the cell membrane after culturing.
根据本申请的技术方案,所述整合素β1的氨基酸序列包括SEQ ID NO:1所示核苷酸序列编码的多肽。According to the technical solution of the present application, the amino acid sequence of integrin β1 includes the polypeptide encoded by the nucleotide sequence shown in SEQ ID NO:1.
根据本申请的技术方案,分离细胞膜的方法为:将细胞消化并裂解后,分离获得细胞膜。According to the technical solution of the present application, the method for separating the cell membrane is as follows: after digesting and lysing the cells, separate to obtain the cell membrane.
优选地,所述消化采用胰酶进行。较佳的,所述胰酶的添加量为(1~4)mL/107个细胞。Preferably, said digestion is performed with trypsin. Preferably, the amount of trypsin added is (1-4) mL/107 cells.
优选地,所述裂解的方法为:将细胞与低渗裂解液混合,固液分离。较佳的,所述低渗裂解液为Tris-HCl缓冲液。较佳的,所述固液分离包括离心。Preferably, the lysing method is: mixing cells with hypotonic lysate, and separating solid and liquid. Preferably, the hypotonic lysate is Tris-HCl buffer. Preferably, the solid-liquid separation includes centrifugation.
本发明的目的之三在于提供一种药物载体,包括上述所述的用于眼部靶向递药的细胞膜。The third object of the present invention is to provide a drug carrier, including the above-mentioned cell membrane for ocular targeted drug delivery.
根据本申请的技术方案,所述药物载体还包括聚合物。According to the technical solution of the present application, the drug carrier further includes a polymer.
优选地,所述聚合物选自聚乳酸-羟基乙酸共聚物(PLGA)、聚乳酸(PLA)、聚乙醇酸(PGA)、聚己内酯(PCL)、聚醚酰亚胺(PEI)、金纳米颗粒或树枝状大分子中的一种或多种。Preferably, the polymer is selected from the group consisting of polylactic-co-glycolic acid (PLGA), polylactic acid (PLA), polyglycolic acid (PGA), polycaprolactone (PCL), polyetherimide (PEI), One or more of gold nanoparticles or dendrimers.
优选地,所述用于眼部靶向递药的细胞膜和聚合物的质量比为1:(2~6)。Preferably, the mass ratio of the cell membrane and the polymer used for ocular targeted drug delivery is 1:(2-6).
本发明的目的之四在于提供一种药物组合物,包括上述所述的用于眼部靶向递药的细胞膜和药物。The fourth object of the present invention is to provide a pharmaceutical composition, including the above-mentioned cell membrane for ocular targeted drug delivery and drugs.
根据本申请的技术方案,所述药物组合物还包括聚合物。According to the technical solution of the present application, the pharmaceutical composition further includes a polymer.
优选地,所述聚合物选自聚乳酸-羟基乙酸共聚物(PLGA)、聚乳酸(PLA)、聚乙醇酸(PGA)、聚己内酯(PCL)、聚醚酰亚胺(PEI)、金纳米颗粒或树枝状大分子中的一种或多种。Preferably, the polymer is selected from the group consisting of polylactic-co-glycolic acid (PLGA), polylactic acid (PLA), polyglycolic acid (PGA), polycaprolactone (PCL), polyetherimide (PEI), One or more of gold nanoparticles or dendrimers.
根据本申请的技术方案,所述药物包括糖皮质激素药物、喹诺酮类药物、大环内酯类药物或抗病毒药物中的一种或多种。According to the technical solution of the present application, the drugs include one or more of glucocorticoid drugs, quinolone drugs, macrolide drugs or antiviral drugs.
优选地,所述糖皮质激素药物包括地塞米松或氟米龙中的一种或两种。Preferably, the glucocorticoid drug includes one or both of dexamethasone or fluorometholone.
优选地,所述喹诺酮类药物包括左氧氟沙星。Preferably, the quinolones include levofloxacin.
优选地,所述大环内酯类药物包括纳他霉素。Preferably, the macrolide drug includes natamycin.
优选地,所述抗病毒药物包括阿昔洛韦和更昔洛韦中的一种或两种。Preferably, the antiviral drug includes one or both of aciclovir and ganciclovir.
本发明的目的之五在于提供上述所述的药物组合物的制备方法,包括如下步骤:The fifth object of the present invention is to provide the preparation method of the above-mentioned pharmaceutical composition, comprising the following steps:
聚合物与药物混合,得到载药聚合物;The polymer is mixed with the drug to obtain a drug-loaded polymer;
用于眼部靶向递药的细胞膜与所述载药聚合物混合,得到所述的组合物。The cell membrane used for ocular targeted drug delivery is mixed with the drug-loaded polymer to obtain the composition.
根据本申请的技术方案,所述混合后还包括后处理。所述后处理为挤压后通过纳米多孔膜。According to the technical solution of the present application, post-processing is also included after the mixing. The post-treatment is passing through a nanoporous membrane after extrusion.
根据本申请的技术方案,所述聚合物与药物的质量比为(0.1~0.5):1。According to the technical solution of the present application, the mass ratio of the polymer to the drug is (0.1-0.5):1.
根据本申请的技术方案,所述用于眼部靶向递药的细胞膜与所述载药聚合物的蛋白质量比为(5~2):1。According to the technical solution of the present application, the protein mass ratio of the cell membrane used for ocular targeted drug delivery to the drug-loaded polymer is (5-2):1.
优选地,所述纳米多孔膜选自聚碳酸酯多孔膜;所述纳米多孔膜的孔径为0.1~0.5μm。Preferably, the nanoporous membrane is selected from polycarbonate porous membranes; the pore size of the nanoporous membrane is 0.1-0.5 μm.
本发明的目的之六在于提供如上述所述的组合物在制备眼部药物中的用途。The sixth object of the present invention is to provide the use of the composition as described above in the preparation of ophthalmic medicines.
根据本申请的技术方案,所述眼部药物用于治疗眼部疾病。According to the technical solution of the present application, the eye medicine is used for treating eye diseases.
优选地,所述眼部疾病包括:葡萄膜炎、干眼、结膜炎和角膜炎。Preferably, the ocular diseases include: uveitis, dry eye, conjunctivitis and keratitis.
更优选地,所述眼部疾病为干眼症。More preferably, the eye disease is dry eye.
优选地,所述药物组合物的剂型为滴剂。Preferably, the dosage form of the pharmaceutical composition is drops.
优选地,当药物为地塞米松时,所述组合物的用量为一天一次,所述组合物的剂量为一次10~50μL,所述组合物的浓度为10mg/mL。Preferably, when the drug is dexamethasone, the dosage of the composition is once a day, the dose of the composition is 10-50 μL once a time, and the concentration of the composition is 10 mg/mL.
本申请通过简单地滴入仿生聚合物在眼表上形成了持久的保护性载药纳米涂层,以达到延长药物停留时间及增强药物生物利用率的目的。The present application forms a durable protective drug-loaded nano-coating on the ocular surface by simply dropping the biomimetic polymer, so as to achieve the purpose of prolonging the residence time of the drug and enhancing the bioavailability of the drug.
与现有技术相比,本发明具有以下有益效果:Compared with the prior art, the present invention has the following beneficial effects:
1)本发明的过表达整合素β1的皮脂腺上皮细胞的细胞膜(SMs)包裹聚合物(NPs)经局部给药后,聚合物表面的整合素β1会与角膜和结膜上皮上表达的纤连蛋白的Arg-Gly-Asp(RGD)序列之间发生特异性结合,从而形成持久的纳米涂层。1) After the cell membrane (SMs) wrapping polymer (NPs) of sebaceous epithelial cells overexpressing integrin β1 of the present invention is administered locally, the integrin β1 on the surface of the polymer will bind to the fibronectin expressed on the corneal and conjunctival epithelium Specific binding occurs between the Arg-Gly-Asp (RGD) sequences of the nanoparticle to form a durable nanocoating.
2)本发明的药物载体显示出良好的舒适度和高的眼表相容性。在体外试验中,NPs表面包载SMs形成的药物载体(SNPs),对兔角膜上皮细胞(RCEC)和结膜上皮细胞(RCjECs)均没有明显的细胞毒性,且对这两种细胞有着强黏附能力。2) The drug carrier of the present invention exhibits good comfort and high ocular surface compatibility. In vitro experiments, the drug carriers (SNPs) formed by SMs on the surface of NPs have no obvious cytotoxicity to rabbit corneal epithelial cells (RCECs) and conjunctival epithelial cells (RCjECs), and have strong adhesion to these two types of cells. .
3)本发明的药物载体(SNPs)负载地塞米松构建的眼部药物(SNPs-Dex),在48h内均能持续释放,并且在两种缓冲盐水(PBS)和透明质酸钠(SH)中持续1个月保持稳定。3) The ophthalmic drug (SNPs-Dex) constructed by the drug carrier (SNPs) of the present invention loaded with dexamethasone can be sustained release within 48h, and can be released in two kinds of buffered saline (PBS) and sodium hyaluronate (SH) remained stable for 1 month.
4)在体内实验中,药物载体(SNPs)可以在眼角膜上形成稳定的纳米涂层,可维持泪膜的脂质层和稳定性。形成的纳米涂层可以在眼表保留长达24h,并在48h内产生足够的局部药物浓度。4) In vivo experiments, the drug carrier (SNPs) can form a stable nanocoating on the cornea, which can maintain the lipid layer and stability of the tear film. The formed nanocoating can remain on the ocular surface for up to 24 h and generate sufficient local drug concentration within 48 h.
5)本发明在干眼症(DED)的小鼠和兔子模型中进行了治疗效果实验,发现,与每天3次滴用临床地塞米松眼药水相比,每隔一天滴一次眼部药物(SNPs-Dex)形成的纳米涂层即可表现出更好的角膜上皮恢复率,减轻角膜混浊度,降低眼表炎性以及恢复泪液分泌功能。5) The present invention has carried out therapeutic effect experiment in the mouse of dry eye disease (DED) and rabbit model, finds, compared with dripping clinical dexamethasone eye drops 3 times every day, every other day drops eye medicine once ( The nanocoating formed by SNPs-Dex) can show better corneal epithelial recovery rate, reduce corneal opacity, reduce ocular surface inflammation and restore tear secretion function.
附图说明Description of drawings
图1a显示为本发明中载药细胞膜、聚合物和药物形成的眼部用药以及眼部用药递送原理示意图。Figure 1a shows a schematic diagram of the ocular drug formed by the drug-loaded cell membrane, polymer and drug and the delivery principle of the ocular drug in the present invention.
图1b显示为本发明中实施例2得到的细胞膜SMs的蛋白质组成图。Figure 1b shows the protein composition diagram of cell membrane SMs obtained in Example 2 of the present invention.
图1c显示为本发明中实施例2得到的细胞膜SMs的脂质组成图。Figure 1c shows the lipid composition diagram of cell membrane SMs obtained in Example 2 of the present invention.
图1d显示为本发明中实施例4得到的药物载体SNPs的TEM图。Figure 1d shows the TEM image of the drug carrier SNPs obtained in Example 4 of the present invention.
图1e显示为本发明中实施例4得到的药物载体SNPs经DLS测量后得到的粒径分布图。Figure 1e shows the particle size distribution of the drug carrier SNPs obtained in Example 4 of the present invention after DLS measurement.
图1f为显示为本发明中实施例4得到的药物载体SNPs经DLS测量后得到的Zeta电位图。Figure 1f shows the Zeta potential diagram obtained after DLS measurement of the drug carrier SNPs obtained in Example 4 of the present invention.
图1g显示为本发明中实施例4得到的药物载体SNPs的电泳图。Figure 1g shows the electrophoresis of the drug carrier SNPs obtained in Example 4 of the present invention.
图1h显示为本发明中实施例4得到的眼部药物SNPs-Dex的TEM图。Figure 1h shows the TEM image of the eye drug SNPs-Dex obtained in Example 4 of the present invention.
图1i显示为本发明中实施例5中眼部药物SNPs-Dex在PBS和SH中的稳定性考察结果图。Fig. 1i is a graph showing the results of the stability investigation of the eye drug SNPs-Dex in PBS and SH in Example 5 of the present invention.
图1j显示为本发明中实施例6中眼部药物SNPs-Dex在PBS中累积释放率图。Fig. 1j is a graph showing the cumulative release rate of eye drug SNPs-Dex in PBS in Example 6 of the present invention.
图2a显示为本发明中实施例7中不同浓度的SNPs分别与原代兔角膜上皮细胞(RCECs)和兔结膜上皮细胞(RCjECs)共同孵育后的死活染色结果图。Fig. 2a shows the results of life-and-death staining after co-incubating primary rabbit corneal epithelial cells (RCECs) and rabbit conjunctival epithelial cells (RCjECs) with different concentrations of SNPs in Example 7 of the present invention.
图2b显示为本发明中实施例7的SNPs分别与RCECs和RCjECs共同孵育后的共聚焦图。Figure 2b shows the confocal images of the SNPs of Example 7 of the present invention after co-incubating with RCECs and RCjECs respectively.
图2c显示为本发明中实施例7的SNPs与RCECs共同孵育后的流式细胞仪定量分析图。Fig. 2c shows the flow cytometry quantitative analysis graph after co-incubating the SNPs of Example 7 and RCECs in the present invention.
图2d显示为本发明中实施例7的SNPs与RCjECs共同孵育后的流式细胞仪定量分析图。Fig. 2d shows the flow cytometry quantitative analysis graph after co-incubating the SNPs of Example 7 and RCjECs in the present invention.
图2e显示为本发明中实施例7的SNPs和NPs给药小鼠后在不同时间点的共聚焦图。Fig. 2e shows the confocal images at different time points after administration of the SNPs and NPs of Example 7 of the present invention to mice.
图2f显示为本发明中实施例7的SNPs和NPs给药小鼠后,角膜冷冻切片上的荧光信号计数图。Fig. 2f is a graph showing the fluorescence signal counts on the cornea cryosections after administration of the SNPs and NPs of Example 7 of the present invention to mice.
图2g显示为本发明中实施例7的SNP-Dex给药兔子后,不同时间点眼泪中的Dex的残留率图。Fig. 2g is a graph showing the residual rate of Dex in tears at different time points after the SNP-Dex of Example 7 of the present invention was administered to rabbits.
图3a为显示为本发明中实施例7的SNP给药兔子后,泪膜脂质层的典型LipidView图。Fig. 3a is a typical LipidView image showing the tear film lipid layer after administration of the SNP of Example 7 in the present invention to rabbits.
图3b显示为本发明中实施例7的SNP给药兔子后,泪膜脂质层厚度图。Fig. 3b is a graph showing the thickness of the tear film lipid layer after the SNP of Example 7 of the present invention is administered to rabbits.
图3c显示为本发明中实施例7的SNP给药兔子后,泪膜的破裂时间图。Fig. 3c is a diagram showing the breakup time of the tear film after administration of the SNP of Example 7 in the present invention to rabbits.
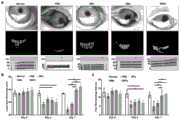
图4a显示为本发明中实施例8的各组小鼠眼球的明场图像。Fig. 4a shows bright-field images of mouse eyeballs in each group in Example 8 of the present invention.
图4b显示为本发明中实施例8的各组小鼠眼球经荧光素染色图。Figure 4b shows the fluorescein staining of eyeballs of mice in each group in Example 8 of the present invention.
图4c显示为本发明中实施例8的各组小鼠眼球的角膜的不透明度分数图。Fig. 4c is a graph showing the opacity fractions of the corneas of the mouse eyes of each group in Example 8 of the present invention.
图4d显示为本发明中实施例8的各组小鼠眼球的角膜的荧光素染色分数图。Fig. 4d is a graph showing the fluorescein staining fractions of the corneas of the mouse eyes of each group in Example 8 of the present invention.
图5a显示为本发明中实施例8的各组小鼠角膜切片经H&E和Masson染色图。Figure 5a shows H&E and Masson staining images of mouse corneal sections of each group in Example 8 of the present invention.
图5b显示为本发明中实施例8的各组小鼠角膜切片的免疫荧光染色图。Figure 5b shows the immunofluorescence staining images of mouse corneal sections of each group in Example 8 of the present invention.
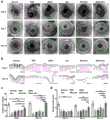
图6a显示为本发明中实施例9的各组兔角膜的明场图。Fig. 6a shows the bright field images of rabbit corneas of each group in Example 9 of the present invention.
图6b显示为本发明中实施例9的各组兔结膜经高碘酸席夫染色后的切片图。Fig. 6b shows the sections of the rabbit conjunctiva of each group in Example 9 of the present invention stained with periodic acid Schiff.
图6c显示为本发明中实施例9的各组兔结膜切片每个视野在显微镜下的杯状细胞数图。Fig. 6c shows the number of goblet cells under the microscope in each field of view of each group of rabbit conjunctival slices in Example 9 of the present invention.
图6d显示为本发明中实施例9的各组兔Schirmer检测结果图。Fig. 6d is a graph showing the Schirmer detection results of each group of rabbits in Example 9 of the present invention.
图7a显示为本发明中实施例9的各组兔在治疗第7天和第14天的角膜经H&E染色图。Figure 7a shows the H&E staining images of the corneas of rabbits in each group in Example 9 of the present invention on the 7th and 14th day of treatment.
图7b显示为本发明中实施例9的各组兔经TUNEL染色后上皮细胞凋亡的水平图。Figure 7b shows the levels of epithelial cell apoptosis after TUNEL staining in each group of rabbits in Example 9 of the present invention.
图8显示为本发明中实施例4得到的眼部药物SNPs-Dex的傅立叶变换红外光谱(FT-IR)光谱图。Fig. 8 shows the Fourier Transform Infrared Spectrum (FT-IR) spectrogram of the eye drug SNPs-Dex obtained in Example 4 of the present invention.
图9显示为本发明中实施例1经载体转染体系前后的细胞中整合素β1表达的Western blot图。Fig. 9 is a Western blot diagram showing the expression of integrin β1 in cells before and after vector transfection system in Example 1 of the present invention.
具体实施方式Detailed ways
以下由特定的具体实施例说明本发明的实施方式,熟悉此技术的人士可由本说明书所揭露的内容轻易地了解本发明的其他优点及功效。The implementation of the present invention will be illustrated by specific specific examples below, and those skilled in the art can easily understand other advantages and effects of the present invention from the contents disclosed in this specification.
在进一步描述本发明具体实施方式之前,应理解,本发明的保护范围不局限于下述特定的具体实施方案;还应当理解,本发明实施例中使用的术语是为了描述特定的具体实施方案,而不是为了限制本发明的保护范围。下列实施例中未注明具体条件的试验方法,通常按照常规条件,或者按照各制造商所建议的条件。Before further describing the specific embodiments of the present invention, it should be understood that the protection scope of the present invention is not limited to the following specific specific embodiments; it should also be understood that the terms used in the examples of the present invention are to describe specific specific embodiments, It is not intended to limit the protection scope of the present invention. The test methods for which specific conditions are not indicated in the following examples are usually in accordance with conventional conditions, or in accordance with the conditions suggested by each manufacturer.
当实施例给出数值范围时,应理解,除非本发明另有说明,每个数值范围的两个端点以及两个端点之间任何一个数值均可选用。除非另外定义,本发明中使用的所有技术和科学术语与本技术领域技术人员通常理解的意义相同。除实施例中使用的具体方法、设备、材料外,根据本技术领域的技术人员对现有技术的掌握及本发明的记载,还可以使用与本发明实施例中所述的方法、设备、材料相似或等同的现有技术的任何方法、设备和材料来实现本发明。When the examples give numerical ranges, it should be understood that, unless otherwise stated in the present invention, the two endpoints of each numerical range and any value between the two endpoints can be selected. Unless defined otherwise, all technical and scientific terms used herein have the same meaning as commonly understood by one of ordinary skill in the art. In addition to the specific methods, equipment, and materials used in the embodiments, according to those skilled in the art's grasp of the prior art and the description of the present invention, the methods, equipment, and materials described in the embodiments of the present invention can also be used Any methods, apparatus and materials of the prior art similar or equivalent to the practice of the present invention.
下面通过实施例对本申请的发明予以进一步说明,但并不因此而限制本申请的范围。The invention of the present application will be further described by the following examples, but the scope of the present application will not be limited thereby.
图1a为本申请的细胞膜、聚合物和药物形成的眼部用药以及眼部用药递送原理示意图。首先,采用基因工程编辑皮脂腺细胞使其过表达整合素β1,然后得到用于眼部靶向递药的细胞膜;聚合物负载药物后形成载药聚合物,然后用于眼部靶向递药的细胞膜包裹在载药聚合物的表面,得到眼部药物。眼部用药物涂在眼表上,其通过表面的整合素β1选择性地结合到眼表上皮中纤连蛋白的Arg-Gly-Asp(RGD)序列上,从而稳定地在角膜和结膜上皮形成由一层薄的、具有粘性的载有治疗药物的纳米膜,来为保护眼表和延长局部药物递送提供一个通用的药物递送平台。Figure 1a is a schematic diagram of the ocular drug formed by the cell membrane, polymer and drug of the present application and the delivery principle of the ocular drug. First, the sebocytes were edited by genetic engineering to overexpress integrin β1, and then the cell membrane for eye-targeted drug delivery was obtained; the drug-loaded polymer was formed after the polymer was loaded with drugs, and then used for eye-targeted drug delivery. The cell membrane wraps on the surface of the drug-loaded polymer to obtain the ocular drug. Ocular drugs are coated on the ocular surface, which selectively binds to the Arg-Gly-Asp (RGD) sequence of fibronectin in the ocular surface epithelium through the integrin β1 on the surface, thereby stably forming in the corneal and conjunctival epithelium A thin, cohesive nanomembrane loaded with therapeutic drugs provides a versatile drug delivery platform for ocular surface protection and prolonged local drug delivery.
本申请下述的具体实施例中,从过表达整合素β1的脂腺上皮细胞分离出的细胞膜,标记为SMs。In the following specific examples of the present application, cell membranes isolated from adipose gland epithelial cells overexpressing integrin β1 are labeled as SMs.
本申请下述的具体实施例中,聚乳酸-羟基乙酸共聚物(PLGA),标记为NPs。聚乳酸-乙醇酸共聚物(Resomer RG 504,MW:38000-54000)。In the following specific examples of the present application, polylactic acid-glycolic acid copolymer (PLGA) is marked as NPs. Polylactic acid-glycolic acid copolymer (Resomer RG 504, MW: 38000-54000).
本申请下述的具体实施例中,磷酸缓冲液,标记为PBS。磷酸缓冲液的配方为:136.89mM NaCl;2.67mM KCl;8.1mM Na2HPO4;1.76mM KH2PO4。In the following specific examples of this application, the phosphate buffer solution is marked as PBS. The formula of the phosphate buffer is: 136.89mM NaCl; 2.67mM KCl; 8.1mM Na2 HPO4 ; 1.76mM KH2 PO4 .
本申请下述的具体实施例中,地塞米松(Dex)(D1756,MW:392.46),标记为Dex。Dex悬浮液由地塞米松溶于PBS得到,浓度为1mg/mL。In the following specific examples of the present application, dexamethasone (Dex) (D1756, MW: 392.46) is marked as Dex. Dex suspension was obtained by dissolving dexamethasone in PBS at a concentration of 1 mg/mL.
本申请下述的具体实施例中,SMs包覆NPs形成的药物载体,标记为SNPs。In the following specific examples of the present application, the drug carrier formed by coating NPs with SMs is labeled as SNPs.
本申请下述的具体实施例中,透明质酸钠,标记为SH。购买于海露公司,浓度为0.1%。In the following specific examples of the present application, sodium hyaluronate is marked as SH. Purchased from Hailu Company, the concentration is 0.1%.
本申请下述的具体实施例中,PLGA负载地塞米松,标记为NPs-Dex,NPs-Dex的浓度为10mg/mL。In the following specific examples of the present application, PLGA is loaded with dexamethasone, labeled as NPs-Dex, and the concentration of NPs-Dex is 10 mg/mL.
本申请下述的具体实施例中,SNPs负载地塞米松,标记为SNPs-Dex,SNPs-Dex的浓度为10mg/mL。In the following specific examples of the present application, SNPs are loaded with dexamethasone, marked as SNPs-Dex, and the concentration of SNPs-Dex is 10 mg/mL.
本申请下述的具体实施例中,SZ95皮脂腺上皮细胞购买于北纳生物科技有限公司。In the following specific examples of this application, SZ95 sebaceous gland epithelial cells were purchased from Beina Biotechnology Co., Ltd.
本申请下述的具体实施例中,脂质体Lipo3000购买于赛默飞世尔科技公司。In the following specific examples of this application, liposome Lipo3000 was purchased from Thermo Fisher Scientific.
实施例1Example 1
本实施例中,进行过表达整合素β1的细胞的构建方法,包括如下:In this embodiment, a method for constructing cells overexpressing integrin β1 is carried out, including the following:
1、构建重组载体1. Construction of recombinant vector
委托纽赫生物科技有限公司将如SEQ ID NO:1所示序列的整合素β1整合于pNHlv-CMV-Mcs-EF1a-Zsgreen-T2A-Puro载体中,得到重组载体。Entrusted Newher Biotechnology Co., Ltd. to integrate integrin β1 with the sequence shown in SEQ ID NO: 1 into the pNHlv-CMV-Mcs-EF1a-Zsgreen-T2A-Puro vector to obtain a recombinant vector.
2、重组载体转染皮脂腺上皮细胞2. Recombinant vector transfection of sebaceous epithelial cells
1)细胞培养:10cm细胞皿中加入2×106个SZ95皮脂腺细胞,37℃CO2培养至70%密度,换新鲜Sebomed培养基。其中Sebomed培养基的配方为:10%胎牛血清(FBS)、5ng/mL人表皮生长因子(EGF),1%L-谷氨酰胺和1mM CaCl2。1) Cell culture: 2×106 SZ95 sebocytes were added to a 10 cm cell dish, cultured in CO2 at 37°C to 70% density, and replaced with fresh Sebomed medium. The formulation of the Sebomed medium is: 10% fetal bovine serum (FBS), 5ng/mL human epidermal growth factor (EGF), 1% L-glutamine and 1mM CaCl2 .
2)转染液制备:在EP管中制备以下两液A液:750μL的Opti MEM和37.5μL的脂质体Lipo3000,B液:750uL的Opti MEM+37.5μL的脂质体P3000和15μg重组载体,吸取B液加入至A液中,轻弹混匀。室温中置15分钟待其充分混匀,得到混合液。2) Preparation of transfection solution: prepare the following two liquids A in EP tube: 750 μL of Opti MEM and 37.5 μL of liposome Lipo3000, B solution: 750 μL of Opti MEM + 37.5 μL of liposome P3000 and 15 μg of recombinant vector , Add liquid B to liquid A, flick to mix. Place it at room temperature for 15 minutes until it is thoroughly mixed to obtain a mixed solution.
3)将上述1.5mL的混合液加入到前述的SZ95皮脂腺上皮细胞中,继续培养约48h,至细胞密度达到约90-100%,得到过表达整合素β1的皮脂腺上皮细胞。3) Add 1.5 mL of the above mixed solution to the aforementioned SZ95 sebaceous epithelial cells, and continue culturing for about 48 hours until the cell density reaches about 90-100%, to obtain sebaceous epithelial cells overexpressing integrin β1.
采用Western blot对皮脂腺上皮细胞经重组质粒转染前后β1整合素的表达进行检测,结果见图9。Western blot was used to detect the expression of β1 integrin in sebaceous gland epithelial cells before and after transfection with the recombinant plasmid, and the results are shown in FIG. 9 .
图9为本实施例的皮脂腺上皮细胞经重组质粒转染后的Western blot图。其中,control代表未加序列的空白载体,GAPDH代表内参蛋白,plasmid代表重组质粒。Fig. 9 is a Western blot diagram of the sebaceous gland epithelial cells transfected with the recombinant plasmid in this example. Among them, control represents a blank vector without sequence addition, GAPDH represents an internal reference protein, and plasmad represents a recombinant plasmid.
从图9可知,转染后,β1整合素的含量是未转染之前的3倍。It can be seen from Figure 9 that after transfection, the content of β1 integrin was 3 times that before non-transfection.
实施例2Example 2
本实施例中,以实施例1得到过表达整合素β1的皮脂腺上皮细胞来制备用于眼部靶向递药的细胞膜,包括如下:In this example, the sebaceous gland epithelial cells overexpressing integrin β1 were obtained in Example 1 to prepare cell membranes for ocular targeted drug delivery, including the following:
采用胰酶消化实施例1得到的皮脂腺上皮细胞,800g离心4min得到沉淀,用PBS洗涤3次,然后用低渗裂解液溶解沉淀,放置在冰上裂解30min;通过手持手持式匀浆器去核(在冰上研磨30次),4℃下以500g离心10min,收集上清液并将沉淀再次悬浮在低渗裂解液中,重复上述步骤两次。其中,胰酶的添加量为2mL/107个细胞。The sebaceous gland epithelial cells obtained in Example 1 were digested with trypsin, centrifuged at 800 g for 4 minutes to obtain a precipitate, washed 3 times with PBS, then dissolved with a hypotonic lysate, placed on ice for 30 minutes, and denucleated by a hand-held homogenizer (grind on
将收集的上清液汇集在一起并在4℃下以18500g离心10min,得到用于眼部靶向递药的细胞膜,标记为SMs。The collected supernatants were pooled together and centrifuged at 18,500 g for 10 min at 4°C to obtain cell membranes for eye-targeted drug delivery, labeled as SMs.
低渗裂解液的成分为:10mM Tris-HCl(pH 7.4)、1mM MgCl2、1mM KCl、25mM蔗糖、0.2mM乙二醇二乙醚二胺四乙酸(EGTA),低渗裂解液中蛋白酶的浓度为1v/v%、磷酸酶的浓度为1v/v%。The composition of the hypotonic lysate is: 10mM Tris-HCl (pH 7.4), 1mM MgCl2 , 1mM KCl, 25mM sucrose, 0.2mM ethylene glycol diethyl ether diamine tetraacetic acid (EGTA), the concentration of protease in the hypotonic lysate The concentration of phosphatase is 1v/v%.
将得到的SMs,采用shotgun法分析其含有的蛋白质组成和脂质组成,结果分别见图1b和图1c所示。The obtained SMs were analyzed by shotgun method for protein composition and lipid composition, and the results are shown in Figure 1b and Figure 1c, respectively.
图1b为本实施例得到的SMs的蛋白质组成图。从图1b可知,按分子功能分,结合蛋白占比为87.12%,催化活性占比为47.15%,分子功能调节剂占比为10.77%,结构分子活性占比为7.11%,转运蛋白活性占比为7.11%、其它占比为12.50%。Fig. 1b is a protein composition diagram of SMs obtained in this example. It can be seen from Figure 1b that, in terms of molecular functions, binding proteins accounted for 87.12%, catalytic activity accounted for 47.15%, molecular function regulators accounted for 10.77%, structural molecular activity accounted for 7.11%, and transporter activity accounted for 7.11%. 7.11%, others accounted for 12.50%.
图1c为本实施例得到的SMs的脂质组成图。从图1c可知,SMs的脂质主要为:磷脂酰胆碱(简记为PC)占比为23.89%,磷脂酰乙醇胺(简记为PE)占比为21.49%,鞘磷脂(简记为SM)占比为19.73%,磷脂酰肌醇(简记为PI)占比为17.31%,磷脂酰丝氨酸(简记为PS)占比为5.85%,磷脂酰甘油(简记为PG)占比为5.43%,心磷脂(简记为CL)占比为3.64%和其他占比为2.66%。这些脂质中,PC、PE和SM是睑脂的重要成分,在维持泪膜的稳定性上发挥着重要作用。Figure 1c is a lipid composition map of SMs obtained in this example. It can be seen from Figure 1c that the lipids of SMs are mainly: phosphatidylcholine (abbreviated as PC) accounting for 23.89%, phosphatidylethanolamine (abbreviated as PE) accounting for 21.49%, sphingomyelin (abbreviated as SM ) accounted for 19.73%, phosphatidylinositol (abbreviated as PI) accounted for 17.31%, phosphatidylserine (abbreviated as PS) accounted for 5.85%, phosphatidylglycerol (abbreviated as PG) accounted for 5.43%, cardiolipin (abbreviated as CL) accounted for 3.64% and others accounted for 2.66%. Among these lipids, PC, PE and SM are important components of meibum and play an important role in maintaining the stability of the tear film.
采用透射电子显微镜(TEM)对SMs进行检测,SMs的直径约为200nm。SMs were detected by transmission electron microscopy (TEM), and the diameter of SMs was about 200 nm.
采用动态光散射(DLS)对SMs进行检测,SMs的ζ电势为-8.5mV。SMs were detected by dynamic light scattering (DLS), and the zeta potential of SMs was -8.5mV.
实施例3Example 3
本实施例中,以PLGA为聚合物,与实施例2的细胞膜制备药物载体,包括如下:In this embodiment, PLGA is used as a polymer to prepare a drug carrier with the cell membrane of Example 2, including the following:
将聚合物NPs与实施例2得到的SMs以2:1的蛋白质量比进行混合,得到混合液,使用微型脂质体挤出仪对上述的混合液进行来回挤压,反复挤压10次,然后通过孔径为0.2μm的聚碳酸酯多孔膜,得到药物载体,药物载体标记为SNPs。The polymer NPs and the SMs obtained in Example 2 were mixed at a protein mass ratio of 2:1 to obtain a mixed solution, and the above-mentioned mixed solution was squeezed back and forth using a micro liposome extruder, and repeatedly extruded 10 times, Then pass through a polycarbonate porous membrane with a pore size of 0.2 μm to obtain a drug carrier, and the drug carrier is marked as SNPs.
检测内容:1)对药物载体SNPs采用透射电子显微镜(TEM)观察形状。2)对药物载体SNPs采用动态光散射(DLS)测量粒径和Zeta电位。3)对药物载体SNPs采用SDS-PAGE进行电泳,来表征SNPs的蛋白组成。Detection content: 1) The shape of the drug carrier SNPs is observed with a transmission electron microscope (TEM). 2) The particle size and Zeta potential of drug carrier SNPs were measured by dynamic light scattering (DLS). 3) SDS-PAGE is used to electrophoresis the drug carrier SNPs to characterize the protein composition of the SNPs.
图1d为本实施例得到的药物载体SNPs的TEM图。从图1d可知,与NPs相比,SNPs的形态显示为球形核-壳结构,在核周围具有单层膜涂层。Figure 1d is a TEM image of the drug carrier SNPs obtained in this example. From Figure 1d, compared with NPs, the morphology of SNPs showed a spherical core–shell structure with a monolayer membrane coating around the core.
图1e为本实施例得到的药物载体SNPs经DLS测量后得到的粒径分布图。从图1e可知,NPs被SMs包被后,直径增加了15nm。Figure 1e is a particle size distribution diagram of the drug carrier SNPs obtained in this example after DLS measurement. It can be seen from Figure 1e that the diameter of NPs increased by 15 nm after being coated with SMs.
图1f为本实施例得到的药物载体SNPs经DLS测量后得到的Zeta电位图。从图1f可知,NPs被SMs包被后,ζ电位降低了7mV。Figure 1f is the Zeta potential diagram obtained after DLS measurement of the drug carrier SNPs obtained in this example. It can be seen from Figure 1f that the zeta potential decreased by 7 mV after NPs were coated with SMs.
图1g为本实施例得到的药物载体SNPs的电泳图。从图1g可知,SNPs上存在目标蛋白整合素β1,证实了SMs含有的整合素β1包被于聚合物NPs的表面。Fig. 1g is the electrophoretic image of the drug carrier SNPs obtained in this example. It can be seen from Figure 1g that the target protein integrin β1 exists on the SNPs, which confirms that the integrin β1 contained in SMs is coated on the surface of polymer NPs.
实施例4Example 4
本实施例中,以地塞米松作为治疗药物,制备眼部药物,包括如下步骤:In the present embodiment, dexamethasone is used as a therapeutic drug to prepare eye medicine, which includes the following steps:
1)将800mg的PLGA溶解在15mL的二氯甲烷中;200mg的地塞米松Dex溶解在15mL的丙酮中。将Dex的丙酮溶液加入到PLGA的二氯甲烷溶液以形成油相。1) 800mg of PLGA was dissolved in 15mL of dichloromethane; 200mg of dexamethasone Dex was dissolved in 15mL of acetone. Dex in acetone was added to PLGA in dichloromethane to form an oil phase.
将上述油相滴加到含有5%PVA的水溶液中,然后用超声波仪(Fisher 500 SonicDismembrator,Fisher Sci.)在冰浴上以60W的恒定功率输出乳化10min。The above oil phase was added dropwise into an aqueous solution containing 5% PVA, and then emulsified with a sonicator (Fisher 500 Sonic Dismembrator, Fisher Sci.) at a constant power output of 60 W for 10 min on an ice bath.
随后,将所得溶液置于旋转蒸发器下直至有机溶剂完全蒸发。通过以15000rpm离心min,使用蒸馏水洗涤两次,得到NPs-Dex。Subsequently, the resulting solution was placed under a rotary evaporator until the organic solvent was completely evaporated. NPs-Dex was obtained by centrifuging at 15000 rpm for min and washing twice with distilled water.
2)将上述的NPs-Dex与实施例2得到的SMs混合,得到混合液,使用微型脂质体挤出仪对上述的混合液进行来回挤压,反复挤压10次,然后通过孔径为0.2μm的聚碳酸酯多孔膜,形成眼部药物,标记为SNPs-Dex。2) Mix the above-mentioned NPs-Dex with the SMs obtained in Example 2 to obtain a mixed solution, use a micro-liposome extruder to squeeze the above-mentioned mixed solution back and forth, repeatedly extrude 10 times, and then pass through a pore size of 0.2 μm polycarbonate porous membranes to form ocular drugs labeled as SNPs-Dex.
检测内容:1)检测NPs-Dex的载药效率(LE)和药物包封率(EE);2)对眼部药物SNPs-Dex采用透射电子显微镜(TEM)观察形状;3)对眼部药物SNPs-Dex采用傅立叶变换红外光谱(FT-IR)进行检测。Test content: 1) Detect the drug loading efficiency (LE) and drug encapsulation efficiency (EE) of NPs-Dex; 2) Observe the shape of the eye drug SNPs-Dex with a transmission electron microscope (TEM); 3) Observe the shape of the eye drug SNPs-Dex SNPs-Dex was detected by Fourier transform infrared spectroscopy (FT-IR).
使用JASCO 6100分光光度计(日本)在525-4000cm-1的范围内。为了计算Dex的加载效率,通过紫外可见分光光度计(DU730,Beckman Coulter)在242nm处测量吸光度。根据预先建立的Dex标准曲线,按以下公式计算载药效率(LE)和药物包封率(EE)。Using a JASCO 6100 spectrophotometer (Japan) in the range of 525-4000 cm−1 . To calculate the loading efficiency of Dex, the absorbance was measured at 242 nm by a UV-Vis spectrophotometer (DU730, Beckman Coulter). According to the pre-established Dex standard curve, the drug loading efficiency (LE) and drug encapsulation efficiency (EE) were calculated according to the following formula.
LE(%)=MDex/MPLGA+MDex×100%LE(%)=MDex /MPLGA +MDex ×100%
EE(%)=MDex/Madded×100%EE(%)=MDex /Madded ×100%
其中MDex是加载到聚合物中的Dex的质量,MPLGA是配方中聚合物的质量,Madded是添加的Dex的质量。where MDex is the mass of Dex loaded into the polymer, MPLGA is the mass of polymer in the formulation, and Madded is the mass of Dex added.
经计算获得,NPs-Dex的负载效率(LE)和包封率(EE)经计算分别为61.0%和30.7%。The calculated loading efficiency (LE) and encapsulation efficiency (EE) of NPs-Dex were calculated to be 61.0% and 30.7%, respectively.
图1h为本实施例得到的眼部药物SNPs-Dex的TEM图。从图1h可知,包裹Dex后,PLGA的直径增加了约50nm;用SMs包被NPs-Dex后,通过TEM进行的形态观察表明,表面有细胞膜包被。Fig. 1h is a TEM image of the eye drug SNPs-Dex obtained in this example. It can be seen from Figure 1h that after wrapping Dex, the diameter of PLGA increased by about 50nm; after wrapping NPs-Dex with SMs, the morphology observation by TEM showed that the surface was coated with cell membrane.
图8为本实施例得到的眼部药物SNPs-Dex的傅立叶变换红外光谱(FT-IR)光谱图。从图可知,PLGA中羰基的特征峰位于1747cm-1,Dex的不饱和六元环内酯的羰基峰位于1664cm-1,证明成功包裹了Dex。Fig. 8 is a Fourier Transform Infrared Spectrum (FT-IR) spectrogram of the eye drug SNPs-Dex obtained in this example. It can be seen from the figure that the characteristic peak of the carbonyl group in PLGA is located at 1747cm-1 , and the carbonyl peak of the unsaturated six-membered ring lactone of Dex is located at 1664cm-1 , which proves that Dex was successfully wrapped.
实施例5Example 5
本实施例中,采用实施例4得到眼部药物SNPs-Dex,进行稳定性考察,包括如下步骤:In this embodiment, the eye drug SNPs-Dex is obtained by using Example 4, and the stability investigation is carried out, including the following steps:
使用Zen 33600 Zetasizer(Malvern)通过DLS测量聚合物的粒径(直径,以纳米为单位)。SNPs-Dex悬浮在1×PBS或透明质酸钠SH中,每种溶液的精确体积储存在4℃。测量3个重复样品,在室温下进行30天。The particle size (diameter in nanometers) of the polymer was measured by DLS using a Zen 33600 Zetasizer (Malvern). SNPs-Dex were suspended in 1× PBS or sodium hyaluronate SH, and precise volumes of each solution were stored at 4°C. Three replicate samples were measured for 30 days at room temperature.
图1i为本实施例中眼部药物SNPs-Dex在PBS和SH中的稳定性考察结果图。从图1i可知,SNPs-Dex在体外放置一个月,SNPs-Dex的粒径也仅略有增加,证实SNPs-Dex是稳定的。Fig. 1i is a graph showing the results of the stability study of the eye drug SNPs-Dex in PBS and SH in this example. It can be seen from Figure 1i that the particle size of SNPs-Dex was only slightly increased after the SNPs-Dex was placed in vitro for one month, confirming that the SNPs-Dex was stable.
实施例6Example 6
本实施例中,采用实施例4得到眼部药物SNPs-Dex,进行释放动力学考察,包括如下步骤:In this example, the eye drug SNPs-Dex was obtained by using Example 4, and the release kinetics was investigated, including the following steps:
分为三组,分别为Dex组、NPs-Dex和SNP-Dex组。将1mL的1mg/mL的Dex悬浮液、10mg/mL的NPs-Dex溶液和10mg/mL的SNPs-Dex溶液添加到一次性透析袋(Slide-A-LyzerMINI Dialysis单位,MWCO:3500Da,Thermo Scientifc)并放置于500mL的PBS中。分别于10min、20min、30min、40min、50min,收集外部药物释放缓冲液并加入等量的PBS。释放的Dex累积量通过HPLC(Agilent 1260Infinity II,美国)定量,并在3个重复样品中测量。使用以下HPLC条件:Diamonsil C18柱(5μm,150×4.6mm)。柱温和吸光度波长分别设置为37℃和240nm。进样体积和流速分别为5μL和1.0mL/min。流动相由乙腈和水以30:70的比例混合而成。Divided into three groups, namely Dex group, NPs-Dex and SNP-Dex group. Add 1 mL of 1 mg/mL Dex suspension, 10 mg/mL NPs-Dex solution, and 10 mg/mL SNPs-Dex solution to a disposable dialysis bag (Slide-A-Lyzer MINI Dialysis unit, MWCO: 3500 Da, Thermo Scientific) And placed in 500mL of PBS. At 10 min, 20 min, 30 min, 40 min, and 50 min, the external drug release buffer was collected and an equal amount of PBS was added. The cumulative amount of released Dex was quantified by HPLC (Agilent 1260 Infinity II, USA) and measured in triplicate samples. The following HPLC conditions were used: Diamonsil C18 column (5 μm, 150×4.6 mm). The column temperature and absorbance wavelength were set to 37 °C and 240 nm, respectively. The injection volume and flow rate were 5 μL and 1.0 mL/min, respectively. The mobile phase was a mixture of acetonitrile and water in a ratio of 30:70.
图1j为本实施例中眼部药物SNPs-Dex在PBS中累积释放率图。从图1j可知,在短短4h的孵育后,Dex组释放了80%的Dex,反映出明显的爆发释放行为;SNPs-Dex组表现出可控的方式,在0.5h、1h、8h和24h内的累积释放率分别为15%,20%,40%和60%。与NPs-Dex组相比,SNPs-Dex组的释放时间更长,可以连续释放负载的药物,并在48h内达到约80%的释放率。Fig. 1j is a graph of the cumulative release rate of the eye drug SNPs-Dex in PBS in this example. It can be seen from Figure 1j that after just 4h of incubation, the Dex group released 80% of Dex, reflecting a clear burst release behavior; the SNPs-Dex group showed a controllable manner, at 0.5h, 1h, 8h and 24h The cumulative release rates within were 15%, 20%, 40% and 60%, respectively. Compared with the NPs-Dex group, the release time of the SNPs-Dex group was longer, the loaded drug could be released continuously, and the release rate reached about 80% within 48h.
药物短时间内快速释放不仅会加快清除速度,甚至还会引起眼表的毒副作用,因此,药物的持续释放对于局部眼用药物的输送尤其重要,本实施例的结果均证实了,整合素β1过表达的SMs的成功制备及其改善包被的聚合物的稳定性和药物缓释的能力。Rapid release of drugs in a short period of time will not only speed up the clearance rate, but may even cause side effects on the ocular surface. Therefore, sustained release of drugs is particularly important for the delivery of local ophthalmic drugs. The results of this example have confirmed that integrin β1 Successful preparation of overexpressed SMs and their ability to improve the stability of coated polymers and sustained drug release.
实施例7Example 7
本实施例中,采用实施例3得到药物载体SNPs,进行细胞生物相容性考察、细胞粘附性考察、对脂质层和泪膜稳定性的保护性考察,包括如下:In this embodiment, the SNPs of the drug carrier were obtained by using Example 3, and the investigation of cell biocompatibility, cell adhesion, and protective investigation of lipid layer and tear film stability were carried out, including the following:
(1)SNPs与细胞的生物相容性考察(1) Biocompatibility study between SNPs and cells
使用CCK-8(Dojindo,日本)和Live/DeadTM(Thermo Fisher Scientific,CA,USA)在原代兔角膜上皮细胞RCECs和兔结膜上皮细胞RCjECs上评估SNPs的生物相容性。The biocompatibility of SNPs was evaluated on primary rabbit corneal epithelial cells RCECs and rabbit conjunctival epithelial cells RCjECs using CCK-8 (Dojindo, Japan) and Live/DeadTM (Thermo Fisher Scientific, CA, USA).
对于CCK-8测定,将细胞以每孔5000个细胞的密度接种到96孔板中,将不同浓度的SNPs(0、2、10μg/mL)添加到培养基中。随后,将板在37℃、90%湿度和5%CO2下孵育。在第0、1、2和3天,通过用100μL PBS轻轻洗涤细胞两次并用含有10μL CCK-8液体的100μL培养基替换培养基,孵育3h后使用酶标仪(ELx800,Bio-Tek,美国)测量450nm处的吸光度。For the CCK-8 assay, cells were seeded into 96-well plates at a density of 5000 cells per well, and different concentrations of SNPs (0, 2, 10 μg/mL) were added to the medium. Subsequently, the plate was incubated at 37 °C, 90% humidity and 5%CO2 . On
使用Live/DeadTM检测进行活力染色,将5×104细胞在24孔板中含有不同浓度SNPs(0、2、10μg/mL)的培养基中培养2天。除去培养基并用PBS润洗后,将细胞在含有乙锭同二聚体2(EthD-2)和钙黄绿素-乙酰氧基甲基酯(CAM)的PBS中于37℃孵育15min,然后用PBS洗涤3次。活细胞用绿色荧光CAM染色,死细胞用红色荧光EthD-2染色。使用荧光显微镜(Olympus BX51;Olympus,Tokyo,Japan)拍照。Live/DeadTM assay was used for viability staining, and 5×104 cells were cultured in medium containing different concentrations of SNPs (0, 2, 10 μg/mL) in a 24-well plate for 2 days. After removing the medium and rinsing with PBS, the cells were incubated in PBS containing ethidium homodimer 2 (EthD-2) and calcein-acetoxymethyl ester (CAM) at 37°C for 15 min, and then washed with PBS Wash 3 times. Live cells were stained with green fluorescent CAM and dead cells were stained with red fluorescent EthD-2. Photographs were taken using a fluorescence microscope (Olympus BX51; Olympus, Tokyo, Japan).
图2a为本实施例不同浓度的SNPs分别与原代兔角膜上皮细胞(RCECs)和兔结膜上皮细胞(RCjECs)共同孵育后的死活染色结果图。Fig. 2a is the results of life and death staining after co-incubating SNPs of different concentrations in this example with primary rabbit corneal epithelial cells (RCECs) and rabbit conjunctival epithelial cells (RCjECs).
从图2a可知,SNPs浓度增加至10μg/mL,死活染色结果显示,细胞活力不受影响。It can be seen from Figure 2a that when the concentration of SNPs was increased to 10 μg/mL, the results of life and death staining showed that the cell viability was not affected.
使用CCK-8测定法未检测到RCECs和RCjECs增殖的显著变化。No significant changes in the proliferation of RCECs and RCjECs were detected using the CCK-8 assay.
上述结果证明,在体外实验中SNPs与RCECs和RCjECs具有很好生物相容性。The above results prove that SNPs have good biocompatibility with RCECs and RCjECs in vitro.
(2)SNPs与细胞的体外黏附性考察(2) In vitro adhesion between SNPs and cells
分为2组,NPs组和SNPs组,NPs和SNPs分别用1,1'-二十八烷基-3,3,3',3'-四甲基茚二碳菁高氯酸盐(DiD)进行荧光标记,然后添加到含有RCECs或RCjECs的培养基中于37℃共同孵育0.5h,孵育后,将细胞用冰冷的PBS洗涤3次,并用含有4,6-二脒基-2-苯基吲哚(DAPI;Vector Laboratories)的vectashield防褪色封固剂封固。使用荧光显微镜(Olympus BX51;Olympus,Tokyo,Japan)捕获图像。对于流式细胞术分析,用PBS洗涤3次后消化收集细胞,然后用Beckman cytoflex S流式细胞仪分析。使用Cytoexpert软件分析结果。。Divided into two groups, NPs group and SNPs group, NPs and SNPs were treated with 1,1'-octadecyl-3,3,3',3'-tetramethylindene dicarbocyanine perchlorate (DiD ) for fluorescent labeling, and then added to the medium containing RCECs or RCjECs and incubated at 37°C for 0.5h. After incubation, the cells were washed 3 times with ice-cold PBS and treated with 4,6-diamidino-2-benzene Mounted with vectashield anti-fade mountant of indole (DAPI; Vector Laboratories). Images were captured using a fluorescence microscope (Olympus BX51; Olympus, Tokyo, Japan). For flow cytometry analysis, cells were collected by digestion after washing 3 times with PBS, and then analyzed with a Beckman cytoflex S flow cytometer. Results were analyzed using Cytoexpert software. .
图2b显示为本实施例的SNPs分别与RCECs和RCjECs共同孵育后的共聚焦图。Fig. 2b shows the confocal images of the SNPs of this example after co-incubating with RCECs and RCjECs respectively.
从图2b可知,SNPs组中细胞RCECs和RCjECs周围有明显的荧光信号。NPs组中细胞RCECs和RCjECs周围的荧光信号非常弱。It can be seen from Figure 2b that there are obvious fluorescent signals around RCECs and RCjECs in the SNPs group. The fluorescent signals around the cells RCECs and RCjECs in the NPs group were very weak.
图2c显示为本实施例的SNPs与RCECs共同孵育后的流式细胞仪定量分析图。Fig. 2c shows the flow cytometry quantitative analysis graph after co-incubating the SNPs and RCECs of this example.
图2d显示为本实施例的SNPs与RCjECs共同孵育后的流式细胞仪定量分析图。Fig. 2d shows the flow cytometry quantitative analysis graph after co-incubating the SNPs of this example and RCjECs.
从图2b和2c可知,流式细胞仪分析进一步证实了用SNPs培养的细胞具有明显更高比例的荧光信号。在定量上,用荧光信号检测到约95%的与SNPs共培养的细胞,而与未包被的NPs共同培养的细胞仅约5%。与SNPs相关的RCECs和RCjECs的平均荧光强度(MFI)分别比NPs高4.6和2.7倍。As can be seen from Figures 2b and 2c, flow cytometry analysis further confirmed that cells cultured with SNPs had a significantly higher proportion of fluorescent signals. Quantitatively, about 95% of cells co-cultured with SNPs were detected with fluorescent signal, while only about 5% of cells co-cultured with uncoated NPs. The mean fluorescence intensity (MFI) of RCECs and RCjECs associated with SNPs was 4.6 and 2.7 times higher than that of NPs, respectively.
为了排除细胞内在化的影响,将细胞与SNPs进一步在4℃孵育,检测到SNPs具有类似的黏附性,表明SNPs在体外具有与RCECs和RCjECs结合的出色能力。In order to rule out the effect of cell internalization, the cells were further incubated with SNPs at 4 °C, and similar adhesion of SNPs was detected, indicating that SNPs have an excellent ability to bind to RCECs and RCjECs in vitro.
(3)SNPs与角膜的体内黏附性考察(3) In vivo adhesion between SNPs and cornea
八周龄雌性C57BL/6小鼠(n=30)购自Charles River。滴1mg/mL DiD标记的NPs和SNPs各5μL于小鼠眼球表面,于0.5h、2h、4h、8h和24h分别死小鼠,取小鼠眼球进行冷冻切片,用DAPI染色细胞核,使用共聚焦激光显微镜(NIKON ECLIPSE TI,日本)拍照来检测SNPs与角膜上皮的粘附性。Eight-week-old female C57BL/6 mice (n=30) were purchased from Charles River.
图2e为本实施例的SNPs和NPs给药小鼠后,不同时间点的共聚焦图。Fig. 2e is a confocal image of mice at different time points after administration of the SNPs and NPs of this example.
从图2e可知,滴加4h后,SNPs组的角膜上形成薄而完整的涂层,且SNPs在角膜上皮上的保留时间可长达24h。It can be seen from Figure 2e that after 4 hours of dripping, a thin and complete coating was formed on the cornea of the SNPs group, and the retention time of SNPs on the corneal epithelium could be as long as 24 hours.
图2f为本实施例的SNPs和NPs给药小鼠后,角膜冷冻切片上的荧光信号计数图。Fig. 2f is a graph of fluorescent signal counts on corneal cryosections after administration of the SNPs and NPs of this example to mice.
从图2f可知,与NPs组相比,SNPs组显著延长了聚合物在角膜前的停留时间。究其原因,SNPs停留时间的延长归因于SMs涂层的存在,SMs涂层可通过整合素β1和纤连蛋白上的RGD序列之间的相互作用而结合到角膜上皮上。From Figure 2f, compared with the NPs group, the SNPs group significantly prolonged the residence time of the polymer in front of the cornea. The reason for this prolongation of the residence time of SNPs was attributed to the presence of SMs coating, which can be bound to the corneal epithelium through the interaction between integrin β1 and the RGD sequence on fibronectin.
(4)通过药物浓度来评估SNPs在眼部的停留时间(4) Evaluate the residence time of SNPs in the eye by drug concentration
新西兰兔子6只分为3组,分别为Dex组、NPs-Dex和SNP-Dex组,分别用20μL的1mg/mL的Dex悬浮液、10mg/mL的NPs-Dex、10mg/mL的SNPs-Dex处理,在滴加0.5h、2h、4h、8h、24h收集眼泪。通过高效液相色谱法(HPLC)测量采集的眼泪中的Dex浓度。Six New Zealand rabbits were divided into 3 groups, namely Dex group, NPs-Dex and SNP-Dex group, respectively, with 20 μL of 1 mg/mL Dex suspension, 10 mg/mL NPs-Dex, and 10 mg/mL SNPs-Dex For treatment, tears were collected after dripping for 0.5h, 2h, 4h, 8h, and 24h. The Dex concentration in the collected tears was measured by high performance liquid chromatography (HPLC).
图2g为本实施例中SNP-Dex给药兔子后,不同时间点眼泪中的Dex的残留率图。Fig. 2g is a diagram of the residual rate of Dex in tears at different time points after SNP-Dex was administered to rabbits in this example.
从图2g可知,与常规滴眼剂相似,施用Dex悬液的兔子眼中Dex残留率在施用后2h显着下降至小于1.0%;NPs-Dex给药后获得了略微增加的保留,但是在给药后6h仍保留不到最初量的Dex的1.0%;但在每个时间点,滴加SNPs-Dex的药物残留浓度均高于Dex组和NPs-Dex组,而且即使给药后时间延长至48h,SNPs-Dex组的Dex残留率仍约为1.5%。说明,即使整个涂层在眼上消失了,细胞中仍残留有药物载体。It can be seen from Figure 2g that, similar to conventional eye drops, the residual rate of Dex in the eyes of rabbits administered with Dex suspension significantly decreased to less than 1.0% 2h after administration; NPs-Dex obtained slightly increased retention after administration, but after administration of 6h after administration, less than 1.0% of the initial amount of Dex was still retained; but at each time point, the drug residual concentration of dripping SNPs-Dex was higher than that of Dex group and NPs-Dex group, and even if the time after administration was extended to At 48h, the residual rate of Dex in the SNPs-Dex group was still about 1.5%. This shows that even though the entire coating is gone on the eye, the drug carrier remains in the cells.
另外,检测了纤连蛋白和整联蛋白-β1在小鼠角膜上皮中的表达。与健康对照相比,尽管纤连蛋白的表达变化可忽略,但损伤后角膜上皮的整合素β1的表达显着降低。即整合素β1的下调可能会限制RGD修饰的聚合物在眼部药物递送的应用。这些结果证实,以眼药水的形式应用SNPs可以大大延长药物在眼部的停留时间。Additionally, the expression of fibronectin and integrin-β1 in mouse corneal epithelium was examined. Integrin β1 expression was significantly reduced in corneal epithelium after injury compared with healthy controls, despite negligible changes in fibronectin expression. That is, the downregulation of integrin β1 may limit the application of RGD-modified polymers in ocular drug delivery. These results confirmed that the application of SNPs in the form of eye drops can greatly prolong the residence time of drugs in the eye.
(5)SNPs对泪膜脂质层的保护性考察(5) The protective effect of SNPs on the tear film lipid layer
分为5组,分别为Normal组、PBS组、NPs组、SMs组和SNPs组。除了Normal组外,其他组的兔子滴加0.1%苯扎氯铵4天,以破坏兔泪膜的稳定性,随后每天滴加PBS、NPs、SMs和SNPs连续3天,每天一次滴眼;然后将5μL2%荧光素钠的无菌PBS滴入下眼睑后,记录从睁眼到观察到角膜上第一个黑点或条纹的时间为兔的泪膜破裂时间TBUT。脂质层厚度LLT使用Lipiview干涉仪(TearScience Inc.,Morrisville,NC)测量,平均LLT在20秒内记录。Divided into 5 groups, namely Normal group, PBS group, NPs group, SMs group and SNPs group. Except for the Normal group, the rabbits in the other groups were dripped with 0.1% benzalkonium chloride for 4 days to destroy the stability of the tear film of the rabbits, followed by daily drops of PBS, NPs, SMs and SNPs for 3 consecutive days, once a day; then After dripping 5 μL of 2% sodium fluorescein in sterile PBS into the lower eyelid, record the time from opening the eyes to observing the first black spot or streak on the cornea as the tear film breakup time TBUT of the rabbit. Lipid layer thickness LLT was measured using a Lipiview interferometer (TearScience Inc., Morrisville, NC), and the average LLT was recorded over 20 seconds.
图3a为本实施例中SNP给药兔子后,泪膜脂质层的典型LipidView图。Fig. 3a is a typical LipidView image of the tear film lipid layer after SNP administration to rabbits in this example.
图3b为本实施例中SNP给药兔子后,泪膜脂质层厚度图。Figure 3b is a diagram of the thickness of the tear film lipid layer after SNP administration in rabbits in this example.
图3c为本实施例中SNP给药兔子后,泪膜的破裂时间图。Fig. 3c is a diagram of tear film breakup time after SNP administration to rabbits in this example.
从图3可知,在苯扎氯氨处理后,兔子的脂质层厚度和泪膜破裂时间均明显减少。与正常对照组相比,用SNPs处理后,兔子在第7天的脂质层厚度的恢复,并恢复了泪膜的稳定性;用SMs治疗导致相似的恢复,表明SMs和相关脂质稳定脂质层的能力。与不含脂质的滴眼液相比,含脂质的泪液补充剂可以改善脂质层的质量,从而保持泪膜稳定性并减少干眼症状。而使用PBS或NPs对脂质层和泪膜没有明显的保护作用。It can be seen from Figure 3 that after the benzalkonium chloride treatment, the lipid layer thickness and tear film break-up time of the rabbits were significantly reduced. Treatment with SNPs restored lipid layer thickness at
实施例8Example 8
本实施例中,采用实施例4得到的SNPs-Dex对小鼠的急性干眼病进行治疗效果考察,包括如下:In this embodiment, the SNPs-Dex obtained in Example 4 is used to investigate the therapeutic effect of acute dry eye disease in mice, including the following:
健康小鼠分为2大组,分别为健康小鼠组和治疗组,每天用PBS的健康小鼠作为正常对照为Normal组;治疗组又分为5组,分别为PBS组、SNPs组、Dex组、NPs-Dex组和SNPs-Dex组。除了Normal组外,各治疗组用0.2%苯扎氯铵滴注诱导7d(定义为第0d)后,再给药治疗7d。PBS组每隔一天用PBS一次;SNPs组每隔一天用5μL10mg/mL的SNPs悬浮液;Dex组每天3次使用1mg/mL的Dex悬浮液;NPs-Dex组每隔一天使用10mg/mL的NPs-Dex悬浮液;SNPs-Dex组每隔一天使用10mg/mL的SNPs-Dex悬浮液。每次每种滴眼液的用量均为5μL。The healthy mice were divided into two groups, namely the healthy mouse group and the treatment group, and the healthy mice with PBS every day were used as the normal control group as the Normal group; the treatment group was divided into 5 groups, respectively, the PBS group, the SNPs group, the Dex group, NPs-Dex group and SNPs-Dex group. Except the Normal group, each treatment group was induced with 0.2% benzalkonium chloride instillation for 7 days (defined as the 0th day), and then administered for another 7 days. The PBS group was given PBS once every other day; the SNPs group was given 5 μL of 10 mg/mL SNPs suspension every other day; the Dex group was given 1 mg/mL Dex suspension three times a day; the NPs-Dex group was given 10 mg/mL NPs every other day -Dex suspension; SNPs-Dex group used 10 mg/mL SNPs-Dex suspension every other day. The dosage of each eye drop is 5 μL each time.
检测内容:1)在第0d和治疗第7天,观察小鼠眼球的明场图像,得到角膜的不透明度分数;并通过荧光素染色后在裂隙灯显微镜下观察,角膜的荧光素染色分数。明场图像由两名角膜专家对彩色数码相机拍摄的图像进行分级,并使用0-4级评分(0=完全清晰,1=轻微模糊,虹膜和瞳孔容易可见,2=轻微不透明,虹膜和瞳孔仍然可检测到,3=不透明,瞳孔几乎检测不到,4=完全不透明,看不到瞳孔)。对于角膜荧光素染色(CFS),将1μL 0.1%荧光素钠滴入结膜囊中,60秒后在裂隙灯显微镜下用钴蓝滤光片捕获角膜。随后,图像被分为五个部分(中央、鼻部、颞部、上部和下部),并使用先前验证的分级系统对每个部分进行评分,如下所示:0=无染色,1=轻微点状染色,2=明显的点状或轻微的聚结染色,3=明显的聚结或轻微的斑片状染色,4=明显的斑片状染色。Test content: 1) On
2)为了评估治疗后的组织学改变,采用苏木精和伊红染色(H&E)以及Masson三色对角膜进行染色,进行组织学检查。2) In order to evaluate the histological changes after treatment, the corneas were stained with hematoxylin and eosin (H&E) and Masson's trichrome for histological examination.
3)为了进一步了解SNPs-Dex的治疗作用,通过免疫荧光染色分析了DED炎症相关细胞因子的表达,以肿瘤坏死因子α(TNF-α)和基质金属蛋白9(MMP-9)为代表性细胞因子。具体操作为:冷冻切片在冷的4%PFA中固定15min,用0.3%Triton X-100透化30min,用PBS中的5%驴血清在室温下封闭1h,并与一抗(TNF-α和MMP-9)在4℃下过夜。洗涤后,切片与生物素化二抗孵育1h,并用DAPI染色细胞核。3) In order to further understand the therapeutic effect of SNPs-Dex, the expressions of DED inflammation-related cytokines were analyzed by immunofluorescence staining, with tumor necrosis factor α (TNF-α) and matrix metalloprotein 9 (MMP-9) as representative cells factor. The specific operation was as follows: frozen sections were fixed in cold 4% PFA for 15 min, permeabilized with 0.3% Triton X-100 for 30 min, blocked with 5% donkey serum in PBS for 1 h at room temperature, and reacted with primary antibodies (TNF-α and MMP-9) overnight at 4°C. After washing, sections were incubated with biotinylated secondary antibody for 1 h, and nuclei were stained with DAPI.
图4a为本实施例的各组小鼠眼球的明场图像。Fig. 4a is the bright-field images of the eyeballs of mice in each group in this embodiment.
图4b为本实施例的各组小鼠眼球经荧光素染色后的图像。Fig. 4b is the images of the eyeballs of mice in each group in this embodiment after being stained with fluorescein.
从图4a和4b可知,Normal组小鼠的角膜是透明的,在荧光素染色后,在裂隙灯显微镜下可显示深蓝色轮廓;而用苯扎氯铵诱导7天(定义为第0天)后,治疗组小鼠的角膜明显不透明,在钴蓝灯下呈绿色。It can be seen from Figures 4a and 4b that the corneas of mice in the Normal group were transparent, and after fluorescein staining, a dark blue outline could be displayed under a slit lamp microscope; Afterwards, the corneas of mice in the treatment group were significantly opaque and appeared green under cobalt blue light.
图4c为本实施例的各组小鼠眼球的角膜的不透明度分数图。Fig. 4c is a graph of the opacity fractions of the corneas of the mouse eyes of each group in this embodiment.
图4d为本实施例的各组小鼠眼球的角膜的荧光素染色分数图。Fig. 4d is a graph of the fluorescein staining fractions of the corneas of the mouse eyeballs in each group in this embodiment.
从图4c和4d可知,在所有治疗组中,SNPs-Dex组小鼠的角膜混浊和荧光素染色得分最低,与Normal组健康小鼠相似。证明,SNPs-Dex具有的优越治疗功效。From Figures 4c and 4d, among all treatment groups, the corneal opacity and fluorescein staining scores of the mice in the SNPs-Dex group were the lowest, similar to those of the healthy mice in the Normal group. It is proved that SNPs-Dex has superior therapeutic efficacy.
图5a为本实施例的各组小鼠角膜切片经H&E和Masson染色图,其中,Epi和End分别代表上皮和内皮,红色箭头表示中性粒细胞浸润,而黑色箭头表示角膜新血管形成。从图5a可知,SNPs-Dex组小鼠的角膜切片显示出多层上皮和规律排列的基质,与Normal组健康小鼠相似。相比之下,PBS组和SNPs组的角膜切片显示上皮细胞排列不良,上皮变薄和不规则纤维化,以及基质中嗜中性粒细胞浸润(红色箭头)和角膜新血管形成(黑色箭头)。NPs-Dex组小鼠的上皮更薄,而中性粒细胞浸润较少。Figure 5a is the H&E and Masson staining images of mouse corneal sections of each group in this example, where Epi and End represent epithelium and endothelium, respectively, red arrows indicate neutrophil infiltration, and black arrows indicate corneal neovascularization. It can be seen from Figure 5a that the corneal sections of the mice in the SNPs-Dex group showed multi-layered epithelium and regularly arranged stroma, similar to the healthy mice in the Normal group. In contrast, corneal sections from the PBS group and the SNPs group showed poor epithelial cell alignment, epithelial thinning, and irregular fibrosis, as well as neutrophil infiltration in the stroma (red arrow) and corneal neovascularization (black arrow) . Mice in the NPs-Dex group had thinner epithelium and less infiltration of neutrophils.
图5b为本实施例的各组小鼠角膜切片的免疫荧光染色图。从图5b可知,苯扎氯铵滴注会导致角膜上皮和基质中TNF-α表达显著增加,以及角膜上皮中MMP-9表达显著增加。与其他组相比,SNPs-Dex组小鼠的角膜中TNF-α和MMP-9表达明显降低。证明,在干眼病DED的小鼠模型中,隔日滴用SNPs-Dex能有效抑制急性角膜炎症,加速角膜上皮修复和恢复角膜透明度。Fig. 5b is the immunofluorescence staining diagram of the mouse corneal sections of each group in this embodiment. It can be seen from Figure 5b that instillation of benzalkonium chloride can lead to a significant increase in the expression of TNF-α in the corneal epithelium and stroma, as well as a significant increase in the expression of MMP-9 in the corneal epithelium. Compared with other groups, the expression of TNF-α and MMP-9 in the cornea of mice in SNPs-Dex group was significantly decreased. It was proved that in the mouse model of dry eye disease DED, every other day drop of SNPs-Dex can effectively inhibit acute corneal inflammation, accelerate corneal epithelial repair and restore corneal transparency.
实施例9Example 9
本实施例中,采用实施例4得到的SNPs-Dex对兔子的慢性干眼病进行治疗效果考察,包括如下:In this embodiment, the SNPs-Dex obtained in Example 4 is used to investigate the therapeutic effect of chronic dry eye disease in rabbits, including the following:
健康兔子分为2大组,分别为健康兔子组和治疗组,健康兔组标记为Normal组;治疗组又分为5组,分别为PBS组、SNPs组、Dex组、NPs-Dex组和SNPs-Dex组。除了Normal组外,各治疗组用0.1%苯扎氯铵连续滴注1个月(定义为第0天)后,再给药治疗14天。Dex组每天给予临床用Dex悬浮液3次,其余各治疗组每隔1d以滴眼剂的形式给药。在对动物实施安乐死后切除角膜和结膜组织。然后将组织固定在4%PFA中并进行石蜡包埋切片。The healthy rabbits were divided into two groups, the healthy rabbit group and the treatment group, and the healthy rabbit group was marked as the Normal group; the treatment group was further divided into 5 groups, namely the PBS group, the SNPs group, the Dex group, the NPs-Dex group and the SNPs group -Dex group. Except the Normal group, each treatment group was instilled with 0.1% benzalkonium chloride continuously for 1 month (defined as day 0), and then administered for another 14 days. The Dex group was given clinical Dex suspension 3 times a day, and the other treatment groups were given eye drops every other day. Corneal and conjunctival tissue were excised after euthanasia of the animals. Tissues were then fixed in 4% PFA and sectioned for paraffin embedding.
检测内容:1)分别在治疗第7天和第14天,观察兔角膜的明场图像。Detection content: 1) Observe the bright-field images of the rabbit cornea on the 7th day and the 14th day of treatment respectively.
2)切片用H&E染色以评估角膜上皮和结膜上皮的形态;采用高碘酸希夫染色料进行可视化和计算杯状细胞数量。2) Sections were stained with H&E to assess the morphology of corneal and conjunctival epithelium; periodate Schiff stain was used for visualization and counting of goblet cell numbers.
3)在第0天、7天和14天,通过Schirmer纸条(天津精明新技术开发有限公司,天津,中国)分别测量各组兔子的泪液分泌功能。3) On
4)用Masson三色染色检测不规则纤维化和胶原沉积。4) Irregular fibrosis and collagen deposition were detected by Masson's trichrome staining.
5)在治疗第7天和第14天,通过末端脱氧核苷酸转移酶dUTP缺口末端标记(TUNEL)染色检查角膜上皮的细胞凋亡水平。5) On the 7th and 14th day of treatment, the apoptosis level of the corneal epithelium was examined by terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) staining.
图6a为本实施例的各组兔角膜的明场图像。Fig. 6a is a bright-field image of rabbit corneas in each group of this embodiment.
从图6a可知,Normal组小鼠的眼球为正常的肌肉血管;而用苯扎氯铵诱导1个月(定义为第0天)后,治疗组各组兔子的眼球可见明显的睫状充血。用SNPs-Dex治疗后,睫状充血明显减少,并在第14天几乎消失;PBS组的兔子在此期间仍然有明显的睫状充血;与PBS相比,Dex组或NPs-Dex组兔子的睫状充血有轻度改善,但在第14天仍然存在明显的睫状充血。It can be seen from Figure 6a that the eyeballs of the mice in the Normal group were normal muscle blood vessels; and after being induced with benzalkonium chloride for 1 month (defined as day 0), the eyeballs of the rabbits in the treatment groups showed obvious ciliary hyperemia. After treatment with SNPs-Dex, the ciliary hyperemia was significantly reduced and almost disappeared on the 14th day; the rabbits in the PBS group still had obvious ciliary hyperemia during this period; There was mild improvement in ciliary hyperemia, but significant ciliary hyperemia remained on
图6b为本实施例的各组兔结膜经高碘酸席夫染色后的切片图。Fig. 6b is a section view of the conjunctiva of rabbits in each group in this embodiment after Schiff staining with periodic acid.
图6c为本实施例的各组兔结膜切片每个视野在显微镜下的杯状细胞数图。Fig. 6c is a diagram of the number of goblet cells under a microscope in each field of view of the rabbit conjunctival slices of each group in this embodiment.
从图6c可知,在第7天,各治疗组的杯状细胞数均显着低于健康组,SNPs-Dex组的杯状细胞数高于PBS组(p<0.01);在第14天,SNPs-Dex组的杯状细胞数量增加,与Normal健康组几乎相同,而其余治疗组的杯状细胞数量仍少于正常组(p<0.001)。It can be seen from Figure 6c that on the 7th day, the number of goblet cells in each treatment group was significantly lower than that in the healthy group, and the number of goblet cells in the SNPs-Dex group was higher than that in the PBS group (p<0.01); on the 14th day, The number of goblet cells in the SNPs-Dex group increased, which was almost the same as that in the Normal healthy group, while the number of goblet cells in the rest of the treatment groups was still less than that in the normal group (p<0.001).
图6d为本实施例的各组兔Schirmer检测结果。Figure 6d shows the Schirmer detection results of rabbits in each group in this embodiment.
从图6d可知,与Normal组相比,各治疗组所有兔子的湿润长度急剧减少(p<0.001);治疗7天后,SNPs-Dex组的兔子的泪液分泌显着增加,且与健康对照无明显差异,但其他治疗组的所有兔子的泪液分泌都严重减少;治疗第14天,其余治疗组的兔子的泪液分泌仍然不足。It can be seen from Figure 6d that compared with the Normal group, the wet length of all rabbits in each treatment group decreased sharply (p<0.001); after 7 days of treatment, the tear secretion of rabbits in the SNPs-Dex group increased significantly, and there was no significant difference from the healthy control However, tear secretion was severely reduced in all rabbits in the other treatment groups; on the 14th day of treatment, the tear secretion in the rabbits in the remaining treatment groups was still insufficient.
图7a为本实施例的各组兔在治疗第7天和第14天的角膜经H&E染色图。Fig. 7a is the H&E staining images of the corneas of rabbits in each group in the present embodiment on the 7th day and the 14th day of treatment.
从图7a可知,在治疗第7天,SNPs-Dex组的角膜切片保持多层上皮和有序的基质层。It can be seen from Figure 7a that on the 7th day of treatment, the corneal slices of the SNPs-Dex group maintained multi-layered epithelium and ordered stroma layers.
图7b为本实施例的各组兔经TUNEL染色后上皮细胞凋亡的水平图。Fig. 7b is a level diagram of epithelial cell apoptosis after TUNEL staining in each group of rabbits in this example.
从图7b可知,在治疗第0天,Normal组兔子的角膜上皮,未观察到细胞凋亡;而在苯扎氯铵诱导后,各治疗组的细胞凋亡水平显著增加。PBS组,经TUNEL染色的阳性细胞数量随时间增加。SNPs-Dex组,可显著抑制细胞凋亡(第7天p<0.01,第14天p<0.001),表明治疗效果显着。Dex组,也可以有效抑制细胞凋亡,但是其角膜上皮在第14天仍然出现了TUNEL染色阳性细胞。It can be seen from Figure 7b that on the 0th day of treatment, no apoptosis was observed in the corneal epithelium of rabbits in the Normal group; however, after benzalkonium chloride induction, the level of apoptosis in each treatment group increased significantly. In PBS group, the number of positive cells stained by TUNEL increased with time. The SNPs-Dex group can significantly inhibit cell apoptosis (p<0.01 on the 7th day, p<0.001 on the 14th day), indicating that the treatment effect is significant. Dex group can also effectively inhibit cell apoptosis, but TUNEL staining positive cells still appear in the corneal epithelium on the 14th day.
综上可知,在DED兔模型中,SNPs-Dex不仅可以有效抑制眼表炎症,而且还可以恢复结膜杯状细胞和泪液分泌功能。更重要的是,SNPs-Dex的治疗功效要优于临床药物,这得益于SNPs-Dex组药物在眼表面的持续释放。In summary, in the DED rabbit model, SNPs-Dex can not only effectively inhibit ocular surface inflammation, but also restore the function of conjunctival goblet cells and tear secretion. More importantly, the therapeutic efficacy of SNPs-Dex is superior to that of clinical drugs, which is due to the sustained release of drugs in the SNPs-Dex group on the ocular surface.
上述实施例仅例示性说明本发明的原理及其功效,而非用于限制本发明。任何熟悉此技术的人士皆可在不违背本发明的精神及范畴下,对上述实施例进行修饰或改变。因此,举凡所属技术领域中具有通常知识者在未脱离本发明所揭示的精神与技术思想下所完成的一切等效修饰或改变,仍应由本发明的权利要求所涵盖。The above-mentioned embodiments only illustrate the principles and effects of the present invention, but are not intended to limit the present invention. Anyone skilled in the art can modify or change the above-mentioned embodiments without departing from the spirit and scope of the present invention. Therefore, all equivalent modifications or changes made by those skilled in the art without departing from the spirit and technical ideas disclosed in the present invention should still be covered by the claims of the present invention.
序列表sequence listing
<110> 上海交通大学医学院附属第九人民医院<110> Ninth People's Hospital Affiliated to Shanghai Jiaotong University School of Medicine
<120> 用于眼部靶向递药的细胞膜、药物载体、组合物及其制备方法和用途<120> Cell membrane, drug carrier, composition, preparation method and use thereof for ocular targeted drug delivery
<160> 1<160> 1
<170> SIPOSequenceListing 1.0<170> SIP Sequence Listing 1.0
<210> 1<210> 1
<211> 2400<211> 2400
<212> DNA<212>DNA
<213> SEQ ID NO:1(Artificial Sequence)<213> SEQ ID NO: 1 (Artificial Sequence)
<400> 1<400> 1
gccaccatga atttacaacc aattttctgg attggactga tcagttcagt ttgctgtgtg 60gccaccatga atttacaacc aattttctgg attggactga tcagttcagt ttgctgtgtg 60
tttgctcaaa cagatgaaaa tagatgttta aaagcaaatg ccaaatcatg tggagaatgt 120tttgctcaaa cagatgaaaa tagatgttta aaagcaaatg ccaaatcatg tggagaatgt 120
atacaagcag ggccaaattg tgggtggtgc acaaattcaa catttttaca ggaaggaatg 180atacaagcag ggccaaattg tgggtggtgc acaaattcaa catttttaca ggaaggaatg 180
cctacttctg cacgatgtga tgatttagaa gccttaaaaa agaagggttg ccctccagat 240cctacttctg cacgatgtga tgatttagaa gccttaaaaa agaagggttg ccctccagat 240
gacatagaaa atcccagagg ctccaaagat ataaagaaaa ataaaaatgt aaccaaccgt 300gacatagaaa atcccagagg ctccaaagat ataaagaaaa ataaaaatgt aaccaaccgt 300
agcaaaggaa cagcagagaa gctcaagcca gaggatatta ctcagatcca accacagcag 360agcaaaggaa cagcagagaa gctcaagcca gaggatatta ctcagatcca accacagcag 360
ttggttttgc gattaagatc aggggagcca cagacattta cattaaaatt caagagagct 420ttggttttgc gattaagatc aggggagcca cagacattta cattaaaatt caagagagct 420
gaagactatc ccattgacct ctactacctt atggacctgt cttactcaat gaaagacgat 480gaagactatc ccattgacct ctactacctt atggacctgt cttactcaat gaaagacgat 480
ttggagaatg taaaaagtct tggaacagat ctgatgaatg aaatgaggag gattacttcg 540ttggagaatg taaaaagtct tggaacagat ctgatgaatg aaatgaggag gattacttcg 540
gacttcagaa ttggatttgg ctcatttgtg gaaaagactg tgatgcctta cattagcaca 600gacttcagaa ttggatttgg ctcatttgtg gaaaagactg tgatgcctta cattagcaca 600
acaccagcta agctcaggaa cccttgcaca agtgaacaga actgcaccag cccatttagc 660acaccagcta agctcaggaa cccttgcaca agtgaacaga actgcaccag cccatttagc 660
tacaaaaatg tgctcagtct tactaataaa ggagaagtat ttaatgaact tgttggaaaa 720tacaaaaatg tgctcagtct tactaataaa ggagaagtat ttaatgaact tgttggaaaa 720
cagcgcatat ctggaaattt ggattctcca gaaggtggtt tcgatgccat catgcaagtt 780cagcgcatat ctggaaattt ggattctcca gaaggtggtt tcgatgccat catgcaagtt 780
gcagtttgtg gatcactgat tggctggagg aatgttacac ggctgctggt gttttccaca 840gcagtttgtg gatcactgat tggctggagg aatgttacac ggctgctggt gttttccaca 840
gatgccgggt ttcactttgc tggagatggg aaacttggtg gcattgtttt accaaatgat 900gatgccgggt ttcactttgc tggagatggg aaacttggtg gcattgtttt accaaatgat 900
ggacaatgtc acctggaaaa taatatgtac acaatgagcc attattatga ttatccttct 960ggacaatgtc acctggaaaa taatatgtac acaatgagcc attattatga ttatccttct 960
attgctcacc ttgtccagaa actgagtgaa aataatattc agacaatttt tgcagttact 1020attgctcacc ttgtccagaa actgagtgaa aataatattc agacaatttt tgcagttact 1020
gaagaatttc agcctgttta caaggagctg aaaaacttga tccctaagtc agcagtagga 1080gaagaatttc agcctgttta caaggagctg aaaaacttga tccctaagtc agcagtagga 1080
acattatctg caaattctag caatgtaatt cagttgatca ttgatgcata caattccctt 1140acattatctg caaattctag caatgtaatt cagttgatca ttgatgcata caattccctt 1140
tcctcagaag tcattttgga aaacggcaaa ttgtcagaag gcgtaacaat aagttacaaa 1200tcctcagaag tcattttgga aaacggcaaa ttgtcagaag gcgtaacaat aagttacaaa 1200
tcttactgca agaacggggt gaatggaaca ggggaaaatg gaagaaaatg ttccaatatt 1260tcttactgca agaacggggt gaatggaaca ggggaaaatg gaagaaaatg ttccaatatt 1260
tccattggag atgaggttca atttgaaatt agcataactt caaataagtg tccaaaaaag 1320tccatggag atgaggttca atttgaaatt agcataactt caaataagtg tccaaaaaag 1320
gattctgaca gctttaaaat taggcctctg ggctttacgg aggaagtaga ggttattctt 1380gattctgaca gctttaaaat taggcctctg ggctttacgg aggaagtaga ggttattctt 1380
cagtacatct gtgaatgtga atgccaaagc gaaggcatcc ctgaaagtcc caagtgtcat 1440cagtacatct gtgaatgtga atgccaaagc gaaggcatcc ctgaaagtcc caagtgtcat 1440
gaaggaaatg ggacatttga gtgtggcgcg tgcaggtgca atgaagggcg tgttggtaga 1500gaaggaaatg ggacatttga gtgtggcgcg tgcaggtgca atgaagggcg tgttggtaga 1500
cattgtgaat gcagcacaga tgaagttaac agtgaagaca tggatgctta ctgcaggaaa 1560cattgtgaat gcagcacaga tgaagttaac agtgaagaca tggatgctta ctgcaggaaa 1560
gaaaacagtt cagaaatctg cagtaacaat ggagagtgcg tctgcggaca gtgtgtttgt 1620gaaaacagtt cagaaatctg cagtaacaat ggagagtgcg tctgcggaca gtgtgtttgt 1620
aggaagaggg ataatacaaa tgaaatttat tctggcaaat tctgcgagtg tgataatttc 1680aggaagagggg ataatacaaa tgaaatttat tctggcaaat tctgcgagtg tgataatttc 1680
aactgtgata gatccaatgg cttaatttgt ggaggaaatg gtgtttgcaa gtgtcgtgtg 1740aactgtgata gatccaatgg cttaatttgt ggaggaaatg gtgtttgcaa gtgtcgtgtg 1740
tgtgagtgca accccaacta cactggcagt gcatgtgact gttctttgga tactagtact 1800tgtgagtgca accccaacta cactggcagt gcatgtgact gttctttgga tactagtact 1800
tgtgaagcca gcaacggaca gatctgcaat ggccggggca tctgcgagtg tggtgtctgt 1860tgtgaagcca gcaacggaca gatctgcaat ggccggggca tctgcgagtg tggtgtctgt 1860
aagtgtacag atccgaagtt tcaagggcaa acgtgtgaga tgtgtcagac ctgccttggt 1920aagtgtacag atccgaagtt tcaagggcaa acgtgtgaga tgtgtcagac ctgccttggt 1920
gtctgtgctg agcataaaga atgtgttcag tgcagagcct tcaataaagg agaaaagaaa 1980gtctgtgctg agcataaaga atgtgttcag tgcagagcct tcaataaagg agaaaagaaa 1980
gacacatgca cacaggaatg ttcctatttt aacattacca aggtagaaag tcgggacaaa 2040gacacatgca cacaggaatg ttcctatttt aacattacca aggtagaaag tcgggacaaa 2040
ttaccccagc cggtccaacc tgatcctgtg tcccattgta aggagaagga tgttgacgac 2100ttaccccagc cggtccaacc tgatcctgtg tcccattgta aggagaagga tgttgacgac 2100
tgttggttct attttacgta ttcagtgaat gggaacaacg aggtcatggt tcatgttgtg 2160tgttggttct attttacgta ttcagtgaat gggaacaacg aggtcatggt tcatgttgtg 2160
gagaatccag agtgtcccac tggtccagac atcattccaa ttgtagctgg tgtggttgct 2220gagaatccag agtgtcccac tggtccagac atcattccaa ttgtagctgg tgtggttgct 2220
ggaattgttc ttattggcct tgcattactg ctgatatgga agcttttaat gataattcat 2280ggaattgttc ttaattggcct tgcattactg ctgatatgga agcttttaat gataattcat 2280
gacagaaggg agtttgctaa atttgaaaag gagaaaatga atgccaaatg ggacacgggt 2340gacagaaggg agtttgctaa atttgaaaag gagaaaatga atgccaaatg ggacacgggt 2340
gaaaatccta tttataagag tgccgtaaca actgtggtca atccgaagta tgagggaaaa 2400gaaaatccta tttataagag tgccgtaaca actgtggtca atccgaagta tgagggaaaa 2400
Claims (10)
Priority Applications (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| CN202111144615.8ACN115869416A (en) | 2021-09-28 | 2021-09-28 | Cell membrane, drug carrier and composition for ocular targeted drug delivery, and preparation method and application thereof |
Applications Claiming Priority (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| CN202111144615.8ACN115869416A (en) | 2021-09-28 | 2021-09-28 | Cell membrane, drug carrier and composition for ocular targeted drug delivery, and preparation method and application thereof |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| CN115869416Atrue CN115869416A (en) | 2023-03-31 |
Family
ID=85763633
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| CN202111144615.8APendingCN115869416A (en) | 2021-09-28 | 2021-09-28 | Cell membrane, drug carrier and composition for ocular targeted drug delivery, and preparation method and application thereof |
Country Status (1)
| Country | Link |
|---|---|
| CN (1) | CN115869416A (en) |
Citations (6)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| EP1279404A1 (en)* | 2001-07-26 | 2003-01-29 | Istituto Superiore di Sanità | Use of HIV-1 tat, fragments or derivatives thereof, to target or to activate antigen-presenting cells, to deliver cargo molecules for vaccination or to treat other diseases |
| US20030207833A1 (en)* | 2002-02-25 | 2003-11-06 | Neil Berkley | Pharmaceutical compositions with minicells |
| US20160346203A1 (en)* | 2013-09-17 | 2016-12-01 | Rijksuniversiteit Groningen | Means and methods for ocular drug delivery |
| CN107961229A (en)* | 2017-11-22 | 2018-04-27 | 西南大学 | Target preparation method of cellular membrane biomimetic delivery system of atherosclerotic lesion and products thereof and application |
| CN108699110A (en)* | 2015-10-23 | 2018-10-23 | 特温特大学 | Integrin-binding peptides and uses thereof |
| CN113425701A (en)* | 2021-04-29 | 2021-09-24 | 赵晨 | Macrophage membrane coated choroid neovascularization targeted nanoparticles and preparation method thereof |
- 2021
- 2021-09-28CNCN202111144615.8Apatent/CN115869416A/enactivePending
Patent Citations (6)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| EP1279404A1 (en)* | 2001-07-26 | 2003-01-29 | Istituto Superiore di Sanità | Use of HIV-1 tat, fragments or derivatives thereof, to target or to activate antigen-presenting cells, to deliver cargo molecules for vaccination or to treat other diseases |
| US20030207833A1 (en)* | 2002-02-25 | 2003-11-06 | Neil Berkley | Pharmaceutical compositions with minicells |
| US20160346203A1 (en)* | 2013-09-17 | 2016-12-01 | Rijksuniversiteit Groningen | Means and methods for ocular drug delivery |
| CN108699110A (en)* | 2015-10-23 | 2018-10-23 | 特温特大学 | Integrin-binding peptides and uses thereof |
| CN107961229A (en)* | 2017-11-22 | 2018-04-27 | 西南大学 | Target preparation method of cellular membrane biomimetic delivery system of atherosclerotic lesion and products thereof and application |
| CN113425701A (en)* | 2021-04-29 | 2021-09-24 | 赵晨 | Macrophage membrane coated choroid neovascularization targeted nanoparticles and preparation method thereof |
Non-Patent Citations (1)
| Title |
|---|
| LIANGBO CHEN ET AL.: ""Therapeutic nanocoating of ocular surface"", 《NANO TODAY》, vol. 41, 2 October 2021 (2021-10-02), pages 1 - 13, XP086878097, DOI: 10.1016/j.nantod.2021.101309* |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| Lopez-Cano et al. | Liposomes as vehicles for topical ophthalmic drug delivery and ocular surface protection | |
| Radwan et al. | Hyaluronic-coated albumin nanoparticles for the non-invasive delivery of apatinib in diabetic retinopathy | |
| Cheng et al. | Thermosensitive chitosan-based hydrogel as a topical ocular drug delivery system of latanoprost for glaucoma treatment | |
| Chen et al. | Tacrolimus loaded cationic liposomes for dry eye treatment | |
| Radwan et al. | Chitosan-coated bovine serum albumin nanoparticles for topical tetrandrine delivery in glaucoma: in vitro and in vivo assessment | |
| Tseng et al. | Cationic gelatin nanoparticles for drug delivery to the ocular surface: in vitro and in vivo evaluation | |
| CN108976288B (en) | Lipophilic derivatives based on wild-type membrane-penetrating peptide penetratin | |
| Wu et al. | Cell penetrating peptide TAT-functionalized liposomes for efficient ophthalmic delivery of flurbiprofen: penetration and its underlying mechanism, retention, anti-inflammation and biocompatibility | |
| Sakai et al. | Prolonged protective effect of basic fibroblast growth factor–impregnated nanoparticles in royal college of surgeons rats | |
| JP2020526574A5 (en) | ||
| Li et al. | Glycopeptide-nanotransforrs eyedrops with enhanced permeability and retention for preventing fundus neovascularization | |
| Eriksen et al. | Multifarious biologic loaded liposomes that stimulate the mammalian target of rapamycin signaling pathway show retina neuroprotection after retina damage | |
| Li et al. | A novel, liposome-loaded, injectable hydrogel for enhanced treatment of choroidal neovascularization by sub-tenon's injection | |
| Wong et al. | Mucin-targeting-aptamer functionalized liposomes for delivery of cyclosporin A for dry eye diseases | |
| CN113425701A (en) | Macrophage membrane coated choroid neovascularization targeted nanoparticles and preparation method thereof | |
| Chen et al. | Therapeutic nanocoating of ocular surface | |
| RU2561585C2 (en) | Local eye peptide composition | |
| Sun et al. | Combined anti-angiogenic and anti-inflammatory nanoformulation for effective treatment of ocular vascular diseases | |
| Wang et al. | Non-viral gene coating modified IOL delivering PDGFR-α shRNA interferes with the fibrogenic process to prevent posterior capsular opacification | |
| CN107638405A (en) | A kind of intraocular passs drug composition and preparation method thereof | |
| Xu et al. | Deliver protein across bio-barriers via hexa-histidine metal assemblies for therapy: a case in corneal neovascularization model | |
| Sun et al. | Topical ophthalmic liposomes dual-modified with penetratin and hyaluronic acid for the noninvasive treatment of neovascular age-related macular degeneration | |
| CN118108804B (en) | A hypoxia-sensitive functionalized anti-angiogenic polypeptide, nano-micelle material, and preparation method and application thereof | |
| CN115869416A (en) | Cell membrane, drug carrier and composition for ocular targeted drug delivery, and preparation method and application thereof | |
| CN118902984A (en) | Sequential controlled release double-drug loaded injectable hydrogel and preparation method and application thereof |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PB01 | Publication | ||
| PB01 | Publication | ||
| SE01 | Entry into force of request for substantive examination | ||
| SE01 | Entry into force of request for substantive examination |